CHEM 110L, Binder. Experiment 1 Carbohydrates
|
|
|
- Collin Wood
- 5 years ago
- Views:
Transcription
1 Experiment 1 Carbohydrates Carbohydrates in our diet are a major source of energy and comfort! Foods high in carbohydrates include the most delicious kinds, such as potatoes, bread, and pasta. If we take in more carbohydrate than needed for energy, the excess is converted to glycogen or fat. The carbohydrate family is organized into three general classes: monosaccharides, disaccharides, and polysaccharides. Monosaccharides contain C,, and in units of (C 2 ) n. The most common monosaccharides have six carbon atoms (hexoses) with five alcohol groups and one carbonyl group. exoses are found in fruit juices, honey, and corn syrup. Either the monosaccharide itself is present, or it is part of a disaccharide and is accessed via hydrolysis. Aldohexoses contain an aldehyde group on C1 while ketoses contain a ketone on C2. Aldohexoses (along with all other aldoses) are known as reducing sugars, since the aldehyde can be further oxidized to a carboxylic acid. Ketoses can isomerize into aldoses and thus are also called reducing sugars. Another key point of hexoses is that they contain 3 or 4 chiral centers. As such, there are 8 possible stereoisomers for ketohexoses and 16 possible for aldohexoses, each of which have a slightly different taste due to different receptors on the tongue. Each of these stereoisomers are metabolized differently, even if the processes differ by only one enzymatic reaction. This is narrowed down by the high prevalence of D-sugars in nature. The D designation is given to the penultimate (next to last) carbon on the chain and limits the configuration of that chiral center to R (Figure 1). An L designation is given to a sugar with an S configuration at the penultimate carbon. Monosaccharides can be shown in their open chain form using a Fischer projection. Recall that while Fischer projections are drawn with straight lines (no wedges or dashes), this convention still implies specific configurations to each chiral center. most oxidized on the top (C1) C reducing sugar - aldehyde can be oxidized to an acid "" is on the right side least oxidized on the on the pentultimate C, bottom (C6) making it a D-sugar D-Glucose Figure 1. Structural features of glucose; conventions for drawing monosaccharides. The main point of interest for hexoses is their metabolic fate: Glycolysis. Metabolism is an organism s way of breaking down nutrients into simpler, more versatile components. Catabolism is the opposite building complex molecules from small molecules. Almost every hexose we ingest in our diet is enzymatically converted to D-glucose, also known as blood sugar, so that glycolysis can occur. Through this process, the energy stored within the sugar is released. The ten steps of glycolysis release a net 8 kj (20 kcal) of energy per mole of glucose. D-Glucose is broken down into two three-carbon units called pyruvate (Figure 2). The fate of pyruvate depends on the presence of oxygen (aerobic vs. anaerobic), type of organism, and the metabolic needs of the organism. In some cases, pyruvate is converted into C 2, which is passed on to plants, wherein C 2 is used as a building block in plant catabolism. Let us take a moment to appreciate the great carbon-circle of life! For frame of reference, running a mile burns about 200 kcal (dietary calories are really kilocalories). Assuming glycolysis is the only source of energy, the body uses almost 10 moles of glucose (1773 g) to run a mile and goes through the steps of glycolysis x times! E1-1
2 C D-Glucose + 2 NAD + 2 ADP 2 P i 10 steps aerobic (with 2 ) 6 C C 3 Lactate (under acidic conditions becomes lactic acid) Figure 2. verview of glycolysis; examples of the fates of pyruvate. 2 Pyruvate + anaerobic (animal) C 2-2 NAD ATP 2 2 anaerobic (yeast) ΔG = -8 kj/mol 2 C C 2 Returning to carbohydrate conventions, the open chain form of hexoses is in a dynamic equilibrium with the closed form. Aldohexoses tend to close into pyranoses (six-membered rings) while ketohexoses form furanoses (five-membered rings). In these cases, the penultimate attacks the carbonyl functionality (pay attention to the numbering in Figure 3). Aldohexoses could also close into furanoses and ketohexoses into pyranoses, but we will leave these possibilities aside for the sake of simplicity. The closed-form sugars are commonly shown as aworth projections, a simpler version of chair conformations. PEN C D-Glucose 6 CLSED - pyranose (anomeric mixture) 1 2 α - down 6 β - up 1 2 Figure 3. Glucose (an aldohexose) in closed (pyranose) form; fructose (a ketohexose) in closed (furanose) form. Disaccharides contain two of the common monosaccharides. Some common disaccharides include maltose, sucrose (table sugar), and lactose (milk sugar). Disaccharide Sources Monosaccharides Maltose Germinating grains, starch hydrolysis Glucose + glucose Lactose Milk, yogurt, ice cream Glucose + galactose Sucrose Sugar cane, sugar beets Glucose + fructose In a disaccharide, two monosaccharides form a glycosidic bond with the loss of water. For example, in maltose, two glucose units are linked by an α-1,4-glycosidic bond. 6 6' 2 1 ' PEN 2 + 4' 1' 1 4' 1' + 2 2' 2 2' Figure 4. Condensation of glucose into α-maltose. 6 ' D-Fructose 6' CLSED - furanose (β form) 6 1 E1-2
3 Polysaccharides are long-chain polymers that contain many thousands of monosaccharides (usually glucose units) joined together by glycosidic bonds. Three important polysaccharides are starch, cellulose, and glycogen. These examples all contain glucose units, but differ in the type of glycosidic bonds and the amount of branching in the molecule. Starch is an insoluble storage form of glucose found in rice, wheat, potatoes, beans, and cereals. Starch is composed of two kinds of polysaccharides, amylose and amylopectin. Amylose, which makes up about 20% of starch, consists of α-d-glucose molecules connected by α-1,4-glycosidic bonds in a continuous chain (Figure a). A typical polymer of amylose may contain from 20 to 4000 glucose units. (a) α-1,4-glycosidic Bonds (b) α-1,6-glycosidic Bond 2 C Figure. Portions of (a) amylose and (b) amylopectin. Amylopectin is a branched-chain polysaccharide that makes up as much as 80% of starch. In amylopectin, α-1,4-glycosidic bonds connect most of the glucose molecules. owever, at about every 2 glucose units, there are branches of glucose molecules attached by α-1,6- glycosidic bond (Figure b). Cellulose is the major structural material of wood and plants. Cotton is almost pure cellulose. In cellulose, glucose molecules form a long unbranched chain similar to amylose except that β-1,4-glycosidic bonds connect the glucose molecules. The β isomers are aligned in parallel rows that are held in place by hydrogen bonds between the rows. This gives a rigid structure for cell walls in wood and fiber and makes cellulose resistant to hydrolysis. Chemical Tests for Carbohydrates The following chemical tests are used to distinguish between the different types of carbohydrates described above. You will be given a 2% solution of an unknown carbohydrate. The chemical tests will be run on the unknown alongside standards: glucose, fructose, sucrose, lactose, starch, and water as a control. Students will work in pairs. Use your observations to determine the type of carbohydrate in your unknown solution. Notebook Preparation Each of Parts A-D should start with a new notebook page containing the following: General reaction scheme for a positive test Reagent table read through procedure for list of chemicals; include amount (# of drops, mg, or ml), MW, bp or mp, and one-word hazards where applicable (refer to Clean-up & Safety Table at the end of the procedures). Provide properties for sugars, unknown, and water, only once then refer to previous pages in the following parts. Procedure hand-written step-wise procedure Data Table re-create tables shown after each procedure Clean-up & Safety copy pertinent notes from the Clean-up & Safety Table Recommended final page with an abbreviated version of the in-lab questions. TA will initial your notebook for completeness before leaving lab. E1-3
4 A. Benedict s Test for Reducing Sugars All of the monosaccharides and most of the disaccharides can be oxidized. When the cyclic structure opens, the aldehyde group is available for oxidation. Reagents such as Benedict s reagent contain a Cu 2+ ion that is reduced. Therefore, all the sugars that react with Benedict s reagent are called reducing sugars. Ketoses also acts as reducing sugars because the ketone group on C2 isomerizes through two keto-enol tautomerizations to give an aldehyde group on C1. A solution of Benedict s solution contains copper (II) sulfate dehydrate, sodium carbonate, and sodium citrate dehydrate. Essentially, this is a basic copper solution. When oxidation of a sugar occurs, the Cu 2+ is reduced to Cu +, which forms a red precipitate of copper (I) oxide (Figure 6). The color of the precipitate varies from green to gold to red depending on the concentration of the reducing sugar. Sucrose is an example of a disaccharide that is not a reducing sugar because it cannot revert to the open-chain form that would provide the aldehyde group needed to reduce the cupric ion. SUGAR + 2 Cu 2+ - SUGAR + Cu 2 (s) (Blue) (Red/Green/Gold) Figure 6. Benedict s test for reducing sugars. PRCEDURE: Place drops of solutions of glucose, fructose, sucrose, lactose, starch, the unknown, and water in separate test tubes. Label each test tube. Add 1 ml of Benedict s reagent to each sample. Place the test tubes in a boiling water bath for 3-4 minutes (careful the labels don t fall off!). Record your observations and classify each as a reducing (R) or nonreducing (NR) sugar. Table A. bservations of Benedict s Test Glucose Fructose Sucrose Lactose Starch Water Unknown B. Seliwanoff s Test for Ketoses Seliwanoff s test is used to distinguish between ketohexoses and aldohexoses. The reagents consist of resorcinol (3-hydroxyphenol) and hydrochloric acid. The acid causes the dehydration of the ketose to eliminate 3 equivalents of water. The dehydrated ketose reacts with resorcinol in another series of condensation (loss of water) reactions to form a highly conjugated molecule with a deep cherry red color (you can find this structure online). With ketoses, a red color is formed rapidly. Aldoses give a light pink color that takes a longer time to develop. The test is most sensitive for fructose, a ketohexose. PRCEDURE: Place drops of each carbohydrate solution in separate test tubes. Add 1 ml of Seliwanoff s reagent to each. The reagent contains concentrated Cl use carefully! Place the test tubes in a boiling hot water bath (careful the labels don t fall off!) and note the time. After 1 minute, observe the colors in the test tubes. Record your observations (fast, slow, or no color change) along with whether the carbohydrate is a ketose (K) or not a ketose (NK). Table B. bservations of Seliwanoff s Test Glucose Fructose Sucrose Lactose Starch Water Unknown E1-4
5 C. Iodine Test for Polysaccharides When molecular iodine is added to amylose, the helical shape of the unbranched polysaccharide traps iodine molecules, producing a deep blue-black complex. Amylopectin and cellulose react with iodine to give red to brown colors. Glycogen produces a reddish-purple color. Monosaccharides and disaccharides are too small to trap iodine molecules and do not form dark colors with iodine. PRCEDURE: Using a spot plate, place drops of each carbohydrate solution and water in the wells. Be sure to carefully label the wells. Add 1 drop of iodine solution to each sample. Record your observations and whether each is a polysaccharide (P) or not (NP). Table C. bservations of Iodine Test Glucose Fructose Sucrose Lactose Starch Water Unknown D. ydrolysis of Disaccharides and Polysaccharides Disaccharides hydrolyze in the presence of an acid to give the individual monosaccharides. In the lab, we use water and acid to hydrolyze starches, which produce smaller saccharides such as maltose. Eventually, the hydrolysis reaction converts maltose to glucose molecules. In the body, enzymes in our saliva and from the pancreas carry out the hydrolysis. Complete hydrolysis produces glucose, which provides about 0% of our nutritional calories. PRCEDURE: Place 3 ml of 2% starch in two test tubes and 3 ml of 2% sucrose solution in two more test tubes. If at this point, you suspect your unknown to be a disaccharide or polysaccharide, do the same with the unknown. To one sample each of sucrose and starch (and the unknown if applicable), add 20 drops of 10% Cl. To the other samples of sucrose and starch, add 20 drops of 2. Label the test tubes and heat in a boiling water bath for 10 minutes. Remove the test tubes from the water bath and let them cool. To the samples containing Cl, add 10% Na (about 20 drops) until one drop of the mixture turns litmus paper blue, indicating the Cl has been neutralized. Test the samples for hydrolysis using the iodine test and Benedict s test. Record your observations and determine whether hydrolysis occurred. Table D. bservations of ydrolysis Tests Results Sucrose/ 2 Sucrose/Cl Starch/ 2 Starch/Cl Unk/ 2 Unk/Cl Iodine test Benedict s test Table 1. Clean-up and Safety Clean-up Safety Clean and return all shared glassware to Wear gloves. Remove gloves before leaving. reagent counters. Liquid waste: contents of test tubes carefully Cl, Benedict s reagent, Seliwanoff s reagent, transfer into waste using a pipet. and Na are corrosive Wipe down benchtops and leave the lab just Benedict s reagent, potassium iodide, and all as your found it. Thank you! sugars are irritants. ALL STUDENTS IN ANY SECTIN WIT GLASSWARE (INCLUDING PIPETS) IN ANY F TE TRASCANS WILL LSE PINTS FRM TEIR LAB REPRT E1-
6 Introduction: Pre-Lab Questions Typed responses in complete sentences with spaces below to draw structures by hand in pen. Turned in at the beginning of lab. 1. Provide the sugar classification and draw representations of D-glucose as indicated. (a) Fischer projection (b) aworth projections: α-d-glucopyranose and β-d-glucopyranose 2. Provide the sugar classification and draw representations of D-fructose as indicated. (a) Fischer projection (b) aworth projections: α-d-fructofuranose and β-d-fructofuranose 3. What is a reducing sugar? Your answer should not be all the sugars that react with Benedict s reagent are called reducing sugars. Be more specific on the criteria of a reducing sugar. Give your answer in one or two sentences. 4. Draw the structure of α-d-maltose and show the complete equation for its hydrolysis with aqueous Cl. Indicate the type of glycosidic bond in maltose.. Briefly describe the expected observation in each chemical test for each sugar (re-create this table in your word processing document). Table x. Expected Chemical Test Results A. Benedict B. Seliwanoff (R / NR) (K / NK) C. Iodine (P / NP) Glucose Fructose Sucrose Lactose Starch 6. What carbohydrate(s) would have the following test results? (a) Produces a reddish-orange solid with the Benedict s test and a red color with Seliwanoff s reagent in 1 minute. (b) Gives a color change with Benedict s test and a light orange color with Seliwanoff s reagent after minutes. (c) Gives no color change with Benedict s or Seliwanoff s test, but turns a blue-black color with the iodine reagent. E1-6
7 Results: In-Lab Questions Typed responses in complete sentences turned in with the lab report. It is recommended that students write the in-lab questions into the notebook before lab. 1. Re-create and complete the following tables in a word processing document to summarize your results. Table x. Expected Chemical Test Results A. Benedict B. Seliwanoff (R / NR) (K / NK) Glucose Fructose Sucrose Lactose Starch C. Iodine (P / NP) Water Unknown: D. ydrolysis Results Results Sucrose/ 2 Sucrose/Cl Starch/ 2 Starch/Cl Unk/ 2 Unk/Cl Iodine test Benedict s test ydrolysis products present? 2. Discuss the results of the Benedict, Seliwanoff, and iodine tests: List the reducing sugars, ketoses, and polysaccharides. Were results as expected? 3. ow do the results of the Benedict s test performed after the hydrolysis test indicate that hydrolysis of sucrose and starch (and the unknown, if applicable) occurred? 4. ow do the results of the iodine test indicate that hydrolysis of starch (and the unknown) occurred?. Provide the unknown code and identity of your unknown (glucose, fructose, sucrose, lactose, or starch). Summarize the results of the chemical tests for the unknown and how this was used to determine the identity of the unknown. 6. Go to pubs.acs.org and perform a search on any one of the tests from this experiment. Choose any journal article (not a book, website, or other type of source) and provide just the citation in the proper ACS format (see Technical Writing Guidelines). Abstract see Technical Writing Guidelines online. Do not write the abstract without using these guidelines! E1-7
8 Exp 1 - Carbohydrates Report Name Section Day Time TA Name CEM 110L GRADING RUBRIC - Use as cover page for report SECTIN INSTRUCTR CMMENTS PINTS ASSIGNED IN-LAB QUIZ / LAB REPRT ABSTRACT ne paragraph, four sentences: Purpose, procedure, main result(s), and conclusion(s). / 10 INTRDUCTIN riginal responses to pre-lab questions with TA initials / 30 RESULTS The main results are stated, as outlined in the in-lab questions, using complete sentences. / 30 DISCUSSIN and CNCLUSIN Reflection of whether results were as expected and whether it is supported by the theory behind the experiment. NNE 0 / 0 EXPERIMENTAL SECTIN The experimental details (including final amount used and obtained) are briefly described in a few sentences. NNE 0 / 0 NTEBK PAGES Proper format: reaction scheme, chemical info table, procedure, waste and clean-up procedure. / 1 NEATNESS, RGANIZATIN, & LAB TECNIQUE Proper order and format (see syllabus for full descriptions of each section). Safety rules followed, equipment used properly. / 10 LAB REPRT TTAL / 100 E1-8
Tests for Carbohydrates
 Goals bserve physical and chemical properties of some common carbohydrates. Use physical and chemical tests to distinguish between monosaccharides, disaccharides, and polysaccharides. Identify an unknown
Goals bserve physical and chemical properties of some common carbohydrates. Use physical and chemical tests to distinguish between monosaccharides, disaccharides, and polysaccharides. Identify an unknown
Chemistry B11 Chapters 13 Esters, amides and carbohydrates
 Chapters 13 Esters, amides and carbohydrates Esters: esters are derived from carboxylic acids (the hydrogen atom in the carboxyl group of carboxylic acid is replaced by an alkyl group). The functional
Chapters 13 Esters, amides and carbohydrates Esters: esters are derived from carboxylic acids (the hydrogen atom in the carboxyl group of carboxylic acid is replaced by an alkyl group). The functional
Fundamentals of Organic Chemistry. CHAPTER 6: Carbohydrates
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 6: Carbohydrates Carbohydrates
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 6: Carbohydrates Carbohydrates
Name: Period: Date: Testing for Biological Macromolecules Lab
 Testing for Biological Macromolecules Lab Introduction: All living organisms are composed of various types of organic molecules, such as carbohydrates, starches, proteins, lipids and nucleic acids. These
Testing for Biological Macromolecules Lab Introduction: All living organisms are composed of various types of organic molecules, such as carbohydrates, starches, proteins, lipids and nucleic acids. These
Ch13. Sugars. What biology does with monosaccharides disaccharides and polysaccharides. version 1.0
 Ch13 Sugars What biology does with monosaccharides disaccharides and polysaccharides. version 1.0 Nick DeMello, PhD. 2007-2015 Ch13 Sugars Haworth Structures Saccharides can form rings. That creates a
Ch13 Sugars What biology does with monosaccharides disaccharides and polysaccharides. version 1.0 Nick DeMello, PhD. 2007-2015 Ch13 Sugars Haworth Structures Saccharides can form rings. That creates a
SPECIFICATION CONTINUED Glucose has two isomers, α-glucose and β-glucose, with structures:
 alevelbiology.co.uk SPECIFICATION Monosaccharides are the monomers from which larger carbohydrates are made. Glucose, galactose and fructose are common monosaccharides. A condensation reaction between
alevelbiology.co.uk SPECIFICATION Monosaccharides are the monomers from which larger carbohydrates are made. Glucose, galactose and fructose are common monosaccharides. A condensation reaction between
BCH302 [Practical] 1
![BCH302 [Practical] 1 BCH302 [Practical] 1](/thumbs/81/83984357.jpg) BCH302 [Practical] 1 Carbohydrates are defined as the polyhydroxy aldehydes or polyhydroxy ketones. Most, but not all carbohydrate have a formula (CH 2 O)n (hence the name hydrate of carbon). Sugars ends
BCH302 [Practical] 1 Carbohydrates are defined as the polyhydroxy aldehydes or polyhydroxy ketones. Most, but not all carbohydrate have a formula (CH 2 O)n (hence the name hydrate of carbon). Sugars ends
EXPERIMENT 6 Properties of Carbohydrates: Solubility, Reactivity, and Specific Rotation
 EXPERIMENT 6 Properties of Carbohydrates: Solubility, Reactivity, and Specific Rotation Materials Needed About 3-5 g each of Glucose, Fructose, Maltose, Sucrose, Starch sodium bicarbonate, NaC 3 (s) 15
EXPERIMENT 6 Properties of Carbohydrates: Solubility, Reactivity, and Specific Rotation Materials Needed About 3-5 g each of Glucose, Fructose, Maltose, Sucrose, Starch sodium bicarbonate, NaC 3 (s) 15
Chapter 18. Carbohydrates with an Introduction to Biochemistry. Carbohydrates with an Introduction to Biochemistry page 1
 Chapter 18 Carbohydrates with an Introduction to Biochemistry Carbohydrates with an Introduction to Biochemistry page 1 Introduction to Proteins, Carbohydrates, Lipids, and Bioenergetics Metabolism and
Chapter 18 Carbohydrates with an Introduction to Biochemistry Carbohydrates with an Introduction to Biochemistry page 1 Introduction to Proteins, Carbohydrates, Lipids, and Bioenergetics Metabolism and
!"#$%&'()*+(!,-./012-,345(
 (!"#$%&'()*+(!,-./012-,345( (!"#"$%&'()$*%#+,'(-(.+/&/*+,%&(01"2+34$5( 6%#+,"(!/$75#38+(92+41( CAPTER 20: Learning Objectives:! >
(!"#$%&'()*+(!,-./012-,345( (!"#"$%&'()$*%#+,'(-(.+/&/*+,%&(01"2+34$5( 6%#+,"(!/$75#38+(92+41( CAPTER 20: Learning Objectives:! >
Carbohydrates. Monosaccharides
 Carbohydrates Carbohydrates (also called saccharides) are molecular compounds made from just three elements: carbon, hydrogen and oxygen. Monosaccharides (e.g. glucose) and disaccharides (e.g. sucrose)
Carbohydrates Carbohydrates (also called saccharides) are molecular compounds made from just three elements: carbon, hydrogen and oxygen. Monosaccharides (e.g. glucose) and disaccharides (e.g. sucrose)
CLASS 11th. Biomolecules
 CLASS 11th 01. Carbohydrates These are the compound of carbon, hydrogen and oxygen having hydrogen and oxygen in the same ratio as that of water, i.e. 2 : 1. They are among the most widely distributed
CLASS 11th 01. Carbohydrates These are the compound of carbon, hydrogen and oxygen having hydrogen and oxygen in the same ratio as that of water, i.e. 2 : 1. They are among the most widely distributed
QUALITATIVE TESTS OF CARBOHYDRATE
 QUALITATIVE TESTS OF CARBOHYDRATE MACROMOLECULE CARBOHYDRATES Are the key source of energy used by living things. Also serve as extracellular structural elements as in cell wall of bacteria and plant.
QUALITATIVE TESTS OF CARBOHYDRATE MACROMOLECULE CARBOHYDRATES Are the key source of energy used by living things. Also serve as extracellular structural elements as in cell wall of bacteria and plant.
Carbohydrates are a large group of organic compounds occurring in and including,, and. They contain hydrogen and oxygen in the same ratio as (2:1).
 Carbohydrates are a large group of organic compounds occurring in and and including,, and. They contain hydrogen and oxygen in the same ratio as (2:1). Why we study carbohydrates 1) carbohydrates are the
Carbohydrates are a large group of organic compounds occurring in and and including,, and. They contain hydrogen and oxygen in the same ratio as (2:1). Why we study carbohydrates 1) carbohydrates are the
Chemical Reactions. Carbohydrate Qualitative Analysis: Foundation Lab. 2012, Sharmaine S. Cady East Stroudsburg University
 hemical Reactions arbohydrate Qualitative Analysis: Foundation Lab 2012, Sharmaine S. ady East Stroudsburg University Skills to build: Doing microscale reactions bserving evidence of a chemical reaction
hemical Reactions arbohydrate Qualitative Analysis: Foundation Lab 2012, Sharmaine S. ady East Stroudsburg University Skills to build: Doing microscale reactions bserving evidence of a chemical reaction
Experiment 27 CARBOHYDRATES. Text Topics and New Techniques. Discussion. Carbohydrate chemistry, qualitative tests for carbohydrates.
 E27-1 Experiment 27 Fig. 27-1 CARBYDRATES Text Topics and New Techniques Discussion Carbohydrate chemistry, qualitative s for carbohydrates. Emil Fischer (1852-1919) received the Nobel Prize in chemistry
E27-1 Experiment 27 Fig. 27-1 CARBYDRATES Text Topics and New Techniques Discussion Carbohydrate chemistry, qualitative s for carbohydrates. Emil Fischer (1852-1919) received the Nobel Prize in chemistry
I (CH 2 O) n or H - C - OH I
 V. ARBYDRATE arbohydrates (glycans) have the following basic composition: I ( ) n or - - I Many carbohydrates are soluble in water. The usual chemical test for the simpler carbohydrates is heating with
V. ARBYDRATE arbohydrates (glycans) have the following basic composition: I ( ) n or - - I Many carbohydrates are soluble in water. The usual chemical test for the simpler carbohydrates is heating with
Structural Polysaccharides
 Carbohydrates & ATP Carbohydrates include both sugars and polymers of sugars. The simplest carbohydrates are the monosaccharides, or simple sugars; these are the monomers from which more complex carbohydrates
Carbohydrates & ATP Carbohydrates include both sugars and polymers of sugars. The simplest carbohydrates are the monosaccharides, or simple sugars; these are the monomers from which more complex carbohydrates
Lab #4: Nutrition & Assays for Detecting Biological Molecules - Introduction
 Lab #4: Nutrition & Assays for Detecting Biological Molecules - Introduction Most biological molecules fall into one of four varieties: proteins, carbohydrates, lipids and nucleic acids. These are sometimes
Lab #4: Nutrition & Assays for Detecting Biological Molecules - Introduction Most biological molecules fall into one of four varieties: proteins, carbohydrates, lipids and nucleic acids. These are sometimes
Carbohydrates. Chapter 12
 Carbohydrates Chapter 12 Educational Goals 1. Given a Fischer projection of a monosaccharide, classify it as either aldoses or ketoses. 2. Given a Fischer projection of a monosaccharide, classify it by
Carbohydrates Chapter 12 Educational Goals 1. Given a Fischer projection of a monosaccharide, classify it as either aldoses or ketoses. 2. Given a Fischer projection of a monosaccharide, classify it by
Qualitative analysis of carbohydrates II
 Qualitative analysis of carbohydrates II C 2 C 2 1 glycogen C 2 C 2 6 C 2 C 2 C 2 5 1 4 4 3 2 Complex carbohydrate Complex sugars consist of more than one unit of monosachride, it could be: -Disaccharides
Qualitative analysis of carbohydrates II C 2 C 2 1 glycogen C 2 C 2 6 C 2 C 2 C 2 5 1 4 4 3 2 Complex carbohydrate Complex sugars consist of more than one unit of monosachride, it could be: -Disaccharides
Biological molecules = Biomolecules = Compounds of life
 Biological molecules = Biomolecules = Compounds of life Carbohydrates Proteins & Amino Acids Mono-saccharides Olego-saccharides Di-saccharides Poly-saccharides Lipids Oils & Fats Amino acids Proteins Enzymes
Biological molecules = Biomolecules = Compounds of life Carbohydrates Proteins & Amino Acids Mono-saccharides Olego-saccharides Di-saccharides Poly-saccharides Lipids Oils & Fats Amino acids Proteins Enzymes
Carbohydrates. Organic compounds which comprise of only C, H and O. C x (H 2 O) y
 Carbohydrates Organic compounds which comprise of only C, H and O C x (H 2 O) y Carbohydrates Monosaccharides Simple sugar Soluble in water Precursors in synthesis triose sugars of other (C3) molecules
Carbohydrates Organic compounds which comprise of only C, H and O C x (H 2 O) y Carbohydrates Monosaccharides Simple sugar Soluble in water Precursors in synthesis triose sugars of other (C3) molecules
Figure 2. Figure 1. Name: Bio AP Lab Organic Molecules
 Name: Bio AP Lab Organic Molecules BACKGROUND: A cell is a living chemistry laboratory in which most functions take the form of interactions between organic molecules. Most organic molecules found in living
Name: Bio AP Lab Organic Molecules BACKGROUND: A cell is a living chemistry laboratory in which most functions take the form of interactions between organic molecules. Most organic molecules found in living
GENERAL TESTS FOR CARBOHYDRATE. By Sandip Kanazariya
 GENERAL TESTS FOR CARBOHYDRATE By Sandip Kanazariya Introduction Carbohydrates are of great importance to human beings. They are major part of our diet, providing 60-70% of total energy required by the
GENERAL TESTS FOR CARBOHYDRATE By Sandip Kanazariya Introduction Carbohydrates are of great importance to human beings. They are major part of our diet, providing 60-70% of total energy required by the
For example, monosaccharides such as glucose are polar and soluble in water, whereas lipids are nonpolar and insoluble in water.
 Biology 4A Laboratory Biologically Important Molecules Objectives Perform tests to detect the presence of carbohydrates, lipids, proteins, and nucleic acids Recognize the importance of a control in a biochemical
Biology 4A Laboratory Biologically Important Molecules Objectives Perform tests to detect the presence of carbohydrates, lipids, proteins, and nucleic acids Recognize the importance of a control in a biochemical
HW #9: 21.36, 21.52, 21.54, 21.56, 21.62, 21.68, 21.70, 21.76, 21.82, 21.88, 21.94, Carbohydrates
 Chemistry 131 Lectures 16 & 17: Carbohydrates Chapter 21 in McMurry, Ballantine, et. al. 7 th edition 05/24/18, 05/25/18 W #9: 21.36, 21.52, 21.54, 21.56, 21.62, 21.68, 21.70, 21.76, 21.82, 21.88, 21.94,
Chemistry 131 Lectures 16 & 17: Carbohydrates Chapter 21 in McMurry, Ballantine, et. al. 7 th edition 05/24/18, 05/25/18 W #9: 21.36, 21.52, 21.54, 21.56, 21.62, 21.68, 21.70, 21.76, 21.82, 21.88, 21.94,
CHAPTER 27 CARBOHYDRATES SOLUTIONS TO REVIEW QUESTIONS
 27 09/17/2013 11:12:35 Page 397 APTER 27 ARBYDRATES SLUTINS T REVIEW QUESTINS 1. In general, the carbohydrate carbon oxidation state determines the carbon s metabolic energy content. The more oxidized
27 09/17/2013 11:12:35 Page 397 APTER 27 ARBYDRATES SLUTINS T REVIEW QUESTINS 1. In general, the carbohydrate carbon oxidation state determines the carbon s metabolic energy content. The more oxidized
Experiment 20 Identification of Some Carbohydrates
 Experiment 20 Identification of Some arbohydrates arbohydrates are the direct product of the photosynthetic combination of carbon dioxide and water. By weight, they are the most common organic compounds
Experiment 20 Identification of Some arbohydrates arbohydrates are the direct product of the photosynthetic combination of carbon dioxide and water. By weight, they are the most common organic compounds
1. Denaturation changes which of the following protein structure(s)?
 Chem 11 Fall 2008 Examination #5 ASWER KEY MULTIPLE CICE (20 pts. total; 2 pts. each) 1. Denaturation changes which of the following protein structure(s)? a. primary b. secondary c. tertiary d. both b
Chem 11 Fall 2008 Examination #5 ASWER KEY MULTIPLE CICE (20 pts. total; 2 pts. each) 1. Denaturation changes which of the following protein structure(s)? a. primary b. secondary c. tertiary d. both b
Number of Carbohydrate Units
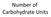 Number of Carbohydrate Units Monosaccharides = single unit Disaccharides = two units Oligiosaccharide = 3 10 units Polysaccharide = 11+ units Bonus: Can you name the most common Mono (4), Di(3), and Poly(4)
Number of Carbohydrate Units Monosaccharides = single unit Disaccharides = two units Oligiosaccharide = 3 10 units Polysaccharide = 11+ units Bonus: Can you name the most common Mono (4), Di(3), and Poly(4)
Chapter 22 Carbohydrates
 Chapter 22 Carbohydrates Introduction Classification of Carbohydrates Carbohydrates have the general formula C x (H 2 O) y Carbohydrates are defined as polyhydroxy aldehydes or ketones or substances that
Chapter 22 Carbohydrates Introduction Classification of Carbohydrates Carbohydrates have the general formula C x (H 2 O) y Carbohydrates are defined as polyhydroxy aldehydes or ketones or substances that
Chem 263 Nov 22, Carbohydrates (also known as sugars or saccharides) See Handout
 hem 263 Nov 22, 2016 arbohydrates (also known as sugars or saccharides) See andout Approximately 0.02% of the sun s energy is used on this planet for photosynthesis in which organisms convert carbon dioxide
hem 263 Nov 22, 2016 arbohydrates (also known as sugars or saccharides) See andout Approximately 0.02% of the sun s energy is used on this planet for photosynthesis in which organisms convert carbon dioxide
Carbohydrates 1. Steven E. Massey, Ph.D. Assistant Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras
 Carbohydrates 1 Steven E. Massey, Ph.D. Assistant Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras Office & Lab: NCN#343B Tel: 787-764-0000 ext. 7798 E-mail: stevenemassey@gmail.com
Carbohydrates 1 Steven E. Massey, Ph.D. Assistant Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras Office & Lab: NCN#343B Tel: 787-764-0000 ext. 7798 E-mail: stevenemassey@gmail.com
Chapter 20 Carbohydrates Chapter 20
 Chapter 20 Carbohydrates Chapter 20 1 Carbohydrates Carbohydrate: A polyhydroxyaldehyde or polyhydroxyketone, or a substance that gives these compounds on hydrolysis. Monosaccharide: A carbohydrate that
Chapter 20 Carbohydrates Chapter 20 1 Carbohydrates Carbohydrate: A polyhydroxyaldehyde or polyhydroxyketone, or a substance that gives these compounds on hydrolysis. Monosaccharide: A carbohydrate that
Lecture 2 Carbohydrates
 Lecture 2 Carbohydrates Sources of CHOs Wholegrains major dietary intake Vegetables, legumes ad fruit contain dietary fibre Milk products provide lactose essential for infants Glycogen is a storage carbohydrate,
Lecture 2 Carbohydrates Sources of CHOs Wholegrains major dietary intake Vegetables, legumes ad fruit contain dietary fibre Milk products provide lactose essential for infants Glycogen is a storage carbohydrate,
24.1 Introduction to Carbohydrates
 24.1 Introduction to Carbohydrates Carbohydrates (sugars) are abundant in nature: They are high energy biomolecules. They provide structural rigidity for organisms (plants, crustaceans, etc.). The polymer
24.1 Introduction to Carbohydrates Carbohydrates (sugars) are abundant in nature: They are high energy biomolecules. They provide structural rigidity for organisms (plants, crustaceans, etc.). The polymer
Topic 4 - #2 Carbohydrates Topic 2
 Topic 4 - #2 Carbohydrates Topic 2 Biologically Important Monosaccharide Derivatives There are a large number of monosaccharide derivatives. A variety of chemical and enzymatic reactions produce these
Topic 4 - #2 Carbohydrates Topic 2 Biologically Important Monosaccharide Derivatives There are a large number of monosaccharide derivatives. A variety of chemical and enzymatic reactions produce these
Biochemistry: Macromolecules
 1 Biology: Macromolecules 2 Carbohydrates Carbohydrate organic compound containing carbon, hydrogen, & oxygen in a 1:2:1 ratio Meaning: hydrated carbon ratio of h:0 is 2:1 (same as in water) Source: plants
1 Biology: Macromolecules 2 Carbohydrates Carbohydrate organic compound containing carbon, hydrogen, & oxygen in a 1:2:1 ratio Meaning: hydrated carbon ratio of h:0 is 2:1 (same as in water) Source: plants
BCH 4053 Spring 2001 Chapter 7 Lecture Notes
 BC 4053 Spring 2001 Chapter 7 Lecture Notes 1 Chapter 7 Carbohydrates 2 Carbohydrates: Nomenclature ydrates of carbon General formula (C 2 ) n (simple sugars) or C x ( 2 0) y Monosaccharides (simple sugars)
BC 4053 Spring 2001 Chapter 7 Lecture Notes 1 Chapter 7 Carbohydrates 2 Carbohydrates: Nomenclature ydrates of carbon General formula (C 2 ) n (simple sugars) or C x ( 2 0) y Monosaccharides (simple sugars)
A Getting-It-On Review and Self-Test. . Carbohydrates are
 A Getting-It-n Review and Self-Test arbohydrates arbohydrates, one of the three principal classes of foods, contain only three elements: (1), (2), and (3). The name carbohydrate is derived from the French
A Getting-It-n Review and Self-Test arbohydrates arbohydrates, one of the three principal classes of foods, contain only three elements: (1), (2), and (3). The name carbohydrate is derived from the French
Questions- Carbohydrates. A. The following structure is D-sorbose. (Questions 1 7) CH 2 OH C = O H C OH HO C H H C OH
 Questions- Carbohydrates A. The following structure is D-sorbose. (Questions 1 7) CH 2 C = O H C HO C H H C CH 2 1. 2. 3. 4. 5. Which characteristic is different when comparing the open-chain forms of
Questions- Carbohydrates A. The following structure is D-sorbose. (Questions 1 7) CH 2 C = O H C HO C H H C CH 2 1. 2. 3. 4. 5. Which characteristic is different when comparing the open-chain forms of
Biological Molecules
 SIM Tuition Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t
SIM Tuition Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t
Carbohydrates. 1. Using the terms provided below, complete the concept map showing the characteristics of organic compounds.
 Name: Class: Date: Grade 10 Science Related Reading/Biology Carbohydrates Biology Gr10 1. Using the terms provided below, complete the concept map showing the characteristics of organic compounds. maltose
Name: Class: Date: Grade 10 Science Related Reading/Biology Carbohydrates Biology Gr10 1. Using the terms provided below, complete the concept map showing the characteristics of organic compounds. maltose
Dr. Nafith Abu Tarboush. Rana N. Talj
 2 Dr. Nafith Abu Tarboush June 19 th 2013 Rana N. Talj Review: Fischer suggested a projection in which the horizontal bonds are projecting towards the viewer and the vertical ones project away from the
2 Dr. Nafith Abu Tarboush June 19 th 2013 Rana N. Talj Review: Fischer suggested a projection in which the horizontal bonds are projecting towards the viewer and the vertical ones project away from the
Carbohydrates CHAPTER SUMMARY
 14 2 cellulose 2 2 arbohydrates 2 amylose APTER SUMMARY 14.1 hemical Nature of arbohydrates - Polyhydroxy Aldehydes and Ketones arbohydrates are a class of organic biopolymers which consist of polyhydroxy
14 2 cellulose 2 2 arbohydrates 2 amylose APTER SUMMARY 14.1 hemical Nature of arbohydrates - Polyhydroxy Aldehydes and Ketones arbohydrates are a class of organic biopolymers which consist of polyhydroxy
Chapter 23 Carbohydrates and Nucleic Acids. Carbohydrates
 Chapter 23 Carbohydrates and Nucleic Acids Carbohydrates Synthesized by plants using sunlight to convert CO 2 and H 2 O to glucose and O 2. Polymers include starch and cellulose. Starch is storage unit
Chapter 23 Carbohydrates and Nucleic Acids Carbohydrates Synthesized by plants using sunlight to convert CO 2 and H 2 O to glucose and O 2. Polymers include starch and cellulose. Starch is storage unit
Carbohydrates. What are they? What do cells do with carbs? Where do carbs come from? O) n. Formula = (CH 2
 Carbohydrates What are they? Formula = (C 2 O) n where n > 3 Also called sugar Major biomolecule in body What do cells do with carbs? Oxidize them for energy Store them to oxidize later for energy Use
Carbohydrates What are they? Formula = (C 2 O) n where n > 3 Also called sugar Major biomolecule in body What do cells do with carbs? Oxidize them for energy Store them to oxidize later for energy Use
I. Carbohydrates Overview A. Carbohydrates are a class of biomolecules which have a variety of functions. 1. energy
 Chapter 22 Carbohydrates Chem 306 Roper I. Carbohydrates Overview A. Carbohydrates are a class of biomolecules which have a variety of functions. 1. energy 2. energy storage 3. structure 4. other functions!
Chapter 22 Carbohydrates Chem 306 Roper I. Carbohydrates Overview A. Carbohydrates are a class of biomolecules which have a variety of functions. 1. energy 2. energy storage 3. structure 4. other functions!
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Carbohydrates. Objectives. Background. Experiment 6
 1 of 6 3/15/2011 7:27 PM Experiment 6 Carbohydrates Objectives During this experiment you will look at some of the physical and chemical properties of carbohydrates. Many of the carbohydrates, especially
1 of 6 3/15/2011 7:27 PM Experiment 6 Carbohydrates Objectives During this experiment you will look at some of the physical and chemical properties of carbohydrates. Many of the carbohydrates, especially
Carbohydrates Chemical Composition and Identification
 Carbohydrates Chemical Composition and Identification Introduction: Today, scientists use a combination of biology and chemistry for their understanding of life and life processes. Thus, an understanding
Carbohydrates Chemical Composition and Identification Introduction: Today, scientists use a combination of biology and chemistry for their understanding of life and life processes. Thus, an understanding
Chem 263 Apr 11, 2017
 hem 263 Apr 11, 2017 arbohydrates- emiacetal Formation You know from previous lectures that carbonyl compounds react with all kinds of nucleophiles. ydration and hemiacetal formation are typical examples.
hem 263 Apr 11, 2017 arbohydrates- emiacetal Formation You know from previous lectures that carbonyl compounds react with all kinds of nucleophiles. ydration and hemiacetal formation are typical examples.
A. Incorrect! No, this is not the description of this type of molecule. B. Incorrect! No, this is not the description of this type of molecule.
 Biochemistry - Problem Drill 08: Carbohydrates No. 1 of 10 1. have one aldehyde (-CHO) or one keto (-C=O) group and many hydroxyl (-OH) groups. (A) Amino acids (B) Proteins (C) Nucleic Acids (D) Carbohydrates
Biochemistry - Problem Drill 08: Carbohydrates No. 1 of 10 1. have one aldehyde (-CHO) or one keto (-C=O) group and many hydroxyl (-OH) groups. (A) Amino acids (B) Proteins (C) Nucleic Acids (D) Carbohydrates
OCR (A) Biology A-level
 OCR (A) Biology A-level Topic 2.2: Biological molecules Notes Water Water is a very important molecule which is a major component of cells, for instance: Water is a polar molecule due to uneven distribution
OCR (A) Biology A-level Topic 2.2: Biological molecules Notes Water Water is a very important molecule which is a major component of cells, for instance: Water is a polar molecule due to uneven distribution
Biomolecule: Carbohydrate
 Biomolecule: Carbohydrate This biomolecule is composed of three basic elements (carbon, hydrogen, and oxygen) in a 1:2:1 ratio. The most basic carbohydrates are simple sugars, or monosaccharides. Simple
Biomolecule: Carbohydrate This biomolecule is composed of three basic elements (carbon, hydrogen, and oxygen) in a 1:2:1 ratio. The most basic carbohydrates are simple sugars, or monosaccharides. Simple
Chapter 24: Carbohydrates
 Chapter 24: Carbohydrates [Sections: 24.1 24.10] 1. Carbohydrates definition naturally occuring compounds derived from carbon, oxygen and hydrogen the net molecular formula comes from each carbon having
Chapter 24: Carbohydrates [Sections: 24.1 24.10] 1. Carbohydrates definition naturally occuring compounds derived from carbon, oxygen and hydrogen the net molecular formula comes from each carbon having
You know from previous lectures that carbonyl react with all kinds of nucleophiles. Hydration and hemiacetal formation are typical examples.
 hem 263 Nov 17, 2009 D,L onfiguration of Sugars Glyceraldehyde has only one stereogenic center and therefore has two enantiomers (mirror image) forms. A D-sugar is defined as one that has configuration
hem 263 Nov 17, 2009 D,L onfiguration of Sugars Glyceraldehyde has only one stereogenic center and therefore has two enantiomers (mirror image) forms. A D-sugar is defined as one that has configuration
2/25/2015. Chapter 6. Carbohydrates. Outline. 6.1 Classes of Carbohydrates. 6.1 Classes of Carbohydrates. 6.1 Classes of Carbohydrates
 Lecture Presentation Chapter 6 Carbohydrates Julie Klare Fortis College Smyrna, GA Outline 6.7 Carbohydrates and Blood The simplest carbohydrates are monosaccharides (mono is Greek for one, sakkhari is
Lecture Presentation Chapter 6 Carbohydrates Julie Klare Fortis College Smyrna, GA Outline 6.7 Carbohydrates and Blood The simplest carbohydrates are monosaccharides (mono is Greek for one, sakkhari is
Review from last lecture
 eview from last lecture D-glucose has the structure shown below (you are responsible for its structure on the exam). It is an aldohexose ( aldo since it contains aldehyde functionality and hexose since
eview from last lecture D-glucose has the structure shown below (you are responsible for its structure on the exam). It is an aldohexose ( aldo since it contains aldehyde functionality and hexose since
BIOMOLECULES & SPECTROSCOPY TABLE OF CONTENTS S.NO. TOPIC PAGE NO. i) Carbohydrates B3. ii) Proteins & Nucleic Acids.
 BIOMOLECULES & SPECTROSCOPY TABLE OF CONTENTS S.NO. TOPIC PAGE NO. 1. Biomolecules i) Carbohydrates B3 ii) Proteins & Nucleic Acids iii) Steroids iv) Terpenes & Cartenoids B27 B61 B65 2. Spectroscopy v)
BIOMOLECULES & SPECTROSCOPY TABLE OF CONTENTS S.NO. TOPIC PAGE NO. 1. Biomolecules i) Carbohydrates B3 ii) Proteins & Nucleic Acids iii) Steroids iv) Terpenes & Cartenoids B27 B61 B65 2. Spectroscopy v)
Chem 60 Takehome Test 2 Student Section
 Multiple choice: 1 point each. Mark only one answer for each question. 1. are composed primarily of carbon and hydrogen, but may also include oxygen, nitrogen, sulfur, phosphorus, and a few other elements.
Multiple choice: 1 point each. Mark only one answer for each question. 1. are composed primarily of carbon and hydrogen, but may also include oxygen, nitrogen, sulfur, phosphorus, and a few other elements.
Sheet #10 Dr. Mamoun Ahram Sec 1,2,3 15/07/2014. Carbohydrates 2
 Carbohydrates 2 A study Guide: Kindly,refer to the slide number,look at the structures and read the sheet notes well,most of the slides content besides all what the doctor said are mentioned here,good
Carbohydrates 2 A study Guide: Kindly,refer to the slide number,look at the structures and read the sheet notes well,most of the slides content besides all what the doctor said are mentioned here,good
CHAPTER 23. Carbohydrates
 CAPTER 23 Carbohydrates 1 Introduction Carbohydrates are naturally occurring compounds of carbon, hydrogen, and oxygen. Carbohydrates have the empirical formula C 2. Carbohydrates have the general formula
CAPTER 23 Carbohydrates 1 Introduction Carbohydrates are naturally occurring compounds of carbon, hydrogen, and oxygen. Carbohydrates have the empirical formula C 2. Carbohydrates have the general formula
Chapter-8 Saccharide Chemistry
 Chapter-8 Saccharide Chemistry Page 217-228 Carbohydrates (Saccharides) are most abundant biological molecule, riginally produced through C 2 fixation during photosynthesis I (C 2 ) n or - C - I where
Chapter-8 Saccharide Chemistry Page 217-228 Carbohydrates (Saccharides) are most abundant biological molecule, riginally produced through C 2 fixation during photosynthesis I (C 2 ) n or - C - I where
LAB 4 Macromolecules
 LAB 4 Macromolecules Overview In addition to water and minerals, living things contain a variety of organic molecules. Most of the organic molecules in living organisms are of 4 basic types: carbohydrate,
LAB 4 Macromolecules Overview In addition to water and minerals, living things contain a variety of organic molecules. Most of the organic molecules in living organisms are of 4 basic types: carbohydrate,
Anomeric carbon Erythritol is achiral because of a mirror plane in the molecule and therefore, the product is optically inactive.
 APTER 22 Practice Exercises 22.1 2 2 2 2 2 2 2 2 D-Ribulose L-Ribulose D-Xyulose L-Xyulose (one pair of enantiomers) (a second pair of enantiomers) 22.3 2 Anomeric carbon Glycosidic bond 3 () Methyl -D-mannopyranoside
APTER 22 Practice Exercises 22.1 2 2 2 2 2 2 2 2 D-Ribulose L-Ribulose D-Xyulose L-Xyulose (one pair of enantiomers) (a second pair of enantiomers) 22.3 2 Anomeric carbon Glycosidic bond 3 () Methyl -D-mannopyranoside
Carbohydrates. Green plants turn H 2 O, CO 2, and sunlight into carbohydrates.
 Chapter 27 Carbohydrates Green plants turn 2 O, CO 2, and sunlight into carbohydrates. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris ein, Scott Pattison, and Susan
Chapter 27 Carbohydrates Green plants turn 2 O, CO 2, and sunlight into carbohydrates. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris ein, Scott Pattison, and Susan
Testing for Biologically Important Molecules
 Testing for Biologically Important Molecules General Principles There are four major classes of organic compounds found in living organisms - arbohydrates, Lipids, Proteins and ucleic Acids. The chemical
Testing for Biologically Important Molecules General Principles There are four major classes of organic compounds found in living organisms - arbohydrates, Lipids, Proteins and ucleic Acids. The chemical
What are Carbohydrates? Aldoses and Ketoses
 What are Carbohydrates? Polyhydroxylated aldehydes and ketones Commonly called sugars General formula of common sugars!glucose: C 6 ( 2 ) 6!Glyceraldehyde: C 3 ( 2 ) 3 Talking points: C 2 ACS Division
What are Carbohydrates? Polyhydroxylated aldehydes and ketones Commonly called sugars General formula of common sugars!glucose: C 6 ( 2 ) 6!Glyceraldehyde: C 3 ( 2 ) 3 Talking points: C 2 ACS Division
Farah Al-Khaled. Razi Kittaneh. Mohammad Omari
 7 Farah Al-Khaled Razi Kittaneh Mohammad Omari Dr. Mamoun Ahram In this lecture we are going to talk about modified sugars. Remember: The Fischer projection can be turned into a ring structure (which is
7 Farah Al-Khaled Razi Kittaneh Mohammad Omari Dr. Mamoun Ahram In this lecture we are going to talk about modified sugars. Remember: The Fischer projection can be turned into a ring structure (which is
Organic Chemistry III
 rganic Chemistry III (Yuki Goto, Bioorganic Chemistry Lab.) rganic chemistry of biomolecules rganic chemistry of radicals 6/6 (Wed) 6/13 (Wed) 6/20 (Wed) 6/27 (Wed) 7/4 (Wed) Examples of biomolecules?
rganic Chemistry III (Yuki Goto, Bioorganic Chemistry Lab.) rganic chemistry of biomolecules rganic chemistry of radicals 6/6 (Wed) 6/13 (Wed) 6/20 (Wed) 6/27 (Wed) 7/4 (Wed) Examples of biomolecules?
Dr. Mahendra P. Bhatt (BMLT, MS-Ph.D., Post-doctorate) Associate Professor Clinical Biochemistry
 Dr. Mahendra P. Bhatt (BMLT, MS-Ph.D., Post-doctorate) Associate Professor Clinical Biochemistry mahendramlt@gmail.com Students will be able to describe: Biochemical organization of the cell Transport
Dr. Mahendra P. Bhatt (BMLT, MS-Ph.D., Post-doctorate) Associate Professor Clinical Biochemistry mahendramlt@gmail.com Students will be able to describe: Biochemical organization of the cell Transport
Chapter 11. Learning objectives: Structure and function of monosaccharides, polysaccharide, glycoproteins lectins.
 Chapter 11 Learning objectives: Structure and function of monosaccharides, polysaccharide, glycoproteins lectins. Carbohydrates Fuels Structural components Coating of cells Part of extracellular matrix
Chapter 11 Learning objectives: Structure and function of monosaccharides, polysaccharide, glycoproteins lectins. Carbohydrates Fuels Structural components Coating of cells Part of extracellular matrix
ANSC 689 PHYSIOLOGICAL CHEMISTRY OF LIVESTOCK SPECIES Carbohydrate Chemistry
 I. General structures A. D-Aldoses ANSC 689 PHYSIOLOGICAL CHEMISTRY OF LIVESTOCK SPECIES 1. All monosaccharides are aldehydes or ketones with multiple hydroxyl groups (i.e., alcohol groups). 2. Smallest
I. General structures A. D-Aldoses ANSC 689 PHYSIOLOGICAL CHEMISTRY OF LIVESTOCK SPECIES 1. All monosaccharides are aldehydes or ketones with multiple hydroxyl groups (i.e., alcohol groups). 2. Smallest
ANSC 619 PHYSIOLOGICAL CHEMISTRY OF LIVESTOCK SPECIES
 I. General structures ANSC 619 PHYSIOLOGICAL CHEMISTRY OF LIVESTOCK SPECIES A. D-Aldoses 1. All monosaccharides are aldehydes or ketones with multiple hydroxyl groups (i.e., alcohol groups). 2. Smallest
I. General structures ANSC 619 PHYSIOLOGICAL CHEMISTRY OF LIVESTOCK SPECIES A. D-Aldoses 1. All monosaccharides are aldehydes or ketones with multiple hydroxyl groups (i.e., alcohol groups). 2. Smallest
6 The chemistry of living organisms
 Living organisms are composed of about 22 different chemical elements. These are combined to form a great variety of compounds. Six major elements make up almost 99% of the mass of the human body, as shown
Living organisms are composed of about 22 different chemical elements. These are combined to form a great variety of compounds. Six major elements make up almost 99% of the mass of the human body, as shown
Biomolecules are organic molecules produced by living organisms which consists mainly of the following elements:
 Biomolecules are organic molecules produced by living organisms which consists mainly of the following elements: These elements are non-metals which combine in various ways to form biomolecules through
Biomolecules are organic molecules produced by living organisms which consists mainly of the following elements: These elements are non-metals which combine in various ways to form biomolecules through
CARBOHYDRATES (SUGARS)
 ARBYDRATES (SUGARS) ARBYDRATES: 1. Most Abundant Molecules on Earth: (100 MILLIN METRI TNS f 2 And 2 0 onverted To ellulose and ther Plant Products/Year) 2. FUNTINS: Diet, Energy, Structural, Signalling
ARBYDRATES (SUGARS) ARBYDRATES: 1. Most Abundant Molecules on Earth: (100 MILLIN METRI TNS f 2 And 2 0 onverted To ellulose and ther Plant Products/Year) 2. FUNTINS: Diet, Energy, Structural, Signalling
BIOCHEMISTRY UNIT 2 Part 4 ACTIVITY #4 (Chapter 5) CARBOHYDRATES
 AP BIOLOGY BIOCHEMISTRY UNIT 2 Part 4 ACTIVITY #4 (Chapter 5) NAME DATE PERIOD CARBOHYDRATES GENERAL CHARACTERISTICS: Polymers of simple sugars Classified according to number of simple sugars Sugars 3
AP BIOLOGY BIOCHEMISTRY UNIT 2 Part 4 ACTIVITY #4 (Chapter 5) NAME DATE PERIOD CARBOHYDRATES GENERAL CHARACTERISTICS: Polymers of simple sugars Classified according to number of simple sugars Sugars 3
IB Biology BIOCHEMISTRY. Biological Macromolecules SBI3U7. Topic 3. Thursday, October 4, 2012
 + IB Biology SBI3U7 BIOCHEMISTRY Topic 3 Biological Macromolecules Essential Questions: 1.What are the 4 main types of biological macromolecules and what is their function within cells? 2.How does the
+ IB Biology SBI3U7 BIOCHEMISTRY Topic 3 Biological Macromolecules Essential Questions: 1.What are the 4 main types of biological macromolecules and what is their function within cells? 2.How does the
Dr. Entedhar Carbohydrates Carbohydrates are carbon compounds that have aldehyde (C-H=0) or ketone (C=O) moiety and comprises polyhyroxyl alcohol
 Dr. Entedhar Carbohydrates Carbohydrates are carbon compounds that have aldehyde (C-H=0) or ketone (C=O) moiety and comprises polyhyroxyl alcohol (polyhydroxyaldehyde or polyhyroxyketone); their polymers,which
Dr. Entedhar Carbohydrates Carbohydrates are carbon compounds that have aldehyde (C-H=0) or ketone (C=O) moiety and comprises polyhyroxyl alcohol (polyhydroxyaldehyde or polyhyroxyketone); their polymers,which
Lab 3 MACROMOLECULES INTRODUCTION I. IDENTIFICATION OF MACROMOLECULES. A. Carbohydrates
 Lab 3 MACROMOLECULES OBJECTIVES Define macromolecule, vitamin, mineral, carbohydrate, monosaccharide, disaccharide, polysaccharide, lipid, protein, amino acid, calorie; Describe the basic structures of
Lab 3 MACROMOLECULES OBJECTIVES Define macromolecule, vitamin, mineral, carbohydrate, monosaccharide, disaccharide, polysaccharide, lipid, protein, amino acid, calorie; Describe the basic structures of
Carbohydrates. Lecture2
 Carbohydrates Lecture2 Disaccharides Consist of two monosaccharides covalently bound to each other. All of which are isomers with the molecular formula C 12 22 O 11. The differences in these disaccharides
Carbohydrates Lecture2 Disaccharides Consist of two monosaccharides covalently bound to each other. All of which are isomers with the molecular formula C 12 22 O 11. The differences in these disaccharides
Name a property of. water why is it necessary for life?
 02.09.18 Name a property of + water why is it necessary for life? n Cohesion n Adhesion n Transparency n Density n Solvent n Heat capacity + Macromolecules (2.3 & some of 2.4) + Organic Molecules All molecules
02.09.18 Name a property of + water why is it necessary for life? n Cohesion n Adhesion n Transparency n Density n Solvent n Heat capacity + Macromolecules (2.3 & some of 2.4) + Organic Molecules All molecules
Definition of a Carbohydrate
 * Atoms held together by covalent bonds Definition of a Carbohydrate * Organic macromolecules * Consist of C, H, & O atoms * Usually in a 1:2:1 ratio of C:H : O Functions Performed by Carbohydrates Used
* Atoms held together by covalent bonds Definition of a Carbohydrate * Organic macromolecules * Consist of C, H, & O atoms * Usually in a 1:2:1 ratio of C:H : O Functions Performed by Carbohydrates Used
Chapter 1. Chemistry of Life - Advanced TABLE 1.2: title
 Condensation and Hydrolysis Condensation reactions are the chemical processes by which large organic compounds are synthesized from their monomeric units. Hydrolysis reactions are the reverse process.
Condensation and Hydrolysis Condensation reactions are the chemical processes by which large organic compounds are synthesized from their monomeric units. Hydrolysis reactions are the reverse process.
among the most important organic compounds in the living organisms;
 CARBOHYDRATES Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine and Pharmacy "Nicolae Testemitanu" CARBOHYDRATESare among the most
CARBOHYDRATES Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine and Pharmacy "Nicolae Testemitanu" CARBOHYDRATESare among the most
Carbohydrates. Haworth Projections
 Carbohydrates There are, for all intents and purposes, 2 classes of carbohydrates: pyranoses and furanoses ( ose is the suffix for a sugar). These two classes are so called because their carbon skeletons
Carbohydrates There are, for all intents and purposes, 2 classes of carbohydrates: pyranoses and furanoses ( ose is the suffix for a sugar). These two classes are so called because their carbon skeletons
Chapter 27 Carbohydrates
 Chapter 27 Carbohydrates Green plants turn 2 O, CO 2, and sunlight into carbohydrates. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris ein, Scott Pattison, and Susan
Chapter 27 Carbohydrates Green plants turn 2 O, CO 2, and sunlight into carbohydrates. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris ein, Scott Pattison, and Susan
I. ROLE OF CARBON IN ORGANISMS: Organic compounds = compounds that contain carbon Ex: Carbohydrates, lipids, proteins
 I. ROLE OF CARBON IN ORGANISMS: Organic compounds = compounds that contain carbon Ex: Carbohydrates, lipids, proteins Inorganic compounds = compounds that DO NOT contain carbon Ex: Vitamins, minerals,
I. ROLE OF CARBON IN ORGANISMS: Organic compounds = compounds that contain carbon Ex: Carbohydrates, lipids, proteins Inorganic compounds = compounds that DO NOT contain carbon Ex: Vitamins, minerals,
Chapter 7 Carbohydrates
 Chapter 7 Carbohydrates Definition of Carbohydrates carbohydrate: hydrate of carbon ; C n ( 2 ) m Examples: glucose (C 6 12 6 or C 6 ( 2 ) 6 ), sucrose (C 12 22 11 or C 12 ( 2 ) 11 ) saccharide: simple
Chapter 7 Carbohydrates Definition of Carbohydrates carbohydrate: hydrate of carbon ; C n ( 2 ) m Examples: glucose (C 6 12 6 or C 6 ( 2 ) 6 ), sucrose (C 12 22 11 or C 12 ( 2 ) 11 ) saccharide: simple
A BEGINNER S GUIDE TO BIOCHEMISTRY
 A BEGINNER S GUIDE TO BIOCHEMISTRY Life is basically a chemical process Organic substances: contain carbon atoms bonded to other carbon atom 4 classes: carbohydrates, lipids, proteins, nucleic acids Chemical
A BEGINNER S GUIDE TO BIOCHEMISTRY Life is basically a chemical process Organic substances: contain carbon atoms bonded to other carbon atom 4 classes: carbohydrates, lipids, proteins, nucleic acids Chemical
2.2: Sugars and Polysaccharides François Baneyx Department of Chemical Engineering, University of Washington
 2.2: Sugars and Polysaccharides François Baneyx Department of hemical Engineering, University of Washington baneyx@u.washington.edu arbohydrates or saccharides are abundant compounds that play regulatory
2.2: Sugars and Polysaccharides François Baneyx Department of hemical Engineering, University of Washington baneyx@u.washington.edu arbohydrates or saccharides are abundant compounds that play regulatory
Basic Biochemistry. Classes of Biomolecules
 Basic Biochemistry ABE 580 Classes of Biomolecules Carbohydrates Lipids Amino Acids Nucleic Acids Other 1 Carbohydrates Sugars Composed of C, H, O (C n H 2n O n ) Biological Uses Energy source/storage
Basic Biochemistry ABE 580 Classes of Biomolecules Carbohydrates Lipids Amino Acids Nucleic Acids Other 1 Carbohydrates Sugars Composed of C, H, O (C n H 2n O n ) Biological Uses Energy source/storage
Moorpark College Chemistry 11 Fall Instructor: Professor Gopal. Examination # 5: Section Five May 7, Name: (print)
 Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Long time ago, people who sacrifice their sleep, family, food, laughter, and other joys of life were called SAINTS. But now, they are called STUDENTS!
 Long time ago, people who sacrifice their sleep, family, food, laughter, and other joys of life were called SAINTS. But now, they are called STUDENTS! Monosaccharaides Q. Can hydrolysis occur at anytime
Long time ago, people who sacrifice their sleep, family, food, laughter, and other joys of life were called SAINTS. But now, they are called STUDENTS! Monosaccharaides Q. Can hydrolysis occur at anytime
May 21 st, 2008 Biochemistry Recitation
 May 21 st, 2008 Biochemistry Recitation MBioS 303 Summer 2008 Outline Carbohydrate basics Aldoses vs. ketoses L and D configurations and anomers Glycosidic bonds, disaccharides Polysaccharides Storage:
May 21 st, 2008 Biochemistry Recitation MBioS 303 Summer 2008 Outline Carbohydrate basics Aldoses vs. ketoses L and D configurations and anomers Glycosidic bonds, disaccharides Polysaccharides Storage:
McMush Lab Testing for the Presence of Biomolecules
 Biology McMush Lab Testing for the Presence of Biomolecules Carbohydrates, lipids, proteins, and nucleic acids are organic molecules found in every living organism. These biomolecules are large carbon-based
Biology McMush Lab Testing for the Presence of Biomolecules Carbohydrates, lipids, proteins, and nucleic acids are organic molecules found in every living organism. These biomolecules are large carbon-based
1. Which of the following contributes to the tertiary structure of proteins?
 Chemistry 11 Spring 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free
Chemistry 11 Spring 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free
