Abstract. Introduction ORIGINAL ARTICLE. G Gross,* K-G Meyer, H Pres, C Thielert, H Tawfik, A Mescheder
|
|
|
- Stephanie McCoy
- 6 years ago
- Views:
Transcription
1 JEADV ISSN Blackwell Publishing Ltd ORIGINAL ARTICLE A randomized, double-blind, four-arm parallel-group, placebo-controlled Phase II/III study to investigate the clinical efficacy of two galenic formulations of Polyphenon E in the treatment of external genital warts G Gross,* K-G Meyer, H Pres, C Thielert, H Tawfik, A Mescheder Private Dermatology Practice, Berlin, Germany Private Dermatology Practice, Berlin, Germany MediGene AG, Planegg/Martinsried, Germany Keywords antioxidant, antitumour, catechins, genital warts, green tea, human comparative study, topical *Corresponding author, Department of Dermatology and Venereology, University of Rostock, Augustenstr , D Rostock, Germany, tel ; fax ; gerd.gross@med.uni-rostock.de Funding/Support: This study was supported by Medigene AG, Munich, Germany. Received: 15 November 2006, accepted 26 June 2007 DOI: /j x Abstract Objective Clinical efficacy and safety of Polyphenon E, a defined green tea extract, in external genital warts. Design Randomized, double-blind, placebo-controlled study for up to 12 weeks with a 12-week treatment-free follow-up. Setting Twenty-eight hospitals and practices in Germany and Russia. Patients Two hundred forty-two outpatients (125 men, 117 women) with 2 to 30 warts (total wart area, mm 2 ). Intervention(s) Topical application of Polyphenon E 10% Cream, Polyphenon E 15% Ointment or placebo to all external genital warts three times a day. Main outcome measure(s) Measurement of total wart area and local reactions/adverse events. Results For 15% ointment, statistically significant differences to placebo were achieved regarding complete clearance of all baseline external genital warts (61.0% vs. 40.5% in males, 56.8% vs. 34.1% in females; combined gender: P = ) and 75% to 100% clearance (80.8% vs. 51.8%; P = ) in both the intent-to-treat and per-protocol populations. For 10% cream, 53.8% males and 39.5% females achieved complete clearance. Recurrence rates 12 weeks after end of treatment were 10.6%, 11.8% and 10.3% for 15% ointment, 10% cream and placebo, respectively. Adverse events were observed in only 7.9% of patients, with no serious adverse events or deaths reported. Local skin reactions were generally mild to moderate and resolved with continued treatment without sequelae. Conclusions Polyphenon E 15% ointment, composed of a defined green tea extract, proved to be efficacious and safe for both gender in the treatment of external genital warts. Introduction External genital warts are non-malignant squamous cell tumours caused by infections of human papillomaviruses (HPV). The low-risk HPV genotypes 6 and 11 are found in > 90% of cases. 1,2 Genital wart infections have one of the fastest growing incidence rates of all sexually transmitted diseases. Overall, 0.5% to 1% of the general population is infected with HPV. 2 Few treatments with varying degrees of efficacy are available. Nevertheless, for topical agents The Authors
2 Gross et al. Polyphenon E treatment of external genital warts such as imiquimod (Aldara ) and podofilox (Condylox, Condyline and Wartec ), common application-site reactions, including erythema, oedema, scaling, erosions, ulcerations, pain, burning, and itching, have been reported. For the provider-administered therapies, such as cryotherapy, curettage and electrodesiccation, laser surgery, podophyllin resin, and trichloroacetic acid, the treatments are often painful and may cause scarring. 2 7 Additionally, because these therapies do not eliminate the source of disease, recurrence of warts is often observed. 8 Polyphenon E is a defined extract of catechins of green tea leaves of Camellia sinensis, a species of the Theaceae family. It contains more than 80% of tea polyphenols/catechins accountable for the major biological properties, an immunomodulatory activity (unpublished data) and protein binding, particularly to enzymes, as well as strong antioxidant activity. 9,10 Antitumour activity has been observed potentially as a result of blocking the mitotic signal transduction pathway, which in turn has an effect on proliferation, on cell cycle progression and even apoptosis. 10 These potential properties are supportive of its use in wart treatment together with the observed anti-inflammatory activities. 10 Initial studies in China have shown that tea polyphenols were effective in the treatment of external genital warts, with minimal pain and inflammation (personal communication from Dr. Shu-Jun Cheng, Cancer Institute of the Chinese Academy of Medical Sciences, Beijing, China). MediGene has developed two new formulations, a 10% cream and a 15% ointment, with enhanced penetration properties to further improve the efficacy of patient-applied Polyphenon E in the treatment of external genital warts. Materials and methods Objective The objective of this study was to investigate the clinical efficacy and safety of Polyphenon E 10% cream and Polyphenon E 15% ointment in comparison with placebo in the treatment of external genital warts in male and female patients. Study design The study was a multicentre, Phase II/III, randomized, double-blind, placebo-controlled, four-arm parallel-group trial. The maximum duration of treatment was 12 weeks or until complete clearance of all baseline warts, whichever came first, followed by a 12-week treatment-free follow-up phase for complete responders. The first patient was enrolled on 27 December 2000, and the last patient completed the study on 18 November Study population Male and female patients, 18 years of age or older, with 2 to 30 clinically diagnosed external genital warts with a total wart area of 12 to 600 mm 2 were enrolled. Data were collected at 20 German and 8 Russian centres, including University hospitals, hospitals and clinical practices. Locations of warts were glans penis, penile shaft, scrotum, and foreskin for men; vulva for women; and the inguinal, perineal, and perianal skin areas for both gender. Female patients and partners of male patients with childbearing potential had negative pregnancy tests and used effective contraception during the treatment period. Patients were not enrolled if they had a current episode of Herpes genitalis or any other current and/or recurrent genital or uncontrolled infection. Patients who had participated in an investigational trial, had treatment of anogenital warts or had systemic intake of acyclovir or immunosuppressive medication within 30 days prior to enrolment were excluded. Patients with organ allograft, with skin conditions that may interfere with the study drug, with internal (vaginal or rectal) warts that required treatment or who were lactating were also excluded. Written informed consent was obtained after providing detailed information about the study and allocating sufficient time to consider whether to participate. Patients with less than 50% clearance of total baseline wart area after 8 weeks of treatment could discontinue treatment and were then regarded as treatment failures. Ethics The responsible local and/or national independent ethics committee approved the study protocol and any other relevant study documentation prior to enrolment. The study was done according to the Declaration of Helsinki (Somerset West, 1996) 11 and the ICH GCP guidelines as well as the demands of national drug and data protection laws and other applicable regulatory requirements. It was fully monitored and audited. Randomization, study medication and treatment Patients were randomly assigned to one of the four treatment groups in a 2 : 1 active: placebo allocation ratio, stratified by gender. The active treatments were Polyphenon E 10% cream and Polyphenon E 15% ointment. In the corresponding placebos, Polyphenon E was replaced by higher amounts of all base ingredients in the cream and of white petrolatum and white wax in the ointment base and inert colours added to ensure blinding. Centers were provided with medication kits that had a pre-assigned 4-digit number, with each patient allocated to the lowest available number. Randomization sequence was generated 2007 The Authors 1405
3 Polyphenon E treatment of external genital warts Gross et al. by Almedica HPS AG, Switzerland. Patients were instructed to apply the medication topically to all external genital warts three times a day, whether they were baseline or new warts. Dosing was anticipated to be < 250 mg of study medication per application. Each tube contained 15 g of ointment, cream or placebo defined for a 2-week application period. Study assessments During the 12-week treatment period, patient visits were scheduled at baseline, at every other week until week 8, with a final visit at week 12. Disease and medical history, demographic data and concomitant medications were recorded during screening. At the baseline visit, the inclusion and exclusion criteria were reviewed, and the treatment was initiated. Wart measurements, as the product of wart length and the rectangular width, were taken at baseline and weeks 2, 4, 6, 8, and 12 and documented in a dermagram. Drug compliance was checked at weeks 2, 4, 6, 8 and 12. At baseline visit and at week 12, patients underwent physical examination, vital signs were measured and photographs including a ruler were taken (optional, for documentation not for evaluation purposes). Blood and urine samples were collected for a standard range of haematology, blood chemistry and urinalysis laboratory evaluations, including pregnancy tests, if applicable. Local reactions at the application site are of special interest for topical treatments. Thus, they were evaluated and described separately from the other adverse events (AE). At baseline and at all subsequent visits, the blinded investigator assessed local signs, including erythema, oedema, induration, vesicles, erosion/ulceration, and global reaction and questioned the patient about local skin symptoms, including burning, itching, pain, other and overall reaction. Intensity of all skin reactions at the site of application were graded as either none, mild, moderate or severe. AEs, concomitant medications and changes to concomitant medications were also recorded at all visits. Follow-up visits Patients with complete clearance of all baseline warts stopped treatment and went into follow-up with assessments after 4 and 12 weeks (if wart-free) to assess recurrence rates. At these visits, local tolerability was evaluated and previously reported AEs and concomitant medications were updated. Number of patients required and statistical analyses Complete clearance rates of baseline warts of 5% (male) and 15% (female) for the placebo group and 35% (male) and 50% (female) for the active treatment groups (10% cream, 15% ointment) were assumed. Using a two-sided Fisher s exact test with a significance level (type I error) of α = 0.025, 80% power required at least 38 patients in each subgroup to compare active treatments with pooled placebo by gender. Thus, allowing a dropout rate of 10%, it was planned to recruit 130 male and 130 female patients. Subsequently, the data were pooled to perform analysis between the active treatment groups and placebo stratified by gender (Mantel & Haenszel test, two-sided, α = 0.05). Descriptive statistics were used to analyse secondary efficacy involving complete/partial clearance, time to complete/partial clearance and recurrence rates as well as safety parameters involving local signs/symptoms, AEs, physical examination, vital signs, or laboratory parameters. Complete clearance of all (baseline and new warts occurring during treatment) warts was additionally analysed as requested for the subsequent pivotal Phase 3 trials by the US Federal Drug Administration. Baseline comparisons were done using Wilcoxon s two-sample test (two-sided, α = 0.05). In addition, an influence analysis (logistic regression model) was done on wart location and area, age and numbers of warts, age of patients, usage of cream and ointment, and previous treatments to investigate contributing prognostic factors. Results Study patients Intent-to-treat (ITT) population included 238 patients (122 men and 116 women). Per-protocol (PP) population consisted of 225 patients (115 men and 110 women), and the safety population included 242 patients (125 male and 117 female). A total of 221 patients completed the study (fig. 1), whereas 21 patients discontinued the study prematurely: 6 patients were lost to follow-up, 3 patients reported AEs (allergic reaction, hypersensitivity/allergic vulvitis, severe itching and burning), 9 dropouts were evenly distributed over lack of efficacy, non-compliance and other reasons, 2 patients withdrew their consent, and 1 patient was withdrawn by the investigator. The first and second follow-up visits were completed by 97.3% and 85.0%, respectively, of all patients with complete clearance. The three treatment groups were comparable with respect to baseline characteristics. Total mean age was 33.2 years (range, years). In the age groups < 20 years, 3 (3.8%), 8 (10.1%) and 8 patients (9.6%), < 25 years, 22 (27.5%), 17 (21.5%) and 13 patients (15.7%), < 30 years, 12 (15.0%), 10 (12.7%) and 13 patients (15.7%), and 30 years, 43 (53.8%), 44 (55.7%) and 49 patients (59.0%) were from the 15% ointment, 10% cream and The Authors
4 Gross et al. Polyphenon E treatment of external genital warts fig. 1 Study patients: flow diagram of patient progress through the phases of this randomized, placebo-controlled study. Patients were randomly assigned to one of the treatment groups in a 2 : 1 active: placebo allocation ratio, stratified by gender. placebo groups, respectively. Mean weight was 72.4 kg (range, kg), and mean height was cm (range, cm). Majority of males were uncircumcised (96 patients, 78.7%). Most patients had never smoked (161 patients, 67.6%), and 105 patients (44.1%) had never consumed alcohol. Most patients (151 patients, 63.4%) had not had any previous episodes of external genital warts, 52 patients (21.8%) previously had one episode, 24 patients (10.1%) two episodes, and 11 patients (4.6%) three or more episodes. Ninety-two patients (38.7%) had warts previously treated (64 males and 28 females). Most males (33 patients, 27.0%) and only 6 females (5.2%) had their warts previously treated with curettage and electrodesiccation. Use of 0.5% podofilox was more common in male patients (24, 19.7%) than in female patients (3, 2.6%). The majority of females who had received previous treatments had chosen laser surgery (11 patients, 9.5%). The number of female patients with previous episodes was much lower than that of male patients. Baseline warts in male patients were mainly located at the penile shaft (70 patients, 57.4%) followed by the glans penis (30 patients, 24.6%), the perianal area (29 patients, 23.8%) and the foreskin (23 patients, 18.9%). Female patients had their baseline warts mainly located at the vulva (75 patients, 64.7%) followed by the perianal area (37 patients, 31.9%) and the perineal area (22 patients, 19.0%). In most instances, the treatment groups were comparable for both male and female patients regarding the locations of baseline warts. Compliance At the end of the study, all used and unused study medication was collected and weighed. As recorded by the investigators, overall assessment of compliance revealed that 94.5% of patients in the ITT population showed an at least adequate compliance ( 75%) at each study visit. Compliance was found to be poor (< 75%) in at least one visit for 13 patients (5.5%), 1 patient in the 10% cream 2007 The Authors 1407
5 Polyphenon E treatment of external genital warts Gross et al. group, 4 patients in the 15% ointment group and 8 patients in the placebo group. Compliance was comparable for both genders. Efficacy analysis: primary endpoint With respect to complete clearance rates of baseline warts (primary efficacy endpoint), differences between the 10% cream placebo group and the 15% ointment placebo group for male and female patients in the ITT as well as in the PP population were negligible (10% cream: 37.2%; 15% ointment: 37.5%; Fisher s exact test, P = ). Thus, pooling of placebo groups was justified. All analyses were also done by individual placebo groups, and the results mimic the results from the pooled placebo group. Differences between the 10% cream and the pooled placebo groups in male and female patients were not statistically significant (Fisher s exact test, P = for males and P = for females). On the other hand, differences between the 15% ointment and the pooled placebo groups in male and female patients were almost statistically significant (Fisher s exact test, P = for males and P = for females). When the data for both gender were pooled, the complete clearance rates of 10% cream (46.8%) and placebo (37.3%) were still not significantly different (Cochran-Mantel-Haenszel test, P = ). In contrast, the complete clearance rate of the 15% ointment group (59.0%) was statistically significantly higher compared with placebo (Cochran- Mantel-Haenszel test, P = ; Table 1). During the treatment period, complete response rates increased gradually only in the 15% ointment group but raised considerably in the last 4 weeks of treatment in all treatment groups. At week 4, complete response rates were 2.6%, 0.0% and 3.6% for the 15% ointment, 10% cream and placebo groups, respectively. At week 8, 6.4%, 0.0% and 2.4%, and at week 12, 41.0%, 37.7% and 31.3% were achieved in these treatment groups. A retrospective analysis of the complete clearance of all warts (baseline and new warts occurring during treatment) showed that 56.4% and 45.5% of patients of the 15% ointment and 10% cream groups were completely wart-free after 12 weeks of treatment compared to 37.5% and 37.2% of patients of the placebo ointment and cream groups, respectively. The difference between the 15% ointment and placebo ointment groups was almost statistically significant (Fisher s exact test, P = 0.079). Efficacy analysis: secondary endpoints Complete clearance rates for the PP population were similar to those of the ITT population and also yielded statistically significant result for the 15% ointment over Table 1 The number of patients with complete clearance rates (ITT population) 10% cream 15% ointment placebo n % n % n % Male Female Total * *Clearance rates statistically significantly higher than in placebo-treated patients (P = ). Table 2 The number of patients with a 75% to 100% reduction of baseline wart area (ITT population) 10% cream 15% ointment* placebo n % n % n % Male Female Total *Males and females significantly superior to placebo (P = and , respectively) and for the combined sexes (P = ). placebo with both gender combined (Cochran-Mantel- Haenszel test, P = ). This was not true for the 10% cream group. No significant differences were found between the 10% cream and 15% ointment groups in terms of complete clearance, irrespective of whether the gender were compared separately or combined (P = for males, P = for females, P = for gender combined). Complete clearance rates seem to be influenced to some extend by circumcision in males: the comparison of 15% ointment to placebo for uncircumcised men was almost statistically significantly different (P = ) compared with P = for circumcised men. Mean time to complete clearance of all baseline warts was 10.6 ± 2.6 weeks and was comparable between gender and active treatment groups. Evaluation of baseline characteristics (baseline wart area, baseline number of warts and average daily dose) and patient compliance showed no significant effect on the complete clearance rates seen in treatment or gender groups. Results for treatment success (75 100% clearance) are shown in Table 2. 15% ointment was statistically superior to placebo in both gender groups and for gender combined (P = for males, P = for females and P = for gender combined) The Authors
6 Gross et al. Polyphenon E treatment of external genital warts fig. 2 Incidence of any local skin reactions (i.e. local skin signs as assessed by the investigator and/or local skin symptoms as assessed by the patient) during the course of treatment. Prior to therapy, about 35% of patients in all three treatment groups presented with local skin reactions comprising local skin signs (overall: 13.6%) and local skin symptoms (overall: 29.3%), mainly itching, burning, and erythema. Shortly after treatment started, local reactions peaked at week 2, before they gradually declined under continued treatment to markedly lower levels at the end of treatment compared to the levels before treatment. Total number of patients with recurring baseline warts during the treatment period was very low at 4.2% (10 patients). For both gender, the 15% ointment group had the highest number of patients with recurring baseline warts (4 males and 2 females). Recurrence of baseline warts during the first 4 weeks of follow-up occurred only in 3 males (2.7%), 1 in the 10% Cream group and 2 in the 15% ointment group in both the ITT and the PP populations. From the first to the second follow-up visit (12 weeks after the end of treatment), recurrence occurred in only three male patients in each of the three treatment groups. There were no recurrences in female patients throughout the follow-up. Thus, overall recurrence rates for the 12-week follow-up period were 10.6%, 11.8% and 10.3% for the Polyphenon E 15% ointment, the Polyphenon E 10% cream and the pooled placebo groups, respectively. Influence of prognostic factors Complete response rate in the placebo group was surprisingly high (37.3%). Analyses were also done to assess the contribution of baseline characteristics of the warts to efficacy rates in the different treatment groups. In addition, a logistic regression procedure was defined with the primary endpoint, complete clearance of all baseline warts, as the dependent variable vs. a set of related explanatory variables (treatment, pretreatment, age of patients, and wart characteristics at baseline) to identify relevant prognostic factors. With the present sample sizes and due to difficulties in satisfying the model assumptions (poor model fit), no parameters could be identified as relevant prognostic factors. Local tolerability as assessed by the investigator and patient At all visits, the blinded investigator assessed local skin signs and questioned the patient about local skin symptoms. An overview of the incidence of any local skin signs and/or symptoms during treatment is shown in fig. 2. Local skin symptoms were present at the baseline visit in 25 (31.3%) and 22 patients (27.8%) in the 15% ointment and 10% cream groups compared with 24 patients (28.0%) in the placebo group. At the same time, 10 (12.5%) and 9 patients (11.4%) in the 15% ointment and 10% cream groups had local skin signs compared to 14 patients (16.9%) in the placebo group. After start of treatment, the incidence of local reactions increased in all three treatment groups to a maximum in week 2 (overall 37.0% and 31.4% local skin signs and symptoms, respectively). Subsequently, all local reactions gradually declined through the treatment period in all three treatment groups to levels well below the ones before start of treatment. A number of local signs and symptoms were most frequent in the 15% ointment group at all visits. The majority of local skin signs were of mild intensity, most local skin symptoms of mild to moderate intensity. The most frequently observed local skin sign was erythema, being most frequent at week 2 (52 patients [22.1%] with mild, 22 patients [9.4%] with moderate and 4 patients [1.7%] with severe erythema). Overall, burning and itching were the most frequent local skin symptoms with their maximum also at week 2. They were reported in 44 (18.7%) and 46 patients (19.6%) with mild, in 14 (6.0%) and 16 patients (6.8%) with moderate and in 2 (0.9%) and 3 patients (1.3%) with severe intensity, respectively. At end of treatment, local signs were present in 13 (17.1%) and 6 patients (7.9%) in the 15% ointment and 10% cream groups, respectively, compared with 7 patients (8.6%) in the placebo group, and local symptoms were reported for 10 (12.5%) and 5 patients (6.3%) in the 15% ointment and 10% cream groups, respectively, compared with 7 patients (8.4%) in the placebo group. During the treatment-free 12-week follow-up, only 1 patient (2.2%) in the 15% ointment group had local reactions at the first follow-up visit (4 weeks after treatment); 2007 The Authors 1409
7 Polyphenon E treatment of external genital warts Gross et al. in addition, 1 patient each in the 15% ointment and 10% cream groups had local skin symptoms at the second follow-up visit (12 weeks after treatment). Complete responders had an increased number of local skin reactions (79 complete responders, 69.9%) when compared to non-responders (58 non-responders, 46.4%), proved by statistical significance (Fisher s exact test, two-sided, P = ). The difference in the complete responders group was mainly based on the difference between the Polyphenon E 15% ointment group and the placebo group. In the 15% ointment group, 38 (82.6%) complete responders had (a) local reaction(s) compared with 15 (48.4%) complete responders in the placebo group (P = ). A similar statistically significant correlation with complete clearance was found when the local skin signs (P = ) and local skin symptoms (P = ) were assessed separately, for 15% ointment vs. placebo for both any local skin signs (P < ) and any local skin symptoms (P = ), and also for the 10% cream vs. placebo for any local skin signs (P < ). However, when only severe local skin reactions, severe signs or severe symptoms were considered, no significant differences were found both in the comparison of complete responders vs. non-complete responders overall and in the comparison of the active treatment groups vs. placebo. Time to first onset analysis of any local sign or symptom with an at least moderate intensity yielded a significant result for the comparison between the two active formulations only in male patients (P = ), indicating that such symptoms were to occur sooner in the 15% ointment group. For female patients, no statistically significant differences were found. tract infection (not otherwise specified; 4 patients, 1.7%) being the most common, followed by skin and subcutaneous tissue disorders (3 patients, 1.2%). The number of female patients (n = 5, 4.3%) with treatment-related AEs was higher than for male patients (n = 1, 0.8%). Withdrawals due to AEs involved three patients in the 15% ointment group. One female patient developed (unproven) allergic dermatitis/vulvitis of severe intensity, which was assessed as probably related to the study drug. Another female patient developed allergic dermatitis of mild intensity and assessed as possibly related to the study drug. In both cases, allergic reactions were not confirmed because no retesting was done. One male patient developed severe itching and burning, assessed as probably study drug-related. During the follow-up period, only 5 patients (2.1%) had a total number of 6 AEs. All AEs occurring during the follow-up period were assessed as not related to study treatment. Other safety assessments Regarding the laboratory values, mean values of laboratory parameters showed only negligible changes during the treatment period. No clinically significant abnormal laboratory values were reported. Mean blood pressure, heart rate and body temperature were also generally within normal range at baseline and at the end of the study, hardly changing in between. Physical examinations revealed normal results in most of the system organ classes of all patients examined. No clinically significant changes were observed during the treatment period. AEs other than local reactions Throughout the study, there were no serious AEs, and no deaths occurred. During the treatment period, 19 patients (7.9%) in the safety population (242 patients) had 21 AEs involving 7 patients (8.9%, 7 AEs) receiving 10% cream, 9 patients (11.3%, 10 AEs) receiving 15% ointment and 3 patients (3.6%, 4 AEs) receiving placebo. Only 6 of these patients (2.5%) had AEs that were considered possibly or probably related to study drug, with 2 patients in the 10% cream group (hyperkeratosis and skin discoloration) and 4 patients in the 15% ointment group (transient local necrosis [not otherwise specified], allergic dermatitis [2 patients], and pain in the foreskin). Of these 21 AEs, 8 were mild, 11 moderate and 2 were of severe intensity, with the latter being probably related to the study drug. Most frequent AEs, experienced by 9 patients (3.7%), were classified as infections and infestations, with respiratory Discussion The study has shown clearly that the 15% ointment preparation was more effective than the 10% cream formulation in significantly increasing the wart clearance rates compared with those of placebo. Following treatment with 15% ointment, complete clearance of baseline warts was observed in 59% of patients (vs. placebo P = ), and a 75% to 100% reduction in wart area (therapeutic success) was seen in 80.8% of patients (vs. placebo P = ). These significant effects were observed in both the ITT and the PP populations, and results were similar for both gender. Clearance rates for the 10% cream treatment group vs. placebo were not statistically significant: total clearance was achieved in 46.8% of patients vs. 37.3% for placebo, and 75% to 100% reduction in wart area was observed in 54.5% of patients vs. 51.8% for placebo. Complete clearance rate of placebo (37.3%) was higher than expected. However, in recent clinical The Authors
8 Gross et al. Polyphenon E treatment of external genital warts studies, rates of spontaneous healing have been reported to be as high as 40%. 12,13 Thorough care and increased hygiene during the treatment period as well as known irritant base excipients might have contributed collaterally to the placebo effect. In previous studies conducted in China (Cheng SJ, Beijing; see Introduction), tea polyphenols achieved an overall complete clearance of external genital warts in 57% of patients. In a number of clinical trials, 0.5% podofilox (marketed as Condylox in Germany and USA and as Condyline in Russia) gave rise to a 30% to 70% complete response rate but had a recurrence rate of approximately 33% to 91%. 2,3,14 Another patient-applied modality, 5% imiquimod cream (marketed as Aldara ), gave a 30% to 62% response rate in men 3,7,15,16 and a 60% to 80% response rate in women, 3,4,15,16 with approximately 13% to 19% recurrence rates in both gender within 6 months after the last administration. 3,4,16 It is worth noting that response rates for Polyphenon E in our study were comparable for both gender. Complete clearance for 15% ointment was 61% for males and 56.8% for females. A 75% to 100% reduction (treatment success) was observed in 80.5% of male patients and in 81.1% of female patients. The relatively poor response to 5% imiquimod cream in males has been linked to greater keratinization of the skin, which influences drug penetration, 7,15 particularly on the penile shaft, which is the most common location of warts in males. The lower degree of keratinization and the semiocclusive effect of the foreskin have also been linked to higher clearance rates observed among uncircumcised men. 7 For Polyphenon E, favourable complete response rates for both gender observed in the study indicate that keratinization of the skin might not be a limiting factor. A strong treatment effect was observed in uncircumcised men treated with the 15% ointment. Given that the majority of men in the world are uncircumcised, this may suggest an additional advantage of Polyphenon E over currently available topical treatments in certain regions. Recurrence rates for the 15% ointment at 3 months after the end of treatment were 10.6% on average, with 20.0% recurrences in males and none in females. The recurrence rate in the placebo group is in the range of the active treatments. Comparison of values did not show any notable differences between the treatment groups. However, due to the limited number of available patients, these findings should be considered as preliminary. Further studies are warranted to compare the recurrence rates between gender and treatments. Overall, the number of patients with study treatment related AEs other than local reactions as well as the number of severe AEs was remarkably low. Discontinuations due to AE were all local reactions at the application site. It is assumed that investigators, not knowing the underlying active principle of the Polyphenon E catechins, assessed the expeditiously flashing local skin reactions after treatment initiation as allergic reactions. An immune-stimulatory mode of action of the Polyphenon E formulations is strengthened by own in vitro and in vivo studies (unpublished data) and the correlation found between complete responders and the incidence of local skin reactions. Most local signs and symptoms observed in the 15% ointment group, many of which were not treatment emergent, were only of mild intensity that subsided over the course of the study. Evaluating the local tolerability of Polyphenon E vs. an available topical agent such as imiquimod, Polyphenon E performed well. The most frequent local sign observed in the 15% ointment group was erythema, with the majority of events of mild intensity and < 2% patients experiencing severe erythema. In comparison, severe erythema has been observed in 5.7% and 11% of patients in two studies of imiquimod 5% cream in populations including both gender, 6,17 in 7% of patients in an all female population, 4 and in up to 9% of patients in two studies of all male populations. 5,7 The results obtained in this study, particularly the favourable safety profile are appreciable given that HPV infections are a major health concern. 1 Currently available treatments (e.g. cryotherapy, laser, podofilox and curettage) are often painful, tissue destructive, expensive, and recurrence is frequent. Local therapy with podophyllin or trichloroacetic acid requires multiple applications done by the physician and often causes considerable problems associated with local skin reactions, such as burning, itching and scarring. 2 8 Other topical treatments such as imiquimod and podofilox are proven to be effective, but podofilox showed high recurrence rates, and both are also frequently associated with application site reactions, which may result in discontinuation of treatment and reduced patient compliance, ultimately affecting overall efficacy. Superiority of the 15% ointment over placebo was established in this study, but not for the 10% cream. Therefore, the 15% ointment will be used for further development. As more than two thirds of the patients had complete clearance relatively late, between 8 and 12 weeks of treatment, it is anticipated that partial responders at week 12 may achieve complete clearance if the maximum treatment period is extended to 16 weeks. In conclusion, the results of this study suggest that Polyphenon E is a favourable new self-applicable treatment for external genital warts with properties that differ from other treatments. In view of the beneficial efficacy and excellent safety profile of the 15% ointment, this formulation will be further evaluated in prospective clinical studies The Authors 1411
9 Polyphenon E treatment of external genital warts Gross et al. Acknowledgements We are indebted to the investigators who participated in this study, and we gratefully acknowledge their contribution, in Germany [Prof. G Gross, Rostock; Dr. KG Meyer, Berlin; Dr. H Pres, Berlin; Dr. C Bayerl, Mannheim; Dr. R Ellringmann, Kirchzarten; Dr. HW Grimm, Gernsheim; Dr. K Ihm, Munich; Dr. H Lupp, Bruchsal; Dr. P Pierchalla, Recklinghausen; Dr. D Quast, Oranienburg; Prof. E Stockfleth, Kiel (now Berlin); Dr. HG Weidenhammer, Freiburg; Dr. RN Bartelt, Frankfurt; Dr. MS El Tobgui, Frankfurt; Dr. N Fischer, Munich; Dr. HW Hanf, Rees; Dr. P Kirschner, Berlin; Dr. M Sebastian, Mahlow; Dr. J Tyagi, Muehlheim; Dr. E Zahn, Berlin] and in Russia [Dr. O Moryleva; Dr. M Umakhanova; Dr. V Stepanov; Dr. V Krasnopolskiy; Dr. N Podzolkova; Dr. A Kubanova; Dr. V Prilepskaya, all Moscow]. The authors would like to thank Quintiles GmbH as the conducting CRO and Omnicare Clinical Research Ltd for medical writing support. References 1 Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med 1997; 102: von Krogh G, Lacey CJ, Gross G, Barrasso R, Schneider A. European course on HPV associated pathology: guidelines for primary care physicians for the diagnosis and management of anogenital warts. Sex Transm Infect 2000; 76: Beutner KR, Tyring SK, Trofatter KF Jr et al. Imiquimod, a patient-applied immune-response modifier for treatment of external genital warts. Antimicrob Agents Chemother 1998; 42: Buck HW, Fortier M, Knudsen J, Paavonen J. Imiquimod 5% cream in the treatment of anogenital warts in female patients. Int J Gynaecol Obstet 2002; 77: Fife KH, Ferenczy A, Douglas JM Jr, Brown DR, Smith M, Owens ML.; HPV Study Group. Treatment of external genital warts in men using 5% imiquimod cream applied three times a week, once daily, twice daily, or three times a day. Sex Transm Dis 2001; 28: Garland SM, Sellors JW, Wikstrom A et al.; Imiquimod Study Group. Imiquimod 5% cream is a safe and effective self-applied treatment for anogenital warts-results of an open-label, multicentre Phase IIIB trial. Int J STD AIDS 2001; 12: Gollnick H, Barasso R, Jappe U et al. Safety and efficacy of imiquimod 5% cream in the treatment of penile genital warts in uncircumcised men when applied three times weekly or once per day. Int J STD AIDS 2001; 12: Gross G. Therapy of human papillomavirus infection and associated epithelial tumors. Intervirology 1997; 40: Chow HH, Cai Y, Alberts DS et al. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev 2001; 10: Lin JK, Liang YC. Cancer chemoprevention by tea polyphenols. Proc Natl Sci Counc Repub China B 2000; 24: World Medical Association Declaration of Helsinki: Ethical Principles For Medical Research Involving Human Subjects. Somerset West (South Africa), Available at: Accessed March 11, Wiley DJ, Douglas J, Beutner K et al. External genital warts: diagnosis, treatment, and prevention. Clin Inf Dis 2002; 35: S210 S Beutner KR, Wiley DJ, Douglas JM et al. Genital warts and their treatment. Clin Inf Dis 1999; 28: S37 S Wiley DJ. Genital warts. Clin Evid 2001; 6: Sauder DN, Skinner RB, Fox TL, Owens ML. Topical imiquimod 5% cream as an effective treatment for external genital and perianal warts in different patient populations. Sex Transm Dis 2003; 30: von Krogh G. Management of anogenital warts (condylomata acuminata). Eur J Dermatol 2001; 11: Edwards L, Ferenczy A, Eron L et al. Self-administered topical 5% imiquimod cream for external anogenital warts. HPV Study Group. Human papilloma virus. Arch Dermatol 1998; 134: The Authors
camellia sinensis (green tea) leaf extract 10% ointment (Catephen ) SMC No. (1133/16) Kora Healthcare
 camellia sinensis (green tea) leaf extract 10% ointment (Catephen ) SMC No. (1133/16) Kora Healthcare 04 March 2016 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
camellia sinensis (green tea) leaf extract 10% ointment (Catephen ) SMC No. (1133/16) Kora Healthcare 04 March 2016 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Efficacy, safety and tolerability of green tea catechins in the treatment of external anogenital warts: a systematic review and meta-analysis
 DOI: 10.1111/j.1468-3083.2010.03796.x JEADV ORIGINAL ARTICLE Efficacy, safety and tolerability of green tea catechins in the treatment of external anogenital warts: a systematic review and meta-analysis
DOI: 10.1111/j.1468-3083.2010.03796.x JEADV ORIGINAL ARTICLE Efficacy, safety and tolerability of green tea catechins in the treatment of external anogenital warts: a systematic review and meta-analysis
Supplementary Table 1: Study and sample characteristics of the included studies
 Supplementary Table 1: Study and sample characteristics of the included studies First author, Imiquimod 5% cream vs. placebo Arican et al. 2004 34 Beutner et al. 1998a 35 Tyring et al. 1998a 36 Beutner
Supplementary Table 1: Study and sample characteristics of the included studies First author, Imiquimod 5% cream vs. placebo Arican et al. 2004 34 Beutner et al. 1998a 35 Tyring et al. 1998a 36 Beutner
TRANSPARENCY COMMITTEE Opinion 17 September 2014
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 17 September 2014 VEREGEN 10%, ointment Tube of 15 g (CIP: 34009 222 531 2 1) Applicant: EXPANSCIENCE INN ATC Code
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 17 September 2014 VEREGEN 10%, ointment Tube of 15 g (CIP: 34009 222 531 2 1) Applicant: EXPANSCIENCE INN ATC Code
AWMSG SECRETARIAT ASSESSMENT REPORT. Green tea leaf extract (Catephen ) 10% Ointment. Reference number: 2739 FULL SUBMISSION
 AWMSG SECRETARIAT ASSESSMENT REPORT Green tea leaf extract (Catephen ) 10% Ointment Reference number: 2739 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics and Toxicology Centre
AWMSG SECRETARIAT ASSESSMENT REPORT Green tea leaf extract (Catephen ) 10% Ointment Reference number: 2739 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics and Toxicology Centre
EXTERNAL ANOGENITAL WARTS
 EXTERNAL ANOGENITAL WARTS Whats new HPV vaccination is now available to MSM under the age of 45 years attending Sexual Health Services. This vaccination is recommended even if a prior/current history of
EXTERNAL ANOGENITAL WARTS Whats new HPV vaccination is now available to MSM under the age of 45 years attending Sexual Health Services. This vaccination is recommended even if a prior/current history of
Effect of adjuvant imiquimod 5% cream on sustained clearance of anogenital warts following laser treatment
 Infect Dis Obstet Gynecol 22;1:79 88 Effect of adjuvant imiquimod 5% cream on sustained clearance of anogenital warts following laser treatment U. B. Hoyme 1, M. Hagedorn 2, A.-E. Schindler 3, P. Schneede
Infect Dis Obstet Gynecol 22;1:79 88 Effect of adjuvant imiquimod 5% cream on sustained clearance of anogenital warts following laser treatment U. B. Hoyme 1, M. Hagedorn 2, A.-E. Schindler 3, P. Schneede
Richard Gilson, Jonathan Ross, Raymond Maw, David Rowen, Christopher Sonnex, Charles Lacey
 A multi-centre, randomised, double-blind, placebo-controlled study of cryotherapy versus cryotherapy and podophyllotoxin cream as treatment for external anogenital warts. Richard Gilson, Jonathan Ross,
A multi-centre, randomised, double-blind, placebo-controlled study of cryotherapy versus cryotherapy and podophyllotoxin cream as treatment for external anogenital warts. Richard Gilson, Jonathan Ross,
STRAIGHT TALK COUNSELING PATIENTS ABOUT EXTERNAL GENITAL AND PERIANAL WARTS (EGW)
 STRAIGHT TALK COUNSELING PATIENTS ABOUT EXTERNAL GENITAL AND PERIANAL WARTS (EGW) Newly Diagnosed Patients and/or Those Who May Become Sexually Active As a Healthcare Provider (HCP), starting a conversation
STRAIGHT TALK COUNSELING PATIENTS ABOUT EXTERNAL GENITAL AND PERIANAL WARTS (EGW) Newly Diagnosed Patients and/or Those Who May Become Sexually Active As a Healthcare Provider (HCP), starting a conversation
HPV Transmission. Rachel Winer, PhD, MPH Department of Epidemiology University of Washington
 HPV Transmission Rachel Winer, PhD, MPH Department of Epidemiology University of Washington rlw@u.washington.edu Disclosure Information I have no financial relationships to disclose. Human Papillomavirus
HPV Transmission Rachel Winer, PhD, MPH Department of Epidemiology University of Washington rlw@u.washington.edu Disclosure Information I have no financial relationships to disclose. Human Papillomavirus
Clinical Trial Study Synopsis
 Clinical Trial Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency
Clinical Trial Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency
Human Papillomavirus. Kathryn Thiessen, ARNP, ACRN The Kansas AIDS Education and Training Center The University of Kansas School of Medicine Wichita
 Human Papillomavirus Kathryn Thiessen, ARNP, ACRN The Kansas AIDS Education and Training Center The University of Kansas School of Medicine Wichita What is Genital HPV Infection Human papillomavirus is
Human Papillomavirus Kathryn Thiessen, ARNP, ACRN The Kansas AIDS Education and Training Center The University of Kansas School of Medicine Wichita What is Genital HPV Infection Human papillomavirus is
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
What you need to know about EGW
 For the treatment of external genital and perianal warts (EGW) What you need to know about EGW Indication VEREGEN is indicated for the topical treatment of external genital and perianal warts (Condylomata
For the treatment of external genital and perianal warts (EGW) What you need to know about EGW Indication VEREGEN is indicated for the topical treatment of external genital and perianal warts (Condylomata
The no t-so-simple cutaneous wart
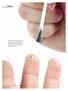 Salicylic acid is a commonly used over-the-counter treatment for nongenital warts. Clearance rates of up to 75% have been reported with salicylic acid. istockphoto 10 Clinical practice guide Dermatology
Salicylic acid is a commonly used over-the-counter treatment for nongenital warts. Clearance rates of up to 75% have been reported with salicylic acid. istockphoto 10 Clinical practice guide Dermatology
HPV Epidemiology and Natural History
 HPV Epidemiology and Natural History Rachel Winer, PhD, MPH Associate Professor Department of Epidemiology University of Washington School of Public Health rlw@uw.edu Human Papillomavirus (HPV) DNA virus
HPV Epidemiology and Natural History Rachel Winer, PhD, MPH Associate Professor Department of Epidemiology University of Washington School of Public Health rlw@uw.edu Human Papillomavirus (HPV) DNA virus
Human Papilloma Viruses HPV Testing and Treatment of STDs
 Human Papilloma Viruses HPV Testing and Treatment of STDs New York State Collaborative Efforts in Medical Evaluations of Child and Adolescent Sexual Offenses. Child and Adolescent Sexual Offense Post-Assault
Human Papilloma Viruses HPV Testing and Treatment of STDs New York State Collaborative Efforts in Medical Evaluations of Child and Adolescent Sexual Offenses. Child and Adolescent Sexual Offense Post-Assault
PFIZER INC. GENERIC DRUG NAME and/or COMPOUND NUMBER: 5% Spironolactone Cream/ PF
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Penis Cancer. What is penis cancer? Symptoms. Patient Information. Pagina 1 / 9. Patient Information - Penis Cancer
 Patient Information English 31 Penis Cancer The underlined terms are listed in the glossary. What is penis cancer? Cancer is abnormal cell growth in the skin or organ tissue. When this cell growth starts
Patient Information English 31 Penis Cancer The underlined terms are listed in the glossary. What is penis cancer? Cancer is abnormal cell growth in the skin or organ tissue. When this cell growth starts
Penis Cancer. What is penis cancer? Symptoms. Patient Information. Pagina 1 / 9. Patient Information - Penis Cancer
 Patient Information English 31 Penis Cancer The underlined terms are listed in the glossary. What is penis cancer? Cancer is abnormal cell growth in the skin or organ tissue. When this cell growth starts
Patient Information English 31 Penis Cancer The underlined terms are listed in the glossary. What is penis cancer? Cancer is abnormal cell growth in the skin or organ tissue. When this cell growth starts
Study Code: Date: 27 July 2007
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
Clinical Trial Report Synopsis. Efficacy and Safety of LEO in Field Treatment of Actinic Keratosis on Face or Chest including 12-month follow-up
 Clinical Trial Report Synopsis Efficacy and Safety of LEO 43204 in Field Treatment of Actinic Keratosis on Face or Chest including 12-month follow-up Design of trial: A phase 3, multi-centre, randomised,
Clinical Trial Report Synopsis Efficacy and Safety of LEO 43204 in Field Treatment of Actinic Keratosis on Face or Chest including 12-month follow-up Design of trial: A phase 3, multi-centre, randomised,
Make Love Not Warts Genital Warts
 Patricia Wong, MD 735 Cowper Street Palo Alto, CA 94301 650-473-3173 www.patriciawongmd.com Make Love Not Warts Genital Warts Genital warts are the most common sexually transmitted disease. The lifetime
Patricia Wong, MD 735 Cowper Street Palo Alto, CA 94301 650-473-3173 www.patriciawongmd.com Make Love Not Warts Genital Warts Genital warts are the most common sexually transmitted disease. The lifetime
Clinical Trial Report Synopsis
 Clinical Trial Report Synopsis A phase 2a, proof of concept trial, testing twice daily application of LEO 124249 ointment 30 mg/g in the treatment of mild to moderate inverse psoriasis Design of trial:
Clinical Trial Report Synopsis A phase 2a, proof of concept trial, testing twice daily application of LEO 124249 ointment 30 mg/g in the treatment of mild to moderate inverse psoriasis Design of trial:
Adalimumab M Clinical Study Report Final R&D/16/0603
 Methodology (Continued): The 70-day safety follow-up period started from the last dose of study drug, but was not required for any subject who initiated commercial Humira after study completion. Additional
Methodology (Continued): The 70-day safety follow-up period started from the last dose of study drug, but was not required for any subject who initiated commercial Humira after study completion. Additional
SYNOPSIS. Issue Date: 25 Oct 2011
 SYNOPSIS Issue Date: 25 Oct 2011 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient(s) Janssen Research & Development STELARA Ustekinumab Protocol No.: Title of Study: Study Name:
SYNOPSIS Issue Date: 25 Oct 2011 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient(s) Janssen Research & Development STELARA Ustekinumab Protocol No.: Title of Study: Study Name:
Genital Human Papillomavirus (HPV) Infections
 Genital Human Papillomavirus (HPV) Infections January 2008 Etiology... 1 Epidemiology... 1 Prevention... 2 Diagnosis... 3 Management... 6 Treatment... 7 Consideration for Other STIs... 9 Reporting and
Genital Human Papillomavirus (HPV) Infections January 2008 Etiology... 1 Epidemiology... 1 Prevention... 2 Diagnosis... 3 Management... 6 Treatment... 7 Consideration for Other STIs... 9 Reporting and
NVESTIGATION Abstract: INTRODUCTION
 236 INVESTIGATION s A prospective, open, comparative study of 5% potassium hydroxide solution versus cryotherapy in the of genital warts in men* Caio Lamunier de Abreu Camargo 1 Walter Belda Junior 1 Luiz
236 INVESTIGATION s A prospective, open, comparative study of 5% potassium hydroxide solution versus cryotherapy in the of genital warts in men* Caio Lamunier de Abreu Camargo 1 Walter Belda Junior 1 Luiz
Primary Endpoint The primary endpoint is overall survival, measured as the time in weeks from randomization to date of death due to any cause.
 CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
Clinical Trial Report Synopsis
 Clinical Trial Report Synopsis Efficacy and Safety of Ingenol Mebutate Gel in Field Treatment of Actinic Keratosis on Full Face, Balding Scalp or Approximately 250 cm 2 on the Chest Design of trial: Part
Clinical Trial Report Synopsis Efficacy and Safety of Ingenol Mebutate Gel in Field Treatment of Actinic Keratosis on Full Face, Balding Scalp or Approximately 250 cm 2 on the Chest Design of trial: Part
EVIDENCE-BASED DERMATOLOGY: ORIGINAL CONTRIBUTION. Direct Medical Costs for Surgical and Medical Treatment of Condylomata Acuminata
 Direct Medical Costs for Surgical and Medical Treatment of Condylomata Acuminata Murad Alam, MD; Matthew Stiller, MD EVIDENCE-BASED DERMATOLOGY: ORIGINAL CONTRIBUTION Objective: To determine which treatment
Direct Medical Costs for Surgical and Medical Treatment of Condylomata Acuminata Murad Alam, MD; Matthew Stiller, MD EVIDENCE-BASED DERMATOLOGY: ORIGINAL CONTRIBUTION Objective: To determine which treatment
Department of Obstetrics & Gynecology, Jersey Shore University Medical Center, Neptune, NJ 07753, USA 4
 Infectious Diseases in Obstetrics and Gynecology Volume 2011, Article ID 806105, 11 pages doi:10.1155/2011/806105 Research Article Imiquimod 3.75% Cream Applied Daily to Treat Anogenital Warts: Combined
Infectious Diseases in Obstetrics and Gynecology Volume 2011, Article ID 806105, 11 pages doi:10.1155/2011/806105 Research Article Imiquimod 3.75% Cream Applied Daily to Treat Anogenital Warts: Combined
Pathology of the Cervix
 Pathology of the Cervix Thomas C. Wright Pathology of the Cervix Topics to Consider Burden of cervical cancer 1 Invasive Cervical Cancer Cervical cancer in world Second cause of cancer death in women Leading
Pathology of the Cervix Thomas C. Wright Pathology of the Cervix Topics to Consider Burden of cervical cancer 1 Invasive Cervical Cancer Cervical cancer in world Second cause of cancer death in women Leading
HPV Management in Special Populations
 HPV Management in Special Populations MELISSA KOTTKE, MD, MPH, MBA Disclosures Merck, Nexplanon trainer CSL Behring, Consultant Evofem, Advisory Board Most treatment options for HPV in special populations
HPV Management in Special Populations MELISSA KOTTKE, MD, MPH, MBA Disclosures Merck, Nexplanon trainer CSL Behring, Consultant Evofem, Advisory Board Most treatment options for HPV in special populations
Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary
 Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-8 Topical Diclofenac Gel Indication
Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-8 Topical Diclofenac Gel Indication
1: : Lifetime risk for prostate cancer 1:27. Lifetime risk for. testicular cancer. Lifetime risk for. penile cancer
 *Based on the National Cancer Registry of 2010 Lifetime risk for prostate cancer 1:27 Lifetime risk for testicular cancer Lifetime risk for penile cancer 1:2 040 1:1 114 Prostate cancer 1 in 27 South African
*Based on the National Cancer Registry of 2010 Lifetime risk for prostate cancer 1:27 Lifetime risk for testicular cancer Lifetime risk for penile cancer 1:2 040 1:1 114 Prostate cancer 1 in 27 South African
Human Papillomaviruses and Cancer: Questions and Answers. Key Points. 1. What are human papillomaviruses, and how are they transmitted?
 CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Human Papillomaviruses
CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Human Papillomaviruses
NDA /S-001. ALDARA [al dar a] (imiquimod) Cream, 5% For Dermatologic Use Only - Not for Ophthalmic Use.
![NDA /S-001. ALDARA [al dar a] (imiquimod) Cream, 5% For Dermatologic Use Only - Not for Ophthalmic Use. NDA /S-001. ALDARA [al dar a] (imiquimod) Cream, 5% For Dermatologic Use Only - Not for Ophthalmic Use.](/thumbs/89/100134179.jpg) ALDARA [al dar a] (imiquimod) Cream, 5% For Dermatologic Use Only - Not for Ophthalmic Use. DESCRIPTION Aldara is the brand name for imiquimod which is an immune response modifier. Each gram of the 5%
ALDARA [al dar a] (imiquimod) Cream, 5% For Dermatologic Use Only - Not for Ophthalmic Use. DESCRIPTION Aldara is the brand name for imiquimod which is an immune response modifier. Each gram of the 5%
Individual Study Table Referring to Part of the Dossier. Volume: Page:
 1 SYNOPSIS (CR002878) Title of Study: The effect of on vasomotor symptoms in healthy postmenopausal women: a double-blind placebo controlled pilot study Investigators: Multiple, see Section 4, Investigators
1 SYNOPSIS (CR002878) Title of Study: The effect of on vasomotor symptoms in healthy postmenopausal women: a double-blind placebo controlled pilot study Investigators: Multiple, see Section 4, Investigators
International Journal of Pharma and Bio Sciences
 Original Research Article Medicine International Journal of Pharma and Bio Sciences ISSN 0975-6299 POSSIBLE EFFECTIVENESS OF ACYCLOVIR AS MEDICAL TREATMENT OPTION FOR VULVAL WARTS DR.BUSHRA J. UMRAN Babylon
Original Research Article Medicine International Journal of Pharma and Bio Sciences ISSN 0975-6299 POSSIBLE EFFECTIVENESS OF ACYCLOVIR AS MEDICAL TREATMENT OPTION FOR VULVAL WARTS DR.BUSHRA J. UMRAN Babylon
Human Papillomavirus (HPV) in Patients with HIV.
 Human Papillomavirus (HPV) in Patients with HIV www.hivguidelines.org Purpose of the Guideline Increase the numbers of NYS residents with HIV who are screened for HPV-related dysplasia and managed effectively.
Human Papillomavirus (HPV) in Patients with HIV www.hivguidelines.org Purpose of the Guideline Increase the numbers of NYS residents with HIV who are screened for HPV-related dysplasia and managed effectively.
Human Papillomavirus
 Human Papillomavirus Dawn Palaszewski, MD Assistant Professor of Obstetrics and Gynecology University of February 18, 2018 9:40 am Dawn Palaszewski, MD Assistant Professor Department of Obstetrics and
Human Papillomavirus Dawn Palaszewski, MD Assistant Professor of Obstetrics and Gynecology University of February 18, 2018 9:40 am Dawn Palaszewski, MD Assistant Professor Department of Obstetrics and
HPV & RELATED DISEASES
 GAY MEN, HPV & ANAL CANCER THEBOTTOMLINE.ORG.AU HPV & RELATED DISEASES WHAT IS HPV? The Human Papilloma Virus (HPV) is not one virus, but a family of about 200 different ones that cause common warts, genital
GAY MEN, HPV & ANAL CANCER THEBOTTOMLINE.ORG.AU HPV & RELATED DISEASES WHAT IS HPV? The Human Papilloma Virus (HPV) is not one virus, but a family of about 200 different ones that cause common warts, genital
Adalimumab M Clinical Study Report Final R&D/14/1263. Page:
 Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study:
Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study:
Clinical Trial Study Synopsis
 Clinical Trial Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency
Clinical Trial Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency
Received 5 December 2003; accepted 21 January 2004
 Journal of Antimicrobial Chemotherapy (2004) 53, 703 707 DOI: 10.1093/jac/dkh154 Advance Access publication 24 March 2004 Clinical significance of antiviral therapy for episodic treatment of herpes labialis:
Journal of Antimicrobial Chemotherapy (2004) 53, 703 707 DOI: 10.1093/jac/dkh154 Advance Access publication 24 March 2004 Clinical significance of antiviral therapy for episodic treatment of herpes labialis:
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Sponsor / Company: Sanofi Drug substance(s): AMARYL M (1/250 mg) / HOE490
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
Sexually Transmitted Diseases. Summary of CDC Treatment Guidelines
 DC 2015 Sexually Transmitted Diseases Summary of CDC Treatment Guidelines These summary guidelines reflect the June 2015 update to the 2010 CDC Guidelines for Treatment of Sexually Transmitted Diseases.
DC 2015 Sexually Transmitted Diseases Summary of CDC Treatment Guidelines These summary guidelines reflect the June 2015 update to the 2010 CDC Guidelines for Treatment of Sexually Transmitted Diseases.
Clinical Study Report SLO-AD-1 Final Version DATE: 09 December 2013
 1. Clinical Study Report RANDOMIZED, OPEN, PARALLEL GROUP, PHASE IIIB STUDY ON THE EVALUATION OF EFFICACY OF SPECIFIC SUBLINGUAL IMMUNOTHERAPY IN PAEDIATRIC PATIENTS WITH ATOPIC DERMATITIS, WITH OR WITHOUT
1. Clinical Study Report RANDOMIZED, OPEN, PARALLEL GROUP, PHASE IIIB STUDY ON THE EVALUATION OF EFFICACY OF SPECIFIC SUBLINGUAL IMMUNOTHERAPY IN PAEDIATRIC PATIENTS WITH ATOPIC DERMATITIS, WITH OR WITHOUT
PRODUCT MONOGRAPH. Sinecatechins Ointment, 10% w/w
 PRODUCT MONOGRAPH Pr VEREGEN Sinecatechins Ointment, 10% w/w Topical Treatment for Genital Warts Canadian Distributor: 100 Alexis-Nihon Blvd., Suite 600 St-Laurent, Québec, Canada H4M 2P2 Date of Revision:
PRODUCT MONOGRAPH Pr VEREGEN Sinecatechins Ointment, 10% w/w Topical Treatment for Genital Warts Canadian Distributor: 100 Alexis-Nihon Blvd., Suite 600 St-Laurent, Québec, Canada H4M 2P2 Date of Revision:
Harleen Chhachhi 1, Anil Kumar Gupta 2, Santosh Kumar Singh 3, Rakesh Kumar Patel 4, Pawan Kumar Patel 5, Raj Kumar 6, Avnish Kumar 7
 THE SOCIODEMOGRAPHIC CHARACTERISTICS, CLINICAL PROFILE AND TREATMENT RECORDS OF ANOGENITAL WARTS IN EASTERN UTTAR PRADESH- A RETROSPECTIVE EVALUATION FROM TREATMENT RECORDS AT A TERTIARY CENTRE Harleen
THE SOCIODEMOGRAPHIC CHARACTERISTICS, CLINICAL PROFILE AND TREATMENT RECORDS OF ANOGENITAL WARTS IN EASTERN UTTAR PRADESH- A RETROSPECTIVE EVALUATION FROM TREATMENT RECORDS AT A TERTIARY CENTRE Harleen
UV-2005/01. Chronic Prostatitis and Chronic Pelvic Pain Syndrom (CP/CPPS) Karl-Bickleder-Str. 44C Straubing - Germany
 SYNOPSIS UV-2005/01 Title: Short Title: Indication: Phase: Study Code: Study Director Co-ordinating Investigator: Study Centres: Multicentre, randomised, double-blind, placebo-controlled clinical study
SYNOPSIS UV-2005/01 Title: Short Title: Indication: Phase: Study Code: Study Director Co-ordinating Investigator: Study Centres: Multicentre, randomised, double-blind, placebo-controlled clinical study
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Timby/Smith: Introductory Medical-Surgical Nursing, 9/e
 Timby/Smith: Introductory Medical-Surgical Nursing, 9/e Chapter 62: Caring for Clients With Sexually Transmitted Diseases Slide 1 Epidemiology Introduction Study of the occurrence, distribution, and causes
Timby/Smith: Introductory Medical-Surgical Nursing, 9/e Chapter 62: Caring for Clients With Sexually Transmitted Diseases Slide 1 Epidemiology Introduction Study of the occurrence, distribution, and causes
A Randomized, Double-Blind, Placebo-Controlled Trial of 5-Fluorouracil for the Treatment of Cervicovaginal Human Papillomavirus
 Infectious Diseases in Obstetrics and Gynecology 7:186-189 (1999) (C) 1999 Wiley-Liss, Inc. A Randomized, Double-Blind, Placebo-Controlled Trial of 5-Fluorouracil for the Treatment of Cervicovaginal Human
Infectious Diseases in Obstetrics and Gynecology 7:186-189 (1999) (C) 1999 Wiley-Liss, Inc. A Randomized, Double-Blind, Placebo-Controlled Trial of 5-Fluorouracil for the Treatment of Cervicovaginal Human
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
2. SYNOPSIS Name of Sponsor/Company:
 in patients with refractory partial seizures 14 Jun 2007 2. SYNOPSIS TITLE OF STUDY: Efficacy and safety of BIA 2-093 as adjunctive therapy for refractory partial seizures in a double-blind, randomized,
in patients with refractory partial seizures 14 Jun 2007 2. SYNOPSIS TITLE OF STUDY: Efficacy and safety of BIA 2-093 as adjunctive therapy for refractory partial seizures in a double-blind, randomized,
2 SYNOPSIS. Study code : MC 9308 FR.
 MC9308 FR Study 19 December 2000 Page 15 of142 2 SYNOPSIS Study code : MC 9308 FR. Title: A comparative study of calcipotriol ointment in combination with narrow-band UVB (TL-01) phototherapy and placebo
MC9308 FR Study 19 December 2000 Page 15 of142 2 SYNOPSIS Study code : MC 9308 FR. Title: A comparative study of calcipotriol ointment in combination with narrow-band UVB (TL-01) phototherapy and placebo
Clearance in vulvar lichen sclerosus: a realistic treatment endpoint or a chimera?
 DOI: 10.1111/jdv.14516 JEADV SHORT REPORT Clearance in vulvar lichen sclerosus: a realistic treatment endpoint or a chimera? A. Borghi,* A. Virgili, S. Minghetti, G. Toni, M. Corazza Dipartimento di Scienze
DOI: 10.1111/jdv.14516 JEADV SHORT REPORT Clearance in vulvar lichen sclerosus: a realistic treatment endpoint or a chimera? A. Borghi,* A. Virgili, S. Minghetti, G. Toni, M. Corazza Dipartimento di Scienze
Synopsis (C0743T09 PHOENIX 2)
 Monoclonal antibody () Synopsis ( PHOENIX 2) Protocol: EudraCT No.: 2005-003530-17 Title of the study: A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Trial Evaluating the Efficacy
Monoclonal antibody () Synopsis ( PHOENIX 2) Protocol: EudraCT No.: 2005-003530-17 Title of the study: A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Trial Evaluating the Efficacy
PFIZER INC. Study Initiation Date and Primary Completion or Completion Dates: 11 November 1998 to 17 September 1999
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Scottish Medicines Consortium
 Scottish Medicines Consortium imiquimod 5% cream (Aldara) No. (385/07) Meda Pharmaceuticals Ltd 04 April 2008 The Scottish Medicines Consortium has completed its assessment of the above product and advises
Scottish Medicines Consortium imiquimod 5% cream (Aldara) No. (385/07) Meda Pharmaceuticals Ltd 04 April 2008 The Scottish Medicines Consortium has completed its assessment of the above product and advises
ClinialTrials.gov Identifier: HOE901_4020 Insulin Glargine Date: Study Code: This was a multicenter study that was conducted at 59 US sites
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
2.0 Synopsis. Adalimumab R&D/04/118. (For National Authority Use Only) Referring to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
2.0 Synopsis Abbott Laboratories Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
STUDY. Self-administered Topical 5% Imiquimod Cream for External Anogenital Warts
 STUDY Self-administered Topical for External Anogenital Warts Libby Edwards, MD; Alex Ferenczy, MD; Lawrence Eron, MD; David Baker, MD; Mary L. Owens, MD; Terry L. Fox, MS; Andrina J. Hougham; Kathy A.
STUDY Self-administered Topical for External Anogenital Warts Libby Edwards, MD; Alex Ferenczy, MD; Lawrence Eron, MD; David Baker, MD; Mary L. Owens, MD; Terry L. Fox, MS; Andrina J. Hougham; Kathy A.
Aldara. Aldara (imiquimod) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.19 Subject: Aldara Page: 1 of 5 Last Review Date: March 17, 2017 Aldara Description Aldara (imiquimod)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.19 Subject: Aldara Page: 1 of 5 Last Review Date: March 17, 2017 Aldara Description Aldara (imiquimod)
Referring to Part of Dossier: Volume: Page:
 Synopsis Abbott Laboratories Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
Synopsis Abbott Laboratories Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
fluorouracil 0.5% / salicylic acid 10% cutaneous solution (Actikerall ) SMC No. (728/11) Almirall S.A.
 fluorouracil 0.5% / salicylic acid 10% cutaneous solution (Actikerall ) SMC No. (728/11) Almirall S.A. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
fluorouracil 0.5% / salicylic acid 10% cutaneous solution (Actikerall ) SMC No. (728/11) Almirall S.A. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
BRL /RSD-101C0D/1/CPMS-704. Report Synopsis
 Report Synopsis Study Title: A Randomized, Multicenter, 10-Week, Double-Blind, Placebo- Controlled, Flexible-Dose Study to Evaluate the Efficacy and Safety of Paroxetine in Children and Adolescents with
Report Synopsis Study Title: A Randomized, Multicenter, 10-Week, Double-Blind, Placebo- Controlled, Flexible-Dose Study to Evaluate the Efficacy and Safety of Paroxetine in Children and Adolescents with
Keratolytics and Other Topical Dermatological Agents
 DRUG POLICY Keratolytics and Other Topical Dermatological Agents BENEFIT APPLICATION Benefit determinations are based on the applicable contract language in effect at the time the services were rendered.
DRUG POLICY Keratolytics and Other Topical Dermatological Agents BENEFIT APPLICATION Benefit determinations are based on the applicable contract language in effect at the time the services were rendered.
Synopsis (C1034T02) CNTO 95 Module 5.3 Clinical Study Report C1034T02
 Module 5.3 Protocol: EudraCT No.: 2004-002130-18 Title of the study: A Phase 1/2, Multi-Center, Blinded, Randomized, Controlled Study of the Safety and Efficacy of the Human Monoclonal Antibody to Human
Module 5.3 Protocol: EudraCT No.: 2004-002130-18 Title of the study: A Phase 1/2, Multi-Center, Blinded, Randomized, Controlled Study of the Safety and Efficacy of the Human Monoclonal Antibody to Human
Clinical Trial Report Synopsis
 This document has been do\vnloaded from \v ww.leo-pharma.com subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or treatment
This document has been do\vnloaded from \v ww.leo-pharma.com subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or treatment
Sponsor. Novartis Pharmaceuticals Corporation Generic Drug Name. Agomelatine Therapeutic Area of Trial. Major depressive disorder Approved Indication
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major depressive disorder Approved Indication Investigational drug Study
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major depressive disorder Approved Indication Investigational drug Study
SYNOPSIS (PROTOCOL WX17796)
 TITLE OF THE STUDY A prospective, randomized, double-blind, placebo-controlled, parallel group, multicenter, 52-week trial to assess the efficacy and safety of adjunct MMF to achieve remission with reduced
TITLE OF THE STUDY A prospective, randomized, double-blind, placebo-controlled, parallel group, multicenter, 52-week trial to assess the efficacy and safety of adjunct MMF to achieve remission with reduced
Anogenital Warts. Questions & Answers
 Anogenital Warts Questions & Answers GLASGOW COLORECTAL CENTRE Ross Hall Hospital 221 Crookston Road Glasgow G52 3NQ e-mail: info@colorectalcentre.co.uk Ph: Main hospital switchboard - 0141 810 3151 Ph.
Anogenital Warts Questions & Answers GLASGOW COLORECTAL CENTRE Ross Hall Hospital 221 Crookston Road Glasgow G52 3NQ e-mail: info@colorectalcentre.co.uk Ph: Main hospital switchboard - 0141 810 3151 Ph.
Synopsis Style Clinical Study Report SAR ACT sarilumab Version number : 1 (electronic 1.0)
 SYNOPSIS Title of the study: A randomized, double-blind, parallel-group, placebo- and active calibrator-controlled study assessing the clinical benefit of SAR153191 subcutaneous (SC) on top of methotrexate
SYNOPSIS Title of the study: A randomized, double-blind, parallel-group, placebo- and active calibrator-controlled study assessing the clinical benefit of SAR153191 subcutaneous (SC) on top of methotrexate
To order reprints or e-prints of JDD articles please contact JDD
 June 2017 534 VOLUME 16 ISSUE 6 Copyright 2017 ORIGINAL ARTICLE Journal of Drugs in Dermatology The Efficacy and Safety of Azelaic Acid 15% Foam in the Treatment of Truncal Acne Vulgaris Lauren K. Hoffman
June 2017 534 VOLUME 16 ISSUE 6 Copyright 2017 ORIGINAL ARTICLE Journal of Drugs in Dermatology The Efficacy and Safety of Azelaic Acid 15% Foam in the Treatment of Truncal Acne Vulgaris Lauren K. Hoffman
Diseases of the vulva
 Diseases of the vulva 1. Bartholin Cyst - Infection of the Bartholin gland produces an acute inflammation within the gland (adenitis) and may result in an abscess. Bartholin duct cysts - Are relatively
Diseases of the vulva 1. Bartholin Cyst - Infection of the Bartholin gland produces an acute inflammation within the gland (adenitis) and may result in an abscess. Bartholin duct cysts - Are relatively
Migraine: Developing Drugs for Acute Treatment Guidance for Industry
 Migraine: Developing Drugs for Acute Treatment Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2018
Migraine: Developing Drugs for Acute Treatment Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2018
Protocol Number: BV-2005/01. OM Pharma OM-85
 Page 3 SYNOPSIS Protocol Number: Name of Finished Product: Broncho-Vaxom (Broncho-Munal ) Title: Double-Blind, Placebo-Controlled, Randomised Clinical Study of Broncho-Vaxom in Children Suffering from
Page 3 SYNOPSIS Protocol Number: Name of Finished Product: Broncho-Vaxom (Broncho-Munal ) Title: Double-Blind, Placebo-Controlled, Randomised Clinical Study of Broncho-Vaxom in Children Suffering from
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 26 November 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 26 November 2008 ALDARA 5%, cream Box of 12 sachets of 250 mg (CIP: 349 204-4) Applicant: MEDA PHARMA imiquimod ATC
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 26 November 2008 ALDARA 5%, cream Box of 12 sachets of 250 mg (CIP: 349 204-4) Applicant: MEDA PHARMA imiquimod ATC
INDIVIDUAL STUDY TABLE REFERRING TO PART OF THE DOSSIER Volume: Page:
 SYNOPSIS Protocol No.: TOPMAT-MIG-303 EudraCT No.: 2005-000321-29 Title of Study: A double-blind, randomised, placebo-controlled, multicentre study to investigate the efficacy and tolerability of in prolonged
SYNOPSIS Protocol No.: TOPMAT-MIG-303 EudraCT No.: 2005-000321-29 Title of Study: A double-blind, randomised, placebo-controlled, multicentre study to investigate the efficacy and tolerability of in prolonged
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
2.0 Synopsis. Adalimumab M Clinical Study Report R&D/13/1011. (For National Authority Use Only)
 2.0 Synopsis AbbVie Inc. Name of Study Drug: Name of Active Ingredient: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study: A Phase 3 Multicenter
2.0 Synopsis AbbVie Inc. Name of Study Drug: Name of Active Ingredient: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study: A Phase 3 Multicenter
Mipomersen (ISIS ) Page 2 of 1979 Clinical Study Report ISIS CS3
 (ISIS 301012) Page 2 of 1979 2 SYNOPSIS ISIS 301012-CS3 synopsis Page 1 Title of Study: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety, Tolerability, Pharmacokinetics,
(ISIS 301012) Page 2 of 1979 2 SYNOPSIS ISIS 301012-CS3 synopsis Page 1 Title of Study: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety, Tolerability, Pharmacokinetics,
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Synopsis of study HBV-314 BST 280 (108988)
 Synopsis of study HBV-314 BST 280 (108988) Pharmaceutical entrepreneur: GlaxoSmithKline GmbH & Co. KG Prinzregentenplatz 9 81675 Munich Germany Personal identifiable data of investigators (name / full
Synopsis of study HBV-314 BST 280 (108988) Pharmaceutical entrepreneur: GlaxoSmithKline GmbH & Co. KG Prinzregentenplatz 9 81675 Munich Germany Personal identifiable data of investigators (name / full
Licensing of Herbal Medicines from Asia in Germany
 Licensing of Herbal Medicines from Asia in Germany Challenges and Experiences TradReg 2017 Regulation of Herbal and Traditional Medicinal Products Challenges of Globalisation International Symposium Bonn,
Licensing of Herbal Medicines from Asia in Germany Challenges and Experiences TradReg 2017 Regulation of Herbal and Traditional Medicinal Products Challenges of Globalisation International Symposium Bonn,
PFIZER INC. Study Initiation Date and Completion Dates: 09 March 2000 to 09 August 2001.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Synopsis (C0743T10) CNTO 1275 Module 5.3 C0743T10. Associated with Module 5.3 of the Dossier
 Module 5.3 Protocol: EudraCT No.: 2005-003525-92 Title of the study: A Phase 2, Multicenter, Randomized, Double-blind, Placebo-controlled Trial of, a Fully Human Anti-IL-12 Monoclonal Antibody, Administered
Module 5.3 Protocol: EudraCT No.: 2005-003525-92 Title of the study: A Phase 2, Multicenter, Randomized, Double-blind, Placebo-controlled Trial of, a Fully Human Anti-IL-12 Monoclonal Antibody, Administered
COMMON VIRAL INFECTIONS. Dr D. Tenea Department of Dermatology University of Pretoria
 COMMON VIRAL INFECTIONS Dr D. Tenea Department of Dermatology University of Pretoria GENERAL Viral infections of the skin important in immunocompromised Pts. Infection: direct inoculation ( warts ) or
COMMON VIRAL INFECTIONS Dr D. Tenea Department of Dermatology University of Pretoria GENERAL Viral infections of the skin important in immunocompromised Pts. Infection: direct inoculation ( warts ) or
ClinialTrials.gov Identifier: Sponsor/company: sanofi-aventis
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
The prevalence of human papilloma virus in the anal region of male Chinese attendees in three public sexually transmitted disease clinics in Hong Kong
 Hong Kong J. Dermatol. Venereol. (2011) 19, 6-13 Original Article The prevalence of human papilloma virus in the anal region of male Chinese attendees in three public sexually transmitted disease clinics
Hong Kong J. Dermatol. Venereol. (2011) 19, 6-13 Original Article The prevalence of human papilloma virus in the anal region of male Chinese attendees in three public sexually transmitted disease clinics
PankoMab-GEX Versus Placebo as Maintenance Therapy in Advanced Ovarian Cancer
 1 von 7 13.01.2014 12:26 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: GEXMab25201 Previous Study Return to List Next Study PankoMab-GEX Versus Placebo as Maintenance Therapy
1 von 7 13.01.2014 12:26 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: GEXMab25201 Previous Study Return to List Next Study PankoMab-GEX Versus Placebo as Maintenance Therapy
Immediate-release Hydrocodone/Acetaminophen M Abbreviated Clinical Study Report R&D/08/1020
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Hydrocodone Bitartrate- Acetaminophen (NORCO ) Name of
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Hydrocodone Bitartrate- Acetaminophen (NORCO ) Name of
Clinical Study Report
 Clinical Study Report No. H 552 000-0902 / 290102BS page 1 of 40 Clinical Study Report Sponsor: Study No.: H 552 000-0902 / 290102BS EudraCT-No.: 2009-009948-23 Title: Study Preparation: A phase IIa single-center,
Clinical Study Report No. H 552 000-0902 / 290102BS page 1 of 40 Clinical Study Report Sponsor: Study No.: H 552 000-0902 / 290102BS EudraCT-No.: 2009-009948-23 Title: Study Preparation: A phase IIa single-center,
VIN/VAIN O C T O B E R 3 RD J M O R G A N
 VIN/VAIN O C T O B E R 3 RD 2 0 1 8 J M O R G A N Vaginal Intraepithelial Neoplasia VAIN I, II, III Incidence 0.1/100,000 women in US Mean age 50s (J Womens Health (Larchmt) 2009:18:1731) (J Obstet Gynaecol
VIN/VAIN O C T O B E R 3 RD 2 0 1 8 J M O R G A N Vaginal Intraepithelial Neoplasia VAIN I, II, III Incidence 0.1/100,000 women in US Mean age 50s (J Womens Health (Larchmt) 2009:18:1731) (J Obstet Gynaecol
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
OPPORTUNISTIC HPV VACCINATION: AN EXPANDED VISION
 OPPORTUNISTIC HPV VACCINATION: AN EXPANDED VISION SUMMARY POSITION Human papillomavirus (HPV) infection is preventable but not adequately prevented. At present, Canada has a robust school vaccination program
OPPORTUNISTIC HPV VACCINATION: AN EXPANDED VISION SUMMARY POSITION Human papillomavirus (HPV) infection is preventable but not adequately prevented. At present, Canada has a robust school vaccination program
