SMC briefing note. The following medicines were accepted for use: The following medicine has not been recommended for use: About SMC.
|
|
|
- Hugh Freeman
- 6 years ago
- Views:
Transcription
1 Monthly briefings are produced in order to help members of the media and other interested people understand the work and advice of the Scottish Medicines Consortium. The detailed advice for each medicine that we have assessed in full can be found at Number 66 May 2013 Scottish Medicines Consortium advice to NHSScotland The following medicines were accepted for use: lisdexamfetamine dimesylate (Elvanse ) mirabegron (Betmiga ) ranibizumab (Lucentis ) The following medicine has not been recommended for use: crizotinib (Xalkori ) About SMC The purpose of the Scottish Medicines Consortium (SMC) is to accept for use those newly licensed medicines that clearly represent good value for money to NHSScotland. SMC analyses information supplied by the medicine's submitting company on the health benefits of the medicine and justification of its price. Because the NHS has limited resources, SMC works to make sure that those medicines which represent good value for money are accepted for routine use as quickly as possible so that they can benefit patients. The Consortium is made up of lead clinicians, pharmacists and health economists together with representatives of health boards, the pharmaceutical industry, the public and the Scottish Government. SMC is part of Healthcare Improvement Scotland. Contact details If you are interested in the work of SMC you can visit our website at: or contact us at: Scottish Medicines Consortium Delta House (8th floor) 50 West Nile Street Glasgow G1 2NP smcsecretariat@nhshealthquality.org Media contact Members of the media should contact Stephen Ferguson on
2 SMC looks at how well medicines work in relation to how much they cost this is to make sure that they provide value for money for the NHS by providing extra benefits over current treatments at an additional cost that is reasonable. Medicines which represent good value for money are accepted for routine use to benefit patients. The following medicines were accepted for use in NHSScotland: lisdexamfetamine dimesylate (Elvanse ) What is lisdexamfetamine used for? Lisdexamfetamine is used as part of a comprehensive treatment programme for attention deficit hyperactivity disorder (ADHD) when the response to previous methylphenidate treatment has been inadequate. ADHD is one of the most commonly diagnosed behavioural disorders among children and adolescents. Symptoms of the disorder include inappropriate levels of activity and impulsiveness, with difficulty concentrating and paying attention. Lisdexamfetamine is converted in the body to an active substance called dexamfetamine and increases the activity of some chemicals in the brain. It is not yet fully understood how this helps to improve symptoms of ADHD. It is given as a capsule once a day. SMC accepted lisdexamfetamine as part of a comprehensive treatment programme for attention deficit/hyperactivity disorder in children aged 6 years of age and over when response to previous methylphenidate treatment is considered clinically inadequate. A study has shown that lisdexamfetamine was associated with a shorter time to first response in improvement in function compared with another commonly used medicine. A greater proportion of lisdexamfetamine -treated patients achieved improvements in symptom scores and functioning compared with the other medicine. An economic analysis compared lisdexamfetamine with another commonly used medicine. There were some uncertainties in the analysis but SMC was satisfied that these did not significantly influence the results. SMC accepted lisdexamfetamine for use because the balance of costs and benefits meant that it was
3 mirabegron (Betmiga ) What is mirabegron used for? Mirabegron is a treatment for the symptoms of overactive bladder syndrome, a condition in which the bladder contracts suddenly without the patient having control, and when the bladder is not full. It is a common condition and usually no cause can be found. Symptoms include increased urinary frequency (need to pass urine often), urgency (sudden urge to pass urine) and urgency incontinence (sudden lack of control resulting in urine leaking before the person can get to the toilet). Mirabegron is a new type of medicine that is thought to work by increasing the bladder s capacity to store urine. It is taken once daily as a tablet, with or without food. SMC accepted mirabegron for symptomatic treatment of urgency, increased urinary frequency and/ or urgency incontinence as may occur in adult patients with overactive bladder syndrome. Studies have shown that mirabegron improved symptoms of overactive bladder syndrome including frequency and incontinence compared with placebo (a dummy medicine containing no active treatment). An economic analysis compared mirabegron with other medicines used for the treatment of overactive bladder syndrome, some of which cost less than mirabegron. SMC accepted mirabegron for use because the balance of costs and benefits meant that it was
4 ranibizumab (Lucentis ) What is ranibizumab used for? Ranibizumab can be used to treat loss of vision caused by macular oedema, which is a build up of fluid in the area of the eye called the macula. Macular oedema can occur due to blockages in the veins of the eye. Such blockages include retinal vein occlusion (RVO); there are two types of retinal vein occlusion, branch RVO or central RVO. The type of RVO refers to where the blockage occurs in the eye. Ranibizumab helps to stop the growth and leakage of new blood vessels in the eye. It is given as a monthly injection into the eye under local anaesthetic. SMC has previously accepted ranibizumab for restricted use in patients with macular oedema secondary to central retinal vein occlusion (CRVO). In this resubmission, the submitting company requested that SMC considers the use of ranibizumab in the treatment of visual impairment due to macular oedema secondary to branch retinal vein occlusion (BRVO). SMC accepted ranibizumab for the treatment of visual impairment due to macular oedema (MO) secondary to retinal vein occlusion (RVO) (branch RVO or central RVO) in adults. This resubmission relates to branch RVO only. One study in patients with BRVO has shown that ranibizumab improved vision over 6 months compared with a dummy injection. This SMC advice takes account of the benefits of a patient access scheme (PAS). A PAS is a scheme proposed by a pharmaceutical company in order to improve the cost effectiveness of a medicine and thus enable patients to receive access to new medicines that may otherwise not have been judged to be a cost-effective use of NHS resources. The proposed PAS gives a discount on the price of the medicine. An economic analysis compared ranibizumab with another medicine given as an intravitreal implant and laser therapy. Although there were some uncertainties in the analysis, SMC was satisfied that these did not significantly influence the results. SMC accepted ranibizumab for the treatment of macular oedema secondary to BRVO because the balance of costs and benefits meant that it was considered to offer value for money when the PAS was taken into account.
5 SMC looks at how well medicines work in relation to how much they cost. Sometimes, when SMC considers the information submitted for a medicine, the committee is not convinced by the health benefit claims made by the company. On other occasions, the health benefits do not justify the cost of the medicine, or the company does not provide appropriate evidence to give SMC assurance that the medicine offers value for money. In any of these circumstances, SMC is unable to recommend that medicine for routine use in NHSScotland. SMC has not recommended the following medicines for use in NHSScotland: crizotinib (Xalkori ) What is crizotinib used for? Crizotinib is a second-line treatment for anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC). Cancer is the term given to a group of abnormal cells which have clumped together and grow to form a tumour. ALK-positive NSCLC is a type of lung cancer which is characterised by a abnormality in a particular gene. Crizotinib is a medicine that blocks the action of an abnormal protein which signals cancer cells to grow and divide, thereby slowing or stopping the spread of cancer cells. It is taken twice daily as a capsule. SMC did not recommend crizotinib for treatment of adults with previously treated ALK-positive NSCLC. A study showed that crizotinib delayed cancer progression by 4.7 months compared with standard chemotherapy. However, it was not clear if this would lead to an increase in overall survival. An economic analysis compared crizotinib with best supportive care or a commonly used medicine for the second-line treatment of patients with ALK-positive NSCLC. However, there were weaknesses in the analysis and the cost in relation to the health benefit was above the limit normally accepted by SMC. SMC did not recommend crizotinib for use because the balance of costs and benefits meant that it was not For medicines that have not been recommended by SMC, all NHS boards have procedures in place to consider individual requests when a doctor feels the medicine would be right for a particular patient. SMC has told the submitting company why the medicine was not recommended and would be pleased to receive any resubmission. For further information and to view the complete advice for the medicines listed above, visit our website at:
SMC briefing note. The following medicines were accepted for use: Contact details. About SMC. Media contact. Number 98 January 2016
 Monthly briefings are produced in order to help members of the media and other interested people understand the work and advice of the Scottish Medicines Consortium. The detailed advice for each medicine
Monthly briefings are produced in order to help members of the media and other interested people understand the work and advice of the Scottish Medicines Consortium. The detailed advice for each medicine
SMC briefing note. The following medicines were accepted for use: The following medicine has not been recommended for use: About SMC.
 Monthly briefings are produced in order to help members of the media and other interested people understand the work and advice of the Scottish Medicines Consortium. The detailed advice for each medicine
Monthly briefings are produced in order to help members of the media and other interested people understand the work and advice of the Scottish Medicines Consortium. The detailed advice for each medicine
A Guide to the Scottish Medicines Consortium
 A Guide to the Scottish Medicines Consortium Providing advice about newly licensed medicines www.scottishmedicines.org About the Scottish Medicines Consortium The Scottish Medicines Consortium (SMC) provides
A Guide to the Scottish Medicines Consortium Providing advice about newly licensed medicines www.scottishmedicines.org About the Scottish Medicines Consortium The Scottish Medicines Consortium (SMC) provides
SMC briefing note. About SMC. cetuximab (Erbitux ) Scottish Medicines Consortium advice to NHSScotland. Number 27 February 2010
 Scottish Medicines Consortium advice to NHSScotland Monthly briefings are produced in order to help members of the media and other interested groups understand the work and advice of the Scottish Medicines
Scottish Medicines Consortium advice to NHSScotland Monthly briefings are produced in order to help members of the media and other interested groups understand the work and advice of the Scottish Medicines
aflibercept 40mg/mL solution for injection (Eylea ) SMC No. (1074/15) Bayer
 aflibercept 40mg/mL solution for injection (Eylea ) SMC No. (1074/15) Bayer 07 August 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS Boards
aflibercept 40mg/mL solution for injection (Eylea ) SMC No. (1074/15) Bayer 07 August 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS Boards
 North of Tyne Area Prescribing Committee DECISION SUMMARY North of Tyne Area Prescribing Committee Summary of decisions made regarding new product requests considered at a meeting of the Committee on Tuesday
North of Tyne Area Prescribing Committee DECISION SUMMARY North of Tyne Area Prescribing Committee Summary of decisions made regarding new product requests considered at a meeting of the Committee on Tuesday
The Trust does not record electronically the number of patients who are treated by laser for a condition.
 Ref: FOI/CAD/ID 3224 06 April 20 Please reply to: FOI Administrator Trust Management Maidstone Hospital Hermitage Lane Maidstone Kent ME 9QQ Email: mtw-tr.foiadmin@nhs.net Freedom of Information Act 2000
Ref: FOI/CAD/ID 3224 06 April 20 Please reply to: FOI Administrator Trust Management Maidstone Hospital Hermitage Lane Maidstone Kent ME 9QQ Email: mtw-tr.foiadmin@nhs.net Freedom of Information Act 2000
Retinal Vein Occlusion (RVO) Treatment pathway- Northeast England. Retinal Vein Occlusion (RVO) with Macular oedema (MO)
 Retinal Vein Occlusion (RVO) Treatment pathway- Northeast England (Royal Victoria Infirmary, Sunderland Eye Infirmary, James Cook University Hospital, Darlington Memorial Hospital, University Hospital
Retinal Vein Occlusion (RVO) Treatment pathway- Northeast England (Royal Victoria Infirmary, Sunderland Eye Infirmary, James Cook University Hospital, Darlington Memorial Hospital, University Hospital
RVO RETINAL VEIN OCCLUSION
 RVO RETINAL VEIN OCCLUSION A guide to understanding RVO Take some time to learn about RVO - it may help you hold on to your vision Retinal vein occlusion is a common disorder of the retina and a leading
RVO RETINAL VEIN OCCLUSION A guide to understanding RVO Take some time to learn about RVO - it may help you hold on to your vision Retinal vein occlusion is a common disorder of the retina and a leading
Consultation on the role of the Scottish Health Council: Strengthening people s voices in health and social care
 Consultation on the role of the Scottish Health Council: Strengthening people s voices in health and social care July 2017 Contents Page Introduction 3 The Scottish Health Council 5 Our Voice 6 Why change?
Consultation on the role of the Scottish Health Council: Strengthening people s voices in health and social care July 2017 Contents Page Introduction 3 The Scottish Health Council 5 Our Voice 6 Why change?
Treatment of Retinal Vein Occlusion (RVO)
 Manchester Royal Eye Hospital Medical Retina Services Information for Patients Treatment of Retinal Vein Occlusion (RVO) What is a Retinal Vein Occlusion (RVO)? The retina is the light sensitive layer
Manchester Royal Eye Hospital Medical Retina Services Information for Patients Treatment of Retinal Vein Occlusion (RVO) What is a Retinal Vein Occlusion (RVO)? The retina is the light sensitive layer
Understanding our report and advice: Antimicrobial wound dressings (AWDs) for chronic wounds
 Understanding our report and advice: Antimicrobial wound dressings (AWDs) for chronic wounds Health technology assessment report 13 December 2015 Healthcare Improvement Scotland 2015 Published December
Understanding our report and advice: Antimicrobial wound dressings (AWDs) for chronic wounds Health technology assessment report 13 December 2015 Healthcare Improvement Scotland 2015 Published December
Updates to the Alberta Drug Benefit List. Effective August 1, 2017
 Updates to the Alberta Drug Benefit List Effective August 1, 2017 Inquiries should be directed to: Pharmacy Services Alberta Blue Cross 10009 108 Street NW Edmonton AB T5J 3C5 Telephone Number: (780) 498-8370
Updates to the Alberta Drug Benefit List Effective August 1, 2017 Inquiries should be directed to: Pharmacy Services Alberta Blue Cross 10009 108 Street NW Edmonton AB T5J 3C5 Telephone Number: (780) 498-8370
Information for Patients undergoing Intravitreal Triamcinolone Acetonide (Kenalog) Injection
 Information for Patients undergoing Intravitreal Triamcinolone Acetonide (Kenalog) Injection Kenalog/SS/ST/04.2012/v1.1 review 05.2013 Page 1 Introduction Your doctor has found that you have leakage of
Information for Patients undergoing Intravitreal Triamcinolone Acetonide (Kenalog) Injection Kenalog/SS/ST/04.2012/v1.1 review 05.2013 Page 1 Introduction Your doctor has found that you have leakage of
Patient and Public Reference Group for Medicines ADTC Sub-group NHS Tayside 17 th February 2015
 Patient and Public Reference Group for Medicines ADTC Sub-group NHS Tayside 17 th February 2015 Claire James, Senior Pharmacist Clinical Effectiveness, Ninewells Hospital Aims To increase awareness and
Patient and Public Reference Group for Medicines ADTC Sub-group NHS Tayside 17 th February 2015 Claire James, Senior Pharmacist Clinical Effectiveness, Ninewells Hospital Aims To increase awareness and
The pcodr review also provided contextual information on ALK mutation testing.
 only approximately 4% of NSCLC patients are expected to have the ALK mutation. perc further discussed these estimates in the context of the feasibility of implementing a funding recommendation for crizotinib.
only approximately 4% of NSCLC patients are expected to have the ALK mutation. perc further discussed these estimates in the context of the feasibility of implementing a funding recommendation for crizotinib.
Regulation of products for Macular Oedema EYE 2011, EMA
 Safeguarding public health Regulation of products for, EMA David Silverman, Clinical Assessor, MHRA 27 October 2011 Points to be covered Length & number of studies Trial population Endpoints Comparators
Safeguarding public health Regulation of products for, EMA David Silverman, Clinical Assessor, MHRA 27 October 2011 Points to be covered Length & number of studies Trial population Endpoints Comparators
nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd
 nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Area Drug and Therapeutics Committee Prescribing Supplement No 47 July 2011
 Area Drug and Therapeutics Committee Prescribing Supplement No 47 In this issue Drugs reviewed by in May / June 2011 ADTC UPDATES ON DRUGS REVIEWED BY THE SMC The following new drugs have been reviewed
Area Drug and Therapeutics Committee Prescribing Supplement No 47 In this issue Drugs reviewed by in May / June 2011 ADTC UPDATES ON DRUGS REVIEWED BY THE SMC The following new drugs have been reviewed
Technology appraisal guidance Published: 24 January 2018 nice.org.uk/guidance/ta500
 Ceritinib for untreated ALK-positive non- small-cell lung cancer Technology appraisal guidance Published: 24 January 2018 nice.org.uk/guidance/ta500 NICE 2018. All rights reserved. Subject to Notice of
Ceritinib for untreated ALK-positive non- small-cell lung cancer Technology appraisal guidance Published: 24 January 2018 nice.org.uk/guidance/ta500 NICE 2018. All rights reserved. Subject to Notice of
EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO
 EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO Yehia Hashad, M.D. Vice President and Global Therapeutic Area Head
EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO Yehia Hashad, M.D. Vice President and Global Therapeutic Area Head
pan-canadian Oncology Drug Review Final Economic Guidance Report Crizotinib (Xalkori) Resubmission for Advanced Non-Small Cell Lung Cancer
 pan-canadian Oncology Drug Review Final Economic Guidance Report Crizotinib (Xalkori) Resubmission for Advanced Non-Small Cell Lung Cancer May 2, 2013 DISCLAIMER Not a Substitute for Professional Advice
pan-canadian Oncology Drug Review Final Economic Guidance Report Crizotinib (Xalkori) Resubmission for Advanced Non-Small Cell Lung Cancer May 2, 2013 DISCLAIMER Not a Substitute for Professional Advice
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Health Technology Appraisal. Aflibercept for treating diabetic macular oedema.
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health Technology Appraisal Aflibercept for treating diabetic macular oedema Final scope Final remit/appraisal objective To appraise the clinical and cost
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health Technology Appraisal Aflibercept for treating diabetic macular oedema Final scope Final remit/appraisal objective To appraise the clinical and cost
29 August 2016 Page 1 of 7. How does the NHS board decide which new medicines to make available for patients?
 NHS Greater Glasgow and Clyde: New Medicines Decisions In Scotland, a newly licensed medicine is routinely available for use in an NHS board only after it has been: accepted for use in the NHSScotland
NHS Greater Glasgow and Clyde: New Medicines Decisions In Scotland, a newly licensed medicine is routinely available for use in an NHS board only after it has been: accepted for use in the NHSScotland
Latest Press Release. sbn network jimmy swaggart comcast
 corp@stantec.com Latest Press Release sbn network jimmy swaggart comcast S i have only been taking optiprostate xts for 8 days and i also take tamsulosin with it. i was taking myrbetriq but it is not all
corp@stantec.com Latest Press Release sbn network jimmy swaggart comcast S i have only been taking optiprostate xts for 8 days and i also take tamsulosin with it. i was taking myrbetriq but it is not all
Overactive bladder. Information for patients from Urogynaecology
 Overactive bladder Information for patients from Urogynaecology An overactive bladder (OAB) is a very common problem. It can cause distressing symptoms that are difficult to control. These can include
Overactive bladder Information for patients from Urogynaecology An overactive bladder (OAB) is a very common problem. It can cause distressing symptoms that are difficult to control. These can include
Medicines in Scotland: What s the right treatment for me? Information for patients and the public
 Medicines in Scotland: What s the right treatment for me? Information for patients and the public You can read and download this document from our website. We are happy to consider requests for other languages
Medicines in Scotland: What s the right treatment for me? Information for patients and the public You can read and download this document from our website. We are happy to consider requests for other languages
Sequential pharmacological therapies in the management of macular oedema secondary to retinal vein occlusion
 Northern (NHS) Treatment Advisory Group Sequential pharmacological therapies in the management of macular oedema secondary to retinal vein occlusion Author: Paul Madill Specialty Registrar in Public Health
Northern (NHS) Treatment Advisory Group Sequential pharmacological therapies in the management of macular oedema secondary to retinal vein occlusion Author: Paul Madill Specialty Registrar in Public Health
Cardiff & Vale (C&V) UHB Corporate Medicines Management Group (c MMG) SHARED CARE DRUG: LISDEXAMFETAMINE PROTOCOL NUMBER: CV 57
 Cardiff & Vale (C&V) UHB Corporate Medicines Management Group (c MMG) SHARED CARE DRUG: LISDEXAMFETAMINE PROTOCOL NUMBER: CV 57 INDICATION: Attention deficit hyperactivity disorder (ADHD) as part of a
Cardiff & Vale (C&V) UHB Corporate Medicines Management Group (c MMG) SHARED CARE DRUG: LISDEXAMFETAMINE PROTOCOL NUMBER: CV 57 INDICATION: Attention deficit hyperactivity disorder (ADHD) as part of a
Medicine Condition being treated NHSGGC Decision Date of decision. 18 June 2018 Page 1 of 5
 NHS Greater Glasgow and Clyde: New Medicines Decisions In Scotland, a newly licensed medicine is routinely available in a health board only after it has been: accepted for use in by the Scottish Medicines
NHS Greater Glasgow and Clyde: New Medicines Decisions In Scotland, a newly licensed medicine is routinely available in a health board only after it has been: accepted for use in by the Scottish Medicines
Summary of Results for Laypersons
 What was the Study Called? Summary of Results for Laypersons A Randomized, Double-blind, Parallel-group, Placebo- and Active-controlled, Multicenter Study to Evaluate the Efficacy, Safety and Tolerability
What was the Study Called? Summary of Results for Laypersons A Randomized, Double-blind, Parallel-group, Placebo- and Active-controlled, Multicenter Study to Evaluate the Efficacy, Safety and Tolerability
Overactive bladder syndrome (OAB)
 Service: Urology Overactive bladder syndrome (OAB) Exceptional healthcare, personally delivered What is OAB? An overactive bladder or OAB is where a person regularly gets a sudden and compelling need or
Service: Urology Overactive bladder syndrome (OAB) Exceptional healthcare, personally delivered What is OAB? An overactive bladder or OAB is where a person regularly gets a sudden and compelling need or
Issue date September 2010 (Reviewed October 2013) Clinicians from Andrew Lang Centre, Mental. Specialist Pharmacist & Formulary Pharmacist
 Title Document Type Issue no Shared care guidelines in the Treatment of Attention Deficit/ Hyperactivity Disorders Shared Care Guidelines and Information for GPs Clinical Governance Support Team Use Issue
Title Document Type Issue no Shared care guidelines in the Treatment of Attention Deficit/ Hyperactivity Disorders Shared Care Guidelines and Information for GPs Clinical Governance Support Team Use Issue
Overactive bladder (OAB) affects approximately 15% of the adult population. Diagnosis is based
 Overactive bladder (OAB) affects approximately 15% of the adult population. Diagnosis is based upon a medical history, and includes a focused physical exam (abdominal, neurological, pelvic in females and
Overactive bladder (OAB) affects approximately 15% of the adult population. Diagnosis is based upon a medical history, and includes a focused physical exam (abdominal, neurological, pelvic in females and
What you can expect with OZURDEX
 Important Information About Macular Edema Following Branch or Central Retinal Vein Occlusion (RVO) and Treatment For patients with RVO What you can expect with OZURDEX Approved Use OZURDEX (dexamethasone
Important Information About Macular Edema Following Branch or Central Retinal Vein Occlusion (RVO) and Treatment For patients with RVO What you can expect with OZURDEX Approved Use OZURDEX (dexamethasone
Technology appraisal guidance Published: 21 December 2016 nice.org.uk/guidance/ta422
 Crizotinib for previously treated anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer Technology appraisal guidance Published: 21 December 2016 nice.org.uk/guidance/ta422 NICE 2018.
Crizotinib for previously treated anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer Technology appraisal guidance Published: 21 December 2016 nice.org.uk/guidance/ta422 NICE 2018.
Laser vaporisation of prostate (Green light laser prostate surgery): procedure-specific information
 PATIENT INFORMATION Laser vaporisation of prostate (Green light laser prostate surgery): procedure-specific information What is the evidence base for this information? This leaflet includes advice from
PATIENT INFORMATION Laser vaporisation of prostate (Green light laser prostate surgery): procedure-specific information What is the evidence base for this information? This leaflet includes advice from
liposomal cytarabine suspension (DepoCyte ) is not recommended for use within NHS Scotland for the intrathecal treatment of lymphomatous meningitis.
 Scottish Medicines Consortium Re-Submission liposomal cytarabine 50mg suspension for injection (DepoCyte) No. (164/05) Napp Pharmaceuticals 6 July 2007 The Scottish Medicines Consortium (SMC) has completed
Scottish Medicines Consortium Re-Submission liposomal cytarabine 50mg suspension for injection (DepoCyte) No. (164/05) Napp Pharmaceuticals 6 July 2007 The Scottish Medicines Consortium (SMC) has completed
Technology appraisal guidance Published: 22 May 2013 nice.org.uk/guidance/ta283
 Ranibizumab for treating visual impairment caused by macular oedema secondary to retinal vein occlusion Technology appraisal guidance Published: 22 May 2013 nice.org.uk/guidance/ta283 NICE 2018. All rights
Ranibizumab for treating visual impairment caused by macular oedema secondary to retinal vein occlusion Technology appraisal guidance Published: 22 May 2013 nice.org.uk/guidance/ta283 NICE 2018. All rights
Understanding our advice ~ December The use of troponin testing in acute coronary syndromes
 Understanding our advice ~ December 2003 The use of troponin testing in acute coronary syndromes The use of troponin testing in acute coronary syndromes Purpose of this document NHS Quality Improvement
Understanding our advice ~ December 2003 The use of troponin testing in acute coronary syndromes The use of troponin testing in acute coronary syndromes Purpose of this document NHS Quality Improvement
eflornithine 11.5% cream (Vaniqa ) No. (159/05) Shire Pharmaceutical Contracts Ltd
 Scottish Medicines Consortium Re-Submission eflornithine 11.5% cream (Vaniqa ) No. (159/05) Shire Pharmaceutical Contracts Ltd 5th August 2005 The Scottish Medicines Consortium (SMC) has completed its
Scottish Medicines Consortium Re-Submission eflornithine 11.5% cream (Vaniqa ) No. (159/05) Shire Pharmaceutical Contracts Ltd 5th August 2005 The Scottish Medicines Consortium (SMC) has completed its
Scottish Medicines Consortium
 Scottish Medicines Consortium atomoxetine capsules 10 mg to 60 mg (Strattera ) (153/05) Eli Lilly and Company Ltd No. 4 February 2005 The Scottish Medicines Consortium has completed its assessment of the
Scottish Medicines Consortium atomoxetine capsules 10 mg to 60 mg (Strattera ) (153/05) Eli Lilly and Company Ltd No. 4 February 2005 The Scottish Medicines Consortium has completed its assessment of the
Area Drug and Therapeutics Committee Prescribing Supplement No 6 December 2004
 Area Drug and Therapeutics Committee Prescribing Supplement No 6 In this issue Drugs currently being considered by the SMC Advice due on 13. Joint Formulary Update. Drugs currently being considered by
Area Drug and Therapeutics Committee Prescribing Supplement No 6 In this issue Drugs currently being considered by the SMC Advice due on 13. Joint Formulary Update. Drugs currently being considered by
Adult Neurodevelopmental Services. ADHD Shared Protocol
 Adult Neurodevelopmental Services ADHD Shared Protocol Issue 1: April 2016 1 2 Adult Neurodevelopmental Service Shared Care Protocol for Adult Attention Deficit Hyperactivity Disorder (ADHD) 1. BACKGROUND
Adult Neurodevelopmental Services ADHD Shared Protocol Issue 1: April 2016 1 2 Adult Neurodevelopmental Service Shared Care Protocol for Adult Attention Deficit Hyperactivity Disorder (ADHD) 1. BACKGROUND
Bladder neck incision: procedure-specific information
 PATIENT INFORMATION Bladder neck incision: procedure-specific information What is the evidence base for this information? This leaflet includes advice from consensus panels, the British Association of
PATIENT INFORMATION Bladder neck incision: procedure-specific information What is the evidence base for this information? This leaflet includes advice from consensus panels, the British Association of
2017/2018 ADHD Guidelines: A Summary of Recommendations for Pharmacological Treatment From Selected Guidelines
 2017/2018 ADHD Guidelines: A Summary of Recommendations for Pharmacological Treatment From Selected Guidelines Supporting patients throughout their lives Date of preparation: December 2018 Job code: C-ANPROM/INT//4324
2017/2018 ADHD Guidelines: A Summary of Recommendations for Pharmacological Treatment From Selected Guidelines Supporting patients throughout their lives Date of preparation: December 2018 Job code: C-ANPROM/INT//4324
CDEC FINAL RECOMMENDATION
 CDEC FINAL RECOMMENDATION RANIBIZUMAB (Lucentis Novartis Pharmaceuticals Canada Inc.) New Indication: Macular Edema Secondary to Retinal Vein Occlusion Recommendation: The Canadian Drug Expert Committee
CDEC FINAL RECOMMENDATION RANIBIZUMAB (Lucentis Novartis Pharmaceuticals Canada Inc.) New Indication: Macular Edema Secondary to Retinal Vein Occlusion Recommendation: The Canadian Drug Expert Committee
Technology appraisal guidance Published: 27 July 2011 nice.org.uk/guidance/ta229
 Dexamethasone intravitreal implant for the treatment of macular oedema secondary to retinal vein occlusion Technology appraisal guidance Published: 27 July 2011 nice.org.uk/guidance/ta229 NICE 2018. All
Dexamethasone intravitreal implant for the treatment of macular oedema secondary to retinal vein occlusion Technology appraisal guidance Published: 27 July 2011 nice.org.uk/guidance/ta229 NICE 2018. All
Area Drug and Therapeutics Committee Prescribing Supplement No 3 November 2003
 Area Drug and Therapeutics Committee Prescribing Supplement No 3 In this issue Drugs currently being considered by the SMC advice due on 8 December 2003. SMC Notice of meeting: Patient power Making a Difference,
Area Drug and Therapeutics Committee Prescribing Supplement No 3 In this issue Drugs currently being considered by the SMC advice due on 8 December 2003. SMC Notice of meeting: Patient power Making a Difference,
Intravesical Botox Injections
 Intravesical Botox Injections Department of Urology Patient Information What What is is Botox? Botox? Botox or Botulinum Type-A is toxin produced by bacteria called Clostridium Botulinum. It is given intravesically
Intravesical Botox Injections Department of Urology Patient Information What What is is Botox? Botox? Botox or Botulinum Type-A is toxin produced by bacteria called Clostridium Botulinum. It is given intravesically
Access to newly licensed medicines. Scottish Medicines Consortium
 Access to newly licensed medicines Scottish Medicines Consortium Modifiers The Committee has previously been provided with information about why the SMC uses modifiers in its appraisal process and also
Access to newly licensed medicines Scottish Medicines Consortium Modifiers The Committee has previously been provided with information about why the SMC uses modifiers in its appraisal process and also
Common Drug Review Patient Group Input Submissions
 Common Drug Review Patient Group Input Submissions Aflibercept (Eylea) for Age-related Macular Degeneration Patient group input submissions were received from the following patient groups. Those with permission
Common Drug Review Patient Group Input Submissions Aflibercept (Eylea) for Age-related Macular Degeneration Patient group input submissions were received from the following patient groups. Those with permission
Patient information Eylea treatment for diabetic macular oedema (DMO)
 Patient information Eylea treatment for diabetic macular oedema (DMO) Introduction The doctor has found that you have swelling affecting the centre of the retina at the back of your eye. This is known
Patient information Eylea treatment for diabetic macular oedema (DMO) Introduction The doctor has found that you have swelling affecting the centre of the retina at the back of your eye. This is known
Uroformation. Instillation of Botox. The Uroformation series is a co-operative venture in patient centered urological information.
 Uroformation Instillation of Botox The Uroformation series is a co-operative venture in patient centered urological information. June 2014 What is Botox? Botox or botulinum toxin, is a purified toxin
Uroformation Instillation of Botox The Uroformation series is a co-operative venture in patient centered urological information. June 2014 What is Botox? Botox or botulinum toxin, is a purified toxin
How is the introduction of a new medicine regulated in the UK?
 Information provided to Duchenne muscular dystrophy patient organisations regarding Raxone (idebenone) and the Early Access to Medicines Scheme in the UK (EAMS 46555/0001) A medicine called Raxone, which
Information provided to Duchenne muscular dystrophy patient organisations regarding Raxone (idebenone) and the Early Access to Medicines Scheme in the UK (EAMS 46555/0001) A medicine called Raxone, which
Treatment of wet macular degeneration by intravitreal injection with Ranibizumab (Lucentis) or Aflibercept (Eylea): Information and consent
 Patient information Treatment of wet macular degeneration by intravitreal injection with Ranibizumab (Lucentis) or Aflibercept (Eylea): Information and consent Introduction You have an eye condition called
Patient information Treatment of wet macular degeneration by intravitreal injection with Ranibizumab (Lucentis) or Aflibercept (Eylea): Information and consent Introduction You have an eye condition called
Scottish Medicines Consortium
 Scottish Medicines Consortium zoledronic acid 5mg/100ml solution for infusion (Aclasta) No. (317/06) Novartis 8 September 2006 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Scottish Medicines Consortium zoledronic acid 5mg/100ml solution for infusion (Aclasta) No. (317/06) Novartis 8 September 2006 The Scottish Medicines Consortium (SMC) has completed its assessment of the
METHYLPHENIDATE AND ATOMOXETINE TREATMENT OF ATTENTION DEFICIT HYPERACTIVITY DISORDER IN CHILDREN AND YOUNG PEOPLE
 NOTTINGHAMSHIRE AREA PRESCRIBING COMMITTEE SHARED CARE PROTOCOL AGREEMENT METHYLPHENIDATE AND ATOMOXETINE TREATMENT OF ATTENTION DEFICIT HYPERACTIVITY DISORDER IN CHILDREN AND YOUNG PEOPLE OBJECTIVES To
NOTTINGHAMSHIRE AREA PRESCRIBING COMMITTEE SHARED CARE PROTOCOL AGREEMENT METHYLPHENIDATE AND ATOMOXETINE TREATMENT OF ATTENTION DEFICIT HYPERACTIVITY DISORDER IN CHILDREN AND YOUNG PEOPLE OBJECTIVES To
Promoting Continence with Physiotherapy
 A Common problem for Men and women Promoting Continence with Physiotherapy This leaflet contains information about physiotherapy advice and treatment for anyone with bladder and bowel problems. This may
A Common problem for Men and women Promoting Continence with Physiotherapy This leaflet contains information about physiotherapy advice and treatment for anyone with bladder and bowel problems. This may
Rising to the Challenge of Satisfying Unmet Medical Needs. Back-of-the-eye. Main Back-of-the-Eye Diseases. Uveitis. Behcet s disease.
 Feature Rising to the Challenge of Satisfying Unmet Medical Needs Eyeing Further Specialization Back-of-the-eye Diseases Main Back-of-the-Eye Diseases Age-related macular degeneration Uveitis Behcet s
Feature Rising to the Challenge of Satisfying Unmet Medical Needs Eyeing Further Specialization Back-of-the-eye Diseases Main Back-of-the-Eye Diseases Age-related macular degeneration Uveitis Behcet s
ADHD. Medicines to help symptoms of adult attention deficit hyperactivity disorder (ADHD)
 ADHD Medicines to help symptoms of adult attention deficit hyperactivity disorder (ADHD) This guide can help you to make a more informed choice about which medication may be best for you if you have been
ADHD Medicines to help symptoms of adult attention deficit hyperactivity disorder (ADHD) This guide can help you to make a more informed choice about which medication may be best for you if you have been
Diabetic Eye Disease
 Manchester Royal Eye Hospital Medical Retinal Services Information for Patients Diabetic Eye Disease This leaflet sets out to answer some of your questions about diabetic eye disease. You may wish to discuss
Manchester Royal Eye Hospital Medical Retinal Services Information for Patients Diabetic Eye Disease This leaflet sets out to answer some of your questions about diabetic eye disease. You may wish to discuss
World Sight Day Case Studies. Mark Frost Screening Manager South East London DESP
 World Sight Day 2015 Case Studies Mark Frost Screening Manager South East London DESP Introduction All of the following cases have been identified in our screening programme over the last 3 years. The
World Sight Day 2015 Case Studies Mark Frost Screening Manager South East London DESP Introduction All of the following cases have been identified in our screening programme over the last 3 years. The
Introduction How the eye works
 1 Introduction Diabetic retinopathy is a condition that can cause permanent loss of eyesight and even blindness. It is a major cause of loss of vision. But if a person with diabetes receives proper eye
1 Introduction Diabetic retinopathy is a condition that can cause permanent loss of eyesight and even blindness. It is a major cause of loss of vision. But if a person with diabetes receives proper eye
ADTC UPDATES ON DRUGS REVIEWED BY THE SMC. The following new drugs have been reviewed by the Scottish Medicines Consortium in July 2013: -
 Area Drug and Therapeutics Committee Prescribing Supplement No 72 In this issue Drugs reviewed by the SMC in July 2013 ADTC UPDATES ON DRUGS REVIEWED BY THE SMC The following new drugs have been reviewed
Area Drug and Therapeutics Committee Prescribing Supplement No 72 In this issue Drugs reviewed by the SMC in July 2013 ADTC UPDATES ON DRUGS REVIEWED BY THE SMC The following new drugs have been reviewed
roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd
 roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd 06 August 2010 (Issued 10 September 2010) The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd 06 August 2010 (Issued 10 September 2010) The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
04 September 2017 Page 1 of 6
 NHS Greater Glasgow and Clyde: New Medicines Decisions In Scotland, a newly licensed medicine is routinely available in a health board only after it has been: accepted for use in by the Scottish Medicines
NHS Greater Glasgow and Clyde: New Medicines Decisions In Scotland, a newly licensed medicine is routinely available in a health board only after it has been: accepted for use in by the Scottish Medicines
Process for End of Life and Very Rare Conditions (orphan and ultra-orphan medicines)
 PACE (Patient & Clinician Engagement) Overview Document Process for End of Life and Very Rare Conditions (orphan and ultra-orphan medicines) Introduction The Scottish Medicines Consortium (SMC) has changed
PACE (Patient & Clinician Engagement) Overview Document Process for End of Life and Very Rare Conditions (orphan and ultra-orphan medicines) Introduction The Scottish Medicines Consortium (SMC) has changed
Resubmission. Scottish Medicines Consortium
 Scottish Medicines Consortium Resubmission aripiprazole 5mg, 10mg, 15mg, 0mg tablets; 10mg, 15mg orodispersible tablets; 1mg/mL oral solution (Abilify ) No. (498/08) Bristol-Myers Squibb Pharmaceuticals
Scottish Medicines Consortium Resubmission aripiprazole 5mg, 10mg, 15mg, 0mg tablets; 10mg, 15mg orodispersible tablets; 1mg/mL oral solution (Abilify ) No. (498/08) Bristol-Myers Squibb Pharmaceuticals
Scottish Medicines Consortium
 Scottish Medicines Consortium ketoprofen/, 100mg/20mg; 200mg/20mg modified release capsules (Axorid ) No. (606/10) Meda Pharmaceuticals 05 February 2010 The Scottish Medicines Consortium (SMC) has completed
Scottish Medicines Consortium ketoprofen/, 100mg/20mg; 200mg/20mg modified release capsules (Axorid ) No. (606/10) Meda Pharmaceuticals 05 February 2010 The Scottish Medicines Consortium (SMC) has completed
The Common Clinical Competency Framework for Non-medical Ophthalmic Healthcare Professionals in Secondary Care
 The Common Clinical Competency Framework for Non-medical Ophthalmic Healthcare Professionals in Secondary Care Medical Retina November 2016 Association of Health Professions in Ophthalmology General basic
The Common Clinical Competency Framework for Non-medical Ophthalmic Healthcare Professionals in Secondary Care Medical Retina November 2016 Association of Health Professions in Ophthalmology General basic
From Outdated to Updated: A Review of Important Clinical Trials in Ocular Disease from 2014
 From Outdated to Updated: A Review of Important Clinical Trials in Ocular Disease from 2014 1. This course is designed to review the important ophthalmic literature that was released between October 2013
From Outdated to Updated: A Review of Important Clinical Trials in Ocular Disease from 2014 1. This course is designed to review the important ophthalmic literature that was released between October 2013
Ophthalmic VEGF Inhibitors. Eylea (aflibercept), Macugen (pegaptanib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Ophthalmic VEGF Inhibitors Page: 1 of 5 Last Review Date: September 20, 2018 Ophthalmic VEGF Inhibitors
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Ophthalmic VEGF Inhibitors Page: 1 of 5 Last Review Date: September 20, 2018 Ophthalmic VEGF Inhibitors
An Update on Branch Retinal Vein Occlusion Treatment Studies. Amiee Ho, O.D. Pacific University College of Optometry
 An Update on Branch Retinal Vein Occlusion Treatment Studies Amiee Ho, O.D. Pacific University College of Optometry Course Description This course focuses on current treatment options available for macular
An Update on Branch Retinal Vein Occlusion Treatment Studies Amiee Ho, O.D. Pacific University College of Optometry Course Description This course focuses on current treatment options available for macular
OCCLUSIVE VASCULAR DISORDERS OF THE RETINA
 OCCLUSIVE VASCULAR DISORDERS OF THE RETINA Learning outcomes By the end of this lecture the students would be able to Classify occlusive vascular disorders (OVD) of the retina. Correlate the clinical features
OCCLUSIVE VASCULAR DISORDERS OF THE RETINA Learning outcomes By the end of this lecture the students would be able to Classify occlusive vascular disorders (OVD) of the retina. Correlate the clinical features
Scottish Medicines Consortium
 Scottish Medicines Consortium rituximab 10mg/ml concentrate for infusion (MabThera ) Roche (No.330/06) 10 November 2006 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
Scottish Medicines Consortium rituximab 10mg/ml concentrate for infusion (MabThera ) Roche (No.330/06) 10 November 2006 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
USE OF UNLICENSED MEDICINES AND OFF-LABEL MEDICINES WHERE A LICENSED MEDICINE IS AVAILABLE
 NHS Scotland Directors of Pharmacy and Scottish Association of Medical Directors USE OF UNLICENSED MEDICINES AND OFF-LABEL MEDICINES WHERE A LICENSED MEDICINE IS AVAILABLE CONSENSUS STATEMENT This consensus
NHS Scotland Directors of Pharmacy and Scottish Association of Medical Directors USE OF UNLICENSED MEDICINES AND OFF-LABEL MEDICINES WHERE A LICENSED MEDICINE IS AVAILABLE CONSENSUS STATEMENT This consensus
Using Physiotherapy to Manage Urinary Incontinence in Women
 Using Physiotherapy to Manage Urinary Incontinence in Women Bladder control problems are common, and affect people of all ages, genders and backgrounds. These problems are referred to as urinary incontinence
Using Physiotherapy to Manage Urinary Incontinence in Women Bladder control problems are common, and affect people of all ages, genders and backgrounds. These problems are referred to as urinary incontinence
17 January 2018 Mary Maclean 50 West Nile Street Glasgow G1 2NP
 17 January 2018 Mary Maclean mary.maclean4@nhs.net 50 West Nile Street Glasgow G1 2NP Dear Colleague Abiraterone in newly diagnosed hormone naive prostate cancer Abiraterone is currently licensed and accepted
17 January 2018 Mary Maclean mary.maclean4@nhs.net 50 West Nile Street Glasgow G1 2NP Dear Colleague Abiraterone in newly diagnosed hormone naive prostate cancer Abiraterone is currently licensed and accepted
Percutaneous Tibial Nerve Stimulation for Overactive Bladder Symptoms. Patient Information Leaflet
 Percutaneous Tibial Nerve Stimulation for Overactive Bladder Symptoms Patient Information Leaflet About this leaflet The information provided in this leaflet should be used as a guide. There may be some
Percutaneous Tibial Nerve Stimulation for Overactive Bladder Symptoms Patient Information Leaflet About this leaflet The information provided in this leaflet should be used as a guide. There may be some
Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta395
 Ceritinib for previously treated anaplastic lymphoma kinase positive non- small-cell lung cancer Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta5 NICE 2018. All rights reserved.
Ceritinib for previously treated anaplastic lymphoma kinase positive non- small-cell lung cancer Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta5 NICE 2018. All rights reserved.
Eye injection treatment costs and rebates
 Eye injection treatment costs and rebates This fact sheet provides general information on the bill you receive from your ophthalmologist for eye injections for the management of wet macular degeneration,
Eye injection treatment costs and rebates This fact sheet provides general information on the bill you receive from your ophthalmologist for eye injections for the management of wet macular degeneration,
diclofenac, 75mg/2ml of solution for intravenous injection (Dyloject ) No. (446/08) Javelin Pharmaceuticals UK Ltd
 Scottish Medicines Consortium diclofenac, 75mg/2ml of solution for intravenous injection (Dyloject ) No. (446/08) Javelin Pharmaceuticals UK Ltd 11 February 2008 The Scottish Medicines Consortium has completed
Scottish Medicines Consortium diclofenac, 75mg/2ml of solution for intravenous injection (Dyloject ) No. (446/08) Javelin Pharmaceuticals UK Ltd 11 February 2008 The Scottish Medicines Consortium has completed
The use of B-type natriuretic peptides in the investigation of patients with suspected heart failure
 Understanding our advice ~ May 2005 The use of B-type natriuretic peptides in the investigation of patients with suspected heart failure The use of B-type natriuretic peptides in the investigation of patients
Understanding our advice ~ May 2005 The use of B-type natriuretic peptides in the investigation of patients with suspected heart failure The use of B-type natriuretic peptides in the investigation of patients
Patient Group Submission Form
 Patient Group Submission Form The Scottish Medicines Consortium (SMC) is committed to working in partnership with patient groups to capture patient and carer experiences, and use them to inform decision-making.
Patient Group Submission Form The Scottish Medicines Consortium (SMC) is committed to working in partnership with patient groups to capture patient and carer experiences, and use them to inform decision-making.
Area Drug and Therapeutics Committee Prescribing
 Area Drug and Therapeutics Committee Prescribing Supplement No 103 In this issue Drugs reviewed by the SMC in March 2016 ADTC UPDATES ON DRUGS REVIEWED BY THE SMC The following new drugs have been reviewed
Area Drug and Therapeutics Committee Prescribing Supplement No 103 In this issue Drugs reviewed by the SMC in March 2016 ADTC UPDATES ON DRUGS REVIEWED BY THE SMC The following new drugs have been reviewed
Information for patients
 Information for patients Intravitreal Anti-VEGF Treatment (vascular endothelial growth factor) This leaflet gives you information that will help you decide whether to have intravitreal treatment. It also
Information for patients Intravitreal Anti-VEGF Treatment (vascular endothelial growth factor) This leaflet gives you information that will help you decide whether to have intravitreal treatment. It also
SHARED CARE PRESCRIBING GUIDELINE
 Approval date: September 2018. Document review date: September 2021 or sooner if evidence/practice changes g SHARED CARE PRESCRIBING GUIDELINE Methylphenidate, atomoxetine, lisdexamfetamine, dexamfetamine
Approval date: September 2018. Document review date: September 2021 or sooner if evidence/practice changes g SHARED CARE PRESCRIBING GUIDELINE Methylphenidate, atomoxetine, lisdexamfetamine, dexamfetamine
Area Drug and Therapeutics Committee Prescribing Supplement No 55 March 2012
 Area Drug and Therapeutics Committee Prescribing Supplement No 55 In this issue Drugs reviewed by the SMC in February 2012 ADTC UPDATES ON DRUGS REVIEWED BY THE SMC The following new drugs have been reviewed
Area Drug and Therapeutics Committee Prescribing Supplement No 55 In this issue Drugs reviewed by the SMC in February 2012 ADTC UPDATES ON DRUGS REVIEWED BY THE SMC The following new drugs have been reviewed
Ophthalmology Macular Pathways
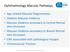 Ophthalmology Macular Pathways Age related Macular Degeneration Diabetic Macular Oedema Macular Oedema secondary to Central Retinal Macular Oedema secondary to Branch Retinal CNV associated with pathological
Ophthalmology Macular Pathways Age related Macular Degeneration Diabetic Macular Oedema Macular Oedema secondary to Central Retinal Macular Oedema secondary to Branch Retinal CNV associated with pathological
Consultation on the role of the Scottish Health Council
 Consultation on the role of the Scottish Health Council What you told us and what we will do next March 2018 Healthcare Improvement Scotland 2018 Published March 2018 This document is licensed under the
Consultation on the role of the Scottish Health Council What you told us and what we will do next March 2018 Healthcare Improvement Scotland 2018 Published March 2018 This document is licensed under the
AWMSG SECRETARIAT ASSESSMENT REPORT. Lisdexamfetamine dimesylate (Elvanse ) 30 mg, 50 mg and 70 mg capsules. Reference number: 188 FULL SUBMISSION
 AWMSG SECRETARIAT ASSESSMENT REPORT Lisdexamfetamine dimesylate (Elvanse ) 30 mg, 50 mg and 70 mg capsules Reference number: 188 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics
AWMSG SECRETARIAT ASSESSMENT REPORT Lisdexamfetamine dimesylate (Elvanse ) 30 mg, 50 mg and 70 mg capsules Reference number: 188 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics
Macular edema (ME) is the most common
 MANAGEMENT OF RETINAL VEIN OCCLUSIONS * Peter A. Campochiaro, MD ABSTRACT Macular edema (ME) is the most common cause of reduced vision in patients with retinal vein occlusions (RVOs). The primary cause
MANAGEMENT OF RETINAL VEIN OCCLUSIONS * Peter A. Campochiaro, MD ABSTRACT Macular edema (ME) is the most common cause of reduced vision in patients with retinal vein occlusions (RVOs). The primary cause
Treatment of Diabetic Macular Oedema by Intravitreal Injection with Ranibizumab (Lucentis)
 Information for Patients Manchester Royal Eye Hospital Medical Retina Services Treatment of Diabetic Macular Oedema by Intravitreal Injection with Ranibizumab (Lucentis) Eye problems are common in people
Information for Patients Manchester Royal Eye Hospital Medical Retina Services Treatment of Diabetic Macular Oedema by Intravitreal Injection with Ranibizumab (Lucentis) Eye problems are common in people
PATIENT INFORMATION LEAFLET. PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM OZURDEX, dexamethasone 700 μg intravitreal implant
 Page 1 of 6 SCHEDULING STATUS Schedule 4 PATIENT INFORMATION LEAFLET PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM, dexamethasone 700 μg intravitreal implant Read all of this leaflet carefully before
Page 1 of 6 SCHEDULING STATUS Schedule 4 PATIENT INFORMATION LEAFLET PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM, dexamethasone 700 μg intravitreal implant Read all of this leaflet carefully before
03 May 2016 Page 1 of 5. How does the NHS board decide which new medicines to make available for patients?
 NHS Greater Glasgow and Clyde: New Medicines Decisions In Scotland, a newly licensed medicine is routinely available for use in an NHS board only after it has been: accepted for use in the NHSScotland
NHS Greater Glasgow and Clyde: New Medicines Decisions In Scotland, a newly licensed medicine is routinely available for use in an NHS board only after it has been: accepted for use in the NHSScotland
FDA approves Roche s Lucentis (ranibizumab injection) for treatment of diabetic retinopathy in people with diabetic macular edema
 Media Release Basel, 9 February 2015 FDA approves Roche s Lucentis (ranibizumab injection) for treatment of diabetic retinopathy in people with diabetic macular edema First eye medicine approved for treatment
Media Release Basel, 9 February 2015 FDA approves Roche s Lucentis (ranibizumab injection) for treatment of diabetic retinopathy in people with diabetic macular edema First eye medicine approved for treatment
Prostate artery embolisation for benign prostatic hyperplasia
 Prostate artery embolisation for benign prostatic Issued: April 2013 guidance.nice.org.uk/ipg NICE has accredited the process used by the NICE Interventional Procedures Programme to produce interventional
Prostate artery embolisation for benign prostatic Issued: April 2013 guidance.nice.org.uk/ipg NICE has accredited the process used by the NICE Interventional Procedures Programme to produce interventional
Cost-effectiveness of apremilast (Otezla )
 Cost-effectiveness of apremilast (Otezla ) alone or in combination with Disease Modifying Antirheumatic Drugs (DMARDs) for the treatment of active psoriatic arthritis in adult patients who have had an
Cost-effectiveness of apremilast (Otezla ) alone or in combination with Disease Modifying Antirheumatic Drugs (DMARDs) for the treatment of active psoriatic arthritis in adult patients who have had an
Treatment of Age Related Macular Degeneration (AMD) by Intravitreal Injection
 Information for Patients Manchester Royal Eye Hospital Medical Retina Services Treatment of Age Related Macular Degeneration (AMD) by Intravitreal Injection What is age related macular degeneration (AMD)?
Information for Patients Manchester Royal Eye Hospital Medical Retina Services Treatment of Age Related Macular Degeneration (AMD) by Intravitreal Injection What is age related macular degeneration (AMD)?
