*smith&nephew. MRI Safety Information & Parameters for Smith & Nephew Orthopaedics AG. Hip Implants
|
|
|
- Harry Francis
- 5 years ago
- Views:
Transcription
1 Hip Implants MRI Safety Information & Parameters for Smith & Nephew Orthopaedics AG Hip Implants *smith&nephew Supporting healthcare professionals for over 150 years
2 Summary All hip implants of Smith & Nephew Orthopaedics AG are considered MR conditional and can be scanned safely if the following criteria are met (1). static magnetic field of 1.5 or 3 T, with spatial gradient field of 14 T/m (value extrapolated) or less spatial gradient field product of 39 T 2 /m (value extrapolated) or less theoretically estimated maximum whole body averaged (WBA) specific absorption rate (SAR) of < 0.3 W/kg at 1.5 T (local SAR < 0.9 W/kg) < 0.2 W/kg at 3 T (local SAR < 1.3 W/kg) for 15 minutes of continuous MR scanning (15 minutes duration given due to recommendations in ASTM F2182) A temperature increase limit of 2 C was used for extrapolation of estimated WBA SAR and recommended local SAR based on in vitro test results. In combination with a Polarcup the following restrictions apply additionally to the general criteria mentioned for hip implants: spatial gradient field of 9.3 T/m (value extrapolated) and less spatial gradient field product of 18.9 T 2 /m (value extrapolated) and less 1 TR a Marking Draft Hip Implant System 2 MRI Safety Information & Parameters for Smith & Nephew Orthopaedics AG Hip Implants
3 Background Magnetic resonance imaging (MRI) is an imaging technique used in medical settings to produce high quality images of the inside of the human body. MRI is based on the principles of nuclear magnetic resonance, a spectroscopic technique to obtain microscopic chemical and physical information about molecules (2). MRI can be used as a powerful diagnostic tool for disease and injury detection throughout the body. In orthopaedics, MRI is a source of accurate information about the structure of the joints, soft tissues as well as bones. Patients with metallic implants can experience adverse effects from the electromagnetic field or radio frequency pulses used for MRI e.g. excessive MRI-related heating. The definitions of MR safety (ASTM F2503) are the following: MR MR MR MR MR Safe is an item that poses no known hazards resulting from exposure to any MR environment. MR Safe items are composed of materials that are electrically nonconductive, nonmetallic and nonmagnetic. MR Conditional is an item with demonstrated safety in the MR environment within defined conditions. MR Unsafe is an item which poses unacceptable risks to the patient, medical staff or other persons within the MR environment. Tested products A hip system contains a stem, a ball head, a cup and an insert. The material composition might vary for each component, depending on combination options. All hip implant components from Smith & Nephew Orthopaedics AG were considered for the tests. The defined worst case combinations were non-clinically tested for radio frequency heating (RF heating) in a MR environment according to ASTM F2182. Before testing mechanically, a computer simulation defined the worst case systems for RF heating. Furthermore, the implants were non-clinically tested for magnetically induced displacement force (ASTM F2052) and torque (ASTM F2213) and image artifacts (ASTM F2119). Field strengths of 1.5 T and 3 T were taken into consideration for the tests. 2 The Basics of MRI J.P. Hornak MRI Safety Information & Parameters for Smith & Nephew Orthopaedics AG Hip Implants 3
4 Results General notice: The whole body or head averaged SAR is inappropriate to scale exact local temperature increases. Local SAR can deviate and result in much higher values than the WBA-SAR generally displayed by the scanner s software. Non-clinical testing has demonstrated that all hip implants of Smith & Nephew Orthopaedics AG are MR conditional. The conditions for MR scanning of Smith & Nephew Orthopaedics AG hip implants are listed in the summary above. MR conditional Smith & Nephew Orthopaedics AG hip implants may cause image artifacts. MR image artifacts may distort the visualisation of the area surrounding the implant as follows (1). Largest artifacts of Spin Echo Gradient Echo 1.5 T 3 T 1.5 T 3 T Test object length 71.8 mm 97.4 mm 87.0 mm 67.5 mm Test object width mm mm mm mm (object long axis parallel to the main magnetic field B0) The width was measured in direction of the worst artifact across the centre of the test object. The length was measured parallel to the test object long axis. The given values represent the artifact extension from the surface of each side of the implant. For example, when imaged with a spin echo pulse sequence and a 1.5 T MRI system, the image artifact caused by the Hip System extends approximately 71.8 mm from the implant in the direction of its long axis. 1 TR a Marking Draft Hip Implant System 4 MRI Safety Information & Parameters for Smith & Nephew Orthopaedics AG Hip Implants
5 Notes MRI Safety Information & Parameters for Smith & Nephew Orthopaedics AG Hip Implants 5
6 6 MRI Safety Information & Parameters for Smith & Nephew Orthopaedics AG Hip Implants
7
8 SAR values should be kept as low as possible in order to minimize any risk for the patient. Before each individual MR scan it might be necessary to discuss the situation with regard to patient benefit, consulting medical experts and MR physicists. Smith & Nephew Orthopaedics AG This safety information applies to the following product groups: ADR BICON-PLUS Ceramic Ball Heads and Metal Ball Heads CPS-PLUS CS-PLUS and CSL-PLUS EP-FIT PLUS Fracture Head and Bipolar Prosthesis Geradschaft-PLUS HI IPA MODULAR-PLUS MPF POLARCUP POLARSTEM SBG SL-PLUS, SLR-PLUS and SL-PLUS MIA Manufacturer Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d 6340 Baar Switzerland Trademark of Smith & Nephew Smith & Nephew en V1 (2183) 0917 Supporting healthcare professionals for over 150 years
*smith&nephew. MRI Safety Information & Parameters for Smith & Nephew Orthopaedics AG. Knee Implants
 Knee Implants MRI Safety Information & Parameters for Smith & Nephew Orthopaedics AG Knee Implants *smith&nephew Supporting healthcare professionals for over 150 years Summary All knee implants of Smith
Knee Implants MRI Safety Information & Parameters for Smith & Nephew Orthopaedics AG Knee Implants *smith&nephew Supporting healthcare professionals for over 150 years Summary All knee implants of Smith
*smith&nephew. MRI Safety Information & Parameters for Smith & Nephew Orthopaedics AG. Shoulder Implants
 Shoulder Implants MRI Safety Information & Parameters for Smith & Nephew Orthopaedics AG Shoulder Implants *smith&nephew Supporting healthcare professionals for over 150 years Summary All shoulder implants
Shoulder Implants MRI Safety Information & Parameters for Smith & Nephew Orthopaedics AG Shoulder Implants *smith&nephew Supporting healthcare professionals for over 150 years Summary All shoulder implants
MRI Guidelines for the Axonics Sacral Neuromodulation System
 MRI Guidelines for the Axonics Sacral Neuromodulation System For Healthcare Professionals Rx only Note: This document is a supplement to the physician manuals provided with the Axonics SNM System. This
MRI Guidelines for the Axonics Sacral Neuromodulation System For Healthcare Professionals Rx only Note: This document is a supplement to the physician manuals provided with the Axonics SNM System. This
Low-Profile Wrist Fixator. For stabilization of fractures of the distal radius.
 Low-Profile Wrist Fixator. For stabilization of fractures of the distal radius. Technique Guide Part of the External Fixation System Low-Profile Wrist Fixator Indications Intended for stabilization of
Low-Profile Wrist Fixator. For stabilization of fractures of the distal radius. Technique Guide Part of the External Fixation System Low-Profile Wrist Fixator Indications Intended for stabilization of
MRI GUIDELINES FOR INSPIRE THERAPY
 MRI GUIDELINES FOR INSPIRE THERAPY Clinician s Manual ONLY The following is a trademark of Inspire Medical Systems, Inc.: Inspire Introduction Read the information in this manual prior to conducting an
MRI GUIDELINES FOR INSPIRE THERAPY Clinician s Manual ONLY The following is a trademark of Inspire Medical Systems, Inc.: Inspire Introduction Read the information in this manual prior to conducting an
Cochlear Implants. Medical Procedures. for MED EL Implant Systems. AW33290_2.0 (English US)
 Cochlear Implants Medical Procedures for MED EL Implant Systems AW33290_2.0 (English US) This manual provides important instructions and safety information for MED EL Implant System users who have to undergo
Cochlear Implants Medical Procedures for MED EL Implant Systems AW33290_2.0 (English US) This manual provides important instructions and safety information for MED EL Implant System users who have to undergo
Algovita. MRI Procedure Guidelines. Spinal Cord Stimulation System. 201x
 Algovita Spinal Cord Stimulation System MRI Procedure Guidelines Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System. ONLY 201x Algovita
Algovita Spinal Cord Stimulation System MRI Procedure Guidelines Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System. ONLY 201x Algovita
Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System.
 Algovita Spinal Cord Stimulation System MRI Procedure Guidelines Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System. ONLY 0300-000175-001
Algovita Spinal Cord Stimulation System MRI Procedure Guidelines Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System. ONLY 0300-000175-001
Cochlear Implants. Medical Procedures. for MED EL CI Systems. AW33290_5.0 (English US)
 Cochlear Implants Medical Procedures for MED EL CI Systems AW33290_5.0 (English US) This manual provides important instructions and safety information for MED EL CI System users who have to undergo a medical
Cochlear Implants Medical Procedures for MED EL CI Systems AW33290_5.0 (English US) This manual provides important instructions and safety information for MED EL CI System users who have to undergo a medical
LCP Metaphyseal Plates. For extra-articular fractures.
 LCP Metaphyseal Plates. For extra-articular fractures. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Image intensifier
LCP Metaphyseal Plates. For extra-articular fractures. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Image intensifier
Medium External Fixator Pediatric Femoral Shaft Frame. Using medium multi-pin clamps.
 Medium External Fixator Pediatric Femoral Shaft Frame. Using medium multi-pin clamps. Technique Guide Part of the Medium External Fixation System MRI Information Synthes Medium External Fixation devices
Medium External Fixator Pediatric Femoral Shaft Frame. Using medium multi-pin clamps. Technique Guide Part of the Medium External Fixation System MRI Information Synthes Medium External Fixation devices
External Distal Radius Fixator. Supplement to the 8 mm rod fixator system
 External Distal Radius Fixator. Supplement to the 8 mm rod fixator system Surgical technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation
External Distal Radius Fixator. Supplement to the 8 mm rod fixator system Surgical technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation
1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System (IPG1000 and IPG1500)
 1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System (IPG1000 and IPG1500). ONLY NEVRO CORP. All questions or concerns about Nevro products should be forwarded to: Nevro
1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System (IPG1000 and IPG1500). ONLY NEVRO CORP. All questions or concerns about Nevro products should be forwarded to: Nevro
MRI Information for Medtronic Spinal and Biologics Devices
 M708348B263E Rev C Release Date: 2013-02 MRI Information for Medtronic Spinal and Biologics Devices Not for Distribution in the U.S or its territories. 1 P age Table of Contents MRI Information for Medtronic
M708348B263E Rev C Release Date: 2013-02 MRI Information for Medtronic Spinal and Biologics Devices Not for Distribution in the U.S or its territories. 1 P age Table of Contents MRI Information for Medtronic
1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System
 1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System ONLY 11096 Rev B 1 NEVRO CORP. All questions or concerns about Nevro products should be forwarded to: Nevro Corp.
1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System ONLY 11096 Rev B 1 NEVRO CORP. All questions or concerns about Nevro products should be forwarded to: Nevro Corp.
Sterile-Packaged Large External Fixator Kits. For treatment of long bone and pelvic fractures that require external fixation.
 Sterile-Packaged Large External Fixator Kits. For treatment of long bone and pelvic fractures that require external fixation. Large External Fixator Ankle Frame Kit Large External Fixator Trauma Kit Large
Sterile-Packaged Large External Fixator Kits. For treatment of long bone and pelvic fractures that require external fixation. Large External Fixator Ankle Frame Kit Large External Fixator Trauma Kit Large
ImageReady MRI Full Body Guidelines for Precision Montage MRI Spinal Cord Stimulator System
 ImageReady MRI Full Body Guidelines for Precision Montage MRI Spinal Cord Stimulator System CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician. 91035972-02
ImageReady MRI Full Body Guidelines for Precision Montage MRI Spinal Cord Stimulator System CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician. 91035972-02
VNS Therapy System Physician s Manual
 VNS Therapy System Physician s Manual Pulse Generator Model 102 Pulse Duo Generator Model 102R Demipulse Generator Model 103 Demipulse Duo Generator Model 104 AspireHC Generator Model 105 AspireSR Generator
VNS Therapy System Physician s Manual Pulse Generator Model 102 Pulse Duo Generator Model 102R Demipulse Generator Model 103 Demipulse Duo Generator Model 104 AspireHC Generator Model 105 AspireSR Generator
Button Plate. Reinforcement for transosseous fixations.
 Button Plate. Reinforcement for transosseous fixations. Product Information This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Image intensifier
Button Plate. Reinforcement for transosseous fixations. Product Information This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Image intensifier
1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System (IPG1000 and IPG1500)
 1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System (IPG1000 and IPG1500) ONLY NEVRO CORP. All questions or concerns about Nevro products should be forwarded to: Nevro
1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System (IPG1000 and IPG1500) ONLY NEVRO CORP. All questions or concerns about Nevro products should be forwarded to: Nevro
MRI GUIDELINES FOR INSPIRE THERAPY
 MRI GUIDELINES FOR INSPIRE THERAPY Clinician s Manual The following is a trademark of Inspire Medical Systems, Inc.: Inspire This product and/or the use of this product in a method may be covered by one
MRI GUIDELINES FOR INSPIRE THERAPY Clinician s Manual The following is a trademark of Inspire Medical Systems, Inc.: Inspire This product and/or the use of this product in a method may be covered by one
Medium External Fixator Humeral Shaft Frame. Modular frame for upper extremity use.
 Medium External Fixator Humeral Shaft Frame. Modular frame for upper extremity use. Technique Guide Part of the Medium External Fixation System MRI Information Synthes Medium External Fixation devices
Medium External Fixator Humeral Shaft Frame. Modular frame for upper extremity use. Technique Guide Part of the Medium External Fixation System MRI Information Synthes Medium External Fixation devices
Product Information. *smith&nephew POLARCUP Dual Mobility System
 APPLYING SCIENCE TO MOBILITY Combined with VERILAST Technology for unmatched performance Product Information *smith&nephew POLARCUP Dual Mobility System APPLYING SCIENCE TO MOBILITY Combined with VERILAST
APPLYING SCIENCE TO MOBILITY Combined with VERILAST Technology for unmatched performance Product Information *smith&nephew POLARCUP Dual Mobility System APPLYING SCIENCE TO MOBILITY Combined with VERILAST
MRI Safety Information for the HiRes Ultra 3D Cochlear Implant
 Advanced Bionics AG Laubisrütistrasse 28 8712 Stäfa, Switzerland +41 58 928 78 00 Manufactured by: Advanced Bionics LLC California, U.S.A. +1 661 362 1400 MRI Safety Information for the HiRes Ultra 3D
Advanced Bionics AG Laubisrütistrasse 28 8712 Stäfa, Switzerland +41 58 928 78 00 Manufactured by: Advanced Bionics LLC California, U.S.A. +1 661 362 1400 MRI Safety Information for the HiRes Ultra 3D
The Calcaneal Plate. The Synthes non-locking solution for the Calcaneus.
 The Calcaneal Plate. The Synthes non-locking solution for the Calcaneus. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation.
The Calcaneal Plate. The Synthes non-locking solution for the Calcaneus. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation.
MRI for Patients with MRI-Conditional Pacing System: Radiographers Role. Lawrance Yip DM, DR, QMH
 MRI for Patients with MRI-Conditional Pacing System: Radiographers Role Lawrance Yip DM, DR, QMH MRI & Pacemaker MRI is a crucial and growing imaging modality Plays an important role in clinical management
MRI for Patients with MRI-Conditional Pacing System: Radiographers Role Lawrance Yip DM, DR, QMH MRI & Pacemaker MRI is a crucial and growing imaging modality Plays an important role in clinical management
Large External Fixator Modular Knee Bridge. Using multi-pin clamps.
 Large External Fixator Modular Knee Bridge. Using multi-pin clamps. Technique Guide Part of the Large External Fixation System Large External Fixator Modular Knee Bridge Technique Overview Insert Schanz
Large External Fixator Modular Knee Bridge. Using multi-pin clamps. Technique Guide Part of the Large External Fixation System Large External Fixator Modular Knee Bridge Technique Overview Insert Schanz
Micra MC1VR01. MRI procedural information for Micra Model MC1VR01 MR Conditional single chamber transcatheter pacing device (VVIR)
 Micra MC1VR01 MRI procedural information for Micra Model MC1VR01 MR Conditional single chamber transcatheter pacing device (VVIR) MRI Technical Manual The following list includes trademarks or registered
Micra MC1VR01 MRI procedural information for Micra Model MC1VR01 MR Conditional single chamber transcatheter pacing device (VVIR) MRI Technical Manual The following list includes trademarks or registered
2014 AAPM 56 th Annual Meeting
 2014 AAPM 56 th Annual Meeting SAM Diagnostic Radiology MR Safety - Deep Brain Stimulator and Other Neurostimulators Yunhong Shu, Ph.D. Mayo Clinic, Rochester, MN Outline Background of Neurostimulator
2014 AAPM 56 th Annual Meeting SAM Diagnostic Radiology MR Safety - Deep Brain Stimulator and Other Neurostimulators Yunhong Shu, Ph.D. Mayo Clinic, Rochester, MN Outline Background of Neurostimulator
WallFlex Biliary RX Partially Covered Stent System Prescriptive Information
 Caution/Rx Only: Federal Law (USA) restricts this device to sale by or on the order of a physician. Intended Use/Indications for Use The WallFlex Biliary RX is indicated for use in the palliative treatment
Caution/Rx Only: Federal Law (USA) restricts this device to sale by or on the order of a physician. Intended Use/Indications for Use The WallFlex Biliary RX is indicated for use in the palliative treatment
Distal Radius Plate 2.4/2.7 dorsal and volar
 Distal Radius Plate 2.4/2.7 dorsal and volar Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Distal Radius Plate
Distal Radius Plate 2.4/2.7 dorsal and volar Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Distal Radius Plate
*smith&nephew EP-FIT PLUS Cementless Press-Fit Cup System. Product Information
 Product Information *smith&nephew EP-FIT PLUS Cementless Press-Fit Cup System EP-FIT PLUS Press-Fit Acetabular Cup System A new generation in press-fit cup technology Proven anchoring concept in combination
Product Information *smith&nephew EP-FIT PLUS Cementless Press-Fit Cup System EP-FIT PLUS Press-Fit Acetabular Cup System A new generation in press-fit cup technology Proven anchoring concept in combination
*smith&nephew POLARCUP Dual Mobility System. Product Information
 Product Information *smith&nephew POLARCUP Dual Mobility System Dual Mobility 30 Years of Clinical Experience Concept combining an extended ROM, a low rate of dislocation and unexpectedly limited wear.
Product Information *smith&nephew POLARCUP Dual Mobility System Dual Mobility 30 Years of Clinical Experience Concept combining an extended ROM, a low rate of dislocation and unexpectedly limited wear.
Building physician referrals and improve patient care for an aging population with
 MR Conditional implants Building physician referrals and improve patient care for an aging population with MR Conditional implants 12.5 million people in the USA are presently carrying an orthopedic or
MR Conditional implants Building physician referrals and improve patient care for an aging population with MR Conditional implants 12.5 million people in the USA are presently carrying an orthopedic or
Warning: Patients should not be exposed to MRI environments until the surgical site following pump implantation is fully healed.
 Prometra and Prometra II Programmable Pumps Magnetic Resonance Imaging (MRI) Safety Information GENERAL MR Conditional WARNING: FAILURE TO EMPTY THE PUMP PRIOR TO EXPOSURE TO MRI ENVIRONMENT COULD RESULT
Prometra and Prometra II Programmable Pumps Magnetic Resonance Imaging (MRI) Safety Information GENERAL MR Conditional WARNING: FAILURE TO EMPTY THE PUMP PRIOR TO EXPOSURE TO MRI ENVIRONMENT COULD RESULT
ImageReady MRI Head Only Guidelines for Spectra WaveWriter Spinal Cord Stimulator System
 ImageReady MRI Head Only Guidelines for Spectra WaveWriter Spinal Cord Stimulator System CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician. 91171762-01
ImageReady MRI Head Only Guidelines for Spectra WaveWriter Spinal Cord Stimulator System CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician. 91171762-01
Wrist Fusion Instrument and Implant Set.
 Wrist Fusion Instrument and Implant Set. Surgical Technique Discontinued December 2016 DSEM/TRM/0815/0479(2) This publication is not intended for distribution in the USA. Instruments and implants approved
Wrist Fusion Instrument and Implant Set. Surgical Technique Discontinued December 2016 DSEM/TRM/0815/0479(2) This publication is not intended for distribution in the USA. Instruments and implants approved
Replacement Heart Valve Product Description (Stented Tissue) Models Reference
 Dear Imaging Center: This letter is in response to your inquiry concerning the safety of performing magnetic resonance (MR) procedures in patients who have been implanted with Edwards Lifesciences LLC
Dear Imaging Center: This letter is in response to your inquiry concerning the safety of performing magnetic resonance (MR) procedures in patients who have been implanted with Edwards Lifesciences LLC
LOW PROFILE NEURO. This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE
 LOW PROFILE NEURO This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 Intended Use, Indications, Contraindications
LOW PROFILE NEURO This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 Intended Use, Indications, Contraindications
Large External Fixator Delta Frame Ankle Bridge. Using pin clamps with outrigger posts.
 Large External Fixator Delta Frame Ankle Bridge. Using pin clamps with outrigger posts. Technique Guide Part of the Large External Fixation System Large External Fixator Delta Frame Ankle Bridge Technique
Large External Fixator Delta Frame Ankle Bridge. Using pin clamps with outrigger posts. Technique Guide Part of the Large External Fixation System Large External Fixator Delta Frame Ankle Bridge Technique
EasyStep. Operative technique
 Operative technique EasyStep - Step staple This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments. It offers guidance that you should heed,
Operative technique EasyStep - Step staple This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments. It offers guidance that you should heed,
Large External Fixator Delta Frame Ankle Bridge. For staged fixation of the distal tibia.
 Large External Fixator Delta Frame Ankle Bridge. For staged fixation of the distal tibia. Technique Guide Part of the Large External Fixation System Large External Fixator Delta Frame Ankle Bridge Technique
Large External Fixator Delta Frame Ankle Bridge. For staged fixation of the distal tibia. Technique Guide Part of the Large External Fixation System Large External Fixator Delta Frame Ankle Bridge Technique
Carry this card with you at all times. Show this card to any medical professional treating you. Patient Implant Card. Option ELITE Vena Cava Filter.
 Patient Guide A Safe Option for a Healthier You! P/N: P-2017-0175-00 Rev B 1. Static magnetic field of 3 Tesla or less. 2. Spatial gradient magnetic field of 720 Gauss/cm or less. 3. Maximum whole body
Patient Guide A Safe Option for a Healthier You! P/N: P-2017-0175-00 Rev B 1. Static magnetic field of 3 Tesla or less. 2. Spatial gradient magnetic field of 720 Gauss/cm or less. 3. Maximum whole body
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
Replacement Heart Valve Product Description (Stented Tissue) Models Reference
 Dear Imaging Center: This letter is in response to your inquiry concerning the safety of performing magnetic resonance (MR) procedures in patients who have been implanted with Edwards Lifesciences LLC
Dear Imaging Center: This letter is in response to your inquiry concerning the safety of performing magnetic resonance (MR) procedures in patients who have been implanted with Edwards Lifesciences LLC
LCP Proximal Radius Plates 2.4. Plates for radial head rim and for radial head neck address individual fracture patterns of the proximal radius.
 LCP Proximal Radius Plates 2.4. Plates for radial head rim and for radial head neck address individual fracture patterns of the proximal radius. Surgical Technique This publication is not intended for
LCP Proximal Radius Plates 2.4. Plates for radial head rim and for radial head neck address individual fracture patterns of the proximal radius. Surgical Technique This publication is not intended for
OBSOLETED. LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation.
 LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation. Surgical Technique LCP Small Fragment System This publication
LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation. Surgical Technique LCP Small Fragment System This publication
MRI-Ready Systems Manual. MRI Procedure Information for the St. Jude Medical MR Conditional System
 MRI-Ready Systems Manual MRI Procedure Information for the St. Jude Medical MR Conditional System CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. WARNING: This
MRI-Ready Systems Manual MRI Procedure Information for the St. Jude Medical MR Conditional System CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. WARNING: This
Advisa MRI and Ensura MRI SureScan pacing systems
 Advisa MRI and Ensura MRI SureScan pacing systems MR Conditional dual chamber and single chamber SureScan pacing systems with SureScan leads MRI Technical Manual The following list includes trademarks
Advisa MRI and Ensura MRI SureScan pacing systems MR Conditional dual chamber and single chamber SureScan pacing systems with SureScan leads MRI Technical Manual The following list includes trademarks
Syllabus References. Resources. Video: MRI Introduction
 MRI Lesson Outline Syllabus References 9.6.4.2.5 Define precessing and relate the frequency of the precessing to the composition of the nuclei and the strength of the applied external magnetic field 9.6.4.2.6
MRI Lesson Outline Syllabus References 9.6.4.2.5 Define precessing and relate the frequency of the precessing to the composition of the nuclei and the strength of the applied external magnetic field 9.6.4.2.6
MEFiSTO. Monolateral External Fixation System for Trauma and Orthopaedics.
 MEFiSTO. Monolateral External Fixation System for Trauma and Orthopaedics. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation.
MEFiSTO. Monolateral External Fixation System for Trauma and Orthopaedics. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation.
Safety Issue of Static Magnetic Field (1)
 MRI Safety Safety Issue of Static Magnetic Field (1) Currently, typical MRI utilizes static field with strengths of 0.5 to 3 Tesla (FDA guideline is max 4 T for infants < 1 month and 8 T for others) 1
MRI Safety Safety Issue of Static Magnetic Field (1) Currently, typical MRI utilizes static field with strengths of 0.5 to 3 Tesla (FDA guideline is max 4 T for infants < 1 month and 8 T for others) 1
For Small-Statured Adults and Pediatric Patients. Medium External Fixator Basic Modular Frame
 For Small-Statured Adults and Pediatric Patients Medium External Fixator Basic Modular Frame Surgical Technique MRI Information DePuy Synthes Medium External Fixation devices are labeled MR Conditional
For Small-Statured Adults and Pediatric Patients Medium External Fixator Basic Modular Frame Surgical Technique MRI Information DePuy Synthes Medium External Fixation devices are labeled MR Conditional
User Manual: Ives MR Conditional Cup Electrodes J.R. Ives, December 22, 2017
 User Manual: Ives MR Conditional Cup Electrodes J.R. Ives, December 22, 2017 Ives EEG Solutions; 25 Storey Ave., #118, Newburyport, MA, 01950; ph: 978-358-8006; fax: 978-358-7825; www.iveseegsolutions.com
User Manual: Ives MR Conditional Cup Electrodes J.R. Ives, December 22, 2017 Ives EEG Solutions; 25 Storey Ave., #118, Newburyport, MA, 01950; ph: 978-358-8006; fax: 978-358-7825; www.iveseegsolutions.com
3.0/3.5/4.0/4.5/6.5/7.0/7.3. Cannulated Screws. Surgical Technique
 3.0/3.5/4.0/4.5/6.5/7.0/7.3 Cannulated Screws Surgical Technique Image intensifier control This description alone does not provide sufficient background for direct use of DePuy Synthes products. Instruction
3.0/3.5/4.0/4.5/6.5/7.0/7.3 Cannulated Screws Surgical Technique Image intensifier control This description alone does not provide sufficient background for direct use of DePuy Synthes products. Instruction
Surgical Technique. *smith&nephew POLARSTEM Cementless Stem System
 Surgical Technique *smith&nephew POLARSTEM Cementless Stem System POLARSTEM Cementless Stem System Contents Introduction... 3 Indications... 4 Contraindications... 4 Case Studies... 5 Preoperative Planning...
Surgical Technique *smith&nephew POLARSTEM Cementless Stem System POLARSTEM Cementless Stem System Contents Introduction... 3 Indications... 4 Contraindications... 4 Case Studies... 5 Preoperative Planning...
Surgical Technique. *smith&nephew POLARSTEM Cementless and Cemented Stem System
 Surgical Technique *smith&nephew POLARSTEM Cementless and Cemented Stem System 2 POLARSTEM Contents Introduction... 4 Indications... 5 Contraindications... 5 Case Studies... 6 Preoperative Planning...
Surgical Technique *smith&nephew POLARSTEM Cementless and Cemented Stem System 2 POLARSTEM Contents Introduction... 4 Indications... 5 Contraindications... 5 Case Studies... 6 Preoperative Planning...
FastFrame External Fixation System. Damage Control Surgical Technique
 FastFrame External Fixation System Damage Control Surgical Technique 1 FastFrame External Fixation System Damage Control Surgical Technique Table of Contents Introduction... 2 Indications and Contradictions...
FastFrame External Fixation System Damage Control Surgical Technique 1 FastFrame External Fixation System Damage Control Surgical Technique Table of Contents Introduction... 2 Indications and Contradictions...
Large External Fixator Modular Knee Bridge
 Using Multi-pin Clamps Large External Fixator Modular Knee Bridge Surgical Technique Large External Fixator Modular Knee Bridge DePuy Synthes Large External Fixation devices are labeled MR Conditional
Using Multi-pin Clamps Large External Fixator Modular Knee Bridge Surgical Technique Large External Fixator Modular Knee Bridge DePuy Synthes Large External Fixation devices are labeled MR Conditional
28 Surgical Technique
 Surgical Technique 10 12 14 16 18 20 22 24 28 26 Technique described by James L. Guyton, MD Campbell Clinic Memphis, Tennessee James W. Harkess, MD Campbell Clinic Memphis, Tennessee David G. LaVelle,
Surgical Technique 10 12 14 16 18 20 22 24 28 26 Technique described by James L. Guyton, MD Campbell Clinic Memphis, Tennessee James W. Harkess, MD Campbell Clinic Memphis, Tennessee David G. LaVelle,
LCP Proximal Radius Plates 2.4. Plates for radial head rim and for radial head neck address individual fracture patterns of the proximal radius.
 LCP Proximal Radius Plates 2.4. Plates for radial head rim and for radial head neck address individual fracture patterns of the proximal radius. Surgical Technique This publication is not intended for
LCP Proximal Radius Plates 2.4. Plates for radial head rim and for radial head neck address individual fracture patterns of the proximal radius. Surgical Technique This publication is not intended for
MRI Procedure Information. For St. Jude Medical MR Conditional Neurostimulation Systems. Clinician's Manual
 MRI Procedure Information For St. Jude Medical MR Conditional Neurostimulation Systems Clinician's Manual Unless otherwise noted, indicates that the name is a trademark of, or licensed to, St. Jude Medical
MRI Procedure Information For St. Jude Medical MR Conditional Neurostimulation Systems Clinician's Manual Unless otherwise noted, indicates that the name is a trademark of, or licensed to, St. Jude Medical
POLARCUP Dual Mobility System. General Safety Instructions. Instructions for Use. Duty to inform the patient. Implant passport
 Important Notice to Users in the United States: This package insert is for product distributed in US only. U.S. Federal law restricts this device to sale by or on the order of a physician. General Safety
Important Notice to Users in the United States: This package insert is for product distributed in US only. U.S. Federal law restricts this device to sale by or on the order of a physician. General Safety
JRI Thompson Hemiarthroplasty
 JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6 English 3 Page 2 of 6 Important Information Please
JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6 English 3 Page 2 of 6 Important Information Please
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
POLARSTEM. Surgical Technique - POLARSTEM Cementless and Cemented Stem System 3
 Surgical Technique POLARSTEM Contents Introduction... 4 Indications... 5 Contraindications... 5 Warnings and precautions... 6 Case Studies... 8 Preoperative Planning... 9 Surgical Technique...10 Postoperative
Surgical Technique POLARSTEM Contents Introduction... 4 Indications... 5 Contraindications... 5 Warnings and precautions... 6 Case Studies... 8 Preoperative Planning... 9 Surgical Technique...10 Postoperative
GUIDELINE 2-G-004 (formerly APPENDIX 3) HSREB (March 2005) Page 1
 HSREB (March 2005) Page 1 Guidelines for Ethics Approval of Research Protocols Involving Human Exposure to Magnetic Resonance Imaging Required Wording in Informed Consent Documentation; The Food & Drug
HSREB (March 2005) Page 1 Guidelines for Ethics Approval of Research Protocols Involving Human Exposure to Magnetic Resonance Imaging Required Wording in Informed Consent Documentation; The Food & Drug
MRI guidelines for Medtronic neurostimulation systems for chronic pain
 MRI guidelines for Medtronic neurostimulation systems for chronic pain See "START HERE" section before conducting MRI. Instructions for use! USA Rx only Explanation of symbols on product or package labeling
MRI guidelines for Medtronic neurostimulation systems for chronic pain See "START HERE" section before conducting MRI. Instructions for use! USA Rx only Explanation of symbols on product or package labeling
In Vitro Magnetic Resonance Imaging Evaluation of Ossicular Implants at 3 T
 Otology & Neurotology 33:871Y877 Ó 2012, Otology & Neurotology, Inc. In Vitro Magnetic Resonance Imaging Evaluation of Ossicular Implants at 3 T *Frank G. Shellock, Lauren N. Meepos, Matthew R. Stapleton,
Otology & Neurotology 33:871Y877 Ó 2012, Otology & Neurotology, Inc. In Vitro Magnetic Resonance Imaging Evaluation of Ossicular Implants at 3 T *Frank G. Shellock, Lauren N. Meepos, Matthew R. Stapleton,
MRI Exams in Patients with Non MR Conditional Pacemakers and with MR Conditional Pacemakers
 MRI Exams in Patients with Non MR Conditional Pacemakers and with MR Conditional Pacemakers Frank G. Shellock, Ph.D., FACC Adjunct Clinical Professor of Radiology and Medicine Keck School of Medicine,
MRI Exams in Patients with Non MR Conditional Pacemakers and with MR Conditional Pacemakers Frank G. Shellock, Ph.D., FACC Adjunct Clinical Professor of Radiology and Medicine Keck School of Medicine,
*smith&nephew SL-PLUS Cementless Femoral Hip System. Product Information
 Product Information *smith&nephew SL-PLUS Cementless Femoral Hip System First Came the Philosophy to develop a universal hip system that could be used in almost every indication, immaterial to the patient
Product Information *smith&nephew SL-PLUS Cementless Femoral Hip System First Came the Philosophy to develop a universal hip system that could be used in almost every indication, immaterial to the patient
Cardiac Rhythm Therapy // Advanced Features // ProMRI. ProMRI. Checklist and Quick Reference Guide
 Cardiac Rhythm Therapy // Advanced Features // ProMRI ProMRI Checklist and Quick Reference Guide PAGE 2 Conditions for MR scans with a BIOTRONIK ProMRI system Usage of an MR scan on a patient having an
Cardiac Rhythm Therapy // Advanced Features // ProMRI ProMRI Checklist and Quick Reference Guide PAGE 2 Conditions for MR scans with a BIOTRONIK ProMRI system Usage of an MR scan on a patient having an
Important Notice to Users in the United States: General Safety Instructions
 POLARSTEM Femoral Stems with Ti/HA (Standard, Lateral, Valgus and Collar) Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340 Baar Phone + 41 41 766 22 22 swiss.implants@smith-nephew.com 02/2017
POLARSTEM Femoral Stems with Ti/HA (Standard, Lateral, Valgus and Collar) Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340 Baar Phone + 41 41 766 22 22 swiss.implants@smith-nephew.com 02/2017
POLARSTEM. Surgical Technique - POLARSTEM Cementless and Cemented Stem System 3
 Surgical Technique POLARSTEM Contents Introduction... 4 Indications... 5 Contraindications... 5 Warnings and precautions... 6 Case Studies... 8 Preoperative Planning... 9 Surgical Technique...10 Postoperative
Surgical Technique POLARSTEM Contents Introduction... 4 Indications... 5 Contraindications... 5 Warnings and precautions... 6 Case Studies... 8 Preoperative Planning... 9 Surgical Technique...10 Postoperative
*smith&nephew SBG Anatomical Hip Stem Prosthesis. Product Information
 Product Information *smith&nephew SBG Anatomical Hip Stem Prosthesis For a Perfect Fit Because Anatomy Matters On account of its anatomical shape the cementless SBG stem, which has been used successfully
Product Information *smith&nephew SBG Anatomical Hip Stem Prosthesis For a Perfect Fit Because Anatomy Matters On account of its anatomical shape the cementless SBG stem, which has been used successfully
Tissue-engineered medical products Evaluation of anisotropic structure of articular cartilage using DT (Diffusion Tensor)-MR Imaging
 Provläsningsexemplar / Preview TECHNICAL REPORT ISO/TR 16379 First edition 2014-03-01 Tissue-engineered medical products Evaluation of anisotropic structure of articular cartilage using DT (Diffusion Tensor)-MR
Provläsningsexemplar / Preview TECHNICAL REPORT ISO/TR 16379 First edition 2014-03-01 Tissue-engineered medical products Evaluation of anisotropic structure of articular cartilage using DT (Diffusion Tensor)-MR
MRI Checklist for MED EL CI and ABI models
 MRI Checklist for MED EL CI and ABI models Mi1200 SYNCHRONY Mi1200 SYNCHRONY PIN Mi1210 SYNCHRONY ST...1 Mi1200 SYNCHRONY ABI Mi1200 SYNCHRONY PIN ABI...2 Mi1000 CONCERTO Mi1000 CONCERTO PIN SONATA...3
MRI Checklist for MED EL CI and ABI models Mi1200 SYNCHRONY Mi1200 SYNCHRONY PIN Mi1210 SYNCHRONY ST...1 Mi1200 SYNCHRONY ABI Mi1200 SYNCHRONY PIN ABI...2 Mi1000 CONCERTO Mi1000 CONCERTO PIN SONATA...3
Magnetic resonance imaging safety of NucleusR 24 cochlear implants at 3.0 T
 International Congress Series 1273 (2004) 394 398 www.ics-elsevier.com Magnetic resonance imaging safety of NucleusR 24 cochlear implants at 3.0 T F. Risi*, A. Saldanha, R. Leigh, P. Gibson Cochlear Limited,
International Congress Series 1273 (2004) 394 398 www.ics-elsevier.com Magnetic resonance imaging safety of NucleusR 24 cochlear implants at 3.0 T F. Risi*, A. Saldanha, R. Leigh, P. Gibson Cochlear Limited,
PATIENT CARE AND MRI SAFETY Module 7
 PATIENT CARE AND MRI SAFETY Module 7 1 Biological Considerations There are no reported adverse biological effects of extended exposure to MRI. However, several inconsequential and reversible effects of
PATIENT CARE AND MRI SAFETY Module 7 1 Biological Considerations There are no reported adverse biological effects of extended exposure to MRI. However, several inconsequential and reversible effects of
Personal use only. MRI Metal Artifact Reduction: Shoulder Implants and Arthroplasty. Reto Sutter, MD
 MRI Metal Artifact Reduction: Shoulder Implants and Arthroplasty Reto Sutter, MD University Hospital Balgrist Zurich University of Zurich Cor PD fat sat 56-year old male patient with positive lift-off
MRI Metal Artifact Reduction: Shoulder Implants and Arthroplasty Reto Sutter, MD University Hospital Balgrist Zurich University of Zurich Cor PD fat sat 56-year old male patient with positive lift-off
*smith&nephew SLR-PLUS
 Surgical Technique *smith&nephew SLR-PLUS Cementless Revision Stem SLR-PLUS Table of Contents Comments from the Author s Clinic... 3 Indications... 4 Contraindications... 5 Preoperative Planning... 5
Surgical Technique *smith&nephew SLR-PLUS Cementless Revision Stem SLR-PLUS Table of Contents Comments from the Author s Clinic... 3 Indications... 4 Contraindications... 5 Preoperative Planning... 5
Version überholt. Nicht verwenden. Version obsolète. Ne pas utiliser. Versione obsoleta. Non utilizzare. Verouderde versie. Niet gebruiken.
 MRI TECHNICAL GUIDE IMAGEREADY MR CONDITIONAL PACING SYSTEM REF J065, J066, J067, J175, J176, J177, J275, J276, J277, J279, L110, L111, L131, L210, L211, L231, L310, L311, L331, 4456, 4457, 4458, 4459,
MRI TECHNICAL GUIDE IMAGEREADY MR CONDITIONAL PACING SYSTEM REF J065, J066, J067, J175, J176, J177, J275, J276, J277, J279, L110, L111, L131, L210, L211, L231, L310, L311, L331, 4456, 4457, 4458, 4459,
MRI SureScan pacemakers & ICDs what you need to know
 MRI SureScan pacemakers & ICDs what you need to know Many variables can affect the device patient s likelihood of risks w/ an MRI Type of imaging sequence Type of scanner MRI scan duration Strength of
MRI SureScan pacemakers & ICDs what you need to know Many variables can affect the device patient s likelihood of risks w/ an MRI Type of imaging sequence Type of scanner MRI scan duration Strength of
VA LOCKING CALCANEAL PLATES 2.7
 VA LOCKING CALCANEAL PLATES 2.7 Instruments and Implants approved by the AO Foundation. This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE Image intensifier control Warning
VA LOCKING CALCANEAL PLATES 2.7 Instruments and Implants approved by the AO Foundation. This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE Image intensifier control Warning
2.4 mm Variable Angle LCP Volar Extra-Articular Distal Radius System. For fragment-specific fracture fixation with variable angle locking technology.
 2.4 mm Variable Angle LCP Volar Extra-Articular Distal Radius System. For fragment-specific fracture fixation with variable angle locking technology. Surgical Technique This publication is not intended
2.4 mm Variable Angle LCP Volar Extra-Articular Distal Radius System. For fragment-specific fracture fixation with variable angle locking technology. Surgical Technique This publication is not intended
RECENT ADVANCES IN CLINICAL MR OF ARTICULAR CARTILAGE
 In Practice RECENT ADVANCES IN CLINICAL MR OF ARTICULAR CARTILAGE By Atsuya Watanabe, MD, PhD, Director, Advanced Diagnostic Imaging Center and Associate Professor, Department of Orthopedic Surgery, Teikyo
In Practice RECENT ADVANCES IN CLINICAL MR OF ARTICULAR CARTILAGE By Atsuya Watanabe, MD, PhD, Director, Advanced Diagnostic Imaging Center and Associate Professor, Department of Orthopedic Surgery, Teikyo
Important Notice to Users in the United States: General Safety Instructions
 SLR-PLUS Revision Stem, SLR-PLUS Revision Stem Lateral Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340 Baar Phone + 41 41 766 22 22 swiss.implants@smith-nephew.com non-sterile cemented non-
SLR-PLUS Revision Stem, SLR-PLUS Revision Stem Lateral Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340 Baar Phone + 41 41 766 22 22 swiss.implants@smith-nephew.com non-sterile cemented non-
SL-PLUS MIA Stem, SL-PLUS MIA Stem Lateral, SL-PLUS/ INTEGRATION-PLUS MIA Stem with Ti/HA, SL-PLUS/INTEGRATION-PLUS MIA Stem Lateral with Ti/HA
 SL-PLUS MIA Stem, SL-PLUS MIA Stem Lateral, SL-PLUS/ INTEGRATION-PLUS MIA Stem with Ti/HA, SL-PLUS/INTEGRATION-PLUS MIA Stem Lateral with Ti/HA Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340
SL-PLUS MIA Stem, SL-PLUS MIA Stem Lateral, SL-PLUS/ INTEGRATION-PLUS MIA Stem with Ti/HA, SL-PLUS/INTEGRATION-PLUS MIA Stem Lateral with Ti/HA Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340
Orthopedic Metal Artifact Reduction Distortion correction in the presence of a orthopedic implant
 O-MAR MR software Orthopedic Metal Artifact Reduction Distortion correction in the presence of a orthopedic implant By S. Hey, D. Hoogenraad, V. Elanchezhian, M. van Meel and F. Admiraal-Behloul In recent
O-MAR MR software Orthopedic Metal Artifact Reduction Distortion correction in the presence of a orthopedic implant By S. Hey, D. Hoogenraad, V. Elanchezhian, M. van Meel and F. Admiraal-Behloul In recent
Hip Revision. Masterclass. Programme. Chairman: Professor Carsten Perka (DE) Hotel Mövenpick, Berlin, Germany May 2016
 Hip Revision Masterclass Programme Chairman: Professor Carsten Perka (DE) Hotel Mövenpick, Berlin, Germany 19-20 May 2016 Page 2 Description This masterclass is a highly focused Healthcare Professional
Hip Revision Masterclass Programme Chairman: Professor Carsten Perka (DE) Hotel Mövenpick, Berlin, Germany 19-20 May 2016 Page 2 Description This masterclass is a highly focused Healthcare Professional
IMF Screw Set. For temporary, peri opera tive stabilisation of the occlusion in adults.
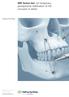 IMF Screw Set. For temporary, peri opera tive stabilisation of the occlusion in adults. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved
IMF Screw Set. For temporary, peri opera tive stabilisation of the occlusion in adults. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved
Magnetic Resonance Imaging on Soft Tissue. Jiten K. Mistry Calvin Gan
 Magnetic Resonance Imaging on Soft Tissue 1 Jiten K. Mistry Calvin Gan Outline Background of Medical Imaging Introduction to MRI How MRI works MRI of Soft Tissue Benefits & Risks Recent Advances 2 The
Magnetic Resonance Imaging on Soft Tissue 1 Jiten K. Mistry Calvin Gan Outline Background of Medical Imaging Introduction to MRI How MRI works MRI of Soft Tissue Benefits & Risks Recent Advances 2 The
Large External Fixator Traveling Traction
 Used to Correct Angular Deformity Large External Fixator Traveling Traction Surgical Technique Large External Fixator Traveling Traction DePuy Synthes Large External Fixation devices are labeled MR Conditional
Used to Correct Angular Deformity Large External Fixator Traveling Traction Surgical Technique Large External Fixator Traveling Traction DePuy Synthes Large External Fixation devices are labeled MR Conditional
VA-Locking Intercarpal Fusion System. Variable angle locking technology for mediocarpal partial arthrodesis.
 VA-Locking Intercarpal Fusion System. Variable angle locking technology for mediocarpal partial arthrodesis. Surgical Technique This publication is not intended for distribution in the USA. Image intensifier
VA-Locking Intercarpal Fusion System. Variable angle locking technology for mediocarpal partial arthrodesis. Surgical Technique This publication is not intended for distribution in the USA. Image intensifier
White Paper for Rotational Stability. *smith&nephew SL-PLUS MIA. Cementless Hip Stem System
 White Paper for Rotational Stability *smith&nephew SL-PLUS MIA Cementless Hip Stem System SL-PLUS MIA Building on the long-term success of the SL-PLUS hip stem, the SL-PLUS MIA hip stem has been developed
White Paper for Rotational Stability *smith&nephew SL-PLUS MIA Cementless Hip Stem System SL-PLUS MIA Building on the long-term success of the SL-PLUS hip stem, the SL-PLUS MIA hip stem has been developed
MRI Procedure Information. For St. Jude Medical MR Conditional Neurostimulation Systems. Clinician's Manual
 MRI Procedure Information For St. Jude Medical MR Conditional Neurostimulation Systems Clinician's Manual Unless otherwise noted, indicates that the name is a trademark of, or licensed to, St. Jude Medical
MRI Procedure Information For St. Jude Medical MR Conditional Neurostimulation Systems Clinician's Manual Unless otherwise noted, indicates that the name is a trademark of, or licensed to, St. Jude Medical
Basics of MRI Part I
 Basics of MRI Part I Mathew J. Dixon, D.O. Chairman Department of Radiology Memorial Health University Medical Center Savannah, GA Objectives Brief History Concept of MRI Creation of a Magnetic Field Concepts
Basics of MRI Part I Mathew J. Dixon, D.O. Chairman Department of Radiology Memorial Health University Medical Center Savannah, GA Objectives Brief History Concept of MRI Creation of a Magnetic Field Concepts
LCP DISTAL TIBIA PLATE
 LCP DISTAL TIBIA PLATE Instruments and implants approved by the AO Foundation. This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE Image intensifier control This description
LCP DISTAL TIBIA PLATE Instruments and implants approved by the AO Foundation. This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE Image intensifier control This description
Understanding Peripheral
 Patient Information Guide Understanding Peripheral Artery Disease Innova Vascular Self-Expanding Stent System Table of Contents Glossary... 2 What is Peripheral Artery Disease?... 4 Treating Peripheral
Patient Information Guide Understanding Peripheral Artery Disease Innova Vascular Self-Expanding Stent System Table of Contents Glossary... 2 What is Peripheral Artery Disease?... 4 Treating Peripheral
