Medical Policy. MP Surgical Treatments for Breast Cancer-Related Lymphedema
|
|
|
- Evelyn Ward
- 5 years ago
- Views:
Transcription
1 Medical Policy MP Surgical Treatments for Breast Cancer-Related Lymphedema BCBSA Ref. Policy: Last Review: 07/25/2018 Effective Date: 10/30/2018 Section: Surgery Related Policies Pneumatic Compression Pumps for Treatment of Lymphedema and Venous Ulcers Bioimpedance Devices for Detection and Management of Lymphedema Experimental / Investigational Services DISCLAIMER Our medical policies are designed for informational purposes only and are not an authorization, explanation of benefits or a contract. Receipt of benefits is subject to satisfaction of all terms and conditions of the coverage. Medical technology is constantly changing, and we reserve the right to review and update our policies periodically. POLICY Lymphatic physiologic microsurgery to treat lymphedema (including, but not limited to, lymphaticolymphatic bypass, lymphovenous bypass, lymphaticovenous anastomosis, autologous lymph node transplantation, and vascularized lymph node transfer) in individuals who have been treated for breast cancer is considered investigational. Lymphatic physiologic microsurgery performed during nodal dissection or breast reconstruction to prevent lymphedema (including, but not limited to, the Lymphatic Microsurgical Preventing Healing Approach) in individuals who are being treated for breast cancer is considered investigational. POLICY GUIDELINES There is no specific CPT code for this procedure. There is an unlisted code: (Unlisted procedure, hemic or lymphatic system). BENEFIT APPLICATION BLUECARD/NATIONAL ACCOUNT ISSUES State or federal mandates (eg, Federal Employee Program) may dictate that certain U.S. Food and Drug Administration approved devices, drugs, or biologics may not be considered investigational, and thus these devices may be assessed only by their medical necessity. BACKGROUND LYMPHEDEMA Lymphedema is an accumulation of fluid due to disruption of lymphatic drainage. Lymphedema can be caused by congenital or inherited abnormalities in the lymphatic system (primary lymphedema) but is most often caused by acquired damage to the lymphatic system (secondary lymphedema).
2 Diagnosis and Staging A diagnosis of secondary lymphedema is based on history (eg, cancer treatment, trauma) and physical examination (localized, progressive edema and asymmetric limb measurements) when other causes of edema can be excluded. Imaging, such as magnetic resonance imaging, computed tomography, ultrasound, or lymphoscintigraphy, may be used to differentiate lymphedema from others causes of edema in diagnostically challenging cases. Table 1 lists International Society of Lymphology guidance for staging lymphedema based on "softness" or "firmness" of the limb and the changes with an elevation of the limb. 1 Table 1. Recommendations for Staging Lymphedema Stage Description Stage 0 Swelling is not evident and most patients are asymptomatic despite impaired (subclinical) lymphatic transport Stage I (mild) Accumulation of fluid that subsides (usually within 24 hours) with limb elevation; soft edema that may pit, without evidence of dermal fibrosis Stage II Does not resolve with limb elevation alone; limb may no longer pit on examination (moderate) Stage III (severe) Lymphostatic elephantiasis; pitting can be absent; skin has trophic changes Breast Cancer Related Lymphedema Breast cancer treatment is one of the most common causes of secondary lymphedema. Both the surgical removal of lymph nodes and radiotherapy are associated with development lymphedema in patients with breast cancer. A systematic review of 72 studies (N=29,612 women), DiSipio et al (2013) reported that approximately 1 in 5 women who survive breast cancer will develop arm lymphedema. 2 Reviewers reported that risk factors for development of lymphedema that had a strong level of evidence were extensive surgery (ie, axillary-lymph-node dissection, greater number of lymph nodes dissected, mastectomy) and being overweight or obese. Management and Treatment Early and ongoing treatment of lymphedema is necessary. Conservative therapy may consist of several features depending on the severity of the lymphedema. Patients are educated on the importance of self-care including hygiene practices to prevent infection, maintaining ideal body weight through diet and exercise, and limb elevation. Compression therapy consists of repeatedly applying padding and bandages or compression garments. Manual lymphatic drainage is a light pressure massage performed by trained physical therapists or by patients designed to move fluid from obstructed areas into functioning lymph vessels and lymph nodes. Complete decongestive therapy is a multiphase treatment program involving all of the previously mentioned conservative treatment components at different intensities. Pneumatic compression pumps may also be considered as an adjunct to conservative therapy or as an alternative to self-manual lymphatic drainage in patients who have difficulty performing self-manual lymphatic drainage. In patients with more advanced lymphedema after fat deposition and tissue fibrosis has occurred, palliative surgery using reductive techniques such as liposuction may be performed. REGULATORY STATUS Physiologic microsurgery for lymphedema is a surgical procedure and, as such, is not subject to regulation by the U.S. Food and Drug Administration. Original Policy Date: July 2018 Page: 2
3 RATIONALE This evidence review was created in July 2018 with a literature search of the MEDLINE database performed through May 17, Evidence reviews assess the clinical evidence to determine whether the use of a technology improves the net health outcome. Broadly defined, health outcomes are length of life, quality of life, and ability to function including benefits and harms. Every clinical condition has specific outcomes that are important to patients and to managing the course of that condition. Validated outcome measures are necessary to ascertain whether a condition improves or worsens; and whether the magnitude of that change is clinically significant. The net health outcome is a balance of benefits and harms. To assess whether the evidence is sufficient to draw conclusions about the net health outcome of a technology, 2 domains are examined: the relevance and the quality and credibility. To be relevant, studies must represent one or more intended clinical use of the technology in the intended population and compare an effective and appropriate alternative at a comparable intensity. For some conditions, the alternative will be supportive care or surveillance. The quality and credibility of the evidence depend on study design and conduct, minimizing bias and confounding that can generate incorrect findings. The randomized controlled trial (RCT) is preferred to assess efficacy; however, in some circumstances, nonrandomized studies may be adequate. RCTs are rarely large enough or long enough to capture less common adverse events and long-term effects. Other types of studies can be used for these purposes and to assess generalizability to broader clinical populations and settings of clinical practice. PHYSIOLOGIC MICROSURGERY TO TREAT LYMPHEDEMA Clinical Context and Therapy Purpose The purpose of physiologic microsurgery treatments for lymphedema in patients who have been treated for breast cancer is to provide a treatment option that is an improvement on existing therapies such as conservative therapy with compression garments or bandages, manual lymph drainage or pneumatic pumps, and decongestive therapy. Both surgical treatment and radiotherapy for breast cancer can lead to lymphedema and is one of the most common causes of secondary peripheral lymphedema. The question addressed in this evidence review is: Does lymphatic physiologic microsurgery for the treatment of breast cancer related lymphedema improve the net health outcome? The following PICOTS were used to select literature to inform this review. Patients The relevant population of interest is individuals who have been treated for breast cancer, who have developed secondary lymphedema, and who have insufficient symptom reduction with conservative therapy, who have recurrent cellulitis or lymphangitis, or who are dissatisfied with conservative therapy. Lymphedema in its late chronic phase is irreversible. The surgical techniques of interest in this review are those performed in individuals who have not reached the irreversible stage, ie, those who have functioning lymphatic channels (stage I, II or early stage III) (see Table 1). Interventions This review focuses on physiologic microsurgical interventions; it does not consider reductive (also known as excisional or ablative) surgical interventions (eg, liposuction). Physiologic microsurgical interventions include several techniques and can be broadly grouped into procedures that (1) reconstruct or bypass the obstructed lymphatic vessels to improve lymphatic drainage and (2) transfer Original Policy Date: July 2018 Page: 3
4 lymph tissue into an obstructed area to reestablish lymphatic flow. Table 2 includes a brief description of the surgeries. Table 2. Physiologic Microsurgical Interventions for Lymphedema Purpose Surgery Description Key Features Bypass or reconstruct obstructed lymph vessels to improve drainage Lymphaticlymphatic bypass Connects functioning lymphatic vessels directly to affected lymphatic vessels; healthy vessels come from donor site Transfer lymph tissue to reestablish lymphatic flow Lymphovenous bypass and lymphaticovenular anastomosis Autologous lymph node transplantation and vascularized lymph node transfer Lymphatic vessels in a affected limb are connected to the venous system Healthy lymph nodes are transferred to the affected limb Lymphedema can develop in donor extremity Scaring at donor site Outpatient procedure or usually discharged within a day Quick return to daily activities Inpatient procedure; requires 2-3 days of hospitalization Lymphedema can develop in donor extremity Comparators Physiological microsurgery may be used as an adjunct to conservative therapy. Conservative therapy is multimodal. It involves meticulous skin hygiene and care, exercise, compression therapy, and physical therapy (manual lymphatic drainage). Complete decongestive therapy and pneumatic compression pumps are also used as adjuncts to conservative therapy. Outcomes Objective outcomes of interest include a reduction in limb circumference and/or volume and reduction in the rates of infections (eg, cellulitis, lymphangitis). Volume is measured using different methods; eg, tape measurements with geometry formulas, perometry, and water displacement. Bioimpedance spectroscopy may be used to detect changes in tissue fluid accumulation; this technology is reviewed in (bioimpedance devices for detection and management of lymphedema). Patient-reported outcomes (PROs) of interest include symptoms, quality of life, and functional measures. A systematic review of PRO instruments and outcomes used to assess quality of life in breast cancer patients with lymphedema, Pusic et al (2013) found that most studies included generic PRO instruments or oncology PRO instruments. 3 Lymphedema-specific instruments are occasionally used; specifically, the Upper Limb Lymphedema 27 was found to have strong psychometric properties. There does not appear to be a consensus on minimally clinically important change for either objective outcomes such as changes in arm volume or subjective measures such as changes to patient symptoms or quality of life. Timing The existing literature supporting conservative therapies for lymphedema has varying lengths of followup, ranging from a few weeks to 1 year. In systematic reviews of microsurgical treatments for lymphedema discussed in the following sections, studies suggest less than a year of follow-up is Original Policy Date: July 2018 Page: 4
5 insufficient to observe outcomes. Therefore, at least 1 year of follow-up is considered necessary to demonstrate efficacy. Setting Microsurgery for lymphedema is performed by surgeons with advanced training in highly specialized microsurgery and lymphology and also requires specialized imaging tools. Study Selection Criteria Methodologically credible studies were selected using the following principles: a. To assess efficacy outcomes, comparative controlled prospective trials were sought, with a preference for RCTs; b. In the absence of such trials, comparative observational studies were sought, with a preference for prospective studies. c. To assess longer term outcomes and adverse events, single-arm studies that capture longer periods of follow-up and/or larger populations were sought. d. Studies with duplicative or overlapping populations were excluded. Because multiple systematic reviews of studies were available for both classes of microsurgery, the focus is on systematic reviews published in 2015 or later. Surgeries That Reconstruct or Bypass Using Donor Lymph Vessels Leung et al (2015) reported on a systematic review of the surgical management of breast cancer related lymphedema. 4 The search included studies reporting on the efficacy of surgical techniques used for the prevention or treatment of breast cancer related lymphedema published between 2000 and Only 1 study on lymphatico-lymphatic bypass was identified and published since The study included 7 patients followed for 2.6 years. One patient had complete recovery as measured by the circumference of the affected limb and the remaining 6 patients had a reasonable outcome. Postsurgery complications were cellulitis, donor-site lymphorrhea, and transient edema of donor leg. Surgeries That Reconstruct or Bypass Using the Venous System Systematic Reviews Two systematic reviews specifically evaluating microsurgical procedures using the venous system (lymphaticovenular anastomosis [LVA], lymphovenous bypass) have been reported. 5,6 Two broader systematic reviews of treatments for lymphedema including several microsurgical procedures have also been reported. 4,7 Corneilissen et al (2018) and Leung et al (2015) were limited to studies of breast cancer related lymphedema but the remaining reviews were not. The overlap between the primary studies included in the systematic reviews is shown in Appendix Table 1. Thirty-four publications on LVA were included across the 4 systematic reviews. Characteristics of the reviews are shown in Table 3. Cornelissen et al (2018) reported on a systematic review assessing the effect of LVA in breast cancer related lymphedema. 5 Fifteen observational studies were identified (11 prospective, 4 retrospective) with follow-up times ranging from 2 months to 8 years. Although LVA surgery was performed in the included studies, the technical procedure differed among studies: 6 studies used only end-to-end anastomoses; 4 studies used both end-to-end and end-to-side anastomoses; 1 study used the Octopus technique ; and 4 studies did not report the LVA technique used. Only 2 studies included a control group (bandaging, decongestive therapy). Scaglioni et al (2017) reported on a systematic review of LVA for the treatment of lymphedema. 6 Reviewers noted significant variations in surgical techniques, numbers of anastomoses, and Original Policy Date: July 2018 Page: 5
6 supplementary interventions (ie, compressive therapy, additional debulking surgery). Nine studies included secondary lymphedema alone, while 8 studies included patients with both primary and secondary lymphedemas. The number of patients with breast cancer related lymphedema was not described. As mentioned, the Carl (2017) and Leung (2015) reviews included multiple surgical techniques. Leung (2015) was limited to breast cancer related lymphedema while Carl (2017) was not. Table 3. Characteristics of Systematic Reviews Assessing Lymphedema Surgeries Using the Venous System Duration Study Dates Studies Participants N (Range) Design (Range) Cornelissen et al (2018) With breast cancer related lymphedema 268 (3-39) Observational or single-arm: 11 Prospective: 20 mo (2 mo to 8 y) Scaglioni et al (2017) 6 Up to 2016 Carl et al (2017) Leung et al (2015) With lymphedema of any cause except filariasisrelated Overall: 69 LVA: 27 a Overall: 13 LVA: 6 With extremity lymphedema of any cause With breast cancer related lymphedema LVA: lymphaticovenular anastomosis; NR: not reported. a Only 12 high-quality LVA studies were discussed. 939 (5-154) (no. with breast cancer related lymphedema NR) NR 4 Observational or single arm: 8 Prospective: 10 Observational or single-arm 146 (6-89) Observational or single-arm 24 mo (5-55 mo) LVA: mo LVA: 17 mo to 8 y Results of the systematic reviews are shown in Table 4. In 3 of the reviews, given the variability in the procedures, metrics for measuring the outcomes, and the time periods of reporting, meta-analyses were not possible and only a narrative synthesis was provided. In the Carl (2017) review, meta-analyses were performed for the outcome measure of percent excess circumference reduction, although only a small subset of studies reported this outcome and could be combined. Risk of bias was assessed in the Cornelissen systematic review and summarized as follows: 9 of 15 studies did not describe whether consecutive patients were included, so selection bias is possible; 9 of 15 studies did not describe the surgery team; 5 of 15 studies did not have sufficient follow-up to evaluate the long-term effects of LVA (ie, <1 year). Table 4. Results of Systematic Reviews Assessing Lymphedema Surgeries Using the Venous System Reduction in Circumference or Study Volume of Affected Limb Reduction in Symptoms Infection Frequency Postoperative Complications Original Policy Date: July 2018 Page: 6
7 Reduction in Circumference or Study Volume of Affected Limb Reduction in Symptoms Infection Frequency Cornelissen et al (2018) 5 n 255 NR NR 205 Narrative Overall reduction in either circumference or volume reported in 13/15 studies Reduction in symptoms reported in 12/15 studies Percent patients with improvements varied from 50% to 100% Scaglioni et al (2017) 6 Total N 939 NR NR NR Narrative All studies reported reductions in circumference measurements Vast majority reported subjective symptom relief based on patient opinion and feeling Reduction in no. of cellulitis episodes present in all cases Postoperative Complications 1 study reported 2 complications (skin irritation on the contrast injection site) 10 studies reported no complications 4 studies did not report whether complications occurred Excess Circumference Reduction (%) Carl et al (2017) 7 n 474 (3 LVA studies) NR (5 studies) NR NR (2 studies) PE (95% CI) or narrative 16.1 (2.6 to 29.6) 1 study reported 92% symptom improvement 2 studies reported average satisfaction rate of 94.5% 2 studies reported improved QOL in 90% of patients and subjective improvement in 50% I 2 (p) 0% (0.17) Leung et al (2015) 4 Total N 146 NR NR 109 Narrative Mean percent reduction in volume at 1 y was 2%, 35%, and 42% in 3 studies Partial skin ulceration (n=1) Wound dehiscence (n=1) No complications in 2 studies Remaining studies did not report on Original Policy Date: July 2018 Page: 7
8 Study Reduction in Circumference or Volume of Affected Limb Mean absolute circumference reduction was 4.1 cm and 0.85 cm in 2 studies Reduction in Symptoms Infection Frequency Postoperative Complications complications CI: confidence interval; LVA: lymphaticovenular anastomosis; NR: not reported; PE: pooled effect; QOL: quality of life. Randomized Controlled Trials No RCTs were identified. Nonrandomized or Observational Studies Additional single-arm studies have been published since the systematic reviews. 8 However, these studies suffer from the same limitations as the studies included in the systematic reviews and do not capture longer periods of follow-up and/or larger populations than the existing studies. Therefore, they are not discussed further. Subsection Summary: Surgeries That Reconstruct or Bypass Using the Venous System No controlled trials were identified evaluating the physiologic microsurgeries using techniques such as lymphovenous bypass or LVA that reconstruct or bypass the obstructed lymphatic vessels using the venous system in patients with breast cancer related lymphedema. Systematic reviews have indicated that most of the available evidence for these procedures comes from uncontrolled studies including fewer than 40 participants each, most of which lack adequate descriptions of how patients were selected for inclusion. Surgical technique, the severity of lymphedema, outcomes metrics, and follow-up times varied across studies making it difficult to synthesize the evidence. Surgical complications have been inconsistently reported but appear to be rare. RCTs of physiologic microsurgeries that bypass the obstructed lymphatic vessels using the venous system plus conservative therapy vs conservative therapy alone are needed. Surgeries That Transfer Lymph Tissue Systematic Reviews Systematic reviews evaluating microsurgical procedures that transfer lymph tissue (autologous lymph node transfer, vascularized lymph node transfer [VLNT]) have been reported. The overlap between the primary studies included in the systematic reviews is shown in Appendix Table 2. Characteristics of systematic reviews of surgeries for lymphedema are shown in Table 5. Ozturk et al (2016) reported on a systematic review of VLNT for treatment of lymphedema. 9 They included treatment for both primary and secondary lymphedema and as such comprised a heterogeneous population. However, 191 of 305 of the surgeries were for breast cancer related lymphedema. Eighteen studies were identified (3 prospective, 15 retrospective). For breast cancer related lymphedema, VLNT with a skin island or VLNT with an autologous flap was used. There was inconsistent reporting of the staging of lymphedema. Reviewers did not state whether any of the studies included a control group. Two systematic reviews of various surgical methods previously described also included a review of lymph node transfer. 4,7 Original Policy Date: July 2018 Page: 8
9 In addition to the systematic reviews of efficacy, Demiri et al (2018) reported on a systematic review of donor-site complications following autologous lymph node transfer for breast cancer related lymphedema. 10 Table 5. Characteristics of Systematic Reviews Assessing Lymphedema Surgeries Using Lymph Tissue Transfer Study Dates Studies Participants N Design Duration (Range) Demiri et al (2018) 10 NR 11 With breast cancer related lymphedema treated with VLNT 189 (8-42) RCT, observational, or single-arm Mean, 38 mo (range, mo) Carl et al (2017) Ozturk et al (2016) 9 Leung et al (2015) to Overall: 69 VLNT: 17 a With extremity lymphedema of any cause 18 With primary or secondary upper- or lower-limb lymphedema (63% breast cancer related) Overall: 13 LNT: 6 With breast cancer related lymphedema NR 305 (6-52) 80 (3-24) Observational or single-arm Observational or single-arm: 3 Prospective: 15 Observational or single-arm NR mo LNT: 6 mo to 8 y NR: not reported; RCT: randomized controlled trial; VLNT: vascularized lymph node transfer; LNT: lymph node transfer. a Only 10 high-quality VLNT studies were discussed. Results of the systematic reviews are shown in Table 6. In Ozturk (2016) and Carl (2017), results in the subgroup of breast cancer related lymphedema were not presented so the table includes all available participants. Due to differences in outcomes metrics and timing of measurements, meta-analyses were not possible and narrative summaries were provided by Ozturk (2016), Demiri (2018), and Leung (2015). Carl (2017) performed meta-analyses for the excess volume-outcome but only a few studies could be pooled in the combined estimate. Risk of bias was assessed in Ozturk (2016) using a checklist from the American Society of Plastic Surgeons guidelines for therapeutic studies. A summary of the assessment follows: 12 of 18 studies did not report whether patients were selected consecutively and one did not include consecutive patients; 13 of 18 studies had insufficient information on the surgical team; 3 of 18 studies had an insufficient follow-up to observe outcomes (ie, <1 year). Table 6. Results of Systematic Reviews Assessing Lymphedema Surgeries Using Lymph Tissue Transfer Reduction in Study Circumference or Volume Reductions in Symptoms Infection Frequency Postoperative Complications Demiri et al (2018) 10 Total N NR NR NR 189 Original Policy Date: July 2018 Page: 9
10 Study Narrative Reduction in Circumference or Volume Reductions in Symptoms Infection Frequency Postoperative Complications Donor limb lymphedema: 3 (1.6%) cases 8 studies reported donor-site complications: o Seroma (n=8) o Lymphocele (n=3) o Lymphorrhea (n=2) o Wound infection (n=2) o Delayed wound healing (n=3) o Donor-site pain, numbness, or discomfort (n=9) o Transient edema of donor site (n=1) o Lymphedema of lower limb (n=3) Excess Circumference Reduction (%) Carl et al (2017) 7 Total N NR (4 studies) a NR NR (4 studies) a NR (7 studies) a PE (95% CI) or narrative 39.5% (36 to 43) Quantitative summaries not given Improved function, appearance, and mood Decreased pain I 2 (p) 0% (0.85) Ozturk et al (2016) 9 Total N 305 a 105 a 106 a 198 a Narrative Overall reduction in either circumference or volume reported in all studies 17/182 patients evaluated by limb circumference showed Various PROs reported in 7 studies 98/105 reported high level of patient Decrease reported in 7 publications using various metrics Remaining publications did not quantify Quantitative summaries not given Cellulitis, lymphocele, donor-site pain, seroma, lymphedema hematoma, wound dehiscence, wound infection, hydrocele, partial skin graft loss, venous congestion Delayed wound healing: 4% Seroma/hematoma: 3% Infection: 2% Abdominal bulge: 0.5% Original Policy Date: July 2018 Page: 10
11 Reduction in Circumference or Study Volume no improvement 16/114 patients evaluated by volume showed no improvement Leung et al (2015) 4 Reductions in Symptoms Infection Frequency Postoperative Complications satisfaction decrease Persistent donor lymphedema: 0% Total N 80 NR NR 52 Narrative Mean percent reduction in circumference was 40% and 51% in 2 studies Reduction in circumference reported in 10/21 (47%), 22/24 (92%), and 7/9 (78%) in 3 studies Donor-site edema (n=1) Wound infection (n=1) Venous congestion (n=1) Seroma (n=3) Delayed wound closure (n=2) 2 studies did not report on complications CI: confidence interval; NR: not reported; PE: pooled effect; PRO: patient-reported outcome. a All etiologies included; results not provided for subgroup of patients with breast cancer related lymphedema. Randomized Controlled Trials Dionyssiou et al (2016) reported on an RCT that evaluated VLNT plus physical therapy vs physical therapy alone for lymphedema in 36 women with stage II breast cancer related lymphedema. 11 Trial characteristics are shown in Table 7. Table 7. Characteristics of RCTs of Lymphedema Surgeries Using Lymph Tissue Transfer Study Countries Sites Dates Participants Interventions Surgery Dionyssiou et Greece al (2016) Women with stage II, unilateral, upper-limb lymphedema related to breast cancer treatment and 1+ infections during last year 18 received VLNT followed by physical therapy a for 6 mo Control 18 received physical therapy a for 6 mo RCT: randomized controlled trial. a Physical therapy included manual lymphatic drainage for 1 month and pressure garments for 5 months. RCT results reported in Dionyssiou (2016) are shown in Table 8. At 18 months, the reduction in the excess volume of the affected limb as a percentage of the intact limb was 57% in the VLNT group and 18% in the physical therapy group (treatment effect not reported, p<0.001). The mean number of lymphedema-related infections per patient per year was lower in the VLNT group (0.28 vs 1.16; Original Policy Date: July 2018 Page: 11
12 treatment effect not reported, p=0.001). The trial had several limitations described in Tables 9 and 10. Notably, there was no description of allocation concealment and the trial was not blinded, possibly introducing both selection and ascertainment bias. The reporting did not describe the power calculations or justify a clinically important difference for the reported outcomes. The trial was not registered, so selective reporting cannot be ruled out. Table 8. Results of RCTs of Lymphedema Surgeries Using Lymph Tissue Transfer Reduction in Circumference of Affected Reduction in Volume Limb of Affected Limb Infections Study Dionyssiou et al (2016) 11 Reduction in Excess Volume of Affected Limb as Percent of Intact Limb at 18 Months Mean Episodes per Patient per Year Function or Quality of Life VAS for Functional Impairment at 18 Months Postoperative Complications N NR Surgery NR 57% a Control NR 18% NA TE (95% CI); p NR NR (NR); <0.001 NR (NR); NR (NR); CI: confidence interval; NA: not applicable; NR: not reported; RCT: randomized controlled trial; TE: treatment effect; VAS: visual analog scale. a Two with mild discomfort at donor side lower limb; 2 with prolonged lymphorrhea at donor area. Table 9. Relevance Gaps of RCTs of Lymphedema Surgeries Using Lymph Tissue Transfer Study Population a Intervention b Comparator c Outcomes d Follow-Up e Dionyssiou et al (2016) Did not use validated measures of quality of life 5, 6. No discussion of clinically important differences The evidence gaps stated in this table are those notable in the current review; this is not a comprehensive gaps assessment. RCT: randomized controlled trial. a Population key: 1. Intended use population unclear; 2. Clinical context is unclear; 3. Study population is unclear; 4. Study population not representative of intended use. b Intervention key: 1. Not clearly defined; 2. Version used unclear; 3. Delivery not similar intensity as comparator; 4.Not the intervention of interest. c Comparator key: 1. Not clearly defined; 2. Not standard or optimal; 3. Delivery not similar intensity as intervention; 4. Not delivered effectively. d Outcomes key: 1. Key health outcomes not addressed; 2. Physiologic measures, not validated surrogates; 3. No CONSORT reporting of harms; 4. Not establish and validated measurements; 5. Clinical significant difference not prespecified; 6. Clinical significant difference not supported. e Follow-Up key: 1. Not sufficient duration for benefit; 2. Not sufficient duration for harms. Original Policy Date: July 2018 Page: 12
13 Study Allocation a Blinding b Table 10. Study Design and Conduct Gaps of RCTs of Lymphedema Surgeries Using Lymph Tissue Transfer Reporting d Follow-Up e Power d Statistical f Selective Dionyssiou et al (2016) No description of allocation concealme nt 1, 2. No blinding of patients, staff, or outcome assessors 1. Registrati on not described Note: flow of participants not described; unclear if any patients lost or crossed 1-3. Power calculati on not describe d 3, 4. Comparative treatment effects and related CIs not provided over The evidence gaps stated in this table are those notable in the current review; this is not a comprehensive gaps assessment. CI: confidence interval; RCT: randomized controlled trial. a Allocation key: 1. Participants not randomly allocated; 2. Allocation not concealed; 3. Allocation concealment unclear; 4. Inadequate control for selection bias. b Blinding key: 1. Not blinded to treatment assignment; 2. Not blinded outcome assessment; 3. Outcome assessed by treating physician. c Selective Reporting key: 1. Not registered; 2. Evidence of selective reporting; 3. Evidence of selective publication. d Follow-Up key: 1. High loss to follow-up or missing data; 2. Inadequate handling of missing data; 3. High number of crossovers; 4. Inadequate handling of crossovers; 5. Inappropriate exclusions; 6. Not intent to treat analysis (per protocol for noninferiority trials). e Power key: 1. Power calculations not reported; 2. Power not calculated for primary outcome; 3. Power not based on clinically important difference. f Statistical key: 1. Intervention is not appropriate for outcome type: (a) continuous; (b) binary; (c) time to event; 2. Intervention is not appropriate for multiple observations per patient; 3. Confidence intervals and/or p values not reported; 4. Comparative treatment effects not calculated. Nonrandomized or Observational Studies Additional single-arm studies have been published since the systematic reviews However, these studies suffer from the same limitations as the studies included in the systematic reviews and do not capture longer periods of follow-up and/or larger populations than the existing studies. Therefore, they are not discussed further. Subsection Summary: Surgeries That Transfer Lymph Tissue One RCT with 36 participants was identified evaluating VLNT that uses lymph tissue transfer in patients with breast cancer related lymphedema. The trial reported reductions in the excess volume of the affected limb and rates of lymphedema-related infections for VLNT plus physical therapy compared with physical therapy alone. Systematic reviews have indicated that most of the remaining available evidence for these procedures comes from uncontrolled studies including fewer than 50 participants each, most of which lacked adequate descriptions of how patients were selected for inclusion. Surgical techniques, the severity of lymphedema, outcomes metrics, and follow-up times varied across studies. Although surgical complications were inconsistently reported, a systematic review of complications estimated that donor-site lymphedema occurs in approximately 2% of surgeries and seroma occurs in Original Policy Date: July 2018 Page: 13
14 approximately 4%. Additional RCTs of physiologic microsurgeries that use lymph tissue transfer with conservative therapy vs conservative therapy alone are needed. PHYSIOLOGIC MICROSURGERY TO PREVENT LYMPHEDEMA Clinical Context and Therapy Purpose The purpose of lymphatic physiologic microsurgery simultaneous to lymphadenectomy for breast cancer (ie, the Lymphatic Microsurgical Preventing Healing Approach [LYMPHA]) is to prevent lymphedema in individuals who are being treated for breast cancer. While recommendations on preventive measures for lymphedema exist, such as avoiding needle sticks, limb constriction, and air travel, most recommendations are based on clinical opinion. A systematic review of preventive measures for lymphedema by Cemal et al (2011) found strong scientific evidence only for the recommendations to maintain a normal body weight or avoid weight gain and to participate in a supervised exercise regimen. 15 LYMPHA is a preventive LVA procedure performed during nodal dissection or reconstructive surgery that involves anastomosing arm lymphatics to a collateral branch of an axillary vein. The question addressed in this evidence review is: Does lymphatic physiological microsurgery for the prevention of breast cancer related lymphedema improve the net health outcome? The following PICOTS were used to select literature to inform this review. Patients The relevant population of interest is individuals who are undergoing a lymphadenectomy or breast reconstruction procedure for breast cancer. Interventions This review focuses on a physiologic microsurgical intervention called LYMPHA. Comparators LYMPHA could be used as an adjunct to standard care. Standard care may involve education regarding lymphedema and recommendations for hygiene, avoidance of blocking the flow of fluids in the body, maintaining a normal body weight and exercise, as well as surveillance for lymphedema during followup with referral as needed. Outcomes Outcomes of interest include diagnosis of lymphedema, lymphedema symptoms, quality of life, and operative and postoperative complications. As discussed, the diagnosis of lymphedema is based on history and physical examination (localized, progressive edema, asymmetric limb measurements). There is no universal agreement on measurement criteria for asymmetric limbs. It may be quantified by a 2 or more centimeters difference in limb girth, a 200 ml difference in limb volume, or a 10% limb volume change from baseline. 16,17 Patient reports of heaviness or swelling, either "now" or "in the past year" may also be used to suggest lymphedema. The estimated incidence of lymphedema varies by the measurement criteria used. 17 Timing Although lymphedema can occur decades after treatment for breast cancer, approximately 80% of patients that eventually develop lymphedema experience onset within 3 years of treatment. The remaining patients develop edema at a rate of about 1% per year. 18 Original Policy Date: July 2018 Page: 14
15 For the purposes of this review, studies with at least 3 years of follow-up to observe cases of lymphedema are of primary interest. Setting Microsurgery for lymphedema is performed by surgeons with advanced training in highly specialized microsurgery and lymphology and also requires specialized imaging tools. Study Selection Criteria Methodologically credible studies were selected as described in the previous section. Systematic Reviews Jorgensen et al (2017) reported on a systematic review of prophylactic LVA and shunts for preventing cancer related lymphedema, not limited to breast cancer. 19 Systematic review characteristics are shown in Table 11. Twelve articles were included in the qualitative analysis (5 specific to breast cancer) and four of those studies (2 specific to breast cancer) were included in a meta-analysis. Table 11. Characteristics of Systematic Reviews of LYMPHA to Prevent Lymphedema Study Dates Studies Participants N (Range) Design Jorgensen (8- et al ) (2017) (5 specific to breast cancer) Underwent lymphadenectomy for cancer treatment and prophylactic LVA for prevention of extremity lymphedema RCT, observational, single-arm Duration, mo 6-69 LVA: lymphaticovenular anastomosis; LYMPHA: Lymphatic Microsurgical Preventing Healing Approach; RCT: randomized controlled trial. Results of the systematic review are shown in Table 12. Jorgensen et al (2017) performed a metaanalysis of the incidence of lymphedema that included 4 studies (2 specific to breast cancer) with a control group consisting of patients without prophylactic LVA. The relative risk for incident lymphedema was 0.33 (95% CI, 0.19 to 0.56) favoring prophylactic LVA vs control; however, because the incidence of lymphedema varies over time and the follow-up times varied across studies, it is not clear whether it would be appropriate to pool the risk including all time points. Table 12. Results of Systematic Reviews of LYMPHA to Prevent Lymphedema Study Incidence of Lymphedema Lymphedema Symptoms Quality of Life Complications Jorgensen et al (2017) 19 Meta-analysis n 176 NR NR NR RR (95% CI) 0.33 (0.19 to 0.56) I 2 (p) 0% (0.74) Qualitative synthesis N range 8-74 NR NR Not clear Original Policy Date: July 2018 Page: 15
16 Study Range estimates Incidence of Lymphedema 0%-30% with varying follow-up times Lymphedema Symptoms Quality of Life Complications 1 study reported lymphorrhea in 1 patient Unclear if other studies reported no events or did not report on complications CI: confidence interval; LYMPHA: Lymphatic Microsurgical Preventing Healing Approach; NR: not reported; RR: relative risk. Jorgensen (2017) also performed a risk of bias assessment of the included studies. They noted the following: None of the studies had allocation concealment or blinding; Only 1 study was randomized; None of the studies were registered; Only 4 studies had a control group. Selection of the control groups was unclear or a potential source of bias in all 4 controlled studies. Randomized Controlled Trials Boccardo et al (2011) reported on results of an RCT including 46 women referred for axillary dissection for breast cancer treatment between 2008 and 2009 who were randomized to LYMPHA or no preventive surgery (control). 20 All LVA procedures were performed by the same surgeon, reported to be skilled in lymphatic microsurgery. The LVA surgeon was not the same surgeon who performed lymph node dissection. The same axillary dissection treatment was performed in the 2 treatment groups. Lymphedema was diagnosed as a difference in excess volume of at least 100 ml compared with preoperative volume measurements. Trial characteristics are shown in Table 13. Table 13. Characteristics of RCTs of LYMPHA to Prevent Lymphedema Diagnosis of Study Countries Sites Dates Participants Lymphedema Boccardo et al (2011) 20 Italy Women referred for complete axillary dissection for breast cancer treatment Difference in excess volume of 100 ml vs preoperative volume Interventions Active Comparator no LYMPHA preventive surgery for lymphedema LYMPHA: lymphatic microsurgical preventing healing approach; RCT: randomized controlled trial. Results of the Boccardo (2011) RCT are shown in Table 14. Lymphedema was diagnosed in 1 (4%) woman in the LYMPHA group and 7 women (30 %) in the control group by 18 months of follow-up. The change in volume with respect to baseline was reportedly higher in the control group than in the LYMPHA group at 1, 3, 6, 12, and 18 months (all p<0.01). The trial had several limitations described in Tables 15 and 16. Notably, the follow-up duration was only 18 months. Methods of randomization and allocation concealment were not described and there was no justification of the sample size. The patients and investigators were not blinded (ie, no sham procedure was performed) and there was no Original Policy Date: July 2018 Page: 16
17 discussion of whether outcome assessors were blinded. There is no indication that the trial was registered. Table 14. Results of RCTs of LYMPHA to Prevent Lymphedema Study Incidence of Lymphedema Change in Volume of Associated Limb, ml Cumulative at 18 At 18 Months Months Symptoms of Lymphedema Quality of Life Boccardo et al (2011) 20 N NR NR NR LYMPHA 4% 10th percentile: -60 ml a 90th percentile: +40 ml a Control 30% 10th percentile: +50 ml a 90th percentile: +130 ml a TE (95% CI); p NR (NR); 0.05 NR CI: confidence interval; LYMPHA: Lymphatic Microsurgical Preventing Healing Approach; NR: not reported; RCT: randomized controlled trial; TE: treatment effect. a Estimated based visual inspection of figure. Complications Table 15. Relevance Gaps of RCTs of LYMPHA to Prevent Lymphedema Study Population a Intervention b Comparator c Outcomes d Follow-Up e Boccardo et al (2011) No patient reported outcomes 3. No reporting of harms 4. Used 100 ml volume displacement to diagnose lymphedema; 200 ml is more commonly used 5, 6. No discussion of clinically important differences 1. Follow-up of 3 y would be needed to assess incidence and durability The evidence gaps stated in this table are those notable in the current review; this is not a comprehensive gaps assessment. LYMPHA: lymphatic microsurgical preventing healing approach; RCT: randomized controlled trial. a Population key: 1. Intended use population unclear; 2. Clinical context is unclear; 3. Study population is unclear; 4. Study population not representative of intended use. b Intervention key: 1. Not clearly defined; 2. Version used unclear; 3. Delivery not similar intensity as comparator; 4.Not the intervention of interest. c Comparator key: 1. Not clearly defined; 2. Not standard or optimal; 3. Delivery not similar intensity as intervention; 4. Not delivered effectively. Original Policy Date: July 2018 Page: 17
18 d Outcomes key: 1. Key health outcomes not addressed; 2. Physiologic measures, not validated surrogates; 3. No CONSORT reporting of harms; 4. Not establish and validated measurements; 5. Clinical significant difference not prespecified; 6. Clinical significant difference not supported. e Follow-Up key: 1. Not sufficient duration for benefit; 2. Not sufficient duration for harms. Table 16. Study Design and Conduct Gaps of RCTs of LYMPHA to Prevent Lymphedema Study Allocation a Blinding b Reporting d Selective Boccardo 1, 2. No et al blindin (2011) 20 g Note that method of randomization was not described 3. Allocation concealmen t not described 1. No discussion of registratio n Data Completeness e Power d Statistical f 1-3. No power calculatio ns discussed 3, 4. Treatment effects and correspondi ng CIs not reported The evidence gaps stated in this table are those notable in the current review; this is not a comprehensive gaps assessment. CI: confidence interval; LYMPHA: lymphatic microsurgical preventing healing approach; RCT: randomized controlled trial. a Allocation key: 1. Participants not randomly allocated; 2. Allocation not concealed; 3. Allocation concealment unclear; 4. Inadequate control for selection bias. b Blinding key: 1. Not blinded to treatment assignment; 2. Not blinded outcome assessment; 3. Outcome assessed by treating physician. c Selective Reporting key: 1. Not registered; 2. Evidence of selective reporting; 3. Evidence of selective publication. d Data completeness key: 1. High loss to follow-up or missing data; 2. Inadequate handling of missing data; 3. High number of crossovers; 4. Inadequate handling of crossovers; 5. Inappropriate exclusions; 6. Not intent to treat analysis (per protocol for noninferiority trials). e Power key: 1. Power calculations not reported; 2. Power not calculated for primary outcome; 3. Power not based on clinically important difference. f Statistical key: 1. Analysis is not appropriate for outcome type: (a) continuous; (b) binary; (c) time to event; 2. Analysis is not appropriate for multiple observations per patient; 3. Confidence intervals and/or p values not reported; 4. Comparative treatment effects not calculated. Nonrandomized or Observational Studies Additional single-arm studies have been published since the systematic reviews. 21 However, these studies suffer from the same limitations as the studies included in the systematic reviews and do not capture longer periods of follow up and/or larger populations than the existing studies. Therefore, they are not discussed further. Section Summary: Physiologic Microsurgery to Prevent Lymphedema One RCT was identified evaluating LYMPHA to prevent lymphedema in 49 patients referred for axillary dissection for breast cancer. The trial reported that lymphedema developed in 4% of women in the LYMPHA group and 30% in the control group by 18 months of follow-up. Longer follow-up is needed to observe incident lymphedema occurring after 18 months and assess the durability of the procedure. The trial had limitations that could have introduced bias: methods of randomization and allocation Original Policy Date: July 2018 Page: 18
19 concealment were not described, and there was no sham procedure or blinding. Systematic reviews have indicated that most of the remaining available evidence for LYMPHA comes from uncontrolled studies, although two controlled observational studies in women with breast cancer have been performed. Selection of the control group was identified as a potential source of bias in both controlled studies. Outcomes metrics and follow-up times varied across studies. Additional RCTs of LYMPHA are needed and 1 such trial is underway (see NCT ). SUMMARY OF EVIDENCE For individuals who have breast cancer related secondary lymphedema who receive physiologic microsurgery to treat lymphedema along with continued conservative therapy, the evidence includes an RCT) observational studies, and systematic reviews. Relevant outcomes are symptoms, morbid events, functional outcomes, health status measures, quality of life, resource utilization, and treatment-related morbidity. Several physiologic microsurgeries have been developed; examples include lymphaticovenular anastomosis and vascularized lymph node transfer. No RCTs of lymphaticovenular anastomosis or similar surgeries involving the venous system were identified. One RCT of vascularized lymph node transfer with 36 participants has been conducted. Systematic reviews have indicated that the preponderance of the available evidence comes from single-arm clinical series from individual institutions. Surgical technique, outcomes metrics, and follow-up time have varied across these studies. These types of studies might be used for preliminary estimates of the amount of volume reduction expected from surgery, the durability of the reduction in volume, and the rates of adverse events. However, these studies are not adequate for determining the comparative efficacy of physiologic microsurgery vs conservative treatment or decongestive therapy, or the comparative efficacy of different microsurgery techniques. RCTs are needed. The evidence is insufficient to determine the effects of the technology on health outcomes. For individuals who are undergoing lymphadenectomy for breast cancer who receive physiologic microsurgery to prevent lymphedema, the evidence includes an RCT, observational studies, and systematic reviews. Relevant outcomes are symptoms, change in disease status, morbid events, quality of life, and treatment-related morbidity. Lymphatic Microsurgical Preventing Healing Approach is a preventive lymphaticovenular anastomosis performed during nodal dissection. One RCT including 46 patients has been conducted. The trial reported that lymphedema developed in 4% of women in the Lymphatic Microsurgical Preventing Healing Approach group and 30% in the control group by 18 months of follow-up. Longer follow-up is needed to observe incident lymphedema occurring after 18 months and assess the durability of the procedure. The trial methods of randomization and allocation concealment were not described and there was no sham procedure or blinding, potentially introducing bias. The remaining evidence consists of 2 controlled observational studies with inadequate description of control selection and uncontrolled studies. The evidence is insufficient to determine the effects of the technology on health outcomes. SUPPLEMENTAL INFORMATION PRACTICE GUIDELINES AND POSITION STATEMENTS National Lymphedema Network The National Lymphedema Network published a position paper on the diagnosis and treatment of lymphedema in The paper stated the following on microsurgical procedures: Microsurgical and supramicrosurgical (much smaller vessels) techniques have been developed to move lymph vessels to congested areas to try to improve lymphatic drainage. Surgeries involve connecting lymph vessels and veins, lymph nodes and veins, or lymph vessels to lymph vessels. Original Policy Date: July 2018 Page: 19
Related Policies None
 Medical Policy MP 7.01.13 BCBSA Ref. Policy: 7.01.13 Last Review: 02/26/2018 Effective Date: 02/26/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 7.01.13 BCBSA Ref. Policy: 7.01.13 Last Review: 02/26/2018 Effective Date: 02/26/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Bioimpedance Devices for Detection and Management of Lymphedema
 Bioimpedance Devices for Detection and Management of Lymphedema Policy Number: 2.01.82 Last Review: 5/2018 Origination: 1/2011 Next Review: 5/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Bioimpedance Devices for Detection and Management of Lymphedema Policy Number: 2.01.82 Last Review: 5/2018 Origination: 1/2011 Next Review: 5/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Bioimpedance Devices for Detection and Management of Lymphedema
 Bioimpedance Devices for Detection and Management of Lymphedema Policy Number: 2.01.82 Last Review: 5/2017 Origination: 1/2011 Next Review: 5/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Bioimpedance Devices for Detection and Management of Lymphedema Policy Number: 2.01.82 Last Review: 5/2017 Origination: 1/2011 Next Review: 5/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Bioimpedance Devices for Detection and Management of Lymphedema
 2.01.82 Bioimpedance Devices for Detection and Management of Lymphedema Section 2.0 Medicine Subsection Effective Date January 30, 2015 Original Policy Date July 2, 2010 Next Review Date January 2015 Medical
2.01.82 Bioimpedance Devices for Detection and Management of Lymphedema Section 2.0 Medicine Subsection Effective Date January 30, 2015 Original Policy Date July 2, 2010 Next Review Date January 2015 Medical
Section: Durable Medical Equipment Last Reviewed Date: June Policy No: 82 Effective Date: September 1, 2014
 Medical Policy Manual Topic: Bioimpedance Devices for Detection and Management of Lymphedema Date of Origin: April 2011 Section: Durable Medical Equipment Last Reviewed Date: June 2014 Policy No: 82 Effective
Medical Policy Manual Topic: Bioimpedance Devices for Detection and Management of Lymphedema Date of Origin: April 2011 Section: Durable Medical Equipment Last Reviewed Date: June 2014 Policy No: 82 Effective
Medical Policy Original Effective Date: 01/23/2019
 Page 1 of 12 Disclaimer Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans or the plan may have broader or more limited benefits
Page 1 of 12 Disclaimer Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans or the plan may have broader or more limited benefits
MP Pneumatic Compression Pumps for Treatment of Lymphedema and Venous Ulcers
 Medical Policy MP 1.01.18 BCBSA Ref. Policy: 1.01.18 Last Review: 03/29/2018 Effective Date: 03/29/2018 Section: Durable Medical Equipment Related Policies 2.01.16 Recombinant and Autologous Platelet-Derived
Medical Policy MP 1.01.18 BCBSA Ref. Policy: 1.01.18 Last Review: 03/29/2018 Effective Date: 03/29/2018 Section: Durable Medical Equipment Related Policies 2.01.16 Recombinant and Autologous Platelet-Derived
4. WHY a Lymphovenous anastomosis can be indicated in Lymphedema?
 LYMPHOVENOUS ANASTOMOSIS TECHNIQUE ( LVA / MLVA / MLVLA ). Alessandro Busetto Wolfsberg March 20-21, 2018 1. Speaer s agreement 2. Title. The aim of our communication is to describe and compare the various
LYMPHOVENOUS ANASTOMOSIS TECHNIQUE ( LVA / MLVA / MLVLA ). Alessandro Busetto Wolfsberg March 20-21, 2018 1. Speaer s agreement 2. Title. The aim of our communication is to describe and compare the various
MP Ovarian and Internal Iliac Vein Embolization as a Treatment of Pelvic Congestion Syndrome
 Medical Policy MP 4.01.18 BCBSA Ref. Policy: 4.01.18 Last Review: 08/20/2018 Effective Date: 08/20/2018 Section: OB/GYN Reproduction Related Policies 4.01.11 Occlusion of Uterine Arteries Using Transcatheter
Medical Policy MP 4.01.18 BCBSA Ref. Policy: 4.01.18 Last Review: 08/20/2018 Effective Date: 08/20/2018 Section: OB/GYN Reproduction Related Policies 4.01.11 Occlusion of Uterine Arteries Using Transcatheter
Catherine Holley, RN Operating Room Nurse Massachusetts General Hospital
 Operating Room Nurse Massachusetts General Hospital March 13, 2019 What is Lymphedema? Swelling (edema) that occurs due to injury or impairment of the lymphatic system Causes: Anything that disrupts the
Operating Room Nurse Massachusetts General Hospital March 13, 2019 What is Lymphedema? Swelling (edema) that occurs due to injury or impairment of the lymphatic system Causes: Anything that disrupts the
SUCTION ASSISTED PROTEIN LIPECTOMY (SAPL) EVEN FOR THE TREATMENT OF CHRONIC FIBROTIC AND SCARIFIED LOWER EXTREMITY LYMPHEDEMA
 36 Lymphology 49 (2016) 36-41 SUCTION ASSISTED PROTEIN LIPECTOMY (SAPL) EVEN FOR THE TREATMENT OF CHRONIC FIBROTIC AND SCARIFIED LOWER EXTREMITY LYMPHEDEMA M. Lee, L. Perry, J. Granzow Emory University
36 Lymphology 49 (2016) 36-41 SUCTION ASSISTED PROTEIN LIPECTOMY (SAPL) EVEN FOR THE TREATMENT OF CHRONIC FIBROTIC AND SCARIFIED LOWER EXTREMITY LYMPHEDEMA M. Lee, L. Perry, J. Granzow Emory University
End Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Lymphedema. Original Policy Date
 MP 2.02.12 End Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Lymphedema Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date
MP 2.02.12 End Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Lymphedema Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date
Liposuction for chronic lymphoedema
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Liposuction for chronic lymphoedema Chronic lymphoedema is the swelling and build-up of body fluid and fat,
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Liposuction for chronic lymphoedema Chronic lymphoedema is the swelling and build-up of body fluid and fat,
MP Magnetic Resonance Imaging for Detection and Diagnosis of Breast Cancer
 Medical Policy MP 6.01.29 BCBSA Ref. Policy: 6.01.29 Last Review: 09/19/2018 Effective Date: 09/19/2018 Section: Radiology Related Policies 6.01.45 Computer-Aided Evaluation of Malignancy With Magnetic
Medical Policy MP 6.01.29 BCBSA Ref. Policy: 6.01.29 Last Review: 09/19/2018 Effective Date: 09/19/2018 Section: Radiology Related Policies 6.01.45 Computer-Aided Evaluation of Malignancy With Magnetic
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Pneumatic Compression Pumps for Treatment of Lymphedema and Venous Ulcers
 Last Review Status/Date: December 2015 Page: 1 of 9 Lymphedema and Venous Ulcers Description Description Pneumatic compression pumps are proposed as a treatment option for patients with lymphedema who
Last Review Status/Date: December 2015 Page: 1 of 9 Lymphedema and Venous Ulcers Description Description Pneumatic compression pumps are proposed as a treatment option for patients with lymphedema who
Surgical Treatment of Bilateral Gynecomastia
 Surgical Treatment of Bilateral Gynecomastia Policy Number: 7.01.13 Last Review: 4/2018 Origination: 4/2006 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Surgical Treatment of Bilateral Gynecomastia Policy Number: 7.01.13 Last Review: 4/2018 Origination: 4/2006 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate surgical options
 A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate
A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate
Treatment of lymphoedema surgical and conservative approaches
 Treatment of lymphoedema surgical and conservative approaches Nina Linnitt RN Clinical Manager medi UK Current Chair British Lymphology Society (BLS) What is lymphoedema? International Societie de Lymphologue
Treatment of lymphoedema surgical and conservative approaches Nina Linnitt RN Clinical Manager medi UK Current Chair British Lymphology Society (BLS) What is lymphoedema? International Societie de Lymphologue
The Standard of Care for Lymphedema: Current Concepts and Physiological Considerations
 The Standard of Care for Lymphedema: Current Concepts and Physiological Considerations Harvey N. Mayrovitz PhD Professor of Physiology College of Medical Sciences Nova Southeastern University mayrovit@nova.edu
The Standard of Care for Lymphedema: Current Concepts and Physiological Considerations Harvey N. Mayrovitz PhD Professor of Physiology College of Medical Sciences Nova Southeastern University mayrovit@nova.edu
Breast Cancer Treatment and Lymphedema
 Breast Cancer Treatment and Lymphedema Over the last decade, major advances have been made in the prevention and treatment of breast cancer in the United States, but minimal attention has been given to
Breast Cancer Treatment and Lymphedema Over the last decade, major advances have been made in the prevention and treatment of breast cancer in the United States, but minimal attention has been given to
Pneumatic Compression Pumps for Treatment of Lymphedema and Venous Ulcers
 Pneumatic Compression Pumps for Treatment of Lymphedema and Venous Ulcers Policy Number: 1.01.18 Last Review: 1/2019 Origination: 10/2000 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas
Pneumatic Compression Pumps for Treatment of Lymphedema and Venous Ulcers Policy Number: 1.01.18 Last Review: 1/2019 Origination: 10/2000 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas
Welcome to Allied Health Telehealth
 Welcome to Allied Health Telehealth Paediatric lymphoedema A challenge for clinicians and families To receive an attendance certificate please complete your online evaluation at: https://www.surveymonkey.com/s/paedlymphoedema
Welcome to Allied Health Telehealth Paediatric lymphoedema A challenge for clinicians and families To receive an attendance certificate please complete your online evaluation at: https://www.surveymonkey.com/s/paedlymphoedema
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Pneumatic Compression Pumps for Treatment Page 1 of 11 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Pneumatic Compression Pumps for Treatment of Lymphedema
Pneumatic Compression Pumps for Treatment Page 1 of 11 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Pneumatic Compression Pumps for Treatment of Lymphedema
PNEUMATIC COMPRESSION DEVICES IN THE HOME SETTING
 Status Active Medical and Behavioral Health Policy Section: Medicine Policy Number: II-60 Effective Date: 05/19/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Status Active Medical and Behavioral Health Policy Section: Medicine Policy Number: II-60 Effective Date: 05/19/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Related Policies None
 Medical Policy MP 7.01.153 BCBSA Ref. Policy: 7.01.153 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 7.01.153 BCBSA Ref. Policy: 7.01.153 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Systematic reviews and meta-analyses of observational studies (MOOSE): Checklist.
 Systematic reviews and meta-analyses of observational studies (MOOSE): Checklist. MOOSE Checklist Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease:
Systematic reviews and meta-analyses of observational studies (MOOSE): Checklist. MOOSE Checklist Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease:
Morbidity after lymph node dissection in patients with cancer: Incidence, risk factors, and prevention Stuiver, M.M.
 UvA-DARE (Digital Academic Repository) Morbidity after lymph node dissection in patients with cancer: Incidence, risk factors, and prevention Stuiver, M.M. Link to publication Citation for published version
UvA-DARE (Digital Academic Repository) Morbidity after lymph node dissection in patients with cancer: Incidence, risk factors, and prevention Stuiver, M.M. Link to publication Citation for published version
Lymphedema Myths Realities - Hope
 Lymphedema Myths Realities - Hope Texas Health Presbyterian Hospital of Dallas Lymphedema Therapy Department 214-345-7680 Lymphatic System Divided into sections - watersheds Lymph nodes act as filtration
Lymphedema Myths Realities - Hope Texas Health Presbyterian Hospital of Dallas Lymphedema Therapy Department 214-345-7680 Lymphatic System Divided into sections - watersheds Lymph nodes act as filtration
CASE REPORT Blocking of the Lymphatic Vessel in Lymphedema
 CASE REPORT Blocking of the Lymphatic Vessel in Lymphedema Hisako Hara, MD, and Makoto Mihara, MD Department of Lymphatic and Reconstructive Surgery, Saiseikai Kawaguchi General Hospital, Saitama, Japan
CASE REPORT Blocking of the Lymphatic Vessel in Lymphedema Hisako Hara, MD, and Makoto Mihara, MD Department of Lymphatic and Reconstructive Surgery, Saiseikai Kawaguchi General Hospital, Saitama, Japan
Medical Policy. MP Hydrogel Spacer use During Radiotherapy for Prostate Cancer
 Medical Policy BCBSA Ref. Policy: 7.01.164 Last Review: 01/24/2019 Effective Date: 04/25/2019 Section: Surgery Related Policies 8.01.47 Intensity-Modulated Radiotherapy of the Prostate 9.01.502 Experimental
Medical Policy BCBSA Ref. Policy: 7.01.164 Last Review: 01/24/2019 Effective Date: 04/25/2019 Section: Surgery Related Policies 8.01.47 Intensity-Modulated Radiotherapy of the Prostate 9.01.502 Experimental
Appropriate Concept of Prevention of Lymphedema at the Initial Treatment
 Global Breast Cancer Conference 2018 Appropriate Concept of Prevention of Lymphedema at the Initial Treatment Zisun Kim Department of Surgery, Soonchunhyang University Bucheon Hospital, Soonchunhyang University
Global Breast Cancer Conference 2018 Appropriate Concept of Prevention of Lymphedema at the Initial Treatment Zisun Kim Department of Surgery, Soonchunhyang University Bucheon Hospital, Soonchunhyang University
End-Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Lymphedema
 End-Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
End-Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Medical Policy. MP Continuous or Intermittent Monitoring of Glucose in Interstitial Fluid. Related Policies None
 Medical Policy BCBSA Ref. Policy: 1.01.20 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Durable Medical Equipment Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy BCBSA Ref. Policy: 1.01.20 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Durable Medical Equipment Related Policies None DISCLAIMER Our medical policies are designed for informational
Vertebral Axial Decompression
 Vertebral Axial Decompression Policy Number: 8.03.09 Last Review: 11/2018 Origination: 11/2005 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Vertebral Axial Decompression Policy Number: 8.03.09 Last Review: 11/2018 Origination: 11/2005 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
National Institute for Health and Care Excellence IP409/2 Liposuction for chronic lymphoedema IPAC date: June 2017
 National Institute for Health and Care Excellence IP409/2 Liposuction for chronic lymphoedema IPAC date: June 2017 Com. 1 Consultee 1 Sec. Comments Response Myself and my mother suffer from primary lymphoedema.
National Institute for Health and Care Excellence IP409/2 Liposuction for chronic lymphoedema IPAC date: June 2017 Com. 1 Consultee 1 Sec. Comments Response Myself and my mother suffer from primary lymphoedema.
EFFICACY OF DECONGESTIVE THERAPY AND INTERMITTENT PNEUMATIC COMPRESSION IN PATIENTS WITH LYMPHEDEMA OF THE ARM AFTER BREAST CANCER TREATMENT
 EFFICACY OF DECONGESTIVE THERAPY AND INTERMITTENT PNEUMATIC COMPRESSION IN PATIENTS WITH LYMPHEDEMA OF THE ARM AFTER BREAST CANCER TREATMENT Dragana Bojinović-Rodić MD, PhD Institute of Physical Medicine
EFFICACY OF DECONGESTIVE THERAPY AND INTERMITTENT PNEUMATIC COMPRESSION IN PATIENTS WITH LYMPHEDEMA OF THE ARM AFTER BREAST CANCER TREATMENT Dragana Bojinović-Rodić MD, PhD Institute of Physical Medicine
Corporate Medical Policy
 Corporate Medical Policy Pneumatic Compression Pumps for Treatment of Lymphedema and File Name: Origination: Last CAP Review: Next CAP Review: Last Review: pneumatic_compression_pumps_for _lymphedema_and_venous_ulcers
Corporate Medical Policy Pneumatic Compression Pumps for Treatment of Lymphedema and File Name: Origination: Last CAP Review: Next CAP Review: Last Review: pneumatic_compression_pumps_for _lymphedema_and_venous_ulcers
Vascularized Lymph Node Transfer From Thoracodorsal Axis for Congenital and Post Filarial Lymphedema of the Lower Limb
 CASE REPORT Vascularized Lymph Node Transfer From Thoracodorsal Axis for Congenital and Post Filarial Lymphedema of the Lower Limb HARI VENKATRAMANI, MCh, DNB, EDHS,* SENTHIL KUMARAN, MCh, SHIVANNAIAH
CASE REPORT Vascularized Lymph Node Transfer From Thoracodorsal Axis for Congenital and Post Filarial Lymphedema of the Lower Limb HARI VENKATRAMANI, MCh, DNB, EDHS,* SENTHIL KUMARAN, MCh, SHIVANNAIAH
REVIEW. Arm Edema in Breast Cancer Patients. Virginia S. Erickson, Marjorie L. Pearson, Patricia A. Ganz, John Adams, Katherine L.
 REVIEW Arm Edema in Breast Cancer Patients Virginia S. Erickson, Marjorie L. Pearson, Patricia A. Ganz, John Adams, Katherine L. Kahn The improvement in the life expectancy of women with breast cancer
REVIEW Arm Edema in Breast Cancer Patients Virginia S. Erickson, Marjorie L. Pearson, Patricia A. Ganz, John Adams, Katherine L. Kahn The improvement in the life expectancy of women with breast cancer
Venous Insufficiency Ulcers. Patient Assessment: Superficial varicosities. Evidence of healed ulcers. Dermatitis. Normal ABI.
 Venous Insufficiency Ulcers Patient Assessment: Superficial varicosities Evidence of healed ulcers Dermatitis Normal ABI Edema Eczematous skin changes 1. Scaling 2. Pruritus 3. Erythema 4. Vesicles Lipodermatosclerosis
Venous Insufficiency Ulcers Patient Assessment: Superficial varicosities Evidence of healed ulcers Dermatitis Normal ABI Edema Eczematous skin changes 1. Scaling 2. Pruritus 3. Erythema 4. Vesicles Lipodermatosclerosis
Risk factors for the initiation and aggravation of lymphoedema after axillary lymph node dissection for breast cancer
 HEALTH SERVICES RESEARCH FUND Risk factors for the initiation and aggravation of lymphoedema after axillary lymph node dissection for breast cancer Key Messages 1. Previous inflammation or infection of
HEALTH SERVICES RESEARCH FUND Risk factors for the initiation and aggravation of lymphoedema after axillary lymph node dissection for breast cancer Key Messages 1. Previous inflammation or infection of
Corporate Medical Policy
 Corporate Medical Policy Spinal Manipulation under Anesthesia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: spinal_manipulation_under_anesthesia 5/1998 10/2018 10/2019 10/2018
Corporate Medical Policy Spinal Manipulation under Anesthesia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: spinal_manipulation_under_anesthesia 5/1998 10/2018 10/2019 10/2018
Intensive Decongestive Lymphatic Therapy for Non-Cancer Secondary Lymphoedema Criteria Based Access Protocol
 NHS Dorset Clinical Commissioning Group Intensive Decongestive Lymphatic Therapy for Non-Cancer Secondary Lymphoedema Criteria Based Access Protocol Supporting people in Dorset to lead healthier lives
NHS Dorset Clinical Commissioning Group Intensive Decongestive Lymphatic Therapy for Non-Cancer Secondary Lymphoedema Criteria Based Access Protocol Supporting people in Dorset to lead healthier lives
Priorities Forum Statement
 Priorities Forum Statement Number 5 Subject Lymphoedema Date of decision November 2015 Date refreshed March 2017 Date of review November 2018 GUIDANCE Lymphoedema is the result of accumulation of fluid
Priorities Forum Statement Number 5 Subject Lymphoedema Date of decision November 2015 Date refreshed March 2017 Date of review November 2018 GUIDANCE Lymphoedema is the result of accumulation of fluid
Medical Policy. MP Absorbable Nasal Implant for Treatment of Nasal Valve Collapse
 Medical Policy MP 7.01.163 BCBSA Ref. Policy: 7.01.163 Last Review: 10/18/2018 Effective Date: 01/25/2019 Section: Surgery Related Policies 9.01.502 Experimental / Investigational Services DISCLAIMER Our
Medical Policy MP 7.01.163 BCBSA Ref. Policy: 7.01.163 Last Review: 10/18/2018 Effective Date: 01/25/2019 Section: Surgery Related Policies 9.01.502 Experimental / Investigational Services DISCLAIMER Our
Early detection and prevention of lymphoedema
 Early detection and prevention of lymphoedema Katie Riches Senior Research Nurse Derby Hospitals NHSF Trust Background: Derby Lymphoedema Service Three lymphoedema services based in Acute Hospitals at
Early detection and prevention of lymphoedema Katie Riches Senior Research Nurse Derby Hospitals NHSF Trust Background: Derby Lymphoedema Service Three lymphoedema services based in Acute Hospitals at
9 Day Certified Lymphedema & Wound Therapist (62.5 CE hrs On-line + 9 Days Live Training (8am 8pm *daily times vary; 153 CE hrs Total!
 1 9 Day Certified Lymphedema & Wound Therapist (62.5 CE hrs On-line + 9 Days Live Training (8am 8pm *daily times vary; 153 CE hrs Total!) On-Line COURSE OUTLINE/Overview of Topics (62.5hrs): Lymphatics
1 9 Day Certified Lymphedema & Wound Therapist (62.5 CE hrs On-line + 9 Days Live Training (8am 8pm *daily times vary; 153 CE hrs Total!) On-Line COURSE OUTLINE/Overview of Topics (62.5hrs): Lymphatics
Submitted by: Date of request: 28 th October 2015
 Submitted by: Frank Vicini, MD, FACR Chief Medical Officer ImpediMed, Inc. 5900 Pasteur Court Suite 125 Carlsbad, CA 92008 Phone: 760 585 2100 Email: fvicini@impedimed.com Date of request: 28 th October
Submitted by: Frank Vicini, MD, FACR Chief Medical Officer ImpediMed, Inc. 5900 Pasteur Court Suite 125 Carlsbad, CA 92008 Phone: 760 585 2100 Email: fvicini@impedimed.com Date of request: 28 th October
Radical excision and free flap transfer in chronic lymphedema
 Radical excision and free flap transfer in chronic lymphedema F. Schwarzl, J. Huracek, J. Farhadi, G. Pierer University Clinic for Plastic and Reconstructive Surgery University Hospital Basel, Switzerland
Radical excision and free flap transfer in chronic lymphedema F. Schwarzl, J. Huracek, J. Farhadi, G. Pierer University Clinic for Plastic and Reconstructive Surgery University Hospital Basel, Switzerland
Systematic reviews: From evidence to recommendation. Marcel Dijkers, PhD, FACRM Icahn School of Medicine at Mount Sinai
 Systematic reviews: From evidence to recommendation Session 2 - June 18, 2014 Going beyond design, going beyond intervention: The American Academy of Neurology (AAN) Clinical Practice Guideline process
Systematic reviews: From evidence to recommendation Session 2 - June 18, 2014 Going beyond design, going beyond intervention: The American Academy of Neurology (AAN) Clinical Practice Guideline process
Absorbable Nasal Implant for Treatment of Nasal Valve Collapse
 NOTE: This policy is not effective until March 1, 2019. Medical Policy Manual Surgery, Policy No. 209 Absorbable Nasal Implant for Treatment of Nasal Valve Collapse Next Review: November 2019 Last Review:
NOTE: This policy is not effective until March 1, 2019. Medical Policy Manual Surgery, Policy No. 209 Absorbable Nasal Implant for Treatment of Nasal Valve Collapse Next Review: November 2019 Last Review:
Facet Arthroplasty. Policy Number: Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019
 Facet Arthroplasty Policy Number: 7.01.120 Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for total facet
Facet Arthroplasty Policy Number: 7.01.120 Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for total facet
TRAINING COURSE IN LYMPHATIC MICROSURGERY
 TRAINING COURSE IN LYMPHATIC MICROSURGERY 28th - 29th June 2018 TRAINING COURSE IN LYMPHATIC MICROSURGERY PROGRAM OVERVIEW The course is available for residents and specialists in plastic surgery (worldwide)
TRAINING COURSE IN LYMPHATIC MICROSURGERY 28th - 29th June 2018 TRAINING COURSE IN LYMPHATIC MICROSURGERY PROGRAM OVERVIEW The course is available for residents and specialists in plastic surgery (worldwide)
Catherine Holley, RN Operating Room Nurse Massachusetts General Hospital
 Operating Room Nurse Massachusetts General Hospital March 15, 2017 Background Nurse 34 years Operating room RN at Massachusetts General Hospital 1979 surgery to remove varicose veins right leg Strong family
Operating Room Nurse Massachusetts General Hospital March 15, 2017 Background Nurse 34 years Operating room RN at Massachusetts General Hospital 1979 surgery to remove varicose veins right leg Strong family
Lymphedema 101. Andréa Leiserowitz, DPT, CLT. Oncology Physical Therapy;
 Lymphedema 101 Andréa Leiserowitz, DPT, CLT Oncology Physical Therapy; oncologypt@gmail.com Note: this information is the property of Dr. Leiserowitz and should not be copied, distributed, or otherwise
Lymphedema 101 Andréa Leiserowitz, DPT, CLT Oncology Physical Therapy; oncologypt@gmail.com Note: this information is the property of Dr. Leiserowitz and should not be copied, distributed, or otherwise
Eunice Jeffs 1,2 Emma Ream 3 Cath Taylor 1,3 Debra Bick 4 ABSTRACT
 SYSTEMATIC REVIEW Clinical effectiveness of decongestive treatments on excess arm volume and patient-centered outcomes in women with early breast cancer-related arm lymphedema: a systematic review Eunice
SYSTEMATIC REVIEW Clinical effectiveness of decongestive treatments on excess arm volume and patient-centered outcomes in women with early breast cancer-related arm lymphedema: a systematic review Eunice
Continuous or Intermittent Glucose Monitoring in Interstitial Fluid
 Continuous or Intermittent Glucose Monitoring in Interstitial Fluid Policy Number: 1.01.20 Last Review: 9/2018 Origination: 11/2001 Next Review: 9/2019 Policy Blue Cross and Blue Shield of Kansas City
Continuous or Intermittent Glucose Monitoring in Interstitial Fluid Policy Number: 1.01.20 Last Review: 9/2018 Origination: 11/2001 Next Review: 9/2019 Policy Blue Cross and Blue Shield of Kansas City
Lymphoedema following treatment for breast cancer: a new approach to an old problem
 Lymphoedema following treatment for breast cancer: a new approach to an old problem Screening breast cancer patients for lymphoedema makes it possible to identify problems early and take action to halt
Lymphoedema following treatment for breast cancer: a new approach to an old problem Screening breast cancer patients for lymphoedema makes it possible to identify problems early and take action to halt
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Continuous or Intermittent Monitoring of Glucose in Interstitial Fluid Page 1 of 39 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Continuous or Intermittent Monitoring
Continuous or Intermittent Monitoring of Glucose in Interstitial Fluid Page 1 of 39 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Continuous or Intermittent Monitoring
Peripheral Subcutaneous Field Stimulation
 Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 9/2018 Origination: 7/2013 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 9/2018 Origination: 7/2013 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
What is Lymphedema? Primary lymphedema: a person is born with the condition where the lymphatic vessels are not formed completely or malformed
 Lymphedema What is Lymphedema? Lymphedema is a chronic health condition which causes localized swelling. There are 2 types: Primary lymphedema: a person is born with the condition where the lymphatic vessels
Lymphedema What is Lymphedema? Lymphedema is a chronic health condition which causes localized swelling. There are 2 types: Primary lymphedema: a person is born with the condition where the lymphatic vessels
LEVEL ONE COURSE CONTENT
 LEVEL ONE COURSE CONTENT Dear Health Practitioner, The level one lymphoedema training course offered by the ALERT program at Macquarie University is open to AHPRA registered health professionals only (such
LEVEL ONE COURSE CONTENT Dear Health Practitioner, The level one lymphoedema training course offered by the ALERT program at Macquarie University is open to AHPRA registered health professionals only (such
THE MANAGEMENT OF THE SWOLLEN ARM IN CARCINOMA OF THE BREAST
 THE MANAGEMENT OF THE SWOLLEN ARM IN CARCINOMA OF THE BREAST NORMAN TREVES, M.D. The terms "brawny arm" and "lymphedema" have been given to the swollen arm which may complicate the inoperable, recurrent,
THE MANAGEMENT OF THE SWOLLEN ARM IN CARCINOMA OF THE BREAST NORMAN TREVES, M.D. The terms "brawny arm" and "lymphedema" have been given to the swollen arm which may complicate the inoperable, recurrent,
Role of free tissue transfer in management of chronic venous ulcer
 Original Article Role of free tissue transfer in management of chronic venous ulcer K. Murali Mohan Reddy, D. Mukunda Reddy Department of Plastic Surgery, Nizams Institute of Medical Sciences, India. Address
Original Article Role of free tissue transfer in management of chronic venous ulcer K. Murali Mohan Reddy, D. Mukunda Reddy Department of Plastic Surgery, Nizams Institute of Medical Sciences, India. Address
Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer
 Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer Policy Number: MM.07.014 Lines of Business: HMO; PPO Place of Service: Outpatient; Inpatient Current Effective Date: February 22, 2019 Original
Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer Policy Number: MM.07.014 Lines of Business: HMO; PPO Place of Service: Outpatient; Inpatient Current Effective Date: February 22, 2019 Original
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Continuous or Intermittent Monitoring of Glucose in Interstitial Fluid Page 1 of 42 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Continuous or Intermittent Monitoring
Continuous or Intermittent Monitoring of Glucose in Interstitial Fluid Page 1 of 42 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Continuous or Intermittent Monitoring
Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery
 7.01.140 Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery Section 7.0 Surgery Subsection Description Effective Date November 26, 2014
7.01.140 Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery Section 7.0 Surgery Subsection Description Effective Date November 26, 2014
MP Hyperthermic Intraperitoneal Chemotherapy for Select IntraAbdominal and Pelvic Malignancies. Related Policies None
 Medical Policy MP 2.03.07 BCBSA Ref. Policy: 2.03.07 Last Review: 10/18/2018 Effective Date: 01/25/2019 Section: Medicine Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 2.03.07 BCBSA Ref. Policy: 2.03.07 Last Review: 10/18/2018 Effective Date: 01/25/2019 Section: Medicine Related Policies None DISCLAIMER Our medical policies are designed for informational
Lymphedema: Early Detection and Quantitative Assessment
 Lymphedema: Early Detection and Quantitative Assessment Harvey N. Mayrovitz PhD Professor of Physiology College of Medical Sciences Nova Southeastern University mayrovit@nova.edu Lymphedema Physiology
Lymphedema: Early Detection and Quantitative Assessment Harvey N. Mayrovitz PhD Professor of Physiology College of Medical Sciences Nova Southeastern University mayrovit@nova.edu Lymphedema Physiology
Velcro Compression Devices
 Velcro Compression Devices Joseph A. Caprini, MD, MS, FACS, RVT, FACCWS Louis W. Biegler Chair of Surgery NorthShore University HealthSystem, Evanston, IL Clinical Professor of Surgery University of Chicago
Velcro Compression Devices Joseph A. Caprini, MD, MS, FACS, RVT, FACCWS Louis W. Biegler Chair of Surgery NorthShore University HealthSystem, Evanston, IL Clinical Professor of Surgery University of Chicago
A. Chronic, progressive swelling in tissues due to insufficient drainage of interstitial fluid
 CHAPTER 26 LYMPHEDEMA Imran Ratanshi, MD, MSc, FRCS(C) and Andres Maldonado, MD, PhD I. DEFINITION A. Chronic, progressive swelling in tissues due to insufficient drainage of interstitial fluid II. EPIDEMIOLOGY
CHAPTER 26 LYMPHEDEMA Imran Ratanshi, MD, MSc, FRCS(C) and Andres Maldonado, MD, PhD I. DEFINITION A. Chronic, progressive swelling in tissues due to insufficient drainage of interstitial fluid II. EPIDEMIOLOGY
Medical Policy. MP Dynamic Spinal Visualization and Vertebral Motion Analysis
 Medical Policy BCBSA Ref. Policy: 6.01.46 Last Review: 09/19/2018 Effective Date: 12/15/2018 Section: Radiology Related Policies 6.01.48 Positional Magnetic Resonance Imaging 9.01.502 Experimental / Investigational
Medical Policy BCBSA Ref. Policy: 6.01.46 Last Review: 09/19/2018 Effective Date: 12/15/2018 Section: Radiology Related Policies 6.01.48 Positional Magnetic Resonance Imaging 9.01.502 Experimental / Investigational
Lymphoedema Level One Course Content
 Lymphoedema Level One Course Content Dear Health Practitioner, The Level One lymphoedema training course offered by the ALERT Education at Macquarie University is open to AHPRA registered health professionals
Lymphoedema Level One Course Content Dear Health Practitioner, The Level One lymphoedema training course offered by the ALERT Education at Macquarie University is open to AHPRA registered health professionals
Bioimpedance Devices for Detection and Management of Lymphedema
 Medical Policy Manual Durable Medical Equipment, Policy No. 82 Bioimpedance Devices for Detection and Management of Lymphedema Next Review: June 2018 Last Review: June 2017 Effective: August 1, 2017 IMPORTANT
Medical Policy Manual Durable Medical Equipment, Policy No. 82 Bioimpedance Devices for Detection and Management of Lymphedema Next Review: June 2018 Last Review: June 2017 Effective: August 1, 2017 IMPORTANT
When is it lymphedema, when is it not lymphedema? UNDERSTANDING EDEMA. John Mulligan, RMT/CLT-LANA
 When is it lymphedema, when is it not lymphedema? UNDERSTANDING EDEMA John Mulligan, RMT/CLT-LANA Learning Objectives How to identify stages of lymphedema The Gold Standard of Treatment for Lymphedema
When is it lymphedema, when is it not lymphedema? UNDERSTANDING EDEMA John Mulligan, RMT/CLT-LANA Learning Objectives How to identify stages of lymphedema The Gold Standard of Treatment for Lymphedema
A comprehensive overview on the surgical management of secondary lymphedema of the upper and lower extremities related to prior oncologic therapies
 Garza et al. BMC Cancer (2017) 17:468 DOI 10.1186/s12885-017-3444-9 REVIEW A comprehensive overview on the surgical management of secondary lymphedema of the upper and lower extremities related to prior
Garza et al. BMC Cancer (2017) 17:468 DOI 10.1186/s12885-017-3444-9 REVIEW A comprehensive overview on the surgical management of secondary lymphedema of the upper and lower extremities related to prior
LYMPHOEDEMA FAISAL GHANI SIDDIQUI MBBS; FCPS; PGDIP-BIOMEDICAL ETHICS; MCPS-HPE
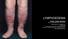 LYMPHOEDEMA FAISAL GHANI SIDDIQUI MBBS; FCPS; PGDIP-BIOMEDICAL ETHICS; MCPS-HPE PROFESSOR OF SURGERY & DIRECTOR, PROFESSIONAL DEVELOPMENT CENTRE J I N N A H S I N D H M E D I C A L U N I V E R S I T Y
LYMPHOEDEMA FAISAL GHANI SIDDIQUI MBBS; FCPS; PGDIP-BIOMEDICAL ETHICS; MCPS-HPE PROFESSOR OF SURGERY & DIRECTOR, PROFESSIONAL DEVELOPMENT CENTRE J I N N A H S I N D H M E D I C A L U N I V E R S I T Y
Medical Policy. MP Sphenopalatine Ganglion Block for Headache
 Medical Policy BCBSA Ref. Policy: 7.01.159 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Surgery Related Policies 2.01.29 Biofeedback as a Treatment of Headache 7.01.125 Occipital Nerve Stimulation
Medical Policy BCBSA Ref. Policy: 7.01.159 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Surgery Related Policies 2.01.29 Biofeedback as a Treatment of Headache 7.01.125 Occipital Nerve Stimulation
Related Policies None
 Medical Policy MP 6.01.57 BCBSA Ref. Policy: 6.01.57 Last Review: 09/19/2018 Effective Date: 09/19/2018 Section: Radiology Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 6.01.57 BCBSA Ref. Policy: 6.01.57 Last Review: 09/19/2018 Effective Date: 09/19/2018 Section: Radiology Related Policies None DISCLAIMER Our medical policies are designed for informational
LYMPHEDEMA GOALS FOR TODAY WELL-INTENTIONED, BUT. the more you know TEDS. Diuretics. Prescribe compression garments without first reducing edema
 LYMPHEDEMA the more you know GOALS FOR TODAY WELL-INTENTIONED, BUT TEDS Recognize full Continuum of Lymphedema stages Identify Range of Lymphedema Diagnoses Differentiate Effective Treatment Options from
LYMPHEDEMA the more you know GOALS FOR TODAY WELL-INTENTIONED, BUT TEDS Recognize full Continuum of Lymphedema stages Identify Range of Lymphedema Diagnoses Differentiate Effective Treatment Options from
Please Provide Responses to the Fields Below Electronically to be Accepted
 Please Provide Responses to the Fields Below Electronically to be Accepted Medicare Red Tape Relief Project Submissions accepted by the Committee on Ways and Means, Subcommittee on Health Date: August
Please Provide Responses to the Fields Below Electronically to be Accepted Medicare Red Tape Relief Project Submissions accepted by the Committee on Ways and Means, Subcommittee on Health Date: August
CLASSIFICATION OF LYMPHOSCINTIGRAPHY AND RELEVANCE TO SURGICAL INDICATION FOR LYMPHATICOVENOUS ANASTOMOSIS IN UPPER LIMB LYMPHEDEMA
 155 Lymphology 44 (2011) 155-167 CLASSIFICATION OF LYMPHOSCINTIGRAPHY AND RELEVANCE TO SURGICAL INDICATION FOR LYMPHATICOVENOUS ANASTOMOSIS IN UPPER LIMB LYMPHEDEMA T. Mikami, M. Hosono, Y. Yabuki, Y.
155 Lymphology 44 (2011) 155-167 CLASSIFICATION OF LYMPHOSCINTIGRAPHY AND RELEVANCE TO SURGICAL INDICATION FOR LYMPHATICOVENOUS ANASTOMOSIS IN UPPER LIMB LYMPHEDEMA T. Mikami, M. Hosono, Y. Yabuki, Y.
Related Policies None
 Medical Policy MP 8.01.62 BCBSA Ref. Policy: 8.01.62 Last Review: 07/25/2018 Effective Date: 07/25/2018 Section: Therapy Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 8.01.62 BCBSA Ref. Policy: 8.01.62 Last Review: 07/25/2018 Effective Date: 07/25/2018 Section: Therapy Related Policies None DISCLAIMER Our medical policies are designed for informational
Deep vein thrombosis and its prevention in critically ill adults Attia J, Ray J G, Cook D J, Douketis J, Ginsberg J S, Geerts W H
 Deep vein thrombosis and its prevention in critically ill adults Attia J, Ray J G, Cook D J, Douketis J, Ginsberg J S, Geerts W H Authors' objectives To systematically review the incidence of deep vein
Deep vein thrombosis and its prevention in critically ill adults Attia J, Ray J G, Cook D J, Douketis J, Ginsberg J S, Geerts W H Authors' objectives To systematically review the incidence of deep vein
Breast Reconstruction Surgery
 Breast Reconstruction Surgery I. Policy University Health Alliance (UHA) will reimburse for Breast Reconstruction Surgery when it is determined to be medically necessary and when it meets the medical criteria
Breast Reconstruction Surgery I. Policy University Health Alliance (UHA) will reimburse for Breast Reconstruction Surgery when it is determined to be medically necessary and when it meets the medical criteria
PRESOR-03 STUDY ON THE EFFECTIVENESS OF USING THE SORISA PRESSURE THERAPY
 PRESOR-03 STUDY ON THE EFFECTIVENESS OF USING THE SORISA PRESSURE THERAPY CONTENTS 1.0. PRESSURE THERAPY 1.1. The physiological effects of pressure therapy 1.2. When pressure therapy is indicated 2.0.
PRESOR-03 STUDY ON THE EFFECTIVENESS OF USING THE SORISA PRESSURE THERAPY CONTENTS 1.0. PRESSURE THERAPY 1.1. The physiological effects of pressure therapy 1.2. When pressure therapy is indicated 2.0.
Intraoperative Fluorescence Imaging Systems
 Intraoperative Fluorescence Imaging Systems Policy Number: 10.01.530 Last Review: 01/2018 Origination: 01/2015 Next Review: 01/2019 Policy Intraoperative fluorescence imaging (SPY Imaging) to evaluate
Intraoperative Fluorescence Imaging Systems Policy Number: 10.01.530 Last Review: 01/2018 Origination: 01/2015 Next Review: 01/2019 Policy Intraoperative fluorescence imaging (SPY Imaging) to evaluate
Systematic Reviews and Meta- Analysis in Kidney Transplantation
 Systematic Reviews and Meta- Analysis in Kidney Transplantation Greg Knoll MD MSc Associate Professor of Medicine Medical Director, Kidney Transplantation University of Ottawa and The Ottawa Hospital KRESCENT
Systematic Reviews and Meta- Analysis in Kidney Transplantation Greg Knoll MD MSc Associate Professor of Medicine Medical Director, Kidney Transplantation University of Ottawa and The Ottawa Hospital KRESCENT
1. Skin care: Cleansing, lubrication, debriding and administration of antimicrobial therapy.
 Lymphedema Treatment Last Review Date: October 13, 2017 Number: MG.MM.ME.05aC9 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider
Lymphedema Treatment Last Review Date: October 13, 2017 Number: MG.MM.ME.05aC9 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider
MP Laparoscopic and Percutaneous Techniques for the Myolysis of Uterine Fibroids
 Medical Policy MP 4.01.19 BCBSA Ref. Policy: 4.01.19 Last Review: 08/20/2018 Effective Date: 08/20/2018 Section: OB/GYN Reproduction Related Policies 4.01.11 Occlusion of Uterine Arteries Using Transcatheter
Medical Policy MP 4.01.19 BCBSA Ref. Policy: 4.01.19 Last Review: 08/20/2018 Effective Date: 08/20/2018 Section: OB/GYN Reproduction Related Policies 4.01.11 Occlusion of Uterine Arteries Using Transcatheter
Cancer Support V.I. Presents
 Cancer Support V.I. Presents Overview & Discussion of Manual Lymph Drainage/Complete Decongestive Therapy (MLD/CDT) Presented by: Christopher A. Borgesen, MS,OTR September 3 rd, 2008 Christopher A. Borgesen,
Cancer Support V.I. Presents Overview & Discussion of Manual Lymph Drainage/Complete Decongestive Therapy (MLD/CDT) Presented by: Christopher A. Borgesen, MS,OTR September 3 rd, 2008 Christopher A. Borgesen,
Phlebo Press. Use In: Phlebo Press
 Phlebo Press Use In: Phlebo Press is a sequential pneumatic compression system specially designed for efficient reduction and management of peripheral edema, such as lymphedema and ulceration associated
Phlebo Press Use In: Phlebo Press is a sequential pneumatic compression system specially designed for efficient reduction and management of peripheral edema, such as lymphedema and ulceration associated
Advances in Breast Surgery. Catherine Campo, D.O. Breast Surgeon Meridian Health System April 17, 2015
 Advances in Breast Surgery Catherine Campo, D.O. Breast Surgeon Meridian Health System April 17, 2015 Objectives Understand the surgical treatment of breast cancer Be able to determine when a lumpectomy
Advances in Breast Surgery Catherine Campo, D.O. Breast Surgeon Meridian Health System April 17, 2015 Objectives Understand the surgical treatment of breast cancer Be able to determine when a lumpectomy
II Dissection techniques in Lymphatic Microsurgery- Hands on live animal model
 II Dissection techniques in Lymphatic Microsurgery- Hands on live animal model 9th - 10th December 2019 II Dissection techniques in Lymphatic Microsurgery- Hands on live animal model PROGRAM OVERVIEW The
II Dissection techniques in Lymphatic Microsurgery- Hands on live animal model 9th - 10th December 2019 II Dissection techniques in Lymphatic Microsurgery- Hands on live animal model PROGRAM OVERVIEW The
Outpatient Pulmonary Rehabilitation
 Outpatient Pulmonary Rehabilitation Policy Number: 8.03.05 Last Review: 7/2018 Origination: 7/1995 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for
Outpatient Pulmonary Rehabilitation Policy Number: 8.03.05 Last Review: 7/2018 Origination: 7/1995 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for
Recent developments for combining evidence within evidence streams: bias-adjusted meta-analysis
 EFSA/EBTC Colloquium, 25 October 2017 Recent developments for combining evidence within evidence streams: bias-adjusted meta-analysis Julian Higgins University of Bristol 1 Introduction to concepts Standard
EFSA/EBTC Colloquium, 25 October 2017 Recent developments for combining evidence within evidence streams: bias-adjusted meta-analysis Julian Higgins University of Bristol 1 Introduction to concepts Standard
USDA Nutrition Evidence Library: Systematic Review Methodology
 USDA Nutrition Evidence Library: Systematic Review Methodology Julie E. Obbagy, PhD, RD USDA Center for Nutrition Policy & Promotion Meeting #2 October 17, 2016 The National Academies of Sciences, Engineering,
USDA Nutrition Evidence Library: Systematic Review Methodology Julie E. Obbagy, PhD, RD USDA Center for Nutrition Policy & Promotion Meeting #2 October 17, 2016 The National Academies of Sciences, Engineering,
