Current treatment options in the management of psoriasis
|
|
|
- Gyles Owens
- 5 years ago
- Views:
Transcription
1 Drug review Current treatment options in the management of psoriasis Eleanor Higgins MB BCh, BAO, MRCPI and Trevor Markham MD, BAO, MRCPI SPL is a common chronic condition and treatment choice primarily centres on disease severity. Our Drug review considers the therapeutic options, their properties and safety profiles, followed by sources of further information and an analysis of prescription data. is a chronic inflammatory condition that can cause significant discomfort, disfigurement and psychological distress for patients. Fewer than half of those affected by psoriasis find their treatment highly satisfactory. Although there is no cure for psoriasis, numerous topical and systemic therapies are available with comprehensive guidelines for these available on the British Association of Dermatologists (BAD) website at Therapeutic modalities are often selected on the basis of disease severity, patient preference, relevant co-morbidities and efficacy. For most patients, the initial treatment plan centres on disease severity and it may be useful to consider patients as having either mild-to-moderate or moderate-to-severe disease. Mildto-moderate psoriasis may often be managed by topical therapy, while more severe disease may require systemic therapy. involving the hands, feet and face may result in more functional debilitation and greater impact on quality of life and warrant more aggressive therapeutic approaches. CPD questions available for this article. See page 44 Prescriber 5 June
2 Treatment regimens may clear the skin but recurrences are frequent and many patients require repeat treatment. This emphasises the importance of considering the risk-benefit profiles of proposed therapies. SPL Topical therapy Topical corticosteroid therapy can be of benefit in the treatment of psoriasis in certain sites such as the scalp, palms and soles. Corticosteroids range in potency from very mild 1 per cent hydrocortisone to very potent clobetasol propionate. Their use has been limited by adverse effects, which include skin atrophy, striae, purpura, skin fragility, telangiectasia and tachyphylaxis. Nevertheless topical corticosteroids are very useful for the treatment of psoriasis of the scalp, palms and soles (see Figure 1) where the thicker skin is more resistant to these adverse effects. Tar has been used in the treatment of psoriasis for many years, usually combined with UV phototherapy. In 1925, Goeckerman introduced daily crude coal tar with UVB phototherapy for generalised psoriasis and this treatment was widely used. 1 Bed shortages and lack of cosmetic appeal have limited patients use of tar-containing agents. Dithranol is another topical therapy with a long history in psoriasis. The standard dithranol regimen is to apply increasing concentrations ( per cent) on a daily basis. This therapy is particularly useful for chronic plaque psoriasis (see Figure 2) but is not recommended for acute forms of the disease. 2 Dithranol is also used in combination with UV photo therapy to improve efficacy and limit side-effects. Topical vitamin D analogues are effective in psoriasis. In a six-week multicentre trial, calcipotriol was more effective than the topical corticosteroid fluocinonide (Metosyn) in plaque psoriasis. 3 Calcitriol (Silkis) 4 and tacalcitol (Curatoderm) 5 have also been shown to be effective in psoriasis, with calcitriol thought to be less irritant on the face and intertriginous sites. These agents can cause local irritation and excessive use can lead to hypercalcaemia. 6 This has led to the use of combinations of vitamin D analogues with other topical therapies, UV phototherapy and systemic therapies. Topical retinoids are another treatment option for mild-to-moderate psoriasis. 7 Figure 1. The use of topical steroids is limited by adverse effects; however, they are useful in treating areas of the body where the skin is thicker, such as the palms Phototherapy Broadband UVB has been used since the early 20th century, but the spectrum of light that is most effective for clearing psoriasis is in the narrow range around 311nm (see Figure 3). Bulbs that have an output in this range have been developed and are used in narrowband UVB (nuvb) therapy. nuvb therapy ( nm) is more effective than conventional broadband UVB with respect to clearing times and remission. 8 Treatment schedules are similar for both, with nuvb administered three times per week. This form of phototherapy is less erythemogenic than broadband UVB and has mainly replaced it as firstline phototherapy for psoriasis. The efficacy of nuvb compared to photochemotherapy (PUVA) has also been demonstrated. 9 In comparison to PUVA, nuvb-treated patients do not have to take tablets or wear protective eyewear. It can also be used during pregnancy and in children. Long-term safety data are awaited but nuvb is thought to be less carcinogenic than PUVA. 10 Remission is variable and patients may require repeat courses. This becomes particularly relevant when one considers the risk of nonmelanoma skin cancers associated with prolonged PUVA therapy. 11 As a result of this concern PUVA therapy is often combined with other treatments such as retinoids and vitamin D analogues to reduce the number of exposures required for clearance. A laser that emits at a wavelength of 308nm is a new therapeutic modality that has been found to be safe and effective in psoriasis in a multicentre study. 12 Compared to conventional phototherapy it has the advantage of targeting only affected skin and thus reduces the risks to uninvolved skin. Prescriber 5 June
3 SPL Systemic therapy Antimetabolites Methotrexate is an antimetabolite agent that has been used for many years in psoriasis treatment and remains one of the most effective therapies. 13 Methotrexate is indicated for recalcitrant disease unresponsive to topical or phototherapy and is particularly useful if the patient has an associated arthropathy. It is usually given orally once a week and folic acid supplements can be given to reduce nausea associated with this agent. Contraindications include liver abnormalities, poor renal function, anaemia, thrombocytopenia, gastritis, active infection and high alcohol intake. 14 Long-term use of methotrexate is associated with liver toxicity so regular liver function tests are required and also regular full blood counts to monitor marrow toxicity. Oral retinoids Oral retinoids are potent antipsoriatic drugs for pustular and erythrodermic psoriasis but are less effective in chronic plaque psoriasis. 16 Acitretin is given in a dose of mg per kg per day, once or twice daily with meals, for 6-12 weeks. Combination therapy with PUVA and UVB allows dose reduction and decreases the incidence of adverse effects. These may include mucocutaneous dryness, alopecia, elevated serum lipids and liver function tests, hyperostosis and teratogenicity. As a result the package insert recommends that acitretin should not be given Figure 2. Dithranol is particularly useful in the treatment of chronic plaque psoriasis and can be used in combination with UV phototherapy to improve outcomes and limit side-effects to women of child-bearing potential who may become pregnant within three years. Despite these adverse effects acitretin is a safe treatment for psoriasis. Immunosuppressors Ciclosporin was developed as an immunosuppressive transplantation drug in the early 1970s. Its efficacy in psoriasis was first described in The longterm risks of therapy include nephrotoxicity 18 and hypertension. However, ciclosporin is excellent for short-term use due to its weak myelosuppressive effect in comparison to methotrexate. Hypertension can be treated with calcium-channel blockers and this has been shown to prevent loss of renal function. 19 Other immunosuppressive agents have been tried in psoriasis with mixed results, either due to lack of efficacy or the development of adverse effects. Hydroxycarbamide is an effective therapy for chronic plaque psoriasis whose main adverse effect is bone marrow toxicity (unlicensed indication). 20 Mycophenolate mofetil is a immunosuppressive transplantation agent that is safe and effective for severe psoriasis using a dose of 2g per day (unlicensed indication). 21 Fumaric acid derivatives (unlicensed indication) are widely used for psoriasis in some European countries. Four months treatment with fumaric acid derivatives in one trial resulted in efficacy in 80 per cent of patients, although adverse effects such as GI upset, flushing and lymphocytopenia occurred in 67 per cent of patients. 22 Biologics The failure of patients to respond to or to tolerate the above treatment modalities has led to the search for better therapies and recently so-called biological therapies have been developed. Biologic therapies are immunomodulators that target specific molecular steps in the pathogenesis of psoriasis, interfering with T-cell function and cytokine activity. Tumour necrosis factor (TNF) is a proinflammatory cytokine that plays a central role in the pathogenesis of psoriasis, psoriatic arthritis and other inflammatory diseases. There are three approved agents that target TNF: anti-tnf alpha monoclonal antibodies adalimumab (Humira) and infliximab (Remicade) and a soluble TNF-alpha receptor fusion protein, etanercept (Enbrel). To be considered eligible to receive a biologic agent under BAD guidelines, patients should have severe psoriasis, defined by a PASI (psoriasis area and severity index score) of 10 or more, have severe, 34 Prescriber 5 June
4 SPL Figure 3. Narrowband UVB is first-line phototherapy for the treatment of psoriasis and is more effective than broadband UVB and comparable to PUVA with respect to clearance times and remission unstable or life-threatening disease and be ineligible for phototherapy or standard systemic therapy because of contraindications, intolerance or unresponsiveness to treatment. 23 Adalimumab is a fully humanised IgG1 monoclonal antibody for used in moderate-to-severe psoriasis. It has shown to be highly effective in three large randomised controlled trials (RCTs). 24 Onset is rapid after 12 weeks in one trial, 53 per cent of patients on adalimumab 40mg every two weeks and 80 per cent on 40mg weekly achieved a 75 per cent reduction in PASI (PASI 75) score. 25 The National Institute for Health and Clinical Excellence (NICE) has approved the use of adalimumab (40mg every two weeks) in severe plaque psoriasis, with continued therapy subject to adequate response at 16 weeks. 26 Infliximab is a chimeric monoclonal anti-tnf antibody and is also highly effective in the treatment of chronic plaque psoriasis. 24 Infliximab has a rapid onset of action, with 79 per cent of patients achieving a PASI 75 by week 10, with a sustained response in a high percentage of patients at one year. Loss of efficacy correlates with development of antibodies, which occurs in 19 per cent of patients treated with infliximab. 27 NICE recommends infliximab to be commenced at initially 5mg per kg² at weeks 0, 2 and 6 and then every eight weeks. Treatment beyond 10 weeks is only recommended in patients who achieve certain response criteria. Etanercept, a TNF-alpha receptor fusion protein, has been shown to be effective in the treatment of chronic plaque psoriasis in three large RCTs. 28 Onset of action appears to be slower than with the monoclonal antibodies. Action appears dose related and overall 34 per cent of patients receiving 25mg etanercept twice weekly achieved PASI 75 after 12 weeks, compared with 49 per cent of patients on 50mg twice weekly. 29 Recent BAD guidelines recommend that etanercept be commenced at 50mg weekly or 25mg twice weekly with disease response to be assessed at three to four months. In patients who respond, therapy may be continued according to clinical need, but longterm data on efficacy are limited to two years. 29 Some smaller studies suggest that its efficacy may be improved by the addition of methotrexate 30 or acitretin. Ustekinumab (Stelara) is a fully human IgG1 K mono clonal antibody that targets the p40 subunit of interleukins IL-12 and IL-23. These cytokines play a role in regulation of immune response and T-cell activation in psoriasis. Ustekinumab is licensed for use in patients with moderate-to-severe psoriasis at 45mg (or 90mg if >100kg) at weeks 0 and 4 and then 12 weekly thereafter. It has shown to be highly effective in the treatment of chronic plaque psoriasis, both at 45mg and 90mg, and its onset of action is evident within two weeks. By 12 weeks, 67 per cent of patients receiving a dose of 45mg and 72 per cent of those receiving 90mg achieved PASI 75. Maximal efficacy is evident between weeks 20 and 24. Factors predictive of a poorer response include higher body weight, previous poor response to at least one biologic agent, longer duration of psoriasis and a history of psoriatic arthritis. 31 NICE has approved the use of ustekinumab in patients with severe plaque psoriasis, with treatment to be continued beyond 16 weeks only in those who respond. 32 In the light of limited patient exposure, 36 Prescriber 5 June
5 ustekinumab should be reserved for use in patients with severe psoriasis and where TNF antagonist therapy has failed or is contraindicated. 30 Potential adverse effects of TNF antagonist therapy include infections, reactivation of tuberculosis, development of demyelinating disease and worsening of cardiac failure. The BAD Biologic Interventions Registry (BAD-BIR) has been established in the UK to collect long-term safety data. This registry aims to follow up all patients on biologic therapy for five years, together with 4000 control subjects on conventional second-line drugs for psoriasis. 23 Etanercept or adalimumab are appropriate for patients with stable chronic plaque psoriasis, based on favourable risk/benefit profile and ease of administration. Where rapid disease control is required, adalimumab or infliximab may be more suitable due to early onset of action and a high chance of achieving a significant improvement in PASI 75 by three months. 33 Treatment decisions around biologics are complex and patients who have failed treatment with one or more biologic may respond to another. 34 Conclusion is a common chronic condition and has been shown to significantly influence a patient s quality of life. Topical treatments remain the mainstay of therapy in psoriasis, with per cent of all patients with psoriasis responding adequately to topical treatments. Vitamin D 3 analogues such calcipotriol and in combination with a corticosteroid are increasingly preferred as first-line topical therapy by patients for convenience and ease of use. Newer systemic agents such as fumaric ester derivatives with less adverse effects and improved patient tolerability are being used successfully. Recent advances in our understanding of its pathophysiology have led to the emergence of biologic agents that specifically target key mechanisms in the pathogenesis of psoriasis. These biologics have led to dramatic clinical responses in patients with psoriasis, and some appear to be more efficacious than older systemic therapies. However, their exact role in the management of psoriasis remains to be fully established, especially as long-term safety data are awaited on the newer biologic agents. References 1. Thami GP, et al. Clin Exp Dermatol 2002;27(2): Mahrle G. Clin Dermatol 1997;15(5): Bruce S, et al. J Am Acad Dermatol 1994;31: Langner A, et al. Br J Dermatol 1996;135: Prescriber 5 June
6 possible psoriasis exclude other disorders eczema, lichen planus, pityriasis rosea, tinea corporis, mycosis fungoides, cutaneous lupus erythematosus and assess degree of psychosocial disability due to psoriasis localised or mild-to-moderate psoriasis (10-15% BSA) trunk and limbs face, flexures and genitalia scalp emollients vitamin D analogue retinoid coal-tar preparation corticosteroid ointment/ cream dithranol incremental strength, short contact emollients mild-to-moderate potency topical corticosteroid vitamin D cream may be used with caution to the hairline and in flexures mild: tar shampoo moderate: corticosteroid or calcipotriol scalp lotion or gel severe: coal tar/salicylic acid/coconut oil ointment if above measures fail, refer for dermatological opinion widespread or moderate-tosevere psoriasis (15-20% BSA) 1st line topical treatment as above +/- dermatological referral 2nd line nuvb/puva methotrexate ciclosporin acitretin hydroxycarbamide mycophenolate mofetil fumaric acid esters 3rd line etanercept infliximab ustekinumab adalimumab Figure 4. Recommended management of psoriasis (BSA = body surface area) 40 Prescriber 5 June
7 Key points topical treatments (emollients, tar, dithranol, steroids, vitamin D analogues) remain the mainstay of treatment preparations combining various topical agents are now available narrowband UVB is the first-line phototherapy for psoriasis first-line systemic agents for psoriasis include methotrexate and most recently fumaric acid esters, which are being increasingly used; ciclosporin remains an excellent therapeutic option for short-term control of psoriasis where rapid disease response is required newer biologic agents have been shown to be effective in psoriasis but long-term safety data are awaited 5. Lambert J, et al. Dermatology 2002;204: Georgiou S, et al. Acta Derm Venereol 1999;79: Lebwohl MG, et al. J Am Acad Dermatol 1998;39: Green C, et al. Br J Dermatol 1988;119: Markham T, et al. Arch Dermatol 2003;139: Ferguson J. Arch Dermatol 1999;135: Stern RS, et al. J Invest Dermatol 1988;91: Feldman SR, et al. J Am Acad Dermatol 2002;46(6): Resources Further reading. Griffiths CEM, et al. In: Burns T, et al, eds. Rook s textbook of dermatology, 8th ed. Oxford: Wiley- Blackwell, 2010; Guidelines Adalimumab for the treatment of adults with psoriasis. TA146. NICE, June Etanercept and efalizumab for the treatment of adults with psoriasis. TA103. NICE, July Infliximab for the treatment of adults with psoriasis. TA134. NICE, January general management. British Association of Dermatologists, Default.aspx. management. NHS Clinical Knowledge Summaries. Ustekinumab for the treatment of adults with moderate to severe psoriasis. TA180. NICE, September Roenigk HH Jr, et al. J Am Acad Dermatol 1998;38(3): Zachariae H, et al. Dermatologica 1987;175(4): Roenigk HH Jr. J Am Acad Dermatol 1999;41:S Muller W, et al. Dtsch Med Wochenschr 1979;104(29): Lowe NJ, et al. J Am Acad Dermatol 1996;35: Raman GV, et al. J Hypertens Suppl 1998;16:S Smith CH. Clin Exp Dermatol 1999;24: Geilen CC, et al. Br J Dermatol 2001;144: Altmeyer PJ, et al. J Am Acad Dermatol 1994;30(6): Smith CH, et al. Br J Dermatol 2009;161: Gorden KB, et al. J Am Acad Dermatol 2006;55: Menter A, et al. J Am Acad Dermatol 2008;38: Saurat JH, et al. Br J Dermatol 2008:158: NICE. Infliximab for the treatment of adults with psoriasis. TA134. January Gottlieb AB, et al. J Am Acad Dermatol 2004: Reich K, et al. Lancet 2005;366: Leonardi CL, et al. N Engl J Med 2003;349: Papp KA, et al. Br J Dermatol 2005;152: Tyring S, et al. Lancet 2006;367: Zachariae C, et al. Acta Derm Venereol 2008;88: Krueger GG. N Engl J Med 2997;35: Dr Higgins is a dermatology registrar and Dr Markham is consultant dermatologist at University Hospital, Galway, Ireland Groups and organisations British Association of Dermatologists. Tel: ; admin@bad.org.uk; website: www. bad.org.uk. Association. Tel: ; mail@psoriasis-association.org.uk; website: www. psoriasis-association.org.uk. and Psoriatic Arthritis Alliance (PAPAA). Tel: ; info@papaa.org; website: Websites This site is provided by the National Foundation and gives information about psoriasis in general and also current research into psoriasis and treatments. The Help Organisation site contains information about treatments, and also provides a discussion forum for psoriasis sufferers to exchange views and advice on living with psoriasis. Prescriber 5 June
8 Prescription review GPs in England wrote 1.2 million prescriptions for psoriasis treatments in 2009 at a total cost of approximately 39 million. Calcipotriol products accounted for 74 per cent of volume and 89 per cent of spending ( 35 million). Prescribing of calcipotriol has been increasing steadily over the past 10 years: in 2009 it was 69 per cent greater than in 2000 whereas costs increased by a factor of 2.7 over the same period, with a slight increase in the rate of cost growth after By contrast, prescribing and spending on other agents has changed little. Coal tar products are the most frequently prescribed in this group but their use has declined by about 13 per cent in the last five years. Trends for Cocois have been similar. Only scrips were dispensed in 2009 for other preparations for psoriasis, such as retinoids, salicylic acid and other vitamin D derivatives, at a total cost of 1.8 million. Items (thousands) 250 calcipotriol coal tar Cocois others Year, by quarter Figure 5. Number of prescriptions for psoriasis treatments in England by quarter, NIC ( millions) 10 calcipotriol coal tar Cocois others Year, by quarter Figure 6. Cost of prescriptions for psoriasis treatments in England by quarter, Prescriber 5 June
9 CPD: management Answer these questions online at Prescriber.co.uk and receive a certificate of completion for your CPD portfolio. Utilise the Learning into Practice form to record how your learning has contributed to your professional development. 1. One of these statements about the treatment of psoriasis is false which is it? a. Treatment is often selected on the basis of disease severity, patient preference, relevant co-morbidities and efficacy b. Mild-to-moderate psoriasis is often managed by topical therapy c. More than half of patients find their treatment highly satisfactory d. affecting the hands, feet and face warrants more aggressive treatment 2. Which of these statements about topical therapy is false? a. The adverse effects of topical steroids on skin thickness are less of a concern on areas such as the scalp, palms and soles b. The standard dithranol regimen is to apply progressively reducing concentrations, from 2 per cent to 0.5 per cent, on a daily basis c. Topical steroids may be associated with tachyphylaxis d. Dithranol is not recommended for the treatment of acute psoriasis 3. Which of these statements about topical vitamin D analogues is false? a. They may cause hypercalcaemia b. Calcipotriol has been shown to be more effective than topical fluocinonide in the treatment of plaque psoriasis c. Calcitriol is thought to be less irritant than tacalcitol when applied to the face d. Vitamin D analogues should not be combined with photo - therapy 4. One of these statements about narrowband UVB (nuvb) is false which is it? a. It is the phototherapy of choice for psoriasis b. The spectrum of nuvb is 350nm c. Patients do not have to wear eye protection during treatment d. It can be used during pregnancy 6. Regarding the treatment of psoriasis with methotrexate, which one of these statements is false? a. It must be given by iv infusion b. It is indicated for recalcitrant disease unresponsive to topical or phototherapy c. Folic acid supplementation reduces nausea d. Regular liver function tests are required during treatment 7. Regarding the treatment of psoriasis with oral retinoids, which one of these statements is false? a. They are more effective for chronic plaque psoriasis than pustular or erythrodermic psoriasis b. Combination with PUVA or UVB allows dose reduction to reduce the incidence of adverse effects c. They are teratogenic d. They may cause mucocutaneous dryness and alopecia 8. Regarding the treatment of psoriasis with immuno - suppressants, which one of these statements is false? a. Loss of renal function associated with ciclosporin can be prevented by treatment with a calcium-channel blocker b. Ciclosporin is an inappropriate choice for short-term control because its onset of action is too slow c. Mycophenolate mofetil is not licensed for the treatment of severe psoriasis d. Fumaric acid derivatives may cause flushing 9. Which one of these statements about treating psoriasis with biological agents is false? a. Adalimumab, infliximab and etanercept target tumour necrosis factor b. Ustekinumab is a monoclonal antibody that targets IL-12 and IL-23 c. Etanercept acts more quickly than infliximab and adalimumab d. Treatment with biological agents should be continued only when an adequate response has been demonstrated after a period of weeks (depending on the agent) 5. One of these statements about phototherapy is false which is it? a. Prolonged PUVA therapy is associated with an increased risk of nonmelanoma skin cancer b. nuvb is administered three times a week c. Laser with a wavelength of 308nm can be targeted at affected skin, reducing the risk to uninvolved skin d. Most patients achieve remission after treatment with nuvb so one course of treatment is usually sufficient 10. One of these statements about biological agents in the treatment of psoriasis is false which is it? a. A history of psoriatic arthritis predicts a poorer response to ustekinumab b. Anti-TNF agents may reactivate latent tuberculosis c. Patients who do not respond to one biological agent will rarely benefit from a second d. 19 per cent of patients develop antibodies to infliximab during treatment and this impairs its efficacy 44 Prescriber 5 June
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Psoriasis: the management of psoriasis 1.1 Short title Psoriasis 2 The remit The Department of Health has asked NICE: 'to produce
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Psoriasis: the management of psoriasis 1.1 Short title Psoriasis 2 The remit The Department of Health has asked NICE: 'to produce
Etanercept: a new option in paediatric plaque psoriasis
 : a new option in paediatric plaque psoriasis Steve Chaplin MSc, MRPharmS, Medical Writer, Dr David Atherton MA, MB, BChir, FRCP, Honorary Consultant in Paediatric Dermatology, Great Ormond Street Hospital
: a new option in paediatric plaque psoriasis Steve Chaplin MSc, MRPharmS, Medical Writer, Dr David Atherton MA, MB, BChir, FRCP, Honorary Consultant in Paediatric Dermatology, Great Ormond Street Hospital
Psoriasis management. A/Prof Amanda Oakley Dermatologist, Waikato
 Psoriasis management A/Prof Amanda Oakley Dermatologist, Waikato AbbVie Breakfast Session, 14 June 2014 Disclosure This breakfast session is sponsored by Abbvie Autoimmune skin disorders Psoriasis Eczema
Psoriasis management A/Prof Amanda Oakley Dermatologist, Waikato AbbVie Breakfast Session, 14 June 2014 Disclosure This breakfast session is sponsored by Abbvie Autoimmune skin disorders Psoriasis Eczema
Horizon Scanning Centre March Tildrakizumab for moderate to severe plaque psoriasis SUMMARY NIHR HSC ID: 6798
 Horizon Scanning Centre March 2015 Tildrakizumab for moderate to severe plaque psoriasis SUMMARY NIHR HSC ID: 6798 This briefing is based on information available at the time of research and a limited
Horizon Scanning Centre March 2015 Tildrakizumab for moderate to severe plaque psoriasis SUMMARY NIHR HSC ID: 6798 This briefing is based on information available at the time of research and a limited
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Psoriasis is a chronic, inflammatory, Prescribing in children
 Psoriasis in children: current approaches to management Laura Proudfoot BSc, MRCP, Elisabeth Higgins MA, FRCP and Judy Davids RGN Our series Prescribing in children gives practical advice for successful
Psoriasis in children: current approaches to management Laura Proudfoot BSc, MRCP, Elisabeth Higgins MA, FRCP and Judy Davids RGN Our series Prescribing in children gives practical advice for successful
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Dimethyl fumarate for treating moderate to severe Draft scope (pre-referral) Draft remit/appraisal objective To appraise
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Dimethyl fumarate for treating moderate to severe Draft scope (pre-referral) Draft remit/appraisal objective To appraise
Scottish Medicines Consortium
 Scottish Medicines Consortium ustekinumab, 45mg solution for injection (Stelara ) No. (572/09) Janssen-Cilag Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of
Scottish Medicines Consortium ustekinumab, 45mg solution for injection (Stelara ) No. (572/09) Janssen-Cilag Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of
Psoriasis. Andrei Metelitsa, MD, FRCPC, FAAD Clinical Associate Professor, Dermatology, U of C Co-Director, Institute for Skin Advancement
 Psoriasis Andrei Metelitsa, MD, FRCPC, FAAD Clinical Associate Professor, Dermatology, U of C Co-Director, Institute for Skin Advancement Copyright 2017 by Sea Courses Inc. All rights reserved. No part
Psoriasis Andrei Metelitsa, MD, FRCPC, FAAD Clinical Associate Professor, Dermatology, U of C Co-Director, Institute for Skin Advancement Copyright 2017 by Sea Courses Inc. All rights reserved. No part
Horizon Scanning Centre March Ixekizumab for moderate to severe chronic plaque psoriasis SUMMARY NIHR HSC ID: 5209
 Horizon Scanning Centre March 2015 Ixekizumab for moderate to severe chronic plaque psoriasis SUMMARY NIHR HSC ID: 5209 This briefing is based on information available at the time of research and a limited
Horizon Scanning Centre March 2015 Ixekizumab for moderate to severe chronic plaque psoriasis SUMMARY NIHR HSC ID: 5209 This briefing is based on information available at the time of research and a limited
KEY MESSAGES. Psoriasis patients are more prone to cardiovascular diseases, stroke, lymphoma and non-melanoma skin cancers, and increased mortality.
 KEY MESSAGES Psoriasis is a genetically determined, systemic immune-mediated chronic inflammatory disease that affects primarily the skin and joints. Psoriasis Vulgaris is characterised by well-demarcated
KEY MESSAGES Psoriasis is a genetically determined, systemic immune-mediated chronic inflammatory disease that affects primarily the skin and joints. Psoriasis Vulgaris is characterised by well-demarcated
It is estimated that about 26,000 new cases of
 Focus on CME at Dalhousie University Set On Soothing Psoriasis A. H. Murray, MD, FRCP(C) Presented at the 76th Annual Dalhousie Refresher Course It is estimated that about 26,000 new cases of psoriasis
Focus on CME at Dalhousie University Set On Soothing Psoriasis A. H. Murray, MD, FRCP(C) Presented at the 76th Annual Dalhousie Refresher Course It is estimated that about 26,000 new cases of psoriasis
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 13 May 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 13 May 2009 STELARA 45 mg, solution for injection B/1 x 0.5 ml vial (CIP code: 392 586-2) JANSSEN-CILAG Ustekinumab
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 13 May 2009 STELARA 45 mg, solution for injection B/1 x 0.5 ml vial (CIP code: 392 586-2) JANSSEN-CILAG Ustekinumab
Phototherapy and Photochemotherapy Treatment (Ultraviolet A [PUVA] and B [UBV])
![Phototherapy and Photochemotherapy Treatment (Ultraviolet A [PUVA] and B [UBV]) Phototherapy and Photochemotherapy Treatment (Ultraviolet A [PUVA] and B [UBV])](/thumbs/89/99382632.jpg) Origination: 09/27/07 Revised: 08/2/17 Annual Review: 11/2/17 Purpose: To provide Phototherapy and Photochemotherapy Treatment (PUVA and UBV) guidelines for the Medical Department staff to reference when
Origination: 09/27/07 Revised: 08/2/17 Annual Review: 11/2/17 Purpose: To provide Phototherapy and Photochemotherapy Treatment (PUVA and UBV) guidelines for the Medical Department staff to reference when
Horizon Scanning Centre May Brodalumab for moderate to severe plaque psoriasis SUMMARY NIHR HSC ID: 5524
 Horizon Scanning Centre May 2014 Brodalumab for moderate to severe plaque psoriasis SUMMARY NIHR HSC ID: 5524 This briefing is based on information available at the time of research and a limited literature
Horizon Scanning Centre May 2014 Brodalumab for moderate to severe plaque psoriasis SUMMARY NIHR HSC ID: 5524 This briefing is based on information available at the time of research and a limited literature
A study of treatment modalities in psoriasis in dermatology outpatient department of a tertiary care teaching hospital
 Original article A study of treatment modalities in psoriasis in dermatology outpatient department of a tertiary care teaching hospital 1Y Roja Ramani, 2 Benu Panigrahy, 3 Sailenkumar Mishra, 4 BTPS Singh
Original article A study of treatment modalities in psoriasis in dermatology outpatient department of a tertiary care teaching hospital 1Y Roja Ramani, 2 Benu Panigrahy, 3 Sailenkumar Mishra, 4 BTPS Singh
An otherwise healthy 12-year-old
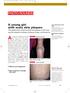 1105 Photo Rounds.finalREV 10/19/05 2:05 PM Page 947 A young girl with scaly skin plaques The patient had numerous thick red plaques on her back and the extensor surfaces of elbows, knees, and forearms
1105 Photo Rounds.finalREV 10/19/05 2:05 PM Page 947 A young girl with scaly skin plaques The patient had numerous thick red plaques on her back and the extensor surfaces of elbows, knees, and forearms
Topical Calcipotriol Algorithm
 Topical Calcipotriol Algorithm Is this patient an adult previously diagnosed with psoriasis by a doctor? Do the skin patches look the same as those diagnosed as psoriasis? Is this psoriasis covering an
Topical Calcipotriol Algorithm Is this patient an adult previously diagnosed with psoriasis by a doctor? Do the skin patches look the same as those diagnosed as psoriasis? Is this psoriasis covering an
Horizon Scanning Centre January Apremilast for psoriasis SUMMARY NIHR HSC ID: 2652
 Horizon Scanning Centre January 2013 Apremilast for psoriasis SUMMARY NIHR HSC ID: 2652 This briefing is based on information available at the time of research and a limited literature search. It is not
Horizon Scanning Centre January 2013 Apremilast for psoriasis SUMMARY NIHR HSC ID: 2652 This briefing is based on information available at the time of research and a limited literature search. It is not
PSORIASIS BEST PRACTICE IN MANAGEMENT
 PSORIASIS BEST PRACTICE IN MANAGEMENT Objectives Discuss pathology of psoriasis Review types of psoriasis Review triggers and factors affecting disease severity Common comorbidity review Review first and
PSORIASIS BEST PRACTICE IN MANAGEMENT Objectives Discuss pathology of psoriasis Review types of psoriasis Review triggers and factors affecting disease severity Common comorbidity review Review first and
Psoriasis: Therapeutic goals
 Psoriasis: Therapeutic goals I want to die 50 45 impetiginization infliximab 600 40 35 30 400 25 20 15 200 10 5 0 22-ene 21-feb 23-mar 22-abr 22- may Efalizumab 6 doses: flare + REBOUND CSA 3 21-jun 21-jul
Psoriasis: Therapeutic goals I want to die 50 45 impetiginization infliximab 600 40 35 30 400 25 20 15 200 10 5 0 22-ene 21-feb 23-mar 22-abr 22- may Efalizumab 6 doses: flare + REBOUND CSA 3 21-jun 21-jul
Psoriasis. Causes of Psoriasis
 Psoriasis Psoriasis is a common, chronic, relapsing/remitting, immune-mediated systemic disease characterized by skin lesions including red, scaly patches, papules, and plaques, which usually itch. The
Psoriasis Psoriasis is a common, chronic, relapsing/remitting, immune-mediated systemic disease characterized by skin lesions including red, scaly patches, papules, and plaques, which usually itch. The
The role of the practice nurse in managing psoriasis in primary care
 The role of the practice nurse in managing psoriasis in primary care Item type Authors Publisher Journal Article Buckley, David Nursing in General Practice Nursing in general practice Downloaded 16-Sep-2016
The role of the practice nurse in managing psoriasis in primary care Item type Authors Publisher Journal Article Buckley, David Nursing in General Practice Nursing in general practice Downloaded 16-Sep-2016
THE THERAPY OF THE REBEL SEVERE PSORIAZIS WITH BIOLOGICAL PREPARATS
 Bulletin of the Transilvania University of Braşov Series VI: Medical Sciences Vol. 5 (54) No. 2-2012 THE THERAPY OF THE REBEL SEVERE PSORIAZIS WITH BIOLOGICAL PREPARATS Mădălina FRÎNCU 1 Abstract: Biological
Bulletin of the Transilvania University of Braşov Series VI: Medical Sciences Vol. 5 (54) No. 2-2012 THE THERAPY OF THE REBEL SEVERE PSORIAZIS WITH BIOLOGICAL PREPARATS Mădălina FRÎNCU 1 Abstract: Biological
Clinical Policy: Ustekinumab (Stelara) Reference Number: ERX.SPMN.167
 Clinical Policy: (Stelara) Reference Number: ERX.SPMN.167 Effective Date: 10/16 Last Review Date: 12/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Stelara) Reference Number: ERX.SPMN.167 Effective Date: 10/16 Last Review Date: 12/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs)
 Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs) January 2010 This technology summary is based on information available at the time of research
Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs) January 2010 This technology summary is based on information available at the time of research
TRANSPARENCY COMMITTEE OPINION. 26 April 2006
 TRANSPARENCY COMMITTEE OPINION 26 April 2006 REMICADE 100 mg powder for concentrate for solution for infusion Box of 1 (CIP code: 562 070.1) Applicant : laboratoires Schering Plough List I Drug for hospital
TRANSPARENCY COMMITTEE OPINION 26 April 2006 REMICADE 100 mg powder for concentrate for solution for infusion Box of 1 (CIP code: 562 070.1) Applicant : laboratoires Schering Plough List I Drug for hospital
Elements of Successful PBS Applications. Barbara Radulski RN. Copyright
 Elements of Successful PBS Applications Barbara Radulski RN PBS Requirements April 1 2006 THE RULES PBS Requirements 18 years and over Psoriasis x 6 months Failed to achieve an adequate response to 3 systemic
Elements of Successful PBS Applications Barbara Radulski RN PBS Requirements April 1 2006 THE RULES PBS Requirements 18 years and over Psoriasis x 6 months Failed to achieve an adequate response to 3 systemic
Psoriatic Arthritis- Second Line Treatments
 Psoriatic Arthritis- Second Line Treatments Second line treatments for Psoriatic Arthritis (PsA) are usually prescribed by a Rheumatologist, Dermatologist, or in a combined clinic where both the Dermatologist
Psoriatic Arthritis- Second Line Treatments Second line treatments for Psoriatic Arthritis (PsA) are usually prescribed by a Rheumatologist, Dermatologist, or in a combined clinic where both the Dermatologist
The Natural History of Psoriasis and Treatment Goals
 The Natural History of Psoriasis and Treatment Goals Psoriasis Epidemiology Prevalence Affects 2 3% of adult population (>7 million in US) Caucasians: 25% 2.5% African Americans: 1.3% (more likely to have
The Natural History of Psoriasis and Treatment Goals Psoriasis Epidemiology Prevalence Affects 2 3% of adult population (>7 million in US) Caucasians: 25% 2.5% African Americans: 1.3% (more likely to have
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Apremilast Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 1/1/2018 Next
Cigna Drug and Biologic Coverage Policy Subject Apremilast Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 1/1/2018 Next
Final Appraisal Report. Etanercept (Enbrel. Pfizer Limited. Advice No: 0210 March Recommendation of AWMSG
 Final Appraisal Report Etanercept (Enbrel ) Pfizer Limited Advice No: 0210 March 2010 Recommendation of AWMSG Etanercept (Enbrel ) is not recommended for use within NHS Wales for the treatment of chronic
Final Appraisal Report Etanercept (Enbrel ) Pfizer Limited Advice No: 0210 March 2010 Recommendation of AWMSG Etanercept (Enbrel ) is not recommended for use within NHS Wales for the treatment of chronic
Single Technology Appraisal (STA) Tildrakizumab for treating moderate to severe plaque psoriasis
 Single Technology Appraisal (STA) Tildrakizumab for treating moderate to severe plaque psoriasis Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) Please
Single Technology Appraisal (STA) Tildrakizumab for treating moderate to severe plaque psoriasis Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) Please
Psoriasis: Causes, Symptoms, And Treatment
 Psoriasis: Causes, Symptoms, And Treatment We all know that a healthy immune system is good. But, do you know that an overactive immune system can cause certain conditions like Psoriasis? Read on to find
Psoriasis: Causes, Symptoms, And Treatment We all know that a healthy immune system is good. But, do you know that an overactive immune system can cause certain conditions like Psoriasis? Read on to find
Psoriatic Arthritis- Secondary Care
 Psoriatic Arthritis- Secondary Care Our Psoriatic Arthritis: First Line Treatments information sheet gives information on the treatments that can be prescribed by a GP, or that might be prescribed if the
Psoriatic Arthritis- Secondary Care Our Psoriatic Arthritis: First Line Treatments information sheet gives information on the treatments that can be prescribed by a GP, or that might be prescribed if the
Psoriasis the latest recommendations for management: where can primary care make a real difference?
 Dermatology Psoriasis the latest recommendations for management: where can primary care make a real difference? Dr Stephen Kownacki Executive chair, Primary Care Dermatology Society (PCDS) This session
Dermatology Psoriasis the latest recommendations for management: where can primary care make a real difference? Dr Stephen Kownacki Executive chair, Primary Care Dermatology Society (PCDS) This session
USTEKINUMAB Generic Brand HICL GCN Exception/Other USTEKINUMAB STELARA GUIDELINES FOR USE
 Generic Brand HICL GCN Exception/Other USTEKINUMAB STELARA 36187 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of psoriatic arthritis (PsA)
Generic Brand HICL GCN Exception/Other USTEKINUMAB STELARA 36187 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of psoriatic arthritis (PsA)
Risankizumab (by subcutaneous injection) for moderate to severe chronic plaque psoriasis
 NIHR Innovation Observatory Evidence Briefing: November 2017 Risankizumab (by subcutaneous injection) for moderate to severe chronic plaque psoriasis NIHRIO (HSRIC) ID: 9708 NICE ID: 9191 LAY SUMMARY Plaque
NIHR Innovation Observatory Evidence Briefing: November 2017 Risankizumab (by subcutaneous injection) for moderate to severe chronic plaque psoriasis NIHRIO (HSRIC) ID: 9708 NICE ID: 9191 LAY SUMMARY Plaque
BJD. Summary. British Journal of Dermatology THERAPEUTICS
 THERAPEUTICS BJD British Journal of Dermatology Efficacy of psoralen plus ultraviolet A therapy vs. biologics in moderate to severe chronic plaque psoriasis: retrospective data analysis of a patient registry
THERAPEUTICS BJD British Journal of Dermatology Efficacy of psoralen plus ultraviolet A therapy vs. biologics in moderate to severe chronic plaque psoriasis: retrospective data analysis of a patient registry
Psoriasis. What is Psoriasis? What causes psoriasis? Medical Topics Psoriasis
 1 Psoriasis What is Psoriasis? Psoriasis is a long standing inflammatory non-contagious skin disease which waxes and wanes with triggering factors. There is a genetic predisposition in psoriasis. Internationally,
1 Psoriasis What is Psoriasis? Psoriasis is a long standing inflammatory non-contagious skin disease which waxes and wanes with triggering factors. There is a genetic predisposition in psoriasis. Internationally,
Original Policy Date
 MP 2.01.07 Psoralens with Ultraviolet A (PUVA) Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed by consensus/12:2013 Return to Medical Policy
MP 2.01.07 Psoralens with Ultraviolet A (PUVA) Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed by consensus/12:2013 Return to Medical Policy
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2018 P 2104-4 Program Prior Authorization/Medical Necessity Medication Taltz (ixekizumab) P&T Approval Date 8/2016, 5/2017, 2/2018 Effective
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2018 P 2104-4 Program Prior Authorization/Medical Necessity Medication Taltz (ixekizumab) P&T Approval Date 8/2016, 5/2017, 2/2018 Effective
Brodalumab for treating moderate to severe plaque psoriasis [ID878]
![Brodalumab for treating moderate to severe plaque psoriasis [ID878] Brodalumab for treating moderate to severe plaque psoriasis [ID878]](/thumbs/82/85279721.jpg) Brodalumab for treating moderate to severe plaque psoriasis [ID878] Thank you for agreeing to give us your organisation s views on this technology and its possible use in the NHS. You can provide a unique
Brodalumab for treating moderate to severe plaque psoriasis [ID878] Thank you for agreeing to give us your organisation s views on this technology and its possible use in the NHS. You can provide a unique
Efficacy of Concomitant Use of PUVA and Methotrexate in Disease Clearance Time in Plaque Type Psoriasis
 Efficacy of Concomitant Use of PUVA and Methotrexate in Disease Clearance Time in Plaque Type Psoriasis T. Shehzad ( Departments of Dermatology Naval Hospital PNS Shifa, Karachi. ) N. R. Dar ( Departments
Efficacy of Concomitant Use of PUVA and Methotrexate in Disease Clearance Time in Plaque Type Psoriasis T. Shehzad ( Departments of Dermatology Naval Hospital PNS Shifa, Karachi. ) N. R. Dar ( Departments
A Retrospective Study on the Risk of Non-Melanoma Skin Cancer in PUVA and Narrowband UVB Treated Patients
 Volume 1, Issue 3 Research Article A Retrospective Study on the Risk of Non-Melanoma Skin Cancer in PUVA and Narrowband UVB Treated Patients Darukarnphut P, Rattanakaemakorn P *, Rajatanavin N Division
Volume 1, Issue 3 Research Article A Retrospective Study on the Risk of Non-Melanoma Skin Cancer in PUVA and Narrowband UVB Treated Patients Darukarnphut P, Rattanakaemakorn P *, Rajatanavin N Division
2 SYNOPSIS. Study code : MC 9308 FR.
 MC9308 FR Study 19 December 2000 Page 15 of142 2 SYNOPSIS Study code : MC 9308 FR. Title: A comparative study of calcipotriol ointment in combination with narrow-band UVB (TL-01) phototherapy and placebo
MC9308 FR Study 19 December 2000 Page 15 of142 2 SYNOPSIS Study code : MC 9308 FR. Title: A comparative study of calcipotriol ointment in combination with narrow-band UVB (TL-01) phototherapy and placebo
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ustekinumab_stelara 02/2010 10/2018 10/2019 10/2018 Description of Procedure or Service Plaque psoriasis
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ustekinumab_stelara 02/2010 10/2018 10/2019 10/2018 Description of Procedure or Service Plaque psoriasis
Psoriasis. Overview. Epidemiology. Epidemiology 08/08/2015. Dr Nigel Burrows Consultant Dermatologist Addenbrooke s Hospital
 Overview Psoriasis 1. Epidemiology of psoriasis 2. Histology Dr Nigel Burrows Consultant Dermatologist Addenbrooke s Hospital Aug 2015 3. Types of psoriasis 4. Assessing severity 5. Treatments Topical
Overview Psoriasis 1. Epidemiology of psoriasis 2. Histology Dr Nigel Burrows Consultant Dermatologist Addenbrooke s Hospital Aug 2015 3. Types of psoriasis 4. Assessing severity 5. Treatments Topical
Clinical Policy: Ustekinumab (Stelara) Reference Number: CP.PHAR.264
 Clinical Policy: (Stelara) Reference Number: CP.PHAR.264 Effective Date: 08/16 Last Review Date: 05/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Stelara) Reference Number: CP.PHAR.264 Effective Date: 08/16 Last Review Date: 05/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Ustekinumab for severe treatment-resistant psoriasis: a 24-week pilot study in Hong Kong Chinese
 Hong Kong J. Dermatol. Venereol. (2011) 19, 59-64 Original Article Ustekinumab for severe treatment-resistant psoriasis: a 24-week pilot study in Hong Kong Chinese Ustekinumab SKF Loo, KH Lau, KM Ho Introduction:
Hong Kong J. Dermatol. Venereol. (2011) 19, 59-64 Original Article Ustekinumab for severe treatment-resistant psoriasis: a 24-week pilot study in Hong Kong Chinese Ustekinumab SKF Loo, KH Lau, KM Ho Introduction:
Guselkumab for treating moderate to severe plaque psoriasis [ID1075]
![Guselkumab for treating moderate to severe plaque psoriasis [ID1075] Guselkumab for treating moderate to severe plaque psoriasis [ID1075]](/thumbs/83/87872235.jpg) Guselkumab for treating moderate to severe plaque psoriasis [ID1075] Thank you for agreeing to give us your organisation s views on this technology and its possible use in the NHS. You can provide a unique
Guselkumab for treating moderate to severe plaque psoriasis [ID1075] Thank you for agreeing to give us your organisation s views on this technology and its possible use in the NHS. You can provide a unique
Combination Nonbiologic Therapy in Psoriasis. Sushil Tahiliani, MBBS, MD
 Combination Nonbiologic Therapy in Psoriasis Sushil Tahiliani, MBBS, MD Agenda Rationale Preferred and less preferred combination Morphology-specific preferred combinations Doses used in combinations Potential
Combination Nonbiologic Therapy in Psoriasis Sushil Tahiliani, MBBS, MD Agenda Rationale Preferred and less preferred combination Morphology-specific preferred combinations Doses used in combinations Potential
Corporate Medical Policy
 Corporate Medical Policy Light Therapy for Dermatologic Conditions File Name: Origination: Last CAP Review: Next CAP Review: Last Review: light_therapy_for_dermatologic_conditions 5/2012 11/2017 11/2018
Corporate Medical Policy Light Therapy for Dermatologic Conditions File Name: Origination: Last CAP Review: Next CAP Review: Last Review: light_therapy_for_dermatologic_conditions 5/2012 11/2017 11/2018
Clinical Policy: Ixekizumab (Taltz) Reference Number: ERX.SPA.122 Effective Date:
 Clinical Policy: (Taltz) Reference Number: ERX.SPA.122 Effective Date: 10.01.16 Last Review Date: 11.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Taltz) Reference Number: ERX.SPA.122 Effective Date: 10.01.16 Last Review Date: 11.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
STELARA (ustekinumab)
 STELARA (ustekinumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
STELARA (ustekinumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
This PDF is available for free download from a site hosted by Medknow Publications
 Net Study Comparison of clinical efficacy of topical tazarotene.1% cream with topical clobetasol propionate.5% cream in chronic plaque psoriasis: A double-blind, randomized, right-left comparison study
Net Study Comparison of clinical efficacy of topical tazarotene.1% cream with topical clobetasol propionate.5% cream in chronic plaque psoriasis: A double-blind, randomized, right-left comparison study
Comparison of the efficacy of PUVA versus BBUVB in the treatment of psoriasis vulgaris
 IJMAMR 5 (2017) 1-6 ISSN 2053-1834 Comparison of the efficacy of PUVA versus BBUVB in the treatment of psoriasis vulgaris Tran Hau Khang* and Le Huu Doanh National Hospital of Dermatology and Venereology,
IJMAMR 5 (2017) 1-6 ISSN 2053-1834 Comparison of the efficacy of PUVA versus BBUVB in the treatment of psoriasis vulgaris Tran Hau Khang* and Le Huu Doanh National Hospital of Dermatology and Venereology,
Certolizumab pegol (Cimzia) for psoriatic arthritis second line
 Certolizumab pegol (Cimzia) for psoriatic arthritis second line This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Certolizumab pegol (Cimzia) for psoriatic arthritis second line This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
C. Assess clinical response after the first three months of treatment.
 Government Health Plan (GHP) of Puerto Rico Authorization Criteria Tumor Necrosis Factor Alpha (TNFα) Adalimumab (Humira ) Managed by MCO Section I. Prior Authorization Criteria A. Physician must submit
Government Health Plan (GHP) of Puerto Rico Authorization Criteria Tumor Necrosis Factor Alpha (TNFα) Adalimumab (Humira ) Managed by MCO Section I. Prior Authorization Criteria A. Physician must submit
Clinical Policy: Apremilast (Otezla) Reference Number: CP.PHAR.245 Effective Date: 08/16 Last Review Date 08/17
 Clinical Policy: (Otezla) Reference Number: CP.PHAR.245 Effective Date: 08/16 Last Review Date 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Otezla) Reference Number: CP.PHAR.245 Effective Date: 08/16 Last Review Date 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
MC 590 ABSTRACT. PageS
 This docwnent has OOen dov,nloaded from 'W'W'\VJ eo-pharma.c-om subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or
This docwnent has OOen dov,nloaded from 'W'W'\VJ eo-pharma.c-om subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or
Biologic Therapies for Psoriasis. A Systematic Review
 Biologic Therapies for Psoriasis. A Systematic Review WOLF-HENNING BOEHNCKE, JÖRG PRINZ, and ALICE B. GOTTLIEB ABSTRACT. Alefacept, efalizumab, etanercept, and infliximab are currently approved for the
Biologic Therapies for Psoriasis. A Systematic Review WOLF-HENNING BOEHNCKE, JÖRG PRINZ, and ALICE B. GOTTLIEB ABSTRACT. Alefacept, efalizumab, etanercept, and infliximab are currently approved for the
ixekizumab 80mg solution for injection (Taltz ) SMC No. (1223/17) Eli Lilly and Company Ltd.
 ixekizumab 80mg solution for injection (Taltz ) SMC No. (1223/17) Eli Lilly and Company Ltd. 10 March 2017 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
ixekizumab 80mg solution for injection (Taltz ) SMC No. (1223/17) Eli Lilly and Company Ltd. 10 March 2017 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
Predicting the Response to Phototherapy for Psoriasis Patients
 A*STAR-NHG-NTU Skin Research Grant Joint Workshop 17 October 2015 Predicting the Response to Phototherapy for Psoriasis Patients Is it possible? Dr Eugene Tan Consultant Dermatologist National Skin Centre
A*STAR-NHG-NTU Skin Research Grant Joint Workshop 17 October 2015 Predicting the Response to Phototherapy for Psoriasis Patients Is it possible? Dr Eugene Tan Consultant Dermatologist National Skin Centre
Clinical Policy: Ustekinumab (Stelara) Reference Number: ERX.SPA.01 Effective Date:
 Clinical Policy: (Stelara) Reference Number: ERX.SPA.01 Effective Date: 04.01.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Stelara) Reference Number: ERX.SPA.01 Effective Date: 04.01.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Stelara. Stelara (ustekinumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.04 Subject: Stelara Page: 1 of 6 Last Review Date: December 8, 2017 Stelara Description Stelara (ustekinumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.04 Subject: Stelara Page: 1 of 6 Last Review Date: December 8, 2017 Stelara Description Stelara (ustekinumab)
Anti-TNF biologic agents Dr Lluís Puig
 Department of Dermatology Hospital de la Santa Creu i Sant Pau IPC NOVARTIS PSORIASIS PRECEPTORSHIP Anti-TNF biologic agents Dr Lluís Puig Barcelona, July 9th-10th, 2013 Anti-TNF therapy in the pathophysiology
Department of Dermatology Hospital de la Santa Creu i Sant Pau IPC NOVARTIS PSORIASIS PRECEPTORSHIP Anti-TNF biologic agents Dr Lluís Puig Barcelona, July 9th-10th, 2013 Anti-TNF therapy in the pathophysiology
ustekinumab (Stelara )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Patients who achieved the primary criterion for response i.e.: complete clearance or a reduction
 MC 9101 F Study Page3 ABSTRACT Background: Cyclosporin A has been shown to be an effective systemic treatment in severe psoriasis but with the disadvantage of dose-dependent toxic effects particularly
MC 9101 F Study Page3 ABSTRACT Background: Cyclosporin A has been shown to be an effective systemic treatment in severe psoriasis but with the disadvantage of dose-dependent toxic effects particularly
Psoriasiform Dermatitis in Children: Calling in the Troops
 Psoriasiform Dermatitis in Children: Calling in the Troops Markus Boos, MD PhD Attending Physician, Dermatology Seattle Children s Hospital Assistant Professor of Pediatrics, University of Washington School
Psoriasiform Dermatitis in Children: Calling in the Troops Markus Boos, MD PhD Attending Physician, Dermatology Seattle Children s Hospital Assistant Professor of Pediatrics, University of Washington School
TREATMENT OPTIONS FOR PSORIASIS. Sandra Hanlon Dermatology Senior Charge Nurse NHS Ayrshire and Arran 07/03/17
 TREATMENT OPTIONS FOR PSORIASIS Sandra Hanlon Dermatology Senior Charge Nurse NHS Ayrshire and Arran 07/03/17 PSORIASIS A chronic, non-infectious inflammatory skin condition that has no cure Characterised
TREATMENT OPTIONS FOR PSORIASIS Sandra Hanlon Dermatology Senior Charge Nurse NHS Ayrshire and Arran 07/03/17 PSORIASIS A chronic, non-infectious inflammatory skin condition that has no cure Characterised
Prior Authorization Conditions for Approval of Enbrel (etanercept) Website Form Submit request via: Fax
 Prior Authorization Conditions for Approval of Enbrel (etanercept) Website Form www.highmarkhealthoptions.com Submit request via: Fax - 1-855-476-4158 All requests for Enbrel (etanercept) require a prior
Prior Authorization Conditions for Approval of Enbrel (etanercept) Website Form www.highmarkhealthoptions.com Submit request via: Fax - 1-855-476-4158 All requests for Enbrel (etanercept) require a prior
PHARMACY POLICY STATEMENT Ohio Medicaid
 DRUG NAME BILLING CODE BENEFIT TYPE SITE OF SERVICE ALLOWED COVERAGE REQUIREMENTS LIST OF DIAGNOSES CONSIDERED NOT MEDICALLY NECESSARY PHARMACY POLICY STATEMENT Ohio Medicaid Enbrel (etanercept) Must use
DRUG NAME BILLING CODE BENEFIT TYPE SITE OF SERVICE ALLOWED COVERAGE REQUIREMENTS LIST OF DIAGNOSES CONSIDERED NOT MEDICALLY NECESSARY PHARMACY POLICY STATEMENT Ohio Medicaid Enbrel (etanercept) Must use
A systematic review of treatments for severe psoriasis Griffiths C E, Clark C M, Chalmers R J, Li Wan Po A, Williams H C
 A systematic review of treatments for severe psoriasis Griffiths C E, Clark C M, Chalmers R J, Li Wan Po A, Williams H C Authors' objectives To compare the effectiveness of currently available treatments
A systematic review of treatments for severe psoriasis Griffiths C E, Clark C M, Chalmers R J, Li Wan Po A, Williams H C Authors' objectives To compare the effectiveness of currently available treatments
Light Therapy for Psoriasis Protocol Medical Benefit Effective Date Next Review Date Preauthorization Review Dates Preauthorization is required.
 Protocol Light Therapy for Psoriasis (20147) Medical Benefit Effective Date: 07/01/16 Next Review Date: 03/18 Preauthorization Yes Review Dates: 03/16, 03/17 Preauthorization is required. The following
Protocol Light Therapy for Psoriasis (20147) Medical Benefit Effective Date: 07/01/16 Next Review Date: 03/18 Preauthorization Yes Review Dates: 03/16, 03/17 Preauthorization is required. The following
Pharmacy Medical Necessity Guidelines: Stelara (ustekinumab)
 Pharmacy Medical Necessity Guidelines: Effective: January 1, 2018 Type of Review Care Prior Authorization Required Management Not Covered Type of Review Clinical Review SQ: RX/ Pharmacy (RX) or Medical
Pharmacy Medical Necessity Guidelines: Effective: January 1, 2018 Type of Review Care Prior Authorization Required Management Not Covered Type of Review Clinical Review SQ: RX/ Pharmacy (RX) or Medical
ETANERCEPT Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW)
 Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL 18830 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL 18830 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
1. Background: Infliximab is administered parenterally; therefore, it is not covered under retail pharmacy benefits.
 Subject: Infliximab (Remicade ) Original Original Committee Approval: October 13, 2006 Revised Last Committee Approval: December 3, 2008 Last Review: October 19, 2007 1. Background: Infliximab is a genetically
Subject: Infliximab (Remicade ) Original Original Committee Approval: October 13, 2006 Revised Last Committee Approval: December 3, 2008 Last Review: October 19, 2007 1. Background: Infliximab is a genetically
Follow this and additional works at: Part of the Skin and Connective Tissue Diseases Commons
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2014 Is the Addition of a Topical Agent to
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2014 Is the Addition of a Topical Agent to
Pediatric Use: Safety and effectiveness of Ustekinumab (STELARA ) in pediatric patients have not been evaluated.
 Original Issue Date (Created): January 1, 2010 Most Recent Review Date (Revised): January 28, 2014 Effective Date: April 1, 2014 I. POLICY Preauthorization Requirements for Ustekinumab (STELARA ) Note:
Original Issue Date (Created): January 1, 2010 Most Recent Review Date (Revised): January 28, 2014 Effective Date: April 1, 2014 I. POLICY Preauthorization Requirements for Ustekinumab (STELARA ) Note:
Clinical Policy: Secukinumab (Cosentyx) Reference Number: CP.PHAR.261 Effective Date: 08/16 Last Review Date: 08/17
 Clinical Policy: (Cosentyx) Reference Number: CP.PHAR.261 Effective Date: 08/16 Last Review Date: 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Cosentyx) Reference Number: CP.PHAR.261 Effective Date: 08/16 Last Review Date: 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
chemotherapeutic agents in
 Use of biologics and chemotherapeutic agents in cutaneous emergencies: Focus on lifethreatening forms of psoriasis Alice Bendix Gottlieb MD, PhD Professor of Dermatology New York Medical College Metropolitan
Use of biologics and chemotherapeutic agents in cutaneous emergencies: Focus on lifethreatening forms of psoriasis Alice Bendix Gottlieb MD, PhD Professor of Dermatology New York Medical College Metropolitan
COSENTYX (secukinumab)
 COSENTYX (secukinumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
COSENTYX (secukinumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
USTEKINUMAB. London New Drugs Group APC/DTC Briefing Document. May 2009
 Page 1 London New Drugs Group APC/DTC Briefing Document USTEKINUMAB Contents Summary 1 Background 4 Clinical efficacy 5 Cost implications 9 Reference list 10 Appendices 11 Produced for the London New Drugs
Page 1 London New Drugs Group APC/DTC Briefing Document USTEKINUMAB Contents Summary 1 Background 4 Clinical efficacy 5 Cost implications 9 Reference list 10 Appendices 11 Produced for the London New Drugs
Clinical Policy: Phototherapy and Photochemotherapy for Dermatological Conditions Reference Number: CP.MP. 441
 Clinical Policy: Phototherapy and Photochemotherapy for Dermatological Conditions Reference Number: CP.MP. 441 Effective Date: November 2008 Last Review Date: January 2017 See Important Reminder at the
Clinical Policy: Phototherapy and Photochemotherapy for Dermatological Conditions Reference Number: CP.MP. 441 Effective Date: November 2008 Last Review Date: January 2017 See Important Reminder at the
Using Your ESP* in Pharmacy: How to Improve Treatment Adherence and Patient Outcomes in Psoriasis (*Expanded Scope of Practice)
 Using Your ESP* in Pharmacy: How to Improve Treatment Adherence and Patient Outcomes in Psoriasis (*Expanded Scope of Practice) Patient Case Study in Psoriasis Patient Case Study in Psoriasis William Smith,
Using Your ESP* in Pharmacy: How to Improve Treatment Adherence and Patient Outcomes in Psoriasis (*Expanded Scope of Practice) Patient Case Study in Psoriasis Patient Case Study in Psoriasis William Smith,
Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS.
 Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Otezla (apremilast) Page 1 of 7 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Otezla (apremilast) Prime Therapeutics will review Prior Authorization requests Prior
Otezla (apremilast) Page 1 of 7 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Otezla (apremilast) Prime Therapeutics will review Prior Authorization requests Prior
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2018 P 1097-7 Program Prior Authorization/Notification Medication *Stelara (ustekinumab) *This program applies to the subcutaneous formulation
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2018 P 1097-7 Program Prior Authorization/Notification Medication *Stelara (ustekinumab) *This program applies to the subcutaneous formulation
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Cosentyx, Cosentyx Sensoready) Reference Number: HIM.PA.SP29 Effective Date: 05/17 Last Review Date: Line of Business: Health Insurance Marketplace Coding Implications Revision Log See
Clinical Policy: (Cosentyx, Cosentyx Sensoready) Reference Number: HIM.PA.SP29 Effective Date: 05/17 Last Review Date: Line of Business: Health Insurance Marketplace Coding Implications Revision Log See
Narrow-band UVB PHOTOTHERAPY for Skin Diseases
 Narrow-band UVB PHOTOTHERAPY for Skin Diseases By Dr. Manal Bosseila Cairo University, Egypt HISTORICAL ASPECT In 1978: Irradiation cabin with broad band UVB tubes was introduced for psoriasis & uremic
Narrow-band UVB PHOTOTHERAPY for Skin Diseases By Dr. Manal Bosseila Cairo University, Egypt HISTORICAL ASPECT In 1978: Irradiation cabin with broad band UVB tubes was introduced for psoriasis & uremic
Clinical Policy: Brodalumab (Siliq) Reference Number: CP.PHAR.375 Effective Date: Last Review Date: 05.18
 Clinical Policy: (Siliq) Reference Number: CP.PHAR.375 Effective Date: 06.01.18 Last Review Date: 05.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Siliq) Reference Number: CP.PHAR.375 Effective Date: 06.01.18 Last Review Date: 05.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
1 P a g e. Systemic Juvenile Idiopathic Arthritis (SJIA) (1.3) Patients 2 years of age and older with active systemic juvenile idiopathic arthritis.
 LENGTH OF AUTHORIZATION: Initial: 3 months for Crohn s or Ulcerative Colitis; 1 year for all other indications. Renewal: 1 year dependent upon medical records supporting response to therapy and review
LENGTH OF AUTHORIZATION: Initial: 3 months for Crohn s or Ulcerative Colitis; 1 year for all other indications. Renewal: 1 year dependent upon medical records supporting response to therapy and review
Treatment for Psoriasis with Modified Goeckerman Regimen -One Year Experience in Southern Taiwan-
 Treatment for Psoriasis with Modified Goeckerman Regimen -One Year Experience in Southern Taiwan- Chih-Sheng Lai Po-Han Huang Ji-Chen Ho Despite the recent advances in treatment for psoriasis, it is still
Treatment for Psoriasis with Modified Goeckerman Regimen -One Year Experience in Southern Taiwan- Chih-Sheng Lai Po-Han Huang Ji-Chen Ho Despite the recent advances in treatment for psoriasis, it is still
Cyltezo (adalimumab-adbm) CG-DRUG-64, CG-DRUG-65
 Market DC Cyltezo (adalimumab-adbm) CG-DRUG-64, CG-DRUG-65 Override(s) Prior Authorization Quantity Limit Medications Cyltezo (adalimumab-adbm) 40 mg/0.8 ml prefilled syringe #* ^ Approval Duration 1 year
Market DC Cyltezo (adalimumab-adbm) CG-DRUG-64, CG-DRUG-65 Override(s) Prior Authorization Quantity Limit Medications Cyltezo (adalimumab-adbm) 40 mg/0.8 ml prefilled syringe #* ^ Approval Duration 1 year
Photochemotherapy MM /09/2004. HMO; PPO; QUEST Integration June 1, 2016 Section: Medicine Place(s) of Service: Home; Office
 Photochemotherapy Policy Number: Original Effective Date: MM.02.015 11/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration June 1, 2016 Section: Medicine Place(s) of Service:
Photochemotherapy Policy Number: Original Effective Date: MM.02.015 11/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration June 1, 2016 Section: Medicine Place(s) of Service:
Pharmacy Medical Necessity Guidelines: Stelara (ustekinumab)
 Pharmacy Medical Necessity Guidelines: Effective: November 14, 2017 Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical Review SQ: RXUM/ RX / Pharmacy (RX) or
Pharmacy Medical Necessity Guidelines: Effective: November 14, 2017 Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical Review SQ: RXUM/ RX / Pharmacy (RX) or
Clinical Policy: Secukinumab (Cosentyx) Reference Number: ERX.SPA.165 Effective Date:
 Clinical Policy: (Cosentyx) Reference Number: ERX.SPA.165 Effective Date: 10.01.16 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Cosentyx) Reference Number: ERX.SPA.165 Effective Date: 10.01.16 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Month/Year of Review: January 2015 Date of Last Review: January 2010
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Copyright 2012 Oregon State University. All Rights
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Copyright 2012 Oregon State University. All Rights
