Guidelines on Chemotherapy-induced Nausea and Vomiting in Pediatric Cancer Patients
|
|
|
- Lorraine Hodge
- 6 years ago
- Views:
Transcription
1 Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For Informational Purposes Only: The information contents offered in or in connection with the Children s Oncology Group Supportive Care Endorsed Guidelines (the Guidelines ) is provided only for informational purposes to children affected by cancer, their families their health care providers. The Guidelines are not intended to substitute for medical advice, medical care, diagnosis or treatment obtained from doctors or other healthcare providers. While the Children s Oncology Group tries to provide accurate up-to-date information, the information in the Guidelines may be or may become out of date or incomplete. The information guidelines may not conform to current stard of care, state-of-the art, or best practices for a particular disease, condition, or treatment. Some information in the Guidelines may be intended to be used by clinical researchers in special clinical settings or situations that may not apply to you, your child or your patient. Special Notice to cancer patients their parents legal guardians: The Children s Oncology Group is a research organization does not provide individualized medical care or treatment. The Guidelines are not intended to replace the independent clinical judgment, medical advice, screening, health counseling, or other intervention performed by your or your child s doctor or other healthcare provider. Please do not rely on this information exclusively seek the care of a doctor or other medical professional if you have any questions regarding the Guidelines or a specific medical condition, disease, diagnosis or symptom. Please contact 911 or your emergency services for any health emergency! Special Notice to physicians other healthcare providers: This document is aimed specifically at members of the Children s Oncology Group or Member affiliates who have agreed to collaborate with the Children s Oncology Group in accordance with the relevant procedures policies for study conduct membership participation. Requirements restrictions applicable to recipients of U.S. governmental funds or restrictions governing certain private donations may apply to the use distribution of the Guidelines the information contained herein. The Guidelines are not intended to replace your independent clinical judgment, medical advice, or to exclude other legitimate criteria for screening, health counseling, or intervention for specific complications of childhood cancer treatment. The Guidelines provided are not intended as a sole source of guidance in the evaluation of childhood cancer patients. Nor are the Guidelines intended to exclude other reasonable alternative care. Specific patient care decisions are the prerogative of the patient, family healthcare provider. Warranty or Liability Assumed by Children s Oncology Group Related Parties: While the Children's Oncology Group has tried to assure that the Guidelines are accurate complete as of the date of publication, no warranty or representation, express or implied, is intended to be made in or with the Guidelines. No liability is assumed by the Children's Oncology Group or any affiliated party or member thereof for damage resulting from the use, review, or access of the Guidelines. 1
2 This document summarizes four clinical practice guidelines on the topic of chemotherapy-induced nausea vomiting: I. The Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients (endorsed by the COG Supportive Care Guideline Committee in August 2014). II. The Guideline for the Prevention of Acute Nausea Vomiting due to Antineoplastic Medication in Pediatric Cancer Patients (endorsed by the COG Supportive Care Guideline Committee in January 2018). III. The Guideline for the Prevention Treatment of Anticipatory Nausea Vomiting due to Chemotherapy in Pediatric Cancer Patients (endorsed by the COG Supportive Care Guideline Committee in August 2014) IV. The Guideline for the Treatment of Breakthrough Treatment of Refractory Chemotherapyinduced Nausea Vomiting in Pediatric Cancer Patients (endorsed by the COG Supportive Care Guideline Committee in October 2016). I. Classification of Chemotherapy Emetogenicity The Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients implementation tools provided by the guideline developers can be found at: A summary of the guideline is published in Pediatric Blood Cancer 2011; 2011; 57: The purpose of this guideline is to provide an evidence-based approach to the assessment of the emetogenic potential of antineoplastic regimens in children. The recommendations of the endorsed guideline are presented below. Summary of s for the Classification of Chemotherapy Emetogenicity RECOMMENDATIONS 1. What risk of acute phase CINV do antineoplastic therapies present to children with cancer? The single antineoplastic agents provided in Table 1 have high, moderate, low or minimal emetogenic potential in children. 2. Is the risk of CINV with multi-agent, single day antineoplastic therapy different than that of the most emetogenic antineoplastic given? With the exceptions noted in Table 2 below, the emetogenicity of multiple agent antineoplastic therapy given to children is classified based on the emetogenic potential of the most highly emetogenic agent in the combination to be given. Very low to low quality of evidence Very low to low quality of evidence 3. Is the risk of CINV with multiple day antineoplastic therapy regimens different than that of the most emetogenic antineoplastic therapy given on any individual day? The emetogenicity of multiple day antineoplastic therapy is classified in children based on the emetogenic potential of the most highly Very low quality of evidence emetogenic agent on each day of therapy. 2
3 Table 1: Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients Given as Single Agents High Level of Emetic Risk (> 90% frequency of emesis in absence of prophylaxis) Altretamine *Cytarabine 3 g/m 2 /dose *Carboplatin Dacarbazine Carmustine > 250 mg/m 2 *Dactinomycin *Cisplatin Mechlorethamine *Cyclophosphamide 1 g/m 2 Moderate Level of Emetic Risk (30-90% frequency of emesis in absence of prophylaxis) Aldesleukin > 12 to 15 million units/m 2 Amifostine > 300 mg/m 2 Arsenic trioxide Azacitidine Bendamustine Busulfan *Carmustine 250 mg/m 2 *Clofarabine *Cyclophosphamide < 1 g/m 2 Cyclophosphamide (oral) Low Level of Emetic Risk Cytarabine > 200 mg to < 3 g/m 2 *Daunorubicin *Doxorubicin Epirubicin Etoposide (oral) Idarubicin Ifosfamide Imatinib (oral) *Intrathecal therapy (methotrexate, hydrocortisone & cytarabine) (10-<30% frequency of emesis in absence of prophylaxis) Amifostine 300 mg/m 2 Fludarabine (oral) Amsacrine 5-Fluorouracil Bexarotene Gemcitabine *Busulfan (oral) Ixabepilone Capecitabine Methotrexate > 50 mg to < 250 Cytarabine 200 mg/m 2 mg/m 2 Docetaxel Mitomycin Doxorubicin (liposomal) Mitoxantrone Etoposide Nilotinib Minimal (<10% frequency of emesis in absence of prophylaxis) Alemtuzumab Erlotinib Alpha interferon Fludarabine Asparaginase (IM or IV) Gefitinib Bevacizumab Gemtuzumab ozogamicin Bleomycin Hydroxyurea (oral) Bortezomib Lapatinib Cetuximab Lenalidomide Chlorambucil (oral) Melphalan (oral low-dose) Cladribine (2- Mercaptopurine (oral) chlorodeoxyadenosine) Methotrexate 50 mg/m 2 Decitabine Nelarabine Denileukin diftitox Panitumumab Dasatinib Pentostatin Dexrazoxane Rituximab *Methotrexate 12 g/m 2 Procarbazine (oral) Streptozocin *Thiotepa 300 mg/m 2 Irinotecan Lomustine Melphalan > 50 mg/m 2 Methotrexate 250 mg to < 12 g/m 2 Oxaliplatin > 75 mg/m 2 Temozolomide (oral) Vinorelbine (oral) Paclitaxel Paclitaxel-albumin Pemetrexed Teniposide Thiotepa < 300 mg/m 2 Topotecan Vorinostat Sorafenib Sunitinib Temsirolimus Thalidomide Thioguanine (oral) Trastuzumab Valrubicin Vinblastine Vincristine Vindesine Vinorelbine * Pediatric evidence available Note: All agents given intravenously (IV) unless stated otherwise. 3
4 Table 2: Classification of the Acute Emetogenic Potential of Specific Antineoplastic Medication in Pediatric Cancer Patients Given in Combination High Level of Emetic Risk (> 90% frequency of emesis in absence of prophylaxis) Cyclophosphamide + anthracycline *Cytarabine 300 mg/m 2 + etoposide *Cyclophosphamide + doxorubicin *Cytarabine 300 mg/m 2 + teniposide *Cyclophosphamide + epirubicin *Doxorubicin + ifosfamide *Cyclophosphamide + etoposide Doxorubicin + methotrexate 5 g/m 2 *Cytarabine mg/m 2 + daunorubicin *Etoposide + ifosfamide * Pediatric evidence available Note: All agents given intravenously (IV) unless stated otherwise. II. Prevention of Acute Chemotherapy-induced Nausea Vomiting The Guideline for the Prevention of Acute Nausea Vomiting due to Antineoplastic Medication in Pediatric Cancer Patients the implementation tools provided by the guideline developers are available at: A summary of the guideline is published in Pediatric Blood Cancer 2013; 60: Pediatric Blood Cancer 2017; 2017; 64: e The purpose of this guideline is to provide evidence-based recommendations for the prevention of acute chemotherapy-induced nausea vomiting in children. The recommendations of the endorsed guideline are presented below. Summary of s for the Prevention of Chemotherapy-induced Nausea Vomiting (CINV) RECOMMENDATIONS 1. How is optimal control of acute CINV defined? We recommend that optimal control of acute CINV be defined as no vomiting, no retching, no nausea, no use of antiemetic agents other than those given for CINV prevention no nausea-related change in the child s usual appetite diet. This level of CINV control is to be achieved on each day that antineoplastic therapy is administered for 24 hours after administration of the last antineoplastic agent of the antineoplastic therapy block 4
5 RECOMMENDATIONS 2a. What pharmacological interventions provide optimal control of acute CINV in children receiving highly emetogenic chemotherapy (HEC)? We recommend that: Children 6 months old receiving HEC which is not known or suspected to interact with aprepitant receive: granisetron, ondansetron or palonosetron + dexamethasone + aprepitant Children < 6 months old receiving HEC receive: granisetron, ondansetron or palonosetron + dexamethasone Children 6 months old receiving HEC which is known or suspected to interact with aprepitant receive: granisetron, ondansetron or palonosetron + dexamethasone Children 6 months old receiving HEC which is not known or suspected to interact with aprepitant who cannot receive dexamethasone for CINV prophylaxis receive: palonosetron + aprepitant We suggest that: Children < 6 months old receiving HEC who cannot receive dexamethasone for CINV prophylaxis receive: palonosetron Children receiving HEC which is known or suspected to interact with aprepitant who cannot receive dexamethasone for CINV prophylaxis receive: palonosetron 2b. What pharmacological interventions provide optimal control of acute CINV in children receiving moderately emetogenic chemotherapy (MEC)? We recommend that: Children receiving MEC receive: granisetron, ondansetron or palonosetron + dexamethasone We suggest that: Children 6 months old receiving MEC who cannot receive dexamethasone for CINV prophylaxis receive: granisetron, ondansetron or palonosetron + aprepitant Children < 6 months old receiving MEC who cannot receive dexamethasone for CINV prophylaxis receive: palonosetron Children receiving MEC which is known or suspected to interact with aprepitant who cannot receive dexamethasone for CINV prophylaxis receive: palonosetron 5
6 RECOMMENDATIONS 2c. What pharmacological interventions provide optimal control of acute CINV in children receiving antineoplastic agents of low emetic risk? We recommend that children receiving antineoplastic agents of low emetic risk receive: ondansetron or granisetron 2d. What pharmacological interventions provide optimal control of acute CINV in children receiving antineoplastic agents of minimal emetic risk? We recommend that children receiving antineoplastic agents of low emetic risk receive: no routine prophylaxis 3. What adjunctive non-pharmacological interventions provide control of acute CINV in children receiving antineoplastic agents of any emetic risk? We suggest that acupuncture, acupressure, guided imagery, music therapy, progressive muscle relaxation psycho-educational support information may be effective in children receiving antineoplastic agents. Virtual reality may convey benefit. We suggest that the following dietary interventions may be effective: eat smaller, more frequent meals; reduce food aromas other stimuli with strong odors; avoid foods that are spicy, fatty or highly salty; take antiemetics prior to meals so that the effect is present during after meals; measures foods (e.g. comfort foods ) that helped to minimize nausea in the past 4. What doses of antiemetic agents are known to be effective in children receiving antineoplastic agents? We suggest the following aprepitant dose for children 6 months old: Day 1: 3 mg/kg/dose (maximum: 125mg) PO x 1; Days 2 3: 2 mg/kg/dose (maximum: 80mg) PO once daily We suggest the following dexamethasone dose for children receiving highly emetogenic antineoplastic therapy: 6 mg/m 2 /dose IV/PO q6h If given concurrently with aprepitant, reduce dexamethasone dose by half. We recommend the following dexamethasone for children receiving moderately emetogenic antineoplastic therapy: 0.6m 2 : 2mg/dose IV/PO q12h > 0.6m 2 : 4mg/dose IV/PO q12h If given concurrently with aprepitant, reduce dexamethasone dose by half 6
7 RECOMMENDATIONS We recommend the following IV granisetron dose for children receiving highly emetogenic antineoplastic therapy: 40 mcg/kg/dose IV as a single daily dose We recommend the following IV granisetron dose for children receiving moderately emetogenic antineoplastic therapy: 40 mcg/kg/dose IV as a single daily dose We suggest the following oral granisetron dose for children receiving moderately emetogenic antineoplastic therapy: 40 mcg/kg/dose PO q12h We recommend the following IV granisetron dose for children receiving antineoplastic therapy of low emetogenicity: 40 mcg/kg/dose IV as a single daily dose We suggest the following oral granisetron dose for children receiving antineoplastic therapy of low emetogenicity: 40 mcg/kg/dose PO q12h We recommend the following ondansetron dose for children receiving highly emetogenic antineoplastic therapy: 5 mg/m 2 /dose (0.15 mg/kg/dose) IV/PO pre-therapy x 1 then q8h We recommend the following ondansetron dose for children receiving moderately emetogenic antineoplastic therapy: 5 mg/m 2 /dose (0.15 mg/kg/dose; maximum 8 mg/dose) IV/PO pre-therapy x 1 then q12h We recommend the following ondansetron dose for children receiving therapy of low emetogenicity: 10 mg/m 2 /dose (0.3 mg/kg/dose; maximum 16 mg/dose IV or 24 mg/dose PO) pre-therapy x 1 We suggest the following palonosetron dose for children: 1 month to < 17 years: 0.02 mg/kg/dose (maximum 1.5 mg) IV once pre-therapy 17 years: 0.5 mg/dose PO once pre-therapy III. Prevention Treatment of Anticipatory Chemotherapy-Induced Nausea Vomiting The Guideline for the Prevention Treatment of Anticipatory Nausea Vomiting due to Chemotherapy in Pediatric Cancer Patients the implementation tools provided by the guideline developers are available at: 7
8 A summary of the guideline is published in Pediatric Blood Cancer 2014; 61: The purpose of this guideline is to provide evidence-based recommendations for the prevention treatment of anticipatory chemotherapy-induced nausea vomiting in children. The recommendations of the endorsed guideline are presented below. Summary of s for the Prevention Treatment of Anticipatory Chemotherapyinduced Nausea Vomiting (CINV) RECOMMENDATIONS 1. What approaches are recommended to prevent the development of anticipatory chemotherapy induced nausea vomiting (CINV) in children? Control of acute delayed CINV should be optimized for each child in order to minimize the risk of the child developing anticipatory CINV. 2. What interventions are recommended to control anticipatory CINV in children who develop it? We suggest that psychological interventions such as hypnosis or systematic desensitization may be offered to children with anticipatory CINV. We suggest that lorazepam in a dose of 0.04 to 0.08 mg/kg/dose (maximum: 2 mg/dose) once at bedtime the night before chemotherapy once the next day prior to administration of chemotherapy may be used to prevent or treat anticipatory CINV in children. IV. Treatment of Breakthrough Prevention of Refractory Chemotherapy-Induced Nausea Vomiting The Guideline for the Treatment of Breakthrough Prevention of Refractory Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients the implementation tools provided by the guideline developers are available at: A summary of the guideline is published in Pediatric Blood Cancer 2016;63: The purpose of this guideline is to provide evidence-based recommendations to optimize breakthrough refractory CINV control in children. The recommendations of the endorsed guideline are presented below. 8
9 Summary of s for the Treatment of Breakthrough the Prevention of Refractory Chemotherapy-induced Nausea Vomiting RECOMMENDATIONS 1. What interventions are recommended to treat breakthrough CINV in children? Breakthrough CINV is defined as nausea /or vomiting presumed to be attributable to antineoplastic chemotherapy with no other pathological cause that occurs during the acute or delayed phase despite CINV prophylaxis. For children receiving acute CINV prophylaxis recommended for minimally, low, or moderately emetogenic chemotherapy, clinicians should upgrade or escalate the acute CINV prophylaxis provided to that recommended for chemotherapy of the next higher level of emetogenic risk. For children receiving acute CINV prophylaxis recommended for highly emetogenic chemotherapy, we suggest that olanzapine be added to guideline-consistent CINV prophylaxis. For children receiving acute CINV prophylaxis recommended for highly emetogenic chemotherapy who cannot receive olanzapine, we suggest that one of the following antiemetic agents be added to guideline-consistent CINV prophylaxis: methotrimeprazine (also known as levomepromazine) or metoclopramide (in children older than 1 year) Given the possibility of extrapyramidal reactions with these agents, the risks benefits of their use should be weighed carefully co-administration of prophylaxis aimed at preventing extrapyramidal symptoms (EPS) should be considered. Patients families should also be educated about the possible occurrence of EPS. 2. What interventions are recommended to prevent CINV in children who have refractory CINV? Refractory CINV is defined as nausea /or vomiting presumed to be attributable to antineoplastic chemotherapy with no other pathological cause which occurs during the acute or delayed phase despite CINV prophylaxis in patients who have experienced breakthrough CINV in a previous chemotherapy block. For children receiving acute CINV prophylaxis recommended for minimally, low, or moderately emetogenic chemotherapy, clinicians should upgrade or escalate the acute CINV prophylaxis provided to that recommended for chemotherapy of the next higher level of emetogenic risk. 9
10 For children receiving acute CINV prophylaxis recommended for highly emetogenic chemotherapy, we suggest that the 5-HT3 antagonist given for CINV prophylaxis be changed from ondansetron or granisetron to palonosetron. In jurisdictions where palonosetron is not available, we suggest that granisetron be substituted for ondansetron. For children experiencing refractory CINV despite initiation of previous recommendations who have not previously received aprepitant because it is known or suspected to interact with the chemotherapeutic agent(s) being given, we suggest that the addition of aprepitant to acute CINV prophylaxis be considered. For children experiencing refractory CINV despite initiation of the previous recommendations, we suggest that one of the following interventions be added to the CINV prophylaxis provided: interventions that were employed successfully for the treatment of breakthrough CINV in previous treatment blocks (olanzapine, methotrimeprazine or metoclopramide); or stimulation of Nei Gaun (P6) by means of acupressure or electroacupuncture. 10
11 Appendix 1: GRADE s: Strong Weak When using GRADE, panels make strong recommendations when they are confident that the desirable effects of adherence to a recommendation outweigh the undesirable effects. s indicate that the desirable effects of adherence to a recommendation probably outweigh the undesirable effects, but the panel is less confident. s Determinants: Factor Balance between desirable undesirable effects Quality of evidence Values preferences Costs (resource allocation) Comment The larger the difference between the desirable undesirable effects, the higher the likelihood that a strong recommendation is warranted. The narrower the gradient, the higher the likelihood that a weak recommendation is warranted The higher the quality of evidence, the higher the likelihood that a strong recommendation is warranted The more values preferences vary, or the greater the uncertainty in values preferences, the higher the likelihood that a weak recommendation is warranted The higher the costs of an intervention that is, the greater the resources consumed the lower the likelihood that a strong recommendation is warranted High Quality Moderate Quality Low Quality Very Low Quality Further research is very unlikely to change our confidence in the estimate of effect Further research is likely to have an important impact on our confidence in the estimate of effect may change the estimate Further research is very likely to have an important impact on our confidence in the estimate of effect is likely to change the estimate Any estimate of effect is very uncertain Guyatt, G.H., et al., GRADE: an emerging consensus on rating quality of evidence strength of recommendations. BMJ, 2008; 336: Guyatt, G.H., et al., GRADE: going from evidence to recommendations. BMJ, 2008; 336:
SCI. SickKids-Caribbean Initiative Enhancing Capacity for Care in Paediatric Cancer and Blood Disorders
 1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer
 APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer 5850/5980 University Avenue, PO Box 9700, Halifax, N.S. B3K 6R8 PEDIATRIC HEMATOLOGY/ONCOLOGY
APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer 5850/5980 University Avenue, PO Box 9700, Halifax, N.S. B3K 6R8 PEDIATRIC HEMATOLOGY/ONCOLOGY
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting
 West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
MEDICAL NECESSITY GUIDELINE
 PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients
 Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic Induced Nausea and Vomiting Guideline Development Panel: L.
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic Induced Nausea and Vomiting Guideline Development Panel: L.
Guideline Update on Antiemetics
 Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY CLASSIFICATION
 GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
MASCC Guidelines for Antiemetic control: An update
 MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
Managements of Chemotherpay Induded Nausea and Vomiting
 REVIEW ARTICLE Managements of Chemotherpay Induded Nausea and Vomiting Department of Surgery, The Catholic University of Korea Sung Geun Kim 23 24 Sung Geun Kim Korean Journal of Clinical Oncology Summer
REVIEW ARTICLE Managements of Chemotherpay Induded Nausea and Vomiting Department of Surgery, The Catholic University of Korea Sung Geun Kim 23 24 Sung Geun Kim Korean Journal of Clinical Oncology Summer
Description The following are synthetic cannabinoids requiring prior authorization: dronabinol (Marinol, Syndros ), nabilone (Cesamet )
 Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Objectives
 Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Susan Urba, M.D. University of Michigan Comprehensive Cancer Center Objectives Mechanisms of
Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Susan Urba, M.D. University of Michigan Comprehensive Cancer Center Objectives Mechanisms of
Guidelines for the Use of Anti-Emetics with Chemotherapy
 Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: CP.CPA.223 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.CPA.223 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
Subject: Palonosetron Hydrochloride (Aloxi )
 09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
COG Supportive Care Endorsed Guidelines Version date: December 11, 2017
 COG Supportive Care Endorsed Guidelines The COG Supportive Care Endorsed Guidelines are comprised of -based guidelines which have been developed by other organizations endorsed by the Children s Oncology
COG Supportive Care Endorsed Guidelines The COG Supportive Care Endorsed Guidelines are comprised of -based guidelines which have been developed by other organizations endorsed by the Children s Oncology
Northern Cancer Alliance
 Northern Cancer Alliance Anti-emetic Guidelines for Chemotherapy Induced Nausea and Vomiting (CINV) Adult Oncology & Haematology Document Control Document Title: Antiemetic Guidelines for CINV NESCN v2.2
Northern Cancer Alliance Anti-emetic Guidelines for Chemotherapy Induced Nausea and Vomiting (CINV) Adult Oncology & Haematology Document Control Document Title: Antiemetic Guidelines for CINV NESCN v2.2
Committee Approval Date: December 12, 2014 Next Review Date: July 2015
 Medication Policy Manual Policy No: dru378 Topic: Akynzeo, netupitant/palonosetron Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: July 2015 Effective Date:
Medication Policy Manual Policy No: dru378 Topic: Akynzeo, netupitant/palonosetron Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: July 2015 Effective Date:
ANTIEMETIC GUIDELINES: MASCC/ESMO
 Open Issues for CINV Do we reliably measure that? Do we control nausea optimally? Are guidelines useful for oral therapies related nausea and vomiting? Breakthrough and refractory nausea and vomiting:
Open Issues for CINV Do we reliably measure that? Do we control nausea optimally? Are guidelines useful for oral therapies related nausea and vomiting? Breakthrough and refractory nausea and vomiting:
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting
 Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Zofran, Zuplenz) Reference Number: CP.CPA.173 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this
Clinical Policy: (Zofran, Zuplenz) Reference Number: CP.CPA.173 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this
Clinical Tools and Resources for Self-Study and Patient Education
 Clinical Tools and Resources for Self-Study and Patient Education CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING CLINICIAN'S RESOURCE GUIDE The clinical tools and resources contained herein are provided as educational
Clinical Tools and Resources for Self-Study and Patient Education CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING CLINICIAN'S RESOURCE GUIDE The clinical tools and resources contained herein are provided as educational
Guideline for the Management of Fever and Neutropenia in Children with Cancer and/or Undergoing Hematopoietic Stem-Cell Transplantation
 Guideline for the Management of Fever Neutropenia in Children with Cancer /or Undergoing Hematopoietic Stem-Cell Transplantation COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive
Guideline for the Management of Fever Neutropenia in Children with Cancer /or Undergoing Hematopoietic Stem-Cell Transplantation COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive
Subject: Fosnetupitant-Palonosetron (Akynzeo) IV
 09-J3000-01 Original Effective Date: 06/15/18 Reviewed: 05/09/18 Revised: 01/01/19 Subject: Fosnetupitant-Palonosetron (Akynzeo) IV THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
09-J3000-01 Original Effective Date: 06/15/18 Reviewed: 05/09/18 Revised: 01/01/19 Subject: Fosnetupitant-Palonosetron (Akynzeo) IV THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
 Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Emetogenicity level 1. Emetogenicity level 2
 Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
Job title: Consultant Pharmacist/Advanced Practice Pharmacist
 Title : Guidelines for the Use of Antiemetics Purpose: To provide trust-wide guidance on the safe and effective use of antiemetics for the prevention and treatment of chemotherapy and radiotherapy induced
Title : Guidelines for the Use of Antiemetics Purpose: To provide trust-wide guidance on the safe and effective use of antiemetics for the prevention and treatment of chemotherapy and radiotherapy induced
Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines
 North of England Cancer Network Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines Adult Oncology & Haematology Quality and safety for every patient every time For more information
North of England Cancer Network Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines Adult Oncology & Haematology Quality and safety for every patient every time For more information
Clinical Practice Guideline on Central Venous Catheter Care for the Patient with Cancer
 Clinical Practice Guideline on Central Venous Catheter Care for the Patient with Cancer COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER
Clinical Practice Guideline on Central Venous Catheter Care for the Patient with Cancer COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER
Clinical Policy: Dolasetron (Anzemet) Reference Number: ERX.NPA.83 Effective Date:
 Clinical Policy: (Anzemet) Reference Number: ERX.NPA.83 Effective Date: 09.01.18 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Anzemet) Reference Number: ERX.NPA.83 Effective Date: 09.01.18 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Guideline for Fertility Preservation for Patients with Cancer
 Guideline for Fertility Preservation for Patients with Cancer COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For Informational Purposes
Guideline for Fertility Preservation for Patients with Cancer COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For Informational Purposes
Subject: NK-1 receptor antagonist injectable therapy (Emend, Cinvanti, Varubi )
 09-J2000-60 Original Effective Date: 06/15/16 Reviewed: 04/11/18 Revised: 01/01/19 Subject: NK-1 receptor antagonist injectable therapy (Emend, Cinvanti, Varubi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT
09-J2000-60 Original Effective Date: 06/15/16 Reviewed: 04/11/18 Revised: 01/01/19 Subject: NK-1 receptor antagonist injectable therapy (Emend, Cinvanti, Varubi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT
Systemic Anti-cancer Therapy Care Pathway Guidelines for the management of SACT induced nausea and vomiting in adult patients
 Systemic Anti-cancer Therapy Care Pathway Guidelines for the management of SACT induced nausea and vomiting in adult patients Pathway of Care Kent & Medway Cancer Collaborative Publication date June 2018
Systemic Anti-cancer Therapy Care Pathway Guidelines for the management of SACT induced nausea and vomiting in adult patients Pathway of Care Kent & Medway Cancer Collaborative Publication date June 2018
Corporate Medical Policy
 Antiemetic Injection Therapy Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: antiemetic_injection_therapy 5/2015 3/2017 3/2018 3/2017 Description of Procedure
Antiemetic Injection Therapy Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: antiemetic_injection_therapy 5/2015 3/2017 3/2018 3/2017 Description of Procedure
Trust Guideline for Prevention and Control of Chemotherapy and Radiotherapy Induced Nausea and Vomiting in Adults
 A Clinical Guideline For Use in: Organisation-wide By: For: Key words: Name of document author: Job title of document author: Name of document author s Line Manager: Job title of author s Line Manager:
A Clinical Guideline For Use in: Organisation-wide By: For: Key words: Name of document author: Job title of document author: Name of document author s Line Manager: Job title of author s Line Manager:
The AngCN Antiemetic Guidelines
 The AngCN Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy AngCN Document Reference: AngCN-CCG-C31 CONTENTS 1.0 Introduction
The AngCN Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy AngCN Document Reference: AngCN-CCG-C31 CONTENTS 1.0 Introduction
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Etiologies:
 VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Incidence: The incidence of acute and delayed N&V was investigated in highly and moderately emetogenic
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Incidence: The incidence of acute and delayed N&V was investigated in highly and moderately emetogenic
ANTIEMETICS UTILIZATION MANAGEMENT CRITERIA
 ANTIEMETICS [Akynzeo, Anzemet, Cesamet, Emend, Sancuso, Varubi, Zuplenz ] DRUG CLASS: UTILIZATION MANAGEMENT CRITERIA 5-HT 3 Receptor Antagonists 5-HT 3 Receptor Antagonist and Substance P/Neurokinin (NK
ANTIEMETICS [Akynzeo, Anzemet, Cesamet, Emend, Sancuso, Varubi, Zuplenz ] DRUG CLASS: UTILIZATION MANAGEMENT CRITERIA 5-HT 3 Receptor Antagonists 5-HT 3 Receptor Antagonist and Substance P/Neurokinin (NK
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Anzemet) Reference Number: CP.PMN.141 Effective Date: 09.01.06 Last Review Date: 08.18 Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the end of this
Clinical Policy: (Anzemet) Reference Number: CP.PMN.141 Effective Date: 09.01.06 Last Review Date: 08.18 Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the end of this
Antiemesis. NCCN Clinical Practice Guidelines in Oncology. Antiemesis. Version Continue
 Table of Contents Clinical in Oncology Version 2.2010 Continue www.nccn.org Version 2.2010, 04/07/2010 2010 National Comprehensive Cancer Network, Inc. All rights reserved. These guidelines and this illustration
Table of Contents Clinical in Oncology Version 2.2010 Continue www.nccn.org Version 2.2010, 04/07/2010 2010 National Comprehensive Cancer Network, Inc. All rights reserved. These guidelines and this illustration
Clinical Policy: Nabilone (Cesamet) Reference Number: ERX.NPA.35 Effective Date:
 Clinical Policy: (Cesamet) Reference Number: ERX.NPA.35 Effective Date: 09.01.17 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Cesamet) Reference Number: ERX.NPA.35 Effective Date: 09.01.17 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Supportive care session 1:
 Board review in oncology pharmacy 2013 Managing Disease or Treatment Related Complication Supportive care session 1: Chemotherapy induced-nausea and vomiting Suthan Chanthawong, B. Pharm, RPh. Objectives
Board review in oncology pharmacy 2013 Managing Disease or Treatment Related Complication Supportive care session 1: Chemotherapy induced-nausea and vomiting Suthan Chanthawong, B. Pharm, RPh. Objectives
REVIEW Guideline for the Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients
 Pediatr Blood Cancer 2011;57:191 198 REVIEW Guideline for the Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients L. Lee Dupuis, MScPhm, ACPR, FCSHP,
Pediatr Blood Cancer 2011;57:191 198 REVIEW Guideline for the Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients L. Lee Dupuis, MScPhm, ACPR, FCSHP,
Clinical Policy: Aprepitant (Emend) Reference Number: CP.PMN.19 Effective Date: 11/06 Last Review Date: 08/17
 Clinical Policy: (Emend) Reference Number: CP.PMN.19 Effective Date: 11/06 Last Review Date: 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Emend) Reference Number: CP.PMN.19 Effective Date: 11/06 Last Review Date: 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: Ondansetron (Zuplenz) Reference Number: CP.PMN.45 Effective Date: Last Review Date: Line of Business: Medicaid
 Clinical Policy: (Zuplenz) Reference Number: CP.PMN.45 Effective Date: 09.01.06 Last Review Date: 02.19 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Zuplenz) Reference Number: CP.PMN.45 Effective Date: 09.01.06 Last Review Date: 02.19 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Prevention and Management of cancer disease and of chemo-and radiotherapyinduced nausea and vomiting
 Prevention and Management of cancer disease and of chemo-and radiotherapyinduced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University
Prevention and Management of cancer disease and of chemo-and radiotherapyinduced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University
Antiemetic Guidelines. Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy
 Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy For approvals and version control see Document Management Record
Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy For approvals and version control see Document Management Record
Defining the Emetogenicity of Cancer Chemotherapy Regimens: Relevance to Clinical Practice
 Defining the Emetogenicity of Cancer Chemotherapy Regimens: Relevance to Clinical Practice PAUL J. HESKETH St. Elizabeth s Medical Center, Boston, Massachusetts, USA Key Words. Chemotherapy Emesis Emetogenicity
Defining the Emetogenicity of Cancer Chemotherapy Regimens: Relevance to Clinical Practice PAUL J. HESKETH St. Elizabeth s Medical Center, Boston, Massachusetts, USA Key Words. Chemotherapy Emesis Emetogenicity
Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A
 Supportive Care in Cancer Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A Systematic Review of the Medicare Database Romina Sosa, Shuling Li, Julia T. Molony, Jiannong
Supportive Care in Cancer Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A Systematic Review of the Medicare Database Romina Sosa, Shuling Li, Julia T. Molony, Jiannong
Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and
 35 Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and Biologically directed therapies ) 1 1- Nausea and vomiting
35 Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and Biologically directed therapies ) 1 1- Nausea and vomiting
Richard J. Gralla, MD Medical Director Quality of Life Research Associates New York, NY
 Oncology Consultations Improving the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) A CE-Certified Activity Featuring Consultations With Supported by an educational grant from Eisai. Dannemiller
Oncology Consultations Improving the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) A CE-Certified Activity Featuring Consultations With Supported by an educational grant from Eisai. Dannemiller
Prevention of chemotherapy induced nausea and vomiting in pediatric cancer patients
 REVIEW ARTICLE Prevention of chemotherapy induced nausea and vomiting in pediatric cancer patients Shubham Roy 1, Rashi Kulshrestha 2, Abhishek Shankar 3, Goura Kishor Rath 4, Vipin Kharade 4 1 Department
REVIEW ARTICLE Prevention of chemotherapy induced nausea and vomiting in pediatric cancer patients Shubham Roy 1, Rashi Kulshrestha 2, Abhishek Shankar 3, Goura Kishor Rath 4, Vipin Kharade 4 1 Department
Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites.
 WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
Primary malignant neoplasms, not lymphatic or hematopoietic. Secondary malignant neoplasms (i.e.metastatic) Malignant neoplasm, unknown site
 Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Cesamet) Reference Number: CP.PMN.160 Effective Date: 11.16.16 Last Review Date: 02.19 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Cesamet) Reference Number: CP.PMN.160 Effective Date: 11.16.16 Last Review Date: 02.19 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Antiemetics: Guidelines, Interactions and more.
 Antiemetics: Guidelines, Interactions and more. Chemotherapy-induced side effects -the patient s view Loss of hair The thought of coming for chemo Affects family/partner Affects work/home duties Jude Lees
Antiemetics: Guidelines, Interactions and more. Chemotherapy-induced side effects -the patient s view Loss of hair The thought of coming for chemo Affects family/partner Affects work/home duties Jude Lees
Patient-Centered Management of Chemotherapy-Induced Nausea and Vomiting
 Clear and accurate communication between patients and health care teams is the key to successfully controlling chemotherapy-induced nausea and vomiting. Roseate Spoonbill_2005. Photograph courtesy of Henry
Clear and accurate communication between patients and health care teams is the key to successfully controlling chemotherapy-induced nausea and vomiting. Roseate Spoonbill_2005. Photograph courtesy of Henry
To help doctors give their patients the best possible care, the American
 Patient Information Resources from ASCO What to Know ASCO s Guideline on Preventing Vomiting Caused by Cancer Treatment SEPTEMBER 2011 KEY MESSAGES The risk of nausea and vomiting depends on the specific
Patient Information Resources from ASCO What to Know ASCO s Guideline on Preventing Vomiting Caused by Cancer Treatment SEPTEMBER 2011 KEY MESSAGES The risk of nausea and vomiting depends on the specific
ECN Protocol Book. Antiemetic Guidelines for Adult Patients Receiving Chemotherapy and Radiotherapy
 ECN Protocol Book Antiemetic Guidelines f Adult Patients Receiving Chemotherapy and Radiotherapy Name of person presenting document: Reason f document development: Names of development team: Specify groups
ECN Protocol Book Antiemetic Guidelines f Adult Patients Receiving Chemotherapy and Radiotherapy Name of person presenting document: Reason f document development: Names of development team: Specify groups
DRUG PROPERTIES YOU NEED TO KNOW
 Dr. Janet Fitzakerley Summer 2013 Med 6541 Hematopoiesis and Host Defences jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 11 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class
Dr. Janet Fitzakerley Summer 2013 Med 6541 Hematopoiesis and Host Defences jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 11 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Antiemesis. Version NCCN.org. Continue
 NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Version 1.2011 NCCN.org Continue Note: All recommendations are category 2A unless otherwise indicated. Clinical Trials: NCCN believes that
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Version 1.2011 NCCN.org Continue Note: All recommendations are category 2A unless otherwise indicated. Clinical Trials: NCCN believes that
Chemotherapy-induced Nausea and Vomiting: The Pharmacist s Role in Integrating Clinical Guidelines into Patient Care
 Chemotherapy-induced Nausea and Vomiting: The Pharmacist s Role in Integrating Clinical Guidelines into Patient Care Presented as a Live Webinar Wednesday, April 13, 2016 12:00 p.m. 1:00 p.m. ET Tuesday,
Chemotherapy-induced Nausea and Vomiting: The Pharmacist s Role in Integrating Clinical Guidelines into Patient Care Presented as a Live Webinar Wednesday, April 13, 2016 12:00 p.m. 1:00 p.m. ET Tuesday,
Chemotherapy induced emesis: Are we doing are best? David Warr University of Toronto
 Chemotherapy induced emesis: Are we doing are best? David Warr University of Toronto david.warr@uhn.on.ca Conflict of interest Merck: speakers bureau and consultant Eisai: consultant Outline What is the
Chemotherapy induced emesis: Are we doing are best? David Warr University of Toronto david.warr@uhn.on.ca Conflict of interest Merck: speakers bureau and consultant Eisai: consultant Outline What is the
1. Purpose Documentation Description.1 4. References 8 5. Cross-References.8 6. Development of the guideline.8
 CLINICAL GUIDELINE For use in (clinical areas): For use by (staff groups): For use for (patients): Document owner: Status: Oncology/Haematology Unit (excluding Paediatrics) Oncologists, Haematologists,
CLINICAL GUIDELINE For use in (clinical areas): For use by (staff groups): For use for (patients): Document owner: Status: Oncology/Haematology Unit (excluding Paediatrics) Oncologists, Haematologists,
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Kytril, Sancuso, Sustol) Reference Number: CP.PMN.74 Effective Date: 11.01.16 Last Review Date: 02.19 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important
Clinical Policy: (Kytril, Sancuso, Sustol) Reference Number: CP.PMN.74 Effective Date: 11.01.16 Last Review Date: 02.19 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important
ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY
 ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY Problem Opening PACS Images on ipads or ibooks has Been Fixed Changes have been made in PROD to enable user credentials to be passed
ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY Problem Opening PACS Images on ipads or ibooks has Been Fixed Changes have been made in PROD to enable user credentials to be passed
Protocol Number Tumour Group Protocol Name on NCCP website 22/02/ Lung Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy
 Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Update on antiemetics, what is new and future directions. Karin Jordan University of Halle
 Update on antiemetics, what is new and future directions Karin Jordan University of Halle History of Antiemetics Controlling Chemotherapy-Induced EMESIS: Progress Over The Past 30 Years: Efficacy 5-Day
Update on antiemetics, what is new and future directions Karin Jordan University of Halle History of Antiemetics Controlling Chemotherapy-Induced EMESIS: Progress Over The Past 30 Years: Efficacy 5-Day
GUIDELINE FOR THE MANAGEMENT OF CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING.
 Page 1 of 20 Guideline for the management of chemotherapy-induced nausea and vomiting, v2.1.1 GUIDELINE FOR THE MANAGEMENT OF CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING. Version: 2.1.0 Ratified by: Date
Page 1 of 20 Guideline for the management of chemotherapy-induced nausea and vomiting, v2.1.1 GUIDELINE FOR THE MANAGEMENT OF CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING. Version: 2.1.0 Ratified by: Date
DOXOrubicin, Cyclophosphamide (AC 60/600) 21 day followed by weekly PACLitaxel (80) Therapy (AC-T) 261 CARBOplatin (AUC4-6) Monotherapy-21 days
 Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
COST CONSIDERATIONS Union for International Cancer Control 2014 Review of Cancer Medicines on the WHO List of Essential Medicines!!!!!!!!!
 UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249
 Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
DRUG EXTRAVASATION. Vesicants. Irritants
 DRUG EXTRAVASATION Vesicants Irritants Vesicants Antineoplastic drugs Amsacrine Dactinomycin Daunorubicin Docetaxel (rare) Doxorubicin Epirubicin Idarubicin Mechlorethamine Mitomycin Oxaliplatin (rare)
DRUG EXTRAVASATION Vesicants Irritants Vesicants Antineoplastic drugs Amsacrine Dactinomycin Daunorubicin Docetaxel (rare) Doxorubicin Epirubicin Idarubicin Mechlorethamine Mitomycin Oxaliplatin (rare)
Medicare Part C Medical Coverage Policy
 Medicare Part C Medical Coverage Policy Oral Antiemetic Medications Origination: June 17, 2009 Review Date: May 17, 2017 Next Review: May, 2019 DESCRIPTION OF PROCEDURE OR SERVICE Oral antiemetic medications
Medicare Part C Medical Coverage Policy Oral Antiemetic Medications Origination: June 17, 2009 Review Date: May 17, 2017 Next Review: May, 2019 DESCRIPTION OF PROCEDURE OR SERVICE Oral antiemetic medications
Chemotherapy-Induced Nausea and Vomiting: Strategies for Achieving Optimal Control
 Chemotherapy-Induced Nausea and Vomiting Strategies for Achieving Learning Objectives Describe the challenges of assessing nausea in patients undergoing chemotherapy and the impact of nausea and also vomiting
Chemotherapy-Induced Nausea and Vomiting Strategies for Achieving Learning Objectives Describe the challenges of assessing nausea in patients undergoing chemotherapy and the impact of nausea and also vomiting
Disclosure. Objectives. Objectives. Oncologic Emergency. Classification of Emergencies 1 3/2/2015
 Disclosure Oncologic Emergencies and Acute Supportive Care of the Critically Ill Oncology Patient By: Kimberly Regis, Pharm.D. Broward Health Medical Center Pharmacy Resident 2014-2015 I do not have a
Disclosure Oncologic Emergencies and Acute Supportive Care of the Critically Ill Oncology Patient By: Kimberly Regis, Pharm.D. Broward Health Medical Center Pharmacy Resident 2014-2015 I do not have a
CLINICAL GUIDANCE FOR THE PREVENTION AND MANAGEMENT OF CHEMOTHERAPY AND RADIOTHERAPY INDUCED NAUSEA AND VOMITING IN ADULTS
 CLINICAL GUIDANCE FOR THE PREVENTION AND MANAGEMENT OF CHEMOTHERAPY AND RADIOTHERAPY INDUCED NAUSEA AND VOMITING IN ADULTS Procedure reference: Document owner: 2196 Version: V2.1 Robert Duncombe, The Director
CLINICAL GUIDANCE FOR THE PREVENTION AND MANAGEMENT OF CHEMOTHERAPY AND RADIOTHERAPY INDUCED NAUSEA AND VOMITING IN ADULTS Procedure reference: Document owner: 2196 Version: V2.1 Robert Duncombe, The Director
Adult Intravenous Systemic Anticancer Therapy (SACT) Section A. SUMMARY of SCHEME QIPP Reference
 CA2 Nationally standardised Dose banding for Adult Intravenous Anticancer Therapy (SACT) Scheme Name CA2: Nationally Standardised Dose Banding for Adult Intravenous Systemic Anticancer Therapy (SACT) Section
CA2 Nationally standardised Dose banding for Adult Intravenous Anticancer Therapy (SACT) Scheme Name CA2: Nationally Standardised Dose Banding for Adult Intravenous Systemic Anticancer Therapy (SACT) Section
Comparing Different Antiemetic Regimens for Chemotherapy Induced Nausea and Vomiting
 Comparing Different Antiemetic Regimens for Chemotherapy Induced Nausea and Vomiting Sayantani Ghosh, Saugat Dey Corresponding author: Sayantani Ghosh (ghoshsayantani@rediffmail.com) Correspondence concerning
Comparing Different Antiemetic Regimens for Chemotherapy Induced Nausea and Vomiting Sayantani Ghosh, Saugat Dey Corresponding author: Sayantani Ghosh (ghoshsayantani@rediffmail.com) Correspondence concerning
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced
 ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
Haematology, Oncology and Palliative Care Directorate.
 Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
Pediatric Trials 23/04/2018. Disclosures. Nausea and Vomiting Control in Adults and Children: Mind the Gap! Learning Objectives
 Nausea and Vomiting Control in Adults and Children: Mind the Gap! Disclosures No relevant conflicts of interest Lee Dupuis, RPh, PhD May 5, 2018 2 Learning Objectives At the end of this presentation, attendees
Nausea and Vomiting Control in Adults and Children: Mind the Gap! Disclosures No relevant conflicts of interest Lee Dupuis, RPh, PhD May 5, 2018 2 Learning Objectives At the end of this presentation, attendees
SURVEY SUL CONFLITTO DI INTERESSE IN ONCOLOGIA PROMOSSO DAL CIPOMO 1-31 MARZO. PARTECIPATE TUTTI nicsonet.it
 SURVEY SUL CONFLITTO DI INTERESSE IN ONCOLOGIA PROMOSSO DAL CIPOMO 1-31 MARZO PARTECIPATE TUTTI www.cipomo.it; nicsonet.it LINEE GUIDA AIOM: LA TERAPIA ANTIEMETICA Fausto Roila S.C. Oncologia Medica, Terni
SURVEY SUL CONFLITTO DI INTERESSE IN ONCOLOGIA PROMOSSO DAL CIPOMO 1-31 MARZO PARTECIPATE TUTTI www.cipomo.it; nicsonet.it LINEE GUIDA AIOM: LA TERAPIA ANTIEMETICA Fausto Roila S.C. Oncologia Medica, Terni
OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY)
 European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref. OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY) Table 1: Organisations
European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref. OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY) Table 1: Organisations
Guideline for Fertility Preservation for Patients with Cancer
 Guideline for Fertility Preservation for Patients with Cancer COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For Informational Purposes
Guideline for Fertility Preservation for Patients with Cancer COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For Informational Purposes
Gastrointestinal Alterations
 Gastrointestinal Alterations Nancy Thompson, RN, MS, AOCNS Swedish Cancer Institute Nausea & Vomiting Mucositis Taste Alterations Anorexia / Cachexia GI Alterations The Chinese do not draw any distinction
Gastrointestinal Alterations Nancy Thompson, RN, MS, AOCNS Swedish Cancer Institute Nausea & Vomiting Mucositis Taste Alterations Anorexia / Cachexia GI Alterations The Chinese do not draw any distinction
Prevention of Antineoplastic Medication induced Nausea and Vomiting in Pediatric Cancer Patients
 Prevention of Antineoplastic Medication induced Nausea and Vomiting in Pediatric Cancer Patients Done by :Meznah Zaid Al-Mutairi Pharm.D Candidate PNU University College of Pharmacy Introduction Nausea
Prevention of Antineoplastic Medication induced Nausea and Vomiting in Pediatric Cancer Patients Done by :Meznah Zaid Al-Mutairi Pharm.D Candidate PNU University College of Pharmacy Introduction Nausea
CLINICAL GUIDELINE FOR ANTIEMETIC USE IN PAEDIATRIC ONCOLOGY 1. Aim/Purpose of this Guideline
 CLINICAL GUIDELINE FOR ANTIEMETIC USE IN PAEDIATRIC ONCOLOGY 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical and nursing staff working with paediatric oncology patients. 2. The
CLINICAL GUIDELINE FOR ANTIEMETIC USE IN PAEDIATRIC ONCOLOGY 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical and nursing staff working with paediatric oncology patients. 2. The
AD regimen, 576 adrenal steroid inhibitor. aminoglutethimide, 23 25
 A 640 Index Index abiraterone acetate, 5 7 Abraxane. See albuminbound paclitaxel ABVD regimen, 57, 540 ABV regimen, 519 520 AC docetaxel regimen, 476, 478 acetaminophen busulfan and, 71 AC regimen, 475,
A 640 Index Index abiraterone acetate, 5 7 Abraxane. See albuminbound paclitaxel ABVD regimen, 57, 540 ABV regimen, 519 520 AC docetaxel regimen, 476, 478 acetaminophen busulfan and, 71 AC regimen, 475,
1 The Cancer Programs Regulation (AR 242/98) is amended by this Regulation.
 Alberta Regulation 18/2005 Cancer Programs Act AMENDMENT REGULATION Filed: February 22, 2005 For information only: Made by the Minister of Health and Wellness (M.O. 9/2005) on February 17, 2005 pursuant
Alberta Regulation 18/2005 Cancer Programs Act AMENDMENT REGULATION Filed: February 22, 2005 For information only: Made by the Minister of Health and Wellness (M.O. 9/2005) on February 17, 2005 pursuant
Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19
 Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19 All Non-Par Provider Requests Requires Authorization Regardless of Service J0178 J0180 J0202 J0205
Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19 All Non-Par Provider Requests Requires Authorization Regardless of Service J0178 J0180 J0202 J0205
Part B payment for drugs in Medicare 0
 Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
clinical practice guidelines
 clinical practice guidelines Annals of Oncology 21 (Supplement 5): v232 v243, 2010 doi:10.1093/annonc/mdq194 Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced
clinical practice guidelines Annals of Oncology 21 (Supplement 5): v232 v243, 2010 doi:10.1093/annonc/mdq194 Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced
Supplementary Figures
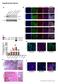 Supplementary Figures Supplementary Figure 1. Characterization of the generated hipsc lines a) Western blot analysis indicating the efficient downregulation of p53 upon knockdown in three hipsc lines (
Supplementary Figures Supplementary Figure 1. Characterization of the generated hipsc lines a) Western blot analysis indicating the efficient downregulation of p53 upon knockdown in three hipsc lines (
CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING
 CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING Jennifer Shamai MS, RN, AOCNS, BMTCN Professional Practice Leader Department of Clinical Practice And Professional Education Click How to the edit Experts Master
CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING Jennifer Shamai MS, RN, AOCNS, BMTCN Professional Practice Leader Department of Clinical Practice And Professional Education Click How to the edit Experts Master
Antiemetics in chemotherapy
 Antiemetics in chemotherapy Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Contact Name and Job Title (author) Directorate & Speciality Date of submission Date
Antiemetics in chemotherapy Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Contact Name and Job Title (author) Directorate & Speciality Date of submission Date
Acute Lymphocytic Leukemia
 Acute Lymphocytic Leukemia Splenectomy (Removal of Spleen) 6-Mercaptopurine (Purinethol, 6-MP) Alemtuzumab (Campath ) Arsenic Trioxide (Trisenox ) Bendamustine (Treanda ) Bexarotene (Targretin ) Bleomycin
Acute Lymphocytic Leukemia Splenectomy (Removal of Spleen) 6-Mercaptopurine (Purinethol, 6-MP) Alemtuzumab (Campath ) Arsenic Trioxide (Trisenox ) Bendamustine (Treanda ) Bexarotene (Targretin ) Bleomycin
Nausea and Vomiting. Symptom Management of Gastrointestinal Alterations. Gastrointestinal Alterations. Nausea/Vomiting Patterns
 Symptom Management of Gastrointestinal Alterations Elise Frans, MN, RN, CWON Oncology Clinical Nurse Specialist University of Washington Medical Center delterzo@uw.edu Nausea and Vomiting Mucositis Taste
Symptom Management of Gastrointestinal Alterations Elise Frans, MN, RN, CWON Oncology Clinical Nurse Specialist University of Washington Medical Center delterzo@uw.edu Nausea and Vomiting Mucositis Taste
