Regeneration Bone Grafting & Soft Tissue Management
|
|
|
- Geoffrey Gregory
- 5 years ago
- Views:
Transcription
1 Regeneration Bone Grafting & Soft Tissue Management
2 OSTEONTMⅡ
3 Table of Contents Bone Graft Material OSTEON TM Collagen 04 OSTEON TM Ⅱ(Sinus & Lifting) 06 OSTEON TM (Sinus & Lifting) 08 ORTHOPEDIC OSTEON TM 12 Membrane OSTEOGuide TM 13 Collagen Membrane 14 Documentation Summaries Bone Graft Material 16
4 04 Application of OSTEON TM Collagen Ridge augmentation Extraction site & osteotomy Cystic cavities Sinus lift Periodontal defect Description OSTEON TM Collagen is a bone void filler composed of synthetic bone, (OSTEON TM ) and natural Type I collagen. Characteristics of OSTEON TM Collagen Collagen coating enables easy handling, and thus shortened operation time Moldable to various defect shape after being wet Collagen dissolves after helping the initial handling Excellent new bone formation and space maintenance Hemostatic function Clinical Case Full mouth rehabilitation
5 05 Cell adhesion test Osteoblasts spread well on the OSTEON TM Collagen Animal Test 8 weeks 1. Animals: New Zealand white rabbit 2. Implantation Area: Calvaria 3. Period: 8 weeks 4. Staining method: Goldner Trichrome Products Type REF Size (mm) Particle Size (mm) Cylinder GOCC0605S Ø6.0 x 5.0 GOCC0610S Ø6.0 x ~ 0.5 Clinical Case Ridge augmentation Ridge preservation
6 06 (Sinus & Lifting) Application of OSTEON TM Ⅱ Ridge augmentation Extraction site & osteotomy Cystic cavities Sinus lift Periodontal defect Composition of OSTEON TM Ⅱ Osteoconductive biphasic calcium phosphate with higher β-tcp = HA 30% + β-tcp 70% Characteristics of OSTEON TM Ⅱ Highly resorbable due to higher β-tcp content Easy manipulation Excellent wettability Osteoconductive synthetic bonegraft Pore size : 250 μm Porosity : 70% Clinical Case Horizontal GBR
7 07 Cell Adhesion Test In vitro dissolution test Osteoblasts attached & spreaded well Animal Test 12-weeks follow up in rabbit calvaria model OSTEON TM OSTEON TM Ⅱ Products Type REF Volume (cc) Particle Size (mm) DT7G DT7G ~0.5 Vial Sinus Lifting DT7G DT7G DT7G DT7G DT7G DT7G DT7G DT7G SS 0.5 DT7G SS 0.5 DT7G LS 0.25 DT7G LS ~ ~ ~ ~ ~ ~1.0
8 08 (Sinus & Lifting) Application of OSTEON TM Ridge augmentation Extraction site Cystic cavities Sinus lift Periodontal defect Composition of OSTEON TM 100% Synthetic bone graft : HA scaffold coated with β-tcp = HA 70% + β-tcp 30% Specification of OSTEON TM 100% Synthetic bone graft Interconnected porous structure similar to that of human cancellous bone Osteoconductive material as a bone growth scaffold OSTEON Human bone Cell Adhesion Test Human Osteoblast cell OSTEON TM X1000 X1000 X5000 Osteoblast cell was well attached and spreaded on OSTEON surface.
9 09 Human Histology 6.5 months after Sinus graft surgery New bone OSTEON TM 0.5mm 0.25mm OSTEON TM area = 1.24mm 2 (17.1%) New bone area = 1.63mm 2 (22.7%) 10 months after Sinus graft surgery OSTEON TM New bone 1.0mm 0.5mm OSTEON TM area = 3.04mm 2 (35.5%) New bone area = 2.38mm 2 (27.7%) 21 months after Sinus graft surgery OSTEON TM New bone 0.5mm 0.25mm OSTEON TM area = 6.30mm 2 (40.4%) New bone area = 5.12mm 2 (33.0%)
10 10 Products Product REF Particle Size (mm) Volume (cc) GBG ~0.5 Vial GBG ~ / 0.5 / 1.0 GBG ~2.0 Sinus GBG0510SS 0.5~1.0 GBG1020SS 1.0~ Lifting GBG0305LS 0.3~0.5 GBG0510LS 0.5~ Syringe Product O.D. I.D. OSTEON TM Sinus Ø7.0mm Ø5.0mm OSTEON TM Lifting Ø5.0mm Ø3.4mm O.D. : Syringe outer diameter I.D. : Syringe inner diameter
11 11 Instruction for OSTEON Sinus & Lifting TM ① Slightly retract the plunger and gently tap to loosen particles. Gently push plunger back into place. ② Place syringe into a sterile dappen dish and retract plunger to draw liquid into the syringe. ③ To optimize delivery, OSTEON should be wetted and loosened sufficiently. ④ Expel excess liquid by applying very gentle pressure on the plunger. ⑤ When sufficiently hydrated, OSTEON will expel with ease from the syringe. Before injecting OSTEON, remove the cap from the syringe. ⑥ Deliver OSTEON directly into the surgical site with the syringe. Clinical Case OSTEONTM Sinus Case (Sinus grafting-lateral approach) After 9 months OSTEONTM Lifting Case (Sinus grafting-crestal approach)
12 12 Application of ORTHOPEDIC OSTEON Bone filling Fractures with bone defects Pseudoarthrosis with or without bone defects Tibial osteotomy In certain cases of arthroplasty revision Products Product REF Particle Size (mm) Volume (cc) ORTHOPEDIC OSTEON GOBG ~4.0 GOBG ~ / 4.0 / 5.0 / 10.0 / 15.0 / 20.0 GOBG0510, GOBG1020, GOBG2030, GOBG3040, GOBG4050 are available. Animal Test - Rabbit Femur Model 12 weeks after bone grafting in rabbit femur. After bone grafting in rabbit femur for 12 weeks, new bone was well formed in the pores and around ORTHOPEDIC OSTEON. Clinical Case Bone void filler
13 13 Description Non-resorbable GBR membrane Biocompatible polymer PCL Thin for easy manipulation Porous membrane Products Product REF Size (mm) Thickness (mm) GPM X 20 OSTEOGuide GPM X GPM X 40 Composition of OSTEOGuide Bio-compatible polymer = 100% PCL (Polycaprolactone) The porous outer surface of the OSTEOGuide allows easy entry of the adjacent cells assuring rapid and good tissue attachment. Good nutrient flow and blood vessel formation supported by the porous structure. Animal Test - Rabbit Calvaria Model New bone was well formed on the bottom of the OSTEOGuide and cells did not penetrate into the defect. Soft tissue OSTEOGuide TM
14 14 Application of Collagen Membrane Biodegradable barrier membrane for guided bone / tissue regeneration Periodontal / infrabony defects Ridge augmentation Extraction sites (implant preparation / placement) Sinus lift Characteristics of Collagen Membrane Easy manipulation Dual-sided usage Barrier function lasting for 6 months Thinner membrane(300 μm ) with multiple layers for easy manipulation and sufficient mechanical strength in surgery. Resorption period of 6 months to provide enough time for stabilizing graft materials and supporting bone growth. Multiple-layered structure enables more effective bone regeneration by sparing enough space for hard tissue formation and facilitates proliferation of osteoblast. x1000 x5000
15 15 Products Product REF Size (mm) Thickness (mm) GCM X 20 Collagen Membrane GCM X 20 GCM X Preclinical Data Rabbit Calvaria Model, 6-12 weeks Degradation character in collagenase solution 6 weeks weeks Relative weight (wt%) A Company (4m) Dentium B Company (6m) Time (h) Clinical Case Horizontal GBR Interpositional bone graft
16 16 Documentation Summaries Bone Graft Material Interconnected Pore
17 17 Kim et al., J. Biomed. Mat. Res. B (2008) Clinical Evaluation of OSTEON as New Alloplastic Material in Sinus Bone Grafting and Effect on Bone Healing Young-Kyun Kim, 1 Pil-Young Yun, 1 Sung-Chul Lim, 2 Su-Gwan Kim, 3 Hyo-Jung Lee, 4 Joo L. Ong 5 Jounal of Biomedical Materials Research Part B. Applied Biomaterials vol 86 page 270~277 This is the summary of Clinical Evaluations OSTEON as a New Alloplastic Material in Sinus Bone Grafting and its effects on Bone Healing which is published at Journal of Biomedical Material Research Part B, written by Prof. Young-Kyun Kim. Introduction. Placement of implant prosthesis in the maxillary posterior region is known to be difficult on many aspects and has lowest success rate. In many clinical situations, the maxillary region is made of type III or IV bone with porous and insufficient bones for implant placement. The advancement in implant surgical techniques, improved bone graft, and recent development in implant surface treatments have resulted in predictable sinus bone graft success, thereby allowing implant placement in the maxillary molar region. However, controversy still exists on what constitute an ideal sinus graft materials. Ideally, the bone graft material used for implant reconstruction should (a) maintain space an optimal period of time to achieve bone in growth and implant healing, (b) remain stable for the period of graft consideration, during implant integration, and after the implants are restored, (c) promote osteoconduction of the neighboring cells to form bone within the graft materials,(d) remodel itself into long-lasting bone, (e) facilitate easy placement to avoid morbidity, and (f) have predictable success rate. Alloplastic materials are recently used as bone substitute. They are biologically acceptable, allowing bone ingrowths and bone remodeling while maintaining volume. Additionally, alloplastic materials have several advantages, such as (a) the lack of required donor site, (b) ample supply, and (c) the nonexistence of disease transmission. OSTEON is one of the alloplastic materials composed of hydroxyapatite (HA) 70% and beta-tricalcium phosphate (β- TCP) 30% which are most close to major mineral components of human bone, and have interconnected porosity structure (scaffolding) which is similar to that of human cancellous bone. Human bone OSTEON Figure 1. SEM morphology of OSTEON with interconnected pore structure (x120). The pore size is from 300 to 500μm, which is similar to human cancellous bone and the porous bone graft is beneficial to osteoblast cell ingrowth to OSTEON
18 18 Kim et al., J. Biomed. Mat. Res. B (2008) Figure 2. Cross-section view of OSTEON (left, x1000) and high magnification of OSTEON surface (right, x3000). The interconnected porous scaffold is comprised from biocompatible HA, while the surface is coated with bioresorbable β-tcp. Purpose The objective of this study was to clinically evaluate the use of OSTEON as a sinus graft material and to measure the effect of healing at 4 and 6 months after surgery. Materials and Methods The two different commercially available OSTEON grafting materials (one with particle size of mm and the other with particle size of mm) were mixed in a ratio of 1:1, hydrated, followed by mixing with 10% autogenous bone chips and stabilized with tissue adhesive. After sinus graft (Fig. 3) using OSTEON in 17 patients, bone specimens were collected from lateral sinus using 2.0-mm trephine bur at the time of 4 or 6 months after surgery. Histology of the bone specimens was prepared and the percentage of newly formed bone fraction, lamellar bone/woven bone ratio (LB/WB), and newly formed bone/graft material ratio (NB/GM) were measured to indicate the suitability of the materials and the successful healing of the graft. Figure 3. The window-forming bone was pushed-on to make the upper border of the graft site, and sinus membrane was elevated carefully. Panorama X-ray images before surgery Panorama X-ray images 7 month after surgery Results & Discussions. The morphology of OSTEON was observed to be interconnected, with 77% porosity and a pore size of μm.this observed architecture was suggested to be similar to human cancellous bone, with the interconnected porosity and pore size capable of providing space for bone cell ingrowth (Fig.1).After implantation, the mean percentage of newly formed bone fraction after 4 months and 6 months surgery was 40.6 and 51.9%, respectively (Table II). Statistical analysis indicated no significant difference (p = 0.135) in the newly formed bone fraction between the two postoperative periods.
19 19 Kim et al., J. Biomed. Mat. Res. B (2008) As described in Table II, the mean LB/WB ratio after 4 months and 6 months surgery was 0.14 and 0.45, respectively, with significant difference observed between the two postoperative periods (p = 0.027). Additionally, the mean NB/GM ratio after 4 months and 6 months surgery was 1.95 and 7.72, respectively, with significant difference observed between the two postoperative periods (p = 0.046). Like most commercially available xenogenic and alloplastic bone grafting materials, bone healing during sinus graft applications using OSTEON is induced via osteoconduction. The host osteoprogenitor and angiogenic cells use the graft as a scaffold to generate new bone across the defect. As the host cells differentiate and mature within the graft, a functional skeletal network develops and replaces the graft through a creeping substitution process. The reported survival rates for grafted xenografts and alloplastic materials are equivalent or better than the survival rates for grafted autogenous materials. Additionally, these studies also indicated that the nonresorbed residual graft materials did not hinder osseointegration but significantly increase the bony density. Caution has to be taken on the mesial side of the sinus wall as the graft material is pressed against it. Too much pressure against the mesial side of the sinus wall causes small particles to obstruct new blood vessel formation and delayed resorbing large particles to retard the formation of new bone. Figure 4. Thickened, focally lamellar trabecular bone (closed asterisks) is seen around the resorbing implant material (open asterisks). H&E staining x40 (after 6 months) Summary and Conclusion In this study, OSTEON, a new alloplastic material was clinically evaluated as a sinus graft material. The morphology was observed to be interconnected, with 77% porosity and a pore size of 300~500 μm No significant difference in the percentage of newly formed bone fraction was observed at 4 months and 6 months after grafting in 17 patients. However, significant differences in mean LB / WB ratio and the mean NB / GM ratio were observed after 4 months and 6 months surgery. As confirmed by observations using the SEM, bone biopsy indicated more lamellar bone after 6 months surgery as compared to biopsy obtained after 4 months surgery. In this short-term study, it was concluded that OSTEON is suitable for use in sinus graft application since desirable time-dependent healing was demonstrated. For full report, please contact DENTIUM website
20 20 Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;107: Analysis of the healing process in sinus bone grafting using various grafting materials Young-Kyun Kim, DDS, PhD, a Pil-Young Yun, DDS, PhD, a Su-Gwan Kim, DDS, PhD, b and Sung-Chul Lim, MD, PhD c SeongNam and GwangJu City, Korea SEOUL NATIONAL UNIVERSITY BUNDANG HOSPITAL AND CHOSUN UNIVERSITY Objectives: The purpose of this study was to compare differences in the healing process in the sinus bone grafting using various grafting materials. Study design: Maxillary sinus bone grafts were divided into 4 groups according to the graft material used: group I, amixture of autogenous bone and BioOss (Osteohealth Co., Shirley, NY); group II, a mixture of BioOss and Orthoblast II (Greencross; Isotis); group III, BioOss only; and group IV, synthetic bone, Osteon (Genoss, Korea), only. To evaluate the healing status of the graft surgery, bone specimens were collected from the lateral sinus using a 2.0-mm trephine bur at 4 and 6 months after surgery. Histology of the bone specimens was prepared, and the percentage of newly formed bone fraction, lamellar bone/woven bone ratio (LB/WB), and newly formed bone/graft material ratio (NB/GM) were measured to indicate the suitability of the materials and the healing of the grafts. Results: The LB/WB ratio and NB/GM ratio were markedly increased at 6 months compared with the values at 4months. It was observed that good bone healing was achieved even for grafts of xenogeneic bone only or synthetic bone only. Cases grafted with a mixture of allogeneic and xenogeneic bone showed no great advantage regarding bone healing. Table 1. Summary of the histomorphometric study Conclusion: The results indicated that grafts of xenogeneic or synthetic bone can be effective for sinus bone grafting. I. Autogenous bone+biooss II. BioOSS +Orthoblast II III. BioOSS IV. OSTEON 4 month 6 month
21 21 Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:e14-e20 Sinus bone graft using new alloplastic bone graft material(osteon) II : clinical evaluation Ji-Hyun Bae, DDS, PhD, a Young-Kyun Kim, DDS, PhD, b Su-Gwan Kim, DDS, PhD, c Pil-Young Yun, DDS, PhD, d and Jae-Seung Kim, DDS, PhD, e Seongnam, Gwangju, and Seoul, Korea SEOUL NATIONAL UNIVERSITY BUNDANG HOSPITAL, CHOSUN UNIVERSITY, AND GUNGUK UNIVERSITY Objectives: The objective of this study was to clinically evaluate the use of Osteon as a sinus bone graft material and to measure the loss of sinus bone graft volume and marginal bone loss around the implants. Study design: Thirty-two implants were placed in 16 patients after maxillary sinus bone grafting. In 7 patients,maxillary sinus bone graft was performed first and 15 implants were placed after 4 months; in 9 patients, 17implants were placed simultaneously with maxillary sinus bone grafting. Based on medical records and radiographs, intraoperative and postoperative complications were examined, and at 1 year after the placement of the upper fixture,the success rate of implants, peri-implant soft tissue condition, and the marginal bone loss were evaluated. Additionally, the sinus bone graft volume loss was evaluated by comparing the residual alveolar bone height of the preoperative maxillary sinus floor with that immediately after the operation and after 1 year. Results: Regarding intraoperative complications, perforation of the maxillary sinus membrane occurred in 6 cases (37.5%), and after surgery maxillary sinusitis developed in 2 cases. During the healing period, 1 implant failed in osseointegration. At the last follow-up observation, none of cases showed marginal bone loss of >1 mm and a 96.9% success rate was seen. The follow-up observation period after placement of the superstructure was months (average 15). Between the simultaneous placement group and the delayed placement group, marginal bone loss showed no statistically significant difference (P=.455). In the entire patient group, the volume of sinus bone graft loss did not correlate with marginal bone loss (P=.568). Preoperative alveolar bone height was mm (mean 4.64), postoperative alveolar bone height was mm (mean 17.67), and the alveolar bone height 1 year after the operation was mm (mean 16.78). Between the group with perforation of the maxillary sinus membrane and the group without, no difference in marginal bone loss was observed (P=.628). Additionally, no difference in the volume of sinus bone graft resorption between the two groups was observed (P=.970). Conclusion: It was concluded that Osteon is suitable for use in sinus graft application.
22 22 Tissue Engineering Regenerative Medicine, Vol.6, pp , Seoul, Korea, Mar.,2009. Effects of 4 Different Alloplastic Materials on Bone Regeneration in Rabbit Calvarial Defects Sun-Jong Kim, Jin-Won Lim, Jae-Jun Ryu, Jin-Soo Ahn, In-ho Han and Sang-Wan Shin * 1 1,2 1 1,2 1,2 2 1,2 Dept of Advan Prosthodontics, Graduate School of Clinical Dentistry, 2 Instit Clinical Dental Research, Korea Univ, 80, Guro-Gu, Seoul , Korea (Received: Jan. 2nd, 2009; Accepted: Jan. 7th, 2009) Objectives: The purpose of this study was to compare bone regeneration in 8-mm defects in 8 New Zealand White rabbit calvaria using 4 different alloplastic bone substitutes. Materials and Methods: Four 8-mm calvarial defects were made in the parietal bone of each animal. The defects were filled with Bongros-HA (Bioalpha, Seongnam, Korea), micro macroporous biphasic calcium phosphate(mbcp, Biomatlante, France), Osteon (Dentium Co, Seoul, Korea) and Cerasorb (Curasan, Kleinsthei, Germany). Two animals died after surgery. Two rabbits were sacrificed after 4 weeks, and the other 4 were sacrificed after 8 weeks. Data analysis included the qualitative assessment of the calvarial specimens. Results and Discussion: Histomorphometric analysis was performed to quantify the amount of new bone within the defects. It was found that Osteon -treated defects had significantly more new bone after 8 weeks than all other groups. Osteon was an effective alloplastic bone substitute which showed reliable osseous healing of critical size defects in the rabbit calvarium. Conclusion: From the results of this study, it is suggested that HA and TCP alloplastic materials can be good bone substitutes for inducing new bone formation in rabbit calvaria in the early stage and that HA coated with TCP may have a better bone regeneration ability than HA, TCP or a mixture of HA and TCP. KEY WORDS: Bongros-HA, MBCP, Osteon, Cerasorb, critical size defect, rabbit model Table 1. For groups of alloplastic materials used in this study. Figure 1. Four different alloplastic materials were placed in the each defect.
23 23 (a) (b) (c) (d) Figure 2. Histologic finding at 4 weeks after healing (x12).(a) BongrosHA, (b) MBCP, (c) Osteon and (d) Cerasorb (a) (b) (c) (d) Figure 3. Histologic finding 8 weeks after healing(x12). (a)bongrosha, (b)mbcp, (c)osteon and (d)cerasorb. Figure 4. Histomorphometric measurement of the percentages of newly formed bone. *p<0.05
24 OSTEONTMⅡ
25 Regeneration Bone Grafting & Soft Tissue Management OSTEON TM Collagen / OSTEON TM Ⅱ(Sinus&Lifting)/OSTEON TM (Sinus&Lifting) ORTHOPEDIC OSTEON TM / OSTEOGuide TM / Collagen Membrane Copyright January, 2012 DENTIUM
26 Regeneration Bone Grafting & Soft Tissue Management Specifications are subject to change without prior notice. Some products to be launched in the market after necessary approvals are also listed in this catalog. CAT-B0401(REV.16,1201) HEAD OFFICE 3105 Trade Tower 159, Samsung-dong, Gangnam-gu, Seoul, Korea T F MANUFACTURER 1F, Gyeonggi R&DB Center / 226, 2F, GSBC Iui-dong, Yeongtong-gu, Suwon-si, Gyeonggi-do, , Korea T F HOMEPAGE /
Regeneration Bone Grafting & Soft Tissue Management
 Regeneration Bone Grafting & Soft Tissue Management OSTEON TM II Table of Contents Bone Graft Material OSTEON TM II Collagen 04 OSTEON TM Collagen 06 OSTEON TM II 08 OSTEON TM 12 ORTHOPEDIC OSTEON TM 14
Regeneration Bone Grafting & Soft Tissue Management OSTEON TM II Table of Contents Bone Graft Material OSTEON TM II Collagen 04 OSTEON TM Collagen 06 OSTEON TM II 08 OSTEON TM 12 ORTHOPEDIC OSTEON TM 14
Regeneration Bone Grafting & Soft Tissue Management
 Regeneration Bone Grafting & Soft Tissue Management 1 Regeneration Bone Grafting & Soft Tissue Management Table of contents Bone Graft Material OSTEON II OSTEON OSTEON II Collagen OSTEON Collagen 5 6
Regeneration Bone Grafting & Soft Tissue Management 1 Regeneration Bone Grafting & Soft Tissue Management Table of contents Bone Graft Material OSTEON II OSTEON OSTEON II Collagen OSTEON Collagen 5 6
Regeneration Bone Grafting & Soft Tissue Management
 Regeneration Bone Grafting & Soft Tissue Management Human Histology OSTEON II 26.5 months Regeneration Products information Table of contents Bone Graft Material OSTEON II OSTEON OSTEON II Collagen OSTEON
Regeneration Bone Grafting & Soft Tissue Management Human Histology OSTEON II 26.5 months Regeneration Products information Table of contents Bone Graft Material OSTEON II OSTEON OSTEON II Collagen OSTEON
Regeneration Bone Grafting & Soft Tissue Management
 Regeneration Bone Grafting & Soft Tissue Management OSTEON TM Table of Contents Bone Graft Material OSTEON TM II Collagen 04 OSTEON TM Collagen 06 OSTEON TM II (Sinus & Lifting) 08 OSTEON TM (Sinus & Lifting)
Regeneration Bone Grafting & Soft Tissue Management OSTEON TM Table of Contents Bone Graft Material OSTEON TM II Collagen 04 OSTEON TM Collagen 06 OSTEON TM II (Sinus & Lifting) 08 OSTEON TM (Sinus & Lifting)
Biomaterials Line. MIS Corporation. All Rights Reserved.
 7. Biomaterials Line MIS Corporation. All Rights Reserved. 6. MIS s Quality System complies with international quality standards: ISO 13485: 2003 - Quality Management System for Medical Devices, ISO 9001:
7. Biomaterials Line MIS Corporation. All Rights Reserved. 6. MIS s Quality System complies with international quality standards: ISO 13485: 2003 - Quality Management System for Medical Devices, ISO 9001:
BioVin Collagen Membrane
 BioVin Collagen Membrane Resorbable cross-linked collagen membrane BioVin Bovine Bone Bovine Bone Substitute OToss Synthetic Bone Synthetic Resorbable Biphasic Calcium Phosphate OToss Synthetic Bone Inject
BioVin Collagen Membrane Resorbable cross-linked collagen membrane BioVin Bovine Bone Bovine Bone Substitute OToss Synthetic Bone Synthetic Resorbable Biphasic Calcium Phosphate OToss Synthetic Bone Inject
C O N T E N T S. Introduction of DASK. Crestal Approach. Lateral Approach. Dentium - For Dentists By Dentists
 C O N T E N T S Introduction of DASK Crestal Approach Lateral Approach OSTEON TM Sinus & Lifting Dentium - For Dentists By Dentists 03 Introduction of DASK Drills for Crestal Approach The distance from
C O N T E N T S Introduction of DASK Crestal Approach Lateral Approach OSTEON TM Sinus & Lifting Dentium - For Dentists By Dentists 03 Introduction of DASK Drills for Crestal Approach The distance from
Welcome to Dentium. If you want to know more detailed documentation in each case, please feel free to contact us by at
 Welcome to Dentium Dentium was founded in June 2000. Since the beginning, we have focused on the quality products and services. As a result, we have obtained a successful clinical results and excellent
Welcome to Dentium Dentium was founded in June 2000. Since the beginning, we have focused on the quality products and services. As a result, we have obtained a successful clinical results and excellent
Innovative Range of Regenerative Solutions
 TM Innovative Range of Regenerative Solutions MIS Implant Technologies Ltd. All rights reserved. Optimal volumes and quality of hard and soft tissue are required to satisfy the goals of oral rehabilitation
TM Innovative Range of Regenerative Solutions MIS Implant Technologies Ltd. All rights reserved. Optimal volumes and quality of hard and soft tissue are required to satisfy the goals of oral rehabilitation
More than bone regeneration. A total solution.
 More than bone regeneration. A total solution. More than a dental implant company. A total solution. When it comes to treatment options, your patients want positive results both functionally and esthetically.
More than bone regeneration. A total solution. More than a dental implant company. A total solution. When it comes to treatment options, your patients want positive results both functionally and esthetically.
Evaluation of different grafting materials in three-wall intra-bony defects around dental implants in beagle dogs
 Current Applied Physics 5 (2005) 507 511 www.elsevier.com/locate/cap Evaluation of different grafting materials in three-wall intra-bony defects around dental implants in beagle dogs Ui-Won Jung a, Hee-Il
Current Applied Physics 5 (2005) 507 511 www.elsevier.com/locate/cap Evaluation of different grafting materials in three-wall intra-bony defects around dental implants in beagle dogs Ui-Won Jung a, Hee-Il
chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute.
 chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute. Osteoconductive Resorbable Synthetic chronos Bone Void Filler chronos granules and preforms are synthetic, porous, osteoconductive,
chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute. Osteoconductive Resorbable Synthetic chronos Bone Void Filler chronos granules and preforms are synthetic, porous, osteoconductive,
DASK-0912 [Rev.3] Distributed by. DASK_rev3_1127: 레이아웃 오? 4:53 페이지 1. Specifications are subject to change without prior notice.
![DASK-0912 [Rev.3] Distributed by. DASK_rev3_1127: 레이아웃 오? 4:53 페이지 1. Specifications are subject to change without prior notice. DASK-0912 [Rev.3] Distributed by. DASK_rev3_1127: 레이아웃 오? 4:53 페이지 1. Specifications are subject to change without prior notice.](/thumbs/90/104476783.jpg) DASK_rev3_1127: 레이아웃 1 09. 12. 02 오? 4:53 페이지 1 Distributed by Specifications are subject to change without prior notice. DASK-0912 [Rev.3] HEAD OFFICE 3105 Trade Tower 159, Samsung-dong, Gangnam-gu, Seoul,
DASK_rev3_1127: 레이아웃 1 09. 12. 02 오? 4:53 페이지 1 Distributed by Specifications are subject to change without prior notice. DASK-0912 [Rev.3] HEAD OFFICE 3105 Trade Tower 159, Samsung-dong, Gangnam-gu, Seoul,
Cerasorb M DENTAL. O:\Zulassung\Cerasorb Dental Kanada 2013\Texte\Cerasorb M Dental final IFU docx
 Cerasorb M DENTAL Resorbable, pure-phase beta-tricalcium phosphate matrix with interconnecting porosity for bone regeneration for use in dental and maxillofacial surgery DESCRIPTION: Cerasorb M DENTAL
Cerasorb M DENTAL Resorbable, pure-phase beta-tricalcium phosphate matrix with interconnecting porosity for bone regeneration for use in dental and maxillofacial surgery DESCRIPTION: Cerasorb M DENTAL
A WIDE RANGE OF REGENERATIVE SOLUTIONS
 A WIDE RANGE OF REGENERATIVE SOLUTIONS INDICATIONS: 1/ SOCKET AND RIDGE PRESERVATION 2/ FILLING OF EXTRACTION SOCKETS Biomaterials offers portfolio of regenerative materials for implantology, aimed at
A WIDE RANGE OF REGENERATIVE SOLUTIONS INDICATIONS: 1/ SOCKET AND RIDGE PRESERVATION 2/ FILLING OF EXTRACTION SOCKETS Biomaterials offers portfolio of regenerative materials for implantology, aimed at
( ) 2009;28(2):89-94
 ( ) 2009;28(2):89-94 Osseointegration is important in the functional aspect, however, esthetics is also important, especially in the maxillary anterior region. An adequate surgical technique is necessary
( ) 2009;28(2):89-94 Osseointegration is important in the functional aspect, however, esthetics is also important, especially in the maxillary anterior region. An adequate surgical technique is necessary
BEGO BIOMATERIALS When the result counts
 BEGO BIOMATERIALS When the result counts Partners in Progress INTRO Challenge what exists get the right answers We practise systematic thinking with a passion and we are never satisfied with the status
BEGO BIOMATERIALS When the result counts Partners in Progress INTRO Challenge what exists get the right answers We practise systematic thinking with a passion and we are never satisfied with the status
Symbios Xenograft Granules Porcine Bone Graft Material
 Symbios Xenograft Granules Porcine Bone Graft Material 32671122-USX-1607_Symbios Highlight Brochure.indd 1 2016-09-27 12:12 Symbios Xenograft Granules Porcine bone graft material Symbios Xenograft Granules
Symbios Xenograft Granules Porcine Bone Graft Material 32671122-USX-1607_Symbios Highlight Brochure.indd 1 2016-09-27 12:12 Symbios Xenograft Granules Porcine bone graft material Symbios Xenograft Granules
botiss dental bone & tissue regeneration biomaterials collacone max Innovative composite matrix socket preservation form-fitting resorbable composite
 dental bone & tissue regeneration botiss biomaterials socket preservation Innovative composite matrix form-fitting resorbable composite 1 botiss regeneration system maxresorb flexbone* collacone.. max
dental bone & tissue regeneration botiss biomaterials socket preservation Innovative composite matrix form-fitting resorbable composite 1 botiss regeneration system maxresorb flexbone* collacone.. max
botiss biomaterials bone & tissue regeneration collacone max Innovative composite matrix socket preservation form-fitting resorbable composite
 bone & tissue regeneration botiss biomaterials socket preservation Innovative composite matrix form-fitting resorbable composite 1 Socket preservation safeguarding your sockets collacone.. max flexbone*
bone & tissue regeneration botiss biomaterials socket preservation Innovative composite matrix form-fitting resorbable composite 1 Socket preservation safeguarding your sockets collacone.. max flexbone*
THE NEW STANDARD OF EXCELLENCE IN BIOMATERIALS. Collagenated heterologous cortico-cancellous bone mix + TSV Gel GTO I N S P I R E D B Y N A T U R E
 GTO THE NEW STANDARD OF EXCELLENCE IN BIOMATERIALS Collagenated heterologous cortico-cancellous bone mix + TSV Gel R E G E N E R A T I O N S C I E N C E I N S P I R E D B Y N A T U R E A unique biotechnology
GTO THE NEW STANDARD OF EXCELLENCE IN BIOMATERIALS Collagenated heterologous cortico-cancellous bone mix + TSV Gel R E G E N E R A T I O N S C I E N C E I N S P I R E D B Y N A T U R E A unique biotechnology
REASONS TO USE R.T.R.
 3 REASONS TO USE R.T.R. AFTER EACH EXTRACTION Fully resorbable ß-TCP material RTR 3raisons 120x280.indd 1 16/06/15 10:52 1AVOID SPONTANEOUS RIDGE RESORPTION After tooth extraction, spontaneous healing
3 REASONS TO USE R.T.R. AFTER EACH EXTRACTION Fully resorbable ß-TCP material RTR 3raisons 120x280.indd 1 16/06/15 10:52 1AVOID SPONTANEOUS RIDGE RESORPTION After tooth extraction, spontaneous healing
Guided Tissue and Bone Regeneration
 Guided Tissue and Bone Regeneration One toolbox for your needs! Tefguide 9000 742 401 12x24 mm pc 1 Osgide 9000 701 520 15 x 20 mm pc 1 Osbone 9000 800 255 250-1000 μm 0.25 cc 5 Product Specifications
Guided Tissue and Bone Regeneration One toolbox for your needs! Tefguide 9000 742 401 12x24 mm pc 1 Osgide 9000 701 520 15 x 20 mm pc 1 Osbone 9000 800 255 250-1000 μm 0.25 cc 5 Product Specifications
OSTEON TM II Collagen. Products from Dentium. Regeneration Implant Digital Dentistry
 OSTEON TM II Collagen Products from Dentium Regeneration Implant Digital Dentistry Overview Since the establishment of Dentium Co., Ltd. in Korea in June 2000, we have been manufacturing high quality
OSTEON TM II Collagen Products from Dentium Regeneration Implant Digital Dentistry Overview Since the establishment of Dentium Co., Ltd. in Korea in June 2000, we have been manufacturing high quality
Inion BioRestore. Bone Graft Substitute. Product Overview
 Inion BioRestore Bone Graft Substitute Product Overview Inion BioRestore Introduction Inion BioRestore is a synthetic bone graft substitute, which remodels into bone and is easy to use. Inion BioRestore
Inion BioRestore Bone Graft Substitute Product Overview Inion BioRestore Introduction Inion BioRestore is a synthetic bone graft substitute, which remodels into bone and is easy to use. Inion BioRestore
Procedure Manual and Catalog
 Procedure Manual and Catalog TM Why SynthoGraft? SynthoGraft offers a unique structure which provides stability, while its micro-porosity allows for rapid vascularization and subsequent resorption. Although
Procedure Manual and Catalog TM Why SynthoGraft? SynthoGraft offers a unique structure which provides stability, while its micro-porosity allows for rapid vascularization and subsequent resorption. Although
Product Information. MIS Corporation. All Rights Reserved.
 Product Information MIS Corporation. All Rights Reserved. MIS Warranty: MIS exercises great care and effort in maintaining the superior quality of its products. All MIS products are guaranteed to be free
Product Information MIS Corporation. All Rights Reserved. MIS Warranty: MIS exercises great care and effort in maintaining the superior quality of its products. All MIS products are guaranteed to be free
Familial tooth bone graft for ridge and sinus augmentation: a report of two cases
 CASE REPORT http://dx.doi.org/10.5125/jkaoms.2014.40.1.37 pissn 2234-7550 eissn 2234-5930 Familial tooth bone graft for ridge and sinus augmentation: a report of two cases Young-Kyun Kim 1, Su-Gwan Kim
CASE REPORT http://dx.doi.org/10.5125/jkaoms.2014.40.1.37 pissn 2234-7550 eissn 2234-5930 Familial tooth bone graft for ridge and sinus augmentation: a report of two cases Young-Kyun Kim 1, Su-Gwan Kim
Product Catalog. Dental Bone & Tissue Regeneration
 Product Catalog Dental Bone & Tissue Regeneration Bone graft regeneration Guided bone substitutes regeneration Osteogenesis Controlled resorption rate Angiogenesis Long term volume stability Remodeling
Product Catalog Dental Bone & Tissue Regeneration Bone graft regeneration Guided bone substitutes regeneration Osteogenesis Controlled resorption rate Angiogenesis Long term volume stability Remodeling
easy-graft TM CRYSTAL
 1 easy-graft TM CRYSTAL Overview 2 Advantages easy-graft : principle & application Material & studies Indications easy-graft CRYSTAL & CLASSIC Advantages of easy-graft CRYSTAL 3 Easy handling: Injectable
1 easy-graft TM CRYSTAL Overview 2 Advantages easy-graft : principle & application Material & studies Indications easy-graft CRYSTAL & CLASSIC Advantages of easy-graft CRYSTAL 3 Easy handling: Injectable
Scientific & Clinical Evidence Jason membrane
 Scientific & Clinical Evidence Jason membrane Pericardium GBR/GTR Membrane Facts - CE since 2009 - so far no serious clinical complication or objection - approx. 250.000 successful clinical treatments
Scientific & Clinical Evidence Jason membrane Pericardium GBR/GTR Membrane Facts - CE since 2009 - so far no serious clinical complication or objection - approx. 250.000 successful clinical treatments
Bone augmentation with biomaterials
 Patient information dental bone & tissue regeneration botiss biomaterials Bone augmentation with biomaterials established safe natural X100 Implantation stability is crucial for success Atrophy of the
Patient information dental bone & tissue regeneration botiss biomaterials Bone augmentation with biomaterials established safe natural X100 Implantation stability is crucial for success Atrophy of the
BONE AUGMENTATION AND GRAFTING
 1 A Computer-Guided Bone Block Harvesting Procedure: A Proof-of-Principle Case Report and Technical Notes Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review
1 A Computer-Guided Bone Block Harvesting Procedure: A Proof-of-Principle Case Report and Technical Notes Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review
Bioactive Glass Biphasic β-tcp & HA Granules Alkylene Oxide Polymer Carrier. MEDLINEUNITE Bioactive Bone Graft
 Bioactive Glass Biphasic β-tcp & HA Granules Alkylene Oxide Polymer Carrier Principles of Bone Healing Reparative Phase Healing Cascade 1. Cellular infiltration and migration to site (fibroblasts, macrophages,
Bioactive Glass Biphasic β-tcp & HA Granules Alkylene Oxide Polymer Carrier Principles of Bone Healing Reparative Phase Healing Cascade 1. Cellular infiltration and migration to site (fibroblasts, macrophages,
FDBA(Freeze Dried Bone Allograft) SureOss Cortical Bone - Powder / Chip. OsteOss Cortical with Cancellous Bone - Powder / Chip
 PERIODONTAL Bone & Skin Allograft Products FDBA(Freeze Dried Bone Allograft) SureOss - Powder / Chip OsteOss Cortical with - Powder / Chip DFDBA(Demineralized Freeze Dried Bone Allograft) SureOss -D Demineralized
PERIODONTAL Bone & Skin Allograft Products FDBA(Freeze Dried Bone Allograft) SureOss - Powder / Chip OsteOss Cortical with - Powder / Chip DFDBA(Demineralized Freeze Dried Bone Allograft) SureOss -D Demineralized
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE
 CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
The Essential Choice. With the Benefits of Biologic Predictability
 The Essential Choice With the Benefits of Biologic Predictability Documented, Reliable, Experienced is the essential choice for your daily regenerative needs. Throughout our long history and dedication
The Essential Choice With the Benefits of Biologic Predictability Documented, Reliable, Experienced is the essential choice for your daily regenerative needs. Throughout our long history and dedication
Versatile grafting Solutions
 Versatile grafting Solutions A Canadian company serving Canadian dentists since 1997 Here s why your colleagues are calling us for their bone regeneration needs Founded in 1997 Citagenix has been providing
Versatile grafting Solutions A Canadian company serving Canadian dentists since 1997 Here s why your colleagues are calling us for their bone regeneration needs Founded in 1997 Citagenix has been providing
CONTENTS. Introduction to DASK. Crestal Approach. Lateral Approach
 Specifications are subject to change without notice. Sales office: 11075 Knott Ave., Suite A Cypress, CA 90630 Tel 1-877-304-6752 Fax 1-714-890-6360 Homepage / E-mail: www.dentiumusa.com / info@dentiumusa.com
Specifications are subject to change without notice. Sales office: 11075 Knott Ave., Suite A Cypress, CA 90630 Tel 1-877-304-6752 Fax 1-714-890-6360 Homepage / E-mail: www.dentiumusa.com / info@dentiumusa.com
Case Study. Case # 1 Author: Dr. Suheil Boutros (USA) 2013 Zimmer Dental, Inc. All rights reserved. 6557, Rev. 03/13.
 Placement of a Zimmer Trabecular Metal Dental Implant with Simultaneous Ridge Augmentation and Immediate Non-Functional Loading Following Tooth Extraction and Orthodontic Treatment for Implant Site Development
Placement of a Zimmer Trabecular Metal Dental Implant with Simultaneous Ridge Augmentation and Immediate Non-Functional Loading Following Tooth Extraction and Orthodontic Treatment for Implant Site Development
The Original remains unique.
 The Original remains unique. Geistlich leading regeneration 2A, 2B Geistlich is the world leader in regenerative dentistry. We transform natural biomaterials into safe and reliable treatment methods that
The Original remains unique. Geistlich leading regeneration 2A, 2B Geistlich is the world leader in regenerative dentistry. We transform natural biomaterials into safe and reliable treatment methods that
Consensus Report Tissue augmentation and esthetics (Working Group 3)
 B. Klinge Thomas F. Flemmig Consensus Report Tissue augmentation and esthetics (Working Group 3) Members of working group: Matteo Chiapasco Jan-Eirik Ellingsen Ronald Jung Friedrich Neukam Isabella Rocchietta
B. Klinge Thomas F. Flemmig Consensus Report Tissue augmentation and esthetics (Working Group 3) Members of working group: Matteo Chiapasco Jan-Eirik Ellingsen Ronald Jung Friedrich Neukam Isabella Rocchietta
B U I L D S T R O N G B O N E F A S T
 B U I L D S T R O N G B O N E F A S T Putty MIS Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
B U I L D S T R O N G B O N E F A S T Putty MIS Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
Alpha-Bio's GRAFT complete portfolio bone substitutes resorbable membranes best combination of quality & value with efficient, easy-to-use products
 Alpha-Bio Tec, a world leader in advanced implantology solutions, is proud to present its growing biomaterials product line: Alpha-Bio's GRAFT, which has taken a leading position in the global dental biomaterials
Alpha-Bio Tec, a world leader in advanced implantology solutions, is proud to present its growing biomaterials product line: Alpha-Bio's GRAFT, which has taken a leading position in the global dental biomaterials
Puros Cancellous Particulate Allograft & Puros Block Allograft
 Puros Cancellous Particulate Allograft & Puros Block Allograft Puros Cancellous Particulate Allograft The Natural Choice For Healthy 1 Bone Growth. 1. Proven, Predictable Regeneration Acts as an osteoconductive
Puros Cancellous Particulate Allograft & Puros Block Allograft Puros Cancellous Particulate Allograft The Natural Choice For Healthy 1 Bone Growth. 1. Proven, Predictable Regeneration Acts as an osteoconductive
ADVANCED BONE GRAFT SYSTEM OVERVIEW
 ADVANCED BONE GRAFT SYSTEM OVERVIEW NANOSS BIOACTIVE ADVANCED BONE GRAFT Table Of Contents INTRODUCTION System Overview... 1 NANOSS BIOACTIVE COMPONENTS Comparison of nanoss Bioactive and Human Bone...
ADVANCED BONE GRAFT SYSTEM OVERVIEW NANOSS BIOACTIVE ADVANCED BONE GRAFT Table Of Contents INTRODUCTION System Overview... 1 NANOSS BIOACTIVE COMPONENTS Comparison of nanoss Bioactive and Human Bone...
The Essential Choice. With the Benefits of Biologic Predictability
 The Essential Choice With the Benefits of Biologic Predictability Documented, Reliable, Experienced is the essential choice for your daily regenerative needs. Throughout our long history and dedication
The Essential Choice With the Benefits of Biologic Predictability Documented, Reliable, Experienced is the essential choice for your daily regenerative needs. Throughout our long history and dedication
botiss maxgraft bonering botiss maxgraft bonebuilder Because one option is not enough. PROCESSED ALLOGENIC BONERING
 Product Information Allograft Biomaterials@Straumann Because one option is not enough. botiss maxgraft bonering PROCESSED ALLOGENIC BONERING botiss maxgraft bonebuilder CUSTOMIZED ALLOGENIC BONE BLOCK
Product Information Allograft Biomaterials@Straumann Because one option is not enough. botiss maxgraft bonering PROCESSED ALLOGENIC BONERING botiss maxgraft bonebuilder CUSTOMIZED ALLOGENIC BONE BLOCK
BONE void FILLER. Beta-tricalcium phosphate ( b-tcp) bone graft substitute. OvERvIEW BROCHURE
 chronos BONE void FILLER Beta-tricalcium phosphate ( b-tcp) bone graft substitute OvERvIEW BROCHURE chronos STRIP chronos Strip is a synthetic bone void filler manufactured from chronos Beta-Tricalcium
chronos BONE void FILLER Beta-tricalcium phosphate ( b-tcp) bone graft substitute OvERvIEW BROCHURE chronos STRIP chronos Strip is a synthetic bone void filler manufactured from chronos Beta-Tricalcium
BIOACTIVE SYNTHETIC GRAFT
 B U I L D S T R O N G B O N E F A S T Putty Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
B U I L D S T R O N G B O N E F A S T Putty Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
Graftys. Cross-selling. Indications. Comparison. Basic Science. Graftys 415 rue Claude Nicolas Ledoux Aix en Provence Cedex 4
 2015 Graftys Cross-selling Indications Comparison Graftys 415 rue Claude Nicolas Ledoux 13854 Aix en Provence Cedex 4 www.graftys.com Basic Science Graftys QuickSet Training 1 Where to use QuickSet? Revision
2015 Graftys Cross-selling Indications Comparison Graftys 415 rue Claude Nicolas Ledoux 13854 Aix en Provence Cedex 4 www.graftys.com Basic Science Graftys QuickSet Training 1 Where to use QuickSet? Revision
P105 Predictable Bone Grafting for Site Preparation for Implants and Prosthetics Workshop JAMES GRISDALE, DDS THURSDAY, FEBRUARY 26
 P105 Predictable Bone Grafting for Site Preparation for Implants and Prosthetics Workshop JAMES GRISDALE, DDS THURSDAY, FEBRUARY 26 Please complete the speaker evaluation form in the Midwinter Meeting
P105 Predictable Bone Grafting for Site Preparation for Implants and Prosthetics Workshop JAMES GRISDALE, DDS THURSDAY, FEBRUARY 26 Please complete the speaker evaluation form in the Midwinter Meeting
Bone augmentation with maxgraft
 Patient information bone & tissue regeneration botiss biomaterials Bone augmentation with maxgraft established safe X100 natural Implantation stability is crucial for success Atrophy of the jaw bone loss
Patient information bone & tissue regeneration botiss biomaterials Bone augmentation with maxgraft established safe X100 natural Implantation stability is crucial for success Atrophy of the jaw bone loss
Dentium Implant System. New Product
 Dentium Implant System New Product S.L.A. Surface S.L.A. (Sandblasting with Large grit and Acid etching) Higher bone-to-implant contact Faster bone formation on the surface Dentium Implant System Contents
Dentium Implant System New Product S.L.A. Surface S.L.A. (Sandblasting with Large grit and Acid etching) Higher bone-to-implant contact Faster bone formation on the surface Dentium Implant System Contents
REGENERATIONTIME. A Case Report by. Ridge Augmentation and Delayed Implant Placement on an Upper Lateral Incisor
 A Case Report by Dr. Daniele Cardaropoli Ridge Augmentation and Delayed Implant Placement on an Upper Lateral Incisor The Situation An adult female patient presented with an endodontic/prosthetic failure
A Case Report by Dr. Daniele Cardaropoli Ridge Augmentation and Delayed Implant Placement on an Upper Lateral Incisor The Situation An adult female patient presented with an endodontic/prosthetic failure
Step-by-Step Step-by-Step. Internal Hex. Implant System MAKE IT SIMLE
 4. 7. Step-by-Step Cemented Step-by-Step Bridge BONDBONE Using Abutments Internal Hex. Implant System MAKE IT SIMLE MIS Corporation. All Rights Reserved. Published by MIS, which reserves the right to ameliorate
4. 7. Step-by-Step Cemented Step-by-Step Bridge BONDBONE Using Abutments Internal Hex. Implant System MAKE IT SIMLE MIS Corporation. All Rights Reserved. Published by MIS, which reserves the right to ameliorate
THE NEXT GENERATION OF REGENERATION
 THE NEXT GENERATION OF REGENERATION Why SynthoGraft? SynthoGraft offers a unique structure which provides stability, while its micro-porosity allows for rapid vascularization and subsequent resorption.
THE NEXT GENERATION OF REGENERATION Why SynthoGraft? SynthoGraft offers a unique structure which provides stability, while its micro-porosity allows for rapid vascularization and subsequent resorption.
Dental Implants: A Predictable Solution for Tooth Loss. Reena Talwar, DDS PhD FRCD(C) Oral & Maxillofacial Surgeon Associate Clinical Professor
 Dental Implants: A Predictable Solution for Tooth Loss Reena Talwar, DDS PhD FRCD(C) Oral & Maxillofacial Surgeon Associate Clinical Professor What are Dental Implants? Titanium posts used to replace missing
Dental Implants: A Predictable Solution for Tooth Loss Reena Talwar, DDS PhD FRCD(C) Oral & Maxillofacial Surgeon Associate Clinical Professor What are Dental Implants? Titanium posts used to replace missing
Inion GTR Biodegradable Membrane System
 Inion GTR Biodegradable Membrane System The concept of GTR/GBR Intended use The Inion GTR Biodegradable Membrane System is intended to be used as a barrier membrane in dental guided tissue regeneration
Inion GTR Biodegradable Membrane System The concept of GTR/GBR Intended use The Inion GTR Biodegradable Membrane System is intended to be used as a barrier membrane in dental guided tissue regeneration
Cytoflex Barrier Membrane Clinical Evaluation
 Cytoflex Barrier Membrane Clinical Evaluation Historical Background Guided tissue regeneration is a well established concept in the repair of oral bone defects. The exclusion of soft tissue epithelial
Cytoflex Barrier Membrane Clinical Evaluation Historical Background Guided tissue regeneration is a well established concept in the repair of oral bone defects. The exclusion of soft tissue epithelial
T he cortical bone of the maxillary
 KIM ET AL IMPLANT DENTISTRY / VOLUME 26, NUMBER 3 2017 351 Sinus Membrane Elevation by the Crestal Approach Using a Novel Drilling System Young-Kyun Kim, DDS, PhD,* Ji-Young Lee, DDS, Jin-Woo Park, DDS,
KIM ET AL IMPLANT DENTISTRY / VOLUME 26, NUMBER 3 2017 351 Sinus Membrane Elevation by the Crestal Approach Using a Novel Drilling System Young-Kyun Kim, DDS, PhD,* Ji-Young Lee, DDS, Jin-Woo Park, DDS,
Surgical Technique Guide. Impact & Inject Formulations
 Surgical Technique Guide Impact & Inject Formulations System Overview OsteoVation QWIK is a bone void filler consisting of a proprietary composite of calcium phosphate and calcium sulfate granules. The
Surgical Technique Guide Impact & Inject Formulations System Overview OsteoVation QWIK is a bone void filler consisting of a proprietary composite of calcium phosphate and calcium sulfate granules. The
Resorbable bilayer synthetic membrane Biomimetic tissue-engineered matrix for GBR and GTR
 Resorbable bilayer synthetic membrane Biomimetic tissue-engineered matrix for GBR and GTR Patented Jet-Spraying technology: Full barrier effect during 4 weeks, and complete resorption in 6 months PATENTED
Resorbable bilayer synthetic membrane Biomimetic tissue-engineered matrix for GBR and GTR Patented Jet-Spraying technology: Full barrier effect during 4 weeks, and complete resorption in 6 months PATENTED
B&B DENTAL. implant company MATERIAL FOR BONE REGENERATION
 B&B DENTAL implant company MATERIAL FOR BONE REGENERATION NOVOCOR PLUS The Novocor Plus medical device comprises grains of natural coral with a low surface/volume, ranging from 200 to 500 mm. The natural
B&B DENTAL implant company MATERIAL FOR BONE REGENERATION NOVOCOR PLUS The Novocor Plus medical device comprises grains of natural coral with a low surface/volume, ranging from 200 to 500 mm. The natural
RELIABLE WHEN IT COUNTS. The unique collagenase-resistant membrane protects bone graft and supports treatment success even when exposed 4
 RELIABLE WHEN IT COUNTS 1 RELIABLE WHEN IT COUNTS RESISTANT TO EXPOSURE The unique collagenase-resistant membrane protects bone graft and supports treatment success even when exposed 4 RELIABLE BARRIER
RELIABLE WHEN IT COUNTS 1 RELIABLE WHEN IT COUNTS RESISTANT TO EXPOSURE The unique collagenase-resistant membrane protects bone graft and supports treatment success even when exposed 4 RELIABLE BARRIER
Horizontal bone augmentation by means of guided bone regeneration
 Periodontology 2000, Vol. 66, 2014, 13 40 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd Printed in Singapore. All rights reserved PERIODONTOLOGY 2000 Horizontal bone augmentation by means
Periodontology 2000, Vol. 66, 2014, 13 40 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd Printed in Singapore. All rights reserved PERIODONTOLOGY 2000 Horizontal bone augmentation by means
SalvinOss Xenograft Bone Graft Material In Vivo Testing Summary
 SalvinOss Xenograft Bone Graft Material In Vivo Testing Summary Summary of In Vivo Use Of Bioresorbable Xenograft Bone Graft Materials In The Treatment Of One-Walled Intrabony Defects In A Canine Model
SalvinOss Xenograft Bone Graft Material In Vivo Testing Summary Summary of In Vivo Use Of Bioresorbable Xenograft Bone Graft Materials In The Treatment Of One-Walled Intrabony Defects In A Canine Model
Guided bone regeneration using two types of non-resorbable barrier membranes
 DOI:10.5125/jkaoms.2010.36.4.275 Guided bone regeneration using two types of non-resorbable barrier membranes Ji-Young Lee 1, Young-Kyun Kim 1, Pil-Young Yun 1, Ji-Su Oh 2, Su-Gwan Kim 2 1 Department of
DOI:10.5125/jkaoms.2010.36.4.275 Guided bone regeneration using two types of non-resorbable barrier membranes Ji-Young Lee 1, Young-Kyun Kim 1, Pil-Young Yun 1, Ji-Su Oh 2, Su-Gwan Kim 2 1 Department of
Sinus elevation with short implant
 Sinus elevation with short implant Authors: Prof. Dr Mauro Marincola, Prof. Dr Dr Rolf Ewers, Prof. Giorgio Lombardo (University of Verona) & Prof. Miguel Simancas Pallares (University of Cartagena), Austria/Italy/Colombia
Sinus elevation with short implant Authors: Prof. Dr Mauro Marincola, Prof. Dr Dr Rolf Ewers, Prof. Giorgio Lombardo (University of Verona) & Prof. Miguel Simancas Pallares (University of Cartagena), Austria/Italy/Colombia
Puros Cancellous Particulate Allograft & Puros Block Allograft
 Puros Cancellous Particulate Allograft & Puros Block Allograft Puros Cancellous Particulate Allograft The Natural Choice For Healthy 1 Bone Growth. 1. Proven, Predictable Regeneration Acts as an osteoconductive
Puros Cancellous Particulate Allograft & Puros Block Allograft Puros Cancellous Particulate Allograft The Natural Choice For Healthy 1 Bone Growth. 1. Proven, Predictable Regeneration Acts as an osteoconductive
Pre op Failed endodontic treatment with sinus involvement.
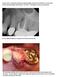 Case #1 of 10 consecutive extraction sockets grafted with Socket Graft Putty, covered with Socket Seal and sealed with Periacryl. I D # HEU This patient is a 66 year old female. Pre op Failed endodontic
Case #1 of 10 consecutive extraction sockets grafted with Socket Graft Putty, covered with Socket Seal and sealed with Periacryl. I D # HEU This patient is a 66 year old female. Pre op Failed endodontic
Alveolar Ridge Preservation:
 Alveolar Ridge Preservation: Preserving and Building up the Bony Structures after Extraction» By: Prof. Roland Hille Konigsallee 49c, 41747 Viersen, Germany E-mail: dr-hille@t-online.de» Prof. Rolf Vollmer
Alveolar Ridge Preservation: Preserving and Building up the Bony Structures after Extraction» By: Prof. Roland Hille Konigsallee 49c, 41747 Viersen, Germany E-mail: dr-hille@t-online.de» Prof. Rolf Vollmer
BONE GRAFTING. Product Catalog
 BONE GRAFTING Product Catalog PRODUCT LINE Titanium Mesh Surgitime Titanium Titanium Foil Surgitime Titanium Seal Polytetraflouroethylene Membrane Surgitime PTFE Bone Screws Fixation Set We innovate to
BONE GRAFTING Product Catalog PRODUCT LINE Titanium Mesh Surgitime Titanium Titanium Foil Surgitime Titanium Seal Polytetraflouroethylene Membrane Surgitime PTFE Bone Screws Fixation Set We innovate to
THE NEXT FRONTIER OF BONE REGENERATION. where Technology meets Nature
 THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
Calcium Phosphate Cement
 Calcium Phosphate Cement Fast-Setting Bone Graft and AutoGraft Extender. * Ossilix is a high performance next generation calcium phosphate cement indicated for filling bony defects in cancellous bone.
Calcium Phosphate Cement Fast-Setting Bone Graft and AutoGraft Extender. * Ossilix is a high performance next generation calcium phosphate cement indicated for filling bony defects in cancellous bone.
Applications of Moldable Autogenous Tooth Bone Graft (M-AutoBT) Mixed with Hydroxypropylmethyl Cellulose for Sinus Lifting
 Clinical Report Young-Kyun Kim et al.:moldable Autogenous Tooth Bone Graft for Sinus Lifting Journal of Hard Tissue Biology 24[4] (2015) 391-396 2015 The Hard Tissue Biology Network Association Printed
Clinical Report Young-Kyun Kim et al.:moldable Autogenous Tooth Bone Graft for Sinus Lifting Journal of Hard Tissue Biology 24[4] (2015) 391-396 2015 The Hard Tissue Biology Network Association Printed
THE NEXT FRONTIER OF BONE REGENERATION. where Technology meets Nature
 THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
Bone Grafting for Socket Preservation
 Bone Grafting for Socket Preservation Dr. Karl R. Koerner Normal extraction facial bone loss. Excessive force. Commonly the thickness of facial bone. Hussain, A. et al. Ridge preservation comparing a nonresorbable
Bone Grafting for Socket Preservation Dr. Karl R. Koerner Normal extraction facial bone loss. Excessive force. Commonly the thickness of facial bone. Hussain, A. et al. Ridge preservation comparing a nonresorbable
In Vivo Evaluation of BioSphere Bioactive Bone Graft Putty: Improved Bone Formation
 In Vivo Evaluation of BioSphere Bioactive Bone Graft : Improved Bone Formation ABSTRACT BioSphere is a novel bone graft product that was developed using spherical particles of bioactive glass with a narrow,
In Vivo Evaluation of BioSphere Bioactive Bone Graft : Improved Bone Formation ABSTRACT BioSphere is a novel bone graft product that was developed using spherical particles of bioactive glass with a narrow,
ctive Bone Bonding Bone Regeneration Osteostimulation*
 www.bonalive.com ctive Bone Bonding Bone Regeneration Osteostimulation* A revolution in bone grafting Osteoconductive bone graft substitute with osteostimulative* properties *non-osteoinductive Before
www.bonalive.com ctive Bone Bonding Bone Regeneration Osteostimulation* A revolution in bone grafting Osteoconductive bone graft substitute with osteostimulative* properties *non-osteoinductive Before
The injectable, self-setting calcium phosphate bone graft substitute.
 The injectable, self-setting calcium phosphate bone graft substitute. With a porous architecture similar to natural bone. STRUCSURE CP Bone Graft Substitute Because simplicity matters STRUCSURE CP Bone
The injectable, self-setting calcium phosphate bone graft substitute. With a porous architecture similar to natural bone. STRUCSURE CP Bone Graft Substitute Because simplicity matters STRUCSURE CP Bone
Symbios. Product Catalog
 Symbios Product Catalog Solutions for all your regenerative needs The comprehensive portfolio for hard and soft tissue regeneration provides ease of use and safety for dental clinicians and their patients.
Symbios Product Catalog Solutions for all your regenerative needs The comprehensive portfolio for hard and soft tissue regeneration provides ease of use and safety for dental clinicians and their patients.
Redefining Regeneration
 Redefining Regeneration Taking Volume to the MAX One Product, One Treatment, Real VOLUME Buccal Bone Loss Socket Preservation Lateral / Vertical Augmentation Grafting Material Scaffold Barrier (4-6 months)
Redefining Regeneration Taking Volume to the MAX One Product, One Treatment, Real VOLUME Buccal Bone Loss Socket Preservation Lateral / Vertical Augmentation Grafting Material Scaffold Barrier (4-6 months)
THE NEXT FRONTIER OF BONE REGENERATION
 THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature swiss made SmartBone is new composite bone substitute specifically developed for bone regeneration in oral and maxillofacial reconstructive
THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature swiss made SmartBone is new composite bone substitute specifically developed for bone regeneration in oral and maxillofacial reconstructive
BIOMATERIAL COLLECTION
 BIOMATERIAL COLLECTION www.implantdirect.com Innovation. Quality. Service. Value. INDEX GRAFTING PAGE Allograft Bone Grafting DirectGen 1-2 Bone Grafting DirectGen Putty 3 Bone Grafting DirectGen FLEX
BIOMATERIAL COLLECTION www.implantdirect.com Innovation. Quality. Service. Value. INDEX GRAFTING PAGE Allograft Bone Grafting DirectGen 1-2 Bone Grafting DirectGen Putty 3 Bone Grafting DirectGen FLEX
A new approach with an in-situ self-hardening grafting material
 74 Bone grafting with simultaneous early implant placement A new approach with an in-situ self-hardening grafting material MINAS LEVENTIS 1,2, PHD; PETER FAIRBAIRN 1,3, BDS; ORESTIS VASILIADIS 2,4, DDS
74 Bone grafting with simultaneous early implant placement A new approach with an in-situ self-hardening grafting material MINAS LEVENTIS 1,2, PHD; PETER FAIRBAIRN 1,3, BDS; ORESTIS VASILIADIS 2,4, DDS
Callos Bone Void Filler
 Surgical Technique Callos Bone Void Filler Acumed is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches that improve
Surgical Technique Callos Bone Void Filler Acumed is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches that improve
Maxillary sinus augmentation without any graft material- A case Report
 A CASE REPORT ISSN: 2321-4988 D.Shiva kumar et al. /JPR:BioMedRx: An International Journal 2013,1(8), Available online through www.jpronline.info Maxillary sinus augmentation without any graft material-
A CASE REPORT ISSN: 2321-4988 D.Shiva kumar et al. /JPR:BioMedRx: An International Journal 2013,1(8), Available online through www.jpronline.info Maxillary sinus augmentation without any graft material-
Use of Bone Regeneration Cement for Bone Grafting in Atrophic Areas Clinical, Radiographic and Histological Analysis
 I user _ report Use of Bone Regeneration Cement for Bone Grafting in Atrophic Areas Clinical, Radiographic and Histological Analysis authors_ Sérgio Alexandre Gehrke*, Bruno König Júnior**, Nara Maria
I user _ report Use of Bone Regeneration Cement for Bone Grafting in Atrophic Areas Clinical, Radiographic and Histological Analysis authors_ Sérgio Alexandre Gehrke*, Bruno König Júnior**, Nara Maria
The Use of Freeze-Dried Bone Allograft as an Alternative to Autogenous Bone Graft in the Atrophic Maxilla: A 3-Year Clinical Follow-up
 643 The Use of Freeze-Dried Bone Allograft as an Alternative to Autogenous Bone Graft in the Atrophic Maxilla: A 3-Year Clinical Follow-up Marco Aurélio Bianchini, DDS, MSc, PhD 1 André R. Buttendorf,
643 The Use of Freeze-Dried Bone Allograft as an Alternative to Autogenous Bone Graft in the Atrophic Maxilla: A 3-Year Clinical Follow-up Marco Aurélio Bianchini, DDS, MSc, PhD 1 André R. Buttendorf,
Extraction with Immediate Implant Placement and Ridge Preservation in the Posterior
 Extraction with Immediate Implant Placement and Ridge Preservation in the Posterior by Timothy F. Kosinski, DDS, MAGD The following case presentation illustrates the diagnosis, planning and treatment for
Extraction with Immediate Implant Placement and Ridge Preservation in the Posterior by Timothy F. Kosinski, DDS, MAGD The following case presentation illustrates the diagnosis, planning and treatment for
THE NEXT FRONTIER OF BONE REGENERATION
 THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature swiss made SmartBone is new composite bone substitute specifically developed for bone regeneration in oral and maxillofacial reconstructive
THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature swiss made SmartBone is new composite bone substitute specifically developed for bone regeneration in oral and maxillofacial reconstructive
BIOMATERIAL COLLECTION
 BIOMATERIAL COLLECTION www.implantdirect.com Innovation. Quality. Service. Value. INDEX GRAFTING PAGE Xenograft Bone Grafting DirectOss 1 Allograft Bone Grafting DirectGen 2-3 Bone Grafting DirectGen Putty
BIOMATERIAL COLLECTION www.implantdirect.com Innovation. Quality. Service. Value. INDEX GRAFTING PAGE Xenograft Bone Grafting DirectOss 1 Allograft Bone Grafting DirectGen 2-3 Bone Grafting DirectGen Putty
Surgical Solutions USA Regenerative Product Catalog USA
 RPC Surgical Solutions Regenerative Product Catalog RESORBABLE COLLAGEN MEMBRANES (BOVINE) CYTOPLAST RTM Collagen Resorption time of 26-38 weeks RTM1520 $185 15mm x 20mm Membranes per box: 2 RTM2030 $230
RPC Surgical Solutions Regenerative Product Catalog RESORBABLE COLLAGEN MEMBRANES (BOVINE) CYTOPLAST RTM Collagen Resorption time of 26-38 weeks RTM1520 $185 15mm x 20mm Membranes per box: 2 RTM2030 $230
PERIODONTAL Bone & Skin Allograft Products
 PERIODONTAL Bone & Skin Allograft Products SureOss Family SureOss Cortical Bone - / SureOss Plus Cortical Bone with CA - / SureOss Collagen Cortical Bone with Collagen - Sponge Block SureOss Paste Cortical
PERIODONTAL Bone & Skin Allograft Products SureOss Family SureOss Cortical Bone - / SureOss Plus Cortical Bone with CA - / SureOss Collagen Cortical Bone with Collagen - Sponge Block SureOss Paste Cortical
Technique Guide. chronos Strip. Osteoconductive betatricalcium phosphate ( -TCP) composite.
 Technique Guide chronos Strip. Osteoconductive betatricalcium phosphate ( -TCP) composite. Table of Contents Introduction chronos Strip 2 Indications and Contraindications 3 Surgical Technique Preparation
Technique Guide chronos Strip. Osteoconductive betatricalcium phosphate ( -TCP) composite. Table of Contents Introduction chronos Strip 2 Indications and Contraindications 3 Surgical Technique Preparation
MASTERGRAFT Putty. Technical Guide
 MASTERGRAFT Putty Technical Guide MASTERGRAFT Putty is a malleable-cohesive, osteoconductive scaffold composed of collagen that is physically mixed with resorbable ceramic granules. Mixing Process Collagen
MASTERGRAFT Putty Technical Guide MASTERGRAFT Putty is a malleable-cohesive, osteoconductive scaffold composed of collagen that is physically mixed with resorbable ceramic granules. Mixing Process Collagen
Sinus Augmentation Studies Methods and Definition
 FDA approved indications for Infuse Bone Graft in the Maxillofacial Skeleton Alveolar Ridge Augmentation (Buccal Wall Defects) in the Maxilla Maxillary Sinus Floor augmentation Sinus Augmentation Studies
FDA approved indications for Infuse Bone Graft in the Maxillofacial Skeleton Alveolar Ridge Augmentation (Buccal Wall Defects) in the Maxilla Maxillary Sinus Floor augmentation Sinus Augmentation Studies
Vertical Bone Augmentation for Implant Placement in the Mandible a Systematic. Review
 Vertical Bone Augmentation for Implant Placement in the Mandible a Systematic Review A dissertation submitted to the University of Manchester for the degree of Master of Science in Dentistry (Oral and
Vertical Bone Augmentation for Implant Placement in the Mandible a Systematic Review A dissertation submitted to the University of Manchester for the degree of Master of Science in Dentistry (Oral and
chronos Synthetic cancellous bone graft substitute ( -tricalcium phosphate) Remodels Replaced by bone in 6 18 months Easy to use
 Synthetic cancellous bone graft substitute ( -tricalcium phosphate) Remodels Replaced by bone in 6 18 months Easy to use Granules, blocks, wedges, cylinders Safe Synthetic origin provides unsurpassed safety
Synthetic cancellous bone graft substitute ( -tricalcium phosphate) Remodels Replaced by bone in 6 18 months Easy to use Granules, blocks, wedges, cylinders Safe Synthetic origin provides unsurpassed safety
