COMMISSION REGULATION (EU) / of XXX
|
|
|
- Kathlyn Sharp
- 5 years ago
- Views:
Transcription
1 EUROPEAN COMMISSION Brussels, XXX SANTE/10530/2015 Rev. 0 [ ](2015) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for boscalid, captan, clothianidin, thiametoxam, folpet and tolclofos-methyl in or on certain products EN EN
2 COMMISSION REGULATION (EU) / of XXX amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for boscalid, captan, clothianidin, thiametoxam, folpet and tolclofos-methyl in or on certain products (Text with EEA relevance) THE EUROPEAN COMMISSION, Having regard to the Treaty on the Functioning of the European Union, Having regard to Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC 1, and in particular Article 14(1)(a), Article 18(1)(b) and Article 49(2) thereof, Whereas: (1) For boscalid, clothianidin, thiamethoxam and tolclofos-methyl maximum residue levels (MRLs) were set in Part A of Annex III to Regulation (EC) No 396/2005. For captan and folpet, MRLs were set in Annex II and Part B of Annex III to that Regulation. (2) For boscalid, the European Food Safety Authority, hereinafter "the Authority", submitted a reasoned opinion on the existing MRLs in accordance with Article 12(2) of Regulation (EC) No 396/2005 in conjunction with Article 12(1) thereof 2. It proposed to change the residue definition. It concluded that concerning all the MRLs under evaluation some information was not available and that further consideration by risk managers was required. It indicated that a potential for accumulation of boscalid residues in crops grown in rotation is expected. It calculated MRLs which take or take not this potential for accumulation into account and left it to risk managers to choose the required option. As there is no risk for consumers, the MRLs should be set in Annex II to Regulation (EC) No 396/2005 at the level identified by the Authority which considers the potential for accumulation. These MRLs will be reviewed; the review will take into account the information available within two years from the publication of this Regulation. (3) For captan, the Authority submitted a reasoned opinion on the existing MRLs in accordance with Article 12(2) of Regulation (EC) No 396/2005 in conjunction with Article 12(1) thereof 3. It proposed to change the residue definition. It recommended raising or keeping the existing MRLs for certain products. It concluded that OJ L 070, , p. 1. EFSA (European Food Safety Authority), Reasoned opinion on the review of the existing maximum residue levels (MRLs) for boscalid according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2014;12(7):3799, 127 pp. EFSA (European Food Safety Authority), Reasoned opinion on the review of the existing maximum residue levels (MRLs) for captan according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2014;12(4):3663, 55 pp. EN 2 EN
3 concerning the MRL for apples, pears, quinces, medlar, loquat, apricots, cherries, peaches, plums, strawberries, blackberries, raspberries, blueberries, currants, gooseberries and tomatoes some information was not available and that further consideration by risk managers was required. As there is no risk for consumers, the MRL for these products should be set in Annex II to Regulation (EC) No 396/2005 at the existing level or the level identified by the Authority. This MRL will be reviewed; the review will take into account the information available within two years from the publication of this Regulation. The Authority concluded that concerning the MRL for almonds, table grapes, wine grapes, potatoes, cucumbers, melons, scarole, leek, maize and sorghum no information was available and that further consideration by risk managers was required. The MRL for these products should be set at the specific limit of determination. (4) For clothianidin, the Authority submitted a reasoned opinion on the existing MRLs in accordance with Article 12(2) of Regulation (EC) No 396/2005 in conjunction with Article 12(1) thereof 4. It recommended lowering the MRLs for pecans, papaya, potatoes, tomatoes, peppers, aubergines, sweet corn, cauliflower, leafy brassica, lettuce, chervil, beans (fresh, without pods), peas (fresh, without pods), fresh lentils, cotton seed, sorghum grain, cocoa and chicory roots. For other products it recommended raising or keeping the existing MRLs. It concluded that concerning the MRLs for citrus fruits, cherries, table and wine grapes, strawberries, pineapples, melons, watermelons, kohlrabi and scarole some information was not available and that further consideration by risk managers was required. As there is no risk for consumers, the MRLs for those products should be set in Annex II to Regulation (EC) No 396/2005 at the existing level or the level identified by the Authority. These MRLs will be reviewed; the review will take into account the information available within two years from the publication of this Regulation. (5) For thiamethoxam, the Authority submitted a reasoned opinion on the existing MRLs in accordance with Article 12(2) of Regulation (EC) No 396/2005 in conjunction with Article 12(1) thereof 5. It proposed to change the residue definition and recommended lowering the MRLs for pecans, pome fruits, peaches, table olives, bananas, papaya, potatoes, swedes, sweet corn, cauliflower, Brussels sprouts, head cabbage, leafy brassica, beans (fresh, with and without pods), peas (fresh, without pods), fresh lentils, pulses, linseed, peanuts, poppy seed, sesame seed, sunflower seed, rape seed, soya bean, mustard seed, cotton seed, pumpkin seeds, safflower, borage, gold of pleasure, hempseed, castor bean, olives for oil production, oats grain, rye grain, cocoa, sugar beet (roots), swine (muscle, liver, kidney), bovine (muscle, liver, kidney), sheep (muscle, liver, kidney) and goat (muscle, liver, kidney). For other products it recommended raising or keeping the existing MRLs. It concluded that concerning the MRLs for citrus fruits, apricots, cherries, table and wine grapes, strawberries, pineapples, melons, watermelons and scarole some information was not available and that further consideration by risk managers was required. As there is no risk for consumers, the MRLs for those products should be set in Annex II to Regulation (EC) No 396/2005 at the existing level or the level identified by the 4 5 EFSA (European Food Safety Authority), Reasoned opinion on the review of the existing maximum residue levels (MRLs) for clothianidin and thiamethoxam according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2014;12(12):3918, 120 pp. doi: /j.efsa EFSA (European Food Safety Authority), Reasoned opinion on the review of the existing maximum residue levels (MRLs) for clothianidin and thiamethoxam according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2014;12(12):3918, 120 pp. doi: /j.efsa EN 3 EN
4 Authority. These MRLs will be reviewed; the review will take into account the information available within two years from the publication of this Regulation. (6) For folpet, the Authority submitted a reasoned opinion on the existing MRLs in accordance with Article 12(2) of Regulation (EC) No 396/2005 in conjunction with Article 12(1) thereof 6. It recommended raising or keeping the existing MRLs for certain products. It concluded that concerning the MRLs for strawberries, table olives, potatoes, radishes, salsify, tomatoes, melons, olives for oil production, barley grain, wheat grain, hops (dried), poultry (meat, fat, liver) and birds eggs some information was not available and that further consideration by risk managers was required. As there is no risk for consumers, the MRLs for those products should be set in Annex II to Regulation (EC) No 396/2005 at the existing level or the level identified by the Authority. These MRLs will be reviewed; the review will take into account the information available within two years from the publication of this Regulation. The Authority concluded that concerning the MRLs for garlic, onions, shallots, spring onions, kohlrabi, lettuce, scarole, spinach and beans (fresh, without pods) no information was available and that further consideration by risk managers was required. The MRLs for these products should be set at the specific limit of determination. (7) For tolclofos-methyl, the Authority, submitted a reasoned opinion on the existing MRLs in accordance with Article 12(2) of Regulation (EC) No 396/2005 in conjunction with Article 12(1) thereof 7. The Authority recommended keeping the MRL for potatoes. It concluded that concerning the MRLs for radishes, broccoli, cauliflower, Brussels sprouts, head cabbage, lamb`s lettuce, lettuce, scarole (broadleave endive), cress, land cress, rocket, rucola, red mustard and leaves and sprouts of Brassica spp. some information was not available and that further consideration by risk managers was required. As there is no risk for consumers, the MRLs for those products should be set in Annex II to Regulation (EC) No 396/2005 at the existing level or the level identified by the Authority. These MRLs will be reviewed; the review will take into account the information available within two years from the publication of this Regulation. The Authority concluded that concerning the MRLs for swedes, turnips, Chinese cabbage, kale, kohlrabi, celery, swine (muscle, fat, liver, kidney), bovine (muscle, fat, liver, kidney), sheep (muscle, fat, liver, kidney), goat (muscle, fat, liver, kidney), poultry (muscle, fat, liver), milk (cattle, sheep, goat) and birds`eggs no information was available and that further consideration by risk managers was required. The MRLs for these products should be set at the specific limit of determination. (8) As regards products on which the use of the plant protection product concerned is not authorised, and for which no import tolerances or CXLs exist, MRLs should, be set at the specific limit of determination or the default MRL should apply, as provided for in Article 18(1)(b) of Regulation (EC) No 396/ EFSA (European Food Safety Authority), Reasoned opinion on the review of the existing maximum residue levels (MRLs) for folpet according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2014;12(5):3700, 55 pp. European Food Safety Authority, Reasoned opinion on the review of the existing maximum residue levels (MRLs) for tolclofos-methyl according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2014;12(12):3920, [42 pp. ] EN 4 EN
5 (9) The Commission consulted the European Union reference laboratories for residues of pesticides as regards the need to adapt certain limits of determination. As regards several substances, those laboratories concluded that for certain commodities technical development requires the setting of specific limits of determination. (10) Based on the reasoned opinions of the Authority and taking into account the factors relevant to the matter under consideration, the appropriate modifications to the MRLs fulfil the requirements of Article 14(2) of Regulation (EC) No 396/2005. (11) Through the World Trade Organisation, the trading partners of the Union were consulted on the new MRLs and their comments have been taken into account. (12) Regulation (EC) No 396/2005 should therefore be amended accordingly. (13) In order to allow for the normal marketing, processing and consumption of products, this Regulation should provide for a transitional arrangement for products which have been produced before the modification of the MRLs and for which information shows that a high level of consumer protection is maintained. (14) A reasonable period should be allowed to elapse before the modified MRLs become applicable, regardless of whether they are raised or lowered, in order to permit Member States, third countries and food business operators to prepare themselves to meet the new requirements which will result from the modification of the MRLs, and in particular to allow the necessary time for any adaptations of the IT systems and tools. The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on Plants, Animals, Food and Feed, HAS ADOPTED THIS REGULATION: Article 1 Annexes II and III to Regulation (EC) No 396/2005 are amended in accordance with the Annex to this Regulation. Article 2 As regards the active substances boscalid, captan, clothianidin, thiametoxam, folpet and tolclofos-methyl in and on all products, Regulation (EC) No 396/2005 as it stood before being amended by this Regulation shall continue to apply to products which were produced by [Office of Publications please insert day before the date of application of this Regulation] Article 3 This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union. It shall apply from [Office of Publication: please insert date 6 months after entry into force]. This Regulation shall be binding in its entirety and directly applicable in all Member States. Done at Brussels, For the Commission The President EN 5 EN
6 Done at Brussels, Jean-Claude JUNCKER For the Commission The President Jean-Claude JUNCKER EN 6 EN
Council of the European Union Brussels, 4 November 2015 (OR. en)
 Council of the European Union Brussels, 4 November 2015 (OR. en) 13706/15 AGRILEG 208 COVER NOTE From: European Commission date of receipt: 30 October 2015 To: No. Cion doc.: D041471/02 Subject: General
Council of the European Union Brussels, 4 November 2015 (OR. en) 13706/15 AGRILEG 208 COVER NOTE From: European Commission date of receipt: 30 October 2015 To: No. Cion doc.: D041471/02 Subject: General
COMMISSION REGULATION (EU) / of XXX
 EUROPEAN COMMISSION Brussels, XXX SANTE/11077/2016 Rev. 1 [ ](2016) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and
EUROPEAN COMMISSION Brussels, XXX SANTE/11077/2016 Rev. 1 [ ](2016) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and
Council of the European Union Brussels, 12 August 2014 (OR. en) Mr Uwe CORSEPIUS, Secretary-General of the Council of the European Union
 Council of the European Union Brussels, 12 August 2014 (OR. en) 12459/14 AGRILEG 168 COVER NOTE From: European Commission date of receipt: 8 August 2014 To: No. Cion doc.: D033914/02 Subject: Mr Uwe CORSEPIUS,
Council of the European Union Brussels, 12 August 2014 (OR. en) 12459/14 AGRILEG 168 COVER NOTE From: European Commission date of receipt: 8 August 2014 To: No. Cion doc.: D033914/02 Subject: Mr Uwe CORSEPIUS,
COMMISSION REGULATION (EU) / of XXX
 EUROPEAN COMMISSION Brussels, XXX SANTE/11715/2017 rev.2 [ ](2018) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament
EUROPEAN COMMISSION Brussels, XXX SANTE/11715/2017 rev.2 [ ](2018) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament
COMMISSION REGULATION (EU) / of XXX
 EUROPEAN COMMISSION Brussels, XXX SANTE/10154/2018 Rev. 2 [ ](2018) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament
EUROPEAN COMMISSION Brussels, XXX SANTE/10154/2018 Rev. 2 [ ](2018) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament
Council of the European Union Brussels, 5 December 2014 (OR. en) Mr Uwe CORSEPIUS, Secretary-General of the Council of the European Union
 Council of the European Union Brussels, 5 December 2014 (OR. en) 16594/14 AGRILEG 254 COVER NOTE From: European Commission date of receipt: 3 December 2014 To: No. Cion doc.: D035772/02 Subject: Mr Uwe
Council of the European Union Brussels, 5 December 2014 (OR. en) 16594/14 AGRILEG 254 COVER NOTE From: European Commission date of receipt: 3 December 2014 To: No. Cion doc.: D035772/02 Subject: Mr Uwe
Setting of new MRLs for fluxapyroxad (BAS 700 F) in various commodities of plant and animal origin 1
 : EFSA Journal 2011;9(6):2196 REASONED OPINION Setting of new MRLs for fluxapyroxad (BAS 700 F) in various commodities of plant and animal origin 1 European Food Safety Authority 2 European Food Safety
: EFSA Journal 2011;9(6):2196 REASONED OPINION Setting of new MRLs for fluxapyroxad (BAS 700 F) in various commodities of plant and animal origin 1 European Food Safety Authority 2 European Food Safety
COMMISSION REGULATION (EU) / of XXX
 EUROPEAN COMMISSION Brussels, XXX SANTE/10893/2018 Rev. 1 [ ](2018) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II, III, IV and V to Regulation (EC) No 396/2005 of the European Parliament
EUROPEAN COMMISSION Brussels, XXX SANTE/10893/2018 Rev. 1 [ ](2018) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II, III, IV and V to Regulation (EC) No 396/2005 of the European Parliament
COMMISSION REGULATION (EU) / of XXX
 EUROPEAN COMMISSION Brussels, XXX SANTE/12049/2017 Rev. 1 [ ](2017) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament
EUROPEAN COMMISSION Brussels, XXX SANTE/12049/2017 Rev. 1 [ ](2017) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament
Council of the European Union Brussels, 28 March 2018 (OR. en)
 Council of the European Union Brussels, 28 March 2018 (OR. en) 7563/18 AGRILEG 47 COVER NOTE From: European Commission date of receipt: 23 March 2018 To: No. Cion doc.: D055526/02 Subject: General Secretariat
Council of the European Union Brussels, 28 March 2018 (OR. en) 7563/18 AGRILEG 47 COVER NOTE From: European Commission date of receipt: 23 March 2018 To: No. Cion doc.: D055526/02 Subject: General Secretariat
COU CIL OF THE EUROPEA U IO. Brussels, 13 March 2012 (14.03) (OR. fr) 7585/12 AGRILEG 37
 COU CIL OF THE EUROPEA U IO Brussels, 13 March 2012 (14.03) (OR. fr) 7585/12 AGRILEG 37 COVER OTE from: European Commission date of receipt: 7 March 2012 to: General Secretariat of the Council No Cion
COU CIL OF THE EUROPEA U IO Brussels, 13 March 2012 (14.03) (OR. fr) 7585/12 AGRILEG 37 COVER OTE from: European Commission date of receipt: 7 March 2012 to: General Secretariat of the Council No Cion
Proposed Revision or Revocation of Maximum Residue Limits for Discontinued Agricultural Pest Control Products: Update 2
 Proposed Maximum Residue Limit PMRL2018-44 Proposed Revision or Revocation of Maximum Residue Limits for Discontinued Agricultural Pest Control Products: Update 2 (publié aussi en français) 8 November
Proposed Maximum Residue Limit PMRL2018-44 Proposed Revision or Revocation of Maximum Residue Limits for Discontinued Agricultural Pest Control Products: Update 2 (publié aussi en français) 8 November
Reasoned opinion on the review of the existing maximum residue levels (MRLs) for diquat according to Article 12 of Regulation (EC) No 396/2005 1
 EFSA Journal 2015;13(1):3972 REASONED OPINION Reasoned opinion on the review of the existing maximum residue levels (MRLs) for according to Article 12 of Regulation (EC) No 396/2005 1 European Food Safety
EFSA Journal 2015;13(1):3972 REASONED OPINION Reasoned opinion on the review of the existing maximum residue levels (MRLs) for according to Article 12 of Regulation (EC) No 396/2005 1 European Food Safety
Review of the existing maximum residue levels (MRLs) for pyraclostrobin according to Article 12 of Regulation (EC) No 396/2005 1
 EFSA Journal 2011;9(8):2344 REASONED OPINION Review of the existing maximum levels (MRLs) for pyraclostrobin according to Article 12 of Regulation (EC) No 396/2005 1 European Food Safety Authority 2, 3
EFSA Journal 2011;9(8):2344 REASONED OPINION Review of the existing maximum levels (MRLs) for pyraclostrobin according to Article 12 of Regulation (EC) No 396/2005 1 European Food Safety Authority 2, 3
Follow up assessment of MRLs for the active substance iprodione. European Food Safety Authority (EFSA)
 Page 1 of 24 EFSA Journal TECHNICAL REPORT APPROVED: 27 March 2018 doi:10.2903/sp.efsa.2018.en-1404 Abstract Follow up assessment of MRLs for the active substance iprodione European Food Safety Authority
Page 1 of 24 EFSA Journal TECHNICAL REPORT APPROVED: 27 March 2018 doi:10.2903/sp.efsa.2018.en-1404 Abstract Follow up assessment of MRLs for the active substance iprodione European Food Safety Authority
COMMISSION REGULATION (EU) No /.. of XXX. amending Regulation (EC) No 1881/2006 as regards maximum levels of lead in certain foodstuffs
 EUROPEAN COMMISSION Brussels, XXX SANCO/10946/2014 [ ](2015) XXX COMMISSION REGULATION (EU) No /.. of XXX amending Regulation (EC) No 1881/2006 as regards maximum levels of lead in certain foodstuffs (Text
EUROPEAN COMMISSION Brussels, XXX SANCO/10946/2014 [ ](2015) XXX COMMISSION REGULATION (EU) No /.. of XXX amending Regulation (EC) No 1881/2006 as regards maximum levels of lead in certain foodstuffs (Text
Review of the existing maximum residue levels (MRLs) for fludioxonil according to Article 12 of Regulation (EC) No 396/2005 1
 EFSA Journal 2011;9(8):2335 REASONED OPINION Review of the existing maximum residue levels (MRLs) for fludioxonil according to Article 12 of Regulation (EC) No 396/2005 1 European Food Safety Authority
EFSA Journal 2011;9(8):2335 REASONED OPINION Review of the existing maximum residue levels (MRLs) for fludioxonil according to Article 12 of Regulation (EC) No 396/2005 1 European Food Safety Authority
WORKING DOCUMENT DOES NOT NECESSARILY REPRESENT THE VIEWS OF THE EUROPEAN COMMISSION SERVICES
 EUROPEAN COMMISSION Brussels, XXX SANTE/11728/2016 [ ](2016) XXX draft of XXX in certain raw apricot kernels and derived products (Text with EEA relevance) WORKING DOCUMT DOES NOT NECESSARILY REPREST THE
EUROPEAN COMMISSION Brussels, XXX SANTE/11728/2016 [ ](2016) XXX draft of XXX in certain raw apricot kernels and derived products (Text with EEA relevance) WORKING DOCUMT DOES NOT NECESSARILY REPREST THE
Council of the European Union Brussels, 24 March 2015 (OR. en)
 Council of the European Union Brussels, 24 March 2015 (OR. en) 7498/15 DLEG 43 AGRI 154 SAN 84 COVER NOTE From: European Commission date of receipt: 24 March 2015 To: No. Cion doc.: D038008/02 Subject:
Council of the European Union Brussels, 24 March 2015 (OR. en) 7498/15 DLEG 43 AGRI 154 SAN 84 COVER NOTE From: European Commission date of receipt: 24 March 2015 To: No. Cion doc.: D038008/02 Subject:
Delegations will find attached document D051559/03.
 Council of the European Union Brussels, 9 November 2017 (OR. en) 14153/17 DLEG 98 AGRI 614 SAN 406 COVER NOTE From: European Commission date of receipt: 8 November 2017 To: No. Cion doc.: D051559/03 Subject:
Council of the European Union Brussels, 9 November 2017 (OR. en) 14153/17 DLEG 98 AGRI 614 SAN 406 COVER NOTE From: European Commission date of receipt: 8 November 2017 To: No. Cion doc.: D051559/03 Subject:
EVEN QUICKER,CHEAPER AND SAFER GERMAN-MADE FOR YOUR MULTIPLE METHODS
 EVEN QUICKER,CHEAPER AND SAFER GERMAN-MADE FOR YOUR MULTIPLE METHODS THE PROCESS AND ITS VARIANTS The QuEChERS method, which concerns the analysis of pesticides and other organic contaminants in foodstuffs
EVEN QUICKER,CHEAPER AND SAFER GERMAN-MADE FOR YOUR MULTIPLE METHODS THE PROCESS AND ITS VARIANTS The QuEChERS method, which concerns the analysis of pesticides and other organic contaminants in foodstuffs
Official Journal of the European Union
 L 79/10 30.3.2016 COMMISSION REGULATION (EU) 2016/452 of 29 March 2016 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue
L 79/10 30.3.2016 COMMISSION REGULATION (EU) 2016/452 of 29 March 2016 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue
Council of the European Union Brussels, 27 March 2017 (OR. en)
 Council of the European Union Brussels, 27 March 2017 (OR. en) 7686/17 DLEG 25 AGRI 162 SAN 121 COVER NOTE From: European Commission date of receipt: 24 March 2017 To: No. Cion doc.: D049176/01 Subject:
Council of the European Union Brussels, 27 March 2017 (OR. en) 7686/17 DLEG 25 AGRI 162 SAN 121 COVER NOTE From: European Commission date of receipt: 24 March 2017 To: No. Cion doc.: D049176/01 Subject:
COMMISSION DELEGATED REGULATION (EU) /... of XXX
 EUROPEAN COMMISSION Brussels, XXX SANTE/11481/2018 CIS (POOL/E1/2018/11481/11481-EN CIS.doc) [ ](2018) XXX draft COMMISSION DELEGATED REGULATION (EU) /... of XXX amending Commission Delegated Regulation
EUROPEAN COMMISSION Brussels, XXX SANTE/11481/2018 CIS (POOL/E1/2018/11481/11481-EN CIS.doc) [ ](2018) XXX draft COMMISSION DELEGATED REGULATION (EU) /... of XXX amending Commission Delegated Regulation
Council of the European Union Brussels, 6 February 2017 (OR. en)
 Council of the European Union Brussels, 6 February 2017 (OR. en) 5966/17 AGRILEG 28 VETER 11 COVER NOTE From: European Commission date of receipt: 3 February 2017 To: General Secretariat of the Council
Council of the European Union Brussels, 6 February 2017 (OR. en) 5966/17 AGRILEG 28 VETER 11 COVER NOTE From: European Commission date of receipt: 3 February 2017 To: General Secretariat of the Council
COMMISSION DELEGATED REGULATION (EU) /... of XXX
 EUROPEAN COMMISSION Brussels, XXX SANTE/11481/2018 CIS (POOL/E1/2018/11481/11481- CIS.doc) [ ](2018) XXX draft COMMISSION DELEGATED REGULATION (EU) /... of XXX amending Commission Delegated Regulation
EUROPEAN COMMISSION Brussels, XXX SANTE/11481/2018 CIS (POOL/E1/2018/11481/11481- CIS.doc) [ ](2018) XXX draft COMMISSION DELEGATED REGULATION (EU) /... of XXX amending Commission Delegated Regulation
Boscalid BOSCALID (221)
 Boscalid 49 5.3 BOSCALID (221) RESIDUE AND ANALYTICAL ASPECTS Boscalid is a systemic fungicide first evaluated by JMPR in 2006 for residues and toxicology as a new active substance. An ADI of 0 0.04 mg/kg
Boscalid 49 5.3 BOSCALID (221) RESIDUE AND ANALYTICAL ASPECTS Boscalid is a systemic fungicide first evaluated by JMPR in 2006 for residues and toxicology as a new active substance. An ADI of 0 0.04 mg/kg
REQUIREMENTS FOR ACCREDITED LABORATORIES APPLYING FOR A FLEXIBLE SCOPE FOR ANALYSIS OF PESTICIDE RESIDUES IN FOOD AND FEED
 BELAC 2-104 Rev 2-2009 REQUIREMENTS FOR ACCREDITED LABORATORIES APPLYING FOR A FLEXIBLE SCOPE FOR ANALYSIS OF PESTICIDE RESIDUES IN FOOD AND FEED English translation for information only French and Dutch
BELAC 2-104 Rev 2-2009 REQUIREMENTS FOR ACCREDITED LABORATORIES APPLYING FOR A FLEXIBLE SCOPE FOR ANALYSIS OF PESTICIDE RESIDUES IN FOOD AND FEED English translation for information only French and Dutch
The Daniel Fast "Fasting For Health And Healing"
 The Daniel Fast "Fasting For Health And Healing" Starting Monday January 3, 2011 at Midnight Ending Sunday January 23, 2011 at 11:59 pm This information was primarily taken from the book "Fasting For Spiritual
The Daniel Fast "Fasting For Health And Healing" Starting Monday January 3, 2011 at Midnight Ending Sunday January 23, 2011 at 11:59 pm This information was primarily taken from the book "Fasting For Spiritual
Reasoned opinion on the review of the existing maximum residue levels (MRLs) for metazachlor according to Article 12 of Regulation (EC) No 396/2005 1
 EFSA Journal 2014;12(4):3634 REASED PII Reasoned opinion on the review of the existing maximum residue levels (MRLs) for metazachlor according to Article 12 of Regulation (EC) o 396/2005 1 European Food
EFSA Journal 2014;12(4):3634 REASED PII Reasoned opinion on the review of the existing maximum residue levels (MRLs) for metazachlor according to Article 12 of Regulation (EC) o 396/2005 1 European Food
COMMISSION REGULATION (EU) / of XXX
 Ref. Ares(2016)5616438-28/09/2016 EUROPEAN COMMISSION Brussels, XXX [ ](2016) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annex III to Directive 2008/98/EC of the European Parliament and of
Ref. Ares(2016)5616438-28/09/2016 EUROPEAN COMMISSION Brussels, XXX [ ](2016) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annex III to Directive 2008/98/EC of the European Parliament and of
Sample results. Actual results may vary. PATIENT INFORMATION DOB: AGE: GENDER: FASTING: Clinical Info: Test Name Result Flag Reference Range Lab
 Sample results. Actual results may vary. SPECIMEN INFORMATION SPECIMEN: REQUISITION: LAB REF NO: PATIENT INFORMATION DOB: AGE: GENDER: FASTING: REPORT STATUS: FINAL ORDERING PHYSICIAN CLIENT INFORMATION
Sample results. Actual results may vary. SPECIMEN INFORMATION SPECIMEN: REQUISITION: LAB REF NO: PATIENT INFORMATION DOB: AGE: GENDER: FASTING: REPORT STATUS: FINAL ORDERING PHYSICIAN CLIENT INFORMATION
COMMISSION REGULATION (EU) / of XXX
 EUROPEAN COMMISSION Brussels, XXX SANTE/11490/2017 [ ](2017) XXX draft COMMISSION REGULATION (EU) / of XXX refusing to authorise certain health claims made on foods, other than those referring to the reduction
EUROPEAN COMMISSION Brussels, XXX SANTE/11490/2017 [ ](2017) XXX draft COMMISSION REGULATION (EU) / of XXX refusing to authorise certain health claims made on foods, other than those referring to the reduction
Official Journal of the European Union
 23.2.2018 EN L 53/69 COMMISSION IMPLEMENTING REGULATION (EU) 2018/243 of 15 February 2018 concerning the authorisation of 3-hydroxybutan-2-one, pentan-2,3-dione, 3,5-dimethyl cyclopentan-1,2-dione, hexan-3,4-dione,
23.2.2018 EN L 53/69 COMMISSION IMPLEMENTING REGULATION (EU) 2018/243 of 15 February 2018 concerning the authorisation of 3-hydroxybutan-2-one, pentan-2,3-dione, 3,5-dimethyl cyclopentan-1,2-dione, hexan-3,4-dione,
Delegations will find attached document D056135/03.
 Council of the European Union Brussels, 1 June 2018 (OR. en) 9586/18 DLEG 46 SAN 175 AGRI 260 COVER NOTE From: European Commission date of receipt: 30 May 2018 To: No. Cion doc.: D056135/03 Subject: General
Council of the European Union Brussels, 1 June 2018 (OR. en) 9586/18 DLEG 46 SAN 175 AGRI 260 COVER NOTE From: European Commission date of receipt: 30 May 2018 To: No. Cion doc.: D056135/03 Subject: General
Du r i n g the past tw^o years there has been a decided increase
 THE VITAMIN A, VITAMIN Bi (THIAMIN), VITAMIN C (ASCORBIC ACID) AND RIBO FLA V IN CO N TEN T OF COMMON FOODS A S U M M A R Y O F R E P R E S E N T A T IV E V A L U E S H a z e l E. M u n s e l l "^ Du r
THE VITAMIN A, VITAMIN Bi (THIAMIN), VITAMIN C (ASCORBIC ACID) AND RIBO FLA V IN CO N TEN T OF COMMON FOODS A S U M M A R Y O F R E P R E S E N T A T IV E V A L U E S H a z e l E. M u n s e l l "^ Du r
COMMISSION DELEGATED REGULATION (EU) /... of XXX
 EUROPEAN COMMISSION Brussels, XXX SANTE/11174/2016 (POOL/E1/2016/E1/11174/11174- EN.doc) [ ](2017) XXX draft COMMISSION DELEGATED REGULATION (EU) /... of XXX amending the Annex to Regulation (EU) No 609/2013
EUROPEAN COMMISSION Brussels, XXX SANTE/11174/2016 (POOL/E1/2016/E1/11174/11174- EN.doc) [ ](2017) XXX draft COMMISSION DELEGATED REGULATION (EU) /... of XXX amending the Annex to Regulation (EU) No 609/2013
Official Journal of the European Union
 L 96/44 COMMISSION REGULATION (EU) 2017/627 of 3 April 2017 amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels
L 96/44 COMMISSION REGULATION (EU) 2017/627 of 3 April 2017 amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels
European Union comments for the. CODEX COMMITTEE ON PESTICIDE RESIDUES 44th Session. Shanghai, China, April 2012.
 - 1-16/04/2012 European Union comments for the CODEX COMMITTEE ON PESTICIDE RESIDUES 44th Session Shanghai, China, 23-28 April 2012 Agenda Item 6 a) Draft and Proposed Draft Maximum Residue Limits for
- 1-16/04/2012 European Union comments for the CODEX COMMITTEE ON PESTICIDE RESIDUES 44th Session Shanghai, China, 23-28 April 2012 Agenda Item 6 a) Draft and Proposed Draft Maximum Residue Limits for
(Text with EEA relevance)
 L 273/10 COMMISSION REGULATION (EU) 2016/1785 of 7 October 2016 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels
L 273/10 COMMISSION REGULATION (EU) 2016/1785 of 7 October 2016 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels
Eat Proteins with.. Acid Forming Proteins. Meats Meat Sub. Nuts (Raw) Oysters Poultry Scallops
 Proper combining of foods optimizes the digestive processes. It is possible to eat a variety of foods which interfere with the digestion of certain others. Likewise, it is possible to eat foods which enhance
Proper combining of foods optimizes the digestive processes. It is possible to eat a variety of foods which interfere with the digestion of certain others. Likewise, it is possible to eat foods which enhance
COMMISSION REGULATION (EU) / of XXX
 EUROPEAN COMMISSION Brussels, XXX SANTE/11992/2017 Rev. 0 [ ](2017) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annex II to Regulation (EC) No 1107/2009 by setting out scientific criteria for
EUROPEAN COMMISSION Brussels, XXX SANTE/11992/2017 Rev. 0 [ ](2017) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annex II to Regulation (EC) No 1107/2009 by setting out scientific criteria for
Combined review of the existing maximum residue levels (MRLs) for the active substances metalaxyl and metalaxyl-m
 REASONED OPINION ADOPTED: 1 April 2015 PUBLISHED: 2 April 2015 doi:10.2903/j.efsa.2015.4076 Combined review of the existing maximum residue levels (MRLs) for the active substances metalaxyl and metalaxyl-m
REASONED OPINION ADOPTED: 1 April 2015 PUBLISHED: 2 April 2015 doi:10.2903/j.efsa.2015.4076 Combined review of the existing maximum residue levels (MRLs) for the active substances metalaxyl and metalaxyl-m
(Text with EEA relevance)
 5.10.2018 L 251/13 COMMISSION REGULATION (EU) 2018/1481 of 4 October 2018 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission
5.10.2018 L 251/13 COMMISSION REGULATION (EU) 2018/1481 of 4 October 2018 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission
Macros and Micros. of a Healthy Diet. Macronutrients. Proteins
 Macros and Micros of a Healthy Diet Macronutrients Nutrients needed in large amounts in the body that provide energy Includes protein, carbohydrates, and fats Proteins Roles in the body: Develops, maintains,
Macros and Micros of a Healthy Diet Macronutrients Nutrients needed in large amounts in the body that provide energy Includes protein, carbohydrates, and fats Proteins Roles in the body: Develops, maintains,
Delegations will find attached document D042445/02.
 Council of the European Union Brussels, 11 December 2015 (OR. en) 15273/15 DLEG 164 AGRI 670 SAN 437 COVER NOTE From: European Commission date of receipt: 11 December 2015 To: No. Cion doc.: D042445/02
Council of the European Union Brussels, 11 December 2015 (OR. en) 15273/15 DLEG 164 AGRI 670 SAN 437 COVER NOTE From: European Commission date of receipt: 11 December 2015 To: No. Cion doc.: D042445/02
LAST NAME FIRST NAME MIDDLE NAME DATE OF BIRTH GENDER PHYSICIAN ID. TESTNAME PATIENT Female For doctor's reference
 FINAL REPORT DATE: ACCESSION ID: 05-04-2017 16:24 SPECIMEN COLLECTED: 11-03-2016 1611040000 SPECIMEN RECEIVED: 11-04-2016 00:00 LAST NAME FIRST NAME MIDDLE NAME DATE OF BIRTH GENDER PHYSICIAN ID TESTNAME
FINAL REPORT DATE: ACCESSION ID: 05-04-2017 16:24 SPECIMEN COLLECTED: 11-03-2016 1611040000 SPECIMEN RECEIVED: 11-04-2016 00:00 LAST NAME FIRST NAME MIDDLE NAME DATE OF BIRTH GENDER PHYSICIAN ID TESTNAME
REASONED OPINION. European Food Safety Authority 2, 3. European Food Safety Authority (EFSA), Parma, Italy
 EFSA Journal 2013;11(7):3339 REASONED OPINION Reasoned opinion on the review of the existing maximum residue levels (MRLs) for methyl bromide according to Article 12 of Regulation (EC) No 396/2005 1 European
EFSA Journal 2013;11(7):3339 REASONED OPINION Reasoned opinion on the review of the existing maximum residue levels (MRLs) for methyl bromide according to Article 12 of Regulation (EC) No 396/2005 1 European
Activity #4: Healthy Food Festival!
 Activity #4: Healthy Food Festival! Activity Overview In this activity, students apply their new learning to create fun ways to eat the rainbow during the week. Students are encouraged to consider the
Activity #4: Healthy Food Festival! Activity Overview In this activity, students apply their new learning to create fun ways to eat the rainbow during the week. Students are encouraged to consider the
My Plate Healthy Eating 1
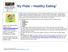 My Plate Healthy Eating 1 Learn more about healthy eating! Go to www.choosemyplate.gov Finding out how many calories YOU need for a day is a first step in managing your weight. Exercise is the key to any
My Plate Healthy Eating 1 Learn more about healthy eating! Go to www.choosemyplate.gov Finding out how many calories YOU need for a day is a first step in managing your weight. Exercise is the key to any
APPROVED: 4 December 2015 PUBLISHED: 9 December 2015
 REASONED OPINION APPROVED: 4 December 2015 PUBLISHED: 9 December 2015 doi:10.2903/j.efsa.2015.4356 Review of the existing maximum residue levels for sodium 5-nitroguaiacolate, sodium o-nitrophenolate and
REASONED OPINION APPROVED: 4 December 2015 PUBLISHED: 9 December 2015 doi:10.2903/j.efsa.2015.4356 Review of the existing maximum residue levels for sodium 5-nitroguaiacolate, sodium o-nitrophenolate and
A FULL SPECTRUM OF NUTRIENTS
 A FULL SPECTRUM OF NUTRIENTS All of us remember being told by our parents Eat your Vegetables. They re good for you. Fruits and vegetables are good for our Budgies too. Each color group has its own nutritional
A FULL SPECTRUM OF NUTRIENTS All of us remember being told by our parents Eat your Vegetables. They re good for you. Fruits and vegetables are good for our Budgies too. Each color group has its own nutritional
Nutrition Essentials Improving your PKU diet through balanced nutrition
 Nutrition Essentials Improving your PKU diet through balanced nutrition Sharon L Ernst, MPH, RD, CSP, FAND Associate Professor Chief Metabolic Dietitian Division of Medical Genetics Department of Pediatrics
Nutrition Essentials Improving your PKU diet through balanced nutrition Sharon L Ernst, MPH, RD, CSP, FAND Associate Professor Chief Metabolic Dietitian Division of Medical Genetics Department of Pediatrics
588G: Dietary Antigen Testing: Sensitivity and Complement 1/5. Dietary Antigen Exposure by Food Group
 PATIENT NAME: CLINIC: DOB: SAMPLE DATE: RECEIVE DATE: REPORT DATE: R //2 /28/27 /29/27 /3/27 Dunwoody Labs 9 Dunwoody Park Suite 2 Dunwoody, GA 3338 USA Phone: 6787366374 Fax: 776747 Nine Dunwoody Park,
PATIENT NAME: CLINIC: DOB: SAMPLE DATE: RECEIVE DATE: REPORT DATE: R //2 /28/27 /29/27 /3/27 Dunwoody Labs 9 Dunwoody Park Suite 2 Dunwoody, GA 3338 USA Phone: 6787366374 Fax: 776747 Nine Dunwoody Park,
Official Journal of the European Union. (Non-legislative acts) REGULATIONS
 27.1.2016 L 20/1 II (Non-legislative acts) REGULATIONS COMMISSION REGULATION (EU) 2016/71 of 26 January 2016 amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and
27.1.2016 L 20/1 II (Non-legislative acts) REGULATIONS COMMISSION REGULATION (EU) 2016/71 of 26 January 2016 amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and
Official Journal of the European Union. (Non-legislative acts) REGULATIONS
 21.6.2017 EN L 159/1 II (Non-legislative acts) REGULATIONS COMMISSION REGULATION (EU) 2017/1016 of 14 June 2017 amending Annexes II, III and IV to Regulation (EC) No 396/2005 of the European Parliament
21.6.2017 EN L 159/1 II (Non-legislative acts) REGULATIONS COMMISSION REGULATION (EU) 2017/1016 of 14 June 2017 amending Annexes II, III and IV to Regulation (EC) No 396/2005 of the European Parliament
Delegations will find attached document D055983/01.
 Council of the European Union Brussels, 2 May 2018 (OR. en) 8547/18 DLEG 34 SAN 130 AGRI 208 COVER NOTE From: European Commission date of receipt: 30 April 2018 To: No. Cion doc.: D055983/01 Subject: General
Council of the European Union Brussels, 2 May 2018 (OR. en) 8547/18 DLEG 34 SAN 130 AGRI 208 COVER NOTE From: European Commission date of receipt: 30 April 2018 To: No. Cion doc.: D055983/01 Subject: General
FRESH AIR FASTING GOD S WAY
 FRESH AIR FASTING GOD S WAY WHAT IS FASTING? Fasting is giving up food or another source of personal gratification for a determined period of time in order to focus on God. Fasting allows us to take our
FRESH AIR FASTING GOD S WAY WHAT IS FASTING? Fasting is giving up food or another source of personal gratification for a determined period of time in order to focus on God. Fasting allows us to take our
Suggested Vegetables and Fruits for a Rabbit Diet
 rabbit.org http://rabbit.org/suggested-vegetables-and-fruits-for-a-rabbit-diet/ Suggested Vegetables and Fruits for a Rabbit Diet By HRS Rabbits in the wild all over the world successfully consume a wide
rabbit.org http://rabbit.org/suggested-vegetables-and-fruits-for-a-rabbit-diet/ Suggested Vegetables and Fruits for a Rabbit Diet By HRS Rabbits in the wild all over the world successfully consume a wide
It is believed that a meal plan that includes low FODMAPs also may help ease symptoms from other health conditions, such as:
 If you have digestive issues, this could be why. You don't have to have IBS (Irritable Bowel Syndrome) or Colitis (a chronically inflammed colon), to experience some of these symptoms. FODMAP The acronym
If you have digestive issues, this could be why. You don't have to have IBS (Irritable Bowel Syndrome) or Colitis (a chronically inflammed colon), to experience some of these symptoms. FODMAP The acronym
Trends of Average Food Supply in the European Union
 Elmadfa I,Weichselbaum E (eds): European Nutrition and Health Report 24. Forum Nutr. Basel, Karger,, vol 8, pp 1 11 Trends of Average Food Supply in the On the Basis of the FAO Food Balance Sheets The
Elmadfa I,Weichselbaum E (eds): European Nutrition and Health Report 24. Forum Nutr. Basel, Karger,, vol 8, pp 1 11 Trends of Average Food Supply in the On the Basis of the FAO Food Balance Sheets The
My Diabetic Meal Plan during Pregnancy
 My Diabetic Meal Plan during Pregnancy When you have diabetes and are pregnant, you need to eat small meals and s throughout the day to help control your blood sugar. This also helps you get in enough
My Diabetic Meal Plan during Pregnancy When you have diabetes and are pregnant, you need to eat small meals and s throughout the day to help control your blood sugar. This also helps you get in enough
Official Journal of the European Union
 L 230/8 EN 25.8.2016 COMMISSION REGULATION (EU) 2016/1413 of 24 August 2016 amending Regulation (EU) No 432/2012 establishing a list of permitted health claims made on foods other than those referring
L 230/8 EN 25.8.2016 COMMISSION REGULATION (EU) 2016/1413 of 24 August 2016 amending Regulation (EU) No 432/2012 establishing a list of permitted health claims made on foods other than those referring
COMMISSION REGULATION (EU) / of XXX. establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food
 EUROPEAN COMMISSION Brussels, XXX SANTE/11059/2016 Rev. 2 (POOL/E2/2016/11059/11059R2- EN.doc) D048379/05 [ ](2017) XXX draft COMMISSION REGULATION (EU) / of XXX establishing mitigation measures and benchmark
EUROPEAN COMMISSION Brussels, XXX SANTE/11059/2016 Rev. 2 (POOL/E2/2016/11059/11059R2- EN.doc) D048379/05 [ ](2017) XXX draft COMMISSION REGULATION (EU) / of XXX establishing mitigation measures and benchmark
COMMISSION REGULATION (EU) / of XXX. establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food
 EUROPEAN COMMISSION Brussels, XXX SANTE/11059/2016 Rev. 2 (POOL/E2/2016/11059/11059R2- EN.doc) D048379/05 [ ](2017) XXX draft COMMISSION REGULATION (EU) / of XXX establishing mitigation measures and benchmark
EUROPEAN COMMISSION Brussels, XXX SANTE/11059/2016 Rev. 2 (POOL/E2/2016/11059/11059R2- EN.doc) D048379/05 [ ](2017) XXX draft COMMISSION REGULATION (EU) / of XXX establishing mitigation measures and benchmark
Protein Carbs. / Healthy Fats Veggie Fruit
 Protein Carbs. / Healthy Fats Veggie Fruit 6 oz. steak 12 oz. Yogurt 1 cup of Kale 1 Banana 4 oz. Peanut Butter ½ Ham Sandwich 1 cup Peas 1 Orange 2 Eggs ½ Turkey Sandwich 1 cup Carrots 1 Apple Cliff Bar
Protein Carbs. / Healthy Fats Veggie Fruit 6 oz. steak 12 oz. Yogurt 1 cup of Kale 1 Banana 4 oz. Peanut Butter ½ Ham Sandwich 1 cup Peas 1 Orange 2 Eggs ½ Turkey Sandwich 1 cup Carrots 1 Apple Cliff Bar
COMMISSION REGULATION (EU) / of XXX. authorising certain health claims made on foods and referring to children's development and health
 EUROPEAN COMMISSION Brussels, XXX SANTE/10891/2015 [ ](2015) XXX draft COMMISSION REGULATION (EU) / of XXX authorising certain health claims made on foods and referring to children's development and health
EUROPEAN COMMISSION Brussels, XXX SANTE/10891/2015 [ ](2015) XXX draft COMMISSION REGULATION (EU) / of XXX authorising certain health claims made on foods and referring to children's development and health
REASONED OPINION. European Food Safety Authority 2, 3. European Food Safety Authority (EFSA), Parma, Italy
 EFSA Journal 215;13(3):45 REASONED OPINION Reasoned opinion on the review of the existing maximum residue levels (MRLs) for fenpropimorph according to Article 12 of Regulation (EC) No 396/25 1 ABSTRACT
EFSA Journal 215;13(3):45 REASONED OPINION Reasoned opinion on the review of the existing maximum residue levels (MRLs) for fenpropimorph according to Article 12 of Regulation (EC) No 396/25 1 ABSTRACT
Eating Healthier: Six Simple Steps
 Eating Healthier: Six Simple Steps Most of us are interested in learning how to eat a healthier diet. As a coach, you can support your client by making simple suggestions as well as giving her resources
Eating Healthier: Six Simple Steps Most of us are interested in learning how to eat a healthier diet. As a coach, you can support your client by making simple suggestions as well as giving her resources
EASY WAYS TO EAT MORE FRUITS AND VEGETABLES AS PART OF A HEALTHY DIET.
 This is a text-only 508 accessible version for the visually impaired. For a full-color brochure, see: www.fruitsandveggiesmatter.gov/downloads/aa_womens_brochure.pdf Page 1- Left column (back cover) EASY
This is a text-only 508 accessible version for the visually impaired. For a full-color brochure, see: www.fruitsandveggiesmatter.gov/downloads/aa_womens_brochure.pdf Page 1- Left column (back cover) EASY
Official Journal of the European Union. (Non-legislative acts) REGULATIONS
 29.6.2016 L 172/1 II (Non-legislative acts) REGULATIONS COMMISSION REGULATION (EU) 2016/1015 of 17 June 2016 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of
29.6.2016 L 172/1 II (Non-legislative acts) REGULATIONS COMMISSION REGULATION (EU) 2016/1015 of 17 June 2016 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of
Official Journal of the European Union
 24.3.16 L 78/51 COMMISSION IMPLEMTING REGULATION (EU) 16/443 of 23 March 16 amending Annex I to Regulation (EC) No 669/09 as regards the list of feed and food of nonanimal subject to an increased level
24.3.16 L 78/51 COMMISSION IMPLEMTING REGULATION (EU) 16/443 of 23 March 16 amending Annex I to Regulation (EC) No 669/09 as regards the list of feed and food of nonanimal subject to an increased level
Pesticide Monitoring Program: Design Assessment 1
 EFSA Journal 2015;13(2):4005 ABSTRACT SCIENTIFIC REPORT OF EFSA Pesticide Monitoring Program: Design Assessment 1 European Food Safety Authority 2, 3 European Food Safety Authority (EFSA), Parma, Italy
EFSA Journal 2015;13(2):4005 ABSTRACT SCIENTIFIC REPORT OF EFSA Pesticide Monitoring Program: Design Assessment 1 European Food Safety Authority 2, 3 European Food Safety Authority (EFSA), Parma, Italy
National reporting 2014 Pesticide residues in food Federal Republic of Germany
 National reporting 2014 Pesticide residues in food Federal Republic of Germany Summary The report presents the results of the analysis of food for pesticide residues. In accordance with Regulation (EC)
National reporting 2014 Pesticide residues in food Federal Republic of Germany Summary The report presents the results of the analysis of food for pesticide residues. In accordance with Regulation (EC)
Part One: Nutrition & Diet
 Healthy Living After Breast Cancer Part One: Nutrition & Diet Lynda McIntyre R.D., L.D 1 What Do the Studies Say Alcohol? Body Weight? Fat? Glycemic Index? Fiber? D airy? Fruits? Vegetables? Sugar? SOY?
Healthy Living After Breast Cancer Part One: Nutrition & Diet Lynda McIntyre R.D., L.D 1 What Do the Studies Say Alcohol? Body Weight? Fat? Glycemic Index? Fiber? D airy? Fruits? Vegetables? Sugar? SOY?
NUTRITIONAL STATUS OP RURAL YOUTH. IV. Sherman County
 AGRICULTURAL EXPERIMENT STATION Oregon State College Irru A. Schoenfeld, Director Corvallis Home Economics Circular of Information No, 349 September 1944 (A Progress Report) NUTRITIONAL STATUS OP RURAL
AGRICULTURAL EXPERIMENT STATION Oregon State College Irru A. Schoenfeld, Director Corvallis Home Economics Circular of Information No, 349 September 1944 (A Progress Report) NUTRITIONAL STATUS OP RURAL
Council of the European Union Brussels, 30 January 2018 (OR. en) Mr Jeppe TRANHOLM-MIKKELSEN, Secretary-General of the Council of the European Union
 Council of the European Union Brussels, 30 January 2018 (OR. en) 5777/18 DLEG 19 AGRI 55 SAN 48 COVER NOTE From: date of receipt: 29 January 2018 To: No. Cion doc.: Secretary-General of the European Commission,
Council of the European Union Brussels, 30 January 2018 (OR. en) 5777/18 DLEG 19 AGRI 55 SAN 48 COVER NOTE From: date of receipt: 29 January 2018 To: No. Cion doc.: Secretary-General of the European Commission,
LESSON 3 E AT A RAINBOW OF SNACKS
 E AT A R A I N B O W O F S N A C K S LESSON 3 E AT A RAINBOW OF SNACKS Objectives for the lesson: 1. Compare the nutrient content and cost of vegetables and fruits as snacks to conventional snack foods.
E AT A R A I N B O W O F S N A C K S LESSON 3 E AT A RAINBOW OF SNACKS Objectives for the lesson: 1. Compare the nutrient content and cost of vegetables and fruits as snacks to conventional snack foods.
Foods Based on Lectin Content
 Foods Based on Lectin Content Lectins are proteins found in many plants which are intended to harm the person consuming them. You can think of them as the way that the plants protect themselves from being
Foods Based on Lectin Content Lectins are proteins found in many plants which are intended to harm the person consuming them. You can think of them as the way that the plants protect themselves from being
Review of the existing maximum residue levels for copper compounds according to Article 12 of Regulation (EC) No 396/2005
 REASONED OPINION ADOPTED: 1 March 2018 doi: 10.2903/j.efsa.2018.5212 Review of the existing imum residue levels for copper compounds according to Article 12 of Regulation (EC) No 396/2005 European Food
REASONED OPINION ADOPTED: 1 March 2018 doi: 10.2903/j.efsa.2018.5212 Review of the existing imum residue levels for copper compounds according to Article 12 of Regulation (EC) No 396/2005 European Food
Fiber In Your Diet. Provided by Hemorrhoid Centers of America Version Fiber
 In Your Diet The lack of dietary fiber and fluids is a contributing factor to the development of hemorrhoids and anal fissures. We recommend consuming 25-35 grams of fiber and drinking 7 glasses of fluids
In Your Diet The lack of dietary fiber and fluids is a contributing factor to the development of hemorrhoids and anal fissures. We recommend consuming 25-35 grams of fiber and drinking 7 glasses of fluids
(Text with EEA relevance)
 12.10.2018 L 256/45 COMMISSION REGULATION (EU) 2018/1516 of 10 October 2018 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue
12.10.2018 L 256/45 COMMISSION REGULATION (EU) 2018/1516 of 10 October 2018 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue
Potassium and Your CKD Diet
 A TO Z HEALTH GUIDE Potassium and Your CKD Diet What is potassium and why is it important to you? Potassium is a mineral found in many of the foods you eat. It plays a role in keeping your heartbeat regular
A TO Z HEALTH GUIDE Potassium and Your CKD Diet What is potassium and why is it important to you? Potassium is a mineral found in many of the foods you eat. It plays a role in keeping your heartbeat regular
5.20 PYRACLOSTROBIN (210)
 Pyraclostrobin 213 5.20 PYRACLOSTROBIN (210) RESIDUE AND ANALYTICAL ASPECTS Pyraclostrobin was first evaluated by JMPR in 2003 when an ADI of 0 0.03mg/kg bw and an ARfD of 0.05 mg/kg bw were established,
Pyraclostrobin 213 5.20 PYRACLOSTROBIN (210) RESIDUE AND ANALYTICAL ASPECTS Pyraclostrobin was first evaluated by JMPR in 2003 when an ADI of 0 0.03mg/kg bw and an ARfD of 0.05 mg/kg bw were established,
lambda-cyhalothrin (S)-(Z)- (1R)-cis-isomer The following abbreviations are used for the metabolites discussed below:
 Cyhalothrin 147 5.8 LAMBDA-CYHALOTHRIN (146) RESIDUE AND ANALYTICAL ASPECTS Lambda-cyhalothrin was scheduled as a priority compound under the periodic re-evaluation programme at the 34 th Session of the
Cyhalothrin 147 5.8 LAMBDA-CYHALOTHRIN (146) RESIDUE AND ANALYTICAL ASPECTS Lambda-cyhalothrin was scheduled as a priority compound under the periodic re-evaluation programme at the 34 th Session of the
European Community Positions for the 41 st Session of the Codex Committee on Pesticide Residues Beijing, China April 2009
 European Community Positions for the 41 st Session of the Codex Committee on Pesticide Residues Beijing, China. 20-25 April 2009 Agenda Items 4a, 4b, 5, 6 and 10 Agenda Item 4a JMPR Report 2008, Point
European Community Positions for the 41 st Session of the Codex Committee on Pesticide Residues Beijing, China. 20-25 April 2009 Agenda Items 4a, 4b, 5, 6 and 10 Agenda Item 4a JMPR Report 2008, Point
Delegations will find attached document D048379/05.
 Council of the European Union Brussels, 17 August 2017 (OR. en) 11651/17 AGRILEG 150 DENLEG 63 COVER NOTE From: European Commission date of receipt: 24 July 2017 To: No. Cion doc.: D048379/05 Subject:
Council of the European Union Brussels, 17 August 2017 (OR. en) 11651/17 AGRILEG 150 DENLEG 63 COVER NOTE From: European Commission date of receipt: 24 July 2017 To: No. Cion doc.: D048379/05 Subject:
The feeding of rhinoceroses - reminder and update
 The feeding of rhinoceroses - reminder and update Marcus Clauss Clinic for Zoo Animals, Exotic Pets and Wildlife, Vetsuisse Faculty, University of Zurich, Switzerland EAZA Conference Belfast 2016 Natural
The feeding of rhinoceroses - reminder and update Marcus Clauss Clinic for Zoo Animals, Exotic Pets and Wildlife, Vetsuisse Faculty, University of Zurich, Switzerland EAZA Conference Belfast 2016 Natural
COMMISSION REGULATION (EU) / of XXX. authorising a health claim made on foods and referring to the reduction of disease risk
 EUROPEAN COMMISSION Brussels, XXX SANTE/12053/2016 [ ](2016) XXX draft COMMISSION REGULATION (EU) / of XXX authorising a health claim made on foods and referring to the reduction of disease risk (Text
EUROPEAN COMMISSION Brussels, XXX SANTE/12053/2016 [ ](2016) XXX draft COMMISSION REGULATION (EU) / of XXX authorising a health claim made on foods and referring to the reduction of disease risk (Text
GREEN YELLOW RED PURPLE WHITE
 GREEN YELLOW RED PURPLE WHITE Broccoli, green cabbage, spinach, romaine lettuce, green peas, kiwi fruit, swiss chard Avoid cancer of the prostate and colon (large intestine) Develop and maintain healthy
GREEN YELLOW RED PURPLE WHITE Broccoli, green cabbage, spinach, romaine lettuce, green peas, kiwi fruit, swiss chard Avoid cancer of the prostate and colon (large intestine) Develop and maintain healthy
Fluopyram FLUOPYRAM (243)
 Fluopyram 163 5.19 FLUOPYRAM (243) RESIDUE AND ANALYTICAL ASPECTS Fluopyram, a pyridylethylamide broad spectrum fungicide was evaluated for the first time by the 2010 JMPR, where an ADI of 0 0.01 mg/kg
Fluopyram 163 5.19 FLUOPYRAM (243) RESIDUE AND ANALYTICAL ASPECTS Fluopyram, a pyridylethylamide broad spectrum fungicide was evaluated for the first time by the 2010 JMPR, where an ADI of 0 0.01 mg/kg
Sample Report Exclusively in the UK and Ireland Contact: Regenerus Laboratories T: +44 (0) E:
 Sample Report Exclusively in the UK and Ireland Contact: Regenerus Laboratories T: + () 7 87 E: info@regeneruslabs.com Patient Information Sheet: Name: Jon Doe Example Date Drawn: //6 Date of Birth: //966
Sample Report Exclusively in the UK and Ireland Contact: Regenerus Laboratories T: + () 7 87 E: info@regeneruslabs.com Patient Information Sheet: Name: Jon Doe Example Date Drawn: //6 Date of Birth: //966
588-Complete Dietary Antigen Testing
 18716_REPORT_COMPETE DUNWOODY ABS 9 Dunwoody Park, Suite 121 Dunwoody, GA 3338 P: 678-736-6374 F: 77-674-171 Email: info@dunwoodylabs.com www.dunwoodylabs.com PATIENT INFO NAME: SAMPE PATIENT REQUISITION
18716_REPORT_COMPETE DUNWOODY ABS 9 Dunwoody Park, Suite 121 Dunwoody, GA 3338 P: 678-736-6374 F: 77-674-171 Email: info@dunwoodylabs.com www.dunwoodylabs.com PATIENT INFO NAME: SAMPE PATIENT REQUISITION
13480/12 PM/ns 1 DG B 4B
 COUNCIL OF THE EUROPEAN UNION Brussels, 10 September 2012 13480/12 DENLEG 80 AGRI 553 COVER NOTE from: European Commission date of receipt: 22 August 2012 to: General Secretariat of the Council No Cion
COUNCIL OF THE EUROPEAN UNION Brussels, 10 September 2012 13480/12 DENLEG 80 AGRI 553 COVER NOTE from: European Commission date of receipt: 22 August 2012 to: General Secretariat of the Council No Cion
Low FODMAP Diet. OK, but what are FODMAPs and who should avoid them?
 Low FODMAP Diet FODMAP? What does that stand for? Fermentable Oligosaccharides (oligo few, saccharide sugar ) Disaccharides ( two sugars ) Monosaccharides ( one sugar ) And Polyols (these are sugar alcohols)
Low FODMAP Diet FODMAP? What does that stand for? Fermentable Oligosaccharides (oligo few, saccharide sugar ) Disaccharides ( two sugars ) Monosaccharides ( one sugar ) And Polyols (these are sugar alcohols)
IgG Food Antibody Assessment (Serum)
 IgG Food Antibody Assessment (Serum) Patient: DOB: Sex: MRN: Order Number: Completed: Received: Collected: IgG Food Antibody Results Dairy Vegetables Fish/Shellfish Nuts and Grains Casein Cheddar cheese
IgG Food Antibody Assessment (Serum) Patient: DOB: Sex: MRN: Order Number: Completed: Received: Collected: IgG Food Antibody Results Dairy Vegetables Fish/Shellfish Nuts and Grains Casein Cheddar cheese
(Text with EEA relevance) (2014/798/EU)
 L 330/50 COMMISSION IMPLEMTING DECISION of 13 November 2014 amending Annex F to Council Directive 64/432/EEC as regards the format of the model health certificates for intra-union trade in bovine animals
L 330/50 COMMISSION IMPLEMTING DECISION of 13 November 2014 amending Annex F to Council Directive 64/432/EEC as regards the format of the model health certificates for intra-union trade in bovine animals
Przeczytaj pełną wersję artykułu:
 Dietary fiber Przeczytaj pełną wersję artykułu: http://www.healthynutritionguide.info/nutrients/dietary-fiber/art,dietary-fiber.html Introduction Functions of dietary fiber Classification Sources in food
Dietary fiber Przeczytaj pełną wersję artykułu: http://www.healthynutritionguide.info/nutrients/dietary-fiber/art,dietary-fiber.html Introduction Functions of dietary fiber Classification Sources in food
Reasoned opinion on the review of the existing maximum residue levels (MRLs) for dodine according to Article 12 of Regulation (EC) No 396/2005 1
 EFSA Journal 2015;13(1):3946 REASONED OPINION Reasoned opinion on the review of the existing maximum residue levels (MRLs) for dodine according to Article 12 of Regulation (EC) No 396/2005 1 European Food
EFSA Journal 2015;13(1):3946 REASONED OPINION Reasoned opinion on the review of the existing maximum residue levels (MRLs) for dodine according to Article 12 of Regulation (EC) No 396/2005 1 European Food
COMMISSION REGULATION (EU) No /.. of XXX. amending Regulation (EC) No 1881/2006 as regards maximum levels of cadmium in foodstuffs
 EUROPEAN COMMISSION Brussels, XXX SANCO/10617/2009 rev.09 [ ](2013) XXX draft COMMISSION REGULATION (EU) No /.. of XXX amending Regulation (EC) No 1881/2006 as regards maximum levels of cadmium in foodstuffs
EUROPEAN COMMISSION Brussels, XXX SANCO/10617/2009 rev.09 [ ](2013) XXX draft COMMISSION REGULATION (EU) No /.. of XXX amending Regulation (EC) No 1881/2006 as regards maximum levels of cadmium in foodstuffs
