Exposure Control Plan for Cytotoxic Substances
|
|
|
- Allen Shaw
- 5 years ago
- Views:
Transcription
1 Exposure Control Plan Department of UBC-RMS-OHS-ECP Effective date: December 8, 2017 Review date: NA Supersedes: NA 1. PURPOSE Exposure Control Plan for Cytotoxic Substances The University of British Columbia (UBC) is committed to providing a safe and healthy workplace by protecting all campus occupants from the potential adverse effects from exposure to cytotoxic substances in accordance with the Occupational Health and Safety (OHS) Regulations. Chapter 6 of the Occupational Health and Safety (OHS) Regulation lists Cytotoxic drugs as substances with specific requirements (Appendix A contains the section of the regulation pertaining to cytotoxic drugs). The requirements of the regulations are incorporated in this ECP. Cytotoxic drugs are primarily used to treat cancer, frequently as part of a chemotherapy regime. Recently, their uses have expanded to treat certain skin conditions, rheumatoid and juvenile rheumatoid arthritis, and steroid-resistant muscle conditions. UBC research projects involving these medical conditions might involve working with cytotoxic drugs. In addition, due to the largely inclusive definition of cytotoxic agents, other UBC employees might be exposed to chemicals classified as cytotoxic agents. Many common techniques in cellular biology, microbiology, biochemistry and molecular biology use reagents that fall into this classification. If a worker is or may be occupationally exposed to a cytotoxic drug, the employer must develop and implement an Exposure Control Plan (ECP) meeting the requirements of section 5.54 of the regulation. This ECP is applicable to all UBC employees, contractors and students carrying out work with cytotoxic substances at UBC and where there is potential for cytotoxic exposure to occur. The document contains the following appendices: Appendix A WorkSafe BC cytotoxic drugs regulation Appendix B Common cytotoxic drugs (WorkSafe BC and BC Cancer Agency lists) Title: Exposure Control Plan for Cytotoxic Substances Page 1 of 13
2 Appendix C Excretion rates of several cytotoxic drugs Appendix D Job tasks and controls table - acceptable control methods for specific job tasks 2. ROLES AND RESPONSIBILITIES Employer Develop, implement and maintain a Cytotoxic Exposure Program to ensure exposure to cytotoxic substances is maintained As Low As Reasonably Achievable (ALARA) as per OHS Regulations Protect workers from hazards in the workplace by considering the hierarchy of controls (e.g. in order of elimination, substitution, engineering, administrative and personal protective equipment (PPE)) Ensure workers are informed on the hazards of cytotoxics and trained on how to minimize exposure to cytotoxic substances Ensure resources are made available to ensure occupational exposure to cytotoxic substances is conducted, when required Ensure exposure control plans (ECPs) are developed and implemented, when required Provide workers with the necessary materials, tools and equipment, PPE and other resources required to minimize exposure to cytotoxic substances If an exposure incident occurs at the workplace, ensure it is investigated and, based on the findings, develop ways to prevent similar incidents from occurring. Ensure workers who exposed to cytotoxic substances participate in health monitoring, when required. Department Manager/Supervisor Responsibilities Attend education and training sessions provided by the employer Identify and evaluate occupations, areas or tasks where the potential for exposure to cytotoxics exists Ensure necessary resources are available to conduct occupational monitoring for cytotoxics exposure, when required Ensure employees have the appropriate education and training for working with cytotoxics and demonstrate competency for identified tasks Ensure employees are able to identify the signs and symptoms associated with cytotoxics exposure Ensure the hierarchy of controls (e.g. elimination/substitution, engineering, administrative, PPE) are considered and implemented accordingly Ensure all equipment, PPE, and materials needed to control against exposure to cytotoxics are available Complete Site-Specific ECPs and provide to workers, when required Title: Exposure Control Plan for Cytotoxic Substances Page 2 of 13
3 Develop and provide safe work procedures to workers Provide workers with adequate supervision to ensure that work practices eliminate or minimize exposure to cytotoxics Ensure exposure to cytotoxics incidents are investigated appropriately. Report any incident via the UBC Centralized Accident / Incident Reporting System (CAIRS). Worker Responsibilities Attend education and training sessions provided by the employer Adhere to the hierarchy of controls for cytotoxics (e.g. elimination / substitution, engineering, administrative, PPE) Follow the ECP and safe work practices established by the employer Ensure all equipment, PPE and materials needed to control against exposure to cytotoxics are in good working condition Understand the importance of evacuating work areas where signs of cytotoxics exposure arise Report unsafe cytotoxics exposure conditions to their supervisor Participate in incident/accident investigations, where required. (RMS) Responsibilities Assist in the implementation of this Cytotoxics Exposure Program through training and consultation Provide education and training on cytotoxics exposure including how to recognize early signs and symptoms associated with the exposure Assist with cytotoxics risk identifications and assessments, as requested Ensure occupational exposure monitoring for cytotoxics is conducted, when required Conduct respirator fit testing sessions for tight-fitting respirators Assist in the development of ECPs and conduct annual reviews of the ECPs Conduct annual reviews of the effectiveness of this Cytotoxics Exposure Program. 3. CYTOTOXIC SUBSTANCES - CHARACTERISTICS This ECP applies to cytotoxic substances defined as any chemical substance confirmed or suspected of having a genotoxic, mutagenic or teratogenic effect in humans. This information can usually be obtained from the SDS of the chemical (sections 2 and 11). A cytotoxic drug means an agent that possesses a specific destructive action on certain cells or that may be genotoxic, oncogenic, mutagenic, teratogenic, or hazardous to cells in any way and includes most anti-cancer drugs. A number of drugs used in health care settings (e.g., hospitals, physician's offices, home healthcare agencies) may pose a risk to workers through acute or chronic occupational exposure. Title: Exposure Control Plan for Cytotoxic Substances Page 3 of 13
4 The American Society of Hospital Pharmacists (ASHP) considers a drug hazardous if it: is genotoxic, is carcinogenic, is teratogenic (a substance that is capable of causing physical defects in a developing embryo) or impairs fertility, or causes serious organ or other toxic manifestations at low doses in experimental animals or treated patients. Some common drugs that meet these criteria are listed in Appendix B. For drugs not included on this list, professional judgement by personnel trained in pharmacology and/or toxicology is needed to designate a drug as cytotoxic. The primary factors to be considered are as follows: Is the drug designated an antineoplastic agent in the American Hospital Formulary Service Drug Information? (An agent is antineoplastic if it inhibits or prevents the development of tumors) Does the manufacturer suggest the use of special isolation techniques in its handling, administration or disposal? Is the drug known to be a human mutagen, carcinogen, teratogen or reproductive toxicant? (An agent is mutagenic if it is capable of inducing genetic mutation.) Is the drug known to be carcinogenic or teratogenic in animals? (A drug known to be mutagenic in multiple bacterial systems or animals should also be considered cytotoxic.) or, Is the drug known to be acutely toxic to an organ system? For any questions and help with identifying cytotoxic substances, contact the RMS Chemical Safety Advisor. 4. HEALTH HAZARDS ASSOCIATED WITH EXPOSURE TO CYTOTOXICS There is evidence that exposure to cytotoxic drugs can cause adverse effects in workers. There are reports that exposure to cytotoxic drugs: can cause an increased frequency of chromosome damage in exposed workers can produce some acute effects in workers which include skin, eyes, and mucous membrane irritations, allergic reactions upon contact with the skin, as well as subjective symptoms including nausea, headache, and dizziness has been associated with adverse reproductive outcomes (including higher incidences of spontaneous abortions and a higher risk of delivering malformed babies) Repeated, long-term occupational exposure to small amounts of cytotoxic drugs has not been identified as a cause of cancer. However, because of the above-mentioned concerns, precautions must be followed to limit occupational exposure to all cytotoxic drugs. Title: Exposure Control Plan for Cytotoxic Substances Page 4 of 13
5 5. RISK IDENTIFICATION AND ASSESSMENT The degree of risk is dependent on the inherent toxicity of the drug, as well as the extent of exposure. Workers may be exposed via inhalation of dusts or aerosols, absorption through the skin, needle stick injuries, and ingestion (e.g., because of contact with contaminated food). Compliance with the personal hygiene requirements (sections 5.82 to 5.84) should eliminate ingestion as a source of exposure. Exposure may occur during preparation and administration and usage of cytotoxic substances, handling of body fluids from animals receiving cytotoxic drugs, handling and disposal of cytotoxic wastes and related trace contaminated material, and transportation of cytotoxic drugs. Some cytotoxic drugs have a direct irritant effect on the mucous membranes, eyes and skin. Spills onto skin surfaces that have cuts or abrasions and punctures of the skin with a contaminated needle or broken glass can lead to severe soft tissue injury. Surface contamination is one of the main sources of occupational exposure to cytotoxic drugs in health care settings. Due to their chemical stability, residue from hazardous drugs can persist in the workplace and be spread far from their point of origin. Residue can also collect on items that are not directly used for handling hazardous drugs, such as pens, door handles, and elevators buttons. Since surfaces throughout the workplace may be contaminated, there is potential risk of exposure for anyone working in the space. Table 1. Main routes of exposure to cytotoxic substances Route of exposure Examples of activities Possible controls Direct dermal contact Indirect dermal contact Contact with eyes Inhalation Handling oral or topical forms of cytotoxic substances Contact with a leak or spill of cytotoxic substances Handling contaminated animal excreta Handling or touching contaminated materials, such as equipment, containers, work surfaces Handling liquid forms of cytotoxic substances Weighing out powders on top loading balances on the open bench Inhaling aerosols or vapours released when breaking open Wearing double chemotherapytested gloves Using a closed-system transfer device Wearing double chemotherapytested gloves Observing a precautionary period for handling animal excreta Wearing eye protection or a face shield Weighing out powders in a fume hood Performing activities inside a biological safety cabinet Title: Exposure Control Plan for Cytotoxic Substances Page 5 of 13
6 Route of exposure Examples of activities Possible controls Percutaneous exposure Ingestion ampules, withdrawing needles from drug vials, transferring drugs with syringes, or expelling air from a drug-filled syringe Inhaling particulate material released when crushing tablets or opening capsules Using nutating mixers for staining and incubations Preparing or administering cytotoxic substances using a needle Handling broken glass or overfilling sharps containers Reusing razor blades, scalpels or dissection pins Eating food that has been contaminated with cytotoxic substances 6. OCCUPATIONAL EXPOSURE LIMITS Wearing an appropriate respirator Performing staining activities inside a fume hood Using cage changing stations for dumping Using a needless system or safety engineered medical sharp Use single use sharps and dispose of directly in a dedicated cytotoxic sharps container Ensuring all food is stored away from areas where hazardous drugs are handled Proper hand washing There are no general occupational exposure limits (OEL) for cytotoxic drugs because it is not possible to determine safe exposure levels. According to the Occupational Health and Safety (OHS) Regulation if a substance identified as carcinogen, reproductive toxin, and/or sensitizer is present in the workplace, the employer must replace it, if practicable, with a material that reduces the risk to workers. If substitution is not practicable, the employer must implement an exposure control plan to maintain workers' exposure as low as reasonably achievable below the exposure limit established. 7. RISK CONTROL Once a risk to cytotoxics has been identified and assessed, the appropriate hierarchy of controls must be implemented to ensure the risk is eliminated or mitigated. The hierarchy of controls is as follows (in priority of most effective to least effective): 1. Elimination or Substitution 2. Engineering 3. Administrative 4. Personal Protective Equipment Title: Exposure Control Plan for Cytotoxic Substances Page 6 of 13
7 The types of controls needed will be dependent on the task being performed. Substitution / Elimination Though the most effective risk control, elimination or substitution are not possible when the cytotoxic substance itself is tested in a research setting (e.g. animal studies). For the cases where the cytotoxic substance is used in a chemical process or as a precursor in a synthesis, the supervisor must replace it, if practicable, with a material, which reduces the risk to workers (WorkSafe BC Regulation 5.57(1)). Engineering Engineering controls are those controls, which aim to minimize the exposure to cytotoxic substances through physical modifications to facilities, equipment and processes. Biological Safety Cabinets (BSC) and Fume Hoods Local Exhaust Ventilation is an engineering control designed to protect against exposure to cytotoxic substances via inhalation route. According to WorkSafe BC Regulation 6.53: All mixing, preparation and priming of administration sets with a cytotoxic drug must be performed in one centralized area in a specially designated Class II Type B biological safety cabinet that; is exhausted to the outside atmosphere in a manner that prevents recirculation into any work area, has exhaust and ventilation systems that remain in operation for a sufficient period of time to ensure that no contaminants escape from the biological safety cabinet into the workplace, and is equipped with a continuous monitoring device to permit confirmation of adequate airflow and cabinet performance. Fume hoods, clean benches and other laminar flow devices such as horizontal flow cabinets that direct the air towards the operator, are not the same as biological safety cabinets. They are not considered appropriate for work with cytotoxic drugs. The BSC must be inspected and certified by a competent person at least annually and when the cabinet is moved. The BSC must be cleaned, maintained, and used according to the manufacturer s recommendations. The exhaust blower on the BSC should be operated continuously even when not in use. The cabinet should be cleaned daily with 70% alcohol and decontaminated weekly or whenever spills occur. Decontamination should consist of surface cleaning with an alkaline detergent followed by thorough rinsing. Personal protective equipment as described later in this document should be used while decontaminating the cabinet. The ductwork attached to the cabinet should be labelled as to its hazardous content. Title: Exposure Control Plan for Cytotoxic Substances Page 7 of 13
8 WorkSafe BC Regulation and related Guidelines detail the exact requirements for BSC operation and certification. When work with cytotoxic substances is part of a chemical synthesis process, the use of a laboratory fume hood is recommended. The fume hood and its related ductwork must be designed, installed and maintained in accordance with the Industrial Ventilation, A Manual of Recommended Practice, published by the American Conference of Governmental Industrial Hygienists, as amended from time to time (WorkSafe BC Regulation 30.8). Safety-engineered sharps All activities involving handling the cytotoxic substance via a syringe (e.g. administration of medication, transfer of chemicals from sealed containers) must be done with safetyengineered sharps. Safety-engineered sharps are devices with a built-in safety feature or mechanism that eliminates or minimizes the risk of accidental parenteral contact while or after the sharp is used. Administrative Administrative controls are those that aim to control or minimize exposure to cytotoxic substances using work methods and work procedures. Several of these administrative controls are part of the regulatory requirements (WorkSafe BC Regulation 6.44 to 6.58) Information - if a cytotoxic drug is received, prepared, administered, stored or disposed of at a workplace, the employer must maintain and make readily available to workers information on its acute and chronic toxicity, acute exposure treatment and safe handling. Labels - a container of a cytotoxic drug and a shelf or bin where a cytotoxic drug is regularly stored must be appropriately labelled. Signs - warning signs which are clearly visible and clearly state the identified hazards must be posted in all areas where cytotoxic drugs are stored or mixed. List - storage and preparation areas for cytotoxic drugs must be posted with a list of all cytotoxic drugs present in the workplace. Safe Work Procedures - when a cytotoxic drug is received, prepared, administered, stored or disposed of, written safe work procedures must be developed and implemented for applicable aspects of receiving, storage, preparation, administration and waste handling. The work safe procedures must be readily available for reference by workers. Reproductive toxins if present in the work place, the policy and procedures must inform workers about the reproductive toxin and identify ways to minimize exposure to the reproductive toxin for a worker who has advised the employer of pregnancy or intent to conceive a child. Title: Exposure Control Plan for Cytotoxic Substances Page 8 of 13
9 Records - the employer must maintain a record of all workers who prepare or administer cytotoxic drugs, including the name of the drugs handled, and when practicable, the number of preparations or administrations per week. The exposure records must be maintained for the duration of employment plus 10 years. Personal Protective Equipment (PPE) and Other Protective Clothing PPE and protective clothing is the least effective control. However, when used in conjunction with other controls (e.g. engineering and administrative), PPE and protective clothing can help further reduce the worker s exposure to cytotoxic substances. The specific PPE that is required for each task in the workplace is determined by a risk assessment. Workers should refer to the safe work procedures at their workplace for determining what types of PPE they are required to wear when handling hazardous drugs. WorkSafe BC regulation (6.55) lists under personal protective equipment: medical gloves that are manufactured and designed for use when handling cytotoxic drugs, a moisture resistant, long-sleeved gown with cuffs, if there is a risk of contact with aerosols, an approved respirator, and if there is a risk of eye contact, eye and face protection. Used gowns and gloves must not be worn outside the preparation, administration or storage area and must be handled as hazardous waste or contaminated linen. All other non-disposable personal protective equipment must be cleaned immediately after use. Gloves Nitrile or natural rubber latex is a preferred basic glove material, while vinyl is considered inappropriate because of its generally increased permeability. For extended exposure to cytotoxic substances, double gloving, the use of thicker gloves and the frequent change of gloves increases their protective power. When following best practices for the use of gloves during handling of hazardous drugs, workers should: Wear double gloves when the risk for dermal contamination with hazardous drugs is high (this is determined as part of your workplace risk assessment) Follow steps to avoid contamination when putting on gloves and during removal, including washing hands before and after wearing gloves Change gloves after 30 minutes of continuous compounding or if they have been contaminated or compromised Remove outer gloves before taking them out of a biological safety cabinet Title: Exposure Control Plan for Cytotoxic Substances Page 9 of 13
10 Gown / lab coat A gown made of low permeability fabric with a closed front (or back), long sleeves, and closed knitted cuffs is recommended. Cuffs should be tucked under gloves. Disposable gowns are recommended when cytotoxic drugs are prepared. Gowns should be immediately changes if contaminated or compromised. Wash hands immediately after removing a gown. Face and eye protection Eye protection, such as splash goggles, should be made available for use in any situation where there is a risk of splashing, which may occur when handling liquid forms of cytotoxic substance or contaminated body fluids and waste. Eye protection should also be used when cleaning up spills. When following best practice for wearing face protection for hazardous drugs, workers should: Wear full-face shields Use disposable face protection whenever practicable Clean non-disposable face protection immediately after use Footwear and shoe covers Workers must ensure their footwear is in a condition to provide protection against exposure to hazardous drugs, such as by wearing closed shoes that are made of a material that prevents liquids from soaking through. Refer to section 8.22 of the WorkSafe BC Regulation for more information on the requirements for footwear. Shoe covers are part of sterile preparation procedures but also help reduce exposure by preventing contamination being spread to other areas of the workplace on workers shoes. Best practices for footwear and shoe covers include, but are not limited to: Having a dedicated set of footwear that is only used in the preparation area Having all workers wear shoe covers when entering a sterile preparation room Removing shoe covers with gloved hands and disposing as hazardous waste upon exiting the preparation room Wearing shoe covers when cleaning up spills or broken containers on the floor Respirators An approved and fit-tested respirator must be worn when there is a risk of exposure to airborne particulates, aerosols, or vapours from hazardous drugs. This type of exposure may occur when workers: Prepare cytotoxic drugs on a counter or some other place that is outside of an approved BSC Clean up spilled cytotoxic substances Title: Exposure Control Plan for Cytotoxic Substances Page 10 of 13
11 Decontaminate a BSC that has the sash raised The respirator selected must provide protection from particulates as well as gases or vapours that can be generated from solid or liquid forms of cytotoxic substance, depending on the activity. This could include a half-or full-face air-purifying respirator that has a particulate filter (such as P100) and a chemical cartridge that removes vapour contaminants from air as it is inhaled. The choice of respirator must be made as part of the risk assessment based on the potential exposure to airborne particulates, aerosols, or vapours from cytotoxic substance for each task in the workplace. Fit tests must also be carried out before a respirator is issued to a worker. The worker must perform a seal check before each use. Respirator fit testing is performed by. More information on how to select an appropriate respirator can be found in the WorkSafe BC publication Breathe Safer: How to Use Respirators Safely and Start a Respirator Program. 8. EDUCATION / TRAINING A worker involved in any aspect of handling a cytotoxic drug must receive pre-job education and on-the-job training on the handling of this substance (WorkSafe BC Regulation 6.50). As necessary, personnel will be trained to a level of demonstrated competency. While not necessarily an exhaustive list, education and training may include: known health risks, including any potential reproductive hazards, relevant techniques and procedures for safe handling, proper use of protective equipment and materials, and spill and waste disposal procedures procedures for reporting known exposures and suspected health effects. The adequacy of instruction must be assessed when required by a change in the substance used, information available on the substance or a change in work procedures. Refresher training is required every 3 years. According to the regulation, training records must be maintained for 3 years from the date that the training occurred. The Occupational & Preventive Health, on Vancouver UBC campus, provides services and programs to help UBC employees manage potential risks in the workplace. 9. WRITTEN WORK PROCEDURES The OHS Regulation requires that an Exposure Control Plan (ECP) for cytotoxics substances be developed and implemented when a worker may be occupationally exposed to a cytotoxic substance. Title: Exposure Control Plan for Cytotoxic Substances Page 11 of 13
12 The ECP must be reviewed at least annually and updated as necessary by the employer, in consultation with the joint committee or the worker health and safety representative, as applicable. The employer must ensure site-specific ECPs and written safe work procedures are developed and implemented to ensure controls are in place to minimize the risk of exposure to cytotoxic substances. The ECPs and safe work procedures must be made readily available to workers. Refer to the following documents: Appendix D: Job Tasks and Controls Table o This table lists job tasks where exposure to cytotoxic substances may occur and respective controls that are to be implemented to avoid exposure to cytotoxic substances. Specific Safe Work Procedure are generated based on the identified tasks. Task-Specific Safe Work Procedures o Cytotoxics Spill Cleanup Procedure (UBC-RMS-OHS-SWP ) o Receiving and Storage of Cytotoxic Substances (UBC-RMS-OHS-SWP ) o Cytotoxic Waste Management (under development) 10. HYGIENE FACILITIES Hygiene facilities must be made available to permit proper hand washing and cleaning of reusable personal protective equipment such as respirators and safety eyewear. Reusable gowns / lab coats must be laundered and changed regularly. Gowns / lab coats must not be worn outside the designated work area (ie. must be removed while eating lunch). Disposable gowns / lab coats must be discarded accordingly. Eating, drinking, smoking, application of cosmetics or storage of food is prohibited in any area where a cytotoxic drug is mixed, administered or stored. 11. HEALTH MONITORING Health monitoring may be required if workers are exposed to cytotoxic substances. The necessity of health monitoring will be decided from case to case by an occupational health professional. Contact Occupational & Preventive Health, on Vancouver UBC campus, with any questions and concerns. All personnel involved in any aspect of the handling of cytotoxic substances should be informed about the risks of occupational exposure to hazardous drugs. A worker who is pregnant or intents to conceive, should advise her supervisor. The supervisor must develop policy and procedures appropriate to the risk, which may include protective reassignment. Title: Exposure Control Plan for Cytotoxic Substances Page 12 of 13
13 12. DOCUMENTATION Documentation associated with the Cytotoxics Exposure Program will need to be maintained. The documentation includes, but not limited to: Cytotoxics Exposure Program, ECP and safe work procedures Project/task specific cytotoxics ECP and safe work procedures Cytotoxics education and training records First aid records pertaining to cytotoxics exposures Incident/accident investigation reports pertaining to cytotoxics exposures WorkSafeBC inspection reports, if applicable Respirator fit test records Safety meeting minutes 13. PROGRAM REVIEW This program is to be reviewed at least annually by the responsible departmental representative in. 14. REFERENCES Occupational Health and Safety Regulations Part 5.54 Exposure Control Plan Occupational Health and Safety Regulations Part 6.42 Cytotoxic Drugs WorkSafe BC Best Practices for the Safe Handling of Hazardous Drugs (2015) Government of South Australia Cytotoxic Drugs and Related Waste (2015) Workplace Health and Safety Queensland Guide for handling cytotoxic drugs and related waste (2017) BC Cancer Agency Cytotoxic chemicals handling and disposal (SOP: CYT-001, 2015) Australian Medicines Handbook (2014) 15. DOCUMENT INFORMATION Written / Reviewed by: Contact: RMS Advisor, Chemical Safety researchsafety@rms.ubc.ca Title: Exposure Control Plan for Cytotoxic Substances Page 13 of 13
14 APPENDIX A: WORKSAFE BC CYTOTOXIC DRUGS REGULATION 6.42 Definition In sections 6.43 to 6.58 "cytotoxic drug" means an agent that possesses a specific destructive action on certain cells or that may be genotoxic, oncogenic, mutagenic, teratogenic, or hazardous to cells in any way and includes most anti-cancer drugs Exposure control plan If a worker is or may be occupationally exposed to a cytotoxic drug, the employer must develop and implement an exposure control plan meeting the requirements of section Information If a cytotoxic drug is received, prepared, administered, stored or disposed of at a workplace, the employer must maintain and make readily available to workers information on its (a) acute and chronic toxicity, including any potential reproductive hazard, (b) acute exposure treatment, and (c) safe handling. [Amended by B.C. Reg. 21/2006, effective May 17, 2006.] 6.45 Labels A container of a cytotoxic drug and a shelf or bin where a cytotoxic drug is regularly stored must be appropriately labelled Signs Warning signs which are clearly visible and clearly state the identified hazards must be posted in all areas where cytotoxic drugs are stored or mixed List Storage and preparation areas for cytotoxic drugs must be posted with a list of all cytotoxic drugs present in the workplace Procedures (1) When a cytotoxic drug is received, prepared, administered, stored or disposed of, written safe work procedures must be developed and implemented for applicable aspects of receiving, storage, preparation, administration and waste handling. APPENDIX A Title: Exposure Control Plan for Cytotoxic Substances Page 1
15 (2) The work procedures required by subsection (1) must be readily available for reference by workers and where practicable, summaries of relevant procedures must be posted in the appropriate work areas Reproductive toxins (1) At any worksite where a worker is occupationally exposed to a cytotoxic drug that is a reproductive toxin, the employer must develop policy and procedures appropriate to the risk, which may include protective reassignment. (2) The policy and procedures must inform workers about the reproductive toxin and identify ways to minimize exposure to the reproductive toxin for a worker who has advised the employer of pregnancy or intent to conceive a child Instruction (1) A worker involved in any aspect of handling a cytotoxic drug must receive pre-job education and on-the-job training on the handling of this substance. (2) The instruction required by subsection (1) must address the (a) known health risks, including any potential reproductive hazards, (b) relevant techniques and procedures for safe handling, (c) proper use of protective equipment and materials, and (d) spill and waste disposal procedures. (3) The adequacy of instruction must be assessed when required by a change in the substance used, information available on the substance or a change in work procedures, and retraining provided where necessary Supervision A worker involved in any aspect of cytotoxic drug handling must be effectively supervised Records (1) The employer must maintain a record of all workers who prepare or administer cytotoxic drugs, including the name of the drugs handled, and when practicable, the number of preparations or administrations per week. (2) Exposure records must be maintained for the duration of employment plus 10 years, and training records for 3 years from the date that the training occurred Drug preparation and administration (1) All mixing, preparation and priming of administration sets with a cytotoxic drug must be performed in one centralized area in a specially designated Class II Type B biological safety cabinet that APPENDIX A Title: Exposure Control Plan for Cytotoxic Substances Page 2
16 (a) is exhausted to the outside atmosphere in a manner that prevents recirculation into any work area, (b) has exhaust and ventilation systems that remain in operation for a sufficient period of time to ensure that no contaminants escape from the biological safety cabinet into the workplace, and (c) is equipped with a continuous monitoring device to permit confirmation of adequate airflow and cabinet performance. (2) The administration of cytotoxic drugs must be done by following safe work procedures Disconnects Syringes and intravenous sets used for cytotoxic drugs must have appropriate fittings, such as Luer locking fittings, which prevent accidental disconnection Personal protective equipment (1) Adequate personal protective equipment must be provided and worn whenever there is a risk of contact with a cytotoxic drug. (2) For the purposes of subsection (1) personal protective equipment includes (a) medical gloves that are manufactured and designed for use when handling cytotoxic drugs, (b) a moisture resistant, long-sleeved gown with cuffs, (c) if there is a risk of contact with aerosols, an approved respirator, and (d) if there is a risk of eye contact, eye and face protection. (3) used gowns and gloves must not be worn outside the preparation, administration or storage area and must be handled as hazardous waste or contaminated linen. (4) all other non-disposable personal protective equipment must be cleaned immediately after use Personal hygiene Eating, drinking, smoking, application of cosmetics or storage of food is prohibited in any area where a cytotoxic drug is mixed, administered or stored Waste disposal (1) Adequate, leak-proof waste disposal containers, including sharps and solids containers, and distinctive plastic waste bags must be available in every area where cytotoxic drugs are prepared, administered or stored, and all cytotoxic drug-related waste must be placed into these containers or bags. (2) Any excreta from a patient being treated with cytotoxic drugs that is handled by a worker must be treated as cytotoxic drug-related waste. APPENDIX A Title: Exposure Control Plan for Cytotoxic Substances Page 3
17 6.58 Spills (1) Written emergency procedures to address spills of a cytotoxic drug must be developed and implemented which address requirements for small spill cleanup, both inside and outside the biological safety cabinet, large spill cleanup, and personal decontamination. (2) Spill kits, clearly labelled, must be kept in or near cytotoxic drug preparation, administration and storage areas and a sign detailing spill procedures must be posted in all such areas. APPENDIX A Title: Exposure Control Plan for Cytotoxic Substances Page 4
18 APPENDIX B: COMMON CYTOTOXIC DRUGS (WORKSAFE BC LIST)* Altretamine Estramustine Mitoxantrone Aminogluthethimide Ethinyl Estradiol Nafarelin Azathioprine Etoposide Pipobroman L-Asparaginase Floxuridine Plicamycin Bleomycin Fluorouracil Procarbazine Busulfan Flutamide Ribavirin Carboplatin Glanciclovir Streptozocin Carmustine Hydroxyurea Tamoxifen Chlorambucil Idarubicin Testolactone Chloramphenicol Ifosfamide Thioguanine Chlorozotocin Interferon-A Thiotepa Cyclosporin Isotretinoin Uracil Mustard Cisplatin Leuprolide Vidarabine Cyclo-phosphamide Levamisole Vinblastine Cytarrabine Lomustine Vincristine Dacarbazine Mechlorethamine Zidovudine Dactinomycin Daunorubicin Diethyl-stilbestrol Doxorubicin Estradiol Medroxy-progesterone Megestrol Melphalan Mercaptopurine Methotrexate * This is not an exhaustive list; consult the (M)SDS of the chemical compound to see if it qualifies as a cytotoxic substance. APPENDIX B Title: Exposure Control Plan for Cytotoxic Substances Page 1
19 BC CANCER AGENCY HAZARDOUS DRUGS LIST Abiraterone 1 Enzalutamide 1 PACLitaxel 1 Acitretin 3 Epirubicin 1 PACLitaxel, nab 4 AFAtinib 1 eribulin 1 Palbociclib 4 Amsacrine 1 Erlotinib 1 PAZOPanib 1 Anastrozole 1 Estramustine 1 Pemetrexed 1 Arsenic trioxide 1 Etoposide 1 PERTuzumab 1 Atezolizumab 1 Everolimus 1 Plerixafor 3 axitinib 1 Exemestane 1 Pomalidomide 1 azacitidine 1 PONATtinib 1 Bacillus Calmette Guerin (BCG)* 1 Fludarabine 1 Bendamustine HCl 1 Fluorouracil 1 Bexarotene 1 Flutamide 1 Procarbazine 1 Bicalutamide 1 Fulvestrant 1 Quinagolide 4 Bleomycin 1 Blinatumomab 4 Gemcitabine 1 Raltitrexed 4 Bortezomib 1 Geserelin 1 Regorafenib 1 Bosutinib 1 Retrovisrus serotype 3 Dearing strain* 4 Brentuximab vedotin 1 romidepsin 1 Buserelin 4 hydroxyurea 1 Ruxolitinib 4 Busulfan 1 Cabazitaxel 1 IDArubicin 1 Cabergoline 3 Ifosfamide 1 SORAfenib 1 Capecitabine 1 imatinib mesylate 1 Streptozocin 1 CARBOplatin 1 Irinotecan HCl 1 SUNItinib malate 1 Carfilzomib 1 Irinotecan, liposome 4 APPENDIX B Title: Exposure Control Plan for Cytotoxic Substances Page 2
20 Carmustine 1 Ixabepilone 1 Chlorambucil 1 Tamoxifen 1 CISplatin 1 Lanhreotide 4 Temozolomide 1 Cladribine 1 Lapatinib 4 Temsirolimus 1 Cobimetinib 4 Lenalitomide 2 Teniposide 1 Crizotinib 1 Lenvatinib 4 Testosterone 3 Cyclophosphamide 1 Letrozole 1 Thalidomide 2 cyclosporine 2 Leuprolide acetate 1 Thioguanidine 1 cytarabine Lomustine 1 Thiotepa 1 Topotecan 1 Mechlorethamine 1 Trametinib 1 medroxyprogesterone acetate 2 Trastuzumab (HERCEPTIN ) 1 dabrafenib 1 Megestrol 1 TRC105 (carotuximab) 4 Dacarbazine 1 Melphalan 1 Tretinoin 3 DACTINomycin 1 Mercaptopurine 1 Daratumumab 4 Methotrexate 1 vandetanib 1 dasatinib 1 Mitomycin 1 vemurafenib 1 DAUNOrubicin HCl 1 Mitotane 1 vinblastine sulfate 1 Degarelix 1 mitoxantrone 1 vincristine sulfate 1 Dexrazoxane 2 Vinorelbine tartrate 1 DOCEtaxel 1 nilotinib 1 Vismodegib 1 DOXOrubicin 1 DOXOrubicin, pegylated 4 Olaparib 4 Zoledronic acid 3 Oxaliplatin 1 * designated as biohazardous (BioHD) at BCCA Numerical superscripts 1, 2, or 3 refer to tables contained within the NIOSH 2014 List as below: 1. NIOSH Table 1 (hazardous antineoplastic drugs) 2. NIOSH Table 2 (hazardous non-antineoplastic drugs) 3. NIOSH Table 3 (hazardous non-antineoplastic drugs with primarily reproductive effects) Numerical superscript 4 refers to drugs evaluated by BCCA per Provincial Pharmacy Directive VI-80 Hazardous Drug List APPENDIX B Title: Exposure Control Plan for Cytotoxic Substances Page 3
21 APPENDIX C: EXCRETION RATES OF SEVERAL CYTOTOXIC DRUGS* Drug name Excretion Rate** Precautionary time period following cytotoxic drug treatment when handling body waste (excreta)*** Urine Feces Bleomycin Urine: up to 68% in first 24 h 3 days Carboplatin Urine: up to 60% in first 24 h 2 days Carmustine Urine: up to 65% in first 24 h 4 days Chlorambucil 2 days Cisplatin Urine: up to 75% in first 5 days 7 days Cyclophosphamide Urine: up to 62% in first 48 h 3 days in urine, sweet and saliva 5 days Cytarabine Urine: up to 90% in first 24 h 1 day Dacarbazine 1 day Daunorubicin 7 days 7 days Docetaxel Urine: up to 60% in first 24 h 1 day 2 days Doxorubicin Urine: up to 15% in first 5 days Feces: up to 85% 6 days 7 days Epirubicin Urine: up to 11% in first 24 h 3 days Etoposide Urine: up to 50% in first 24 h Feces: up to 15% in first 24 h 3 days 5 days Fludarabine Urine: up to 60% in first 24 h 3 days Fluorouracil (5-FU) Urine: up to 15% in first 24 h 2 days 5 days Gemcitabine 1 day Idarubicin 3 days 2 days Ifosfamide 2 days Melphalan Urine: up to 60% over first 24 h 2 days 7 days Mercaptopurine Up to 40% in first 24 h 2 days 5 days Methotrexate Urine: up to 90% in first 48 h Feces: up to 9% 3 days 7 days APPENDIX C Title: Exposure Control Plan for Cytotoxic Substances Page 1
22 Mitomycin Mitozantrone Urine: up to 6.5% over first 5 days Feces: up to 18% 1 day 6 days 7 days Oxaliplatin Urine: up to 50% in first 24 h 3 days Paclitaxel Urine and feces: at least 13% in first 24 h 3 days 5 days Procarbazine Urine: up o 70% in first 30 days 3 days Teniposide Thioguanine Topotecan Vinblastine Vincristine Urine: up to 33% in first 3 days Feces: up to 41% in first 3 days Urine: up to 8% in first 3 days Feces: up to 40% in first 3 days 3 days 1 day 2 days 4 days 7 days 4 days 7 days Vinorelbine 4 days 7 days * Data from Australian Medicines Handbook July 2014 ( ) ** The majority of cytotoxic drugs will be excreted within 7 days however some may require longer precautionary periods. If the cytotoxic drug excretion rate is not known use precautions for at least 48 hours following treatment. *** May be unchanged drug or metabolite APPENDIX C Title: Exposure Control Plan for Cytotoxic Substances Page 2
23 APPENDIX D: JOB TASKS AND CONTROL TABLE - ACCEPTABLE CONTROL METHODS FOR SPECIFIC JOB TASKS Work Activity Control Methods Personal Protective Equipment (PPE) Comments Receiving and storage of cytotoxic substances Transport of cytotoxic substances Restricted access area Negative pressure with respect to surrounding rooms Storage shelves with fall guards Transport cytotoxic substances in equipment that reduces spills Use equipment that is easy to clean Recommended gloves for use with cytotoxic substances Gowns / lab coats An approved and fit-tested respirator (if handling damaged packages containing cytotoxic substances) Recommended gloves for use with cytotoxic substances Gowns / lab coats Surface contamination of containers and packaging is a potential source of exposure for workers as they unpack and store incoming shipments of cytotoxic substances (drugs). Workers who transport cytotoxic substances throughout a facility may be exposed if they come in contact with contaminated surfaces or a cytotoxic substances leak or spill. Cytotoxic drug preparation Preparation is done in a designated and controlled area, all air should be exhausted externally A closed-system transfer device is used when appropriate Safety engineered needles are used to reduce the risk of percutaneous exposure Furniture and equipment is made of materials that are easily cleaned and decontaminated (i.e. stainless steel) Drug preparation is done only inside a BSC Type II B Follow recommended procedures for handling waste and/or cleaning spills Recommended gloves for use with cytotoxic substances Disposable gown Shoe covers Eye and, if needed, face protection An approved and fit-tested respirator (if necessary) Workers who prepare (compound, dilute, mix, etc.) cytotoxic drugs can be exposed APPENDIX D Title: Exposure Control Plan for Cytotoxic Substances Page 1
24 Work Activity Control Methods Personal Protective Equipment (PPE) Comments Cytotoxic drug administration Handling cytotoxic substances in a research lab (synthesis, tissue culture, etc.) Non-invasive monitoring of animals treated with cytotoxic drugs (during the precautionary period) Treatment of animals should be done in an area kept under neutral or negative air pressure; a BSC Type II B is recommended A closed-system transfer device is used when appropriate Safety engineered needles are used to reduce the risk of percutaneous exposure Use disposable, absorbent pads to capture any possible spills Use bandages over areas where a topical medication has been applied Follow recommended procedures for handling waste and/or cleaning spills All handling should be done inside a fume hood or a BSC Use disposable, absorbent pads to capture any possible spills Use safety engineered needles and disposable syringes when necessary Follow recommended procedures for handling waste and/or cleaning spills Animals should be housed in microisolator type cages during the precautionary period (the period for which the animal excretes the drug or metabolites of the drug, see Appendix C) Removal of animal from the cage should be done inside a BSC Type II B Recommended gloves for use with cytotoxic substances Gowns / lab coats Eye and, if needed, face protection Recommended gloves for use with cytotoxic substances Lab coat Eye protection Recommended gloves for use with cytotoxic substances Gown or lab coat Administration of cytotoxic drugs can take many forms (i.e. IV, inhalation, injections, oral or topical) and workers can be exposed by using equipment that may leak or spill, by directly handling medication, by exposure to body fluids, etc. Working with cytotoxic substances in a research laboratory requires Handling the container containing the substance Transfer of the substance from original container to a secondary vessel Cleaning the glassware used Non-invasive monitoring tasks include observing the animal without direct contact as well as procedures requiring physically handling the animal (i.e. weighing, injection site evaluation, and electrocardiogram). APPENDIX D Title: Exposure Control Plan for Cytotoxic Substances Page 2
25 Work Activity Control Methods Personal Protective Equipment (PPE) Comments Invasive monitoring of animals treated with cytotoxic drugs (during the precautionary period) Bed changing and cage cleaning Waste disposal Spill response Procedures should be done in a BSC Type II B Use disposable, absorbent pads to capture any possible spills Use safety engineered needles and disposable syringes when necessary Follow recommended procedures for handling waste and/or cleaning spills Identify animals who have received cytotoxic drugs (name of drug and day/time of administration on cage) The use of pressure washers for cleaning purposes is prohibited Cleaning of contaminated cages (during the precautionary time) should be done inside a fume hood or BSC See Appendix C. Contaminated waste should be disposed off according to procedures Adequate, leak-proof waste disposal containers, including sharps and solids containers are available and labelled as cytotoxic waste Procedures for disposal of contaminated waste are in place Animal carcases are disposed of as biohazardous waste Supply of spill kits are available in area where cytotoxic substances are handled Regular training on emergency spill procedures is provided Recommended gloves for use with cytotoxic substances Disposable gown Eye protection Heavy duty cleaning gloves on top of disposable gloves Gown / lab coat Eye protection An approved and fit-tested respirator (if task is not completed inside a fume hood or BSC during the precautionary period) Recommended gloves for use with cytotoxic substances Gown / lab coat Heavy duty cleaning gloves on top of disposable gloves Disposable gown Eye and face protection An approved and fit-tested respirator Invasive monitoring tasks involve direct contact with bodily fluids (i.e. surgery, IV treatment) Cleaning cages or changing bedding of animals receiving cytotoxic drugs exposes the worker to contaminated residues resulted from the animals excreting the drug or metabolites. Workers can be exposed to cytotoxic substances via contact with contaminated waste and/or waste containers While responding to a spill, workers can be exposed to a leak, spill, or can inhale aerosols, vapours or particulates released. APPENDIX D Title: Exposure Control Plan for Cytotoxic Substances Page 3
MEDICAL NECESSITY GUIDELINE
 PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
British Columbia Cancer Agency
 Page 1 of 12 A. SCOPE This Policy covers the preparation, administration, and disposal of hazardous drugs. See Cancer Drug Manual Appendix 5 for the Hazardous Drug List. (www.bccancer.bc.ca/hpi/drugdatabase/appendices)
Page 1 of 12 A. SCOPE This Policy covers the preparation, administration, and disposal of hazardous drugs. See Cancer Drug Manual Appendix 5 for the Hazardous Drug List. (www.bccancer.bc.ca/hpi/drugdatabase/appendices)
Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites.
 WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
SAM HOUSTON STATE UNIVERSITY ENVIRONMENTAL HEALTH, SAFETY & RISK MANAGMENT
 BLOODBORNE PATHOGENS PROGRAM I. PURPOSE The SHSU Bloodborne Pathogens program ensures SHSU compliance with Occupational Safety and Health (OSHA) Standard, 29 CFR 1910.1030, Blood Borne Pathogens. II. SCOPE
BLOODBORNE PATHOGENS PROGRAM I. PURPOSE The SHSU Bloodborne Pathogens program ensures SHSU compliance with Occupational Safety and Health (OSHA) Standard, 29 CFR 1910.1030, Blood Borne Pathogens. II. SCOPE
STANDARD OPERATING PROCEDURE #714 USE OF MPTP IN NHPS
 STANDARD OPERATING PROCEDURE #714 USE OF MPTP IN NHPS 1. PURPOSE This Standard Operating Procedure (SOP) describes the guidelines for the use of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) in
STANDARD OPERATING PROCEDURE #714 USE OF MPTP IN NHPS 1. PURPOSE This Standard Operating Procedure (SOP) describes the guidelines for the use of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) in
REFERENCES American Society of Health-System Pharmacists. ASHP guidelines on handling hazardous drugs. Am J Health-Syst Pharm. 2006; 63:
 Ordering and Handling Applies To: UNM Hospitals Responsible Department: Pharmacy Revised: 8/2017 Procedure Patient Age Group: ( ) N/A (X) All Ages ( )Newborns ( )Pediatric ( )Adult DESCRIPTION/OVERVIEW
Ordering and Handling Applies To: UNM Hospitals Responsible Department: Pharmacy Revised: 8/2017 Procedure Patient Age Group: ( ) N/A (X) All Ages ( )Newborns ( )Pediatric ( )Adult DESCRIPTION/OVERVIEW
METHOTREXATE CLINICAL PRACTICE GUIDELINES FOR STAFF
 Mary Pack Arthritis Program METHOTREXATE CLINICAL PRACTICE GUIDELINES FOR STAFF Handling of premixed injectable Methotrexate (MTX) by employees who are pregnant, breast-feeding or attempting to reproduce
Mary Pack Arthritis Program METHOTREXATE CLINICAL PRACTICE GUIDELINES FOR STAFF Handling of premixed injectable Methotrexate (MTX) by employees who are pregnant, breast-feeding or attempting to reproduce
 Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Hazardous Medication List
 Hazardous Medication List REDUCING OCCUPATIONAL EXPOSURE TO HAZARDOUS MEDICATION FOR ALL STAFF Table of Contents Hazardous Medication List Key Points... 1 Hazardous Medication List... 2 Special Handling
Hazardous Medication List REDUCING OCCUPATIONAL EXPOSURE TO HAZARDOUS MEDICATION FOR ALL STAFF Table of Contents Hazardous Medication List Key Points... 1 Hazardous Medication List... 2 Special Handling
PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1
 PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1 RELATED POLICY: 5.21REV PAGE NO.: 1 OF 9 RESPONSIBLE ADMINISTRATOR(S): VPF&A/EHS EFECTIVE DATE: 07/11/14 NEXT REVIEW DATE:
PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1 RELATED POLICY: 5.21REV PAGE NO.: 1 OF 9 RESPONSIBLE ADMINISTRATOR(S): VPF&A/EHS EFECTIVE DATE: 07/11/14 NEXT REVIEW DATE:
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting
 West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
Guidelines on Chemotherapy-induced Nausea and Vomiting in Pediatric Cancer Patients
 Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
Harvard University Exposure Control Plan
 Harvard University Exposure Control Plan Harvard University is committed to providing a safe and healthy work environment. In accordance with this goal, the Occupational Health and Safety Administration
Harvard University Exposure Control Plan Harvard University is committed to providing a safe and healthy work environment. In accordance with this goal, the Occupational Health and Safety Administration
Bloodborne Pathogens Training (OHS_BIO500) Course Material
 Introduction (OHS_BIO500) Course Material Welcome to the Bloodborne Pathogens (BBP) Training Course (OHS_BIO500). UAB Campus Employees whose job duties put them at increased risk for exposure to bloodborne
Introduction (OHS_BIO500) Course Material Welcome to the Bloodborne Pathogens (BBP) Training Course (OHS_BIO500). UAB Campus Employees whose job duties put them at increased risk for exposure to bloodborne
TIP NO AEROSOLIZED DRUGS TECHNICAL INFORMATION PAPER NO PURPOSE.
 AEROSOLIZED DRUGS TECHNICAL INFORMATION PAPER NO. 56-088-0618 PURPOSE. Healthcare workers risk exposure to exhaled aerosolized drugs along with infectious airborne pathogens during administration and routine
AEROSOLIZED DRUGS TECHNICAL INFORMATION PAPER NO. 56-088-0618 PURPOSE. Healthcare workers risk exposure to exhaled aerosolized drugs along with infectious airborne pathogens during administration and routine
Assessing the Risk of Laboratory Acquired Allergies
 Guideline Created By: Stephanie Thomson, Kelly Eaton, Sonam Uppal & Hollie Burrage Edited By: N/A Workplace Health Services Occupational & Preventive Health Unit Effective date: January 8, 2015 Review
Guideline Created By: Stephanie Thomson, Kelly Eaton, Sonam Uppal & Hollie Burrage Edited By: N/A Workplace Health Services Occupational & Preventive Health Unit Effective date: January 8, 2015 Review
Safety Committee Prototypical Safety Program Manual
 1 Bloodborne Pathogens Exposure Control Plan Policy The Department Bloodborne Pathogens Exposure Control Plan is designed to comply with the requirements of the OSHA Bloodborne Pathogens Standard, 29 CFR
1 Bloodborne Pathogens Exposure Control Plan Policy The Department Bloodborne Pathogens Exposure Control Plan is designed to comply with the requirements of the OSHA Bloodborne Pathogens Standard, 29 CFR
Protocol Number Tumour Group Protocol Name on NCCP website 22/02/ Lung Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy
 Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
DOXOrubicin, Cyclophosphamide (AC 60/600) 21 day followed by weekly PACLitaxel (80) Therapy (AC-T) 261 CARBOplatin (AUC4-6) Monotherapy-21 days
 Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Infection Control. Chapter 11 Intro to HST
 Infection Control Chapter 11 Intro to HST All health care workers must understand basic infection control Key terms Pathogen: germ Microorganism: small, living organism that is not visible to the naked
Infection Control Chapter 11 Intro to HST All health care workers must understand basic infection control Key terms Pathogen: germ Microorganism: small, living organism that is not visible to the naked
LEAD SAFETY PROGRAM. Purpose. Scope. Responsibilities. Southern Heat Exchanger Services Safety Program
 Page: Page 1 of 5 Purpose The purpose of this procedure is to identify the controls and actions necessary to prevent adverse health effects to employees from occupational exposure to lead, and to ensure
Page: Page 1 of 5 Purpose The purpose of this procedure is to identify the controls and actions necessary to prevent adverse health effects to employees from occupational exposure to lead, and to ensure
"BLOODBORNE PATHOGENS IN COMMERCIAL AND LIGHT INDUSTRIAL FACILITIES"
 MAJOR PROGRAM POINTS "BLOODBORNE PATHOGENS IN COMMERCIAL AND LIGHT INDUSTRIAL FACILITIES" Training for THE OSHA BLOODBORNE PATHOGENS STANDARD Quality Safety and Health Products, for Today... and Tomorrow
MAJOR PROGRAM POINTS "BLOODBORNE PATHOGENS IN COMMERCIAL AND LIGHT INDUSTRIAL FACILITIES" Training for THE OSHA BLOODBORNE PATHOGENS STANDARD Quality Safety and Health Products, for Today... and Tomorrow
Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249
 Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
Description The following are synthetic cannabinoids requiring prior authorization: dronabinol (Marinol, Syndros ), nabilone (Cesamet )
 Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
Naval Support Activity Monterey / Naval Postgraduate School
 Background: Bloodborne pathogens are viruses present in human blood and body fluids that can cause disease in humans. Diseases like the hepatitis B virus (HBV), human immunodeficiency virus (HIV) and others
Background: Bloodborne pathogens are viruses present in human blood and body fluids that can cause disease in humans. Diseases like the hepatitis B virus (HBV), human immunodeficiency virus (HIV) and others
LeadingAge Florida February 24, 2016
 BLOODBORNE PATHOGENS EMPLOYEE HANDBOOK 1 POLICY EMPLOYEE ORIENTATION AND IN-SERVICE TO BLOODBORNE PATHOGENS AWARENESS It is the policy of Healthcare Services Group, Inc., to ensure that all employees with
BLOODBORNE PATHOGENS EMPLOYEE HANDBOOK 1 POLICY EMPLOYEE ORIENTATION AND IN-SERVICE TO BLOODBORNE PATHOGENS AWARENESS It is the policy of Healthcare Services Group, Inc., to ensure that all employees with
Drew University Bloodborne Pathogens Exposure Control Plan and Procedures
 PURPOSE To provide a written plan for preventing and/or minimizing exposure to bloodborne pathogens for those Drew University personnel who may be involved in the handling of human blood, blood products,
PURPOSE To provide a written plan for preventing and/or minimizing exposure to bloodborne pathogens for those Drew University personnel who may be involved in the handling of human blood, blood products,
Bloodborne Pathogens
 Bloodborne Pathogens Session Objectives Identify bloodborne pathogens (BBPs) Understand how diseases are transmitted Risk of exposure Protecting yourself from exposure through prevention Responding appropriately
Bloodborne Pathogens Session Objectives Identify bloodborne pathogens (BBPs) Understand how diseases are transmitted Risk of exposure Protecting yourself from exposure through prevention Responding appropriately
Subject: Palonosetron Hydrochloride (Aloxi )
 09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
Personal Protective Equipment:
 have, at a minimum a legible label to identify the contents even if it is water or benign material. Hazardous chemical containers must have the following: - Original manufacturer label with the date received
have, at a minimum a legible label to identify the contents even if it is water or benign material. Hazardous chemical containers must have the following: - Original manufacturer label with the date received
What employees should know about UNIVERSAL PRECAUTIONS. They re work practices that help prevent contact with blood and certain other body fluids.
 What are Universal Precautions? What employees should know about UNIVERSAL PRECAUTIONS They re work practices that help prevent contact with blood and certain other body fluids. Universal precautions are:
What are Universal Precautions? What employees should know about UNIVERSAL PRECAUTIONS They re work practices that help prevent contact with blood and certain other body fluids. Universal precautions are:
IARC - International Agency for Research on Cancer - Classifies carcinogens in the following manner:
 ESHD 5008, Section 8, Chapter 2 Carcinogens, Mutagens, and Teratogens Rev. 3 Page 1 of 10 Approved 10/21/98 CHAPTER 2 CARCINOGENS, MUTAGENS, AND TERATOGENS 2.1 INTRODUCTION The purpose of this chapter
ESHD 5008, Section 8, Chapter 2 Carcinogens, Mutagens, and Teratogens Rev. 3 Page 1 of 10 Approved 10/21/98 CHAPTER 2 CARCINOGENS, MUTAGENS, AND TERATOGENS 2.1 INTRODUCTION The purpose of this chapter
Bloodborne Pathogens. General
 Bloodborne Pathogens General Session Objectives Identify bloodborne pathogens (BBPs) Understand how diseases are transmitted Determine your risk of exposure Protect yourself from exposure through prevention
Bloodborne Pathogens General Session Objectives Identify bloodborne pathogens (BBPs) Understand how diseases are transmitted Determine your risk of exposure Protect yourself from exposure through prevention
ADMINISTRATIVE SERVICES MANUAL
 1 of 10 Purpose Scope University of Alaska Anchorage departments will develop plans and procedures to limit occupational exposure to blood and other potentially infectious materials (PIM) in compliance
1 of 10 Purpose Scope University of Alaska Anchorage departments will develop plans and procedures to limit occupational exposure to blood and other potentially infectious materials (PIM) in compliance
SUBJECT: Management of Human Body Fluids/Waste (Bloodborne Pathogens)
 Page 1 of 6 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 PURPOSE To establish uniform procedures for the safe management of human body fluids
Page 1 of 6 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 PURPOSE To establish uniform procedures for the safe management of human body fluids
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting
 Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
Infection Prevention and Control - General Orientation
 Infection Prevention and Control - General Orientation Hand Hygiene-CDC Isolation Precautions - CDC Medical Waste - OSHA Environmental Cleaning - CDC Safe Injection Practices - CDC Bloodborne Pathogens
Infection Prevention and Control - General Orientation Hand Hygiene-CDC Isolation Precautions - CDC Medical Waste - OSHA Environmental Cleaning - CDC Safe Injection Practices - CDC Bloodborne Pathogens
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced
 ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
Textile Chemist and Colorist & American Dyestuff Reporter. Vol. 32, No. 1, January Safe Handling of Enzymes
 Textile Chemist and Colorist & American Dyestuff Reporter Vol. 32, No. 1, January 2000 Safe Handling of Enzymes By the Enzyme Technical Association, Washington, D.C. Enzymes have been used for over 35
Textile Chemist and Colorist & American Dyestuff Reporter Vol. 32, No. 1, January 2000 Safe Handling of Enzymes By the Enzyme Technical Association, Washington, D.C. Enzymes have been used for over 35
EASTERN ILLINOIS UNIVERSITY OCCUPATIONAL HEALTH FOR STUDENTS
 EASTERN ILLINOIS UNIVERSITY OCCUPATIONAL HEALTH FOR STUDENTS The Public Health Service of the U.S. Department of Health and Human Services has directed research/teaching institutions to develop programs
EASTERN ILLINOIS UNIVERSITY OCCUPATIONAL HEALTH FOR STUDENTS The Public Health Service of the U.S. Department of Health and Human Services has directed research/teaching institutions to develop programs
STANDARD OPERATING PROCEDURES (SOP) FOR CARCINOGENS
 STANDARD OPERATING PROCEDURES (SOP) FOR CARCINOGENS INTRODUCTION: Standard operating procedures (SOP) are intended to provide you with general guidance on how to safely work with a specific class of chemical
STANDARD OPERATING PROCEDURES (SOP) FOR CARCINOGENS INTRODUCTION: Standard operating procedures (SOP) are intended to provide you with general guidance on how to safely work with a specific class of chemical
Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Objectives
 Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Susan Urba, M.D. University of Michigan Comprehensive Cancer Center Objectives Mechanisms of
Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Susan Urba, M.D. University of Michigan Comprehensive Cancer Center Objectives Mechanisms of
Safe Practices in Oral Anticancer Medications: Implications for Community Pharmacy
 Safe Practices in Oral Anticancer Medications: Implications for Community Pharmacy Faculty Disclosures Dr. Holle has provided content expertise on patient education materials for Innocrin Pharmaceuticals
Safe Practices in Oral Anticancer Medications: Implications for Community Pharmacy Faculty Disclosures Dr. Holle has provided content expertise on patient education materials for Innocrin Pharmaceuticals
Bloodborne Pathogens Training
 Bloodborne Pathogens Training OSHA S Bloodborne Pathogen Standard 29CFR 1910.1030 Employers must: Develop an Exposure Control Plan (ECP) that details their Bloodborne Pathogens (BBP) Program Provide employees
Bloodborne Pathogens Training OSHA S Bloodborne Pathogen Standard 29CFR 1910.1030 Employers must: Develop an Exposure Control Plan (ECP) that details their Bloodborne Pathogens (BBP) Program Provide employees
Guidelines for the Use of Anti-Emetics with Chemotherapy
 Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Bloodborne Pathogens, Title 29 Code of Federal Regulations (CFR) Part
 STANDARDS Bloodborne Pathogens, Title 29 Code of Federal Regulations (CFR) Part 1910.1030 1.0 PROCEDURE The Illinois Wesleyan University Physical Plant is committed to providing a safe and healthful work
STANDARDS Bloodborne Pathogens, Title 29 Code of Federal Regulations (CFR) Part 1910.1030 1.0 PROCEDURE The Illinois Wesleyan University Physical Plant is committed to providing a safe and healthful work
Biosafety Level 2 Standard Practices and Containment Office of Research Safety Affairs
 Biosafety Level 2 Standard Practices and Containment Office of Research Safety Affairs Purpose and Objectives Purpose To promote the safe conduct of research with biohazardous materials known to present
Biosafety Level 2 Standard Practices and Containment Office of Research Safety Affairs Purpose and Objectives Purpose To promote the safe conduct of research with biohazardous materials known to present
Biosafety Level 3 (BL3)
 Biosafety Level 3 (BL3) NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (Appendix G-II-C) - April 2016 Biosafety Level 3 (BL3) Biosafety Level 3 is applicable to clinical,
Biosafety Level 3 (BL3) NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (Appendix G-II-C) - April 2016 Biosafety Level 3 (BL3) Biosafety Level 3 is applicable to clinical,
Lab Animal Allergens: Exposure Control Plan Revised November 2016
 Lab Animal Allergens: Exposure Control Plan Revised November 2016 Contents Purpose of Document... 2 Lab Animal Allergies... 2 Roles and Responsibilities... 2 UBC Departmental Managers/Supervisors/PIs will:...
Lab Animal Allergens: Exposure Control Plan Revised November 2016 Contents Purpose of Document... 2 Lab Animal Allergies... 2 Roles and Responsibilities... 2 UBC Departmental Managers/Supervisors/PIs will:...
Welcome to this training session about bloodborne pathogens. This session is intended for any employee who is likely to be exposed to blood or
 Welcome to this training session about bloodborne pathogens. This session is intended for any employee who is likely to be exposed to blood or potentially infectious bodily fluids. These employees include
Welcome to this training session about bloodborne pathogens. This session is intended for any employee who is likely to be exposed to blood or potentially infectious bodily fluids. These employees include
PANDEMIC INFLUENZA PHASE 6 INFECTION CONTROL RECOMMENDATIONS TEMPLATE
 PANDEMIC INFLUENZA PHASE 6 INFECTION CONTROL RECOMMENDATIONS TEMPLATE (Updated September 7, 2006) Information and concept courtesy Of the San Francisco Public Health Department Table of Contents Pandemic
PANDEMIC INFLUENZA PHASE 6 INFECTION CONTROL RECOMMENDATIONS TEMPLATE (Updated September 7, 2006) Information and concept courtesy Of the San Francisco Public Health Department Table of Contents Pandemic
Biosafety & Biosecurity in the Laboratory. Based on the OIE Terrestrial Manual for Diagnostic Tests and Vaccines
 Biosafety & Biosecurity in the Laboratory Based on the OIE Terrestrial Manual for Diagnostic Tests and Vaccines Keeping people and animals safe Keeping samples safe Definitions Biosafety: The measures
Biosafety & Biosecurity in the Laboratory Based on the OIE Terrestrial Manual for Diagnostic Tests and Vaccines Keeping people and animals safe Keeping samples safe Definitions Biosafety: The measures
Bloodborne Pathogens Exposure Control Plan
 San Bernardino County Superintendent of Schools Bloodborne Pathogens Exposure Control Plan BIOHAZARD 2018/2019 Title 8 California Code of Regulations Section 5193 Table of Contents PURPOSE OF THE PLAN...3
San Bernardino County Superintendent of Schools Bloodborne Pathogens Exposure Control Plan BIOHAZARD 2018/2019 Title 8 California Code of Regulations Section 5193 Table of Contents PURPOSE OF THE PLAN...3
AMBULANCE DECONTAMINATION GUIDELINES SUSPECTED INFLUENZA PATIENT
 AMBULANCE DECONTAMINATION GUIDELINES SUSPECTED INFLUENZA PATIENT Reprinted with the Permission of John Hill, President Iowa EMS Association Following are general guidelines for cleaning or maintaining
AMBULANCE DECONTAMINATION GUIDELINES SUSPECTED INFLUENZA PATIENT Reprinted with the Permission of John Hill, President Iowa EMS Association Following are general guidelines for cleaning or maintaining
Hazardous Drug Safety
 Annual Compliance Education This course contains annual compliance education necessary to meet compliance and regulatory requirements. Instructions: To receive credit for completion: 1. Read the content
Annual Compliance Education This course contains annual compliance education necessary to meet compliance and regulatory requirements. Instructions: To receive credit for completion: 1. Read the content
BLOODBORNE PATHOGEN 29 CFR !!
 Sample Written Program For Your Company For BLOODBORNE PATHOGEN 29 CFR 1910.1030 Provided By: P.O. Box 2136 Slidell, LA 70458 Phone: 985-781-1444 Fax: 985-718-4957 Email: info@se-safety.com Purpose Bloodborne
Sample Written Program For Your Company For BLOODBORNE PATHOGEN 29 CFR 1910.1030 Provided By: P.O. Box 2136 Slidell, LA 70458 Phone: 985-781-1444 Fax: 985-718-4957 Email: info@se-safety.com Purpose Bloodborne
Chapter 12. Preventing Infection. Elsevier items and derived items 2014, 2010 by Mosby, an imprint of Elsevier Inc. All rights reserved.
 Chapter 12 Preventing Infection Infection Infection is a major safety and health hazard. The health team follows certain practices and procedures to protect patients, residents, visitors, and staff from
Chapter 12 Preventing Infection Infection Infection is a major safety and health hazard. The health team follows certain practices and procedures to protect patients, residents, visitors, and staff from
FOR INFECTION TO OCCUR: Bloodborne Pathogens are viral diseases that can infect a person if they are exposed Hepatitis B Hepatitis C HIV
 OCCUPATIONAL EXPOSURE TO BLOODBORNE PATHOGENS Bloodborne Pathogens are viral diseases that can infect a person if they are exposed Hepatitis B Hepatitis C HIV FOR INFECTION TO OCCUR: A germ Bloodborne
OCCUPATIONAL EXPOSURE TO BLOODBORNE PATHOGENS Bloodborne Pathogens are viral diseases that can infect a person if they are exposed Hepatitis B Hepatitis C HIV FOR INFECTION TO OCCUR: A germ Bloodborne
Supplementary Figures
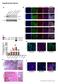 Supplementary Figures Supplementary Figure 1. Characterization of the generated hipsc lines a) Western blot analysis indicating the efficient downregulation of p53 upon knockdown in three hipsc lines (
Supplementary Figures Supplementary Figure 1. Characterization of the generated hipsc lines a) Western blot analysis indicating the efficient downregulation of p53 upon knockdown in three hipsc lines (
Primary malignant neoplasms, not lymphatic or hematopoietic. Secondary malignant neoplasms (i.e.metastatic) Malignant neoplasm, unknown site
 Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
2002, ERI PRODUCTIONS INC.
 ERI Safety Videos Videos for Safety Meetings PREVENTING EXPOSURE TO BLOODBORNE PATHOGENS Leader s Guide 2002, ERI PRODUCTIONS INC. PREVENTING EXPOSURE TO BLOODBORNE PATHOGENS This easy-to-use Leader s
ERI Safety Videos Videos for Safety Meetings PREVENTING EXPOSURE TO BLOODBORNE PATHOGENS Leader s Guide 2002, ERI PRODUCTIONS INC. PREVENTING EXPOSURE TO BLOODBORNE PATHOGENS This easy-to-use Leader s
Chapter 11 PREVENTING INFECTION. Elsevier items and derived items 2010 by Mosby, Inc. an affiliate of Elsevier Inc. All rights reserved
 Chapter 11 PREVENTING INFECTION Infection is a major safety and health hazard. The health team follows certain practices and procedures to protect patients, residents, visitors, and staff from infection.
Chapter 11 PREVENTING INFECTION Infection is a major safety and health hazard. The health team follows certain practices and procedures to protect patients, residents, visitors, and staff from infection.
The Bloodborne Pathogen Standard. An Overview
 The Bloodborne Pathogen Standard An Overview The Standard l In 1990, OSHA (Occupational Safety and Health Administration), developed the Bloodborne Pathogen Standard to protect workers by limiting occupational
The Bloodborne Pathogen Standard An Overview The Standard l In 1990, OSHA (Occupational Safety and Health Administration), developed the Bloodborne Pathogen Standard to protect workers by limiting occupational
TOPIC 4 HANDLING HEALTH PROTECTION & SAFETY PRACTICES FOR MEDICAL STAFF & WASTE HANDLERS TRAINING & PUBLIC EDUCATION
 TOPIC 4 HANDLING HEALTH PROTECTION & SAFETY PRACTICES FOR MEDICAL STAFF & WASTE HANDLERS TRAINING & PUBLIC EDUCATION Who Is AT RISK? Basic Questions Key Points General Principles Waste Handling Minimum
TOPIC 4 HANDLING HEALTH PROTECTION & SAFETY PRACTICES FOR MEDICAL STAFF & WASTE HANDLERS TRAINING & PUBLIC EDUCATION Who Is AT RISK? Basic Questions Key Points General Principles Waste Handling Minimum
BLOODBORNE PATHOGENS IN FIRST RESPONSE ENVIRONMENTS
 1708 BLOODBORNE PATHOGENS IN FIRST RESPONSE ENVIRONMENTS Leader s Guide Marcom Group Ltd. Structure and Organization Information in this program is presented in a definite order so that employees will
1708 BLOODBORNE PATHOGENS IN FIRST RESPONSE ENVIRONMENTS Leader s Guide Marcom Group Ltd. Structure and Organization Information in this program is presented in a definite order so that employees will
A. Background for Trainer: B. What OSHA Requires: Bloodborne Pathogens. Lesson Plan 6080a
 Lesson Plan 6080a This training session outline is designed to follow the accompanying booklet, OSHA s Bloodborne Pathogens Standard. The booklet reviews what employees who are potentially exposed to the
Lesson Plan 6080a This training session outline is designed to follow the accompanying booklet, OSHA s Bloodborne Pathogens Standard. The booklet reviews what employees who are potentially exposed to the
"BLOODBORNE PATHOGENS IN FIRST RESPONSE ENVIRONMENTS"
 MAJOR PROGRAM POINTS "BLOODBORNE PATHOGENS IN FIRST RESPONSE ENVIRONMENTS" Training for THE OSHA BLOODBORNE PATHOGENS STANDARD Quality Safety and Health Products, for Today... and Tomorrow Outline of Major
MAJOR PROGRAM POINTS "BLOODBORNE PATHOGENS IN FIRST RESPONSE ENVIRONMENTS" Training for THE OSHA BLOODBORNE PATHOGENS STANDARD Quality Safety and Health Products, for Today... and Tomorrow Outline of Major
P 1 Summary P 2 3 Part 1 General Information P 4 7 Part 2 Exposure among healthcare workers P 8 11 Part 3 Health Effects
 TOXICOLOGY PROFILE METHOTREXATE IN THE HEALTH CARE INDUSTRY P1Summary P23Part1GeneralInformation P47Part2Exposureamonghealthcareworkers P811Part3HealthEffects P8PulmonaryEffects P8EffectsonBone P8Hepatotoxicity
TOXICOLOGY PROFILE METHOTREXATE IN THE HEALTH CARE INDUSTRY P1Summary P23Part1GeneralInformation P47Part2Exposureamonghealthcareworkers P811Part3HealthEffects P8PulmonaryEffects P8EffectsonBone P8Hepatotoxicity
Center for Vaccine Biology and Immunology. Cell Sorting Facility Laboratory Safety Manual. Updated: January 25, 2012
 Center for Vaccine Biology and Immunology Cell Sorting Facility Laboratory Safety Manual Updated: January 25, 2012 I. Standard Laboratory Rules The standard BSL-2 precautions are outlined in the University
Center for Vaccine Biology and Immunology Cell Sorting Facility Laboratory Safety Manual Updated: January 25, 2012 I. Standard Laboratory Rules The standard BSL-2 precautions are outlined in the University
Occupational exposure to bloodborne pathogens
 Occupational exposure to bloodborne pathogens Dr. Sadeghniiat Professor of Tehran University of Medical Sciences Director of Iranian Occupational Medicine Association Head of Imam Khomeini complex hospital
Occupational exposure to bloodborne pathogens Dr. Sadeghniiat Professor of Tehran University of Medical Sciences Director of Iranian Occupational Medicine Association Head of Imam Khomeini complex hospital
Chapter 13. Preventing Infection. Copyright 2019 by Elsevier, Inc. All rights reserved.
 Chapter 13 Preventing Infection Copyright 2019 by Elsevier, Inc. All rights reserved. Lesson 13.1 Define the key terms and key abbreviations in this chapter. Identify what microbes need to live and grow.
Chapter 13 Preventing Infection Copyright 2019 by Elsevier, Inc. All rights reserved. Lesson 13.1 Define the key terms and key abbreviations in this chapter. Identify what microbes need to live and grow.
Cytotoxic Drugs. Table of Contents
 Cytotoxic Drugs Table of Contents Introduction 3 Reasons for concern 3 Sources of exposure 4 Responsibilities for controlling exposure 5 Guidelines for controlling exposures 6 The written program 6 Preparation
Cytotoxic Drugs Table of Contents Introduction 3 Reasons for concern 3 Sources of exposure 4 Responsibilities for controlling exposure 5 Guidelines for controlling exposures 6 The written program 6 Preparation
SCI. SickKids-Caribbean Initiative Enhancing Capacity for Care in Paediatric Cancer and Blood Disorders
 1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
AMENDATORY SECTION (Amending WSR , filed 10/10/95, effective 11/10/95)
 AMENDATORY SECTION (Amending WSR 95-21-041, filed 10/10/95, effective 11/10/95) WAC 246-817-601 Purpose. The purpose of WAC 246-817-601 through ((246-817-630)) 246-817-660 is to establish requirements
AMENDATORY SECTION (Amending WSR 95-21-041, filed 10/10/95, effective 11/10/95) WAC 246-817-601 Purpose. The purpose of WAC 246-817-601 through ((246-817-630)) 246-817-660 is to establish requirements
Bloodborne Pathogens in the Workplace
 Bloodborne Pathogens in the Workplace 1 What Are Bloodborne Pathogens? They are viruses, bacteria, and other microorganisms that: Are carried in a person s bloodstream Cause disease If a person comes in
Bloodborne Pathogens in the Workplace 1 What Are Bloodborne Pathogens? They are viruses, bacteria, and other microorganisms that: Are carried in a person s bloodstream Cause disease If a person comes in
"LEAD EXPOSURE IN GENERAL INDUSTRY"
 PRESENTER'S GUIDE "LEAD EXPOSURE IN GENERAL INDUSTRY" Training For THE OSHA LEAD STANDARD Quality Safety and Health Products, for Today... and Tomorrow OUTLINE OF MAJOR PROGRAM POINTS OUTLINE OF MAJOR
PRESENTER'S GUIDE "LEAD EXPOSURE IN GENERAL INDUSTRY" Training For THE OSHA LEAD STANDARD Quality Safety and Health Products, for Today... and Tomorrow OUTLINE OF MAJOR PROGRAM POINTS OUTLINE OF MAJOR
CITY OF CHESTERFIELD POLICE DEPARTMENT GENERAL ORDER 7-02 EFFECTIVE: DECEMBER 1, 2002 CANCELS: GENERAL ORDER 94-7
 CITY OF CHESTERFIELD POLICE DEPARTMENT GENERAL ORDER 7-02 EFFECTIVE: DECEMBER 1, 2002 CANCELS: GENERAL ORDER 94-7 TO: ALL PERSONNEL INDEX AS: AIDS BLOODBORNE PATHOGENS SUBJECT: EXPOSURE TO BLOODBORNE PATHOGENS
CITY OF CHESTERFIELD POLICE DEPARTMENT GENERAL ORDER 7-02 EFFECTIVE: DECEMBER 1, 2002 CANCELS: GENERAL ORDER 94-7 TO: ALL PERSONNEL INDEX AS: AIDS BLOODBORNE PATHOGENS SUBJECT: EXPOSURE TO BLOODBORNE PATHOGENS
ANTIEMETICS UTILIZATION MANAGEMENT CRITERIA
 ANTIEMETICS [Akynzeo, Anzemet, Cesamet, Emend, Sancuso, Varubi, Zuplenz ] DRUG CLASS: UTILIZATION MANAGEMENT CRITERIA 5-HT 3 Receptor Antagonists 5-HT 3 Receptor Antagonist and Substance P/Neurokinin (NK
ANTIEMETICS [Akynzeo, Anzemet, Cesamet, Emend, Sancuso, Varubi, Zuplenz ] DRUG CLASS: UTILIZATION MANAGEMENT CRITERIA 5-HT 3 Receptor Antagonists 5-HT 3 Receptor Antagonist and Substance P/Neurokinin (NK
Procedure for Working with MPTP or MPTP-Treated Animals
 Procedure for Working with MPTP or MPTP-Treated Animals Introduction MPTP, or 1-methyl-4 phenyl-1,2,3,6-tetrahydropyridine, is a neurotoxic agent that is commonly used in Parkinson s Disease (PD) research
Procedure for Working with MPTP or MPTP-Treated Animals Introduction MPTP, or 1-methyl-4 phenyl-1,2,3,6-tetrahydropyridine, is a neurotoxic agent that is commonly used in Parkinson s Disease (PD) research
Adult Intravenous Systemic Anticancer Therapy (SACT) Section A. SUMMARY of SCHEME QIPP Reference
 CA2 Nationally standardised Dose banding for Adult Intravenous Anticancer Therapy (SACT) Scheme Name CA2: Nationally Standardised Dose Banding for Adult Intravenous Systemic Anticancer Therapy (SACT) Section
CA2 Nationally standardised Dose banding for Adult Intravenous Anticancer Therapy (SACT) Scheme Name CA2: Nationally Standardised Dose Banding for Adult Intravenous Systemic Anticancer Therapy (SACT) Section
USP 800 and Hazardous Medications
 USP 800 and Hazardous Medications Trung H. Nguyen, PharmD and Alesha Davis, CPhT Dayton VA Medical Center Trung.Nguyen610@va.gov Alesha.Davis@va.gov The speaker has no actual or potential conflict of interest
USP 800 and Hazardous Medications Trung H. Nguyen, PharmD and Alesha Davis, CPhT Dayton VA Medical Center Trung.Nguyen610@va.gov Alesha.Davis@va.gov The speaker has no actual or potential conflict of interest
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan RIM of the World Unified School District 27315 North Bay Road Blue Jay, CA 92352 (909) 336-4100 July 2016 Safety and Risk Management Department RIM of the World
Bloodborne Pathogens Exposure Control Plan RIM of the World Unified School District 27315 North Bay Road Blue Jay, CA 92352 (909) 336-4100 July 2016 Safety and Risk Management Department RIM of the World
B. Tasks and Procedures where employees, students or contractors can be exposed to bloodborne pathogens:
 Page 1 of 6 BLOODBORNE PATHOGEN PROGRAM INTRODUCTION The intended purpose of this document is to comply with OSHA s Occupational Exposures to Bloodborne Pathogens in Title 29 Code of Federal Regulations
Page 1 of 6 BLOODBORNE PATHOGEN PROGRAM INTRODUCTION The intended purpose of this document is to comply with OSHA s Occupational Exposures to Bloodborne Pathogens in Title 29 Code of Federal Regulations
Doc No: BLOOD Midland Engineering Co., Inc. Initial Issue Date 12/04/15 Safety Management System
 Revision Preparation: Safety Mgr Authority: President Issuing Dept: Safety Page: Page 1 of 15 INTRODUCTION The Occupational Safety and Health Administration (OSHA) has a variety of regulations that all
Revision Preparation: Safety Mgr Authority: President Issuing Dept: Safety Page: Page 1 of 15 INTRODUCTION The Occupational Safety and Health Administration (OSHA) has a variety of regulations that all
INTRODUCTION TO THE OSHA BLOODBORNE PATHOGENS STANDARD
 INTRODUCTION TO THE OSHA BLOODBORNE PATHOGENS STANDARD Background On December 6, 1991 the Occupational Safety and Health Administration (OSHA) published the "Occupational Exposure to Bloodborne Pathogens"
INTRODUCTION TO THE OSHA BLOODBORNE PATHOGENS STANDARD Background On December 6, 1991 the Occupational Safety and Health Administration (OSHA) published the "Occupational Exposure to Bloodborne Pathogens"
Medication Review. Cancer Chemotherapy Drugs. Pharmacy Technician Training Systems Passassured, LLC
 Medication Review Cancer Chemotherapy Drugs Pharmacy Technician Training Systems Passassured, LLC Medication Review, Cancer Chemotherapy Drugs PassAssured's Pharmacy Technician Training Program Medication
Medication Review Cancer Chemotherapy Drugs Pharmacy Technician Training Systems Passassured, LLC Medication Review, Cancer Chemotherapy Drugs PassAssured's Pharmacy Technician Training Program Medication
The term Routine Practices is used to describe practices that were previously known as Universal Precautions.
 Health & Safety Manual Health Promotion & Wellness ROUTINE PRACTICES PROCEDURES INTRODUCTION The term Routine Practices is used to describe practices that were previously known as Universal Precautions.
Health & Safety Manual Health Promotion & Wellness ROUTINE PRACTICES PROCEDURES INTRODUCTION The term Routine Practices is used to describe practices that were previously known as Universal Precautions.
Bloodborne Pathogens. Montclair Kimberley Academy 1
 Bloodborne Pathogens Montclair Kimberley Academy 1 Introduction! Approximately 5.6 million workers in health care and other facilities are at risk of exposure to bloodborne pathogens such as human immunodeficiency
Bloodborne Pathogens Montclair Kimberley Academy 1 Introduction! Approximately 5.6 million workers in health care and other facilities are at risk of exposure to bloodborne pathogens such as human immunodeficiency
Bloodborne Pathogens and Universal Precautions
 Bloodborne Pathogens and Universal Precautions Parkway School District 2012-2013 Revised 9/19/2012 What Are Bloodborne Pathogens(BBPs) Bloodborne pathogens (BBPs) are disease causing microorganisms carried
Bloodborne Pathogens and Universal Precautions Parkway School District 2012-2013 Revised 9/19/2012 What Are Bloodborne Pathogens(BBPs) Bloodborne pathogens (BBPs) are disease causing microorganisms carried
Intrathecal. By the end of this chapter, you will be able to: Introduction. Chapter 20
 Chapter 20 Intrathecal Preparations By the end of this chapter, you will be able to: l Describe the specific risks with intrathecal cytotoxic products l List five checks that need to be carried out on
Chapter 20 Intrathecal Preparations By the end of this chapter, you will be able to: l Describe the specific risks with intrathecal cytotoxic products l List five checks that need to be carried out on
Standard Operating Procedure (SOP)
 INTRODUCTION This SOP applies to HYDROFLUORIC ACID (HF). HYDROFLUORIC ACID is one of the strongest acids. It is extremely destructive to tissue and potentially fatal. HF causes such severe burns because
INTRODUCTION This SOP applies to HYDROFLUORIC ACID (HF). HYDROFLUORIC ACID is one of the strongest acids. It is extremely destructive to tissue and potentially fatal. HF causes such severe burns because
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: CP.CPA.223 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.CPA.223 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
City of Montpelier, Vermont The Smallest Capital City in the United States BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN AND PROCEDURES
 City of Montpelier, Vermont The Smallest Capital City in the United States BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN AND PROCEDURES Last Updated June 19, 2003 1 Bloodborne Pathogens Exposure Control Plan
City of Montpelier, Vermont The Smallest Capital City in the United States BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN AND PROCEDURES Last Updated June 19, 2003 1 Bloodborne Pathogens Exposure Control Plan
Bloodborne Pathogen Exposure Control Plan
 Bloodborne Pathogen Exposure Control Plan Table of Contents I. Purpose II. Exposure Determination III. Methods of Compliance A. Universal Precautions B. Engineering Controls C. Work Practice Controls D.
Bloodborne Pathogen Exposure Control Plan Table of Contents I. Purpose II. Exposure Determination III. Methods of Compliance A. Universal Precautions B. Engineering Controls C. Work Practice Controls D.
Network Guidance for Handling the Spillage of Cytotoxic and Anti-Cancer Drug
 Network Guidance for Handling the Spillage of Cytotoxic and Anti-Cancer Drug West Midlands Expert Advisory Group for Systemic Anti-Cancer Therapy (SACT) Page 1 of 8 West Midlands Clinical Networks and
Network Guidance for Handling the Spillage of Cytotoxic and Anti-Cancer Drug West Midlands Expert Advisory Group for Systemic Anti-Cancer Therapy (SACT) Page 1 of 8 West Midlands Clinical Networks and
Subject: Fosnetupitant-Palonosetron (Akynzeo) IV
 09-J3000-01 Original Effective Date: 06/15/18 Reviewed: 05/09/18 Revised: 01/01/19 Subject: Fosnetupitant-Palonosetron (Akynzeo) IV THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
09-J3000-01 Original Effective Date: 06/15/18 Reviewed: 05/09/18 Revised: 01/01/19 Subject: Fosnetupitant-Palonosetron (Akynzeo) IV THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
Task Risk Assessment (Detailed)
 Task/Process Details Task/Process ID: 62317 Name: Working with toxins/venoms in the laboratory Effective Risk Level: Low Author: Amanda Jones Audited By: Workplace Location of the Task/Process Last Updated
Task/Process Details Task/Process ID: 62317 Name: Working with toxins/venoms in the laboratory Effective Risk Level: Low Author: Amanda Jones Audited By: Workplace Location of the Task/Process Last Updated
Universal Precautions
 Universal Precautions emphasizes the need for workers and students to consider all blood and body fluids as potentially infected with HIV, HBV, and / or other blood-borne pathogens, and to adhere rigorously
Universal Precautions emphasizes the need for workers and students to consider all blood and body fluids as potentially infected with HIV, HBV, and / or other blood-borne pathogens, and to adhere rigorously
Bloodborne Pathogens Training For School Personnel
 Bloodborne Pathogens Training For School Personnel OSHA Defined: Occupational Safety and Health Administration Published a standard to reduce or eliminate health risk, resulting in: Annual training of
Bloodborne Pathogens Training For School Personnel OSHA Defined: Occupational Safety and Health Administration Published a standard to reduce or eliminate health risk, resulting in: Annual training of
