Critique of evidence put forward by Servier suggesting an association between acid-suppressive medication and fracture risk. Dr Myfanwy Lloyd Jones
|
|
|
- Hannah Pope
- 5 years ago
- Views:
Transcription
1 Critique of evidence put forward by Servier suggesting an association between acid-suppressive medication and fracture risk Dr Myfanwy Lloyd Jones February 2008
2 Background Proton pump inhibitors (PPIs) and histamine 2 receptor antagonists (H2RAs) are both acid-suppressive medications. They are licensed for use both as short-term treatments for gastric and duodenal ulcers, and to relieve gastro-oesophageal reflux disease and also, in the case of PPIs, dyspepsia. 1 Gastrointestinal disorders are the adverse events most commonly reported in connection with bisphosphonate therapy for osteoporosis or osteopenia; they appear to be more commonly reported in patients taking alendronate or risedronate rather than etidronate. 2 An Australian case-control study found that, after controlling for previous NSAID use, new users with no recent H2RA or PPI use who were prescribed bisphosphonates (primarily alendronate) in general practice were significantly more likely than matched controls who were prescribed other medications to require acid suppression agents (H2RAs, PPIs or other antacids) within 6 weeks of their prescription (odds ratio 3.21, 95% CI ). 3 Servier claim that acid-suppressing medication significantly reduces, if not completely negates, the anti-fracture benefits of bisphosphonate treatment. 4 They refer to three papers in support of this claim: A case-control study by Vestergaard et al. 5 A nested case-control study by Yang et al. 6 A prospective cohort study by Yu et al. 7 They also present some results from a retrospective cohort study using the General Practice Research Database. 4 This study has been published in abstract form by de Vries et al. 8 All four studies are controlled observational studies. This is appropriate: most RCTs are too small to detect adverse events which are either rare or take a long time to develop, 9 and it is unlikely that any RCT of a PPI would have been powered to detect a significant difference in the incidence of clinical fracture. However, observational studies suffer from the inherent drawback that, because the subjects select themselves, or are deliberately selected by a physician, for exposure to the putative harmful agent, they may differ from nonexposed persons with respect to important determinants of outcome other than, or additional to, the exposure of interest. Attempts to document subject characteristics and either demonstrate comparability or use statistical techniques to adjust for differences will only account for those prognostic factors (confounders) which the investigators thought of and also measured, 10 and thus all observational studies are susceptible to unmeasured confounders. In the current example, it is impossible to rule out the theoretical possibility that the risk of fracture in patients prescribed acid-suppressive medication is associated with the condition which required the use of such medication rather than with the medication itself. Thus, if the condition which required acid-suppressive medication predated bisphosphonate use, any associated risk of fracture would not be reduced by use of an anti-osteoporosis agent other than a bisphosphonate. There is a hierarchy of evidence within observational studies, with cohort studies generally recognised as providing higher quality evidence than case-control studies.
3 Those cohort studies which are planned in advance, and which follow their subjects prospectively, are likely to be more reliable than those which are undertaken retrospectively, both because the data collection is planned and is therefore more likely to be uniformly reliable and complete, and because the selection of participants is unlikely to be influenced by their outcomes. 11 Because case-control studies identify people who have already developed the outcome of interest (cases), and match them with people who do not have that outcome but who are otherwise similar to cases in respect to important determinants of outcome such as age, sex, concurrent medical conditions (controls), their quality depends largely on the appropriateness of the choice of control group. 11. Bias may also be introduced because the relative frequency of exposure to the putative harmful agent in cases and controls is assessed respectively. Studies which depend on asking subjects about past exposure are particularly susceptible to recall bias (whereby cases may be more likely than controls to remember exposure to potentially harmful agents) and interviewer bias (whereby the interviewer may probe for information more actively in cases than in controls). However, such bias may be avoided if the information is obtained from routinely collected data, 10 as in both case-control studies assessed here. In a nested case-control studies, the cases are identified within a cohort which was defined before the case-control study began. Because information relating to the cohort is collected prospectively, such case-control studies are potentially less susceptible to recall bias than non-nested case-control studies. However, they have the drawback that, because of deaths and losses to follow-up, the controls may not be fully representative of the original cohort. As noted above, in both cohort and case-control studies statistical techniques may be used to adjust for differences between cases and controls in the prevalence of confounders (potential prognostic factors, other than the exposure being studied, which may affect the outcome of interest). Variables which are known securely, without the possibility of conceptual or measurement error, (eg age or sex) can be used with more confidence than those such as smoking or diabetes which are measured less well because duration and severity are not taken into account: the latter will leave unquantifiable residual confounding. It has therefore been suggested that ideally, to minimise the degree of residual confounding, analyses should include only those cases and controls who do not have any such insecurely-known potential prognostic factors. 9
4 Summary and critique of study design Study quality was assessed using quality criteria based on the CRD criteria for cohort and case-control studies, 11 the CASP checklists for cohort 12 and case-control 13 studies, and the SIGN methodology checklists for cohort 14 and case-control 15 studies. The key features of each study are summarised and critiqued below. Vestergaard et al This case-control study addressed the question whether the risk of fracture was different in users of PPIs, H2RAs, and other types of acid-suppressive medication from that in never-users of antacids. It thus cannot distinguish between any difference in risk associated with acid-suppressive medication or with the underlying conditions which necessitated the use of such medication. The cases were all 124,655 men and women who sustained any clinical fracture in Denmark in the year They were identified from routine hospital discharge diagnoses of fracture in the National Hospital Discharge Register. These diagnoses had previously been shown to have a validity of around 93% in relation to hip fractures; their validity in relation to other fracture sites is not known. As fracture diagnosis was independent of any inquiry into the use of acid-suppressive medication, it seems unlikely that it could have been influenced by knowledge of exposure to such substances. Three controls per case, matched only for age and gender, who were not recorded in the National Hospital Discharge Register as having had a fracture during the relevant period, were randomly selected from the background population. It is not clear from what database or register they were sampled. As the validity of fracture discharge diagnoses was not 100%, it is possible that some controls may have suffered a fracture during the relevant period. It is not clear from the study as published whether any cases or controls refused to participate in the study. However, it seems likely that only routinely-collected data were used, and that therefore consent and active participation were not required, and participation rates may have been 100% in both groups. Because cases and controls were only matched for age and gender, they were not wholly comparable with respect to potential confounding factors. Cases were more likely than controls to be retired, and to be living alone; they had higher comorbidity and were more likely to use drugs associated with an increased risk of fracture (corticosteroids, antiepileptics, anxiolytics and sedatives, neuroleptics, and antidepressants). Exposure to acid-suppressive medications was measured using the proxy measure of the cumulated number of defined daily dosages (DDDs) redeemed over a 4 to 5 year period (from 1 st January 1996 to the occurrence of fracture or equivalent date for controls in 2000), using routinely-collected prescription data from the Danish Medicines Agency. This is an objective measure, with no possibility of recall bias. However, it could not account for any patients who redeemed but did not take the medication, although such non-compliance was assumed to be relatively rare because
5 patients paid part of the drug cost when they redeemed the prescription. It also could not take into account drugs administered during episodes of hospitalisation, but this was felt to be a minor factor as the study population did not spend many days in hospital. More seriously, it could not take into account medications available over the counter (OTC) as these could not be linked to a named individual. The investigators sought to overcome this problem (which affected all acid-suppressive medications other than the prescription-only PPIs) by comparing consumption of prescribed acidsuppressive medications among the study population with sales of prescription and OTC acid-suppressive medication among the general population. Data on other potentially relevant exposures (the occurrence of other diseases, and of alcoholism) were obtained from national registers. However, the authors recognised that the fact that H2RAs and other acid-suppressive medications were available over the counter may have led to an underestimation of the risk associated with those drugs. The measure of effect used was the odds ratio, adjusted for alcoholism, working or not, the Charlson index (an index of 19 comorbid conditions), ever-use of antiepileptics, ever-use of anxiolytics or sedatives, ever-use of antidepressants, everuse of neuroleptics, ever-use of corticosteroids, number of bed days in 1999, number of contacts with a GP or specialist in 1999, living with someone or living alone, prior fracture, education level, and income in Potentially important confounding factors which were not adjusted for include smoking, physical activity, differences in body weight, use of calcium/vitamin D supplements, and sun exposure. The actual numbers of cases and controls who received PPIs, H2RAs or other acid-suppressive medications (ie the numbers underlying the crude odds ratio) were not reported. Yang et al This was a nested case-control study which sought to explore the association between PPI therapy and risk of hip fracture, and compare it with the risk associated with H2RA therapy. It was conducted using data from the General Practice Research Database (GPRD) for the period 1987 to The GPRD contains the computerised medical records of 683 general practices in the UK, and represents about 6% of the UK population, 16 of which it is broadly representative in terms of age and gender. 17 The GPRD contains demographic data, information about prescriptions, clinical events, preventive care, specialist referrals, hospital admissions, and major outcomes for each patient. 16 The cohort within which the case-control study was nested consisted of men and women who were aged 50 years or over at the time of database enrolment; who had at least 365 days of up-to-standard database follow-up; who had either received at least one prescription for PPIs, or at least one prescription for H2RAs but none for PPIs, during their up-to-standard database follow-up, or had no documented prescription for PPI or H2RA therapy; who had not received PPI or H2RA therapy exclusively during non-up-to-standard periods of database follow-up; and who had not had a documented hip fracture before, or during the first year of, up-to-standard database follow-up. The 13,556 cases were variously said to be all patients within that cohort who suffered a first incident hip fracture (other than distal femoral fracture) at least a year after the start of their up-to-standard database follow-up, or to have been sampled from the cohort using incidence density sampling. The GPRD diagnoses of hip fracture had previously been shown to have a validity of over 90%. As fracture diagnosis was
6 independent of any inquiry into the use of acid-suppressive medications, it seems unlikely that it could have been influenced by knowledge of exposure to such medications. One to ten controls per case were drawn from the same cohort as the cases, using incidence density sampling and matching for sex, index date, year of birth, and both calendar period and duration of up-to-standard database follow-up before the index date. It is not clear why the number of controls per case varied. As the validity of fracture diagnosis was not 100%, it is possible that some controls may have suffered a fracture during the relevant period. As only pre-existing data were used, informed consent and active participation were not required. Participation rates may therefore in theory have been as high as 100% in both groups. In practice, however, patients may have died or moved away from their GPRD-participating practice, and the number lost to follow-up in this way is not quantified. As drop-out rates are not presented, it is not possible to determine whether they were similar in exposed and unexposed groups. Although cases and controls were matched for a number of potential confounding factors, they were not wholly comparable: cases were more likely than controls to have received medications or to have medical diagnoses with known associations with either osteoporosis or the risk of falling. The primary exposures of interest were PPI or H2RA therapy of more than one year duration before the index date. Individual periods of PPI or H2RA exposure were determined by a proxy measure, namely the intended duration of each prescription recorded in the GPRD. Participants who had received both PPIs and H2RAs were considered in the primary analysis for PPIs only. PPI and H2RA use was thus measured objectively, with no possibility of recall bias. However, it did not take into account any patients who either failed to redeem the prescription, or who redeemed it but did not then take the medication. The possibility that the PPI and H2RA groups contain antacid non-users is therefore higher than in the study by Vestergaard et al. 5 in which the treatment groups did not include patients who failed to redeem their prescription. As in the study by Vestergaard et al., it was not possible to take into account the possible use of OTC acid-suppressive medications, and this may have led to an underestimation of the risk associated with PPIs and H2RAs. The measure of effect used was the odds ratio. Conditional logistic regression was used to estimate the unadjusted and adjusted odds ratios. The potential confounders which were included in the analysis included BMI, smoking history, alcoholism, health conditions (congestive heart failure, cerebrovascular accident, dementia, impaired mobility, myocardial infarction, COPD or asthma, peptic ulcer disease, PVD, rheumatoid arthritis, vision loss, coeliac disease, Paget disease, osteomalacia, chronic renal failure, Cushing disease, inflammatory bowel disease, seizure disorder, or fracture >3 months before the index date), and use of specific medications (anxiolytics, antidepressants, antiparkinsonian drugs, thiazide diuretics, statins, corticosteroids, hormone therapy, calcitonin, NSAIDs, anticonvulsants, thyroxine, and calcium and vitamin D supplementation). Potentially important confounding factors which were not adjusted for include physical activity, sun exposure, and supplementation with OTC calcium/vitamin D. The actual numbers of participants in
7 each group who received PPIs, H2RAs or other acid-suppressive medications (ie the numbers underlying the crude odds ratio) were not reported. de Vries et al This was a retrospective cohort study which sought to compare the fracture risk in patients taking a bisphosphonate plus acid-suppressive medication with that in those taking a bisphosphonate alone. As this study has been published only in abstract form, although Servier have provided some additional findings 4 very little detail is available, and a number of questions remain unanswered. The study used data from the General Practice Research Database (GPRD) on 67,309 men and women aged 40 or over who were starting treatment with bisphosphonates; 20.1% of these had been prescribed PPIs and 7.5% H2RAs. The time period within which bisphosphonate therapy was initiated is not clear, nor is it clear whether the cohort represented all men and women in the GPRD who were prescribed bisphosphonates during that time period. As only pre-existing data were used, informed consent and active participation were presumably not required, and participation rates may in theory have been as high as 100% in both groups. In practice, however, patients may have died or moved away from their GPRDparticipating practice, and the number lost to follow-up in this way is not quantified. There is insufficient information to determine whether the two groups were comparable in terms of potential prognostic factors other than use of acid-suppressive medication. In particular, it is not clear whether they were balanced in terms of disease progression: bisphosphonates may be prescribed for the primary or secondary prevention of osteoporosis, and as the risk of future fracture is lower in primary than in secondary prevention, if the proportion of patients being prescribed bisphosphonates for secondary osteoporosis was not comparable in each group, this would potentially invalidate the study findings. Similarly, patients prescribed bisphosphonates to prevent osteoporotic fracture secondary to other conditions (eg those requiring treatment with corticosteroids) will be at particularly high risk of fracture, and evidence is required, but not available, that they formed a comparable proportion of each of the two groups. Drop-out rates are not presented, and it is therefore not possible to determine whether they were similar in exposed and unexposed groups. It is not specified how exposure to acid-suppressive medication was measured. Presumably, however, as in the study by Yang et al., 6 it was determined by a proxy measure, namely prescriptions for PPIs and H2RAs recorded in the GPRD. Although this is an objective measure, it will not take into account any patients who either failed to redeem the prescription, or who redeemed it but did not then take the medication. It also fails to address the possible use of OTC acid-suppressive medication. No indication is given regarding length of therapy. The outcome of interest was not clearly specified, but was presumably any clinical fracture recorded in the GPRD during the relevant (unspecified) time period. The length of the follow-up period is not clear. The measure of effect used was the relative rate, adjusted using time-dependent Cox regression. The actual numbers of women in
8 each group who suffered fractures (ie the numbers underlying the relative rate) were not reported. GPRD data can be used to quantify the absolute risks of adverse events, which casecontrol studies cannot. 17 However, neither de Vries et al. nor Servier did so. Yu et al This was a prospective cohort study which addressed the question of whether PPI and/or H2RA use was associated with changes in BMD and in the risk of nonvertebral fracture in postmenopausal women who were not taking bisphosphonates or other antiosteoporotic agents. The cohort of 3432 women was recruited from women enrolled in the Study of Osteoporotic Fractures (ie 9704 non-black women aged >65 years recruited from population-based listings and health maintenance membership lists at four sites in the USA between Oct 1986 and Oct ). Within this cohort, comparisons were made between PPI users, H2RA users, and non-users of acid-suppressive medication. As no information is provided regarding participation rates at enrolment, it is not clear how representative the cohort is of the population of interest. There is insufficient information to determine whether the two groups were comparable in terms of potential prognostic factors. Exposure was measured by assessment of current PPI and/or H2RA use. It is not clear how this was done, and no indication is given as to how long, on average, current users had been using PPIs or H2RAs. The outcomes of interest were bone loss (apparently assessed by total hip BMD), and hip and other nonvertebral fractures. The fractures were confirmed by central adjudication of x-ray reports; it would appear from another article 18 that they were initially reported by the participants themselves. Thus, while central confirmation of fracture could have been blinded to exposure status, initial reporting of the fracture would not have been. Mean follow-up for BMD data was 4.9 years, when only 72% of the cohort were available to provide information about medication use. Mean follow-up for fracture data was 7.5 years; no information is provided about the number of participants who provided data at that point. Implicitly, drop-out was mainly due to the unavailability of repeat BMD measurements and information about medication use. No information is provided as to whether drop-out rates were comparable in the exposed and unexposed groups. 188 women who had not dropped out were excluded at the follow-up visit because they reported use of bisphosphonates or other osteoporosis medications; as they were women who had either suffered a fracture or were at increased risk of doing so, if they were not distributed between the study cohorts in equal proportions, their exclusion would distort the study findings. The measure of effect used was the relative hazard. Linear regression and timedependent proportional hazards regression were used to adjust for age, ethnicity, BMI, calcium intake, health status, exercise, alcohol intake, and use of oestrogens or corticosteroids. It is not clear what comorbidities or medications might be included
9 under the heading of health status. Potentially important confounding factors which were not adjusted for include smoking and sun exposure. The actual numbers of participants in each group who suffered fractures (ie the numbers underlying the relative rate) were not reported. Summary and interpretation of study findings Vestergaard et al Vestergaard et al. found that the use of PPIs within the last year was associated with a small increase in the risk of any fracture (adjusted OR 1.18, 95% CI 1.12, 1.43), as were other antacids (adjusted OR 1.33, 95% CI 1.24, 1.43). This increased risk disappeared if more than a year had elapsed since last use of PPIs (AOR 1.01, 95% CI 0.96, 1.06) or other antacids (AOR 1.02, 95% CI 0.96, 1.08). H2RA use within the last year was associated with a decrease in fracture risk (adjusted OR 0.88, 95% CI 0.82, 0.95). Because the study design could not distinguish between any difference in risk associated with acid-suppressive medication or with the underlying conditions which necessitated the use of such medication, it is not certain from these whether the risk of fracture is increased by the use of PPIs and other acid-suppressive medication, or whether it is increased by the underlying condition but reduced by the use of H2RAs. However, an analysis which stratified antacid use by average daily dose in the year 2000 may support the latter interpretation: it indicated a dose-response relationship for H2RAs and for other antacids, but not for PPIs, suggesting that there may be no causal relationship between PPI use and changes in fracture risk, but that H2RAs may be associated with a decrease, and other antacids with an increase, in fracture risk. As noted earlier, the authors recognise that the availability of H2RAs and other antacids over the counter may have led to an underestimation of the changes in risk associated with those drugs: in other words, H2RAs may be associated with a greater reduction, and other antacids with a greater increase, in fracture risk than indicated above. However, as Yang et al. 6 observe, Vestergaard et al. assessed the dose-response relationship regardless of length of exposure, although short-term use, even at a high dose, is unlikely to have a significant impact on fracture rates. An analysis which stratified antacid use among those who had used such drugs during the previous year by the number of defined daily dosages redeemed from 1 st January 1996 to the date of fracture or censoring failed to demonstrate a duration-response relationship. Yang et al Yang et al. found that more than one year of PPI therapy was associated with an increase in the risk of hip fracture compared with no acid-suppressive therapy (multivariable adjusted OR 1.44, 95% CI 1.30, 1.59), as was more than one year of H2RA use (multivariable adjusted OR 1.23, 95% CI 1.14, 1.39). As some of the PPI users also used H2RAs, a separate analysis was conducted comparing PPI-only users with acid-suppressive medication non-users; this yielded an adjusted odds ratio for hip fracture of 1.62 (95% CI 1.41, 1.89). As noted earlier, the inability of the study design
10 to take into account the use of OTC acid-suppressive medications may have led to an underestimation of the increase in risk associated with those drugs. Long-term PPI use (>1 year of cumulative use) was associated with a higher fracture risk than long-term H2RA use (adjusted OR 1.34, 95% CI 1.14, 1.38). The risk increased with increasing duration of PPI therapy (adjusted OR for one year s therapy 1.22, 95% CI 1.15, 1.30; for 4 years therapy 1.59, 95% CI 1.39, 1.80); it is not clear whether the comparator here is H2RA use or acid-suppressive medication non-use. The association between long-term PPI use and hip fracture was stronger in men (OR 1.78, 95% CI 1.42, 2.22) than in women (OR 1.36, 95% CI 1.22, 1.53). Compared with acid-suppressive medication non-users, a significant dose-response effect was observed among patients who had been prescribed PPI therapy for more than one year: the adjusted OR was 1.40 (95% CI 1.26, 1.54) in those prescribed <1.75 average daily dose but 2.65 (95% CI 1.80, 3.90) for those prescribed >1.75 average daily dose. No such dose-response effect was observed in relation to H2RA use. To exclude the possibility that increased fracture risk was associated with gastrooesophageal reflux disease (GERD), rather than with the medications used to treat it, the investigators conduced an additional analysis restricted to patients with documented chronic GERD (defined as 3 or more diagnoses recorded on different dates in the database). In these patients, the multivariable adjusted OR for hip fracture associated with at least one year s PPI therapy was 1.41 (95% CI 1.02, 1.94), while the corresponding OR for H2RA was 1.21 (95% CI 0.67, 2.22). A dose-response effect was again observed with PPI use. de Vries et al de Vries et al. found that concomitant use of PPIs and bisphosphonates was associated with an increased risk of any fracture (adjusted relative rate 1.08 (1.01, 1.15)) and of hip fracture (ARR 1.21, 95% CI 1.05, 1.38) but not of vertebral fracture (ARR 1.11, 95% CI 0.94, 1.31) compared to bisphosphonate use alone. In contrast, concomitant use of H2RAs and bisphosphonates was associated with an increased risk of vertebral fracture (ARR 1.48, 95% CI 1.17, 1.87) but not of any fracture (ARR 1.07, 95% CI 0.93, 1.22) or of hip fracture (ARR 1.04, 95% CI 0.83, 1.32), compared with bisphosphonate use alone. The inability of the study design to take into account the use of OTC acid-suppressive medications may have led to an underestimation of these changes in fracture risk. However, as no information is available regarding the factors used to produce the adjusted relative rate, other than that they include *********** [in confidence, Servier] and other unspecified factors, 4 it is possible that the findings are invalidated by imbalances between the groups in the proportions of patients receiving bisphosphonates for primary or secondary fracture prevention, and for primary or secondary osteoporosis. De Vries et al stated that consistent dose-dependent trends were observed when data were stratified by daily dose of acid-suppressive medication. Further detail is available in the data submitted by Servier. 4 ********************************** *********************************************************************
11 ********************************************************************* ********************************************** [in confidence, Servier] Yu et al Yu et al. found that, over a mean follow-up period of 7.5 years, using a mulltivariate adjusted relative hazard model, users of acid-suppressant medications were at increased risk of nonvertebral fracture compared with non-users of such medications (1.18, 95% CI 1.01, 1.39). Findings were said to be similar in stratified analyses of PPI users (n=264) and H2RA users (n=594) compared with non-users of acidsuppressant medications. However, statistical significance was not achieved for PPI users. Moreover, without confirmation that the 188 women who were excluded for use of bisphosphonates or other osteoporosis medications were proportionately distributed between the three groups, no confidence can be placed in the findings. The statement that with prolonged follow-up, there was an 18% increase in the risk of non-spine fracture among users of acid-suppressive medications may suggest that no significant increase in fracture risk was seen when BMD outcomes were assessed, after a mean of 4.9 years follow-up. Conclusions The quality of evidence regarding any possible association between the use of acidsuppressive medications and increased risk of fracture is generally poor. Of the two case-control studies, that by Yang et al. 6 found that more than one year of either PPI or H2RA therapy was associated with an increased risk of hip fracture. The risk was higher for long-term PPI use than for long-term H2RA use. However, an analysis conducted to exclude the possibility that the increased fracture risk was associated with the underlying disease for which PPI or H2RA therapy was prescribed failed to demonstrate that H2RA therapy was associated with an increase in fracture risk. The case-control study by Vestergaard et al. 5 did not demonstrate convincingly that fracture risk was increased by PPIs rather than the underlying disease for which they were prescribed, though it did suggest that H2RAs might reduce, and other acidsuppressive medications increase, such risk. Neither of the cohort studies have been reported in full. No confidence may be placed in the results of the study by de Vries et al. 8 because of its failure to demonstrate comparability between exposure groups in terms of key prognostic factors, in particular whether bisphosphonates were prescribed bisphosphonates for primary or secondary fracture prevention, and for primary or secondary osteoporosis. Similarly, confidence cannot be placed in the results of the study by Yu et al. 7 because of their failure to demonstrate that the exposure groups were comparable in terms of exclusions for use of bisphosphonates or other osteoporosis medications.
12 Reference List (1) Anonymous. British National Formulary. Internet [ 2008 Available from: URL: (2) Lloyd Jones M, Wilkinson A. Adverse effects and persistence with therapy in patients taking oral alendronate, etidronate or risedronate: systematic reviews (3) Roughead EE, McGeechan K, Sayer GP. Bisphosphonate use and subsequent prescription of acid suppressants. British Journal of Clinical Pharmacology 2004; 57: (4) Jones T. Letter to NICE (5) Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcified Tissue International 2006; 79: (6) Yang Y-X, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296(24): (7) Yu EW, Shinoff C, Blackwell T, Ensrud K, Hillier T, Bauer DC. Use of acid-suppressive medications and risk of bone loss and fracture in postmenopausal women. Journal of Bone & Mineral Research 2006; 21:S281. (8) de Vries F, Cooper A, Logan R, Cockle S, van Staa T, Cooper C. Fracture risk in pateints receiving concomitant bisphosphonate and acid-suppressive medication on bisphosphonates alone. Osteoporosis International 2007; 18(Suppl 3):S261. (9) Vandenbroucke JP. When are observational studies as credible as randomised trials? Lancet 2004; 363: (10) Levine M, Walter S, Lee H, Holbrook A, Moyer V. Users' guides to the medical literature. IV. How to use an article about harm. JAMA 1994; 271(20): (11) Centre for Reviews and Dissemination. Undertaking systematic reviews of research on effectiveness. CRD's guidance for those carrying out or commissioning reviews (12) Critical Skills Appraisal Programme. 12 questions to help you make sense of a cohort study (13) Critical Skills Appraisal Programme. 11 questions to help you make sense of a case control study
13 (14) SIGN. SIGN 50: A guideline developers' handbook. Methodology checklist 3: cohort studies (15) SIGN. SIGN 50: A guideline developers' handbook. Methodology checklist 4: case-control studies (16) van Staa TP, Dennison EM, Leufkens HGM, Cooper C. Epidemiology of fractures in England and Wales. Bone 2001; 29(6): (17) Walley T, Mantgani A. The UK General Practice Research Database. Lancet 1997; 350: (18) Wainwright SA, Marshall LM, Ensrud KE, Cauley JA, Black DM, Hillier TA et al. Hip fracture in women without osteoporosis. Journal of Clinical Endocrinology and Metabolism 2005; 90(5):
Prescribing of anti-osteoporotic therapies following the use of Proton Pump Inhibitors in general practice
 Prescribing of anti-osteoporotic therapies following the use of Proton Pump Inhibitors in general practice B. McGowan, K. Bennett, J. Feely Department of Pharmacology & Therapeutics, Trinity Centre for
Prescribing of anti-osteoporotic therapies following the use of Proton Pump Inhibitors in general practice B. McGowan, K. Bennett, J. Feely Department of Pharmacology & Therapeutics, Trinity Centre for
Analyses of cost-effective BMD scanning and treatment strategies for generic alendronate, and the costeffectiveness
 Analyses of cost-effective BMD scanning and treatment strategies for generic alendronate, and the costeffectiveness of risedronate and strontium ranelate in those people who would be treated with generic
Analyses of cost-effective BMD scanning and treatment strategies for generic alendronate, and the costeffectiveness of risedronate and strontium ranelate in those people who would be treated with generic
The Incidence of Hip Fracture Associated with Proton Pump Inhibitor (PPI) and/or H2 Receptor Antagonist (H2RA) Use in the Kentucky Medicaid Population
 University of Kentucky UKnowledge MPA/MPP Capstone Projects Martin School of Public Policy and Administration 2011 The Incidence of Hip Fracture Associated with Proton Pump Inhibitor (PPI) and/or H2 Receptor
University of Kentucky UKnowledge MPA/MPP Capstone Projects Martin School of Public Policy and Administration 2011 The Incidence of Hip Fracture Associated with Proton Pump Inhibitor (PPI) and/or H2 Receptor
Risk of Fractures Following Cataract Surgery in Medicare Beneficiaries
 Risk of Fractures Following Cataract Surgery in Medicare Beneficiaries Victoria L. Tseng, MD, Fei Yu, PhD, Flora Lum, MD, Anne L. Coleman, MD, PhD JAMA. 2012;308(5):493-501 Background Visual impairment
Risk of Fractures Following Cataract Surgery in Medicare Beneficiaries Victoria L. Tseng, MD, Fei Yu, PhD, Flora Lum, MD, Anne L. Coleman, MD, PhD JAMA. 2012;308(5):493-501 Background Visual impairment
Osteoporosis/Fracture Prevention
 Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
RESEARCH. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database
 Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database Julia Hippisley-Cox, professor of clinical epidemiology and general practice,
Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database Julia Hippisley-Cox, professor of clinical epidemiology and general practice,
Complications of Proton Pump Inhibitor Therapy. Gastroenterology 2017; 153:35-48 발표자 ; F1 김선화
 Complications of Proton Pump Inhibitor Therapy Gastroenterology 2017; 153:35-48 발표자 ; F1 김선화 Background Proton pump inhibitors (PPIs) are among the most commonly prescribed medicines for gastroesophageal
Complications of Proton Pump Inhibitor Therapy Gastroenterology 2017; 153:35-48 발표자 ; F1 김선화 Background Proton pump inhibitors (PPIs) are among the most commonly prescribed medicines for gastroesophageal
Use of a-blockers and the risk of hip/femur fractures
 Journal of Internal Medicine 2003; 254: 548 554 Use of a-blockers and the risk of hip/femur fractures P. C. SOUVEREIN 1,T.P.VANSTAA 1,2,A.C.G.EGBERTS 1,J.J.M.C.H.DELAROSETTE 3, C. COOPER 2 & H. G. M. LEUFKENS
Journal of Internal Medicine 2003; 254: 548 554 Use of a-blockers and the risk of hip/femur fractures P. C. SOUVEREIN 1,T.P.VANSTAA 1,2,A.C.G.EGBERTS 1,J.J.M.C.H.DELAROSETTE 3, C. COOPER 2 & H. G. M. LEUFKENS
Case Finding and Risk Assessment for Osteoporosis
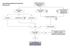 Case Finding and Risk Assessment for Osteoporosis Patient may present as a fragility fracture or risk fracture Fragility fracture age 50 Clinical risk factors aged 50 Very strong clinical risk factors
Case Finding and Risk Assessment for Osteoporosis Patient may present as a fragility fracture or risk fracture Fragility fracture age 50 Clinical risk factors aged 50 Very strong clinical risk factors
The Cost-Effectiveness of Bisphosphonates in Postmenopausal Women Based on Individual Long-Term Fracture Risks
 Volume ** Number ** ** VALUE IN HEALTH The Cost-Effectiveness of Bisphosphonates in Postmenopausal Women Based on Individual Long-Term Fracture Risks Tjeerd-Peter van Staa, MD, MA, PhD, 1,2 John A. Kanis,
Volume ** Number ** ** VALUE IN HEALTH The Cost-Effectiveness of Bisphosphonates in Postmenopausal Women Based on Individual Long-Term Fracture Risks Tjeerd-Peter van Staa, MD, MA, PhD, 1,2 John A. Kanis,
Analyses of the cost-effectiveness of pooled alendronate and risedronate, compared with strontium ranelate, raloxifene, etidronate and teriparatide
 Analyses of the cost-effectiveness of pooled alendronate and risedronate, compared with strontium ranelate, raloxifene, etidronate and teriparatide Dr Matt Stevenson Sarah Davis July 2006 1 Page Executive
Analyses of the cost-effectiveness of pooled alendronate and risedronate, compared with strontium ranelate, raloxifene, etidronate and teriparatide Dr Matt Stevenson Sarah Davis July 2006 1 Page Executive
Family Medicine Clinical Pharmacy Forum Vol. 3, Issue 1 (January/February 2007)
 1 Family Medicine Clinical Pharmacy Forum Vol. 3, Issue 1 (January/February 2007) Family Medicine Clinical Pharmacy Forum is a brief bi-monthly publication from the Family Medicine clinical pharmacists
1 Family Medicine Clinical Pharmacy Forum Vol. 3, Issue 1 (January/February 2007) Family Medicine Clinical Pharmacy Forum is a brief bi-monthly publication from the Family Medicine clinical pharmacists
Horizon Scanning Technology Briefing. Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal. National Horizon Scanning Centre
 Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
Guideline for the investigation and management of osteoporosis. for hospitals and General Practice
 Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Submission to the National Institute for Clinical Excellence on
 Submission to the National Institute for Clinical Excellence on Strontium ranelate for the prevention of osteoporotic fractures in postmenopausal women with osteoporosis by The Society for Endocrinology
Submission to the National Institute for Clinical Excellence on Strontium ranelate for the prevention of osteoporotic fractures in postmenopausal women with osteoporosis by The Society for Endocrinology
Effective Health Care
 Number 12 Effective Health Care Comparative Effectiveness of Treatments To Prevent Fractures in Men and Women With Low Bone Density or Osteoporosis Executive Summary Background Osteoporosis is a systemic
Number 12 Effective Health Care Comparative Effectiveness of Treatments To Prevent Fractures in Men and Women With Low Bone Density or Osteoporosis Executive Summary Background Osteoporosis is a systemic
Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464
 Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Issue date: October Review date: July 2010
 Issue date: October 2008 Review date: July 2010 Alendronate, etidronate, risedronate, raloxifene and strontium ranelate for the primary prevention of osteoporotic fragility fractures in postmenopausal
Issue date: October 2008 Review date: July 2010 Alendronate, etidronate, risedronate, raloxifene and strontium ranelate for the primary prevention of osteoporotic fragility fractures in postmenopausal
PFIZER INC. What is the difference in incidence of fracture in women who ever or never used DMPA for contraception?
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
SCHEDULE 2 THE SERVICES. A. Service Specifications
 SCHEDULE 2 THE SERVICES A. Service Specifications Service Specification No. 04/MSKT/0013 Service PAN DORSET FRACTURE LIAISON SERVICE Commissioner Lead CCP for Musculoskeletal & Trauma Provider Lead Deputy
SCHEDULE 2 THE SERVICES A. Service Specifications Service Specification No. 04/MSKT/0013 Service PAN DORSET FRACTURE LIAISON SERVICE Commissioner Lead CCP for Musculoskeletal & Trauma Provider Lead Deputy
Technology appraisal guidance Published: 27 October 2008 nice.org.uk/guidance/ta160
 Raloxifene for the primary prevention ention of osteoporotic fragility fractures in postmenopausal women Technology appraisal guidance Published: 27 October 2008 nice.org.uk/guidance/ta160 NICE 2018. All
Raloxifene for the primary prevention ention of osteoporotic fragility fractures in postmenopausal women Technology appraisal guidance Published: 27 October 2008 nice.org.uk/guidance/ta160 NICE 2018. All
DECLARATION OF CONFLICT OF INTEREST
 DECLARATION OF CONFLICT OF INTEREST Warfarin and the risk of major bleeding events in patients with atrial fibrillation: a population-based study Laurent Azoulay PhD 1,2, Sophie Dell Aniello MSc 1, Teresa
DECLARATION OF CONFLICT OF INTEREST Warfarin and the risk of major bleeding events in patients with atrial fibrillation: a population-based study Laurent Azoulay PhD 1,2, Sophie Dell Aniello MSc 1, Teresa
RESEARCH. Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores
 Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores Julia Hippisley-Cox, professor of clinical epidemiology and general
Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores Julia Hippisley-Cox, professor of clinical epidemiology and general
Proton Pump Inhibitors, Histamine H 2 Receptor Antagonists, and Other Antacid Medications and the Risk of Fracture
 Calcif Tissue Int (2006) 79:76 83 DOI: 10.1007/s00223-006-0021-7 Proton Pump Inhibitors, Histamine H 2 Receptor Antagonists, and Other Antacid Medications and the Risk of Fracture P. Vestergaard, 1 L.
Calcif Tissue Int (2006) 79:76 83 DOI: 10.1007/s00223-006-0021-7 Proton Pump Inhibitors, Histamine H 2 Receptor Antagonists, and Other Antacid Medications and the Risk of Fracture P. Vestergaard, 1 L.
Management of postmenopausal osteoporosis
 Management of postmenopausal osteoporosis Yeap SS, Hew FL, Chan SP, on behalf of the Malaysian Osteoporosis Society Committee Working Group for the Clinical Guidance on the Management of Osteoporosis,
Management of postmenopausal osteoporosis Yeap SS, Hew FL, Chan SP, on behalf of the Malaysian Osteoporosis Society Committee Working Group for the Clinical Guidance on the Management of Osteoporosis,
An audit of osteoporotic patients in an Australian general practice
 professional Darren Parker An audit of osteoporotic patients in an Australian general practice Background Osteoporosis is a major contributor to morbidity and mortality in Australia, and is predicted to
professional Darren Parker An audit of osteoporotic patients in an Australian general practice Background Osteoporosis is a major contributor to morbidity and mortality in Australia, and is predicted to
Summary of the risk management plan by product
 Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks
Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks
Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464
 Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Online Supplementary Material
 Section 1. Adapted Newcastle-Ottawa Scale The adaptation consisted of allowing case-control studies to earn a star when the case definition is based on record linkage, to liken the evaluation of case-control
Section 1. Adapted Newcastle-Ottawa Scale The adaptation consisted of allowing case-control studies to earn a star when the case definition is based on record linkage, to liken the evaluation of case-control
Horizon Scanning Centre March Denosumab for glucocorticoidinduced SUMMARY NIHR HSC ID: 6329
 Horizon Scanning Centre March 2014 Denosumab for glucocorticoidinduced osteoporosis SUMMARY NIHR HSC ID: 6329 This briefing is based on information available at the time of research and a limited literature
Horizon Scanning Centre March 2014 Denosumab for glucocorticoidinduced osteoporosis SUMMARY NIHR HSC ID: 6329 This briefing is based on information available at the time of research and a limited literature
DENOSUMAB SHARED CARE GUIDLINES
 DENOSUMAB LICENSING Denosumab (PROLIA ) is licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fractures and for bone loss associated with hormone ablation in men with
DENOSUMAB LICENSING Denosumab (PROLIA ) is licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fractures and for bone loss associated with hormone ablation in men with
Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis. Date: Date:
 Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis Section 1: Shared care arrangements and responsibilities Section 1.1 Agreement for transfer of prescribing to
Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis Section 1: Shared care arrangements and responsibilities Section 1.1 Agreement for transfer of prescribing to
Use of proton pump inhibitors and risk of hip/femur fracture: a population-based case-control study
 Osteoporos Int (2011) 22:903 910 DOI 10.1007/s00198-010-1337-8 ORIGINAL ARTICLE Use of proton pump inhibitors and risk of hip/femur fracture: a population-based case-control study S. Pouwels & A. Lalmohamed
Osteoporos Int (2011) 22:903 910 DOI 10.1007/s00198-010-1337-8 ORIGINAL ARTICLE Use of proton pump inhibitors and risk of hip/femur fracture: a population-based case-control study S. Pouwels & A. Lalmohamed
nogg Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK
 nogg NATIONAL OSTEOPOROSIS GUIDELINE GROUP Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK Produced by J Compston, A Cooper,
nogg NATIONAL OSTEOPOROSIS GUIDELINE GROUP Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK Produced by J Compston, A Cooper,
Tool to Assess Risk of Bias in Cohort Studies
 Tool to Assess Risk of Bias in Cohort Studies Contributed by the CLARITY Group at McMaster University 1. Was selection of exposed and non-exposed cohorts drawn from the same population? Exposed and unexposed
Tool to Assess Risk of Bias in Cohort Studies Contributed by the CLARITY Group at McMaster University 1. Was selection of exposed and non-exposed cohorts drawn from the same population? Exposed and unexposed
Oral Alendronate Vs. Three-Monthly Iv Ibandronate In The Treatment Of Postmenopausal Osteoporosis
 Oral Alendronate Vs. Three-Monthly Iv Ibandronate In The Treatment Of Postmenopausal Osteoporosis Miriam Silverberg A. Study Purpose and Rationale More than 70% of fractures in people after the age of
Oral Alendronate Vs. Three-Monthly Iv Ibandronate In The Treatment Of Postmenopausal Osteoporosis Miriam Silverberg A. Study Purpose and Rationale More than 70% of fractures in people after the age of
Outline. Switching treatment. Evidence from randomized trials. The effects of switching 7/8/2016. When and for whom? Steven Cummings, MD
 Outline Switching treatment When and for whom? Steven Cummings, MD Focus on switching from alendronate or risedronate Evidence about the effects of switching on BMD Purposes of switching Symptoms Poor
Outline Switching treatment When and for whom? Steven Cummings, MD Focus on switching from alendronate or risedronate Evidence about the effects of switching on BMD Purposes of switching Symptoms Poor
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 : assessing the risk of fragility fracture bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new
: assessing the risk of fragility fracture bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new
CAN EFFECTIVENESS BE MEASURED OUTSIDE A CLINICAL TRIAL?
 CAN EFFECTIVENESS BE MEASURED OUTSIDE A CLINICAL TRIAL? Mette Nørgaard, Professor, MD, PhD Department of Clinical Epidemiology Aarhus Universitety Hospital Aarhus, Denmark Danish Medical Birth Registry
CAN EFFECTIVENESS BE MEASURED OUTSIDE A CLINICAL TRIAL? Mette Nørgaard, Professor, MD, PhD Department of Clinical Epidemiology Aarhus Universitety Hospital Aarhus, Denmark Danish Medical Birth Registry
Observational Study Designs. Review. Today. Measures of disease occurrence. Cohort Studies
 Observational Study Designs Denise Boudreau, PhD Center for Health Studies Group Health Cooperative Today Review cohort studies Case-control studies Design Identifying cases and controls Measuring exposure
Observational Study Designs Denise Boudreau, PhD Center for Health Studies Group Health Cooperative Today Review cohort studies Case-control studies Design Identifying cases and controls Measuring exposure
Rapid appraisal of the literature: Identifying study biases
 Rapid appraisal of the literature: Identifying study biases Rita Popat, PhD Clinical Assistant Professor Division of Epidemiology Stanford University School of Medicine August 7, 2007 What is critical
Rapid appraisal of the literature: Identifying study biases Rita Popat, PhD Clinical Assistant Professor Division of Epidemiology Stanford University School of Medicine August 7, 2007 What is critical
MANAGEMENT OF DYSPEPSIA AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
 DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT OF DYSPEPSIA AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD) Routine endoscopic investigation of patients of any age, presenting with dyspepsia
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT OF DYSPEPSIA AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD) Routine endoscopic investigation of patients of any age, presenting with dyspepsia
Proton Pump Inhibitor De-prescribing Guidance
 Amendment History Proton Pump Inhibitor De-prescribing Guidance VERSION DATE AMENDMENT HISTORY 1.0 2013 Previous version 2.0 September 2015 Comments Amendment to Flow chart and addition of Rationale page
Amendment History Proton Pump Inhibitor De-prescribing Guidance VERSION DATE AMENDMENT HISTORY 1.0 2013 Previous version 2.0 September 2015 Comments Amendment to Flow chart and addition of Rationale page
Costing statement: Denosumab for the prevention of osteoporotic fractures in postmenopausal women
 Costing statement: Denosumab for the prevention of osteoporotic fractures in postmenopausal women Resource impact The guidance Denosumab for the prevention of osteoporotic fractures in postmenopausal women
Costing statement: Denosumab for the prevention of osteoporotic fractures in postmenopausal women Resource impact The guidance Denosumab for the prevention of osteoporotic fractures in postmenopausal women
Database of Abstracts of Reviews of Effects (DARE) Produced by the Centre for Reviews and Dissemination Copyright 2017 University of York.
 A comparison of the cost-effectiveness of five strategies for the prevention of non-steroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling Brown
A comparison of the cost-effectiveness of five strategies for the prevention of non-steroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling Brown
RESEARCH. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort
 1 Cancer Epidemiology Unit, University of Oxford, Oxford OX3 7LF 2 Medicines and Healthcare products Regulatory Agency, Pharmacoepidemiology Research Unit, London SW8 5NQ Correspondence to: J Green jane.green@ceu.ox.ac.uk
1 Cancer Epidemiology Unit, University of Oxford, Oxford OX3 7LF 2 Medicines and Healthcare products Regulatory Agency, Pharmacoepidemiology Research Unit, London SW8 5NQ Correspondence to: J Green jane.green@ceu.ox.ac.uk
LONG -TERM USE OF PPIS: INDICATIONS, BENEFITS AND HARMS. Jihane Naous, M.D.
 LONG -TERM USE OF PPIS: INDICATIONS, BENEFITS AND HARMS Jihane Naous, M.D. Objectives Identify the conditions supported by AGA/ACG guidelines necessitating long-term use of daily PPIs, Recognize which
LONG -TERM USE OF PPIS: INDICATIONS, BENEFITS AND HARMS Jihane Naous, M.D. Objectives Identify the conditions supported by AGA/ACG guidelines necessitating long-term use of daily PPIs, Recognize which
PROCTER & GAMBLE and SANOFI-AVENTIS v ROCHE and GLAXOSMITHKLINE
 CASES AUTH/1803/2/06 and AUTH/1804/2/06 PROCTER & GAMBLE and SANOFI-AVENTIS v ROCHE and GLAXOSMITHKLINE Bonviva Once Monthly slide kits Procter & Gamble and Sanofi-Aventis complained jointly about two
CASES AUTH/1803/2/06 and AUTH/1804/2/06 PROCTER & GAMBLE and SANOFI-AVENTIS v ROCHE and GLAXOSMITHKLINE Bonviva Once Monthly slide kits Procter & Gamble and Sanofi-Aventis complained jointly about two
Appendix. TABLE E-1 Study Variables and Associated ICD-9-CM, HCPCS, and CPT Codes. Codes. (1) Fracture locations
 Page 1 Appendix TABLE E-1 Study Variables and Associated ICD-9-CM, HCPCS, and CPT Codes (1) Fracture locations Vertebral fracture Codes ICD-9-CM Diagnosis codes: 733.13, 805.xx, 806.xx ICD-9-CM Procedure
Page 1 Appendix TABLE E-1 Study Variables and Associated ICD-9-CM, HCPCS, and CPT Codes (1) Fracture locations Vertebral fracture Codes ICD-9-CM Diagnosis codes: 733.13, 805.xx, 806.xx ICD-9-CM Procedure
Validation of QFracture. Analysis prepared for NICE 2011
 Validation of QFracture compared with FRAX Analysis prepared for NICE 2011 Authors: Julia Hippisley-Cox & Carol Coupland Email: Julia.hippisley-cox@nottingham.ac.uk Julia Hipisley-Cox, University of Nottingham,
Validation of QFracture compared with FRAX Analysis prepared for NICE 2011 Authors: Julia Hippisley-Cox & Carol Coupland Email: Julia.hippisley-cox@nottingham.ac.uk Julia Hipisley-Cox, University of Nottingham,
A response by Servier to the Statement of Reasons provided by NICE
 A response by Servier to the Statement of Reasons provided by NICE Servier gratefully acknowledges the Statement of Reasons document from NICE, and is pleased to provide information to assist NICE in its
A response by Servier to the Statement of Reasons provided by NICE Servier gratefully acknowledges the Statement of Reasons document from NICE, and is pleased to provide information to assist NICE in its
The 2017 CMS Merit-based Incentive Payment System includes 2 inflammatory bowel disease (IBD) measures.
 The 2017 CMS Merit-based Incentive Payment System includes 2 inflammatory bowel disease (IBD) measures. Measure Specifications for Registry Reporting MIPS #271: Inflammatory Bowel Disease (IBD): Preventive
The 2017 CMS Merit-based Incentive Payment System includes 2 inflammatory bowel disease (IBD) measures. Measure Specifications for Registry Reporting MIPS #271: Inflammatory Bowel Disease (IBD): Preventive
Use of inhaled and oral glucocorticoids, severity of inflammatory disease and risk of hip/femur fracture: a population-based case control study
 Original Article doi: 10.1111/j.1365-2796.2006.01754.x Use of inhaled and oral glucocorticoids, severity of inflammatory disease and risk of hip/femur fracture: a population-based case control study F.
Original Article doi: 10.1111/j.1365-2796.2006.01754.x Use of inhaled and oral glucocorticoids, severity of inflammatory disease and risk of hip/femur fracture: a population-based case control study F.
Bisphosphonate Step Therapy Criteria
 ϯ ϯ ϯ A Division of Health Care Service Corporation, a Mutual Legal Reserve Company, an Independent Licensee of the Blue Cross and Blue Shield Association Bisphosphonate Step Therapy Criteria Program may
ϯ ϯ ϯ A Division of Health Care Service Corporation, a Mutual Legal Reserve Company, an Independent Licensee of the Blue Cross and Blue Shield Association Bisphosphonate Step Therapy Criteria Program may
Osteoporosis challenges
 Osteoporosis challenges Osteoporosis challenges Who should have a fracture risk assessment? Who to treat? Drugs, holidays and unusual adverse effects Fracture liaison service? The size of the problem 1
Osteoporosis challenges Osteoporosis challenges Who should have a fracture risk assessment? Who to treat? Drugs, holidays and unusual adverse effects Fracture liaison service? The size of the problem 1
Prevention of osteoporosis: cost-effectiveness of different pharmaceutical treatments Ankjaer-Jensen A, Johnell O
 Prevention of osteoporosis: cost-effectiveness of different pharmaceutical treatments Ankjaer-Jensen A, Johnell O Record Status This is a critical abstract of an economic evaluation that meets the criteria
Prevention of osteoporosis: cost-effectiveness of different pharmaceutical treatments Ankjaer-Jensen A, Johnell O Record Status This is a critical abstract of an economic evaluation that meets the criteria
Bisphosphonates for preventing osteoporotic fragility fracture
 Bisphosphonates for preventing osteoporotic fragility fracture An example of a multi-dimensional modelling problem Sarah Davis, ScHARR Background Project was part of ongoing NICE Multiple Technology Appraisal
Bisphosphonates for preventing osteoporotic fragility fracture An example of a multi-dimensional modelling problem Sarah Davis, ScHARR Background Project was part of ongoing NICE Multiple Technology Appraisal
Fragile Bones and how to recognise them. Rod Hughes Consultant physician and rheumatologist St Peter s hospital Chertsey
 Fragile Bones and how to recognise them Rod Hughes Consultant physician and rheumatologist St Peter s hospital Chertsey Osteoporosis Osteoporosis is a skeletal disorder characterised by compromised bone
Fragile Bones and how to recognise them Rod Hughes Consultant physician and rheumatologist St Peter s hospital Chertsey Osteoporosis Osteoporosis is a skeletal disorder characterised by compromised bone
Osteoporosis Agents Drug Class Prior Authorization Protocol
 Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
THIS TALK STATINS REDUCE: Immortal time bias Immeasurable time bias Time-window bias TIME-RELATED BIASES IN PHARMACOEPIDEMIOLOGY
 TIME-RELATED BIASES IN PHARMACOEPIDEMIOLOGY Samy Suissa McGill University and Jewish General Hospital Montreal, Canada ISPE mid-year meeting, Miami April 22, 2012 STATINS REDUCE: JAMA 2000: Fracture rate
TIME-RELATED BIASES IN PHARMACOEPIDEMIOLOGY Samy Suissa McGill University and Jewish General Hospital Montreal, Canada ISPE mid-year meeting, Miami April 22, 2012 STATINS REDUCE: JAMA 2000: Fracture rate
Antidepressant use and risk of adverse outcomes in people aged years: cohort study using a primary care database
 Coupland et al. BMC Medicine (2018) 16:36 https://doi.org/10.1186/s12916-018-1022-x RESEARCH ARTICLE Open Access Antidepressant use and risk of adverse outcomes in people aged 20 64 years: cohort study
Coupland et al. BMC Medicine (2018) 16:36 https://doi.org/10.1186/s12916-018-1022-x RESEARCH ARTICLE Open Access Antidepressant use and risk of adverse outcomes in people aged 20 64 years: cohort study
Interpreting DEXA Scan and. the New Fracture Risk. Assessment. Algorithm
 Interpreting DEXA Scan and the New Fracture Risk Assessment Algorithm Prof. Samir Elbadawy *Osteoporosis affect 30%-40% of women in western countries and almost 15% of men after the age of 50 years. Osteoporosis
Interpreting DEXA Scan and the New Fracture Risk Assessment Algorithm Prof. Samir Elbadawy *Osteoporosis affect 30%-40% of women in western countries and almost 15% of men after the age of 50 years. Osteoporosis
New 2010 Osteoporosis Guidelines: What you and your health provider need to know QUESTIONS&ANSWERS
 New 2010 Osteoporosis Guidelines: What you and your health provider need to know QUESTIONS&ANSWERS Wednesday, December 1, 2010 1:00 p.m. to 2:00 p.m. ET 1. I m 55 years old. I ve been taking Fosavance
New 2010 Osteoporosis Guidelines: What you and your health provider need to know QUESTIONS&ANSWERS Wednesday, December 1, 2010 1:00 p.m. to 2:00 p.m. ET 1. I m 55 years old. I ve been taking Fosavance
Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures
 APper apc15-0avgfh7 Shared Care Guideline Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures For the latest information on interactions and adverse effects,
APper apc15-0avgfh7 Shared Care Guideline Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures For the latest information on interactions and adverse effects,
Understanding NICE guidance. NICE technology appraisal guidance advises on when and how drugs and other treatments should be used in the NHS.
 Understanding NICE guidance Information for people who use NHS services Alendronate, etidronate, risedronate, strontium ranelate and raloxifene for preventing bone fractures in postmenopausal women with
Understanding NICE guidance Information for people who use NHS services Alendronate, etidronate, risedronate, strontium ranelate and raloxifene for preventing bone fractures in postmenopausal women with
Name of Policy: Boniva (Ibandronate Sodium) Infusion
 Name of Policy: Boniva (Ibandronate Sodium) Infusion Policy #: 266 Latest Review Date: April 2010 Category: Pharmacology Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Name of Policy: Boniva (Ibandronate Sodium) Infusion Policy #: 266 Latest Review Date: April 2010 Category: Pharmacology Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Use of combined oral contraceptives and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases
 open access Use of combined oral contraceptives and risk of venous thromboembolism: nested case-control studies using the and databases Yana Vinogradova, Carol Coupland, Julia Hippisley-Cox Division of
open access Use of combined oral contraceptives and risk of venous thromboembolism: nested case-control studies using the and databases Yana Vinogradova, Carol Coupland, Julia Hippisley-Cox Division of
1. Draft checklist for judging on quality of animal studies (Van der Worp et al., 2010)
 Appendix C Quality appraisal tools (QATs) 1. Draft checklist for judging on quality of animal studies (Van der Worp et al., 2010) 2. NICE Checklist for qualitative studies (2012) 3. The Critical Appraisal
Appendix C Quality appraisal tools (QATs) 1. Draft checklist for judging on quality of animal studies (Van der Worp et al., 2010) 2. NICE Checklist for qualitative studies (2012) 3. The Critical Appraisal
Summary. Background. Diagnosis
 March 2009 Management of post-menopausal osteoporosis This bulletin focuses on the pharmacological management of patients with post-menopausal osteoporosis both those with clinically evident disease (e.g.
March 2009 Management of post-menopausal osteoporosis This bulletin focuses on the pharmacological management of patients with post-menopausal osteoporosis both those with clinically evident disease (e.g.
Osteoporosis: fragility fracture risk. Costing report. Implementing NICE guidance
 Osteoporosis: fragility fracture risk Costing report Implementing NICE guidance August 2012 NICE clinical guideline 146 1 of 15 This costing report accompanies the clinical guideline: Osteoporosis: assessing
Osteoporosis: fragility fracture risk Costing report Implementing NICE guidance August 2012 NICE clinical guideline 146 1 of 15 This costing report accompanies the clinical guideline: Osteoporosis: assessing
PRESCRIBING SUPPORT TEAM AUDIT: PROTON PUMP INHIBITOR PRESCRIBING REVIEW
 PRESCRIBING SUPPORT TEAM AUDIT: PROTON PUMP INHIBITOR PRESCRIBING REVIEW DATE OF AUTHORISATION: AUTHORISING GP: PRESCRIBING SUPPORT TECHNICIAN: SUMMARY Dyspepsia refers to a broad range of symptoms related
PRESCRIBING SUPPORT TEAM AUDIT: PROTON PUMP INHIBITOR PRESCRIBING REVIEW DATE OF AUTHORISATION: AUTHORISING GP: PRESCRIBING SUPPORT TECHNICIAN: SUMMARY Dyspepsia refers to a broad range of symptoms related
May Professor Matt Stevenson School of Health and Related Research, University of Sheffield
 ASSESSING THE FEASIBILITY OF TRANSFORMING THE RECOMMENDATIONS IN TA160, TA161 AND TA204 INTO ABSOLUTE 10-YEAR RISK OF FRACTURE A REPORT PRODUCED BY THE DECISION SUPPORT UNIT IN THE CONTEXT OF THE REVIEW
ASSESSING THE FEASIBILITY OF TRANSFORMING THE RECOMMENDATIONS IN TA160, TA161 AND TA204 INTO ABSOLUTE 10-YEAR RISK OF FRACTURE A REPORT PRODUCED BY THE DECISION SUPPORT UNIT IN THE CONTEXT OF THE REVIEW
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health and social care directorate Quality standards and indicators Briefing paper
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health and social care directorate Quality standards and indicators Briefing paper Quality standard topic: Osteoporosis Output: Prioritised quality improvement
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health and social care directorate Quality standards and indicators Briefing paper Quality standard topic: Osteoporosis Output: Prioritised quality improvement
Challenging the Current Osteoporosis Guidelines. Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA
 Challenging the Current Osteoporosis Guidelines Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Whom to screen Which test How to diagnose Whom to treat Benefits
Challenging the Current Osteoporosis Guidelines Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Whom to screen Which test How to diagnose Whom to treat Benefits
Osteoporosis Clinical Guideline. Rheumatology January 2017
 Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
Page 1
 Osteoporosis Osteoporosis is a condition characterised by weakened bones that fracture easily. After menopause many women are at risk of developing osteoporosis. Peak bone mass is usually reached during
Osteoporosis Osteoporosis is a condition characterised by weakened bones that fracture easily. After menopause many women are at risk of developing osteoporosis. Peak bone mass is usually reached during
Clinical Specialist Statement Template
 Clinical Specialist Statement Template Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can
Clinical Specialist Statement Template Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can
Bones & Joints/CVD risk assessment
 Quiz feedback: Bones & Joints/CVD risk assessment Bones and Joints Osteoporosis Who should receive calcium supplementation and what interactions are important (i.e. what drug/food combinations should be
Quiz feedback: Bones & Joints/CVD risk assessment Bones and Joints Osteoporosis Who should receive calcium supplementation and what interactions are important (i.e. what drug/food combinations should be
OSTEOPOROSIS: PREVENTION AND MANAGEMENT
 OSTEOPOROSIS: OVERVIEW OSTEOPOROSIS: PREVENTION AND MANAGEMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Key Risk factors Screening and Monitoring
OSTEOPOROSIS: OVERVIEW OSTEOPOROSIS: PREVENTION AND MANAGEMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Key Risk factors Screening and Monitoring
Purpose. Study Designs. Objectives. Observational Studies. Analytic Studies
 Purpose Study Designs H.S. Teitelbaum, DO, PhD, MPH, FAOCOPM AOCOPM Annual Meeting Introduce notions of study design Clarify common terminology used with description and interpretation of information collected
Purpose Study Designs H.S. Teitelbaum, DO, PhD, MPH, FAOCOPM AOCOPM Annual Meeting Introduce notions of study design Clarify common terminology used with description and interpretation of information collected
Module 5 - Speaking of Bones Osteoporosis For Health Professionals: Fracture Risk Assessment. William D. Leslie, MD MSc FRCPC
 Module 5 - Speaking of Bones Osteoporosis For Health Professionals: Fracture Risk Assessment William D. Leslie, MD MSc FRCPC Case #1 Age 53: 3 years post-menopause Has always enjoyed excellent health with
Module 5 - Speaking of Bones Osteoporosis For Health Professionals: Fracture Risk Assessment William D. Leslie, MD MSc FRCPC Case #1 Age 53: 3 years post-menopause Has always enjoyed excellent health with
Scottish Medicines Consortium
 Scottish Medicines Consortium ketoprofen/, 100mg/20mg; 200mg/20mg modified release capsules (Axorid ) No. (606/10) Meda Pharmaceuticals 05 February 2010 The Scottish Medicines Consortium (SMC) has completed
Scottish Medicines Consortium ketoprofen/, 100mg/20mg; 200mg/20mg modified release capsules (Axorid ) No. (606/10) Meda Pharmaceuticals 05 February 2010 The Scottish Medicines Consortium (SMC) has completed
Administration of Denosumab (PROLIA ) for the treatment of osteoporosis in postmenopausal women and in men at increased risk of fractures
 Administration of Denosumab (PROLIA ) for the treatment of osteoporosis in postmenopausal women and in men at increased risk of fractures 2017 18 1. Purpose of Agreement This agreement outlines the expectations
Administration of Denosumab (PROLIA ) for the treatment of osteoporosis in postmenopausal women and in men at increased risk of fractures 2017 18 1. Purpose of Agreement This agreement outlines the expectations
Treatments for Osteoporosis Expected Benefits, Potential Harms and Drug Holidays. Suzanne Morin MD FRCP FACP McGill University May 2014
 Treatments for Osteoporosis Expected Benefits, Potential Harms and Drug Holidays Suzanne Morin MD FRCP FACP McGill University May 2014 Learning Objectives Overview of osteoporosis management Outline efficacy
Treatments for Osteoporosis Expected Benefits, Potential Harms and Drug Holidays Suzanne Morin MD FRCP FACP McGill University May 2014 Learning Objectives Overview of osteoporosis management Outline efficacy
Pathway from Fracture or Risk Factor to Treatment
 Appendix 6A - Guidance on Diagnosis and Management of Osteoporosis Pathway from Fracture or Risk Factor to Treatment Fragility Fracture = fracture sustained from a low energy fall from standing height
Appendix 6A - Guidance on Diagnosis and Management of Osteoporosis Pathway from Fracture or Risk Factor to Treatment Fragility Fracture = fracture sustained from a low energy fall from standing height
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women
 S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
1. UK List Price of Zoledronic acid (Zoledronate) 5 mg (Aclasta )
 Novartis Pharmaceuticals UK Ltd Frimley Business Park Frimley Camberley Surrey GU16 7SR Dr C M Longson Director, Centre for Health Technology Evaluation National Institute for Health and Clinical Excellence
Novartis Pharmaceuticals UK Ltd Frimley Business Park Frimley Camberley Surrey GU16 7SR Dr C M Longson Director, Centre for Health Technology Evaluation National Institute for Health and Clinical Excellence
The Healthy User Effect: Ubiquitous and Uncontrollable S. R. Majumdar, MD MPH FRCPC FACP
 The Healthy User Effect: Ubiquitous and Uncontrollable S. R. Majumdar, MD MPH FRCPC FACP Professor of Medicine, Endowed Chair in Patient Health Management, Health Scholar of the Alberta Heritage Foundation,
The Healthy User Effect: Ubiquitous and Uncontrollable S. R. Majumdar, MD MPH FRCPC FACP Professor of Medicine, Endowed Chair in Patient Health Management, Health Scholar of the Alberta Heritage Foundation,
Management of dyspepsia and of Helicobacter pylori infection
 Management of dyspepsia and of Helicobacter pylori infection The University of Nottingham John Atherton Wolfson Digestive Diseases Centre University of Nottingham, UK Community management of dyspepsia
Management of dyspepsia and of Helicobacter pylori infection The University of Nottingham John Atherton Wolfson Digestive Diseases Centre University of Nottingham, UK Community management of dyspepsia
Misuse of Bisphosphonates. Dr Rukhsana Parvin Associate Professor Department of Medicine Enam Medical College & Hospital
 Misuse of Bisphosphonates Dr Rukhsana Parvin Associate Professor Department of Medicine Enam Medical College & Hospital Introduction Bisphosphonates are chemically stable analogues of pyrophosphate compounds.
Misuse of Bisphosphonates Dr Rukhsana Parvin Associate Professor Department of Medicine Enam Medical College & Hospital Introduction Bisphosphonates are chemically stable analogues of pyrophosphate compounds.
Characteristics of selective and non-selective NSAID use in Scotland
 Characteristics of selective and non-selective NSAID use in Scotland Alford KMG 1, Simpson C 1, Williams D 2 1 Department of General Practice & Primary Care, The University of Aberdeen. 2 Department of
Characteristics of selective and non-selective NSAID use in Scotland Alford KMG 1, Simpson C 1, Williams D 2 1 Department of General Practice & Primary Care, The University of Aberdeen. 2 Department of
COPD-Related Musculoskeletal Disease. Jessica Bon Field, MD, MS 2017 Update in Internal Medicine October 20, 2017
 COPD-Related Musculoskeletal Disease Jessica Bon Field, MD, MS 2017 Update in Internal Medicine October 20, 2017 A 60-year old man with COPD comes into your office for a routine office visit. He is a former
COPD-Related Musculoskeletal Disease Jessica Bon Field, MD, MS 2017 Update in Internal Medicine October 20, 2017 A 60-year old man with COPD comes into your office for a routine office visit. He is a former
Appendix 2 Quality assessment tools. Cochrane risk of bias tool for RCTs. Support for judgment
 Appendix 2 Quality assessment tools Cochrane risk of bias tool for RCTs Item Judgment (H/L/Unclear) Random sequence generation (selection bias) Allocation concealment (selection bias) Blinding of participants
Appendix 2 Quality assessment tools Cochrane risk of bias tool for RCTs Item Judgment (H/L/Unclear) Random sequence generation (selection bias) Allocation concealment (selection bias) Blinding of participants
HIV and your Bones Osteopenia and Osteoporosis
 Osteopenia and Osteoporosis Background information For reasons not yet fully understood, higher rates of bone disease are starting to be seen in people living with HIV. These bone diseases include osteopenia
Osteopenia and Osteoporosis Background information For reasons not yet fully understood, higher rates of bone disease are starting to be seen in people living with HIV. These bone diseases include osteopenia
GSK Medicine: salmeterol, Salmeterol+Fluticasone proprionate, fluticasone propionate, beclomethasone
 GSK Medicine: salmeterol, Salmeterol+Fluticasone proprionate, fluticasone propionate, beclomethasone Study No.: WWE111984/WEUSRTP2640/EPI40528 Title: The Asthma Death Case Control Study (ADCCS): Association
GSK Medicine: salmeterol, Salmeterol+Fluticasone proprionate, fluticasone propionate, beclomethasone Study No.: WWE111984/WEUSRTP2640/EPI40528 Title: The Asthma Death Case Control Study (ADCCS): Association
Differentiating Pharmacological Therapies for Osteoporosis
 Differentiating Pharmacological Therapies for Osteoporosis Socrates E Papapoulos Department of Endocrinology & Metabolic Diseases Leiden University Medical Center The Netherlands Competing interests: consulting/speaking
Differentiating Pharmacological Therapies for Osteoporosis Socrates E Papapoulos Department of Endocrinology & Metabolic Diseases Leiden University Medical Center The Netherlands Competing interests: consulting/speaking
Osteoporosis: An Overview. Carolyn J. Crandall, MD, MS
 Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Objectives Review osteoporosis
Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Objectives Review osteoporosis
A Population-Based Study of the Effectiveness of Bisphosphonates at Reducing Hip Fractures among High Risk Women
 A Population-Based Study of the Effectiveness of Bisphosphonates at Reducing Hip Fractures among High Risk Women APHA Conference Washington, DC November 2, 2011 Presenter Disclosures Kathy Schneider, PhD
A Population-Based Study of the Effectiveness of Bisphosphonates at Reducing Hip Fractures among High Risk Women APHA Conference Washington, DC November 2, 2011 Presenter Disclosures Kathy Schneider, PhD
Sponsor / Company: sanofi-aventis and Proctor & Gamble Drug substance(s): Risedronate (HMR4003)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: sanofi-aventis and
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: sanofi-aventis and
