National Medical Policy
|
|
|
- George Fisher
- 6 years ago
- Views:
Transcription
1 National Medical Policy Subject: Policy Number: Breast Reconstructive Surgery NMP492 Effective Date*: February 2013 Updated: April 2016 This National Medical Policy is subject to the terms in the IMPORTANT NOTICE at the end of this document For Medicaid Plans: Please refer to the appropriate State's Medicaid manual(s), publication(s), citations(s) and documented guidance for coverage criteria and benefit guidelines prior to applying Health Net Medical Policies The Centers for Medicare & Medicaid Services (CMS) For Medicare Advantage members please refer to the following for coverage guidelines first: Use Source Reference/Website Link X National Coverage Determination (NCD) Breast Reconstruction (140.2): X National Coverage Manual Citation Local Coverage Determination (LCD)* Cosmetic and Reconstructive Surgery; Plastic Surgery: X Article (Local)* Cosmetic vs. Reconstructive Surgery: Other None Use Health Net Policy Instructions Medicare NCDs and National Coverage Manuals apply to ALL Medicare members in ALL regions. Medicare LCDs and Articles apply to members in specific regions. To access your specific region, select the link provided under Reference/Website and follow the search instructions. Enter the topic and your specific state to find the coverage determinations for your region. *Note: Health Net must follow local coverage determinations (LCDs) of Medicare Administration Contractors (MACs) located outside their service area when those MACs have exclusive coverage of an item or service. (CMS Manual Chapter 4 Section 90.2) Breast Reconstructive Surgery Apr 16 1
2 If more than one source is checked, you need to access all sources as, on occasion, an LCD or article contains additional coverage information than contained in the NCD or National Coverage Manual. If there is no NCD, National Coverage Manual or region specific LCD/Article, follow the Health Net Hierarchy of Medical Resources for guidance. Current Policy Statement (Please Refer to HN NMP 169 Cosmetic and Reconstructive Surgery and HN NMP 323 Lymphedema and Venous Stasis Ulcer Treatments for additional information) and/or state mandates). *Note Women s Health & Cancer Rights Act of 1998 (WHCRA) is a federal law that provides protections to patients who choose to have breast reconstruction in connection with a mastectomy. In accordance with WHCRA, all stages of reconstruction of the breast on which a mastectomy was performed, surgery and reconstruction of the other breast to produce symmetrical appearance, prostheses and treatment of physical complications of the mastectomy, including lymphedema are considered medically necessary. Health Net, Inc. considers reconstructive breast surgery medically necessary after a mastectomy or a lumpectomy which results in a significant deformity. Medically necessary procedures include any of the following: 1. Implantation of U.S. Food and Drug Administration (FDA)-approved internal breast prosthesis; 2. The use of tissue expanders; 3. Use of AlloDerm Regenerative Tissue Matrix, FlexHD Acelluar Hydrated Dermis, AlloMax, NeoForm Dermis; 4. Tissue/muscle reconstruction procedures (e.g. flaps); 5. Oncoplastic reconstruction; 6. Reconstructive surgical revisions; 7. Breast reconstruction of the affected breast, including reconstruction of the nipple and areolar complex; 8. Tattooing in conjunction with reconstructive breast surgery post-mastectomy; 9. Removal of a breast implant, periprosthetic capsulotomy or capsulectomy is considered medically necessary when documentation in the patient s record indicates any of the following mechanical complications of breast prosthesis: Ruptured implant Implant extrusion Painful capsular contracture with disfigurement Infection or inflammatory reaction due to breast prosthesis Siliconoma Granuloma Interference with diagnosis of breast cancer 10. Autologous fat/graft transfer (e.g., lipoinjection, lipofilling, lipopmodelling) postmastectomy, when no native breast tissue is present. Health Net considers breast reconstructive surgery to correct breast asymmetry in the nondiseased, unaffected, or contralateral breast, medically necessary only in the following situations: 1. Surgical correction due to a medically necessary mastectomy or a medically necessary lumpectomy that results in a significant deformity; 2. Repair of breast asymmetry due to trauma. 3. Breast implant for Poland s syndrome (congenital absence of breast). Breast Reconstructive Surgery Apr 16 2
3 4. Pectus excavatum repair when documented functional impairment exists (i.e., decreased cardiac output and/or abnormal pulmonary function during exercise) or when future cardiovascular compromise is anticipated. Not Medically Necessary Health Net, Inc. considers the following not medically necessary when used in association with breast reconstruction procedures, since there is a lack of randomized, controlled, prospective studies (list may not be all inclusive): 1. Permacol 2. Radiesse Note: Breast reconstruction services following mastectomy and lumpectomy is available to both females and males. In addition, a diagnosis of breast cancer is not required for breast reconstruction services, and the timing of reconstructive services is not a factor. Codes Related To This Policy NOTE: The codes listed in this policy are for reference purposes only. Listing of a code in this policy does not imply that the service described by this code is a covered or noncovered health service. Coverage is determined by the benefit documents and medical necessity criteria. This list of codes may not be all inclusive. On October 1, 2015, the ICD-9 code sets used to report medical diagnoses and inpatient procedures have been replaced by ICD-10 code sets. \ ICD-9 Codes (not an inclusive list) Malignant neoplasm of breast Secondary malignant neoplasm of breast Diffuse cystic mastopathy [severe fibrocystic disease] Specified anomalies of breast (absence) V10.3 Personal history of malignant neoplasm of breast V45.71 Acquired absence of breast [following medically necessary mastectomy or lumpectomy resulting in significant deformity] ICD-10 Codes (not an inclusive list) C50 Malignant neoplasm of breast N60.1 Diffuse cystic mastopathy(fibrocystic disease) Q83 Congenital malformations of the breast Z85.3 Personal history of malignant neoplasm of breast Z90.1 Acquired absence of breast(s) CPT Codes (not an inclusive list) Tattooing, intradermal introduction of insoluble opaque pigments to correct color defects of skin, including micropigmentation; 6.0 sq cm or less Tattooing, intradermal introduction of insoluble opaque pigments to correct color defects of skin, including micropigmentation; 6.1 to 20.0 sq cm Replacement of tissue expander with permanent prosthesis Removal of tissue expander(s) without insertion of prosthesis Reduction mammaplasty Mammaplasty, augmentation; without prosthetic implant Breast Reconstructive Surgery Apr 16 3
4 19325 Mammaplasty, augmentation; with prosthetic implant Removal of intact mammary implant Removal of mammary implant material Immediate insertion of breast prosthesis following mastopexy, mastectomy or in reconstruction Delayed insertion of breast prosthesis following mastopexy, mastectomy or in reconstruction Nipple/areola reconstruction Breast reconstruction, immediate or delayed, with tissue expander, Including subsequent expansion Breast reconstruction with latissimus dorsi flap, with or without prosthetic implant Breast reconstruction with free flap Breast reconstruction with other technique Breast reconstruction with transverse rectus abdominis myocutaneous flap (TRAM), single pedicle, including closure of donor site Breast reconstruction with transverse rectus abdominis myocutaneous flap (TRAM), single pedicle, including closure of donor site; with microvascular anastomosis (supercharging) Breast reconstruction with transverse rectus abdominis myocutaneous flap (TRAM), double pedicle, including closure of donor site Open periprosthetic capsulotomy, breast Periprosthetic capsulectomy, breast Revision of reconstructed breast HCPCS Codes (not an all inclusive list) L8600 Implantable breast prosthesis, silicone or equal Q4100 Skin substitute, not otherwise specified Q4116 Skin substitute, alloderm, per square centimeter Q4128 Flex HD, Allopatch HD, Or Matrix HD, Per Square Centimeter Scientific Rationale Update April 2016 NCCN guidelines on Breast Cancer (1.2016) note that breast reconstruction may be an option for any women receiving surgical treatment for breast cancer. The guidelines state that all women undergoing breast cancer treatment should be educated about breast reconstruction options as adapted to their individual clinical situation. However, breast reconstruction should not interfere with the appropriate surgical management of the cancer. The guidelines also note that it is sometimes necessary to perform surgery on the contralateral breast (e.g. breast reduction, implantation) to achieve optimal symmetry between the ipsilateral reconstructed breast and the contralateral breast. Kronowitz et al (2016) assessed the risk of locoregional and systemic recurrence in patients who underwent lipofilling for breast reconstruction. The authors identified all patients who underwent segmental or total mastectomy for breast cancer (719 breasts) (i.e., cases) or breast cancer risk reduction or benign disease (305 cancerfree breasts) followed by breast reconstruction with lipofilling as an adjunct or primary procedure between June of 1981 and February of They also then identified matched patients with breast cancer treated with segmental or total mastectomy followed by reconstruction without lipofilling (670 breasts) (i.e., controls). The probability of locoregional recurrence was estimated by the Kaplan- Breast Reconstructive Surgery Apr 16 4
5 Meier method. Mean follow-up times after mastectomy were 60 months for cases, 44 months for controls, and 73 months for cancer-free breasts. Locoregional recurrence was observed in 1.3 percent of cases (nine of 719 breasts) and 2.4 percent of controls (16 of 670 breasts). Breast cancer did not develop in any cancerfree breast. The cumulative 5-year locoregional recurrence rates were 1.6 percent and 4.1 percent for cases and controls, respectively. Systemic recurrence occurred in 2.4 percent of cases and 3.6 percent of controls (p = 0.514). There was no primary breast cancer in healthy breasts reconstructed with lipofilling. The authors concluded the study results showed no increase in locoregional recurrence, systemic recurrence, or second breast cancer. These findings support the oncologic safety of lipofilling in breast reconstruction. Lindegren et al (2016) reported autologous fat transplantation (AFT) to the breast can correct defects and be a part of a breast reconstruction to achieve a better aesthetic result. The impact of AFT on the radiological evaluation and detection of cancer remains unclarified. The authors sought to investigate whether AFT induces lasting modifications. In this study, a valuation was performed of 44 breasts from 37 patients examined with mammography and ultrasound before and after autologous fat transplantation. Breast radiologists evaluated the images using a study specific protocol. AFT did not hinder post-operative assessment of mammograms or ultrasound. No detectable changes with serious clinical impact were found after injections of mean 177 ml (34-516) of fat in one to four sessions. The rate of oil cysts was significantly higher after AFT than pre-operatively (2.3 vs % p = ). Significantly more post-operative oil cysts were detected after injection of larger volumes of fat (144 vs. 243 ml, p = 0.013). No significant differences were found in the post-operative images regarding age at surgery, follow-up time, or time from previous breast surgery. The authors concluded AFT does not impair assessment of mammograms and ultrasound in patients who have a history of breast cancer surgery or prophylactic mastectomy. Frey et al (2015) reported acellular dermal matrices are used in implant-based breast reconstruction. The introduction of contour fenestrated AlloDerm (Life-Cell, Branchburg, N.J.) offers sterile processing, a crescent shape, and prefabricated fenestrations. However, any evidence comparing reconstructive outcomes between this newer generation acellular dermal matrices and earlier versions is lacking. Patients undergoing implant-based breast reconstruction from 2010 to 2014 were identified. Reconstructive outcomes were stratified by 4 types of implant coverage: aseptic AlloDerm, sterile "ready-to-use" AlloDerm, contour fenestrated AlloDerm, or total submuscular coverage. Outcomes were compared with significance set at P < A total of 620 patients (1019 reconstructions) underwent immediate, implantbased breast reconstruction; patients with contour fenestrated AlloDerm were more likely to have nipple-sparing mastectomy (P = , , and ) and immediate permanent implant reconstructions (P = ). Those with contour fenestrated AlloDerm coverage had lower infection rates requiring oral (P = ) and intravenous antibiotics (P = ) compared with aseptic AlloDerm coverage. Compared with sterile "ready-to-use" AlloDerm coverage, those with contour fenestrated AlloDerm had similar infection outcomes but significantly more minor mastectomy flap necrosis (P = ). Compared with total submuscular coverage, those with contour fenestrated AlloDerm coverage had similar infection outcomes but significantly more explantations (P = ), major (P = ) and minor mastectomy flap necrosis (P = ). Significant independent risk factors for increased infection were also identified. The authors concluded contour fenestrated AlloDerm reduces infections compared with aseptic AlloDerm, but infection rates are similar to those of sterile, ready-to-use AlloDerm and total submuscular coverage. Breast Reconstructive Surgery Apr 16 5
6 Scientific Rationale April 2015 On The U.S. FDA site it is noted that the Biosciences SurgiMend was issued a 510(k) Number of K083898, on 2/4/09. Per the U.S. FDA SurgiMend is intended for implantation to reinforce soft tissue where weakness exists and for the surgical repair of damaged or ruptured soft tissue membranes. SurgiMend is specifically indicated for plastic and reconstructive surgery, muscle flap reinforcement, hernia repair including abdominal, inguinal, femoral, diaphragmatic, scrotal, umbilical, and incisional x`. The FDA site includes 67 adverse events associated with SurgiMend. Per the Manufacturer s site: SurgiMend is a unique acellular collagen matrix derived from fetal and neonatal bovine dermis. SurgiMend offers clear advantages over synthetic and other biologic products for soft tissue repair and reconstruction. There is a Clinical Trial on Acellular Dermal Matrix in Tissue Expander Breast Reconstruction: A Prospective, Randomized, Clinical Trial Comparing SurgiMend PRS and AlloDerm RTU which is currently recruiting participants. The ClinicalTrials.gov Identifier is NCT and it was last updated June The purpose of this study is to assess the relative performance and complication rates between the AlloDerm RTU and SurgiMend PRS products as well as the relative economics of these two treatment options. The estimated primary completion date is September There is another Clinical Trial on SurgiMend in Two-stage Implant-based Breast Reconstruction in Patients With Pre-Mastectomy Radiotherapy, and the study is not yet open for participant recruitment. The ClinicalTrials.gov Identifier is NCT , and it was last updated in October This is a prospective clinical study comparing two-stage breast reconstruction following mastectomy with and without the use of SurgiMend PRS fetal bovine collagen matrix in patients with premastectomy radiation therapy. The estimated primary completion date is September The sponsor of the trial is TEI Biosciences Inc., the manufacturer of Surgimend. Scientific Rationale Update April 2013 Autologous fat grafting (AFG) is used as an adjunct to standard breast reconstruction following mastectomy or breast-conserving surgery (lumpectomy or partial mastectomy). Autologous fat grafting consists of 3 phases - harvesting of fat tissue (e.g. lower abdomen, back, or thighs), processing, and application of the graft to the surgical site. Fat harvesting is similar to classic liposuction. In 2009, the American Society of Plastic Surgeons (ASPS) Executive Committee approved Guiding Principles that did not provide specific recommendations for the clinical use of fat grafts, citing a lack of strong data and literature. The review of the scientific evidence by the Task Force indicated that there were no reports suggesting an increased risk of malignancy associated with fat grafting and limited data suggested that fat grafts may not interfere with radiologic imaging for breast cancer detection. In 2011, a joint ASPS & American Society for Aesthetic Plastic Surgery (ASAPS) Position Statement: Stem Cells and Fat Grafting, addressed concerns regarding stem cells and fat grafting in clinical practice. They noted that standard fat grafting procedures which do transfer some stem cells naturally present within the tissue should be described as a fat grafting procedure, not a stem cell procedure. They stated that the marketing and promotion of stem cell procedures in aesthetic surgery is not adequately supported by clinical evidence. A substantial body of clinical data to assess plastic surgery applications of stem cell therapies still needs to be collected. Until further evidence is available, stem cell therapies in aesthetic and Breast Reconstructive Surgery Apr 16 6
7 reconstructive surgery should be conducted within clinical studies under Institutional Review Board approval, including compliance with all guidelines for human medical studies Post-Mastectomy Fat Graft/Fat Transfer (ASPS) Guiding Principles, provides recommendations regarding fat transfer to the post-mastectomy breast with no native breast tissue present. Per the ASPS, An evaluation of available literature on autologous fat grafting following mastectomy with no remaining native breast tissue indicates that the body of evidence is comprised mostly of case series, and when combined, the studies provide consistent evidence, thus resulting in grade B recommendations. A grade B recommendation encourages clinicians to employ the available information while remaining cognizant of newer, evidence-based findings. The existing evidence suggests autologous fat grafting as an effective option in breast reconstruction following mastectomy while demonstrating moderate to significant aesthetic improvement. In addition, the available evidence also cites autologous fat grafting as a useful modality for alleviating post mastectomy pain syndrome. Furthermore, the evidence suggests autologous fat grafting as a viable option for improving the quality of irradiated skin present in the setting of breast reconstruction. ASPS GUIDING PRINCIPLES 1. Aesthetic Outcome: Studies indicate that breast cancer patients undergoing fat grafting as an adjunct to post-mastectomy breast reconstruction experience moderate to significant aesthetic improvement, particularly for volume, contour and superomedial fullness. The evidence also suggests that cosmetic outcome is significantly enhanced after serial fat grafting and that, overall, patients are satisfied with aesthetic results. 2. Breast Cancer Recurrence: Evidence suggests that in post-mastectomy breast reconstruction patients, fat grafting does not increase the risk of breast cancer recurrence. As surveillance is integral for the management of any breast cancer patient, fat grafting to post-mastectomy reconstructed breasts does not delay diagnosis of breast cancer recurrence. When reviewed by experienced radiologists, the presence of oil cysts and fat necrosis on mammography, ultrasound and MRI imaging is distinguishable from suspicious lesions. Surveillance should continue to be rigorous and it is encouraged that radiologists who are experienced in breast imaging work with plastic surgeons to facilitate imaging accuracy. 3. Complications: Although there is variability in physician technique for fat grafting, the evidence suggests that post-mastectomy breast reconstruction with fat grafting is effective and is associated with a low risk of complications. Furthermore, there is increasing evidence that fat grafting is an effective surgical technique for treating post-mastectomy pain syndrome. 4. Radiation Therapy: There is a growing body of evidence that suggests no increased risk of complications when fat grafting is employed in the presence of previously irradiated tissue. 5. Technique: The number of fat grafting sessions required varies per patient. Studies suggest that a majority of patients require more than one fat grafting session to achieve adequate aesthetic results, and that each additional session will contribute to gradual improvement of the overall outcome Sarfati et al (2013) investigated sixty-eight patients who had mastectomy and breast irradiation who had one or more sessions of lipofilling prior to breast implant reconstruction. These patients were prospectively followed up in order to collect the following data: postoperative complications; cosmetic result; local breast cancer recurrences. The mean number of fat grafting sessions was 2.3 (range 1-6). An average volume of 115mL (70-275) was injected each time. The mean volume of Breast Reconstructive Surgery Apr 16 7
8 breast implants was 300mL ( ). The mean follow-up was 23 months. No breast cancer local recurrence was diagnosed during follow-up. Implant explantation was performed in one case (1.47%) The mean cosmetic result was 4.5/5. Investigators concluded fat grafting to the irradiated chest wall prior to implant placement might be an alternative to flap reconstruction for patients who are not suitable or who refuse this option. Irani et al (2012) sought to determine whether fat grafting prior to breast reconstruction could improve thoracic tissue trophicity enough to perform a simple prosthetic reconstruction, avoiding a flap procedure. A total of 25 patients who had undergone a modified radical mastectomy followed by radiotherapy were retrieved. Fat was injected according to Coleman's technique. Prosthetic reconstruction was performed three to six months after the fat grafting procedure. Reconstruction of the nipple-areola complex was performed 3 months after implant positioning. Median follow-up interval was two years. Patient satisfaction was ascertained with a selfassessment questionnaire. Three independent blinded physician observers judged preoperative and postoperative photographs to determine the quality of reconstruction. The mean refined fat injected volume was 160ml. In all cases except two, a unique fat grafting procedure was necessary. Prosthetic reconstruction was achieved in 23 cases. The technique failed in two cases and breast reconstruction was achieved by a latissimus dorsi flap with implant. During the follow-up interval, two complications occurred in two patients presenting with fat necrosis and oil cysts. The mean number of total surgical procedures was 2.5 per patient. Quality of reconstruction was judged as good by both physicians and patients. Authors concluded autologous fat grafting in radiated tissue prior to breast reconstruction is a safe and reliable technique. In selected cases, a simple prosthetic reconstruction can be achieved avoiding a flap procedure. Seth et al (2012) reported that long-term oncologic implications of autologous fat grafting following breast reconstruction remains unclear. The authors evaluated longterm outcomes following tissue expander breast reconstruction with and without fat grafting in a retrospective review of consecutive patients undergoing mastectomy with immediate tissue expander reconstruction. Demographic, operative, oncologic, and postoperative factors were recorded, including the use of fat grafting. Mean follow-up was 42.1 ± 28.8 and 43.6 ± 27.2 months for non-fat-grafting and fatgrafting patients, respectively, including 24.8 ± 5.9 months after the first fatgrafting procedure. Fisher's exact test, t test, and regression analysis were used for statistics. A review of 886 patients (n = 1202 breasts) revealed no significant differences in demographics, operative characteristics, tumor staging, or radiation therapy exposure between fat-grafting (n = 90 breasts) and non-fat-grafting (n = 1112 breasts) patients. Ninety-nine fat-grafting procedures were performed an average of 18.3 months after reconstruction, with one complication (fat necrosis). Grafting did not affect local tumor recurrence or survival when compared with nonfat-grafted breasts. Complication following reconstruction, including a poor cosmetic result, was an independent predictor of undergoing subsequent fat grafting.. The authors concluded the analysis suggests that fat grafting after breast reconstruction does not adversely affect local tumor recurrence or survival on long-term follow-up. Autologous fat grafting can be used as an aesthetic adjunct to prosthetic reconstruction with minimal complications. These results also indicate the need for multi-institutional, prospective studies to definitively establish its oncologic safety. de Blacam et al (2011) reviewed their early experience of fat grafting in the correction of acquired contour deformities after postmastectomy breast reconstruction in a retrospective review of 49 patients who underwent fat grafting to 68 reconstructed breasts was carried out. Clinical outcomes were analyzed and aesthetic results were assessed with objective grading of preoperative and Breast Reconstructive Surgery Apr 16 8
9 postoperative photographs by two independent, blinded plastic surgeons. On average, 67 cc of fat was injected into each breast per session. There were 111 fat injection procedures, as more than one injection was required in 51.5 percent of cases. Average follow-up time was 2.4 years. Complications occurred in 6.3 percent of procedures, including fat necrosis (3.6 percent), oil cysts (1.8 percent), and infection (0.9 percent). Aesthetic outcome was significantly improved across all measurements, including volume, contour, placement, and superomedial fullness (p<0.001 for all). The reviewers concluded although further studies are required to provide surgeons with definitive guidelines for the implementation of this technique, fat injection is a safe intervention and significantly improves the aesthetic results in patients with contour deformities of the reconstructed breast. Losken et al (2011) reviewed their experience with fat grafting for the correction of acquired breast deformities. A retrospective review was performed on 107 patients with a history of breast cancer between 1996 and 2010, who had autologous fat grafting at the time of secondary breast reconstruction. The indications were for improvement in contour, shape, and volume of the breast following transverse rectus abdominis myocutaneous (TRAM) flap reconstruction (n = 55), latissimus dorsi with or without implant (n = 20), implant reconstruction (n = 20), and breast conservation therapy deformity (n = 12). The average volume of injection was 40 ml (range, ml), the most common location being upper and medial quadrants. Fat was harvested mainly from the abdomen, thighs, and flanks. Complications occurred in 11% of the patients, and included fat necrosis, erythema, keloid scarring, and pain. Complications were higher when performed with implant reconstructions. Repeat fat injection was performed in 25% (n = 27/107), which increased with the length of follow-up. Patients with a history of radiation therapy had an increased incidence of repeat injections (36% vs. 18%). Patients with >6 months follow-up reported an improvement of about 83%. The authors concluded autologous fat grafting is a safe and effective tool for secondary breast reconstruction. It is helpful in all types of reconstructions to improve contour, volume, and overall breast shape and symmetry. Repeat injections are often required and this is more common in patients with longer follow-up and in those with a history of radiation therapy. The popularity of this approach in reconstructive breast surgery will likely continue to increase. Breast Reconstructive Surgery Apr 16 9
10 Rigotti et al (2010) compared the incidence of local and regional recurrence of breast cancer between two contiguous time windows in a homogeneous population of 137 patients who underwent fat tissue transplant after modified radical mastectomy. Median follow-up time was 7.6 years and the follow-up period was divided into two contiguous time windows, the first starting at the date of the radical mastectomy and ending at the first lipoaspirate grafting session and the second beginning at the time of the first lipoaspirate grafting session and ending at the end of the total follow-up time. Although this study did not employ an independent control group, the incidence of local recurrence of breast cancer was found to be comparable between the two periods and in line with data from similar patient populations enrolled in large multicenter clinical trials and who did not undergo postsurgical fat tissue grafting. Statistical comparison of disease-free survival curves revealed no significant differences in relapse rate between the two patient subgroups before fat grafting and after fat grafting. Although further confirmation is needed from multicenter randomized clinical trials, our results support the hypothesis that autologous lipoaspirate transplant combines striking regenerative properties with no or marginal effects on the probability of post-mastectomy locoregional recurrence of breast cancer. Data on long-term outcomes of fat grafting to the breast are lacking. To confirm the safety of lipotransfer in breast cancer patients clinical studies with control group based on long term follow up are needed. Scientific Rationale - Update February 2013 Claro et al (2012) conducted a systematic review of the clinical applicability and safety of autologous fat grafting to the breast for cosmetic and reconstructive purposes. Studies included in the review were original articles of autologous liposuctioned fat grafting to the female breast, with description of clinical complications and/or radiographic changes and/or local breast cancer recurrence. The review included 60 articles with 4601 patients. Thirty studies used fat grafting for augmentation and 41 for reconstructive procedures. The incidence of clinical complications, identified in 21 studies, was 3 9 per cent (117 of 3015); the majority were induration and/or palpable nodularity. Radiographic abnormalities occurred in 332 (13 0 per cent) of 2560 women (17 studies); more than half were consistent with cysts. Local recurrence of breast cancer (14 of 616, 2 3 per cent) was evaluated in three studies, of which only one was prospective. Reviewers concluded there is broad clinical applicability of autologous fat grafting for breast reconstruction. Complications were few and there was no evidence of interference with follow-up after treatment for breast cancer. Oncological safety remains unclear. Pérez-Cano et al (2012) reported results of the RESTORE-2, the first prospective clinical trial using autologous adipose-derived regenerative cell (ADRC)-enriched fat grafting for reconstruction of such defects. This single-arm, prospective, multi-center clinical trial enrolled 71 patients post- breast conservation therapy (BCT) with defects 150 ml. Adipose tissue was collected via syringe lipoharvest and then processed during the same surgical procedure using a closed automated system that isolates ADRCs and prepares an ADRC-enriched fat graft for immediate reimplantation. ADRC-enriched fat graft injections were performed in a fan-shaped pattern to prevent pooling of the injected fat. Overall procedure times were less than 4 h. The RESTORE-2 protocol allowed for up to two treatment sessions and 24 patients elected to undergo a second procedure following the six month follow-up visit. Of the 67 patients treated, 50 reported satisfaction with treatment results through 12 months. Using the same metric, investigators reported satisfaction with 57 out of 67 patients. Independent radiographic core laboratory assessment reported improvement in the breast contour of 54 out of 65 patients based on blinded Breast Reconstructive Surgery Apr 16 10
11 assessment of MRI sequence. There were no serious adverse events associated with the ADRC-enriched fat graft injection procedure. There were no reported local cancer recurrences. Injection site cysts were reported as adverse events in ten patients. Investigators concluded the prospective trial demonstrates the safety and efficacy of the treatment of BCT defects utilizing ADRC-enriched fat grafts. Kijima et al (2012) investigated 22 breasts in 21 patients who underwent partial mastectomy for early breast cancer involving mainly the inner upper quadrant were enrolled in this study. The defect was reconstructed immediately by filling it with an autologous free dermal fat graft (FDFG). At 6 months and 1, 2, 3, 4, and 5 years postoperatively, the width (horizontal length) and thickness (distance perpendicular to skin) of the FDFG on computed tomography (CT) scans were measured. Histologic samples from the implanted FDFG were collected by core needle biopsy (CNB). The correlations between clinical findings and volume of the FDFG on CT and the proportion of fatty tissue in the CNB were examined statistically. Cosmetic results were also evaluated. On CT, the mean width of the FDFG was 95%, 81%, 79%, 73%, 68%, and 47% and the mean thickness were 125%, 121%, 120%, 115%, 104%, and 103% at 6 months, and 1-5 years postoperatively, respectively. In the CNB samples, the mean fatty tissue distribution was 76%, 63%, and 54% at 1 year, 1-4 years, and >4 years postoperatively, respectively. The percent change in the width of the FDFG at 6 months after the operation displayed a significant negative correlation with the postmenopausal period and a significant positive correlation with the maximum surgical margin. On CT, thickness was negatively correlated with the size of the resected breast tissue, and thickness of the implanted FDFG. No clinicopathologic factors or technical factors were related to FDFG outcome and the proportion of fat tissue in the CNB sample, except for axillary dissection. Nineteen of 21 patients had good to excellent cosmesis. Authors concluded FDFG implanted into breast defects after partial mastectomy undergo mild resorption and degeneration to fibrous tissue, but most patients have good to excellent cosmesis. The available evidence on the use of FlexHD to assist one- or two-staged breast reconstruction following mastectomy for breast cancer is very limited. Institutional experiences reported in four retrospective studies address the efficacy and safety of FlexHD and other biologic meshes; however, none of the studies presented or analyzed outcomes separately for FlexHD. The studies included 210 women (258 reconstructed breasts). Reported complications affected 0 to 46% of patients, including infection, necrosis, hematoma, serosa, and pain. The need for reoperation ranged from 0 to 25%. One comparative study (n=119) compared the efficacy of staged biologic mesh-assisted breast reconstruction with breast reconstruction without the use of mesh. In this study, the use of biologic mesh was associated with a significantly higher rate of infection, reoperation, expander explantation, and overall complications, especially in patients with larger breasts (weighing 600 grams) compared with reconstruction without mesh. The patients were generally satisfied after surgery with respect to breast size, shape, and firmness. However, limited evidence showed a discrepancy in patient satisfaction related to symmetry issues between bilateral and unilateral reconstructions. Scientific Rationale Update December 2009 Most breast reconstruction currently performed today utilizes tissue expanders and implants. Breast reconstruction with implants can be performed as a one-step or multiple-step procedure. For example, in a staged procedure, a tissue expander is placed into a surgically created pocket either at the time of mastectomy or after the mastectomy has healed. Over several months, the expander is filled at regular intervals by weekly injections of saline. Tissue expansion is needed to accommodate the permanent implant if an insufficient amount of skin is left after mastectomy. The remaining tissues are stretched over several weeks or months, and the expander is Breast Reconstructive Surgery Apr 16 11
12 exchanged for a permanent silicone or saline implant during a second surgery when the expansion is complete. Breast reconstruction may also be accomplished in a single stage with the breast implant inserted during a procedure immediately after the mastectomy without tissue expansion, however, the success of this approach depends upon compliance of the subpectoral muscles, adequacy of the subpectoral pocket, and health of the mastectomy flap, etc. Breast reconstruction can be challenging if the patient s skin-pectoral muscle pocket cannot adequately support tissue expansion or if it cannot hold the expander or implant in place. A weakened muscle layer overlying the implant can cause visible rippling and implant extrusion. Autologous flap reconstruction (i.e., a portion of skin and fat, with or without muscle, is harvested from another part of the body and transplanted to the chest where it is shaped into a breast) are generally considered to give a more long-lasting and natural result than implants with fewer revision surgeries and may eliminate the need for a prosthetic implant, however, flap reconstruction is complex, and is associated with longer operating room time, longer recovery period and increased morbidity. In an attempt to improve outcomes following breast reconstruction, AlloDerm (LifeCell Corp.), has been investigated to provide for additional coverage and support of implants and other tissues at the surgical site for either one or two-stage reconstruction procedures. AlloDerm is an acellular dermal matrix derived from human cadaver skin that has been processed and sterilized to remove all cells and antigenic components that could cause inflammation and rejection. This dermal matrix serves as scaffolding for normal tissue ingrowth and cellular repopulation, which promotes tissue regeneration and neovascularization. AlloDerm is available in sheets ranging in size from 4 x 12 to 6 x 16 cm, with a thickness from approximately 0.8 to 3.3 mm. The sheets must be rehydrated in sterile saline before use and trimmed to fit the defect. For breast reconstruction using expanders and/or implants, 1 to 3 sheets of AlloDerm are rehydrated, sized, and cut to match the size and shape of the defect to cover and support the exposed implant beneath the lower edge and to the side of the pectoralis muscle. The allograft is sutured to the pectoralis major muscle superiorly, to the chest wall inferiorly, and to the serratus anterior muscle laterally. The characteristic shape of the AlloDerm graft for this indication is referred to as a hammock or sling. AlloDerm can be used to cover the exposed portion of the implant at the lower pole, which completes and secures the submuscular pocket, and defines the inframammary fold. After completion of expansion and adjuvant therapy if needed, the patient is scheduled for device exchange. Proponents of AlloDerm claim it reduces postoperative pain and musculoskeletal morbidity in breast reconstruction since the allograft eliminates the need for or reduces the extent of dissection and elevation of abdominal muscles during the creation of the inferior-lateral portion of the submuscular pocket for the implant. In addition, the graft may allow for greater and more rapid expansion, and for the immediate placement of a permanent implant. Proposed benefits of Alloderm include supplementation of the pectoralis major muscle deficiency at the breast lower pole, supporting and holding the prosthesis in place and thereby helping to define the shape and contour of the reconstructed breast and serves to cushion the mastectomy skin envelope from direct contact with the implant/expander. Preliminary data indicate that the use of AlloDerm in facilitating breast reconstruction with implant/expander is safe, with no major complications. AlloDerm also contributes to improving the aesthetic outcome of the reconstruction, by achieving good breast projection and symmetry. Breast Reconstructive Surgery Apr 16 12
13 According to the manufacturer, the following are potential adverse effects, among others, that may result from placement of an implant or graft: wound or systemic infection; seroma; dehiscence; hypersensitive, allergic, or other immune response; sloughing or failure of the graft; and disease transmission. As previously noted in the initial scientific rationale of this policy, published peer review literature evaluating AlloDerm is limited to three nonrandomized comparative studies that includes 2 retrospective studies, Preminger et al (2008), and Becker et al (2009), and 1 prospective study, Spear et al. (2008). Several small case series, one of which evaluated the efficacy of AlloDerm for nipple reconstruction, have also been published. In the comparative studies, no significant differences in tissue expansion measures (fill volume, fill time, etc.) were observed between patients treated with and without AlloDerm. No serious complications or instances of contracture were observed. One study that evaluated aesthetic outcomes found that blinded assessors scored AlloDerm-reconstructed breasts the same as the contralateral breasts. One study found that the revision rate for AlloDerm-treated breasts was significantly lower than that for historical data on breasts that were reconstructed without the graft. The case series found that patients were satisfied with the treatment results and that there were few complications specific to AlloDerm; however, as in the comparative trials, there was little to no objective assessment of clinical outcomes. Although studies have been relatively small, non-randomized studies without long term results, AlloDerm appears to be relatively safe and well tolerated. Histological analysis of tissues showed good incorporation of AlloDerm into the host tissues. No major complications have been reported. According the American Cancer Society, Alloderm is fairly new in breast reconstruction and has been used to extend and support natural tissues and may be used with expanders and implants as well as in nipple reconstruction. The ACS notes that outcome studies are still in progress, but have been promising. Researchers at Memorial Sloan-Kettering Cancer Center are sponsoring a randomized controlled trial assessing the use of Alloderm in two-stage, tissue expander/implant reconstruction. Estimated study completion date is March Several other clinical trials are currently recruiting patients in an effort to further evaluate Alloderm. Scientific Rationale Initial Breast cancer is the most common malignant neoplasm in women, affecting about 1 in 8 women in the United States. Because it has been conclusively shown that postmastectomy reconstruction does not adversely influence survival outcomes or recurrence rates, preoperative consultation is offered to all individuals desiring breast reconstruction, and the option of not reconstructing the breast is also discussed. Reconstructive breast surgery is defined as surgical procedures that are intended to restore the normal appearance of the breast after surgery, accidental injury, or trauma. The most common indication for reconstructive breast surgery is mastectomy or lumpectomy. These procedures could also be done for chronic, severe fibrocystic breast disease, known as cystic mastitis, unresponsive to medical therapy. Modern breast reconstruction began in 1964 with the introduction of the silicone breast implant. The most common and simplest form of reconstructive breast surgery has been the insertion of a silicone gel-filled or saline-filled breast implant, either inserted immediately at the time of mastectomy, or sometime afterward in conjunction with the previous use of a tissue expander. Significant local Breast Reconstructive Surgery Apr 16 13
14 complications of breast implants, such as contracture, may require removal of the implant. The art of breast reconstruction has undergone evolution over the last 20 years. Reconstruction selection is based on an assessment of cancer treatment, patient body habitus, smoking history, co-morbidities and patient concerns. Other types of reconstruction include nipple/areola reconstruction, nipple tattooing, and/or the use of autologous tissue, such as a transverse rectus abdominis myocutaneous flap (TRAM procedure), a latissimus dorsi flap, or additional flaps as noted below. In addition, mastopexy, reduction mammoplasty, or implant on the contralateral breast may be performed in order to achieve symmetry with the reconstructed breast. The development of musculocutaneous flaps and microsurgical tissue transplantation paved the way for modern autogenous tissue breast reconstruction. These flaps may be transposed into position with their vascular origin intact ("pedicled" flaps). Tissue flap surgery, or autologous tissue reconstruction, is a way to rebuild the shape of a breast using skin, fat, blood vessels and muscle from another part of the body (upper back, abdomen, or buttocks). This type of flap procedure is usually done to reconstruct the appearance of a breast with a blood supply, after mastectomy. Compared to breast reconstruction with implants, tissue flap procedures require a longer surgery and recovery time but result in a more natural-looking breast. Although there are several different types of tissue flap procedures, the most common are the TRAM flap and the latissimus dorsi flap. Complications can occur with any type of breast reconstruction, however, the most significant effect is delay of initiation of adjuvant therapy. Partial or complete flap loss, wound breakdown, and infection are all reasons why chemotherapy or radiation therapy would be delayed. Complication rates are higher in patients who require radiotherapy postoperatively. In patients who require postoperative radiotherapy, TRAM reconstructions can provide better cosmetic outcomes and lower complication rates than implant reconstructions can. Because postoperative radiotherapy has been recognized to worsen the outcome of an immediate TRAM reconstruction, there has been a movement to delay reconstruction in patients who are expected to need postoperative radiotherapy. However, the advantages of immediate reconstruction outweigh the risk for late complications seen with irradiated immediate TRAM reconstructions. In general, radiotherapy after mastectomy and immediate TRAM flap reconstruction is well tolerated and not associated with an increased rate of acute complications or interruption of radiation therapy. Alloderm AlloDerm Regenerative Tissue Matrix is an acellular dermal matrix derived from human cadaver skin that has been processed and sterilized to remove all cells and antigenic components that could cause inflammation and rejection. Tissue banks procure fresh human cadaveric skin following American Association of Tissue Bank (AATB) guidelines in accordance with the AATB and FDA guidelines. The resulting product contains an extracellular mix of connective tissue components such as collagen, elastin, and hyaluronic acid. When introduced into viable tissue, the matrix serves as scaffolding for normal tissue ingrowth and cellular repopulation, which promotes tissue regeneration and neovascularization. AlloDerm has been proposed as a graft for burn patients, and for reconstructive applications such as facial reconstruction, abdominal wall reconstruction, and breast reconstruction. Prosthetic breast reconstruction with implant or expander is currently the standard in immediate breast reconstruction postmastectomy. Proposed as an alternative to traditional reconstruction, proponents claim AlloDerm reduces postoperative pain and musculoskeletal morbidity since the allograft Breast Reconstructive Surgery Apr 16 14
15 eliminates the need for or reduces the extent of dissection and elevation of abdominal muscles during the creation of the inferior-lateral portion of the submuscular pocket for the implant. The graft may allow for greater and more rapid expansion, and for the immediate placement of a permanent implant. The evidence base on the efficacy and safety of AlloDerm for postmastectomy breast reconstruction is limited to 3 nonrandomized comparative studies, 2 retrospective studies, (Preminger et al. [2008], and Becker et al. [2009], and 1 prospective study, Spear et al. [2008]), as well as 5 small case series, Breuing et al. [2007], Zienowicz et al. [2007], Salzberg et al. [2006], Gamboa-Bobadilla [2006], and Bindingnavele et al. [2007]. One small case series evaluated the efficacy of AlloDerm for nipple reconstruction. Alloderm has been evaluated and found that patients were satisfied with the treatment results and that there were few complications specific to AlloDerm; however, there was little to no objective assessment of clinical outcomes in any study, and outcomes were undefined or poorly defined. The three-nonrandomized comparative trials (n=30 to 90 patients) and 5 case series (n=11 to 49 patients) evaluated the efficacy and safety of AlloDerm in breast reconstruction following mastectomy for breast cancer and prophylactic mastectomy. In the comparative studies, no significant differences in tissue expansion measures (fill volume, fill time, etc.) were observed between patients treated with and without AlloDerm. No serious complications or instances of contracture were observed. One study that evaluated aesthetic outcomes found that blinded assessors scored AlloDerm reconstructed breasts the same as the contralateral breasts. One study found that the revision rate for AlloDerm-treated breasts was significantly lower than that for historical data on breasts that were reconstructed without the graft. The case series found that patients were satisfied with the treatment results and that there were few complications specific to AlloDerm; however, as in the comparative trials, there was little to no objective assessment of clinical outcomes. AlloDerm appears to be relatively safe and well tolerated. Histological analysis of tissues showed good incorporation of AlloDerm into the host tissues. No major complications have been reported. Reported complications that affected 0 to 25% of patients included infection, necrosis, hematoma, seroma, and flap loss. The studies are all limited by weak designs, lack of randomization, and inadequate follow-up times, which makes it difficult to draw conclusions about the long-term efficacy and safety of AlloDerm in postmastectomy breast reconstruction. Permacol Permacol Porcine Dermal Collagen Surgical Mesh is proposed for use in breast reconstruction. Permacol is comprised of acellular cross-linked porcine dermal collagen and elastin. According to the U.S. Food and Drug Administration (FDA), Permacol is intended for use as a soft tissue patch to reinforce soft tissue where weakness exists and for the surgical repair of damaged or ruptured soft tissue membranes. Evidence in the published, peer-reviewed scientific literature supporting the use of this product in breast reconstruction is lacking and its role is unclear. Radiesse Radiesse injections consist of very small, smooth calcium hydroxylapatite (CaHA) microspheres that are suspended in a water-based gel carrier. Radiesse has been proposed to reshape nipples after reconstruction of the breast following mastectomy. Radiesse has received PMA approval by the FDA as a medical device for subdermal implantation for two indications: correction of moderate to severe facial wrinkles and folds such as nasolabial folds and the correction of facial fat loss in people with human immunodeficiency virus (FDA, 2006). There remains a lack of evidence in the Breast Reconstructive Surgery Apr 16 15
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Breast Reconstructive Surgery After Mastectomy Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Breast Reconstructive Surgery After Mastectomy PRE-DETERMINATION
Breast Reconstructive Surgery After Mastectomy Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Breast Reconstructive Surgery After Mastectomy PRE-DETERMINATION
Medical Policy Original Effective Date: Revised Date: Page 1 of 8
 Page 1 of 8 Disclaimer Description Coverage Determination Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans, or the plan
Page 1 of 8 Disclaimer Description Coverage Determination Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans, or the plan
Breast Reconstruction Surgery
 Breast Reconstruction Surgery I. Policy University Health Alliance (UHA) will reimburse for Breast Reconstruction Surgery when it is determined to be medically necessary and when it meets the medical criteria
Breast Reconstruction Surgery I. Policy University Health Alliance (UHA) will reimburse for Breast Reconstruction Surgery when it is determined to be medically necessary and when it meets the medical criteria
BREAST RECONSTRUCTION POST MASTECTOMY
 UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: January 1, 2019 Table of Contents Page INSTRUCTIONS FOR USE...
UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: January 1, 2019 Table of Contents Page INSTRUCTIONS FOR USE...
BREAST RECONSTRUCTION POST MASTECTOMY
 UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: February 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...
UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: February 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...
Medical Review Criteria Breast Surgeries
 Medical Review Criteria Breast Surgeries Effective Date: November 8, 2016 Subject: Breast Surgeries Policy: HPHC covers medically necessary breast surgeries including mastectomy, breast reconstruction,
Medical Review Criteria Breast Surgeries Effective Date: November 8, 2016 Subject: Breast Surgeries Policy: HPHC covers medically necessary breast surgeries including mastectomy, breast reconstruction,
Breast Reconstruction Following Mastectomy or Lumpectomy
 Breast Reconstruction Following Mastectomy or Lumpectomy [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr go to Comunicados a Proveedores, and click Cartas
Breast Reconstruction Following Mastectomy or Lumpectomy [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr go to Comunicados a Proveedores, and click Cartas
Medical Review Criteria Breast Surgeries
 Medical Review Criteria Breast Surgeries Subject: Breast Surgeries Authorization: Prior authorization is required for the following procedures requested for members enrolled in HPHC commercial (HMO, POS,
Medical Review Criteria Breast Surgeries Subject: Breast Surgeries Authorization: Prior authorization is required for the following procedures requested for members enrolled in HPHC commercial (HMO, POS,
BREAST RECONSTRUCTION/REMOVAL AND REPLACEMENT OF IMPLANTS
 Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION
 NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION 42 yo female healthy athlete Right breast mass. Past medical history: none Family history: aunt with Breast cancer Candidates for nipple-sparing mastectomy
NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION 42 yo female healthy athlete Right breast mass. Past medical history: none Family history: aunt with Breast cancer Candidates for nipple-sparing mastectomy
Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer. Oncoplastic and Reconstructive Surgery
 Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Oncoplastic and Reconstructive Surgery Plastic-reconstructive aspects after mastectomy Versions 2002 2017: Audretsch / Bauerfeind
Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Oncoplastic and Reconstructive Surgery Plastic-reconstructive aspects after mastectomy Versions 2002 2017: Audretsch / Bauerfeind
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander.
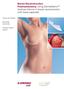 Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Breast Reconstruction Surgery after Mastectomy or Lumpectomy
 Breast Reconstruction Surgery after Mastectomy or Lumpectomy Date of Origin: 11/1998 Last Review Date: 11/25/2017 Effective Date: 11/25/2017 Dates Reviewed: 08/2000, 09/2001, 11/2003, 11/2004, 12/2005,
Breast Reconstruction Surgery after Mastectomy or Lumpectomy Date of Origin: 11/1998 Last Review Date: 11/25/2017 Effective Date: 11/25/2017 Dates Reviewed: 08/2000, 09/2001, 11/2003, 11/2004, 12/2005,
Infectious Complications Leading to Explantation in Implant-Based Breast Reconstruction With AlloDerm
 Infectious Complications Leading to Explantation in Implant-Based Breast Reconstruction With AlloDerm Minh-Doan Nguyen, MD, PhD, a Chen Chen, MS, b Salih Colakoğlu, MD, b Donald J. Morris, MD, b Adam M.
Infectious Complications Leading to Explantation in Implant-Based Breast Reconstruction With AlloDerm Minh-Doan Nguyen, MD, PhD, a Chen Chen, MS, b Salih Colakoğlu, MD, b Donald J. Morris, MD, b Adam M.
Reconstruction of the Breast after Cancer An Overview of Procedures and Options by Karen M. Horton, MD, MSc, FRCSC
 Downloaded from Reconstruction of the Breast after Cancer An Overview of Procedures and Options by Karen M. Horton, MD, MSc, FRCSC What is Breast Reconstruction? Reconstruction of the breast involves recreating
Downloaded from Reconstruction of the Breast after Cancer An Overview of Procedures and Options by Karen M. Horton, MD, MSc, FRCSC What is Breast Reconstruction? Reconstruction of the breast involves recreating
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons
 Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP
 INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify
INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify
Pocket Conversion Made Easy: A Simple Technique Using Alloderm to Convert Subglandular Breast Implants to the Dual-Plane Position
 Breast Surgery Pocket Conversion Made Easy: A Simple Technique Using Alloderm to Convert Subglandular Breast Implants to the Dual-Plane Position M. Mark Mofid, MD; and Navin K. Singh, MD Background: The
Breast Surgery Pocket Conversion Made Easy: A Simple Technique Using Alloderm to Convert Subglandular Breast Implants to the Dual-Plane Position M. Mark Mofid, MD; and Navin K. Singh, MD Background: The
Reconstructive Breast Surgery and Management of Breast Implants
 Reconstructive Breast Surgery and Management of Breast Implants Policy Number: 7.01.22 Last Review: 1/2018 Origination: 3/1993 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Reconstructive Breast Surgery and Management of Breast Implants Policy Number: 7.01.22 Last Review: 1/2018 Origination: 3/1993 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Advances in Breast Surgery. Catherine Campo, D.O. Breast Surgeon Meridian Health System April 17, 2015
 Advances in Breast Surgery Catherine Campo, D.O. Breast Surgeon Meridian Health System April 17, 2015 Objectives Understand the surgical treatment of breast cancer Be able to determine when a lumpectomy
Advances in Breast Surgery Catherine Campo, D.O. Breast Surgeon Meridian Health System April 17, 2015 Objectives Understand the surgical treatment of breast cancer Be able to determine when a lumpectomy
Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate surgical options
 A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate
A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate
Breast Surgery Corporate Medical Policy
 File name: Breast Surgery File code: UM.SURG.17 Origination: 2016 Last Review: 11/2018 (PA List Review) Next Review: 11/2019 Effective Date: 04/01/2018 Breast Surgery Corporate Medical Policy Description/Summary
File name: Breast Surgery File code: UM.SURG.17 Origination: 2016 Last Review: 11/2018 (PA List Review) Next Review: 11/2019 Effective Date: 04/01/2018 Breast Surgery Corporate Medical Policy Description/Summary
Prophylactic Mastectomy & Reconstructive Implications
 Prophylactic Mastectomy & Reconstructive Implications Minas T Chrysopoulo, MD PRMA Center For Advanced Breast Reconstruction Prophylactic Mastectomy Surgical removal of one or both breasts to reduce the
Prophylactic Mastectomy & Reconstructive Implications Minas T Chrysopoulo, MD PRMA Center For Advanced Breast Reconstruction Prophylactic Mastectomy Surgical removal of one or both breasts to reduce the
BREAST RECONSTRUCTION FOLLOWING PROPHYLACTIC OR THERAPEUTIC MASTECTOMY FOR BREAST CANCER
 BREAST RECONSTRUCTION FOLLOWING PROPHYLACTIC OR THERAPEUTIC MASTECTOMY FOR BREAST CANCER Effective Date: September 2013 The recommendations contained in this guideline are a consensus of the Alberta Provincial
BREAST RECONSTRUCTION FOLLOWING PROPHYLACTIC OR THERAPEUTIC MASTECTOMY FOR BREAST CANCER Effective Date: September 2013 The recommendations contained in this guideline are a consensus of the Alberta Provincial
CLINICAL MEDICAL POLICY
 CLINICAL MEDICAL POLICY Policy Name: Reconstructive Breast Surgery Policy Number: MP-052-MD-DE Approved By: Medical Management Medical Policy Provider Notice Date: 08/01/2017 Original Effective Date: 09/01/2017
CLINICAL MEDICAL POLICY Policy Name: Reconstructive Breast Surgery Policy Number: MP-052-MD-DE Approved By: Medical Management Medical Policy Provider Notice Date: 08/01/2017 Original Effective Date: 09/01/2017
Current Strategies in Breast Reconstruction
 Current Strategies in Breast Reconstruction Hani Sbitany, MD Assistant Professor of Surgery University of California, San Francisco Division of Plastic and Reconstructive Surgery 12 th Annual School of
Current Strategies in Breast Reconstruction Hani Sbitany, MD Assistant Professor of Surgery University of California, San Francisco Division of Plastic and Reconstructive Surgery 12 th Annual School of
Goals of Care. Restore shape and function after cancer
 Goals of Care Restore shape and function after cancer Aid in physiological and psychological benefit Relationship with significant other Self esteem and positive body image Feeling of a whole body Avoid
Goals of Care Restore shape and function after cancer Aid in physiological and psychological benefit Relationship with significant other Self esteem and positive body image Feeling of a whole body Avoid
Breast Reconstruction Options
 Breast Reconstruction Options Natural reconstruction using your ABDOMINAL tissue: TRAM Flap (Transverse Rectus Abdominis Myocutaneous) There are various forms of TRAM flap reconstruction that are commonly
Breast Reconstruction Options Natural reconstruction using your ABDOMINAL tissue: TRAM Flap (Transverse Rectus Abdominis Myocutaneous) There are various forms of TRAM flap reconstruction that are commonly
Breast Reconstruction. Westmead Breast Cancer Institute
 Breast Reconstruction Westmead Breast Cancer Institute What is breast reconstruction? Breast reconstruction is a surgical procedure that creates a shape on the chest wall following a mastectomy. Occasionally,
Breast Reconstruction Westmead Breast Cancer Institute What is breast reconstruction? Breast reconstruction is a surgical procedure that creates a shape on the chest wall following a mastectomy. Occasionally,
Related Policies None
 Medical Policy MP 7.01.153 BCBSA Ref. Policy: 7.01.153 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 7.01.153 BCBSA Ref. Policy: 7.01.153 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Breast Reconstruction: Current Strategies and Future Opportunities
 Breast Reconstruction: Current Strategies and Future Opportunities Hani Sbitany, MD Assistant Professor of Surgery University of California, San Francisco Division of Plastic and Reconstructive Surgery
Breast Reconstruction: Current Strategies and Future Opportunities Hani Sbitany, MD Assistant Professor of Surgery University of California, San Francisco Division of Plastic and Reconstructive Surgery
How To Make a Good Mastectomy for Reconstruction Based on the Anatomy. Zhang Jin, Ph.D MD
 How To Make a Good Mastectomy for Reconstruction Based on the Anatomy Zhang Jin, Ph.D MD Deputy Director and Professor Tianjin Medical University Cancer Institute and Hospital People s Republic of China
How To Make a Good Mastectomy for Reconstruction Based on the Anatomy Zhang Jin, Ph.D MD Deputy Director and Professor Tianjin Medical University Cancer Institute and Hospital People s Republic of China
Medical Policy Original Effective Date: Revised Date: 09/26/2018 Page 1 of 26
 Contents: This includes the following items: 1. Breast Reconstruction Following Mastectomy: Page 1 of 26 2. Breast Implant Removal and/or Replacement and Capsulectomy: 3. Breast Reduction Mammaplasty for
Contents: This includes the following items: 1. Breast Reconstruction Following Mastectomy: Page 1 of 26 2. Breast Implant Removal and/or Replacement and Capsulectomy: 3. Breast Reduction Mammaplasty for
The Effect of Acellular Dermal Matrix in Implant-Based Immediate Breast Reconstruction with Latissimus Dorsi Flap
 ORIGINAL ARTICLE https://doi.org/10.14730/.2017.23.1.17 Arch Aesthetic Plast Surg 2017;23(1):17-23 pissn: 2234-0831 eissn: 2288-9337 The Effect Acellular Dermal Matrix in Implant-Based Immediate Breast
ORIGINAL ARTICLE https://doi.org/10.14730/.2017.23.1.17 Arch Aesthetic Plast Surg 2017;23(1):17-23 pissn: 2234-0831 eissn: 2288-9337 The Effect Acellular Dermal Matrix in Implant-Based Immediate Breast
In a second stage or a second operation that tissue expander is removed through the same incision and the implant is placed within the chest pocket.
 Hello, I m Summer Hanson. I m an assistant professor in the Department of Plastics & Reconstructive Surgery at The University of Texas MD Anderson Cancer Center and today I m going to talk about the role
Hello, I m Summer Hanson. I m an assistant professor in the Department of Plastics & Reconstructive Surgery at The University of Texas MD Anderson Cancer Center and today I m going to talk about the role
Protocol. Reconstructive Breast Surgery/Management of Breast Implants
 Protocol Reconstructive Breast Surgery/Management of Breast Implants Medical Benefit Effective Date: 04/01/14 Next Review Date: 11/18 Preauthorization Yes Review Dates: 02/07, 02/08, 01/09, 01/10, 01/11,
Protocol Reconstructive Breast Surgery/Management of Breast Implants Medical Benefit Effective Date: 04/01/14 Next Review Date: 11/18 Preauthorization Yes Review Dates: 02/07, 02/08, 01/09, 01/10, 01/11,
BREAST AUGMENTATION TECHNIQUES
 BREAST AUGMENTATION TECHNIQUES Breast Augmentation Top Surgical Procedure in 2015 (Worldwide) Surgical Procedure : Breast Augmentation Rank : 1 Total : 1,488,992 Percent of Total Surgical Procedures :
BREAST AUGMENTATION TECHNIQUES Breast Augmentation Top Surgical Procedure in 2015 (Worldwide) Surgical Procedure : Breast Augmentation Rank : 1 Total : 1,488,992 Percent of Total Surgical Procedures :
Advances in Localized Breast Cancer
 Advances in Localized Breast Cancer Melissa Camp, MD, MPH and Fariba Asrari, MD June 18, 2018 Moderated by Elissa Bantug 1 Advances in Surgery for Breast Cancer Melissa Camp, MD June 18, 2018 2 Historical
Advances in Localized Breast Cancer Melissa Camp, MD, MPH and Fariba Asrari, MD June 18, 2018 Moderated by Elissa Bantug 1 Advances in Surgery for Breast Cancer Melissa Camp, MD June 18, 2018 2 Historical
Neil J. Zemmel, MD, FACS Steven J. Montante, MD Megan J. Russell, PA-C. Your Guide To BREAST RECONSTRUCTION
 Neil J. Zemmel, MD, FACS Steven J. Montante, MD Megan J. Russell, PA-C Your Guide To BREAST RECONSTRUCTION Introduction The diagnosis of breast cancer begins a journey of making many informed decisions
Neil J. Zemmel, MD, FACS Steven J. Montante, MD Megan J. Russell, PA-C Your Guide To BREAST RECONSTRUCTION Introduction The diagnosis of breast cancer begins a journey of making many informed decisions
Post-mastectomy breast reconstruction
 Follow the link from the online version of this article to obtain certified continuing medical education credits Post-mastectomy breast reconstruction Paul T R Thiruchelvam, 1 Fiona McNeill, 2 Navid Jallali,
Follow the link from the online version of this article to obtain certified continuing medical education credits Post-mastectomy breast reconstruction Paul T R Thiruchelvam, 1 Fiona McNeill, 2 Navid Jallali,
The decision to repair a partial mastectomy CME. State of the Art and Science in Postmastectomy Breast Reconstruction.
 CME State of the Art and Science in Postmastectomy Breast Reconstruction Steven J. Kronowitz, M.D. Houston, Texas Learning Objectives: After reading this article, the participant should be able to: 1.
CME State of the Art and Science in Postmastectomy Breast Reconstruction Steven J. Kronowitz, M.D. Houston, Texas Learning Objectives: After reading this article, the participant should be able to: 1.
Strattice Reconstructive Tissue Matrix used in the repair of rippling
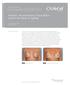 Clinical case study Strattice Tissue Matrix Strattice Reconstructive Tissue Matrix used in the repair of rippling Steven Teitelbaum, MD* Santa Monica, CA Case summary A 48-year-old woman with a history
Clinical case study Strattice Tissue Matrix Strattice Reconstructive Tissue Matrix used in the repair of rippling Steven Teitelbaum, MD* Santa Monica, CA Case summary A 48-year-old woman with a history
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference
 2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
Adipose-Derived Stem Cells in Autologous Fat Grafting to the Breast
 Adipose-Derived Stem Cells in Autologous Fat Grafting to the Breast Policy Number: 7.01.153 Last Review: 12/2018 Origination: 6/2015 Next Review: 12/2019 Policy Blue Cross and Blue Shield of Kansas City
Adipose-Derived Stem Cells in Autologous Fat Grafting to the Breast Policy Number: 7.01.153 Last Review: 12/2018 Origination: 6/2015 Next Review: 12/2019 Policy Blue Cross and Blue Shield of Kansas City
Breast Restoration Surgery After a mastectomy
 UW MEDICINE PATIENT EDUCATION Breast Restoration Surgery After a mastectomy This handout explains the most common procedures that are used at University of Washington Medical Center (UWMC) to restore a
UW MEDICINE PATIENT EDUCATION Breast Restoration Surgery After a mastectomy This handout explains the most common procedures that are used at University of Washington Medical Center (UWMC) to restore a
Allograft Based Breast Reconstruction: Opportunity for a Second Look
 Allograft Based Breast Reconstruction: Opportunity for a Second Look Martin I. Newman, MD, FACS Director of Resident Education and Associate Program Director Department of Plastic and Reconstructive Surgery
Allograft Based Breast Reconstruction: Opportunity for a Second Look Martin I. Newman, MD, FACS Director of Resident Education and Associate Program Director Department of Plastic and Reconstructive Surgery
ASPS Recommended Insurance Coverage Criteria for Third- Party Payers
 ASPS Recommended Insurance Coverage Criteria for Third- Party Payers Breast Implant Associated Anaplastic Large Cell Lymphoma BACKGROUND Anaplastic Large Cell Lymphoma (ALCL) is a rare type of cancer of
ASPS Recommended Insurance Coverage Criteria for Third- Party Payers Breast Implant Associated Anaplastic Large Cell Lymphoma BACKGROUND Anaplastic Large Cell Lymphoma (ALCL) is a rare type of cancer of
Frederick J. Duffy, Jr., MD, FACS and Brice W. McKane, MD, FACS BREAST RECONSTRUCTION
 Frederick J. Duffy, Jr., MD, FACS and Brice W. McKane, MD, FACS BREAST RECONSTRUCTION BREAST RECONSTRUCTION: A WOMAN S DECISION Options and Information Our approach to breast reconstruction entails a very
Frederick J. Duffy, Jr., MD, FACS and Brice W. McKane, MD, FACS BREAST RECONSTRUCTION BREAST RECONSTRUCTION: A WOMAN S DECISION Options and Information Our approach to breast reconstruction entails a very
Breast Reconstruction
 Steven E. Copit, M.D. Chief- Division of Plastic Surgery Thomas Jefferson University Hospital Philadelphia, PA analysis of The Defect Skin Breast Volume Nipple Areola Complex analysis of The Defect the
Steven E. Copit, M.D. Chief- Division of Plastic Surgery Thomas Jefferson University Hospital Philadelphia, PA analysis of The Defect Skin Breast Volume Nipple Areola Complex analysis of The Defect the
National Mastectomy & Breast Reconstruction Audit Datasheet - Mastectomy +/- Immediate Reconstruction
 Patient Registration data Surname Forename NHS/Private Hospital Number Date of birth Postcode Ethnicity Patient-reported outcomes consent Has this patient consented to being sent outcome questionnaires?
Patient Registration data Surname Forename NHS/Private Hospital Number Date of birth Postcode Ethnicity Patient-reported outcomes consent Has this patient consented to being sent outcome questionnaires?
Chapter 11 Worksheet Code It
 Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
AESTHETIC SURGERY OF THE BREAST: MASTOPEXY, AUGMENTATION & REDUCTION
 CHAPTER 18 AESTHETIC SURGERY OF THE BREAST: MASTOPEXY, AUGMENTATION & REDUCTION Ali A. Qureshi, MD and Smita R. Ramanadham, MD Aesthetic surgery of the breast aims to either correct ptosis with a mastopexy,
CHAPTER 18 AESTHETIC SURGERY OF THE BREAST: MASTOPEXY, AUGMENTATION & REDUCTION Ali A. Qureshi, MD and Smita R. Ramanadham, MD Aesthetic surgery of the breast aims to either correct ptosis with a mastopexy,
ANNEX 1 OBJECTIVES. At the completion of the training period, the fellow should be able to:
 1 ANNEX 1 OBJECTIVES At the completion of the training period, the fellow should be able to: 1. Breast Surgery Evaluate and manage common benign and malignant breast conditions. Assess the indications
1 ANNEX 1 OBJECTIVES At the completion of the training period, the fellow should be able to: 1. Breast Surgery Evaluate and manage common benign and malignant breast conditions. Assess the indications
A Comparative Study of CG CryoDerm and AlloDerm in Direct-to-Implant Immediate Breast Reconstruction
 A Comparative Study of CG CryoDerm and AlloDerm in Direct-to-Implant Immediate Breast Reconstruction Original Article Jun Ho Lee 1, Ki Rin Park 1, Tae Gon Kim 1, Ju-Ho Ha 1, Kyu-Jin Chung 1, Yong-Ha Kim
A Comparative Study of CG CryoDerm and AlloDerm in Direct-to-Implant Immediate Breast Reconstruction Original Article Jun Ho Lee 1, Ki Rin Park 1, Tae Gon Kim 1, Ju-Ho Ha 1, Kyu-Jin Chung 1, Yong-Ha Kim
Case Study. TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis.
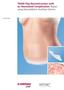 Case Study TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis. TRAM Flap Reconstruction with an Associated Complication Challenge Insulin-dependent diabetes
Case Study TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis. TRAM Flap Reconstruction with an Associated Complication Challenge Insulin-dependent diabetes
rupture, you may notice silicone in their lymph nodes on radiographs. This may be seen and help us detect that there is a rupture.
 Hello. I m Melissa Crosby. I m an Associate Professor at The University of Texas MD Anderson Cancer Center in the Department of Plastic Surgery. I d like to discuss with you the Late Effects of Breast
Hello. I m Melissa Crosby. I m an Associate Professor at The University of Texas MD Anderson Cancer Center in the Department of Plastic Surgery. I d like to discuss with you the Late Effects of Breast
Cigna Medical Coverage Policy
 Cigna Medical Coverage Policy Subject Breast Reconstruction Following Mastectomy or Lumpectomy Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 19 References...
Cigna Medical Coverage Policy Subject Breast Reconstruction Following Mastectomy or Lumpectomy Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 19 References...
The Case FOR Oncoplastic Surgery in Small Breasts. Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA
 The Case FOR Oncoplastic Surgery in Small Breasts Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA Changing issues in breast cancer management Early detection
The Case FOR Oncoplastic Surgery in Small Breasts Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA Changing issues in breast cancer management Early detection
Tackling challenging revision breast augmentation cases
 the BREAST Careful preoperative consultations can reduce the need for revision breast surgery. Second Time Around Tackling challenging revision breast augmentation cases By Adam D. Schaffner, MD, FACS
the BREAST Careful preoperative consultations can reduce the need for revision breast surgery. Second Time Around Tackling challenging revision breast augmentation cases By Adam D. Schaffner, MD, FACS
Updates in Breast Care. Truth or Hype. History of Breast Cancer Surgery. Dr Karen Barbosa 5/3/2017 4/20/2017
 Updates in Breast Care Dr Karen Barbosa 4/20/2017 Truth or Hype Princess Bust Developer Sears, Roebuck and Co. 1897 Promised to make the breast round, firm and beautiful History of Breast Cancer Surgery
Updates in Breast Care Dr Karen Barbosa 4/20/2017 Truth or Hype Princess Bust Developer Sears, Roebuck and Co. 1897 Promised to make the breast round, firm and beautiful History of Breast Cancer Surgery
Reduction Mammaplasty and Mastopexy in Previously Irradiated Breasts
 Breast Surgery Reduction Mammaplasty and Mastopexy in Previously Irradiated Breasts Scott L. Spear, MD; Samir S. Rao, MD; Ketan M. Patel, MD; and Maurice Y. Nahabedian, MD The combination of lumpectomy
Breast Surgery Reduction Mammaplasty and Mastopexy in Previously Irradiated Breasts Scott L. Spear, MD; Samir S. Rao, MD; Ketan M. Patel, MD; and Maurice Y. Nahabedian, MD The combination of lumpectomy
Pre-pectoral Breast Reconstruction in Nipple Sparing Mastectomy
 September 2017 Issue 9 Pre-pectoral Breast Reconstruction in Nipple Sparing Mastectomy Aldona J. Spiegel, MD Director and Founder of the Center for Breast Restoration at the Institute for Reconstructive
September 2017 Issue 9 Pre-pectoral Breast Reconstruction in Nipple Sparing Mastectomy Aldona J. Spiegel, MD Director and Founder of the Center for Breast Restoration at the Institute for Reconstructive
Breast Augmentation - Silicone Implants
 Breast Augmentation - Silicone Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics,
Breast Augmentation - Silicone Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics,
Breast Reconstruction: Patient Information Document
 breastreconstructioncanada.ca Breast Reconstruction: Patient Information Document By Dr. Nicolas Guay Dr. Haemi Lee STANDARDIZED BREAST RECONSTRUCTION PATIENT INFORMATION TABLE OF CONTENTS Glossary...
breastreconstructioncanada.ca Breast Reconstruction: Patient Information Document By Dr. Nicolas Guay Dr. Haemi Lee STANDARDIZED BREAST RECONSTRUCTION PATIENT INFORMATION TABLE OF CONTENTS Glossary...
can see several late effects. Asymmetry is probably the most common and the thing that patients notice the most. We can also see implant wrinkling or
 Hello, I am Summer Hanson. I m an assistant professor with the Department of Plastic and Reconstructive Surgery at the University of Texas MD Anderson Cancer Center. And today I m going to talk to you
Hello, I am Summer Hanson. I m an assistant professor with the Department of Plastic and Reconstructive Surgery at the University of Texas MD Anderson Cancer Center. And today I m going to talk to you
Breast debridement and closure cpt
 Breast debridement and closure cpt Close Breast debridement cpt code Medicare Billing Guidelines, Medicare payment and reimbursment, Medicare codes. Here is a list of CPT codes and Diagnoses that are.
Breast debridement and closure cpt Close Breast debridement cpt code Medicare Billing Guidelines, Medicare payment and reimbursment, Medicare codes. Here is a list of CPT codes and Diagnoses that are.
Is Unilateral Implant or Autologous Breast Reconstruction Better in Obtaining Breast Symmetry?
 ORIGINAL ARTICLE Is Unilateral Implant or Autologous Breast Reconstruction Better in Obtaining Breast Symmetry? Oriana Cohen, MD, Kevin Small, MD, Christina Lee, BA, Oriana Petruolo, MD, Nolan Karp, MD,
ORIGINAL ARTICLE Is Unilateral Implant or Autologous Breast Reconstruction Better in Obtaining Breast Symmetry? Oriana Cohen, MD, Kevin Small, MD, Christina Lee, BA, Oriana Petruolo, MD, Nolan Karp, MD,
Why Do Patients Seek Revisionary Breast Surgery?
 Breast Surgery Why Do Patients Seek Revisionary Breast Surgery? Navanjun S. Grewal, MD; and Jack Fisher, MD In 2011, according to the American Society for Aesthetic Plastic Surgery (ASAPS), 316 848 American
Breast Surgery Why Do Patients Seek Revisionary Breast Surgery? Navanjun S. Grewal, MD; and Jack Fisher, MD In 2011, according to the American Society for Aesthetic Plastic Surgery (ASAPS), 316 848 American
The biplanar oncoplastic technique case series: a 2-year review
 Original Article The biplanar oncoplastic technique case series: a 2-year review Alexander J. Kaminsky 1, Ketan M. Patel 2, Costanza Cocilovo 1, Maurice Y. Nahabedian 2, Reza Miraliakbari 3 1 INOVA Fairfax
Original Article The biplanar oncoplastic technique case series: a 2-year review Alexander J. Kaminsky 1, Ketan M. Patel 2, Costanza Cocilovo 1, Maurice Y. Nahabedian 2, Reza Miraliakbari 3 1 INOVA Fairfax
BREAST RECONSTRUCTION FOLLOWING PROPHYLACTIC OR THERAPEUTIC MASTECTOMY FOR BREAST CANCER
 Page 1 of 44 BREAST RECONSTRUCTION FOLLOWING PROPHYLACTIC OR THERAPEUTIC MASTECTOMY FOR BREAST CANCER Effective Date: February, 2017 The recommendations contained in this guideline are a consensus of the
Page 1 of 44 BREAST RECONSTRUCTION FOLLOWING PROPHYLACTIC OR THERAPEUTIC MASTECTOMY FOR BREAST CANCER Effective Date: February, 2017 The recommendations contained in this guideline are a consensus of the
Recent Advances in Breast Cancer Treatment
 Recent Advances in Breast Cancer Treatment Pornchai O-charoenrat MD, PhD, FRCST, FICS Professor Chief, Division of Head-Neck & Breast Surgery Department of Surgery, Siriraj Hospital, THAILAND Recent Advances
Recent Advances in Breast Cancer Treatment Pornchai O-charoenrat MD, PhD, FRCST, FICS Professor Chief, Division of Head-Neck & Breast Surgery Department of Surgery, Siriraj Hospital, THAILAND Recent Advances
Chest Wall Tumors and Reconstruction: Lateral Chest Wall. Dr. Robert Kelly
 Chest Wall Tumors and Reconstruction: Lateral Chest Wall Dr. Robert Kelly THORACIC PROGRAMME: ADVANCES IN CHEST WALL SURGERY AND OSTEOSYNTHESIS Dr. José Ribas Milanez de Campos Assistant, Professor, Department
Chest Wall Tumors and Reconstruction: Lateral Chest Wall Dr. Robert Kelly THORACIC PROGRAMME: ADVANCES IN CHEST WALL SURGERY AND OSTEOSYNTHESIS Dr. José Ribas Milanez de Campos Assistant, Professor, Department
Modified Radical Mastectomy
 Modified Radical Mastectomy Valerie L. Staradub, MD, and Monica Morrow, MD S urgical management options for breast cancer include modified radical mastectomy (MRM), MRM with immediate reconstruction, and
Modified Radical Mastectomy Valerie L. Staradub, MD, and Monica Morrow, MD S urgical management options for breast cancer include modified radical mastectomy (MRM), MRM with immediate reconstruction, and
Breast Augmentation - Saline Implants
 Breast Augmentation - Saline Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics, pregnancy,
Breast Augmentation - Saline Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics, pregnancy,
NEW TECHNIQUES IN BREAST RECONSTRUCTION
 NEW TECHNIQUES IN BREAST RECONSTRUCTION J Van Geertruyden and J-V Berthe Plastic Surgery Erasme University Hospital and Clinique Edith Cavell Brussels What s new in breast reconstruction? New materials
NEW TECHNIQUES IN BREAST RECONSTRUCTION J Van Geertruyden and J-V Berthe Plastic Surgery Erasme University Hospital and Clinique Edith Cavell Brussels What s new in breast reconstruction? New materials
Vertical mammaplasty has been developed
 BREAST Y-Scar Vertical Mammaplasty David A. Hidalgo, M.D. New York, N.Y. Background: Vertical mammaplasty is an effective alternative to inverted-t methods. Among other benefits, it results in a significantly
BREAST Y-Scar Vertical Mammaplasty David A. Hidalgo, M.D. New York, N.Y. Background: Vertical mammaplasty is an effective alternative to inverted-t methods. Among other benefits, it results in a significantly
The success of breast conservation protocols BREAST. Implant Reconstruction in Breast Cancer Patients Treated with Radiation Therapy
 BREAST Implant Reconstruction in Breast Cancer Patients Treated with Radiation Therapy Jeffrey A. Ascherman, M.D. Matthew M. Hanasono, M.D. Martin I. Newman, M.D. Duncan B. Hughes, M.D. New York, N.Y.
BREAST Implant Reconstruction in Breast Cancer Patients Treated with Radiation Therapy Jeffrey A. Ascherman, M.D. Matthew M. Hanasono, M.D. Martin I. Newman, M.D. Duncan B. Hughes, M.D. New York, N.Y.
Institute of Reconstructive Plastic Surgery, New York University Medical Center, New York, NY USA
 Free full text on www.ijps.org Sheel Sharma, Gordon Kaplan Institute of Reconstructive Plastic Surgery, New York University Medical Center, New York, NY 10016 USA Address for correspondence: Dr. Sheel
Free full text on www.ijps.org Sheel Sharma, Gordon Kaplan Institute of Reconstructive Plastic Surgery, New York University Medical Center, New York, NY 10016 USA Address for correspondence: Dr. Sheel
Motiva Implant Matrix Silicone Breast Implants Summary of Clinical Data 5-Year Follow Up
 Motiva Implant Matrix Silicone Breast Implants Summary of Clinical Data 5-Year Follow Up October 21 - February 216 Motiva Implant Matrix Silicone Breast Implants Prospective Clinical Evaluation: 5-Year
Motiva Implant Matrix Silicone Breast Implants Summary of Clinical Data 5-Year Follow Up October 21 - February 216 Motiva Implant Matrix Silicone Breast Implants Prospective Clinical Evaluation: 5-Year
Technique Guide. A natural product for a natural repair. Post-Mastectomy Breast Reconstruction
 A natural product for a natural repair. Acellular Dermal Matrix Tissue In Conjunction With Soft Tissue Repair Technique Guide Post-Mastectomy Breast Reconstruction This Technique Guide contains the opinions
A natural product for a natural repair. Acellular Dermal Matrix Tissue In Conjunction With Soft Tissue Repair Technique Guide Post-Mastectomy Breast Reconstruction This Technique Guide contains the opinions
INFORMED-CONSENT- AUGMENTATION MAMMOPLASTY
 INFORMED-CONSENT- AUGMENTATION MAMMOPLASTY Instructions This is an informed-consent document that has been prepared to help inform you about augmentation mammoplasty, its risks, and alternative treatments.
INFORMED-CONSENT- AUGMENTATION MAMMOPLASTY Instructions This is an informed-consent document that has been prepared to help inform you about augmentation mammoplasty, its risks, and alternative treatments.
Reconstructive Breast Surgery following Mastectomy for Breast Cancer: A Review
 Research Article http://www.alliedacademies.org/advanced-surgical-research/ Reconstructive Breast Surgery following Mastectomy for Breast Cancer: A Review Gurnam Virdi* Department of surgery, Queen Elizabeth
Research Article http://www.alliedacademies.org/advanced-surgical-research/ Reconstructive Breast Surgery following Mastectomy for Breast Cancer: A Review Gurnam Virdi* Department of surgery, Queen Elizabeth
Breast Cancer Reconstruction
 Breast Cancer Jerome H. Liu, MD Tom S. Liu, MD Jerome H. Liu, MD Undergraduate: Brown University Medical School: University of California, Los Angeles Residency: UCLA Medical Center Fellowship:UCLA Medical
Breast Cancer Jerome H. Liu, MD Tom S. Liu, MD Jerome H. Liu, MD Undergraduate: Brown University Medical School: University of California, Los Angeles Residency: UCLA Medical Center Fellowship:UCLA Medical
Contralateral Prophylactic Mastectomy with Immediate Reconstruction: Added Benefits, Added Risks
 Contralateral Prophylactic Mastectomy with Immediate Reconstruction: Added Benefits, Added Risks Grant W. Carlson Wadley R. Glenn Professor of Surgery Divisions of Plastic Surgery & Surgical Oncology Emory
Contralateral Prophylactic Mastectomy with Immediate Reconstruction: Added Benefits, Added Risks Grant W. Carlson Wadley R. Glenn Professor of Surgery Divisions of Plastic Surgery & Surgical Oncology Emory
A Combined Practice. Why Its Worked. Barriers to Breast Reconstruction. As a breast oncologist the patient gets seemless care
 A Combined Practice A Combined Breast Oncology and Plastic Surgery Practice Why It Works Anne M. Wallace, MD, FACS Director, Comprehensive Breast Health Center Professor of Clinical Surgery, Surgical Oncology
A Combined Practice A Combined Breast Oncology and Plastic Surgery Practice Why It Works Anne M. Wallace, MD, FACS Director, Comprehensive Breast Health Center Professor of Clinical Surgery, Surgical Oncology
Outcomes Evaluation Following Bilateral Breast Reconstruction Using Latissimus Dorsi Myocutaneous Flaps
 BREAST SURGERY Outcomes Evaluation Following Bilateral Breast Reconstruction Using Latissimus Dorsi Myocutaneous Flaps Albert Losken, MD, FACS, Claire S. Nicholas, MD, Ximena A. inell, MD, and Grant W.
BREAST SURGERY Outcomes Evaluation Following Bilateral Breast Reconstruction Using Latissimus Dorsi Myocutaneous Flaps Albert Losken, MD, FACS, Claire S. Nicholas, MD, Ximena A. inell, MD, and Grant W.
MICHAEL R. ZENN, M.D. INFORMATION ABOUT BREAST RECONSTRUCTION
 MICHAEL R. ZENN, M.D. INFORMATION ABOUT BREAST RECONSTRUCTION The purpose of breast reconstruction is to restore body image and to enable you to wear all types of clothes without restriction. Most women
MICHAEL R. ZENN, M.D. INFORMATION ABOUT BREAST RECONSTRUCTION The purpose of breast reconstruction is to restore body image and to enable you to wear all types of clothes without restriction. Most women
Breast Reconstruction Following Mastectomy or Lumpectomy
 Medical Coverage Policy Effective Date... 1/15/2018 Next Review Date... 1/15/2019 Coverage Policy Number... 0178 Breast Reconstruction Following Mastectomy or Lumpectomy Table of Contents Related Coverage
Medical Coverage Policy Effective Date... 1/15/2018 Next Review Date... 1/15/2019 Coverage Policy Number... 0178 Breast Reconstruction Following Mastectomy or Lumpectomy Table of Contents Related Coverage
INFORMED-CONSENT-AUGMENTATION MAMMAPLASTY
 INFORMED-CONSENT-AUGMENTATION MAMMAPLASTY 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein
INFORMED-CONSENT-AUGMENTATION MAMMAPLASTY 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein
Supplementary Online Content
 Supplementary Online Content Abt NB, Flores JM, Baltodano PA, et al. Neoadjuvant chemotherapy and short-term in patients undergoing mastectomy with and without breast reconstruction. JAMA Surg. Published
Supplementary Online Content Abt NB, Flores JM, Baltodano PA, et al. Neoadjuvant chemotherapy and short-term in patients undergoing mastectomy with and without breast reconstruction. JAMA Surg. Published
ONCOPLASTIC SURGERY. Dr. Sadir Alrawi Director of Surgical Oncology Services. Dr. Humaa Darr Surgical Oncology Fellow
 Hessa St ONCOPLASTIC SURGERY Dr. Sadir Alrawi Director of Surgical Oncology Services Dr. Humaa Darr Surgical Oncology Fellow Al Sufouh Rd AL SUFOUH AL SUFOUH Sharaf DG Mall of the Emirates Mall Of the
Hessa St ONCOPLASTIC SURGERY Dr. Sadir Alrawi Director of Surgical Oncology Services Dr. Humaa Darr Surgical Oncology Fellow Al Sufouh Rd AL SUFOUH AL SUFOUH Sharaf DG Mall of the Emirates Mall Of the
Prepectoral breast reconstruction and radiotherapy a closer look
 Original Article Prepectoral breast reconstruction and radiotherapy a closer look Steven Sigalove Scottsdale Center for Plastic Surgery, Scottsdale, AZ, USA Correspondence to: Steven Sigalove, MD, FACS.
Original Article Prepectoral breast reconstruction and radiotherapy a closer look Steven Sigalove Scottsdale Center for Plastic Surgery, Scottsdale, AZ, USA Correspondence to: Steven Sigalove, MD, FACS.
Autologous Grafts for the Correction of Breast Contour Deformities after Breast Reconstruction
 24 Journal of Advanced Plastic Surgery Research, 2015, 1, 24-28 Autologous Grafts for the Correction of Breast Contour Deformities after Breast Reconstruction M. Massa 1,2*, T. Massa 3, A. Peirano 1, P.L.
24 Journal of Advanced Plastic Surgery Research, 2015, 1, 24-28 Autologous Grafts for the Correction of Breast Contour Deformities after Breast Reconstruction M. Massa 1,2*, T. Massa 3, A. Peirano 1, P.L.
2017 PHYSICIAN CODING GUIDE
 2017 PHYSICIAN CODING GUIDE Breast Repair and/or Reconstruction Surgeon s Procedure Codes 11970 Replacement of tissue expander with permanent prosthesis 8.01 $628.05 +15777 Implantation of biologic implant
2017 PHYSICIAN CODING GUIDE Breast Repair and/or Reconstruction Surgeon s Procedure Codes 11970 Replacement of tissue expander with permanent prosthesis 8.01 $628.05 +15777 Implantation of biologic implant
Selective salvage of zones 2 and 4 in the pedicled TRAM flap: a focus on reducing fat necrosis and improving aesthetic outcomes
 DOI 10.1186/s40064-016-1714-7 RESEARCH Open Access Selective salvage of zones 2 and 4 in the pedicled TRAM flap: a focus on reducing fat necrosis and improving aesthetic outcomes Chi Sun Yoon and Kyu Nam
DOI 10.1186/s40064-016-1714-7 RESEARCH Open Access Selective salvage of zones 2 and 4 in the pedicled TRAM flap: a focus on reducing fat necrosis and improving aesthetic outcomes Chi Sun Yoon and Kyu Nam
ARTICLE COVERSHEET SERVER-BASED
 ARTICLE COVERSHEET LWW_CONDENSED-FLA(7.75X10.75) SERVER-BASED Template version : 8.1 Revised: 08/23/2012 Article :SAP22670 Creator : jteves Date : Saturday September 8th 2012 Time : 05:19:34 Number of
ARTICLE COVERSHEET LWW_CONDENSED-FLA(7.75X10.75) SERVER-BASED Template version : 8.1 Revised: 08/23/2012 Article :SAP22670 Creator : jteves Date : Saturday September 8th 2012 Time : 05:19:34 Number of
Fat Grafting Technique, A Paradigm Shift in the Treatment of Tuberous Breast
 7 Fat grafting in tuberous breast Original Article Fat Grafting Technique, A Paradigm Shift in the Treatment of Tuberous Breast Downloaded from wjps.ir at 5:45 +040 on Sunday September 9th 08 Claudio Silva-Vergara*,
7 Fat grafting in tuberous breast Original Article Fat Grafting Technique, A Paradigm Shift in the Treatment of Tuberous Breast Downloaded from wjps.ir at 5:45 +040 on Sunday September 9th 08 Claudio Silva-Vergara*,
inding the fit that s right for you. Your Surgery Planner For Breast Augmentation or Reconstruction with NATRELLE Saline-Filled Breast Implants
 inding the fit that s right for you. Your Surgery Planner For Breast Augmentation or Reconstruction with NATRELLE Saline-Filled Breast Implants L Place Your Device Identification Card(s) Here R INTRODUCTION
inding the fit that s right for you. Your Surgery Planner For Breast Augmentation or Reconstruction with NATRELLE Saline-Filled Breast Implants L Place Your Device Identification Card(s) Here R INTRODUCTION
COPE Library Sample
 Breast Anatomy LOBULE LOBE ACINI (MILK PRODUCING UNITS) NIPPLE AREOLA COMPLEX ENLARGEMENT OF DUCT AND LOBE LOBULE SUPRACLAVICULAR NODES INFRACLAVICULAR NODES DUCT DUCT ACINI (MILK PRODUCING UNITS) 8420
Breast Anatomy LOBULE LOBE ACINI (MILK PRODUCING UNITS) NIPPLE AREOLA COMPLEX ENLARGEMENT OF DUCT AND LOBE LOBULE SUPRACLAVICULAR NODES INFRACLAVICULAR NODES DUCT DUCT ACINI (MILK PRODUCING UNITS) 8420
