Product Information Kit
|
|
|
- Gwenda Whitehead
- 5 years ago
- Views:
Transcription
1 Ultrapro Comfort Plug TM Value Analysis Committee Kit I want a product that works with my technique My patients don t want to feel the mesh Minimize the amount of foreign material implanted Color sticky notes represent customer insights Third-party trademarks used herein are trademarks of their respective owners.
2 Table of Contents a and Codes (k) Clearance Letter Clinical Literature Brochures
3 a The first and only macroporous, partially absorbable mesh plug made from ULTRAPRO Partially Absorbable Lightweight Mesh ULTRAPRO Mesh provides long-term improvement in quality of life, with significant relief from movement limitations at 1-year postsurgery versus presurgery (P < 0.001) 1 The ULTRAPRO Portfolio offers low rates of recurrence (<2%) for a lasting repair, based on data from the IHMR and a multicentric German quality control study 1-3* ULTRAPRO Partially Absorbable Lightweight Mesh ULTRAPRO Hernia System ULTRAPRO Plug N= % recurrence-free at 1 year 1 N= % recurrence-free at 1 year 2 N= % recurrence-free at 1 year 3 * 0.7% recurrence rate 0.4% recurrence rate 1.4% recurrence rate * Data from a prospective, longitudinal study of 71 patients receiving open hernia repair with ULTRAPRO Plug from the IHMR. Two additional patient-reported recurrences could not be medically confirmed by a clinician Data from a prospective, longitudinal study of 151 patients receiving open hernia repair with ULTRAPRO Partially Absorbable Lightweight Mesh from the IHMR Data from a prospective, longitudinal study of 2792 patients receiving hernia repair with ULTRAPRO Hernia System from the IHMR s 1. Berrevoet F, Tollens T, Romanowski C, Jones P, McRoy L. Open macroporous partially absorbable flat mesh 12 month outcomes. Poster presented at 15th Annual Hernia Repair; March 13-16, 2013; Orlando, FL. 2. Lorenz R, Koch A, Wiese M, Born H. Gilbert repair a new gold standard? First results of 2792 patients of a multicentric German quality control study. Poster presented at 15th Annual Hernia Repair; March 13-16, 2013; Orlando, FL Doerhoff C, Lydon P, Hammond J, Romanowski C, Jones P. 12 month outcomes following open hernia repair with a partially absorbable plug and patch device. Abstract presented at the 36th Annual International Congress of the European Hernia Society; May 28-31, 2014; Edinburgh, Scotland. 3
4 Improved patient comfort without changing your technique Relies on familiar, easily reproducible plug & patch technique 1,2 No need to create a preperitoneal space Orientation markings aid placement, alignment, and fixation Large-pore, partially absorbable mesh promotes good tissue in-growth 3 * Leaves behind 65% less foreign material compared with heavier-weight mesh after partial absorption 4 *Evidence shown in an animal model. s 1. ULTRAPRO COMFORT PLUG Partially Absorbable Hernia Repair. Ethicon Inc. 2. UPP Design Validation Results. UPP March Ethicon, Inc. 3. Klosterhalfen B. The ULTRAPRO Plug - a next generation device for hernia plug-repair. February Ethicon, Inc. 4. Hernia Mesh Sheet. April 29, Ethicon, Inc. 4
5 ULTRAPRO COMFORT PLUG is a sterile plug device made from ULTRAPRO Partially Absorbable Lightweight Mesh for open repair of groin hernias The device consists of a three-dimensional plug and a flat, preshaped onlay patch manufactured from nonabsorbable polypropylene monofilament and absorbable poliglecaprone 25 monofilament fibers. The structure of the plug contains ribs made from dyed (D&C Violet No. 2) polydioxanone polymer film 1 Trimmable filler and plug for a customized fit 1 Polydioxanone (PDS) ribs to ease placement 1 Offers appropriate strength with minimal foreign-body mass, for natural healing 2 Designed for excellent intraoperative handling 3 Relies on familiar surgical techniques 1,3 Rounded, soft conical tip and low profile in the abdomen-peritoneum designed to minimize foreign-body sensation The plug is composed of undyed ULTRAPRO Mesh. Dyed ribs are laminated to the plug mesh Ribs are made from dyed (D&C Violet No. 2) polydioxanone polymer film The onlay patch is a pre-shaped, dyed ULTRAPRO Mesh After absorption of the poliglecaprone 25 components, only the polypropylene mesh remains in the body, which is 65% less foreign material compared to heavyweight mesh after partial absorption. 4 The structure and size of this remaining mesh is designed to withstand the physiological forces of the abdominal wall 1 s 1. ULTRAPRO COMFORT PLUG Partially Absorbable Hernia Repair. Ethicon Inc. 2. Klosterhalfen B. The ULTRAPRO Plug - a next generation device for hernia plug-repair. February Ethicon, Inc. 3. UPP Design Validation Results. UPP March Ethicon, Inc. 4. Hernia Mesh Sheet. April 29, Ethicon, Inc. 5
6 and Codes Code Size Shape How Supplied UPLUG401 Plug diameter: 40 mm Sterile, 1 per box Onlay mesh: 7 cm x 14 cm UPLUG403 Plug diameter: 40 mm Sterile, 3 per box Onlay mesh: 7 cm x 14 cm UPLUG406 Plug diameter: 40 mm Sterile, 6 per box Onlay mesh: 7 cm x 14 cm UPLUG551 Plug diameter: 55 mm Sterile, 1 per box Onlay mesh: 7 cm x 14 cm UPLUG553 Plug diameter: 55 mm Sterile, 3 per box Onlay mesh: 7 cm x 14 cm UPLUG556 Plug diameter: 55 mm Sterile, 6 per box Onlay mesh: 7 cm x 14 cm How supplied ULTRAPRO COMFORT PLUG is available in two sizes of plug (40 mm and 55 mm) with one size of onlay mesh (7 cm x 14 cm). ULTRAPRO COMFORT PLUG is supplied in boxes of one, three, or six devices. Each unit is sterile and individually wrapped in sterile packaging Indications for use 1 The ULTRAPRO COMFORT PLUG is indicated for reinforcement of soft tissue, where weakness exists, in procedures involving soft tissue repair, such as groin hernia defects For full Prescribing, see page 16 s 1. ULTRAPRO COMFORT PLUG Partially Absorbable Hernia Repair. Ethicon Inc. 6
7 Product can be ordered direct through Johnson & Johnson Health Care Systems, Inc. (JJHCS) and through distributor channels Electronic ordering options Note: Placing orders electronically avoids minimum order fees for hospitals Order 360 : order360.jnjgateway.com For questions about your order, visit the website or call Global Healthcare Exchange: ghx.com For questions about your order, visit the website or call YOUR-GHX Electronic Data Interchange Call JJHCS EDI Help Line: Nonelectronic/Manual ordering options Call JJHCS at (option 1) between 8:30 a.m. and 8:00 p.m. eastern time, or fax your order to or For more information or product support, call ETHICON ( ) Customer support For product use assistance, clinical guidelines, service and repair, emergency assistance, copy of 510(k) clearance letter or complaints, please contact our Customer Support Center at customersupport@eesus.jnj.com or by calling ETHICON ( ). Our support center is staffed 24 hours a day, 7 days a week by qualified nurses to answer your product-related questions For complete product details, see. For more information, contact your local Ethicon representative or call ETHICON ( ) 7
8 Competitive Code Conversions ULTRAPRO COMFORT PLUG 40 mm codes Plug Mnfr Code # Description diameter QTY Category Mesh material Ethicon UPLUG401 ULTRAPRO COMFORT 40mm 1 Semi- Macroporous, PLUG Absorbable Thin Filament Polypropylene Ethicon UPLUG403 ULTRAPRO COMFORT 40mm 3 Semi- Macroporous, PLUG Absorbable Thin Filament Polypropylene Ethicon UPLUG406 ULTRAPRO COMFORT 40mm 6 Semi- Macroporous, PLUG Absorbable Thin Filament Polypropylene 8
9 Competitive Codes - Comparable to 40mm ULTRAPRO COMFORT PLUG Plug Mnfr Code # Description diameter QTY Category Mesh material BARD PerFix Plug - Small 25mm 2 Non-Absorbable Polypropylene BARD PerFix Plug - Small 25mm 6 Non-Absorbable Polypropylene BARD PerFix Plug - Medium 33mm 2 Non-Absorbable Polypropylene BARD PerFix Plug - Medium 33mm 6 Non-Absorbable Polypropylene BARD PerFix Light Plug - Small 25mm 1 Non-Absorbable Polypropylene BARD PerFix Light Plug - Small 25mm 6 Non-Absorbable Polypropylene BARD PerFix Light Plug - Medium 33mm 1 Non-Absorbable Polypropylene BARD PerFix Light Plug - Medium 33mm 6 Non-Absorbable Polypropylene BARD PHASIX Plug and Patch - Small 25mm 1 Fully-resorbable Poly-4- Hydroxybutyrate (P4HB) BARD PHASIX Plug and Patch - Medium 33mm 1 Fully-resorbable Poly-4- Hydroxybutyrate (P4HB) 9
10 Competitive Codes - Comparable to 40mm ULTRAPRO COMFORT PLUG Plug Mnfr Code # Description diameter QTY Category Mesh material ATRIUM ProLite Ultra Mesh - 25mm 5 Non-Absorbable Polypropylene Self Forming Onlay ATRIUM ProLite Ultra Mesh - 32mm 5 Non-Absorbable Polypropylene Self Forming Onlay ATRIUM ProLite Ultra Mesh - 38mm 5 Non-Absorbable Polypropylene Self Forming Onlay ATRIUM ProLite Ultra Mesh - 25mm 5 Non-Absorbable Polypropylene Self Forming Keyhole Slit Onlay ATRIUM ProLite Ultra Mesh - 32mm 5 Non-Absorbable Polypropylene Self Forming Keyhole Slit Onlay ATRIUM ProLite Ultra Mesh - 38mm 5 Non-Absorbable Polypropylene Self Forming Keyhole Slit Onlay 10
11 Competitive Codes - Comparable to 40mm ULTRAPRO COMFORT PLUG Plug Mnfr Code # Description diameter QTY Category Mesh material ATRIUM ProLoop Mesh - 28mm 2 Non-Absorbable Polypropylene ProLoop with Onlay ATRIUM ProLoop Mesh - 39mm 2 Non-Absorbable Polypropylene ProLoop with Onlay ATRIUM ProLoop Mesh - 28mm 2 Non-Absorbable Polypropylene ProLoop with Keyhole Slit Onlay ATRIUM ProLoop Mesh - 39mm 2 Non-Absorbable Polypropylene ProLoop with Keyhole Slit Onlay ATRIUM ProLoop Mesh - 28mm 2 Non-Absorbable Polypropylene No Onlay ATRIUM ProLoop Mesh - 39mm 2 Non-Absorbable Polypropylene No Onlay GORE HP01 GORE BIO-A Hernia Plug Unknown 1 Fully resorbable Poly (glycolide: trimethylene carbonate) copolymer GORE HP02 GORE BIO-A Hernia Plug Unknown 1 Fully resorbable Poly (glycolide: trimethylene carbonate) copolymer) 11
12 ULTRAPRO COMFORT PLUG 55 mm codes Plug Mnfr Code # Description diameter QTY Category Mesh material Ethicon UPLUG551 ULTRAPRO COMFORT 55mm 1 Semi- Macroporous, PLUG Absorbable Thin Filament Polypropylene Ethicon UPLUG553 ULTRAPRO COMFORT 55mm 3 Semi- Macroporous, PLUG Absorbable Thin Filament Polypropylene Ethicon UPLUG556 ULTRAPRO COMFORT 55mm 6 Semi- Macroporous, PLUG Absorbable Thin Filament Polypropylene 12
13 Competitive Codes - Comparable to 55mm ULTRAPRO COMFORT PLUG Plug Mnfr Code # Description diameter QTY Category Mesh material BARD PerFix Plug - Large 41mm 2 Non-Absorbable Polypropylene BARD PerFix Plug - Large 41mm 6 Non-Absorbable Polypropylene BARD PerFix Plug X-Large 41mm 2 Non-Absorbable Polypropylene BARD PerFix Plug X-Large 41mm 6 Non-Absorbable Polypropylene BARD PerFix Light Plug - Large 41mm 1 Non-Absorbable Polypropylene BARD PerFix Light Plug - Large 41mm 6 Non-Absorbable Polypropylene BARD PerFix Light Plug X-Large 38mm 1 Non-Absorbable Polypropylene BARD PerFix Light Plug X-Large 38mm 6 Non-Absorbable Polypropylene BARD PHASIX Plug and Patch - Large 41mm 1 Fully resorbable Poly-4- Hydroxybutyrate (P4HB) BARD PHASIX Plug and Patch X-Large 38mm 1 Fully resorbable Poly-4- Hydroxybutyrate (P4HB) 13
14 Competitive Codes - Comparable to 55mm ULTRAPRO COMFORT PLUG Plug Mnfr Code # Description diameter QTY Category Mesh material ATRIUM ProLite Ultra Mesh 44mm 5 Non-Absorbable Polypropylene Self Forming Onlay ATRIUM ProLite Ultra Mesh 44mm 5 Non-Absorbable Polypropylene Self Forming Keyhole Slit Onlay ATRIUM ProLoop Mesh 48mm 2 Non-Absorbable Polypropylene ProLoop with Onlay ATRIUM ProLoop Mesh 50mm 2 Non-Absorbable Polypropylene ProLoop with Onlay ATRIUM ProLoop Mesh 48mm 2 Non-Absorbable Polypropylene ProLoop with Keyhole Slit Onlay ATRIUM ProLoop Mesh 50mm 2 Non-Absorbable Polypropylene ProLoop with Keyhole Slit Onlay ATRIUM ProLoop Mesh - No Onlay 48mm 2 Non-Absorbable Polypropylene ATRIUM ProLoop Mesh - No Onlay 50mm 2 Non-Absorbable Polypropylene 14
15 Competitive Codes - Comparable to 55mm ULTRAPRO COMFORT PLUG Plug Mnfr Code # Description diameter QTY Category Mesh material COVIDIEN PNP6X3 Parietex Plug and Patch System 65mm (flat) 3 Non-Absorbable Polyester 6.5cm round plug COVIDIEN PNP8X3 Parietex Plug and Patch System 80mm (flat) 3 Non-Absorbable Polyester 8cm round plug 15
16
17 17
18 for use DESCRIPTION ULTRAPRO COMFORT PLUG Partially Absorbable Hernia Repair en For indirect inguinal hernia repair, a high dissection of the neck of the hernia sac can be performed allowing the Plug to expand. Surgical manipulation may be used to facilitate deployment. For direct inguinal hernia repair, the defect is circumscribed at its base and the content is fully may be used to facilitate the deployment. the femoral vessels and nerves. ADVERSE REACTIONS Potential adverse reactions with device implantation are those typically associated with surgically STERILITY The device is sterilized by Ethylene Oxide. Do not resterilize. Do not use if package is opened or damaged. Discard open, unused product. STORAGE Store at or below 30 C. Do not use after expiry date. TRACEABILITY The Plug is composed of an undyed ULTRAPRO Partially Absorbable Lightweight Mesh. Dyed ribs are laminated to the Plug mesh. Ribs are made from dyed (D&C Violet No. 2) polydioxanone The onlay patch is a pre-shaped, dyed ULTRAPRO Mesh. The ULTRAPRO Mesh is manufactured from approximately equal parts of absorbable Onlay patch No ) is identical to the material used for dyed/undyed PROLENE Polypropylene Suture. copolymer is identical to the material used for MONOCRYL (poliglecaprone 25) Suture. After absorption of the poliglecaprone 25 components, only the polypropylene mesh remains. The structure and size of this remaining mesh is designed to withstand the physiological forces of the abdominal wall. INDICATIONS Plug { reinforcement of soft tissue, where weakness exists, in procedures involving soft tissue repair, such as groin hernia defects. APPLICATION layer can be cut or trimmed. outer layer ribs accommodate the cord structures. Adequate overlap needs to be considered to cover the defect on all sides. The onlay mesh should be positioned tension- and fold-free with the longer side parallel Adequate onlay patch fixation, e.g., with sutures, is required to minimize postoperative complications and recurrence. be maintained. The tails created to accommodate the cord structures must be brought together ACTIONS/PERFORMANCE and following wound healing. When implanted subcutaneously in animals, the poliglecaprone 25 copolymer and the The device elicits a transient mild to moderate foreign body reaction. In an animal study, the not potentiate infections. CONTRAINDICATIONS The device must be separated from the abdominal cavity by peritoneum. WARNINGS If this product is used in patients with the potential for growth or tissue expansion (such as infants or children or women who may become pregnant), the surgeon should be aware that the device Users should be familiar with open surgical procedures and techniques involving non-absorbable meshes. Do not use if package is opened or damaged. Reuse of this device (or portions of this device) may create a risk of product degradation and cross-contamination, which may lead to infection or transmission of bloodborne pathogens to patients and users. PRECAUTIONS In inguinal hernia repair, the device should always be used as a whole, i.e., Plug plus onlay patch. subsequent infection may require removal of the device. In order to minimize the risk of hernia recurrence, the following precautions should be observed: Care should be taken to avoid intra-operative damaging of the device, e.g., with sharp instruments or thermal devices. The onlay patch should adequately overlap the defect on all sides. must be maintained. to clearly identify the device that was implanted. CAUTION Federal (U.S.A.) Law restricts this device to sale by or on the order of a licensed healthcare practitioner. SYMBOLS USED ON LABELING Do not reuse Do not resterilize Use By Year and Month Sterile. Method of sterilization: Ethylene Oxide Recommended storage conditions: below 30 C (86 F) product meets the essential requirements of Medical Directive 93/42/EEC. See Catalogue number Manufacturer Batch number Do not use if the product sterilization barrier or its packaging is compromised. CAUTION: Federal (U.S.A.) law restricts this device to sale by or on the order of a licensed healthcare practitioner. 06/ Ethicon, Inc
19 510(k) Clearance Letter Clinical Literature 510(k) Clearance Letter 19
20 510(k) Clearance Letter Clinical Literature 510(k) Clearance Letter 20
21 510(k) Clearance Letter Clinical Literature 510(k) Clearance Letter 21
22 510(k) Clearance Letter Clinical Literature Clinical Literature Gilbert Repair a New Gold Standard? First Results of 2792 Patients of a Multicentric German Quality Control Study Ralph Lorenz MD, Andreas Koch MD, Martin Wiese, Henry Born MD for the working group In collaboration with AN- Institute for quality management in the operative medicine Otto-von-Guericke-Universität Magdeburg 26 ambulatory Hernia centers in Germany Location of Participating Hernia centers Arnsberg Bad Aibling Berlin 3 x Cottbus Dortmund 2 x Fürstenfeldbruck Jena Kelkheim Krefeld Lampertheim Leipzig 2 x Meißen München 2 x Münster Neumünster Neustadt/Coburg Nürnberg Pforzheim Tübingen Wahlstedt Weimar Online project since 2009 Pilot phase for 2 years 16 hernia centres in Germany now joining 26 hernia centers in Germany criteria: pain and recurrence only Hernia surgery with 3-D-meshes Follow up after 4, 12 and 52 weeks (examination) Independent patient questionaire with Carolina Comfort Scale after 4, 12 and 52 weeks EHS Classification and using UHS as Tailored approach. The highest rate (for Gilbert Repair selected cases) are large size mostly direct or combined hernias. The rate of intraoperative and postoperative complications is very low intraoperative complications Follow up after 4,12,and 52 weeks (Recurrence rate) postoperative complications Number of Hernia Procedures in different Hernia centers per year 2009/2010/2011/ Follow up after 4,12,and 52 weeks (Patients satisfaction with Carolina Comfort Scale) ASA Classification Day cases Ultrapro Hernia System = UHS size M = round 7,5cm L = round 10 cm OVAL = 10 x12 cm with retromuscular mesh part (colour rings) Conclusion: Gilbert repair using the partly absorbable Ultrapro Hernia System combines a simple and safe open access with a secure posterior mesh placement The Gilbert Repair is usable in most of all groin hernias as well in recurrent cases, in our study it was mostly used in large size direct or combined hernias With 3 available sizes of the UHS there is a possibility for a tailored concept adapted on the size of the hernia A high rate of day cases is possible The rate of intra- and postoperative complications is very low The recurrence rate after 1 year is very low The patient satisfaction is very high Our results are comparable with other current comparative studies (1),(2),(3) Literature (1) Persson K, Rimback G, Dalenback J The Lichtenstein Perfix Plug and Prolene Hernia System techniques for inguinal hernia repair Long time follow up of a RCT Hernia (2012) 16 (suppl.1) : (2) Tsirline T, Colavit P,Belyansky I et al. Ultrapro Hernia System versus conventional Lichtenstein repair for inguinal hernia system: results from the multinational registry Presentation New York 5th Hernia Congress (3) Tollens T. et al Ultrapro Hernia System: Toward an ideal solution? Surg Techn Intern (2011) Authors Ralph Lorenz MD, 3CHIRURGEN Klosterstrasse 34/35, D Berlin, lorenz@3chirurgen.de Andreas Koch MD, FACS, Chirurgische Praxis Cottbus, Thiem Strasse 112, D Cottbus Martin G. Wiese, GesundheitsZentrum Kelkheim Frankenallee 1, D Kelkheim Henry Born MD, Medizinisches Zentrum Leipzig-Lausen, Zschochersche Allee 68, D Leipzig 22
23 Table of Contents 510(k) Clearance Letter Clinical Literature Clinical Literature 23
24 510(k) Clearance Letter Clinical Literature Clinical Literature 12 Month Outcomes Following Open Hernia Repair with a Partially Absorbable Plug and Patch Carl Doerhoff¹, Peter Lydon², Jeffrey Hammond³, Christine Romanowski ³ Pete Jones³ 1 Surgicare of Missouri, 1705 Christy Drive, Suite 215, Jefferson City, Missouri ² Eastern Massachusetts Surgery Center, 100 Morse Street, Norwood, MA ³ Clinical Development & Medical Affairs, ETHICON Surgical Care, Johnson & Johnson Global Surgery Group Background: The popularity of the plug and patch technique for hernia repair stems from the ease of performing this surgical procedure. A two-component, partially absorbable device was developed primarily for groin hernias to fill or reinforce hernia defects and to provide support during wound healing. Methods: The International Hernia Mesh Registry, a prospective multi-center registry collects data on hernia mesh products. Patients complete Carolinas Comfort Scale (CCS), validated QOL questionnaire specific to herniorrhaphy at baseline and 1, 6, 12 and 24 months postoperatively. Symptomatic patient is defined as one providing a response of >1 to any CCS question. Patients who underwent open herniorrhaphy with a partially absorbable plug and patch device (ULTRPRO Plug, Ethicon, Somerville, NJ) and completed up to 12 months postoperative follow-up were included. McNemar tests were used to compare symptomatic events. Results: 92 patients enrolled across 7 centers with 12 month data available for 71 patients. Mean age 57.6 years (15.2 SD)) and mean BMI kg/m² (3.9 SD). Hernia types: 90.2% inguinal; 8.7% umbilical; 1.1% femoral, 94.6% were primary repairs. Fixation methods: 97.8% sutures; 1.1 % sutures and glue; 1.1% none. Anesthesia utilized; 57.6% general, 42.4% local. Most procedures (78.3%) were < 1 hour long and median duration in hospital; 0.0 (0.0, 3.0) nights. Preoperatively, incidence of patient reported symptomatic pain (56.5%) decreased (7.5%) p<0.001; symptomatic movement limitations (35.9%) decreased (4.5%) p < at 12 months postoperatively, respectively. Most common adverse event was seroma, 7.6 % patients. There were 4 patient reported recurrences; 1 medically confirmed, 1 confirmed as not a recurrence and 2 yet to be confirmed, due to patients not returning for assessment. Conclusion: These results indicate that inguinal hernia repair with this plug and patch is associated with significant improvement compared to baseline in pain and movement limitations at 12 months postoperatively. 24
25 Brochures Brochures ULTRAPRO COMFORT PLUG Partially Absorbable Hernia Repair The first and only macroporous, partially absorbable mesh plug for open inguinal hernia repair New technology without the need to learn a new technique Trimmable filler and plug for a customized fit 1 Polydioxanone (PDS) ribs to ease placement 1,2A Offers appropriate strength with minimal foreign body mass, for natural healing 1,2B Designed for excellent intraoperative handling 2A Relies on familiar surgical techniques 1,2A Macroporous, partially absorbable mesh allows for excellent tissue in-growth* 2B 65% less foreign material versus heavyweight mesh after partial absorption 2C Rounded, soft conical tip, and low profile in abdomen-peritoneum designed to minimize foreign body sensation 3 25
26 For complete indications, contraindications, warnings, precautions, and adverse reactions, please reference full package insert Ethicon US, LLC. All rights reserved
II.- PLUG. NAME of the products. Premilene Mesh Plug MANUFACTURER. B Braun DESCRIPTION. Polypropylene mesh for plug technique
 II.- PLUG Premilene Mesh Plug B Braun Polypropylene mesh for plug technique Premilene Mesh Plug is a monofilament polypropylene mesh plug designed for the repair of recurrent hernias and can also be used
II.- PLUG Premilene Mesh Plug B Braun Polypropylene mesh for plug technique Premilene Mesh Plug is a monofilament polypropylene mesh plug designed for the repair of recurrent hernias and can also be used
Designed to help advance patient outcomes and ease of use
 Introducing ULTRAPRO ADVANCED Macroporous Partially Absorbable Mesh for inguinal and ventral hernia repair Designed to help advance patient outcomes and ease of use t n rese rep otes n y k. r stic ights
Introducing ULTRAPRO ADVANCED Macroporous Partially Absorbable Mesh for inguinal and ventral hernia repair Designed to help advance patient outcomes and ease of use t n rese rep otes n y k. r stic ights
Technique Guide. Bard MK Hernia Repair. Featuring Modified Onflex Mesh SOFT TISSUE REPAIR. Anterior Approach to a Preperitoneal Inguinal Hernia Repair
 Bard MK Hernia Repair Featuring Modified Onflex Mesh Technique Guide Anterior Approach to a Preperitoneal Inguinal Hernia Repair SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. The opinions
Bard MK Hernia Repair Featuring Modified Onflex Mesh Technique Guide Anterior Approach to a Preperitoneal Inguinal Hernia Repair SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. The opinions
Tissue-Separating Mesh A Comparative Guide
 Ethicon provides comprehensive solutions to advance hernia repair PROCEED Surgical Mesh with macroporous, partially absorbable monofilament construction has been trusted by surgeons for more than 10 years
Ethicon provides comprehensive solutions to advance hernia repair PROCEED Surgical Mesh with macroporous, partially absorbable monofilament construction has been trusted by surgeons for more than 10 years
Ventralex ST Hernia Patch featuring Sepra Technology
 Ventralex ST Hernia Patch featuring Sepra Technology Proven Sepra Technology in a Low Profile, Lightweight Mesh Sepra Technology An extensively studied barrier with more than 10 publications and used clinically
Ventralex ST Hernia Patch featuring Sepra Technology Proven Sepra Technology in a Low Profile, Lightweight Mesh Sepra Technology An extensively studied barrier with more than 10 publications and used clinically
Ultrapro Hernia System Bi Layer Dr Cosmas Gora T SpB-KBD. dffdfdfxxgfxgfxgffxgxgxg
 Bi Layer Dr Cosmas Gora T SpB-KBD dffdfdfxxgfxgfxgffxgxgxg Why UHS? Lightweight Mesh Covering entire myopectineal orifices with underlay mesh in preperitoneal space (posterior repair) Covering the inguinal
Bi Layer Dr Cosmas Gora T SpB-KBD dffdfdfxxgfxgfxgffxgxgxg Why UHS? Lightweight Mesh Covering entire myopectineal orifices with underlay mesh in preperitoneal space (posterior repair) Covering the inguinal
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
TiLENE. Plug Set Titanized Polymers. Surgical Technique
 Titanized Polymers Surgical Technique The only Plug Implant with a Titanium Coating Excellent biocompatibility through a unique combination of a compound material with a covalent bonded titanium layer
Titanized Polymers Surgical Technique The only Plug Implant with a Titanium Coating Excellent biocompatibility through a unique combination of a compound material with a covalent bonded titanium layer
Meshes. Meshes. Non-absorbable meshes. Absorbable meshes
 Meshes Meshes Non-absorbable meshes Absorbable meshes Non-absorbable meshes hernia Premilene Mesh Premilene Mesh Plug Optilene Mesh Optilene Mesh LP Optilene Mesh Elastic Omyra Mesh Non-absorbable meshes
Meshes Meshes Non-absorbable meshes Absorbable meshes Non-absorbable meshes hernia Premilene Mesh Premilene Mesh Plug Optilene Mesh Optilene Mesh LP Optilene Mesh Elastic Omyra Mesh Non-absorbable meshes
Evidence Summary. Ethicon Hernia Portfolio. Better surgery for a better world
 Ethicon Hernia Portfolio Ethicon HerniaSummary Portfolio Evidence Evidence Summary Color Better surgery for a better world sticky notes repre s ent cu stome r insig ht s. The third party trademarks used
Ethicon Hernia Portfolio Ethicon HerniaSummary Portfolio Evidence Evidence Summary Color Better surgery for a better world sticky notes repre s ent cu stome r insig ht s. The third party trademarks used
Duracryl sutures should be selected and implanted depending on the patient condition, surgical experience, suturing technique and wound size.
 FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: DURACRYL Material: Monofilament Polydioxanone. Synthetic absorbable surgical suture (Dyed) Description: DURACRYL is a sterile synthetic absorbable
FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: DURACRYL Material: Monofilament Polydioxanone. Synthetic absorbable surgical suture (Dyed) Description: DURACRYL is a sterile synthetic absorbable
VALUE ANALYSIS COMMITTEE PRODUCT INFORMATION KIT
 VALUE ANALYSIS COMMITTEE PRODUCT INFORMATION KIT Versatex Monofilament Mesh Macroporous flatsheet for preperitoneal hernia repair HERNIA CARE MESH FIXATION BIOLOGICS DISSECTION HERNIA REPAIR We have established
VALUE ANALYSIS COMMITTEE PRODUCT INFORMATION KIT Versatex Monofilament Mesh Macroporous flatsheet for preperitoneal hernia repair HERNIA CARE MESH FIXATION BIOLOGICS DISSECTION HERNIA REPAIR We have established
Doc no: IFU/CPL Issue date: Rev no: 00 Rev date: 1
 FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: PROGUT Material: Catgut Plain. Absorbable surgical suture U.S.P Description: Progut surgical catgut suture is a sterile absorbable suture composed
FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: PROGUT Material: Catgut Plain. Absorbable surgical suture U.S.P Description: Progut surgical catgut suture is a sterile absorbable suture composed
Bard CapSure. Technique Guide. Permanent Fixation System SOFT TISSUE REPAIR. Laparoscopic Inguinal, Open and Laparoscopic Ventral Hernia Repair
 Bard CapSure Permanent Fixation System Technique Guide Laparoscopic Inguinal, Open and Laparoscopic Ventral Hernia Repair SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. The opinions
Bard CapSure Permanent Fixation System Technique Guide Laparoscopic Inguinal, Open and Laparoscopic Ventral Hernia Repair SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. The opinions
Doc no: IFU/CC Issue date: Rev no: 04 Rev date:
 FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: PROGUT Material: Catgut Chromic Absorbable surgical suture U.S.P Description: Progut surgical catgut suture is a sterile absorbable suture composed
FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: PROGUT Material: Catgut Chromic Absorbable surgical suture U.S.P Description: Progut surgical catgut suture is a sterile absorbable suture composed
B. Braun Mesh Range It s All about Prevention. Experts in Abdominal Wall Health. Hernia Repair
 B. Braun Mesh Range It s All about Prevention. Experts in Abdominal Wall Health Hernia Repair B. Braun Mesh Range It s All about Prevention Experts in Abdominal Wall Health Welcome to B. Braun Closure
B. Braun Mesh Range It s All about Prevention. Experts in Abdominal Wall Health Hernia Repair B. Braun Mesh Range It s All about Prevention Experts in Abdominal Wall Health Welcome to B. Braun Closure
Echo PS Positioning System with Ventralight ST Mesh or Composix L/P Mesh
 Ventralight ST Mesh with Echo PS Positioning System Catalog Number Shape Mesh Size 5955450 Circle 4.5" (11.4 cm) 5955460 Ellipse 4" x 6" (10.2 cm x 15.2 cm) 5955600 Circle 6" (15.2 cm) 5955680 Ellipse
Ventralight ST Mesh with Echo PS Positioning System Catalog Number Shape Mesh Size 5955450 Circle 4.5" (11.4 cm) 5955460 Ellipse 4" x 6" (10.2 cm x 15.2 cm) 5955600 Circle 6" (15.2 cm) 5955680 Ellipse
Ventral Hernia Repair
 Ventral Hernia Repair Ventrio ST Hernia Patch Ventrio Hernia Patch Technique Guide Open and Laparoscopic Ventral Hernia Repair SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. This Technique
Ventral Hernia Repair Ventrio ST Hernia Patch Ventrio Hernia Patch Technique Guide Open and Laparoscopic Ventral Hernia Repair SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. This Technique
OptiFix Absorbable Fixation System
 OptiFix Absorbable Fixation System Absorbable Fixation Redefined Advancing the Fixation Experience. SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Challenges and
OptiFix Absorbable Fixation System Absorbable Fixation Redefined Advancing the Fixation Experience. SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Challenges and
Positioning System. Laparoscopic ventral hernia repair KEY BENEFITS SOFT TISSUE REPAIR
 Echo PS Positioning System with Ventralight ST Mesh or Composix L/P Mesh Laparoscopic ventral hernia repair Echo PS Positioning System with Ventralight ST Mesh Echo PS Positioning System with Composix
Echo PS Positioning System with Ventralight ST Mesh or Composix L/P Mesh Laparoscopic ventral hernia repair Echo PS Positioning System with Ventralight ST Mesh Echo PS Positioning System with Composix
with Laparoscopic Ventral Hernia Repair Positioning System SOFT TISSUE REPAIR Designed for Accurate Mesh Centering
 with Laparoscopic Ventral Hernia Repair Positioning System Designed for Accurate Mesh Centering SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. with A Consistent, Reproducible Technique
with Laparoscopic Ventral Hernia Repair Positioning System Designed for Accurate Mesh Centering SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. with A Consistent, Reproducible Technique
The AperFix II System
 The AperFix II System A Complete Anatomic Solution Transtibial Surgical Technique 2 AperFix II System Transtibial Surgical Technique Figure 1 A Complete Anatomic Solution The Cayenne Medical AperFix and
The AperFix II System A Complete Anatomic Solution Transtibial Surgical Technique 2 AperFix II System Transtibial Surgical Technique Figure 1 A Complete Anatomic Solution The Cayenne Medical AperFix and
Open Tension-Free Mesh Repair for Adult Inguinal Hernia: Eight Years of Experience in a Community Hospital
 Original Articles Asian Journal of Surgery Excerpta Medica Asia Ltd Open Tension-Free Mesh Repair for Adult Inguinal Hernia: Eight Years of Experience in a Community Hospital Shunji Yamamoto, Toshiki Maeda,
Original Articles Asian Journal of Surgery Excerpta Medica Asia Ltd Open Tension-Free Mesh Repair for Adult Inguinal Hernia: Eight Years of Experience in a Community Hospital Shunji Yamamoto, Toshiki Maeda,
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
HeliMEND Advanced. Absorbable Collagen Membrane. Instructions for Use
 HeliMEND Advanced Absorbable Collagen Membrane Instructions for Use 2 Indications HeliMEND Advanced absorbable collagen membrane is an absorbable, implantable material that is indicated for guided tissue
HeliMEND Advanced Absorbable Collagen Membrane Instructions for Use 2 Indications HeliMEND Advanced absorbable collagen membrane is an absorbable, implantable material that is indicated for guided tissue
Desara TV and Desara Blue TV
 Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE
 CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
3/21/2011. Advances in laparoscopic ventral hernia repair. Laparoscopic approach well-suited for simple hernias:
 Advances in laparoscopic ventral hernia repair Topics Technique of laparoscopic ventral hernia repair Patient selection Is laparoscopic any better than open? Recent advances (or, should we say, advances?)
Advances in laparoscopic ventral hernia repair Topics Technique of laparoscopic ventral hernia repair Patient selection Is laparoscopic any better than open? Recent advances (or, should we say, advances?)
SYNPOR POROUS POLYETHYLENE IMPLANTS. For craniofacial and orbital augmentation and reconstruction
 SYNPOR POROUS POLYETHYLENE IMPLANTS For craniofacial and orbital augmentation and reconstruction SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SYNPOR Porous Polyethylene Implants 2 Indications and
SYNPOR POROUS POLYETHYLENE IMPLANTS For craniofacial and orbital augmentation and reconstruction SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SYNPOR Porous Polyethylene Implants 2 Indications and
7/2/2015. Incidence. *Mudge M et al, Br. J. Surg, 1985, 72:70-71
 Ventral Hernia Repair: Revisonal Surgery Natan Zundel MD FACS Professor of Surgery Vice-Chairman Department of Surgery FIU Herbert Wertheim College of Medicine. Miami Florida DISCLOSURE Ethicon Endosurgery
Ventral Hernia Repair: Revisonal Surgery Natan Zundel MD FACS Professor of Surgery Vice-Chairman Department of Surgery FIU Herbert Wertheim College of Medicine. Miami Florida DISCLOSURE Ethicon Endosurgery
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
2013 myresearch Public Health Internship Program
 2013 myresearch Public Health Internship Program Joo Kim Public Health Internship Program Open Repair: Significance of Mesh Type on Inguinal Herniorrhaphy Joo Kim Richard T. Guttman Jr., MD, FACS Abstract
2013 myresearch Public Health Internship Program Joo Kim Public Health Internship Program Open Repair: Significance of Mesh Type on Inguinal Herniorrhaphy Joo Kim Richard T. Guttman Jr., MD, FACS Abstract
What you should know about your diagnosis of incontinence
 What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
CrossFix II. All-Suture, All-Inside Meniscal Repair System. Surgical Technique
 CrossFix II All-Suture, All-Inside Meniscal Repair System Surgical Technique 3 CrossFix II All- Suture Repair System Surgical Technique All-Inside Repair with Inside-Out Results The CrossFix II Meniscal
CrossFix II All-Suture, All-Inside Meniscal Repair System Surgical Technique 3 CrossFix II All- Suture Repair System Surgical Technique All-Inside Repair with Inside-Out Results The CrossFix II Meniscal
Early View Article: Online published version of an accepted article before publication in the final form.
 : Online published version of an accepted article before publication in the final form. Journal Name: Journal of Case Reports and Images in Surgery doi: To be assigned Early view version published: November
: Online published version of an accepted article before publication in the final form. Journal Name: Journal of Case Reports and Images in Surgery doi: To be assigned Early view version published: November
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
CLOSES WITH SECURITY. LEAVES WITHOUT A TRACE.
 CLOSES WITH SECURITY. LEAVES WITHOUT A TRACE. Close with Confidence. Leave Nothing Behind. The innovative design and predictable deployment of MYNX CONTROL Vascular Closure Device (VCD) delivers outstanding
CLOSES WITH SECURITY. LEAVES WITHOUT A TRACE. Close with Confidence. Leave Nothing Behind. The innovative design and predictable deployment of MYNX CONTROL Vascular Closure Device (VCD) delivers outstanding
MEDPOR. Oral maxillofacial surgery
 MEDPOR Oral maxillofacial surgery MEDPOR biomaterial MEDPOR has been a trusted name in the industry since 1985, with hundreds of thousands of procedures performed, and hundreds of published clinical reports
MEDPOR Oral maxillofacial surgery MEDPOR biomaterial MEDPOR has been a trusted name in the industry since 1985, with hundreds of thousands of procedures performed, and hundreds of published clinical reports
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP
 RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
UNDERSTANDING EPISIOTOMY C-SECTION AND RECTOCELE. Our suture portfolio meets all your procedural needs
 UNDERSTANDING EPISIOTOMY C-SECTION AND RECTOCELE Our suture portfolio meets all your procedural needs GYNECOLOGY Episiotomy A surgically planned incision on the perineum and the posterior vaginal wall,
UNDERSTANDING EPISIOTOMY C-SECTION AND RECTOCELE Our suture portfolio meets all your procedural needs GYNECOLOGY Episiotomy A surgically planned incision on the perineum and the posterior vaginal wall,
Laparoscopic Hernia Repair, Indications, Superiority and Outcome
 Laparoscopic Hernia Repair, Indications, Superiority and Outcome Mr. Amir Morgan MBBCh; MSc; MD; FICS; JAG; FRCS Consultant Laparoscopic Colorectal & General Surgeon Lead of medical education and surgical
Laparoscopic Hernia Repair, Indications, Superiority and Outcome Mr. Amir Morgan MBBCh; MSc; MD; FICS; JAG; FRCS Consultant Laparoscopic Colorectal & General Surgeon Lead of medical education and surgical
INGUINAL HERNIA REPAIR PROCEDURE GUIDE
 ROOM CONFIGURATION The following figure shows an overhead view of the recommended OR configuration for a da Vinci Inguinal Hernia Repair (Figure 1). NOTE: Configuration of the operating room suite is dependent
ROOM CONFIGURATION The following figure shows an overhead view of the recommended OR configuration for a da Vinci Inguinal Hernia Repair (Figure 1). NOTE: Configuration of the operating room suite is dependent
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
Biosynthe*c meshes in hernia repair «Newer class of materials»
 Biosynthe*c meshes in hernia repair «Newer class of materials» Pr C.Barrat Chirurgie diges*ve et métabolique Pôle des Ac*vités Interven*onnelles Ambulatoires et Nutri*onnelles Hôpitaux Universitaire de
Biosynthe*c meshes in hernia repair «Newer class of materials» Pr C.Barrat Chirurgie diges*ve et métabolique Pôle des Ac*vités Interven*onnelles Ambulatoires et Nutri*onnelles Hôpitaux Universitaire de
PROCEED Surgical Mesh Product Information Kit
 Value Analysis Committee PROCEED Surgical Mesh Product Information Kit Create an IPOM mesh optimized for strength and performance Color sticky note represents customer insight. Third-party trademarks used
Value Analysis Committee PROCEED Surgical Mesh Product Information Kit Create an IPOM mesh optimized for strength and performance Color sticky note represents customer insight. Third-party trademarks used
RIGIDfix. Soft Tissue. Surgical Technique for Mitek RIGIDfix ACL Reconstruction PRODUCTS. Daniel J. McKernan, M.D. TISSUE SOFT.
 RIGIDfix A C L C R O S S P I N S Y S T E M Daniel J. McKernan, M.D. Toledo, Ohio SOFT TISSUE Surgical Technique for Mitek RIGIDfix ACL Reconstruction Soft Tissue PRODUCTS SURGICAL TECHNIQUE RIGIDfix A
RIGIDfix A C L C R O S S P I N S Y S T E M Daniel J. McKernan, M.D. Toledo, Ohio SOFT TISSUE Surgical Technique for Mitek RIGIDfix ACL Reconstruction Soft Tissue PRODUCTS SURGICAL TECHNIQUE RIGIDfix A
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6
 Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
ThruPort Ergonic Minimal Incision Instrumentation
 I SEE MINIMAL INCISIONS * THRU Ergonic Minimal Incision Instrumentation. ThruPort Ergonic Minimal Incision Instrumentation *When compared to median sternotomy MIVS Redefined > THRUPORT SYSTEMS > TECHNOLOGY
I SEE MINIMAL INCISIONS * THRU Ergonic Minimal Incision Instrumentation. ThruPort Ergonic Minimal Incision Instrumentation *When compared to median sternotomy MIVS Redefined > THRUPORT SYSTEMS > TECHNOLOGY
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
Case Report. XCM Biologic Tissue Matrix. Components separation using sandwich technique for reconstruction of abdominal wall defect.
 Case Report XCM Biologic Tissue Matrix. Components separation using sandwich technique for reconstruction of abdominal wall defect. XCM Biologic Tissue Matrix. Components separation using sandwich technique
Case Report XCM Biologic Tissue Matrix. Components separation using sandwich technique for reconstruction of abdominal wall defect. XCM Biologic Tissue Matrix. Components separation using sandwich technique
Stop Coping. Start Living. Talk to your doctor about pelvic organ prolapse and sacrocolpopexy
 Stop Coping. Start Living Talk to your doctor about pelvic organ prolapse and sacrocolpopexy Did you know? One in three women will suffer from a pelvic health condition in her lifetime. Four of the most
Stop Coping. Start Living Talk to your doctor about pelvic organ prolapse and sacrocolpopexy Did you know? One in three women will suffer from a pelvic health condition in her lifetime. Four of the most
The following languages are included in this packet:
 OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Integra. TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE
 Integra TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE Table of Contents Description...2 Indications...2 Contraindications...2 Surgical Technique...4 Step 1 Surgical Approach...4 Step 2 Remove TenoGlide
Integra TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE Table of Contents Description...2 Indications...2 Contraindications...2 Surgical Technique...4 Step 1 Surgical Approach...4 Step 2 Remove TenoGlide
Precautionary Statement ( )
 Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
TissueMend. Arthroscopic Surgical Technique. Arthroscopic Insertion of a Biologic Rotator Cuff Tissue Augment After Rotator Cuff Repair
 TissueMend Arthroscopic Surgical Technique Arthroscopic Insertion of a Biologic Rotator Cuff Tissue Augment After Rotator Cuff Repair Table of Contents Placement of suture anchors Placement of anteromedial
TissueMend Arthroscopic Surgical Technique Arthroscopic Insertion of a Biologic Rotator Cuff Tissue Augment After Rotator Cuff Repair Table of Contents Placement of suture anchors Placement of anteromedial
Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology
 Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
Technique Guide. SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction.
 Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
The lateral incisional hernia: anatomical considerations for a standardized retromuscular sublay repair
 Hernia (2009) 13:293 297 DOI 10.1007/s10029-009-0479-0 ORIGINAL ARTICLE The lateral incisional hernia: anatomical considerations for a standardized retromuscular sublay repair M. Stumpf J. Conze A. Prescher
Hernia (2009) 13:293 297 DOI 10.1007/s10029-009-0479-0 ORIGINAL ARTICLE The lateral incisional hernia: anatomical considerations for a standardized retromuscular sublay repair M. Stumpf J. Conze A. Prescher
BIOKNOTLESSRC ROTATOR CUFF REPAIR SUTURE ANCHOR SURGICAL TECHNIQUE. Surgical Technique for Arthroscopic Rotator Cuff Repair. Raymond Thal, M.D.
 SURGICAL TECHNIQUE ROTATOR CUFF REPAIR BIOKNOTLESSRC SUTURE ANCHOR Surgical Technique for Arthroscopic Rotator Cuff Repair Raymond Thal, M.D. Town Center Orthopaedic Associates Reston, Virginia Surgical
SURGICAL TECHNIQUE ROTATOR CUFF REPAIR BIOKNOTLESSRC SUTURE ANCHOR Surgical Technique for Arthroscopic Rotator Cuff Repair Raymond Thal, M.D. Town Center Orthopaedic Associates Reston, Virginia Surgical
Small umbilical hernias and mesh repair: a big challenge
 Small umbilical hernias and mesh repair: a big challenge René H.Fortelny Allgemein-, Viszeral- und Tumorchirurgie Wilhelminenspital, Wien, Österreich Speakers Bureau: Bard Baxter B.Braun Johnson&Johnson
Small umbilical hernias and mesh repair: a big challenge René H.Fortelny Allgemein-, Viszeral- und Tumorchirurgie Wilhelminenspital, Wien, Österreich Speakers Bureau: Bard Baxter B.Braun Johnson&Johnson
AFX. Femoral Implant. System. The AperFix. AM Portal Surgical Technique Guide. with the. The AperFix System with the AFX Femoral Implant
 The AperFix System AFX with the Femoral Implant AM Portal Surgical Technique Guide The Cayenne Medical AperFix system with the AFX Femoral Implant is the only anatomic system for soft tissue ACL reconstruction
The AperFix System AFX with the Femoral Implant AM Portal Surgical Technique Guide The Cayenne Medical AperFix system with the AFX Femoral Implant is the only anatomic system for soft tissue ACL reconstruction
TrueSight Personalized Planning & Targeting System. Operative technique
 TrueSight Personalized Planning & Targeting System Operative technique TrueSight Personalized Planning & Targeting System Contents Introduction.... 2 Indications and contraindications... 3 Design rationale...
TrueSight Personalized Planning & Targeting System Operative technique TrueSight Personalized Planning & Targeting System Contents Introduction.... 2 Indications and contraindications... 3 Design rationale...
Duraloc CONSTRAINED LINER
 SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
SYNTHECEL Dura Repair. Assurance and Versatility for Dura Reconstruction.
 SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction. SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice
SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction. SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 20FR Guidewire PEG kit REORDER NO:
 Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
CAT FOR TREATMENT. Clinical Scenario:
 CAT FOR TREATMENT Clinical Scenario: A 30-year old male footballer presented at surgical OPD with clinical of painful, reducible groin hernia. I advice him for surgical management and gave him the possible
CAT FOR TREATMENT Clinical Scenario: A 30-year old male footballer presented at surgical OPD with clinical of painful, reducible groin hernia. I advice him for surgical management and gave him the possible
MULTIFIX S Knotless Implants
 Technique Guide MULTIFIX S Knotless Implants The MULTIFIX S system offers 5.5mm and 6.5mm knotless, all-peek, poundin implants. The system provides multiple fixation options, a streamlined technique, and
Technique Guide MULTIFIX S Knotless Implants The MULTIFIX S system offers 5.5mm and 6.5mm knotless, all-peek, poundin implants. The system provides multiple fixation options, a streamlined technique, and
Laparoscopic Repair of Inguinal Hernia with Biomimetic Matrix
 SCIENTIFIC PAPER Laparoscopic Repair of Inguinal Hernia with Biomimetic Matrix Arthur Fine, MD ABSTRACT Background and Objectives: Materials utilized for the repair of hernias fall into 2 broad categories,
SCIENTIFIC PAPER Laparoscopic Repair of Inguinal Hernia with Biomimetic Matrix Arthur Fine, MD ABSTRACT Background and Objectives: Materials utilized for the repair of hernias fall into 2 broad categories,
This information is intended as an overview only
 This information is intended as an overview only Please refer to the INSTRUCTIONS FOR USE included with this device for indications, contraindications, warnings, precautions and other important information
This information is intended as an overview only Please refer to the INSTRUCTIONS FOR USE included with this device for indications, contraindications, warnings, precautions and other important information
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
LAPAROSCOPIC HERNIA REPAIR
 LAPAROSCOPIC HERNIA REPAIR Treating Your Hernia with Laparoscopy When You Have a Hernia Anyone can have a hernia. This is a weakness or tear in the wall of the abdomen. It often results from years of wear
LAPAROSCOPIC HERNIA REPAIR Treating Your Hernia with Laparoscopy When You Have a Hernia Anyone can have a hernia. This is a weakness or tear in the wall of the abdomen. It often results from years of wear
30+ MEDPOR biomaterial. years of proven clinical history
 MEDPOR ENT surgery MEDPOR biomaterial MEDPOR has been a trusted name in the industry since 1985, with hundreds of thousands of procedures performed, and hundreds of published clinical reports in reconstructive,
MEDPOR ENT surgery MEDPOR biomaterial MEDPOR has been a trusted name in the industry since 1985, with hundreds of thousands of procedures performed, and hundreds of published clinical reports in reconstructive,
Integra. Silicone PIP Implant SURGICAL TECHNIQUE
 Integra Silicone PIP Implant SURGICAL TECHNIQUE Table of Contents Indications For Use...2 Contraindications...2 Warnings and Precautions...2 Surgical Technique Preoperative Assessment... 3 Step 1: Initial
Integra Silicone PIP Implant SURGICAL TECHNIQUE Table of Contents Indications For Use...2 Contraindications...2 Warnings and Precautions...2 Surgical Technique Preoperative Assessment... 3 Step 1: Initial
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
REINFORCED BIOSCAFFOLDS
 REINFORCED BIOSCAFFOLDS Midline Incisional Open OviTex 1S Resorbable Clinical Case Study: Open Abdomen Incisional Herniorrhaphy in Contaminated (CDC Class IV) Operative Field Performed by Dr. Michael Sawyer,
REINFORCED BIOSCAFFOLDS Midline Incisional Open OviTex 1S Resorbable Clinical Case Study: Open Abdomen Incisional Herniorrhaphy in Contaminated (CDC Class IV) Operative Field Performed by Dr. Michael Sawyer,
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
Gallery Laminoplasty Spine System
 Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Strattice Reconstructive Tissue Matrix used in the repair of rippling
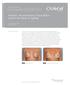 Clinical case study Strattice Tissue Matrix Strattice Reconstructive Tissue Matrix used in the repair of rippling Steven Teitelbaum, MD* Santa Monica, CA Case summary A 48-year-old woman with a history
Clinical case study Strattice Tissue Matrix Strattice Reconstructive Tissue Matrix used in the repair of rippling Steven Teitelbaum, MD* Santa Monica, CA Case summary A 48-year-old woman with a history
eptfe Vascular Graft Instructions for Use
 TM eptfe Vascular Graft Instructions for Use R Instructions For Use Caution: Federal (U.S.A) law restricts this device to sale by or on the order of a physician. Device Description, Indications, Contraindications,
TM eptfe Vascular Graft Instructions for Use R Instructions For Use Caution: Federal (U.S.A) law restricts this device to sale by or on the order of a physician. Device Description, Indications, Contraindications,
Ruby Coil. Large Volume Detachable Coils
 Ruby Coil Ruby Case Examples 38 mm Hepatic Artery Aneurysm Pulmonary AVM 2 Coils Dr. James Benenati Miami Cardiac and Vascular Institute, FL Y90 Embolization Type 2 Endoleak Dr. J Moskovitz Florida Hospital,
Ruby Coil Ruby Case Examples 38 mm Hepatic Artery Aneurysm Pulmonary AVM 2 Coils Dr. James Benenati Miami Cardiac and Vascular Institute, FL Y90 Embolization Type 2 Endoleak Dr. J Moskovitz Florida Hospital,
Early Outcome Of Inguinal Hernia Repair Using Ultrapro Mesh In University Of Calabar Teaching Hospital, Nigeria
 ISPUB.COM The Internet Journal of Third World Medicine Volume 6 Number 2 Early Outcome Of Inguinal Hernia Repair Using Ultrapro Mesh In University Of Calabar Teaching N Usoro, C Agbor, K Emelike, A Bamidele
ISPUB.COM The Internet Journal of Third World Medicine Volume 6 Number 2 Early Outcome Of Inguinal Hernia Repair Using Ultrapro Mesh In University Of Calabar Teaching N Usoro, C Agbor, K Emelike, A Bamidele
Information from the MAUDE Database
 Information from the MAUDE Database Device mesh, surgical, polymeric Regulation Description Surgical mesh. Product Code FTL Regulation Number 878.3300 Device Class 2 Manufacturer ETHICON Medical Device
Information from the MAUDE Database Device mesh, surgical, polymeric Regulation Description Surgical mesh. Product Code FTL Regulation Number 878.3300 Device Class 2 Manufacturer ETHICON Medical Device
JRI Thompson Hemiarthroplasty
 JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6 English 3 Page 2 of 6 Important Information Please
JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6 English 3 Page 2 of 6 Important Information Please
SureLock All-Suture Anchor System
 SureLock All-Suture Anchor System Guide for Bankart Repair Surgical Technique 2 SureLock All-Suture System Bankart Repair Surgical Technique Figure 1 *Curved and straight drill guides are available for
SureLock All-Suture Anchor System Guide for Bankart Repair Surgical Technique 2 SureLock All-Suture System Bankart Repair Surgical Technique Figure 1 *Curved and straight drill guides are available for
Tibial Fixation. with TunneLoc Device. Surgical Technique by Mark J. Albritton, M.D. and Sherwin Ho, M.D.
 Tibial Fixation with TunneLoc Device Surgical Technique by Mark J. Albritton, M.D. and Sherwin Ho, M.D. Table of Contents Surgical Technique... 4 Ordering Information... 11 Indications For Use... 12 Contraindications...
Tibial Fixation with TunneLoc Device Surgical Technique by Mark J. Albritton, M.D. and Sherwin Ho, M.D. Table of Contents Surgical Technique... 4 Ordering Information... 11 Indications For Use... 12 Contraindications...
PREVENTION OF INCISIONAL HERNIA AFTER MIDLINE LAPAROTOMY FOR ABDOMINAL AORTIC ANEURYSM TREATMENT: A RANDOMIZED CONTROLLED TRIAL
 PREVENTION OF INCISIONAL HERNIA AFTER MIDLINE LAPAROTOMY FOR ABDOMINAL AORTIC ANEURYSM TREATMENT: A RANDOMIZED CONTROLLED TRIAL F. Muysoms 1, T. Vierendeels 2, M. Huyghe 3, M. Miserez 4, M. Ruppert 5,
PREVENTION OF INCISIONAL HERNIA AFTER MIDLINE LAPAROTOMY FOR ABDOMINAL AORTIC ANEURYSM TREATMENT: A RANDOMIZED CONTROLLED TRIAL F. Muysoms 1, T. Vierendeels 2, M. Huyghe 3, M. Miserez 4, M. Ruppert 5,
Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use. Polyurethane Dual Lumen Occlusion Catheter
 Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use Polyurethane Dual Lumen Occlusion Catheter Polyurethane Dual Lumen Occlusion Catheter (Model Numbers 4F-2L-40, 4F-2L-80, 5F-2L-40,
Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use Polyurethane Dual Lumen Occlusion Catheter Polyurethane Dual Lumen Occlusion Catheter (Model Numbers 4F-2L-40, 4F-2L-80, 5F-2L-40,
ADVANCED BONE GRAFT SYSTEM OVERVIEW
 ADVANCED BONE GRAFT SYSTEM OVERVIEW NANOSS BIOACTIVE ADVANCED BONE GRAFT Table Of Contents INTRODUCTION System Overview... 1 NANOSS BIOACTIVE COMPONENTS Comparison of nanoss Bioactive and Human Bone...
ADVANCED BONE GRAFT SYSTEM OVERVIEW NANOSS BIOACTIVE ADVANCED BONE GRAFT Table Of Contents INTRODUCTION System Overview... 1 NANOSS BIOACTIVE COMPONENTS Comparison of nanoss Bioactive and Human Bone...
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE REF LOT STERILE EO. Manufactured for: 824 Twelfth Avenue Bethlehem, PA
 ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
C-THRU Anterior Spinal System
 C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
