GLOBALTRAINING. IMPLANON : Reference Guide
|
|
|
- Alison Horn
- 5 years ago
- Views:
Transcription
1 GLOBALTRAINING IMPLANON : Reference Guide
2 IMPLANON : Clinical Information IMPLANON is a subdermal, long-acting, progestagen-only contraceptive effective for up to 3 years. The mechanisms of action of IMPLANON include inhibition of ovulation and increases in the viscosity of cervical mucus. Efficacy does not depend on daily, weekly, or monthly self-administration. IMPLANON is effective from day one, when inserted according to instructions in the label. No method of contraception is 100% effective IMPLANON is over 99% effective when inserted correctly. 1 Studies show a continuation rate of 82% after 1 year of use. 2 11% of women studied discontinued IMPLANON due to bleeding irregularities. Bleeding irregularities may include amenorrhea or infrequent, frequent, and/or prolonged bleeding. Information, counseling, and the use of a bleeding diary can improve the woman s acceptance of a bleeding pattern. Following removal of IMPLANON, the hormone is below detectable levels within 7 days. 3 Bone Mineral Density A comparative study of IMPLANON and a non-hormonal IUD showed that bone density remained unaltered over 2 years, with no detectable difference between users of each contraceptive method. Breast Milk Available data indicate that IMPLANON may be used during lactation since it does not influence the production or the quality of breast milk. However, it is important to be aware that small amounts of etonogestrel are excreted in breast milk. In clinical studies use of IMPLANON had no effect on the production or quality of breast milk in nursing mothers. Adverse Events Headache Weight increase Acne Breast pain Irregular menstruation Vaginal infections Contraindications Use of IMPLANON is contraindicated in patients with: Known or suspected pregnancy Active venous thromboembolic disorder Presence of history of liver tumors (benign or malignant) Presence or history of severe hepatic disease as long as liver function values have not returned to normal Known or suspected sex-steroid sensitive malignancies Undiagnosed vaginal bleeding Hypersensitivity to the active substance or to any of the excipients of IMPLANON Drug Interactions Interactions can occur with medicinal products that induce microsomal enzymes, specifically cytochrome P450 enzymes, which can result in increased clearance of sex hormones (eg, phenytoin, barbiturates, primidone, bosentan, carbamazepine, rifampicin, and HIV medication [eg, ritonavir, nelfinavir, nevirapine, efavirenz], and possibly also oxcarbazepine, topiramate, felbamate, griseofulvin, and the herbal remedy St. John s wort). Women on treatment with any of these drugs should temporarily use a barrier method in addition to IMPLANON. Please refer to the regulatory-approved full Prescribing Information for additional interactions. Pregnancy IMPLANON is not indicated during pregnancy. If pregnancy occurs during use of IMPLANON, the implant should be removed. Animal studies have shown that very high doses of progestagenic substances may cause masculinization of female fetuses. Extensive epidemiological studies have revealed neither an increased risk of birth defects in children born to women who used oral contraceptives (OCs) prior to pregnancy, nor of a teratogenic effect when OCs were inadvertently used during pregnancy. Although this probably applies to all OCs, it is not clear whether this is also the case for IMPLANON. Key Points for Patient Counseling Women are likely to have changes in their menstrual bleeding pattern with IMPLANON. These may include changes in bleeding frequency, intensity, or duration; however, the bleeding pattern experienced during the first 3 months is broadly predictive of future bleeding patterns for many women. Amenorrhea was reported in about 1 of 5 women while another 1 of 5 women reported frequent and/or prolonged bleeding. Dysmenorrhea tended to improve while using IMPLANON. Appropriate counseling may make bleeding changes more acceptable for women. Key counseling points include: Discussion of the likelihood of alterations in bleeding patterns Discussion of the risks, benefits, and possible side effects Explain the insertion and removal procedures; emphasize that the implant should always be palpable and that scars or complications may occur. If possible, provide patient education materials. Allow sufficient time for the patient to review the educational materials, consider options, and ask questions. Applicator for IMPLANON How to Insert IMPLANON applicator seal applicator obturator support obturator Insertion of IMPLANON should be performed under aseptic conditions, and only by a health care provider who is familiar with the procedure. Insertion of IMPLANON is performed with the specially designed applicator. The use of this applicator differs substantially from that of a classical syringe. A drawing of a dismantled applicator and its individual components (eg, cannula, obturator, and needle with double-angled bevel) is shown in this leaflet to clarify their specific functions. The procedure used for insertion of IMPLANON is opposite to giving an injection. When inserting IMPLANON, the obturator must remain fixed while the cannula (needle) is retracted from the arm. Allow the subject to lie on her back with her non-dominant arm (the arm which the woman does not use for writing) turned outward and bent at the elbow. To minimize the risk of neural or vascular damage, IMPLANON should be inserted at the inner side of the non-dominant upper arm about 8 to 10 cm above the medial epicondyle of the humerus. IMPLANON should be inserted subdermally, ie, just under the skin (subcutaneously). When IMPLANON is inserted too deeply (intramuscularly or in the fascia), this may cause neural or vascular damage. Too deep insertions have been associated with paresthesia (due to neural damage) and migration of the implant (due to intramuscular or fascial insertion), and in rare cases with intravascular insertion. Moreover, when the implant is inserted too deeply, it may not be palpable and localization and/or removal can be difficult later. needle location of IMPLANON 4 cm Mark the insertion site. IMPLANON Clean the insertion site with an antiseptic. cannula Anesthetize with an anesthetic spray, or with 2 ml of lidocaine (1%) applied just under the skin along the insertion canal. Remove the sterile disposable applicator carrying IMPLANON from its blister. While keeping the shield on the needle, visually verify the presence of the implant, seen as a white body inside the needle tip. If the implant is not seen, tap the top of the needle shield against a firm surface to bring the implant into the needle tip. Following visual confirmation, the implant should be lowered back into the needle by tapping it back into the needle tip. The needle shield can now be removed. Please note that the implant can fall out of the needle prior to insertion. Therefore, always hold the applicator in the upward position (ie, with the needle pointed upward) until the time of insertion. This is to prevent the implant from dropping out. Keep the needle and the implant sterile. If contamination occurs, a new package with a new sterile applicator must be used. PAGE: 1 References: 1 Graesslin 2008, 2 Blumenthal 2008, 3 Davies 1993 PAGE: 2
3 Figure 1 Figure 2 Figure 3 Figure 4 Stretch the skin around the insertion site with thumb and index finger (Figure 1). Insert first only the tip of the needle, slightly angled (~20 ) (Figure 2). Release the skin. Lower the applicator to a horizontal position (Figure 3). While lifting the skin, gently insert the needle to its full length. Do not exert force. The needle should be inserted parallel to the skin to ensure that IMPLANON is inserted superficially just under the skin (Figure 4). Keep the applicator parallel to the surface of the skin. When the implant is placed too deeply, paresthesia and migration of the implant may occur. Moreover, removal can be difficult later. Break the seal of the applicator (Figure 5). Turn the obturator 90 (Figure 6). Fix the obturator with 1 hand parallel to the arm and with the other hand slowly retract the cannula (needle) out of the arm (Figure 7). Never push against the obturator. Check the needle for the absence of the implant. After retraction of the cannula, the grooved tip of the obturator should be visible (Figure 8). Always verify the presence of the implant by palpation and also have the woman palpate it herself. In case the implant cannot be palpated or when the presence of the implant is doubtful, other methods must be applied to confirm its presence. Suitable methods to locate the implant are first of all ultrasound (USS) and secondly magnetic resonance imaging (MRI). Prior to the application of USS or MRI for the localization of IMPLANON, it is recommended to consult MSD for instructions. Localizing IMPLANON Localization is an essential component of the insertion and removal process. Palpation is the first step in the localization process. Always localize by palpation: Immediately after insertion Immediately prior to removal If the implant is not palpable after insertion, confirm its presence in the arm with imaging techniques (USS, MRI) as soon as possible. The patient must use a back-up method of contraception until the presence of IMPLANON has been confirmed. Exploratory surgery for the purpose of removing IMPLANON without knowledge of the exact location of the implant is strictly discouraged. IMPLANON is not radiopaque and is not visible on X-ray or CT images. Although IMPLANON is visible on MRI images, ultrasound is the preferred imaging method because it is least invasive. After localizing the implant using USS, removal can be completed with the assistance of USS guidance. Characteristics of IMPLANON on USS: Sharp acoustic shadow below the implant in the transverse position Implant is a small echogenic spot (2 mm) when viewed in the transverse position MRI - Implant appears as a hypodense area. It is especially important to differentiate the implant from blood vessels. How to Remove IMPLANON Removal of IMPLANON should be performed only by a health care provider who is familiar with the procedure. Prior to removal carefully read the full Prescribing Information. Indications for removal Patient request Medical indication At the end of 3 years of use If the woman does not wish to become pregnant, another contraceptive method should be started immediately (return to normal menstrual cycle may be very rapid). Counsel the patient thoroughly prior to removal of IMPLANON. A non-palpable implant should always first be localized by either USS or MRI before removal is attempted. In case of doubt, the presence of IMPLANON can be verified by etonogestrel determination. Exploratory surgery without knowledge of the exact localization of the implant is strictly discouraged. Removal of deeply inserted implants should be conducted with caution in order to prevent damage to deeper neural or vascular structures in the arm and be performed by health care providers familiar with the anatomy of the arm. Figure 5 Figure 6 In case these imaging methods fail, it is advised to verify the presence of the implant by measuring the etonogestrel level in a blood sample of the subject. In this case, MSD will also provide the appropriate procedure. Until the presence of IMPLANON has been confirmed, a contraceptive barrier method must be used. Apply sterile gauze with a pressure bandage to prevent bruising. Fill out the User Card and hand it to the patient to facilitate removal of the implant later. Figure 7 Figure 8 The applicator is for single use only and must be adequately disposed of, in accordance with local regulations for the handling of biohazardous waste. PAGE: 3 PAGE: 4
4 How to Remove IMPLANON Figure A Figure B The precise location of the implant is indicated on the User Card. Locate the implant by palpation and mark the distal end. (Figure A). Wash the area and apply an antiseptic. Anesthetize the arm with ml lidocaine (1%) at the site of incision, which is just below the distal end of the implant. Note: Apply the anesthetic under the implant. Application above the implant makes the skin swell, which may cause difficulties in locating the implant (Figure B). Push down the proximal tip to fix the implant; a bulge may appear, indicating the distal end of the implant. Starting below the distal tip to the implant, make a longitudinal incision of 2 mm toward the distal tip of the implant (Figure C). Gently push the implant towards the incision until the tip is visible. Grasp the implant with forceps (preferably mosquito forceps) and remove it (Figure D). If the tip of the implant is not visible, there might be formation of fibrotic tissue around the implant. The fibrotic tissue can be split by continuing to cut toward the distal tip until the tip is clearly visible. Remove the implant with forceps (Figures E and F). Figure G Figure H If the tip of the implant is not visible, gently insert a forceps into the incision and grasp the implant (Figures G and H). With a second forceps, carefully dissect the tissue around the implant. The implant can then be removed (Figure I). Close the incision with a butterfly closure. Apply sterile gauze with a pressure bandage to prevent bruising. There have been occasional reports of displacement of the implant; usually this involves minor movement relative to the original position. This may complicate localization of the implant by palpation, USS, and/or MRI, and removal may require a larger incision and more time. If the woman would like to continue using IMPLANON, a new implant may be inserted immediately after the old implant is removed (see How to replace IMPLANON ). If the woman does not wish to continue using IMPLANON and does not want to become pregnant, another contraceptive method should be recommended. Figure I Figure C Figure D Figure E Figure F How to Replace IMPLANON Replacement of IMPLANON should only be performed under aseptic conditions and only by a health care provider who is familiar with the insertion and removal procedure. Immediate replacement can be done after removal of the previous implant as described in How to Remove IMPLANON. The procedure to replace IMPLANON is similar to the insertion procedure described in the section How to Insert IMPLANON. The new implant can be inserted in the same arm and often through the same incision from which the previous implant was removed. If the same incision is being used, the following instructions must also be taken into account. The small incision of the removal procedure can be used as the entrance for the needle of the new applicator. Anesthetize the insertion site with 2 ml lidocaine (1%) applied just under the skin commencing at the removal incision along the insertion canal. During replacement, inserting the needle to its full length is crucial; failure to do so will result in a partly visible implant in the removal incision in the skin. PAGE: 5 PAGE: 6
5 Before administering IMPLANON, please read the accompanying Prescribing Information. Copyright 2013 Merck Sharp & Dohme B.V., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA. All rights reserved. WOMN /13
GLOBALTRAINING. IMPLANON NXT : Reference Guide
 GLOBALTRAINING IMPLANON NXT : Reference Guide IMPLANON NXT (etonogestrel implant): Clinical Information Applicator for IMPLANON NXT A double-blind, parallel-group, randomized trial in 108 women has conclusively
GLOBALTRAINING IMPLANON NXT : Reference Guide IMPLANON NXT (etonogestrel implant): Clinical Information Applicator for IMPLANON NXT A double-blind, parallel-group, randomized trial in 108 women has conclusively
Jadelle Contraceptive Implants up to 5 years Insertion and Removal
 Contraceptive Implants up to 5 years Bayer AG Global HealthCare Programs Family Planning 13342 Berlin, Germany www.bayer.com September 2017 Global HealthCare Programs Family Planning Contraceptive Independence»»
Contraceptive Implants up to 5 years Bayer AG Global HealthCare Programs Family Planning 13342 Berlin, Germany www.bayer.com September 2017 Global HealthCare Programs Family Planning Contraceptive Independence»»
Emergency contraception is an occasional method. It should in no instance replace a regular contraceptive method.
 1. NAME OF THE MEDICINAL PRODUCT: Levonorgestrel Tablets 1.5 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION: Each tablet contains levonorgestrel 1.5 mg. Excipient with known effect: Each tablet contains
1. NAME OF THE MEDICINAL PRODUCT: Levonorgestrel Tablets 1.5 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION: Each tablet contains levonorgestrel 1.5 mg. Excipient with known effect: Each tablet contains
Implanon NXT Insertion and Removal Training Course for Registered Nurses & Midwives Course Guide
 Implanon NXT Insertion and Removal Training Course for Registered Nurses & Midwives Course Guide DELIVERED BY 2 July 2015 CONTENTS Learning Outcomes 4 Course Structure 5 Workplace Scope of Practice 5 Assessment
Implanon NXT Insertion and Removal Training Course for Registered Nurses & Midwives Course Guide DELIVERED BY 2 July 2015 CONTENTS Learning Outcomes 4 Course Structure 5 Workplace Scope of Practice 5 Assessment
ATI Skills Modules Checklist for Central Venous Access Devices
 For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
ARROW EZ-IO Intraosseous Vascular Access System Procedure Template
 ARROW EZ-IO Intraosseous Vascular Access System Procedure Template PURPOSE To provide procedural guidance for establishment of intraosseous vascular access using the ARROW EZ-IO Intraosseous Vascular Access
ARROW EZ-IO Intraosseous Vascular Access System Procedure Template PURPOSE To provide procedural guidance for establishment of intraosseous vascular access using the ARROW EZ-IO Intraosseous Vascular Access
Stratis INSTRUCTIONS FOR USE. Needle-free Injection System. 0.5mL volume (+/-5%)
 Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
EndoRelease ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM
 EndoRelease ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM SURGICAL TECHNIQUE Up p e r Ex t r e m i t y So l u t i o n s ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM Description: The EndoRelease Endoscopic Cubital
EndoRelease ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM SURGICAL TECHNIQUE Up p e r Ex t r e m i t y So l u t i o n s ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM Description: The EndoRelease Endoscopic Cubital
KINGSTON GENERAL HOSPITAL NURSING POLICY AND PROCEDURE
 KINGSTON GENERAL HOSPITAL NURSING POLICY AND PROCEDURE SUBJECT Sample (Adult): Advanced Competency (AC) for Nurses (Registered Nurses and Registered Practical Nurses) PAGE 1 of 5 ORIGINAL ISSUE 1985 January
KINGSTON GENERAL HOSPITAL NURSING POLICY AND PROCEDURE SUBJECT Sample (Adult): Advanced Competency (AC) for Nurses (Registered Nurses and Registered Practical Nurses) PAGE 1 of 5 ORIGINAL ISSUE 1985 January
New Zealand Datasheet
 New Zealand Datasheet 1 PRODUCT NAME POSTINOR-1 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Levonorgestrel 1.5 mg 3 PHARMACEUTICAL FORM Each round white tablet contains 1.5 mg of levonorgestrel. The tablet
New Zealand Datasheet 1 PRODUCT NAME POSTINOR-1 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Levonorgestrel 1.5 mg 3 PHARMACEUTICAL FORM Each round white tablet contains 1.5 mg of levonorgestrel. The tablet
SELF-INJECTION GUIDE
 SELF-INJECTION GUIDE (asfotase alfa) 40 mg/ml solution for injection 18 mg/0.45 ml 28 mg/0.7 ml 40 mg/1 ml 100 mg/ml solution for injection 80 mg/0.8 ml asfotase alfa Introduction Contents This Self-Injection
SELF-INJECTION GUIDE (asfotase alfa) 40 mg/ml solution for injection 18 mg/0.45 ml 28 mg/0.7 ml 40 mg/1 ml 100 mg/ml solution for injection 80 mg/0.8 ml asfotase alfa Introduction Contents This Self-Injection
Patient Information Publications Warren Grant Magnuson Clinical Center National Institutes of Health
 Warren Grant Magnuson Clinical Center National Institutes of Health What is a subcutaneous injection? A subcutaneous injection is given in the fatty layer of tissue just under the skin. A subcutaneous
Warren Grant Magnuson Clinical Center National Institutes of Health What is a subcutaneous injection? A subcutaneous injection is given in the fatty layer of tissue just under the skin. A subcutaneous
Integra. Endoscopic Gastrocnemius Release System SURGICAL TECHNIQUE
 Integra Endoscopic Gastrocnemius Release System SURGICAL TECHNIQUE Table of Contents Indications... 2 Contraindications... 2 System Description... 2 Features and Benefits... 2 Surgical Site Preparation...3
Integra Endoscopic Gastrocnemius Release System SURGICAL TECHNIQUE Table of Contents Indications... 2 Contraindications... 2 System Description... 2 Features and Benefits... 2 Surgical Site Preparation...3
SARASOTA MEMORIAL HOSPITAL. NURSING PROCEDURE INTRAOSSEOUS NEEDLE: INSERTION, CARE, AND REMOVAL (inv08) 12/18 12/18 1 of 7 RESPONSIBILITY:
 SARASOTA MEMORIAL HOSPITAL TITLE: ISSUED FOR: NURSING PROCEDURE INTRAOSSEOUS NEEDLE: INSERTION, CARE, AND REMOVAL (inv08) Nursing DATE: REVIEWED: PAGES: 12/18 12/18 1 of 7 RESPONSIBILITY: PS1094 Insertion-
SARASOTA MEMORIAL HOSPITAL TITLE: ISSUED FOR: NURSING PROCEDURE INTRAOSSEOUS NEEDLE: INSERTION, CARE, AND REMOVAL (inv08) Nursing DATE: REVIEWED: PAGES: 12/18 12/18 1 of 7 RESPONSIBILITY: PS1094 Insertion-
PROBUPHINE is available only through a restricted program called the PROBUPHINE REMS Program. (5.2)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PROBUPHINE safely and effectively. See full prescribing information for PROBUPHINE. PROBUPHINE (buprenorphine)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PROBUPHINE safely and effectively. See full prescribing information for PROBUPHINE. PROBUPHINE (buprenorphine)
Contraceptives. Kim Dawson October 2010
 Contraceptives Kim Dawson October 2010 Objectives: You will learn about: The about the different methods of birth control. How to use each method of birth control. Emergency contraception What are they?
Contraceptives Kim Dawson October 2010 Objectives: You will learn about: The about the different methods of birth control. How to use each method of birth control. Emergency contraception What are they?
Home Health Foundation, Inc. To create more permanent IV access for patients undergoing long term IV therapy.
 PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
Package leaflet: Information for the user. Levonorgestrel 1.5 mg Tablet. levonorgestrel
 Package leaflet: Information for the user Levonorgestrel 1.5 mg Tablet levonorgestrel Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package leaflet: Information for the user Levonorgestrel 1.5 mg Tablet levonorgestrel Read all of this leaflet carefully before you start taking this medicine because it contains important information
Patient Guide Levonorgestrel and Ethinyl Estradiol Tablets USP (0.1 mg/0.02 mg) and Ethinyl Estradiol Tablets USP (0.
 Patient Guide Levonorgestrel and Ethinyl Estradiol Tablets USP (0.1 mg/0.02 mg) and Ethinyl Estradiol Tablets USP (0.01 mg) Rx Only This product (like all oral contraceptives) is intended to prevent pregnancy.
Patient Guide Levonorgestrel and Ethinyl Estradiol Tablets USP (0.1 mg/0.02 mg) and Ethinyl Estradiol Tablets USP (0.01 mg) Rx Only This product (like all oral contraceptives) is intended to prevent pregnancy.
NHS Lothian Patient Group Direction Version: 007. Title: Levonorgestrel 1500mcg for Emergency Contraception
 PATIENT GROUP DIRECTION FOR THE ADMINISTRATION OF LEVORGESTREL 1500mcg FOR EMERGENCY CONTRACEPTION BY DESIGNATED HEALTHCARE PROFESSIONALS PROVIDING SEXUAL HEALTH SERVICES, UNSCHEDULED CARE SERVICES OR
PATIENT GROUP DIRECTION FOR THE ADMINISTRATION OF LEVORGESTREL 1500mcg FOR EMERGENCY CONTRACEPTION BY DESIGNATED HEALTHCARE PROFESSIONALS PROVIDING SEXUAL HEALTH SERVICES, UNSCHEDULED CARE SERVICES OR
The tablet is white, round, biconvex and 5 mm in diameter. On one side it is coded KV above 2 and on
 1. NAME OF THE MEDICINAL PRODUCT Cerazette 75 microgram film-coated tablets. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 75 microgram desogestrel. Excipient (s) with known effect:
1. NAME OF THE MEDICINAL PRODUCT Cerazette 75 microgram film-coated tablets. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 75 microgram desogestrel. Excipient (s) with known effect:
Gammanorm, 165 mg/ml, solution for injection Human normal immunoglobulin
 PACKAGE LEAFLET: INFORMATION FOR THE USER Gammanorm, 165 mg/ml, solution for injection Human normal immunoglobulin Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
PACKAGE LEAFLET: INFORMATION FOR THE USER Gammanorm, 165 mg/ml, solution for injection Human normal immunoglobulin Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
Guidance for Pharmacists on the Safe Supply of Non-Prescription Levonorgestrel 1500mcg for Emergency Hormonal Contraception
 Guidance for Pharmacists on the Safe Supply of Non-Prescription Levonorgestrel 1500mcg for Emergency Hormonal Contraception Pharmaceutical Society of Ireland Version 4 December 2016 Updates made following
Guidance for Pharmacists on the Safe Supply of Non-Prescription Levonorgestrel 1500mcg for Emergency Hormonal Contraception Pharmaceutical Society of Ireland Version 4 December 2016 Updates made following
Package leaflet: Information for the patient. Orgalutran 0.25 mg/0.5 ml solution for injection Ganirelix
 Package leaflet: Information for the patient Orgalutran 0.25 mg/0.5 ml solution for injection Ganirelix Read all of this leaflet carefully before you start using this medicine because it contains important
Package leaflet: Information for the patient Orgalutran 0.25 mg/0.5 ml solution for injection Ganirelix Read all of this leaflet carefully before you start using this medicine because it contains important
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
Title: EZ-IO. Effective Date: January SOG Number: EMS Rescinds:
 S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
MODUS TABLETS. Medroxyprogesterone Tablets IP
 For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory MODUS TABLETS Medroxyprogesterone Tablets IP QUALITATIVE AND QUANTITATIVE COMPOSITION MODUS TABLET 2.5 mg Each uncoated
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory MODUS TABLETS Medroxyprogesterone Tablets IP QUALITATIVE AND QUANTITATIVE COMPOSITION MODUS TABLET 2.5 mg Each uncoated
WARNINGS AND PRECAUTIONS
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PROBUPHINE safely and effectively. See full prescribing information for PROBUPHINE. PROBUPHINE (buprenorphine)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PROBUPHINE safely and effectively. See full prescribing information for PROBUPHINE. PROBUPHINE (buprenorphine)
FDA-Approved Patient Labeling Patient Information Mirena (mur-ā-nah) (levonorgestrel-releasing intrauterine system)
 FDA-Approved Patient Labeling Patient Information Mirena (mur-ā-nah) (levonorgestrel-releasing intrauterine system) Mirena does not protect against HIV infection (AIDS) and other sexually transmitted infections
FDA-Approved Patient Labeling Patient Information Mirena (mur-ā-nah) (levonorgestrel-releasing intrauterine system) Mirena does not protect against HIV infection (AIDS) and other sexually transmitted infections
Draft Label HIGHLIGHTS OF PRESCRIBING INFORMATION INDICATIONS AND USAGE
 1 2 3 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PROBUPHINE safely and effectively. See full prescribing information for PROBUPHINE. PROBUPHINE
1 2 3 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PROBUPHINE safely and effectively. See full prescribing information for PROBUPHINE. PROBUPHINE
YASMIN 0.03 mg/3 mg BAYER MIDDLE EAST
 08-15 YASMIN 0.03 mg/3 mg BAYER MIDDLE EAST film-coated tablets Ethinylestradiol / Drospirenone 1. WHAT YASMIN IS AND WHAT IT IS USED FOR Yasmin is a contraceptive pill and is used to prevent pregnancy.
08-15 YASMIN 0.03 mg/3 mg BAYER MIDDLE EAST film-coated tablets Ethinylestradiol / Drospirenone 1. WHAT YASMIN IS AND WHAT IT IS USED FOR Yasmin is a contraceptive pill and is used to prevent pregnancy.
Normal Immunoglobulin (Human) 16%, solution for subcutaneous administration.
 Normal Immunoglobulin (Human) 16%, solution for subcutaneous administration. Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It does not contain
Normal Immunoglobulin (Human) 16%, solution for subcutaneous administration. Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It does not contain
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Tips for Successful Venipuncture
 Page 1 of 5 Patient Positioning Have patient lie or sit down. Never draw blood on a patient who is standing. Make the patient as comfortable as you possibly can. Always watch and ask patient if he/she
Page 1 of 5 Patient Positioning Have patient lie or sit down. Never draw blood on a patient who is standing. Make the patient as comfortable as you possibly can. Always watch and ask patient if he/she
Package leaflet: Information for the user
 Package leaflet: Information for the user Aimovig 70 mg solution for injection in pre-filled pen erenumab This medicine is subject to additional monitoring. This will allow quick identification of new
Package leaflet: Information for the user Aimovig 70 mg solution for injection in pre-filled pen erenumab This medicine is subject to additional monitoring. This will allow quick identification of new
How to use your gonal-f pre-filled pen
 How to use your gonal-f pre-filled pen Information in this user guide is based on the European product information for Gonal-f. It is not country specific and may vary from country to country. Please always
How to use your gonal-f pre-filled pen Information in this user guide is based on the European product information for Gonal-f. It is not country specific and may vary from country to country. Please always
17. Preventing pregnancy
 17. Preventing pregnancy Objectives By the end of this session, group members will be able to: Define contraception. List ways young people can prevent pregnancy. Background notes What is contraception?
17. Preventing pregnancy Objectives By the end of this session, group members will be able to: Define contraception. List ways young people can prevent pregnancy. Background notes What is contraception?
How to use your Pergoveris pre-filled pen
 How to use your Pergoveris pre-filled pen Information in this user guide is based on the European product information for Pergoveris. It is not country specific and may vary from country to country. Please
How to use your Pergoveris pre-filled pen Information in this user guide is based on the European product information for Pergoveris. It is not country specific and may vary from country to country. Please
Training slides for Tuberculin Skin Testing (TST)
 Disclaimer This presentation is produced by McGill International TB Centre solely for educational purposes. This presentation content is intended to train health care providers to perform Tuberculin skin
Disclaimer This presentation is produced by McGill International TB Centre solely for educational purposes. This presentation content is intended to train health care providers to perform Tuberculin skin
TREMFYA (guselkumab) Information for patients
 TREMFYA (guselkumab) Information for patients Pre-filled syringe Please read this booklet carefully as it contains important information about TREMFYA, the treatment that your doctor has prescribed to
TREMFYA (guselkumab) Information for patients Pre-filled syringe Please read this booklet carefully as it contains important information about TREMFYA, the treatment that your doctor has prescribed to
Example CLINICAL GUIDELINES for Postpartum IUD insertion
 Example CLINICAL GUIDELINES for Postpartum IUD insertion Postpartum Intrauterine Device Insertion 1.0 Indications: 1.1 Insertion of an intrauterine device (IUD) for long-acting reversible contraception
Example CLINICAL GUIDELINES for Postpartum IUD insertion Postpartum Intrauterine Device Insertion 1.0 Indications: 1.1 Insertion of an intrauterine device (IUD) for long-acting reversible contraception
INSTRUCTIONS FOR PREPARING AND GIVING AN INJECTION OF ENBREL POWDER
 INSTRUCTIONS FOR PREPARING AND GIVING AN INJECTION OF ENBREL POWDER Introduction The following instructions explain how to prepare and inject Enbrel powder for injection. Please read the instructions carefully
INSTRUCTIONS FOR PREPARING AND GIVING AN INJECTION OF ENBREL POWDER Introduction The following instructions explain how to prepare and inject Enbrel powder for injection. Please read the instructions carefully
Pfizer, BeneFIX R2 Recombinant Factor IX
 Pfizer, BeneFIX R2 Recombinant Factor IX BeneFIX, Recombinant Factor IX, is indicated for the prevention and control of bleeding episodes in people with hemophilia B (Christmas Disease). BeneFIX is also
Pfizer, BeneFIX R2 Recombinant Factor IX BeneFIX, Recombinant Factor IX, is indicated for the prevention and control of bleeding episodes in people with hemophilia B (Christmas Disease). BeneFIX is also
1 Before You Begin. Injection Instructions. Safety Tips. Disposing of Used Syringes, Needles, and Supplies. Having Someone Help You With Injections
 Injection Instructions (Logo) FUZEON (R) enfuvirtide 1 Before You Begin This is a step-by-step guide to injecting FUZEON (enfuvirtide) that helps remind you about what you learned at your healthcare provider
Injection Instructions (Logo) FUZEON (R) enfuvirtide 1 Before You Begin This is a step-by-step guide to injecting FUZEON (enfuvirtide) that helps remind you about what you learned at your healthcare provider
If viewing a printed copy of this policy, please note it could be expired. Got to to view current policies.
 If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5575 Entity: Fairview Pharmacy Services
If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5575 Entity: Fairview Pharmacy Services
Prescriber s Guide IMPORTANT INFORMATION ON MINIMISING THE RISK OF ADVERSE EVENTS
 MAVENCLAD 10 mg Tablets (cladribine) Prescriber s Guide IMPORTANT INFORMATION ON MINIMISING THE RISK OF ADVERSE EVENTS Reporting Adverse Events Adverse events should be reported. Reporting forms and information
MAVENCLAD 10 mg Tablets (cladribine) Prescriber s Guide IMPORTANT INFORMATION ON MINIMISING THE RISK OF ADVERSE EVENTS Reporting Adverse Events Adverse events should be reported. Reporting forms and information
A patient guide to administration of subcutaneous immunoglobulin replacement therapy - using manual push technique
 A patient guide to administration of subcutaneous immunoglobulin replacement therapy - using manual push technique This piece of patient information is a step by step guide to administering your immunoglobulin
A patient guide to administration of subcutaneous immunoglobulin replacement therapy - using manual push technique This piece of patient information is a step by step guide to administering your immunoglobulin
If viewing a printed copy of this policy, please note it could be expired. Got to to view current policies.
 If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5555 Entity: Fairview Pharmacy Services
If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5555 Entity: Fairview Pharmacy Services
Family Planning UNMET NEED. The Nurse Mildred Radio Talk Shows
 Family Planning UNMET NEED The Nurse Mildred Radio Talk Shows TOPIC 9: IUD/COIL Guests FP counsellor from MSU, RHU& UHMG Nurse Mildred Nurse Betty Objectives of the programme: To inform listeners about
Family Planning UNMET NEED The Nurse Mildred Radio Talk Shows TOPIC 9: IUD/COIL Guests FP counsellor from MSU, RHU& UHMG Nurse Mildred Nurse Betty Objectives of the programme: To inform listeners about
Pregnyl 1500 I.U. powder for solution for injection (Human Chorionic Gonadotrophin) INFORMATION FOR THE USER
 Pregnyl 1500 I.U. powder for solution for injection (Human Chorionic Gonadotrophin) INFORMATION FOR THE USER Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet.
Pregnyl 1500 I.U. powder for solution for injection (Human Chorionic Gonadotrophin) INFORMATION FOR THE USER Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet.
THE WOMAN-FRIENDLY STERILIZATION METHOD
 THE WOMAN-FRIENDLY STERILIZATION METHOD Urogyn BV Transistorweg 5a 6534 AT Nijmegen The Netherlands t +31(0) 24 711 41 30 info@urogynbv.com www.urogynbv.com THE MOST WOMAN-FRIENDLY STERILIZATION METHOD
THE WOMAN-FRIENDLY STERILIZATION METHOD Urogyn BV Transistorweg 5a 6534 AT Nijmegen The Netherlands t +31(0) 24 711 41 30 info@urogynbv.com www.urogynbv.com THE MOST WOMAN-FRIENDLY STERILIZATION METHOD
How to Use ENBREL : Vial Adapter Method
 How to Use ENBREL : Vial Adapter Method SETTING UP FOR AN INJECTION Select a clean, well-lit, flat working surface, such as a table. Take the ENBREL dose tray out of the refrigerator and place it on your
How to Use ENBREL : Vial Adapter Method SETTING UP FOR AN INJECTION Select a clean, well-lit, flat working surface, such as a table. Take the ENBREL dose tray out of the refrigerator and place it on your
LONG-ACTING REVERSIBLE CONTRACEPTION. Summary Tables
 LONG-ACTING REVERSIBLE CONTRACEPTION Summary Tables Bridging the Divide: A Project of the Jacobs Institute of Women s Health June 2016 Table 1. Summary of LARC Methods Available Years Since Effective Copper
LONG-ACTING REVERSIBLE CONTRACEPTION Summary Tables Bridging the Divide: A Project of the Jacobs Institute of Women s Health June 2016 Table 1. Summary of LARC Methods Available Years Since Effective Copper
Standard operating procedures for preparation and administration of intramuscular injections. No Action Rationale
 Standard operating procedures for preparation and administration of intramuscular injections Preparation Overview No Action Rationale 1 Collect and check all equipment 2 Check that the packaging of all
Standard operating procedures for preparation and administration of intramuscular injections Preparation Overview No Action Rationale 1 Collect and check all equipment 2 Check that the packaging of all
Acknowledgement goes to WHO for use of their hand washing technique
 How to administer third party insulin injections safely with a pen device, safety pen needle and syringe 1 Hand washing It takes at least 15 seconds to wash your hands. This is about how long it takes
How to administer third party insulin injections safely with a pen device, safety pen needle and syringe 1 Hand washing It takes at least 15 seconds to wash your hands. This is about how long it takes
Things you need to know about injections
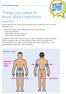 www.trend-uk.org Things you need to know about injections Injection sites: You can see from the picture below the best sites for injecting insulin or GLP-1 medications are: Back of the upper arms (difficult
www.trend-uk.org Things you need to know about injections Injection sites: You can see from the picture below the best sites for injecting insulin or GLP-1 medications are: Back of the upper arms (difficult
Table of Contents: CARPAL TUNNEL RELEASE SURGICAL TECHNIQUE Knife Assembly... Page 6 VERSION 2.2
 www.s2ssurgical.com Table of Contents: Preparation... Page 1 Dissection... Page 2 Tunneling... Page 3 Release... Page 4 Closure... Page 6 Knife Assembly... Page 6 CARPAL TUNNEL RELEASE SURGICAL TECHNIQUE
www.s2ssurgical.com Table of Contents: Preparation... Page 1 Dissection... Page 2 Tunneling... Page 3 Release... Page 4 Closure... Page 6 Knife Assembly... Page 6 CARPAL TUNNEL RELEASE SURGICAL TECHNIQUE
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. AIM-oh-vig Pr AIMOVIG (erenumab injection)
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION AIM-oh-vig Pr AIMOVIG (erenumab injection) Single-Use Pre-filled SureClick Autoinjector Read this carefully before you
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION AIM-oh-vig Pr AIMOVIG (erenumab injection) Single-Use Pre-filled SureClick Autoinjector Read this carefully before you
Intramuscular injections
 Intramuscular injections BENEFITS OF TREATMENT WITH INJECTION With injections of subcutaneous and intramuscular injection is possible to: - the administration of drugs to patients with impaired consciousness
Intramuscular injections BENEFITS OF TREATMENT WITH INJECTION With injections of subcutaneous and intramuscular injection is possible to: - the administration of drugs to patients with impaired consciousness
The following lesson on contraception (birth control) is not intended to infer that you will be sexually active as a teen. This is information that
 The following lesson on contraception (birth control) is not intended to infer that you will be sexually active as a teen. This is information that may be used in the future Abstinence Choosing not to
The following lesson on contraception (birth control) is not intended to infer that you will be sexually active as a teen. This is information that may be used in the future Abstinence Choosing not to
Example Clinical Guideline for Immediate Postpartum LARC Insertion
 Example Clinical Guideline for Immediate Postpartum LARC Insertion RATIONALE Delay in contraceptive provision until the six week postpartum appointment can leave some women at risk for rapid repeat pregnancy.
Example Clinical Guideline for Immediate Postpartum LARC Insertion RATIONALE Delay in contraceptive provision until the six week postpartum appointment can leave some women at risk for rapid repeat pregnancy.
Patient/Carer instructions for the administration of Subcutaneous Cytarabine
 Patient/Carer instructions for the administration of Subcutaneous Cytarabine This document covers the following information: What cytarabine is What subcutaneous means What happens if you decide to inject
Patient/Carer instructions for the administration of Subcutaneous Cytarabine This document covers the following information: What cytarabine is What subcutaneous means What happens if you decide to inject
IMPORTANT: PLEASE READ. Don t
 PATIENT/CAREGIVER INSTRUCTIONS FOR USE - ORENCIA (ABATACEPT) PREFILLED SYRINGE WITH BD ULTRASAFE PASSIVE TM NEEDLE GUARD WITH FLANGE EXTENDERS Getting started with ORENCIA therapy Did you receive self-injection
PATIENT/CAREGIVER INSTRUCTIONS FOR USE - ORENCIA (ABATACEPT) PREFILLED SYRINGE WITH BD ULTRASAFE PASSIVE TM NEEDLE GUARD WITH FLANGE EXTENDERS Getting started with ORENCIA therapy Did you receive self-injection
TRANSCRIPT for Lantus SoloSTAR pen injection for your patients
 TRANSCRIPT for Lantus SoloSTAR pen injection for your patients SUPER: Prescription Lantus is a long-acting insulin used to treat adults with type 2 diabetes and adults and pediatric patients (children
TRANSCRIPT for Lantus SoloSTAR pen injection for your patients SUPER: Prescription Lantus is a long-acting insulin used to treat adults with type 2 diabetes and adults and pediatric patients (children
BLOOD COLLECTION GUIDELINES
 I. Patient Identification Lee Memorial Health System Lee County, FL CLINICAL LABORATORY BLOOD COLLECTION GUIDELINES A. Inpatient / Outpatient with armband 1. When possible, ask patient to state their name
I. Patient Identification Lee Memorial Health System Lee County, FL CLINICAL LABORATORY BLOOD COLLECTION GUIDELINES A. Inpatient / Outpatient with armband 1. When possible, ask patient to state their name
Standard Operating Procedure for cannulation
 Standard Operating Procedure for cannulation Effective date: 26.07.2017 Review due date: 31.03.2019 Original Author Name: Richard Metcalfe Position: PhD Student Date: 05.12.2012 Reviewer Name: Pippa Heath
Standard Operating Procedure for cannulation Effective date: 26.07.2017 Review due date: 31.03.2019 Original Author Name: Richard Metcalfe Position: PhD Student Date: 05.12.2012 Reviewer Name: Pippa Heath
Central venous access devices for children with lysosomal storage disorders
 Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Central venous access devices for children with lysosomal storage disorders This information explains about central
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Central venous access devices for children with lysosomal storage disorders This information explains about central
In the Republic of Ireland, side effects can be reported online at or directly to the HPRA by calling (01)
 MAVENCLAD 10 mg Tablets (cladribine) Patient Guide IMPORTANT INFORMATION ON MINIMISING THE RISK OF ADVERSE EVENTS Reporting of side effects If you get any side effects, talk to your doctor, pharmacist
MAVENCLAD 10 mg Tablets (cladribine) Patient Guide IMPORTANT INFORMATION ON MINIMISING THE RISK OF ADVERSE EVENTS Reporting of side effects If you get any side effects, talk to your doctor, pharmacist
Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Syringe (75 mg/ml)
 Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Syringe (75 mg/ml) Important Information The device is a single-dose pre-filled syringe.
Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Syringe (75 mg/ml) Important Information The device is a single-dose pre-filled syringe.
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
Instructions for Use HEMLIBRA (hem-lee-bruh) (emicizumab-kxwh) injection, for subcutaneous use
 Instructions for Use HEMLIBRA (hem-lee-bruh) (emicizumab-kxwh) injection, for subcutaneous use Be sure that you read, understand, and follow the Instructions for Use before injecting HEMLIBRA. Your healthcare
Instructions for Use HEMLIBRA (hem-lee-bruh) (emicizumab-kxwh) injection, for subcutaneous use Be sure that you read, understand, and follow the Instructions for Use before injecting HEMLIBRA. Your healthcare
EVOGAM. Information for patients Evogam 2014 NZ Patient Brochure Update v11
 EVOGAM Information for patients 11881 Evogam 2014 NZ Patient Brochure Update v11 Information for patients and caregivers about EVOGAM This booklet is designed to help you follow the training you will have
EVOGAM Information for patients 11881 Evogam 2014 NZ Patient Brochure Update v11 Information for patients and caregivers about EVOGAM This booklet is designed to help you follow the training you will have
EPF Endoscopic Plantar Fasciotomy. Operative technique
 Endoscopic Plantar Fasciotomy Operative technique Endoscopic Plantar Fasciotomy Table of contents Introduction 3 Operative technique 4 This publication sets forth detailed recommended procedures for using
Endoscopic Plantar Fasciotomy Operative technique Endoscopic Plantar Fasciotomy Table of contents Introduction 3 Operative technique 4 This publication sets forth detailed recommended procedures for using
Femme-Tab ED 20/100 Levonorgestrel 100 μg and Ethinyloestradiol 20 μg tablets Consumer Medicine Information
 Levonorgestrel 100 μg and Ethinyloestradiol 20 μg tablets Consumer Medicine Information What is in this leaflet Please read this leaflet carefully before you start using. It will advise you about how to
Levonorgestrel 100 μg and Ethinyloestradiol 20 μg tablets Consumer Medicine Information What is in this leaflet Please read this leaflet carefully before you start using. It will advise you about how to
LESSON 9. How to counsel clients on Sayana Press self-injection
 LESSON 9 How to counsel clients on Sayana Press self-injection 1 LESSON 9 Learning objectives In this session, you will learn how to advise clients on: Proper storage of Sayana Press. Proper disposal of
LESSON 9 How to counsel clients on Sayana Press self-injection 1 LESSON 9 Learning objectives In this session, you will learn how to advise clients on: Proper storage of Sayana Press. Proper disposal of
PRE-FILLED PEN 300 IU/0.5 ml N F
 PRE-FILLED PEN 300 IU/0.5 ml N1990207F PRE-FILLED PEN 300 IU/0.5 ml Instructions for use 300 IU/0.5 ml (22 micrograms/0.5 ml) Contents 1. How to use the GONAL-f pre-filled pen 2. How to use your GONAL-f
PRE-FILLED PEN 300 IU/0.5 ml N1990207F PRE-FILLED PEN 300 IU/0.5 ml Instructions for use 300 IU/0.5 ml (22 micrograms/0.5 ml) Contents 1. How to use the GONAL-f pre-filled pen 2. How to use your GONAL-f
ASSESSMENT AND CARE Monitor for the following and remove catheter if there is: redness, pain, swelling, firm tissue, exudates, bleeding or leakage.
 POLICY An Indwelling subcutaneous catheter may be inserted when an infant requires regular subcutaneous injections (At least one subcutaneous injection per day). SUPPORTIVE DATA In the NICU medications
POLICY An Indwelling subcutaneous catheter may be inserted when an infant requires regular subcutaneous injections (At least one subcutaneous injection per day). SUPPORTIVE DATA In the NICU medications
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
Unintended Pregnancy is Common LEARNING OBJECTIVES. Distribution Of Contraception Use By Women In The Us. Unintended Pregnancy And Contraceptive Use
 3:45 4:30 pm Beyond the Pill: Long Acting Contraceptives and IUDs Presenter Disclosure Information The following relationships exist related to this presentation: Christine L. Curry, MD, PhD: No financial
3:45 4:30 pm Beyond the Pill: Long Acting Contraceptives and IUDs Presenter Disclosure Information The following relationships exist related to this presentation: Christine L. Curry, MD, PhD: No financial
IO considerations. Daniel Dunham
 IO considerations Daniel Dunham If patient is conscious Advise of EMERGENT NEED for this procedure and obtain informed consent Rule out contraindications Fracture. Excessive tissue and/or absence of adequate
IO considerations Daniel Dunham If patient is conscious Advise of EMERGENT NEED for this procedure and obtain informed consent Rule out contraindications Fracture. Excessive tissue and/or absence of adequate
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Levonorgestrel and Ethinyl Estradiol Tablets USP 0.1 mg/0.02 mg Lupin Limited Pithampur (M.P.)
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Levonorgestrel and Ethinyl Estradiol Tablets USP 0.1 mg/0.02 mg Lupin Limited Pithampur (M.P.)
INSTRUCTIONS FOR USE
 Comfort Injects 50 Comfort-in INSTRUCTIONS FOR USE English user manual 1 Introduction Thank you for choosing the Comfort in Needle free Injection system, developed with your comfort and convenience in
Comfort Injects 50 Comfort-in INSTRUCTIONS FOR USE English user manual 1 Introduction Thank you for choosing the Comfort in Needle free Injection system, developed with your comfort and convenience in
SCIG INFUSIONS A PRACTICAL GUIDE FOR PATIENTS
 PRIMARY IMMUNODEFICIENCIES SCIG INFUSIONS: A PRACTICAL GUIDE FOR PATIENTS SCIG INFUSIONS A PRACTICAL GUIDE FOR PATIENTS 1 PRIMARY IMMUNODEFICIENCIES ABBREVIATIONS IG IVIG PID SCIG Immunoglobulin Intravenous
PRIMARY IMMUNODEFICIENCIES SCIG INFUSIONS: A PRACTICAL GUIDE FOR PATIENTS SCIG INFUSIONS A PRACTICAL GUIDE FOR PATIENTS 1 PRIMARY IMMUNODEFICIENCIES ABBREVIATIONS IG IVIG PID SCIG Immunoglobulin Intravenous
Femme-Tab ED 20/100. What is in this leaflet. Before you use Femme- Tab ED 20/100. What is Femme-Tab ED 20/100 used for and how does it work
 Levonorgestrel 100 μg and Ethinyloestradiol 20 μg tablets Consumer Medicine Information What is in this leaflet Please read this leaflet carefully before you start using Femme-Tab ED 20/100. It will advise
Levonorgestrel 100 μg and Ethinyloestradiol 20 μg tablets Consumer Medicine Information What is in this leaflet Please read this leaflet carefully before you start using Femme-Tab ED 20/100. It will advise
Subacromial Bursa Injection
 Subacromial Bursa Injection 5 cc syringe, 21 gauge 1.5 inch needle 1% lidocaine - 4cc 40mg triamcinolone - 1 cc of 40mg/ml identify site-seat the patient with weight of arm hanging down, palpate the lateral
Subacromial Bursa Injection 5 cc syringe, 21 gauge 1.5 inch needle 1% lidocaine - 4cc 40mg triamcinolone - 1 cc of 40mg/ml identify site-seat the patient with weight of arm hanging down, palpate the lateral
THE HUMERUS 20 THE HUMERUS* CROSS SECTION CROSS SECTION SUPERIOR VIEW
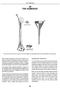 20 THE HUMERUS* CROSS SECTION CROSS SECTION SUPERIOR VIEW The marrow canal of the humerus is funnel-shaped. Its successful pinning is influenced by many factors. With a few exceptions, the entire humerus
20 THE HUMERUS* CROSS SECTION CROSS SECTION SUPERIOR VIEW The marrow canal of the humerus is funnel-shaped. Its successful pinning is influenced by many factors. With a few exceptions, the entire humerus
Important Safety Information for Adlyxin (lixisenatide) injection
 A GLP-1 receptor agonist (RA) Adlyxin (lixisenatide) injection 20 mcg Information To Help Answer Patients Questions This booklet contains information about Adlyxin to help you answer patients questions
A GLP-1 receptor agonist (RA) Adlyxin (lixisenatide) injection 20 mcg Information To Help Answer Patients Questions This booklet contains information about Adlyxin to help you answer patients questions
BACTISEAL Endoscopic Ventricular Catheter (REF & )
 BACTISEAL Endoscopic Ventricular Catheter (REF 82-3087 & 82-3088) LCN 206966-001/E 2011 2017 DePuy Synthes. All rights reserved. Revised 07/17 206966-001-E.indd 1 ENGLISH IMPORTANT INFORMATION Please Read
BACTISEAL Endoscopic Ventricular Catheter (REF 82-3087 & 82-3088) LCN 206966-001/E 2011 2017 DePuy Synthes. All rights reserved. Revised 07/17 206966-001-E.indd 1 ENGLISH IMPORTANT INFORMATION Please Read
Inspection Window. Syringe Body
 Guide to Parts Before use Instructions for Use UDENYCA (yoo-den-i-kah) (pegfilgrastim-cbqv) Injection Single-Dose Prefilled Syringe Inspection Window Needle Cap Needle Label Plunger Plunger Head Needle
Guide to Parts Before use Instructions for Use UDENYCA (yoo-den-i-kah) (pegfilgrastim-cbqv) Injection Single-Dose Prefilled Syringe Inspection Window Needle Cap Needle Label Plunger Plunger Head Needle
Breast Cancer Screening
 Scan for mobile link. Breast Cancer Screening What is breast cancer screening? Screening examinations are tests performed to find disease before symptoms begin. The goal of screening is to detect disease
Scan for mobile link. Breast Cancer Screening What is breast cancer screening? Screening examinations are tests performed to find disease before symptoms begin. The goal of screening is to detect disease
EVOGAM. Information for patients Evogam NZ Patient Brochure Update FA3
 EVOGAM Information for patients 11179 Evogam NZ Patient Brochure Update FA3 Information for patients and caregivers about EVOGAM This booklet is designed to help you follow the training you will have been
EVOGAM Information for patients 11179 Evogam NZ Patient Brochure Update FA3 Information for patients and caregivers about EVOGAM This booklet is designed to help you follow the training you will have been
JvD/PL/ PACKAGE LEAFLET
 JvD/PL/20160721 1 PACKAGE LEAFLET PACKAGE LEAFLET: INFORMATION FOR THE USER GammaQuin, 160 g/l, solution for injection Human normal immunoglobulin Read all of this leaflet carefully before you start using
JvD/PL/20160721 1 PACKAGE LEAFLET PACKAGE LEAFLET: INFORMATION FOR THE USER GammaQuin, 160 g/l, solution for injection Human normal immunoglobulin Read all of this leaflet carefully before you start using
Contains the active ingredient levonorgestrel. For a copy of a large print leaflet, Ph:
 Postella-1 Tablet Emergency Contraception Contains the active ingredient levonorgestrel Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195 055 What is in this leaflet Read
Postella-1 Tablet Emergency Contraception Contains the active ingredient levonorgestrel Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195 055 What is in this leaflet Read
Phlebotomy Blood Sampling From the Arm by Venipuncture
 REB SOP 03 Page 1 of 9 Short Title Arm Venipuncture Effective Date May 4, 2017 Approved by REB May 4, 2017 Version Number 1 A. PURPOSE AND BACKGROUND 1. Venipuncture is the transcutaneous puncture of a
REB SOP 03 Page 1 of 9 Short Title Arm Venipuncture Effective Date May 4, 2017 Approved by REB May 4, 2017 Version Number 1 A. PURPOSE AND BACKGROUND 1. Venipuncture is the transcutaneous puncture of a
Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet, you may need to read it again.
 Yasmin PIL Page: 1 of 16 PACKAGE LEAFLET: INFORMATION FOR THE USER Yasmin 0.03 mg / 3 mg film-coated tablets Ethinylestradiol / Drospirenone Read all of this leaflet carefully before you start taking this
Yasmin PIL Page: 1 of 16 PACKAGE LEAFLET: INFORMATION FOR THE USER Yasmin 0.03 mg / 3 mg film-coated tablets Ethinylestradiol / Drospirenone Read all of this leaflet carefully before you start taking this
Children's (Pediatric) PICC Line Placement
 Scan for mobile link. Children's (Pediatric) PICC Line Placement A peripherally inserted central catheter (PICC line) is most often used to deliver medication over a long period. The doctor or nurse inserts
Scan for mobile link. Children's (Pediatric) PICC Line Placement A peripherally inserted central catheter (PICC line) is most often used to deliver medication over a long period. The doctor or nurse inserts
TAFEN NASAL 50mcg nasal spray, suspension
 PACKAGE LEAFLET: INFORMATION FOR THE USER TAFEN NASAL 50mcg nasal spray, suspension BUDESONIDE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine,
PACKAGE LEAFLET: INFORMATION FOR THE USER TAFEN NASAL 50mcg nasal spray, suspension BUDESONIDE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine,
