fenestrae, glomerular basement membrane, and filtration slits. Using as inputs certain morphometric data from rats and the human glomerular diseases.
|
|
|
- Abigayle Franklin
- 5 years ago
- Views:
Transcription
1 Structural Basis for Reduced Glomerular Filtration Capacity in Nephrotic Humans M. Claudia Drumond,* Batya Kristal,* Bryan D. Myers, and William M. Deen* *Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139; and Division, Stanford University School of Medicine, Stanford, California Nephrology Abstract Previous studies have established that in a variety of human glomerulopathies the reduced glomerular filtration rate (GFR) is due to a marked lowering of the ultrafiltration coefficient (Kf). To identify the factors which lower Kf, we measured the filtering surface area per glomerulus, filtration slit frequency, basement membrane thickness, and GFR and its determinants in patients with minimal change and membranous nephropathies and in age-matched healthy controls. Overall values of Kf for the two kidneys were calculated from GFR, renal plasma flow rate, systemic colloid osmotic pressure, and three assumed values for the transcapillary pressure difference. "Experimental" values of the glomerular hydraulic permeability (kep) were then calculated from Kf, glomerular filtering surface area, and estimates of the total number of nephrons of the two kidneys. Independent estimates of the glomerular hydraulic permeability (kmodh) were obtained using a recent mathematical model that is based on analyses of viscous flow through the various structural components of the glomerular capillary wall. Individual values of basement membrane thickness and filtration slit frequency were used as inputs in this model. The results indicate that the reductions of Kf in both nephropathies can be attributed entirely to reduced glomerular hydraulic permeability. The mean values of kp and ko.de, were very similar in both disorders and much smaller in the nephrotic groups than in healthy controls. There was good agreement between k.ep and k..md for any given group of subjects. It was shown that, in both groups of nephrotics, filtration slit frequency was a more important determinant of the water flow resistance than was basement membrane thickness. The decrease in filtration slit frequency observed in both disorders caused the average path length for the filtrate to increase, thereby explaining the decreased hydraulic permeability. (J. Clin. Invest : ) Key words: membranous nephropathy - iinimal change nephropathy * ultrafiltration coefficient. hydraulic permeability * glomerular capillary morphometry Address correspondence to William M. Deen, Department of Chemical Engineering, , Massachusetts Institute of Technology, Cambridge, MA Received for publication 6 December 1993 and in revised form 31 May J. Clin. Invest. The American Society for Clinical Investigation, Inc /94/09/1187/09 $2.00 Volume 94, September 1994, Introduction Studies of a variety of human glomerulopathies have demonstrated that the overall ultrafiltration coefficient (Kf)1 for the two kidneys is reduced relative to that in healthy controls (1-5). Although the inability to measure the glomerular transcapillary hydraulic pressure difference (AP) in humans precludes precise calculations of Kf, the reductions in Kf computed for nephrotic individuals are typically so large that there is little doubt that glomerular ultrafiltration capacity is severely compromised in such disorders. Because Kf as determined in these human studies is the product of the hydraulic permeability of the glomerular capillary wall (k) and the total capillary surface area available for filtration in the two kidneys, the observed reductions in Kf might be due to decreased k, decreased surface area per glomerulus, a decreased number of functioning glomeruli, or some combination of these factors. Measurements have been reported for some of the key quantities in humans which should influence Kf, including surface area per glomerulus (1, 2, 4, 6), basement membrane thickness (1-4, 6, 7), and filtration slit frequency, which is the number of filtration slits per unit length of peripheral capillary wall (1-4). Reductions in filtration slit frequency reflect broadening and "effacement" of the epithelial foot processes, a uniform finding in virtually all humans with the nephrotic syndrome, regardless of its etiology. The most consistent inference from morphometric analysis of glomeruli of nephrotic individuals has been a strong correlation between decreases in estimated Kf and reductions in filtration slit frequency ( 8-11 ). Efforts to ascertain whether changes in filtration slit frequency or other factors can account for the observed alterations in Kf have been hampered by an inadequate understanding of the relationship between the various structural quantities and k. These relationships have been clarified recently by a mathematical model of the glomerular capillary wall, which is based on detailed analyses of viscous flow across the various structural components (12, 13). The structures include the endothelial fenestrae, glomerular basement membrane, and filtration slits. Using as inputs certain morphometric data from rats and the hydraulic permeability measured for isolated rat glomerular basement membrane, this "ultrastructural model" was able to predict a value for k that is in good agreement with micropuncture measurements in normal rats (13). It was also able to explain changes in k in an experimental rat model of glomerular injury, suggesting that it might be useful in interpreting data in human glomerular diseases. 1. Abbreviations used in this paper: k, hydraulic permeability of the glomerular capillary wall; Kf, ultrafiltration coefficient; MAP, mean arterial pressure; PAH, para-aminohippuric acid; RPF, rate of plasma flow. Structural Basis for Reduced Glomerular Filtration Capacity 1187
2 The objective of this study was to apply the ultrastructural model to membranous nephropathy and minimal change nephropathy, two human glomerulopathies in which Kf is estimated to decline markedly, despite preservation of the surface area available for filtration ( 1, 10). To elucidate the biophysical basis for reduced GFR and Kf in these disorders, we combined an assessment of glomerular filtration dynamics with a morphometric analysis of glomeruli obtained by renal biopsy. Methods Patient population The subjects of our study were 34 consecutive adult patients below 60 yr of age, who were referred to one of us (B. D. Myers) because of a nephrotic syndrome and a histopathological diagnosis of either membranous nephropathy (n = 23 ) or minimal change nephropathy (n = 11 ). They were between 18 and 59 yr old, and 20 of the 34 were male. 36 healthy volunteers, matched for age (21-54) and gender (19 males), served as controls. They were divided into two groups. Healthy control group 1 (n = 24) underwent a physiological evaluation of glomerular function. Healthy control group 2 (n = 12) were living kidney transplant donors, who underwent a morphometric analysis of glomeruli obtained by kidney biopsy at the time of transplantation. Each control subject denied a history of renal disease, hypertension, or diabetes and was found to be normotensive and normoglycemic and to have a negative dipstick test for urinary protein at the time of evaluation. Physiologic evaluation Patients and healthy control group 1 consented to undergo differential solute clearances according to a protocol which had been approved previously by the Institutional Review Board at the Stanford University School of Medicine. Each was admitted to a Clinical Research Center on the morning of the study. Antihypertensive agents were withdrawn 48 h before admission in all patients receiving such therapy. Urine was voided spontaneously after diuresis had been established with an oral water load (10-15 mg/kg). A priming dose of inulin (50 mg/kg) and para-aminohippuric acid (PAH, 12 mg/kg) was then administered. Thereafter, inulin and PAH were given by continuous infusion to maintain plasma levels at 20 and 1.5 mg/dl, respectively. 60 min after the priming dose, arterial blood pressure was determined, and blood was sampled for examination of systemic colloid osmotic pressure (1rA) and plasma concentrations of albumin and IgG. Four timed urine collections were then made, each of which was bracketed by a blood sample drawn from a peripheral vein. GFR was expressed as the average value for the four timed inulin clearances. The rate of plasma flow (RPF) was estimated by dividing the corresponding clearance of PAH by an estimate of the prevailing renal arteriovenous extraction ratio for PAH. We have shown previously that reductions of GFR and peritubular capillary protein concentration exert an additive effect to lower the extraction ratio for PAH in patients with glomerular disease (14). Based on the observed relationships, we assigned a value for the extraction ratio of PAH of 0.9 to healthy controls and 0.8 or 0.7 to the nephrotic patients with a normalized GFR above or below 80 ml/ min per 1.73 m2, respectively. The concentrations of inulin and PAH were determined with an automated assay (10). The concentrations of endogenous albumin and IgG in serum and urine were determined immunochemically, and the colloid osmotic pressure of plasma was determined by membrane osmometry, as described previously (15). The GFR, RPF, and 1rA were used together with the model of Deen et al. (16) to calculate values of Kf for each individual, expressed as the total Kf for all nephrons in the two kidneys. Because the glomerular transcapillary hydraulic pressure difference (AsP) could not be measured, we used assumed values of 35, 40, and 45 mmhg; the lower values are representative of micropuncture results in normal rats (17), whereas the highest value corresponds to moderate glomerular hypertension. The fractional clearances of albumin (0ib) and IgG (6jG) were determined by dividing the clearances of albumin and IgG, respectively, by that of inulin. The fractional clearances of albumin and IgG were measured in only 21 of the 24 individuals of healthy control group 1. Morphometric measurements Light microscopy. The biopsies of all nephrotic patients were performed contemporaneously with clearance determinations and before the initiation of specific treatment. Paraffin-embedded tissue was sectioned at 1- /sm intervals and stained with periodic acid-schiff reagent. On average, 19 glomeruli per biopsy were analyzed at the light microscopic level in each nephrotic patient (range 7-30). The average number of glomeruli among the 12 control biopsies was also 19 (range 13-30). A dedicated computer system (Southern Micro Instruments, Inc., Atlanta, GA), consisting of a video camera, screen, microscope, and digitizing tablet, was used to perform measurements ( 1, 2). The outline of each glomerular tuft in the cross-section was traced onto the digitizing tablet at a magnification of 900, and the cross-sectional area of the tuft (AG) was computed using area perimeter analysis. Glomerular volume (VG) was calculated from the measured AG and corrected to account for tissue shrinkage associated with paraffin embedding, using a linear shrinkage factor (f,) (18): VG - dag s (1) where 63 is a dimensionless "shape coefficient" (,/ = 1.38 for spheres) and d is a "size distribution coefficient" which is introduced to account for variations in glomerular size (18). We used d = 1.1 as in previous studies ( 1-4), which corresponds to a distribution of glomerular sizes with a standard deviation of 25% of the mean size (18). We determined that, in our experimental procedure for tissue fixation, the value - of the shrinkage factor is Af = 0.86, and used this value in Eq. 1. The numbers of patent (np) and globally sclerosed (ns) glomeruli were counted in sections of cortical tissue. The percentage of sclerosed glomeruli (G) was calculated by G = Ds *100 ns + n Ds (2) where Dp and Ds are the diameters of patent and sclerosed glomeruli, respectively, which are proportional to the square root of the tuft crosssectional area. The ratio Dp/Ds accounts for the differences in size between patent and sclerosed glomeruli and the consequent difference in the probability of encountering a glomerulus of either type in a random cross-section. Electron microscopy. For transmission electron microscopy, the tissue was fixed in 2.5% glutaraldehyde and embedded in epon. Toluidine blue-stained sections were then surveyed to locate the two patent glomeruli closest to the center of each section. Ultrathin sections (60-70 nm) of the selected glomeruli were next stained with uranyl acetate and lead citrate and photographed. A complete montage of each glomerulus was prepared, and point and intercept counting at a magnification of 2,820 was used to determine the peripheral capillary surface area (S), which was defined as the interface between the peripheral capillary wall and epithelium, and calculated as S = SVVG (3) where sv is the surface density of peripheral capillary wall (expressed as length of peripheral capillary wall per unit cross-sectional area of glomerulus). Six to eight high-power electron photomicrographs ( x 1 1,280) were then obtained from each of the two glomerular profiles to evaluate the thickness of the glomerular basement membrane and the frequency of epithelial filtration slits. The harmonic mean basement membrane thickness (6bt.) was calcu M. C. Drumond, B. Kristal, B. D. Myers, and W. M. Deen
3 6 bm Epithelium Basement membrane Endothelium Table I. Inputs for Ultrastructural Model Quantity Value Permeability of endothelium (ken) 2.0 x 1O- m/s/pa Permeability of epithelial slits (Az) 7.9 X 10-8 mrs/pa Width of structural unit (W) Individual data Width of epithelial slits (W1) 39 nm Basement membrane thickness (6bm) Individual data Darcy permeability of basement membrane (KD) 2.7 nm2 Fractional area of fenestrae (Ef) 0.2 Number density of fenestrae (n1iw) 8.33 x 106 m-' W Figure 1. Schematic representation of the glomerular capillary wall showing the fenestrated endothelium, the basement membrane (of thickness 6b.), and the epithelial foot processes connected by the slit diaphragms. The width of a repeating structural unit is W, and the width of the slit channel at the level of the slit diaphragm is Ws. lated for each individual from the measured (apparent) harmonic mean thickness (6lt ) by 6t. 867 = 3ir bm (4) where 8/(37r) is a correction factor derived by Jensen et al. (19) to account for the random angle of sectioning. The filtration slit frequency was determined by counting the total number of slits and dividing it by the total length of peripheral capillary wall captured on the electron micrographs ( 1, 10). Calculation of k from hemodynamic and morphometric data k is given by the ultrafiltration coefficient divided by the peripheral capillary surface area. The experimental estimate of k from the hemodynamic data (k,,p) was obtained from Kf, the surface area per glomerulus (S), the percentage of sclerosed glomeruli (G), and the total number of glomeruli in the two kidneys (n) by k K, (5) ns(i - G/l00) We assumed n = 2 x 106 (20) for all subjects. The value of kep was calculated for each individual with membranous nephropathy or minimal change nephropathy. To obtain kap for healthy controls, we used the individual morphometric data from healthy control group 2 together with the mean hemodynamic data from healthy control group 1. Calculation of k using ultrastructural model Estimates of k independent of those given by Eq. 5 were obtained from filtration slit frequency and basement membrane thickness by using the model of Drumond and Deen (13), in which the capillary wall is approximated as consisting of a large number of repeating structural units. As illustrated in Fig. 1, each structural unit is based on a single filtration slit. The width of a structural unit (W) was calculated from the filtration slit frequency (FSF) by W = 2 1 (6) 7r FSF I where 2/ir is a correction factor, derived in the Appendix, which accounts for the random angle of sectioning. Using the concept of resistances in series, the overall hydraulic permeability of the capillary wall was calculated from the permeabilities of each layer by I -1 I I - (7) k. kb. kp where ks,, kbm, and kep are, respectively, the hydraulic permeabilities of the endothelium, basement membrane, and epithelium. Many of the structural parameters needed appear not to have been measured for the human glomerular capillary wall, so that we used certain values derived from data for rats, as described in detail by Drumond and Deen (12, 13). The resulting permeabilities of the endothelium (k,,) and of the filtration slits (ka) were, respectively, 2.0 x 10-7 and 7.9 x 10-8 mlsl Pa (12, 13). The permeability of the epithelial layer was calculated using wk= ks where E. is the fraction of the basement membrane area occupied by filtration slits, and W, is the slit width; e, = WJ/W. To be consistent with the use of rat data to compute ka, we used W, = 39 nm as for the rat (12, 13, 21). This value is not very different from an estimate obtained using the data of Ellis et al. (9) for healthy humans (W.' 43 nra). The permeability of the basement membrane (kbm) was calculated using Eq. 21 of Drumond and Deen (13). In addition to EC, this equation requires knowledge of the basement membrane thickness (6bm), the Darcy permeability (KD) of the basement membrane, the fraction of the basement membrane area occupied by endothelial fenestrae (Cf), and the number of fenestrae per structural unit (nf ). Whereas 6bb was measured in this study, KD, ECf, and nf were estimated using values for the rat glomerular capillary wall (13). The inputs for the calculations of k with the ultrastructural model are listed in Table I. Statistical analysis Tabulated results are given as meantstandard error. Paired Student t tests and an ANOVA between groups were used to assess the significance of differences in mean values. In all cases, differences between means were judged significant when P < Linear regression analysis was used to test the strength of the relationship between kmwe, and kep. Results The results of the hemodynamic measurements and calculations are shown in Table II. There were no statistically significant differences between the two nephrotic disorders in any of the hemodynamic quantities. In both nephrotic groups the values of (8) Structural Basis for Reduced Glomerular Filtration Capacity 1189
4 Table II. Mean Values of Functional Results Group Minimal Quantity (units) Healthy controls Membranous nephropathy change nephropathy GFR (ml/min) 113±3* 75±81 88±12$ RPF (mlmin) 618±22* 736±85* 635±70* Filtration fraction 0.185±0.005* 0.117±0.009$ 0.140±0.018$ MAP (mmhg) 88±2* 108±3$ 102±4$ 7rA (mmhg) 23.2±0.4* 15.0±0.9* 12.9±1.3* K35) (mi/min/mmhg) 18.4±1.6* 4.7±0.7t 5.1±1.0* K) (mlmin/mmhg) 9.3±0.4* 3.5± ±0.6* 4W5) (ml/minimmhg) 6.5±0.2* 2.8±0.3$ 3.1±0.5* Oalb (2.8±1.0) X 10-6* 0.010±0.003* 0.017±0.009* OISG (1.2±0.1) X 10-6* 0.004±0.001$ 0.011±0.008* *t Different symbols indicate means that are different by ANOVA (P < 0.05). GFR, filtration fraction, and.ra were all significantly depressed relative to those in healthy controls. Conversely, mean arterial pressure (MAP) was elevated in the nephrotic groups. Differences in RPF between nephrotics and controls were not statistically significant. As a consequence of the low GFR and the reduced colloid osmotic pressure opposing filtration, Kf was calculated to be much lower in either nephrotic group than in controls. If AP is assumed to have remained constant at mmhg, then the reductions in Kf were approximately twoto fourfold. If the systemic hypertension in the nephrotic groups is assumed to have caused a 5-mmHg increase in AP (from 35 to 40 or from 40 to 45), then there is as much as a fivefold reduction in the calculated value of Kf. The virtually identical values of Kf in membranous nephropathy and minimal change nephropathy are of particular interest, given the marked differences in morphometry between these groups (see below). Also shown in Table II are fractional clearances of albumin and IgG, which were three to four orders of magnitude higher in the two nephrotic groups than in healthy controls. Results for the morphometric quantities are shown in Table III. The percentage of globally sclerosed glomeruli was low in Table III. Mean Values of Morphometric Results Group Minimal Quantity (units) Healthy Membranous controls nephropathy change nephropathy Sclerosed glomeruli (%) 2.3±1.2* 6.2±1.8* 0.73±0.73* Glomerular volume (106 im3) 1.98±0.20* 4.91±0.34t 3.27±0.59 Surface area per glomerulus (10 Om2) 2.97±0.32* 5.37±0.46* 4.06±0.61*t Filtration slit frequency (I/mm) 1370±49* 384±46t 315±60t Basement membrane thickness (nm) 518±16* 1145± 106t 513±29* **l Different symbols indicate means that are different by ANOVA (P < 0.05). Table IV. Experimental and Predicted Values of Hydraulic Permeabilities (Units = 10' r/s/pa) Group Healthy Membranous Minimal change controls nephropathy nephropathy 43x.p) 4.54±0.52** 0.748± ±0.121 CTx~p) 2.31±0.27* 0.561± ± *p 1.61 ±0.19** 0.451±0.079t* 0.511±0.081 k,, 2.58±0.08* 0.733± ±0.140 * For comparisons between groups, different symbols indicate means that are different by ANOVA (P < 0.05). * P < 0.05 for comparisons of k,,p with kayo within a given group. all three groups, and the numerical differences in the mean values between groups were not significant. Due to a significant increase in glomerular volume in membranous nephropathy, the value for the surface area per glomerulus in this disorder was markedly enhanced. The numerical differences between the mean value of surface area in minimal change nephropathy and that in healthy controls and membranous nephropathy were not significant, while the differences in the mean values of glomerular volume were marginal (P = for minimal change nephropathy versus healthy controls and P = for minimal change nephropathy versus membranous nephropathy). In membranous nephropathy, there was an approximately threefold reduction in the filtration slit frequency relative to controls, accompanied by a doubling of the basement membrane thickness. The reduction in filtration slit frequency measured in minimal change nephropathy was very similar to that seen in membranous nephropathy, but in minimal change nephropathy the value of the basement membrane thickness remained normal. Thus, the morphometric characteristics of membranous nephropathy differed from those in minimal change nephropathy in that glomerular volume, filtration surface area, and basement membrane thickness were all markedly increased in the former. The "experimental" estimates of hydraulic permeability (kep, Eq. 5) and the predicted values from the ultrastructural model (kmodej, Eq. 7) are compared in Table IV. The mean values of kexp were very similar for membranous nephropathy and minimal change nephropathy at each of the assumed pressures and were much smaller in the nephrotic groups than in healthy controls. There was good agreement between kep and kmodjl for the two nephrotic groups. The differences between k,,p and km,, were statistically significant only for healthy controls when kexp was evaluated at AP = 35 or 45 mmhg, and for membranous nephropathy when k,,p was evaluated at AP = 45 mmhg. Individual values of k5,p (evaluated at AP = 40 mmhg, k(4)) and k5.i, for minimal change nephropathy and membranous nephropathy are shown in Fig. 2. There was a significant positive correlation between ke5p and km,, in both groups. The correlation coefficients (r) between km,,o and k(35), between k,,,, and ke4p and between and kxp were, respectively, 0.45, 0.42, and 0.40 for membranous nephropathy; 0.72, 0.65, and 0.62 for minimal change nephropathy; and 0.49, 0.46, and 0.44 for the combined data of membranous nephropathy and minimal change nephropathy. Meaningful correlation coeffi M. C. Drumond, B. Kristal, B. D. Myers, and W. M. Deen
5 1-- Iz oil 7: r x v kmodel (109 mstpa') Figure 2. Individual values of km:ojel and kexp in membranous (open symbols) and minimal change (filled symbols) nephropathies. For each individual, kexp was computed at an assumed transcapillary hydraulic pressure difference of 40 mrmhg. The solid line is the identity line, and the dashed line is the regression line for the two groups. The r value for the two groups is also shown. cients for healthy controls could not be calculated because the functional and morphometric data were obtained in separate groups. Much of the scatter of the points shown in Fig. 2 is likely due to the assumptions regarding AP and n, which significantly affect kexp. Specifically, differences in AP between individuals within a given group were not taken into account, and n was assumed to be the same for all individuals in all groups. Another potential source of scatter in Fig. 2 is the method by which S was calculated in the present study. Using the Cavalieri method as a gold standard, Lane et al. (22) have reported that determination of VG by the Weibel method may be unreliable when fewer than 15 glomeruli are available in the biopsy sample. To examine this possible source of error, we calculated kexp separately in the individuals whose biopsies contained : 15 glomeruli (9 healthy controls, 16 with membranous nephropathy, and 7 with minimal change nephropathy). In these subsets k40 was similar to the values given in Table IV for the entire groups: vs for healthy controls; vs 0.56±0.10 for membranous nephropathy; vs 0.62±0.10 in minimal change nephropathy (all 10-' m/s/pa). None of these differences were statistically significant. Thus, whereas errors in calculated S from biopsies with < 15 glomeruli could have contributed to the differences between kmode, and kexp, such a contribution is likely to have been small. Finally, the assumptions regarding the uniformity of some of the parameters used in the calculation of kmodel are also likely to have contributed to the differences between this quantity and kexp. Thus, many factors are likely to account for the scatter in Fig. 2, and better quantitative agreement between individual values of kmde, and kexp would have been difficult to achieve. Values of k40 and kmodel for the controls and the two nephrotic groups are displayed as box plots in Fig. 3. Using either estimate of hydraulic permeability, there was little or no overlap between the ranges of k obtained for the healthy controls and the ranges for either nephrotic group. The patterns obtained with k(i5) and k'i5 (not shown) were similar. These plots clearly illustrate that the hydraulic permeability was very similar in membranous and minimal change nephropathies and was strikingly lower in those groups than in healthy controls. The fractional clearances of albumin (Oalb) and IgG (9IgG) for membranous nephropathy and minimal change nephropathy are plotted as functions of kmode, in Fig. 4. There were significant negative correlations between either fractional clearance and kmode,. The correlation coefficients between log 6alb and kmilel were r = for membranous nephropathy, r = for minimal change nephropathy, and r = for the combined data of minimal change nephropathy and membranous nephropathy. The corresponding relationships between log 9IgG and kmodel were even stronger (r = -0.47, -0.60, and -0.51, respectively). Thus, the individuals with the highest fractional clearances of albumin and IgG tended to have the lowest hydraulic permeabilities. The same conclusion is reached if 9alb and 0IgG are plotted against kexp. Discussion In both groups of nephrotic individuals, GFR was reduced by about 30% relative to an age-matched group of healthy controls. Among the determinants of GFR, TA was depressed in the nephrotic patients while RPF was not significantly altered. These findings are in keeping with previous observations in membranous and minimal change nephropathies ( 1, 2, 10, 11). Given that MAP was higher in each nephrotic group than in controls, it is unlikely that glomerular capillary pressure and hence AP were lower in nephrotics than in controls. Thus, the only factor that can explain the reduced GFR is a decrease in the overall Kf for the two kidneys. The precise magnitude of the calculated reduction in Kf depends on what is assumed to have happened to AP. If AP remained constant at either 35 or 40 mmhg, then Kf was reduced by roughly a factor of two to four, the greater reduction in Kf corresponding to AP = 35 mmhg. If AP increased by 5 mmhg in nephrotics, then there were approximately three- to fivefold reductions in Kf. Modest increases in AP in the nephrotic individuals are consistent with micropuncture measurements in rats with Heymann nephritis or with nephropathies induced by puromycin or adriamycin, which are regarded as experimental models for membranous nephropathy and minimal change nephropathy (23-26). Changes in peripheral capillary surface area per glomerulus (S) or in the number of functioning glomeruli are incapable of explaining the two- to fivefold reductions in Kf. The percentage of globally sclerosed glomeruli measured in nephrotic individuals was similar to that observed in controls, and this sclerosis is likely due to aging. This suggests that there was little or no reduction in the number of functioning glomeruli in the membranous nephropathy and minimal change nephropathy groups. Indeed, 2 94% of glomerular tufts appeared nonsclerosed and widely patent by light microscopy in all groups studied. There was a tendency toward increased S in both nephrotic groups, although the increase relative to controls reached statistical significance only for membranous nephropathy. Because the changes in the number of functioning glomeruli appeared to Structural Basis for Reduced Glomerular Filtration Capacity' 1191
6 ad b - 3 a 2 IC K 3 7o j2 HC MN MCN HC MN MCN 9 Figure 3. Quartile box plots of experimental estimates (kip) and model predictions (kmode) of the hydraulic permeability of the glomerular capillary wall in healthy controls (HC) and in membranous (MN) and minimal change (MCN) nephropathies. The horizontal lines comprising each box are the 25th (lower), 50th (middle), and 75th (top) percentiles, respectively. In all but one case, the vertical lines represent the remainder of the range. In that case, the outlier is shown as an open symbol. be quantitatively insignificant, and because S tended to remain constant or to increase, we infer that the depression of Kf must be attributable entirely to reductions in the effective hydraulic permeability of the capillary wall (k). Using GFR and its determinants to calculate the hydraulic permeability, the resulting values (kexp) were virtually identical in membranous nephropathy and minimal change nephropathy. An alternative way to examine changes in hydraulic permeability is provided by the mathematical model which combines structural information and hydrodynamic calculations at the level of individual components of the glomerular capillary wall (13). This ultrastructural model does not use any information about GFR or its hemodynamic determinants, so that the resulting values of hydraulic permeability (kmodel) are entirely independent of kexp. There are three principal assumptions of the ultrastructural model. One is that the capillary wall can be represented as having many repeating structural units as shown in Fig. 1. A second assumption is that the intrinsic hydraulic permeability (Darcy permeability) of the basement membrane is the same as that which has been measured for the isolated rat glomerular basement membrane (27). The third principal assumption is that the hydraulic resistance of the slit diaphragm can be calculated from an analysis of viscous flow through the zipperlike structure first described in rats by Rodewald and Karnovsky (21). These assumptions are supported by the fact that the predicted hydraulic permeabilities for rats are in excellent agreement with values measured by micropuncture (13). The applicability of this model to human data is supported by the finding that kmoel accurately predicts not only the trends in kexp in the present study but also the absolute values in each group of subjects. Perhaps the main value of the ultrastructural model is that it can explain findings which are not intuitively obvious. The similarity of kexp in minimal change nephropathy and membranous nephropathy, despite the twofold difference in basement membrane thickness (ONb), is one such finding. To explain this result, we consider now the individual factors which contribute to k. First of all, the calculations using the ultrastructural model suggest that, in healthy controls as well as in subjects with membranous nephropathy and minimal change nephropathy, the hydraulic resistance of the endothelium is negligible, the basement membrane resistance is about 60% of the total, and the epithelial resistance accounts for the remaining 40%. To examine in more detail the factors which contribute to the basement membrane resistance, a good approximation to kbm under most conditions2 is 2. Except for one subject with membranous nephropathy and four with minimal change nephropathy, Eq. 9 yielded values of kbm within 2% of those obtained from the more rigorous formula (Eq. 23 of Drumond and Deen [13]). For those exceptional cases, in which 6bm/W c 0.08, the discrepancies were 19-37% ' 10-2 Oalb lo o 'o 00 0 o - 'Q * 6oo - *._ : 0-6 L 0.( (109 ms'pat) kmodel 0~~~~ r= igg l ' 4 * * O 60 clearances of albumin (9alb, left) and IgG 10l , * & o 0 o ot r=-0.51 ~o'-~~- * Figure 4. Individual values of the fractional 0 - (0g, right) in membranous (open symbols) 0 0 6, and minimal change (filled symbols) nephropathies, plotted as a function of kmodel In each plot, the dashed line is the regression kmdei (lo0-' ms-'pa-') line for the two groups. The corresponding r km0do (e values are also shown M. C. Drumond, B. Kristal, B. D. Myers, and W. M. Deen
7 B Xl/l_ fl" (C A e(la-( C'.a) \J h"e \A)\..AI\.A)VAL. I Figure 5. Streamlines in the basement membrane, illustrating the effect of changes in filtration slit frequency. A is an example representative of healthy controls. In B the filtration slit frequency is four times smaller than that in A. The basement membrane thickness, the frequency and fractional area of the fenestrae, and the width of the slit opening are the same in both panels. There are 4 fenestrae in the structural unit of A and 16 in that of B. kbm Ḵ { bm + 1 [1.5 - In (27ref)],iW IW 7r f [1.5 - In (27rEs)]}. (9) 7r The three main contributions to kbm are the terms separated by "+" signs. The first term, involving 6bm, represents the resistance of "bare" basement membrane (i.e., if endothelial cells and foot processes did not cover the basement membrane surfaces). The second term, involving nf and Ef, gives the increased basement membrane resistance due to coverage by endothelial cells; and the third term, containing Es, is the increased resistance due to coverage by podocytes. It is worth noting that elementary membrane models yield only the first term in Eq. 9 and that a two- or three-dimensional representation of the flow field in the basement membrane is needed to account for the surface blockage effects (13). In healthy controls, the first term in Eq. 9 was found to be the most important, accounting for - 60% of the basement membrane resistance. That is, the basement membrane thickness is an important determinant of kbm and k in healthy individuals. By contrast, in nephrotic subjects the filtration slit frequency was reduced enough that the third term became dominant (- 60% in membranous nephropathy and 80% in minimal change nephropathy). Accordingly, kbm in membranous nephropathy and minimal change nephropathy was determined primarily by filtration slit frequency and not by basement membrane thickness. The reason that kbm becomes almost independent of basement membrane thickness is that, with widely separated slits, much of the flow path for filtrate within the basement membrane is parallel to the basement membrane surfaces, as is illustrated by the streamlines (i.e., lines which represent the local direction of flow) shown in Fig. 5. Fig. 5 A shows streamlines in a structural unit representative of healthy controls, and B shows streamlines in a structural unit with a much smaller slit frequency, similar to that of the nephrotic subjects. For this illustration, we assumed that the basement membrane thickness is the same in both cases but that the filtration slit frequency is four times smaller in B than in A. Consequently, the width of the structural unit in B is four times that in A. The path length for the filtrate, which ultimately dictates the pressure drop across the basement membrane, tends to be much larger in B than in A, even with identical basement membrane thickness. Thus, in the nephrotic groups, where the filtration slit frequency is considerably reduced relative to that in controls, the average path length and the resulting pressure drop are determined more by the slit spacing than by basement membrane thickness. Assuming no major differences in filtration slit width or slit diaphragm structure, the hydraulic permeability of the epithelial layer (k4p) is governed mainly by filtration slit frequency. With both kb, and kp in the nephrotic groups being determined primarily by filtration slit frequency, so is the overall value of k. This provides an attractive explanation for the aforementioned fact that reductions in GFR and Kf in nephrotic subjects tend to be correlated much more strongly with reductions in filtration slit frequency than with changes in basement membrane thickness (8-1I1 ). For lack of specific data, we assumed that the total number of nephrons (n) was the same in all individuals of the present study, although differences in n are likely to exist. For example, it has been observed that n decreases with age (20, 28). To minimize the effects of age differences on our results, we, excluded individuals with age 2 60 yr. If we had excluded all subjects with ages 2 50 yr, the results would not have been significantly changed. Recently, it has been observed that n in diabetic patients with advanced nephropathy is smaller than in healthy controls (29). If such differences exist in membranous nephropathy and minimal change nephropathy, it is possible that the correction for glomerular sclerosis used in Eq. 5 is not enough to account for the differences in n between groups. The most widely used method to determine n (as well as glomerular volume, VG) is that proposed by Weibel (18, 30). Using this method, estimates of n in healthy humans have been 2 x 106 (20). Recently, however, this method was criticized as being biased (29, 31 ), and a new method to determine n and glomerular volume was proposed. Using this new method, a value of n = 1.2 x 106 was obtained (28). Since the theoretical basis of the relationships involved in the calculations of VG (which is related to the glomerular filtering surface area, S, by Eq. 3) and n are the same, and because in this study we used the method of Weibel ( 18, 30) to determine glomerular volume, we chose to use n = 2 X 106 in Eq. 5. In fact, with the method of Weibel, the product ns (which appears in Eq. 5) does not involve the shape coefficient (/6) and distribution coefficient (d), which are sources of bias. The reductions in k in membranous nephropathy and minimal change nephropathy appear at first to be inconsistent with the finding of considerable proteinuria, as reflected by the values of fractional albumin clearance and fractional IgG clearance. That is, one might expect that any structural changes which retard filtration of water would at least equally retard the transmural passage of proteins. One reasonable hypothesis to explain these findings is that the broadening of the foot processes (decrease of filtration slit frequency) causes changes in the structure or even entirely disrupts some of the slit diaphragms. Such changes could augment considerably the filtration of albumin and IgG, without significantly affecting the value of k. For example, if 5% of the slit Structural Basis for Reduced Glomerular Filtration Capacity 1193
8 diaphragms are assumed to be disrupted in membranous nephropathy and minimal change nephropathy, and if this causes the resistance of the epithelium to water flow to be negligible in 5% of the capillary wall, the mean values of keel would be 7.57 X and 7.22 X 1010 rns/pa in membranous nephropathy and minimal change nephropathy, respectively. These values are < 4% higher than those given in Table IV, not a very noticeable difference. However, given the observation that the cellular component of the capillary wall accounts for much of the overall size selectivity (32), it is entirely possible that the rupture of this small fraction of the slit diaphragms might allow the filtration of large quantities of albumin and IgG. Another possible explanation is that in membranous nephropathy and minimal change nephropathy there might have been changes in the fixed charge content of the capillary wall which, while not affecting k, could have greatly reduced the resistance to filtration of albumin. Charge selectivity of the glomerular barrier in healthy humans and its impairment in nephrotic subjects with either membranous or minimal change nephropathy has been demonstrated recently (33). Another potential application of the ultrastructural model would be to use the predicted values of k to calculate Kf and, together with the hemodynamic data (GFR, RPF, and W7A), to estimate AP for each individual. Because of the assumptions involved in the model, as well as in the value of nephron number used to calculate Kf, any such estimates of AP at present are highly tentative. Nonetheless, with the present data and assumptions, the mean values of the predicted AP were 39 mmhg in healthy controls, 36 mmhg in membranous nephropathy, and 42 mmhg in minimal change nephropathy (neglecting the value for one patient with minimal change nephropathy, which was larger than 100 mmhg). The value of AP for healthy controls is quite similar to that calculated for healthy humans by an indirect method (AP = 36 mmhg), one based on curve fitting of the measured sieving coefficients of neutral dextran of graded size (34). Moreover, mean values for AP predicted by km, for all three groups are remarkably similar to corresponding values measured directly by micropuncture in normal rats, and in rats with induced glomerular diseases which are analogues of membranous and minimal change nephropathy in humans (23-26). To summarize, our analysis suggests that a reduction of glomerular hydraulic permeability is a major determinant of the impaired ultrafiltration capacity in membranous nephropathy and minimal change nephropathy. To elucidate the basis for the reduced hydraulic permeability, we used a recent hydrodynamic model of flow across the structural components of the glomerular capillary wall ( 12, 13). We concluded that, in both disorders, the hydraulic permeability is determined mainly by the slit spacing rather than by the basement membrane thickness. These findings point to an injury to epithelial foot processes, with an ensuing reduction in the frequency of intervening filtration slits, as the predominant cause of hypofiltration during the acute, nephrotic stage of each of these glomerular injuries. Appendix Because of the random angle of sectioning the glomerular capillary, the measured apparent width of a structural unit (W') (i.e., the sum of foot process width and filtration slit width) will generally exceed the true width (W). As illustrated in Fig. 6, W' depends on the angle (4) between the filtration slits and the line of intersection of the sample plane (a9) with the outer surface of the capillary wall (2). It can be seen that for 0 < 4 c 7r, W'. W, the equality holding when 4) = r/2. Filtration Slits Figure 6 Schematic representation of the outer surface of the capillary wall (9) and sample plane (p), showing the relationship between the measured apparent width of a structural unit (W') and the true width (W). To calculate W from the measured mean width of a structural unit (W ), we used principles of geometrical probability. In particular, our problem is closely related to that of calculating the expected length (af) of the chord formed by the intersection of a plane (CA) with a closed convex figure lying in another plane (i ). Using results given in Kendall and Moran (35) we obtained 7rA (Al) p where A is the area of the closed convex curve, and P is its perimeter. This result is equivalent to that derived by Solomon (36) for the case of a straight line intersecting a closed convex curve in a plane. Eq. Al can be applied directly to our problem by defining the closed curve as a rectangle of width W and (arbitrary) length L, corresponding to the area occupied by one foot process and one filtration slit. Then, a = W'. Neglecting edge effects (i.e., assuming L > W), Eq. Al yields w = - w 2 (A2) which is the desired relationship between the apparent and true widths of a structural unit. This is the basis for the factor 2/ir in Eq. 6. Eq. A2 can be derived also as follows. As indicated by Fig. 6, the apparent width W' is related to W by wi= w. sin 4 The arithmetic mean of W' is then calculated by f W'f(4)d) WI = o f(4)do (A3) (A4) where f(4) is the probability density of the angle 4. As shown by Solomon (36), the probability density of the angle formed by two random lines that are known to intersect within some finite region in space is given by f(4)) =-Isin4). 2 (AS) Using Eqs. A3 and AS in Eq. A4, and performing the required integrations, one obtains again Eq. A M. C. Drumond, B. Kristal, B. D. Myers, and W. M. Deen
9 Similarly, one can also calculate W from the apparent harmonic mean width (W') which is defined by wh The result is 4 W>=-W. f(o)do =ax * ~~~~~~~~~(A6) (A7) 7r The derivations presented here to obtain relationships between W' (or W') and W are different from that of Jensen et al. ( 19), who obtained the relationship we have used to calculate the "true" thickness of the basement membrane (6bm, Eq. 4). To determine 6bm one measures the distance between two lines obtained by the intersection of the sample plane (SO) with two parallel planes (O' and 2) corresponding to the "inner" and "outer" surfaces of the basement membrane. The angle of interest in that case (denoted as 9) is that between 9 and 2 (or O'), and the probability density for that angle is proportional to sin2 9 (19). In contrast, W' is the distance between two points in the line of intersection of the sample plane (S) with the plane (2) corresponding to the outer surface of the basement membrane. The angle of interest is now the angle (4) formed by the line of intersection of the two planes with the parallel lines in 2 corresponding to the filtration slits. This is why, contrary to the suggestion of Gundersen et al. (37), we did not use the same correction factors for W and 6bbm Acknowledgments This work was supported by grants from the National Institutes of Health (DK and DK-29985). M. C. Drumond is the recipient of a fellowship from Junta Nacional de Investigagio Cientifica e Tecnol6gica, Programa CIENCIA, Portugal, and B. Kristal is the recipient of a fellowship from the Juvenile Diabetes Foundation International. References 1. Guasch, A., R. K. Sibley, P. Huie, and B. D. Myers Extent and course of glomerular injury in human membranous glomerulopathy. Anm J. Physiol. 263 (Renal Fluid Electrolyte Physiol. 32) :F1034-F Guasch, A., H. Hashimoto, R. K. Sibley, W. M. Deen, and B. D. Myers Glomerular dysfunction in nephrotic humans with minimal changes or focal glomerulosclerosis. Am. J. Physiol. 260 (Renal Fluid Electrolyte Physiol. 29) :F728-F Myers, B. D., A. Chagnac, H. Golbetz, L. Newton, S. Strober, and R. K. Sibley Extent of glomerular injury in active and resolving lupus nephritis: a theoretical analysis. Am. J. Physiol. 260 (Renal Fluid Electrolyte Physiol. 29) :F717-F Austin, S. M., J. S. Lieberman, L. D. Newton, M. Mejia, W. A. Peters, and B. D. Myers Slope of serial GFR and the progression of diabetic glomerular disease. J. Am. Soc. Nephrol. 3: Scandling, J. D., V. M. Black, W. M. Deen, and B. D. Myers Glomerular permselectivity in healthy and nephrotic humans. Necker's Seminar in Nephrology. Adv. Nephrol. 21: Mauer, S. M., M. W. Steffes, E. N. Ellis, D. E. R. Sutherland, D. M. Brown, and F. C. Goetz Structural-functional relationships in diabetic nephropathy. J. Clin. Invest. 74: Osterby, R., H. J. G. Gundersen, A. Horlyck, J. P. Koustrup, G. Nyberg, and G. Westberg Diabetic glomerulopathy: structural characteristics of the early and advanced stages. Diabetes. 32(Suppl. 2): Bohman, S. O., G. Jaremko, A. B. Bohlin, and U. Berg Foot process fusion and glomerular filtration rate in minimal change nephrotic syndrome. Kidney Int. 25: Ellis, E. N., M. W. Steffes, B. Chavers, and S. M. Mauer Observations of glomerular epithelial cell structure in patients with type I diabetes mellitus. Kidney Int. 32: Guasch, A., and B. D. Myers Determinants of glomerular hypofiltration in nephrotic patients with minimal change nephropathy. J. Am. Soc. Nephrol 4: Shemesh, O., J. C. Ross, W. M. Deen, G. W. Grant, and B. D. Myers Nature of the glomerular capillary injury in human membranous glomerulopathy. J. Clin. Invest. 77: Drumond, M. C., and W. M. Deen Stokes flow through a row of cylinders between parallel walls: model for the glomerular slit diaphragm. J. Biomech. Eng. 116: Drumond, M. C., and W. M. Deen Structural determinants of glomerular hydraulic permeability. Am. J. Physiol. 266 (Renal Fluid Electrolyte Physiol. 35):Fl-F Battilana, C., H. Zhang, R. Olshen, L. Wexler, and B. D. Myers PAH extraction and the estimation of plasma flow in the diseased human kidney. Am. J. Physiol. 261 (Renal Fluid Electrolyte Physiol. 30):F726-F Cannan-Kuhl, S., E. S. Venkatraman, S. I. B. Ernst, R. A. Olshen, and B. D. Myers Relationships among protein and albumin concentrations and oncotic pressure in nephrotic plasma. Am. J. Physiol. 264 (Renal Fluid Electrolyte Physiol. 33) :F1052-F Deen, W. M., C. R. Robertson, and B. M. Brenner A model of glomerular ultrafiltration in the rat. Am. J. Physiol. 223: Maddox, D. A., W. M. Deen, and B. M. Brenner Glomerular Filtration. In Handbook of Physiology: Renal Physiology. E. E. Windhager, editor. American Physiological Society, Oxford University Press, New York Weibel, E. R Stereological Methods. Vol. 1: Practical Methods for Biological Morphometry. Academic Press, London. 19. Jensen, E. B., H. J. G. Gundersen, and R. Osterby Determination of membrane thickness distribution from orthogonal intercepts. J. Microsc. (04) 115: Dunnill, M. S., and W. Halley Some observations on the quantitative anatomy of the kidney. J. Pathol. 110: Rodewald, R., and M. J. Karnovsky Porous substructure of the glomerular slit diaphragm in the rat and mouse. J. Cell Biol. 60: Lane, P. H., M. W. Steffes, and S. M. Maurer Estimation of glomerular volume: a comparison of four methods. Kidney Int. 41: Ichikawa, I., J. R. Hoyer, W. M. Seiler, and B. M. Brenner Mechanism of glomerulotubular balance in the setting of heterogeneous glomerular injury. J. Clin. Invest. 69: Yoshioka, T., H. G. Rennke, D. J. Salant, W. M. Deen, and I. Ichikawa Role of abnormally high transmural pressure in the permselectivity defect of glomerular capillary wall: a study in early passive Heymann nephritis. Circ. Res. 61: Scholey, J. W., P. L. Miller, H. G. Rennke, and T. W. Meyer Effect of converting enzyme inhibition on the course of adriamycin-induced nephropathy. Kidney Int. 36: Anderson, S., J. R. Diamond, M. J. Karnovsky, and B. M. Brenner Mechanisms underlying transition from acute glomerular injury to late glomerular sclerosis in a rat model of nephrotic syndrome. J. Clin. Invest. 82: Daniels, B. S., E. B. Hauser, W. M. Deen, and T. H. Hostetter Glomerular basement membrane: in vitro studies of water and protein permeability. Am. J. Physiol. 262 (Renal Fluid Electrolyte Physiol. 31):F919-F Nyengaard, J. R., and T. F. Bendtsen Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat. Rec. 232: Bendtsen, T. F., and J. R. Nyengaard The number of glomeruli in type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 35: Weibel, E. R., and D. M. Gomez A principle for counting tissue structures on random sections. J. Appl. Physiol. 17: Bendtsen, T. F., and J. R. Nyengaard Unbiased estimation of particle number using sections: an historical perspective with special reference to the stereology of glomeruli. J. Microsc. 153: Daniels, B. S., W. M. Deen, G. Mayer, T. Meyer, and T. H. Hostetter Glomerular permeability barrier in the rat. Functional assessment by in vitro methods. J. Clin. Invest. 92: Guasch, A., W. M. Deen, and B. D. Myers Charge selectivity of the glomerular filtration barrier in healthy and nephrotic humans. J. Clin. Invest. 92: Myers, B. D., C. Peterson, C. R. Molina, S. J. Tomlanovich, L. D. Newton, R. Nitkin, H. Snadler, and F. Murad Role of cardiac atria in the human renal response to changing plasma volume. Am. J. Physiol. 254 (Renal Fluid Electrolyte Physiol. 23) :F562-F Kendall, M. G., and P. A. P. Moran Geometrical Probability. Charles Griffin & Co., London Solomon, H Geometric Probability. Society for Industrial and Applied Mathematics, Philadelphia. 174 pp. 37. Gundersen, H. J. G., T. Seefeldt, and R. 0sterby Glomerular epithelial foot processes in normal man and rats. Cell Tissue Res. 205: Structural Basis for Reduced Glomerular Filtration Capacity 1195
MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER
 MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER The glomerular filtration barrier consists of three elements: (1) endothelial
MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER The glomerular filtration barrier consists of three elements: (1) endothelial
Actualités néphrologiques Jean Hamburger 23 Avril Marie Courbebaisse, Service de Physiologie Hôpital Européen Georges Pompidou, Paris
 Single nephron GFR Actualités néphrologiques Jean Hamburger 23 Avril 2018 Marie Courbebaisse, Service de Physiologie Hôpital Européen Georges Pompidou, Paris Introdution Glomerular filtration and SNGFR
Single nephron GFR Actualités néphrologiques Jean Hamburger 23 Avril 2018 Marie Courbebaisse, Service de Physiologie Hôpital Européen Georges Pompidou, Paris Introdution Glomerular filtration and SNGFR
Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION
 3 Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION David Shirley, Giovambattista Capasso and Robert Unwin The kidney has three homeostatic functions that can broadly be described as excretory, regulatory
3 Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION David Shirley, Giovambattista Capasso and Robert Unwin The kidney has three homeostatic functions that can broadly be described as excretory, regulatory
Fine structural appearances of glomerular capillaries in a case of malignant hypertension
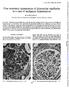 J. clin. Path. (1969), 22, 579-583 Fine structural appearances of glomerular capillaries in a case of malignant hypertension R. F. MACADAM From the University Department of Pathology, Western Infirmary,
J. clin. Path. (1969), 22, 579-583 Fine structural appearances of glomerular capillaries in a case of malignant hypertension R. F. MACADAM From the University Department of Pathology, Western Infirmary,
Glomerular filtration rate (GFR)
 LECTURE NO (2) Renal Physiology Glomerular filtration rate (GFR) Faculty Of Medicine Dept.Of Physiology The glomerulus Is a tuft of capillaries enclosed within a Bowman capsule. It is supplied by an afferent
LECTURE NO (2) Renal Physiology Glomerular filtration rate (GFR) Faculty Of Medicine Dept.Of Physiology The glomerulus Is a tuft of capillaries enclosed within a Bowman capsule. It is supplied by an afferent
MAJOR FUNCTIONS OF THE KIDNEY
 MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
Overview of glomerular diseases
 Overview of glomerular diseases *Endothelial cells are fenestrated each fenestra: 70-100nm in diameter Contractile, capable of proliferation, makes ECM & releases mediators *Glomerular basement membrane
Overview of glomerular diseases *Endothelial cells are fenestrated each fenestra: 70-100nm in diameter Contractile, capable of proliferation, makes ECM & releases mediators *Glomerular basement membrane
Longitudinal study of living kidney donor glomerular dynamics after nephrectomy
 The Journal of Clinical Investigation Clinical Medicine Longitudinal study of living kidney donor glomerular dynamics after nephrectomy Colin R. Lenihan, 1 Stephan Busque, 2 Geraldine Derby, 1 Kristina
The Journal of Clinical Investigation Clinical Medicine Longitudinal study of living kidney donor glomerular dynamics after nephrectomy Colin R. Lenihan, 1 Stephan Busque, 2 Geraldine Derby, 1 Kristina
Filtration and Reabsorption Amount Filter/d
 Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD Contact me at lharris@lsuhsc.edu Renal Physiology Lecture 3 Renal Clearance and Glomerular Filtration Filtration and Reabsorption Amount Filter/d Amount
Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD Contact me at lharris@lsuhsc.edu Renal Physiology Lecture 3 Renal Clearance and Glomerular Filtration Filtration and Reabsorption Amount Filter/d Amount
Glomerular perm-selective function
 Kidney International, Vol. 45 (1994), pp. 398-402 Glomerular perm-selective function ANDREA REMUZZI and GIUSEPPE REMUZZI "Mario Negri" Institute for Pharmacological Research and Division of Nephrology
Kidney International, Vol. 45 (1994), pp. 398-402 Glomerular perm-selective function ANDREA REMUZZI and GIUSEPPE REMUZZI "Mario Negri" Institute for Pharmacological Research and Division of Nephrology
A Simpli ed Method for Measuring the Thickness of Glomerular Basement Membranes
 Ultrastructural Pathology, 27:409 416, 2003 Copyright # Taylor & Francis Inc. ISSN: 0191-3123 print/1521-0758 online DOI: 10.1080/01913120390248728 A Simpli ed Method for Measuring the Thickness of Glomerular
Ultrastructural Pathology, 27:409 416, 2003 Copyright # Taylor & Francis Inc. ISSN: 0191-3123 print/1521-0758 online DOI: 10.1080/01913120390248728 A Simpli ed Method for Measuring the Thickness of Glomerular
Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy
 original article http://www.kidney-international.org & 2012 International Society of Nephrology see commentary on page 949 Podocyte detachment and reduced glomerular capillary endothelial fenestration
original article http://www.kidney-international.org & 2012 International Society of Nephrology see commentary on page 949 Podocyte detachment and reduced glomerular capillary endothelial fenestration
Basic Functions of the Kidneys
 Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Glomerular Pathology- 1 Nephrotic Syndrome. Dr. Nisreen Abu Shahin
 Glomerular Pathology- 1 Nephrotic Syndrome Dr. Nisreen Abu Shahin The Nephrotic Syndrome a clinical complex resulting from glomerular disease & includes the following: (1) massive proteinuria (3.5 gm /day
Glomerular Pathology- 1 Nephrotic Syndrome Dr. Nisreen Abu Shahin The Nephrotic Syndrome a clinical complex resulting from glomerular disease & includes the following: (1) massive proteinuria (3.5 gm /day
The functions of the kidney:
 The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
Lecture-2 Review of the previous lecture:
 Lecture-2 Review of the previous lecture: -Kidney s function is to clean the blood by the removing of the waste plus adding some valuable substances -kidney failure will lead to death for many reasons,
Lecture-2 Review of the previous lecture: -Kidney s function is to clean the blood by the removing of the waste plus adding some valuable substances -kidney failure will lead to death for many reasons,
RENAL HISTOPATHOLOGY
 RENAL HISTOPATHOLOGY Peter McCue, M.D. Department of Pathology, Anatomy & Cell Biology Sidney Kimmel Medical College There are no conflicts of interest. 1 Goals and Objectives! Goals Provide introduction
RENAL HISTOPATHOLOGY Peter McCue, M.D. Department of Pathology, Anatomy & Cell Biology Sidney Kimmel Medical College There are no conflicts of interest. 1 Goals and Objectives! Goals Provide introduction
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
Collin County Community College RENAL PHYSIOLOGY
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Surgical Pathology Report
 Louisiana State University Health Sciences Center Department of Pathology Shreveport, Louisiana Accession #: Collected: Received: Reported: 6/1/2012 09:18 6/2/2012 09:02 6/2/2012 Patient Name: Med. Rec.
Louisiana State University Health Sciences Center Department of Pathology Shreveport, Louisiana Accession #: Collected: Received: Reported: 6/1/2012 09:18 6/2/2012 09:02 6/2/2012 Patient Name: Med. Rec.
By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences. Body fluids and.
 By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences Body fluids and Renal physiology 25 Volume and Osmolality of Extracellular and Intracellular Fluids
By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences Body fluids and Renal physiology 25 Volume and Osmolality of Extracellular and Intracellular Fluids
Comparison of volume estimation methods for pancreatic islet cells
 Comparison of volume estimation methods for pancreatic islet cells Jiří Dvořák a,b, Jan Švihlíkb,c, David Habart d, and Jan Kybic b a Department of Probability and Mathematical Statistics, Faculty of Mathematics
Comparison of volume estimation methods for pancreatic islet cells Jiří Dvořák a,b, Jan Švihlíkb,c, David Habart d, and Jan Kybic b a Department of Probability and Mathematical Statistics, Faculty of Mathematics
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
Functional morphology of kidneys Clearance
 Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
PHSI2006/2906: Integrated Physiology B
 PHSI2006/2906: Integrated Physiology B TOPIC 1: RESPIRATION 1. The Mechanics of Breathing...2 2. Work of Breathing....5 3. Pulmonary Gas Exchange.. 10 4. Transport of Oxygen...16 5. Control of Respiration...20
PHSI2006/2906: Integrated Physiology B TOPIC 1: RESPIRATION 1. The Mechanics of Breathing...2 2. Work of Breathing....5 3. Pulmonary Gas Exchange.. 10 4. Transport of Oxygen...16 5. Control of Respiration...20
Diabetologia 9 by Springer-Verlag 1980
 Diabetologia 18,493-500 (1980) Diabetologia 9 by Springer-Verlag 1980 Fast Accumulation of Basement Membrane Material and the Rate of Morphological Changes in Acute Experimental Diabetic Glomerular Hypertrophy
Diabetologia 18,493-500 (1980) Diabetologia 9 by Springer-Verlag 1980 Fast Accumulation of Basement Membrane Material and the Rate of Morphological Changes in Acute Experimental Diabetic Glomerular Hypertrophy
Case Presentation Turki Al-Hussain, MD
 Case Presentation Turki Al-Hussain, MD Director, Renal Pathology Chapter Saudi Society of Nephrology & Transplantation Consultant Nephropathologist & Urological Pathologist Department of Pathology & Laboratory
Case Presentation Turki Al-Hussain, MD Director, Renal Pathology Chapter Saudi Society of Nephrology & Transplantation Consultant Nephropathologist & Urological Pathologist Department of Pathology & Laboratory
Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph
 The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
Dietary protein restriction and glomerular permselectivity in nephrotoxic serum nephritis
 Kidney International, Vol. 40 (1991), pp. 57 61 Dietary protein restriction and glomerular permselectivity in nephrotoxic serum nephritis JOEL NEUGARTEN, ARTHUR K0zIN, ROBERT GAYNER, ROBERT G. SCHACHT,
Kidney International, Vol. 40 (1991), pp. 57 61 Dietary protein restriction and glomerular permselectivity in nephrotoxic serum nephritis JOEL NEUGARTEN, ARTHUR K0zIN, ROBERT GAYNER, ROBERT G. SCHACHT,
Renal Physiology. April, J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine.
 Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
The kidneys are excretory and regulatory organs. By
 exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
The topic of normal vascular and glomerular anatomy is introduced
 Normal Vascular and Glomerular Anatomy Arthur H. Cohen Richard J. Glassock The topic of normal vascular and glomerular anatomy is introduced here to serve as a reference point for later illustrations of
Normal Vascular and Glomerular Anatomy Arthur H. Cohen Richard J. Glassock The topic of normal vascular and glomerular anatomy is introduced here to serve as a reference point for later illustrations of
Nephritic vs. Nephrotic Syndrome
 Page 1 of 18 Nephritic vs. Nephrotic Syndrome Terminology: Glomerulus: A network of blood capillaries contained within the cuplike end (Bowman s capsule) of a nephron. Glomerular filtration rate: The rate
Page 1 of 18 Nephritic vs. Nephrotic Syndrome Terminology: Glomerulus: A network of blood capillaries contained within the cuplike end (Bowman s capsule) of a nephron. Glomerular filtration rate: The rate
CHAPTER 2 PRIMARY GLOMERULONEPHRITIS
 CHAPTER 2 Sunita Bavanandan Lim Soo Kun 19 5th Report of the 2.1: Introduction This chapter covers the main primary glomerulonephritis that were reported to the MRRB from the years 2005-2012. Minimal change
CHAPTER 2 Sunita Bavanandan Lim Soo Kun 19 5th Report of the 2.1: Introduction This chapter covers the main primary glomerulonephritis that were reported to the MRRB from the years 2005-2012. Minimal change
Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note
![Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note](/thumbs/77/75431210.jpg) Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1)
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional role of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional role of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
Diabetologia 9. Impact of metabolic control in progression of clinical diabetic nephropathy
 Diabetologia (1987) 3:82-86 Diabetologia 9 Impact of metabolic control in progression of clinical diabetic nephropathy G. Nyberg a, G. Blohm6 z and G. Nord6n 1 Departments of 1Nephrology and 2Medieine
Diabetologia (1987) 3:82-86 Diabetologia 9 Impact of metabolic control in progression of clinical diabetic nephropathy G. Nyberg a, G. Blohm6 z and G. Nord6n 1 Departments of 1Nephrology and 2Medieine
Microcirculation and Edema- L1 L2
 Microcirculation and Edema- L1 L2 Faisal I. Mohammed MD, PhD. University of Jordan 1 Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchanged
Microcirculation and Edema- L1 L2 Faisal I. Mohammed MD, PhD. University of Jordan 1 Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchanged
Nature of the Glomerular Capillary Injury in Human Membranous Glomerulopathy
 Nature of the Glomerular Capillary Injury in Human Membranous Glomerulopathy. Shemesh, J. C. Ross, W. M. Deen, G. W. Grant, and B. D. Myers Departments ofmedicine and Pathology, Stanford University School
Nature of the Glomerular Capillary Injury in Human Membranous Glomerulopathy. Shemesh, J. C. Ross, W. M. Deen, G. W. Grant, and B. D. Myers Departments ofmedicine and Pathology, Stanford University School
CAPILLARY FLUID EXCHANGE
 CAPILLARY FLUID EXCHANGE Aubrey E. Taylor and Timothy M. Moore Department of Physiology, University of South Alabama, College of Medicine, Mobile, Alabama 36688-0002 AM. J. PHYSIOL. 277 (ADV. PHYSIOL.
CAPILLARY FLUID EXCHANGE Aubrey E. Taylor and Timothy M. Moore Department of Physiology, University of South Alabama, College of Medicine, Mobile, Alabama 36688-0002 AM. J. PHYSIOL. 277 (ADV. PHYSIOL.
Diabetologia 9 Springer-Verlag 1981
 Diabetologia (1981) 2:451-456 Diabetologia 9 Springer-Verlag 1981 Originals Increased Kidney Size, Glomerular Filtration Rate and Renal Plasma Flow in Short-Term Insulin-Dependent Diabetics J. Sandahl
Diabetologia (1981) 2:451-456 Diabetologia 9 Springer-Verlag 1981 Originals Increased Kidney Size, Glomerular Filtration Rate and Renal Plasma Flow in Short-Term Insulin-Dependent Diabetics J. Sandahl
Chapter 25 The Urinary System
 Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Functions of the kidney
 Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
REVERSAL OF LESIONS OF DIABETIC NEPHROPATHY AFTER PANCREAS TRANSPLANTATION REVERSAL OF LESIONS OF DIABETIC NEPHROPATHY AFTER PANCREAS TRANSPLANTATION
 REVERSAL OF LESIONS OF DIABETIC NEPHROPATHY AFTER PANCREAS TRANSPLANTATION PAOLA FIORETTO, M.D., PH.D., MICHAEL W. STEFFES, M.D., PH.D., DAVID E.R. SUTHERLAND, M.D., PH.D., FREDERICK C. GOETZ, M.D., AND
REVERSAL OF LESIONS OF DIABETIC NEPHROPATHY AFTER PANCREAS TRANSPLANTATION PAOLA FIORETTO, M.D., PH.D., MICHAEL W. STEFFES, M.D., PH.D., DAVID E.R. SUTHERLAND, M.D., PH.D., FREDERICK C. GOETZ, M.D., AND
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM
 RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION
 LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
Chapter 1: Exploring Data
 Chapter 1: Exploring Data Key Vocabulary:! individual! variable! frequency table! relative frequency table! distribution! pie chart! bar graph! two-way table! marginal distributions! conditional distributions!
Chapter 1: Exploring Data Key Vocabulary:! individual! variable! frequency table! relative frequency table! distribution! pie chart! bar graph! two-way table! marginal distributions! conditional distributions!
Microcirculation and Edema. Faisal I. Mohammed MD, PhD.
 Microcirculation and Edema Faisal I. Mohammed MD, PhD. Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchang in capillaries. Outline what
Microcirculation and Edema Faisal I. Mohammed MD, PhD. Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchang in capillaries. Outline what
Assessment of glomerular filtration rate in healthy subjects and normoalbuminuric diabetic patients: validity of a new (MDRD) prediction equation
 Nephrol Dial Transplant (2002) 17: 1909 1913 Original Article Assessment of glomerular filtration rate in healthy subjects and normoalbuminuric diabetic patients: validity of a new () prediction equation
Nephrol Dial Transplant (2002) 17: 1909 1913 Original Article Assessment of glomerular filtration rate in healthy subjects and normoalbuminuric diabetic patients: validity of a new () prediction equation
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Diabetologia 9 Springer-Verlag 1995
 Diabetologia (1995) 38:1197-1204 Diabetologia 9 Springer-Verlag 1995 Glomerular epithelial foot processes and filtration slits in IDDM patients S.F. Bjorn ~, H-J. Bangstad 2, K. E Hanssen 2, G. Nyberg
Diabetologia (1995) 38:1197-1204 Diabetologia 9 Springer-Verlag 1995 Glomerular epithelial foot processes and filtration slits in IDDM patients S.F. Bjorn ~, H-J. Bangstad 2, K. E Hanssen 2, G. Nyberg
The standard structural changes seen in type 1
 Podocyte Number in Normotensive Type 1 Diabetic Patients With Albuminuria Kathryn E. White, 1 Rudolf W. Bilous, 1 Sally M. Marshall, 1 Meguid El Nahas, 2 Giuseppe Remuzzi, 3 Giampiero Piras, 4 Salvatore
Podocyte Number in Normotensive Type 1 Diabetic Patients With Albuminuria Kathryn E. White, 1 Rudolf W. Bilous, 1 Sally M. Marshall, 1 Meguid El Nahas, 2 Giuseppe Remuzzi, 3 Giampiero Piras, 4 Salvatore
Quantitative protein estimation of Urine
 Quantitative protein estimation of Urine 1 In a healthy renal and urinary tract system, the urine contains no protein or only trace amounts. The presence of increased amounts of protein in the urine can
Quantitative protein estimation of Urine 1 In a healthy renal and urinary tract system, the urine contains no protein or only trace amounts. The presence of increased amounts of protein in the urine can
..." , \ I \ ... / ~\ \ : \\ ~ ... I CD ~ \ CD> 00\ CD) CD 0. Relative frequencies of numerical responses in ratio estimation1
 Relative frequencies of numerical responses in ratio estimation1 JOHN C. BAIRD.2 CHARLES LEWIS, AND DANIEL ROMER3 DARTMOUTH COLLEGE This study investigated the frequency of different numerical responses
Relative frequencies of numerical responses in ratio estimation1 JOHN C. BAIRD.2 CHARLES LEWIS, AND DANIEL ROMER3 DARTMOUTH COLLEGE This study investigated the frequency of different numerical responses
Diabetic nephropathy affects 25 30% of type 1
 Low Glomerular Filtration Rate in Normoalbuminuric Type 1 Diabetic Patients An Indicator of More Advanced Glomerular Lesions M. Luiza Caramori, 1 Paola Fioretto, 2 and Michael Mauer 1 Increased urinary
Low Glomerular Filtration Rate in Normoalbuminuric Type 1 Diabetic Patients An Indicator of More Advanced Glomerular Lesions M. Luiza Caramori, 1 Paola Fioretto, 2 and Michael Mauer 1 Increased urinary
Course of preeclamptic glomerular injury after delivery
 Am J Physiol Renal Physiol 294: F614 F620, 2008. First published January 16, 2008; doi:10.1152/ajprenal.00470.2007. Course of preeclamptic glomerular injury after delivery M. A. Hladunewich, 1 B. D. Myers,
Am J Physiol Renal Physiol 294: F614 F620, 2008. First published January 16, 2008; doi:10.1152/ajprenal.00470.2007. Course of preeclamptic glomerular injury after delivery M. A. Hladunewich, 1 B. D. Myers,
Nature Neuroscience: doi: /nn Supplementary Figure 1. Behavioral training.
 Supplementary Figure 1 Behavioral training. a, Mazes used for behavioral training. Asterisks indicate reward location. Only some example mazes are shown (for example, right choice and not left choice maze
Supplementary Figure 1 Behavioral training. a, Mazes used for behavioral training. Asterisks indicate reward location. Only some example mazes are shown (for example, right choice and not left choice maze
Sebastião Rodrigues Ferreira-Filho, Camila Caetano Cardoso, Luiz Augusto Vieira de Castro, Ricardo Mendes Oliveira, and Renata Rodrigues Sá
 SAGE-Hindawi Access to Research International Nephrology Volume 211, Article ID 626178, 4 pages doi:1.461/211/626178 Research Article Comparison of Measured Creatinine Clearance and Clearances Estimated
SAGE-Hindawi Access to Research International Nephrology Volume 211, Article ID 626178, 4 pages doi:1.461/211/626178 Research Article Comparison of Measured Creatinine Clearance and Clearances Estimated
CHAPTER 2. Primary Glomerulonephritis
 2nd Report of the PRIMARY GLOMERULONEPHRITIS CHAPTER 2 Primary Glomerulonephritis Sunita Bavanandan Lee Han Wei Lim Soo Kun 21 PRIMARY GLOMERULONEPHRITIS 2nd Report of the 2.1 Introduction This chapter
2nd Report of the PRIMARY GLOMERULONEPHRITIS CHAPTER 2 Primary Glomerulonephritis Sunita Bavanandan Lee Han Wei Lim Soo Kun 21 PRIMARY GLOMERULONEPHRITIS 2nd Report of the 2.1 Introduction This chapter
Unit 1 Exploring and Understanding Data
 Unit 1 Exploring and Understanding Data Area Principle Bar Chart Boxplot Conditional Distribution Dotplot Empirical Rule Five Number Summary Frequency Distribution Frequency Polygon Histogram Interquartile
Unit 1 Exploring and Understanding Data Area Principle Bar Chart Boxplot Conditional Distribution Dotplot Empirical Rule Five Number Summary Frequency Distribution Frequency Polygon Histogram Interquartile
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
You should know the T max for any substance that you use and for PAH ; T max = mg / min
 Tubular function - What is clearance? o clearance referred to the theoretical volume of plasma from which a substance is cleared ( cleaned ) over a period of time and so its unit would be ((ml/min)) -
Tubular function - What is clearance? o clearance referred to the theoretical volume of plasma from which a substance is cleared ( cleaned ) over a period of time and so its unit would be ((ml/min)) -
PHGY210 Renal Physiology
 PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
BIOL 2402 Renal Function
 BIOL 2402 Renal Function Dr. Chris Doumen Collin County Community College 1 Renal Clearance and GFR Refers to the volume of blood plasma from which a component is completely removed in one minute by all
BIOL 2402 Renal Function Dr. Chris Doumen Collin County Community College 1 Renal Clearance and GFR Refers to the volume of blood plasma from which a component is completely removed in one minute by all
** Accordingly GFR can be estimated by using one urine sample and do creatinine testing.
 This sheet includes the lecture and last year s exam. When a patient goes to a clinic, we order 2 tests: 1) kidney function test: in which we measure UREA and CREATININE levels, and electrolytes (Na+,
This sheet includes the lecture and last year s exam. When a patient goes to a clinic, we order 2 tests: 1) kidney function test: in which we measure UREA and CREATININE levels, and electrolytes (Na+,
3. PODOCYTE INJURY IN GLOMERULAR DISEASES
 How to Cite this article: Podocyte Injury in Glomerular Diseases - ejifcc 20/01 2009 http://www.ifcc.org 3. PODOCYTE INJURY IN GLOMERULAR DISEASES Mirjana Sabljar Matovinović Podocytes are injured in diabetic
How to Cite this article: Podocyte Injury in Glomerular Diseases - ejifcc 20/01 2009 http://www.ifcc.org 3. PODOCYTE INJURY IN GLOMERULAR DISEASES Mirjana Sabljar Matovinović Podocytes are injured in diabetic
Structural-Functional Relationships
 Structural-Functional Relationships in Diabetic Nephropathy S. Michael Mauer, Michael W. Steffes, Eileen N. Ellis, David E. R. Sutherland, David M. Brown, and Fredrick C. Goetz Departments ofpediatrics,
Structural-Functional Relationships in Diabetic Nephropathy S. Michael Mauer, Michael W. Steffes, Eileen N. Ellis, David E. R. Sutherland, David M. Brown, and Fredrick C. Goetz Departments ofpediatrics,
Year 2004 Paper one: Questions supplied by Megan
 QUESTION 53 Endothelial cell pathology on renal biopsy is most characteristic of which one of the following diagnoses? A. Pre-eclampsia B. Haemolytic uraemic syndrome C. Lupus nephritis D. Immunoglobulin
QUESTION 53 Endothelial cell pathology on renal biopsy is most characteristic of which one of the following diagnoses? A. Pre-eclampsia B. Haemolytic uraemic syndrome C. Lupus nephritis D. Immunoglobulin
Serum and urinary markers of early impairment of GFR in chronic kidney disease patients: diagnostic accuracy of urinary -trace protein
 Am J Physiol Renal Physiol 299: F1407 F1423, 2010. First published September 15, 2010; doi:10.1152/ajprenal.00507.2009. Serum and urinary markers of early impairment of GFR in chronic kidney disease patients:
Am J Physiol Renal Physiol 299: F1407 F1423, 2010. First published September 15, 2010; doi:10.1152/ajprenal.00507.2009. Serum and urinary markers of early impairment of GFR in chronic kidney disease patients:
Prof. Andrzej Wiecek Department of Nephrology, Endocrinology and Metabolic Diseases Medical University of Silesia Katowice, Poland.
 What could be the role of renal denervation in chronic kidney disease? Andrzej Wiecek, Katowice, Poland Chairs: Peter J. Blankestijn, Utrecht, The Netherlands Jonathan Moss, Glasgow, UK Prof. Andrzej Wiecek
What could be the role of renal denervation in chronic kidney disease? Andrzej Wiecek, Katowice, Poland Chairs: Peter J. Blankestijn, Utrecht, The Netherlands Jonathan Moss, Glasgow, UK Prof. Andrzej Wiecek
Case # 2 3/27/2017. Disclosure of Relevant Financial Relationships. Clinical history. Clinical history. Laboratory findings
 Case # 2 Christopher Larsen, MD Arkana Laboratories Disclosure of Relevant Financial Relationships USCAP requires that all planners (Education Committee) in a position to influence or control the content
Case # 2 Christopher Larsen, MD Arkana Laboratories Disclosure of Relevant Financial Relationships USCAP requires that all planners (Education Committee) in a position to influence or control the content
INVESTIGATION OF THE ULTRAFINE STRUCTURE OF THE KIDNEY BY MEANS OF SCANNING ELECTRON MICROSCOPE
 THE KURUME MEDICAL JOURNAL 1975 Vol.22, No.3, P.135-141 INVESTIGATION OF THE ULTRAFINE STRUCTURE OF THE KIDNEY BY MEANS OF SCANNING ELECTRON MICROSCOPE I. THE GLOMERULUS SHINSHI NODA Department of Urology,
THE KURUME MEDICAL JOURNAL 1975 Vol.22, No.3, P.135-141 INVESTIGATION OF THE ULTRAFINE STRUCTURE OF THE KIDNEY BY MEANS OF SCANNING ELECTRON MICROSCOPE I. THE GLOMERULUS SHINSHI NODA Department of Urology,
Elevated Serum Creatinine, a simplified approach
 Elevated Serum Creatinine, a simplified approach Primary Care Update Creighton University School of Medicine. April 27 th, 2018 Disclosure Slide I have no disclosures and have no conflicts with this presentation.
Elevated Serum Creatinine, a simplified approach Primary Care Update Creighton University School of Medicine. April 27 th, 2018 Disclosure Slide I have no disclosures and have no conflicts with this presentation.
URINARY SYSTEM. Urinary System
 URINARY SYSTEM Urinary System Kidney Functions Excretion Regulation of blood volume and pressure Regulation of electrolyte and ph levels Kidney Structure Gross Anatomy Fibrous Capsule Renal Cortex Renal
URINARY SYSTEM Urinary System Kidney Functions Excretion Regulation of blood volume and pressure Regulation of electrolyte and ph levels Kidney Structure Gross Anatomy Fibrous Capsule Renal Cortex Renal
Renal Pathology 1: Glomerulus. With many thanks to Elizabeth Angus PhD for EM photographs
 Renal Pathology 1: Glomerulus With many thanks to Elizabeth Angus PhD for EM photographs Anatomy of the Kidney http://www.yalemedicalgroup.org/stw/page.asp?pageid=stw028980 The Nephron http://www.beltina.org/health-dictionary/nephron-function-kidney-definition.html
Renal Pathology 1: Glomerulus With many thanks to Elizabeth Angus PhD for EM photographs Anatomy of the Kidney http://www.yalemedicalgroup.org/stw/page.asp?pageid=stw028980 The Nephron http://www.beltina.org/health-dictionary/nephron-function-kidney-definition.html
Section 3.2 Least-Squares Regression
 Section 3.2 Least-Squares Regression Linear relationships between two quantitative variables are pretty common and easy to understand. Correlation measures the direction and strength of these relationships.
Section 3.2 Least-Squares Regression Linear relationships between two quantitative variables are pretty common and easy to understand. Correlation measures the direction and strength of these relationships.
KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin
![KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin](/thumbs/87/96174332.jpg) Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
BY: Bernard L. Cohen University of Pittsburgh. Pittsburgh, PA ABSTRACT
 CORRELATION BETWEEN MEAN RADON LEVELS AND LUNG CANCER RATES IN U.S. COUNTIES A TEST OF THE LINEAR - NO THRESHOLD THEORY BY: Bernard L. Cohen University of Pittsburgh. Pittsburgh, PA 15260 ABSTRACT Mean
CORRELATION BETWEEN MEAN RADON LEVELS AND LUNG CANCER RATES IN U.S. COUNTIES A TEST OF THE LINEAR - NO THRESHOLD THEORY BY: Bernard L. Cohen University of Pittsburgh. Pittsburgh, PA 15260 ABSTRACT Mean
Indexes derived from non-linear ESPVR for evaluation of ventricular performance
 Modelling in Medicine and Biology X 133 Indexes derived from non-linear ESPVR for evaluation of ventricular performance R. M. Shoucri Department of Mathematics and Computer Science, Royal Military College
Modelling in Medicine and Biology X 133 Indexes derived from non-linear ESPVR for evaluation of ventricular performance R. M. Shoucri Department of Mathematics and Computer Science, Royal Military College
Evaluation of the Cockroft Gault, Jelliffe and Wright formulae in estimating renal function in elderly cancer patients
 Original article Annals of Oncology 15: 291 295, 2004 DOI: 10.1093/annonc/mdh079 Evaluation of the Cockroft Gault, Jelliffe and Wright formulae in estimating renal function in elderly cancer patients G.
Original article Annals of Oncology 15: 291 295, 2004 DOI: 10.1093/annonc/mdh079 Evaluation of the Cockroft Gault, Jelliffe and Wright formulae in estimating renal function in elderly cancer patients G.
THE PREVALENCE AND INCIDENCE of
 Podocyte Lesions in Patients With Obesity-Related Glomerulopathy Hui-Mei Chen, PhD, Zhi-Hong Liu, MD, Cai-Hong Zeng, PhD, Shi-Jun Li, PhD, Qing-Wen Wang, MD, and Lei-Shi Li, MD Background: Obesity-related
Podocyte Lesions in Patients With Obesity-Related Glomerulopathy Hui-Mei Chen, PhD, Zhi-Hong Liu, MD, Cai-Hong Zeng, PhD, Shi-Jun Li, PhD, Qing-Wen Wang, MD, and Lei-Shi Li, MD Background: Obesity-related
man of the effects of diabetes and of insulin on the maximum ability of the tubules to reabsorb glucose.
 EFFECT OF DIABETES AND INSULIN ON THE MAXIMUM CA- PACITY OF THE RENAL TUBULES TO REABSORB GLUCOSE t By SAUL J. FARBER, EUGENE Y. BERGER, AND DAVID P. EARLE (From the Department of Medicine, New York University
EFFECT OF DIABETES AND INSULIN ON THE MAXIMUM CA- PACITY OF THE RENAL TUBULES TO REABSORB GLUCOSE t By SAUL J. FARBER, EUGENE Y. BERGER, AND DAVID P. EARLE (From the Department of Medicine, New York University
RENAL EVENING SPECIALTY CONFERENCE
 RENAL EVENING SPECIALTY CONFERENCE Harsharan K. Singh, MD The University of North Carolina at Chapel Hill Disclosure of Relevant Financial Relationships No conflicts of interest to disclose. CLINICAL HISTORY
RENAL EVENING SPECIALTY CONFERENCE Harsharan K. Singh, MD The University of North Carolina at Chapel Hill Disclosure of Relevant Financial Relationships No conflicts of interest to disclose. CLINICAL HISTORY
2.75: 84% 2.5: 80% 2.25: 78% 2: 74% 1.75: 70% 1.5: 66% 1.25: 64% 1.0: 60% 0.5: 50% 0.25: 25% 0: 0%
 Capstone Test (will consist of FOUR quizzes and the FINAL test grade will be an average of the four quizzes). Capstone #1: Review of Chapters 1-3 Capstone #2: Review of Chapter 4 Capstone #3: Review of
Capstone Test (will consist of FOUR quizzes and the FINAL test grade will be an average of the four quizzes). Capstone #1: Review of Chapters 1-3 Capstone #2: Review of Chapter 4 Capstone #3: Review of
Diabetologia 9 Springer-Verlag 1991
 Diabetologia (i991) 34:668-674 0012186X9100181Y Diabetologia 9 Springer-Verlag 1991 Kidney transplantation in Type 1 (insulin-dependent) diabetic patients Early glomerulopathy R. f~sterby 1, G. Nyberg
Diabetologia (i991) 34:668-674 0012186X9100181Y Diabetologia 9 Springer-Verlag 1991 Kidney transplantation in Type 1 (insulin-dependent) diabetic patients Early glomerulopathy R. f~sterby 1, G. Nyberg
Urinary bladder provides a temporary storage reservoir for urine
 Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
I. General Features of Renal Function -Objectives II. Blood Flow and Filtration -Objectives III. Tubular Anatomy and Function -Objectives IV.
 ORGANIZATION This syllabus is divided into ten sections or topics. Each section is divided into objectives. On the contents page, clicking on the section title takes you directly to that section. Clicking
ORGANIZATION This syllabus is divided into ten sections or topics. Each section is divided into objectives. On the contents page, clicking on the section title takes you directly to that section. Clicking
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Nephron Anatomy Nephron Anatomy
 Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Ανάπτυξη Βιοτράπεζας για την Ανίχνευση Πρώιμων Βιοδεικτών σε Ασθενείς με Χρόνια Νεφρική Νόσο
 Ανάπτυξη Βιοτράπεζας για την Ανίχνευση Πρώιμων Βιοδεικτών σε Ασθενείς με Χρόνια Νεφρική Νόσο ΔΗΜΗΤΡΙΟΣ Σ. ΓΟΥΜΕΝΟΣ Νεφρολογικό και Μεταμοσχευτικό Κέντρο Πανεπιστημιακό Νοσοκομείο Πατρών Causes of chronic
Ανάπτυξη Βιοτράπεζας για την Ανίχνευση Πρώιμων Βιοδεικτών σε Ασθενείς με Χρόνια Νεφρική Νόσο ΔΗΜΗΤΡΙΟΣ Σ. ΓΟΥΜΕΝΟΣ Νεφρολογικό και Μεταμοσχευτικό Κέντρο Πανεπιστημιακό Νοσοκομείο Πατρών Causes of chronic
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
URINARY SYSTEM. Primary functions. Major organs & structures
 URINARY SYSTEM Primary functions Excretion of metabolic wastes Regulation of water and ion balances Regulation of blood pressure Vitamin D activation Regulation of rbc s (erythropoietin) Gluconeogenesis
URINARY SYSTEM Primary functions Excretion of metabolic wastes Regulation of water and ion balances Regulation of blood pressure Vitamin D activation Regulation of rbc s (erythropoietin) Gluconeogenesis
Pressure Diuresis 9 Sample Student Essays
 Pressure Diuresis 9 Sample Student Essays Below please find assembled consecutively in one document the brief analyses submitted by nine students in Mammalian Physiology 08 to the Teach Yourself Pressure
Pressure Diuresis 9 Sample Student Essays Below please find assembled consecutively in one document the brief analyses submitted by nine students in Mammalian Physiology 08 to the Teach Yourself Pressure
Introduction to Statistical Data Analysis I
 Introduction to Statistical Data Analysis I JULY 2011 Afsaneh Yazdani Preface What is Statistics? Preface What is Statistics? Science of: designing studies or experiments, collecting data Summarizing/modeling/analyzing
Introduction to Statistical Data Analysis I JULY 2011 Afsaneh Yazdani Preface What is Statistics? Preface What is Statistics? Science of: designing studies or experiments, collecting data Summarizing/modeling/analyzing
we usually use PAH - a substance called para-amino-hippouric acid to measure Clearance because it has the following characteristics :
 مرحبا.. Last Lecture we where talking about How to measure Renal Plasma Flow (aka RPF), Recall that we used a substrance that is 100 % CLEARED from the plasma ONCE it enter the kidney. Today s lecture
مرحبا.. Last Lecture we where talking about How to measure Renal Plasma Flow (aka RPF), Recall that we used a substrance that is 100 % CLEARED from the plasma ONCE it enter the kidney. Today s lecture
Evolution of incipient nephropathy in type 2 diabetes mellitus
 Kidney International, Vol. 58 (2000), pp. 1228 1237 CLINICAL NEPHROLOGY EPIDEMIOLOGY CLINICAL TRIALS Evolution of incipient nephropathy in type 2 diabetes mellitus KEVIN V. LEMLEY, ISHA ABDULLAH, BRYAN
Kidney International, Vol. 58 (2000), pp. 1228 1237 CLINICAL NEPHROLOGY EPIDEMIOLOGY CLINICAL TRIALS Evolution of incipient nephropathy in type 2 diabetes mellitus KEVIN V. LEMLEY, ISHA ABDULLAH, BRYAN
