including some caused by gram-negative bacilli. These studies
|
|
|
- Barnard Boone
- 5 years ago
- Views:
Transcription
1 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Feb. 1985, p /85/ $02.00/0 Copyright C 1985, American Society for Microbiology Vol. 27, No. 2 Efficacy and Safety of Aztreonam-Clindamycin Versus Tobramycin- Clindamycin in the Treatment of Lower Respiratory Tract Infections Caused by Aerobic Gram-Negative Bacilli JULIE R. RODRIGUEZ,',2* CARLOS H. RAMIREZ-RONDA,12 AND MINERVA NEVAREZ1 Infectious Disease Program and Departments of Research' and Medicine,2 Veterans Administration Medical Center and University of Puerto Rico School of Medicine, San Juan, Puerto Rico Received 24 May 1984/Accepted 6 November 1984 A total of 80 patients were randomized to receive either aztreonam or tobramycin for the treatment of lower respiratory tract infections caused by gram-negative bacilli; all these patients received clindamycin concomitantly. A total of 53 patients were randomized to receive aztreonam-clindamycin; of these, 46 were clinically evaluable and 39 were bacteriologically evaluable. Of the 46 clinically evaluable patients, 41 were considered cured, 3 failed to be cured, and 2 died during the study period of unrelated causes. Of the 39 bacteriologically evaluable patients, 36 were considered cured, and 3 failed to be cured. There were 26 clinically evaluable patients in the group randomized to receive tobramycin-clindamycin. Of them, 22 patients were considered cured, 3 failed to be cured, and 1 died of unrelated causes during the study period. There were 18 bacteriologically evaluable patients in the tobramycin-clindamycin group; 17 were cured, and 1 failed to be cured. The most common pathogens isolated from the patients were Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa. All of the isolated organisms were susceptible to both tested antibiotics, except for a strain of Pseudomonas cepacia resistant to both tested antimicrobial agents and a strain of Enterobacter aerogenes and one of P. aeruginosa that were resistant to aztreonam. Very few adverse reactions related to the antibiotics were seen. These effects, when present, were transient and comparable in both studied groups, except for renal-function tests, which were altered in 7.7% of the patients randomized to receive tobramycinclindamycin and in none of the patients randomized to receive aztreonam-clindamycin. Aztreonam-clindamycin is safe and effective for the treatment of lower respiratory tract infections caused by aerobic gram-negative bacilli when the organisms are susceptible. Infections of the lower respiratory tract, when acquired in the hospital, are associated with a very high mortality rate (8, 13). Many hospital-acquired lower respiratory tract infections are caused by aerobic gram-negative bacilli, and the high mortality rate is a reflection of this etiology. Lower respiratory tract infections caused by gram-negative bacilli, even when not hospital acquired, are associated with a significant mortality, and are most frequently seen in patients with underlying medical conditions like diabetes mellitus, alcoholism, chronic obstructive pulmonary disease, and others. The probability of a patient developing a gramnegative type of pneumonia has been associated with the pharyngeal colonization at the time the insult or aspiration, or both, take place (14). The colonization of the pharynx by gram-negative organisms has been shown to be increased in alcoholic patients (5, 17), diabetic patients (17), elderly patients (28), seriously ill patients (12), subjects during a viral illness (21), and others (11-13, 29). This colonization has been shown to be transient in healthy subjects (21) but to persist and even increase in sick patients (13). The management of lower respiratory tract infections caused by gram-negative bacilli usually involves the use of an aminoglycoside antibiotic with a beta-lactam antibiotic. Some authors have questioned the penetration of aminoglycosides into bronchial secretions (10) and have raised the question of the need of other agents to treat such infections because of the nephrotoxic-ototoxic potential of aminoglycosides (24). This prompted studies with newer beta-lactam agents for the treatment of lower respiratory tract infections, * Corresponding author. 246 including some caused by gram-negative bacilli. These studies have demonstrated variable effectiveness with cefotaxime (20), ceftizoxime (23), moxalactam (4), cefoperazone (6), piperacillin (9), and mezlocillin (22, 26). Aztreonam is a monobactam agent with specific activity against aerobic gram-negative bacilli and looks promising for the treatment of gram-negative pneumonia, not only because of its specific spectrum of activity, but because of its few expected side effects. Gram-negative bacillary pneumonias, either hospital acquired or community acquired, are common in our hospital. This aroused our interest in performing a study to compare the safety and efficacy of aztreonam-clindamycin with that of tobramycin-clindamycin in a group of patients with lower respiratory tract infections caused by gram-negative bacilli. Since many of these patients developed pneumonia after aspiration and on enrollment, we could not rule out a mixed infection with aerobic and anaerobic microorganisms. Every patient received clindamycin on enrollment. MATERIALS AND METHODS Patients. Hospitalized patients at the San Juan Veterans Administration Medical Center, 18 years of age or older, were requested to participate in the study. The patients were enrolled and randomized to receive either aztreonam or tobramycin after signing the informed written consent. A computer-generated randomization schedule designed to have a 2:1 aztreonam-to-tobramycin ratio provided by the sponsor was utilized. All patients received clindamycin in addition to the study antibiotics. The requirements for enrollment included radiological demonstration of a new pul-
2 VOL. 27, 1985 AZTREONAM-CLINDAMYCIN FOR TREATMENT OF PNEUMONIA 247 TABLE 1. Clinical data on patients enrolled in the lower respiratory tract infection study No. of patients treated with: Clinical data' Aztreonam Tobramycin (n = 53) (n = 26) Underlying disease Diabetes mellitus 12 5 Cardiovascular disease 15 5 Malignancy 6 4 Neurological disease 11 9 COPDb 9 8 Alcoholism 4 2 Trauma 3 1 Gas aspiration 1 Parkinson's 4 1 Organic brain syndrome 3 2 Pulmonary embolism 2 Source of sputum for culture Transtracheal or double lumen 18 7 Expectorated sputum Classification of pneumonia Community acquired Hospital acquired The mean age of the patients treated with aztreonam was 67.8 years (range, 35 to 97 years), and that of the patients treated with tobramycin was 63.4 years (range, 22 to 100 years). bcopd, Chronic obstructive pulmonary disease. monary infiltrate, clinical findings compatible with pneumonia, the production of purulent sputum, with microscopic examination revealing more than 25 polymorphonuclear leukocytes and less than 10 squamous epithelial cells per lower-power (10x) field, and a predominant population of gram-negative bacteria in the Gram stain. Microbiological confirmation of the diagnosis with sputum, pleural fluid, or blood cultures followed enrollment. Patients were excluded from the study if: (i) there was a history of hypersensitivity to aminoglycosides or clindamycin, (ii) there was a history of type I reaction to penicillins or cephalosporins, or both, (iii) there was hepatic dysfunction defined as serum glutamic oxalacetic transaminase or serum glutamic pyruvic transaminase more than twice the upper limits of normal or a total serum bilirubin concentration greater than 3 mg/dl, (iv) there was a condition which required treatment with an antiinfective agent other than the randomly assigned antibiotic and clindamycin, (v) there was concurrent severe disease that might limit survival during the period of study, (vi) the neutrophil count was less than 1,000/mm3, (vii) the patients required hemodialysis or peritoneal dialysis, and (viii) if the patients were psychotic, addicted to drugs, mentally or legally incapacitated, or otherwise unreliable. All candidates for the study were examined before and after enrollment by one of the authors (J. Rodrfguez). They were examined on a daily basis, and all clinical data were recorded on standardized forms. The following laboratory studies were done on all patients before and during therapy and, when indicated, on the last day of treatment and posttreatment: testing of lower respiratory tract secretions for Gram stains, culture, and sensitivity, blood cultures, complete blood cell count and differential, platelet count, direct and indirect Coomb's test, urinalysis, liver and renal function tests, and chest radiological studies. Bacteriological studies. The microorganisms isolated from cultures of an adequate expectorated sputum as previously defined (1, 7, 18), transtracheal aspirate, double-lumen aspirate, and blood or pleural fluid were identified by standard methods (15). The MICs of aztreonam and tobramycin were determined by a twofold serial microdilution (Dynatech MIC 2000) technique, with an inoculum of 105 to 5 x 105 CFU/ml for each strain tested. Resistance was defined as a MIC of -8 p.g/ml for aztreonam or tobramycin. Administration of antibiotics. All antibiotics were administered intravenously and were infused over a period of 20 to 60 min. A total of 53 patients were randomly assigned to receive aztreonam. Of these, 52 patients received 2 g every 8 h, and 1 patient received 1 g every 8 h. A total of 26 patients were randomly assigned to receive 80 mg of tobramycin every 8 h. All patients received clindamycin at a dose of 600 mg every 6 h. The mean duration of treatment for the patients receiving aztreonam was 12 days (range, 7 to 27 days), and for the patients receiving tobramycin it was 11 days (range, 7 to 26 days). Patients receiving antibiotic treatment for 72 h or less were considered nonevaluable. Assessment of therapeutic response. The therapeutic efficacy of aztreonam and tobramycin by clinical and bacteriological criteria was defined as follows. A clinical cure was defined as defervescence and resolution of signs and symptoms of lower respiratory tract infections, together with roentgenographic improvement or resolution. A partial response was defined as a substantial or temporary improvement of signs and symptoms of lower respiratory tract infection without complete or sustained resolution. Treatment was considered a failure if there was an absence of any substantial clinical improvement. A microbiological cure was defined as eradication of the causative organisms from sputum, blood, and pleural fluid if initial cultures were positive. A microbiological failure represented the persistance of causative organisms. Superinfection was defined as the emergence during treatment of infection due to organisms of different species. Assessment of toxicity. Patients were examined daily by one of the investigators for evidence of adverse reactions to any of the study medications. Laboratory tests, as mentioned previously, were performed before, during, and at the end of therapy for any evidence of alterations that could be associated to the antibiotic. RESULTS Clinical course. The study was conducted between February 1981 and August During the 19-month study period 80 patients with gram-negative bacillary lower respiratory tract infections were enrolled in the study. A total of 53 patients were randomized to receive aztreonam, and 27 were randomized to receive tobramycin. One patient in the tobramycin-clindamycin group refused the medications after signing the informed consent. All patients included in the study were males. The ages of the patients treated with aztreonamclindamycin ranged from 35 to 97 years (mean, 67.8 years), and those treated with tobramycin-clindamycin ranged from 22 to 100 years (mean, 63.4 years). The presence of underlying diseases which might predispose patients to pneumonia was comparable between the two groups and included diabetes mellitus, malignancy, chronic obstructive pulmonary disease, and alcoholism, among others (See Table 1). In the aztreonam-clindamycin group there were 34 patients with hospital-acquired pneumonia and 19 with community-acquired pneumonia. In the group receiving tobra-
3 248 RODRiGUEZ, RAMIREZ-RONDA, AND NEVAREZ ANTIMICROB. AGENTS CHEMOTHER. TABLE 2. Analysis of the evaluable patients receiving aztreonam whose responses were considered failures or who died during the study p'eriod, or both. Aztreonam Duration Case Age dose of Underlying Organism(s) isolated Comments no. (years) (g/day) therapy (as disease(s) Aspiration king Diffuse bilateral pneumonia; GNBb in double-lumen pine aspirate; started on aztreonam and clindamycin; responded to treatment; infiltrates disappeared after 10 days of treatment. Patient developed myocardial infarct while on therapy; several days later developed cardiogenic shock and died. Autopsy not granted. Death while in study period, possible clinical failure; most likely death not related to pneumonia Cerebrovascular accident COPDc Smoker, rectal adenocarcinoma, abdominal resection Arterial hypertension, chronic smoker, trauma (subdural hematoma) a *, Gram-negative b bacilli seen in Gram stain. GNB, Gram-negative bacilli. c COPD, Chronic obstructive pulmonary disease. mycin-clindamycin, 11 patients had hospital-acquired pneumonia, and 15 had community-acquired pneumonia. A total of 22 patients receiving aztreonam-clindamycin and 17 patients receiving tobramycin-clindamycin were moderately ill: that is, with clinical pneumonia but able to give a history by themselves and only requiring nasal oxygen. A total of 22 patients in the aztreonam-clindamycin group and 9 patients in the tobramycin-clindamycin group were severely ill; the patients were so ill that they were usually on a respirator, unable to give a history and with evidence of severe hypoxemia. Bacteriological documentation in the 53 patients randomized to receive aztreonam-clindamycin was based on sputum samples obtained with transtracheal or double-lumen aspiration in 18 patients and from expectorated sputum P. aeruginosa (double lumen); superinfection with a resistant P. aeruginosa strain Serratia marcescens (expectorated); superinfection with S. epidermidis and C. albicans (B/C) E. aerogenes, Staphylococcus aureus (double lumen) Right upper lobe pneumonia; GNB in expectorated sputum; started on aztreonam and clindamycin; clinical and radiological improveme'nt of pneumonia after 12 days of therapy. The same day therapy was stopped developed gastrointestinal bleeding; he also had eviden'ce of subcutaneous hematomas; bleeding diathesis work-up started; patient died. No cause for bleeding determined. May have been related to antibiotic. Possible clinical failure. No autopsy granted. Lower lobe infiltrate; P. aeruginosa from double lumen; started on aztreonam and clindamycin; after 6 days clinically deteriorated; culture of respiratory secretions grew P. aeruginosa resistant now to aztreonam. Antibiotics changed; patient died subsequently. No autopsy granted. Clinical bacteriological failure and superinfection. Lower lobe infiltrate; Serratia marcesens in expectorated sputum susceptible to aztreonam; after 4 days on therapy deteriorated clinically; intraabdominal infection in addition to pneumonia. Antibiotics changed; prior to antibiotic change sputum culture grew Staphylococcus epidermidis and C. albicans; blood culture grew C. albicans. Patient died several weeks later of intraabdominal complication. No autopsy. Clinical and bacteriological failure, and superinfection. Lower lung infiltrates; E. aerogenes. samples in 35 patients. In patients assigned to receive tobramycin-clindamycin, bacteriological documentation was based on sputum samples obtained from transtracheal or double-lumen aspiration in 7 patients and from an adequate expectorated sputum sample in 19 patients. All these sputum samples contained more than 25 polymorphonuclear leukocytes and less than 10 squamous epithelial cells per lowerpower (10 x) field and had a predominance of gram-negative bacilli in the Gram stain. All 80'patients had pretherapy roentgenographic findings consistent with lower respiratory tract infections. More than 70% of the patients in both groups had lower pulmonary infiltrates. There were 72 evaluable patients of 80 patients enrolled. Of 53 patients treated with aztreonam-clindamycin, 46 were
4 VOL. 27, 1985 AZTREONAM-CLINDAMYCIN FOR TREATMENT OF PNEUMONIA 249 TABLE 3. Analysis of the evaluable patients receiving clindamycin whose responses were considered failures or who died during the study period, or both Duration Case Age Tobramycin of Comments no. (years) dose (g/day) therapy Underlying disease(s) Organism Isolated (days) Organic brain syndrome E. coli Right lower lobe infiltrates; E. coli in expectorated spu- (expectorated) tum. Treated with tobramycin and clindamycin; there is recovery, lung infiltrates clear; patient on the ninth day of therapy develops a pulmonary embolism and dies; pulmonary embolism confirmed by autopsy; no evidence of pneumonia. Death while in study Pernicious anemia, right Bilateral basal infiltrates; GNBb in sputum; no growth hemiplegia, thymoma on culture. Started on tobramycin and clindamycin. Six days later was not improving and started to deteriorate clinically. Sputum still with GNB; no growth on culture; antibiotics changed. After several days on new antibiotics died. Autopsy performed: necrotizing pneumonia. Clinical failure Atrial diabetes mnellitus * Right middle lung infiltrate; GNB in sputum; no growth on culture. Started on tobramycin and clindamycin; after 7 days on therapy, patient clinically not responding and died. Autopsy performed: pulmonary embolism and necrotizing pneumonia. Clinical failure COPD,C carcinoma of K. pneumonia Necrotizing pneumonia, right lower lobe, with K. pneularynx (expectorated) moniae susceptible to tobramycin (MIC, <4,ug/ml). Patient did not improve in 48 h and antibiotics were changed, considering the response a clinical failure. Antibiotics changed; pneumonia progressed and patient died. No autopsy granted. Clinical and bacteriological failure. a * Gram-negative bacilli seen in Gram stain. bgnb, Gram-negative bacilli. COPD, Chronic obstructive pulmonary disease. evaluable for clinical response. Seven patients who were considered nonevaluable received less than 72 h of antibiotic therapy. Two of the seven patients had an aztreonam-resistant organism recovered from the sputum; therefore, aztreonam was discontinued. All seven patients died of underlying diseases or pneumonia, or both. There were 27 patients randomized to received tobramycin-clindamycin: 26 were evaluable for clinical response, and 1 refused medication after enrollment. The overall response rate was as follows: 46 patients were clinically evaluable and 39 were bacteriologically and clinically evaluable in the group randomized to receive aztreonam-clindarmycin. In this group, the responses of 41 of the 46 clinically evaluable patients were considered clinical cures, and the responses of 36 of the 39 bacteriologically evaluable patients were considered bacteriological cures. Three patients (7%) in the aztreonam-clindamycin group were considered to have failed to respond to therapy; that is, aztreonam-clindamycin had to be discontinued and an alternate antibiotic therapy given because of the clinical deterioration of the patient (Table 2). The responses of two additional patients were also classified as failures because the patients died within the study period of causes probably unrelated to infection. In the group randomized to receive tobramycin-clindamycin, the responses of 22 of 26 clinically evaluable patients were considered clinical cures, and the responses of 17 of 18 clinically and bacteriologically evaluable patients were considered bacteriological cures. The responses of three patients in the tobramycin-clindamycin group were considered "true" failures (Table 3), and the response of one patient who died of unrelated causes was classified as a failure. The clinical and bacteriological cure rates are not statistically different (P = and P = , respectively) by the X2 test. Among the 79 studied patients, we documented six episodes of bacteremia. There were four patients with bacteremia in the group randomized to receive aztreonam-clindamycin, and two with bacteremia in the group randomized to receive tobramycin-clindamycin. The responses of all these patients were considered clinical and bacteriological cures (Table 4). This table depicts the clinical and bacteriological outcome by microorganism in each of the treatment groups. Among the patients with bacteriologically documented infections in the group randomized to receive aztreonam-clindamycin, 36 of 39 showed a clinical cure and bacteriological eradication. Two patients had Staphylococcus aureus recovered from sputum, in each case mixed with gram-negative bacilli. In the group randomized to receive tobramycin-clindamycin, 17 of 18 patients showed clinical cure and bacteriological eradication. The bacteriological failures in the aztreonam-clindamycin group included the responses of those patients with pneumonia secondary to Pseudomonas aeruginosa, Serratia rnarcescens, and Enterobacter aerogenes infections. One patient treated with aztreonam-clindamycin had an E. aerogenes strain with a
5 250 RODRIGUEZ, RAMiREZ-RONDA, AND NEVAREZ ANTIMICROB. AGENTS CHEMO'THER. TABLE 4. Clinical and bacteriological outcome by microorganism No. of cases treated by: MIC (pg/ml) of: Outcome (cure/total) with: Isolate Aztreonam Tobramycin Aztreonam Tobramycin Aztreonam Tobramycin Klebsiella pneumoniaea 13b 7b ' /13 6/7 Klebsiella oxytocac /1 Escherichia coli gb 4b /9 4/4 Pseudomonas aeruginosac 5b /5 4/4 Pseudomonas spp /2 1/1 Pseudomonas cepacea 1 32 >128 d Enterobacter aerogenese > /3 Enterobacter spp /1 2/2 Serratia spp /2 Proteus stuartii /1 Citrobacter diversus 1 0 ' /1 Proteus mirabilis /1 Staphylococcus aureusae 2 0 a Mixed infection-patient had S. aureus recovered with K. pneumoniae. bthere were four episodes of bacteremias in the aztreonam group: two K. pneumoniae, one E. coli, and one P. aeruginosa. There were two episodes of bacteremia in the tobramycin group: one E. coli and one K. pneumoniae. Bacteremia was with same pathogen obtained from sputum. c Mixed infection-patient had K. oxytoca and P. aeruginosa isolated from sputum. d The patient had a P. cepacea strain resistant to aztreonam recovered from sputum, although the patient was considered nonevaluable. emixed infection-patient had S. aureus recovered with E. aerogenes. MIC of >128 jg/ml for aztreonam and 4 [Lg/ml for tobramycin recovered from sputum. This patient recovered from his pneumonia. The two patients whose responses were considered failures are also analyzed in Table 2. In the tobramycinclindamycin group, the bacteriological failure occurred in a patient with pneumonia secondary to infection with Klebsiella pneumfoniae susceptible to both studied drugs. Bacteriological superinfection was seen in two patients who received aztreonam-clindamycin. The first patient (case 57) was a 69-year-old male with pneumonia caused by a strain of P. aeruginosa susceptible to both aztreonam and tobramycin. He was randomized to receive aztreonam and while on therapy developed a superinfection with a multiresistant strain of P. aeruginosa (the MICs of aztreonam and tobramycin were greater than 128,ug/ml) (Table 4). The second patient (case 59) was a 65-year-old male with pneumonia caused by Serratia marcescens and randomized to receive aztreonam. While on antibiotic therapy with aztreonam and clindamycin, he developed a bacteremic superinfection with Staphylococcus epidermidis and Candida albicans. There were few drug-related side effects. Elevation of serum glutamic oxalacetic transanimase occurred in 4 of 49 patients receiving aztreonam-clindamycin and in 3 of 26 patients receiving tobramycin-clindamycin. Thrombocytosis (>400,000 platelets per mm3) was present in nine and five patients of the aztreonam-clindamycin and tobramycinclindamycin groups, respectively. Eosinophilia (>1,500/ mm3) occurred in two patients in the aztreonamclindamycin group and in one patient in the tobramycin group. A skin rash was present in only one patient who was receiving tobramycin-clindamycin. All these parameters returned to pretherapy values after discontinuation of drug therapy. Abnormal renal function tests, defined as an increase of at least 0.5 mg of serum creatinine per dl, with no evidence of any other underlying disorder to explain it, was found in 2 of 26 patients (7.7%) on tobramycin-clindamycin therapy. DISCUSSION Aztreonam has specific activity against aerobic gram-negative bacilli (3, 16). It has been demonstrated that aztreonam penetrates well into meninges, lungs, heart, kidneys, spleen, bile, and genital tract (R. B. Sykes, D. P. Bonner, and C. M. Cimarusti, Proc. Int. Congr. Chemother. 13th, Vienna, Austria, p. 15/2-15/10, 1983) and that its antimicrobial activity is not affected by changes in ph (2). These characteristics and the few side effects seen (B. Swelly and H. C. Neu, Proc. Int. Congr. Chemother. 13th, Vienna, Austria, p. 15/37-15/42, 1983) make this compound an interesting one to be considered in the treatment of lower respiratory tract infections caused by gram-negative bacilli. This study points toward this potential use. Mortality associated with lower respiratory tract infections caused by gram-negative bacilli, as reported by other authors (8, 13, 19), is similar to that reported in the present study, and there are reports published that if fluctuates from 15% (8) to as high as 50% (25). The mortality rates found in both studied groups were comparable. The questions raised in many studies of lower respiratory tract infections are whether the diagnosis or pneumonia is based on sound clinical and laboratory grounds and whether the bacteriology is a true reflection of the etiological agent or just a mere colonizer. Although we do not claim to have a foolproof system, an effort was made to include only patients with adequate and true sputum as previously defined. There are reports that if the definition we used is adhered to, there is a 94% correlation with transtracheal aspiration (7, 18, 27). In our study, a transtracheal aspirate or double-lumen aspirate in intubated patients was performed in 25 of 79 patients, and bacteriological confirmation was seen in 23 of 25 of them (92%). An adequate sputum sample was used in the rest of the patients, 54 of 79, and bacteriological confirmation of Gram stain was found in 35 of 54 of them (64.8%). In the six patients with bacteremia, the organisms recovered from blood were the same as those in the sputum culture. This gives support to the bacteriological findings of the present study. The bacteriology found is a reflection of the type of patients selected and the criteria required for enrollment: a respiratory tract secretion (sputum, transtracheal, etc.) or Gram stain with predominance of gram-negative bacilli. The most frequent pathogens were K. pneumonia, Escherichia coli, and Pseudomonas spp., a reflection of the organisms which are frequently found colonizing the pharynx of some of these patients. Based on this, we want to emphasize the need of not only looking at sputum cultures
6 VOL. 27, 1985 AZTREONAM-CLINDAMYCIN FOR TREATMENT OF PNEUMONIA 251 but also at the sputum cellularity to help separate colonization from infection. A finding which merits consideration and that deserves attention in-future clinical studies, when aztreonam becomes commercially available, is the phenomenon of superinfection. Two patients were superinfected while receiving aztreonam-clindamycin therapy. In one patient the organism which caused the superinfection was a multiresistant strain of P. aeruginosa which developed while the patient was receiving therapy. The other patient developed a superinfection with Staphylococcus epidermidis and C. albicans. Although the serious conditions and underlying diseases of the patients and the broad-spectrum agents utilized may be a reasonable explanation for the superinfection, this must be observed closely in the future (29). The adverse effects were few and comparable in both studied groups, except for nephrotoxicity. No nephrotoxicity was attributed to therapy with aztreonam-clindamycin, whereas 2 of 26 (7.7%) patients receiving tobramycin-clindamycin had significant deterioration in renal-function parameters. These results are consistent with what has been previously reported (24). The therapeutic success observed for aztreonam-clindamycin and the lack of significant adverse effects associated with their use makes aztreonam-clindamycin an antimicrobial combination to be seriously considered in the treatment of serious nosocomial pulmonary infections associated with aspiration and subsequently documented to be caused by susceptible aerobic gram-negative bacilli. This is especially true in patients whose underlying diseases preclude the use of aminoglycosides or in whom the physician desires to use a monobactamic agent. ACKNOWLEDGMENTS This work was supported in part by the Squibb Institute for Medical Research, Princeton, N.J. We acknowledge the secretarial assistance of Carmen D. Camareno. LITERATURE CITED 1. Bartlett, J. G., and N. S. Brewer, and K. J. Ryan Cumitech 7, Laboratory diagnosis of lower respiratory tract infections. Coordinating ed., J. A. Washington II. American Society for Microbiology, Washington, D.C. 2. Breuer, H., C. M. Cimarusti, T. H. Denzel, W. H. Koster, W. A. Slusarchyk, and U. D. Treuner Monobactams-structureactivity relationships leading to SQ 26,776. J. Antimicrob. Chemother. 89(Suppl. E): Fainstein, V., S. Weaver, and G. P. Bodey Comparative in vitro study of SQ26,776. Antimicrob. Agents Chemother. 21: Fuxench-Chiesa, Z. Z., and C. H. Ramirez-Ronda Moxalactam therapy in the treatment of soft tissue and lower respiratory tract infections. Curr. Ther. Res. Clin. Exp. 33: Fuxench-L6pez, Z. Z., and C. H. Ramirez-Ronda Pharyngeal flora in ambulatory alcoholic patients: prevalence of gramnegative bacilli. Arch. Intern. Med. 138: Gardner, W. G Multicentered clinical evaluation of cefoperazone for the treatment of lower respiratory tract infections. Rev. Infect. Dis. 5(Suppl. 1): Geckler, R. W., D. Gremillion, K. McAllister, and C. Ellenbogen Microscopic and bacteriological comparison of paired sputa and transtracheal aspirates. J. Clin. Microbiol. 6: Gross, P. A., H. C. Neu, P. Aswapokee, C. van Antwerpen, and N. Aswapokee Deaths from nosocomial infections: experience in a university hospital and a community hospital. Am. J. Med. 68: Harrington, P. T., C. H. Ramirez-Ronda, and M. Nevarez Comparative clinical trial of piperacillin and carbenicillin. Curr. Ther. Res. Clin. Exp. 34: Hawley, H. B., R. M. Lewis, D. R. Swartz, and D. W. Gump Tobramycin therapy of pulmonary infections in patients with cystic fibrosis. Curr. Ther. Res. Clin. Exp. 16: Higuchi, J. H., T. R. Chaudhuri, D. E. Woods, and W. G. Johanson Bacterial adherence to epithelial cells in bacillary colonization of respiratory tract. Am. Rev. Respir. Dis. 121: Johanson, W. G., A. K. Pierce, and J. P. Sanford Changing pharyngeal bacterial flora of hospitalized patients. Emergence of gram-negative bacilli. N. Engl. J. Med. 281: La Force, F. M Hospital-acquired gram-negative rods pneumonias: an overview. Am. J. Med. 70: La Force, F. M., J. Hopkins, R. Trow, and L. Wang Human oral defenses against gram-negative rods. Am. Rev. Respir. Dis. 114: Lennette, E. H., A. Balows, W. J. Hausler, Jr., and J. P. Truant (ed.) Manual of clinical microbiology, 3rd ed., p American Society for Microbiology, Washington, D.C. 16. Livermore, D. M., and J. D. William In vitro activity of the monobactams SQ,776 against gram-negative bacteria and its stability to their P-lactamase. J. Antimicrob. Chemother. 8(Suppl. E): Mackowiak, P. A., R. M. Martin, and J. W. Smith The role of bacterial interference in the increase prevalence of oropharyngeal gram-negative bacilli among alcoholics and diabetics. Am. Rev. Respir. Dis. 120: Murray, P. R., and J. A. Washington III Microscopic and bacteriologic analysis of expectorated sputum. Mayo Clin. Proc. 50: Pierce, A. K., and J. P. Sanford Aerobic gram-negative bacillary pneumoniae. Am. Rev. Respir. Dis. 110: Quinlones-Soto, R. A., Z. Z. Fuxench-Chiesa, and C. H. Ramirez- Ronda Cefotaxime in the treatment of serious infections. Curr. Ther. Res. Clin. Exp. 33: Ramirez-Ronda, C. H., Z. Z. Fuxench-L6pez, and M. Nevarez Increased pharyngeal bacterial colonization during viral illness. Arch. Intern. Med. 141: Ramirez-Ronda, C. H., J. Gutierrez, and R. H. Bermgndez Comparative effectiveness, safety and tolerance of mezlocillin and ticarcillin: a prospective randomized trial. J. Antimicrob. Chemother. 9(Suppl. A): Rodriguez, J., G. J. Vazquez, R. H. Bermudez, A. Luinia, and C. H. Ramirez-Ronda A randomized clinical trial of ceftizoxime and cefamandole in the treatment of serious lower respiratory tract infections. J. Antimicrob. Chemother. IO(Suppl. C): Schentag, J. J., M. E. Plaut, and F. B. Cerra Comparative nephrotoxicity of gentamicin and tobramycin: pharmacokinetics and clinical studies in 201 patients. Antimicrob. Agents Chemother. 19: Stevens, R. M., D. Teres, J. J. Skillman, and D. S. Feingold Pneumonia in an intensive care unit. Arch. Intern. Med. 134: Thadepalli, H., and B. Rao Clinical evaluation of mezlocillin. Antimicrob. Agents Chemother. 16: Thorsteinsson, S. B., D. M. Musher, and T. Fagan The diagnostic value of sputum culture in acute pneumoniae. J. Am. Med. Assoc. 233: Valente, W. M., R. G. Trudele, and D. W. Bentley Factors predisposing to oropharyngeal colonization ofgram-negative bacilli in the aged. N. Engl. J. Med. 298: Van Sevy, R. E Bacterial sputum cultures, a clinician viewpoint. Mayo Clin. Proc. 52:39-41.
Ceftizoxime in the treatment of infections in patients with cancer
 Journal of Antimicrobial Chemotherapy (98), Suppl. C, 67-73 Ceftizoxime in the treatment of infections in patients with cancer V. Fainstein, R. Bolivar,. Elting, M. Valdivieso and G. P. Bodey Department
Journal of Antimicrobial Chemotherapy (98), Suppl. C, 67-73 Ceftizoxime in the treatment of infections in patients with cancer V. Fainstein, R. Bolivar,. Elting, M. Valdivieso and G. P. Bodey Department
Against Aerobic Gram-Negative Bacilli
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Dec. 1979, p. 6-6 0066-0/79/1-06/05$0.00/0 Vol., No. 6 In Vitro Activity of LY17935, a New 1-Oxa Cephalosporin, Against Aerobic Gram-Negative Bacilli DENNIS G. DELGADO,
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Dec. 1979, p. 6-6 0066-0/79/1-06/05$0.00/0 Vol., No. 6 In Vitro Activity of LY17935, a New 1-Oxa Cephalosporin, Against Aerobic Gram-Negative Bacilli DENNIS G. DELGADO,
Clinical Comparison of Cefotaxime with Gentamicin plus Clindamycin in the Treatment of Peritonitis and Other Soft-Tissue Infections
 REVIEWS OF INFECTIOUS DISEASES. VOL. 4, SUPPLEMENT. SEPTEMBER-OCTOBER 982 982 by The University of Chicago. All rights reserved. 062-0886/82/0405-022$02.00 Clinical Comparison of with Gentamicin plus Clindamycin
REVIEWS OF INFECTIOUS DISEASES. VOL. 4, SUPPLEMENT. SEPTEMBER-OCTOBER 982 982 by The University of Chicago. All rights reserved. 062-0886/82/0405-022$02.00 Clinical Comparison of with Gentamicin plus Clindamycin
Treatment of serious Pseudomonas infections with azlocillin
 Journal of Antimicrobial Chemotherapy (983), Suppl. B, 53-58 Treatment of serious Pseudomonas infections with azlocillin S. Olive, W. J. Mogabgab, B. Holmes, B. Pollock, B. Pauling and R. Beville Tulane
Journal of Antimicrobial Chemotherapy (983), Suppl. B, 53-58 Treatment of serious Pseudomonas infections with azlocillin S. Olive, W. J. Mogabgab, B. Holmes, B. Pollock, B. Pauling and R. Beville Tulane
Evaluation of Aztreonam in the Treatment of Severe Bacterial Infections
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 1985, p. 222-226 0066-4804/85/020222-05$02.00/0 Copyright 1985, American Society for Microbiology Vol. 28, No. 2 Evaluation of Aztreonam in the Treatment of
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 1985, p. 222-226 0066-4804/85/020222-05$02.00/0 Copyright 1985, American Society for Microbiology Vol. 28, No. 2 Evaluation of Aztreonam in the Treatment of
Sputum Screening by Nomarski Interference Contrast
 JOURNAL OF CLINICAL MICROBIOLOGY, Apr. 1979, p. 520-524 0095-1137/79/04-0520/05$02.00/0 Vol. 9, No. 4 Sputum Screening by Nomarski Interference Contrast Microscopy DAVID F. WELCH AND MICHAEL T. KELLYt*
JOURNAL OF CLINICAL MICROBIOLOGY, Apr. 1979, p. 520-524 0095-1137/79/04-0520/05$02.00/0 Vol. 9, No. 4 Sputum Screening by Nomarski Interference Contrast Microscopy DAVID F. WELCH AND MICHAEL T. KELLYt*
AXITAB-CV TAB. COMPOSITION :
 AXITAB-CV TAB. COMPOSITION : Each film coated tablet contains: Cefuroxime Axetil I.P. Eq. to Anhydrous 500mg. Potassium Clavulanate Diluted I.P. Eq. to Clavulanic Acid 125mg DESCRIPTION : Cefuroxime Axetil
AXITAB-CV TAB. COMPOSITION : Each film coated tablet contains: Cefuroxime Axetil I.P. Eq. to Anhydrous 500mg. Potassium Clavulanate Diluted I.P. Eq. to Clavulanic Acid 125mg DESCRIPTION : Cefuroxime Axetil
Efficacy of Ceftriaxone in Serious Bacterial Infections
 ANTIMIROBIAL AGENTS AND HEMOTHERAPY, Mar 1982, p 402-406 0066-4804/82/030402-05$0200/0 Vol 21, No 3 Efficacy of eftriaxone in Serious Bacterial Infections JAY S EPSTEIN, SUSAN M HASSELQUIST, AND GARY L
ANTIMIROBIAL AGENTS AND HEMOTHERAPY, Mar 1982, p 402-406 0066-4804/82/030402-05$0200/0 Vol 21, No 3 Efficacy of eftriaxone in Serious Bacterial Infections JAY S EPSTEIN, SUSAN M HASSELQUIST, AND GARY L
Online Supplement for:
 Online Supplement for: INFLUENCE OF COMBINED INTRAVENOUS AND TOPICAL ANTIBIOTIC PROPHYLAXIS ON THE INCIDENCE OF INFECTIONS, ORGAN DYSFUNCTIONS, AND MORTALITY IN CRITICALLY ILL SURGICAL PATIENTS A PROSPECTIVE,
Online Supplement for: INFLUENCE OF COMBINED INTRAVENOUS AND TOPICAL ANTIBIOTIC PROPHYLAXIS ON THE INCIDENCE OF INFECTIONS, ORGAN DYSFUNCTIONS, AND MORTALITY IN CRITICALLY ILL SURGICAL PATIENTS A PROSPECTIVE,
Comparative Activity of Cefotaxime and Selected f3-lactam Antibiotics Against Haemophilus Influenzae and Aerobic Gram-Negative Bacilli
 REVIEWS OF INFECTIOUS DISEASES VOL. 4, SUPPLEMENT SEPTEMBER-OCTOBER 1982 1982 by The University of Chicago. All rights reserved. 0162-0886/82/0405-0015$02.00 Comparative Activity of Cefotaxime and Selected
REVIEWS OF INFECTIOUS DISEASES VOL. 4, SUPPLEMENT SEPTEMBER-OCTOBER 1982 1982 by The University of Chicago. All rights reserved. 0162-0886/82/0405-0015$02.00 Comparative Activity of Cefotaxime and Selected
Acceptability of Sputum Specimens
 JOURNAL OF CLINICAL MICROBIOLOGY, Oct. 1982, p. 627-631 0095-1137/82/100627-05$02.00/0 Copyright C 1982, American Society for Microbiology Vol. 16, No. 4 Comparison of Six Different Criteria for Judging
JOURNAL OF CLINICAL MICROBIOLOGY, Oct. 1982, p. 627-631 0095-1137/82/100627-05$02.00/0 Copyright C 1982, American Society for Microbiology Vol. 16, No. 4 Comparison of Six Different Criteria for Judging
JAC Efficacy and tolerance of roxithromycin versus clarithromycin in the treatment of lower respiratory tract infections
 Journal of Antimicrobial Chemotherapy (1998) 41, Suppl. B, 69 73 JAC Efficacy and tolerance of roxithromycin versus clarithromycin in the treatment of lower respiratory tract infections G. Tatsis*, G.
Journal of Antimicrobial Chemotherapy (1998) 41, Suppl. B, 69 73 JAC Efficacy and tolerance of roxithromycin versus clarithromycin in the treatment of lower respiratory tract infections G. Tatsis*, G.
Randomized Prospective Study Comparing Moxalactam and Cefoxitin with or without Tobramycin for the Treatment of Serious Surgical Infections
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Feb. 1986, p. 244-249 0066-4804/86/020244-06$02.00/0 Copyright C 1986, American Society for Microbiology Vol. 29, No. 2 Randomized Prospective Study Comparing Moxalactam
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Feb. 1986, p. 244-249 0066-4804/86/020244-06$02.00/0 Copyright C 1986, American Society for Microbiology Vol. 29, No. 2 Randomized Prospective Study Comparing Moxalactam
ZINEX. Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg
 ZINEX Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg Tablets Action Cefuroxime axetil owes its bactericidal activity to the parent compound cefuroxime. Cefuroxime is a well-characterized
ZINEX Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg Tablets Action Cefuroxime axetil owes its bactericidal activity to the parent compound cefuroxime. Cefuroxime is a well-characterized
Affinity of Doripenem and Comparators to Penicillin-Binding Proteins in Escherichia coli and ACCEPTED
 AAC Accepts, published online ahead of print on February 00 Antimicrob. Agents Chemother. doi:./aac.01-0 Copyright 00, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
AAC Accepts, published online ahead of print on February 00 Antimicrob. Agents Chemother. doi:./aac.01-0 Copyright 00, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
Cefotaxime Rationale for the EUCAST clinical breakpoints, version th September 2010
 Cefotaxime Rationale for the EUCAST clinical breakpoints, version 1.0 26 th September 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the European
Cefotaxime Rationale for the EUCAST clinical breakpoints, version 1.0 26 th September 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the European
Ceftomax TM S (Cefoperazone Sodium plus Sulbactam Sodium Injection)
 COMPOSITION Ceftomax TM S (Cefoperazone Sodium plus Sulbactam Sodium Injection) CEFTOMAX - S Injection 1.5 gm Each vial contains: Cefoperazone Sodium equivalent to Cefoperazone IP. 1,000 mg Sulbactam Sodium
COMPOSITION Ceftomax TM S (Cefoperazone Sodium plus Sulbactam Sodium Injection) CEFTOMAX - S Injection 1.5 gm Each vial contains: Cefoperazone Sodium equivalent to Cefoperazone IP. 1,000 mg Sulbactam Sodium
TRANSPARENCY COMMITTEE OPINION. 15 October Date of Marketing Authorisation (national procedure): 16 April 1997, variation of 18 February 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 October 2008 MERONEM 1 g, powder for solution for IV Injection Box of 10 vials (CIP: 387 830-6) Applicant: ASTRAZENECA
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 October 2008 MERONEM 1 g, powder for solution for IV Injection Box of 10 vials (CIP: 387 830-6) Applicant: ASTRAZENECA
Susceptibility of Cephalothin-Resistant Gram-Negative Bacilli
 ANTIMICROBIAL AGENTS AND CHEmOTHERAPY, Mar. 1978, p. 484489 0066-4804/8/0013-0484$02.00/0 Copyright 1978 American Society for Microbiology Vol. 13, No. 3 Printed in U.S.A. Susceptibility of Cephalothin-Resistant
ANTIMICROBIAL AGENTS AND CHEmOTHERAPY, Mar. 1978, p. 484489 0066-4804/8/0013-0484$02.00/0 Copyright 1978 American Society for Microbiology Vol. 13, No. 3 Printed in U.S.A. Susceptibility of Cephalothin-Resistant
Clinical experience with ceftazidime in urology in Japan
 Journal of Antimicrobial Chemotherapy (98), Suppl. A, 6- Clinical experience with ceftazidime in urology in Japan Noboo Kawamura Department of Urology, Tokai University, School of Medicine, Bosei-dai,
Journal of Antimicrobial Chemotherapy (98), Suppl. A, 6- Clinical experience with ceftazidime in urology in Japan Noboo Kawamura Department of Urology, Tokai University, School of Medicine, Bosei-dai,
Hospital-acquired Pneumonia
 Hospital-acquired Pneumonia Hospital-acquired pneumonia (HAP) Pneumonia that occurs at least 2 days after hospital admission. The second most common and the leading cause of death due to hospital-acquired
Hospital-acquired Pneumonia Hospital-acquired pneumonia (HAP) Pneumonia that occurs at least 2 days after hospital admission. The second most common and the leading cause of death due to hospital-acquired
Aciphin Ceftriaxone Sodium
 Aciphin Ceftriaxone Sodium Only for the use of Medical Professionals Description Aciphin is a bactericidal, long-acting, broad spectrum, parenteral cephalosporin preparation, active against a wide range
Aciphin Ceftriaxone Sodium Only for the use of Medical Professionals Description Aciphin is a bactericidal, long-acting, broad spectrum, parenteral cephalosporin preparation, active against a wide range
PULMONARY EMERGENCIES
 EMERGENCIES I. Pneumonia A. Bacterial Pneumonia (most common cause of a focal infiltrate) 1. Epidemiology a. Accounts for up to 10% of hospital admissions in the U.S. b. Most pneumonias are the result
EMERGENCIES I. Pneumonia A. Bacterial Pneumonia (most common cause of a focal infiltrate) 1. Epidemiology a. Accounts for up to 10% of hospital admissions in the U.S. b. Most pneumonias are the result
Pharmacologyonline 1: (2010) ewsletter Singh and Kochbar. Optimizing Pharmacokinetic/Pharmacodynamics Principles & Role of
 Optimizing Pharmacokinetic/Pharmacodynamics Principles & Role of Cefoperazone Sulbactam Singh M*, Kochhar P* Medical & Research Division, Pfizer India. Summary Antimicrobial resistance is associated with
Optimizing Pharmacokinetic/Pharmacodynamics Principles & Role of Cefoperazone Sulbactam Singh M*, Kochhar P* Medical & Research Division, Pfizer India. Summary Antimicrobial resistance is associated with
Mesporin TM. Ceftriaxone sodium. Rapid onset, sustained action, for a broad spectrum of infections
 Ceftriaxone sodium Rapid onset, sustained action, for a broad spectrum of infections 1, 2, 3 Antibiotic with a broad spectrum of activity Broad spectrum of activity against gram-positive* and gram-negative
Ceftriaxone sodium Rapid onset, sustained action, for a broad spectrum of infections 1, 2, 3 Antibiotic with a broad spectrum of activity Broad spectrum of activity against gram-positive* and gram-negative
Treatment of febrile neutropenia in patients with neoplasia
 Treatment of febrile neutropenia in patients with neoplasia George Samonis MD, PhD Medical Oncologist Infectious Diseases Specialist Professor of Medicine The University of Crete, Heraklion,, Crete, Greece
Treatment of febrile neutropenia in patients with neoplasia George Samonis MD, PhD Medical Oncologist Infectious Diseases Specialist Professor of Medicine The University of Crete, Heraklion,, Crete, Greece
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for
Cefepime versus Ceftriaxone for Empiric Treatment of Hospitalized Patients with Community-Acquired Pneumonia
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Apr. 1998, p. 729 733 Vol. 42, No. 4 0066-4804/98/$04.00 0 Copyright 1998, American Society for Microbiology Cefepime versus Ceftriaxone for Empiric Treatment of
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Apr. 1998, p. 729 733 Vol. 42, No. 4 0066-4804/98/$04.00 0 Copyright 1998, American Society for Microbiology Cefepime versus Ceftriaxone for Empiric Treatment of
Correlation of Sputum Gram Stain and Sputum Culture for Respiratory Tract Infections in a Tertiary Care Hospital, Ballari, India
 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 6 Number 6 (2017) pp. 3008-3012 Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2017.606.357
International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 6 Number 6 (2017) pp. 3008-3012 Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2017.606.357
Mezlocillin for Treatment of Infections in Cancer Patients
 ANTIMICROBIAL AGENTs A CHEMOTHERAPY, June 98, p. 8- -8/8/-8/$./ Vol. 7,. Mezlocillin for Treatment of Infections in Cancer Patients BRIAN F. ISSELLt A GERALD P. BODEY* Department ofdevelopmental Therapeutics,
ANTIMICROBIAL AGENTs A CHEMOTHERAPY, June 98, p. 8- -8/8/-8/$./ Vol. 7,. Mezlocillin for Treatment of Infections in Cancer Patients BRIAN F. ISSELLt A GERALD P. BODEY* Department ofdevelopmental Therapeutics,
Aminoglycosides John A. Bosso, Pharm.D.
 AMINOGLYCOSIDES Therapeutics/PHRMP-73 Aminoglycoside Mechanism of Action Aminoglycosides bind to 30s ribosomal subunit resulting in mistranslation of mrna thus disrupting protein synthesis. They are rapidly
AMINOGLYCOSIDES Therapeutics/PHRMP-73 Aminoglycoside Mechanism of Action Aminoglycosides bind to 30s ribosomal subunit resulting in mistranslation of mrna thus disrupting protein synthesis. They are rapidly
Work up of Respiratory & Wound Cultures:
 Work up of Respiratory & Wound Cultures: Culture work up 2 Systematic approaches 1 Work up of Respiratory & Wound Cultures Resident flora Colonizing organisms Pathogens 2 Work up of Respiratory & Wound
Work up of Respiratory & Wound Cultures: Culture work up 2 Systematic approaches 1 Work up of Respiratory & Wound Cultures Resident flora Colonizing organisms Pathogens 2 Work up of Respiratory & Wound
UTI IN ELDERLY. Zeinab Naderpour
 UTI IN ELDERLY Zeinab Naderpour Urinary tract infection (UTI) is the most frequent bacterial infection in elderly populations. While urinary infection in the elderly person is usually asymptomatic, symptomatic
UTI IN ELDERLY Zeinab Naderpour Urinary tract infection (UTI) is the most frequent bacterial infection in elderly populations. While urinary infection in the elderly person is usually asymptomatic, symptomatic
Respiratory Tract Infections
 JOURNAL OF CLINICAL MICROBIOLOGY, May 1987, p. 758-762 0095-1137/87/050758-05$02.00/0 Copyright 1987, American Society for Microbiology Vol. 25, No. 5 Nonvalue of Sputum Culture in the Management of Lower
JOURNAL OF CLINICAL MICROBIOLOGY, May 1987, p. 758-762 0095-1137/87/050758-05$02.00/0 Copyright 1987, American Society for Microbiology Vol. 25, No. 5 Nonvalue of Sputum Culture in the Management of Lower
Development of C sporins. Beta-lactam antibiotics - Cephalosporins. Second generation C sporins. Targets - PBP s
 Beta-lactam antibiotics - Cephalosporins Development of C sporins Targets - PBP s Activity - Cidal - growing organisms (like the penicillins) Principles of action - Affinity for PBP s Permeability properties
Beta-lactam antibiotics - Cephalosporins Development of C sporins Targets - PBP s Activity - Cidal - growing organisms (like the penicillins) Principles of action - Affinity for PBP s Permeability properties
Work-up of Respiratory Specimens Now you can breathe easier
 34 th Annual Meeting Southwestern Association of Clinical Microbiology Work-up of Respiratory Specimens Now you can breathe easier Yvette S. McCarter, PhD, D(ABMM) Director, Clinical Microbiology Laboratory
34 th Annual Meeting Southwestern Association of Clinical Microbiology Work-up of Respiratory Specimens Now you can breathe easier Yvette S. McCarter, PhD, D(ABMM) Director, Clinical Microbiology Laboratory
University of Alberta Hospital Antibiogram for 2007 and 2008 Division of Medical Microbiology Department of Laboratory Medicine and Pathology
 University of Alberta Hospital Antibiogram for 2007 and 2008 Division of Medical Microbiology Department of Laboratory Medicine and Pathology This material is supported in part by unrestricted educational
University of Alberta Hospital Antibiogram for 2007 and 2008 Division of Medical Microbiology Department of Laboratory Medicine and Pathology This material is supported in part by unrestricted educational
MINISTRY OF HEALTH OF THE REPUBLIC OF BELARUS. Patient Information Leaflet
 No. 851 of August 19, 2014 КЛС No. 10 of July 31, 2014 Invented trade name: Cefosulbactam MINISTRY OF HEALTH OF THE REPUBLIC OF BELARUS Patient Information Leaflet CEFOSULBACTAM (ЦЕФОСУЛЬБАКТАМ) Powder
No. 851 of August 19, 2014 КЛС No. 10 of July 31, 2014 Invented trade name: Cefosulbactam MINISTRY OF HEALTH OF THE REPUBLIC OF BELARUS Patient Information Leaflet CEFOSULBACTAM (ЦЕФОСУЛЬБАКТАМ) Powder
Severe β-lactam allergy. Alternative (use for mild-moderate β-lactam allergy) therapy
 Recommended Empirical Antibiotic Regimens for MICU Patients Notes: The antibiotic regimens shown are general guidelines and should not replace clinical judgment. Always assess for antibiotic allergies.
Recommended Empirical Antibiotic Regimens for MICU Patients Notes: The antibiotic regimens shown are general guidelines and should not replace clinical judgment. Always assess for antibiotic allergies.
Cerebrospinal Fluid Penetration of Imipenem and Cilastatin (Primaxin) in Children with Central Nervous System Infections
 ANTIMIWROBIAL AGENTS AND CHEMOTHERAPY, Apr. 1986, p. 670-674 0066-4804/86/040670-05$02.00/0 Copyright X 1986, American Society for Microbiology Vol. 29, No. 4 Cerebrospinal Fluid Penetration of Imipenem
ANTIMIWROBIAL AGENTS AND CHEMOTHERAPY, Apr. 1986, p. 670-674 0066-4804/86/040670-05$02.00/0 Copyright X 1986, American Society for Microbiology Vol. 29, No. 4 Cerebrospinal Fluid Penetration of Imipenem
Outpatient treatment in women with acute pyelonephritis after visiting emergency department
 LETTER TO THE EDITOR Korean J Intern Med 2017;32:369-373 Outpatient treatment in women with acute pyelonephritis after visiting emergency department Hee Kyoung Choi 1,*, Jin-Won Chung 2, Won Sup Oh 3,
LETTER TO THE EDITOR Korean J Intern Med 2017;32:369-373 Outpatient treatment in women with acute pyelonephritis after visiting emergency department Hee Kyoung Choi 1,*, Jin-Won Chung 2, Won Sup Oh 3,
Hospital Acquired Pneumonias
 Hospital Acquired Pneumonias Hospital Acquired Pneumonia ( HAP ) Hospital acquired pneumonia ( HAP ) is defined as an infection of the lung parenchyma developing during hospitalization and not present
Hospital Acquired Pneumonias Hospital Acquired Pneumonia ( HAP ) Hospital acquired pneumonia ( HAP ) is defined as an infection of the lung parenchyma developing during hospitalization and not present
Healthcare-associated infections acquired in intensive care units
 SURVEILLANCE REPORT Annual Epidemiological Report for 2015 Healthcare-associated infections acquired in intensive care units Key facts In 2015, 11 788 (8.3%) of patients staying in an intensive care unit
SURVEILLANCE REPORT Annual Epidemiological Report for 2015 Healthcare-associated infections acquired in intensive care units Key facts In 2015, 11 788 (8.3%) of patients staying in an intensive care unit
Cephalosporin, Against Cephalosporin-Resistant Bacteria, and
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Nov. 1979, P. 59-553 Vol. 16, No. 5 66-/79/11-59/$2./ Antibacterial Activity of Ceftizoxime (FK 79), a New Cephalosporin, Against Cephalosporin-Resistant Bacteria,
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Nov. 1979, P. 59-553 Vol. 16, No. 5 66-/79/11-59/$2./ Antibacterial Activity of Ceftizoxime (FK 79), a New Cephalosporin, Against Cephalosporin-Resistant Bacteria,
ROSOBAC-1GM / ROSOBAC-FORT
 ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
JMSCR Vol 04 Issue 12 Page December 2016
 www.jmscr.igmpublication.org Impact Factor 5.244 Index Copernicus Value: 83.27 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v4i12.124 COPD: Microbial Pattern in Acute Exacerbation
www.jmscr.igmpublication.org Impact Factor 5.244 Index Copernicus Value: 83.27 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v4i12.124 COPD: Microbial Pattern in Acute Exacerbation
Ailyn T. Isais-Agdeppa, MD*, Lulu Bravo, MD*
 A FIVE-YEAR RETROSPECTIVE STUDY ON THE COMMON MICROBIAL ISOLATES AND SENSITIVITY PATTERN ON BLOOD CULTURE OF PEDIATRIC CANCER PATIENTS ADMITTED AT THE PHILIPPINE GENERAL HOSPITAL FOR FEBRILE NEUTROPENIA
A FIVE-YEAR RETROSPECTIVE STUDY ON THE COMMON MICROBIAL ISOLATES AND SENSITIVITY PATTERN ON BLOOD CULTURE OF PEDIATRIC CANCER PATIENTS ADMITTED AT THE PHILIPPINE GENERAL HOSPITAL FOR FEBRILE NEUTROPENIA
Pneumonia Community-Acquired Healthcare-Associated
 Pneumonia Community-Acquired Healthcare-Associated Edwin Yu Clin Infect Dis 2007;44(S2):27-72 Am J Respir Crit Care Med 2005; 171:388-416 IDSA / ATS Guidelines Microbiology Principles and Practice of Infectious
Pneumonia Community-Acquired Healthcare-Associated Edwin Yu Clin Infect Dis 2007;44(S2):27-72 Am J Respir Crit Care Med 2005; 171:388-416 IDSA / ATS Guidelines Microbiology Principles and Practice of Infectious
PULMONARY MEDICINE BOARD REVIEW. Financial Conflicts of Interest. Question #1: Question #1 (Cont.): None. Christopher H. Fanta, M.D.
 PULMONARY MEDICINE BOARD REVIEW Christopher H. Fanta, M.D. Pulmonary and Critical Care Division Brigham and Women s Hospital Partners Asthma Center Harvard Medical School Financial Conflicts of Interest
PULMONARY MEDICINE BOARD REVIEW Christopher H. Fanta, M.D. Pulmonary and Critical Care Division Brigham and Women s Hospital Partners Asthma Center Harvard Medical School Financial Conflicts of Interest
A Snapshot of Colistin Use in South-East Europe and Particularly in Greece
 A Snapshot of Colistin Use in South-East Europe and Particularly in Greece Helen Giamarellou 02.05.2013 When Greek Physicians Prescribe Colistin? It is mainly prescribed in the ICU for VAP, bacteremia
A Snapshot of Colistin Use in South-East Europe and Particularly in Greece Helen Giamarellou 02.05.2013 When Greek Physicians Prescribe Colistin? It is mainly prescribed in the ICU for VAP, bacteremia
without the permission of the author Not to be copied and distributed to others
 Emperor s Castle interior-prato What is the Role of Inhaled Polymyxins for Treatment of Respiratory Tract Infections? Helen Giamarellou CONCLUSIONS: Patients with Pseudomonas and Acinetobacter VAP may
Emperor s Castle interior-prato What is the Role of Inhaled Polymyxins for Treatment of Respiratory Tract Infections? Helen Giamarellou CONCLUSIONS: Patients with Pseudomonas and Acinetobacter VAP may
Cefuroxime iv Rationale for the EUCAST clinical breakpoints, version th September 2010
 Cefuroxime iv Rationale for the EUCAST clinical breakpoints, version 1.0 26 th September 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the
Cefuroxime iv Rationale for the EUCAST clinical breakpoints, version 1.0 26 th September 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the
MAGNEX Injection (Sulbactam Sodium/Cefoperazone Sodium 1:1)
 MAGNEX Injection (Sulbactam Sodium/Cefoperazone Sodium 1:1) 1. NAME OF MEDICINAL PRODUCT MAGNEX 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Sulbactam sodium/cefoperazone sodium combination is available
MAGNEX Injection (Sulbactam Sodium/Cefoperazone Sodium 1:1) 1. NAME OF MEDICINAL PRODUCT MAGNEX 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Sulbactam sodium/cefoperazone sodium combination is available
Dithiothreitol as a Mucolytic Agent
 JOURNAL OF CLINICAL MICROBIOLOGY, June 1980, p. 552-557 0095-1137/80/06-0552/06$02.00/0 Vol. 11, No. 6 Bacteriology of Sputum in Cystic Fibrosis: Evaluation of Dithiothreitol as a Mucolytic Agent MARGARET
JOURNAL OF CLINICAL MICROBIOLOGY, June 1980, p. 552-557 0095-1137/80/06-0552/06$02.00/0 Vol. 11, No. 6 Bacteriology of Sputum in Cystic Fibrosis: Evaluation of Dithiothreitol as a Mucolytic Agent MARGARET
Routine endotracheal cultures for the prediction of sepsis in ventilated babies
 Archives of Disease in Childhood, 1989, 64, 34-38 Routine endotracheal cultures for the prediction of sepsis in ventilated babies T A SLAGLE, E M BIFANO, J W WOLF, AND S J GROSS Department of Pediatrics,
Archives of Disease in Childhood, 1989, 64, 34-38 Routine endotracheal cultures for the prediction of sepsis in ventilated babies T A SLAGLE, E M BIFANO, J W WOLF, AND S J GROSS Department of Pediatrics,
Unit II Problem 2 Pathology: Pneumonia
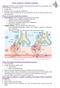 Unit II Problem 2 Pathology: Pneumonia - Definition: pneumonia is the infection of lung parenchyma which occurs especially when normal defenses are impaired such as: Cough reflex. Damage of cilia in respiratory
Unit II Problem 2 Pathology: Pneumonia - Definition: pneumonia is the infection of lung parenchyma which occurs especially when normal defenses are impaired such as: Cough reflex. Damage of cilia in respiratory
Blood culture 壢新醫院 病理檢驗科 陳啟清技術主任
 Blood culture 壢新醫院 病理檢驗科 陳啟清技術主任 A Positive Blood Culture Clinically Important Organism Failure of host defenses to contain an infection at its primary focus Failure of the physician to effectively eradicate,
Blood culture 壢新醫院 病理檢驗科 陳啟清技術主任 A Positive Blood Culture Clinically Important Organism Failure of host defenses to contain an infection at its primary focus Failure of the physician to effectively eradicate,
ISF criteria (International sepsis forum consensus conference of infection in the ICU) Secondary peritonitis
 Appendix with supplementary material. This appendix was part of the submitted manuscript and has been peer reviewed. It is posted as supplied by the authors. Supplementary Tables Table S1. Definitions
Appendix with supplementary material. This appendix was part of the submitted manuscript and has been peer reviewed. It is posted as supplied by the authors. Supplementary Tables Table S1. Definitions
Successful treatment of pan - resistant pseudomonas Aerugınosa menıngıtıs wıth ıntrathecal polymyxın b therapy
 ISPUB.COM The Internet Journal of Infectious Diseases Volume 7 Number 2 Successful treatment of pan - resistant pseudomonas Aerugınosa menıngıtıs wıth ıntrathecal polymyxın b F Simsek, T Yildirmak, G Cetmeli,
ISPUB.COM The Internet Journal of Infectious Diseases Volume 7 Number 2 Successful treatment of pan - resistant pseudomonas Aerugınosa menıngıtıs wıth ıntrathecal polymyxın b F Simsek, T Yildirmak, G Cetmeli,
Received 30 March 2005; returned 16 June 2005; revised 8 September 2005; accepted 12 September 2005
 Journal of Antimicrobial Chemotherapy (2005) 56, 1047 1052 doi:10.1093/jac/dki362 Advance Access publication 20 October 2005 Evaluation of PPI-0903M (T91825), a novel cephalosporin: bactericidal activity,
Journal of Antimicrobial Chemotherapy (2005) 56, 1047 1052 doi:10.1093/jac/dki362 Advance Access publication 20 October 2005 Evaluation of PPI-0903M (T91825), a novel cephalosporin: bactericidal activity,
Consultation on the Revision of Carbapenem Breakpoints
 Consultation on the Revision of Carbapenem Breakpoints July 2018 Please send comments to the EUCAST Scientific Secretary at jturnidge@gmail.com by September 15. EUCAST revision of carbapenem breakpoints
Consultation on the Revision of Carbapenem Breakpoints July 2018 Please send comments to the EUCAST Scientific Secretary at jturnidge@gmail.com by September 15. EUCAST revision of carbapenem breakpoints
Discrepancies in the recovery of bacteria from multiple sinuses in acute and chronic sinusitis
 Journal of Medical Microbiology (2004), 53, 879 885 DOI 10.1099/jmm.0.45655-0 Short Communication Correspondence Itzhak Brook ib6@georgetown.edu Received 1 March 2004 Accepted 18 May 2004 Discrepancies
Journal of Medical Microbiology (2004), 53, 879 885 DOI 10.1099/jmm.0.45655-0 Short Communication Correspondence Itzhak Brook ib6@georgetown.edu Received 1 March 2004 Accepted 18 May 2004 Discrepancies
Fig 1. p-lactamase activity of cell extract from the culture of E. cloacae H-27
 CPZ or CMZ was added to the cultures at the start of cultivation. Ĉ-lactamase activity was determined by spectrophotometry using CER(100pM) as a substrate. Fig 1. p-lactamase activity of cell extract
CPZ or CMZ was added to the cultures at the start of cultivation. Ĉ-lactamase activity was determined by spectrophotometry using CER(100pM) as a substrate. Fig 1. p-lactamase activity of cell extract
Management of Catheter Related Bloodstream Infection (CRBSI), including Antibiotic Lock Therapy.
 Management of Catheter Related Bloodstream Infection (CRBSI), including Antibiotic Lock Therapy. Written by: Dr K Gajee, Consultant Microbiologist Date: June 2017 Approved by: Drugs & Therapeutics Committee
Management of Catheter Related Bloodstream Infection (CRBSI), including Antibiotic Lock Therapy. Written by: Dr K Gajee, Consultant Microbiologist Date: June 2017 Approved by: Drugs & Therapeutics Committee
Clinical Evaluation of Moxalactam
 ANTIUCROBIAL AGENTS AND CHEMOTHERAPY, JUly 1981, p. 88-97 0066-4804/81/070088-10$02.00/0 Vol. 20, No. 1 Clinical Evaluation of Moxalactam W. K. LIVINGSTON, A. M. ELLIOTT, W. E. DISMUKES, C. K. AVENT, AND
ANTIUCROBIAL AGENTS AND CHEMOTHERAPY, JUly 1981, p. 88-97 0066-4804/81/070088-10$02.00/0 Vol. 20, No. 1 Clinical Evaluation of Moxalactam W. K. LIVINGSTON, A. M. ELLIOTT, W. E. DISMUKES, C. K. AVENT, AND
Twice daily ceftriaxone therapy for serious bacterial infections in children
 Journal of Antimicrobial Chemotherapy (1984) 13, 511-516 Twice daily ceftriaxone therapy for serious bacterial infections in children Tasnee Chonmaitree*, Blaise L. Congenif, Jose Munoz+, Tamara A. Rakusan*,
Journal of Antimicrobial Chemotherapy (1984) 13, 511-516 Twice daily ceftriaxone therapy for serious bacterial infections in children Tasnee Chonmaitree*, Blaise L. Congenif, Jose Munoz+, Tamara A. Rakusan*,
ESCMID Online Lecture Library. by author
 Hospital Universitario Virgen Macarena, Seville New drugs against MRSA and VRE L. Eduardo López Cortés Seville, 8th July Tedizolid Oxazolidinone Ceftaroline // Ceftobiprole 5 th gen cephalosporin Overview
Hospital Universitario Virgen Macarena, Seville New drugs against MRSA and VRE L. Eduardo López Cortés Seville, 8th July Tedizolid Oxazolidinone Ceftaroline // Ceftobiprole 5 th gen cephalosporin Overview
PHARMACOKINETICS OF COLISTIN IN
 PHARMACOKINETICS OF COLISTIN IN CRITICALLY ILL PATIENTS WITH MULTIDRUG-RESISTANT GRAM- NEGATIVE BACILLI INFECTION JOURNAL CLUB PRESENTATION Amal M. Al-Anizi, PharmD Candidate KSU, Infectious disease rotation
PHARMACOKINETICS OF COLISTIN IN CRITICALLY ILL PATIENTS WITH MULTIDRUG-RESISTANT GRAM- NEGATIVE BACILLI INFECTION JOURNAL CLUB PRESENTATION Amal M. Al-Anizi, PharmD Candidate KSU, Infectious disease rotation
Overcoming the PosESBLities of Enterobacteriaceae Resistance
 Overcoming the PosESBLities of Enterobacteriaceae Resistance Review of current treatment options Jamie Reed, PharmD Pharmacy Grand Rounds August 28, 2018 Rochester, MN 2018 MFMER slide-1 Disclosure No
Overcoming the PosESBLities of Enterobacteriaceae Resistance Review of current treatment options Jamie Reed, PharmD Pharmacy Grand Rounds August 28, 2018 Rochester, MN 2018 MFMER slide-1 Disclosure No
Chapter 10 Respiratory System J00-J99. Presented by: Jesicca Andrews
 Chapter 10 Respiratory System J00-J99 Presented by: Jesicca Andrews 1 Respiratory System 2 Respiratory Infections A respiratory infection cannot be assumed from a laboratory report alone; physician concurrence
Chapter 10 Respiratory System J00-J99 Presented by: Jesicca Andrews 1 Respiratory System 2 Respiratory Infections A respiratory infection cannot be assumed from a laboratory report alone; physician concurrence
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Prevalence of Extended Spectrum -Lactamases In E.coli and Klebsiella spp. in a Tertiary Care Hospital
 ISSN: 2319-7706 Volume 3 Number 10 (2014) pp. 474-478 http://www.ijcmas.com Original Research Article Prevalence of Extended Spectrum -Lactamases In E.coli and Klebsiella spp. in a Tertiary Care Hospital
ISSN: 2319-7706 Volume 3 Number 10 (2014) pp. 474-478 http://www.ijcmas.com Original Research Article Prevalence of Extended Spectrum -Lactamases In E.coli and Klebsiella spp. in a Tertiary Care Hospital
Skin reactivity to autologous bacteria isolated from respiratory tract of patients with obstructive pulmonary disease
 Skin reactivity to autologous bacteria 149 Original Article Skin reactivity to autologous bacteria isolated from respiratory tract of patients with obstructive pulmonary disease J. Halasa 1, M. Halasa
Skin reactivity to autologous bacteria 149 Original Article Skin reactivity to autologous bacteria isolated from respiratory tract of patients with obstructive pulmonary disease J. Halasa 1, M. Halasa
Brice Taylor Assistant Professor Division of Pulmonary and Critical Care Medicine
 Brice Taylor Assistant Professor Division of Pulmonary and Critical Care Medicine Discuss advances in predicting prognosis Understand dwhat we know (and don t know) about the Microbiology Recognize important
Brice Taylor Assistant Professor Division of Pulmonary and Critical Care Medicine Discuss advances in predicting prognosis Understand dwhat we know (and don t know) about the Microbiology Recognize important
Bacteriemia and sepsis
 Bacteriemia and sepsis Case 1 An 80-year-old man is brought to the emergency room by his son, who noted that his father had become lethargic and has decreased urination over the past 4 days. The patient
Bacteriemia and sepsis Case 1 An 80-year-old man is brought to the emergency room by his son, who noted that his father had become lethargic and has decreased urination over the past 4 days. The patient
THE USE OF THE PENICILLINASE-RESISTANT
 Therapeutic problems THE USE OF THE PENICILLINASE-RESISTANT PENICILLIN IN THE PNEUMONIAS OF CHILDREN MARTHA D. Yow, MARY A. SOUTH AND CHARLES G. HESS From the Department of Pediatrics, Baylor University
Therapeutic problems THE USE OF THE PENICILLINASE-RESISTANT PENICILLIN IN THE PNEUMONIAS OF CHILDREN MARTHA D. Yow, MARY A. SOUTH AND CHARLES G. HESS From the Department of Pediatrics, Baylor University
Pathology of Pneumonia
 Pathology of Pneumonia Dr. Atif Ali Bashir Assistant Professor of Pathology College of Medicine Majma ah University Introduction: 5000 sq meters of area.! (olympic track) Filters >10,000 L of air / day!
Pathology of Pneumonia Dr. Atif Ali Bashir Assistant Professor of Pathology College of Medicine Majma ah University Introduction: 5000 sq meters of area.! (olympic track) Filters >10,000 L of air / day!
Marcos I. Restrepo, MD, MSc, FCCP
 Thank you for viewing this presentation. We would like to remind you that this material is the property of the author. It is provided to you by the ERS for your personal use only, as submitted by the author.
Thank you for viewing this presentation. We would like to remind you that this material is the property of the author. It is provided to you by the ERS for your personal use only, as submitted by the author.
Cefsulodin Therapy for Osteomyelitis Due to Pseudomonas aeruginosa
 REVIEWS OF INFECTIOUS DISEASES VOL. 6, SUPPLEMENT SEPTEMBER-OCTOBER 984 984 by The University of Chicago. All rights reserved. 062-0886/84/0605-004$02.00 Cefsulodin Therapy for Osteomyelitis Due to Pseudomonas
REVIEWS OF INFECTIOUS DISEASES VOL. 6, SUPPLEMENT SEPTEMBER-OCTOBER 984 984 by The University of Chicago. All rights reserved. 062-0886/84/0605-004$02.00 Cefsulodin Therapy for Osteomyelitis Due to Pseudomonas
WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS
 DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
Amphotericin B or Ketoconazole Therapy of Fungal Infections in Neutropenic Cancer Patients
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Jan. 1987, p. 11-15 0066-4804/87/010011-05$02.00/0 Copyright 1987, American Society for Microbiology Vol. 31, No. 1 or Therapy of Fungal Infections in Neutropenic
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Jan. 1987, p. 11-15 0066-4804/87/010011-05$02.00/0 Copyright 1987, American Society for Microbiology Vol. 31, No. 1 or Therapy of Fungal Infections in Neutropenic
Surveillance of antimicrobial susceptibility of Enterobacteriaceae pathogens isolated from intensive care units and surgical units in Russia
 Feb. 2016 THE JAPANESE JOURNAL OF ANTIBIOTICS 69 1 41 41 Surveillance of antimicrobial susceptibility of Enterobacteriaceae pathogens isolated from intensive care units and surgical units in Russia IRINA
Feb. 2016 THE JAPANESE JOURNAL OF ANTIBIOTICS 69 1 41 41 Surveillance of antimicrobial susceptibility of Enterobacteriaceae pathogens isolated from intensive care units and surgical units in Russia IRINA
Community Acquired Pneumonia. Abdullah Alharbi, MD, FCCP
 Community Acquired Pneumonia Abdullah Alharbi, MD, FCCP A 68 y/ male presented to the ED with SOB and productive coughing for 2 days. Reports poor oral intake since onset due to nausea and intermittent
Community Acquired Pneumonia Abdullah Alharbi, MD, FCCP A 68 y/ male presented to the ED with SOB and productive coughing for 2 days. Reports poor oral intake since onset due to nausea and intermittent
NETSPAN Injection (Netilmicin sulfate)
 Published on: 22 Sep 2014 NETSPAN Injection (Netilmicin sulfate) Composition NETSPAN 10 Each ml contains: netilmicin... 10 mg Benzyl alcohol BP...1% v/v (as preservative) Water for Injection BP...q.s NETSPAN
Published on: 22 Sep 2014 NETSPAN Injection (Netilmicin sulfate) Composition NETSPAN 10 Each ml contains: netilmicin... 10 mg Benzyl alcohol BP...1% v/v (as preservative) Water for Injection BP...q.s NETSPAN
Bacterial Adherence to Pharyngeal Cells in Smokers, Nonsmokers, and Chronic Bronchitics
 INFECTION AND IMMUNITY, Oct. 1979, p. 178-182 0019-9567/79/10-0178/05$02.00/0 Vol. 26, No. 1 Bacterial Adherence to Pharyngeal Cells in Smokers, Nonsmokers, and Chronic Bronchitics VICTOR FAINSTEIN AND
INFECTION AND IMMUNITY, Oct. 1979, p. 178-182 0019-9567/79/10-0178/05$02.00/0 Vol. 26, No. 1 Bacterial Adherence to Pharyngeal Cells in Smokers, Nonsmokers, and Chronic Bronchitics VICTOR FAINSTEIN AND
Anaerobes Bacteroides species, Clostridium species (Note: most strains of C.difficile are resistant).
 CEFIXON Composition Each vial contains 1 g Ceftriaxone (as sodium). Vial Action The bactericidal activity of Ceftriaxone results from inhibition of cell wall Synthesis. Ceftriaxone has a high degree of
CEFIXON Composition Each vial contains 1 g Ceftriaxone (as sodium). Vial Action The bactericidal activity of Ceftriaxone results from inhibition of cell wall Synthesis. Ceftriaxone has a high degree of
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME TOBREX TM Eye Drops 0.3% TOBREX TM Eye Ointment 0.3 % 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Tobrex Eye Drops contains the active ingredient tobramycin
NEW ZEALAND DATA SHEET 1. PRODUCT NAME TOBREX TM Eye Drops 0.3% TOBREX TM Eye Ointment 0.3 % 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Tobrex Eye Drops contains the active ingredient tobramycin
FAECAL WELL D-ONE. System for the presumptive identification and antibiotic susceptibility of pathogenic microorganisms of the intestinal tract.
 System for the presumptive identification and antibiotic susceptibility of pathogenic microorganisms of the intestinal tract. 1. INTRODUCTION Intestinal and gastrointestinal infections are a fairly common
System for the presumptive identification and antibiotic susceptibility of pathogenic microorganisms of the intestinal tract. 1. INTRODUCTION Intestinal and gastrointestinal infections are a fairly common
ORIGINAL ARTICLE SUSCEPTIBILITY PATTERNS IN GRAM NEGATIVE URINARY ISOLATES TO CIPROFLOXACIN, CO-TRIMOXAZOLE AND NITROFURANTOIN
 SUSCEPTIBILITY PATTERNS IN GRAM NEGATIVE URINARY ISOLATES TO CIPROFLOXACIN, CO-TRIMOXAZOLE AND NITROFURANTOIN Anoop Sinha 1, Benny P V 2 HOW TO CITE THIS ARTICLE: Anoop Sinha, Benny PV. Susceptibility
SUSCEPTIBILITY PATTERNS IN GRAM NEGATIVE URINARY ISOLATES TO CIPROFLOXACIN, CO-TRIMOXAZOLE AND NITROFURANTOIN Anoop Sinha 1, Benny P V 2 HOW TO CITE THIS ARTICLE: Anoop Sinha, Benny PV. Susceptibility
Bacteraemia in patients receiving human cadaveric
 J. clin. Path., 1971, 24, 295-299 Bacteraemia in patients receiving human cadaveric renal transplants D. A. LEIGH1 From the Department of Bacteriology, The Wright-Fleming Institute, St Mary's Hospital,
J. clin. Path., 1971, 24, 295-299 Bacteraemia in patients receiving human cadaveric renal transplants D. A. LEIGH1 From the Department of Bacteriology, The Wright-Fleming Institute, St Mary's Hospital,
NEONATAL SEPSIS. Dalima Ari Wahono Astrawinata Departemen Patologi Klinik, FKUI-RSCM
 NEONATAL SEPSIS Dalima Ari Wahono Astrawinata Departemen Patologi Klinik, FKUI- Background Neonatal sepsis : Early-onset Late-onset Early-onset : mostly premature neonates Within 24 hours 85% 24-48 hours
NEONATAL SEPSIS Dalima Ari Wahono Astrawinata Departemen Patologi Klinik, FKUI- Background Neonatal sepsis : Early-onset Late-onset Early-onset : mostly premature neonates Within 24 hours 85% 24-48 hours
Plazomicin Versus Meropenem for the Treatment of Complicated Urinary Tract Infection and Acute Pyelonephritis: Results of the EPIC Study
 Plazomicin Versus Meropenem for the Treatment of Complicated Urinary Tract Infection and Acute Pyelonephritis: Results of the EPIC Study Daniel J. Cloutier 1, Loren G. Miller 2, Allison S. Komirenko 1,
Plazomicin Versus Meropenem for the Treatment of Complicated Urinary Tract Infection and Acute Pyelonephritis: Results of the EPIC Study Daniel J. Cloutier 1, Loren G. Miller 2, Allison S. Komirenko 1,
CHEST VOLUME 117 / NUMBER 4 / APRIL, 2000 Supplement
 CHEST VOLUME 117 / NUMBER 4 / APRIL, 2000 Supplement Evidence-Based Assessment of Diagnostic Tests for Ventilator- Associated Pneumonia* Executive Summary Ronald F. Grossman, MD, FCCP; and Alan Fein, MD,
CHEST VOLUME 117 / NUMBER 4 / APRIL, 2000 Supplement Evidence-Based Assessment of Diagnostic Tests for Ventilator- Associated Pneumonia* Executive Summary Ronald F. Grossman, MD, FCCP; and Alan Fein, MD,
Chapter 22. Pulmonary Infections
 Chapter 22 Pulmonary Infections Objectives State the incidence of pneumonia in the United States and its economic impact. Discuss the current classification scheme for pneumonia and be able to define hospital-acquired
Chapter 22 Pulmonary Infections Objectives State the incidence of pneumonia in the United States and its economic impact. Discuss the current classification scheme for pneumonia and be able to define hospital-acquired
Dose-dependent effects of tobramycin in an animal model of Pseudomonas sinusitis Am J Rhino Jul-Aug; 21(4):423-7
 AMINOGLYCOSIDES Dose-dependent effects of tobramycin in an animal model of Pseudomonas sinusitis Am J Rhino. 2007 Jul-Aug; 21(4):423-7 http://www.ncbi.nlm.nih.gov/pubmed/17882910 Evaluation of the in-vivo
AMINOGLYCOSIDES Dose-dependent effects of tobramycin in an animal model of Pseudomonas sinusitis Am J Rhino. 2007 Jul-Aug; 21(4):423-7 http://www.ncbi.nlm.nih.gov/pubmed/17882910 Evaluation of the in-vivo
PACKAGE INSERT USP ANTIBIOTIC
 Pr AMPICILLIN for Injection USP ANTIBIOTIC ACTIONS AND CLINICAL PHARMACOLOGY Ampicillin has a broad spectrum of bactericidal activity against many gram-positive and gramnegative aerobic and anaerobic bacteria.
Pr AMPICILLIN for Injection USP ANTIBIOTIC ACTIONS AND CLINICAL PHARMACOLOGY Ampicillin has a broad spectrum of bactericidal activity against many gram-positive and gramnegative aerobic and anaerobic bacteria.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Doribax 250 mg powder for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains doripenem monohydrate
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Doribax 250 mg powder for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains doripenem monohydrate
MDR AGENTS: RISK FACTORS AND THERAPEUTIC STRATEGIES
 MDR AGENTS: RISK FACTORS AND THERAPEUTIC STRATEGIES 1 Marin H. Kollef, MD Professor of Medicine Virginia E. and Sam J. Golman Chair in Respiratory Intensive Care Medicine Washington University School of
MDR AGENTS: RISK FACTORS AND THERAPEUTIC STRATEGIES 1 Marin H. Kollef, MD Professor of Medicine Virginia E. and Sam J. Golman Chair in Respiratory Intensive Care Medicine Washington University School of
December 3, 2015 Severe Sepsis and Septic Shock Antibiotic Guide
 Severe Sepsis and Septic Shock Antibiotic Guide Surviving Sepsis: The choice of empirical antimicrobial therapy depends on complex issues related to the patient s history, including drug intolerances,
Severe Sepsis and Septic Shock Antibiotic Guide Surviving Sepsis: The choice of empirical antimicrobial therapy depends on complex issues related to the patient s history, including drug intolerances,
