Surgical Technique Guide
|
|
|
- Aleesha Atkins
- 5 years ago
- Views:
Transcription
1 Surgical Technique Guide Flow-FX, LLC Darvin Drive, Mokena, IL
2
3 Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique 5 1. Fracture Reduction 5 2. Screw Prep 5 3. Screw Placement 7 4. Flow-Screw - Delivery of Bone Void Filler 7 5. Close 8 Instruction for Use 9
4 Implant OVERVIEW Washer 13mm 6.5mm, Partially Threaded - Short mm (5mm Increments) 7.3mm, Partially Threaded - Short mm (5mm Increments) 6.5mm, Partially Threaded - Long mm (5mm Increments) 7.3mm, Partially Threaded - Long mm (5mm Increments) 6.5mm, Fully Threaded 20-60mm (5mm Increments) 7.3mm, Fully Threaded 20-60mm (5mm Increments) Flow-Screw 1
5 Features & BENEFITS The Biologics Era has officially begun, allowing dramatic alterations in orthopedic fracture care, just in time to address the steadily rising number of osteoporotic fractures as well as complex non-healing fractures. The patented Flow-Screw System with the Flow-Fx Side-Port Cannula enables precise delivery of bone void filler throughout the body. Cannulated, fenestrated screws available in 7.3mm, 6.5mm, 5.5mm, 4.5mm and 3.5mm in partially threaded and fully threaded versions. Aggressive thread and wide range of lengths make it appropriate for most situations. A capable stand-alone fixation system, Flow-Screw can work alongside other systems for improved outcomes. Flow-Screw 2
6 Surgical TECHNIQUE Cannulated Screw Sheath Parallel Guide-Wire Guide Multiple Guide-Wire Guide Angled Guide-Wire Guide Washer Sheath T-Handle Driver Handle Side-Port Cannula Flow-Screw 3
7 SPC Plunger Threaded Guide-wire Guide-Wire Guide Trocar Guide-Wire Guide Screw-Length Gauge Drill, Cannulated Tap, Cannulated Screw Driver Screw Driver Bolt Flow-Screw 4
8 Surgical TECHNIQUE Fracture Reduction 1. Reduce the fracture as required. Cannulated Screw Prep 2. Determine the screw diameter proper for the indication. (Some instruments vary with screw diameter) Sets include 3.5, 4.5, 5.5, 6.5, and 7.3mm diameter screws of varying lengths and thread length. Screw Diameter (mm) Guide-wire Diameter (mm) Pilot hole (mm) Guide-wire Drill Taps Cannula Incise for the cannulated screw. The incision will be approximately 2cm. Parallel Guide-Wire Guide Angle Guide Multiple Guide-Wire Guide 4. Install the cannulated screw guide-wire. Choose the correct guide-wire for the screw diameter. Choose the correct guide-wire guide for the application. Drive the guide-wire through the guide using a surgical drill with guide-wire attachment until the proper depth is reached (using fluoroscopy as needed). Remove the guide-wire guide when complete. Flow-Screw 5
9 Cannulated Screw Prep (cont) 5. Measure the cannulated screw length. Measure the distance from the tip of the guide-wire to the bone surface by sliding the cannulated screw length gauge over the guide-wire until it contacts the bone and reading the number adjacent to the back end of the guide-wire. Remove cannulated screw length gauge when complete. 6. Drill the cannulated screw. Attach the correct size drill bit to the surgical drill. Drill to the desired depth. Remove the cannulated screw drill. 7. Tap for the cannulated screw (optional). Attach the T-Handle to the correct size tap Tap over the guide-wire. Remove the tap. Flow-Screw 6
10 Surgical TECHNIQUE Cannulated Screw Placement 8. Install the cannulated Flow-Screw. Fasten the correct diameter and length cannulated Flow-Screw to the correct cannulated screw driver. Attach the Driver Handle (or the T-Handle) to the driver. Install the cannulated Flow-Screw. Remove the Handle (or optionally remove the entire driver/fastener). Remove the guide-wire. Washer Sheath Option Cannulated Flow Screw - Delivery of Bone Void Filler (BVF) 9. Prepare the BVF. The Flow-Screw can also be used for the delivery of injectable bone void fillers capable of being delivered via a 16 gauge needle. Prepare the BVF per the manufacturer s instructions. Attach BVF syringe to the side-port cannula when complete. Flow-Screw 7
11 Cannulated Flow Screw - Delivery of BVF (cont) 10. Dispense the BVF. Insert the side-port cannula into the driver/ fastener (or directly into the screw head cannulation). Slide the side-port cannula in until the tip is just past the void to be filled. The depth of the side-port cannula can be judged under imaging as the cannula is radiopaque. The BVF will flow out of the side-port cannula in all directions in the vicinity of the tip and into the void through the nearest fenestrations in the screw. 11. Plunge the BVF (5.5/6.5/7.3mm only). Remove the syringe and replace it with the side-port plunger. Dispense the BVF that remains in the cannula by pressing the plunger forward until it comes to the end of the cannula. Remove the side-port plunger and side-port cannula. Close 12. Remove the driver/fastener (if present). 13. Clean the BVF from the cannulated screw head and bone exterior. 14. Close. Flow-Screw 8
12 IFU 102 rev A Flow-Screw Cannulated Screw System Instructions for Use Description The Flow-FX Flow-Screw is designed as a variety of fractures and is composed of a cortical screw and accompanying instruments. The Flow- Screw can also be used for the delivery ofinjectable gauge needle. The Flow-Screw is not designed to be coupled to other metal implants. INDICATIONS fractures and bone reconstructions. When used for these indications, the Flow-Screw can also be used site to treat fractures throughout the skeletal anatomy as deemed appropriate by the surgeon. MATERIALS The Flow-Screw implant components are made of titanium alloy (Ti-6Al-4V) conforming to ASTM F136 or stainless steel conforming to ASTM F138 or ASTM F2229. CONTRAINDICATIONS conditions that retard healing (not including pathological fractures) and conditions causing poor blood supply. Foreign body sensitivity. Where material sensitivity is suspected, appropriate tests should be made and sensitivity ruled out prior to implantation. Any active or suspected latent infection or Compromised vascularity that would inhibit adequate blood supply to the fracture or the operative site. Material sensitivity documented or suspected. Patients having inadequate tissue coverage over the operative site. Implant utilization that would interfere with anatomical structures or physiological performance. Any mental or neuromuscular disorder which failure or complications in postoperative care. Other medical or surgical conditions which Use with Polymethyl methacrylate (PMMA) WARNINGS For professional use only. Do not use this system without fully reading the instructions for use. The surgeon should be familiar with the general principles and technique of treating fractures using cannulated screws. The surgeon should be familiar with the general principles and technique of delivering bone void designated in its labeling. Surgeons should follow the surgical technique. Selection of the correct screw diameter and length is very important. Determination of the alignment of the fractured bones should be done load bearing prior to good callus formation. Patients who are either noncompliant or predisposed to delayed union or non-union, must have auxiliary support. Periodic x-rays are recommended for at least six months to detect any changes in position, nonunion, loosening, bending or cracking of components. Patients should be cautioned that even after complete healing there is a higher risk for refractures while the screw is in position. Postoperative care and physical therapy should be structured to prevent excessive loading of the operated extremity. The implant system has not been evaluated for safety and compatibility in the MR environment, nor has it been tested for heating or migration in the MR environment. Most parts, excepting specially packaged disposable kits, are delivered non-sterile. These must be sterilized prior to surgery in accordance with the Sterilization Section. attachment to the posterior elements (pedicles) of the cervical, thoracic, or lumbar spine. Flow-Screw implants should only be used for validated for use with the device. PRECAUTIONS/INSPECTION The Flow-Screw implants, the guide-wire and the Side Port Cannula for the application of Port Cannula and Guidewire will be disposed of in accordance with local requirements. The implants are intended to remain in the patient. The Flow-Screw Instrument set, with exception of the Side Port Cannula and Trocar-Tipped Guide-Wire, is intended to be reusable and will be cleaned and sterilized in accordance with the Sterilization Section. The Side Port Cannula has been demonstrated to survive 5 steam sterilization cycles but should be examined for structural integrity before use. Proper handling and storage of the system components is mandatory. Damage or alterations may produce stresses and cause defects, which could become the focal point for failure. All implants and instruments must be inspected prior to use for any damage. Do not use if damage exceeds normal standards for implants and instruments. ADVERSE EFFECTS The adverse events include but are not limited to: Implants may migrate, cause pain or stress shield bone even after a fracture has healed. Pain, discomfort, nerve or soft tissue damage etc. Metal sensitivity or allergies. Trauma. A pulmonary embolism may result from using this implant with an injectable bone STERILITY The Side Port Cannula and the Trocar-Tipped Guide-Wires are single use instruments and should be discarded after each use. Cleaning Scope These cleaning instructions apply to the orthopedic surgical implants and instruments used to prepare the bone and implant the Flow-Screw Cannulated Screw implant set. The Side Port Cannula and the Trocar-Tipped Guide-Wires are single use instruments and should be discarded after each use. Both the Implants and the Instruments are contained in the same case/tray. The Implants are further contained within the case inascrew caddy. The intent is to clean the implants within their caddy separately from the instruments. Implant cleaning may be automated. Instruments are recommended to be cleaned manually. Warnings and Limitations A thorough, manual cleaning process is recommended. Cleaning agents with chlorine or chloride as the active ingredient are corrosive to stainless steel and must not be used. Enzymatic and cleaning Flow-Screw 9
13 agents with neutral ph are recommended. Repeated processing, according to these reusable manual instruments. End oflife is normally determined by wear and damage due to use. Instructions at Point of Use Separate any nested instruments and disassemble any instruments that can be disassembled without using tools. There are no instruments that will require disassembly using tools. Please refer to the surgical tray graphics for the expected state of disassembly. a disposable, non-shedding wipe and cover with a damp cloth or submerge in a basin of not be allowed to dry on instruments prior to cleaning. Containment/Transportation Universal precautions for handling contaminated/bio-hazardous materials should be observed. Instruments and implants should be cleaned within 30 minutes of use to minimize the potential for drying prior to cleaning. Manual Cleaning Procedure 1. Prepare neutral ph enzyme and cleaning agents at the use-dilution and temperature recommended by the manufacturer. 2. Completely submerge the instrument in enzyme solution and allow it to soak for 20 minutes. Use a soft-bristled brush to gently clean the device (paying close attention to all threads, crevices and other hard-to-clean areas) until all visible soil has been removed. Any instruments with lumens or cannulas should be cleaned with a long, narrow, softbristled brush (i.e. pipe cleaner brush). The enzyme solution should be changed when it becomes grossly contaminated. 3. Remove the device from the enzyme one or any combination of the following distilled) for a minimum of 3 minutes. 4. Prepare the neutral ph cleaning (detergent) solution and place in a sonication unit. 5. Completely submerge device in cleaning solution and sonicate for 10 minutes, preferably at khz. one or any combination of the following distilled) thoroughly for at least 3 minutes or until there is no sign of blood or soil in the rinse stream. 7. Repeat steps 5 and 6 with freshly prepared cleaning solution until the instruments are thoroughly clean. 8. Dry instrument with a clean, disposable, absorbent, non-shedding wipe. Automated Cleaning Procedure Automated washer/disinfector systems are not recommended as the sole cleaning method for complex surgical instruments. These instruments should be cleaned following the manual cleaning procedure above. An automated system may be used as a follow-up method but is not required. Automated washer/disinfector systems are recommended for cleaning the Flow-Screw automated cleaning of the screw caddy, remove the tray cover, remove the removable screw caddies. Disinfection Disinfection is only acceptable as an adjunct to full sterilization for reusable surgical instruments. See sterilization section below. Inspection/Function Testing 1. Carefully inspect each instrument and implant to ensure that there is no visible contamination and that all visible blood and soil has been removed. If visible contamination is evident, repeat the Manual Cleaning Procedure above. 2. Visually inspect for damage and/or wear, corrosion, pitting, discoloration or separation of parts. If there is any evidence of damage, do not use the instrument or implant. 3. Check the action of moving parts (such as hinges and box-locks) to ensure smooth operation throughout the intended range of motion. 4. Check instruments with long slender features (particularly rotating instruments) for distortion. 5. When instruments form part of a larger assembly, check that the devices assemble readily with mating components.. Inspection/Function Testing Failure If an instrument fails inspection or a functional test due to improper cleaning, repeat the cleaning procedure. If the instrument continues to fail after repeated cleanings, discard the instrument. If an instrument fails inspection or a functional test due to wear or damage, discard the instrument. Maintenance Lubricate hinges, threads and other moving parts with a commercial water-based surgical grade instrument lubricant (such as instrument milk) to reduce friction and wear. Packaging 1. Individually a standard polyethylene/tyvek (or equivalent) sterilization pouch of the appropriate size may be used for single instruments. Ensure that the pack is large enough to contain the instrument without stressing the seals or tearing the packaging. 2. In Sets - sets of instruments may be loaded into dedicated instrument trays or general purpose sterilization trays for sterilization. If applicable, use standard medical grade steam sterilization wrap following the AAMI wrap method (ANSI/ AAMI ST ). Sterilization Scope Both the implants within their caddies and the instruments are intended to be sterilized together in their case/tray The following steam sterilization method should be performed: PROCEDURE FRACTIONATED VACUUM PROCEDURE Exposure Time 4 minutes Temperature 132 C Drying Time 30 minutes STORAGE INSTRUCTIONS 1. Implants and instruments should be stored at room temperature. 2. Sterile, packaged instruments should be stored in a designated, limited access area that is well ventilated and provides protection from dust, moisture, insects, vermin, and temperature and humidity extremes. 3. Sterile instrument packages should be examined closely prior to opening to ensure that there has been no loss of package integrity. These instructions have been validated by Flow-FX, LLC as being capable of preparing manual orthopaedic surgical instruments for re-use. It is the responsibility of the reprocessor to ensure that reprocessing is performed using the appropriate equipment and materials, and those personnel in the reprocessing facility have been adequately trained in order to achieve the desired result. Any deviation by the reprocessor from these instructions should be properly consequences. CAUTION Federal law restricts this device to sale by or on order of a physician. MANUFACTURER CONTACT INFORMATION Flow-FX, LLC Darvin Dr. Mokena, Il Phone: (815) Flow-Screw 10
14
15 Patented - Flow-FX, LLC Darvin Drive Mokena, IL P by Flow-FX, LLC. Products referenced with TM are trademarks of Flow-Fx.
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Asnis. Micro Cannulated screw system. Xpress operative technique
 Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
Spartan S 3 Facet Screw System PACKAGE INSERT
 LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
SI-BONE ifuse Implant System Instruments Hospital Cleaning and Sterilization Instructions 0344
 HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
Reprocessing Guide. Autoclavable Arthroscope and Hardware Sterilization Tray
 Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Zeus Intervertebral Body Fusion Devices PACKAGE INSERT
 LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
OBSOLETE. Visit for the latest version. Ankle Plating System 3 PKGI-79-B EFFECTIVE
 Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Lapidus Arthrodesis System
 Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
Headless Compession Screw 2.5 / 3.0
 SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Headless Compession Screw 4.5 / 6.5 SURGICAL TECHNIQUE. Titanium or Stainless Steel. Cannulated Headless Design. Multiple Thread Options.
 Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON
 ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Spider Cervical Plating System
 X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
Integra Total Foot System 2
 Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
REDUCT Headless Compression Screw System
 REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System
 X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
2. Active systemic infection or infection localized to the site of the proposed implantation is contraindications to implantation.
 OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
Zimmer Anterior Buttress Plate System. Surgical Technique
 Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System
 X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
X-spine Systems, Inc. Certex Spinal Fixation System
 X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
DARCO MIS FOREFOOT SCREW
 DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
Surgical Technique & Product Catalogue. Guide for Open & MIS Procedures
 Surgical Technique & Product Catalogue Guide for Open & MIS Procedures INTRODUCTION The VIPER Cortical Fix Fenestrated Screw System is the first pedicle screw implant to offer enhanced fixation in both
Surgical Technique & Product Catalogue Guide for Open & MIS Procedures INTRODUCTION The VIPER Cortical Fix Fenestrated Screw System is the first pedicle screw implant to offer enhanced fixation in both
TTA Wedge System INSTRUCTIONS FOR USE
 TTA Wedge System INSTRUCTIONS FOR USE 1 INSTRUCTIONS FOR USE The OssAbility TTA Wedge System consists of the following products: Wedge Implants Osteotomy Guide Advancement Levers Osteotomy Planning Overlay
TTA Wedge System INSTRUCTIONS FOR USE 1 INSTRUCTIONS FOR USE The OssAbility TTA Wedge System consists of the following products: Wedge Implants Osteotomy Guide Advancement Levers Osteotomy Planning Overlay
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
3.5 mm LCP Olecranon Plates
 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Olecranon Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Olecranon Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications
The following languages are included in this packet:
 DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
X-spine Systems, Inc. Fixcet Spinal Facet Screw System
 X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
ACP1 CERVICAL PLATE SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE II.
 I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
Zavation Z-Link Cervical
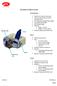 Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE
 OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
Surgical Technique Guide
 Sacroiliac Joint Fusion System Surgical Technique Guide Moving Life Forward Table of Contents SiCure Implant Overview...2 SiCure System Information...3 X-ray Basics...4 Patient Positioning....5 Surgical
Sacroiliac Joint Fusion System Surgical Technique Guide Moving Life Forward Table of Contents SiCure Implant Overview...2 SiCure System Information...3 X-ray Basics...4 Patient Positioning....5 Surgical
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE
 English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
MetaFix Ludloff Plate
 Merete MetaFix Ludloff Plate Low Profile Locking Bone Plate System Surgical Technique and Ordering Information - Content - Content 1. Description.................................................. 3 2.
Merete MetaFix Ludloff Plate Low Profile Locking Bone Plate System Surgical Technique and Ordering Information - Content - Content 1. Description.................................................. 3 2.
Cervical Screw System
 Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
The Omega LIF System devices are made from Titanium alloy Ti6Al4V ELI, ASTM F136.
 Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
PediLoc 3.5mm and 4.5mm Contour Femur Plate Surgical Technique
 PediLoc 3.5mm and 4.5mm Contour Femur Plate Surgical Technique Surgical Technique Contour Femur Plate The technique description herein is made available to the healthcare professional to illustrate the
PediLoc 3.5mm and 4.5mm Contour Femur Plate Surgical Technique Surgical Technique Contour Femur Plate The technique description herein is made available to the healthcare professional to illustrate the
3.5 mm Locking Attachment Plate
 For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
Velocity Orthopedics Instrument Cleaning and Sterilization
 Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
INTELLIGENT SPINAL SYSTEM
 INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
Endoscopic Technology. Operating Manual. Cannula system
 Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
Page 1 of 7 Doc. No.: IU/15 Rev. No.: 05 Rev. Date:03MAY2018. Instructions for use for ATLAS Tibial Fracture(TF) Nail
 Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
AxSOS Locking Plate System
 AxSOS Locking Plate System Operative Technique Small Fragment Basic Fragment 1 2 Contents Page 1. Introduction 4 2. Features & Benefits 5 4 and 5mm Compression Plates 5 Reconstruction and 1/3 Tubular Locking
AxSOS Locking Plate System Operative Technique Small Fragment Basic Fragment 1 2 Contents Page 1. Introduction 4 2. Features & Benefits 5 4 and 5mm Compression Plates 5 Reconstruction and 1/3 Tubular Locking
Preparation Instructions for the CAMLOG /CONELOG Implant System
 J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
Table of Contents.
 surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
Flexible Fragment Fixation. Surgical Technique
 Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Aesculap Implant Systems
 Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. ACDF Anterior Cervical Discectomy and Fusion
 SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
Gallery Laminoplasty Spine System
 Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Customized Cranial and Craniofacial Implants. Instructions For Use. 7000revD_Instructions_For_Use. Page 1 of 8
 Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
ATEC Porous Ti System
 ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
Amendia Interbody Fusion Devices PACKAGE INSERT
 LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE
 ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
PERFORATED CLICK X Augmentable pedicle screws for osteoporotic bone
 PERFORATED CLICK X Augmentable pedicle screws for osteoporotic bone Instruments and implants approved by the AO Foundation. This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE
PERFORATED CLICK X Augmentable pedicle screws for osteoporotic bone Instruments and implants approved by the AO Foundation. This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE
Technique Guide. 3.5 mm LCP Olecranon Plates. Part of the Synthes locking compression plate (LCP) system.
 Technique Guide 3.5 mm LCP Olecranon Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications 3 Clinical
Technique Guide 3.5 mm LCP Olecranon Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications 3 Clinical
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System
 X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
Straumann ProClean Cassette. Basic Information on the
 Straumann ProClean Cassette Basic Information on the Straumann ProClean Cassette Contents 1. Straumann ProClean Cassette Uncompromised hygiene 2 2. Straumann ProClean Cassette product at a glance 3 2.1
Straumann ProClean Cassette Basic Information on the Straumann ProClean Cassette Contents 1. Straumann ProClean Cassette Uncompromised hygiene 2 2. Straumann ProClean Cassette product at a glance 3 2.1
MICA SCREWS For additional information and translations please contact the manufacturer or local distributor.
 MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
OPERATIVE TECHNIQUE. anterior cervical plating system
 OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine.
 Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation
Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation
3.0 mm Cannulated Screw System. Surgical Technique
 3.0 mm Cannulated Screw System Surgical Technique 3 3.0 mm Cannulated Screw System Surgical Technique 3.0 mm Cannulated Screw System The 3.0 mm Cannulated Screw System is part of a series of cannulated
3.0 mm Cannulated Screw System Surgical Technique 3 3.0 mm Cannulated Screw System Surgical Technique 3.0 mm Cannulated Screw System The 3.0 mm Cannulated Screw System is part of a series of cannulated
The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental fixation is used.
 Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Cervical Spacer System surgical technique
 Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
AxSOS. Locking Plate System. Operative Technique. Small Fragment Basic Fragment
 AxSOS Locking Plate System Operative Technique Small Fragment Basic Fragment Stryker Plating Contents Page 1. Introduction 4 2. Features & Benefits 5 4 and 5 Compression Plates 5 Reconstruction and 1/3
AxSOS Locking Plate System Operative Technique Small Fragment Basic Fragment Stryker Plating Contents Page 1. Introduction 4 2. Features & Benefits 5 4 and 5 Compression Plates 5 Reconstruction and 1/3
3. Insert Tocar Sleeves Insert the NCB tissue protection sleeve assembly 1.6 to 10mm through a skin incision (Fig. 38).
 NCB Proximal Humerus Plating System Surgical Technique 19 2. Temporary Plate Fixation The plate can be temporary fixed to the bone with 1.6mm K-wire through the proximal cannulated fixation screw of the
NCB Proximal Humerus Plating System Surgical Technique 19 2. Temporary Plate Fixation The plate can be temporary fixed to the bone with 1.6mm K-wire through the proximal cannulated fixation screw of the
SACROILIAC JOINT FIXATION WITH SAMBA SCREW SYSTEM SURGICAL PROCEDURE MANUAL
 SACROILIAC JOINT FIXATION WITH TM SAMBA SURGICAL PROCEDURE MANUAL SAMBATM Contents: Introduction Indications Contraindications Warnings Potential Adverse Events Implant Device Overview Instrument Overview
SACROILIAC JOINT FIXATION WITH TM SAMBA SURGICAL PROCEDURE MANUAL SAMBATM Contents: Introduction Indications Contraindications Warnings Potential Adverse Events Implant Device Overview Instrument Overview
EXTERNAL FIXATION SYSTEMS The following languages are included in this packet:
 EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
SMV Scientific Bone Plate and Screw System Surgical Technique
 SMV Scientific Bone Plate and Screw System Surgical Technique Description: The SMV Scientific Bone Plate and Screw System consists of non-locking plates and bone screw fasteners in a variety of lengths,
SMV Scientific Bone Plate and Screw System Surgical Technique Description: The SMV Scientific Bone Plate and Screw System consists of non-locking plates and bone screw fasteners in a variety of lengths,
SURGICAL TECHNIQUE GUIDE: JONES FRACTURE USING THE PRECISION JONES FRACTURE SCREW SYSTEM
 PRODUCT DESCRIPTION The PRECISION Jones Fracture Screw System offers extensive options of Type II Anodized Titanium screws. System-specific instrumentation is designed to address procedural challenges
PRODUCT DESCRIPTION The PRECISION Jones Fracture Screw System offers extensive options of Type II Anodized Titanium screws. System-specific instrumentation is designed to address procedural challenges
Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac.
 Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac. This document is intended as guidance for decontamination and sterilization for medical
Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac. This document is intended as guidance for decontamination and sterilization for medical
Instructions for use for Bone Plates, Bone Screws, Intramedullary Nails, Pins and Wires
 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
Single-Thread Screw Available in 25-45mm lengths (5mm Increments) Dual-Thread Screw Available in 30-45mm lengths (5mm Increments)
 Single-Thread Screw Available in 25-45mm lengths (5mm Increments) Dual-Thread Screw Available in 30-45mm lengths (5mm Increments) 4.5mm diameter screws All screws cannulated 12mm percutaneous incision
Single-Thread Screw Available in 25-45mm lengths (5mm Increments) Dual-Thread Screw Available in 30-45mm lengths (5mm Increments) 4.5mm diameter screws All screws cannulated 12mm percutaneous incision
Cannulated Angled Blade Plate 3.5 and 4.5, 90.
 Cannulated Angled Blade Plate 3.5 and 4.5, 90. Technique Guide This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Table of Contents Introduction
Cannulated Angled Blade Plate 3.5 and 4.5, 90. Technique Guide This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Table of Contents Introduction
Technique Guide. 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system.
 Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
A U X I L I A R Y C O N N E C T O R S Surgical Technique
 A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
Visit Linvatec.com today to learn more. Surgical Technique: Sequential Meniscal Running Stitch
 ORDERING INFORMATION Sequent Meniscal Repair System MR004S MR007S MR004C MR007C SC047D Straight Needle, 4 Implants Straight Needle, 7 Implants Curved Needle, 4 Implants Curved Needle, 7 implants Sequent
ORDERING INFORMATION Sequent Meniscal Repair System MR004S MR007S MR004C MR007C SC047D Straight Needle, 4 Implants Straight Needle, 7 Implants Curved Needle, 4 Implants Curved Needle, 7 implants Sequent
SURGICAL TECHNIQUE GUIDE TRESTLE. Anterior Cervical Plating System
 SURGICAL TECHNIQUE GUIDE TRESTLE Anterior Cervical Plating System 2 SURGICAL TECHNIQUE GUIDE SURGICAL TECHNIQUE GUIDE System Features Large window enables visualization of graft site and end plates Screw
SURGICAL TECHNIQUE GUIDE TRESTLE Anterior Cervical Plating System 2 SURGICAL TECHNIQUE GUIDE SURGICAL TECHNIQUE GUIDE System Features Large window enables visualization of graft site and end plates Screw
PROXIMAL TIBIAL PLATE
 SURGICAL NÁSTROJE TECHNIQUE PRO ARTROSKOPII PROXIMAL INSTRUMENTS TIBIAL FOR PLATE ARTHROSCOPY Proximal Tibial Plate Description of medical device The Proximal Tibial Plate is used in epyphyseal and metaphyseal
SURGICAL NÁSTROJE TECHNIQUE PRO ARTROSKOPII PROXIMAL INSTRUMENTS TIBIAL FOR PLATE ARTHROSCOPY Proximal Tibial Plate Description of medical device The Proximal Tibial Plate is used in epyphyseal and metaphyseal
ACETABULAR CUP SURGICAL TECHNIQUE
 ACETABULAR CUP SURGICAL TECHNIQUE ACETABULAR CUP DEVICE INDICATIONS FOR USE The ICONACY I-Hip total hip replacement is indicated for the following conditions: 1. A severely painful and/or disabled hip
ACETABULAR CUP SURGICAL TECHNIQUE ACETABULAR CUP DEVICE INDICATIONS FOR USE The ICONACY I-Hip total hip replacement is indicated for the following conditions: 1. A severely painful and/or disabled hip
