SNAQ BCR- ABL PILOT. AccuGenomics Inc.
|
|
|
- Anastasia George
- 5 years ago
- Views:
Transcription
1 SNAQ BCR- ABL PILOT AccuGenomics Inc.
2 Unmet Need for Molecular Testing Standardization Currently, the quantitative definition of a significant rise in BCR-ABL RNA (to warrant mutation analysis) is controversial and varies among laboratories. The Oncologist 2010;15: However, because PCR negativity is poorly standardized among laboratories and varies depending on the sensitivity of PCR assays, the definition of CMR continues to evolve, and confirming a consistent prognostic value for CMR has been problematic. RE: International Standardization : e111-e117 While this process has generally worked well, it is apparent that the establishment of conversion factors is time-consuming, complex, expensive, and open to only a limited number of laboratories at any given time. Furthermore, it is unclear how frequently any individual CF will need to be revalidated. Today most molecular tests are self regulated leading to a patchwork of tests, interpretations and outcomes that arise from lack of standardized measurements and poor QA/QC necessary to deliver accurate results. 4
3 COMPETATIVE PCR (OLD SCHOOL) Controls for variation in PCR efficiency through spike in of a Competitive Template kinetically identical to Native Template Procedure: Add known quantity of CT PCR (CT:NT ratio constant) Measure CT:NT ratio Calculate NT Issue Cumbersome Open tube contamination Limited dynamic range Poor load control Cornelia Piper, et. al. J Am Coll Cardiol. 2000;36(1):
4 Fluorescence Standardized Nucleic Acid Quantification (SNAQ) qpcr Native Template Internal Standard G C C G G C A T Native Template Both Internal Standard Temperature Melting Curve Closed tube Microplate throughput Optical correction Specificity control >600-fold reporting range Load Control Mixtures of competitive templates for targets & reference genes Sufficient for millions of assays
5 SNAQ qpcr BENEFITS Benefits Real-time SNAQ QA/QC Reproducibility Fluidic Handling Pre amplification Meet Unmet Needs + +++
6 SNAQ BCR-ABL1 Pilot Study Workflow QIAamp RNA SuperScript VILO 1.5 ml Blood Mini Kit cdna Synthesis Kit COLLECT BLOOD SNAQ SOFTWARE CALCULATES COPY NUMBER %BCR-ABL/GUSB QA METRICS EXTRACT RNA SYNTHESIZE cdna (20 m l) HotStarTaq Plus PCR & MELTING CURVE Dilute SNAQ REAGENTS Divide cdna into SIX 10uL PCR wells containing master mix and 2 LOW MIS wells 2 MEDIUM MIS wells 2 HIGH MIS wells 7500 Fast DxReal - Time PCR Instrument
7 SNAQ BCR-ABL1 SOFTWARE Over 20 QA tests Reagent setup Instrument setup Assay performance CFR-part 11 compliant
8 SNAQ BCR-ABL1 Linearity BCR-ABL linearity met goal of 7 logs linearity NCCLS EP6-A Evaluation of Linearity of Quantitative Measurement Procedures
9 ABUNDANCE Limit of Detection Limit of Quantification 1.b2a2 2.b2a2 3.b2a2 1.b3a2 2.b3a2 3.b3a2 DILUTION Assay met goal of 10 copies LOD & LOQ EP17-A Protocols for Determination of Limits of Detection and Limits of Quantitation
10 MEASURED B2A2/GUSB (LOG) MEASURED B3A2/GUSB (LOG) Interday Reproducibility A. 2 B DAYS x 2 SAMPLES DAYS x 2 sample -2-3 y = x R² = y = x R² = EXPECTED B2A2/GUSB (LOG) EXPECTED B3A2/GUSB (LOG) Copies CV 24% 24% 17% 12% 6% 6% 5% Copies CV 18% 19% 16% 8% 4% 3% 3% BCR-ABL1 Assays met an internal goal of better than 2-fold accuracy, leaving room for additional bias and impression introduced when testing real-world samples.
11 SNAQ BCR-ABL1 Kit Stability Kit -20 C (ongoing) over 30 months
12 Pilot Study Goals Primary Goal Clinical Validation Secondary Goal Estimate Limit of Agreement (half-log) Estimate clinical LOQ (MR4.5) Further tweaking?
13 Pilot Study Protocol Collect blood from up to 50 MD Anderson CML patients expected to be PCR positive 5 ml EDTA blood draws, kept at 4ºC until processed to lysates within 48 hours Samples shipped to two CLIA laboratories, each measured %BCR-ABL using CLIA regulated laboratory developed test and SNAQ BCR-ABL1 reported in International Scale
14 Sample Summary Blood samples collected over 6 months 37 samples had measurements by all four methods 27 with quantitative results Three outliers detected in Lab #2 LDT were removed from analysis
15 LAB 2(LOG %BCR-ABL) %BCR-ABL METHOD CORRELATIONS LDT -2 SNAQ -2.5 LAB 1 (LOG %BCR-ABL) SNAQ correlation with LDT >0.95 R 2
16 Limits of Agreement Comparisons with Lab 1 LDT Remaining Comparisons LDT methods demonstrated 7-fold bias and possible differential response SNAQ demonstrated no bias with a ±3-fold limit of agreement SNAQ BCR-ABL1 met goal of half log accuracy.
17 Clinical Sensitivity Estimate Sensitivity estimated from GusB pilot study measurements MR4.5 sensitivity possible
18 Summary SNAQ BCR-ABL method demonstrated significant correlation with two laboratory developed tests SNAQ BCR-ABL method supports ±3-fold Limit of Agreement LDTs Limit of Agreement suffered from outliers, bias and differential response SNAQ BCR-ABL method has an estimated LOQ of MR4.5
19 Acknowledgements MD Anderson Jorge Cortes Rajyalakshmi Luthra Talha Badar Hagop Kantarjian Elias Jabbour Gautam Borthakur Guillermo Garcia- Manero Xuelin Huang Rajesh Singh Brittany Alvarez Keyur Patel AccuGenomics Bradley Austermiller Tom B Morrison
Molecular Detection of BCR/ABL1 for the Diagnosis and Monitoring of CML
 Molecular Detection of BCR/ABL1 for the Diagnosis and Monitoring of CML Imran Mirza, MD, MS, FRCPC Pathology & Laboratory Medicine Institute Sheikh Khalifa Medical City, Abu Dhabi, UAE. imirza@skmc.ae
Molecular Detection of BCR/ABL1 for the Diagnosis and Monitoring of CML Imran Mirza, MD, MS, FRCPC Pathology & Laboratory Medicine Institute Sheikh Khalifa Medical City, Abu Dhabi, UAE. imirza@skmc.ae
Phosphate buffered saline (PBS) for washing the cells TE buffer (nuclease-free) ph 7.5 for use with the PrimePCR Reverse Transcription Control Assay
 Catalog # Description 172-5080 SingleShot Cell Lysis Kit, 100 x 50 µl reactions 172-5081 SingleShot Cell Lysis Kit, 500 x 50 µl reactions For research purposes only. Introduction The SingleShot Cell Lysis
Catalog # Description 172-5080 SingleShot Cell Lysis Kit, 100 x 50 µl reactions 172-5081 SingleShot Cell Lysis Kit, 500 x 50 µl reactions For research purposes only. Introduction The SingleShot Cell Lysis
Single Cell Quantitative Polymer Chain Reaction (sc-qpcr)
 Single Cell Quantitative Polymer Chain Reaction (sc-qpcr) Analyzing gene expression profiles from a bulk population of cells provides an average profile which may obscure important biological differences
Single Cell Quantitative Polymer Chain Reaction (sc-qpcr) Analyzing gene expression profiles from a bulk population of cells provides an average profile which may obscure important biological differences
Storage: Logix Smart Zika Virus Master Mix and Logix Smart Zika Virus Positive Control must be stored at -20 ⁰C and can last up to 60 days.
 Logix Smart Zika Virus (ZIKV-K-003; ZIKV-PC-003; GEN-NTC-001) Description The Logix Smart Zika Virus Test developed by Co-Diagnostics, Inc. detects ribonucleic acid (RNA) of Zika Virus in a single step
Logix Smart Zika Virus (ZIKV-K-003; ZIKV-PC-003; GEN-NTC-001) Description The Logix Smart Zika Virus Test developed by Co-Diagnostics, Inc. detects ribonucleic acid (RNA) of Zika Virus in a single step
MRD in CML (BCR-ABL1)
 MRD in CML (BCR-ABL1) Moleculaire Biologie en Cytometrie cursus Barbara Denys LAbo Hematologie UZ Gent 6 mei 2011 2008 Universitair Ziekenhuis Gent 1 Myeloproliferative Neoplasms o WHO classification 2008:
MRD in CML (BCR-ABL1) Moleculaire Biologie en Cytometrie cursus Barbara Denys LAbo Hematologie UZ Gent 6 mei 2011 2008 Universitair Ziekenhuis Gent 1 Myeloproliferative Neoplasms o WHO classification 2008:
Analytical and economic evaluation of the fully automated Xpert BCR-ABL assay from Cepheid
 Analytical and economic evaluation of the fully automated Xpert BCR-ABL assay from Cepheid Jean-Michel Cayuela Saint-Louis hospital Paris July 5, 2012 Talk outline Chronic myeloid leukemia > Genetic bases,
Analytical and economic evaluation of the fully automated Xpert BCR-ABL assay from Cepheid Jean-Michel Cayuela Saint-Louis hospital Paris July 5, 2012 Talk outline Chronic myeloid leukemia > Genetic bases,
Ambient Temperature Stabilization of RNA derived from Jurkat, HeLa and HUVEC Cell Lines for Use in RT-qPCR Assays
 Ambient Temperature Stabilization of RNA derived from Jurkat, HeLa and HUVEC Cell Lines for Use in RT-qPCR Assays C. Litterst 1, H. Martinez, B. Iverson and R. Nuňez 1 Bio-Rad Laboratories, Life Science
Ambient Temperature Stabilization of RNA derived from Jurkat, HeLa and HUVEC Cell Lines for Use in RT-qPCR Assays C. Litterst 1, H. Martinez, B. Iverson and R. Nuňez 1 Bio-Rad Laboratories, Life Science
Clinical Utility of Droplet ddpcr, moving to diagnostics. Koen De Gelas, PhD, CRIG ddpcr mini symposium, 15/05/2018
 1 Clinical Utility of Droplet ddpcr, moving to diagnostics 2 Koen De Gelas, PhD, CRIG ddpcr mini symposium, 15/05/2018 Disclaimer Disclaimer: all consumables, instruments, applications and software covered
1 Clinical Utility of Droplet ddpcr, moving to diagnostics 2 Koen De Gelas, PhD, CRIG ddpcr mini symposium, 15/05/2018 Disclaimer Disclaimer: all consumables, instruments, applications and software covered
MolecularMD. One-Step qrt-pcr BCR-ABL Kit. Product Description and User Manual. For Quantitative RT-PCR Analysis of BCR-ABL. Contact Us.
 Contact Us If you have any questions for or comments about MolecularMD, please feel free to contact us. Email Customer Service CustomerService@MolecularMD.com Technical Support TechSupport@MolecularMD.com
Contact Us If you have any questions for or comments about MolecularMD, please feel free to contact us. Email Customer Service CustomerService@MolecularMD.com Technical Support TechSupport@MolecularMD.com
HIV-1 Viral Load Real Time (RG)
 -1 Viral Load Real Time (RG) Real Time RT-PCR type 1 RNA quantification assay MSP Reg. pending Valdense 3616. 11700. Montevideo. Uruguay. phone (598) 2 336 83 01. Fax (598) 2 336 71 60. Info@atgen.com.uy
-1 Viral Load Real Time (RG) Real Time RT-PCR type 1 RNA quantification assay MSP Reg. pending Valdense 3616. 11700. Montevideo. Uruguay. phone (598) 2 336 83 01. Fax (598) 2 336 71 60. Info@atgen.com.uy
For in vitro Veterinary Diagnostics only. Kylt Rotavirus A. Real-Time RT-PCR Detection.
 For in vitro Veterinary Diagnostics only. Kylt Rotavirus A Real-Time RT-PCR Detection www.kylt.eu DIRECTION FOR USE Kylt Rotavirus A Real-Time RT-PCR Detection A. General Kylt Rotavirus A products are
For in vitro Veterinary Diagnostics only. Kylt Rotavirus A Real-Time RT-PCR Detection www.kylt.eu DIRECTION FOR USE Kylt Rotavirus A Real-Time RT-PCR Detection A. General Kylt Rotavirus A products are
Technical Bulletin No. 161
 CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 161 cobas 6800 HIV-1 Viral Load Assay - New Platform - June 1, 2017 Contact: Heather Habig, MLS (ASCP) CM, MB CM, 717-851-1422 Operations
CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 161 cobas 6800 HIV-1 Viral Load Assay - New Platform - June 1, 2017 Contact: Heather Habig, MLS (ASCP) CM, MB CM, 717-851-1422 Operations
Human Neurology 3-Plex A
 Human Neurology 3-Plex A SUMMARY AND EXPLANATION OF THE TEST The Human N3PA assay is a digital immunoassay for the quantitative determination of total Tau, Aβ42, and Aβ40 in human plasma and CSF. Determination
Human Neurology 3-Plex A SUMMARY AND EXPLANATION OF THE TEST The Human N3PA assay is a digital immunoassay for the quantitative determination of total Tau, Aβ42, and Aβ40 in human plasma and CSF. Determination
Leukemia BCR-ABL Fusion Gene Real Time RT-PCR Kit
 Revision No.: ZJ0003 Issue Date: Aug 7 th, 2008 Leukemia BCR-ABL Fusion Gene Real Time RT-PCR Kit Cat. No.: TR-0126-02 For use with ABI Prism 7000/7300/7500/7900(96 well); Smart Cycler II; icycler iq 4/iQ
Revision No.: ZJ0003 Issue Date: Aug 7 th, 2008 Leukemia BCR-ABL Fusion Gene Real Time RT-PCR Kit Cat. No.: TR-0126-02 For use with ABI Prism 7000/7300/7500/7900(96 well); Smart Cycler II; icycler iq 4/iQ
Droplet Digital PCR, the new tool in HIV reservoir quantification? Ward De Spiegelaere
 Droplet Digital PCR, the new tool in HIV reservoir quantification? Ward De Spiegelaere Droplet Digital PCR, the new tool in HIV reservoir quantification? Content: - Digital PCR - Applications - Total HIV
Droplet Digital PCR, the new tool in HIV reservoir quantification? Ward De Spiegelaere Droplet Digital PCR, the new tool in HIV reservoir quantification? Content: - Digital PCR - Applications - Total HIV
Chlamydia pneumoniae PCR reagents Detection with real time PCR reagents
 Chlamydia pneumoniae PCR reagents Detection with real time PCR reagents Overview:... 1 Products... 2 C. pneumoniae FAM-BHQ1 Primer-probe PP3400 0.055ml... 2 AttoMaster 2X Mix for qpcr AM10 1.25 ml... 2
Chlamydia pneumoniae PCR reagents Detection with real time PCR reagents Overview:... 1 Products... 2 C. pneumoniae FAM-BHQ1 Primer-probe PP3400 0.055ml... 2 AttoMaster 2X Mix for qpcr AM10 1.25 ml... 2
HbA1c (Human) ELISA Kit
 HbA1c (Human) ELISA Kit Cat. No.:DEIA3509 Pkg.Size:96T Intended use GHbA1c (Human) ELISA Kit is a sandwich enzyme immunoassay for the quantitative measurement of human GHbA1c. General Description vhemoglobin,
HbA1c (Human) ELISA Kit Cat. No.:DEIA3509 Pkg.Size:96T Intended use GHbA1c (Human) ELISA Kit is a sandwich enzyme immunoassay for the quantitative measurement of human GHbA1c. General Description vhemoglobin,
Microsart Calibration Reagent
 Instructions for Use Microsart Calibration Reagent Prod. No. SMB95-2021 Mycoplasma arginini Prod. No. SMB95-2022 Mycoplasma orale Prod. No. SMB95-2023 Mycoplasma gallisepticum Prod. No. SMB95-2024 Mycoplasma
Instructions for Use Microsart Calibration Reagent Prod. No. SMB95-2021 Mycoplasma arginini Prod. No. SMB95-2022 Mycoplasma orale Prod. No. SMB95-2023 Mycoplasma gallisepticum Prod. No. SMB95-2024 Mycoplasma
(DNA) Real-time PCR. Exicycler 96 Rotor-Gene Q/6000 PCR
 Real-Time (DNA) Real-time Exicycler 96 Rotor-Gene Q/6000 IU Mix1 Mix2 IU/μl IU/μl IU/μl IU/μl IU/μl IPC NTC C 1 Lot# 2 Freeze & thawing 1 MSDS: Material Safety Data Sheets (TaqMan Real-time ' FAM ' BHQ1
Real-Time (DNA) Real-time Exicycler 96 Rotor-Gene Q/6000 IU Mix1 Mix2 IU/μl IU/μl IU/μl IU/μl IU/μl IPC NTC C 1 Lot# 2 Freeze & thawing 1 MSDS: Material Safety Data Sheets (TaqMan Real-time ' FAM ' BHQ1
For purification of viral DNA and RNA from a wide range of sample materials
 QIAamp virus kits For purification of viral DNA and RNA from a wide range of sample materials Automatable on QIAGEN s proven QIAamp Kits set the standard for purification of viral DNA and RNA. QIAamp virus
QIAamp virus kits For purification of viral DNA and RNA from a wide range of sample materials Automatable on QIAGEN s proven QIAamp Kits set the standard for purification of viral DNA and RNA. QIAamp virus
Technical Bulletin No. 162
 CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 162 cobas 6800 HCV Viral Load Assay - New Platform - June 1, 2017 Contact: Heather Habig, MLS (ASCP) CM, MB CM, 717-851-1422 Operations
CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 162 cobas 6800 HCV Viral Load Assay - New Platform - June 1, 2017 Contact: Heather Habig, MLS (ASCP) CM, MB CM, 717-851-1422 Operations
Circulating Cell-Free DNA Pre-analytics: Importance of ccfdna Stabilization and Extraction for Liquid Biopsy Applications
 Circulating Cell-Free DNA Pre-analytics: Importance of ccfdna Stabilization and Extraction for Liquid Biopsy Applications Martin Schlumpberger, Ass.Director R&D, QIAGEN GmbH IQNpath 2017 - Circulating
Circulating Cell-Free DNA Pre-analytics: Importance of ccfdna Stabilization and Extraction for Liquid Biopsy Applications Martin Schlumpberger, Ass.Director R&D, QIAGEN GmbH IQNpath 2017 - Circulating
Supplementary webappendix
 Supplementary webappendix This webappendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Kratz JR, He J, Van Den Eeden SK, et
Supplementary webappendix This webappendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Kratz JR, He J, Van Den Eeden SK, et
RealLine HIV quantitative Str-Format
 Instructions for use DETECTION AND QUANTIFICATION OF THE HUMAN IMMUNODEFICIENCY VIRUS RNA BY REAL TIME PCR Research Use Only (RUO) Attention! Please read the information about quantification process carefully!
Instructions for use DETECTION AND QUANTIFICATION OF THE HUMAN IMMUNODEFICIENCY VIRUS RNA BY REAL TIME PCR Research Use Only (RUO) Attention! Please read the information about quantification process carefully!
Gentian Canine CRP Immunoassay Application Note for Abbott Architect * c4000
 v2-jan214 Gentian Canine CRP Immunoassay Application Note for Abbott Architect * c4 Intended Use The canine CRP immunoassay on Abbott's Architect c4 is an in vitro diagnostic test for quantitative determination
v2-jan214 Gentian Canine CRP Immunoassay Application Note for Abbott Architect * c4 Intended Use The canine CRP immunoassay on Abbott's Architect c4 is an in vitro diagnostic test for quantitative determination
ipsogen BCR-ABL1 Mbcr Kit Handbook
 March 2015 ipsogen BCR-ABL1 Mbcr Kit Handbook 52 Version 1 Quantitative in vitro diagnostics For use with Rotor-Gene Q, ABI PRISM 7000, 7700, or 7900HT SDS, Applied Biosystems 7500 Real-Time PCR System,
March 2015 ipsogen BCR-ABL1 Mbcr Kit Handbook 52 Version 1 Quantitative in vitro diagnostics For use with Rotor-Gene Q, ABI PRISM 7000, 7700, or 7900HT SDS, Applied Biosystems 7500 Real-Time PCR System,
Supplemental Materials and Methods Plasmids and viruses Quantitative Reverse Transcription PCR Generation of molecular standard for quantitative PCR
 Supplemental Materials and Methods Plasmids and viruses To generate pseudotyped viruses, the previously described recombinant plasmids pnl4-3-δnef-gfp or pnl4-3-δ6-drgfp and a vector expressing HIV-1 X4
Supplemental Materials and Methods Plasmids and viruses To generate pseudotyped viruses, the previously described recombinant plasmids pnl4-3-δnef-gfp or pnl4-3-δ6-drgfp and a vector expressing HIV-1 X4
Norgen s HIV proviral DNA PCR Kit was developed and validated to be used with the following PCR instruments: Qiagen Rotor-Gene Q BioRad icycler
 3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com HIV Proviral DNA PCR Kit Product # 33840 Product Insert Background Information
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com HIV Proviral DNA PCR Kit Product # 33840 Product Insert Background Information
Do Your Flow Cytometric LDTs. Validation Guidelines? Fiona E. Craig, MD University of Pittsburgh School of Medicine
 Do Your Flow Cytometric LDTs Conform to the ICSH ICCS Validation Guidelines? Fiona E. Craig, MD University of Pittsburgh School of Medicine How should LDTs be validated? Accuracy Specificity Sensitivity
Do Your Flow Cytometric LDTs Conform to the ICSH ICCS Validation Guidelines? Fiona E. Craig, MD University of Pittsburgh School of Medicine How should LDTs be validated? Accuracy Specificity Sensitivity
Human diagnostics. Better be Sure: Quantify HDV & HBV viral load. RoboGene product family
 Human diagnostics Better be Sure: Quantify HDV & HBV viral load. RoboGene product family 2 RoboGene Product Family Improved patient management: Standardized monitoring of HBV DNA and HDV RNA viral load.
Human diagnostics Better be Sure: Quantify HDV & HBV viral load. RoboGene product family 2 RoboGene Product Family Improved patient management: Standardized monitoring of HBV DNA and HDV RNA viral load.
A novel isothermal amplification approach for rapid identification of BCR-ABL fusion genes at onset:
 A novel isothermal amplification approach for rapid identification of BCR-ABL fusion genes at onset: Josh Glason: Sales Manager, DiaSorin Australia Pty Ltd September 6, 2014 This product is not currently
A novel isothermal amplification approach for rapid identification of BCR-ABL fusion genes at onset: Josh Glason: Sales Manager, DiaSorin Australia Pty Ltd September 6, 2014 This product is not currently
ipsogen BCR-ABL1 Mbcr Kit Handbook
 March 2015 ipsogen BCR-ABL1 Mbcr Kit Handbook 24 Version 1 Quantitative in vitro diagnostics For use with Rotor-Gene Q, ABI PRISM, LightCycler, and SmartCycler instruments 670123 QIAGEN GmbH, QIAGEN Strasse
March 2015 ipsogen BCR-ABL1 Mbcr Kit Handbook 24 Version 1 Quantitative in vitro diagnostics For use with Rotor-Gene Q, ABI PRISM, LightCycler, and SmartCycler instruments 670123 QIAGEN GmbH, QIAGEN Strasse
RealLine Mycoplasma genitalium Str-Format
 Instructions for use ASSAY KIT FOR THE QUALITATIVE DETECTION OF MYCOPLASMA GENITALIUM DNA BY REAL-TIME PCR METHOD In vitro Diagnostics () VBD4396 96 Tests valid from December 2018 Rev06_1218_EN Page 1
Instructions for use ASSAY KIT FOR THE QUALITATIVE DETECTION OF MYCOPLASMA GENITALIUM DNA BY REAL-TIME PCR METHOD In vitro Diagnostics () VBD4396 96 Tests valid from December 2018 Rev06_1218_EN Page 1
Influenza A viruses Detection with real time RT-PCR reagents
 Influenza A viruses Detection with real time RT-PCR reagents Overview:... 1 Products... 2 Influenza A matix FAM-BHQ1 PP500 0.055ml... 2 Influenza A Plasmid 200 pg/ml PLAS500 0.25ml... 2 Detection Influenza
Influenza A viruses Detection with real time RT-PCR reagents Overview:... 1 Products... 2 Influenza A matix FAM-BHQ1 PP500 0.055ml... 2 Influenza A Plasmid 200 pg/ml PLAS500 0.25ml... 2 Detection Influenza
RayBio Human Granzyme B ELISA Kit
 RayBio Human Granzyme B ELISA Kit Catalog #: ELH-GZMB User Manual Last revised April 15, 2016 Caution: Extraordinarily useful information enclosed ISO 13485 Certified 3607 Parkway Lane, Suite 100 Norcross,
RayBio Human Granzyme B ELISA Kit Catalog #: ELH-GZMB User Manual Last revised April 15, 2016 Caution: Extraordinarily useful information enclosed ISO 13485 Certified 3607 Parkway Lane, Suite 100 Norcross,
APOB (Human) ELISA Kit
 APOB (Human) ELISA Kit Catalog Number KA4330 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
APOB (Human) ELISA Kit Catalog Number KA4330 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Allergen Analysis : Customer Solution Case Studies. ILSI Madrid 2018 Adrian Rogers
 Allergen Analysis : Customer Solution Case Studies ILSI Madrid 2018 Adrian Rogers Immunoassay Challenges Every food is different and presents it s own unique challenges with immunoassay analysis If there
Allergen Analysis : Customer Solution Case Studies ILSI Madrid 2018 Adrian Rogers Immunoassay Challenges Every food is different and presents it s own unique challenges with immunoassay analysis If there
METHOD VALIDATION CASE
 METHOD VALIDATION CASE METHOD VALIDATION PROTOCOL CLIA Regulation 493.1253 (2) 1.Accuracy (closeness to true/comparative method) 2.Precision (reproducibility) 3.Reference Interval 4.Reportable range (linearity,
METHOD VALIDATION CASE METHOD VALIDATION PROTOCOL CLIA Regulation 493.1253 (2) 1.Accuracy (closeness to true/comparative method) 2.Precision (reproducibility) 3.Reference Interval 4.Reportable range (linearity,
Validation Case studies: the good, the bad and the molecular
 Validation Case studies: the good, the bad and the molecular Patti Jones PhD Professor of Pathology UT Southwestern Medical Center Director of Chemistry Children s Medical Center Dallas Presented by AACC
Validation Case studies: the good, the bad and the molecular Patti Jones PhD Professor of Pathology UT Southwestern Medical Center Director of Chemistry Children s Medical Center Dallas Presented by AACC
AmoyDx TM BRAF V600E Mutation Detection Kit
 AmoyDx TM BRAF V600E Mutation Detection Kit Detection of V600E mutation in the BRAF oncogene Instructions For Use Instructions Version: B3.1 Date of Revision: April 2012 Store at -20±2 o C 1/5 Background
AmoyDx TM BRAF V600E Mutation Detection Kit Detection of V600E mutation in the BRAF oncogene Instructions For Use Instructions Version: B3.1 Date of Revision: April 2012 Store at -20±2 o C 1/5 Background
INTRODUCTION PRODUCT DESCRIPTION
 INTRODUCTION Mycoplasma are known as important contaminants of biological products derived from cell lines in the biopharmaceutical industry affecting every parameter of a cell culture system. Contaminated
INTRODUCTION Mycoplasma are known as important contaminants of biological products derived from cell lines in the biopharmaceutical industry affecting every parameter of a cell culture system. Contaminated
Supplementary Figure 1: Experimental design. DISCOVERY PHASE VALIDATION PHASE (N = 88) (N = 20) Healthy = 20. Healthy = 6. Endometriosis = 33
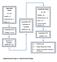 DISCOVERY PHASE (N = 20) Healthy = 6 Endometriosis = 7 EAOC = 7 Quantitative PCR (mirnas = 1113) a Quantitative PCR Verification of Candidate mirnas (N = 24) VALIDATION PHASE (N = 88) Healthy = 20 Endometriosis
DISCOVERY PHASE (N = 20) Healthy = 6 Endometriosis = 7 EAOC = 7 Quantitative PCR (mirnas = 1113) a Quantitative PCR Verification of Candidate mirnas (N = 24) VALIDATION PHASE (N = 88) Healthy = 20 Endometriosis
(DNA) Real-time PCR. Exicycler 96 Rotor-Gene Q/6000 PCR
 Real-Time (DNA) Real-time Exicycler 96 Rotor-Gene Q/6000 IU Mix1 Mix2 IU/μl IU/μl IU/μl IU/μl IU/μl IPC NTC C 1 Lot# 2 Freeze & thawing 1 MSDS: Material Safety Data Sheets Real- (TaqMan time ' FAM ' BHQ1
Real-Time (DNA) Real-time Exicycler 96 Rotor-Gene Q/6000 IU Mix1 Mix2 IU/μl IU/μl IU/μl IU/μl IU/μl IPC NTC C 1 Lot# 2 Freeze & thawing 1 MSDS: Material Safety Data Sheets Real- (TaqMan time ' FAM ' BHQ1
Product Manual. Human LDLR ELISA Kit. Catalog Number. FOR RESEARCH USE ONLY Not for use in diagnostic procedures
 Product Manual Human LDLR ELISA Kit Catalog Number STA-386 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cholesterol is an essential component of cellular membranes,
Product Manual Human LDLR ELISA Kit Catalog Number STA-386 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cholesterol is an essential component of cellular membranes,
SensoLyte 520 HIV-1 Protease Assay Kit *Fluorimetric*
 SensoLyte 520 HIV-1 Protease Assay Kit *Fluorimetric* Catalog # 71147 Kit Size 100 assays (96-well) or 500 assays (384-well) Convenient Format: Complete kit including all the assay components. Optimized
SensoLyte 520 HIV-1 Protease Assay Kit *Fluorimetric* Catalog # 71147 Kit Size 100 assays (96-well) or 500 assays (384-well) Convenient Format: Complete kit including all the assay components. Optimized
Instructions for Use. RealStar Influenza Screen & Type RT-PCR Kit /2017 EN
 Instructions for Use RealStar Influenza Screen & Type RT-PCR Kit 4.0 05/2017 EN RealStar Influenza Screen & Type RT-PCR Kit 4.0 For research use only! (RUO) 164003 INS-164000-EN-S01 96 05 2017 altona
Instructions for Use RealStar Influenza Screen & Type RT-PCR Kit 4.0 05/2017 EN RealStar Influenza Screen & Type RT-PCR Kit 4.0 For research use only! (RUO) 164003 INS-164000-EN-S01 96 05 2017 altona
Norgen s HIV Proviral DNA PCR Kit was developed and validated to be used with the following PCR instruments: Qiagen Rotor-Gene Q BioRad T1000 Cycler
 3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com HIV Proviral DNA PCR Kit Product# 33840 Product Insert Intended
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com HIV Proviral DNA PCR Kit Product# 33840 Product Insert Intended
ipsogen BCR-ABL1 Mbcr IS-MMR Kit Handbook
 July 2016 ipsogen BCR-ABL1 Mbcr IS-MMR Kit Handbook 24 Version 1 Quantitative in vitro diagnostics For use with Rotor-Gene Q, Applied Biosystems, ABI PRISM, and LightCycler instruments 670723 QIAGEN GmbH,
July 2016 ipsogen BCR-ABL1 Mbcr IS-MMR Kit Handbook 24 Version 1 Quantitative in vitro diagnostics For use with Rotor-Gene Q, Applied Biosystems, ABI PRISM, and LightCycler instruments 670723 QIAGEN GmbH,
Human influenza A virus subtype (H3)
 PCRmax Ltd TM qpcr test Human influenza A virus subtype (H3) Haemoglutinin H3 gene 150 tests For general laboratory and research use only 1 Introduction to Human influenza A virus subtype (H3) Influenza,
PCRmax Ltd TM qpcr test Human influenza A virus subtype (H3) Haemoglutinin H3 gene 150 tests For general laboratory and research use only 1 Introduction to Human influenza A virus subtype (H3) Influenza,
EXO-DNAc Circulating and EV-associated DNA extraction kit
 Datasheet EXO-DNAc Circulating and EV-associated DNA extraction kit This product is for research use only. It is highly recommended to read this users guide in its entirety prior to using this product.
Datasheet EXO-DNAc Circulating and EV-associated DNA extraction kit This product is for research use only. It is highly recommended to read this users guide in its entirety prior to using this product.
ipsogen BCR-ABL1 mbcr Kit Handbook
 March 2015 ipsogen BCR-ABL1 mbcr Kit Handbook 24 Version 1 Quantitative in vitro diagnostics For use with Rotor-Gene Q, ABI PRISM, LightCycler, and SmartCycler instruments 670023 QIAGEN GmbH, QIAGEN Strasse
March 2015 ipsogen BCR-ABL1 mbcr Kit Handbook 24 Version 1 Quantitative in vitro diagnostics For use with Rotor-Gene Q, ABI PRISM, LightCycler, and SmartCycler instruments 670023 QIAGEN GmbH, QIAGEN Strasse
ONE STEP MULTIPLEX RT-PCR FOR BCRlABL GENE IN MALAY PATIENTS DIAGNOSED AS LEUKAEMIA
 ONE STEP MULTIPLEX RT-PCR FOR BCRlABL GENE IN MALAY PATIENTS DIAGNOSED AS LEUKAEMIA 1Rosline H, 1Majdan R, 1Wan Zaidah A, 1Rapiaah M, 1Selamah G, 2A A Baba, 3D M Donald 1Department of Haematology, 2Department
ONE STEP MULTIPLEX RT-PCR FOR BCRlABL GENE IN MALAY PATIENTS DIAGNOSED AS LEUKAEMIA 1Rosline H, 1Majdan R, 1Wan Zaidah A, 1Rapiaah M, 1Selamah G, 2A A Baba, 3D M Donald 1Department of Haematology, 2Department
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT. Product: Alere q HIV-1/2 Detect WHO reference number: PQDx
 WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: Alere q HIV-1/2 Detect WHO reference number: PQDx 0226-032-00 Alere q HIV-1/2 Detect with product codes 270110050, 270110010 and 270300001,
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: Alere q HIV-1/2 Detect WHO reference number: PQDx 0226-032-00 Alere q HIV-1/2 Detect with product codes 270110050, 270110010 and 270300001,
Human Rotavirus A. genesig Standard Kit. Non structural protein 5 (NSP5) 150 tests. Primerdesign Ltd. For general laboratory and research use only
 TM Primerdesign Ltd Human Rotavirus A Non structural protein 5 (NSP5) genesig Standard Kit 150 tests For general laboratory and research use only 1 Introduction to Human Rotavirus A Rotavirus is a genus
TM Primerdesign Ltd Human Rotavirus A Non structural protein 5 (NSP5) genesig Standard Kit 150 tests For general laboratory and research use only 1 Introduction to Human Rotavirus A Rotavirus is a genus
VARIATION IN MEASUREMENT OF HIV RNA VIRAL LOAD
 VARIATION IN MEASUREMENT OF HIV RNA VIRAL LOAD SOURCES OF VARIATION (RANDOM VS SYSTEMATIC) MAGNITUDE OF EACH SOURCE CONSEQUENCES FOR CONFIDENCE LIMITS AROUND MEASUREMENTS AND CHANGES DATA FROM ROCHE HIV
VARIATION IN MEASUREMENT OF HIV RNA VIRAL LOAD SOURCES OF VARIATION (RANDOM VS SYSTEMATIC) MAGNITUDE OF EACH SOURCE CONSEQUENCES FOR CONFIDENCE LIMITS AROUND MEASUREMENTS AND CHANGES DATA FROM ROCHE HIV
Vitamin A-E Serum HPLC Analysis Kit
 Vitamin A-E Serum HPLC Analysis Kit ZV-4013-0200-10 200 2-8 C ZIVAK TEKNOLOJI SANAYI VE TICARET LIMITED SIRKETI Gursel Mh. Eski Besiktas Cd. No:44/1-2 KAGITHANE-ISTANBUL-TURKEY www.zivak.com info@zivak.com
Vitamin A-E Serum HPLC Analysis Kit ZV-4013-0200-10 200 2-8 C ZIVAK TEKNOLOJI SANAYI VE TICARET LIMITED SIRKETI Gursel Mh. Eski Besiktas Cd. No:44/1-2 KAGITHANE-ISTANBUL-TURKEY www.zivak.com info@zivak.com
AVENIO family of NGS oncology assays ctdna and Tumor Tissue Analysis Kits
 AVENIO family of NGS oncology assays ctdna and Tumor Tissue Analysis Kits Accelerating clinical research Next-generation sequencing (NGS) has the ability to interrogate many different genes and detect
AVENIO family of NGS oncology assays ctdna and Tumor Tissue Analysis Kits Accelerating clinical research Next-generation sequencing (NGS) has the ability to interrogate many different genes and detect
Product # Kit Components
 3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Pneumocystis jirovecii PCR Kit Product # 42820 Product Insert Background Information
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Pneumocystis jirovecii PCR Kit Product # 42820 Product Insert Background Information
altona RealStar Instructions for Use RealStar CMV PCR Kit /2017 EN DIAGNOSTICS
 altona DIAGNOSTICS Instructions for Use RealStar CMV PCR Kit 1.2 08/2017 EN RealStar RealStar CMV PCR Kit 1.2 For research use only! (RUO) 021202 INS-021200-EN-S01 48 08 2017 altona Diagnostics GmbH Mörkenstr.
altona DIAGNOSTICS Instructions for Use RealStar CMV PCR Kit 1.2 08/2017 EN RealStar RealStar CMV PCR Kit 1.2 For research use only! (RUO) 021202 INS-021200-EN-S01 48 08 2017 altona Diagnostics GmbH Mörkenstr.
VQA Control SOP Version 4.0 Roche Amplicor HIV-1 DNA Test, v August 2007
 1. PRINCIPLE 1.1. The Virology Quality Assurance (VQA) Laboratory provides external cell pellet controls for use in the validation of assays that detect HIV proviral DNA. 1.2. HIV seronegative peripheral
1. PRINCIPLE 1.1. The Virology Quality Assurance (VQA) Laboratory provides external cell pellet controls for use in the validation of assays that detect HIV proviral DNA. 1.2. HIV seronegative peripheral
ab HIV-1 Protease Inhibitor Screening Kit (Fluorometric)
 Version 1 Last updated 2 March 2017 ab211106 HIV-1 Protease Inhibitor Screening Kit (Fluorometric) For the rapid, sensitive and accurate screening of potential HIV-1 Protease inhibitors. This product is
Version 1 Last updated 2 March 2017 ab211106 HIV-1 Protease Inhibitor Screening Kit (Fluorometric) For the rapid, sensitive and accurate screening of potential HIV-1 Protease inhibitors. This product is
CoQ10(Coenzyme Q10) ELISA Kit
 CoQ10(Coenzyme Q10) ELISA Kit Catalogue No.: EU0196 Size: 48T/96T Reactivity: Universal Detection Range: 0.781-50ng/ml Sensitivity:
CoQ10(Coenzyme Q10) ELISA Kit Catalogue No.: EU0196 Size: 48T/96T Reactivity: Universal Detection Range: 0.781-50ng/ml Sensitivity:
Avian influenza A virus subtype (H7)
 TM Primerdesign Ltd Avian influenza A virus subtype (H7) Haemoglutinin H7 gene genesig Advanced Kit 150 tests For general laboratory and research use only 1 Introduction to Avian influenza A virus subtype
TM Primerdesign Ltd Avian influenza A virus subtype (H7) Haemoglutinin H7 gene genesig Advanced Kit 150 tests For general laboratory and research use only 1 Introduction to Avian influenza A virus subtype
Human Hemoglobin Colorimetric Detection Kit
 Human Hemoglobin Colorimetric Detection Kit CATALOG NO: IRAAKT2522 LOT NO: SAMPLE INTENDED USE The Hemoglobin detection kit is designed to quantitatively measure all forms of hemoglobin present in blood
Human Hemoglobin Colorimetric Detection Kit CATALOG NO: IRAAKT2522 LOT NO: SAMPLE INTENDED USE The Hemoglobin detection kit is designed to quantitatively measure all forms of hemoglobin present in blood
BÜHLMANN fcal turbo. Calprotectin turbidimetric assay for professional use. Reagent Kit B-KCAL-RSET. Revision date:
 BÜHLMANN fcal turbo Calprotectin turbidimetric assay for professional use Reagent Kit B-KCAL-RSET Revision date: 2017-02-24 BÜHLMANN LABORATORIES AG Baselstrasse 55 4124 Schönenbuch, Switzerland Tel.:
BÜHLMANN fcal turbo Calprotectin turbidimetric assay for professional use Reagent Kit B-KCAL-RSET Revision date: 2017-02-24 BÜHLMANN LABORATORIES AG Baselstrasse 55 4124 Schönenbuch, Switzerland Tel.:
THE USE OF WT1-QPCR TO MEASURE AND DETECT MINIMAL RESIDUAL DISEASE IN ACUTE MYELOID LEUKEMIA. Ava Greco
 THE USE OF WT1-QPCR TO MEASURE AND DETECT MINIMAL RESIDUAL DISEASE IN ACUTE MYELOID LEUKEMIA Ava Greco REVIEW OF LITERATURE What is Leukemia? Abnormal growth of cells in the blood stream Progresses rapidly
THE USE OF WT1-QPCR TO MEASURE AND DETECT MINIMAL RESIDUAL DISEASE IN ACUTE MYELOID LEUKEMIA Ava Greco REVIEW OF LITERATURE What is Leukemia? Abnormal growth of cells in the blood stream Progresses rapidly
HEK293FT cells were transiently transfected with reporters, N3-ICD construct and
 Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Human Rotavirus A. genesig Advanced Kit. Non structural protein 5 (NSP5) 150 tests. Primerdesign Ltd. For general laboratory and research use only
 TM Primerdesign Ltd Human Rotavirus A Non structural protein 5 (NSP5) genesig Advanced Kit 150 tests For general laboratory and research use only 1 Introduction to Human Rotavirus A Rotavirus is a genus
TM Primerdesign Ltd Human Rotavirus A Non structural protein 5 (NSP5) genesig Advanced Kit 150 tests For general laboratory and research use only 1 Introduction to Human Rotavirus A Rotavirus is a genus
Human Rotavirus C. genesig Advanced Kit. DNA testing. Everything... Everyone... Everywhere... Non structural protein 5 (NSP5) 150 tests
 TM Primerdesign Ltd TM Primerdesign Ltd Human Rotavirus C Non structural protein 5 (NSP5) genesig Advanced Kit 150 tests DNA testing Everything... Everyone... Everywhere... For general laboratory and research
TM Primerdesign Ltd TM Primerdesign Ltd Human Rotavirus C Non structural protein 5 (NSP5) genesig Advanced Kit 150 tests DNA testing Everything... Everyone... Everywhere... For general laboratory and research
Simple Solutions for Patient Monitoring. Maximum Flexibility 2 Configuration: 8 to 1000 viral load tests/day 2 Sample: Plasma/DBS
 Simple Solutions for Patient Monitoring What is the interest of having a high level of sensitivity? Higher sensitivity means you are able to offer better patient monitoring, particularly in the low viral
Simple Solutions for Patient Monitoring What is the interest of having a high level of sensitivity? Higher sensitivity means you are able to offer better patient monitoring, particularly in the low viral
Molecular Diagnosis Future Directions
 Molecular Diagnosis Future Directions Philip Cunningham NSW State Reference Laboratory for HIV/AIDS & Molecular Diagnostic Medicine Laboratory, SydPath St Vincent s Hospital Sydney Update on Molecular
Molecular Diagnosis Future Directions Philip Cunningham NSW State Reference Laboratory for HIV/AIDS & Molecular Diagnostic Medicine Laboratory, SydPath St Vincent s Hospital Sydney Update on Molecular
Update on the development of clinical diagnostic run controls at NIBSC. Neil Almond Division of Virology
 Update on the development of clinical diagnostic run controls at NIBSC Neil Almond Division of Virology BACKGROUND Molecular techniques are increasingly being used for microbiological diagnoses Assays
Update on the development of clinical diagnostic run controls at NIBSC Neil Almond Division of Virology BACKGROUND Molecular techniques are increasingly being used for microbiological diagnoses Assays
METHOD VALIDATION: WHY, HOW AND WHEN?
 METHOD VALIDATION: WHY, HOW AND WHEN? Linear-Data Plotter a,b s y/x,r Regression Statistics mean SD,CV Distribution Statistics Comparison Plot Histogram Plot SD Calculator Bias SD Diff t Paired t-test
METHOD VALIDATION: WHY, HOW AND WHEN? Linear-Data Plotter a,b s y/x,r Regression Statistics mean SD,CV Distribution Statistics Comparison Plot Histogram Plot SD Calculator Bias SD Diff t Paired t-test
altona DIAGNOSTICS altona DIAGNOSTICS RealStar CMV PCR Kit 1.0 always a drop ahead. 11/2012
 altona DIAGNOSTICS altona DIAGNOSTICS RealStar CMV PCR Kit 1.0 11/2012 altona Diagnostics GmbH Moerkenstr. 12 22767 Hamburg Germany phone +49 40 548 06 76-0 fax +49 40 548 06 76-10 e-mail info@altona-diagnostics.com
altona DIAGNOSTICS altona DIAGNOSTICS RealStar CMV PCR Kit 1.0 11/2012 altona Diagnostics GmbH Moerkenstr. 12 22767 Hamburg Germany phone +49 40 548 06 76-0 fax +49 40 548 06 76-10 e-mail info@altona-diagnostics.com
ab Homocysteine Assay Kit (Fluorometric) 1
 Version 1 Last updated 25 May 2018 ab208559 Homocysteine Assay Kit (Fluorometric) For the measurement of homocysteine in mammalian plasma and serum. This product is for research use only and is not intended
Version 1 Last updated 25 May 2018 ab208559 Homocysteine Assay Kit (Fluorometric) For the measurement of homocysteine in mammalian plasma and serum. This product is for research use only and is not intended
Molecular diagnosis of infectious disease - Method validation and environmental setting 傳染病的分子診斷 - 方法確認與環境背景. WC Yam 任永昌
 Molecular diagnosis of infectious disease - Method validation and environmental setting 傳染病的分子診斷 - 方法確認與環境背景 WC Yam 任永昌 Dept of Microbiology Queen Mary Hospital The University of Hong Kong Clinical and
Molecular diagnosis of infectious disease - Method validation and environmental setting 傳染病的分子診斷 - 方法確認與環境背景 WC Yam 任永昌 Dept of Microbiology Queen Mary Hospital The University of Hong Kong Clinical and
EXO-DNA Circulating and EV-associated DNA extraction kit
 Datasheet EXO-DNA Circulating and EV-associated DNA extraction kit This product is for research use only. It is highly recommended to read this users guide in its entirety prior to using this product.
Datasheet EXO-DNA Circulating and EV-associated DNA extraction kit This product is for research use only. It is highly recommended to read this users guide in its entirety prior to using this product.
Avian influenza A virus subtype (H7)
 TM Primerdesign Ltd Avian influenza A virus subtype (H7) Haemoglutinin H7 gene genesig Standard Kit 150 tests For general laboratory and research use only 1 Introduction to Avian influenza A virus subtype
TM Primerdesign Ltd Avian influenza A virus subtype (H7) Haemoglutinin H7 gene genesig Standard Kit 150 tests For general laboratory and research use only 1 Introduction to Avian influenza A virus subtype
AVENIO ctdna Analysis Kits The complete NGS liquid biopsy solution EMPOWER YOUR LAB
 Analysis Kits The complete NGS liquid biopsy solution EMPOWER YOUR LAB Analysis Kits Next-generation performance in liquid biopsies 2 Accelerating clinical research From liquid biopsy to next-generation
Analysis Kits The complete NGS liquid biopsy solution EMPOWER YOUR LAB Analysis Kits Next-generation performance in liquid biopsies 2 Accelerating clinical research From liquid biopsy to next-generation
SensoLyte 490 HIV-1 Protease Assay Kit *Fluorimetric*
 SensoLyte 490 HIV-1 Protease Assay Kit *Fluorimetric* Catalog # 71127 Unit Size Kit Size 1 kit 500 assays (96-well) or 1250 assays (384-well) This kit is optimized to detect the activity of human immunodeficiency
SensoLyte 490 HIV-1 Protease Assay Kit *Fluorimetric* Catalog # 71127 Unit Size Kit Size 1 kit 500 assays (96-well) or 1250 assays (384-well) This kit is optimized to detect the activity of human immunodeficiency
Sponsored and reviewed by ICCS Quality and Standards Committee Title: Verification of PNH assay sensitivity through spiking experiment Written by:
 Sponsored and reviewed by ICCS Quality and Standards Committee Title: Verification of PNH assay sensitivity through spiking experiment Written by: A Illingworth, T Oldaker, R Sutherland, S Kotanchiyev,
Sponsored and reviewed by ICCS Quality and Standards Committee Title: Verification of PNH assay sensitivity through spiking experiment Written by: A Illingworth, T Oldaker, R Sutherland, S Kotanchiyev,
Instructions for Use. RealStar Influenza S&T RT-PCR Kit /2017 EN
 Instructions for Use RealStar Influenza S&T RT-PCR Kit 3.0 01/2017 EN RealStar Influenza S&T RT-PCR Kit 3.0 For research use only! (RUO) 163003 INS-163000-EN-S02 96 01 2017 altona Diagnostics GmbH Mörkenstr.
Instructions for Use RealStar Influenza S&T RT-PCR Kit 3.0 01/2017 EN RealStar Influenza S&T RT-PCR Kit 3.0 For research use only! (RUO) 163003 INS-163000-EN-S02 96 01 2017 altona Diagnostics GmbH Mörkenstr.
LDL (Human) ELISA Kit
 LDL (Human) ELISA Kit Cat. No.:DEIA3864 Pkg.Size:96T Intended use This immunoassay kit allows for the specific measurement of human low density lipoprotein, LDL concentrations in cell culture supernates,
LDL (Human) ELISA Kit Cat. No.:DEIA3864 Pkg.Size:96T Intended use This immunoassay kit allows for the specific measurement of human low density lipoprotein, LDL concentrations in cell culture supernates,
Validation Report: VERSA Mini PCR Workstation Reverse Transcription of Avian Flu RNA and Amplification of cdna & Detection of H5N1
 I. Objectives Validation Report: VERSA Mini PCR Workstation Reverse Transcription of Avian Flu RNA and Amplification of cdna & Detection of H5N1 1. To ensure stability of RNA (highly thermolabile and degradatively
I. Objectives Validation Report: VERSA Mini PCR Workstation Reverse Transcription of Avian Flu RNA and Amplification of cdna & Detection of H5N1 1. To ensure stability of RNA (highly thermolabile and degradatively
QUANTIFICATION RAPID VIRUS WHAT S YOUR APPLICATION?
 VIRUS COUNTER 3100 RAPID VIRUS QUANTIFICATION A novel approach for determining Total Virus Particle concentration that provides precise results in minutes, not days. MORE MEANINGFUL RESULTS Get a quantitative
VIRUS COUNTER 3100 RAPID VIRUS QUANTIFICATION A novel approach for determining Total Virus Particle concentration that provides precise results in minutes, not days. MORE MEANINGFUL RESULTS Get a quantitative
Hyaluronan Quantification Kit
 Product manual (ELISA-like assay for HA / Hyaluronic Acid) Updated on Jan. 6th, 207 - Background Hyaluronan (HA) is an unbranched glycosaminoglycan composed of repeating disaccharide units of D-glucronic
Product manual (ELISA-like assay for HA / Hyaluronic Acid) Updated on Jan. 6th, 207 - Background Hyaluronan (HA) is an unbranched glycosaminoglycan composed of repeating disaccharide units of D-glucronic
STELLUX C-peptide Chemiluminescence ELISA
 STELLUX C-peptide Chemiluminescence ELISA For the quantitative determination of Human C-peptide in Human Serum, Heparin Plasma, EDTA plasma, and Tissue Culture Supernatants. For In Vitro Diagnostic use
STELLUX C-peptide Chemiluminescence ELISA For the quantitative determination of Human C-peptide in Human Serum, Heparin Plasma, EDTA plasma, and Tissue Culture Supernatants. For In Vitro Diagnostic use
SensoLyte 520 HDAC Activity Assay Kit *Fluorimetric*
 SensoLyte 520 HDAC Activity Assay Kit *Fluorimetric* Catalog # 72084 Kit Size 100 Assays (96-well plate) Optimized Performance: This kit is optimized to detect HDAC activity. Enhanced Value: It provides
SensoLyte 520 HDAC Activity Assay Kit *Fluorimetric* Catalog # 72084 Kit Size 100 Assays (96-well plate) Optimized Performance: This kit is optimized to detect HDAC activity. Enhanced Value: It provides
virellatbe real time RT-PCR Kit LC
 Instruction for Use virellatbe real time RT-PCR Kit LC For the in-vitro detection of TBE Virus RNA in clinical specimens and in ticks. G01065-32 G01065-96 32 96 gerbion gmbh & Co. KG Remsstr. 1 70806 Kornwestheim
Instruction for Use virellatbe real time RT-PCR Kit LC For the in-vitro detection of TBE Virus RNA in clinical specimens and in ticks. G01065-32 G01065-96 32 96 gerbion gmbh & Co. KG Remsstr. 1 70806 Kornwestheim
VQA HIV DNA Control SOP Version 5.0 HIV DNA Testing 13 March 2012
 1. PRINCIPLE 1.1. The Virology Quality Assurance (VQA) Laboratory provides external cell pellet controls for use in the validation of assays that detect HIV proviral DNA. 1.2. HIV seronegative peripheral
1. PRINCIPLE 1.1. The Virology Quality Assurance (VQA) Laboratory provides external cell pellet controls for use in the validation of assays that detect HIV proviral DNA. 1.2. HIV seronegative peripheral
ab ORAC Assay Kit
 Version 1 Last updated 10 April 2018 ab233473 ORAC Assay Kit For the measurement of ORAC activity in cell lysate, plasma, serum, tissue homogenates and food extracts. This product is for research use only
Version 1 Last updated 10 April 2018 ab233473 ORAC Assay Kit For the measurement of ORAC activity in cell lysate, plasma, serum, tissue homogenates and food extracts. This product is for research use only
RayBio Human ENA-78 ELISA Kit
 RayBio Human ENA-78 ELISA Kit Catalog #: ELH-ENA78 User Manual Last revised April 15, 2016 Caution: Extraordinarily useful information enclosed ISO 13485 Certified 3607 Parkway Lane, Suite 100 Norcross,
RayBio Human ENA-78 ELISA Kit Catalog #: ELH-ENA78 User Manual Last revised April 15, 2016 Caution: Extraordinarily useful information enclosed ISO 13485 Certified 3607 Parkway Lane, Suite 100 Norcross,
Nature Protocols: doi: /nprot Supplementary Figure 1. Fluorescent titration of probe CPDSA.
 Supplementary Figure 1 Fluorescent titration of probe CPDSA. Fluorescent titration of probe CPDSA (10 um) upon addition of GSH in HEPES (10 mm, ph = 7.4) containing 10% DMSO. Each spectrum was recorded
Supplementary Figure 1 Fluorescent titration of probe CPDSA. Fluorescent titration of probe CPDSA (10 um) upon addition of GSH in HEPES (10 mm, ph = 7.4) containing 10% DMSO. Each spectrum was recorded
Hepatitis B Virus Genemer
 Product Manual Hepatitis B Virus Genemer Primer Pair for amplification of HBV Viral Specific Fragment Catalog No.: 60-2007-10 Store at 20 o C For research use only. Not for use in diagnostic procedures
Product Manual Hepatitis B Virus Genemer Primer Pair for amplification of HBV Viral Specific Fragment Catalog No.: 60-2007-10 Store at 20 o C For research use only. Not for use in diagnostic procedures
Evaluation Report: Eurolyser CRP test (ST0100 and ST0102) on. CUBE analyser (CA0100)
 Evaluation Report: Eurolyser CRP test (ST0100 and ST0102) on CUBE analyser (CA0100) Location Location: Eurolyser Diagnostica GmbH Operators: Simone Wieser; Franz Helminger; Michael Gruber Date: July-November
Evaluation Report: Eurolyser CRP test (ST0100 and ST0102) on CUBE analyser (CA0100) Location Location: Eurolyser Diagnostica GmbH Operators: Simone Wieser; Franz Helminger; Michael Gruber Date: July-November
Swine H1N1 Influenza Human Pandemic Strain
 TM Primerdesign Ltd Swine H1N1 Influenza Human Pandemic Strain M1 - global Influenza A & N1- specific for Swine H1N1 Influenza Human Pandemic Strain genesig Standard Kit 150 tests For general laboratory
TM Primerdesign Ltd Swine H1N1 Influenza Human Pandemic Strain M1 - global Influenza A & N1- specific for Swine H1N1 Influenza Human Pandemic Strain genesig Standard Kit 150 tests For general laboratory
Gentian Canine CRP Immunoassay Application Note for scil VitroVet*
 v02-jan2014 Gentian Canine CRP Immunoassay Application Note for scil VitroVet* Intended Use The canine CRP immunoassay on scil VitroVet is an in vitro diagnostic test for quantitative determination of
v02-jan2014 Gentian Canine CRP Immunoassay Application Note for scil VitroVet* Intended Use The canine CRP immunoassay on scil VitroVet is an in vitro diagnostic test for quantitative determination of
Human Rotavirus B. Non structural protein 5 (NSP5) 150 tests. Quantification of Human Rotavirus B genomes Advanced kit handbook HB10.01.
 PCR Max Ltd TM qpcr test Human Rotavirus B Non structural protein 5 (NSP5) 150 tests For general laboratory and research use only 1 Introduction to Human Rotavirus B Rotavirus is a genus of double-stranded
PCR Max Ltd TM qpcr test Human Rotavirus B Non structural protein 5 (NSP5) 150 tests For general laboratory and research use only 1 Introduction to Human Rotavirus B Rotavirus is a genus of double-stranded
