Echo PS Positioning System with Ventralight ST Mesh or Composix L/P Mesh
|
|
|
- Clyde Griffin
- 6 years ago
- Views:
Transcription
1 Ventralight ST Mesh with Echo PS Positioning System Catalog Number Shape Mesh Size Circle 4.5" (11.4 cm) Ellipse 4" x 6" (10.2 cm x 15.2 cm) Circle 6" (15.2 cm) Ellipse 6" x 8" (15.2 cm x 20.3 cm) Oval 6" x 10" (15.2 cm x 25.4 cm) Ellipse 7" x 9" (17.8 cm x 22.9 cm) Circle 8" (20.3 cm) Ellipse 8" x 10" (20.3 cm x 25.4 cm) Ellipse 10" x 13" (25.4 cm x 33.0 cm) Rectangle 12" x 14" (30.5 cm x 35.6 cm) Reorder Code for Echo PS Positioning System Inflation Assembly (6/cs) Please add the Ventralight ST Mesh with Echo PS Positioning System to my preference card. I would like to have the Ventralight ST Mesh with Echo PS Positioning System in stock. I would like to trial the Ventralight ST Mesh with Echo PS Positioning System. Composix L/P Mesh with Echo PS Positioning System Catalog Number Shape Mesh Size Ellipse 6.2" x 8.2" (15.9 cm x 21.0 cm) Oval 6.2" x 10.2" (15.9 cm x 26.1 cm) Ellipse 7.2" x 9.2" (18.4 cm x 23.5 cm) Ellipse 8.2" x 10.2" (21.0 cm x 26.1 cm) Ellipse 10.2" x 13.2" (26.1 cm x 33.7 cm) Rectangle 10.2" x 14.2" (26.1 cm x 36.2 cm) Please add the Composix L/P Mesh with Echo PS Positioning System to my preference card. I would like to have the Composix L/P Mesh with Echo PS Positioning System in stock. I would like to trial the Composix L/P Mesh with Echo PS Positioning System. Surgeon s Signature Purchase Order Number Catalog Number Date Quantity Echo PS Positioning System with Ventralight ST Mesh or Composix L/P Mesh Technique Guide Bard, Davol, Composix, Echo and Ventralight are trademarks and/or registered trademarks of C. R. Bard, Inc. Davol Inc. Subsidiary of C. R. Bard, Inc. 100 Crossings Boulevard Warwick, RI Medical Services & Support Copyright 2014, C. R. Bard, Inc. All Rights Reserved. MMECHOTG6 DAV/ECST/0614/0002
2 This information contains the opinions of and personal surgical techniques practiced by the individual physicians named herein (use as applicable). The opinion and techniques presented herein are for informational purposes only and the decision of which technique to use in a particular surgical application should be made by the physician based on the individual facts and circumstances of the patient and previous surgical experience. Please consult product inserts and labels for any indications, contraindications, hazards, warnings, precautions and instructions for use. Table of Contents Mesh Features and Benefits Echo PS Positioning System Overview Trocar Size Recommendations... 6 Note on Transfascial Sutures... 7 Device Preparation and Hydration Device Insertion Balloon Inflation Mesh Positioning Initial Mesh Fixation Balloon Deflation and Removal Final Mesh Fixation Device Indications, Contraindications, Warnings and Precautions
3 absorbable barrier Ventralight ST MESH permanent barrier Composix L/P MESH Featuring an absorbable barrier based on proven Sepra Technology on one side of the mesh Based on the technology used in Seprafilm Minimizes tissue attachment to the visceral side of the mesh Featuring a permanent eptfe barrier on one side of the mesh... Minimizes tissue attachment to the prosthesis Submicronic eptfe used in general surgery for many years with demonstrated clinical success and a low profile design with a lightweight monofilament polypropylene mesh on the other. Insertion through trocar and mechanical fixation made easier Allows for rapid and complete tissue ingrowth Approximately 50% lighter than traditional polypropylene mesh and a low profile design with lightweight monofilament polypropylene mesh on the other. Easy handling and laparoscopic insertion Allows for rapid and complete tissue ingrowth Approximately 60% lighter than traditional polypropylene mesh 2 3
4 Echo PS P OSITIONING SYSTEM Posterior Side: 6 The Echo PS Positioning System comes pre-attached to Ventralight ST or Composix L/P Mesh, requiring no assembly or specialty instruments. 7 Anterior Side: Low profile, thermoplastic polyurethane (TPU) coated nylon balloon 7 Logo identifies long axis 8 Removal points marked by arrows 3 9 Tabs clearly identify the connector locations Shown on Ventralight ST Mesh Marked center of proximal ends represent the midline of the mesh 1 2 Inflation tube Connectors keep the Echo PS Positioning System attached to the mesh, but are simultaneously removed from the body with the positioning system Anchor allows for a secure connection between the inflation tube and inflation assembly Tube cut location Retrieval loop Also Included: Pre-packaged inflation assembly inflates balloon Introducer Tool, provided with most sizes, facilitates rolling and insertion of mesh. 4 5
5 trocar size recommendations note on transfascial sutures Ventralight ST Mesh with Echo PS Positioning System Catalog Number Shape Mesh Size # of Mesh Connectors Introducer Tool Included Recommended Minimum Trocar/ Incision Size * Circle 4.5" (11.4 cm) 2 No 10 mm Ellipse 4" x 6" (10.2 cm x 15.2 cm) 2 No 10 mm Circle 6" (15.2 cm) 4 Yes 12 mm Ellipse 6" x 8" (15.2 cm x 20.3 cm) 4 Yes 12 mm Oval 6" x 10" (15.2 cm x 25.4 cm) 4 Yes 12 mm Ellipse 7" x 9" (17.8 cm x 22.9 cm) 4 Yes 12 mm Circle 8" (20.3 cm) 4 Yes 12 mm Ellipse 8" x 10" (20.3 cm x 25.4 cm) 4 Yes 12 mm Ellipse 10" x 13" (25.4 cm x 33.0 cm) 8 Yes 15 mm Rectangle 12" x 14" (30.5 cm x 35.6 cm) 8 No 12 mm trocar incision site Composix L/P Mesh with Echo PS Positioning System Catalog Number Shape Mesh Size # of Mesh Connectors Introducer Tool Included Recommended Minimum Trocar/ Incision Size * Ellipse 6.2" x 8.2" (15.9 cm x 21.0 cm) 4 Yes 12 mm Oval 6.2" x 10.2" (15.9 cm x 26.1 cm) 4 Yes 12 mm Ellipse 7.2" x 9.2" (18.4 cm x 23.5 cm) 4 Yes 12 mm Ellipse 8.2" x 10.2" (21.0 cm x 26.1 cm) 4 Yes 12 mm Ellipse 10.2" x 13.2" (26.1 cm x 33.7 cm) 8 Yes 15 mm Rectangle 10.2" x 14.2" (26.1 cm x 36.2 cm) 8 Yes 15 mm The Echo PS Positioning System eliminates the need for transfascial orientation sutures. If transfascial sutures are to be used for fixation, place sutures after removing the Echo PS Positioning System and after completing all mechanical fixation. * If a proximal cap is available on the trocar, removing the proximal cap can help facilitate deployment. Deployment capability may vary depending on rolled patch size and graspers/trocars used. 6 7
6 1 Device Preparation Remove mesh with Echo PS Positioning System (the device*) and included Introducer Tool (when provided) from the sterile pouch. Set inflation assembly pouch aside. 2 Device Hydration For Ventralight ST Mesh Only: Hydrate the mesh in sterile saline for no more than 1-3 seconds just prior to rolling. The device refers to either Ventralight ST Mesh with Echo PS Positioning System or Composix L/P Mesh with Echo PS Positioning System. 8 9
7 2a Device Insertion with Introducer Tool * Place the device lengthwise between the metal tines approximately 2.5 to 5 cm from the long edge of the device (The Bard logo and dark shaded areas on the device represent the long axis). Ensure that the device is centered on the tines and that the inflation tube is laying flat and facing the proximal end of the tool in parallel to the rolling tines. Grasp the center of the device/tines to provide counter pressure against the device/tines. With one hand roll the device, polypropylene side out and bioresorbable coated side with Echo PS Positioning System on the inside, by turning the handle until the device is completely wrapped around the tines. Ensure the inflation tube is not wrapped around the mesh. Remove the T-cap. Ensure the device is positioned such that at least 1 /2 cm of the tines extends beyond the mesh edge. Place T-cap on the end of the tines. For sizes where Introducer Tool is not included, please refer to Device Insertion with Grasper step 2b, on page
8 Deliver the device through the trocar under sufficient visualization of the device and the surrounding anatomy. As the device is being deployed through the trocar, rotate the handle in the direction that the device was rolled. This will keep the device tight around the tines, thus facilitating deployment. To release the device from the Introducer Tool, rotate the handle approximately ½ turn in the opposite direction the device was rolled and partially slide the tines out of the device. Do not completely remove the tines from the device until the device has passed through the trocar entirely. Note: If the tines are removed from the device before it is completely deployed through the trocar, use the laparoscope to push the mesh through the trocar, or use a grasper from an opposing trocar location to pull the mesh into the abdomen. Under visualization, continue to advance the device and the tines through the trocar. Repeat the previous steps and rotate the handle in the direction the device was rolled to completely deploy the patch through the trocar. After the device has cleared the trocar, remove the Introducer Tool from the trocar and discard appropriately
9 Utilize the grasper technique for 4.5, 4 x 6 and 12 x 14 Ventralight Mesh sizes. No Introducer Tools are included with these sizes. 2b Device Insertion with Grasper Only for Ventralight ST Mesh sizes 4.5", 4" x 6" and 12" x 14" Roll the device tightly on itself starting at the long outside edge (The Bard logo and dark shaded areas of the device represent the long axis). Continue across with the polypropylene side on the outside and bioresorbable coated side with the Echo PS Positioning System on the inside. Utilize the grasper technique for 4.5, 4 x 6 and 12 x 14 Ventralight Mesh sizes. No Introducer Tools are included with these sizes. While holding the rolled device, grasp the leading edge with the grasper as shown. Ensure that the inflation tube is laying flat and facing the proximal end of the grasper. Place through the trocar incision site. Note: If the grasper slips from the device before the device is completely deployed through the trocar incision site, use a grasper from an opposing trocar location to pull the mesh into the abdomen
10 3 Balloon Inflation Once the device is inserted into the abdomen, use a grasper to locate the blue retrieval loop on the inflation tube, ensuring that the blue inflation tube is not wrapped around the mesh and is clearly visible. Place an atraumatic clamp or hemostat on the inflation tube at the level of the skin to temporarily hold the device in place. Cut the inflation tube on the dashed line between the retrieval loop and the yellow anchor with surgical scissors to open the tube for inflation. Discard the retrieval loop. Pass a suture passer device through healthy skin at the center of the hernia defect (avoid going directly through the umbilicus). Grasp the blue retrieval loop and pull the retrieval loop and inflation tube out of the abdominal cavity
11 Remove the inflation assembly from the sterile pouch and screw the inflation adapter tightly to the syringe. Connect the inflation tube and inflation assembly as follows: a. Ensure the clear cap of the adapter is pushed downward to open the inflation tube channel b. Insert the inflation tube as far as possible into the cap opening c. Pull the clear cap of the adapter upward to lock in place To inflate the device, release the clamp or hemostat and pull the inflation tube upward to lift the mesh off the viscera. Be sure to always grab and pull the tube directly. Do not lift up using the inflation adapter/assembly. Inflate the device by pumping the syringe until the balloon is fully inflated. The syringe may be filled while attached to the inflation assembly. One to three pumps will be required to fully inflate the device depending on the size of the mesh. A slight high pitch sound may occur; this is normal and indicates the inflation assembly is working properly. A fully inflated balloon will not accept more air. Once the device is inflated, if desired, remove the syringe by unscrewing from the adapter and setting aside
12 4 Mesh Positioning Raise the inflation tube to properly adjust the mesh to the desired position. Clamp the inflation tube to hold the device in place. Use a grasper to orient the mesh in relation to the defect. The Bard logo and dark shaded areas on the device represent the long axis. The two marked areas at both ends of the long axis indicate the midline of the mesh
13 5 Initial Mesh Fixation 6 Balloon Deflation Once the device has been properly positioned and before the Echo PS Positioning System is deflated, ensure that no tissue is entrapped between the device and the abdominal wall and then fixate around the entire perimeter of the mesh with fasteners placed 1-2 cm apart. For Composix L/P, fasteners must be placed at least 1 /2 cm inside the outermost row of stitches. Ensure that no fasteners are placed through the Echo PS Positioning System. To deflate the Echo PS Positioning System, release the clamp on the inflation tube, cut the tube as close to the skin as possible, and then discard
14 7 Balloon Removal 8 Final Mesh Fixation Begin removal of the Echo PS Positioning System by grasping one of the two removal points marked by the dark arrows adjacent to the Bard logo and pulling the positioning system off the mesh in one smooth motion. Reinsert the trocar, apply final fixation, and complete the procedure. Care should be taken to ensure that the mesh is adequately fixated to the abdominal wall. If necessary, additional fasteners and/or sutures should be placed. Continue removing the Echo PS Positioning System by pulling it up to the tip of the trocar. Remove both the Echo PS Positioning System and trocar simultaneously. Verify that the device is fully intact after removal. * Discard the Echo PS Positioning System appropriately. * Refer to the chart on page 6 for the number of connectors for each catalog number
15 Ventralight ST MESH WITH Echo PS P OSITIONING SYSTEM INDICATIONS: Ventralight ST Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias. The Echo PS Positioning System is intended to be used to facilitate the delivery of soft tissue prostheses during laparoscopic hernia repair. CONTRAINDICATIONS: 1. Do not use the Ventralight ST Mesh with Echo PS Positioning System in infants or children whereby future growth will be compromised by use of such material. 2. Do not use Ventralight ST Mesh with Echo PS Positioning System for the reconstruction of cardiovascular defects. 3. Literature reports there is a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera. WARNINGS: 1. This device is supplied sterile. Inspect the packaging to be sure it is intact and undamaged prior to use. 2. This device has been designed for single use only. Reuse, resterilization, reprocessing and/or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the device and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, resterilization, or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the device may lead to injury, illness, or death of the patient or end user. 3. If unused prosthesis has been in contact with instruments or supplies used on a patient or contaminated with body fluids, discard with care to prevent risk of transmission of viral infections. 4. Ensure proper orientation; the coated side of Ventralight ST Mesh with Echo PS Positioning System should be oriented against the bowel or sensitive organs. Do not place the polypropylene side against the bowel. There is a possibility for adhesion formation when the polypropylene side is placed in direct contact with the bowel or viscera. 5. The use of any permanent mesh or patch in a contaminated or infected wound could lead to infection, fistula formation and/or extrusion of the prosthesis. 6. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the prosthesis. An unresolved infection may require removal of the prosthesis. 7. To prevent recurrences when repairing hernias, it is recommended that the prosthesis be large enough to extend at least 3 to 5 cm beyond the margins of the defect. 8. Do not apply sharp, heat emitting, or ultrasonic tools (such as scissors, needles, tackers, diathermic tools, etc.) to the Echo PS Positioning System. 9. The Echo PS Positioning System should not be used with any other hernia prosthesis aside from those with which it comes pre-attached/packaged. 10. Ventralight ST Mesh is the only permanent implant component of the device. The inflation adapter and syringe are to be kept external to the patient and discarded after use. The Echo PS Positioning System (including the balloon, all connectors, and inflation tube) is to be removed from the patient and appropriately discarded as it is not part of the permanent implant
16 11. Discard Introducer Tool and all components of the Echo PS Positioning System (including the inflation adapter and syringe) after use. This product may be a potential biohazard. Handle and dispose in accordance with accepted medical practice and applicable local, state and federal laws and regulations. PRECAUTIONS: 1. Please read all instructions prior to use. 2. Only physicians qualified in the appropriate surgical techniques should use this device. 3. The safety and effectiveness of Ventralight ST Mesh with Echo PS Positioning System has not been evaluated in clinical studies for the presence of malignancies in the abdominopelvic cavity. 4. Visualization should be maintained throughout the course of the entire procedure. Additionally, laparoscopic removal of the Echo PS Positioning System must be performed under sufficient visualization of the entire device and surrounding anatomy, to ensure proper removal. 5. Do not trim the mesh. This will affect the interface between the mesh and positioning system. FIXATION: Bard fixation devices or nonabsorbable monofilament sutures are recommended to properly secure Ventralight ST Mesh. If other fixation devices are used, they must be indicated for use in hernia repair. If transfascial sutures are to be used for fixation, place sutures after removing the Echo PS Positioning System from the body and after completing all mechanical fixation. Care should be taken to ensure that the patch is adequately fixated to the abdominal wall. If necessary, additional fasteners and/or sutures should be placed. ADVERSE REACTIONS: Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction and recurrence of the hernia or soft tissue defect. INDICATIONS: Composix L/P Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias and chest wall defects. The Echo PS Positioning System is intended to be used to facilitate the delivery of soft tissue prostheses during laparoscopic hernia repair. CONTRAINDICATIONS: 1. Literature reports there is a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera. 2. Do not use the Composix L/P Mesh with Echo PS Positioning System in infants or children whereby future growth will be compromised by use of such material. 3. Do not use Composix L/P Mesh with Echo PS Positioning System for the reconstruction of cardiovascular defects. WARNINGS: Composix L/P MESH WITH Echo PS P OSITIONING SYSTEM 1. This device is supplied sterile. Inspect the packaging to be sure it is intact and undamaged prior to use. 2. This device has been designed for single use only. Reuse, resterilization, reprocessing and/or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the device and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, resterilization, or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the device may lead to injury, illness, or death of the patient or end user. PK
17 3. Ensure proper orientation; the solid white surface (eptfe) of Composix L/P Mesh with Echo PS Positioning System must be oriented against the bowel or sensitive organs. Do not place the polypropylene mesh surface against the bowel. There is a possibility for adhesion formation when polypropylene mesh is placed in direct contact with the bowel or viscera. 4. The use of any permanent mesh or patch in a contaminated or infected wound could lead to infection, fistula formation and/or extrusion of the prosthesis. 5. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the prosthesis. An unresolved infection may require removal of the prosthesis. 6. To prevent recurrences when repairing hernias, it is recommended that the prosthesis be large enough to extend at least 3 to 5 cm beyond the margins of the defect. 7. Do not apply sharp, heat emitting, or ultrasonic tools (such as scissors, needles, tackers, diathermic tools, etc.) to the Echo PS Positioning System. 8. The Echo PS Positioning System should not be used with any other hernia prosthesis aside from those with which it comes pre-attached/packaged. 9. Composix L/P Mesh is the only permanent implant component of the device. The inflation adapter and syringe are to be kept external to the patient and discarded after use. The Echo PS Positioning System (including the balloon, all connectors, and inflation tube) is to be removed from the patient and appropriately discarded as it is not part of the permanent implant. 10. Discard Introducer Tool and all components of the Echo PS Positioning System (including the inflation adapter and syringe) after use. This product may be a potential biohazard. Handle and dispose in accordance with accepted medical practice and applicable local, state and federal laws and regulations. PRECAUTIONS: 1. Please read all instructions prior to use. 2. Only physicians qualified in the appropriate surgical techniques should use this device. 3. Visualization should be maintained throughout the course of the entire procedure. Additionally, laparoscopic removal of the balloon device must be performed under sufficient visualization of the device and surrounding anatomy, to ensure proper device removal. 4. Do not trim the mesh. This will affect the interface between the mesh and positioning system. FIXATION: Bard fixation devices or nonabsorbable monofilament sutures are recommended to properly secure Composix L/P Mesh. If other fixation devices are used, they must be indicated for use in hernia repair. If transfascial sutures are to be used for fixation, place sutures after removing the Echo PS Positioning System from the body and after completing all mechanical fixation. Care should be taken to ensure that the mesh is adequately fixated to the abdominal wall. If necessary, additional fasteners and/or sutures should be placed. ADVERSE REACTIONS: Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, and recurrence of the hernia or soft tissue defect. PK
18 NOTES: NOTES: 32 33
Positioning System. Laparoscopic ventral hernia repair KEY BENEFITS SOFT TISSUE REPAIR
 Echo PS Positioning System with Ventralight ST Mesh or Composix L/P Mesh Laparoscopic ventral hernia repair Echo PS Positioning System with Ventralight ST Mesh Echo PS Positioning System with Composix
Echo PS Positioning System with Ventralight ST Mesh or Composix L/P Mesh Laparoscopic ventral hernia repair Echo PS Positioning System with Ventralight ST Mesh Echo PS Positioning System with Composix
Bard CapSure. Technique Guide. Permanent Fixation System SOFT TISSUE REPAIR. Laparoscopic Inguinal, Open and Laparoscopic Ventral Hernia Repair
 Bard CapSure Permanent Fixation System Technique Guide Laparoscopic Inguinal, Open and Laparoscopic Ventral Hernia Repair SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. The opinions
Bard CapSure Permanent Fixation System Technique Guide Laparoscopic Inguinal, Open and Laparoscopic Ventral Hernia Repair SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. The opinions
with Laparoscopic Ventral Hernia Repair Positioning System SOFT TISSUE REPAIR Designed for Accurate Mesh Centering
 with Laparoscopic Ventral Hernia Repair Positioning System Designed for Accurate Mesh Centering SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. with A Consistent, Reproducible Technique
with Laparoscopic Ventral Hernia Repair Positioning System Designed for Accurate Mesh Centering SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. with A Consistent, Reproducible Technique
OptiFix Absorbable Fixation System
 OptiFix Absorbable Fixation System Absorbable Fixation Redefined Advancing the Fixation Experience. SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Challenges and
OptiFix Absorbable Fixation System Absorbable Fixation Redefined Advancing the Fixation Experience. SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Challenges and
Ventral Hernia Repair
 Ventral Hernia Repair Ventrio ST Hernia Patch Ventrio Hernia Patch Technique Guide Open and Laparoscopic Ventral Hernia Repair SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. This Technique
Ventral Hernia Repair Ventrio ST Hernia Patch Ventrio Hernia Patch Technique Guide Open and Laparoscopic Ventral Hernia Repair SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. This Technique
Ventralex ST Hernia Patch featuring Sepra Technology
 Ventralex ST Hernia Patch featuring Sepra Technology Proven Sepra Technology in a Low Profile, Lightweight Mesh Sepra Technology An extensively studied barrier with more than 10 publications and used clinically
Ventralex ST Hernia Patch featuring Sepra Technology Proven Sepra Technology in a Low Profile, Lightweight Mesh Sepra Technology An extensively studied barrier with more than 10 publications and used clinically
Technique Guide. Bard MK Hernia Repair. Featuring Modified Onflex Mesh SOFT TISSUE REPAIR. Anterior Approach to a Preperitoneal Inguinal Hernia Repair
 Bard MK Hernia Repair Featuring Modified Onflex Mesh Technique Guide Anterior Approach to a Preperitoneal Inguinal Hernia Repair SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. The opinions
Bard MK Hernia Repair Featuring Modified Onflex Mesh Technique Guide Anterior Approach to a Preperitoneal Inguinal Hernia Repair SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. The opinions
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
Desara TV and Desara Blue TV
 Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 20FR Guidewire PEG kit REORDER NO:
 Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
The Ceterix NovoStitch Disposable Suture Passer. Do Not Reuse
 The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedics 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241-1748 IK Sterile D Do Not Reuse i See Instructions for Use THE CETERIX
The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedics 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241-1748 IK Sterile D Do Not Reuse i See Instructions for Use THE CETERIX
Passer. Sterile. D i. Do Not PASSER DESCRIPTION. The Ceterix. Page 1
 The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedic cs 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241 1748 IK D i Sterile Do Not Reusee See Instructions for Use THE
The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedic cs 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241 1748 IK D i Sterile Do Not Reusee See Instructions for Use THE
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE
 RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE FIG. 1 RD180 SP - THE RUNNING DEVICE 1 2 6 3 7 4 5 1 NEEDLE 2 FERRULE 3 SUTURE 4 DEVICE TIP 5 ANGLED SHAFT 6 HANDLE 7 PINK LEVER
RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE FIG. 1 RD180 SP - THE RUNNING DEVICE 1 2 6 3 7 4 5 1 NEEDLE 2 FERRULE 3 SUTURE 4 DEVICE TIP 5 ANGLED SHAFT 6 HANDLE 7 PINK LEVER
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
The AperFix II System
 The AperFix II System A Complete Anatomic Solution Transtibial Surgical Technique 2 AperFix II System Transtibial Surgical Technique Figure 1 A Complete Anatomic Solution The Cayenne Medical AperFix and
The AperFix II System A Complete Anatomic Solution Transtibial Surgical Technique 2 AperFix II System Transtibial Surgical Technique Figure 1 A Complete Anatomic Solution The Cayenne Medical AperFix and
Technique Guide. *smith&nephew SPEEDSCREW Fully Threaded Knotless Implant
 Technique Guide *smith&nephew SPEEDSCREW Fully Threaded Knotless Implant SPEEDSCREW system Fully threaded knotless implant system The SPEEDSCREW system is specifically designed for rotator cuff repair
Technique Guide *smith&nephew SPEEDSCREW Fully Threaded Knotless Implant SPEEDSCREW system Fully threaded knotless implant system The SPEEDSCREW system is specifically designed for rotator cuff repair
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
AFX. Femoral Implant. System. The AperFix. AM Portal Surgical Technique Guide. with the. The AperFix System with the AFX Femoral Implant
 The AperFix System AFX with the Femoral Implant AM Portal Surgical Technique Guide The Cayenne Medical AperFix system with the AFX Femoral Implant is the only anatomic system for soft tissue ACL reconstruction
The AperFix System AFX with the Femoral Implant AM Portal Surgical Technique Guide The Cayenne Medical AperFix system with the AFX Femoral Implant is the only anatomic system for soft tissue ACL reconstruction
Aspira* Peritoneal Drainage Catheter
 Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use
 Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Desara and Desara Blue
 Desara and Desara Blue Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide M Manufactured by: Caldera Medical, Inc.
Desara and Desara Blue Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide M Manufactured by: Caldera Medical, Inc.
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6
 Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
MULTIFIX S Knotless Implants
 Technique Guide MULTIFIX S Knotless Implants The MULTIFIX S system offers 5.5mm and 6.5mm knotless, all-peek, poundin implants. The system provides multiple fixation options, a streamlined technique, and
Technique Guide MULTIFIX S Knotless Implants The MULTIFIX S system offers 5.5mm and 6.5mm knotless, all-peek, poundin implants. The system provides multiple fixation options, a streamlined technique, and
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas. Reprocessed Device for Single Use.
 Reprocessed by Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by
Reprocessed by Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by
This information is intended as an overview only
 This information is intended as an overview only Please refer to the INSTRUCTIONS FOR USE included with this device for indications, contraindications, warnings, precautions and other important information
This information is intended as an overview only Please refer to the INSTRUCTIONS FOR USE included with this device for indications, contraindications, warnings, precautions and other important information
Technique Guide. *smith&nephew MAGNUM 2 Knotless Implant
 Technique Guide *smith&nephew MAGNUM 2 Knotless Implant MAGNUM 2 knotless implant The MAGNUM 2 implant increases surgeon control by combining independent cortical bone lock, suture lock, and incremental
Technique Guide *smith&nephew MAGNUM 2 Knotless Implant MAGNUM 2 knotless implant The MAGNUM 2 implant increases surgeon control by combining independent cortical bone lock, suture lock, and incremental
GENERAL TECHNIQUE GUIDE
 GENERAL TECHNIQUE GUIDE PRODUCT OVERVIEW The Eclipse Soft Tissue Anchor employs MedShape s shape memory PEEK Altera technology for soft tissue fixation procedures in the shoulder, elbow, knee, hand & wrist,
GENERAL TECHNIQUE GUIDE PRODUCT OVERVIEW The Eclipse Soft Tissue Anchor employs MedShape s shape memory PEEK Altera technology for soft tissue fixation procedures in the shoulder, elbow, knee, hand & wrist,
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Technique Guide. MULTIFIX P PEEK 4.5mm Knotless Fixation Implant
 Technique Guide MULTIFIX P PEEK 4.5mm Knotless Fixation Implant MULTIFIX P system With the ability to accommodate 2-4 suture strands and lock suture internally, the 4.5mm MULTIFIX P system gives the surgeon
Technique Guide MULTIFIX P PEEK 4.5mm Knotless Fixation Implant MULTIFIX P system With the ability to accommodate 2-4 suture strands and lock suture internally, the 4.5mm MULTIFIX P system gives the surgeon
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology
 Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE REF LOT STERILE EO. Manufactured for: 824 Twelfth Avenue Bethlehem, PA
 ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Integra. Endoscopic Gastrocnemius Release System SURGICAL TECHNIQUE
 Integra Endoscopic Gastrocnemius Release System SURGICAL TECHNIQUE Table of Contents Indications... 2 Contraindications... 2 System Description... 2 Features and Benefits... 2 Surgical Site Preparation...3
Integra Endoscopic Gastrocnemius Release System SURGICAL TECHNIQUE Table of Contents Indications... 2 Contraindications... 2 System Description... 2 Features and Benefits... 2 Surgical Site Preparation...3
What you should know about your diagnosis of incontinence
 What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
Stratis INSTRUCTIONS FOR USE. Needle-free Injection System. 0.5mL volume (+/-5%)
 Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Technique Guide. A natural product for a natural repair. Post-Mastectomy Breast Reconstruction
 A natural product for a natural repair. Acellular Dermal Matrix Tissue In Conjunction With Soft Tissue Repair Technique Guide Post-Mastectomy Breast Reconstruction This Technique Guide contains the opinions
A natural product for a natural repair. Acellular Dermal Matrix Tissue In Conjunction With Soft Tissue Repair Technique Guide Post-Mastectomy Breast Reconstruction This Technique Guide contains the opinions
DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS
 DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS HALYARD* MIC* AND MIC-KEY* INTRODUCER KITS HALYARD* MIC* and MIC-KEY* Introducer Kits provide physicians with an innovative solution to facilitate the primary
DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS HALYARD* MIC* AND MIC-KEY* INTRODUCER KITS HALYARD* MIC* and MIC-KEY* Introducer Kits provide physicians with an innovative solution to facilitate the primary
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device. Instructions for Use
 The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
LOWER EXTREMITIES SINGLE-USE INSTRUMENTATION SURGICAL IMPLANTATION TECHNIQUE. First Metatarsal Phalangeal Joint FOOT THE DIFFERENCE IS MOVING.
 LOWER EXTREMITIES SINGLE-USE INSTRUMENTATION SURGICAL IMPLANTATION TECHNIQUE First Metatarsal Phalangeal Joint FOOT THE DIFFERENCE IS MOVING. CARTIVA SYNTHETIC CARTILAGE IMPLANT SURGICAL IMPLANTATION TECHNIQUE
LOWER EXTREMITIES SINGLE-USE INSTRUMENTATION SURGICAL IMPLANTATION TECHNIQUE First Metatarsal Phalangeal Joint FOOT THE DIFFERENCE IS MOVING. CARTIVA SYNTHETIC CARTILAGE IMPLANT SURGICAL IMPLANTATION TECHNIQUE
INGUINAL HERNIA REPAIR PROCEDURE GUIDE
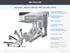 ROOM CONFIGURATION The following figure shows an overhead view of the recommended OR configuration for a da Vinci Inguinal Hernia Repair (Figure 1). NOTE: Configuration of the operating room suite is dependent
ROOM CONFIGURATION The following figure shows an overhead view of the recommended OR configuration for a da Vinci Inguinal Hernia Repair (Figure 1). NOTE: Configuration of the operating room suite is dependent
EXACTECH SPINE. Operative Technique. Cervical Spacer System. Surgeon focused. Patient driven. TM
 EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
ARROW EZ-IO Intraosseous Vascular Access System Procedure Template
 ARROW EZ-IO Intraosseous Vascular Access System Procedure Template PURPOSE To provide procedural guidance for establishment of intraosseous vascular access using the ARROW EZ-IO Intraosseous Vascular Access
ARROW EZ-IO Intraosseous Vascular Access System Procedure Template PURPOSE To provide procedural guidance for establishment of intraosseous vascular access using the ARROW EZ-IO Intraosseous Vascular Access
Title: EZ-IO. Effective Date: January SOG Number: EMS Rescinds:
 S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
ASEPT Pleural Drainage System
 ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE. Rx only REF LOT. STERILE EO Sterilized using ethylene oxide
 ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
Duracryl sutures should be selected and implanted depending on the patient condition, surgical experience, suturing technique and wound size.
 FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: DURACRYL Material: Monofilament Polydioxanone. Synthetic absorbable surgical suture (Dyed) Description: DURACRYL is a sterile synthetic absorbable
FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: DURACRYL Material: Monofilament Polydioxanone. Synthetic absorbable surgical suture (Dyed) Description: DURACRYL is a sterile synthetic absorbable
TissueMend. Arthroscopic Surgical Technique. Arthroscopic Insertion of a Biologic Rotator Cuff Tissue Augment After Rotator Cuff Repair
 TissueMend Arthroscopic Surgical Technique Arthroscopic Insertion of a Biologic Rotator Cuff Tissue Augment After Rotator Cuff Repair Table of Contents Placement of suture anchors Placement of anteromedial
TissueMend Arthroscopic Surgical Technique Arthroscopic Insertion of a Biologic Rotator Cuff Tissue Augment After Rotator Cuff Repair Table of Contents Placement of suture anchors Placement of anteromedial
fassier DUVal TelescoPIc IM system s RESCUE SYSTEM 2.0
 fassier DUVal TelescoPIc IM system s RESCUE SYSTEM 2.0 The Rescue System was conceived to retrieve components of the Fassier- Duval telescopic IM System after the treatment has been completed or in case
fassier DUVal TelescoPIc IM system s RESCUE SYSTEM 2.0 The Rescue System was conceived to retrieve components of the Fassier- Duval telescopic IM System after the treatment has been completed or in case
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
Integra Flowable Wound Matrix
 Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Arthroscopic Shoulder Repair Using the Smith & Nephew KINSA Suture Anchor
 Shoulder Series Technique Guide *smith&nephew KINSA Suture Anchor Arthroscopic Shoulder Repair Using the Smith & Nephew KINSA Suture Anchor Reviewed by: Larry D. Field, MD Mississippi Sports Medicine and
Shoulder Series Technique Guide *smith&nephew KINSA Suture Anchor Arthroscopic Shoulder Repair Using the Smith & Nephew KINSA Suture Anchor Reviewed by: Larry D. Field, MD Mississippi Sports Medicine and
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
CARTIVA. Synthetic Cartilage Implant SURGICAL IMPLANTATION TECHNIQUE. First Metatarsal Phalangeal Joint THE DIFFERENCE IS MOVING.
 CARTIVA Synthetic Cartilage Implant SURGICAL IMPLANTATION TECHNIQUE First Metatarsal Phalangeal Joint THE DIFFERENCE IS MOVING. CARTIVA SYNTHETIC CARTILAGE IMPLANT TABLE OF CONTENTS Introduction... 3 Cartiva
CARTIVA Synthetic Cartilage Implant SURGICAL IMPLANTATION TECHNIQUE First Metatarsal Phalangeal Joint THE DIFFERENCE IS MOVING. CARTIVA SYNTHETIC CARTILAGE IMPLANT TABLE OF CONTENTS Introduction... 3 Cartiva
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
FAST1 Intraosseous Infusion System. Training Session
 FAST1 Intraosseous Infusion System Training Session Why IO? Peripheral IV is often difficult to obtain Requires an average of 3-12 minutes Failure rate ranges between 10-40% AHA & ILCOR guidelines now
FAST1 Intraosseous Infusion System Training Session Why IO? Peripheral IV is often difficult to obtain Requires an average of 3-12 minutes Failure rate ranges between 10-40% AHA & ILCOR guidelines now
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
RECOMMENDED INSTRUCTIONS FOR USE
 Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Cardiva Catalyst III INSTRUCTIONS FOR USE
 Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Doc no: IFU/CPL Issue date: Rev no: 00 Rev date: 1
 FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: PROGUT Material: Catgut Plain. Absorbable surgical suture U.S.P Description: Progut surgical catgut suture is a sterile absorbable suture composed
FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: PROGUT Material: Catgut Plain. Absorbable surgical suture U.S.P Description: Progut surgical catgut suture is a sterile absorbable suture composed
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Wide-Span Balloon Retractor ( Laprotract ) Narrative
 ( Laprotract ) Narrative I. ABSTRACT A inflatable balloon retractor for minimally invasive surgery is described. The retractor consists of a triple-headed balloon which provides a much wider footprint
( Laprotract ) Narrative I. ABSTRACT A inflatable balloon retractor for minimally invasive surgery is described. The retractor consists of a triple-headed balloon which provides a much wider footprint
EndoRelease ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM
 EndoRelease ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM SURGICAL TECHNIQUE Up p e r Ex t r e m i t y So l u t i o n s ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM Description: The EndoRelease Endoscopic Cubital
EndoRelease ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM SURGICAL TECHNIQUE Up p e r Ex t r e m i t y So l u t i o n s ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM Description: The EndoRelease Endoscopic Cubital
Technique Guide. *smith&nephew N8TIVE ACL Anatomic ACL Reconstruction System
 Technique Guide *smith&nephew N8TIVE ACL Anatomic ACL Reconstruction System N8TIVE ACL System The N8TIVE ACL Anatomic Reconstruction System provides a novel and simple approach to ACL repair. The N8TIVE
Technique Guide *smith&nephew N8TIVE ACL Anatomic ACL Reconstruction System N8TIVE ACL System The N8TIVE ACL Anatomic Reconstruction System provides a novel and simple approach to ACL repair. The N8TIVE
Trilogy Acetabular System
 Trilogy Acetabular System Surgical Technique Versatility in a proven design Trilogy Acetabular System 1 Trilogy Acetabular System Surgical Technique Table of Contents Acetabular Reaming 2 Component Sizing
Trilogy Acetabular System Surgical Technique Versatility in a proven design Trilogy Acetabular System 1 Trilogy Acetabular System Surgical Technique Table of Contents Acetabular Reaming 2 Component Sizing
Zimmer Trabecular Metal Ankle Interpositional Spacer and Trabecular Metal Ankle Fusion Spacer
 Zimmer Trabecular Metal Ankle Interpositional Spacer and Trabecular Metal Ankle Fusion Spacer Surgical Technique 2 Zimmer Trabecular Metal Ankle Interpositional Spacer and Trabecular Metal Ankle Fusion
Zimmer Trabecular Metal Ankle Interpositional Spacer and Trabecular Metal Ankle Fusion Spacer Surgical Technique 2 Zimmer Trabecular Metal Ankle Interpositional Spacer and Trabecular Metal Ankle Fusion
Doc no: IFU/CC Issue date: Rev no: 04 Rev date:
 FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: PROGUT Material: Catgut Chromic Absorbable surgical suture U.S.P Description: Progut surgical catgut suture is a sterile absorbable suture composed
FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: PROGUT Material: Catgut Chromic Absorbable surgical suture U.S.P Description: Progut surgical catgut suture is a sterile absorbable suture composed
II.- PLUG. NAME of the products. Premilene Mesh Plug MANUFACTURER. B Braun DESCRIPTION. Polypropylene mesh for plug technique
 II.- PLUG Premilene Mesh Plug B Braun Polypropylene mesh for plug technique Premilene Mesh Plug is a monofilament polypropylene mesh plug designed for the repair of recurrent hernias and can also be used
II.- PLUG Premilene Mesh Plug B Braun Polypropylene mesh for plug technique Premilene Mesh Plug is a monofilament polypropylene mesh plug designed for the repair of recurrent hernias and can also be used
ARROW ENDURANCE. Extended Dwell Peripheral Catheter System. Rx only.
 ARROW ENDURANCE Extended Dwell Peripheral Catheter System Rx only. Product Description: The ARROW Endurance catheter system is a sterile, single use peripheral intravascular device designed to permit access
ARROW ENDURANCE Extended Dwell Peripheral Catheter System Rx only. Product Description: The ARROW Endurance catheter system is a sterile, single use peripheral intravascular device designed to permit access
EX-PRESS Glaucoma Filtration Device Surgical Procedure
 EX-PRESS Glaucoma Filtration Device Surgical Procedure Surgical Recommendations EX-PRESS Glaucoma Filtration Device preloaded device (plus 1 back-up) Sterile caliper Lid speculum Corneal forceps 0.12 forceps
EX-PRESS Glaucoma Filtration Device Surgical Procedure Surgical Recommendations EX-PRESS Glaucoma Filtration Device preloaded device (plus 1 back-up) Sterile caliper Lid speculum Corneal forceps 0.12 forceps
Biologically-Assisted ACL Reconstruction. Surgical Technique
 Biologically-Assisted ACL Reconstruction Surgical Technique One Surgeon. One Patient. Over 1 million times per year, Biomet helps one surgeon provide personalized care to one patient. The science and art
Biologically-Assisted ACL Reconstruction Surgical Technique One Surgeon. One Patient. Over 1 million times per year, Biomet helps one surgeon provide personalized care to one patient. The science and art
Peritoneal Drainage System
 ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
Visit Linvatec.com today to learn more. Surgical Technique: Sequential Meniscal Running Stitch
 ORDERING INFORMATION Sequent Meniscal Repair System MR004S MR007S MR004C MR007C SC047D Straight Needle, 4 Implants Straight Needle, 7 Implants Curved Needle, 4 Implants Curved Needle, 7 implants Sequent
ORDERING INFORMATION Sequent Meniscal Repair System MR004S MR007S MR004C MR007C SC047D Straight Needle, 4 Implants Straight Needle, 7 Implants Curved Needle, 4 Implants Curved Needle, 7 implants Sequent
Flexible Fragment Fixation. Surgical Technique
 Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Technique Manual Technique Manual Rev. A 01/11/2011
 Technique Manual Technique Manual Table of Contents Introduction 4 Indications 5 Instrumentation 6 Frame Assembly 7 Position Guide 8-9 Tips and Tricks 10-13 Cleaning 14 Sterilization 14 Storage Instructions
Technique Manual Technique Manual Table of Contents Introduction 4 Indications 5 Instrumentation 6 Frame Assembly 7 Position Guide 8-9 Tips and Tricks 10-13 Cleaning 14 Sterilization 14 Storage Instructions
BIOKNOTLESSRC ROTATOR CUFF REPAIR SUTURE ANCHOR SURGICAL TECHNIQUE. Surgical Technique for Arthroscopic Rotator Cuff Repair. Raymond Thal, M.D.
 SURGICAL TECHNIQUE ROTATOR CUFF REPAIR BIOKNOTLESSRC SUTURE ANCHOR Surgical Technique for Arthroscopic Rotator Cuff Repair Raymond Thal, M.D. Town Center Orthopaedic Associates Reston, Virginia Surgical
SURGICAL TECHNIQUE ROTATOR CUFF REPAIR BIOKNOTLESSRC SUTURE ANCHOR Surgical Technique for Arthroscopic Rotator Cuff Repair Raymond Thal, M.D. Town Center Orthopaedic Associates Reston, Virginia Surgical
FASSIER DUVAL TELESCOPIC IM SYSTEM S RESCUE SYSTEM 2.0
 FASSIER DUVAL TELESCOPIC IM SYSTEM S RESCUE SYSTEM 2.0 The Rescue System was conceived to retrieve components of the Fassier- Duval telescopic IM System after the treatment has been completed or in case
FASSIER DUVAL TELESCOPIC IM SYSTEM S RESCUE SYSTEM 2.0 The Rescue System was conceived to retrieve components of the Fassier- Duval telescopic IM System after the treatment has been completed or in case
Rotator Cuff Repair using JuggerKnot Soft Anchor 2.9mm Surgical Technique
 Rotator Cuff Repair using JuggerKnot Soft Anchor 2.9mm Surgical Technique It s small. It s strong. And it's all suture. The JuggerKnot Soft Anchor represents the next generation of suture anchor technology.
Rotator Cuff Repair using JuggerKnot Soft Anchor 2.9mm Surgical Technique It s small. It s strong. And it's all suture. The JuggerKnot Soft Anchor represents the next generation of suture anchor technology.
ARTHROTUNNELER TUNNELPRO SYSTEM
 TORNIER ARTHROTUNNELER TUNNELPRO SYSTEM Transosseous Rotator Cuff Repair SURGICAL TECHNIQUE ARTHROTUNNELER TUNNELPRO SYSTEM ARTHROTUNNELER Surgical Technique Step 1. Drill medial tunnel(s) to a positive
TORNIER ARTHROTUNNELER TUNNELPRO SYSTEM Transosseous Rotator Cuff Repair SURGICAL TECHNIQUE ARTHROTUNNELER TUNNELPRO SYSTEM ARTHROTUNNELER Surgical Technique Step 1. Drill medial tunnel(s) to a positive
DISCOVER NEW HORIZONS IN FLUID DRAINAGE. Bringing Safety and Convenience to Fluid Drainage Management
 DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
eptfe Vascular Graft Instructions for Use
 TM eptfe Vascular Graft Instructions for Use R Instructions For Use Caution: Federal (U.S.A) law restricts this device to sale by or on the order of a physician. Device Description, Indications, Contraindications,
TM eptfe Vascular Graft Instructions for Use R Instructions For Use Caution: Federal (U.S.A) law restricts this device to sale by or on the order of a physician. Device Description, Indications, Contraindications,
Tibial Fixation. with TunneLoc Device. Surgical Technique by Mark J. Albritton, M.D. and Sherwin Ho, M.D.
 Tibial Fixation with TunneLoc Device Surgical Technique by Mark J. Albritton, M.D. and Sherwin Ho, M.D. Table of Contents Surgical Technique... 4 Ordering Information... 11 Indications For Use... 12 Contraindications...
Tibial Fixation with TunneLoc Device Surgical Technique by Mark J. Albritton, M.D. and Sherwin Ho, M.D. Table of Contents Surgical Technique... 4 Ordering Information... 11 Indications For Use... 12 Contraindications...
RIGIDfix. Soft Tissue. Surgical Technique for Mitek RIGIDfix ACL Reconstruction PRODUCTS. Daniel J. McKernan, M.D. TISSUE SOFT.
 RIGIDfix A C L C R O S S P I N S Y S T E M Daniel J. McKernan, M.D. Toledo, Ohio SOFT TISSUE Surgical Technique for Mitek RIGIDfix ACL Reconstruction Soft Tissue PRODUCTS SURGICAL TECHNIQUE RIGIDfix A
RIGIDfix A C L C R O S S P I N S Y S T E M Daniel J. McKernan, M.D. Toledo, Ohio SOFT TISSUE Surgical Technique for Mitek RIGIDfix ACL Reconstruction Soft Tissue PRODUCTS SURGICAL TECHNIQUE RIGIDfix A
Integra. TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE
 Integra TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE Table of Contents Description...2 Indications...2 Contraindications...2 Surgical Technique...4 Step 1 Surgical Approach...4 Step 2 Remove TenoGlide
Integra TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE Table of Contents Description...2 Indications...2 Contraindications...2 Surgical Technique...4 Step 1 Surgical Approach...4 Step 2 Remove TenoGlide
Desara Blue OV D I. Sling for Female Stress Urinary Incontinence. Instructions For Use
 Desara Blue OV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide M Manufactured by: Caldera Medical, Inc. 5171 Clareton
Desara Blue OV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide M Manufactured by: Caldera Medical, Inc. 5171 Clareton
BLOOD COLLECTION GUIDELINES
 I. Patient Identification Lee Memorial Health System Lee County, FL CLINICAL LABORATORY BLOOD COLLECTION GUIDELINES A. Inpatient / Outpatient with armband 1. When possible, ask patient to state their name
I. Patient Identification Lee Memorial Health System Lee County, FL CLINICAL LABORATORY BLOOD COLLECTION GUIDELINES A. Inpatient / Outpatient with armband 1. When possible, ask patient to state their name
Zimmer NexGen MIS Tibial Component. Cemented Surgical Technique IMAGE TO COME
 Zimmer NexGen MIS Tibial Component Cemented Surgical Technique IMAGE TO COME Zimmer NexGen MIS Tibial Component Cemented Surgical Technique 1 Zimmer NexGen MIS Tibial Component Cemented Surgical Technique
Zimmer NexGen MIS Tibial Component Cemented Surgical Technique IMAGE TO COME Zimmer NexGen MIS Tibial Component Cemented Surgical Technique 1 Zimmer NexGen MIS Tibial Component Cemented Surgical Technique
Integra. Silicone PIP Implant SURGICAL TECHNIQUE
 Integra Silicone PIP Implant SURGICAL TECHNIQUE Table of Contents Indications For Use...2 Contraindications...2 Warnings and Precautions...2 Surgical Technique Preoperative Assessment... 3 Step 1: Initial
Integra Silicone PIP Implant SURGICAL TECHNIQUE Table of Contents Indications For Use...2 Contraindications...2 Warnings and Precautions...2 Surgical Technique Preoperative Assessment... 3 Step 1: Initial
SYNTHECEL Dura Repair. Assurance and Versatility for Dura Reconstruction.
 SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction. SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice
SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction. SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice
Meshes. Meshes. Non-absorbable meshes. Absorbable meshes
 Meshes Meshes Non-absorbable meshes Absorbable meshes Non-absorbable meshes hernia Premilene Mesh Premilene Mesh Plug Optilene Mesh Optilene Mesh LP Optilene Mesh Elastic Omyra Mesh Non-absorbable meshes
Meshes Meshes Non-absorbable meshes Absorbable meshes Non-absorbable meshes hernia Premilene Mesh Premilene Mesh Plug Optilene Mesh Optilene Mesh LP Optilene Mesh Elastic Omyra Mesh Non-absorbable meshes
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Per-Q-Cath* PICC Catheters with Excalibur Introducer* System
 Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Look For These Other Products by PARÉ Surgical, Inc. INNOVATIVE MEDICAL DEVICES
 Look For These Other Products by PARÉ Surgical, Inc. INNOVATIVE MEDICAL DEVICES Quik-Stitch-Endoscopic Suturing Device Disposable & Reusable The giflx- Disposable Rat tooth Jaw grasping Forceps with Ratcheting
Look For These Other Products by PARÉ Surgical, Inc. INNOVATIVE MEDICAL DEVICES Quik-Stitch-Endoscopic Suturing Device Disposable & Reusable The giflx- Disposable Rat tooth Jaw grasping Forceps with Ratcheting
