DARCO HEADED CANNULATED SCREWS
|
|
|
- Cynthia Haynes
- 5 years ago
- Views:
Transcription
1 DARCO HEADED CANNULATED SCREWS The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk) For additional languages, visit our website Then click on the Prescribing Information option. For additional information and translations please contact the manufacturer or local distributor. M C 0086* P Wright Medical Technology, Inc. Wright Medical UK Ltd Cherry Rd. 3rd Avenue Memphis, TN Letchworth U.S.A. Hertfordshire, SG6 2JF UK * The CE-Marking of Conformity is applied per catalog number and appears on the outer label, if applicable. April 2014 Printed in U.S.A
2
3 Attention Operating Surgeon IMPORTANT MEDICAL INFORMATION DARCO HEADED CANNULATED SCREWS ( ) EN OUTLINE: I. GENERAL PRODUCT INFORMATION A. PATIENT SELECTION B. INDICATIONS C. CONTRAINDICATIONS D. POTENTIAL COMPLICATIONS AND ADVERSE REACTIONS E. PRECAUTIONS F. HANDLING & STERILIZATION G. STORAGE CONDITIONS
4 DEFINITIONS Symbols and abbreviations may be used on the package label. The following table provides the definition of these symbols and abbreviations. Table 1. Definitions of Symbols and Abbreviations Symbol g h D Y i H l p Definition Batch code Catalog number Do not re-use Caution, consult accompanying documents Consult operating instructions Use by Temperature limitation Keep dry Keep away from sunlight
5 N M P I K Date of manufacture Manufacturer Authorized EC Representative in the European Community Sterilized using ethylene oxide Sterilized using radiation STERILE GAS Sterilized using gas plasma J Sterilized using aseptic processing techniques For prescription use only Abbreviation Ti Ti6Al4V CoCr SS UHMWPE Material Titanium Titanium Alloy Cobalt Chrome Alloy Stainless Steel Ultra High Molecular Weight Polyethylene
6 I. GENERAL PRODUCT INFORMATION Through the advancement of surgical fusion hardware, the surgeon has been provided a means of correcting deformity and reducing pain for many patients. While the implants used are largely successful in attaining these goals, it must be recognized that they are manufactured from metal, and that no implant can be expected to withstand the activity levels and loads as would normal, healthy bone after fusion occurs. The surgeon must evaluate each situation individually based on the patient s clinical presentation in making any decisions regarding implant selection. Surgeons must be familiar with the applicable operative technique and instructions for use for each product. This package insert and immediate package label contain essential warnings and precautions for each surgery. Additionally the surgical technique should be referenced for detailed information about implant selection, relevant product details, proposed surgical instructions, and/or assembly use. The surgeon should contact Wright for the proposed product-specific surgical technique. In using fusion implants, the surgeon should be aware of the following: The correct selection and sizing of the implant is extremely important. Selection of the proper size, shape, and design of the implant increases the potential for success. The implants require careful seating and adequate bone support. In selecting patients for surgery, the following factors can be critical to the eventual success of the procedure: 1. Patient s occupation or activity. If the patient is involved in an occupation or activity which includes substantial lifting or muscle strain, the resultant forces can cause failure of the fixation, the device, or both. The implant will not restore function to the level expected with normal healthy bone, and the patient should not have unrealistic functional expectations. 2. Condition of senility, mental illness, or alcoholism. These conditions, among others, may cause the patient to ignore certain necessary limitations and precautions in the use of the implant, leading to failure or other complications.
7 3. Foreign body sensitivity. Where material sensitivity is suspected, appropriate tests should be made prior to material selection or implantation. A. PATIENT SELECTION Use of surgical fusion hardware requires consideration of the following general indications: Good condition of the patient Good neurovascular status Adequate skin coverage Possibility of a functional musculotendinous system Adequate bone stock to receive implant Availability of post-operative therapy Cooperative patient B. INDICATIONS DARCO HEADED CANNULATED SCREWS DESCRIPTION The DARCO Headed Cannulated Screws are available in various sizes, thread types, and lengths. The screws contain a hex drive interface and an optional washer is available with the system. The screws and washers are manufactured from titanium alloy. INDICATIONS The DARCO Headed Cannulated Screws are intended for use over a guide pin or wire for bone fracture fixation and bone fragment fixation. Wright s washers may be used with the screws in cases where the patient has poor bone quality.
8 Minimally invasive fracture/ joint reconstruction Multiple-fragment joint fractures Simple metaphyseal fractures Simple epiphyseal fractures Fractures of the head of the humerus Fractures of the head of the tibia Cooper fractures of the tibia Fractures of the radius Fractures of the wrist, ankle, elbow and shoulder Scaphoid fractures and other fractures of the hand Metatarsal fractures and other fractures of the foot Ligament fixation of the proximal humerus Ligament avulsion injuries (Apohysis) Fractures of small joint bones Malleolar fractures Navicular fractures Fractures of the calcaneus and talus Arthrodesis of the ankle joint Avulsion fracture and metatarsal V Fractures of the tarsal region
9 C. CONTRAINDICATION Inflammation, sepsis and osteomyelitis are absolute contraindications. All applications that are not defined by the indications are contraindicated. In addition, surgical success can be adversely affected by: Acute or chronic infections, local or systemic Vascular, muscular or neurological pathologies that compromise the concerned extremity All concomitant pathologies that could affect the function of the implant Osteopathies with reduced bone substance that could affect the function of the implant Any mental or neuromuscular disorder that could result in an unacceptable risk of failure at the time of fixation or complications in post-operative treatment. Known or suspected sensitivity to metal Corpulence; an overweight or corpulent patient can strain the implant to such a degree that stabilization or implant failure can occur Whenever the use of the implant comes into conflict with the anatomical structures of physiological status Other medical or surgical pre-conditions that could compromise the potentially beneficial procedure, such as: The presence of tumors Congenital abnormalities Immunosuppressive pathologies Increased sedimentation rates that cannot be explained by other pathologies Increased leukocyte (WBC) count Pronounced left shift in the differential leukocyte count
10 D. POTENTIAL COMPLICATIONS AND ADVERSE REACTIONS In any surgical procedure, the potential for complications exists. The risks and complications with these implants include: loosening, deformation or fracture of the implant acute post-operative wound infections and late infections with possible sepsis migration, subluxation of the implant with resulting reduction in range of movement fractures resulting from unilateral joint loading thrombosis and embolism wound hematoma and delayed wound healing temporary and protracted functional neurological perturbation tissue reactions as the result of allergy or foreign body reaction to dislodged particles. corrosion with localized tissue reaction and pain pain, a feeling of malaise or abnormal sensations due to the implant used bone loss due to stress shielding All possible complications listed here are not typical of WMT products but are in principle observed with any implant. Promptly inform WMT as soon as complications occur in connection with the implants or surgical instruments used. In the event of premature failure of an implant in which a causal relationship with its geometry, surface quality or mechanical stability is suspected, please provide WMT with the explant(s) in a cleaned, disinfected and sterile condition. The manufacturer cannot accept any other returns of used implants. The surgeon is held liable for complications associated with inadequate asepsis, inadequate preparation of the osseous implant bed in the case of implants, incorrect indication or surgical technique or incorrect patient information and consequent incorrect patient behavior.
11 E. PRECAUTIONS Following the instructions for use provided in product literature can minimize the potential for complications or adverse reactions with any implant. It is the responsibility of each surgeon using implants to consider the clinical and medical status of each patient and to be knowledgeable about all aspects of implant procedure and the potential complications that may occur. The benefits derived from implant surgery may not meet the patient s expectations or may deteriorate with time, necessitating revision surgery to replace the implant or to carry out alternative procedures. The patient s mental status must also be considered. Willingness and/or ability to follow post-operative instructions may also impact the surgical outcome. Surgeons must balance many considerations to achieve the best result in individual patients. Instructions on combining implants can be found in the corresponding surgical technique. Wright has tested combinations using implants and instruments for the manufacturers Wright and aap; any other combination is at the risk and hazard of the surgeon. IF EXCESSIVE LOADING CANNOT BE PREVENTED, AN IMPLANT SHOULD NOT BE USED. The main goal of surgery with this implant is to establish bony fusion. Abnormal or excessive forces could lead to delayed union, non-union, or failure of the implant. Abnormal force loading and subsequent wear may be caused by: Uncorrected instability Improperly sized implant Inadequate soft tissue support Implant malposition Excessive motion Uncorrected or recurrent deformity Patient misuse or overactivity
12 Proper fixation at the time of surgery is critical to the success of the procedure. Bone stock must be adequate to support the device. Some preventative measures to consider minimizing the potential for complications: Follow guidelines for indications and contraindications Identify prior pathology Stabilize collapse deformities Bone graft pre-existing cysts Use a properly sized implant Avoid K-wires and sutures through the implant Avoid flawing implant surfaces or excessive bending to minimize the potential for early fatigue failure. If complications develop, possible corrective procedures include: Implant removal Synovectomy Bone grafting of cysts Replacement of the implant Removal of the implant with fusion of the joint Over time, metallic implants may loosen, fracture, or cause pain after the bone fracture or osteotomy is healed. Removal of metallic implants is at the surgeon s discretion, and the appropriateness of the selected procedure will be based on the surgeon s personal medical training and experience. It is imperative that adequate post-operative care and protection be provided by the surgeon.
13 Recommendations Regarding Device Fragments Use medical devices in accordance with their labeled indications and the manufacturer s instructions for use, especially during insertion and removal. Inspect devices prior to use for damage during shipment or storage or any out-of-box defects that might increase the likelihood of fragmentation during a procedure. If the device is damaged, retain it to assist with the manufacturer s analysis of the event. Carefully consider and discuss with the patient (if possible) the risks and benefits of retrieving vs. leaving the fragment in the patient. Advise the patient of the nature and safety of unretrieved device fragments including the following information: a. The material composition of the fragment (if known); b. The size of the fragment (if known); c. The location of the fragment; d. The potential mechanisms for injury, e.g., migration, infection; e. Procedures or treatments that should be avoided such as MRI exam in case of metallic fragments. This may help reduce the possibility of serious injury from the fragment. Clinical results depend on surgeon and technique, pre-operative and post-operative care, the implant, patient pathology and daily activity. It is important that surgeons obtain appropriate informed consent and discuss the potential for complications with each patient prior to surgery. This may include a review of alternative, non-implant procedures such as soft tissue reconstruction or arthrodesis.
14 Concerning Magnetic Resonance Environments There are inherent risks associated with the use of metallic implants in the MR environment; including component migration, heat induction, and signal interference or distortion near the component(s). Heat induction of metallic implants is a risk related to component geometry and material, as well as the MR power, duration, and pulse sequence. Since MR equipment is not standardized, the severity and likelihood of occurrence are unknown for these implants. DARCO Headed Cannulated Screws have not been evaluated for safety and compatibility in the MR environment. DARCO Headed Cannulated Screws have not been tested for heating or migration in the MR environment. Since these devices have not been tested, Wright cannot make a recommendation for the use of MRIs with these implants, neither for safety considerations nor imaging accuracy. These components are passive metallic devices, and as with all passive devices, there is potential for reciprocal interference with certain imaging modalities; including image distortion for MR and X-ray scatter in CT. F. HANDLING AND STERILIZATION When removing the medical devices from the packaging, inspect the integrity of the medical devices and the correspondence of the device type and size with the labeling. Damaged medical devices must not be used. WMT is solely responsible for the medical devices and their supply presentation. Any change made to these results in a new medical device for which WMT assumes no responsibility. The required instrument set can be ordered from WMT. Please see the Surgical Technique for further details on implantation of the components and on the instrument set. IMPLANTS The implants described in this package insert are provided non-sterile as indicated on the individual product s label. Implants that are presented in instrument trays are provided non-sterile.
15 Implants provided non-sterile should be processed according to the recommended parameters for instruments (below). This product is for single use only. An implant should never be re-sterilized after contact with body tissues or fluids. Devices labeled for single-use only should never be reused. Reuse of these devices may potentially result in, but are not limited to, significant degradation in device performance, cross-infection, or contamination which may result in serious patient harm. INSTRUMENTS Surgical instruments (and non-sterile implants) should be cleaned and sterilized according to the following parameters: Cleaning 1. Disassemble all components as per manufacturer instructions (if appropriate). 2. Rinse with cold tap water to remove gross contamination. 3. Bathe in an enzymatic detergent solution prepared per manufacturer directions for 5 minutes. 4. Scrub thoroughly with a soft brush and/or pipe cleaner; repeatedly flush any very narrow lumens with enzymatic detergent solution using a syringe. 5. Rinse with cold tap water for a minimum of one minute; use a syringe to repeatedly flush any very narrow lumens. 6. Bathe in a detergent solution prepared per manufacturer directions for 5 minutes. 7. Scrub thoroughly with a soft brush and/or pipe cleaner; repeatedly flush any very narrow lumens with detergent solution using a syringe. 8. Rinse thoroughly/flush with deionized/reverse osmosis (RO/DI) water.
16 9. Sonicate for a minimum of 10 minutes in an enzymatic detergent solution prepared per manufacturer directions. 10. Rinse thoroughly/flush with RO/DI water. 11. Dry with a clean, soft, absorbent, disposable cloth. 12. Visually inspect for cleanliness. All visible surfaces, internal and external, should be visually inspected. If necessary re-clean until it is visibly clean. Note: Brushes (i.e. pipe cleaners) could be used for cleaning most lumens, however, the use of a syringe to flush narrow lumens with diameters less than or equal to inches is recommended. Sterilization The minimum recommended steam sterilization conditions for Wright reusable instruments are as follows: 1. Double wrap the component in an FDA-cleared CSR wrap or similar type non-woven medical grade wrapping material. 2. Autoclave according to the following parameters: Steam Sterilization Cycle Type Parameter Minimum Set Point Prevacuum Exposure Temperature 270 F (132 C) 270 F (132 C) Exposure Time 4 minutes Dry Time 20 minutes
17 3. After sterilization, remove the component from its wrapping using accepted sterile technique with powder-free gloves. Ensure that implants are at room temperature prior to implantation. Avoid contact with hard objects that may cause damage. These recommendations are consistent with AAMI ST79 Table 5 guidelines and have been developed and tested using specific equipment to achieve a Sterility Assurance Level (SAL) of Due to variations in environment and equipment, it must be demonstrated that these recommendations produce sterility in your environment. If processing conditions, wrapping materials, or equipment changes occur, the effectiveness of the sterilization process must be demonstrated. For additional information see Wright s Cleaning and Handling of Wright Medical Instruments. G. STORAGE CONDITIONS All implants must be stored in a clean, dry environment and be protected from sunlight and extremes in temperature. DARCO is a licensed trademark of Wright Medical Technology, Inc.
The following languages are included in this packet:
 DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO MIS FOREFOOT SCREW
 DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
MICA SCREWS For additional information and translations please contact the manufacturer or local distributor.
 MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE
 ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
BIOFOAM BONE WEDGE
 BIOFOAM BONE WEDGE 150837-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
BIOFOAM BONE WEDGE 150837-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
CANNULATED SCREW SYSTEM The following languages are included in this packet:
 CANNULATED SCREW SYSTEM 137183-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -Chinese (sch) Türkçe
CANNULATED SCREW SYSTEM 137183-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -Chinese (sch) Türkçe
CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM
 EN CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM 150871-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
EN CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM 150871-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
EXTERNAL FIXATION SYSTEMS The following languages are included in this packet:
 EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
Metallic Internal Fixation Devices The following languages are included in this packet:
 Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
STABILIZATION AND FRACTURE FIXATION
 STABILIZATION AND FRACTURE FIXATION 150838-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
STABILIZATION AND FRACTURE FIXATION 150838-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM The following languages are included in this packet:
 ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM
 METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
METAL HEMI SYSTEM
 METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM
 ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM 150884-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM 150884-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
SALVATION EXTERNAL FIXATION SYSTEM
 SALVATION EXTERNAL FIXATION SYSTEM 151660-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
SALVATION EXTERNAL FIXATION SYSTEM 151660-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
The following languages are included in this packet:
 OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
SALVATION EXTERNAL FIXATION SYSTEM
 SALVATION EXTERNAL FIXATION SYSTEM 151660-2 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
SALVATION EXTERNAL FIXATION SYSTEM 151660-2 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
Fracture Fixation The following languages are included in this packet:
 Fracture Fixation 150846-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
Fracture Fixation 150846-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE
 CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
INVISION TOTAL ANKLE REVISION SYSTEM The following languages are included in this packet:
 INVISION TOTAL ANKLE REVISION SYSTEM 151678-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 -
INVISION TOTAL ANKLE REVISION SYSTEM 151678-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 -
SURGICAL TECHNIQUE GUIDE: JONES FRACTURE USING THE PRECISION JONES FRACTURE SCREW SYSTEM
 PRODUCT DESCRIPTION The PRECISION Jones Fracture Screw System offers extensive options of Type II Anodized Titanium screws. System-specific instrumentation is designed to address procedural challenges
PRODUCT DESCRIPTION The PRECISION Jones Fracture Screw System offers extensive options of Type II Anodized Titanium screws. System-specific instrumentation is designed to address procedural challenges
BIOFOAM ANKLE SPACER BLOCK
 BIOFOAM ANKLE SPACER BLOCK 145782-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
BIOFOAM ANKLE SPACER BLOCK 145782-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
BIOARCH SUBTALAR IMPLANT SYSTEM
 BIOARCH SUBTALAR IMPLANT SYSTEM 137308-4 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
BIOARCH SUBTALAR IMPLANT SYSTEM 137308-4 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
TOTAL ELBOW SYSTEM The following languages are included in this packet:
 TOTAL ELBOW SYSTEM 115298-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch) Türkçe
TOTAL ELBOW SYSTEM 115298-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch) Türkçe
TOTAL ANKLE SYSTEMS
 TOTAL ANKLE SYSTEMS 150864-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
TOTAL ANKLE SYSTEMS 150864-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
MICROPORT KNEE SYSTEMS The following languages are included in this packet:
 EN English (en) MICROPORT KNEE SYSTEMS 151251-0 The following languages are included in this packet: For additional languages, visit our website ortho.microport.com Then click on the Prescribing Information
EN English (en) MICROPORT KNEE SYSTEMS 151251-0 The following languages are included in this packet: For additional languages, visit our website ortho.microport.com Then click on the Prescribing Information
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
SJO SILICONE IMPLANTS The following languages are included in this packet:
 SJO SILICONE IMPLANTS 150823-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
SJO SILICONE IMPLANTS 150823-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
INTELLIGENT SPINAL SYSTEM
 INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
FUSEFORCE. Hand Fixation System SURGICAL TECHNIQUE
 FUSEFORCE Hand Fixation System SURGICAL TECHNIQUE Contents Chapter 1 3 Chapter 2 4 Appendix A 7 Intended Use Surgical Technique Ordering Information Indication Sizing Reference Proper surgical procedures
FUSEFORCE Hand Fixation System SURGICAL TECHNIQUE Contents Chapter 1 3 Chapter 2 4 Appendix A 7 Intended Use Surgical Technique Ordering Information Indication Sizing Reference Proper surgical procedures
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
D. The following factors are of extreme importance to the eventual success of the procedure.
 Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE. This Package Insert is for product distributed in the US only.
 TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
WristMotion Wrist Hemiarthroplasty System Instructions for Use
 WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
INBONE II. Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT TIBIAL REAMING SPECIFICATION
 INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT TIBIAL REAMING SPECIFICATION INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT Tibial Reaming Specification Contents Chapter 1 4 Product
INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT TIBIAL REAMING SPECIFICATION INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT Tibial Reaming Specification Contents Chapter 1 4 Product
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
Page 1 of 7 Doc. No.: IU/15 Rev. No.: 05 Rev. Date:03MAY2018. Instructions for use for ATLAS Tibial Fracture(TF) Nail
 Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
Doc. No.: IU/8 for. Rev. No.: 07 ResTOR Prosthesis
 0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
Precautionary Statement ( )
 Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Surgical Technique Guide
 Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Technique Guide Wrist Hemiarthroplasty System
 Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
BONE GRAFT WASHER _Rev A IMPORTANT INFORMATION ON THE BONE GRAFT WASHER
 0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
Technique Guide Lapidus Arthrodesis System
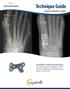 Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
Important notice: the device(s) can be prescribed and implanted only by a doctor legally authorized to perform this type of surgery.
 rev.10 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH - EVOLIS/GMK KNEE PROSTHESIS - INSTRUCTIONS FOR USE Important notice: the device(s) can be prescribed
rev.10 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH - EVOLIS/GMK KNEE PROSTHESIS - INSTRUCTIONS FOR USE Important notice: the device(s) can be prescribed
Salto Talaris Total Ankle Prosthesis
 Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
OBSOLETE. Visit for the latest version. Ankle Plating System 3 PKGI-79-B EFFECTIVE
 Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Instructions for use for Bone Plates, Bone Screws, Intramedullary Nails, Pins and Wires
 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
Spartan S 3 Facet Screw System PACKAGE INSERT
 LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
Integra Total Foot System 2
 Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
SI-BONE ifuse Implant System Instruments Hospital Cleaning and Sterilization Instructions 0344
 HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Compression Screw 3.2 Bioabsorbable. Product information
 Compression Screw 3.2 Bioabsorbable Product information Caution The current product description is not sufficient for the immediate application of instruments and implants. Instructional training by an
Compression Screw 3.2 Bioabsorbable Product information Caution The current product description is not sufficient for the immediate application of instruments and implants. Instructional training by an
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
Important Information on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only
 0434 Page 1 of 6 Instructions use Important Inmation on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: Atlas Hip Fracture (HF) Nail is an intramedullary interlocking
0434 Page 1 of 6 Instructions use Important Inmation on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: Atlas Hip Fracture (HF) Nail is an intramedullary interlocking
EasyStep. Operative technique
 Operative technique EasyStep - Step staple This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments. It offers guidance that you should heed,
Operative technique EasyStep - Step staple This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments. It offers guidance that you should heed,
Reprocessing Guide. Autoclavable Arthroscope and Hardware Sterilization Tray
 Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Headless Compession Screw 4.5 / 6.5 SURGICAL TECHNIQUE. Titanium or Stainless Steel. Cannulated Headless Design. Multiple Thread Options.
 Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Document No Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE
 Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
Implantable K-wire SURGIC A L T ECHNIQUE
 Implantable K-wire SURGIC A L T ECHNIQUE Contents Chapter 1 4 Product Information 4 Device Description 4 Indications 4 Contraindications Chapter 2 5 Surgical Technique 5 Hammertoe Correction 6 MTP Joint
Implantable K-wire SURGIC A L T ECHNIQUE Contents Chapter 1 4 Product Information 4 Device Description 4 Indications 4 Contraindications Chapter 2 5 Surgical Technique 5 Hammertoe Correction 6 MTP Joint
For use by an Accredited Orthopaedic Surgeon only
 Instructions use Page 1 of 6 For use by an Accredited Orthopaedic Surgeon only 1. DEVICE DESCRIPTION: The advancement of partial and total hip replacement has provided surgeons with the means of restoring
Instructions use Page 1 of 6 For use by an Accredited Orthopaedic Surgeon only 1. DEVICE DESCRIPTION: The advancement of partial and total hip replacement has provided surgeons with the means of restoring
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
Hip Resurfacing System
 Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
REPIPHYSIS LIMB SALVAGE SYSTEM
 REPIPHYSIS LIMB SALVAGE SYSTEM 150800-0 For additional information please contact the manufacturer or local distributor. M MicroPort Orthopedics Inc. 5677 Airline Rd Arlington, TN 38002 U.S.A. i Y October
REPIPHYSIS LIMB SALVAGE SYSTEM 150800-0 For additional information please contact the manufacturer or local distributor. M MicroPort Orthopedics Inc. 5677 Airline Rd Arlington, TN 38002 U.S.A. i Y October
Headless Compession Screw 2.5 / 3.0
 SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
TriboFit Hip System. Issue 3 - August 2013 English
 Important Information: Please read prior to use in a clinical setting. The surgeon should be familiar with the operative technique and all the information in this insert. Description: The TriboFit Hip
Important Information: Please read prior to use in a clinical setting. The surgeon should be familiar with the operative technique and all the information in this insert. Description: The TriboFit Hip
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
JRI Thompson Hemiarthroplasty
 JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6 English 3 Page 2 of 6 Important Information Please
JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6 English 3 Page 2 of 6 Important Information Please
2. Active systemic infection or infection localized to the site of the proposed implantation is contraindications to implantation.
 OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System
 X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Fixcet Spinal Facet Screw System
 X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
X-spine Systems, Inc. Spider Cervical Plating System
 X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
STRYKER SPINE Spinal Fixation Systems XIA 3
 STRYKER SPINE Spinal Fixation Systems XIA 3 NON STERILE PRODUCT The STRYKER Spine Spinal Fixation Systems are made of devices for fixation of the non-cervical spine. They include smooth rods, screws, hooks,
STRYKER SPINE Spinal Fixation Systems XIA 3 NON STERILE PRODUCT The STRYKER Spine Spinal Fixation Systems are made of devices for fixation of the non-cervical spine. They include smooth rods, screws, hooks,
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE
 OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
ACP1 CERVICAL PLATE SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE II.
 I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
A U X I L I A R Y C O N N E C T O R S Surgical Technique
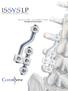 A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
REDUCT Headless Compression Screw System
 REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
Asnis. Micro Cannulated screw system. Xpress operative technique
 Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
SURGICAL TECHNIQUE GUIDE: LAPIDUS ARTHRODESIS USING THE PHANTOM INTRAMEDULLARY NAIL INTRAMEDULLARY NAIL
 LAPIDUS ARTHRODESIS USING THE PHANTOM INTRAMEDULLARY NAIL INTRAMEDULLARY NAIL LAPIDUS ARTHRODESIS USING THE PHANTOM INTRAMEDULLARY NAIL Acknowledgment: Paragon 28 would like to thank James T. Clancy, DPM
LAPIDUS ARTHRODESIS USING THE PHANTOM INTRAMEDULLARY NAIL INTRAMEDULLARY NAIL LAPIDUS ARTHRODESIS USING THE PHANTOM INTRAMEDULLARY NAIL Acknowledgment: Paragon 28 would like to thank James T. Clancy, DPM
QIN4432TRICREV01. IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage. Version 1 1 STERILE PRODUCT
 IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage STERILE PRODUCT DESCRIPTION The Stryker Spine Tritanium C Anterior Cervical Cage is a hollow, ring shaped titanium alloy (Ti-6Al- 4V) interbody
IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage STERILE PRODUCT DESCRIPTION The Stryker Spine Tritanium C Anterior Cervical Cage is a hollow, ring shaped titanium alloy (Ti-6Al- 4V) interbody
