THE EFFECT OF MUTATIONS IN THE MEQ ONCOPROTEIN OF MAREK S DISEASE VIRUS (MDV) ON LYMPHOMAS COMPOSITION. Wachen Peters
|
|
|
- Jordan Patterson
- 5 years ago
- Views:
Transcription
1 THE EFFECT OF MUTATIONS IN THE MEQ ONCOPROTEIN OF MAREK S DISEASE VIRUS (MDV) ON LYMPHOMAS COMPOSITION by Wachen Peters A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for the degree of Master of Science in Biological Sciences Summer Wachen Peters All Rights Reserved
2 UMI Number: All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. UMI Published by ProQuest LLC (2014). Copyright in the Dissertation held by the Author. Microform Edition ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, MI
3 THE EFFECT OF MUTATIONS IN THE MEQ ONCOPROTEIN OF MAREK S DISEASE VIRUS (MDV) ON LYMPHOMAS COMPOSITION by Wachen Peters Approved: Mark S. Parcells, Ph.D. Professor in charge of thesis on behalf of the Advisory Committee Approved: Randall L. Duncan, Ph.D. Chair of the Department of Biological Sciences Approved: George H. Watson, Ph.D. Dean of the College of Arts and Sciences Approved: James G. Richards, Ph.D. Vice Provost for Graduate and Professional Education
4 ACKNOWLEDGMENTS To God Be the Glory! Thank you Jesus! I would like to express my sincere appreciation to my advisor, Dr. Mark Parcells for not only giving me a great project but also most importantly, providing me with an invaluable guidance I will use as my roadmap for the rest of my life. I would also like to thank my committee members, Dr. David Usher and Dr. Deni Galileo for accepting to serve on my committee and also providing me with guidance during my project. A special thanks to Phaedra Tavlarides-Hontz for the tremendous laboratory and personal support in terms of guidance and future advise. To all my past and present lab-mates Upendra Katneni, Juliana Rojas Amortegui, Deb Yannessa, Sabri Nath Neerukonda, Nick Siano and classmates Ann Terrell, Kaitlyn Read, Casey Lumpkins, Amanda, Richard, I will like to say a huge thank you for giving me all the support I needed to make it to the finish line. I will also like to thank the Department of Biological Sciences for giving me this opportunity and Betty Cowgill for all her services and support. My sincere thanks go to my family in Sierra Leone and Nigeria who have devoted everything they can to ensure that I reach my goal. I will like to thank my uncle, Arthur Cummings my role model for believing in me, my loving aunty, Mrs. Junia de-winton Cummings, my mom and step-dad, Mr. & Mrs. C.O.M Palmer, my brother, Mr. Albert Cole, my sisters, Precious Cummings and Wakaygo Cummings, and Hans Lewis. I will also like to thank my Uncle and aunt, Dr. & Mrs Blake for all their love and support. Most importantly, I will like to thank my son, James, for iii
5 allowing me to pursue my goal even though it means being away from him for 7 years. I love you son!! iv
6 TABLE OF CONTENTS LIST OF TABLES... viii LIST OF FIGURES... ix ABSTRACT... xi Chapter 1 INTRODUCTION Marek s Disease Virus MDV Pathogenesis Cytolytic Phase... 5 Latent Phase... 6 Secondary Cytolytic Phase... 7 Transformation... 7 MDV Oncogene The MDV-1 Genome... 3 The Proline-Rich Region (PRR) of Meq Protein Vaccine-induced Immunity to MD MDV Pathotypes Hypothesis MATERIALS AND METHODS Cell Lines MDV Lymphomas Antibodies Cell Treatments Prior to Staining Flow Cytometry Statistical Analysis TCR Spectratype Analysis Proteomic Analysis of Meq-Associated Proteins from MDV Cell Lines v
7 2.9 PCR Cloning of Meq Proteins (Prohibitin and RPL26) Subcloning of Chicken Prohibitin and RPL26 Genes Transfection of HTC Cells with Expression of Vectors RESULTS Macrophage Cell lines Show Positive Surface Antigen Expression Against Monoclonal Antibodies Across Treatments (Fixed, Frozen, Live Cells) Surface Antigen Expression of B Cells Against Monoclonal Antibodies Across Treatments Surface Antigen Expression of T cells Expression of Activation-associated Antigens on MDV-Transformed T Cell Lines Flow Cytometric Analysis of MDV-induced Tumor Cellular Population Analysis of the Activated state of Cells in MD lymphomas Analysis of the Activated Cellular Population of MD Lymphomas via Dual Staining TCR Spectratyping of MDV cell lines and lymphomas Cloning and Subcloning of Genes Associated with Meq Proteins DISCUSSION The Effect of Treatments on Antibody-staining of Cell Lines The Effect of Amino Acid Aubstitution in the PRR of Meq on Cellular Composition of MD Tumors TCR Clonality of MDV-induced Lymphomas Identification of Meq-associated Proteins: Meq Mutations and New Binding Sites REFERENCES Appendix A CO-IMMUNOPRECIPITATION PULL-DOWN OF MEQ-ASSOCIATED PROTEINS FROM MDV-TRANSFORMED CELL LINES; MSB-1, UD35, UA A.1 Meq-Asociated proteins pull-owned from Meq in UA53 only and also UD35(last 2 rows) A.2 Meq-Associated proteins pull-downed from Meq in UD35 only A.3 Meq-Associated Proteins Pull-downed from Meq in MSB-1 Only vi
8 A.4 Meq-Associated proteins pull-downed from Meq in MSB-1 and all cells..79 vii
9 LIST OF TABLES Table 1.1 Amino Acid Sequence Alignment of MDV-1 Meq-coding Sequence. Amino Acid mutations in the Proline-Rich region (position 153,176,217), the retinoblastoma protein binding-pocket (LaChE, position ), and the DNA binding basic region (BR2, position 77) of the Meq protein of the MDV1 pathotypes adapted from (72) Table 1.2 Expression of activation and tumor-associated antigens of RB-1B, rmdv-1137, and rmd5 induced primary lymphoma cells (from (33)).. 19 Table 2.1 ALV-, REV-, MDV-Transformed Cell lines Table 2.2 MDV-Transformed Cell Lines Table 2.3 MDV-induced lymphomas used to determine relationship between cell type and population Table 2.4 Antibodies used for Immunophenotype Analysis, their specificities, and Functional significance Table 2.5 Phases of Flow Cytometric Analysis and Antibodies Used Table 2.6 Primers used to amplify cellular genes identified through interactions with Meq proteins by mass spectrometry Table 3.1 Flow Cytometric Analysis of ALV-A- ALV-J-, and ALV-REVtransformed macrophage cell linesg Table 3.2 Comparison of Surface Antigens expressed on pre-treated ALV- and REV- transformed B CellsC Table 3.3 Comparison of pre-treated MDV-and REV-transformed T cells Surface antigen expressiong Table 3.4 Meq-Associated Proteins Isolated from Immunoprecipitation Pulldown from MDV-transformed Cell lines viii
10 LIST OF FIGURES Figure 1.1 The Genomic Structure of Marek s Disease Virus (MDV) showing the location of genes specific to MDV-1. MDV consists of long and short unique sequences (UL and Us respectively), flanked by long and short internal repeats (IRL and IRs respectively), and long and short inverted repeat terminals (TRL and TRs respectively)...4 Figure 1.2 Relationship of year of virus isolation to virulence. MDV strains associated with distinct epidemiology patterns of the disease in the field. vmdv= strains associated with the explosive increases in MD losses during the 1960s; vvmdv= strains associated with the decreasing efficacy of HVT vaccination in the 1980s; vv+mdv= maybe associated with the decease efficacy of the bivalent, in ovo, CVI988 + trivalent vaccination (adapted from (87)) Figure 1.3 Amino-Acid alignments of Meq coding sequences ( ) from strains rmd5, rmdv-1137, and RB-1B. The Meq encoded by rmdv1137 is identical to RB-1B despite having the genomic background of rmd5 (40).. 18 Figure 3.1 Flow cytometry analysis of MDV-transformed cell lines with selected monoclonal antibodies. (A) CD25, CD30, and CD44 (B) CD86, MHCI, and MHCII (C) CSAT, MATSA, and CD45. a,b,c denotes statistical difference between group (Tukey-Kramer HSD comparison) Figure 3.2 Flow cytometry analysis of MD lymphomas cellular population. Surface antigen represents B-, T-, Macrophage, and Thrombocytes Figure 3.3 Flow Cytometric analysis of the activation state of MD lymphomas cellular populations Figure 3.4 Flow cytometric analysis of the activated population of MD lymphomas ix
11 Figure 3.5 Clonality of MDV-transformed cell lines determined by PCR analysis of TCRβ. (A) 1% agarose gel showing TCRβ Amplicons from PCR (B) ImageJ graphical profile of the Vβ1 and Vβ2 repertoire (respectively) of MDV-transformed cell lines from PCR gel Figure 3.6 Confirmed Amino Acid sequence Alignment of (A) Prohibitin and (B) RPL26 showing and 100% homology respectively using ensemble.org and MeqAlign Figure 3.7 Transfected HTC cells showing localization Prohibitin and RPL26 proteins. HTC cells transfected with 200ng of each protein previously cloned into the ecfp-n1 expression vector having Bright field, DAPI, CFP and YFP filters. Images were acquired using a CCD and analyzed using NIS-Elements software Figure 3.8 Co-transfection of HTC cells with either (A) RPL26-eCFP or (B) prohibitin-ecfp and Meq proteins: CU210 Meq (JM102 isoform), RB1B Meq, and MK (N strain) Meq. Note change in prohibitinlocalization with MK Meq derived from a vv+mdv strain (bottom row) Figure 3.9 Localization of prohibitin in cell co-transfected with Meq isoform expression vectors. Representative Fields are shown for each set of transfections. HTC cells were co-transfected with T7 epitope-tagged Meq expression vectors: (top row) Rispens (CVI988) Meq, small form (339 aa), Rispens (CVI988) Meq, large form (398 aa), vmdv strain 617A Meq, and RB/MK Meq (a composite of RB-1B and MK forms, essentially the MK form with a re-inserted LACHE domain). Consult Table 1.1 for point mutations within the coding sequences of these strains. (Note: some nuclear localization is observed for 617A and RB/MK form) x
12 ABSTRACT Marek s Disease (MD) is a highly transmissible immunosuppressive alphaherpes-virus infection of chickens caused by Marek s disease virus (MDV). MDV infects chickens and can cause immunosuppression, paralysis, and rapid induction of T-cell lymphomas. The exact mechanism of tumor formation is still unknown, but involves the expression of Meq, a basic-leucine zipper (bzip) protein encoded by the virus. Losses due to MD are currently controlled through the near ubiquitous use of vaccines. Despite the success of these vaccines, field strains of MDV have increased in virulence since the early 1970s. A main focus of our laboratory is the molecular basis of the increase in virulence of MDV field strains. We have found that mutations in the proline-rich repeat (PRR) domains of Meq correlate with increased virulence. To determine the effect of these mutations on virulence, we examined the composition (in terms of cell populations) of lymphomas induced by strains RB-1B, rmd5, and rmdv-1137 (a recombinant having the meq gene of RB-1B in the background strain rmd5). We found that RB-1B and rmdv-1137-induced lymphomas had greater numbers of CD30+ CD4+ T-cells, compared to rmd5. As the meq genes of RB-1B and rmd5 have only 3 amino acid changes in the PRR region, our data suggest that mutations in Meq may affect tumor development and progression. xi
13 To extend these results, we used flow cytometry to identify the cellular composition of archived tumor suspension samples and cell lines induced by MDVs of different virulence levels (virulent, very virulent and very virulent plus MDVs). We first validated this technique using defined cell lines that were fixed prior to staining, thawed from frozen storage and stained prior to fixation, or were actively growing at the time of staining. Next, we determined the cellular composition and surface antigen expression of selected MDV induced lymphomas and MDV-transformed cell lines of different pathotypes (vmdv, vvmdv, and vv+mdv) respectively. Our results suggested that MDV induced lymphomas are heterogeneous and changes in tumor composition appear unrelated to virus pathotype. Since we did not see pathotype-associated effects on tumor composition, we performed two additional sets of experiments to test the effect of Meq mutations on: (1) the T-cell receptor (TCR) spectratype of defined MDVtransformed cell lines and tumors induced by different pathotypes MDVs via PCR analysis of the rearranged TCR to determine whether lymphomas induced by vv+mdvs were comprised of polyclonal or monoclonal expansion events; and (2) the interaction of Meq proteins with specific and common cellular proteins detected by proteomic analysis of Meq-co-immunoprecipitations from MDV transformed cell lines MSB-1 (transformed by vmdv strain,bc-1), UD35 (transformed by vvmdv strain, RB-1B) and UA53 (transformed by a vv+mdv strain, TK). xii
14 From our analysis, we have concluded that MDV-mediated transformation is somewhat stochastic, in that there does not seem to be a pathotype-specific difference in lymphoma development as seen by changes in cell populations. We did see differences in the affinity of Meq isoforms for cellular proteins associated with DNA repair (KIN17) and tumor suppression (prohibitin, annexin A2), suggesting that the observed mutations in the Meq coding sequence directly affect the spectrum and affinity of Meq-binding proteins, causing increased cellular proliferation and perhaps a decrease in sensitivity to DNA damage. xiii
15 Chapter 1 INTRODUCTION Marek s disease (MD) is the most common clinical neoplastic condition of any animal, including man, on the globe (71). MD is caused by a cell-associated αherpesvirus called Marek s disease virus (MDV). MD was first described in the early 1900s by a Hungarian veterinary pathologist, Dr. Joszef Marek. He described a polyneuritis of laying hens, a condition of lymphoid infiltration into peripheral nerves resulting in paralysis. Decades later, the course and severity of the disease changed with clinical manifestations from a mild paralytic disease to a highly contagious neoplastic infectious disease in chickens worldwide (59). By the 1960 s, the disease was termed acute Marek s disease or virulent Marek s disease and this described the manifestation of the disease contrary to classical Marek s disease, which described a milder, primarily paralytic form of the disease (99) The clinical signs of MD include transient paralysis, depression, skin leukosis, lymphomas, persistent neurological disease, and death (52, 99). The clinical picture of the disease is increasingly changing, even in vaccinated chickens, and MD remains an economic threat to the poultry industry, due to mortality and the cost of vaccination. Prior to the introduction of vaccines, MD accounted for about 60% of losses in poultry production (10), and these losses totaled about $12 million in the United States in 1984 (72). Even though vaccines have been very successful in reducing the losses due to MD-associated mortality and condemnation, poultry producers must continually 1
16 vaccinate for MD, and immunosuppression, unevenness of vaccine-induced protection, and sporadic field breaks still cause significant economic losses. 1.1 Marek s Disease Virus The causative agent of MD is Marek s Disease Virus (MDV), a double- stranded DNA, cell-associated virus of the family Herpesviridae, subfamily Alphaherpesvirinae. MDV belongs to the Mardivirus genus which has been grouped into 3 distinctly related species: Mardivirus 1 (formerly Gallid herpesvirus 2, GaHV2) also known as MDV serotype 1 (MDV-1), Mardivirus 2 (formerly Gallid herpesvirus 3, GaHV-3) also known as MDV serotype 2 (MDV-2), and Meleagrid herpesvirus-1, or herpesvirus of turkeys (HVT, also known as MDV serotype 3, MDV-3) (54). The term serotype was used because MDVs could be distinguished by monoclonal antibodies to specific antigens, despite the cross-reactivity of antibodies generated in the chicken to many proteins of each of the viruses (42). GaHV-2 (MDV-1) viruses are comprised of the oncogenic MDVs and their attenuated derivatives, and have the largest dsdna genomes (~185 kbp), while nononcogenic strains GaHV-3 (MDV-2) and HVT were isolated from chickens and turkeys respectively, and have smaller genomes (~170 and ~165 kbp, respectively) (62). All MDV strains resemble the alphaherpesviruses in genomic organization and gene content (1). Both MDV-2 and HVT are used as vaccines against Marek s disease (19, 102).In infected cell cultures, icosahedral nucleocapsids nm in diameter and enveloped particles nm in diameter can be seen in the nucleus and very rarely in the cytoplasm of infected cells (82). MDVs are enveloped, highly cell-associated viruses with lymphotropic properties similar to gammaherpesviruses, such as Epstein-Barr virus (EBV), but have 2
17 genomic organization that is similar to the human herpesvirus 1 (HHV-1, a.k.a., herpes simplex virus 1, HSV-1) and human herpesvirus 3 (HHV-3, a.k.a., varicella-zoster virus, VZV). Contrary to other herpesvirus-associated diseases, MDV can be readily studied in its natural host as well as induce T cell lymphomas and visceral tumors within two to three weeks post-infection, making it a valuable model for understanding virus-induce lymphomagenesis and human tumorigenesis (62) The MDV-1 Genome The sequences of all three MDV genomes (GaHV-2, GaHV-3 and HVT) have been determined, confirming that they are linear double-stranded DNA molecules ranging in size kbp (1, 44). The MDV genome has a buoyant density that is very close to that of chicken genomic DNA, making it very difficult to separate these by classic density gradient centrifugation. The genomic structure of all three serotypes consists of unique long (UL) and short (Us) sequences flanked by inverted repeat sequences (TRL, IRL and TRS, IRS) (28) (Figure 1). The UL and US sequences contain most of the structural and replication-associated genes of MDV, which are largely homologous to HSV-1 and VZV (44). Sequences that are unique to the alphaherpesvirus are found at the terminal ends TRL and IRL and between the IRL and the TRS regions. These sequences are believed to be important for cleavage and packaging of the viral DNA into virions. MDV Genes with homologues in alphaherpesvirus include the immediate early genes (ICP4, ICP22, and ICP27) important for transcriptional regulation of virus gene expression, and the late genes that include the nucleocapsid protein, tegument proteins (UL36, UL47, UL48, UL49, etc), and glycoproteins (gb, gc, gd, ge, gh, gi, gl, gk, 3
18 gm and gn) associated with the infection of cells, viral transmission, and immune responses (48). MDV-specific genes are (LATS, pp14, Meq, v-il8, viral lipase vlip, pp38/pp24, and a 1.8 kb gene family) located in the repeats flanking the unique long (RLORFs), the repeats flanking the unique short (RSORFs), the UL (LORFs) and US (SORFs) regions of the MDV-1 genome. Several of these genes are encoded by all three serotypes of MDV (vlip, pp24/pp38, SORFs 2, 3 and 4, etc.). Among the genes unique to MDV-1 strains, however, are clusters within the repeats flanking the UL region of the genome (TRL, IRL). These include pp14, Meq (Marek s EcoRI Q fragment-encoded protein, Meq), RLORF4, and vil-8 (44, 90). Figure 1.1 The Genomic Structure of Marek s Disease Virus (MDV) showing the location of genes specific to MDV-1. MDV consists of long and short unique sequences (UL and Us respectively), flanked by long and short internal repeats (IRL and IRs respectively), and long and short inverted repeat terminals (TRL and TRs respectively). 4
19 1.2 MDV Pathogenesis MDV infection is initiated in the chicken via inhalation of cell-free virus associated with dander from the feather follicle. The current model of pathogenesis predicts that after inhalation, the enveloped virus binds to receptors on cells of the respiratory tract and infects them, although no cellular receptor has been identified and lung epithelial cells do not support MDV infection. Subsequently, phagocytic cells (Bcells, macrophages, dendritic cells) are infected directly via transfer of infection from cells of the respiratory tract (5, 35, 62). The mechanism by which the MDV virus is transmitted to the lymphocytes after host invasion is unknown; however, phagocytic cells have been implicated (34). Macrophages are the main phagocytes that have been implicated in the transmission of MDV, but B-cells have been identified as being directly infected (5). By three to five days post-infection, virus can be readily detected in primary lymphoid tissues where it targets the B-cells and later activated T-cells and rarely, CD4-CD8- T or CD8+ T cells (62). Upon infection of the lymphocytes, four characterized phases are observed: early cytolytic (semi-productive), latent, secondary cytolytic, and transformation. Distinction of these phases varies with the genetic susceptibility of the host, the age at infection, virus strain and challenge dose (21) Cytolytic Phase The early cytolytic phase of infection is characterized by viral replication in B- cells in the bursa of Fabricius and T-cells in the thymus. During this phase, the viral DNA replicates, synthesizes proteins, and produces viral particles. Consequently, the lymphoid organs undergo hypertrophy followed by apoptosis and necrosis of infected cells. Following infection of the primary lymphoid organs, the spleen becomes the 5
20 major site of viral replication (68). The early cytolytic infection peaks within 5 to 7 days and induces a host innate immune response. This immune response inhibits viral replication, presents antigen to resting CD4+ T cells, thereby activating the T cells, and promotes latency (7 to 10 days) in activated CD4+ T-cells (86). In chickens infected at one day of age, early cytolytic infection is somewhat delayed and peaks at days post-infection. One hallmark of the cytolytic phase is the downregulation of major histocompatibility complex class I (MHC I) in infected cells (62). The downregulation of MHC I enables virus-infected cells to evade cytotoxic CD8+ T cells. During the early cytolytic phase, both CD4+ and CD8+ T-cells can be cytolytically infected, and T cells can either support lytic or latent infection (20) Latent Phase Latency defines the presence and maintenance of the MDV genome in a cell without production of infectious progeny virus particles. Latency occurs approximately 7 days post-infection (dpi), in chickens infected at 2 3 weeks old or older (62, 63). In chickens infected at hatch, latency typically occurs dpi (63). Latency is induced by an early immune response of the host during lytic replication, but virus can be rescued via inoculation into chickens or co-cultivation on cells in culture (72). The sequence of events that leads to latency is unknown; however, activated CD4+ are the primary target T-cells for MDV latency (62). Furthermore, immunocompetence may be necessary for the establishment and maintenance of latency and involve the use of both the host and viral factors to regulate repression of the MDV genome (15, 91). The immune suppression during this phase is likely due to 6
21 (a) the loss of lymphocytes by the early cytolytic infection, and (b) the expression of immunosuppressive factors and surface antigens from latently-infected cells (11, 84). Chickens that are genetically resistant to MD overcome this immune suppression, survive the infection, but acquire a lifelong latent infection. However, genetically susceptible chickens maintain a permanent immunosuppression after latent infection develop lymphomas and reactivate virus at peripheral sites (13, 18). Since CD4+ T-cells are the target for latency and transformation, it has been difficult to identify the transition from latency to transformation. Most of the studies done on latency have been focused on antisense transcripts to ICP4, multiply-spliced transcripts in the BamHI-H fragment of the genome, and the meq gene transcripts in the EcoRI Q fragment of the genome (62) Secondary Cytolytic Phase Following latency, a secondary cytolytic phase occurs at peripheral sites. This phase occurs at varying levels in all infected chickens. Secondary cytolytic infection occurs in lymphoid organs, Schwann cells surrounding peripheral nerves, and the feather follicle epithelium (FFE), the only site where cell-free infectious MDV is produced and shed to the environment (22, 52) Transformation During or following latency, primarily activated CD4+ T-cells with T-cell receptors 2 (TCR2) or 3 (TCR3) become transformed and form lymphomas. The transformed component of lymphomas is CD4+ T-cells expressing high levels of CD30, a TNF family receptor, common to Hodgkin s Lymphomas (13) Double negative (CD4-, CD8-) and CD8+ T-cells can also be transformed but appear to be 7
22 less permissive to transformation (81). No chicken-derived B-cell lines or TCR1 (γδ) T-cell lines have ever been established from MDV-induced lymphomas. In addition to CD30 and MHC-II (Ia) surface expression, MD lymphoma-derived cells also express Marek s disease-associated tumor surface antigens (MATSAs) detected on cells from MD-induced lymphomas and lymphoblastoid cells. To date only CD30, a TNFR associated with TH2 polarization is expressed at high levels on MD-transformed CD4+ T-cells has been identified (14, 100). MATSAs are activation-associated antigens, some of which can also be expressed by cells stimulated by mitogen (50). CD30 is a tumor necrotic factor receptor initially identified as a marker of malignant Hodgkin and Reed-Sternberg cells in Hodgkin s lymphoma (3). It regulates proliferation, cell survival, as well as growth inhibition and cell death depending on the cell type and stimuli via NF-κβ, MAPKs, JNK, or p38 signaling pathways. Presently, it is targeted as an immunotherapy agent for Hodgkin s lymphomas. On normal cells in mammals, CD30 expression is restricted to activated T, B, and eosinophil cells, while it is overexpressed on malignant Hodgkin s lymphoma cells. According to Al-Shamkhani, as a survival mechanism, malignant cells expressing CD30 produce cytokines and chemokines that recruit leukocytes expressing CD30L (CD153), binds the CD30, and stimulate growth and survival of the malignant cells (3). Moreover, CD30 can be cleaved from the cell surface by cellular proteases and released into the circulation as a soluble protein where it binds to the CD30 ligand and inhibit cell death. In MDV-induced lymphomas, CD30 hi -expressing cells represent the transformed component, which are typically comprised of proliferative and inflammatory cells (13). Hodgkin s Lymphoma cells may regulate the CD30 promoter 8
23 via transcription factors. The promoter was found to be directly up-regulated by Meq, suggesting that Meq not only induces proliferation and blocks apoptosis in latently infected cells, but also may be involved in T-cell polarization during lymphomagenesis (14). Moreover, CD30 hi -expressing cells in lymphomas resemble T- helper (TH2) or T-regulatory (Treg) cells, as determined by proteomic and gene ontogeny analyses (84). In MDV-induced tumors, CD30 hi -expressing cells typically show low expression of co-stimulatory protein CD28, as an immune evasion mechanism (13). MDV tumors rapidly develop as early as 14 d.p.i (33). The lymphomas are predominantly T cell lymphomas that express neoplastically transformed markers known as MD-associated tumor surface antigen (MATSA) (13). Several techniques have been used to address whether MDV lymphomas develop from single transformation events (monoclonal) or multiple different events (polyclonal). In 1993, Delecluse et al., using fluorescent in situ hybridization (FISH) to analyze MDV genome integration, reported that MDV lymphomas are monoclonal (26). In contrast, Burgess and Davison in 2002 used flow cytometry to analyze CD30 and TCRβ gene family expression in single and multiple lymphomas and the report suggested that MD lymphomas are polyclonal (14). Recent studies on the clonality of MDV lymphomas using either PCR/CDR3 spectratyping or fluorescent in situ hybridization, however, suggested that MDV lymphomas are predominantly but not exclusively monoclonal (56, 77) The exact mechanism of MDV lymphoma formation is not known; however, several studies in humans have shown that autoimmune diseases play a major role in lymphomagenesis, since both are characterized by immune dysregulation (29). These 9
24 studies have shown that patients with autoimmune diseases develop lymphomas either concurrent with or after the emergence of the autoimmune disease (88). The possible mechanism suggests that dysfunction of B- and T-cell regulatory pathways, due to primary immune stimulation (autoimmunity, infection), secondary immune response (viral reactivation, weakened control of infectious agents), followed by a secondary inflammation leads to cellular transformation and subsequently lymphomagenesis (29). In the case of MDV, the initial immune stimulation/damage is caused by the primary cytolytic infection, the transformation events during latency in the CD4+ Tcell and the further secondary inflammation by the secondary cytolytic infection. Thus, MDV may be an example of this immune dysfunction-mediated mechanism of oncogenesis. 1.3 The MDV Oncogene, meq Marek s EcoRI-Q-encoded protein (Meq) is the main MDV oncogene encoding a 339 amino acid, basic leucine-zipper (bzip) protein that has a transactivation domain at its C terminus (36). Meq is expressed in all MDV-induced lymphomas and derived T-cell lines. The bzip domain consists of the basic regions (BR1 and 2) and a leucine zipper that bears homology to cellular proto-oncogenes such as c-jun and c-fos (36). The basic regions are essential for DNA binding and nuclear/nucleolar localization, while the leucine zipper domain is essential for dimerization and interaction with Meq or c-jun or other cellular bzip proteins. The meq gene is encoded approximately 3 4 kbp downstream from the origin of lytic replication (ORI), and is essential for MDV tumor formation, as is its ability to form 10
25 both homo- and heterodimers (47, 89). Deletion of meq attenuates the MDV-1 oncogenicity but not its ability to replicate in vivo. Meq is localized to the nucleus and nucleolus during infection and in transfected cells and binds numerous cell-cycle regulatory and transcriptional regulatory proteins (reviewed recently by Parcells et al., 2012). Meq has the capacity to transactivate and transrepress gene expression depending on its dimerization partners and phosphorylation state (46). The proline-rich region and bzip domains are essential for transformation by Meq. Thus, mutations within these domains may selectively determine Meq s ability to transform MDV infected cells and develop lymphomas The Proline-Rich Region (PRR) of Meq Protein The C-terminus of Meq is comprised of three 21 amino acid proline-rich repeats (PRR) that are associated with transrepression, and a C-terminal 33 amino acid transactivation domain (73). Prolines account for 36.8% of the amino acid residues in the PRR region and 21.5% of the total amino acid residue in the C-terminal domain. PRR s are flexible structures associated with regulation of cell signaling via mediation of protein-protein interaction (49). According to Mwimanzi et al., mutations within the PRR of the Nef protein are associated with immune evasion, viral replication, and pathogenicity of HIV (57). Similarly, Wessels et al., showed that mutations in the PRR reduces the 3A protein s ability to inhibit transport of proteins from the ER to the Golgi thereby enhancing protein secretion (27). The C terminal domain of Meq is similar to the WT-1 tumor suppressor protein that also has a high content of proline and regulates transcription (92). 11
26 Sequence analysis of the meq genes of MDV field strains of different virulence levels identified distinct mutations in the amino acid sequence of the proline-rich region (85). MDV strains of lower virulent (CU-2, BC-1, etc.) and attenuated (CVI988) strains showed amplification of the proline-rich region. The more virulent strains (RB1B, rmd5, N strain, TK, etc.) showed mutations of the second proline in a set of four tandem prolines in the PRR at positions 153, 176, and 217 (85) (Figure 2). The transcriptional activity of Meq can be affected by the diversity and point mutations within the PRR region (23). Substitution of proline-to-alanine at position 217 of the proline region enhanced the transcriptional activity of rmd5-meq (55). In follow-up work, Murata et al., showed that changes in Meq coding sequence directly affected the transcriptional activity of the Meq protein and its ability to dimerize with c-jun (54). Table 1.1 Amino Acid Sequence Alignment of MDV-1 Meq-coding Sequence. Amino Acid mutations in the Proline-Rich region (position 153,176,217), the retinoblastoma protein binding-pocket (LaChE, position ), and the DNA binding basic region (BR2, position 77) of the Meq protein of the MDV1 pathotypes adapted from (85). Virulence Level Strains 71 NoLs LACHE PPPP PPPP PPPP VV+ 660A A K D Q V R Q A A A P A I T VV+ 648A A K D Q V R Q A A A P A I T VV+ U A K D Q V R Q A A A P A I T VV+ MK A K D Q V R Q A A A P A I T VV+ 643P A K D Q V R Q A A A F A I T VV+ CD A K D Q V R Q A A A L A I T VV+ RL A K D Q V R Q A A A L A I T VV+ TK A K D Q V R Q A A A L A I T VV+ NEW A K D Q V R Q A T A L V T T VV+ W A K D Q V C P P T A L V T T VV+ 595 A K D Q V R Q A A A L A I T VV MD5 A K D Q V C P P T A L V T T VV RB1B A K D Q V C P P T P L A I T V GA A K D Q V C P P T P L A I T 326 /383 12
27 V V Vacc vacc 617A CU210 RISP339 RISP A S S S E A E E Y D D D Q R Q Q V A V V R C C C P P P P P P P P T T T T A P P P L L L L A A A A Vaccine-induced Immunity to MD MDVs stimulate both innate and acquired immune responses in chickens (66). Upon MDV infection, the host s innate immune response is immediately elicited so that macrophages and natural killer (NK) cells are activated (Schats et al, 2000). Activated NK cells provide the first line of defense by directly inducing death of MDV-infected cells or through indirectly stimulating activated macrophages to produce nitric oxide (NO) (66, 83). Macrophages play a very important role in MD pathogenesis and immunity. Activated macrophages induce anti-viral responses by releasing nitric oxide and pro-inflammatory cytokines such as IL-1β, IL-6, and IL-18 (83). During MDV infection, pro-inflammatory cytokines such as IFN-γ, upregulate the inducible form of nitric oxide synthase (inos) resulting in enzymatic production of NO and inhibition of viral replication. Macrophages also control the acquired (humoral and cell-mediated) immune response by presenting viral antigens to B and T lymphocytes in the primary lymphoid organs. The host specifically respond to MDV infections via innate defense mechanism immediately whereas the adaptive immune responses emerges by 5 to 7 d.p.i through the secretion of antibodies against MDV proteins and the mounting CD4+ T helper (Th) and CD8+ cytotoxic T-cells (CTL) against virus-infected or tumor cells (38, 66). Even though antibodies have been identified against a wide range of MDV proteins, including the surface glycoproteins, and antibodies play a pivotal role in reducing MD mortality, clinical representation, and tumor formation, humoral 13 I I I I T T I I
28 responses against MDV are not regarded as protective (66). Most importantly, T cellmediated immune responses, particularly CD8+ CTLs, appear to be essential for immunity against the cell-associated MDV (61). MD vaccines are the most successful vaccines against a transmissible neoplastic disease in any species (17). In comparison to other poultry disease vaccines, MD vaccines are more effective, and have been essential in reducing losses due to MD in the poultry industry, worldwide. Most importantly, vaccines have been successful in preventing lymphoma formation, thus, it has been proposed that the vaccines provide anti-tumor more so than anti-viral protection (53). The exact mechanism underlying MD vaccine-mediated immunity is not understood; however, several studies have attempted to propose a possible mechanism. According to Morimura et al., several T cells namely CD8+, γδ T cells and minor lymphocytes namely CD8-CD4-, Natural Killer (NK) cells, and Natural Killer T (NKT) cells may be involved in the anti-viral and anti-tumor effect of vaccines. This model proposed that CD8+ T cells may be essential in eliminating MDVinfected cells (mainly activated CD4+ T cells expressing viral antigens) while γδ T cells, CD8-CD4-, Natural Killer (NK) cells, and Natural Killer T (NKT) cells may be essential in preventing transformation of MDV infected cells and lymphoma formation. Other models propose that the vaccine reduces tumor formation by either limiting early oncogenic virus replication or by limiting the amount of CD4+ cells infected by the virus (31, 58). Despite its valuable impact in the poultry industry, MD vaccines have apparently been an important driver of the evolution of MDV virulence (58). MD vaccines confer non-sterilizing immunity meaning the vaccines prevent disease but not viral infection, replication, and transmission. In addition to non- 14
29 sterilizing immunity, several factors; namely, poultry industry management practices, improper storage or administration of vaccine, the presence of maternal antibodies, the presence of other avian pathogens, and emergence of higher virulent MDV strains affect the efficacy of the vaccine (7, 58). Decrease in the vaccine efficacy enhances MDV to selectively evolve into more virulent pathotypes, evade immunity, and spread from vaccinated to unvaccinated chickens (66). Hence, new vaccine strategies are required to prevent MDV increasing virulence and outbreak. 1.5 MDV Pathotypes Prior to the 1940 s, poultry production was comprised of backyard and low density farming where the number of chickens genetically susceptible to MDV infection mirrored the infected chicken immune response, and MDV infections were controlled with little clinical impact (58). Thus, the strains of MDV isolated in the 1950s have subsequently been characterized as mild (mmdvs) as they caused nerve inflammation and occasional visceral organ lymphomas, but a low frequency of paralysis in infected chickens after extended infection (i.e., > 12 weeks)(9). However, during the late 1950s, poultry production transformed into a large-scale industry. This change to industrial farming introduced an imbalance between the MDV-susceptible chickens and their immune response to natural exposure. This imbalance promoted more serious disease, and the virus evolved into more virulent strains characterized by about 40% mortality in layers, as well as increased lesion frequency in broilers (8). This form of Marek s disease was classified as acute MD based on the increase virulence of the disease. Acute MDV strains (or virulent MDVs, vmdvs) have been identified with increased lymphoma frequency in various visceral organs such as the liver, spleen, gonads, heart, lungs, and kidneys compared to mmdvs. Virulent MDV 15
30 strains also caused lymphomas more rapidly, typically in less than 12 weeks. The vmdv strains caused a high incidence of MD in unvaccinated chickens prior to the introduction of the turkey herpesvirus vaccine (HVT) (97). Subsequently, the preferred method of control for Marek s disease worldwide has been vaccination (101). MD is one of the few herpesvirus diseases controlled by live attenuated vaccines (69).Widespread vaccine use apparently lead to the evolution of very virulent MDVs (vvmdvs) in the early 1980s, and very virulent plus (vv+mdv) in the late 1980s and early 1990s in the US (95, 96). Prior to the identification of the acute MD (vmdv) only lower virulence, classical MDV strains were known. The only surviving isolate from this time is HPRS-41. Representative of the vmdvs are several viral isolates obtained from unvaccinated chickens (CU-2, JM-16, GA, and HPRS-16). The first MD vaccine introduced was an attenuated HPRS-16 serotype 1 MDV (HPRS-16att) followed by a serotype 3, HVT strain vaccine (FC-126) (68). vvmdv are strains that overcame HVT-only vaccination and are typified by ALA-1, Md5, and RB-1B. These strains were all isolated from HVT-vaccinated chickens (96). Over time, field isolates have developed the ability to overcome the protection elicited by the predominant vaccine program used at the time; thereby, increasing the severity of the disease (Figure 1.3). To combat this evolution of virulence a succession of other vaccines has been introduced: a bivalent vaccine from HVT-SB-1 in the mid-1980s, and CVI988 (Rispens) introduced in the US in the early 1990s in the United States (69, 78)Following bivalent vaccine, in ovo vaccine administered to 18- day old embryos was introduced as a labor-saving device and to provide more rapid development of the immune response. vv+mdv overcame both the bivalent and in 16
31 ovo vaccination and strains were isolated from chickens vaccinated with these vaccines. Since the evolution of vv+mdvs (MK, T.King, 645, etc.) in the field, trivalent vaccine use has also been introduced. Presently, different vaccine strategies are employed for different birds: (1) broilers are typically monovalently (HVT only) or bivalent vaccinated (HVT/SB1) in ovo; (2) layers and broiler breeders typically receive CVI988 + HVT; and (3) in areas of high challenge, broilers receive bivalent vaccine in ovo and CVI988, at hatch. CVI988+trivalent vaccination HVT/SB-1 vaccination In ovo vaccination vv+mdvs HVT vaccination High density brooding vvmdvs vmdv 1960s 1970s 1980s 1990s
32 Figure 1.2 Relationship of year of virus isolation to virulence. MDV strains associated with distinct epidemiology patterns of the disease in the field. vmdv= strains associated with the explosive increases in MD losses during the 1960s; vvmdv= strains associated with the decreasing efficacy of HVT vaccination in the 1980s; vv+mdv= maybe associated with the decease efficacy of the bivalent, in ovo, CVI988 + trivalent vaccination (adapted from (98). We recently described a recombinant MDV (rmdv-1137) having the meq gene of the RB-1B strain in the background of the rmd5 strain (40). rmdv-1137 was selected in vivo through the infection of chickens with cells co-transfected with meq loci of different MDV pathotypes (v, vv and vv+) and rmd5 Meq, a non-oncogenic derivative of the rmd5 strain from which both copies of meq had been deleted (47). RB-1B, rmdv-1137 and rmd5 were lethal to chickens and induced lymphomas; however we found significant differences in the cell populations comprising these lymphomas, suggesting that point mutations in the Meq PRR region could affect tumor composition (Figure 1.3 and Table 1). Figure 1.3 Amino-Acid alignments of Meq coding sequences ( ) from strains rmd5, rmdv-1137, and RB-1B. The Meq encoded by rmdv1137 is identical to RB-1B despite having the genomic background of rmd5 (40). 18
33 Table 1.2 Expression of activation and tumor-associated antigens of RB-1B, rmdv-1137, and rmd5 induced primary lymphoma cells, from (40) rmdv-1137-induced rmd5-induced (%) (%) Unstained BU CD CD CD4+CD KUL CD CD MATSA-1 (A35.5) MATSA-2 (Hyb 6) MATSA-3 (143B) A Statistically different from RB1B-induced ( P<0.05) and statistically different from RB1B-induced lymphomas ( P<0.05) Antigen 1.6 RB1B-induced (%) Hypothesis Since Meq-target genes include genes involved in cell signaling, metastasis and recruitment (45),(16), and since mutations are implicated in increasing transcriptional potential (55), we hypothesize that these mutations may directly affect MDV pathogenicity through affecting: (a) tumor composition via differences in cell recruitment and immune polarization, (b) the efficiency of cellular transformation (monoclonal vs polyclonal expansion of transformed cells), and/or (c) through affecting Meq-interacting proteins. To address this hypothesis we have developed three aims employing Marek s disease virus (MDV)-, reticuloendoliosis virus (REV)-, and avian leukosis virus (ALV)-transformed cell lines, archived MDV tumor cells, and proteomic data provided by our collaborators, Drs. Shane Burgess and Fiona McCarthy. These aims are: 19
34 1. Perform flow cytometric analysis of archived MDV-transformed cell lines and tumors to determine possible differences in composition associated with different pathotype MDVs (v, vv and vv+mdv). 2. Perform T-cell receptor spectratyping via PCR analysis of cell lines and tumor samples to determine if high virulence tumors (vv and vv+mdv-derived) represent polyclonal events, suggesting that mutations in Meq have affected the efficiency of MDV-mediated transformation. 3. From proteomics data of Meq Co-immunoprecipitation studies, determine if the mutations in Meq confer novel binding sites for select cellular proteins. 20
35 Chapter 2 MATERIALS AND METHODS 2.1 Cell Lines Lymphoblastoid cell lines (DT40, CU60, CU91, UD35, UD37, UA53, UD32, and UD31) were grown at 41 C, 5% CO2, in modified Iscove s Dulbecco medium IDM supplemented with 20% fetal bovine serum (Atlanta Biologicals), 10% Chicken Serum (Life Technologies, [LT]), 10% tryptose phosphate broth (TPB), 1X Insulin/Transferrin/Selenium (ITS, LT), 1X Non-essential amino acides (NEAA, LT), 4 mm L-glutamine (LT), 2 mm sodium pyruvate (LT), 2 µm 2-mercaptoethanol (LT), 1X PSN antibiotics (LT) and 1X fungizone (LT). To validate all monoclonal antibodies we examined surface antigen expression of chicken ALV-, REV-, and MDV-transformed cell lines (Table 2.1). Meanwhile, to determine possible differences associated with surface antigen expression with different pathotypes of MDVs, we examined MDV-transformed cell lines (Table 2.2). Macrophage cell lines HD11, HTC and MQ-NCSU (Table 2.1) were grown at 37 C, 5% CO2, in DMEM (high glucose) medium supplemented with 10% FBS, 4 mm L-glutamine, 2 mm sodium pyruvate, 1X PSN and 1X fungizone. 21
36 Table 2.1 Cell Line DT 40 CU60 CU91 UD35 UD37 HD11 HTC MQ-NCSU Table 2.2 Cell Line MSB-1 UD35 UD37 UA53 UD32 UD ALV-, REV-, MDV-Transformed Cell lines Cell Type B-Cell (immature) B-Cell T-Cell T Cell T-Cell Macrophages Macrophages Macrophages Transforming Virus ALV-A (RAV-O) REV (REV A/T) REV (REV A/T) MDV (RB-1B) MDV (RB-1B) ALV-A REV ALV-A/REV Reference (6) (93) (70) (40) (39) (39) (72) (75) MDV-Transformed Cell Lines Cell Type T-Cell T Cell T-Cell T-Cell T-Cell T-Cell Transforming Virus MDV MDV (RB-1B) MDV (RB-1B) MDV (TK) MDV (CD) MDV (N) Reference (2) (39) (39) (76) (60) (60) MDV Lymphomas The tumor suspensions used for analysis had been isolated from several Marek s disease virus pathogenesis trials ( ). The virus strains that elicited these lymphomas were: m/vmdv strains (low passage CVI988, GA-22); vvmdv strains (rmd5, RB-1B,) and vv+mdvs (MK/N strain, TK, 645) (Table 2.3). Dr. Isabel Gimeno (NCSU) provided the 645 strain-induced tumors as a frozen suspension. 22
37 Table 2.3 MDV-induced lymphomas used to determine relationship between cell type and population. Virus Strain Used CVI988 GA-22 rmd5 RB-1B TKing MK M Pathotype vacc/vmdv v vv vv vv+ vv+ vv+ # of Lymphomas used Spleen 3 Kidney 3 2Spleen/1Kidney 3 Spleen 3 Spleen 3 Spleen 3 Spleen 3 Tumor Site # of Time Stained Antibodies For immunophenotypic analysis, monoclonal antibodies to chicken lymphocyte antigens (unconjugated and directly conjugated to FITC and PE) were purchased from the Southern Biotechnology Associates, Birmingham, AL (SBT) see Table 2.4 below). All primary antibodies were diluted 1:50 and secondary 1:100 in 1XPBS containing 0.1% sodium Azide, 3%goat serum, and 1% BSA. Table 2.4 Antibodies used for immunophenotype analysis, their specificities, and functional significance Functional Significance Of Antigen Transduces activation of TCR Co-receptor for MHC-II restricted T-cells activation Source Antibody Anti CD3 Antigen Chicken CD3 Anti CD4 Chicken CD4 Anti CD5 Chicken CD5 Regulates T-cell proliferation SBT1 Anti CD8 Chicken CD8 Co-receptor of cytolytic Tcells SBT1 23 SBT1 SBT1
38 Anti CD25 IL2Rα Activation of T cells Anti CD28 Activation antigen on T- cells Binds CD80 (B71) and CD86 (B72) Dr. Hyunn Lillehoj SBT 1 Anti CD44 Hyaluronic acid-binding Essential for extravasation SBT 1 Anti CD45 LFA T cell activation via antigen receptor SBT 1 CD86 B7 molecules Co-stimulation Abcam Anti-BU KUL01 Surface antigen on avian B-cells Surface antigen on avian macrophages Differentiation antigen Non-specific and specific defense SBT 1 SBT 1 Anti-TCR1 TCR ( ) Cytotoxic activity SBT 1 Anti-TCR2 TCR aß (Vß1) Chicken TCR ß (Vß1). SBT 1 Anti-TCR3 TCR aß (Vß2) Chicken TCR ß (Vß2) SBT 1 MHC I MHCII K1 MHC I ( 2 microglobin) MHC II Surface antigens on monocytes and thrombocytes Antigen presenting molecule for CD8 + T-cells Antigen presenting molecule for CD4 + T-cells and DCs Recognizes thrombocytes C6B12 Recognize MHCI Antigen presentation SBT 1 SBT 1 Dr. Hyun Lillehoj, Dr. Marcia Miller, City of Hope AV37 Chicken CD30 TNFR family member, Ross et al, 1997 CSAT Integrin 1 Binds ECM, essential for motility MATSAs MD tumor associated Recognize MD-induced 1-5 antigens antigens Anti- Immunospecific for chicken Chicken IgG Chicken Ig IgG 1 SBT, Southern Biotechnology Associates, Birmingham, AL DSHB Lucy Lee, USDA, ADOL Sigma Aldrich 24
39 Table 2.5 Phases of Flow Cytometric Analysis and Antibodies Used Phase 1: Composition of Lymphoma Analysis Anti-BU Anti-CD4 Anti-CD8 Anti-TCR1 ( ) Anti-TCR2 ( 1) Anti-TCR3 ( 2) KUL01 K1 (Monocyte/thrombocyte) C6B12 (MHC-I) 2.4 Phase 2: Analysis of Activation State of Lymphoma Cells Anti-CD25 Anti-CD28 Anti-CD30 Anti-CD44 Anti-CD45 Anti-CD86 MATSA 1 (14B36.7) MATSA 2 (14G) MATSA 3 (G152.6) MATSA 4 (A35.5) MATSA 5 (B94.5) CSAT (Integrin 1) Phase 3: Assignment of Activation State to Leukocyte Populations BU-FITC/CD3-PE CD4-FITC/CD8-PE KUL01-FITC/MHC-II-PE TCR1-FITC/CD44-PE TCR2-FITC/CD44-PE TCR3/CD44-PE CD3-FITC/CD28-PE CD4-FITC/MHC-II-PE CD8-FITC/MHC-II-PE CD4-FITC/MHC-I-PE Cell Treatments Prior to Staining Since we planned to use archived, frozen tumor suspension samples for flow cytometric analysis, we first performed a series of studies to test whether cell lines stained directly from being thawed differed from actively growing cell lines or cell lines fixed with paraformaldehyde prior to staining. To assess the consistency of antigen staining, we stained live cells, live cells just thawed from frozen storage, and cells fixed with paraformaldehyde prior to staining. For the staining of live cells, cultures were collected by centrifugation (1,200 rpm for 6 min at RT), washed with cold 1X PBS, ph % BSA + 0.1% NaN3 (FACS wash buffer), and resuspended at 1 2 x 107 cells per ml in antibody diluent (1X PBS, ph 7.4, 3% goat serum, 1% BSA, 0.1% NaN3) prior to staining. For the staining of cells directly from liquid nitrogen storage, vials were thawed rapidly at 37 C and cells were resuspended in growth medium (DMEM, or 25
40 IDM, depending on cell type), and spun through a 1 ml cushion of filtered calf serum at 1,200 rpm for 6 min at RT. Cells were then resuspended either in antibody diluent, or fixed directly (see below) prior to staining. To assess the effect of fixation on surface antigen detection, cells resuspended in 1 ml of 2 % paraformaldehyde in 1X PBS, ph 7.4. The cells were fixed at RT for 30 min with agitation. Cells were then washed three times with 1X PBS, and resuspended in antibody diluent and stored at 4 C prior to staining. Since we observed variability between antigen staining of live, thawed from frozen, and fixed cells for some antibodies, we carried out a fixation study using macrophage cell line HTC and four paraformaldehyde concentrations: 0.1%, 0.5%, 1%, and 2%. The cells were fixed with each concentration of paraformaldehyde for 30 mins with agitation at RT, washed with 1XPBS and resuspended as above, prior to antibody staining. For our tumor composition study, archived frozen MDV tumors were thawed, washed, and fixed in 1 ml of 2% paraformaldehyde, and for 30 min on ice. Following fixation, the cells were washed with 10 mls of FACs wash buffer at 1,200 rpm for 6 mins and resuspended in 1 ml of antibody diluent prior to staining. 2.5 Flow Cytometry For staining, cells were adjusted to ~1-2 x 107 cells per ml and stained according to established methods (65). Phase 1: To determine the cellular composition of lymphoma suspensions, the antibodies listed in Table 2.5 (left column) were used. The antibodies used were diluted 1:25 1:50 (per manufacturers recommendations) and were detected using fluorescein isothiocyanate (FITC)-conjugated (or in some cases Alexa Fluor 488)- 26
41 conjugated goat anti-mouse Ig (whole molecule) at 1:100 dilution. These antibodies were chosen to assess relative percentages of B- (immature and mature), T-cell (CD4+, CD8+ and T-cell receptor class) and macrophages in each tumor sampl Phase 2: To determine the relative activation state of lymphoma cells, the antibodies listed in Table 2.5 (center column) were used. As in Phase 1, these antibodies were not directly conjugated but were detected using goat anti-mouse Ig conjugated antibodies (either FITC or Alexa Fluor 488). The purpose of Phase 2 was to determine which of this activation-associated antigen were upregulated on lymphoma cells for follow-up in Phase 3. Phase 3: To determine activated population of MDV tumors, cells were incubated with combinations of 50µl PE and FITC-conjugated chicken monoclonal antibodies as seen in Table 2.5 for 1hr then centrifuged at 1200rpm for 6mins thrice with 4mls of FACS wash buffer. Following tumor staining, MDV-transformed cell lines were incubated with chicken monoclonal antibodies as seen in Table 2.4 as described in phases 1 and 2. Following direct or indirect antibody-labelling, cells were fixed with 100µl of 2% paraformaldehyde, and suspended in 250ml of FACS wash buffer and stored at 4 C prior to flow cytometry analysis. Cells were acquired using FACscalibur flow cytometry and BD Accuri C6 flow cytometers (Becton-Dickincson, San Jose, CA) (UD-BISC and Medical Technology respectively). For analysis, 10,000 ungated events were acquired for each sample. Cells were gated based on light scattering profiles of unstained, as well as secondary antibody-only stained cells. Analyses for all cell lines were performed using CellQuest software and analysis for tumor samples were performed using Cflow software (Becton and Dickinson). 27
42 2.6 Statistical Analysis For each set of analyses, experiments were carried out three separate times with each cell line or tumor sample stained in triplicate Acquired data were analyzed statistically via One-Way ANOVA using JMP statistical discovery software. A value of 0.05 was used as the threshold for statistical significance. 2.7 TCR Spectratype Analysis To test the hypothesis that MDVs of increased virulence may have evolved to induce polyclonal tumors, we examined the T-cell receptor (TCR) rearrangement via spectratype analysis essentially as described (94). We first examined the TCR spectratype of established MDV-1 transformed cell lines, MSB1 (BC-1-transformed), UD35 (RB-1B-transformed), and UA53 (TK-transformed). Cultured cells were harvested from a T25 flask into a 15cc tube, snap frozen in liquid nitrogen, lysed with 350 µl RLT buffer (QIAGEN), homogenized with silicon beads, and then pelleted by centrifugation at 13,000 rpm for 2 min. DNA and RNA was extracted from each sample using the All Prep DNA/RNA/Protein Qiagen Kit and eluted with 50 µl of RNase-free water. Reverse transcription was performed using the Applied Biosystem cdna Reverse Transcription (High Capacity) kit with 2 µg of total RNA as a template in a reaction volume of 20 µl. PCR was performed using forward primers Vβ1 and Vβ2 with a common Cβ reverse primer (Table 2.6). A 50µl total volume reaction was prepared with 1 µl cdna, 2 µl of each primers (2 µm), 45 µl Platinum Taq DNA polymerase (Invitrogen) using the following conditions; Primary denaturing 96 C for 5 min for 1 cycle, followed by 35 cycles of 96 C for 15s, 55 C for 40s, 72 C for 1 min and a final cycle for 10 min at 72 C (Eppendorf Mastercycler Gradient). PCR products 28
43 were separated by electrophoresis using a 1% agarose submarine gel (Fisher Scientific FB300). Images were scanned for peak analysis using ImageJ software. 2.8 Proteomic Analysis of Meq-Associated Proteins from MDV Cell Lines As part of a previous study, the Meq proteins from three MDV cell lines were immunoprecipitated using a polyclonal antibody to the first 106 amino acids of Meq (43). The cell lines were: MSB-1 cells, transformed by the BC-1 virus (a vmdv strain, Meq protein accession #: AY362707), UD35 cells (RB-1B-transformed Meq protein accession #AY571783) and UA53 cells (TK strain-transformed, Meq protein: AY362721) (85). As a negative control, CU91 cells, an REV-transformed T-cell line was used for immunoprecipitation. Nuclear lysates each cell line were immunoprecipitated using the anti-meq protein and pelleted protein A/G agarose beads were submitted for proteomic analysis. Protein samples were denatured and reduced prior to trypsin digestion using standard proteomic methods. Following digestion, samples were desalted, concentrated using a peptide (reverse phase) microtrap (Michrom BioResources) and resuspended in 2% acetonitrile, 0.1% formic acid using low retention vials in preparation for analysis using 1D-LC-MS/MS. Resulting peptide mixtures were separated using an in-line HPLC system with a gradient of 2% to 50% acetonitrile over one hour and a flow rate of 333 nl per minute. Eluate from the HPLC system was passed to a Thermo LTQ Velos Pro mass spectrometer using a nanospray Flex ion source. Data was collected using one MS scan followed by 10 MS/MS scans of the 5 most intense peaks. MS/MS scans were performed in pairs, with a CID fragmentation scan followed a HCD fragmentation scan. A mass exclusion time of three minutes was 29
44 used with a repeat count of one within 30 seconds of initial M/Z measurement. Spectra were converted to MGF format for spectrum matching using the X! tandem software ( and searched against a custom database of protein sequences containing all Gallus gallus and Marek s Disease Virus RefSeq proteins. Matches were filtered to remove peptides identified from a single spectrum and peptides identified with an E-value > In order to evaluate the quality of the data set, decoy searches were done using a randomized version of the target database and the false discovery rate was set at 4.4% (minimum 2.4%). For follow-up to proteins identified by mass spectrometry, CU91-identified peptides were subtracted from MDV Meq protein hits in order to remove non-meqassociated proteins. Messenger RNA sequences from selected Meq-interacting proteins were used for designing primers for cloning and expression of these proteins. These primers are presented in Table 2.6, below. Table 2.6 Gene Primers used to amplify cellular genes identified through interactions with Meq proteins by mass spectrometry Access. # Vβ1-For Vβ2-For Cβ-Rev Annexin A2 NP_ Amplification Primers 5 ACAGGTCGACCTGGGAGACTCTCTGA CTCTGAACTG-3 5 CACGGTCGACGATGAGAACGCTACCCTGAG ATGC-3 5 ACAGGTCGACGTACCAAA GCATCATCCCCATCACAA- 3 Amplicon size 800bp1 (For) GCTAGCatgtctactgtccatgaaattttaagcaag 1068 bp (Rev) ggatcctcaaagcttcagatcctcttct GAGATGAGTTTTTGTTCgtcctctccaccacacaggttcag 30
45 ELMO-2 XM_ (For) gctagcatgtacccatacgatgttcca GATTACGCTatgccaccaccttcagacattgtg 2203 bp (Rev) GAATTCCgccgtagtggtacacgaagtcgtag ACI HSP70 (For) gctagcatgtacccatacgatgttccaga TTACGCTATGTCTGGCAAAGGGCCGGCCATCGGCA 1947 bp (Rev) tggatccgcatctacttcttcaatggtt GGGCCAC XM_ XM_ XM_ KIN17 (For) gctagcatgtacccatacgatgttccagattacgctatg gggaagtcggatttcct 1218 bp (Rev-1) TGGATCCCCggcaagtttggaaatatcttc 1193 bp (Rev-2) GAATTCAAAGCTTatgatagcat Ctctctttgtgaaag Prohibitin NM_ (For) gctagcatgtacccatacgatgttccaga TTACGCTatggctgccaaagtgtttgaaag 857 bp (Rev) tggatccccctgtggcaactggaggagt ACAGA RPL26 NM_ (For) gctagcatgtacccatacgatgttccagattacgctatg aagttcaacccgttcgtaactt 476 bp (Rev) TGGATCctcttgcatcttttcgattgtttct 1 -Amplicon size based on result on MSB-1 using the same primers (Mwangi et al, 1994) 2.9 PCR Cloning of Meq Proteins (Prohibitin and RPL26) PCR was performed using previously prepared cdna from MSB-1, UD35, and UA53 cells (Section 2.4.1). A 50 µl total volume reaction was prepared with 1 µl cdna, 2 µl of each primer (Table 2.6), 45 µl Platinum Taq DNA polymerase (Invitrogen). The reaction was ran with the following conditions; 96 C for 5 mins for 1 cycle, followed by 35 cycles of 96 C for 15s, 55 C for 40s, 72 C for 1min and a final cycle for 10mins at 72 C (Eppendorf Mastercycler Gradient). 5 µl of the 31
46 amplicons were analyzed using 1% ethidium bromide stained agarose gel through electrophoresis (Fisher Scientific FB300) at 60volts. Amplicons of the predicted size were cloned into a pcr2.1 TOPO vector (TOPO TA cloning Kit, Invitrogen Inc), transformed into a TOP 10 E. Coli competent cells, and incubated on LB agar plates prepared with 50µg/ml kanamycin + 40 µg/ml X-gal overnight. For each cloned gene products, eight white colonies were selected for each gene and screened for mini-prep analysis. The insert size of the selected clones was screened via restriction digestion. Clones having the correct sized inserts were grown for midi prep (Qiagen kit), and DNAs were subjected to DNA sequence analysis using M13 forward and reverse primers at Genewiz Inc., South Plainfield, NJ Subcloning of Chicken Prohibitin and RPL26 Genes Sequence verified inserts were isolated for two of the genes selected (prohibitin and ribosomal protein L26, RPL26) for subcloning into enhanced cyan fluorescence protein (ecfp) and enhanced yellow fluorescence protein (eyfp) expression vectors. The chicken prohibitin (PHB) gene was amplified using primers that introduced a unique Nhe I site and an amino terminal fusion of the HA epitope of influenza virus (MYPYDVPDYA) at the 5 end and a unique BamHI site at the 3 end removing the stop codon from each gene. Hence, both genes were subcloned as Nhe I BamHI fragments into Nhe I and BamHI-digested pecfp-n1 and peyfp-n1 directionally. This resulted in fluorescent protein fusions of prohibitin and RPL26 downstream of the CMV promoter. Subcloning was performed using standard procedures and positive clones were subjected to DNA sequence analysis using a FP universal reverse primer and each of the forward amplification primers. 32
47 2.11 Transfection of HTC Cells with Expression of Vectors To examine the localization of prohibitin- and RPL26-fluorescent protein fusions, and their co-localization with Meq isoforms HTC cells were transfected in 12well dishes using Lipofectamine 2000 (invitrogen) and expression vector DNAs (200 ng/well) using manufacturers procedure. Cells were transfected with each of the ecfp-based expression vectors (prohibitin-ecfp and RPL26-eCFP) and cotransfected with untagged Meq proteins associated with different pathotypes (Table 2.3) of MDV (Rispens 339, Rispens 398, 617A, CU210, RB1B, RB/MK, Meq/vIL8, MeqvIL8 exon3). Cells were examined and imaged using a Nikon TE2000 epifluorescence microscope having Bright field, DAPI, GFP, Rhodamine, CFP and YFP filters. Images were acquired using a CCD and analyzed using NIS-Elements software (Nikon, Tokyo). 33
48 Chapter 3 RESULTS 3.1 Macrophage Cell lines Show Positive Surface Antigen Expression Against Monoclonal Antibodies Across Treatments (Fixed, Frozen, Live Cells) All macrophage cell lines tested (HD11, HTC and MQ-NCSU) were negative or expressed very low levels of CD5 and CD28 expression. All of these lines were positive for CD44, CD45, MHC-I, MHC-II, and KUL01 expression (Figures 3.1). Paraformaldehyde-fixed NCSU and HTC cell lines showed significantly decreased CD44 expression compared to the other treatments (thawed from frozen and liveactively growing cells), suggesting that fixation may mask the epitope for this antibody (Figures 3.1). Since this was not observed for HD11 cells, our data suggest a cell line-specific difference in CD44 epitope presentation on HTC and NCSU cells. Similarly, CD45 expression on all macrophage cell lines was undetectable on cells stained after fixation, again suggesting that fixation may block certain epitopes. For MHC-I expression we saw no such masking effect of fixation, in fact in each case, MHC-I expression was higher than in unfixed live or thawed cells (Figure 3.1). Since the monoclonal antibody to MHC-I used (F22-2, Southern Biotech) recognizes an epitope that appears to require stabilization by 2 microglobulin, fixation may stabilize this interaction. Interestingly, MHC-I expression was undetectable or very low on actively growing macrophage cell lines, which is uncharacteristic. MHC-II on the other hand was highly expressed by all cell lines 34
49 regardless of treatment, particularly HD11 cells, which showed 95% expression for all treatments. The antigen detected by the mab KUL01, common to chicken macrophage monocytes was consistently expressed (except from frozen HTC cells, Figure 3), however the phycoerythrin- (PE) conjugated antibody showed a greater percentage of KUL01+ cells compared to the FITC conjugate. This may have been due to differences in the antibody concentration between the two conjugates, or in the quantum yield from PE vs FITC since PE 50X brighter than FITC (32). Table 3.1 Flow Cytometric Analysis of ALV-A- ALV-J-, and ALV-REVtransformed macrophage cell lines G Antibody HD11 HTC MQ-NCSU Fixed Frozen Live Fixed Frozen Live Fixed Frozen Live CD5 C CD28 C CD44 D B ++++ A ++++ A ++ B ++++ A ++++ A CD45 E + A +++ A ++ AB KUL01 FITC C KUL01 PE E +++ B +++ A ++++ A MHCI D A + B ++ B ++++ A + B + B MHCII D A +++ B +++ B ++++ A ++ B ++++ AB G % levels of expression as follows; (-) indicates negative expression; (±) positive but <5% expression; (+) indicates 5-25% expression; (++) indicates 26-50% expression; (+++) indicates 51-75% expression; (++++) indicates % expression A, B Indicates Statistical difference between treatments (P<0.05; Tukey-Kramer comparison) C No Statistical difference between treatments of all cells D No Statistical difference between treatments of HD11 cells E No Statistical difference between treatments of HTC and MQ-NCSU cells 35
50 3.2 Surface Antigen Expression of B Cells Against Monoclonal Antibodies Across Treatments To identify surface antigens of B cells and validate reagents with varying treatments (fixed, frozen, live), we selected antibodies against B-cell surface antigens conjugated with either FITC or PE. All cell lines showed consistently high expression for monoclonal antibodies BU, CD44, and CD45. Neither cell line showed significant expression for MHC-I, contrary to our prior report (76). In this study, however, antigens were detected via indirect staining (mab followed by a conjugate) which amplifies the signal. In this previous report, less than 20% of cells showed MHC-I expression. Similarly, MHC-II showed very low to no expression, similar to the previous report. CD5 expression was only observed on living and thawed from frozen DT40 cells (but not CU60 cells). Expression of CD5 was not observed for fixed DT40 cells, suggesting that this antigen is sensitive to fixation. It is interesting to know that DT40 cells are immature B-cells (used for class-switching studies), while CU60 cells are more mature and do not express CD5. CD5 expression on B-cells is associated with the downregulation of self-reactive B-cell receptors (BCRs), and therefore its expression would be on immature as opposed to mature B-cells. Neither cell line was positive for surface immunoglobulin (Ig), which differs from our previous report (76). In this case 20 50% of each cell line were positive for surface IgM. The antibody used in this case (anti-chicken Ig whole molecule) does not appear to react to IgM (Table 3.2). 36
51 Table 3.2 Antibody CD5 CD44 CD45 BU PE BU FITC MHC I MHC II Comparison of Surface Antigens expressed on pre-treated ALV- and REV- transformed B CellsC. Fixed CU60 Frozen Live Fixed B ++++B DT40 Frozen ++++A ++++A Live +++A ++++A c % levels of expression as follows; (-) indicates negative expression; (±) positive but <5% expression; (+) indicates 5-25% expression; (++) indicates 26-50% expression; (+++) indicates 51-75% expression; (++++) indicates % expression A, B Indicates Statistical difference between treatments (P<0.05, Tukey-Kramer Comparison) 3.3 Surface Antigen Expression of T cells Since MDV is associated with the induction of T-cell lymphomas and T-cells are the transformed component of the lymphomas, the examination of fixation and/or freezing/thawing effects on antigen detection and expression was essential for our examination of archived lymphoma samples. Previous study has reported the immunophenotypes of MDV-transformed cell lines, UD35 and UD37, as well as REV-transformed CU91 T-cell line (40) for UD35 and UD37 (4, 76). CU91 cells were initially established through in vitro infection of thymocytes in cell culture with a mixture of REV-A and REV-T, the replication defective retrovirus having the v-rel oncogene (93). The Parcells lab has previously characterized these cells in several publications (76) (4, 64, 80). In the present study, we extend these results to cells stained post-fixation and cells stained directly upon 37
52 thawing from frozen stocks. Our data are largely consistent with the previous results, CU91 cells are positive for CD3, TCR3 ( V 2), and CD4, albeit weakly positive (Figure 3.3A). In terms of auxiliary antigens, we saw no CD28 expression (reported to be weakly positive for this antigen previously), but we observed high levels of CD44, CD45 and MHC-II. These high levels of expression were also reported previously. Interestingly, for several antigens, fixation enhanced the level of staining, although this was generally not significantly higher than that expression seen on actively growing cells. UD35, established from an RB-1B-induced lymphoma, cells were previously reported to be CD3+, CD4+, TCR2+, and expressed high levels of MHC-I and moderate levels of MHC-II (40). In our current study, we detected CD3 and CD4 using FITC-conjugated antibodies, but not with PE-conjugated antibodies. Oddly, we observed higher expression on fixed and cells from frozen stocks, but not actively growing cultures (Figure 3.3B). We also observed no expression of TCR2, but expression of TCR3 only on fixed cells, contrary to our previous results. In terms of co-stimulatory antigen CD28, our results are consistent with prior results in that UD35 showed little to no expression (40). CD44 expression was also consistently high with this cell line and was not affected by fixation or being stained directly from being thawed (Figure 3.3B). Our present analysis found higher expression of CD44 than previously reported, but since a PE conjugate was used, this may have increased the sensitivity to this antigen. 38
53 CD45 (Leucocyte Common Antigen, Protein Tyrosine Phosphatase receptor type C) expression was undetectable on UD35 cells in these studies, regardless of treatment. A low level for this protein tyrosine phosphatase was reported previously, again using indirect staining (40). UD35 cells showed high levels of MHC-I and MHC-II, and this was unaffected by treatment, unlike macrophage cell lines HTC and NCSU (see Figure 3.1 A and B, above). These results were consistent with our previous report. Finally, UD35 cells were negative for CD5 expression, an antigen common to immature and naïve T-cells, lost in many leukemias and notably in human Cutaneous T-cell lymphomas. The loss of CD5 expression is associated with a poor prognosis for patients. UD37 cells, also established from an RB-1B lymphoma, showed consistently high expression of most antigens (Figure 3.3). The level of CD3 expression was higher than previously reported, and cells stained directly from being thawed showed higher expression than actively growing or fixed. This is likely due to cells being somewhat permeable just after being thawed and residual DMSO may have allowed antibodies to stain CD3 intracellularly. Similarly, UD37 showed high levels of CD4 expression, regardless of treatment. This was consistent with our previous report for these cells (40). UD37 cells were previously shown to be highly positive for TCR2 ( V 1) and weakly positive for TCR2 ( V 2) (40), a strange immunophenotype that we previously reported for UD14 cells, another RB-1B-transformed T-cell line (65). In this study, however, we found them to be only positive for TCR2, and again that cells stained after freezing showed higher expression. 39
54 In terms of auxiliary antigens, UD37 cells were positive for CD28, and high levels of CD44 and CD45. All of these antigens were previously reported as being expressed on these cells. UD37 cells expressed high levels of MHC-I and II, consistent with our previous results, and also express high levels of CD5. This is particularly true in the actively growing and thawed from frozen treatments. This may indicate that UD37 cells were from a lymphoma induced in an immature T-cell lineage. Table 3.3 Comparison of pre-treated MDV-and REV-transformed T cells Surface antigen expressiong Fixed CU91 Frozen CD3 FITC +++A +B CD3 PE A +AB +B ++C ++++A CD4 FITC +AB ++A +B +++A +B +C +++C ++++A CD4 PE B +A B B A B ++++C ++++A CD5 B +A B -B A -B ++C ++++A CD A B +++C ++++A +++A ++++A A B ++++A A +++A ++++A AB +B +B B B +A +++A B ++B AB +++B ++++B +++A ++++A ++++B Antibody CD44 A ++B CD45 +++A +A TCR2 TCR MHC I MHC II A ++++ A ++B ++A Fixed UD35 Frozen Live B ++A ++B Live ++A B A +++ +B B ++++B ++++A ++++B 40 Fixed UD37 Frozen Live +B ++C ++++A +++B +++B A ++++ A ++++ B ++++ B ++++ B +++B ++++ B ++++ AB
55 3.4 Expression of Activation-associated Antigens on MDV-Transformed T Cell Lines To examine whether there were differences in surface antigen expression that could be associated with the different pathotypes of MDV (vmdv, vvmdv, and vv+mdv), we selected monoclonal antibodies that identify activation state-associated surface antigens. The cell lines used were established using different pathotype viruses: MSB-1 (BC-1, a vmdv), UD35, UD37 (RB-1B, a vvmdv), UD31, UD32 and UA53 (MK or N, CD or X, and TK strains, respectively, all vv+mdvs). All cell line showed very low to no expression of CD25 (IL-2R ), despite the fact that MDV-transformed cells are thought to be polarized as Treg cells (which are usually CD25+). Oddly, during this analysis, all lines were negative for CSAT (CD29, 1 integrin), however this may have been due to the lot of CSAT we were using, as previously, all MDV and REV cell lines showed high levels of 1 integrin (40, 64, 80). All cell lines showed positive expression of Marek s Disease associated marker CD30; however, the vv+mdv cell lines UA53 (TK-transformed), UD31 (MK or N strain transformed) showed the highest expression that was significantly different from the other cell lines (Figure 3.1). UD32 cells, however, established from the CD or X strain showed very low levels of CD30 expression. UD31, UD32 and UA53 cells are odd, in that these do not express a TCR (80, 85). The UD31 cell line was established directly from a spleen tumor and did not undergo crisis during establishment (Parcells, personal communication). CD44 was highly expressed by all cell lines, and MHC-I and II were expressed on all but UD32 cells. This line showed low expression of CD30, MATSA and CD86, but showed high levels of CD45 expression. Since these cells do not 41
56 express a TCR class, surface CD3 or CD4 expression, it was unclear that they were, in fact T-cells, however, do express CD28 and are negative for BU and surface IgM (85) suggesting that they are, in fact T-cell derived. MSB-1 (vmdv) and UD31 (vv+mdv) showed significantly higher expression of MATSA A35.5 compared to the other cell lines (Figure 3.1C). The identity of this MATSA, as for most of the antigens referred to as MATSAs are currently unknown. These were described by monoclonal antibodies generated against MDV-induced tumors (42). We found that all MDV cell lines tested were at least weakly positive for CD86 expression. CD86 (B-72 antigen) is typically expressed on antigen presenting cells where along with CD80, it engages T-cell auxiliary receptors CD28 and CTLA- 4. The expression of CD86 on mammalian T-cells is an indicator of memory effector cell function (67) consequently this helps define MDV cell lines as transformed effector memory cells. CD45 expression was quite variable among the cell lines, with MSB-1, UD37 and UD32 expressing the highest levels. No expression was detected on UA53 cells, although a low level of CD45 expression was noted previously (80). Based on our examination of cell line activation-associated antigens, we observed no consistent trend with respect to viral pathotype and antigen expression. If anything, the absence of common T-cell antigen expression on the vv+mdv-derived cell lines suggests that either these viruses allow the outgrowth of tumors from aberrant T-cells or that expression of these antigens is affected during transformation. 42
57 43
58 Figure 3.1 Flow cytometric analysis of MDV-transformed cell lines with selected monoclonal antibodies. (A) CD25, CD30, and CD44 (B) CD86, MHCI, and MHCII (C) CSAT, MATSA, and CD45. a,b,c denotes statistical difference between group (Tukey-Kramer HSD comparison). 3.5 Flow Cytometric Analysis of MDV-induced Tumor Cellular Population To address our central hypothesis, namely that tumors induced by different pathotypes of MDV have pathotype-common cell population changes during lymphogenesis, we examined archived tumor suspensions induced by vmdv, vvmdv, and vv+mdv strains. We first examined leukocyte populations comprising lymphomas via flow cytometry. Somewhat surprising was that all MD lymphomas examined in this series of studies showed low percentages of B-, T-, and macrophage cells (Figure 3.2). Meanwhile, all lymphomas showed above 30% expression of C6B12 with the exception of the lymphoma induced by 645 (Figure 3.2). Despite the low expression CD4 cells across lymphomas, lymphomas appear to express higher levels of TCR2 44
59 compared to other T cell receptors. Given the previous body of literature regarding MDV lymphomas, for instance (12)and our own work (40) these data suggest that many of these antigens were not readily detected in lymphoma cells stores in liquid nitrogen. Figure 3.2 Flow cytometric analysis of MD lymphomas cellular population. Surface antigen represents B-, T-, Macrophage, and Thrombocytes (K1). 3.6 Analysis of the Activated state of Cells in MD lymphomas We next sought to determine pathotype-specific changes in the activation state of MD lymphoma cells. Again, we observed very low expression of almost all surface antigens studied (Figure 3.3), however, all tumors were positive for and showed high expression of CD44. Despite the low expression, Rb-1B and TK-induced lymphomas showed higher level of CD30, MATSA4 (A35.5), MATSA2 (14G) and MATSA5 45
60 (B94.5) compared to the other lymphomas. RB-1B also showed higher expression of CD28 and CD45, indicating higher levels of activation antigens. In terms of pathotype-specific changes in expression, the consistent change that w saw was an increase in CD44 expression, but this was more of a general trend. Again, we saw very little CSAT ( 1 integrin), which had been described as being highly-expressed previously for all cell lines. Figure 3.3 Flow Cytometric analysis of the activation state of MD lymphomas cellular populations. 3.7 Analysis of the Activated Cellular Population of MD Lymphomas via Dual Staining To determine cells that were double positive for antigens (i.e., CD4+/CD8+ cells), as well as activated populations of cells, we performed dual staining using antibodies directly conjugated with either FITC or PE. 46
61 We observed somewhat higher double positive (DP, CD4+/CD8+) cells in tumors from lower virulence viruses (CVI988, GA22) (Figure 3.4). We saw low levels of MHC-II+ macrophages across all infections. Interestingly, we did observe the highest percentage of TCR1 positive cells in the CVI988-induced lymphomas by this method, compared to the single-staining method (compare Figures 3.2 and 3.4). We also observed that the percentage of CD44+ cells was much lower for dual-stained cells than for single-staining, which is reasonable, since the double staining identifies a subset of CD44+ cells. Again, the number of CD4+ T-cells (those expressing MHC-II and ostensibly activated) was much lower than had been reported previously for lymphomas and cell lines (40, 51), suggesting that our conditions were not optimized for identifying the cell populations. One aspect we did note in our previous work was a large variation in the number unstained cells in lymphomas (40), suggesting that surface antigen expression may be downregulated on transformed cells. In terms of the staining that we did observe, we found that GA22 showed higher percentages of DP T-cells (CD4+, CD8+), CD3+, CD28+, and CD4+, MHCII+ (activated T-cells). The lower virulence strains CVI988 and GA22 showed higher percentages of CD8+, MHC-I+ and CD8+, MHC-II+, suggesting that these tumors contained more inflammatory components than those induced by higher virulence strains. 47
62 Figure 3.4 Flow cytometric analysis of the activated population of MD lymphomas 3.8 TCR Spectratyping of MDV cell lines and lymphomas Since we were unable to determine if MDV pathotypes induced tumors of distinct populations via flow cytometric analysis, we attempted to analyze whether there was a difference in the clonality of T-cell receptors for tumors induced by different pathotypes of MDV. We hypothesized that tumors of vv+mdvs may show an increase in being polyclonal, that is comprised of distinct transformation events in separate T-cells. To test this method, we analyzed three cell lines representing the three pathotypes of MDV: MSB-1 (established from vmdv strain, BC-1-induced lymphoma), UD35 (established using a vvmdv strain, RB-1B), and UA53 (established using a vv+mdv strain, TK). 48
63 Using primers designed against the V 1 and V 2 chains, we used PCR to amplify across the rearranged TCR loci of each cell line. Our results suggested that all cell lines encoded a commonly rearranged V 1 with an approximate band size of 800 bp whereas only MSB-1 expressed V 2 (Figure 3.5A). Meanwhile, while MSB-1 showed a single graphical peak profile both UD35 and UA53 showed two peaks of different sizes (Figure 3.5 B). 49
64 Figure 3.5 Clonality of MDV-transformed cell lines determined by TCR Specratyping. (A) 1% agarose gel showing TCRβ Amplicons from PCR (B) ImageJ graphical profile of the Vβ1 and Vβ2 repertoire (respectively) of MDV-transformed cell lines from PCR gel 3.9 Cloning and Subcloning of Genes Associated with Meq Proteins Since we did not identify changes in lymphoma populations characteristic of MDV pathotype, nor did we find differences in tumor clonality via TCR spectratyping, we hypothesized that the changes in Meq coding sequence could confer different binding sites for different cellular proteins. These differences in Meqbinding proteins may therefore provide insight into the acquisition of increased virulence through new pathways being affected within the latently infected/transformed cell. 50
65 As part of a previous study, we had used an anti-meq (amino terminal 106 aa) to immunoprecipitate Meq from cell lines MSB-1, UD35 and UA53. These immunoprecipitated proteins, and all proteins bound were subjected to proteomic analysis at the University of Arizona (Drs. Fiona McCarthy and Shane Burgess). As a control for a chicken T-cell line, not transformed by MDV, we used CU91 cells (REVtransformed). These samples had actually been lossed when the investigators moved from Mississippi State University to Arizona and were found this Spring (2014). After these samples were found, they were digested and analyzed by mass spectrometry. We then received a list of proteins identified by peptides that had been immunoprecipitated by the rabbit anti-meq antibody (see Appendix 1, list of proteins). We analyzed these putative Meq-interacting proteins and sorted them into groups: (1) vmdv-specific (MSB-1 only), (2) vmdv+vvmdv common (MSB-1 and UD35), (3) vvmdv-specific (UD35 only), (4) vvmdv + vv+mdv common (UD35 and UA53), (5) UA53-specific, (6) MDV common (seen in all cell lines) and (7) common to MSB-1 and UA53. The number of peptides identified for each group is given in the table, below. 51
66 Table 3.4 Meq-Associated Proteins Isolated from Immunoprecipitation Pulldown from MDV-transformed Cell lines Group Cell Lines # of unique proteins Representative Proteins 1 MSB-1 only kd HSP, ELMO2, RNA-bp14 2 MSB-1 + UD35 12 HSC71, RPL6, RPL7a, peptidyl-prolyl isomerase 3 UD35 only 13 HSP70, GRP78, RSP24 4 UD35 + UA53 1 RSP2 5 UA53 only 11 Annexin A2, KIN17, RPL26 6 MDV common 2 Histone H1.10, Prohibitin 7 MSB-1 + UA53 1 Protein disulfide isomerase In addition to subtracting all proteins identified in CU91 cells, we also examined the chicken genome for proteins homologous to the Meq amino terminal 106 aa. None of the hits matched any of the peptides identified (Parcells, personal communication). We then selected several of the genes for follow-up (Annexin A2 Prohibitin, RPL26, ELMO2, HSP70 and KIN17) identified as co-immunoprecipitating with the Meq proteins. We designed a series of primers (Table 2.6 above) for amplifying and epitope-tagging each of the target genes. The primers were used to amplify the genes from cdna libraries prepared from MSB-1, UD35, and UA53 cell lines. Of the genes we have attempted to clone, we have been successful in the amplification of: Annexin A2, Prohibitin, and RPL26, and each of these have been cloned into pcr2.1topo and had their sequences confirmed (see Figure 3.6A and 52
67 3.6B, below). Prohibitin and RPL26 sequences showed (Figure 3.7A) and 100% (Figure 3.7B) amino acid identity with the GenBank sequences, respectively. A 53
68 B Figure 3.6 Confirmed Deduced Amino Acid sequences of (A) Prohibitin and (B) RPL26. These proteins show and 100% sequence identity to those in GenBank, respectively. The prohibitin was amplified from UA53 and consisted of an open reading frame of 816 bp encoding 272 amino acids with a predicted molecular weight of 29.8 kda. RPL26 was isolated from the MSB-1 cell line and consisted of an open reading frame of 435 bp encoding 145 amino acids with a predicted molecular weight of 17.3kDa (Figure 3.6 A& B). Since these were identified as interacting with Meq via mass spectrometry, we sought to confirm that these proteins interact with Meq and whether there were differences in these interactions that corresponded to pathotype. 54
69 We have subcloned the epitope-tagged chicken Prohibitin and RPL26 genes into fluorescence protein (ecfp and eyfp) vectors. These were then transfected into the chicken macrophage cell line HTC, with and without co-transfection of T7 epitope-tagged Meq expression vectors. The Meq expression vectors encoded the Meq proteins of JM102 (large, 398 aa, vmdv form), RB-1B (339 aa, vvmdv form), and MK strain (a.k.a. N strain, 339 aa, vv+mdv form). Transfection of HTC cells with the fluorescent protein-tagged prohibitin showed a distinctive cytoplasmic pattern (Figure 3.7). When co-transfected with Meq isoform expression vectors, we found that prohibitin remained cytoplasmic in localization when co-expressed with the JM102-Meq and RB-1B-Meq but was localized to the nucleus and nucleolus when co-expressed with MK-Meq (Figure 3.8B). These data suggest that the higher virulence form of Meq directly or indirectly interacts with Prohibitin and affects cellular localization. The 60s ribosomal protein L26 (RPL26) localized to the nucleus and nucleolus both without and with co-expression of Meq proteins. Therefore, expression with Meq did not appear to alter the cellular localization of RPL26 (Figure 3.7 A), however they do co-localize. Thus we need to perform additional analyses to determine if these proteins physically interact and if there are differences in these interactions corresponding to mutations. 55
70 Figure 3.7 Transfected HTC cells showing localization Prohibitin and RPL26 proteins. HTC cells transfected with 200ng of each protein previously cloned into the ecfp-n1 expression vector having Bright field, DAPI, CFP and YFP filters. Images were acquired using a CCD and analyzed using NIS-Elements software. 56
71 Figure 3.8 Localization of Prohibitin-eCFP in Co-transfected HTC cells. HTC cultures were co-transfected with either (A) RPL26-eCFP or (B) prohibitin-ecfp and Meq proteins: CU210 Meq (JM102 isoform), RB1B Meq, and MK (N strain) Meq. Note change in prohibitin-localization with MK Meq derived from a vv+mdv strain (bottom row). 57
72 58
73 Figure 3.9 Localization of prohibitin in cell co-transfected with Meq isoform expression vectors. Representative Fields are shown for each set of transfections. HTC cells were co-transfected with T7 epitope-tagged Meq expression vectors: (top row) Rispens (CVI988) Meq, small form (339 aa), Rispens (CVI988) Meq, large form (398 aa), vmdv strain 617A Meq, and RB/MK Meq (a composite of RB-1B and MK forms, essentially the MK form with a re-inserted LACHE domain). Consult Table 1.1 for point mutations within the coding sequences of these strains. (Note: some nuclear localization is observed for 617A and RB/MK form). 59
74 Chapter 4 DISCUSSION Marek s disease is a lymphoproliferative disease that has been successfully controlled by vaccinations for the past few decades. However, the success of the vaccines has been limited since the virulence of the disease has increased in terms of the clinical picture as well as the time of incidence in unvaccinated as well as vaccinated chickens. Consequently, MDV1 has been characterized into three pathotypes (m/vmdv, vvmdv, vv+mdv) and sequencing of the meq genes of MDV1 isolates have shown amino acid substitutions in the Proline-rich region that has been associated with increasing virulence. Several attempts have been made to study the role of substitution or deletion in the PRR of meq on transactivation (55), (74); however, there has been no study to address the role of PRR amino acid substitutions in meq in lymphoma composition, clonality of lymphomas or in differences in Meqinteractions with cellular proteins. In an attempt to construct MDV recombinants, a previous study in our lab successfully constructed rmdv-1137 which had the background of rmd5 and the meq gene of RB-1B, and flow cytometry immunophenotyping of the tumors acquired from this recombinant showed intermediate tumor composition compared to the parent strains. Since RB-1B and rmd5 are both vvmdvs, but the rmd5 meq contains amino acid substitutions in the PRR that are not present in RB-1B, we theorized that the differences in cellular composition was associated with the mutations. Hence, in this study we hypothesized that mutations in the PRR region may affect tumor 60
75 composition. Determination of differences in cellular composition of MDV tumors correlating with different pathotypes could provide more information on the mechanism of MDV pathogenesis and prevention. To address this study, we tested the antibodies to be used on pre-treated cell lines and determined if fixation or staining cells directly from thawing would affect the affinity of these antibodies to stain the cell lines. 4.1 The Effect of Treatments on Antibody-staining of Cell Lines Our results were generally consistent with previous immunophenotyping reports showing that B cell lines (DT40 and CU60) expressed BU, MHC-I, and MHC-II. Similarly, T cells lines expressed CD3, CD4, MHC-I, and MHC-II, and macrophage cell lines expressed varying amounts of KUL01, CD44, MHC-I, and MHC-II (4, 41, 76). We observed some inconsistencies in our data when compared with previous reports, however. This may be due to the differences in cell conditions prior to staining, consistency of staining conditions and methods, since some of the reports used indirect compared to the direct method used in our experiment. Secondly, we suggest that B cell and macrophage surface antigen expression is not affected by thawed-from frozen and un-fixed live cell conditions but can be affected by fixing. Availability of antigen after paraformaldehyde fixation, which may be due to antigen masking, may be associated with differences observed. However, a dose-dependent fixation study with HTC cells showed no differences in staining (results not shown). Contrary to B cells and macrophages, conditions provided to the cells prior to staining may affect surface antigen expression. Since differences vary across 61
76 treatments and cell lines, we suggest that differences may be due to (1) variation in the surface antigen expression due to passage level and subcloning history since previous immunophenotyping of cell lines show varying levels of staining; (2) thawfrom frozen and paraformaldehyde fixation may further reduce or mask the surface antigens and this may be true for only certain antigens; or (3) variability in staining technique. The staining of vv+mdv-derived cell lines (UD31, UD32, UA51, UA53) in previous reports (80, 85) and in this work showed that many of the putative T-cell lines were atypical in their antigen expression. Several of these did not show expression of a T-cell receptor (TCR) class, the CD3 complex, CD4 or CD8, and the only inference that suggested that they were, in fact, T-cells was expression of auxiliary activation antigen CD28 (85). Our cell line data present the first reports on the expression of CD5 by avian B- cell (DT40) and T-cell (UD37) lines. This antigen appeared to have some sensitivity to fixation since for both of these cell lines paraformaldehyde treatment appeared to decrease the level of detection (Table 3.2 and 3.3). Additionally, this is the first report of chicken CD86 expression for MDV cell lines (Figure 3.1B) and MDV-induced lymphomas (Figure 3.3). In mice, the expression of CD86 by a T-cell is indicative of a Memory Effector cell. If this is the case in chickens, then our data suggest that some MDV lymphomas are derived from memory effector cells generated following inflammation (such as generated during early replication) and may not in fact be polarized to being regulatory T-cells (Tregs). Both of these observations require additional experimentation, but present important insight into the lineages susceptible to MDV-transformation. 62
77 4.2 The Effect of Amino Acid Substitution in the PRR of Meq on Cellular Composition of MD Tumors The main goal of this study was to analyze lymphomas induced by different pathotypes of MDV for differences in cellular composition and possible correlation. MDV lymphomas have been characterized as a CD4+ T cell lymphoma expressing high levels of CD30 (13) surface antigen. Almost all T cells found in MD-induced lymphomas contain the MDV genome (79). Based on the incidence of lymphomas, time and duration of the cytolytic phase, and severity of immunosuppression, MDV has been subdivided into three pathotypes. Sequencing of the principle oncoprotein, Meq loci of the different MDV pathotypes showed amino acid mutations in the proline-rich repeat region that correlates with virulence (85). Therefore, we predicted that mutations might affect the transcriptional activity of Meq and regulate cells enlisted into each lymphoma. Hence, lymphomas induced by more virulent strains of MDV will contain more CD4+CD30+ cells. The overall expression of surface antigen was very low across all lymphomas. In our examination of lymphomas induced by MDVs of different pathotype, we saw uncharacteristically low expression of many antigens. We could not extend our previous work in which we saw relatively consistent changes in tumor composition between RB-1B and rmd5, with an rmd5-based recombinant, harboring the meq gene of RB-1B showed an intermediate phenotype in terms of cell populations (40). Based on our current analysis, we feel the main conclusion of this work, therefore, is that no specific change in antigen expression appears to consistently correlate with MDV pathotype. 63
78 4.3 TCR Clonality of MDV-induced Lymphomas MD lymphomas predominantly consist of neoplastically transformed T cells, which are the usual targets of MDV-transformation (13). Work by Delecluse and Hammerschmidt (26) and subsequent work by Robinson et al., (77)has shown that MDV integrates within the chicken genome and that these integration sites are distinctive within lymphomas and cell lines. The conclusion from these works is that MDV lymphomas arise primarily from expansion of monoclonal transformation events. Similarly, TCR spectratyping work also suggested that despite a high efficiency in inducing lymphomas, tumors arising in birds were typically monoclonal in origin (94). Previous studies have identified expression of TCR chains in MD-induced cell lines (65, 80)showing that both of these TCR classes are. These studies suggested that MDV may target a single T-cell for transformation and induce a lymphoma. Since we did not see a difference in surface antigen expression that was characteristic of MDV pathotype, we hypothesized that changes in Meq coding sequence may affect the efficiency with which lymphomas are induced and that tumors may be arising through multiple independent transformation events. The complementaritydetermining region 3 (CDR3) generates the antigenic diversity to T cells; thereby, varying among clones of T cells. Thus, the amino acid sequence of CDR3 varies among clones of TCR and if MDV targets a single T cell for transformation, then a study of the TCR rearrangements in MD lymphomas will show only one sequence of CDR3. Our result suggested that MSB-1 (vmdv) contained predominantly a rearranged TCRV 1 gene but a minor population of re-arranged TCRV 2. UD35 (vvmdv) and UA53 (vv+mdv) cell lines, however both showed only TCRV 1 64
79 rearrangement, although more than one TCRV 1 product was evident. To identify the origin of the TCR, a previous study looked at the sizes of CDR3 via spectratyping and it was reported that some tumors showed only one size of CDR3, while a few tumors showed more than one rearrangement (94). In an attempt to mimic spectratyping, we further analyzed the gel using ImageJ gel graphical analysis. We report that two peak sizes were observed from UD35 and UA53 amplicons and only one for MSB-1. Although we did not sequence these amplicons, the presence of more than one peak in cell lines UD35 and UA53 suggested that this method may be problematic for examining primary lymphomas, which not only contain transformed CD4+ T-cells, but responding CD8+ T-cell populations. Consequently, we did not address this aspect of MDV lymphomagenesis. 4.4 Identification of Meq-associated Proteins: Meq Mutations and New Binding Sites As we were performing the TCR rearrangement analysis, we serendipitously received data back from Meq immunoprecipitations from CU91 cells (negative control), MSB-1 cells, UD35 cells and UA53 cells (see Appendix 1). Although the number of peptides identified was somewhat limited, several proteins appeared to be pathotype MDV-specific in their identification (see table 3.4, above). In an attempt to follow-up on specific protein-meq interactions, we selected five genes to be amplified, cloned, and sub-cloned into fluorescent protein expression vectors. These were chicken Annexin A2 precipitated by the Meq protein of UA53 cells (vv+mdv), engulfment and motility factor 2 (ELMO2) precipitated by the Meq of MSB-1 cells (vmdv), HSP70 precipitated by the RB1B form of Meq and 65
80 previously described as a Meq-binding protein (104), the chicken prohibitin homolog, precipitated by all three isoforms of Meq (vmdv, vvmdv and vv+mdv) and large ribosomal subunit protein 26 (RPL26). We have so far successfully amplified two of these genes that are both implicated in other forms of cancer, prohibitin and RPL26 respectively. Prohibitin is from a highly conserved family of primarily mitochondrial-localized proteins that also has a function in transcriptional control in mammalian cells (reviewed recently in (25)). Prohibitin has recently been identified as a potential target in drug-resistant malignancies (30). Structurally, prohibitin has an amphipathic amino terminal domain, a central PHB domain (homologous region in all prohibitin proteins), a coiled-coil domain and C-terminal nuclear localization signal. We have found that when fused to ecfp or eyfp at its C-terminus, prohibitin is localized primarily to cytoplasm in a punctate pattern ostensibly mitochondrial localized (see figure 3.7). Although prohibitin was found to be immunoprecipitated along with the Meq proteins from all of the MDV cell lines tested (MSB-1, UD35 and UA53), when we co-expressed T7-epitope-tagged forms of Meq with prohibitin-ecfp, we found that only the MK Meq isoform (from a vv+mdv) altered the localization of this protein from the cytoplasm to the nucleus (Figure 3.8B). In follow-up, we co-transfected prohibitin-ecfp with Meq isoforms to try and map the mutations that confer the observed nuclear localization. We found that both the Meq protein from 617A and the RB/MK isoforms conferred some level of nuclear localization of the prohibitin-ecfp suggesting that mutations within the proline-rich repeats and the cysteine -> arginine mutation at position 119 appear to be involved in this putative higher affinity for prohibitin. 66
81 Most provocatively, the nuclear and nucleolar localization of prohibitin, as seen during co-expression with MK Meq, has also been described for leukemic pre-bcell lines (37) so this acquisition of binding affinity for the MK Meq may confer some of the increased dissemination of vv+mdv tumors cells. These results require further corroboration, but suggest that mutations in Meq may functionally affect tumor formation and/or metastasis. Regarding RPL26, we found that the ecfp and eyfp tagged versions of this protein localize to the nucleus and nucleolus. Furthermore, we found that Meq coexpression did not affect RPL26 nuclear and nucleolar localization. Since both proteins localizes to the nucleus and nucleolus, we will follow-up with other methods to characterize the putative interactions of Meq isoforms with RPL26. The exact role RPL26 and prohibitin in Marek s disease has never been described. These proteins are both implicated as proteins involved in tumor suppression (24) (87). In mammals, RPL26 binds to the tumor suppressor protein, P53 and inhibits the proliferation of cells during stress that may be caused by the virus. Similarly, prohibitin binds to the retinoblastoma (Rb) protein and suppress the bound E2F transcription factor (87). According to Li, et al., RPL26 plays a crucial role in the proliferation of pancreatic cancer (PANC-1) cells as silencing of this gene promoted apoptosis (24). Studies on the role and function of prohibitin suggest that it is involved in both anti-tumorigenic and pro-tumorigenic activities. It has been identified in breast, prostate, stomach, lungs and other cancers where it is suggested that it suppresses tumor formation by binding to Rb or p53 (80, 83, 86). As a pro-tumor protein, prohibitin is suggested to play a role in transformation as it binds to the oncogene Ras and activate c-raf and mediates their stimulation of MEK/ERK 67
82 activation. Thus, we suggest that RPL26 and prohibitin binds to meq, the MDV oncogene, and stimulate transformation of cells. In conclusion, firstly, we found that most, if not all of the commerciallyavailable lymphocyte and macrophage surface antigen antibodies worked on fixed, actively growing and thawed from frozen cells. Secondly, we found expression of CD5 and CD86 on at least one MDVtransformed cell line having implications on the lineage of cells transformed. Thirdly, despite technical problems, our data suggests that MDV-induced lymphomas arise in a somewhat stochastic manner and depend perhaps to a greater extent on the lineages infected and the level of immune suppression caused by the virus during early replication. Fourthly, Meq isoforms bind cellular proteins in the nucleus and nucleolus and changes in Meq coding sequences may be driven by the acquisition of binding sites for these factors and their effects on the transformed cell. Of the putative Meq-binding proteins we report on the expression of two (prohibitin and RPL26), both of which have been implicated in other malignancies. The binding of MK (vv+mdv) isoform of Meq to prohibitin either directly or indirectly affects its localization to the nucleus of transfected cells. Since, CD30 the surface antigen associated with immunosuppression in Marek s disease showed no correlation with changes in Meq and Meq binding partners show pathotype specificity, our data suggest that meq may selectively bind proteins that either directly or indirectly activate proteases that cleave the CD30 anchored to the membrane, and increase intracellular CD30. According to work by Zhang et al., prohibitin may be involved in the regulation of protease-activated receptor 1 which is 68
83 irreversibly activated by thrombin and metalloprotease 1 (103). This finding may further suggest the association of Meq-binding proteins with proteases and perhaps increased generation of soluble CD30. Thus, expression observed during staining may be due to intracellular CD30 that inhibits CD30L (CD153) and enhance immunosuppression followed by cell survival. Finally, to follow-up on this work (1) we will optimize flow cytometry technique to eliminate or at least reduce any technical that will interfere with staining to confirm stochastic pattern observed in MD lymphomas in this study (2) to further address MDV lymphomas clonality, we will analyze TCR CDR3 using spectratype followed by sequencing of the CDR3 genes which will help to confirm spectratype profile, (3) address Meq interactions with putative Meq-binding proteins, using Förster resonance energy transfer (FRET) which will efficiently measure the distance between two fluorophores ( ecfp and eyfp) using energy transfer (4) to evaluate the level of soluble CD30 proteins expressed by different MDV strains using MDV-infected serum and MDV-transformed cell lines. 69
84 REFERENCES 1. Afonso, C. L., E. R. Tulman, Z. Lu, L. Zsak, D. L. Rock, and G. F. Kutish The genome of turkey herpesvirus. J Virol 75: Akiyama, Y., and S. Kato Two cell lines from lymphomas of Marek's disease. Biken J 17: Al-Shamkhani, A The role of CD30 in the pathogenesis of haematopoietic malignancies. Pharmacology 4: Arumugaswami, V., P. M. Kumar, V. Konjufca, R. L. Dienglewicz, S. M. Reddy, and M. S. Parcells Latency of Marek's disease virus (MDV) in a reticuloendotheliosis virus-transformed T-cell line. II: expression of the latent MDV genome. Avian Dis 53: Baaten, B. J., C. Butter, and T. F. Davison Study of host-pathogen interactions to identify sustainable vaccine strategies to Marek's disease. Vet Immunol Immunopathol 100: Baba, T. W., B. P. Giroir, and E. H. Humphries Cell lines derived from avian lymphomas exhibit two distinct phenotypes. Virology 144: Baigent, S. J., L. J. Petherbridge, L. P. Smith, Y. Zhao, P. M. Chesters, and V. K. Nair Herpesvirus of turkey reconstituted from bacterial artificial chromosome clones induces protection against Marek's disease. J Gen Virol 87: Benton, W. J., and M.S Cover The increased incidence of visceral lymphomatosis in broiler and replacement birds. Avian Diseases 1: Biggs, P. M Marek's disease--the disease and its prevention by vaccination. Br J Cancer 31: Biggs, P. M The Leeuwenhoek Lecture, Marek's disease herpesvirus: oncogenesis and prevention. Philos Trans R Soc Lond B Biol Sci 352: Bumstead, J. M., and L. N. Payne Production of an immune suppressor factor by Marek's disease lymphoblastoid cell lines. Vet Immunol Immunopathol 16: Burgess, S. C., and T. F. Davison A quantitative duplex PCR technique for measuring amounts of cell- associated Marek's disease virus: differences in two populations of lymphoma cells [In Process Citation]. J Virol Methods 82: Burgess, S. C., and T. F. Davison Identification of the neoplastically transformed cells in Marek's disease herpesvirus-induced lymphomas: recognition by the monoclonal antibody AV37. J Virol 76:
85 14. Burgess, S. C., J. R. Young, B. J. Baaten, L. Hunt, L. N. Ross, M. S. Parcells, P. M. Kumar, C. A. Tregaskes, L. F. Lee, and T. F. Davison Marek's disease is a natural model for lymphomas overexpressing Hodgkin's disease antigen (CD30). Proc Natl Acad Sci U S A 101: Buscaglia, C., B. W. Calnek, and K. A. Schat Effect of immunocompetence on the establishment and maintenance of latency with Marek's disease herpesvirus. J Gen Virol 69: Buza, T. J., F. M. McCarthy, and S. C. Burgess Experimentalconfirmation and functional-annotation of predicted proteins in the chicken genome. BMC Genomics 8: Calnek, B. W Marek's disease--a model for herpesvirus oncology. Crit Rev Microbiol 12: Calnek, B. W Pathogenesis of Marek's disease virus infection. Curr Top Microbiol Immunol 255: Calnek, B. W., J. Fabricant, K. A. Schat, and K. K. Murthy Rejection of a transplantable Marek's disease lymphoma in normal versus immunologically deficient chickens. J Neurol Sci 36: Calnek, B. W., K. A. Schat, L. J. Ross, and C. L. Chen Further characterization of Marek's disease virus-infected lymphocytes. II. In vitro infection. Int J Cancer 33: Calnek, B. W., K. A. Schat, L. J. Ross, W. R. Shek, and C. L. Chen Further characterization of Marek's disease virus-infected lymphocytes. I. In vivo infection. Int J Cancer 33: Calnek, B. W., T. Ubertini, and H. K. Adldinger Viral antigen, virus particles, and infectivity of tissues from chickens with Marek's disease. J Natl Cancer Inst 45: Chang, K. S., K. Ohashi, and M. Onuma Suppression of transcription activity of the MEQ protein of oncogenic Marek's disease virus serotype 1 (MDV1) by L-MEQ of non-oncogenic MDV1. J Vet Med Sci 64: Chen D, C. L., M Ge, Y YIn, M Luo, D Chen Silencing expression of ribosomal protein L26 and L29 by RNA interfering inhibits proliferation of human pancreatic cancer PANC-1 cells Chowdhury, I., W. E. Thompson, and K. Thomas. Prohibitins role in cellular survival through Ras-Raf-MEK-ERK pathway. J Cell Physiol 229: Delecluse, H. J., S. Schuller, and W. Hammerschmidt Latent Marek's disease virus can be activated from its chromosomally integrated state in herpesvirus-transformed lymphoma cells. Embo J 12: Els Wessels, D. l. D., Richard A. Notebaart, Willem J. G. Melchers,, and a. F. J. M. v. Kuppeveld A Proline-Rich Region in the Coxsackievirus 3A Protein Is Required for the Protein To Inhibit Endoplasmic 71
86 Reticulum-to-Golgi Transport. American Society for Microbiology 79: Fukuchi, K., M. Sudo, Y. S. Lee, A. Tanaka, and M. Nonoyama Structure of Marek's disease virus DNA: detailed restriction enzyme map. J Virol 51: Goldin, L. R., and O. Landgren Autoimmunity and lymphomagenesis. International Journal of Cancer Guo, S., J. Zou, and G. Wang. Advances in the proteomic discovery of novel therapeutic targets in cancer. Drug Des Devel Ther 7: Hunt, H. D., and J. R. Dunn Serial Transfer of a Transplantable Tumor: Implications for Marek's Vaccine Mechanisms. Avian Diseases 55: Jain P, and T. kalina Phenotype Analysis 33. Jarosinski, K. W., S. Arndt, B. B. Kaufer, and N. Osterrieder. Fluorescently tagged pul47 of Marek's disease virus reveals differential tissue expression of the tegument protein in vivo. J Virol 86: Jeurissen, S. H., and G. F. de Boer Chicken anaemia virus influences the pathogenesis of Marek's disease in experimental infections, depending on the dose of Marek's disease virus. Vet Q 15: Jeurissen, S. H., E. M. Janse, G. L. Kok, and G. F. De Boer Distribution and function of non-lymphoid cells positive for monoclonal antibody CVI-ChNL-68.2 in healthy chickens and those infected with Marek's disease virus. Vet Immunol Immunopathol 22: Jones, D., L. Lee, J. L. Liu, H. J. Kung, and J. K. Tillotson Marek disease virus encodes a basic-leucine zipper gene resembling the fos/jun oncogenes that is highly expressed in lymphoblastoid tumors [published erratum appears in Proc Natl Acad Sci U S A 1993 Mar 15;90(6):2556]. Proc Natl Acad Sci U S A 89: Kaisa J. Teittinena, P. K., Johanna Salonenb, Gunilla Rönnholmc,, and M. V. Hanna Korkeamäkia, Nisse Kalkkinenc, Olli Lohia Nucleolar proteins with altered expression in leukemic cell lines. Elsevier 36: Kindt, T. J., R.A Goldsby, and B.A Osborne Chapter 2: Cells and organs of the immune system. In Immunology, 6th end. 39. Klasing K.C, and P. R.K INFLUENCE OF CELL SOURCES, STIMULATING AGENTS, AND INCUBATION CONDITIONS ON RELEASE OF INTERLEUKIN-1 FROM CHICKEN MACROPHAGES. Developmental and Comparative Immunology 11: Kumar, P., H. Dong, D. Lenihan, S. Gaddamanugu, U. Katneni, S. Shaikh, P. Tavlarides-Hontz, S. M. Reddy, W. Peters, and M. S. Parcells Selection of a recombinant Marek's disease virus in vivo through expression of the Marek's EcoRI-Q (Meq)-encoded oncoprotein: characterization of an rmd5-based mutant expressing the Meq of strain RB-1B. Avian Dis 56:
87 41. Kumar, P. M., V. Arumugaswami, H. Dong, Y. Farnell, R. L. Dienglewicz, P. Tavlarides-Hontz, and M. S. Parcells Spliced gene products of the Meq oncoprotein of Marek's disease virus (MDV) are expressed during latency, bind to the MDV genome, are potent transcriptional repressors, and induce cellular proliferation. J Virol In press. 42. Lee, L. F., and L. D. Bacon Ontogeny and line differences in the mitogenic response of chicken lymphocytes. Poult Sci 62: Lee, L. F., J. L. Liu, X. P. Cui, and H. J. Kung Marek's disease virus latent protein MEQ: delineation of an epitope in the BR1 domain involved in nuclear localization. Virus Genes 27: Lee, L. F., P. Wu, D. Sui, D. Ren, J. Kamil, H. J. Kung, and R. L. Witter The complete unique long sequence and the overall genomic organization of the GA strain of Marek's disease virus. Proc Natl Acad Sci U S A 97: Levy, A. M., O. Gilad, L. Xia, Y. Izumiya, J. Choi, A. Tsalenko, Z. Yakhini, R. Witter, L. Lee, C. J. Cardona, and H. J. Kung Marek's disease virus Meq transforms chicken cells via the v-jun transcriptional cascade: a converging transforming pathway for avian oncoviruses. Proc Natl Acad Sci U S A 102: Liu, J. L., and H. J. Kung Marek's disease herpesvirus transforming protein MEQ: a c-jun analogue with an alternative life style. Virus Genes 21: Lupiani, B., L. F. Lee, X. Cui, I. Gimeno, A. Anderson, R. W. Morgan, R. F. Silva, R. L. Witter, H. J. Kung, and S. M. Reddy Marek's disease virus-encoded Meq gene is involved in transformation of lymphocytes but is dispensable for replication. Proc Natl Acad Sci U S A 101: Epub 2004 Aug Lupiani, B., L. F. Lee, and S. M. Reddy Protein-coding content of the sequence of Marek's disease virus serotype 1. Curr Top Microbiol Immunol 255: Macdonald, A., K. Crowder, A. Street, C. McCormick, K. Saksela, and M. Harris The hepatitis C virus non-structural NS5A protein inhibits activating protein-1 function by perturbing Ras-ERK pathway signaling. Journal of Biological Chemistry 278: McColl, K. A., B. W. Calnek, W. V. Harris, K. A. Schat, and L. F. Lee Expression of a putative tumor-associated surface antigen on normal versus Marek's disease virus-transformed lymphocytes. J Natl Cancer Inst 79: Moore, F. R., K. A. Schat, N. Hutchison, C. Leciel, and S. E. Bloom Consistent chromosomal aberration in cell lines transformed with Marek's disease herpesvirus: evidence of genomic DNA amplification. Int J Cancer 54:
88 Morimura, T., M. Hattori, K. Ohashi, C. Sugimoto, and M. Onuma Immunomodulation of peripheral T cells in chickens infected with Marek's disease virus: involvement in immunosuppression. J Gen Virol 76: Morimura, T., K. Ohashi, Y. Kon, M. Hattori, C. Sugimoto, and M. Onuma Apoptosis in peripheral CD4+T cells and thymocytes by Marek's disease virus-infection. Leukemia 11: Murata, S., T. Hashiguchi, Y. Hayashi, Y. Yamamoto, A. MatsuyamaKato, S. Takasaki, M. Isezaki, M. Onuma, S. Konnai, and K. Ohashi. Characterization of Meq proteins from field isolates of Marek's disease virus in Japan. Infect Genet Evol 16: Murata, S., Tsukasa Okada, Rika Kano, Yuko Hayashi, Tomoyuki Hashiguchi, Misao Onuma, Satoru Konnai, and Kazuhiko Ohashi Analysis of transcriptional activities of the Meq proteins present in highly virulent Marek's disease virus strains, RB1B and Md : Mwangi, W. N., L. P. Smith, S. J. Baigent, A. L. Smith, and V. Nair Induction of lymphomas by inoculation of Marek's disease virus-derived lymphoblastoid cell lines: prevention by CVI988 vaccination. Avian Pathol 41: Mwimanzi, P., Z. Hasan, R. Hassan, S. Suzu, M. Takiguchi, and T. Ueno Effects of naturally-arising HIV Nef mutations on cytotoxic T lymphocyte recognition and Nef's functionality in primary macrophages. Retrovirology 8. Nair, V Evolution of Marek's disease - A paradigm for incessant race between the pathogen and the host. Vet J 170: Nair, V Successful control of Marek's disease by vaccination. Dev Biol (Basel) 119: Olmeda-Miro, N In vivo characterization of very virulent Marek's disease virus isolates. Masters. University of Delaware, Newark. Omar, A. R., and K. A. Schat Syngeneic Marek's disease virus (MDV)-specific cell-mediated immune responses against immediate early, late, and unique MDV proteins. Virology 222: Osterrieder, N., J. P. Kamil, D. Schumacher, B. K. Tischer, and S. Trapp Marek's disease virus: from miasma to model. Nat Rev Microbiol 4: Parcells, M. S., and S. C. Burgess Immunological Aspects of Marek's disease virus (MDV)-induced Lymphoma Progression, p In H. E. a. A. N. Kaiser (ed.), Selected Aspects of Cancer Progression: Metastasis, Apoptosis and Immune Response, vol. 11. Springer, The Netherlands. Parcells, M. S., J. Burnside, and R. W. Morgan Marek's disease virus (MDV)-induced T-cell Lymphomas, p In E. Robertson (ed.), Cancer Associated Viruses. Springer, New York. Parcells, M. S., R. L. Dienglewicz, A. S. Anderson, and R. W. Morgan Recombinant Marek's disease virus (MDV)-derived lymphoblastoid cell 74
89 lines: regulation of a marker gene within the context of the MDV genome. J Virol 73: Parvizi, P., M. F. Abdul-Careem, K. Haq, N. Thanthrige-Don, K. A. Schat, and S. Sharif. Immune responses against Marek's disease virus. Anim Health Res Rev 11: Pascale Jeannin, N. H., Yves Delneste, GiovanniMagistrelli, Sybille Lecoanet-Henchoz, Gersende Caron,Jean-Pierre Aubry and Jean-Yves Bonnefoy Human Effector Memory T Cells Express CD86: A Functional Role in Naive T Cell Priming. The Journal Of Immunology 162: Payne, L. N Pathology., p In L. N. Payne (ed.), Marek's Disease: Scientific Basis and Methods of Control, vol. xiii. Martinus Nijhoff Publishing, Boston. Petherbridge, L., K. Howes, S. J. Baigent, M. A. Sacco, S. Evans, N. Osterrieder, and V. Nair Replication-competent bacterial artificial chromosomes of Marek's disease virus: novel tools for generation of molecularly defined herpesvirus vaccines. J Virol 77: Pratt, W. D., R. W. Morgan, and K. A. Schat Characterization of reticuloendotheliosis virus-transformed avian T- lymphoblastoid cell lines infected with Marek's disease virus. J Virol 66: Purchase, H. G Recent advances in the knowledge of Marek's disease. Nippon Rinsho 30: Purchase, H. G Role of herpesviruses in Marek's disease, a malignant lymphoma of chickens. Am J Vet Res 33: Qian, Z., P. Brunovskis, L. Lee, P. K. Vogt, and H. J. Kung Novel DNA binding specificities of a putative herpesvirus bzip oncoprotein. J Virol 70: Qian, Z., P. Brunovskis, F. Rauscher, 3rd, L. Lee, and H. J. Kung Transactivation activity of Meq, a Marek's disease herpesvirus bzip protein persistently expressed in latently infected transformed T cells. J Virol 69: Qureshi, M. A., L. Miller, H. S. Lillehoj, and M. D. Ficken Establishment and characterization of a chicken mononuclear cell line. Vet Immunol Immunopathol 26: Rath, N. C., M. S. Parcells, H. Xie, and E. Santin Characterization of a spontaneously transformed chicken mononuclear cell line. Vet Immunol Immunopathol 96: Robinson, C. M Chromosomal integration of an avian oncogenic herpesvirus reveals telomeric preferences and evidence for lymphoma clonality. Herpesviridae 1:5. Rosenberger, J. K., S. S. Cloud, and N. Olmeda-Miro Presented at the Avian Tumor Virus Symposium, Reno, NV, July
90 Ross, N. L., W. DeLorbe, H. E. Varmus, J. M. Bishop, M. Brahic, and A. Haase Persistence and expression of Marek's disease virus DNA in tumour cells and peripheral nerves studied by in situ hybridization. J Gen Virol 57: Santin, E. R., C. E. Shamblin, J. T. Prigge, V. Arumugaswami, R. L. Dienglewicz, and M. S. Parcells Examination of the effect of a naturally occurring mutation in glycoprotein L on Marek's disease virus pathogenesis. Avian Dis 50: Schat, K. A Importance of cell-mediated immunity in Marek's disease and other viral tumor diseases. Poult Sci 70: Schat, K. A Marek's disease: a model for protection against herpesvirus-induced tumours. Cancer Surv 6:1-37. Schat, K. A., and Z. Xing Specific and nonspecific immune responses to Marek's disease virus. Dev Comp Immunol 24: Shack, L. A., J. J. Buza, and S. C. Burgess The neoplastically transformed (CD30hi) Marek's disease lymphoma cell phenotype most closely resembles T-regulatory cells. Cancer Immunol Immunother 57: Shamblin, C. E., N. Greene, V. Arumugaswami, R. L. Dienglewicz, and M. S. Parcells Comparative analysis of Marek's disease virus (MDV) glycoprotein-, lytic antigen pp38- and transformation antigen Meq-encoding genes: association of meq mutations with MDVs of high virulence. Vet Microbiol 102: Shek, W. R., B. W. Calnek, K. A. Schat, and C. H. Chen Characterization of Marek's disease virus-infected lymphocytes: discrimination between cytolytically and latently infected cells. J Natl Cancer Inst 70: SHENG WANG, N. N., GINA FUSARO, SRIKUMAR CHELLAPPAN Rb and Prohibitin Target Distinct Regions of E2F1 for Repression and Respond to Different Upstream Signals. MOLECULAR AND CELLULAR BIOLOGY, 19: Smedby, K. E., H. Hjalgrim, J. Askling, E. T Chang, H. Gregersen, A. Porwit-MacDonald, C. Sundstrom, M. Akerman, M. Melbye, B. Glimelius, and H. O. Adami Autoimmune and chronic inflammatory disorders and risk of non-hodgkin lymphoma by subtype. Journal of the National Cancer Institute 98: Suchodolski, P. F., Y. Izumiya, B. Lupiani, D. K. Ajithdoss, L. F. Lee, H. J. Kung, and S. M. Reddy Both homo and heterodimers of Marek's disease virus encoded Meq protein contribute to transformation of lymphocytes in chickens. Virology 399: Tulman, E. R., C. L. Afonso, Z. Lu, L. Zsak, D. L. Rock, and G. F. Kutish The genome of a very virulent Marek's disease virus. J Virol 74: Volpini, L. M., B. W. Calnek, M. J. Sekellick, and P. I. Marcus Stages of Marek's disease virus latency defined by variable sensitivity to interferon modulation of viral antigen expression. Vet Microbiol 47:
91 92. Wang, Z. Y., Q. Q. Qiu, and T. F. Deuel THE WILMS-TUMOR GENE-PRODUCT WT1 ACTIVATES OR SUPPRESSES TRANSCRIPTION THROUGH SEPARATE FUNCTIONAL DOMAINS. Journal of Biological Chemistry 268: Weinstock, D., K. A. Schat, and B. W. Calnek Cytotoxic T lymphocytes in reticuloendotheliosis virus-infected chickens. Eur J Immunol 19: William N. Mwangi, L. P. S., Susan J. Baigent, Richard K. Beal, Venugopal Nair, Adrian L., and Smith Clonal Structure of Rapid- Onset MDV-Driven CD4+ Lymphomas and Responding CD8+ T Cells. Pathogens Witter, R. L Avian tumor viruses: persistent and evolving pathogens. Acta Vet Hung 45: Witter, R. L Characteristics of Marek's disease viruses isolated from vaccinated commercial chicken flocks: association of viral pathotype with lymphoma frequency. Avian Dis 27: Witter, R. L Epidemiological studies relating to the control of Marek's Disease. J Gen Virol 9: Witter, R. L Increased virulence of Marek's disease virus field isolates. Avian Dis 41: Witter, R. L Marek's disease: The continuing struggle between pathogen and host. Vet J 170: Witter, R. L., B. W. Calnek, C. Buscaglia, I. M. Gimeno, and K. A. Schat Classification of Marek's disease viruses according to pathotype: philosophy and methodology. Avian Pathol 34: Witter, R. L., J. M. Sharma, W. B. Chase, D. A. Halvorson, and V. Sivanandan Field trials to test the efficacy of polyvalent Marek's disease vaccines in layer and broiler breeder chickens. Poult Sci 64: Witter, R. L., J. M. Sharma, and L. Offenbecker Turkey herpesvirus infection in chickens: induction of lymphoproliferative lesions and characterization of vaccinal immunity against Marek's disease. Avian Dis 20: Yan-JieWang, X.-L. G., Sheng-An Li, Yu-Qi Zhao, Zi-Chao Liu,Wen- Hui Lee,, and Y. Z. Yang Xiang Prohibitin is involved in the activated internalization and degradation of protease-activated receptor 1. Biochimica et Biophysica Acta 1843: Zhang, Z., W. Chen, C. Ma, P. Zhao, L. Duan, F. Zhang, A. Sun, Y. Li, H. Su, S. Li, H. Cui, and Z. Cui. Construction of recombinant Marek's disease virus (MDV) lacking the meq oncogene and co-expressing AIV-H9N2 HA and NA genes under control of exogenous promoters. J Biotechnol 181:
92 Appendix A CO-IMMUNOPRECIPITATION PULL-DOWN OF MEQ-ASSOCIATED PROTEINS FROM MDV-TRANSFORMED CELL LINES; MSB-1, UD35, UA53 A.1 Meq-Asociated proteins pull-owned from Meq in UA53 only and also UD35(last 2 rows) A.2 Meq-Associated proteins pull-downed from Meq in UD35 only 78
93 A.3 Meq-Associated Proteins Pull-downed from Meq in MSB-1 Only A.4 Meq-Associated proteins pull-downed from Meq in MSB-1 and all cells 79
Marek s disease and vaccination Presented by Dr Peter Makang a
 Challenge poultry Marek s disease and vaccination Presented by Dr Peter Makang a Economic impact of MD on the global poultry industry: 1 to 2 billion USD / year Jozsef MAREK (1868 1952) Multiple Nervenenzündung
Challenge poultry Marek s disease and vaccination Presented by Dr Peter Makang a Economic impact of MD on the global poultry industry: 1 to 2 billion USD / year Jozsef MAREK (1868 1952) Multiple Nervenenzündung
An update on Marek s disease. Isabel M. Gimeno
 An update on Marek s disease Isabel M. Gimeno Content Marek s disease (MD) and Marek s disease virus (MDV) evolution MDV-IS: the newest challenge of MDV infection MDV-induced tumors: Analysis of a MD outbreak-
An update on Marek s disease Isabel M. Gimeno Content Marek s disease (MD) and Marek s disease virus (MDV) evolution MDV-IS: the newest challenge of MDV infection MDV-induced tumors: Analysis of a MD outbreak-
Advances in Marek s Disease Vaccine Development:
 Advances in Marek s Disease Vaccine Development: Michel Bublot, DVM, PhD - Merial R&D Asian Avian Forum 2016, Tokyo, July 12-13 Marek s Disease Control Good flock management Cleaning, disinfection Adequate
Advances in Marek s Disease Vaccine Development: Michel Bublot, DVM, PhD - Merial R&D Asian Avian Forum 2016, Tokyo, July 12-13 Marek s Disease Control Good flock management Cleaning, disinfection Adequate
AviagenBrief. Marek s Disease Control in Broiler Breeders
 AviagenBrief January 2018 Marek s Disease Control in Broiler Breeders Author: A. Gregorio Rosales DVM, MS, PhD, DACPV - Poultry Health Consultant Introduction Marek s Disease Virus (MDV), a highly infectious
AviagenBrief January 2018 Marek s Disease Control in Broiler Breeders Author: A. Gregorio Rosales DVM, MS, PhD, DACPV - Poultry Health Consultant Introduction Marek s Disease Virus (MDV), a highly infectious
Corporation obtaining approval, the name of its representative, and the address of its main office
 Corporation obtaining approval, the name of its representative, and the address of its main office Name: The Chemo-Sero-Therapeutic Research Institute Applicant: Akinobu Funatsu, Director General Address:
Corporation obtaining approval, the name of its representative, and the address of its main office Name: The Chemo-Sero-Therapeutic Research Institute Applicant: Akinobu Funatsu, Director General Address:
ABSTRACT. oncogenic herpesvirus, Marek s disease virus (MDV). In addition to lymphoma, MDV induces
 ABSTRACT NIK MOHD AZMI, NIK MOHD FAIZ BIN. Characterization of Marek s Disease Virus- Induced Immunosuppression in Meat Type Chickens using Infectious Laryngotracheitis Challenge Model. (Under the direction
ABSTRACT NIK MOHD AZMI, NIK MOHD FAIZ BIN. Characterization of Marek s Disease Virus- Induced Immunosuppression in Meat Type Chickens using Infectious Laryngotracheitis Challenge Model. (Under the direction
EBV infection B cells and lymphomagenesis. Sridhar Chaganti
 EBV infection B cells and lymphomagenesis Sridhar Chaganti How EBV infects B-cells How viral genes influence the infected B cell Differences and similarities between in vitro and in vivo infection How
EBV infection B cells and lymphomagenesis Sridhar Chaganti How EBV infects B-cells How viral genes influence the infected B cell Differences and similarities between in vitro and in vivo infection How
Herpesviruses. Virion. Genome. Genes and proteins. Viruses and hosts. Diseases. Distinctive characteristics
 Herpesviruses Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Virion Enveloped icosahedral capsid (T=16), diameter 125 nm Diameter of enveloped virion 200 nm Capsid
Herpesviruses Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Virion Enveloped icosahedral capsid (T=16), diameter 125 nm Diameter of enveloped virion 200 nm Capsid
Medical Virology Immunology. Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University
 Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
INNATE PATTERNING OF THE IMMUNE RESPONSE TO MAREK'S DISEASE VIRUS (MDV) DURING PATHOGENESIS AND VACCINATION. Upendra Kumar Katneni
 INNATE PATTERNING OF THE IMMUNE RESPONSE TO MAREK'S DISEASE VIRUS (MDV) DURING PATHOGENESIS AND VACCINATION by Upendra Kumar Katneni A dissertation submitted to the Faculty of the University of Delaware
INNATE PATTERNING OF THE IMMUNE RESPONSE TO MAREK'S DISEASE VIRUS (MDV) DURING PATHOGENESIS AND VACCINATION by Upendra Kumar Katneni A dissertation submitted to the Faculty of the University of Delaware
Immunology - Lecture 2 Adaptive Immune System 1
 Immunology - Lecture 2 Adaptive Immune System 1 Book chapters: Molecules of the Adaptive Immunity 6 Adaptive Cells and Organs 7 Generation of Immune Diversity Lymphocyte Antigen Receptors - 8 CD markers
Immunology - Lecture 2 Adaptive Immune System 1 Book chapters: Molecules of the Adaptive Immunity 6 Adaptive Cells and Organs 7 Generation of Immune Diversity Lymphocyte Antigen Receptors - 8 CD markers
The Threat of Marek s Disease Virus Is Expanding
 The Threat of Marek s Disease Virus Is Expanding The virus responsible for this lymphoproliferative disease is growing more virulent and evading control by available vaccines Kyle S. MacLea and Hans H.
The Threat of Marek s Disease Virus Is Expanding The virus responsible for this lymphoproliferative disease is growing more virulent and evading control by available vaccines Kyle S. MacLea and Hans H.
COURSE: Medical Microbiology, PAMB 650/720 - Fall 2008 Lecture 16
 COURSE: Medical Microbiology, PAMB 650/720 - Fall 2008 Lecture 16 Tumor Immunology M. Nagarkatti Teaching Objectives: Introduction to Cancer Immunology Know the antigens expressed by cancer cells Understand
COURSE: Medical Microbiology, PAMB 650/720 - Fall 2008 Lecture 16 Tumor Immunology M. Nagarkatti Teaching Objectives: Introduction to Cancer Immunology Know the antigens expressed by cancer cells Understand
Third line of Defense
 Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
Avian Disease & Oncology Lab (ADOL) Research Update. John Dunn, Hans Cheng, Mohammad Heidari, Huanmin Zhang USDA-ARS-USPNRC
 Avian Disease & Oncology Lab (ADOL) Research Update John Dunn, Hans Cheng, Mohammad Heidari, Huanmin Zhang USDA-ARS-USPNRC Research Programs at ADOL 1) Genomics (Cheng, Zhang, Vacant SY) Title: Employing
Avian Disease & Oncology Lab (ADOL) Research Update John Dunn, Hans Cheng, Mohammad Heidari, Huanmin Zhang USDA-ARS-USPNRC Research Programs at ADOL 1) Genomics (Cheng, Zhang, Vacant SY) Title: Employing
FEATHER TIP MONITORING OF MAREK S DISEASE VIRUS IN EXPERIMENTAL AND COMMERCIAL SETTINGS. Milos Markis
 FEATHER TIP MONITORING OF MAREK S DISEASE VIRUS IN EXPERIMENTAL AND COMMERCIAL SETTINGS by Milos Markis A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements
FEATHER TIP MONITORING OF MAREK S DISEASE VIRUS IN EXPERIMENTAL AND COMMERCIAL SETTINGS by Milos Markis A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements
Overview of primary HHV-8 infection
 Overview of primary HHV-8 infection HHV-8, also known as Kaposi sarcoma-associated herpesvirus (KSHV), is a gamma herpesvirus primarily transmitted through saliva. The virus initially replicates in epithelial
Overview of primary HHV-8 infection HHV-8, also known as Kaposi sarcoma-associated herpesvirus (KSHV), is a gamma herpesvirus primarily transmitted through saliva. The virus initially replicates in epithelial
The Adaptive Immune Response. T-cells
 The Adaptive Immune Response T-cells T Lymphocytes T lymphocytes develop from precursors in the thymus. Mature T cells are found in the blood, where they constitute 60% to 70% of lymphocytes, and in T-cell
The Adaptive Immune Response T-cells T Lymphocytes T lymphocytes develop from precursors in the thymus. Mature T cells are found in the blood, where they constitute 60% to 70% of lymphocytes, and in T-cell
Fayth K. Yoshimura, Ph.D. September 7, of 7 HIV - BASIC PROPERTIES
 1 of 7 I. Viral Origin. A. Retrovirus - animal lentiviruses. HIV - BASIC PROPERTIES 1. HIV is a member of the Retrovirus family and more specifically it is a member of the Lentivirus genus of this family.
1 of 7 I. Viral Origin. A. Retrovirus - animal lentiviruses. HIV - BASIC PROPERTIES 1. HIV is a member of the Retrovirus family and more specifically it is a member of the Lentivirus genus of this family.
Chapter 1. Full file at
 Chapter 1 1. Which is the best definition of immunity? Answer: B A. The state of having been exposed to a pathogen repeatedly B. The state of being resistant to reinfection with a pathogen C. When an individual
Chapter 1 1. Which is the best definition of immunity? Answer: B A. The state of having been exposed to a pathogen repeatedly B. The state of being resistant to reinfection with a pathogen C. When an individual
AVIAN VIRAL TUMORS I. MAREK'S DISEASE
 DEFINITION The several viral neoplastic diseases of chickens and turkeys, although previously considered a "complex", are actually distinct disease entities. In some cases a single tumor virus strain can
DEFINITION The several viral neoplastic diseases of chickens and turkeys, although previously considered a "complex", are actually distinct disease entities. In some cases a single tumor virus strain can
GENERATION OF RECOMBINANT MAREK S DISEASE VIRUSES IN VIVO. Dawn Lenihan
 GENERATION OF RECOMBINANT MAREK S DISEASE VIRUSES IN VIVO by Dawn Lenihan A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for the degree of Honors
GENERATION OF RECOMBINANT MAREK S DISEASE VIRUSES IN VIVO by Dawn Lenihan A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for the degree of Honors
Diagnostic Considerations for Marek s Disease. Frederic J. Hoerr, DVM, PhD AMEVEA - Argentina Colon, Entre Rios May 15, 2013
 Diagnostic Considerations for Marek s Disease Frederic J. Hoerr, DVM, PhD AMEVEA - Argentina Colon, Entre Rios May 15, 2013 Control of Marek s Disease Accurate diagnosis Apply appropriate procedures to
Diagnostic Considerations for Marek s Disease Frederic J. Hoerr, DVM, PhD AMEVEA - Argentina Colon, Entre Rios May 15, 2013 Control of Marek s Disease Accurate diagnosis Apply appropriate procedures to
Principles of Adaptive Immunity
 Principles of Adaptive Immunity Chapter 3 Parham Hans de Haard 17 th of May 2010 Agenda Recognition molecules of adaptive immune system Features adaptive immune system Immunoglobulins and T-cell receptors
Principles of Adaptive Immunity Chapter 3 Parham Hans de Haard 17 th of May 2010 Agenda Recognition molecules of adaptive immune system Features adaptive immune system Immunoglobulins and T-cell receptors
Large DNA viruses: Herpesviruses, Poxviruses, Baculoviruses and Giant viruses
 Large DNA viruses: Herpesviruses, Poxviruses, Baculoviruses and Giant viruses Viruses are the only obstacles to the domination of the Earth by mankind. -Joshua Lederberg Recommended reading: Field s Virology
Large DNA viruses: Herpesviruses, Poxviruses, Baculoviruses and Giant viruses Viruses are the only obstacles to the domination of the Earth by mankind. -Joshua Lederberg Recommended reading: Field s Virology
The Adaptive Immune Responses
 The Adaptive Immune Responses The two arms of the immune responses are; 1) the cell mediated, and 2) the humoral responses. In this chapter we will discuss the two responses in detail and we will start
The Adaptive Immune Responses The two arms of the immune responses are; 1) the cell mediated, and 2) the humoral responses. In this chapter we will discuss the two responses in detail and we will start
Research Update: Avian Disease & Oncology Lab (ADOL) and SEPRL Endemic Poultry Virus Diseases (EPVD)
 Research Update: Avian Disease & Oncology Lab (ADOL) and SEPRL Endemic Poultry Virus Diseases (EPVD) John Dunn, Hans Cheng, Mohammad Heidari, Huanmin Zhang, Taejoong Kim, Stephen Spatz, Qingzhong Yu USDA-ARS-USPNRC
Research Update: Avian Disease & Oncology Lab (ADOL) and SEPRL Endemic Poultry Virus Diseases (EPVD) John Dunn, Hans Cheng, Mohammad Heidari, Huanmin Zhang, Taejoong Kim, Stephen Spatz, Qingzhong Yu USDA-ARS-USPNRC
Determination of the temporal pattern and importance of BALF1 expression in Epstein-Barr viral infection
 Determination of the temporal pattern and importance of BALF1 expression in Epstein-Barr viral infection Melissa Mihelidakis May 6, 2004 7.340 Research Proposal Introduction Apoptosis, or programmed cell
Determination of the temporal pattern and importance of BALF1 expression in Epstein-Barr viral infection Melissa Mihelidakis May 6, 2004 7.340 Research Proposal Introduction Apoptosis, or programmed cell
CELL BIOLOGY - CLUTCH CH THE IMMUNE SYSTEM.
 !! www.clutchprep.com CONCEPT: OVERVIEW OF HOST DEFENSES The human body contains three lines of against infectious agents (pathogens) 1. Mechanical and chemical boundaries (part of the innate immune system)
!! www.clutchprep.com CONCEPT: OVERVIEW OF HOST DEFENSES The human body contains three lines of against infectious agents (pathogens) 1. Mechanical and chemical boundaries (part of the innate immune system)
ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS. Choompone Sakonwasun, MD (Hons), FRCPT
 ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS Choompone Sakonwasun, MD (Hons), FRCPT Types of Adaptive Immunity Types of T Cell-mediated Immune Reactions CTLs = cytotoxic T lymphocytes
ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS Choompone Sakonwasun, MD (Hons), FRCPT Types of Adaptive Immunity Types of T Cell-mediated Immune Reactions CTLs = cytotoxic T lymphocytes
The Immune System. These are classified as the Innate and Adaptive Immune Responses. Innate Immunity
 The Immune System Biological mechanisms that defend an organism must be 1. triggered by a stimulus upon injury or pathogen attack 2. able to counteract the injury or invasion 3. able to recognise foreign
The Immune System Biological mechanisms that defend an organism must be 1. triggered by a stimulus upon injury or pathogen attack 2. able to counteract the injury or invasion 3. able to recognise foreign
Understanding Marek s Disease Immunity: A Continuing Challenge
 International Journal of Poultry Science 3 (1): 89-95, 2004 Asian Network for Scientific Information 2004 Understanding Marek s Disease Immunity: A Continuing Challenge K.A. Schat Unit of Avian Medicine,
International Journal of Poultry Science 3 (1): 89-95, 2004 Asian Network for Scientific Information 2004 Understanding Marek s Disease Immunity: A Continuing Challenge K.A. Schat Unit of Avian Medicine,
7.012 Quiz 3 Answers
 MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
The Adaptive Immune Response: T lymphocytes and Their Functional Types *
 OpenStax-CNX module: m46560 1 The Adaptive Immune Response: T lymphocytes and Their Functional Types * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
OpenStax-CNX module: m46560 1 The Adaptive Immune Response: T lymphocytes and Their Functional Types * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
Persistent Infections
 Persistent Infections Lecture 17 Biology 3310/4310 Virology Spring 2017 Paralyze resistance with persistence WOODY HAYES Acute vs persistent infections Acute infection - rapid and self-limiting Persistent
Persistent Infections Lecture 17 Biology 3310/4310 Virology Spring 2017 Paralyze resistance with persistence WOODY HAYES Acute vs persistent infections Acute infection - rapid and self-limiting Persistent
Third line of Defense. Topic 8 Specific Immunity (adaptive) (18) 3 rd Line = Prophylaxis via Immunization!
 Topic 8 Specific Immunity (adaptive) (18) Topics - 3 rd Line of Defense - B cells - T cells - Specific Immunities 1 3 rd Line = Prophylaxis via Immunization! (a) A painting of Edward Jenner depicts a cow
Topic 8 Specific Immunity (adaptive) (18) Topics - 3 rd Line of Defense - B cells - T cells - Specific Immunities 1 3 rd Line = Prophylaxis via Immunization! (a) A painting of Edward Jenner depicts a cow
Immunology Lecture 4. Clinical Relevance of the Immune System
 Immunology Lecture 4 The Well Patient: How innate and adaptive immune responses maintain health - 13, pg 169-181, 191-195. Immune Deficiency - 15 Autoimmunity - 16 Transplantation - 17, pg 260-270 Tumor
Immunology Lecture 4 The Well Patient: How innate and adaptive immune responses maintain health - 13, pg 169-181, 191-195. Immune Deficiency - 15 Autoimmunity - 16 Transplantation - 17, pg 260-270 Tumor
THE ROLE OF MAREK S DISEASE VIRUS MEQ GENE PRODUCTS ON MDV PATHOGENICITY AND ONCOGENICITY. Huimin Dong
 THE ROLE OF MAREK S DISEASE VIRUS MEQ GENE PRODUCTS ON MDV PATHOGENICITY AND ONCOGENICITY by Huimin Dong A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements
THE ROLE OF MAREK S DISEASE VIRUS MEQ GENE PRODUCTS ON MDV PATHOGENICITY AND ONCOGENICITY by Huimin Dong A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements
ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY
 ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY The recognition of specific antigen by naïve T cell induces its own activation and effector phases. T helper cells recognize peptide antigens through
ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY The recognition of specific antigen by naïve T cell induces its own activation and effector phases. T helper cells recognize peptide antigens through
Adaptive immune responses: T cell-mediated immunity
 MICR2209 Adaptive immune responses: T cell-mediated immunity Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will discuss the T-cell mediated immune response, how it is activated,
MICR2209 Adaptive immune responses: T cell-mediated immunity Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will discuss the T-cell mediated immune response, how it is activated,
Identification and Molecular Cloning of Marek s Disease Virus Type 3 Glycoprotein M and Glycoprotein K
 International Journal of Genetic Engineering and Biotechnology. ISSN 0974-3073 Volume 1, Number 1 (2010), pp. 57-64 International Research Publication House http://www.irphouse.com Identification and Molecular
International Journal of Genetic Engineering and Biotechnology. ISSN 0974-3073 Volume 1, Number 1 (2010), pp. 57-64 International Research Publication House http://www.irphouse.com Identification and Molecular
Immune System AP SBI4UP
 Immune System AP SBI4UP TYPES OF IMMUNITY INNATE IMMUNITY ACQUIRED IMMUNITY EXTERNAL DEFENCES INTERNAL DEFENCES HUMORAL RESPONSE Skin Phagocytic Cells CELL- MEDIATED RESPONSE Mucus layer Antimicrobial
Immune System AP SBI4UP TYPES OF IMMUNITY INNATE IMMUNITY ACQUIRED IMMUNITY EXTERNAL DEFENCES INTERNAL DEFENCES HUMORAL RESPONSE Skin Phagocytic Cells CELL- MEDIATED RESPONSE Mucus layer Antimicrobial
1. Overview of Adaptive Immunity
 Chapter 17A: Adaptive Immunity Part I 1. Overview of Adaptive Immunity 2. T and B Cell Production 3. Antigens & Antigen Presentation 4. Helper T cells 1. Overview of Adaptive Immunity The Nature of Adaptive
Chapter 17A: Adaptive Immunity Part I 1. Overview of Adaptive Immunity 2. T and B Cell Production 3. Antigens & Antigen Presentation 4. Helper T cells 1. Overview of Adaptive Immunity The Nature of Adaptive
Human Immunodeficiency Virus
 Human Immunodeficiency Virus Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Viruses and hosts Lentivirus from Latin lentis (slow), for slow progression of disease
Human Immunodeficiency Virus Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Viruses and hosts Lentivirus from Latin lentis (slow), for slow progression of disease
Adaptive Immunity: Humoral Immune Responses
 MICR2209 Adaptive Immunity: Humoral Immune Responses Dr Allison Imrie 1 Synopsis: In this lecture we will review the different mechanisms which constitute the humoral immune response, and examine the antibody
MICR2209 Adaptive Immunity: Humoral Immune Responses Dr Allison Imrie 1 Synopsis: In this lecture we will review the different mechanisms which constitute the humoral immune response, and examine the antibody
Random Sample Pages for Preview
 MAREK S DISEASE Slide study set # 26 Prepared by ISABEL M. GIMENO A, RICHARD L. WITTER A AND ANDREA MILES B A USDA-ARS Avian Disease and Oncology Laboratory. East Lansing. MI B USDA APHIS Veterinary Services.
MAREK S DISEASE Slide study set # 26 Prepared by ISABEL M. GIMENO A, RICHARD L. WITTER A AND ANDREA MILES B A USDA-ARS Avian Disease and Oncology Laboratory. East Lansing. MI B USDA APHIS Veterinary Services.
VIRUSES. Biology Applications Control. David R. Harper. Garland Science Taylor & Francis Group NEW YORK AND LONDON
 VIRUSES Biology Applications Control David R. Harper GS Garland Science Taylor & Francis Group NEW YORK AND LONDON vii Chapter 1 Virus Structure and 2.2 VIRUS MORPHOLOGY 26 Infection 1 2.3 VIRAL CLASSIFICATION
VIRUSES Biology Applications Control David R. Harper GS Garland Science Taylor & Francis Group NEW YORK AND LONDON vii Chapter 1 Virus Structure and 2.2 VIRUS MORPHOLOGY 26 Infection 1 2.3 VIRAL CLASSIFICATION
Investigation of the genetic differences between bovine herpesvirus type 1 variants and vaccine strains
 Investigation of the genetic differences between bovine herpesvirus type 1 variants and vaccine strains Name: Claire Ostertag-Hill Mentor: Dr. Ling Jin Bovine herpesvirus Bovine herpesvirus-1 (BHV-1) Pathogen
Investigation of the genetic differences between bovine herpesvirus type 1 variants and vaccine strains Name: Claire Ostertag-Hill Mentor: Dr. Ling Jin Bovine herpesvirus Bovine herpesvirus-1 (BHV-1) Pathogen
Section Lectures: Immunology/Virology Time: 9:00 am 10:00 am LRC 105 A & B
 Section Director: Cliff Bellone, Ph.D. Office: Doisy Hall - R 405 Phone: 577-8449 E-Mail: bellonec@slu.edu Lecturers: James Swierkosz, Ph.D. Office: Medical School Rm. 412 Phone: 577-8430 E-Mail: swierkoszje@slu.edu
Section Director: Cliff Bellone, Ph.D. Office: Doisy Hall - R 405 Phone: 577-8449 E-Mail: bellonec@slu.edu Lecturers: James Swierkosz, Ph.D. Office: Medical School Rm. 412 Phone: 577-8430 E-Mail: swierkoszje@slu.edu
Immunology for the Rheumatologist
 Immunology for the Rheumatologist Rheumatologists frequently deal with the immune system gone awry, rarely studying normal immunology. This program is an overview and discussion of the function of the
Immunology for the Rheumatologist Rheumatologists frequently deal with the immune system gone awry, rarely studying normal immunology. This program is an overview and discussion of the function of the
Physiology Unit 3. ADAPTIVE IMMUNITY The Specific Immune Response
 Physiology Unit 3 ADAPTIVE IMMUNITY The Specific Immune Response In Physiology Today The Adaptive Arm of the Immune System Specific Immune Response Internal defense against a specific pathogen Acquired
Physiology Unit 3 ADAPTIVE IMMUNITY The Specific Immune Response In Physiology Today The Adaptive Arm of the Immune System Specific Immune Response Internal defense against a specific pathogen Acquired
Supplemental Table I.
 Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
Complete nucleotide sequence analysis of oncogenicity associated Gene pp38 of Serotype 1 Marek s disease virus isolates from India
 2018; SP1: 2197-2204 E-ISSN: 2278-4136 P-ISSN: 2349-8234 JPP 2018; SP1: 2197-2204 P Suresh Assistant Professor, Department of Veterinary Microbiology, Veterinary College and Research Institute, Namakkal,
2018; SP1: 2197-2204 E-ISSN: 2278-4136 P-ISSN: 2349-8234 JPP 2018; SP1: 2197-2204 P Suresh Assistant Professor, Department of Veterinary Microbiology, Veterinary College and Research Institute, Namakkal,
Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells
 ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
Polyomaviridae. Spring
 Polyomaviridae Spring 2002 331 Antibody Prevalence for BK & JC Viruses Spring 2002 332 Polyoma Viruses General characteristics Papovaviridae: PA - papilloma; PO - polyoma; VA - vacuolating agent a. 45nm
Polyomaviridae Spring 2002 331 Antibody Prevalence for BK & JC Viruses Spring 2002 332 Polyoma Viruses General characteristics Papovaviridae: PA - papilloma; PO - polyoma; VA - vacuolating agent a. 45nm
LESSON 2: THE ADAPTIVE IMMUNITY
 Introduction to immunology. LESSON 2: THE ADAPTIVE IMMUNITY Today we will get to know: The adaptive immunity T- and B-cells Antigens and their recognition How T-cells work 1 The adaptive immunity Unlike
Introduction to immunology. LESSON 2: THE ADAPTIVE IMMUNITY Today we will get to know: The adaptive immunity T- and B-cells Antigens and their recognition How T-cells work 1 The adaptive immunity Unlike
Influenza viruses. Virion. Genome. Genes and proteins. Viruses and hosts. Diseases. Distinctive characteristics
 Influenza viruses Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Virion Enveloped particles, quasi-spherical or filamentous Diameter 80-120 nm Envelope is derived
Influenza viruses Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Virion Enveloped particles, quasi-spherical or filamentous Diameter 80-120 nm Envelope is derived
I. INTRODUCTION. The White revolution in India during the past three decades has revived the Indian
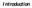 Introduction I. INTRODUCTION The White revolution in India during the past three decades has revived the Indian rural economy by giving its populace the much needed sustainable monetary security and a
Introduction I. INTRODUCTION The White revolution in India during the past three decades has revived the Indian rural economy by giving its populace the much needed sustainable monetary security and a
Parallel session IVB. Chair: Hans Wildiers
 Parallel session IVB Biology of ageing and cancer Chair: Hans Wildiers IMMUNOSENESCENCE AND AGEING Tamas Fulop M.D., PhD Research Center on Aging, Division of Geriatrics, University of Sherbrooke, Sherbrooke,
Parallel session IVB Biology of ageing and cancer Chair: Hans Wildiers IMMUNOSENESCENCE AND AGEING Tamas Fulop M.D., PhD Research Center on Aging, Division of Geriatrics, University of Sherbrooke, Sherbrooke,
How T cells recognize antigen: The T Cell Receptor (TCR) Identifying the TCR: Why was it so hard to do? Monoclonal antibody approach
 How T cells recognize antigen: The T Cell Receptor (TCR) Identifying the TCR: Why was it so hard to do By the early 1980s, much about T cell function was known, but the receptor genes had not been identified
How T cells recognize antigen: The T Cell Receptor (TCR) Identifying the TCR: Why was it so hard to do By the early 1980s, much about T cell function was known, but the receptor genes had not been identified
A second type of TCR TCR: An αβ heterodimer
 How s recognize antigen: The T Cell Receptor (TCR) Identifying the TCR: Why was it so hard to do By the early 1980s, much about function was known, but the receptor genes had not been identified Recall
How s recognize antigen: The T Cell Receptor (TCR) Identifying the TCR: Why was it so hard to do By the early 1980s, much about function was known, but the receptor genes had not been identified Recall
Microbiology 204: Cellular and Molecular Immunology
 Microbiology 204: Cellular and Molecular Immunology Class meets MWF 1:00-2:30PM (*exceptions: no class Fri Sept 23, Fri Oct 14, Nov 11, or Wed Nov 23) Lectures are open to auditors and will be live-streamed
Microbiology 204: Cellular and Molecular Immunology Class meets MWF 1:00-2:30PM (*exceptions: no class Fri Sept 23, Fri Oct 14, Nov 11, or Wed Nov 23) Lectures are open to auditors and will be live-streamed
Immunity to Viruses. Patricia Fitzgerald-Bocarsly September 25, 2008
 Immunity to Viruses Patricia Fitzgerald-Bocarsly September 25, 2008 The Immune System Deals with a Huge Range of Pathogens Roitt, 2003 Immune Responses to Viruses Viruses are dependent on the host cell
Immunity to Viruses Patricia Fitzgerald-Bocarsly September 25, 2008 The Immune System Deals with a Huge Range of Pathogens Roitt, 2003 Immune Responses to Viruses Viruses are dependent on the host cell
Viruses. Poxviridae. DNA viruses: 6 families. Herpesviridae Adenoviridae. Hepadnaviridae Papovaviridae Parvoviridae
 Viruses DNA viruses: 6 families Poxviridae Herpesviridae Adenoviridae Hepadnaviridae Papovaviridae Parvoviridae Human herpesviruses Three subfamilies (genome structure, tissue tropism, cytopathologic effect,
Viruses DNA viruses: 6 families Poxviridae Herpesviridae Adenoviridae Hepadnaviridae Papovaviridae Parvoviridae Human herpesviruses Three subfamilies (genome structure, tissue tropism, cytopathologic effect,
RAISON D ETRE OF THE IMMUNE SYSTEM:
 RAISON D ETRE OF THE IMMUNE SYSTEM: To Distinguish Self from Non-Self Thereby Protecting Us From Our Hostile Environment. Innate Immunity Acquired Immunity Innate immunity: (Antigen nonspecific) defense
RAISON D ETRE OF THE IMMUNE SYSTEM: To Distinguish Self from Non-Self Thereby Protecting Us From Our Hostile Environment. Innate Immunity Acquired Immunity Innate immunity: (Antigen nonspecific) defense
General Overview of Immunology. Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center
 General Overview of Immunology Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center Objectives Describe differences between innate and adaptive immune responses
General Overview of Immunology Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center Objectives Describe differences between innate and adaptive immune responses
Host Genetic Resistance Sustains HVT Protective Efficacy Comparable to CVI988/Rispens in Lines of Chickens Relatively Resistant to Marek s Disease
 Host Genetic Resistance Sustains HVT Protective Efficacy Comparable to CVI988/Rispens in Lines of Chickens Relatively Resistant to Marek s Disease Huanmin Zhang 1, Shuang Chang 1, 2, John R. Dunn 1 Mohammad
Host Genetic Resistance Sustains HVT Protective Efficacy Comparable to CVI988/Rispens in Lines of Chickens Relatively Resistant to Marek s Disease Huanmin Zhang 1, Shuang Chang 1, 2, John R. Dunn 1 Mohammad
Infectious Bursal Disease, Immunosuppression and the role of VAXXITEK HVT+ IBD
 Research note Infectious Bursal Disease, Immunosuppression and the role of VAXXITEK HVT+ IBD Grogan K. 1 1 Poultry Chicken Scratch, LLC, 30019 Dacula GA United States of America [from Hoerr F.J., 2010,
Research note Infectious Bursal Disease, Immunosuppression and the role of VAXXITEK HVT+ IBD Grogan K. 1 1 Poultry Chicken Scratch, LLC, 30019 Dacula GA United States of America [from Hoerr F.J., 2010,
chapter 17: specific/adaptable defenses of the host: the immune response
 chapter 17: specific/adaptable defenses of the host: the immune response defense against infection & illness body defenses innate/ non-specific adaptable/ specific epithelium, fever, inflammation, complement,
chapter 17: specific/adaptable defenses of the host: the immune response defense against infection & illness body defenses innate/ non-specific adaptable/ specific epithelium, fever, inflammation, complement,
Bihong Zhao, M.D, Ph.D Department of Pathology
 Bihong Zhao, M.D, Ph.D Department of Pathology 04-28-2009 Is tumor self or non-self? How are tumor antigens generated? What are they? How does immune system respond? Introduction Tumor Antigens/Categories
Bihong Zhao, M.D, Ph.D Department of Pathology 04-28-2009 Is tumor self or non-self? How are tumor antigens generated? What are they? How does immune system respond? Introduction Tumor Antigens/Categories
Tumors arise from accumulated genetic mutations. Tumor Immunology (Cancer)
 Tumor Immunology (Cancer) Tumors arise from accumulated genetic mutations Robert Beatty MCB150 Mutations Usually have >6 mutations in both activation/growth factors and tumor suppressor genes. Types of
Tumor Immunology (Cancer) Tumors arise from accumulated genetic mutations Robert Beatty MCB150 Mutations Usually have >6 mutations in both activation/growth factors and tumor suppressor genes. Types of
VIRUSES AND CANCER Michael Lea
 VIRUSES AND CANCER 2010 Michael Lea VIRAL ONCOLOGY - LECTURE OUTLINE 1. Historical Review 2. Viruses Associated with Cancer 3. RNA Tumor Viruses 4. DNA Tumor Viruses HISTORICAL REVIEW Historical Review
VIRUSES AND CANCER 2010 Michael Lea VIRAL ONCOLOGY - LECTURE OUTLINE 1. Historical Review 2. Viruses Associated with Cancer 3. RNA Tumor Viruses 4. DNA Tumor Viruses HISTORICAL REVIEW Historical Review
Differential susceptibility to Marek s disease is associated with differences in number, but not phenotype or location, of pp38 M lymphocytes
 Journal of General Virology (1998), 79, 2795 2802. Printed in Great Britain...... Differential susceptibility to Marek s disease is associated with differences in number, but not phenotype or location,
Journal of General Virology (1998), 79, 2795 2802. Printed in Great Britain...... Differential susceptibility to Marek s disease is associated with differences in number, but not phenotype or location,
BIT 120. Copy of Cancer/HIV Lecture
 BIT 120 Copy of Cancer/HIV Lecture Cancer DEFINITION Any abnormal growth of cells that has malignant potential i.e.. Leukemia Uncontrolled mitosis in WBC Genetic disease caused by an accumulation of mutations
BIT 120 Copy of Cancer/HIV Lecture Cancer DEFINITION Any abnormal growth of cells that has malignant potential i.e.. Leukemia Uncontrolled mitosis in WBC Genetic disease caused by an accumulation of mutations
IMMUNE CELL SURFACE RECEPTORS AND THEIR FUNCTIONS
 LECTURE: 07 Title: IMMUNE CELL SURFACE RECEPTORS AND THEIR FUNCTIONS LEARNING OBJECTIVES: The student should be able to: The chemical nature of the cellular surface receptors. Define the location of the
LECTURE: 07 Title: IMMUNE CELL SURFACE RECEPTORS AND THEIR FUNCTIONS LEARNING OBJECTIVES: The student should be able to: The chemical nature of the cellular surface receptors. Define the location of the
Lecture 2: Virology. I. Background
 Lecture 2: Virology I. Background A. Properties 1. Simple biological systems a. Aggregates of nucleic acids and protein 2. Non-living a. Cannot reproduce or carry out metabolic activities outside of a
Lecture 2: Virology I. Background A. Properties 1. Simple biological systems a. Aggregates of nucleic acids and protein 2. Non-living a. Cannot reproduce or carry out metabolic activities outside of a
T Cell Effector Mechanisms I: B cell Help & DTH
 T Cell Effector Mechanisms I: B cell Help & DTH Ned Braunstein, MD The Major T Cell Subsets p56 lck + T cells γ δ ε ζ ζ p56 lck CD8+ T cells γ δ ε ζ ζ Cα Cβ Vα Vβ CD3 CD8 Cα Cβ Vα Vβ CD3 MHC II peptide
T Cell Effector Mechanisms I: B cell Help & DTH Ned Braunstein, MD The Major T Cell Subsets p56 lck + T cells γ δ ε ζ ζ p56 lck CD8+ T cells γ δ ε ζ ζ Cα Cβ Vα Vβ CD3 CD8 Cα Cβ Vα Vβ CD3 MHC II peptide
T cell maturation. T-cell Maturation. What allows T cell maturation?
 T-cell Maturation What allows T cell maturation? Direct contact with thymic epithelial cells Influence of thymic hormones Growth factors (cytokines, CSF) T cell maturation T cell progenitor DN DP SP 2ry
T-cell Maturation What allows T cell maturation? Direct contact with thymic epithelial cells Influence of thymic hormones Growth factors (cytokines, CSF) T cell maturation T cell progenitor DN DP SP 2ry
Hepatitis virus immunity. Mar 9, 2005 Rehermann and Nascimbeni review Crispe review
 Hepatitis virus immunity Mar 9, 2005 Rehermann and Nascimbeni review Crispe review HBV & HCV infection outcomes Both viruses cause immune-mediated active and chronic hepatitis HBV Vertical transmission
Hepatitis virus immunity Mar 9, 2005 Rehermann and Nascimbeni review Crispe review HBV & HCV infection outcomes Both viruses cause immune-mediated active and chronic hepatitis HBV Vertical transmission
Response of the Embryo to in ovo
 Response of the Embryo to in ovo Vaccination with IBDV Jagdev Sharma a The Biodesign Institute Arizona State University Tempe, Arizona, U.S.A. BUDAPEST 37 Billion chickens produced yearly y IBDV A highly
Response of the Embryo to in ovo Vaccination with IBDV Jagdev Sharma a The Biodesign Institute Arizona State University Tempe, Arizona, U.S.A. BUDAPEST 37 Billion chickens produced yearly y IBDV A highly
Chapter 13 Lymphatic and Immune Systems
 The Chapter 13 Lymphatic and Immune Systems 1 The Lymphatic Vessels Lymphoid Organs Three functions contribute to homeostasis 1. Return excess tissue fluid to the bloodstream 2. Help defend the body against
The Chapter 13 Lymphatic and Immune Systems 1 The Lymphatic Vessels Lymphoid Organs Three functions contribute to homeostasis 1. Return excess tissue fluid to the bloodstream 2. Help defend the body against
C. Incorrect! MHC class I molecules are not involved in the process of bridging in ADCC.
 Immunology - Problem Drill 13: T- Cell Mediated Immunity Question No. 1 of 10 1. During Antibody-dependent cell mediated cytotoxicity (ADCC), the antibody acts like a bridge between the specific antigen
Immunology - Problem Drill 13: T- Cell Mediated Immunity Question No. 1 of 10 1. During Antibody-dependent cell mediated cytotoxicity (ADCC), the antibody acts like a bridge between the specific antigen
Chapter 22: The Lymphatic System and Immunity
 Bio40C schedule Lecture Immune system Lab Quiz 2 this week; bring a scantron! Study guide on my website (see lab assignments) Extra credit Critical thinking questions at end of chapters 5 pts/chapter Due
Bio40C schedule Lecture Immune system Lab Quiz 2 this week; bring a scantron! Study guide on my website (see lab assignments) Extra credit Critical thinking questions at end of chapters 5 pts/chapter Due
VMC-221: Veterinary Immunology and Serology (1+1) Question Bank
 VMC-221: Veterinary Immunology and Serology (1+1) Objective type Questions Question Bank Q. No. 1 - Fill up the blanks with correct words 1. The British physician, who developed the first vaccine against
VMC-221: Veterinary Immunology and Serology (1+1) Objective type Questions Question Bank Q. No. 1 - Fill up the blanks with correct words 1. The British physician, who developed the first vaccine against
The humoral immune responses to IBV proteins.
 The humoral immune responses to IBV proteins. E. Dan Heller and Rosa Meir The Hebrew University of Jerusalem, Israel COST FA1207 meeting WG2 + WG3, Budapest, Jan. 2015 1 IBV encodes four major structural
The humoral immune responses to IBV proteins. E. Dan Heller and Rosa Meir The Hebrew University of Jerusalem, Israel COST FA1207 meeting WG2 + WG3, Budapest, Jan. 2015 1 IBV encodes four major structural
The development of T cells in the thymus
 T cells rearrange their receptors in the thymus whereas B cells do so in the bone marrow. The development of T cells in the thymus The lobular/cellular organization of the thymus Immature cells are called
T cells rearrange their receptors in the thymus whereas B cells do so in the bone marrow. The development of T cells in the thymus The lobular/cellular organization of the thymus Immature cells are called
Alec Steep Submitted: July 28 th, Committee: Eran Andrechek Titus Brown Hans Cheng Jerry Dodgson Michele Fluck
 Discovery of Somatic Driver Variations of MDV-induced Transformations and Genetic Elements Demonstrating Resistance to Marek s Disease via Cytogenetic Analysis, Genome-Wide Selection, and Next-Generation
Discovery of Somatic Driver Variations of MDV-induced Transformations and Genetic Elements Demonstrating Resistance to Marek s Disease via Cytogenetic Analysis, Genome-Wide Selection, and Next-Generation
VZV, EBV, and HHV-6-8
 VZV, EBV, and HHV-6-8 Anne Gershon Common Features of Herpesviruses Morphology Basic mode of replication Primary infection followed by latency Ubiquitous Ability to cause recurrent infections (reactivation
VZV, EBV, and HHV-6-8 Anne Gershon Common Features of Herpesviruses Morphology Basic mode of replication Primary infection followed by latency Ubiquitous Ability to cause recurrent infections (reactivation
Effector Mechanisms of Cell-Mediated Immunity
 Effector Mechanisms of Cell-Mediated Immunity Dr. Julia Rempel Section of Hepatology 789-3825 jdrempel@cc.umanitoba.ca 804D JBRC Topics: I. Types of Cell-Mediated Immunity II. Migration of Effector T Lymphocytes
Effector Mechanisms of Cell-Mediated Immunity Dr. Julia Rempel Section of Hepatology 789-3825 jdrempel@cc.umanitoba.ca 804D JBRC Topics: I. Types of Cell-Mediated Immunity II. Migration of Effector T Lymphocytes
Fluid movement in capillaries. Not all fluid is reclaimed at the venous end of the capillaries; that is the job of the lymphatic system
 Capillary exchange Fluid movement in capillaries Not all fluid is reclaimed at the venous end of the capillaries; that is the job of the lymphatic system Lymphatic vessels Lymphatic capillaries permeate
Capillary exchange Fluid movement in capillaries Not all fluid is reclaimed at the venous end of the capillaries; that is the job of the lymphatic system Lymphatic vessels Lymphatic capillaries permeate
otherwise known as Cytotoxic T lymphocytes (CTLs)
 MIT Biology Department 7.012: Introductory Biology - Fall 200 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel NAME TA SEC 7.012 Problem Set 5 FRIDAY October 29, 2004
MIT Biology Department 7.012: Introductory Biology - Fall 200 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel NAME TA SEC 7.012 Problem Set 5 FRIDAY October 29, 2004
The Innate Immune Response
 The Innate Immune Response FUNCTIONS OF THE IMMUNE SYSTEM: Recognize, destroy and clear a diversity of pathogens. Initiate tissue and wound healing processes. Recognize and clear damaged self components.
The Innate Immune Response FUNCTIONS OF THE IMMUNE SYSTEM: Recognize, destroy and clear a diversity of pathogens. Initiate tissue and wound healing processes. Recognize and clear damaged self components.
Innate Immunity. Chapter 3. Connection Between Innate and Adaptive Immunity. Know Differences and Provide Examples. Antimicrobial peptide psoriasin
 Chapter Know Differences and Provide Examples Innate Immunity kin and Epithelial Barriers Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive Immunity
Chapter Know Differences and Provide Examples Innate Immunity kin and Epithelial Barriers Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive Immunity
Chapter 10 (pages ): Differentiation and Functions of CD4+ Effector T Cells Prepared by Kristen Dazy, MD, Scripps Clinic Medical Group
 FIT Board Review Corner September 2015 Welcome to the FIT Board Review Corner, prepared by Andrew Nickels, MD, and Sarah Spriet, DO, senior and junior representatives of ACAAI's Fellows-In-Training (FITs)
FIT Board Review Corner September 2015 Welcome to the FIT Board Review Corner, prepared by Andrew Nickels, MD, and Sarah Spriet, DO, senior and junior representatives of ACAAI's Fellows-In-Training (FITs)
Follicular Lymphoma. ced3 APOPTOSIS. *In the nematode Caenorhabditis elegans 131 of the organism's 1031 cells die during development.
 Harvard-MIT Division of Health Sciences and Technology HST.176: Cellular and Molecular Immunology Course Director: Dr. Shiv Pillai Follicular Lymphoma 1. Characterized by t(14:18) translocation 2. Ig heavy
Harvard-MIT Division of Health Sciences and Technology HST.176: Cellular and Molecular Immunology Course Director: Dr. Shiv Pillai Follicular Lymphoma 1. Characterized by t(14:18) translocation 2. Ig heavy
Scott Abrams, Ph.D. Professor of Oncology, x4375 Kuby Immunology SEVENTH EDITION
 Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 13 Effector Responses: Cell- and Antibody-Mediated Immunity Copyright 2013 by W. H.
Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 13 Effector Responses: Cell- and Antibody-Mediated Immunity Copyright 2013 by W. H.
Immune Regulation and Tolerance
 Immune Regulation and Tolerance Immunoregulation: A balance between activation and suppression of effector cells to achieve an efficient immune response without damaging the host. Activation (immunity)
Immune Regulation and Tolerance Immunoregulation: A balance between activation and suppression of effector cells to achieve an efficient immune response without damaging the host. Activation (immunity)
Central tolerance. Mechanisms of Immune Tolerance. Regulation of the T cell response
 Immunoregulation: A balance between activation and suppression that achieves an efficient immune response without damaging the host. Mechanisms of Immune Tolerance ACTIVATION (immunity) SUPPRESSION (tolerance)
Immunoregulation: A balance between activation and suppression that achieves an efficient immune response without damaging the host. Mechanisms of Immune Tolerance ACTIVATION (immunity) SUPPRESSION (tolerance)
Mechanisms of Immune Tolerance
 Immunoregulation: A balance between activation and suppression that achieves an efficient immune response without damaging the host. ACTIVATION (immunity) SUPPRESSION (tolerance) Autoimmunity Immunodeficiency
Immunoregulation: A balance between activation and suppression that achieves an efficient immune response without damaging the host. ACTIVATION (immunity) SUPPRESSION (tolerance) Autoimmunity Immunodeficiency
Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 24 The Immune System Multiple-Choice Questions
 Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 24 The Immune System 24.1 Multiple-Choice Questions 1) The body's innate defenses against infection include A) several nonspecific
Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 24 The Immune System 24.1 Multiple-Choice Questions 1) The body's innate defenses against infection include A) several nonspecific
