Review Article Cutaneous Side Effects of BRAF Inhibitors in Advanced Melanoma: Review of the Literature
|
|
|
- Janis Sharp
- 5 years ago
- Views:
Transcription
1 Dermatology Research and Practice Volume 2016, Article ID , 6 pages Review Article Cutaneous Side Effects of BRAF Inhibitors in Advanced Melanoma: Review of the Literature Bilgen Gençler and Müzeyyen Gönül Department of Dermatology, Ministry of Health Diskapi Yildirim Beyazit Education and Research Hospital, Ankara, Turkey Correspondence should be addressed to Bilgen Gençler; bilgen16@gmail.com Received 30 November 2015; Revised 7 February 2016; Accepted 15 February 2016 Academic Editor: Jean Kanitakis Copyright 2016 B. Gençler and M. Gönül. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The incidence of melanoma has recently been increasing. BRAF mutations have been found in 40 60% of melanomas. The increased activity of BRAF V600E leads to the activation of downstream signaling through the mitogen-activated protein kinase (MAPK) pathway, which plays a key role as a regulator of cell growth, differentiation, and survival. The use of BRAF inhibitors in metastatic melanoma with BRAF mutation ensures clinical improvement of the disease. Vemurafenib and dabrafenib are two selective BRAF inhibitors approved by the Food and Drug Administration (FDA). Both drugs are well tolerated and successfully used in clinical practice. However, some adverse reactions have been reported in patients in the course of treatment. Cutaneous side effects are the most common adverse events among them with a broad spectrum. Both the case reports and several original clinical trials reported cutaneous reactions during the treatment with BRAF inhibitors. In this review, the common cutaneous side effects of BRAF inhibitors in the treatment of metastatic melanoma with BRAF V600E mutation were reviewed. 1. Introduction Melanoma is a lethal type of skin cancer that is derived from melanocytes. The incidence of melanoma has been increasing in recent decades and the mortality is approximately 10% [1]. Although the patients with early stage melanoma can be cured with surgery, the prognosis of patients with inoperable metastatic melanoma is poor, with a 5-year survival rate of <10% and a median survival of <1year[2]. Until recently, dacarbazine and interleukin-2 were the only agents approved by the Food and Drug Administration (FDA) for the standard treatment of metastatic melanoma [3]. The clinical development of targeted therapies of mitogen-activated protein kinase (MAPK) pathway is a milestone in the management of advanced melanoma. 2. MAPK Pathway The mitogen-activated protein kinase (MAPK) is an important signaling pathway that plays a key role as a regulator of cell growth, differentiation, and survival. When an extracellular ligand binds to specific plasma membrane receptor tyrosine kinase, a series of phosphorylation including RAS, RAF, MEK, and ERK mediates the growth signals to the nucleus to promote cell proliferation, differentiation, and survival [4]. The mutation of the MAPK pathway is the critical point at the pathogenesis of melanoma. BRAF mutations were found in approximately 40 60% of cutaneous melanomas andv600eisthemostcommontypeofthesemutations. It was shown that valine is substituted by glutamic acid at position 600 in 90% of BRAF mutant melanomas [5, 6]. The increased activity of BRAF V600E leads to the activation of downstream signaling through the MAPK pathway. Constitutive oncogenic signaling causes apoptosis prevention and excessive cell proliferation [6]. Additionally, BRAF mutations were associated with a poor prognosis in patients with metastatic disease [7]. 3. BRAF Inhibitors After the discovery of BRAF mutations, clinical trials of targeted therapies of advanced melanoma show significant improvement. The selective inhibitors of mutant BRAF
2 2 Dermatology Research and Practice kinase have become the key component of the treatment of metastatic disease. Vemurafenib and dabrafenib are two BRAF inhibitors (BRAFi) that have been licensed by FDA for the treatment of metastatic melanoma with mutant BRAF V600 [2]. Vemurafenib was the first selective tyrosine kinase inhibitor that demonstrated antitumor activity by blocking the activation of MAPK kinase pathway [2]. The antitumor activity of vemurafenib was observed in melanoma cell lines with BRAF V600E mutation, but not in wild-type melanomas [8]. Dabrafenib was the second reversible and potent selective inhibitor of BRAF V600 kinase approved by the FDA [2]. The use of BRAFi significantly increases the response rate, and prolonged progression-free and overall survival in melanoma patients with BRAF mutation [8, 9]. These oral agents are well tolerated, but some adverse events can occur due to paradoxical reactivation of MAPK signaling [10]. This review aimed to determine the most common cutaneous side effects duetobrafithathasbeenusedinadvancedmelanomas. 4. Cutaneous Side Effects Dermatologic reactions related to the treatment of BRAFi in advanced melanoma are well known common side effects. The rate of cutaneous adverse events associated with vemurafenib was 92% to 95% of patients in the BRAF inhibitor melanoma (BRIM) studies [11]. However, the cutaneous adverse events related to dabrafenib in BREAK studies were similar to those due to vemurafenib in BRIM studies, and the percentages of these side effects varied in both of the studies [9, 12, 13]. Skin reactions usually occur within days of undergoing treatment. Adverse events (AEs) can be classified in five grades: grades 1-2 as mild to moderate, grade 3 as severe adverse event, grade 4 as life-threatening adverse event, and grade 5 as fatal adverse event [14]. The most seen adverse events previously reported were grade 1 or 2, so patients could continue treatment without dose modifications [11]. Percentages of common (>5%) cutaneous adverse events with vemurafenib and dabrafenib treatment are shown in Tables 1 and Inflammatory Dermatoses. Skin rash was one of the most commonly reported AEs [11]. The incidence of skin rash was reported as 75% in the BRIM 2 study and 64% in the BRIM 3study,mostlywithgrade1or2severity[6,8,11].Itwas revealed in the BRIM 3 study that 10% of patients developed grade 2 skin rash and 8% of patients developed grade 3 skin rash [8]. Skin rash usually occurred on face, neck, trunk, and extremities and appeared with a mean time of 1.6 weeks after vemurafenib treatment. Many subtypes of rash were seen, but the most common was not otherwise specified with a range of 37% 54%. Maculopapular rash due to vemurafenib therapy was another clinical feature and linked to a hypersensitivity reaction. Serious cutaneous adverse events such as Stevens- Johnson syndrome, toxic epidermal necrolysis, cellulitis, drug reaction with eosinophilia, and systemic symptoms (DRESS) were rarely seen but could cause drug discontinuation [5, 11, 15, 16]. Table 1: Percentage of common (>5%) cutaneous adverse events with vemurafenib treatment. Adverse events Percentage (%) Verrucous papilloma/wart 22.2 [14] 79 [17] Rash [11] Photosensitivity 22.2 [14] 66.7 [18] Hand-foot skin reaction (PPD) 5.6 [14] 60 [17] Hair growth modification 45 [17] Actinic keratosis 40 [18] 44.4 [14] Alopecia 11.1 [14] 36 [6] Pruritus 29 [6] 33.3 [14] Xerosis 11.1 [14] 33 [17] Milia 26.7 [18] 31 [17] cscc and KA 22.2 [14] 26.7 [18] Panniculitis 14 [17] 16.7 [14] Keratosis pilaris 16.7 [14] Cheilitis 14 [17] BCC 13.3 [18] Nipple hyperkeratosis 12 [17] Nevi changes 5.6 [14] 10 [17] PPD: palmar-plantar dysesthesia; cscc: cutaneous squamous cell carcinoma; KA: keratoacanthoma; BCC: basal cell carcinoma. In various studies, rash was categorised as erythema, maculopapular rash, folliculitis, and not otherwise specified; however some authors pointed out rash as a general term. Indicated numbers beside the percentages denote the related references. Boussemart et al. reported grade 1 xerosis in 33% of patients with a median time of 57 days. Xerosis could provoke mild pruritus [17]. Pruritus was observed in patients treated with vemurafenib with an incidence of 29% in the BRIM 2 study [6]. Six percent of patients with grade 2 and 1% of patients with grade 3 pruritus were reported in the BRIM 3 study [8]. In the BREAK-2 study with dabrafenib, 10% of patients experienced pruritus, while Sanlorenzo et al. reported pruritus both in vemurafenib and in dabrafenib treatment groups with a percentage of 33.3% [13, 14]. Anforth et al. reported acneiform eruptions in 3% of patients who were treated with BRAFi longer than 52 weeks. Theselesionswereseenatareassuchasface,trunk,andupper limbs [19]. The incidence of follicular papulopustular rash was6%inthestudybymatteietal.[18]. Boussemart et al. described grade 1 or 2 erythematous hyperkeratotic follicular papules on the arms and thighs that wereusuallyassociatedwithbilateralnipplehyperkeratosis. The incidence of these eruptions was 55% of patients with a mean time to onset of 32 days. The histopathological examination of the skin biopsy revealed pilar dystrophy and folliculitis [17]. Keratosis pilaris like eruptions presenting asymptomatic spinous hair follicle openings was seen during BRAFi treatment with a range of 6% 10% [11]. Sanlorenzo et al. reported that patients receiving dabrafenib developed keratosis pilaris (33.3%) more frequently than the vemurafenib group (16.7%) [14]. Topical steroid creams or exfoliants can be used for treatment [11]. Wang et al. also described a patient that developed diffuse folliculocentric papules with
3 Dermatology Research and Practice 3 Table 2: Percentage of common (>5%) cutaneous adverse events with dabrafenib treatment. Adverse events Percentage (%) Actinic keratosis 10.7 [9] 66.7 [14] Hyperkeratosis 27 [13] 39.4 [9] Pruritus 5.35 [9] 33.3 [14] Photosensitivity 2.67 [9] 33.3 [14] Panniculitis 33.3 [14] Keratosis pilaris 33.3 [14] Alopecia 28.8 [9] Skin papilloma 15 [13] [9] Palmar-plantar dysesthesia [9] Rash [9] Dry skin 10.7 [9] Seborrheic keratosis 8.56 [9] Hair texture abnormal 6.42 [9] cscc 1.6 [9] cscc: cutaneous squamous cell carcinoma. Indicated numbers beside the percentages denote the related references. tiny keratotic plugs during vemurafenib treatment. They considered that this was a result of dysfunctional keratinocyte proliferation and treated the patient with ammonium lactate 12% cream [20]. Grade 1 and 2 hand-foot skin reactions were observed with the mean time to onset of 61 days in 60% of patients in the study by Boussemart et al. Hyperkeratotic, yellowish, and painful plaques were localized on the soles [17]. Lacouture et al.reportedthatpalmar-plantarerythrodysesthesiaoccurred in 8% 10% of patients undergoing vemurafenib treatment. Topical moisturizers or keratolytic agents can be used for the treatment [11]. Hyperkeratosis as the most common cutaneous side effect was noted in 27% of patients in BREAK- 2 [13]. Plantar, mucosal, vulvar, and gingival hyperkeratosis were also reported during BRAFi treatment [17, 19]. Boussemart et al. reported that, three weeks after the drug initiation, one patient developed greasy, scaly papules on the back with gingival lesions that indicated Darier s disease with distinctive histopathological findings [17]. Anforth et al. reported Grover s disease in 45% of patients treated with BRAF inhibitors longer than 52 weeks [19]. Chu et al. described Grover s disease such as a reaction with histopathological findings of acantholytic dyskeratosis during treatment of both BRAF inhibitors [21]. Cutaneous granulomatous eruption is a very rare side effectduetobrafitherapy.parketal.reportedtwocases of granulomatous reactions during BRAFi treatment. The first patient developed multiple erythematous and violaceous papules and erythematous indurated plaque after two months of dabrafenib and trametinib (MEK inhibitor) initiation. The lesions occurred on the areas of the metastatic subcutaneous disease. While the first biopsy revealed granulomatous inflammation with no melanoma cells, the second biopsy revealed granulomatous inflammation surrounding melanoma cells. It was speculated that the cause of the reaction was an immune response or activation against melanoma cells and indicated a positive therapeutic sign. The eruption resolved completely with clobetasol ointment use within two weeks. The second patient developed multiple erythematous, violaceous papules on his extremities after five months of vemurafenib treatment. The biopsy revealed granulomatous dermatitis with focal necrosis. The lesions disappeared spontaneously after the cessation of treatment and did not appear again after the resume of vemurafenib [22]. Garrido et al. reported that a patient developed erythematous nonpruritic plaques on his trunk and arm during dabrafenib and trametinib (MEK inhibitor) treatment. The histopathological findings of the first biopsy revealed granulomatous inflammation, admixed with melanophages. The second biopsy demonstrated granulomatous reaction. Atypical cells were not seen in either biopsy. The lesions improved spontaneously within few weeks [23]. Boussemart et al. described that 14% of patients developed panniculitis on the lower extremities. Lesions occurred with the mean time to onset of 78 days [17]. Sanlorenzo et al. found that the dabrafenib treatment group developed panniculitis more frequently than the vemurafenib treatment group, at rates of 33.3% and 16.7%, respectively [14]. Vasculitis, erythema nodosum were rarely seen AEs (<2%) [11]. Hair growth changes were observed in patients during BRAFi treatment such as cymotrichous, alopecia, or slower and thinner scalp hair growth [17, 19]. Alopecia was found in 11.1% 36% of patients in BRIM 2 and the study of Sanlorenzo et al., respectively [6, 14]. Boussemart et al. described alopecia with grade 1 or 2 in seven patients (16.6%) and thinner and slower scalp hair growth in 12 patients (28.5%) in their study [17]. Pigmentary changes such as vitiligo, nail changes, psoriasis flare, and urticaria were also seen during BRAFi treatment [18, 19, 24] Photosensitivity. Photosensitivity reaction was one of the most reported adverse events related to BRAFi. The photosensitivity incidence was 52% in the BRIM 2 study, 7% with grade 2, and 1% with grade 3 in the BRIM 1 study of patients treated with vemurafenib [5, 6]. However, there was a broad range of percentages in several studies (22.2% 66.7%) [14, 18]. It was experienced more frequently in patients using vemurafenib during the summer time [17]. The cutaneous eruptions usually appeared on sun-exposed areas oftheskinwithinhoursofsunexposure,andin3%ofthem with grade 3 or higher severity [6, 17, 25]. Photosensitivity developed within days of drug initiation and the median time to onset was 1.7 weeks [11]. Grade 1 cheilitis with a mean time to onset of 32 days, predominantly on the lower lip, was observed in patients with photosensitivity (14%). Facial erythematous eruption was reported with incidence of 17% and a mean time to onset of 62 days and mostly associated with photosensitivity [17]. Photosensitivity was less frequent for patients treated with dabrafenib compared to vemurafenib, so it can be used as an alternative treatment. Other skin lesions usually regressed after a couple of months, but photosensitivity could insist during treatment [26]. It was estimated that the cause of photosensitivity was dependent
4 4 Dermatology Research and Practice on the drug s chemical structure and ultraviolet A [27]. Broad spectrum sunscreens including UVA protection and protective clothing must be advised for all patients to avoid photosensitivity Malign and Benign Lesions. Both benign and malignant skin lesions occurred as side effects during BRAFi treatment. Cutaneous squamous cell carcinoma (cscc) and keratoacanthoma(ka)wereseenmorefrequently. The incidence rate of cscc or KA development was 22.2% 26% in Sanlorenzo et al. s study and the BRIM 2 study, respectively [6, 14]. cscc was reported in 12% of patients with grade 3 severity in the BRIM 3 study [8]. Cutaneous SCC incidence ranged from 16% to 26.7% in different studies [18, 19]. The mean time to first onset of cscc was revealed as 7.1 weeks during vemurafenib treatment [11]. In the BREAK- 1 study, cscc occurred with a rate of 11% [12]. KAs were observed in 2% (grade 2) and 6% (grade 3) of patients in BRIM 3 study and 14% of patients in the study of Boussemart et al. [8, 17]. The underlying mechanism of cutaneous neoplasia was paradoxical activation of the MAPK pathway in sundamagedskincellswithpreexistingrasmutations.brafi activated CRAF signaling in wild-type cells that induce ERK signaling, which promoted the development of cscc [6, 8,11].TheresultofmutationanalysesofresectedcSCC lesions confirmed RAS mutations with a rate of 41% [11]. Su et al. reported that the prevalence of RAS mutations in patients treated with vemurafenib was 60%. The lesions tend to appear within the first weeks after vemurafenib initiation on sun-exposed areas of the body such as the face, neck, trunk, and thigh, which indicates that chronic sun exposure was a risk factor for cscc development [11, 14, 17,28].Becauseoftheearlyoccurrenceofthelesions,Su et al. suggested that vemurafenib may potentiate preexisting subclinical oncogenic lesions [28]. The treatment of cscc and KA is simple surgical resection without dose interruptions or reductions [17]. KAs can regress spontaneously, as well [29]. Basal cell carcinoma was also observed in patients during treatment with both vemurafenib (13.3%) and dabrafenib (4%) [13, 18]. Actinic keratoses (AKs) were well-described lesions in patients during BRAFi treatment. Sanlorenzo et al. reported AKs in 44.4% of patients during vemurafenib treatment and 66.7% of patients during dabrafenib treatment [14]. Anforth et al. reported that 26% of patients developed AK after 52 weeks of BRAFi treatment, while Mattei et al. noted AK in 40%ofpatientsintheirstudy[18,19].Theselesionsareknown as precursors of cscc; therefore, surgical removal must be completed as soon as possible [18]. Changes in melanocytic lesions were seen in patients with advanced melanoma during treatment with BRAF inhibitors [30]. These changes were seen as involution of nevi, changes of nevi color, and size and development of new melanoma from preexisting nevi and also new primary melanoma [31]. Anforth et al. reported changes in nevi in 11% of patients in the course of BRAFi therapy. While changes in size and color of melanocytic nevi such as hyperpigmentation and regression and the development of new nevi occurred in their study, no new primary melanomas were observed [19]. Haenssle et al. described a patient who developed involution of preexisting melanocytic nevi during vemurafenib treatment. This situation occurred because the nevi also harbored the BRAF V600E mutation and were affected by the treatment. They also performed a biopsy on preexisting nevi with wild-type BRAF, which showed increased pigmentation and size. The findings demonstrated cytologic dysplasia, melanophages, and lymphohistiocytic inflammation [32]. Zimmer et al. analyzed 22 cutaneous melanocytic lesions in 19 patients with metastatic melanoma, undergoing treatment with selective BRAF inhibitors. Seven invasive and five in situ melanomas developed in 11 patients with a mean time of eight weeks. None of these 12 new primary melanomas harbored the BRAF V600 mutation. Five of these new melanomas appeared on sun-exposed areas. Nine of ten preexisting nevi were classified as dysplastic nevi and one lesion was classified as a common nevus. The mean time of change was 17.5 weeks [30]. Dalle et al. reported that primary melanomas occurred four to twelve weeks after vemurafenib initiation. These melanomas were BRAF wildtype and diagnosed from atypical melanocytic lesions [33]. Anforth et al. reported a patient who developed eruptive nevi during the treatment of a new selective BRAF inhibitor LGX818. Multiple lesions occurred on the back, chest, and leg, two months after the treatment. The histopathological examination revealed pigmented compound nevus [34]. Sanlorenzo et al. observed warts in the course of BRAFi treatment in 22.2% of patients [14]. Anforth et al. reported verruca vulgaris in 5% of patients after 52 weeks of treatment with BRAFi. The histopathological examination of these lesions confirmed viral inclusions such as koilocytes and keratohyalin granules [19]. Mattei et al. reported that 46.7% of patients developed warts with a median time of four weeks in their study [18]. Verrucal keratoses were seen in patients during BRAFi therapy. These premalignant papules were precursors to cutaneous SCC and were reported in 18% of patients with BRAFi treatment longer than 52 weeks in the study of Anforth et al. [19]. Boussemart et al. described polypoid lesions with a hyperkeratotic surface as benign verrucous papillomas on the face, limbs, and trunk during vemurafenib treatment. They occurred with a mean time to onsetof35daysin79%ofpatients.itwasthemostcommon cutaneous side effect in their study [17]. Mattei et al. reported 26.7% of patients developed milia and one patient developed infundibular occlusion cysts [18]. Boussemart et al. also observed milia cysts on the face of the 31% of patients, which occurred in a range of days. Epidermoid cysts were also reported in 33% of patients with a mean time to onset of 108 days in their study [17]. Gebhardt et al.publishedasinglecaseofapatientthatdevelopedmultiple, asymptomatic superficial keratinous cysts presenting milia. The lesions occurred on sun-damaged areas within seven weeks after vemurafenib initiation and disappeared after discontinuation [35]. Treatment is unnecessary unless physical symptoms or cosmetic concerns insist. Houriet et al. reported a patient who developed localized epidermal cysts during combined treatment with vemurafenib and radiotherapy.
5 Dermatology Research and Practice 5 Lesions appeared on previously irradiated localized areas after two months of vemurafenib initiation. It was estimated that the cause was radiosensitivity [36]. It was advised that vemurafenib should be interrupted seven days before and after radiotherapy [37]. Garrido et al. described a patient that developed multiple nodular lesions on his back during vemurafenib treatment for advanced melanoma. The lesions occurred after four months of treatment. The biopsy was performed and histopathological findings revealed primary, cutaneous small/medium CD4 T-cell lymphoma. The authors believed that the activation of immunity that was induced by vemurafenib was the cause of this situation [38]. 5. Conclusion BRAF inhibitors have a crucial role in patients with inoperable metastatic melanoma. They have significant benefits in the prognosis, but some cutaneous adverse reactions can occur in their clinical use. The combination therapies with MEK inhibitors reduce the side effects that occur with monotherapy alone. The patients undergoing BRAFi treatment should beexaminedinthecourseofthetreatmentperiodicallyby selective dermatologic working groups. Early diagnosis and treatment of these cutaneous side effects can improve the patient s quality of life. Conflict of Interests The authors declare that there is no conflict of interests regarding the publication of this paper. References [1] D. A. M. Heideman, I. Lurkin, M. Doeleman et al., KRAS and BRAF mutation analysis in routine molecular diagnostics: comparison of three testing methods on formalin-fixed, paraffinembedded tumor-derived DNA, The Molecular Diagnostics,vol.14,no.3,pp ,2012. [2]L.A.Dossett,R.R.Kudchadkar,andJ.S.Zager, BRAFand MEKinhibitioninmelanoma, ExpertOpiniononDrugSafety, vol. 14, no. 4, pp , [3] B.HagenandV.A.Trinh, Managingsideeffectsofvemurafenib therapy for advanced melanoma, the Advanced Practitioner in Oncology,vol.5,no.6,pp ,2014. [4] M.S.ChapmanandJ.N.Miner, Novelmitogen-activatedprotein kinase kinase inhibitors, Expert Opinion on Investigational Drugs,vol.20,no.2,pp ,2011. [5]K.T.Flaherty,I.Puzanov,K.B.Kimetal., Inhibitionof mutated, activated BRAF in metastatic melanoma, The New England Medicine,vol.363,no.9,pp ,2010. [6] J. A. Sosman, K. B. Kim, L. Schuchter et al., Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib, The New England Medicine, vol.366,no.8,pp , [7] R. Halaban, W. Zhang, A. Bacchiocchi et al., PLX4032, a selective BRAF V600E kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF WT melanoma cells, Pigment Cell & Melanoma Research, vol. 23, no. 2, pp , [8]P.B.Chapman,A.Hauschild,C.Robertetal., Improved survival with vemurafenib in melanoma with BRAF V600E mutation, The New England Medicine, vol. 364, no. 26, pp , [9] A. Hauschild, J.-J. Grob, L. V. Demidov et al., Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, openlabel, phase 3 randomised controlled trial, The Lancet,vol.380, no.9839,pp ,2012. [10] C. H. Johansson and S. E. Brage, BRAF inhibitors in cancer therapy, Pharmacology and Therapeutics,vol.142,no.2,pp , [11] M. E. Lacouture, M. Duvic, A. Hauschild et al., Analysis of dermatologic events in vemurafenib-treated patients with melanoma, Oncologist,vol.18,no.3,pp ,2013. [12] G. S. Falchook, G. V. Long, R. Kurzrock et al., Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial, The Lancet, vol. 379, no. 9829, pp , [13] P. A. Ascierto, D. Minor, A. Ribas et al., Phase II trial (BREAK- 2) of the BRAF inhibitor dabrafenib (GSK ) in patients with metastatic melanoma, Clinical Oncology,vol.31, no. 26, pp , [14] M. Sanlorenzo, A. Choudhry, I. Vujic et al., Comparative profile of cutaneous adverse events: BRAF/MEK inhibitor combination therapy versus BRAF monotherapy in melanoma, the American Academy of Dermatology,vol.71,no.6, pp , [15] L.Peuvrel,G.Quéreux, M. Saint-Jean et al., Profile of vemurafenib-induced severe skin toxicities, the European Academy of Dermatology and Venereology, vol.30,no.2,pp , [16] M. Munch, L. Peuvrel, A. Brocard et al., Early-onset vemurafenib-induced DRESS syndrome, Dermatology, vol. 232, no. 1, pp , [17] L.Boussemart,E.Routier,C.Mateusetal., Prospectivestudy of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients, Annals of Oncology, vol. 24,no.6,pp ,2013. [18]P.L.Mattei,M.B.Alora-Palli,S.Kraft,D.P.Lawrence,K.T. Flaherty, and A. B. Kimball, Cutaneous effects of BRAF inhibitor therapy: a case series, Annals of Oncology, vol. 24, no. 2, pp , [19] R. Anforth, G. Carlos, A. Clements, R. Kefford, and P. Fernandez-Peñas, Cutaneous adverse events in patients treated with BRAF inhibitor-based therapies for metastatic melanoma for longer than 52 weeks, The British Dermatology, vol.172,no.1,pp ,2015. [20] C.M.Wang,K.F.Fleming,andS.Hsu, Acaseofvemurafenibinduced keratosis pilaris-like eruption, Dermatology Online Journal,vol.18,no.4,p.7,2012. [21] E. Y. Chu, K. A. Wanat, C. J. Miller et al., Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study, the American Academy of Dermatology, vol.67,no.6,pp ,2012. [22]J.J.Park,E.B.Hawryluk,S.R.Tahan,K.Flaherty,andC. C. Kim, Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma, JAMA Dermatology,vol.150,no.3,pp ,2014. [23] M. C. Garrido, C. Gutierrez, E. Riveiro-Falkenbach, P. Ortiz, and J. L. Rodriguez-Peralto, BRAF inhibitor induced
6 6 Dermatology Research and Practice antitumoral granulomatous dermatitis eruption in advanced melanoma, The American Dermatopathology, vol. 37, no. 10, pp , [24] J.D.Rinderknecht,S.M.Goldinger,S.Rozatietal., RASopathic skin eruptions during vemurafenib therapy, PLoS ONE,vol.8, no. 3, Article ID e58721, [25] C. M. Reyes-Habito and E. K. Roh, Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: part II. Targeted therapy, the American Academy of Dermatology,vol.71,no.2,pp.217.e1 217.e11,2014. [26] S. J. Welsh and P. G. Corrie, Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma, Therapeutic Advances in Medical Oncology,vol.7,no.2,pp , [27] R. Dummer, J. Rinderknecht, and S. M. Goldinger, Ultraviolet a and photosensitivity during vemurafenib therapy, The New England Medicine,vol.366,no.5,pp ,2012. [28]F.Su,A.Viros,C.Milagreetal., RASmutationsincutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors, The New England Medicine, vol. 366, no. 3, pp , [29] J. C. Mandrell and D. J. Santa Cruz, Keratoacanthoma: hyperplasia, benign neoplasm, or a type of squamous cell carcinoma? Seminars in Diagnostic Pathology, vol.26,no.3,pp , [30] L. Zimmer, U. Hillen, E. Livingstone et al., Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF Inhibition, Clinical Oncology,vol.30,no.19,pp , [31] P. R. Cohen, A. Y. Bedikian, and K. B. Kim, Appearance of new vemurafenib-associated melanocytic nevi on normal-appearing skin: case series and a review of changing or new pigmented lesionsinpatientswithmetastaticmalignantmelanomaafter initiating treatment with vemurafenib, The Clinical and Aesthetic Dermatology,vol.6,no.5,pp.27 37,2013. [32] H. A. Haenssle, S. L. Kraus, F. Brehmer et al., Dynamic changes in nevi of a patient with melanoma treated with vemurafenib: importance of sequential dermoscopy, Archives of Dermatology,vol.148,no.10,pp ,2012. [33] S. Dalle, N. Poulalhon, and L. Thomas, Vemurafenib in melanoma with BRAF V600E mutation, The New England Journal of Medicine, vol. 365, no. 15, pp , [34] R. M. Anforth, G. R. M. Carlos, R. A. Scolyer, S. Chou, and P. Fernandez-Peñas, Eruptive naevi in a patient treated with LGX818 for BRAF mutant metastatic melanoma, Melanoma Research,vol.25,no.1,pp.91 94,2015. [35] C. Gebhardt, J. Staub, A. Schmieder, S. Utikal, and J. Utikal, Multiple white cysts on face and trunk of a melanoma patient treatedwithvemurafenib, Acta Dermato-Venereologica,vol.95, no. 1, pp , [36] C. Houriet, N. D. Klass, H. Beltraminelli, L. Borradori, and P. A. Oberholzer, Localized epidermal cysts as a radiation recall phenomenon in a melanoma patient treated with radiotherapy and the BRAF inhibitor vemurafenib, Case Reports in Dermatology,vol.6,no.3,pp ,2014. [37] C. J. Anker, A. Ribas, A. H. Grossmann et al., Severe liver and skin toxicity after radiation and vemurafenib in metastatic melanoma, Clinical Oncology,vol.31,no.17,pp.e283 e287, [38]M.C.Garrido,E.Riveiro-Falckenbach,Y.Ruano,P.Ortiz, and J. L. Rodriguez-Peralto, Primary cutaneous small/medium CD4 + T-Cell lymphoma occurring during treatment with vemurafenib for advanced melanoma, American Dermatopathology,vol.37,no.6,pp ,2015.
7 MEDIATORS of INFLAMMATION The Scientific World Journal Gastroenterology Research and Practice Diabetes Research International Endocrinology Immunology Research Disease Markers Submit your manuscripts at BioMed Research International PPAR Research Obesity Ophthalmology Evidence-Based Complementary and Alternative Medicine Stem Cells International Oncology Parkinson s Disease Computational and Mathematical Methods in Medicine AIDS Behavioural Neurology Research and Treatment Oxidative Medicine and Cellular Longevity
BRAF Inhibition in Melanoma
 BRAF Inhibition in Melanoma New York City, Mar 22-23, 2013 Bartosz Chmielowski, MD, PhD Assistant Clinical Professor University of California Los Angeles Disclosures Speaker Bureau: BMS, Genentech, Prometheus
BRAF Inhibition in Melanoma New York City, Mar 22-23, 2013 Bartosz Chmielowski, MD, PhD Assistant Clinical Professor University of California Los Angeles Disclosures Speaker Bureau: BMS, Genentech, Prometheus
Prospective Case Series of Cutaneous Adverse Effects Associated With Dabrafenib and Trametinib
 670368CMSXXX10.1177/1203475416670368Journal of Cutaneous Medicine and SurgeryLacroix and Wang research-article2016 Original Article Prospective Case Series of Cutaneous Adverse Effects Associated With
670368CMSXXX10.1177/1203475416670368Journal of Cutaneous Medicine and SurgeryLacroix and Wang research-article2016 Original Article Prospective Case Series of Cutaneous Adverse Effects Associated With
17/01/2017. ckit NRAS BRAF MEK ERK. ANTITUMOR IMMUNE RESPONSE PROLIFERATION
 BRAF inhibitors: vemurafenib (Zelboraf ) dabrafenib (Tafinlar ) Universitair Ziekenhuis Gent MEK inhibitors: cobimetinib trametinib(mekinist ) selumetinib CUTANEOUS SIDE EFFECTS OF BRAF-INHIBITORS Lieve
BRAF inhibitors: vemurafenib (Zelboraf ) dabrafenib (Tafinlar ) Universitair Ziekenhuis Gent MEK inhibitors: cobimetinib trametinib(mekinist ) selumetinib CUTANEOUS SIDE EFFECTS OF BRAF-INHIBITORS Lieve
Case Report A Rare Cutaneous Adnexal Tumor: Malignant Proliferating Trichilemmal Tumor
 Case Reports in Medicine Volume 2015, Article ID 742920, 4 pages http://dx.doi.org/10.1155/2015/742920 Case Report A Rare Cutaneous Adnexal Tumor: Malignant Proliferating Trichilemmal Tumor Omer Alici,
Case Reports in Medicine Volume 2015, Article ID 742920, 4 pages http://dx.doi.org/10.1155/2015/742920 Case Report A Rare Cutaneous Adnexal Tumor: Malignant Proliferating Trichilemmal Tumor Omer Alici,
The Development of Encorafenib (LGX818) and Binimetinib (MEK162) in Patients With Metastatic Melanoma
 The Development of Encorafenib (LGX818) and Binimetinib (MEK162) in Patients With Metastatic Melanoma Reinhard Dummer, 1 Keith Flaherty, 2 Richard Kefford, 3 Paolo A. Ascierto, 4 Laure Moutouh-de Parseval,
The Development of Encorafenib (LGX818) and Binimetinib (MEK162) in Patients With Metastatic Melanoma Reinhard Dummer, 1 Keith Flaherty, 2 Richard Kefford, 3 Paolo A. Ascierto, 4 Laure Moutouh-de Parseval,
Dermatopathology: The tumor is composed of keratinocytes which show atypia, increase mitoses and abnormal mitoses.
 Squamous cell carcinoma (SCC): A common malignant tumor of keratinocytes arising in the epidermis, usually from a precancerous condition: 1- UV induced actinic keratosis, usually of low grade malignancy.
Squamous cell carcinoma (SCC): A common malignant tumor of keratinocytes arising in the epidermis, usually from a precancerous condition: 1- UV induced actinic keratosis, usually of low grade malignancy.
Skin cancer is the most common
 Section Editors: Christopher J. Campen and Beth Eaby-Sandy Vemurafenib: First-in-Class BRAF- Mutated Inhibitor for the Treatment of Unresectable or Metastatic Melanoma LINDSAY SHELLEDY, PharmD, and DANIELLE
Section Editors: Christopher J. Campen and Beth Eaby-Sandy Vemurafenib: First-in-Class BRAF- Mutated Inhibitor for the Treatment of Unresectable or Metastatic Melanoma LINDSAY SHELLEDY, PharmD, and DANIELLE
Case Report Long Term Survival and Continued Complete Response of Vemurafenib in a Metastatic Melanoma Patient with BRAF V600K Mutation
 Case Reports in Oncological Medicine Volume 2016, Article ID 2672671, 4 pages http://dx.doi.org/10.1155/2016/2672671 Case Report Long Term Survival and Continued Complete Response of Vemurafenib in a Metastatic
Case Reports in Oncological Medicine Volume 2016, Article ID 2672671, 4 pages http://dx.doi.org/10.1155/2016/2672671 Case Report Long Term Survival and Continued Complete Response of Vemurafenib in a Metastatic
Dermatologists & Oncologists: Two important reasons we are getting closer. Ioanna Panoutsopoulou, MD. GAMC June 1 st 2016
 Dermatologists & Oncologists: Two important reasons we are getting closer Ioanna Panoutsopoulou, MD GAMC June 1 st 2016 Points of presentation Cutaneous adverse events from: Epidermal Growth Factor Receptor
Dermatologists & Oncologists: Two important reasons we are getting closer Ioanna Panoutsopoulou, MD GAMC June 1 st 2016 Points of presentation Cutaneous adverse events from: Epidermal Growth Factor Receptor
Normal RAS-RAF (MAPK) pathway signaling
 BRAF-Mutations in Melanomas L. Mazzucchelli Istituto Cantonale di Patologia, Locarno 77. Annual Meeting Swiss Society of Pathology, Lucerne 2011 Sponsored by Roche Pharma Switzerland Melanoma has increased
BRAF-Mutations in Melanomas L. Mazzucchelli Istituto Cantonale di Patologia, Locarno 77. Annual Meeting Swiss Society of Pathology, Lucerne 2011 Sponsored by Roche Pharma Switzerland Melanoma has increased
Cutaneous reactions to targeted therapies. Stavonnie Patterson, MD, FAAD Northwestern University Feinberg School of Medicine March 6, 2017
 Cutaneous reactions to targeted therapies Stavonnie Patterson, MD, FAAD Northwestern University Feinberg School of Medicine March 6, 2017 Disclosures I have no relevant disclosures Papulopustular Eruption
Cutaneous reactions to targeted therapies Stavonnie Patterson, MD, FAAD Northwestern University Feinberg School of Medicine March 6, 2017 Disclosures I have no relevant disclosures Papulopustular Eruption
Clinical characteristics
 Skin Cancer Fernando Vega, MD Seattle Healing Arts Clinical characteristics Precancerous lesions Common skin cancers ACTINIC KERATOSIS Precancerous skin lesions Actinic keratoses Dysplastic melanocytic
Skin Cancer Fernando Vega, MD Seattle Healing Arts Clinical characteristics Precancerous lesions Common skin cancers ACTINIC KERATOSIS Precancerous skin lesions Actinic keratoses Dysplastic melanocytic
AperTO - Archivio Istituzionale Open Access dell'università di Torino
 AperTO - Archivio Istituzionale Open Access dell'università di Torino Comparative profile of cutaneous adverse events: BRAF/MEK inhibitor combination therapy versus BRAF monotherapy in melanoma. This is
AperTO - Archivio Istituzionale Open Access dell'università di Torino Comparative profile of cutaneous adverse events: BRAF/MEK inhibitor combination therapy versus BRAF monotherapy in melanoma. This is
Cutaneous Malignancies: A Primer COPYRIGHT. Marissa Heller, M.D.
 Cutaneous Malignancies: A Primer Marissa Heller, M.D. Associate Director of Dermatologic Surgery Department of Dermatology Beth Israel Deaconess Medical Center December 10, 2016 Skin Cancer Non-melanoma
Cutaneous Malignancies: A Primer Marissa Heller, M.D. Associate Director of Dermatologic Surgery Department of Dermatology Beth Israel Deaconess Medical Center December 10, 2016 Skin Cancer Non-melanoma
What You Need to Know about Advanced Melanoma Therapies Targeted Approaches
 2018 AAD Annual Meeting, San Diego, CA What You Need to Know about Advanced Melanoma Therapies Targeted Approaches Susan M. Swetter, MD, FAAD Professor of Dermatology Director, Pigmented Lesion & Melanoma
2018 AAD Annual Meeting, San Diego, CA What You Need to Know about Advanced Melanoma Therapies Targeted Approaches Susan M. Swetter, MD, FAAD Professor of Dermatology Director, Pigmented Lesion & Melanoma
Erdheim-Chester disease and Skin issues
 Erdheim-Chester disease and Skin issues STÉPHANE BARETE, MD, PHD UNIT OF DERMATOLOGY PITIÉ -SALPÊTRIÈRE HOSPITAL PARIS stephane.barete@aphp.fr Introduction Erdheim-Chester (ECD) is an orphan disease included
Erdheim-Chester disease and Skin issues STÉPHANE BARETE, MD, PHD UNIT OF DERMATOLOGY PITIÉ -SALPÊTRIÈRE HOSPITAL PARIS stephane.barete@aphp.fr Introduction Erdheim-Chester (ECD) is an orphan disease included
MEK/BRAF inhibitors and the implications on patients and health care providers
 MEK/BRAF inhibitors and the implications on patients and health care providers Tara McKeown NP Paediatric Neuro Oncology Hospital for Sick Children Adjunct Lecturer, Lawrence S. Bloomberg, Faculty of Nursing,
MEK/BRAF inhibitors and the implications on patients and health care providers Tara McKeown NP Paediatric Neuro Oncology Hospital for Sick Children Adjunct Lecturer, Lawrence S. Bloomberg, Faculty of Nursing,
NEOPLASMS OF THE SURFACE EPITHELIUM (KERATINOCYTES)
 NEOPLASMS OF THE SURFACE EPITHELIUM (KERATINOCYTES) Papillary Lesions Precancerous Lesions Keratinocyte Proliferations Carcinomas Melanotic Lesions Melanomas Normal Mucosa Keratin layer Spinous layer Basal
NEOPLASMS OF THE SURFACE EPITHELIUM (KERATINOCYTES) Papillary Lesions Precancerous Lesions Keratinocyte Proliferations Carcinomas Melanotic Lesions Melanomas Normal Mucosa Keratin layer Spinous layer Basal
Actinic keratosis (AK): Dr Sarma s simple guide
 Actinic keratosis (AK): Dr Sarma s simple guide Actinic keratosis is a very common lesion that you will see in your day-to-day practice. First, let me explain the name Actinic keratosis. It means keratosis
Actinic keratosis (AK): Dr Sarma s simple guide Actinic keratosis is a very common lesion that you will see in your day-to-day practice. First, let me explain the name Actinic keratosis. It means keratosis
NCCP Chemotherapy Regimen. Vemurafenib Monotherapy
 INDICATIONS FOR USE: Vemurafenib Monotherapy INDICATION ICD10 Regimen Code *Reimbursement Indicator Treatment of adult patients with BRAF V600 mutation-positive unresectable or metastatic melanoma. C43
INDICATIONS FOR USE: Vemurafenib Monotherapy INDICATION ICD10 Regimen Code *Reimbursement Indicator Treatment of adult patients with BRAF V600 mutation-positive unresectable or metastatic melanoma. C43
Benign versus Cancerous Lesions How to tell the difference FMF 2014 Christie Freeman MD, CCFP, DipPDerm, MSc
 1 Benign versus Cancerous Lesions How to tell the difference FMF 2014 Christie Freeman MD, CCFP, DipPDerm, MSc Benign lesions Seborrheic Keratoses: Warty, stuck-on Genetics and birthdays Can start in late
1 Benign versus Cancerous Lesions How to tell the difference FMF 2014 Christie Freeman MD, CCFP, DipPDerm, MSc Benign lesions Seborrheic Keratoses: Warty, stuck-on Genetics and birthdays Can start in late
Dermoscopy: Recognizing Top Five Common In- Office Diagnoses
 Dermoscopy: Recognizing Top Five Common In- Office Diagnoses Vu A. Ngo, DO Department of Family Medicine and Dermatology Choctaw Nation Health Services Authority Learning Objectives Introduction to dermoscopy
Dermoscopy: Recognizing Top Five Common In- Office Diagnoses Vu A. Ngo, DO Department of Family Medicine and Dermatology Choctaw Nation Health Services Authority Learning Objectives Introduction to dermoscopy
Acute drug reactions associated with the novel targeted therapies used in treating skin cancer
 Acute drug reactions associated with the novel targeted therapies used in treating skin cancer Dr Rishika Sinha Consultant Dermatologist Chelsea & Westminster Hospital NHS Foundation Trust West Middlesex
Acute drug reactions associated with the novel targeted therapies used in treating skin cancer Dr Rishika Sinha Consultant Dermatologist Chelsea & Westminster Hospital NHS Foundation Trust West Middlesex
Case Report A Case of Cystic Basal Cell Carcinoma Which Shows a Homogenous Blue/Black Area under Dermatoscopy
 Volume 20, Article ID 450472, 4 pages doi:0.55/20/450472 Case Report A Case of Cystic Basal Cell Carcinoma Which Shows a Homogenous Blue/Black Area under Dermatoscopy Akihiro Yoneta, Kohei Horimoto, Keiko
Volume 20, Article ID 450472, 4 pages doi:0.55/20/450472 Case Report A Case of Cystic Basal Cell Carcinoma Which Shows a Homogenous Blue/Black Area under Dermatoscopy Akihiro Yoneta, Kohei Horimoto, Keiko
Skin Side Effects U N I V E R S I T Y OF V I E N N A, D E P A R T M E N T OF O N C O L O G Y, G E N E R A L H O S P I T A L V I E N N A
 Skin Side Effects Christiane Thallinger, M D U N I V E R S I T Y OF V I E N N A, D E P A R T M E N T OF O N C O L O G Y, G E N E R A L H O S P I T A L V I E N N A Targets Substances 1 Multi Kinase Inhibitors
Skin Side Effects Christiane Thallinger, M D U N I V E R S I T Y OF V I E N N A, D E P A R T M E N T OF O N C O L O G Y, G E N E R A L H O S P I T A L V I E N N A Targets Substances 1 Multi Kinase Inhibitors
Basal cell carcinoma 5/28/2011
 Goal of this Presentation A practical approach to the diagnosis of cutaneous carcinomas and their mimics Thaddeus Mully, MD University of California San Francisco To review common non-melanoma skin cancers
Goal of this Presentation A practical approach to the diagnosis of cutaneous carcinomas and their mimics Thaddeus Mully, MD University of California San Francisco To review common non-melanoma skin cancers
BCCA Protocol Summary for the Treatment of BRAF V600 Mutation- Positive Unresectable or Metastatic Melanoma using vemurafenib and cobimetinib
 BCCA Protocol Summary for the Treatment of BRAF V600 Mutation- Positive Unresectable or Metastatic Melanoma using vemurafenib and cobimetinib Protocol Code Tumour Group Contact Physician USMAVVC Skin and
BCCA Protocol Summary for the Treatment of BRAF V600 Mutation- Positive Unresectable or Metastatic Melanoma using vemurafenib and cobimetinib Protocol Code Tumour Group Contact Physician USMAVVC Skin and
BRAF Inhibitors in Metastatic disease. Grant McArthur MB BS PhD Peter MacCallum Cancer Centre Melbourne, Australia
 Inhibitors in Metastatic disease Grant McArthur MB BS PhD Peter MacCallum Cancer Centre Melbourne, Australia Disclosures Research Support Pfizer & Cellgene Consultant Provectus Mortality from Melanoma
Inhibitors in Metastatic disease Grant McArthur MB BS PhD Peter MacCallum Cancer Centre Melbourne, Australia Disclosures Research Support Pfizer & Cellgene Consultant Provectus Mortality from Melanoma
Dermatology for the PCP Deanna G. Brown, MD, FAAD Susong Dermatology Consulting Staff at CHI Memorial
 Dermatology for the PCP Deanna G. Brown, MD, FAAD Susong Dermatology Consulting Staff at CHI Memorial Cutaneous Oncology for the PCP Deanna G. Brown, MD, FAAD Susong Dermatology Consulting Staff at CHI
Dermatology for the PCP Deanna G. Brown, MD, FAAD Susong Dermatology Consulting Staff at CHI Memorial Cutaneous Oncology for the PCP Deanna G. Brown, MD, FAAD Susong Dermatology Consulting Staff at CHI
Clinical Study Mucosal Melanoma in the Head and Neck Region: Different Clinical Features and Same Outcome to Cutaneous Melanoma
 ISRN Dermatology Volume 2013, Article ID 586915, 5 pages http://dx.doi.org/10.1155/2013/586915 Clinical Study Mucosal Melanoma in the Head and Neck Region: Different Clinical Features and Same Outcome
ISRN Dermatology Volume 2013, Article ID 586915, 5 pages http://dx.doi.org/10.1155/2013/586915 Clinical Study Mucosal Melanoma in the Head and Neck Region: Different Clinical Features and Same Outcome
Skin lesions The Good and the Bad. Dr Virginia Hubbard Ipswich Hospital NHS Trust Barts and the London School of Medicine and Dentistry
 Skin lesions The Good and the Bad Dr Virginia Hubbard Ipswich Hospital NHS Trust Barts and the London School of Medicine and Dentistry Case 1 32 year old woman Australian Lesion on back New hair growing
Skin lesions The Good and the Bad Dr Virginia Hubbard Ipswich Hospital NHS Trust Barts and the London School of Medicine and Dentistry Case 1 32 year old woman Australian Lesion on back New hair growing
Benign and malignant epithelial lesions: Seborrheic keratosis: A common benign pigmented epidermal tumor occur in middle-aged or older persons more
 Benign and malignant epithelial lesions: Seborrheic keratosis: A common benign pigmented epidermal tumor occur in middle-aged or older persons more common on the trunk; but extremities, head and neck are
Benign and malignant epithelial lesions: Seborrheic keratosis: A common benign pigmented epidermal tumor occur in middle-aged or older persons more common on the trunk; but extremities, head and neck are
Contents. Part I Genodermatoses
 Contents Part I Genodermatoses 1 Hyperkeratotic Palms and Soles with Periorificial Keratosis............... 3 2 Indurated, Dark, Hairy Plaques, with Arthritis and Deafness.............. 9 3 Cleft Palate,
Contents Part I Genodermatoses 1 Hyperkeratotic Palms and Soles with Periorificial Keratosis............... 3 2 Indurated, Dark, Hairy Plaques, with Arthritis and Deafness.............. 9 3 Cleft Palate,
LUMPS AND BUMPS: AN ORGANIZED APPROACH TO DIAGNOSIS AND MANAGEMENT
 LUMPS AND BUMPS: AN ORGANIZED APPROACH TO DIAGNOSIS AND MANAGEMENT Tammy P. Than, M.S., O.D., F.A.A.O. The University of Alabama at Birmingham / School of Optometry 1716 University Blvd. Birmingham, AL
LUMPS AND BUMPS: AN ORGANIZED APPROACH TO DIAGNOSIS AND MANAGEMENT Tammy P. Than, M.S., O.D., F.A.A.O. The University of Alabama at Birmingham / School of Optometry 1716 University Blvd. Birmingham, AL
Know who is at risk: LOOK! for ABCDs, rapidly changing lesions, do a biopsy when indicated
 Lindy P. Fox, MD Associate Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
Lindy P. Fox, MD Associate Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
Large majority caused by sun exposure Often sun exposure before age 20 Persons who burn easily and tan poorly are at greatest risk.
 Basics of Skin Cancer Detection and Treatment of Non- Melanoma Skin Cancers Large majority caused by sun exposure Often sun exposure before age 20 Persons who burn easily and tan poorly are at greatest
Basics of Skin Cancer Detection and Treatment of Non- Melanoma Skin Cancers Large majority caused by sun exposure Often sun exposure before age 20 Persons who burn easily and tan poorly are at greatest
Living Beyond Cancer Skin Cancer Detection and Prevention
 Living Beyond Cancer Skin Cancer Detection and Prevention Cutaneous Skin Cancers Identification Diagnosis Treatment options Prevention What is the most common cancer in people? What is the most common
Living Beyond Cancer Skin Cancer Detection and Prevention Cutaneous Skin Cancers Identification Diagnosis Treatment options Prevention What is the most common cancer in people? What is the most common
IT S FUNDAMENTAL MY DEAR WATSON! A SHERLOCKIAN APPROACH TO DERMATOLOGY
 IT S FUNDAMENTAL MY DEAR WATSON! A SHERLOCKIAN APPROACH TO DERMATOLOGY Skin, Bones, and other Private Parts Symposium Dermatology Lectures by Debra Shelby, PhD, DNP, FNP-BC, FADNP, FAANP Debra Shelby,
IT S FUNDAMENTAL MY DEAR WATSON! A SHERLOCKIAN APPROACH TO DERMATOLOGY Skin, Bones, and other Private Parts Symposium Dermatology Lectures by Debra Shelby, PhD, DNP, FNP-BC, FADNP, FAANP Debra Shelby,
Multikinase inhibitors: Multikinase inhibitors: Regorafenib skin toxicity. Cutaneous side effects of multikinase-inhibitors and their management
 TARGETED THERAPIES AND THEIR CUTANEOUS TOXICITIES Brussels, 14/1/2017 Cutaneous side effects of multikinase-inhibitors and their management Siegfried Segaert Dermatology Dept. Uinversity Hospital Leuven
TARGETED THERAPIES AND THEIR CUTANEOUS TOXICITIES Brussels, 14/1/2017 Cutaneous side effects of multikinase-inhibitors and their management Siegfried Segaert Dermatology Dept. Uinversity Hospital Leuven
DESCRIPTIONS FOR MED 3 ROTATIONS Dermatology A3S
 Regardless of your future field of practice, you will be exposed to a considerable amount of dermatology and this rotation provides you the chance to see a range of skin diseases. You will have the opportunity
Regardless of your future field of practice, you will be exposed to a considerable amount of dermatology and this rotation provides you the chance to see a range of skin diseases. You will have the opportunity
Common Benign Lesions and Skin Cancers. 22nd May 2015 Dr Mark Foley
 Common Benign Lesions and Skin Cancers 22nd May 2015 Dr Mark Foley Thank you for downloading this file. This intended to supplement the presentation given at the NZ Wound Care Conference, it is not intended
Common Benign Lesions and Skin Cancers 22nd May 2015 Dr Mark Foley Thank you for downloading this file. This intended to supplement the presentation given at the NZ Wound Care Conference, it is not intended
Case Report Metastatic Malignant Melanoma of Parotid Gland with a Regressed Primary Tumor
 Case Reports in Otolaryngology Volume 2016, Article ID 5393404, 4 pages http://dx.doi.org/10.1155/2016/5393404 Case Report Metastatic Malignant Melanoma of Parotid Gland with a Regressed Primary Tumor
Case Reports in Otolaryngology Volume 2016, Article ID 5393404, 4 pages http://dx.doi.org/10.1155/2016/5393404 Case Report Metastatic Malignant Melanoma of Parotid Gland with a Regressed Primary Tumor
Dermatology GP Referral Guidelines
 Austin Health Dermatology Department holds 5 Clinic sessions to discuss and plan the treatment of with Dermatology conditions. Department of Health clinical urgency categories for specialist clinics Urgent:
Austin Health Dermatology Department holds 5 Clinic sessions to discuss and plan the treatment of with Dermatology conditions. Department of Health clinical urgency categories for specialist clinics Urgent:
Subject: Cobimetinib (Cotellic ) Tablet
 09-J2000-53 Original Effective Date: 03/15/16 Reviewed: 11/14/18 Revised: 12/15/18 Subject: Cobimetinib (Cotellic ) Tablet THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J2000-53 Original Effective Date: 03/15/16 Reviewed: 11/14/18 Revised: 12/15/18 Subject: Cobimetinib (Cotellic ) Tablet THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
Case Report Micromelanomas: A Review of Melanomas 2mmand a Case Report
 Case Reports in Oncological Medicine, Article ID 206260, 4 pages http://dx.doi.org/10.1155/2014/206260 Case Report Micromelanomas: A Review of Melanomas 2mmand a Case Report Sharad P. Paul 1,2,3 1 Skin
Case Reports in Oncological Medicine, Article ID 206260, 4 pages http://dx.doi.org/10.1155/2014/206260 Case Report Micromelanomas: A Review of Melanomas 2mmand a Case Report Sharad P. Paul 1,2,3 1 Skin
Initial Results from an Open-label, Doseescalation Phase I Study of the Oral BRAF Inhibitor LGX818 in BRAF V600 mutant Advanced Melanoma
 Initial Results from an Open-label, Doseescalation Phase I Study of the Oral BRAF Inhibitor LGX818 in BRAF V600 mutant Advanced Melanoma Reinhard Dummer, 1 Caroline Robert, 2 Marta Nyakas, 3 Grant McArthur,
Initial Results from an Open-label, Doseescalation Phase I Study of the Oral BRAF Inhibitor LGX818 in BRAF V600 mutant Advanced Melanoma Reinhard Dummer, 1 Caroline Robert, 2 Marta Nyakas, 3 Grant McArthur,
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal (STA) Dabrafenib for treating unresectable, advanced or metastatic
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal (STA) Dabrafenib for treating unresectable, advanced or metastatic BRAF V600 mutation-positive melanoma mutation-positive melanoma
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal (STA) Dabrafenib for treating unresectable, advanced or metastatic BRAF V600 mutation-positive melanoma mutation-positive melanoma
Learning Objectives. Tanning. The Skin. Classic Features. Sun Reactive Skin Type Classification. Skin Cancers: Preventing, Screening and Treating
 Learning Objectives Skin Cancers: Preventing, Screening and Treating Robert A. Baldor, MD, FAAFP Professor, Family Medicine & Community Health University of Massachusetts Medical School Distinguish the
Learning Objectives Skin Cancers: Preventing, Screening and Treating Robert A. Baldor, MD, FAAFP Professor, Family Medicine & Community Health University of Massachusetts Medical School Distinguish the
A dinical and histopathologic entity associated with an increased risk of nonmelanoma skin cancer
 PUVA keratosis A dinical and histopathologic entity associated with an increased risk of nonmelanoma skin cancer M. C. G. van Praag, MD, a J. N. Bouwes Bavinck, MD, a W. Bergman, MD, PhD, a F. R. Rosendaal,
PUVA keratosis A dinical and histopathologic entity associated with an increased risk of nonmelanoma skin cancer M. C. G. van Praag, MD, a J. N. Bouwes Bavinck, MD, a W. Bergman, MD, PhD, a F. R. Rosendaal,
Targeted Therapies 5/21/2018. Iatrogenic Dermatopathology: When Therapy Goes Wrong
 Iatrogenic Dermatopathology: When Therapy Goes Wrong Tammie Ferringer, MD Geisinger Medical Center, Danville, PA tferringer@geisinger.edu I do not have any relevant relationships with industry Targeted
Iatrogenic Dermatopathology: When Therapy Goes Wrong Tammie Ferringer, MD Geisinger Medical Center, Danville, PA tferringer@geisinger.edu I do not have any relevant relationships with industry Targeted
Undergraduate Dermatology Curriculum July 2016
 Undergraduate Dermatology Curriculum July 2016 British Association of Dermatologists Introduction This document is the 2016 revised dermatology undergraduate curriculum (UK) from the British Association
Undergraduate Dermatology Curriculum July 2016 British Association of Dermatologists Introduction This document is the 2016 revised dermatology undergraduate curriculum (UK) from the British Association
Research Article Clinical Outcome of a Novel Anti-CD6 Biologic Itolizumab in Patients of Psoriasis with Comorbid Conditions
 Dermatology Research and Practice Volume 2, Article ID 13326, 4 pages http://dx.doi.org/.1155/2/13326 Research Article Clinical Outcome of a Novel Anti-CD6 Biologic Itolizumab in Patients of Psoriasis
Dermatology Research and Practice Volume 2, Article ID 13326, 4 pages http://dx.doi.org/.1155/2/13326 Research Article Clinical Outcome of a Novel Anti-CD6 Biologic Itolizumab in Patients of Psoriasis
Know who is at risk: LOOK! for ABCDs, rapidly changing lesions, do a biopsy when indicated
 Lindy P. Fox, MD Assistant Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
Lindy P. Fox, MD Assistant Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
Modern therapy in oncology Metastatic melanoma
 Modern therapy in oncology Metastatic melanoma Anna Buda-Nowak Oncology Department; University Hospital in Cracow Melanoma Malignant skin neoplasm derived from neuroectodermal melanomatous cells. The incidence:
Modern therapy in oncology Metastatic melanoma Anna Buda-Nowak Oncology Department; University Hospital in Cracow Melanoma Malignant skin neoplasm derived from neuroectodermal melanomatous cells. The incidence:
CONDITIONS OF THE SKIN
 CONDITIONS OF THE SKIN UCSF/SFGH Family & Community Medicine Residency Program Educational Objectives I. Knowledge The resident will be able to discuss the definition, diagnosis, and initial management
CONDITIONS OF THE SKIN UCSF/SFGH Family & Community Medicine Residency Program Educational Objectives I. Knowledge The resident will be able to discuss the definition, diagnosis, and initial management
Research Article A Clinicopathological and Immunohistochemical Correlation in Cutaneous Metastases from Internal Malignancies: A Five-Year Study
 Skin Cancer, Article ID 793937, 5 pages http://dx.doi.org/10.1155/2014/793937 Research Article A Clinicopathological and Immunohistochemical Correlation in Cutaneous Metastases from Internal Malignancies:
Skin Cancer, Article ID 793937, 5 pages http://dx.doi.org/10.1155/2014/793937 Research Article A Clinicopathological and Immunohistochemical Correlation in Cutaneous Metastases from Internal Malignancies:
Pathology of the skin. Dr Fónyad László, 1sz. Patológiai és Kísérleti Rákkutató Intézet, SE
 Pathology of the skin Dr Fónyad László, 1sz. Patológiai és Kísérleti Rákkutató Intézet, SE The skin Biggest organ Kb. 1.8 nm Kb. 10 kg Most frequent site for tumor development (BCC) Pathology of the skin
Pathology of the skin Dr Fónyad László, 1sz. Patológiai és Kísérleti Rákkutató Intézet, SE The skin Biggest organ Kb. 1.8 nm Kb. 10 kg Most frequent site for tumor development (BCC) Pathology of the skin
Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM
 Dilemmas and Challenges in Skin Cancer Therapies and Management Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM Roger I. Ceilley, M.D. Clinical Professor of Dermatology The University of Iowa
Dilemmas and Challenges in Skin Cancer Therapies and Management Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM Roger I. Ceilley, M.D. Clinical Professor of Dermatology The University of Iowa
VACAVILLE DERMATOLOGY
 Connecting the Dots on those Spots NANDAN V. KAMATH, M.D. VACAVILLE DERMATOLOGY Sources All of the photos were taken with permission from the Dermnet NZ website - Dermnet New Zealand after communicating
Connecting the Dots on those Spots NANDAN V. KAMATH, M.D. VACAVILLE DERMATOLOGY Sources All of the photos were taken with permission from the Dermnet NZ website - Dermnet New Zealand after communicating
vemurafenib 240mg film-coated tablet (Zelboraf ) SMC No. (792/12) Roche Products Ltd.
 Resubmission vemurafenib 240mg film-coated tablet (Zelboraf ) SMC No. (792/12) Roche Products Ltd. 08 November 2013 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Resubmission vemurafenib 240mg film-coated tablet (Zelboraf ) SMC No. (792/12) Roche Products Ltd. 08 November 2013 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
المركب النموذج--- سبيتز وحمة = Type Spitz's Nevus, Compound SPITZ NEVUS 1 / 7
 SPITZ NEVUS 1 / 7 Epidemiology An annual incidence rate of 1.4 cases of Spitz nevus per 100,000 individuals has been estimated in Australia, compared with 25.4 per 100,000 individuals for cutaneous melanoma
SPITZ NEVUS 1 / 7 Epidemiology An annual incidence rate of 1.4 cases of Spitz nevus per 100,000 individuals has been estimated in Australia, compared with 25.4 per 100,000 individuals for cutaneous melanoma
Lid Lesions: Relax or Refer
 Lid Lesions: Relax or Refer Blair Lonsberry, MS, OD, MEd., FAAO Professor of Optometry Pacific University College of Optometry blonsberry@pacificu.edu Agenda Benign vs. Malignant lesions Benign Eyelid
Lid Lesions: Relax or Refer Blair Lonsberry, MS, OD, MEd., FAAO Professor of Optometry Pacific University College of Optometry blonsberry@pacificu.edu Agenda Benign vs. Malignant lesions Benign Eyelid
Update on Targeted Therapy in Melanoma
 Update on Targeted Therapy in Melanoma Seville June 2013 James Larkin FRCP PhD London UK Overview What are the targets in melanoma? BRAF / KIT / NRAS / GNAQ / MEK DNA / microtubules CTLA4 / PD1 / PDL1
Update on Targeted Therapy in Melanoma Seville June 2013 James Larkin FRCP PhD London UK Overview What are the targets in melanoma? BRAF / KIT / NRAS / GNAQ / MEK DNA / microtubules CTLA4 / PD1 / PDL1
Chapter 6 Squamous Cell Carcinoma: Variants and Challenges
 Chapter 6 Squamous Cell Carcinoma: Variants and Challenges Michael B. Morgan EPIDEMIOLOGY: Second most common skin cancer, rare in the dark-skinned races. ETIOLOGY: Ultraviolet light, HPV infection. PATHOGENESIS:
Chapter 6 Squamous Cell Carcinoma: Variants and Challenges Michael B. Morgan EPIDEMIOLOGY: Second most common skin cancer, rare in the dark-skinned races. ETIOLOGY: Ultraviolet light, HPV infection. PATHOGENESIS:
I have a skin lump doc! What s next? 12 th August 2017 Dr. Sue-Ann Ho Ju Ee
 I have a skin lump doc! What s next? 12 th August 2017 Dr. Sue-Ann Ho Ju Ee Some thoughts Is this skin cancer? How common is this? How likely is this in this patient? What happens next if it s something
I have a skin lump doc! What s next? 12 th August 2017 Dr. Sue-Ann Ho Ju Ee Some thoughts Is this skin cancer? How common is this? How likely is this in this patient? What happens next if it s something
UNIVERSITY OF MEDICINE AND PHARMACY OF CRAIOVA FACULTY OF MEDICINE DOCTORAL THESIS SUMMARY
 UNIVERSITY OF MEDICINE AND PHARMACY OF CRAIOVA FACULTY OF MEDICINE DOCTORAL THESIS SUMMARY CLINICAL, HISTOPATHOLOGICAL AND IMMUNOHISTOCHEMICAL STUDY OF THE EPITHELIAL PRECANCEROUS LESIONS PRECURSORS OF
UNIVERSITY OF MEDICINE AND PHARMACY OF CRAIOVA FACULTY OF MEDICINE DOCTORAL THESIS SUMMARY CLINICAL, HISTOPATHOLOGICAL AND IMMUNOHISTOCHEMICAL STUDY OF THE EPITHELIAL PRECANCEROUS LESIONS PRECURSORS OF
Vemurafenib (Zelboraf ) BRAF V600 mutation positive Metastatic Melanoma
 Vemurafenib (Zelboraf ) BRAF V600 mutation positive Metastatic Melanoma Background: Vemurafenib has been approved by NICE for the treatment of BRAF V600 mutation positive unresectable or metastatic melanoma.
Vemurafenib (Zelboraf ) BRAF V600 mutation positive Metastatic Melanoma Background: Vemurafenib has been approved by NICE for the treatment of BRAF V600 mutation positive unresectable or metastatic melanoma.
Early View Article: Online published version of an accepted article before publication in the final form.
 : Online published version of an accepted article before publication in the final form. Journal Name: International Journal of Case Reports and Images (IJCRI) Type of Article: Case Report Title: A Case
: Online published version of an accepted article before publication in the final form. Journal Name: International Journal of Case Reports and Images (IJCRI) Type of Article: Case Report Title: A Case
What's New in Oncodermatopathology: Immunotherapy Reactions
 What's New in Oncodermatopathology: Immunotherapy Reactions Emily Y. Chu, M.D., Ph.D. Assistant Professor of Dermatology & Pathology and Laboratory Medicine Hospital of the University of Pennsylvania March
What's New in Oncodermatopathology: Immunotherapy Reactions Emily Y. Chu, M.D., Ph.D. Assistant Professor of Dermatology & Pathology and Laboratory Medicine Hospital of the University of Pennsylvania March
Corporate Medical Policy
 Corporate Medical Policy BRAF Gene Variant Testing to Select Melanoma or Glioma Patients File Name: Origination: Last CAP Review: Next CAP Review: Last Review: braf_gene_variant_testing_to_select_melanoma_or_glioma_patients_for_targeted_
Corporate Medical Policy BRAF Gene Variant Testing to Select Melanoma or Glioma Patients File Name: Origination: Last CAP Review: Next CAP Review: Last Review: braf_gene_variant_testing_to_select_melanoma_or_glioma_patients_for_targeted_
Sudden Appearance of Indurated Erythematous Plaques on a Man's Face
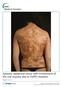
 Touro College and University System Touro Scholar NYMC Student Publications Students 2016 Sudden Appearance of Indurated Erythematous Plaques on a Man's Face Adam Carter New York Medical College Karthikeyan
Touro College and University System Touro Scholar NYMC Student Publications Students 2016 Sudden Appearance of Indurated Erythematous Plaques on a Man's Face Adam Carter New York Medical College Karthikeyan
Advances in Melanoma
 Advances in Melanoma The Blue, the Black and the Ugly 1 Outline History of Melanoma Why be concerned? Skin cancer updates What s old? What s new (and why are Skin Tumor Group med oncs excited again)? What
Advances in Melanoma The Blue, the Black and the Ugly 1 Outline History of Melanoma Why be concerned? Skin cancer updates What s old? What s new (and why are Skin Tumor Group med oncs excited again)? What
Pathology of the skin. 2nd Department of Pathology, Semmelweis University
 Pathology of the skin 2nd Department of Pathology, Semmelweis University Histology of the skin Epidermis: Stratum corneum Stratum granulosum Stratum spinosum Stratum basale Dermis: papillary and reticular
Pathology of the skin 2nd Department of Pathology, Semmelweis University Histology of the skin Epidermis: Stratum corneum Stratum granulosum Stratum spinosum Stratum basale Dermis: papillary and reticular
Premalignant skin tumours
 Chapter 14: Premalignant skin tumours page: 434 Premalignant skin tumours page: 435 Solar keratoses (senile keratoses) Raised red and well-defined plaques with a rough surface covered in scales of varying
Chapter 14: Premalignant skin tumours page: 434 Premalignant skin tumours page: 435 Solar keratoses (senile keratoses) Raised red and well-defined plaques with a rough surface covered in scales of varying
Glenn D. Goldman, MD. Fletcher Allen Health Care. University of Vermont College of Medicine
 Glenn D. Goldman, MD Fletcher Allen Health Care University of Vermont College of Medicine Recognize and identify the main types of skin cancer Understand how and why Mohs surgery is utilized for the treatment
Glenn D. Goldman, MD Fletcher Allen Health Care University of Vermont College of Medicine Recognize and identify the main types of skin cancer Understand how and why Mohs surgery is utilized for the treatment
Case Report Two Cases of Small Cell Cancer of the Maxillary Sinus Treated with Cisplatin plus Irinotecan and Radiotherapy
 Case Reports in Otolaryngology Volume 2013, Article ID 893638, 4 pages http://dx.doi.org/10.1155/2013/893638 Case Report Two Cases of Small Cell Cancer of the Maxillary Sinus Treated with Cisplatin plus
Case Reports in Otolaryngology Volume 2013, Article ID 893638, 4 pages http://dx.doi.org/10.1155/2013/893638 Case Report Two Cases of Small Cell Cancer of the Maxillary Sinus Treated with Cisplatin plus
Table of Contents: Part 1 Medical Dermatology. Chapter 1 Acneiform Disorders. Acne. Acne Vulgaris. Pomade Acne. Steroid Acne
 Table of Contents: Part 1 Medical Dermatology Chapter 1 Acneiform Disorders Acne Acne Vulgaris Pomade Acne Steroid Acne Infantile Acne Pediatric Perspectives Neonatal Acne (Acne Neonatorum) Pediatric Perspectives
Table of Contents: Part 1 Medical Dermatology Chapter 1 Acneiform Disorders Acne Acne Vulgaris Pomade Acne Steroid Acne Infantile Acne Pediatric Perspectives Neonatal Acne (Acne Neonatorum) Pediatric Perspectives
Reference 1 ID:
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ZELBORAF safely and effectively. See full prescribing information for ZELBORAF. ZELBORAF (vemurafenib)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ZELBORAF safely and effectively. See full prescribing information for ZELBORAF. ZELBORAF (vemurafenib)
Systemic epidermal nevus with involvement of the oral mucosa due to FGFR3 mutation
 Systemic epidermal nevus with involvement of the oral mucosa due to FGFR3 mutation Bygum et al. Bygum et al. BMC Medical Genetics 2011, 12:79 (5 June 2011) CASE REPORT Open Access Systemic epidermal nevus
Systemic epidermal nevus with involvement of the oral mucosa due to FGFR3 mutation Bygum et al. Bygum et al. BMC Medical Genetics 2011, 12:79 (5 June 2011) CASE REPORT Open Access Systemic epidermal nevus
Update on Genetic Testing for Melanoma
 Update on Genetic Testing for Melanoma Emily Y. Chu, M.D., Ph.D. Assistant Professor of Dermatology & Pathology and Laboratory Medicine Hospital of the University of Pennsylvania February 18, 2018 AAD
Update on Genetic Testing for Melanoma Emily Y. Chu, M.D., Ph.D. Assistant Professor of Dermatology & Pathology and Laboratory Medicine Hospital of the University of Pennsylvania February 18, 2018 AAD
Research Article Prognostic Factors in Advanced Non-Small-Cell Lung Cancer Patients: Patient Characteristics and Type of Chemotherapy
 SAGE-Hindawi Access to Research Lung Cancer International Volume 2011, Article ID 152125, 4 pages doi:10.4061/2011/152125 Research Article Prognostic Factors in Advanced Non-Small-Cell Lung Cancer Patients:
SAGE-Hindawi Access to Research Lung Cancer International Volume 2011, Article ID 152125, 4 pages doi:10.4061/2011/152125 Research Article Prognostic Factors in Advanced Non-Small-Cell Lung Cancer Patients:
Case Report Clear Cell Basal Cell Carcinoma
 SAGE-Hindawi Access to Research Volume 2011, Article ID 386921, 4 pages doi:10.4061/2011/386921 Case Report Clear Cell Basal Cell Carcinoma Deba P. Sarma, 1 Daniel Olson, 1 Jennifer Olivella, 1 Tracey
SAGE-Hindawi Access to Research Volume 2011, Article ID 386921, 4 pages doi:10.4061/2011/386921 Case Report Clear Cell Basal Cell Carcinoma Deba P. Sarma, 1 Daniel Olson, 1 Jennifer Olivella, 1 Tracey
Corporate Medical Policy
 Corporate Medical Policy BRAF Gene Mutation Testing to Select Melanoma or Glioma Patients File Name: Origination: Last CAP Review: Next CAP Review: Last Review: braf_gene_mutation_testing_to_select_melanoma_or_glioma_patients_for_targeted_
Corporate Medical Policy BRAF Gene Mutation Testing to Select Melanoma or Glioma Patients File Name: Origination: Last CAP Review: Next CAP Review: Last Review: braf_gene_mutation_testing_to_select_melanoma_or_glioma_patients_for_targeted_
R. F. Falkenstern-Ge, 1 S. Bode-Erdmann, 2 G. Ott, 2 M. Wohlleber, 1 and M. Kohlhäufl Introduction. 2. Histology
 Case Reports in Oncological Medicine Volume 2013, Article ID 167585, 4 pages http://dx.doi.org/10.1155/2013/167585 Case Report Late Lung Metastasis of a Primary Eccrine Sweat Gland Carcinoma 10 Years after
Case Reports in Oncological Medicine Volume 2013, Article ID 167585, 4 pages http://dx.doi.org/10.1155/2013/167585 Case Report Late Lung Metastasis of a Primary Eccrine Sweat Gland Carcinoma 10 Years after
Identifying Benign and Malignant Skin Lesions. No Disclosures. Common Benign Lesions. Benign Lesions 2/25/2018. Stucco Keratoses.
 Dermatology in Primary Care Identifying Benign and Malignant Skin Lesions Christy Quire Baker, APRN, FNP-BC, DCNP Dermatology Certified Nurse Practitioner No Disclosures Common Benign Lesions Seborrheic
Dermatology in Primary Care Identifying Benign and Malignant Skin Lesions Christy Quire Baker, APRN, FNP-BC, DCNP Dermatology Certified Nurse Practitioner No Disclosures Common Benign Lesions Seborrheic
Diploma examination. Dermatopathology: First paper. Tuesday 21 March Candidates must answer FOUR questions ONLY. Time allowed: Three hours
 Dermatopathology: First paper Tuesday 21 March 2017 1. Discuss the role of fluorescent in-situ hybridization (FISH) and emerging molecular techniques in the diagnosis of cutaneous melanocytic lesions,
Dermatopathology: First paper Tuesday 21 March 2017 1. Discuss the role of fluorescent in-situ hybridization (FISH) and emerging molecular techniques in the diagnosis of cutaneous melanocytic lesions,
Diploma Examination. Dermatopathology: First paper. Tuesday 20 March Candidates must answer FOUR questions. Time allowed: 3 hours
 Dermatopathology: First paper Tuesday 20 March 2018 Candidates must answer FOUR questions Time allowed: 3 hours 1. Give an account of the genetic aberrations encountered in Spitzoid neoplasms and how these
Dermatopathology: First paper Tuesday 20 March 2018 Candidates must answer FOUR questions Time allowed: 3 hours 1. Give an account of the genetic aberrations encountered in Spitzoid neoplasms and how these
THE IDENTIFICATION OF BRAF
 ONLINE FIRST OSERVATION Panniculitis With Arthralgia in Patients With Melanoma Treated With Selective RAF Inhibitors and Its Management Lisa Zimmer, MD; Elisabeth Livingstone, MD; Uwe Hillen, MD; Stephanie
ONLINE FIRST OSERVATION Panniculitis With Arthralgia in Patients With Melanoma Treated With Selective RAF Inhibitors and Its Management Lisa Zimmer, MD; Elisabeth Livingstone, MD; Uwe Hillen, MD; Stephanie
Histopathology: skin pathology
 Histopathology: skin pathology These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information
Histopathology: skin pathology These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information
Non-melanocytic Patterns
 Non-melanocytic Lesions Non-melanocytic Patterns Michelle Tarbox, MD Assistant Professor of Dermatology and Dermatopathology Texas Tech University Health Sciences Center 2018 Seborrheic keratoses Acanthotic
Non-melanocytic Lesions Non-melanocytic Patterns Michelle Tarbox, MD Assistant Professor of Dermatology and Dermatopathology Texas Tech University Health Sciences Center 2018 Seborrheic keratoses Acanthotic
Identifying Skin Cancer. Mary S. Stone MD Professor of Dermatology and Pathology University of Iowa Carver College of Medicine March, 2018
 Identifying Skin Cancer Mary S. Stone MD Professor of Dermatology and Pathology University of Iowa Carver College of Medicine March, 2018 American Cancer Society web site Skin Cancer Melanoma Non-Melanoma
Identifying Skin Cancer Mary S. Stone MD Professor of Dermatology and Pathology University of Iowa Carver College of Medicine March, 2018 American Cancer Society web site Skin Cancer Melanoma Non-Melanoma
Case Report Denosumab Chemotherapy for Recurrent Giant-Cell Tumor of Bone: A Case Report of Neoadjuvant Use Enabling Complete Surgical Resection
 Case Reports in Oncological Medicine Volume 2013, Article ID 496351, 4 pages http://dx.doi.org/10.1155/2013/496351 Case Report Denosumab Chemotherapy for Recurrent Giant-Cell Tumor of Bone: A Case Report
Case Reports in Oncological Medicine Volume 2013, Article ID 496351, 4 pages http://dx.doi.org/10.1155/2013/496351 Case Report Denosumab Chemotherapy for Recurrent Giant-Cell Tumor of Bone: A Case Report
Glenn D. Goldman, MD. University of Vermont Medical Center. University of Vermont College of Medicine
 Glenn D. Goldman, MD University of Vermont Medical Center University of Vermont College of Medicine Recognize and identify the main types of skin cancer and their precursors Identify and understand new
Glenn D. Goldman, MD University of Vermont Medical Center University of Vermont College of Medicine Recognize and identify the main types of skin cancer and their precursors Identify and understand new
Periocular Malignancies
 Periocular Malignancies Andrew Gurwood, O.D., F.A.A.O., Dipl. Marc Myers, O.D., F.A.A.O. Drs. Myers and Gurwood have no financial interests to disclose. Course Description Discussion of the most common
Periocular Malignancies Andrew Gurwood, O.D., F.A.A.O., Dipl. Marc Myers, O.D., F.A.A.O. Drs. Myers and Gurwood have no financial interests to disclose. Course Description Discussion of the most common
Approaches To Treating Advanced Melanoma
 Approaches To Treating Advanced Melanoma Suraj Venna, MD Medical Director, Melanoma and Cutaneous Oncology Inova Schar Cancer Institute Associate Professor, VCU Fairfax VA Disclosures No relevant disclosures
Approaches To Treating Advanced Melanoma Suraj Venna, MD Medical Director, Melanoma and Cutaneous Oncology Inova Schar Cancer Institute Associate Professor, VCU Fairfax VA Disclosures No relevant disclosures
Lumps and Bumps: An Organized Approach to Diagnosis and Management. Disclosure. Introduction. References. Structure of Skin.
 Lumps and Bumps: An Organized Approach to Diagnosis and Management Nothing to disclose Disclosure Tammy Pifer Than, MS, OD, FAAO Carl Vinson VAMC tammythan@bellsouth.net References Fitzpatrick's Color
Lumps and Bumps: An Organized Approach to Diagnosis and Management Nothing to disclose Disclosure Tammy Pifer Than, MS, OD, FAAO Carl Vinson VAMC tammythan@bellsouth.net References Fitzpatrick's Color
Questions. Answers. Share your photos and diagnoses with us!
 Illustrated quizzes on problems seen in everyday practice CASE 1 A 66-year-old male presents with ruddy-brown, pruritic papules on his chest and back that have been present for several years. The patient
Illustrated quizzes on problems seen in everyday practice CASE 1 A 66-year-old male presents with ruddy-brown, pruritic papules on his chest and back that have been present for several years. The patient
Skin Cancers Emerging Trends and Treatment Approaches
 Skin Cancers Emerging Trends and Treatment Approaches Andrei Metelitsa, MD, FRCPC, FAAD Clinical Associate Professor, Dermatology, U of C Co-Director, Institute for Skin Advancement Copyright 2017 by Sea
Skin Cancers Emerging Trends and Treatment Approaches Andrei Metelitsa, MD, FRCPC, FAAD Clinical Associate Professor, Dermatology, U of C Co-Director, Institute for Skin Advancement Copyright 2017 by Sea
