Customized Cranial and Craniofacial Implants. Instructions For Use. 7000revD_Instructions_For_Use. Page 1 of 8
|
|
|
- Austen Hudson
- 5 years ago
- Views:
Transcription
1 Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8
2 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL INC. CUSTOMIZED CRANIAL/CRANIOFACIAL IMPLANT... 4 POTENTIAL ADVERSE REACTIONS... 5 INTRAOPERATIVE AND EARLY POSTOPERATIVE COMPLICATIONS CAN INCLUDE:... 5 LATE POSTOPERATIVE COMPLICATIONS CAN INCLUDE:... 5 INSPECTION... 5 CLEANING... 6 STERILIZATION... 6 RECOMMENDATIONS FOR STEAM STERILIZATION... 6 STORAGE... 6 DO NOT USE IF THE PACKAGE IS OPEN OR DAMAGED... 6 MAGNETIC RESONANCE IMAGING... 6 CAUTION... 6 SYMBOLS CONTAINED IN DEVICE LABELING... 7 NOTES... 7 INFORMATION... 8 Page 2 of 8
3 INDICATIONS Patient Specific Implants are intended for the replacement of bony voids in the cranial/craniofacial skeleton. DESCRIPTION OF DEVICE The Kelyniam Global Inc. customized cranial/craniofacial implants are a non-load bearing, single use device and are: Manufactured using Polyether ether ketone (PEEK) implantable grade materials (i.e. PEEK-OPTIMA or Vestakeep i4 ). Designed individually for each patient to correct defects in cranial/facial bone. Individually sized and shaped implantable prosthetic cranioplasty plates intended to fill defects in a specific patient s cranial skeleton. Fabricated using the patient s CT imaging data. Provided non-sterile for sterilization prior to implantation. Attached to the native bone using commercially available, non-biodegradable fixation screws or any commercially available cranioplasty fixation system. CONDITIONS OF USE CAUTION - The Kelyniam Global Inc. customized cranial/craniofacial implants should only be implanted by surgeons who are fully trained and licensed in the use of such implants and the required specialized cranial surgical techniques. CONTRAINDICATIONS This device is contraindicated under any of the following conditions: Infection and sepsis; Degenerative bone disease which would render the device or the treatment unjustifiable; Distant foci of infection which can spread to the implant site; Uncooperative patients or patients with neurologic or psychiatric/psychological dysfunction who are incapable or unwilling to follow postoperative instructions. Page 3 of 8
4 KELYNIAM GLOBAL INC. CUSTOMIZED CRANIAL/CRANIOFACIAL IMPLANT These devices are used for augmenting and contouring bone. They are not intended or designed for full or partial load bearing. Do not use these devices for replacement of bone within articulating surfaces. Patients who engage in contact sports or other activities that risk facial injury are to be warned that facial injury may lead to damage of the implant and a subsequent failure of treatment. The patient is to be warned that the device does not replace normal healthy bone and that traumatic injury could necessitate surgical treatment. The patient must be advised of surgical risks and the possible adverse effects. CAUTION - This device has been designed to fit the defect existing at the time of the CT Scan and implant fabrication. changes in the patient s anatomy occurring after the CT Scan as well as the use of the implant after such changes may result in a sub-optimal fit within the defected area. Improper selection, placement, positioning, and fixation of the Kelyniam Global Inc. Customized Cranial/Craniofacial Implant can cause a subsequent undesirable result. The surgeon is to be familiar with the implant and the surgical procedure prior to performing the surgery. If a commercially available, non-biodegradable fixation system are to be used, a minimum of 3 fixation points is recommended. The screw holes must be pre-drilled (away from the surgical site) and should be at least 4mm from the edge of the implant. The screws should not protrude past the underside of the implant to avoid tissue damage. Due to the customized nature of the implant, the appropriate selection of FDA approved fixation devices are left to the surgeon s discretion. All instructions for fixation (drilling, tapping of holes, and insertion of screws) should be followed by FDA approved commercially available, non-biodegradable manufacturer s instructions and specifications. Optional pre-surgical models may have been provided to allow for detailed orientation and inspection of form, fit, & function (proper alignment and orientation of the device). Intra-operative shaping and sizing of the implant can be critical to the cosmetic success of the procedure. Improper shape and size can result in a noticeable undesirable prominence or possible disfigurement at or near the implant site. All intra-operative shaping and sizing should be performed away from the surgical site. Kelyniam Customized Cranial/Craniofacial Implants placed, positioned and fixated over or near air containing sinuses could result in infection. Kelyniam Global Inc. Customized Cranial/Craniofacial Implants are not recommended for patients that have had radiation therapy. Should the surgeon determine that use of this implant is necessary and appropriate for a patient that has had previous radiation therapy; the surgeon should be familiar with all applicable surgical techniques. To prevent dehiscence at the incision site, a firm primary closure of the incision is required. If the surgeon deems necessary, the re-shaping, sizing, or contouring of the Kelyniam Global Inc. Customized Cranial/Craniofacial Implant, it is best accomplished using high-speed rotating instruments. After the implant has been shaped, they must be rinsed in sterile saline to remove any loose particles. Particularly in instances where implants are shaped, intra-operative damage to the Page 4 of 8
5 molded implant may occur. It is recommended that the implant be examined for damage or disfigurement prior to implantation. Pediatric use is not recommended. Rapid remodeling of the pediatric skull may cause dehiscence of the incision, prominence or disfigurement at the implant site, or related complications that could result in the need to remove the implant. The surgeon should weigh the risks versus benefits when deciding to remove the implant. Implant removal should be followed by adequate postoperative management. Do not reuse Kelyniam Global Inc. Customized Cranial/Craniofacial Implants. Discard any unused portion. The device must be sterilized prior to use per ANSI/AAMI ST79. Surgical models, if provided, are to be used for pre-operative planning analysis only and are not intended to come in contact with the Kelyniam Customized Cranial/Craniofacial Implants or enter the Operating Room. POTENTIAL ADVERSE REACTIONS While rare, implantation of foreign materials may result in sensitivity reactions. Peripheral neuropathies have been reported in conjunction with surgical procedures involving implantation of various types of devices. Sub-clinical nerve damage occurs more frequently, usually as a result of surgical exposure/trauma. Kelyniam Global Inc. Customized Cranial/Craniofacial Implants can loosen/migrate due to loss of fixation or trauma. Infection can lead to failure of the procedure. INTRAOPERATIVE AND EARLY POSTOPERATIVE COMPLICATIONS CAN INCLUDE: Fracture of the implant; Fracture of bone or soft tissue damage; Dehiscence of the incision; Prominence or disfigurement at the implant site; and Infection. LATE POSTOPERATIVE COMPLICATIONS CAN INCLUDE: Fracture of the device due to traumatic injury; Loosening or migration due to loss of fixation or trauma; and Prominence or disfigurement over time at or near the implant site. INSPECTION All implantable devices are shipped NON-STERILE from Kelyniam Global Inc. in a sealed polyethylene bag to be repackaged at the hospital prior to steam sterilization. The seals along with the entire pouch should be inspected to ensure they have not been tampered with or damaged during shipping. All devices must be checked to include a twelve (12) digit alpha-numeric case number which matches the case number on the product label. Page 5 of 8
6 CLEANING This implant was produced in a non-sterile cleanroom environment and has been manually cleaned using a 70% alcohol solution. The implant MUST BE sterilized before use. STERILIZATION The Kelyniam Customized Cranial/Craniofacial Implants are supplied clean but non-sterile and should be repackaged before steam sterilization. Prior to sterilization, the Kelyniam Customized Implant together with an FDA approved biological Indicator should be double pouched in FDA approved steam sterilization pouches. All materials used should be applicable to the method of sterilization to be performed. The recommended parameters listed below are based on Laboratory testing conducted to establish sterility. These are in accordance with ANSI/AAMI ST79 - Comprehensive Guide to Steam Sterilization and Sterility Assurance in Health Care Facilities RECOMMENDATIONS FOR STEAM STERILIZATION Minimum Exposure Method Cycle Time Minimum Exposure Temperature Drying Steam Gravity Displacement 10 minutes 132 C (270 F) 30 minutes Steam Pre-vacuum 4 minutes 132 C (270 F) 30 minutes Figure 7 - Sterilization Recommendations STORAGE Kelyniam Customized Cranial/Craniofacial Implants should be stored in a controlled environment at approximately 24 C (75 F) in accordance with AAMI ST79. DO NOT USE IF THE PACKAGE IS OPEN OR DAMAGED Following sterilization, the package should again be inspected to ensure the integrity of the packaging was maintained. Product not scheduled for immediate use should be stored in a cool dry place. MAGNETIC RESONANCE IMAGING The Kelyniam Customized Cranial/Craniofacial Implants described in this Instruction For Use (IFU) have not been evaluated for safety and compatibility in the MR environment by Kelyniam Global, Inc. These implants have not been tested for heating or migration in the MR environment by Kelyniam Global, Inc. **Kelyniam Global, Inc. shall not be liable for any data supplied by any third party.** CAUTION Federal (USA) law restrictions require these devices be sold by or on the order of a licensed physician. Page 6 of 8
7 SYMBOLS CONTAINED IN DEVICE LABELING REF Part Number Expiry Date Non-sterile LOT Part Number S/N Part Number Single Patient Use Prescription Manufactured By Instructions For Use Figure 7 - Symbols Referenced On Device Labeling. NOTES Page 7 of 8
8 INFORMATION For further information, please contact Customer Service at: Kelyniam Global Inc. 97 River Road, Suite A Canton, Connecticut Toll Free: (800) Fax: (501) info@kelyniam.com URL: Proudly Designed & Manufactured in Canton, Connecticut, USA Page 8 of 8
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Precautionary Statement ( )
 Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
Low Profile Neuro Plating System. Surgical Technique
 Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
SYNPOR POROUS POLYETHYLENE IMPLANTS. For craniofacial and orbital augmentation and reconstruction
 SYNPOR POROUS POLYETHYLENE IMPLANTS For craniofacial and orbital augmentation and reconstruction SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SYNPOR Porous Polyethylene Implants 2 Indications and
SYNPOR POROUS POLYETHYLENE IMPLANTS For craniofacial and orbital augmentation and reconstruction SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SYNPOR Porous Polyethylene Implants 2 Indications and
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
Cranial System. Neuro Plating and Screw System
 Cranial System Neuro Plating and Screw System Innovasis Cranial System INDICATIONS FOR USE The Innovasis Cranial System (ICS) is intended for use in selective trauma of cranial skeleton, cranial surgery
Cranial System Neuro Plating and Screw System Innovasis Cranial System INDICATIONS FOR USE The Innovasis Cranial System (ICS) is intended for use in selective trauma of cranial skeleton, cranial surgery
The following languages are included in this packet:
 OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
TABLE OF CONTENTS. ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2. Implant Specifications 3. Instrument Features 4
 Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
WristMotion Wrist Hemiarthroplasty System Instructions for Use
 WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
OPERATIVE TECHNIQUE. CONSTRUX Mini PTC. Mini PTC Spacer System
 OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM The following languages are included in this packet:
 ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
low ProfIle neuro PlaTIng system
 low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
INTELLIGENT SPINAL SYSTEM
 INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
D. The following factors are of extreme importance to the eventual success of the procedure.
 Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP
 RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
Flexible Fragment Fixation. Surgical Technique
 Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
METAL HEMI SYSTEM
 METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
Hip Resurfacing System
 Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE. This Package Insert is for product distributed in the US only.
 TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
ACP1 CERVICAL PLATE SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE II.
 I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
MEDPOR. Oral maxillofacial surgery
 MEDPOR Oral maxillofacial surgery MEDPOR biomaterial MEDPOR has been a trusted name in the industry since 1985, with hundreds of thousands of procedures performed, and hundreds of published clinical reports
MEDPOR Oral maxillofacial surgery MEDPOR biomaterial MEDPOR has been a trusted name in the industry since 1985, with hundreds of thousands of procedures performed, and hundreds of published clinical reports
Document No Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE
 Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
Headless Compession Screw 2.5 / 3.0
 SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Headless Compession Screw 4.5 / 6.5 SURGICAL TECHNIQUE. Titanium or Stainless Steel. Cannulated Headless Design. Multiple Thread Options.
 Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
BIOFOAM ANKLE SPACER BLOCK
 BIOFOAM ANKLE SPACER BLOCK 145782-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
BIOFOAM ANKLE SPACER BLOCK 145782-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
Cranial idtm. Cranial restoration. id SolutionsTM. Individually designed. Personalized care.
 Cranial idtm Cranial restoration id SolutionsTM Individually designed. Personalized care. id Solutions Individually designed cranial implants for the restoration of cranial defects. Our id Solutions, Cranial
Cranial idtm Cranial restoration id SolutionsTM Individually designed. Personalized care. id Solutions Individually designed cranial implants for the restoration of cranial defects. Our id Solutions, Cranial
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
Technique Guide. SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction.
 Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use
 Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE
 CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician.
 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH Mpact 3D Metal Implants and Augments 3D Metal INSTRUCTION FOR USE Important notice: the device(s) can
CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH Mpact 3D Metal Implants and Augments 3D Metal INSTRUCTION FOR USE Important notice: the device(s) can
STERILE IMPLANT PRODUCT INSERT PATIENT-FITTED TEMPOROMANDIBULAR JOINT RECONSTRUCTION PROSTHESIS SYSTEM
 STERILE IMPLANT PRODUCT INSERT PATIENT-FITTED TEMPOROMANDIBULAR JOINT RECONSTRUCTION PROSTHESIS SYSTEM CAUTION United States Federal Law restricts this device to sale by or on the order of a physician.
STERILE IMPLANT PRODUCT INSERT PATIENT-FITTED TEMPOROMANDIBULAR JOINT RECONSTRUCTION PROSTHESIS SYSTEM CAUTION United States Federal Law restricts this device to sale by or on the order of a physician.
CRANIAL RECONSTRUCTION SOLUTIONS
 CRANIAL RECONSTRUCTION SOLUTIONS CRANIAL RECONSTRUCTION SOLUTIONS Your partner of CHOiCE at depuy Synthes CMF, we are dedicated to providing solutions for your individual patient needs. We do this through
CRANIAL RECONSTRUCTION SOLUTIONS CRANIAL RECONSTRUCTION SOLUTIONS Your partner of CHOiCE at depuy Synthes CMF, we are dedicated to providing solutions for your individual patient needs. We do this through
REDUCT Headless Compression Screw System
 REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Asnis. Micro Cannulated screw system. Xpress operative technique
 Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
Duraloc CONSTRAINED LINER
 SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
Surgical Technique Guide
 Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Gallery Laminoplasty Spine System
 Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM
 ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM 150884-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM 150884-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
Metallic Internal Fixation Devices The following languages are included in this packet:
 Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
JuggerLoc Bone-to-Bone System for Ankle Syndesmosis Fixation. Surgical Technique
 JuggerLoc Bone-to-Bone System for Ankle Syndesmosis Fixation Surgical Technique Table of Contents Position and Preparation... 2 Incision... 2 Fracture Reduction... 2 Drill Fibula and Tibia... 3 JuggerLoc
JuggerLoc Bone-to-Bone System for Ankle Syndesmosis Fixation Surgical Technique Table of Contents Position and Preparation... 2 Incision... 2 Fracture Reduction... 2 Drill Fibula and Tibia... 3 JuggerLoc
Technique Guide Wrist Hemiarthroplasty System
 Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
C-THRU Anterior Spinal System
 C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
Important Information on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only
 0434 Page 1 of 6 Instructions use Important Inmation on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: Atlas Hip Fracture (HF) Nail is an intramedullary interlocking
0434 Page 1 of 6 Instructions use Important Inmation on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: Atlas Hip Fracture (HF) Nail is an intramedullary interlocking
Olecranon Locking Plate II
 INDEX Indications Patient Position Fracture Reduction and Fixation Surgical Technique Step 1 Surgical Approach Step 2 Implantation Step 3 Proximal Locking Screw Insertion Step 4 Distal Screw Insertion
INDEX Indications Patient Position Fracture Reduction and Fixation Surgical Technique Step 1 Surgical Approach Step 2 Implantation Step 3 Proximal Locking Screw Insertion Step 4 Distal Screw Insertion
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Visit Linvatec.com today to learn more. Surgical Technique: Sequential Meniscal Running Stitch
 ORDERING INFORMATION Sequent Meniscal Repair System MR004S MR007S MR004C MR007C SC047D Straight Needle, 4 Implants Straight Needle, 7 Implants Curved Needle, 4 Implants Curved Needle, 7 implants Sequent
ORDERING INFORMATION Sequent Meniscal Repair System MR004S MR007S MR004C MR007C SC047D Straight Needle, 4 Implants Straight Needle, 7 Implants Curved Needle, 4 Implants Curved Needle, 7 implants Sequent
Technique Guide. Orthodontic Bone Anchor (OBA) System. Skeletal implants for the orthodontic movement of the teeth.
 Technique Guide Orthodontic Bone Anchor (OBA) System. Skeletal implants for the orthodontic movement of the teeth. Table of Contents Introduction Orthodontic Bone Anchor (OBA) System 2 Indications and
Technique Guide Orthodontic Bone Anchor (OBA) System. Skeletal implants for the orthodontic movement of the teeth. Table of Contents Introduction Orthodontic Bone Anchor (OBA) System 2 Indications and
Dentoalveolar Bone Fixation System. Bone fixation sets for the office.
 Dentoalveolar Bone Fixation System. Bone fixation sets for the office. Compact Modular Customized Dentoalveolar Bone Fixation System. Bone fixation sets for the office. Who we are Synthes is a global medical
Dentoalveolar Bone Fixation System. Bone fixation sets for the office. Compact Modular Customized Dentoalveolar Bone Fixation System. Bone fixation sets for the office. Who we are Synthes is a global medical
Technique Guide Hip Resurfacing System
 Technique Guide Hip Resurfacing System Approximately 5%-18% of all hip arthroplasties are completed on patients with a primary diagnosis of osteonecrosis. Patients are generally younger adults age 35 years
Technique Guide Hip Resurfacing System Approximately 5%-18% of all hip arthroplasties are completed on patients with a primary diagnosis of osteonecrosis. Patients are generally younger adults age 35 years
nvp Posterior Lumbar Interbody Fusion System
 nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvt Transforaminal Lumbar Interbody Fusion System
 nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
MetaFix Ludloff Plate
 Merete MetaFix Ludloff Plate Low Profile Locking Bone Plate System Surgical Technique and Ordering Information - Content - Content 1. Description.................................................. 3 2.
Merete MetaFix Ludloff Plate Low Profile Locking Bone Plate System Surgical Technique and Ordering Information - Content - Content 1. Description.................................................. 3 2.
Table of Contents.
 surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
Apache Cervical Interbody Fusion Device. Surgical Technique. Page of 13. LC-005 Rev F
 LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
Instructions for use for Bone Plates, Bone Screws, Intramedullary Nails, Pins and Wires
 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
ALIGN Radial Head System INSTRUCTIONS FOR USE
 ALIGN Radial Head System INSTRUCTIONS FOR USE R x: For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may lead to patient
ALIGN Radial Head System INSTRUCTIONS FOR USE R x: For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may lead to patient
nva Anterior Lumbar Interbody Fusion System
 nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
CRANIOMAXILLOFACIAL. Rethinking Possibilities, Reshaping Lives
 CRANIOMAXILLOFACIAL Rethinking Possibilities, Reshaping Lives TABLE OF CONTENTS PREOPERATIVE PLANNING 5 DISTRACTOR IMPLANTATION 6 ACTIVATION WIRE REMOVAL 9 DISTRACTOR REMOVAL 10 INSTRUMENTS 12 ORGANIZER
CRANIOMAXILLOFACIAL Rethinking Possibilities, Reshaping Lives TABLE OF CONTENTS PREOPERATIVE PLANNING 5 DISTRACTOR IMPLANTATION 6 ACTIVATION WIRE REMOVAL 9 DISTRACTOR REMOVAL 10 INSTRUMENTS 12 ORGANIZER
PPS P I N P O S I T I O N I N G S Y S T E M GMK EFFICIENCY VERSION. Hip Knee Spine Navigation
 PPS P I N P O S I T I O N I N G S Y S T E M D ESIGNED FOR YOU BY YOU GMK EFFICIENCY VERSION Surgical Surgical Technique Hip Knee Spine Navigation MyKnee Surgical Technique Hip Knee Spine Navigation I N
PPS P I N P O S I T I O N I N G S Y S T E M D ESIGNED FOR YOU BY YOU GMK EFFICIENCY VERSION Surgical Surgical Technique Hip Knee Spine Navigation MyKnee Surgical Technique Hip Knee Spine Navigation I N
TABLE OF CONTENTS. Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview. Implants 3. Instruments 5. Surgical Technique 10
 Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
PediLoc Tibia Surgical Technique
 PediLoc Tibia PediLoc Tibia Surgical Technique The technique description herein is made available to the healthcare professional to illustrate the author s suggested treatment for an uncomplicated procedure.
PediLoc Tibia PediLoc Tibia Surgical Technique The technique description herein is made available to the healthcare professional to illustrate the author s suggested treatment for an uncomplicated procedure.
Instructions for Use DENTAL IMPLANTS
 HAHN TAPERED IMPLANT GUIDED SURGERY SYSTEM Instructions for Use English General Information IMPORTANT INFORMATION PLEASE READ Caution: U.S. federal law restricts this device to sale by, or on the order
HAHN TAPERED IMPLANT GUIDED SURGERY SYSTEM Instructions for Use English General Information IMPORTANT INFORMATION PLEASE READ Caution: U.S. federal law restricts this device to sale by, or on the order
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
OBSOLETE. Visit for the latest version. Ankle Plating System 3 PKGI-79-B EFFECTIVE
 Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
Hinged Laminoplasty System surgical technique
 BlackbirdTM Hls Hinged Laminoplasty System surgical technique Blackbird TM Hls The ChoiceSpine Blackbird Hinged Laminoplasty System (HLS) design eliminates fitting plates through trial and error bending.
BlackbirdTM Hls Hinged Laminoplasty System surgical technique Blackbird TM Hls The ChoiceSpine Blackbird Hinged Laminoplasty System (HLS) design eliminates fitting plates through trial and error bending.
HawkeyeTM Peek. surgical technique
 HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
Mini External Fixator
 Stabilize the Phalanges and Metacarpals Mini External Fixator Surgical Technique Table of Contents Introduction Mini External Fixator 2 Indications 4 Surgical Technique Technique Overview 5 Product Information
Stabilize the Phalanges and Metacarpals Mini External Fixator Surgical Technique Table of Contents Introduction Mini External Fixator 2 Indications 4 Surgical Technique Technique Overview 5 Product Information
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
Ceramic Femoral Heads and Acetabular Cup Liners
 Important Information: Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution: Federal (U.S.A) law restricts this device to sale by or on the
Important Information: Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution: Federal (U.S.A) law restricts this device to sale by or on the
ACETABULAR CUP SURGICAL TECHNIQUE
 ACETABULAR CUP SURGICAL TECHNIQUE ACETABULAR CUP DEVICE INDICATIONS FOR USE The ICONACY I-Hip total hip replacement is indicated for the following conditions: 1. A severely painful and/or disabled hip
ACETABULAR CUP SURGICAL TECHNIQUE ACETABULAR CUP DEVICE INDICATIONS FOR USE The ICONACY I-Hip total hip replacement is indicated for the following conditions: 1. A severely painful and/or disabled hip
EVOS MINI with IM Nailing
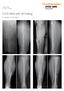 Case Series Dr. John A. Scolaro EVOS MINI with IM Nailing A series of studies Introduction Intramedullary nailing has become the standard for many long bone fractures. Fracture reduction prior to nail
Case Series Dr. John A. Scolaro EVOS MINI with IM Nailing A series of studies Introduction Intramedullary nailing has become the standard for many long bone fractures. Fracture reduction prior to nail
The following languages are included in this packet:
 DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Technique Guide. 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system.
 Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
NEODENT GUIDED SURGERY
 NEODENT GUIDED SURGERY MANUAL Grand Morse Connection Helix Implant GRAND POSSIBILITIES WITH A LIMITLESS SOLUTION. GRAND MORSE NEODENT GUIDED SURGERY. 4 5 6 CONTENTS 1. CLINICAL STEP BY STEP OF GRAND
NEODENT GUIDED SURGERY MANUAL Grand Morse Connection Helix Implant GRAND POSSIBILITIES WITH A LIMITLESS SOLUTION. GRAND MORSE NEODENT GUIDED SURGERY. 4 5 6 CONTENTS 1. CLINICAL STEP BY STEP OF GRAND
OPERATIVE TECHNIQUE. anterior cervical plating system
 OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation.
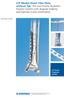 LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation. Technique Guide LCP Small Fragment System Table of Contents Introduction
LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation. Technique Guide LCP Small Fragment System Table of Contents Introduction
Technique Guide. Midface Distractor System. For the temporary stabilization and gradual lengthening of the cranial or midfacial bones.
 Technique Guide Midface Distractor System. For the temporary stabilization and gradual lengthening of the cranial or midfacial bones. Table of Contents Introduction Midface Distractor System 2 Indications
Technique Guide Midface Distractor System. For the temporary stabilization and gradual lengthening of the cranial or midfacial bones. Table of Contents Introduction Midface Distractor System 2 Indications
Page 1 of 7 Doc. No.: IU/15 Rev. No.: 05 Rev. Date:03MAY2018. Instructions for use for ATLAS Tibial Fracture(TF) Nail
 Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
KASM Knee Articulating Spacer Mold. Surgical Summary
 KASM Knee Articulating Spacer Mold Surgical Summary KASM Designing Surgeons: Michael H. Bourne, M.D. Salt Lake Orthopedic Clinic E. Marc Mariani, M.D. Salt Lake Orthopedic Clinic U.S. Patent 6,942,475
KASM Knee Articulating Spacer Mold Surgical Summary KASM Designing Surgeons: Michael H. Bourne, M.D. Salt Lake Orthopedic Clinic E. Marc Mariani, M.D. Salt Lake Orthopedic Clinic U.S. Patent 6,942,475
TRUMATCH PERSONALIZED SOLUTIONS with the SIGMA High Performance Instruments
 TRUMATCH PERSONALIZED SOLUTIONS with the SIGMA High Performance Instruments Resection Guide System SURGICAL TECHNIQUE RESECTION GUIDE SURGICAL TECHNIQUE The following steps are an addendum to the SIGMA
TRUMATCH PERSONALIZED SOLUTIONS with the SIGMA High Performance Instruments Resection Guide System SURGICAL TECHNIQUE RESECTION GUIDE SURGICAL TECHNIQUE The following steps are an addendum to the SIGMA
