Switch from systemic steroids to budesonide in steroid dependent patients with inactive Crohn s disease
|
|
|
- Rudolf Rose
- 5 years ago
- Views:
Transcription
1 86 Service de Gastroenterologie, Hôpital Claude Huriez, Lille, France A Cortot J-F Colombel University Hospital of Leuven, Belgium P Rutgeerts Medical University Hospital of Odense, Denmark K Lauritsen Medizinische Klinik II, Städtisches Krankenhaus Leverkusen, Germany H Malchow Krankenhaus Tabea, Hamburg, Germany J Hämling Groote Schuur Hospital, Cape Town, South Africa T Winter Service de Gastroentérologie, Hôpital Erasme, Bruxelles, Belgium A Van Gossum Clinical Research and Development, AstraZeneca R&D, Lund, Sweden T Persson Eva Pettersson Members of the study group are listed in the appendix. Correspondence to: A Cortot, Service de Gastroenterologie, Hôpital Claude Huriez F-5937 Lille, France. a-cortot@chru-lille.fr Accepted for publication 8 August Switch from systemic steroids to budesonide in steroid dependent patients with inactive Crohn s disease A Cortot, J-F Colombel, P Rutgeerts, K Lauritsen, H Malchow, J Hämling, T Winter, A Van Gossum, T Persson, E Pettersson, for the International Study Group Abstract Background Steroid dependent patients with Crohn s disease are at high risk of developing glucocorticosteroid induced side evects. Aims We evaluated the possibility of switching from systemic steroids to budesonide (Entocort) in prednisolone/ prednisone dependent patients with inactive Crohn s disease avecting the ileum and/or ascending colon. Patients Steroid dependent patients with a Crohn s disease activity index < were included. Methods In a double blind multicentre trial, patients were randomly assigned to receive budesonide 6 mg once daily or placebo. Prednisolone was tapered to zero during the first weeks and budesonide or placebo was given concomitantly and for a further weeks. Relapse was defined as an index > and an increase of 6 points from baseline or withdrawal due to disease deterioration. Results After one and 3 weeks without prednisolone, relapse rates were 7% and 3%, respectively, in the budesonide group, and % and 65% in the placebo group (95% confidence intervals for the diverence in percentages %, 8% and 5%, 6%; p=. and p<., respectively). The number of glucocorticosteroid side evects was reduced by 5% by switching from prednisolone and was similar in the budesonide and placebo groups. Basal plasma cortisol increased in both groups. Conclusions The majority of patients with steroid dependent ileocaecal Crohn s disease may be switched to budesonide controlled ileal release capsules 6 mg without relapse, resulting in a sharp decrease in glucocorticosteroid side effects similar to placebo, and with an increase in plasma cortisol levels. (Gut ;8:86 9) Keywords: budesonide; Crohn s disease; steroid dependent; prednisolone Crohn s disease (CD) is an inflammatory bowel disorder often initiated by an acute attack and may become chronic in some patients. Systemic glucocorticosteroids (GCS), such as prednisolone and prednisone, are commonly used in the treatment of acute attacks of the disease. Approximately one third of Gut ;8:86 9 patients initially treated with GCS become GCS dependent: they remain in remission while receiving GCS but relapse when they are stopped. 3 However, this treatment is often associated with serious side evects: metabolic (diabetes, hypertension, osteopenia) or cosmetic (moon face, hirsutism, acne). is a GCS with a marked topical anti-inflammatory evect and low systemic activity compared with conventional GCS because of its marked potency combined with a high first pass metabolism (about 9%) to metabolites with minimal or no steroid activity. (Entocort) controlled ileal release (CIR) capsules deliver the drug mainly to the ileum and ascending colon. This overs a new topical steroid treatment for patients with ileocolic CD, with a reduced risk of GCS induced side evects. In a placebo controlled dose finding study, budesonide CIR capsules were significantly more evective than placebo in inducing remission of active CD avecting the ileum and/or ascending colon, with an optimal dose of 9 mg daily. 5 9 mg daily has also been found to be as evective as oral prednisolone 6 7 but with fewer GCS induced side evects. In addition, budesonide CIR capsules have been shown to significantly prolong the time to relapse in patients with quiescent disease. 8 We evaluated the evect of replacing prednisolone/prednisone with budesonide in steroid dependent patients with the possibility of improved tolerability. Materials and methods PATIENT SELECTION The study was performed between April 996 and October 998. Eligible patients were at least 8 years of age and had inactive CD, as defined by a Crohn s disease activity index (CDAI) <. The index assesses eight variables: number of liquid stools, extent of abdominal pain, general well being, occurrence of extraintestinal symptoms, need for antidiarrhoeal drugs, presence of abdominal masses, haematocrit, and body weight. Relapse was defined as a score > and an increase of at least 6 points from baseline. Disease extent had to be confined mainly to the distal ileum, Abbreviations used in this paper: 5-ASA, 5-aminosalicylate; CD, Crohn s disease; CDAI, Crohn s disease activity index; CIR, controlled ileal release; GCS, glucocorticosteroids; IBDQ, inflammatory bowel disease questionnaire; QoL, quality of life.
2 in steroid dependent Crohn s disease 87 ileocaecal region, and/or ascending colon, except for scattered aphthous ulcers, and verified (by colonoscopy, small bowel follow through, or barium enema) within months before randomisation. Patients with any major manifestations of CD in the rectum were not eligible, nor were those with septic complications, abscesses, perforations or active fistulae, ileostomy, pouch or colostomy, resection of the ileum of more than cm, or who required immediate surgery. All patients had been receiving prednisolone or prednisone 3 mg/day, six months prior to the study, and at least two attempts to taper the dose with subsequent relapses had occurred. Patients receiving immunosuppressive or 5-aminosalicylate (5-ASA) treatment had started this treatment at least six months or one month, respectively, prior to the study and the dose had to be kept constant during the study. Patients treated with steroids in combination with azathioprine were initially excluded. However, to speed up inclusion of patients into the study, the inclusion criteria were changed during the study to allow the combination of steroids and azathioprine. Pregnant or breast feeding women and patients allergic to GCS were excluded. The study was approved by the institutional review board at each centre and was conducted according to the principles of the second declaration of Helsinki. All patients gave written informed consent. STUDY MEDICATION The budesonide formulation used (Entocort capsules, 3 mg, AstraZeneca R&D, Lund, Sweden) is a gelatin capsule containing acid stable microgranules (each approximately mm). The microgranules are composed of an inner sugar core surrounded by a layer of budesonide in ethylcellulose and an outer acrylic based resin coating (Eudragit L 55) that dissolves at a ph of 5.5 or higher. capsules were identical in appearance and taste. TRIAL DESIGN The trial was a randomised double blind study performed at centres in six countries (Belgium, Denmark, France, Germany, Israel, and South Africa). Randomisation of patients in permuted blocks of four was performed separately for each centre using sealed and opaque treatment code envelopes. Treatment was scheduled for 6 weeks. Tapering of prednisolone (5 mg per week until mg and thereafter.5 mg per week until the dose was reduced to zero) started concomitantly with intake of budesonide 6 mg once daily or placebo. Compliance was assessed by pill count. At entry, patient demographics and medical history, current and past diagnoses, and current medication were recorded. Sigmoidoscopy was performed to exclude major sigmoidorectal inflammation. CDAI was assessed before treatment and every four weeks until the prednisolone dose had been zero for a week, and then after a further six and weeks or at the time of withdrawal for patients who discontinued the study. At the visits, a physical examination, quality of life (QoL) assessment, laboratory tests, physician s global evaluation, and an adverse events check were also performed. A short Synacthen test was performed at 8 am within hours of the start of the study and after the last intake of study medication. Loperamide or other opiates to control diarrhoea were allowed. QoL was assessed using the self administered inflammatory bowel disease questionnaire (IBDQ) which contains 3 questions each with seven possible answers numbered 7, where 7 is the most favourable. Ten questions are related to bowel movements, five to systemic symptoms, to emotional functions, and five to social functions. The total score of the index is 3 (an increase in score is an improvement). QoL was also assessed by SF-36 with 36 questions where, in this study, the physical component summary and the mental component summary were used. 3 The questionnaire is constructed to give a value of 5 (for mental and physical component summary scores) for the average US population. A high value is favourable. Patients recorded on diary cards their intake of study medication, frequency of loose stools, abdominal pain, and general well being during the seven days before each visit. Blood samples were obtained for laboratory assessments: haematology, clinical chemistry, liver function tests, and indicators of inflammatory activity (erythrocyte sedimentation rate and C-reactive protein). Blood was drawn for measurements of plasma cortisol at the time of intravenous Synacthen (tetracosactrin, Ciba-Geigy) administration and 3 and 6 minutes later. A normal response was defined as a baseline plasma cortisol concentration of at least 5. µg/dl (5 nmol/l) and an increment above baseline of at least 7. µg/dl ( nmol/l), or an absolute value above. µg/dl ( nmol/l) at 3 or 6 minutes. All adverse events were recorded, whether or not they were considered to be related to the study medication. GCS related side evects were actively asked for (moon face, buvalo hump, acne, hirsutism, purple skin striae, bruises easily, swelling of ankles, hair loss, mood swings, depression, and insomnia). A serious adverse event was defined as one which suggested a significant hazard or handicap to the patient (death, permanent disability, cancer, hospitalisation) or was life threatening. Adverse events were assessed for intensity using the scale mild, moderate, or severe (where severe was incapacitating with inability to work or to take part in normal activities). The number of possibly GCS related side evects was recorded at each visit and their intensity graded from to 3. Patients could be withdrawn from the study at any time if their physicians believed their condition had deteriorated substantially. In such cases, CDAI was assessed. STATISTICAL ANALYSIS It was estimated that 5 patients per group would have to be studied to detect a 3%
3 88 Cortot, Colombel, Rutgeerts, et al Table Characteristic Baseline characteristics of the patients group (n=59) group (n=58) Mean Range Mean Range Sex (M/F) 8/3 /38 Race (Caucasian/mixed) 5/5 53/5 Age (y) 35 (9 7) 3 (8 66) Height (cm) 69 (5 93) 68 (8 87) Weight (kg) 67 (6 9) 65 ( 6) CDAI 3 ( 8) 9 ( 5 9) Disease duration (y) 8.9 ( 5) 8. ( 6) Disease site (ileum/ileocaecal/colon) 8/9/ 3/8/ Previous resection (yes/no) 8/ /37 Time since resection (y) 3.7 ( ).5 ( 5) Mean length of resection (cm) 3 ( 8) 6 (5 ) Use of 5-ASA 9 8 Mean dose among users (g).8.7 Use of azathioprine 9 5 Mean dose among users (mg) 39 5 Initial prednisolone dose (mg) 6.5 ( 3) 5.7 ( 3) No of patients with initial dose <5/5 </ 3 mg 8/7/ 9/8/ Figure n = 59 Analysed for efficacy n = 59 Analysed for safety n = 59 Treatment failure Adverse events Other reason Randomised n = 3 Flow chart of patients and reasons for discontinuation. Never treated n = n = 59 (Tuberculosis incl.) Analysed for efficacy n = 58 Analysed for safety n = 59 Treatment failure Adverse events Other reason 3 diverence in relapse rates, assuming a relapse rate of 5% with budesonide treatment. The primary outcome was relapse rate, with relapse defined as a score of > and an increase of 6 points from baseline in CDAI, or withdrawal due to disease deterioration. The primary analysis was based on all patients treated with at least one dose of study medication. Exploratory analyses (determined in advance) investigated the influence of prognostic factors on relapse rates by two way analysis of variance, with initial prednisolone dose, treatment, subgroup (for example, sex), and their interaction as factors. Secondary variables were () changes in CDAI, () time to relapse, (3) quantitative changes in QoL index, and () changes in safety variables (such as plasma cortisol, GCS side evects, and adverse events). The last value carried forward approach was used for missing data that is, the value last recorded for the patient was used in the analysis. If a patient suvered a relapse according to the above definition, they were registered as having relapsed for the remaining study period. The χ test was used to compare proportions. Time to discontinuation and time to relapse were analysed by Kaplan-Meier curves and a generalised Wilcoxon test. The Student s t test, Wilcoxon test, and analysis of variance were used for quantitative variables. All tests. 6 mg No of days in study Figure Time to relapse in the budesonide and placebo groups. Fraction of patients without relapse IBD questionaire score mg 3 Study start week 3 weeks Figure 3 Scores from the inflammatory bowel disease (IBD) questionnaire for the budesonide and placebo groups. were two sided; p values not exceeding 5% were considered significant. The adverse events profile was analysed by means of descriptive statistics and qualitative analysis. Results A total of patients were randomly assigned to receive budesonide (6 patients) or placebo (6 patients). One hundred and eighteen patients took at least one dose of study drug. One patient was excluded from the eycacy analysis because of tuberculosis, which was discovered after the patient had completed the study. Baseline characteristics were similar in the two groups (table ). The mean time during which patients had taken prednisolone before the start of the study was 5 days in the budesonide group and 36 days in the placebo group. A flow chart describing the patients in the study is shown in fig. No of glucocorticoid symptoms 3 Study start 7 3 weeks Figure Number of glucocorticosteroid related symptoms in the budesonide and placebo groups.
4 in steroid dependent Crohn s disease 89 Table Moon face intensity Study start 7 3 weeks Figure 5 Average intensity of moon face at each visit in the budesonide and placebo groups. Acne intensity Study start 7 3 weeks Figure 6 Average intensity of acne at each visit in the budesonide and placebo groups. CLINICAL EFFICACY Relapse rates were lower in the budesonide group than in the placebo group; after one and 3 weeks without prednisolone, and 9 patients, respectively, had suvered a relapse (7% and 3%) in the budesonide group and and 38 patients (% and 65%) in the placebo group (p=. and p<.). Sex, previous intestinal resection, initial prednisolone dose, and use of azathioprine or 5-ASA treatment had no influence on relapse rates. Median time to relapse was significantly longer for budesonide (>6 days) than for placebo (75 days) (p<.) (fig ). Total averages for IBDQ scores are given in fig 3. The scores were similar in the two treatment groups at the start of the study. IBDQ score was always better in the budesonide Adverse events in the budesonide and placebo groups No of treated patients Treatment failure 3 3 Discontinuations due to adverse events Discontinuations due to other reasons Most frequent adverse events (tapering period/after tapering of prednisolone) Cushing syndrome 68/53 53/59 Arthralgia 7/6 / C-reactive protein increased /3 7/ Abdominal pain 8/ /5 CD aggravated 7/3 7/6 Fever 7/6 /5 Arthritis 7/6 /3 Headache 7/9 3/3 Diarrhoea 5/ /3 Back pain 5/6 7/8 group than in the placebo group either at one or 3 weeks, achieving a significant diverence (p=.5) at week 3. Improvement occurred in emotional and social function scores. For SF-36, there was a discrepancy between the evolution of the physical component summary which improved significantly (p=.5) at week 3 and the mental component summary which did not diver between treatment groups during the study. No clinically important diverences between treatments were found in the assessments of haematological and biochemical variables. ADVERSE EVENTS There was a positive correlation between initial prednisolone dose and baseline GCS side evects (p<.). The number of side evects was reduced from the start of the study (when patients were receiving prednisolone) in both the budesonide and placebo groups (fig ). For individual side evects, average intensity observed at each visit decreased mainly in moon face (fig 5), acne (fig 6), insomnia, and mood swings. An intensity score of represents mild intensity. The number of patients with adverse events was similar in the two groups; the most frequent events are shown in table. All serious adverse events were due to hospitalisation of patients. No serious adverse events were considered causally related to the study drug. ADRENAL FUNCTION Mean morning unstimulated plasma cortisol increased by 5. µg/dl (39 nmol/l) from the first to the last visit in the budesonide group (n=37) and by 9.3 µg/dl (57 nmol/l) in the placebo group (n=3). This increase in unstimulated plasma cortisol from baseline (prednisolone treatment) was statistically significant for both budesonide (p=.7) and placebo (p<.). According to our criteria (including basal and stimulated cortisol level), in the budesonide group, 3/37 patients had abnormal adrenal function at entry. Of those 3, (5%) recovered normal function at the last visit. In the placebo group of 3 patients, 3 had abnormal adrenal function of whom 9 (83%) recovered normal function at the last visit. Discussion In this study, budesonide capsules (6 mg once daily) were more evective than placebo in maintaining remission in steroid dependent patients with inactive ileocaecal CD when systemic steroid was tapered to zero. Thus systemic steroid dependent patients could be switched to budesonide with a limited risk of relapse (68% without relapse after 3 weeks of budesonide). The majority of flare ups due to a change in steroids should appear within three months given the tough criterion for steroid dependency which implied that patients were in constant need of steroids. Sex, previous intestinal resection, initial prednisolone dose, and use of azathioprine or 5-ASA treatment did not influence relapse rate. Including patients receiving steroids in combination with
5 9 Cortot, Colombel, Rutgeerts, et al azathioprine was initially regarded as inadvisable because of the risk of confusing the study. To speed up inclusions into the study however, patients who were receiving azathioprine were eventually included, resulting in a few patients in both groups. Previous experience of replacement of systemic steroids with budesonide in CD was limited to a shorter study using a diverent formulation and no placebo group. 5 There is greater experience of the use of budesonide in patients achieving remission after treatment, and subsequently being treated with budesonide 3 or 6 mg/day or placebo. 6 mg/day significantly prolonged the time in remission by 9 days compared with placebo. In chronic systemic steroid dependent asthmatics, a similar finding (inhaled budesonide had a prednisolone sparing evect) was reported. 6 Systemic steroids are an important part of the therapy alternatives in CD but their use is limited by side evects, particularly in steroid dependent patients. The use of systemic steroids has a negative impact on QoL in patients with CD, correlated with the dose received by the patient. 7 One of the motives behind switching from systemic steroids to budesonide was to reduce the steroid induced side evects, particularly the so called cosmetic evects (moon face, acne, easy bruising, hirsutism) and the intensity of mood swing and insomnia. These in part resulted in a global improvement in QoL in both groups. However, QoL improved more in the budesonide group than in the placebo group, probably reflecting the better control of disease activity in the budesonide group. At the start of the study there were more patients with abnormal than with normal adrenal function and this changed towards a higher number of patients with normal than with abnormal function in both groups at the last visit (numerically higher in the placebo group). Average plasma cortisol levels increased in both groups over the course of the study. Switching from systemic steroids to budesonide (6 mg/day) may be proposed in steroid dependent patients with inactive ileocaecal CD and such a switch has the potential to decrease GCS associated side evects and produce a beneficial evect on the HPA axis in these patients. Azathioprine/6-mercaptopurine and methotrexate are currently regarded as the standard therapy in this situation. 8 could however be proposed in patients receiving steroids in whom immunosuppressors are temporarily inevective or contraindicated. In the light of the results of our study, budesonide could be evaluated as an alternative to standard therapy. Supported by AstraZeneca R&D, Lund, Sweden. Appendix In addition to the authors, the following participated in the international budesonide study group: Hopital Nord, Amiens, France (J Dupas); Hopital Charles Nicolle, Rouen, France (E Lerebours); Hopital Saint- Louis, Paris, France (R Modigliani); Hopital Hautepierre, Strasbourg, France (B Duclos); Hopital Rotschild, Paris, France (J Gendre); Hopital Cochin, Paris, France (S Chaussade); Hopital Henri Mondor, Creteil, France (J Delchier); Hospital OLV Middelares, Antwerpen, Belgium (O Peters); Tygerberg Hospital, Cape Town, South Africa (C van Rensburg); Soroka Hospital, Beer- Sheva, Israel (A Fich); Asaf-Harofeh Medical centre, Zrifin, Israel (E Scapa); Haemek Medical Centre, Afula, Israel (E Nussinson); Kaplan Hospital, Rehovot, Israel (D Bass); Medizinische Universität, Lubeck, Germany (E Stange); Medizinische Hochschule, Hannover, Germany (M Göke); Aalborg Sygehus, Aalborg, Denmark (J Fallingborg); Herlev Hospital, University of Copenhagen, Denmark (O Ø Thomsen); Hillerod Sygehus, Hillerod, Denmark (S Kiilerich). Meyers S, Sachar DB, Medical management of Crohn s disease. Hepatogastroenterology 99;37: 55. Summers RW, Switz DM, Sessions JT, et al. National Cooperative Crohn s Disease Study. Gastroenterology 979;77: Munkholm P, Langholz E, Davidsen M, et al. Frequency of glucocorticoid resistance and dependency in Crohn s disease. Gut 99;35:36. Brattsand R. Overview of newer glucocorticosteroid preparations for inflammatory bowel disease. Can J Gastroenterol 99;:7. 5 Greenberg GR, Feagan BG, Martin F, et al. Oral budesonide for active Crohn s disease. N Engl J Med 99; 33: Rutgeerts P, Löfberg R, Malchow H, et al. A comparison of budesonide with prednisolone for active Crohn s disease. N Engl J Med 99;33: Campieri M, Ferguson A, Doe W, et al. Oral budesonide is as evective as oral prednisolone in active Crohn s disease. Gut 997;:9. 8 Löfberg R, Rutgeerts P, Malchow H, et al. prolongs time to relapse in ileal and ileocaecal Crohn s disease. A placebo controlled one year study. Gut 996;39: Greenberg GR, Feagan BG, Martin F, et al. Oral budesonide as maintenance treatment for Crohn s disease: A placebo-controlled, dose-ranging study. Gastroenterology 996;:5 5. Feagan B, Greenberg GR, Löfberg R, et al. controlled ileal release prolongs remission in Crohn s disease: A pooled analysis. Gastroenterology 997;:A97. Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn s disease activity index. National Cooperative Crohn s Disease Study. Gastroenterology 976;7:39. Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 989;96:8. 3 Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). Med Care 99;3: Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley & Sons Inc., 98: 9. 5 Gross V, Caesar I, Andus T, et al. Replacement of systemic steroids by oral budesonide in patients with post-active or chronic Crohn s ileocolitis. A dose-finding study. Gastroenterology 997;:A Shapiro G, Mendelson L, Kraemer MJ, et al. EYcacy and safety of budesonide inhalation suspension (Pulmicort Respules) in young children with inhaled steroiddependent persistent asthma. J Allergy Clin Immunol 998; : Colombel JF, Chircop C, Blondel-Kucharski F, et al. Quality of life in Crohn s disease: a prospective longitudinal study in 3 patients. Gastroenterology 999;6:A Pearson DC, May GR, Fick GH, et al. Azathioprine and 6-mercaptopurine in Crohn s disease. A meta-analysis. Ann Intern Med 995;:3. 9 Feagan B, Rochon J, Fedorak RN, et al. Methotrexate for the treatment of Crohn s disease. N Engl J Med 995;33: 9 7. Rutgeerts P. Medical therapy of inflammatory bowel disease. Digestion 998;59:53 69.
The New England Journal of Medicine A COMPARISON OF BUDESONIDE AND MESALAMINE FOR ACTIVE CROHN S DISEASE. Selection of Patients
 A COMPARISON OF BUDESONIDE AND MESALAMINE FOR ACTIVE CROHN S DISEASE OLE ØSTERGAARD THOMSEN, M.D., D.M.SCI., ANTOINE CORTOT, M.D., PH.D., DEREK JEWELL, M.A., D.PHIL., JOHN P. WRIGHT, M.D., PH.D., TREVOR
A COMPARISON OF BUDESONIDE AND MESALAMINE FOR ACTIVE CROHN S DISEASE OLE ØSTERGAARD THOMSEN, M.D., D.M.SCI., ANTOINE CORTOT, M.D., PH.D., DEREK JEWELL, M.A., D.PHIL., JOHN P. WRIGHT, M.D., PH.D., TREVOR
Efficacy and Safety of Budesonide CIR versus Prednisolone in Children with Active Crohn s Disease.
 CLINICAL STUDY REPORT DRUG SUBSTANCE DOCUMENT NO. VERSION NO. 01 STUDY CODE Budesonide SD-008-CR-3037 SD-008-3037 DATE 23 April, 2001 FINAL Efficacy and Safety of Budesonide CIR versus Prednisolone in
CLINICAL STUDY REPORT DRUG SUBSTANCE DOCUMENT NO. VERSION NO. 01 STUDY CODE Budesonide SD-008-CR-3037 SD-008-3037 DATE 23 April, 2001 FINAL Efficacy and Safety of Budesonide CIR versus Prednisolone in
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 3 October 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 October 2012 REMICADE 100 mg, powder for concentrate for solution for infusion B/1 vial (CIP code: 562 070-1) Applicant:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 October 2012 REMICADE 100 mg, powder for concentrate for solution for infusion B/1 vial (CIP code: 562 070-1) Applicant:
Budesonide in the management of patients with Crohn s disease.
 REVIEW Budesonide in the management of patients with Crohn s disease ABR THOMSON MD PhD FRCPC FACG, DANIEL SADOWSKI MD FRCPC, ROBERT JENKINS PhD, GARY WILD MDCM PhD FRCPC FACP ABR THOMSON, D SADOWSKI,
REVIEW Budesonide in the management of patients with Crohn s disease ABR THOMSON MD PhD FRCPC FACG, DANIEL SADOWSKI MD FRCPC, ROBERT JENKINS PhD, GARY WILD MDCM PhD FRCPC FACP ABR THOMSON, D SADOWSKI,
Until the late 1990s, treatment of Crohn s disease was primarily aimed at
 CHALLENGES IN CROHN S DISEASE An Historical Overview of the Treatment of Crohn s Disease: Why Do We Need Biological Therapies? Paul J. Rutgeerts, MD, PhD, FRCP Faculty of Medicine, Gastroenterology Section,
CHALLENGES IN CROHN S DISEASE An Historical Overview of the Treatment of Crohn s Disease: Why Do We Need Biological Therapies? Paul J. Rutgeerts, MD, PhD, FRCP Faculty of Medicine, Gastroenterology Section,
Moderately to severely active ulcerative colitis
 Adalimumab in the Treatment of Moderate-to-Severe Ulcerative Colitis: ULTRA 2 Trial Results Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients
Adalimumab in the Treatment of Moderate-to-Severe Ulcerative Colitis: ULTRA 2 Trial Results Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients
Crohn's Disease. The What, When, and Why of Treatment
 Crohn's Disease The What, When, and Why of Treatment Gary R. Lichtenstein, MD, FACG Professor of Medicine Director, Inflammatory Bowel Disease Program University of Pennsylvania Philadelphia, PA In my
Crohn's Disease The What, When, and Why of Treatment Gary R. Lichtenstein, MD, FACG Professor of Medicine Director, Inflammatory Bowel Disease Program University of Pennsylvania Philadelphia, PA In my
Efficacy and Safety of Treatment for Pediatric IBD
 Efficacy and Safety of Treatment for Pediatric IBD Andrew B. Grossman MD Co-Director, Center for Pediatric Inflammatory Bowel Disease Assistant Professor of Clinical Pediatrics Division of Gastroenterology,
Efficacy and Safety of Treatment for Pediatric IBD Andrew B. Grossman MD Co-Director, Center for Pediatric Inflammatory Bowel Disease Assistant Professor of Clinical Pediatrics Division of Gastroenterology,
Clinical guideline Published: 10 October 2012 nice.org.uk/guidance/cg152
 Crohn's disease: management Clinical guideline Published: 10 October 2012 nice.org.uk/guidance/cg152 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Crohn's disease: management Clinical guideline Published: 10 October 2012 nice.org.uk/guidance/cg152 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Old and new steroids: hitting the target
 Oxford Inflammatory Bowel Disease & Hepatology MasterClass Old and new steroids: hitting the target Silvio Danese, MD, PhD IBD Center Division of Gastroenterology Istituto Clinico Humanitas, Milan, Italy
Oxford Inflammatory Bowel Disease & Hepatology MasterClass Old and new steroids: hitting the target Silvio Danese, MD, PhD IBD Center Division of Gastroenterology Istituto Clinico Humanitas, Milan, Italy
Review article: medical treatment of mild to moderately active Crohn s disease
 Aliment Pharmacol Ther 2003; 17 (Suppl. 2): 18 22. Review article: medical treatment of mild to moderately active Crohn s disease R. LÖFBERG Karolinska Institutet, IBD-unit at HMQ Sophia Hospital, Stockholm,
Aliment Pharmacol Ther 2003; 17 (Suppl. 2): 18 22. Review article: medical treatment of mild to moderately active Crohn s disease R. LÖFBERG Karolinska Institutet, IBD-unit at HMQ Sophia Hospital, Stockholm,
Efficacy and Safety of Treatment for Pediatric IBD
 Efficacy and Safety of Treatment for Pediatric IBD Andrew B. Grossman MD Co-Director, Center for Pediatric Inflammatory Bowel Disease Associate Professor of Clinical Pediatrics Division of Gastroenterology,
Efficacy and Safety of Treatment for Pediatric IBD Andrew B. Grossman MD Co-Director, Center for Pediatric Inflammatory Bowel Disease Associate Professor of Clinical Pediatrics Division of Gastroenterology,
Doncaster & Bassetlaw Medicines Formulary
 Doncaster & Bassetlaw Medicines Formulary Section 1.5 Chronic Bowel Disorders (including IBD) Aminosalicylates: Mesalazine 400mg and 800mg MR Tablets (Octasa) Mesalazine 1.2g MR Tablets (Mezavant XL) Mesalazine
Doncaster & Bassetlaw Medicines Formulary Section 1.5 Chronic Bowel Disorders (including IBD) Aminosalicylates: Mesalazine 400mg and 800mg MR Tablets (Octasa) Mesalazine 1.2g MR Tablets (Mezavant XL) Mesalazine
Mucosal Healing in Crohn s Disease. Geert D Haens MD, PhD University Hospital Gasthuisberg University of Leuven Leuven, Belgium
 Mucosal Healing in Crohn s Disease Geert D Haens MD, PhD University Hospital Gasthuisberg University of Leuven Leuven, Belgium Mucosal Lesions in CD: General Features CD can affect the entire GI tract
Mucosal Healing in Crohn s Disease Geert D Haens MD, PhD University Hospital Gasthuisberg University of Leuven Leuven, Belgium Mucosal Lesions in CD: General Features CD can affect the entire GI tract
T he treatment strategy for Crohn s disease (CD) is
 237 INFLAMMATORY BOWEL DISEASE Impact of the increasing use of immunosuppressants in Crohn s disease on the need for intestinal surgery J Cosnes, I Nion-Larmurier, L Beaugerie, P Afchain, E Tiret, J-P
237 INFLAMMATORY BOWEL DISEASE Impact of the increasing use of immunosuppressants in Crohn s disease on the need for intestinal surgery J Cosnes, I Nion-Larmurier, L Beaugerie, P Afchain, E Tiret, J-P
Budesonide Use and Hospitalization Rate in Crohn s Disease: Results From a Cohort at a Tertiary Care IBD Referral Center
 Elmer ress Original Article J Clin Med Res. 2016;8(10):705-709 Budesonide Use and Hospitalization Rate in Crohn s Disease: Results From a Cohort at a Tertiary Care IBD Referral Center Jordan Orr a, d,
Elmer ress Original Article J Clin Med Res. 2016;8(10):705-709 Budesonide Use and Hospitalization Rate in Crohn s Disease: Results From a Cohort at a Tertiary Care IBD Referral Center Jordan Orr a, d,
Crohn's disease. Appendix J. Clinical Guideline < > 10 October Review of Cochrane ASA review
 Crohn's disease Clinical Guideline < > Review of Cochrane ASA review 0 October 202 Commissioned by the National Institute for Health and Clinical Excellence Review of Cochrane 5-ASA review Contents Published
Crohn's disease Clinical Guideline < > Review of Cochrane ASA review 0 October 202 Commissioned by the National Institute for Health and Clinical Excellence Review of Cochrane 5-ASA review Contents Published
Mucosal healing: does it really matter?
 Oxford Inflammatory Bowel Disease MasterClass Mucosal healing: does it really matter? Professor Jean-Frédéric Colombel, New York, USA Oxford Inflammatory Bowel Disease MasterClass Mucosal healing: does
Oxford Inflammatory Bowel Disease MasterClass Mucosal healing: does it really matter? Professor Jean-Frédéric Colombel, New York, USA Oxford Inflammatory Bowel Disease MasterClass Mucosal healing: does
Crohn's Disease. The What, When, and Why of Treatment
 Crohn's Disease The What, When, and Why of Treatment Brian Feagan, MD, FACG Professor of Medicine and Epidemiology and Biostatistics Director, Robarts Clinical Trials Robarts Research Institute University
Crohn's Disease The What, When, and Why of Treatment Brian Feagan, MD, FACG Professor of Medicine and Epidemiology and Biostatistics Director, Robarts Clinical Trials Robarts Research Institute University
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 20 October 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 20 October 2010 MEZAVANT LP 1200 mg, prolonged-release gastro-resistant tablets B/60 (CIP code: 378 689-2) Applicant
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 20 October 2010 MEZAVANT LP 1200 mg, prolonged-release gastro-resistant tablets B/60 (CIP code: 378 689-2) Applicant
What is ulcerative colitis?
 What is ulcerative colitis? Ulcerative colitis is a disease that causes inflammation and sores, called ulcers, in the lining of the rectum and colon. Ulcers form where inflammation has killed the cells
What is ulcerative colitis? Ulcerative colitis is a disease that causes inflammation and sores, called ulcers, in the lining of the rectum and colon. Ulcers form where inflammation has killed the cells
WHAT IS ULCERATIVE COLITIS?
 235 60th Street, West New York, NJ 07093 T: (201) 854-4646 F: (201) 854-4647 810 Main Street, Hackensack, NJ 07601 T: (201) 488-0095 Ulcerative Colitis WHAT IS ULCERATIVE COLITIS? Ulcerative colitis is
235 60th Street, West New York, NJ 07093 T: (201) 854-4646 F: (201) 854-4647 810 Main Street, Hackensack, NJ 07601 T: (201) 488-0095 Ulcerative Colitis WHAT IS ULCERATIVE COLITIS? Ulcerative colitis is
How do I choose amongst medicines for inflammatory bowel disease. Maria T. Abreu, MD
 How do I choose amongst medicines for inflammatory bowel disease Maria T. Abreu, MD Overview of IBD Pathogenesis Bacterial Products Moderately Acutely Inflamed Chronic Inflammation = IBD Normal Gut Mildly
How do I choose amongst medicines for inflammatory bowel disease Maria T. Abreu, MD Overview of IBD Pathogenesis Bacterial Products Moderately Acutely Inflamed Chronic Inflammation = IBD Normal Gut Mildly
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency London, 22 February 2007 Doc. Ref. CPMP/EWP/2284/99 Rev. 1 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON THE DEVELOPMENT OF NEW MEDICINAL PRODUCTS FOR
European Medicines Agency London, 22 February 2007 Doc. Ref. CPMP/EWP/2284/99 Rev. 1 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON THE DEVELOPMENT OF NEW MEDICINAL PRODUCTS FOR
CLINICAL TRIAL PROTOCOL
 CLINICAL TRIAL PROTOCOL Azathioprine maintenance therapy in steroid-refractory Ulcerative Colitis responsive to i.v. Cyclosporine A: Is a therapeutic bridge with oral Cyclosporine A necessary? Miquel A.
CLINICAL TRIAL PROTOCOL Azathioprine maintenance therapy in steroid-refractory Ulcerative Colitis responsive to i.v. Cyclosporine A: Is a therapeutic bridge with oral Cyclosporine A necessary? Miquel A.
Position of Biologics in IBD Circa 2006: Top Down vs. Step Up Therapy
 Position of Biologics in IBD Circa 2006: Top Down vs. Step Up Therapy Stephen B. Hanauer, MD University of Chicago Potential Conflicts: Centocor/Schering, Abbott, UCB, Elan, Berlex, PDL Goals of Treatment
Position of Biologics in IBD Circa 2006: Top Down vs. Step Up Therapy Stephen B. Hanauer, MD University of Chicago Potential Conflicts: Centocor/Schering, Abbott, UCB, Elan, Berlex, PDL Goals of Treatment
Budesonide Versus Mesalamine for Maintaining Remission in Patients Refusing Other Immunomodulators for Steroid-Dependent Crohn s Disease
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2003;1:122 128 Budesonide Versus Mesalamine for Maintaining Remission in Patients Refusing Other Immunomodulators for Steroid-Dependent Crohn s Disease GERASSIMOS
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2003;1:122 128 Budesonide Versus Mesalamine for Maintaining Remission in Patients Refusing Other Immunomodulators for Steroid-Dependent Crohn s Disease GERASSIMOS
INFLAMMATORY BOWEL DISEASE
 1. Medical Condition INFLAMMATORY BOWEL DISEASE (IBD) specifically includes Crohn s disease (CD) and ulcerative colitis (UC) but also includes IBD unclassified (IBDu), seen in about 10% of cases. These
1. Medical Condition INFLAMMATORY BOWEL DISEASE (IBD) specifically includes Crohn s disease (CD) and ulcerative colitis (UC) but also includes IBD unclassified (IBDu), seen in about 10% of cases. These
New treatment options in UC. Rob Bryant IBD Consultant Royal Adelaide Hospital
 New treatment options in UC Rob Bryant IBD Consultant Royal Adelaide Hospital Talk Outline 1. Raising expectations 2. Optimising UC therapy 3. Clinical trials 4. What s new on the PBS? 5. Questions 1.
New treatment options in UC Rob Bryant IBD Consultant Royal Adelaide Hospital Talk Outline 1. Raising expectations 2. Optimising UC therapy 3. Clinical trials 4. What s new on the PBS? 5. Questions 1.
Predicting the natural history of IBD. Séverine Vermeire, MD, PhD Department of Gastroenterology University Hospital Leuven Belgium
 Predicting the natural history of IBD Séverine Vermeire, MD, PhD Department of Gastroenterology University Hospital Leuven Belgium Patient 1 Patient 2 Age 22 Frequent cramps and diarrhea for 6 months Weight
Predicting the natural history of IBD Séverine Vermeire, MD, PhD Department of Gastroenterology University Hospital Leuven Belgium Patient 1 Patient 2 Age 22 Frequent cramps and diarrhea for 6 months Weight
2. SYNOPSIS. Clinical Study Report CD-LAQ-201. November 2012
 2. SYNOPSIS Protocol No.: Study Title A Phase IIA, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Sequential Cohorts, Dose Range Finding Study to Evaluate the Safety, Tolerability and Clinical
2. SYNOPSIS Protocol No.: Study Title A Phase IIA, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Sequential Cohorts, Dose Range Finding Study to Evaluate the Safety, Tolerability and Clinical
The London Gastroenterology Partnership CROHN S DISEASE
 CROHN S DISEASE What is Crohn s disease? Crohn s disease is a condition, in which inflammation develops in parts of the gut leading to symptoms such as diarrhoea, abdominal pain and tiredness. The inflammation
CROHN S DISEASE What is Crohn s disease? Crohn s disease is a condition, in which inflammation develops in parts of the gut leading to symptoms such as diarrhoea, abdominal pain and tiredness. The inflammation
IBD Case Studies. David Rowbotham. Clinical Director & Consultant Gastroenterologist Dept of Gastroenterology & Hepatology Auckland City Hospital
 IBD Case Studies David Rowbotham Clinical Director & Consultant Gastroenterologist Dept of Gastroenterology & Hepatology Auckland City Hospital Dr David Rowbotham The Leeds Teaching Hospitals NHS Trust
IBD Case Studies David Rowbotham Clinical Director & Consultant Gastroenterologist Dept of Gastroenterology & Hepatology Auckland City Hospital Dr David Rowbotham The Leeds Teaching Hospitals NHS Trust
Crohn's disease CAUSES COURSE OF CROHN'S DISEASE TREATMENT. Sulfasalazine
 Crohn's disease Crohn's disease is an inflammatory condition of the digestive tract that affects children and adults. Common features of Crohn's disease include mouth sores, diarrhea, abdominal pain, weight
Crohn's disease Crohn's disease is an inflammatory condition of the digestive tract that affects children and adults. Common features of Crohn's disease include mouth sores, diarrhea, abdominal pain, weight
Azathioprine for Induction and Maintenance of Remission in Crohn s Disease
 Azathioprine for Induction and Maintenance of Remission in Crohn s Disease William J. Sandborn, MD Chief, Division of Gastroenterology Director, UCSD IBD Center Objectives Azathioprine as induction and
Azathioprine for Induction and Maintenance of Remission in Crohn s Disease William J. Sandborn, MD Chief, Division of Gastroenterology Director, UCSD IBD Center Objectives Azathioprine as induction and
Treatment of Inflammatory Bowel Disease. Michael Weiss MD, FACG
 Treatment of Inflammatory Bowel Disease Michael Weiss MD, FACG What is IBD? IBD is an immune-mediated chronic intestinal disorder, characterized by chronic or relapsing inflammation within the GI tract.
Treatment of Inflammatory Bowel Disease Michael Weiss MD, FACG What is IBD? IBD is an immune-mediated chronic intestinal disorder, characterized by chronic or relapsing inflammation within the GI tract.
budesonide, 3mg, gastro-resistant capsules (Budenofalk ) SMC No. (1043/15) Dr Falk Pharma UK Ltd
 budesonide, 3mg, gastro-resistant capsules (Budenofalk ) SMC No. (1043/15) Dr Falk Pharma UK Ltd 10 April 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
budesonide, 3mg, gastro-resistant capsules (Budenofalk ) SMC No. (1043/15) Dr Falk Pharma UK Ltd 10 April 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Understanding Inflammatory Bowel Diseases (IBD):
 Understanding Inflammatory Bowel Diseases (IBD): What Every Patient Needs to Know William H Holderman, MD Digestive Health Specialists Tacoma, WA Today s Objectives Define IBD, its potential causes and
Understanding Inflammatory Bowel Diseases (IBD): What Every Patient Needs to Know William H Holderman, MD Digestive Health Specialists Tacoma, WA Today s Objectives Define IBD, its potential causes and
Medical Therapy for Pediatric IBD: Efficacy and Safety
 Medical Therapy for Pediatric IBD: Efficacy and Safety Betsy Maxwell, MD Assistant Professor of Clinical Pediatrics Division of Gastroenterology, Hepatology, and Nutrition Pediatric IBD: Defining Remission
Medical Therapy for Pediatric IBD: Efficacy and Safety Betsy Maxwell, MD Assistant Professor of Clinical Pediatrics Division of Gastroenterology, Hepatology, and Nutrition Pediatric IBD: Defining Remission
Ulcerative Colitis. ulcerative colitis usually only affects the colon.
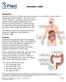 Ulcerative Colitis Introduction Ulcerative colitis is an inflammatory bowel disease. It is one of the 2 most common inflammatory bowel diseases. The other one is Crohn s disease. Ulcerative colitis and
Ulcerative Colitis Introduction Ulcerative colitis is an inflammatory bowel disease. It is one of the 2 most common inflammatory bowel diseases. The other one is Crohn s disease. Ulcerative colitis and
DENOMINATOR: All patients aged 18 and older with a diagnosis of inflammatory bowel disease
 Measure #270: Inflammatory Bowel Disease (IBD): Preventive Care: Corticosteroid Sparing Therapy National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY
Measure #270: Inflammatory Bowel Disease (IBD): Preventive Care: Corticosteroid Sparing Therapy National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY
Dr David Epstein Vincent Pallotti Hospital and University of Cape Town
 Inflammatory Bowel Disease Management in South Africa in 2016 Pharmaceutical Care Management Association Dr David Epstein Vincent Pallotti Hospital and University of Cape Town Inflammatory Bowel Disease
Inflammatory Bowel Disease Management in South Africa in 2016 Pharmaceutical Care Management Association Dr David Epstein Vincent Pallotti Hospital and University of Cape Town Inflammatory Bowel Disease
Scottish Medicines Consortium
 Scottish Medicines Consortium infliximab 100mg powder for intravenous infusion (Remicade ) No. (364/07) Schering-Plough UK Ltd 6 April 2007 The Scottish Medicines Consortium (SMC) has completed its assessment
Scottish Medicines Consortium infliximab 100mg powder for intravenous infusion (Remicade ) No. (364/07) Schering-Plough UK Ltd 6 April 2007 The Scottish Medicines Consortium (SMC) has completed its assessment
Tumor necrosis factor-alpha antibody for maintenace of remission in Crohn s disease (Review)
 Tumor necrosis factor-alpha antibody for maintenace of remission in Crohn s disease (Review) Behm BW, Bickston SJ This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration
Tumor necrosis factor-alpha antibody for maintenace of remission in Crohn s disease (Review) Behm BW, Bickston SJ This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration
Synopsis (C0168T37 ACT 1)
 () Module 5.3 Protocol: CR004777 EudraCT No.: Not Applicable Title of the study: A Randomized, Placebo-controlled, Double-blind Trial to Evaluate the Safety and Efficacy of Infliximab in Patients with
() Module 5.3 Protocol: CR004777 EudraCT No.: Not Applicable Title of the study: A Randomized, Placebo-controlled, Double-blind Trial to Evaluate the Safety and Efficacy of Infliximab in Patients with
Oral contraceptive use and the clinical course of Crohn s disease: a prospective cohort study
 218 Gut 1999;45:218 222 Service d Hépatogastroentérologie et Nutrition, Hôpital Rothschild, Paris, France J Cosnes F Carbonnel L Beaugerie J-P Gendre Unité de Biostatistique et d Informatique Médicale,
218 Gut 1999;45:218 222 Service d Hépatogastroentérologie et Nutrition, Hôpital Rothschild, Paris, France J Cosnes F Carbonnel L Beaugerie J-P Gendre Unité de Biostatistique et d Informatique Médicale,
Which is the Safest Strategy to Treat Moderate to Severe IBD?
 Which is the Safest Strategy to Treat Moderate to Severe IBD? David G. Binion, M.D. Co-Director, Inflammatory Bowel Disease Center Director, Translational Inflammatory Bowel Disease Research Visiting Professor
Which is the Safest Strategy to Treat Moderate to Severe IBD? David G. Binion, M.D. Co-Director, Inflammatory Bowel Disease Center Director, Translational Inflammatory Bowel Disease Research Visiting Professor
Diseases in which appear swelling, redness, pain and heat; aspects of the immune system (Inflammatory and immunologic conditions-imid)
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prednisolone is generally preferred amongst glucocorticoids for anti-inflammatory and immunosuppressive treatment. There are three
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prednisolone is generally preferred amongst glucocorticoids for anti-inflammatory and immunosuppressive treatment. There are three
U of Cape Town, South Africa, 10 U of Washington, Seattle, WA,USA, 11 CHRU de Lille, Hôpital Claude Huriez, Lille, France, 12
 A Multicenter, Double-blind, Placebo-controlled Phase 3 Study of Ustekinumab, a Human IL-12/23p40 Monoclonal Antibody, in Moderate-severe Crohn s Disease Refractory to Anti-TNFα: UNITI-1 WJ Sandborn 1,
A Multicenter, Double-blind, Placebo-controlled Phase 3 Study of Ustekinumab, a Human IL-12/23p40 Monoclonal Antibody, in Moderate-severe Crohn s Disease Refractory to Anti-TNFα: UNITI-1 WJ Sandborn 1,
Indications for use of Infliximab
 Indications for use of Infliximab Moscow, June 10 th 2006 Prof. Dr. Dr. Gerhard Rogler Klinik und Poliklinik für Innere Medizin I Universität Regensburg Case report 1989: Diagnosis of Crohn s disease of
Indications for use of Infliximab Moscow, June 10 th 2006 Prof. Dr. Dr. Gerhard Rogler Klinik und Poliklinik für Innere Medizin I Universität Regensburg Case report 1989: Diagnosis of Crohn s disease of
FERRING PHARMACEUTICALS. Enjoy The simple COR/939/2014/CH3
 Enjoy The simple pleasures of life COR/939/2014/CH3 Ulcerative colitis disrupts the simple pleasures in life Patients with ulcerative colitis may live with a considerable symptom burden despite medical
Enjoy The simple pleasures of life COR/939/2014/CH3 Ulcerative colitis disrupts the simple pleasures in life Patients with ulcerative colitis may live with a considerable symptom burden despite medical
Crohn's disease. Appendix I. Clinical Guideline < > 10 October Research recommendations
 Crohn's disease Clinical Guideline < > 10 October 2012 Commissioned by the National Institute for Health and Clinical Excellence Contents Published by the National Clinical Guideline Centre at The Royal
Crohn's disease Clinical Guideline < > 10 October 2012 Commissioned by the National Institute for Health and Clinical Excellence Contents Published by the National Clinical Guideline Centre at The Royal
5-ASA Therapy, Steroids and Antibiotics in Inflammatory Bowel Disease
 5-ASA Therapy, Steroids and Antibiotics in Inflammatory Bowel Disease David T. Rubin, MD Associate Professor of Medicine Co-Director, Inflammatory Bowel Disease Center University it of Chicago Medical
5-ASA Therapy, Steroids and Antibiotics in Inflammatory Bowel Disease David T. Rubin, MD Associate Professor of Medicine Co-Director, Inflammatory Bowel Disease Center University it of Chicago Medical
INDIVIDUAL STUDY TABLE REFERRING TO PART OF THE DOSSIER Volume: Page:
 SYNOPSIS Protocol No.: TOPMAT-MIG-303 EudraCT No.: 2005-000321-29 Title of Study: A double-blind, randomised, placebo-controlled, multicentre study to investigate the efficacy and tolerability of in prolonged
SYNOPSIS Protocol No.: TOPMAT-MIG-303 EudraCT No.: 2005-000321-29 Title of Study: A double-blind, randomised, placebo-controlled, multicentre study to investigate the efficacy and tolerability of in prolonged
National Institute for Health and Care Excellence
 National Institute for Health and Care Excellence 4-year surveillance (2017) Ulcerative colitis: management (2013) NICE guideline CG166 Appendix A.2: Summary of new evidence from surveillance Patient information
National Institute for Health and Care Excellence 4-year surveillance (2017) Ulcerative colitis: management (2013) NICE guideline CG166 Appendix A.2: Summary of new evidence from surveillance Patient information
This information explains the advice about Crohn's disease that is set out in NICE guideline CG152.
 Information for the public Published: 1 October 2012 nice.org.uk About this information NICE guidelines provide advice on the care and support that should be offered to people who use health and care services.
Information for the public Published: 1 October 2012 nice.org.uk About this information NICE guidelines provide advice on the care and support that should be offered to people who use health and care services.
Synopsis. Clinical Report Synopsis for Protocol Name of Company: Otsuka Pharmaceutical Development & Commercialization, Inc.
 Synopsis Clinical Report Synopsis for Protocol 197-02-220 Name of Company: Otsuka Pharmaceutical Development & Commercialization, Inc. Name of Product: Tetomilast (OPC-6535) Study Title: A Phase 3, Multicenter,
Synopsis Clinical Report Synopsis for Protocol 197-02-220 Name of Company: Otsuka Pharmaceutical Development & Commercialization, Inc. Name of Product: Tetomilast (OPC-6535) Study Title: A Phase 3, Multicenter,
Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn s disease
 42 Gut 2001;49:42 46 Gastroenterology Unit, Hopital Claude Huriez, Lille, France J-F Colombel P Desreumaux Medicine, University of Leuven, Leuven, Belgium P Rutgeerts Klinikum Leverkusen, Leverkusen, Germany
42 Gut 2001;49:42 46 Gastroenterology Unit, Hopital Claude Huriez, Lille, France J-F Colombel P Desreumaux Medicine, University of Leuven, Leuven, Belgium P Rutgeerts Klinikum Leverkusen, Leverkusen, Germany
Assessment of disease activity in inflammatory bowel disease; relevance for clinical trials
 REVIEW Assessment of disease activity in inflammatory bowel disease; relevance for clinical trials A.H.J. Naber *, D.J. de Jong Department of Gastroenterology and Hepatology, University Medical Centre
REVIEW Assessment of disease activity in inflammatory bowel disease; relevance for clinical trials A.H.J. Naber *, D.J. de Jong Department of Gastroenterology and Hepatology, University Medical Centre
Crohn's Disease. What causes Crohn s disease? What are the symptoms?
 Crohn's Disease Crohn s disease is an ongoing disorder that causes inflammation of the digestive tract, also referred to as the gastrointestinal (GI) tract. Crohn s disease can affect any area of the GI
Crohn's Disease Crohn s disease is an ongoing disorder that causes inflammation of the digestive tract, also referred to as the gastrointestinal (GI) tract. Crohn s disease can affect any area of the GI
Therapy for Inflammatory Bowel Disease
 Therapy for Inflammatory Bowel Disease Jonathan P. Terdiman, MD Professor of Clinical Medicine Clinical Director, Center for Colitis and Crohn s Disease University of California San Francisco, CA UC: Current
Therapy for Inflammatory Bowel Disease Jonathan P. Terdiman, MD Professor of Clinical Medicine Clinical Director, Center for Colitis and Crohn s Disease University of California San Francisco, CA UC: Current
NON INVASIVE MONITORING OF MUCOSAL HEALING IN IBD. THE ROLE OF BOWEL ULTRASOUND. Fabrizio Parente
 NON INVASIVE MONITORING OF MUCOSAL HEALING IN IBD. THE ROLE OF BOWEL ULTRASOUND Fabrizio Parente Gastrointestinal Unit, A.Manzoni Hospital, Lecco & L.Sacco School of Medicine,University of Milan - Italy
NON INVASIVE MONITORING OF MUCOSAL HEALING IN IBD. THE ROLE OF BOWEL ULTRASOUND Fabrizio Parente Gastrointestinal Unit, A.Manzoni Hospital, Lecco & L.Sacco School of Medicine,University of Milan - Italy
INTESTINAL DISEASE MEETING BERLIN Topical steroids rectal application
 INTESTINAL DISEASE MEETING BERLIN 2006 Topical steroids rectal application Prof. Dr. med. T. Andus Department of Internal Medicine, Gastroenterology, Hepatology, and Oncology Krankenhaus Bad Cannstatt,
INTESTINAL DISEASE MEETING BERLIN 2006 Topical steroids rectal application Prof. Dr. med. T. Andus Department of Internal Medicine, Gastroenterology, Hepatology, and Oncology Krankenhaus Bad Cannstatt,
Definitions. Clinical remission: Resolution of symptoms (stool frequency 3/day, no bleeding and no urgency)
 CROHN S DISEASE Definitions Clinical remission: Resolution of symptoms (stool frequency 3/day, no bleeding and no urgency) Recurrence: The reappearance of lesions after surgical resection Endoscopic remission:
CROHN S DISEASE Definitions Clinical remission: Resolution of symptoms (stool frequency 3/day, no bleeding and no urgency) Recurrence: The reappearance of lesions after surgical resection Endoscopic remission:
Dr. Elmer Schabel, MD. Bundesinstitut für Arzneimittel und Medizinprodukte, Bonn, Germany (No conflicts of interest)
 EMA workshop on the development of new medicinal products for the treatment of ulcerative colitis and Crohn s disease Overview of authorised medicines for IBD in Europe - previous regulatory positions
EMA workshop on the development of new medicinal products for the treatment of ulcerative colitis and Crohn s disease Overview of authorised medicines for IBD in Europe - previous regulatory positions
What is Crohn's disease?
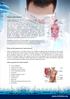 What is Crohn's disease? Crohn's disease is a chronic inflammatory disorder that causes inflammation of the digestive tract. It can affect any area of the GI tract, from the mouth to the anus, but it most
What is Crohn's disease? Crohn's disease is a chronic inflammatory disorder that causes inflammation of the digestive tract. It can affect any area of the GI tract, from the mouth to the anus, but it most
Inflammatory Bowel Disease. Your Illness and Its Treatment
 Inflammatory Bowel Disease Your Illness and Its Treatment What Is Inflammatory Bowel Disease? Inflammatory bowel disease (IBD) is inflammation (irritation and swelling) of the digestive tract. Your digestive
Inflammatory Bowel Disease Your Illness and Its Treatment What Is Inflammatory Bowel Disease? Inflammatory bowel disease (IBD) is inflammation (irritation and swelling) of the digestive tract. Your digestive
Azathioprine (AZA), and its active metabolite 6-mercaptopurine,
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2009;7:80 85 Azathioprine Withdrawal in Patients With Crohn s Disease Maintained on Prolonged Remission: A High Risk of Relapse XAVIER TRETON,* YORAM BOUHNIK,*
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2009;7:80 85 Azathioprine Withdrawal in Patients With Crohn s Disease Maintained on Prolonged Remission: A High Risk of Relapse XAVIER TRETON,* YORAM BOUHNIK,*
September 12, 2015 Millie D. Long MD, MPH, FACG
 Update on Biologic Therapy in 2015 September 12, 2015 Millie D. Long MD, MPH, FACG Assistant Professor of Medicine Inflammatory Bowel Disease Center University of North Carolina-Chapel Hill Outline Crohn
Update on Biologic Therapy in 2015 September 12, 2015 Millie D. Long MD, MPH, FACG Assistant Professor of Medicine Inflammatory Bowel Disease Center University of North Carolina-Chapel Hill Outline Crohn
Perianal and Fistulizing Crohn s Disease: Tough Management Decisions. Jean-Paul Achkar, M.D. Kenneth Rainin Chair for IBD Research Cleveland Clinic
 Perianal and Fistulizing Crohn s Disease: Tough Management Decisions Jean-Paul Achkar, M.D. Kenneth Rainin Chair for IBD Research Cleveland Clinic Talk Overview Background Assessment and Classification
Perianal and Fistulizing Crohn s Disease: Tough Management Decisions Jean-Paul Achkar, M.D. Kenneth Rainin Chair for IBD Research Cleveland Clinic Talk Overview Background Assessment and Classification
ORIGINAL ARTICLE. Volume 63, No. 3 : 2006 GASTROINTESTINAL ENDOSCOPY 433
 ORIGINAL ARTICLE maintenance treatment with infliximab is superior to episodic treatment for the of mucosal associated with Crohn s disease Paul Rutgeerts, MD, PhD, Robert H. Diamond, MD, Mohan Bala, PhD,
ORIGINAL ARTICLE maintenance treatment with infliximab is superior to episodic treatment for the of mucosal associated with Crohn s disease Paul Rutgeerts, MD, PhD, Robert H. Diamond, MD, Mohan Bala, PhD,
ULCERATIVE COLITIS. Sean Lynch, MD and Richard Bloomfeld, MD Wake Forest University School of Medicine Winston-Salem, NC
 ULCERATIVE COLITIS Sean Lynch, MD and Richard Bloomfeld, MD Wake Forest University School of Medicine Winston-Salem, NC What is Ulcerative Colitis? Ulcerative colitis (UC) is a disease marked by inflammation
ULCERATIVE COLITIS Sean Lynch, MD and Richard Bloomfeld, MD Wake Forest University School of Medicine Winston-Salem, NC What is Ulcerative Colitis? Ulcerative colitis (UC) is a disease marked by inflammation
Treatment Goals. Current Therapeutic Pyramids Crohn s Disease Ulcerative Colitis 11/14/10
 Current Management of IBD: From Conventional Agents to Biologics Stephen B. Hanauer, M.D. University of Chicago Treatment Goals Induce and maintain response/ remission Prevent complications Improve quality
Current Management of IBD: From Conventional Agents to Biologics Stephen B. Hanauer, M.D. University of Chicago Treatment Goals Induce and maintain response/ remission Prevent complications Improve quality
To help protect your privacy, PowerPoint prevented this external picture from being automatically downloaded. To download and display this picture,
 To help protect your privacy, PowerPoint prevented this external picture from being automatically downloaded. To download and display this picture, click Options in the Message Bar, and then click Enable
To help protect your privacy, PowerPoint prevented this external picture from being automatically downloaded. To download and display this picture, click Options in the Message Bar, and then click Enable
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
International Co-ordinating investigator None appointed.
 Drug product: Budesonide/formoterol SYNOPSIS Drug substance(s): Budesonide/formoterol Document No.: SD-039-CR-0673 Referring to part Edition No.: 1 of the dossier Study code: SD-039-0673 Date: 13 June,
Drug product: Budesonide/formoterol SYNOPSIS Drug substance(s): Budesonide/formoterol Document No.: SD-039-CR-0673 Referring to part Edition No.: 1 of the dossier Study code: SD-039-0673 Date: 13 June,
Achieving Success in Ulcerative Colitis: the Role of Infliximab
 Achieving Success in Ulcerative Colitis: the Role of Infliximab Dr Gill Watermeyer IBD clinic Groote Schuur Hospital 17 th August 2012 Inflammatory Bowel Disease Crohn s disease and ulcerative colitis
Achieving Success in Ulcerative Colitis: the Role of Infliximab Dr Gill Watermeyer IBD clinic Groote Schuur Hospital 17 th August 2012 Inflammatory Bowel Disease Crohn s disease and ulcerative colitis
Medical management of mild to moderate Crohn s disease: evidence-based treatment algorithms for induction and maintenance of remission
 Alimentary Pharmacology & Therapeutics Medical management of mild to moderate Crohn s disease: evidence-based treatment algorithms for induction and maintenance of remission W. J. SANDBORN*, B. G. FEAGAN
Alimentary Pharmacology & Therapeutics Medical management of mild to moderate Crohn s disease: evidence-based treatment algorithms for induction and maintenance of remission W. J. SANDBORN*, B. G. FEAGAN
Treatment of ulcerative colitis with adalimumab or infliximab: long-term follow-up of a single-centre cohort
 Alimentary Pharmacology and Therapeutics Treatment of ulcerative colitis with adalimumab or infliximab: long-term follow-up of a single-centre cohort N. Gies, K. I. Kroeker, K. Wong & R. N. Fedorak Division
Alimentary Pharmacology and Therapeutics Treatment of ulcerative colitis with adalimumab or infliximab: long-term follow-up of a single-centre cohort N. Gies, K. I. Kroeker, K. Wong & R. N. Fedorak Division
ENTYVIO (VEDOLIZUMAB)
 ENTYVIO (VEDOLIZUMAB) UnitedHealthcare Community Plan Medical Benefit Drug Policy Policy Number: CS2017D0053F Effective Date: July 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
ENTYVIO (VEDOLIZUMAB) UnitedHealthcare Community Plan Medical Benefit Drug Policy Policy Number: CS2017D0053F Effective Date: July 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
Withdrawal of corticosteroids in inflammatory bowel disease patients after dependency periods ranging from 2 to 45 years: a proposed method
 Withdrawal of corticosteroids in inflammatory bowel disease patients after dependency periods ranging from 2 to 45 years: a proposed method Murphy, S. J., Wang, L., Anderson, L., Steinlauf, A., Present,
Withdrawal of corticosteroids in inflammatory bowel disease patients after dependency periods ranging from 2 to 45 years: a proposed method Murphy, S. J., Wang, L., Anderson, L., Steinlauf, A., Present,
Lessons to learn from Crohn's disease clinical trials: implications for ulcerative colitis
 Lessons to learn from Crohn's disease clinical trials: implications for ulcerative colitis Aránzazu Jáuregui Amézaga, Elena Ricart, Julián Panés Department of Gastroenterology, Hospital Clínic de Barcelona,
Lessons to learn from Crohn's disease clinical trials: implications for ulcerative colitis Aránzazu Jáuregui Amézaga, Elena Ricart, Julián Panés Department of Gastroenterology, Hospital Clínic de Barcelona,
Medical therapies and IBD
 Medical therapies and IBD Although there is no cure for IBD, there are many treatment options available. There is no standard treatment for IBD that is effective in all situations or for all patients,
Medical therapies and IBD Although there is no cure for IBD, there are many treatment options available. There is no standard treatment for IBD that is effective in all situations or for all patients,
The Spectrum of IBD. Inflammatory Bowel Disease. Symptoms. Epidemiology. Tests for IBD. CD or UC? Inflamatory Bowel Disease. Fernando Vega, M.D.
 The Spectrum of IBD Inflammatory Bowel Disease Fernando Vega, M.D. Epidemiology CD and UC together 1:400 UC Prevalence 1:500 UC Incidence 6-12K/annum CD Prevalence 1:1000 CD Incidence 3-6K/annum Symptoms
The Spectrum of IBD Inflammatory Bowel Disease Fernando Vega, M.D. Epidemiology CD and UC together 1:400 UC Prevalence 1:500 UC Incidence 6-12K/annum CD Prevalence 1:1000 CD Incidence 3-6K/annum Symptoms
Ali Keshavarzian MD Rush University Medical Center
 Treatment: Step Up or Top Down? Ali Keshavarzian MD Rush University Medical Center Questions What medication should IBD be treated with? Can we predict which patients with IBD are high risk? Is starting
Treatment: Step Up or Top Down? Ali Keshavarzian MD Rush University Medical Center Questions What medication should IBD be treated with? Can we predict which patients with IBD are high risk? Is starting
Maintenance therapy for inflammatory bowel disease: What really works
 REVIEW Maintenance therapy for inflammatory bowel disease: What really works LLOYD RSUTHERLAND MDCM MSc FRCPC FACP LR SUTHERLAND. Maintenance therapy for inflammatory bowel disease: What really works.
REVIEW Maintenance therapy for inflammatory bowel disease: What really works LLOYD RSUTHERLAND MDCM MSc FRCPC FACP LR SUTHERLAND. Maintenance therapy for inflammatory bowel disease: What really works.
Beyond Anti TNFs: positioning of other biologics for Crohn s disease. Christina Ha, MD Cedars Sinai Inflammatory Bowel Disease Center
 Beyond Anti TNFs: positioning of other biologics for Crohn s disease Christina Ha, MD Cedars Sinai Inflammatory Bowel Disease Center Objectives: To define high and low risk patient and disease features
Beyond Anti TNFs: positioning of other biologics for Crohn s disease Christina Ha, MD Cedars Sinai Inflammatory Bowel Disease Center Objectives: To define high and low risk patient and disease features
ustekinumab 130mg concentrate for solution for infusion and 90mg solution for injection (Stelara ) SMC No. (1250/17) Janssen-Cilag Ltd
 ustekinumab 130mg concentrate for solution for infusion and 90mg solution for injection (Stelara ) SMC No. (1250/17) Janssen-Cilag Ltd 09 June 2017 The Scottish Medicines Consortium (SMC) has completed
ustekinumab 130mg concentrate for solution for infusion and 90mg solution for injection (Stelara ) SMC No. (1250/17) Janssen-Cilag Ltd 09 June 2017 The Scottish Medicines Consortium (SMC) has completed
Technology appraisal guidance Published: 19 May 2010 nice.org.uk/guidance/ta187
 Infliximab and adalimumab for the treatment of Crohn's disease Technology appraisal guidance Published: 19 May 2010 nice.org.uk/guidance/ta187 NICE 2018. All rights reserved. Subject to Notice of rights
Infliximab and adalimumab for the treatment of Crohn's disease Technology appraisal guidance Published: 19 May 2010 nice.org.uk/guidance/ta187 NICE 2018. All rights reserved. Subject to Notice of rights
Ulcerative Colitis: State of the Art 2006
 Ulcerative Colitis: State of the Art David T. Rubin, MD Assistant Professor of Medicine Inflammatory Bowel Disease Center University of Chicago Improving Management of Ulcerative Colitis (UC) Better classification/diagnostic
Ulcerative Colitis: State of the Art David T. Rubin, MD Assistant Professor of Medicine Inflammatory Bowel Disease Center University of Chicago Improving Management of Ulcerative Colitis (UC) Better classification/diagnostic
An Update on the Biologic Treatment for Patients with Inflammatory Bowel Disease. David A. Schwartz, MD
 An Update on the Biologic Treatment for Patients with Inflammatory Bowel Disease David A. Schwartz, MD Director, Inflammatory Bowel Disease Center Associate Professor of Medicine Vanderbilt University
An Update on the Biologic Treatment for Patients with Inflammatory Bowel Disease David A. Schwartz, MD Director, Inflammatory Bowel Disease Center Associate Professor of Medicine Vanderbilt University
Guideline Ulcerative colitis: management
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline Ulcerative colitis: management Draft for consultation, December 0 This guideline covers the care and treatment of adults, children and young
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline Ulcerative colitis: management Draft for consultation, December 0 This guideline covers the care and treatment of adults, children and young
Corticosteroid therapy in inflammatory bowel diseases. The informed patient
 Corticosteroid therapy in inflammatory bowel diseases The informed patient Publisher 201 Falk Foundation e.v. All rights reserved. U2 12th revised edition 2017 The informed patient Corticosteroid therapy
Corticosteroid therapy in inflammatory bowel diseases The informed patient Publisher 201 Falk Foundation e.v. All rights reserved. U2 12th revised edition 2017 The informed patient Corticosteroid therapy
INFLAMMATORY BOWEL DISEASE. Jean-Paul Achkar, MD Center for Inflammatory Bowel Disease Cleveland Clinic
 INFLAMMATORY BOWEL DISEASE Jean-Paul Achkar, MD Center for Inflammatory Bowel Disease Cleveland Clinic WHAT IS INFLAMMATORY BOWEL DISEASE (IBD)? Chronic inflammation of the intestinal tract Two related
INFLAMMATORY BOWEL DISEASE Jean-Paul Achkar, MD Center for Inflammatory Bowel Disease Cleveland Clinic WHAT IS INFLAMMATORY BOWEL DISEASE (IBD)? Chronic inflammation of the intestinal tract Two related
Technology appraisal guidance Published: 26 August 2015 nice.org.uk/guidance/ta352
 Vedolizumab for treating moderately to severely erely active Crohn's disease after prior therapy Technology appraisal guidance Published: 26 August 2015 nice.org.uk/guidance/ta352 NICE 2017. All rights
Vedolizumab for treating moderately to severely erely active Crohn's disease after prior therapy Technology appraisal guidance Published: 26 August 2015 nice.org.uk/guidance/ta352 NICE 2017. All rights
The Best of IBD at UEGW (Crohn s)
 The Best of IBD at UEGW (Crohn s) Iyad Issa MD Head of Gastroenterology, Rafik Hariri Univ Hosp Adjunct Faculty, School of Medicine, Leb Univ Founding Faculty, School Of Medicine, Leb Am Univ 1 The Best
The Best of IBD at UEGW (Crohn s) Iyad Issa MD Head of Gastroenterology, Rafik Hariri Univ Hosp Adjunct Faculty, School of Medicine, Leb Univ Founding Faculty, School Of Medicine, Leb Am Univ 1 The Best
Personalized Medicine in IBD
 Personalized Medicine in IBD Anita Afzali MD, MPH Assistant Professor of Medicine Director, Inflammatory Bowel Diseases Program University of Washington Harborview Medical Center CCFA April 2 nd, 2016
Personalized Medicine in IBD Anita Afzali MD, MPH Assistant Professor of Medicine Director, Inflammatory Bowel Diseases Program University of Washington Harborview Medical Center CCFA April 2 nd, 2016
I nflammatory bowel diseases (IBD) are chronic intestinal
 364 INFLAMMATORY BOWEL DISEASE Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn s disease F Costa, M G Mumolo, L Ceccarelli, M Bellini, M R Romano, C Sterpi,
364 INFLAMMATORY BOWEL DISEASE Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn s disease F Costa, M G Mumolo, L Ceccarelli, M Bellini, M R Romano, C Sterpi,
Clinical Study Clinical Study of the Relation between Mucosal Healing and Long-Term Outcomes in Ulcerative Colitis
 Hindawi Publishing Corporation Gastroenterology Research and Practice Volume 2013, Article ID 192794, 6 pages http://dx.doi.org/10.1155/2013/192794 Clinical Study Clinical Study of the Relation between
Hindawi Publishing Corporation Gastroenterology Research and Practice Volume 2013, Article ID 192794, 6 pages http://dx.doi.org/10.1155/2013/192794 Clinical Study Clinical Study of the Relation between
