8 Influence of permeation modulators on the behaviour of a SC lipid model mixture
|
|
|
- Brendan Hood
- 5 years ago
- Views:
Transcription
1 8 Influence of permeation modulators on the behaviour of a SC lipid model mixture 8.1 Introduction In the foregoing parts of this thesis, a model membrane system of SC lipids has been developed and characterized. The methods used allowed to elucidate the role of CHOL, as one constituent of the original membrane, in the SC lipid model and herewith in the native SC. The final part of the thesis attends to the question whether the developed membrane model can be used to describe interactions with other substances, which are not components of the original model membrane, and whether the mode of action of permeation modulators can be studied using the SC lipid model system. For the present study, three different substances, namely urea, oleic acid (OA), and N-lauroylglycine lauryl ester (12G12) were chosen. Urea is believed to be a permeation enhancer which affects the hydrophilic parts of the SC membranes [8,9], while OA and 12G12 should enhance the transport of substances due to influencing the hydrocarbon tails. Urea is a hydrophilic and well water-soluble compound, which is supposed to be concentrated in the water phase of the system. It is a physiological substance occurring in mammalian tissues. In the human SC, urea plays an important role as a component of natural moisturizing factor [190]. The effect of urea can be summarized as proteo- and keratolytic, water-binding, and itching alleviative. The decreased urea levels in SC were found in the dry skin syndrome [191]. Urea is used in the treatment of many skin diseases as psoriasis, ichthyosis, dry skin, dystrophic nails, etc. (for a review see [53,192]). Urea and especially its derivatives were described to enhance transdermal permeation of several drugs by facilitating hydration of the SC and by the formation of hydrophilic diffusion channels within the barrier [193]. For these reasons it is sometimes classified as enhancer for the hydrophilic pathway [8]. The keratolysis induced by urea plays also a role in its transdermal penetration enhancement mode of action. 87
2 Unlike urea, OA and 12G12 are of lipid type and probably incorporate into the membrane. The physiological presence of OA in human SC is controversially discussed. While some earlier studies have detected OA in its free fatty acid form as an important constituent of the SC lipid matrix [194]; more recently, this assumption has not been confirmed [20,195]. Indeed, some OA is generated in the stratum granulosum as a product of phospholipid degradation; it is, however, esterified by CHOL before it reaches SC [196]. Thus only very small trace amounts of free OA are detected in SC. Nevertheless, OA is known as an efficient transdermal permeation enhancer which interacts with and modifies the lipid domains of the SC due to the cis double bond at C9. The presence of the double bond causes a kink in the hydrocarbon chain of OA, which is likely to disrupt the ordered array of the predominantly saturated highly ordered skin lipid chains and increase the fluidity of the SC lipid membranes [57]. In SC lipid mixtures, OA lowers the main phase transition temperature and forms a separate domain with disordered chains within the SC lipid membranes [197]. Ceramides and OA are immiscible in the solid state and their mixtures show a monotectic behaviour [62]. It has been suggested that such a domain formation of OA and some SC lipids is responsible for the decreased capacity of skin barrier function [198]. In essential fatty acid deficiency, acylceramides are produced, where linoleate is replaced by oleate. It has been suggested that the modified acylceramide acts as an endogenous permeation enhancer [21]. Ceramide analogue 12G12 has been synthesized and evaluated as a potential permeation enhancer. According to recent studies, the substance shows a pronounced enhancing effect on human skin [199,200]. The thermotropic phase behaviour of 12G12 in the bulk phase has been studied within the framework of the present thesis and the main results are summarized in Appendix C. The mode of action of 12G12 is proposed to be the interaction with SC lipid matrix. The present study should bring more information about the mode of action of this novel enhancer. 8.2 Methods Material Cer[AP] was a gift from Cosmoferm (Delft, The Netherland). Cholesterol (CHOL), cholesterol sulphate (CS), and palmitic acid (PA) as well oleic acid (OA) and urea were purchased from Sigma-Aldrich (Taufkirchen, Germany). N-lauroylglycine lauryl ester (12G12) was synthesised and purified by Dr. Vávrová, Dept. of Inorganic and Organic Chemistry, Faculty of Pharmacy, Charles University, Prague. Water, chloroform and methanol used were of HPLC grade. 88
3 8.2.2 Sample preparation The MLVs (10% of lipids in water) were prepared by the thin layer method as described previously (Chapter 5.2.2). The basic composition of all the samples is 55% ceramide[ap], 25% CHOL, 15% PA, and 5% CS. To the samples, OA or 12G12 in various molar concentrations were added. A part of the samples with the basic composition was prepared in buffers with various urea concentrations SAXD measurements The time-resolved X-ray diffraction patterns from the MLVs (10% w/w of lipid in water) in the small-angle region were collected on the D22 line at DCI synchrotron source (LURE, Orsay, France) at the wavelength of 1.39 Å. The samples were placed in a quartz capillary. In order to eliminate the phase separation, the samples were heated for 15 min at 90 C before the measurement. The acquisition time was 10 min for the static measurements at 32 C and 1 min for the continuous measurements during the heating. The heating rate was 1 C min -1. The linear position sensitive detector was arranged in the sample-to-detector distance of 1813 mm. X- ray diffraction from DMPC or DPPC MLVs was used as known calibration standards. The obtained diffraction peaks were fitted with Gaussian functions in order to minimize the statistical error. The lamellar repeat distance was calculated from the position of the first diffraction peak according to: D = 2π q. 8.3 Results and discussion Influence of urea on the SC lipid system Fig. 8.1 shows the X-ray diffraction patterns from the MLVs with the basic composition prepared in buffers with various urea concentrations at 32 C after the heating. As can be seen, the position of the diffraction peak is not affected by the presence of urea in the environment. The determined repeat distance is very similar in all the samples and amounts to about 46.3 Å. The obtained information is striking, because the effect of urea on the hydration of SC is well known and the influence on the water layer between the SC lipid membranes can be assumed. The present results, however, bring the evidence that the thickness of the water layer between the membranes is not affected by urea. During the heating of the samples, the membrane repeat distance changes. As it has been described previously (Chapter 6), the thermotropic behaviour of the system with the basic 89
4 composition without the presence of urea in the environment shows two phase transitions. During the first transition at about 45 C, the lamellar repeat distance decreases abruptly from 46.3 to 43.6 Å. The first transition seems to be connected with the melting of a part of the SC lipids. The other phase transition starts at 70 C, when a further decrease in the periodicity occurs indicating the melting of all hydrocarbon chains % urea Intensity [a.u.] % urea 10% urea 5% urea 2% urea 1% urea 0 0% urea q [Å -1 ] Fig. 8.1 X-ray diffraction patterns from the SC lipid MLVs in buffers with various urea concentrations at 32 C. As can be seen in Fig. 8.2, the presence of urea in the water environment influences the thermotropic phase behaviour of the SC lipid system. When 2-5% of urea in the buffer is present, the first phase transition shifts to higher temperatures and becomes less distinct. Further increase in the urea concentration to 10% causes that both phase transitions merge into a broad one. The system starts to melt continuously at about 42 C and no gel phase can be observable. There is an apparent influence of urea on the phase behaviour of the SC lipid membranes. The smoothing of the first phase transition and the merging of both transitions into the broad one indicates that in the state below the first phase transition, the particular lipid components became more miscible in the presence of urea and do not melt separately. This effect could be explained by displacement of some water molecules bound by the hydrogen bonds to the polar headgroups of the SC lipids by urea. Herewith, the miscibility of the system can increase. 90
5 47 Lamellar repeat distance [Å] % urea 2% urea 5% urea 10% urea Temperature [ C] Fig. 8.2 Lamellar repeat distance of the membranes with various urea concentrations vs. temperature. Urea induces an up-shift in the first phase transition temperature and merging of both phase transitions together Influence of oleic acid on the SC lipid system The X-ray diffractograms from the SC lipid system with various OA concentrations measured at 32 C are shown in Fig. 8.3a. When only 2-5 molar % of OA is incorporated in the membrane, the systems show one diffraction peak at nearly the same position. The calculated repeat distance (Fig. 8.3b) decreases by about 0.5 Å in comparison to that of the neat SC lipid system. A different situation arises when higher concentrations of OA (10-15 molar %) are present in the mixture. In that case, the membrane separates into two phases: the longer one (the L-phase) with the periodicity of about 46.6 Å and the shorter one (the S-phase) with the repeat distance of 43.5 Å. As described previously (Chapter 5), a similar phase separation occurs also in the pure SC lipid model system, but the process requires much longer time (several days). Evidently, OA enhances the formation of a new domain (the S-phase) in the SC lipid system at 32 C. 91
6 phase separation % OA % OA Int. [a.u.] % OA D [Å] % OA 0 [a] 0% OA 43.5 [b] q [Å -1 ] mol % oleic acid Fig. 8.3 [a] X-ray diffraction patterns from the SC lipid MLVs with various oleic acid molar concentrations at 32 C. [b] The determined lamellar repeat distance of the membranes. The thermotropic response of the lamellar repeat distance of the systems with various OA concentrations is shown in Fig All the systems show two phase transitions during the heating; the first one at about 45 C, the other one starting at about 70 C. The phase behaviour of the system with 2% OA is very similar to that one without OA. In the course of the first phase transition, the periodicity of the membrane decreases suddenly to about 43.3 Å. In the membrane with 5% OA, a new phase with a D-value of 43.3 Å can be detected besides the L-phase at 40 C. During the first phase transition, the phases merge together into a resulting phase with 43.8 Å. Increasing the OA concentration in the membrane to 10%, the phase separation occurs already at 30 C. During the first phase transition at about 50 C, the phases mix together into another one with a repeat distance of about 44 Å. In all the samples, the second phase transition at about 70 C is connected with melting of the remaining hydrocarbon chains. From the present data, it is apparent that the miscibility of OA with the SC lipids is very limited in the state below the main phase transition. Only a small amount of OA (up to 2%) can be fully incorporated into the membrane. Higher amounts of OA in the system start to create a new domain that predominantly consists of OA presumably in the fluid phase. This phase, however, does not consist only of OA, but most probably also of the other lipid components as PA. This assumption is supported by the fact that the periodicity 92
7 of the L-phase in the separated state increases compared to the SC lipid system without OA. Unexpectedly, OA decreases the temperature of neither the first nor the second phase transition. This again hints to the strong immiscibility of the SC lipid system with OA. 47 Lamellar repeat distance [Å] % OA 2% OA 5% OA 10% OA Temperature [ C] Fig. 8.4 Lamellar repeat distance of the membranes with various molar concentrations of oleic acid vs. temperature. The phase separation is distinct in the samples with 10% and 5%OA at 32 and 40 C, respectively. The phase separation of OA from SC lipids has also been described earlier [197]. The mode of action of OA as the permeation enhancer is most probably connected with the phase separation effect. The new fluid domain will be more permeable for a drug as the more rigid regions Influence of 12G12 on the SC lipid system The diffractograms from the SC lipid systems including various concentrations of 12G12 measured at 32 C are shown in Fig. 8.5a. As can be seen, there is a shift in the peak position to higher q-values with increasing amount of 12G12 in the system. The corresponding repeat distances are plotted in Fig. 8.5b. The values decrease with the increasing 12G12 concentration. In the concentration range used, the dependence has an exponential character. This decay in the periodicity can be connected with the fact that the hydrocarbon chains of 12G12 are shorter than the other chains in the system. 93
8 Interestingly, one diffraction peak only is detectable in all the patterns. This indicates that 12G12 incorporates into the SC lipid membrane and does not create its own domain, as it Intensity [a.u.] % 12G12 15% 12G12 D [Å] Exponential decay: y = x/ [a] 5% 12G12 0% 12G [b] q [A -1 ] mol % 12G12 Fig. 8.5 [a] X-ray diffraction patterns from the SC lipid MLVs with various 12G12 molar concentrations at 32 C. [b] The determined lamellar repeat distance of the SC lipid membrane decreases with the increasing 12G12 concentration. can be presumable for the lipid mixtures with mismatch in the hydrocarbon chain lengths. Simultaneously with the increasing concentration of 12G12 in the system, the intensity of the diffraction signal becomes weaker. As it has been suggested earlier [103,184], such weakening in the diffraction intensity can be related to the increasing membrane undulations due to higher concentrations of 12G12 in the membrane. The dependence of the repeat distance on the temperature for the systems with various 12G12 concentrations is presented in Fig As mentioned above, the SC lipid system without the permeation enhancer shows two phase transitions at about 45 and 70 C. When 5% of 12G12 is incorporated into the membrane, the first phase transition shifts to higher temperatures. The repeat distance of the membrane starts to decrease continuously at about 50 C. Between 57 and 61 C, the downshift of the repeat distance is not so rapid as before. Presumably, the gel phase can be detectable in this temperature range. Above 61 C, the periodicity decreases more abruptly again. The sample with 15% 12G12 shows quite different behaviour. The lamellar repeat distance starts to decrease continuously at about 40 C in one broad phase transition. The process does not show a separation into two phase transitions. 94
9 47 Lamellar repeat distance [Å] % 12G12 5% 12G12 15% 12G Temperature [ C] Fig. 8.6 Lamellar repeat distance of the membranes with various molar concentrations of 12G12 vs. temperature. 12G12 shifts the first phase transition to higher temperatures. The shift of the first phase transition to higher temperatures in the sample with 5% 12G12 indicates that the SC lipids become more miscible after the addition of enhancer. Assuming that the first transition is due to separated melting of a domain including predominantly PA (Chapters 5, 6), this domain is affected by 12G12. Probably, the enhancer interacts with PA and causes an increase in the melting temperature compared to neat PA. The fact that 12G12 affects the miscibility of the SC lipids can be important for the mode of action of the enhancing effect. 8.4 Conclusions In the present study, the effect of three permeation modulators and/or skin moisturizers as urea, oleic acid and 12G12 has been studied. Urea is a very hydrophilic and well watersoluble compound with moisturizing and enhancing effect, which is supposed to be concentrated in the water phase of the system. The permeation enhancers, oleic acid (OA) and 12G12 are of lipid type and probably incorporate into the lipid bilayers. Urea does not affect the lamellar repeat distance of the SC lipid system at 32 C; however, its influence on the thermotropic phase behaviour of the SC lipids is confirmed. Probably, displacement of some water molecules bound to the SC lipid headgroups by the hydrogen bonds causes a higher miscibility between the lipids. 95
10 Oleic acid shows a very limited miscibility with the SC lipid system. Higher concentrations of OA induce a phase separation. A new domain with a repeat distance of about 43.3 Å consists predominantly of OA. The phase transitions temperatures are not affected by OA. This behaviour seems to be connected with the enhancing effect of OA. 12G12 incorporates into the SC lipid matrix and does not induce a phase separation of the membrane. The increasing concentration of 12G12 in the system decreases the lamellar repeat distance. 12G12 affects the miscibility properties of the SC lipids. The presented study confirms that the SC lipid model system developed and characterized in the framework of this thesis is convenient to describe the mode of action of permeation enhancers and other agents influencing the SC lipid organization. 96
morphological and molecular structure of the Stratum corneum
 New insights into the morphological and molecular structure of the Stratum corneum Prof. Dr. Dr. h.c.. Reinhard H. H. Neubert Institute of Pharmacy of the Martin Luther University, Halle-Wittenberg Content:
New insights into the morphological and molecular structure of the Stratum corneum Prof. Dr. Dr. h.c.. Reinhard H. H. Neubert Institute of Pharmacy of the Martin Luther University, Halle-Wittenberg Content:
Lipids and Membranes
 Lipids Lipids are hydrophobic or amphiphilic insoluble in water soluble in organic solvents soluble in lipids Lipids are used as energy storage molecules structural components of membranes protective molecules
Lipids Lipids are hydrophobic or amphiphilic insoluble in water soluble in organic solvents soluble in lipids Lipids are used as energy storage molecules structural components of membranes protective molecules
Life Sciences 1a. Practice Problems 4
 Life Sciences 1a Practice Problems 4 1. KcsA, a channel that allows K + ions to pass through the membrane, is a protein with four identical subunits that form a channel through the center of the tetramer.
Life Sciences 1a Practice Problems 4 1. KcsA, a channel that allows K + ions to pass through the membrane, is a protein with four identical subunits that form a channel through the center of the tetramer.
Structure and Phase Behaviour of Binary Mixtures of Cholesterol with DPPC and DMPC
 Chapter 3 Structure and Phase Behaviour of Binary Mixtures of Cholesterol with DPPC and DMPC 3.1 Introduction As discussed in chapter 1, phospholipids and cholesterol are important constituents of plasma
Chapter 3 Structure and Phase Behaviour of Binary Mixtures of Cholesterol with DPPC and DMPC 3.1 Introduction As discussed in chapter 1, phospholipids and cholesterol are important constituents of plasma
Chapter 1 Membrane Structure and Function
 Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
AFM In Liquid: A High Sensitivity Study On Biological Membranes
 University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2006 AFM In Liquid: A High Sensitivity Study On Biological Membranes Michael J. Higgins
University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2006 AFM In Liquid: A High Sensitivity Study On Biological Membranes Michael J. Higgins
Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson
 Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson Link download full: http://testbankair.com/download/test-bank-forlehninger-principles-of-biochemistry-5th-edition-by-nelson/ Chapter
Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson Link download full: http://testbankair.com/download/test-bank-forlehninger-principles-of-biochemistry-5th-edition-by-nelson/ Chapter
THE INS AND OUTS OF YOUR SKIN. Emma Sparr Physical Chemistry Lund University
 THE INS AND OUTS OF YOUR SKIN Emma Sparr Physical Chemistry Lund University The skin - A Responding Barrier Membrane stratum corneum (10 20 µm) Water CO 2 Temperature ph 5.5 O 2 Moisturizers, Drugs etc
THE INS AND OUTS OF YOUR SKIN Emma Sparr Physical Chemistry Lund University The skin - A Responding Barrier Membrane stratum corneum (10 20 µm) Water CO 2 Temperature ph 5.5 O 2 Moisturizers, Drugs etc
Millicient A. Firestone, Amanda C. Wolf, and Sonke Seifert. Chris Bianchi 4/30/12
 Small-Angle X-ray Scattering of the Interaction of Poly(ethylene oxide)-b-poly(propylene oxide)-b- Poly(ethylene oxide)triblock Copolymers with Lipid Bilayers Millicient A. Firestone, Amanda C. Wolf, and
Small-Angle X-ray Scattering of the Interaction of Poly(ethylene oxide)-b-poly(propylene oxide)-b- Poly(ethylene oxide)triblock Copolymers with Lipid Bilayers Millicient A. Firestone, Amanda C. Wolf, and
Cellular membranes are fluid mosaics of lipids and proteins.
 Study Guide e Plasma Membrane You should be able to write out the definitions to each of the following terms in your own words: plasma membrane fluid mosaic integral proteins peripheral proteins receptor
Study Guide e Plasma Membrane You should be able to write out the definitions to each of the following terms in your own words: plasma membrane fluid mosaic integral proteins peripheral proteins receptor
Chapter 2 The Chemistry of Life Part 2
 Chapter 2 The Chemistry of Life Part 2 Carbohydrates are Polymers of Monosaccharides Three different ways to represent a monosaccharide Carbohydrates Carbohydrates are sugars and starches and provide
Chapter 2 The Chemistry of Life Part 2 Carbohydrates are Polymers of Monosaccharides Three different ways to represent a monosaccharide Carbohydrates Carbohydrates are sugars and starches and provide
Methods and Materials
 a division of Halcyonics GmbH Anna-Vandenhoeck-Ring 5 37081 Göttingen, Germany Application Note Micostructured lipid bilayers ANDREAS JANSHOFF 1), MAJA GEDIG, AND SIMON FAISS Fig.1: Thickness map of microstructured
a division of Halcyonics GmbH Anna-Vandenhoeck-Ring 5 37081 Göttingen, Germany Application Note Micostructured lipid bilayers ANDREAS JANSHOFF 1), MAJA GEDIG, AND SIMON FAISS Fig.1: Thickness map of microstructured
Physical Cell Biology Lecture 10: membranes elasticity and geometry. Hydrophobicity as an entropic effect
 Physical Cell Biology Lecture 10: membranes elasticity and geometry Phillips: Chapter 5, Chapter 11 and Pollard Chapter 13 Hydrophobicity as an entropic effect 1 Self-Assembly of Lipid Structures Lipid
Physical Cell Biology Lecture 10: membranes elasticity and geometry Phillips: Chapter 5, Chapter 11 and Pollard Chapter 13 Hydrophobicity as an entropic effect 1 Self-Assembly of Lipid Structures Lipid
Membranes & Membrane Proteins
 School on Biomolecular Simulations Membranes & Membrane Proteins Vani Vemparala The Institute of Mathematical Sciences Chennai November 13 2007 JNCASR, Bangalore Cellular Environment Plasma membrane extracellular
School on Biomolecular Simulations Membranes & Membrane Proteins Vani Vemparala The Institute of Mathematical Sciences Chennai November 13 2007 JNCASR, Bangalore Cellular Environment Plasma membrane extracellular
2.3 Carbon-Based Molecules CARBON BASED MOLECULES
 CARBON BASED MOLECULES KEY CONCEPTS Carbon-based molecules are the foundation of life. Lipids are one class of organic molecules. This group includes fats, oils, waxes, and steroids. Lipids are made of
CARBON BASED MOLECULES KEY CONCEPTS Carbon-based molecules are the foundation of life. Lipids are one class of organic molecules. This group includes fats, oils, waxes, and steroids. Lipids are made of
Role of ceramide 1 in the molecular organization of the stratum corneum lipids
 Role of ceramide 1 in the molecular organization of the stratum corneum lipids J. A. Bouwstra, 1, * G. S. Gooris,* F. E. R. Dubbelaar,* A. M. Weerheim, A. P. IJzerman* and M. Ponec Leiden/Amsterdam Center
Role of ceramide 1 in the molecular organization of the stratum corneum lipids J. A. Bouwstra, 1, * G. S. Gooris,* F. E. R. Dubbelaar,* A. M. Weerheim, A. P. IJzerman* and M. Ponec Leiden/Amsterdam Center
Lipids and Membranes
 Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Biological membranes are composed of lipid bilayers
Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Biological membranes are composed of lipid bilayers
Classification, functions and structure
 Classification, functions and structure Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine and Pharmacy "Nicolae Testemitanu" Lipids
Classification, functions and structure Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine and Pharmacy "Nicolae Testemitanu" Lipids
Supplement 2 - Quantifying sample mosaicity
 Supplement 2 - Quantifying sample mosaicity Supplementary information for: Order parameters and areas in fluid-phase oriented lipid membranes using wide angle x-ray scattering Thalia T. Mills, Gilman E.
Supplement 2 - Quantifying sample mosaicity Supplementary information for: Order parameters and areas in fluid-phase oriented lipid membranes using wide angle x-ray scattering Thalia T. Mills, Gilman E.
A: All atom molecular simulation systems
 Cholesterol level affects surface charge of lipid membranes in physiological environment Aniket Magarkar a, Vivek Dhawan b, Paraskevi Kallinteri a, Tapani Viitala c, Mohammed Elmowafy c, Tomasz Róg d,
Cholesterol level affects surface charge of lipid membranes in physiological environment Aniket Magarkar a, Vivek Dhawan b, Paraskevi Kallinteri a, Tapani Viitala c, Mohammed Elmowafy c, Tomasz Róg d,
3.1.3 Lipids. Source: AQA Spec
 alevelbiology.co.uk SPECIFICATION Triglycerides and phospholipids are two groups of lipid. Triglycerides are formed by the condensation of one molecule of glycerol and three molecules of fatty acid. A
alevelbiology.co.uk SPECIFICATION Triglycerides and phospholipids are two groups of lipid. Triglycerides are formed by the condensation of one molecule of glycerol and three molecules of fatty acid. A
BIOB111_CHBIO - Tutorial activity for Session 12
 BIOB111_CHBIO - Tutorial activity for Session 12 General topic for week 6 Session 12 Lipids Useful Links: 1. Animations on Cholesterol (its synthesis, lifestyle factors, LDL) http://www.wiley.com/college/boyer/0470003790/animations/cholesterol/cholesterol.htm
BIOB111_CHBIO - Tutorial activity for Session 12 General topic for week 6 Session 12 Lipids Useful Links: 1. Animations on Cholesterol (its synthesis, lifestyle factors, LDL) http://www.wiley.com/college/boyer/0470003790/animations/cholesterol/cholesterol.htm
Chemical Surface Transformation 1
 Chemical Surface Transformation 1 Chemical reactions at Si H surfaces (inorganic and organic) can generate very thin films (sub nm thickness up to µm): inorganic layer formation by: thermal conversion:
Chemical Surface Transformation 1 Chemical reactions at Si H surfaces (inorganic and organic) can generate very thin films (sub nm thickness up to µm): inorganic layer formation by: thermal conversion:
Chapter 7: Membranes
 Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
Cell Membrane Structure (1.3) IB Diploma Biology
 Cell Membrane Structure (1.3) IB Diploma Biology Essential idea: The structure of biological membranes makes them fluid and dynamic http://www.flickr.com/photos/edsweeney/6346198056/ 1.3.1 Phospholipids
Cell Membrane Structure (1.3) IB Diploma Biology Essential idea: The structure of biological membranes makes them fluid and dynamic http://www.flickr.com/photos/edsweeney/6346198056/ 1.3.1 Phospholipids
Biological role of lipids
 Lipids Lipids Organic compounds present in living organisms, insoluble in water but able to be extracted by organic solvents such as: chloroform, acetone, benzene. Extraction = the action of taking out
Lipids Lipids Organic compounds present in living organisms, insoluble in water but able to be extracted by organic solvents such as: chloroform, acetone, benzene. Extraction = the action of taking out
CH 3. Lipids CHAPTER SUMMARY
 H 3 C H 3 C 15 H 3 C H Views of Cholesterol APTER SUMMARY 15.1 The Nature of can best be defined as biomolecules which are soluble to a great extent in solvents. In contrast to carbohydrates, proteins
H 3 C H 3 C 15 H 3 C H Views of Cholesterol APTER SUMMARY 15.1 The Nature of can best be defined as biomolecules which are soluble to a great extent in solvents. In contrast to carbohydrates, proteins
The Interaction between Lipid Bilayers and Biological Membranes. Chapter 18
 The Interaction between Lipid Bilayers and Biological Membranes Chapter 18 Introduction Membrane & Phospholipid Bilayer Structure Membrane Lipid bilayer 2 Introduction Forces Acting between Surfaces in
The Interaction between Lipid Bilayers and Biological Membranes Chapter 18 Introduction Membrane & Phospholipid Bilayer Structure Membrane Lipid bilayer 2 Introduction Forces Acting between Surfaces in
Lipids: Membranes Testing Fluid Mosaic Model of Membrane Structure: Cellular Fusion
 Models for Membrane Structure NEW MODEL (1972) Fluid Mosaic Model proposed by Singer & Nicholson Lipids form a viscous, twodimensional solvent into which proteins are inserted and integrated more or less
Models for Membrane Structure NEW MODEL (1972) Fluid Mosaic Model proposed by Singer & Nicholson Lipids form a viscous, twodimensional solvent into which proteins are inserted and integrated more or less
Fluid Mozaic Model of Membranes
 Replacement for the 1935 Davson Danielli model Provided explanation for Gortner-Grendel lack of lipid and permitted the unit membrane model. Trans membrane protein by labelling Fry & Edidin showed that
Replacement for the 1935 Davson Danielli model Provided explanation for Gortner-Grendel lack of lipid and permitted the unit membrane model. Trans membrane protein by labelling Fry & Edidin showed that
Experiment 12 Lipids. Structures of Common Fatty Acids Name Number of carbons
 Experiment 12 Lipids Lipids are a class of biological molecules that are insoluble in water and soluble in nonpolar solvents. There are many different categories of lipids and each category has different
Experiment 12 Lipids Lipids are a class of biological molecules that are insoluble in water and soluble in nonpolar solvents. There are many different categories of lipids and each category has different
AC Flax Seed Oil. Conditioning + Nourishing + Moisturizing. Tomorrow s Vision Today!
 AC Flax Seed Oil Conditioning + Nourishing + Moisturizing Tomorrow s Vision Today! AC Flax Seed Oil Technical Information: Product Code: 15002 INCI Name: Linum Usitatissimum (Linseed) Seed Oil INCI Status:
AC Flax Seed Oil Conditioning + Nourishing + Moisturizing Tomorrow s Vision Today! AC Flax Seed Oil Technical Information: Product Code: 15002 INCI Name: Linum Usitatissimum (Linseed) Seed Oil INCI Status:
Chem 60 Takehome Test 2 Student Section
 Multiple choice: 1 point each. Mark only one answer for each question. 1. are composed primarily of carbon and hydrogen, but may also include oxygen, nitrogen, sulfur, phosphorus, and a few other elements.
Multiple choice: 1 point each. Mark only one answer for each question. 1. are composed primarily of carbon and hydrogen, but may also include oxygen, nitrogen, sulfur, phosphorus, and a few other elements.
Nafith Abu Tarboush DDS, MSc, PhD
 Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush Lipids (cholesterol, cholesterol esters, phospholipids & triacylglycerols) combined with proteins (apolipoprotein) in
Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush Lipids (cholesterol, cholesterol esters, phospholipids & triacylglycerols) combined with proteins (apolipoprotein) in
The Cell Membrane (Ch. 7)
 The Cell Membrane (Ch. 7) Phospholipids Phosphate head hydrophilic Fatty acid tails hydrophobic Arranged as a bilayer Phosphate attracted to water Fatty acid repelled by water Aaaah, one of those structure
The Cell Membrane (Ch. 7) Phospholipids Phosphate head hydrophilic Fatty acid tails hydrophobic Arranged as a bilayer Phosphate attracted to water Fatty acid repelled by water Aaaah, one of those structure
OPTION GROUP: BIOLOGICAL MOLECULES 2 LIPIDS & PHOSPHOLIPIDS WORKBOOK
 NAME: OPTION GROUP: BIOLOGICAL MOLECULES 2 LIPIDS & PHOSPHOLIPIDS WORKBOOK Instructions REVISION CHECKLIST AND ASSESSMENT OBJECTIVES Regular revision throughout the year is essential. It s vital you keep
NAME: OPTION GROUP: BIOLOGICAL MOLECULES 2 LIPIDS & PHOSPHOLIPIDS WORKBOOK Instructions REVISION CHECKLIST AND ASSESSMENT OBJECTIVES Regular revision throughout the year is essential. It s vital you keep
OBJECTIVE. Lipids are largely hydrocarbon derivatives and thus represent
 Paper 4. Biomolecules and their interactions Module 20: Saturated and unsaturated fatty acids, Nomenclature of fatty acids and Essential and non-essential fatty acids OBJECTIVE The main aim of this module
Paper 4. Biomolecules and their interactions Module 20: Saturated and unsaturated fatty acids, Nomenclature of fatty acids and Essential and non-essential fatty acids OBJECTIVE The main aim of this module
Effect of Cholesterol on the Properties of Phospholipid Membranes. 2. Free Energy Profile of Small Molecules
 5322 J. Phys. Chem. B 2003, 107, 5322-5332 Effect of Cholesterol on the Properties of Phospholipid Membranes. 2. Free Energy Profile of Small Molecules Pál Jedlovszky*, Department of Colloid Chemistry,
5322 J. Phys. Chem. B 2003, 107, 5322-5332 Effect of Cholesterol on the Properties of Phospholipid Membranes. 2. Free Energy Profile of Small Molecules Pál Jedlovszky*, Department of Colloid Chemistry,
Cell Membrane: a Phospholipid Bilayer. Membrane Structure and Function. Fluid Mosaic Model. Chapter 5
 Membrane Structure and Function Chapter 5 Cell Membrane: a Phospholipid Bilayer Phospholipid Hydrophilic Head Hydrophobic Tail Lipid Bilayer Fluid Mosaic Model Mixture of saturated and unsaturated fatty
Membrane Structure and Function Chapter 5 Cell Membrane: a Phospholipid Bilayer Phospholipid Hydrophilic Head Hydrophobic Tail Lipid Bilayer Fluid Mosaic Model Mixture of saturated and unsaturated fatty
Factors to Consider in the Study of Biomolecules
 Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General structure and functional groups
Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General structure and functional groups
(multiple answers) This strain of HIV uses a different chemokine coreceptor for entry into cells.
 LS1a Fall 06 Problem Set #4 100 points total all questions including the (*extra*) one should be turned in TF 1. (20 points) To further investigate HIV infection you decide to study the process of the
LS1a Fall 06 Problem Set #4 100 points total all questions including the (*extra*) one should be turned in TF 1. (20 points) To further investigate HIV infection you decide to study the process of the
Week 5 Section. Junaid Malek, M.D.
 Week 5 Section Junaid Malek, M.D. HIV: Anatomy Membrane (partiallystolen from host cell) 2 Glycoproteins (proteins modified by added sugar) 2 copies of RNA Capsid HIV Genome Encodes: Structural Proteins
Week 5 Section Junaid Malek, M.D. HIV: Anatomy Membrane (partiallystolen from host cell) 2 Glycoproteins (proteins modified by added sugar) 2 copies of RNA Capsid HIV Genome Encodes: Structural Proteins
GUTS Lecture Syllabus for Lipid Structure and Nomenclature
 GUTS Lecture Syllabus for Lipid Structure and Nomenclature For Questions or Assistance contact: Dr. Gwen Sancar, gsancar@ad.unc.edu Learning bjectives After completing the GUTS lecture and associated self-
GUTS Lecture Syllabus for Lipid Structure and Nomenclature For Questions or Assistance contact: Dr. Gwen Sancar, gsancar@ad.unc.edu Learning bjectives After completing the GUTS lecture and associated self-
Novel lipid mixtures based on synthetic ceramides reproduce the unique stratum corneum lipid organization
 Novel lipid mixtures based on synthetic ceramides reproduce the unique stratum corneum lipid organization Miranda W. de Jager,* Gert S. Gooris,* Igor P. Dolbnya, Wim Bras, Maria Ponec, and Joke A. Bouwstra
Novel lipid mixtures based on synthetic ceramides reproduce the unique stratum corneum lipid organization Miranda W. de Jager,* Gert S. Gooris,* Igor P. Dolbnya, Wim Bras, Maria Ponec, and Joke A. Bouwstra
and controllable behavior - Supplementary Information
 Metastability in lipid based particles exhibits temporally deterministic and controllable behavior - Supplementary Information Guy Jacoby, Keren Cohen, Kobi Barkan, Yeshayahu Talmon, Dan Peer, Roy Beck
Metastability in lipid based particles exhibits temporally deterministic and controllable behavior - Supplementary Information Guy Jacoby, Keren Cohen, Kobi Barkan, Yeshayahu Talmon, Dan Peer, Roy Beck
Supporting Information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 018 Supporting Information Modulating Interactions between Ligand-Coated Nanoparticles and Phase-Separated
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 018 Supporting Information Modulating Interactions between Ligand-Coated Nanoparticles and Phase-Separated
Lipids fatty, oily, or waxy hydrophobic organic compounds.
 Lipids Lipids Lipids fatty, oily, or waxy hydrophobic organic compounds. u long hydrocarbon chain u composed of CHO Diverse group u fats u oils u waxes u steroids Do not form polymers u big molecules made
Lipids Lipids Lipids fatty, oily, or waxy hydrophobic organic compounds. u long hydrocarbon chain u composed of CHO Diverse group u fats u oils u waxes u steroids Do not form polymers u big molecules made
Supplementary Information: Liquid-liquid phase coexistence in lipid membranes observed by natural abundance 1 H 13 C solid-state NMR
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the wner Societies 28 Supplementary Information: Liquid-liquid phase coexistence in lipid membranes observed
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the wner Societies 28 Supplementary Information: Liquid-liquid phase coexistence in lipid membranes observed
Lipids and Membranes
 Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Lipids and Membranes I. overview Lipids are related
Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Lipids and Membranes I. overview Lipids are related
Lipids are macromolecules, but NOT polymers. They are amphipathic composed of a phosphate head and two fatty acid tails attached to a glycerol
 d 1 2 Lipids are macromolecules, but NOT polymers. They are amphipathic composed of a phosphate head and two fatty acid tails attached to a glycerol backbone. The phosphate head group is hydrophilic water
d 1 2 Lipids are macromolecules, but NOT polymers. They are amphipathic composed of a phosphate head and two fatty acid tails attached to a glycerol backbone. The phosphate head group is hydrophilic water
1.4 Page 1 Cell Membranes S. Preston 1
 AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.3 Cell Membranes and Transport Page 1 1.3 Cell Membranes and Transport from your syllabus l. Cell Membrane Structure 1. Read and
AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.3 Cell Membranes and Transport Page 1 1.3 Cell Membranes and Transport from your syllabus l. Cell Membrane Structure 1. Read and
Application Note. Authors. Abstract. Petrochemical
 Fast screening of impurities in biodiesel using the Agilent 160 Infinity Analytical SFC System in combination with evaporative light scattering detection Application Note Petrochemical Authors Maria Rambla-Alegre,
Fast screening of impurities in biodiesel using the Agilent 160 Infinity Analytical SFC System in combination with evaporative light scattering detection Application Note Petrochemical Authors Maria Rambla-Alegre,
Chapter 2 Transport Systems
 Chapter 2 Transport Systems The plasma membrane is a selectively permeable barrier between the cell and the extracellular environment. It permeability properties ensure that essential molecules such as
Chapter 2 Transport Systems The plasma membrane is a selectively permeable barrier between the cell and the extracellular environment. It permeability properties ensure that essential molecules such as
Interactions of Polyethylenimines with Zwitterionic and. Anionic Lipid Membranes
 Interactions of Polyethylenimines with Zwitterionic and Anionic Lipid Membranes Urszula Kwolek, Dorota Jamróz, Małgorzata Janiczek, Maria Nowakowska, Paweł Wydro, Mariusz Kepczynski Faculty of Chemistry,
Interactions of Polyethylenimines with Zwitterionic and Anionic Lipid Membranes Urszula Kwolek, Dorota Jamróz, Małgorzata Janiczek, Maria Nowakowska, Paweł Wydro, Mariusz Kepczynski Faculty of Chemistry,
CHAPTER 28 LIPIDS SOLUTIONS TO REVIEW QUESTIONS
 28 09/16/2013 17:44:40 Page 415 APTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents
28 09/16/2013 17:44:40 Page 415 APTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents
Lecture-3. Water and Phospholipid
 Lecture-3 Water and Phospholipid Life on earth began in water and evolved there for three billion years before spreading onto land. Although most of the water in liquid form, it is also in solid form and
Lecture-3 Water and Phospholipid Life on earth began in water and evolved there for three billion years before spreading onto land. Although most of the water in liquid form, it is also in solid form and
Bell Work The stomach is located between the esophagus and the small intestine. The stomach secretes digestive enzymes and strong acids to aid in the
 Bell Work The stomach is located between the esophagus and the small intestine. The stomach secretes digestive enzymes and strong acids to aid in the digestion of food, playing a very important role in
Bell Work The stomach is located between the esophagus and the small intestine. The stomach secretes digestive enzymes and strong acids to aid in the digestion of food, playing a very important role in
Photochemical Applications to the Study of Complexity Phospholipid Bilayer Environments
 Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2006 Photochemical Applications to the Study of Complexity Phospholipid Bilayer Environments Christopher John
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2006 Photochemical Applications to the Study of Complexity Phospholipid Bilayer Environments Christopher John
Ch7: Membrane Structure & Function
 Ch7: Membrane Structure & Function History 1915 RBC membranes studied found proteins and lipids 1935 membrane mostly phospholipids 2 layers 1950 electron microscopes supported bilayer idea (Sandwich model)
Ch7: Membrane Structure & Function History 1915 RBC membranes studied found proteins and lipids 1935 membrane mostly phospholipids 2 layers 1950 electron microscopes supported bilayer idea (Sandwich model)
Phase Behavior of Stratum Corneum Lipid Mixtures Based on Human Ceramides: The Role of Natural and Synthetic Ceramide 1
 Phase Behavior of Stratum Corneum Lipid Mixtures Based on Human Ceramides: The Role of Natural and Synthetic Ceramide 1 Joke A. Bouwstra,* Gert S. Gooris,* Frank E. R. Dubbelaar,* and Maja Ponec² *Leiden/Amsterdam
Phase Behavior of Stratum Corneum Lipid Mixtures Based on Human Ceramides: The Role of Natural and Synthetic Ceramide 1 Joke A. Bouwstra,* Gert S. Gooris,* Frank E. R. Dubbelaar,* and Maja Ponec² *Leiden/Amsterdam
Cell membrane & Transport. Dr. Ali Ebneshahidi Ebneshahidi
 Cell membrane & Transport Dr. Ali Ebneshahidi Cell Membrane To enclose organelles and other contents in cytoplasm. To protect the cell. To allow substances into and out of the cell. To have metabolic reactions
Cell membrane & Transport Dr. Ali Ebneshahidi Cell Membrane To enclose organelles and other contents in cytoplasm. To protect the cell. To allow substances into and out of the cell. To have metabolic reactions
The main biological functions of the many varied types of lipids include: energy storage protection insulation regulation of physiological processes
 Big Idea In the biological sciences, a dehydration synthesis (condensation reaction) is typically defined as a chemical reaction that involves the loss of water from the reacting molecules. This reaction
Big Idea In the biological sciences, a dehydration synthesis (condensation reaction) is typically defined as a chemical reaction that involves the loss of water from the reacting molecules. This reaction
Model for measurement of water layer thickness under lipid bilayers by surface plasmon resonance
 Model for measurement of water layer thickness under lipid bilayers by surface plasmon resonance Koyo Watanabe Unit of Measurement Technology, CEMIS-OULU, University of Oulu, PO Box 51, 87101 Kajaani,
Model for measurement of water layer thickness under lipid bilayers by surface plasmon resonance Koyo Watanabe Unit of Measurement Technology, CEMIS-OULU, University of Oulu, PO Box 51, 87101 Kajaani,
Inorganic compounds: Usually do not contain carbon H 2 O Ca 3 (PO 4 ) 2 NaCl Carbon containing molecules not considered organic: CO 2
 Organic Chemistry The study of carbon-containing compounds and their properties. Biochemistry: Made by living things All contain the elements carbon and hydrogen Inorganic: Inorganic compounds: All other
Organic Chemistry The study of carbon-containing compounds and their properties. Biochemistry: Made by living things All contain the elements carbon and hydrogen Inorganic: Inorganic compounds: All other
CHAPTER 28 LIPIDS SOLUTIONS TO REVIEW QUESTIONS
 HAPTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents and their insolubility
HAPTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents and their insolubility
Dr. Nafith Abu Tarboush
 4 Dr. Nafith Abu Tarboush June 24 th 2013 Ahmad Moayd 1 Definition and general properties refer to slide no. 2 Lipids: macromolecules made from Alcohol and Fatty acid bonded by ester linkage. Amphipathic
4 Dr. Nafith Abu Tarboush June 24 th 2013 Ahmad Moayd 1 Definition and general properties refer to slide no. 2 Lipids: macromolecules made from Alcohol and Fatty acid bonded by ester linkage. Amphipathic
Cell Boundaries. Chapter 7.3 Strand: B2.5h
 Cell Boundaries Chapter 7.3 Strand: B2.5h Review: Cell Membrane What is the role of the cell membrane within a cell? The cell membrane regulates what enters and leaves the cell and also provides protection
Cell Boundaries Chapter 7.3 Strand: B2.5h Review: Cell Membrane What is the role of the cell membrane within a cell? The cell membrane regulates what enters and leaves the cell and also provides protection
What do you remember about the cell membrane?
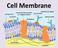 Cell Membrane What do you remember about the cell membrane? Cell (Plasma) Membrane Separates the internal environment of the cell from the external environment All cells have a cell membrane Selectively
Cell Membrane What do you remember about the cell membrane? Cell (Plasma) Membrane Separates the internal environment of the cell from the external environment All cells have a cell membrane Selectively
MCQS ON LIPIDS. Dr. RUCHIKA YADU
 MCQS ON LIPIDS Dr. RUCHIKA YADU Q1. THE FATS AND OILS ARE RESPECTIVELY RICH IN a) Unsaturated fatty acids b) Saturated fatty acids c) Saturated and unsaturated fatty acids d) None of these Q2. ESSENTIAL
MCQS ON LIPIDS Dr. RUCHIKA YADU Q1. THE FATS AND OILS ARE RESPECTIVELY RICH IN a) Unsaturated fatty acids b) Saturated fatty acids c) Saturated and unsaturated fatty acids d) None of these Q2. ESSENTIAL
15.1 Lipids 15.2 Fatty Acids. Copyright 2009 by Pearson Education, Inc.
 Chapter 15 Lipids 15.1 Lipids 15.2 Fatty Acids Copyright 2009 by Pearson Education, Inc. 1 Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but
Chapter 15 Lipids 15.1 Lipids 15.2 Fatty Acids Copyright 2009 by Pearson Education, Inc. 1 Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but
Liquid crystals; biological and artificial membranes
 Liquid crystals; biological and artificial membranes Dr. István Voszka Liquid crystals: Intermediate state between liquids and crystalline solids anisotropic liquids. (anisotropy = the physical properties
Liquid crystals; biological and artificial membranes Dr. István Voszka Liquid crystals: Intermediate state between liquids and crystalline solids anisotropic liquids. (anisotropy = the physical properties
Paper 4. Biomolecules and their interactions Module 22: Aggregates of lipids: micelles, liposomes and their applications OBJECTIVE
 Paper 4. Biomolecules and their interactions Module 22: Aggregates of lipids: micelles, liposomes and their applications OBJECTIVE The main aim of this module is to introduce the students to the types
Paper 4. Biomolecules and their interactions Module 22: Aggregates of lipids: micelles, liposomes and their applications OBJECTIVE The main aim of this module is to introduce the students to the types
Cell Membranes and Signaling
 5 Cell Membranes and Signaling Concept 5.1 Biological Membranes Have a Common Structure and Are Fluid A membrane s structure and functions are determined by its constituents: lipids, proteins, and carbohydrates.
5 Cell Membranes and Signaling Concept 5.1 Biological Membranes Have a Common Structure and Are Fluid A membrane s structure and functions are determined by its constituents: lipids, proteins, and carbohydrates.
Lipids. Lipids: a Diverse group of chemicals. Storage Lipids: derivatives of fatty acids. 11/21/10
 1 Lipids Lehninger 3 rd ed. Chapter 11 (For biosynthesis see Chapter 21) 2 Lipids: a Diverse group of chemicals Insolubility in water. Fats and oils: energy stores. Phospholipids and sterols: structural
1 Lipids Lehninger 3 rd ed. Chapter 11 (For biosynthesis see Chapter 21) 2 Lipids: a Diverse group of chemicals Insolubility in water. Fats and oils: energy stores. Phospholipids and sterols: structural
Chem 431A-L25-F 07 admin: Last time: : soaps, DG s and phospholipids, sphingolipids and cholesterol.
 Chem 431A-L25-F'07 page 1 of 5 Chem 431A-L25-F 07 admin: Last time: : soaps, DG s and phospholipids, sphingolipids and cholesterol. Today: distinguish between various lipids specific lipids and their structures.
Chem 431A-L25-F'07 page 1 of 5 Chem 431A-L25-F 07 admin: Last time: : soaps, DG s and phospholipids, sphingolipids and cholesterol. Today: distinguish between various lipids specific lipids and their structures.
2.2 Properties of Water
 2.2 Properties of Water I. Water s unique properties allow life to exist on Earth. A. Life depends on hydrogen bonds in water. B. Water is a polar molecule. 1. Polar molecules have slightly charged regions
2.2 Properties of Water I. Water s unique properties allow life to exist on Earth. A. Life depends on hydrogen bonds in water. B. Water is a polar molecule. 1. Polar molecules have slightly charged regions
Biology. Chapter 3. Molecules of Life. Concepts and Applications 9e Starr Evers Starr
 Biology Concepts and Applications 9e Starr Evers Starr Chapter 3 Molecules of Life 2015 3.1 What Are the Molecules of Life? The molecules of life contain a high proportion of carbon atoms: Complex carbohydrates
Biology Concepts and Applications 9e Starr Evers Starr Chapter 3 Molecules of Life 2015 3.1 What Are the Molecules of Life? The molecules of life contain a high proportion of carbon atoms: Complex carbohydrates
Membrane Structure and Function
 Membrane Structure and Function Chapter 7 Objectives Define the following terms: amphipathic molecules, aquaporins, diffusion Distinguish between the following pairs or sets of terms: peripheral and integral
Membrane Structure and Function Chapter 7 Objectives Define the following terms: amphipathic molecules, aquaporins, diffusion Distinguish between the following pairs or sets of terms: peripheral and integral
Topical Preparations
 Topical Preparations One of the functions of the skin is to protect the internal body components against the external environment and thus to control the passage of chemicals into and out of the body.
Topical Preparations One of the functions of the skin is to protect the internal body components against the external environment and thus to control the passage of chemicals into and out of the body.
Acid/Base chemistry. NESA Biochemistry Fall 2001 Review problems for the first exam. Complete the following sentences
 1 NESA Biochemistry Fall 2001 eview problems for the first exam Acid/Base chemistry 1. 2 3 is a weak acid. 2. The anion of a weak acid is a weak base 3. p is the measure of a solutions acidity. 4. 3 and
1 NESA Biochemistry Fall 2001 eview problems for the first exam Acid/Base chemistry 1. 2 3 is a weak acid. 2. The anion of a weak acid is a weak base 3. p is the measure of a solutions acidity. 4. 3 and
The Cell Membrane. Usman Sumo Friend Tambunan Arli Aditya Parikesit. Bioinformatics Group Faculty of Mathematics and Science University of Indonesia
 The Cell Membrane Usman Sumo Friend Tambunan Arli Aditya Parikesit Bioinformatics Group Faculty of Mathematics and Science University of Indonesia Overview Cell membrane separates living cell from nonliving
The Cell Membrane Usman Sumo Friend Tambunan Arli Aditya Parikesit Bioinformatics Group Faculty of Mathematics and Science University of Indonesia Overview Cell membrane separates living cell from nonliving
Lipids are used to store and excess energy from extra carbohydrates in animals
 Lipids Lipids are a major source of energy used by cells, however lipids are more difficult for your body to break down. They produce nearly twice the amount of energy than proteins or carbohydrates. Lipids
Lipids Lipids are a major source of energy used by cells, however lipids are more difficult for your body to break down. They produce nearly twice the amount of energy than proteins or carbohydrates. Lipids
Membrane Structure and Membrane Transport of Small Molecules. Assist. Prof. Pinar Tulay Faculty of Medicine
 Membrane Structure and Membrane Transport of Small Molecules Assist. Prof. Pinar Tulay Faculty of Medicine Introduction Cell membranes define compartments of different compositions. Membranes are composed
Membrane Structure and Membrane Transport of Small Molecules Assist. Prof. Pinar Tulay Faculty of Medicine Introduction Cell membranes define compartments of different compositions. Membranes are composed
What is the intermolecular force present in these molecules? A) London B) dipole-dipole C) hydrogen bonding D) ion-dipole E) None. D.
 REVIEW SHEET CHP 7, FRST AND DEAL 1. (7.1) Types of Attractive Forces (Intermolecular forces (IMF)). IMF s are attractive forces between molecules due to electrostatic attraction. Therefore a molecule
REVIEW SHEET CHP 7, FRST AND DEAL 1. (7.1) Types of Attractive Forces (Intermolecular forces (IMF)). IMF s are attractive forces between molecules due to electrostatic attraction. Therefore a molecule
Introduction to the Study of Lipids
 Introduction to the Study of Lipids Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General
Introduction to the Study of Lipids Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General
Boundary Lipid bilayer Selectively Permeable Fluid mosaic of lipids and proteins Contains embedded proteins
 1 Boundary Lipid bilayer Selectively Permeable Fluid mosaic of lipids and proteins Contains embedded proteins 2 Phosphate head hydrophilic Fatty acid tails hydrophobic Amphipathic Phosphate attracted to
1 Boundary Lipid bilayer Selectively Permeable Fluid mosaic of lipids and proteins Contains embedded proteins 2 Phosphate head hydrophilic Fatty acid tails hydrophobic Amphipathic Phosphate attracted to
TUTORIAL IN SMALL ANGLE X-RAY SCATTERING ANALYSIS
 TUTORIAL IN SMALL ANGLE X-RAY SCATTERING ANALYSIS at the Abdus Salam International Center of Theoretical Physics (ICTP) Heinz Amenitsch Sigrid Bernstorff Michael Rappolt Trieste, 15. May 2006 (14:30-17:15)
TUTORIAL IN SMALL ANGLE X-RAY SCATTERING ANALYSIS at the Abdus Salam International Center of Theoretical Physics (ICTP) Heinz Amenitsch Sigrid Bernstorff Michael Rappolt Trieste, 15. May 2006 (14:30-17:15)
Chemical Level Of Organization
 Chemical Level Of Organization List the Four Chemical Elements that Make Up Most of the Body s Mass Oxygen Carbon Hydrogen Nitrogen Distinguish Between Organic and Inorganic Compounds Organic Compounds:
Chemical Level Of Organization List the Four Chemical Elements that Make Up Most of the Body s Mass Oxygen Carbon Hydrogen Nitrogen Distinguish Between Organic and Inorganic Compounds Organic Compounds:
MEMBRANE STRUCTURE. Lecture 8. Biology Department Concordia University. Dr. S. Azam BIOL 266/
 1 MEMBRANE STRUCTURE Lecture 8 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University Plasma Membrane 2 Plasma membrane: The outer boundary of the cell that separates it from the world
1 MEMBRANE STRUCTURE Lecture 8 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University Plasma Membrane 2 Plasma membrane: The outer boundary of the cell that separates it from the world
Define the terms biopharmaceutics and bioavailability.
 Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Chapter Sections: 3.1 Carbon s Place in the Living World 3.2 Functional Groups 3.3 Carbohydrates 3.4 Lipids 3.5 Proteins 3.
 Chapter Sections: 3.1 Carbon s Place in the Living World 3.2 Functional Groups 3.3 Carbohydrates 3.4 Lipids 3.5 Proteins 3.6 Nucleic Acids Student Goals: By the end of this lecture series, students should
Chapter Sections: 3.1 Carbon s Place in the Living World 3.2 Functional Groups 3.3 Carbohydrates 3.4 Lipids 3.5 Proteins 3.6 Nucleic Acids Student Goals: By the end of this lecture series, students should
The Cell Membrane & Movement of Materials In & Out of Cells PACKET #11
 1 February 26, The Cell Membrane & Movement of Materials In & Out of Cells PACKET #11 Introduction I 2 Biological membranes are phospholipid bilayers with associated proteins. Current data support a fluid
1 February 26, The Cell Membrane & Movement of Materials In & Out of Cells PACKET #11 Introduction I 2 Biological membranes are phospholipid bilayers with associated proteins. Current data support a fluid
Application of Skin-Identical Ceramide 3 for Enhanced Skin Moisturization and Smoothness: Latest Results
 Application of Skin-Identical Ceramide 3 for Enhanced Skin Moisturization and Smoothness: Latest Results By Ute Wollenweber* and Dr. Mike Farwick* Keywords: Ceramides, Stratum corneum, skin moisturization,
Application of Skin-Identical Ceramide 3 for Enhanced Skin Moisturization and Smoothness: Latest Results By Ute Wollenweber* and Dr. Mike Farwick* Keywords: Ceramides, Stratum corneum, skin moisturization,
Chapter - V RESULTS AND DISCUSSION
 Chapter - V RESULTS AND DISCUSSION ANALYTICAL STUDY SCANNING OF DRUG Pure Ketoconazole was scanned in phosphate buffer saline (PBS) ph 7.4 and 10% methanol between 200 nm and 400 nm using uv-visible spectrophotometer.
Chapter - V RESULTS AND DISCUSSION ANALYTICAL STUDY SCANNING OF DRUG Pure Ketoconazole was scanned in phosphate buffer saline (PBS) ph 7.4 and 10% methanol between 200 nm and 400 nm using uv-visible spectrophotometer.
Insights into the Molecular Organization of Lipids in the Skin Barrier from Infrared Spectroscopy Studies of Stratum Corneum Lipid Models
 Acta Derm Venereol 2000; Supp 208: 16±22 Insights into the Molecular Organization of Lipids in the Skin Barrier from Infrared Spectroscopy Studies of Stratum Corneum Lipid Models DAVID J. MOORE and MARK
Acta Derm Venereol 2000; Supp 208: 16±22 Insights into the Molecular Organization of Lipids in the Skin Barrier from Infrared Spectroscopy Studies of Stratum Corneum Lipid Models DAVID J. MOORE and MARK
Chemistry 304B Spring 1999 Lecture 24 1
 Chemistry 304B Spring 1999 Lecture 24 1 Exam: Monday evening, 7:30-10 pm, McCosh 50 pen book, etc. eview sessions: Today: 5-6 pm m 124 Frick Sunday: 8-9:30 pm m 324 Frick Monday lecture 9 am, m 120 Frick
Chemistry 304B Spring 1999 Lecture 24 1 Exam: Monday evening, 7:30-10 pm, McCosh 50 pen book, etc. eview sessions: Today: 5-6 pm m 124 Frick Sunday: 8-9:30 pm m 324 Frick Monday lecture 9 am, m 120 Frick
Draw and label a diagram to show the structure of membranes
 2.4 Membranes 2.4.1 - Draw and label a diagram to show the structure of membranes Phospholipid Bilayer - This is arranged with the hydrophilic phosphate heads facing outwards, and the hydrophobic fatty
2.4 Membranes 2.4.1 - Draw and label a diagram to show the structure of membranes Phospholipid Bilayer - This is arranged with the hydrophilic phosphate heads facing outwards, and the hydrophobic fatty
Lipids do not like water! (aka: hydrophobic) Generally insoluble
 Lipids Lipids Lipids do not like water! (aka: hydrophobic) Generally insoluble Lipids They act like this because of their molecular structure (non-polar) Lipids are made mostly of C and H atoms, with O
Lipids Lipids Lipids do not like water! (aka: hydrophobic) Generally insoluble Lipids They act like this because of their molecular structure (non-polar) Lipids are made mostly of C and H atoms, with O
Structure of Dipalmitoylphosphatidylcholine/Cholesterol Bilayer at Low and High Cholesterol Concentrations: Molecular Dynamics Simulation
 Biophysical Journal Volume 77 October 1999 2075 2089 2075 Structure of Dipalmitoylphosphatidylcholine/Cholesterol Bilayer at Low and High Cholesterol Concentrations: Molecular Dynamics Simulation Alexander
Biophysical Journal Volume 77 October 1999 2075 2089 2075 Structure of Dipalmitoylphosphatidylcholine/Cholesterol Bilayer at Low and High Cholesterol Concentrations: Molecular Dynamics Simulation Alexander
