Design Verification IMTEC-TSH R -A C Intended Use... 2 Diagnostic Sensitivity and Diagnostic Specificity... 2 Interferences... 4 Imprecision...
|
|
|
- Hector Gray
- 5 years ago
- Views:
Transcription
1 Design Verification IMTEC-TSH RECEPTOR-ANTIBODIES CONTENTS 1 Intended Use Diagnostic Sensitivity and Diagnostic Specificity Determination of Diagnostic Sensitivity and Diagnostic Specificity Evaluation Data Calculation of Diagnostic Sensitivity and Diagnostic Specificity Conclusion Analytical Sensitivity (Detection Limit) Interferences Imprecision Within-run Imprecision (Intra-Assay) Between-run Imprecision (Inter-Assay) Between-lot Imprecision (Inter-Lot) Linearity Standardisation Stability Conclusion... 6 Form: /6 valid of
2 1 Intended Use IMTEC-TSH Receptor-Antibodies is an enzyme immunoassay (ELISA) for the quantitative measurement of autoantibodies against TSH receptor in human serum. The assay is intended for in vitro diagnostic use only as an aid in the diagnosis of Graves disease. Thyroid dysfunction may have a variable clinical presentation. Laboratory tests help to determine thyroid function and to differentiate common causes for hypothyroidism and hyperthyroidism. Low or undetectable TSH levels are diagnostic for hyperthyroidism and may be caused by Graves Disease, toxic multinodular goiter (MNG), toxic adenoma and subacute thyroiditis. Graves Disease is the most common cause of hyperthyroidism, accounting for 60 to 80 percent of all cases. Hyperthyroidism in Graves disease is attributable to autoantibodies to the thyroid-stimulating hormone receptor (TSHR), and measurement of these TSHR autoantibodies can help in the diagnosis and management of the disease. Second generation TSHR antibody assays measure the ability to inhibit receptor binding of TSH and are a more sensitive and specific alternative to first generation assays. The IMTEC-TSH Receptor-Antibodies ELISA is a nonisotopic and automatable second generation assay. 1. Rees Smith B. et al., A new assay for thyroid receptor, Thyoid : Kamijo K.,TSH receptor antibody measurement [ ], Endocrine J 50, (2003) 2. Bolton et al., Measurement of thyroid stimulating hormone receptor autoantibodies by ELISA, Clin. Chem. 45, (1999) 2 Diagnostic Sensitivity and Diagnostic Specificity 2.1 Determination of Diagnostic Sensitivity and Diagnostic Specificity Diagnostic sensitivity and diagnostic specificity were calculated according to DIN : Diagnostic Sensitivity = Number of correctly positive determined samples Number of true positive + false negative determined samples Diagnostic Specificity = Number of correctly negative determined samples Number of true negative + false positive determined samples Design Verification and Product Data for IMTEC-TSH Receptor-Antibodies 2/6
3 2.1.1 Evaluation Data A total of 154 apparently healthy donors, 35 Disease controls and 50 patients with Graves disease were assayed. The following table summarises the results. Control and Disease groups Total number Negative < 1.0 IU/L Equivocal IU/L Positive 1.5 IU/L Blood Donors Graves Disease Calculation of Diagnostic Sensitivity and Diagnostic Specificity Diagnostic Sensitivity (Graves Disease) = 49/50 = 98 % Diagnostic Specificity (Blood Donors) = 152/154 = 98.7 % Conclusion The data support a sensitivity of 98 % and a specificity > 95 % for the IMTEC-TSH Receptor-Antibodies ELISA. 2.2 Analytical Sensitivity (Detection Limit) The analytical sensitivity (detection limit [IU/L]) was determined using the negative control. The absolute mean was obtained from 32 determinations. The detection limit of 0.21 IU/L was calculated from the absolute mean plus two standard deviations. Design Verification and Product Data for IMTEC-TSH Receptor-Antibodies 3/6
4 3 Interferences No interferences was observed by spiking three low positive samples with bilirubin at 0.2 mg/ml, hemoglobin at 5 mg/ml, lipid at 30 mg/dl and TSH at 3.35 mu/ml. The following table lists the tested substances in their final concentration which has been set to a level above physiologically relevant concentration. Substance Bilirubin Hemoglobin Lipids TSH 0.2 mg/ml 5.0 mg/ml 30 mg/dl 3.35 mu/ml 4 Imprecision 4.1 Within-run Imprecision (Intra-Assay) For determination of the intra-assay imprecision two controls were assayed twenty-five times each in one assay. 1 2 IU/l IU/l Mean SD CV [%] The obtained data confirm an intra-assay reproducibility of CV %. 4.2 Between-run Imprecision (Inter-Assay) For determination of the inter-assay imprecision two controls were assayed in single determination on twenty different runs. 1 2 IU/l IU/l Mean SD CV [%] The obtained data confirm an inter-assay reproducibility of CV %. 4.3 Between-lot Imprecision (Inter-Lot) For determination of the inter-lot imprecision four controls were assayed twenty times each in twenty batches IU/l IU/l IU/l IU/l Mean SD CV [%] The obtained data confirm an inter-lot reproducibility of CV %. Design Verification and Product Data for IMTEC-TSH Receptor-Antibodies 4/6
5 5 Linearity For determination of the linearity one positive sample was diluted using a negative pool and assayed in the IMTEC- TSH Receptor-Antibodies ELISA. The assay shows linearity within the measuring range. Sample Dilution Observed Mean Expected Mean Recovery [O/E] 1 1 in % 1 in % 1 in % 1 in % 1 in % 1 in % 1 in % 1 in % 1 in % 1 in % 6 Standardisation The IMTEC-TSH Receptor-Antibodies ELISA is calibrated against the 1 st international reference preparation for thyroid stimulating antibodies (NIBSC 90/672). Batch NIBSC 90/672 standard Observed Mean Recovery [O/N] % % % % % % % % Design Verification and Product Data for IMTEC-TSH Receptor-Antibodies 5/6
6 7 Stability The stability of the IMTEC-TSH Receptor-Antibodies ELISA has been evaluated by real time studies of all test kit components. A function test was applied with the whole kit employing all standards and controls. LOT 2ATERK358A: Time/ Sample 0 months 6 months 15 months Absorbance U/L Absorbance U/L Absorbance U/L LP MP HP LOT 2ATERK359: Time /Sample 0 months 6 months 15 months Absorbance U/L Absorbance U/L Absorbance U/L LP MP HP LOT 2ATERK360B: Time /Sample 0 months 6 months 15 months Absorbance U/L Absorbance U/L Absorbance U/L LP MP HP Conclusion The stability data support the shelf life claim of 14 months after production. Design Verification and Product Data for IMTEC-TSH Receptor-Antibodies 6/6
Design Verification. Form: /5 Rev. 007 valid of
 Design Verification g 1 Determination of Diagnostic Sensitivity and Diagnostic Specificity... 2 Crossreactivity... 3 Potentially interfering substances... 3 2 Imprecision... 4 3 Standardisation... 4 4
Design Verification g 1 Determination of Diagnostic Sensitivity and Diagnostic Specificity... 2 Crossreactivity... 3 Potentially interfering substances... 3 2 Imprecision... 4 3 Standardisation... 4 4
TSH Receptor Autoantibody ELISA
 Li StarFish S.r.l. Via Cavour, 35-20063 Cernusco S/N (MI), Italy Tel. +39-02-92150794 - Fax. +39-02-92157285 info@listarfish.it -www.listarfish.it TSH Receptor Autoantibody ELISA Product Data Sheet Cat.
Li StarFish S.r.l. Via Cavour, 35-20063 Cernusco S/N (MI), Italy Tel. +39-02-92150794 - Fax. +39-02-92157285 info@listarfish.it -www.listarfish.it TSH Receptor Autoantibody ELISA Product Data Sheet Cat.
liquicolor (AMP Buffer, IFCC) Design Verification
 Design Verification (AMP Buffer, IFCC) liquicolor 1 Introduction... 2 2 Imprecision... 2 3 arity and Detection Limit... 2 3.1 arity... 2 3.2 Detection Limit... 3 4 Comparison of Methods... 3 5 Stability...
Design Verification (AMP Buffer, IFCC) liquicolor 1 Introduction... 2 2 Imprecision... 2 3 arity and Detection Limit... 2 3.1 arity... 2 3.2 Detection Limit... 3 4 Comparison of Methods... 3 5 Stability...
Total Thyroxine ELISA (T4)
 Design Verification Total Thyroxine ELISA (T4) Contents 1 Assay Principle... 2 2 Imprecision... 2 Within-run Imprecision... 2 Between run Imprecision... 2 3 Comparison of Methods, Accuracy... 2 4 Linearity...
Design Verification Total Thyroxine ELISA (T4) Contents 1 Assay Principle... 2 2 Imprecision... 2 Within-run Imprecision... 2 Between run Imprecision... 2 3 Comparison of Methods, Accuracy... 2 4 Linearity...
IMTEC-ANA-LIA MAXX. Design Verification
 Design Verification IMTEC-ANA-LIA MAXX CONTENTS 1 Intended Use... 2 2 Diagnostic Sensitivity and Specificity... 2 3 Interferences... 4 4 Imprecision... 6 4.1 Within-Run Imprecision... 6 4.2 Between-Run
Design Verification IMTEC-ANA-LIA MAXX CONTENTS 1 Intended Use... 2 2 Diagnostic Sensitivity and Specificity... 2 3 Interferences... 4 4 Imprecision... 6 4.1 Within-Run Imprecision... 6 4.2 Between-Run
LIPASE liquicolor. Design Verification. Multipurpose Reagent
 Design Verification LIPASE liquicolor Multipurpose Reagent CONTENTS 1 Introduction... 2 2 Imprecision... 2 3 Linearity and Detection Limit... 2 3.1 Linearity... 2 3.2 Detection Limit... 3 4 Recovery of
Design Verification LIPASE liquicolor Multipurpose Reagent CONTENTS 1 Introduction... 2 2 Imprecision... 2 3 Linearity and Detection Limit... 2 3.1 Linearity... 2 3.2 Detection Limit... 3 4 Recovery of
liquicolor Design Verification
 Design Verification liquicolor 1 Introduction... 2 2 Imprecision... 2 2.1 Imprecision for... 2 2.2 Imprecision for... 2 3 Linearity and Detection Limit... 2 3.1 Linearity... 2 3.2 Detection Limit... 4
Design Verification liquicolor 1 Introduction... 2 2 Imprecision... 2 2.1 Imprecision for... 2 2.2 Imprecision for... 2 3 Linearity and Detection Limit... 2 3.1 Linearity... 2 3.2 Detection Limit... 4
Instructions for Use. Tg Antibody ELISA
 Instructions for Use Tg Antibody ELISA Enzyme Immunoassay for the Quantitative Determination of Autoantibodies to Thyroglobulin (Tg) in Serum and Plasma I V D REF EA618/96 12 x 8 2 8 C DLD Gesellschaft
Instructions for Use Tg Antibody ELISA Enzyme Immunoassay for the Quantitative Determination of Autoantibodies to Thyroglobulin (Tg) in Serum and Plasma I V D REF EA618/96 12 x 8 2 8 C DLD Gesellschaft
Anti-TSH-Receptor ELISA
 Instructions for Use Anti-TSH-Receptor ELISA Enzyme Immuno Assay for the Quantitative Determination of TSH Receptor Autoantibodies (TRAb) in Serum I V D REF EA101/96 12 x 8 2 8 C DLD Gesellschaft für Diagnostika
Instructions for Use Anti-TSH-Receptor ELISA Enzyme Immuno Assay for the Quantitative Determination of TSH Receptor Autoantibodies (TRAb) in Serum I V D REF EA101/96 12 x 8 2 8 C DLD Gesellschaft für Diagnostika
hc Step Serum/Urine, hc Step Detector and HumaPreg Serum/Urine
 Design Verification hc Step Serum/Urine, hc Step Detector and HumaPreg Serum/Urine 1 Function... 2 2 Sensitivity and Dynamic Range... 2 2.1 Analytical Sensitivity... 2 3 Validation of Efficacy... 3 3.1
Design Verification hc Step Serum/Urine, hc Step Detector and HumaPreg Serum/Urine 1 Function... 2 2 Sensitivity and Dynamic Range... 2 2.1 Analytical Sensitivity... 2 3 Validation of Efficacy... 3 3.1
HumaTex CRP. Design Verification. Contents
 Design Verification HumaTex CRP Contents 1 Function... 2 2 Reproducibility... 2 3 Sensitivity and dynamic range... 2 Preparation of serum control panel... 2 Sensitivity test results... 2 Prozone check...
Design Verification HumaTex CRP Contents 1 Function... 2 2 Reproducibility... 2 3 Sensitivity and dynamic range... 2 Preparation of serum control panel... 2 Sensitivity test results... 2 Prozone check...
3 Linearity and Detection Limit Linearity Detection limit... 4
 Design Verification liquicolor 1 Introduction... 2 2 Imprecision, Reproducibility... 2 2.1 Data from internal study... 2 2.2 Date from external study (Cholesterol Reference Method Laboratory Network)...
Design Verification liquicolor 1 Introduction... 2 2 Imprecision, Reproducibility... 2 2.1 Data from internal study... 2 2.2 Date from external study (Cholesterol Reference Method Laboratory Network)...
ab TSH Receptor Autoantibody ELISA Kit
 ab178661 TSH Receptor Autoantibody ELISA Kit Instructions for Use For the quantitative measurement of TSH Receptor Autoantibodies in Human serum. This product is for research use only and is not intended
ab178661 TSH Receptor Autoantibody ELISA Kit Instructions for Use For the quantitative measurement of TSH Receptor Autoantibodies in Human serum. This product is for research use only and is not intended
2 Screen Islet Cell Autoantibody Human ELISA
 2 Screen Islet Cell Autoantibody Human ELISA Cat. No.:DEIA4453 Pkg.Size:96T Intended use The 2 Screen Islet Cell autoantibody (2 Screen) ELISA kit is intended for use by professional persons only, for
2 Screen Islet Cell Autoantibody Human ELISA Cat. No.:DEIA4453 Pkg.Size:96T Intended use The 2 Screen Islet Cell autoantibody (2 Screen) ELISA kit is intended for use by professional persons only, for
1 Introduction Imprecision Within-run imprecision, results Day-to-day imprecision, results... 2
 Design Verification liquicolor 1 Introduction... 2 2 Imprecision... 2 2.1 Within-run imprecision, results... 2 2.2 Day-to-day imprecision, results... 2 3 Linearity and Detection Limit... 2 3.1 Linearity...
Design Verification liquicolor 1 Introduction... 2 2 Imprecision... 2 2.1 Within-run imprecision, results... 2 2.2 Day-to-day imprecision, results... 2 3 Linearity and Detection Limit... 2 3.1 Linearity...
Hexagon PSA. Design Verification. Contents
 Design Verification Hexagon PSA Contents 1. Function...2 2. Sensitivity, Dynamic Range and Traceability...2 Description of Control Materials...2 Analytical Sensitivity...2 3. Clinical Evaluation...3 Evaluation
Design Verification Hexagon PSA Contents 1. Function...2 2. Sensitivity, Dynamic Range and Traceability...2 Description of Control Materials...2 Analytical Sensitivity...2 3. Clinical Evaluation...3 Evaluation
IDEXX Catalyst One Chemistry Analyzer for In-house Measurement of Total Thyroxine (TT 4 ) Concentration in Serum from Dogs and Cats
 IDEXX Catalyst One Chemistry Analyzer for In-house Measurement of Total Thyroxine (TT 4 ) Concentration in Serum from Dogs and Cats Authors: Kate Cote, Ph.D., Graham Bilbrough, MA, VetMB, CertVA, MRCVS,
IDEXX Catalyst One Chemistry Analyzer for In-house Measurement of Total Thyroxine (TT 4 ) Concentration in Serum from Dogs and Cats Authors: Kate Cote, Ph.D., Graham Bilbrough, MA, VetMB, CertVA, MRCVS,
Grave s disease (1 0 )
 THYROID DYSFUNCTION Grave s disease (1 0 ) Autoimmune - activating AB s to TSH receptor High concentrations of circulating thyroid hormones Weight loss, tachycardia, tiredness Diffuse goitre - TSH stimulating
THYROID DYSFUNCTION Grave s disease (1 0 ) Autoimmune - activating AB s to TSH receptor High concentrations of circulating thyroid hormones Weight loss, tachycardia, tiredness Diffuse goitre - TSH stimulating
Gentian Canine CRP Immunoassay Application Note for Abbott Architect * c4000
 v2-jan214 Gentian Canine CRP Immunoassay Application Note for Abbott Architect * c4 Intended Use The canine CRP immunoassay on Abbott's Architect c4 is an in vitro diagnostic test for quantitative determination
v2-jan214 Gentian Canine CRP Immunoassay Application Note for Abbott Architect * c4 Intended Use The canine CRP immunoassay on Abbott's Architect c4 is an in vitro diagnostic test for quantitative determination
FinTest IgG4 Screen 20 ELISA KIT
 FinTest IgG4 Screen 20 ELISA KIT Cat. No.:DEIA6196 Pkg.Size:96T Intended use Enzyme immunoassay (microtiter strips) for the detection and the quantitative determination of IgG4 antibodies against 20 Food
FinTest IgG4 Screen 20 ELISA KIT Cat. No.:DEIA6196 Pkg.Size:96T Intended use Enzyme immunoassay (microtiter strips) for the detection and the quantitative determination of IgG4 antibodies against 20 Food
Gentian Canine CRP Immunoassay Application Note for scil VitroVet*
 v02-jan2014 Gentian Canine CRP Immunoassay Application Note for scil VitroVet* Intended Use The canine CRP immunoassay on scil VitroVet is an in vitro diagnostic test for quantitative determination of
v02-jan2014 Gentian Canine CRP Immunoassay Application Note for scil VitroVet* Intended Use The canine CRP immunoassay on scil VitroVet is an in vitro diagnostic test for quantitative determination of
TSH ELISA Kit Medical Device Licence No.: 16419
 TSH ELISA Kit Medical Device Licence No.: 16419 Enzyme immunoassay kit for the quantitative determination of TSH concentration in serum. Catalog Number: SL100305 Catalog Number: SL100306 Catalog Number:
TSH ELISA Kit Medical Device Licence No.: 16419 Enzyme immunoassay kit for the quantitative determination of TSH concentration in serum. Catalog Number: SL100305 Catalog Number: SL100306 Catalog Number:
TSH Receptor Autoantibody
 TSH Receptor Autoantibody Enzyme immunoassay for the quantitative determination of TRAb in human serum Only for research use only Product Number: GWB-521200 (96 Determinations) 1. INTRODUCTION The hyperthyroidism
TSH Receptor Autoantibody Enzyme immunoassay for the quantitative determination of TRAb in human serum Only for research use only Product Number: GWB-521200 (96 Determinations) 1. INTRODUCTION The hyperthyroidism
Design Verification. Form:
 Design Verification SYPHILIS TPHA liquid CONTENTS 1 Function... 2 2 Sensitivity... 2 Preparation of Serum Control Panel... 2 Kit Controls Positive and Negative... 3 3 Traceability... 3 4 Specificity and
Design Verification SYPHILIS TPHA liquid CONTENTS 1 Function... 2 2 Sensitivity... 2 Preparation of Serum Control Panel... 2 Kit Controls Positive and Negative... 3 3 Traceability... 3 4 Specificity and
Evaluation Report. Eurolyser HbA1c Test Kit on Smart and Cube Analyser
 Evaluation Report Eurolyser HbA1c Test Kit on Smart and Cube Analyser Locations Location 1: Klinisk kemi University Hospital MAS Malmö Operator: Dr. Benny Larsson Date: June/July 2007 Location 2: Eurolyser
Evaluation Report Eurolyser HbA1c Test Kit on Smart and Cube Analyser Locations Location 1: Klinisk kemi University Hospital MAS Malmö Operator: Dr. Benny Larsson Date: June/July 2007 Location 2: Eurolyser
Analytical and Clinical Validation of Two Commercially Available Immunoassays Used in the Detection of TSHR Antibodies
 Analytical and Clinical Validation of Two Commercially Available Immunoassays Used in the Detection of TSHR Antibodies David J. Kemble, 1,2 Tara Jackson, 1,2 Mike Morrison, 1,2 Mark A. Cervinski, 1,2 and
Analytical and Clinical Validation of Two Commercially Available Immunoassays Used in the Detection of TSHR Antibodies David J. Kemble, 1,2 Tara Jackson, 1,2 Mike Morrison, 1,2 Mark A. Cervinski, 1,2 and
Insulin ELISA. For the quantitative determination of insulin in serum and plasma
 Insulin ELISA For the quantitative determination of insulin in serum and plasma For In Vitro Diagnostic use within the United States of America. This product is for Research Use Only outside of the United
Insulin ELISA For the quantitative determination of insulin in serum and plasma For In Vitro Diagnostic use within the United States of America. This product is for Research Use Only outside of the United
Instructions for use. TSH rat ELISA. Please use only the valid version of the Instructions for Use provided with the kit AR E-8600
 Instructions for use TSH rat ELISA AR E-8600 TSH rat ELISA 1. INTRODUCTION 1.1 Intended use The TSH rat ELISA is an enzyme immunoassay for the quantitative measurement of TSH in rat serum. For research
Instructions for use TSH rat ELISA AR E-8600 TSH rat ELISA 1. INTRODUCTION 1.1 Intended use The TSH rat ELISA is an enzyme immunoassay for the quantitative measurement of TSH in rat serum. For research
Human Thyroid Stimulating Hormone CLIA kit
 Human Thyroid Stimulating Hormone CLIA kit Cat. No.:DEEL0223 Pkg.Size:96 tests Intended use For the direct quantitative determination of Thyroid Stimulating Hormone in human serum by chemiluminescence
Human Thyroid Stimulating Hormone CLIA kit Cat. No.:DEEL0223 Pkg.Size:96 tests Intended use For the direct quantitative determination of Thyroid Stimulating Hormone in human serum by chemiluminescence
GAD 65 Antibody ELISA
 Instructions for Use GAD 65 Antibody ELISA Enzyme Immuno Assay for the Quantitative Determination of Antibodies against Glutamic Acid Decarboxylase in Serum I V D REF EA104/96 12 x 8 2 8 C DLD Gesellschaft
Instructions for Use GAD 65 Antibody ELISA Enzyme Immuno Assay for the Quantitative Determination of Antibodies against Glutamic Acid Decarboxylase in Serum I V D REF EA104/96 12 x 8 2 8 C DLD Gesellschaft
USING THE ACCESS AMH ASSAY IN YOUR LABORATORY
 INFORMATION BULLETIN USING THE ACCESS AMH ASSAY IN YOUR LABORATORY ///////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////
INFORMATION BULLETIN USING THE ACCESS AMH ASSAY IN YOUR LABORATORY ///////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////
TSH. 01 English - Ref.: 903. Ref.:903. Insert. Intended use. System for quantitative determination of the thyroid stimulating hormone (TSH) in serum.
 TSH Insert Intended use. System for quantitative determination of the thyroid stimulating hormone (TSH) in serum. [Only for in vitro diagnostic use.] Test principle. The TSH present in the sample is specifically
TSH Insert Intended use. System for quantitative determination of the thyroid stimulating hormone (TSH) in serum. [Only for in vitro diagnostic use.] Test principle. The TSH present in the sample is specifically
Human Thyroglobulin EIA
 Human Thyroglobulin EIA For the quantitative determination of human thyroglobulin (htg) in plasma and serum. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 30-7510 Size: 96
Human Thyroglobulin EIA For the quantitative determination of human thyroglobulin (htg) in plasma and serum. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 30-7510 Size: 96
Validation Report for the Neogen Fentanyl Kit for ELISA Screening of Whole Blood and Urine Specimens
 Validation Report for the Neogen Fentanyl Kit for ELISA Screening of Whole Blood and Urine Specimens This document describes the validation of a Neogen Fentanyl kit for the semi-quantitative analysis of
Validation Report for the Neogen Fentanyl Kit for ELISA Screening of Whole Blood and Urine Specimens This document describes the validation of a Neogen Fentanyl kit for the semi-quantitative analysis of
PERIOSTIN ELISA CONTENTS
 PERIOSTIN ELISA for the quantitative determination of Periostin in human serum, EDTA plasma, heparin plasma, and citrate plasma Cat. No. BI-20433. 12 x 8 tests CONTENTS ASSAY CHARACTERISTICS Summary...
PERIOSTIN ELISA for the quantitative determination of Periostin in human serum, EDTA plasma, heparin plasma, and citrate plasma Cat. No. BI-20433. 12 x 8 tests CONTENTS ASSAY CHARACTERISTICS Summary...
HbA1c (Human) ELISA Kit
 HbA1c (Human) ELISA Kit Cat. No.:DEIA3509 Pkg.Size:96T Intended use GHbA1c (Human) ELISA Kit is a sandwich enzyme immunoassay for the quantitative measurement of human GHbA1c. General Description vhemoglobin,
HbA1c (Human) ELISA Kit Cat. No.:DEIA3509 Pkg.Size:96T Intended use GHbA1c (Human) ELISA Kit is a sandwich enzyme immunoassay for the quantitative measurement of human GHbA1c. General Description vhemoglobin,
AIA-600II Assay Specifications TgAb Test Code 048
 AIA-600II Assay Specifications TgAb Test Code 048 Screen Item Data Screen 1 Lot ***Enter current cal. lot no. Cal 1 0 IU/mL Cal 2 2 IU/mL (example) Cal 3 4 IU/mL (example) Cal 4 10 IU/mL (example) Cal
AIA-600II Assay Specifications TgAb Test Code 048 Screen Item Data Screen 1 Lot ***Enter current cal. lot no. Cal 1 0 IU/mL Cal 2 2 IU/mL (example) Cal 3 4 IU/mL (example) Cal 4 10 IU/mL (example) Cal
Four rules to remember: 1) Addition, subtraction, multiplication and division of SD and/or CV.
 Analytical Quality Specifications for Thyroid Hormones Bayer Nordic Users Meeting, 17. September 2001. Frode Engbaek, Ph. D., Department of Clinical Biochemistry, Aarhus Kommunehospital, DK-8000 Aarhus
Analytical Quality Specifications for Thyroid Hormones Bayer Nordic Users Meeting, 17. September 2001. Frode Engbaek, Ph. D., Department of Clinical Biochemistry, Aarhus Kommunehospital, DK-8000 Aarhus
02006B 1 vial 02006B 1 vial Store at -20 C. Lyophilized recombinant IL-2
 For detection and measurement of human interleukin 2 Catalog #02006 Catalog #02007 2 Plates 10 Plates Product Description The Human Interleukin 2 (IL-2) ELISA Kit is designed for the quantitative detection
For detection and measurement of human interleukin 2 Catalog #02006 Catalog #02007 2 Plates 10 Plates Product Description The Human Interleukin 2 (IL-2) ELISA Kit is designed for the quantitative detection
DBC s panel of thyroid hormone immunoassays is now more comprehensive with the new Reverse T3 ELISA kit. Test Your Hormones
 DBC s panel of thyroid hormone immunoassays is now more comprehensive with the new Reverse T3 ELISA kit Test Your Hormones INTRODUCTION For years thyroid hormone testing has been concentrated on TSH and
DBC s panel of thyroid hormone immunoassays is now more comprehensive with the new Reverse T3 ELISA kit Test Your Hormones INTRODUCTION For years thyroid hormone testing has been concentrated on TSH and
Insulin ELISA. For the quantitative determination of insulin in serum and plasma.
 Insulin ELISA For the quantitative determination of insulin in serum and plasma. For In Vitro Diagnostic use within the United States of America. This product is for Research Use Only outside of the United
Insulin ELISA For the quantitative determination of insulin in serum and plasma. For In Vitro Diagnostic use within the United States of America. This product is for Research Use Only outside of the United
B-Resistance to the action of hormones, Hormone resistance characterized by receptor mediated, postreceptor.
 Disorders of the endocrine system 38 Disorders of endocrine system mainly are caused by: A-Deficiency or an excess of a single hormone or several hormones: - deficiency :can be congenital or acquired.
Disorders of the endocrine system 38 Disorders of endocrine system mainly are caused by: A-Deficiency or an excess of a single hormone or several hormones: - deficiency :can be congenital or acquired.
DKK-1 ELISA, Cat.No. BI For the quantitative determination of DKK-1 in human serum
 DKK-1 ELISA, Cat.No. BI-20413 For the quantitative determination of DKK-1 in human serum ASSAY CHARACTERISTICS Method Standard range Conversion factor Sample volume / well Incubation time, temp. Sensitivity
DKK-1 ELISA, Cat.No. BI-20413 For the quantitative determination of DKK-1 in human serum ASSAY CHARACTERISTICS Method Standard range Conversion factor Sample volume / well Incubation time, temp. Sensitivity
Thyroid Stimulating Hormone (TSH) ELISA Catalog No. GWB , legacy id (96 Tests)
 For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. TSH ELISA Kit is intended for the quantitative measurement of TSH in human serum or plasma. For research use only.
For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. TSH ELISA Kit is intended for the quantitative measurement of TSH in human serum or plasma. For research use only.
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 21 September 2006 EMEA/CHMP/BWP/298390/2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 21 September 2006 EMEA/CHMP/BWP/298390/2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON
Evaluation of the EUROLYSER-SOLO Total T4 assay. for veterinary samples
 Evaluation of the EUROLYSER-SOLO Total T4 assay for veterinary samples Dr. rer.-nat. Volker Schlüter Zacherlweg 18 D-82061 Neuried Date: 2011 April 27 th Scope of the study Goal of this study was the validation
Evaluation of the EUROLYSER-SOLO Total T4 assay for veterinary samples Dr. rer.-nat. Volker Schlüter Zacherlweg 18 D-82061 Neuried Date: 2011 April 27 th Scope of the study Goal of this study was the validation
Med Chem 535P ~ Diagnostic Medicinal Chemistry. General Comments
 Med Chem 535P ~ Diagnostic Medicinal Chemistry General Comments Most blood chemistry and serology assays are performed automatically. Larger clinical laboratories often use sophisticated analyzers that
Med Chem 535P ~ Diagnostic Medicinal Chemistry General Comments Most blood chemistry and serology assays are performed automatically. Larger clinical laboratories often use sophisticated analyzers that
Forum for Collaborative HIV Research External Validation of CD4 and Viral Load Assays Paris, France June 29, 2007
 Forum for Collaborative HIV Research External Validation of CD4 and Viral Load Assays Paris, France June 29, 2007 Thomas J. Spira, M.D. International Laboratory Branch Global AIDS Program Centers for Disease
Forum for Collaborative HIV Research External Validation of CD4 and Viral Load Assays Paris, France June 29, 2007 Thomas J. Spira, M.D. International Laboratory Branch Global AIDS Program Centers for Disease
Instructions for use TSH ELISA. 2nd Generation
 Instructions for use TSH ELISA 2nd Generation TF E2000 TSH ELISA 2nd Generation INTENDED USE For the direct quantitative determination of Thyroid Stimulating Hormone by enzyme immunoassay in human serum.
Instructions for use TSH ELISA 2nd Generation TF E2000 TSH ELISA 2nd Generation INTENDED USE For the direct quantitative determination of Thyroid Stimulating Hormone by enzyme immunoassay in human serum.
BÜHLMANN fcal turbo. Calprotectin turbidimetric assay for professional use. Reagent Kit B-KCAL-RSET. Revision date:
 BÜHLMANN fcal turbo Calprotectin turbidimetric assay for professional use Reagent Kit B-KCAL-RSET Revision date: 2017-02-24 BÜHLMANN LABORATORIES AG Baselstrasse 55 4124 Schönenbuch, Switzerland Tel.:
BÜHLMANN fcal turbo Calprotectin turbidimetric assay for professional use Reagent Kit B-KCAL-RSET Revision date: 2017-02-24 BÜHLMANN LABORATORIES AG Baselstrasse 55 4124 Schönenbuch, Switzerland Tel.:
EliKine Free Thyroxine (ft4) ELISA Kit
 EliKine Free Thyroxine (ft4) ELISA Kit Booklet Item NO. KET0005 Product Name EliKine Free Thyroxine (ft4) ELISA Kit ATTENTION For laboratory research use only. Not for clinical or diagnostic use TABLE
EliKine Free Thyroxine (ft4) ELISA Kit Booklet Item NO. KET0005 Product Name EliKine Free Thyroxine (ft4) ELISA Kit ATTENTION For laboratory research use only. Not for clinical or diagnostic use TABLE
Human Thyreoglobulin (htg, recovery) ELISA Kit, human
 Human Thyreoglobulin (htg, recovery) ELISA Kit, human 2 3 Contents Intended Use 3 Introduction 3 Material Supplied 4 Material Required But Not Supplied 4 Preparation and Storage of Reagents 5 Sample Preparation
Human Thyreoglobulin (htg, recovery) ELISA Kit, human 2 3 Contents Intended Use 3 Introduction 3 Material Supplied 4 Material Required But Not Supplied 4 Preparation and Storage of Reagents 5 Sample Preparation
VGKC-Autoantibody Assay RIA
 Instructions for Use VGKC-Autoantibody Assay RIA 125I-Radio Immuno Assay for the Quantitative Determination of Antibodies to the Voltage-Gated Potassium Channel (VGKC) in Serum I V D REF RA115/25 25 2
Instructions for Use VGKC-Autoantibody Assay RIA 125I-Radio Immuno Assay for the Quantitative Determination of Antibodies to the Voltage-Gated Potassium Channel (VGKC) in Serum I V D REF RA115/25 25 2
Human T4-HRP ELISA Kit Medical Device Licence No.: 21177
 Human T4-HRP ELISA Kit Medical Device Licence No.: 21177 Enzyme immunoassay kit for the quantitative determination of T4 concentration in serum. Catalog Number: SL100303 96 tests For in vitro diagnostic
Human T4-HRP ELISA Kit Medical Device Licence No.: 21177 Enzyme immunoassay kit for the quantitative determination of T4 concentration in serum. Catalog Number: SL100303 96 tests For in vitro diagnostic
Human Neurology 3-Plex A
 Human Neurology 3-Plex A SUMMARY AND EXPLANATION OF THE TEST The Human N3PA assay is a digital immunoassay for the quantitative determination of total Tau, Aβ42, and Aβ40 in human plasma and CSF. Determination
Human Neurology 3-Plex A SUMMARY AND EXPLANATION OF THE TEST The Human N3PA assay is a digital immunoassay for the quantitative determination of total Tau, Aβ42, and Aβ40 in human plasma and CSF. Determination
METHOD VALIDATION CASE
 METHOD VALIDATION CASE METHOD VALIDATION PROTOCOL CLIA Regulation 493.1253 (2) 1.Accuracy (closeness to true/comparative method) 2.Precision (reproducibility) 3.Reference Interval 4.Reportable range (linearity,
METHOD VALIDATION CASE METHOD VALIDATION PROTOCOL CLIA Regulation 493.1253 (2) 1.Accuracy (closeness to true/comparative method) 2.Precision (reproducibility) 3.Reference Interval 4.Reportable range (linearity,
Thyroid Peroxidase IgG ELISA Kit
 Thyroid Peroxidase IgG ELISA Kit Catalog Number KA0952 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Thyroid Peroxidase IgG ELISA Kit Catalog Number KA0952 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Instructions for use. TSH canine ELISA. Please use only the valid version of the Instructions for Use provided with the kit AR E-8500
 Instructions for use TSH canine ELISA AR E-8500 TSH canine ELISA INTRODUCTION INTENDED USE The TSH canine ELISA is an enzyme immunoassay for the quantitative measurement of canine TSH (thyrotropin). SUMMARY
Instructions for use TSH canine ELISA AR E-8500 TSH canine ELISA INTRODUCTION INTENDED USE The TSH canine ELISA is an enzyme immunoassay for the quantitative measurement of canine TSH (thyrotropin). SUMMARY
Insulin (Porcine/Canine) ELISA
 Insulin (Porcine/Canine) ELISA For the quantitative measurement of insulin in Porcine/Canine serum and plasma (EDTA) For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSPO-E01
Insulin (Porcine/Canine) ELISA For the quantitative measurement of insulin in Porcine/Canine serum and plasma (EDTA) For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSPO-E01
Standard Operating Procedure
 Subject Alkaline Phosphatase C111 Index Number Lab-1504 Section Laboratory Subsection Regional Clinic/Affiliate Hospital Laboratories Category Departmental Contact Kamprud, Elizabeth Last Revised 4/12/2017
Subject Alkaline Phosphatase C111 Index Number Lab-1504 Section Laboratory Subsection Regional Clinic/Affiliate Hospital Laboratories Category Departmental Contact Kamprud, Elizabeth Last Revised 4/12/2017
Triiodothyronine (T3) ELISA
 For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. Triiodothyronine (T3) ELISA Kit is intended for the detection of total T3 in human serum or plasma. For research
For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. Triiodothyronine (T3) ELISA Kit is intended for the detection of total T3 in human serum or plasma. For research
Thyroid Stimulating Hormone (TSH) Canine ELISA
 Thyroid Stimulating Hormone (TSH) Canine ELISA For the quantitative measurement of canine TSH For Research use Only. Not For Use In Diagnostic Procedures. Catalog Number: Size: 55-TSHCA-E01 96 Wells Version:
Thyroid Stimulating Hormone (TSH) Canine ELISA For the quantitative measurement of canine TSH For Research use Only. Not For Use In Diagnostic Procedures. Catalog Number: Size: 55-TSHCA-E01 96 Wells Version:
The analytical phase
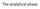 The analytical phase Result interpretation Test request Result Sampling Black box: the lab ANALYTICAL PHASE The CASE Uncle Pete, 67 years old Marked abdominal pain 8 pm, ED Acute abdomen? Assessment (+
The analytical phase Result interpretation Test request Result Sampling Black box: the lab ANALYTICAL PHASE The CASE Uncle Pete, 67 years old Marked abdominal pain 8 pm, ED Acute abdomen? Assessment (+
Contents of the Kit. Instruction Manual B R A H M S anti-tg n LIA. B R A H M S Service
 B R A H M S GmbH Neuendorfstr. 25 D-16761 Hennigsdorf Luminescence immunoassay (LIA) for the quantitative determination of autoantibodies to thyroglobulin (Tg) in human serum (Coated Tube System) Article
B R A H M S GmbH Neuendorfstr. 25 D-16761 Hennigsdorf Luminescence immunoassay (LIA) for the quantitative determination of autoantibodies to thyroglobulin (Tg) in human serum (Coated Tube System) Article
In Vitro Diagnostic Glucose Test System
 CDRH Final Guidance 2-Page Cover Sheet Guidance for Industry In Vitro Diagnostic Glucose Test System Document issued on: July 6, 1998 U.S. Department Of Health and Human Services Food and Drug Administration
CDRH Final Guidance 2-Page Cover Sheet Guidance for Industry In Vitro Diagnostic Glucose Test System Document issued on: July 6, 1998 U.S. Department Of Health and Human Services Food and Drug Administration
Thyroid Function. Thyroid Antibodies. Analyte Information
 Thyroid Function Thyroid Antibodies Analyte Information - 1-2013-04-30 Thyroid Antibodies Determination of thyroid autoantibodies are, besides TSH and FT4, one of the most important diagnostic parameters.
Thyroid Function Thyroid Antibodies Analyte Information - 1-2013-04-30 Thyroid Antibodies Determination of thyroid autoantibodies are, besides TSH and FT4, one of the most important diagnostic parameters.
EXOTESTTM. ELISA assay for exosome capture, quantification and characterization from cell culture supernatants and biological fluids
 DATA SHEET EXOTESTTM ELISA assay for exosome capture, quantification and characterization from cell culture supernatants and biological fluids INTRODUCTION Exosomes are small endosome-derived lipid nanoparticles
DATA SHEET EXOTESTTM ELISA assay for exosome capture, quantification and characterization from cell culture supernatants and biological fluids INTRODUCTION Exosomes are small endosome-derived lipid nanoparticles
The gold standard in Graves disease diagnosis. Thermo Scientific B R A H M S TRAK human Immunodiagnostic Assays
 Thermo Scientific B R A H M S TRAK human Immunodiagnostic Assays The gold standard in Graves disease diagnosis Is it really Graves disease? Will my patient relapse? Which course of Graves ophthalmopathy
Thermo Scientific B R A H M S TRAK human Immunodiagnostic Assays The gold standard in Graves disease diagnosis Is it really Graves disease? Will my patient relapse? Which course of Graves ophthalmopathy
Thyroxine (T4) Human ELISA Kit
 ab108662 Thyroxine (T4) Human ELISA Kit Instructions for Use For the quantitative measurement of Human Thyroxine (T4) concentrations in serum. This product is for research use only and is not intended
ab108662 Thyroxine (T4) Human ELISA Kit Instructions for Use For the quantitative measurement of Human Thyroxine (T4) concentrations in serum. This product is for research use only and is not intended
THE THYROID GLAND AND YOUR HEALTH
 THE THYROID GLAND AND YOUR HEALTH Your Thyroid is a gland located at the base of your neck, just below your Adam s apple. It is shaped like a butterfly each wing or lobe, of your thyroid lies on either
THE THYROID GLAND AND YOUR HEALTH Your Thyroid is a gland located at the base of your neck, just below your Adam s apple. It is shaped like a butterfly each wing or lobe, of your thyroid lies on either
Instructions for use. TSH canine ELISA. Please use only the valid version of the Instructions for Use provided with the kit AR E-8500R
 Instructions for use TSH canine ELISA AR E-8500R TSH canine ELISA 1. INTRODUCTION 1.1 INTENDED USE The TSH canine ELISA is an enzyme immunoassay for the quantitative measurement of canine TSH (thyrotropin).
Instructions for use TSH canine ELISA AR E-8500R TSH canine ELISA 1. INTRODUCTION 1.1 INTENDED USE The TSH canine ELISA is an enzyme immunoassay for the quantitative measurement of canine TSH (thyrotropin).
Endocrine system pathology
 Endocrine system pathology Central endocrine system peripheral endocrine system: thyroid gland parathyroid gland pancreas adrenal glands Thyroid gland. the weight of normal thyroid gland is about 15 grams.
Endocrine system pathology Central endocrine system peripheral endocrine system: thyroid gland parathyroid gland pancreas adrenal glands Thyroid gland. the weight of normal thyroid gland is about 15 grams.
Hexagon OBTI. Design Verification. Contents
 Design Verification Hexagon OBTI Contents 1 Function... 2 2 Analytical sensitivity... 2 2.1 Description of control materials... 2 2.2 Results with human haemoglobin... 2 3 Specificity, cross-reactivity
Design Verification Hexagon OBTI Contents 1 Function... 2 2 Analytical sensitivity... 2 2.1 Description of control materials... 2 2.2 Results with human haemoglobin... 2 3 Specificity, cross-reactivity
Table 1: Thyroid panel. Result (reference interval) TSH 89.5 miu/l ( ) Total T4 5.2 µg/dl ( ) T3 uptake 39% (22-35)
 Introduction Thyroid disease is the second most common endocrine disorder (behind diabetes), and its prevalence increases with increasing age. The incidence of newly diagnosed thyroid cancer is increasing
Introduction Thyroid disease is the second most common endocrine disorder (behind diabetes), and its prevalence increases with increasing age. The incidence of newly diagnosed thyroid cancer is increasing
Evaluation of the analytical performances of six measurands for thyroid functions of Mindray CL-2000i system
 Original Article Page 1 of 7 Evaluation of the analytical performances of six measurands for thyroid functions of Mindray CL-2i system Andrea Padoan, Chiara Cosma, Mario Plebani Department of Laboratory
Original Article Page 1 of 7 Evaluation of the analytical performances of six measurands for thyroid functions of Mindray CL-2i system Andrea Padoan, Chiara Cosma, Mario Plebani Department of Laboratory
See external label 96 tests ULTRASENSITIVE THYROID STIMULATING HORMONE (u-tsh) TSH Ultra Sensitive
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
Instructions for use. TSH rat ELISA. Please use only the valid version of the Instructions for Use provided with the kit AR E-8600
 Instructions for use TSH rat ELISA AR E8600 TSH rat ELISA INTRODUCTION INTENDED USE The TSH rat ELISA is an enzyme immunoassay for the quantitative measurement of TSH in rat serum. For research use only.
Instructions for use TSH rat ELISA AR E8600 TSH rat ELISA INTRODUCTION INTENDED USE The TSH rat ELISA is an enzyme immunoassay for the quantitative measurement of TSH in rat serum. For research use only.
Canine Thyroid Stimulating Hormone, TSH ELISA Kit
 Canine Thyroid Stimulating Hormone, TSH ELISA Kit Catalog No: E0463c 96 Tests Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Canine Thyroid Stimulating Hormone, TSH ELISA Kit Catalog No: E0463c 96 Tests Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
GLUH PRINCIPLE REF B ANNUAL REVIEW Reviewed by. Date. Date INTENDED USE
 SYNCHRON System(s) Chemistry Information Sheet Copyright 2014 Beckman Coulter, Inc. GLUH Glucose REF B24985 For In Vitro Diagnostic Use Rx Only ANNUAL REVIEW Reviewed by Date Reviewed by Date PRINCIPLE
SYNCHRON System(s) Chemistry Information Sheet Copyright 2014 Beckman Coulter, Inc. GLUH Glucose REF B24985 For In Vitro Diagnostic Use Rx Only ANNUAL REVIEW Reviewed by Date Reviewed by Date PRINCIPLE
Herpes Simplex Virus 2 IgM HSV 2 IgM
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
S4. Summary of the GALNS assay validation. Intra-assay variation (within-run precision)
 S4. Summary of the GALNS assay validation (i.) Intra-assay variation (within-run precision) Intra-assay variation was determined by measuring standard blood samples (low activity standard; medium activity
S4. Summary of the GALNS assay validation (i.) Intra-assay variation (within-run precision) Intra-assay variation was determined by measuring standard blood samples (low activity standard; medium activity
YY/T Translated English of Chinese Standard: YY/T PHARMACEUTICAL INDUSTRY STANDARD
 Translated English of Chinese Standard: YY/T1518-2017 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA
Translated English of Chinese Standard: YY/T1518-2017 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE
 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k103542 B. Purpose for Submission: Alternative blood glucose test strips for use with LifeScan OneTouch
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k103542 B. Purpose for Submission: Alternative blood glucose test strips for use with LifeScan OneTouch
Anti-TSH Receptor (TRAb) ELISA
 SUMMARY Anti-TSH Receptor ELISA Assay Kit is used for the quantitative determination of Thyrotropin (TSH) receptor autoantibodies in human serum. Anti-TSH Receptor (TRAb) ELISA Catalog Number: TRA31-K01
SUMMARY Anti-TSH Receptor ELISA Assay Kit is used for the quantitative determination of Thyrotropin (TSH) receptor autoantibodies in human serum. Anti-TSH Receptor (TRAb) ELISA Catalog Number: TRA31-K01
Annex 1 Instruction for Use
 Annex 1 Instruction for Use THYROID STIMULATING HORMONE (TSH) ELISA KIT Catalog No. E1003 INTENDED USE The Autobio TSH assay is designed for the quantitative determination of thyroid stimulating hormone
Annex 1 Instruction for Use THYROID STIMULATING HORMONE (TSH) ELISA KIT Catalog No. E1003 INTENDED USE The Autobio TSH assay is designed for the quantitative determination of thyroid stimulating hormone
Chorionic Gonadotropin Human ELISA Kit
 ab108637 Chorionic Gonadotropin Human ELISA Kit Instructions for Use For the qualitative measurement of Human Chorionic Gonadotropin concentrations in serum and urine. This product is for research use
ab108637 Chorionic Gonadotropin Human ELISA Kit Instructions for Use For the qualitative measurement of Human Chorionic Gonadotropin concentrations in serum and urine. This product is for research use
Enzyme Immunoassay for the Quantitative Determination of Total Thyroxine (T4) in Human Serum
 of total T4, TSH, Free T3 and Free T4 by immunoassay are reliable and convenient methods to determine the presence of thyroid disorders in patients.4,5 Increased levels of T4 have been found in hyperthyroidism
of total T4, TSH, Free T3 and Free T4 by immunoassay are reliable and convenient methods to determine the presence of thyroid disorders in patients.4,5 Increased levels of T4 have been found in hyperthyroidism
See external label 2 C-8 C Σ=96 tests Cat # 3122Z MICROWELL ELISA THYROID STIMULATING HORMONE (TSH) ENZYME IMMUNOASSAY TEST KIT TSH.
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Bovine Insulin ELISA
 Bovine Insulin ELISA For quantitative determination of insulin in bovine serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSBO-E01 Size: 96 wells Version:
Bovine Insulin ELISA For quantitative determination of insulin in bovine serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSBO-E01 Size: 96 wells Version:
HYPERTHYROIDISM. Hypothalamus. Thyrotropin-releasing hormone (TRH) Anterior pituitary gland. Thyroid-stimulating hormone (TSH) Thyroid gland T4, T3
 HYPERTHYROIDISM Hypothalamus Thyrotropin-releasing hormone (TRH) Anterior pituitary gland Thyroid-stimulating hormone (TSH) Thyroid gland T4, T3 In hyperthyroidism, there is an increased production of
HYPERTHYROIDISM Hypothalamus Thyrotropin-releasing hormone (TRH) Anterior pituitary gland Thyroid-stimulating hormone (TSH) Thyroid gland T4, T3 In hyperthyroidism, there is an increased production of
Porcine/Canine Insulin ELISA
 Porcine/Canine Insulin ELISA For the quantitative determination of insulin in porcine or canine serum and plasma. Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research
Porcine/Canine Insulin ELISA For the quantitative determination of insulin in porcine or canine serum and plasma. Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research
colorimetric sandwich ELISA kit datasheet
 colorimetric sandwich ELISA kit datasheet For the quantitative detection of human TNF-alpha in serum, plasma and cell culture supernatants. general information Catalogue Number Product Name Species cross-reactivity
colorimetric sandwich ELISA kit datasheet For the quantitative detection of human TNF-alpha in serum, plasma and cell culture supernatants. general information Catalogue Number Product Name Species cross-reactivity
Human Thyroid-Peroxidase Antibody, TPO-Ab ELISA Kit
 Human Thyroid-Peroxidase Antibody, TPO-Ab ELISA Kit Catalog No: E0442h 96 Tests Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Human Thyroid-Peroxidase Antibody, TPO-Ab ELISA Kit Catalog No: E0442h 96 Tests Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Turbidos. Design Verification. Contents
 Design Verification Turbidos Contents 1 adjustment... 2 2 Target value determination... 2 3 Stability... 2 3.1 Accelerated stress data... 2 3.2 Open vial stability... 3 3.3 Real Time Stability... 4 4 Result
Design Verification Turbidos Contents 1 adjustment... 2 2 Target value determination... 2 3 Stability... 2 3.1 Accelerated stress data... 2 3.2 Open vial stability... 3 3.3 Real Time Stability... 4 4 Result
HumaTex Febrile Antigens
 Design Verification HumaTex Febrile Antigens Contents 1 Function... 2 2 Product Line... 2 3 Sensitivity... 2 Description of Control Materials... 2 Kit controls Positive and Negative... 3 4 Interferences...
Design Verification HumaTex Febrile Antigens Contents 1 Function... 2 2 Product Line... 2 3 Sensitivity... 2 Description of Control Materials... 2 Kit controls Positive and Negative... 3 4 Interferences...
METHOD VALIDATION: WHY, HOW AND WHEN?
 METHOD VALIDATION: WHY, HOW AND WHEN? Linear-Data Plotter a,b s y/x,r Regression Statistics mean SD,CV Distribution Statistics Comparison Plot Histogram Plot SD Calculator Bias SD Diff t Paired t-test
METHOD VALIDATION: WHY, HOW AND WHEN? Linear-Data Plotter a,b s y/x,r Regression Statistics mean SD,CV Distribution Statistics Comparison Plot Histogram Plot SD Calculator Bias SD Diff t Paired t-test
Standard Operating Procedure
 Subject Free Thyroxine (FT4-II) cobas e411 Index Number Lab-1594 Section Regional/Affiliate Subsection Laboratory Category Departmental Contact Goplin, Darcy Last Revised 2/2/2017 References Required document
Subject Free Thyroxine (FT4-II) cobas e411 Index Number Lab-1594 Section Regional/Affiliate Subsection Laboratory Category Departmental Contact Goplin, Darcy Last Revised 2/2/2017 References Required document
Design Verification Function... 2 Analytical sensitivity... 2 Specificity, cross-reactivity and interferences... 3
 Design Verification 1 Function... 2 2 Analytical sensitivity... 2 2.1 Description of control materials... 2 2.2 Results with human haemoglobin... 2 3 Specificity, cross-reactivity and interferences...
Design Verification 1 Function... 2 2 Analytical sensitivity... 2 2.1 Description of control materials... 2 2.2 Results with human haemoglobin... 2 3 Specificity, cross-reactivity and interferences...
Suppl. Table 1: CV of pooled lipoprotein fractions analysed by ESI-MS/MS
 Supplement VLDL LDL HDL PC 3.3 1.77 1.3 LPC 4.82 2.5.35 SM 3.1 4.6 1.92 CER 2.17 6.3 4.15 PE 3.18 1.93 2.79 PE-pl 13.18 1.9 2.32 CE 2.9.65.4 FC.36 3.5 2.54 Suppl. Table 1: CV of pooled lipoprotein fractions
Supplement VLDL LDL HDL PC 3.3 1.77 1.3 LPC 4.82 2.5.35 SM 3.1 4.6 1.92 CER 2.17 6.3 4.15 PE 3.18 1.93 2.79 PE-pl 13.18 1.9 2.32 CE 2.9.65.4 FC.36 3.5 2.54 Suppl. Table 1: CV of pooled lipoprotein fractions
ELEGANCE Chlamydia pneumoniae IgG ELISA KIT
 INTENDED USE The ELEGANCE Chlamydia pneumoniae IgG ELISA has been designed for the in vitro diagnostic measurement of anti- Chlamydia pneumoniae IgG in the screening of human serum. PRINCIPLES OF THE ELEGANCE
INTENDED USE The ELEGANCE Chlamydia pneumoniae IgG ELISA has been designed for the in vitro diagnostic measurement of anti- Chlamydia pneumoniae IgG in the screening of human serum. PRINCIPLES OF THE ELEGANCE
