COMPLIANCE. Individual Highlights: Advance Beneficiary Notices Medical Necessity IL Requisition Reflex Test List Panel Test. September 2018.
|
|
|
- Todd Simpson
- 5 years ago
- Views:
Transcription
1 September 2018 COMPLIANCE Dear Physician: Inova Laboratories (IL) is proud to serve the Northern Virginia community as the only full-service reference laboratory. Each year we disclose information about our billing practices and compliance policies as required. This letter provides healthcare professionals with written information addressing various policies that affect ordering, performing and billing clinical laboratory tests. Details regarding IL policies are attached. Dr. Nam is our Medical Director and Clinical Consultant. She can be reached at for questions about testing. If you have any questions, or would like more information about the topics covered in this compliance communication, I may be contacted at or by at beth.deaton@inova.org. If you have questions about any services we offer, please contact our Client Service department at and they can connect you with a Marketing Representative. Additionally more information is available on our website at lab. Individual Highlights: Advance Beneficiary Notices Medical Necessity IL Requisition Reflex Test List Panel Test Sincerely, Beth Deaton Director, Administrator Reference Lab Inova Laboratories Page 1
2 Advanced Beneficiary Notices An Advanced Beneficiary Notice (ABN) should be completed if any of the laboratory tests ordered for a Medicare patient are not accompanied by a diagnosis code eligible for coverage by Medicare. Medicare will only pay for tests that it determines are reasonable and necessary. Before the Laboratory testing is performed, the beneficiary should be notified in writing with an ABN if any testing will not be paid for by Medicare. After reviewing the ABN, the beneficiary may sign the ABN agreeing to receive the service and pay for it, or not receive services. The ABN must clearly identify the test, the estimated cost, and give the reason that payment is likely to be denied. It must also be signed and dated. Requesting an ABN from all Medicare patients or requesting beneficiaries to sign a blank ABN are unacceptable practices. Medical Necessity Claims submitted for laboratory testing will only be paid by Medicare if the service is covered, reasonable and necessary for the beneficiary given their clinical condition. Medicare may deny payment for tests a physician believes is appropriate, but does not meet the Medicare coverage criteria, such as for screening. ICD-10 CM diagnosis codes must be provided for each test ordered. A full list of limited coverage policies and approved by diagnosis codes can be found at: National Coverage Decisions (NCD) Local Coverage Decisions (LCD) l&policytype=final&s=all&advsearchname=6&datetag=c&kq =true&bc=iaaaaaaaaaaa& Page 2
3 IL Requisition Inside Story Headline Page 3
4 Reflex Test List Test Order CPT Code Reflex Test CPT Code 2018 Medicare Reimb ANA screen with reflex Autoimmune Profile (includes SSA, SSB, Jo 1, RNP, SM, Histone, Centromere, DsDNA, SCL 70) x x 8 HCV Antibody HCV PCR Hepatitis B Surface Antigen Hepatitis B Surface Ag Neutralization HIV differentiation, if HIV HIV Ag/AB, 4th Generation Ag/Ab 4th generation is reactive HIV-1 RNA Quant, if HIV differentiation is invalid ELECTROPHORESIS, SERUM Immunofixation Electrophoresis ELECTROPHORESIS, URINE Immunofixation Electrophoresis LYME DISEASE (IgG, IgM) X2 WESTERN BLOT PSA Total with reflex PSA Free TSH with reflex T4 Free CBC with Differential CBC with Manual Differential CBC WITH DIFF + RBC No charge CBC with Differential MORPHOLOGY BODY FLUID CELL COUNT & Pathology Review SMEAR MALARIA PARASITE SMEAR Pathology Review Bacterial Cultures Various Fungal Cultures AFB Cultures Susceptibility Testing Organism Identification Culture Typing PBP2 Testing Fungal Smear Fungal Identification Specimen Concentration Specimen Homogenization Acid Fast Smear Susceptibility Testing Specimen Concentration Specimen Homogenization M.tb by TMA Mycobacterial Identification Stool Cultures Campylobacter Ag Dection Testing Shiga-like Toxin Cryptococcal AG Cryptococcal antigen titer Strep Screen Throat Culture Page 4
5 Test Order CPT Code Reflex Test CPT Code 2018 Medicare Reimb RPR RPP Titer FTA-Abs Wound/Body Fluid/Biopsy Gram Stain Culture CSF Culture Gram Stain Sputum Culture Gram Stain Bronchial Culture Gram Stain Urinalysis Microscopic Exam UAMRX- Urinalysis with reflex Urine Culture to culture Pathologist interpretation with written report will be added based on laboratory reflex criteria Body fluid smear Crystal ID Malaria / Parasite Identification Peripheral blood smear interpretation Platelet aggregation / alloimmunization CSF electrophoresis Immunofixation of serum, urine or CSF Protein electrophoresis Page 5
6 Panel Test ORDERSET NAME DISPLAY NAME CPT IHS AMB INOVA LAB FEMALE HORMONE PANEL Female Hormone Panel (E2, Prog, FSH, LH, Testo, DHEA) MEDICARE REIMBURSEMENT IHS AMB INOVA LAB IMMUNOGLOBULINS A/E/G/M Immunoglobulins A/E/G/M X X IHS AMB INOVA LAB MALE HORMONE PANEL Male Hormone Panel IHS CSF FLUID LAB PANEL TUBE 2 Inova CSF Tube IHS LAB PANEL CSF LABS CSF Labs x x x x Inova Laboratories 2832 Juniper Street Fairfax, VA PHONE: FAX: We re on the Web! See us at: Page 6
2014 Notice to Physicians
 2014 Notice to Physicians August 20, 2014 Dear Physician, will only pay for tests that are deemed medically necessary. These regulations impact physicians who order the tests as well as the laboratories
2014 Notice to Physicians August 20, 2014 Dear Physician, will only pay for tests that are deemed medically necessary. These regulations impact physicians who order the tests as well as the laboratories
Annual Notice to Providers (2014)
 8901 West Lincoln Avenue, West Allis, WI 53227 5400 Pearl, Rosemont, IL 60018 Annual Notice to Providers (2014) May 2014 Dear Physician/Client: The Medicare Program encourages clinical laboratories to
8901 West Lincoln Avenue, West Allis, WI 53227 5400 Pearl, Rosemont, IL 60018 Annual Notice to Providers (2014) May 2014 Dear Physician/Client: The Medicare Program encourages clinical laboratories to
Reflex Test Protocols
 UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
REFLEX TESTING LIST March 2012
 Aeromonas/Plesiomonas Positive findings Organism ID & Susceptibility April 2011 Culture *AFB Culture and Stain Positive findings Organism ID & Susceptibility Nov. 1999 *AFB Culture Only Positive findings
Aeromonas/Plesiomonas Positive findings Organism ID & Susceptibility April 2011 Culture *AFB Culture and Stain Positive findings Organism ID & Susceptibility Nov. 1999 *AFB Culture Only Positive findings
Reflex Test Protocols
 Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive polyspecific DAT IgG DAT, C3 DAT Fetal Cell
Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive polyspecific DAT IgG DAT, C3 DAT Fetal Cell
2015 Annual Notice to Providers
 8901 West Lincoln Avenue, West Allis, WI 53227 5400 Pearl, Rosemont, IL 60018 2015 Annual Notice to Providers April 2015 Dear Physician/Client: ACL Laboratories maintains an active compliance program that
8901 West Lincoln Avenue, West Allis, WI 53227 5400 Pearl, Rosemont, IL 60018 2015 Annual Notice to Providers April 2015 Dear Physician/Client: ACL Laboratories maintains an active compliance program that
Current September 2017
 1 Lab Service PRICE 87077 PATHOGEN ID 6 87088 PRESUMP ID, URINE CULTUR 6 87140 HSV CULTURE TYPING 13 87186 MIC (MIN INHIB CONC) 7 AMYLASE, SERUM 3 ANA SCREEN/RFX TITER AND DSDNA 4 ANA SCREEN/RFX TITER/COMPREHEN
1 Lab Service PRICE 87077 PATHOGEN ID 6 87088 PRESUMP ID, URINE CULTUR 6 87140 HSV CULTURE TYPING 13 87186 MIC (MIN INHIB CONC) 7 AMYLASE, SERUM 3 ANA SCREEN/RFX TITER AND DSDNA 4 ANA SCREEN/RFX TITER/COMPREHEN
Reflex Test Protocols
 University of Colorado Hospital Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test
University of Colorado Hospital Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test
A written order to request the performance of a laboratory test can be initiated by the following parties:
 Requests/Reporting Requests for Laboratory Services A written order to request the performance of a laboratory test can be initiated by the following parties: Staff physicians and dentists who have been
Requests/Reporting Requests for Laboratory Services A written order to request the performance of a laboratory test can be initiated by the following parties: Staff physicians and dentists who have been
IMMEDIATE HOT LINE: Effective January 6, 2014
 MEDICARE COVERAGE OF LABORATORY TESTING Please remember when ordering laboratory tests that are billed to Medicare/Medicaid or other federally funded programs, the following requirements apply: 1. Only
MEDICARE COVERAGE OF LABORATORY TESTING Please remember when ordering laboratory tests that are billed to Medicare/Medicaid or other federally funded programs, the following requirements apply: 1. Only
The Medical Laboratory
 The Medical Laboratory Purpose of a Laboratory testing Record an individuals state of health Satisfy employment, Insurance or legal requirements Research and clinical trials Detect asymptomatic conditions
The Medical Laboratory Purpose of a Laboratory testing Record an individuals state of health Satisfy employment, Insurance or legal requirements Research and clinical trials Detect asymptomatic conditions
December Metanephrines, Fractionated, Free, Plasma Plasminogen Activator Inhibitor Type 1 Stool Cultures and Sensitivities
 University of Washington December 2012 TEST change 1,25 Dihydroxy Vitamin D Frequency AFB Blood Cultures Alcohol Screen Anti Deaminated Gliadin, IgG Anti Gliadin Package Anti Mullerian Hormone Package
University of Washington December 2012 TEST change 1,25 Dihydroxy Vitamin D Frequency AFB Blood Cultures Alcohol Screen Anti Deaminated Gliadin, IgG Anti Gliadin Package Anti Mullerian Hormone Package
1. Purpose 1.1. To define testing locations, schedule and order priority for each test performed in the core laboratory.
 Department Of Pathology GEN.1017.03 Integrated Test Schedule Version# 5 Department Specimen Processing POLICY NO. 1938 PAGE NO. 1 OF 6 Printed copies are for reference only. Please refer to the electronic
Department Of Pathology GEN.1017.03 Integrated Test Schedule Version# 5 Department Specimen Processing POLICY NO. 1938 PAGE NO. 1 OF 6 Printed copies are for reference only. Please refer to the electronic
Pathology and Laboratory
 Pathology and Laboratory CPT CPT copyright 2011 American Medical Association. All rights reserved. Fee schedules, relative value units, conversion factors and/or related components are not assigned by
Pathology and Laboratory CPT CPT copyright 2011 American Medical Association. All rights reserved. Fee schedules, relative value units, conversion factors and/or related components are not assigned by
Clinical Microbiology CLS 2019 Clinical Microbiology Laboratory Scheme Application Form
 Laboratory Scheme Application Form complete all sections below and return to LGC Standards Proficiency Testing by email, fax or post. Returning customer Lab ID: Purchase order no.: (compulsory) Round Despatch
Laboratory Scheme Application Form complete all sections below and return to LGC Standards Proficiency Testing by email, fax or post. Returning customer Lab ID: Purchase order no.: (compulsory) Round Despatch
TEST LIST SAMPLE REQUIREMENT. 1 ml serum None
 ALBUMIN TEST NAME ALKALINE PHOSPHATASE ALLERGY PROFILE, FOOD 30 allergens ALLERGY PROFILE, INHALANT 30 Allergens ALT AMYLASE ANA ANTI- TG ANTI-GLIADIN IGG ANTI-GLIADIN IGA ANTI-HBS ANTI-HCV ANTI-TPO APOLIPOPROTEIN
ALBUMIN TEST NAME ALKALINE PHOSPHATASE ALLERGY PROFILE, FOOD 30 allergens ALLERGY PROFILE, INHALANT 30 Allergens ALT AMYLASE ANA ANTI- TG ANTI-GLIADIN IGG ANTI-GLIADIN IGA ANTI-HBS ANTI-HCV ANTI-TPO APOLIPOPROTEIN
Collection (Specimen Source Required on all tests) Sputum: >5 ml required. First morning specimen preferred.
 Type Acid Fast (Mycobacteria) Sputum: >5 ml required. First morning specimen preferred. For blood, sodium heparin tube preferred. Lithium heparin acceptable. Do not centrifuge.. delay. Swabs are not appropriate
Type Acid Fast (Mycobacteria) Sputum: >5 ml required. First morning specimen preferred. For blood, sodium heparin tube preferred. Lithium heparin acceptable. Do not centrifuge.. delay. Swabs are not appropriate
Management of Needlestick and Mucous Membrane Exposures To Blood/Body Fluids
 Management of Needlestick and Mucous Membrane Exposures To Blood/Body Fluids August 2004 Table Of Contents Protocol - Management of Needlestick and Mucous Membrane Exposures to Blood/Body Fluids Assessment
Management of Needlestick and Mucous Membrane Exposures To Blood/Body Fluids August 2004 Table Of Contents Protocol - Management of Needlestick and Mucous Membrane Exposures to Blood/Body Fluids Assessment
AccuSet Autoimmune Performance Panel
 PACKAGE INSERT 0840-0001 INTENDED USE The 0840-0001 is intended for use by diagnostic manufacturers, researchers, and clinical laboratories to develop, evaluate, or troubleshoot autoimmune related test
PACKAGE INSERT 0840-0001 INTENDED USE The 0840-0001 is intended for use by diagnostic manufacturers, researchers, and clinical laboratories to develop, evaluate, or troubleshoot autoimmune related test
Submitted by: Fred Medrano, Director, Department of Health Services
 Office of the City Manager ACTION CALENDAR November 17, 2009 To: From: Honorable Mayor and Members of the City Council Phil Kamlarz, City Manager Submitted by: Fred Medrano, Director, Department of Health
Office of the City Manager ACTION CALENDAR November 17, 2009 To: From: Honorable Mayor and Members of the City Council Phil Kamlarz, City Manager Submitted by: Fred Medrano, Director, Department of Health
The most current laboratory testing information can be obtained at
 About Adventist Lab Partners Reference Laboratory Adventist Lab Partners (ALPs) Reference Laboratory is affiliated with Adventist Health System. Adventist Lab Partners has been a hospital-based laboratory
About Adventist Lab Partners Reference Laboratory Adventist Lab Partners (ALPs) Reference Laboratory is affiliated with Adventist Health System. Adventist Lab Partners has been a hospital-based laboratory
Request Forms/Forms. Anatomic Pathology Request Form ANATOMIC PATHOLOGY REQUISITION PATIENT INFORMATION CASE NUMBER SPECIMEN TYPE AND SITE
 Request Forms/Forms Anatomic Pathology Request Form Your resource for life. MaineGeneral Medical Center Augusta & Waterville, Maine ANATOMIC PATHOLOGY REQUISITION Pathology Associates J. Benziger, W. Kindig,
Request Forms/Forms Anatomic Pathology Request Form Your resource for life. MaineGeneral Medical Center Augusta & Waterville, Maine ANATOMIC PATHOLOGY REQUISITION Pathology Associates J. Benziger, W. Kindig,
Laboratory Bulletin...
 Laboratory Bulletin... Updates and Information from Rex Healthcare and Rex Outreach March 1998 Issue Number 30 More on Prestorage Leukocyte Reduced Blood Products Allogeneic cellular blood component transfusions
Laboratory Bulletin... Updates and Information from Rex Healthcare and Rex Outreach March 1998 Issue Number 30 More on Prestorage Leukocyte Reduced Blood Products Allogeneic cellular blood component transfusions
Recommendations for Celiac Disease Testing. IgA & ttg IgA
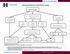 Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
HEALTH ASSESSMENT FOR NEW ZEALAND VISA APPLICANTS
 INFORMATION SHEET HEALTH ASSESSMENT FOR NEW ZEALAND VISA APPLICANTS Health assessments of New Zealand visa applicants are conducted by the International Organization for Migration (IOM) on behalf of Immigration
INFORMATION SHEET HEALTH ASSESSMENT FOR NEW ZEALAND VISA APPLICANTS Health assessments of New Zealand visa applicants are conducted by the International Organization for Migration (IOM) on behalf of Immigration
Notification for Outpatient Injectable Chemotherapy for Medicare Advantage Plans Frequently Asked Questions
 Notification for Outpatient Injectable Chemotherapy for Medicare Advantage Plans Frequently Asked Questions Key Points Physicians and facilities are required to submit notification to UnitedHealthcare
Notification for Outpatient Injectable Chemotherapy for Medicare Advantage Plans Frequently Asked Questions Key Points Physicians and facilities are required to submit notification to UnitedHealthcare
Coding for Preventive Services A Guide for HIV Providers
 Coding for Preventive Services A Guide for HIV Providers Jessie Murphy, MPH and Michelle Cataldo, LCSW, April 2016 Implementation of the Patient Protection and Affordable Care Act and other regulatory
Coding for Preventive Services A Guide for HIV Providers Jessie Murphy, MPH and Michelle Cataldo, LCSW, April 2016 Implementation of the Patient Protection and Affordable Care Act and other regulatory
July Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
New Test Announcements
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com New Test Announcements Dear Colleague: We are pleased to announce many new test offerings this month. January 25, 2007 In oncology,
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com New Test Announcements Dear Colleague: We are pleased to announce many new test offerings this month. January 25, 2007 In oncology,
Chapter 17 Worksheet Code It
 Class: Date: Chapter 17 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. CPT laboratory codes include collection of the specimen. 2. A dipstick is a small piece of
Class: Date: Chapter 17 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. CPT laboratory codes include collection of the specimen. 2. A dipstick is a small piece of
Electrical Stimulation Device Used for Cancer Treatment
 Electrical Stimulation Device Used for Cancer Treatment OPTUNE (NOVOTTF 100A SYSTEM) For any item to be covered by The Health Plan, it must: 1. Be eligible for a defined Medicare or The Health Plan benefit
Electrical Stimulation Device Used for Cancer Treatment OPTUNE (NOVOTTF 100A SYSTEM) For any item to be covered by The Health Plan, it must: 1. Be eligible for a defined Medicare or The Health Plan benefit
Patient Reimbursement Guide. Brainsway Deep Transcranial Magnetic Stimulation (TMS) Treatment. Obtain Coverage - the Right Way
 Patient Reimbursement Guide Brainsway Deep Transcranial Magnetic Stimulation (TMS) Treatment Obtain Coverage - the Right Way Ever since Brainsway Deep TMS was approved by the FDA in 2013 for the treatment
Patient Reimbursement Guide Brainsway Deep Transcranial Magnetic Stimulation (TMS) Treatment Obtain Coverage - the Right Way Ever since Brainsway Deep TMS was approved by the FDA in 2013 for the treatment
MICROBIOLOGY SPECIMEN COLLECTION MANUAL
 Lee Memorial Health System Lee County, FL CLINICAL LABORATORY MICROBIOLOGY SPECIMEN COLLECTION MANUAL ACID FAST CULTURE Specimen Type see Specimen Chart ACID FAST STAIN see Specimen Chart Acid Fast stain
Lee Memorial Health System Lee County, FL CLINICAL LABORATORY MICROBIOLOGY SPECIMEN COLLECTION MANUAL ACID FAST CULTURE Specimen Type see Specimen Chart ACID FAST STAIN see Specimen Chart Acid Fast stain
October Billing and compliance. Coagulation. Immunohistochemistry. Immunology. Referral testing ICD 10
 October 2015 Billing and compliance ICD 10 Coagulation Processing, transport and stability changes for multiple coagulation tests Immunohistochemistry IHC stain updates Immunology Lyme Western Blot testing
October 2015 Billing and compliance ICD 10 Coagulation Processing, transport and stability changes for multiple coagulation tests Immunohistochemistry IHC stain updates Immunology Lyme Western Blot testing
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
Icd 10 codes for free t4 lab to get paid
 Icd 10 codes for free t4 lab to get paid Medically Supportive ICD Codes are listed by determination of thyroid hormone levels [free thyroxine (ft-4) or total thyroxine (T4) with Triiodothyronine ( T3)
Icd 10 codes for free t4 lab to get paid Medically Supportive ICD Codes are listed by determination of thyroid hormone levels [free thyroxine (ft-4) or total thyroxine (T4) with Triiodothyronine ( T3)
Clinical Laboratory. [None
 Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Double-Stranded DNA (dsdna) Ab IgG ELISA Detected * [None 18-289-900151 Detected] Double-Stranded DNA (dsdna) Ab IgG
Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Double-Stranded DNA (dsdna) Ab IgG ELISA Detected * [None 18-289-900151 Detected] Double-Stranded DNA (dsdna) Ab IgG
IP Lab Webinar 8/23/2012
 2 What Infection Preventionists need to know about the Laboratory Anne Maher, MS, M(ASCP), CIC Richard VanEnk PhD, CIC 1 Objectives Describe what the laboratory can do for you; common laboratory tests
2 What Infection Preventionists need to know about the Laboratory Anne Maher, MS, M(ASCP), CIC Richard VanEnk PhD, CIC 1 Objectives Describe what the laboratory can do for you; common laboratory tests
Counseling to Prevent Tobacco Use
 News Flash Vaccination is the Best Protection Against the Flu. This year, the Centers for Disease Control and Prevention (CDC) is encouraging everyone 6 months of age and older to get vaccinated against
News Flash Vaccination is the Best Protection Against the Flu. This year, the Centers for Disease Control and Prevention (CDC) is encouraging everyone 6 months of age and older to get vaccinated against
2019 Patient Price Information List
 2019 Patient Price Information List In compliance with state law, Genesis Healthcare System is providing this price list containing our charges for room and board, emergency department, operating room,
2019 Patient Price Information List In compliance with state law, Genesis Healthcare System is providing this price list containing our charges for room and board, emergency department, operating room,
WPS Medicare Part B - Quarterly CERT Error Findings Report ~ MICHIGAN ~
 WPS Medicare Part B - Quarterly CERT Error Findings Report ~ MICHIGAN ~ This report provides details of Comprehensive Error Rate Testing (CERT) errors assessed July 2014 through September 2014 for Michigan
WPS Medicare Part B - Quarterly CERT Error Findings Report ~ MICHIGAN ~ This report provides details of Comprehensive Error Rate Testing (CERT) errors assessed July 2014 through September 2014 for Michigan
Payment Policy Drug Testing EFFECTIVE DATE: POLICY LAST UPDATED:
 Payment Policy Drug Testing EFFECTIVE DATE: 05 23 2013 POLICY LAST UPDATED: 06 05 2018 OVERVIEW This policy documents the criteria and documentation requirements for immunoassay (IA) testing (also called
Payment Policy Drug Testing EFFECTIVE DATE: 05 23 2013 POLICY LAST UPDATED: 06 05 2018 OVERVIEW This policy documents the criteria and documentation requirements for immunoassay (IA) testing (also called
Services provided beyond a Member s benefit limit are not covered unless a BLE is requested and approved by Avesis.
 April 1, 2012 Dear Provider: Avesis would like to thank you for your continued participation in the Avesis UPMC for You dental network. This notice is to inform you of some upcoming changes to benefits
April 1, 2012 Dear Provider: Avesis would like to thank you for your continued participation in the Avesis UPMC for You dental network. This notice is to inform you of some upcoming changes to benefits
WALNUT CREEK FAMILY PRACTICE 4303 JODECO ROAD MCDONOUGH, GA
 WALNUT CREEK FAMILY PRACTICE 4303 JODECO ROAD MCDONOUGH, GA 30253 770-898-7840 Dear Walnut Creek Family Practice Patient, Your physical appointment is scheduled for you and no one else at that time. If
WALNUT CREEK FAMILY PRACTICE 4303 JODECO ROAD MCDONOUGH, GA 30253 770-898-7840 Dear Walnut Creek Family Practice Patient, Your physical appointment is scheduled for you and no one else at that time. If
July Billing and Compliance. Chemistry. Help Us Help You. Referral Testing
 July 2018 Billing and Compliance New Medicare card mailings have begun for Minnesota and Wisconsin residents Chemistry Beta hydroxybutyrate, whole blood specimen requirement change Help Us Help You Preventing
July 2018 Billing and Compliance New Medicare card mailings have begun for Minnesota and Wisconsin residents Chemistry Beta hydroxybutyrate, whole blood specimen requirement change Help Us Help You Preventing
Medicare Updates Part 2. Tracy Cole, D.C.
 Medicare Updates Part 2 Tracy Cole, D.C. tcoledc@gmail.com Tracy Cole, D.C., Bio u u u u CCA representative to Noridian Contractor Advisory Committee for California Member, ACA Medicare Committee Member,
Medicare Updates Part 2 Tracy Cole, D.C. tcoledc@gmail.com Tracy Cole, D.C., Bio u u u u CCA representative to Noridian Contractor Advisory Committee for California Member, ACA Medicare Committee Member,
Clinical Laboratory. 14:41:00 Complement Component 3 50 mg/dl Oct-18
 Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Thyroid Peroxidase (TPO) Antibody 5.0 IU/mL [0.0-9.0] 18-289-900139 16-Oct-18 Complement Component 3 50 mg/dl 18-289-900139
Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Thyroid Peroxidase (TPO) Antibody 5.0 IU/mL [0.0-9.0] 18-289-900139 16-Oct-18 Complement Component 3 50 mg/dl 18-289-900139
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
Title: Fecal Occult Blood Test Number: TCFHT-MD01 Activation Date: Reviewed: Next Review:
 MEDICAL DIRECTIVE Title: Fecal Occult Blood Test Number: TCFHT-MD01 Activation Date: 01-01-2014 Reviewed: 01-01-2015 Next Review: 01-01-2016 Sponsoring/Contact Person(s) (name, position, contact particulars):
MEDICAL DIRECTIVE Title: Fecal Occult Blood Test Number: TCFHT-MD01 Activation Date: 01-01-2014 Reviewed: 01-01-2015 Next Review: 01-01-2016 Sponsoring/Contact Person(s) (name, position, contact particulars):
Bloodborne Pathogen Exposure STUDENT. Blood Borne Pathogen Exposure. Student. Rev:
 Blood Borne Pathogen Exposure Student INSTRUCTIONS 1. Student: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
Blood Borne Pathogen Exposure Student INSTRUCTIONS 1. Student: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
Title: Fecal Occult Blood Test Number: TCFHT-MD01 Activation Date: 01-January-2014 Review Date: 08-October-2017 Next Review Date: 08-October-2018
 MEDICAL DIRECTIVE Title: Fecal Occult Blood Test Number: TCFHT-MD01 Activation Date: 01-January-2014 Review Date: 08-October-2017 Next Review Date: 08-October-2018 Sponsoring/Contact Person(s) (name, position,
MEDICAL DIRECTIVE Title: Fecal Occult Blood Test Number: TCFHT-MD01 Activation Date: 01-January-2014 Review Date: 08-October-2017 Next Review Date: 08-October-2018 Sponsoring/Contact Person(s) (name, position,
2009 Pain Coding Update and Pain Industry Business Trends
 2009 Pain Coding Update and Pain Industry Business Trends Linda Van Horn, MBA June 13, 2009 2009 Pain Coding Update and Pain Industry Trends Agenda 2009 CPT Coding Updates Pay For Incentives ICD-10 American
2009 Pain Coding Update and Pain Industry Business Trends Linda Van Horn, MBA June 13, 2009 2009 Pain Coding Update and Pain Industry Trends Agenda 2009 CPT Coding Updates Pay For Incentives ICD-10 American
ALPS Screen Order Set B-Cell Panel Order Set (% and absolute #) (% and Absolute #) ALPS (CD3+/CD4-/CD8-/TCR αβ+)
 All test indicated in a Panel or are available individually. Version 1/1/2018 ALPS Screen B-Cell Panel (% and absolute #) (% and Absolute #) ALPS (/CD4-/CD8-/TCR αβ+) CD5+/ ANA Profile * Anti-Nuclear Ab*
All test indicated in a Panel or are available individually. Version 1/1/2018 ALPS Screen B-Cell Panel (% and absolute #) (% and Absolute #) ALPS (/CD4-/CD8-/TCR αβ+) CD5+/ ANA Profile * Anti-Nuclear Ab*
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
Integrating HIV Screening Into
 MaxiMizing Third ParTy reimbursement for hiv TesTing Integrating HIV Screening Into Title X Services IntroductIon HIV screening services are a core family planning service, and all individuals aged 13-64
MaxiMizing Third ParTy reimbursement for hiv TesTing Integrating HIV Screening Into Title X Services IntroductIon HIV screening services are a core family planning service, and all individuals aged 13-64
Successful strategies for reporting TB results to public health officials. Max Salfinger, MD Mycobacteriology and Pharmacokinetics Denver, Colorado
 Successful strategies for reporting TB results to public health officials Max Salfinger, MD Mycobacteriology and Pharmacokinetics Denver, Colorado Alternative titles Which TB result needs to be reported?
Successful strategies for reporting TB results to public health officials Max Salfinger, MD Mycobacteriology and Pharmacokinetics Denver, Colorado Alternative titles Which TB result needs to be reported?
LOUISIANA MEDICAID PROGRAM ISSUED: 02/01/12 REPLACED: 02/01/94 CHAPTER 5: PROFESSIONAL SERVICES SECTION 5.1: COVERED SERVICES PAGE(S) 5
 Immunizations Vaccine Codes Providers should refer to the Immunization Fee Schedules to determine covered vaccines and any restriction to the use of the vaccine codes. (See Appendix A for information on
Immunizations Vaccine Codes Providers should refer to the Immunization Fee Schedules to determine covered vaccines and any restriction to the use of the vaccine codes. (See Appendix A for information on
DEPARTMENT OF MICROBIOLOGY IMPORTANT NOTICE TO USERS Turnaround Times (TATs) for Microbiology Investigations
 Dear User, ISSUE: M008 DEPARTMENT OF MICROBIOLOGY IMPORTANT NOTICE TO USERS Turnaround Times (TATs) for Microbiology Investigations In order to comply with national quality guidance and as part of our
Dear User, ISSUE: M008 DEPARTMENT OF MICROBIOLOGY IMPORTANT NOTICE TO USERS Turnaround Times (TATs) for Microbiology Investigations In order to comply with national quality guidance and as part of our
BID ANNUAL CONTRACT FOR LAB TESTING
 1 ACID FAST BACILLISTAIN 100 10.48 1,048.00 8.00 800.00 2 AFP, TUMOR (CHIRON) 10 9.04 90.40 7.00 70.00 3 AMYLASE 160 3.13 500.80 2.75 440.00 4 BASIC METAB PNL 1,800 1.44 2,592.00 1.95 3,510.00 5 BZV IGGAB
1 ACID FAST BACILLISTAIN 100 10.48 1,048.00 8.00 800.00 2 AFP, TUMOR (CHIRON) 10 9.04 90.40 7.00 70.00 3 AMYLASE 160 3.13 500.80 2.75 440.00 4 BASIC METAB PNL 1,800 1.44 2,592.00 1.95 3,510.00 5 BZV IGGAB
DCO Registration. If you have any questions contact the DCO help desk at:
 DCO Registration Please register using the two simple steps below: 1. Log-in or create a CME account: https://tiny.army.mil/r/zb8a/cme **Tip: If your facility is not listed as an option on the registration
DCO Registration Please register using the two simple steps below: 1. Log-in or create a CME account: https://tiny.army.mil/r/zb8a/cme **Tip: If your facility is not listed as an option on the registration
Taking Part B Therapy Beyond the $3,700 Threshold New Manual Medical Review Process Effective date October 1, 2012
 Taking Part B Therapy Beyond the $3,700 Threshold New Manual Medical Review Process Effective date October 1, 2012 Presented by: Leigh Ann Frick, PT, MBA Vice President of Clinical Services Heritage Healthcare
Taking Part B Therapy Beyond the $3,700 Threshold New Manual Medical Review Process Effective date October 1, 2012 Presented by: Leigh Ann Frick, PT, MBA Vice President of Clinical Services Heritage Healthcare
Thyroid Care & Diagnostic Centre
 Thyroid Care & Diagnostic Centre Laboratory Test List DEPARTMENT OF CLINICAL PATHOLOGY BLOOD : CBC HCT/ PCV Hb, TC, DC & ESR MCV CE MCH PC MCHC MP Reticulocyte count PBF LE Cell Hb-Electrophoresis Protin
Thyroid Care & Diagnostic Centre Laboratory Test List DEPARTMENT OF CLINICAL PATHOLOGY BLOOD : CBC HCT/ PCV Hb, TC, DC & ESR MCV CE MCH PC MCHC MP Reticulocyte count PBF LE Cell Hb-Electrophoresis Protin
FDA Reentry Guidance
 FDA Reentry Guidance T. cruzi - Chagas, Hepatitis C and HIV Wednesday 6/6/18 Doug Denyer O Dina Hurlburt, SBB(ASCP)CM Outline Impact to Clients Review of FDA Guidance Documents Reentry Request Process
FDA Reentry Guidance T. cruzi - Chagas, Hepatitis C and HIV Wednesday 6/6/18 Doug Denyer O Dina Hurlburt, SBB(ASCP)CM Outline Impact to Clients Review of FDA Guidance Documents Reentry Request Process
Routine HIV Testing in Healthcare Settings: Reimbursement & Sustainability Updated October, 2017
 Routine HIV Testing in Healthcare Settings: Reimbursement & Sustainability Updated October, 2017 Overview Importance of routine testing Current recommendations for testing Reimbursement for HIV counseling
Routine HIV Testing in Healthcare Settings: Reimbursement & Sustainability Updated October, 2017 Overview Importance of routine testing Current recommendations for testing Reimbursement for HIV counseling
Managed Health Services (MHS) Candace V. Ervin Market Manager, Indiana Provider Relations October 18, 2017
 Managed Health Services (MHS) Candace V. Ervin Market Manager, Indiana Provider Relations Candace.Ervin@Envolvehealth.com October 18, 2017 1 Today s Agenda MHS ID Card Samples Provider Visits D1110 (Prophylaxis
Managed Health Services (MHS) Candace V. Ervin Market Manager, Indiana Provider Relations Candace.Ervin@Envolvehealth.com October 18, 2017 1 Today s Agenda MHS ID Card Samples Provider Visits D1110 (Prophylaxis
Saskatchewan ND Price List
 Saskatchewan ND Price List Note: There is a $55 per requisition documentation fee. Prices valid only in Saskatchewan. PANEL Healthy Living Assessment Panel $115.00 Enhanced Healthy Living Assessment Panel
Saskatchewan ND Price List Note: There is a $55 per requisition documentation fee. Prices valid only in Saskatchewan. PANEL Healthy Living Assessment Panel $115.00 Enhanced Healthy Living Assessment Panel
Positive Airway Pressure (PAP) Devices Physician Frequently Asked Questions December 2008
 Positive Airway Pressure (PAP) Devices Physician Frequently Asked Questions December 2008 Based on questions received from the clinical community, the following Frequently Asked Questions will address
Positive Airway Pressure (PAP) Devices Physician Frequently Asked Questions December 2008 Based on questions received from the clinical community, the following Frequently Asked Questions will address
HML Update. Summer 2009 Volume 15, No. 3. Keeping Our Clients Informed Questions or Comments: Call
 HML Update Summer 2009 Volume 15, No. 3 Keeping Our Clients Informed Questions or Comments: Call 651-232-3500 Inside: Panel Changes...1-2 New Panels...2-3 H1N1... 3 Note of Appreciation... 3 BD Vacutainer
HML Update Summer 2009 Volume 15, No. 3 Keeping Our Clients Informed Questions or Comments: Call 651-232-3500 Inside: Panel Changes...1-2 New Panels...2-3 H1N1... 3 Note of Appreciation... 3 BD Vacutainer
Washington County-Johnson City Health Department Christen Minnick, MPH, Director 219 Princeton Road Johnson City, Tennessee Phone:
 Washington County-Johnson City Health Department Christen Minnick, MPH, Director 219 Princeton Road Johnson City, Tennessee 37601 Phone: 423-975-2200 Dear Parent: The Washington County Health Department
Washington County-Johnson City Health Department Christen Minnick, MPH, Director 219 Princeton Road Johnson City, Tennessee 37601 Phone: 423-975-2200 Dear Parent: The Washington County Health Department
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 Schedule of Accreditation 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK National Infection Service, Public Health England Colindale 61 Colindale Avenue London NW9 5EQ Contact: Dr Sanjiv
Schedule of Accreditation 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK National Infection Service, Public Health England Colindale 61 Colindale Avenue London NW9 5EQ Contact: Dr Sanjiv
IU Health Pathology Laboratory. DOS Updates
 IU Health Pathology Laboratory News Bulletin February 23, 2012 DOS Updates Apolipoprotein B QN Ship refrigerated plasma instead of frozen; Frozen plasma is acceptable however. Myoglobin, Serum Discontinued
IU Health Pathology Laboratory News Bulletin February 23, 2012 DOS Updates Apolipoprotein B QN Ship refrigerated plasma instead of frozen; Frozen plasma is acceptable however. Myoglobin, Serum Discontinued
Patient Price Information List January 1, 2018
 In compliance with state law, Western Reserve Hospital is providing this price list containing our charges for Room and Board, Emergency Department, Operating Room, Physical Therapy, Pain Medicine and
In compliance with state law, Western Reserve Hospital is providing this price list containing our charges for Room and Board, Emergency Department, Operating Room, Physical Therapy, Pain Medicine and
1-Appropriate Use Criteria for Advanced Diagnostic Imaging Services
 The Honorable Seema Verma, Administrator Centers for Medicare and Medicaid Services Attention: CMS 1676 P P.O. Box 8016 Baltimore, MD 21244 1850 Dear Administrator Verma: September 10, 2017 On behalf of
The Honorable Seema Verma, Administrator Centers for Medicare and Medicaid Services Attention: CMS 1676 P P.O. Box 8016 Baltimore, MD 21244 1850 Dear Administrator Verma: September 10, 2017 On behalf of
H. PYLORI AB, IGG HPYL
 H. PYLORI AB, IGG HPYL Specimen Required: 3 ml blood, Serum gel tube Analytical Time: 1 day CPT Code: 86677 HAPTOGLOBIN HAP Specimen Required: 1.5 ml blood, gel tube Reference Range: 30-200 mg/dl CPT Code:
H. PYLORI AB, IGG HPYL Specimen Required: 3 ml blood, Serum gel tube Analytical Time: 1 day CPT Code: 86677 HAPTOGLOBIN HAP Specimen Required: 1.5 ml blood, gel tube Reference Range: 30-200 mg/dl CPT Code:
Hepatitis A Antibody Testing Seldom Required.: An Article From: Family Practice News [HTML] [Digital] READ ONLINE
![Hepatitis A Antibody Testing Seldom Required.: An Article From: Family Practice News [HTML] [Digital] READ ONLINE Hepatitis A Antibody Testing Seldom Required.: An Article From: Family Practice News [HTML] [Digital] READ ONLINE](/thumbs/81/84399133.jpg) Hepatitis A Antibody Testing Seldom Required.: An Article From: Family Practice News [HTML] [Digital] READ ONLINE If you are searching for a book Hepatitis A Antibody Testing Seldom Required.: An article
Hepatitis A Antibody Testing Seldom Required.: An Article From: Family Practice News [HTML] [Digital] READ ONLINE If you are searching for a book Hepatitis A Antibody Testing Seldom Required.: An article
Contractor Name: Novitas Solutions, Inc. Contractor Number: Contractor Type: MAC B. LCD ID Number: L34834 Status: A-Approved
 LCD for Blood Glucose Monitoring in a Skilled Nursing Facility (SNF) (L34834) Contractor Name: Novitas Solutions, Inc. Contractor Number: 12502 Contractor Type: MAC B LCD ID Number: L34834 Status: A-Approved
LCD for Blood Glucose Monitoring in a Skilled Nursing Facility (SNF) (L34834) Contractor Name: Novitas Solutions, Inc. Contractor Number: 12502 Contractor Type: MAC B LCD ID Number: L34834 Status: A-Approved
Medical Necessity and the Retrospective Review Process
 Medical Necessity and the Retrospective Review Process Medicaid Retrospective Therapy Review Medicaid contracted with QSource of Arkansas to perform post-payment audits Random quarterly selection across
Medical Necessity and the Retrospective Review Process Medicaid Retrospective Therapy Review Medicaid contracted with QSource of Arkansas to perform post-payment audits Random quarterly selection across
Claim Submission. Agenda 1/31/2013. Payment Basics
 February 2013 Jean C. Russell, MS, RHIT jrussell@epochhealth.com Richard Cooley, BA, CCS rcooley@epochhealth.com 518-430-1144 2 Payment Basics Agenda 2013 PT / OT / SP Codes Deleted Codes New Codes Significant
February 2013 Jean C. Russell, MS, RHIT jrussell@epochhealth.com Richard Cooley, BA, CCS rcooley@epochhealth.com 518-430-1144 2 Payment Basics Agenda 2013 PT / OT / SP Codes Deleted Codes New Codes Significant
Local Coverage Determination for Colorectal Cancer Screening (L29796)
 Page 1 of 15 Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & E People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
Page 1 of 15 Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & E People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
Medicare Adds New Screening Services for Human Papillomavirus (HPV) and Human Immunodeficiency Virus (HIV) March 2016
 Medicare Adds New Screening Services for Human Papillomavirus (HPV) and Human Immunodeficiency Virus (HIV) March 2016 On February 5, 2016, CMS released change requests announcing the addition of HPV and
Medicare Adds New Screening Services for Human Papillomavirus (HPV) and Human Immunodeficiency Virus (HIV) March 2016 On February 5, 2016, CMS released change requests announcing the addition of HPV and
Quarterly CERT Error Findings Report WPS GHA Part B J5 MAC ~ Iowa, Kansas, Missouri and Nebraska ~
 Quarterly CERT Error Findings Report WPS GHA Part B J5 MAC ~ Iowa, Kansas, Missouri and Nebraska ~ This report provides details of Comprehensive Error Rate Testing (CERT) errors assessed January 1, 2018,
Quarterly CERT Error Findings Report WPS GHA Part B J5 MAC ~ Iowa, Kansas, Missouri and Nebraska ~ This report provides details of Comprehensive Error Rate Testing (CERT) errors assessed January 1, 2018,
Reject Code Reason for Rejection What to do
 Reject Code Reason for Rejection What to do 10 Hospital where services rendered missing or invalid. Input the Hospital where services were rendered on the HCFA. 11 Patient first name missing or invalid.
Reject Code Reason for Rejection What to do 10 Hospital where services rendered missing or invalid. Input the Hospital where services were rendered on the HCFA. 11 Patient first name missing or invalid.
Budsakorn Darawankul, MD. Maharat Nakhon Ratchasima Hospital
 Budsakorn Darawankul, MD. Maharat Nakhon Ratchasima Hospital Outline What is ANA? How to detect ANA? Clinical application Common autoantibody in ANA diseases Outline What is ANA? How to detect ANA? Clinical
Budsakorn Darawankul, MD. Maharat Nakhon Ratchasima Hospital Outline What is ANA? How to detect ANA? Clinical application Common autoantibody in ANA diseases Outline What is ANA? How to detect ANA? Clinical
RACE (SEE BACK) ETHNICITY (SEE BACK) DIAGNOSIS CODE ADDRESS OF RESPONSIBLE PARTY APT # DATE OF BIRTH
 Date } ACCOUNT INFORMATION NAME ADDRESS PHONE ORDERING PHYSICIAN BILL TO: ACCOUNT PATIENT/INSURANCE ALTERNATE PATIENT DEPARTMENT OF LABORATORIES St. Louis, Missouri 63110 PHONE: (314) 362-1470 FAX: (314)
Date } ACCOUNT INFORMATION NAME ADDRESS PHONE ORDERING PHYSICIAN BILL TO: ACCOUNT PATIENT/INSURANCE ALTERNATE PATIENT DEPARTMENT OF LABORATORIES St. Louis, Missouri 63110 PHONE: (314) 362-1470 FAX: (314)
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION LANCET KENYA LIMITED UPPERHILL NAIROBI LABORATORY Co. Reg. No.: C168507 Facility Accreditation Number: is a South African National Accreditation System accredited laboratory
CERTIFICATE OF ACCREDITATION LANCET KENYA LIMITED UPPERHILL NAIROBI LABORATORY Co. Reg. No.: C168507 Facility Accreditation Number: is a South African National Accreditation System accredited laboratory
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates September 25, 2009 Dear Colleague: Specialty Laboratories is pleased to announce a new assay, PT and PTT-LA Mixing
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates September 25, 2009 Dear Colleague: Specialty Laboratories is pleased to announce a new assay, PT and PTT-LA Mixing
AMERICAN ASSOCIATION OF BIOANALYSTS PROFICIENCY TESTING SERVICE APPLICATION
 AMERICAN ASSOCIATION OF BIOANALYSTS PROFICIENCY TESTING SERVICE APPLICATION INSTRUCTIONS FOR USERS OF THE FASTPACK IP SYSTEM SELECT THE FOLLOWING PROGRAM CATALOG NUMBERS BASED ON THE TEST TYPES RUN: TESTING
AMERICAN ASSOCIATION OF BIOANALYSTS PROFICIENCY TESTING SERVICE APPLICATION INSTRUCTIONS FOR USERS OF THE FASTPACK IP SYSTEM SELECT THE FOLLOWING PROGRAM CATALOG NUMBERS BASED ON THE TEST TYPES RUN: TESTING
What s New. Don t Forget! There are 2 different influenza vaccines available. Flu Vaccine. Michigan Newsletter Fall 2009
 What s New Michigan Newsletter Fall 2009 Flu Vaccine Don t Forget! There are 2 different influenza vaccines available this year (one for seasonal flu and one for Novel H1N1 or swine flu). Both vaccines
What s New Michigan Newsletter Fall 2009 Flu Vaccine Don t Forget! There are 2 different influenza vaccines available this year (one for seasonal flu and one for Novel H1N1 or swine flu). Both vaccines
WATCHMAN. For questions regarding WATCHMAN reimbursement, please contact:
 WATCHMAN IMPORTANCE OF DOCUMENTATION & THE IMPACT ON MS- DRG ASSIGNMENT This guide stresses the importance of documentation in capturing the appropriate acuity level for patients considered WATCHMAN candidates.
WATCHMAN IMPORTANCE OF DOCUMENTATION & THE IMPACT ON MS- DRG ASSIGNMENT This guide stresses the importance of documentation in capturing the appropriate acuity level for patients considered WATCHMAN candidates.
Reporting Periods in 2010
 Reporting Periods in 2010 1. Full Year (January 1, 2010 December 31, 2010) eligible professionals (EP) whose PQRI quality measure information is successfully submitted (via claims, measures group, or registry)
Reporting Periods in 2010 1. Full Year (January 1, 2010 December 31, 2010) eligible professionals (EP) whose PQRI quality measure information is successfully submitted (via claims, measures group, or registry)
TEST REQUEST INFORMATION- VIROLOGY
 Chlamydia/ Gonorrhea Nucleic Acid Amplification VC75 Qualitative Nucleic Acid Amplification SPECIMEN: Genital swab or first catch urine CONTAINER:GEN-PROBE APTIMA 2 Combo swab transport tube or urine transport
Chlamydia/ Gonorrhea Nucleic Acid Amplification VC75 Qualitative Nucleic Acid Amplification SPECIMEN: Genital swab or first catch urine CONTAINER:GEN-PROBE APTIMA 2 Combo swab transport tube or urine transport
Laboratory Values, Critical. ThedaCare. Date Last Reviewed: 4/5/2018 Reviewing Body(s): Quality; Laboratory Leadership
 Laboratory Values, Critical ThedaCare Policy & Procedure Policy Title: Laboratory Values, Critical Policy Number: 694 Location(s): All ThedaCare Department(s): Laboratory Date Last Reviewed: 4/5/2018 Reviewing
Laboratory Values, Critical ThedaCare Policy & Procedure Policy Title: Laboratory Values, Critical Policy Number: 694 Location(s): All ThedaCare Department(s): Laboratory Date Last Reviewed: 4/5/2018 Reviewing
PLEASE PRINT PLEASE CHECK THE BOX AFTER THE PHONE NUMBER THAT YOU WANT AS YOUR PREFERRED NUMBER
 NORTHERN VIRGINIA CENTER FOR ARTHRITIS PLEASE PRINT PATIENT REGISTRATION Patient s Name: DOB: Sex: Address: PLEASE CHECK THE BOX AFTER THE PHONE NUMBER THAT YOU WANT AS YOUR PREFERRED NUMBER Home#( ) [
NORTHERN VIRGINIA CENTER FOR ARTHRITIS PLEASE PRINT PATIENT REGISTRATION Patient s Name: DOB: Sex: Address: PLEASE CHECK THE BOX AFTER THE PHONE NUMBER THAT YOU WANT AS YOUR PREFERRED NUMBER Home#( ) [
The Facts about Reimbursement for Self-administered Drugs. By William L. Malm, N.D., R.N.
 The Facts about Reimbursement for Self-administered Drugs By William L. Malm, N.D., R.N. Table of Contents Introduction 3 Background 3 Coverage 3 Definition of Self-administered 4 SADs that are Integral
The Facts about Reimbursement for Self-administered Drugs By William L. Malm, N.D., R.N. Table of Contents Introduction 3 Background 3 Coverage 3 Definition of Self-administered 4 SADs that are Integral
Part D Benefits & Clinical Research Trials: What's Covered
 Part D Benefits & Clinical Research Trials: What's Covered Presented by: Ryan Meade, JD Meade & Roach, LLP 312.498.7004 RMeade@MeadeRoach.com Health Care Compliance Association December 12, 2005 Baltimore,
Part D Benefits & Clinical Research Trials: What's Covered Presented by: Ryan Meade, JD Meade & Roach, LLP 312.498.7004 RMeade@MeadeRoach.com Health Care Compliance Association December 12, 2005 Baltimore,
Detail PRINCIPLE: Body fluids other than blood and urine will be analyzed according to their site of origin, and the providers specific orders.
 Subject Body Fluid Analysis - Affiliate Index Number Lab-8760 Section Laboratory Subsection Regional/Affiliates Category Departmental Contact Munson, Karen Last Revised 6/28/2017 References Required document
Subject Body Fluid Analysis - Affiliate Index Number Lab-8760 Section Laboratory Subsection Regional/Affiliates Category Departmental Contact Munson, Karen Last Revised 6/28/2017 References Required document
DEPARTMENT: Regulatory Compliance Support
 PAGE: 1 of 5 REPLACES POLICY DATED: 1/16/98, 3/1/99, SCOPE: All Company-affiliated hospitals performing and/or billing laboratory services. Specifically, the following departments: Business Office Admitting/Registration
PAGE: 1 of 5 REPLACES POLICY DATED: 1/16/98, 3/1/99, SCOPE: All Company-affiliated hospitals performing and/or billing laboratory services. Specifically, the following departments: Business Office Admitting/Registration
Specimen Collection Requirements
 The following is a job aid listing the specimen collection requirements for laboratory testing at Colchester East Hants Health Center. Specimens must be accompanied by the Patient Information Form G09.
The following is a job aid listing the specimen collection requirements for laboratory testing at Colchester East Hants Health Center. Specimens must be accompanied by the Patient Information Form G09.
TEST NAME/ PSYCHE CODE COLLECTION GUIDELINES REFERENCE VALUES ROOM TEMP. REFRIGERATED FROZEN
 TEST NAME/ PSYCHE CODE COLLECTION GUIDELINES REFERENCE VALUES ROOM TEMP. REFRIGERATED FROZEN Clinical Utility CA19-9 (CA-GI) 8 hours 48 hours UNDETERMINED CA 19-9 may be ordered along with other tests,
TEST NAME/ PSYCHE CODE COLLECTION GUIDELINES REFERENCE VALUES ROOM TEMP. REFRIGERATED FROZEN Clinical Utility CA19-9 (CA-GI) 8 hours 48 hours UNDETERMINED CA 19-9 may be ordered along with other tests,
