CLINICAL MEDICAL POLICY
|
|
|
- Leo Lane
- 6 years ago
- Views:
Transcription
1 CLINICAL MEDICAL POLICY Policy Name: Reconstructive Breast Surgery Policy Number: MP-052-MD-DE Approved By: Medical Management Medical Policy Provider Notice Date: 08/01/2017 Original Effective Date: 09/01/2017 Annual Approval Date: 07/01/2018 Revision Date: N/A Products: Highmark Health Options Application: All participating hospitals and providers Page Number(s): 1 of 15 DISCLAIMER Highmark Health Options medical policy is intended to serve only as a general reference resource regarding payment and coverage for the services described. This policy does not constitute medical advice and is not intended to govern or otherwise influence medical decisions. POLICY STATEMENT Highmark Health Options provides coverage under the medical surgical benefits of the Company s Medicaid products for medically necessary breast reconstructive surgical procedures. This policy is designed to address medical necessity guidelines that are appropriate for the majority of individuals with a particular disease, illness or condition. Each person s unique clinical circumstances warrant individual consideration, based upon review of applicable medical records. The qualifications of the policy will meet the standards of the National Committee for Quality Assurance (NCQA) and the Delaware Department of Health and Social Services (DHSS) and all applicable state and federal regulations. Policy No. MP-052-MD-DE Page 1 of 15
2 DEFINITIONS Acellular Skin Substitutes Products that contain a matrix or scaffold composed of materials such as collagen, hyaluronic acid, and fibronectin. These materials are obtained from either human (dermis, amniotic membrane or placenta) or nonhuman (bovine, porcine and ovine) sources. Reconstructive Breast Surgery Surgical procedures performed to correct or repair abnormal structures of the breast that are designed to restore the normal appearance of one breast or both breasts. Reconstructive Surgery Surgical procedures performed on abnormal structures of the body caused by congenital deformity, trauma, infection, tumors, or disease. These procedures are performed to improve function, but may also be done to approximate a normal appearance. Reduction Mammoplasty A surgical procedure to decrease breast size. Cosmetic Surgery Procedures are considered cosmetic when intended to change a physical appearance that would be considered within normal human anatomic variation. Cosmetic services are often described as those that are primarily intended to preserve or improve appearance and self-esteem. Congenital Anomaly A physical developmental defect that is present at the time of birth and that is identified within the first 12 months of birth. Functional/Physical Impairment A functional/physical or physiological impairment causes deviation from normal function of a tissue or organ. This results in a significantly limited, impaired, or delayed capacity to move, coordinate actions, or perform physical activities, and is exhibited by difficulties in one or more of the following areas: physical and motor tasks; independent movement; performance of basic life functions. Prophylactic Mastectomy A surgical procedure to remove a breast or both breasts with the purpose of reducing the risk of breast cancer in women determined to be at intermediate or high risk for developing breast cancer. Poland Syndrome A rare development disorder that is present at birth. It is characterized by absence or underdevelopment of certain muscles in the chest and abnormally short, webbed fingers. Other findings associated with this syndrome can include underdevelopment or absence of one nipple (including the darkened area around the nipple (areola) and/or patchy axilla hair growth. Typically the physical abnormalities are unilateral and affect the right side primarily. Deep Inferior Epigastric Perforator (DIEP) Flap A type of breast reconstruction in which blood vessels called deep inferior epigastric perforators, as well as the skin and fat connected to them, are removed from the lower abdomen and transferred to the chest to reconstruct a breast after mastectomy, without the sacrifice of any of the abdominal muscles. Policy No. MP-052-MD-DE Page 2 of 15
3 PROCEDURES Reconstructive breast surgery is covered for patients who have had a mastectomy with or without a diagnosis of cancer. A mastectomy includes partial (lumpectomy, tylectomy, quadrantectomy, and segmentectomy), simple, and radical. Breast surgery is considered reconstructive, and therefore medically necessary, when there are abnormalities related to trauma, congenital defects, infection, or other nonmalignant disease such as Poland s syndrome. In accordance with federal and state mandates, the following breast reconstruction services are covered: Reconstruction of the breast where the mastectomy was performed; Surgery and reconstruction of the other breast to produce a symmetrical appearance, including nipple tattooing; Prosthesis (implanted and/or external) Treatment of physical complications of mastectomy (including lymphedema); Timing of the reconstructive services is not a factor in coverage; Cancer does not have to be the reason for the mastectomy; The mandate applies to men and women; Requires coverage for all stages of breast reconstruction; There is no mandated coverage for revision of a completed breast reconstruction to improve appearance 1. Breast reconstruction will be performed on the diseased/affected breast, including: A. Areolar and nipple reconstruction; OR B. Areolar and nipple tattooing; OR C. Autologous fat transplant (e.g., lipoinjection, lipofilling, lipomodeling); OR D. Breast implant removal and subsequent reimplantation for implants done postmastectomy or other covered reconstructive procedures; OR E. Capsulectomy; OR F. Capsulotomy; OR G. Implantation of tissue expander; OR H. Implantation of U.S. FDA-approved internal breast prosthesis; OR I. Reconstructive surgical revisions; OR J. Tissue/muscle reconstruction procedures (e.g., flaps), including but not limited to the following: 1) Deep inferior epigastric perforator (DIEP) flap 2) Latissimus dorsi (LD) myocutaneous flap 3) Ruben s flap 4) Superficial inferior epigastric perforator/artery (SEIP/SIEA) flap 5) Superior or inferior gluteal free flap 6) Transverse rectus abdominus myocutaneous (TRAM) flap 7) Transverse upper gracilis (TUG) flap Note: There must be clear documentation in the operative report on the size of the resection, the size of the defect, incisions made, the nature of the flap(s), the size of the flap(s), and how they are rotated or transposed. K. FDA-approved tissue expanders post-mastectomy with/without skin substitutes that have also been approved by the FDA. Policy No. MP-052-MD-DE Page 3 of 15
4 2. Breast reconstruction procedures performed on the non-diseased/unaffected breast after having a mastectomy/lumpectomy in order to produce a symmetrical appearance are considered medically necessary, including: A. Areolar and nipple reconstruction; OR B. Areolar and nipple tattooing; OR C. Augmentation mammoplasty with implantation of FDA-approved internal breast prosthesis; OR D. Autologous fat transplant (e.g., lipoinjection, lipofilling, lipomodeling); OR E. Breast implant removal and subsequent reimplantation when performed to produce a symmetrical appearance; OR F. Breast reduction by mammoplasty or mastopexy; OR G. Capsulectomy; OR H. Capsulotomy; OR I. Reconstructive surgery revisions to produce a symmetrical appearance 3. Breast reconstruction may require the use of skin substitutes. Skin substitutes are considered medically necessary when any one of the following medically necessary criteria are met: A. When there is insufficient tissue expander or implant coverage by the pectoralis major muscle, and additional coverage is required; OR B. When there is viable but compromised or thin post-mastectomy flaps that are at risk of dehiscence or necrosis; OR C. The infra-mammary fold and lateral mammary folds have been undermined during mastectomy, and re-establishment of these landmarks is needed The following FDA-approved skin substitutes used in breast reconstruction following a mastectomy for breast cancer are considered medically necessary: A. AlloDerm (acellular dermal matrix) B. Cortiva (formerly known as AlloMax ) (acellular dermal matrix) C. FlexHD (acellular dermal matrix) All other skin substitutes used in breast reconstruction following a mastectomy are considered not medically necessary. 4. Removal, Revision, or Replacement of Breast Implants Removal, revision, or replacement of FDA-approved breast implants will be considered medically necessary when: A. The breast implant was originally implanted for reconstruction following a mastectomy for breast cancer and covered surgical reconstruction for breast abnormalities listed above; AND B. There is implant rupture, failure, Baker Class III or IV contracture, implant exposure or extrusion; OR C. There is infection or inflammatory reaction to a breast prosthesis including siliconoma, granuloma, or painful capsular contracture with disfigurement; OR D. There is interference with the diagnosis or treatment of breast cancer E. Removal, revision, or replacement of breast implants is not considered medically necessary for complications, immediate or delayed, from a prior cosmetic breast implant. Policy No. MP-052-MD-DE Page 4 of 15
5 5. Capsulectomy/Capsulotomy A. Capsulectomy and/or capsulotomy is considered medically necessary when the original implant was placed during a covered breast reconstruction procedure. B. Capsulectomy and/or capsulotomy is required due to a related complication of a covered medical condition or procedure. C. Capsulectomy and/or capsulotomy is not considered medically necessary for complications, immediate or delayed, from a prior cosmetic breast implant. 6. Nonsurgical Option An external breast prosthesis and mastectomy bra are covered benefits for women who choose not to have breast reconstruction or are poor candidates for reconstruction. 7. Treatment of Post-Mastectomy Complications Coverage will be provided for the treatment of the following complications of a mastectomy: A. Lymphedema 1) Lymphedema pumps 2) Compression lymphedema sleeves 3) Complex decongestive physiotherapy (CDP) B. Infection C. Removal of a breast implant due to rupture D. Replacement of new breast implant 8. Precautions The following conditions have been identified as risk factors for breast reconstructive surgery and should be adequately addressed by the performing provider: A. Active smoking B. A body mass index (BMI) of 25 or greater C. Poorly managed diabetes 9. Non-covered Services Aspirations Biopsy (open or core) Excision of cysts, fibroadenomas or other benign or malignant tumors, aberrant breast tissue, duct lesions, nipple or areolar lesions Treatment of gynecomastia Lipectomy (suction or ultrasonically-assisted suction) for correction of surgically-induced donor site asymmetry that results from one or more flap breast reconstruction procedure(s) is not covered as it is not medically necessary Removal and/or replacement of an existing breast implant if the earlier breast implant was performed for cosmetic reasons when there are no local breast complications Implantation or reimplantation of breast implant(s) for cosmetic reasons Breast reconstruction using adipose-derived stem cells in autologous fat grafting (ADSC) Vascularized lymph node transfer (VLNTx) Xenograft cartilage grafting Breast surgery for cosmetic reasons Policy No. MP-052-MD-DE Page 5 of 15
6 10. Contraindications There are no known contraindications for breast reconstructive surgery. 11. When Breast Reconstruction services are not covered For conditions other than those listed above, scientific evidence has not been established. 12. Post-payment Audit Statement The medical record must include documentation that reflects the medical necessity criteria and is subject to audit by Highmark Health Options at any time pursuant to the terms of your provider agreement. 13. Place of Service The place of service for breast reconstructive surgery can be inpatient, outpatient, or the provider office. All of the procedures in this medical policy are considered to be outpatient procedures except for the following: Breast reconstruction with latissimus dorsi flap, without prosthetic implant Breast reconstruction with free flap Breast reconstruction with transverse rectus abdominis myocutaneous flap (TRAM), single pedicle, including closure of donor site Breast reconstruction with transverse rectus abdominis myocutaneous flap (TRAM), single pedicle, including closure of donor site; with microvascular anastomosis (supercharging) Breast reconstruction with transverse rectus abdominis myocutaneous flap (TRAM), double pedicle, including closure of donor site Reconstructive repair of pectus excavatum or carinatum; open GOVERNING BODIES APPROVAL The FDA published the regulatory history of breast implants in the United States. mplants/ucm htm Federal The Women s Health and Cancer Rights Act of 1998 (WHCRA) is a federal law that provides protections to patients who choose to have breast reconstruction in connection with a mastectomy. Patients can elect to have breast reconstruction, and coverage must be provided for: All stages of reconstruction of the breast on which the mastectomy has been performed; Surgery and reconstruction of the other breast to produce a symmetrical appearance; and Prostheses and treatment of physical complications of all stages of the mastectomy, including lymphedema. Policy No. MP-052-MD-DE Page 6 of 15
7 Pennsylvania 1997 Pa.ALS51; 1977 Pa.SB 176 Scope: Reimbursement for Length of Stay/Inpatient Care Following Mastectomy Reimbursement for Breast Reconstruction and Prosthesis Breast Cancer Reconstructive Surgery Coverage Act Bill signed into law October 21, 1998 and effective October 1, 1998 Provides mandatory insurance coverage for a 48-hour hospital stay and related breast reconstruction following a mastectomy. Provides for opposite breast reconstruction for symmetry, and requires home health care visits for mastectomy patients discharged before 48 hours. Provides protection to women who need time to recover from the trauma of major surgery. Note the following: Cancer does not have to be the reason for the mastectomy The mandate applies to men and women Mandates coverage for all stages of breast reconstruction Does not mandate coverage for revision of a completed breast reconstruction to improve appearance Extension of Medicaid for Breast Cancer Treatment Effective January 1, 2002 Executive Order by Governor Ridge (PA) in June 2001 Extends the state s Medicaid coverage to uninsured women diagnosed with breast or cervical cancer through the Centers for Disease Control s National Breast and Cervical Cancer Early Detection Program Covers any other medical needs through the period of treatment Entitles qualified women to immediate free treatment paid for through Medicaid Guarantees coverage to women in Pennsylvania of their full treatment costs through Pennsylvania s Medicaid program Covers the expenses of patients with breast and cervical cancer whose incomes are too high to meet the traditional guidelines for Medicaid, a federal-state health coverage program Act 81 of 2002 No time limit on reconstructive surgery Effective June 28, 2002, removed the 6-year time limit on mandatory insurance coverage for reconstructive surgery following mastectomy. It directs that all health care policies also cover physical complications from breast cancer, including lymphedema, and provides for prosthetics. CODING REQUIREMENTS Procedure Codes CPT Codes Description Tattooing, intradermal introduction of insoluble opaque pigments to correct color defects of skin, including micropigmentation; 6.0 sq. cm or less Tattooing, intradermal introduction of insoluble opaque pigments to correct color defects of skin, including micropigmentation; 6.1 to 20.0 sq. cm. Policy No. MP-052-MD-DE Page 7 of 15
8 11922 Tattooing, intradermal introduction of insoluble opaque pigments to correct color defects of skin, including micropigmentation; each additional 20 sq. cm, or thereof (List separately in addition to code for primary procedure) Replacement of tissue expander with permanent prosthesis Removal of tissue expander(s) without insertion of prosthesis Application of skin substitute graft to trunk, arms, legs, total wound surface area up to 100 sq. cm; first 25 sq.cm or less wound surface area Application of skin substitute graft to trunk, arms, legs, total wound surface area up to 100 sq. cm; each additional 25 sq. cm wound surface area, or part thereof (List separately in addition to code for primary procedure) Implantation of biologic implant (e.g., acellular dermal matrix) for soft tissue reinforcement (i.e., breast, trunk) (List separately in addition to code for primary procedure) Suction assisted lipectomy; trunk [when specified as a breast reconstruction procedure following breast surgery] Mastopexy Reduction mammaplasty Mammaplasty, augmentation; without prosthetic implant Mammaplasty, augmentation; with prosthetic implant Removal of intact mammary implant Removal of mammary implant material Immediate insertion of breast prosthesis following mastopexy, mastectomy or in reconstruction Delayed insertion of breast prosthesis following mastopexy, mastectomy or in reconstruction Nipple/areola reconstruction Correction of inverted nipples Breast reconstruction, immediate or delayed, with tissue expander, including subsequent expansion Breast reconstruction with latissimus dorsi flap, without prosthetic implant Breast reconstruction with free flap Breast reconstruction with other technique (e.g., autologous fat transplant without adipose derived stem cells or xenograft cartilage grafting) Breast reconstruction with transverse rectus abdominis myocutaneous flap (TRAM), single pedicle, including closure of donor site Breast reconstruction with transverse rectus abdominis myocutaneous flap (TRAM), single pedicle, including closure of donor site; with microvascular anastomosis (supercharging) Breast reconstruction with transverse rectus abdominis myocutaneous flap (TRAM), double pedicle, including closure of donor site Open periprosthetic capsulotomy, breast Periprosthetic capsulectomy, breast Revision of reconstructed breast Preparation of moulage for custom breast implant Policy No. MP-052-MD-DE Page 8 of 15
9 21740 Reconstructive repair of pectus excavatum or carinatum; open Reconstructive repair of pectus excavatum or carinatum; minimally invasive approach (Nuss procedure), without thoracoscopy Reconstructive repair of pectus excavatum or carinatum; minimally invasive approach (Nuss procedure), with thoracoscopy HCPCS Codes C1789 Prosthesis, breast (implantable) L8000 Breast prosthesis; mastectomy bra L8001 Breast prosthesis; mastectomy bra, with integrated breast prosthesis form, unilateral L8002 Breast prosthesis; mastectomy bra, with integrated breast prosthesis form, bilateral L8015 External breast prosthesis garment, with mastectomy form, post mastectomy L8020 Breast prosthesis; mastectomy form L8030 Breast prosthesis; silicone or equal, without integral adhesive L8031 Breast prosthesis; silicone or equal, with integral adhesive L8032 Nipple prosthesis, reusable, any type, each L8035 Custom breast prosthesis, post mastectomy, molded to patient model L8039 Breast prosthesis, not otherwise classified L8600 Implantable breast prosthesis, silicone or equal Q4100 Skin substitute, not otherwise specified Q4116 AlloDerm, per square centimeter Q4128 FlexHD or AllopathHD, per sq. cm S2066 Breast reconstruction with gluteal artery perforator (GAP) flap, including harvesting of the flap, microvascular transfer, closure of donor site and shaping the flap into a breast, unilateral S2067 Breast reconstruction of a single breast with stacked deep inferior epigastric perforator (IEP) flaps(s) and/or gluteal artery perforator (GAP) flap(s), including harvesting of the flap(s), microvascular transfer, closure of donor site(s) and shaping the flap into a breast, unilateral S2068 Breast reconstruction with deep inferior epigastric perforator *DIEP) flap or superficial inferior epigastric artery (SIEA) flap, including harvesting of the flap, microvascular transfer, closure of donor site and shaping of the flap into a breast, unilateral Procedure Codes Not Applicable to Breast Reconstruction Services CPT Codes Description Biopsy of breast; percutaneous, needle core, not using imaging guidance Biopsy of breast; open, incisional Excision of cyst, fibroadenomas, or other benign or malignant tumor, aberrant breast tissue, duct lesion, nipple or areolar lesion (Except 19300), open male or female, one or more lesions Excision of breast lesion identified by preoperative placement of radiological marker, open; single lesion Excision of breast lesion identified by preoperative placement of radiological marker, open; each additional lesion separately identified by a preoperative radiological marker Excision tumor, soft tissue of neck or thorax, subcutaneous Policy No. MP-052-MD-DE Page 9 of 15
10 21556 Excision tumor, soft tissue of neck or thorax; deep subfascial, intramuscular Diagnosis Codes ICD-10 Codes Description C Unspecified malignant neoplasm of skin of breast C Basal Cell carcinoma of skin of breast C Squamous cell carcinoma of skin of breast C Other specified malignant neoplasm of skin of breast C Malignant neoplasm of nipple and areola right female breast C Malignant neoplasm of nipple and areola left female breast C Malignant neoplasm of nipple and areola right male breast C Malignant neoplasm of nipple and areola left male breast C Malignant neoplasm of central portion of right female breast C Malignant neoplasm of central portion of left female breast C Malignant neoplasm of central portion of right male breast C Malignant neoplasm of central portion of left male breast C Malignant neoplasm of upper-inner quadrant of right female breast C Malignant neoplasm of upper-inner quadrant of left female breast C Malignant neoplasm of upper-inner quadrant of right male breast C Malignant neoplasm of upper-inner quadrant of left male breast C Malignant neoplasm of lower-inner quadrant of right female breast C Malignant neoplasm of lower-inner quadrant of left female breast C Malignant neoplasm of lower-inner quadrant of right male breast C Malignant neoplasm of lower-inner quadrant of left male breast C Malignant neoplasm of upper-outer quadrant of right female breast C Malignant neoplasm of upper-outer quadrant of left female breast C Malignant neoplasm of upper-outer quadrant of right male breast C Malignant neoplasm of upper-outer quadrant of left male breast C Malignant neoplasm of lower-outer quadrant of right female breast C Malignant neoplasm of lower-outer quadrant of left female breast C Malignant neoplasm of lower-outer quadrant of right male breast C Malignant neoplasm of lower-outer quadrant of left male breast C Malignant neoplasm of axillary tail of right female breast C Malignant neoplasm of axillary tail of left female breast C Malignant neoplasm of axillary tail of right male breast C Malignant neoplasm of axillary tail of left male breast C Malignant neoplasm of overlapping sites of right female breast C Malignant neoplasm of overlapping sites of left female breast C Malignant neoplasm of overlapping sites of right male breast C Malignant neoplasm of overlapping sites of left male breast C Malignant neoplasm of unspecified site of right female breast C Malignant neoplasm of unspecified site of left female breast C Malignant neoplasm of unspecified site of right male breast Policy No. MP-052-MD-DE Page 10 of 15
11 C Malignant neoplasm of unspecified site of left male breast C79.2 Secondary malignant neoplasm of skin C79.81 Secondary malignant neoplasm of breast D04.5 Carcinoma in situ of skin of trunk D05.01 Lobular carcinoma in situ of right breast D05.02 Lobular carcinoma in situ of left breast D05.11 Intraductal carcinoma in site of right breast D05.12 Intraductal carcinoma in site of left breast D48.61 Neoplasm of uncertain behavior of right breast D48.62 Neoplasm of uncertain behavior of left breast D05.81 Other specified type of carcinoma in situ of right breast D05.82 Other specified type of carcinoma in situ of left breast D05.91 Unspecified type of carcinoma in situ of right breast D05.92 Unspecified type of carcinoma in situ of left breast N60.11 Diffuse cystic mastopathy of right breast N60.12 Diffuse cystic mastopathy of left breast N60.21 Fibroadenosis of right breast N60.22 Fibroadenosis of left breast N60.31 Fibrosclerosis of right breast N60.32 Fibrosclerosis of left breast N65.0 Deformity of reconstructed breast N65.1 Disproportion of reconstructed breast Q67.6 Pectus excavatum Q67.7 Pectus carinatum Q79.8 Other congenital malformations of musculoskeletal system Q83.0 Congenital absence of breast with absent nipple Q83.2 Absent nipple T85.41XA Breakdown (mechanical) of breast prosthesis and implant, initial encounter T85.41XD T85.41XS T85.42XA T85.42XD T85.42XS T85.43XA T85.43XD T85.43XS T85.44XA T85.44XD T85.44XS T85.49XA T85.49XD T85.49XS Breakdown (mechanical) of breast prosthesis and implant, subsequent encounter Breakdown (mechanical) of breast prosthesis and implant, sequela Displacement of breast prosthesis and implant, initial encounter Displacement of breast prosthesis and implant, subsequent encounter Displacement of breast prosthesis and implant, sequela Leakage of breast prosthesis and implant, initial encounter Leakage of breast prosthesis and implant, subsequent encounter Leakage of breast prosthesis and implant, sequela Capsular contracture of breast implant, initial encounter Capsular contracture of breast implant, subsequent encounter Capsular contracture of breast implant, sequela Other mechanical complication of breast prosthesis and implant, initial encounter Other mechanical complication of breast prosthesis and implant, subsequent encounter Other mechanical complication of breast prosthesis and implant, sequela Policy No. MP-052-MD-DE Page 11 of 15
12 T85.79XA Infection and inflammatory reaction due to other internal prosthetic devices, implants and grafts, initial encounter Z40.01 Encounter for prophylactic removal of breast Z42.1 Encounter for breast reconstruction following mastectomy Z42.8 Encounter for other plastic and reconstructive surgery following medical procedure or healed injury Z44.30 Encounter for fitting and adjustment of external breast prosthesis, unspecified breast Z44.31 Encounter for fitting and adjustment of external right breast prosthesis Z44.32 Encounter for fitting and adjustment of external left breast prosthesis Z Encounter for adjustment or removal of right breast implant Z Encounter for adjustment or removal of left breast implant Z Encounter for adjustment or removal of unspecified breast implant Z48.3 Aftercare following surgery for neoplasm Z85.3 Personal history of malignant neoplasm of breast Z90.10 Acquire absence of unspecified breast and nipple Z90.11 Acquired absence of right breast and nipple Z90.12 Acquired absence of left breast and nipple Z90.13 Acquired absence of bilateral breasts and nipples Z98.82 Breast implant status REIMBURSEMENT Participating facilities will be reimbursed per their Highmark Health Options contract. POLICY SOURCE(S) American Society of Plastic Surgeons (ASPS). Evidence-Based Clinical Practice Guideline: Breast Reconstruction with Expanders and Implants. March Accessed on March 17, 2017 and available at: American Society of Plastic Surgeons (ASPS). Practice Parameter. Treatment Principles of Silicone Breast Implants. March Accessed on March 20, 2017 and available at: Barber MD, Williams L, Anderson ED, Neades GT, Raine C, Young O, et al. Outcome of the use of acellular-dermal matrix to assist implant-based breast reconstruction in a single centre. Eur J Surg Oncol Jan; 41(1): Accessed on March 17, 2017 and abstract available at: Policy No. MP-052-MD-DE Page 12 of 15
13 Centers for Medicare and Medicaid Services (CMS). National Coverage Determination: Breast reconstruction following mastectomy. NCD # Effective January 1, 1997; revised October 3, Accessed on March 17, 2017 and available at: Davila AA, Seth AK, Wang E, et al. Human acellular dermis versus submuscular tissue expander breast reconstruction: A multivariate analysis of short-term complications. Arch Plast Surg Jan; 40(1): Accessed on March 16, 2017 and available at: Department of Health and Human Services (HHS). Proposed Rule on Nondiscrimination in Health Programs and Activities of Affordable Care Act (ACA); Proposed 45 C.F.R ; 80 Fed. Reg RIN 0945-AA02. The Kaiser Commission on Medicaid and the Uninsured. Issue Brief. October Final Rule. Published May 18, Effective July 18, Accessed on March 14, 2017 and available at: Brooke S, Mesa J, Uluer M, Michelotti B, Moyer K, Neves RI, et al. Complications in Tissue Expander Breast Reconstruction: A Comparison of AlloDerm, DermaMatrix, and FlexHD Acellular Inferior Pole Dermal Slings. Ann Plast Surg Aug 3. Accessed on March 17, 2017, and abstract available at: Complications_in_Tissue_ Expander.30.aspx. Breast Cancer (V1.2016). Revised November 18, The Women's Health and Cancer Rights Act (WHCRA), 713; October 21, Accessed on March 20, 2017 and available at: Reform/HealthInsReformforConsume/downloads/WHCRA_Statute.pdf. The American Cancer Society. Women s Health and Cancer Rights Act: The Federal law. Effective October 21, Accessed on April 12, 2107 and available at: The American Medical Association (AMA). Definitions of cosmetic and reconstructive surgery. H Accessed on March 17, 2017 and available at: Chun YS. Nipple-Areola Reconstruction. Updated April 29, Accessed March 17, 2017 and available at: The American Cancer Society. Cancer Fats & Figures No Accessed on March 17, 2017 and available at: Policy No. MP-052-MD-DE Page 13 of 15
14 National Institute for Health and Clinical Excellence (NICE). Breast reconstruction using lipomodeling after breast cancer treatment. Interventional Procedure Guidance 417. London, UK: NICE; January National Institute for Health and Clinical Excellence (NICE). Laparoscopic mobilization of the greater omentum for breast reconstruction. Interventional Procedure Guidance 253. London, UK: NICE; Valdatta L, Cattaneo AG, Pellegatta I, et al. Acellular dermal matrices and radiotherapy in breast reconstruction: A systematic review and meta-analysis of the literature. Plast Surg Int. 2014; 2014: Accessed on March 20, 2017 and available at: Perez-Cano R, Vranckx JJ, Lasso JM, et al. Prospective trial adipose-derived regenerative cell (ADRC)- enriched fat grafting for partial mastectomy defects: the RESTORE-2 trial. Eur J Surg Oncol. March 2012; 38(5): Accessed on March 21, 2017 and abstract available at: Derived_Regenerative_Cell_ADRC enriched_fat_grafting_for_partial_mastectomy_defects_the_restore-2_trial. National Institute for Health and Care Excellence (2012, January) Interventional procedure guidance: Breast reconstruction using lipomodeling after breast cancer treatment. Accessed on March 21, 2017 and available at: Kamat, P., Schweizer, R., Kaenel, P., Salemi, S., Calcagni, M., Giovanoli, P., et al. (2015). Human adiposederived mesenchymal stromal cells may promote breast cancer progression and metastatic spread. Plastic and Reconstructive Surgery, 136(1), Accessed on March 21, 2017 and available at: _Stromal_Cells.13.aspx. Centers for Medicare & Medicaid Services. CMS.gov. NCD for breast reconstruction following mastectomy (140.2). Accessed on March 21, 2017 and available at: Women s Health and Cancer Rights Act (WHCRA) of Centers for Medicare and Medicaid Services. (1998). The Women's Health and Cancer Rights Act. Accessed on March 21, 2107 from Women s Health and Cancer Rights conforming Amendments of Congressional Bills 109th Congress 1st Session H. R Women's Health and Cancer Rights Conforming Amendments of Accessed on March 20, 2017 and available at: 109hr437ih/html/BILLS-109hr437ih.htm. Baker Classification Breast Implant Contractures Grade I: Augmented breast feels as soft as normal breast Grade II: Breast is less soft and the implant can be palpated but it is not visible Grade III: Breast is firm, palpable, and the implant (or its distortion) is visible Grade IV: Breast is hard, painful, cold, tender, and distorted Policy No. MP-052-MD-DE Page 14 of 15
15 Policy History Date Activity 04/14/2017 Initial policy developed 06/27/2017 QI/UM Committee approval 09/01/2017 Provider effective date Policy No. MP-052-MD-DE Page 15 of 15
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Breast Reconstructive Surgery After Mastectomy Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Breast Reconstructive Surgery After Mastectomy PRE-DETERMINATION
Breast Reconstructive Surgery After Mastectomy Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Breast Reconstructive Surgery After Mastectomy PRE-DETERMINATION
Breast Reconstruction Surgery
 Breast Reconstruction Surgery I. Policy University Health Alliance (UHA) will reimburse for Breast Reconstruction Surgery when it is determined to be medically necessary and when it meets the medical criteria
Breast Reconstruction Surgery I. Policy University Health Alliance (UHA) will reimburse for Breast Reconstruction Surgery when it is determined to be medically necessary and when it meets the medical criteria
Medical Policy Original Effective Date: Revised Date: Page 1 of 8
 Page 1 of 8 Disclaimer Description Coverage Determination Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans, or the plan
Page 1 of 8 Disclaimer Description Coverage Determination Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans, or the plan
BREAST RECONSTRUCTION POST MASTECTOMY
 UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: January 1, 2019 Table of Contents Page INSTRUCTIONS FOR USE...
UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: January 1, 2019 Table of Contents Page INSTRUCTIONS FOR USE...
BREAST RECONSTRUCTION POST MASTECTOMY
 UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: February 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...
UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: February 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...
Breast Reconstruction Following Mastectomy or Lumpectomy
 Breast Reconstruction Following Mastectomy or Lumpectomy [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr go to Comunicados a Proveedores, and click Cartas
Breast Reconstruction Following Mastectomy or Lumpectomy [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr go to Comunicados a Proveedores, and click Cartas
Medical Review Criteria Breast Surgeries
 Medical Review Criteria Breast Surgeries Subject: Breast Surgeries Authorization: Prior authorization is required for the following procedures requested for members enrolled in HPHC commercial (HMO, POS,
Medical Review Criteria Breast Surgeries Subject: Breast Surgeries Authorization: Prior authorization is required for the following procedures requested for members enrolled in HPHC commercial (HMO, POS,
Medical Review Criteria Breast Surgeries
 Medical Review Criteria Breast Surgeries Effective Date: November 8, 2016 Subject: Breast Surgeries Policy: HPHC covers medically necessary breast surgeries including mastectomy, breast reconstruction,
Medical Review Criteria Breast Surgeries Effective Date: November 8, 2016 Subject: Breast Surgeries Policy: HPHC covers medically necessary breast surgeries including mastectomy, breast reconstruction,
Breast Reconstruction Surgery after Mastectomy or Lumpectomy
 Breast Reconstruction Surgery after Mastectomy or Lumpectomy Date of Origin: 11/1998 Last Review Date: 11/25/2017 Effective Date: 11/25/2017 Dates Reviewed: 08/2000, 09/2001, 11/2003, 11/2004, 12/2005,
Breast Reconstruction Surgery after Mastectomy or Lumpectomy Date of Origin: 11/1998 Last Review Date: 11/25/2017 Effective Date: 11/25/2017 Dates Reviewed: 08/2000, 09/2001, 11/2003, 11/2004, 12/2005,
Breast Surgery Corporate Medical Policy
 File name: Breast Surgery File code: UM.SURG.17 Origination: 2016 Last Review: 11/2018 (PA List Review) Next Review: 11/2019 Effective Date: 04/01/2018 Breast Surgery Corporate Medical Policy Description/Summary
File name: Breast Surgery File code: UM.SURG.17 Origination: 2016 Last Review: 11/2018 (PA List Review) Next Review: 11/2019 Effective Date: 04/01/2018 Breast Surgery Corporate Medical Policy Description/Summary
Medical Policy Original Effective Date: Revised Date: 09/26/2018 Page 1 of 26
 Contents: This includes the following items: 1. Breast Reconstruction Following Mastectomy: Page 1 of 26 2. Breast Implant Removal and/or Replacement and Capsulectomy: 3. Breast Reduction Mammaplasty for
Contents: This includes the following items: 1. Breast Reconstruction Following Mastectomy: Page 1 of 26 2. Breast Implant Removal and/or Replacement and Capsulectomy: 3. Breast Reduction Mammaplasty for
BREAST RECONSTRUCTION/REMOVAL AND REPLACEMENT OF IMPLANTS
 Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Breast Reconstruction Options
 Breast Reconstruction Options Natural reconstruction using your ABDOMINAL tissue: TRAM Flap (Transverse Rectus Abdominis Myocutaneous) There are various forms of TRAM flap reconstruction that are commonly
Breast Reconstruction Options Natural reconstruction using your ABDOMINAL tissue: TRAM Flap (Transverse Rectus Abdominis Myocutaneous) There are various forms of TRAM flap reconstruction that are commonly
Reconstruction of the Breast after Cancer An Overview of Procedures and Options by Karen M. Horton, MD, MSc, FRCSC
 Downloaded from Reconstruction of the Breast after Cancer An Overview of Procedures and Options by Karen M. Horton, MD, MSc, FRCSC What is Breast Reconstruction? Reconstruction of the breast involves recreating
Downloaded from Reconstruction of the Breast after Cancer An Overview of Procedures and Options by Karen M. Horton, MD, MSc, FRCSC What is Breast Reconstruction? Reconstruction of the breast involves recreating
CMS Limitations Guide Mammography & Bone Mass Measurement
 Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations guide provides you with the latest changes.
Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations guide provides you with the latest changes.
Current Strategies in Breast Reconstruction
 Current Strategies in Breast Reconstruction Hani Sbitany, MD Assistant Professor of Surgery University of California, San Francisco Division of Plastic and Reconstructive Surgery 12 th Annual School of
Current Strategies in Breast Reconstruction Hani Sbitany, MD Assistant Professor of Surgery University of California, San Francisco Division of Plastic and Reconstructive Surgery 12 th Annual School of
Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate surgical options
 A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate
A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate
Reconstructive Breast Surgery and Management of Breast Implants
 Reconstructive Breast Surgery and Management of Breast Implants Policy Number: 7.01.22 Last Review: 1/2018 Origination: 3/1993 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Reconstructive Breast Surgery and Management of Breast Implants Policy Number: 7.01.22 Last Review: 1/2018 Origination: 3/1993 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
CLINICAL MEDICAL POLICY
 CLINICAL MEDICAL POLICY Policy Name: Faslodex (fulvestrant) Policy Number: MP-044-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
CLINICAL MEDICAL POLICY Policy Name: Faslodex (fulvestrant) Policy Number: MP-044-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
Breast Reconstruction: Current Strategies and Future Opportunities
 Breast Reconstruction: Current Strategies and Future Opportunities Hani Sbitany, MD Assistant Professor of Surgery University of California, San Francisco Division of Plastic and Reconstructive Surgery
Breast Reconstruction: Current Strategies and Future Opportunities Hani Sbitany, MD Assistant Professor of Surgery University of California, San Francisco Division of Plastic and Reconstructive Surgery
Chapter 11 Worksheet Code It
 Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
Prophylactic Mastectomy & Reconstructive Implications
 Prophylactic Mastectomy & Reconstructive Implications Minas T Chrysopoulo, MD PRMA Center For Advanced Breast Reconstruction Prophylactic Mastectomy Surgical removal of one or both breasts to reduce the
Prophylactic Mastectomy & Reconstructive Implications Minas T Chrysopoulo, MD PRMA Center For Advanced Breast Reconstruction Prophylactic Mastectomy Surgical removal of one or both breasts to reduce the
Advances in Breast Surgery. Catherine Campo, D.O. Breast Surgeon Meridian Health System April 17, 2015
 Advances in Breast Surgery Catherine Campo, D.O. Breast Surgeon Meridian Health System April 17, 2015 Objectives Understand the surgical treatment of breast cancer Be able to determine when a lumpectomy
Advances in Breast Surgery Catherine Campo, D.O. Breast Surgeon Meridian Health System April 17, 2015 Objectives Understand the surgical treatment of breast cancer Be able to determine when a lumpectomy
National Medical Policy
 National Medical Policy Subject: Policy Number: Breast Reconstructive Surgery NMP492 Effective Date*: February 2013 Updated: April 2016 This National Medical Policy is subject to the terms in the IMPORTANT
National Medical Policy Subject: Policy Number: Breast Reconstructive Surgery NMP492 Effective Date*: February 2013 Updated: April 2016 This National Medical Policy is subject to the terms in the IMPORTANT
Breast Reconstruction. Westmead Breast Cancer Institute
 Breast Reconstruction Westmead Breast Cancer Institute What is breast reconstruction? Breast reconstruction is a surgical procedure that creates a shape on the chest wall following a mastectomy. Occasionally,
Breast Reconstruction Westmead Breast Cancer Institute What is breast reconstruction? Breast reconstruction is a surgical procedure that creates a shape on the chest wall following a mastectomy. Occasionally,
Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer. Oncoplastic and Reconstructive Surgery
 Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Oncoplastic and Reconstructive Surgery Plastic-reconstructive aspects after mastectomy Versions 2002 2017: Audretsch / Bauerfeind
Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Oncoplastic and Reconstructive Surgery Plastic-reconstructive aspects after mastectomy Versions 2002 2017: Audretsch / Bauerfeind
Breast Restoration Surgery After a mastectomy
 UW MEDICINE PATIENT EDUCATION Breast Restoration Surgery After a mastectomy This handout explains the most common procedures that are used at University of Washington Medical Center (UWMC) to restore a
UW MEDICINE PATIENT EDUCATION Breast Restoration Surgery After a mastectomy This handout explains the most common procedures that are used at University of Washington Medical Center (UWMC) to restore a
Breast Reconstruction
 Steven E. Copit, M.D. Chief- Division of Plastic Surgery Thomas Jefferson University Hospital Philadelphia, PA analysis of The Defect Skin Breast Volume Nipple Areola Complex analysis of The Defect the
Steven E. Copit, M.D. Chief- Division of Plastic Surgery Thomas Jefferson University Hospital Philadelphia, PA analysis of The Defect Skin Breast Volume Nipple Areola Complex analysis of The Defect the
Wound & Burn. Reimbursement & Coding Guide
 Wound & Burn Reimbursement & Coding Guide Wound & Burn Reimbursement and Coding Guide MicroMatrix and Cytal devices facilitate the remodeling of functional, site-appropriate tissue. Comprised of ACell
Wound & Burn Reimbursement & Coding Guide Wound & Burn Reimbursement and Coding Guide MicroMatrix and Cytal devices facilitate the remodeling of functional, site-appropriate tissue. Comprised of ACell
2017 PHYSICIAN CODING GUIDE
 2017 PHYSICIAN CODING GUIDE Breast Repair and/or Reconstruction Surgeon s Procedure Codes 11970 Replacement of tissue expander with permanent prosthesis 8.01 $628.05 +15777 Implantation of biologic implant
2017 PHYSICIAN CODING GUIDE Breast Repair and/or Reconstruction Surgeon s Procedure Codes 11970 Replacement of tissue expander with permanent prosthesis 8.01 $628.05 +15777 Implantation of biologic implant
Breast debridement and closure cpt
 Breast debridement and closure cpt Close Breast debridement cpt code Medicare Billing Guidelines, Medicare payment and reimbursment, Medicare codes. Here is a list of CPT codes and Diagnoses that are.
Breast debridement and closure cpt Close Breast debridement cpt code Medicare Billing Guidelines, Medicare payment and reimbursment, Medicare codes. Here is a list of CPT codes and Diagnoses that are.
Neil J. Zemmel, MD, FACS Steven J. Montante, MD Megan J. Russell, PA-C. Your Guide To BREAST RECONSTRUCTION
 Neil J. Zemmel, MD, FACS Steven J. Montante, MD Megan J. Russell, PA-C Your Guide To BREAST RECONSTRUCTION Introduction The diagnosis of breast cancer begins a journey of making many informed decisions
Neil J. Zemmel, MD, FACS Steven J. Montante, MD Megan J. Russell, PA-C Your Guide To BREAST RECONSTRUCTION Introduction The diagnosis of breast cancer begins a journey of making many informed decisions
Selected Operative Procedure Categories for KNHSS SSI Surveillance
 Selected Operative Procedure Categories for KNHSS SSI Surveillance Breast Surgery Excision of lesion or tissue of breast including radical, modified, or quadrant resection, lumpectomy, incisional biopsy,
Selected Operative Procedure Categories for KNHSS SSI Surveillance Breast Surgery Excision of lesion or tissue of breast including radical, modified, or quadrant resection, lumpectomy, incisional biopsy,
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander.
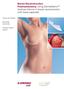 Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): July 26, 2011 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Original Issue Date (Created): July 26, 2011 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
In a second stage or a second operation that tissue expander is removed through the same incision and the implant is placed within the chest pocket.
 Hello, I m Summer Hanson. I m an assistant professor in the Department of Plastics & Reconstructive Surgery at The University of Texas MD Anderson Cancer Center and today I m going to talk about the role
Hello, I m Summer Hanson. I m an assistant professor in the Department of Plastics & Reconstructive Surgery at The University of Texas MD Anderson Cancer Center and today I m going to talk about the role
Goals of Care. Restore shape and function after cancer
 Goals of Care Restore shape and function after cancer Aid in physiological and psychological benefit Relationship with significant other Self esteem and positive body image Feeling of a whole body Avoid
Goals of Care Restore shape and function after cancer Aid in physiological and psychological benefit Relationship with significant other Self esteem and positive body image Feeling of a whole body Avoid
The Case FOR Oncoplastic Surgery in Small Breasts. Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA
 The Case FOR Oncoplastic Surgery in Small Breasts Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA Changing issues in breast cancer management Early detection
The Case FOR Oncoplastic Surgery in Small Breasts Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA Changing issues in breast cancer management Early detection
Reconstructive and Cosmetic Services
 Reconstructive and Cosmetic Services Policy Number: 10.01.09 Last Review: 4/2018 Origination: 2/2006 Next Review: 4/2019 Policy Determination of whether a proposed therapy would be considered reconstructive
Reconstructive and Cosmetic Services Policy Number: 10.01.09 Last Review: 4/2018 Origination: 2/2006 Next Review: 4/2019 Policy Determination of whether a proposed therapy would be considered reconstructive
Classification System
 Classification System A graduate of the Breast Oncology training program should be able to care for all aspects of disease and/or provide comprehensive management. When referring to a discipline of training
Classification System A graduate of the Breast Oncology training program should be able to care for all aspects of disease and/or provide comprehensive management. When referring to a discipline of training
How To Make a Good Mastectomy for Reconstruction Based on the Anatomy. Zhang Jin, Ph.D MD
 How To Make a Good Mastectomy for Reconstruction Based on the Anatomy Zhang Jin, Ph.D MD Deputy Director and Professor Tianjin Medical University Cancer Institute and Hospital People s Republic of China
How To Make a Good Mastectomy for Reconstruction Based on the Anatomy Zhang Jin, Ph.D MD Deputy Director and Professor Tianjin Medical University Cancer Institute and Hospital People s Republic of China
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons
 Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
Exercise & Breast Cancer Recovery
 Exercise & Breast Cancer Recovery LEARNING OBJECTIVES Demonstrate an understanding of the diagnosis and treatment of breast cancer Demonstrate an understanding of how breast cancer surgery and treatment
Exercise & Breast Cancer Recovery LEARNING OBJECTIVES Demonstrate an understanding of the diagnosis and treatment of breast cancer Demonstrate an understanding of how breast cancer surgery and treatment
Supplementary Online Content
 Supplementary Online Content Abt NB, Flores JM, Baltodano PA, et al. Neoadjuvant chemotherapy and short-term in patients undergoing mastectomy with and without breast reconstruction. JAMA Surg. Published
Supplementary Online Content Abt NB, Flores JM, Baltodano PA, et al. Neoadjuvant chemotherapy and short-term in patients undergoing mastectomy with and without breast reconstruction. JAMA Surg. Published
Frederick J. Duffy, Jr., MD, FACS and Brice W. McKane, MD, FACS BREAST RECONSTRUCTION
 Frederick J. Duffy, Jr., MD, FACS and Brice W. McKane, MD, FACS BREAST RECONSTRUCTION BREAST RECONSTRUCTION: A WOMAN S DECISION Options and Information Our approach to breast reconstruction entails a very
Frederick J. Duffy, Jr., MD, FACS and Brice W. McKane, MD, FACS BREAST RECONSTRUCTION BREAST RECONSTRUCTION: A WOMAN S DECISION Options and Information Our approach to breast reconstruction entails a very
2012 Head and Neck Reconstruction/ENT Repair Coding Observations
 Health Policy, Economics & Reimbursement Reimbursement Hotline Tel: 888.543.3656 Fax: 866.262.6977 reimbursement@lifecell.com www.lifecell.com 2012 Head and Neck Reconstruction/ENT Repair Coding Observations
Health Policy, Economics & Reimbursement Reimbursement Hotline Tel: 888.543.3656 Fax: 866.262.6977 reimbursement@lifecell.com www.lifecell.com 2012 Head and Neck Reconstruction/ENT Repair Coding Observations
Position Statement Treatments that primarily affect the appearance are considered medically necessary only in the following circumstances:
 Policy Name: Cosmetic Services Policy Number: CMO 500 Effective Date of current policy: 9/1/2018 Description and Scope This policy applies to procedures that primarily affect the appearance of the member.
Policy Name: Cosmetic Services Policy Number: CMO 500 Effective Date of current policy: 9/1/2018 Description and Scope This policy applies to procedures that primarily affect the appearance of the member.
Corporate Medical Policy
 Corporate Medical Policy Breast Surgeries File Name: Origination: Last CAP Review: Next CAP Review: Last Review: breast_surgeries 1/2000 8/2017 8/2018 8/2017 Description of Procedure or Service Mastectomy
Corporate Medical Policy Breast Surgeries File Name: Origination: Last CAP Review: Next CAP Review: Last Review: breast_surgeries 1/2000 8/2017 8/2018 8/2017 Description of Procedure or Service Mastectomy
National Mastectomy & Breast Reconstruction Audit Datasheet - Mastectomy +/- Immediate Reconstruction
 Patient Registration data Surname Forename NHS/Private Hospital Number Date of birth Postcode Ethnicity Patient-reported outcomes consent Has this patient consented to being sent outcome questionnaires?
Patient Registration data Surname Forename NHS/Private Hospital Number Date of birth Postcode Ethnicity Patient-reported outcomes consent Has this patient consented to being sent outcome questionnaires?
COPE Library Sample
 Breast Anatomy LOBULE LOBE ACINI (MILK PRODUCING UNITS) NIPPLE AREOLA COMPLEX ENLARGEMENT OF DUCT AND LOBE LOBULE SUPRACLAVICULAR NODES INFRACLAVICULAR NODES DUCT DUCT ACINI (MILK PRODUCING UNITS) 8420
Breast Anatomy LOBULE LOBE ACINI (MILK PRODUCING UNITS) NIPPLE AREOLA COMPLEX ENLARGEMENT OF DUCT AND LOBE LOBULE SUPRACLAVICULAR NODES INFRACLAVICULAR NODES DUCT DUCT ACINI (MILK PRODUCING UNITS) 8420
Cigna Medical Coverage Policy
 Cigna Medical Coverage Policy Subject Breast Reconstruction Following Mastectomy or Lumpectomy Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 19 References...
Cigna Medical Coverage Policy Subject Breast Reconstruction Following Mastectomy or Lumpectomy Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 19 References...
Breast Reconstruction: Patient Information Document
 breastreconstructioncanada.ca Breast Reconstruction: Patient Information Document By Dr. Nicolas Guay Dr. Haemi Lee STANDARDIZED BREAST RECONSTRUCTION PATIENT INFORMATION TABLE OF CONTENTS Glossary...
breastreconstructioncanada.ca Breast Reconstruction: Patient Information Document By Dr. Nicolas Guay Dr. Haemi Lee STANDARDIZED BREAST RECONSTRUCTION PATIENT INFORMATION TABLE OF CONTENTS Glossary...
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Breast Reconstruction Following Mastectomy or Lumpectomy
 Medical Coverage Policy Effective Date... 1/15/2018 Next Review Date... 1/15/2019 Coverage Policy Number... 0178 Breast Reconstruction Following Mastectomy or Lumpectomy Table of Contents Related Coverage
Medical Coverage Policy Effective Date... 1/15/2018 Next Review Date... 1/15/2019 Coverage Policy Number... 0178 Breast Reconstruction Following Mastectomy or Lumpectomy Table of Contents Related Coverage
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Extending breast conservation and other new oncoplastic techniques
 Extending breast conservation and other new oncoplastic techniques Dick Rainsbury BSBR 11-12 November 2013 Liverpool What s the maximum volume of the breast which can be resected during lumpectomy without
Extending breast conservation and other new oncoplastic techniques Dick Rainsbury BSBR 11-12 November 2013 Liverpool What s the maximum volume of the breast which can be resected during lumpectomy without
Post-mastectomy breast reconstruction
 Follow the link from the online version of this article to obtain certified continuing medical education credits Post-mastectomy breast reconstruction Paul T R Thiruchelvam, 1 Fiona McNeill, 2 Navid Jallali,
Follow the link from the online version of this article to obtain certified continuing medical education credits Post-mastectomy breast reconstruction Paul T R Thiruchelvam, 1 Fiona McNeill, 2 Navid Jallali,
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference
 2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
Protocol. Reconstructive Breast Surgery/Management of Breast Implants
 Protocol Reconstructive Breast Surgery/Management of Breast Implants Medical Benefit Effective Date: 04/01/14 Next Review Date: 11/18 Preauthorization Yes Review Dates: 02/07, 02/08, 01/09, 01/10, 01/11,
Protocol Reconstructive Breast Surgery/Management of Breast Implants Medical Benefit Effective Date: 04/01/14 Next Review Date: 11/18 Preauthorization Yes Review Dates: 02/07, 02/08, 01/09, 01/10, 01/11,
Case Study. TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis.
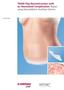 Case Study TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis. TRAM Flap Reconstruction with an Associated Complication Challenge Insulin-dependent diabetes
Case Study TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis. TRAM Flap Reconstruction with an Associated Complication Challenge Insulin-dependent diabetes
Clinical Policy Title: Cosmetic, plastic, and scar revision surgery
 Clinical Policy Title: Cosmetic, plastic, and scar revision surgery Clinical Policy Number: CCP.1184 Effective Date: October 1, 2015 Initial Review Date: August 19, 2015 Most Recent Review Date: September
Clinical Policy Title: Cosmetic, plastic, and scar revision surgery Clinical Policy Number: CCP.1184 Effective Date: October 1, 2015 Initial Review Date: August 19, 2015 Most Recent Review Date: September
INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP
 INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify
INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify
ASPS Recommended Insurance Coverage Criteria for Third- Party Payers
 ASPS Recommended Insurance Coverage Criteria for Third- Party Payers Breast Implant Associated Anaplastic Large Cell Lymphoma BACKGROUND Anaplastic Large Cell Lymphoma (ALCL) is a rare type of cancer of
ASPS Recommended Insurance Coverage Criteria for Third- Party Payers Breast Implant Associated Anaplastic Large Cell Lymphoma BACKGROUND Anaplastic Large Cell Lymphoma (ALCL) is a rare type of cancer of
CLINICAL MEDICATION POLICY
 CLINICAL MEDICATION POLICY Policy Name: Opdivo (nivolumab) injection Policy Number: Approved By: Medical Management, Clinical Pharmacy Products: Highmark Health Options Application: All participating hospitals
CLINICAL MEDICATION POLICY Policy Name: Opdivo (nivolumab) injection Policy Number: Approved By: Medical Management, Clinical Pharmacy Products: Highmark Health Options Application: All participating hospitals
CROSS CODER. Sample page. Anesthesia. codes to ICD-10-CM and HCPCS. Essential links from CPT. Power up your coding optum360coding.
 CROSS CODER 2019 Anesthesia Essential links from CPT codes to ICD-10-CM and HCPCS Power up your coding optum360coding.com Contents Introduction...i CPT Anesthesia to Procedure Code Crosswalk... i Format...
CROSS CODER 2019 Anesthesia Essential links from CPT codes to ICD-10-CM and HCPCS Power up your coding optum360coding.com Contents Introduction...i CPT Anesthesia to Procedure Code Crosswalk... i Format...
(https://www.aetna.com/) Number: Policy *Please see amendment for Pennsylvania Medicaid at the end
 (https://www.aetna.com/) Number: 0185 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. Aetna considers reconstructive breast surgery medically necessary after a medically
(https://www.aetna.com/) Number: 0185 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. Aetna considers reconstructive breast surgery medically necessary after a medically
Related Policies None
 Medical Policy MP 7.01.153 BCBSA Ref. Policy: 7.01.153 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 7.01.153 BCBSA Ref. Policy: 7.01.153 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Author's response to reviews
 Author's response to reviews Title: Classifying breast cancer surgery: a novel, complexity-based system for oncological, oncoplastic and reconstructive procedures, and proof of principle by analysis of
Author's response to reviews Title: Classifying breast cancer surgery: a novel, complexity-based system for oncological, oncoplastic and reconstructive procedures, and proof of principle by analysis of
Advances in Localized Breast Cancer
 Advances in Localized Breast Cancer Melissa Camp, MD, MPH and Fariba Asrari, MD June 18, 2018 Moderated by Elissa Bantug 1 Advances in Surgery for Breast Cancer Melissa Camp, MD June 18, 2018 2 Historical
Advances in Localized Breast Cancer Melissa Camp, MD, MPH and Fariba Asrari, MD June 18, 2018 Moderated by Elissa Bantug 1 Advances in Surgery for Breast Cancer Melissa Camp, MD June 18, 2018 2 Historical
Coding Companion for Podiatry. A comprehensive illustrated guide to coding and reimbursement
 Coding Companion for Podiatry comprehensive illustrated guide to coding and reimbursement 2016 Contents Contents Foot and Toes 28043-28045 [28039, 28041] 28043 28039 28045 28041 Excision, tumor, soft tissue
Coding Companion for Podiatry comprehensive illustrated guide to coding and reimbursement 2016 Contents Contents Foot and Toes 28043-28045 [28039, 28041] 28043 28039 28045 28041 Excision, tumor, soft tissue
Blepharoptosis repair is covered as functional/reconstructive surgery to correct: Visual impairment due to droop or displacement of the upper lid.
 Premier Health Insuring Corporation POLICY AND PROCEDURE MANUAL MP.074.PC - Blepharoplasty This policy applies to the following line(s) of business: Premier Health Insuring Corporation MA DSNP Premier
Premier Health Insuring Corporation POLICY AND PROCEDURE MANUAL MP.074.PC - Blepharoplasty This policy applies to the following line(s) of business: Premier Health Insuring Corporation MA DSNP Premier
rupture, you may notice silicone in their lymph nodes on radiographs. This may be seen and help us detect that there is a rupture.
 Hello. I m Melissa Crosby. I m an Associate Professor at The University of Texas MD Anderson Cancer Center in the Department of Plastic Surgery. I d like to discuss with you the Late Effects of Breast
Hello. I m Melissa Crosby. I m an Associate Professor at The University of Texas MD Anderson Cancer Center in the Department of Plastic Surgery. I d like to discuss with you the Late Effects of Breast
The progress in microsurgical procedures has led
 Original Article Breast reconstruction with free anterolateral thigh flap Ranjit Raje, Ramesh Chepauk, Kanti Shetty, Rajendra Prasad J. S. Plastic & Reconstructive Services, Department of Surgical Oncology,
Original Article Breast reconstruction with free anterolateral thigh flap Ranjit Raje, Ramesh Chepauk, Kanti Shetty, Rajendra Prasad J. S. Plastic & Reconstructive Services, Department of Surgical Oncology,
Sponsored by: INOVA August 19, Presented by: Teri Romano, RN, MBA, CPC, CMDP CONNECT WITH US AT
 Sponsored by: INOVA August 19, 2015 Presented by: Teri Romano, RN, MBA, CPC, CMDP CONNECT WITH US AT WWW.KARENZUPKO.COM 2 1 FOCUS Documentation! Diagnosis codes that get paid. Diagnosis codes that accurately
Sponsored by: INOVA August 19, 2015 Presented by: Teri Romano, RN, MBA, CPC, CMDP CONNECT WITH US AT WWW.KARENZUPKO.COM 2 1 FOCUS Documentation! Diagnosis codes that get paid. Diagnosis codes that accurately
MICHAEL R. ZENN, M.D. INFORMATION ABOUT BREAST RECONSTRUCTION
 MICHAEL R. ZENN, M.D. INFORMATION ABOUT BREAST RECONSTRUCTION The purpose of breast reconstruction is to restore body image and to enable you to wear all types of clothes without restriction. Most women
MICHAEL R. ZENN, M.D. INFORMATION ABOUT BREAST RECONSTRUCTION The purpose of breast reconstruction is to restore body image and to enable you to wear all types of clothes without restriction. Most women
AESTHETIC SURGERY OF THE BREAST: MASTOPEXY, AUGMENTATION & REDUCTION
 CHAPTER 18 AESTHETIC SURGERY OF THE BREAST: MASTOPEXY, AUGMENTATION & REDUCTION Ali A. Qureshi, MD and Smita R. Ramanadham, MD Aesthetic surgery of the breast aims to either correct ptosis with a mastopexy,
CHAPTER 18 AESTHETIC SURGERY OF THE BREAST: MASTOPEXY, AUGMENTATION & REDUCTION Ali A. Qureshi, MD and Smita R. Ramanadham, MD Aesthetic surgery of the breast aims to either correct ptosis with a mastopexy,
Quality ID #263: Preoperative Diagnosis of Breast Cancer National Quality Strategy Domain: Effective Clinical Care
 Quality ID #263: Preoperative Diagnosis of Breast Cancer National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION:
Quality ID #263: Preoperative Diagnosis of Breast Cancer National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION:
Lesion Imaging Characteristics Mass, Favoring Benign Circumscribed Margins Intramammary Lymph Node
 Lesion Imaging Characteristics Mass, Favoring Benign Circumscribed Margins Intramammary Lymph Node Oil Cyst Mass, Intermediate Concern Microlobulated Margins Obscured Margins Mass, Favoring Malignant Indistinct
Lesion Imaging Characteristics Mass, Favoring Benign Circumscribed Margins Intramammary Lymph Node Oil Cyst Mass, Intermediate Concern Microlobulated Margins Obscured Margins Mass, Favoring Malignant Indistinct
The decision to repair a partial mastectomy CME. State of the Art and Science in Postmastectomy Breast Reconstruction.
 CME State of the Art and Science in Postmastectomy Breast Reconstruction Steven J. Kronowitz, M.D. Houston, Texas Learning Objectives: After reading this article, the participant should be able to: 1.
CME State of the Art and Science in Postmastectomy Breast Reconstruction Steven J. Kronowitz, M.D. Houston, Texas Learning Objectives: After reading this article, the participant should be able to: 1.
Reconstructive Breast Surgery following Mastectomy for Breast Cancer: A Review
 Research Article http://www.alliedacademies.org/advanced-surgical-research/ Reconstructive Breast Surgery following Mastectomy for Breast Cancer: A Review Gurnam Virdi* Department of surgery, Queen Elizabeth
Research Article http://www.alliedacademies.org/advanced-surgical-research/ Reconstructive Breast Surgery following Mastectomy for Breast Cancer: A Review Gurnam Virdi* Department of surgery, Queen Elizabeth
Figure 1. Anatomy of the breast
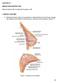 CHAPTER 12 BREAST RECONSTRUCTION Mihaela Rapolti, MD and Michelle Roughton, MD I. BREAST ANATOMY A. Mastering breast anatomy is essential for understanding how the breast changes with aging and principles
CHAPTER 12 BREAST RECONSTRUCTION Mihaela Rapolti, MD and Michelle Roughton, MD I. BREAST ANATOMY A. Mastering breast anatomy is essential for understanding how the breast changes with aging and principles
Payment Policy: Cosmetic Procedures Reference Number: CC.PP.024 Product Types: ALL
 Payment Policy: Cosmetic Procedures Reference Number: CC.PP.024 Product Types: ALL Effective Date: 01/01/2014 Last Review Date: 06/20/2018 Coding Implications Revision Log See Important Reminder at the
Payment Policy: Cosmetic Procedures Reference Number: CC.PP.024 Product Types: ALL Effective Date: 01/01/2014 Last Review Date: 06/20/2018 Coding Implications Revision Log See Important Reminder at the
ONCOPLASTIC SURGERY. Dr. Sadir Alrawi Director of Surgical Oncology Services. Dr. Humaa Darr Surgical Oncology Fellow
 Hessa St ONCOPLASTIC SURGERY Dr. Sadir Alrawi Director of Surgical Oncology Services Dr. Humaa Darr Surgical Oncology Fellow Al Sufouh Rd AL SUFOUH AL SUFOUH Sharaf DG Mall of the Emirates Mall Of the
Hessa St ONCOPLASTIC SURGERY Dr. Sadir Alrawi Director of Surgical Oncology Services Dr. Humaa Darr Surgical Oncology Fellow Al Sufouh Rd AL SUFOUH AL SUFOUH Sharaf DG Mall of the Emirates Mall Of the
SIMPOSIO Ricostruzione mammaria ed implicazioni radioterapiche Indicazioni
 SIMPOSIO Ricostruzione mammaria ed implicazioni radioterapiche Indicazioni Icro Meattini, MD Radiation Oncology Department - University of Florence Azienda Ospedaliero Universitaria Careggi Firenze Breast
SIMPOSIO Ricostruzione mammaria ed implicazioni radioterapiche Indicazioni Icro Meattini, MD Radiation Oncology Department - University of Florence Azienda Ospedaliero Universitaria Careggi Firenze Breast
MEDICAL POLICY No R10 BREAST RELATED PROCEDURES*
 BREAST RELATED PROCEDURES* Effective Date: June 19, 2017 Review Dates: 8/07, 8/08, 8/09, 4/10, 6/10, 8/10, 8/11, 8/12, 6/13, 8/14, 8/15, 2/16, 2/17 Date of Origin: August 8, 2007 Status: Current *This
BREAST RELATED PROCEDURES* Effective Date: June 19, 2017 Review Dates: 8/07, 8/08, 8/09, 4/10, 6/10, 8/10, 8/11, 8/12, 6/13, 8/14, 8/15, 2/16, 2/17 Date of Origin: August 8, 2007 Status: Current *This
2017 Hospital Coding and Payment Guide
 Reimbursement Men s Health 2017 Hospital Coding and Payment Guide This coding reference guide is intended to illustrate the common coding and payment groups for male prosthetic urology procedures and related
Reimbursement Men s Health 2017 Hospital Coding and Payment Guide This coding reference guide is intended to illustrate the common coding and payment groups for male prosthetic urology procedures and related
NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION
 NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION 42 yo female healthy athlete Right breast mass. Past medical history: none Family history: aunt with Breast cancer Candidates for nipple-sparing mastectomy
NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION 42 yo female healthy athlete Right breast mass. Past medical history: none Family history: aunt with Breast cancer Candidates for nipple-sparing mastectomy
Breast Cancer Reconstruction
 Breast Cancer Jerome H. Liu, MD Tom S. Liu, MD Jerome H. Liu, MD Undergraduate: Brown University Medical School: University of California, Los Angeles Residency: UCLA Medical Center Fellowship:UCLA Medical
Breast Cancer Jerome H. Liu, MD Tom S. Liu, MD Jerome H. Liu, MD Undergraduate: Brown University Medical School: University of California, Los Angeles Residency: UCLA Medical Center Fellowship:UCLA Medical
CLINICAL MEDICAL POLICY
 CLINICAL MEDICAL POLICY Policy Name: Avastin (bevacizumab) Policy Number: MP-030-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
CLINICAL MEDICAL POLICY Policy Name: Avastin (bevacizumab) Policy Number: MP-030-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
Departmental Segregated Total Form for Plastic and Reconstructive Surgery
 Departmental Segregated Total Form for Plastic and Reconstructive Surgery American Osteopathic Association and the American College of Osteopathic Surgeons Revised, COPT 11/2001 Revised, BOT 2/2006, Effective,
Departmental Segregated Total Form for Plastic and Reconstructive Surgery American Osteopathic Association and the American College of Osteopathic Surgeons Revised, COPT 11/2001 Revised, BOT 2/2006, Effective,
TOTAL Head and Neck Congenital Defects 50
 Operative Minimums Effective July 1, 2014 Review Committee for Plastic Surgery NOTE: The index procedure number for Laser is tracked by Total Laser and not by the subcategories of Aesthetic Laser and Reconstructive
Operative Minimums Effective July 1, 2014 Review Committee for Plastic Surgery NOTE: The index procedure number for Laser is tracked by Total Laser and not by the subcategories of Aesthetic Laser and Reconstructive
PROS AND CONS OF IMMEDIATE PROSTHETIC IMPLANTS VS USE OF EXPANDER FOR POST MASTECTOMY BREAST RECONSTRUCTIONS
 PROS AND CONS OF IMMEDIATE PROSTHETIC IMPLANTS VS USE OF EXPANDER FOR POST MASTECTOMY BREAST Dr Tienie van Rooyen Mediclinic Kloof Hospital Pretoria IMMEDIATE Since 1990 s Skin sparing mastectomies proven
PROS AND CONS OF IMMEDIATE PROSTHETIC IMPLANTS VS USE OF EXPANDER FOR POST MASTECTOMY BREAST Dr Tienie van Rooyen Mediclinic Kloof Hospital Pretoria IMMEDIATE Since 1990 s Skin sparing mastectomies proven
Breast Augmentation - Silicone Implants
 Breast Augmentation - Silicone Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics,
Breast Augmentation - Silicone Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics,
Endoscopic assisted harvest of the pedicled pectoralis major muscle flap
 British Journal of Plastic Surgery (2005) 58, 170 174 Endoscopic assisted harvest of the pedicled pectoralis major muscle flap Arif Turkmen*, A. Graeme B. Perks Plastic Surgery Department, Nottingham City
British Journal of Plastic Surgery (2005) 58, 170 174 Endoscopic assisted harvest of the pedicled pectoralis major muscle flap Arif Turkmen*, A. Graeme B. Perks Plastic Surgery Department, Nottingham City
Medical Policy Breast Surgeries
 Medical Policy Breast Surgeries Document Number: 006 Commercial and MassHealth Connector/Qualified Health Plans Authorization required Breast Reconstruction Surgeries; Reduction Mammoplasty, Female Members;
Medical Policy Breast Surgeries Document Number: 006 Commercial and MassHealth Connector/Qualified Health Plans Authorization required Breast Reconstruction Surgeries; Reduction Mammoplasty, Female Members;
Mitchell Buller, MEng, a Adee Heiman, BA, a Jared Davis, MD, b ThomasJ.Lee,MD, b Nicolás Ajkay, MD, FACS, c and Bradon J. Wilhelmi, MD, FACS b
 Immediate Breast Reconstruction of a Nipple Areolar Lumpectomy Defect With the L-Flap Skin Paddle Breast Reduction Design and Contralateral Reduction Mammoplasty Symmetry Procedure: Optimizing the Oncoplastic
Immediate Breast Reconstruction of a Nipple Areolar Lumpectomy Defect With the L-Flap Skin Paddle Breast Reduction Design and Contralateral Reduction Mammoplasty Symmetry Procedure: Optimizing the Oncoplastic
DermACELL AWM Comprehensive Reimbursement Resource Guide. Prepared by Musculoskeletal Clinical Regulatory Advisers, LLC. Version 01/2018.
 2018 Comprehensive Reimbursement Resource Guide Prepared by Musculoskeletal Clinical Regulatory Advisers, LLC. Version 01/2018. DermACELL AWM Disclaimer: This information is for educational/informational
2018 Comprehensive Reimbursement Resource Guide Prepared by Musculoskeletal Clinical Regulatory Advisers, LLC. Version 01/2018. DermACELL AWM Disclaimer: This information is for educational/informational
Plastic surgery of the breast includes; augmentation, reduction, Plastic Surgery of the Breast. Abstract. Continuing Education Column
 Plastic Surgery of the Breast Keuk Shun Shin, M.D. Keuk SHUN SHIN s Asthetic Plastic Surgery E mail: drsks@drsks.co.kr Abstract Plastic surgery of the breast includes; augmentation, reduction, reconstruction
Plastic Surgery of the Breast Keuk Shun Shin, M.D. Keuk SHUN SHIN s Asthetic Plastic Surgery E mail: drsks@drsks.co.kr Abstract Plastic surgery of the breast includes; augmentation, reduction, reconstruction
