Impact of radiologically stratified exacerbations: insights into pneumonia aetiology in COPD
|
|
|
- Zoe Gilbert
- 5 years ago
- Views:
Transcription
1 Williams et al. Respiratory Research (2018) 19:143 RESEARCH Open Access Impact of radiologically stratified exacerbations: insights into pneumonia aetiology in COPD Nicholas P. Williams 1,2*, Kristoffer Ostridge 1,2, Jeanne-Marie Devaster 3, Viktoriya Kim 1,2, Ngaire A. Coombs 4, Simon Bourne 1,9, Stuart C. Clarke 1,5, Stephen Harden 6, Ausami Abbas 6, Emmanuel Aris 3, Christophe Lambert 3, Andrew Tuck 7, Anthony Williams 5, Stephen Wootton 8, Karl J. Staples 1,5, Tom M. A. Wilkinson 1,2,5 and on behalf of the AERIS Study Group Abstract Background: COPD patients have increased risk of developing pneumonia, which is associated with poor outcomes. It can be symptomatically indistinguishable from exacerbations, making diagnosis challenging. Studies of pneumonia in COPD have focused on hospitalised patients and are not representative of the ambulant COPD population. Therefore, we sought to determine the incidence and aetiology of acute exacerbation events with evidence of pneumonic radiographic infiltrates in an outpatient COPD cohort. Methods: One hundred twenty-seven patients with moderate to very severe COPD aged years underwent blood and sputum sampling over one year, at monthly stable visits and within 72 h of exacerbation symptom onset. 343 exacerbations with chest radiographs were included. Results: 20.1% of exacerbations had pneumonic infiltrates. Presence of infiltrate was highly seasonal (Winter vs summer OR 3.056, p = 0.027). In paired analyses these exacerbation events had greater increases in systemic inflammation. Bacterial detection rate was higher in the pneumonic group, with Haemophilus influenzae the most common bacteria in both radiological groups. Viral detection and sputum microbiota did not differ with chest radiograph appearance. Conclusions: In an outpatient COPD cohort, pneumonic infiltrates at exacerbation were common, and associated with more intense inflammation. Bacterial pathogen detection and lung microbiota were not distinct, suggesting that exacerbations and pneumonia in COPD share common infectious triggers and represent a continuum of severity rather than distinct aetiological events. Trial registration: Trial registration Number: NCT Keywords: COPD, Pneumonia, Infiltrates, Exacerbations * Correspondence: NPWilliams@doctors.org.uk 1 Clinical and Experimental Sciences, University of Southampton Faculty of Medicine, Southampton General Hospital, Southampton, UK 2 Southampton NIHR Respiratory Biomedical Research Unit, Southampton General Hospital, Southampton, UK Full list of author information is available at the end of the article The Author(s) Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( applies to the data made available in this article, unless otherwise stated.
2 Williams et al. Respiratory Research (2018) 19:143 Page 2 of 12 Background Acute exacerbations of COPD are associated with impaired quality of life, accelerated lung function decline and place considerable burden on health-care resources [1 3]. They are typically inflammatory events [4, 5], triggered by bacterial and viral infection[6], often requiring treatment with antibiotics and oral corticosteroids (OCS) either in an ambulatory setting, or less frequently in hospital [7]. As a result,theyhavebecomeakeytargetforinterventionin COPD. With this recognition, the emergence of symptom, pathogen and biomarker-guided strategies now offers scope to phenotype exacerbations, potentially allowing for tailored management approaches [8]. Pneumonia is more common in patients with COPD [9], and can be clinically indistinguishable from exacerbations. Numerous factors associated with pneumonia risk have been reported in COPD, including inhaled corticosteroid (ICS) use, comorbidity and nutritional status [10, 11]. COPD patients requiring hospitalisation for acute respiratory tract illness, often have complicating pneumonic infiltrate evident on chest radiograph (CXR) [12]. Moreover, these hospitalised episodes are associated with more intense inflammatory responses, differing pathogen profiles and often lead to longer hospital stays and increased rates of readmission [13 15]. This raises important treatment dilemmas, particularly around the use of OCS, risk-stratification for ICS and antibiotic usage. This is particularly relevant in the era of emerging antimicrobial resistance, where clinical strategies to better stratify and target antibiotics effectively are vital. Validating a diagnosis of pneumonia requires the radiographic identification of pulmonary infiltrate in the context of symptoms of a respiratory tract infection which to date, has largely limited its study to hospitalised events. Given that only a comparatively small proportion of exacerbations require hospitalisation [16], our understanding of the frequency, nature and impact of pneumonic infiltrates complicating non-hospitalised exacerbations is currently lacking. In a secondary analysis of the Acute Exacerbation and Respiratory InfectionS in COPD (AERIS) cohort, a prospective, longitudinal study of outpatients with COPD, we used radiological stratification to provide a detailed insight into the aetiology and character of exacerbations complicated by pneumonic change. Methods Study design and measurements The AERIS study (ClinicalTrials.gov NCT ) was a prospective, observational cohort study conducted at the University Hospital Southampton, UK. The study protocol has been described previously [17]. Eligible patients with 1 treated exacerbation in the year prior to enrolment, and a confirmed diagnosis of COPD from a post-bronchodilator forced expiratory volume in 1 s (FEV1)/Forced vital capacity (FVC) ratio of < 0.7, were recruited. Patients on long-term antibiotic and/or OCS therapy were not eligible for inclusion. For full inclusion/exclusion criteria see Additional file 1. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice, and was approved by the Southampton and South West Hampshire Research Ethics Committee. All participants provided written informed consent. Patients were followed monthly in the stable-state and reviewed within 72 h of exacerbation symptom onset. Exacerbations were detected using daily electronic diary cards. The definition and severity of exacerbations, has been previously described [6, 17]. All events were managed as an exacerbation from the outset, as judged appropriate by the clinical team, in accordance with local guidelines. Exacerbation onset date was used to describe seasonality with winter defined as December February, spring as March May, summer as June August and autumn as September November. Posterior-anterior CXRs performed at acute visits, were reported by a blinded, designated thoracic radiologist (SH), with a randomly selected subset (n = 100) reported independently by a second, blinded thoracic radiologist (AA). Pneumonic infiltrates were characterised as acinar (lobar) or reticulo-nodular. Multiple exacerbations with pneumonic infiltrate in the same individual, were included if the infiltrate was new. Sample collection and analysis Spontaneous or induced sputum samples were processed as previously described [6, 18, 19]. Sputum was cultured for bacteria and analysed by polymerase chain reaction (PCR) for bacterial and viral detection, monthly and at exacerbation (See Additional file 1: Supplementary methods). Blood analysis included differential white cell count, C-reactive protein (CRP), fibrinogen and procalcitonin (PCT) at study entry, exacerbation and at quarterly visits throughout the year. We have extensively analysed the microbiome of the AERIS cohort and the in-depth analysis of correlations with disease severity and exacerbation phenotype have been previously reported [20]. In the current sub-analysis, we investigated whether there were differences in the abundance of specific phyla and genera in exacerbations with and without pneumonic infiltrate. For the microbiome analysis, genomic DNA was extracted from sputum using the MagNA Pure 96 DNA Volume Kit (Roche Diagnostics), according to the manufacturer s instructions. The conserved V4 region of the 16S rrna gene was amplified and sequenced on an Illumina MiSeq sequencer. Sequenced reads were filtered for quality and processed using the QIIME (quantitative insights into microbial ecology) pipeline version Operational taxonomic
3 Williams et al. Respiratory Research (2018) 19:143 Page 3 of 12 units (OTUs) were subsequently clustered from chimera-cleaned reads at a 97% identity threshold and assigned taxonomy using the GreenGenes 16S RNA sequence database (version 13.8). For further methodological detail, see Additional file 1. Statistical analysis Analyses were performed using SPSS version 22 (Armonk, NY:IBM Corp) and Stata version 14 (Stata Corporation, College Station, TX, USA). The sample size calculation for the AERIS study has been described previously [17]. Categorical data are presented as frequency, percentage (%) and were compared using the Chi-square test or Fisher s exact test as appropriate. Continuous data are presented as mean, standard deviation (SD) if normally distributed and median, interquartile range (IQR) if not. Means were compared using the independent sample t-test and medians using the Mann-Whitney U test. For paired analyses comparing pre-exacerbation with exacerbation samples and stratifying by presence/ absence of infiltrate, the Wilcoxon signed rank test was used. Clustered multivariate logistic regression, including the subject as a random effect, was used to account for individual patients having multiple exacerbations. Presence of pneumonic infiltrate at exacerbation was designated the dependent variable. Other factors judged by univariate analysis or, a priori to be of clinical importance were included as independent variables. P-values of < 0.05 were considered statistically significant. All analyses should be considered post hoc as they were not pre-specified in the AERIS statistical analysis plan. Results Patient characteristics and exacerbations One hundred twenty-seven patients were enrolled (Fig. 1). The mean (SD) age was 66.8 ± 8.6 and 68 (53.5%) were male. At study entry 45, 40 and 15% had GOLD stage II, III and IV respectively, with a mean (SD) post-bronchodilator FEV1% predicted of 46.4 ± 15.2 Bronchiectasis, which was assessed at enrolment by high resolution CT, was identified in only a minority of patients (10 of 127; 7.9%) (Table 1). Three hundred fifty-five exacerbation visits from 108 patients were studied. The mean annual exacerbation rate was 3.04 (95% CI ) per patient. 8 exacerbations did not have a CXR and 4 had evidence of persisting infiltrate and were therefore excluded, leaving 343 exacerbations for analysis. Only 19 (5.5%) of these events required hospitalisation. The Kappa agreement for the subset of CXRs analysed independently by two clinical reviewers was Fig. 1 Flow chart of subject enrolment and sub-groupings in the study
4 Williams et al. Respiratory Research (2018) 19:143 Page 4 of 12 Table 1 Baseline characteristics of the study cohort at the time of recruitment Characteristic n = 127 Age (years) 66.8 ± 8.6 Male sex, n (%) 68 (53.5) Body mass index (kg/m 2 ) 27.7 (5.5) Smoking status (n, %) Current smoker 54 (43.0) Ex-smoker 73 (57.0) Smoking history pack-years a 47.0 (26.3) Post-bronchodilator FEV1 (% predicted) 46.4 ± 15.2 FEV1 (Litres) 1.20 ± 0.47 FVC (Litres) 2.82 ± 0.82 GOLD Stage, n (%) II (moderate) 57 (44.9) III (severe) 51 (40.2) IV (very severe) 19 (15.0) Bronchiectasis evident on enrolment HRCT, n (%) 10 (7.9) Frequency of exacerbation reporting in the preceding 12 months, n (%) 1 exacerbation 28 (22.0) 2 exacerbations 37 (29.1) 3 or more exacerbations 62 (48.8) Number of exacerbations in the preceding 12 months 3.1 ± 2.3 ICS use, n (%) 113 (89.0) LABA use, n (%) 104 (81.9) LAMA use, n (%) 93 (73.2) Influenza vaccination in the preceding 12 months, n (%) 114 (89.8) Pneumococcal vaccination in the preceding 12 months, n (%) 12 (9.4) Data presented as mean ± standard deviation or n (%) unless otherwise stated a presented as median (IQR) FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, GOLD Global Initiative for Chronic Obstructive Lung Disease, HRCT high resolution CT, ICS inhaled corticosteroid, LABA long acting beta agonist, LAMA long acting muscarinic antagonist Overall, 69 (20.1%) exacerbations had a new pneumonic infiltrate (For other radiological findings, see Additional file 2: Table S1). 61 (88%) infiltrates were considered reticulo-nodular and 8 (12%) lobar in character. 95.7% of exacerbations with pneumonic infiltrate were outpatient managed. 43 were treated with both antibiotics and OCS, 11 with antibiotics alone, 10 with OCS alone and 5 required no systemic treatment (Additional file 2: Table S2). Table 2 shows the clinical characteristics of exacerbations. Forty-six (36.2%) patients had 1 exacerbation with pneumonic infiltrate and 81 (63.8%) did not. In order to identify baseline factors that may predict risk of future exacerbations with infiltrate, these groups were compared. No significant differences in age, sex or smoking status were observed and ICS use was similar. However, greater disease severity (FEV1 (%) 41.7 versus 49.2; p = 0.007) and poorer six minute walk distance (256.6 m versus m; p = 0.002) were associated with having one or more exacerbations with pneumonic infiltrate during the study (Table 3). Seasonality of exacerbation groups Considering all exacerbations (n = 343), significant variability was observed across the seasons, with 33.8% occurring in winter, 25.9% in autumn, 23.0% in spring and 17.2% in summer (p < 0.001) (Fig. 2a). Exacerbations with infiltrate were highly prevalent in winter, constituting almost a third of all exacerbations during that season (Fig. 2b). Pneumonic infiltrates and microbial aetiology 308 (89.8%) exacerbations had available sputum for bacterial culture, 295 (86.0%) for bacterial PCR and 294 (85.7%) for viral PCR. Overall, 69.8% of exacerbations with pneumonic infiltrate and 57.1% of exacerbations without had bacteria detected by culture (p = 0.067). For PCR-detected bacteria, corresponding proportions were 77.0 and 65.4% respectively for exacerbations with and without pneumonic infiltrate (p = 0.082). Haemophilus influenzae (HI) was the most frequently identified bacteria by both culture (52.4% of exacerbations with, versus 38.0% without infiltrate, p = 0.038) and PCR (62.3% of exacerbations with, versus 52.4% without infiltrate, p = 0.165). A separate analysis, including only sputum considered high quality (fewer than 30% squamous cells) yielded similar results (Additional file 2: Table S3). Streptococcus pneumoniae (SP) was detected by culture in 11.1% (n = 7) of exacerbations with, and 16.3% (n = 40) without pneumonic infiltrate (p = 0.305) (Additional file 2: Table S4 and Figure S1). PCR detected only 19 (8.2%) cases of SP at exacerbations without infiltrate, accounted for by the identification of the closely related species, Streptococcus pseudopneumoniae by PCR, which was not differentiated by culture [6]. Of those with lobar infiltrate (n =8),5 (62.5%) were associated with bacterial detection, 4 of which were HI, whilst SP was not detected. Viruses were identified in similar proportions between exacerbation groups (n = 25 (41.7%) with, versus n =101 (43.2%) without infiltrate; p = 0.835), with rhinovirus predominating. There were no differences in bacterial-viral co-infection rates by either bacterial culture-viral PCR (n = 18 (30.0%) with, versus n = 58 (24.8%) without infiltrate; p = 0.411) or bacterial PCR-viral PCR (n =21 (35.0%) with, versus n = 68 (29.1%) without; p =0.372). Using a subset of 205 exacerbations with paired preceding stable visits and available sputum, (n = 38 (18.5%) with and n = 167 (81.5%) without infiltrate), acquisition of bacteria not seen at the previous stable visit occurred in 37.6%. There were no differences in the rate of new
5 Williams et al. Respiratory Research (2018) 19:143 Page 5 of 12 Table 2 Clinical characteristics of all exacerbations, stratified by the presence or absence of pneumonic infiltrate All exacerbations (n = 343) Exacerbations without infiltrate (n = 274) Exacerbations with infiltrate (n = 69) n a n a n a FEV1 (Litres) (0.62) (0.62) (0.68) FEV1 (% predicted) (18.7) (19.1) (19.4) FEV1/FVC ratio (0.17) (0.18) (0.15) CAT score (10) (10) (10) P-value* EXACT score (10) (9) (11) Blood WCC (cellsx10 9 L) (3.98) (3.60) (4.90) < Blood neutrophils (cellsx10 9 L) (3.50) (3.10) (4.50) < Blood eosinophils (cellsx10 9 L) (0.20) (0.20) (0.20) % blood eosinophils (2.43) (2.43) (2.28) CRP (mg/l) (23.0) (16.8) (61.0) < Fibrinogen (g/l) (1.8) (1.5) (2.0) < Procalcitonin (μg/l) (0.0400) (0.0400) (0.0400) Sputum bacterial detection (culture) n, (%) (59.7) (57.1) (69,8) Sputum bacterial detection (PCR) n, (%) Sputum viral detection (PCR) n, (%) (67.8) (65.4) (77.0) (42.9) (43.2) (41.7) Continuous variables presented as median (IQR) and categorical variables as n, (%) unless otherwise stated FEV1 Forced expiratory volume in 1 s, FVC Forced vital capacity, CAT COPD Assessment Test, EXACT EXAcerbations of Chronic pulmonary disease Tool, PCR Polymerase chain reaction, WCC white blood cell count, CRP C-reactive protein a indicates the number of subjects with available data/samples *P-values comparing exacerbations with and without infiltrate using Mann Whitney U test for continuous variables and Chi-square test for categorical variables Table 3 Baseline characteristics of subjects grouped by the presence/absence of at least one exacerbation with pneumonic infiltrate Characteristic Subjects with no pneumonic infiltrate identified at any exacerbation (n = 81) Subjects with 1 pneumonic infiltrate identified at exacerbation (n = 46) P-value n a Age (years) (8.4) (9.1) Male sex, n (%) (54.3) (52.2) Current smokers, n (%) (42.0) (43.5) Body mass index (kg/m 2 ) (5.5) (5.5) FEV1 (Litres) (0.99) FEV1 (% predicted) (15.7) (13.0) TLCO (mmol/kpa/min)* (2.78) (1.30) MWD (meters) (110.8) (100.9) CAT score (7.8) (7.3) Prior history of pneumonia (before study enrolment) (27.8) (45.7) Influenza vaccination during the year prior to enrolment (88.9) (91.3) ICS use (85.2) (95.7) Bacteria detected (culture) (49.3) (56.1) Bacteria detected (PCR) (56.5) (70.0) Continuous variables presented as mean (SD) and categorical variables as n (%) unless otherwise stated. * presented as median (IQR) FEV1 forced expiratory volume in 1 s, TLCO transfer factor for carbon monoxide, 6MWD 6 min walk distance, CAT COPD Assessment Test, ICS inhaled corticosteroid, PCR polymerase chain reaction a indicates the number of subjects with available data/samples for each characteristic *P-values comparing the subject groups using the independent sample t-test or Mann Whitney U test where appropriate for continuous variables and the Chisquare test for categorical variables n a
6 Williams et al. Respiratory Research (2018) 19:143 Page 6 of 12 a b Fig. 2 The seasonal distribution of all exacerbations and exacerbations with infiltrate. a.) Total number of exacerbations, and number of exacerbations with infiltrate by month; b.) Proportion of total exacerbations, with infiltrate by month bacterial acquisition between exacerbations with and without pneumonic infiltrate (n = 15 (39.5%) versus n = 62 (37.1%) respectively; p = 0.787). Pneumonic infiltrates and the microbiome Firmicutes, Proteobacteria and Bacteroidetes were the most abundant phyla and Veillonella, Haemophilus, Streptococcus and Prevotella the most abundant genera identified at exacerbation. Stratifying by presence or absence of pneumonic infiltrate did not identify any significant differences in the main phyla (p = 0.350), genera (p = 0.540) nor in Shannon diversity between exacerbation groups. (Fig. 3 and Additional file 2: Figure S2). Pneumonic infiltrates and inflammatory responses When considering all exacerbations, those with pneumonic infiltrates had significantly greater systemic inflammation (p < for CRP, fibrinogen and neutrophil count), than those without (Table 2). In exacerbations with available high quality sputum (n = 209), pneumonic infiltrate was associated with an increased sputum neutrophil percentage (89.6 versus 79.1, p = 0.007). CRP had the greatest area under the receiver operator curve (0.692) for distinguishing exacerbations with infiltrate (Additional file 2: FigureS3). Inflammation can vary at baseline, therefore a paired analysis comparing pre-exacerbation (stable visit) with exacerbation samples was performed for 193 exacerbations. The median (IQR) time between stable and exacerbation samples was 42 (39) days for those with pneumonic infiltrate and 38 (46) days for those without (p = 0.328). All markers analysed, increased at exacerbation. However, exacerbations with pneumonic infiltrate had a significantly higher median CRP (19.0 versus 7.0 mg/l; p < 0.001), fibrinogen (5.8 versus 5.0 g/l; p < 0.005), blood neutrophil count (7.7 versus L; p <0.001) (Fig. 4 and Additional file 2: Table S5) and greater median changes from stable-state than those without (Additional file 2: Table S6). For PCT, although greater increases were observed from stable-state in those exacerbations with infiltrate compared to those without, PCT was otherwise not a good discriminatory marker. A separate paired analysis (Additional file 2: Table S7), using only the first infiltrate-associated exacerbation where available, or the first non-infiltrative exacerbation where not (subjects were therefore represented only once), yielded similar results. Infiltrate character did not appear to influence inflammatory responses. Lung function changes at exacerbation Paired lung function was available for 239 exacerbations. To highlight clinical severity, infiltrate-associated exacerbations were associated with greater falls in FEV1 than those without ( 125 ml versus -40 ml, p = 0.010). A similar trend was also seen for FVC, although this did not reach significance (Additional file 2: Table S8). Predicting pneumonic infiltrate at exacerbation Factors significantly associated with increased odds of having pneumonic infiltrate at exacerbation were winter season (OR 3.056, 95%CI , p =0.027) and greater CRP levels (categorised by tertiles) at exacerbation (OR 5.723, 95%CI , p < for a CRP > 18
7 Williams et al. Respiratory Research (2018) 19:143 Page 7 of 12 a b Fig. 3 The lung microbiome of exacerbations, stratified by the presence or absence of pneumonic infiltrate. a.) The Shannon diversity index did not show any significant difference between radiological groups (p = 0.34). b.) The genus-level abundances showed no significant differences between groups (p = 0.54) versus < 6 mg/l). Cold and/or sore throat symptoms were associated with reduced odds of having pneumonic infiltrate (OR 0.435, 95%CI , p =0.014)(Table4). Discussion In this prospective study of outpatients with moderate to very severe COPD, we show that exacerbations with new pneumonic infiltrates were associated with heightened levels of systemic inflammation, greater lung function deterioration and similar microbiological signatures, compared to exacerbations without. Importantly, more than 94% of exacerbations were managed as an outpatient, therefore providing a unique insight into the incidence and significance of pneumonic infiltrate in this clinical setting. Recent interest has focused on understanding the heterogeneity of exacerbations [8]. Although the use of CXR has been reported in hospitalised COPD patients [13, 21, 22], few prospective studies report on its use in such a well-characterised outpatient COPD cohort, and none addressing the microbiological and inflammatory complexities of exacerbations to the extent of the AERIS study. The only other large COPD study collecting radiological data at exacerbation, did not report on inflammatory or microbiological indices [23]. Although having a similar patient demographic, the differing primary objectives and aims make direct comparisons to our study difficult. Pneumonic infiltrates were a feature in approximately 20% of exacerbations. Furthermore, half of all infiltrate-associated exacerbations occurred during the winter, and proportionally were a feature in around a third of all exacerbations occurring during that period, therefore contributing considerably to the exacerbation-burden during this time of year. In multivariate analysis seasonality remained an important determinant, with greater odds of having an exacerbation with infiltrate in winter, compared to summer. The seasonal nature of exacerbations and of pneumonia in COPD has previously been described [6, 11, 24, 25]. Population behaviours, changes in temperature and humidity, as well as cyclical patterns of respiratory pathogens have all been proposed as key determinants [26]. Understanding whether seasonality could direct exacerbation treatment approaches remains to be seen, but characterising exacerbations using methods such as CXR could guide this approach. Exacerbations are typically inflammatory events [5]. In this study, exacerbations with pneumonic infiltrate were associated with greater airway neutrophillic inflammation
8 Williams et al. Respiratory Research (2018) 19:143 Page 8 of 12 a Fig. 4 Blood inflammatory marker levels at exacerbations, stratified by the presence or absence of pneumonic infiltrate. Box and whisker plots showing a.) Blood C-reactive protein (CRP) levels; b.) Blood fibrinogen levels and c.) Blood neutrophil counts, in paired stable and acute exacerbation (AE) samples. White bars represent paired stable, non-infiltrate associated AE visits and grey bars represent paired stable, infiltrate-associated AE visits. Data are presented as median (interquartile range) b c and showed more intense systemic inflammatory responses than exacerbations without. Additionally, significant decreases in FEV1 at exacerbation onset were seen in those with pneumonic infiltrate. This observed decline, provides a measure of exacerbation severity and may be directly associated with the greater inflammatory responses identified. Neutrophilic inflammation has previously been shown to correlate with greater decreases in lung function during exacerbation [27, 28], but these studies did not utilise radiological stratification. The intensity of systemic inflammation at exacerbation has previously been linked to the presence of bacteria [4], bacterial strain changes [29] and bacterial-viral co-infection [28]. In agreement with prior studies [30, 31], we have previously reported a substantial number of exacerbations associated with viral detection within this cohort [6]. However, overall the proportion of viral-associated exacerbations with and without pneumonic infiltrate was broadly similar and viral symptoms (cold and/or sore throat) occurred less frequently in exacerbations with pneumonic infiltrate. Bacteria, particularly HI, have long been regarded as an important cause of exacerbations [32]. Conversely, studies of hospitalised pneumonia in COPD patients consistently report SP as the most frequently implicated bacteria [13, 22], similarly for hospitalised pneumonia in patients without COPD [15]. Like previous studies, our data re-confirms HI as the most frequently detected bacteria at exacerbation, but additionally suggests a potential association between HI and pneumonic infiltrate. By culture, HI was implicated in 52% of exacerbations with infiltrate compared to 38% of those without. However, as HI commonly causes chronic infection in COPD even at stable-state, establishing a causal link between HI detection at exacerbation and risk of pneumonic infiltrate is challenging. In this regard, although new bacterial acquisition did not appear to be associated with pneumonic infiltrate, bacterial strain changes and increasing load of existing pathogens could be key mechanisms, having previously been implicated in exacerbation development and more profound inflammatory responses [28, 29]. This study adds to the evolving exploration of exacerbation heterogeneity, using radiological stratification in an outpatient COPD population. Current practise varies on whether an acute respiratory illness, complicated by
9 Williams et al. Respiratory Research (2018) 19:143 Page 9 of 12 Table 4 Risk factors associated with the odds of pneumonic infiltrate at exacerbation Characteristic OR (95% CI) P-value Age (per year) ( ) Male sex ( ) BMI (per 1 kg/m 2 ) ( ) Current smoker ( ) ICS use ( Maintenance bronchodilator use a ( ) FEV1% predicted 50% Reference 30- < 50% ( ) < 30% ( ) Season Spring (Mar May) ( ) Summer (Jun Aug) Reference Autumn (Sep Nov) ( ) Winter (Dec Feb) ( ) Sputum purulence ( ) Fever ( ) Cold and/or sore throat ( ) Blood eosinophils < 2% at exacerbation ( ) CRP < 6 mg/l Reference CRP 6 18 mg/l ( ) CRP > 18 mg/l ( ) < BMI body mass index, FEV1 forced expiratory volume in 1 s, CRP C-reactive protein Clustered multivariate logistic regression analysis, including the patient as a random effect, for 313 exacerbations (63 with infiltrate, 250 without) with complete data for all the variables included in the model. Other independent variables included in the regression model were influenza and pneumococcal vaccine in the year prior to enrolment a refers to the use of either a long acting beta agonist or long acting muscarinic antagonist, alone or in combination with other inhaled medication CRP ranges based on categorisation by tertiles pneumonic infiltrate in COPD patients is included under the diagnosis of exacerbation or considered as pneumonia. There is some evidence to suggest that exacerbations and pneumonia in COPD differ in their aetiology and inflammatory profile and should perhaps be considered distinct, despite being clinically hard to distinguish [13, 33]. Our findings suggest that the infective aetiology of exacerbations is similar to that of acute events classified as pneumonia in an outpatient clinical setting. The results suggest common mechanisms between pneumonia and exacerbations in patients with COPD and will help to inform exacerbation management. We recognise that this study has limitations. The use of CXR remains the radiological standard for diagnosing pneumonia, and yet false-positive and false-negative results can occur [34]. CT scanning, may improve diagnostic accuracy, but is currently more expensive, less readily available and associated with greater radiation dose than plain CXR, making its utilisation currently unfeasible in a study of frequent exacerbators. The mean exacerbation rate in AERIS reflects the selected cohort of patients with a history of frequent exacerbations. We accept that our findings may not be generalizable to more heterogeneous groups, or those that infrequently exacerbate. Nevertheless, like other studies, the exacerbation rate for the cohort was similar to that in the year prior to enrolment, suggesting a fairly constant phenotype rather than an overestimation of exacerbations [35, 36]. There are well-established links between ICS use and increased pneumonia risk in COPD [10, 37]. In our cohort, the majority of patients were already prescribed ICS at enrolment, limiting our ability to assess the impact of ICS use on the risk of developing pneumonic infiltrates. We also acknowledge that differing methodological approaches for determining aetiology, may limit direct comparisons with other studies. However, like a recent report of hospitalised pneumonia, not limited to COPD patients [38], we utilised sputum for microbiological analysis and similarly identified HI as the most frequently detected bacteria. This contrasts with many other studies of COPD patients hospitalised
10 Williams et al. Respiratory Research (2018) 19:143 Page 10 of 12 with pneumonia, where an aetiological diagnosis was predominantly made from blood culture, urinary antigen testing or serological analysis and was typically found to be SP [13, 21]. However, due to the early identification and treatment of exacerbations, we cannot exclude the possibility that bacteria such as SP may have a role in the later stages of pneumonic exacerbations. Additionally, the use of electronic diary cards and consequently the early capture of exacerbations, may underestimate the frequency of complicating pneumonic infiltrates, as radiological changes in the context of acute lower respiratory tract illness can lag behind clinical symptoms [39]. Conclusions This is the first prospective study to radiologically stratify exacerbations and demonstrate the incidence, seasonality and character of acute events characterised as pneumonia in an outpatient COPD cohort. That pneumonia is common in this clinical context, associated with heightened levels of systemic inflammation, greater lung function deterioration and a similar infective aetiology to exacerbations, is highly relevant clinically. These results suggest that exacerbations and pneumonia in COPD share common infectious triggers and represent a continuum of severity rather than distinct aetiological events. This knowledge will help to inform exacerbation management, particularly important in an era of emerging antimicrobial resistance and antibiotic governance. Based on these findings, interventional studies, testing the utility of radiological and inflammatory stratification of exacerbations to assess optimal response to standard treatments are required. Additional files Additional file 1: AERIS study subject inclusion and exclusion criteria and supplementary methods. (DOCX 58 kb) Additional file 2: Table S1. Radiological findings at exacerbation. Table S2. Exacerbation treatment stratified by the presence or absence of radiographic pneumonic infiltrate. Table S3. Bacterial identification by culture and PCR in all exacerbation sputum samples and those exacerbation sputum samples with fewer than 30% squamous cells (considered high quality). Table S4. Bacterial and viral identification at exacerbation by culture (bacteria) and PCR (bacteria/viral). Table S5. Levels of inflammatory markers at paired stable and exacerbation visits. Table S6. Changes in levels of serum inflammatory markers between stable (pre-exacerbation) and exacerbation samples. Table S7. Levels of serum inflammatory markers at paired stable and exacerbation visits. The occurrence of the first infiltrate-associated exacerbation where available was prioritised, or first non-infiltrative exacerbation if not (subjects are therefore only represented once). Table S8. Lung function changes between nearest stable-state and exacerbation visits, stratified by the presence/absence of pneumonic infiltrate. Figure S1. The proportion of bacterial positive sputum samples at exacerbation by both culture and PCR. Figure S2. The lung microbiome (phylum) of exacerbations stratified by the presence or absence of pneumonic infiltrate. Figure S3. Area under the receiver operator curve analysis for CRP, fibrinogen and neutrophil count. (DOCX 232 kb) Abbreviations AERIS: Acute Exacerbations and Respiratory Infections in COPD study; CI: Confidence interval; COPD: Chronic obstructive pulmonary disease; CRP: C-reactive protein; CT: Computed tomography; CXR: Chest radiograph; FEV1: Forced expiratory volume in 1 s; FVC: Forced vital capacity; GOLD: Global initiative for chronic obstructive lung disease; HI: Haemophilus influenzae; ICS: Inhaled corticosteroid; IQR: Interquartile range; OCS: Oral corticosteroids; OR: Odds ratio; OTU: Operational taxanomic units; PCR: Polymerase chain reaction; PCT: Procalcitonin; SD: Standard deviation; SP: Streptococcus pneumoniae Acknowledgements The authors would like to thank all the study volunteers for their invaluable contribution towards furthering COPD knowledge and each team member for their assistance conducting the study. We acknowledge all members of the AERIS study group. The authors would also like to thank Geraldine Drevon and Regis Azizieh (XPE Pharma & Science, on behalf of GSK Vaccines) for coordination and editorial support. The study was funded by GlaxoSmithKline Biologicals SA. The AERIS Study Group: J Alnajar, R Anderson, E Aris, WR Ballou, A Barton, S Bourne, M Caubet, SC Clarke, D Cleary, C Cohet, NA Coombs, K Cox, J-M Devaster, V Devine, N Devos, E Dineen, T Elliot, R Gladstone, S Harden, J Jefferies, V Kim, C Lambert, S Mesia-Vela, P Moris, K Ostridge, TG Pascal, M Peeters, S Schoonbroodt, KJ Staples, A Tuck, L Welsh, V Weynants, TMA Wilkinson, AP Williams, NP Williams, C Woelk, M Wojtas, S Wootton. All members of the AERIS Study Group were involved in the planning, conduct, and/or reporting of the work described in the article. Funding The study was funded by GlaxoSmithKline Biologicals SA. No restrictions were placed on authors regarding the statements made in the manuscript. Authors contributions NPW had full access to the data and takes responsibility for the accuracy of the data analysis. JMD, SB, SW, AT, VK, SCC, AW and TMAW conceived and designed the AERIS study. EA, JMD, CL, SB, SW, AT, NPW, KO, KJS, SCC, VK, AW, SH and TMAW collected or generated the data. EA, JMD, CL SB, SW, NPW, KO, KJS, SCC, NAC, VK, AW and TMAW analysed or interpreted the data, All authors contributed to the development of the manuscript and approved the final version. Ethics approval and consent to participate The study was approved by the Southampton and South West Hampshire Research Ethics Committee. All participants provided written informed consent. Consent for publication Not applicable. Competing interests TMAW has received reimbursement for travel and meeting attendance from Boehringer Ingelheim and AstraZeneca, outside of the submitted work. SB received grants and assistance in travel to conferences from GSK outside of the submitted work. SCC received a grant from Pfizer outside of the submitted work. KJS received grants from Asthma UK (08/026) and BMA HC Roscoe Award outside of the submitted work, and he has a patent PCT/ GB2010/ Ex Vivo Modelling of Therapeutic Interventions pending. EA, JMD and CL are employees of the GSK group of companies. EA and JMD hold shares/restricted shares in the GSK group of companies. KJS, VK, NPW, KO, SW, and TMAW received an institutional grant from the GSK group of companies to conduct this study. AW, NAC, SH and AT declare no conflict of interest. Publisher s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Author details 1 Clinical and Experimental Sciences, University of Southampton Faculty of Medicine, Southampton General Hospital, Southampton, UK. 2 Southampton
11 Williams et al. Respiratory Research (2018) 19:143 Page 11 of 12 NIHR Respiratory Biomedical Research Unit, Southampton General Hospital, Southampton, UK. 3 GSK Vaccines, Rixensart, Belgium. 4 Primary Care and Population Sciences, Faculty of Medicine, University of Southampton, Southampton, UK. 5 Wessex Investigational Sciences Hub, University of Southampton Faculty of Medicine, Southampton General Hospital, Southampton, UK. 6 Department of Radiology, University Hospital Southampton NHS Foundation Trust, Southampton General Hospital, Tremona Road, Southampton, UK. 7 Faculty of Medicine and Institute for Life Sciences, University of Southampton, Southampton, UK. 8 Southampton NIHR Biomedical Research Centre, University Hospital Southampton NHS Foundation Trust, Southampton, UK. 9 Present address: Portsmouth Hospitals NHS Trust, Queen Alexandra Hospital, Portsmouth, UK. Received: 8 May 2018 Accepted: 10 July 2018 References 1. Miravitlles M, Ferrer M, Pont A, Zalacain R, Alvarez-Sala JL, Masa F, Verea H, Murio C, Ros F, Vidal R. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax. 2004;59: Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57: Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. 2010;19: Hurst JR, Perera WR, Wilkinson TM, Donaldson GC, Wedzicha JA. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173: Bathoorn E, Kerstjens H, Postma D, Timens W, MacNee W. Airways inflammation and treatment during acute exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2008;3: Wilkinson TMA, Aris E, Bourne S, Clarke SC, Peeters M, Pascal TG, Schoonbroodt S, Tuck AC, Kim V, Ostridge K, et al. A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax. 2017; 7. Miravitlles M, Murio C, Guerrero T. Factors associated with relapse after ambulatory treatment of acute exacerbations of chronic bronchitis. DAFNE Study Group Eur Respir J. 2001;17: Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, McCormick M, Haldar K, Kebadze T, Duvoix A, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184: Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for communityacquired pneumonia in adults in Europe: a literature review. Thorax. 2013; 68: Crim C, Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Willits LR, Yates JC, Vestbo J. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34: Williams NP, Coombs NA, Johnson MJ, Josephs LK, Rigge LA, Staples KJ, Thomas M, Wilkinson TM. Seasonality, risk factors and burden of community-acquired pneumonia in COPD patients: a population database study using linked health care records. Int J Chron Obstruct Pulmon Dis. 2017;12: Royal College of Physicians. National Chronic Obstructive Pulmonary Disease Audit Programme: clinical audit of COPD exacerbations admitted to acute units in England and Wales In: Royal College of Physicians; Huerta A, Crisafulli E, Menendez R, Martinez R, Soler N, Guerrero M, Montull B, Torres A. Pneumonic and nonpneumonic exacerbations of COPD: inflammatory response and clinical characteristics. Chest. 2013;144: Andreassen SL, Liaaen ED, Stenfors N, Henriksen AH. Impact of pneumonia on hospitalizations due to acute exacerbations of COPD. Clin Respir J. 2014; 8: Crisafulli E, Menendez R, Huerta A, Martinez R, Montull B, Clini E, Torres A. Systemic inflammatory pattern of patients with community-acquired pneumonia with and without COPD. Chest. 2013;143: Donaldson GC, Goldring JJ, Wedzicha JA. Influence of season on exacerbation characteristics in patients with COPD. Chest. 2012;141: Bourne S, Cohet C, Kim V, Barton A, Tuck A, Aris E, Mesia-Vela S, Devaster JM, Ballou WR, Clarke SC, Wilkinson T. Acute exacerbation and respiratory InfectionS in COPD (AERIS): protocol for a prospective, observational cohort study. BMJ Open. 2014;4:e Kim VL, Coombs NA, Staples KJ, Ostridge KK, Williams NP, Wootton SA, Devaster JM, Aris E, Clarke SC, Tuck AC, et al. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir J. 2017; Ostridge K, Williams NP, Kim V, Harden S, Bourne S, Clarke SC, Aris E, Mesia- Vela S, Devaster JM, Tuck A, et al. Relationship of CT-quantified emphysema, small airways disease and bronchial wall dimensions with physiological, inflammatory and infective measures in COPD. Respir Res. 2018;19: Mayhew D, Devos N, Lambert C, Brown JR, Clarke SC, Kim VL, Magid-Slav M, Miller BE, Ostridge KK, Patel R, et al. Longitudinal profiling of the lung microbiome in the AERIS study demonstrates repeatability of bacterial and eosinophilic COPD exacerbations. Thorax. 2018; 21. Molinos L, Clemente MG, Miranda B, Alvarez C, del Busto B, Cocina BR, Alvarez F, Gorostidi J, Orejas C. Community-acquired pneumonia in patients with and without chronic obstructive pulmonary disease. J Infect. 2009;58: Liapikou A, Polverino E, Ewig S, Cilloniz C, Marcos MA, Mensa J, Bello S, Martin-Loeches I, Menendez R, Torres A. Severity and outcomes of hospitalised community-acquired pneumonia in COPD patients. Eur Respir J. 2012;39: Crim C, Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, Wachtel A, Martinez FJ, Barnhart F, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12: Jenkins CR, Celli B, Anderson JA, Ferguson GT, Jones PW, Vestbo J, Yates JC, Calverley PM. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur Respir J. 2012;39: Rabe KF, Fabbri LM, Vogelmeier C, Kogler H, Schmidt H, Beeh KM, Glaab T. Seasonal distribution of COPD exacerbations in the prevention of exacerbations with tiotropium in COPD trial. Chest. 2013;143: Donaldson GC, Wedzicha JA. The causes and consequences of seasonal variation in COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2014;9: Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173: Wilkinson TM, Hurst JR, Perera WR, Wilks M, Donaldson GC, Wedzicha JA. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest. 2006;129: Sethi S, Wrona C, Eschberger K, Lobbins P, Cai X, Murphy TF. Inflammatory profile of new bacterial strain exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177: Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, Maccallum P, Meade TW, Jeffries DJ, Johnston SL, Wedzicha JA. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164: McManus TE, Marley AM, Baxter N, Christie SN, O'Neill HJ, Elborn JS, Coyle PV, Kidney JC. Respiratory viral infection in exacerbations of COPD. Respir Med. 2008;102: Sethi S. Bacteria in exacerbations of chronic obstructive pulmonary disease: phenomenon or epiphenomenon? Proc Am Thorac Soc. 2004;1: Gutierrez P, Closa D, Piner R, Bulbena O, Menendez R, Torres A. Macrophage activation in exacerbated COPD with and without community-acquired pneumonia. Eur Respir J. 2010;36: Claessens YE, Debray MP, Tubach F, Brun AL, Rammaert B, Hausfater P, Naccache JM, Ray P, Choquet C, Carette MF, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192: Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363: Mullerova H, Shukla A, Hawkins A, Quint J. Risk factors for acute exacerbations of COPD in a primary care population: a retrospective observational cohort study. BMJ Open. 2014;4:e
12 Williams et al. Respiratory Research (2018) 19:143 Page 12 of Calverley PM, Stockley RA, Seemungal TA, Hagan G, Willits LR, Riley JH, Wedzicha JA. Reported pneumonia in patients with COPD: findings from the INSPIRE study. Chest. 2011;139: Gadsby NJ, Russell CD, McHugh MP, Mark H, Conway Morris A, Laurenson IF, Hill AT, Templeton KE. Comprehensive molecular testing for respiratory pathogens in community-acquired pneumonia. Clin Infect Dis. 2016;62: Hagaman JT, Rouan GW, Shipley RT, Panos RJ. Admission chest radiograph lacks sensitivity in the diagnosis of community-acquired pneumonia. Am J Med Sci. 2009;337:
Consolidation and Exacerbation of COPD
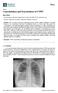 Review Consolidation and Exacerbation of COPD John R Hurst UCL Respiratory, University College London, London, UK, NW3 2PF, UK; j.hurst@ucl.ac.uk Received: 7 March 2018; Accepted: 31 May 2018; Published:
Review Consolidation and Exacerbation of COPD John R Hurst UCL Respiratory, University College London, London, UK, NW3 2PF, UK; j.hurst@ucl.ac.uk Received: 7 March 2018; Accepted: 31 May 2018; Published:
An Update in COPD John Hurst PhD FRCP
 An Update in COPD John Hurst PhD FRCP Reader in Respiratory Medicine / Honorary Consultant University College London / Royal Free London NHS Foundation Trust j.hurst@ucl.ac.uk What s new in COPD papers
An Update in COPD John Hurst PhD FRCP Reader in Respiratory Medicine / Honorary Consultant University College London / Royal Free London NHS Foundation Trust j.hurst@ucl.ac.uk What s new in COPD papers
Time course and pattern of COPD exacerbation onset
 < An additional material is published online only. To view this file please visit the journal online (http://thorax.bmj.com/ content/67/3.toc). 1 Department of Medicine, The Ottawa Hospital Research Institute,
< An additional material is published online only. To view this file please visit the journal online (http://thorax.bmj.com/ content/67/3.toc). 1 Department of Medicine, The Ottawa Hospital Research Institute,
To describe the impact of COPD exacerbations and the importance of the frequent exacerbator phenotype.
 Educational aims To describe the impact of COPD exacerbations and the importance of the frequent exacerbator phenotype. To describe the spectrum of pharmacological and non-pharmacological interventions
Educational aims To describe the impact of COPD exacerbations and the importance of the frequent exacerbator phenotype. To describe the spectrum of pharmacological and non-pharmacological interventions
Update on COPD John Hurst PhD FRCP
 Update on COPD John Hurst PhD FRCP Reader / Consultant in Respiratory Medicine UCL / Royal Free London NHS Foundation Trust Director, UCL Royal Free COPD Centre j.hurst@ucl.ac.uk Questions 1. What is the
Update on COPD John Hurst PhD FRCP Reader / Consultant in Respiratory Medicine UCL / Royal Free London NHS Foundation Trust Director, UCL Royal Free COPD Centre j.hurst@ucl.ac.uk Questions 1. What is the
COPD EXACERBATIONS AND HOSPITAL ADMISSIONS HOW CAN WE PREVENT THEM? Wisia Wedzicha National Heart and Lung Institute, Imperial College London, UK
 COPD EXACERBATIONS AND HOSPITAL ADMISSIONS HOW CAN WE PREVENT THEM? Wisia Wedzicha National Heart and Lung Institute, Imperial College London, UK Presenter Disclosures Wisia Wedzicha All disclosures prior
COPD EXACERBATIONS AND HOSPITAL ADMISSIONS HOW CAN WE PREVENT THEM? Wisia Wedzicha National Heart and Lung Institute, Imperial College London, UK Presenter Disclosures Wisia Wedzicha All disclosures prior
What s new in COPD? Apichart Khanichap MD. Department of Medicine, Faculty of Medicine, Thammasat university
 What s new in COPD? Apichart Khanichap MD. Department of Medicine, Faculty of Medicine, Thammasat university Management stable COPD Relieve symptoms Improve exercise tolerance Improve health status Prevent
What s new in COPD? Apichart Khanichap MD. Department of Medicine, Faculty of Medicine, Thammasat university Management stable COPD Relieve symptoms Improve exercise tolerance Improve health status Prevent
Turning Science into Real Life Roflumilast in Clinical Practice. Roland Buhl Pulmonary Department Mainz University Hospital
 Turning Science into Real Life Roflumilast in Clinical Practice Roland Buhl Pulmonary Department Mainz University Hospital Therapy at each stage of COPD I: Mild II: Moderate III: Severe IV: Very severe
Turning Science into Real Life Roflumilast in Clinical Practice Roland Buhl Pulmonary Department Mainz University Hospital Therapy at each stage of COPD I: Mild II: Moderate III: Severe IV: Very severe
Francesco Blasi Head Respiratory Medicine Section Cardio-Thoracic Department University of Milan, Italy
 COPD EXACERBATIONS Francesco Blasi Head Respiratory Medicine Section Cardio-Thoracic Department University of Milan, Italy COPD OUTCOMES Cazzola M et al. ERJ 2008 COPD AND CARDIOVASCULAR DISEASE Cumulative
COPD EXACERBATIONS Francesco Blasi Head Respiratory Medicine Section Cardio-Thoracic Department University of Milan, Italy COPD OUTCOMES Cazzola M et al. ERJ 2008 COPD AND CARDIOVASCULAR DISEASE Cumulative
Circulating eosinophil levels do not predict severe exacerbations in COPD: a retrospective study
 ORIGINAL ARTICLE COPD Circulating eosinophil levels do not predict severe exacerbations in COPD: a retrospective study Yochai Adir 1, Omar Hakrush 1, Michal Shteinberg 1, Sonia Schneer 1 and Alvar Agusti
ORIGINAL ARTICLE COPD Circulating eosinophil levels do not predict severe exacerbations in COPD: a retrospective study Yochai Adir 1, Omar Hakrush 1, Michal Shteinberg 1, Sonia Schneer 1 and Alvar Agusti
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Calverley P M A, Anzueto A R, Carter K, et
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Calverley P M A, Anzueto A R, Carter K, et
The role of blood eosinophil level in acute exacerbation of Chronic Obstructive Pulmonary Disease
 112 Original Article The role of blood eosinophil level in acute exacerbation of Chronic Obstructive Pulmonary Disease Department of Pulmonology and Critical Care Medicine, Tribhuvan University Teaching
112 Original Article The role of blood eosinophil level in acute exacerbation of Chronic Obstructive Pulmonary Disease Department of Pulmonology and Critical Care Medicine, Tribhuvan University Teaching
Is Acute Exacerbation of COPD (AECOPD) Related to Viral Infection Associated with Subsequent Mortality or Exacerbation Rate? KHERAD, Omar, et al.
 Article Is Acute Exacerbation of COPD (AECOPD) Related to Viral Infection Associated with Subsequent Mortality or Exacerbation Rate? KHERAD, Omar, et al. Abstract There is a growing interest in better
Article Is Acute Exacerbation of COPD (AECOPD) Related to Viral Infection Associated with Subsequent Mortality or Exacerbation Rate? KHERAD, Omar, et al. Abstract There is a growing interest in better
Blood Eosinophils and Response to Maintenance COPD Treatment: Data from the FLAME Trial. Online Data Supplement
 Blood Eosinophils and Response to Maintenance COPD Treatment: Data from the FLAME Trial Nicolas Roche, Kenneth R. Chapman, Claus F. Vogelmeier, Felix JF Herth, Chau Thach, Robert Fogel, Petter Olsson,
Blood Eosinophils and Response to Maintenance COPD Treatment: Data from the FLAME Trial Nicolas Roche, Kenneth R. Chapman, Claus F. Vogelmeier, Felix JF Herth, Chau Thach, Robert Fogel, Petter Olsson,
The Relationship among COPD Severity, Inhaled Corticosteroid Use, and the Risk of Pneumonia.
 The Relationship among COPD Severity, Inhaled Corticosteroid Use, and the Risk of Pneumonia. Rennard, Stephen I; Sin, Donald D; Tashkin, Donald P; Calverley, Peter M; Radner, Finn Published in: Annals
The Relationship among COPD Severity, Inhaled Corticosteroid Use, and the Risk of Pneumonia. Rennard, Stephen I; Sin, Donald D; Tashkin, Donald P; Calverley, Peter M; Radner, Finn Published in: Annals
Advances in the management of chronic obstructive lung diseases (COPD) David CL Lam Department of Medicine University of Hong Kong October, 2015
 Advances in the management of chronic obstructive lung diseases (COPD) David CL Lam Department of Medicine University of Hong Kong October, 2015 Chronic obstructive pulmonary disease (COPD) COPD in Hong
Advances in the management of chronic obstructive lung diseases (COPD) David CL Lam Department of Medicine University of Hong Kong October, 2015 Chronic obstructive pulmonary disease (COPD) COPD in Hong
Surveillance report Published: 6 April 2016 nice.org.uk. NICE All rights reserved.
 Surveillance report 2016 Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2010) NICE guideline CG101 Surveillance report Published: 6 April 2016 nice.org.uk NICE 2016. All rights
Surveillance report 2016 Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2010) NICE guideline CG101 Surveillance report Published: 6 April 2016 nice.org.uk NICE 2016. All rights
The distribution of COPD in UK general practice using the new GOLD classification
 ERJ Express. Published on October 31, 2013 as doi: 10.1183/09031936.00065013 The distribution of COPD in UK general practice using the new GOLD classification John Haughney 1, Kevin Gruffydd-Jones 2, June
ERJ Express. Published on October 31, 2013 as doi: 10.1183/09031936.00065013 The distribution of COPD in UK general practice using the new GOLD classification John Haughney 1, Kevin Gruffydd-Jones 2, June
Research in Real Life
 Research in Real Life Study 1: Exploratory study - identifying the benefits of pmdi versus Diskus for delivering fluticasone/salmeterol combination therapy in patients with chronic obstructive pulmonary
Research in Real Life Study 1: Exploratory study - identifying the benefits of pmdi versus Diskus for delivering fluticasone/salmeterol combination therapy in patients with chronic obstructive pulmonary
TORCH: Salmeterol and Fluticasone Propionate and Survival in COPD
 TORCH: and Propionate and Survival in COPD April 19, 2007 Justin Lee Pharmacy Resident University Health Network Outline Overview of COPD Pathophysiology Pharmacological Treatment Overview of the TORCH
TORCH: and Propionate and Survival in COPD April 19, 2007 Justin Lee Pharmacy Resident University Health Network Outline Overview of COPD Pathophysiology Pharmacological Treatment Overview of the TORCH
The distribution of COPD in UK general practice using the new GOLD classification
 ORIGINAL ARTICLE COPD The distribution of COPD in UK general practice using the new GOLD classification John Haughney 1, Kevin Gruffydd-Jones 2, June Roberts 3, Amanda J. Lee 4, Alison Hardwell 5 and Lorcan
ORIGINAL ARTICLE COPD The distribution of COPD in UK general practice using the new GOLD classification John Haughney 1, Kevin Gruffydd-Jones 2, June Roberts 3, Amanda J. Lee 4, Alison Hardwell 5 and Lorcan
The Journal Club: COPD Exacerbations
 252 The Journal Club: COPD Exacerbations Chronic Obstructive Pulmonary Diseases: Journal of the COPD Foundation Journal Club The Journal Club: COPD Exacerbations Ron Balkissoon, MD, MSc, DIH, FRCPC 1 Abbreviations:
252 The Journal Club: COPD Exacerbations Chronic Obstructive Pulmonary Diseases: Journal of the COPD Foundation Journal Club The Journal Club: COPD Exacerbations Ron Balkissoon, MD, MSc, DIH, FRCPC 1 Abbreviations:
Outcomes: Initially, our primary definitions of pneumonia was severe pneumonia, where the subject was hospitalized
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Predictors of exacerbation frequency in chronic obstructive pulmonary disease
 Yang et al. European Journal of Medical Research 2014, 19:18 EUROPEAN JOURNAL OF MEDICAL RESEARCH RESEARCH Open Access Predictors of exacerbation frequency in chronic obstructive pulmonary disease Hui
Yang et al. European Journal of Medical Research 2014, 19:18 EUROPEAN JOURNAL OF MEDICAL RESEARCH RESEARCH Open Access Predictors of exacerbation frequency in chronic obstructive pulmonary disease Hui
Title: Objective measurement of cough frequency during COPD exacerbation convalescence
 The final publication is available at Springer via http://dx.doi.org/10.1007/s00408-015-9782-y Title: Objective measurement of cough frequency during COPD exacerbation convalescence Michael G Crooks 1,
The final publication is available at Springer via http://dx.doi.org/10.1007/s00408-015-9782-y Title: Objective measurement of cough frequency during COPD exacerbation convalescence Michael G Crooks 1,
Potential risks of ICS use
 Potential risks of ICS use Randomised controlled trial Observational study Systematic review Pneumonia Tuberculosis Bone fracture Skin thinning/easy bruising Cataract Diabetes No effect on fracture risk
Potential risks of ICS use Randomised controlled trial Observational study Systematic review Pneumonia Tuberculosis Bone fracture Skin thinning/easy bruising Cataract Diabetes No effect on fracture risk
roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd
 roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd 06 August 2010 (Issued 10 September 2010) The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd 06 August 2010 (Issued 10 September 2010) The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Chronic obstructive pulmonary disease (COPD) is characterized
 RESEARCH Impact of COPD Exacerbation Frequency on Costs for a Managed Care Population Anand A. Dalal, PhD, MBA; Jeetvan Patel, MS; Anna D Souza, BPharm, PhD; Eileen Farrelly, MPH; Saurabh Nagar, MS; and
RESEARCH Impact of COPD Exacerbation Frequency on Costs for a Managed Care Population Anand A. Dalal, PhD, MBA; Jeetvan Patel, MS; Anna D Souza, BPharm, PhD; Eileen Farrelly, MPH; Saurabh Nagar, MS; and
Prevention of COPD exacerbations: medications and other controversies
 REVIEW COPD Prevention of COPD exacerbations: medications and other controversies Jørgen Vestbo 1 and Peter Lange 2,3 Affiliations: 1 Centre for Respiratory Medicine and Allergy, Institute of Inflammation
REVIEW COPD Prevention of COPD exacerbations: medications and other controversies Jørgen Vestbo 1 and Peter Lange 2,3 Affiliations: 1 Centre for Respiratory Medicine and Allergy, Institute of Inflammation
Shaping a Dynamic Future in Respiratory Practice. #DFResp
 Shaping a Dynamic Future in Respiratory Practice #DFResp www.dynamicfuture.co.uk Inhaled Therapy in COPD: Past, Present and Future Richard Russell Chest Physician West Hampshire Integrated Respiratory
Shaping a Dynamic Future in Respiratory Practice #DFResp www.dynamicfuture.co.uk Inhaled Therapy in COPD: Past, Present and Future Richard Russell Chest Physician West Hampshire Integrated Respiratory
COPD Bronchiectasis Overlap Syndrome.
 COPD Bronchiectasis Overlap Syndrome. John R Hurst 1, J Stuart Elborn 2, and Anthony De Soyza 3 on Behalf of the BRONCH-UK Consortium (D Bilton, J Bradley, JS Brown, J Duckers, F Copeland, A Floto, J Foweraker,
COPD Bronchiectasis Overlap Syndrome. John R Hurst 1, J Stuart Elborn 2, and Anthony De Soyza 3 on Behalf of the BRONCH-UK Consortium (D Bilton, J Bradley, JS Brown, J Duckers, F Copeland, A Floto, J Foweraker,
ASTHMA-COPD OVERLAP SYNDROME 2018: What s All the Fuss?
 ASTHMA-COPD OVERLAP SYNDROME 2018: What s All the Fuss? Randall W. Brown, MD MPH AE-C Association of Asthma Educators Annual Conference July 20, 2018 Phoenix, Arizona FACULTY/DISCLOSURES Randall Brown,
ASTHMA-COPD OVERLAP SYNDROME 2018: What s All the Fuss? Randall W. Brown, MD MPH AE-C Association of Asthma Educators Annual Conference July 20, 2018 Phoenix, Arizona FACULTY/DISCLOSURES Randall Brown,
Chronic obstructive pulmonary disease
 0 Chronic obstructive pulmonary disease Implementing NICE guidance June 2010 NICE clinical guideline 101 What this presentation covers Background Scope Key priorities for implementation Discussion Find
0 Chronic obstructive pulmonary disease Implementing NICE guidance June 2010 NICE clinical guideline 101 What this presentation covers Background Scope Key priorities for implementation Discussion Find
SGRQ Questionnaire assessing respiratory disease-specific quality of life. Questionnaire assessing general quality of life
 SUPPLEMENTARY MATERIAL e-table 1: Outcomes studied in present analysis. Outcome Abbreviation Definition Nature of data, direction indicating adverse effect (continuous only) Clinical outcomes- subjective
SUPPLEMENTARY MATERIAL e-table 1: Outcomes studied in present analysis. Outcome Abbreviation Definition Nature of data, direction indicating adverse effect (continuous only) Clinical outcomes- subjective
C hronic obstructive pulmonary disease (COPD) is one of
 589 RESPIRATORY INFECTIONS Time course of recovery of health status following an infective exacerbation of chronic bronchitis S Spencer, P W Jones for the GLOBE Study Group... Thorax 2003;58:589 593 See
589 RESPIRATORY INFECTIONS Time course of recovery of health status following an infective exacerbation of chronic bronchitis S Spencer, P W Jones for the GLOBE Study Group... Thorax 2003;58:589 593 See
Defining COPD. Georgina Grantham Community Respiratory Team Leader/ Respiratory Nurse Specialist
 Defining COPD Georgina Grantham Community Respiratory Team Leader/ Respiratory Nurse Specialist Defining COPD Chronic Obstructive Pulmonary Disease (COPD) is a common, preventable and treatable disease
Defining COPD Georgina Grantham Community Respiratory Team Leader/ Respiratory Nurse Specialist Defining COPD Chronic Obstructive Pulmonary Disease (COPD) is a common, preventable and treatable disease
Journal of the COPD Foundation
 132 Predictors of Change in SGRQ Score Chronic Obstructive Pulmonary Diseases: Journal of the COPD Foundation Original Research Baseline Severity as Predictor of Change in St George s Respiratory Questionnaire
132 Predictors of Change in SGRQ Score Chronic Obstructive Pulmonary Diseases: Journal of the COPD Foundation Original Research Baseline Severity as Predictor of Change in St George s Respiratory Questionnaire
Supplementary Online Content
 Supplementary Online Content Regan EA, Lynch DA, Curran-Everett D, et al; Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and radiologic disease in smokers with normal spirometry. Published
Supplementary Online Content Regan EA, Lynch DA, Curran-Everett D, et al; Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and radiologic disease in smokers with normal spirometry. Published
Clinical features and determinants of COPD exacerbation in the Hokkaido COPD cohort study
 ORIGINAL ARTICLE COPD Clinical features and determinants of COPD exacerbation in the Hokkaido COPD cohort study Masaru Suzuki 1, Hironi Makita 1, Yoichi M. Ito 2, Katsura Nagai 1, Satoshi Konno 1 and Masaharu
ORIGINAL ARTICLE COPD Clinical features and determinants of COPD exacerbation in the Hokkaido COPD cohort study Masaru Suzuki 1, Hironi Makita 1, Yoichi M. Ito 2, Katsura Nagai 1, Satoshi Konno 1 and Masaharu
RESPIRATORY CARE IN GENERAL PRACTICE
 RESPIRATORY CARE IN GENERAL PRACTICE Definitions of Asthma and COPD Asthma is due to inflammation of the air passages in the lungs and affects the sensitivity of the nerve endings in the airways so they
RESPIRATORY CARE IN GENERAL PRACTICE Definitions of Asthma and COPD Asthma is due to inflammation of the air passages in the lungs and affects the sensitivity of the nerve endings in the airways so they
Randomized double-blind controlled trial of roflumilast at acute exacerbations of
 Page 1 of 12 AJRCCM Articles in Press. Published on 01-February-2017 as 10.1164/rccm.201612-2518LE Randomized double-blind controlled trial of roflumilast at acute exacerbations of COPD Authors: Alex J
Page 1 of 12 AJRCCM Articles in Press. Published on 01-February-2017 as 10.1164/rccm.201612-2518LE Randomized double-blind controlled trial of roflumilast at acute exacerbations of COPD Authors: Alex J
COPD exacerbation. Dr. med. Frank Rassouli
 Definition according to GOLD report: - «An acute event - characterized by a worsening of the patients respiratory symptoms - that is beyond normal day-to-day variations - and leads to a change in medication»
Definition according to GOLD report: - «An acute event - characterized by a worsening of the patients respiratory symptoms - that is beyond normal day-to-day variations - and leads to a change in medication»
Clinical and radiographic predictors of GOLD-Unclassified smokers in COPDGene
 Clinical and radiographic predictors of GOLD-Unclassified smokers in COPDGene Emily S. Wan, John E. Hokanson, James R. Murphy, Elizabeth A. Regan, Barry J. Make, David A. Lynch, James D. Crapo, Edwin K.
Clinical and radiographic predictors of GOLD-Unclassified smokers in COPDGene Emily S. Wan, John E. Hokanson, James R. Murphy, Elizabeth A. Regan, Barry J. Make, David A. Lynch, James D. Crapo, Edwin K.
C-reactive protein level and microbial aetiology in patients hospitalised with acute exacerbation of COPD
 ERJ Express. Published on September 3, 214 as doi: 1.1183/931936.92214 ORIGINAL ARTICLE IN PRESS CORRECTED PROOF C-reactive protein level and microbial aetiology in patients hospitalised with acute exacerbation
ERJ Express. Published on September 3, 214 as doi: 1.1183/931936.92214 ORIGINAL ARTICLE IN PRESS CORRECTED PROOF C-reactive protein level and microbial aetiology in patients hospitalised with acute exacerbation
exacerbation has greater impact on functional status than frequency of exacerbation episodes.
 Original Article Singapore Med J 2011, 52(12) 894 Changes in the BODE index, exacerbation duration and hospitalisation in a cohort of COPD patients Bu X N, Yang T, Thompson M A, Hutchinson A F, Irving
Original Article Singapore Med J 2011, 52(12) 894 Changes in the BODE index, exacerbation duration and hospitalisation in a cohort of COPD patients Bu X N, Yang T, Thompson M A, Hutchinson A F, Irving
Journal of the COPD Foundation. Journal Club. Chronic Obstructive Pulmonary Diseases:
 85 Journal Club Chronic Obstructive Pulmonary Diseases: Journal of the COPD Foundation Journal Club Ron Balkissoon, MD, MSc, DIH, FRCPC 1 Abbreviations: inhaled corticosteroid, ICS; long acting β 2 -agonist,
85 Journal Club Chronic Obstructive Pulmonary Diseases: Journal of the COPD Foundation Journal Club Ron Balkissoon, MD, MSc, DIH, FRCPC 1 Abbreviations: inhaled corticosteroid, ICS; long acting β 2 -agonist,
THE PHARMA INNOVATION - JOURNAL Acute exacerbation of chronic obstructive pulmonary disease, caused by viruses: the need of combined antiinfective
 Received: 19-11-2013 Accepted: 28-12-2013 ISSN: 2277-7695 CODEN Code: PIHNBQ ZDB-Number: 2663038-2 IC Journal No: 7725 Vol. 2 No. 11. 2014 Online Available at www.thepharmajournal.com THE PHARMA INNOVATION
Received: 19-11-2013 Accepted: 28-12-2013 ISSN: 2277-7695 CODEN Code: PIHNBQ ZDB-Number: 2663038-2 IC Journal No: 7725 Vol. 2 No. 11. 2014 Online Available at www.thepharmajournal.com THE PHARMA INNOVATION
Choosing an inhaler for COPD made simple. Dr Simon Hart Castle Hill Hospital
 Choosing an inhaler for COPD made simple Dr Simon Hart Castle Hill Hospital 1 Declaration of interests I have received speaker fees, sponsorship to attend conferences, and funding for research from companies
Choosing an inhaler for COPD made simple Dr Simon Hart Castle Hill Hospital 1 Declaration of interests I have received speaker fees, sponsorship to attend conferences, and funding for research from companies
Bronchiectasis Domiciliary treatment. Prof. Adam Hill Royal Infirmary and University of Edinburgh
 Bronchiectasis Domiciliary treatment Prof. Adam Hill Royal Infirmary and University of Edinburgh Plan of talk Background of bronchiectasis Who requires IV antibiotics Domiciliary treatment Results to date.
Bronchiectasis Domiciliary treatment Prof. Adam Hill Royal Infirmary and University of Edinburgh Plan of talk Background of bronchiectasis Who requires IV antibiotics Domiciliary treatment Results to date.
UPDATE IN CHRONIC OBSTRUCTIVE PULMONARY DISEASE
 UPDATE IN CHRONIC OBSTRUCTIVE PULMONARY DISEASE Radhika Shah, MD Erlanger Health System University of Tennessee College of Medicine Chattanooga Respiratory, Critical Care, and Sleep medicine No disclosures
UPDATE IN CHRONIC OBSTRUCTIVE PULMONARY DISEASE Radhika Shah, MD Erlanger Health System University of Tennessee College of Medicine Chattanooga Respiratory, Critical Care, and Sleep medicine No disclosures
Life-long asthma and its relationship to COPD. Stephen T Holgate School of Medicine University of Southampton
 Life-long asthma and its relationship to COPD Stephen T Holgate School of Medicine University of Southampton Definitions COPD is a preventable and treatable disease with some significant extrapulmonary
Life-long asthma and its relationship to COPD Stephen T Holgate School of Medicine University of Southampton Definitions COPD is a preventable and treatable disease with some significant extrapulmonary
Antimicrobial Stewardship in Community Acquired Pneumonia
 Antimicrobial Stewardship in Community Acquired Pneumonia Medicine Review Course 2018 Dr Lee Tau Hong Consultant Department of Infectious Diseases National Centre for Infectious Diseases Scope 1. Diagnosis
Antimicrobial Stewardship in Community Acquired Pneumonia Medicine Review Course 2018 Dr Lee Tau Hong Consultant Department of Infectious Diseases National Centre for Infectious Diseases Scope 1. Diagnosis
Three s Company - The role of triple therapy in chronic obstructive pulmonary disease (COPD)
 Three s Company - The role of triple therapy in chronic obstructive pulmonary disease (COPD) Zahava Picado, PharmD PGY1 Pharmacy Practice Resident Central Texas Veterans Healthcare System Temple, TX October
Three s Company - The role of triple therapy in chronic obstructive pulmonary disease (COPD) Zahava Picado, PharmD PGY1 Pharmacy Practice Resident Central Texas Veterans Healthcare System Temple, TX October
Re-Submission. roflumilast, 500 microgram, film-coated tablet (Daxas ) SMC No 635/10 AstraZeneca UK Ltd. Published 11 September
 Re-Submission roflumilast, 500 microgram, film-coated tablet (Daxas ) SMC No 635/10 AstraZeneca UK Ltd 4 August 2017 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Re-Submission roflumilast, 500 microgram, film-coated tablet (Daxas ) SMC No 635/10 AstraZeneca UK Ltd 4 August 2017 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Pneumococcal pneumonia
 Pneumococcal pneumonia Wei Shen Lim Consultant Respiratory Physician & Honorary Professor of Medicine Nottingham University Hospitals NHS Trust University of Nottingham Declarations of interest Unrestricted
Pneumococcal pneumonia Wei Shen Lim Consultant Respiratory Physician & Honorary Professor of Medicine Nottingham University Hospitals NHS Trust University of Nottingham Declarations of interest Unrestricted
Roflumilast: Οι κλινικές μελέτες
 Roflumilast: Οι κλινικές μελέτες Επαμεινώνδας Ν. Κοσμάς Δ/ντής Πνευμονολογικού Τμήματος Νοσοκομείου Metropolitan PDE4 PLAYS AN IMPORTANT ROLE IN INFLAMMATION PDE4 inhibition P P P PDE4 P Adapted from Rabe
Roflumilast: Οι κλινικές μελέτες Επαμεινώνδας Ν. Κοσμάς Δ/ντής Πνευμονολογικού Τμήματος Νοσοκομείου Metropolitan PDE4 PLAYS AN IMPORTANT ROLE IN INFLAMMATION PDE4 inhibition P P P PDE4 P Adapted from Rabe
Optimum treatment for chronic obstructive pulmonary disease exacerbation prevention
 Commentary Page 1 of 5 Optimum treatment for chronic obstructive pulmonary disease exacerbation prevention Pradeep Karur, Dave Singh Centre for Respiratory Medicine and Allergy, Medicines Evaluation Unit,
Commentary Page 1 of 5 Optimum treatment for chronic obstructive pulmonary disease exacerbation prevention Pradeep Karur, Dave Singh Centre for Respiratory Medicine and Allergy, Medicines Evaluation Unit,
Asthma COPD Overlap (ACO)
 Asthma COPD Overlap (ACO) Dr Thomas Brown Consultant Respiratory Physician Thomas.Brown@porthosp.nhs.uk Dr Hitasha Rupani Consultant Respiratory Physician Hitasha.rupani@porthosp.nhs.uk What is Asthma
Asthma COPD Overlap (ACO) Dr Thomas Brown Consultant Respiratory Physician Thomas.Brown@porthosp.nhs.uk Dr Hitasha Rupani Consultant Respiratory Physician Hitasha.rupani@porthosp.nhs.uk What is Asthma
UNDERSTANDING COPD MEDIA BACKGROUNDER
 UNDERSTANDING COPD MEDIA BACKGROUNDER What is COPD? Chronic Obstructive Pulmonary Disease (COPD) also called emphysema and/or chronic obstructive bronchitis* is a preventable lung disease caused by the
UNDERSTANDING COPD MEDIA BACKGROUNDER What is COPD? Chronic Obstructive Pulmonary Disease (COPD) also called emphysema and/or chronic obstructive bronchitis* is a preventable lung disease caused by the
Asthma and COPD in older people lumping or splitting? Christine Jenkins Concord Hospital Woolcock Institute of Medical Research
 Asthma and COPD in older people lumping or splitting? Christine Jenkins Concord Hospital Woolcock Institute of Medical Research Concord Hospital Woolcock Institute of Medical Research Joe has asthma What
Asthma and COPD in older people lumping or splitting? Christine Jenkins Concord Hospital Woolcock Institute of Medical Research Concord Hospital Woolcock Institute of Medical Research Joe has asthma What
National COPD Audit Programme
 National COPD Audit Programme Planning for every breath National Chronic Obstructive Pulmonary Disease (COPD) Audit Programme: Primary care audit () 2015 17 Data analysis and methodology Section 4: Providing
National COPD Audit Programme Planning for every breath National Chronic Obstructive Pulmonary Disease (COPD) Audit Programme: Primary care audit () 2015 17 Data analysis and methodology Section 4: Providing
Outline FEF Reduced FEF25-75 in asthma. What does it mean and what are the clinical implications?
 Reduced FEF25-75 in asthma. What does it mean and what are the clinical implications? Fernando Holguin MD MPH Director, Asthma Clinical & Research Program Center for lungs and Breathing University of Colorado
Reduced FEF25-75 in asthma. What does it mean and what are the clinical implications? Fernando Holguin MD MPH Director, Asthma Clinical & Research Program Center for lungs and Breathing University of Colorado
Roflumilast (Daxas) for chronic obstructive pulmonary disease
 Roflumilast (Daxas) for chronic obstructive pulmonary disease August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Roflumilast (Daxas) for chronic obstructive pulmonary disease August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Inhaled Corticosteroid vs. Add-On Long-Acting Beta-Agonist for Step-Up Therapy in Asthma
 Online Data Supplement Inhaled Corticosteroid vs. Add-On Long-Acting Beta-Agonist for Step-Up Therapy in Asthma Elliot Israel, Nicolas Roche, Richard J. Martin, Gene Colice, Paul M. Dorinsky, Dirkje S.
Online Data Supplement Inhaled Corticosteroid vs. Add-On Long-Acting Beta-Agonist for Step-Up Therapy in Asthma Elliot Israel, Nicolas Roche, Richard J. Martin, Gene Colice, Paul M. Dorinsky, Dirkje S.
Chronic obstructive pulmonary disease in over 16s: diagnosis and management
 National Institute for Health and Care Excellence Draft for consultation Chronic obstructive pulmonary disease in over 16s: diagnosis and management [D] Diagnosing COPD and predicting outcomes NICE guideline
National Institute for Health and Care Excellence Draft for consultation Chronic obstructive pulmonary disease in over 16s: diagnosis and management [D] Diagnosing COPD and predicting outcomes NICE guideline
T he prevalence of chronic obstructive pulmonary
 164 REVIEW SERIES COPD exacerbations? 1: Epidemiology G C Donaldson, J A Wedzicha... The epidemiology of exacerbations of chronic obstructive pulmonary disease (COPD) is reviewed with particular reference
164 REVIEW SERIES COPD exacerbations? 1: Epidemiology G C Donaldson, J A Wedzicha... The epidemiology of exacerbations of chronic obstructive pulmonary disease (COPD) is reviewed with particular reference
COPD: From Phenotypes to Endotypes. MeiLan K Han, M.D., M.S. Associate Professor of Medicine University of Michigan, Ann Arbor, MI
 COPD: From Phenotypes to Endotypes MeiLan K Han, M.D., M.S. Associate Professor of Medicine University of Michigan, Ann Arbor, MI Presenter Disclosures MeiLan K. Han Consulting Research support Novartis
COPD: From Phenotypes to Endotypes MeiLan K Han, M.D., M.S. Associate Professor of Medicine University of Michigan, Ann Arbor, MI Presenter Disclosures MeiLan K. Han Consulting Research support Novartis
THE CHALLENGES OF COPD MANAGEMENT IN PRIMARY CARE An Expert Roundtable
 THE CHALLENGES OF COPD MANAGEMENT IN PRIMARY CARE An Expert Roundtable This activity is supported by an educational grant from Sunovion Pharmaceuticals Inc. COPD in the United States Third leading cause
THE CHALLENGES OF COPD MANAGEMENT IN PRIMARY CARE An Expert Roundtable This activity is supported by an educational grant from Sunovion Pharmaceuticals Inc. COPD in the United States Third leading cause
11/19/2012. The spectrum of pulmonary diseases in HIV-infected persons is broad.
 The spectrum of pulmonary diseases in HIV-infected persons is broad. HIV-associated Opportunistic infections Neoplasms Miscellaneous conditions Non HIV-associated Antiretroviral therapy (ART)-associated
The spectrum of pulmonary diseases in HIV-infected persons is broad. HIV-associated Opportunistic infections Neoplasms Miscellaneous conditions Non HIV-associated Antiretroviral therapy (ART)-associated
aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A.
 aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A. 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A. 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
Online supplementary material
 Online supplementary material Add-on long-acting β2-agonist (LABA) in a separate inhaler as asthma step-up therapy versus increased dose of inhaled corticosteroid (ICS) or ICS/LABA combination inhaler
Online supplementary material Add-on long-acting β2-agonist (LABA) in a separate inhaler as asthma step-up therapy versus increased dose of inhaled corticosteroid (ICS) or ICS/LABA combination inhaler
COPD or not COPD, that is the question.
 COPD or not COPD, that is the question. Asthma-COPD Overlap Syndrome: ACOS Do we really need this? Michelle Harkins Disclosure Slide Slide help - William Busse, MD Organizational Interests ATS, ACCP, ACP
COPD or not COPD, that is the question. Asthma-COPD Overlap Syndrome: ACOS Do we really need this? Michelle Harkins Disclosure Slide Slide help - William Busse, MD Organizational Interests ATS, ACCP, ACP
Comparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classification
 ORIGINAL ARTICLE COPD Comparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classification Paul W. Jones 1, Lukasz Adamek 2, Gilbert Nadeau 2 and Norbert Banik 3 Affiliations:
ORIGINAL ARTICLE COPD Comparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classification Paul W. Jones 1, Lukasz Adamek 2, Gilbert Nadeau 2 and Norbert Banik 3 Affiliations:
Title: Real-life use of inhaled corticosteroids in COPD patients vs. GOLD proposals: a paradigm shift in GOLD 2011?
 ERJ Express. Published on October 31, 2013 as doi: 10.1183/09031936.00162313 Letter to the Editor Title: Real-life use of inhaled corticosteroids in COPD patients vs. GOLD proposals: a paradigm shift in
ERJ Express. Published on October 31, 2013 as doi: 10.1183/09031936.00162313 Letter to the Editor Title: Real-life use of inhaled corticosteroids in COPD patients vs. GOLD proposals: a paradigm shift in
The impacts of cognitive impairment on acute exacerbations of chronic obstructive pulmonary disease
 The impacts of cognitive impairment on acute exacerbations of chronic obstructive pulmonary disease Dr. Lo Iek Long Department of Respiratory Medicine C.H.C.S.J. Chronic Obstructive Pulmonary Disease (COPD)
The impacts of cognitive impairment on acute exacerbations of chronic obstructive pulmonary disease Dr. Lo Iek Long Department of Respiratory Medicine C.H.C.S.J. Chronic Obstructive Pulmonary Disease (COPD)
Differential diagnosis
 Differential diagnosis The onset of COPD is insidious. Pathological changes may begin years before symptoms appear. The major differential diagnosis is asthma, and in some cases, a clear distinction between
Differential diagnosis The onset of COPD is insidious. Pathological changes may begin years before symptoms appear. The major differential diagnosis is asthma, and in some cases, a clear distinction between
Factors associated with change in exacerbation frequency in COPD
 Donaldson et al. Respiratory Research 2013, 14:79 RESEARCH Open Access Factors associated with change in exacerbation frequency in COPD Gavin C Donaldson 1*, Hanna Müllerova 2, Nicholas Locantore 3, John
Donaldson et al. Respiratory Research 2013, 14:79 RESEARCH Open Access Factors associated with change in exacerbation frequency in COPD Gavin C Donaldson 1*, Hanna Müllerova 2, Nicholas Locantore 3, John
Sydney, AUSTRALIA Beijing, CHINA Hyderabad, INDIA Oxford, UK. Affiliated with
 Sydney, AUSTRALIA Beijing, CHINA Hyderabad, INDIA Oxford, UK Affiliated with COPD and Comorbidities Norbert Berend Professor Emeritus University of Sydney Head, Respiratory Research The George Institute
Sydney, AUSTRALIA Beijing, CHINA Hyderabad, INDIA Oxford, UK Affiliated with COPD and Comorbidities Norbert Berend Professor Emeritus University of Sydney Head, Respiratory Research The George Institute
Characterisation and impact of reported and unreported exacerbations: results from ATTAIN
 ORIGINAL ARTICLE COPD Characterisation and impact of reported and unreported exacerbations: results from ATTAIN Paul W. Jones 1, Rosa Lamarca 2, Ferran Chuecos 2, Dave Singh 3, Alvar Agustí 4,5, Eric D.
ORIGINAL ARTICLE COPD Characterisation and impact of reported and unreported exacerbations: results from ATTAIN Paul W. Jones 1, Rosa Lamarca 2, Ferran Chuecos 2, Dave Singh 3, Alvar Agustí 4,5, Eric D.
Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984]
![Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984]](/thumbs/85/91889043.jpg) Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] 1 st Appraisal Committee meeting Background & Clinical Effectiveness John McMurray 11 th January 2016 For
Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] 1 st Appraisal Committee meeting Background & Clinical Effectiveness John McMurray 11 th January 2016 For
Dr Stephen Child. General Physician Auckland. 14:20-14:40 Secondary Care Perspective
 Dr Stephen Child General Physician Auckland 14:20-14:40 Secondary Care Perspective Wheeze Witchery Stephen Child MD, FRACP, FRCPC General Physician Respiratory Interest Director of Clinical Training Auckland
Dr Stephen Child General Physician Auckland 14:20-14:40 Secondary Care Perspective Wheeze Witchery Stephen Child MD, FRACP, FRCPC General Physician Respiratory Interest Director of Clinical Training Auckland
Airway Bacterial Concentrations and Exacerbations of Chronic Obstructive Pulmonary Disease
 Airway Bacterial Concentrations and Exacerbations of Chronic Obstructive Pulmonary Disease Sanjay Sethi 1,2, Rohin Sethi 2, Karen Eschberger 2, Phyllis Lobbins 3, Xueya Cai 4, Brydon J. B. Grant 1,2, and
Airway Bacterial Concentrations and Exacerbations of Chronic Obstructive Pulmonary Disease Sanjay Sethi 1,2, Rohin Sethi 2, Karen Eschberger 2, Phyllis Lobbins 3, Xueya Cai 4, Brydon J. B. Grant 1,2, and
Decramer 2014 a &b [21]
![Decramer 2014 a &b [21] Decramer 2014 a &b [21]](/thumbs/90/101390504.jpg) Buhl 2015 [19] Celli 2014 [20] Decramer 2014 a &b [21] D Urzo 2014 [22] Maleki-Yazdi 2014 [23] Inclusion criteria: Diagnosis of chronic obstructive pulmonary disease; 40 years of age or older; Relatively
Buhl 2015 [19] Celli 2014 [20] Decramer 2014 a &b [21] D Urzo 2014 [22] Maleki-Yazdi 2014 [23] Inclusion criteria: Diagnosis of chronic obstructive pulmonary disease; 40 years of age or older; Relatively
Disclosure and Conflict of Interest 8/15/2017. Pharmacist Objectives. At the conclusion of this program, the pharmacist will be able to:
 Digging for GOLD Rebecca Young, PharmD, BCACP, Roosevelt University College of Pharmacy Assistant Professor of Clinical Sciences Practice Site Advocate Medical Group-Nesset Pavilion Disclosure and Conflict
Digging for GOLD Rebecca Young, PharmD, BCACP, Roosevelt University College of Pharmacy Assistant Professor of Clinical Sciences Practice Site Advocate Medical Group-Nesset Pavilion Disclosure and Conflict
รศ. นพ. ว ชรา บ ญสว สด M.D., Ph.D. ภาคว ชาอาย รศาสตร คณะแพทยศาสตร มหาว ทยาล ยขอนแก น
 รศ. นพ. ว ชรา บ ญสว สด M.D., Ph.D. ภาคว ชาอาย รศาสตร คณะแพทยศาสตร มหาว ทยาล ยขอนแก น COPD Guideline Changing concept in COPD management Evidences that we can offer COPD patients better life COPD Guidelines
รศ. นพ. ว ชรา บ ญสว สด M.D., Ph.D. ภาคว ชาอาย รศาสตร คณะแพทยศาสตร มหาว ทยาล ยขอนแก น COPD Guideline Changing concept in COPD management Evidences that we can offer COPD patients better life COPD Guidelines
Treatment Responses. Ronald Dahl, Aarhus University Hospital, Denmark
 Asthma and COPD: Are They a Spectrum Treatment Responses Ronald Dahl, Aarhus University Hospital, Denmark Pharmacological Treatments Bronchodilators Inhaled short-acting β -Agonist (rescue) Inhaled short-acting
Asthma and COPD: Are They a Spectrum Treatment Responses Ronald Dahl, Aarhus University Hospital, Denmark Pharmacological Treatments Bronchodilators Inhaled short-acting β -Agonist (rescue) Inhaled short-acting
Late diagnosis of influenza in adult patients during a seasonal outbreak
 ORIGINAL ARTICLE Korean J Intern Med 2018;33:391-396 Late diagnosis of influenza in adult patients during a seasonal outbreak Seong-Ho Choi 1, Jin-Won Chung 1, Tark Kim 2, Ki-Ho Park 3, Mi Suk Lee 3, and
ORIGINAL ARTICLE Korean J Intern Med 2018;33:391-396 Late diagnosis of influenza in adult patients during a seasonal outbreak Seong-Ho Choi 1, Jin-Won Chung 1, Tark Kim 2, Ki-Ho Park 3, Mi Suk Lee 3, and
umeclidinium, 55 micrograms, powder for inhalation (Incruse ) SMC No. (1004/14) GlaxoSmithKline
 umeclidinium, 55 micrograms, powder for inhalation (Incruse ) SMC No. (1004/14) GlaxoSmithKline 07 November 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
umeclidinium, 55 micrograms, powder for inhalation (Incruse ) SMC No. (1004/14) GlaxoSmithKline 07 November 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Research in Real Life. Real Life Effectiveness of Aclidinium Bromide. Version Date: 26 September 2013
 Research in Real Life Real Life Effectiveness of Aclidinium Bromide Version Date: 26 September 2013 Project for Almirall Real-life effectiveness evaluation of the long-acting muscarinic antagonist aclidinium
Research in Real Life Real Life Effectiveness of Aclidinium Bromide Version Date: 26 September 2013 Project for Almirall Real-life effectiveness evaluation of the long-acting muscarinic antagonist aclidinium
Supplementary Online Content
 Supplementary Online Content Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory
Supplementary Online Content Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory
Disclosures. Update on COPD & Asthma. Update on the Management of COPD. No Pharma Disclosures. NHLBI - Asthma Clinical Research Network
 Update on COPD & Asthma Michael C. Peters, M.D. MAS Division of Pulmonary & Critical Care Medicine Cardiovascular Research Institute University of California San Francisco UCSF Primary Care Medicine San
Update on COPD & Asthma Michael C. Peters, M.D. MAS Division of Pulmonary & Critical Care Medicine Cardiovascular Research Institute University of California San Francisco UCSF Primary Care Medicine San
Management of Acute Exacerbations
 15 Management of Acute Exacerbations Cenk Kirakli Izmir Dr. Suat Seren Chest Diseases and Surgery Training Hospital Turkey 1. Introduction American Thoracic Society (ATS) and European Respiratory Society
15 Management of Acute Exacerbations Cenk Kirakli Izmir Dr. Suat Seren Chest Diseases and Surgery Training Hospital Turkey 1. Introduction American Thoracic Society (ATS) and European Respiratory Society
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Chronic obstructive pulmonary disease in over 16s: diagnosis and management
 National Institute for Health and Care Excellence Final Chronic obstructive pulmonary disease in over 16s: diagnosis and management [D] Diagnosing COPD and predicting outcomes NICE guideline NG115 Evidence
National Institute for Health and Care Excellence Final Chronic obstructive pulmonary disease in over 16s: diagnosis and management [D] Diagnosing COPD and predicting outcomes NICE guideline NG115 Evidence
COPD in primary care: reminder and update
 COPD in primary care: reminder and update Managing COPD continues to be a major feature of primary care, particularly in practices with a high proportion of M ori and Pacific peoples. COPDX clinical practice
COPD in primary care: reminder and update Managing COPD continues to be a major feature of primary care, particularly in practices with a high proportion of M ori and Pacific peoples. COPDX clinical practice
Pharmacotherapy for COPD
 10/3/2017 Topics to be covered Pharmacotherapy for chronic treatment Pharmacotherapy for COPD Dr. W C Yu 3rd September 2017 Commonly used drugs Guidelines for their use Inhaled corticosteroids (ICS) in
10/3/2017 Topics to be covered Pharmacotherapy for chronic treatment Pharmacotherapy for COPD Dr. W C Yu 3rd September 2017 Commonly used drugs Guidelines for their use Inhaled corticosteroids (ICS) in
Blood eosinophil count: a biomarker of an important treatable trait in patients with airway disease
 EDITORIAL COPD Blood eosinophil count: a biomarker of an important treatable trait in patients with airway disease Ian D. Pavord 1 and Alvar Agusti 2 Affiliations: 1 Respiratory Medicine Unit, Nuffield
EDITORIAL COPD Blood eosinophil count: a biomarker of an important treatable trait in patients with airway disease Ian D. Pavord 1 and Alvar Agusti 2 Affiliations: 1 Respiratory Medicine Unit, Nuffield
Design - Multicentre prospective cohort study. Setting UK Community Pharmacies within one CCG area within the UK
 Enabling Patient Health Improvements through COPD (EPIC) Medicines Optimisation within Community Pharmacy: a prospective cohort study Abstract Objectives To improve patients ability to manage their own
Enabling Patient Health Improvements through COPD (EPIC) Medicines Optimisation within Community Pharmacy: a prospective cohort study Abstract Objectives To improve patients ability to manage their own
Impact of inhaled corticosteroid use on outcome in COPD patients admitted with pneumonia
 Eur Respir J 2011; 38: 36 41 DOI: 10.1183/09031936.00077010 CopyrightßERS 2011 Impact of inhaled corticosteroid use on outcome in COPD patients admitted with pneumonia A. Singanayagam, J.D. Chalmers, A.R.
Eur Respir J 2011; 38: 36 41 DOI: 10.1183/09031936.00077010 CopyrightßERS 2011 Impact of inhaled corticosteroid use on outcome in COPD patients admitted with pneumonia A. Singanayagam, J.D. Chalmers, A.R.
Yuriy Feschenko, Liudmyla Iashyna, Ksenia Nazarenko and Svitlana Opimakh
 2018; 7(1): 74-78 ISSN (E): 2277-7695 ISSN (P): 2349-8242 NAAS Rating: 5.03 TPI 2018; 7(1): 74-78 2018 TPI www.thepharmajournal.com Received: 11-11-2017 Accepted: 12-12-2017 Yuriy Feschenko Liudmyla Iashyna
2018; 7(1): 74-78 ISSN (E): 2277-7695 ISSN (P): 2349-8242 NAAS Rating: 5.03 TPI 2018; 7(1): 74-78 2018 TPI www.thepharmajournal.com Received: 11-11-2017 Accepted: 12-12-2017 Yuriy Feschenko Liudmyla Iashyna
