CiPA, Pre-clinical Testing. Derek Leishman PhD DSP & ICH S7B
|
|
|
- Evan Martin
- 5 years ago
- Views:
Transcription
1 CiPA, Pre-clinical Testing Derek Leishman PhD DSP & ICH S7B
2 Outline Introduction Scenarios Conclusion Expressing an opinion What is not covered? Analysis/critique of the components too little time to try for ourselves.
3 TdP and QTc CPMP/986/96 The assessment for the potential for QT prolongation by noncardiovascular medicinal products The issue was drug-induced TdP Focus shifted quickly to the surrogate of QTc prolongation CiPA is shifting the focus back towards TdP Improved QTc measurement and concentration-qtc modeling have enabled assessment in early clinical studies
4 ICH S7B and E14 QTc QTc QTc QTc They work no unanticipated cases of drug-induced TdP with molecules approved after 2005 If it ain t broke why fix it? Nonclinical assessment had little impact on clinical development The scheme lacks specificity overall, leading to a lost opportunity cost for everyone Companies generally doing more any way Focusing on FIH study entry was the point of lowest overall value Abernathy & Leishman, SPS Mtg Berlin 2017
5 How Has CiPA Already Changed SP & S7B? CiPA has injected science in to a topic susceptible to becoming a check list of assays to conduct CiPA has brought new technology to proarrhythmia assessment Too new? Too different? CiPA has signaled an era of model-informed drug development Along with PBPK modeling it was the first example of PBPD modeling highlighted in a March 2017 FDA Advisory Committee Meeting Graaf, P. H. (2012), CPT: Pharmacometrics and Systems Pharmacology. CPT: Pharmacometrics & Systems Pharmacology, 1: doi: /psp
6 Softer Value Of CIPA A recent survey suggests many companies do additional assays rather than just GLP ICH S7B assessments CIPA creates a common framework of assays and a consistent basis for regulatory discussions Greater control and standardization of assay protocols under CIPA Appreciation of the value of earlier screening Nonclinical approaches used to address proarrhythmia issues. What best describes the nonclinical approach used to address proarrhythmia issues in your company? Answer options Conduct a battery of in vitro (i.e., binding studies, ion channel studies, etc.), and in vivo CV safety pharmacology assays to screen drug candidates and establish risk during drug discovery Conduct fit-for-purpose nonclinical safety pharmacology tests based on an integration of observations derived from chemistry (SAR), toxicology findings and other scientific considerations (e.g., drug indication, drug class, pharmacology, PK/PD etc.) Response percent Response count 75% 58 31% 24 Perform safety testing based on GLP findings from safety pharmacology and toxicology studies only 20% 15 I do not know 4% 3 Authier et al. (2017) Proarrhythmia liability assessment and the comprehensive in vitro Proarrhythmia Assay (CiPA): An industry survey on current practice. Journal of Pharmacological and Toxicological Methods, Volume 86, 34 43
7 My Opinion These are my opinions and not those of Eli Lilly and Company or the ICH E14/S7B discussion group Ion Channel In Silico Myocytes Clinical ECGs it is a positive to have effective standardized assays, I worry about the burden of the herg assay required to get the kinetic information, but believe it may not always be necessary this is a nice robust validation of modifications to a mature model, I worry about there being few ion channel neutral compounds in the training set a complex package with many moving parts, I worry about there being two methods each of which will give distinct concentration-response relationships and, more than one supplier of cells which will also gives distinct concentration-response relationships. I also worry about an assay to be used to identify mechanistic gaps but validated as a classification tool. a nice set of prospective mechanistic studies. I worry that these have been designed and tested to give mechanistic insight but may be used as classification tools. I also worry that they may not be sensitive enough when QTc changes are around 10ms. Decision-Making risk is a continuum but decisions usually become binary, will the practical cut-offs advance the scheme from where it is now?
8 Scenarios #1 A large pharma new small molecule target team #2 A large pharma team with a mature project with compound properties in the ion channel space #3 Biotech company with the GLP pre-fih data on a molecule which works at the target but had limited frontloaded safety work #4 A compound which had unexpected effects in the clinic Many scenarios are a blend between some of these
9 Large Pharma New Project This is the scenario familiar to safety pharmacologists for the last 2 decades ICH S7B works, therefore.. Select compounds which have a wide enough separation between concentrations associated with herg block and predicted efficacious concentrations Example criteria >1000-fold separation between target and herg IC 50, or a margin between efficacious free plasma concentrations and herg IC 50 >30-fold
10 Scenario #1 - What do we require from CiPA? Understand the relationship between conventional screening herg assay and the CiPA kinetic herg assay Agreed definition of no effect at clinically relevant concentrations Caveats allowed given very early stage e.g. if clinical concentrations increase a re-assessment would be necessary or taking in to account drug-drug interaction Would it be more generous than currently used? Agreed herg assay quality at FIH Result Assessment of Low Risk of TdP
11 Relationship Between herg Assays A kinetic model of drug-channel interaction allows simulation of patch clamp protocols Requires more elaborate experimental protocol to capture kinetics Can be used to understand differences between assays Overall it should provide a more robust assessment of drugchannel interaction Protocol Cisapride IC50 (nm) Fast patch 10s 31 Ramp 5s 38 VAP 12s 237 VAP 1s 38 0 mv mv 461
12 Large Pharma Mature Project In Suspect Space The intended target is perhaps another ion channel, or is a CNS target where good brain penetration is essential Having tried the conventional screening paradigm (#1) only molecules with modest selectivity or margins have desired efficacy and pharmacokinetic profiles Selected compound has a separation between efficacy target and herg between fold Selected compound has good brain penetration Note the if efficacy is CNS and TdP is a peripheral issue the plasma to brain ratio erodes margin
13 Scenario #2 What do we require from CiPA? The herg driven concentration-qtc analysis is key Predict from in vitro data (empirical or OHRd Model) Test in vivo in safety pharmacology assessments, conduct concentration-qtc analysis Conduct concentration-qtc analysis in early clinical assessments Understanding PBPK for molecule likely also critical Conduct herg-driven In Silico CiPA assessment metric likely to bridge the Low to Intermediate Risk threshold Additional ion channels may modify the risk metric but QTc prolongation remains possible Need to agree on clinical pharmacology and PBPK assessment Question Are the herg protocols robust enough for this context of use? Question Is the concentration-response information strong enough in isolated cardiomyocytes to reduce the reliance on the in vivo assessment? Question What might the label look like?
14 Predicting QTc Concentration-QTc relationship can be predicted from in vitro data using an empirically derived PKPD model or a quantitative systems pharmacology model The latter allows unique combinations of ion channel properties The range of predictions in these figures is based on the range of herg IC 50 s and the range of plasma protein binding values Focus on the herg assay and other sources of variability will allow distributions to be used rather than ranges
15 Biotech Company Molecule has positive findings in S7B assays Limited front-loading of safety assessment The GLP herg assay has a potency close enough to predicted efficacious range to suggest QTc prolongation likely in the clinic The a GLP in vivo study demonstrated no QTc prolongation in a low sensitivity assessment (power to detect 20ms change) Elements of this scenario resemble taking forward compounds in oncology Overall package of information is limited to required studies Question what is the torsadogenic risk?
16 Scenario #3 What do we require from CiPA? Based on current practice this compound may be discarded Conduct ion channel and in silico assessment need the additional ion channel data Make an in silico prediction of TdP risk Scan across multiples of predicted efficacious concentrations to see how risk metric changes Consider PBPK and likelihood of variability in exposure levels through drug-drug interactions etc Need consensus on likely risk level Need consensus on the overall risk benefit
17 Useful Space Heatmap Heat map illustrates that QTc prolongation is likely whenever there is appreciable herg block, despite calcium channel block The useful space can be restricted Below the white dashed lines are properties likely to be torsadogenic Jonker et al (2005) suggests focusing on less than 30% herg block Consideration of the reduction in the calcium transient AUC would restrict levels of calcium block which could be considered Heat Maps are a work in progress
18 Learn and Confirm Scenario #4 A compound believed to be in scenarios #1, 2 or 3 when tested in vivo or in man shows unexpected effects, for example. A concentration QTc effect larger than expected A JTc effect larger than anticipated for a low or intermediate risk This is a situation where what was learned nonclinically was not confirmed in the clinic Need to reassess where the potential gap is based on what is observed
19 Causes of Discrepancy Solubility issues with compounds more potent in vivo A metabolite appearing in animals or man which hadn t been accounted for A metabolite unique to man An indirect effect on QTc e.g. hypokalemia, hypoglycemia or hypothermia In this scenario, where ion channel and in vitro data has a low risk of TdP metric what are the implications What if the QTc and JTc are both prolonged? Does it change the risk assessment? How important is concentration-qtc assessment? How important are other biomarkers?
20 Can Q&As Based on CiPA to ICH S7B and E14 Address Scenarios? Scenario #1 Yes, by adding clarity on herg study quality and defining what is sufficient margin to be considered no effect Scenario #2 Yes, S7B already includes consideration of pharmacokinetics and drug interactions in the integrated risk assessment Scenario #3 Yes, S7B already suggests follow up assays. The CiPA paradigm is designed to address the question regarding TdP risk for such compounds and offers a structured way to do the integrated risk assessment Scenario #4 CiPA still appears to fall short on indirect effects on QTc but these do fit as a component of the integrated risk assessment in S7B (Gap to be filled)
21 To Conclude ICH S7B was focused solidly on predicting QTc prolongation while leaving unaddressed the question of distinguishing nontorsadogenic from torsadogenic effects There was discussion of additional considerations relevant to proarrhythmia risk CiPA appears to offer a supplemental package for S7B which could clarify some scenarios Gaps? Yes. Indirect effects may still be unresolved. The role and quality of in vivo QTc assessment unclear.
22 Acknowledgements Matt Abernathy Evan Wang Irene Bae
MEA assays using human ipsc-derived cardiomyocytes; challenges and opportunities
 MEA assays using human ipsc-derived cardiomyocytes; challenges and opportunities Event Presenter Date EMA Workshop 2017 Tessa de Korte, MSc 2017, October 5 Page 1 Ncardia at a glance Foundation Ncardia
MEA assays using human ipsc-derived cardiomyocytes; challenges and opportunities Event Presenter Date EMA Workshop 2017 Tessa de Korte, MSc 2017, October 5 Page 1 Ncardia at a glance Foundation Ncardia
Developing and Validating an In Silico Model for Proarrhythmia Risk Assessment Under the CiPA Initiative
 Developing and Validating an In Silico Model for Proarrhythmia Risk Assessment Under the CiPA Initiative May 2018 Zhihua Li, PhD for the CiPA In Silico Working Group US Food and Drug Administration This
Developing and Validating an In Silico Model for Proarrhythmia Risk Assessment Under the CiPA Initiative May 2018 Zhihua Li, PhD for the CiPA In Silico Working Group US Food and Drug Administration This
EXPOSURE RESPONSE ANALYSIS TO EVALUATE A DRUG'S EFFECT ON ECG PARAMETERS
 EXPOSURE RESPONSE ANALYSIS TO EVALUATE A DRUG'S EFFECT ON ECG PARAMETERS Christine Garnett, PharmD DCRP, FDA April 6, 2016 CSRC/FDA Workshop: The Proarrhythmic Assessment of New Chemical Entities Disclosures
EXPOSURE RESPONSE ANALYSIS TO EVALUATE A DRUG'S EFFECT ON ECG PARAMETERS Christine Garnett, PharmD DCRP, FDA April 6, 2016 CSRC/FDA Workshop: The Proarrhythmic Assessment of New Chemical Entities Disclosures
In Vitro Assessment to Replace the Clinical TQT Study: The Comprehensive In Vitro ProArrhythmia Assay (CiPA) Initiative
 In Vitro Assessment to Replace the Clinical TQT Study: The Comprehensive In Vitro ProArrhythmia Assay (CiPA) Initiative Gary Gintant, AbbVie for the Comprehensive in Vitro ProArrhythmia Assay Group Hot
In Vitro Assessment to Replace the Clinical TQT Study: The Comprehensive In Vitro ProArrhythmia Assay (CiPA) Initiative Gary Gintant, AbbVie for the Comprehensive in Vitro ProArrhythmia Assay Group Hot
Successes and Evolving Challenges Posed by the Comprehensive In Vitro Proarrhythmia (CiPA) Initiative
 Successes and Evolving Challenges Posed by the Comprehensive In Vitro Proarrhythmia (CiPA) Initiative Gary Gintant Dept. Integrative Pharmacology Integrated Sciences and Technology AbbVie For the CiPA
Successes and Evolving Challenges Posed by the Comprehensive In Vitro Proarrhythmia (CiPA) Initiative Gary Gintant Dept. Integrative Pharmacology Integrated Sciences and Technology AbbVie For the CiPA
Preclinical Perspectives of QT Outlook from ICH Guidelines
 Biometry in Early Clinical Research QT/QTc Interval Workshop in Heidelberg, November 17-19, 2005 Preclinical Perspectives of QT Outlook from ICH Guidelines Gerd Bode, M.D.,Ph.D. Frelance Consultant, e-mail
Biometry in Early Clinical Research QT/QTc Interval Workshop in Heidelberg, November 17-19, 2005 Preclinical Perspectives of QT Outlook from ICH Guidelines Gerd Bode, M.D.,Ph.D. Frelance Consultant, e-mail
Benchmarking In Silico Models and Candidate Metrics for Assessing the Risk of Torsade de Pointes
 Benchmarking In Silico Models and Candidate Metrics for Assessing the Risk of Torsade de Pointes Sara Dutta Division of Applied Regulatory Science/OCP/CDER CRSC/HESI/SPS/FDA Meeting December 11, 2014 Silver
Benchmarking In Silico Models and Candidate Metrics for Assessing the Risk of Torsade de Pointes Sara Dutta Division of Applied Regulatory Science/OCP/CDER CRSC/HESI/SPS/FDA Meeting December 11, 2014 Silver
Definitive QTc Assessment in Early Phase Trials: Expectations from FDA s Interdisciplinary Review Team
 Definitive QTc Assessment in Early Phase Trials: Expectations from FDA s Interdisciplinary Review Team Jiang Liu, Ph.D. Scientific Lead for QT-IRT Division of Pharmacometrics Office of Clinical Pharmacology
Definitive QTc Assessment in Early Phase Trials: Expectations from FDA s Interdisciplinary Review Team Jiang Liu, Ph.D. Scientific Lead for QT-IRT Division of Pharmacometrics Office of Clinical Pharmacology
Breakout #4 Phase 1 ECG:
 Breakout #4 Phase 1 ECG: Potential Role of ECG under CiPA HESI CSRC CiPA Meeting Washington, DC - May 22, 2018 Jose Vicente, PhD Christine Garnett, PharmD Division of Cardiovascular and Renal Products
Breakout #4 Phase 1 ECG: Potential Role of ECG under CiPA HESI CSRC CiPA Meeting Washington, DC - May 22, 2018 Jose Vicente, PhD Christine Garnett, PharmD Division of Cardiovascular and Renal Products
Drug-induced cardiotoxicity: screening methods, regulatory context, commercial environment. Beremans Ltd / Insight Pharma Reports April 2008
 Drug-induced cardiotoxicity: screening methods, regulatory context, commercial environment. Overview Beremans Ltd / Insight Pharma Reports April 2008 This report identifies and discusses methods, products
Drug-induced cardiotoxicity: screening methods, regulatory context, commercial environment. Overview Beremans Ltd / Insight Pharma Reports April 2008 This report identifies and discusses methods, products
Appropriate Use of Recovery Groups in Nonclinical Toxicity Studies: Value in a Science-Driven Case-by-Case Approach
 Appropriate Use of Recovery Groups in Nonclinical Toxicity Studies: Value in a Science-Driven Case-by-Case Approach Veterinary Pathology 49(2) 357-361 ª The Author(s) 2012 Reprints and permission: sagepub.com/journalspermissions.nav
Appropriate Use of Recovery Groups in Nonclinical Toxicity Studies: Value in a Science-Driven Case-by-Case Approach Veterinary Pathology 49(2) 357-361 ª The Author(s) 2012 Reprints and permission: sagepub.com/journalspermissions.nav
Safety Pharmacology Society Webinar: Simon Authier, DVM, MBA, PhD Director - Safety Pharmacology and Veterinary Science CIToxLAB North America
 Safety Pharmacology Society Webinar: Simon Authier, DVM, MBA, PhD Director - Safety Pharmacology and Veterinary Science CIToxLAB North America Agenda Introduction GI presentation rescheduled Upcoming SPS
Safety Pharmacology Society Webinar: Simon Authier, DVM, MBA, PhD Director - Safety Pharmacology and Veterinary Science CIToxLAB North America Agenda Introduction GI presentation rescheduled Upcoming SPS
Interpreting Adult Human Thorough QT Studies: Are They Relevant to Pediatric Safety?
 Interpreting Adult Human Thorough QT Studies: Are They Relevant to Pediatric Safety? Hao Zhu, Ph.D. Scientific Lead, QT-IRT, FDA (Dec 10, 2010) Disclaimer: The views expressed in this talk are those of
Interpreting Adult Human Thorough QT Studies: Are They Relevant to Pediatric Safety? Hao Zhu, Ph.D. Scientific Lead, QT-IRT, FDA (Dec 10, 2010) Disclaimer: The views expressed in this talk are those of
DOSE SELECTION FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS *)
 DOSE SELECTION FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS *) Guideline Title Dose Selection for Carcinogenicity Studies of Pharmaceuticals *) Legislative basis Directive 75/318/EEC as amended Date
DOSE SELECTION FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS *) Guideline Title Dose Selection for Carcinogenicity Studies of Pharmaceuticals *) Legislative basis Directive 75/318/EEC as amended Date
Recent insights from the FDA QT-IRT on concentration-qtc analysis and requirements for TQT study ( waiver ) substitution
 Recent insights from the FDA QT-IRT on concentration-qtc analysis and requirements for TQT study ( waiver ) substitution Dhananjay D. Marathe, Ph.D. QT-IRT Lead, Office of Clinical Pharmacology US FDA
Recent insights from the FDA QT-IRT on concentration-qtc analysis and requirements for TQT study ( waiver ) substitution Dhananjay D. Marathe, Ph.D. QT-IRT Lead, Office of Clinical Pharmacology US FDA
TCP Transl Clin Pharmacol
 TCP 2018;26(4):145-149 https://doi.org/10.12793/tcp.2018.26.4.145 Five years of the CiPA project (2013 2018) - what did we learn? Dong-Seok Yim* Department of Clinical Pharmacology and Therapeutics, Seoul
TCP 2018;26(4):145-149 https://doi.org/10.12793/tcp.2018.26.4.145 Five years of the CiPA project (2013 2018) - what did we learn? Dong-Seok Yim* Department of Clinical Pharmacology and Therapeutics, Seoul
InPulse: Development of an ipsc cardiomyocyte (ipsc-cm) platform to assess drug-induced contractility liabilities.
 InPulse: Development of an ipsc cardiomyocyte (ipsc-cm) platform to assess drug-induced contractility liabilities. Sponsors: Arun Sridhar, Peter J. Clements, Brian Berridge, Drug induced contractility
InPulse: Development of an ipsc cardiomyocyte (ipsc-cm) platform to assess drug-induced contractility liabilities. Sponsors: Arun Sridhar, Peter J. Clements, Brian Berridge, Drug induced contractility
IMPLEMENTATION OF NOVEL ECG BIOMARKERS
 IMPLEMENTATION OF NOVEL ECG BIOMARKERS CSRC CiPA meeting May 21, 2018 Washington DC Borje Darpo, MD, PhD, FESC Chief Scientific Officer, Cardiac Safety, ERT 1 Disclosures I work as a consultant for ERT,
IMPLEMENTATION OF NOVEL ECG BIOMARKERS CSRC CiPA meeting May 21, 2018 Washington DC Borje Darpo, MD, PhD, FESC Chief Scientific Officer, Cardiac Safety, ERT 1 Disclosures I work as a consultant for ERT,
TQT studies - this is the end!
 TQT studies - this is the end! Borje Darpo MD, PhD Associate Professor of Cardiology Chief Scientific Officer icardiac Technologies Yes as the only way to confidently exclude that a new drug has a clinically
TQT studies - this is the end! Borje Darpo MD, PhD Associate Professor of Cardiology Chief Scientific Officer icardiac Technologies Yes as the only way to confidently exclude that a new drug has a clinically
Preparing for Changing Cardiac Safety Regulations. Joy Olbertz, PharmD, PhD
 Preparing for Changing Cardiac Safety Regulations Joy Olbertz, PharmD, PhD Questions? Do I still have to assess proarrhythmic potential of my compound in a Thorough QT (TQT) study? If I have a TQT to conduct,
Preparing for Changing Cardiac Safety Regulations Joy Olbertz, PharmD, PhD Questions? Do I still have to assess proarrhythmic potential of my compound in a Thorough QT (TQT) study? If I have a TQT to conduct,
Preclinical Requirements for Therapeutic Studies in Humans with Advanced Cancer
 Preclinical Requirements for Therapeutic Studies in Humans with Advanced Cancer Scott Laurie MD, FRCPC The Ottawa Hospital Cancer Centre Associate Professor, University of Ottawa Disclosures I have no
Preclinical Requirements for Therapeutic Studies in Humans with Advanced Cancer Scott Laurie MD, FRCPC The Ottawa Hospital Cancer Centre Associate Professor, University of Ottawa Disclosures I have no
DOWNLOAD OR READ : UPDATE ON BIOMARKERS IN ASTHMA AN ISSUE OF IMMUNOLOGY AND ALLERGY CLINICS PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : UPDATE ON BIOMARKERS IN ASTHMA AN ISSUE OF IMMUNOLOGY AND ALLERGY CLINICS PDF EBOOK EPUB MOBI Page 1 Page 2 update on biomarkers in asthma an issue of immunology and allergy clinics
DOWNLOAD OR READ : UPDATE ON BIOMARKERS IN ASTHMA AN ISSUE OF IMMUNOLOGY AND ALLERGY CLINICS PDF EBOOK EPUB MOBI Page 1 Page 2 update on biomarkers in asthma an issue of immunology and allergy clinics
Reflection paper on the use of extrapolation in the development of medicines for paediatrics
 7 October 2018 EMA/189724/2018 Reflection paper on the use of extrapolation in the development of medicines for paediatrics Final Draft agreed by Biostatistics Working Party, Modelling and Simulation September
7 October 2018 EMA/189724/2018 Reflection paper on the use of extrapolation in the development of medicines for paediatrics Final Draft agreed by Biostatistics Working Party, Modelling and Simulation September
Evolution of strategies to improve preclinical cardiac safety testing
 OPINION Evolution of strategies to improve preclinical cardiac safety testing Gary Gintant, Philip T. Sager and Norman Stockbridge Abstract The early and efficient assessment of cardiac safety liabilities
OPINION Evolution of strategies to improve preclinical cardiac safety testing Gary Gintant, Philip T. Sager and Norman Stockbridge Abstract The early and efficient assessment of cardiac safety liabilities
Industry Experience of the Concentration-QTc Relationship in Phase 1 Studies. Corina Dota, AstraZeneca
 Industry Experience of the Concentration-QTc Relationship in Phase 1 Studies Corina Dota, AstraZeneca CSRC-2 th February 2012 ICH E14 2.2.5 Alternative Strategies to Assess QT/QTc Interval Effects Alternatives
Industry Experience of the Concentration-QTc Relationship in Phase 1 Studies Corina Dota, AstraZeneca CSRC-2 th February 2012 ICH E14 2.2.5 Alternative Strategies to Assess QT/QTc Interval Effects Alternatives
QT prolongation and drug-drug interactions. Filip Josephson M.D., Ph.D Clinical Assessor Swedish Medical Products Agency
 QT prolongation and drug-drug interactions Filip Josephson M.D., Ph.D Clinical Assessor Swedish Medical Products Agency Disposition Introduction to QT prolongation Three relevant DDI scenarios Concluding
QT prolongation and drug-drug interactions Filip Josephson M.D., Ph.D Clinical Assessor Swedish Medical Products Agency Disposition Introduction to QT prolongation Three relevant DDI scenarios Concluding
Can the Thorough QT Study Be Replaced? Norman Stockbridge Division of Cardiovascular and Renal Products FDA/CDER
 Can the Thorough QT Study Be Replaced? Norman Stockbridge Division of Cardiovascular and Renal Products FDA/CDER Can the TQT study be replaced? Yes. Thank you. Any questions? Four genre of replacement
Can the Thorough QT Study Be Replaced? Norman Stockbridge Division of Cardiovascular and Renal Products FDA/CDER Can the TQT study be replaced? Yes. Thank you. Any questions? Four genre of replacement
Safety Pharmacology Post Meeting Survey 2014
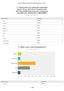 Q1 Please enter your personal information below. (If you want to be included in the 2014 Annual Meeting survey for a chance to win $200, this information is REQUIRED) Answered: 132 Skipped: 14 Name: Company:
Q1 Please enter your personal information below. (If you want to be included in the 2014 Annual Meeting survey for a chance to win $200, this information is REQUIRED) Answered: 132 Skipped: 14 Name: Company:
The ICHS1 Regulatory Testing Paradigm of Carcinogenicity in rats - Status Report
 INTERNATIONAL COUNCIL FOR HARMONISATION OF TECHNICAL REQUIREMENTS FOR PHARMACEUTICALS FOR HUMAN USE The ICHS1 Regulatory Testing Paradigm of Carcinogenicity in rats - Status Report Introduction The ICH
INTERNATIONAL COUNCIL FOR HARMONISATION OF TECHNICAL REQUIREMENTS FOR PHARMACEUTICALS FOR HUMAN USE The ICHS1 Regulatory Testing Paradigm of Carcinogenicity in rats - Status Report Introduction The ICH
DMPK. APRIL 27 TH 2017 Jan Neelissen Scientific Adviser Science & Technology
 DMPK APRIL 27 TH 2017 Jan Neelissen Scientific Adviser Science & Technology What I learned is a good DMPK profile have acceptable water solubility for development be completely absorbed, preferably via
DMPK APRIL 27 TH 2017 Jan Neelissen Scientific Adviser Science & Technology What I learned is a good DMPK profile have acceptable water solubility for development be completely absorbed, preferably via
The regulatory landscape Stephen White on behalf of the EBF
 The regulatory landscape Stephen White on behalf of the EBF http://www.europeanbioanalysisforum.eu Overview 1. MIST and DDI Guideline 2. Regulatory landscape for BA 3. Tiered Approach on BA of metabolites
The regulatory landscape Stephen White on behalf of the EBF http://www.europeanbioanalysisforum.eu Overview 1. MIST and DDI Guideline 2. Regulatory landscape for BA 3. Tiered Approach on BA of metabolites
ICH Topic S1C(R2) Dose Selection for Carcinogenicity Studies of Pharmaceuticals. Step 5
 European Medicines Agency October 2008 EMEA/CHMP/ICH/383/1995 ICH Topic S1C(R2) Dose Selection for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON DOSE SELECTION FOR CARCINOGENICITY
European Medicines Agency October 2008 EMEA/CHMP/ICH/383/1995 ICH Topic S1C(R2) Dose Selection for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON DOSE SELECTION FOR CARCINOGENICITY
Metrion Biosciences: the ion channel specialists
 Metrion Biosciences: the ion channel specialists In depth profiling of human ipsc cardiomyocytes: From electrophysiology to phenotypic assays Saïd El-Haou, PhD 30 November 2017 www.metrionbiosciences.com
Metrion Biosciences: the ion channel specialists In depth profiling of human ipsc cardiomyocytes: From electrophysiology to phenotypic assays Saïd El-Haou, PhD 30 November 2017 www.metrionbiosciences.com
Potential Best-In-Class Potent, Selective & Orally Active S1P 1 Agonists for Multiple Sclerosis
 Potential Best-In-Class Potent, Selective & Orally Active S1P 1 Agonists for Multiple Sclerosis Art Taveras, PhD March 25, 2009 Therapeutic Hypothesis of S1P Agonism Multiple Sclerosis (MS): A chronic
Potential Best-In-Class Potent, Selective & Orally Active S1P 1 Agonists for Multiple Sclerosis Art Taveras, PhD March 25, 2009 Therapeutic Hypothesis of S1P Agonism Multiple Sclerosis (MS): A chronic
Cardiac Safety Assessment. In-Vitro/In-Vivo Extrapolation
 Cardiac Safety Assessment In-Vitro/In-Vivo Extrapolation Drugs removed from the market for arrhythmia risk Courtesy of Dr. Norman Stockbridge Copyright 2014 Certara, L.P. All rights reserved. 2 Let s have
Cardiac Safety Assessment In-Vitro/In-Vivo Extrapolation Drugs removed from the market for arrhythmia risk Courtesy of Dr. Norman Stockbridge Copyright 2014 Certara, L.P. All rights reserved. 2 Let s have
Guidance for Industry
 Reprinted from FDA s website by Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs Questions and Answers (R1) U.S. Department
Reprinted from FDA s website by Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs Questions and Answers (R1) U.S. Department
The Opportunity: c-ibs and pain relief with confidence YKP10811
 The Opportunity: c-ibs and pain relief with confidence YKP10811 1 TABLE OF CONTENTS Profile Summary Clinical Data Mode of Action Pharmacologic Profile Safety and Toxicity Profile ADME Overview vs. Competitors
The Opportunity: c-ibs and pain relief with confidence YKP10811 1 TABLE OF CONTENTS Profile Summary Clinical Data Mode of Action Pharmacologic Profile Safety and Toxicity Profile ADME Overview vs. Competitors
PKPD modelling to optimize dose-escalation trials in Oncology
 PKPD modelling to optimize dose-escalation trials in Oncology Marina Savelieva Design of Experiments in Healthcare, Issac Newton Institute for Mathematical Sciences Aug 19th, 2011 Outline Motivation Phase
PKPD modelling to optimize dose-escalation trials in Oncology Marina Savelieva Design of Experiments in Healthcare, Issac Newton Institute for Mathematical Sciences Aug 19th, 2011 Outline Motivation Phase
FDA Use of Big Data in Modeling and Simulations
 FDA Use of Big Data in Modeling and Simulations Jeffry Florian, Ph.D., Division of Pharmacometrics, CDER/OTS/OCP/DPM DISCLAIMER: The views expressed in this presentation are that of the author and do not
FDA Use of Big Data in Modeling and Simulations Jeffry Florian, Ph.D., Division of Pharmacometrics, CDER/OTS/OCP/DPM DISCLAIMER: The views expressed in this presentation are that of the author and do not
Discussion of Changes in the Draft Preamble
 Discussion of Changes in the Draft Preamble Prepared by the staff of the IARC Monographs programme 31 August 2005 This paper describes the major changes that appear in the draft Preamble that will be reviewed
Discussion of Changes in the Draft Preamble Prepared by the staff of the IARC Monographs programme 31 August 2005 This paper describes the major changes that appear in the draft Preamble that will be reviewed
The QT interval as it relates to the safety of non-cardiac drugs
 European Heart Journal Supplements (2007) 9 (Supplement G), G3 G8 doi:10.1093/eurheartj/sum047 The QT interval as it relates to the safety of non-cardiac drugs Peter R. Kowey 1 * and Marek Malik 2,3 1
European Heart Journal Supplements (2007) 9 (Supplement G), G3 G8 doi:10.1093/eurheartj/sum047 The QT interval as it relates to the safety of non-cardiac drugs Peter R. Kowey 1 * and Marek Malik 2,3 1
Conflict of Interest Statement
 Specific Aspects and Approaches for Regulatory Evaluation of Pharmaceuticals in Two-Year Rodent Carcinogenicity Studies James A. Popp Stratoxon LLC Morgantown, PA Tel: 610.286.7592 popp@stratoxon.com Conflict
Specific Aspects and Approaches for Regulatory Evaluation of Pharmaceuticals in Two-Year Rodent Carcinogenicity Studies James A. Popp Stratoxon LLC Morgantown, PA Tel: 610.286.7592 popp@stratoxon.com Conflict
Challenges and Strategies to Facilitate Formulation Development of Pediatric Drug Products
 Challenges and Strategies to Facilitate Formulation Development of Pediatric Drug Products Session 5: Safety Qualification of Excipients Session Chairs: Darren Fegley (FDA) Lorrene Buckley (Eli Lilly &
Challenges and Strategies to Facilitate Formulation Development of Pediatric Drug Products Session 5: Safety Qualification of Excipients Session Chairs: Darren Fegley (FDA) Lorrene Buckley (Eli Lilly &
New ECG Biomarkers and their Potential Role in CiPA: Results and Implications
 New ECG Biomarkers and their Potential Role in CiPA: Results and Implications HESI CSRC CiPA Meeting Washington, DC - May 21, 2018 Jose Vicente, PhD for the ECG Biomarker Working Group Division of Cardiovascular
New ECG Biomarkers and their Potential Role in CiPA: Results and Implications HESI CSRC CiPA Meeting Washington, DC - May 21, 2018 Jose Vicente, PhD for the ECG Biomarker Working Group Division of Cardiovascular
The Structure/Function Relationship: What do we know? What would we like to know?
 The Structure/Function Relationship: What do we know? What would we like to know? Brian R. Berridge, DVM, PhD, DACVP GlaxoSmithKline Research Triangle Park, NC brian.x.berridge@gsk.com 919-315-6592 Outline
The Structure/Function Relationship: What do we know? What would we like to know? Brian R. Berridge, DVM, PhD, DACVP GlaxoSmithKline Research Triangle Park, NC brian.x.berridge@gsk.com 919-315-6592 Outline
Pharmacometric Modelling to Support Extrapolation in Regulatory Submissions
 Pharmacometric Modelling to Support Extrapolation in Regulatory Submissions Kristin Karlsson, PhD Pharmacometric/Pharmacokinetic assessor Medical Products Agency, Uppsala,Sweden PSI One Day Extrapolation
Pharmacometric Modelling to Support Extrapolation in Regulatory Submissions Kristin Karlsson, PhD Pharmacometric/Pharmacokinetic assessor Medical Products Agency, Uppsala,Sweden PSI One Day Extrapolation
DRAFT (Final) Concept Paper On choosing appropriate estimands and defining sensitivity analyses in confirmatory clinical trials
 DRAFT (Final) Concept Paper On choosing appropriate estimands and defining sensitivity analyses in confirmatory clinical trials EFSPI Comments Page General Priority (H/M/L) Comment The concept to develop
DRAFT (Final) Concept Paper On choosing appropriate estimands and defining sensitivity analyses in confirmatory clinical trials EFSPI Comments Page General Priority (H/M/L) Comment The concept to develop
Concepts and Case Study Template for Surrogate Endpoints Workshop. Lisa M. McShane, Ph.D. Biometric Research Program National Cancer Institute
 Concepts and Case Study Template for Surrogate Endpoints Workshop Lisa M. McShane, Ph.D. Biometric Research Program National Cancer Institute Medical Product Development GOAL is to improve how an individual
Concepts and Case Study Template for Surrogate Endpoints Workshop Lisa M. McShane, Ph.D. Biometric Research Program National Cancer Institute Medical Product Development GOAL is to improve how an individual
Use of Target Tissue Concentrations in PK-PD Modeling of Inhaled Drugs Ramon Hendrickx, AstraZeneca, RIA IMed Gothenburg, Sweden
 Use of Target Tissue Concentrations in PK-PD Modeling of Inhaled Drugs Ramon Hendrickx, AstraZeneca, RIA IMed Gothenburg, Sweden IQ DMPK Leadership Group 31 May 2017 What s On? Lung as target organ Benefit
Use of Target Tissue Concentrations in PK-PD Modeling of Inhaled Drugs Ramon Hendrickx, AstraZeneca, RIA IMed Gothenburg, Sweden IQ DMPK Leadership Group 31 May 2017 What s On? Lung as target organ Benefit
NASH Regulatory Landscape. Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH
 NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
Risk and Benefit Assessments for Optimal Dose Selection Based on Exposure Response
 Risk and Benefit Assessments for Optimal Dose Selection Based on Exposure Response 2003 FDA/Industry Statistics Workshop Peter I. Lee, PhD Associate Director, Pharmacometrics OCPB, CDER, FDA Disclaimers
Risk and Benefit Assessments for Optimal Dose Selection Based on Exposure Response 2003 FDA/Industry Statistics Workshop Peter I. Lee, PhD Associate Director, Pharmacometrics OCPB, CDER, FDA Disclaimers
Is a Maximal Tolerated Dose in Human useful for drug development?
 Is a Maximal Tolerated Dose in Human useful for drug development? How to define an acceptable highest dose to be tested? EuFeMED, London Pre-conference Workshop 17-May-2017 Eric Legangneux Philippe Grosjean
Is a Maximal Tolerated Dose in Human useful for drug development? How to define an acceptable highest dose to be tested? EuFeMED, London Pre-conference Workshop 17-May-2017 Eric Legangneux Philippe Grosjean
Tracer studies in GSK for Discovery and Development
 Tracer studies in GSK for Discovery and Development 3 rd June 2010...a ripple or a wavefront? Graeme Young, DMPK, GlaxoSmithKline R&D, Ware, UK Outline Historical use of AMS at GSK variety of ADME studies;
Tracer studies in GSK for Discovery and Development 3 rd June 2010...a ripple or a wavefront? Graeme Young, DMPK, GlaxoSmithKline R&D, Ware, UK Outline Historical use of AMS at GSK variety of ADME studies;
COMMENTS. Submitted by The International Pharmaceutical Aerosol Consortium
 COMMENTS on a draft Guidance for Industry Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products Chemistry, Manufacturing, and Controls Documentation (Docket No. 99D-1454) Submitted by
COMMENTS on a draft Guidance for Industry Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products Chemistry, Manufacturing, and Controls Documentation (Docket No. 99D-1454) Submitted by
Preclinical Approaches to Assess Potential Small Molecule Kinase Inhibitor-Induced Cardiac Toxicity: Past, Present, and Future
 Preclinical Approaches to Assess Potential Small Molecule Kinase Inhibitor-Induced Cardiac Toxicity: Past, Present, and Future Baichun Yang, MD, PhD, DABT FDA/CDER/OND/DCRP Opinions presented here are
Preclinical Approaches to Assess Potential Small Molecule Kinase Inhibitor-Induced Cardiac Toxicity: Past, Present, and Future Baichun Yang, MD, PhD, DABT FDA/CDER/OND/DCRP Opinions presented here are
Discussion Meeting for MCP-Mod Qualification Opinion Request. Novartis 10 July 2013 EMA, London, UK
 Discussion Meeting for MCP-Mod Qualification Opinion Request Novartis 10 July 2013 EMA, London, UK Attendees Face to face: Dr. Frank Bretz Global Statistical Methodology Head, Novartis Dr. Björn Bornkamp
Discussion Meeting for MCP-Mod Qualification Opinion Request Novartis 10 July 2013 EMA, London, UK Attendees Face to face: Dr. Frank Bretz Global Statistical Methodology Head, Novartis Dr. Björn Bornkamp
METHODOLOGIES FOR INTERMITTENT AND SHORT-TERM EXPOSURE TO CARCINOGENS SUBCOMMITTEE
 METHODOLOGIES FOR INTERMITTENT AND SHORT-TERM EXPOSURE TO CARCINOGENS SUBCOMMITTEE Mission The mission of the MISTEC Subcommittee is to develop methodologies for establishing appropriate dose-metrics to
METHODOLOGIES FOR INTERMITTENT AND SHORT-TERM EXPOSURE TO CARCINOGENS SUBCOMMITTEE Mission The mission of the MISTEC Subcommittee is to develop methodologies for establishing appropriate dose-metrics to
Concentration-QTc analysis to obviate the need for a dedicated QTc study in cancer patients: ixazomib, an oral proteasome inhibitor, as a case study
 Concentration-QTc analysis to obviate the need for a dedicated QTc study in cancer patients: ixazomib, an oral proteasome inhibitor, as a case study Neeraj Gupta, Ph.D. Outline Concentration-QTc analysis
Concentration-QTc analysis to obviate the need for a dedicated QTc study in cancer patients: ixazomib, an oral proteasome inhibitor, as a case study Neeraj Gupta, Ph.D. Outline Concentration-QTc analysis
Masterstudiengang TOXIKOLOGIE
 Masterstudiengang TOXIKOLOGIE Kolloquium Toxikologie Präklinische Untersuchungsstrategien in der Europäischen Arzneimittelzulassung 21. Januar 2009 Rolf Baß, Berlin Arzneimittelzulassung Gesetze (Europa,
Masterstudiengang TOXIKOLOGIE Kolloquium Toxikologie Präklinische Untersuchungsstrategien in der Europäischen Arzneimittelzulassung 21. Januar 2009 Rolf Baß, Berlin Arzneimittelzulassung Gesetze (Europa,
HESI Technical Committee on Cardiovascular Safety: Advancing the clinical relevance of preclinical assessments
 HESI Technical Committee on Cardiovascular Safety: Advancing the clinical relevance of preclinical assessments Brian R. Berridge, DVM, PhD, DACVP Committee Co-Chair ILSI Health and Environmental Sciences
HESI Technical Committee on Cardiovascular Safety: Advancing the clinical relevance of preclinical assessments Brian R. Berridge, DVM, PhD, DACVP Committee Co-Chair ILSI Health and Environmental Sciences
NOTE FOR GUIDANCE ON TOXICOKINETICS: THE ASSESSMENT OF SYSTEMIC EXPOSURE IN TOXICITY STUDIES S3A
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NOTE FOR GUIDANCE ON TOXICOKINETICS: THE ASSESSMENT
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NOTE FOR GUIDANCE ON TOXICOKINETICS: THE ASSESSMENT
Use of Bridging Justifications to Support the Safety of Excipients in Generic Drug Products
 Use of Bridging Justifications to Support the Safety of Excipients in Generic Drug Products Sruthi King, Ph.D. Pharmacology/Toxicology Team Leader Division of Clinical Review, Office of Generic Drugs Center
Use of Bridging Justifications to Support the Safety of Excipients in Generic Drug Products Sruthi King, Ph.D. Pharmacology/Toxicology Team Leader Division of Clinical Review, Office of Generic Drugs Center
PKPD models to describe the growth and inhibition of xenograft tumors by
 Applying mechanistic c PKPD models to describe the growth and inhibition of xenograft tumors by targeted anti-cancer agents. James WT Yates, Neil Evans, RD Owen Jones, Mike Walker, Patricia Schroeder,
Applying mechanistic c PKPD models to describe the growth and inhibition of xenograft tumors by targeted anti-cancer agents. James WT Yates, Neil Evans, RD Owen Jones, Mike Walker, Patricia Schroeder,
Use of quantitative tools for study planning purposes and study design optimisation
 Use of quantitative tools for study planning purposes and study design optimisation Valeria Gigante AIFA - Italian Medicines Agency EMA MSWG 12.06.2016 Public Declaration of transparency/interests* The
Use of quantitative tools for study planning purposes and study design optimisation Valeria Gigante AIFA - Italian Medicines Agency EMA MSWG 12.06.2016 Public Declaration of transparency/interests* The
Section 5.3: Preclinical safety data
 Section 5.3: Preclinical safety data SmPC training presentation Note: for full information refer to the European Commission s Guideline on summary of product characteristics (SmPC) SmPC Advisory Group
Section 5.3: Preclinical safety data SmPC training presentation Note: for full information refer to the European Commission s Guideline on summary of product characteristics (SmPC) SmPC Advisory Group
The Comet Assay Tails of the (Un)expected
 The Comet Assay Tails of the (Un)expected Use of the in vivo comet assay in pharmaceutical development Bas-jan van der Leede Janssen R&D Johnson & Johnson Pharmaceutical Companies Discovery Sciences Genetic
The Comet Assay Tails of the (Un)expected Use of the in vivo comet assay in pharmaceutical development Bas-jan van der Leede Janssen R&D Johnson & Johnson Pharmaceutical Companies Discovery Sciences Genetic
Druggable Future: What it takes and how we can get there
 Precision Medicine: Present challenges for Future Cures Druggable Future: What it takes and how we can get there Guido Guidi Venice - 18 September 2015 1 Precision Medicine: Where and how to go there?
Precision Medicine: Present challenges for Future Cures Druggable Future: What it takes and how we can get there Guido Guidi Venice - 18 September 2015 1 Precision Medicine: Where and how to go there?
Modeling and Simulation Approaches for Cardiovascular Function and Their Role in Safety Assessment
 Citation: CPT Pharmacometrics Syst. Pharmacol. (2015) 4, 175 188; VC 2015 ASCPT All rights reserved doi:10.1002/psp4.18 REVIEW Modeling and Simulation Approaches for Cardiovascular Function and Their Role
Citation: CPT Pharmacometrics Syst. Pharmacol. (2015) 4, 175 188; VC 2015 ASCPT All rights reserved doi:10.1002/psp4.18 REVIEW Modeling and Simulation Approaches for Cardiovascular Function and Their Role
BIOMARKER DRIVEN EARLY PHASE ONCOLOGY CLINICAL TRIALS; CHALLENGES IN THE REAL WORLD SID KATUGAMPOLA CENTRE FOR DRUG DEVELOPMENT CANCER RESEARCH UK
 BIOMARKER DRIVEN EARLY PHASE ONCOLOGY CLINICAL TRIALS; CHALLENGES IN THE REAL WORLD SID KATUGAMPOLA CENTRE FOR DRUG DEVELOPMENT CANCER RESEARCH UK Cancer; Some Sobering Thoughts Getting cancer is one of
BIOMARKER DRIVEN EARLY PHASE ONCOLOGY CLINICAL TRIALS; CHALLENGES IN THE REAL WORLD SID KATUGAMPOLA CENTRE FOR DRUG DEVELOPMENT CANCER RESEARCH UK Cancer; Some Sobering Thoughts Getting cancer is one of
Voltage-Gated Ion Channels as Drug Targets
 Voltage-Gated Ion Channels as Drug Targets Edited by David J. Triggle, Murali Gopalakrishnan, David Rampe, and Wei Zheng WILEY- VCH WILEY-VCH Verlag GmbH & Co. KGaA Preface XI 1 Introduction - On Ion Channels
Voltage-Gated Ion Channels as Drug Targets Edited by David J. Triggle, Murali Gopalakrishnan, David Rampe, and Wei Zheng WILEY- VCH WILEY-VCH Verlag GmbH & Co. KGaA Preface XI 1 Introduction - On Ion Channels
REVISITING THE PHARMACOKINETICS IN PATIENTS
 REVISITING THE PHARMACOKINETICS IN PATIENTS WITH IMPAIRED RENAL FUNCTION IN THE LIGHT OF THE LATEST FDA AND EMA GUIDELINES 3 RD CONFERENCE OF EUROPEAN PHARMACOLOGICAL SOCIETIES EUROPEAN COMPETITIVENESS
REVISITING THE PHARMACOKINETICS IN PATIENTS WITH IMPAIRED RENAL FUNCTION IN THE LIGHT OF THE LATEST FDA AND EMA GUIDELINES 3 RD CONFERENCE OF EUROPEAN PHARMACOLOGICAL SOCIETIES EUROPEAN COMPETITIVENESS
Patient Susceptibilities in Preclinical Drug Safety Assessment
 Patient Susceptibilities in Preclinical Drug Safety Assessment B. R. Berridge GlaxoSmithKline ILAR Roundtable October, 2017 Animal studies represent an apical preclinical modeling system in drug development
Patient Susceptibilities in Preclinical Drug Safety Assessment B. R. Berridge GlaxoSmithKline ILAR Roundtable October, 2017 Animal studies represent an apical preclinical modeling system in drug development
PK-PD modelling to support go/no go decisions for a novel gp120 inhibitor
 PK-PD modelling to support go/no go decisions for a novel gp120 inhibitor Phylinda LS Chan Pharmacometrics, Pfizer, UK EMA-EFPIA Modelling and Simulation Workshop BOS1 Pharmacometrics Global Clinical Pharmacology
PK-PD modelling to support go/no go decisions for a novel gp120 inhibitor Phylinda LS Chan Pharmacometrics, Pfizer, UK EMA-EFPIA Modelling and Simulation Workshop BOS1 Pharmacometrics Global Clinical Pharmacology
Changing the paradigm: Removing barriers, building bridges
 Changing the paradigm: Removing barriers, building bridges Jeffrey S. Hyams, MD Mandell Braunstein Family Endowed Chair in Pediatric IBD Head, Division of Digestive Diseases, Hepatology, and Nutrition
Changing the paradigm: Removing barriers, building bridges Jeffrey S. Hyams, MD Mandell Braunstein Family Endowed Chair in Pediatric IBD Head, Division of Digestive Diseases, Hepatology, and Nutrition
Building innovative drug discovery alliances. Hepatic uptake and drug disposition o in vitro and in silico approaches
 Building innovative drug discovery alliances Hepatic uptake and drug disposition o in vitro and in silico approaches Dr Beth Williamson Evotec AG, 2017 Outline Importance of predicting clearance In vitro
Building innovative drug discovery alliances Hepatic uptake and drug disposition o in vitro and in silico approaches Dr Beth Williamson Evotec AG, 2017 Outline Importance of predicting clearance In vitro
Drug-Disease Modeling Applied to Drug Development and Regulatory Decision Making in the Type 2 Diabetes Mellitus Arena
 Drug-Disease Modeling Applied to Drug Development and Regulatory Decision Making in the Type 2 Diabetes Mellitus Arena Tokyo, Japan, December 8, 2015 Stephan Schmidt, Ph.D. Assistant Professor Center for
Drug-Disease Modeling Applied to Drug Development and Regulatory Decision Making in the Type 2 Diabetes Mellitus Arena Tokyo, Japan, December 8, 2015 Stephan Schmidt, Ph.D. Assistant Professor Center for
Addressing Evolving CHMP Guidance of Phase I Study Designs
 Addressing Evolving CHMP Guidance of Phase I Study Designs BIA 10-2474 background Lutz Müller Project Leader in Pharmaceutical Science Roche Innovation Center Basel Context: Modern Ph I Studies Are Generally
Addressing Evolving CHMP Guidance of Phase I Study Designs BIA 10-2474 background Lutz Müller Project Leader in Pharmaceutical Science Roche Innovation Center Basel Context: Modern Ph I Studies Are Generally
Can non-clinical repolarization assays predict the results of clinical thorough QT studies? Results from a research consortium
 BJP British Journal of Pharmacology RESEARCH PAPER British Journal of Pharmacology (2018) 175 606 617 606 Can non-clinical repolarization assays predict the results of clinical thorough QT studies? Results
BJP British Journal of Pharmacology RESEARCH PAPER British Journal of Pharmacology (2018) 175 606 617 606 Can non-clinical repolarization assays predict the results of clinical thorough QT studies? Results
Alex Dmitrienko (Eli Lilly and Company), Chair of Distance Training, Biopharmaceutical Section of ASA
 Spring 2008 series Introduction Alex Dmitrienko (Eli Lilly and Company), Chair of Distance Training, Biopharmaceutical Section of ASA Assessment of QTc prolongation in clinical drug development Alex Dmitrienko
Spring 2008 series Introduction Alex Dmitrienko (Eli Lilly and Company), Chair of Distance Training, Biopharmaceutical Section of ASA Assessment of QTc prolongation in clinical drug development Alex Dmitrienko
IQ-CSRC Prospective Study - Background and Objectives
 IQ-CSRC Prospective Study - Background and Objectives December 12, 2014 Borje Darpo MD, PhD icardiac Technologies Co-chair SoC, CSRC Objectives of the IQ-CSRC Prospective Study The objective of the initiative
IQ-CSRC Prospective Study - Background and Objectives December 12, 2014 Borje Darpo MD, PhD icardiac Technologies Co-chair SoC, CSRC Objectives of the IQ-CSRC Prospective Study The objective of the initiative
General Chapter/Section: <232> Elemental Impurities - Limits Expert Committee(s): General Chapters Chemical Analysis No.
 General Chapter/Section: Elemental Impurities - Limits Expert Committee(s): General Chapters Chemical Analysis No. of Commenters: 18 Editorial changes suggested by commenters have been reviewed by
General Chapter/Section: Elemental Impurities - Limits Expert Committee(s): General Chapters Chemical Analysis No. of Commenters: 18 Editorial changes suggested by commenters have been reviewed by
Model Informed Drug Development: Is there a need to repaint the canvas?
 Model Informed Drug Development: Is there a need to repaint the canvas? Regulatory Perspectives Vikram Sinha, PhD Director, Division of Pharmacometrics Office of Clinical Pharmacology Office of Translational
Model Informed Drug Development: Is there a need to repaint the canvas? Regulatory Perspectives Vikram Sinha, PhD Director, Division of Pharmacometrics Office of Clinical Pharmacology Office of Translational
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/19939 holds various files of this Leiden University dissertation. Author: Chain, Anne S.Y. Title: Mind the gap : predicting cardiovascular risk during drug
Cover Page The handle http://hdl.handle.net/1887/19939 holds various files of this Leiden University dissertation. Author: Chain, Anne S.Y. Title: Mind the gap : predicting cardiovascular risk during drug
FDA's Analysis and Interpretation of the IQ/CSRC Clinical Study
 FDA's Analysis and Interpretation of the IQ/CSRC Clinical Study Jiang Liu Scientific Lead of QT-IRT Division of Pharmacometrics OCP/OTS/CDER/FDA Disclaimer: My remarks today do not necessarily reflect
FDA's Analysis and Interpretation of the IQ/CSRC Clinical Study Jiang Liu Scientific Lead of QT-IRT Division of Pharmacometrics OCP/OTS/CDER/FDA Disclaimer: My remarks today do not necessarily reflect
Nonclinical Safety Evaluation of Inhalation Drug Products
 Nonclinical Safety Evaluation of Inhalation Drug Products February 13, 2002 Life Science Research Organization Bethesda, Maryland Luqi Pei, Ph.D. Division of Pulmonary and Allergy Drug Products Center
Nonclinical Safety Evaluation of Inhalation Drug Products February 13, 2002 Life Science Research Organization Bethesda, Maryland Luqi Pei, Ph.D. Division of Pulmonary and Allergy Drug Products Center
E11(R1) Addendum to E11: Clinical Investigation of Medicinal Products in the Pediatric Population Step4
 E11(R1) Addendum to E11: Clinical Investigation of Medicinal Products in the Step4 October 2017 International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use 1 Legal
E11(R1) Addendum to E11: Clinical Investigation of Medicinal Products in the Step4 October 2017 International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use 1 Legal
The Importance of Early Interaction with FDA: EOP2A Experiences
 The Importance of Early Interaction with FDA: EOP2A Experiences Yaning Wang, Ph.D. Office of Clinical Pharmacology Center of Drug Evaluation and Research Food and Drug Administration Schematic of Development
The Importance of Early Interaction with FDA: EOP2A Experiences Yaning Wang, Ph.D. Office of Clinical Pharmacology Center of Drug Evaluation and Research Food and Drug Administration Schematic of Development
Ncardia. Assessment of pro-arrhythmic effects in Pluricyte Cardiomyocytes. using the Axion BioSystems Maestro TM MEA system
 Ncardia Stem cell experts Assessment of pro-arrhythmic effects in Pluricyte Cardiomyocytes using the Axion BioSystems Maestro TM MEA system Application note Version 2.0 Contents 1. Introduction 1 2. Assessment
Ncardia Stem cell experts Assessment of pro-arrhythmic effects in Pluricyte Cardiomyocytes using the Axion BioSystems Maestro TM MEA system Application note Version 2.0 Contents 1. Introduction 1 2. Assessment
Migraine: Developing Drugs for Acute Treatment Guidance for Industry
 Migraine: Developing Drugs for Acute Treatment Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2018
Migraine: Developing Drugs for Acute Treatment Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2018
When does exposure matching require additional consideration?
 When does exposure matching require additional consideration? Jeffrey S Barrett, PhD, FCP Vice-President, Interdisciplinary Pharmacometrics Program Global Head, Pediatric Clinical Pharmacology Sanofi Pharmaceuticals
When does exposure matching require additional consideration? Jeffrey S Barrett, PhD, FCP Vice-President, Interdisciplinary Pharmacometrics Program Global Head, Pediatric Clinical Pharmacology Sanofi Pharmaceuticals
Workshop # 3 : Application of Bayesian Statistics in early development studies
 Workshop # 3 : Application of Bayesian Statistics in early development studies Francois Vandenhende, ClinBAY Patricia Sanwald Ducray, Roche Joint Conference of European Human Pharmacological Societies
Workshop # 3 : Application of Bayesian Statistics in early development studies Francois Vandenhende, ClinBAY Patricia Sanwald Ducray, Roche Joint Conference of European Human Pharmacological Societies
Effects of reference compounds on impedance signals from stem cellderived human cardiomyocytes
 Effects of reference compounds on impedance signals from stem cellderived human cardiomyocytes Herbert M. Himmel, Safety Pharmacology, Bayer Pharma AG, Wuppertal, Germany SPS Webinar Cardiac Safety Testing
Effects of reference compounds on impedance signals from stem cellderived human cardiomyocytes Herbert M. Himmel, Safety Pharmacology, Bayer Pharma AG, Wuppertal, Germany SPS Webinar Cardiac Safety Testing
PQRI PODP Extractables & Leachables Workshop Leachable Evaluation of a Container Closure System - What to do When Above the Threshold
 PQRI PODP Extractables & Leachables Workshop Leachable Evaluation of a Container Closure System - What to do When Above the Threshold William P. Beierschmitt, PhD, DABT, FATS Drug Safety Research and Development
PQRI PODP Extractables & Leachables Workshop Leachable Evaluation of a Container Closure System - What to do When Above the Threshold William P. Beierschmitt, PhD, DABT, FATS Drug Safety Research and Development
The Maximum Mean Difference
 The Maximum Mean Difference Statistical Problems in Assessing Cardiac Safety Georg Ferber, Novartis Pharma AG, Basel ROeS Seminar Bern, 10-13 Sep 2007 Overview QT prolongation some background The Thorough
The Maximum Mean Difference Statistical Problems in Assessing Cardiac Safety Georg Ferber, Novartis Pharma AG, Basel ROeS Seminar Bern, 10-13 Sep 2007 Overview QT prolongation some background The Thorough
Analytical Performance in the The Test Evaluation Cycle
 Analytical Performance Specifications based on Outcome Studies: Is it possible? Test Evaluation Working Group of EFLM www.efcclm.eu SEALS Department of Clinical Chemistry, Prince of Wales Hospital, Sydney
Analytical Performance Specifications based on Outcome Studies: Is it possible? Test Evaluation Working Group of EFLM www.efcclm.eu SEALS Department of Clinical Chemistry, Prince of Wales Hospital, Sydney
ICH Topic S1B Carcinogenicity: Testing for Carcinogenicity of Pharmaceuticals. Step 5
 European Medicines Agency March 1998 CPMP/ICH/299/95 ICH Topic S1B Carcinogenicity: Testing for Carcinogenicity of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON CARCINOGENICITY: TESTING FOR CARCINOGENICITY
European Medicines Agency March 1998 CPMP/ICH/299/95 ICH Topic S1B Carcinogenicity: Testing for Carcinogenicity of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON CARCINOGENICITY: TESTING FOR CARCINOGENICITY
The EU PIP - a step in Pediatric Drug Development. Thomas Severin Bonn,
 The EU PIP - a step in Pediatric Drug Development Thomas Severin Bonn, 13.01.2009 Agenda Implications for Industry Company Preparation Time of PIP Submission Content of the PIP The PIP Process and first
The EU PIP - a step in Pediatric Drug Development Thomas Severin Bonn, 13.01.2009 Agenda Implications for Industry Company Preparation Time of PIP Submission Content of the PIP The PIP Process and first
Design and Analysis of QT/QTc Studies Conceptional and Methodical Considerations Based on Experience
 Design and Analysis of QT/QTc Studies Conceptional and Methodical Considerations Based on Experience Dr. Manfred Wargenau, Institute, Düsseldorf OVERVIEW Clinical background The ICH E14 guideline / review
Design and Analysis of QT/QTc Studies Conceptional and Methodical Considerations Based on Experience Dr. Manfred Wargenau, Institute, Düsseldorf OVERVIEW Clinical background The ICH E14 guideline / review
MODELING MECHANISM BASED INACTIVATION USING PBPK JAN WAHLSTROM DIRECTOR, PRECLINICAL
 MODELING MECHANISM BASED INACTIVATION USING PBPK JAN WAHLSTROM DIRECTOR, PRECLINICAL ABSTRACT Quantitative prediction of the magnitude of drug-drug interactions (DDI) is critical to underwriting patient
MODELING MECHANISM BASED INACTIVATION USING PBPK JAN WAHLSTROM DIRECTOR, PRECLINICAL ABSTRACT Quantitative prediction of the magnitude of drug-drug interactions (DDI) is critical to underwriting patient
Sub Population: The elderly
 Sub Population: The elderly How can Modelling and Simulation inform understanding of safety and efficacy? Amy S. Y. Cheung, Senior Clinical Pharmacometrician Early Clinical Development, AstraZeneca On
Sub Population: The elderly How can Modelling and Simulation inform understanding of safety and efficacy? Amy S. Y. Cheung, Senior Clinical Pharmacometrician Early Clinical Development, AstraZeneca On
