Medics Home Health Services 1075 South Main St. Ste. 450 Madison, GA P F
|
|
|
- Loren Jordan
- 5 years ago
- Views:
Transcription
1 Medics Home Health Services 1075 South Main St. Ste. 450 Madison, GA P F Medicare Coverage Criteria for DME WE HAVE THIS AVAILBIE IN DIGITAL FORM IF YOU D PREFER LET US KNOW All durable medical equipment ordered by a physician will need a face to face prior to the order and within the last 6 months that details what is required to be covered by Medicare, Medicaid, and most private insurance. An order or Rx should be written as well. The order should always have: the beneficiaries name, item, diagnosis, physician s signature, date, and NPI. The ordering provider (MD, DO, PA-C, Nurse Practitioner) must be PECOS certified. PECOS certification can be Table of Contents Canes & Walkers... 2 Walkers... 2 Manual Wheelchairs... 3 Hoyer Lift... 4 Commodes... 4 Hospital Beds... 5 Group 1 Support Surfaces (APP and Gel Overlay)... 5 Group 2 Support Surface... 6 Nebulizers... 7 Oxygen... 7 PAPs (CPAP and BIPAP)
2 Canes & Walkers Canes (E0100, E0105) and crutches (E E0116) are covered if all of the following criteria (1-3) are met: 1. The beneficiary has a mobility limitation that significantly impairs his/her ability to participate in one or more mobility-related activities of daily living (MRADL) in the home. The MRADLs to be considered in this and all other statements in this policy are toileting, feeding, dressing, grooming, and bathing performed in customary locations in the home. A mobility limitation is one that: a. Prevents the beneficiary from accomplishing the MRADL entirely, or, b. Places the beneficiary at reasonably determined heightened risk of morbidity or mortality secondary to the attempts to perform an MRADL; or, c. Prevents the beneficiary from completing the MRADL within a reasonable time frame; And, 2. The beneficiary is able to safely use the cane or crutch; and, 3. The functional mobility deficit can be sufficiently resolved by use of a cane or crutch. If all of the criteria are not met, the cane or crutch will be denied as not reasonable and necessary. Walkers A standard walker (E0130, E0135, E0141, E0143) and related accessories are covered if all of the following criteria (1-3) are met: 1. The beneficiary has a mobility limitation that significantly impairs his/her ability to participate in one or more mobility-related activities of daily living (MRADL) in the home. A mobility limitation is one that: a. Prevents the beneficiary from accomplishing the MRADL entirely, or b. Places the beneficiary at reasonably determined heightened risk of morbidity or mortality secondary to the attempts to perform the MRADL, or c. Prevents the beneficiary from completing the MRADL within a reasonable time frame; and 2. The beneficiary is able to safely use the walker; and 3. The functional mobility deficit can be sufficiently resolved with use of a walker. If all the criteria are not met, the walker will be denied as not reasonable and necessary 2
3 Manual Wheelchairs - The Rx needs to have standard weight or light weight A manual wheelchair for use inside the home (E E1039, E1161, K0001 K0009) is covered if: Criteria A, B, C, D, and E are met; and Criterion F or G is met. A. The beneficiary has a mobility limitation that significantly impairs his/her ability to participate in one or more mobility-related activities of daily living (MRADLs) such as toileting, feeding, dressing, grooming, and bathing in customary locations in the home. A mobility limitation is one that: 1. Prevents the beneficiary from accomplishing an MRADL entirely, or 2. Places the beneficiary at reasonably determined heightened risk of morbidity or mortality secondary to the attempts to perform an MRADL; or 3. Prevents the beneficiary from completing an MRADL within a reasonable time frame. B. The beneficiary s mobility limitation cannot be sufficiently resolved by the use of an appropriately fitted cane or walker. C. The beneficiary s home provides adequate access between rooms, maneuvering space, and surfaces for use of the manual wheelchair that is provided. D. Use of a manual wheelchair will significantly improve the beneficiary s ability to participate in MRADLs and the beneficiary will use it on a regular basis in the home. E. The beneficiary has not expressed an unwillingness to use the manual wheelchair that is provided in the home. F. The beneficiary has sufficient upper extremity function and other physical and mental capabilities needed to safely self-propel the manual wheelchair that is provided in the home during a typical day. Limitations of strength, endurance, range of motion, or coordination, presence of pain, or deformity or absence of one or both upper extremities are relevant to the assessment of upper extremity function. G. The beneficiary has a caregiver who is available, willing, and able to provide assistance with the wheelchair. ADDITIONAL CRITERIA FOR SPECIFIC MANUAL WHEELCHAIRS (E1037, E1038, E1039, E1161, K0002 K0008) In addition to the general manual wheelchair criteria above, the specific criteria below must be met for each manual wheelchair. If the specific criteria are not met, the manual wheelchair will be denied as not reasonable and necessary. A transport chair (E1037, E1038 or E1039) is covered as an alternative to a standard manual wheelchair (K0001) and if basic coverage criteria A-E and G above are met. A standard hemi-wheelchair (K0002) is covered when the beneficiary requires a lower seat height (17" to 18") 3
4 because of short stature or to enable the beneficiary to place his/her feet on the ground for propulsion. A lightweight wheelchair (K0003) is covered when a beneficiary meets both criteria (1) and (2): 1. Cannot self-propel in a standard wheelchair in the home; and 2. The beneficiary can and does self-propel in a lightweight wheelchair. A high strength lightweight wheelchair (K0004) is covered when a beneficiary meets the criteria in (1) or (2): 1. The beneficiary self-propels the wheelchair while engaging in frequent activities in the home that cannot be performed in a standard or lightweight wheelchair. 2. The beneficiary requires a seat width, depth, or height that cannot be accommodated in a standard, lightweight or hemi-wheelchair, and spends at least two hours per day in the wheelchair. We no longer offer power mobility, but we can help you find a provider if you d like to call Hoyer Lift The office note needs say something very similar to: A patient lift is necessary because transfer between bed and a chair, wheelchair, or commode is required and, without the use of a lift, the beneficiary would be bed confined Commodes A commode is covered when the beneficiary is physically incapable of utilizing regular toilet facilities. This would occur in the following situations: 1. The beneficiary is confined to a single room, or 2. The beneficiary is confined to one level of the home environment and there is no toilet on that level, or 3. The beneficiary is confined to the home and there are no toilet facilities in the home. An extra wide/heavy duty commode chair (E0168) is covered for a beneficiary who weighs 300 pounds or more. If an E0168 commode is ordered and the beneficiary does not weigh more than 300 pounds, it will be denied as not reasonable and necessary. A commode chair with detachable arms (E0165) is covered if the detachable arms feature is necessary to facilitate transferring the beneficiary or if the beneficiary has a body configuration that requires extra width. If coverage criteria are not met payment will be denied as not reasonable and necessary. 4
5 Hospital Beds The Rx or written order must say Semi-Electric Hospital Bed A fixed height hospital bed (E0250, E0251, E0290, E0291, and E0328) is covered if one or more of the following criteria (1-4) are met: 1. The beneficiary has a medical condition which requires positioning of the body in ways not feasible with an ordinary bed. Elevation of the head/upper body less than 30 degrees does not usually require the use of a hospital bed, or 2. The beneficiary requires positioning of the body in ways not feasible with an ordinary bed in order to alleviate pain, or 3. The beneficiary requires the head of the bed to be elevated more than 30 degrees most of the time, due to congestive heart failure, chronic pulmonary disease, or problems with aspiration, and pillows or a wedge have been tried and failed, or 4. The beneficiary requires traction equipment, which can only be attached to a hospital bed. 5. A semi-electric hospital bed (E0260, E0261, E0294, E0295, and E0329) is covered if the beneficiary meets one of the criteria for a fixed height bed and requires frequent changes in body position and/or has an immediate need for a change in body position. (so one or more of the conditions listed in 1-4 AND #5) A heavy duty extra wide hospital bed (E0301, E0303) is covered if the beneficiary meets one of the criteria for a fixed height hospital bed and the beneficiary's weight is more than 350 pounds, but does not exceed 600 pounds. An extra heavy-duty hospital bed (E0302, E0304) is covered if the beneficiary meets one of the criteria for a hospital bed and the beneficiary's weight exceeds 600 pounds. A total electric hospital bed (E0265, E0266, E0296, and E0297) is not covered; the height adjustment feature is a convenience feature. Total electric beds will be denied as not reasonable and necessary. Group 1 Support Surfaces (APP and Gel Overlay) A Group 1 mattress overlay or mattress (E0181-E0189, E0196-E0199, and A4640) is covered if one of the following three criteria are met: 1. The beneficiary is completely immobile - i.e., beneficiary cannot make changes in body position without assistance, or 2. The beneficiary has limited mobility - i.e., beneficiary cannot independently make changes in body position significant enough to alleviate pressure and at least one of conditions A-D below, or 3. The beneficiary has any stage pressure ulcer on the trunk or pelvis and at least one of conditions A-D below. Conditions for criteria 2 and 3 (in each case the medical record must document the severity of the condition sufficiently to demonstrate the medical necessity for a pressure reducing support surface): 5
6 A. Impaired nutritional status B. Fecal or urinary incontinence C. Altered sensory perception D. Compromised circulatory status When the coverage criteria for a Group 1 mattress overlay or mattress are not met, the claim will be denied as not reasonable and necessary Group 2 Support Surface A group 2 support surface is covered if the beneficiary meets at least one of the following three Criteria (1, 2 or 3): 1. The beneficiary has multiple stage II pressure ulcers located on the trunk or pelvis (ICD ) which have failed to improve over the past month, during which time the beneficiary has been on a comprehensive ulcer treatment program including each of the following: a. Use of an appropriate group 1 support surface, and b. Regular assessment by a nurse, physician, or other licensed healthcare practitioner, and c. Appropriate turning and positioning, and d. Appropriate wound care, and e. Appropriate management of moisture/incontinence, and f. Nutritional assessment and intervention consistent with the overall plan of care 2. The beneficiary has large or multiple stage III or IV pressure ulcer(s) on the trunk or pelvis (ICD ), 3. The beneficiary had a myocutaneous flap or skin graft for a pressure ulcer on the trunk or pelvis within the past 60 days (ICD ), and has been on a group 2 or 3 support surface immediately prior to discharge from a hospital or nursing facility within the past 30 days. 6
7 Nebulizers Dx of J41.0-J70.9 for medications albuterol, arformoterol, budesonide, cromolyn, formoterol, ipratropium, levalbuterol, or metaproterenol Nebs and these medicines are typically necessary for chronic conditions if the diagnosis doesn t fall into this range - an inhaler is typically considered what s necessary. The following elements are necessary on the Rx: beneficiaries name, nebulizer, diagnosis (see above), LON, medication and dosage, physician signature, date, and NPI#. Rx example: (We are not a pharmacy and do not fill the drug, but we must know the drug and frequency) Oxygen Full time 24/7 Diagnosis causing hypoxemia (COPD causing hypoxemia is an example) So if Hypoxemia is diagnosed we need to know what is causing it 1. An arterial PO 2 at or below 55 mm Hg or an arterial oxygen saturation at or below 88 percent taken at rest (awake), or 2. A decrease in arterial PO 2 more than 10 mm Hg, or a decrease in arterial oxygen saturation more than 5 percent from baseline saturation, for at least 5 minutes taken during sleep associated with symptoms (e.g., impairment of cognitive processes and [nocturnal restlessness or insomnia]) or signs (e.g., cor pulmonale, "P" pulmonale on EKG, documented pulmonary hypertension and erythrocytosis) reasonably attributable to hypoxemia, or 3. An arterial PO 2 at or below 55 mm Hg or an oxygen saturation at or below 88 percent, taken during exercise for a beneficiary who demonstrates an arterial PO 2 at or above 56 mm Hg or an arterial oxygen saturation at or above 89 percent during the day while at rest. In this case, a 6 minute walk test would commonly be done. Below is a description of what is necessary during an exercise test. 7
8 Exercise testing When oxygen is covered based on an oximetry study obtained during exercise, there must be documentation of three (3) oximetry studies in the beneficiary s medical record. (1) Testing at rest without oxygen, (2) testing during exercise without oxygen, and (3) testing during exercise with oxygen applied (to demonstrate the improvement of the hypoxemia) are required. All 3 tests must be performed within the same testing session. Exercise testing must be performed in-person by a physician or other medical professional qualified to conduct exercise oximetry testing. Unsupervised or remotely supervised home exercise testing does not qualify as a valid test for purposes of Medicare reimbursement of home oxygen and oxygen equipment. Only the testing during exercise without oxygen is used for qualification and reported on the CMN. The other two results do not have to be routinely submitted but must be available on request. OR An arterial PO 2 of mm Hg or an arterial blood oxygen saturation of 89 percent at rest (awake), during sleep for at least 5 minutes, or during exercise (as described under Group I criteria), and Any of the following: 1. Dependent edema suggesting congestive heart failure, or 2. Pulmonary hypertension or cor pulmonale, determined by measurement of pulmonary artery pressure, gated blood pool scan, echocardiogram, or "P" pulmonale on EKG (P wave greater than 3 mm in standard leads II, III, or AVF), or 3. Erythrocythemia with a hematocrit greater than 56 percent. This will qualify the patient for 90 days of oxygen. The patient will need to schedule a follow-up within 90 days Oxygen DURING SLEEP ONLY - without a diagnosis of obstructive sleep apnea Overnight Oximetry Studies: Overnight sleep oximetry may be performed in a facility or at home. For home overnight oximetry studies, the oximeter provided to the beneficiary must be tamper-proof and must have the capability to download data that allows documentation of the duration of oxygen desaturation below a specified value. For all the overnight oximetry criteria described above, the 5 minutes does not have to be continuous. Baseline saturation is defined as the mean saturation level during the duration of the test. For purposes of meeting criterion 3 described in Group I above there must be a minimum of 2 hours test time recorded for sleep oximetry. The result must reach a qualifying test value otherwise the Group III presumption of non-coverage applies. 8
9 Home overnight oximetry is limited solely to stand-alone overnight pulse oximetry performed in the beneficiary s home under the conditions specified below. Overnight oximetry performed as part of home sleep testing or as part of any other home testing is not considered to be eligible under this provision to be used for qualification for reimbursement of home oxygen and oxygen equipment even if the testing was performed in compliance with the requirements of this section. Beneficiaries may self-administer home based overnight oximetry tests under the direction of a Medicareenrolled Independent Diagnostic Testing Facility (IDTF). A DME supplier or another shipping entity may deliver a pulse oximetry test unit and related technology to a beneficiary s home under the following circumstances: 1. The beneficiary s treating physician has contacted the IDTF to order an overnight pulse oximetry test before the test is performed. 2. The test is performed under the direction and/or instruction of a Medicare-approved IDTF. Because it is the beneficiary who self-administers this test, the IDTF must provide clear written instructions to the beneficiary on proper operation of the test equipment and must include access to the IDTF in order to address other concerns that may arise. The DME supplier may not create this written instruction, provide verbal instructions, answer questions from the beneficiary, apply or demonstrate the application of the testing equipment to the beneficiary, or otherwise participate in the conduct of the test. 3. The test unit is sealed and tamper-proof such that test results cannot be accessed by anyone other than the IDTF which is responsible for transmitting a test report to the treating physician. The DME supplier may use related technology to download test results from the testing unit and transmit those results to the IDTF. In no case may the DME supplier access or manipulate the test results in any form. The IDTF must send the test results to the physician. The IDTF may send the test results to the supplier if the supplier is currently providing or has an order to provide oxygen or other respiratory services to the beneficiary or if the beneficiary has signed a release permitting the supplier to receive the report. Oximetry test results obtained through a similar process as described for home overnight oximetry (see above) while the beneficiary is awake, either at rest or with exercise, may not be used for purposes of qualifying the beneficiary for home oxygen therapy. Overnight oximetry does not include oximetry obtained during polysomnography or other sleep testing for sleep apnea, regardless of the location the testing was performed. See below for information on sleep testing that may be used to qualify for oxygen coverage. Oxygen DURING SLEEP ONLY - with a diagnosis of obstructive sleep apnea Some beneficiaries may require the simultaneous use of home oxygen therapy with a PAP device. To be considered for simultaneous coverage, all requirements in the Coverage Indications, Limitations and/or Medical Necessity for both the Oxygen and Oxygen Equipment and Positive Airway Pressure (PAP) Devices for the Treatment of Obstructive Sleep Apnea LCDs must be met. Consequently, in addition to this Oxygen LCD, suppliers should refer to the Positive Airway Pressure (PAP) Devices for the Treatment of Obstructive Sleep Apnea LCD and related Policy Article for additional coverage, coding and documentation requirements. 9
10 Coverage of home oxygen therapy requires that the beneficiary be tested in the chronic stable state. Chronic stable state is a requirement of the National Coverage Determination (CMS Internet-only Manual, Pub , Section 240.2) and is one of the key criteria when determining coverage of home oxygen therapy. The NCD defines chronic stable state as not during a period of an acute illness or an exacerbation of their underlying disease. Based on this NCD definition, all co-existing diseases or conditions that can cause hypoxia must be treated and the beneficiary must be in a chronic stable state before oxygen therapy is considered eligible for payment. In addition, the beneficiary must have a severe lung disease, such as chronic obstructive pulmonary disease, diffuse interstitial lung disease, cystic fibrosis, bronchiectasis, widespread pulmonary neoplasm, or hypoxia-related symptoms or findings that might be expected to improve with oxygen therapy. In the case of OSA, it is required that the OSA be appropriately and sufficiently treated such that the beneficiary is in the chronic stable state before oxygen saturation results obtained during sleep testing are considered qualifying for oxygen therapy (see PAP LCD for additional information). For beneficiaries with OSA, this means that the OSA must be sufficiently treated such that the underlying severe lung disease is unmasked. This must be demonstrated before oxygen saturation results obtained during polysomnography are considered qualifying for oxygen therapy. For beneficiaries with OSA, a qualifying oxygen saturation test may only occur during a titration polysomnographic study (either split night or stand-alone). The titration PSG is one in which all of the following criteria are met: 1. The titration is conducted over a minimum of two (2) hours; and 2. During titration: A. The AHI/RDI is reduced to less than or equal to an average of ten (10) events per hour; or B. If the initial AHI/RDI was less than an average of ten (10) events per hour, the titration demonstrates further reduction in the AHI/RDI; and 3. Nocturnal oximetry conducted for the purpose for oxygen reimbursement qualification may only be performed after optimal PAP settings have been determined and the beneficiary is using the PAP device at those settings; and 4. The nocturnal oximetry conducted during the PSG demonstrates an oxygen saturation 88% for 5 minutes total (which need not be continuous) If all of the above criteria are met, for the purposes of a qualifying oxygen saturation test, the beneficiary is considered to be in the chronic stable state. To be eligible for Medicare coverage and payment for home oxygen therapy for concurrent use with PAP therapy, in addition to being in the chronic stable state, the beneficiary must meet all other coverage requirements for oxygen therapy. Beneficiaries that qualify for oxygen therapy based on testing conducted only during the course of a sleep test are eligible only for reimbursement of stationary equipment. 10
11 Rx needs: beneficiaries name oxygen at liters per minute via nasal cannula (or via cpap if qualified during titration sleep study) Dx (what s causing hypoxemia) doctor s signature, date, and NPI# We will need the office note qualifying the patient and/or sleep study qualifying the patient along with the Rx faxed to PAPs (CPAP and BIPAP) Cpap INITIAL COVERAGE: In this policy, the term PAP (positive airway pressure) device will refer to both a single-level continuous positive airway pressure device (E0601) and a bi-level respiratory assist device without back-up rate (E0470) when it is used in the treatment of obstructive sleep apnea. 1. An E0601 CPAP device is covered for the treatment of obstructive sleep apnea (OSA) if criteria A C are met: A. The beneficiary has a face-to-face clinical evaluation by the treating physician prior to the sleep test to assess the beneficiary for obstructive sleep apnea. B. The beneficiary has a sleep test (as defined below) that meets either of the following criteria (1 or 2): 1. The apnea-hypopnea index (AHI) or Respiratory Disturbance Index (RDI) is greater than or equal to 15 events per hour with a minimum of 30 events; or, 2. The AHI or RDI is greater than or equal to 5 and less than or equal to 14 events per hour with a minimum of 10 events and documentation of: a. Excessive daytime sleepiness, impaired cognition, mood disorders, or insomnia; or, b. Hypertension, ischemic heart disease, or history of stroke. 2. A BIPAP device is covered for those beneficiaries with OSA who meet criteria A-C above, in addition to criterion D: 11
12 D. A CPAP has been tried and proven ineffective based on a therapeutic trial conducted in either a facility or in a home setting. 3. A RAD (E0470 BIPAP, E0471 BIPAP with respiratory assist device) is covered for those beneficiaries with one of the following clinical disorders: restrictive thoracic disorders (i.e., neuromuscular diseases or severe thoracic cage abnormalities), severe chronic obstructive pulmonary disease (COPD), CSA or CompSA, or hypoventilation syndrome, as described in the following section. Restrictive Thoracic Disorders An E0470 Bipap or E0471 BipapASV device is covered when criteria A C are met. A. There is documentation in the beneficiary s medical record of a neuromuscular disease (for example, amyotrophic lateral sclerosis) or a severe thoracic cage abnormality (for example, post-thoracoplasty for TB). B. One of the following: a. An arterial blood gas PaCO2, done while awake and breathing the beneficiary s prescribed FIO2 is greater than or equal to 45 mm Hg, or b. Sleep oximetry demonstrates oxygen saturation less than or equal to 88% for greater than or equal to 5 minutes of nocturnal recording time (minimum recording time of 2 hours), done while breathing the beneficiary s prescribed recommended FIO2, or c. For a neuromuscular disease (only), either i or ii, i. Maximal inspiratory pressure is less than 60 cm H20, or ii. Forced vital capacity is less than 50% predicted C. Chronic obstructive pulmonary disease does not contribute significantly to the beneficiary s pulmonary limitation. If all of the above criteria are met, either an E0470 Bipap or an E0471BipapASV device (based upon the judgment of the treating physician) will be covered for the first three months of therapy. If all of the above criteria are not met, then E0470 Bipap or E0471 BipapASV and related accessories will be denied as not reasonable and necessary. See CONTINUED COVERAGE CRITERIA FOR E0470 Bipap AND E0471 BipapASV DEVICES BEYOND THE FIRST THREE MONTHS for information on more than three months use. Severe COPD An E0470 Bipap device is covered if criteria A - C are met. A. An arterial blood gas PaCO2, done while awake and breathing the beneficiary s prescribed FIO2, is greater than or equal to 52 mm Hg. 12
13 B. Sleep oximetry demonstrates oxygen saturation less than or equal to 88% for greater than or equal to a cumulative 5 minutes of nocturnal recording time (minimum recording time of 2 hours), done while breathing oxygen at 2 LPM or the beneficiary s prescribed FIO2 (whichever is higher). C. Prior to initiating therapy, sleep apnea and treatment with a continuous positive airway pressure device (CPAP) has been considered and ruled out. (Note: Formal sleep testing is not required if there is sufficient information in the medical record to demonstrate that the beneficiary does not suffer from some form of sleep apnea (Obstructive Sleep Apnea (OSA), CSA and/or CompSA) as the predominant cause of awake hypercapnia or nocturnal arterial oxygen desaturation). If all of the above criteria for beneficiaries with COPD are met, an E0470 device will be covered for the first three months of therapy. If all of the above criteria are not met, E0470 and related accessories will be denied as not reasonable and necessary. An E0471 device will be covered for a beneficiary with COPD in either of the two situations below, depending on the testing performed to demonstrate the need. Situation 1. For Group II beneficiaries (COPD) who qualified for an E0470 Bipap device, an E0471 BipapASV started any time after a period of initial use of an E0470 device is covered if both criteria A and B are met. A. An arterial blood gas PaCO2, done while awake and breathing the beneficiary s prescribed FIO2, shows that the beneficiary s PaCO2 worsens greater than or equal to 7 mm HG compared to the original result from criterion A, (above). B. A facility-based PSG demonstrates oxygen saturation less than or equal to 88% for greater than or equal to a cumulative 5 minutes of nocturnal recording time (minimum recording time of 2 hours) while using an E0470 Bipap device that is not caused by obstructive upper airway events i.e., AHI less than 5. (Refer to the Positive Airway Pressure Devices LCD for information about E0470 coverage for obstructive sleep apnea). Situation 2. For Group II beneficiaries (COPD) who qualified for an E0470 Bipap device, an E0471 BipapASV device will be covered if, at a time no sooner than 61 days after initial issue of the E0470 Bipap device, both of the following criteria A and B are met: A. An arterial blood gas PaCO2 is done while awake and breathing the beneficiary s prescribed FIO2, still remains greater than or equal to 52 mm Hg. B. Sleep oximetry while breathing with the E0470 device, demonstrates oxygen saturation less than or equal to 88% for greater than or equal to a cumulative 5 minutes of nocturnal recording time (minimum recording time of 2 hours), done while breathing oxygen at 2 LPM or the beneficiary s prescribed FIO2 [whichever is higher]. If E0471 is billed but the criteria described in either situation 1 or 2 are not met, it will be denied as not reasonable and necessary. See CONTINUED COVERAGE CRITERIA FOR E0470 AND E0471 DEVICES BEYOND THE FIRST THREE MONTHS for information on more than three months use. 13
14 Central Sleep Apnea or Complex Sleep Apnea An E0470 Bipap or E0471 BipapASV device is covered when, prior to initiating therapy, a complete facility- based, attended PSG is performed documenting the following (A and B): A. The diagnosis of CSA or CompSA; and B. Significant improvement of the sleep-associated hypoventilation with the use of an E0470 or E0471 device on the settings that will be prescribed for initial use at home, while breathing the beneficiary s prescribed FIO2. If all of the above criteria are met, either an E0470 Bipap or an E0471 BipapASV device (based upon the judgment of the treating physician) will be covered for beneficiaries with documented CSA or CompSA for the first three months of therapy. If all of the above criteria are not met, then E0470 Bipap or E0471BipapASV and related accessories will be denied as not reasonable and necessary. See CONTINUED COVERAGE CRITERIA FOR E0470 Bipap AND E0471 BipapASV DEVICES BEYOND THE FIRST THREE MONTHS for information on more than three months use. Hypoventilation Syndrome An E0470 Bipap device is covered if both criteria A and B and either criterion C or D are met. A. An initial arterial blood gas PaCO2, done while awake and breathing the beneficiary s prescribed FIO2, is greater than or equal to 45 mm Hg. B. Spirometry shows an FEV1/FVC greater than or equal to 70%. (Refer to SEVERE COPD (above) for information about device coverage for beneficiaries with FEV1/FVC less than 70%.) C. An arterial blood gas PaCO2, done during sleep or immediately upon awakening, and breathing the beneficiary s prescribed FIO2, shows the beneficiary's PaCO2 worsened greater than or equal to 7 mm HG compared to the original result in criterion A (above). D. A facility-based PSG or HST demonstrates oxygen saturation less than or equal to 88% for greater than or equal to 5 minutes of nocturnal recording time (minimum recording time of 2 hours) that is not caused by obstructive upper airway events i.e., AHI less than 5. (Refer to the Positive Airway Pressure Devices LCD for information about E0470 coverage for obstructive sleep apnea.) If the above criteria are not met, E0470 Bipap and related accessories will be denied as not reasonable and necessary. 14
15 An E0471 BipapASV device is covered for a beneficiary with hypoventilation syndrome if both criteria A, B, and either criterion C or D are met: A. A covered E0470 Bipap device is being used. B. Spirometry shows an FEV1/FVC greater than or equal to 70%. (Refer to SEVERE COPD (above) for information about device coverage for beneficiaries with FEV1/FVC less than 70%). C. An arterial blood gas PaCO2, done while awake, and breathing the beneficiary s prescribed FIO2, shows that the beneficiary s PaCO2 worsens greater than or equal to 7 mm HG compared to the ABG result performed to qualify the beneficiary for the E0470 device (criterion A under E0470 bipap). D. A facility-based PSG or HST demonstrates oxygen saturation less than or equal 88% for greater than or equal to 5 minutes of nocturnal recording time (minimum recording time of 2 hours) that is not caused by obstructive upper airway events i.e., AHI less than 5 while using an E0470 device. (Refer to the Positive Airway Pressure Devices LCD for information about E0470 coverage for obstructive sleep apnea.) All durable medical equipment ordered by a physician will need a face to face prior to the order and within the last 6 months that details what is required to be covered by Medicare, Medicaid, and most private insurance. An order or Rx should be written as well. The order should always have: the beneficiaries name, item, diagnosis, physician s signature, date, and NPI. The ordering provider (MD, DO, PA-C, Nurse Practitioner) must be PECOS certified. PECOS certification can be If you ever have questions simply call. I put most of the popular ones on here if there is something you want added let me know. Thanks so much!! Medics Home Health Services 1075 South Main St. Ste. 450 Madison, GA P F 15
MEDICARE Quick Reference Guide. Reimbursement Guidelines for Referral Sources
 MEDICARE Quick Reference Guide Reimbursement Guidelines for Referral Sources What s inside? Face-to-Face Rules Patient Lifts/Lift Chairs/ Commodes Support Surfaces and Beds Mobility Equipment Non-invasive
MEDICARE Quick Reference Guide Reimbursement Guidelines for Referral Sources What s inside? Face-to-Face Rules Patient Lifts/Lift Chairs/ Commodes Support Surfaces and Beds Mobility Equipment Non-invasive
RESPIRATORY ASSIST DEVICE E0470
 JURISDICTIONS B &C Bi-Level Pressure Capacity WITHOUT Backup Rate REQUIRED DOCUMENTATION All Claims for E0470 Initial Coverage (1st Three Months) 5 Element Order (5EO) obtained prior to delivery for the
JURISDICTIONS B &C Bi-Level Pressure Capacity WITHOUT Backup Rate REQUIRED DOCUMENTATION All Claims for E0470 Initial Coverage (1st Three Months) 5 Element Order (5EO) obtained prior to delivery for the
Policy Specific Section: October 1, 2010 January 21, 2013
 Medical Policy Bi-level Positive Airway Pressure (BPAP/NPPV) Type: Medical Necessity/Not Medical Necessity Policy Specific Section: Durable Medical Equipment Original Policy Date: Effective Date: October
Medical Policy Bi-level Positive Airway Pressure (BPAP/NPPV) Type: Medical Necessity/Not Medical Necessity Policy Specific Section: Durable Medical Equipment Original Policy Date: Effective Date: October
Respiratory Assist Device E0470:
 Respiratory Assist Device E0470: Bi-Level Pressure Capacity WITHOUT Backup Rate REQUIRED DOCUMENTATION IN SUPPLIER S FILE All Claims for E0470 Initial Coverage (1st Three Months) 5 Element Order obtained
Respiratory Assist Device E0470: Bi-Level Pressure Capacity WITHOUT Backup Rate REQUIRED DOCUMENTATION IN SUPPLIER S FILE All Claims for E0470 Initial Coverage (1st Three Months) 5 Element Order obtained
RESPIRATORY ASSIST DEVICE E0471
 JURISDICTIONS B &C Bi-Level Pressure Capacity WITH Backup Rate REQUIRED DOCUMENTATION All Claims for E0471 Initial Coverage (1st Three Months) 5 Element Order (5EO) obtained prior to delivery for the E0470
JURISDICTIONS B &C Bi-Level Pressure Capacity WITH Backup Rate REQUIRED DOCUMENTATION All Claims for E0471 Initial Coverage (1st Three Months) 5 Element Order (5EO) obtained prior to delivery for the E0470
RESPIRATORY ASSIST DEVICE E0471
 JURISDICTIONS B &C Bi-Level Pressure Capacity WITH Backup Rate REQUIRED DOCUMENTATION All Claims for E0471 Initial Coverage (1st Three Months) 5 Element Order (5EO) obtained prior to delivery for the E0470
JURISDICTIONS B &C Bi-Level Pressure Capacity WITH Backup Rate REQUIRED DOCUMENTATION All Claims for E0471 Initial Coverage (1st Three Months) 5 Element Order (5EO) obtained prior to delivery for the E0470
Medicare C/D Medical Coverage Policy. Respiratory Assist Devices for Obstructive Sleep Apnea and Breathing Related Sleep Disorders
 Medicare C/D Medical Coverage Policy Respiratory Assist Devices for Obstructive Sleep Apnea and Breathing Related Sleep Disorders Origination: June 26, 2000 Review Date: January 18, 2017 Next Review January,
Medicare C/D Medical Coverage Policy Respiratory Assist Devices for Obstructive Sleep Apnea and Breathing Related Sleep Disorders Origination: June 26, 2000 Review Date: January 18, 2017 Next Review January,
Helpful hints for filing
 Helpful hints for filing Respiratory Assist Devices HCPCS Code E0470 E0471 Overview The following information describes the Durable Medical Equipment Medicare Administrative Contractors' (DME MACs) medical
Helpful hints for filing Respiratory Assist Devices HCPCS Code E0470 E0471 Overview The following information describes the Durable Medical Equipment Medicare Administrative Contractors' (DME MACs) medical
Oxygen and Oxygen Equipment
 Oxygen and Oxygen Equipment I. Policy University Health Alliance (UHA) will reimburse for home oxygen and oxygen equipment when it is determined to be medically necessary and when it meets the medical
Oxygen and Oxygen Equipment I. Policy University Health Alliance (UHA) will reimburse for home oxygen and oxygen equipment when it is determined to be medically necessary and when it meets the medical
HOME OXYGEN THERAPY (AT REST) Ht: Wt: Home Oxygen with Portability X LPM Continuous Pulse Ox XX% on Room Rest Evaluate for conserving device*
 HOME OXYGEN THERAPY (AT REST) HOME OXYGEN THERAPY (DURING EXCERCISE) HOME OXYGEN THERAPY (DURING SLEEP) VENTILATOR AND SUPPLIES VENT WITH NONINVASIVE INTERFACES (VENT VS BI-LEVEL PAP) TRACHEOSTOMY CARE
HOME OXYGEN THERAPY (AT REST) HOME OXYGEN THERAPY (DURING EXCERCISE) HOME OXYGEN THERAPY (DURING SLEEP) VENTILATOR AND SUPPLIES VENT WITH NONINVASIVE INTERFACES (VENT VS BI-LEVEL PAP) TRACHEOSTOMY CARE
Oxygen and Oxygen Equipment
 Oxygen and Oxygen Equipment Policy Number: Original Effective Date: MM.01.008 12/01/2010 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 08/25/2017 Section: DME Place(s) of Service:
Oxygen and Oxygen Equipment Policy Number: Original Effective Date: MM.01.008 12/01/2010 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 08/25/2017 Section: DME Place(s) of Service:
New Government O2 Criteria and Expert Panel. Jennifer Despain, RPSGT, RST, AS
 New Government O2 Criteria and Expert Panel Jennifer Despain, RPSGT, RST, AS Lead Sleep Technologist, Central Utah Clinic Sleep Disorders Center; Provo, Utah Objectives: Review new government O2 criteria
New Government O2 Criteria and Expert Panel Jennifer Despain, RPSGT, RST, AS Lead Sleep Technologist, Central Utah Clinic Sleep Disorders Center; Provo, Utah Objectives: Review new government O2 criteria
Medicare CPAP/BIPAP Coverage Criteria
 Medicare CPAP/BIPAP Coverage Criteria For any item to be covered by Medicare, it must 1) be eligible for a defined Medicare benefit category, 2) be reasonable and necessary for the diagnosis or treatment
Medicare CPAP/BIPAP Coverage Criteria For any item to be covered by Medicare, it must 1) be eligible for a defined Medicare benefit category, 2) be reasonable and necessary for the diagnosis or treatment
Oxygen and Oxygen Equipment
 Oxygen and Oxygen Equipment Policy Number: Original Effective Date: MM.01.008 12/01/2010 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 09/01/2013 Section: DME Place(s) of Service: Home I.
Oxygen and Oxygen Equipment Policy Number: Original Effective Date: MM.01.008 12/01/2010 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 09/01/2013 Section: DME Place(s) of Service: Home I.
(To be filled by the treating physician)
 CERTIFICATE OF MEDICAL NECESSITY TO BE ISSUED TO CGHS BENEFICIAREIS BEING PRESCRIBED BILEVEL CONTINUOUS POSITIVE AIRWAY PRESSURE (BI-LEVEL CPAP) / BI-LEVEL VENTILATORY SUPPORT SYSTEM Certification Type
CERTIFICATE OF MEDICAL NECESSITY TO BE ISSUED TO CGHS BENEFICIAREIS BEING PRESCRIBED BILEVEL CONTINUOUS POSITIVE AIRWAY PRESSURE (BI-LEVEL CPAP) / BI-LEVEL VENTILATORY SUPPORT SYSTEM Certification Type
CERT Oxygen Errors: The DME CERT Outreach and Education Task Force Responds
 CERT Oxygen Errors: The DME CERT Outreach and Education Task Force Responds DME CERT Outreach and Education Task Force National Oxygen Webinar, July 22, 2014 1 Today s Presenters Michael Hanna, CERT Task
CERT Oxygen Errors: The DME CERT Outreach and Education Task Force Responds DME CERT Outreach and Education Task Force National Oxygen Webinar, July 22, 2014 1 Today s Presenters Michael Hanna, CERT Task
CPAP. The CPAP will be covered
 CPAP CPAP Did your patient have a face to face visit with the physician prior to having a sleep study that documented (1) Sleep History and symptoms and/or (2) Epworth Scale and/or (3) Physical Examination?
CPAP CPAP Did your patient have a face to face visit with the physician prior to having a sleep study that documented (1) Sleep History and symptoms and/or (2) Epworth Scale and/or (3) Physical Examination?
Clinical Policy: Oxygen Therapy in the Home Reference Number: CP.MP.485
 Clinical Policy: Reference Number: CP.MP.485 Effective Date: 09/04 Last Review Date: 09/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.485 Effective Date: 09/04 Last Review Date: 09/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Cues for Coding & Coverage
 Cues for Coding & Coverage Last Updated: September, 2010 www.medgroup.com Table of Contents Introduction 1 Testing Specifications 1 Initial Coverage Criteria 2 Upgrades 4 Continued Coverage 5 RAD Replacements
Cues for Coding & Coverage Last Updated: September, 2010 www.medgroup.com Table of Contents Introduction 1 Testing Specifications 1 Initial Coverage Criteria 2 Upgrades 4 Continued Coverage 5 RAD Replacements
Oxygen Therapy Coverage Guidelines for Non-Medicare Advantage Members
 Origination: 05/06/04 Revised: 08/02/17 Annual Review: 11/02/17 Purpose: To provide oxygen therapy guidelines for non-medicare Advantage Members for the Medical Department staff to reference when making
Origination: 05/06/04 Revised: 08/02/17 Annual Review: 11/02/17 Purpose: To provide oxygen therapy guidelines for non-medicare Advantage Members for the Medical Department staff to reference when making
CERT PAP Errors: The DME CERT Outreach and Education Task Force Responds
 CERT PAP Errors: The DME CERT Outreach and Education Task Force Responds DME CERT Outreach and Education Task Force National PAP Webinar, December 17, 2014 PAP CERT Errors Medical Records: Face-to-Face
CERT PAP Errors: The DME CERT Outreach and Education Task Force Responds DME CERT Outreach and Education Task Force National PAP Webinar, December 17, 2014 PAP CERT Errors Medical Records: Face-to-Face
Medicare Part C Medical Coverage Policy
 Nebulizer Medications Origination: June 17, 2009 Review Date: October 18, 2017 Next Review: October, 2019 Medicare Part C Medical Coverage Policy DESCRIPTION Nebulizer medications are used to prevent and
Nebulizer Medications Origination: June 17, 2009 Review Date: October 18, 2017 Next Review: October, 2019 Medicare Part C Medical Coverage Policy DESCRIPTION Nebulizer medications are used to prevent and
A Clinician s Guide To Prescribing Home Oxygen and Home Medical Equipment for the Medicare Beneficiary
 KEENE MEDICAL PRODUCTS, LLC. HOME RENTAL MEDICAL SALES KEENE MEDICAL PRODUCTS Bringing Healthcare Home Since 1975 HOME CARE IS OUR BUSINESS A Clinician s Guide To Prescribing Home Oxygen and Home Medical
KEENE MEDICAL PRODUCTS, LLC. HOME RENTAL MEDICAL SALES KEENE MEDICAL PRODUCTS Bringing Healthcare Home Since 1975 HOME CARE IS OUR BUSINESS A Clinician s Guide To Prescribing Home Oxygen and Home Medical
About VirtuOx. Was marketed exclusively by Phillips Healthcare division, Respironics for 3 years
 About VirtuOx VirtuOx, Inc. assists physicians and Durable Medical Equipment (DME)( companies diagnose respiratory diseases and qualify patients for home respiratory equipment under the guidelines of CMS
About VirtuOx VirtuOx, Inc. assists physicians and Durable Medical Equipment (DME)( companies diagnose respiratory diseases and qualify patients for home respiratory equipment under the guidelines of CMS
Itamar Medical 2016 Reimbursement Coding Guide
 Itamar Medical 2016 Reimbursement Coding Guide Continuous positive airway pressure (CPAP) and associated devices for Obstructive Sleep Apnea (OSA) DISCLAIMER: The information contained in this guide is
Itamar Medical 2016 Reimbursement Coding Guide Continuous positive airway pressure (CPAP) and associated devices for Obstructive Sleep Apnea (OSA) DISCLAIMER: The information contained in this guide is
Positive Airway Pressure and Oral Devices for the Treatment of Obstructive Sleep Apnea
 Positive Airway Pressure and Oral Devices for the Treatment of Obstructive Sleep Apnea Policy Number: Original Effective Date: MM.01.009 11/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO;
Positive Airway Pressure and Oral Devices for the Treatment of Obstructive Sleep Apnea Policy Number: Original Effective Date: MM.01.009 11/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO;
Jurisdiction B, C and D Combined Council Questions Sorted by A-Team May, 2015
 A CMS Medicare Administrative Contractor http://www.ngsmedicare.com Jurisdiction B, C and D Combined Council Questions Sorted by A-Team May, 2015 Disclaimer: This Q&A document is not an official publication
A CMS Medicare Administrative Contractor http://www.ngsmedicare.com Jurisdiction B, C and D Combined Council Questions Sorted by A-Team May, 2015 Disclaimer: This Q&A document is not an official publication
TOPIC: Continuing Coverage of CPAP Machines and Supplies for the Treatment of Obstructive Sleep Apnea
 These documents are not used to determine benefits or reimbursement. Please reference the appropriate certificate or contract for benefit information. BLUE CROSS BLUE SHIELD of MI MEDICAL POLICY Enterprise:
These documents are not used to determine benefits or reimbursement. Please reference the appropriate certificate or contract for benefit information. BLUE CROSS BLUE SHIELD of MI MEDICAL POLICY Enterprise:
MEDICAL POLICY SUBJECT: HOME AND COMMUNITY OXYGEN THERAPY
 MEDICAL POLICY PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
Medical Affairs Policy
 Medical Affairs Policy Service: Sleep Disorder Treatment: Positive Airway Pressure Devices and Oral Appliances (CPAP, BPAP, BiPAP, BiPAP ST, BiPAP with backup, BiPAP -Auto SV, VPAP, VPAP Adapt, VPAP adapt
Medical Affairs Policy Service: Sleep Disorder Treatment: Positive Airway Pressure Devices and Oral Appliances (CPAP, BPAP, BiPAP, BiPAP ST, BiPAP with backup, BiPAP -Auto SV, VPAP, VPAP Adapt, VPAP adapt
Positive Airway Pressure (PAP) Devices Physician Frequently Asked Questions December 2008
 Positive Airway Pressure (PAP) Devices Physician Frequently Asked Questions December 2008 Based on questions received from the clinical community, the following Frequently Asked Questions will address
Positive Airway Pressure (PAP) Devices Physician Frequently Asked Questions December 2008 Based on questions received from the clinical community, the following Frequently Asked Questions will address
Sleep Apnea: Diagnosis and Treatment
 Coverage Summary Sleep Apnea: Diagnosis and Treatment Policy Number: S-003 Products: UnitedHealthcare Medicare Advantage Plans Original Approval Date: 08/23/2007 Approved by: UnitedHeatlhcare Medicare
Coverage Summary Sleep Apnea: Diagnosis and Treatment Policy Number: S-003 Products: UnitedHealthcare Medicare Advantage Plans Original Approval Date: 08/23/2007 Approved by: UnitedHeatlhcare Medicare
DMEPOS: hospital beds, bed accessories, and pressurereducing
 ACTION: Final DATE: 07/02/2018 10:03 AM 5160-10-18 DMEPOS: hospital beds, bed accessories, and pressurereducing support surfaces. (A) Definitions and explanations. (1) "Group 1," "group 2," and "group
ACTION: Final DATE: 07/02/2018 10:03 AM 5160-10-18 DMEPOS: hospital beds, bed accessories, and pressurereducing support surfaces. (A) Definitions and explanations. (1) "Group 1," "group 2," and "group
RESPIRATORY EQUIPMENT AND SUPPLIES CSHCN SERVICES PROGRAM PROVIDER MANUAL
 RESPIRATORY EQUIPMENT AND SUPPLIES CSHCN SERVICES PROGRAM PROVIDER MANUAL APRIL 2018 CSHCN PROVIDER PROCEDURES MANUAL APRIL 2018 RESPIRATORY EQUIPMENT AND SUPPLIES Table of Contents 36.1 Enrollment......................................................................
RESPIRATORY EQUIPMENT AND SUPPLIES CSHCN SERVICES PROGRAM PROVIDER MANUAL APRIL 2018 CSHCN PROVIDER PROCEDURES MANUAL APRIL 2018 RESPIRATORY EQUIPMENT AND SUPPLIES Table of Contents 36.1 Enrollment......................................................................
Respiratory Equipment and Supplies
 Respiratory Equipment and Supplies Chapter.1 Enrollment..................................................................... -2.2 Benefits, Limitations, and Authorization Requirements...........................
Respiratory Equipment and Supplies Chapter.1 Enrollment..................................................................... -2.2 Benefits, Limitations, and Authorization Requirements...........................
MEDICAL POLICY SUBJECT: HOME AND COMMUNITY OXYGEN THERAPY
 MEDICAL POLICY SUBJECT: HOME AND COMMUNITY OXYGEN PAGE: 1 OF: 6 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: HOME AND COMMUNITY OXYGEN PAGE: 1 OF: 6 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
and Supplies Amended Date: November 1, 2016 Table of Contents
 Durable Medical Equipment and Supplies Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1... 1 1.2 Categories of... 1 2.0 Eligibility Requirements... 2 2.1 Provisions... 2
Durable Medical Equipment and Supplies Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1... 1 1.2 Categories of... 1 2.0 Eligibility Requirements... 2 2.1 Provisions... 2
Name of Policy: Noninvasive Positive Pressure Ventilation
 Name of Policy: Noninvasive Positive Pressure Ventilation Policy #: 203 Latest Review Date: April 2014 Category: Durable Medical Equipment Policy Grade: Effective July 31, 2013: Active Policy but no longer
Name of Policy: Noninvasive Positive Pressure Ventilation Policy #: 203 Latest Review Date: April 2014 Category: Durable Medical Equipment Policy Grade: Effective July 31, 2013: Active Policy but no longer
Positive Airway Pressure and Oral Devices for the Treatment of Obstructive Sleep Apnea
 Positive Airway Pressure and Oral Devices for the Treatment of Obstructive Sleep Apnea Policy Number: Original Effective Date: MM.01.009 11/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO
Positive Airway Pressure and Oral Devices for the Treatment of Obstructive Sleep Apnea Policy Number: Original Effective Date: MM.01.009 11/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO
CSHCN Services Program Benefits Have Changed for Some Durable Medical Equipment (DME) CSHCN Services Program Documentation of Receipt
 CSHCN Services Program Benefits Have Changed for Some Durable Medical Equipment (DME) Information posted February 6, 2009 Effective for dates of service on or after April 1, 2009, benefit criteria for
CSHCN Services Program Benefits Have Changed for Some Durable Medical Equipment (DME) Information posted February 6, 2009 Effective for dates of service on or after April 1, 2009, benefit criteria for
LCD for Oxygen and Oxygen Equipment (L27221)
 Page 1 of 16 LCD for Oxygen and Oxygen Equipment (L27221) Contractor Information Contractor Name National Government Services, Inc. Contractor Number 17003 Contractor Type DME MAC LCD Information LCD ID
Page 1 of 16 LCD for Oxygen and Oxygen Equipment (L27221) Contractor Information Contractor Name National Government Services, Inc. Contractor Number 17003 Contractor Type DME MAC LCD Information LCD ID
Pulse Oximetry Test: Summary Report
 Test Condition : Overnight on Room Air Pulse Oximetry Test: Summary Report Patient Information Referring/Ordering Physician DME/HME Courier John Sample Insurance ID : 123456789B DOB : 1/1/1909 Gender :
Test Condition : Overnight on Room Air Pulse Oximetry Test: Summary Report Patient Information Referring/Ordering Physician DME/HME Courier John Sample Insurance ID : 123456789B DOB : 1/1/1909 Gender :
CPAP. Respiratory and General DME Mission Do you Accept? /14/2016
 Respiratory and General DME Mission Do you Accept? 3-10-2016 Ronda Buhrmester, CRT O: 888-665-6518 F: 855-262-3821 ronda.buhrmester@vgm.com Twitter @RondaBuhrmester CPAP 1 2 Top Denial Reasons for PAP
Respiratory and General DME Mission Do you Accept? 3-10-2016 Ronda Buhrmester, CRT O: 888-665-6518 F: 855-262-3821 ronda.buhrmester@vgm.com Twitter @RondaBuhrmester CPAP 1 2 Top Denial Reasons for PAP
LCD for Oxygen and Oxygen Equipment (L11457)
 Page 1 of 19 LCD for Oxygen and Oxygen Equipment (L11457) Contractor Name Noridian Administrative Services Contractor Number 19003 Contractor Type DME MAC Contractor Information LCD ID Number L11457 LCD
Page 1 of 19 LCD for Oxygen and Oxygen Equipment (L11457) Contractor Name Noridian Administrative Services Contractor Number 19003 Contractor Type DME MAC Contractor Information LCD ID Number L11457 LCD
7 Element Order. elsewhere classified, Spinal stenosis, lumbar region, without neurogenic claudication. Physician signature:
 7 Element Order Medicare national and local policy specify that following completion of the face-to-face examination, the physician or treating practitioner must complete a written order containing seven
7 Element Order Medicare national and local policy specify that following completion of the face-to-face examination, the physician or treating practitioner must complete a written order containing seven
Positive Airway Pressure and Oral Devices for the Treatment of Obstructive Sleep Apnea
 Positive Airway Pressure and Oral Devices for the Treatment of Obstructive Sleep Apnea Policy Number: Original Effective Date: MM.01.009 11/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO;
Positive Airway Pressure and Oral Devices for the Treatment of Obstructive Sleep Apnea Policy Number: Original Effective Date: MM.01.009 11/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO;
Enclosed on Page 5 is an authorization form to release your health information.
 Monitor Medical, Inc. "The CPAP Co." Ph: (877) 569-9436 Fax: (888) 773-2854 www.monitormedical.com Dear Medicare Beneficiary: Thank you for selecting Monitor Medical, Inc. to provide you with all of your
Monitor Medical, Inc. "The CPAP Co." Ph: (877) 569-9436 Fax: (888) 773-2854 www.monitormedical.com Dear Medicare Beneficiary: Thank you for selecting Monitor Medical, Inc. to provide you with all of your
Airway Clearance Devices
 Print Page 1 of 11 Wisconsin.gov home state agencies subject directory department of health services Search Welcome» August 2, 2018 5:18 PM Program Name: BadgerCare Plus and Medicaid Handbook Area: Durable
Print Page 1 of 11 Wisconsin.gov home state agencies subject directory department of health services Search Welcome» August 2, 2018 5:18 PM Program Name: BadgerCare Plus and Medicaid Handbook Area: Durable
CMS Reimbursement of CPAP, Oxygen, BPAP, HMV for Sleep Disordered Breathing Peter C Gay Professor of Medicine Mayo Clinic Rochester, MN
 45th Annual New Mexico Thoracic Society Lung Disease Symposium CMS Reimbursement of CPAP, Oxygen, BPAP, HMV for Sleep Disordered Breathing Peter C Gay Professor of Medicine Mayo Clinic Rochester, MN Conflicts?
45th Annual New Mexico Thoracic Society Lung Disease Symposium CMS Reimbursement of CPAP, Oxygen, BPAP, HMV for Sleep Disordered Breathing Peter C Gay Professor of Medicine Mayo Clinic Rochester, MN Conflicts?
PORTABLE OR HOME SLEEP STUDIES FOR ADULT PATIENTS:
 Sleep Studies: Attended Polysomnography and Portable Polysomnography Tests, Multiple Sleep Latency Testing and Maintenance of Wakefulness Testing MP9132 Covered Service: Prior Authorization Required: Additional
Sleep Studies: Attended Polysomnography and Portable Polysomnography Tests, Multiple Sleep Latency Testing and Maintenance of Wakefulness Testing MP9132 Covered Service: Prior Authorization Required: Additional
Polysomnography and Sleep Studies
 Polysomnography and Sleep Studies Policy Number: Original Effective Date: MM.02.016 09/14/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 05/01/2014 Section: Medicine Place(s)
Polysomnography and Sleep Studies Policy Number: Original Effective Date: MM.02.016 09/14/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 05/01/2014 Section: Medicine Place(s)
Sleep Studies: Attended Polysomnography and Portable Polysomnography Tests, Multiple Sleep Latency Testing and Maintenance of Wakefulness Testing
 Portable Polysomnography Tests, Multiple Sleep Latency Testing and Maintenance of Wakefulness Testing MP9132 Covered Service: Yes when meets criteria below Prior Authorization Required: Yes as indicated
Portable Polysomnography Tests, Multiple Sleep Latency Testing and Maintenance of Wakefulness Testing MP9132 Covered Service: Yes when meets criteria below Prior Authorization Required: Yes as indicated
Durable Medical Equipment Providers
 August 2009 Provider Bulletin Number 974 Durable Medical Equipment Providers Vacuum Assisted Wound Closure Therapy Negative pressure wound therapy (NPWT) must be requested and supplied by an enrolled durable
August 2009 Provider Bulletin Number 974 Durable Medical Equipment Providers Vacuum Assisted Wound Closure Therapy Negative pressure wound therapy (NPWT) must be requested and supplied by an enrolled durable
Sleep & Respiratory. Qualifying Criteria Guidebook
 Sleep & Respiratory Qualifying Criteria Guidebook Sleep & Respiratory Qualifying Criteria Guidebook Respiratory Therapy Services & Equipment Respiratory Care Practitioners at HME are industry top performers
Sleep & Respiratory Qualifying Criteria Guidebook Sleep & Respiratory Qualifying Criteria Guidebook Respiratory Therapy Services & Equipment Respiratory Care Practitioners at HME are industry top performers
DECISION AND ORDER. After due notice, a telephone hearing was held on. , Medical Director, also testified as a witness for the MHP.
 STATE OF MICHIGAN MICHIGAN ADMINISTRATIVE HEARING SYSTEM FOR THE DEPARTMENT OF HEALTH AND HUMAN SERVICES P.O. Box 30763, Lansing, MI 48909 (517) 373-0722; Fax: (517) 373-4147 IN THE MATTER OF:, MAHS Docket
STATE OF MICHIGAN MICHIGAN ADMINISTRATIVE HEARING SYSTEM FOR THE DEPARTMENT OF HEALTH AND HUMAN SERVICES P.O. Box 30763, Lansing, MI 48909 (517) 373-0722; Fax: (517) 373-4147 IN THE MATTER OF:, MAHS Docket
Polysomnography (PSG) (Sleep Studies), Sleep Center
 Policy Number: 1036 Policy History Approve Date: 07/09/2015 Effective Date: 07/09/2015 Preauthorization All Plans Benefit plans vary in coverage and some plans may not provide coverage for certain service(s)
Policy Number: 1036 Policy History Approve Date: 07/09/2015 Effective Date: 07/09/2015 Preauthorization All Plans Benefit plans vary in coverage and some plans may not provide coverage for certain service(s)
CLINICAL GUIDELINES. Sleep Apnea And Treatment. Version Effective May 1, 2018
 CLINICAL GUIDELINES Sleep Apnea And Treatment Version 6.0.2018 Effective May 1, 2018 evicore healthcare Clinical Decision Support Tool Diagnostic Strategies: This tool addresses common symptoms and symptom
CLINICAL GUIDELINES Sleep Apnea And Treatment Version 6.0.2018 Effective May 1, 2018 evicore healthcare Clinical Decision Support Tool Diagnostic Strategies: This tool addresses common symptoms and symptom
OSA and COPD: What happens when the two OVERLAP?
 2011 ISRC Seminar 1 COPD OSA OSA and COPD: What happens when the two OVERLAP? Overlap Syndrome 1 OSA and COPD: What happens when the two OVERLAP? ResMed 10 JAN Global leaders in sleep and respiratory medicine
2011 ISRC Seminar 1 COPD OSA OSA and COPD: What happens when the two OVERLAP? Overlap Syndrome 1 OSA and COPD: What happens when the two OVERLAP? ResMed 10 JAN Global leaders in sleep and respiratory medicine
Coding for Sleep Disorders Jennifer Rose V. Molano, MD
 Practice Coding for Sleep Disorders Jennifer Rose V. Molano, MD Accurate coding is an important function of neurologic practice. This section of is part of an ongoing series that presents helpful coding
Practice Coding for Sleep Disorders Jennifer Rose V. Molano, MD Accurate coding is an important function of neurologic practice. This section of is part of an ongoing series that presents helpful coding
Corporate Medical Policy
 Corporate Medical Policy Noninvasive Respiratory Assist Devices File Name: Origination: Last CAP Review: Next CAP Review: Last Review: noninvasive_respiratory_assist_devices 12/2009 2/2017 2/2018 2/2017
Corporate Medical Policy Noninvasive Respiratory Assist Devices File Name: Origination: Last CAP Review: Next CAP Review: Last Review: noninvasive_respiratory_assist_devices 12/2009 2/2017 2/2018 2/2017
LCD for Positive Airway Pressure (PAP) Devices for the Treatment of Obstructive Sleep Apnea (L171)
 Page 1 of 20 LCD for Positive Airway Pressure (PAP) Devices for the Treatment of Obstructive Sleep Apnea (L171) Contractor Name Noridian Administrative Services Contractor Number 19003 Contractor Type
Page 1 of 20 LCD for Positive Airway Pressure (PAP) Devices for the Treatment of Obstructive Sleep Apnea (L171) Contractor Name Noridian Administrative Services Contractor Number 19003 Contractor Type
CLINICAL GUIDELINES. Sleep Apnea and Treatment. Version Effective February 15, 2019
 CLINICAL GUIDELINES Sleep Apnea and Treatment Version 1.0.2019 Effective February 15, 2019 evicore healthcare Clinical Decision Support Tool Diagnostic Strategies: This tool addresses common symptoms and
CLINICAL GUIDELINES Sleep Apnea and Treatment Version 1.0.2019 Effective February 15, 2019 evicore healthcare Clinical Decision Support Tool Diagnostic Strategies: This tool addresses common symptoms and
Challenging Cases in Pediatric Polysomnography. Fauziya Hassan, MBBS, MS Assistant Professor Pediatric Pulmonary and Sleep
 Challenging Cases in Pediatric Polysomnography Fauziya Hassan, MBBS, MS Assistant Professor Pediatric Pulmonary and Sleep Conflict of Interest None pertaining to this topic Will be using some slides from
Challenging Cases in Pediatric Polysomnography Fauziya Hassan, MBBS, MS Assistant Professor Pediatric Pulmonary and Sleep Conflict of Interest None pertaining to this topic Will be using some slides from
Home Pulse Oximetry for Infants and Children
 Last Review Date: April 21, 2017 Number: MG.MM.DM.12aC2v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: April 21, 2017 Number: MG.MM.DM.12aC2v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Sleep 101. Kathleen Feeney RPSGT, RST, CSE Business Development Specialist
 Sleep 101 Kathleen Feeney RPSGT, RST, CSE Business Development Specialist 2016 Why is Sleep Important More than one-third of the population has trouble sleeping (Gallup) Obstructive Sleep Apnea Untreated
Sleep 101 Kathleen Feeney RPSGT, RST, CSE Business Development Specialist 2016 Why is Sleep Important More than one-third of the population has trouble sleeping (Gallup) Obstructive Sleep Apnea Untreated
Ron Hosp, MS-HSA, RRT Regional Respiratory Specialist. This program has been approved for 1 hour of continuing education credit.
 Ron Hosp, MS-HSA, RRT Regional Respiratory Specialist This program has been approved for 1 hour of continuing education credit. Course Objectives Identify at least four goals of home NIV Identify candidates
Ron Hosp, MS-HSA, RRT Regional Respiratory Specialist This program has been approved for 1 hour of continuing education credit. Course Objectives Identify at least four goals of home NIV Identify candidates
MEDICAL POLICY. SUBJECT: POSITIVE AIRWAY PRESSURE DEVICES: CPAP, BiPAP, APAP AND NONINVASIVE POSITIVE PRESSURE VENTILATORS
 MEDICAL POLICY SUBJECT: POSITIVE AIRWAY PRESSURE DEVICES: CPAP, BiPAP, APAP AND (DELETED: 07/16/09-04/28/11) PAGE: 1 OF: 10 If a product excludes coverage for a service, it is not covered, and medical
MEDICAL POLICY SUBJECT: POSITIVE AIRWAY PRESSURE DEVICES: CPAP, BiPAP, APAP AND (DELETED: 07/16/09-04/28/11) PAGE: 1 OF: 10 If a product excludes coverage for a service, it is not covered, and medical
The Complex Rehab Technology Company. Focused on Providing Specialized Products and Related Services to People with Disabilities
 The Complex Rehab Technology Company Focused on Providing Specialized Products and Related Services to People with Disabilities The Complex Rehab Technology Company What is Complex Rehab Technology and
The Complex Rehab Technology Company Focused on Providing Specialized Products and Related Services to People with Disabilities The Complex Rehab Technology Company What is Complex Rehab Technology and
Up to 50% of continuous flow oxygen therapy patients experience clinically significant nocturnal desaturation. 1
 Up to 50% of continuous flow oxygen therapy patients experience clinically significant nocturnal desaturation. 1 Continuous Flow Oxygen Delivery & Sleep A number of theories and studies are published surrounding
Up to 50% of continuous flow oxygen therapy patients experience clinically significant nocturnal desaturation. 1 Continuous Flow Oxygen Delivery & Sleep A number of theories and studies are published surrounding
2019 COLLECTION TYPE: MIPS CLINICAL QUALITY MEASURES (CQMS) MEASURE TYPE: Process
 Quality ID #277: Sleep Apnea: Severity Assessment at Initial Diagnosis National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Management of Chronic Conditions 2019 COLLECTION
Quality ID #277: Sleep Apnea: Severity Assessment at Initial Diagnosis National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Management of Chronic Conditions 2019 COLLECTION
PEDIATRIC SLEEP GUIDELINES Version 1.0; Effective
 MedSolutions, Inc. Clinical Decision Support Tool Diagnostic Strategies This tool addresses common symptoms and symptom complexes. Requests for patients with atypical symptoms or clinical presentations
MedSolutions, Inc. Clinical Decision Support Tool Diagnostic Strategies This tool addresses common symptoms and symptom complexes. Requests for patients with atypical symptoms or clinical presentations
MEDICAL POLICY. SUBJECT: POSITIVE AIRWAY PRESSURE DEVICES: CPAP, BiPAP, APAP AND NONINVASIVE POSITIVE PRESSURE VENTILATORS
 MEDICAL POLICY SUBJECT: POSITIVE AIRWAY PRESSURE DEVICES: CPAP, BiPAP, APAP AND (DELETED: 07/16/09-04/28/11) PAGE: 1 OF: 9 If a product excludes coverage for a service, it is not covered, and medical policy
MEDICAL POLICY SUBJECT: POSITIVE AIRWAY PRESSURE DEVICES: CPAP, BiPAP, APAP AND (DELETED: 07/16/09-04/28/11) PAGE: 1 OF: 9 If a product excludes coverage for a service, it is not covered, and medical policy
Premier Health Plan considers Oral Appliances for Obstructive Sleep Apnea (OSA) medically necessary for the following indications:
 Premier Health Plan POLICY AND PROCEDURE MANUAL MP.063.PH - al Appliances for Obstructive Sleep Apnea This policy applies to the following lines of business: Premier Commercial Premier Employee Premier
Premier Health Plan POLICY AND PROCEDURE MANUAL MP.063.PH - al Appliances for Obstructive Sleep Apnea This policy applies to the following lines of business: Premier Commercial Premier Employee Premier
Enhanced. Patient Care SAMPLE
 Enhanced Patient Care Your Health is Our Priority In an effort to help facilitate an increased quality of life for you our valued customer, we have provided this simple reference guide for your convenience.
Enhanced Patient Care Your Health is Our Priority In an effort to help facilitate an increased quality of life for you our valued customer, we have provided this simple reference guide for your convenience.
(HST) (95806, G0398, G0399)
 95800 Sleep study, unattended, simultaneous recording of, heart rate, oxygen saturation, respiratory analysis (e.g. by airflow or peripheral arterial tone), and sleep time 95801 Sleep study, unattended,
95800 Sleep study, unattended, simultaneous recording of, heart rate, oxygen saturation, respiratory analysis (e.g. by airflow or peripheral arterial tone), and sleep time 95801 Sleep study, unattended,
Identification and Treatment of the Patient with Sleep Related Hypoventilation
 Identification and Treatment of the Patient with Sleep Related Hypoventilation Hillary Loomis-King, MD Pulmonary and Critical Care of NW MI Munson Sleep Disorders Center X Conflict of Interest Disclosures
Identification and Treatment of the Patient with Sleep Related Hypoventilation Hillary Loomis-King, MD Pulmonary and Critical Care of NW MI Munson Sleep Disorders Center X Conflict of Interest Disclosures
11/20/2015. Beyond CPAP. No relevant financial conflicts of interest. Kristie R Ross, M.D. November 12, Describe advanced ventilation options
 Beyond CPAP Kristie R Ross, M.D. November 12, 2015 No relevant financial conflicts of interest Sponsored by The Warren Alpert Medical School of Brown University Describe advanced ventilation options Compare
Beyond CPAP Kristie R Ross, M.D. November 12, 2015 No relevant financial conflicts of interest Sponsored by The Warren Alpert Medical School of Brown University Describe advanced ventilation options Compare
Positive Airway Pressure Systems for Sleep Disordered Breathing
 Positive Airway Pressure Systems for Sleep Disordered Breathing Lori Pickrell, RRT Account Manager Roberts Home Medical Lpickrell@robertshomemedical.com Objectives Upon completion of the session, attendees
Positive Airway Pressure Systems for Sleep Disordered Breathing Lori Pickrell, RRT Account Manager Roberts Home Medical Lpickrell@robertshomemedical.com Objectives Upon completion of the session, attendees
Referral Forms for TYVASO and REMODULIN
 Referral Forms for TYVASO and REMODULIN HOW TO GET STARTED Tyvaso and Remodulin are available only through select Specialty Pharmacy Services (SPS) providers. Follow these 5 simple steps to complete each
Referral Forms for TYVASO and REMODULIN HOW TO GET STARTED Tyvaso and Remodulin are available only through select Specialty Pharmacy Services (SPS) providers. Follow these 5 simple steps to complete each
BTS Guideline for Home Oxygen use in adults Appendix 9 (online only) Key Questions - PICO 10 December 2012
 BTS Guideline for Home Oxygen use in adults Appendix 9 (online only) Key Questions - PICO 10 December 2012 Evidence base for Home Oxygen therapy in COPD, non-copd respiratory disease and nonrespiratory
BTS Guideline for Home Oxygen use in adults Appendix 9 (online only) Key Questions - PICO 10 December 2012 Evidence base for Home Oxygen therapy in COPD, non-copd respiratory disease and nonrespiratory
Premier Health Plan considers Negative Pressure Wound Therapy (NPWT) in the home setting medically necessary for the following indications:
 Premier Health Plan POLICY AND PROCEDURE MANUAL PA.009.PH Negative Pressure Wound Therapy This policy applies to the following lines of business: Premier Commercial Premier Employee Premier Health Plan
Premier Health Plan POLICY AND PROCEDURE MANUAL PA.009.PH Negative Pressure Wound Therapy This policy applies to the following lines of business: Premier Commercial Premier Employee Premier Health Plan
Oral Appliances for Obstructive Sleep Apnea Response to Comments
 Oral Appliances for Obstructive Sleep Apnea Response to Comments November 11, 2010 1. There are no randomized, controlled crossover trials that show efficacy of any prefabricated oral appliance. As the
Oral Appliances for Obstructive Sleep Apnea Response to Comments November 11, 2010 1. There are no randomized, controlled crossover trials that show efficacy of any prefabricated oral appliance. As the
Treatment of Obstructive Sleep Apnea (OSA)
 MP9239 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria below Yes as shown below None Prevea360 Health Plan Medical Policy: 1.0 A continuous positive airway
MP9239 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria below Yes as shown below None Prevea360 Health Plan Medical Policy: 1.0 A continuous positive airway
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL Policy Number: PA.010.MH Last Review Date: 05/11/2017 Effective Date: 07/01/2017
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL Last Review Date: 05/11/2017 Effective Date: 07/01/2017 PA.010.MH Durable Medical Equipment, Corrective Appliances and This policy applies to the following
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL Last Review Date: 05/11/2017 Effective Date: 07/01/2017 PA.010.MH Durable Medical Equipment, Corrective Appliances and This policy applies to the following
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Home Oxygen Therapy (HOT) MP-069-MD-PA Medical Management Provider Notice Date: 11/15/2018; 11/01/2017 Issue Date: 12/15/2018;
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Home Oxygen Therapy (HOT) MP-069-MD-PA Medical Management Provider Notice Date: 11/15/2018; 11/01/2017 Issue Date: 12/15/2018;
OXYGEN USE IN PHYSICAL THERAPY PRACTICE. Rebecca H. Crouch, PT,DPT,MS,CCS,FAACVPR
 OXYGEN USE IN PHYSICAL THERAPY PRACTICE Rebecca H. Crouch, PT,DPT,MS,CCS,FAACVPR Supplemental Oxygen Advantages British Medical Research Council Clinical Trial Improved survival using oxygen 15 hrs/day
OXYGEN USE IN PHYSICAL THERAPY PRACTICE Rebecca H. Crouch, PT,DPT,MS,CCS,FAACVPR Supplemental Oxygen Advantages British Medical Research Council Clinical Trial Improved survival using oxygen 15 hrs/day
FAQ CODING & REIMBURSEMENT. WatchPAT TM Home Sleep Test
 FAQ CODING & REIMBURSEMENT WatchPAT TM Home Sleep Test TABLE OF CONTENTS PATIENT SELECTION CRITERIA 3 CODING & MODIFIERS 4-6 PLACE OF SERVICE 6 FREQUENCY 7 ACCREDITATION 7 SLEEP MEDICINE GLOSSARY AND ACRONYMS
FAQ CODING & REIMBURSEMENT WatchPAT TM Home Sleep Test TABLE OF CONTENTS PATIENT SELECTION CRITERIA 3 CODING & MODIFIERS 4-6 PLACE OF SERVICE 6 FREQUENCY 7 ACCREDITATION 7 SLEEP MEDICINE GLOSSARY AND ACRONYMS
InterQual Care Planning Durable Medical Equipment Criteria 2011 Clinical Revisions
 InterQual Care Planning Durable Medical Equipment Criteria 2011 Clinical Revisions The Clinical Revisions provide details of changes to InterQual Clinical Criteria. They do not provide information on changes
InterQual Care Planning Durable Medical Equipment Criteria 2011 Clinical Revisions The Clinical Revisions provide details of changes to InterQual Clinical Criteria. They do not provide information on changes
Medical Policy Original Effective Date:01/23/2019
 Page 1 of 17 Disclaimer Description Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans or the plan may have broader or more
Page 1 of 17 Disclaimer Description Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans or the plan may have broader or more
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Home Oxygen Therapy (HOT) MP-069-MD-WV Medical Management Provider Notice Date: 01/15/2018 Issue Date: 02/15/2018 Effective
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Home Oxygen Therapy (HOT) MP-069-MD-WV Medical Management Provider Notice Date: 01/15/2018 Issue Date: 02/15/2018 Effective
Jurisdictions B, C and D Councils Combined A-Team Questions Jurisdiction D Host December 2017
 1 Final to Council Chairs January 25, 2018 Jurisdictions B, C and D Councils Combined A-Team Questions Jurisdiction D Host December 2017 Enteral/Parenteral/IV Therapy 1. When a beneficiary is receiving
1 Final to Council Chairs January 25, 2018 Jurisdictions B, C and D Councils Combined A-Team Questions Jurisdiction D Host December 2017 Enteral/Parenteral/IV Therapy 1. When a beneficiary is receiving
Dental Sleep Medicine Basics
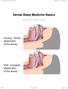 Dental Sleep Medicine Basics Written By: Patrick Tessier 2018 www.tap.wiki/ Page 1 of 8 INTRODUCTION Here are some basic aspect of Dental Sleep Medicine. This viewpoint is from an industry participant,
Dental Sleep Medicine Basics Written By: Patrick Tessier 2018 www.tap.wiki/ Page 1 of 8 INTRODUCTION Here are some basic aspect of Dental Sleep Medicine. This viewpoint is from an industry participant,
Drug Prior Authorization Form Alertec (modafinil)
 This document contains both information and form fields. To read information, use the Down Arrow from a form field. Drug Prior Authorization Form The purpose of this form is to obtain information required
This document contains both information and form fields. To read information, use the Down Arrow from a form field. Drug Prior Authorization Form The purpose of this form is to obtain information required
Glucose Monitors Policy Pearls
 Glucose Monitors Policy Pearls Length: 20:31 Date Recorded: 1.1.17 Hello and welcome to Medicare Minute MD, a video and podcast series produced by the DME MACs for the benefit of physicians and healthcare
Glucose Monitors Policy Pearls Length: 20:31 Date Recorded: 1.1.17 Hello and welcome to Medicare Minute MD, a video and podcast series produced by the DME MACs for the benefit of physicians and healthcare
Asthma Coding Fact Sheet for Primary Care Pediatricians
 01/01/2017 Asthma Coding Fact Sheet for Primary Care Pediatricians Physician Evaluation & Management Services Outpatient 99201 99202 99203 99204 99205 Office or other outpatient visit, new patient; self
01/01/2017 Asthma Coding Fact Sheet for Primary Care Pediatricians Physician Evaluation & Management Services Outpatient 99201 99202 99203 99204 99205 Office or other outpatient visit, new patient; self
5/3/2012 PRESENTATION GOALS RESPIRATORY THERAPISTS ROLE IN END OF LIFE CARE FOR THE PULMONARY PATIENT
 RESPIRATORY THERAPISTS ROLE IN END OF LIFE CARE FOR THE PULMONARY PATIENT Presented by Carrie Black Bourassa, LRT, RRT PRESENTATION GOALS Define palliative care Define hospice care Discuss pulmonary hospice
RESPIRATORY THERAPISTS ROLE IN END OF LIFE CARE FOR THE PULMONARY PATIENT Presented by Carrie Black Bourassa, LRT, RRT PRESENTATION GOALS Define palliative care Define hospice care Discuss pulmonary hospice
Triennial Pulmonary Workshop 2012
 Triennial Pulmonary Workshop 2012 Rod Richie, M.D., DBIM Medical Director Texas Life Insurance Company, Waco, TX EMSI, Waco, TX Lisa Papazian, M.D., DBIM Assistant Vice President and Medical Director Sun
Triennial Pulmonary Workshop 2012 Rod Richie, M.D., DBIM Medical Director Texas Life Insurance Company, Waco, TX EMSI, Waco, TX Lisa Papazian, M.D., DBIM Assistant Vice President and Medical Director Sun
