SI-BONE ifuse Implant System Instruments Hospital Cleaning and Sterilization Instructions 0344
|
|
|
- Isabel Walters
- 5 years ago
- Views:
Transcription
1 HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System Instructions for Use. SI-BONE INSTRUMENT STERILIZATION SI-BONE Instruments are provided non-sterile and must be cleaned and sterilized prior to use. The following instructions apply to instrument cleaning and sterilization. Note: Single-use devices that have been used should be disposed of immediately after use. WARNINGS 1. Follow the instructions and warnings issued by the suppliers of any cleaning solutions and equipment used. 2. Avoid exposure to hypochlorite solutions and solutions containing iodine or high chlorine content, as these will promote corrosion. 3. Cleaning agents with a ph of 7 9 are recommended. 4. Highly alkaline conditions (ph > 11) can damage instruments (esp. aluminum parts). 5. Manual Pre-cleaning must be performed prior to Automated Cleaning for all instruments. 6. Soiled or used devices should not be loaded into the instrument tray for cleaning in a mechanical washer. Soiled instruments must be processed separate from trays and cases. SI-BONE instrument trays are designed to be an organization tool for steam sterilization process, a storage tool for all medical devices and an organizational tool for surgery. 7. The parameters listed are only valid for properly installed, maintained, calibrated and compliant reprocessing equipment in accordance with ISO and ISO CLEANING THE INSTRUMENTS: Clean Using Automated or Manual Process as Indicated Below Pre-Cleaning Instructions: 1. Clean instruments as soon as possible after use, thoroughly rinsing the instruments with warm tap water to remove gross contamination. Do not allow blood and debris to dry on the instruments. 2. Disassemble Navigation Impactor/Removal Adapter (see Figure 15). 3. DO NOT disassemble the pinned soft tissue protector assembly (see Figure 1). 4. DO NOT disassemble the screw from the Adjustable Parallel Pin Guide (see Figure 5). 5. Dispose of any pins (e.g., guide wires, Steinmann pins, K-wires) and drill bits used in the procedure. 6. Fully immerse all instruments in a mildly alkaline, enzymatic cleaner, such as MediClean at 0.5-2%, at < 40 C. Remove gross soil using a soft bristled brush. 7. Soak instruments for minutes. Flush all lumens and hard to reach areas of the instruments with the prepared detergent using an appropriately sized syringe or pipette filled with the prepared cleaning solution a minimum of 3 times. 8. Rinse the instruments with cold tap water for 1 minute. Remove remaining gross soil using a soft-bristled, nylon brush. Pay particular attention to crevices, lumens, mated AUTOMATED CLEANING PROCESS surfaces, connectors and other hard-to-clean areas. Lumens and hard to reach areas should be cleaned with a long, narrow, soft-bristled brush (i.e. pipe cleaner brush). Do not use metal scouring pads. 9. Flush lumens with water a minimum of 3 times using a syringe or pipette. Automated Cleaning Process Instructions: 10. Load the instruments so that the lumens/blind holes can drain. 11. Using a validated washer disinfector and a mildly alkaline enzymatic cleaning agent intended for use in an automated cleaning process, use the minimum cycle parameter set points below in Table After the completion of the washer cycle, visually inspect the instruments for remaining soil in a well-lit area; no visible soil should be left on the device. 13. If devices are still wet after the automated cleaning cycle, thoroughly dry the devices using a clean, lint-free cloth. If needed, use filtered pressurized air at <40 psi to aid in drying. 14. Return instruments to their designated locations in the tray as applicable. Page 1 of 5
2 Table 1: Automated Washer/Disinfector Cleaning Cycle Parameters Time Cycle (minutes:seconds) Temperature Type of Detergent/ Water Pre-Cleaning 2:00 Cold Tap Cleaning 5:00 Rinse 2:00 Thermal Rinse 1:00 Dry 26:00 Tap (55 C) (70 C) (90 C) (85 C) END OF AUTOMATED CLEANING PROCESS MediClean (per the manufacturer s instructions) Reverse Osmosis (RO) Water RO Water NA 1. Clean instruments as soon as possible after use, thoroughly rinsing the instruments under running warm tap water to remove gross contamination. Do not allow blood and debris to dry on the instruments. 2. Disassemble Navigation Impactor/Removal Adapter (see Figure 15). 3. DO NOT disassemble the pinned soft tissue protector assembly (see Figure 1). 4. DO NOT disassemble the screw from the Adjustable Parallel Pin Guide (see Figure 5). 5. Dispose of any pins (e.g., guide wires, Steinmann pins, K-wires) and drill bits used in the procedure. 6. Prepare a neutral ph enzymatic detergent or equivalent such as Enzol per manufacturer s directions at 1 oz/gallon using lukewarm tap water. 7. Fully immerse the instruments in the prepared detergent. 8. Flush all hard to reach areas of the instruments with the prepared detergent using an appropriately sized syringe and allow them to soak for a minimum of 10 minutes. MANUAL CLEANING PROCESS 9. Following the minimum 10 minute soak, use a softbristled, nylon brush to gently scrub the instruments until all visible soil has been removed. Pay particular attention to crevices, lumens, mated surfaces, connectors and other hard-to-clean areas. Lumens and hard to reach areas should be cleaned with a long, narrow, soft-bristled brush (i.e. pipe cleaner brush). Do not use metal scouring pads. 10. After brushing, use an appropriately sized syringe to thoroughly flush the prepared detergent through all lumens, holes and other difficult-to-reach areas. 11. Thoroughly rinse the instruments under running tap water for a minimum of 1 minute. Flush all lumens, holes and hard to reach areas using an appropriately sized syringe filled with tap water. 12. Thoroughly dry the instruments using a clean lint-free cloth. 13. Visually inspect the instruments for cleanliness. If soil is visible, repeat the cleaning procedures outlined above. 14. Return instruments to their designated locations in the tray as applicable. END OF MANUAL CLEANING PROCESS Page 2 of 5
3 VERIFY THAT THE INSTRUMENTS ARE CLEAN PRIOR TO STERILIZATION A. Visually inspect the instruments for corrosion, pitting, discoloration or cracking, which might indicate excessive wear. If an instrument is damaged or shows signs of unusual wear, SI-BONE will replace the instrument(s). B. For disposable parts (Pins and Drill Bits), visually inspect and dispose if parts show signs of prior use. C. Visually inspect the tray and content surfaces for dry blood and tissue D. Check the assembly of mating parts as follows. For all instruments: Check the inner canal and threads of the, Drill/Pin Sleeve and Broach (See Figures 2, 3 and 11). For Radiolucent instruments: For Revision instruments (7.5 mm and mm implants): For Navigation instruments: Check the inside of the handle (See examples in Figures 4 and 6). Check sliding mechanisms and/or actuating parts of the Drill/Pin Sleeve, Adjustable Parallel Pin Guide, Variable Parallel Pin Guide, Orientation Guide, Implant Orientation Guide. (See Figures 3, 5, 7, 8, 9, 12). Check the slot and cutting surfaces of the Broach (See example in Figure 11 and 16). Check the inner canal and threads of the, Variable Parallel Pin Guide, Orientation Guide, Broach Stop and Pin Sleeve (See Figures 6 10). Check the threads of the Triangular and Circular s and Dilators. Check the inner canal and threads of the Implant Orientation Guide and Navigation Broach 7.0 (See Figures 12 and 16). Check the inner cannula and threads for the Navigation Impactor/Removal Adapter (See Figure 13, 14 and 15). E. Return instruments to their designated locations in the tray as applicable. F. Instruments not stored in the tray should be sterilized individually per validated hospital procedures. STANDARD, XL AND NAVIGATION INSTRUMENTS Figure 1 Figure 2 Figure 3 Figure 4 Figure 5 Pinned Soft Tissue Protector inner canal Drill/Pin Sleeve Inner & Outer threads Handle Adjustable Parallel Pin Guide RADIOLUCENT INSTRUMENTS Figure 6 Figure 7 Figure 8 Figure 9 Figure 10 Variable Parallel Pin Guide (View 1) Variable Parallel Pin Guide (View 2) Orientation Guide Adjustable Broach Stop Page 3 of 5
4 Figure 11 Broach NAVIGATION INSTRUMENTS Figure 12 Figure 13 Figure 14 Nav. Implant Orientation Guide Nav. Impactor/ Removal Adapter, Tube Nav. Impactor/Removal Adapter, Cap Figure 15 Nav. Impactor/Removal Adapter Assembly Figure 16 Nav. Broach STERILIZATION PROCEDURE The SI-BONE instrument set should be covered in a wrap and the instruments must be steam sterilized prior to use, using the following parameters: TABLE 1: XL INSTRUMENTS / REMOVAL SYSTEM INSTRUMENTS Sterilizer Type Preconditioning Pulses Temperature Full Cycle Time Dry Time Pre Vacuum C 3 minutes 40 minutes Page 4 of 5
5 TABLE 2: STANDARD INSTRUMENTS / RADIOLUCENT INSTRUMENTS / REVISION INSTRUMENTS (7.5 MM & MM IMPLANTS) / NAVIGATION INSTRUMENTS Sterilizer Type Preconditioning Pulses Temperature Full Cycle Time Dry Time Pre Vacuum C 3 minutes 30 minutes Indications for Use: The ifuse Implant System is intended for sacroiliac joint fusion. Headquarters SI-BONE, INC Olin Avenue, Suite 2200 San Jose, CA USA t f info@si-bone.com SI-BONE S.r.l. Via Postcastello, Gallarate (VA), Italy t f infoeurope@si-bone.com SI-BONE Deutschland GmbH Soldnerstraße 11 D Mannheim, Germany t +49 (621) f +49 (621) infodeutschland@si-bone.com SI-BONE UK Monkswell House Manse Lane Knaresborough, North Yorkshire HG5 8NQ, UK Authorized Representative MedPass International Ltd. Windsor House Bretforton, Evesham Worcs.WR11 7JJ United Kingdom SI-BONE and ifuse Implant System are registered trademarks of SI-BONE, Inc SI-BONE, Inc. All rights reserved. Patents: Page 5 of 5
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
Reprocessing Guide. Autoclavable Arthroscope and Hardware Sterilization Tray
 Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Endoscopic Technology. Operating Manual. Cannula system
 Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
M Manufacturer by: Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants
 Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants M Manufacturer by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S.
Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants M Manufacturer by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S.
Surgical Technique Guide
 Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Hygienic Reprocessing
 Hygienic Reprocessing HEINE UniSpec Instrument head General warning and safety information: WARNING! This symbol draws attention to a potentially dangerous situation. Non-observance can result in moderate
Hygienic Reprocessing HEINE UniSpec Instrument head General warning and safety information: WARNING! This symbol draws attention to a potentially dangerous situation. Non-observance can result in moderate
RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON
 ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Preparation Instructions for the CAMLOG /CONELOG Implant System
 J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
OBSOLETE. Visit for the latest version. Ankle Plating System 3 PKGI-79-B EFFECTIVE
 Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Straumann ProClean Cassette. Basic Information on the
 Straumann ProClean Cassette Basic Information on the Straumann ProClean Cassette Contents 1. Straumann ProClean Cassette Uncompromised hygiene 2 2. Straumann ProClean Cassette product at a glance 3 2.1
Straumann ProClean Cassette Basic Information on the Straumann ProClean Cassette Contents 1. Straumann ProClean Cassette Uncompromised hygiene 2 2. Straumann ProClean Cassette product at a glance 3 2.1
Manufacturer s information
 WARNINGS: Observe the standard German accident prevention regulations (UVV) We are not aware of any warnings if the instructions for the devices and disinfection and cleaning agents to be used are followed.
WARNINGS: Observe the standard German accident prevention regulations (UVV) We are not aware of any warnings if the instructions for the devices and disinfection and cleaning agents to be used are followed.
Velocity Orthopedics Instrument Cleaning and Sterilization
 Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Integra Total Foot System 2
 Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
REDUCT Headless Compression Screw System
 REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
Scope This policy applies to all personnel and departments that clean, prepare and/or sterilize items intended for patient care use.
 Dental Sterilization Procedures Policy Number VIM4(4)-10 Purpose The purpose of this policy is to ensure patient and employee safety when using instruments with potential for exposure to bloodborne pathogens
Dental Sterilization Procedures Policy Number VIM4(4)-10 Purpose The purpose of this policy is to ensure patient and employee safety when using instruments with potential for exposure to bloodborne pathogens
Aesculap Implant Systems
 Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
MATERIAL SAFETY DATA SHEET (MSDS) Information about stainless steel for Surgical/Dental/Orthodontic Instruments.
 MATERIAL SAFETY DATA SHEET (MSDS) THE INFORMATION BELOW IS BELIEVED TO BE ACCURATE AND REPRESENTS THE BEST INFORMATION CURRENTYLY AVAILABLE TO US. HOWEVER, WE MAKE NO WARRANTY OF MERCHANTABILITY OR ANY
MATERIAL SAFETY DATA SHEET (MSDS) THE INFORMATION BELOW IS BELIEVED TO BE ACCURATE AND REPRESENTS THE BEST INFORMATION CURRENTYLY AVAILABLE TO US. HOWEVER, WE MAKE NO WARRANTY OF MERCHANTABILITY OR ANY
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. Oblique Lumbar Interbody Fusion
 SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
TABLE OF CONTENTS. PAL-650 Instrument System Instruction Manual. Applicable Part Numbers Introduction General Warnings...
 1 NOTES 19 TABLE OF CONTENTS PAL-650 Instrument System Instruction Manual Applicable Part Numbers................................................................... 1-4 Introduction...............................................................................
1 NOTES 19 TABLE OF CONTENTS PAL-650 Instrument System Instruction Manual Applicable Part Numbers................................................................... 1-4 Introduction...............................................................................
How clean are your instruments?
 How clean are your instruments? Pre cleaning & preparation Samuel Morais Technical Director January 25 th 2015 Overview 1. Correct Preparation will result on good cleaning results that are visible and
How clean are your instruments? Pre cleaning & preparation Samuel Morais Technical Director January 25 th 2015 Overview 1. Correct Preparation will result on good cleaning results that are visible and
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
The following languages are included in this packet:
 DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Cleaning and sterilization instructions for Astra Tech Implant System EV
 Astra Tech Implant System Cleaning and sterilization instructions for Astra Tech Implant System EV Astra Tech Implant System CONTENTS Introduction Astra Tech Implant System EV 4 Pre cleaning 4 Cleaning
Astra Tech Implant System Cleaning and sterilization instructions for Astra Tech Implant System EV Astra Tech Implant System CONTENTS Introduction Astra Tech Implant System EV 4 Pre cleaning 4 Cleaning
Zavation Z-Link Cervical
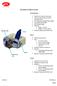 Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental fixation is used.
 Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
ATEC Porous Ti System
 ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
DARCO MIS FOREFOOT SCREW
 DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
X-spine Systems, Inc. Spider Cervical Plating System
 X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
Zimmer Biomet Instrument Kit System
 Zimmer Biomet Instrument Kit System Reference Guide Tapered Screw-Vent (TSV ) Implant System Trabecular Metal Dental Implants. mmd Eztetic Dental Implants Staging Block NP Surgical Module for. mmd Eztetic
Zimmer Biomet Instrument Kit System Reference Guide Tapered Screw-Vent (TSV ) Implant System Trabecular Metal Dental Implants. mmd Eztetic Dental Implants Staging Block NP Surgical Module for. mmd Eztetic
X-spine Systems, Inc. Fixcet Spinal Facet Screw System
 X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
INFORMATION TO THE PRODUCT RANGES. Instructions for Cleaning, Disinfection, Sterilization, Inspection and Maintenance of Medartis Products APTUS MODUS
 INFORMATION TO THE PRODUCT RANGES Instructions for Cleaning, Disinfection, Sterilization, Inspection and Maintenance of Medartis Products APTUS MODUS Instructions for Cleaning, Disinfection, Sterilization,
INFORMATION TO THE PRODUCT RANGES Instructions for Cleaning, Disinfection, Sterilization, Inspection and Maintenance of Medartis Products APTUS MODUS Instructions for Cleaning, Disinfection, Sterilization,
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System
 X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Certex Spinal Fixation System
 X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE
 ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac.
 Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac. This document is intended as guidance for decontamination and sterilization for medical
Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac. This document is intended as guidance for decontamination and sterilization for medical
TJF-160F/VF Cleaning and Disinfection Checklist
 TJF-160F/VF Cleaning and Disinfection Checklist TJF-160F/VF Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-160F/VF has been performed
TJF-160F/VF Cleaning and Disinfection Checklist TJF-160F/VF Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-160F/VF has been performed
Dentium Surgery Kit Dental Implant Surgical Instrument Set
 www.dentium.com Dentium Surgery Kit Dental Implant Surgical Instrument Set Dentium Surgery Kit is a cassette of surgical instruments for periodontal and dental implant surgery. The cassette is divided
www.dentium.com Dentium Surgery Kit Dental Implant Surgical Instrument Set Dentium Surgery Kit is a cassette of surgical instruments for periodontal and dental implant surgery. The cassette is divided
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
EXTERNAL FIXATION SYSTEMS The following languages are included in this packet:
 EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System
 X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
ATHLET VBR. The real winner. VBR in PEEK-OPTIMA PRODUCT INFORMATION
 ATHLET VBR in PEEK-OPTIMA The real winner PRODUCT INFORMATION VBR PRODUCT INFORMATION The Concept A vertebral body replacement requires top performance Simple intra- and postoperative control Anatomic
ATHLET VBR in PEEK-OPTIMA The real winner PRODUCT INFORMATION VBR PRODUCT INFORMATION The Concept A vertebral body replacement requires top performance Simple intra- and postoperative control Anatomic
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. ACDF Anterior Cervical Discectomy and Fusion
 SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
Aesculap Implant Systems
 Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for Use / Technical Description Arcadius XP C Instruments Page 1 of 28 USA Aesculap Implant Systems Arcadius XP C Instruments Page
Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for Use / Technical Description Arcadius XP C Instruments Page 1 of 28 USA Aesculap Implant Systems Arcadius XP C Instruments Page
Spartan S 3 Facet Screw System PACKAGE INSERT
 LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
Battalion TM Universal Spacer System
 Battalion TM Universal Spacer System GENERAL INFORMATION: The Battalion Universal Spacer System (Battalion System) is an intervertebral body fusion device with implants of various lengths, widths, heights,
Battalion TM Universal Spacer System GENERAL INFORMATION: The Battalion Universal Spacer System (Battalion System) is an intervertebral body fusion device with implants of various lengths, widths, heights,
Zeus Intervertebral Body Fusion Devices PACKAGE INSERT
 LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE
 English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM
 EN CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM 150871-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
EN CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM 150871-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
INSTRUCTIONS FOR CLEANING, DISINFECTION AND STERILIZATION OF DFS INSTRUMENTS
 Preparation (cleaning, disinfection and sterilization) of reusable rotating dental instruments General principles All instruments must be cleaned, disinfected and sterilized before each use; this applies
Preparation (cleaning, disinfection and sterilization) of reusable rotating dental instruments General principles All instruments must be cleaned, disinfected and sterilized before each use; this applies
TONTARRA Medizintechnik GmbH. Surgical Instruments in Demand. Laminectomy Punches and Rongeurs
 TONTARRA Medizintechnik GmbH Surgical Instruments in Demand Laminectomy Punches and Rongeurs KERRISON; Laminectomy Punches; clean wave KERRISON with Standard footplate Art.Nr. Size FL 240-072-02A-CW 2
TONTARRA Medizintechnik GmbH Surgical Instruments in Demand Laminectomy Punches and Rongeurs KERRISON; Laminectomy Punches; clean wave KERRISON with Standard footplate Art.Nr. Size FL 240-072-02A-CW 2
BIOFOAM BONE WEDGE
 BIOFOAM BONE WEDGE 150837-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
BIOFOAM BONE WEDGE 150837-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
 EN MANUFACTURER S INFORMATION regarding the preparation of resterilisable medical devices complies with EN ISO 17664 Please read carefully! Medical devices which may be re-processed are tools for abutments
EN MANUFACTURER S INFORMATION regarding the preparation of resterilisable medical devices complies with EN ISO 17664 Please read carefully! Medical devices which may be re-processed are tools for abutments
MICA SCREWS For additional information and translations please contact the manufacturer or local distributor.
 MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
LOCATOR F-Tx SEATING AND REMOVAL TOOL PROCEDURES ADDENDUM
 1 LOCATOR F-Tx SEATING AND REMOVAL TOOL PROCEDURES ADDENDUM INSTRUCTIONS FOR USE DESCRIPTION Wire and Lever Wrench Tip Wrench Seating and Removal Tool Seating Tip Removal Tip with Threaded Adapter The
1 LOCATOR F-Tx SEATING AND REMOVAL TOOL PROCEDURES ADDENDUM INSTRUCTIONS FOR USE DESCRIPTION Wire and Lever Wrench Tip Wrench Seating and Removal Tool Seating Tip Removal Tip with Threaded Adapter The
Technique Guide Lapidus Arthrodesis System
 Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
TMJ Fossa-Eminence Prosthesis System. Instructions for Use. For partial reconstruction (hemiarthroplasty) of the temporomandibular joint
 TMJ Fossa-Eminence Prosthesis System Instructions for Use For partial reconstruction (hemiarthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF: FER-01
TMJ Fossa-Eminence Prosthesis System Instructions for Use For partial reconstruction (hemiarthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF: FER-01
CANNULATED SCREW SYSTEM The following languages are included in this packet:
 CANNULATED SCREW SYSTEM 137183-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -Chinese (sch) Türkçe
CANNULATED SCREW SYSTEM 137183-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -Chinese (sch) Türkçe
UNICLIP DIRECTIONS FOR USE REF C226U - 0,8 MM / 1 MM FOR DENTAL USE ONLY. FISDR / F EN / 11 / updated 06/2016 1/7
 UNICLIP FOR DENTAL USE ONLY DIRECTIONS FOR USE REF C226U - 0,8 MM / 1 MM EN FISDR / F19 02 31.EN / 11 / 2001 - updated 06/2016 1/7 1) Indications for use These products have to be used only in hospital
UNICLIP FOR DENTAL USE ONLY DIRECTIONS FOR USE REF C226U - 0,8 MM / 1 MM EN FISDR / F19 02 31.EN / 11 / 2001 - updated 06/2016 1/7 1) Indications for use These products have to be used only in hospital
Zimmer Biomet Instrument Kit System
 Zimmer Biomet Instrument Kit System Reference Guide Tapered Screw-Vent (TSV ) Implant System Trabecular Metal Dental Implants. mmd Eztetic Dental Implants Staging Block NP Surgical Module for. mmd Eztetic
Zimmer Biomet Instrument Kit System Reference Guide Tapered Screw-Vent (TSV ) Implant System Trabecular Metal Dental Implants. mmd Eztetic Dental Implants Staging Block NP Surgical Module for. mmd Eztetic
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
TTA Wedge System INSTRUCTIONS FOR USE
 TTA Wedge System INSTRUCTIONS FOR USE 1 INSTRUCTIONS FOR USE The OssAbility TTA Wedge System consists of the following products: Wedge Implants Osteotomy Guide Advancement Levers Osteotomy Planning Overlay
TTA Wedge System INSTRUCTIONS FOR USE 1 INSTRUCTIONS FOR USE The OssAbility TTA Wedge System consists of the following products: Wedge Implants Osteotomy Guide Advancement Levers Osteotomy Planning Overlay
ProUltra ULTRASONIC SURGICAL TIPS
 ProUltra ULTRASONIC SURGICAL TIPS EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC SURGICAL TIPS) A0640 A0650 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Surgical tips Ref. A0640 Ref. A0650 1) INDICATIONS
ProUltra ULTRASONIC SURGICAL TIPS EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC SURGICAL TIPS) A0640 A0650 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Surgical tips Ref. A0640 Ref. A0650 1) INDICATIONS
Surgical Technique Guide
 Sacroiliac Joint Fusion System Surgical Technique Guide Moving Life Forward Table of Contents SiCure Implant Overview...2 SiCure System Information...3 X-ray Basics...4 Patient Positioning....5 Surgical
Sacroiliac Joint Fusion System Surgical Technique Guide Moving Life Forward Table of Contents SiCure Implant Overview...2 SiCure System Information...3 X-ray Basics...4 Patient Positioning....5 Surgical
The Omega LIF System devices are made from Titanium alloy Ti6Al4V ELI, ASTM F136.
 Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
Disinfection and Decontamination Core Subject. The Process of Instrument Decontamination
 Disinfection and Decontamination Core Subject The Process of Instrument Decontamination Aims: To give an overview of the processes involved in dental instrument decontamination using essential quality
Disinfection and Decontamination Core Subject The Process of Instrument Decontamination Aims: To give an overview of the processes involved in dental instrument decontamination using essential quality
2. Active systemic infection or infection localized to the site of the proposed implantation is contraindications to implantation.
 OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
Longevity Offset and Oblique Liners. Surgical Technique
 Longevity Offset and Oblique Liners Surgical Technique Longevity Offset & Oblique Liners Surgical Technique 1 Introduction Complete Acetabular System Joint instability is an important issue in both primary
Longevity Offset and Oblique Liners Surgical Technique Longevity Offset & Oblique Liners Surgical Technique 1 Introduction Complete Acetabular System Joint instability is an important issue in both primary
Page 1 of 7 Doc. No.: IU/15 Rev. No.: 05 Rev. Date:03MAY2018. Instructions for use for ATLAS Tibial Fracture(TF) Nail
 Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE
 OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
Cleaning and sterilization. Guidelines for Nobel Biocare products including MRI information
 Cleaning and sterilization Guidelines for Nobel Biocare products including MRI information Note: In order to improve readability, Nobel Biocare does not use or in the running text. By doing so, however,
Cleaning and sterilization Guidelines for Nobel Biocare products including MRI information Note: In order to improve readability, Nobel Biocare does not use or in the running text. By doing so, however,
a perfect fit INSTRUCTION MANUAL CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT Handling Sterilization Care
 a perfect fit INSTRUCTION MANUAL CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT Handling Sterilization Care CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT INTRODUCTION The torque wrench
a perfect fit INSTRUCTION MANUAL CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT Handling Sterilization Care CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT INTRODUCTION The torque wrench
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
ALIGN Radial Head System INSTRUCTIONS FOR USE
 ALIGN Radial Head System INSTRUCTIONS FOR USE R x: For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may lead to patient
ALIGN Radial Head System INSTRUCTIONS FOR USE R x: For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may lead to patient
ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM
 ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM 150884-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM 150884-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
1) INDICATIONS FOR USE These products have to be used only in hospital environments, clinics or dental offices by qualified dental personnel.
 ProFile 04/06 & O.S. EN DENTAL USE ONLY DIRECTIONS FOR USE PROFILE 0) COMPOSITION The cutting part of these instruments is made of a nickel-titanium alloy. 1) INDICATIONS FOR USE These products have to
ProFile 04/06 & O.S. EN DENTAL USE ONLY DIRECTIONS FOR USE PROFILE 0) COMPOSITION The cutting part of these instruments is made of a nickel-titanium alloy. 1) INDICATIONS FOR USE These products have to
EXPEDIUM Spine System VIPER System
 0902-90-105 Rev. H EXPEDIUM Spine System VIPER System Revised January 2017 DePuy Synthes 2013-2017. All rights reserved. en EXPEDIUM Spine System VIPER System CAUTION: USA law restricts this device to
0902-90-105 Rev. H EXPEDIUM Spine System VIPER System Revised January 2017 DePuy Synthes 2013-2017. All rights reserved. en EXPEDIUM Spine System VIPER System CAUTION: USA law restricts this device to
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM
 METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
SURGICAL TECHNIQUE MANUAL
 SURGICAL TECHNIQUE MANUAL TABLE OF CONTENTS TABLE OF CONTENTS System Values and Mechanism of Action 4 Indications and Contraindications 5 Implant 6 Instrument Set 7 Instrument Assembly 8 Precautions 10
SURGICAL TECHNIQUE MANUAL TABLE OF CONTENTS TABLE OF CONTENTS System Values and Mechanism of Action 4 Indications and Contraindications 5 Implant 6 Instrument Set 7 Instrument Assembly 8 Precautions 10
Aesculap Spine Instructions for use
 Aesculap Spine S 4 Spondylolisthesis Reduction Instrument (SRI) Instructions for use S 4 Spondylolisthesis Reduction Instrument (SRI) 5 4 3 2 Fig. 1 1 9 10 11 8 6 7 Fig. 2 Aesculap Spine S 4 Spondylolisthesis
Aesculap Spine S 4 Spondylolisthesis Reduction Instrument (SRI) Instructions for use S 4 Spondylolisthesis Reduction Instrument (SRI) 5 4 3 2 Fig. 1 1 9 10 11 8 6 7 Fig. 2 Aesculap Spine S 4 Spondylolisthesis
ProUltra Ultrasonic Non Surgical Endo Tips
 ProUltra Ultrasonic Non Surgical Endo Tips EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC NON SURGICAL TIPS) A0620 A0621 A0630 A0631 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Endo tips, Zirconium Ref.
ProUltra Ultrasonic Non Surgical Endo Tips EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC NON SURGICAL TIPS) A0620 A0621 A0630 A0631 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Endo tips, Zirconium Ref.
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System
 X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
TMJ Fossa-Eminence and Condylar Prosthesis System. Instructions for Use. For total reconstruction (arthroplasty) of the temporomandibular joint
 TMJ Fossa-Eminence and Condylar Prosthesis System Instructions for Use For total reconstruction (arthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF:
TMJ Fossa-Eminence and Condylar Prosthesis System Instructions for Use For total reconstruction (arthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF:
Double Engine Orthopedic Bone Nail System Universal Humeral Nail
 Double Engine Orthopedic Bone Nail System ----------- Universal Humeral Nail Surgical Technique Manual Note: The surgical procedures should be performed under the guidance of qualified skilled orthopedic
Double Engine Orthopedic Bone Nail System ----------- Universal Humeral Nail Surgical Technique Manual Note: The surgical procedures should be performed under the guidance of qualified skilled orthopedic
Failure to follow instructions may lead to patient injury.
 STABLYX CMC Arthroplasty System INSTRUCTIONS FOR USE R x : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions
STABLYX CMC Arthroplasty System INSTRUCTIONS FOR USE R x : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions
Convalescent Kelly. Directions for Use
 Convalescent Kelly Directions for Use 302-00001 2 Table of Contents Recommends 4 Items Included 4 Skills Taught 4 Airway System 5 Arms 6 Legs 6 Colon Reservoir 7 Genitalia 7 Urinary Catheterization and
Convalescent Kelly Directions for Use 302-00001 2 Table of Contents Recommends 4 Items Included 4 Skills Taught 4 Airway System 5 Arms 6 Legs 6 Colon Reservoir 7 Genitalia 7 Urinary Catheterization and
Instruction Manual Aerogen, Inc. Part No. AG-AL1010 Rev. C
 Instruction Manual 2004 Aerogen, Inc. Part No. AG-AL1010 Rev. C Table of Contents Introduction... 3 System description... 4 Warnings... 5 Cautions... 5 Electromagnetic Susceptibility... 5 Symbols... 6
Instruction Manual 2004 Aerogen, Inc. Part No. AG-AL1010 Rev. C Table of Contents Introduction... 3 System description... 4 Warnings... 5 Cautions... 5 Electromagnetic Susceptibility... 5 Symbols... 6
Astra Tech Implant System. Manual and product catalog. Repair procedures. Removal and retrieval of screw fragment, abutment and implant
 Astra Tech Implant System Manual and product catalog Repair procedures Removal and retrieval of screw fragment, abutment and implant Astra Tech Implant System CONTENTS Introduction 5 Remove/retrieve on
Astra Tech Implant System Manual and product catalog Repair procedures Removal and retrieval of screw fragment, abutment and implant Astra Tech Implant System CONTENTS Introduction 5 Remove/retrieve on
STABILIZATION AND FRACTURE FIXATION
 STABILIZATION AND FRACTURE FIXATION 150838-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
STABILIZATION AND FRACTURE FIXATION 150838-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
Aesculap Long and Short Ventriculoscope Systems Instructions for Use
 Aesculap Long and Short Ventriculoscope Systems Instructions for Use INDICATIONS FOR USE Aesculap s short and long ventriculoscopes and associated instruments are designed for visual orientation and therapeutic
Aesculap Long and Short Ventriculoscope Systems Instructions for Use INDICATIONS FOR USE Aesculap s short and long ventriculoscopes and associated instruments are designed for visual orientation and therapeutic
