Minor Crop Farmer Alliance
|
|
|
- Kristopher Chase
- 5 years ago
- Views:
Transcription
1 Minor Crop Farmer Alliance By mail and electronically Mr. Jack E. Housenger Director, Office of Pesticide Programs C/o OPP Docket Environmental Protection Agency Docket Center (EPA/DC), (28221T) Environmental Protection Agency 1200 Pennsylvania Ave., N.W. Washington, DC Dear Mr. Housenger: Re: Chlorpyrifos; Tolerance Revocations; Proposed Rule; Docket ID No. EPA-HQ-OPP Dear Mr. Housenger: These comments are submitted by the Minor Crop Farmer Alliance ( MCFA ) on the subject notice published in the Federal Register on November 6, 2015, 80 Fed. Reg MCFA is an alliance of national and regional organizations and individuals representing growers, shippers, packers, handlers and processors of various agricultural commodities, including food, fiber, turf grass, nursery and landscape crops, and organizations involved with public health pesticides. Our members are extremely interested in the development and safe use of pest management tools including crop protection chemicals that are environmentally sound, 1 Many stakeholders including members of MCFA timely filed requests for a modest extension of time to file comments on the Agency s proposed Chlorpyrifos tolerance revocation. Despite the significant impacts on stakeholders in the agricultural community if the proposed action was finalized and also the significant policy issues involved in this matter, the Agency chose to deny those requests. EPA primarily relied on the rationale that it was under a court imposed deadline to make a final decision on the Chlorpyrifos tolerances by December 30, 2016 and that the comment period for the Revised Human Health Risk Assessment (RHHRA), originally published in January 2015 (Docket No. EPA-HQ-OPP ) and which is an essential part of the Agency s proposed tolerance revocation decision, had previously been briefly extended. Apparently the Agency believed that any modest extension of time on the tolerance revocation proposal would adversely affect its schedule and EPA was not inclined to advise the 9 th Circuit that a brief extension of the comment period was needed. Although as EPA acknowledges in the subject notice it has not been able to complete its review of the comments that were received on the RHHRA, the Agency still feels compelled to proceed with the revocation proposal.
2 Page 2 safe for applicators and workers, and do not represent an unreasonable adverse risk to the environment, including humans. While our commodities are often called minor crops or specialty crops, they contribute to the diversity and highly nutritious diets available for the global population and to safe and aesthetic surroundings for our homes, schools, and places of business. U.S. farmers grow more than 500 types of fruit, vegetable, tree nut, flower, ornamental nursery and turf grass crops in addition to the major bulk (row) commodity crops. Specialty crop production accounts for more than $60 billion, or approximately 40% of total U.S. crop receipts. On behalf of our members, MCFA objects to the revocation of the Chlorpyrifos tolerances (40 CFR ). Those tolerances should remain in effect. Chlorpyrifos is the only viable option in certain pest management situations and plays a very important role in the production of various crops produced by some of our members. For example, it has been the primary response to several newly emerging insect pests such as vine mealybug when it first attacked grape vines in California, as well as the brown marmorated stinkbug in other regions of the country. The revocation of the tolerances would essentially eliminate the use of the product, adversely affecting our members interests. The affected crops include alfalfa, asparagus, beets (sugar), Cole crops, carrots, citrus, nectarine, clover, corn, cotton, cranberry, cucumber, fig, ginseng, grapes, legume vegetables (beans, peas), mint, peppermint, spearmint, onions, peanut, peppers, pineapple, apple, cherries, peach, pear, plum, prune, pumpkin, radish, rutabaga, sorghum (grain), soybeans, strawberries, sunflower, sweet potatoes, tobacco, turnip, tree nuts (almonds, hazelnut, pecans, walnuts), wheat, and triticale. 2 The factual underpinnings, policy and scientific issues involved in this matter are significant and warrant a more complete and comprehensive investigation and review by the Agency. MCFA is extremely disappointed with the process being followed in this matter. Apparently for administrative convenience, the Agency decided to bypass the registration cancellation 2 Growers need the current list of tolerances for these crops and for any processed fraction or food tolerance, animal feed tolerance, and animal commodity tolerance (such as, but not limited to milk, meat, or eggs) that would be associated with the use on these crops, to be maintained. 2
3 Page 3 procedures contained in Section 6 of the Federal Insecticide, Fungicide and Rodenticide Act, as amended ( FIFRA ) in favor of the tolerance revocation pathway offered by the Federal Food, Drug and Cosmetic Act ( FFDCA ) as amended by the Food Quality Protection Act ( FQPA ), thereby avoiding the procedural safeguards established under Section 6 of FIFRA. The Agency appears to have adopted a predetermined conclusion namely that Chlorpyrifos should not be available for the agricultural community. That conclusion is after-the-fact being supported with information cobbled together from various sources, but most notably certain epidemiological papers. For example, despite EPA acknowledging that it has not completed its review of the comments on the RHHRA 3 which, in turn, involves those papers, the Agency continues to press forward with the tolerance revocation. 4 This calls into question EPA s objectiveness and transparency when reviewing the comments submitted on the RHHRA, as well as the comments being submitted challenging the Agency s proposed tolerance revocation decision. 5 Many of MCFA s members are submitting information directly to the Agency in response to the proposed tolerance revocation. That information will include the parameters concerning their use of Chlorpyrifos and the importance to them of maintaining the current necessary tolerances Fed. Reg. at It is understood that while EPA is relying on the epidemiological papers, the Agency has not secured or reviewed all the raw data upon which the papers are based. This is a fundamental problem. At the very least, the Agency should secure that information so it can critically evaluate the analysis and conclusions in the cited epidemiology papers, if the Agency intends to continue to rely on these papers to justify the retention of the 10X FQPA safety factor. In our view, it is unlawful for the Agency to default to the views of authors of these papers in making a regulatory decision. The Agency does not know in fact that the reported conclusions are actually consistent with the data collected for the papers. Additionally, the Agency is required to determine itself: (1) whether the participants were actually exposed to Chlorpyrifos, and if so, (2) at what dose, (3) over what time period, (4) whether the reported effects actually occurred, (5) that the measurements were accurate, and, (6) if the measurements were accurate, whether there were factors other than exposure to Chlorpyrifos which caused the purported effect. Apparently the Agency believes that the researchers evaluation of the underlying data (assuming its veracity) must have been objective, and the Agency can default to the researchers conclusions. Under this novel approach, adherence to Good Laboratory Practices ( GLP ) and scientific merit are irrelevant for purposes of justifying a regulatory decision that will significantly adversely affect many agricultural producers. 5 While it may prove to be a futile exercise because the Agency appears to have already made up its mind regarding the proposed tolerance revocation, MCFA incorporates by reference herein the comments submitted in Docket No. EPA-HQ-OPP identifying issues with the RHHRA, including those submitted by the American Farm Bureau, Dow AgroSciences, LLC, Michigan Farm Bureau, Western Growers, CropLife America, California Citrus Quality Council, US Department of Agriculture, Northwest Horticultural Council, National Agriculture Aviation Association, California Specialty Crop Council, Western Agricultural Processors Association, California Fresh Fruit Agreement, National Cotton Council, American Mosquito Control Association, United Fresh Produce Association, California Walnut Commission, the Almond Hullers & Processors Association, Florida Department of Agriculture and Consumer Services, California Grape and Tree Fruit League, Cheminova and the Cranberry Institute. 3
4 Page 4 Recognizing that providing a scientific critique of all the underlying information involved in this matter is beyond the expertise of MCFA, nevertheless there are certain issues involved that we are commenting on. These include the substantial weight that the Agency accords the epidemiological evidence to justify its proposed revocation decision. In addition, comments are provided herein on EPA s substantial reliance on screening-level drinking water models to estimate drinking water risks from potential exposures to Chlorpyrifos and EPA s expressed intention to provide the public an opportunity to comment only to the extent practicable on the Agency s new or modified analysis of the relevant action before it issues a final rule. See 80 Fed. Reg MCFA is also concerned that the assessment approaches reflected in the Chlorpyrifos tolerance revocation proposal may serve as a precedent for the future evaluation of other pesticides. Consequently, this magnifies the importance of the issues involved, including for MCFA members that do not use Chlorpyrifos. EPA should reconsider its reliance on the epidemiology papers in this instance EPA should reconsider its approaches used in its revised Chlorpyrifos human health risk assessment, particularly the emphasis given to the Columbia University epidemiology paper. The information in that paper, in conjunction with two other epidemiological papers, underpins EPA s decision to increase the FQPA safety factor by an additional 10X. This additional 10X is crucial in determining whether Chlorpyrifos exceeds the total aggregate/dietary risk under FQPA. Essentially, the Agency is choosing to set aside the results of carefully conducted Chlorpyrifos laboratory animal exposure studies and instead rely on these limited epidemiological papers. Prior to this time, it was understood that the Chlorpyrifos toxicological database was very extensive and the endpoints were well understood. In this instance, however, the Agency has decided to give undue weight to the epidemiological papers, using the conclusions of those papers to redefine toxicity endpoints. 6 6 It is understood that there are other epidemiological publications and studies the results of which are not consistent with the conclusions of the three studies in question. Those other studies appear to indicate that at the measured levels of exposure, the evidence is insufficient to show causality between Chlorpyrifos and adverse neurological effects in infants and children. Further, a review of approximately 600 studies contracted by the EU European Food Safety Agency concluded that there is no evidence to suggest an association between pesticide exposure, including Chlorpyrifos, and neurodevelopment effects. These publications and references were identified in previous comments submitted on the RHHRA by CropLife America as referenced supra. MCFA respectfully requests the 4
5 Page 5 MCFA agrees with those commentators who have previously advised the Agency that it is critically important that exposures referenced in epidemiological studies, including the abovementioned three papers, be evaluated against exposures and internal doses in toxicological studies when assessing environmental exposures to a pesticide and the potential to cause harm. This is particularly important for human health conditions that can be influenced by a myriad of factors including lifestyle. When the Agency intends to override the results of carefully constructed GLP laboratory animal toxicology studies in favor of epidemiological studies, it should be assured that the exposures to the pesticide are clearly documented. Otherwise, the Agency is replacing scientific results with guesswork. By giving epidemiology studies such primacy in its decision making without having the raw data available and public consultation or discussion, EPA is reordering the hierarchy of information it uses to make regulatory decisions. This raises a host of questions that need to be considered including: What is the relationship between animal studies and human epidemiology in determining risk? Will the Agency issue guidelines for conducting epidemiology studies that will be used for regulatory decision-making? What criteria will EPA use to choose between animal studies or epidemiology studies when the results are conflicting? Based on the current record, can the Agency objectively conclude that the three epidemiological papers are rigorous enough to override the vast array of existing GLP animal toxicology studies? We think not. As mentioned above, MCFA is concerned that EPA is making a major shift in its decision-making process in a way that will lead away from science-based decisions and to the loss of many more crop protection tools. Certainly MCFA supports efforts to protect human health and the environment. If, in its analysis, EPA determines that the science establishes that a specific use is unsafe, we will support the regulatory decision. However, the process must be scientifically reliable, robust, transparent, and objective. In this instance, basing regulatory decisions on the three epidemiology studies introduces considerable uncertainty into the regulatory process and appears to be inconsistent with sound science. Rather than advancing a Agency to review those 600 studies to determine whether under a weight of evidence approach continued reliance on the three epidemiological papers is appropriate. 5
6 Page 6 science-based decision, the proposed tolerance revocation decision moves away from the reliable scientific data reflected in GLP animal toxicology studies. EPA should refine its drinking water models in this instance EPA s screening-level drinking water model is an important driver in EPA s human health risk assessment. Modeling can be a useful tool in risk assessment, but a model must be based on realistic scenarios if a credible exposure estimate is desired. Certainly, the modeling should be compared with available monitoring data to see whether or not the model is robust and reliable. At the very least, monitoring data can help refine drinking water assessments. In this case, EPA acknowledges the existence of monitoring data and suggests since the results of its modeling are within one order of magnitude of the monitoring results, the models results are not overly conservative and are suitable for risk assessment purposes. With all due respect, it is understood that a difference of one order of magnitude (10X) is not considered acceptable in the scientific community. What is more typical is a 3-4X difference. A difference of 10X indicates that the models results are not reflective of the monitoring results. The drinking water modeling used to estimate the dietary risk from Chlorpyrifos appears unrealistic because of the inaccurate assumptions used. For example, the Agency assumes that the entire watershed area is treated with Chlorpyrifos at the maximum rate on a single day, using the maximum possible amount of runoff from the treated area and that all of the runoff is drinking water. The Agency does not identify even one watershed in which all of these assumptions are true at the same time. It is understood that for Chlorpyrifos, there are almost 47,000 water data monitoring points available to the Agency that should be considered. Further, we understand that data on actual application rates and use patterns have not been used to refine modeling inputs. Rather, the models appear to rely on unrealistically exaggerated worst-case scenarios. MCFA urges EPA to refine its drinking water modeling by using more realistic assumptions and using available monitoring data as an input in the models. The modeling is recognized as overly 6
7 Page 7 conservative and does not reflect the extensive real-world monitoring data for Chlorpyrifos. EPA highlights the high level of refinement of their dietary food (residue) assessment and should require the same level of refinement for their drinking water assessments before relying on them for any regulatory decision. We believe a more refined modeling will show that there is an acceptable level of dietary risk from continued Chlorpyrifos use. Conclusion Chlorpyrifos is an important tool that many MCFA members rely on to control serious insect pests. Finalizing the proposed tolerance revocation will result in growers not being able to use Chlorpyrifos. In such circumstance, EPA will have unreasonably made the growers ability to manage various pests much more difficult. 7 Reliance on unsubstantiated epidemiology papers and results from unrefined drinking water models that reflect unrealistic assumptions, is not a basis for a robust science-based decision. Before EPA finalizes its tolerance decision, the Agency should reevaluate its approach and revise it in accordance with the comments presented above. Further, the Agency should unconditionally commit to allow the public to comment on its new or modified analysis of the relevant action before it issues a final rule. The Agency s inclination to provide an opportunity for public comment only to the extent practicable is simply inappropriate and inconsistent with the principles reflected in the Administrative Procedure Act. The policy issues involved in this action are too significant to not allow full public comment on the analysis, including when that analysis is modified by the Agency. If the 7 It should be noted that while EPA would be hurting our Nation s growers by eliminating the Chlorpyrifos tolerances, growers in foreign countries that use Chlorpyrifos on the commodities they export to the US should be able to continue to use the chemical, since the Agency acknowledges in the subject notice that the risk from residues in foods is acceptable. This unfair competitive advantage would not exist but for the unreasonable action that EPA is proposing. 7
8 Page 8 rulemaking process requires additional time, then the Agency can so advise the 9 th Circuit that the obligation to permit the interested public meaningful opportunity to comment fully on the Agency s analysis before a final decision is made necessitates an extension. Sincerely Daniel A. Botts Vice President Industry Resources, Florida Fruit & Vegetable Association & Chairman, Minor Crop Farmer Alliance Technical Committee DM_US
Proposed Revision or Revocation of Maximum Residue Limits for Discontinued Agricultural Pest Control Products: Update 2
 Proposed Maximum Residue Limit PMRL2018-44 Proposed Revision or Revocation of Maximum Residue Limits for Discontinued Agricultural Pest Control Products: Update 2 (publié aussi en français) 8 November
Proposed Maximum Residue Limit PMRL2018-44 Proposed Revision or Revocation of Maximum Residue Limits for Discontinued Agricultural Pest Control Products: Update 2 (publié aussi en français) 8 November
BUNDESINSTITUT FÜR RISIKOBEWERTUNG
 BUNDESINSTITUT FÜR RISIKOBEWERTUNG Mehrfachrückstände von Pflanzenschutzmitteln in Lebensmitteln Teil III Internationale Bewertungskonzepte für Mehrfachrückstände 10.11.2005 Cumulative Risk Assessment:
BUNDESINSTITUT FÜR RISIKOBEWERTUNG Mehrfachrückstände von Pflanzenschutzmitteln in Lebensmitteln Teil III Internationale Bewertungskonzepte für Mehrfachrückstände 10.11.2005 Cumulative Risk Assessment:
Setting of new MRLs for fluxapyroxad (BAS 700 F) in various commodities of plant and animal origin 1
 : EFSA Journal 2011;9(6):2196 REASONED OPINION Setting of new MRLs for fluxapyroxad (BAS 700 F) in various commodities of plant and animal origin 1 European Food Safety Authority 2 European Food Safety
: EFSA Journal 2011;9(6):2196 REASONED OPINION Setting of new MRLs for fluxapyroxad (BAS 700 F) in various commodities of plant and animal origin 1 European Food Safety Authority 2 European Food Safety
RE: Application of the Solely Engaged Exemptions in Parts 117 and 507; Draft Guidance for Industry; Availability: Docket No.
 1400 Crystal Drive, Suite 260 Arlington, VA 22202 P: (202) 289-0873 F: (202) 289-5388 April 18, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane Room 1061 Rockville,
1400 Crystal Drive, Suite 260 Arlington, VA 22202 P: (202) 289-0873 F: (202) 289-5388 April 18, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane Room 1061 Rockville,
Re: National Bioengineered Food Disclosure Standard; Proposed Rule; Request for Comments, 83 Fed. Reg (May 4, 2018), Docket No.
 VIA ELECTRONIC SUBMISSION July 3, 2018 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: National Bioengineered Food Disclosure Standard;
VIA ELECTRONIC SUBMISSION July 3, 2018 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: National Bioengineered Food Disclosure Standard;
COMMISSION REGULATION (EU) / of XXX
 EUROPEAN COMMISSION Brussels, XXX SANTE/10530/2015 Rev. 0 [ ](2015) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and
EUROPEAN COMMISSION Brussels, XXX SANTE/10530/2015 Rev. 0 [ ](2015) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and
SFIREG Issue Paper: Pesticide Use on Cannabis State Established Pesticide Residue Action Levels
 SFIREG Issue Paper: Pesticide Use on Cannabis State Established Pesticide Residue Action Levels Introduction to Issue: In recent years, with the approval of medical and recreational use of marijuana in
SFIREG Issue Paper: Pesticide Use on Cannabis State Established Pesticide Residue Action Levels Introduction to Issue: In recent years, with the approval of medical and recreational use of marijuana in
There is a clear link between
 University of California Number 27 A u g u s t 2 0 0 4 Agricultural Issues Center AIC Issues Brief Does 5-a-Day Pay? Potential Gains to Growers from Increasing Consumption of Fruits and Vegetables to Recommended
University of California Number 27 A u g u s t 2 0 0 4 Agricultural Issues Center AIC Issues Brief Does 5-a-Day Pay? Potential Gains to Growers from Increasing Consumption of Fruits and Vegetables to Recommended
Council of the European Union Brussels, 4 November 2015 (OR. en)
 Council of the European Union Brussels, 4 November 2015 (OR. en) 13706/15 AGRILEG 208 COVER NOTE From: European Commission date of receipt: 30 October 2015 To: No. Cion doc.: D041471/02 Subject: General
Council of the European Union Brussels, 4 November 2015 (OR. en) 13706/15 AGRILEG 208 COVER NOTE From: European Commission date of receipt: 30 October 2015 To: No. Cion doc.: D041471/02 Subject: General
The Federal Insecticide, Fungicide and Rodenticide Act (FIFRA)
 July 14, 2015 Mitchell Yergert Director Division of Plant Industry Colorado Department of Agriculture 305 Interlocken Parkway Broomfield, CO 80021 Dear Mr. Yergert, On behalf of Beyond Pesticides, we are
July 14, 2015 Mitchell Yergert Director Division of Plant Industry Colorado Department of Agriculture 305 Interlocken Parkway Broomfield, CO 80021 Dear Mr. Yergert, On behalf of Beyond Pesticides, we are
2. Bone fractures:... the majority of the committee concluded that the MCLG is not likely to be protective against bone fractures.
 Fluoride Action Network The Pesticides Project http://www.fluoridealert.org/f-pesticides.htm 82 Judson Street, Canton NY 13617 Tel: 315-379-9200 Email: pesticides@fluoridealert.org Food and Drug Administration
Fluoride Action Network The Pesticides Project http://www.fluoridealert.org/f-pesticides.htm 82 Judson Street, Canton NY 13617 Tel: 315-379-9200 Email: pesticides@fluoridealert.org Food and Drug Administration
NUTRITIONAL STATUS OP RURAL YOUTH. IV. Sherman County
 AGRICULTURAL EXPERIMENT STATION Oregon State College Irru A. Schoenfeld, Director Corvallis Home Economics Circular of Information No, 349 September 1944 (A Progress Report) NUTRITIONAL STATUS OP RURAL
AGRICULTURAL EXPERIMENT STATION Oregon State College Irru A. Schoenfeld, Director Corvallis Home Economics Circular of Information No, 349 September 1944 (A Progress Report) NUTRITIONAL STATUS OP RURAL
Dietary Risk Assessment (Food Only)
 Dietary Risk Assessment (Food Only) Sheila Piper EPA OPP/HED Jennifer Lantz Bayer CropScience CARES Training Workshop January 28-29, 2009 Washington DC 1 Objectives Brief Overview of Dietary Assessment
Dietary Risk Assessment (Food Only) Sheila Piper EPA OPP/HED Jennifer Lantz Bayer CropScience CARES Training Workshop January 28-29, 2009 Washington DC 1 Objectives Brief Overview of Dietary Assessment
June 9, U.S. Food and Drug Administration Division of Dockets Management, HFA Fishers Lane, Room 1061 Rockville, MD 20852
 June 9, 2014 U.S. Food and Drug Administration Division of Dockets Management, HFA-305 5630 Fishers Lane, Room 1061 Rockville, MD 20852 Re: Implementation of the Food and Drug Administration Food Safety
June 9, 2014 U.S. Food and Drug Administration Division of Dockets Management, HFA-305 5630 Fishers Lane, Room 1061 Rockville, MD 20852 Re: Implementation of the Food and Drug Administration Food Safety
5.9 DIFLUBENZURON (130)
 Diflubenzuron 79 5.9 DIFLUBENZURON (130) RESIDUE AND ANALYTICAL ASPECTS Diflubenzuron [1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea] is an agricultural insect growth regulator. It was originally evaluated
Diflubenzuron 79 5.9 DIFLUBENZURON (130) RESIDUE AND ANALYTICAL ASPECTS Diflubenzuron [1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea] is an agricultural insect growth regulator. It was originally evaluated
Pesticide Risk Assessment-- Dietary Exposure
 Pesticide Risk Assessment-- Dietary Exposure Allan Felsot Department of Entomology, WSU-TC Food & Environmental Quality Lab afelsot@tricity.wsu.edu Lecture for 11/17/03 Mandates of the FQPA All tolerances
Pesticide Risk Assessment-- Dietary Exposure Allan Felsot Department of Entomology, WSU-TC Food & Environmental Quality Lab afelsot@tricity.wsu.edu Lecture for 11/17/03 Mandates of the FQPA All tolerances
LAWS, STANDARDS & REGULATIONS
 Appendixes APPENDIX 1: LAWS, STANDARDS & REGULATIONS The United States enjoys one of the safest food supplies in the world. The laws and regulations required to achieve that safe food supply are lengthy
Appendixes APPENDIX 1: LAWS, STANDARDS & REGULATIONS The United States enjoys one of the safest food supplies in the world. The laws and regulations required to achieve that safe food supply are lengthy
5.17 METHOXYFENOZIDE (209)
 Methoxyfenozide 239 5.17 METHOXYFENOZIDE (209) RESIDUE AND ANALYTICAL ASPECTS Methoxyfenozide was evaluated by the JMPR for residues and toxicology in 2003, when an ADI of 0-0.1 mg/kg bw and an ARfD of
Methoxyfenozide 239 5.17 METHOXYFENOZIDE (209) RESIDUE AND ANALYTICAL ASPECTS Methoxyfenozide was evaluated by the JMPR for residues and toxicology in 2003, when an ADI of 0-0.1 mg/kg bw and an ARfD of
May 7, Dear Mr. Landa:
 Ralph A. Simmons 202 429 6459 rsimmons@steptoe.com 1330 Connecticut Avenue, NW Washington, DC 20036-1795 202 429 3000 main www.steptoe.com May 7, 2013 Michael M. Landa Director, Center for Food Safety
Ralph A. Simmons 202 429 6459 rsimmons@steptoe.com 1330 Connecticut Avenue, NW Washington, DC 20036-1795 202 429 3000 main www.steptoe.com May 7, 2013 Michael M. Landa Director, Center for Food Safety
Potassium and Your CKD Diet
 A TO Z HEALTH GUIDE Potassium and Your CKD Diet What is potassium and why is it important to you? Potassium is a mineral found in many of the foods you eat. It plays a role in keeping your heartbeat regular
A TO Z HEALTH GUIDE Potassium and Your CKD Diet What is potassium and why is it important to you? Potassium is a mineral found in many of the foods you eat. It plays a role in keeping your heartbeat regular
588G: Dietary Antigen Testing: Sensitivity and Complement 1/5. Dietary Antigen Exposure by Food Group
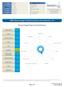 PATIENT NAME: CLINIC: DOB: SAMPLE DATE: RECEIVE DATE: REPORT DATE: R //2 /28/27 /29/27 /3/27 Dunwoody Labs 9 Dunwoody Park Suite 2 Dunwoody, GA 3338 USA Phone: 6787366374 Fax: 776747 Nine Dunwoody Park,
PATIENT NAME: CLINIC: DOB: SAMPLE DATE: RECEIVE DATE: REPORT DATE: R //2 /28/27 /29/27 /3/27 Dunwoody Labs 9 Dunwoody Park Suite 2 Dunwoody, GA 3338 USA Phone: 6787366374 Fax: 776747 Nine Dunwoody Park,
Boscalid BOSCALID (221)
 Boscalid 49 5.3 BOSCALID (221) RESIDUE AND ANALYTICAL ASPECTS Boscalid is a systemic fungicide first evaluated by JMPR in 2006 for residues and toxicology as a new active substance. An ADI of 0 0.04 mg/kg
Boscalid 49 5.3 BOSCALID (221) RESIDUE AND ANALYTICAL ASPECTS Boscalid is a systemic fungicide first evaluated by JMPR in 2006 for residues and toxicology as a new active substance. An ADI of 0 0.04 mg/kg
Chlorantraniliprole 67
 Chlorantraniliprole 67 5.5 CHLORANTRANILIPROLE (230) RESIDUE AND ANALYTICAL ASPECTS Chlorantraniliprole is a novel insecticide belonging to the class of selective ryanodine receptor agonists and was evaluated
Chlorantraniliprole 67 5.5 CHLORANTRANILIPROLE (230) RESIDUE AND ANALYTICAL ASPECTS Chlorantraniliprole is a novel insecticide belonging to the class of selective ryanodine receptor agonists and was evaluated
COMMISSION REGULATION (EU) / of XXX
 EUROPEAN COMMISSION Brussels, XXX SANTE/11715/2017 rev.2 [ ](2018) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament
EUROPEAN COMMISSION Brussels, XXX SANTE/11715/2017 rev.2 [ ](2018) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament
LAST NAME FIRST NAME MIDDLE NAME DATE OF BIRTH GENDER PHYSICIAN ID. TESTNAME PATIENT Female For doctor's reference
 FINAL REPORT DATE: ACCESSION ID: 05-04-2017 16:24 SPECIMEN COLLECTED: 11-03-2016 1611040000 SPECIMEN RECEIVED: 11-04-2016 00:00 LAST NAME FIRST NAME MIDDLE NAME DATE OF BIRTH GENDER PHYSICIAN ID TESTNAME
FINAL REPORT DATE: ACCESSION ID: 05-04-2017 16:24 SPECIMEN COLLECTED: 11-03-2016 1611040000 SPECIMEN RECEIVED: 11-04-2016 00:00 LAST NAME FIRST NAME MIDDLE NAME DATE OF BIRTH GENDER PHYSICIAN ID TESTNAME
SUMMARY: EPA is proposing, in follow-up to canceled product registrations or uses, to revoke
 This document is scheduled to be published in the Federal Register on 07/22/2015 and available online at http://federalregister.gov/a/2015-17628, and on FDsys.gov BILLING CODE 6560-50-P ENVIRONMENTAL PROTECTION
This document is scheduled to be published in the Federal Register on 07/22/2015 and available online at http://federalregister.gov/a/2015-17628, and on FDsys.gov BILLING CODE 6560-50-P ENVIRONMENTAL PROTECTION
National reporting 2014 Pesticide residues in food Federal Republic of Germany
 National reporting 2014 Pesticide residues in food Federal Republic of Germany Summary The report presents the results of the analysis of food for pesticide residues. In accordance with Regulation (EC)
National reporting 2014 Pesticide residues in food Federal Republic of Germany Summary The report presents the results of the analysis of food for pesticide residues. In accordance with Regulation (EC)
5.20 PYRACLOSTROBIN (210)
 Pyraclostrobin 213 5.20 PYRACLOSTROBIN (210) RESIDUE AND ANALYTICAL ASPECTS Pyraclostrobin was first evaluated by JMPR in 2003 when an ADI of 0 0.03mg/kg bw and an ARfD of 0.05 mg/kg bw were established,
Pyraclostrobin 213 5.20 PYRACLOSTROBIN (210) RESIDUE AND ANALYTICAL ASPECTS Pyraclostrobin was first evaluated by JMPR in 2003 when an ADI of 0 0.03mg/kg bw and an ARfD of 0.05 mg/kg bw were established,
An Overview of EPA/FDA Jurisdiction of Food Contact Antimicrobial Products
 An Overview of EPA/FDA Jurisdiction of Food Contact Antimicrobial Products John Wood Sr. Director, Agency Relations Regulatory Affairs Ecolab Inc. Antimicrobial Regulation: The Statutory Framework FIFRA
An Overview of EPA/FDA Jurisdiction of Food Contact Antimicrobial Products John Wood Sr. Director, Agency Relations Regulatory Affairs Ecolab Inc. Antimicrobial Regulation: The Statutory Framework FIFRA
September 30, Mr. Chuck Rosenberg Acting Administrator U.S. Drug Enforcement Administration 8701 Morrissette Drive Springfield, Virginia 22152
 Mr. Chuck Rosenberg Acting Administrator U.S. Drug Enforcement Administration 8701 Morrissette Drive Springfield, Virginia 22152 Re: Docket No. DEA-442 Temporary Placement of Mitragynine and 7- Hydroxymitragynine
Mr. Chuck Rosenberg Acting Administrator U.S. Drug Enforcement Administration 8701 Morrissette Drive Springfield, Virginia 22152 Re: Docket No. DEA-442 Temporary Placement of Mitragynine and 7- Hydroxymitragynine
FRESH AIR FASTING GOD S WAY
 FRESH AIR FASTING GOD S WAY WHAT IS FASTING? Fasting is giving up food or another source of personal gratification for a determined period of time in order to focus on God. Fasting allows us to take our
FRESH AIR FASTING GOD S WAY WHAT IS FASTING? Fasting is giving up food or another source of personal gratification for a determined period of time in order to focus on God. Fasting allows us to take our
COMMISSION REGULATION (EU) / of XXX
 EUROPEAN COMMISSION Brussels, XXX SANTE/11077/2016 Rev. 1 [ ](2016) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and
EUROPEAN COMMISSION Brussels, XXX SANTE/11077/2016 Rev. 1 [ ](2016) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and
Alignment of FSMA with Existing Food Safety Programs International Citrus & Beverage Conference
 Alignment of FSMA with Existing Food Safety Programs International Citrus & Beverage Conference Donald Kautter US Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Food
Alignment of FSMA with Existing Food Safety Programs International Citrus & Beverage Conference Donald Kautter US Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Food
Fluopyram FLUOPYRAM (243)
 Fluopyram 163 5.19 FLUOPYRAM (243) RESIDUE AND ANALYTICAL ASPECTS Fluopyram, a pyridylethylamide broad spectrum fungicide was evaluated for the first time by the 2010 JMPR, where an ADI of 0 0.01 mg/kg
Fluopyram 163 5.19 FLUOPYRAM (243) RESIDUE AND ANALYTICAL ASPECTS Fluopyram, a pyridylethylamide broad spectrum fungicide was evaluated for the first time by the 2010 JMPR, where an ADI of 0 0.01 mg/kg
MICRO NUTRIENTS AND SECONDARY NUTRIENTS
 BR Global, LLC. P.O. Box 8164 Rocky Mount, NC 27804 Tel: 252-442-0700 / Fax: 252-442-0787 Sales@BRGLimited.com www.brglimited.com MICRO NUTRIENTS AND SECONDARY NUTRIENTS Trace elements or micronutrients
BR Global, LLC. P.O. Box 8164 Rocky Mount, NC 27804 Tel: 252-442-0700 / Fax: 252-442-0787 Sales@BRGLimited.com www.brglimited.com MICRO NUTRIENTS AND SECONDARY NUTRIENTS Trace elements or micronutrients
The Daniel Fast "Fasting For Health And Healing"
 The Daniel Fast "Fasting For Health And Healing" Starting Monday January 3, 2011 at Midnight Ending Sunday January 23, 2011 at 11:59 pm This information was primarily taken from the book "Fasting For Spiritual
The Daniel Fast "Fasting For Health And Healing" Starting Monday January 3, 2011 at Midnight Ending Sunday January 23, 2011 at 11:59 pm This information was primarily taken from the book "Fasting For Spiritual
A FULL SPECTRUM OF NUTRIENTS
 A FULL SPECTRUM OF NUTRIENTS All of us remember being told by our parents Eat your Vegetables. They re good for you. Fruits and vegetables are good for our Budgies too. Each color group has its own nutritional
A FULL SPECTRUM OF NUTRIENTS All of us remember being told by our parents Eat your Vegetables. They re good for you. Fruits and vegetables are good for our Budgies too. Each color group has its own nutritional
BACKGROUND + GENERAL COMMENTS
 Response on behalf of Sobi (Swedish Orphan Biovitrum AB) to the European Commission s Public Consultation on a Commission Notice on the Application of Articles 3, 5 and 7 of Regulation (EC) No. 141/2000
Response on behalf of Sobi (Swedish Orphan Biovitrum AB) to the European Commission s Public Consultation on a Commission Notice on the Application of Articles 3, 5 and 7 of Regulation (EC) No. 141/2000
National Organic Program (NOP); Sunset 2017 Amendments to the National List
 This document is scheduled to be published in the Federal Register on 07/06/2017 and available online at https://federalregister.gov/d/2017-14006, and on FDsys.gov DEPARTMENT OF AGRICULTURE Agricultural
This document is scheduled to be published in the Federal Register on 07/06/2017 and available online at https://federalregister.gov/d/2017-14006, and on FDsys.gov DEPARTMENT OF AGRICULTURE Agricultural
EASY WAYS TO EAT MORE FRUITS AND VEGETABLES AS PART OF A HEALTHY DIET.
 This is a text-only 508 accessible version for the visually impaired. For a full-color brochure, see: www.fruitsandveggiesmatter.gov/downloads/aa_womens_brochure.pdf Page 1- Left column (back cover) EASY
This is a text-only 508 accessible version for the visually impaired. For a full-color brochure, see: www.fruitsandveggiesmatter.gov/downloads/aa_womens_brochure.pdf Page 1- Left column (back cover) EASY
Becoming A Healthier You!!
 Eating right and staying active will enhance your quality of life Learning For The University of Georgia Cooperative Contact your local office at Becoming A Healthier You!! hoices you make today determine
Eating right and staying active will enhance your quality of life Learning For The University of Georgia Cooperative Contact your local office at Becoming A Healthier You!! hoices you make today determine
January 5, Comments on EPA Proposal To Revoke Chlorpyrifos Tolerances EPA-HQ-OPP
 ALASKA CALIFORNIA FLORIDA MID- PACIFIC NORTHEAST NORTHERN ROCKIES NORTHWEST ROCKY MOUNTAIN WASHINGTON, D.C. INTERNATIONAL Federal erulemaking Portal: http://www.regulations.gov OPP Docket Environmental
ALASKA CALIFORNIA FLORIDA MID- PACIFIC NORTHEAST NORTHERN ROCKIES NORTHWEST ROCKY MOUNTAIN WASHINGTON, D.C. INTERNATIONAL Federal erulemaking Portal: http://www.regulations.gov OPP Docket Environmental
Client will make 2 specific goals to decrease her potassium intake. Client will make 1 specific goal to decrease her fluid retention.
 Title: Renal Diet Target Audience: Renal Dialysis Patients LESSON PLAN Name: Heidi Washburn Terminal Objective Client will be able to plan an appropriate renal dialysis diet for him/herself. Domain Cognitive
Title: Renal Diet Target Audience: Renal Dialysis Patients LESSON PLAN Name: Heidi Washburn Terminal Objective Client will be able to plan an appropriate renal dialysis diet for him/herself. Domain Cognitive
Family Smoking Prevention and Tobacco Control Act. Comments regarding crop protection agents
 Family Smoking Prevention and Tobacco Control Act (PUBLIC LAW 111-31 JUNE 22, 2009) Comments regarding crop protection agents Presentation Objectives What is the intent of FDA in regulating CPAs? What
Family Smoking Prevention and Tobacco Control Act (PUBLIC LAW 111-31 JUNE 22, 2009) Comments regarding crop protection agents Presentation Objectives What is the intent of FDA in regulating CPAs? What
Council of the European Union Brussels, 12 August 2014 (OR. en) Mr Uwe CORSEPIUS, Secretary-General of the Council of the European Union
 Council of the European Union Brussels, 12 August 2014 (OR. en) 12459/14 AGRILEG 168 COVER NOTE From: European Commission date of receipt: 8 August 2014 To: No. Cion doc.: D033914/02 Subject: Mr Uwe CORSEPIUS,
Council of the European Union Brussels, 12 August 2014 (OR. en) 12459/14 AGRILEG 168 COVER NOTE From: European Commission date of receipt: 8 August 2014 To: No. Cion doc.: D033914/02 Subject: Mr Uwe CORSEPIUS,
August 3, RE: Specially Designed Definition (Federal Register Notice of June 19, 2012; RIN 0694-AF66)
 August 3, 2012 Mr. Timothy Mooney Regulatory Policy Division Bureau of Industry and Security U.S. Department of Commerce 14 th Street Pennsylvania Ave., N.W. Washington, D.C. 20230 RE: Specially Designed
August 3, 2012 Mr. Timothy Mooney Regulatory Policy Division Bureau of Industry and Security U.S. Department of Commerce 14 th Street Pennsylvania Ave., N.W. Washington, D.C. 20230 RE: Specially Designed
LESSON 3 E AT A RAINBOW OF SNACKS
 E AT A R A I N B O W O F S N A C K S LESSON 3 E AT A RAINBOW OF SNACKS Objectives for the lesson: 1. Compare the nutrient content and cost of vegetables and fruits as snacks to conventional snack foods.
E AT A R A I N B O W O F S N A C K S LESSON 3 E AT A RAINBOW OF SNACKS Objectives for the lesson: 1. Compare the nutrient content and cost of vegetables and fruits as snacks to conventional snack foods.
Council of the European Union Brussels, 5 December 2014 (OR. en) Mr Uwe CORSEPIUS, Secretary-General of the Council of the European Union
 Council of the European Union Brussels, 5 December 2014 (OR. en) 16594/14 AGRILEG 254 COVER NOTE From: European Commission date of receipt: 3 December 2014 To: No. Cion doc.: D035772/02 Subject: Mr Uwe
Council of the European Union Brussels, 5 December 2014 (OR. en) 16594/14 AGRILEG 254 COVER NOTE From: European Commission date of receipt: 3 December 2014 To: No. Cion doc.: D035772/02 Subject: Mr Uwe
SUPPLY BALANCE SHEETS
 SUPPLY BALANCE SHEETS Artūras Vaitkevičius Senior specialist Agriculture and Environment Statistics Division Agriculture and environment statistics division compiles Supply balance sheets from 1988. From
SUPPLY BALANCE SHEETS Artūras Vaitkevičius Senior specialist Agriculture and Environment Statistics Division Agriculture and environment statistics division compiles Supply balance sheets from 1988. From
FARM TO TABLE: PESTICIDE RESIDUES AND RISK ASSESSMENT
 FARM TO TABLE: PESTICIDE RESIDUES AND RISK ASSESSMENT JANET V. COWINS, Ph.D. CHEMIST U.S. ENVIRONMENTAL PROTECTION AGENCY HEALTH EFFECTS DIVISION/RISK ASSESSMENT BRANCH 2 DISCUSSION OUTLINE Overview of
FARM TO TABLE: PESTICIDE RESIDUES AND RISK ASSESSMENT JANET V. COWINS, Ph.D. CHEMIST U.S. ENVIRONMENTAL PROTECTION AGENCY HEALTH EFFECTS DIVISION/RISK ASSESSMENT BRANCH 2 DISCUSSION OUTLINE Overview of
Human Health Risk Assessment Overview [For the APS/OPP Roundtable]
![Human Health Risk Assessment Overview [For the APS/OPP Roundtable] Human Health Risk Assessment Overview [For the APS/OPP Roundtable]](/thumbs/74/69622095.jpg) Human Health Risk Assessment Overview [For the APS/OPP Roundtable] Christina Swartz, USEPA Nov. 12, 2008 [Slides Courtesy of Mike Metzger, USEPA] The Risk Assessment Paradigm: The Red Book Hazard Identification
Human Health Risk Assessment Overview [For the APS/OPP Roundtable] Christina Swartz, USEPA Nov. 12, 2008 [Slides Courtesy of Mike Metzger, USEPA] The Risk Assessment Paradigm: The Red Book Hazard Identification
Before the OFFICE OF MANAGEMENT AND BUDGET Washington, D.C.
 Before the OFFICE OF MANAGEMENT AND BUDGET Washington, D.C. In the Matter of ) ) ) Notice of Information Collection Being ) OMB Control No. 3060-0761 Submitted to the Office of Management and ) Budget
Before the OFFICE OF MANAGEMENT AND BUDGET Washington, D.C. In the Matter of ) ) ) Notice of Information Collection Being ) OMB Control No. 3060-0761 Submitted to the Office of Management and ) Budget
REASONED OPINION. European Food Safety Authority 2, 3. European Food Safety Authority (EFSA), Parma, Italy
 EFSA Journal 2013;11(7):3339 REASONED OPINION Reasoned opinion on the review of the existing maximum residue levels (MRLs) for methyl bromide according to Article 12 of Regulation (EC) No 396/2005 1 European
EFSA Journal 2013;11(7):3339 REASONED OPINION Reasoned opinion on the review of the existing maximum residue levels (MRLs) for methyl bromide according to Article 12 of Regulation (EC) No 396/2005 1 European
Mono- and Di-Potassium Salts of Phosphorous Acid
 Registration Decision RD2013-10 Mono- and Di-Potassium Salts of Phosphorous Acid (publié aussi en français) 9 July 2013 This document is published by the Health Canada Pest Management Regulatory Agency.
Registration Decision RD2013-10 Mono- and Di-Potassium Salts of Phosphorous Acid (publié aussi en français) 9 July 2013 This document is published by the Health Canada Pest Management Regulatory Agency.
Mandates of the FQPA. ES/RP 532 Applied Environmental Toxicology. Tolerance. Mandate of FIFRA. FQPA Mandate. How Tolerance Is Developed
 Mandates of the FQPA ES/RP 532 Applied Environmental Toxicology Lecture 11 Pesticides: Human Health Risk Assessment (How EPA Assesses Aggregate & Cumulative & Characterizes Risk) All tolerances are safe
Mandates of the FQPA ES/RP 532 Applied Environmental Toxicology Lecture 11 Pesticides: Human Health Risk Assessment (How EPA Assesses Aggregate & Cumulative & Characterizes Risk) All tolerances are safe
Basic Information About Food and Facility
 The Farm to School project goal is to increase the use of seasonal, fresh, New York grown foods in school meals. We want your active voice and participation in planning and implementing this project. Please
The Farm to School project goal is to increase the use of seasonal, fresh, New York grown foods in school meals. We want your active voice and participation in planning and implementing this project. Please
Inter-Agency Overlap and Jurisdictional Boundaries
 Inter-Agency Overlap and Jurisdictional Boundaries FDLI Food Advertising, Labeling, and Litigation Conference September 14, 2017 Jessica P. O Connell Covington & Burling LLP jpoconnell@cov.com 1 FDA Approach/Perspective
Inter-Agency Overlap and Jurisdictional Boundaries FDLI Food Advertising, Labeling, and Litigation Conference September 14, 2017 Jessica P. O Connell Covington & Burling LLP jpoconnell@cov.com 1 FDA Approach/Perspective
My Diabetic Meal Plan during Pregnancy
 My Diabetic Meal Plan during Pregnancy When you have diabetes and are pregnant, you need to eat small meals and s throughout the day to help control your blood sugar. This also helps you get in enough
My Diabetic Meal Plan during Pregnancy When you have diabetes and are pregnant, you need to eat small meals and s throughout the day to help control your blood sugar. This also helps you get in enough
EXPOSURE ASSESSMENT: CURRENT SITUATION IN THE PHILIPPINES
 EXPOSURE ASSESSMENT: CURRENT SITUATION IN THE PHILIPPINES INTRODUCTION Risk assessments has grown significantly, even in developing countries, in light of the WTO s Agreement on the Application of Sanitary
EXPOSURE ASSESSMENT: CURRENT SITUATION IN THE PHILIPPINES INTRODUCTION Risk assessments has grown significantly, even in developing countries, in light of the WTO s Agreement on the Application of Sanitary
SUPPLEMENT TO NATURAL RESOURCES DEFENSE COUNCIL S PETITION TO CANCEL PET COLLAR USES FOR THE PESTICIDE PROPOXUR
 January 18, 2011 SUPPLEMENT TO NATURAL RESOURCES DEFENSE COUNCIL S PETITION TO CANCEL PET COLLAR USES FOR THE PESTICIDE PROPOXUR On November 26, 2007, the Natural Resources Defense Council (NRDC) filed
January 18, 2011 SUPPLEMENT TO NATURAL RESOURCES DEFENSE COUNCIL S PETITION TO CANCEL PET COLLAR USES FOR THE PESTICIDE PROPOXUR On November 26, 2007, the Natural Resources Defense Council (NRDC) filed
Problems with the 1906 Act
 Problems with the 1906 Act failed to provide clear-cut meanings and specific means for enforcement insufficient funding for enforcement USDA was responsible for testing, but no standards for foods were
Problems with the 1906 Act failed to provide clear-cut meanings and specific means for enforcement insufficient funding for enforcement USDA was responsible for testing, but no standards for foods were
Nutrition Essentials Improving your PKU diet through balanced nutrition
 Nutrition Essentials Improving your PKU diet through balanced nutrition Sharon L Ernst, MPH, RD, CSP, FAND Associate Professor Chief Metabolic Dietitian Division of Medical Genetics Department of Pediatrics
Nutrition Essentials Improving your PKU diet through balanced nutrition Sharon L Ernst, MPH, RD, CSP, FAND Associate Professor Chief Metabolic Dietitian Division of Medical Genetics Department of Pediatrics
How Much Pesticide is on My Plate?
 How Much Pesticide is on My Plate? Dr. Jeff Miller https://desdaughter.com 1 The Dirty Dozen, from the Environmental Working Group Dirty Dozen PLUS 51 produce items Potatoes = #12 Beginning Statements
How Much Pesticide is on My Plate? Dr. Jeff Miller https://desdaughter.com 1 The Dirty Dozen, from the Environmental Working Group Dirty Dozen PLUS 51 produce items Potatoes = #12 Beginning Statements
Food Sources of Soluble Fibre
 Food Sources of Soluble Fibre Dietary fibre comes from plant foods. There are two types: soluble and insoluble fibre. Most fibre containing foods have a mix of both. Insoluble fibre is found in the skins
Food Sources of Soluble Fibre Dietary fibre comes from plant foods. There are two types: soluble and insoluble fibre. Most fibre containing foods have a mix of both. Insoluble fibre is found in the skins
BODY RESPONSIBLE FOR IMPLEMENTING THE SCHEME
 TITLE OF THE SCHEME School Fruit and Vegetables Scheme 2015-2016 Page 1 of 7 LEGAL BASIS 1. Council Regulation (EU) No 1370/2013 of 16 December 2013. 2. Regulation (EU) No 1308/2013 of the European Parliament
TITLE OF THE SCHEME School Fruit and Vegetables Scheme 2015-2016 Page 1 of 7 LEGAL BASIS 1. Council Regulation (EU) No 1370/2013 of 16 December 2013. 2. Regulation (EU) No 1308/2013 of the European Parliament
ECPA position paper on the criteria for the determination of endocrine disrupting properties under Regulation
 POSITION PAPER 09/06/2016 PP/14/PD/23734 ECPA position paper on the criteria for the determination of endocrine disrupting properties under Regulation The European Commission is currently developing new
POSITION PAPER 09/06/2016 PP/14/PD/23734 ECPA position paper on the criteria for the determination of endocrine disrupting properties under Regulation The European Commission is currently developing new
Objections to March 29, 2017 Order Denying PAN/NRDC Petition to Revoke All Tolerances and Cancel All Registrations for the Pesticide Chlorpyrifos
 Objections to March 29, 2017 Order Denying PAN/NRDC Petition to Revoke All Tolerances and Cancel All Registrations for the Pesticide Chlorpyrifos Submitted by: Earthjustice June 5, 2017 Docket No. EPA-HQ-OPP-2007-1005
Objections to March 29, 2017 Order Denying PAN/NRDC Petition to Revoke All Tolerances and Cancel All Registrations for the Pesticide Chlorpyrifos Submitted by: Earthjustice June 5, 2017 Docket No. EPA-HQ-OPP-2007-1005
IgG Food Antibody Assessment (Serum)
 IgG Food Antibody Assessment (Serum) Patient: DOB: Sex: MRN: Order Number: Completed: Received: Collected: IgG Food Antibody Results Dairy Vegetables Fish/Shellfish Nuts and Grains Casein Cheddar cheese
IgG Food Antibody Assessment (Serum) Patient: DOB: Sex: MRN: Order Number: Completed: Received: Collected: IgG Food Antibody Results Dairy Vegetables Fish/Shellfish Nuts and Grains Casein Cheddar cheese
Residue Chemistry Test Guidelines OPPTS Processed Food/Feed
 United States Environmental Protection Agency Prevention, Pesticides and Toxic Substances (7101) EPA 712 C 96 184 August 1996 Residue Chemistry Test Guidelines OPPTS 860.1520 Processed Food/Feed INTRODUCTION
United States Environmental Protection Agency Prevention, Pesticides and Toxic Substances (7101) EPA 712 C 96 184 August 1996 Residue Chemistry Test Guidelines OPPTS 860.1520 Processed Food/Feed INTRODUCTION
2. DEFINING REASONABLE CERTAINTY OF NO HARM
 2. DEFINING REASONABLE CERTAINTY OF NO HARM HOW HAS EPA DEFINED ACCEPTABLE EXPOSURE TO MEET THE SAFETY STANDARD OF THE FQPA, AND HOW WELL HAS EPA USED THE ACT S INNOVATIVE SAFETY-FACTOR PROVISIONS? To
2. DEFINING REASONABLE CERTAINTY OF NO HARM HOW HAS EPA DEFINED ACCEPTABLE EXPOSURE TO MEET THE SAFETY STANDARD OF THE FQPA, AND HOW WELL HAS EPA USED THE ACT S INNOVATIVE SAFETY-FACTOR PROVISIONS? To
European Union comments for the. CODEX COMMITTEE ON PESTICIDE RESIDUES 44th Session. Shanghai, China, April 2012.
 - 1-16/04/2012 European Union comments for the CODEX COMMITTEE ON PESTICIDE RESIDUES 44th Session Shanghai, China, 23-28 April 2012 Agenda Item 6 a) Draft and Proposed Draft Maximum Residue Limits for
- 1-16/04/2012 European Union comments for the CODEX COMMITTEE ON PESTICIDE RESIDUES 44th Session Shanghai, China, 23-28 April 2012 Agenda Item 6 a) Draft and Proposed Draft Maximum Residue Limits for
Eating Healthier: Six Simple Steps
 Eating Healthier: Six Simple Steps Most of us are interested in learning how to eat a healthier diet. As a coach, you can support your client by making simple suggestions as well as giving her resources
Eating Healthier: Six Simple Steps Most of us are interested in learning how to eat a healthier diet. As a coach, you can support your client by making simple suggestions as well as giving her resources
School Meal Programs Lessons Learned
 School Meal Programs Lessons Learned Presentation to Institute of Medicine Food and Nutrition Board Committee on Nutrition Standards in Schools April 21, 2006 1 Child Nutrition Division Stanley Garnett,
School Meal Programs Lessons Learned Presentation to Institute of Medicine Food and Nutrition Board Committee on Nutrition Standards in Schools April 21, 2006 1 Child Nutrition Division Stanley Garnett,
Cypermethrins CYPERMETHRINS (INCLUDING ALPHA- AND ZETA-CYPERMETHRIN) (118)
 Cypermethrins 51 5.5 CYPERMETHRINS (INCLUDING ALPHA- AND ZETA-CYPERMETHRIN) (118) RESIDUE AND ANALYTICAL ASPECTS Cypermethrins was evaluated by JMPR 1979 (T, R), 1981 (T, R), 1982 (R), 1983 (R), 1984 (R),
Cypermethrins 51 5.5 CYPERMETHRINS (INCLUDING ALPHA- AND ZETA-CYPERMETHRIN) (118) RESIDUE AND ANALYTICAL ASPECTS Cypermethrins was evaluated by JMPR 1979 (T, R), 1981 (T, R), 1982 (R), 1983 (R), 1984 (R),
April 30, National Organic Standards Board Spring 2012 Meeting Albuquerque, NM. Re. Vaccines from Excluded Methods. Dear Board Members:
 National Organic Standards Board Spring 2012 Meeting Albuquerque, NM Dear Board Members: These comments are submitted on behalf of Beyond Pesticides. Beyond Pesticides, founded in 1981 as a national, grassroots,
National Organic Standards Board Spring 2012 Meeting Albuquerque, NM Dear Board Members: These comments are submitted on behalf of Beyond Pesticides. Beyond Pesticides, founded in 1981 as a national, grassroots,
Sample Report Exclusively in the UK and Ireland Contact: Regenerus Laboratories T: +44 (0) E:
 Sample Report Exclusively in the UK and Ireland Contact: Regenerus Laboratories T: + () 7 87 E: info@regeneruslabs.com Patient Information Sheet: Name: Jon Doe Example Date Drawn: //6 Date of Birth: //966
Sample Report Exclusively in the UK and Ireland Contact: Regenerus Laboratories T: + () 7 87 E: info@regeneruslabs.com Patient Information Sheet: Name: Jon Doe Example Date Drawn: //6 Date of Birth: //966
Principles of the DASH Diet
 DASH Diet Lower your blood pressure by changing your eating habits. The DASH diet is based on findings from the "Dietary Approaches to Stop Hypertension" clinical study that found that high blood pressure
DASH Diet Lower your blood pressure by changing your eating habits. The DASH diet is based on findings from the "Dietary Approaches to Stop Hypertension" clinical study that found that high blood pressure
Fruit Juice and Vegetable Juice as Color Additives in Food: Guidance for Industry
 Fruit Juice and Vegetable Juice as Color Additives in Food: Guidance for Industry Draft Guidance This guidance is being distributed for comment purposes only. Although you can comment on any guidance at
Fruit Juice and Vegetable Juice as Color Additives in Food: Guidance for Industry Draft Guidance This guidance is being distributed for comment purposes only. Although you can comment on any guidance at
Fiber In Your Diet. Provided by Hemorrhoid Centers of America Version Fiber
 In Your Diet The lack of dietary fiber and fluids is a contributing factor to the development of hemorrhoids and anal fissures. We recommend consuming 25-35 grams of fiber and drinking 7 glasses of fluids
In Your Diet The lack of dietary fiber and fluids is a contributing factor to the development of hemorrhoids and anal fissures. We recommend consuming 25-35 grams of fiber and drinking 7 glasses of fluids
25* or higher Underweight. 240 mg/dl and above High (More than twice the risk as desirable level.) OK, but higher is better
 Biometrics Screening Biometric Health Indicators The charts below provide a summary of the ranges for each of the biometric screening tests. Be sure to check with your doctor if your results are outside
Biometrics Screening Biometric Health Indicators The charts below provide a summary of the ranges for each of the biometric screening tests. Be sure to check with your doctor if your results are outside
588-Complete Dietary Antigen Testing
 18716_REPORT_COMPETE DUNWOODY ABS 9 Dunwoody Park, Suite 121 Dunwoody, GA 3338 P: 678-736-6374 F: 77-674-171 Email: info@dunwoodylabs.com www.dunwoodylabs.com PATIENT INFO NAME: SAMPE PATIENT REQUISITION
18716_REPORT_COMPETE DUNWOODY ABS 9 Dunwoody Park, Suite 121 Dunwoody, GA 3338 P: 678-736-6374 F: 77-674-171 Email: info@dunwoodylabs.com www.dunwoodylabs.com PATIENT INFO NAME: SAMPE PATIENT REQUISITION
SUMMARY: This Interim final rule amends the Department of Agriculture s (USDA) National
 DEPARTMENT OF AGRICULTURE Agricultural Marketing Service 7 CFR Part 205 [Docket No. AMS-TM-07-0062; TM-07-06IF] RIN 0581-AC71 National Organic Program (NOP) Amendments to the National List of Allowed and
DEPARTMENT OF AGRICULTURE Agricultural Marketing Service 7 CFR Part 205 [Docket No. AMS-TM-07-0062; TM-07-06IF] RIN 0581-AC71 National Organic Program (NOP) Amendments to the National List of Allowed and
COMMISSION REGULATION (EU) / of XXX
 EUROPEAN COMMISSION Brussels, XXX SANTE/10154/2018 Rev. 2 [ ](2018) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament
EUROPEAN COMMISSION Brussels, XXX SANTE/10154/2018 Rev. 2 [ ](2018) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament
Contribution to the European Commission s Public Consultation on. 15 January 2015
 Contribution to the European Commission s Public Consultation on Defining criteria for identifying Endocrine Disruptors in the context of the implementation of the Plant Protection Product Regulation and
Contribution to the European Commission s Public Consultation on Defining criteria for identifying Endocrine Disruptors in the context of the implementation of the Plant Protection Product Regulation and
European Community Positions for the 41 st Session of the Codex Committee on Pesticide Residues Beijing, China April 2009
 European Community Positions for the 41 st Session of the Codex Committee on Pesticide Residues Beijing, China. 20-25 April 2009 Agenda Items 4a, 4b, 5, 6 and 10 Agenda Item 4a JMPR Report 2008, Point
European Community Positions for the 41 st Session of the Codex Committee on Pesticide Residues Beijing, China. 20-25 April 2009 Agenda Items 4a, 4b, 5, 6 and 10 Agenda Item 4a JMPR Report 2008, Point
SUMMARY: We are amending the fruits and vegetables regulations to allow the importation of
 This document is scheduled to be published in the Federal Register on 04/08/2016 and available online at http://federalregister.gov/a/2016-08191, and on FDsys.gov BILLING CODE: 3410-34-P DEPARTMENT OF
This document is scheduled to be published in the Federal Register on 04/08/2016 and available online at http://federalregister.gov/a/2016-08191, and on FDsys.gov BILLING CODE: 3410-34-P DEPARTMENT OF
Activity #4: Healthy Food Festival!
 Activity #4: Healthy Food Festival! Activity Overview In this activity, students apply their new learning to create fun ways to eat the rainbow during the week. Students are encouraged to consider the
Activity #4: Healthy Food Festival! Activity Overview In this activity, students apply their new learning to create fun ways to eat the rainbow during the week. Students are encouraged to consider the
Before the FEDERAL COMMUNICATIONS COMMISSION Washington, DC REPLY COMMENTS OF THE TELECOMMUNICATIONS INDUSTRY ASSOCIATION
 Before the FEDERAL COMMUNICATIONS COMMISSION Washington, DC 20554 In the Matter of Closed Captioning of Internet Protocol-Delivered Video Programming: Implementation of the Twenty- First Century Communications
Before the FEDERAL COMMUNICATIONS COMMISSION Washington, DC 20554 In the Matter of Closed Captioning of Internet Protocol-Delivered Video Programming: Implementation of the Twenty- First Century Communications
Food. Food Groups & Nutrients
 Food Food Groups & Nutrients Grains Group Grains Group Defined: Foods made from wheat, rice, oats, barley, etc. Grains Group Defined: Foods made from wheat, rice, oats, barley, etc. Examples: bread,
Food Food Groups & Nutrients Grains Group Grains Group Defined: Foods made from wheat, rice, oats, barley, etc. Grains Group Defined: Foods made from wheat, rice, oats, barley, etc. Examples: bread,
Connect with schools. Meet with the school nutrition director
 Connect with schools Start to build relationships with nutrition directors and school community members by searching for schools in your area: www.dpi.state.wi.us/schldist.html. Organizations listed in
Connect with schools Start to build relationships with nutrition directors and school community members by searching for schools in your area: www.dpi.state.wi.us/schldist.html. Organizations listed in
Registration Decision. Metconazole
 Registration Decision RD2015-05 Metconazole (publié aussi en français) 21 April 2015 This document is published by the Health Canada Pest Management Regulatory Agency. For further information, please contact:
Registration Decision RD2015-05 Metconazole (publié aussi en français) 21 April 2015 This document is published by the Health Canada Pest Management Regulatory Agency. For further information, please contact:
OVERVIEW OF FOOD SAFETY REGULATION IN THE UNITED STATES. Presented By William C. Balek International Sanitary Supply Association March 30, 2001
 OVERVIEW OF FOOD SAFETY REGULATION IN THE UNITED STATES Presented By William C. Balek International Sanitary Supply Association March 30, 2001 I INTRODUCTION The regulation of food safety in the United
OVERVIEW OF FOOD SAFETY REGULATION IN THE UNITED STATES Presented By William C. Balek International Sanitary Supply Association March 30, 2001 I INTRODUCTION The regulation of food safety in the United
Quality Certification in Italy and in the Mediterranean countries: a way for accessing foreign markets. Davide Pierleoni
 Quality Certification in Italy and in the Mediterranean countries: a way for accessing foreign markets Davide Pierleoni Vice President Istituto Mediterraneo di Certificazione srl Presentation of IMC IMC
Quality Certification in Italy and in the Mediterranean countries: a way for accessing foreign markets Davide Pierleoni Vice President Istituto Mediterraneo di Certificazione srl Presentation of IMC IMC
JMPR Review and MRL Recommendations Prof. Dr. Árpád Ambrus
 JMPR Review and MRL Recommendations Prof. Dr. Árpád Ambrus Deputy Director General Hungarian Food Safety Office Budapest Outline Structure and operation of JMPR Type of evaluations Data and information
JMPR Review and MRL Recommendations Prof. Dr. Árpád Ambrus Deputy Director General Hungarian Food Safety Office Budapest Outline Structure and operation of JMPR Type of evaluations Data and information
APPROVED: 4 December 2015 PUBLISHED: 9 December 2015
 REASONED OPINION APPROVED: 4 December 2015 PUBLISHED: 9 December 2015 doi:10.2903/j.efsa.2015.4356 Review of the existing maximum residue levels for sodium 5-nitroguaiacolate, sodium o-nitrophenolate and
REASONED OPINION APPROVED: 4 December 2015 PUBLISHED: 9 December 2015 doi:10.2903/j.efsa.2015.4356 Review of the existing maximum residue levels for sodium 5-nitroguaiacolate, sodium o-nitrophenolate and
Chemical food safety in the U.S. analysis of FDA s scientific basis for assessing chemical risk. Tom Neltner October 9, 2014
 Chemical food safety in the U.S. analysis of FDA s scientific basis for assessing chemical risk Tom Neltner October 9, 2014 Topics 1. Current focus of U.S. public interest community 2. Comparison of U.S.
Chemical food safety in the U.S. analysis of FDA s scientific basis for assessing chemical risk Tom Neltner October 9, 2014 Topics 1. Current focus of U.S. public interest community 2. Comparison of U.S.
December 4, 2017 VIA ELECTRONIC SUBMISSION
 VIA ELECTRONIC SUBMISSION December 4, 2017 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Development of a List of pre-dietary Supplement
VIA ELECTRONIC SUBMISSION December 4, 2017 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Development of a List of pre-dietary Supplement
REQUIREMENTS FOR ACCREDITED LABORATORIES APPLYING FOR A FLEXIBLE SCOPE FOR ANALYSIS OF PESTICIDE RESIDUES IN FOOD AND FEED
 BELAC 2-104 Rev 2-2009 REQUIREMENTS FOR ACCREDITED LABORATORIES APPLYING FOR A FLEXIBLE SCOPE FOR ANALYSIS OF PESTICIDE RESIDUES IN FOOD AND FEED English translation for information only French and Dutch
BELAC 2-104 Rev 2-2009 REQUIREMENTS FOR ACCREDITED LABORATORIES APPLYING FOR A FLEXIBLE SCOPE FOR ANALYSIS OF PESTICIDE RESIDUES IN FOOD AND FEED English translation for information only French and Dutch
Food Sensations Quiz (Primary Schools 2010)
 Food Sensations Quiz (Primary Schools 2010) January 2011 Christina Mills Research and Evaluation Consultant christina.mills@westnet.com.au 0404159241 INTRODUCTION The Food Sensations program is run by
Food Sensations Quiz (Primary Schools 2010) January 2011 Christina Mills Research and Evaluation Consultant christina.mills@westnet.com.au 0404159241 INTRODUCTION The Food Sensations program is run by
Du r i n g the past tw^o years there has been a decided increase
 THE VITAMIN A, VITAMIN Bi (THIAMIN), VITAMIN C (ASCORBIC ACID) AND RIBO FLA V IN CO N TEN T OF COMMON FOODS A S U M M A R Y O F R E P R E S E N T A T IV E V A L U E S H a z e l E. M u n s e l l "^ Du r
THE VITAMIN A, VITAMIN Bi (THIAMIN), VITAMIN C (ASCORBIC ACID) AND RIBO FLA V IN CO N TEN T OF COMMON FOODS A S U M M A R Y O F R E P R E S E N T A T IV E V A L U E S H a z e l E. M u n s e l l "^ Du r
