Reversing the defective induction of IL-10 secreting regulatory T cells in glucocorticoid-resistant asthma patients
|
|
|
- Gavin Harrison
- 5 years ago
- Views:
Transcription
1 Research article Reversing the defective induction of IL-10 secreting regulatory T cells in glucocorticoid-resistant asthma patients Emmanuel Xystrakis, 1 Siddharth Kusumakar, 1 Sandra Boswell, 1 Emma Peek, 1 Zoë Urry, 1 David F. Richards, 1 Tonye Adikibi, 1 Carol Pridgeon, 2 Margaret Dallman, 2 Tuck-Kay Loke, 1 Douglas S. Robinson, 2,3 Franck J. Barrat, 4 Anne O Garra, 5 Paul Lavender, 1 Tak H. Lee, 1 Christopher Corrigan, 1 and Catherine M. Hawrylowicz 1 1 Medical Research Council and Asthma-UK Centre in Allergic Mechanisms of Asthma at King s College London, Strand, London, United Kingdom. 2 Life Sciences and 3 Medical Research Council and Asthma-UK Centre in Allergic Mechanisms of Asthma at Imperial College, Imperial College, London, United Kingdom. 4 Dynavax Technologies Corp., Berkeley, California, USA. 5 Laboratory of Immunoregulation, National Institute for Medical Research, Mill Hill, London, United Kingdom. We previously reported that human CD4 + Tregs secrete high levels of IL-10 when stimulated in the presence of dexamethasone and calcitriol (vitamin D3). We now show that following stimulation by allergen, IL-10 secreting Tregs inhibit cytokine secretion by allergen-specific Th2 cells in an IL-10 dependent manner. A proportion of patients with severe asthma fail to demonstrate clinical improvement upon glucocorticoid therapy, and their asthma is characterized as glucocorticoid resistant (SR, abbreviation derived from steroid resistant ). Dexamethasone does not enhance secretion of IL-10 by their CD4 + T cells. Addition of vitamin D3 with dexamethasone to cultures of SR CD4 + T cells enhanced IL-10 synthesis to levels observed in cells from glucocorticoid-sensitive patients cultured with dexamethasone alone. Furthermore, pretreatment with IL-10 fully restored IL-10 synthesis in these cells in response to dexamethasone. Vitamin D3 significantly overcame the inhibition of glucocorticoid-receptor expression by dexamethasone while IL-10 upregulated glucocorticoid-receptor expression by CD4 + T cells, suggesting potential mechanisms whereby these treatments may overcome poor glucocorticoid responsiveness. We show here that administration of vitamin D3 to healthy individuals and SR asthmatic patients enhanced subsequent responsiveness to dexamethasone for induction of IL-10. This strongly suggests that vitamin D3 could potentially increase the therapeutic response to glucocorticoids in SR patients. Introduction CD4 + Th2 cells are implicated in the pathogenesis of asthma and allergic diseases through the actions of IL-4 and IL-13, which switch B cells to IgE synthesis; IL-5, which plays a role in eosinophil maturation and survival; and IL-13, which regulates airway hyperresponsiveness and mucus hyperplasia (1). Glucocorticoids are the first-line antiinflammatory treatment for asthma (2). Their multiple inhibitory properties, including the inhibition of Th2 cytokine synthesis, are likely to contribute to clinical efficacy. Glucocorticoids also enhance IL-10 production in vitro by human CD4 + and CD8 + T cells (3), and glucocorticoid treatment induces the synthesis of IL-10 by airway cells in asthmatic patients (4). IL-10 is a potent antiinflammatory and immunosuppressive cytokine that mediates its major immunosuppressive function by inhibiting APC function and cytokine production by macrophages and dendritic cells, leading to profound inhibition of Th1 cell mediated immunity (5). IL-10 also regulates effector responses associated with established allergic and asthmatic disease, including inhibition of cytokine production by Th2 cells as well as mast cell and eosinophil function and modulation of IgG4:IgE ratios (5, 6). There appears to be an inverse correlation between IL-10 levels and the incidence and/or severity of asthmatic Nonstandard abbreviations used: FEV 1, forced expired volume in 1 second; GR, glucocorticoid receptor; SR, glucocorticoid resistant; SS, glucocorticoid sensitive. Conflict of interest: The authors have declared that no conflict of interest exists. Citation for this article: J. Clin. Invest. 116: (2006). doi: /jci and allergic disease (7 10). Furthermore, studies in animal models of transfer of the IL-10 gene directly to the lung (11) or adoptive transfer of IL-10 transfected CD4 + T cells (12) demonstrate that IL-10 can block allergic airway inflammation (reviewed in ref. 6). These studies imply a central role for IL-10 in disease control and suggest that the induction of IL-10 synthesis may contribute to the clinical efficacy of glucocorticoids in allergy and asthma. A proportion of asthmatic patients fails to benefit from oral glucocorticoid therapy and are thus denoted as having glucocorticoid-resistant (SR, derived from steroid resistant ) or insensitive asthma (13). SR is associated with in vitro and in vivo alterations in cellular responses to exogenous glucocorticoids. We have previously demonstrated that CD4 + T cells from SR asthma patients fail to induce IL-10 synthesis following in vitro stimulation in the presence of dexamethasone as compared with their glucocorticoid-sensitive counterparts (SS, derived from steroid sensitive ) (14), suggesting a link between induction of IL-10 synthesis and clinical efficacy of glucocorticoids. Resistance to the inhibitory effects of glucocorticoids can be induced in T cells in vitro, for example, by incubation with IL-2 and IL-4 (15). It may, therefore, be possible to modulate the function of SR CD4 + T cells in vitro and restore IL-10 synthesis. One potential source of IL-10 is Tregs, which control the function of effector T cells, thereby preventing responsiveness to self antigens and regulating immune responses. The major Treg populations described to date include naturally occurring CD4 + CD25 + Tregs and inducible IL-10 or TGF-β secreting Tregs (16 18). 146 The Journal of Clinical Investigation Volume 116 Number 1 January 2006
2 Figure 1 Polyclonal activation of human CD4 + T cells in the presence of dexamethasone plus vitamin D3 induces IL-10 expression, which does not correlate with expression of the transcription factor Foxp3. (A) CD4 + T cells from an SS asthma patient were cultured with APCs, anti-cd3, IL-2, and IL-4 alone (medium) or together with 10 7 M Vitamin D3 and 10 7 M dexamethasone (vit/dex). Cells were restimulated at 7 days with PMA and ionomycin for 4 hours, and coexpression of IL-10 with IL-2, IL-4, IFN-γ, or GM-CSF was analyzed by flow cytometry. Data are representative of 5 experiments. (B) Real-time RT-PCR was performed on PBMCs, freshly isolated CD4 + CD25 and CD4 + CD25 + T cells, and IL-10 secreting Tregs harvested after 14 days in culture to compare expression of Foxp3 and IL-10. Data are representative of 3 experiments. HPRT, hypoxanthine-guanine phosphoribosyl transferase. IL-10 secreting Tregs have been described following different regimens of antigenic administration both in vitro and in vivo (19 24). Our initial studies showed that glucocorticoids enhanced the production of IL-10 by polyclonally stimulated T cells and that these cells inhibited IFN-γ production by human CD4 + T cells in an IL-10 dependent manner (3). More recently, we demonstrated in both mouse and human that a combination of dexamethasone and calcitriol, the active form of vitamin D3, induced high numbers of IL-10 producing T cells that made negligible amounts of Th1 and Th2 cytokines (25). The inhibitory activity of these cells was demonstrated in vivo, where adoptive transfer of IL-10 secreting Tregs inhibited the development of murine experimental allergic encephalomyelitis (25); and in vitro, where they inhibited the proliferation of naive T cells (26). However, no data are yet available on the capacity of drug-induced IL-10 secreting Tregs to inhibit previously activated T cells, specifically Th2 cells, a major therapeutic target in allergic asthma. In vitro suppression by CD4 + CD25 + T cells appears to be independent of IL-10 (27) whereas in vivo regulation by these cells is IL-10 dependent in some models (28 30). The transcription factor Foxp3 has recently been reported to be specifically expressed by CD4 + CD25 + Tregs, controlling their development and function (31). To date, the relationship between naturally occurring CD4 + CD25 + Tregs and IL-10 secreting Treg subsets is unclear. In this study, we describe the in vitro inhibitory potential of human IL-10 secreting Tregs, derived by stimulation in the presence of vitamin D3 and dexamethasone, to inhibit cytokine production by allergen-specific Th2 cells associated with immune pathology in allergic asthma. We further demonstrate that impaired induction of IL-10 by glucocorticoids in T cells from SR patients can be reversed by vitamin D3 and IL-10, leading to levels of IL-10 production comparable to those observed in SS asthmatic patients, and examine the mechanisms by which this may occur. Results Short-term culture of CD4 + T cells with glucocorticoids plus vitamin D3 is sufficient to generate IL-10 secreting Tregs. One week of culture of CD4 + T cells with anti-cd3, APCs, IL-2, and IL-4 in the pres- The Journal of Clinical Investigation Volume 116 Number 1 January
3 Figure 2 TGF-β is not upregulated by vitamin D3 and dexamethasone treatment and does not play a role in IL-10 secreting Treg mediated suppression of Th1 and Th2 cytokine production. (A) CD4 + T cells were stimulated for 7 days with (T vit/dex) or without (T neutral) vitamin D3 and dexamethasone, as for Figure 1, and restimulated without drugs for 48 hours. TGF-β and IL-10 levels in culture supernatants were determined by ELISA. Mean data of 3 experiments are shown. (B) CD4 + T cells were cultured for two 7-day cycles with or without vitamin D3 and dexamethasone and then activated with PMA and ionomycin for 4 hours. PBMCs were cultured without or with PMA and ionomycin for 4 hours. TGF-β and IL-10 mrna were determined by real-time RT-PCR and expressed relative to unactivated PBMCs. A representative experiment of 4 is shown. (C) CD4 + T cell lines were cultured for 7 days with vitamin D3 and dexamethasone and then used in cocultures with fresh autologous T cells (1:4 ratio) and irradiated APCs. Day 3 supernatants were assessed for IFN-γ and IL-13 content. Mean data from 4 experiments is shown. *P < 0.05 using 2-tailed Student s t test. ence of dexamethasone and vitamin D3 promoted an IL-10 expressing phenotype and a reduction in Th1- and Th2-associated cytokines as well as GM-CSF, a key cytokine implicated in allergic airway inflammation (32) (6% IL-10 positive T cells in medium versus 26% IL-10 positive T cells in the presence of vitamin D3 plus dexamethasone; Figure 1A). In the absence of IL-4, T cells stimulated in the presence of vitamin D3 and dexamethasone showed reduced cell survival (data not shown) but still demonstrated enhanced IL-10 synthesis (4% IL-10 positive T cells in medium versus 15% IL-10 positive T cells in presence of vitamin D3 plus dexamethasone). IL-10 secreting Tregs express low levels of Foxp3 and are distinct from Th3 regulatory cells. To determine a possible relationship between naturally occurring CD4 + CD25 + Tregs and antigen-stimulated IL-10 secreting Tregs, we compared the expression of the transcription factor Foxp3 and IL-10 using real-time PCR analysis. In vitro derived IL-10 secreting Tregs harvested at the end of 14 days of culture expressed comparatively low amounts of Foxp3 mrna compared with unstimulated CD4 + CD25 + Tregs isolated directly ex vivo (Figure 1B). In contrast, IL-10 secreting Tregs expressed markedly higher amounts of mrna encoding IL-10 than CD4 + CD25 + Tregs, suggesting that these 2 regulatory populations may repre- 148 The Journal of Clinical Investigation Volume 116 Number 1 January 2006
4 Figure 3 IL-10 secreting Tregs inhibit polyclonal T cell proliferative responses to anti-cd3 and cytokine production by allergen-stimulated Th2 cells. This inhibition is reversed by an IL-10 antagonist. (A F). Freshly isolated CFSE-labeled CD4 + T cells (CD4 + T-CFSE) were cultured with irradiated APCs and (A) medium or (B) anti-cd3. Alternatively, they were (C) cocultured at a ratio of 1 T IL-4 to 4 CD4 + T-CFSE or (D F) 1 IL-10 secreting Treg (IL-10 Treg) to 4 CD4 + T-CFSE. (E) Isotype control or (F) anti IL-10 receptor monoclonal antibodies were added as indicated. Cell-cycle progression was analyzed at day 6. (G) Allergen-specific IL-10 secreting Tregs or Th2 cells were cultured alone or together (1 IL-10 secreting Treg to 4 Th2 cells) with APCs in medium or with cat allergen extract. Day 3 culture supernatants were analyzed by ELISA and cytometric bead array. Data are representative of 5 experiments. (H) Grass pollen allergen extract stimulated cultures were established as above with the addition of a control isotype or anti IL-10 receptor monoclonal antibody as indicated. Cytokine levels detected in the absence of allergen were 200 pg/ml or less and were subtracted in all groups. Data are representative of 3 experiments. sent 2 distinct lineages (Figure 1B). Similarly, to permit a comparison of drug-induced IL-10 secreting Tregs with TGF-β secreting Th3 cells, CD4 + T cells were cultured in the presence or absence of vitamin D3 and dexamethasone in serum-free media to facilitate analysis of TGF-β secretion. The drug treatment enhanced IL-10 production, as assessed by both ELISA and quantitative PCR analysis, but no increase in TGF-β was observed in the same cultures, suggesting drug-induced IL-10 secreting Tregs are distinct from Th3-type regulatory cells (Figure 2, A and B). Human IL-10 secreting Tregs inhibit the proliferation of autologous T cells in an IL-10 dependent manner. To determine whether druginduced IL-10 secreting Tregs manifest regulatory function for inhibition of CD4 + T cell proliferation, autologous CD4 + T cells were isolated and labeled with the fluorescent dye CFSE. Cell cycle progression of CFSE-labeled CD4 + T cells was analyzed on day 6 by flow cytometry after stimulation with anti-cd3 and APCs (Figure 3B; the ModFit-generated proliferation index of CFSE CD4 + T cells in the absence or presence of anti-cd3 was 1.1 versus 5.95). IL-10 secreting Tregs blocked the cell cycle progression of the freshly isolated CD4 + T cells at a ratio of 1 IL-10 secreting Treg to 4 CFSE CD4 + T cells (Figure 3D; proliferation index, 1.55). This inhibition of proliferation by IL-10 secreting Tregs was reversed by the addition of anti IL-10R mabs (Figure 3F; proliferation index, 5.12), in contrast to an isotype control, which showed little effect (Figure 3E; proliferation index, 2.03). As few as 1 IL-10 secreting Treg could ablate the proliferative response of 16 CFSE-labeled responder T cells (data not shown). T cell lines generated with IL-4 in the absence of drugs had little or no effect on cell cycle progression of the autologous freshly derived CFSE- CD4 + T cells (Figure 3C; proliferation index, 5.3). IL-10 secreting Tregs inhibit cytokine synthesis from allergen-specific Th2 cell lines. Allergen-specific Th2 cells and IL-10 secreting Tregs were generated in parallel cultures using T cells from the same atopic donor. After two 7-day cycles of stimulation with cat allergen presented by autologous, irradiated T cell depleted PBMC, the cells were stimulated alone or together at a ratio of 1 IL-10 secreting Treg to 4 Th2 cells with APCs and with or without allergen. IL-10 secreting Tregs inhibited IL-5 and IL-13 synthesis by allergenstimulated Th2 cells almost to the levels of cytokine production detected in supernatants of unstimulated Th2 cells (Figure 3G). As predicted from independent studies demonstrating IL-10 synthesis by Th2 cells, addition of anti IL-10R mabs to cultures of cat allergen stimulated Th2 cells enhanced IL-13 cytokine production. Furthermore, the marked inhibition of IL-13 production by The Journal of Clinical Investigation Volume 116 Number 1 January
5 Figure 4 Enhancement of glucocorticoid-induced IL-10 synthesis by T cells from SR asthma patients with vitamin D3 and pretreatment with IL-10. (A) CD4 + T cells from SS (n = 4) and GR (n = 8) asthma patients were cultured with anti-cd3, APCs, IL-2, IL-4, and the indicated combinations of 10 7 M dexamethasone and M vitamin D3 for 7 days. Cells were recultured at cells/ml with anti-cd3 and IL-2 alone, and 48-hour culture supernatants were assayed for IL-10 content by ELISA. One patient was tested 2 different times at an interval of 1 year. (B) CD4 + T cells from SS (n = 4) and SR (n = 6) asthma patients were cultured with APCs overnight in medium or with 2 ng/ml IL-10, washed and cultured with anti-cd3, IL-2, IL-4, and the indicated concentrations of dexamethasone, M vitamin D3, or 10 7 M dexamethasone plus vitamin D3 for 7 days. Cells were recultured with anti-cd3 and IL-2 alone and 48-hour culture supernatants assayed by ELISA. *P < 0.05; **P < 0.01 using 2-tailed Student s t test. Th2 cells observed in the presence of IL-10 secreting Tregs was completely overcome by the addition of anti IL-10R mabs but not by isotype-matched control antibodies (Figure 3H). In addition to their ability to inhibit Th2 responses, IL-10 secreting Tregs also inhibited IFN-γ production by T cells in anti-cd3 driven stimulation cultures. Again, this inhibition was reversed by an anti IL-10 receptor antibody but not by a neutralizing anti TGF-β antibody (Figure 2C). Anti TGF-β also failed to reverse IL-10 secreting Treg mediated inhibition of Th2 cytokine production in this anti-cd3 driven culture system (Figure 2C). These data further emphasize that IL-10 secreting Tregs represent a population distinct from previously described naturally occurring and Th3 Treg populations. Vitamin D3 enhances IL-10 synthesis by CD4 + T cells from SR patients. We previously demonstrated that CD4 + T cells from SR patients show a statistically significant reduction in their capacity to respond to dexamethasone for induction of IL-10 synthesis in comparison with SS patients (14). We therefore tested whether vitamin D3 could enhance IL-10 synthesis in the SR cultures stimulated with anti-cd3 and APCs. Mean data from 8 SR patient cell cultures is shown in Figure 4A. The poor response of CD4 + T cells from SR patients to dexamethasone in producing IL-10 was greatly enhanced by the addition of Vitamin D3 to the cultures, restoring IL-10 levels to those seen in cultures of cells from SS patients stimulated with dexamethasone alone such that these 2 groups were not significantly different. However, under none of the experimental conditions tested was IL-10 synthesis in the SR patient cultures fully restored to the levels observed in CD4 + T cells derived from SS patients. Pretreatment with IL-10 fully restores the capacity of CD4 + T cells from SR patients to respond to dexamethasone for induction of IL-10 synthesis. Since the initial development of murine IL-10 secreting Tregs in the presence of dexamethasone and vitamin D3 is IL-10 dependent (25), we investigated whether pretreatment with IL-10 could further enhance the capacity of SR CD4 + T cells to produce IL-10 when stimulated in the presence of dexamethasone. T cells were incubated overnight either in medium or with 2 ng/ml IL-10 and then washed prior to polyclonal stimulation in the presence of the indicated combinations of dexamethasone and vitamin D3 for 7 days. All culture groups were then restimulated for 48 hours with anti-cd3 and IL-2 in the absence of the drugs. Following overnight preculture in medium prior to stimulation with anti-cd3 and dexamethasone plus vitamin D3, a clear difference in IL-10 synthesis was still maintained between the SR and SS patient groups (P < 0.01; Figure 4B). In contrast, overnight pulsing with IL-10 prior to stimulation in the presence of dexamethasone alone fully restored IL-10 synthesis by CD4 + T cells from SR patients to the levels seen in SS patients. In addition, IL-10 pretreatment and subsequent stimulation in the presence of dexamethasone plus vitamin D3 further enhanced levels of IL-10 synthesis in SR CD4 + T cell cultures. IL-10 pretreatment did not, however, significantly affect the production of IL-10 by CD4 + T cells from SS patients stimulated in the presence of the 2 drugs (Figure 4B). Figure 5 IL-10 enhances GR expression in CD4 + T cells. Purified CD4 + T cells were cultured in (a) medium (0 hours) or with 2 ng/ml IL-10 for the indicated time periods (in hours) or (b) medium or the indicated concentrations of IL-10 for 18 hours prior to analysis of GR and GAPDH levels by Western blotting. Data shown are from 2, and representative of 4, healthy nonasthmatic donors. Identification of the immunoreactive protein as GRα was permitted by comparison of migration of native extract with extracts of cells transiently transfected with cdnas encoding FLAG-tagged GRα and GRβ. CMV, cytomegalovirus. CMV.GRa. Flag, CMV promotor driving the expression of full-length GRa engineered to include the 8-aa FLAG epitope. 150 The Journal of Clinical Investigation Volume 116 Number 1 January 2006
6 Figure 6 Vitamin D3 does not increase GR expression in CD4 + T cells but prevents downregulation by dexamethasone. (A) CD4 + T cells were cultured in medium ( ) or with 10 7 M vitamin D3 (+) for 12, 24, or 48 hours and then analyzed for GR and GAPDH by Western blot. Data from a representative donor (P1) and densitometry analysis of the ratio of GR to GAPDH is shown in the left panel; mean data from 4 individuals at 48 hours is shown in the right panel. (B) CD4 + T cells were cultured without or with dexamethasone for 48 hours prior to Western blot analysis. Data from 2 representative individuals (P2 and P3, left panel) and mean data from 4 individuals (right panel) are shown. (C) Culture of CD4 + T cells with vitamin D3, dexamethasone, or both drugs for 48 hours. Western blot analysis from 2 representative individuals (P4 and P5; left panel) and mean data from 7 individuals (right panel) are shown. *P < 0.05; **P < We investigated whether vitamin D3 might also act to increase GR expression since topical administration of calcitriol to patients with psoriasis has been shown to increase IL-10 locally in the skin (33). Although this was not the case (Figure 6A), we observed, as independently reported in certain cell lines (34), that the culture of CD4 + T cells with glucocorticoids leads to a dose-dependent decrease in GRα (Figure 6B). Strikingly, vitamin D3 abrogated the profound downregulation of GRα caused by dexamethasone (Figure 6C), suggesting a potential mechanism whereby vitamin D3 may contribute to the efficacy of dexamethasone in promoting glucocorticoid-induced IL-10 synthesis. Oral administration of vitamin D3 enhances the responsiveness to glucocorticoids for induction of IL-10 synthesis. In a proof of concept study, the effect of ingestion of vitamin D3 in SR asthmatic patients was investigated. Subjects were bled prior to vitamin D3 ingestion and on days 1, 3, and 7 after ingestion of 0.5 µg vitamin D3 daily. T cells were stimulated with anti-cd3 in the presence or absence of dexamethasone for 7 days, using methodology identical to that used in earlier experiments, to determine any differences in reactivity to dexamethasone. Cell pellets were prepared at this time for mrna extraction, or cells were recultured in the absence of drugs and analyzed for changes in IL-10 protein at 48 hours. In all SR individuals tested, IL-10 expression by CD4 + T cells was enhanced following vitamin D3 ingestion as assessed by intracellular cytokine staining, ELISA, and quantitative PCR (Figure 7). In 2 donors (SR1 and SR2), this enhancement was sustained on days 1 and 3 as well as day 7 in SR2 over a range of dexamethasone concentrations. In the third donor, SR3, enhancement was observed on day 3 alone. Similar protein data were observed in 4 healthy individuals tested in a pilot study (Supplemental Figure 1; supplemental material available online with this article; doi: /jci21759ds1; RNA not assayed in this earlier study). These preliminary data provide encouraging evidence of the capacity of vitamin D3 to promote responsiveness to glucocorticoids for the induction of IL-10 synthesis. Effects of IL-10 and vitamin D3 on the expression of the glucocorticoid receptor. Since we have implicated deficient IL-10 production by T cells in the mechanism of clinical glucocorticoid resistance, we hypothesized that IL-10 may regulate responsiveness of T cells to glucocorticoids and therefore tested to determine whether IL-10 modulates the expression of the glucocorticoid receptor (GR). Western blotting studies of total cell lysates from CD4 + T cells from nonasthmatic healthy individuals demonstrated an IL-10 concentration dependent induction of GRα expression in comparison with cells cultured in medium alone (Figure 5A). Induction was already detectable at 6 hours but most marked following 18 and 24 hours of culture with IL-10. GRβ expression was not detected in the T cell lysates (Figure 5B) although it could be detected in U293 cells transfected with cdna encoding FLAG-tagged GRβ, indicating very low expression of GRβ in human peripheral CD4 + T cells. Discussion The present study demonstrates that human IL-10 secreting Tregs induced ex vivo following stimulation in the presence of vitamin D3 and dexamethasone secrete high levels of IL-10 that is associated with a potent capacity to inhibit immune responses implicated in the pathogenesis of allergic and asthmatic disease. Specifically, allergen-induced cytokine production by Th2 cells was inhibited by allergen-induced IL-10 secreting Tregs in an IL-10 dependent manner. Furthermore, we demonstrated that by combining preincubation with IL-10 and addition of vitamin D3 to the T cell stimulation cultures, a defect in glucocorticoid-induced IL-10 production by CD4 + T cells from SR asthma patients was fully overcome. This may result from our findings that IL-10 increased GR expression by human CD4 + T cells while vitamin D3 overcame ligand-induced downregulation of GR. Strikingly, ingestion of vitamin D3 by SR asthmatic patients enhanced subsequent responsiveness to dexa- The Journal of Clinical Investigation Volume 116 Number 1 January
7 Figure 7 Ingestion of vitamin D3 enhances responsiveness to dexamethasone for induction of IL-10 synthesis. CD4 + T cells isolated prior to (day 0 [d 0]) or 1, 3, or 7 days after ingestion of vitamin D3 were cultured with anti-cd3 and IL-2 with or without the indicated concentrations of dexamethasone for 7 days. Cells were then assayed for mrna at this time or recultured for 48 hours with anti-cd3 and IL-2 and analyzed for expression of IL-10 by intracellular cytokine staining and ELISA. (A) IL-10 responses to a range of dexamethasone concentrations were assessed, and data are shown at the time of maximal induction for each donor (SR1 and SR3, day 3; SR2, day 7). mrna levels are expressed relative to day 0, medium ( ). (B) IL-10 responses to a single dose of dexamethasone (10 6 or 10 7 M) over time. methasone for the induction of IL-10 synthesis, highlighting the potential clinical relevance of these observations. IL-10 secreting Tregs may hold therapeutic potential for asthma and allergic diseases, and our findings offer potential novel strategies for reversing glucocorticoid resistance. We have previously described the capacity of vitamin D3 and dexamethasone to induce IL-10 producing T cells from both murine and human CD4 + T cells (25) and show here that the human IL-10 producing Tregs exhibit regulatory activity in vitro. Importantly, human IL-10 secreting Tregs could suppress previously activated antigen-specific Th2 cells, the therapeutic target in allergy and asthma. This inhibition of cytokine secretion from allergen-specific Th2 cells by IL-10 secreting Tregs was IL-10 dependent in this system. Since these cultures included APCs, this IL-10 dependent suppression may have been mediated via the well established effects of IL-10 on APCs (5). CD4 + CD25 + Tregs suppress allergen-induced proliferation and Th2 cytokine production by peripheral CD4 + T cells independently of IL-10 secretion (35). Here, we compared human IL-10 secreting Tregs and CD4 + CD25 + T cells and show that unlike IL-10 secreting Tregs, expression of IL-10 by CD4 + CD25 + Tregs was minimal. Furthermore, unlike CD4 + CD25 + Tregs, IL-10 secreting Tregs did not express high levels of mrna encoding the forkhead winged helix transcription factor FoxP3. Thus, although FoxP3 appears to be important for the development and function of naturally occurring CD4 + CD25 + T cells (31), in vitro derived IL-10 secreting Tregs appear to have regulatory function despite low levels of Foxp3 expression. We further demonstrate that drug-induced IL-10 secreting Tregs are distinct for TGF-β synthesizing Th3 cells, as they show no increased synthesis of TGF-β, and neutralizing antibody to this cytokine does not modify their inhibitory function (17). Although inhaled glucocorticoids are very effective for the treatment of most patients with asthma, an important subgroup of patients require oral glucocorticoids to control their disease at the risk of considerable side effects, and a subgroup of these patients fail to show clinical improvement even with high-dose glucocorticoid therapy (13). These patients represent an important healthcare problem and, consequently, if the response to glucocorticoid treatment could be enhanced in such patients, this would represent a major therapeutic advance. Our data imply that induction of IL-10 synthesis may contribute to the clinical efficacy of glucocorticoid therapy in asthma since IL-10 plays an essential role in the functional activities of IL-10 secreting Tregs and patients who fail to respond clinically to glucocorticoids also fail to respond ex vivo to glucocorticoids for induction of IL-10 synthesis (14). We now present evidence that it is possible to reverse this defect. First, addition of vitamin D3 to T cell cultures polyclonally stimulated with anti-cd3 and dexamethasone enhanced IL-10 synthesis in the SR cultures, restoring them to levels observed in SS cultures stimulated in the presence of dexamethasone alone. Secondly, administration of vitamin D3 orally to SR patients and healthy donors modified subsequent responsiveness of their CD4 + T cells to glucocorticoids for induction of IL-10 synthesis in all individuals tested. Vitamin D3 is at present occasionally administered to severe asthmatic patients as part of a prophylactic regimen against glucocorticoid-induced osteoporosis (36), and our studies suggest 152 The Journal of Clinical Investigation Volume 116 Number 1 January 2006
8 an additional potential benefit of such treatment. These data with vitamin D3 establish an important precedent showing that it is possible to boost IL-10 synthesis in this important patient group. However, it may also be possible to achieve similar cooperative effects between glucocorticoids and other drugs for induction of IL-10 secreting Tregs. Indeed, our recent studies demonstrate a similar capacity upon combining glucocorticoids with long-acting β 2 -agonists to induce an IL-10 secreting Treg profile (37). The mechanisms by which IL-10 and vitamin D3 enhance glucocorticoid-induced IL-10 synthesis were investigated. We hypothesized that IL-10 itself may be regulating responsiveness to glucocorticoids and now demonstrate a concentration-dependent effect of IL-10 on GRα expression by human CD4 + T cells. The possible functional significance of this observation is supported by an independent study that demonstrated that IL-10 increased the sensitivity of human monocytes to dexamethasone and increased the number of tritiated dexamethasone binding sites in U937 cells (38). The antibody used in our Western blotting detects both a and b spliced variants of GR, and these forms are readily distinguishable in Western blotting by virtue of their size difference. We consistently observed enhancement of GRα in IL-10 treated T cells whereas the GRβ variant remained undetectable by Western blot in agreement with several independent studies (ref. 39 and references therein). Two studies have used immunohistochemistry to detect GR expression in tissues, with both suggesting that less GRα is available in SR. One study demonstrated that glucocorticoid resistance in asthma is associated with elevated in vivo expression of GRβ relative to GRα in skin biopsies (40) while the second showed that airway T cells from SR patients showed increased GRβ expression in comparison with cells from glucocorticoid-sensitive asthmatics or control subjects (41). Notably, however, although elevated GRβ was also reported in blood T cells from this patient group, the levels were much lower than those observed in airway T cells. Thus GRβ is expressed at extremely low levels in normal cells but may be upregulated in SR asthma (40, 42). GRβ has been proposed to act as a dominant-negative form of GR operative at glucocorticoid-response elements (43). Thus, the upregulation of GRα observed by Western blot may alter the GRα to GRβ ratio and therefore partially restore responsiveness. In studies into the mechanism of vitamin D3 action, we observed that dexamethasone induces a dose-dependent and profound downregulation of GR in primary human CD4 + T cells. Strikingly, coincubation of CD4 + T cells with vitamin D3 and dexamethasone was observed to rescue GR expression and lead to a less severe decrease of GR expression in the CD4 + T cell population. We found no direct effect of vitamin D3 on GR expression by human CD4 + T cells. We propose that these observations may, in part, explain the capacity of vitamin D3 to promote glucocorticoid-induced IL-10 synthesis. It will be important to determine whether the defect in glucocorticoid-induced IL-10 synthesis observed in some asthmatic patients is a common feature of other immune-mediated inflammatory diseases that exhibit clinical refractoriness to glucocorticoid therapy, e.g., inflammatory bowel disease and rheumatoid arthritis (44). If failure to induce IL-10 ex vivo also correlates with clinical refractoriness to glucocorticoid therapy in other inflammatory conditions, then analysis of IL-10 synthesis may ultimately prove useful in disease management. For example, it could act as a useful predictive tool in determining which patients will be unresponsive to glucocorticoid therapy so that alternative treatment regimens can be introduced sooner. Both our own and independent studies suggest the capacity of both glucocorticoids and vitamin D3 to promote IL-10 synthesis in vivo. Other regimens, such as peptide therapy in murine models or allergen immunotherapy in allergic patients, may also induce IL-10 secreting Tregs (45 47). However, allergen immunotherapy carries a risk of severe allergic reactions or anaphylaxis (48), which precludes its use for severe asthma in current dose regimens, and peptide therapy for autoimmune diseases carries similar risks (49, 50). Combination with drug therapy might allow safer use of immunotherapy and promote the induction of IL-10 secreting Tregs. Other strategies have been adopted for investigating IL-10 secreting Tregs in vitro in animal models and support a therapeutic role of IL-10 synthesizing T cells in alleviating allergic symptoms (6, 51 53). For example, 1 recent study demonstrated that CD4 + T cells engineered to express IL-10 prevent Th2-driven airway hyperresponsiveness and inflammation (12). Finally, although 2 groups suggest that IL-10 may be required in the development of murine airway hyperresponsiveness (54, 55), these represent extremes of either transgenic overexpression or the complete absence of IL-10 in the airways and therefore do not represent physiological conditions. We would argue that the challenge for the future will be to harness these beneficial drug-mediated effects and induce allergen-specific IL-10 synthesis in a local and controlled manner at the time of allergen exposure (6). In conclusion, we show that human IL-10 secreting Tregs are distinct from CD4 + CD25 + T cells and TGF-β secreting Th3 cells and show in vitro suppressive activity against allergen-specific Th2 cells. Furthermore, we show that both vitamin D3 and IL-10 can overcome the defect in IL-10 production by CD4 + T cells from SR asthma patients. Induction of antigen-specific IL-10 secreting Tregs is an appealing future therapeutic area for asthma and allergic diseases. Methods Patient details. PBMCs were obtained from healthy individuals and patients attending the Asthma Clinic at Guy s Hospital, London, United Kingdom. Asthma was defined by American Thoracic Society criteria as reversible obstruction ( 15%) of the airways (14). Glucocorticoid resistance (SR) was defined as the failure of forced expired volume in 1 second (FEV 1) to improve by 15% or more from a baseline of 75% or less after 14 days of 40 mg/day oral prednisolone, and glucocorticoid sensitivity (SS) was defined as an improvement of 25% or more in FEV 1 following an identical course of prednisolone treatment. PBMCs were obtained from 7 SR patients (2 female, 5 male; mean age ± SD, 54 ± 15; mean basal FEV 1, 55% ± 20% of predicted; and range of prednisolone reversibility, 0 14%) and from 5 SS patients (2 female, 3 male; mean age, 50 ± 10; mean basal FEV 1, 46% ± 24% of predicted; and range of prednisolone reversibility, 26% to > 100%). All patients were nonsmokers and using inhaled ( µg) glucocorticoids daily and β2-agonists as required. Patients taking oral glucocorticoids within 1 month of study were excluded. All donors signed a consent form approved by the Ethics Committee at Guy s Hospital. Cell purification and culture. PBMCs were isolated as previously described (3). CD4 + T cells were purified by positive selection using Dynabeads (Dynal Biotech; typical purity, 99.5%). Samples containing CD4 + T cells plus APCs, unless otherwise specified, were prepared by negative depletion using CD8 + Dynabeads (3). CD4 + CD25 + T cells were isolated as previously described (35). We stimulated cells/ml CD4 + T cells or CD4 + T cells plus APCs with 1 µg/ml plate-bound or soluble anti-cd3 (OKT-3), respectively, 50 U/ml IL-2 (EuroCetus), 10 ng/ml IL-4 (NBS), calcitriol (Vitamin D3; BIOMOL), and dexamethasone (Sigma-Aldrich) as indicated. Cells were restimulated with cross-linked anti-cd3 and the same stimuli as in the The Journal of Clinical Investigation Volume 116 Number 1 January
9 first culture (3, 25). Where indicated, cells were recultured after 7 days with cross-linked anti-cd3 and IL-2 alone and supernatants harvested at 48 hours for cytokine analysis. To generate allergen-specific T cell lines, PBMCs were obtained from atopic volunteers. Atopy was defined as previously described (3). We cultured cells/ml CD4 plus APCs with 10 µg/ml of allergen and 10 ng/ml IL-4. The Th2 group also received 5 µg/ml anti IFN-γ and anti IL-12 (both from BD Biosciences Pharmingen), and the regulatory group received 10 7 M dexamethasone and 10 7 M vitamin D3. On day 7, cells were washed and recultured under the same initial culture conditions plus fresh allergen and irradiated APCs (T cell depleted PBMCs; 300 Gy). T cell lines were also derived from healthy volunteers by stimulating CD4 and APCs with anti-cd3 and IL-2 in the presence of IL-4 (T IL-4) or IL-4, vitamin D3, and dexamethasone (IL-10 secreting Tregs). Functional assays of regulatory function. On day 14, fresh autologous CD4 + T cells were purified and labeled with 2 µm CFSE (Invitrogen Corp.), and cells were cocultured with autologous irradiated APCs and T IL-4 or IL-10 secreting Tregs as indicated. Cultures were stimulated with soluble anti-cd3, with or without 5 µg/ml anti IL-10 receptor (3F9-2; DNAX), 5 µg/ml anti TGF-β (DNAX), or 5 µg/ml isotype control rat IgG2a (R35-9S; BD Biosciences Pharmingen) as indicated for 6 days. Propidium iodide (Sigma-Aldrich) was then added to exclude dead cells and 10,000 CD4-CFSE positive cells analyzed using a FACSCaliber flow cytometer (BD). The proliferation index was generated using ModFit LT software version 3.0 (Verity Software House). Alternatively, supernatants were harvested on day 3 and assayed by ELISA for cytokine content. For analysis of allergen-specific regulatory function, 14-day Th2 and IL-10 secreting Treg lines were generated. Cocultures contained the indicated combinations of Th2, IL-10 secreting Tregs, and autologous irradiated APCs, stimulated with 10 µg/ml allergen and, where indicated, 5 µg/ml anti IL-10 receptor or 5 µg/ml isotype control rat IgG 2a added. Supernatants were harvested on day 3 for analysis of cytokine content. Intracellular staining. On day 7 or day 7 plus 48 hours of a further anti-cd3 induced stimulation, cells were restimulated for 4 hours with 0.25 ng/ml PMA (Sigma-Aldrich) and 25 ng/ml ionomycin (Calbiochem), with monensin (Sigma-Aldrich) added for the final 2 hours. Cells were washed, fixed, and permeabilized using Cytofix/Cytoperm kit (BD Biosciences Pharmingen) and triple-stained with fluorescently labeled monoclonal antibodies to IL-10, IL-2, IL-4, IL-13, GM-CSF (all at 100 ng/sample), or IFN-γ (250 ng/sample) (BD Biosciences Pharmingen) (3, 25). Staining with isotype control antibodies (BD Biosciences Pharmingen) was performed in all experiments. Dead cells (7-aminoactinomycin D [7-AAD] positive; Sigma-Aldrich) were gated out and 10,000 live cells analyzed by flow cytometry. Cytokine analysis. IL-5, IL-10, IL-13, and IFN-γ were measured using ELISA and matched antibody pairs (BD Biosciences Pharmingen), with reference to commercial standards (R&D Systems). The lower limit of detection for IFN-γ and IL-10 was 50 pg/ml, for IL-5, 100 pg/ml, and for IL-13, 100 pg/ml. When less than 100 µl supernatant was available, a human Th1/Th2 cytometric bead array kit (BD Biosciences Pharmingen) was used. TGF-β1 was measured using the TGF-β1 Emax Immunoassay System (Promega). For assay of total active TGF-β1, samples were diluted 1:5, treated with 1 N HCl, then neutralized with 1 N NaOH. Vitamin D3 treatment of healthy and SR asthmatic volunteers. The study was approved by the Research Ethics Committee of Guy s Hospital and informed consent obtained from volunteers. Four healthy volunteers (1 female, 3 male; years old) and 3 SR patients (1 female, 2 male; years old) were given 0.5 µg/day ( µg) oral calcitriol for 7 days. PBMCs were obtained at days 0 (before treatment) and days 1, 3, and 7. Donors were always bled between 9:30 am and 10:30 am to control for any influence of diurnal changes. CD4 + T lymphocytes plus APCs were stimulated for 7 days with 1 µg/ml plate-bound anti-cd3, 50 U/ml IL-2, and varying concentrations of dexamethasone. On day 7, cells were washed and restimulated with PMA-ionomycin for 4 hours (cell pellets kept for mrna extraction) or cross-linked with anti-cd3 and IL-2 for a further 48 hours. Cells were harvested at 48 hours for intracellular staining for IL-10 and culture supernatants harvested for cytokine analysis. Western analysis of GR protein. CD4 + T cells were incubated with human recombinant IL-10 (R&D Systems), vitamin D3, and/or dexamethasone for the indicated periods (0 to 72 hours), then they were washed, soluble cell proteins were extracted, and Western blot was performed as previously described (56). GR was detected using a mouse monoclonal antibody (BD Transduction Laboratories; BD Biosciences Pharmingen) and goat antimouse horseradish peroxidase conjugated secondary antibody. Immunoreactive complexes were revealed by ECL (Amersham Biosciences) and visualized by autoradiography. Discrimination between GRα and GRβ was facilitated by the use of FLAG epitope tagged GRα and GRβ cdnas. An empty vector (pcdna3; Invitrogen Corp.) was used as a negative control. Overexpression of these cdnas in U293 cells permitted Western blotting with either anti-flag (Sigma-Aldrich; not shown) or anti-gr (56). The different migration of these 2 proteins and comparison with native protein allowed discrimination among spliced variants. Real-time RT-PCR. RNA was extracted from cell pellets using either the Absolutely RNA kit (Stratagene) or the RNeasy Mini kit (QIAGEN) according to the manufacturers instructions. The RNA was quantified using Ribogreen RNA quantification kit (Molecular Probes). For each sample, 250 ng of RNA was reverse transcribed in a total volume of 30 µl using random hexamer primers (Fermentas Life Sciences). Real-time RT- PCR was performed in triplicate using an Applied Biosystems 7900 HT system and carboxy fluorescein labeled (FAM-labeled) Assay-on-Demand reagent sets (Applera Corp.) for the following: FoxP3, Hs _m1; IL-10, Hs _m1; and TGF-β, Hs _m1. Real-time RT-PCR reactions were multiplexed using VIC-labeled 18s primers and probes (Hs _s1) as endogenous control and analyzed using SDS software version 2.1 (Applied Biosystems) according to the 2 ( Ct) method. Statistics. Results are presented as mean ± SEM. Differences between groups were assessed using the 2-tailed paired Student s t test. Differences were considered significant at the 95% confidence level. Acknowledgments E. Xystrakis and S. Kusumakar were funded by Asthma-UK (00/023 and 03/065). T. Adikibi was funded by a UK Medical Research Council Realising Our Potential Award. T.H. Lee and P. Lavender were funded by a UK Medical Research Council Programme Grant. Received for publication March 31, 2004, and accepted in revised form October 11, Address correspondence to: Catherine M. Hawrylowicz, Medical Research Council and Asthma-UK Centre in Allergic Mechanisms of Asthma, Department of Asthma, Allergy and Respiratory Science, Guy s Hospital, King s College London, London SE1 9RT, United Kingdom. Phone: ; Fax: ; catherine.hawrylowicz@kcl.ac.uk. Sandra Boswell and Emma Peek contributed equally to this work. Emmanuel Xystrakis and Siddharth Kusumakar are joint first authors. 154 The Journal of Clinical Investigation Volume 116 Number 1 January 2006
10 1. Wills-Karp, M Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 17: Umland, S.P., Schleimer, R.P., and Johnston, S.L Review of the molecular and cellular mechanisms of action of glucocorticoids for use in asthma. Pulm. Pharmacol. Ther. 15: Richards, D.F., Fernandez, M., Caulfield, J., and Hawrylowicz, C.M Glucocorticoids drive human CD8(+) T cell differentiation towards a phenotype with high IL-10 and reduced IL-4, IL-5 and IL-13 production. Eur. J. Immunol. 30: John, M., et al Inhaled corticosteroids increase interleukin-10 but reduce macrophage inflammatory protein-1a, granulocyte-macrophage colony stimulating factor and interferon-g release from alveolar macrophages in asthma. Am. J. Respir. Crit. Care Med. 157: Moore, K.W., de Waal Malefyt, R., Coffman, R.L., and O Garra, A Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19: Hawrylowicz, C.M., and O Garra, A Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 5: Borish, L., et al Interleukin-10 regulation in normal subjects and patients with asthma. J. Allergy Clin. Immunol. 97: Lim, S., Crawley, E., Woo, P., and Barnes, P.J Haplotype associated with low interleukin-10 production in patients with severe asthma [letter]. Lancet. 352: Akdis, M., et al Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 199: Heaton, T., et al An immunoepidemiological approach to asthma: identification of in-vitro T-cell response patterns associated with different wheezing phenotypes in children. Lancet. 365: Stampfli, M.R., et al Interleukin-10 gene transfer to the airway regulates allergic mucosal sensitization in mice. Am. J. Respir. Cell Mol. Biol. 21: Oh, J.W., et al CD4 T-helper cells engineered to produce IL-10 prevent allergen-induced airway hyperreactivity and inflammation. J. Allergy Clin. Immunol. 110: Corrigan, C.J., et al Glucocorticoid resistance in chronic asthma. Glucocorticoid pharmacokinetics, glucocorticoid receptor characteristics, and inhibition of peripheral blood T cell proliferation by glucocorticoids in vitro. Am. Rev. Respir. Dis. 144: Hawrylowicz, C., Richards, D., Loke, T.K., Corrigan, C., and Lee, T A defect in corticosteroid-induced IL-10 production in T lymphocytes from corticosteroid-resistant asthmatic patients. J. Allergy Clin. Immunol. 109: Kam, J.C., Szefler, S.J., Surs, W., Sher, E.R., and Leung, D.Y Combination IL-2 and IL-4 reduces glucocorticoid receptor-binding affinity and T cell response to glucocorticoids. J. Immunol. 151: Maloy, K.J., and Powrie, F Regulatory T cells in the control of immune pathology. Nat. Immunol. 2: Weiner, H.L Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol. Rev. 182: Sakaguchi, S., et al Immunologic self tolerance maintained by T-cell-mediated control of selfreactive T cells: implications for autoimmunity and tumor immunity. Microbes Infect. 3: Bacchetta, R., et al High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J. Exp. Med. 179: Groux, H., et al A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389: Sundstedt, A., et al Immunoregulatory role of IL-10 during superantigen-induced hyporesponsiveness in vivo. J. Immunol. 158: Papiernik, M., et al T cell deletion induced by chronic infection with mouse mammary tumor virus spares a CD25-positive, IL-10-producing T cell population with infectious capacity. J. Immunol. 158: Jonuleit, H., Schmitt, E., Schuler, G., Knop, J., and Enk, A.H Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192: Sundstedt, A., O Neill, E.J., Nicolson, K.S., and Wraith, D.C Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo. J. Immunol. 170: Barrat, F.J., et al In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 195: Vieira, P.L., et al IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J. Immunol. 172: Thornton, A.M., and Shevach, E.M CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188: Asseman, C., Mauze, S., Leach, M.W., Coffman, R.L., and Powrie, F An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190: Hara, M., et al IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J. Immunol. 166: Belkaid, Y., Piccirillo, C.A., Mendez, S., Shevach, E.M., and Sacks, D.L CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420: Ramsdell, F Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 19: Ritz, S.A., Stampfli, M.R., Davies, D.E., Holgate, S.T., and Jordana, M On the generation of allergic airway diseases: from GM-CSF to Kyoto. Trends Immunol. 23: Kang, S., et al Calcipotriene-induced improvement in psoriasis is associated with reduced interleukin-8 and increased interleukin- 10 levels within lesions. Br. J. Dermatol. 138: Burnstein, K.L., Jewell, C.M., and Cidlowski, J.A Human glucocorticoid receptor cdna contains sequences sufficient for receptor down-regulation. J. Biol. Chem. 265: Ling, E.M., et al Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 363: Lindgren, U., Lindholm, S., and Sarby, B Short-term effects of 1-alpha-hydroxy-vitamin D3 in patients on corticosteroid treatment and in patients with senile osteoporosis. Acta Med. Scand. 204: Peek, E.J., et al Interleukin-10-secreting regulatory T cells induced by glucocorticoids and beta2- agonists. Am. J. Respir. Cell Mol. Biol. 33: Franchimont, D., et al Tumor necrosis factor alpha decreases, and interleukin-10 increases, the sensitivity of human monocytes to dexamethasone: potential regulation of the glucocorticoid receptor. J. Clin. Endocrinol. Metab. 84: Torrego, A., et al Glucocorticoid receptor isoforms alpha and beta in in vitro cytokineinduced glucocorticoid insensitivity. Am. J. Respir. Crit. Care Med. 170: Sousa, A.R., Lane, S.J., Cidlowski, J.A., Staynov, D.Z., and Lee, T.H Glucocorticoid resistance in asthma is associated with elevated in vivo expression of the glucocorticoid receptor beta-isoform. J. Allergy Clin. Immunol. 105: Hamid, Q.A., et al Increased glucocorticoid receptor beta in airway cells of glucocorticoidinsensitive asthma. Am. J. Respir. Crit. Care Med. 159: Leung, D.Y., et al Association of glucocorticoid insensitivity with increased expression of glucocorticoid receptor beta. J. Exp. Med. 186: Schaaf, M.J., and Cidlowski, J.A Molecular mechanisms of glucocorticoid action and resistance. J. Steroid Biochem. Mol. Biol. 83: Chikanza, I.C Mechanisms of corticosteroid resistance in rheumatoid arthritis: a putative role for the corticosteroid receptor beta isoform. Ann. N. Y. Acad. Sci. 966: Akdis, C.A., Blesken, T., Akdis, M., Wuthrich, B., and Blaser, K Role of interleukin 10 in specific immunotherapy. J. Clin. Invest. 102: Burkhart, C., Liu, G.Y., Anderton, S.M., Metzler, B., and Wraith, D.C Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: a role for IL-10. Int. Immunol. 11: Faith, A., et al Impaired secretion of interleukin-4 and interleukin-13 by allergen-specific T cells correlates with defective nuclear expression of NF-AT2 and jun B: relevance to immunotherapy. Clin. Exp. Allergy. 33: Abramson, M.J., Puy, R.M., and Weiner, J.M Allergen immunotherapy for asthma. Cochrane Database Syst. Rev. 2003:CD doi: / cd Kappos, L., et al Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The Altered Peptide Ligand in Relapsing MS Study Group. Nat. Med. 6: Pedotti, R., et al Severe anaphylactic reactions to glutamic acid decarboxylase (GAD) self peptides in NOD mice that spontaneously develop autoimmune type 1 diabetes mellitus. BMC Immunol. 4:2. doi: / Cottrez, F., Hurst, S.D., Coffman, R.L., and Groux, H T regulatory cells 1 inhibit a Th2-specific response in vivo. J. Immunol. 165: Akbari, O., DeKruyff, R.H., and Umetsu, D.T Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2: Akbari, O., et al Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 8: Makela, M.J., et al The failure of interleukin- 10-deficient mice to develop airway hyperresponsiveness is overcome by respiratory syncytial virus infection in allergen-sensitized/challenged mice. Am. J. Respir. Crit. Care Med. 165: Lee, C.G., et al Transgenic overexpression of interleukin (IL)-10 in the lung causes mucus metaplasia, tissue inflammation, and airway remodeling via IL-13-dependent and -independent pathways. J. Biol. Chem. 277: Smith, P.J., et al Suppression of granulocytemacrophage colony-stimulating factor expression by glucocorticoids involves inhibition of enhancer function by the glucocorticoid receptor binding to composite NF-AT/activator protein-1 elements. J. Immunol. 167: The Journal of Clinical Investigation Volume 116 Number 1 January
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/23854 holds various files of this Leiden University dissertation. Author: Marel, Sander van der Title: Gene and cell therapy based treatment strategies
Cover Page The handle http://hdl.handle.net/1887/23854 holds various files of this Leiden University dissertation. Author: Marel, Sander van der Title: Gene and cell therapy based treatment strategies
Optimizing Intracellular Flow Cytometry:
 Optimizing Intracellular Flow Cytometry: Simultaneous Detection of Cytokines and Transcription Factors An encore presentation by Jurg Rohrer, PhD, BD Biosciences 10.26.10 Outline Introduction Cytokines
Optimizing Intracellular Flow Cytometry: Simultaneous Detection of Cytokines and Transcription Factors An encore presentation by Jurg Rohrer, PhD, BD Biosciences 10.26.10 Outline Introduction Cytokines
Human and mouse T cell regulation mediated by soluble CD52 interaction with Siglec-10. Esther Bandala-Sanchez, Yuxia Zhang, Simone Reinwald,
 Human and mouse T cell regulation mediated by soluble CD52 interaction with Siglec-1 Esther Bandala-Sanchez, Yuxia Zhang, Simone Reinwald, James A. Dromey, Bo Han Lee, Junyan Qian, Ralph M Böhmer and Leonard
Human and mouse T cell regulation mediated by soluble CD52 interaction with Siglec-1 Esther Bandala-Sanchez, Yuxia Zhang, Simone Reinwald, James A. Dromey, Bo Han Lee, Junyan Qian, Ralph M Böhmer and Leonard
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
MATERIALS AND METHODS. Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All
 MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice
 Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
PBMC from each patient were suspended in AIM V medium (Invitrogen) with 5% human
 Anti-CD19-CAR transduced T-cell preparation PBMC from each patient were suspended in AIM V medium (Invitrogen) with 5% human AB serum (Gemini) and 300 international units/ml IL-2 (Novartis). T cell proliferation
Anti-CD19-CAR transduced T-cell preparation PBMC from each patient were suspended in AIM V medium (Invitrogen) with 5% human AB serum (Gemini) and 300 international units/ml IL-2 (Novartis). T cell proliferation
Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD-
 Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Effector T Cells and
 1 Effector T Cells and Cytokines Andrew Lichtman, MD PhD Brigham and Women's Hospital Harvard Medical School 2 Lecture outline Cytokines Subsets of CD4+ T cells: definitions, functions, development New
1 Effector T Cells and Cytokines Andrew Lichtman, MD PhD Brigham and Women's Hospital Harvard Medical School 2 Lecture outline Cytokines Subsets of CD4+ T cells: definitions, functions, development New
Nature Medicine: doi: /nm.3922
 Title: Glucocorticoid-induced tumor necrosis factor receptor-related protein co-stimulation facilitates tumor regression by inducing IL-9-producing helper T cells Authors: Il-Kyu Kim, Byung-Seok Kim, Choong-Hyun
Title: Glucocorticoid-induced tumor necrosis factor receptor-related protein co-stimulation facilitates tumor regression by inducing IL-9-producing helper T cells Authors: Il-Kyu Kim, Byung-Seok Kim, Choong-Hyun
DNA vaccine, peripheral T-cell tolerance modulation 185
 Subject Index Airway hyperresponsiveness (AHR) animal models 41 43 asthma inhibition 45 overview 41 mast cell modulation of T-cells 62 64 respiratory tolerance 40, 41 Tregs inhibition role 44 respiratory
Subject Index Airway hyperresponsiveness (AHR) animal models 41 43 asthma inhibition 45 overview 41 mast cell modulation of T-cells 62 64 respiratory tolerance 40, 41 Tregs inhibition role 44 respiratory
Optimizing Intracellular Flow Cytometry:
 Optimizing Intracellular Flow Cytometry: Simultaneous Detection of Cytokines and Transcription Factors Presented by Jurg Rohrer, PhD, BD Biosciences 23-10780-00 Outline Introduction Cytokines Transcription
Optimizing Intracellular Flow Cytometry: Simultaneous Detection of Cytokines and Transcription Factors Presented by Jurg Rohrer, PhD, BD Biosciences 23-10780-00 Outline Introduction Cytokines Transcription
SUPPLEMENTARY INFORMATION
 Supplemental Figure 1. Furin is efficiently deleted in CD4 + and CD8 + T cells. a, Western blot for furin and actin proteins in CD4cre-fur f/f and fur f/f Th1 cells. Wild-type and furin-deficient CD4 +
Supplemental Figure 1. Furin is efficiently deleted in CD4 + and CD8 + T cells. a, Western blot for furin and actin proteins in CD4cre-fur f/f and fur f/f Th1 cells. Wild-type and furin-deficient CD4 +
of whole cell cultures in U-bottomed wells of a 96-well plate are shown. 2
 Supplementary online material Supplementary figure legends Supplementary Figure 1 Exposure to T reg cells causes loss of T resp cells in co-cultures. T resp cells were stimulated with CD3+CD28 alone or
Supplementary online material Supplementary figure legends Supplementary Figure 1 Exposure to T reg cells causes loss of T resp cells in co-cultures. T resp cells were stimulated with CD3+CD28 alone or
Bead Based Assays for Cytokine Detection
 Bead Based Assays for Cytokine Detection September 27, 2014 6 th EFIS-EJI South East European Immunology School SEEIS 2014 Timisoara, Romania The Cells of the Immune System The Immune Reaction (Th2) (Th1)
Bead Based Assays for Cytokine Detection September 27, 2014 6 th EFIS-EJI South East European Immunology School SEEIS 2014 Timisoara, Romania The Cells of the Immune System The Immune Reaction (Th2) (Th1)
Supplementary Data Table of Contents:
 Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to
 Class II tetramer staining Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to PE were combined with dominant HIV epitopes (DRB1*0101-DRFYKTLRAEQASQEV, DRB1*0301- PEKEVLVWKFDSRLAFHH,
Class II tetramer staining Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to PE were combined with dominant HIV epitopes (DRB1*0101-DRFYKTLRAEQASQEV, DRB1*0301- PEKEVLVWKFDSRLAFHH,
Mechanisms of allergen-specific immunotherapy
 2012 KAAACI/EAAS Spring Mechanisms of allergen-specific immunotherapy Woo-Jung Song, MD Division of Allergy and Clinical Immunology Department of Internal Medicine Seoul National University Hospital, Seoul,
2012 KAAACI/EAAS Spring Mechanisms of allergen-specific immunotherapy Woo-Jung Song, MD Division of Allergy and Clinical Immunology Department of Internal Medicine Seoul National University Hospital, Seoul,
NK cell flow cytometric assay In vivo DC viability and migration assay
 NK cell flow cytometric assay 6 NK cells were purified, by negative selection with the NK Cell Isolation Kit (Miltenyi iotec), from spleen and lymph nodes of 6 RAG1KO mice, injected the day before with
NK cell flow cytometric assay 6 NK cells were purified, by negative selection with the NK Cell Isolation Kit (Miltenyi iotec), from spleen and lymph nodes of 6 RAG1KO mice, injected the day before with
Central tolerance. Mechanisms of Immune Tolerance. Regulation of the T cell response
 Immunoregulation: A balance between activation and suppression that achieves an efficient immune response without damaging the host. Mechanisms of Immune Tolerance ACTIVATION (immunity) SUPPRESSION (tolerance)
Immunoregulation: A balance between activation and suppression that achieves an efficient immune response without damaging the host. Mechanisms of Immune Tolerance ACTIVATION (immunity) SUPPRESSION (tolerance)
Mechanisms of Immune Tolerance
 Immunoregulation: A balance between activation and suppression that achieves an efficient immune response without damaging the host. ACTIVATION (immunity) SUPPRESSION (tolerance) Autoimmunity Immunodeficiency
Immunoregulation: A balance between activation and suppression that achieves an efficient immune response without damaging the host. ACTIVATION (immunity) SUPPRESSION (tolerance) Autoimmunity Immunodeficiency
Supplementary Figure 1. Characterization of basophils after reconstitution of SCID mice
 Supplementary figure legends Supplementary Figure 1. Characterization of after reconstitution of SCID mice with CD4 + CD62L + T cells. (A-C) SCID mice (n = 6 / group) were reconstituted with 2 x 1 6 CD4
Supplementary figure legends Supplementary Figure 1. Characterization of after reconstitution of SCID mice with CD4 + CD62L + T cells. (A-C) SCID mice (n = 6 / group) were reconstituted with 2 x 1 6 CD4
Defining Asthma: Clinical Criteria. Defining Asthma: Bronchial Hyperresponsiveness
 Defining Asthma: Clinical Criteria Atopy 34% Recent wheeze 20% Asthma 11% AHR 19% n = 807 From: Woolcock, AJ. Asthma in Textbook of Respiratory Medicine, 2nd ed. Murray, Nadel, eds.(saunders:philadelphia)
Defining Asthma: Clinical Criteria Atopy 34% Recent wheeze 20% Asthma 11% AHR 19% n = 807 From: Woolcock, AJ. Asthma in Textbook of Respiratory Medicine, 2nd ed. Murray, Nadel, eds.(saunders:philadelphia)
Defining Asthma: Bronchial Hyperresponsiveness. Defining Asthma: Clinical Criteria. Impaired Ventilation in Asthma. Dynamic Imaging of Asthma
 Defining Asthma: Clinical Criteria Defining Asthma: Bronchial Hyperresponsiveness Atopy 34% Recent wheeze 20% Asthma 11% AHR 19% n = 807 From: Woolcock, AJ. Asthma in Textbook of Respiratory Medicine,
Defining Asthma: Clinical Criteria Defining Asthma: Bronchial Hyperresponsiveness Atopy 34% Recent wheeze 20% Asthma 11% AHR 19% n = 807 From: Woolcock, AJ. Asthma in Textbook of Respiratory Medicine,
Defining Asthma: Clinical Criteria. Defining Asthma: Bronchial Hyperresponsiveness
 Defining Asthma: Clinical Criteria Atopy 34% Recent wheeze 20% Asthma 11% AHR 19% n = 807 From: Woolcock, AJ. Asthma in Textbook of Respiratory Medicine, 2nd ed. Murray, Nadel, eds.(saunders:philadelphia)
Defining Asthma: Clinical Criteria Atopy 34% Recent wheeze 20% Asthma 11% AHR 19% n = 807 From: Woolcock, AJ. Asthma in Textbook of Respiratory Medicine, 2nd ed. Murray, Nadel, eds.(saunders:philadelphia)
Ex vivo Human Antigen-specific T Cell Proliferation and Degranulation Willemijn Hobo 1, Wieger Norde 1 and Harry Dolstra 2*
 Ex vivo Human Antigen-specific T Cell Proliferation and Degranulation Willemijn Hobo 1, Wieger Norde 1 and Harry Dolstra 2* 1 Department of Laboratory Medicine - Laboratory of Hematology, Radboud University
Ex vivo Human Antigen-specific T Cell Proliferation and Degranulation Willemijn Hobo 1, Wieger Norde 1 and Harry Dolstra 2* 1 Department of Laboratory Medicine - Laboratory of Hematology, Radboud University
Supplementary Fig. 1 p38 MAPK negatively regulates DC differentiation. (a) Western blot analysis of p38 isoform expression in BM cells, immature DCs
 Supplementary Fig. 1 p38 MAPK negatively regulates DC differentiation. (a) Western blot analysis of p38 isoform expression in BM cells, immature DCs (idcs) and mature DCs (mdcs). A myeloma cell line expressing
Supplementary Fig. 1 p38 MAPK negatively regulates DC differentiation. (a) Western blot analysis of p38 isoform expression in BM cells, immature DCs (idcs) and mature DCs (mdcs). A myeloma cell line expressing
The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells
 1 SUPPLEMENTARY INFORMATION The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells Karin Loser 1,2,6, Thomas Vogl 2,3, Maik Voskort 1, Aloys
1 SUPPLEMENTARY INFORMATION The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells Karin Loser 1,2,6, Thomas Vogl 2,3, Maik Voskort 1, Aloys
Searching for Targets to Control Asthma
 Searching for Targets to Control Asthma Timothy Craig Distinguished Educator Professor Medicine and Pediatrics Penn State University Hershey, PA, USA Inflammation and Remodeling in Asthma The most important
Searching for Targets to Control Asthma Timothy Craig Distinguished Educator Professor Medicine and Pediatrics Penn State University Hershey, PA, USA Inflammation and Remodeling in Asthma The most important
The Adaptive Immune Responses
 The Adaptive Immune Responses The two arms of the immune responses are; 1) the cell mediated, and 2) the humoral responses. In this chapter we will discuss the two responses in detail and we will start
The Adaptive Immune Responses The two arms of the immune responses are; 1) the cell mediated, and 2) the humoral responses. In this chapter we will discuss the two responses in detail and we will start
- 1 - Cell types Monocytes THP-1 cells Macrophages. LPS Treatment time (Hour) IL-6 level (pg/ml)
 Supplementary Table ST1: The dynamic effect of LPS on IL-6 production in monocytes and THP-1 cells after GdA treatment. Monocytes, THP-1 cells and macrophages (5x10 5 ) were incubated with 10 μg/ml of
Supplementary Table ST1: The dynamic effect of LPS on IL-6 production in monocytes and THP-1 cells after GdA treatment. Monocytes, THP-1 cells and macrophages (5x10 5 ) were incubated with 10 μg/ml of
Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and
 Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and Thy1 in NH cells derived from the lungs of naïve mice.
Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and Thy1 in NH cells derived from the lungs of naïve mice.
Optimizing Intracellular Flow Cytometry
 Optimizing Intracellular Flow Cytometry Detection of Cytokines, Transcription Factors, and Phosphoprotein by Flow Cytometry Presented by Erika O Donnell, PhD, BD Biosciences 23-14876-00 Outline Basic principles
Optimizing Intracellular Flow Cytometry Detection of Cytokines, Transcription Factors, and Phosphoprotein by Flow Cytometry Presented by Erika O Donnell, PhD, BD Biosciences 23-14876-00 Outline Basic principles
Supplementary Figure 1. IL-12 serum levels and frequency of subsets in FL patients. (A) IL-12
 1 Supplementary Data Figure legends Supplementary Figure 1. IL-12 serum levels and frequency of subsets in FL patients. (A) IL-12 serum levels measured by multiplex ELISA (Luminex) in FL patients before
1 Supplementary Data Figure legends Supplementary Figure 1. IL-12 serum levels and frequency of subsets in FL patients. (A) IL-12 serum levels measured by multiplex ELISA (Luminex) in FL patients before
The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23- induced production of the cytokine GM-CSF
 CORRECTION NOTICE Nat.Immunol. 12, 568 575 (2011) The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23- induced production of the cytokine GM-CSF Mohamed El-Behi, Bogoljub Ciric, Hong
CORRECTION NOTICE Nat.Immunol. 12, 568 575 (2011) The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23- induced production of the cytokine GM-CSF Mohamed El-Behi, Bogoljub Ciric, Hong
Tolerance, autoimmunity and the pathogenesis of immunemediated inflammatory diseases. Abul K. Abbas UCSF
 Tolerance, autoimmunity and the pathogenesis of immunemediated inflammatory diseases Abul K. Abbas UCSF Balancing lymphocyte activation and control Activation Effector T cells Tolerance Regulatory T cells
Tolerance, autoimmunity and the pathogenesis of immunemediated inflammatory diseases Abul K. Abbas UCSF Balancing lymphocyte activation and control Activation Effector T cells Tolerance Regulatory T cells
T Cell Effector Mechanisms I: B cell Help & DTH
 T Cell Effector Mechanisms I: B cell Help & DTH Ned Braunstein, MD The Major T Cell Subsets p56 lck + T cells γ δ ε ζ ζ p56 lck CD8+ T cells γ δ ε ζ ζ Cα Cβ Vα Vβ CD3 CD8 Cα Cβ Vα Vβ CD3 MHC II peptide
T Cell Effector Mechanisms I: B cell Help & DTH Ned Braunstein, MD The Major T Cell Subsets p56 lck + T cells γ δ ε ζ ζ p56 lck CD8+ T cells γ δ ε ζ ζ Cα Cβ Vα Vβ CD3 CD8 Cα Cβ Vα Vβ CD3 MHC II peptide
Detailed step-by-step operating procedures for NK cell and CTL degranulation assays
 Supplemental methods Detailed step-by-step operating procedures for NK cell and CTL degranulation assays Materials PBMC isolated from patients, relatives and healthy donors as control K562 cells (ATCC,
Supplemental methods Detailed step-by-step operating procedures for NK cell and CTL degranulation assays Materials PBMC isolated from patients, relatives and healthy donors as control K562 cells (ATCC,
Supplemental Information. T Cells Enhance Autoimmunity by Restraining Regulatory T Cell Responses via an Interleukin-23-Dependent Mechanism
 Immunity, Volume 33 Supplemental Information T Cells Enhance Autoimmunity by Restraining Regulatory T Cell Responses via an Interleukin-23-Dependent Mechanism Franziska Petermann, Veit Rothhammer, Malte
Immunity, Volume 33 Supplemental Information T Cells Enhance Autoimmunity by Restraining Regulatory T Cell Responses via an Interleukin-23-Dependent Mechanism Franziska Petermann, Veit Rothhammer, Malte
colorimetric sandwich ELISA kit datasheet
 colorimetric sandwich ELISA kit datasheet For the quantitative detection of human IL5 in serum, plasma, cell culture supernatants and urine. general information Catalogue Number Product Name Species cross-reactivity
colorimetric sandwich ELISA kit datasheet For the quantitative detection of human IL5 in serum, plasma, cell culture supernatants and urine. general information Catalogue Number Product Name Species cross-reactivity
Supplemental Materials for. Effects of sphingosine-1-phosphate receptor 1 phosphorylation in response to. FTY720 during neuroinflammation
 Supplemental Materials for Effects of sphingosine-1-phosphate receptor 1 phosphorylation in response to FTY7 during neuroinflammation This file includes: Supplemental Table 1. EAE clinical parameters of
Supplemental Materials for Effects of sphingosine-1-phosphate receptor 1 phosphorylation in response to FTY7 during neuroinflammation This file includes: Supplemental Table 1. EAE clinical parameters of
Tolerance 2. Regulatory T cells; why tolerance fails. Abul K. Abbas UCSF. FOCiS
 1 Tolerance 2. Regulatory T cells; why tolerance fails Abul K. Abbas UCSF FOCiS 2 Lecture outline Regulatory T cells: functions and clinical relevance Pathogenesis of autoimmunity: why selftolerance fails
1 Tolerance 2. Regulatory T cells; why tolerance fails Abul K. Abbas UCSF FOCiS 2 Lecture outline Regulatory T cells: functions and clinical relevance Pathogenesis of autoimmunity: why selftolerance fails
W/T Itgam -/- F4/80 CD115. F4/80 hi CD115 + F4/80 + CD115 +
 F4/8 % in the peritoneal lavage 6 4 2 p=.15 n.s p=.76 CD115 F4/8 hi CD115 + F4/8 + CD115 + F4/8 hi CD115 + F4/8 + CD115 + MHCII MHCII Supplementary Figure S1. CD11b deficiency affects the cellular responses
F4/8 % in the peritoneal lavage 6 4 2 p=.15 n.s p=.76 CD115 F4/8 hi CD115 + F4/8 + CD115 + F4/8 hi CD115 + F4/8 + CD115 + MHCII MHCII Supplementary Figure S1. CD11b deficiency affects the cellular responses
Mouse Total IgA Antibody Detection Kit
 Mouse Total IgA Antibody Detection Kit Catalog # 3019 For Research Use Only - Not Human or Therapeutic Use INTRODUCTION The total IgA levels in specimens are often determined in mouse disease models involving
Mouse Total IgA Antibody Detection Kit Catalog # 3019 For Research Use Only - Not Human or Therapeutic Use INTRODUCTION The total IgA levels in specimens are often determined in mouse disease models involving
Supplemental Figure 1. Signature gene expression in in vitro differentiated Th0, Th1, Th2, Th17 and Treg cells. (A) Naïve CD4 + T cells were cultured
 Supplemental Figure 1. Signature gene expression in in vitro differentiated Th0, Th1, Th2, Th17 and Treg cells. (A) Naïve CD4 + T cells were cultured under Th0, Th1, Th2, Th17, and Treg conditions. mrna
Supplemental Figure 1. Signature gene expression in in vitro differentiated Th0, Th1, Th2, Th17 and Treg cells. (A) Naïve CD4 + T cells were cultured under Th0, Th1, Th2, Th17, and Treg conditions. mrna
Supplemental Table I.
 Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
Cytokines modulate the functional activities of individual cells and tissues both under normal and pathologic conditions Interleukins,
 Cytokines http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter22/animation the_immune_response.html Cytokines modulate the functional activities of individual cells and tissues both under
Cytokines http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter22/animation the_immune_response.html Cytokines modulate the functional activities of individual cells and tissues both under
Supporting Information
 Supporting Information van der Windt et al. 10.1073/pnas.1221740110 SI Materials and Methods Mice and Reagents. C57BL/6 and major histocompatibility complex class I-restricted OVA-specific T-cell receptor
Supporting Information van der Windt et al. 10.1073/pnas.1221740110 SI Materials and Methods Mice and Reagents. C57BL/6 and major histocompatibility complex class I-restricted OVA-specific T-cell receptor
E-1 Role of IgE and IgE receptors in allergic airway inflammation and remodeling
 E-1 Role of IgE and IgE receptors in allergic airway inflammation and remodeling Ruby Pawankar, MD, Ph.D. FRCP, FAAAAI Prof. Div of Allergy, Dept of Pediatrics Nippon Medical School Tokyo, Japan pawankar.ruby@gmail.com
E-1 Role of IgE and IgE receptors in allergic airway inflammation and remodeling Ruby Pawankar, MD, Ph.D. FRCP, FAAAAI Prof. Div of Allergy, Dept of Pediatrics Nippon Medical School Tokyo, Japan pawankar.ruby@gmail.com
Supplementary Figure 1. Ex vivo IFNγ production by Tregs. Nature Medicine doi: /nm % CD127. Empty SSC 98.79% CD25 CD45RA.
 SSC CD25 1.8% CD127 Empty 98.79% FSC CD45RA CD45RA Foxp3 %IFNγ + cells 4 3 2 1 + IL-12 P =.3 IFNγ p=.9 %IL-4+ cells 3 2 1 IL-4 P =.4 c %IL-1 + cells IFNγ 4 3 2 1 Control Foxp3 IL-1 P =.41.64 4.76 MS 2.96
SSC CD25 1.8% CD127 Empty 98.79% FSC CD45RA CD45RA Foxp3 %IFNγ + cells 4 3 2 1 + IL-12 P =.3 IFNγ p=.9 %IL-4+ cells 3 2 1 IL-4 P =.4 c %IL-1 + cells IFNγ 4 3 2 1 Control Foxp3 IL-1 P =.41.64 4.76 MS 2.96
Role of Tyk-2 in Th9 and Th17 cells in allergic asthma
 Supplementary File Role of Tyk-2 in Th9 and Th17 cells in allergic asthma Caroline Übel 1*, Anna Graser 1*, Sonja Koch 1, Ralf J. Rieker 2, Hans A. Lehr 3, Mathias Müller 4 and Susetta Finotto 1** 1 Laboratory
Supplementary File Role of Tyk-2 in Th9 and Th17 cells in allergic asthma Caroline Übel 1*, Anna Graser 1*, Sonja Koch 1, Ralf J. Rieker 2, Hans A. Lehr 3, Mathias Müller 4 and Susetta Finotto 1** 1 Laboratory
Human Immunodeficiency Virus Type-1 Myeloid Derived Suppressor Cells Inhibit Cytomegalovirus Inflammation through Interleukin-27 and B7-H4
 Human Immunodeficiency Virus Type-1 Myeloid Derived Suppressor Cells Inhibit Cytomegalovirus Inflammation through Interleukin-27 and B7-H4 Ankita Garg, Rodney Trout and Stephen A. Spector,,* Department
Human Immunodeficiency Virus Type-1 Myeloid Derived Suppressor Cells Inhibit Cytomegalovirus Inflammation through Interleukin-27 and B7-H4 Ankita Garg, Rodney Trout and Stephen A. Spector,,* Department
Principles of Adaptive Immunity
 Principles of Adaptive Immunity Chapter 3 Parham Hans de Haard 17 th of May 2010 Agenda Recognition molecules of adaptive immune system Features adaptive immune system Immunoglobulins and T-cell receptors
Principles of Adaptive Immunity Chapter 3 Parham Hans de Haard 17 th of May 2010 Agenda Recognition molecules of adaptive immune system Features adaptive immune system Immunoglobulins and T-cell receptors
B6/COLODR/SPL/11C/83/LAP/#2.006 B6/COLODR/SPL/11C/86/LAP/#2.016 CD11C B6/COLODR/SPL/11C/80/LAP/#2.011 CD11C
 CD3-specific antibody-induced immune tolerance and suppression of autoimmune encephalomyelitis involves TGF-β production through phagocytes digesting apoptotic T cells Sylvain Perruche 1,3, Pin Zhang 1,
CD3-specific antibody-induced immune tolerance and suppression of autoimmune encephalomyelitis involves TGF-β production through phagocytes digesting apoptotic T cells Sylvain Perruche 1,3, Pin Zhang 1,
Supplemental Figure 1
 Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Supplemental Figures: Supplemental Figure 1
 Supplemental Figures: Supplemental Figure 1 Suppl. Figure 1. BM-DC infection with H. pylori does not induce cytotoxicity and treatment of BM-DCs with H. pylori sonicate, but not heat-inactivated bacteria,
Supplemental Figures: Supplemental Figure 1 Suppl. Figure 1. BM-DC infection with H. pylori does not induce cytotoxicity and treatment of BM-DCs with H. pylori sonicate, but not heat-inactivated bacteria,
HEK293FT cells were transiently transfected with reporters, N3-ICD construct and
 Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Naive, memory and regulatory T lymphocytes populations analysis
 Naive, memory and regulatory T lymphocytes populations analysis Jaen Olivier, PhD ojaen@beckmancoulter.com Cellular Analysis application specialist Beckman Coulter France Introduction Flow cytometric analysis
Naive, memory and regulatory T lymphocytes populations analysis Jaen Olivier, PhD ojaen@beckmancoulter.com Cellular Analysis application specialist Beckman Coulter France Introduction Flow cytometric analysis
Reviewers' comments: Reviewer #1 Expert in EAE and IL-17a (Remarks to the Author):
 Reviewers' comments: Reviewer #1 Expert in EAE and IL-17a (Remarks to the Author): This study shows that the inducible camp early repressor (ICER) is involved in development of Th17 cells that are pathogenic
Reviewers' comments: Reviewer #1 Expert in EAE and IL-17a (Remarks to the Author): This study shows that the inducible camp early repressor (ICER) is involved in development of Th17 cells that are pathogenic
ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS. Choompone Sakonwasun, MD (Hons), FRCPT
 ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS Choompone Sakonwasun, MD (Hons), FRCPT Types of Adaptive Immunity Types of T Cell-mediated Immune Reactions CTLs = cytotoxic T lymphocytes
ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS Choompone Sakonwasun, MD (Hons), FRCPT Types of Adaptive Immunity Types of T Cell-mediated Immune Reactions CTLs = cytotoxic T lymphocytes
Canberra, Australia). CD11c-DTR-OVA-GFP (B6.CD11c-OVA), B6.luc + and. Cancer Research Center, Germany). B6 or BALB/c.FoxP3-DTR-GFP mice were
 Supplemental Materials and Methods Mice Female C57BL/6 (B6, I-E null, H-2 b ), BALB/c (H-2 d ) + ), FVB/N (H-2 q, I-E null, CD45.1 + ), and B6D2F1 (H-2 b/d ) mice were purchased from the Animal Resources
Supplemental Materials and Methods Mice Female C57BL/6 (B6, I-E null, H-2 b ), BALB/c (H-2 d ) + ), FVB/N (H-2 q, I-E null, CD45.1 + ), and B6D2F1 (H-2 b/d ) mice were purchased from the Animal Resources
Immunology of Asthma. Kenneth J. Goodrum,Ph. Ph.D. Ohio University College of Osteopathic Medicine
 Immunology of Asthma Kenneth J. Goodrum,Ph Ph.D. Ohio University College of Osteopathic Medicine Outline Consensus characteristics/incidence data Immune/inflammatory basis Etiology/Genetic basis Hygiene
Immunology of Asthma Kenneth J. Goodrum,Ph Ph.D. Ohio University College of Osteopathic Medicine Outline Consensus characteristics/incidence data Immune/inflammatory basis Etiology/Genetic basis Hygiene
SUPPLEMENTARY INFORMATION. Involvement of IL-21 in the epidermal hyperplasia of psoriasis
 SUPPLEMENTARY INFORMATION Involvement of IL-21 in the epidermal hyperplasia of psoriasis Roberta Caruso 1, Elisabetta Botti 2, Massimiliano Sarra 1, Maria Esposito 2, Carmine Stolfi 1, Laura Diluvio 2,
SUPPLEMENTARY INFORMATION Involvement of IL-21 in the epidermal hyperplasia of psoriasis Roberta Caruso 1, Elisabetta Botti 2, Massimiliano Sarra 1, Maria Esposito 2, Carmine Stolfi 1, Laura Diluvio 2,
IMMUNOTHERAPY IN ALLERGIC RHINITIS
 Rhinology research Chair Weekly Activity, King Saud University IMMUNOTHERAPY IN ALLERGIC RHINITIS E V I D E N C E D - B A S E O V E R V I E W O F T H E R U L E O F I M M U N O T H E R A P Y I N A L L E
Rhinology research Chair Weekly Activity, King Saud University IMMUNOTHERAPY IN ALLERGIC RHINITIS E V I D E N C E D - B A S E O V E R V I E W O F T H E R U L E O F I M M U N O T H E R A P Y I N A L L E
Diagnosis and Management of Fungal Allergy Monday, 9-139
 Diagnosis and Management of Fungal Allergy Monday, 9-139 13-2010 Alan P. Knutsen,, MD Director, Pediatric Allergy & Immunology Director, Jeffrey Modell Diagnostic Center for Primary Immunodeficiencies
Diagnosis and Management of Fungal Allergy Monday, 9-139 13-2010 Alan P. Knutsen,, MD Director, Pediatric Allergy & Immunology Director, Jeffrey Modell Diagnostic Center for Primary Immunodeficiencies
Determinants of Immunogenicity and Tolerance. Abul K. Abbas, MD Department of Pathology University of California San Francisco
 Determinants of Immunogenicity and Tolerance Abul K. Abbas, MD Department of Pathology University of California San Francisco EIP Symposium Feb 2016 Why do some people respond to therapeutic proteins?
Determinants of Immunogenicity and Tolerance Abul K. Abbas, MD Department of Pathology University of California San Francisco EIP Symposium Feb 2016 Why do some people respond to therapeutic proteins?
TITLE: MODULATION OF T CELL TOLERANCE IN A MURINE MODEL FOR IMMUNOTHERAPY OF PROSTATIC ADENOCARCINOMA
 AD Award Number: DAMD17-01-1-0085 TITLE: MODULATION OF T CELL TOLERANCE IN A MURINE MODEL FOR IMMUNOTHERAPY OF PROSTATIC ADENOCARCINOMA PRINCIPAL INVESTIGATOR: ARTHUR A HURWITZ, Ph.d. CONTRACTING ORGANIZATION:
AD Award Number: DAMD17-01-1-0085 TITLE: MODULATION OF T CELL TOLERANCE IN A MURINE MODEL FOR IMMUNOTHERAPY OF PROSTATIC ADENOCARCINOMA PRINCIPAL INVESTIGATOR: ARTHUR A HURWITZ, Ph.d. CONTRACTING ORGANIZATION:
Tolerance 2. Regulatory T cells; why tolerance fails. FOCiS. Lecture outline. Regulatory T cells. Regulatory T cells: functions and clinical relevance
 1 Tolerance 2. Regulatory T cells; why tolerance fails Abul K. Abbas UCSF FOCiS 2 Lecture outline Regulatory T cells: functions and clinical relevance Pathogenesis of autoimmunity: why selftolerance fails
1 Tolerance 2. Regulatory T cells; why tolerance fails Abul K. Abbas UCSF FOCiS 2 Lecture outline Regulatory T cells: functions and clinical relevance Pathogenesis of autoimmunity: why selftolerance fails
Lecture outline. Immunological tolerance and immune regulation. Central and peripheral tolerance. Inhibitory receptors of T cells. Regulatory T cells
 1 Immunological tolerance and immune regulation Abul K. Abbas UCSF 2 Lecture outline Central and peripheral tolerance Inhibitory receptors of T cells Regulatory T cells 1 The immunological equilibrium:
1 Immunological tolerance and immune regulation Abul K. Abbas UCSF 2 Lecture outline Central and peripheral tolerance Inhibitory receptors of T cells Regulatory T cells 1 The immunological equilibrium:
CD25-PE (BD Biosciences) and labeled with anti-pe-microbeads (Miltenyi Biotec) for depletion of CD25 +
 Supplements Supplemental Materials and Methods Depletion of CD25 + T-cells from PBMC. Fresh or HD precultured PBMC were stained with the conjugate CD25-PE (BD Biosciences) and labeled with anti-pe-microbeads
Supplements Supplemental Materials and Methods Depletion of CD25 + T-cells from PBMC. Fresh or HD precultured PBMC were stained with the conjugate CD25-PE (BD Biosciences) and labeled with anti-pe-microbeads
B220 CD4 CD8. Figure 1. Confocal Image of Sensitized HLN. Representative image of a sensitized HLN
 B220 CD4 CD8 Natarajan et al., unpublished data Figure 1. Confocal Image of Sensitized HLN. Representative image of a sensitized HLN showing B cell follicles and T cell areas. 20 µm thick. Image of magnification
B220 CD4 CD8 Natarajan et al., unpublished data Figure 1. Confocal Image of Sensitized HLN. Representative image of a sensitized HLN showing B cell follicles and T cell areas. 20 µm thick. Image of magnification
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Chapter 13: Cytokines
 Chapter 13: Cytokines Definition: secreted, low-molecular-weight proteins that regulate the nature, intensity and duration of the immune response by exerting a variety of effects on lymphocytes and/or
Chapter 13: Cytokines Definition: secreted, low-molecular-weight proteins that regulate the nature, intensity and duration of the immune response by exerting a variety of effects on lymphocytes and/or
Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538
 Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538 Background: TIGIT is a co-inhibitory receptor that is highly expressed in Natural Killer (NK) cells, activated CD4+, CD8+ and regulatory
Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538 Background: TIGIT is a co-inhibitory receptor that is highly expressed in Natural Killer (NK) cells, activated CD4+, CD8+ and regulatory
BD CBA on the BD Accuri C6: Bringing Multiplexed Cytokine Detection to the Benchtop
 BD CBA on the BD Accuri C6: Bringing Multiplexed Cytokine Detection to the Benchtop Maria Dinkelmann, PhD Senior Marketing Applications Specialist BD Biosciences, Ann Arbor, MI 23-14380-00 Cellular Communication
BD CBA on the BD Accuri C6: Bringing Multiplexed Cytokine Detection to the Benchtop Maria Dinkelmann, PhD Senior Marketing Applications Specialist BD Biosciences, Ann Arbor, MI 23-14380-00 Cellular Communication
Following T-cell activation and differentiation with HTRF reagents: IL-2, IFN-γ and IL-17
 Following T-cell activation and differentiation with HTRF reagents: IL-2, IFN-γ and IL-17 4 th HTRF Symposium for Drug Discovery Avignon, Sept. 24-26, 28 Introduction: T-cells have effector and helper
Following T-cell activation and differentiation with HTRF reagents: IL-2, IFN-γ and IL-17 4 th HTRF Symposium for Drug Discovery Avignon, Sept. 24-26, 28 Introduction: T-cells have effector and helper
SUPPLEMENTARY INFORMATION
 Complete but curtailed T-cell response to very-low-affinity antigen Dietmar Zehn, Sarah Y. Lee & Michael J. Bevan Supp. Fig. 1: TCR chain usage among endogenous K b /Ova reactive T cells. C57BL/6 mice
Complete but curtailed T-cell response to very-low-affinity antigen Dietmar Zehn, Sarah Y. Lee & Michael J. Bevan Supp. Fig. 1: TCR chain usage among endogenous K b /Ova reactive T cells. C57BL/6 mice
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supporting Information
 Supporting Information Bellora et al. 10.1073/pnas.1007654108 SI Materials and Methods Cells. NK cells purified from peripheral blood mononuclear cells (PBMC) of healthy donors (Human NK Cell Isolation
Supporting Information Bellora et al. 10.1073/pnas.1007654108 SI Materials and Methods Cells. NK cells purified from peripheral blood mononuclear cells (PBMC) of healthy donors (Human NK Cell Isolation
well for 2 h at rt. Each dot represents an individual mouse and bar is the mean ±
 Supplementary data: Control DC Blimp-1 ko DC 8 6 4 2-2 IL-1β p=.5 medium 8 6 4 2 IL-2 Medium p=.16 8 6 4 2 IL-6 medium p=.3 5 4 3 2 1-1 medium IL-1 n.s. 25 2 15 1 5 IL-12(p7) p=.15 5 IFNγ p=.65 4 3 2 1
Supplementary data: Control DC Blimp-1 ko DC 8 6 4 2-2 IL-1β p=.5 medium 8 6 4 2 IL-2 Medium p=.16 8 6 4 2 IL-6 medium p=.3 5 4 3 2 1-1 medium IL-1 n.s. 25 2 15 1 5 IL-12(p7) p=.15 5 IFNγ p=.65 4 3 2 1
Impact of Asthma in the U.S. per Year. Asthma Epidemiology and Pathophysiology. Risk Factors for Asthma. Childhood Asthma Costs of Asthma
 American Association for Respiratory Care Asthma Educator Certification Prep Course Asthma Epidemiology and Pathophysiology Robert C. Cohn, MD, FAARC MetroHealth Medical Center Cleveland, OH Impact of
American Association for Respiratory Care Asthma Educator Certification Prep Course Asthma Epidemiology and Pathophysiology Robert C. Cohn, MD, FAARC MetroHealth Medical Center Cleveland, OH Impact of
Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel
 Supplementary Figures 1-8 Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel Marc Schmidt 1,2, Badrinarayanan Raghavan 1,2, Verena Müller 1,2, Thomas Vogl 3, György
Supplementary Figures 1-8 Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel Marc Schmidt 1,2, Badrinarayanan Raghavan 1,2, Verena Müller 1,2, Thomas Vogl 3, György
Mouse Anti-OVA IgM Antibody Assay Kit
 Mouse Anti-OVA IgM Antibody Assay Kit Catalog # 3017 For Research Use Only - Not Human or Therapeutic Use INTRODUCTION Ovalbumin (OVA) is a widely used antigen for inducing allergic reactions in experimental
Mouse Anti-OVA IgM Antibody Assay Kit Catalog # 3017 For Research Use Only - Not Human or Therapeutic Use INTRODUCTION Ovalbumin (OVA) is a widely used antigen for inducing allergic reactions in experimental
Supplemental Information. CD4 + CD25 + Foxp3 + Regulatory T Cells Promote. Th17 Cells In Vitro and Enhance Host Resistance
 Immunity, Volume 34 Supplemental Information D4 + D25 + + Regulatory T ells Promote Th17 ells In Vitro and Enhance Host Resistance in Mouse andida albicans Th17 ell Infection Model Pushpa Pandiyan, Heather
Immunity, Volume 34 Supplemental Information D4 + D25 + + Regulatory T ells Promote Th17 ells In Vitro and Enhance Host Resistance in Mouse andida albicans Th17 ell Infection Model Pushpa Pandiyan, Heather
Vasoactive intestinal peptide inhibits IFN-α secretion and modulates the immune function of plasmacytoid dendritic cells
 Excerpt from MACS&more Vol 12 1/21 Vasoactive intestinal peptide inhibits IFN-α secretion and modulates the immune function of plasmacytoid dendritic cells Dorit Fabricius 1, 2,, M. Sue O Dorisio 1, 2,
Excerpt from MACS&more Vol 12 1/21 Vasoactive intestinal peptide inhibits IFN-α secretion and modulates the immune function of plasmacytoid dendritic cells Dorit Fabricius 1, 2,, M. Sue O Dorisio 1, 2,
Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells
 ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
CD90 + Human Dermal Stromal Cells Are Potent Inducers of FoxP3 + Regulatory T Cells
 CD90 + Human Dermal Stromal Cells Are Potent Inducers of FoxP3 + Regulatory T Cells Karin Pfisterer, Karoline M Lipnik, Erhard Hofer and Adelheid Elbe-Bürger Journal of Investigative Dermatology (2015)
CD90 + Human Dermal Stromal Cells Are Potent Inducers of FoxP3 + Regulatory T Cells Karin Pfisterer, Karoline M Lipnik, Erhard Hofer and Adelheid Elbe-Bürger Journal of Investigative Dermatology (2015)
Supplementary Figures
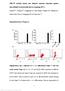 MiR-29 controls innate and adaptive immune responses against intracellular bacterial infection by targeting IFN-γ Feng Ma 1,2,5, Sheng Xu 1,5, Xingguang Liu 1, Qian Zhang 1, Xiongfei Xu 1, Mofang Liu 3,
MiR-29 controls innate and adaptive immune responses against intracellular bacterial infection by targeting IFN-γ Feng Ma 1,2,5, Sheng Xu 1,5, Xingguang Liu 1, Qian Zhang 1, Xiongfei Xu 1, Mofang Liu 3,
Cytokine Responses during Exacerbation Compared with Stable Phase in Asthmatic Children
 ASIAN PACIFIC JOURNAL OF ALLERGY AND IMMUNOLOGY (2010) 28: 35-40 Cytokine Responses during Exacerbation Compared with Stable Phase in Asthmatic Children Yingwan Charoenying 1, Wasu Kamchaisatian 2, Kalayanee
ASIAN PACIFIC JOURNAL OF ALLERGY AND IMMUNOLOGY (2010) 28: 35-40 Cytokine Responses during Exacerbation Compared with Stable Phase in Asthmatic Children Yingwan Charoenying 1, Wasu Kamchaisatian 2, Kalayanee
Simultaneous correlation of cytokine production with Treg and Th17 cell proliferation
 Simultaneous correlation of cytokine production with Treg and Th17 cell proliferation Jurg Rohrer, PhD Director, R&D BD Biosciences 23-11773-00 For Research Use Only. Not for use in diagnostic or therapeutic
Simultaneous correlation of cytokine production with Treg and Th17 cell proliferation Jurg Rohrer, PhD Director, R&D BD Biosciences 23-11773-00 For Research Use Only. Not for use in diagnostic or therapeutic
Immunology of Asthma. Kenneth J. Goodrum,Ph. Ph.D. Ohio University College of Osteopathic Medicine
 Immunology of Asthma Kenneth J. Goodrum,Ph Ph.D. Ohio University College of Osteopathic Medicine Outline! Consensus characteristics! Allergens:role in asthma! Immune/inflammatory basis! Genetic basis!
Immunology of Asthma Kenneth J. Goodrum,Ph Ph.D. Ohio University College of Osteopathic Medicine Outline! Consensus characteristics! Allergens:role in asthma! Immune/inflammatory basis! Genetic basis!
Therapeutic effect of baicalin on experimental autoimmune encephalomyelitis. is mediated by SOCS3 regulatory pathway
 Therapeutic effect of baicalin on experimental autoimmune encephalomyelitis is mediated by SOCS3 regulatory pathway Yuan Zhang 1,2, Xing Li 1,2, Bogoljub Ciric 1, Cun-gen Ma 3, Bruno Gran 4, Abdolmohamad
Therapeutic effect of baicalin on experimental autoimmune encephalomyelitis is mediated by SOCS3 regulatory pathway Yuan Zhang 1,2, Xing Li 1,2, Bogoljub Ciric 1, Cun-gen Ma 3, Bruno Gran 4, Abdolmohamad
Supplementary Figure 1
 d f a IL7 b IL GATA RORγt h HDM IL IL7 PBS Ilra R7 PBS HDM Ilra R7 HDM Foxp Foxp Ilra R7 HDM HDM Ilra R7 HDM. 9..79. CD + FOXP + T reg cell CD + FOXP T conv cell PBS Ilra R7 PBS HDM Ilra R7 HDM CD + FOXP
d f a IL7 b IL GATA RORγt h HDM IL IL7 PBS Ilra R7 PBS HDM Ilra R7 HDM Foxp Foxp Ilra R7 HDM HDM Ilra R7 HDM. 9..79. CD + FOXP + T reg cell CD + FOXP T conv cell PBS Ilra R7 PBS HDM Ilra R7 HDM CD + FOXP
York criteria, 6 RA patients and 10 age- and gender-matched healthy controls (HCs).
 MATERIALS AND METHODS Study population Blood samples were obtained from 15 patients with AS fulfilling the modified New York criteria, 6 RA patients and 10 age- and gender-matched healthy controls (HCs).
MATERIALS AND METHODS Study population Blood samples were obtained from 15 patients with AS fulfilling the modified New York criteria, 6 RA patients and 10 age- and gender-matched healthy controls (HCs).
Supplementary Figures. T Cell Factor-1 initiates T helper 2 fate by inducing GATA-3 and repressing Interferon-γ
 Supplementary Figures T Cell Factor-1 initiates T helper 2 fate by inducing GATA-3 and repressing Interferon-γ Qing Yu, Archna Sharma, Sun Young Oh, Hyung-Geun Moon, M. Zulfiquer Hossain, Theresa M. Salay,
Supplementary Figures T Cell Factor-1 initiates T helper 2 fate by inducing GATA-3 and repressing Interferon-γ Qing Yu, Archna Sharma, Sun Young Oh, Hyung-Geun Moon, M. Zulfiquer Hossain, Theresa M. Salay,
Supplementary Figure 1 IL-27 IL
 Tim-3 Supplementary Figure 1 Tc0 49.5 0.6 Tc1 63.5 0.84 Un 49.8 0.16 35.5 0.16 10 4 61.2 5.53 10 3 64.5 5.66 10 2 10 1 10 0 31 2.22 10 0 10 1 10 2 10 3 10 4 IL-10 28.2 1.69 IL-27 Supplementary Figure 1.
Tim-3 Supplementary Figure 1 Tc0 49.5 0.6 Tc1 63.5 0.84 Un 49.8 0.16 35.5 0.16 10 4 61.2 5.53 10 3 64.5 5.66 10 2 10 1 10 0 31 2.22 10 0 10 1 10 2 10 3 10 4 IL-10 28.2 1.69 IL-27 Supplementary Figure 1.
Immunology Lecture 4. Clinical Relevance of the Immune System
 Immunology Lecture 4 The Well Patient: How innate and adaptive immune responses maintain health - 13, pg 169-181, 191-195. Immune Deficiency - 15 Autoimmunity - 16 Transplantation - 17, pg 260-270 Tumor
Immunology Lecture 4 The Well Patient: How innate and adaptive immune responses maintain health - 13, pg 169-181, 191-195. Immune Deficiency - 15 Autoimmunity - 16 Transplantation - 17, pg 260-270 Tumor
Statins and control of MHC2TA gene transcription
 Statins and CIITA 4 4 Statins and control of MHC2TA gene transcription 4 Hedwich F. Kuipers and Peter J. van den Elsen Nature Medicine, 2005, 11: 365-366 Statins and control of MHC2TA gene transcription
Statins and CIITA 4 4 Statins and control of MHC2TA gene transcription 4 Hedwich F. Kuipers and Peter J. van den Elsen Nature Medicine, 2005, 11: 365-366 Statins and control of MHC2TA gene transcription
<10. IL-1β IL-6 TNF + _ TGF-β + IL-23
 3 ns 25 ns 2 IL-17 (pg/ml) 15 1 ns ns 5 IL-1β IL-6 TNF
3 ns 25 ns 2 IL-17 (pg/ml) 15 1 ns ns 5 IL-1β IL-6 TNF
Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell?
 Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
