ANTIBIOTICS AND OTHER NATURALLY OCCURRING HYDROXAMIC ACIDS AND HYDROXAMATES HUBERT MAEHR. Hoff,nann-La Roche Inc., Nutley, N.J , USA ABSTRACT
|
|
|
- Allyson Bennett
- 6 years ago
- Views:
Transcription
1 ANTIBIOTICS AND OTHER NATURALLY OCCURRING HYDROXAMIC ACIDS AND HYDROXAMATES HUBERT MAEHR Hoff,nann-La Roche Inc., Nutley, N.J , USA ABSTRACT Naturally occurring hydroxamic acids and hydroxamates are reviewed and classified according to the chemistry of the hydroxamic acid functions. The identity of the Fe(m) trihydroxamate albomycin ö2 with antibiotic Ro is established. TABLE OF CONTENTS 1. Monohydroxamic acids 1.1. Linear hydroxamic acids Primary hydroxamic acids Secondary hydroxamic acids 1.2. Cyclic hydroxamic acids Pyrazine-type skeleton Oxazine-type skeleton HO HO 2. Dihydroxamic acids 2.1. Two linear hydroxamic acid functions Head-to-tail connection via ester bond. O co U 603
2 HUBERT MAEHR Head-to-head connection via amide bond. NH CO 2.2. Two cyclic hydroxamic acid functions 2.3. Linear and cyclic hydroxamic acid functions 3. Trihydroxamic acids 3.1. Three linear hydroxamic acid functions Head-to-tail connections Connections via ester bonds 0 co I-. o co--l Connections via amide bonds -. NH CO ' NH CO Head-to-head connections via amide bonds COLNFI0 (CO NH) Head-to-head connection via amide bonds and head-to-tail connection via ester bond'0 CO 3.2. Three hydroxamic acid functions in a cyclic skeleton Head-to-tail connections via ester bonds OO Head-to-tail connections via amide bonds 604
3 NATURALLY OCCURRING HYDROXAMIC ACIDS AND HYDROXAMATES Flydroxamic acid containing compounds are ubiquitous and frequently bewildering in structural variety and biological implications. Although details of their physiological significance in nature are yet largely unknown, hydroxamic acid moieties are constituents of antibiotics, growth factors, tumour inhibitors, cell-division factors and pigments, and are intimately associated with iron-transport phenomena. Various general and specific aspects of hydroxamic acids have been reviewed previously,' but intensified research interest due to the apparent connection of biological activities with hydroxamic acid functions has resulted in the discovery of an everincreasing number of hydroxamic acid containing compounds. Hence a survey of this class of substances within the framework of a detailed chemical classification is of interest. Hydroxamic acids may be regarded as derivatives of both hydroxylamines and carboxylic acids. The hydroxylamine part of the more complex hydroxamic acids is almost exclusively derived from hydroxylamino acids or their derivatives; the acyl portion is usually simple and is often acetyl or originates biogenetically from acetate. Classifying the natural hydroxamic acids according to the number of hydroxamic acid units per molecule5 differentiates three major classes with one, two or three units per molecule. Subdivisions are provided by distinguishing between primary (I) and secondary hydroxamic acids (II) which may be abbreviated by the symbols IA lila. Secondary hydroxamic acids may be linear (II, ha) or cyclic (III, lila). Di- and trihydroxamic acids are further differentiated according to the types of linkages connecting the hydroxamic acid moieties and according to the relative orientation of the hydroxatnic acid functions with respect to each other. If the hydroxylamine part of a given hydroxamic acid is designated as the head and the acyl part as the tail (cf. TA lila), one can distinguish between head to tail and head to head connections of two hydroxamic acid functions. Compounds with more than three hydroxamic acid functions per molecule, as well as tail to tail connected hydroxamic acids, have not as yet been reported. H N C R R' N C R I II I II I II HO 0 HO 0 HO 0 (I) (11) (III) R R'.--. R (IA) (ha) (lila) A striking feature of hydroxamic acids is the ability to form coordination compounds. As a bidentate group, the anionic hydroxamate function resembles the acetylacetonato ligand in its behaviour towards various chelate ions. The fact that the corresponding chelate compounds contain 605
4 HUBERT MAEHR five (IV) and six (V) membered chelate rings obviously is not reflected in the observed stability data8. = CH3 R ---M--- HC ---M--- (iv) / \\ / 0 /C0 CH3 The monohydroxamato ligand exhibits little cation selectivity with the exception of a distinct preference for Fe(iii). Acethydroxamic acid, for example, forms a 3: 1 chelate with ferric ions which is more stable than the corresponding Al and Yb complexes by factors of io and 1012, respectively8. As complex formation is a ph dependent process, protons effectively compete with chelate ions at low ph. The stepwise destruction of Fe(iii) complexes is accompanied by bathochromic shifts and hypsochromic effects in the visible light absorption spectra. The preference for Fe(In) is more pronounced in trihydroxamic acids where three hydroxamate groups create a sexadentate ligand. The corresponding chelations with ferric ions proceed with favourable entropy changes giving rise to positive chelate effects. Accordingly, the Fe(m) complex of the linear trihydroxamic acid ferrioxamine B is 200times as stable as the tris-acethydroxamato complex9. Utilizing this difference between relative stabilities, Fe(m) chelates of trihydroxamic acids can be distinguished spectroscopically from monoand dihydroxamic acids1 Oa The 1:1 complex of a trihydroxamic acid is stable at ph 2.5 and hence will exhibit the same visible light absorption spectrum at ph 2.5 and 7. In contrast, a tris-monohydroxamato complex at ph 2.5 is extensively dissociated" and can be recognized by bathochromic and hypsochromic changes in the spectrum. Hydroxamate functions, as unsymmetrical bidentate ligands, give rise to hexa-coordinated Fe(iii) complexes with octahedral configurations: thus, three molecules of a monohydroxamic acid can be arranged to form four isomeric structures. As represented in Figure 1, structures A and C are enantiomers as are B and D. The A-C pair is the one with the greater degree of order; all nitrogen atoms are equidistant from each other, as are the carbon atoms. Both members of this pair are readily recognized by their facial orientation of those corners of the 5-membered chelate rings which contain either nitrogen (second smallest atoms) or carbon (smallest atoms). As a result of specific Fe(iii) selectivity it is not surprising to find hydroxamic acids, particularly trihydroxamic acids, in association with ferric ions. This distinct preference of Fe(iii) masks the affinity of hydroxamate ligands toward other bio-active cations and may thus account for the widespread distribution and accumulation of hydroxamic acids in microbial cells. The low order of selectivity towards Fe(ii), however, explains the function of certain hydroxamic acids as iron-transporters in microbial systems (v)
5 NATURALLY OCCURRING HYDROXAMIC ACIDS AND HYDROXAMATES A B In this report, we attempt to summarize and classify naturally occurring hydroxamic acids according to structural features previously elaborated. Comparative chemistry should illustrate structure-activity relationships, and brief outlines of pertinent degradation reactions will demonstrate progress in a complex and fascinating field of natural products Linear monohydroxamic acids C Figure 1. Isomeric structures of a Fe(III) trihydroxamate. 1. MONOHYDROXAMIC ACIDS Primary hydroxamic acids Simple hydroxamic acids. The simplest hydroxamic acids have been prepared enzymatically3. Incubation of acetate with ATP and hydroxylamine in fresh pigeon liver gave acethydroxamic acid: higher fatty acid homologues could be prepared by lipase-catalysed condensations in various liver extracts. Cysteine-activated papain catalysed the replacement of the amide amino-group of a number of amino acid derivatives by hydroxylamine. Similar transamidations could be achieved by cell-free extracts of Proteus vulgaris with asparagine and glutamine. Actinonin. The antibiotic actinonin, produced by a Streptomnyces species and active against a number of Gram positive and Gram negative bacteria, mycobacteria and phages, represents a more complex member of this group. Acid hydrolysis of actinonin yielded L-prOlinOl, L-valifle and D-cx-n-pentyl succinic acid: Lossen rearrangement of the O-acetyl derivative VI followed by hydrolysis yielded compound VII. Actinonin could thus be formulated as VIII' R NH C CH -CH2 - NH O C- CH3-4 HOOC H -CH2 NH2 C5H11 C5H11 (VI) 607 (VII) D
6 HUBERT MAEHR C5 NHOH CH (Cl-I1)2 CH,OH Actinonin (VIII) Secondary hydroxainic acids Hadacidin. Produced by several Penicilliuin species, hadacidin (X) is active as tumour inhibitor and plant growth retardant. It was identified by hydrolysis yielding formic acid and hydroxylaminoacetic acid and synthesized'6 by formylation of hydroxylaminoacetic acid, which in turn was prepared from ethyl acetoacetate and nitric oxide. A more recent synthesis17 involved N-alkylation of anti-benzaldoxime' 8 19 to give N-(carboxymethyI)-xphenyl nitrone (IX) which was decomposed and formylated to yield N- formyihydroxyamino acetic acid, X. NCOOH HCOOH CHO (IX) AC2O + HANCOOH OH Hadacidin (X) Fusarinines. Although Fusarium species produce cis-fusarininet in abundance, this compound is without apparent growth factor or antibiotic properties-0'. Its presence in nature is significant in that it constitutes the monomeric unit of fusarinines A and B2 and fusigen2' and is contained in ferrirhodin22. cis-fusarinine is one of the simple representatives containing N5-hydroxyornithine, XII, a hydroxylamine derivative frequently found in natural trihydroxamic acids. N5-hydroxyornithine was originally described as a constituent of ferrichrome23 and was synthesized in eight steps from glutamic acid XVI A via the corresponding nitro- and hydroxylaminohydantoins in 7 per cent yield24. An improved synthesis via nitrone XVI C was achieved in our laboratory with 40 per cent yield, starting with 5- hydroxy-2-aminovaleric acid XVI B25. Treatment of XII with methanolic hydrogen chloride afforded the hydrochloride of l-hydroxy-3-amino-2- piperidone, XV26. This compound was originally called fusarinine, but should be termed cis-fusarinine to differentiate it from 3-butyipyridine, the decarboxylation product of fusaric acid, which is also named fusarinine (dl P1. A. Plattner, W. Keller and A. Boiler. Helv. Chi!n. Acta 37, 1379, 1954) and from the recently discovered trans-fusarinine (cf. ref. lob). 608
7 NATURALLY OCCURRING HYDROXAMIC ACIDS AND HYDROXAMATES Structure determination loa of cis-fusarinine (XI) is primarily based on degradation reactions summarized below. Reductive hydrolysis with 47 per cent hydriodic acid gave ornithine, Xlii, whereas 6 N hydrochloric acid OH N 0 0 MeOH HCI (XIV) NHOH 8steps (XV) ('OOH NH2 H,N cis-fusarinine (XI) HI NH2 HI HUOOH (XII) N) IHCI 4 steps H2cOOH (XVIA) OH H,N COOH Ac HN COOCH3 H2N COOH (XIII) (XVIC) (XVIB) hydrolysis yielded N5 hydroxyornithine, XII. 3-Methylpent-2-eno--5-lactone (XIV) was obtained upon periodic acid oxidation27. Lactone XIV, which can be regarded as A2-anhydromevalonic acid lactone, had previously been identified as a metabolite of various fungi2sa. trans-5-hydroxy-3-methylpent-2-enoic acid is found in substrates of various moulds2sb: the geometric isomer of fusarinine, trans-fusarinine, has recently been described as a naturally occurring productiob and constituent of more complex compounds containing hydroxamate functions Cyclic hydroxamic acids Pyrazine-type skeleton Aspergillic acid. One of the first antimicrobial mould metabolites described is aspergillic acid29 with activity against certain Gram positive and many Gram negative bacteria and known as a microbial growth factor30' 31 J is presumably identical with an antibiotic isolated by Glister32' 609
8 HUBERT MAEHR Aspergillic acid, elaborated by Aspergillus flavus, was recognized as a pyrazine cyclic hydroxamic acid34, the first example of a naturally occurring hydroxamic acid. Aspergillic acid exhibits weakly acidic and basic properties and gives a wine-red colour with ferric chloride. Reduction with either hydriodic acid in acetic acid or with hydrazine, yielded deoxyaspergillic acid, 3,6-dialkyl-2(1H)-pyrazinone, to which tentative structure XIX was assigned. Bromine oxidation of both aspergillic and deoxyaspergillic acids, followed by reduction, gave a diketopiperazine which did not depress the melting point of isoleucine anhydride, XXII. Aspergillic acid was therefore thought to be XXV or the tautomeric hydroxamic acid35. Flavacol (XVII) H Deoxyaspergillic acid (XVIII) H (XIX) H H Leu anhydride (XX) Leu-isoleu anhydride (XXI) Isoleu anhydride (XXII) Neoaspergillic acid (XXIII) Aspergillic acid (XXIV) (XXV) A depression of a mixture melting point36 of the synthetic racemate of X1X37 by racemized deoxyaspergillic acid, however, indicated that the two compounds were different. Hydrolysis of the diketopiperazine derived from aspergillic acid indeed yielded Jeucine and isoleucine and could thus be formulated more correctly as XX138. To distinguish between the two possible structures for deoxyaspergillic acid, racemic XVIII was synthesized and found to be identical with racemized deoxyaspergillic acid. This established the structure of aspergillic acid as 610
9 NATURALLY OCCURRING FIYDROXAMIC ACIDS AND HYDROXAMATES 6-sec-butyl-1.-hydroxy-3-isobutyl-2(IH)pyrazinofle or its tautomer XXIV, 6-sec-butyl-3-isobutyl-2-pyrazinol-1-oxide39 racemic aspergillic acid was ultimately synthesized40. Granegillin. Isomeric with aspergillic acid, granegillin is also produced by Aspergillus flavus but differs from aspergillic acid in several chemical and biological aspects41. No structural formula has been proposed for this antibiotic. Neoaspergillic acid. Another isomeric aspergillic acid, neoaspergillic acid, is elaborated by Aspergillus sclerotiurn42. The close chemical relationship to aspergillic acid is reflected in very similar biopotencies. The structure of neoaspergillic acid (XXIII) was deduced from the great similarity to aspergillic acid and n.m.r. spectroscopy and was proved by reduction to pyrazi none XV1143 and synthesis40. Interestingly, compound XVII was previously found with aspergillic acid in culture filtrates of Aspergillus flavus and was termed flavacol44, although it would now, more appropriately, be regarded as deoxyneoaspergillic acid. Hydroxyaspergillic acid. Shortly after the discovery of aspergillic acid, it was observed that Aspergillusfiavus, under modified fermentation conditions, produced a new antibiotic33. This substance was named hydroxyaspergillic acid45. Treatment of hydroxyaspergillic acid with hydriodic acid in phosphoric acid afforded racemic deoxyaspergillic acid XVIII and established the close relationship to aspergillic acid. Hydriodic acid in acetic acid, however, yielded a deoxyaspergillic acid (XXVII) with an additional double bond in conjugation with the pyrazinone ring. Cleavage of XXVII with ozone gave optically inactive XXVIII and acetaldehyde, confining the hydroxyl groups to the sec-butyl side chain. The assignment of a tertiary hydroxyl group is supported by the observed reluctance towards esterification and a HPc (XXVI) (XVIII) HI ACOH HC-IlCXN' (XXVII) XN (XX VIII) negative iodoform reaction, establishing the structure of hydroxyaspergillic acid as XXVI. Neohydroxyaspergillic acid. The second example of a hydroxyaspergillic 611
10 HUBERT MAEHR acid was provided by Aspergillus scierotiorum which produced an antibiotic with antiphage and antibacterial activity. The compound was identified as a hydroxyaspergillic acid and was termed neohydroxyaspergillic acid46. Several years later the same compound was reported to be coproduced with aspergillic acid by the same mould42. Hydroxy- and neohydroxyaspergillic acids have similar biological activities but are less active antibiotics than aspergillic and neoaspergillic acids. Assignment of probable structure XXIX43 to neohydroxyaspergillic acid is based on biogenetic considerations as well as on n.m.r. spectra, whose interpretations were facilitated by the availability of previously defined aspergillic acids. Hydroxyaspergillic Neohydroxyaspergillic Mutaaspergillic acid acid acid (XXVI) (XXIX) (XXX) Mutaaspergillic acid. A third variation of hydroxylated aspergillic acids, mutaaspergillic acid, was obtained from a culture of Aspergillus oryzae47. Guided by the investigations of Dutcher on hydroxyaspergillic acid, hydriodic acid in phosphoric acid treatment yielded dideoxymutaaspergillic acid, 3-isobutyl-6-isopropyl-2(1H)-pyrazinone, whose constitution was confirmed by synthesis48 and, subsequently, by degradation to valine and leucine49 following the example of previous studies35' 38, The side chain hydroxyl group was assigned to the tertiary position of the isopropyl chain in view of optical inactivity of mutaaspergillic acid, a negative iodoform reaction and the reluctance to form a dehydro compound which could be obtained from hydroxyaspergillic acid45. Structure XXX for mutaaspergillic acid was also confirmed by synthesis Oxazine-type skeleton 2,4-Dihydroxy-1,4-benzoxazin-3-one; 2,4-dihydroxy- 7-met hoxy- 1,4-benzoxazin-3-one and the two corresponding glucosides. The immediate precursors of benzoxazoline-2-one (BOA, XXXI) and 6-methoxybenzoxazoline- 2-one (MBOA, XXXII) found in rye, wheat and maize plants were identified as two cyclic hydroxamic acids, 2,4-dihydroxy-1,4-benzoxazin-3-one XXXIII and the corresponding 7-methoxy derivative XXXIV. The latter two compounds are derived enzymatically from the corresponding glucosides XXXV and XXX V!51. R 0 \, R = H (BOA) (XXXI) R = CH3O (MBOA) (XXXII) N H 612
11 NATURALLY OCCURRING HYDROXAMIC ACIDS AND HYDROXAMATES R.OOR' R = H, R' = H (XXXIII) N"O OH R = CH3O, R' = H (XXXIV) R=H,R=C6H11O5(XXXV) R = CH3O, R' = CH1O5 (XXXVI) BOA is found in crushed rye plants, whereas MBOA originates from maize plants. There is strong evidence that neither of the two compounds is present in uninjured tissue52. Thus, apparently stable hydroxamic acids occurring in living plants decompose upon plant injury to yield substances with modified biological activity. In contrast to the glucosides XXXV and XXXVI, the corresponding aglucones as well as BOA and BMOA exhibit growth-inhibiting properties with pronounced fungistatic activities51. Whereas the decomposition of the glucosides to the corresponding aglucones occurs enzymatically, the ring contraction appears to be a relatively simple process and occurs readily within the ph range 4 8.Prooffor the structure of glucoside and aglucone was ascertained by the reactions summarized below amino-phenol accompanied by hydroxylamine or glycine, depending upon the reaction conditions, was obtained from compounds XXXV and XXXIII. Hydrogenolysis yielded XXXVII and XXXVIII which no longer gave positive tests with ferric chloride. As expected, XXXVIII could not be converted to benzoxazolinone XXXI upon treatment with hot water. The structures of aglucone XXXIII and its reduction product, XXXVIII, were confirmed by syntheses IMHYDROXAMIC ACIDS 2.1. Compounds with two linear hydroxamic acid functions Hydroxarnic acid functions connected head to tail via ester bond Fusarinine A. A depsipeptide composed of two cis-fusarinine units was obtained from a Fusariurn roseurn culture and termed Fusarinine A, XXXIX20. Hydrolysis of one mole of fusarinine A in water cleaved the ester bond selectively giving rise to two moles of cis-fusarinine, XI. Dilute acid, protonating the free amino group and thus protecting the vicinal ester linkage against hydrolytic cleavage, liberated 3-methylpent-2-eno-5-lactone, XIV. Periodic acid oxidation cleaved both hydroxamic acid functions selectively and resulted in lactone XIV: the incipient nitrogen-bearing terminals combined to the dimeric nitroso compound XL which, upon hydrogenolysis, yielded ornithine XIII and 5-O-(L-ornithyl)-DL-3-methylpentanoic acid, XLI. The structure of XLI was confirmed by synthesis, providing definite proof for the existence of the ester linkage in fusarinine A Hydroxarnic acid functions connected head to head via arnide bonds Rhodotorulic acid. This compound was isolated from iron-deficient cultures of a red yeast, Rhodotorula pilirnanae57, and other organisms58 and showed potent growth-stimulating activity for Arthrobacter species5. Rhodotorulic acid was identified as the diketopiperazine of N5-acetyl-L-N5-hydroxyornithine, XLII, and is thus chemically related to sideramines of the fernchrome type, but does not show the antagonism against albomycin, which is characteristic of the sideramine growth factors. 613
12 GLUCOSE dii. HC1, A C6H1105 O C6H1105 conc. Zn/ACOH IIIIII:O NH2 H I OH (XXX VII) (XXXV) KOH Enzyme Zn/HCI H (XXX VIII) ti1ii1ixo N OH (XXXIII) OH HI/P OH 2 /H20, A BOA (XXXI) +NH2OH + NH, CH2---COOH tn
13 NATURALLY OCCURRING HYDROXAMIC ACIDS AND HYDROXAMATES >< z Q4-7 -J 0 Z C x 0 C C 615
14 HUBERT MAEHR Hydrolyses of rhodotorulic acid with hydrochloric acid and hydriodic acid yielded XII and XIII, respectively, whereas hydrogenolysis59 afforded N5-acetyl-L-ornithine anhydride, identical with a synthetic specimen. Ditneric acid. Closely related to rhodotorulic acid, dimeric acid, XLIII, is produced by Fusariurn dirneruin and represents the diketopiperazine of trans$usarinine, N5-(trans-5-hydroxy-3-methylpent-2-enoyl)-N5-hydroxyornithinebo, lob In contrast to rhodotorulic acid, dimeric acid is reported to antagonize the action of ferrimycin and albomycin60. 2XN OH Rhodotorulic acid: R CH3 H Dimeric acid: R =,CH2 CH2OH.. ' CH3 (XLII) Aerobactin. A pentaprotic acid with two hydroxamic acid moieties, produced by Aerobacter aerogenes and related strains, to which structure XLIV was assigned61, was named aerobactin. Hydriodic acid hydrolysis yielded L-lysine, whereas hydrochloric acid treatment liberated citric acid and N6-hydroxylysine, XLV, an amino acid originally discovered by Snow62 as a constituent of mycobactins. The presence of N6-acetyl-N6-hydroxylysine was indicated by periodate oxidation27 yielding acetic acid and the n.m.r. spectrum of aerobactin was in satisfactory agreement with assignment XLIV. OH 0 HOOC H COOH H COOH H2N COOH Aerobactin (XLIV) (XLV) Ferribactin. Cultures of Pseudornonas fluorescens Migula produce a polypeptide, called ferribactin, which appears to be a dihydroxamic acid63. The hydroxamic acid functions are due to two N5-acetyl-N5-hydroxyornithine moieties. Since ferribactin forms a 1: 1 complex with Fe(iii), it was concluded that additional ligands, other than hydroxamates, must be present. The structure of this compound is as yet unknown, a molecular weight between has been estimated and its listing in this section is tentative. 616
15 NATURALLY OCCURRING HYDROXAMIC ACIDS AND HYDROXAMATES 2.2. Compounds with two cyclic hydroxamic acid functions Puicherrirninic acid. Under certain conditions Candida pulcherriina produces a red pigment which was described as an Fe(m) containing polymer, pulcherrimin64. To the iron-free monomer, termed puicherriminic acid65, tentative structure XLVI was assigned50, for reductive pyrolysis of pulcherrimin yielded leucine anbydride, XX. Furthermore, the Fe(iii) complex of aspergillic acid resembled puicherrimin according to visible and ultraviolet light absorption spectra, but there appeared to be two hydroxamic acid functions in puicherriminic acid as judged from the N :0 ratio and the insolubility of puicherrimin due to the high molecular weight. Puicherriminic acid was thus regarded as a quadridentate ligand. Structure XLVI subsequently gained support when the diprotic pulcherriminic acid could be degraded with zinc and acetic acid to give L- leucine anhydride65. A comparison of puicherriminic acid65 with synthetic 1,4-dihydroxy- 3,6-diisobutyl-2,5-dioxopiperazine, XLVI, however, showed the two compounds to be different and alternative tautomer XLVII was proposed for puicherriminic acid66. Realizing the inability of XLVII to give rise to optically active leucine, MacDonald sought to resolve this discrepancy67. OH 0 0 (XLVI) (XLVII) (xlvih) He isolated puicherrimin from three different strains of Candida puicherritna but, although the resulting three samples of puicherriminic acid were identical, they differed from the previously described66 puleherriminic acid. Nevertheless, MacDonald's puicherriminic acid was reduced to DL-leucine anhydride with zinc and acetic acid and n.m.r. spectra indicated two isobutyl side chains and no ring hydrogen atoms. Thus structure XLVIII, or possibly its tautomer XLVII, was postulated for MacDonald's puicherriminic acid. This assignment was supported by analysis of reduction products of puicherriminic acid67 and by synthesis of 2,5-dihydroxy-3,6- diisobutylpyrazine 1,4-dioxide (XLVIII) and its Fe(iii) complex, which proved to be identical with puicherriminic acid and puicherrimin, respectively68. Mycelianarnide. Preferentially active against Gram positive bacteria, this weakly acidic, laevorotatory antibiotic is elaborated by Penicilliutn griseofulvum Dierckx69. Mild hydrolytic conditions led to the optically inactive p-myceloxybenzoic acid (L) and its amide (LI), whereas vigorous acid hydrolysis yielded ammonia, carbon dioxide and aminoacetophenone, LII. 617
16 HUBERT MAEHR The hydrolytic instability of the ether function of the p-myceloxybenzoic acid moiety was correctly attributed to an allylic ether69. In an extension of this work70, mycelianamide was reduced to the ferric chloride negative dideoxymycelianamide LIV which, in turn, yielded alanine LIII and p- hydroxyphenylpyruvic acid LV upon acid hydrolysis. Alanine was also detected in acid hydrolysates of XLIX. These results suggested a cyclic dihydroxamic acid XLIX, an assignment which was supported by the similarity of the u.v. spectra of mycelianamide and the dideoxy compound and by a strong band at 1675 cm' in the i.r. spectrum, indicating an unstrained cyclic amide. The correct structure of the mevalonic acid derived C10H17 side chain was established in the course of a reexamination several years later71. The n.m.r. spectra strongly suggested a geranoxy or neroxy structure which was supported by oxonolysis products LVI, 4-carbamoylphenoxyacetic acid, and LVII, laevulinic acid. The two possible geometrical isomers of p-myceloxybenzamide were synthesized establishing the structure of LI as p-geranoxybenzamide and hence the total structure of mycelianamide (XLIX). A synthesis of racemic deoxymycelianamide has been reported recently Compounds with linear and cyclic hydroxamic acid functions Mycobactins. The present knowledge of the chemistry of mycobactins is largely due to the contributions of Snow which were summarized recently7. Mycobactins, apparently produced exclusively by mycobacteria, are highly mycobacteria-specific growth factors. Mycobactins are chemically closely related; the chemical differences are characteristic for certain groups of the producing microorganism. The basic formula LIX pertains to all mycobactins; the chemical variations are due to different substituents R1 to R5 and may be further complicated by stereoisomeric possibilities. Cleaving the ester linkage, mild alkaline treatment of mycobactins yielded the corresponding mycobactic acids LX and cobactins LXI. Further acid degradation of mycobactic acid liberated a substituted benzoic acid of transient existence giving rise to carbon dioxide and LXII, which was either phenol (R2 H) or rn-cresol (R2 = CH3), depending upon the mycobactin investigated. In addition to this aromatic fragment the acid hydrolysate of LX always contained a hydroxyamino acid LXIII, which was serine (R3 = H) or threonine (R3 CH3), N6-hydroxylysine (XLV) and a more or less complex fatty acid LXIV. The presence of the hydroxyphenyloxazoline nucleus was confirmed by u.v. spectral data and the hydroxamic acid linkage of the fatty acid side chain R1 was demonstrated by periodate oxidation, suggesting structure LX for mycobactic acid. Acid hydrolysis of cobactin LXI again yielded N6-hydroxylysine as well as a hydroxyamino acid. LXV, which, in the case of most mycobactins, was either 3-hydroxybutyric acid (R4 = CH3; R5 = CH3) or 3-hydroxy-2- methylpentanoic acid (R4 = C2H5, R5 = CH3). Similar to mycobactins and mycobactic acid, cobactin gives a wine-red ferric chloride colour, is not reducing and possesses only one ionizing group (PKa 9.1), indicating that both carboxyl groups as well as the amino group are masked. The resulting assignment LXI for cobactin provides a hydroxyl group which is involved 618
17 H o N OH cn_( N CH NH3 z H00C_((J 1117 H NO H OH Zn/AcOH NH4OH Myceloxybenzoic acid (L) H2N CO ' [LJ O C1OH (LI) c-i 17 Dideoxymycelianamide Mycelianamide (LIV) (XLIX).< H2N_CO_fj 0 C dii. HC\ /. HC1 H2N HO'O '. OH 0 HOX HOYJ O (LVI) 0 I NH2 (LV) (LIII) (LII) (LVII) 03 rid C H rn
18 HUBERT MAEHR in the formation of the ester linkage with the carboxyl group of mycobactic acid to yield mycobactin, LIX. R2 Mycobactin (LIX) OH 0 H R3 R2 Mycobactic acid (LX) Cobactin (LXI) H,N COOH CO2 HO R3 H,N HOOC R1 (LXIV) (LXII) (LXIII) (XLV) HOCOOH (LXV) HN - COOH YJNHOH (XLV) Although they are dihydroxamic acids, mycobactins form extremely stable 1:1 complexes with Fe(iii) ions, a third bidentate ligand being contributed by the phenolic hydroxyl group and the oxazoline ring nitrogen. 3. TRIHYDROXAMIC ACIDS Trihydroxamic acids of natural origin which are able to form stable Fe(m) trihydroxamate complexes and exhibit absorption maxima between 620
19 NATURALLY OCCURRING HYDROXAMIC ACIDS AND HYDROXAMATES nm,were originally designated as siderochromes73' 2Siderochromes are subdivided according to biological activity into sideromycins, designating siderochromes with antibiotic activity, and sideramines, representing growth-factor siderochromes. More specifically, therefore, the term siderochrome should be limited to natural trihydroxamic acids or their Fe(III) chelates which either lack sideromycin or sideramine activity, which can exhibit both of these activities, or whose biological activities are undetermined776. Sideromycins have been differentiated according to biological activity and paper chromatographic behaviour7. Rapid development of resistant colonies with cross-resistance toward all sideromycins appears to be peculiar to these antibiotics. Antagonism between sideromycins and sideramines is of diagnostic value74 and considerable biological significance14 Although sideromycins have as yet only been found as metabolites of streptomycetes, sideramines have been demonstrated in cultures of streptomycetes and other actinomycetes, fungi and bacteria74' Compounds with three linear hydroxamic acid functions Hydroxarnic acid functions connected head to tail Connection via ester bond Fusarinine B. Total acid hydrolyses of all cis-fusarinines yielded the same product profiles consisting of N5- hydroxyornithine, XII, and 3-methylpent-2-eno-5-lactone, XIV. Brief treatment of fusarinine B in hot water yielded cis-fusarinine (XI) and fusarinine A (XXXIX). On continued hydrolysis cis-fusarinine is the principal degradation product. The trihydroxamic acid nature of fusarinine B was demonstrated by the relative stability of the Fe(m) complex in acidic solution. Closely related to and coproduced with fusarinines A and C, fusarinine B can thus be formulated as LXVI and regarded as a sideramine, for fusarinines A, B and C exhibit slight growth-factor activity with Arthrobacter JG-920. Fusarinine B CLXVI) 621
20 HUBERT MAEHR Connections via anide bonds Ferrioxainines and ferrirnycins. Ferrimycins, metabolites of Streptornyces griseoflavus, exhibit pronounced activity against Gram positive microorganisms75' 8 Extended efforts to isolate related substances resulted in the discovery of a new class of sideramines, the ferrioxamines79' 1, which are chemically related to ferrimycins and were found in a large variety of microorganisms77' 80 In spite of the chemical similarity to ferrimycins, ferrioxamines, as a rule, do now show antibiotic activity, but antagonize the antibiotic activity of ferrimycins75 and other sideromycins77 against Gram positive organisms. An acid hydrolysis product, common to both ferrimycins and ferrioxamines, was soon recognized as 1-amino-5-hydroxylamino pentane, LXVII8. Ninhydrin-positive and reducing, LXVII is dibasic with pk*mcs 5.30 and Hydrogenolysis of LX VII gave 1,5-diaminopentane, LX VIII. In analogy to the work of Snow62, LXVIII could be obtained directly from ferrioxamines and ferrimycins as a result of reductive hydrolysis with hydriodic acid. The structure of LXVII was confirmed by synthesis starting with 1-phthalimido-5-bromopentane8' and later by a reaction sequence starting with 1-benzoyl-5-chloropentane82. Among the ferrioxamines, ferrioxamine B (LXIX) is the most abundant representative. It is basic (pk*mcs 8.79) and upon acid hydrolysis yielded acetic acid, succinic acid and 1-amino-5-hydroxylaminopentane in a molar ratio of 1:2:3. To account for the observed stability of the Fe(iii) complex, the sequence depicted in structure LXIX was postulated83' 84, an assignment later confirmed by synthesi85. N-Acetylfèrrioxamine B occurs in nature and was designated ferrioxamine D1 (LXX)86: the structure was readily proved by N-acetylation of LXIX85' 86 The corresponding iron-free N-methanesulphonyl derivative (Desferal ) is used in the treatment of iron intoxication or iron-storage diseases. The structure of the zwitter-ionic Ferrioxamine 0 (LXXI) was confirmed by conversion to nocardamine87 and total synthesis88. Diverging from the basic theme, ferrioxamines A1 and A2 contain 1- amino-4-hydroxylaminobutane residues. The structure of ferrioxamine A1, LXXII, was elucidated89. Ferrioxamine A2 differs from A1 in that it contains two 1-amino-4-hydroxylaminobutane residues of unknown sequence. The structures of the two basic ferrioxamines C and F are still unknown. Among the ferrimycins, component A1, LXXIII, was studied most extensively90. The iron-binding centre of ferrimycin A1 is identical to LXIX and LXX. Controlled hydrochloric acid hydrolysis of ferrimycin gave LXXIV and LXXV in a molar ratio of 2: 1. The structure of fragment LXXV was deduced by further degradation reactions yielding LXVII, ammonia, and 3-amino-5-hydroxybenzoic acid, and by n.m.r. studies and mass spectrometry Three hydroxamic acid functions connected head to head via amide bonds. Ferrichrorne, ferrichroine A and C. Cultures of the smut fungus Ustilago sphaerogena produced two microbial growth factors, ferrichrome and the less active ferricbrome A'2'. Two factors, properties of N6-hydroxylysine known due to the investiga- 622
21 NATURALLY OCCURRING HYDROXAMIC ACIDS AND HYDROXAMATES Ferrioxamine B: R = H Ferrioxamine D1: R = COd-I3 (LXIX) (LXX) H H CF CF Ferrimycin (LXXIII) NH2 HO H NHOH (LX VIII) (LX VII) (LXXIV) + CIH2N H3C( Cl (LX XV) 623 P.A.C. 28/4 1
22 HUBERT MAEHR Ferrioxamine G (LXXI) 0 Ferrioxamine (LXXII) tions of Snow62 and the discovery of the cleavability of hydroxamic acid C N bonds by periodate9 1, facilitated the elucidation of the iron-binding centres as trihydroxamic acids consisting of three N5-acyl-N5-L-hydroxyornithine moieties24. One molecule of ferrichrome contains three molecules of N5- acetyl-n5-hydroxyornithine and 3 molecules of glycine. To account for the observed neutrality, a cyclic hexapeptide was proposed whose sequence was determined as depicted in LXXVI92. Ferrichrome has now been synthesized93, confirming earlier structural assignments. Ferrichrome A (LXXVII) is an acidic substance and differs from fernchrome in that two of the glycine moieties are replaced by serine and the acyl portions of the three hydroxamic acid functions are derived from 2-methylpropene-1,3-dicarboxylic acid with the double bond x-13 to the hydroxamate function23. The amino acid sequence92 assigned was confirmed by crystallographic analysis94, which also revealed the absolute configuration C (Figure 1) about the Fe(IiI) ion. Ferrichrome C resembles ferrichrome. Acid hydrolysis indicated that one of the glycine moieties is replaced by alanine58, suggesting structure LXXVIII for ferrichrome C where the hydroxymethyl group of serine is replaced by a methyl group. Ferrichrocin, ferrichrysin, ferrirubin and ferrirhodin. An extended search for sideramines among Aspergiilaceae revealed not only the presence of ferrichrome and coprogen95 but a series of new, ferrichrome-like sideramines75. In regard to the amino acid constituents, Ferricrocin, LXXVIII, assumes a position between ferrichrome and fernichrome A since it contains two molecules of glycine, one of serine and three molecules of N5-acetyl- N5-hydroxyornithine96. The amino acid sequence is assumed to be analogous to that of ferrichrome A94. Ferrichrysin96'97 (LXXIX), ferrirubin22 (LXXX), and ferrirhodin22 (LXXXI) possess the hexapeptide moiety of ferrichrome A: the differences are localized in the acyl portions of the hydroxamate functions. Cleavage of the hydroxamate functions of fernirhodin by periodate yielded lactone XIV, demonstrating the similarity to cisfusarinineboa and 624
23 C' ci' Ferrichrome (LXX VI) 0 Ferricrocin (LXX VIII) HN. H A Ferriclirome R = Ferricbrysin R Cl-I3 (LXX VII) HOOC H3C = H (LXIX) Ferrirubin R = (LXXX) Ferrirbodin R = (LXXXI) H3CN,, HOH2CH2C'ThH H3CH HOH2C H2C"'
24 HUBERT MAEHR establishing the acyl portions of the hydroxamate groupings as cis-5-hydroxy- 3-methylpent-2-enoyl. Similarly, ferrirubin gave rise to trans-5-hydroxy- 3-methylpent-2-enoic acid281' which did not lactonize. Both ferrirhodin and ferrirubin gave identical hexahydro derivatives upon catalytic hydrogenation confirming structures LXXX and LXXXI as geometric isomers. Ferrichrysin possesses the hydroxamate arrangement of ferrichrome (LXX VI) and ferricrocin (LXX VIII). Mild acid hydrolysis was found to cleave hydroxamic acids to liberate carboxylic acids and hydroxylamine moieties. Reacylation followed by O-deacylation in methanol-ammonia reconstituted hydroxamic acids96. Thus, ferrirubin (LXXX) was converted to ferrichrysin as was ferrichrome A establishing the identity of the hexapeptide moieties involved22. Albotnycin, grisein, alveornycin, antibiotics A-i 787, LA 5352, LA 5937, 10073, Ro , Ro and Ro Albomycin, produced by Actinornyces subtropicus98, is an antibiotic with broad-spectrum in vitro activity3. Initial separation studies revealed heterogeneity of albomycin preparations99: gradient elution chromatography on a sulphonated polystyrene resin column and electrophoresis demonstrated the presence of one major antibiotic, albomycin ö2. Antibiotics and c were recognized as products resulting from degradation of the unstable albomycin Albomycin 2 in dilute acid, at room temperature, was completely converted to the basic albomycin 8; UOfl heating in neutral, aqueous solution albomycin E was the major product' 1. Curiously, albomycin 8 at 28 acts as an antibiotic against B. subtilis, but at 38 it is a sideramine and antagonizes the activity of albomycins and 102, 103, The latter are also subject to competitive inhibition by sideramines of the ferrichrome and ferrioxamine types7. Hydrochloric acid hydrolysis of a mixture of albomycin ö1 and 2 was reported to yield serine and ornithine in an equimolar ratio, as deduced by quantitative evaluation of paper chromatograms99. In an independent investigation by Poddubnaya and Krysin' 4, albomycin was claimed to contain N5-hydroxyornithine, serine and iron in molar ratios of 3:3:1, but experimental details were not disclosed. In the meantime it was demonstrated that hydrochloric acid hydrolysis in the presence of Fe(iii) ions was unsuitable for the determination of hydroxylamine moieties but quantitation could be achieved by reductive hydrolysis23'75' 96 Alternatively, catalytic reduction of a hydrochloric acid hydrolysate, obtained after removal of Fe(iii) ions, yielded reliable identification of the corresponding amines22. Yet, even in view of these findings, the postulates of equimolar amounts of serine and N5-hydroxyornithine in albomycin hydrolysates were retained'05' 102, A cyclic hexapeptide structure was inferred in view of the absence of terminal amino groups and three free serine hydroxyl groupings were postulated on the basis of partial esterification with sulphuric acid104. Three acetyl residues at the N5-positions of the N5-hydroxyornithine moieties were found to contribute to the Fe(iii) trihydroxamate part' 2' 104, providing a structural arrangement resembling sideramines of the ferrichrysin type. In addition to these structural features, albomycin was disclosed to contain a pyrimidine and one sulphur atom, both attached to the same 626
25 NATURALLY OCCURRING HYDROXAMIC ACIDS AND HYDROXAMATES serine moiety' 6. Partial hydrochloric acid hydrolyis reportedly gave compounds which, upon total hydrolysis, yielded 3-methyluracil (LXXX VI) and serine in molar ratios of 1:1, 1:2 and 1 :3b0'7, 1O8 Degradative oxidation of the hydroxamic acid functions of albomycin, followed by partial hydrolysis, allegedly gave six peptides whose composition indicated a peptide moiety as depicted in LXXXII-LXXXIV' 8' The same hexapeptides were Fragment H2 113C ON-S-O N ('OOH JHOH (LXXXV) H3C 0 HO. 0 OH Albomycin Aibomycin Albomycin (5 = N CO -CH3 R 0 R = NH H (LXXXII) (LXXXIII) (LXXXIV) proposed for all albomycins: the differences were assumed to be localized in the pyrimidine moieties 106,llo 3-Methyluracil (LXXXVI) was obtained upon hydrochloric acid hydrolysis of a mixture of albomycins' whereas perchioric acid hydrolysis of albomycins and ö2 gave 3-methylcytosine (LXXXVII)' 1. A comparison of the u.v. spectra of 1,3-dimethyluracil (LXXXVIII) and 1,3-dimethylcytosine (LXXXIX) with the spectra of albomycins ö1 and suggested that the 3-methylpyrimidines were linked to the serine moiety via position A similar conclusion was reached after isolation of 3,N4-dimethylcytosine (XC) from a perchioric acid hydrolysate 627
26 HUBERT MAEHR of albomycin c, previously treated with dimethyl sulphate' 12 Diazomethane and dimethyl sulphate failed to methylate the pyrimidine moiety of albomycin ö2. The absence of brominated pyrimidines in albomycins and 2' after treatment with bromine in acetic acid, was taken as indication that the albomycins contained 5-substituted pyrimidines"3. Implying u.v.-spectral similarity between 1,3-dimethyl-N4-acetylcytosine (XCI) and albomycin 2' Sorm and co-workers' 6 proposed a 1-substituted 3-methyl-N4-acetylcytosine moiety as the chromophore of albomycin 2 as illustrated in LXXXII. 0 NH, 0 (LXXXVI) NO (LXXXVII) LNO CH1 (Lxxxviii) NH NH CH2 N C0 CH2 NO CH3 CH, (LXXXIX) (XC) (xci) The albomycins each contain one molecule of sulphur and alkaline hydrolyses were reported to give nearly quantitative yields of sulphate' 6. Similarly, a fragment 112 (LXXXV), derived from partial acid hydrolysis of albomycin, was reported to yield sulphate upon alkaline hydrolysis106. No desulphurized reaction product could be isolated after treatment of albomycm with Raney nickel and, in an audacious proposal, the sulphur atom was thus assigned to a sulphone bridge connecting the pyrimidine moiety with a hydroxyl group of serine, giving rise to a sulpharnate. The structures of albomycins 2' and c proposed by Sorm and co-workers are depicted by formulae LXXXII LXXXIV' 8" 6 Although among the earliest antibiotics to be discovered 114 very little regarding the chemistry of grisein has been published1 15 The antibiotic is produced by Streptornyces griseus and was never prepared in pure form. Nevertheless, a comparison of albomycin and grisein in terms of biological properties and partition chromatographic behaviour revealed their great similarity and possible identity'16' 117, These concltisions were supported by later studies73' 118, but, due to the lack of pure grisein, rigorous proof of identity with albomycin could never be established. A similar dilemma prevails for alveomycin"9, antibiotics A , LA 5352 and LA , and 1OO73 which are similar to and perhaps identical with, albomycin. 628
27 NATURALLY OCCURRING HYDROXAMIC ACIDS AND HYDROXAMATES Recently, we have isolated an antibiotic complex from the culture fluid of Streptoinyces griseus var. X-2455 which closely resembled albomycin122. Hydriodic acid hydrolysis of this antibiotic complex, however, yielded serine and ornithine in a molar ratio of 1:3 and it was therefore concluded that this complex was different from albomycin. Ion exchange and partition chromatography resolved the complex into three antibiotics, designated Ro , Ro and Ro The u.v.-spectra of these three antibiotics were qualitatively identical with those of albomycins 2. and 106, although a quantitative comparison was not possible since absorbancies for albomycins were never reported. Hydriodic acid hydrolysis of antibiotics Ro , Ro and Ro confirmed the molar ratio of serine to ornithine as 1:3 in each and performic acid oxidations, followed by hydrochloric acid hydrolyses, indicated molar ratios of serine to glutamic acid of 1:3123. An in vitro comparison of Ro , Ro and Ro with albomycin complex further confirmed similarity122. Identity with albomycin was again suspected when it was observed that Ro could act as antibiotic, at 35, against a Serratia sp. but antagonized the antibiotic activity of compounds Ro and Ro at 22. With Bacillus subtilis as test organism this antagonism was clearly observed at A similar peculiarity of albomycin c had previously been noted' 2. Upon thin layer chromatographic comparison in several solvent systems albomycin 2 and Ro , as well as albomycin ö, and Ro , could not be differentiated123 To re-investigate published hydrolysis data, the albomycin complexi was separated in our laboratory into its components 2' and 8 and hydriodic acid hydrolyses of albomycins, and ö2 indeed revealed molar ratios of serine to ornithine of 1:3 in both preparations. Similarly, both albomycins yielded, after Fe(iii) removal, performic acid oxidations and hydrochloric acid hydrolyses, serine and glutamic acid in molar ratios of 1:3. Identical n.m.r. spectra of iron-free preparations of the major antibiotics, albomycin 2 and Ro , established definite proof of identity'23. Iron-free antibiotic Ro yielded N'-hydroxy-L-ornithine and L- serine upon hydrochloric acid hydrolysis and catalytic hydrogenation of the hydrolysate afforded serine and ornithine in a molar ratio of 1:3. Periodate oxidation and hydriodic acid hydrolysis gave acetic acid and ornithine, respectively, in equimolar amounts. Similarly, iron and sulphur were present in equimolar amounts and the molar ratio of acetic acid to iron was 3: 1. The iron-binding centre is thus composed of three N5-acetyl-N5-hydroxyornithine moieties. The assignment of a trihydroxamate structure was compatible with the corresponding acid stability of the Fe(iii) trihydroxamate complex, whereas n.m.r. spectra confirmed the presence of three N5-acetyl-N5-hydroxyornithine moieties and only one serine residue. The presence of an additional acetyl group was ruled out by n.m.r. spectroscopy, eliminating the possibility of an N4-acetylcytosine moiety, as proposed for albomycin 2 by Sorm and co-workers'06 A re-investigation of the u.v. t Albomycin samples were obtained from the Soviet Union through the courtesy of Dr R. Morf and from the laboratories of Prof. orrn. 629
28 HUBERT MAEHR spectra of compound XCI which, in part, were responsible for orm's proposed structure LXXXII and a consideration of dissociation data confirmed the disagreement between LXXXII and XC Substituted pyrimidines, proposed by Poddubnaya'13, are ruled out on the basis of clearly discernible AB patterns in the n.m.r. spectra of Ro ,albomycin 2' Ro and Ro , due to olefinic protons 5 and 6 of the pyrimi moieties. The assignment of the sulphur atom proposed by Sorm and collaborators106 (LXXXII LXXXV) appears to be incorrect in two respects. The recovery of sulphate from albomycin after alkaline hydrolysis could not be duplicated with antibiotic Ro In contrast, pyrolysis of antibiotic Ro at 2500 liberated hydrogen sulphide, as did treatment with zinc and dilute hydrochloric acid. Secondly, the sulphur moiety cannot be attached to the pyrimidine part as depicted by orm and co-workers106 (LXXXII LXXXV) in view of CD spectra of iron-free compounds Ro , Ro and Ro , which suggested the attachement of the pyrimidine moieties to asymmetric carbon atoms25. The many discrepancies observed between albomycin 2 and antibiotic Ro indicate the necessity for a critical reappraisal of the published structures for albomycins. It was observed previously that similar iron-binding sites appertain to both ferrimycin and ferrimycin-antagonists, the ferrioxamines. Similarly, ferrimycin decomposes under mild conditions creating ferrimycin-antagonists, most probably with unchanged iron-binding centres. It appears, therefore, that effective sideromycin antagonism requires close chemical similarity of the iron-binding sites between both sideromycin and sideramine. In further agreement, ferrichrome, ferrichrysin and ferricrocin, distinguished by N5-acetyl-N5-hydroxyornithine moieties, resemble albomycin closely and antagonize the antibiotic activity of albomycin against Gram positive and Gram negative organisms more effectively than the more distant relatives LXXVII, LXXX and LXXXI. The iron-binding centres of ferrimycins and ferrichrome-type sideramines are quite different and, as a result, antagonism prevails only with Staph. aureus. Similar differences among the iron-binding centres exist between albomycin, with ferrichrome-iike trihydroxamate arrangement, and ferrioxamines, hence, limited antagonism between albomycm and ferrioxamines is observed. Albomycin 8 and siderochrome Ro can act as antibiotics or albomycin antagonists. Clearly, this special behaviour is explained by a similarity between the sideramine and sideromycin component which is not limited to the iron-binding site but embraces the majority of the molecules Hydroxarnic acid functions connected head to head via ainide bonds and head to tail via ester bond Coprogen. This compound is a metabolite of various fungi and acts as a growth factor for Pilobolus species. Structure elucidation9 " was achieved nearly two decades after the discovery of this sideramine9sa. Coprogen is a neutral compound and contains L-N5-hydroxyornithine (XII) as the only amino acid and hydroxylamino moiety. A ferrirubin-type trihydroxamate grouping was suspected because of spectroscopic similarity with compounds LXXVII, LXXX and LXXXI and the liberation of 5-630
29 NATURALLY OCCURRING HYDROXAMIC ACIDS AND HYDROXAMATES hydroxy-3-methylpent-2-enoic acid2sb upon periodic acid oxidation of deferricoprogen. Acetic acid was obtained upon acid hydrolysis of coprogen and n.m.r. spectra suggested its origin in an N-acetyl function. Three trans-fusarinine units and one acetic acid molecule were indicated as the only components of coprogen based on elemental analysis and n.m.r. Coprogen (XCII) spectra. The ester bond (1730 cm 1) was ammonolized resulting in the addition of one mole of ammonia; removal of iron from the ammonolysis product gave dimeric acid, XLIII. Two free hydroxyl groupings were suggested by acetylation studies leading to structure XCII for coprogen. Coprogen B is desacetyl coprogen and is produced by a variety of fungi60. The antibiotic activity of ferrimycin A and albomycin against Staph. aureus is antagonized to the same extent by coprogen, coprogen B, fusigen (XCIII) and dimeric acid60. Possibly related to coprogen and ferrichromes121' is Terregens Factor, a bacterial growth factor produced by Arthrobacter pascens Compounds with three hydroxamic acid functions in a cyclic skeleton Hydroxarnic acid functions connected head to tail via ester bonds Fusigen. A close relative of fusarinine B20, fusigen was isolated from culture fluids of fungi21. As a trimer of cis-fusarinine' Oa structure XCIII was assigned to fusigen based on physico-chemical data and degradation products. Fusigen exhibits growth-factor activity and is probably identical with fusarinine C20. The terms fusigen B and fusarinine B are synonymous as are fusigen C and fusarinine A. 631
30 HUBERT MAEHR o OCHN NH2 N' -- Fe(iiij. Fusigen (XCIII) Hydroxarnic acid functions connected head to tail via ainide bonds Nocardainine (Ferrioxainine E). This compound was originally described'24 as a narrow-spectrum antibiotic with activity against mycobacteria. Hydrolysis with hydrochloric acid yielded succinic acid and a hitherto unknown compound'25. Some ten years later8' 1-amino-5 -hydroxylaminopentane (LX VII) was isolated as a hydrolysis product of ferrioxamines and ferrimycins and it was not until then that the structure of the new nocardamine-derived hydrolysis product could be correctly identified as LXVII82. Nocardamine (Ferrioxamine E) (XCIV) The representation of nocardamine as a cyclic, eleven-membered monohydroxamic acid involving one molecule each of succinic acid and 1-amino- 5-hydroxylaminopentane82 was untenable on the basis of polarographic data'26 which favoured the trihydroxamate structure XCIV. This assignment 632
31 NATURALLY OCCURRING HYDROXAMIC ACIDS AND HYDROXAMATES was supported by molecular weight determinations'26 and later by the availability of stability constants of various Fe(m) complexes. Whereas ferrioxamine B is 200 times more stable than the tris-aceth.ydroxamatocomplex, nocardamine is 1000 times more stable9. The relative difference in chelate effects of ferrioxamine B and nocardamine is explained by linearity and cyclicity, respectively, of the molecules, giving rise to greatly different entropy changes upon complex formation. Ferrioxainine D2. Ferrioxamines B and A1 are in the same homologous relationship as are nocardamine and ferrioxamine D2. Similar to previous examples, structure XCV was deduced for ferrioxamine D2 by titration data, hydrochloric acid hydrolysis and n.m.r. studies89. o NH / Ferrioxamine D2 (XCV) 4. UNCLASSIFIED COMPOUNDS CONTAINING HYDROXAMATE FUNCTIONS Succinimycin, produced by Streptornyces oiivochrorno genes, is active against certain Gram positive bacteria and shows cross-resistance with grisein. Ammonia, methylamine, succinic acid and LXVIII are among the products of hydrolysis with dilute alkali'28. Similarly, danomycin, a metabolite of Streptoinyces albadancus, is active preferentially against Gram positive bacteria and, upon acid hydrolysis, yields acetic acid, succinic acid, LXVIII and 17 amino acids'29. Like succinimycin, danomycin is crossresistant with grisein. Little is known about the toxic gluconimycin which is elaborated by Streptoinyces AS9 and shows activity against Gram positive and Gram negative bacteria and fungi' Antibiotic ASK-753, derived from Streptoinyces AS-K-753, is active mainly against Gram positive bacteria and to a lesser extent against Gram negative organisms and non-filamentous fungi. The antibacterial activity is not antagonized by ferrioxamine B131. Antibiotics and 2293 i have received little attention. Hydroxamic acid functions are suspected in conalbumin of egg white, in siderophylin, the iron-binding 3-pseudoglobulin of human plasma
32 HUBERT MAEHR and in ferritin', a brown pigment occurring in spleen, bone marrow and liver. Schizokinen, an iron transport factor produced by Pxicillus megatheriurn Texas, is responsible for the initiation and maintenance of exponential cell division of certain microorganisms' 34aSchizokinen antagonized ferrimycin- A activity against three Bacillus species; it was isolated as an Fe(uI) complex tion. A schematic model of the conjugal transfer of R factors is depicted in REFERENCES 1 V. Prelog. Pure App!. Chem. 6, 327 (1963). 2 w Keller-Schierlein, V. Prelog and H. Zahner. Fortschr. Chem. Org. Naturstoffe 22, 279 (1964). 0. Mikei and J. Turková. Chem. Listy 58, 65 (1964). J. B. Neilands. Structure and Bonding Springer: New York 1, 59 (1966). J. B. Neilands. Science 156, 1443 (1967). 6 B. Bapat, D. St. C. Black and R. F. C. Brown. Advan. Heterocyc!!c Chem. 10, 199 (1969). G. A. Snow. Bacterio!. Rev. 34, 99 (1970). G. Anderegg, F. I'Eplattenier and G. Schwarzenbach. Helv. Chim. Acta 46, 1400 (1963). G. Anderegg, F. I'Eplattenier and G. Schwarzenbach. He/v. Chim. Acta 46, 1409 (1963). 10(a) T. F. Emery. Biochemistry 4, 1410 (1965). H. Diekmann. Arch. Mikrobiol. In press. 0. Schwarzenbach and K. Schwarzenbach. He/v. Chim. Acta 46, 1390 (1963). (b) 12(a) J. B. Neilands. Bacterio!. Rev. 21, 101 (1957). (10 B. F. Burnham and J. B. Neilands. J. B/al. Cliem. 236, 554 (1961). 13 H. Zahner. Natur w. Rundschau 17, 391 (1964). 14 ' F. KnUsel, J. NUesch and H. J. Treichier. Naturwiss. 54, 242 (1967). W. D. Ollis, A. J. East, J. J. Gordon and I. 0. Sutherland. In Chemistry of Microbia! Products p. 204 (1964), Institute of AppI. Microbiology, Univ. Tokyo. 16 E. A. Kacaka, C. 0. Gitterman, E. L. Dulaney and K. Folkers. Biochemistry 1, 340 (1962). E. F. Schoenewaldt, R. B. Kinnel and P. Davis. J. Org. Chem. 33, 4270 (1968). 'E. Buehler. J. Org. Chein. 32, 261 (1967). 19(a) E. Buehler and G. B. Brown. J. Org. Chein (1967). (b1 E. Falco and 0. B. Brown. J. Med. Chem. 11, 142 (1968). 20 M. Sayer and T. F. Emery. Biochemistry 7, 184 (1968). 21 H. Dieckmann. Angew. Chem. 7, 551 (1968). 22 w Keller-Schierlein. He/v. Chim. Acta 46, 1920 (1963). 23 T. F. Emery and 3. B. Neilands..1. Am. Chem. Soc. 83, 1626 (1961) Rogers and 3. B. Neilands. Biochemistry 2, 6 (1963). 25 H. Maehr. Unpublished results. 26 T. F. Emery. Biochemistry 5, 3694 (1966). 27(a) T. F. Emery and J. B. Neilands. J. Am. Chem. Soc. 82, 4903 (1960). (10 1. F. Emery and J. B. Neilands. J. Org. Chem. 27, 1075 (1962). 28(a) w. Keller-Schierlein, H. Zahner, V. PUnter-Streit and H. Bar. Biochemn. Z. 341, 378 (1965). hi H. Diekmann. Arch. Mikrobio!. 62, 322 (1968). 29 F. C. White and 3. H. Hill. J. Bacterial. 45, 433 (1943). 30 M. 0. Burton, F. J. Sowden and A. 0. Lochhead. Can. J. Biochem. Physiol. 32, 400 (1954). 31 A. L. Demain and D. Hendlin. J. Gen. Microbial. 21, 72 (1959) A. Glister. Nature 148, 470 (1941). A. F. 0. Menzel, 0. Wintersteiner and 0. Rake. J. BacteriaL 46, 109 (1943). J. D. Dutcher and 0. Wintersteiner. J. Biol. Chem. 155, 359 (1944). " 3. D. Dutcher. J. Biol. Chem. 171, 321 and 341 (1947) Dunn, 3. J. Gallagher, 0. T. Newbold and F. S. Spring. J. Chem. Soc. S 126 (1949). 0. T. Newbold and F. S. Spring. J. Chem. Soc (1947). 38 G. Dunn, G. T. Newbold and F. S. Spring. J. Chem. Soc. S 131 (1949). 0. T. Newbold, W. Sharp and F. S. Spring. J. Chem. Soc (1951). 634
33 NATURALLY OCCURRING HYDROXAMIC ACIDS AND HYDROXAMATES 40(a) M. Masaki, Y. Chigira and M. Ohta. J. Org. Chetn. 31, 4143 (1966). )b) A. Ohta. Chein. Phann. Bull. (Tokyo) 16, 1160 (1968). 41 A. Csillag..4cta Microbiol. Acad. Sci. Hung. 1, 321 (1954); Cheni. Abstr. 49, 13356c (1955). 42 LI. C. MacDonald, R. G. Micetich and R. H. Haskins. Can. J. Microbiol. 10, 90 (1964). R. G. Micetich and LI. C. MacDonald. J. Chetn. Soc (1964). G. Dunn, G. T. Newbold and F. S. Spring. J. Chem. Soc (1949). LI. D. Dutcher. J. Biol. Chem. 232, 785 (1958). 46 u Weiss, F. Strelitz, H. Flon and I. N. Asheshov. Arch. Biochein. Biophys. 74, 150 (1958). S. Nakamura. BulL Agr. Chem. Soc. (Japan) 24, 629 (1960). 48 Nakamura. Agr. Biol. Chem. (Tokyo) 25, 74 (1961). S. Nakamura. Agr. Biol. Chein. (Tokyo) 25, 658 (1961). M. Sugiyama, M. Masaki and M. Otha. Tetrahedron Letters 845 (1967). 51(a) O. Wahiroos and A. I. Virtanen. Acta Chem. Scand. 13, 1906 (1959). 51(b) LI. Hoffman and 0. Hoffmanova. Fur. J. Biochem. 8, 109 (1969). 52 A. 1. Virtanen and 0. Wahlroos. J. Pharm. Sd. 52, 713 (1963). LI. B. Bredenberg, E. Honkanen and A. I. Virtanen. Acta Chem. Scand. 16, 135 (1962). A. I. Virtanen and P. K. Hietala. Acta Chem. Scand. 14, 499 (1960). P. K. Hietala and A. I. Virtanen. Acta Chein. Scand. 14, 502 (1960). 56 E. Honkanen and A. 1. Virtanen. Acta Chem. Scand. 14, 504 (1960). C. L. Atkin and J. B. Neilands. Biochemistry 7, 3734 (1968). 58 C. L. Atkin, J. B. Neilands and H. J. Phaff. J. Bacteriol 103, 722 (1970). R. M. Gipson, F. H. Pettit, C. G. Skinner and W. Shive. J. Org. Chem. 28, 1425 (1963). 60 H. Diekmann. Arch. Mikrobiol. 73, 65 (1970). 61 F. Gibson and D. I. Magrath. Biochim. Biophys. Acta 192, 175 (1969). 62 G. A. Snow. J. Chem. Soc (1954) B. Maurer, A. MUller, W. Keller-Schierlein and H. Zahner. Arch. Mikrobiol. 60, 326 (1968). A. J. Kluyver. J. P. van der Walt and A. LI. van Triet. Proc. NatL Acad. Sci. US 39, 583 (1953). 65 A. H. Cook and C. A. Slater. J. Inst. Brewing 60, 213 (1954). 66 A. H. Cook and C. A. Slater. J. Cheni. Soc and 4133 (1956). 67 J. C. MacDonald. Can. J. Chem. 41, 165 (1963) A. Ohta. Chem. Pharin. BulL (Tokyo) 12, 125 (1964). A. E. Oxford and H. Raistrick. Biochein. J. 42, 323 (1948). 70 A. J. Birch, R. A. Massy-Westropp and R. W. Rickards. J. Chem. Soc (1956). 71 R. B. Bates, J. H. Schamble and M. Souèek. Tetrahedron Letters 1683 (1963). 72 C. Gallina, A. Romeo, V. Tortôrella and G. D'Angelo. Chein. md. (London) 1300 (1966). H. Bickel, E. Gäumann, W. Keller-Schierlein, V. Prelog, E. Vischer, A. Wettstein and H. Zhhner. Experientia 16, 129 (1960). H. Zahner, E. Bachmann, R. Hutter and J. Niiesch. PathoL Microbiol. 25, 708 (1962). H. Zahner, W. Keller-Schierlein, R. HUtter, K. Hess-Leisinger and A. Deer. Arch. Mikrobiol. 45, 119 (1963). 76 H. Zähner. Angew. Chem. 6, 469 (1967). H. Zahner, R. HUtter and E. Bachmann. Arch. Mikrobiol. 36, 325 (1960). 78 H. Bickel, E. Gaumann, G. Nussberger, P. Reusser, E. Vischer, W. Voser, A. Wettstein and H. Zahner. Help. Chim. Acta 43, 2105 (1960). H. Bickel, R. Bosshardt, E. Gãumann, P. Reusser, E. Vischer, W. Voser, A. Wettstein and H. Zahner. Help. Chim. Acta 43, 2118 (1960). 80 A. Muller and H. Zahner. Arch. Mikrobiol. 62, 257 (1968). 81 H. Bickel, B. Fechtig, G. E. Hall, W. Keller-Schierlein, V. Prelog and F. Vischer. Help. Chim. Acta 43, 901 (1960). 82 R. F. C. Brown, G. Buchi, W. Keller-Schierlein, V. Prelog and LI. Renz. Help. Chim. Acta 43, 1868 (1960). 83 H. Bickel, G. F. Hall, W. Keller-Schierlein, V. Prelog and F. Vischer. Help. Chins. Acta 43, 2129 (1960). 84 H. Bickel, H. Keberle and F. Vischer. Help. Chins. Acta 46, 1385 (1963). 85 V. Prelog and A. Walser. Help. Chins. Acta4S, 631 (1962). 86 W. Keller-Schierlein and V. Prelog. Help. Chins. Acta 44, 709 (1961). 87 W. Keller-Schierlein and V. Prelog. Help. Chins. Acta 45, 590 (1962). 88 V. Prelog and A. Walser. Help. Chins. Acta 45, 1732 (1962). 89 W. Keller-Schierlein, P. Mertens, V. Prelog and A. Walser. Help. Chins. Acta 48, 710(1965). H. Bickel, P. Mertens, V. Prelog, LI. Seibl and A. Walser. Tetrahedron 22, Suppl. 8, 171 (1966). 635
34 HUBERT MAEHR ' T. F. Emery and J. B. Neilands. J. Am. Chem. Soc. 82, 3658 (1960). 92 5, j, Rogers, R. A. J. Warren and J. B. Neilands. Nature 200, 167 (1963). W. Keller-Schierlein and B. Maurer. He/v. Chim. Acta 52, 603 (1969). A. Zalkin, J. D. Forrester and D. H. Templeton. J. Am. Chem. Soc. 88, 1810 (1966). 95(a) c, Pidacks, A. R. Whitehill, L. M. Pruess, C. W. Hesseltine, B. L. Hutchings, N. Bohonos and J. H. Williams. J. Am. Chem. Soc. 75, 6064 (1953). (b( W. Keller-Schierlein and H. Diekmann. He/v. Chim. Acta 53, 2035 (1970). W. Keller-Schierlein and A. Deer. He/v. Chim. Acta 46, 1907 (1963). 'M. Tadenuma and S. Sato. Agr. Biol. Chem. (Tokyo) 31, 1482 (1967). 98 G. F. Gauze and M. G. BraThikova. Novosti Med. Akad. Med. Nauk SSSR 23, 1, 3 (1951). M. G. Branikova, 0. Mike and N. N. Lomakina. Biochemistry USSR 22, 104 (1957). ' J. Turková, 0. Mike and F. orm. Antibiotiki 7, 878 (1962). to' G. I. Lavrenova, L. P. Revina and N. A. Poddubnaya. J. Gen. Chem. USSR 36, 2088 (1966). 102 o Mikel, J. Turková and F. Sorm. Co//ection Czech. Chem. Cominun. 28, 1747 (1963). 103 A. RiCicová. Co//ection Czech. Chem. Commun. 28, 1761 (1963). 104 N. A. Poddubnaya and F. P. Krysin. J. Gen. Chem. USSR 32, 990 (1962). 105 F. P. Krysin and N. A. Poddubnaya. J. Gen. Chem. USSR 33, 1339 (1963). 106 j Turkovk, 0. Mike and F. orm. Co//ection Czech. Chem. Coininun. 30, 118 (1965). 107 o MikS, J. Turkovk and F. Sorm. Co/lection Czech. Chem. Commun. 28, 1747 (1963). 108 j Turková, 0. Mike and F. orm. Experientia 19, 633 (1963). 109 J Turková, 0. MikS and F. orm. Co//ection Czech. Chem. Commun. 29, 280(1964). 110 j Turková, 0. Mikel and F. Sorm. Proc. Congr. Antibiotics, Prague, June 1964, p J. Turková, 0. Mike and F. gorm. Co/lection Czech. Chem. Commun. 27, 591 (1962). 112 G. I. Lavrenova, L. P. Revina and N. A. Poddubnaya..1. Gen. Chem. USSR 36, 2091 (1966). 113 G. I. Lavrenova and N. A. Poddubnaya, J. Gen. Chem. USSR 38, 63 (1968). " D. M. Reynolds, A. Schatz and S. A. Waksman. Proc. Soc. Expt/. BioL Med. 64, 50(1947). 115 F. A. Kuehl, Jr., M. N. Bishop, L. Chaiet and K. Folkers. J. Am. Chem. Soc. 73, 1770 (1951). 116 S. A. Waksman. Science 125, 585 (1957). 117 F. 0. Stapley and R. F. Ormond. Science 125, 587 (1957). 118 j Turková, 0. Mikei and F. orm. Co//ection Czech. Chem. Commun. 31, 2444 (1966). 119 G. Schmidt-Kastner and J. Schmid. Med. und Chem., Abhandl. Med-C hem. Forschungsstaetten Farbenfabriken Bayer AG, VII, 528 (1963), Verlag Chemie: Weinheim/Bergstrasse. 120 H. Thrum. Naturuiss. 44, 561 (1957). 121 P. Sensi and M. T. Timbal. Antibiot. Chemotherapy 9, 160 (1959). 122 H. Maehr and J. Berger. Biotechnol. Bioeng. 11, 1111 (1969). 123 H. Maehr. J. Antibiotics, In press. 124 A. Stoll, A. Brack and J. Renz. Schweiz. Z. A//gem. Patho/. Bacterio/. 14, 225 (1951). 125 A. Stoll, J. Renz and A. Brack. He/v. Chi:n. Acta 34, 862 (1951). 126 W. Keller-Schierlein and V. Prelog. He/v. Chim. Acta 44, 1981 (1961). 12 7(a) M. 0. Burton, F. J. Sowden and A. G. Lochhead. Can..1. Biochem. 32, 400 (1954). (b) A. G. Lochhead and M. 0. Burton. Soil Sci. 82, 237 (1956). 128 T. H. Haskell, R. H. Bunge, J. C. French and Q. R.Bartz. J. Antibiotics(Tokyo) 16A, 67(1963). 129(a) H. Tsukiura, M. Okanishi, T. Ohmori, H. Koshiyama, T. Miyaki, H. Kitazima and H. Kawaguchi..1. Antibiotics (Tokyo) 17A, 39 (1964). (b( H. Ogawara, K. Maeda and H. Umezawa. J. Antibiotics (Tokyo) 19A, 190 (1966). 130 I. R. Shimi and A. Dewedar. Arch. Mikrobio/. 54, 246 (1966). 131 I. R. Shimi, G. M. Imam and B. M. Haroun. J. Antibiotics (Tokyo) 22, 106 (1969) Fiala and D. Burk. Arch. Biochem (1949). 133 H. N. Munro and J. W. Drysdale. Federation Proc. 29, 1469 (1970). 1 34)a( J. L. Arceneaux and C. F. Lankford. Biochem. Biophys. Res. Commun. 24, 370 (1966). (b( B. R. Byers, M. V. Powell and C. F. Lankford. J. Bacteriot 93, 286 (1967). 636
Calisthenics for Adults
 017 American Sokol Slet & Festival Adult Calisthenics Page 1 of 1 Calisthenics for Adults Composed by Bro. Howie Maskill and Sis. Alex Zahrobsky, Sokol Spirit Terminology by Sis. Patricia Satek Music:
017 American Sokol Slet & Festival Adult Calisthenics Page 1 of 1 Calisthenics for Adults Composed by Bro. Howie Maskill and Sis. Alex Zahrobsky, Sokol Spirit Terminology by Sis. Patricia Satek Music:
III 1 Left sidestride moderate bend reararm downslant inward, left hand grasping right wrist; 2-4 Hold.
 THE CHILDHOOD YEARS ENERGY, GROWTH & DISCOVERY A composition of Combined Calisthenics for Sokolettes and Sokolads Composed by Sister Ellen Kovac Sokol USA Lodge 12 Music: Arthur Fiedler and the Boston
THE CHILDHOOD YEARS ENERGY, GROWTH & DISCOVERY A composition of Combined Calisthenics for Sokolettes and Sokolads Composed by Sister Ellen Kovac Sokol USA Lodge 12 Music: Arthur Fiedler and the Boston
Menopause. from an Ayurvedic perspective
 Menopause from an Ayurvedic perspective DevaKhalsa May2012 Table of Contents Introduction. 2 Whatismenopause?... 3 HotFlashes 5 TheDoshas 8 Dhatus.. 10 Herbs. 15 PathologyofMenopause 18 HormoneReplacementTherapy
Menopause from an Ayurvedic perspective DevaKhalsa May2012 Table of Contents Introduction. 2 Whatismenopause?... 3 HotFlashes 5 TheDoshas 8 Dhatus.. 10 Herbs. 15 PathologyofMenopause 18 HormoneReplacementTherapy
Chapter 18. Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
Lecture 11 AMINO ACIDS AND PROTEINS
 Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
The Effect of County-level Smoke-free Air (SFA) Policies. on Smoking and Drinking
 The Effect of County-level Smoke-free Air (SFA) Policies on Smoking and Drinking BY Yawen Liu B.S., Jilin University, China, 2008 M.A., University of Illinois at Chicago, Chicago, 2010 THESIS Submitted
The Effect of County-level Smoke-free Air (SFA) Policies on Smoking and Drinking BY Yawen Liu B.S., Jilin University, China, 2008 M.A., University of Illinois at Chicago, Chicago, 2010 THESIS Submitted
Reactions and amino acids structure & properties
 Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Prelab 6: Carboxylic Acids
 The Structure of Carboxylic Acids Prelab 6: Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group,
The Structure of Carboxylic Acids Prelab 6: Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group,
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1)
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 7. CARBOXYLIC ACIDS AND THEIR
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 7. CARBOXYLIC ACIDS AND THEIR
H 2 C H 2 N C CH O N C CH 3 CH 2 H O. aspartame
 1 The addition of sucrose, table sugar, to food and drink has been linked to the increased risk of obesity and insulin resistance. Aspartame is used as an alternative to sugar. The structure of aspartame
1 The addition of sucrose, table sugar, to food and drink has been linked to the increased risk of obesity and insulin resistance. Aspartame is used as an alternative to sugar. The structure of aspartame
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay
 Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
Biomolecules: amino acids
 Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS
 AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
Lakeland Health Simulation Training Center
 Lakeland Health Simulation Training Center www.lakelandhealth.org The Lakeland Health Simulation Training Center currently offers the following simulations for resident physicians, medical students, nurses,
Lakeland Health Simulation Training Center www.lakelandhealth.org The Lakeland Health Simulation Training Center currently offers the following simulations for resident physicians, medical students, nurses,
Chemical Nature of the Amino Acids. Table of a-amino Acids Found in Proteins
 Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
1/3/2011. Chapter 17 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon
 Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl compounds or carboxylic acid derivatives Chapter 17 Carboxylic Acids and Their Derivatives. Nucleophilic
Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl compounds or carboxylic acid derivatives Chapter 17 Carboxylic Acids and Their Derivatives. Nucleophilic
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl
Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids.
 Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Amino acids. (Foundation Block) Dr. Essa Sabi
 Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
REACTIONS OF CARBOXYLIC ACID DERIVATIVES WITH NUCLEOPHILES A. Reactions of Acid Chlorides with Nucleophiles
 1016 CHAPTER 1 THE CHEMITRY F CARBXYLIC ACID DERIVATIVE 1.8 REACTI F CARBXYLIC ACID DERIVATIVE WITH UCLEPHILE ection 1.7 showed that all carboxylic acid derivatives hydrolyze to carboxylic acids. Water
1016 CHAPTER 1 THE CHEMITRY F CARBXYLIC ACID DERIVATIVE 1.8 REACTI F CARBXYLIC ACID DERIVATIVE WITH UCLEPHILE ection 1.7 showed that all carboxylic acid derivatives hydrolyze to carboxylic acids. Water
I) Choose the best answer: 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine.
 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
Physiology, Owens College *.
 THE ACTION OF CALCIUM, STRONTIUM AND BARIUM SALTS IN PREVENTING COAGULATION OF THE BLOOD. BY R. M. HORNE, M.D., Senior Denonstrator of Physiology, Owens College *. (From the Physiological Laboratory, Owens
THE ACTION OF CALCIUM, STRONTIUM AND BARIUM SALTS IN PREVENTING COAGULATION OF THE BLOOD. BY R. M. HORNE, M.D., Senior Denonstrator of Physiology, Owens College *. (From the Physiological Laboratory, Owens
From Atoms to Cells: Fundamental Building Blocks. Models of atoms. A chemical connection
 From Atoms to Cells: A chemical connection Fundamental Building Blocks Matter - all materials that occupy space & have mass Matter is composed of atoms Atom simplest form of matter not divisible into simpler
From Atoms to Cells: A chemical connection Fundamental Building Blocks Matter - all materials that occupy space & have mass Matter is composed of atoms Atom simplest form of matter not divisible into simpler
For more info visit
 Carbohydrates Classification of carbohydrates: Monosaccharides: Polyhydroxy aldehydes or polyhydroxy ketones which cannot be decomposed by hydrolysis to give simpler carbohydrates.examples: Glucose, Fructose,
Carbohydrates Classification of carbohydrates: Monosaccharides: Polyhydroxy aldehydes or polyhydroxy ketones which cannot be decomposed by hydrolysis to give simpler carbohydrates.examples: Glucose, Fructose,
For more important question's visit :
 For more important question's visit : www.4ono.com Unit - 14 BIOMOLECULES POINTS TO REMEMBER 1. Carbohydrates are optically active polyhydroxy aldehydes or ketones or molecules which provide such units
For more important question's visit : www.4ono.com Unit - 14 BIOMOLECULES POINTS TO REMEMBER 1. Carbohydrates are optically active polyhydroxy aldehydes or ketones or molecules which provide such units
1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids
 Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Carboxylic Acids. The Importance of Carboxylic Acids (RCO 2 H)
 Carboxylic Acids The Importance of Carboxylic Acids (RCO 2 H) Starting materials for acyl derivatives (esters, amides, and acid chlorides) Abundant in nature from oxidation of aldehydes and alcohols in
Carboxylic Acids The Importance of Carboxylic Acids (RCO 2 H) Starting materials for acyl derivatives (esters, amides, and acid chlorides) Abundant in nature from oxidation of aldehydes and alcohols in
Gentilucci, Amino Acids, Peptides, and Proteins. Peptides and proteins are polymers of amino acids linked together by amide bonds CH 3
 Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
ORGANIC SYNTHESIS VIA ENOLATES
 1 ORGANIC SYNTHESIS VIA ENOLATES Aldehydes and ketones undergo nucleophilic addition reaction at the carbonyl group. Further, α-hydrogen containing compounds are acidic in nature. In addition to carbonyl
1 ORGANIC SYNTHESIS VIA ENOLATES Aldehydes and ketones undergo nucleophilic addition reaction at the carbonyl group. Further, α-hydrogen containing compounds are acidic in nature. In addition to carbonyl
Young Homeless Mothers & Homeless Children Literature Review (Citations at Appendix 1)
 Young Homeless Mothers & Homeless Children Literature Review (Citations at Appendix 1) Dr. Austin O Carroll of Safetynet with whom Anew have consulted with over the past year undertook this Literature
Young Homeless Mothers & Homeless Children Literature Review (Citations at Appendix 1) Dr. Austin O Carroll of Safetynet with whom Anew have consulted with over the past year undertook this Literature
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
COO - l. H 3 N C a H l R 1
 COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
POSITION PAPER ON COVERAGE FOR NRTS AND TOBACCO CESSATION MEDICATIONS
 POSITION PAPER ON COVERAGE FOR NRTS AND TOBACCO CESSATION MEDICATIONS Prepared by the NEWFOUNDLAND AND LABRADOR MEDICAL ASSOCIATION November 2012 Introduction The Newfoundland and Labrador Medical Association
POSITION PAPER ON COVERAGE FOR NRTS AND TOBACCO CESSATION MEDICATIONS Prepared by the NEWFOUNDLAND AND LABRADOR MEDICAL ASSOCIATION November 2012 Introduction The Newfoundland and Labrador Medical Association
9/6/2011. Amino Acids. C α. Nonpolar, aliphatic R groups
 Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Metabolic Classification of the Amino Acids
 Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
10. CARBOXYLIC ACIDS AND THEIR DERIVATIVES 10.1 Nomenclature of Carboxylic Acids 10.2 Physical Properties of Carboxylic Acids 10.
 BOOKS 1) Organic Chemistry Structure and Function, K. Peter C. Vollhardt, Neil Schore, 6th Edition 2) Organic Chemistry, T. W. Graham Solomons, Craig B. Fryhle 3) Organic Chemistry: A Short Course, H.
BOOKS 1) Organic Chemistry Structure and Function, K. Peter C. Vollhardt, Neil Schore, 6th Edition 2) Organic Chemistry, T. W. Graham Solomons, Craig B. Fryhle 3) Organic Chemistry: A Short Course, H.
O H 2 N. α H. Chapter 4 - Amino Acids
 hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
BCH302 [Practical] 1
![BCH302 [Practical] 1 BCH302 [Practical] 1](/thumbs/78/76808179.jpg) BCH302 [Practical] 1 Amino acids play a central role: i. As building blocks of proteins. ii. As intermediates in metabolism, converted to specialized products. There are 20 natural amino acids that are
BCH302 [Practical] 1 Amino acids play a central role: i. As building blocks of proteins. ii. As intermediates in metabolism, converted to specialized products. There are 20 natural amino acids that are
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
AMINO ACIDS. Qualitative Tests
 AMINO ACIDS Qualitative Tests AMINO ACIDS Amino acid play A central role as building block of proteins. Amino acids also converted to specialized products. More than 300 different amino acids have been
AMINO ACIDS Qualitative Tests AMINO ACIDS Amino acid play A central role as building block of proteins. Amino acids also converted to specialized products. More than 300 different amino acids have been
Chapter 22 Carbohydrates
 Chapter 22 Carbohydrates Introduction Classification of Carbohydrates Carbohydrates have the general formula C x (H 2 O) y Carbohydrates are defined as polyhydroxy aldehydes or ketones or substances that
Chapter 22 Carbohydrates Introduction Classification of Carbohydrates Carbohydrates have the general formula C x (H 2 O) y Carbohydrates are defined as polyhydroxy aldehydes or ketones or substances that
EXPERIMENT 8 (Organic Chemistry II) Carboxylic Acids Reactions and Derivatives
 EXPERIMENT 8 (rganic Chemistry II) Carboxylic Acids Reactions and Derivatives Pahlavan/Cherif Materials Medium test tubes (6) Test tube rack Beakers (50, 150, 400 ml) Ice Hot plate Graduated cylinders
EXPERIMENT 8 (rganic Chemistry II) Carboxylic Acids Reactions and Derivatives Pahlavan/Cherif Materials Medium test tubes (6) Test tube rack Beakers (50, 150, 400 ml) Ice Hot plate Graduated cylinders
Carboxylic Acid Derivatives Reading Study Problems Key Concepts and Skills Lecture Topics: Structures and reactivity of carboxylic acid derivatives
 Carboxylic Acid Derivatives Reading: Wade chapter 21, sections 21-1- 21-16 Study Problems: 21-45, 21-46, 21-48, 21-49, 21-50, 21-53, 21-56, 21-58, 21-63 Key Concepts and Skills: Interpret the spectra of
Carboxylic Acid Derivatives Reading: Wade chapter 21, sections 21-1- 21-16 Study Problems: 21-45, 21-46, 21-48, 21-49, 21-50, 21-53, 21-56, 21-58, 21-63 Key Concepts and Skills: Interpret the spectra of
Lecture 10: MS Interpretation Part 4 Postulation of Molecular Structures
 Lecture 10: MS Interpretation Part 4 Postulation of Molecular Structures CU- Boulder CHEM 5181 Mass Spectrometry & Chromatography Prof. Jose-Luis Jimenez Postulation of Molecular Structures There are several
Lecture 10: MS Interpretation Part 4 Postulation of Molecular Structures CU- Boulder CHEM 5181 Mass Spectrometry & Chromatography Prof. Jose-Luis Jimenez Postulation of Molecular Structures There are several
United States Patent (19) Motz
 United States Patent (19) Motz 54 I75 73 21 22 (63) (51) (52) 58 TRANSPARENT CMPST NS F BISPHENL-A-PLYCARBNATE, PLYALKYLENE TEREPHTHALATE AND AN ARMATIC PLYESTER CARBNATE Inventor: Assignee: Gary S. Motz,
United States Patent (19) Motz 54 I75 73 21 22 (63) (51) (52) 58 TRANSPARENT CMPST NS F BISPHENL-A-PLYCARBNATE, PLYALKYLENE TEREPHTHALATE AND AN ARMATIC PLYESTER CARBNATE Inventor: Assignee: Gary S. Motz,
Carboxylic Acids and their Derivatives I
 2302272 Org Chem II Part I Lecture 5 Carboxylic Acids and their Derivatives I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 20 in Organic Chemistry,
2302272 Org Chem II Part I Lecture 5 Carboxylic Acids and their Derivatives I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 20 in Organic Chemistry,
13. Carboxylic Acids (text )
 2009, Department of Chemistry, The University of Western ntario 13.1 13. Carboxylic Acids (text 14.1 14.9) A. Structure and Nomenclature The carboxylic acid functional group results from the connection
2009, Department of Chemistry, The University of Western ntario 13.1 13. Carboxylic Acids (text 14.1 14.9) A. Structure and Nomenclature The carboxylic acid functional group results from the connection
AMINO-ACID SYNTHESIS IN THE ANIMAL ORGANISM.
 AMINO-ACID SYNTHESIS IN THE ANIMAL ORGANISM. CAN NOR-LEUCINE REPLACE LYSINE FOR THE NUTRITIVE REQUIREMENTS OF THE WHITE RAT? BY HOWARD B. LEWIS AND LUCIE E. ROOT. (From the Laboratory of Physiological
AMINO-ACID SYNTHESIS IN THE ANIMAL ORGANISM. CAN NOR-LEUCINE REPLACE LYSINE FOR THE NUTRITIVE REQUIREMENTS OF THE WHITE RAT? BY HOWARD B. LEWIS AND LUCIE E. ROOT. (From the Laboratory of Physiological
PHAR3316 Pharmacy biochemistry Exam #2 Fall 2010 KEY
 1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
Identification of novel endophenaside antibiotics produced by Kitasatospora sp. MBT66
 SUPPORTING INFORMATION belonging to the manuscript: Identification of novel endophenaside antibiotics produced by Kitasatospora sp. MBT66 by Changsheng Wu 1, 2, Gilles P. van Wezel 1, *, and Young Hae
SUPPORTING INFORMATION belonging to the manuscript: Identification of novel endophenaside antibiotics produced by Kitasatospora sp. MBT66 by Changsheng Wu 1, 2, Gilles P. van Wezel 1, *, and Young Hae
Chapter Three (Biochemistry)
 Chapter Three (Biochemistry) 1 SECTION ONE: CARBON COMPOUNDS CARBON BONDING All compounds can be classified in two broad categories: organic compounds and inorganic compounds. Organic compounds are made
Chapter Three (Biochemistry) 1 SECTION ONE: CARBON COMPOUNDS CARBON BONDING All compounds can be classified in two broad categories: organic compounds and inorganic compounds. Organic compounds are made
Catabolism of Carbon skeletons of Amino acids. Amino acid metabolism
 Catabolism of Carbon skeletons of Amino acids Amino acid metabolism Carbon skeleton Carbon Skeleton a carbon skeleton is the internal structure of organic molecules. Carbon Arrangements The arrangement
Catabolism of Carbon skeletons of Amino acids Amino acid metabolism Carbon skeleton Carbon Skeleton a carbon skeleton is the internal structure of organic molecules. Carbon Arrangements The arrangement
Carboxylic Acids, Esters and Acyl Chlorides
 R hemistry A 432 arboxylic Acids, Esters and Acyl hlorides arboxylic Acids, Esters and Acyl hlorides arboxylic acids contain the functional group, attached to an alkyl stem. They are widely found in nature,
R hemistry A 432 arboxylic Acids, Esters and Acyl hlorides arboxylic Acids, Esters and Acyl hlorides arboxylic acids contain the functional group, attached to an alkyl stem. They are widely found in nature,
Chapter 20 Carboxylic Acids. Introduction
 hapter 20 arboxylic Acids Introduction arbonyl (-=) and hydroxyl (-H) on the same carbon is carboxyl group. arboxyl group is usually written -H or 2 H. Aliphatic acids have an alkyl group bonded to -H.
hapter 20 arboxylic Acids Introduction arbonyl (-=) and hydroxyl (-H) on the same carbon is carboxyl group. arboxyl group is usually written -H or 2 H. Aliphatic acids have an alkyl group bonded to -H.
Proteins are sometimes only produced in one cell type or cell compartment (brain has 15,000 expressed proteins, gut has 2,000).
 Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Esters of Carboxylic Acids These are derivatives of carboxylic acids where the hydroxyl group is replaced by an alkoxy group.
 Carboxylic acid Derivatives Carboxylic acid derivatives are described as compounds that can be converted to carboxylic acids via simple acidic or basic hydrolysis. The most important acid derivatives are
Carboxylic acid Derivatives Carboxylic acid derivatives are described as compounds that can be converted to carboxylic acids via simple acidic or basic hydrolysis. The most important acid derivatives are
1 C 2 C 3 C 4 C 5 C 6 C 7 C 8 C
 I. Carbon atoms form an enormous variety of structures A. Carbon has 4 valence electrons in the outer shell and therefore may form up to 4 covalent bonds B. Carbon tends to bond to C, H, O, N, S, and P
I. Carbon atoms form an enormous variety of structures A. Carbon has 4 valence electrons in the outer shell and therefore may form up to 4 covalent bonds B. Carbon tends to bond to C, H, O, N, S, and P
Alehydes, Ketones and Carboxylic Acid
 Alehydes, Ketones and Carboxylic Acid Aldehydes and Ketones: Introduction Aldedydes and ketones are organic compounds that contain carbon-oxygen doule bonds. The general formula for aldehydes is O C R
Alehydes, Ketones and Carboxylic Acid Aldehydes and Ketones: Introduction Aldedydes and ketones are organic compounds that contain carbon-oxygen doule bonds. The general formula for aldehydes is O C R
Introduction to Biochemistry Midterm exam )ومن أحياها(
 Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
THE JOURNAL OF ANTIBIOTICS. Polyketomycin, a New Antibiotic from Streptomyces sp. MK277-AF1. II. Structure Determination
 THE JOURNAL OF ANTIBIOTICS Polyketomycin, a New Antibiotic from Streptomyces sp. MK277-AF1 II. Structure Determination ISAO MOMOSE, WEI CHEN, HIKARU NAKAMURA, HIROSHI NAGANAWA, HIRONOBU IINUMA and TOMIO
THE JOURNAL OF ANTIBIOTICS Polyketomycin, a New Antibiotic from Streptomyces sp. MK277-AF1 II. Structure Determination ISAO MOMOSE, WEI CHEN, HIKARU NAKAMURA, HIROSHI NAGANAWA, HIRONOBU IINUMA and TOMIO
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy
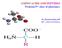 AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON
 CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON RED ANT WAS SOURCE OF FORMIC ACID (RCOOH) Lecture 8 ORGANIC CHEMISTRY 2 Introduction The carboxyl group (-CO
CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON RED ANT WAS SOURCE OF FORMIC ACID (RCOOH) Lecture 8 ORGANIC CHEMISTRY 2 Introduction The carboxyl group (-CO
Lecture 3: 8/24. CHAPTER 3 Amino Acids
 Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur. Lecture -02 Amino Acids II
 Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture -02 Amino Acids II Ok, we start off with the discussion on amino acids. (Refer Slide Time: 00:48)
Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture -02 Amino Acids II Ok, we start off with the discussion on amino acids. (Refer Slide Time: 00:48)
Organic Chemistry Diversity of Carbon Compounds
 Organic Chemistry Diversity of Carbon Compounds Hydrocarbons The Alkanes The Alkenes The Alkynes Naming Hydrocarbons Cyclic Hydrocarbons Alkyl Groups Aromatic Hydrocarbons Naming Complex Hydrocarbons Chemical
Organic Chemistry Diversity of Carbon Compounds Hydrocarbons The Alkanes The Alkenes The Alkynes Naming Hydrocarbons Cyclic Hydrocarbons Alkyl Groups Aromatic Hydrocarbons Naming Complex Hydrocarbons Chemical
ON TEA TANNIN ISOLATED FROM GREEN TEA.
 70 [Vol. 6 ON TEA TANNIN ISOLATED FROM GREEN TEA. By MICHIYO TSUJIMIIRA. (Received September 8th., 1930). The author(1) has recently isolated Tea catechin from green tea and pro posed the following formula
70 [Vol. 6 ON TEA TANNIN ISOLATED FROM GREEN TEA. By MICHIYO TSUJIMIIRA. (Received September 8th., 1930). The author(1) has recently isolated Tea catechin from green tea and pro posed the following formula
Chem 1120 Final 210 points Dr. Luther Giddings
 Chem 1120 Final 210 points Dr. Luther Giddings Name Phone or E-Mail Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam,
Chem 1120 Final 210 points Dr. Luther Giddings Name Phone or E-Mail Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam,
Soil organic matter composition, decomposition, mineralization and immobilization
 Soil organic matter composition, decomposition, mineralization and immobilization SOIL ORGANIC MATTER Substances containing carbon are organic matter. Soil organic matter consists of decomposing plant
Soil organic matter composition, decomposition, mineralization and immobilization SOIL ORGANIC MATTER Substances containing carbon are organic matter. Soil organic matter consists of decomposing plant
INTRODUCTION TO BIOCHEMISTRY/POLYMERS. 3. With respect to amino acids, polypeptides, and proteins, know:
 INTRDUCTIN T BICEMISTRY/PLYMERS A STUDENT SULD BE ABLE T: 1. With respect to lipids, know: The characteristic common to members of the class (solubility in nonpolar solvents) The functional groups most
INTRDUCTIN T BICEMISTRY/PLYMERS A STUDENT SULD BE ABLE T: 1. With respect to lipids, know: The characteristic common to members of the class (solubility in nonpolar solvents) The functional groups most
Radicals. Structure and Stability of Radicals. Radicals are formed from covalent bonds by adding energy in the form of heat (Δ) or light (hν).
 Radicals Chapter 15 A small but significant group of reactions involve radical intermediates. A radical is a reactive intermediate with a single unpaired electron, formed by homolysis of a covalent bond.
Radicals Chapter 15 A small but significant group of reactions involve radical intermediates. A radical is a reactive intermediate with a single unpaired electron, formed by homolysis of a covalent bond.
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2.
 BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
CH 3 CH 2 CH 2 CH 2 OH
 1 The alcohols form a homologous series. The first member is methanol and the fourth is butanol. 3 O methanol 3 2 2 2 O butanol (a) Give two general characteristics of a homologous series. (ii) alculate
1 The alcohols form a homologous series. The first member is methanol and the fourth is butanol. 3 O methanol 3 2 2 2 O butanol (a) Give two general characteristics of a homologous series. (ii) alculate
Macromolecules of Life -3 Amino Acids & Proteins
 Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Page 8/6: The cell. Where to start: Proteins (control a cell) (start/end products)
 Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Model 2: Aldohexoses, aldopentoses, ketohexoses and ketopentoses
 Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
Content Lecture 1 Lecture 2 - Amino acid Peptide and Polypeptide Lecture 3 Lecture 4
 Good Morning Class! Content Lecture 1 - Basic knowledge for Biochemistry - Cell: Structure and Function - Water Lecture 2 - Amino acid Peptide and Polypeptide Lecture 3 - Protein Structure and Function
Good Morning Class! Content Lecture 1 - Basic knowledge for Biochemistry - Cell: Structure and Function - Water Lecture 2 - Amino acid Peptide and Polypeptide Lecture 3 - Protein Structure and Function
Org/Biochem Final Lec Form, Spring 2012 Page 1 of 6
 Page 1 of 6 Missing Complete Protein and Question #45 Key Terms: Fill in the blank in the following 25 statements with one of the key terms in the table. Each key term may only be used once. Print legibly.
Page 1 of 6 Missing Complete Protein and Question #45 Key Terms: Fill in the blank in the following 25 statements with one of the key terms in the table. Each key term may only be used once. Print legibly.
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Identification of Aromatic Fatty Acid Ethyl Esters
 Chapter 3.2 Identification of Aromatic Fatty Acid Ethyl Esters The only use of gas chromatography is not sufficient to determine which compounds are eluting from the catalytic bed. At the beginning of
Chapter 3.2 Identification of Aromatic Fatty Acid Ethyl Esters The only use of gas chromatography is not sufficient to determine which compounds are eluting from the catalytic bed. At the beginning of
Organic Chemistry. Chapter 23. Hill, Petrucci, McCreary & Perry 4 th. Ed. Alkane to Substituent Group methane CH 4 methyl CH 3
 hapter 23 rganic hemistry ill, Petrucci, Mcreary & Perry 4 th Ed. Alkane to Substituent Group methane 4 methyl 3 ethane 3 3 ethyl 3 2 propane 3 2 3 propyl 3 2 2 isopropyl ( 3 ) 2 or 3 3 butyl 3 2 2 2 butane
hapter 23 rganic hemistry ill, Petrucci, Mcreary & Perry 4 th Ed. Alkane to Substituent Group methane 4 methyl 3 ethane 3 3 ethyl 3 2 propane 3 2 3 propyl 3 2 2 isopropyl ( 3 ) 2 or 3 3 butyl 3 2 2 2 butane
MBB 694:407, 115:511. Please use BLOCK CAPITAL letters like this --- A, B, C, D, E. Not lowercase!
 MBB 694:407, 115:511 First Test Severinov/Deis Tue. Sep. 30, 2003 Name Index number (not SSN) Row Letter Seat Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions
MBB 694:407, 115:511 First Test Severinov/Deis Tue. Sep. 30, 2003 Name Index number (not SSN) Row Letter Seat Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions
Biomolecules Amino Acids & Protein Chemistry
 Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
The Structure and Function of Macromolecules
 The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
Pathways to Biomolecules
 Week 12 Pathways to Biomolecules Are molecules such as fats and oils, carbohydrates, proteins and nucleic acids that are found in all living things. Have an essential role in the supply of energy to the
Week 12 Pathways to Biomolecules Are molecules such as fats and oils, carbohydrates, proteins and nucleic acids that are found in all living things. Have an essential role in the supply of energy to the
KMnO 4 1 O 4'' Apigenin. 1 In the following reactions draw the structures of products B and C. 1. NaH/DMF 2. excess MeI. acetic anhydride(excess)
 Problem 26: hemical and Stereochemical Structure of oniine oniine is a toxic compound found in the plant hemlock (conium maculatum), with which the ancient Greek philosopher Socrates was poisoned. oniine
Problem 26: hemical and Stereochemical Structure of oniine oniine is a toxic compound found in the plant hemlock (conium maculatum), with which the ancient Greek philosopher Socrates was poisoned. oniine
Short polymer. Dehydration removes a water molecule, forming a new bond. Longer polymer (a) Dehydration reaction in the synthesis of a polymer
 HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
not to be republished NCERT BIOMOLECULES CHAPTER 9 BIOMOLECULES 43 MULTIPLE CHOICE QUESTIONS
 BIOMOLECULES 43 43 CHAPTER 9 BIOMOLECULES MULTIPLE CHOICE QUESTIONS 1. It is said that elemental composition of living organisms and that of inanimate objects (like earth s crust) are similar in the sense
BIOMOLECULES 43 43 CHAPTER 9 BIOMOLECULES MULTIPLE CHOICE QUESTIONS 1. It is said that elemental composition of living organisms and that of inanimate objects (like earth s crust) are similar in the sense
Amino Acids in Cervical Mucus
 Amino Acids in Cervical Mucus D. P. Pederson, A.B., and W. T. Pommerenke, Ph.D., M.D. DURING THE ovulatory phase of the menstrual cycle, the secretions of the cervix are abundant and fluid. At this time
Amino Acids in Cervical Mucus D. P. Pederson, A.B., and W. T. Pommerenke, Ph.D., M.D. DURING THE ovulatory phase of the menstrual cycle, the secretions of the cervix are abundant and fluid. At this time
Emory Global Health Case Competition
 Emory Global Health Case Competition Connecting students from diverse fields to address a global health challenge Addressing Tobacco Burdens in Gujarat, India March 16 20, 2010 Douglas & Barbara Engmann
Emory Global Health Case Competition Connecting students from diverse fields to address a global health challenge Addressing Tobacco Burdens in Gujarat, India March 16 20, 2010 Douglas & Barbara Engmann
PAPER No. : 16 Bioorganic and biophysical chemistry MODULE No. : 25 Coenzyme-I Coenzyme A, TPP, B12 and biotin
 Subject Paper No and Title Module No and Title Module Tag 16, Bio organic and Bio physical chemistry 25, Coenzyme-I : Coenzyme A, TPP, B12 and CHE_P16_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction
Subject Paper No and Title Module No and Title Module Tag 16, Bio organic and Bio physical chemistry 25, Coenzyme-I : Coenzyme A, TPP, B12 and CHE_P16_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction
Amino acids. You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures.
 Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Carboxylic Acids and Nitriles. Chapters 20, 21 Organic Chemistry, 8th Edition John McMurry
 Carboxylic Acids and Nitriles Chapters 20, 21 Organic Chemistry, 8th Edition John McMurry 1 Carboxylic Acid Derivatives 2 Carboxylic Acid Derivatives nitrile R = CH 3 acetonitrile 3 Structure and Bonding
Carboxylic Acids and Nitriles Chapters 20, 21 Organic Chemistry, 8th Edition John McMurry 1 Carboxylic Acid Derivatives 2 Carboxylic Acid Derivatives nitrile R = CH 3 acetonitrile 3 Structure and Bonding
Biology 12 - Biochemistry Practice Exam
 Biology 12 - Biochemistry Practice Exam Name: Water: 1. The bond between water molecules is a (n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Biology 12 - Biochemistry Practice Exam Name: Water: 1. The bond between water molecules is a (n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
2. Which of the following is NOT true about carbohydrates
 Chemistry 11 Fall 2011 Examination #5 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response questions
Chemistry 11 Fall 2011 Examination #5 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response questions
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Lecture 10 - Protein Turnover and Amino Acid Catabolism
 Lecture 10 - Protein Turnover and Amino Acid Catabolism Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction 2 Proteins are degraded into amino acids. Protein
Lecture 10 - Protein Turnover and Amino Acid Catabolism Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction 2 Proteins are degraded into amino acids. Protein
Chapter 3: Amino Acids and Peptides
 Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Carbohydrates. Learning Objective
 , one of the four major classes of biomolecules, are aldehyde or ketone compounds with multiple hydroxyl groups. They function as energy stores, metabolic intermediates and important fuels for the body.
, one of the four major classes of biomolecules, are aldehyde or ketone compounds with multiple hydroxyl groups. They function as energy stores, metabolic intermediates and important fuels for the body.
