actin-troponin-tropomyosin complex (muscle relaxation/cooperativity/regulated actin)
|
|
|
- Belinda Stanley
- 6 years ago
- Views:
Transcription
1 Proc. Nati. Acad. Sci. USA Vol. 77, No. 5, pp , May 1980 Biochemistry Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex (muscle relaxation/cooperativity/regulated actin) Lois E. GREENE AND EVAN EISENBERG Laboratory of Cell Biology, National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, Maryland Communicated by Terrell L. Hill, February 22, 1980 ABSTRACT The binding of myosin subfragment-1 (S-i) to the F-actin-troponin-tropomyosin complex (regulated F-actin). was examined in the presence of ADP (ionic strength, 0.23 M; 220C) by using the ultracentrifuge and S-1 blocked at SHI with iodo["4c]acetamide. S-1ADP binds with positive cooperativity to regulated F-actin, both in the presence and absence of calcium; it binds independently to unregulated actin. With and without CaO+ at very low levels of occupancy of the regulated actin by S-19ADP, S-1*ADP binds to the regulated actin with <1% of the strength that it binds to unregulated actin, whereas at high levels of occupancy of the regulated actin by S-1-ADP, S-1ADP binds about 3-fold more strongly to the regulated actin than it does to unregulated actin. The major difference between the results obtained in the presence and absence of Ca2+ with regulated actin is that, in the absence of Ca2+, the binding of S-1'ADP remains weak until a higher free S-1ADP concentration is reached and the transition to strong binding is much more cooperative. These results are consistent with a model that is basically similar to the cooperative binding model of Hill [Hill, T. L. (1952) 1. Chem. Phys. 20, and of Monod et al. [Monod, J., Wyman,. & Changeux, J. (1965)1. Mol. Biol. 12, ]: The regulated actin filament can exist in two forms, a weak-binding and a strong-binding form; and Ca2+ and S-1.ADP, acting as allosteric effectors, shift the equilibrium between the two forms. It is now generally accepted that muscle contraction is caused by myosin and actin filaments sliding past each other as ATP is hydrolyzed. In skeletal muscle the regulation of muscle contraction appears to be controlled by the troponin-tropomyosin complex which binds to the actin filament to form regulated F-actin. The most widely accepted mechanism of troponin-tropomyosin action is the steric blocking model (1-3) which suggests that the position of the tropomyosin molecule on the actin filament controls the actin-myosin interaction. This model proposes that, in the absence of Ca2+, tropomyosin takes a position where it blocks the binding of myosin to the thin filament whereas, when Ca2+ binds to troponin, the tropomyosin is thought to move toward the central groove of the actin filament, enabling the myosin to interact with actin. Because the tropomyosin molecule spans seven F-actin monomers (4), the position of the tropomyosin is thought to be effective over the entire actin unit and, therefore, this model suggests that cooperativity is an inherent part of regulation. On a biochemical level, the removal of Ca2+ from the troponin-tropomyosin complex generally causes marked inhibition of the actomyosin ATPase activity. However, this is not always the case, as was observed by Bremel and Weber (5). At low ATP concentration and high ratios of subfragment 1 of myosin (S-i) to actin, they found that the ATPase activity of regulated actin-s-1 complex (acto-s-1) is no longer sensitive to Ca2+. They suggested that this cooperative response was due to the binding The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" in accordance with 18 U. S. C solely to indicate this fact. of a few S-1 molecules, free of ATP, to the actin filament and pushing the tropomyosin away from its inhibitory position, thus preventing inhibition of the ATPase activity even in the absence of Ca2+. Cooperative responses have also been observed in the presence of Ca2+. Weber and coworkers (6) found that at high S-1 concentration the ATPase activity of regulated acto-s-1 can be potentiated so that it is higher than the ATPase activity of acto*s-1 in the absence of troponin-tropomyosin. The cooperative responses observed with regulated actin are fundamental to our understanding of the biochemical basis of regulation. Up to the present time, there have not been any studies on the binding of S-1 to regulated actin in the absence of ATP. Equilibrium binding studies are generally easier to interpret than steady-state ATPase studies. Therefore, in the present study, using a method previously used in our studies on the binding of S-1-nucleotide complexes to unregulated F-actin (7), we investigated the binding of S-1-ADP to regulated F-actin both in the presence and in the absence of Ca2+. At ionic strength = 0.23 M at 220C, although S-1-ADP binds independently to unregulated actin, it binds with positive cooperativity to regulated F-actin, both in the presence and absence of Ca2+. With and without Ca2+ at very low levels of occupancy of the regulated actin by S-1-ADP, S-1-ADP binds to the regulated actin with <1% of the strength that it binds to unregulated actin, whereas at high levels of occupancy of the regulated actin by S-1-ADP, S-1-ADP binds about 3-fold more strongly to the regulated actin than it does to unregulated actin. Our results are consistent with a typical cooperative binding model (8-10) in which the regulated actin filament can exist in two forms, a weak-binding and a strong-binding form. Ca2+ and S-1-ADP, acting as allosteric effectors, shift the equilibrium toward the strong form. MATERIALS AND METHODS Rabbit skeletal myosin, S-1, and actin were prepared as described by Stein et al. (11). The troponin-tropomyosin complex was prepared according to Eisenberg and Kielley (12). The molecular weights used for S-1, actin, and troponin-tropomyosin complex were 120,000, 42,000, and 150,000, respectively. Protein concentrations were determined by UV absorption and the following extinction coefficients: 750 cm2/g at 280 nm for S-1, 1150 cm2/g at 280 nm for F-actin, and 380 cm2/g at 278 nm for the troponin-tropomyosin complex. The rabbit myosin re- was labeled with iodo['4c]acetamide, as described (13), sulting in mol of iodoacetamide incorporated per mol of S-1. Binding studies were conducted in the preparative centrifuge (Beckman L2-65B). The SHI-blocked S-1 and regulated actin in a total volume of 4 ml were stirred for several minutes and Abbreviations: S-1, subfragment 1 of myosin; acto-s-1, complex of actin with S-1; EGTA, ethylene glycol bis(f-aminoethyl ether)-n,n,- N',N'-tetraacetic acid. 2616
2 then allowed to remain for 30 min at 220C before centrifugation. All binding studies were conducted at ionic strength 0.2 M and 220C in the presence of 2.5 mm ADP to saturate both the S-1 and acto-s-1 with ADP. Diadenosine pentaphosphate (3,M) was added to inhibit myokinase activity. The order of addition of the proteins typically was actin, S-1, and then the troponin-tropomyosin complex. Aliquots (3-ml) were then centrifuged for 1 hr at 80,000 X g. An 0.5-ml aliquot removed prior to centrifugation and the supernatant after centrifugation were assayed for radioactivity in a Beckman LS-250 liquid scintillation counter to determine the total and free S-1 concentrations, respectively. In the absence of actin, >97% of the S-1 remained in the supernatant after centrifugation. Centrifugation of the actin alone showed that >96% sedimented, as determined by absorbance. The binding data were plotted by using the Scatchard equation (14) and the value of the slope for linear regions of the Scatchard plots was determined by linear regression analysis. ADP and diadenosine pentaphosphate were from PL Biochemicals and iodo[14c]acetamide was from Amersham. RESULTS We first compared the binding of S-1-ADP to regulated and unregulated actin in the absence of Ca2, using SHI-blocked S-I modified with iodo[14c]acetamide. These studies were done at an ionic strength of 0.23 M to obtain detectable amounts of dissociation of acto-s-i by ADP. The binding of S-1iADP to unregulated actin gave a linear Scatchard plot (Fig. 1), confirming that there is no cooperativity in the binding of S-1iADP to unregulated F-actin. In contrast, with regulated actin, the to 4 A Biochemistry: Greene and Eisenberg Free S-1, um FIG. 1. Binding of S-1-ADP to regulated (-) and unregulated (A) F-actin in the absence of Ca2. Varying amounts of SH,-blocked S-i were added to a fixed concentration of either unregulated or regulated actin. Conditions were 0.2 M KCl, 12 mm imidazole, 5 mm MgCl2, 5 mm KPi, 2.5 mm ADP, 0.5 mm dithiothreitol, 3,M diadenosine pentaphosphate, and 1 mm ethylene glycol bis(,6-aminoethyl ether)- N,N,N',N'-tetraacetic acid (EGTA) at ph 7.0 and 22 C. In the binding study using regulated actin, um SH1-blocked S-1 was added to 5.5 MM F-actin and 1.6 MAM troponin-tropomyosin complex. In the binding study using unregulated actin, ,JM SHI-blocked S-1 was added to 5.5MgM F-actin. (A) Data are plotted according to the Scatchard equation. (B) Same data are plotted as fraction of actin saturated with S-1 (0) vs. free S-1 for values of 0 obtained at free S-1 < 5 MuM. 0 is mol of S-1 bound per mol of F-actin monomer. Proc. Natl. Acad. Sci. USA 77 (1980) 2617 Scatchard plot shows a marked convex curvature, indicating that the binding of S-i ADP is highly cooperative in the absence of Ca2. This cooperativity is shown more clearly in Fig. lb where the fraction of actin saturated with S-I is plotted as a function of free S-1 concentration. In contrast to the situation with unregulated actin, at a free S-1 concentration less than 1,M there was almost no binding of S-1iADP to regulated actin. Then, over a narrow range of S-1 concentration (1 to 1.5 MM), the fraction of regulated actin saturated with S-I increased from about 0.05 to 0.5 which is about twice the level of saturation observed with unregulated actin at 1.5 MM S-1. Finally, above a free S-1 concentration of 1.5 MM, the binding of S-1 to regulated actin no longer was cooperative but was about 3 times stronger than the binding of S-I to unregulated actin. This can best be seen by comparing the slopes of the Scatchard plots for regulated and unregulated actin at 0 > 0.5 in Fig. IA. In general, for cooperative binding systems, noncooperative regions of binding occur at both very low and very high levels of saturation and, therefore, binding constants can be obtained for these regions. The binding constant of S-1 to regulated actin at high levels of saturation can be obtained from the slope of the Scatchard plot at 0 > 0.5. This binding constant has a value of 7 X 105 M-1, about 3 times that obtained with unregulated actin, 2 X 105 M-1. To obtain an accurate binding constant at very low levels of saturation, binding experiments were conducted at a higher actin concentration (56,uM). Much less S-I bound to regulated actin than to unregulated actin at low free S-1 concentration (Table 1). From the concentrations of unbound S-1, bound S-i, and regulated actin, a value of 103 M-1 was calculated for the binding constant of S-1iADP to regulated actin at very low levels of saturation in the absence of Ca2+. However, because only a small amount of S-1 bound to the regulated actin, this value is probably an upper limit; the binding constant could be even weaker than 103 M-1. To make certain that the cooperative binding of S-I to regulated actin is a real effect, several control experiments were carried out (Table 2). First, the amount of dissociated S-I was not significantly different at 1.2 and 2.5 mm ADP. Therefore, at 2.5 mm ADP both the S-1 and acto-s-l are saturated with ADP. Second, our results were unaffected by a 5-fold increase in the concentration of the troponin-tropomyosin complex, which shows that the actin was saturated with the troponintropomyosin complex. Third, to test whether the system was in equilibrium, the order of addition of S-1, troponin-tropomyosin, and EGTA was varied. The 55% of the added S-I Table 1. Binding of S-1-ADP to unregulated and regulated actin at low levels of saturation Total S-1,,uM Bound S-1, AM Regulated actin + 1 mm EGTA Regulated actin mm CaCl Unregulated actin Conditions were: 0.2 M KCl, 12 mm imidazole, 5mM MgCl2, 5mM KPi, 0.5 mm dithiothreitol, 3 mm ADP, and 3 AM diadenosine pentaphosphate at 220C and ph 7.0. The actin concentration was 56 MM. In the experiments-using regulated actin, the troponin-tropomyosin complex was at 16 MM.
3 2618 Biochemistry: Greene and Eisenberg Table 2. Factors affecting the binding of S-1-ADP to regulated actin Condition % S-1 bound ADP at 1.2 mm 10 ADP at 2.5mM 8 Troponin-tropomyosin complex at 2.2 AM 4 Troponin-tropomyosin complex at 11,gM 4 Actin + S-1 55 Actin + troponin-tropomyosin, then S-1 added 8 Actin + S-1, then troponin-tropomyosin added 8 CaCl2 at mm, no added EGTA 55 CaCl2 at mm, then 5 mm EGTA added* 5 Conditions were: 0.2 M KC1, 12 mm imidazole, 5 mm KPi, 5 mm MgCl2, 0.5 mm dithiothreitol, and 3MuM diadenosine pentaphosphate at 22 C and ph 7.0. Free S-1 < 1.0 1,M; actin = 6-8 sm; and troponin-tropomyosin complex 2,uM (except line 4). ADP was at 2.5 mm (except line 1) and EGTA was at 1 mm (except lines 8 and 9). * EGTA was added 10 min after the addition of CaCl2 (solution at 22 C); the mixture was centrifuged 30 min later. that bound to unregulated F-actin was almost completely dissociated upon addition of the troponin-tropomyosin complex. Thus, the amount of S-1 bound decreased to the same low level (-5%) as occurred when S-1 was added directly to the troponin-tropomyosin-actin complex. Similarly, the 55% of the added S-1 that bound to F-actin in the presence of Ca2+ (see below) was almost completely dissociated when EGTA was added. Therefore, the binding of the S-1 to regulated actin is reversible and the system appears to be in equilibrium. Finally, although SH1-blocked S-1 was used in these binding studies, the same results were obtained in preliminary experiments using unmodified S-1. We next examined the binding of S-1-ADP to regulated actin in the presence of Ca2+. Because the regulated actomyosin ATPase activity is at a high level in the presence of Ca2+ both in vivo and in vitro (15), it was expected that the binding of S-1-ADP to regulated actin would not show cooperativity. However, contrary to expectations, the results obtained in the presence of Ca2+ were similar to the results obtained in the presence of EGTA; the convex Scatchard plot in Fig. 2A shows that the binding of S-1-ADP to regulated F-actin is quite cooperative. However, there are important differences between the binding in Ca2+ and EGTA. Comparison of Fig. 2B with Fig. 1B shows that the transition from weak to strong S-1 binding occurred at a lower free S-1 concentration with Ca2+ than with EGTA. In addition, the cooperative transition was not as steep as that obtained in the presence of EGTA. Nevertheless, the binding in the presence of Ca2+ clearly was cooperative. As in the presence of EGTA, binding constants can be obtained for the noncooperative regions at very low and very high levels of saturation of actin with S-1-ADP. At high levels of occupancy of the regulated actin by S-1, the value of the binding constant obtained from the slope of the Scatchard plot is 6 X 105 M-1. This is almost identical to the value obtained in the presence of EGTA at high levels of occupancy. Because the cooperative transition occurs at such a low free S-1 concentration in the presence of Ca2+, only an approximate value could be obtained for the upper limit of the binding constant of S-1iADP to actin at very low levels of occupancy. As with EGTA, this binding constant was estimated at a regulated actin concentration of 56,uM. With 0.29 AtM S-1 added, the value of the binding constant is about 103 M-1 (Table 1). At higher added S-1 concentration, much more S-1 is bound, probably because there is cooperativity at these S-1 concentrations. Therefore, both in the presence and absence of Ca2+, tu) 0 0 x Cl) a1) - DZ Proc. Natt. Acad. Sci. USA 77 (1980) Free S-1, pm FIG. 2. Binding of S-1-ADP to regulated (0) and unregulated (A) F-actin in the presence of Ca2+. The conditions were as in Fig. 1, except 0.5 mm CaCl2 was added instead of EGTA. In the binding study using regulated actin, ,AM SHI-blocked S-1 was added to 5.5 ACM F-actin and 1.6 MM troponin-tropomyosin complex. In the binding study using unregulated actin, MM SH1-blocked S-1 was added to 5.5,uM F-actin. (A) Data are plotted according to the Scatchard equation. (B) Same data are plotted as fraction of actin saturated with S-1 (0) vs. free S-1 for values of 0 obtained at free S-1 < 5,uM. S-1-ADP binds to regulated actin with 1% of the strength that it binds to unregulated actin at very low levels of occupancy of the actin sites by S-1 and about 3-fold more strongly at high levels of occupancy. DISCUSSION The results presented in this paper show that the binding of the S-1-ADP complexto regulated F-actin is a highly cooperative phenomenon. Because cooperative binding occurs both in the presence and absence of Ca2+, it seems reasonable to use as a framework for analyzing our data one of the classic allosteric models for cooperative binding (8-10). Monod et al. (10) have applied such a cooperative model to the regulatory properties of allosteric enzymes. We use their simple formulation, which is a special case of Hill's treatment (8, 9), to present the basic elements of a cooperative model that accounts for our data. Both in the presence and absence of Ca2+, each cooperative unit along the regulated actin filament is assumed to occur in two forms, a "weak-binding" and a "strong-binding" form. The equilibrium constant L between the two forms of the cooperative unit is defined as L = [weak form]/[strong form]. When the cooperative unit is in the weak form, each of the actin monomers in the cooperative unit is assumed to bind S-1 with an association constant Kw; when the cooperative unit is in the strong form, each of the monomers is assumed to bind S-1 with an association constant K. In agreement with the model of Monod et al., the values of Kw and K, are assumed to be independent of the amount of S-1 bound to the F-actin. On this basis, we have the following model: * AwMn-* AwMn An * Aw-M1; Aw M2. L It It It l 1 AMn Ans _ ' Asn- Ml As M2Z As~-' As
4 in which A' and An, are cooperative units in the weak and strong forms, respectively, M is S-1, and n is the number of actin monomers in a cooperative unit. In applying this model to our data, n was set equal to 7 because one tropomyosin molecule binds to seven actin monomers. Kw was taken to be 103 M-1 and Ks, 7 X 105 M-1 in both the presence and absence of Ca2+ (Figs. 1 and 2; Table 1). The data were then fitted to the above model by varying the value of L. As shown in Fig. 3 (a replot of the data in Figs. 1B and 2B), the data obtained in the presence of Ca2+ can be fitted reasonably well to a theoretical plot with L = 7 (the solid line). However, we could not duplicate the high degree of cooperativity observed in the absence of Ca2+ with any value of L. The best fit we could obtain is shown by the dashed line in Fig. 3, which is the theoretical plot with L = 60. Although it was assumed in our modeling that Kw = 103 M-1, a decrease in this association constant would not significantly affect the theoretical plots. A change in Ks (e.g., 10%) would affect the shape of the theoretical plots, but it would still not be possible to fit the data obtained in the absence of Ca2. It is possible to fit these highly cooperative data if we use a value of n much greater than 7. However, there is no physical basis for using a larger value of n because an indefinite array of tropomyosin molecules along the actin filament could be cooperatively linked. A more realistic (and somewhat more complex) cooperative model in which the interaction between adjacent tropomyosin molecules is taken into consideration has recently been developed by Hill et al. (16). It is essentially identical with the simple cooperative model presented above except that a function has been added which allows for variable interaction between adjacent tropomyosin molecules. By making this interaction stronger in the absence than in the presence of Ca2+, it is possible to obtain an excellent fit to our data, in particular the high level of cooperativity observed in the absence of Ca2+. Therefore, the basic concept that, both in the presence and absence of Ca2+, the regulated actin filament can occur in either a weak form or a strong form appears to be a reasonable way to account for the cooperative data presented in this paper. It is of interest to consider the implications of our data for the mechanism of skeletal muscle relaxation. Our data show that I Biochemistry: Greene and Eisenberg 0 / Free S-1, pm FIG. 3. Binding of S-1-ADP to regulated actin in the presence (0) and absence (@) of Ca2+. The data obtained in the absence of Ca2+ in Fig. 1B and in the presence of Ca2+ in Fig. 2B are replotted here. The solid line is the theoretical line obtained when Kw = 103 M-1, K, - 7 X 105 M-1, n = 7, and L = 7; and the dashed line is the theoretical line obtained when Kw = 103 M-1, K8 = 7 X 105M-1, n = 7, and L = 60. Proc. Natl. Acad. Sci. USA 77 (1980) 2619 the troponin-tropomyosin complex blocks the binding of S-1ADP to regulated actin in the weak form. It might then be expected, on the basis of the steric blocking model (1-3), that the myosin cross-bridge with bound ATP or ADP + Pi would also be prevented from binding to regulated actin in the weak form but would bind quite well to regulated actin in the strong form. However, by using stopped-flow turbidity measurements (11) to determine the binding of S-1 to regulated actin in the presence of ATP, Chalovich et al. (17) found that troponin-tropomyosin has very little effect on the binding of S-1-ATP or S-1-ADP-Pi to actin, in either the presence or absence of Ca2+. This suggests that the troponin-tropomyosin complex may cause relaxation not by blocking the binding of S-1ATP or S-1-ADP-Pi to actin but rather by inhibiting the release of Pi from the acto-s-ladp-pi complex. The inhibition of Pi release and the weakening of S-1ADP binding to actin by the troponin-tropomyosin complex may have a common origin: an increase in the free energy (decrease in stability) of the acto-s-1-adp complex when the actin filament is in the weak form. An increase in the free energy of acto.s-iadp relative to acto-s-i'adp-pi would occur if the rate constant for Pi release decreased without a corresponding decrease in the rate constant for Pi binding. At the same time, an increase in the free energy of acto-s-i'adp relative to S-1*ADP would decrease the association constant of S-1ADP to actin. On this basis, weak binding of S-1 ADP to regulated actin in the weak form would always be linked to an inability of the weak form to activate the S-I-ATPase. The major advantage of this model is that the properties of the weak form are the same in the presence and absence of Ca2+; Ca2+ acts only as an allosteric effector, shifting the equilibrium between the weak and strong forms. The weak and strong forms are synonymous in this model with the "relaxed" and "active" states observed in vivo. Unfortunately, there are data that may not be compatible with this simple model. It may be necessary to assume that Ca2+ actually affects the properties of the weak form so that, in the presence of Ca2+, regulated actin in the weak form is able to activate the S-I-ATPase activity. The major finding that suggests a need for this more complex approach is that considerable ATPase activity is observed in the presence of Ca2+, even at low ratios of S- to actin where our data suggest that most of the actin remains in the weak form. In addition, the x-ray diffraction studies by Haselgrove (2) suggest that, even when the actin and myosin filaments are stretched out of overlap, the binding of Ca2+ to troponin shifts the position of the tropomyosin on the actin filament from the "relaxed" to the "active" position, although again our data suggest that under these conditions much of the actin remains in the weak form. Of course, it is possible that the binding of Ca2+ shifts enough of the regulated actin into the strong form to account for these x-ray diffraction results and for the increased ATPase activity that occurs in the presence of Ca2+. However, if this turns out not to be the case, the most likely explanation for these data is that Ca2+ can induce a conformational change in the weak form which causes a change in the x-ray diffradion pattern and allows the actin, still in the weak form, to activate the S-1ATPase activity. Whether or not Ca2+ affects the kinetic properties of the weak form, it does appear that regulated actin must be in the weak form in order for relaxation to occur. At low ATP concentration, when S-i or S-1-ADP binds to actin, removal of Ca2+ does not cause inhibition in vitro (5) or relaxation in vivo (18, 19). Based on our results, it seems likely that this is because at low ATP concentration, even in the absence of Ca2+, most of the actin occurs in the strong form, shifted from the weak form by the binding of the allosteric effector S-1-ADP. It therefore
5 2620 Biochemistry: Greene and Eisenberg appears that the weak form of regulated actin is necessary for relaxation to occur. We thank Mr. Louis Dobkin for technical assistance. 1. Huxley, H. E. (1972) Cold Spring Harbor Symp. Quant. Biol. 37, Haselgrove, J. C. (1972) Cold Spring Harbor Synp. Quant. Biol. 37, Parry, D. A. D. & Squire, J. M. (1973) J. Mol. Biol. 75, Ebashi, S., Endo, M. & Ohtsuki, I. (1969) Quart. Rev. Biophys. 2, Bremel, R. D. & Weber, A. (1972) Nature (London) New Biol. 238, Bremel, R. D., Murray, J. M. & Weber, A. (1972) Cold Spring Harbor Symp. Quant. Biol. 37, Greene, L. E. & Eisenberg, E. (1980) J. Biol. Chem. 255, Hill, T. L. (1952) J. Chem. Phys. 20, Proc. Nati. Acad. Sci. USA 77 (1980) 9. Hill, T. L. (1960) Introduction to Statistical Thermodynamics (Addison-Wesley, Reading, MA), pp Monod, J., Wyman, J. & Changeux, J. (1965) J. Mol. Biol. 12, Stein, L. A., Schwarz, R. P. Chock, P. B. & Eisenberg, E. (1978) Biochemistry 18, Eisenberg, E. & Kielley, W. W. (1974) J. Biol. Chem. 249, Greene, L. E. & Eisenberg, E. (1980) J. Biol. Chem. 255, Scatchard, G. (1949) Ann. N. Y. Acad. Sci. 51, Weber, A. & Murray, J. M. (1973) Physiol. Rev., Hill, T. L., Eisenberg, E. & Greene, L. E. (1980) Proc. Natl. Acad. Sci. USA, in press. 17. Chalovich, J. M., Chock, P. B. & Eisenberg, E. (1980) Fed. Proc. Fed. Am. Soc. Exp. Biol. 39, in press. 18. White, D. C. S. (1970) J. Physiol. 208, Reuben, J. P., Brandt, P. W., Berman, M. & Grundfest, H. (1971) J. Gen. Physiol. 57,
2, 6, 3, 7, 8, 9, 10, 5
 A novel regulatory element in cardiac muscle troponin T. Striated muscle contraction is regulated by the position of tropomyosin on actin. That position, in turn, is regulated by the troponin complex (troponin
A novel regulatory element in cardiac muscle troponin T. Striated muscle contraction is regulated by the position of tropomyosin on actin. That position, in turn, is regulated by the troponin complex (troponin
ANSC (FSTC) 607 Physiology and Biochemistry of Muscle as a Food MUSCLE CONTRACTION
 I. Basic model of muscle contraction A. Overall ANSC (FSTC) 607 Physiology and Biochemistry of Muscle as a Food MUSCLE CONTRACTION 1. Calcium is released from sarcoplasmic reticulum. 2. Myosin globular
I. Basic model of muscle contraction A. Overall ANSC (FSTC) 607 Physiology and Biochemistry of Muscle as a Food MUSCLE CONTRACTION 1. Calcium is released from sarcoplasmic reticulum. 2. Myosin globular
Rate of force generation in muscle: Correlation with actomyosin ATPase activity in solution
 Proc. Natl. Acad. Sci. USA Vol. 83, pp. 3542-3546, May 1986 Physiological Sciences Rate of force generation in muscle: Correlation with actomyosin ATPase activity in solution (myosin/actin/contraction/velocity/force
Proc. Natl. Acad. Sci. USA Vol. 83, pp. 3542-3546, May 1986 Physiological Sciences Rate of force generation in muscle: Correlation with actomyosin ATPase activity in solution (myosin/actin/contraction/velocity/force
Mechanism of Muscular Contraction
 ~ Sorin2:er Jack A. Rail Mechanism of Muscular Contraction Contents 1 Setting the Stage: Myosin, Actin, Actomyosin and ATP... 1.1 Introduction... 1 1.2 Muscle Structure as Observed by Nineteenth Century
~ Sorin2:er Jack A. Rail Mechanism of Muscular Contraction Contents 1 Setting the Stage: Myosin, Actin, Actomyosin and ATP... 1.1 Introduction... 1 1.2 Muscle Structure as Observed by Nineteenth Century
Muscle Dr. Ted Milner (KIN 416)
 Muscle Dr. Ted Milner (KIN 416) Muscles are biological motors which actively generate force and produce movement through the process of contraction. The molecular mechanism responsible for muscle contraction
Muscle Dr. Ted Milner (KIN 416) Muscles are biological motors which actively generate force and produce movement through the process of contraction. The molecular mechanism responsible for muscle contraction
Calcium Regulation in Squid Mantle and Scallop Adductor Muscles 1
 . Biochem. 89, 581-589 (1981) Calcium Regulation in Squid Mantle and Scallop Adductor Muscles 1 Kunihiko KONNO,* Ken-ichi ARM,* Mikiharu YOSHIDA,** and Shizuo WATANABE*** Department of Food Science, Faculty
. Biochem. 89, 581-589 (1981) Calcium Regulation in Squid Mantle and Scallop Adductor Muscles 1 Kunihiko KONNO,* Ken-ichi ARM,* Mikiharu YOSHIDA,** and Shizuo WATANABE*** Department of Food Science, Faculty
Muscle Cells & Muscle Fiber Contractions. Packet #8
 Muscle Cells & Muscle Fiber Contractions Packet #8 Skeletal muscle is attached to bones and is responsible for movement. Introduction Introduction II Skeletal muscle is composed of bundles of muscle fibers
Muscle Cells & Muscle Fiber Contractions Packet #8 Skeletal muscle is attached to bones and is responsible for movement. Introduction Introduction II Skeletal muscle is composed of bundles of muscle fibers
108. Time.Resolved X.Ray Diffraction from Frog Skeletal Muscle during an Isotonic Twitch under a Small Load
 No. 9] Proc. Japan Acad., 54, Ser. B (1978) 559 108. Time.Resolved X.Ray Diffraction from Frog Skeletal Muscle during an Isotonic Twitch under a Small Load By Haruo SUGI,*> Yoshiyuki AMEMIYA,**> and Hiroo
No. 9] Proc. Japan Acad., 54, Ser. B (1978) 559 108. Time.Resolved X.Ray Diffraction from Frog Skeletal Muscle during an Isotonic Twitch under a Small Load By Haruo SUGI,*> Yoshiyuki AMEMIYA,**> and Hiroo
Ch. 6: Contraction of Skeletal Muscle Physiological Anatomy of Skeletal Muscle
 Ch. 6: Contraction of Skeletal Muscle 40% skeletal muscle + 10% smooth and cardiac muscle Ch. 7: Excitation of Skeletal Muscle Ch. 9: Contraction and Excitation of Smooth Muscle Physiological Anatomy of
Ch. 6: Contraction of Skeletal Muscle 40% skeletal muscle + 10% smooth and cardiac muscle Ch. 7: Excitation of Skeletal Muscle Ch. 9: Contraction and Excitation of Smooth Muscle Physiological Anatomy of
Some Properties of Glycerinated Skeletal Muscle Fibres Containing Phosphorylated Myosin
 Gen. Physiol. Biophys. (1989), 8, 569-578 569 Some Properties of Glycerinated Skeletal Muscle Fibres Containing Phosphorylated Myosin M. WROTEK', YU. S. BOROVIKOV 2, N. N. LEBEDEVA 2 and I. K^KOĽ 1 Department
Gen. Physiol. Biophys. (1989), 8, 569-578 569 Some Properties of Glycerinated Skeletal Muscle Fibres Containing Phosphorylated Myosin M. WROTEK', YU. S. BOROVIKOV 2, N. N. LEBEDEVA 2 and I. K^KOĽ 1 Department
ATP Hydrolysis. Presented by: Rami Amro Instructor: Dr. Neiman
 ATP Hydrolysis Presented by: Rami Amro Instructor: Dr. Neiman Ch. 13: Direct movement of the motors along the filament thought to be a result of conformational changes while it is attached. Ch.14: How
ATP Hydrolysis Presented by: Rami Amro Instructor: Dr. Neiman Ch. 13: Direct movement of the motors along the filament thought to be a result of conformational changes while it is attached. Ch.14: How
Skeletal Muscle : Structure
 1 Skeletal Muscle : Structure Dr.Viral I. Champaneri, MD Assistant Professor Department of Physiology 2 Learning objectives 1. Gross anatomy of the skeletal muscle 2. Myofilaments & their molecular structure
1 Skeletal Muscle : Structure Dr.Viral I. Champaneri, MD Assistant Professor Department of Physiology 2 Learning objectives 1. Gross anatomy of the skeletal muscle 2. Myofilaments & their molecular structure
2692 Biophysical Journal Volume 77 November
 2692 Biophysical Journal Volume 77 November 1999 2692 2708 Kinetics of Thin Filament Activation Probed by Fluorescence of N-((2- (Iodoacetoxy)ethyl)-N-methyl)amino-7-nitrobenz-2-oxa-1,3-diazole- Labeled
2692 Biophysical Journal Volume 77 November 1999 2692 2708 Kinetics of Thin Filament Activation Probed by Fluorescence of N-((2- (Iodoacetoxy)ethyl)-N-methyl)amino-7-nitrobenz-2-oxa-1,3-diazole- Labeled
YASUSHI OHIZUMI, KIMIHIRO MATSUNAGA, KEIGO NAKATANI and JUN ICHI KOBAYASHI
 0022-3565/98/2852-0695$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 285, No. 2 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/98/2852-0695$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 285, No. 2 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
Movement of Scallop Myosin on Nitella Actin Filaments: Regulation by Calcium. Ronald D. Vale, Andrew G. Szent-Gyorgyi, and Michael P.
 Movement of Scallop Myosin on Nitella Actin Filaments: Regulation by Calcium Ronald D. Vale, Andrew G. Szent-Gyorgyi, and Michael P. Sheetz PNAS 1984;81;6775-6778 doi:10.1073/pnas.81.21.6775 This information
Movement of Scallop Myosin on Nitella Actin Filaments: Regulation by Calcium Ronald D. Vale, Andrew G. Szent-Gyorgyi, and Michael P. Sheetz PNAS 1984;81;6775-6778 doi:10.1073/pnas.81.21.6775 This information
1458 Biophysical Journal Volume 78 March
 1458 Biophysical Journal Volume 78 March 2000 1458 1473 Cross-Bridge Attachment during High-Speed Active Shortening of Skinned Fibers of the Rabbit Psoas Muscle: Implications for Cross-Bridge Action during
1458 Biophysical Journal Volume 78 March 2000 1458 1473 Cross-Bridge Attachment during High-Speed Active Shortening of Skinned Fibers of the Rabbit Psoas Muscle: Implications for Cross-Bridge Action during
The Major Component of Metin from Rabbit Skeletal and Bovine Cardiac Muscle*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 24, No. 1, October 1965 Printed in U.S.A. The Major Component of Metin from Rabbit Skeletal and Bovine Cardiac Muscle* NAOMI AzUMAt AND SHIZUO WATANABE~ From the
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 24, No. 1, October 1965 Printed in U.S.A. The Major Component of Metin from Rabbit Skeletal and Bovine Cardiac Muscle* NAOMI AzUMAt AND SHIZUO WATANABE~ From the
EXCITATION- CONTRACTION COUPLING IN SKELETAL MUSCLES 1
 EXCITATION- CONTRACTION COUPLING IN SKELETAL MUSCLES 1 Summary: The sequence of events from the movement of an AP moving down a neuron to the completion of a contraction is examined. These events are referred
EXCITATION- CONTRACTION COUPLING IN SKELETAL MUSCLES 1 Summary: The sequence of events from the movement of an AP moving down a neuron to the completion of a contraction is examined. These events are referred
Nerve Muscle Relationship and Neural Muscular Junction Quiz. Remember, you need to know the structure and the function!
 Nerve Muscle Relationship and Neural Muscular Junction Quiz Remember, you need to know the structure and the function! What is this called? What is this? Schwann cell What is this called? Basal lamina
Nerve Muscle Relationship and Neural Muscular Junction Quiz Remember, you need to know the structure and the function! What is this called? What is this? Schwann cell What is this called? Basal lamina
Regulatory Proteins from Dorsal Muscle of the Carp
 J. Biochent. 82, 931-938 (1977) Regulatory Proteins from Dorsal Muscle of the Carp Kunihiko KONNO,* Ken-ichi ARAI,* and Shizuo WATANABE** *The Department of Food Science, Faculty of Fisheries, Hokkaido
J. Biochent. 82, 931-938 (1977) Regulatory Proteins from Dorsal Muscle of the Carp Kunihiko KONNO,* Ken-ichi ARAI,* and Shizuo WATANABE** *The Department of Food Science, Faculty of Fisheries, Hokkaido
Self-association of α-chymotrypsin: Effect of amino acids
 J. Biosci., Vol. 13, Number 3, September 1988, pp. 215 222. Printed in India. Self-association of α-chymotrypsin: Effect of amino acids T. RAMAKRISHNA and M. W. PANDIT* Centre for Cellular and Molecular
J. Biosci., Vol. 13, Number 3, September 1988, pp. 215 222. Printed in India. Self-association of α-chymotrypsin: Effect of amino acids T. RAMAKRISHNA and M. W. PANDIT* Centre for Cellular and Molecular
Organismic Biology Bio 207. Lecture 6. Muscle and movement; sliding filaments; E-C coupling; length-tension relationships; biomechanics. Prof.
 Organismic Biology Bio 207 Lecture 6 Muscle and movement; sliding filaments; E-C coupling; length-tension relationships; biomechanics Prof. Simchon Today s Agenda Skeletal muscle Neuro Muscular Junction
Organismic Biology Bio 207 Lecture 6 Muscle and movement; sliding filaments; E-C coupling; length-tension relationships; biomechanics Prof. Simchon Today s Agenda Skeletal muscle Neuro Muscular Junction
SKELETAL MUSCLE CHARACTERISTICS
 THE MUSCULAR SYSTEM SKELETAL MUSCLE CHARACTERISTICS Most are attached by tendons to bones Cells are multinucleate Striated have visible banding Voluntary subject to conscious control Cells are surrounded
THE MUSCULAR SYSTEM SKELETAL MUSCLE CHARACTERISTICS Most are attached by tendons to bones Cells are multinucleate Striated have visible banding Voluntary subject to conscious control Cells are surrounded
The organization of skeletal muscles. Excitation contraction coupling. Whole Skeletal Muscles contractions. Muscle Energetics
 Muscle and Movement The organization of skeletal muscles Excitation contraction coupling Whole Skeletal Muscles contractions Muscle Energetics The molecular bases of movement Muscular cells use molecular
Muscle and Movement The organization of skeletal muscles Excitation contraction coupling Whole Skeletal Muscles contractions Muscle Energetics The molecular bases of movement Muscular cells use molecular
DIDS INHIBITION OF SARCOPLASMIC RETICULUM ANION EFFLUX AND CALCIUM TRANSPORT
 DIDS INHIBITION OF SARCOPLASMIC RETICULUM ANION EFFLUX AND CALCIUM TRANSPORT Kevin P. Campbell and David H. MacLennan Reprinted from ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Volume 358 Pages 328-331
DIDS INHIBITION OF SARCOPLASMIC RETICULUM ANION EFFLUX AND CALCIUM TRANSPORT Kevin P. Campbell and David H. MacLennan Reprinted from ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Volume 358 Pages 328-331
THE pca-tension CHARACTERISTICS OF SINGLE SKINNED FIBRES ISOLATED FROM THE ANTERIOR AND POSTERIOR LATISSIMUS DORSI MUSCLES OF THE CHICKEN
 exp. Biol. 05, 4-46 (983) 4 \ in Great Britain The Company of Biologist} Limited 983 THE pca-tension CHARACTERISTICS OF SINGLE SKINNED FIBRES ISOLATED FROM THE ANTERIOR AND POSTERIOR LATISSIMUS DORSI MUSCLES
exp. Biol. 05, 4-46 (983) 4 \ in Great Britain The Company of Biologist} Limited 983 THE pca-tension CHARACTERISTICS OF SINGLE SKINNED FIBRES ISOLATED FROM THE ANTERIOR AND POSTERIOR LATISSIMUS DORSI MUSCLES
Myosin-ATP Binding to Actin
 892 Biophysical Journal Volume 65 August 1993 892-898 Characterization of a Caldesmon Fragment That Competes with Myosin-ATP Binding to Actin Laly Velaz,* Yi-der Chen,t and Joseph M. Chalovich* *Department
892 Biophysical Journal Volume 65 August 1993 892-898 Characterization of a Caldesmon Fragment That Competes with Myosin-ATP Binding to Actin Laly Velaz,* Yi-der Chen,t and Joseph M. Chalovich* *Department
Skeletal muscle in the light of its structure
 Mechanism of contraction of Skeletal muscle in the light of its structure By Dr. Mudassar Ali Roomi (MBBS, M. Phil) Muscle Tissue Skeletal Muscle Cardiac Muscle Smooth Muscle Skeletal Muscle Long cylindrical
Mechanism of contraction of Skeletal muscle in the light of its structure By Dr. Mudassar Ali Roomi (MBBS, M. Phil) Muscle Tissue Skeletal Muscle Cardiac Muscle Smooth Muscle Skeletal Muscle Long cylindrical
PhosFree TM Phosphate Assay Biochem Kit
 PhosFree TM Phosphate Assay Biochem Kit (Cat. # BK050) ORDERING INFORMATION To order by phone: (303) - 322-2254 To order by Fax: (303) - 322-2257 To order by e-mail: cservice@cytoskeleton.com Technical
PhosFree TM Phosphate Assay Biochem Kit (Cat. # BK050) ORDERING INFORMATION To order by phone: (303) - 322-2254 To order by Fax: (303) - 322-2257 To order by e-mail: cservice@cytoskeleton.com Technical
Lecture #15. Energy of transformation of one molecule is ~ktln(p e /S e ) ktln(p e /10S e ) = =ktln10=2.3kt
 Lecture #14 Problems 1. If the K d for the actin subunit-subunit interactions along a strand is 0.1 mm and the K d for subunits at the ends of two-stranded filaments is 0.03 mm, then what is the K d for
Lecture #14 Problems 1. If the K d for the actin subunit-subunit interactions along a strand is 0.1 mm and the K d for subunits at the ends of two-stranded filaments is 0.03 mm, then what is the K d for
NIH Public Access Author Manuscript Biochemistry. Author manuscript; available in PMC 2006 January 25.
 NIH Public Access Author Manuscript Published in final edited form as: Biochemistry. 2004 December 7; 43(48): 15276 15285. The 14 Mutation of Human Cardiac Troponin T Enhances ATPase Activity and Alters
NIH Public Access Author Manuscript Published in final edited form as: Biochemistry. 2004 December 7; 43(48): 15276 15285. The 14 Mutation of Human Cardiac Troponin T Enhances ATPase Activity and Alters
Muscle Contraction A BIOCHEMICAL PERSPECTIVE
 Muscle Contraction A BIOCHEMICAL PERSPECTIVE This presentation was last edited Oct 2016, by Koni Stone, Professor of Chemistry California State University, Stanislaus Muscle cells! Myoblasts are large
Muscle Contraction A BIOCHEMICAL PERSPECTIVE This presentation was last edited Oct 2016, by Koni Stone, Professor of Chemistry California State University, Stanislaus Muscle cells! Myoblasts are large
Calcium Ion-Insensitive Contraction of Glycerinated Porcine Cardiac Muscle Fibers by Mg-Inosine Triphosphate
 1350 Calcium Ion-Insensitive Contraction of Glycerinated Porcine Cardiac Muscle Fibers by Mg-Inosine Triphosphate FTP as a Tool to Dissociate the Contraction Mechanism from the Regulatory Mechanism TERUHIKO
1350 Calcium Ion-Insensitive Contraction of Glycerinated Porcine Cardiac Muscle Fibers by Mg-Inosine Triphosphate FTP as a Tool to Dissociate the Contraction Mechanism from the Regulatory Mechanism TERUHIKO
5. What component of the sarcomere is not attached to the Z line?
 Model 2: Anatomy of a Sarcomere 1. Label the thick filament and the thin filament in Model 2. 2. How many sarcomeres are shown in Model 2? 3. Using Model 2, based on the locations of thick and thin filaments,
Model 2: Anatomy of a Sarcomere 1. Label the thick filament and the thin filament in Model 2. 2. How many sarcomeres are shown in Model 2? 3. Using Model 2, based on the locations of thick and thin filaments,
TA Review. Neuronal Synapses. Steve-Felix Belinga Neuronal synapse & Muscle
 TA Review Steve-Felix Belinga sbelinga@wustl.edu Neuronal synapse & Muscle Neuronal Synapses 1 Things you should know beyond the obvious stuff 1. Differences between ionotropic and metabotropic receptors.
TA Review Steve-Felix Belinga sbelinga@wustl.edu Neuronal synapse & Muscle Neuronal Synapses 1 Things you should know beyond the obvious stuff 1. Differences between ionotropic and metabotropic receptors.
GENERAL THOUGHTS ON REGULATION. Lecture 16: Enzymes & Kinetics IV Regulation and Allostery REGULATION IS KEY TO VIABILITY
 GENERAL THOUGHTS ON REGULATION Lecture 16: Enzymes & Kinetics IV Regulation and Allostery Margaret A. Daugherty Fall 2004 1). Enzymes slow down as product accumulates 2). Availability of substrates determines
GENERAL THOUGHTS ON REGULATION Lecture 16: Enzymes & Kinetics IV Regulation and Allostery Margaret A. Daugherty Fall 2004 1). Enzymes slow down as product accumulates 2). Availability of substrates determines
J. Biosci., Vol. 7, Number 2, March 1985, pp Printed in India.
 J. Biosci., Vol. 7, Number 2, March 1985, pp. 123 133. Printed in India. Irreversibility of the interaction of human growth hormone with its receptor and analysis of irreversible reactions in radioreceptor
J. Biosci., Vol. 7, Number 2, March 1985, pp. 123 133. Printed in India. Irreversibility of the interaction of human growth hormone with its receptor and analysis of irreversible reactions in radioreceptor
Subfragment-1 ATPase in Skeletal, Cardiac,
 1067 Product Inhibition of the Actomyosin Subfragment-1 ATPase in Skeletal, Cardiac, and Smooth Muscle Jean S. Drew, Vijay A. Harwalkar, and Leonard A. Stein We studied product inhibition of the actin-activated
1067 Product Inhibition of the Actomyosin Subfragment-1 ATPase in Skeletal, Cardiac, and Smooth Muscle Jean S. Drew, Vijay A. Harwalkar, and Leonard A. Stein We studied product inhibition of the actin-activated
Animal Locomotion. Lots of ways to get around. What are the advantages of locomotion? Why Do We Need All That ATP?
 Animal Locomotion What are the advantages of locomotion? Motor Mechanisms sessile (Muscles & Motor Locomotion) motile Why Do We Need All That ATP? 2006-2007 mollusk mammal bird reptile We saw how some
Animal Locomotion What are the advantages of locomotion? Motor Mechanisms sessile (Muscles & Motor Locomotion) motile Why Do We Need All That ATP? 2006-2007 mollusk mammal bird reptile We saw how some
#1 20. physiology. Muscle tissue 30/9/2015. Ahmad Adel Sallal. Mohammad Qudah
 # 20 physiology Muscle tissue Ahmad Adel Sallal 30/9/205 Mohammad Qudah MUSCLES PHYSIOLOGY Awn, welcome to the first physiology lecture in the MSS, I wish you a perfect exams with high grades, and never
# 20 physiology Muscle tissue Ahmad Adel Sallal 30/9/205 Mohammad Qudah MUSCLES PHYSIOLOGY Awn, welcome to the first physiology lecture in the MSS, I wish you a perfect exams with high grades, and never
ENHANCEMENT BY F-ACTIN OF MGATP-DEPENDENT DOPAMINE UPTAKE INTO ISOLATED CHROMAFFIN GRANULES
 Vol. 4, No. 1, September 1996 BIOCHEMISTRY and MOLECULAR BIOLOGY INTERNATIONAL Pages 61-66 ENHANCEMENT BY F-ACTIN OF MGATP-DEPENDENT DOPAMINE UPTAKE INTO ISOLATED CHROMAFFIN GRANULES Kyoji Morita ~)*,
Vol. 4, No. 1, September 1996 BIOCHEMISTRY and MOLECULAR BIOLOGY INTERNATIONAL Pages 61-66 ENHANCEMENT BY F-ACTIN OF MGATP-DEPENDENT DOPAMINE UPTAKE INTO ISOLATED CHROMAFFIN GRANULES Kyoji Morita ~)*,
Ch 12 can be done in one lecture
 Ch 12 can be done in one lecture Developed by John Gallagher, MS, DVM Chapter 12: Muscles Review muscle anatomy (esp. microanatomy of skeletal muscle) Terminology: sarcolemma t-tubules sarcoplasmic reticulum
Ch 12 can be done in one lecture Developed by John Gallagher, MS, DVM Chapter 12: Muscles Review muscle anatomy (esp. microanatomy of skeletal muscle) Terminology: sarcolemma t-tubules sarcoplasmic reticulum
Chapter 50. You re on your own for: Sensory Reception Mechanoreceptors Gravity, Hearing and Equilibrium. Chemoreception taste and smell
 1 Sensory and Motor Mechanisms 2 Chapter 50 You re on your own for: Sensory Reception Mechanoreceptors Gravity, Hearing and Equilibrium Chemoreception taste and smell Photoreceptors vision It s interesting.
1 Sensory and Motor Mechanisms 2 Chapter 50 You re on your own for: Sensory Reception Mechanoreceptors Gravity, Hearing and Equilibrium Chemoreception taste and smell Photoreceptors vision It s interesting.
Name: Student Number
 UNIVERSITY OF GUELPH CHEM 454 ENZYMOLOGY Winter 2003 Quiz #1: February 13, 2003, 11:30 13:00 Instructor: Prof R. Merrill Instructions: Time allowed = 80 minutes. Total marks = 34. This quiz represents
UNIVERSITY OF GUELPH CHEM 454 ENZYMOLOGY Winter 2003 Quiz #1: February 13, 2003, 11:30 13:00 Instructor: Prof R. Merrill Instructions: Time allowed = 80 minutes. Total marks = 34. This quiz represents
Name: Teacher: Olsen Hour:
 Name: Teacher: Olsen Hour: Dem Bones Textbook p84-85, 88-99 61 In all exercises, quizzes and tests in this class, always answer in your own words. That is the only way that you can show that you understand
Name: Teacher: Olsen Hour: Dem Bones Textbook p84-85, 88-99 61 In all exercises, quizzes and tests in this class, always answer in your own words. That is the only way that you can show that you understand
Muscles Muscles are effectors which enable movement to be carried out
 Muscles 13.8 Muscles are effectors which enable movement to be carried out Muscle Is responsible for almost all the movements in animals 3 types Cardiac muscle Smo oth muscle Skeletal mus cle (aka striped
Muscles 13.8 Muscles are effectors which enable movement to be carried out Muscle Is responsible for almost all the movements in animals 3 types Cardiac muscle Smo oth muscle Skeletal mus cle (aka striped
Enzymes: The Catalysts of Life
 Chapter 6 Enzymes: The Catalysts of Life Lectures by Kathleen Fitzpatrick Simon Fraser University Activation Energy and the Metastable State Many thermodynamically feasible reactions in a cell that could
Chapter 6 Enzymes: The Catalysts of Life Lectures by Kathleen Fitzpatrick Simon Fraser University Activation Energy and the Metastable State Many thermodynamically feasible reactions in a cell that could
OF LIGHT CHAINS OF CARDIAC MYOSIN ISOZYMES: ATRIAL AND VENTRICULAR MYOSINS
 CROSS-HYBRIDIZATION OF LIGHT CHAINS OF CARDIAC MYOSIN ISOZYMES: ATRIAL AND VENTRICULAR MYOSINS Gabor HOLLGSI*, Sudhir SRIVASTAVA** and Joan WIKMAN-COFFELT University of California, San Francisco Cardiovascular
CROSS-HYBRIDIZATION OF LIGHT CHAINS OF CARDIAC MYOSIN ISOZYMES: ATRIAL AND VENTRICULAR MYOSINS Gabor HOLLGSI*, Sudhir SRIVASTAVA** and Joan WIKMAN-COFFELT University of California, San Francisco Cardiovascular
Dual nucleotide specificity of bovine glutamate dehydrogenase
 Biochem J. (1980) 191, 299-304 Printed in Great Britain 299 Dual nucleotide specificity of bovine glutamate dehydrogenase The role of negative co-operativity Stephen ALX and J. llis BLL Department ofbiochemistry,
Biochem J. (1980) 191, 299-304 Printed in Great Britain 299 Dual nucleotide specificity of bovine glutamate dehydrogenase The role of negative co-operativity Stephen ALX and J. llis BLL Department ofbiochemistry,
Table of contents. Author's preface. Part 1: Structure and function of enzymes
 Author's preface xvii Part 1: Structure and function of enzymes 1 An introduction to enzymes 1.1 What are enzymes 3 1.2 A brief history of enzymes 3 1.3 The naming and classification of enzymes 4 1.3.1
Author's preface xvii Part 1: Structure and function of enzymes 1 An introduction to enzymes 1.1 What are enzymes 3 1.2 A brief history of enzymes 3 1.3 The naming and classification of enzymes 4 1.3.1
The Sliding Filament Theory
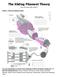 The Sliding Filament Theory Model 1: Muscle Histology Review How do muscle cells contract? Use your knowledge of muscle tissue histology to fill in the blanks numbered 1-11 with the following terms: Fasicle,
The Sliding Filament Theory Model 1: Muscle Histology Review How do muscle cells contract? Use your knowledge of muscle tissue histology to fill in the blanks numbered 1-11 with the following terms: Fasicle,
A Kinetic Study of Glucose-6-phosphate Dehydrogenase
 A Kinetic Study of Glucose-6-phosphate Dehydrogenase (Received for publication, September 10, 1975) MOHAMMED. KANJ, MYRON L. TOEWS, AND W. ROBERT CARPER* From the Department of Chemistry, Wichita State
A Kinetic Study of Glucose-6-phosphate Dehydrogenase (Received for publication, September 10, 1975) MOHAMMED. KANJ, MYRON L. TOEWS, AND W. ROBERT CARPER* From the Department of Chemistry, Wichita State
About This Chapter. Skeletal muscle Mechanics of body movement Smooth muscle Cardiac muscle Pearson Education, Inc.
 About This Chapter Skeletal muscle Mechanics of body movement Smooth muscle Cardiac muscle Skeletal Muscle Usually attached to bones by tendons Origin: closest to the trunk or to more stationary bone Insertion:
About This Chapter Skeletal muscle Mechanics of body movement Smooth muscle Cardiac muscle Skeletal Muscle Usually attached to bones by tendons Origin: closest to the trunk or to more stationary bone Insertion:
Concept 50.5: The physical interaction of protein filaments is required for muscle function
 Concept 50.5: The physical interaction of protein filaments is required for muscle function Muscle activity is a response to input from the nervous system The action of a muscle is always to contract Vertebrate
Concept 50.5: The physical interaction of protein filaments is required for muscle function Muscle activity is a response to input from the nervous system The action of a muscle is always to contract Vertebrate
Margaret A. Daugherty Fall 2003
 Enzymes & Kinetics IV Regulation and Allostery ENZYME-SUBSTRATE INTERACTIONS THE LOCK & KEY MODEL Margaret A. Daugherty Fall 2003 A perfect match between enzyme and substrate can explain enzyme specificity
Enzymes & Kinetics IV Regulation and Allostery ENZYME-SUBSTRATE INTERACTIONS THE LOCK & KEY MODEL Margaret A. Daugherty Fall 2003 A perfect match between enzyme and substrate can explain enzyme specificity
Abstract. The Distribution Of Regulated Actomyosin States Is Central To Cardiac Muscle
 Abstract The Distribution Of Regulated Actomyosin States Is Central To Cardiac Muscle Regulation And Disturbance Of This Distribution Leads To Congenital Cardiomyopathies by Mohit C. Mathur July, 2009
Abstract The Distribution Of Regulated Actomyosin States Is Central To Cardiac Muscle Regulation And Disturbance Of This Distribution Leads To Congenital Cardiomyopathies by Mohit C. Mathur July, 2009
Skeletal Muscle Contraction and ATP Demand
 Skeletal Muscle Contraction and ATP Demand Anatomy & Structure Contraction Cycling Calcium Regulation Types of Contractions Force, Power, and Contraction Velocity Epimysium - separates fascia and muscle
Skeletal Muscle Contraction and ATP Demand Anatomy & Structure Contraction Cycling Calcium Regulation Types of Contractions Force, Power, and Contraction Velocity Epimysium - separates fascia and muscle
Supplementary Figure 1. Overview of steps in the construction of photosynthetic protocellular systems
 Supplementary Figure 1 Overview of steps in the construction of photosynthetic protocellular systems (a) The small unilamellar vesicles were made with phospholipids. (b) Three types of small proteoliposomes
Supplementary Figure 1 Overview of steps in the construction of photosynthetic protocellular systems (a) The small unilamellar vesicles were made with phospholipids. (b) Three types of small proteoliposomes
Skeletal Muscle. Connective tissue: Binding, support and insulation. Blood vessels
 Chapter 12 Muscle Physiology Outline o Skeletal Muscle Structure o The mechanism of Force Generation in Muscle o The mechanics of Skeletal Muscle Contraction o Skeletal Muscle Metabolism o Control of Skeletal
Chapter 12 Muscle Physiology Outline o Skeletal Muscle Structure o The mechanism of Force Generation in Muscle o The mechanics of Skeletal Muscle Contraction o Skeletal Muscle Metabolism o Control of Skeletal
Perspectives in Physiology
 Perspectives in Physiology Published on behalf of The American Physiological Society by Springer More information about this series at http://www.springer.com/series/11779 Integrating the Life Sciences
Perspectives in Physiology Published on behalf of The American Physiological Society by Springer More information about this series at http://www.springer.com/series/11779 Integrating the Life Sciences
Muscle Contraction and (Microscopic) Anatomy. Some copyright issues but I won t tell if you
 Muscle Contraction and (Microscopic) Anatomy Some copyright issues but I won t tell if you don t What is a contraction? It is so important to visualise the fact that muscles work their magic by simply
Muscle Contraction and (Microscopic) Anatomy Some copyright issues but I won t tell if you don t What is a contraction? It is so important to visualise the fact that muscles work their magic by simply
Muscular System Module 3: Contraction and Relaxation *
 OpenStax-CNX module: m48498 1 Muscular System Module 3: Contraction and Relaxation * Donna Browne Based on Muscle Fiber Contraction and Relaxation by OpenStax This work is produced by OpenStax-CNX and
OpenStax-CNX module: m48498 1 Muscular System Module 3: Contraction and Relaxation * Donna Browne Based on Muscle Fiber Contraction and Relaxation by OpenStax This work is produced by OpenStax-CNX and
Molecular Mechanism of Actin Myosin Motor in Muscle
 ISSN 0006-2979, Biochemistry (Moscow), 2011, Vol. 76, No. 13, pp. 1484-1506. Pleiades Publishing, Ltd., 2011. Original Russian Text N. A. Koubassova, A. K. Tsaturyan, 2011, published in Uspekhi Biologicheskoi
ISSN 0006-2979, Biochemistry (Moscow), 2011, Vol. 76, No. 13, pp. 1484-1506. Pleiades Publishing, Ltd., 2011. Original Russian Text N. A. Koubassova, A. K. Tsaturyan, 2011, published in Uspekhi Biologicheskoi
ROLE OF CALCIUM IN DRUG ACTION ON SMOOTH MUSCLE 1, 2 NORIKO YUKISADA AND FUMIKO EBASHI
 Jap. J. Pharmacol. 11, 46-53 (1961) ROLE OF CALCIUM IN DRUG ACTION ON SMOOTH MUSCLE 1, 2 NORIKO YUKISADA AND FUMIKO EBASHI Department of Pharmacology, Faculty of Medicine, University of Tokyo, Tokyo Received
Jap. J. Pharmacol. 11, 46-53 (1961) ROLE OF CALCIUM IN DRUG ACTION ON SMOOTH MUSCLE 1, 2 NORIKO YUKISADA AND FUMIKO EBASHI Department of Pharmacology, Faculty of Medicine, University of Tokyo, Tokyo Received
Interaction of Flavonoid Compounds with Contractile Proteins of Skeletal Muscle
 Gen. Physiol. Biophys. (1988). 7,165 175 165 Interaction of Flavonoid Compounds with Contractile Proteins of Skeletal Muscle V. L. ZYMA, N. S. MIROSHNICHENKO, V. M. DANILOVA and E. En GIN Department of
Gen. Physiol. Biophys. (1988). 7,165 175 165 Interaction of Flavonoid Compounds with Contractile Proteins of Skeletal Muscle V. L. ZYMA, N. S. MIROSHNICHENKO, V. M. DANILOVA and E. En GIN Department of
Chapter 9 Muscle. Types of muscle Skeletal muscle Cardiac muscle Smooth muscle. Striated muscle
 Chapter 9 Muscle Types of muscle Skeletal muscle Cardiac muscle Smooth muscle Striated muscle Chapter 9 Muscle (cont.) The sliding filament mechanism, in which myosin filaments bind to and move actin
Chapter 9 Muscle Types of muscle Skeletal muscle Cardiac muscle Smooth muscle Striated muscle Chapter 9 Muscle (cont.) The sliding filament mechanism, in which myosin filaments bind to and move actin
CHAPTER 6 2/9/2016. Learning Objectives List the four traits that all muscle types have in common.
 Learning Objectives List the four traits that all muscle types have in common. CHAPTER 6 The Muscular System Demonstrate and explain the use of antagonistic muscle pairs. Describe the attachment of muscle
Learning Objectives List the four traits that all muscle types have in common. CHAPTER 6 The Muscular System Demonstrate and explain the use of antagonistic muscle pairs. Describe the attachment of muscle
Chapter 8: Skeletal Muscle: Structure and Function
 Chapter 8: Skeletal Muscle: Structure and Function Objectives Draw & label the microstructure of skeletal muscle Outline the steps leading to muscle shortening Define the concentric and isometric Discuss:
Chapter 8: Skeletal Muscle: Structure and Function Objectives Draw & label the microstructure of skeletal muscle Outline the steps leading to muscle shortening Define the concentric and isometric Discuss:
User s Manual and Instructions
 User s Manual and Instructions Mitochondria Activity Assay (Cytochrome C Oxidase Activity Assay) Kit Catalog Number: KC310100 Introduction Mitochondria are the eukaryotic subcellular organelles that contain
User s Manual and Instructions Mitochondria Activity Assay (Cytochrome C Oxidase Activity Assay) Kit Catalog Number: KC310100 Introduction Mitochondria are the eukaryotic subcellular organelles that contain
Hydrolysis of Irradiated Ovalbumin by Pepsin
 Hydrolysis of Irradiated Ovalbumin by Pepsin HECTOR A. DIEU and V. DESREUX From the Department of Physical Chemistry, University of Liege, Liege, Belgium ABSTRACT Solid ovalbumin has been irradiated at
Hydrolysis of Irradiated Ovalbumin by Pepsin HECTOR A. DIEU and V. DESREUX From the Department of Physical Chemistry, University of Liege, Liege, Belgium ABSTRACT Solid ovalbumin has been irradiated at
The Musculoskeletal System. Chapter 46
 The Musculoskeletal System Chapter 46 Types of Skeletal Systems Changes in movement occur because muscles pull against a support structure Zoologists recognize three types: 1. Hydrostatic skeletons a fluid
The Musculoskeletal System Chapter 46 Types of Skeletal Systems Changes in movement occur because muscles pull against a support structure Zoologists recognize three types: 1. Hydrostatic skeletons a fluid
Mechanics of Cardiac Tissue
 Bioengineering 6000: System Physiology I Mechanics of Cardiac Tissue PD Dr.-Ing. Frank B. Sachse Overview Recapitulation Excitation Propagation II Force Development Experimental Studies Anatomy Cross Bridge
Bioengineering 6000: System Physiology I Mechanics of Cardiac Tissue PD Dr.-Ing. Frank B. Sachse Overview Recapitulation Excitation Propagation II Force Development Experimental Studies Anatomy Cross Bridge
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
Exercise Science (Muscle Anatomy and Physiology) PPL10 Date: May 11 th, 2015
 Exercise Science (Muscle Anatomy and Physiology) PPL10 Date: May 11 th, 2015 Examples of Strength and Physique Athletes The Principle of Muscles The most important principle for muscles is the use it or
Exercise Science (Muscle Anatomy and Physiology) PPL10 Date: May 11 th, 2015 Examples of Strength and Physique Athletes The Principle of Muscles The most important principle for muscles is the use it or
Skeletal Muscle and the Molecular Basis of Contraction. Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry
 Skeletal Muscle and the Molecular Basis of Contraction Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Like neurons, all muscle cells can be excited chemically, electrically, and
Skeletal Muscle and the Molecular Basis of Contraction Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Like neurons, all muscle cells can be excited chemically, electrically, and
X-ray Diffraction Studies of the Thick Filament in Permeabilized Myocardium from Rabbit
 3768 Biophysical Journal Volume 91 November 2006 3768 3775 X-ray Diffraction Studies of the Thick Filament in Permeabilized Myocardium from Rabbit Sengen Xu,* Donald Martyn, y Jessica Zaman,* and Leepo
3768 Biophysical Journal Volume 91 November 2006 3768 3775 X-ray Diffraction Studies of the Thick Filament in Permeabilized Myocardium from Rabbit Sengen Xu,* Donald Martyn, y Jessica Zaman,* and Leepo
AN INTRODUCTION TO INVOLUNTARY (ESPECIALLY SMOOTH) MUSCLES 1
 AN INTRODUCTION TO INVOLUNTARY (ESPECIALLY SMOOTH) MUSCLES 1 Summary: This section is an introduction to a fascinating and extremely important group of tissue, the smooth muscles. As you will see, their
AN INTRODUCTION TO INVOLUNTARY (ESPECIALLY SMOOTH) MUSCLES 1 Summary: This section is an introduction to a fascinating and extremely important group of tissue, the smooth muscles. As you will see, their
New Concepts in the Control of Muscle Contraction
 New Concepts in the Control of Muscle Contraction Gerry Smith PhD (Cantab) Gerry is a synthetic organic chemist who has collaborated in many biological fields ranging from pharmaceutical synthesis at Glaxo
New Concepts in the Control of Muscle Contraction Gerry Smith PhD (Cantab) Gerry is a synthetic organic chemist who has collaborated in many biological fields ranging from pharmaceutical synthesis at Glaxo
Physiology sheet #2. The heart composed of 3 layers that line its lumen and cover it from out side, these layers are :
 Physiology sheet #2 * We will talk in this lecture about cardiac muscle physiology, the mechanism and the energy sources of their contraction and intracellular calcium homeostasis. # Slide 4 : The heart
Physiology sheet #2 * We will talk in this lecture about cardiac muscle physiology, the mechanism and the energy sources of their contraction and intracellular calcium homeostasis. # Slide 4 : The heart
Session 3-Part 2: Skeletal Muscle
 Session 3-Part 2: Skeletal Muscle Course: Introduction to Exercise Science-Level 2 (Exercise Physiology) Presentation Created by Ken Baldwin, M.ED, ACSM-H/FI Copyright EFS Inc. All Rights Reserved. Skeletal
Session 3-Part 2: Skeletal Muscle Course: Introduction to Exercise Science-Level 2 (Exercise Physiology) Presentation Created by Ken Baldwin, M.ED, ACSM-H/FI Copyright EFS Inc. All Rights Reserved. Skeletal
Collin County Community College BIOL Muscle Physiology. Muscle Length-Tension Relationship
 Collin County Community College BIOL 2401 Muscle Physiology 1 Muscle Length-Tension Relationship The Length-Tension Relationship Another way that muscle cells can alter their force capability, is determined
Collin County Community College BIOL 2401 Muscle Physiology 1 Muscle Length-Tension Relationship The Length-Tension Relationship Another way that muscle cells can alter their force capability, is determined
On which skeletal muscle filament is troponin located? What is the function of the sarcoplasmic reticulum (SR)?
 CASE 6 A 21-year-old man presents to a rural emergency center with a 1-day history of progressive stiffness of the neck and jaw, difficulty swallowing, stiff shoulders and back, and a rigid abdomen. Upon
CASE 6 A 21-year-old man presents to a rural emergency center with a 1-day history of progressive stiffness of the neck and jaw, difficulty swallowing, stiff shoulders and back, and a rigid abdomen. Upon
Muscular System- Part 1. Unit 5 Miss Wheeler
 Muscular System- Part 1 Unit 5 Miss Wheeler Fun Facts! The tongue is the strongest muscle in your body The smallest muscles in the body are in the middle ear The largest muscle in the body is the gluteus
Muscular System- Part 1 Unit 5 Miss Wheeler Fun Facts! The tongue is the strongest muscle in your body The smallest muscles in the body are in the middle ear The largest muscle in the body is the gluteus
HASPI Medical Anatomy & Physiology 09b Lab Activity
 HASPI Medical Anatomy & Physiology 09b Lab Activity Name(s): Period: Date: Muscle Cell Structure Muscle cells are specialized to contract. An individual muscle is actually a bundle of hundreds to thousands
HASPI Medical Anatomy & Physiology 09b Lab Activity Name(s): Period: Date: Muscle Cell Structure Muscle cells are specialized to contract. An individual muscle is actually a bundle of hundreds to thousands
Chapter Skeletal Muscle Structure and Function
 Chapter 10.2 Skeletal Muscle Structure and Function Introduction to Muscle Physiology Movement is a fundamental characteristic of all living things All muscle cells (skeletal, cardiac, and smooth) are
Chapter 10.2 Skeletal Muscle Structure and Function Introduction to Muscle Physiology Movement is a fundamental characteristic of all living things All muscle cells (skeletal, cardiac, and smooth) are
The Action of Thiamine Phosphates on the Contraction of Glycerinated Psoas Muscle
 THE JOURNAL OF VITAMINOLOGY 14, 101-105 (1968) The Action of Thiamine Phosphates on the Contraction of Glycerinated Psoas Muscle ATSUSHI MURAI AND EISUKE KATSURA2 Nutrition Laboratory, Kyoto University
THE JOURNAL OF VITAMINOLOGY 14, 101-105 (1968) The Action of Thiamine Phosphates on the Contraction of Glycerinated Psoas Muscle ATSUSHI MURAI AND EISUKE KATSURA2 Nutrition Laboratory, Kyoto University
B108 BC Muscle Contraction and Locomotion *
 OpenStax-CNX module: m62420 1 B108 BC Muscle Contraction and Locomotion * Melodye Gold Based on Human Biology Chapter 16: Muscle Contraction and Locomotion by OpenStax Willy Cushwa This work is produced
OpenStax-CNX module: m62420 1 B108 BC Muscle Contraction and Locomotion * Melodye Gold Based on Human Biology Chapter 16: Muscle Contraction and Locomotion by OpenStax Willy Cushwa This work is produced
Skeletal Muscle Qiang XIA (
 Skeletal Muscle Qiang XIA ( 夏强 ), PhD Department of Physiology Rm C518, Block C, Research Building, School of Medicine Tel: 88208252 Email: xiaqiang@zju.edu.cn Course website: http://10.71.121.151/physiology
Skeletal Muscle Qiang XIA ( 夏强 ), PhD Department of Physiology Rm C518, Block C, Research Building, School of Medicine Tel: 88208252 Email: xiaqiang@zju.edu.cn Course website: http://10.71.121.151/physiology
Ca'+ CALMODULIN, TROPONIN, PARVALBUMIN, AND MYOSIN IN RESPONSE TO TRANSIENT INCREASES. THE TIME-COURSE OF Ca2+ IN Ca2`
 THE TIME-COURSE OF Ca2+ EXCHANGE WITH CALMODULIN, TROPONIN, PARVALBUMIN, AND MYOSIN IN RESPONSE TO TRANSIENT INCREASES IN Ca2` STEVEN P. ROBERTSON, J. DAVID JOHNSON, AND JAMES D. POTrER Department of Pharmocology
THE TIME-COURSE OF Ca2+ EXCHANGE WITH CALMODULIN, TROPONIN, PARVALBUMIN, AND MYOSIN IN RESPONSE TO TRANSIENT INCREASES IN Ca2` STEVEN P. ROBERTSON, J. DAVID JOHNSON, AND JAMES D. POTrER Department of Pharmocology
BIO 130 Anatomy and Physiology Spring, 2016 Exam 3 Name: Course ID Number. Section 1 Answer questions 1 40 on the scan sheet.
 BIO 130 Anatomy and Physiology Spring, 2016 Exam 3 Name: Course ID Number Section 1 Answer questions 1 40 on the scan sheet. 1. Which of the following is NOT a characteristic of epithelial tissue? a. It
BIO 130 Anatomy and Physiology Spring, 2016 Exam 3 Name: Course ID Number Section 1 Answer questions 1 40 on the scan sheet. 1. Which of the following is NOT a characteristic of epithelial tissue? a. It
Role of Actin C-Terminus in Regulation of Striated Muscle Thin Filament
 Biophysical Journal Volume 94 February 2008 1341 1347 1341 Role of Actin C-Terminus in Regulation of Striated Muscle Thin Filament Ma1gorzata Śliwińska, Rados1aw Skórzewski, and Joanna Moraczewska Kazimierz
Biophysical Journal Volume 94 February 2008 1341 1347 1341 Role of Actin C-Terminus in Regulation of Striated Muscle Thin Filament Ma1gorzata Śliwińska, Rados1aw Skórzewski, and Joanna Moraczewska Kazimierz
Lecture 6: Allosteric regulation of enzymes
 Chem*3560 Lecture 6: Allosteric regulation of enzymes Metabolic pathways do not run on a continuous basis, but are regulated according to need Catabolic pathways run if there is demand for ATP; for example
Chem*3560 Lecture 6: Allosteric regulation of enzymes Metabolic pathways do not run on a continuous basis, but are regulated according to need Catabolic pathways run if there is demand for ATP; for example
Chapter 10 -Muscle Tissue
 Chapter 10 -Muscle Tissue Muscles: 1. Overview of Muscle Tissue A. Review 5 functions of muscle tissue. B. Review the 5 properties of muscle tissue. WHICH do they share with nervous tissue? (2, plus the
Chapter 10 -Muscle Tissue Muscles: 1. Overview of Muscle Tissue A. Review 5 functions of muscle tissue. B. Review the 5 properties of muscle tissue. WHICH do they share with nervous tissue? (2, plus the
BIOMECHANICS 2 Origins and consequences of forces in biological systems
 BIOMECHANICS 2 Origins and consequences of forces in biological systems MOLECULAR MECHANISMS OF BIOLOGICAL MOVEMENT AT THE MOLECULAR LEVEL MOTOR PROTEINS DR. BEÁTA BUGYI - BIOPHYSICS UP MEDICAL SCHOOL
BIOMECHANICS 2 Origins and consequences of forces in biological systems MOLECULAR MECHANISMS OF BIOLOGICAL MOVEMENT AT THE MOLECULAR LEVEL MOTOR PROTEINS DR. BEÁTA BUGYI - BIOPHYSICS UP MEDICAL SCHOOL
Ch 12: Muscles sarcolemma, t-tubules, sarcoplasmic reticulum, myofibrils, myofilaments, sarcomere...
 Ch 12: Muscles Review micro-anatomy of muscle tissue Terminology examples: sarcolemma, t-tubules, sarcoplasmic reticulum, myofibrils, myofilaments, sarcomere... SLOs Differentiate levels of muscle structure:
Ch 12: Muscles Review micro-anatomy of muscle tissue Terminology examples: sarcolemma, t-tubules, sarcoplasmic reticulum, myofibrils, myofilaments, sarcomere... SLOs Differentiate levels of muscle structure:
Chapter 10: Muscles. Vocabulary: aponeurosis, fatigue
 Chapter 10: Muscles 37. Describe the structural components of skeletal muscle tissue from the molecular to the organ level. 38. Describe the structure, function, and importance of sarcomeres. 39. Identify
Chapter 10: Muscles 37. Describe the structural components of skeletal muscle tissue from the molecular to the organ level. 38. Describe the structure, function, and importance of sarcomeres. 39. Identify
atively poor response of adenylate cyclase in Leydig cell
 Proc. Nati. Acad. Sci. USA Vol. 77, No. 10, pp. 5837-5841, October 1980 Biochemistry Hormone-induced guanyl nucleotide binding and activation of adenylate cyclase in the Leydig cell (hormone action/testicular
Proc. Nati. Acad. Sci. USA Vol. 77, No. 10, pp. 5837-5841, October 1980 Biochemistry Hormone-induced guanyl nucleotide binding and activation of adenylate cyclase in the Leydig cell (hormone action/testicular
BCH 450 Biochemistry of Specialized Tissues. V. Muscle Tissues
 BCH 450 Biochemistry of Specialized Tissues V. Muscle Tissues Nomenclature Sarcolemma = plasma membrane Sarcoplasmic reticulum = endoplasmic reticulum Muscle fiber = cell Myofibril = subcellular fibers
BCH 450 Biochemistry of Specialized Tissues V. Muscle Tissues Nomenclature Sarcolemma = plasma membrane Sarcoplasmic reticulum = endoplasmic reticulum Muscle fiber = cell Myofibril = subcellular fibers
STUDIES ON THE CALCIUM-PROTEIN RELATIONSHIP WITH THE AID OF THE ULTRACENTRIFUGE
 STUDIES ON THE CALCIUM-PROTEIN RELATIONSHIP WITH THE AID OF THE ULTRACENTRIFUGE II. OBSERVATIONS ON SERUM BY STEPHAN LUDEWIG, ALFRED CHANUTIN, AND A. V. MASKETt (From the Biochemical Laboralory, University
STUDIES ON THE CALCIUM-PROTEIN RELATIONSHIP WITH THE AID OF THE ULTRACENTRIFUGE II. OBSERVATIONS ON SERUM BY STEPHAN LUDEWIG, ALFRED CHANUTIN, AND A. V. MASKETt (From the Biochemical Laboralory, University
10 - Muscular Contraction. Taft College Human Physiology
 10 - Muscular Contraction Taft College Human Physiology Muscular Contraction Sliding filament theory (Hanson and Huxley, 1954) These 2 investigators proposed that skeletal muscle shortens during contraction
10 - Muscular Contraction Taft College Human Physiology Muscular Contraction Sliding filament theory (Hanson and Huxley, 1954) These 2 investigators proposed that skeletal muscle shortens during contraction
