Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course
|
|
|
- Geraldine Hawkins
- 6 years ago
- Views:
Transcription
1 Southern Adventist Univeristy Graduate Research Projects Nursing Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University, Dawn Frerichs Follow this and additional works at: Part of the Nursing Commons Recommended Citation Hart, Sharon K. and Frerichs, Dawn, "Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course" (2015). Graduate Research Projects. Paper This Article is brought to you for free and open access by the Nursing at It has been accepted for inclusion in Graduate Research Projects by an authorized administrator of For more information, please contact
2 Running head: STEVENS JOHNSON SYNDROME 1 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Dawn Frerichs & Sharon Hart December 4, 2015 Translational Evidence Based Practice Manuscript with Matrix A Paper Presented to Meet Partial Requirements For NRSG-527-A Nursing Research Southern Adventist University School of Nursing
3 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course 2 Stevens Johnson syndrome (SJS) is a rare, potentially life threatening, severe adverse drug reaction involving the skin and mucous membranes. SJS affects only one to five patients per million annually (Rehmus, n.d.), therefore providing a challenge to medical providers for timely diagnosis and appropriate treatment. Even after the patient battles SJS for their lives, their struggle is long from over. Complications of SJS include living with scars and complications related to eye injury and corneal tears that their bodies sustained from this rare condition. By facilitating the knowledge base of the Advanced Practice Nurse (APN), an expedient diagnosis of SJS may be achieved in order to prevent delay in diagnosis that can increase complications from infections and mortality. Background SJS was first noted in 1922 as an acute mucocutaneous syndrome in which cell death causes the epidermis to separate from the dermis (Harr & French, 2010). Two American physicians named Stevens and Johnson first noted this syndrome in two young boys with symptoms described as an acute mucocutaneous syndrome characterized by severe purulent conjunctivitis and severe stomatitis with extensive mucosal necrosis (Naveen, Pai, Rai, & Athanikar, 2013, p. 80). Presenting symptoms typically include fever, sore throat, and fatigue that can be misdiagnosed and treated with a course of antibiotics, which could possibly continue or worsen the condition. Any mucosal membrane has the potential of being affected. SJS has been linked to agents including NSAIDS (non-steroid anti-inflammatory drugs), allopurinol, phenytoin, carbamazepine, barbiturates, anticonvulsants, and sulfa antibiotics, but almost any medication can be a cause (DeClerck, Jhun, Bright & Herbert, 2015). Individuals who experience SJS have usually completed a course of antibiotics or drug therapy and present with a
4 3 STEVENS JOHNSON SYNDROME rash, fever, lesions in the mouth, and/or eye irritation. Unlike typical allergic drug reactions, the presentation of SJS is delayed by one to four weeks after the initiation of the medication. SJS and Toxic Epidermal Necrolysis (TEN) present as severe mucosal erosions with widespread erythematous, cutaneous macules or atypical targets (Ghislain & Roujeau, 2002), SJS and TEN are considered to be the same syndrome, but on opposite ends of the spectrum. SJS differs from TEN by the degree of body surface involvement. In SJS the detachment of the epidermis is less than 10%, with the SJS/TEN overlap skin detachment range is 10-30%, and more than 30% of body surface involvement happens with TEN. When there is more skin surface involvement there is a corresponding higher mortality rate. The mortality rate for SJS is 5-15% and 30-35% for TEN (Ghislain & Roujeau, 2002). Risk factors for both SJS and TEN are increased when there is continued exposure to the predisposing factor, an autoimmune disorder, or a human leukocyte antigen (HLA)-linked genetic susceptibility. This HLA-linked factor affects individuals of Asian descent and makes them more susceptible to SJS. The genetic variations most strongly associated with SJS/TEN occur in the HLA-B gene (Genetics Home Reference, n.d.). The HLA complex helps the body to tell the difference between proteins the body makes and proteins that are foreign substances like those made by viruses and bacteria. When the body has a reaction to drugs, a substance called granulysin is released by the immune cells cytotoxic T cells and natural killer (NK) cells which destroys skin and mucous membrane cells (Genetics Home Reference, n.d.). When the cells are destroyed or die that is what causes the peeling and blistering skin associated with SJS/TEN patients. Research Question Due to SJS being a rare reaction, it is crucial to provide primary and emergency care providers with the appropriate tools to aid in early recognition of SJS symptoms. Early
5 recognition and subsequent diagnosis along with removal of the offending pharmacological 4 agent will assist in minimizing mucocutaneous damage. Many SJS and TEN patients present with skin lesions that are inflamed and resemble partial thickness burns or scalded skin. Primarily, the goal of treatment is to reduce the devastating impact of SJS while minimizing avoidable long-term effects of therapy. Does early diagnosis shorten the disease course in patients with SJS? Ultimately, a strong working knowledge by the Advanced Practice Nurse regarding SJS is imperative in order to reduce additional risks and facilitate a return of homeostasis by the patient. Literature Review The focus of this literature review is to provide information regarding how diagnosis impacts the disease course in patients with SJS and to facilitate understanding regarding current treatment plans. Even though SJS and TEN affect only a small number of individuals, it is important for health care providers to understand what can be done to improve the process in diagnosing these individuals, improve the recovery time and eliminate any lasting injury from the disease process. The following eight literature reviews attempt to determine what current evidence shows regarding how diagnosis from time of symptom onset impacts the disease process and additional long-term effects from SJS. One study by Naveen, Pai, Rai & Athanikar supported that early identification of the drug will help in prompt withdrawal of the drug thus reducing mortality and morbidity among the patients with SJS/TEN (Naveen, et al., 2013, p. 80). A focus of the study was to help those prescribing and treating patients to use caution when prescribing medications and to understand the risk of the drugs that cause the life threatening reactions. In this retrospective analysis, antiepileptics, antibiotics, antipsychotics, analgesics and antiretrovirals were the causal drugs. More
6 patients were affected by antiepileptics in this study (50%) followed by antibiotics. The drug 5 that had a higher morbidity and mortality rate was ofloxacin instead of the anti-epileptic that had a higher usage rate. The drug ofloxacin had a longer recovery time of 9.6 days and anticonvulsants had 6 days. The study emphasized drugs that were the possible trigger of SJS/TEN. With polypharmacy becoming more common it is important to find the culprit drugs early to ensure removal is performed accurately and to avoid removing effective drug therapies. The importance of this study will help APNs and physicians to understand which drugs could be possible triggers of SJS/TEN and what treatment plans could be effective. Another aspect that came out of this study was the use of steroids and how steroids could affect the outcomes of SJS/TEN if given early in the treatment process. The limitations of the study are that it had a small sample size of 22 individuals, 14 males and 8 females. The age group that was most affected in this study was years old, but the mean age in other studies varied, so age could play a factor in reaction times of the offending drugs. In a multicenteric, retrospective study completed by Barvaliya, Sanmukhani, Patel, Paliwal, Shah, & Tripathi (2011) data for patients with SJS and TEN were evaluated to find out the proportion of individual drugs involved, major complications, and cost of management (p.116) associated with these diagnoses. Of the 32 cases included, antimicrobials were the most common causative drug followed by nonsteroidal anti-inflammatory drugs (NSAIDS) and antiepileptic drugs. Secondary infections were the most common complication and the cost of management was higher in patients with a SJS/TEN overlap or TEN diagnoses in comparison to other adverse drug reactions (ADR s) while SJS remained similar in cost. Barvaliya et al. (2011)
7 6 STEVENS JOHNSON SYNDROME also compared SCORTEN scores with actual patient mortality. The research findings showed a higher mortality rate with a higher SCORTEN score. Limitations noted within this study include 13 patients with co-existing HIV and a small sample size. Patients with co-existing HIV decrease generalizability of the findings while enhancing the knowledge of potential causes of SJS in this vulnerable population. The findings increase knowledge concerning the most common causative drugs in patients with SJS and TEN for the stated population and continue to validate the value of SCORTEN for predicting mortality. A small study by Mehta, Prajapati, Mittal, Joshi, Sheth, Patel, & Goyal (2009) examined the association of human leucocyte antigen (HLA)-B*1502 allele and carbamazepine induced SJS in Indian patients. This research was based on the strong association between the HLA-B*1502 allele and carbamazepine induced SJS in Han Chinese patients. Previous research suggests that carbamazepine induced SJS and its association with the HLA-B*1502 allele is ethnicity specific for Asian patients. Carbamazepine is a commonly prescribed antiepileptic in India and is responsible for many cases of SJS/TEN in Indian patients. Mehta et al. (2009) found that six of the eight Indian patients with carbamazepine induced SJS had the HLA-B*1502 gene, while none of the ten patients in the control group had the gene. The association between the HLA-B*1502 gene is not as strong in Indian patients in comparison to Han Chinese patients, but this research does show a significant association (P=0.0014) between the HLA-B*1502 allele and carbamazepine induced SJS in Indian patients. The main limitation within this study was the small sample size. The findings suggest the need for more research concerning genetic susceptibility and its association with the
8 development of SJS/TEN. This research promotes knowledge concerning potential causes of 7 SJS in Indian patients which is useful for any health care provider caring for an ethnically diverse group of patients. All providers should have an understanding of genetic susceptibility when prescribing medications for ethnically diverse patients. Bansal, Garg, Sardana, and Sarkar (2015) completed an analysis on the predictive value and accuracy of the SCORe of Toxic Epidermal Necrolysis (SCORTEN) scale in predicting disease severity and patient prognosis. SCORTEN was developed and validated in 2000 by Bastuji-Garin et al. as a way for health care providers to predict disease severity and mortality based on seven parameters in patients with SJS and TEN (Bansal et al., 2015). This study evaluated SCORTEN values on days 1, 3, and 5 following diagnosis and compared them to actual mortality. Of the 60 patients in the study, the discriminative ability of SCORTEN values was appropriate at each time point with day 5 being the most significant (P< 0.001). Bansal et al. (2015) also reported that of the SCORTEN parameters only heart rate, blood urea, and serum bicarb were statistically significant (P< 0.05) concerning mortality. Other risk factors including delayed hospitalization, hypernatremia, and skin colonization were evaluated and did not show statistical significance with patient mortality. The researchers shared how the first eight to twelve days of SJS/TEN is considered the acute phase during which the disease progresses. During this phase, time of symptom onset influences SCORTEN s predictive ability greater than length of hospital stay. Ethnicity must also be considered when evaluating patients with SJS/TEN due to the noted differences in causative factors, genetics and comorbidities. All 60 patients received conservative treatment which included withdrawal of the causative agent, fluid replacement, nutritional support, temperature regulation, provision of daily dressings and prophylactic antibiotics (Bansal et al, 2015, p. e19). The mortality rate of
9 16.7% noted in this study presents conservative treatment, as previously described, as a valid 8 option for SJS/TEN. Limitations noted in this research included a participant mean age of 23.3 years (Bansal et al.) while the original study, completed by Bastuji-Garin et al., had a mean age of 42.3 years. With the SJS and TEN being a rare reaction, 60 participants is a large number therefore strengthening the validity of this study. SCORTEN s predictive value remains valid and continues to be a useful tool for providers when caring for patients with SJS. A large cohort study of 513 patients with SJS or TEN from six countries Austria, France, Germany, Israel, Italy, and the Netherlands were enrolled in EuroSCAR from a network of about 1800 hospitals. This is a registry of a multinational collaborative research team that was established in 1988 to study severe cutaneous adverse reactions (SCAR), recently changed to RegiSCAR-group. Collaborating members include specialists in dermatology, epidemiology, genealogy and immunology where they studied three different varieties of SCAR, which include SJS/TEN. The majority of enrolled patients (80%) were from France and Germany. There were 379 patients that had confirmed diagnosis of SJS or TEN who developed a reaction outside the hospital. The patients were classified by any treatment after hospitalization that used systemic corticosteroids, intravenous immunoglobulin (IVIG) or immunosuppressive agents. The study analyzed differences in outcomes from three different drug treatment plans in relation to earlier and later treatment of a combination of IVIG only, IVIG and corticosteroids or corticosteroids only. The research supports that age is an important factor in mortality with the death rate being lower in children than adults. Other factors in this study that contributed to a poor prognosis, were related to new diagnosis of cancer or other possible underlying diseases like hyperglycemia, elevated urea, and decreased bicarbonates (Schneck et al., 2007, p. 33). The
10 SCORTEN scale which is a predictor of mortality and severity of illness was not used in this 9 study because not enough data were collected in the EuroSCAR study. There were 134 patients with SJS (35%), 136 patients with SJS/TEN overlap (36%), and 109 patients with TEN (29%). Authors concluded that the mortality rate was 21% or 81 patients who did not survive their disease process. There was a strong correlation of death risk related to the patient s age and severity of disease. The limitation of this study were differences in two main countries, France and Germany, and there were no significant benefit from any one medication treatment, neither was there any difference in outcomes related to early or late treatment. The study pointed out that there were many challenging aspects to control in this study to get a definitive answer on treatment plans of corticosteroid and IVIG medication benefits. Another study would be necessary to observe a uniform treatment plan especially studying a low or high dose regimen to provide a better outcome analysis and the effects on the disease course of SJS/TEN. The importance of the study was the finding that no treatment modality was better in treating SJS/TEN, than simple early detection and removal of the offending drug and starting supportive care early. The strengths of the study include a well-defined population, the largest ever studied. Patients were assessed by an expert committee and information was obtained in a way to assure correct classification of the disease stage. A study examined by Kardaun and Jonkman (2007) found Dexamethasone Pulse Therapy (DPT), if given early in the treatment process, can possibly reduce the mortality risk. DPT is the administration of large doses of drugs in an intermittent manner to enhance the therapeutic effect and reduce side effects ( What is Pulse Therapy, n.d.). DPT was started early and contributed to a relatively quick stabilization and healing, may even have halted the process of apoptosis or cell
11 10 STEVENS JOHNSON SYNDROME death. If DPT is given for longer periods of time it increases the risk of infection. On the other hand corticosteroids usually are not given because it can delay wound healing and cause sepsis. DPT is used with corticosteroids in severe, sometimes autoimmune, diseases. The only definitive way to diagnose SJS/TEN is to take a skin biopsy that verifies sub epidermal involvement. In this study, DPT treatment was started as soon as a definitive diagnosis was made. The strength of the study showed that using DPT early in the disease course could possibly stop the cell damage and could reduce mortality in SJS/TEN patients. The limitations were a small sample size of 12 patients over a 10 year period and it was difficult to show statistical relevance because of the small number of patients. Another retrospective study of treating patients with IVIG therapy was completed by Gravante, Delogu, Marianetti, Trombetta, Esposito and Montone in The study consisted of understanding how late referral to their center increased the risk of mortality in patients because IVIG therapy treatment was not started early and the risk of infection increased. There was a 10 day delay in referral in starting the IVIG therapy and supportive care. This delay of referral increased mortality probability of times, with the absence of IVIG therapy of 67.5 times and more than 2-types of bacteria 84 times (Gravante, et al., 2007, p. 121). Late referral and total body surface area of detached skin are two factors that contributed to an increased death rate. Out of 35 patients that had an initial diagnosis of TEN, 32 patients were included in this study. The 32 patients that were evaluated, there was one case of SJS and seven patients with SJS/TEN overlap. This made up 25% with SJS involvement and a total mortality rate of 34.4% which is 11 out of 32 patients studied (Gravante, et al., 2007).
12 11 STEVENS JOHNSON SYNDROME The limitations in this retrospective study show that it is difficult to determine how sick patients were at time of referral. This could have an impact on mortality rates and what the treatment regimens were at the time of the study. Another limitation was the small sample size. The importance of the study showed that septicemia played an important role in mortality, and if there were more than three different bacteria present during hospitalization than this increased the risk of death for patients. This study showed an encouraging outcome of giving IVIG even if there is late referral to treatment. Further studies are recommended to understand current treatment modalities and the specific effect on IVIG therapy. Even though IVIG therapy is not well established for SJS/TEN in this study, the delay in introducing specific immunoglobulin-blocking therapy and specific support therapy contributed to an increased risk of death. A study completed by Singh, Chatterjee, and Verma (2013) compared the use of cyclosporine versus steroids for treatment of patients with SJS and TEN. The researchers reported a significant reduction in the amount of time to the arrest of progression of SJS/TEN (P = ), total re-epithelization time (P = ) and hospitalization stay (P = ) in those treated with cyclosporine in comparison to those treated with corticosteroids. There were no statistically significant differences noted between the cyclosporine and corticosteroid groups concerning age, total body surface area (TBSA) and onset of disease to admission time. Singh et al. also reported on other studies showing that early withdrawal of the causative drug positively influenced prognosis and causative drugs with a long half-life increase the risk of mortality. Identification of the causative drug can be challenging in patients receiving multiple medications that have the potential to cause SJS/TEN.
13 12 STEVENS JOHNSON SYNDROME This research was conducted over a one year time span on eleven patients who has SJS or TEN. Once again the number of participants is small for research purposes, but when combined with other studies, the evidence does promote the use of cyclosporine in uncomplicated cases of SJS and TEN. Timely diagnosis from onset of symptoms and removal of the causal agent combined with cyclosporine treatment provides hope for providers who aim to decrease mortality in patients with SJS and TEN. Recommendations One recommendation to practice is to have a better understanding of how certain medications are a leading cause of SJS and TEN in over 75% of cases affecting both adults and children. When patients present with symptoms resembling SJS, a thorough drug history and identification of any causative medications must be identified. The causative agent should be eliminated and supportive care started immediately following diagnosis. If the reaction resulted from a sulfonamide, early diagnosis will provide avoidance of drugs that have a sulfa-base, such as silver sulfadiazine ointment, which is commonly used for burn patients. The Advanced Practice Nurse must have a strong working knowledge of SJS/TEN symptoms and causative medications to reduce additional risks and facilitate early treatment plans for the patient. Conclusion The reviewed literature consistently stated that apart from intensive supportive therapy, there is no accepted guideline for treatment or standard practice that exists besides supportive care. Most of the literature states that larger cohorts need to be studied and that there still is a lack of information regarding SJS/TEN and the benefits of IVIG, cyclosporine and corticosteroid treatment. When there is a delay in early diagnosis, there is an increased risk in mortality because the causative drug is still triggering injury and supportive care has not been initiated.
14 In order to advance knowledge about SJS/TEN and prevent delay in diagnosis, it is 13 important to have concise drug information given to all physicians and primary care providers. Adverse drug reactions are the leading cause of SJS and TEN and a multidisciplinary approach is needed to manage any complications of the syndrome. More than 100 drugs are associated with SJS/TEN, and an improved system of warnings is needed in drug dictionaries regarding any associated risk. It is important for health care providers to understand what can be done to improve the process of diagnosing these individuals, shorten their disease course and eliminate lasting injury from the disease process.
15 References 14 Bansal, S., Garg, V. K., Sardana, K., Sarkar, R. (2015). A clinicotherapeutic analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis with an emphasis on the predictive value and accuracy of SCORe of Toxic Epidermal Necrolysis. International Journal of Dermatology, 54, e18-e26. Barvaliya, M., Sanmukhani, J., Patel, T., Paliwal, N., Shah, H., & Tripathi, C. (2011). Druginduced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS- TEN overlap: a multicentric retrospective study. Journal of Postgraduate Medicine, 57(2), doi: / Declerck, B., Jhun, P., Bright, A., & Herbert, M. (2015). Trust me, this is the worst acne of your life! Annals of Emergency Medicine, 65(2), Genetics Home Reference (n.d.). Stevens-Johnson syndrome/toxic epidermal necrolysis. Retrieved from Ghislain, P., & Roujeau, J. (2002). Treatment of severe drug reactions: Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis and Hypersensitivity syndrome. Dermatology Online Journal, 8(1), 5. Gravante, G. Delogu, D., Marianetti, M., Trombetta, M., Esposito, G., & Montone, A. (2007, March 1). Toxic epidermal necrolysis and Stevens Johnson syndrome: 11 year s experience and outcome. European Review for Medical and Pharmacological Sciences, 11(2), Harr, T., & French, L. (2010). Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet Journal of Rare Diseases, 5(1), 39. doi: /
16 Kardaun, S., & Jonkman, M. (2007). Dexamethasone pulse therapy for Stevens-Johnson 15 syndrome/toxic epidermal necrolysis. Acta Dermato-Venereologica, 87(2), / Mehta, T., Prajapati, L., Mittal, B., Joshi, C., Sheth, J., Patel, D., & Goyal, R. (2009). Association of HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian Journal of Dermatology, Venereology & Leprology, 75(6), p. doi: / Naveen, K., Pai, V., Rai, V., & Athanikar, S. (2013). Retrospective analysis of Stevens Johnson syndrome and toxic epidermal necrolysis over a period of 5 years from northern Karnataka, India. Indian Journal of Pharmacology, 45(1), doi: / Rehmus, W. (n.d.). Steven-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN). Retrieved from hypersensitivity-and-inflammatory-disorders/acute-febrile-neutrophilic-dermatosis Schneck, J., Fagot, J., Sekula, P., Sassolas, B., Roujeau, J., & Mockenhaupt, M. (2008). Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: a retrospective study on patients included in the prospective EuroSCAR study. Journal of the American of Dermatology, 45(1), Retrieved from Singh, G. K., Chatterjee, M., & Verma, R. (2013). Cyclosporine in Stevens Johnson syndrome and toxic epidermal necrolysis and retrospective comparison with systemic corticosteroid. Indian Journal of Dermatology, Venereology & Leprology, 79(5), doi: /
17 What is Pulse Therapy? (n.d.). Retrieved from 16 therapy_as_a_cure_for_auto.htm (See Appendix A for Matrix Evaluation Table for Stevens Johnson Syndrome)
Correspondence should be addressed to Wanjarus Roongpisuthipong; rr
 Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
allergy Asia Pacific Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from
 pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
Dilantin (phenytoin) ROBERT A. SCHWARTZ
 Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome
 Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital
 Original article: Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital *Arjun R 1,Mahalingeshwara Bhat K P 2, Sara C 1 1 PG student in General Medicine, KS Hegde Medical Acadamy,
Original article: Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital *Arjun R 1,Mahalingeshwara Bhat K P 2, Sara C 1 1 PG student in General Medicine, KS Hegde Medical Acadamy,
CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
Skin Manifestations of Drug Reactions
 Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES. R e g i S C A R PATIENT'S DATA. Age country of birth
 REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update
 Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update Robert G. Micheletti, MD Assistant Professor of Dermatology and Medicine Director, Cutaneous Vasculitis Clinic, Penn Vasculitis Center
Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update Robert G. Micheletti, MD Assistant Professor of Dermatology and Medicine Director, Cutaneous Vasculitis Clinic, Penn Vasculitis Center
To update the use of IVIG and CORTICOIDS IN management of SJS/ TEN To remind Doctors being careful when giving
 Present : Dr Pham Thi Minh Rang Internal Department No2-Hospital for children No2 AIMS To update the use of IVIG and CORTICOIDS IN management of SJS/ TEN To remind Doctors being careful when giving To
Present : Dr Pham Thi Minh Rang Internal Department No2-Hospital for children No2 AIMS To update the use of IVIG and CORTICOIDS IN management of SJS/ TEN To remind Doctors being careful when giving To
SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW
 SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW *Anderson Isaac, PharmD Candidate, 2019 Pooja Patel, PharmD Candidate, 2019 Katelyn Thomasson, PharmD Candidate, 2019 Erika Tillery, PharmD, BCPP,
SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW *Anderson Isaac, PharmD Candidate, 2019 Pooja Patel, PharmD Candidate, 2019 Katelyn Thomasson, PharmD Candidate, 2019 Erika Tillery, PharmD, BCPP,
Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review
 140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
Pediatric Dermatology
 Pediatric Dermatology --------- Emergencies & Urgencies Nicholas V. Nguyen, M.D. Director, Pediatric Dermatology Disclosures In the past 12 months, I have had the following financial relationships with
Pediatric Dermatology --------- Emergencies & Urgencies Nicholas V. Nguyen, M.D. Director, Pediatric Dermatology Disclosures In the past 12 months, I have had the following financial relationships with
Emergency Dermatology Dr Melissa Barkham
 Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
Sidney Miller, MD, FACS Professor of Surgery Director of Research and Development Ohio State University Burn Center
 Management of the Burn Patient Sidney Miller, MD, FACS Professor of Surgery Director of Research and Development Ohio State University Burn Center American Burn Association Transfer Criteria Burn > 10%
Management of the Burn Patient Sidney Miller, MD, FACS Professor of Surgery Director of Research and Development Ohio State University Burn Center American Burn Association Transfer Criteria Burn > 10%
PedsCases Podcast Scripts
 PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Drug Allergy. These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio
PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Drug Allergy. These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio
A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive Patients
 Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
Original Article. Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah H 1, Tripathi C
 Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah
Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah
Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis: a case report
 Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT
 OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review
 Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review Review question(s) To carry out a systematic review of the published evidence of the cutaneous adverse drug reactions in
Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review Review question(s) To carry out a systematic review of the published evidence of the cutaneous adverse drug reactions in
Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
 Case Reports in Immunology Volume 2012, Article ID 931528, 4 pages doi:10.1155/2012/931528 Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
Case Reports in Immunology Volume 2012, Article ID 931528, 4 pages doi:10.1155/2012/931528 Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
Drug Allergy A Guide to Diagnosis and Management
 Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
Scratching the Surface: A Review of SJS/TEN
 Scratching the Surface: A Review of SJS/TEN Sarah Smith, PharmD Pharmacy Grand Rounds 2018 November 27, 2018 2018 MFMER slide 1 Toxic Epidermal Necrolysis Cutaneous Anthrax Stevens Johnson Syndrome Necrotizing
Scratching the Surface: A Review of SJS/TEN Sarah Smith, PharmD Pharmacy Grand Rounds 2018 November 27, 2018 2018 MFMER slide 1 Toxic Epidermal Necrolysis Cutaneous Anthrax Stevens Johnson Syndrome Necrotizing
Emergency Dermatology. Emergency Dermatology
 Emergency Dermatology These are rapidly progressive skin conditions and some are potentially lifethreatening. Early recognition is important to implement prompt supportive care and therapy. Some are drug
Emergency Dermatology These are rapidly progressive skin conditions and some are potentially lifethreatening. Early recognition is important to implement prompt supportive care and therapy. Some are drug
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review
 Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Department of Pharmacology & NIPER-Guwahati, Gauhati Medical College & Hospital, Guwahati , Assam, INDIA. 3
 J Young Pharm, 2016; 8(2): 149-153 A multifaceted peer reviewed journal in the field of Pharmacy www.jyoungpharm.org www.phcog.net Short Communication A study on drug induced Stevens-Johnson Syndrome (SJS),
J Young Pharm, 2016; 8(2): 149-153 A multifaceted peer reviewed journal in the field of Pharmacy www.jyoungpharm.org www.phcog.net Short Communication A study on drug induced Stevens-Johnson Syndrome (SJS),
Prevalence and pattern of adverse cutaneous drug reactions presenting to a tertiary care hospital
 International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate
 Original Research Article Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate Manab Nandy 1, Sangeeta De 2*, Mustafa Asad 2, Nirmal
Original Research Article Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate Manab Nandy 1, Sangeeta De 2*, Mustafa Asad 2, Nirmal
Cutaneous Conditions Associated with Systemic Disease
 Cutaneous Conditions Associated with Systemic Disease Johnnie M Woodson, M.D., F.A.A.D. Assistant Professor of Dermatology University of Nevada School of Medicine Director of J. Woodson Dermatology & Associates,
Cutaneous Conditions Associated with Systemic Disease Johnnie M Woodson, M.D., F.A.A.D. Assistant Professor of Dermatology University of Nevada School of Medicine Director of J. Woodson Dermatology & Associates,
Bugs and Drugs: What s New in Hypersensitivity Reactions?
 Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
Cutaneous Drug Reactions
 Cutaneous Drug Reactions Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada Copyright 2017 by Sea Courses
Cutaneous Drug Reactions Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada Copyright 2017 by Sea Courses
Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients
 original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening
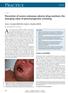 CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
DERMATOLOGICAL EMERGENCIES. DR. Ian Hoyle MBBS DIP IMC RCS (Ed), DA (UK),FRACGP,FACRRM,DIP DERM(Wales) TASMANIAN SKIN AND BODY CENTRE
 DERMATOLOGICAL EMERGENCIES DR. Ian Hoyle MBBS DIP IMC RCS (Ed), DA (UK),FRACGP,FACRRM,DIP DERM(Wales) TASMANIAN SKIN AND BODY CENTRE Dermatological Emergencies INFECTIONS ERYTHRODERMA DRUG ERUPTIONS STEVENS-JOHNSON
DERMATOLOGICAL EMERGENCIES DR. Ian Hoyle MBBS DIP IMC RCS (Ed), DA (UK),FRACGP,FACRRM,DIP DERM(Wales) TASMANIAN SKIN AND BODY CENTRE Dermatological Emergencies INFECTIONS ERYTHRODERMA DRUG ERUPTIONS STEVENS-JOHNSON
1 Introduction. Kenneth Irungu 1 David Nyamu
 Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Cutaneous Adverse Drug Reactions in Domestic Animals. Katherine Doerr, DVM, Dip. ACVD. Veterinary Dermatology Center
 Cutaneous Adverse Drug Reactions in Domestic Animals Katherine Doerr, DVM, Dip. ACVD Veterinary Dermatology Center Maitland, Rockledge, Waterford Lakes, FL Not highly studied in veterinary medicine Unknown
Cutaneous Adverse Drug Reactions in Domestic Animals Katherine Doerr, DVM, Dip. ACVD Veterinary Dermatology Center Maitland, Rockledge, Waterford Lakes, FL Not highly studied in veterinary medicine Unknown
STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSIS IN CHILDREN: A LITERATURE REVIEW OF CURRENT TREATMENTS
 STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSIS IN CHILDREN: A LITERATURE REVIEW OF CURRENT TREATMENTS *Blanca R. Del Pozzo-Magaña, 1 Alejandro Lazo-Langner 2,3 1. Department of Pediatrics, University
STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSIS IN CHILDREN: A LITERATURE REVIEW OF CURRENT TREATMENTS *Blanca R. Del Pozzo-Magaña, 1 Alejandro Lazo-Langner 2,3 1. Department of Pediatrics, University
Five things not to miss in Dermatology. Dr Judy Wismer Associate Clinical Professor Michael G DeGroote School of Medicine
 Five things not to miss in Dermatology Dr Judy Wismer Associate Clinical Professor Michael G DeGroote School of Medicine Key Descriptives Fever, skin pain Purpura, necrosis Bullae, Mucosal, Skin sloughing
Five things not to miss in Dermatology Dr Judy Wismer Associate Clinical Professor Michael G DeGroote School of Medicine Key Descriptives Fever, skin pain Purpura, necrosis Bullae, Mucosal, Skin sloughing
Future of Pediatrics: Blisters, Hives and Other Tales from the Emergency Room June 14 th, 2016
 A. Yasmine Kirkorian MD Assistant Professor of Dermatology & Pediatrics Children s National Health System George Washington University School of Medicine & Health Sciences Future of Pediatrics: Blisters,
A. Yasmine Kirkorian MD Assistant Professor of Dermatology & Pediatrics Children s National Health System George Washington University School of Medicine & Health Sciences Future of Pediatrics: Blisters,
A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash
 CLINICAL CASE OF THE MONTH A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash Patrick Njoku, MD; Temeka Tate, MD; Sousan Zadeh, MD; Erin Hauck, MD; Anila Chaudhry, MD; Betty Lo-Blais, MD; Lee
CLINICAL CASE OF THE MONTH A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash Patrick Njoku, MD; Temeka Tate, MD; Sousan Zadeh, MD; Erin Hauck, MD; Anila Chaudhry, MD; Betty Lo-Blais, MD; Lee
GOOD MORNING! AUGUST 5, 2014
 GOOD MORNING! AUGUST 5, 2014 PREP QUESTION During the health supervision visit of a term newborn boy, his mother relates that a cousins child died at age 4 months from sudden infant death syndrome. She
GOOD MORNING! AUGUST 5, 2014 PREP QUESTION During the health supervision visit of a term newborn boy, his mother relates that a cousins child died at age 4 months from sudden infant death syndrome. She
Accepted Manuscript. Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need
 Accepted Manuscript Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need Lee Haur Yueh, MBBS, MRCP, MMed, FAMS PII: S0190-9622(18)32138-8 DOI: 10.1016/j.jaad.2018.05.1258
Accepted Manuscript Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need Lee Haur Yueh, MBBS, MRCP, MMed, FAMS PII: S0190-9622(18)32138-8 DOI: 10.1016/j.jaad.2018.05.1258
Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes
 Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
Diagnosis and Management of Drug-induced Stevens-Johnson Syndrome: Report of Two Cases
 10.5005/jp-journals-10011-1189 CASE REPORT JIAOMR Diagnosis and Management of Drug-induced Stevens-Johnson Syndrome: Report of Two Cases 1 M Venkateshwarlu, 2 B Radhika 1 Professor and Head, Department
10.5005/jp-journals-10011-1189 CASE REPORT JIAOMR Diagnosis and Management of Drug-induced Stevens-Johnson Syndrome: Report of Two Cases 1 M Venkateshwarlu, 2 B Radhika 1 Professor and Head, Department
PACKAGE LEAFLET: INFORMATION FOR THE USER
 PACKAGE LEAFLET: INFORMATION FOR THE USER Fucidin 250 mg Tablets sodium fusidate Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
PACKAGE LEAFLET: INFORMATION FOR THE USER Fucidin 250 mg Tablets sodium fusidate Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW
 STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW G. Ramya sree*, Sreeram Vandavasi Guru 1, E. Sam Jeeva Kumar 2 * Pharm D, Intern, P. Rami Reddy Memorial College of Pharmacy, Kadapa. 1 Pharm-D Intern, P. Rami
STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW G. Ramya sree*, Sreeram Vandavasi Guru 1, E. Sam Jeeva Kumar 2 * Pharm D, Intern, P. Rami Reddy Memorial College of Pharmacy, Kadapa. 1 Pharm-D Intern, P. Rami
Your letter Your reference The Hague, Subject Request for change in the product information following the PhVWP/CMDh decision
 Registratiehouder ( t.a.v. registratieafdeling) Your letter Your reference The Hague, -- --.. Case number Our reference../../ Case manager Telephone number Subject Request for change in the product information
Registratiehouder ( t.a.v. registratieafdeling) Your letter Your reference The Hague, -- --.. Case number Our reference../../ Case manager Telephone number Subject Request for change in the product information
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
 BRIEF COMMUNICATION http://dx.doi.org/10.4110/in.2016.16.4.256 pissn 1598-2629 eissn 2092-6685 Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
BRIEF COMMUNICATION http://dx.doi.org/10.4110/in.2016.16.4.256 pissn 1598-2629 eissn 2092-6685 Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC
 SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
PHARMACOVIGILANCE CELL Government Medical College, Bhavnagar, Gujarat.
 PHARMACOVIGILANCE CELL Introduction Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drugrelated problems.
PHARMACOVIGILANCE CELL Introduction Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drugrelated problems.
Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/ Toxic Epidermal Necrolysis
 Acta Derm Venereol 2007; 87: 144 148 CLINICAL REPORT Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/ Toxic Epidermal Necrolysis Sylvia H. Kardaun and Marcel F. Jonkman Center for Blistering Diseases,
Acta Derm Venereol 2007; 87: 144 148 CLINICAL REPORT Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/ Toxic Epidermal Necrolysis Sylvia H. Kardaun and Marcel F. Jonkman Center for Blistering Diseases,
Elements for a public summary
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Nevirapine is used for antiretroviral combination therapy of Human Immunodeficiency Virus (HIV) infection. Human immunodeficiency
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Nevirapine is used for antiretroviral combination therapy of Human Immunodeficiency Virus (HIV) infection. Human immunodeficiency
Understanding PIDD. Primary Immunodeficiency Disease (PIDD)
 Understanding PIDD Primary Immunodeficiency Disease (PIDD) Understanding PIDD Primary Immunodeficiency Disease (PIDD) What is Primary Immunodeficiency? Primary Immunodeficiency (PIDD or PID) is a disease
Understanding PIDD Primary Immunodeficiency Disease (PIDD) Understanding PIDD Primary Immunodeficiency Disease (PIDD) What is Primary Immunodeficiency? Primary Immunodeficiency (PIDD or PID) is a disease
Severe cutaneous adverse reactions (SCAR) are life-threatening
 Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management Maja Mockenhaupt, MD, PhD n Abstract Severe cutaneous adverse
Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management Maja Mockenhaupt, MD, PhD n Abstract Severe cutaneous adverse
Stevens-Johnson syndrome (SJS) and toxic epidermal. necrolysis in Asian children
 Stevens-Johnson syndrome and toxic epidermal necrolysis in Asian children Mark Jean-Aan Koh, MD, and Yong-Kwang Tay, MD Singapore Background: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis
Stevens-Johnson syndrome and toxic epidermal necrolysis in Asian children Mark Jean-Aan Koh, MD, and Yong-Kwang Tay, MD Singapore Background: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis
PACKAGE LEAFLET: INFORMATION FOR THE USER
 PACKAGE LEAFLET: INFORMATION FOR THE USER Fucidin 250 mg/5 ml Oral Suspension fusidic acid Read all of this leaflet carefully before you start taking this medicine because it contains important information
PACKAGE LEAFLET: INFORMATION FOR THE USER Fucidin 250 mg/5 ml Oral Suspension fusidic acid Read all of this leaflet carefully before you start taking this medicine because it contains important information
Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics
 MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
FDA Approves Carnexiv (carbamazepine) injection as Intravenous Short-Term Replacement Therapy for Certain Seizure Types
 FOR IMMEDIATE RELEASE FDA Approves Carnexiv (carbamazepine) injection as Intravenous Short-Term Replacement Therapy for Certain Seizure Types Carnexiv is the first FDA-approved intravenous carbamazepine
FOR IMMEDIATE RELEASE FDA Approves Carnexiv (carbamazepine) injection as Intravenous Short-Term Replacement Therapy for Certain Seizure Types Carnexiv is the first FDA-approved intravenous carbamazepine
Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015)
 1 of 5 Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015) Co-chairs: Dr. Neil H. Shear at the 23rd World Congress of Dermatology in Vancouver, Canada. Division of Dermatology,
1 of 5 Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015) Co-chairs: Dr. Neil H. Shear at the 23rd World Congress of Dermatology in Vancouver, Canada. Division of Dermatology,
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME
 EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
Summary of the risk management plan (RMP) for Pemetrexed Actavis (pemetrexed)
 EMA/58503/2016 Summary of the risk management plan (RMP) for Pemetrexed Actavis (pemetrexed) This is a summary of the risk management plan (RMP) for Pemetrexed Actavis, which details the measures to be
EMA/58503/2016 Summary of the risk management plan (RMP) for Pemetrexed Actavis (pemetrexed) This is a summary of the risk management plan (RMP) for Pemetrexed Actavis, which details the measures to be
Package Leaflet: Information for the User Zyloric 100 mg and 300 mg tablets allopurinol
 Package Leaflet: Information for the User Zyloric 100 mg and 300 mg tablets allopurinol Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read
Package Leaflet: Information for the User Zyloric 100 mg and 300 mg tablets allopurinol Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read
EU RISK MANAGEMENT PLAN (EU RMP)
 EU RISK MANAGEMENT PLAN (EU RMP) Active substance(s) (INN or common name): Esomeprazole Pharmaco-therapeutic group (ATC Code): A02B C05 Name of Marketing Authorisation Holder or Applicant: Strength and
EU RISK MANAGEMENT PLAN (EU RMP) Active substance(s) (INN or common name): Esomeprazole Pharmaco-therapeutic group (ATC Code): A02B C05 Name of Marketing Authorisation Holder or Applicant: Strength and
New product information wording Extracts from PRAC recommendations on signals
 20 July 2017 EMA/PRAC/406976/2017 Pharmacovigilance Risk Assessment Committee (PRAC) New product information wording Extracts from PRAC recommendations on signals Adopted at the 3-6 July 2017 PRAC The
20 July 2017 EMA/PRAC/406976/2017 Pharmacovigilance Risk Assessment Committee (PRAC) New product information wording Extracts from PRAC recommendations on signals Adopted at the 3-6 July 2017 PRAC The
Severe cutaneous reactions caused by barbiturates in seven Iranian children
 Pharmacology and therapeutics Severe cutaneous reactions caused by barbiturates in seven Iranian children Setareh Mamishi, MD, Fatemeh Fattahi, MD, Zahra Pourpak, MD, PhD, Farzaneh Mirza Aghaee, MD, Zeinab
Pharmacology and therapeutics Severe cutaneous reactions caused by barbiturates in seven Iranian children Setareh Mamishi, MD, Fatemeh Fattahi, MD, Zahra Pourpak, MD, PhD, Farzaneh Mirza Aghaee, MD, Zeinab
Toxic Epidermal Necrolysis - A Case Report
 The Journal of Critical Care Medicine 2017;3(1):29-33 CASE REPORT Toxic Epidermal Necrolysis - A Case Report Laura Stătescu¹, Magda Constantin², Horia Silviu Morariu³ *, Laura Gheucă Solovăstru¹ ¹ Dermatology
The Journal of Critical Care Medicine 2017;3(1):29-33 CASE REPORT Toxic Epidermal Necrolysis - A Case Report Laura Stătescu¹, Magda Constantin², Horia Silviu Morariu³ *, Laura Gheucă Solovăstru¹ ¹ Dermatology
Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis.
 Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Supplementary online-material Supplementary Table S1: Detailed descriptive analysis
Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Supplementary online-material Supplementary Table S1: Detailed descriptive analysis
PEER REVIEW HISTORY ARTICLE DETAILS VERSION 1 - REVIEW
 PEER REVIEW HISTORY BMJ Paediatrics Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form and are provided with free text boxes to elaborate
PEER REVIEW HISTORY BMJ Paediatrics Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form and are provided with free text boxes to elaborate
Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients
 Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients Original Article Journal of Epilepsy Research pissn 2233-6249 / eissn 2233-6257 Bhargavi
Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients Original Article Journal of Epilepsy Research pissn 2233-6249 / eissn 2233-6257 Bhargavi
Herbal and homeopathic products, often considered natural and non-toxic, can also cause adverse drug reactions.
 Idiosyncratic and potentially serious cutaneous adverse drug reactions (CADRs), although relatively rare, account for significant morbidity and mortality. RANNAKOE J LEHLOENYA, BSc, MB ChB, FCDerm (SA)
Idiosyncratic and potentially serious cutaneous adverse drug reactions (CADRs), although relatively rare, account for significant morbidity and mortality. RANNAKOE J LEHLOENYA, BSc, MB ChB, FCDerm (SA)
A Case Report on Paracetamol Induced Generalized Fixed Drug Eruptions
 Human Journals Case report November 2016 Vol.:7, Issue:4 All rights are reserved by Dr. C Sugunakar Raju et al. A Case Report on Paracetamol Induced Generalized Fixed Drug Eruptions Keywords: Skin Eruptions,
Human Journals Case report November 2016 Vol.:7, Issue:4 All rights are reserved by Dr. C Sugunakar Raju et al. A Case Report on Paracetamol Induced Generalized Fixed Drug Eruptions Keywords: Skin Eruptions,
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Actavis is a cancer medicine used to treat two types of lung cancer, malignant pleural mesothelioma and advanced non-small-cell
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Actavis is a cancer medicine used to treat two types of lung cancer, malignant pleural mesothelioma and advanced non-small-cell
8/8/2016. Overview. Back to Basics: Immunology. Adverse Reactions to Drugs: Dispelling Myths
 Adverse Reactions to Drugs: Dispelling Myths Allison Ramsey, MD NPA Annual Conference September 30, 2016 Overview Review of types of hypersensitivity reactions Penicillin allergy IV contrast allergy Local
Adverse Reactions to Drugs: Dispelling Myths Allison Ramsey, MD NPA Annual Conference September 30, 2016 Overview Review of types of hypersensitivity reactions Penicillin allergy IV contrast allergy Local
New product information wording Extracts from PRAC recommendations on signals
 12 October 2017 EMA/PRAC/610988/2017 Pharmacovigilance Risk Assessment Committee (PRAC) New product information wording Extracts from PRAC recommendations on signals Adopted at the 25-29 September 2017
12 October 2017 EMA/PRAC/610988/2017 Pharmacovigilance Risk Assessment Committee (PRAC) New product information wording Extracts from PRAC recommendations on signals Adopted at the 25-29 September 2017
Stevens-Johnson Syndrome in patients receiving cranial irradiation and concomitant diphenylhydantoin
 Turkish Journal of Cancer Vol. 32/ No. 4/2002 Stevens-Johnson Syndrome in patients receiving cranial irradiation and concomitant diphenylhydantoin ERKAN TOPKAN, FARUK ZORLU, MURAT GÜRKAYNAK Hacettepe University
Turkish Journal of Cancer Vol. 32/ No. 4/2002 Stevens-Johnson Syndrome in patients receiving cranial irradiation and concomitant diphenylhydantoin ERKAN TOPKAN, FARUK ZORLU, MURAT GÜRKAYNAK Hacettepe University
The liver and skin are two of the commonest
 AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 3, 2016 LIVER INJURY/REGENERATION Drug-Induced Liver Injury Associated With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis:
AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 3, 2016 LIVER INJURY/REGENERATION Drug-Induced Liver Injury Associated With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis:
RHEUMATIC FEVER AND POST-STREPTOCOCCAL REACTIVE ARTHRITIS
 www.pediatric-rheumathology.printo.it RHEUMATIC FEVER AND POST-STREPTOCOCCAL REACTIVE ARTHRITIS What is it? Rheumatic fever has been defined as a disease triggered by infection caused by streptococcus.
www.pediatric-rheumathology.printo.it RHEUMATIC FEVER AND POST-STREPTOCOCCAL REACTIVE ARTHRITIS What is it? Rheumatic fever has been defined as a disease triggered by infection caused by streptococcus.
Burns. A Comprehensive Review Assessment & Management
 Burns A Comprehensive Review Assessment & Management 1 Objectives Understand types of Burns Understand the pathophysiology of the Burns Understand Rule of Nine Understand Classification of Burns Identify
Burns A Comprehensive Review Assessment & Management 1 Objectives Understand types of Burns Understand the pathophysiology of the Burns Understand Rule of Nine Understand Classification of Burns Identify
Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from 2000 to 2006 Yumiko Yamane 1, Michiko Aihara 1 and Zenro Ikezawa 1
 Allergology International. 7;:9- DOI:. allergolint.o-7- ORIGINAL ARTICLE Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from to Yumiko Yamane, Michiko Aihara and Zenro Ikezawa
Allergology International. 7;:9- DOI:. allergolint.o-7- ORIGINAL ARTICLE Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from to Yumiko Yamane, Michiko Aihara and Zenro Ikezawa
HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review
 HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
Drug eruptions and hepatic involvement: a study
 International Journal of Research in Dermatology http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20183819 Drug eruptions and hepatic involvement:
International Journal of Research in Dermatology http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20183819 Drug eruptions and hepatic involvement:
Genetic susceptibility for Stevens-Johnson syndrome/toxic epidermal necrolysis with mucosal involvements
 249 Special Issue: Inflammation in Ophthalmology Review Article Genetic susceptibility for Stevens-Johnson syndrome/toxic epidermal necrolysis with mucosal involvements 1, 2, ) Mayumi Ueta 1) Department
249 Special Issue: Inflammation in Ophthalmology Review Article Genetic susceptibility for Stevens-Johnson syndrome/toxic epidermal necrolysis with mucosal involvements 1, 2, ) Mayumi Ueta 1) Department
Summary of the risk management plan (RMP) for Cresemba (isavuconazole)
 EMA/576866/2015 Summary of the risk management plan (RMP) for Cresemba (isavuconazole) This is a summary of the risk management plan (RMP) for Cresemba, which details the measures to be taken in order
EMA/576866/2015 Summary of the risk management plan (RMP) for Cresemba (isavuconazole) This is a summary of the risk management plan (RMP) for Cresemba, which details the measures to be taken in order
In the past few years, beta-lactam-resistant gram-positive
 Early Diagnosis Is Key in Vancomycin-Induced Linear IgA Bullous Dermatosis and Stevens-Johnson Syndrome Douglas H. Jones, MS Michael Todd, MD Timothy J. Craig, DO Background: With the emergence of highly
Early Diagnosis Is Key in Vancomycin-Induced Linear IgA Bullous Dermatosis and Stevens-Johnson Syndrome Douglas H. Jones, MS Michael Todd, MD Timothy J. Craig, DO Background: With the emergence of highly
Immune System. Presented by Kazzandra Anton, Rhea Chung, Lea Sado, and Raymond Tanaka
 Immune System Presented by Kazzandra Anton, Rhea Chung, Lea Sado, and Raymond Tanaka Content Standards 35.1 In innate immunity, recognition and response rely on traits common to groups of pathogens 35.2
Immune System Presented by Kazzandra Anton, Rhea Chung, Lea Sado, and Raymond Tanaka Content Standards 35.1 In innate immunity, recognition and response rely on traits common to groups of pathogens 35.2
Early View Article: Online published version of an accepted article before publication in the final form.
 : Online published version of an accepted article before publication in the final form. Journal Name: International Journal of Case Reports and Images (IJCRI) Type of Article: Case Report Title: A Case
: Online published version of an accepted article before publication in the final form. Journal Name: International Journal of Case Reports and Images (IJCRI) Type of Article: Case Report Title: A Case
cipro loxacin; stevens-johnson syndrome; urinary tract infection; adverse drug reaction
 Open Journal of Clinical & Medical Case Reports Volume 3 (2017) Issue 7 ISSN 2379-1039 Cipro loxacin induced Stevens Johnson syndrome: A case report on Dermatology department P Mohammed Sha i*; K Narotham
Open Journal of Clinical & Medical Case Reports Volume 3 (2017) Issue 7 ISSN 2379-1039 Cipro loxacin induced Stevens Johnson syndrome: A case report on Dermatology department P Mohammed Sha i*; K Narotham
Mark A. Bechtel, MD Clinical Associate Professor Division Director, Dermatology Ohio State University Medical Center
 Dermatologic Emergencies Mark A. Bechtel, MD Clinical Associate Professor Division Director, Dermatology Ohio State University Medical Center Clinical Features of SJS/TEN Initial symptoms Fever, stinging
Dermatologic Emergencies Mark A. Bechtel, MD Clinical Associate Professor Division Director, Dermatology Ohio State University Medical Center Clinical Features of SJS/TEN Initial symptoms Fever, stinging
Cutaneous drug reactions
 Maintenance of Certification clinical management series Series editor: James T. Li, MD, PhD Cutaneous drug reactions David A. Khan, MD Dallas, Tex INSTRUCTIONS Credit can now be obtained, free for a limited
Maintenance of Certification clinical management series Series editor: James T. Li, MD, PhD Cutaneous drug reactions David A. Khan, MD Dallas, Tex INSTRUCTIONS Credit can now be obtained, free for a limited
Inpatient Consultative Dermatology and Severe Cutaneous Adverse Reactions
 Inpatient Consultative Dermatology and Severe Cutaneous Adverse Reactions Jeremy A. Schneider, MD Assistant Clinical Professor UC San Diego Department of Dermatology Objectives Brief overview of some of
Inpatient Consultative Dermatology and Severe Cutaneous Adverse Reactions Jeremy A. Schneider, MD Assistant Clinical Professor UC San Diego Department of Dermatology Objectives Brief overview of some of
Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection
 Case Report Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection J Med Assoc Thai 2015; 98 (Suppl. 7): S243-S247 Full text. e-journal:
Case Report Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection J Med Assoc Thai 2015; 98 (Suppl. 7): S243-S247 Full text. e-journal:
Adverse drug reactions
 Medications William Smith Adverse drug reactions Allergy? Side-effect? Intolerance? Background Adverse drug reactions (ADRs) vary from life-threatening anaphylaxis to minor common side-effects. Objective
Medications William Smith Adverse drug reactions Allergy? Side-effect? Intolerance? Background Adverse drug reactions (ADRs) vary from life-threatening anaphylaxis to minor common side-effects. Objective
Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review
 Case Report Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review Deepali P. Mohite*, Satyajitraje Tekade*, Amol Gadbail*, M. S. Chaudhary** Abstract : Toxic epidermal necrolysis (TEN) and Stevens
Case Report Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review Deepali P. Mohite*, Satyajitraje Tekade*, Amol Gadbail*, M. S. Chaudhary** Abstract : Toxic epidermal necrolysis (TEN) and Stevens
NEOFEN 60 mg suppository
 PACKAGE LEAFLET: INFORMATION FOR THE USER NEOFEN 60 mg suppository IBUPROFEN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PACKAGE LEAFLET: INFORMATION FOR THE USER NEOFEN 60 mg suppository IBUPROFEN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
Package leaflet: Information for the patient UNITED KINGDOM Feldene 5mg/g Gel piroxicam
 Package leaflet: Information for the patient UNITED KINGDOM Feldene 5mg/g Gel piroxicam Read all of this leaflet carefully before you start using this medicine because it contains important information
Package leaflet: Information for the patient UNITED KINGDOM Feldene 5mg/g Gel piroxicam Read all of this leaflet carefully before you start using this medicine because it contains important information
STEVENS-JOHNSON SYNDROME: A CASE REPORT
 STEVENS-JOHNSON SYNDROME: A CASE REPORT Castana O., Rempelos G., Anagiotos G., Apostolopoulou C., Dimitrouli A., Alexakis D. Department of Plastic and Reconstructive Surgery, Evangelismos General Hospital,
STEVENS-JOHNSON SYNDROME: A CASE REPORT Castana O., Rempelos G., Anagiotos G., Apostolopoulou C., Dimitrouli A., Alexakis D. Department of Plastic and Reconstructive Surgery, Evangelismos General Hospital,
DERMATOLOGIC EMERGENCIES. Mary Evers D.O., F.A.O.C.D. Georgetown, Texas
 DERMATOLOGIC EMERGENCIES Mary Evers D.O., F.A.O.C.D. Georgetown, Texas SKIN EMERGENCIES??? Subclassifications: Autoimmune (Anaphylaxis, Vasculitis, Pemphigus) Erythroderma (AGEP, DRESS, SJS, TEN) Infectious
DERMATOLOGIC EMERGENCIES Mary Evers D.O., F.A.O.C.D. Georgetown, Texas SKIN EMERGENCIES??? Subclassifications: Autoimmune (Anaphylaxis, Vasculitis, Pemphigus) Erythroderma (AGEP, DRESS, SJS, TEN) Infectious
PHM142 Autoimmune Disorders + Idiosyncratic Drug Reactions
 PHM142 Autoimmune Disorders + Idiosyncratic Drug Reactions 1 Autoimmune Disorders Auto-reactivity: low physiological levels (e.g. tolerance) vs. pathogenic levels 80+ types of autoimmune diseases affect
PHM142 Autoimmune Disorders + Idiosyncratic Drug Reactions 1 Autoimmune Disorders Auto-reactivity: low physiological levels (e.g. tolerance) vs. pathogenic levels 80+ types of autoimmune diseases affect
