medicines_management/correspondence/pathway-for-the-managed- Access-of-FreeStyle-Libre.
|
|
|
- Loren Welch
- 5 years ago
- Views:
Transcription
1 Pathway for the Managed Access of FreeStyle Libre (Flash Glucose monitoring) for Adults and Children in the care of Trust Specialist Diabetes Clinics in Northern Ireland medicines_management/correspondence/pathway-for-the-managed- Access-of-FreeStyle-Libre.docx This pathway is for use by secondary care diabetes health care professionals, to assess if patients are suitable for a trial period of the FreeStyle Libre system or for supply of sensors on prescription (for those who have previously been self-funding). It does not cover assessment of patients for continuous glucose monitoring. The pathway provides criteria and additional points for consideration to support clinical decision making processes. The application of the points for consideration will be dependent upon clinical judgement and individual patient circumstances. Note: The use of FreeStyle Libre in pregnancy is outside the scope of this pathway Criteria for a 3-6 month trial of FreeStyle Libre (NOTE: These criteria must also be met when establishing if patients who have been selffunding are now eligible for supply of sensors on prescription). 1) Type 1 Diabetes. 2) Have undergone (or willing to undertake) previous advanced insulin selfmanagement education e.g. DAFNE, BERTIE, CHOICE. Additional clinical points to be considered: 3) Utilising effective basal bolus insulin self-management with evidence of CHO counting and correction insulin use with an SMBG frequency of >4 tests daily. 4) Problematic BG Control or difficulties maintaining good control, despite the above e.g. a. Variable SMBG control with episodic hypoglycaemia impacting on lifestyle. b. Recurrent hypoglycaemia (> 3 episodes per week or >2 severe episodes in a year). c. Loss of hypoglycaemia Awareness Symptoms ( Modified Clarke and Gold Score >4; NICE NG17. See Appendix 1). d. Persistent Elevation of HbA1c despite insulin dose adjustments. e. Severe physical, psychological or occupational barriers to effective SMBG. 1
2 5) Substantial evidence of benefit in maintaining good glucose control with a sensor device. 6) Regular attendance at secondary care clinic. Action Pathway (for patients assessed as suitable for a trial of FreeStyle Libre ) Initial assessments. Full examination and biochemical assessment of potential confounders of control (e.g. thyroid/adrenal/coeliac assessment). SMBG control or Diagnostic (Professional) CGM (ipro or equivalent) as a baseline assessment. All outcomes of assessments and clinical results should be recorded in the patient s medical notes. Consent and Training For first time users of FreeStyle Libre Patient consent obtained to ensure that they wish to trial the FreeStyle Libre system. Patient referred to clinic for training. Attendance at training confirmed and patient still wishes to proceed. Patient supplied with FreeStyle Libre reader and starter pack of sensors at training clinic. For ALL patients (including those who have previously self-funded FreeStyle Libre ) Patients to be informed that they will be reviewed in 3-6 months, to assess if they have met the criteria for ongoing use of FreeStyle Libre. Patient s current method of testing blood glucose and/or blood ketones to be reviewed. Note: The FreeStyle Libre reader has a built in port to enable blood glucose and ketone testing. This requires use of FreeStyle Optium blood glucose test strips and FreeStyle Optium β Ketone strips, which are NOT cost effective choices of blood glucose and ketone strips. (see price lists in appendix 6). Patients should therefore be given separate glucometer(s), which, where possible use cost effective blood glucose strips; < 10 for 50 and cost effective ketone test strips < 10 for 10, to use alongside FreeStyle Libre. Patient provided with patient information leaflet (see sample leaflets; appendix 4 and 5). This should explain the process for review, so that the patients understand, from the outset that prescribing may not continue if criteria are 2
3 not met and also details of what to do if they receive a faulty sensor i.e. contact Abbott Manufacturer; Customer Service Telephone number: (Mon Fri 8am -8pm) excluding bank holidays). GP Communication Letter to be issued from the training clinic to patients GP. Letter to outline the following: (see sample letters in appendix; 2 and 3). Patient has met the criteria for a 3-6 month trial of FreeStyle Libre or is now eligible to receive sensors on prescription, after previously self- funding. Patient has received training on the device. If the patient is less than 4 years old, GP to be informed that use of FreeStyle Libre is off licence. Clarification obtained from GP that they are willing to undertake prescribing. First time users have been supplied with FreeStyle Libre reader and starter pack of sensors Request GP to issue prescriptions for FreeStyle Libre sensors (maximum 2 per month). Inform GP of the patient s review date for assessment of continued use. Inform GP of the name of blood glucose test strips (and ketone strips, if appropriate), which will be used alongside the FreeStyle Libre system. Inform GP that they should contact secondary care at any time, if they are concerned as to the appropriateness of the continued use of FreeStyle Libre system for a particular patient. Review criteria Ideally 3 monthly assessments (with regular data upload) whilst on trial to confirm: 1) Use of each sensor for at least 70% of the time. 2) Effect of sensor use on reduction of BG variability and increase of percentage of time spent in target BG range (4-10mmol/L) compared to baseline 3) Improvement in the initial problem triggering the trial. If all 3 criteria are achieved, then approve continued use of sensors (reviewed annually) with expectation of ongoing regular contact and sensor upload by diabetes specialist team. 3
4 If use is suboptimal or does not result in effective change then sensor supply withdrawn and alternative avenues for the assistance of the individual are assessed. 4) Patient s GP to be informed by letter of outcome of the review. Advised to cease or continue prescribing of FreeStyle Libre sensors. Specialist Clinic Reporting to Diabetes Network Technology sub group 1) Quarterly report of numbers of patients assessed by GP practice 2) Quarterly report of numbers of patients offered a 3-6 month trials. 3) Quarterly report of numbers of trial successful and numbers of established users continuing effective use (after year 1). Audit Trusts will undertake to carry out regular audits to ensure that the pathway has been appropriately applied, with results reported to the Technology Subgroup. Initial audit to be undertaken after 6 months and annually thereafter. Review This pathway will be reviewed as further clinical evidence for FreeStyle Libre becomes available or guidance is issued by NICE. 4
5 5
6 Appendix 1: Clarke Hypoglycaemic Index Score 1. Pick the category that best describes you: (pick only one) I always have symptoms when my blood sugar is low 0 total I sometimes have symptoms when my blood sugar is low 1 I no longer have symptoms when my blood sugar is low 2. Have you lost some of the symptoms that used to occur when your blood sugar was low? Yes 1 total No 0 3. In the past 6 months how often have you had moderate hypoglycemia episodes? (Episodes where you might feel confused, disoriented, or lethargic and were unable to treat yourself) Never 0 total once or twice in 6 months every other month 1 once a month more than once a month 4. In the past year how often have you had severe hypoglycemia episodes? (Episodes where you were unconscious or had a seizure and needed glucagon or intravenous glucose) Never 0 total 1 time 2 times 1 3 times 4 times 5. How often in the last month have you had readings < 3.5mmol/L with symptoms? never 1 time/week 4 to 5 times/week 1 to 3 times 2 to 3 times/week almost daily 6. How often in the last month have you had readings < 3.5 mmol/l without symptoms? S c never 1 time/week 4 to 5 times/week o 1 to 3 times 2 to 3 times/week almost daily 7. How low does your blood sugar need to go before you feel symptoms? total < To what extent can you tell by your symptoms that your blood sugar is low? Always 0 total often sometimes 1 rarely never Score 1 if reading <3.5 with no symptoms Total = 6
7 Appendix 2: Sample letter to send to GP for first time users of FreeStyle Libre (following patient s attendance at training clinic) Dear Dr {insert name}, Your patient, {insert name} has been assessed in line with regional guidance, as eligible for a trial of the flash glucose monitoring System, FreeStyle Libre. {Insert name} has attended a training clinic on {insert date}. We have supplied the patient with a FreeStyle Libre reader and starter pack of sensors. We would be grateful if you could continue the prescribing of FreeStyle Libre sensors on a monthly basis for the patient. Each sensor lasts for 14 days, so a supply of 2 sensors per month is necessary. Patients have been advised that should they receive a faulty sensor, they should NOT request additional supplies via prescription but contact the company to obtain a replacement. (Abbott UK General Customer Service telephone number is It is open from 8am to 8pm, Monday to Friday; excluding bank holidays). {Insert name} will be reviewed on {insert date} to assess if they have met the criteria to continue long term use of the FreeStyle Libre system. We will write to you, following this review, to inform you of the outcome. In some cases ongoing prescribing of FreeStyle Libre sensors will not be required. If, at any time, you are concerned about the appropriateness of the use of FreeStyle Libre for your patient, please feel free to contact us directly. Patients will still, on occasions (e.g. to meet DVLA requirements, during periods of sickness) be required to perform blood glucose {*delete as appropriate and/or ketone blood} testing. Please prescribe {insert name of blood glucose test strip and/or ketone test strips} for use alongside the FreeStyle Libre system. Yours sincerely, {Insert prescriber s name} 7
8 Appendix 3: Sample letter to send to GP for patients who have already been using the FreeStyle Libre system. Dear Dr {insert name}, Your patient, {insert name} has been self- funding the FreeStyle Libre system and has been assessed in line with regional guidance, as now being eligible to obtain FreeStyle Libre sensors on prescription. The patient has their own FreeStyle Libre reader. We would be grateful if you could prescribe FreeStyle Libre sensors on a monthly basis for the patient. Each sensor lasts for 14 days, so a supply of 2 sensors per month is necessary. Patients have been advised, that should they should receive a faulty sensor, they should NOT request additional supplies via prescription but contact the company to obtain a replacement. (Abbott UK General Customer Service telephone number is It is open from 8am to 8pm, Monday to Friday; excluding bank holidays). {Insert name} will be reviewed on {insert date} to assess if they continue to meet the criteria for ongoing long term use of the FreeStyle Libre system. We will write to you, following this review, to inform you of the outcome. In some cases ongoing prescribing of FreeStyle Libre sensors will not be required. If at any time you are concerned about the appropriateness of the use of FreeStyle Libre for your patient, please feel free to contact us directly. Patients will still, on occasions (e.g. to meet DVLA requirements, during periods of sickness) be required to perform blood glucose {*delete as appropriate and/or ketone blood} testing. Please prescribe {insert name of blood glucose test strip and/or ketone test strips} for use alongside the FreeStyle Libre system. Yours sincerely, {Insert prescriber s name} 8
9 Appendix 4: Sample Patient Information Leaflet FreeStyle Libre (for first time users) Following assessment by your specialist diabetes team, you have been assessed as being eligible for a trial of FreeStyle Libre system. You will have received the appropriate training on the system and have been provided with a FreeStyle Libre reader and starter pack of sensors. We have written to your GP to request that they prescribe a maximum of 2 FreeStyle Libre sensors per month for you. Please note that should you receive a faulty sensor, DO NOT contact your GP for another prescription. Please contact the manufacturer (Abbott) directly for a replacement. Abbott Customer Service Telephone number: (Mon Fri 8am -8pm) excluding bank holidays). You will be reviewed at your next hospital appointment (usually in 3-6 months) where you will be assessed to check that you have met criteria to continue the prescribing of FreeStyle Libre sensors on prescription. If you meet the review criteria, prescribing of the sensors will continue and you will be reviewed on annual basis, to assess if FreeStyle Libre is still beneficial to help to manage your diabetes. In cases where the review criteria are not met, it may be necessary for your diabetes specialist to write to your GP to request that prescribing of the sensors is no longer appropriate. Your diabetes specialist will discuss with you other available options, to help manage your diabetes. You will have been made aware at your FreeStyle Libre training session that a finger-prick test using a glucometer is still required e.g. during times of rapidly changing glucose levels (i.e. acute illness), if hypoglycaemia (low blood sugars) or impending hypoglycaemia is reported, or the symptoms do not match the system readings and also prior to, and during driving, to meet current DVLA requirements. Your diabetes specialist will discuss with you the most suitable glucometer for you to use alongside your FreeStyle Libre system. If you have any queries, please feel free to contact {insert name} for further information. 9
10 Appendix 5: Sample Patient Information Leaflet FreeStyle Libre (for patients who have previously self-funded) Following assessment by your specialist diabetes team, you have been assessed as being eligible to receive FreeStyle Libre sensors on prescription. We have written to your GP to request that they prescribe a maximum of 2 FreeStyle Libre sensors per month for you. Please note that should you receive a faulty sensor, DO NOT contact your GP for another prescription. Please contact the manufacturer (Abbott) directly for a replacement. Abbott Customer Service Telephone number: (Mon Fri 8am -8pm) excluding bank holidays). You will be reviewed at your next hospital appointment (usually in 3-6 months) where you will be assessed to check that you have met criteria to continue the prescribing of FreeStyle Libre sensors on prescription. If you meet the review criteria, prescribing of the sensors will continue and you will be reviewed on annual basis, to assess if FreeStyle Libre is still beneficial to help to manage your diabetes. In cases where the review criteria are not met, it may be necessary for your diabetes specialist to write to your GP to request that prescribing of the sensors is no longer appropriate. Your diabetes specialist will discuss with you other available options, to help manage your diabetes. You will be aware when using the FreeStyle Libre system, that a finger-prick test using a glucometer is still required e.g. during times of rapidly changing glucose levels (i.e. acute illness), if hypoglycaemia (low blood sugars) or impending hypoglycaemia is reported, or the symptoms do not match the system readings and also prior to, and during driving, to meet current DVLA requirements. Your diabetes specialist will discuss with you the most suitable glucometer for you to use alongside your FreeStyle Libre system. If you have any queries, please feel free to contact {insert name} for further information. 10
THE SHEFFIELD AREA PRESCRIBING GROUP. Position Statement for Prescribing of Freestyle Libre In Type 1 Diabetes. Date: March 2018.
 THE SHEFFIELD AREA PRESCRIBING GROUP Position Statement for Prescribing of Freestyle Libre In Type 1 Diabetes Date: March 2018 Overview Freestyle Libre is a flash glucose sensor device that measures *interstitial
THE SHEFFIELD AREA PRESCRIBING GROUP Position Statement for Prescribing of Freestyle Libre In Type 1 Diabetes Date: March 2018 Overview Freestyle Libre is a flash glucose sensor device that measures *interstitial
NHS GG&C Introduction of Freestyle Libre flash glucose monitoring system
 NHS GG&C Introduction of Freestyle Libre flash glucose monitoring system The Freestyle Libre flash glucose monitoring system is a sensor based, factory-calibrated system that measures interstitial fluid
NHS GG&C Introduction of Freestyle Libre flash glucose monitoring system The Freestyle Libre flash glucose monitoring system is a sensor based, factory-calibrated system that measures interstitial fluid
Commissioning Statement. Flash Glucose Monitoring system (FreeStyle Libre ) March 2018
 Technology Commissioning Statement Flash Glucose Monitoring system (FreeStyle Libre ) March 2018 FreeStyle Libre (Abbott) Flash Glucose Monitoring System for use in adults, young people and children. Recommendation
Technology Commissioning Statement Flash Glucose Monitoring system (FreeStyle Libre ) March 2018 FreeStyle Libre (Abbott) Flash Glucose Monitoring System for use in adults, young people and children. Recommendation
Commissioning Statement
 Commissioning Statement Treatment/ device For the treatment of Commissioning position Flash Glucose Monitoring Systems (including Freestyle Libre ) Monitoring glucose levels in adults and children over
Commissioning Statement Treatment/ device For the treatment of Commissioning position Flash Glucose Monitoring Systems (including Freestyle Libre ) Monitoring glucose levels in adults and children over
Placename CCG. Policies for the Commissioning of Healthcare
 Placename CCG Policies for the Commissioning of Healthcare Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus. This document is part
Placename CCG Policies for the Commissioning of Healthcare Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus. This document is part
Implementation of Freestyle Libre prescribing guidance across the NHS in London
 Implementation of FreeStyle Libre prescribing guidance across the NHS in London Contents Section 1 Background to the document (pages 2-5) Section 2 Implementation guidance pathways 1. Recommended implementation
Implementation of FreeStyle Libre prescribing guidance across the NHS in London Contents Section 1 Background to the document (pages 2-5) Section 2 Implementation guidance pathways 1. Recommended implementation
NHS Leeds CCG. Policy for the Funding of Flash Glucose Monitoring (FlashGM) in Paediatrics and Adults
 NHS Leeds CCG Policy for the Funding of Flash Glucose Monitoring (FlashGM) in Paediatrics and Adults Produced by: Jo Alldred, Medicines Effectiveness Lead, NHS Leeds CCG Dr Bryan Power, Long Term Conditions
NHS Leeds CCG Policy for the Funding of Flash Glucose Monitoring (FlashGM) in Paediatrics and Adults Produced by: Jo Alldred, Medicines Effectiveness Lead, NHS Leeds CCG Dr Bryan Power, Long Term Conditions
FreeStyle Libre for glucose monitoring: Interim Position Statement for GPs & Patient FAQ
 North Central London Joint Formulary Committee FreeStyle Libre for glucose monitoring: Interim Position Statement for GPs & Patient FAQ GPs should not prescribe FreeStyle Libre sensors on the NHS until
North Central London Joint Formulary Committee FreeStyle Libre for glucose monitoring: Interim Position Statement for GPs & Patient FAQ GPs should not prescribe FreeStyle Libre sensors on the NHS until
Description of the technology
 Advice Note 2017/ 001 What is the clinical effectiveness, safety and budget impact of the Freestyle Libre System compared with current glucose monitoring methods for people aged 4 years and over with diabetes
Advice Note 2017/ 001 What is the clinical effectiveness, safety and budget impact of the Freestyle Libre System compared with current glucose monitoring methods for people aged 4 years and over with diabetes
Flash Glucose Monitoring (Flash GM) Frequently asked questions (November 2018)
 Flash Glucose Monitoring (Flash GM) Frequently asked questions (November 2018) What is Flash Glucose Monitoring (Flash GM)? Flash glucose monitoring is a small sensor that you wear on your skin (usually
Flash Glucose Monitoring (Flash GM) Frequently asked questions (November 2018) What is Flash Glucose Monitoring (Flash GM)? Flash glucose monitoring is a small sensor that you wear on your skin (usually
How can I access flash glucose monitoring if I need it? Support pack. This pack will help you to find out more about flash and how you can access it.
 How can I access flash glucose monitoring if I need it? Support pack This pack will help you to find out more about flash and how you can access it. Reviewed March 2019 Introduction Following several major
How can I access flash glucose monitoring if I need it? Support pack This pack will help you to find out more about flash and how you can access it. Reviewed March 2019 Introduction Following several major
Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus
 Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus Version No. Changes Made Version of 05.10.2018 V1 Policy ratified by Healthier
Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus Version No. Changes Made Version of 05.10.2018 V1 Policy ratified by Healthier
In keeping with the Scottish Diabetes Group criteria, use should be restricted to those who:
 Advice Statement 009-18 July 2018 Advice Statement What is the clinical and cost effectiveness of Freestyle Libre flash glucose monitoring for patients with diabetes mellitus treated with intensive insulin
Advice Statement 009-18 July 2018 Advice Statement What is the clinical and cost effectiveness of Freestyle Libre flash glucose monitoring for patients with diabetes mellitus treated with intensive insulin
Policy for Continuous Glucose Monitoring for Type 1 Diabetic Paediatric Patients (<18 years of age)
 Policy for Continuous Glucose Monitoring for Type 1 Diabetic Paediatric Patients (
Policy for Continuous Glucose Monitoring for Type 1 Diabetic Paediatric Patients (
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) JAPC Briefing FreeStyle Libre
 DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) JAPC Briefing FreeStyle Libre JAPC has classified Freestyle Libre as BROWN after diabetes consultant/specialist initiation within a Derbyshire diabetes
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) JAPC Briefing FreeStyle Libre JAPC has classified Freestyle Libre as BROWN after diabetes consultant/specialist initiation within a Derbyshire diabetes
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
Blood Glucose monitoring during extra-corporeal renal therapy and plasmapheresis.
 Blood Glucose monitoring during extra-corporeal renal therapy and plasmapheresis. Lead Clinician: Dr. R. Diwakar Implementation date: July 2013 Last updated: August 2017 Last review date: Planned review
Blood Glucose monitoring during extra-corporeal renal therapy and plasmapheresis. Lead Clinician: Dr. R. Diwakar Implementation date: July 2013 Last updated: August 2017 Last review date: Planned review
Placename CCG. Policies for the Commissioning of Healthcare
 Placename CCG Policies for the Commissioning of Healthcare Policy for the funding of insulin pumps and continuous glucose monitoring devices for patients with diabetes 1 Introduction 1.1 This document
Placename CCG Policies for the Commissioning of Healthcare Policy for the funding of insulin pumps and continuous glucose monitoring devices for patients with diabetes 1 Introduction 1.1 This document
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose should be offered to all newly diagnosed type 2 diabetic patients as an integral part
Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose should be offered to all newly diagnosed type 2 diabetic patients as an integral part
Information leaflet for MDI patients. Improving Diabetes using Diasend
 Information leaflet for MDI patients Improving Diabetes using Diasend Contents Easy Steps for Setting up Diasend Account 2 Finding the resources on the internet 3 VIP Patient Diasend Assessment Template
Information leaflet for MDI patients Improving Diabetes using Diasend Contents Easy Steps for Setting up Diasend Account 2 Finding the resources on the internet 3 VIP Patient Diasend Assessment Template
INFORMATION ON DRIVING AND DIABETES: GROUP 2 VEHICLES
 INFORMATION ON DRIVING AND DIABETES: GROUP 2 VEHICLES This information leaflet offers information on what is now required if you wish to hold a Group 2 Lorries, bus, taxi and HGV driving licence and to
INFORMATION ON DRIVING AND DIABETES: GROUP 2 VEHICLES This information leaflet offers information on what is now required if you wish to hold a Group 2 Lorries, bus, taxi and HGV driving licence and to
Healthcare professionals involved in care of children and young people with Type 1 Diabetes Mellitus. Children and young people with diabetes mellitus
 ssociation of Children s Diabetes Clinical Guideline A Practical Approach to the Management of Continuous Glucose Monitoring (CGM) / Real-Time Flash Glucose Scanning (FGS) in Type 1 Diabetes Mellitus in
ssociation of Children s Diabetes Clinical Guideline A Practical Approach to the Management of Continuous Glucose Monitoring (CGM) / Real-Time Flash Glucose Scanning (FGS) in Type 1 Diabetes Mellitus in
Country Health SA Local Health Network. Title: Continuous Glucose Monitoring (CGM) and Flash Glucose Monitoring (FGM) Ambulatory Service
 Protocol (Clinical) Title: Continuous Glucose Monitoring (CGM) and Flash Glucose Monitoring (FGM) Ambulatory Service Protocol developed by: CHSALHN Diabetes Service Protocol Sponsor: CHSALHN, Executive
Protocol (Clinical) Title: Continuous Glucose Monitoring (CGM) and Flash Glucose Monitoring (FGM) Ambulatory Service Protocol developed by: CHSALHN Diabetes Service Protocol Sponsor: CHSALHN, Executive
Preconception advice for women with type 1 and 2 diabetes. Points to consider before or as soon as you learn that you are pregnant.
 Preconception advice for women with type 1 and 2 diabetes Points to consider before or as soon as you learn that you are pregnant. General advice for women planning pregnancy Folic acid tablets: Doctors
Preconception advice for women with type 1 and 2 diabetes Points to consider before or as soon as you learn that you are pregnant. General advice for women planning pregnancy Folic acid tablets: Doctors
Healthcare professionals involved in care of children and young people with Type 1 Diabetes Mellitus
 Clinical Guideline A Practical Approach to the Management of Continuous Glucose Monitoring (CGM) / Real-Time Flash Glucose Scanning (FGS) in Type 1 Diabetes Mellitus in Children and Young People Under
Clinical Guideline A Practical Approach to the Management of Continuous Glucose Monitoring (CGM) / Real-Time Flash Glucose Scanning (FGS) in Type 1 Diabetes Mellitus in Children and Young People Under
Integrated Community Diabetes Services (ICDS) GP Referral Guide Version 3 - October 2014
 Integrated Community Diabetes Services (ICDS) GP Referral Guide Version 3 - October 2014 Introduction The Integrated Community Diabetes Service (ICDS) will deliver high quality care to individuals who
Integrated Community Diabetes Services (ICDS) GP Referral Guide Version 3 - October 2014 Introduction The Integrated Community Diabetes Service (ICDS) will deliver high quality care to individuals who
Consultation Group: Dr Amalia Mayo, Paediatric Consultant. Review Date: March Uncontrolled when printed. Version 2. Executive Sign-Off
 Policy For The Adjustment Of Insulin Injections By Paediatric Diabetes Specialist Nurses/Community Paediatric Nurses Diabetes Working With Children Within NHS Grampian Co-ordinators: Lead Paediatric Diabetes
Policy For The Adjustment Of Insulin Injections By Paediatric Diabetes Specialist Nurses/Community Paediatric Nurses Diabetes Working With Children Within NHS Grampian Co-ordinators: Lead Paediatric Diabetes
Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes
 Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Standard Operating Procedure Completion of DAFNE data collection: Core baseline form F04.007
 Standard Operating Procedure Completion of DAFNE data collection: Core baseline form F04.007 DAFNE P01.013 version 10, February 2016 1 of 10 CONTENTS Introduction... 3 Patient DAFNE number... 3 Type of
Standard Operating Procedure Completion of DAFNE data collection: Core baseline form F04.007 DAFNE P01.013 version 10, February 2016 1 of 10 CONTENTS Introduction... 3 Patient DAFNE number... 3 Type of
24 Hour Support. Telephone Available 24 hours a day, 7 days a week
 Contents Page What is SHAIRE? 1 What is basal-bolus regimen? 2 Why do I need a basal-bolus regimen? 3 How does basal insulin work? 3 How does rapid-acting insulin work? 4 How often should I test my Blood
Contents Page What is SHAIRE? 1 What is basal-bolus regimen? 2 Why do I need a basal-bolus regimen? 3 How does basal insulin work? 3 How does rapid-acting insulin work? 4 How often should I test my Blood
TYPE 1 DIABETES on a pump
 TYPE 1 DIABETES on a pump Advice from the Cedar Centre Patient information leaflet How do I look after myself if I am unwell and have TYPE 1 DIABETES? We all get ill occasionally with the flu or a tummy
TYPE 1 DIABETES on a pump Advice from the Cedar Centre Patient information leaflet How do I look after myself if I am unwell and have TYPE 1 DIABETES? We all get ill occasionally with the flu or a tummy
In Hospital Management of Hypoglycaemia in Adults with Diabetes Mellitus
 In Hospital Management of Hypoglycaemia in Adults with Diabetes Mellitus This procedural document supersedes any previous guidelines in relation to this subject: PAT/T 49 v.2 In Hospital Management of
In Hospital Management of Hypoglycaemia in Adults with Diabetes Mellitus This procedural document supersedes any previous guidelines in relation to this subject: PAT/T 49 v.2 In Hospital Management of
Commissioning Policy
 Commissioning Policy Abbott FreeStyle Libre Flash Glucose Monitoring System Individual Funding Request Date Adopted: 09 September 2018 Version: 1819.1.02 Title of document: Authors job title(s): Document
Commissioning Policy Abbott FreeStyle Libre Flash Glucose Monitoring System Individual Funding Request Date Adopted: 09 September 2018 Version: 1819.1.02 Title of document: Authors job title(s): Document
Continuous Glucose Monitoring
 Continuous Glucose Monitoring Information about fully-subsidised continuous glucose monitoring for children and young people with type 1 diabetes Continuous glucose monitoring (CGM) can help in managing
Continuous Glucose Monitoring Information about fully-subsidised continuous glucose monitoring for children and young people with type 1 diabetes Continuous glucose monitoring (CGM) can help in managing
Policy for the Provision of Insulin Pumps for Patients with Diabetes Mellitus
 Policy for the Provision of Insulin Pumps for Patients with Diabetes Mellitus Version No. Changes Made Version of July 2018 V0.5 Changes made to the policy following patient engagement including: - the
Policy for the Provision of Insulin Pumps for Patients with Diabetes Mellitus Version No. Changes Made Version of July 2018 V0.5 Changes made to the policy following patient engagement including: - the
Agenda Item 10 - Glucose Monitoring Device for Individuals with Diabetes
 Dorset Health Scrutiny Committee 17 October 2018 Public Participation Agenda Item 10 - Glucose Monitoring Device for Individuals with Diabetes Questions 1. Rosie and Kirsty Edwardes, Bridport Residents
Dorset Health Scrutiny Committee 17 October 2018 Public Participation Agenda Item 10 - Glucose Monitoring Device for Individuals with Diabetes Questions 1. Rosie and Kirsty Edwardes, Bridport Residents
Control of Glycemic Variability for Reducing Hypoglycemia Jae Hyeon Kim
 Control of Glycemic Variability for Reducing Hypoglycemia Jae Hyeon Kim Division of Endocrinology and Metabolism, Samsung Medical Center, Sungkyunkwan University School of Medicine Conflict of interest
Control of Glycemic Variability for Reducing Hypoglycemia Jae Hyeon Kim Division of Endocrinology and Metabolism, Samsung Medical Center, Sungkyunkwan University School of Medicine Conflict of interest
Continuous Glucose Monitoring (CGM)/Real-Time Flash Glucose Scanning (FGS) Training for Healthcare Professionals and Patients
 ssociation of Children s Diabetes Continuous Glucose Monitoring (CGM)/Real-Time Flash Glucose Scanning (FGS) Training for Healthcare Professionals and Patients 1 ssociation of Children s Diabetes Contents
ssociation of Children s Diabetes Continuous Glucose Monitoring (CGM)/Real-Time Flash Glucose Scanning (FGS) Training for Healthcare Professionals and Patients 1 ssociation of Children s Diabetes Contents
02/GMS/0030 ADULT EPILEPSY SERVICE CCP for General Medical and Surgical POOLE HOSPITAL NHS FOUNDATION TRUST
 Service Specification No. Service Commissioner Leads 02/GMS/0030 ADULT EPILEPSY SERVICE CCP for General Medical and Surgical Provider Lead POOLE HOSPITAL NHS FOUNDATION TRUST Period 1 April 2013 to 31
Service Specification No. Service Commissioner Leads 02/GMS/0030 ADULT EPILEPSY SERVICE CCP for General Medical and Surgical Provider Lead POOLE HOSPITAL NHS FOUNDATION TRUST Period 1 April 2013 to 31
Type 2 Diabetes Recommended SMBG
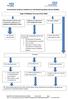 Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
Trust Protocol for the Administration of Intravenous Methylprednisolone for Thyroid Eye Disease A Protocol. The Clinical Investigation Unit (CIU)
 A Protocol For Use in: By: For: Division responsible for document: Key words: Name of document author: Job title of document author: Name and job title of document author s Line Manager: Supported by:
A Protocol For Use in: By: For: Division responsible for document: Key words: Name of document author: Job title of document author: Name and job title of document author s Line Manager: Supported by:
Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes
 Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
A GUIDE TO STARTING HUMALOG
 A GUIDE TO STARTING HUMALOG This booklet is intended only for those who have been prescribed Humalog. It is intended to be used in addition to the Patient Information Leaflet (PIL) which is included in
A GUIDE TO STARTING HUMALOG This booklet is intended only for those who have been prescribed Humalog. It is intended to be used in addition to the Patient Information Leaflet (PIL) which is included in
Monitoring in Type 2 Diabetes. Learning Outcomes. Type 2 Diabetes. Senga Hunter Community Diabetes Specialist Nurse
 Monitoring in Type 2 Diabetes Senga Hunter Community Diabetes Specialist Nurse Learning Outcomes Understand why blood monitoring is necessary Understand the blood tests for monitoring diabetes Understand
Monitoring in Type 2 Diabetes Senga Hunter Community Diabetes Specialist Nurse Learning Outcomes Understand why blood monitoring is necessary Understand the blood tests for monitoring diabetes Understand
Pathway for Adult Patients with Diabetes attending the Emergency Department (ED) with Hypoglycaemia
 Leicestershirediabetes Guidelines Pathway for Adult Patients with Diabetes attending the Emergency Department (ED) with Hypoglycaemia Patient attends ED Support available from Diabetes Specialist Nurses
Leicestershirediabetes Guidelines Pathway for Adult Patients with Diabetes attending the Emergency Department (ED) with Hypoglycaemia Patient attends ED Support available from Diabetes Specialist Nurses
Audit support for continuous subcutaneous insulin infusion for the treatment of diabetes mellitus (review of technology appraisal guidance 57)
 Audit support for continuous subcutaneous insulin (review of technology appraisal guidance 57) Issue date: 2008 Audit support Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus
Audit support for continuous subcutaneous insulin (review of technology appraisal guidance 57) Issue date: 2008 Audit support Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus
FLIPS FreeStyle Libre in Pregnancy Study
 FLIPS FreeStyle Libre in Pregnancy Study Evaluation of the Accuracy of the FreeStyle Libre Flash Glucose Monitoring System Use in Pregnancy Label Extension Study (CE) Section 1: PARTICIPANT INFORMATION
FLIPS FreeStyle Libre in Pregnancy Study Evaluation of the Accuracy of the FreeStyle Libre Flash Glucose Monitoring System Use in Pregnancy Label Extension Study (CE) Section 1: PARTICIPANT INFORMATION
Pre-Dialysis Insulin Information for Patients with Type 2 Diabetes receiving haemodialysis
 Pre-Dialysis Insulin Information for Patients with Type 2 Diabetes receiving haemodialysis Exceptional healthcare, personally delivered 2 This leaflet is for patients with Type 2 diabetes who require insulin
Pre-Dialysis Insulin Information for Patients with Type 2 Diabetes receiving haemodialysis Exceptional healthcare, personally delivered 2 This leaflet is for patients with Type 2 diabetes who require insulin
REPORT INTERPRETATION
 REPORT INTERPRETATION: Interpreting ipro Professional Continuous Glucose Monitoring (CGM) Reports and Making Therapy Adjustments TARGET AUDIENCE The audience for this section is physicians, mid-level practitioners,
REPORT INTERPRETATION: Interpreting ipro Professional Continuous Glucose Monitoring (CGM) Reports and Making Therapy Adjustments TARGET AUDIENCE The audience for this section is physicians, mid-level practitioners,
June 2016 Review: June 2019
 BEDFORDSHIRE & LUTON JOINT PRESCRIBING COMMITTEE June 2016 Review: June 2019 Bulletin 241 : Guidelines on self-monitoring of blood glucose (SMBG) in non-insulin treated Type 2 diabetic patients for maintaining
BEDFORDSHIRE & LUTON JOINT PRESCRIBING COMMITTEE June 2016 Review: June 2019 Bulletin 241 : Guidelines on self-monitoring of blood glucose (SMBG) in non-insulin treated Type 2 diabetic patients for maintaining
NICE to. know... The facts you need to make the most of your diabetes care.
 NICE to know... 3 The facts you need to make the most of your diabetes care. NICE to know... The National Institute for Health and Care Excellence (NICE) provides advice on the care and support that should
NICE to know... 3 The facts you need to make the most of your diabetes care. NICE to know... The National Institute for Health and Care Excellence (NICE) provides advice on the care and support that should
Medicines Optimisation Team Standard Operating procedure for Alogliptin Audit:
 Medicines Optimisation Team Standard Operating procedure for Alogliptin Audit: To review type 2 diabetic patients prescribed dipedtidylpeptidase-4 (DPP-4) inhibitors (linagliptin (Trajenta, saxagliptin
Medicines Optimisation Team Standard Operating procedure for Alogliptin Audit: To review type 2 diabetic patients prescribed dipedtidylpeptidase-4 (DPP-4) inhibitors (linagliptin (Trajenta, saxagliptin
Executive Summary Management of Type 1 Diabetes Mellitus during illness in children and young people under 18 years (Sick Day Rules)
 Executive Summary Management of Type 1 Diabetes Mellitus during illness in children and young people under 18 years (Sick Day Rules) SETTING FOR STAFF PATIENTS Medical and nursing staff Children and young
Executive Summary Management of Type 1 Diabetes Mellitus during illness in children and young people under 18 years (Sick Day Rules) SETTING FOR STAFF PATIENTS Medical and nursing staff Children and young
Diabetes Subcommittee of PTAC meeting held 3 March (minutes for web publishing)
 Diabetes Subcommittee of PTAC meeting held 3 March 2011 (minutes for web publishing) Diabetes Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology and Therapeutics
Diabetes Subcommittee of PTAC meeting held 3 March 2011 (minutes for web publishing) Diabetes Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology and Therapeutics
EFFECTIVE SHARE CARE AGREEMENT. FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY
 Specialist details Patient identifier Name Tel: EFFECTIVE SHARE CARE AGREEMENT FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY The aim of Effective Shared Care Guidelines
Specialist details Patient identifier Name Tel: EFFECTIVE SHARE CARE AGREEMENT FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY The aim of Effective Shared Care Guidelines
Standard Operating Procedure 11. Completion of DAFNE Data Collection: Full Post Course Form F04.010, Version 8
 Standard Operating Procedure 11 Completion of DAFNE Data Collection: Full Post Course Form F04.010, Version 8 Date Version Issue Review Contact Approved Date Date Person October 2013 9 October 2013 March
Standard Operating Procedure 11 Completion of DAFNE Data Collection: Full Post Course Form F04.010, Version 8 Date Version Issue Review Contact Approved Date Date Person October 2013 9 October 2013 March
DIABETES: SAFE DRIVING AND THE DVLA
 LIFESTYLE DIABETES: SAFE DRIVING AND THE DVLA WHY IS THIS LEAFLET FOR YOU? Having diabetes does not mean that you need to give up driving. It does mean that you have a responsibility to inform certain
LIFESTYLE DIABETES: SAFE DRIVING AND THE DVLA WHY IS THIS LEAFLET FOR YOU? Having diabetes does not mean that you need to give up driving. It does mean that you have a responsibility to inform certain
DIABETES STRUCTURED EDUCATION IN WORCESTERSHIRE Information for Healthcare Professionals May 2011
 DIABETES STRUCTURED EDUCATION IN WORCESTERSHIRE Information for Healthcare Professionals May 2011 What is Structured Education? Diabetes Structured Education is referred to in the Diabetes NSF standards
DIABETES STRUCTURED EDUCATION IN WORCESTERSHIRE Information for Healthcare Professionals May 2011 What is Structured Education? Diabetes Structured Education is referred to in the Diabetes NSF standards
Trust Guideline for the Management of: Insulin Pump Therapy in Children with Type 1 Diabetes
 A clinical guideline recommended for use In: By: For: Key words: Written by: Supported by: Approved by: Jenny Lind Children s Department Jenny Lind Diabetes Team Children on CSII therapy CSII, Insulin
A clinical guideline recommended for use In: By: For: Key words: Written by: Supported by: Approved by: Jenny Lind Children s Department Jenny Lind Diabetes Team Children on CSII therapy CSII, Insulin
National Diabetes Inpatient Audit (NaDIA) 2016
 National Diabetes Inpatient Audit (NaDIA) 2016 DIABETES A summary report for people with diabetes and anyone interested in the quality of care for people with diabetes when they stay in hospital. Based
National Diabetes Inpatient Audit (NaDIA) 2016 DIABETES A summary report for people with diabetes and anyone interested in the quality of care for people with diabetes when they stay in hospital. Based
The management of diabetes
 MEDICINE DIGEST STATINS The management of diabetes should include efforts to prevent, or delay, the onset of cardiovascular disease. Statins have been shown to lower cardiovascular risk in diabetes and
MEDICINE DIGEST STATINS The management of diabetes should include efforts to prevent, or delay, the onset of cardiovascular disease. Statins have been shown to lower cardiovascular risk in diabetes and
Parkinson s Specialist Practitioner
 Parkinson s Specialist Practitioner Delivered by The Royal Marsden NHS Foundation Trust 2 The Parkinson s Specialist is experienced in caring for people affected by Parkinson s. The Specialist will work
Parkinson s Specialist Practitioner Delivered by The Royal Marsden NHS Foundation Trust 2 The Parkinson s Specialist is experienced in caring for people affected by Parkinson s. The Specialist will work
For Safe and Effective Diabetes Management
 For Safe and Effective Diabetes Management How FreeStyle Libre Works FreeStyle Libre is a system for continuous glucose monitoring (CGM) that gives you easy access to your glucose numbers. CGM has been
For Safe and Effective Diabetes Management How FreeStyle Libre Works FreeStyle Libre is a system for continuous glucose monitoring (CGM) that gives you easy access to your glucose numbers. CGM has been
Connecting to Children s Diabetes
 Children s Diabetes Nurses How do I contact my diabetes team? Michelle, Nicky, Julia, Birgit 01823 343666 Monday - Friday, excluding Bank Holidays Office hours plus answerphone facility (Note same day
Children s Diabetes Nurses How do I contact my diabetes team? Michelle, Nicky, Julia, Birgit 01823 343666 Monday - Friday, excluding Bank Holidays Office hours plus answerphone facility (Note same day
Community Sleep Studies Hub and Spoke service Community first attends (schedule 1) and Community follow-ups (schedule 2)
 Community Sleep Studies Hub and Spoke service 2018-2019 Community first attends (schedule 1) and Community follow-ups (schedule 2) 1. Purpose of Agreement This local service agreement is for patients with
Community Sleep Studies Hub and Spoke service 2018-2019 Community first attends (schedule 1) and Community follow-ups (schedule 2) 1. Purpose of Agreement This local service agreement is for patients with
New NICE guidance: Changes in practice for multidisciplinary teams. Part 1: Type 1 diabetes in children and young people
 Article New NICE guidance: Changes in practice for multidisciplinary teams. Part 1: Type 1 diabetes in children and young people Helen Thornton This is the first of two articles on the 2015 NICE NG18 guideline,
Article New NICE guidance: Changes in practice for multidisciplinary teams. Part 1: Type 1 diabetes in children and young people Helen Thornton This is the first of two articles on the 2015 NICE NG18 guideline,
NHS Greater Glasgow and Clyde Adult Blood Glucose Meter and Test Strip Formulary
 NHS Greater Glasgow and Clyde Adult Blood Glucose and Test Strip Formulary 2016-17 Primary Care and Acute Joint Formulary Blood Glucose formulary developed by the Diabetes MCN Blood Glucose Formulary Review
NHS Greater Glasgow and Clyde Adult Blood Glucose and Test Strip Formulary 2016-17 Primary Care and Acute Joint Formulary Blood Glucose formulary developed by the Diabetes MCN Blood Glucose Formulary Review
University College Hospital. Blood glucose and HbA 1 c targets
 University College Hospital Blood glucose and HbA 1 c targets Children & Young People s Diabetes Service The diabetes team at UCLH aims to help every child and young person with Type 1 Diabetes to safely
University College Hospital Blood glucose and HbA 1 c targets Children & Young People s Diabetes Service The diabetes team at UCLH aims to help every child and young person with Type 1 Diabetes to safely
Optimising Insulin Pen Needles
 Optimising Insulin Pen Needles Following a review of insulin pen needles NHS Telford and Wrekin CCG now recommends the use of GlucoRx FinePoint Pen Needles. GlucoRx FinePoint Pen Needles are a high quality
Optimising Insulin Pen Needles Following a review of insulin pen needles NHS Telford and Wrekin CCG now recommends the use of GlucoRx FinePoint Pen Needles. GlucoRx FinePoint Pen Needles are a high quality
DIABETES MANAGEMENT POLICY
 DIABETES MANAGEMENT POLICY 1. PURPOSE St Hilda s is committed to providing a safe, healthy and supportive environment for all students. For students with diabetes, additional care must be taken to ensure
DIABETES MANAGEMENT POLICY 1. PURPOSE St Hilda s is committed to providing a safe, healthy and supportive environment for all students. For students with diabetes, additional care must be taken to ensure
State of California Health and Human Services Agency Department of Health Care Services
 State of California Health and Human Services Agency Department of Health Care Services JENNIFER KENT Director EDMOND G. BROWN JR Governor DATE: N.L.: 03-0317 Index: Benefits TO: ALL COUNTY CALIFORNIA
State of California Health and Human Services Agency Department of Health Care Services JENNIFER KENT Director EDMOND G. BROWN JR Governor DATE: N.L.: 03-0317 Index: Benefits TO: ALL COUNTY CALIFORNIA
Endoscopy or colonoscopy guidelines
 Endoscopy or colonoscopy guidelines for patients with diabetes The development and audit of local pilot guidelines for patients with diabetes undergoing an endoscopy or colonoscopy as an out-patient D
Endoscopy or colonoscopy guidelines for patients with diabetes The development and audit of local pilot guidelines for patients with diabetes undergoing an endoscopy or colonoscopy as an out-patient D
RELEASED. first steps. Icon Icon name What it means
 Icon Icon name What it means Connection The connection icon appears green when the Sensor feature is on and your transmitter is successfully communicating with your pump. The connection icon appears gray
Icon Icon name What it means Connection The connection icon appears green when the Sensor feature is on and your transmitter is successfully communicating with your pump. The connection icon appears gray
home monitoring diary
 home monitoring diary Jennifer Cohen lives in the UK and has Type 2 diabetes If this diary is lost please return it to: Novo Nordisk durable injection devices have a three year warranty Should you need
home monitoring diary Jennifer Cohen lives in the UK and has Type 2 diabetes If this diary is lost please return it to: Novo Nordisk durable injection devices have a three year warranty Should you need
Calgary Diabetes Centre Insulin Pump Therapy: Preparation and Expectations
 Calgary Diabetes Centre Insulin Pump Therapy: Preparation and Expectations This is a long and important document. It lists the steps for starting insulin pump therapy at the Calgary Diabetes Centre. It
Calgary Diabetes Centre Insulin Pump Therapy: Preparation and Expectations This is a long and important document. It lists the steps for starting insulin pump therapy at the Calgary Diabetes Centre. It
QOF Indicator DM013:
 QOF Indicator DM013: The percentage of patients with diabetes, on the register, who have a record of a dietary review by a suitably competent professional in the preceding 12 months Note: the bold signposts
QOF Indicator DM013: The percentage of patients with diabetes, on the register, who have a record of a dietary review by a suitably competent professional in the preceding 12 months Note: the bold signposts
A new model for prescribing varenicline
 Pharmacist Independent Prescribers in partnership with A new model for prescribing varenicline Dear Stop Smoking Advisor You will be aware of the stop smoking drug varenicline that goes under the brand
Pharmacist Independent Prescribers in partnership with A new model for prescribing varenicline Dear Stop Smoking Advisor You will be aware of the stop smoking drug varenicline that goes under the brand
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE)
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA151 Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus This guidance was issued in
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA151 Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus This guidance was issued in
Guidelines for managing the health care needs of children and young persons with diabetes in education
 Guidelines for managing the health care needs of children and young persons with diabetes in education Contents Page: Page Number 1.0 Introduction 1 2.0 The Care Management Plan 2 2.1 Blood Glucose monitoring
Guidelines for managing the health care needs of children and young persons with diabetes in education Contents Page: Page Number 1.0 Introduction 1 2.0 The Care Management Plan 2 2.1 Blood Glucose monitoring
Quick Reference Guide
 FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide
FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide
ESCA: Cinacalcet (Mimpara )
 ESCA: Cinacalcet (Mimpara ) Effective Shared Care Agreement for the Treatment of Primary hyperparathyroidism when parathyroidectomy is contraindicated or not clinically appropriate. Specialist details
ESCA: Cinacalcet (Mimpara ) Effective Shared Care Agreement for the Treatment of Primary hyperparathyroidism when parathyroidectomy is contraindicated or not clinically appropriate. Specialist details
CASE 8 Unwell insulin-dependent diabetic
 50 CASE 8 Unwell insulin-dependent diabetic INFORMATION FOR THE DOCTOR This is a telephone consultation. Name Michael Ede Age 29 Past medical history Type 1 diabetes 2 years ago Patello-femoral knee joint
50 CASE 8 Unwell insulin-dependent diabetic INFORMATION FOR THE DOCTOR This is a telephone consultation. Name Michael Ede Age 29 Past medical history Type 1 diabetes 2 years ago Patello-femoral knee joint
ssociation of Children s Diabetes Clinicians Clinicians Continuous Glucose Monitoring (CGM) Training for Healthcare Professionals and Patients
 ssociation of Children s Diabetes Continuous Glucose Monitoring (CGM) Training for Healthcare Professionals and Patients 1 ssociation of Children s Diabetes Contents Chapter Page no. STEP 1...3 Getting
ssociation of Children s Diabetes Continuous Glucose Monitoring (CGM) Training for Healthcare Professionals and Patients 1 ssociation of Children s Diabetes Contents Chapter Page no. STEP 1...3 Getting
CIT-06 Eligibility Questionnaire
 Today s Date: Last Name: First Name: Middle Name: Date of Birth: Height: Weight (lbs): PERSONAL CONTACT INFORMATION Street Address: City: State: Zip code: Home Phone: Cell Phone: Work Phone: Email Address:
Today s Date: Last Name: First Name: Middle Name: Date of Birth: Height: Weight (lbs): PERSONAL CONTACT INFORMATION Street Address: City: State: Zip code: Home Phone: Cell Phone: Work Phone: Email Address:
DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER
 DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER 2017-404 Question/s to be Answered Can you please provide the following
DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER 2017-404 Question/s to be Answered Can you please provide the following
Guidelines for slow loading of patients on warfarin for Atrial Fibrillation (AF) in the non acute setting
 ANTICOAGULANT SERVICE Guidelines for slow loading of patients on warfarin for Atrial Fibrillation (AF) in the non acute setting Introduction Fast loading of warfarin carries a risk of over anticoagulation
ANTICOAGULANT SERVICE Guidelines for slow loading of patients on warfarin for Atrial Fibrillation (AF) in the non acute setting Introduction Fast loading of warfarin carries a risk of over anticoagulation
Clinical News and Innovation in Type 1 Diabetes and Technology. Parth Narendran University Hospitals Birmingham
 Clinical News and Innovation in Type 1 Diabetes and Technology Parth Narendran University Hospitals Birmingham Adjunctive therapy to insulin Accurate diagnosis of T1D Early intensive treatment Technology
Clinical News and Innovation in Type 1 Diabetes and Technology Parth Narendran University Hospitals Birmingham Adjunctive therapy to insulin Accurate diagnosis of T1D Early intensive treatment Technology
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 Gestational diabetes: risk assessment, testing, diagnosis and management bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They
Gestational diabetes: risk assessment, testing, diagnosis and management bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They
Policy for Supporting. Children and Young People with. Diabetes in Education
 Policy for Supporting Children and Young People with Diabetes in Education December 2011 POLICY FOR SUPPORTING CHILDREN AND YOUNG PEOPLE WITH DIABETES IN EDUCATION Contents 1. Introduction 2. Before a
Policy for Supporting Children and Young People with Diabetes in Education December 2011 POLICY FOR SUPPORTING CHILDREN AND YOUNG PEOPLE WITH DIABETES IN EDUCATION Contents 1. Introduction 2. Before a
Quick Reference Guide
 FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide
FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide
Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181)
 Putting NICE guidance into practice Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181) Published: July 2014 This costing report accompanies Lipid modification:
Putting NICE guidance into practice Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181) Published: July 2014 This costing report accompanies Lipid modification:
Diabetes Medical Management Plan
 Diabetes Medical Management Plan This plan should be completed by the student's personal health care team and parents/guardian. It should be reviewed with relevant school staff and copies should be kept
Diabetes Medical Management Plan This plan should be completed by the student's personal health care team and parents/guardian. It should be reviewed with relevant school staff and copies should be kept
Children & Young People Travelling with Type 1 Diabetes
 Children & Young People Travelling with Type 1 Diabetes Advice on what to do to prepare for your holiday when you have type 1 diabetes Paediatric Diabetes Patient Information Leaflet Preparation Before
Children & Young People Travelling with Type 1 Diabetes Advice on what to do to prepare for your holiday when you have type 1 diabetes Paediatric Diabetes Patient Information Leaflet Preparation Before
This Diabetes Policy should be read in conjunction with the Dealing with Medical Conditions Policy of Goulburn Region Preschool Association Inc.
 DIABETES POLICY Mandatory Quality Area 2 The content of this policy was developed for KPV by advocacy and diabetes educators at Diabetes Australia Vic and the Royal Children s Hospital Melbourne s manager
DIABETES POLICY Mandatory Quality Area 2 The content of this policy was developed for KPV by advocacy and diabetes educators at Diabetes Australia Vic and the Royal Children s Hospital Melbourne s manager
Medicines Optimisation Team Standard Operating Procedure Insulin Quantities Audit
 Medicines Optimisation Team Standard Operating Procedure Insulin Quantities Audit Aim Review and rationalise the insulin quantities based on dosage instructions for diabetic patients in order to align
Medicines Optimisation Team Standard Operating Procedure Insulin Quantities Audit Aim Review and rationalise the insulin quantities based on dosage instructions for diabetic patients in order to align
