Multimodal analgesic therapy has gained widespread
|
|
|
- Tyrone Eaton
- 5 years ago
- Views:
Transcription
1 TOPICAL REVIEW Analgesia for Anesthetized Patients Kip A. Lemke, DVM, MSc, Dipl. ACVA, and Catherine M. Creighton, DVM Many perioperative pain management protocols for cats and dogs are overly complex, some are inive, and still others expose patients to unnecessary risk. The purpose of this article is to provide clinicians with a basic understanding of the pathophysiology of perioperative pain and a working knowledge of the principles of ive therapy. First, the concept of multimodal analgesic therapy is discussed. Next, the pathophysiology of perioperative pain and the clinical pharmacology of the major classes of analgesic drugs are reviewed. And last, a simplified approach to managing perioperative pain in cats and dogs is presented Elsevier Inc. All rights reserved. Keywords: analgesia,, pain, dogs, cats From the Atlantic Veterinary College, University of Prince Edward Island, Charlottetown, Prince Edward Island, Canada. Address reprint requests to: Kip A. Lemke, DVM, MSc, Dipl. ACVA, Atlantic Veterinary College, University of Prince Edward Island, 550 University Ave, Charlottetown, PE, Canada, C1A 4P3. klemke@upei.ca Elsevier Inc. All rights reserved /06/ \.00/0 doi: /j.tcam Multimodal analgesic therapy has gained widespread acceptance in the management of perioperative pain in both dogs and cats. 1-3 In recent years, a clearer understanding of the pathophysiology of perioperative pain has provided the conceptual framework for a more rational use of analgesic drugs and techniques. Surgical trauma and inflammation produce sensitization of the peripheral nervous system, and the subsequent barrage of nociceptive input produces sensitization of neurons in the dorsal horn of the spinal cord. Blockade or attenuation of ascending nociceptive pathways or activation of descending antinociceptive pathways by different classes of analgesic drugs usually provides better analgesia with fewer side s than unimodal therapy with a single class of analgesic drugs. Because peripheral and central neural blockade with local anesthetics are the only analgesic techniques that can produce complete blockade of peripheral nociceptive input, these techniques are the most ive way to attenuate sensitization of the central nervous system and the development of pathological pain. 4,5 Clinicians should also remember that atraumatic surgical technique is always the most ive method to prevent peripheral and central sensitization and the development of pain The neuroendocrine or stress response to surgical trauma compromises hemostatic, metabolic, and immunological function, which increases perioperative morbidity and mortality. 6,7 Early studies in human infants undergoing cardiac surgery demonstrated that the neuroendocrine response was significantly reduced by intraoperative administration of halothane and fentanyl when compared with administration of halothane alone. 8 In a subsequent clinical study, the mortality rate dropped from 25% to 0% in a similar group of infants when sufentanil was given intraoperatively and additional opioids were given 9 Perioperative use of neural blockade, in particular central neural blockade, also attenuates the neuroendocrine response and dramatically reduces mortality and the incidence of major complications in human patients undergoing a wide variety of surgical procedures. 10 In this analysis of 141 clinical trials, patients with central neural blockade had a 30% reduction in mortality and a 40% to 60% reduction in major complications (thromboembolism, hemorrhage, respiratory depression, pneumonia) when compared with those without central neural blockade. Intraoperative use of multimodal analgesic therapy also reduces inhalation anesthetic requirements and autonomic responses to noxious surgical stimuli. These reductions improve cardiopulmonary function intraoperatively and facilitate a rapid, smooth recovery from Alpha-2 agonists, opioids, N-methyl-D-aspartate (NMDA) antagonists, cyclooxygenase (COX) inhibitors, and neural blockade with local anesthetics are often used perioperatively as part of a multimodal strategy to manage pain. 1-3 These classes of analgesic drugs can be incorporated easily into anesthetic and pain management plans for cats and dogs undergoing most types of surgical procedures. Alpha-2 agonists can be used preoperatively and postoperatively to provide sedation and analgesia. Opioids can be used throughout the perioperative period to reduce anesthetic requirements and to manage pain. NMDA antagonists (ketamine) can be used throughout the perioperative period to manage pain in patients with significant central sensitization and pathological pain. COX inhibitors can be used postoperatively to reduce opioid requirements and provide more ive analgesia with fewer side s. Peripheral and central neural blockade with local anesthetics can be used intraoperatively to reduce anesthetic requirements and attenuate the development of central sensitization. Neural blockade can also be used postoperatively to manage pain in selected patients. A clear understanding of the pathophysiology of pain and the 70
2 Volume 25, Number 2, May clinical pharmacology of the major classes of analgesic drugs is required to use multimodal analgesic therapy safely and ively in anesthetized patients. Pathophysiology The terminology used to describe the pathophysiology of pain is confusing, so defining a few terms relevant to the management of perioperative pain is important. Nociception is defined as the neural response to a noxious stimulus. Specifically, nociception includes signal transduction and nerve conduction in the peripheral nervous system, and synaptic transmission, projection, and modulation of nociceptive input in the central nervous system (Fig 1). Pain, on the other hand, is a complex sensation that requires integration of nociceptive and other sensory input at the cortical level. Pain is defined as an unpleasant sensory or emotional experience that is associated with actual or potential tissue damage. Pain can be further classified anatomically as somatic or visceral pain, or temporally as acute or chronic pain. Recent neuroanatomical and functional imaging studies suggest that pain is one component of an interoceptive system that is primarily responsible for maintaining internal homeostasis, and that there are significant differences among species in the neural components that make up this system. 11 Most perioperative pain is due to surgical trauma and inflammation. Some patients may have preexisting tissue trauma and inflammation, and others may have pain associated with nerve injury. Although a small number of surgical patients may experience both inflammatory and neuropathic pain, inflammatory pain is by far the most common type of perioperative pain. The mechanisms of inflammatory pain are reasonably well understood and form the basis for rational, ive, multimodal analgesic therapy. Neuropathic pain is relatively uncommon in surgical patients, and the mechanisms of neuropathic pain are similar to those of inflammatory pain. 12,13 Consequently, clinicians should focus on understanding the pathophysiology and management of inflammatory pain. Nociceptive Pathways Ascending nociceptive pathways begin in the peripheral tissues and project to the dorsal horn of the spinal cord, brain stem, thalamus, and cerebral cortex (Fig 1). The nociceptive pathways are composed of 3 general types of neurons. The first-order neurons are primary afferent neurons, and these neurons are responsible for transduction of noxious stimuli and conduction of electrical signals to the dorsal horn of the spinal cord. The second-order neurons are projection neurons, and these neurons receive input from the primary afferent neurons and project to the medulla, pons, midbrain, thalamus, and hypothalamus. Third-order supraspinal neurons integrate input from spinal neurons and project to subcortical and cortical areas where pain is finally perceived. Supraspinal processing of afferent nociceptive input is also Figure 1. Overview of nociceptive and antinociceptive pathways. Surgical trauma activates mechanical, chemical, and thermal nociceptors. Action potentials are conducted to the dorsal horn of the spinal cord by primary afferent nerve fibers. Second-order projection neurons encode and relay signals to the brainstem and thalamus. Third-order neurons in the thalamus project to the limbic system and somatosensory cortex where pain is perceived. Descending antinociceptive pathways modulate nociceptive processing at the level of the thalamus, brainstem, and spinal cord. Different classes of analgesic drugs act at different sites in the nociceptive and antinociceptive pathways. Multimodal analgesic therapy inhibits processing of nociceptive input at 2 or more sites. PAG, periaqueductal gray; RVM, rostroventral medulla.
3 72 Topics in Companion Animal Medicine closely integrated with regulation of the autonomic nervous system. Primary afferent neurons are bipolar neurons. The cell bodies of these bipolar neurons are located in the trigeminal and dorsal root ganglia, and their axons project peripherally to somatic and visceral tissues and centrally to the dorsal horn of the spinal cord. Some primary afferent neurons respond to noxious or high-threshold stimuli, and others respond to non-noxious or low-threshold stimuli (touch). Afferent nociceptive neurons have free nerve endings that change or transduce noxious mechanical, thermal, or chemical stimuli into electrical signals. Somatic tissues have a higher density of nociceptive nerve fibers and smaller receptive fields, whereas visceral tissues have a lower density of nociceptive nerve fibers and larger receptive fields. These anatomical differences may account for some of the qualitative differences between somatic (discrete) and visceral (diffuse) pain. Primary afferent neurons are classified by axon diameter, the presence or absence of myelination, and their response to mechanical, thermal, and chemical stimuli. A afferent neurons have large, myelinated axons that conduct impulses at a velocity of greater than 30 m/sec. The free nerve endings of these fibers respond to non-noxious mechanical stimuli (touch) but do not respond to noxious stimuli directly. A nociceptive neurons have small, myelinated axons that conduct impulses at a velocity of 3 to 30 m/sec. The free nerve endings of these fibers contain membrane-bound receptors that respond primarily to intense mechanical and thermal stimuli and are called mechanothermal nociceptors. C nociceptive neurons have small, unmyelinated axons that conduct nerve impulses at a velocity of less than 3 m/sec. The free nerve endings of these fibers contain membrane-bound receptors that respond to chemical as well as thermal and mechanical stimuli and are called polymodal nociceptors. Small, myelinated A fibers carry the nociceptive input responsible for the fast, sharp pain that occurs immediately after injury. The nociceptive input responsible for the prolonged dull pain that occurs several seconds later is carried by small, unmyelinated C fibers. Silent nociceptive neurons are also present in somatic tissues. The free nerve endings of these neurons only respond to mechanical and thermal stimuli after they are activated by chemical (inflammatory) mediators. This classification scheme is derived from analysis of fibers that innervate the somatic tissues (skin). Visceral pain is qualitatively different from somatic pain and is typically dull and poorly localized. Visceral pain also lacks the fast and slow components that are characteristic of somatic pain. Transduction of mechanical, thermal, and chemical stimuli by the free nerve endings of A and C nociceptive fibers is mediated by membrane-bound receptors Most of these receptors are nonselective cation channels that are gated by temperature, chemical ligands, or mechanical shearing forces. Activation of these channels increases inward conduction of Na and Ca 2 ions, which ultimately depolarizes the membrane and generates a burst of action potentials. The mechanisms of mechanical signal transduction are not well defined. Noxious mechanical stimuli may activate a mechanically gated ion channel directly, or shearing forces may release adenosine triphosphate, which acts on purine receptors (P2X). Noxious chemical stimuli (H ) activate acid-sensing ion channels and transient receptor potential vanilloid (TRPV1) channels. Noxious heat also activates TRPV1 channels as well as related channels (TRPV2), and noxious cold activates transient receptor potential menthol (TRPM8) channels. Nociceptive afferent neurons synapse with second-order neurons in the dorsal horn of the spinal cord. Projection neurons and interneurons are the 2 major types of nociceptive neurons in the dorsal horn, and these neurons are organized in layers or laminae. Neurons that mediate nociception are located in laminae I, II, and V. Projection neurons are located in laminae I and V, and they have axons that cross midline and project to third-order supraspinal neurons. Projection neurons located in lamina I receive input directly from A and C nociceptive fibers and are classified as nociceptivespecific and polymodal nociceptive neurons, respectively. Projection neurons in lamina V receive input from both nociceptive and non-nociceptive (A ) fibers and are classified as wide, dynamic-range neurons. Interneurons are located in lamina II and also receive input from nociceptive and nonnociceptive (A ) fibers. Inhibitory and excitatory interneurons play a central role in gating and modulating nociceptive input. Propriospinal neurons that project across several dermatomes are also present in the dorsal horn and are responsible for segmental reflexes associated with nociception. Glutamate is the primary excitatory neurotransmitter in the dorsal horn of the spinal cord. Nociceptive as well as non-nociceptive fibers co-release glutamate and neuropeptides (substance P, neurokinin A, calcitonin gene related peptide). With normal afferent input, glutamate binds to -amino-3-hydroxy-5-methyl-4-isoxazolepropionoic acid (AMPA) receptors located on the postsynaptic membrane of projection neurons. Neuropeptides bind to several types of receptors on the postsynaptic membrane. With intensive afferent input, prolonged activation of AMPA and neuropeptide receptors leads to progressive depolarization of the postsynaptic membrane and activation of additional types of glutamate receptors. Activation of a specific type of glutamate receptor, the NMDA receptor, plays a key role in the development of central sensitization. The spinothalamic tract (STT) is the major ascending nociceptive pathway in carnivores and primates. 11,16 Lamina I, nociceptive (nociceptive-specific, polymodal nociceptive) neurons are somatotopically organized, modality-selective neurons with small receptive fields that convey discrete noxious mechanical, thermal, and chemical afferent input. These neurons project to the ventromedial nucleus of the lateral thalamus, which projects to the insular cortex and the secondary somatosensory cortex. These neurons also project to the mediodorsal nucleus of the medial thalamus, which projects to the anterior cingulate cortex. Like the dorsal horn, glutamate is an important excitatory neurotransmitter within the thalamic nuclei. Lamina V, wide, dynamic-range
4 Volume 25, Number 2, May neurons have large receptive fields, are not somatotopically organized or modality selective, and are responsible for integration of all afferent input to the dorsal horn. These neurons project to the motor thalamus (ventrodorsal and ventrolateral nuclei), which projects to the basal ganglia and the primary somatosensory cortex. Axons from lamina I are concentrated in the lateral STT, and axons from lamina V are concentrated in the ventral STT. The thalamus relays afferent input from the STT and integrates this information with afferent input from the autonomic nervous system. Projection from neurons in the lateral thalamus to neurons in the insular and secondary somatosensory cortex appears to be responsible for the sensory-discriminative aspects of pain. Projection from neurons in the medial thalamus to neurons in the anterior cingulate cortex appears to be responsible for the motivational-affective aspects of pain. Projection from neurons in the motor thalamus to neurons in the primary somatosensory cortex appears to be responsible for sensory and motor integration. Direct connections between the lateral thalamus and the dorsal margin of the insular cortex (interoceptive cortex) are more developed in primates than in carnivores. Antinociceptive Pathways Mammals also have descending antinociceptive pathways that modulate nociceptive input at spinal and supraspinal levels (Fig 1). The antinociceptive pathways begin at the supraspinal level and project to neurons in the dorsal horn of the spinal cord. The periaqueductal gray matter (midbrain), locus ceruleus (pons), and nucleus raphe magnus (medulla) are all important structures in the modulation of nociceptive input. The periaqueductal gray matter receives direct input from the thalamus and the hypothalamus, and indirect input from the insular cortex and the anterior cingulate cortex. These neurons in the periaqueductal gray matter send projections to neurons in the nucleus raphe magnus, which project to the dorsal horn of the spinal cord. Neurons in the locus ceruleus project directly to the dorsal horn, and they may also receive input from the periaqueductal gray matter. Endogenous opioids (endorphins, enkephalins, dynorphins), serotonin, and norepinephrine are the primary neurotransmitters in the descending antinociceptive pathways. Axons that originate in the nucleus raphe magnus release serotonin in the dorsal horn of the spinal cord and are called serotonergic neurons. Similarly, neurons that originate in the locus ceruleus release norepinephrine in the dorsal horn and are called noradrenergic neurons. Supraspinal release of endogenous opioid peptides activates both types of neurons, whereas supraspinal release of release of -aminobutyric acid (GABA) inhibits both types of neurons. Supraspinal inhibition of descending antinociceptive pathways is mediated by GABA A receptors. At the supraspinal level, endogenous opioids not only activate descending antinociceptive pathways, but inhibit GABA-mediated inhibition of these same pathways, which is called disinhibition. Release of norepinephrine and serotonin in the dorsal horn of the spinal cord, and subsequent release of enkephalins and GABA by local interneurons, inhibit presynaptic calcium channels that modulate neurotransmitter release. This inhibition, mediated by presynaptic noradrenergic ( 2 ), opioid ( ), and GABA B receptors, limits release of glutamate and neuropeptides from primary afferent neurons, which inhibits nociceptive transmission. Release of norepinephrine, enkephalins, and GABA in the dorsal horn hyperpolarizes projection neurons, which also inhibits nociceptive transmission. This inhibition is mediated by postsynaptic noradrenergic ( 2 ) and opioid ( ) receptors that activate potassium channels, which leads to an outward flux of potassium ions and hyperpolarization of the postsynaptic membrane. This inhibition is also mediated by postsynaptic GABA A receptors that activate chloride channels, which leads to an inward flux of chloride ions and hyperpolarization of the postsynaptic membrane. In summary, release of norepinephrine, endogenous opioids, and GABA inhibits synaptic transmission between primary afferent neurons and projection neurons by inhibiting neurotransmitter release and hyperpolarizing the postsynaptic membrane, which ively shuts down the key synapse in the dorsal horn. Peripheral and Central Sensitization Neural plasticity is defined as the ability of the nervous system to modify its function in response to different environmental stimuli. Surgical trauma and inflammation produce sensitization of the peripheral nervous system, and the subsequent barrage of nociceptive input produces sensitization of neurons in the dorsal horn of the spinal cord. Peripheral and central sensitization of nociceptive pathways plays a central role in the development of pathological pain. 17 Patients with no preexisting tissue trauma and inflammation experience pain that is physiological or protective in nature (Table 1). This type of inflammatory pain is well localized, proportionate to the peripheral stimulus, and subsides once the inflammatory process resolves. Patients with significant tissue trauma and inflammation experience pain that is pathological or debilitating in nature (Table 2). This type of inflammatory pain is diffuse, disproportionate to the peripheral stimulus, and continues beyond resolution of the inflammatory process. A key concept that is often lost when trying to understand the clinical relevance of neural plasticity is that central sensitization occurs secondary to surgical trauma, inflammation, and the development of peripheral sensitiza- Table 1. Characteristics of Physiological Pain Protective Discrete or well localized No peripheral or central sensitization Proportionate to the peripheral stimulus Subsides once the inflammatory response resolves Pain can be differentiated from touch Responds well to conventional analgesic therapy
5 74 Topics in Companion Animal Medicine Table 2. Characteristics of Pathological Pain Debilitating Diffuse or poorly localized Significant peripheral and central sensitization Disproportionate or exaggerated response to the peripheral stimulus Continues beyond resolution of the inflammatory response Pain cannot be differentiated from touch Does not respond well to conventional analgesic therapy tion. Consequently, atraumatic surgical technique and neural blockade are far more ive than other types of analgesic therapy in limiting the development of pathological pain, blunting the stress response, preventing major complications, and reducing mortality. 18,19 Peripheral sensitization occurs as a direct consequence of tissue trauma and inflammation. Tissue trauma leads to the release of inflammatory mediators from damaged cells (H, K, prostaglandins), plasma (bradykinin), platelets (serotonin), mast cells (histamine), and macrophages (cytokines). Some inflammatory mediators activate nociceptors directly (bradykinin), whereas others sensitize nociceptors (prostaglandins). Activation of nociceptors also leads to antidromal or retrograde activation of nociceptive nerve fibers and release of substance P and other neuropeptides. Release of these neuropeptides leads to mast cell degranulation, vasodilation, edema, and further activation and sensitization of nociceptors. Peripheral sympathetic nerve terminals also contribute to activation and sensitization of nerve terminals by releasing norepinephrine and prostaglandins. Ultimately, tissue trauma and inflammation produce a sensitizing soup of chemical mediators that convert high-threshold nociceptors to low-threshold nociceptors. In other words, peripheral sensitization is characterized by a reduction in the activation threshold of peripheral nociceptors. Central sensitization occurs as an indirect consequence of tissue trauma and inflammation and is contingent to a large degree on the development of peripheral sensitization. Constant activation of sensitized peripheral nociceptors leads to sustained release of glutamate and neuropeptides from afferent fibers. Constant activation of AMPA and neuropeptide receptors on dorsal horn projection neurons leads to progressive cellular depolarization and activation of additional types of glutamate receptors (NMDA, metabotropic). Normally, the channel of NMDA receptor complex is blocked by a magnesium plug. Progressive depolarization of the postsynaptic membrane releases this plug, activates the receptor complex, and increases inward conduction of Ca 2 ions. This influx of calcium, along with activation of other types of receptors, leads to activation of phospholipases, polyphosphoinosites, kinases, and other intracellular messengers. These activity-dependent changes lead to an increase in the excitability of dorsal horn projection neurons. This initial phase of central sensitization is called short-term sensitization. If the inflammatory process continues for several days, gene regulatory proteins are activated, new types receptors are expressed, and dorsal horn projection neurons become even more reactive to subsequent nociceptive input. This phase of central sensitization is called long-term sensitization. Activation of NMDA receptors and the subsequent influx of Ca 2 also leads to the release of arachidonic acid, which is converted to prostaglandins by COX. Prostaglandins act presynaptically and postsynaptically to facilitate the development of central sensitization. Glial cells also appear to facilitate the development of central sensitization. Microglia and astrocytes normally play a supportive role in neurotransmission, but they are also activated by glutamate and neuropeptides released from primary afferent fibers. Activated glial cells release adenosine triphosphate, glutamate, nitric oxide, and cytokines, which facilitate release of neurotransmitters from afferent fibers and further sensitization of projection neurons. Clinical Pharmacology The primary goal of perioperative multimodal analgesic therapy is to limit the development of peripheral and central sensitization, and prevent the development of pathological pain. Effective analgesic therapy also blunts the neuroendocrine response, reduces major complications, and improves outcome. There are 5 major classes of analgesic drugs, and each class blocks or modulates nociceptive input at one or more sites of action (Fig 1). Alpha-2 agonists and opioids alter the central perception of pain. Activation of supraspinal and spinal alpha-2 receptors and opioid receptors also inhibits synaptic transmission in the dorsal horn of the spinal cord. Dissociative anesthetics (ketamine) block NMDA receptors on projection neurons, which inhibit the development of central sensitization. Peripheral and central neural blockade with local anesthetics also inhibits the development of central sensitization. COX inhibitors reduce inflammation, which limits the development of peripheral sensitization. COX inhibitors also reduce the synthesis of prostaglandins in the dorsal horn of the spinal cord, which limits the development of central sensitization. Selective Alpha-2 Agonists Norepinephrine is the endogenous ligand for spinal and supraspinal alpha-2 adrenergic receptors. Medetomidine and dexmedetomidine are the selective alpha-2 agonists currently available in North America. Medetomidine is supplied as a racemic mixture of 2 optical enantiomers. Dexmedetomidine is the active enantiomer, whereas levomedetomidine has no apparent pharmacological activity. As a result, dexmedetomidine is approximately twice as potent as medetomidine. The clinical s of medetomidine and dexmedetomidine are comparable when equivalent sedative doses are administered to cats and dogs. Medetomidine has a rapid onset after parenteral injection, and the drug has a duration of action of
6 Volume 25, Number 2, May approximately 1 hour in dogs. The alpha-2 to alpha-1 adrenergic receptor selectively ratios for medetomidine and xylazine are 1620:1 and 160:1, respectively. 20 Medetomidine and dexmedetomidine are approved for use in dogs in Canada and the United States, and studies evaluating the use of these drugs in cats have been completed Selective alpha-2 agonists induce reliable dose-dependent sedation, analgesia, and muscle relaxation in cats and dogs. 25,26 The sedative and analgesic s of selective alpha-2 agonists are mediated by activation of alpha-2 adrenergic receptors located in the pons (locus ceruleus) and the dorsal horn of the spinal cord, respectively. In addition to providing sedation and analgesia, preoperative administration of medetomidine reduces the amount of intravenous and inhalation anesthetic required to induce and maintain Perioperative administration of selective alpha-2 agonists also blunts the neuroendocrine response and reduces catecholamine and cortisol levels. 30,31 Blood pressure increases, and heart rate and cardiac output decrease after medetomidine administration. Bradycardia and atrioventricular blockade are potential complications, and heart rate and rhythm should be monitored closely. Medetomidine does not sensitize the myocardium to catecholamines or facilitate development of ventricular arrhythmias in patients anesthetized with isoflurane. 32,33 Respiratory rate and minute ventilation are well maintained after administration of medetomidine or dexmedetomidine in conscious and anesthetized dogs. 34,35 Blood glucose and urine production increase significantly after administration of medetomidine. Selective alpha-2 agonists are used perioperatively to sedate and calm patients, provide muscle relaxation, and potentiate the analgesic and anesthetic s of other drugs. Medetomidine and dexmedetomidine are administered at relatively low doses, and the use of these drugs is usually limited to healthy, hemodynamically stable patients. Preoperatively, selective alpha-2 agonists are often given in combination with opioids. The preanesthetic intramuscular dose ranges for medetomidine in healthy dogs and cats are to 0.01 mg/kg and 0.01 to 0.02 mg/kg, respectively. Ultralow doses of medetomidine can be given postoperatively to provide sedation. The postanesthetic intramuscular dose ranges for medetomidine in healthy for healthy dogs and cats are to mg/kg and to mg/kg, respectively. Dexmedetomidine can be given at approximately half of the medetomidine dose. Atropine or glycopyrrolate can be used to manage bradycardia associated with administration of low and ultra-low doses of selective alpha-2 agonists. Opioids Endorphins, enkephalins, and dynorphins are the endogenous ligands for spinal and supraspinal opioid receptors. Currently, 3 major classes of opioid receptors have been cloned: OP1 ( ), OP2 ( ) and OP3 ( ). OP1, OP2, and OP3 receptors modulate supraspinal and spinal analgesia, as well as sedation, bradycardia, respiratory depression, and gastrointestinal motility. Receptor subtypes of each of these classes have been identified, and expression of these subtypes varies among tissues. There are also significant differences in the expression of opioid receptors among species. Parenteral administration of opioids usually causes sedation in dogs, whereas administration of high doses of opioids can cause excitation and dysphoria in cats. Respiratory depression is a significant side in human patients, but ventilation is better maintained in cats and dogs. Butorphanol, morphine, hydromorphone, and fentanyl are the opioids used most commonly in small animals. Buprenorphine and tramadol have also been used perioperatively to a limited extent. Opioid agonists are the safest and most ive class of analgesic drugs used for the management of perioperative pain in cats and dogs. Morphine, hydromorphone, and fentanyl are the opioid agonists used most commonly in small animals. These analgesic drugs are agonists at all 3 types of opioid receptors. Opioid agonists are equally efficacious in treating moderate to severe pain, but differ in potency and duration of action. Intraoperative administration of morphine, hydromorphone, or fentanyl reduces isoflurane requirements by 30% to 50% in cats and dogs Significant respiratory depression can occur intraoperatively and postoperatively if the additive s of opioid agonists and general anesthetics are not considered. Morphine and hydromorphone are often administered as preanesthetics, and vomiting can occur with either drug. High doses of hydromorphone ( 0.1 mg/kg) can also cause significant postoperative hyperthermia in cats Morphine and hydromorphone have an intermediate duration of action (2-4 hours) after parenteral administration. The preanesthetic intramuscular dose range for morphine in cats and dogs is 0.2 to 0.4 mg/kg. Hydromorphone is approximately 5 times more potent than morphine, and the preanesthetic intramuscular dose range for hydromorphone in cats and dogs is 0.05 to 0.1 mg/kg. Approximately half of the preanesthetic dose of morphine or hydromorphone can be given intraoperatively or Morphine can also be given epidurally alone or in combination with local anesthetics. The dose range for preoperative or postoperative epidural administration of morphine is mg/kg. At this dose range, the onset time is slow (1-2 h), but the duration of action in long (12-24 h). Morphine can also be given postoperatively as an intravenous constant-rate infusion at a dose range of 0.1 to 0.2 mg/kg/h. Morphine has a relatively long duration of action and the drug will accumulate over time. As a result, patients should be monitored closely, and the infusion rate should be reduced as needed. Fentanyl can also be given intravenously as a preanesthetic. The drug is approximately 100 times more potent than morphine and has a relatively short duration of action (0.5 hour). The preanesthetic intravenous dose range for fentanyl in cats and dogs is to mg/kg. Fentanyl is less likely to accumulate over time, and the drug is often given intraoperatively to dogs as an intravenous constant rate infusion. The amount of inhalation anesthetic required to maintain adequate anesthetic depth is reduced by 30% to 50%, which improves cardiovascular function. Minute ventilation may decrease, and positive-pressure ven-
7 76 Topics in Companion Animal Medicine tilation may be required to prevent significant hypoventilation (P a CO 2 60 mm Hg). Intraoperatively, the dose range for fentanyl in dogs is to 0.01 mg/kg/h. Fentanyl can also be given postoperatively as an intravenous constant-rate infusion at a dose range of to mg/kg/h. Objective clinical data on the use of intraoperative and postoperative fentanyl infusions in cats are limited. Fentanyl patches were designed to be applied to human skin. Even in healthy cats and dogs, systemic absorption of fentanyl from transdermal patches is erratic. 43,44 In surgical patients, altered body temperature, peripheral circulation, and hydration can all compromise transdermal absorption of fentanyl. On the other hand, heating pads and forced-air warmers can dramatically increase fentanyl absorption intraoperatively and Consequently, preoperative and intraoperative use of fentanyl patches should be avoided. Once normal body temperature, peripheral circulation, and hydration are restored, fentanyl patches can be used postoperatively with reasonable efficacy. Onset time is very slow, and there is a 12- to 24-hour lag before ive plasma concentrations are reached. If the patch stays properly adhered to on the patient, plasma fentanyl concentrations are maintained for 3 to 5 days. Transdermal fentanyl is dosed at a rate of to mg/kg/h in cats and dogs. Opioid agonist-antagonists are also used to manage perioperative pain in cats and dogs. Butorphanol is the opioid agonist-antagonist used most commonly in small animals. Butorphanol is an agonist at the OP2 ( ) receptor and an antagonist or partial agonist at the OP3 ( ) receptor. The analgesia produced by butorphanol is not as profound as that produced by opioid agonists, and its duration of action is relatively short (1-2 hours). However, side s (vomiting, respiratory depression, bradycardia) are less likely to occur after administration of butorphanol than after administration of morphine, hydromorphone, or fentanyl. Intraoperative administration of butorphanol also reduces isoflurane requirements by 10% to 20% in cats and dogs. 36,45 Butorphanol can be used perioperatively to manage mild to moderate pain. The preanesthetic intramuscular dose range for butorphanol in cats and dogs is 0.2 to 0.4 mg/kg. Approximately half of the preanesthetic dose can be given intraoperatively or Butorphanol can be given postoperatively as an intravenous constant-rate infusion at a dose range of 0.1 to 0.2 mg/kg/h. Small intravenous doses of butorphanol (0.1 mg/kg) can also be given postoperatively to reverse sedation and respiratory depression caused by administration of excessive doses of opioid agonists. NMDA Antagonists Glutamate is the endogenous agonist for spinal and supraspinal NMDA receptors. Blockade of NMDA receptors in the dorsal horn of the spinal cord prevents windup and the development of central sensitization. Ketamine is the most widely used NMDA antagonist in veterinary medicine. Ketamine is supplied as a racemic mixture of 2 optical enantiomers. S( ) ketamine has 4 times the affinity of L( ) ketamine for the NMDA receptor and has a shorter duration of action. The clinical analgesic potency of S( ) ketamine is approximately twice that of the racemic mixture. Nonracemic mixtures of ketamine may be available for use in small animals in the near future. Ketamine can be used to manage pain throughout the perioperative period at anesthetic and subanesthetic doses. 46 Intravenous or intramuscular administration of anesthetic doses produces dissociative with poor muscle relaxation in both cats and dogs. Cardiovascular s are limited, and ventilation is better maintained than with other anesthetic drugs. Dysphoria and seizures can also occur after administration of high doses of ketamine, but dysphoria is less severe or absent at low subanesthetic or analgesic doses. The anesthetic s of ketamine last for approximately 30 minutes, but motor s can persist for several hours. Intravenous administration of ketamine at an infusion rate of 0.6 mg/kg/h decreases isoflurane requirements by 25%. 37 Intraoperative and postoperative administration of ketamine also reduces pain scores in dogs after forelimb amputation. 47 Because of the potential for dysphoria, opioid infusions are a better choice for most patients than opioid-ketamine infusions or ketamine infusions alone. However, patients with significant preexisting central sensitization or those undergoing major procedures with significant surgical trauma may benefit from intraoperative and postoperative opioid-ketamine infusions. Intraoperatively, the intravenous infusion dose range for cats and dogs is 0.4 to 0.6 mg/kg/h. Postoperatively, a lower intravenous infusion dose range of 0.2 to 0.3 mg/kg/h is used to avoid dysphoria and motor s. An intravenous bolus of 0.5 to 1.0 mg/kg can be given as a loading dose or to provide short-term analgesia. Local Anesthetics Local anesthetics have the unique ability to produce complete blockade of sensory nerve fibers and prevent the development of central sensitization. Consequently, peripheral and central neural blockade are often used in combination with other analgesic and anesthetic drugs to manage perioperative pain. Local anesthetics block the generation and conduction of nerve impulses by inhibiting voltage-gated sodium channels in nerve fibers. Lidocaine is also given systemically to manage pain and to reduce ileus after abdominal surgery. The mechanism of action of systemic administration of lidocaine is unclear, but peripheral, central, and antiinflammatory mechanisms have been proposed. 48 Lidocaine, mepivacaine, and bupivacaine are the local anesthetics used most commonly in cats and dogs. Lidocaine has a fast onset (10 minutes) and a short duration of action (1-2 hours) and is used for short diagnostic and surgical procedures. Mepivacaine is similar to lidocaine in potency and onset, but has a longer duration of action (2-3 hours), causes less tissue irritation, and has a higher therapeutic index. Bupivacaine is approximately 4 times the potency of lidocaine and mepivacaine, has a slow onset (20 minutes), and a long duration of action (4-6 hours) and is used for most
8 Volume 25, Number 2, May surgical procedures. Aminoamide local anesthetics are highly protein-bound and are metabolized primarily by the liver. Consequently, anemic and hypoproteinemic patients are more susceptible to local anesthetic toxicity. In most patients, the clearance of lidocaine is dependent on blood flow rather than hepatic metabolism. As a result, the clearance of lidocaine is significantly reduced in patients with low cardiac output. Local anesthetics are relatively safe if they are used correctly. Administration of an excessive dose and accidental intravenous administration are the most common causes of systemic toxicity in small animals. As a general rule, the total dose of lidocaine or bupivacaine should not exceed 8 mg/kg or 2 mg/kg, respectively. Clinicians should always consider including peripheral or central neural blockade in their perioperative pain management plans. These techniques reduce inhalation anesthetic requirements, attenuate the neuroendocrine response to surgical trauma, and improve outcome. Local anesthetic techniques for cats and dogs are described in detail in several recent articles. 4,49,50 Systemic administration of lidocaine can be used perioperatively to reduce inhalation anesthetic requirements, to provide analgesia, to control ventricular arrhythmias, and to manage postoperative ileus. Intravenous administration of lidocaine at a rate of 3 mg/kg/h reduces isoflurane requirements by 20% to 30%. 37,51 Intravenous loading doses of 1 to 2 mg/kg are appropriate for most dogs. Intraoperatively, the intravenous infusion dose range for dogs is 4 to 6 mg/kg/h. Postoperatively, a lower intravenous infusion dose range of 2 to 3 mg/kg/h is used to provide analgesia and to improve gastrointestinal motility. Objective clinical data on the use of intraoperative and postoperative lidocaine infusions in cats are limited. COX Inhibitors Prostaglandins play a central role in inflammation and the development of peripheral sensitization. Production of prostaglandins in the dorsal horn of the spinal cord also contributes to the development of central sensitization. Inhibition of constitutive (COX-1) and inducible (COX-2) cyclooxygenase inhibits the conversion of arachadonic acid to prostaglandins and thromboxanes and produces a peripheral antiinflammatory. In the dorsal horn, inhibition of COX produces a central analgesic. COX inhibitors vary in their selectivity for the different isoenzymes, as well as their ability to produce central analgesic and antiinflammatory s. 52,53 Several COX inhibitors are approved for perioperative use in cats and dogs. COX inhibitors are usually given postoperatively alone or in combination with opioids. Parenteral formulations of ketoprofen, meloxicam, and carprofen are available in Canada and the United States and are better suited for perioperative administration. Ketoprofen has a rapid onset (0.5 hour), a short half-life, and an intermediate duration of action (12 hours). The drug inhibits platelet function but does not appear to prolong bleeding time in healthy animals undergoing elective surgery. 54 Ketoprofen can be given subcutaneously to cats and dogs immediately after surgery at a dose of 2 mg/kg. Therapy with ketoprofen can be continued at a dose of 0.5 mg/kg twice daily for 3 to 5 days. Meloxicam and carprofen have a slow onset time (1-2 hours) and a long duration of action (24 hours). These drugs do not appear to inhibit platelet function and do not prolong bleeding time in healthy animals. Meloxicam can be given subcutaneously to dogs immediately after surgery at a dose of 0.2 mg/kg. Therapy with meloxicam can be continued at a dose of 0.1 mg/kg once daily for 3 to 5 days. Carprofen can be given subcutaneously to dogs immediately after surgery at a dose of 4 mg/kg. Therapy with carprofen can be continued at a dose of 4 mg/kg once daily for 3 to 5 days. Deracoxib is also labeled for perioperative use in dogs, but no parenteral formulation is available. Side s are not usually a problem in healthy animals with normal platelet, gastrointestinal, and renal function. There is little benefit to preoperative administration of COX inhibitors, and there is significant risk for patients with compromised platelet and renal function. Figure 2. Components of balanced. General anesthetics (propofol, isoflurane) are used to induce hypnosis or a loss of consciousness, but these drugs have very low therapeutic indices and produce significant cardiopulmonary depression at clinically relevant doses. Concurrent administration of analgesic drugs not only attenuates autonomic responses (increased heart rate and arterial pressure, increased respiratory rate) to surgical trauma, but reduces the amount of intravenous and inhalation anesthetics required to induce and maintain. Administration of alpha-2 agonists and local anesthetics can also improve muscle relaxation.
9 Table 3. Examples of Anesthetic and Pain Management Protocols for Healthy Cats* Surgical Procedure Preoperative Management Intraoperative Management Postoperative Management Comments Castration (Protocol 1) Castration (Protocol 2) Ovariohysterectomy (Protocol 1) Ovariohysterectomy (Protocol 2) Onychectomy (Protocol 1) Acepromazine: Butorphanol: Medetomidine: Butorphanol: Acepromazine: Hydromorphone: Medetomidine: Hydromorphone: Glycopyrrolate: 0.01 mg/ kg, IM Acepromazine: Hydromorphone: and maintenance Ketamine: and maintenance Ketamine: 5-10 Thiopental: 8-12 mg/kg, IV to Isoflurane: 1.5%-2.5% Thiopental: 4-6 mg/kg, IV to Isoflurane: 1.0%-2.0% Propofol: 4-6 mg/kg, IV to Isoflurane: 1.5%-2.5% Digital nerve block 0.5% bupivacaine: ml Do not exceed a total dose of 2 mg/kg. Ketoprofen: 1.0 mg/kg, PO Ketoprofen: 1.0 mg/kg, PO Ketoprofen: 1.0 mg/kg, PO Mild pain Mild pain Some patients may require additional hydromorphone Some patients may require additional hydromorphone Digital nerve block reduces anesthetic requirements and improves analgesia 78 Topics in Companion Animal Medicine
10 Volume 25, Number 2, May Table 3. Continued Surgical Procedure Preoperative Management Intraoperative Management Postoperative Management Comments Digital nerve block reduces anesthetic requirements and improves analgesia Ketoprofen: 1.0 mg/kg, PO Propofol: 2-3 mg/kg, IV to Isoflurane: 1.0%-2.0% Digital nerve block 0.5% Bupivacaine: ml Do not exceed a total dose of 2 mg/kg. Medetomidine: Hydromorphone: Glycopyrrolate: 0.01 mg/ kg, IM Onychectomy (Protocol 2) Abbreviations: IM, Intramuscularly; IV, intravenously; SC, subcutaneously; PO, by mouth. *Some of these drugs are not approved for use in cats in Canada or the United States. Dexmedetomidine can be substituted at approximately half the medetomidine dose. Clinical Management Multimodal analgesia is not a difficult concept to understand, nor is it a difficult concept to put into practice. In the perioperative setting, a multimodal analgesic protocol is simply a balanced anesthetic and pain management protocol (Fig 2). At first glance, the notion that analgesic drugs should be given to anesthetized patients that are unable to consciously perceive pain seems irrational. However, most intravenous (propofol) and inhalation (isoflurane, sevoflurane) anesthetics simply produce unconsciousness and do not substantially alter nociceptive processing. In fact, autonomic responses (sudden changes in respiratory rate, heart rate, and blood pressure) that occur during surgery as a result of noxious stimulation usually reflect inadequate analgesia rather than insufficient anesthetic depth. The safest approach for most patients is to limit the amount of inhalation anesthetic required by providing supplemental intraoperative analgesic therapy with opioids and local anesthetic techniques. After initial assessment of analgesic requirements and identification of major anesthetic risk factors, drugs are selected to minimize risk and to provide optimal anesthetic and pain management throughout the perioperative period. Patient monitoring and supportive care are also selected to optimize cardiopulmonary function and to minimize anesthetic risk. Hypoventilation (P a CO 2 60 mm Hg), hypotension (mean arterial pressure 60 mm Hg), and bradycardia are common perioperative complications. Care should be taken to avoid or reduce doses of anesthetic and analgesic drugs that have similar side s. Opioids and inhalation anesthetics can induce significant respiratory depression. Acepromazine, inhalation anesthetics, and epidural can cause significant hypotension. Alpha-2 agonists and opioids can cause bradycardia and enhance oculovagal and viscerovagal reflexes triggered by surgical manipulation. Given the potential for significant intraoperative complications, there is no substitute for diligent patient monitoring by a qualified, experienced anesthetist. The type of surgery (invasive vs minimally invasive) and the surgical site (somatic vs visceral) should also be considered when developing anesthetic and pain management plans. Pain must be managed aggressively throughout the perioperative period in patients scheduled for invasive surgical procedures, whereas postoperative administration of COX inhibitors may be appropriate for patients scheduled for minimally invasive procedures. Peripheral or central neural blockade is appropriate for most patients scheduled for surgical procedures of the front or hind limbs and for those scheduled for dental procedures. Conversely, complete blockade of nociceptive input from somatic and visceral afferents may be impossible to achieve in patients scheduled for thoracic or abdominal surgical procedures. Perioperative use of analgesic drugs and techniques reduces the doses of intravenous and inhalation anesthetics required to induce and maintain, improves cardiopulmonary function during surgery, and promotes a smooth recovery from after surgery. These analgesic drugs
Receptors and Neurotransmitters: It Sounds Greek to Me. Agenda. What We Know About Pain 9/7/2012
 Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Pharmacology of Pain Transmission and Modulation
 Pharmacology of Pain Transmission and Modulation 2 Jürg Schliessbach and Konrad Maurer Nociceptive Nerve Fibers Pain is transmitted to the central nervous system via thinly myelinated Aδ and unmyelinated
Pharmacology of Pain Transmission and Modulation 2 Jürg Schliessbach and Konrad Maurer Nociceptive Nerve Fibers Pain is transmitted to the central nervous system via thinly myelinated Aδ and unmyelinated
211MDS Pain theories
 211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
Somatosensory Physiology (Pain And Temperature) Richard M. Costanzo, Ph.D.
 Somatosensory Physiology (Pain And Temperature) Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture the student should be familiar with: 1. The relationship between nociception
Somatosensory Physiology (Pain And Temperature) Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture the student should be familiar with: 1. The relationship between nociception
Chapter 16. Sense of Pain
 Chapter 16 Sense of Pain Pain Discomfort caused by tissue injury or noxious stimulation, and typically leading to evasive action important /// helps to protect us lost of pain in diabetes mellitus = diabetic
Chapter 16 Sense of Pain Pain Discomfort caused by tissue injury or noxious stimulation, and typically leading to evasive action important /// helps to protect us lost of pain in diabetes mellitus = diabetic
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress REDUCING THE PAIN FACTOR AN UPDATE ON PERI-OPERATIVE ANALGESIA Sandra Forysth, BVSc DipACVA Institute of Veterinary,
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress REDUCING THE PAIN FACTOR AN UPDATE ON PERI-OPERATIVE ANALGESIA Sandra Forysth, BVSc DipACVA Institute of Veterinary,
San Francisco Chronicle, June 2001
 PAIN San Francisco Chronicle, June 2001 CONGENITAL INSENSITIVITY TO PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional)
PAIN San Francisco Chronicle, June 2001 CONGENITAL INSENSITIVITY TO PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional)
Pain Pathways. Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH
 Pain Pathways Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH Objective To give you a simplistic and basic concepts of pain pathways to help understand the complex issue of pain Pain
Pain Pathways Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH Objective To give you a simplistic and basic concepts of pain pathways to help understand the complex issue of pain Pain
CHAPTER 4 PAIN AND ITS MANAGEMENT
 CHAPTER 4 PAIN AND ITS MANAGEMENT Pain Definition: An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. Types of Pain
CHAPTER 4 PAIN AND ITS MANAGEMENT Pain Definition: An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. Types of Pain
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Pathophysiology of Pain
 Pathophysiology of Pain Wound Inflammatory response Chemical mediators Activity in Pain Path PAIN http://neuroscience.uth.tmc.edu/s2/chapter08.html Chris Cohan, Ph.D. Dept. of Pathology/Anat Sci University
Pathophysiology of Pain Wound Inflammatory response Chemical mediators Activity in Pain Path PAIN http://neuroscience.uth.tmc.edu/s2/chapter08.html Chris Cohan, Ph.D. Dept. of Pathology/Anat Sci University
PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus. MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional) Cognitive
 PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional) Cognitive MEASUREMENT OF PAIN: A BIG PROBLEM Worst pain ever
PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional) Cognitive MEASUREMENT OF PAIN: A BIG PROBLEM Worst pain ever
PERIOPERATIVE PAIN MANAGEMENT: WHAT S UP WITH METHADONE?
 PERIOPERATIVE PAIN MANAGEMENT: WHAT S UP WITH METHADONE? Sandra Z Perkowski, VMD, PhD, DACVAA University of Pennsylvania, School of Veterinary Medicine, Philadelphia, PA Pre-emptive and multimodal use
PERIOPERATIVE PAIN MANAGEMENT: WHAT S UP WITH METHADONE? Sandra Z Perkowski, VMD, PhD, DACVAA University of Pennsylvania, School of Veterinary Medicine, Philadelphia, PA Pre-emptive and multimodal use
What is Pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. Pain is always subjective
 Pain & Acupuncture What is Pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. NOCICEPTION( the neural processes of encoding and processing noxious stimuli.)
Pain & Acupuncture What is Pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. NOCICEPTION( the neural processes of encoding and processing noxious stimuli.)
Pathophysiology of Pain. Ramon Go MD Assistant Professor Anesthesiology and Pain medicine NYP-CUMC
 Pathophysiology of Pain Ramon Go MD Assistant Professor Anesthesiology and Pain medicine NYP-CUMC Learning Objectives Anatomic pathway of nociception Discuss the multiple target sites of pharmacological
Pathophysiology of Pain Ramon Go MD Assistant Professor Anesthesiology and Pain medicine NYP-CUMC Learning Objectives Anatomic pathway of nociception Discuss the multiple target sites of pharmacological
Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007)
 Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007) Introduction Adrian s work on sensory coding Spinal cord and dorsal root ganglia Four somatic sense modalities Touch Mechanoreceptors
Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007) Introduction Adrian s work on sensory coding Spinal cord and dorsal root ganglia Four somatic sense modalities Touch Mechanoreceptors
The anatomy and physiology of pain
 The anatomy and physiology of pain Charlotte E Steeds Abstract Pain is an unpleasant experience that results from both physical and psychological responses to injury. A complex set of pathways transmits
The anatomy and physiology of pain Charlotte E Steeds Abstract Pain is an unpleasant experience that results from both physical and psychological responses to injury. A complex set of pathways transmits
ANAT2010. Concepts of Neuroanatomy (II) S2 2018
 ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
Sensory coding and somatosensory system
 Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
Pain and impulse conduction
 1 Pain and impulse conduction L.H.D.J. Booij According to the World Health Organisation pain is defined as an unpleasant sensation that occurs from imminent tissue damage. From a physiological perspective,
1 Pain and impulse conduction L.H.D.J. Booij According to the World Health Organisation pain is defined as an unpleasant sensation that occurs from imminent tissue damage. From a physiological perspective,
General Anesthesia. Mohamed A. Yaseen
 General Anesthesia Mohamed A. Yaseen M.S,c Surgery Before Anesthesia General Anesthesia ( GA ) Drug induced absence of perception of all sensation allowing surgery or other painful procedure to be carried
General Anesthesia Mohamed A. Yaseen M.S,c Surgery Before Anesthesia General Anesthesia ( GA ) Drug induced absence of perception of all sensation allowing surgery or other painful procedure to be carried
UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL), SPRING 2014
 UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL), SPRING 2014 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition 2011 Eman Al-Khateeb,
UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL), SPRING 2014 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition 2011 Eman Al-Khateeb,
Pain. Pain. Pain: One definition. Pain: One definition. Pain: One definition. Pain: One definition. Psyc 2906: Sensation--Introduction 9/27/2006
 Pain Pain Pain: One Definition Classic Paths A new Theory Pain and Drugs According to the international Association for the Study (Merskey & Bogduk, 1994), Pain is an unpleasant sensory and emotional experience
Pain Pain Pain: One Definition Classic Paths A new Theory Pain and Drugs According to the international Association for the Study (Merskey & Bogduk, 1994), Pain is an unpleasant sensory and emotional experience
1 The Physiology of Pain
 1 The Physiology of Pain Rohit Juneja and Siân Jaggar Introduction Definitions Key Messages Pain is still underdiagnosed and undertreated. Pain is a subjective experience and may even be present in the
1 The Physiology of Pain Rohit Juneja and Siân Jaggar Introduction Definitions Key Messages Pain is still underdiagnosed and undertreated. Pain is a subjective experience and may even be present in the
MOLECULAR AND CELLULAR NEUROSCIENCE
 MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
SOMATOSENSORY SYSTEMS AND PAIN
 SOMATOSENSORY SYSTEMS AND PAIN A 21 year old man presented with a stab wound of the right side of the neck (Panel A). Neurological examination revealed right hemiplegia and complete right-sided loss of
SOMATOSENSORY SYSTEMS AND PAIN A 21 year old man presented with a stab wound of the right side of the neck (Panel A). Neurological examination revealed right hemiplegia and complete right-sided loss of
AKA a painful lecture by Colleen Blanchfield, MD Full Circle Neuropsychiatric Wellness Center
 A lecture on PAIN AKA a painful lecture by Colleen Blanchfield, MD Full Circle Neuropsychiatric Wellness Center 1 Overview of the lecture Anatomy of the Pain Tract How a painful stimulus travels from the
A lecture on PAIN AKA a painful lecture by Colleen Blanchfield, MD Full Circle Neuropsychiatric Wellness Center 1 Overview of the lecture Anatomy of the Pain Tract How a painful stimulus travels from the
ANAT2010. Concepts of Neuroanatomy (II) S2 2018
 ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE
 Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Chapter 7. The Nervous System: Structure and Control of Movement
 Chapter 7 The Nervous System: Structure and Control of Movement Objectives Discuss the general organization of the nervous system Describe the structure & function of a nerve Draw and label the pathways
Chapter 7 The Nervous System: Structure and Control of Movement Objectives Discuss the general organization of the nervous system Describe the structure & function of a nerve Draw and label the pathways
Chapter 7. Objectives
 Chapter 7 The Nervous System: Structure and Control of Movement Objectives Discuss the general organization of the nervous system Describe the structure & function of a nerve Draw and label the pathways
Chapter 7 The Nervous System: Structure and Control of Movement Objectives Discuss the general organization of the nervous system Describe the structure & function of a nerve Draw and label the pathways
Medical Neuroscience Tutorial
 Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Chapter 9. Nervous System
 Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
The Nervous System. Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output
 The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
Lecture 22: A little Neurobiology
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
Pain classifications slow and fast
 Pain classifications slow and fast Fast Pain Slow Pain Sharp, pricking (Aδ) fiber Short latency Well localized Short duration Dull, burning (C) fiber Slower onset Diffuse Long duration Less emotional Emotional,
Pain classifications slow and fast Fast Pain Slow Pain Sharp, pricking (Aδ) fiber Short latency Well localized Short duration Dull, burning (C) fiber Slower onset Diffuse Long duration Less emotional Emotional,
Module H NERVOUS SYSTEM
 Module H NERVOUS SYSTEM Topic from General functions of the nervous system Organization of the nervous system from both anatomical & functional perspectives Gross & microscopic anatomy of nervous tissue
Module H NERVOUS SYSTEM Topic from General functions of the nervous system Organization of the nervous system from both anatomical & functional perspectives Gross & microscopic anatomy of nervous tissue
Spinal Cord Injury Pain. Michael Massey, DO CentraCare Health St Cloud, MN 11/07/2018
 Spinal Cord Injury Pain Michael Massey, DO CentraCare Health St Cloud, MN 11/07/2018 Objectives At the conclusion of this session, participants should be able to: 1. Understand the difference between nociceptive
Spinal Cord Injury Pain Michael Massey, DO CentraCare Health St Cloud, MN 11/07/2018 Objectives At the conclusion of this session, participants should be able to: 1. Understand the difference between nociceptive
CHAPTER 15 LECTURE OUTLINE
 CHAPTER 15 LECTURE OUTLINE I. INTRODUCTION A. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. B. Operation of the ANS to maintain homeostasis,
CHAPTER 15 LECTURE OUTLINE I. INTRODUCTION A. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. B. Operation of the ANS to maintain homeostasis,
CHAPTER 10 THE SOMATOSENSORY SYSTEM
 CHAPTER 10 THE SOMATOSENSORY SYSTEM 10.1. SOMATOSENSORY MODALITIES "Somatosensory" is really a catch-all term to designate senses other than vision, hearing, balance, taste and smell. Receptors that could
CHAPTER 10 THE SOMATOSENSORY SYSTEM 10.1. SOMATOSENSORY MODALITIES "Somatosensory" is really a catch-all term to designate senses other than vision, hearing, balance, taste and smell. Receptors that could
Name Biology 125 Midterm #2 ( ) Total Pages: 9
 Name - 1 - Biology 125 Midterm #2 (11-15-07) Part 1: (30 pts) Part 2: (40 pts) Part 3: (6 pts) Part 4: (12 pts) Part 5: (12 pts) Total Pages: 9 Total Score: (100 pts) 1 Name - 2 - Part 1: True or False.
Name - 1 - Biology 125 Midterm #2 (11-15-07) Part 1: (30 pts) Part 2: (40 pts) Part 3: (6 pts) Part 4: (12 pts) Part 5: (12 pts) Total Pages: 9 Total Score: (100 pts) 1 Name - 2 - Part 1: True or False.
The Nervous System -The master controlling and communicating system of the body
 The Nervous System -The master controlling and communicating system of the body Functions: -Sensory input -Integration -Motor output Organization of the Nervous System Central nervous system (CNS) -Brain
The Nervous System -The master controlling and communicating system of the body Functions: -Sensory input -Integration -Motor output Organization of the Nervous System Central nervous system (CNS) -Brain
Chapter 12 Nervous Tissue. Copyright 2009 John Wiley & Sons, Inc. 1
 Chapter 12 Nervous Tissue Copyright 2009 John Wiley & Sons, Inc. 1 Terms to Know CNS PNS Afferent division Efferent division Somatic nervous system Autonomic nervous system Sympathetic nervous system Parasympathetic
Chapter 12 Nervous Tissue Copyright 2009 John Wiley & Sons, Inc. 1 Terms to Know CNS PNS Afferent division Efferent division Somatic nervous system Autonomic nervous system Sympathetic nervous system Parasympathetic
THE NERVOUS SYSTEM. Homeostasis Strand
 THE NERVOUS SYSTEM Homeostasis Strand Introduction In general, a nervous system has three overlapping functions : 1. Sensory input conduction of signals from sensory receptors to integration centres 2.
THE NERVOUS SYSTEM Homeostasis Strand Introduction In general, a nervous system has three overlapping functions : 1. Sensory input conduction of signals from sensory receptors to integration centres 2.
Chapter 17. Nervous System Nervous systems receive sensory input, interpret it, and send out appropriate commands. !
 Chapter 17 Sensory receptor Sensory input Integration Nervous System Motor output Brain and spinal cord Effector cells Peripheral nervous system (PNS) Central nervous system (CNS) 28.1 Nervous systems
Chapter 17 Sensory receptor Sensory input Integration Nervous System Motor output Brain and spinal cord Effector cells Peripheral nervous system (PNS) Central nervous system (CNS) 28.1 Nervous systems
Primary Functions. Monitor changes. Integrate input. Initiate a response. External / internal. Process, interpret, make decisions, store information
 NERVOUS SYSTEM Monitor changes External / internal Integrate input Primary Functions Process, interpret, make decisions, store information Initiate a response E.g., movement, hormone release, stimulate/inhibit
NERVOUS SYSTEM Monitor changes External / internal Integrate input Primary Functions Process, interpret, make decisions, store information Initiate a response E.g., movement, hormone release, stimulate/inhibit
Neural Communication. Central Nervous System Peripheral Nervous System. Communication in the Nervous System. 4 Common Components of a Neuron
 Neural Communication Overview of CNS / PNS Electrical Signaling Chemical Signaling Central Nervous System Peripheral Nervous System Somatic = sensory & motor Autonomic = arousal state Parasympathetic =
Neural Communication Overview of CNS / PNS Electrical Signaling Chemical Signaling Central Nervous System Peripheral Nervous System Somatic = sensory & motor Autonomic = arousal state Parasympathetic =
Pain Mechanisms. Prof Michael G Irwin MD, FRCA, FANZCA FHKAM Head Department of Anaesthesiology University of Hong Kong. The Somatosensory System
 ain Mechanisms rof Michael G Irwin MD, FRCA, FANZCA FHKAM Head Department of Anaesthesiology University of Hong Kong The Somatosensory System Frontal cortex Descending pathway eriaqueductal gray matter
ain Mechanisms rof Michael G Irwin MD, FRCA, FANZCA FHKAM Head Department of Anaesthesiology University of Hong Kong The Somatosensory System Frontal cortex Descending pathway eriaqueductal gray matter
Neural Basis of Motor Control
 Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
Nervous System. Master controlling and communicating system of the body. Secrete chemicals called neurotransmitters
 Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
*Anteriolateral spinothalamic tract (STT) : a sensory pathway that is positioned anteriorly and laterally in the spinal cord.
 *somatic sensations : PAIN *Anteriolateral spinothalamic tract (STT) : a sensory pathway that is positioned anteriorly and laterally in the spinal cord. *This pathway carries a variety of sensory modalities:
*somatic sensations : PAIN *Anteriolateral spinothalamic tract (STT) : a sensory pathway that is positioned anteriorly and laterally in the spinal cord. *This pathway carries a variety of sensory modalities:
General anesthesia. No single drug capable of achieving these effects both safely and effectively.
 General anesthesia General anesthesia is essential to surgical practice, because it renders patients analgesic, amnesia, and unconscious reflexes, while causing muscle relaxation and suppression of undesirable
General anesthesia General anesthesia is essential to surgical practice, because it renders patients analgesic, amnesia, and unconscious reflexes, while causing muscle relaxation and suppression of undesirable
Nervous System, Neuroanatomy, Neurotransmitters
 Nervous System, Neuroanatomy, Neurotransmitters Neurons Structure of neurons Soma Dendrites Spines Axon Myelin Nodes of Ranvier Neurons Structure of neurons Axon collaterals 1 Neurons Structure of neurons
Nervous System, Neuroanatomy, Neurotransmitters Neurons Structure of neurons Soma Dendrites Spines Axon Myelin Nodes of Ranvier Neurons Structure of neurons Axon collaterals 1 Neurons Structure of neurons
Pain and Temperature Objectives
 Pain and Temperature Objectives 1. Describe the types of sensory receptors that transmit pain and temperature. 2. Understand how axon diameter relates to transmission of pain and temp information. 3. Describe
Pain and Temperature Objectives 1. Describe the types of sensory receptors that transmit pain and temperature. 2. Understand how axon diameter relates to transmission of pain and temp information. 3. Describe
By the end of this lecture the students will be able to:
 UNIT VII: PAIN Objectives: By the end of this lecture the students will be able to: Review the concept of somatosensory pathway. Describe the function of Nociceptors in response to pain information. Describe
UNIT VII: PAIN Objectives: By the end of this lecture the students will be able to: Review the concept of somatosensory pathway. Describe the function of Nociceptors in response to pain information. Describe
The Nervous System. Chapter 4. Neuron 3/9/ Components of the Nervous System
 Chapter 4 The Nervous System 1. Components of the Nervous System a. Nerve cells (neurons) Analyze and transmit information Over 100 billion neurons in system Four defined regions Cell body Dendrites Axon
Chapter 4 The Nervous System 1. Components of the Nervous System a. Nerve cells (neurons) Analyze and transmit information Over 100 billion neurons in system Four defined regions Cell body Dendrites Axon
CNS part 2 & Intro to Sensory Systems
 CNS part 2 & Intro to Sensory Systems Brain Function Important Concepts Functional areas of the cerebral cortex Sensory, Motor, Association Cerebral lateralization each hemisphere has functions not shared
CNS part 2 & Intro to Sensory Systems Brain Function Important Concepts Functional areas of the cerebral cortex Sensory, Motor, Association Cerebral lateralization each hemisphere has functions not shared
CHAPTER 48: NERVOUS SYSTEMS
 CHAPTER 48: NERVOUS SYSTEMS Name I. AN OVERVIEW OF NERVOUS SYSTEMS A. Nervous systems perform the three overlapping functions of sensory input, integration, and motor output B. Networks of neurons with
CHAPTER 48: NERVOUS SYSTEMS Name I. AN OVERVIEW OF NERVOUS SYSTEMS A. Nervous systems perform the three overlapping functions of sensory input, integration, and motor output B. Networks of neurons with
Nervous System. 2. Receives information from the environment from CNS to organs and glands. 1. Relays messages, processes info, analyzes data
 Nervous System 1. Relays messages, processes info, analyzes data 2. Receives information from the environment from CNS to organs and glands 3. Transmits impulses from CNS to muscles and glands 4. Transmits
Nervous System 1. Relays messages, processes info, analyzes data 2. Receives information from the environment from CNS to organs and glands 3. Transmits impulses from CNS to muscles and glands 4. Transmits
10.1: Introduction. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial cells) Dendrites.
 10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
PAIN. Physiology of pain relating to pain management
 PAIN Physiology of pain relating to pain management What is pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. (Melzac and Wall) The generation of pain
PAIN Physiology of pain relating to pain management What is pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. (Melzac and Wall) The generation of pain
biological psychology, p. 40 The study of the nervous system, especially the brain. neuroscience, p. 40
 biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
Somatosensory System. Steven McLoon Department of Neuroscience University of Minnesota
 Somatosensory System Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Dr. Riedl s review session this week: Tuesday (Oct 10) 4-5pm in MCB 3-146B 2 Sensory Systems Sensory
Somatosensory System Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Dr. Riedl s review session this week: Tuesday (Oct 10) 4-5pm in MCB 3-146B 2 Sensory Systems Sensory
CHAPTER 4 PAIN AND ITS MANAGEMENT
 CHAPTER 4 PAIN AND ITS MANAGEMENT Pain Definition: An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. Types of Pain
CHAPTER 4 PAIN AND ITS MANAGEMENT Pain Definition: An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. Types of Pain
Human Anatomy. Autonomic Nervous System
 Human Anatomy Autonomic Nervous System 1 Autonomic Nervous System ANS complex system of nerves controls involuntary actions. Works with the somatic nervous system (SNS) regulates body organs maintains
Human Anatomy Autonomic Nervous System 1 Autonomic Nervous System ANS complex system of nerves controls involuntary actions. Works with the somatic nervous system (SNS) regulates body organs maintains
The Nervous System: Neural Tissue Pearson Education, Inc.
 13 The Nervous System: Neural Tissue Introduction Nervous System Characteristics Controls and adjust the activity of the body Provides swift but brief responses The nervous system includes: Central Nervous
13 The Nervous System: Neural Tissue Introduction Nervous System Characteristics Controls and adjust the activity of the body Provides swift but brief responses The nervous system includes: Central Nervous
Psychophysical laws. Legge di Fechner: I=K*log(S/S 0 )
 Psychophysical laws Legge di Weber: ΔS=K*S Legge di Fechner: I=K*log(S/S 0 ) Sensory receptors Vision Smell Taste Touch Thermal senses Pain Hearing Balance Proprioception Sensory receptors Table 21-1 Classification
Psychophysical laws Legge di Weber: ΔS=K*S Legge di Fechner: I=K*log(S/S 0 ) Sensory receptors Vision Smell Taste Touch Thermal senses Pain Hearing Balance Proprioception Sensory receptors Table 21-1 Classification
Pain Protocols for Common Elective Surgical Procedures
 Most opioids used in veterinary medicine are controlled drugs and are generally administered by injection because of po al bioavailability; however, fentanyl patches have become a popupatient suppt PAIN
Most opioids used in veterinary medicine are controlled drugs and are generally administered by injection because of po al bioavailability; however, fentanyl patches have become a popupatient suppt PAIN
Basic Neuroscience. Sally Curtis
 The Physiology of Pain Basic Neuroscience Sally Curtis sac3@soton.ac.uk The behaviour of humans is a result of the actions of nerves. Nerves form the basis of Thoughts, sensations and actions both reflex
The Physiology of Pain Basic Neuroscience Sally Curtis sac3@soton.ac.uk The behaviour of humans is a result of the actions of nerves. Nerves form the basis of Thoughts, sensations and actions both reflex
ACTIVITY2.15 Text:Campbell,v.8,chapter48 DATE HOUR NERVOUS SYSTEMS NEURON
 AP BIOLOGY ACTIVITY2.15 Text:Campbell,v.8,chapter48 NAME DATE HOUR NERVOUS SYSTEMS NEURON SIMPLE REFLEX RESTING POTENTIAL ACTION POTENTIAL ACTION POTENTIAL GRAPH TRANSMISSION ACROSS A SYNAPSE QUESTIONS:
AP BIOLOGY ACTIVITY2.15 Text:Campbell,v.8,chapter48 NAME DATE HOUR NERVOUS SYSTEMS NEURON SIMPLE REFLEX RESTING POTENTIAL ACTION POTENTIAL ACTION POTENTIAL GRAPH TRANSMISSION ACROSS A SYNAPSE QUESTIONS:
2018 Learning Outcomes
 I. Pain Physiology and Anatomy (20%) A. Describe the basic anatomy of the nervous system. B. Describe the physiological mechanisms of neuronal function (eg- action potentials). C. Review the nociceptive
I. Pain Physiology and Anatomy (20%) A. Describe the basic anatomy of the nervous system. B. Describe the physiological mechanisms of neuronal function (eg- action potentials). C. Review the nociceptive
Organization of the nervous system. [See Fig. 48.1]
![Organization of the nervous system. [See Fig. 48.1] Organization of the nervous system. [See Fig. 48.1]](/thumbs/90/103926552.jpg) Nervous System [Note: This is the text version of this lecture file. To make the lecture notes downloadable over a slow connection (e.g. modem) the figures have been replaced with figure numbers as found
Nervous System [Note: This is the text version of this lecture file. To make the lecture notes downloadable over a slow connection (e.g. modem) the figures have been replaced with figure numbers as found
NERVOUS SYSTEM C H A P T E R 2 8
 NERVOUS SYSTEM C H A P T E R 2 8 CAN AN INJURED SPINAL CORD BE FIXED? Injuries to the spinal cord disrupt communication between the central nervous system (brain and spinal cord) and the rest of the body
NERVOUS SYSTEM C H A P T E R 2 8 CAN AN INJURED SPINAL CORD BE FIXED? Injuries to the spinal cord disrupt communication between the central nervous system (brain and spinal cord) and the rest of the body
Neural Integration I: Sensory Pathways and the Somatic Nervous System
 15 Neural Integration I: Sensory Pathways and the Somatic Nervous System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to Sensory Pathways and
15 Neural Integration I: Sensory Pathways and the Somatic Nervous System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to Sensory Pathways and
The Fifth Vital Sign.
 Recognizing And Monitoring The Painful Patient Susan Clark, LVT, VTS(ECC) The Fifth Vital Sign. Pain control is part of the accepted standard of care in veterinary medicine. The ability to recognize the
Recognizing And Monitoring The Painful Patient Susan Clark, LVT, VTS(ECC) The Fifth Vital Sign. Pain control is part of the accepted standard of care in veterinary medicine. The ability to recognize the
Functions of Nervous System Neuron Structure
 Chapter 10 Nervous System I Divisions of the Nervous System Cell Types of Neural Tissue neurons neuroglial cells Central Nervous System brain spinal cord Peripheral Nervous System nerves cranial nerves
Chapter 10 Nervous System I Divisions of the Nervous System Cell Types of Neural Tissue neurons neuroglial cells Central Nervous System brain spinal cord Peripheral Nervous System nerves cranial nerves
Neurons, Synapses, and Signaling
 Neurons, Synapses, and Signaling The Neuron is the functional unit of the nervous system. Neurons are composed of a cell body, which contains the nucleus and organelles; Dendrites which are extensions
Neurons, Synapses, and Signaling The Neuron is the functional unit of the nervous system. Neurons are composed of a cell body, which contains the nucleus and organelles; Dendrites which are extensions
LECTURE STRUCTURE ASC171 NERVOUS SYSTEM PART 1: BACKGROUND 26/07/2015. Module 5
 LECTURE STRUCTURE PART 1: Background / Introduction PART 2: Structure of the NS, how it operates PART 3: CNS PART 4: PNS Why did the action potential cross the synaptic junction? To get to the other side
LECTURE STRUCTURE PART 1: Background / Introduction PART 2: Structure of the NS, how it operates PART 3: CNS PART 4: PNS Why did the action potential cross the synaptic junction? To get to the other side
Anatomical Substrates of Somatic Sensation
 Anatomical Substrates of Somatic Sensation John H. Martin, Ph.D. Center for Neurobiology & Behavior Columbia University CPS The 2 principal somatic sensory systems: 1) Dorsal column-medial lemniscal system
Anatomical Substrates of Somatic Sensation John H. Martin, Ph.D. Center for Neurobiology & Behavior Columbia University CPS The 2 principal somatic sensory systems: 1) Dorsal column-medial lemniscal system
Neurons, Synapses and Signaling. Chapter 48
 Neurons, Synapses and Signaling Chapter 48 Warm Up Exercise What types of cells can receive a nerve signal? Nervous Organization Neurons- nerve cells. Brain- organized into clusters of neurons, called
Neurons, Synapses and Signaling Chapter 48 Warm Up Exercise What types of cells can receive a nerve signal? Nervous Organization Neurons- nerve cells. Brain- organized into clusters of neurons, called
PAIN MODULATION. numerical value. adjectives. DR SYED SHAHID HABIB Professor & Consultant Dept. of Physiology College of Medicine & KKUH
 PAIN MODULATION numerical value adjectives DR SYED SHAHID HABIB Professor & Consultant Dept. of Physiology College of Medicine & KKUH OBJECTIVES At the end of this lecture you should be able to describe:
PAIN MODULATION numerical value adjectives DR SYED SHAHID HABIB Professor & Consultant Dept. of Physiology College of Medicine & KKUH OBJECTIVES At the end of this lecture you should be able to describe:
CHAPTER 13&14: The Central Nervous System. Anatomy of the CNS
 CHAPTER 13&14: The Central Nervous System Anatomy of the CNS in human consists of brain and spinal cord as stated earlier neurons have little support from their extracellular matrix and depend on glial
CHAPTER 13&14: The Central Nervous System Anatomy of the CNS in human consists of brain and spinal cord as stated earlier neurons have little support from their extracellular matrix and depend on glial
Analgesia for Small Animals Pharmacology & Clinical Practice. Jill Maddison The Royal Veterinary College Hawkshead Lane, North Mymms, AL9 7TA, UK
 Analgesia for Small Animals Pharmacology & Clinical Practice Jill Maddison The Royal Veterinary College Hawkshead Lane, North Mymms, AL9 7TA, UK Colin Dunlop Advanced Anaesthesia Specialists Unit 13, 46-48
Analgesia for Small Animals Pharmacology & Clinical Practice Jill Maddison The Royal Veterinary College Hawkshead Lane, North Mymms, AL9 7TA, UK Colin Dunlop Advanced Anaesthesia Specialists Unit 13, 46-48
-Ensherah Mokheemer. -Amani Nofal. -Loai Alzghoul
 -1 -Ensherah Mokheemer -Amani Nofal -Loai Alzghoul 1 P a g e Today we will start talking about the physiology of the nervous system and we will mainly focus on the Central Nervous System. Introduction:
-1 -Ensherah Mokheemer -Amani Nofal -Loai Alzghoul 1 P a g e Today we will start talking about the physiology of the nervous system and we will mainly focus on the Central Nervous System. Introduction:
Fundamentals of the Nervous System and Nervous Tissue: Part C
 PowerPoint Lecture Slides prepared by Janice Meeking, Mount Royal College C H A P T E R 11 Fundamentals of the Nervous System and Nervous Tissue: Part C Warm Up What is a neurotransmitter? What is the
PowerPoint Lecture Slides prepared by Janice Meeking, Mount Royal College C H A P T E R 11 Fundamentals of the Nervous System and Nervous Tissue: Part C Warm Up What is a neurotransmitter? What is the
Posterior White Column-Medial Lemniscal Pathway
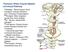 Posterior White Column-Medial Lemniscal Pathway Modality: Discriminative Touch Sensation (include Vibration) and Conscious Proprioception Receptor: Most receptors except free nerve endings Ist Neuron:
Posterior White Column-Medial Lemniscal Pathway Modality: Discriminative Touch Sensation (include Vibration) and Conscious Proprioception Receptor: Most receptors except free nerve endings Ist Neuron:
Chapter 25. General Anesthetics
 Chapter 25 1. Introduction General anesthetics: 1. Analgesia 2. Amnesia 3. Loss of consciousness 4. Inhibition of sensory and autonomic reflexes 5. Skeletal muscle relaxation An ideal anesthetic: 1. A
Chapter 25 1. Introduction General anesthetics: 1. Analgesia 2. Amnesia 3. Loss of consciousness 4. Inhibition of sensory and autonomic reflexes 5. Skeletal muscle relaxation An ideal anesthetic: 1. A
Nervous System C H A P T E R 2
 Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
What it Takes to be a Pain
 What it Takes to be a Pain Pain Pathways and the Neurophysiology of pain Dennis S. Pacl, MD, FACP, FAChPM Austin Palliative Care/ Hospice Austin A Definition of Pain complex constellation of unpleasant
What it Takes to be a Pain Pain Pathways and the Neurophysiology of pain Dennis S. Pacl, MD, FACP, FAChPM Austin Palliative Care/ Hospice Austin A Definition of Pain complex constellation of unpleasant
Department of Neurology/Division of Anatomical Sciences
 Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
EE 791 Lecture 2 Jan 19, 2015
 EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
What are the 6 types of neuroglia and their functions?!
 Warm Up! Take out your 11C Notes What are the 6 types of neuroglia and their functions?! Astrocytes Microglia Ependymal Cells Satellite Cells Schwann Cells Oligodendrocytes Support, brace, & nutrient transfer
Warm Up! Take out your 11C Notes What are the 6 types of neuroglia and their functions?! Astrocytes Microglia Ependymal Cells Satellite Cells Schwann Cells Oligodendrocytes Support, brace, & nutrient transfer
ABSTRACT the first chapter,
 ABSTRACT To induce the anesthesia, specially injectable general anesthesia, it supposes the use of an increased number of substances, that usually overcome 4 to 8 number, and which may reach in certain
ABSTRACT To induce the anesthesia, specially injectable general anesthesia, it supposes the use of an increased number of substances, that usually overcome 4 to 8 number, and which may reach in certain
Mechanism of Pain Production
 Mechanism of Pain Production Pain conducting nerve fibers are small myelinated (A-delta) or unmyelinated nerve fibers (C-fibers). Cell bodies are in the dorsal root ganglia (DRG) or sensory ganglia of
Mechanism of Pain Production Pain conducting nerve fibers are small myelinated (A-delta) or unmyelinated nerve fibers (C-fibers). Cell bodies are in the dorsal root ganglia (DRG) or sensory ganglia of
NEURAL TISSUE (NEUROPHYSIOLOGY) PART I (A): NEURONS & NEUROGLIA
 PART I (A): NEURONS & NEUROGLIA Neural Tissue Contains 2 kinds of cells: neurons: cells that send and receive signals neuroglia (glial cells): cells that support and protect neurons Neuron Types Sensory
PART I (A): NEURONS & NEUROGLIA Neural Tissue Contains 2 kinds of cells: neurons: cells that send and receive signals neuroglia (glial cells): cells that support and protect neurons Neuron Types Sensory
QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY]
![QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY] QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY]](/thumbs/86/94924826.jpg) QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY] Learning Objectives: Explain how neurons communicate stimulus intensity Explain how action potentials are conducted along
QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY] Learning Objectives: Explain how neurons communicate stimulus intensity Explain how action potentials are conducted along
Somatic Sensory System I. Background
 Somatic Sensory System I. Background A. Differences between somatic senses and other senses 1. Receptors are distributed throughout the body as opposed to being concentrated at small, specialized locations
Somatic Sensory System I. Background A. Differences between somatic senses and other senses 1. Receptors are distributed throughout the body as opposed to being concentrated at small, specialized locations
number Done by Corrected by Doctor
 number 13 Done by Tamara Wahbeh Corrected by Doctor Omar Shaheen In this sheet the following concepts will be covered: 1. Divisions of the nervous system 2. Anatomy of the ANS. 3. ANS innervations. 4.
number 13 Done by Tamara Wahbeh Corrected by Doctor Omar Shaheen In this sheet the following concepts will be covered: 1. Divisions of the nervous system 2. Anatomy of the ANS. 3. ANS innervations. 4.
Pain. Pain and management of pain. Henning Andreas Haga Associate professor Norwegian School of Veterinary Science
 Pain and management of pain Henning Andreas Haga Associate professor Norwegian School of Veterinary Science Pain An unpleasant sensory and emotional experience associated with actual or potential tissue
Pain and management of pain Henning Andreas Haga Associate professor Norwegian School of Veterinary Science Pain An unpleasant sensory and emotional experience associated with actual or potential tissue
Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons. Chad Smurthwaite & Jordan Shellmire
 Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons Chad Smurthwaite & Jordan Shellmire The Chemical Synapse The most common type of synapse used for signal transmission in the central
Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons Chad Smurthwaite & Jordan Shellmire The Chemical Synapse The most common type of synapse used for signal transmission in the central
