Parallel Event-Driven Neural Network Simulations Using the Hodgkin-Huxley Neuron Model
|
|
|
- August Tucker
- 5 years ago
- Views:
Transcription
1 Parallel Event-Driven Neural Network Simulations Using the Hodgkin-Huxley Neuron Model Collin J. Lobb 1, Zenas Chao 2, Richard M. Fujimoto 1, Steve M. Potter 2 1 College of Computing Georgia Institute of Technology {collin,fujimoto)@cc.gatech.edu Abstract Neural systems are composed of a large number of highly-connected neurons and are widely simulated within the neurological community. In this paper, we examine the application of parallel discrete event simulation techniques to networks of a complex model called the Hodgkin-Huxley neuron[1]. We describe the conversion of this model into an event-driven simulation, a technique that offers the potential of much greater performance in parallel and distributed simulations compared to timestepped techniques. We report results of an initial set of experiments conducted to determine the feasibility of this parallel event-driven Hodgkin-Huxley model and analyze its viability for large-scale neural simulations. 1. Introduction Much work in parallel and distributed simulation focuses on synchronizing time in hundreds or thousands of entities. Researchers have been able to create scalable algorithms that efficiently coordinate the temporal activities of these entities. However, there are many domains in which one must synchronize a massive number of entities. Many of these domains have not been investigated. In this paper, we will examine a relatively new domain in parallel and distributed simulation whose scale could eventually reach billions of entities making trillions of connections: the nervous system. In parallel and distributed simulation, there are two main techniques for incrementing time: a time-stepped approach and an event-driven approach. Event-driven simulations have been shown to offer better performance. Thus, many efforts are devoted to creating suitable eventdriven models. This allows one to abstract out the partitioning and synchronizing algorithms. In this paper, we will discuss the transformation of the Hodgkin-Huxley 2 Laboratory for Neuroengineering Georgia Institute of Technology zenas@neuro.gatech.edu stpotter@ece.gatech.edu model [1] of a neuron, hence referred to as the HH model, into an event-driven process and discuss some of our preliminary findings in simulating a large scalable number of these neurons. We are investigating how network scale and interconnectivity affect the emergence of a large synchronous burst of neural activity called a superburst [2]. In order to investigate these network-level questions, we need the ability to run large network simulations. The computational requirements for such networks are much greater than those currently available on typical desktop computers. The effective use of parallel and distributed computation can help address these ever-increasing computational demands. This paper is organized into eight sections. In section 2, we provide a brief introduction to neural systems. In section 3, we discuss previous work in simulating these neuron models. In section 4, we describe how we converted the continuous HH model into a discrete eventdriven simulation model. In section 5, we discuss the implementation of the HH model using a parallel discrete event simulation engine called sik [3] and the methods by which we create networks of these HH neurons. In section 6, we describe efforts to verify this model. In section 7, we discuss some initial scalability experiments on this eventdriven HH model. Finally in section 8, we provide some concluding thoughts on these results and how one might go about improving them. 2. Neural Systems Like most biological systems, neural systems are typically comprised of cells and the fluid that they inhabit (extracellular medium). These cells are able to communicate with one another. For many cells, these communication mechanisms involve the movement of ions, which are atoms with an electric charge. Most cells, including neurons, have a charged membrane but only a few types of cells (e.g. neurons and cardiac cells) are
2 considered to be electrically excitable and are able to produce electrical responses that can be transmitted from cell to cell. It is important to remember that these electrical responses are in the form of ion flows, not flows of electrons. A neuron (depicted below in Figure 1) is an electrically-excitable cell found in the nervous system. A neuron lives in a medium similar to seawater, which contains various ions. The soma is the cell body and has a charge due to the presence of these ions. The soma is surrounded by a selectively permeable cell membrane. The soma of a pre-synaptic neuron can produce an electrical response called an action potential that is transmitted down its axon to its synaptic terminals with some transmission delay. This output charge is then received from the synaptic cleft by the post-synaptic neuron via its dendrites. potential of the neuron is governed by a combination of how open each of these gates is and thus what ion flow is permitted across the membrane (i.e. its permeability). This combination is modeled by a set of differential equations that many experimenters such as Hodgkin and Huxley have quantified. One of the most important processes that a neuron undergoes is the action potential, shown in Figure 2 (henceforth called an AP ). It is characterized electrically as a very large increase in the membrane potential (on the order of 100mV) followed by a sharp decrease in potential that sends the membrane potential below its equilibrium value, followed by a slow return to equilibrium. This also defines the shape of the AP. Consider this in terms of gates and gatekeepers. The gatekeepers manipulate these gates, much like an operator controlling floodgates at a reservoir, trying to maintain equilibrium. For the neuron, there are two main types of gatekeepers: sodium gatekeepers that permit sodium flow into the neuron, and potassium gatekeepers that permit potassium flow out of the cell. Since both sodium and potassium ions are positive, all of these gatekeepers must work together in order to maintain equilibrium. Figure 1: A schematized neuron In the absence of any outside forces (i.e., at equilibrium), there will be differences in the amount of each ion on either side of the neuron s membrane. When we account for all of the forces due to (a) differences in concentration of each type of ion (i.e., diffusion ) and (b) the differences in the amount of positive and negative ions on each side (i.e., electrostatic pressure ), we find that typically a neuron at equilibrium has a negative membrane potential (the charge difference on the neuron s membrane) in the range of -65 mv to -90 mv. In the presence of outside forces, e.g., due to an injected current or the reception of a transmitted electrical response from another neuron, ions will flow into or out of the neuron through pores in the membrane called ion channels. One can think of these ion channels as small gates that are each operated by a gatekeeper according to the laws of chemistry and physics. A gatekeeper will only allow the flow of certain types of ions at certain times at certain rates and in certain conditions. The membrane Figure 2: An action potential generated from a HH neuron in our simulation In response to a sufficiently large positive input (e.g., injecting current into the cell) the sodium ion gatekeepers begin to open their gates to let sodium ions into the cell. This starts to increase the membrane potential, which in turn tells the sodium gatekeepers to let more sodium ions in. Meanwhile, there is a large number of potassium gatekeepers that prior to this sodium influx have been inactive and kept their gates closed. One of their jobs is to monitor the membrane potential and open up their gates when the membrane potential becomes too high. As the sodium level (and thus the membrane potential) begins to rise these potassium gatekeepers begin to take notice and open their gates to let potassium out of the cell. This would lower the membrane potential and return the cell to equilibrium. However, these potassium gatekeepers (letting potassium out) operate more slowly than the sodium gatekeepers (letting sodium in) so the membrane
3 potential keeps rising. When the sodium ion gatekeepers let in enough sodium ions to raise the membrane potential above some threshold, a flood of additional sodium ions rush into the cell and quickly raise the membrane potential (the upstroke ). The sodium gatekeepers try to quickly close the gates to control the flood of incoming sodium ions and are only able to effectively do so after letting in a large number of sodium ions. The potassium gatekeepers try to compensate by opening their gates further. Through the combination of the sodium gates closing and the potassium gates opening the large influx of positive ions is controlled and the membrane potential stops rising (the peak of the AP). However, at this point much potassium efflux is being allowed through the open potassium gates and the membrane potential begins to drop dramatically (the falling phase ). The potassium gatekeepers slowly begin to notice that the membrane potential is dropping rapidly (this is their other responsibility) and that they need to close their gates. By the time they close their gates, the membrane potential has shot well below its equilibrium value ( hyperpolarization ). The sodium gatekeepers, however, quickly recover and begin to open their gates up just enough to increase the membrane potential to its equilibrium value. It is this process that experimenters such as Hodgkin and Huxley have mathematically modeled with a set of differential equations. Neuroscientists have created a wide range of neuronal models but are split concerning how to deal with the action potential. One class of models called integrate-andfire (henceforth called IF ) or spiking neuron models [4, 5], does not model the AP itself. These models effectively model the relationship between the neuron and its responses to various input currents but when this threshold is crossed they assume that a spike is generated and the neuron is instantaneously reset to some reset voltage. Think of this spike as taking the action potential from Figure 2 and compressing it from both ends until it approaches a vertical line. They are unable to capture the shape of the AP or accurately capture how the neuron responds to input during the action potential and during recovery. Despite being biologically unrealistic, the differential equations that govern their behavior are much simpler, easier to analyze, and are solvable. Models such as the HH model that are more biologically realistic are typically governed by a more complex set of differential equations that cannot be solved explicitly and therefore must be approximated using numerical analysis. To put it more quantitatively, 1 ms. of simulation time of the IF model requires around 5 FLOPS while the HH requires 1200 FLOPS [6]. For reasons such as these, large network simulations have been primarily limited to IF models. How might we be able to efficiently run large simulations of these more complex models? This is the driving question behind this work. 3. Related Work Since IF models are primarily used in large scale network models, many iteratively-stepped parallel simulators have been created to efficiently simulate them: NEOSIM[13], the Neocortex simulator[11], SPIKELAB[14], SPIKENET [15] and Brettle and Niebur [16] to mention a few. Using an iterative approach, one would iterate the governing differential equations for each dt and determine if its threshold has been crossed. If so, it would fire a spike. However, since the governing equations of the IF model can be solved, one can determine when the next time the IF neuron will fire. One could just keep an estimate of this firing time and update it if any more input is received. This is an event-driven approach and can be used to dramatically speed up the simulation[10, 17-19]. There are several existing parallel simulators capable of running networks of HH neurons: PNEURON, a parallel version of NEURON [7], PGENESIS [8], PARALLEL NEUROSYS[9], and the Neocortex simulator [10, 11]. Thomas [12] completed some work on running HH networks, which parallelizes time-stepped subsystems through waveform relaxation. As far as the authors are aware, only the Neocortex simulator has looked into partitioning algorithms. All of these simulators use an iterative approach. This paper explores the use of an event-driven framework on such HH networks. 4. Model Design Muresan [10] states that ideally a large-scale neural simulator would support a high degree of interoperability in both the types of neurons that can be used and the techniques that each neuron employs. It would allow for both implicit and explicit synaptic representations. An explicit representation is one that says that neuron A connects to neuron B; in contrast, an implicit representation is one where neuron A connects to some other neuron as defined by some rule of connectivity [10]. This is in addition to the stereotypical desires of high accuracy, computational efficiency (speed), and minimal memory requirements. Two major, interrelated aspects that one must consider when designing a parallel, event-driven HH simulation are (a) how to define the distinct entities making up the network and (b) the time intervals inherent in the model that can be used to extract lookahead. It is well known that good lookahead properties are desirable for optimistic synchronization mechanisms and essential for conservative techniques. The means by which network entities are defined will determine the types of events that are exchanged between entities, greatly affecting the
4 partitioning strategy, and the types of optimizations that might be later applied. One can define the neural system as being composed of at least four components: the soma, the axon, the synapse (i.e., the synaptic terminals, the dendrites and the synaptic cleft) and the extracellular medium (environment). One important question is how one would represent each of these neural components in such a way that performance is maximized for the types of experiments that will be performed. There are two things that we should keep in mind as we go about defining these components. First, for scalability purposes, a fairly compact representation for these components is desirable as memory requirements will be large. It is also important that we keep in mind that there are many simplifying assumptions that one makes in order to investigate network behavior. These can be made for a general class of neural systems or can be made purely on the nature of the experiment at hand. Foremost, we describe several general simplifications that one might make when looking at network-level questions. The first simplification is that we are not modeling changes in the environment. We assume isopotentiality in the neuron, i.e., space is uniform and does not have to be explicitly considered in our equations. We are not modeling the interactions of the AP as it travels down the axon (e.g. by cable equations). We characterize the axon purely in terms of the delay in which an AP is transmitted. This delay is typically much larger than the timesteps that are used in these types of simulations. This transmission is also one-way. These two facts suggest that the axon would provide a good means of separating the neuron components from the synapse components. Other important considerations arise when we consider a model for our synapses. The interactions between the post-synaptic neuron and the synapse itself are assumed to be instantaneous; i.e., the dendrites are not explicitly modeled. After an AP has been transmitted down the axon with some delay, it is considered to be instantly transmitted to the receiving neuron. Learning mechanisms inherent in a typical synaptic model (e.g., [20, 21]) describe instantaneous messages to be passed between the post-synaptic neuron and the synapse model. Unfortunately, this means that separating the neuron model from the synapse model would result in a zerolookahead simulation. To avoid this and to improve the efficiency of interaction between these components, the representations of the neuron model and the synaptic model are combined into a single synapse-neuron simulation entity (i.e., are mapped to a single logical process), allowing instantaneous messages to be implemented as manipulations of shared state. However, combining these representations can be potentially disastrous if one has to replicate and coordinate multiple synaptic models. Because of our desire to obtain good lookahead, we wish to partition the network by splitting a neural connection by its axon and base our lookahead on the axonal transmission time. Fortunately, the standard synaptic models employed here are unidirectional, meaning that the synapse only affects the post-synaptic neuron and does not need to pass messages back to the pre-synaptic neuron. Therefore, we incorporate the synaptic model into the post-synaptic neuron s representation. The pre-synaptic neuron only needs to know to which synapses it should fire and what delays are involved in these firings. On the other hand, the postsynaptic neuron doesn t need to know this delay since it will receive this action potential as an event. The only replication of the synapse that is needed is the unique synaptic identifier. An important consideration that arises from this combined representation is a hierarchical notion of variable scope. A neuron owns many synaptic representations. Thus, variables that are common to all of its synapses may be abstracted from the synaptic representation and put in the neuron representation. As this is a one-to-many relationship this can result in large memory savings. Computationally, a common means of speeding up various synaptic calculations (e.g. how much current is being received by a neuron at this moment) is to allow these calculations to be performed in batch for all of the synapses thus making the calculation more computational efficient (see [22] for an excellent example of this technique). One can extend this variable abstraction technique two more levels and say that state common to all neurons in a simulation process can be abstracted to that simulation process itself and that variables common to all simulation processes can become constants in the simulation. Such abstraction is a powerful means for limiting memory consumption. One of the most challenging issues concerns how to define the action potential event and how to determine not only what it looks like but exactly when it occurred. Typically, they can be defined in terms of timing, kinetic models, and sometimes their waveforms (i.e., shape). Here we have chosen to use the synaptic model described in [22], which uses kinetic models to exhibit STDP[21] and short-term depression[20], but are not currently using any of the optimizations that they describe. STDP is a Hebbian learning mechanism based purely on timings of received and fired action potentials. Short-term depression describes a synapse as having limited resources and its output is affected by recent synaptic activity. We can effectively model the AP here as a singular event where we only need to know the time it is received. The advantage of this is that we are effectively modeling the relationship between the voltage on the soma and any input current at all times. The disadvantage of this is that any small variation in AP shape is not transmitted to a receiving neuron. We could effectively transmit a
5 waveform to a receiving neuron by knowing where it starts and stops. This event-driven approach can theoretically handle all three types of synaptic models and thus promotes interoperability. Since we are measuring the AP purely in terms of timing, we need to determine when the AP needs to be received. If we know the axonal delay then we need to determine when the AP was fired. This is essentially the same issue as the problem of finding the exact moment threshold crossing for an IF neuron (e.g. see the work in [17, 19, 24, 25]). For most IF models this is determined by explicitly solving the governing equations (see Brette [19] for an unsolvable IF model). The HH model does not explicitly have any notion of a threshold. However, this notion is quite useful here. For an event-driven HH model, one could set the threshold to be a voltage on the action potential with the idea that if this threshold was reached then a neuron must be firing an action potential (by definition, we must because the threshold is on the action potential). By experimentally varying the amount of a constant input current we can determine that the HH neuron will fire if the voltage is increased above -50mV. Thus we can say that -50mV is our threshold. This leaves us with the problem of determining the exact moment in which the threshold was crossed. One possible solution lies in the fact that the APs generated from a neuron generally have a narrow range of possible shapes. Given this, it seems plausible that we could better interpolate the exact crossing from the range of slopes that will occur at threshold crossing. As the reader may have noticed it is not absolutely necessary to determine when this HH threshold was crossed. In the above paragraphs, this notion of threshold was used because it is a convenient place to define the moment when the action potential event occurred. However, one must keep in mind that the focus of events in discrete event simulation is not when an event actually is sent but when it is received. Instead of calculating the threshold and using that to determine when the action potential event should be received, one could determine this received time from the current stepped value of time directly. This would conflate the issue of what lookahead would be used but it is indeed possible especially for optimistic protocols. This type of approach may be very useful for more complex neurons where the shape of the AP is not as well defined. The above description of an event-driven HH model affords several advantages. One such advantage is that it allows one to make various modeling optimizations concerning when the state of the neural components need not be updated. As an illustrative example, consider the synapse-neuron representation at equilibrium. In the absence of noise, none of the values are changing so there is no point in updating it during these times. This is essentially the same type of rationale that led to the creation of event-driven IF models. The next time that it must be updated is when it comes out of equilibrium. Or do we? If the input is small and enough time would pass that the system would quickly respond back to equilibrium, one might be able to ignore it completely. This type of optimizations would be more acceptable for many network-level questions where the concern is typically about the generation of action potentials and not minute changes in a neuron s membrane potential. A vital point to be made here is that in these neural systems there is a significant amount of noise. This noise is accounted for in many of the models as a noise current. How might we be able to efficiently have random noise in the system? Does this make it a highly irregular problem and inherently more difficult to solve using this type of an approach? Does this dismiss the possible optimizations that are discussed above? These types of questions deserve further exploration (see [18] for an account of how to deal random noise in IF neurons). 5. Model Implementation We chose to implement the neural system using the simulation engine sik [3] (pronounced Musik ), which is written in C++. sik was developed to be able to efficiently simulate large-scale discrete-event simulations while allowing for maximum flexibility in its synchronization algorithm. It uses a micro-kernel design analogous to the one employed in operating systems. These inherent advantages are reminiscent of the design goals that were discussed in the previous section. As such, sik is well-suited as a simulation engine for modeling this neural system. It also will provide a seamless way for testing different synchronization methodologies and comparing the performance advantages and disadvantages of each. In sik, a physical process is referred to as a Simulator and is composed of many logical processes. Each logical process has its own synchronization algorithm. Logical processes communicate via discrete events. For conservative approaches, time is synchronized by determining the next event for each logical process via a global lower bound timestamp (LBTS) computation. Lookahead is important in these approaches for efficient synchronization as it allows the simulation engine to know when the federate will not schedule any new events. The neural simulation is implemented using the synapse-neuron representation as the logical process. Presently, a conservative protocol is used for synchronization. LPs send action potential events when their threshold is crossed. In order to meet the constraints of the conservative approach, each neuron updates its state at regular intervals. The reasoning behind this is outlined below.
6 In sik, the physical process, called NeuralSimulator, is defined as a subclass of Simulator. Inside this federate, there are pointers to output files for recording data. The logical processes are implemented as the class Neuron, a subclass of NormalSimProcess. Inside Neuron, several state variables are defined including the current timestep, the threshold of excitation, the membrane potential, the time the neuron was last updated, and two ids. One id serves as the unique LP identifier for a NeuralSimulator instance. The other id is defined to be unique over the entire simulation and is used for determining noise current. In the previous section, the state of the synapse was described as being an explicit component in the representation for the post-synaptic neuron and only an identifier and a delay for the pre-synaptic neuron. We have implemented this as two C++ maps to which Neuron holds a pointer: one that holds a list of pointers to this Synapse class and the other that holds a list of neurons, their federate ids and the appropriate axonal transmission delay (in a ConnectionInfo class) to whom this neuron fires. Both maps are keyed on the unique synapse id. The Neuron class sends and receives two types of messages. The first is an event called an AP_ARRIVAL, that signals the neuron that an action potential has arrived at the neuron. When this event is received, the neuron will update its time from the last time it was updated and then update the appropriate state variables. An adaptive Runge-Kutta timestep method with error control, as described in [23], is used to perform the necessary numerical approximations. This algorithm allows one to set a tolerance on the calculated error and lets the timestep grow or shrink as needed in order to minimally meet this tolerance. This timestep is bound to a certain range and will grow/shrink at a constant rate. Each neuron LP has its own timestep value which is retained between events. This adaptive timestep method is also consistent with the event-driven philosophy. One challenge is determining when this action potential event will actually be received. If the exact firing time were known then the AP event would need to be received at the firing time plus the axonal transmission time. For this implementation we simply see if the threshold was crossed during some timestep dt. If it was crossed then a neuron will fire an AP_ARRIVAL event at the end of this dt. We perform no interpolation to get a more exact crossing moment (within dt). Unlike the IF event models, it is not known deterministically when this threshold will be crossed and one cannot employ a simpler LBTS-maintained systemwide spike estimate priority queue with conservative synchronization. To meet the constraints of the conservative protocol that we are currently using, we update the neuron periodically with a WAKEUP event. If the threshold has not been crossed then we could theoretically fire at any time (e.g. a supra-threshold input current arrives). In order to ensure that the received event occurs in the LPs future and meets the constraints of the conservative protocol we require that the WAKEUP event occurs at one-half the minimum axonal transmission delay. As a result of this, we must define our lookahead as also being one-half the minimum axonal transmission delay. However, if a threshold has been crossed and we are currently generating the AP we know that we cannot fire again until the AP finishes and a period of time that the neuron cannot fire again (called the absolute refractory period ) has passed. We have experimentally calculated a very conservative estimate of the sum of these two periods as 10ms. Thus, one-half the minimal transmission time plus 10ms is the next time that our neuron would have to be updated. The use of this WAKEUP event has an interesting consequence in that the more the neuron fires the less often we need to update its state via a WAKEUP event. This suggests that the simulation may get better performance if there is a moderate amount of activity. We investigate this quantitatively in section seven. To model noise in the system, we added a noise current to the HH model. The value used is obtained by a Gaussian distribution with a mean of 700nA and standard deviation of 7500 and is sampled upon every time increment. This was defined with the intuition that most of the noise will generate sub-threshold responses but occasionally a neuron will receive supra-threshold noise, generating an action potential. Experimentally, if these large noise currents did not exist then the dynamics of our ion channels would simply converge to a steady-state range of a few millivolts above equilibrium. The creation of these networks and its partitioning is done by a separate C++ program. This program will generate a network configuration based on [26]. The network generated is consistent with biological data in that synaptic connections between two neurons are much more likely if those neurons are near each other. It is important to note that the number of synapses generated will grow as the amount of neurons in the simulation grows! This relationship is shown in Figure 3. The current version of this program is extremely memory intensive and limits us to creating a network below 10,000 neurons. Mean synapses per neuron Network Size Figure 3: Neural connectivity in the generated HH networks
7 The partitioning of these networks is currently done through a simple scoring system where each synapse is given a weighted score based on the transmission delay and the number of synapses that are firing to the same neuron. Qualitatively, this weighted score is a measure of how often events will be received by the post-synaptic neuron. We iterate through the synapses from highest score to lowest score assigning neurons to processors. If both neurons for a synapse are unassigned then both are assigned in a round robin order to the next processor. If only one of the neurons is unassigned then it is either assigned to the same processor as the already assigned neuron or to the next processor. The greater the score of the synapse the more likely it is that it will be assigned to the same processor. The partitioned network for each processor is written to a unique input file that a NeuralSimulator reads in when it is initialized. 6. Model Verification It is very difficult to verify that the network that we have created will generate reasonable neuronal activity in the absence of experimental data. Such verification requires a fair amount of parameter tuning. In the present paper, we are more concerned with if the basic mechanisms are correct and how different parameters (e.g. those that increase the chance that a neuron will fire an AP) will affect simulator performance. In order to investigate this, we have conducted several efforts to verify the neuron and synaptic models used in our implementation. In order to verify our model of the HH neuron, we compared the event-driven model to a standard timestepped HH model. The event-driven model appears to generate a normal action potential shape (see Figure 2) and respond similarly to sub-threshold input currents. It is worth noting here that since we are using numerical approximation the results of the comparison will not be exact. We have completed some preliminary verification on our synaptic model. Initial testing on the mechanisms of STDP in our synaptic model and each of these variables indicates it appears to be responding correctly. We have done no testing with our short-term depression implementation so we have not included it in the experiments below. Verification of the biological accuracy of the networks that we generated remains an area of future work. 7. Initial Results An initial set of simulation runs were conducted in order to test the feasibility of this event-driven Hodgkin- Huxley model and to access its scalability and performance. The simulations were run on a single cluster node consisting of MHz PIII Xeon SMP processors with an available 4GB RAM. All simulations were conducted on a single node and thus there is no overhead due to intern-node communications. Networks of size 25, 50, 100, 200, and 400 neurons were created according to the description in section five and simulated for 15 seconds of simulation time using 1, 2, 4, and 8 processors. Each neuron initially received between zero and four action potentials at random times in the first twenty milliseconds of the simulation. These inputs were designed to augment the spontaneous activity of these networks. All synapses in these networks were excitatory, meaning that input currents increase the membrane potential of the neuron. The speedups for these networks are shown in Figure 4. Speedup Number of Processors Size 25 Size 50 Size 100 Size 200 Size 400 Figure 4: Speedup for networks of 25, 50, 100, 200, and 400 neurons on 1, 2, 4 and 8 processors. As can be seen from the results in Figure 4, we are able to achieve good speedup in our neural networks. The network of 50 neurons offers particularly good speedup here because there are fewer remote events due to the way the network is partitioned. One might be able to achieve better speedup with other networks with better partitioning. These results suggest that one may be able to effectively run larger networks of these event-driven Hodgkin-Huxley neurons. Next, we conducted an experiment to determine how simulation performance changes as network activity increases. In order to test this, we varied the maximal synaptic conductance (g max ) in a set of 15 second simulations on a network of size 100 on a single processor. The results are shown in Table 1. This experiment demonstrates that we are able to achieve better performance when there is a moderate amount of activity.
8 Table 1: Effects of Network Activity on Execution Time of a 100 HH Neuron Network g max APs Fired Total Elapsed Time (s) Important factors that determine the execution time are the number of events that must be processed and the amount of computation required to process each event. In our model there are two types of events: a WAKEUP event and an AP_ARRIVAL event. In Table 2 below, we list the percentage of each event that we process in our simulations. As one can see the number of WAKEUP events is far greater than the number of AP_ARRIVAL events. Efforts to reduce the number of WAKEUP events needed or the regularity in which they occur would greatly reduce the number of events that need to be processed and could yield much better performance. Table 2: Percentage of Each Event in Simulations % AP_ARRIVAL Events % WAKEUP Events It is important to be able to characterize the computation time of each of these events. In order to determine this, the time taken to process each type of event was recorded in a separate set of simulations that were setup as described above but ran for only 1s of simulation time. In Figure 5, we can see the amount of time to process each event increases as the size of our network increases. The WAKEUP event updates the state of the neuron to the timestamp in that event. The time to process this event grows roughly linearly with network size because the number of synapses for which we must calculate the synaptic input grows linearly (see Figure 3). Optimizations that try to pull out computations from individual synapses may greatly help speed up the computation here (e.g., see [22]). Avg Processing Time (ms) WC Time per Event Network Size (in Neurons) WAKEUP AP_ARRIVAL Figure 5: Wall clock processing time for WAKEUP and AP_ARRIVAL events for networks of size 25, 50, 100, 200, and 400 neurons The AP_ARRIVAL event updates the state of our neuron to the timestamp of that event and then updates the state of the synapses for the arrival of an action potential. The time to process an AP_ARRIVAL event will grow similar to the WAKEUP event because of linear synaptic growth. In addition, we must find and update the appropriate synapse in our synaptic representation (a C++ map). This computation is logarithmic and accounts for the difference between the two types of events. 8. Conclusion In this paper, we have described an event-driven Hodgkin-Huxley neuron that offers promise for effective parallel simulation of larger networks. This technique offers good performance as the networks size increases, better performance when there is moderate activity in the network, and meets several of the design criteria described in [10] for large scale neural simulators. From the simulation side, there are many future directions that could be pursued. These include: improving the partitioning algorithm; investigating the use of other synchronization algorithms, experimentation on larger networks with a larger number of processors; achieving a lookahead based on action potential events only; characterizing and limiting the memory consumption of the simulation; and performing a comparison with a timestepped approach to determine what, if any, savings result from using this event-driven approach. From the modeling side, there are equally as many directions that deserve further investigation. These include: further verification of the neuron and synapse models and networks for biological realism and parameter sensitivity; a better characterization of the noise current and the questions that it begs; and investigating other techniques and optimizations that may be employed to
9 improve the computational speed in which events are processed. Appendix: Model Details The Hodgkin-Huxley model is a set of four differential equations (1-4). The synaptic model is also a set of four differential equations (5-8). (1) -C * dv/dt = I noise (I Na + I K + I leak ) a. I Na = g na *m(t) 3 *h(t)* (V E Na ) b. I K = g K * n(t) 4 * (V E K ) c. I leak = g leak * (V E leak ) (2) dm/dt = (m inf m) / (3) dn/dt = (n inf n) / (4) dh/dt = (h inf h) / (5) dg ex /dt = -g ex / ex (Excitatory Synapses) (6) dg in /dt = -g in / in (Inhibitory Synapses) (7) dm/dt = -M/ - (8) dp a /dt = -P a / + Equation 1 was solved using adaptive Runge-Kutta using a tolerance of Equations 2-8 were formulated in terms of an update rule (of the form x(t + dt) = ) and evaluated for the appropriate value of V. These parameters resulted in average timesteps in the range of ms. to ms. for the first set of experiments described in section seven. Parameter values for these equations were given in their respective papers. Similar to [21], we set the initial value of g a to g max in order to maximize network activity (g max = 150 ps). Acknowledgements This research was supported in part by NSF grant ATM References [1] A. L. Hodgkin and A. F. Huxley, "A quantitative description of membrane current and its application to conduction and excitation in nerve." Journal of physiology, vol. 117, pp , [2] Nadasdy Z., W. D. A., Potter S.M. (2003). Attractor dynamics of superbursts in living neural networks. SFN 2003, New Orleans, LA. [3] K.Perumalla, Musik A Micro-kernel for Parallel/Distributed Simulation Systems, PADS [4] L. Lapicque, "Recherches quantitatives sur l'excitation electrique des nerfs traitée comme une polarisation," vol. 9, pp , [5] W. Gerstner and W. M. Kistler, Spiking neuron models: single neurons, populations, plasticity. New York: Cambridge University Press, [6] E. M. Izhikevich, "Which model to use for cortical spiking neurons?" IEEE transactions on neural networks, vol. 15, pp , [7] M. Hines and N. T. Carnevale, "The NEURON Simulation Environment," Neural Computation, vol. 9, pp , [8] J. M. Bower and D. Beeman, The book of GENESIS: exploring realistic neural models with the GEneral NEural SImulation System. Santa Clara, Calif.: Telos, [9] P. Pacheco, M. Camperi, and T. Uchino, "PARALLEL NEUROSYS: a system for the simulation of very large networks of biologically accurate neurons on parallel computers," Neurocomputing, July 1999, vol , pp , [10]I. I. Muresan R.C., "Principles of Design for Large Scale Neural Simulators," presented at International Conference on Automation, Quality and Testing, Robotics, Cluj-Napoca, [11]I. I. Muresan R.C., "The "Neocortex" Neural Simulator. A Modern Design," presented at International Conference on Intelligent Engineering Systems, Cluj-Napoca, [12]E. A. Thomas, "A parallel algorithm for simulation of large neural networks," Journal of Neuroscience Methods, vol. 98, pp , [13]N. Goddard, G. Hood, F. Howell, M. Hines, and E. De Schutter, "NEOSIM: portable large-scale plug and play modelling," Neurocomputing, vol , pp , [14]C. Grassman and J. K. Anlauf, "Fast digital simulation of spiking neural networks and neuromorphic integration with SPIKELAB," International Journal of Neural Systems, vol. 9, pp , [15]A. Delorme, J. Gautrais, R. van Rullen, and S. Thorpe, "SpikeNET: a simulator for modeling large networks of integrate and fire neurons," Neurocomputing, vol , pp , [16]D. Brettle and E. Niebur, "Detailed parallel simulation of a biological neuronal network," IEEE Computational Science & Engineering, vol. 1, pp , [17]M. Mattia and P. Del Giudice, "Efficient event-driven simulation of large networks of spiking neurons and dynamical synapses," Neural Computation, vol. 12, pp , [18]J. Reutimann, M. Giugliano, and S. Fusi, "Event-driven simulation of spiking neurons with stochastic dynamics," Neural Computation, vol. 15, pp , [19]R. Brette, "Event-driven simulation of integrate-and-fire neurons with exponential synaptic conductances," Submitted, [20]H. Markram, Y. Wang, and M. Tsodyks, "Differential signaling via the same axon of neocortical pyramidal neurons," Proceedings of the National Academy of Sciences of the United States of America, vol. 95, pp. 5323, [21]S. Song, Miller, K.D. and Abbott, L.F., "Competitive Hebbian Learning Through Spike-Timing Dependent Synaptic Plasticity," Nature Neurosci, vol. 3, pp , [22]M. Giugliano, M. Bove, and M. Grattarola, "Fast calculation of short-term depressing synaptic conductances," Neural Computation, vol. 11, pp , [23]W. H. Press, Numerical recipes in C: the art of scientific computing, 2nd ed. Cambridge; New York: Cambridge University Press, 1992.
10 [24]M. J. Shelley and L. Tao, "Efficient and accurate timestepping schemes for integrate-and-fire neuronal networks," Journal of Computational Neuroscience, vol. 11, pp [25]T. Makino, "A discrete-event neural network simulator for general neuron models," Neural Computing & Applications, vol. 11, pp , [26]R. Segev and E. Ben-Jacob, "Generic modeling of chemotactic based self-wiring of neural networks," Neural Networks, vol. 13, pp , 2000.
RetinotopicNET: An Efficient Simulator for Retinotopic Visual Architectures.
 RetinotopicNET: An Efficient Simulator for Retinotopic Visual Architectures. Dipl. Eng. RAUL C. MUREŞAN Nivis Research Gh. Bilascu, Nr. 85, Cluj-Napoca, Cod 3400 ROMANIA raulmuresan@personal.ro Abstract.
RetinotopicNET: An Efficient Simulator for Retinotopic Visual Architectures. Dipl. Eng. RAUL C. MUREŞAN Nivis Research Gh. Bilascu, Nr. 85, Cluj-Napoca, Cod 3400 ROMANIA raulmuresan@personal.ro Abstract.
Neuromorphic computing
 Neuromorphic computing Robotics M.Sc. programme in Computer Science lorenzo.vannucci@santannapisa.it April 19th, 2018 Outline 1. Introduction 2. Fundamentals of neuroscience 3. Simulating the brain 4.
Neuromorphic computing Robotics M.Sc. programme in Computer Science lorenzo.vannucci@santannapisa.it April 19th, 2018 Outline 1. Introduction 2. Fundamentals of neuroscience 3. Simulating the brain 4.
Evaluating the Effect of Spiking Network Parameters on Polychronization
 Evaluating the Effect of Spiking Network Parameters on Polychronization Panagiotis Ioannou, Matthew Casey and André Grüning Department of Computing, University of Surrey, Guildford, Surrey, GU2 7XH, UK
Evaluating the Effect of Spiking Network Parameters on Polychronization Panagiotis Ioannou, Matthew Casey and André Grüning Department of Computing, University of Surrey, Guildford, Surrey, GU2 7XH, UK
Basics of Computational Neuroscience: Neurons and Synapses to Networks
 Basics of Computational Neuroscience: Neurons and Synapses to Networks Bruce Graham Mathematics School of Natural Sciences University of Stirling Scotland, U.K. Useful Book Authors: David Sterratt, Bruce
Basics of Computational Neuroscience: Neurons and Synapses to Networks Bruce Graham Mathematics School of Natural Sciences University of Stirling Scotland, U.K. Useful Book Authors: David Sterratt, Bruce
Introduction to Computational Neuroscience
 Introduction to Computational Neuroscience Lecture 6: Single neuron models Lesson Title 1 Introduction 2 Structure and Function of the NS 3 Windows to the Brain 4 Data analysis I 5 Data analysis II 6 Single
Introduction to Computational Neuroscience Lecture 6: Single neuron models Lesson Title 1 Introduction 2 Structure and Function of the NS 3 Windows to the Brain 4 Data analysis I 5 Data analysis II 6 Single
Applied Neuroscience. Conclusion of Science Honors Program Spring 2017
 Applied Neuroscience Conclusion of Science Honors Program Spring 2017 Review Circle whichever is greater, A or B. If A = B, circle both: I. A. permeability of a neuronal membrane to Na + during the rise
Applied Neuroscience Conclusion of Science Honors Program Spring 2017 Review Circle whichever is greater, A or B. If A = B, circle both: I. A. permeability of a neuronal membrane to Na + during the rise
Scaling a slow-wave sleep cortical network model using NEOSIM*
 NEUROCOMPUTING ELSEVIER Neurocomputing 44-46 (2002) 453-458 Scaling a slow-wave sleep cortical network model using NEOSIM* adivision of Informatics, Institute for Adaptive and Neural Computation, University
NEUROCOMPUTING ELSEVIER Neurocomputing 44-46 (2002) 453-458 Scaling a slow-wave sleep cortical network model using NEOSIM* adivision of Informatics, Institute for Adaptive and Neural Computation, University
Ameen Alsaras. Ameen Alsaras. Mohd.Khatatbeh
 9 Ameen Alsaras Ameen Alsaras Mohd.Khatatbeh Nerve Cells (Neurons) *Remember: The neural cell consists of: 1-Cell body 2-Dendrites 3-Axon which ends as axon terminals. The conduction of impulse through
9 Ameen Alsaras Ameen Alsaras Mohd.Khatatbeh Nerve Cells (Neurons) *Remember: The neural cell consists of: 1-Cell body 2-Dendrites 3-Axon which ends as axon terminals. The conduction of impulse through
Investigation of Physiological Mechanism For Linking Field Synapses
 Investigation of Physiological Mechanism For Linking Field Synapses Richard B. Wells 1, Nick Garrett 2, Tom Richner 3 Microelectronics Research and Communications Institute (MRCI) BEL 316 University of
Investigation of Physiological Mechanism For Linking Field Synapses Richard B. Wells 1, Nick Garrett 2, Tom Richner 3 Microelectronics Research and Communications Institute (MRCI) BEL 316 University of
Recognition of English Characters Using Spiking Neural Networks
 Recognition of English Characters Using Spiking Neural Networks Amjad J. Humaidi #1, Thaer M. Kadhim *2 Control and System Engineering, University of Technology, Iraq, Baghdad 1 601116@uotechnology.edu.iq
Recognition of English Characters Using Spiking Neural Networks Amjad J. Humaidi #1, Thaer M. Kadhim *2 Control and System Engineering, University of Technology, Iraq, Baghdad 1 601116@uotechnology.edu.iq
CHAPTER I From Biological to Artificial Neuron Model
 CHAPTER I From Biological to Artificial Neuron Model EE543 - ANN - CHAPTER 1 1 What you see in the picture? EE543 - ANN - CHAPTER 1 2 Is there any conventional computer at present with the capability of
CHAPTER I From Biological to Artificial Neuron Model EE543 - ANN - CHAPTER 1 1 What you see in the picture? EE543 - ANN - CHAPTER 1 2 Is there any conventional computer at present with the capability of
LESSON 3.3 WORKBOOK. Why does applying pressure relieve pain? Workbook. Postsynaptic potentials
 Depolarize to decrease the resting membrane potential. Decreasing membrane potential means that the membrane potential is becoming more positive. Excitatory postsynaptic potentials (EPSP) graded postsynaptic
Depolarize to decrease the resting membrane potential. Decreasing membrane potential means that the membrane potential is becoming more positive. Excitatory postsynaptic potentials (EPSP) graded postsynaptic
Electrophysiology. General Neurophysiology. Action Potentials
 5 Electrophysiology Cochlear implants should aim to reproduce the coding of sound in the auditory system as closely as possible, for best sound perception. The cochlear implant is in part the result of
5 Electrophysiology Cochlear implants should aim to reproduce the coding of sound in the auditory system as closely as possible, for best sound perception. The cochlear implant is in part the result of
PSY 215 Lecture 3 (1/19/2011) (Synapses & Neurotransmitters) Dr. Achtman PSY 215
 Corrections: None needed. PSY 215 Lecture 3 Topic: Synapses & Neurotransmitters Chapters 2 & 3, pages 40-57 Lecture Notes: SYNAPSES & NEUROTRANSMITTERS, CHAPTER 3 Action Potential (above diagram found
Corrections: None needed. PSY 215 Lecture 3 Topic: Synapses & Neurotransmitters Chapters 2 & 3, pages 40-57 Lecture Notes: SYNAPSES & NEUROTRANSMITTERS, CHAPTER 3 Action Potential (above diagram found
Intro. Comp. NeuroSci. Ch. 9 October 4, The threshold and channel memory
 9.7.4 The threshold and channel memory The action potential has a threshold. In figure the area around threshold is expanded (rectangle). A current injection that does not reach the threshold does not
9.7.4 The threshold and channel memory The action potential has a threshold. In figure the area around threshold is expanded (rectangle). A current injection that does not reach the threshold does not
Omar Sami. Muhammad Abid. Muhammad khatatbeh
 10 Omar Sami Muhammad Abid Muhammad khatatbeh Let s shock the world In this lecture we are going to cover topics said in previous lectures and then start with the nerve cells (neurons) and the synapses
10 Omar Sami Muhammad Abid Muhammad khatatbeh Let s shock the world In this lecture we are going to cover topics said in previous lectures and then start with the nerve cells (neurons) and the synapses
LESSON 3.3 WORKBOOK. Why does applying pressure relieve pain?
 Postsynaptic potentials small changes in voltage (membrane potential) due to the binding of neurotransmitter. Receptor-gated ion channels ion channels that open or close in response to the binding of a
Postsynaptic potentials small changes in voltage (membrane potential) due to the binding of neurotransmitter. Receptor-gated ion channels ion channels that open or close in response to the binding of a
Neurons: Structure and communication
 Neurons: Structure and communication http://faculty.washington.edu/chudler/gall1.html Common Components of a Neuron Dendrites Input, receives neurotransmitters Soma Processing, decision Axon Transmits
Neurons: Structure and communication http://faculty.washington.edu/chudler/gall1.html Common Components of a Neuron Dendrites Input, receives neurotransmitters Soma Processing, decision Axon Transmits
Computational cognitive neuroscience: 2. Neuron. Lubica Beňušková Centre for Cognitive Science, FMFI Comenius University in Bratislava
 1 Computational cognitive neuroscience: 2. Neuron Lubica Beňušková Centre for Cognitive Science, FMFI Comenius University in Bratislava 2 Neurons communicate via electric signals In neurons it is important
1 Computational cognitive neuroscience: 2. Neuron Lubica Beňušková Centre for Cognitive Science, FMFI Comenius University in Bratislava 2 Neurons communicate via electric signals In neurons it is important
International Journal of Advanced Computer Technology (IJACT)
 Abstract An Introduction to Third Generation of Neural Networks for Edge Detection Being inspired by the structure and behavior of the human visual system the spiking neural networks for edge detection
Abstract An Introduction to Third Generation of Neural Networks for Edge Detection Being inspired by the structure and behavior of the human visual system the spiking neural networks for edge detection
Dynamics of Hodgkin and Huxley Model with Conductance based Synaptic Input
 Proceedings of International Joint Conference on Neural Networks, Dallas, Texas, USA, August 4-9, 2013 Dynamics of Hodgkin and Huxley Model with Conductance based Synaptic Input Priyanka Bajaj and Akhil
Proceedings of International Joint Conference on Neural Networks, Dallas, Texas, USA, August 4-9, 2013 Dynamics of Hodgkin and Huxley Model with Conductance based Synaptic Input Priyanka Bajaj and Akhil
AP Biology Unit 6. The Nervous System
 AP Biology Unit 6 The Nervous System Branches of the Nervous System There are 2 main branches of the nervous system Central Nervous System Brain Spinal Cord Peripheral Nervous System All nerves leading
AP Biology Unit 6 The Nervous System Branches of the Nervous System There are 2 main branches of the nervous system Central Nervous System Brain Spinal Cord Peripheral Nervous System All nerves leading
What is Anatomy and Physiology?
 Introduction BI 212 BI 213 BI 211 Ecosystems Organs / organ systems Cells Organelles Communities Tissues Molecules Populations Organisms Campbell et al. Figure 1.4 Introduction What is Anatomy and Physiology?
Introduction BI 212 BI 213 BI 211 Ecosystems Organs / organ systems Cells Organelles Communities Tissues Molecules Populations Organisms Campbell et al. Figure 1.4 Introduction What is Anatomy and Physiology?
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM
 STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
Thursday, January 22, Nerve impulse
 Nerve impulse Transmembrane Potential caused by ions moving through cell membrane at different rates Two main ions of concern Na + - Sodium K + - potassium Cell membrane not freely permeable therefore
Nerve impulse Transmembrane Potential caused by ions moving through cell membrane at different rates Two main ions of concern Na + - Sodium K + - potassium Cell membrane not freely permeable therefore
Introduction to Neurobiology
 Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
Neurons, Synapses, and Signaling
 Neurons, Synapses, and Signaling The Neuron is the functional unit of the nervous system. Neurons are composed of a cell body, which contains the nucleus and organelles; Dendrites which are extensions
Neurons, Synapses, and Signaling The Neuron is the functional unit of the nervous system. Neurons are composed of a cell body, which contains the nucleus and organelles; Dendrites which are extensions
Chapter 7 Nerve Cells and Electrical Signaling
 Chapter 7 Nerve Cells and Electrical Signaling 7.1. Overview of the Nervous System (Figure 7.1) 7.2. Cells of the Nervous System o Neurons are excitable cells which can generate action potentials o 90%
Chapter 7 Nerve Cells and Electrical Signaling 7.1. Overview of the Nervous System (Figure 7.1) 7.2. Cells of the Nervous System o Neurons are excitable cells which can generate action potentials o 90%
Scaling a slow-wave sleep cortical network model using NEOSIM
 Neurocomputing 44 46 (2002) 453 458 www.elsevier.com/locate/neucom Scaling a slow-wave sleep cortical network model using NEOSIM F. Howell a;, M. Bazhenov b, P. Rogister a, T. Sejnowski b, N. Goddard a
Neurocomputing 44 46 (2002) 453 458 www.elsevier.com/locate/neucom Scaling a slow-wave sleep cortical network model using NEOSIM F. Howell a;, M. Bazhenov b, P. Rogister a, T. Sejnowski b, N. Goddard a
Chapter 2: Cellular Mechanisms and Cognition
 Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Na + K + pump. The beauty of the Na + K + pump. Cotransport. The setup Cotransport the result. Found along the plasma membrane of all cells.
 The beauty of the Na + K + pump Na + K + pump Found along the plasma membrane of all cells. Establishes gradients, controls osmotic effects, allows for cotransport Nerve cells have a Na + K + pump and
The beauty of the Na + K + pump Na + K + pump Found along the plasma membrane of all cells. Establishes gradients, controls osmotic effects, allows for cotransport Nerve cells have a Na + K + pump and
Mike Davies Director, Neuromorphic Computing Lab Intel Labs
 Mike Davies Director, Neuromorphic Computing Lab Intel Labs Loihi at a Glance Key Properties Integrated Memory + Compute Neuromorphic Architecture 128 neuromorphic cores supporting up to 128k neurons and
Mike Davies Director, Neuromorphic Computing Lab Intel Labs Loihi at a Glance Key Properties Integrated Memory + Compute Neuromorphic Architecture 128 neuromorphic cores supporting up to 128k neurons and
Temporally asymmetric Hebbian learning and neuronal response variability
 Neurocomputing 32}33 (2000) 523}528 Temporally asymmetric Hebbian learning and neuronal response variability Sen Song*, L.F. Abbott Volen Center for Complex Systems and Department of Biology, Brandeis
Neurocomputing 32}33 (2000) 523}528 Temporally asymmetric Hebbian learning and neuronal response variability Sen Song*, L.F. Abbott Volen Center for Complex Systems and Department of Biology, Brandeis
Chapter 4 Neuronal Physiology
 Chapter 4 Neuronal Physiology V edit. Pg. 99-131 VI edit. Pg. 85-113 VII edit. Pg. 87-113 Input Zone Dendrites and Cell body Nucleus Trigger Zone Axon hillock Conducting Zone Axon (may be from 1mm to more
Chapter 4 Neuronal Physiology V edit. Pg. 99-131 VI edit. Pg. 85-113 VII edit. Pg. 87-113 Input Zone Dendrites and Cell body Nucleus Trigger Zone Axon hillock Conducting Zone Axon (may be from 1mm to more
Neurobiology: The nerve cell. Principle and task To use a nerve function model to study the following aspects of a nerve cell:
 Principle and task To use a nerve function model to study the following aspects of a nerve cell: INTRACELLULAR POTENTIAL AND ACTION POTENTIAL Comparison between low and high threshold levels Comparison
Principle and task To use a nerve function model to study the following aspects of a nerve cell: INTRACELLULAR POTENTIAL AND ACTION POTENTIAL Comparison between low and high threshold levels Comparison
Rolls,E.T. (2016) Cerebral Cortex: Principles of Operation. Oxford University Press.
 Digital Signal Processing and the Brain Is the brain a digital signal processor? Digital vs continuous signals Digital signals involve streams of binary encoded numbers The brain uses digital, all or none,
Digital Signal Processing and the Brain Is the brain a digital signal processor? Digital vs continuous signals Digital signals involve streams of binary encoded numbers The brain uses digital, all or none,
Chapter 3 Neurotransmitter release
 NEUROPHYSIOLOGIE CELLULAIRE CONSTANCE HAMMOND Chapter 3 Neurotransmitter release In chapter 3, we proose 3 videos: Observation Calcium Channel, Ca 2+ Unitary and Total Currents Ca 2+ and Neurotransmitter
NEUROPHYSIOLOGIE CELLULAIRE CONSTANCE HAMMOND Chapter 3 Neurotransmitter release In chapter 3, we proose 3 videos: Observation Calcium Channel, Ca 2+ Unitary and Total Currents Ca 2+ and Neurotransmitter
The Nervous System. Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output
 The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
35-2 The Nervous System Slide 1 of 38
 1 of 38 35-2 The Nervous System The nervous system controls and coordinates functions throughout the body and responds to internal and external stimuli. 2 of 38 Neurons Neurons The messages carried by
1 of 38 35-2 The Nervous System The nervous system controls and coordinates functions throughout the body and responds to internal and external stimuli. 2 of 38 Neurons Neurons The messages carried by
Spiking neural network simulator: User s Guide
 Spiking neural network simulator: User s Guide Version 0.55: December 7 2004 Leslie S. Smith, Department of Computing Science and Mathematics University of Stirling, Stirling FK9 4LA, Scotland lss@cs.stir.ac.uk
Spiking neural network simulator: User s Guide Version 0.55: December 7 2004 Leslie S. Smith, Department of Computing Science and Mathematics University of Stirling, Stirling FK9 4LA, Scotland lss@cs.stir.ac.uk
Branches of the Nervous System
 The Nervous System Branches of the Nervous System There are 2 main branches of the nervous system Central Nervous System Brain Spinal Cord Peripheral Nervous System All nerves leading to rest of body Anatomy
The Nervous System Branches of the Nervous System There are 2 main branches of the nervous system Central Nervous System Brain Spinal Cord Peripheral Nervous System All nerves leading to rest of body Anatomy
Neurons. Pyramidal neurons in mouse cerebral cortex expressing green fluorescent protein. The red staining indicates GABAergic interneurons.
 Neurons Pyramidal neurons in mouse cerebral cortex expressing green fluorescent protein. The red staining indicates GABAergic interneurons. MBL, Woods Hole R Cheung MSc Bioelectronics: PGEE11106 1 Neuron
Neurons Pyramidal neurons in mouse cerebral cortex expressing green fluorescent protein. The red staining indicates GABAergic interneurons. MBL, Woods Hole R Cheung MSc Bioelectronics: PGEE11106 1 Neuron
Cognitive Neuroscience History of Neural Networks in Artificial Intelligence The concept of neural network in artificial intelligence
 Cognitive Neuroscience History of Neural Networks in Artificial Intelligence The concept of neural network in artificial intelligence To understand the network paradigm also requires examining the history
Cognitive Neuroscience History of Neural Networks in Artificial Intelligence The concept of neural network in artificial intelligence To understand the network paradigm also requires examining the history
3) Most of the organelles in a neuron are located in the A) dendritic region. B) axon hillock. C) axon. D) cell body. E) axon terminals.
 Chapter 48 Neurons, Synapses, and Signaling Multiple-Choice Questions 1) A simple nervous system A) must include chemical senses, mechanoreception, and vision. B) includes a minimum of 12 ganglia. C) has
Chapter 48 Neurons, Synapses, and Signaling Multiple-Choice Questions 1) A simple nervous system A) must include chemical senses, mechanoreception, and vision. B) includes a minimum of 12 ganglia. C) has
1. What are the two basic types of cells in the nervous system? Neurons and Glial Cells
 Biological Psychology Basic Structure of a Neuron 1. What are the two basic types of cells in the nervous system? Neurons and Glial Cells a. Cells that process incoming signals and respond by sending out
Biological Psychology Basic Structure of a Neuron 1. What are the two basic types of cells in the nervous system? Neurons and Glial Cells a. Cells that process incoming signals and respond by sending out
THE EFFECT OF EXCITATION CURRENT ON REFRACTORY PERIOD OF ACTION POTENTIAL AND ITS SIMULATION BY MATLAB SOFTWARE
 THE EFFECT OF EXCITATION CURRENT ON REFRACTORY PERIOD OF ACTION POTENTIAL AND ITS SIMULATION BY MATLAB SOFTWARE Mostafa Mohammadi Department of Electrical Engineering Islamic Azad University of Central
THE EFFECT OF EXCITATION CURRENT ON REFRACTORY PERIOD OF ACTION POTENTIAL AND ITS SIMULATION BY MATLAB SOFTWARE Mostafa Mohammadi Department of Electrical Engineering Islamic Azad University of Central
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February ISSN THINKING CIRCUIT
 International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 1 THINKING CIRCUIT Mr.Mukesh Raju Bangar Intern at Govt. Dental College and hospital, Nagpur Email: Mukeshbangar008@gmail.com
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 1 THINKING CIRCUIT Mr.Mukesh Raju Bangar Intern at Govt. Dental College and hospital, Nagpur Email: Mukeshbangar008@gmail.com
Introduction to Computational Neuroscience
 Introduction to Computational Neuroscience Lecture 7: Network models Lesson Title 1 Introduction 2 Structure and Function of the NS 3 Windows to the Brain 4 Data analysis 5 Data analysis II 6 Single neuron
Introduction to Computational Neuroscience Lecture 7: Network models Lesson Title 1 Introduction 2 Structure and Function of the NS 3 Windows to the Brain 4 Data analysis 5 Data analysis II 6 Single neuron
Heterogeneous networks of spiking neurons: self-sustained activity and excitability
 Heterogeneous networks of spiking neurons: self-sustained activity and excitability Cristina Savin 1,2, Iosif Ignat 1, Raul C. Mureşan 2,3 1 Technical University of Cluj Napoca, Faculty of Automation and
Heterogeneous networks of spiking neurons: self-sustained activity and excitability Cristina Savin 1,2, Iosif Ignat 1, Raul C. Mureşan 2,3 1 Technical University of Cluj Napoca, Faculty of Automation and
9/28/2016. Neuron. Multipolar Neuron. Astrocytes Exchange Materials With Neurons. Glia or Glial Cells ( supporting cells of the nervous system)
 Neuron Multipolar Neuron https://www.youtube.com/watch?v=lw-psbnu5xago to :38 Glia or Glial Cells ( supporting cells of the nervous system) 10X more numerous than neurons but one-tenth the size make up
Neuron Multipolar Neuron https://www.youtube.com/watch?v=lw-psbnu5xago to :38 Glia or Glial Cells ( supporting cells of the nervous system) 10X more numerous than neurons but one-tenth the size make up
ANATOMY AND PHYSIOLOGY OF NEURONS. AP Biology Chapter 48
 ANATOMY AND PHYSIOLOGY OF NEURONS AP Biology Chapter 48 Objectives Describe the different types of neurons Describe the structure and function of dendrites, axons, a synapse, types of ion channels, and
ANATOMY AND PHYSIOLOGY OF NEURONS AP Biology Chapter 48 Objectives Describe the different types of neurons Describe the structure and function of dendrites, axons, a synapse, types of ion channels, and
CAS Seminar - Spiking Neurons Network (SNN) Jakob Kemi ( )
 CAS Seminar - Spiking Neurons Network (SNN) Jakob Kemi (820622-0033) kemiolof@student.chalmers.se November 20, 2006 Introduction Biological background To be written, lots of good sources. Background First
CAS Seminar - Spiking Neurons Network (SNN) Jakob Kemi (820622-0033) kemiolof@student.chalmers.se November 20, 2006 Introduction Biological background To be written, lots of good sources. Background First
Information Processing During Transient Responses in the Crayfish Visual System
 Information Processing During Transient Responses in the Crayfish Visual System Christopher J. Rozell, Don. H. Johnson and Raymon M. Glantz Department of Electrical & Computer Engineering Department of
Information Processing During Transient Responses in the Crayfish Visual System Christopher J. Rozell, Don. H. Johnson and Raymon M. Glantz Department of Electrical & Computer Engineering Department of
Portions from Chapter 6 CHAPTER 7. The Nervous System: Neurons and Synapses. Chapter 7 Outline. and Supporting Cells
 CHAPTER 7 The Nervous System: Neurons and Synapses Chapter 7 Outline Neurons and Supporting Cells Activity in Axons The Synapse Acetylcholine as a Neurotransmitter Monoamines as Neurotransmitters Other
CHAPTER 7 The Nervous System: Neurons and Synapses Chapter 7 Outline Neurons and Supporting Cells Activity in Axons The Synapse Acetylcholine as a Neurotransmitter Monoamines as Neurotransmitters Other
DIGITIZING HUMAN BRAIN: BLUE BRAIN PROJECT
 DIGITIZING HUMAN BRAIN: BLUE BRAIN PROJECT Diwijesh 1, Ms. Pooja Khanna 2 1 M.tech CSE, Amity University 2 Associate Professor, ASET ABSTRACT Human brain is most complex and unique creation of nature which
DIGITIZING HUMAN BRAIN: BLUE BRAIN PROJECT Diwijesh 1, Ms. Pooja Khanna 2 1 M.tech CSE, Amity University 2 Associate Professor, ASET ABSTRACT Human brain is most complex and unique creation of nature which
Physiology of the nerve
 Physiology of the nerve Objectives Transmembrane potential Action potential Relative and absolute refractory period The all-or-none law Hoorweg Weiss curve Du Bois Reymond principle Types of nerve fibres
Physiology of the nerve Objectives Transmembrane potential Action potential Relative and absolute refractory period The all-or-none law Hoorweg Weiss curve Du Bois Reymond principle Types of nerve fibres
6.5 Nerves, Hormones and Homeostasis
 6.5 Nerves, Hormones and Homeostasis IB Biology SL Part 1 - Nerves Outcomes Part 1 6.5.1State that the nervous system consists of the central nervous system (CNS) and peripheral nerves, and is composed
6.5 Nerves, Hormones and Homeostasis IB Biology SL Part 1 - Nerves Outcomes Part 1 6.5.1State that the nervous system consists of the central nervous system (CNS) and peripheral nerves, and is composed
THE HISTORY OF NEUROSCIENCE
 1. Historically, how have neuroscientists determined the function of various brain regions? 2. Describe the impact of the Phineas Gage case on the field of neuroscience. 3. Explain neuron theory. THE HISTORY
1. Historically, how have neuroscientists determined the function of various brain regions? 2. Describe the impact of the Phineas Gage case on the field of neuroscience. 3. Explain neuron theory. THE HISTORY
Period: Date: Module 28: Nervous System, Student Learning Guide
 Name: Period: Date: Module 28: Nervous System, Student Learning Guide Instructions: Work in pairs (share a computer). Make sure that you log in for the first quiz so that you get credit. Go to www.sciencemusicvideos.com.
Name: Period: Date: Module 28: Nervous System, Student Learning Guide Instructions: Work in pairs (share a computer). Make sure that you log in for the first quiz so that you get credit. Go to www.sciencemusicvideos.com.
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
BIOLOGICAL PROCESSES
 BIOLOGICAL PROCESSES CHAPTER 3 1 LEARNING GOALS Discuss how the nervous system communicates internally. Describe the structure and function of neurons Describe how the neuron transmits information Describe
BIOLOGICAL PROCESSES CHAPTER 3 1 LEARNING GOALS Discuss how the nervous system communicates internally. Describe the structure and function of neurons Describe how the neuron transmits information Describe
Signal detection in networks of spiking neurons with dynamical synapses
 Published in AIP Proceedings 887, 83-88, 7. Signal detection in networks of spiking neurons with dynamical synapses Jorge F. Mejías and Joaquín J. Torres Dept. of Electromagnetism and Physics of the Matter
Published in AIP Proceedings 887, 83-88, 7. Signal detection in networks of spiking neurons with dynamical synapses Jorge F. Mejías and Joaquín J. Torres Dept. of Electromagnetism and Physics of the Matter
Synaptic Transmission: Ionic and Metabotropic
 Synaptic Transmission: Ionic and Metabotropic D. Purves et al. Neuroscience (Sinauer Assoc.) Chapters 5, 6, 7. C. Koch. Biophysics of Computation (Oxford) Chapter 4. J.G. Nicholls et al. From Neuron to
Synaptic Transmission: Ionic and Metabotropic D. Purves et al. Neuroscience (Sinauer Assoc.) Chapters 5, 6, 7. C. Koch. Biophysics of Computation (Oxford) Chapter 4. J.G. Nicholls et al. From Neuron to
Shock-induced termination of cardiac arrhythmias
 Shock-induced termination of cardiac arrhythmias Group members: Baltazar Chavez-Diaz, Chen Jiang, Sarah Schwenck, Weide Wang, and Jinglei Zhang Cardiac arrhythmias, also known as irregular heartbeat, occur
Shock-induced termination of cardiac arrhythmias Group members: Baltazar Chavez-Diaz, Chen Jiang, Sarah Schwenck, Weide Wang, and Jinglei Zhang Cardiac arrhythmias, also known as irregular heartbeat, occur
THE HISTORY OF NEUROSCIENCE
 THE HISTORY OF NEUROSCIENCE BIOLOGICAL ASPECTS OF BEHAVIOR: THE NEURON & NEURAL COMMUNICATION NERVOUS SYSTEM Combined activity of the brain, spinal cord & other nerve fibers Acts as an information processing
THE HISTORY OF NEUROSCIENCE BIOLOGICAL ASPECTS OF BEHAVIOR: THE NEURON & NEURAL COMMUNICATION NERVOUS SYSTEM Combined activity of the brain, spinal cord & other nerve fibers Acts as an information processing
A general error-based spike-timing dependent learning rule for the Neural Engineering Framework
 A general error-based spike-timing dependent learning rule for the Neural Engineering Framework Trevor Bekolay Monday, May 17, 2010 Abstract Previous attempts at integrating spike-timing dependent plasticity
A general error-based spike-timing dependent learning rule for the Neural Engineering Framework Trevor Bekolay Monday, May 17, 2010 Abstract Previous attempts at integrating spike-timing dependent plasticity
Electrical Properties of Neurons. Steven McLoon Department of Neuroscience University of Minnesota
 Electrical Properties of Neurons Steven McLoon Department of Neuroscience University of Minnesota 1 Neuronal Communication Neurons communicate with other cells, often over long distances. The electrical
Electrical Properties of Neurons Steven McLoon Department of Neuroscience University of Minnesota 1 Neuronal Communication Neurons communicate with other cells, often over long distances. The electrical
MOLECULAR AND CELLULAR NEUROSCIENCE
 MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
A hardware implementation of a network of functional spiking neurons with hebbian learning
 A hardware implementation of a network of functional spiking neurons with hebbian learning Andrés Upegui, Carlos Andrés Peña-Reyes, Eduardo Sánchez Swiss Federal Institute of Technology, Logic Systems
A hardware implementation of a network of functional spiking neurons with hebbian learning Andrés Upegui, Carlos Andrés Peña-Reyes, Eduardo Sánchez Swiss Federal Institute of Technology, Logic Systems
-Ensherah Mokheemer. -Amani Nofal. -Loai Alzghoul
 -1 -Ensherah Mokheemer -Amani Nofal -Loai Alzghoul 1 P a g e Today we will start talking about the physiology of the nervous system and we will mainly focus on the Central Nervous System. Introduction:
-1 -Ensherah Mokheemer -Amani Nofal -Loai Alzghoul 1 P a g e Today we will start talking about the physiology of the nervous system and we will mainly focus on the Central Nervous System. Introduction:
Lab 4: Compartmental Model of Binaural Coincidence Detector Neurons
 Lab 4: Compartmental Model of Binaural Coincidence Detector Neurons Introduction The purpose of this laboratory exercise is to give you hands-on experience with a compartmental model of a neuron. Compartmental
Lab 4: Compartmental Model of Binaural Coincidence Detector Neurons Introduction The purpose of this laboratory exercise is to give you hands-on experience with a compartmental model of a neuron. Compartmental
EE 791 Lecture 2 Jan 19, 2015
 EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
Neurophysiology scripts. Slide 2
 Neurophysiology scripts Slide 2 Nervous system and Endocrine system both maintain homeostasis in the body. Nervous system by nerve impulse and Endocrine system by hormones. Since the nerve impulse is an
Neurophysiology scripts Slide 2 Nervous system and Endocrine system both maintain homeostasis in the body. Nervous system by nerve impulse and Endocrine system by hormones. Since the nerve impulse is an
Active Control of Spike-Timing Dependent Synaptic Plasticity in an Electrosensory System
 Active Control of Spike-Timing Dependent Synaptic Plasticity in an Electrosensory System Patrick D. Roberts and Curtis C. Bell Neurological Sciences Institute, OHSU 505 N.W. 185 th Avenue, Beaverton, OR
Active Control of Spike-Timing Dependent Synaptic Plasticity in an Electrosensory System Patrick D. Roberts and Curtis C. Bell Neurological Sciences Institute, OHSU 505 N.W. 185 th Avenue, Beaverton, OR
Chapter 1. Introduction
 Chapter 1 Introduction Artificial neural networks are mathematical inventions inspired by observations made in the study of biological systems, though loosely based on the actual biology. An artificial
Chapter 1 Introduction Artificial neural networks are mathematical inventions inspired by observations made in the study of biological systems, though loosely based on the actual biology. An artificial
Anatomy Review. Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (
 Anatomy Review Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Neurons communicate with other cells at junctions
Anatomy Review Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Neurons communicate with other cells at junctions
Running PyNN Simulations on SpiNNaker
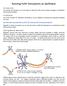 Introduction Running PyNN Simulations on SpiNNaker This manual will introduce you to the basics of using the PyNN neural network language on SpiNNaker neuromorphic hardware. Installation The PyNN toolchain
Introduction Running PyNN Simulations on SpiNNaker This manual will introduce you to the basics of using the PyNN neural network language on SpiNNaker neuromorphic hardware. Installation The PyNN toolchain
A HMM-based Pre-training Approach for Sequential Data
 A HMM-based Pre-training Approach for Sequential Data Luca Pasa 1, Alberto Testolin 2, Alessandro Sperduti 1 1- Department of Mathematics 2- Department of Developmental Psychology and Socialisation University
A HMM-based Pre-training Approach for Sequential Data Luca Pasa 1, Alberto Testolin 2, Alessandro Sperduti 1 1- Department of Mathematics 2- Department of Developmental Psychology and Socialisation University
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed.,
 Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by B.-W. Ku,
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by B.-W. Ku,
Hierarchical dynamical models of motor function
 ARTICLE IN PRESS Neurocomputing 70 (7) 975 990 www.elsevier.com/locate/neucom Hierarchical dynamical models of motor function S.M. Stringer, E.T. Rolls Department of Experimental Psychology, Centre for
ARTICLE IN PRESS Neurocomputing 70 (7) 975 990 www.elsevier.com/locate/neucom Hierarchical dynamical models of motor function S.M. Stringer, E.T. Rolls Department of Experimental Psychology, Centre for
Rule-based firing for network simulations
 Neurocomputing 69 (2006) 1160 1164 www.elsevier.com/locate/neucom Rule-based firing for network simulations William W. Lytton, Mark Stewart Departments of Physiology, Pharmacology & Neurology, SUNY Downstate,
Neurocomputing 69 (2006) 1160 1164 www.elsevier.com/locate/neucom Rule-based firing for network simulations William W. Lytton, Mark Stewart Departments of Physiology, Pharmacology & Neurology, SUNY Downstate,
Realization of Visual Representation Task on a Humanoid Robot
 Istanbul Technical University, Robot Intelligence Course Realization of Visual Representation Task on a Humanoid Robot Emeç Erçelik May 31, 2016 1 Introduction It is thought that human brain uses a distributed
Istanbul Technical University, Robot Intelligence Course Realization of Visual Representation Task on a Humanoid Robot Emeç Erçelik May 31, 2016 1 Introduction It is thought that human brain uses a distributed
Simulation of myelinated neuron with focus on conduction speed and changeable excitability
 Simulation of myelinated neuron with focus on conduction speed and changeable excitability Pengfei Chen Sung Min Kim Abstract In this paper we focus on the two particular properties of myelinated neuron,
Simulation of myelinated neuron with focus on conduction speed and changeable excitability Pengfei Chen Sung Min Kim Abstract In this paper we focus on the two particular properties of myelinated neuron,
Faris Haddad. Dania Alkouz. Mohammad-Khatatbeh
 9 Faris Haddad Dania Alkouz Mohammad-Khatatbeh Revision of previous ideas I. The Action potential stages are mainly controlled by Na+ and K+ channels II. These channels can be either pumps (chemical gated)
9 Faris Haddad Dania Alkouz Mohammad-Khatatbeh Revision of previous ideas I. The Action potential stages are mainly controlled by Na+ and K+ channels II. These channels can be either pumps (chemical gated)
Chapter 3 subtitles Action potentials
 CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
Part 11: Mechanisms of Learning
 Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Lecture 22: A little Neurobiology
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
Nervous System. 2. Receives information from the environment from CNS to organs and glands. 1. Relays messages, processes info, analyzes data
 Nervous System 1. Relays messages, processes info, analyzes data 2. Receives information from the environment from CNS to organs and glands 3. Transmits impulses from CNS to muscles and glands 4. Transmits
Nervous System 1. Relays messages, processes info, analyzes data 2. Receives information from the environment from CNS to organs and glands 3. Transmits impulses from CNS to muscles and glands 4. Transmits
The nervous system is responsible for most of the functions that characterize
 3 E X E R C I S E Neurophysiology of Nerve Impulses O B J E C T I V E S 1. To define the following: irritability, conductivity, resting membrane potential, polarized, sodium-potassium pump, threshold stimulus,
3 E X E R C I S E Neurophysiology of Nerve Impulses O B J E C T I V E S 1. To define the following: irritability, conductivity, resting membrane potential, polarized, sodium-potassium pump, threshold stimulus,
Computing with Spikes in Recurrent Neural Networks
 Computing with Spikes in Recurrent Neural Networks Dezhe Jin Department of Physics The Pennsylvania State University Presented at ICS Seminar Course, Penn State Jan 9, 2006 Outline Introduction Neurons,
Computing with Spikes in Recurrent Neural Networks Dezhe Jin Department of Physics The Pennsylvania State University Presented at ICS Seminar Course, Penn State Jan 9, 2006 Outline Introduction Neurons,
NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3
 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES Neurons communicate with other neurons or target cells at synapses. Chemical synapse: a very narrow
NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES Neurons communicate with other neurons or target cells at synapses. Chemical synapse: a very narrow
VHDL implementation of Neuron based classification for future artificial intelligence applications
 VHDL implementation of Neuron based classification for future artificial intelligence applications 1Reasearch Scholar, Deptof ECEAURORAEngineering College, Hyderabad,India. 2Assoc Prof,Dept of ECE AURORA
VHDL implementation of Neuron based classification for future artificial intelligence applications 1Reasearch Scholar, Deptof ECEAURORAEngineering College, Hyderabad,India. 2Assoc Prof,Dept of ECE AURORA
Input-speci"c adaptation in complex cells through synaptic depression
 0 0 0 0 Neurocomputing }0 (00) } Input-speci"c adaptation in complex cells through synaptic depression Frances S. Chance*, L.F. Abbott Volen Center for Complex Systems and Department of Biology, Brandeis
0 0 0 0 Neurocomputing }0 (00) } Input-speci"c adaptation in complex cells through synaptic depression Frances S. Chance*, L.F. Abbott Volen Center for Complex Systems and Department of Biology, Brandeis
MAC Sleep Mode Control Considering Downlink Traffic Pattern and Mobility
 1 MAC Sleep Mode Control Considering Downlink Traffic Pattern and Mobility Neung-Hyung Lee and Saewoong Bahk School of Electrical Engineering & Computer Science, Seoul National University, Seoul Korea
1 MAC Sleep Mode Control Considering Downlink Traffic Pattern and Mobility Neung-Hyung Lee and Saewoong Bahk School of Electrical Engineering & Computer Science, Seoul National University, Seoul Korea
Chapter 2 The Brain or Bio Psychology
 Chapter 2 The Brain or Bio Psychology 1 2 3 1 Glial Cells Surround neurons and hold them in place Make Myelin (covering for neurons) Manufacture nutrient chemicals neurons need Absorb toxins and waste
Chapter 2 The Brain or Bio Psychology 1 2 3 1 Glial Cells Surround neurons and hold them in place Make Myelin (covering for neurons) Manufacture nutrient chemicals neurons need Absorb toxins and waste
Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons
 Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons Peter Osseward, Uri Magaram Department of Neuroscience University of California, San Diego La Jolla, CA 92092 possewar@ucsd.edu
Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons Peter Osseward, Uri Magaram Department of Neuroscience University of California, San Diego La Jolla, CA 92092 possewar@ucsd.edu
Problem Set 3 - Answers. -70mV TBOA
 Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey HST 131/ Neuro 200 18 September 05 Explanation in text below graphs. Problem
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey HST 131/ Neuro 200 18 September 05 Explanation in text below graphs. Problem
BIOLOGY 2050 LECTURE NOTES ANATOMY & PHYSIOLOGY I (A. IMHOLTZ) FUNDAMENTALS OF THE NERVOUS SYSTEM AND NERVOUS TISSUE P1 OF 5
 P1 OF 5 The nervous system controls/coordinates the activities of cells, tissues, & organs. The endocrine system also plays a role in control/coordination. The nervous system is more dominant. Its mechanisms
P1 OF 5 The nervous system controls/coordinates the activities of cells, tissues, & organs. The endocrine system also plays a role in control/coordination. The nervous system is more dominant. Its mechanisms
100 Million, Billion, Trillion What s the diff? *Shrug*
 Brain Facts 100 billion neurons A typical neuron has about 1,000 to 10,000 synapses (that is, it communicates with 1,000 10,000 other neurons, muscle cells, glands, etc.). 100 trillion synapses Weight
Brain Facts 100 billion neurons A typical neuron has about 1,000 to 10,000 synapses (that is, it communicates with 1,000 10,000 other neurons, muscle cells, glands, etc.). 100 trillion synapses Weight
Chapter 2. The Cellular and Molecular Basis of Cognition
 Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga,, R. B. Ivry,, and G. R. Mangun,, Norton, 2002. Summarized by B.-W. Ku,
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga,, R. B. Ivry,, and G. R. Mangun,, Norton, 2002. Summarized by B.-W. Ku,
