UvA-DARE (Digital Academic Repository) Regulation of integrin function and trafficking Margadant, C. Link to publication
|
|
|
- Claude Strickland
- 5 years ago
- Views:
Transcription
1 UvA-DARE (Digital Academic Repository) Regulation of integrin function and trafficking Margadant, C. Link to publication Citation for published version (APA): Margadant, C. (2013). Regulation of integrin function and trafficking General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam ( Download date: 09 Jan 2019
2 Regulation of integrin function and trafficking Coert Margadant Regulation of integrin function and trafficking Coert Margadant
3 Regulation of integrin function and trafficking Coert Margadant
4 ISBN: Cover: Cells migrating on fibronectin. Green, integrin-mediated adhesion sites; red, actin filaments; blue, cell nuclei. Lay-out and printing: Off Page, Copyright 2013 by C. Margadant. All rights reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, without prior permission of the author.
5 Regulation of integrin function and trafficking ACADEMISCH PROEFSCHRIFT ter verkrijging van de graad van doctor aan de Universiteit van Amsterdam op gezag van de Rector Magnificus prof. dr. D.C. van den Boom ten overstaan van een door het college voor promoties ingestelde commissie, in het openbaar te verdedigen in de Agnietenkapel op dinsdag 29 januari 2013, te 14:00 uur door Coert Margadant geboren te Hengelo
6 Promotor: Copromotor: Overige leden: Prof. Dr. A.J.M. Berns Dr. A. Sonnenberg Prof. Dr. C.J.F. van Noorden Prof. Dr. S.T. Pals Prof. Dr. P.L. Hordijk Prof. Dr. J.C. Norman Dr. E.H.J. Danen Faculteit der Geneeskunde
7 I am grateful to everybody that has contributed to this thesis.
8
9 table of contents Chapter 1 Introduction: functions and regulation of integrins Chapter 1.1 Unique and redundant functions of integrins in the epidermis 9 FASEB Journal Chapter 1.2 Regulation of hemidesmosme disassembly by growth factor receptors 45 Current Opinion in Cell Biology Chapter 1.3 Integrin-TGF-β crosstalk in fibrosis, cancer and wound healing 59 EMBO Reports Chapter 2 Chapter 3 Gain-of-glycosylation in integrin α3β1 causes lung disease and nephrotic syndrome 79 Journal of Clinical Investigation Integrin α3β1 inhibits directional migration and wound re-epithelialization in the skin 105 Journal of Cell Science Chapter 4 Mechanisms of integrin activation and trafficking 129 Current Opinion in Cell Biology Chapter 5 Kindlin-1 regulates integrin dynamics and adhesion turnover 143 Submitted for publication Chapter 6 Chapter 7 Chapter 8 Chapter 9 Kindlin-1 mutant zebrafish as an in vivo model sytem to study adhesion mechanisms in the epidermis 161 Submitted for publication Distinct roles of talin and kindlin in regulating integrin α5β1 function and trafficking 183 Current Biology Epigenetic regulation of galectin-3 expression by β1 integrins promotes cell adhesion and migration 205 Journal of Biological Chemistry MAPK uncouples cell cycle progression from cell spreading and cytoskeletal organization in cycling cells 227 Cellular and Molecular Life Sciences Chapter 10 Summarizing Discussion 251 Addendum Nederlandse Samenvatting 263 List of publications 267
10
11 Unique and redundant functions of integrins in the epidermis Coert Margadant, Rabab A. Charafeddine #, and Arnoud Sonnenberg Division of Cell Biology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands. # Present address: Albert Einstein College of medicine, Bronx, NY 10461, USA. FASEB J 24, (2010)
12
13 ABSTRAct The skin forms a barrier against the environment and protects us from mechanical trauma, pathogens, radiation, dehydration, and dangerous temperature fluctuations. The epithelium of the skin, the epidermis, is in a continuous equilibrium of growth and differentiation and has the remarkable capacity to completely self-renew, which relies on reservoirs of stem cells. Epidermal homeostasis is further dependent on proper repair after injury, and on tight adhesion to the underlying basement membrane. Epidermal adhesion is primarily mediated by integrins, cell-surface receptors that connect the extracellular matrix to the cytoskeleton. In addition, numerous in vitro reports have implicated integrins, integrin-associated proteins, or downstream integrin effectors in the regulation of a plethora of cellular processes other than adhesion. Over the past decade, a wealth of information on the function of these proteins has been gathered both from (conditional) knockout mice and from human skin disorders, allowing for a reconstruction of integrin signaling pathways in vivo. Here, we address how epidermal integrins and integrin-associated proteins regulate keratinocyte adhesion, proliferation, and differentiation, as well as signal transduction, re-epithelialization during wound healing, hair growth, and stem cell maintenance. Furthermore, we discuss human pathologies associated with altered integrin functions in the epidermis. 1 IntRODUctIon The largest organ of our body, the skin, protects us from the outside world. It serves as a barrier against radiation and pathogens, maintains body temperature, and prevents water loss. In addition, it functions as a sensory organ. The skin consists of two layers, the epidermis and the underlying dermis (Figure 1A). The epidermis is a stratified squamous epithelium comprising several layers of keratinocytes, which give rise to appendages such as nails, sweat glands, and hairs. In between the periodically spaced hairs, the epidermis is referred to as the interfollicular epidermis (IFE). The IFE contains a variety of immigrant cells in addition to keratinocytes, including Langerhans cells, Merkel cells, and melanocytes. The dermis is a mesenchymally-derived connective tissue, consisting of nerves, fibroblasts, and blood and lymphatic vessels embedded in an extracellular matrix (ECM) of predominantly collagens and elastins. The dermis provides the epidermis with mechanical support and nutrients. Epidermis and dermis are separated from one another by the basement membrane (BM), a meshwork of proteoglycans, growth factors, and ECM proteins, including laminins and collagen (Col)-IV that are deposited by the keratinocytes, and collagens and nidogen synthesized by the dermal fibroblasts. Anchoring fibrils, which consist of Col-VII, project from the BM in U-shaped bows that entrap interstitial Col fibers, providing for stable anchorage of the BM to the dermis (Figure 1A). In the epidermis, proliferation occurs under normal conditions only in the basal layer. Differentiation is initiated when basal keratinocytes withdraw from the cell cycle and detach from the BM, allowing Integrins in the epidermis 11
14 migration towards the suprabasal spinous and granular layers. Proliferation and differentiation are accompanied by spatiotemporal differences in the expression of specific markers. For instance, whereas suprabasal layers express keratin-1 and -10, keratin-5 and -14 are only expressed by basal keratinocytes. Similarly, the spinous layer expresses involucrin, while filaggrin and loricrin are expressed in the granular layer. Eventually, keratinocytes end their lives in the stratum corneum as e-nucleated, keratinized squames, which are sloughed off every day. This perpetual cell loss requires continuous replenishment, which is provided for by epidermal stem cells. The skin has to withstand great mechanical stress, and it is therefore important to tightly regulate both intercellular adhesion and adhesion of the epidermis to the BM. Whereas the barrier function of the epidermis is maintained by intercellular adhesion molecules in adherens junctions, tight junctions, and keratin-associated desmosomes, adhesion of the epidermis to the BM is mediated by cell-matrix receptors, the most important of which are integrins. Integrins are heterodimeric transmembrane receptors consisting of an a and a b subunit that link the ECM to the cytoskeleton, thus integrating the extracellular environment with the cell interior (1). Integrins and their associated proteins have traditionally been implicated in the regulation of a wide variety of cellular processes. The (conditional) ablation in mice of the genes encoding either integrin subunits or integrin-associated and -interacting proteins has shed new light on the functions of epidermal integrins in vivo, and has generated some surprising insights that could not have been predicted from in vitro studies. Whereas there is considerable functional redundancy among integrins in certain processes such as wound healing, some epidermal integrins also seem to have unique functions. Here, we discuss the functions of integrins and integrin-regulated pathways in the epidermis, both in normal physiology and in human disease. OVERVIEW of InteGRIns IN THE EPIDERMIS To date, 18 a and 8 b integrin subunits have been identified in mammals, which can combine to form 24 different heterodimers with different affinities toward specific ECM components (1). Integrins in the epidermis can be classified into b1-containing integrins, av-containing integrins, and the integrin a6b4 (Table I). Whereas some integrins are constitutively expressed in the epidermis, the expression of others is induced during wound healing and in pathological conditions. The constitutively expressed integrin a6b4 is a laminin receptor which preferentially binds to laminin-332 (Ln-332; previously known as Ln-5), and is localized exclusively at the basal surface in large adhesive complexes designated hemidesmososmes (HDs). Intracellularly, a6b4 is connected to the intermediate filament (IF) system. Integrins sharing the b1-subunit are found basally in clusters surrounding the HDs, but also at the apicolateral cell surfaces. They are connected intracellularly to the 12
15 Table 1 (Putative) epidermal integrins and their ligands in the basement membrane. Integrin Ligand Expression in epidermis b1 av a2 Collagens Constitutive, upregulated during wound healing a3 Laminins, mainly Ln-332 and Ln-511/521 in the HF, b-ig-h3 Constitutive, upregulated during wound healing a5 FN Induced during wound healing a6 laminins Uncertain a8 RGD In developing HFs and the arrector pili muscle a9 RGD, mainly of FN and TN Weak constitutive, upregulated during wound healing b1 RGD Uncertain b5 RGD, mainly of VN Weak constitutive, upregulated during wound healing b6 RGD, mainly of FN, TN, and LAP-1/-3 b8 RGD, mainly of VN and LAP-1/-3 Weak, suprabasal a6b4 Laminins, mainly Ln-332 Constitutive In stem cells in the HF, induced during wound healing in interfollicular epidermis 1 b-ig-h3, transforming growth factor-b-inducible gene-h3; FN, fibronectin; HF, hair follicle; LAP, latency-associated protein; Ln-332, laminin-332; Ln-511/521, laminin-511/521; RGD, arginine-glycine-aspartate; TN, tenascin; VN, vitronectin. actin cytoskeleton via talin and vinculin, and in culture they mediate cell spreading over the substrate, which is accompanied by the assembly of large multimolecular adhesion complexes known as focal adhesions (FAs) (Figure 1C). The constitutively expressed b1 integrins are a2b1, which binds to collagens, a3b1, which binds primarily to Ln-332 and Ln-511/521 (previously Ln-10/11) in the hair follicle (HF), and a9b1, which binds to proteins containing an arginine-glycineaspartate (RGD) motif, predominantly fibronectin (FN) and tenascin (TN) (2). Furthermore, the RGD-binding integrin a8b1 is found in developing HFs and in the arrector pili muscle, which is associated with the HF and is responsible for the erection of hairs at low temperatures. The a5b1 integrin binds to FN, and is de novo expressed upon wounding (3-5). The av-containing integrins also bind to RGD motifs, and include avb5, avb6, and avb8. Integrins avb5 and avb8 bind mainly to vitronectin (VN), and are expressed constitutively in intact adult epidermis -albeit at very low levelswhereas avb6 is expressed in stem cells in the HF, in keratinocytes in culture, and in IFE during wound healing and under pathological conditions (2,6). Last, the presence of the lamininbinding integrin a6b1 as well as the RGDbinding integrin avb1 in the epidermis is unclear, while they are occasionally detected on keratinocytes in culture. In normal epidermis, integrin expression is restricted to the basal layer and the outer root sheath of HFs, with the exception of avb8, which is expressed suprabasally (7). However, suprabasal expression of other Integrins in the epidermis 13
16 integrins can occur under circumstances of hyperproliferation, for instance during wound healing, in squamous cell carcinomas, or in certain pathologies associated with inflammation such as psoriasis (3-5). REGUlatIon of EPIDERMal HOMeostasIS (ADHesIon, PRolIfeRATIon, DIffeRentIatIon and SURVIVal) BY InteGRIns Integrin α6β4 HDs were originally identified in ultrastructural studies as electron-dense structures in the plasma membrane of basal epithelial cells. Below the HD is an electron-lucent zone called the lamina lucida, and the electron-dense zone between the lamina lucida and the dermis is called the lamina densa. The lamina lucida and the lamina densa collectively comprise the BM (Figure 1B). Whereas simple epithelia such as that of the intestine express type II HDs consisting solely of the integrin a6b4 and plectin (HD-1), the epidermis contains type I (classical) HDs that are composed of a6b4, plectin, tetraspanin CD151, and the bullous pemphigoid (BP) antigens 180 (also called BPAG-2 or type XVII collagen) and 230 (BPAG-1). Binding of a6b4 and BP180 to Ln-332 is stabilized intracellularly via their association with keratin-5 and -14, through the two plakins plectin and BP230. Furthermore, the extracellular domain of the a6 subunit interacts with BP180 and CD151 (Figure 1C) (8,9). Stabilization of a6b4- mediated epidermal adhesion to Ln-332 is further achieved by the binding of the anchoring fibrils to Ln-332. The importance of this adhesion axis in maintaining dermal-epidermal integrity is evident from two lines of evidence. First, ablation of the gene encoding any of its components in mice causes neonatal trauma-induced blistering, and second, mutations in any of the genes encoding these proteins can cause a skin blistering disorder known as epidermolysis bullosa (EB) in humans (Table II) (10-29). The connection of a6b4 to keratin filaments is vital for the integrity of the HD, as either the targeted deletion of the cytoplasmic domain of b4 in mice, or b4 mutations that prevent the binding to plectin in humans cause blistering as well (18,30,31). Whereas a6b4 is thus crucial for stable adhesion of basal keratinocytes to the BM, it is controversial whether it also regulates proliferation, survival, and differentiation in the epidermis. High levels of a6b4 are expressed in proliferating basal keratinocytes but not in differentiating suprabasal layers, which may suggest that a6b4 supports proliferation but is not involved in differentiation. Although one study reported an increase of differentiation markers in a6-null epidermis, most reports demonstrate normal stratification and differentiation in mice lacking either a6 or b4 (14-18,32,33). It has been suggested that a6b4 regulates cell cycle progression through a domain in its cytoplasmic tail (34). In line with this, reduced numbers of proliferating cells were observed in the epidermis of mice expressing a mutant b4 either lacking this domain or the entire C-tail, however differences in pup size or in the total number 14
17 1 Figure 1 Structure of the skin, hemidesmosomes, and focal adhesions. (A) Haematoxylin/eosin staining (left) and schematic representation (right) of human interfollicular epidermis, the basement membrane, and the dermis. (B) Electron micrograph (left) and schematic representation (right) of the hemidesmosome and the dermal-epidermal junction. (C) Molecular composition of hemidesmosomes and focal adhesions. AF, anchoring fibril; CF, collagen fiber; BM, basement membrane; FA, focal adhesion; FAK, focal adhesion kinase; HD, hemidesmosome; IF, intermediate filaments; IFE, interfollicular epidermis; ILK, integrin-linked kinase; K5/14, keratin-5/-14; LD, lamina densa; LL, lamina lucida; Ln-332, laminin-332; Ln-511, laminin-511. Integrins in the epidermis 15
18 Table II Skin phenotypes of mutant mice and associated human diseases. Gene Type Human disease Skin phenotype Akt1 Con A Epidermal hyperplasia Akt1/2 Con KO Reduced proliferation, reduced number of HFs, thin epidermis Bp180 KO BP, nh-jeb Blistering, hair loss Bp230 KO BP, EB Blistering, hair loss Cd151 KO EB (?) Normal, but blistering has been observed in humans Col7a1 KO DEB Severe blistering Fak Con KO SG hypoplasia, thin epidermis, HF abnormalities, hair loss Con KO HF and hair cycle abnormalities Ilk Con KO Blistering, BM abnormalities, inflammation, hair loss, abnormal pigmentation Con KO Blistering, inflammation, abnormal HF morphogenesis, hair loss, abnormal pigmentation Itga2 KO Normal Itga3 KO Blistering, BM abnormalities Itga3 Con KO Occasional microblistering, BM duplication, hair loss, inflammation KO / Graft Hair loss, abnormal HF morphogenesis, IFE normal Itga6 KO JEB-PA Severe blistering due to the absence of HDs Itga3/ Itga6 KO Severe blistering as in the absence of a6 alone, proliferation, differentiation and HF morphogenesis normal in adherent areas Itga9 KO Normal Con KO Normal Itgb1 Con KO Blistering, reduced proliferation, BM abnormalities, inflammation, hair loss, erythema Con KO Blistering, reduced proliferation, BM abnormalities, inflammation, hair loss Ind KO Normal Hypo Like con KO but less severe T188I Normal Sup Psoriasis Hyperproliferation, perturbed differentiation, inflammation Itgb4 KO JEB-PA Severe blistering due to the absence of HDs KO Severe blistering due to the absence of HDs Con KO Blistering due to the absence of HDs Tail-less Severe blistering due to the absence of HDs, reduced proliferation 16
19 Table II Continued from previous page. Gene Type Itgb4 1355T Normal, but reduced proliferation Itgb5 KO Normal KO Human disease Itgb6 Overexp Chronic fibrotic ulcers Itgb5/ Itgb6 KO Normal Skin phenotype Juvenile hair loss due to macrophage infiltration Itga2/Itgb1 Sup Psoriasis Hyperproliferation, perturbed differentiation, inflammation Itga5/Itgb1 Sup Psoriasis Hyperproliferation, perturbed differentiation, inflammation Kind1 KO KS In mice mild skin atrophy, but in humans blistering, erythema, abnormal pigmentation, occassional hair loss, atrophy Krt5 KO EBS Severe blistering Krt14 KO EBS Severe blistering Lama3 KO H-JEB Severe blistering Lama5 KO / Graft BM and HF defects Lamb3 Natural H-JEB Severe blistering Lamc2 KO H-JEB Blistering, reduced HDs MEK1 Sup CA Psoriasis Hyperproliferation, perturbed differentiation, inflammation MEK1 CA Psoriasis Hyperproliferation, suppressed differentiation Plec1 KO EBS-MD/ EBS-PA Blistering, reduced HDs Con KO EBS Blistering, reduced HDs rac1 Con KO Defects in IFE, SGs and HFs Con KO Hair loss, IFE normal Con DN Normal 1 BM, basement membrane; BP, bullous pemphigoid; CA, constitutively active; Con A, conditionally active; Con DN, conditional dominant-negative; Con KO, conditional knockout; DEB, dystrophic epidermolysis bullosa; EB, epidermolysis bullosa; EBS, epidermolysis bullosa simplex; EBS-MD, epidermolysis bullosa simplex associated with muscular dystrophy; EBS-PA, epidermolysis bullosa simplex associated with pyloric atresia; HD, hemidesmosome; H-JEB, Herlitz-junctional epidermolysis bullosa; HF, hair follicle; Hypo, hypomorph; Ind KO, inducible knockout; IFE, interfollicular epidermis; JEB-PA, junctional epidermolysis bullosa associated with pyloric atresia; KO, knockout; KS, Kindler syndrome; nh-jeb, nonherlitz-junctional epidermolysis bullosa; Overexp, overexpression; SG, sebaceous gland; Sup, suprabasal expression. Integrins in the epidermis 17
20 of cells in the epidermis were not detected (18,35). Furthermore, in mice in which the b4 subunit was deleted only in small areas of the epidermis, keratinocyte proliferation and survival were normal, also in the b4-null areas (17). Perhaps the most convincing evidence suggesting that a6b4 does not affect differentiation and is not required for keratinocyte proliferation stems from observations in human patients carrying mutations in either one of the genes encoding the a6 or b4 subunit. In areas that are not too severely affected by blistering, differentiation and stratification seem normal and proliferation is not impaired. In fact, as in many bullous disorders, hyperproliferation is commonly observed, probably as a response to chronic injury. b1 integrins Initial observations gave rise to the concept that b1-mediated adhesion in keratinocytes promotes proliferation while inhibiting differentiation. For instance, expression of b1 integrins is high in proliferating basal keratinocytes, whereas a loss of expression is observed in the suprabasal layers. In addition, integrin crosslinking supports proliferation in vitro and inhibits terminal differentiation induced by transfer to suspension (36,37). Moreover, a gainof-function mutation in b1 (T188I) that was originally detected in a squamous cell carcinoma of the tongue, reduces differentiation while increasing adhesion through super-activation of b1 (38). As the b1 subunit can pair with a dozen a subunits, it is not surprising that its targeted deletion causes early embryonic death (39,40). The functions of b1 integrins in the epidermis have therefore been investigated by confining the expression or deletion of b1 to the basal layer of the epidermis using keratin-5 or keratin-14 driven promoters. These studies have not confirmed the supposed role of b1 integrins as negative regulators of differentiation. For instance, basal expression of the T188I mutant neither affected proliferation nor differentiation in the epidermis (41). Moreover, only a slight increase in terminally differentiated cells was reported upon the targeted deletion of b1 from basal keratinocytes, whereas in a parallel study no differences were observed (42,43). Thus, whereas cultured keratinocytes that are transferred to suspension massively undergo differentiation, deletion of b1 integrins from the epidermis is not sufficient to induce differentiation in vivo, probably because cell-cell interactions and a6b4-mediated adhesion to the BM are not disrupted. Hence, suspensioninduced differentiation is probably a consequence of a general loss of adhesion, rather than a loss of b1 ligation. In line with the impaired adhesion and proliferation of β1-null keratinocytes in vitro, keratinocyte proliferation in vivo is dramatically reduced in the absence of b1 and extensive blistering at the dermal-epidermal junction is observed, albeit not as severe as in the absence of a6b4 (42-44). Likely, a6b4 rescues adhesion in the absence of b1 integrins although the numbers of HDs are lower, suggesting that b1 integrins may facilitate efficient HD formation by a6b4. As b1 integrins mediate cell spreading in vitro, it is conceivable that in tissue, they also provide cells with a surface area optimal for HD formation. The combined loss of all b1 integrins from the epidermis is not phenocopied by the deletion of either individual b1-con- 18
21 taining heterodimer. In fact, although a2b1 is absolutely required for keratinocyte adhesion to collagens in vitro, mice lacking a2b1 develop normally and do not display any epidermal defects (45-47). In addition, the targeted deletion of the a9 subunit causes respiratory failure and postnatal death but no epidermal defects are observed, which is confirmed by the epidermis-specific deletion of a9 (48,49). Hence, it appears that a2b1 and a9b1 do not have a unique function in normal intact epidermis. The phenotype of a3-null mice partially resembles that of b1-null mice. Integrin a3b1 is not required for epidermal morphogenesis during development but for postnatal integrity, as newborn a3-deficient mice develop mild, trauma-induced blisters along the epidermal-dermal junction. Furthermore, the BM is disorganized, which is also observed in b1-null epidermis (50,42,43). It remains to be determined whether this is simply a consequence of decreased ligand clustering in the absence of integrin-mediated adhesion, or whether integrins play a more active role in BM assembly. At least, the synthesis and deposition of Ln-332 are not impaired in b1-null or a3-null keratinocytes (42,43,50-52). As a3-deficient mice die from lung and kidney defects shortly after birth, the role of a3b1 in adult epidermis has been studied either by transplanting full-thickness skin biopts from a3-null mice onto nude mice, or by restricting a3 deletion to the basal layer of the epidermis (52-55). In the absence of a3b1, proliferation, survival, and differentiation in the IFE are all normal, whereas microblistering between the epidermis and the dermis and a marked duplication of the BM are occasionally observed, particularly in neonatal animals. Thus, although a number of in vitro studies have suggested that a3b1 is required for proliferation, survival, and cytoskeletal organization in keratinocytes, the physiological significance of these observations seems limited (56-59). Furthermore, while a3b1 is required for in vitro adhesion and cell spreading over Ln-332, the observed blistering in a3-null epidermis is mild, as compared to that after a total loss of b1 integrins. Possibly, whereas a2b1 and a9b1 under steadystate conditions hardly contribute to epidermal homeostasis, they may play a more active role in the absence of a3b1 and at least partially compensate for its absence. Indeed, it has been suggested that a3b1 trans-dominantly inhibits the functions of other integrins (57). In addition, a6b4 can largely maintain adhesion to Ln-332 in the absence of a3b1 but not vice versa, as blistering observed in mice lacking both a3b1 and a6b4 is not more severe than in mice lacking a6b4 alone (33). The latter study also demonstrates that a6b1 that may be formed in the absence of a3b1 and a6b4 cannot compensate for the loss of these integrins. Furthermore, apoptosis occurred in these mice only in blistered but not in non-blistered epidermis, indicating that keratinocyte survival does not critically depend on signals from either a3b1 or a6b4, as long as adhesion is maintained. Thus, a general loss of adhesion rather than the loss of a specific integrin may trigger exaggerated effects including reduced proliferation, apoptosis, and an inflammatory response, as observed in a3- and b1-null epidermis. Indeed, in a study in which b1 deletion from adult mouse epidermis did not cause blistering, probably because adhesion was rescued by 1 Integrins in the epidermis 19
22 a6b4, neither inflammation nor reduced proliferation were observed (60). av integrins Genetic ablation of the av subunit in mice causes embryonic or perinatal lethality, and mice that lack av only in the epidermis have not been generated (61). However, the b5 and b6 subunits have been ablated in mice. Deletion of the b5 subunit had no effect on epidermal development nor on epidermal homeostasis in adult mice, despite impaired adhesion of b5-null keratinocytes to VN in vitro, probably because the expression of avb5 and its ligands are expressed at very low levels in normal epidermis (62). Similarly, deletion of b6 did not affect the IFE but some hair loss was observed in juvenile mice, which will be discussed below (63,64). The role of the weakly expressed integrin avb8 in the epidermis has been poorly defined. Genetic ablation of the b8 subunit causes embryonic or perinatal lethality with severe defects in vascular development, and there are no epidermisspecific b8 knockout mice (65). In vitro, avb8 has been described to inhibit proliferation of airway epithelial cells (66-68). Because avb8 is only expressed in the suprabasal layers of the epidermis and not in the proliferating basal layer, it may also inhibit proliferation of keratinocytes, and possibly even stimulate differentiation. InteGRIN-REGUlateD PROGRessIon THROUGH THE HAIR CYcle In many mammals, a dense hair coat maintains body temperature and provides protection against the environment. Each hair is a fully keratinized appendage that emerges from a highly specialized canal designated the HF, which is continuous with the epidermis and penetrates deeply into the dermis (Figure 2A). The HF consists of several concentric cylinders, including the outer root sheath (ORS), the inner root sheath (IRS), and the innermost hair shaft (HS). In turn, the IRS comprises the Henley, Huxley and cuticle layers, whereas the HS is composed of the cuticle, cortex and medulla (69,70). The ORS and the IRS are separated from each other by a layer of cells called the companion cell layer. In the upper segment of the HF, where the IFE invaginates into the dermis, the infundibulum and the isthmus can be distinguished. In addition, the upper segment contains a glandular appendage, the sebaceous gland (SG), which secretes sebum into the hair canal to lubricate the skin. Just below the SG is the bulge, a reservoir for pluripotent stem cells, whereas the base of the HF or bulb contains the stem cells progeny, which are referred to as transit amplifying cells (TACs) or matrix keratinocytes. In addition, the bulb contains dermal fibroblasts in the dermal papilla (DP), which is crucial for the epithelial-mesenchymal interactions that shape the HF during morphogenesis (Figure 2B). During postnatal life, the hair coat is constantly renewed. However, new HFs are not generated. Instead, the lower portion of the HF cycles through alternating phases of growth (anagen), regression or involution (catagen), and rest (telogen). The parts that remain stable 20
23 1 Figure 2 Structural organization of the hair follicle and the stem cell compartments. (A) Haematoxylin/eosin staining of a hair follicle in late catagen/telogen phase from mouse back skin. (B) Schematic representation of the hair follicle and location of the stem cell compartments in the epidermis. AP, arrector pili; CCL, companion cell layer; DP, dermal papilla; HF, hair follicle; HS, hair shaft; IFE, interfollicular epidermis; Inf, infundibulum; IRS, inner root sheath; Ist, Isthmus; ORS, outer root sheath, SC, stem cells; SG, sebaceous gland; TACs, transit-amplifying cells. are the infundibulum, the isthmus, the bulge and the SG. During anagen, matrix cells proliferate and differentiate while moving upwards. The differentiated cells form the IRS and the HS. Both proliferation and differentiation are halted during catagen, and the HS stops growing. Matrix keratinocytes undergo apoptosis, which is accompanied by regression of the HS. Moreover, the bulb moves upward until it reaches the bulge. In the subsequent telogen phase, follicles become quiescent but a new anagen phase is initiated when the DP interacts with the stem cells in the bulge. New matrix keratinocytes are then generated, which will produce a new HS (Figure 3) (69-71). Several observations implicate keratinocyte integrins in HF morphogenesis and progression through the hair cycle. For instance, deletion of the b1-subunit in mouse epidermis causes progressive hair loss, due to a reduced proliferation of matrix keratinocytes. HFs are morphologically aberrant, fail to invaginate, and are low in number. After the hair is shed from the follicle, a new hair cycle is not initiated, and the degenerating HFs are subsequently eliminated by infiltrating macrophages (42,43). In addition, treatment of human scalp HFs in vitro with b1-activating antibodies promotes hair growth and inhibits its spontaneous regression, suggesting that b1 integrins are equally Integrins in the epidermis 21
24 important for hair growth in human HFs (72). Intriguingly, mice lacking the a3 but not the a2 or a9 subunit in the epidermis also develop local progressive hair loss (alopecia), indicating a unique function for a3b1 as an essential regulator of the hair cycle (45,49,52,54,55). The main BM ligand in the HF is Ln-511, both during development and during the hair cycle in postnatal life. Hair growth in vitro is stimulated by Ln-511, whereas Ln-332 antagonizes it (73,74). In addition, the targeted deletion in mice of the a5 chain of Ln-511, which leads to a loss of Ln-511, causes -besides BM abnormalities- also a failure in hair germ elongation, followed by complete regression. The defects can be rescued by application of purified Ln-511, which depends on its b1-binding domain. Conversely, blocking antibodies against b1 or Ln-511 cause alopecia in human scalp xenografts transplanted onto mice (73,75). Although Ln-511 is a ligand for both a3b1 and a6b4, a3b1 binds Ln-511 with higher affinity (76-78). In line with this, alopecia has not been reported in mice lacking either a6 or b4, or in EB patients carrying mutations in either gene (14-17,26-27). Collectively, these data suggest that HF morphogenesis and the regulation of hair growth in mammals are crucially dependent on a3b1-mediated adhesion to Ln-511, which is conceivable for several reasons. By anchoring the stem cells to Ln-511 in the bulge, a3b1 may simply protect against stem cell depletion, thus maintaining the stem cells in their niche. In addition, it is possible that a3b1-mediated adhesion regulates stem cell proliferation. Alternatively, a3b1 may regulate stem cell migration over Ln-511 from the bulge towards the bulb. Hence, in its absence, migration is impaired and stem cells differentiate before reaching the bulb (79). It should be mentioned that some local hair loss and inflammation is observed in juvenile mice upon deletion of the b6 subunit (64). Although avb6 is expressed in stem cells in the HF, its function is unclear, and there is ample evidence to suggest that the hair loss in b6-null mice is a secondary effect of inflammation due to reduced TGF-b signaling, which will be detailed below (6,64). Figure 3 The hair cycle. Hair follicles cycle through alternating phases of anagen, catagen, and telogen. DP, dermal papilla; IRS, inner root sheath; ORS, outer root sheath; SG, sebaceous gland. 22
25 STEM cell MAIntenance and InteGRIns The epidermis has the remarkable potential to self-renew throughout the entire life span of the animal. In humans, the entire epidermis is renewed approximately every two weeks. The regenerative capacity of the epidermis relies on reservoirs of epidermal stem cells, mainly in the bulge of the HF, although additional stem cells are located in the SG and in the IFE (Figure 2). Only the bulge stem cells have been shown to be multipotent, i.e. they can contribute to all keratinocyte lineages of the IFE, the SG, and the HF. Although they repopulate the epidermis after wounding, bulge stem cells do not contribute to epidermal homeostasis under steady-state conditions (80). Instead, epidermal homeostasis is thought to be regulated by stem cells residing in the IFE. Bulge stem cells are defined by a number of markers, including p51/p63, CD34, keratin-15, keratin-19, Lgr5, and Lgr6 (81-83). Moreover, stem cells have an unlimited clonogenic potential, while the progenitor cells or TACs are proliferatively restricted and are destined for a limited number of mitotic divisions. Stem cells also express integrins a2b1, a3b1, and a6b4 at high levels, whereas integrin expression is much weaker in TACs (84-86). Accordingly, stem cells are characterized by very rapid adhesion in vitro, whereas TACs adhere more slowly (85). It is crucial for the maintenance of the epidermis and the HFs that stem cells are maintained in their niche. As hypothesized above, there is good evidence that stem cell maintenance is dependent on integrin-mediated adhesion, and loss of adhesion may lead to stem cell depletion. Support for this hypothesis is the hair loss that occurs in the absence of a3, b1, or Ln-511. In addition, although the IFE is not essentially disturbed in the absence of b1 integrins, wound closure occurs only by those cells that have escaped deletion, indicating that b1 is essential for the expansion and/or the maintenance of stem cells (87). In contrast, expansion of the stem cell compartment is not observed in transgenic mice expressing the super-adherent T188I mutant in the epidermis (41). Moreover, whereas b1 is required for the in vitro expansion of stem cells and their progenitors, embryonic stem cells devoid of b1 do not differentiate, probably because they fail to assemble a BM (88). Manipulation of transcriptional regulators of integrins underscores that integrins are required for stem cell maintenance. For example, activation or overexpression of c-myc causes stem cells to exit from their niche and terminally differentiate into sebocytes and IFE keratinocytes but not HF keratinocytes, leading to hair loss (89,90). A large number of genes encoding proteins involved in adhesion and cytoskeletal organization are repressed by c-myc, thus altering adhesive interactions with the local microenvironment (91). Indeed, c-myc downregulates the expression of b1 integrins and of a6b4, leading to decreased adhesion and spreading of primary keratinocytes in vitro. The failure of hair differentiation probably results from impaired keratinocyte migration along the ORS to the bulb. Repression of integrin expression by c-myc is mediated via association with miz-1, and the resultant complex binds directly to the promoters of the genes encoding a6 and b1. The cause for c-mycinduced premature differentiation is most 1 Integrins in the epidermis 23
26 likely the downregulation of b1, as it can be prevented by b1 overexpression in the epidermis (92). Expression of the a3 subunit and of a6b4 is directly regulated by the p53 homologue p51/63 (93,94). Interestingly, this transcription factor is essential for skin development and maintains stem cell immaturity (94,95). It is therefore tempting to believe that p51/ p63 maintains the stem cell compartment at least partially through its effects on integrin expression. EPIDERMal InteGRIns as SIGnalING MODUlatoRS Integrin signaling in keratinocytes Integrin-ligand binding induces massive changes in cell shape and cytoskeletal organization, phenomena generally referred to as integrin signaling. As the cytoplasmic tails of integrins lack enzymatic activity, they rely on accessory proteins to propagate signals across the membrane. A notable exception is a6b4, since in HDs only structural and no signaling proteins are detected. In contrast, FAs contain a wide variety of structural, adaptor, and signaling proteins (Figure 1C). In fact, over 150 proteins have been identified to date as components of the integrin adhesome in vitro, and FAs thus constitute large signaling platforms in which integrins connect to proteins involved in the regulation of cytoskeletal organization, or to signal transduction pathways including the mitogen-activated protein kinase (MAPK) or phosphatidylinositol- 3OH kinase (PI3K)/Akt routes (96,97). In addition, integrins not only associate with transmembrane proteins including tetraspanins and growth factor receptors, but also with cell surface heparin sulfate proteoglycans such as syndecans. Numerous interactions and signaling events initiated by b1 integrins have been reported in a variety of cell systems and organisms, and the targeted deletion of integrinassociated proteins in mice has resulted in several cases in early embryonic defects comparable to those in b1-null mice, emphasizing that the interactions between b1 and these proteins are equally important in vivo. We will focus here on a number of proteins that seem to have a functional role in integrin signaling in the epidermis, including focal adhesion kinase (FAK), integrin-linked kinase (ILK), kindlin-1, and CD151. Although FAK may contribute to the physical link between b1 integrins and the cytoskeleton, its primary role is to regulate FA turnover, and to initiate signaling events in FAs (98). Keratinocyte-restricted deletion of FAK only partially mimics the loss of a3 or b1; whereas epidermal blistering or BM irregularities are not observed, the SGs are hypoplastic and the hair cycle is disturbed, leading to spatiotemporal hair loss (99,100). Thus, whereas not required for epidermal integrity, FAK may mediate integrin-regulated events downstream of adhesion. The pseudokinase ILK can also bind the cytoplasmic domain of integrins and link them to the actin cytoskeleton. Because ILK lacks an actin-binding domain, its association with actin is indirect, via PINCH and parvin (101). Intriguingly, genetic ablation of ILK in the epidermis has the same consequences as the loss of b1 integrins. Not only is HF morphogenesis aberrant as a consequence of impaired downward 24
27 migration of progenitor cells to the bulb, but there is also epidermal blistering and disorganization of the BM, indicating that in contrast to FAK, ILK is required for the full repertoire of b1 integrin functions in the epidermis (102,103). In addition to ILK, the b1-binding protein kindlin-1 is of crucial importance for integrin functions in the epidermis. The kindlins have received vast attention in the past few years as integrin activators, but they also reorganize the cytoskeleton and may reinforce integrin-actin connections (104). Kindlins have no actin-binding domain, but at least kindlin-2 can associate indirectly with actin via migfilin (105). Although kindlin-1 knockout mice develop only minor skin defects, mutations in the gene encoding kindlin-1 in humans cause the skin blistering disorder Kindler syndrome (KS), which will be discussed in detail below (106). Hallmarks of KS include duplication of the BM, (micro-)blistering, and occasionally alopecia, thus resembling the defects seen upon loss of b1 integrins and in particular of a3b1 (52). It is conceivable that genetic ablation of additional structural components of the integrinactin link in the epidermis (e.g. migfilin, parvin, PINCH) will cause similar blistering and hair defects. Finally, the tetraspanin CD151 is particularly interesting, as it can associate both with a3b1 and a6b4 in keratinocytes, and is thought to strengthen integrin-mediated adhesions. A mutation in the CD151 gene in humans has been described to cause localized (i.e. in the tibia) skin blistering in addition to deafness and renal failure (12). However, the targeted deletion of CD151 in mice did not cause any epidermal defects while severe kidney dysfunction was observed, reminiscent of that observed in the absence of a3b1 (107,108). Thus, whereas the function of CD151 in the epidermis remains to be clarified, it seems likely that CD151 mainly strengthens adhesions formed by a3b1, rather than a6b4-mediated adhesions. Integrin regulation of Rho GTPases The organization of the actin cytoskeleton is controlled by Rho, Rac, and Cdc42, members of the family of Rho GTPases, which cycle between an active GTPbound form and an inactive GDP-bound form. Whereas Rho increases cytoskeletal tension through actomyosin-based contractility, Rac and Cdc42 stimulate the formation of actin-directed membrane protrusions called lamellipodia and filopodia, respectively. Initial studies reported activation of the Rho GTPases by receptors for soluble factors, but later observations suggested that they can also be activated by integrins. For instance, lamellipodia formation is associated with integrinmediated cell spreading and occurs only on integrin ligands, as opposed to substrates that do not support integrin-mediated adhesion. Furthermore, integrin ligation targets Rac1 to the membrane and induces GTP-loading of Rac1 and Cdc42 (109,110). In keratinocytes, several integrins have been linked to Rac1 activation. For example, an a3b1-fak/src axis was implicated in Rac-mediated lamellipodia formation and directional migration on Ln-332 (111,112). However, directional migration and the formation of leading lamellipodia can also occur on Ln-332 in keratinocytes that lack a3b1 but express a6b1, or on other b1 ligands including Col and FN, demonstrating 1 Integrins in the epidermis 25
28 that these events are not dependent on a specific b1-containing integrin (52,57). In addition, a number of studies have reported that a6b4 can support Rac activation, both under steady-state conditions or following EGF stimulation ( ). Whether integrin ligation simply creates the conditions for GTPase activation, or whether there exists a more direct connection between integrins and GTPases has been largely unclear. Recent observations suggest that talin can bind the Rac1- activator Tiam1 (K. Kaibuchi, personal communication). Although talin-tiam1 binding provides a physical link between b1 integrins and Rac1, it can not provide a rationale for Rac1 activation by a6b4, as talin does not bind to the b4 tail. The deletion of Rac1 in mouse epidermis has generated some clues about the integrin-rac1 connection in vivo, although there are phenotypic differences between mice generated by different groups. In one report, epidermal deletion of Rac1 did not cause defects in the IFE or SGs but resulted in a marked degeneration of the nonpermanent portion of HFs, leading to hair loss and the removal of mutant HFs by macrophages (116). In a different study, HF degeneration was also observed, along with a failure to maintain the IFE and SGs, which was associated with an initial stimulation of stem cell proliferation followed by terminal differentiation (117). Blistering was not observed despite reduced levels of a6b4, indicating that b1 integrins maintain epidermal adhesion in the absence of Rac1. Hence, Rac1 deletion in the epidermis seems to at least partially recapitulate the a3- and b1-null phenotypes downstream of adhesion, whereas it does not cause defects reminiscent of b4 loss. The differences in phenotypes probably arise from differences in deletion efficiency, as residual Rac activity may (partially) rescue the phenotype. Along this line, the expression of a dominantnegative mutant of Rac1 in mouse epidermis did not cause any abnormalities, possibly because endogenous Rac was not completely inhibited (118). In addition to Rac1, Cdc42 has been shown to be involved in the terminal differentiation of HF progenitor cells, through the regulation of b-catenin turnover (119). Integrin crosstalk with RTK signaling in keratinocytes A number of polypeptide growth factors, including epidermal growth factor (EGF), fibroblast growth factor (FGF), hepatocyte growth factor (HGF), keratinocyte growth factor (KGF), macrophage-stimulating protein (MSP), and transforming growth factor (TGF)-a and TGF-b, have been implicated in the maintenance of epidermal homeostasis, based on in vitro studies and on observations in mice in which the expression or function of their cognate receptors has been altered ( ). All of these factors, except for TGF-b, signal through receptor tyrosine kinases (RTKs). Growth factor binding typically induces dimerization of the RTK and autophosphorylation on tyrosines, initiating signaling through the MAPK and PI3K/Akt pathways, which are essential for survival and growth. Indeed, both the targeted deletion of MEK1/2 in mouse epidermis and the knockdown of MAPK1/2 in human epidermis grafted onto mice result in epidermal hypoproliferation and hypoplasia. Activation of the MAPK pathway in mouse or human epidermis leads to epidermal hyperproliferation and hyperplasia, concomitant with 26
29 reduced differentiation ( ). Similarly, constitutive activation of PI3K promotes cell proliferation and inhibits differentiation in both keratinocyte monolayers and organotypic skin cultures, deletion of Akt1/2 in mice impairs skin development, and conditional activation of Akt in the epidermis results in epidermal hyperplasia ( ). Although numerous studies indicate that there is cooperative signaling between b1 integrins and RTKs in a variety of cell types including fibroblasts and endothelial cells, there is no evidence that the same mechanisms are active in keratinocytes (132,133). In contrast, crosstalk between b4 and RTK signaling has been described extensively. A number of reports demonstrate that the cytoplasmic tail of b4 is phosphorylated following stimulation with HGF, MSP, and primarily EGF. Serine phosphorylations induced by EGF or MSP lead to disruption of the bonds between plectin and b4, causing at least partial disassembly of HDs when required, for instance during wound healing (9, ). EGF has also been reported to induce tyrosine phosphorylations in the b4 tail, which proposedly connects b4 to the PI3K and MAPK pathways. Furthermore, crosstalk between b4 and growth factor signaling has been reported to occur through physical association between b4 and a variety of RTKs, including the EGF receptor, ErbB2, and c-met, thereby creating a signaling platform that regulates multiple phenomena such as migration, invasion, survival, and proliferation (9,137). The physiological significance of these observations requires further investigation, as the vast majority of the reports focuses on transformed cells or carcinoma cells instead of normal keratinocytes. As proteins with enzymatic activity have not been detected in HDs, a6b4 is -unlike b1 integrins- not associated with signaling proteins under normal conditions. However, in carcinoma cells, HDs are often rudimentary and a6b4 is no longer confined to the basal surface but is diffusely distributed over the membrane, which may facilitate association with a growth factor receptor and/or tyrosine phosphorylations by hyperactive RTKs existing commonly in transformed cells. Indeed, there is no evidence to suggest that b4 association with growth factor receptors occurs in normal epidermis in vivo. In line with this, lossof-function of a6b4 is partially or fully phenocopied by mutations in genes that encode for (structural) proteins residing within the HD or that are associated with it, whereas to date no genetic disruption of a gene encoding a signaling protein has resembled a6b4 loss (Table II). Integrin crosstalk with TGF-b signaling in keratinocytes Pleiotropic cytokines belonging to the TGF-b superfamily exert a wide range of effects on a variety of cell types. They bind to a heterodimeric receptor complex consisting of a type I and type II receptor serine/threonine kinase, leading to the recruitment, phosphorylation, and dimerization of proteins called receptor-regulated Smads. These subsequently bind to a co-mediator Smad, and the heterotrimeric complex is translocated to the nucleus to initiate transcription of target genes. TGF-b signaling is negatively regulated by inhibitory Smads (138). In addition to Smad-dependent signaling, TGF-β also activates Smad-independent signal transduction pathways. Several members of the TGF-b superfamily, including the bone 1 Integrins in the epidermis 27
30 morphogenetic proteins (BMPs) and the TGF-b isoforms TGF-b1, TGF-b2, and TGF-b3 play important roles in the epidermis. BMPs regulate epidermal proliferation and differentiation during development, as well as the hair cycle in postnatal HFs (81,139). TGF-b1 is strongly involved in the maintenance of epidermal homeostasis, as it inhibits keratinocyte proliferation and promotes their differentiation and apoptosis (122,140). Furthermore, TGF-b1 also regulates progression through the hair cycle, and stimulates a migratory phenotype during wound healing by inducing the expression of FN and FN-binding integrins ( ). Accumulating data indicate that several integrins including avb3, avb5, avb6, and avb8 can activate TGF-b1 and TGF-b3 (146). TGF-bs are secreted in an inactive form in complex with latency-associated protein (LAP) and latent TGF-b binding protein. The LAPs of TGF-b1 and TGF-b3, but not that of TGF-b2, contain an RGD site to which av integrins can bind. Integrins can release TGF-b either proteolytically by recruiting a protease, or non-proteolytically by inducing a conformational change of the LAP. Integrin-mediated activation of TGF-b1/-3 accounts for a large part of TGF-b1/-3 activation in vivo, as suggested by several lines of evidence. First, mutation of the RGD site of the LAP of TGF-b1 causes defects similar to those observed in TGF-b1-null mice (147). Second, deletion of the b6 subunit or conditional deletion of b8 from dendritic cells causes exaggerated inflammation as a result of impaired TGF-b signaling ( ). Third, mice lacking both the avb6 and avb6 integrins recapitulate the whole array of abnormalities observed in TGF-b1 and TGF-b3, but not in TGFb2-knockout mice (151). Evidence that TGF-b activation by avb6 is important in the epidermis stems from observations in mice lacking the b6 subunit. Young b6-deficient mice develop local epidermal inflammation in areas subject to mechanical trauma, including the inner thighs and the head and neck region, which is used by mother mice to lift and move them (64). As integrin avb6 is only expressed on injured or irritated epidermis, it may locally regulate TGF-b activation to combat inflammation at a site of repeated injury. Loss of local avb6-mediated TGF-b activation then leads to exaggerated inflammation. Conversely, constitutive expression of b6 in the basal layer of the epidermis induces persistent TGF-b1 activation, leading to the development of chronic ulcers with severe fibrosis (152). TGF-b activation by avb6 and possibly also avb8 has implications for the healing of skin wounds, as we will discuss below. Besides directly activating TGF-b, integrins can also regulate TGF-b signaling in a variety of cell types (146). In keratinocytes, observations describing the regulation of TGF-b signaling by a3b1 are only beginning to emerge. The integrin a3b1 potentiates TGF-b-mediated induction of matrix metalloproteinase-9 via a Smadindependent mechanism in immortalized but not in primary keratinocytes, which may be relevant for tumorigenesis (153,154). In another study, it was proposed that a3b1 regulates wound healing by enhancing TGF-b signaling by increasing the levels of the inhibitory Smad7, which will be outlined below (155). 28
31 Table III Re-epithelialization phenotypes of mutant mice. Gene Type Re-epithelialization phenotype BP230 KO Delayed re-epithelialization Fak Con KO Normal Itga2 KO Normal Itga3 KO / graft Delayed re-epithelialization due to repressed TGF-b signaling Con KO Normal / faster re-epithelialization; normal re-epithelialization but impaired angiogenesis Itga9 Con KO Normal migration, but thinner epidermis because of reduced proliferation Itgb1 Con KO Delayed re-epithelialization, poor fusion of epidermal edges, normal proliferation Itgb5 KO Normal Itgb6 KO Normal, impaired under conditions of aberrant TGF-b signaling Itgb5 / Itgb6 KO Normal Rac1 Con DN / Con KO Impaired keratinocyte proliferation and migration 1 Con DN, conditional dominant-negative; Con KO, Conditional knockout; KO, knockout; TGF-b, transforming growth factor-b. REGUlatIon of KERATInocYte MIGRATIon and WOUND HealING BY InteGRIns Injury to the skin must be efficiently and rapidly repaired, which is achieved by the concerted efforts of multiple cell types (Figures 4A,B). Upon wounding, blood from damaged vessels leaks into the wound bed, forming a clot that temporarily plugs the wound. The clot consists of degranulating platelets embedded in a provisional matrix of FN, VN, fibrin, and thrombospondin. Growth factors and cytokines released by activated platelets attract inflammatory cells such as macrophages and neutrophils. They eradicate contaminating micro-organisms present at the wound site, and release additional growth factors and cytokines. Fibroblasts and endothelial cells are then recruited to synthesize new ECM and capillaries, forming a connective tissue referred to as granulation tissue ( ). The granulation tissue is contractile, due to the differentiation of fibroblasts into myofibroblasts. Wound contraction facilitates the covering of the wound bed by new epidermis to provide for permanent wound repair, a process referred to as re-epithelialization. Re-epithelialization starts with keratinocyte migration over the provisional matrix, and is followed by keratinocyte hyperproliferation. TGF-b is an important player in multiple aspects of the wound healing process. TGF-b is released by degranulating platelets, infiltrating neutrophils and macrophages, dermal fibroblasts, and migrating keratinocytes. It limits the inflammatory Integrins in the epidermis 29
32 response and stimulates the formation of granulation tissue, by inducing proliferation and subsequent differentiation of fibroblasts, expression and deposition of ECM proteins, and angiogenesis ( ). Tight control over TGF-b activity in the granulation tissue is important, as persistent or exaggerated TGF-b signaling induces the formation of excess connective tissue or scar tissue, a condition known as fibrosis. Because TGF-b inhibits keratinocyte proliferation but promotes migration, its effects on re-epithelialization seem paradoxical and are a topic of controversy. However, most evidence suggests that TGF-b inhibits the rate of re-epithelialization (158). Keratinocyte migration in a twodimensional environment occurs in four basic sequential steps: (1) Rac and Cdc42-driven protrusions at the leading edge, (2) stabilization of these protrusions by integrin-mediated adhesion, (3) translocation of the cell body, and (4) adhesion disassembly and actomyosin-mediated retraction at the cell rear (Figures 4C,D) (160). Migrating keratinocytes deposit large amounts of Ln-332, both in vitro and in vivo, which regulates their adhesion and polarization (Figure 4C) (161,162). Whereas Ln-332 has been reported to have both positive and negative effects on the speed of keratinocyte migration, it is essential for repair of the damaged BM, and for the re-establishment of HDs after wound closure (162). The importance of Ln-332 for proper wound healing is illustrated by the development of chronic wounds with excessive granulation tissue in EB patients who lack Ln-332 (163). In line with the important role of integrins during migration, integrin expression is dramatically upregulated during wound healing, and several integrins are de novo expressed (Table I) (3-5, ). Without b1 integrins, reepithelialization is delayed for days due to defective keratinocyte migration, whereas proliferation is not impaired (44). The inhibition of proliferation in b1-deficient epidermis is apparently overcome during wound healing, probably because of the large amount of growth factors that are released into the wound. Whereas it was initially believed that av integrins were upregulated to drive migration in the absence of b1, it was shown recently that eventual repopulation of the wound is due to the expansion of cells that have escaped b1 deletion, underlining that b1-containing integrins are indispensable for reepithelialization (87). Little is known about the roles of integrin effectors during wound healing, and concepts obtained from keratinocyte migration in culture are not always applicable in vivo. For example, although FAK is required for migration in vitro, wound healing occurs normally in its absence (99). In contrast, Rac1 is crucial for both keratinocyte migration and proliferation during wound healing (118). Despite the crucial importance of b1 integrins, accumulating data indicate that individual b1-containing heterodimers are dispensable for wound healing. In fact, loss of neither αβ1 heterodimer causes a delay in keratinocyte migration, in contrast to predictions made on the basis of in vitro results. For instance, although a2-null keratinocytes or keratinocytes treated with blocking antibodies against the a2 subunit can not migrate over collagens in vitro, re-epithelialization in a2 knockout mice is normal and even slightly 30
33 1 Figure 4 Cutaneous wound repair in vivo and keratinocyte migration in vitro. (A) Schematic representation of wound healing. (B) Immunofluorescence image of mouse epidermis migrating into a wound. Red, plet-1; green, integrin a6; blue, nuclei. (C) Representation of keratinocyte migration in two dimensions. (D) Immunofluorescence image of a migrating mouse keratinocyte in vitro. Green, paxillin; red, actin; blue, integrin b4. BM, basement membrane; FA, focal adhesion; Ln-332, laminin-332. faster than in wild-type mice (45-47, ). Furthermore, re-epithelialization is not impaired in mice lacking the a9b1 integrin in the epidermis, although proliferation is slightly decreased resulting in a thinner neo-epidermis (49). The role of the a3b1 integrin seems more complicated. In vitro studies using blocking antibodies against a3 or a3-null keratinocytes indicate that keratinocyte migration on collagens and FN is faster whereas migration on Ln-332 is impaired in the absence of a3b1 (51,57,111,112,171). This has led to the idea that a3b1 is a trans-dominant inhibitor, suppressing the functions of FN- and Col-binding integrins in nonwounded epidermis. In a wound, the inhibition is then relieved to allow these integrins to mediate migration over the dermis and the provisional matrix (57). However, the upregulation of a3b1 expression in wounds suggests a more active role in keratinocyte migration, and it has been generally assumed that a3b1 mediates adhesion to and migration over the newly synthesized Ln-332 during re-epithelial- Integrins in the epidermis 31
34 ization. Interestingly, two recent reports show that deletion of a3 from the epidermis does not impair re-epithelialization, despite decreased wound angiogenesis in one study (52,55). Consistent with previous observations, migration in the absence of a3 was increased (51,52,57,171). This notion is further supported by very recent findings in human keratinocytes in which the expression of the a3 subunit was silenced (172). In a different study, delayed re-epithelialization was observed in a3-null skin explants grafted onto a3-expressing mice (155). TGF-b signaling in the a3-null wounds was repressed, and the delay in re-epithelialization was reversed when TGF-b signaling was restored. Therefore, it was concluded that a3b1 is not required for re-epithelialization as a mediator of keratinocyte migration, but as a modulator of TGF-b signaling. However, repression of TGF-b signaling in the absence of a3b1 can not explain the delay in re-epithelialization, since it is generally accepted that TGF-b signaling inhibits this process (158). Furthermore, the physiological relevance of these observations is unclear in light of the aforementioned studies. The observed defect is probably inherent to the used system. In the skin grafts, not only the epidermis but also the dermis is a3-deficient. In addition, the grafts are surrounded by a wildtype environment. Moreover, grafting may create aberrant TGF-b signaling from the start, because of the creation of a wound upon grafting. Altogether, it appears that the integrins a2b1, a3b1, and a9b1 are redundant during re-epithelialization, and that ligation of any of these heterodimers alone is not per se required for keratinocyte migration. This is conceivable since several different ligands are available in the wound bed, i.e. the provisional matrix and the dermis. The expression of many different integrins upon injury may thus be a safekeeping mechanism ensuring adhesion of the epidermis to any component of the provisional matrix during re-epithelialization. Possibly, combinations of two or three b1 integrins cooperate in re-epithelialization. Alternatively, re-epithelialization may be mediated by a5b1. However, it is unlikely that a5b1 is indispensable for re-epithelialization since several av-containing integrins that also recognize FN are expressed in wounds, indicating functional redundancy. Indeed, whereas keratinocytes harvested from mice lacking either the b5 or the b6 subunit exhibit impaired migration on FN and VN in vitro, re-epithelialization and wound healing are normal in b5(-/-), b6(-/-), and double knockout mice, suggesting that avb5 and avb6 are not required (62,64). Interestingly, a delay in wound closure is observed in b6-null mice under conditions of repressed TGF-b signaling in the wound, for example upon hydrocortisone treatment (173). TGF-b and avb6 thus seem to play additive roles, and the primary function of avb6 in wounds may be to modulate TGF-b signaling rather than to maintain adhesion and migration of keratinocytes. Expression of avb6 in wounds is absent in the first days after injury when keratinocyte migration is important, and peak expression is not observed until the epidermal edges fuse, after which it remains high for days (174). Possibly, avb6 controls TGF-b activation at this stage, to inhibit proliferation in the epidermis and stop the re-epithelialization process. Wound healing experiments in the absence of avb8 have not been performed 32
35 in vivo, but a prominent role in re-epithelialization is unlikely given that avb8 is expressed at low levels and restricted to the suprabasal layers, while upregulation during wound healing has not been reported. In fact, avb8 was found to delay scratch closure in airway epithelial cells in vitro, due to inhibition of proliferation by TGF-b activation (68). It remains to be determined whether regulation of TGF-b signaling by avb8 plays a role in the reepithelialization of skin wounds in vivo. Finally, the role of the a6b4 integrin during re-epithelialization is obscure, as the severe blistering in its absence renders in vivo wound healing experiments impossible. HDs are disassembled during wound healing to allow for keratinocyte migration, and a6b4 is diffusely distributed in migrating cells in vitro (Figure 4D). Association of a6b4 with actin-rich protrusions has been described in carcinoma cells, and a body of evidence indicates that a6b4 drives tumor cell migration and invasion (9,137,175,176). In keratinocytes, however, a6b4 is connected to the IF system, and there is no evidence for its association with actin filaments, although it is theoretically possible that a6b4 connects to actin via a plectin dimer. It is therefore unlikely that a6b4 directly promotes migration of normal keratinocytes. Indeed, a6b4 remains concentrated in the keratin-rich trailing edge in re-epithelializing wounds (177). Moreover, a6b4 can not rescue re-epithelialization in the absence of b1 integrins (44). Nevertheless, it can not be excluded that a6b4 is indirectly involved in migration, for instance by establishing internal polarity in the cell (i.e. a6b4 binds endogenous Ln-332 in the trailing edge, while a promigratory integrin at the front binds to a ligand available in the matrix). Interestingly, keratinocyte migration in mice lacking BP230 is delayed, suggesting that BP230 promotes keratinocyte migration. It is unclear to date by which mechanism this occurs and whether it involves a6b4 (11). 1 SKIN DIsoRDERS ResUltING FROM aberrant InteGRIN FUnctIon Epidermolysis Bullosa (eb) EB comprises a heterogeneous group of either autosomally dominant or autosomally recessive inherited skin fragility disorders characterized clinically by skin blistering. Worldwide, around 1 in 5000 babies is diagnosed with EB. The blistering results from compromised adhesion along the IFs-HD-Ln-332-anchoring fibrils axis, and is caused by mutations in either gene encoding its components (Figure 1C; Table II) (10-29). EB can be classified into three subcategories, based on the microscopic level at which tissue cleavage occurs; (1) EB simplex (EBS), in which a split is observed within the basal keratinocytes; (2) junctional EB ( JEB), characterized by blistering within the BM, most often in the lamina lucida; and (3) dystrophic EB (DEB), in which rupture occurs in the dermis, at the level of the anchoring fibrils. Different forms of EB can be associated with epithelial fragility in the respiratory, urogenital, and gastrointestinal tracts. In addition, EB can be associated with late-onset muscular Integrins in the epidermis 33
36 dystrophy (EB-MD), or with congenital pyloric atresia (EB-PA). Although the mechanisms underlying EB are thus clearly defined, there is currently no cure or effective treatment. However, molecular approaches such as gene therapy and protein replacement therapy are under development and may prove valuable strategies to treat EB in the future (178). Kindler syndrome (KS) KS is a rare genodermatosis first described by Theresa Kindler in a 14-year old girl in England (179). Whereas KS is primarily a skin blistering disorder, blistering of gastro-intestinal and urinary epithelia has also been reported in some patients. In addition, typical symptoms of KS include photosensitivity, progressive atrophy, erythema (unusual redness), poikiloderma (telangiectases and abnormal pigmentation), and webbing of the fingers and toes (180). Furthermore, a number of patients develop alopecia. Defects in HD assembly, anchoring fibrils, or Ln-332 are not observed, but (micro-)blistering and multiplication of the BM are common, reminiscent of mice that lack b1 integrins, in particular a3b1, in the epidermis. Thus, the symptoms of KS point to a defect in the BM-b1 integrinactin cytoskeleton connection, instead of a defect in the BM-a6b4-keratin filament axis. KS is caused by loss-of-function mutations in the gene encoding kindlin-1 (kindlerin, fermitin family homologue-1), a member of a small family further comprising kindlin-2 (mitogen-inducible gene-2) and kindlin-3 (UNC-112 related protein-2). While accumulating evidence establishes the kindlins as modulators of talin-mediated integrin activation, at least kindlin-2 can strengthen integrinmediated adhesions, by connecting to the actin cytoskeleton via migfilin and filamin (105,181). Kindlin-1 possibly acts in a similar manner. Alternatively, kindlin-1 may connect integrins to actin filaments via ILK, PINCH, and parvin (104). It is thus conceivable that the blistering caused by the loss of kindlin-1 or ILK is due to defective connection to the cytoskeleton, leading to decreased resistance of the epidermis to mechanical stress. Interestingly, a functional link between b1 integrins, ILK, and kindlin-1 is suggested by observations of erythema in b1-null epidermis, or the abnormal pigmentation in the absence of ILK, both of which are common in KS patients (43,102). Bullous phemphigoid (BP) The pemphigoid group of diseases is characterized by subepidermal blistering as a result of circulating autoantibodies and includes, among other disorders, cicatricial pemphigoid, pemphigoid gestationatis, mucous membrane pemphigoid, and BP. Whereas antibodies against desmogleins in desmosomes cause related disorders collectively known as pemphigus, pemphigoid is caused by antibodies targeting components of the HD. BP is the most common acquired blistering disorder, and is induced by IgG autoantibodies against BP180 and BP230. Although targeting the BP proteins may destabilize the HD on its own, it is generally associated with the release of pro-inflammatory cytokines and proteases, activation of the complement system, and the accumulation of inflammatory cells. The combined effect of these events is thought to result in blister formation (182,183). Interestingly, 34
37 BP is associated with alopecia, indicating that BP180 and BP230 may regulate hair cycling, which is further suggested by the hair loss in mice lacking either BP180 or BP230 (10,11). In the absence of the BP proteins, HDs may become mechanically unstable, causing detachment of ORS keratinocytes. However, hair loss has not been described in mice deficient for a6, b4, plectin, or keratin-5 and -14, or in EB patients carrying mutations in either one of these genes, probably because mortality occurs before defects in the hair cycle become apparent. An alternative hypothesis, for which there is no evidence to date, is that the BPs may have functions independent from those in the HD. Psoriasis Psoriasis is a chronic and debilitating inflammatory disease affecting 2-3% of the population worldwide. Although the pathogenesis is unknown, psoriasis may result from a combination of both environmental and genetic factors. Symptoms include dilatation of skin blood vessels, hyperproliferation and loss of differentiation in the epidermis, and infiltration in both the dermis and epidermis of cytokinereleasing inflammatory cells including T-lymphocytes, macrophages and monocytes (184,185). As vasodilatation and infiltration of inflammatory cells precede epidermal hyperproliferation, aberrant keratinocyte behaviour is likely triggered by inflammatory cells. However, there is also evidence to suggest that psoriasis is caused by integrin-induced suprabasal keratinocyte proliferation. For instance, psoriatic keratinocytes produce cytokines that can activate T-cells in culture (186). Furthermore, suprabasal expression of human integrins a2b1, a5b1, or only the b1-subunit in mice induces symptoms of psoriasis, including keratinocyte hyperproliferation, perturbed keratinocyte differentiation, and inflammation (187). Suprabasal keratinocytes derived from these mice release high levels of interleukin-1a, which may trigger the inflammatory response through T-cell activation. Moreover, suprabasal integrin expresssion is associated with constitutive MAPK signaling, stimulating hyperproliferation (188). Indeed, similar hyperproliferation and loss of differentiation are induced upon either basal or suprabasal expression of constitutively active MEK1, or by activation of Ras (126,128,189). Interestingly, constitutive MAPK signaling in turn stimulates integrin expression, suggesting a self-amplifying loop between integrins and the MAPK pathway (126,128). In conclusion, whereas psoriasis is most likely triggered by inflammatory cells infiltrating in the epidermis, subsequent disease progression may be mediated by keratinocyte hyperproliferation and cytokine release from keratinocytes, driven by cooperative signaling between integrins and the MAPK pathway. 1 Integrins in the epidermis 35
38 FInal REMARKS Studies of human disorders, transplantation studies, and studies using transgenic and knockout mice have generated valuable information about the functions of integrins and associated proteins in vivo. Drawbacks of skin grafting may include aberrant signaling events following transplantation, and the presence of a normal environment surrounding the graft. On the other hand, knockout mice do not always recapitulate the phenotype of patients carrying mutations in the corresponding gene, which may be due to compensation mechanisms that exist in mouse but not in man, or to intrinsic differences between mouse and human epidermis. For instance, whereas mouse epidermis is typically 2 to 3 cell layers thick, human skin is much thicker and obviously contains less hair. In addition, the phenotypes of genetically manipulated mice from different labs are not always similar, probably because of differences in genetic background and/or environmental factors. Further studies will be required to explain the differences in phenotypes, and to determine the functions of more integrin-associated proteins and integrin effectors. This will not only increase our understanding of integrin functions in skin homeostasis, stem cell biology, hair cycling or wound healing, but may also lead to new treatment strategies for several human pathologies. ACKnoWleDGEMents We apologize to all authors whose work may have been omitted due to space restrictions. We thank Hans Janssen for the preparation of electron-microscopic images. ABBREVIatIons BM, basement membrane; BMP, bone morphogenetic protein; BP, bullous pemphigoid; Col, collagen; DEB, dystrophic epidermolysis bullosa; DP, dermal papilla; EB, epidermolysis bullosa; EBS, epidermolysis bullosa simplex; ECM, extracellular matrix; EGF, epidermal growth factor; FA, focal adhesion; FAK, focal adhesion kinase; FN, fibronectin; FGF, fibroblast growth factor; HD, hemidesmosome; HF, hair follicle; HGF, hepatocyte growth factor; HS, hair shaft; IF, intermediate filaments; IFE, interfollicular epidermis; ILK, integrin-linked kinase; IRS, inner root sheath; JEB, junctional epidermolysis bullosa; LAP, latency-associated protein; Ln, laminin; KGF, keratinocyte growth factor; KS, Kindler syndrome; MAPK, mitogen-activated protein kinase; MSP, macrophagestimulating protein; ORS, outer root sheath; PI3K, phosphatidylinositol-3-oh kinase; RGD, arginine-glycine-aspartate; RTK, receptor tyrosine kinase; SG, sebaceous gland; TAC, transit-amplifying cells; TGF-a, transforming growth factor-a; TGF-β, transforming growth factor-β; TN, tenascin; VN, vitronectin 36
39 RefeRences 1. Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, Watt FM (2002) Role of integrins in regulating epidermal adhesion, growth, and differentiation. EMBO J 21, Hertle MD, et al (1992) Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J Clin Invest 89, Cavani A, et al (1993) Distinctive integrin expression in the newly forming epidermis during wound healing in humans. J Invest Dermatol 101, Juhasz I, et al (1993) Regulation of extracellular matrix proteins and integrin cell substratum adhesion receptors on epithelium during cutaneous wound healing in vivo. Am J Pathol 143, Tumbar T, et al (2004) Defining the epithelial stem cell niche in skin. Science 303, Stepp MA (1999) a9 and b8 integrin expression correlates with the merger of the developing mouse eyelids. Dev Dyn 214, Litjens SH, et al (2006) Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol 16, Margadant C, et al (2008) Regulation of hemidesmosome disassembly by growth factor receptors. Curr Opin Cell Biol 20, Nishie W, et al (2007) Humanization of autoantigen. Nat Med 13, Guo L, et al (1995) Gene targeting of BPAG1: abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell 81, Karamatic Crew V, et al (2004) CD151, the first member of the tetraspanin (TM4) superfamily detected on erythrocytes, is essential for the correct assembly of human basement membranes in kidney and skin. Blood 104, Heinonen S, et al (1999) Targeted inactivation of the type VII collagen gene (Col7a1) in mice results in severe blistering phenotype: a model for recessive dystrophic epidermolysis bullosa. J Cell Sci 112, Georges-Labouesse E, et al (1996) Absence of the a6 integrin leads to epidermolysis bullosa and neonatal death in mice. Nat Genet 13, Dowling J, et al (1996) b4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol 134, van der Neut R, et al (1996) Epithelial detachment due to absence of hemidesmosomes in integrin b4 null mice. Nat Genet 13, Raymond K, et al (2005) Keratinocytes display normal proliferation, survival and differentiation in conditional b4-integrin knockout mice. J Cell Sci 118, Murgia C, et al (1998) Cell cycle and adhesion defects in mice carrying a targeted deletion of the integrin b4-cytoplasmic domain. EMBO J 17, Peters B, et al (2001) Complete cytolysis and neonatal lethality in keratin-5 knockout mice reveal its fundamental role in skin integrity and in epidermolysis bullosa simplex. Mol Biol Cell 12, Lloyd C, et al (1995) The basal keratin network of stratified squamous epithelia: defining K15 function in the absence of K14. J Cell Biol 129, Ryan MC, et al (1999) Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J Cell Biol 145, Kuster JE, et al (1997) IAP insertion in the murine LamB3 gene results in junctional epidermolysis bullosa. Mamm Genome 8, Meng X, et al (2003) Targeted inactivation of murine laminin gamma2- chain gene recapitulates human junctional epidermolysis bullosa. J Invest Dermatol 121, Andrä K, et al (1997) Targeted inactivation of plectin reveals essential function in maintaining the integrity of skin, muscle, and heart cytoarchitecture. Genes Dev 11, Ackerl R, et al (2007) Conditional targeting of plectin in prenatal and adult mouse stratified epithelia causes keratinocyte fragility and lesional epidermal barrier defects. J Cell Sci 120, Integrins in the epidermis 37
40 26. Ruzzi L, et al (1997) A homozygous mutation in the integrin a6 gene in junctional epidermolysis bullosa with pyloric atresia. J Clin Invest 99, Vidal F, et al (1995) Integrin b4 mutations associated with junctional epidermolysis bullosa with pyloric atresia. Nat Genet 10, Ashton GHS (2003) Kindler syndrome. Clin Exp Dermatol 29, Uitto J (2004) Epidermolysis bullosa: the expanding mutation database. J Invest Dermatol 123, Koster J, et al (2001) Two different mutations in the cytoplasmic domain of the integrin b4 subunit in nonlethal forms of epidermolysis bullosa prevent interaction of b4 with plectin. J Invest Dermatol 117, Nakano A, et al (2001) Epidermolysis bullosa with congenital pyloric atresia: novel mutations in the b4 integrin gene (ITGB4) and genotype/phenotype correlations. Pediatr Res 49, Rodius S, et al (2007) Loss of a6 integrins in keratinocytes leads to an increase in TGF-b and AP-1 signaling and in expression of differentiation genes. J Cell Physiol 212, DiPersio CM, et al (2000) a3b1 and a6b4 integrin receptors for Ln-5 are not essential for epidermal morphogenesis and homeostasis during skin development. J Cell Sci 113, Mainiero F, et al (1997) The coupling of a6b4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J 16, Nikolopoulos SN, et al (2005) Targeted deletion of the integrin b4 signaling domain suppresses laminin-5-dependent nuclear entry of mitogen-activated protein kinases and NF-kappaB, causing defects in epidermal growth and migration. Mol Cell Biol 25, Zhu AJ, et al (1999) Signaling via b1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc Natl Acad Sci 96, Levy L, et al (2000) β1 integrins regulate keratinocyte adhesion and differentiation by distinct mechanisms. Mol Biol Cell 11, Evans RD, et al (2003) A tumor-associated b1 integrin mutation that abrogates epithelial differentiation control. J Cell Biol 160, Fässler R, and M Meyer (1995) Consequences of lack of b1 integrin gene expression in mice. Genes Dev 9, Stephens LE, et al (1995) Deletion of b1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev 9, Ferreira M, et al (2009) An activating b1 integrin mutation increases the conversion of benign to malignant skin tumors. Cancer Res 69, Raghavan S, et al (2000) Conditional ablation of b1 integrin in skin. Severe defects in epidermal proliferation, BM formation, and hair follicle invagination. J Cell Biol 150, Brakebusch C, et al (2000) Skin and hair follicle integrity is crucially dependent on b1 integrin expression on keratinocytes. EMBO J 19, Grose R, et al (2002) A crucial role of b1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 129, Chen J, et al (2002) The a2 integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol 161, Holtkötter O, et al (2002) Integrin a2- deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J Biol Chem 277, Zhang ZG, et al (2006) Interactions of primary fibroblasts and keratinocytes with extracellular matrix proteins: contribution of a2b1 integrin. J Cell Sci 119, Huang XZ, et al (2000b) Fatal bilateral chylothorax in mice lacking the integrin a9b1. Mol Cell Biol 20, Singh P, et al (2009) Loss of integrin a9b1 results in defects in proliferation, causing poor re-epithelialization during cutaneous wound healing. J Invest Dermatol 129, DiPersio CM, et al (1997) a3b1 integrin is required for normal development of the epidermal BM. J Cell Biol 137, DeHart GW, et al (2003) The role of a3b1 integrin in determining the 38
41 supramolecular organization of Ln-5 in the extracellular matrix of keratinocytes. Exp Cell Res 283, Margadant C, et al (2009) Integrin a3b1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci 122, Kreidberg JA, et al (1996) a3b1 integrin has a crucial role in kidney and lung organogenesis. Development 122, Conti FJ, et al (2003) a3b1 integrin regulates hair follicle but not interfollicular morphogenesis in adult epidermis. J Cell Sci 116, Mitchell K, et al (2009) Integrin a3b1 in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial cell crosstalk through induction of MRP3/ Prl2c4. J Cell Sci 122, Gonzales M, et al (1999) A cell signal pathway involving laminin-5, a3b1 integrin, and mitogen-activated protein kinase can regulate epithelial cell proliferation. Mol Biol Cell 10, Hodivala-Dilke KM, et al (1998) Novel roles for a3b1 integrin as a regulator of cytoskeletal assembly and as a transdominant inhibitor of integrin receptor function in mouse keratinocytes. J Cell Biol 142, Wang Z, et al (1999) a3b1 integrin regulates epithelial cytoskeletal organization. J Cell Sci 112, Manohar A, et al (2004) a3b1 integrin promotes keratinocyte cell survival through activation of a MEK/ERK signaling pathway. J Cell Sci 117, López-Rovira T, et al (2005) Different consequences of b1 integrin deletion in neonatal and adult mouse epidermis reveal a context-dependent role of integrins in regulating proliferation, differentiation, and intercellular communication. J Invest Dermatol 125, Bader BL, et al (1998) Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all av integrins. Cell 95, Huang X, et al (2000a) Normal development, wound healing, and adenovirus susceptibility in b5-deficient mice. Mol Cell Biol 20, Huang X, et al (1998) The integrin avb6 is critical for keratinocyte migration on both its known ligand, fibronectin, and on vitronectin. J Cell Sci 1, Huang XZ, et al (1996) Inactivation of the integrin b6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol 133, Zhu J, et al (2002) b8 integrins are required for vascular morphogenesis in mouse embryos. Development 129, Fjellbirkeland L, et al (2003) Integrin avb8-mediated activation of TGFb inhibits human airway epithelial proliferation in intact bronchial tissue. Am J Pathol 163, Araya J, et al (2006) Integrin-mediated TGF-b activation regulates homeostasis of the pulmonary epithelial-mesenchymal trophic unit. Am J Pathol 169, Neurohr C, et al (2006) Activation of TGF-b by the integrin avb8 delays epithelial wound closure. Am J Respir Cell Mol Biol 35, Paus R, and G Cotsarelis (1999) The biology of hair follicles. N Engl J Med 341, Alonso L, and E Fuchs (2006) The hair cycle. J Cell Sci 119, Fuchs E, et al (2001) At the roots of a never-ending cycle. Dev Cell 1, Kloepper JE, et al (2008) Functional role of b1 integrin-mediated signalling in the human hair follicle. Exp Cell Res 314, Li J, et al (2003) Ln-10 is crucial for hair follicle morphogenesis. EMBO J 22, Sugawara K, et al (2007) Spatial and temporal control of laminin-332 (5) and -511 (10) expression during induction of anagen hair growth. J Histochem Cytochem 55, Gao J, et al (2008) Ln-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev 22, Kikkawa Y, et al (2000) Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by a3b1, a6b1 and a6b4 integrins. J Cell Sci 113, Pouliot N, et al (2002) Laminin 10/11: an alternative adhesive ligand for epidermal keratinocytes with a functional role in promoting proliferation and migration. Exp Dermatol 11, Integrins in the epidermis 39
42 78. Delwel GO, et al (1994) Distinct and overlapping ligand specificities of the a3ab1 and a6ab1 integrins: recognition of laminin isoforms. Mol Biol Cell 5, Brakebusch C, and R Fässler (2005) b1 integrin function in vivo: Adhesion, migration and more. Cancer Metast Rev 24, Ito M, et al (2005) Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11, Blanpain C, and E Fuchs (2006) Epidermal stem cells of the skin. Annu Rev Cell Dev Biol 22, Snippert HJ, et al (2010) Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327, Jaks V, et al (2008) Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet 40, Akiyama M, et al (2000) Changing patterns of localization of putative stem cells in developing human hair follicles. J Invest Dermatol 114, Kaur P, and A Li (2000) Adhesive properties of human basal epidermal cells: an analysis of keratinocyte stem cells, transit amplifying cells, and postmitotic differentiating cells. J Invest Dermatol 114, Jones PH, et al (1995) Stem cell patterning and fate in human epidermis. Cell 80, Piwko-Czuchra A, et al (2009) b1 integrin-mediated adhesion signalling is essential for epidermal progenitor cell expansion. PLoS One 4, e Bagutti C, et al (1996) Differentiation of embryonal stem cells into keratinocytes: comparison of wild-type and b1 integrindeficient cells. Dev Biol 179, Gandarillas A, and FM Watt (1997) c-myc promotes differentiation of human epidermal stem cells. Genes Dev 11, Waikel RL, et al (2001) Deregulated expression of c-myc depletes epidermal stem cells. Nat Genet 28, Frye M, et al (2003) Evidence that Myc activation depletes the epidermal stem cell compartment by modulating adhesive interactions with the local microenvironment. Development 130, Gebhardt A, et al (2006) Myc regulates keratinocyte adhesion and differentiation via complex formation with Miz1. J Cell Biol 172, Kurata S, et al (2004) p51/p63 controls subunit a3 of the major epidermis integrin anchoring the stem cells to the niche. J Biol Chem 279, Okuyama R, et al (2007) p53 homologue, p51/p63, maintains the immaturity of keratinocyte stem cells by inhibiting Notch1 activity. Oncogene 26, Mills AA, et al (1999) p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398, Zaidel-Bar R, et al (2007) Functional atlas of the integrin adhesome. Nat Cell Biol 9, Legate KR, et al (2009) Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev 23, Ilić D, et al (1995) Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377, Essayem S, et al (2006) Hair cycle and wound healing in mice with a keratinocyterestricted deletion of FAK. Oncogene 25, Schober M, et al (2007) Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J Cell Biol 176, Legate KR, et al (2006) ILK, PINCH and parvin: the tipp of integrin signalling. Nat Rev Mol Cell Biol 7, Lorenz K, et al (2007) Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J Cell Biol 177, Nakrieko KA, et al (2008) Impaired hair follicle morphogenesis and polarized keratinocyte movement upon conditional inactivation of integrin-linked kinase in the epidermis. Mol Biol Cell 19, Meves A, et al (2009) The kindlin protein family: new members to the club of focal adhesion proteins. Trends Cell Biol 19, Tu Y, et al (2003) Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell 113, Ussar S, et al (2008) Loss of kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet 4, e
43 107. Wright MD, et al (2004) Characterization of mice lacking the tetraspanin superfamily member CD151. Mol Cell Biol 24, Sachs N, et al (2006) Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol 175, Price LS, et al (2003) Calcium signaling regulates translocation and activation of Rac. J Biol Chem. 278, del Pozo MA, et al (2004) Integrins regulate Rac targeting by internalization of membrane domains. Science 303, Choma DP, et al (2004) Integrin a3b1 directs the stabilization of a polarized lamellipodium in epithelial cells through activation of Rac1. J Cell Sci 117, Choma DP, et al (2007) Integrin a3b1- dependent activation of FAK/Src regulates Rac1-mediated keratinocyte polarization on laminin-5. J Invest Dermatol 127, Pullar CE, et al (2006) b4 integrin and epidermal growth factor coordinately regulate electric field-mediated directional migration via Rac1. Mol Biol Cell 17, Russell AJ, et al (2003) a6b4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of a3b1 integrin. J Cell Sci 116, Sehgal BU, et al (2006) Integrin b4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J Biol Chem 281, Chrostek A, et al (2006) Rac1 is crucial for hair follicle integrity but is not essential for maintenance of the epidermis. Mol Cell Biol 26, Benitah SA, et al (2005) Stem cell depletion through epidermal deletion of Rac1. Science 309, Tscharntke M, et al (2007) Impaired epidermal wound healing in vivo upon inhibition or deletion of Rac1. J Cell Sci 120, Wu X, et al (2006) Cdc42 controls progenitor cell differentiation and b-catenin turnover in skin. Genes Dev 20, Miettinen PJ, et al (1995) Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature 376, Vassar R, and E Fuchs (1991) Transgenic mice provide new insights into the role of TGF-a during epidermal development and differentiation. Genes Dev 5, Sellheyer K, et al (1993) Inhibition of skin development by overexpression of TGFb1 in the epidermis of transgenic mice. Proc Natl Acad Sci 90, Werner S, et al (1993) Targeted expression of a dominant-negative FGF receptor mutant in the epidermis of transgenic mice reveals a role of FGF in keratinocyte organization and differentiation. EMBO J 12, Guo L, et al (1996) Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev 10, Dumesic PA, et al (2009) Erk1/2 MAP kinases are required for epidermal G2/M progression. J Cell Biol 185, Tarutani M, et al (2003) Inducible activation of Ras and Raf in adult epidermis. Cancer Res 63, Scholl FA, et al (2007) Mek1/2 MAPK kinases are essential for mammalian development, homeostasis, and Rafinduced hyperplasia. Dev Cell 12, Scholl FA, et al (2004) Mek1 alters epidermal growth and differentiation. Cancer Res 64, Pankow S, et al (2006) Regulation of epidermal homeostasis and repair by phosphoinositide-3-kinase. J Cell Sci 119, Peng XD, et al (2003) Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev 17, Murayama K, et al (2007) Akt activation induces epidermal hyperplasia and proliferation of epidermal progenitors. Oncogene 26, Assoian RK, and MA Schwartz (2001) Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev 11, Howe AK, et al (2002) Anchoragedependent ERK signaling-mechanisms and consequences. Curr Opin Genet Dev 12, Rabinovitz I, et al (2004) Protein kinase C-a phosphorylation of specific serines in the connecting segment of the b4 integrin regulates the dynamics of type II 1 Integrins in the epidermis 41
44 hemidesmosomes. Mol Cell Biol 24, Wilhelmsen K, et al (2007) Serine phosphorylation of the integrin b4 subunit is necessary for epidermal growth factor receptor induced hemidesmosome disruption. Mol Biol Cell 18, Santoro MM, et al (2003) The MSP receptor regulates a6b4 and a3b1 integrins via proteins in keratinocyte migration. Dev Cell 5, Wilhelmsen K, et al (2006) Multiple functions of the integrin a6b4 in epidermal homeostasis and tumorigenesis. Mol Cell Biol 26, Massagué J, and YG Chen (2000) Controlling TGF-b signaling. Genes Dev 14, Botchkarev VA, and AA Sharov (2004) BMP signaling in the control of skin development and hair follicle growth. Differentiation 72, Coffey RJ, et al (1988) Selective inhibition of growth-related gene expression in murine keratinocytes by TGF-b. Mol Cell Biol 8, Foitzik K, et al (2000) Control of murine hair follicle regression (catagen) by TGFb1 in vivo. FASEB J 14, Hashiro M, et al (1991) Stimulation of fibronectin secretion in cultured human keratinocytes by TGF-b not by other growth inhibitory substances. J Dermatol 18, Zambruno G, et al (1995) TGF-b1 modulates b1 and b5 integrin receptors and induces the de novo expression of the avb6 heterodimer in normal human keratinocytes-implications for wound healing. J Cell Biol 129, Gailit J, et al (1994) TGF-b1 stimulates expression of keratinocyte integrins during re-epithelialization of cutaneous wounds. J Invest Dermatol 103, Jeong HW, and IS Kim (2004) TGF-b1 enhances big-h3-mediated keratinocyte cell migration through the a3b1 integrin and P13K. J Cell Biochem 92, Margadant C, and A Sonnenberg (2010) Integrin-TGF-b crosstalk in fibrosis, cancer, and wound healing. EMBO Rep 11, Yang Z, et al (2007) Absence of integrinmediated TGF-b1 activation in vivo recapitulates the phenotype of TGF-b1- null mice. J Cell Biol 176, Munger JS, et al (1999) The integrin avb6 binds and activates latent TGF-b1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, Travis MA, et al (2007) Loss of integrin avb8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449, Lacy-Hulbert A, et al (2007) Ulcerative colitis and autoimmunity induced by loss of myeloid av integrins. Proc Natl Acad Sci 104, Aluwihare P, et al (2008) Mice that lack activity of avb6- and avb8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci 122, Häkkinen L, et al (2004) Increased expression of b6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol 164, Lamar JM, et al (2008) An immortalizationdependent switch in integrin function upregulates MMP-9 to enhance tumor cell invasion. Cancer Res 68, Lamar JM et al (2008) Integrin a3b1 potentiates TGF-b-mediated induction of MMP-9 in immortalized keratinocytes. J Invest Dermatol 128, Reynolds LE, et al (2008) a3b1 integrin-controlled Smad7 regulates reepithelialization during wound healing in mice. J Clin Invest 118, Martin, P. Wound healing: aiming for perfect skin regeneration. (1997) Science 276, Singer AJ, and RA Clark (1999) Cutaneous wound healing. N Engl J Med 341, Werner S, and R Grose (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83, Verrecchia F, and A Mauviel (2002) TGFb signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol 118, Ridley AJ, et al (2003) Cell migration: integrating signals from front to back. Science 302, Frank DE, and WG Carter (2004) Laminin-5 deposition regulates keratinocyte polarization and persistent migration. J Cell Sci 117, Nguyen BP, et al (2000) Deposition of laminin-5 in epidermal wounds regulates integrin signaling and adhesion. Curr Opin Cell Biol 12,
45 163. Schneider H, et al (2007) Biological function of laminin-5 and pathogenic impact of its deficiency. Eur J Cell Biol 86, Larjava H, et al (1993) Expression of integrins and BM components by wound keratinocytes. J Clin Invest 92, Häkkinen L, et al (2000) Immunolocalization of tenascin-c, a9 integrin subunit and avb6 integrin during wound healing in human oral mucosa. J Histochem Cytochem 48, Breuss JM, et al (1995) Expression of the b6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 108, Singh P, et al (2004) The spatial and temporal expression patterns of integrin a9b1 and one of its ligands, the EIIIA segment of fibronectin, in cutaneous wound healing. J Invest Dermatol 123, Pilcher BK, et al (1997) The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol 137, Zweers MC, et al (2007) Integrin a2b1 is required for regulation of murine wound angiogenesis but is dispensable for reepithelialization. J Invest Dermatol 127, Grenache DG, et al (2007) Wound healing in the a2b1 integrin-deficient mouse: altered keratinocyte biology and dysregulated matrix metalloproteinase expression. J Invest Dermatol 127, Kim JP, et al (1992) Integrin receptors and RGD sequences in human keratinocyte migration: unique anti-migratory function of a3b1 epiligrin receptor. J Invest Dermatol 98, Wen T, et al (2010) Integrin a3 subunit regulates events linked to epithelial repair, including keratinocyte migration and protein expression. Wound Repair Regen 18, AlDahlawi S, et al (2006) The avb6 integrin plays a role in compromised epidermal wound healing. Wound Repair Regen 14, Thomas GJ, et al (2006) avb6 integrin in wound healing and cancer of the oral cavity. J Oral Pathol Med 35, Mercurio AM, et al (2001) The a6b4 integrin and epithelial cell migration. Curr Opin Cell Biol 13, Rabinovitz I, et al (1999) Protein kinase C-dependent mobilization of the a6b4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J Cell Biol 146, Underwood RA, et al (2009) Ultrastructural localization of integrin subunits b4 and a3 within the migrating epithelial tongue of in vivo human wounds. J Histochem Cytochem 57, Mavilio F, et al (2006) Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med 12, Kindler T (1954) Congenital poikiloderma with traumatic bulla formation and progressive cutaneous atrophy. Br J Dermatol 66, White SJ, and WHI McLean (2005) Kindler surprise: mutations in a novel actin-associated protein cause Kindler syndrome. J Dermatol Sci 38, Larjava H, et al (2008) Kindlins: essential regulators of integrin signalling and cellmatrix adhesion. EMBO Rep 9, Kasperkiewicz M, and D Zillikens (2007) The pathophysiology of bullous pemphigoid. Clin Rev Allergy Immunol 33, Olasz EB, and KB Yancey (2008) Bullous pemphigoid and related subepidermal autoimmune blistering disease. Curr Dir Autoimmun 10, Baadsgaard O, et al (1990) The role of immune system in the pathogenesis of psoriasis. J Invest Dermatol 95, 32S-34S 185. Nickoloff BJ, et al (2007) Immunopathogenesis of psoriasis. Clinic Rev Allerg Immunol 33, Chang, et al (1992) T-cell activation is potentiated by cytokines released by lesional psoriatic, but not normal, epidermis. Arch Dermatol 128, Carroll, JM, et al (1995) Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell 83, Haase I, et al (2001) A role for mitogenactivated protein kinase activation by integrins in the pathogenesis of psoriasis. J Clin Invest 108, Hobbs RM, et al (2004) Expression of activated MEK1 in differentiating epidermal cells is sufficient to generate hyperproliferative and inflammatory skin lesions. J Invest Dermatol 123, Integrins in the epidermis 43
46
47 Regulation of hemidesmosme disassembly by growth factor receptors Coert Margadant, Evelyne Frijns, Kevin Wilhelmsen #, and Arnoud Sonnenberg Division of Cell Biology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands. # Present address: Department of Anesthesia and Perioperative Care, University of California, San Francisco, CA Curr Opin Cell Biol 20, (2008)
48
49 ABSTRAct Hemidesmosomes (HDs) promote stable adhesion of basal epithelial cells to the underlying basement membrane (BM). Critical for the mechanical stability of the HD is the interaction between the integrin a6b4 and plectin, which is destabilized when HD disassembly is required, for instance, to allow keratinocyte migration during wound healing. Growth factors such as epidermal growth factor (EGF) can trigger HD disassembly and induce phosphorylation of the b4 intracellular domain. Whereas tyrosine phosphorylation appears to mediate cooperation with growth factor signaling pathways and invasion in carcinoma cells, serine phosphorylation seems the predominant mechanism to regulate HD destabilization. Here, we discuss recent advances that shed light on the involved residues, the identity of the kinases that phosphorylate them, and the interactions that are targeted by these phosphorylations. 1 IntRODUctIon Hemidesmosomes (HDs) are specialized multiprotein complexes that provide for stable adhesion of basal epithelial cells to the underlying basement membrane (BM) in (pseudo-) stratified as well as certain complex and simple epithelia (1). Two types of HDs can be distinguished on the basis of their components (Figure 1). Type II HDs are found in simple epithelia including that of the intestine, and consist of the integrin a6b4 and plectin (HD1). Type I (classical) HDs are found in (pseudo-) stratified epithelium, such as in the skin, and consist of a6b4, plectin, the tetraspanin CD151 and the bullous pemphigoid (BP) antigens 180 (type XVII collagen) and 230 (BPAG1) (1,2). Integrin a6b4 and BP180 bind with high and low affinity, respectively, to laminin-332 (Ln- 332; previously called laminin-5) in the BM, and intracellular stabilization occurs via association of plectin and BP230 with keratin intermediate filaments, thus creating a stable anchoring complex (1-4). The importance of HDs in maintaining epithelial integrity is illustrated by two lines of evidence. Firstly, ablation of the genes encoding a6, b4, or plectin in mice results in severe blistering of the skin, causing neonatal death because of an epithelial barrier defect; however, knockout mice lacking BP180 or BP230 display only a mild form of skin blistering (1,2). Secondly, human patients carrying mutations in any of the HD components suffer from a skin blistering disorder known as epidermolysis bullosa. The severity of the disease depends on the type and location of the mutations, and their consequences at the mrna and protein levels (5,6). Despite the role of HDs in mediating stable adhesion, they are highly dynamic structures that can quickly disassemble under conditions where (partial) detachment from the BM is required, for example during cell division, differentiation or migration (7,8). Upon disassembly, HD components are no longer concentrated at the basal surface but are instead diffusely distributed over the plasma membrane or in the cytoplasm, or become translocated to lamellipodia (9-11). Although Regulation of hemidesmosome disassembly 47
50 the precise mechanisms that lead to HD disassembly remain obscure, it is at least partially triggered by, and dependent on, phosphorylation events of HD components elicited by growth factor stimulation. Phosphorylation of the b4 intracellular domain has been documented in response to hepatocyte growth factor (HGF), macrophage-stimulating protein (MSP), and primarily epidermal growth factor (EGF). However, significant controversies exist in the literature concerning the residues that are phosphorylated, their role in the regulation of HD destabilization, and the intracellular responses that are triggered by these phosphorylations independent of HD disassembly. In this review, we first focus on protein-protein interactions governing HD assembly, and then discuss recent insights into how growth factor-induced phosphorylation events impact these interactions to regulate HD disassembly and a6b4-dependent functions in normal keratinocytes and carcinoma cells. Figure 1 Schematic drawing of Type I and Type II HDs. Type II HDs are present in simple epithelia such as that of the intestine and consist solely of the integrin a6b4 and the plakin plectin (HD1). Type I HDs are found in (pseudo-)stratified epithelia such as that of the skin and additionally contain the tetraspanin CD151, the Type XVII collagen BP180, and the plakin BP230. BP230 and plectin mediate intracellular stabilization of the HD by binding to intermediate filament keratins. K, keratin; Ln, laminin. 48
51 PRoteIN-PRoteIN InteRActIons INVolVED IN HD DIsasseMblY The cytoplasmic tail of b4a is 1017 amino acids long and consists of a membraneproximal Na + -Ca 2+ (CalX) exchanger motif and 2 pairs of fibronectin type III (FNIII) repeats, which are separated by a connecting segment (CS) (Figure 2). The cytoskeletal linker protein plectin can associate with either b4 or actin filaments, and these binding events are mutually exclusive (12-14). The interaction of the actin binding domain (ABD) of plectin with the first pair of FNIII repeats and the N-terminal 27 amino acids of the CS of b4 (residues ) is thought to be the initial step in HD assembly, which is strengthened by additional interactions of the plectin plakin domain with the CS and the C-tail (Figures 2 and 3). Subsequently, BP180 interacts extracellularly with Ln-332, and intracellularly with plectin and the third FNIII repeat of b4. Lastly, BP230 is recruited through associations with BP180 and a region on b4 comprising the C-terminal 21 amino acids of the CS and the second pair of FNIII repeats (15-19). In addition to the multiple associations exerted by the cytoplasmic domain of the b4 subunit, the extracellular domain of the a6 subunit interacts with BP180 and CD151 (20). The crucial determinant for HD assembly is the interaction between b4 and plectin, as indicated both by the existence of type II HDs which can apparently form in the absence of BP180 and BP230 recruitment, and the hypoplastic nature of HDs that are observed in patients with mutations in b4 (R1281W or R1225H) that prevent this interaction (21,22). Furthermore, in vitro evidence indicates that disruption of the plectin-b4 interaction is sufficient to disrupt HD formation (17). It is therefore likely that HD disassembly in response to growth factor stimulation is primarily achieved by destabilization of the plectinb4 interaction. 1 Figure 2 Structural organization of integrin α6β4 and plectin. Indicated are the various domains, the regions that are involved in the plectin-b4 interaction, and the positions of important tyrosine and serine phosphorylation sites as reported in the literature. Regulation of hemidesmosome disassembly 49
52 GROWTH factor-induced TYRosIne PHosPHORYlatIon of THE β4-subunit Several tyrosines located in the b4 cytoplasmic tail have been implicated in processes typically regulated by growth factor receptors (Figure 2). However, it is an area of many conflicting results. For instance, association of a6b4 with ErbB2 was reported in transformed keratinocytes, carcinoma cells, and ErbB2- transformed fibroblasts, resulting in ErbB2 autophosphorylation, activation of phosphatidylinositol 3-kinase (PI3-K), tumorigenesis and enhanced invasiveness (23-27). Activation of PI3-K and increased invasion were induced by ligation of a6b4, and the subsequent phosphorylation of primarily tyrosines 1257 and 1494 (28,29). Nevertheless, while one study determined Y1494 as the crucial residue, another study reported that the region spanning residues 854 to 1183 was required (27,29). Association of a6b4 with the EGF receptor or c-met (the HGF receptor) has also been reported in carcinoma cells (30-36). Upon stimulation with HGF, tyrosine phosphorylations in b4 elicit activation of both the PI3-K and extracellular signalregulated kinase (ERK) pathways, leading to enhanced HGF-dependent tumorigenesis and invasion (33-35). Phosphorylation of Y1257, Y1440, Y1494, and Y1526 is responsible for coupling b4 to the Ras-ERK pathway, either via binding of Shp2 to b4, which results in the stimulation of Src and the subsequent phosphorylation of Gab1 on residues that promote Grb2 binding, or via the binding of Shc, which when phosphorylated also recruits Grb2 to the membrane (36,37). Cooperation between a6b4 and c-met was independent of the extracellular domain, giving rise to the idea that the b4 cytoplasmic domain functions as a signaling platform for growth factor signaling pathways (33-36). However, a c-met-a6b4 association was not detected by other researchers in the same cells. In addition, b4-enhanced invasion was not specific to c-met, and c-met could mediate invasion independently of b4 (38). Furthermore, the role of the b4 intracellular domain as a signaling adaptor is questioned by a recent study showing that b4-shp2 association was only slightly increased by HGF, and HGF-induced invasion, as well as ERK and PI3-K signaling, were not enhanced by dimerization of the b4 intracellular domain (39). It therefore remains unclear exactly how b4 and c-met cooperate in carcinoma cells. The role of EGF-induced tyrosine phosphorylation events is also controversial; whereas initial studies suggested that phosphorylation of Y1422 and Y1440 in the CS of b4 mediates HD assembly, a later study by the same group confusingly reported the opposite, namely that these phosphorylations antagonize HD formation (37,40,41). A subsequent report then again implicated these residues in HD assembly based on the observation that phenylalanine substitutions impaired HD formation in an in vitro organotypic culture model (42). However, it remains ambiguous whether it is the inability to phosphorylate these residues or the mutations themselves that caused this effect. Given the available data, the contribution of tyrosine phosphorylation to HD disassembly under physiological conditions 50
53 1 Figure 3 Hypothetical models for HD disassembly induced by serine phosphorylation. When not phosphorylated, the b4 intracellular domain interacts with the ABD and the plakin domain of plectin. Upon serine phosphorylation of the b4 CS, binding of the plectin ABD is prevented either by (A) a conformational change leading to intramolecular folding of the b4 cytoplasmic domain, or (B) binding of an alternative protein to the phosphorylated CS of b4. (i.e. in normal untransformed keratinocytes) is disputable. This is underscored by the observation that a b4 mutant that was not tyrosine-phosphorylated in response to EGF was not impaired in mediating EGF-stimulated migration and thus HD disassembly in keratinocytes (43). In addition, in normal keratinocytes as well as the same transformed cell lines used in the aforementioned studies, tyrosine phosphorylation was absent or only marginally detected by several groups, both in unstimulated conditions and under conditions when HDs are disassembled such as during EGF stimulation. Instead, serine phosphorylation of b4 was evident under steady state conditions, and increased in the presence of EGF (44-47). Altogether, the functional relevance of tyrosine phosphorylation of the b4 cytoplasmic domain may be restricted to processes such as carcinoma invasion. Association of a6b4 with a growth factor receptor and tyrosine phosphorylation of b4 in carcinoma cells may represent aberrant phenomena that are induced by overexpression of growth factor receptors or the constitutive signaling by hyperactive receptor tyrosine kinases, as commonly observed in transformed cells. Moreover, the HDs in carcinoma cells are often rudimentary and structurally inferior due to decreased expression levels of BP180 and Regulation of hemidesmosome disassembly 51
54 BP230, and a6b4 localization is no longer confined to the basal surface but is in fact diffusely distributed over the membrane, which may increase its susceptibility for active kinases (2,48). GROWTH factor-induced serine PHosPHORYlatIons of HD components Early reports documented a redistribution of HD components from the basal surface to the cytosol upon phorbol esterinduced activation of members of the protein kinase C (PKC) family of serine/ threonine kinases, suggesting that PKCs regulate HD disruption (49). This was confirmed in later studies demonstrating the breakdown of HDs in carcinoma cells and normal keratinocytes after activation of PKC-family members or overexpression of PKC isoforms. In particular PKCa and PKCd have been implicated in this process, with the specific isoform involved seemingly cell type-dependent (44-47). The b4 cytoplasmic domain is phosphorylated on serines under steady-state conditions, which is augmented after phorbol myristate actetate-stimulated PKC activation or, physiologically more relevant, EGF stimulation. Serine phosphorylations occur primarily in the CS and the C-tail, and phosphopeptide mapping experiments identified S1356, S1360, and S1364 in the CS as the most prominent sites (Figure 2) (46,47). They are embedded in an amino acid context that is highly conserved, in mammals as well as in evolutionarily more distant species such as fish (Table 1), which suggests a critical role for this region. Indeed, studies using mutants carrying either phosphomimic aspartic acid or nonphosphorylatable alanine substitutions pointed out that phosphorylation of two or more of these serines prevents binding of the plectin ABD to b4. Accordingly, b4 mutants with triple aspartic acid substitutions were significantly impaired in HD formation under steady-state conditions, whereas mutants carrying triple alanine substitutions formed robust HDs which were resistant to EGF-induced disruption (47). Although PKC is undoubtedly involved, it may not account for the phosphorylation of all three residues. Whereas in one study it was reported that at least two of the three moieties were PKC targets, we found that S1360 is the only PKC site on b4, at least in keratinocytes (46,47). In search for additional kinases involved, S1364 was identified as a site for protein kinase A (47). However, there is no evidence for protein kinase A activation downstream of the EGF receptor in keratinocytes, whereas the EGF-induced activation of PKC is well established. The exact identity of all kinases triggering b4 serine phosphorylations in response to EGF remains to be determined. Interestingly, S1356, S1360 and S1364 are not directly involved in plectin binding, and are located in a region that can be deleted without compromising HD formation (15,18). There is evidence suggesting that the C-tail of b4 can bind intramolecularly to a 321 amino acid segment including the first pair of FNIII repeats and part of the CS (16,19). These regions of b4 also bind to a segment of the plectin 52
55 Table 1 Sequence conservation of the region containing serines 1356, 1360 and plakin domain (17), thereby enforcing the interaction between the two proteins. Possibly, this complex is disrupted upon serine phosphorylation of b4, allowing two of the three phosphorylated serines to interact with arginines 1225 and 1281 in the second FNIII repeat (Figure 3). Since the arginines are essential for plectin binding (21,22), the segment of the CS containing the phosphorylated serines thus competes for binding with plectin. Alternatively, b4 phosphorylation may increase its affinity for a third protein, that when bound to b4 prevents plectin binding by steric hindrance (Figure 3) (1). Although the main determinant for HD stability is the plectin-b4 interaction, additional associations must be broken for full HD dissolution, including the interactions of b4 with both BP180 and BP230. In this respect, it is noteworthy that BP180 is also phosphorylated by PKC, leading to its translocation out of HDs (49). It is conceivable that other HD components are subject to a similar mode of regulation. In fact, PKC-mediated phosphorylation of a6 has also been reported (45). Moreover, whereas the emphasis has been on the effects of EGF, it should be noted that EGF alone does not induce complete HD disruption. It is likely that in an in vivo situation such as during wound healing, additional growth factors known to modulate keratinocyte migration and proliferation induce the activity of other kinases that contribute to HD disassembly. These factors may Regulation of hemidesmosome disassembly 53
56 include MSP and transforming growth factor-a and -b. For the latter factors, no evidence exists to date with respect to their involvement in HD disassembly, but an interesting report has highlighted the role of MSP, a ligand for the receptor tyrosine kinase Ron, in the breakdown of HDs. MSP-Ron signaling regulates multiple processes in keratinocytes including proliferation, survival, and migration. Keratinocyte stimulation with MSP results in the serine phosphorylation of a6b4, causing protein-dependent mobilization to lamellipodia where it associates with Ron, and the partial breakdown of HDs (50). Furthermore, although the role of S1356, S1360 and S1364 is emphasized, they are not the only serines phosphorylated. Phosphorylation of additional serines on b4 may play a role to achieve full HD destabilization. The complete dissolution of HDs is likely to be the result of the concerted efforts of multiple kinases activated by distinct extracellular stimuli. CONCLUSIons We have discussed recent findings on the mechanisms of HD disassembly by growth factor receptors, both in normal keratinocytes and carcinoma cells. The mechanisms involved may differ on the cell type investigated: tyrosine phosphorylation seems to mediate activation of growth factor signaling pathways involved in migration and invasion in carcinoma cells, while serine phosphorylation appears more relevant under physiological conditions in normal keratinocytes to destabilize HDs. It is possible that serine phosphorylation is also the primary mechanism to disrupt HDs in carcinoma cells, which then releases b4 to become phosphorylated on tyrosines. Serine phosphorylations primarily target the plectin-b4 interaction and may result in an intramolecular binding of the b4 cytoplasmic domain which prevents the interaction with plectin. Alternatively, a third protein may bind b4 when phosphorylated, thus preventing plectin binding through competition. Though an important role is established for EGF-induced PKC activation, it does not account for complete HD disassembly. Additional kinases and extracellular stimuli governing complete HD dissolution remain to be identified. ACKnoWleDGEMents We thank Allan Sonnenberg for excellent artwork. We are grateful to DEBRA (UK) and the Netherlands Organization for Scientific Research for financial support. ABBREVIatIons ABD, actin-binding domain; BM, basement membrane; BP, bullous pemphigoid; CS, connecting segment; EGF, epidermal growth factor; ERK, extracellular signal regulated kinase; FNIII, fibronectin-type III; HD, hemidesmosome; HGF, hepatocyte growth factor; Ln-332, laminin-332; MSP, macrophage-stimulating protein; PI3-K, phosphatidylinositol 3- kinase; PKC, protein kinase C 54
57 RefeRences 1. Litjens SHM, et al (2006) Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol 16, Wilhelmsen K, et al (2006) Multiple functions of integrin a6b4 in epidermal homeostasis and tumorigenesis. Mol Cell Biol 26, Tasanen K, et al (2004) Keratinocytes from patients lacking collagen XVII display a migratory phenotype. Am J Pathol 164, Sonnenberg A, and RK Liem (2007) Plakins in development and disease. Exp Cell Res 313, Pulkkinen L, and J Uitto (1999) Mutation analysis and molecular genetics of epidermolysis bullosa. Matrix Biol 18, Pfendner E, et al (2005) Progress in epidermolysis bullosa: the phenotypic spectrum of plectin mutations. Exp Dermatol 14, Geuijen CA, and A Sonnenberg (2002) Dynamics of the a6b4 integrin in keratinocytes. Mol Biol Cell 13, Tsuruta D, et al (2003) Hemidesmosome protein dynamics in live epithelial cells. Cell Motil Cytoskeleton 54, Kurpakus MA, et al (1991) Surface relocation of a6b4 integrins and assembly of hemidesmosomes in an in vitro model of wound healing. J Cell Biol 115, Gipson IK, et al (1993) Redistribution of the hemidesmosome components a6b4 and bullous phemphigoid antigens during epithelial wound healing. Exp Cell Res 207, Mercurio AM, et al (2001) The a6b4 integrin and cell migration. Curr Opin Cell Biol 13, Geerts D, et al (1999) Binding of integrin a6b4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding. J Cell Biol 147, Litjens SH, et al (2003) Specificity of binding of the plectin actin-binding domain to b4 integrin. Mol Biol Cell 14, Litjens SHM, (2005) Modeling and experimental validation of the binary complex of the plectin actin-binding domain and the first pair of fibronectin type III (FNIII) domains of the b4 integrin. J Biol Chem 280, Niessen CM, et al (1997) A minimal region on the integrin b4-subunit that is critical to its localization in hemidesmosomes regulates the distribution of HD-1/plectin in COS-7 cells. J Cell Sci 110, Schaapveld RQJ, et al (1998) Hemidesmosme formation is initiated by the b4-integrin subunit, requires complex formation of b4 and HD1/plectin, and involves a direct interaction between b4 and the bullous pemphigoid antigen 180. J Cell Biol 142, Koster J, et al (2004) Role of binding of plectin to the integrin b4 subunit in the assembly of hemidesmosomes. Mol Biol Cell 15, Nikolopoulos SN, et al (2005) Targeted deletion of the integrin b4-cytoplasmic domain suppresses laminin-5 dependent nuclear entry of mitogen activated protein kinases and NF-kB, causing defects in epidermal growth and migration. Mol Cell Biol 25, Rezniczek GA, et al (1999) Linking integrin a6b4-based cell adhesion to the intermediate filament cytoskeleton: direct interaction between the b4-subunit and plectin at multiple molecular sites. J Cell Biol 141, Sterk L, et al (2000) The tetraspan molecule CD151, a novel constituent of hemidesmosomes, associates with the integrin a6b4 and may regulate the spatial organization of hemidesmosomes. J Cell Biol 149, Nakano A, et al (2001) Epidermolysis bullosa with congenital pyloric atresia: novel mutations in the b4 integrin gene (ITGB4) and genotype/phenotype correlations. Pediatr Res 49, Koster J, et al (2001) Two different mutations in the cytoplasmic domain of the integrin b4 subunit in nonlethal forms of epidermolysis bullosa prevent interaction of b4 with plectin. J Invest Dermatol 117, Hintermann E, et al (2001) Inhibitory role of a6b4-associated erbb2 and phosphoinositide 3-kinase in keratinocyte haptotactic migration dependent on a3b1 integrin. J Cell Biol 153, Regulation of hemidesmosome disassembly 55
58 24. Hintermann E, (2005) Integrin a6b4-erbb2 complex inhibits haptotaxis by upregulating E-cadherin cell-cell juntions in keratinocytes. J Biol Chem 280, Guo W, et al (2006) b4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 126, Falcioni R, et al (1997) a6b4 and a6b1 integrins associate with ErbB-2 in human carcinoma cell lines. Exp Cell Res 236, Gambaletta D, et al (2000) Cooperative signaling between the a6b4 integrin and ErbB-2 receptor is required to promote phosphatidylinositol 3-kinase-dependent invasion. J Biol Chem 275, Shaw LM (1997) Activation of phosphoinositide-3-oh kinase by the a6b4 integrin promotes carcinoma invasion. Cell 26, Shaw LM (2001) Identification of Insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the a6b4 integrindependent activation of phosphoinositide 3- OH kinase and promotion of invasion. Mol Cell Biol 21, Mariotti A, et al (2001) EGF-R signaling through Fyn kinase disrupts the function of a6b4 integrin at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J Cell Biol 155, Gagnoux-Palacios L, et al (2003) Compartmentalization of integrin a6b4 signaling in lipid rafts. J Cell Biol 162, Giancotti FG (2007) Targeting integrin b4 for cancer and anti-angiogenic therapy. Trends Pharmacol Sci 28, Trusolino L (2001) A signaling adaptor function for a6b4 integrin in the control of HGF-dependent invasive growth. Cell 107, Comoglio PM (2003) Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr Opin Cell Biol 15, Bertotti A (2005) b4 integrin is a transforming molecule that unleashes Met tyrosine kinase tumorigenesis. Cancer Res 65, Bertotti A (2006) b4 integrin activates a Shp2-Src signaling pathway that sustains HGF-induced anchorage-independent growth. J Cell Biol 175, Dans M, (2001) Tyrosine phosphorylation of the b4 integrin cytoplasmic domain mediates Shc signaling to extracellular signal-regulated kinase and antagonizes formation of hemidesmosomes. J Biol Chem 276, Chung J, et al (2004) The Met receptor and a6b4 integrin can function independently to promote carcinoma invasion. J Biol Chem 279, Merdek KD, et al (2007) Intrinsic signaling functions of the b4 integrin intracellular domain. J Biol Chem 282, Mainiero F, et al (1995) Signal transduction by the a6b4 integrin: distinct b4-subunit sites mediate recruitment of Shc/Grb2 and association with the cytoskeleton of hemidesmosomes. EMBO J 14, Mainiero F, et al (1996) The intracellular functions of a6b4 integrin are regulated by EGF. J Cell Biol 134, Dellambra E, et al (2001) Gene correction of integrin b4-dependent pyloric atresia-juntional epidermolysis bullosa keratinocytes estableshes a role for b4 tyrosines 1422 and 1440 in hemidesmosome assembly. J Biol Chem 276, Russell AJ, et al (2003) a6b4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of a3b1 integrin. J Cell Sci 116, Rabinovitz I, et al (1999) Protein kinase C-dependent mobilization of the a6b4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J Cell Biol 146, Alt A, et al (2001) Protein kinase Cdmediated phosphorylation of a6b4 is associated with reduced integrin localization to the hemidesmosome and decreased keratinocyte attachment. Cancer Res 61, Rabinovitz I, et al (2004) Protein kinase C-a phosphorylation of specific serines in the connecting segment of the b4 integrin regulates the dynamics of type II hemidesmosomes. Mol Cell Biol 24, Wilhelmsen K, et al (2007) Serine phosphorylation of the integrin b4- subunit is necessary for epidermal growth factor-induced hemidesmosome disruption. Mol Biol Cell 18, Lipscomb EA and AM Mercurio (2005) Mobilization and activation of a signaling 56
59 competent a6b4 integrin underlies its contribution to carcinoma progression. Cancer Metastasis Rev 24, Kitajima Y, et al (1999) Transmembrane signaling for adhesive regulation of desmosomes and hemidesmosomes, and for cell-cell detachment induced by pemphigus IgG in cultures keratinocytes: involvement of protein kinase C. J Invest Dermatol Symp Proc 4, Santoro MM, et al (2003) The MSP receptor regulates a6b4 and a3b1 integrins via proteins in keratinocyte migration. Dev Cell 5, Regulation of hemidesmosome disassembly 57
60
61 Integrin-TGF-β crosstalk in fibrosis, cancer and wound healing Coert Margadant and Arnoud Sonnenberg Division of Cell Biology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands. EMBO Rep 11, (2010)
62
63 ABSTRAct Accumulating evidence indicates that there is an extensive crosstalk between integrins and TGF-b signalling. TGF-b affects integrin-mediated cell adhesion and migration by regulating the expression of integrins, their ligands, and integrin-associated proteins. Conversely, several integrins control TGF-b activation directly. In addition, a number of integrins can interfere with both Smad-dependent and Smad-independent TGF-b signalling in different ways, including the regulation of the expression of TGF-b signalling pathway components, the physical association of integrins with TGF-b receptors, or the modulation of downstream effectors. Reciprocal TGF-b-integrin signalling is implicated in normal physiology, as well as in a wide variety of pathological processes including systemic sclerosis, idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease, and cancer thus, integrins could provide attractive therapeutic targets to interfere with TGF-b signalling in these processes. 1 IntRODUctIon Integrins which consist of an a and a b subunit constitute a family of transmembrane receptors that bind extracellularly to the ECM and intracellularly to the cytoskeleton, thereby integrating the extracellular environment with the cell interior (1). Integrins transduce signals from the outside into the cell and vice versa to regulate cell adhesion and cell spreading, as well as migration, proliferation, differentiation, and remodeling of the ECM. In addition, integrins can modulate the signalling cascade elicited by several growth factors, including TGF-b. The TGF-b isoforms TGF-b1, TGF-b2, and TGF-b3 are pleiotropic cytokines that mediate a variety of effects on a wide range of cell types. TGF-bs bind to a heterodimeric serine/threonine kinase receptor complex which consists of a type I and type II receptor (TGF-bRI and TGF-bRII) leading to the recruitment and phosphorylation of the intracellular effector proteins Smad2 and Smad3. Phosphorylated Smad2 and Smad3 subsequently bind to Smad4 and translocate to the nucleus to initiate gene expression. TGF-b signalling is negatively regulated by inhibitory Smads, including Smad6 and Smad7 (2). In addition, TGF-b can affect numerous signal transduction pathways in a Smad-independent manner. Although the effect of TGF-b signalling depends on the context and cell type, TGF-b clearly controls a vast number of transcriptional targets, many of which are integrins and their ligands. The connection between integrins and TGF-b is therefore bidirectional, and it is becoming increasingly clear that it is relevant in many physiological and pathological phenomena. Here, we will discuss the integrin-tgf-b interplay and highlight its importance in the context of fibrosis, cancer, and wound repair. Integrin-TGF-b crosstalk 61
64 InteGRIN REGUlatIon BY TGF-β TGF-b controls the transcription of genes that encode numerous integrins (Table 1) in several cell types and tissues, as well as in various human cancers. Although the downregulation of integrin expression mostly laminin receptors has also been reported, in the majority of cases TGF-b stimulates integrin expression. Intriguingly, the induction of integrin expression by TGF-b can be driven by cooperative signalling between the integrin and TGF-b, thereby creating a feedforward loop (3). TGF-b not only regulates the expression of integrin ligands including big-h3, tenascin, vitronectin, fibronectin, and several members of the laminin and collagen families but also stimulates the expression of integrin-associated proteins, including disabled-2, ILK, kindlin-1, paxillin, and PINCH, which could increase integrin activation. Therefore, the transcriptional control exerted by TGF-b can strongly affect integrin-mediated processes. Finally, TGF-b could also regulate integrin activation directly, by a still unidentified inside-out mechanism (4). REGUlatIon of TGF-β activation BY InteGRIns TGF-b is secreted in an inactive (latent) form in a complex with two proteins LAP and LTBP. Its activation requires the dissociation from the complex, which occurs at low ph, or through the action of reactive oxygen species, proteases, thrombospondin-1, or several integrins. The LAPs of TGF-b1 and TGF-b3 but not that of TGF-b2 contain an Arg- Gly-Asp (RGD) motif that can potentially be bound by the five av-containing integrins, aiibb3, a5b1, and a8b1. Integrin binding to LAP has been formally demonstrated for a8b1 and all av integrins, although binding of a8b1 does not seem to lead to activation and whether avb1 can activate TGF-b is also unclear (Table 2) (5-7). Integrin-mediated TGF-b activation seems to be possible in a protease-dependent or protease-independent manner. Protease-dependent TGF-b activation has only been demonstrated for avb8 and depends on the binding of the integrin to the RGD site in LAP and simultaneous recruitment of MMP-14, which then releases TGF-b by proteolytic cleavage (Figure 1A) (8). This mode of activation does not require that the activating cell and the target cell be in close proximity. Interestingly, avb3 can serve as a docking site for MMP-2 and MMP-9 (9,10), although whether this also leads to proteolytic activation of TGF-b remains to be seen. Notably, the genes for these MMPs are TGF-b targets and, therefore, a self-amplifying TGF-b feedforward loop could be envisioned. Non-proteolytic TGF-b activation occurs through cell traction forces exerted by the actin cytoskeleton. These forces are translated by integrins into a conformational change of the TGF-b/LAP/LTBP complex, leading to the presentation of active TGF-b to its receptor (11-14). Hence, non-proteolytic activation requires cytoskeletal integrity, the connection of the b-tail of the integrin to the cytoskeleton, a mechanically-resistant matrix, and the interaction between 62
65 Table 1 Overview of the regulation of integrin expression by TGF-β. Integrin Main ligand Effect of TGF-β Cell type α1β1 Collagens Upregulation Fibroblasts α2β1 α3β1 Collagens Laminins Upregulation, Downregulation Fibroblasts, Keratinocytes Upregulation, Downregulation α5β1 Fibronectin Upregulation α6β1 Laminins Upregulation α8β1 RGD Upregulation α6β4 Laminins αvβ3 RGD Upregulation αvβ5 RGD Upregulation αvβ6 RGD Upregulation αlβ2 ICAM-1 Upregulation Keratinocytes, Fibroblasts, Carcinoma cells, Lung alveolar epithelial cells Keratinocytes, Fibroblasts, Carcinoma cells, Endothelial cells Lung alveolar epithelial cells, Promonocytic leukemia cells, Carcinoma cells Fibroblasts, Vascular smooth muscle cells Upregulation, Downregulation Keratinocytes, Carcinoma cells Fibroblasts, Endothelial cells, Carcinoma cells Keratinocytes, Fibroblasts Keratinocytes, Carcinoma cells, Fibroblasts Promonocytic leukemia cells αεβ7 E-cadherin Upregulation T-lymphocytes Context Collagen remodeling and contraction, myofibroblast differentiation during wound healing and fibrosis Collagen remodeling and contraction, myofibroblast differentiation during wound healing and fibrosis, re-epithelialization during wound healing Re-epithelialization during wound healing, EMT, cancer cell migration and invasion Re-epithelialization during wound healing, EMT, cancer cell migration and invasion, endothelial cell migration and tube formation Macrophage maturation, cancer cell migration and invasion Myofibroblast differentiation, vascular smooth muscle cell contraction Re-epithelialization during wound healing, EMT, cancer cell migration and invasion Myofibroblast differentiation during wound healing and fibrosis, angiogenesis, carcinoma cell migration and invasion Myofibroblast differentiation during fibrosis, re-epithelialization during wound healing, EMT, cancer cell migration and invasion Myofibroblast differentiation during fibrosis and in tumors, re-epithelialization during wound healing, EMT, cancer cell migration and invasion Macrophage maturation T-lymphocyte infiltration into epithelia 1 Col, collagen; EMT, epithelial-to-mesenchymal transition; ICAM-1, intercellular adhesion molecule-1; RGD, arginine-glycine-aspartate; TGF-β, transforming growth factor-β. Integrin-TGF-b crosstalk 63
66 LAP and the ECM through LTBP (Figure 1B), and that the target cell be in the direct vicinity of the activating cell. Non-proteolytic activation has been demonstrated in vitro for avb3, avb5 and avb6, as well as a b1 integrin, the a subunit of which has not been identified (13). Whether activation of TGF-b by a b1 integrin is physiologically relevant remains controversial. The activation of TGF-b by integrins can also be initiated by G-protein coupled receptors. For example, the stimulation of PAR-1 with thrombin leads to RhoAdependent and ROCK-dependent TGF-b activation by integrin avb6 in vitro and in vivo (15). Similarly, PAR-1 stimulation with coagulation factor X induces avb5- regulated TGF-b activation through ROCK signalling (16). Furthermore, avb6-mediated TGF-b activation can be induced by lysophosphatidic acid signalling to RhoA and ROCK, through the lysophosphatidic acid receptor coupled to small G-protein, Gaq (Figure 1B) (17). Whether other integrins mediate TGF-b activation through similar signalling pathways remains to be established. The importance of integrin-mediated activation of TGF-b in vivo is evident, as mutation of the RGD site of LAP leads to defects similar to those observed in TGFb1-null mice (18). In addition, genetic ablation of the b6 subunit or conditional deletion of av or b8 from dendritic cells causes exaggerated inflammation as a result of impaired TGF-b signalling (19,20). The phenotype of mice lacking both the avb6 and avb8 integrins recapitulates the whole array of abnormalities observed in TGF-b1 and TGF-b3 but not in TGF-b2 knockout mice, indicating that the integrins avb6 and avb8 can account for the full activation of TGF-b1 and TGF-b3 in vivo (21). Indeed, mice lacking b3, b5, or both do not develop abnormalities similar to those due to deficient TGF-b signalling (22-24). Nevertheless, avb3- or avb5-mediated TGF-b activation could be important in pathological conditions, as increased expression of both of these integrins is observed in the dermis of scleroderma patients, and these integrins elicit autocrine TGF-b signalling in patient fibroblasts in vitro (25-28). In addition, TGF-b activation by avb5 is important in pulmonary fibrosis, as discussed below. However, a causal effect of avb3-mediated TGF-b activation in human pathology has not yet been established. REGUlatIon of TGF-β SIGnallING BY InteGRIns In addition to the direct activation of TGF-b, several integrins seem to influence TGF-b-induced signal transduction (Table 2). The effect is almost exclusively an amplification of the signal, that is, increased activation of signalling proteins, and/or increased expression of TGF-b target genes. The regulation of TGF-b signalling by integrins occurs at multiple levels. Integrin-mediated adhesion can potentiate TGF-b-induced signalling and gene expression, in an analogous manner to how integrins regulate growth factor signalling through receptor tyrosine kinases. Indeed, TGF-b-induced collagen expression through p42/p44 MAPK requires integrin-mediated FAK activation in mesangial cells (29). Furthermore, b1 64
67 1 Figure 1 TGF-b activation by integrins. (A) Protease-dependent activation by integrin avb8 and MMP-14. (B) Protease-independent activation results from a conformational change of LAP-TGF-b induced by cell traction forces. FXa, coagulation factor X; Gaq, G-protein aq; LAP, latency-associated protein; LPA, lysophosphatidic acid; LPAR2, lysophosphatidic acid receptor 2; LTBP, latent TGF-b binding protein; MMP-14, matrix metalloproteinase-14; PAR-1, protease-activated receptor-1; ROCK; rho-associated kinase; TGF-b, transforming growth factor-b; TGF-bR, TGF-b receptor. integrins induce TGF-b-dependent p38 MAPK activity during EMT in mammary epithelial cells, and TGF-b-stimulated MMP-9 expression in keratinocytes is enhanced by the integrin a3b1 (30,31). Integrins can also control the expression of components of the TGF-b pathway indirectly. For example, the ectopic expression of the integrin a5 subunit induces TGF-bRII expression, which is further potentiated by a5b1 ligation to fibronectin, rendering cells responsive to TGF-b (32). In fibroblasts deficient for the integrin subunit b3, TGF-b signalling is enhanced due to an increased expression of both TGF-bRI and TGF-bRII, suggesting that the expression of these receptors is repressed by avb3 (33). In addition, TGF-b signalling is repressed in a3-deficient keratinocytes due to an elevated expression of the inhibitory Smad7, which could mean that a3b1 can downregulate Smad7 to enhance TGF-b signalling (34). Integrin-TGF-b crosstalk 65
68 Integrins may also regulate TGF-b signalling in a synergistic manner, through their physical interaction with TGF-bRs. For example, TGF-b stimulation induces the association of integrin avb3 with TGF-bRII in both breast cancer cells and lung fibroblasts, initiating cooperative signalling to c-src and MAPKs (35,36). Similarly, TGF-bRII associates with avb5 in sclerodermal fibroblasts, and integrin signalling through FAK is necessary for TGF-b-induced myofibroblastic differentiation (27,28). Furthermore, a3b1 association with E-cadherin and TGF-bRs mediates the TGF-b-stimulated phosphorylation of b-catenin and its association with phosphorylated Smad2, as well as the subsequent nuclear translocation of the Smad2/b-catenin complex. Interestingly, both phenomena are independent of ligand binding by a3b1 (37,38). Finally, in mammary epithelial cells overexpressing HER2, TGF-b stimulates integrin clustering with HER2 and their association with the cytoskeleton, leading to PI3-K signalling through c-src and FAK (39). In conclusion, integrins can control TGF-b signalling directly by TGF-b activation, or indirectly by affecting Smaddependent and Smad-independent signal- Table 2 Overview of integrin-mediated TGF-β activation and signalling. Integrin Regulation of TGF-β activation or signalling Binding of LAP-1 and LAP-3, activation of αvβ1 TGF-β is unclear αvβ3 αvβ5 αvβ6 αvβ8 α8β1 α5β1 α3β1 TGF-β activation in vitro, modulation of TGF-β signalling by physical association with TGF-βRII, control of expression of TGF-βRI and II TGF-β activation in vitro and in vivo, enhancement of TGF-β signalling by physical association with TGF-βRII TGF-β activation in vitro and in vivo TGF-β activation in vitro and in vivo Binding of LAP-1 and LAP-3, but no activation of TGF-β Binding and activation of LAP: NA, control of TGF-βRII expression Modulation of TGF-β signalling by enabling formation of a β-catenin/smad2 complex, or by repressing Smad7 expression Context NA Regulation of granulation tissue during wound healing, carcinoma cell migration and invasion, possible role in SS/scleroderma Pulmonary fibrosis, possible role in SS/scleroderma Development, IPF, kidney and renal fibrosis, SS, wound healing, EMT, carcinoma migration and invasion Development, suppression of T-cell-mediated immunity, possible role in COPD or wound healing NA NA EMT during IPF, re-epithelialization during wound healing? COPD, chronic obstructive pulmonary disease; EMT, epithelial-to-mesenchymal transition; IPF, idiopathic pulmonary fibrosis; LAP, latency-associated protein; NA, not assessed; SS, systemic sclerosis; TGF-β, transforming growth factor-β; TGF-βR, TGF-β receptor. 66
69 ling pathways at various levels (Table 2). Although the physiological relevance of some of the proposed mechanisms needs to be clarified, others are clearly important in the context of EMT, cancer, fibrosis, and wound healing, as will be described below. 1 InteGRIN-TGF-β CRosstalK IN FIBRosIS Fibrosis results from an aberrant response to organ injury and is characterized by the proliferation of fibroblasts, their differentiation into myofibroblasts, and excessive ECM production and deposition; all of these processes are mediated by TGF-b. Fibrosis can ultimately lead to major organ failure and even death. Increasing evidence points to the integrin-tgf-b crosstalk as crucial for the development and pathogenesis of fibrosis. TGF-b induces the expression of the integrins a1b1 and a2b1, which mediate collagen remodeling and myofibroblast contraction (Figure 2A). Furthermore, the integrins a3b1, avb5 and most notably avb6 control TGF-b activity or signalling in fibrosis. The first clue that the integrin-tgf-b interplay was important in fibrosis came from the observation that mice lacking the b6 subunit are protected from bleomycin-induced pulmonary fibrosis (5). The importance of avb6 for fibrogenesis has been subsequently demonstrated in several models; avb6 is normally not expressed in healthy epithelia but its expression is induced in many human fibrotic disorders in the kidney (diabetes mellitus, progressive fibrosing glomerulonephritis, Alport syndrome), the liver (acute biliary fibrosis), and in the lung (sclerosis and idiopathic pulmonary fibrosis (IPF)). In mice, the constitutive expression of avb6 in the basal layer of the epidermis leads to elevated TGF-b1 activation and the development of spontaneous chronic ulcers with severe fibrosis (40). Conversely, b6 knockout mice are partially or completely protected from pulmonary fibrosis induced by radiation, tubulointerstitial fibrosis as a response to kidney obstruction, or acute biliary fibrosis caused by bile duct ligation. In wildtype mice, fibrosis can be equally inhibited by treatment with antagonists of TGF-b signalling or by using a blocking antibody against avb6 (41-44). In fact, given that blocking the TGF-b pathway has serious adverse effects such as the development of autoimmunity the specific inhibition of avb6-induced TGF-b activation at sites of injury is a promising therapeutic tool to combat TGF-b-mediated fibrosis. Indeed, low doses of antibodies against avb6 prevent radiation-induced or bleomycin-induced pulmonary fibrosis without causing inflammation in mice (45,46). Various observations suggest that the integrins avb3, avb5, and avb8 provide additional therapeutic targets for this pathology. As mentioned above, avb3 and avb5 are thought to contribute to the pathogenesis of systemic sclerosis and scleroderma through TGF-b activation (25-28). In human fibrotic lungs, epithelial cells expressing avb5 and PAR-1 colocalize with myofibroblasts, and TGFb-mediated pulmonary fibrosis is reduced by blockade of avb5 in a mouse model (16). Furthermore, TGF-b activation by avb8 can induce the differentiation of Integrin-TGF-b crosstalk 67
70 airway fibroblasts into myofibroblasts, and the expression of avb8 is increased in the airways of chronic obstructive pulmonary disease patients, correlating with severity of the obstruction (47,48). However, the importance of avb8 in this process has not been corroborated by knockout or targeting studies. Finally, a3b1 also contributes to the development of IPF through a b-catenin/smad2 dependent mechanism, as described above (Figure 2B). In IPF, a subset of differentiating fibroblasts is initially derived from alveolar epithelial cells by EMT (49). The lung-specific deletion of the a3 subunit in a mouse model of IPF reduces myofibroblast accumulation, collagen deposition, expression of EMTassociated genes, and progression to fibrosis, suggesting that blocking a3b1 could also be effective against fibrosis (37,38). Collectively, these results show that several integrins aggravate TGF-b-mediated fibrotic disorders, either by direct activation of TGF-b, or by affecting downstream signalling. Thus, targeting these integrins could prove a valuable antifibrotic therapy in humans. Alternatively, integrin-associated proteins might also represent targets for therapeutic intervention. For example, ILK is essential for TGF-binduced kidney and liver fibrosis, although whether this depends on the modulation of integrin activity or is an integrin-independent effect of ILK is still unknown (50). InteGRIN-TGF-β CRosstalK IN carcinoma PROGRessIon TGF-b has a dual role in the development and progression of epithelial tumors: it initially acts as a tumor suppressor for epithelial cells, but can also promote growth, invasion, and metastasis at a later stage. The ability of TGF-b to promote or suppress carcinoma progression is at least partially dependent on the tumor microenvironment (51,52). The interactions between TGF-b and integrins can affect tumorigenesis and malignant progression in a number of ways. For example, an inappropriate suprabasal expression of a6b4 in stratified squamous epithelia inhibits TGF-b signalling, thereby enhancing tumorigenesis by relieving the inhibitory effects of TGF-b on epithelial proliferation (53). In addition, squamous cell carcinomas develop in stratified epithelia after the abrogation of TGF-b signalling, which could be associated with enhanced integrin activity and would suggest that under normal circumstances TGF-b has a suppressive effect on integrins (54). However, it should be noted that most studies support a role for TGF-b in inducing the de novo expression of several integrins that are normally not expressed in epithelial cells such as a5b1, avb3, avb5, and avb6, thereby enhancing the migratory and invasive behaviour of carcinoma cells, particularly in conjunction with newly expressed MMPs and ECM components such as fibronectin (Figure 2C). Indeed, antagonizing the TGF-b pathway blocks the induction of the expression of these integrins, as well as TGF-b-mediated invasion and metastasis, without affecting the growth of the primary tumor, suggesting that inhibiting integrin upregulation by TGF-b is sufficient to block metastasis (55,56). 68
71 A 1 B C Figure 2 Integrin-TGF-b crosstalk mechanisms. (A) In fibrosis and sclerosis, TGF-b signalling induces fibroblast differentiation into contractile myofibroblasts. The myofibroblasts express and deposit collagen (1), express a1b1 and a2b1 integrins that mediate collagen remodeling and contraction (2), and express av integrins that activate latent TGF-b from the matrix (3). (B) During TGF-b-mediated EMT of alveolar epithelial cells, integrin a3b1 forms a complex with TGF-bRs and E-cadherin, facilitating b-catenin/smad2 complex formation and nuclear translocation. (C) During malignant progression, TGF-b frequently represses the expression of laminin and/or laminin-binding integrins a3b1 and a6b4, while inducing the expression of fibronectin and integrins a5b1 and avb6. avb6 mediates migration and invasion and generates new active TGF-b, stimulating other tumor cells as well as myofibroblast differentiation in the tumor stroma. b-cat, b-catenin; Col, collagen; E-cadh, E-cadherin; EMT, epithelial-to-mesenchymal transition; FN, fibronectin; LN-332, laminin-332; TGF-b, transforming growth factor-b; TGF-bR, transforming growth factor-b receptor. Integrin-TGF-b crosstalk 69
72 As in fibrosis, avb6 appears to have a crucial role in the TGF-b-integrin crosstalk in carcinomas. TGF-b induces the expression of avb6 during EMT in vitro and in vivo, and avb6 is upregulated at the tumor-stromal interface of several aggressive squamous cell carcinomas including cervical, colorectal, esophageal, head and neck, and skin carcinomas and its upregulation is a prognostic factor for decreased survival (57-60). avb6 can mediate migration and invasion, but also establish a self-amplifying loop by activating TGF-b; the interruption of this feedforward mechanism could be an important step to arrest malignant progression. Although blockade of avb6 had no effect on TGF-b-mediated proliferation of tumor cells in vitro, it did successfully inhibit the growth of xenograft tumors in vivo, suggesting that the tumor microenvironment has an important regulatory role (59). Indeed, avb6-mediated TGF-b activation in an organotypic culture system for basal cell carcinoma induced differentiation of fibroblasts into myofibroblasts, which subsequently induced tumor cell invasion by the secretion of hepatocyte growth factor. Interestingly, the stroma of highrisk basal cell carcinomas is rich in myofibroblasts that express hepatocyte growth factor, while its receptor c-met is expressed on the tumor cells, suggesting that a similar tumor-stroma interaction can occur in patients (60). Therefore, although the blockade of several TGF-binduced integrins might inhibit the migratory and invasive behavior of tumor cells, antagonizing avb6 could also be important for interfering with self-amplifying, TGF-b-mediated tumor-stromal interactions. This approach could ultimately become an effective treatment for various carcinomas. InteGRIN-TGF-β CRosstalK DURING WOUND HealING The repair of cutaneous wounds is achieved through the concerted efforts of multiple cell types (Figure 3A) (61). TGF-b is involved in every phase of wound repair and is released by platelets, neutrophils, macrophages, fibroblasts, and migrating keratinocytes. TGF-b suppresses the inflammatory response and promotes the formation of granulation tissue by inducing fibroblast proliferation and differentiation, the expression of integrins and deposition of ECM proteins by fibroblasts, as well as endothelial cell migration and angiogenesis (Figure 3B) (62). However, there are conflicting results as to the role of TGF-b during re-epithelialization. On the one hand, TGF-b stimulates the expression of fibronectin and the integrins a5b1, avb5, and avb6 in keratinocytes, thereby inducing a migratory phenotype (Figure 3C). On the other hand, TGF-b strongly inhibits keratinocyte proliferation, and there is evidence to indicate that the net result of TGF-b signalling on re-epithelialization is inhibitory. For example, re-epithelialization is delayed in mice that overexpress TGF-b1 in the basal layer of the epidermis (63-65), whereas it is accelerated and keratinocyte proliferation is increased in mice that express a dominant negative TGF-bRII in basal keratinocytes or that 70
73 1 Figure 3 Overview of proposed integrin-tgf-b interactions during wound healing. (A) Schematic representation of the major phases in wound healing, which are explained in the figure key. (B) In the granulation tissue, TGF-b induces expression of integrins a1b1 and a2b1, which mediate fibroblast contraction, and of av integrins, which activate latent TGF-b. Furthermore, avb3 may repress TGF-b signalling by inhibiting TGF-bR expression. (C) During re-epithelialization, TGF-b stimulates the expression of fibronectin and integrins, which mediate keratinocyte migration or activate latent TGF-b. Integrin a3b1 could enhance TGF-b signalling by controlling the expression of Smad7. BM, basement membrane; Col, collagen; FN, fibronectin; LN-332, laminin-332. Integrin-TGF-b crosstalk 71
74 lack TGF-bRII (54,66). In addition, reepithelialization is accelerated in Smad3 knockout mice (67,68). Integrins mediate adhesion and migration during re-epithelialization (69), and emerging evidence suggests that several can modulate TGF-b signalling during wound healing, although the precise mechanisms are controversial and poorly understood. Re-epithelialization is accelerated in b3-null mice, which is accompanied by enhanced fibroblast infiltration, fibronectin deposition and neoangiogenesis, and elevated TGF-β levels in the granulation tissue, suggesting that avb3 suppresses TGF-β signalling (33). However, this is clearly inconsistent with both the activation of TGF-b by avb3 and the inhibitory effects of TGF-b on re-epithelialization. In addition, although the targeted deletion of the b6 subunit does not affect wound healing, abnormal wound healing is observed in b6-null mice when TGF-b signalling is disturbed for example in the presence of glucocorticoids (23,70-72) suggesting that rather than maintaining adhesion and mediating migration, avb6 functions as a safekeeper in wounds, ensuring sufficient supply of TGF-b when required. The activation of TGF-b by avb8 has been also seen to delay the closure of scratch wounds in vitro, although whether it has a physiological role during re-epithelialization in vivo is unknown (73,74). Finally, delayed wound re-epithelialization has been observed in full-thickness skin explants from a3-null mice, supposedly owing to repressed TGF-b signalling caused by an upregulation of Smad7 in the absence of integrin a3b1 (Figure 3C) (34). However, these data are controversial in light of the evidence that TGF-b signalling inhibits re-epithelialization, and because the targeted deletion of a3 from the basal layer of the epidermis has been recently shown not to inhibit re-epithelialization (75,76). Therefore, although regulation of TGF-b signalling by inte grins is potentially important in multiple aspects of the wound healing process, it is currently incompletely understood. Future studies should shed more light on the exact mechanisms involved. CONCLUSIons Extensive interactions undoubtedly exist between integrins and the TGF-b pathway. Although our knowledge of the wide implications of this crosstalk and the underlying mechanisms has greatly increased in recent years, there are still several outstanding questions to address in the coming years. A clarification of these issues is important as it will not only increase our understanding of integrin signalling, TGF-b signalling, and the integrin-tgf-b crosstalk, but importantly could also lead to new treatment strategies for several human pathologies. ACKnoWleDGEMents We thank Rabab Charafeddine for stimulating discussions. We apologize to colleagues whose work may have been omitted, due to space constraints. This study was supported 72
75 by grants from the Dystrophic Epidermolysis Bullosa Research Association (DEBRA Foundation, Crowthorne, UK) and the Dutch Cancer Society. ABBREVIatIons big-h3, TGF-β inducible gene-h3; ECM, extracellular matrix; EMT, epithelial-to-mesenchymal transition; FAK, focal adhesion kinase; ILK, integrin-linked kinase; LAP, latency-associated protein; LTBP, latent TGF-b binding protein; MAPK, mitogenactivated protein kinase; MMP, matrix metalloproteinase; PAR-1, protease-activated receptor-1; PI3-K, phosphoinositide-3-kinase; RGD, arginine-glycine-aspartate; ROCK, rho-associated kinase; TGF-b, transforming growth factor-b; TGF-bRI, TGF-b type I receptor; TGF-bRII, TGF-b type II receptor 1 RefeRences 1. Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, Massagué J, and YG Chen (2000) Controlling TGF-b signaling. Genes Dev 14, Pechkovsky DV, et al (2008) TGF-b1 induces avb3 integrin expression in human lung fibroblasts via a b3 integrin-, c-src-, and p38 MAPK-dependent pathway. J Biol Chem 283, Fransvea E, et al (2009) TGF-bRI inhibits activation of b1 integrin and blocks vascular invasion in hepatocellular carcinoma. Hepatology 49, Munger JS, et al (1999) The integrin avb6 binds and activates latent TGF-b1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, Lu M, et al (2002) Integrin a8b1 mediates adhesion to LAP-TGF-b1. J Cell Sci 115, Ludbrook SB, et al (2003) The integrin avb3 is a receptor for the latencyassociated peptides of TGF-b1 and -b3. Biochem J 369, Mu D, et al (2002) The integrin avb8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-b1. J Cell Biol 157, Brooks PC, et al (1996) Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin avb3. Cell 85, Rolli M, et al (2003) Activated integrin avb3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci USA 100, Annes JP, et al (2004) Integrin avb6- mediated activation of latent TGFb requires the latent TGF-b binding protein-1. J Cell Biol 165, Fontana L, et al (2005). Fibronectin is required for integrin avb6-mediated activation of latent TGF-b complexes containing LTBP-1 (2005). FASEB J 19, Wipff PJ, et al (2007) Myofibroblast contraction activates latent TGF-b1 from the extracellular matrix. J Cell Biol 179, Wipff PJ and B Hinz (2008) Integrins and activation of latent TGF-b1: an intimate relationship. Eur J Cell Biol 87, Jenkins RG, et al (2006) Ligation of protease-activated receptor 1 enhances avb6 integrin-dependent TGF-b activation and promotes acute lung injury. J Clin Invest 116, Scotton CJ, et al (2005) Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J Clin Invest 119, Xu MY, et al (2009) Lysophosphatidic acid induces avb6 integrin-mediated TGF-b activation via the LPA2 receptor Integrin-TGF-b crosstalk 73
76 and the small G protein Ga(q). Am J Pathol 174, Yang Z, et al (2007) Absence of integrinmediated TGF-b1 activation in vivo recapitulates the phenotype of TGF-b1- null mice. J Cell Biol 176, Lacy-Hulbert A, et al (2007) Ulcerative colitis and autoimmunity induced by the loss of myeloid av integrins. Proc Natl Acad Sci USA 104, Travis MA, et al (2007) Loss of integrin avb8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449, Aluwihare P, et al (2008) Mice that lack activity of avb6- and avb8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci 122, Hodivala-Dilke KM, et al (1999) b3- integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest 103, Huang X, et al (2000) Normal development, wound healing, and adenovirus susceptibility in b5-deficient mice. Mol Cell Biol 20, Reynolds LE, et al (2002) Enhanced pathological angiogenesis in mice lacking b3 integrin or b3 and b5 integrins. Nat Med 8, Asano Y, et al (2005) Involvement of avb5 integrin-mediated activation of latent TGF-b1 in autocrine TGF-b signaling in systemic sclerosis fibroblasts. Arthritis Rheum 52, Asano Y, et al (2005) Increased expression of integrin avb3 contributes to the establishment of autocrine TGFb signaling in scleroderma fibroblasts. J Immunol 175, Asano Y, et al (2006) Involvement of avb5 integrin in the establishment of autocrine TGF-b signaling in dermal fibroblasts derived from localized scleroderma. J Invest Dermatol 126, Asano Y, et al (2006) Increased expression of integrin avb5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol 168, Hayashida T, et al (2007) MAPkinase activity necessary for TGF-b1- stimulated mesangial cell type I collagen expression requires adhesion-dependent phosphorylation of FAK tyrosine 397. J Cell Sci 120, Bhowmick NA, et al (2001) Integrin b1 signaling is necessary for TGF-b activation of p38mapk and epithelial plasticity. J Biol Chem 276, Lamar JM, et al (2008) Integrin a3b1 potentiates TGF-b-mediated induction of MMP-9 in immortalized keratinocytes. J Invest Dermatol 128, Wang D, et al (1999) Control of type II TGF-b receptor expression by integrin ligation. J Biol Chem 274, Reynolds LE, et al (2005) Accelerated reepithelialization in b3 integrin-deficient mice is associated with enhanced TGF-b1 signaling. Nat Med 11, Reynolds LE, et al (2008) a3b1 integrin-controlled Smad7 regulates reepithelialization during wound healing in mice. J Clin Invest 118, Scaffidi AK, et al (2004) avb3 integrin interacts with the TGF-b type II receptor to potentiate the proliferative effects of TGF-b1 in living human lung fibroblasts. J Biol Chem 279, Galliher AJ and WP Schiemann (2006) b3 integrin and Src facilitate transforming growth factor-b mediated induction of EMT in mammary epithelial cells. Breast Cancer Res 8, R Kim KK, et al (2009a) Epithelial cell a3b1 integrin links b-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest 119, Kim Y, et al (2009b) Integrin a3b1- dependent b-catenin phosphorylation links epithelial Smad signaling to cell contacts. J Cell Biol 184, Wang SE, et al (2009) TGF-b induces clustering of HER2 and integrins by activating Src-FAK and receptor association to the cytoskeleton. Cancer Res 69, Häkkinen L, et al (2004) Increased expression of b6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol 164, Ma LJ, et al (2003) TGF-b-dependent and -independent pathways of induction of tubulointerstitial fibrosis in b6(-/-) mice. Am J Pathol 163, Hahm K, et al (2007) avb6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol 170,
77 43. Wang B, et al (2007) Role of avb6 integrin in acute biliary fibrosis. Hepatology 46, Patsenker E, et al (2008) Inhibition of integrin avb6 on cholangiocytes blocks TGF-b activation and retards biliary fibrosis progression. Gastroenterology 135, Horan GS, et al (2008) Partial inhibition of integrin avb6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med 177, Puthawala K, et al (2008) Inhibition of integrin avb6, an activator of latent TGF-b, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med 177, Araya J, et al (2006) Integrin-mediated TGF-b activation regulates homeostasis of the pulmonary epithelial-mesenchymal trophic unit. Am J Pathol 169, Araya J, et al (2007) Squamous metaplasia amplifies pathologic epithelialmesenchymal interactions in COPD patients. J Clin Invest 117, Kim KK, et al (2006) Alveolar EMT develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 103, Li Y, et al (2003) Role for integrin-linked kinase in mediating tubular EMT and renal interstitial fibrogenesis. J Clin Invest 112, Bierie B and HL Moses (2006) Tumour microenvironment: TGF-b: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer 6, Massagué J (2008) TGF-b in Cancer. Cell 134, Owens DM, et al (2003) Suprabasal a6b4 integrin expression in epidermis results in enhanced tumourigenesis and disruption of TGF-b signalling. J Cell Sci 116, Guasch G, et al (2007) Loss of TGF-b signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell 12, Bandyopadhyay A, et al (2006) Inhibition of pulmonary and skeletal metastasis by a TGF-b type I receptor kinase inhibitor. Cancer Res 66, Kawajiri H, et al (2008) A novel TGF-b receptor kinase inhibitor, A-77, prevents the peritoneal dissemination of scirrhous gastric carcinoma. Clin Cancer Res 14, Bates RC, et al (2005) Transcriptional activation of integrin b6 during EMT defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest 115, Hazelbag S, et al (2007) Overexpression of the avb6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J Pathol 212, Van Aarsen LA, et al (2008) Antibodymediated blockade of integrin avb6 inhibits tumor progression in vivo by a TGF-b-regulated mechanism. Cancer Res 68, Marsh D, et al (2008) avb6 integrin promotes the invasion of morphoeic basal cell carcinoma through stromal modulation. Cancer Res 68, Singer AJ and RA Clark (1999) Cutaneous wound healing. N Engl J Med 341, Werner S and R Grose (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83, Yang L, et al (2001) Healing of burn wounds in transgenic mice overexpressing TGF-b1 in the epidermis. Am J Path 159, Chan T, et al (2002) Development, characterization, and wound healing of the keratin 14 promoted TGF-b1 transgenic mouse. Wound Repair Regen 10, Tredget EB, et al (2005) TGF-b and its effect on re-epithelialization of partialthickness ear wounds in transgenic mice. Wound Repair Regen 13, Amendt C, et al (2002) Resistance of keratinocytes to TGF-b-mediated growth restriction and apoptosis induction accelerates re-epithelialization in skin wounds. J Cell Sci 115, Ashcroft GS, et al (1999) Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol 1, Falanga V, et al (2004) Full-thickness wounding of the mouse tail as a model for delayed wound healing: accelerated wound closure in Smad2 knockout mice. Wound Repair Regen 12, Grose R, et al (2002) A crucial role of b1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 129, Huang XZ, et al (1996) Inactivation of the integrin b6 subunit gene reveals a role of epithelial integrins in regulating 1 Integrin-TGF-b crosstalk 75
78 inflammation in the lung and skin. J Cell Biol 133, AlDahlawi S, et al (2006) The avb6 integrin plays a role in compromised epidermal wound healing. Wound Repair Regen 14, Xie Y, et al (2009) Mice lacking b6 integrin in skin show accelerated wound repair in dexamethasone impaired wound healing model. Wound Repair Regen 17, Fjellbirkeland L, et al (2003) Integrin avb8-mediated activation of TGFb inhibits human airway epithelial proliferation in intact bronchial tissue. Am J Pathol 163, Neurohr C, et al (2006) Activation of TGF-b by the integrin avb8 delays epithelial wound closure. Am J Respir Cell Mol Biol 35, Margadant C, et al (2009) Integrin a3b1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci 122, Mitchell K, et al (2009) Integrin a3b1 in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial cell crosstalk through induction of MRP3/ Prl2c4. J Cell Sci 122,
79
80
81 Gain-of-glycosylation in integrin α3β1 causes lung disease and nephrotic syndrome Coert Margadant 1*, Nayia Nicolaou 2*, Sietske H. Kevelam 2, Marc R. Lilien 3, Michiel J.S. Oosterveld 3, Maaike Kreft 1, Albertien M. van Eerde 2, Rolph Pfundt 4, Paulien A. Terhal 2, Bert van der Zwaag 2, Peter G.J. Nikkels 5, Norman Sachs 1, Roel Goldschmeding 5, Nine V.A.M. Knoers 2, Kirsten Renkema 2#, and Arnoud Sonnenberg 1# 1 Division of Cell Biology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX, Amsterdam, The Netherlands. 2 Department of Medical Genetics, University Medical Center Utrecht, Utrecht, The Netherlands. 3 Department of Pediatric Nephrology, University Medical Center Utrecht, Wilhelmina Children s Hospital, Utrecht, The Netherlands. 4 Department of Human Genetics, Radboud University Nijmegen Medical Center, Nijmegen, The Netherlands. 5 Department of Pathology, University Medical Center Utrecht, Utrecht, The Netherlands. *,# equal contribution. J Clin Invest 122, (2012)
82
83 ABSTRAct Integrins are transmembrane αβ glycoproteins that connect the extracellular matrix to the cytoskeleton. The laminin-binding integrin a3b1 is highly expressed in lung epithelium and in kidney podocytes. In podocytes, a3b1 associates with the tetraspanin CD151 to maintain a functional filtration barrier. Here, we report a novel homozygous missense mutation in the human ITGA3 gene, causing a fatal multi-organ condition comprising interstitial lung disease and congenital nephrotic syndrome. The mutation causes an alanine to serine substitution in the integrin a3-subunit, thereby introducing an N-glycosylation motif at amino acid position 349. Hyperglycosylation of the a3 precursor prevents its heterodimerization with b1, whereas CD151 association with the a3 subunit occurs normally. Consequently, the b1 precursor accumulates in the endoplasmic reticulum, and the mutant a3 precursor is degraded by the ubiquitin-proteasome system. Thus, these findings uncover a gain-of-glycosylation mutation in ITGA3 that prevents the biosynthesis of functional a3b1, causing a fatal multi-organ disorder. 2 IntRODUctIon The integrin family consists of 24 transmembrane heterodimeric αβ glycoproteins that link the extracellular matrix to the cytoskeleton (1). Most integrins connect to actin filaments, and reside in cellular adhesion structures designated focal adhesions (FAs), which are highly enriched in tyrosine-phosphorylated proteins and serve as major hubs for signal transduction (2). Integrin-ligand binding can be controlled by conformational changes that tune integrin affinity (3). Furthermore, integrin function depends strongly on trafficking events, which include endocytosis, intracellular sorting and recycling, and delivery of de novo synthesized integrins to the plasma membrane by the biosynthetic route (4, 5). Both a- and b-subunits are synthesized as precursors. After N-linked glycosylation, folding, and association of the a- and b-subunits in the endoplasmic reticulum (ER), the heterodimer is transported to the Golgi network, where the N-linked high-mannose oligosaccharides are further processed into complex oligosaccharides. Subsequently, several of the a-subunit precursors including a3, are cleaved by proprotein convertases such as furin into a heavy and a light chain, which are held together by a disulphide bond to generate the mature form that is expressed at the plasma membrane (6, 7). A number of human congenital disorders have been associated with defective integrin-mediated adhesion, including the blistering disorder epidermolysis bullosa (integrin a6b4 in epithelia), the bleeding disorder Glanzmann s thrombasthaenia (integrin aiibb3 in platelets), leukocyte adhesion deficiency-i (b2-integrins in leukocytes), and muscular dystrophy (integrin a7b1 in striated muscle) (8-11). Furthermore, the importance of integrins for cell adhesion in a variety of organ systems has been demonstrated by numerous mouse models targeting either integrin subunits or integrin-associated proteins (12). For example, genetic deletion of Mutation in a3b1 causes lung and kidney disease 81
84 the laminin (LN)-binding epithelial integrin a3b1 in mice causes severe lung and kidney defects, as well as abnormalities in the epidermis (13-17). In podocytes, which are the epithelial cells of the kidney glomerulus, a3b1 maintains cell adhesion against great dynamic stress by binding to LN-511/521 in the glomerular basement membrane (GBM). Integrin-LN binding is reinforced by intracellular connection to the actin cytoskeleton via linker proteins such as a-actinin or integrin-linked kinase, and by lateral association with the tetraspanin CD151 (18). The pivotal role of this multi-component adhesion unit in regulating the barrier function of the kidney is illustrated by the severe glomerular defects observed in mice upon (podocyte-specific) deletion of a3, b1, integrinlinked kinase, CD151, or the a5-chain of LN-511/521 (19-25). In addition, mutations in the genes encoding the b2-chain of LN-521, a-actinin-4, and CD151 have been identified in patients suffering from renal disorders including Pierson syndrome, focal segmental glomerulosclerosis, and hereditary nephritis (26-28). Here, we disclose a mutation in the human ITGA3 gene, in a patient with interstitial lung disease and congenital nephrotic syndrome. The mutation leads to a gain-of-glycosylation, which impedes heterodimerization of the a3 precursor with b1 but not the association with CD151. As a consequence, the b1 precursor accumulates in the ER, and the mutant a3 precursor is cleared by ubiquitination and degradation by the proteasome. Thus, we have identified a gain-of-glycosylation mutation in ITGA3 that prevents the biosynthesis of functional a3b1, leading to severe kidney and lung defects. ResUlts A point mutation in ITGA3 causes congenital nephrotic syndrome and interstitial lung disease The patient was born prematurely at a gestational age of 36 weeks from unaffected non-consanguineous Dutch parents. She had one unaffected sibling (Figure 1A). At birth, the patient presented with respiratory distress. In addition, renal ultrasound revealed unilateral kidney hypoplasia with hydronephrosis on the left side, and the patient was diagnosed with congenital nephrotic syndrome. Growth retardation was prominent, which could in part be attributed to prolonged steroid treatment and intermittent feeding difficulties. The patient died at the age of 7 months due to respiratory insufficiency. We first screened known genes implicated in nephrotic syndrome (NPHS1, NPHS2, PLCE1, WT1, LAMB2, TRPC6, ACTN4, and INF2) and surfactant metabolism dysfunction, which is implicated in perinatal respiratory distress (ABCA3, SFTP-B and SFTP- C). No mutations were detected in these genes (data not shown). Subsequently, we conducted genome-wide screening in the patient and both parents for copy number variations (CNVs), using Affymetrix 250K single-nucleotide polymorphism (SNP) array analysis. Whereas no clinically relevant CNVs were detected, a ~19.2 Mb long continuous stretch of homozygosity (LCSH) ( Mb) was identified on chromosome 17, encompassing 1261 SNP probes, with SNP_A_
85 and SNP_A_ as bordering SNPs (Figures 1B,C). The region contained 566 genes, among which the ITGA3 gene that encodes the integrin a3-subunit (Figures 1B,C). The possibility of uniparental disomy of chromosome 17 was excluded by investigating the Mendelian inheritance pattern of 4854 SNPs that are located on chromosome 17 and which are present on the array (data not shown). We then directly sequenced the ITGA3 coding sequence, which revealed a homozygous missense variant c.1045g>t, for which both parents and the healthy sibling were heterozygous (Figure 1D). In contrast, the variant was not observed in 384 chromosomes of ethnically matched (Dutch) individuals. In addition, it has not been annotated in dbsnp, the NHLBI Exome Sequencing Project, or our in-house database of 100 exome-sequencing projects. The ITGA3 gene extends over 34.5 kb and contains 26 exons. The identified mutation is located in exon 7 (Figure 1E). At the protein level, the mutation causes the substitution of alanine 349 for serine (A349S), in an extracellular domain of a3 designated the β-propeller (Figure 1F). In summary, we have identified a missense mutation in ITGA3 in a patient with interstitial lung disease and congenital nephrotic syndrome. The A349S mutation leads to a lack of integrin a3b1 expression in vivo and causes severe kidney and lung abnormalities Congenital nephrotic syndrome is characterized by proteinuria, resulting from defects in the filtration barrier of the glomerulus. To characterize the defects that caused congenital nephrotic syndrome in our patient, we performed histological examination of kidney biopsies. Severe abnormalities were observed, including aberrant glomerular morphology and cystic dilation of tubuli, local protein casts, as well as interstitial fibrosis and some inflammatory infiltrate. The GBM was irregularly thickened, and the glomeruli consisted of fewer and wider capillary loops covered by highly variable numbers of podocytes. In addition, mesangiolysis and mesangial hypercellularity, occasional extracapillary proliferation reflecting a loss of capillary integrity, and focal segmental glomerulosclerosis were observed (Figure 2A; Figure S1; and data not shown). Ultrastructural analysis revealed partial foot process effacement and a disorganized and thickened GBM, displaying a multi-layered and pseudoreticular structure with loose fragments, suggestive of disturbance of the fusion process (Figure 2B and data not shown). We then investigated the expression and distribution of individual glomerular components by immunofluorescence analysis of kidney cryosections. In the patient glomeruli the integrin a3-subunit could not be detected, neither using antibodies J143 or P1B5, which are directed against the extracellular domain of a3, nor with a home-made antibody that recognizes the cytoplasmic tail (Figure 2C and data not shown). Some aspecific immunoreactivity was observed in focal patches of cells. These also reacted with control IgG and consist most likely of activated mesangial cells, which are known to express Fc receptors. In addition to the lack of a3, a dramatic decrease in the levels of the b1-subunit was visible, confirming that a3b1 is the main b1-integrin 2 Mutation in a3b1 causes lung and kidney disease 83
86 Figure 1 Identification of a homozygous ITGA3 mutation in a patient with interstitial lung disease and nephrotic syndrome. (A) Pedigree of the patient s family. Both parents and the patient s sister were unaffected. (B) Results from the 250K SNP array performed on patient s DNA. Upper panel; log2 test-overreference ratio values (y-axis) for SNP loci plotted against the position on chromosome 17 (x-axis). Red dots represent the log2 ratio for each SNP locus. Middle panel; the effective hidden Markov model outcome with a normal test-over-reference ratio of 0. Blue dots represent the mean log2 ratio of neighboring SNPs on the array, indicating no significant copy number gains or losses. Lower panel; idiogram of chromosome 17. Green marks represent heterozygous SNP calls at particular DNA loci. The red box indicates the 19.2 Mb region of homozygosity, and the thickness of the blue line below represents the likelihood of loss of heterozygosity. (C) Idiogram of chromosome 17. The homozygous region from 17q12 to 17q23.2 is boxed. (D) DNA sequences of part of the ITGA3 gene in the parents (I:1 and I:2) the patient (II:2), and her sister (II:1). The patient (II:2) carries a homozygous missense mutation (c.1045g>t), for which both parents are heterozygous. (E) Schematic overview of the 26 exons of the ITGA3 gene. The location of the c.1045g>t mutation in exon 7 is indicated by an asterisk. (F) Domain organization of the integrin a3-subunit. The A349S mutation is located between two FG-GAP repeats in the extracellular b-propeller domain. in the glomerulus. Also the expression of podocin and CD151 seemed reduced in patient glomeruli, whereas expression of the integrin a6 subunit appeared to be slightly increased. Furthermore, an increase in expression and deposition of the GBM proteins LN, nidogen, and collagen (Col)-IV was observed, confirming the thickening of the GBM observed by electron microscopy (Figure 2C). Next, we analyzed lung biopsies from the patient. Also in the lungs, multiple abnormalities were observed, including widened alveolar septa lined with reactive type 2 pneumocytes and increased numbers of alveolar macrophages, 84
87 2 Figure 2 Abnormalities in patient kidneys. (A) Jones silver staining of a section of patient kidney, showing highly irregular mesangium, and fewer and wider capillary loops with highly variable numbers of podocytes. Inset shows a fibrocellular crescent in a glomerulus undergoing glomerulosclerosis. (B) Ultrastructural analysis of the filtration barrier of patient kidney, demonstrating abnormal foot processes and thickening of the GBM with local protrusions. Ec, endothelial cell; Fp, foot processes; GBM, glomerular basement membrane; Pdc, podocyte. (C) Kidney cryosections were subjected to indirect immunofluorescence analysis. The images show a3 e (J143 antibody against the extracellular domain; green) with podocin (red), CD151 (green) with nidogen (red), a6 (green) with a3 c (polyclonal antibody against the cytoplasmic domain; red), b1 (green) with laminin (red), and control IgG (green) with Col-IV (red). Nuclei were counter-stained with DAPI (blue). whereas the alveolar walls in an agematched healthy individual are lined with flat type I pneumocytes, contain virtually no reactive type 2 pneumocytes, and no alveolar macrophages (Figures 3A,B,C). Pronounced pulmonary interstitial glycogenesis was detected by PAS staining (Figure 3D). In addition, we observed abnormal deposition of surfactant by electron microscopy (data not shown). As in the kidney, a3 could not be detected by immunofluorescence analysis of lung cryosections, whereas the expression of a6 seemed to be increased (Figure 3E). Together, these data show that the A349S mutation leads to the lack of integrin a3b1 expression, causing severe abnormalities in the kidneys and the lungs. Mutation in a3b1 causes lung and kidney disease 85
88 86 Figure 3 Abnormalities in patient lung tissue. (A) Haematoxylin/eosin staining of a lung section from a healthy individual. (B) Haematoxylin/eosin staining of a lung section from the patient. (C) Keratin staining highlighting lining of the thickened alveolar septa with reactive type 2 pneumocytes in patient lungs. (D) PAS-staining demonstrating glycogen deposits in interstitial cells in patient lungs. (E) Lung cryosections of patient and control were subjected to indirect immunofluorescence analysis using our home-made antibody against the cytoplasmic tail of a3 (green) and Col-IV (red), or a6 (green) and nidogen (red). IgG was included as a control for antibody specificity. Nuclei were counter-stained with DAPI (blue).
89 The A349S mutation impairs the expression of mature a3 at the cell-surface To investigate the effect of the A349S mutation in vitro, we isolated glomeruli from Itga3 flox/flox ;Cd151 flox/flox ;Trp53 +/- mice, according to the Dynabead perfusion method as described previously (29). Outgrowing epithelial cells were sorted for expression of podocalyxin and absence of the endothelial cell marker CD31. The floxed Itga3 and Cd151 genes were deleted by adenoviral delivery of Cre-recombinase, and absence of both proteins was confirmed by flow cytometry (Figure S2). We then generated the A349S mutation in cdna encoding human a3, and stably expressed either wild-type human a3 or mutant human a3 A349S in the a3/cd151- deficient podocytes by retroviral transduction, followed by selection with zeocin. The podocytes were also reconstituted with FLAG-tagged human CD151, to avoid possible differences in binding-efficiency of murine CD151 to human a3 because of sequence variability in the a3-binding QRD motif, which is KRD in mouse (30). We first determined the cell-surface expression of various integrin subunits and CD151 by flow cytometry. In line with the immunofluorescence analysis of the patient tissue, a3 was completely absent from the cell-surface of podocytes expressing a3 A349S, and b1-levels were strongly reduced (Figure 4A). In contrast, cell-surface expression of CD151, as well as that of the integrin subunits a2, a5, and a6 was similar in podocytes expressing a3 wt or a3 A349S. Next, we analyzed the expression of precursor and mature a3 by Western blotting. The a3-subunit is synthesized as a precursor of ~150 kda. After N-linked glycosylation in the ER and association with b1, the a3b1 heterodimer is transported to the Golgi. Here, processing of the N-linked oligosaccharides occurs, and the a3 precursor is cleaved into a heavy (~115 kda) and a light chain (~35 kda) (6, 7). The b1 subunit is synthesized as a partially glycosylated precursor of ~105 kda, and a more heavily glycosylated mature form of ~125 kda that is expressed at the cell-surface. Intriguingly, we clearly detected precursor a3 A349S, though to a lesser extent than a3 wt, suggesting that the mutant is either less expressed or that some degradation occurs. The mature product was nevertheless completely absent, as evidenced by the lack of the a3 light chain (Figure 4B). Consistently, there was a large pool of b1 precursor, but a dramatic reduction of mature b1, as compared to podocytes expressing wildtype a3. This observation supports the results obtained by flow cytometry, and suggests that the mutant a3 precursor fails to heterodimerize, leading to accumulation of b1 precursor and a strong reduction of mature b1. These data indicate that whereas the A349S mutation does not impair the expression of precursor a3, it leads to a lack of expression of the mature, cell-surface form of a3, probably because heterodimerization with b1 is prevented. The A349S mutation impairs a3b1 heterodimerization but not the association of precursor a3 with CD151 To determine whether the A349S mutation indeed prevents heterodimerization of the a3 and b1 subunits, we immunoprecipitated a3 from lysates of podocytes expressing a3 wt or a3 A349S. The a3 subunit was precipitated with either an antibody against its cytoplasmic tail 2 Mutation in a3b1 causes lung and kidney disease 87
90 Figure 4 The α3 A349S mutation disturbs expression of mature but not precursor α3 in podocytes. (A) Murine a3/cd151-deficient podocytes were retrovirally transduced with human CD151, as well as either with human wild-type a3 (a3 wt ; blue) or human a3 carrying the patient mutation (a3 A349S ; red), and positive cells were selected with zeocin. Cell-surface expression of integrin subunits a2, a3, a5, a6, and b1, as well as CD151 was determined by flow cytometry. Green; cells incubated with secondary antibody only. (B) Expression of the precursor (~150 kda) and mature (~35 kda) a3 subunit, as well as of b1, a2, and a6 was investigated by Western blotting in whole-cell lysates of podocytes expressing either a3 wt or a3 A349S. (29A3), or antibodies that recognize its extracellular domain ( J143 and P1B5), whereas detection of a3 was performed using our home-made antibody directed against its cytoplasmic tail. On reducing gels, this antibody recognizes the precursor of a3, as well as the 35 kda light chain of the mature a3 subunit. In lysates from podocytes expressing a3 wt, all antibodies precipitated both mature a3 and the precursor, although the latter was recognized to a lesser extent by J143 and P1B5 than by 29A3. Precursor a3 wt appeared as two bands, which likely represent the high-mannose form in the ER, and the complex form after modification of the N-linked oligosaccharides in the Golgi apparatus. As expected, the b1 subunit was co-precipitated with a3 wt. In contrast, neither J143 nor P1B5 precipitated the a3 A349S precursor, whereas 29A3 did precipitate precursor a3 A349S, but not the mature a3 or b1 subunits (Figure 5A). These results suggest that the a3 A349S precursor does not associate with b1, and that J143 and P1B5 recognize a complexdependent epitope on the a3 subunit. We then precipitated the b1 subunit from podocyte lysates, using either the MB1.2 or the 9EG7 antibody. Intriguingly, 9EG7 almost exclusively precipitated the b1 precursor but little or no mature b1, whereas MB1.2 precipitated both the precursor and mature b1, together with mature a3 wt. In contrast, mainly precursor b1 and hardly any mature b1 were precipitated from lysates of podocytes expressing the mutant a3 subunit (Figure 5B). Furthermore, neither of the two antibodies against b1 co-precipitated a3 A349S. These results confirm that the A349S mutation prevents association of the a3-subunit with b1 in cultured podocytes. To analyze whether heterodimerization was also impaired in the patient, we precipitated a3 with 29A3 from a lysate of patient lung tissue. Con- 88
91 2 Figure 5 The α3 A349S mutation disrupts α3 heterodimerization with β1, but not CD151 binding. (A) Podocytes expressing a3 or a3 A349S were lysed and the a3-subunit was precipitated using antibodies against the cytoplasmic tail (29A3) or the extracellular domain (J143 and P1B5). Precipitated a3 and b1 were detected by Western blotting. (B) b1 was precipitated from podocyte lysates using 9EG7 or MB1.2, and a3 and b1 were detected by Western blotting. (C) The a3-subunit was precipitated using 29A3 from tissue lysates of the patient and a healthy individual, and a3 and b1 were detected by Western blotting. (D) FLAG-tagged CD151 was precipitated from podocyte lysates using M2 against the FLAG-tag, 11G5 against CD151, or TS151R that recognizes the QRD-sequence in CD151 when not in complex with a3. J143 was included as a control. CD151, a3, and b1 were subsequently detected by Western blotting. Mutation in a3b1 causes lung and kidney disease 89
92 sistent with the observations in podocytes, both precursor and mature a3, as well as b1 were co-precipitated with 29A3 from tissue of an unaffected individual, whereas no mature a3 or b1, and only a small amount of precursor a3 was precipitated from patient tissue (Figure 5C). We then investigated the association of a3 wt and a3 A349S with CD151 in podocytes. For this purpose, we performed immunoprecipitation experiments using either the 11G5 antibody against CD151, or M2 which is directed against the FLAG-tag. As a negative control, we included the TS151R antibody that recognizes the QRD-sequence in CD151 that interacts with a3, and thus can only bind to CD151 when not in complex with a3b1 (31, 32). Both M2 and 11G5 co-precipitated CD151 with precursor and mature a3 wt, as well as with b1, suggesting that CD151 can associate both with the a3 precursor and mature a3. In addition, both antibodies clearly co-precipitated CD151 with a3 A349S but not b1, indicating that CD151 association with the mutant precursor is not impaired, and that CD151-a3 association can occur prior to the association of a3 with b1 (Figure 5D). As expected, the TS151R antibody only precipitated CD151 and no integrin subunits. These data suggest that CD151 can associate with precursor a3 prior to a/b heterodimerization, and that the A349S mutation prevents a3 heterodimerization with b1, but not its association with CD151. Gain-of-glycosylation disrupts heterodimerization and cell-surface expression To understand the mechanism by which the A349S mutation impairs a/b heterodimerization, we first determined the position of alanine 349 in the three-dimensional structure of a3b1, by homology modeling based on the recently resolved crystal structure of a5b1 (33). Alanine 349 is located on a loop opposite to the a/b-interface, suggesting that a mutation at this site does not directly impair a3b1 heterodimerization by steric hindrance (Figure 6A). The A349S substitution introduces a novel N-glycosylation motif in a3 (N-x-A > N-x-S), indicating that it leads to a gain-of-glycosylation. This was further suggested by the reduced mobility of the a3 A349S precursor in gel electrophoresis on low-percentage gels, whereas both the a3 wt and the a3 A349S precursor migrated with similar mobility after incubation of the precipitated proteins with N-glycanase, an enzyme that cleaves all N-linked high-mannose oligosaccharides (Figure 6B). Alternatively, treatment of cells with tunicamycin, a pharmacological inhibitor of N-linked glycosylation, also abolished the difference in mobility between a3 wt and a3 A349S precursors, confirming that a3 A349S is indeed hyperglycosylated (Figure 6C). However, the removal of all N-linked sugars by tunicamycin did not rescue heterodimerization but instead induced degradation of both the a3 and b1 subunits, reflecting the essential role of N-glycosylation in protein stability. To demonstrate that the addition of a sugar moiety prevents a/b heterodimerization, alanine 349 in a3 was substituted for glycine (a3 A349G ), which cannot be glycosylated, and the a3 A349G mutant was introduced in a3/cd151- deficient podocytes as described above. Intriguingly, flow cytometry indicated that a3 A349G was expressed at normal levels on 90
93 the cell surface (Figure 6D). Consistently, immunoprecipitation followed by Western blotting demonstrated that the a3 A349G precursor was normally cleaved to generate the mature product, and that a3 A349G indeed associates with the b1 subunit (Figure 6E). Thus, whereas the A349S substitution has dramatic consequences, substitution with glycine has no effect. In line with this result, alignment of the amino acid sequence of a3 from different species revealed that a glycine at this position occurs naturally in some organisms, as well as an arginine or asparagine but not a serine or threonine, further suggesting that sequence variation at this site is not detrimental per se, whereas the introduction of a novel oligosaccharide is (Figure S3). Taken together, these data suggest that the A349S mutation leads to a gain-of-glycosylation, which prevents a3b1 heterodimerization and cell-surface expression. 2 Figure 6 The a3 A349S mutation introduces a novel glycosylation motif that impairs heterodimerization and cell-surface expression. (A) Model of the ectodomain of a5β1 (left) and a3β1 (right). The N-x-A motif is indicated by an arrow. (B) The a3-subunit was precipitated with 29A3, the precipitates were treated with N-glycanase, and a3 and b1 were detected by Western blotting. (C) Podocytes expressing either a3 wt or a3 A349S were cultured for 16 hrs in the absence or the presence of tunicamycin (1 mg/ml), whereafter expression of the a3 subunit was analyzed by Western blotting. (D) Alanine 349 was mutated to glycine in human a3, and the a3 A349G mutant was expressed in a3-deficient podocytes by retroviral transduction and selection with zeocin. Cell-surface expression of a3 and b1 in a3 wt (blue) or a3 A349G (red) expressing cells was then investigated by flow cytometry. Green represents cells incubated with secondary antibody only. (E) Expression of precursor and mature a3 A349G, as well as heterodimerization with b1 were investigated by precipitation of a3 with antibody 29A3 from lysates of podocytes expressing either a3 wt or a3 A349G, followed by Western blotting. Mutation in a3b1 causes lung and kidney disease 91
94 Heterodimerization and cell-surface expression of a3 A349S b1 are not impaired by CD151 association Complex formation with CD151 requires an extracellular region in the a3 subunit comprising amino acids , adjacent to the loop containing alanine 349 (32). Because binding of CD151 to the a3 A349S precursor is not impaired, we hypothesized that CD151 association with a3 A349S may affect the orientation of the oligosaccharide such to induce a conformational change that prevents heterodimerization with b1. To explore this possibility, we introduced either a3 wt or a3 A349S, but not CD151, in a3/cd151-deficient podocytes, and investigated their expression at the cellsurface by flow cytometry. Similarly to in podocytes that express CD151, a3 wt but not a3 A349S was expressed at the cellsurface in CD151(-/-) cells (Figure 7A). Accordingly, in lysates of CD151(-/-) cells reconstituted with a3 wt, both the precursor and mature a3 subunits were detected by Western blotting, while in lysates of CD151(-/-) cells expressing the mutant a3 A349S, only the precursor a3 subunit was present (Figure 7B). These results were further confirmed by the lack of expression of mature a3 A349S, but not of a3 wt, in podocytes that contain CD151 in which the a3-binding QRD motif at position was mutated to INF (Figure 7B). This demonstrates that CD151 association with a3 A349S is not the cause for defective a3b1 heterodimerization, cleavage of precursor a3, and cell-surface expression, and suggest that the introduction of an additional oligosaccharide side-chain is by itself sufficient to disturb proper folding of the a3-subunit. The A349S mutation causes accumulation of the a3 and b1 precursors in the ER, a3 ubiquitination, and proteasomal degradation We next investigated the subcellular distribution of a3 wt b1 and a3 A349S b1 in podocytes by confocal microscopy. First, we used the J143 antibody against the extracellular domain of a3. As expected, a3 wt was clearly distributed over the plasma membrane, and was enriched laterally at cell-cell contact sites and basally in FAs, as indicated by co-localization with the FA marker phospho-paxillin. In contrast, no a3 A349S was detected at the plasma membrane, consistent with the biochemical and flow cytometry data. Furthermore, J143 did not react with precursor a3 A349S in intracellular compartments, supporting the idea that this antibody recognizes only the a3 subunit when it is associated with b1 (Figure 8A). In the presence of a3 wt, b1 was distributed over the plasma membrane, laterally in cell-cell contacts, and basally in FAs, as determined using an antibody directed against phosphotyrosines. However, in cells expressing a3 A349S, b1 was hardly detected at the plasma membrane, although the FA pool was retained. Instead, a dramatic accumulation was observed in a perinuclear compartment, which was barely visible in cells expressing a3 wt (Figure 8B). These observations are consistent with the dramatic reduction in b1 surface expression observed by flow cytometry, and the large pool of b1 precursor found in lysates of a3 A349S. The perinuclear fraction represents most likely the ER-localized excess of b1 that is unable to heterodimerize with a3 A349S. We then analyzed a3 localization using our antibody directed against the 92
95 2 Figure 7 CD151 binding does not impair a3b1 heterodimerization or transport of a3 A349S to the plasma membrane. (A) a3/cd151-deficient podocytes were reconstituted with a3 wt or a3 A349S, and cellsurface expression of a3 (left) and b1 (right) was analyzed by flow cytometry. a3 wt (blue), a3 A349S (red), a3 wt with secondary antibody only (green). (B) CD151-deficient podocytes expressing either a3 wt or a3 A349S were lysed, and precursor and mature a3 were detected by Western blotting (left panel). Alternatively, a3/ CD151-deficient podocytes were reconstituted with a3 wt or a3 A349S, as well as with CD151 in which the QRD-motif was mutated to INF. Podocytes were lysed, and precursor and mature a3 were detected by Western blotting (right panel). cytoplasmic domain of a3. Much like the staining pattern obtained with J143, this antibody detected a3 wt at the plasma membrane, in cell-cell contacts, and in cell-matrix adhesions. Intriguingly, it also revealed accumulation of a3 A349S around Mutation in a3b1 causes lung and kidney disease 93
96 the nucleus, where it co-localized with b1 (Figures 8C,D). Finally, we examined the distribution of CD151. In cells expressing a3 wt, CD151 co-localized with a3 at the plasma membrane and in adhesions. In cells expressing a3 A349S, CD151 was also localized at the plasma membrane, consistent with observations that CD151 can traffic to the cell-surface independently of a3b1 (34). However, a small perinuclear pool co-localized with a3 A349S, which probably represents CD151 bound to Figure 8 The a3 A349S mutation causes accumulation of b1 precursor in the ER, and ubiquitination and proteosomal degradation of a3. (A) Subcellular localization of a3 was investigated in podocytes expressing either a3 wt or a3 A349S, using J143 against the extracellular domain of a3 (a3 e ; blue). Filamentous actin (F-actin) was stained with phalloidin (red), and FAs were visualized using an antibody against phosphorylated paxillin (P(Y)pax; green). (B) Localization of b1 in podocytes expressing either a3 wt or a3 A349S (blue). P(Y)pax; green, F-actin; red. (C) Distribution of a3 was investigated using an antibody directed against the cytoplasmic domain (a3 c ; blue), and FAs were detected with an antibody against phosphotyrosines (P(Y); green). F-actin; red. (D) Co-localization of b1 (green) and a3 (blue). F-actin; red. (E) Distribution of CD151 (green) and a3 (blue). F-actin; red. (F) Co-localization of a3 A349S with protein disulphide isomerase (PDI; upper panel), and b1 with calnexin (lower panel). Nuclei were stained with DAPI. (G) Podocytes expressing either a3 wt or a3 A349S were treated for 6 hrs with the proteasomal inhibitor lactacystin (10 mm), lysed, and ubiquitinated a3b1 was detected by precipitation of the indicated subunits followed by Western blotting. 94
97 precursor a3 in the ER (Figure 8E). Costaining of a3 or b1 with the ER markers protein disulphide isomerase or calnexin confirmed that the perinuclear compartment to which a3 A349S b1 localized is indeed the ER (Figure 8F). Calnexin is a chaperone protein that is involved in ER retention and folding of nascent glycoproteins (35). Both the integrin a and b subunit precursors undergo several cycles of folding and unfolding, during which calnexin associates and dissociates with both to ensure that they reach their proper conformation before transport to the Golgi (36). To investigate whether a3 A349S could still associate with calnexin, we precipitated either b1 or a3 from podocytes expressing a3 wt or a3 A349S. Both wild-type and mutant a3, as well as b1, co-precipitated from podocyte lysates with calnexin, indicating that the interaction of calnexin with neither subunit was impaired (Figure S4). In an attempt to circumvent the quality control mechanism in the ER, we cultured podocytes at 30ºC or incubated them with the pharmacological chaperones curcumin or 4-phenylbutyrate. Compounds that can act as chaperones have been employed to evade the ER-response to misfolded G-protein-coupled receptors including the vasopressin receptor, gondotropin-releasing hormone receptor, and cystic fibrosis transmembrane conductance regulator (37, 38). Therefore, pharmacological chaperones are suggested as treatment for a variety of diseases due to protein misfolding (39). However, neither strategy induced cellsurface expression of a3 A349S, whereas the cell-surface expression of a3 wt was increased (data not shown). Misfolded proteins are disposed of by the ER-associated degradation pathway, which involves ubiquitination and transport from the ER to the cytosol for proteasomal degradation (40, 41). To inhibit the proteasome, podocytes were incubated for 6 hrs with lactacystin (10 mm). Treatment with lactacystin induced a striking accumulation of poly-ubiquitinated a3 A349S precursor, but much less so of a3 wt, indicating that indeed a large fraction of the mutant precursor is ubiquitinated and proteasomally degraded (Figure 8G). Together, these data demonstrate that the misfolded a3 A349S precursor is cleared by ubiquitination and subsequent proteasomal degradation, whereas the excess of b1 that is unable to associate with a3 A349S is mostly retained in the ER. 2 DIscUssIon Here, we report a novel homozygous missense mutation in the ITGA3 gene, which disturbs the biosynthesis of integrin a3b1, resulting in a multi-organ disorder consisting of interstitial lung disease and congenital nephrotic syndrome. The mutation causes a gain-of-glycosylation in the a3 subunit, which prevents the heterodimerization with b1 but not the association with CD151. Although the mutant a3 subunit still interacts with calnexin, its misfolding is terminal, leading to rapid clearance by the ubiquitin/proteasome system while the non-complexed b1 accumulates in the ER (Figure 9). The kidney and lung abnormalities observed in the patient are consistent with the defects in kidney and lung morphogen- Mutation in a3b1 causes lung and kidney disease 95
98 esis observed in a3-deficient mice (13). In addition, we find extracapillary proliferation, focal segmental glomerulosclerosis, and interstitial fibrosis in the patient glomeruli, which was not observed in a3-null mice. The latter observations likely reflect the progressive nature of glomerular damage, as a3-deficient mice die much earlier after birth. In addition to the kidney and lung defects, absence of a3 in the epidermis causes skin abnormalities including (micro-)blistering, basement membrane duplication, inflammation, and alopecia (13-17). Although obvious macroscopic skin abnormalities were not observed in the patient described here, we cannot exclude that microblistering or aberrant epidermal basement membrane organization occurred. During the revision of this manuscript, a report was published describing mutations Figure 9 Model summarizing the biosynthetic route of CD151, and wild-type and mutant integrin a3b1. Newly synthesized a3 and b1 precursors, as well as CD151, undergo N-glycosylation and folding in the ER, facilitated by the chaperone calnexin. Thereafter, CD151 can associate with other tetraspanins, or associate with the a3 subunit prior to a/b heterodimerization. Properly folded and assembled complexes then traffic to the Golgi apparatus either as integrin a3b1 heterodimers, a3b1 heterodimers in association with CD151, or CD151 in complex with other tetraspanins. In the Golgi, the high-mannose oligosaccharides are processed to complex type sugars, and the a3-subunit is cleaved into a heavy and a light chain to generate the mature a3b1 heterodimer (a). Gain-of-glycosylation mutations in an integrin subunit can cause a failure to reach the final conformation, and thereby prevent a/b heterodimerization. The affected subunits are then cleared by the ubiquitin/proteasome system, whereas their partners accumulate in the ER (b). 96
99 in ITGA3. While the kidney and lung defects observed in these patients are largely similar to those described here, also a skin phenotype was reported (42). It should be noted that the skin defects caused by a3 deficiency in mice are relatively minor, because adhesion of epidermal keratinocytes to laminins is rescued by other integrins, most notably the hemidesmosome-based integrin a6b4 (17, 43). Thus, redundant mechanisms exist in epidermal keratinocytes to ensure adhesion, which is probably why skin defects are not always detected in the absence of a3 (44). Similarly, a mutation in CD151 has been described to cause epidermolysis bullosa, whereas skin defects have not been observed in Cd151-deficient mice (19, 24, 25, 28). Nevertheless, a6 integrins can clearly not compensate for the absence of a3b1 in the kidneys and the lungs, even though a6 expression seemed to be increased in these organs in the patient described here. Our immunoprecipitation experiments yield some important conclusions. Firstly, the mutant a3 subunit does not heterodimerize with b1, as shown by pulldown of either the a3 subunit with 29A3 or the b1 subunit with MB1.2. Intriguingly, the 9EG7 antibody, which is widely used as a marker for b1 integrins in the active, high-affinity conformation (45), exclusively precipitated precursor b1 but no mature b1. In addition, 9EG7 did not recognize b1 on the cell-surface by flow cytometry (data not shown). These observations would suggest that there is no b1 in the active conformation at the cellsurface, questioning the use of 9EG7 as a bona fide marker for active b1 integrins in general. Secondly, the J143 and P1B5 antibodies, both directed against the extracellular domain of a3, apparently recognize a complex-dependent epitope on the a3 subunit, because they do not precipitate precursor a3 A349S, and precipitate much smaller amounts of the a3 wt precursor than 29A3, but comparable amounts of mature a3 or b1. The fraction of precursor a3 that is not recognized by these antibodies is presumably not associated with b1. Thirdly, our data show that CD151 can associate with both the a3 precursor and mature a3, which is consistent with previous observations indicating that CD151-a3 association occurs early during biosynthesis of a3b1 (34). In addition, it has been shown that CD151 mutants that are not expressed at the cell surface can be co-precipitated with a3, suggesting that CD151 binds intracellular a3, of which at least a fraction consists of precursor (34). In fact, the observation that CD151 binds precursor a3 A349S even though the latter does not associate with b1, suggests that CD151-a3 association occurs prior to a/b heterodimerization. The A349S mutation leads to hyperglycosylation of the a3 precursor, which is likely the mechanism for its disruptive effects, as (i) considerable sequence variability exists at this position among eukaryotes but a serine or threonine is never encountered, and (ii) a mutant carrying a glycine at this position, which cannot be glycosylated, associates normally with b1 and is expressed at the cell-surface. As the novel sugar moiety is oriented away from the a/b interface, a direct effect on a/b subunit interaction by steric hindrance seems unlikely. However, it is located in between two FG-GAP repeats, which form b-strands in the b-propeller domain. This region is very important for the proper folding of integrin a subunits, and numerous mutations in the b-propeller 2 Mutation in a3b1 causes lung and kidney disease 97
100 of aiib have been identified that disturb its folding and the heterodimerization with b3 (46). Similar to what we describe here, such mutations lead to proteasomal degradation of the mutant aiib precursor, whereas the non-complexed b3 precursor is retained in the ER (47). The repertoire of gain-of-glycosylation disorders is ever-expanding, and gainof-glycosylation mutations that disrupt integrin biosynthesis as the cause for a human disorder have previously been identified in the genes encoding integrin subunits b2 (causing leukocyte adhesion deficiency), and b3 (causing Glanzmann s thrombasthaenia) (48-50). We now report a new gain-of-glycosylation mutation that prevents the biosynthesis of integrin a3b1, causing interstitial lung disease and congenital nephrotic syndrome. It will be important to implement ITGA3 gene sequencing in DNA diagnostics for newborns presenting with severe respiratory distress and/or congenital nephrotic syndrome of unknown etiology, to facilitate early diagnosis in patients and provide recurrence risk estimation for patients and their relatives. MateRIals and MetHODS Materials The cdna encoding CD151 was provided by M. Hemler (Dana Farber Cancer Institute, Boston, MA), and the cdna encoding human a3 has been described previously (51). Mouse mabs directed against human a3 were J143 (hybridoma from American Type Culture Collection) and P1B5 (W. Carter, Fred Hutchinson Cancer Research center, Seattle, WA). Mouse Abs against human CD151 were 5C11 (F. Berditchevski, University of Birmingham, Birmingham, UK), TS151R (E. Rubinstein, Hôpital Paul Brousse, Villejuif, France), and 8C3 (K. Sekiguchi, Osaka University, Osaka, Japan). The mouse mab 29A3 against the cytoplasmic domain of a3a has been described previously (52). Rat mabs were: GoH3 against a6 and MB1.2 against β1 from B. Chan (University of Ontario, Ontario, Canada). Rabbit polyclonal Abs were directed against the cytoplasmic domain of a3a (19), the extracellular domain of a6 (AA6NT, 53) from A. Cress (University of Arizona, AZ), the cytoplasmic domains of β1a and a2 from G. Tarone (University of Turin, Italy), calnexin from I. Braakman (University of Utrecht, Utrecht, The Netherlands), Col-IV from E. Engvall (The Burnham Institute, La Jolla, CA), LN and nidogen from T. Sasaki (Shriners Hospital for Children Research Center, Portland, OR), and podocin from C. Antignac (Cochin Biomedical Research Institute, Paris, France). The goat Ab used against mouse a3 was AF2787 from R&D Systems Inc. TRITCand FITC-conjugated secondary Abs, phalloidin and DAPI were from Molecular Probes (Eugene). HRP-conjugated secondary Abs were from Amersham, curcumin, 4-phenylbutyrate, tunicamycin and zeocin were from Sigma, and Col-I was obtained from Vitrogen (Nutacon). Patient material and Dna diagnostics Peripheral blood samples were obtained from the patient, parents and sibling after informed consent was given. Genomic 98
101 DNA was extracted from these samples and known genes implicated in nephrotic syndrome (NPHS1, WT1, PLCE1, LAMB2, NPHS2, TRPC6, ACTN4, INF2, ABCA3, TTF1, TTF2) and neonatal respiratory distress (ABCA3, SFTP-B, and SFTP-C) were screened for variants by Sanger sequencing. Tissue samples used in this study were derived from kidney and lung autopsy or biopsy specimens, obtained with informed parental consent. snp array analysis Genomic DNA was obtained from peripheral blood samples of the patient and the parents. CNV screening by means of microarray analyses was carried out on the Affymetrix GeneChip 250k (NspI) SNP array platform (Affymetrix, Inc.), which contains 25-mer oligonucleotides representing a total of 262,264 SNPs. The average resolution of this array platform is kb. Hybridizations were performed according to the manufacturer s protocols. CNVs and LCSH were determined using Copy Number Analyzer for Affymetrix GeneChip mapping software (version 2.0). The average resolution of this array platform is Kb. To investigate uniparental disomy, genotypes were called by Affymetrix Genotype Console Software v2.1. Mutation analysis of ITGA3 To sequence the complete coding region of ITGA3 in genomic DNA, exon-flanking primers were designed with the online available Primer3 program (Table S1). All coding exons of ITGA3 were amplified by PCR in a total reaction volume of 20 μl, which contained 50 ng DNA, 10 pmol of each primer, 50 nmol of MgCl2, 1x Taq buffer (Applied Biosystems, Inc.) and 1 U Taq polymerase (Applied Biosystems, Inc.). PCR products were analyzed forward and reverse on a 3730 DNA analyzer (Applied Biosystems, Inc.) with dye-termination chemistry (Big Dye Terminator Cycle sequencing Kit version 1.1, Applied Biosystems, Inc.). Sequence analysis was performed using Sequencher 4.8 software. The identified gene variant was confirmed by bidirectional sequencing. Segregation analysis was performed by Sanger sequencing. Furthermore, we sequenced the ITGA3 gene in 192 healthy blood donors from the Netherlands. To determine whether the mutation was previously detected in reference populations we used our in-house database of ~100 whole-exome sequencing experiments, dbsnp, and the online available Exome Variant Server of the NHLBI Exome Sequencing Project. Generation of cell lines, cell culture, cloning, and retroviral transduction Glomeruli were isolated from Itga3 flox/ flox ; Cd151 flox/flox ; Trp53+/- mice, according to the Dynabead perfusion method as described previously (29), and cultured on 3 μg/ml Col-I at 37 C and 5% CO2 in keratinocyte serum-free medium (K-SFM; Gibco BRL) supplemented with 50 μg/ml bovine pituitary extract, 5 ng/ml epidermal growth factor, 100 U/ml penicillin and 100 U/ml streptomycin. Outgrowing epithelial cells were sorted for expression of podocalyxin and absence of CD31. The Itga3 and Cd151 genes were deleted by adenoviral delivery of Cre-recombinase. The A349S and A349G mutations were generated by PCR overlap extension method using a cdna encoding human full-length a3a as a template. Wild-type ITGA3 was isolated by digestion with SacI 2 Mutation in a3b1 causes lung and kidney disease 99
102 and ligated into puc18-a3. After digestion with SphI, ITGA3 was ligated into LZRS- IRES-zeo, which was transfected into Phoenix packaging cells using the Calcium Phosphate method. Virus-containing supernatant was isolated after 48 hrs and stable expression in the a3/cd151-deficient podocytes was achieved by retroviral transduction, followed by selection with zeocin (200 μg/ml). The podocytes were also reconstituted with a cdna encoding FLAG-tagged human CD151. Immunoprecipitations and Western blotting Cell lysis and immunoprecipitations were performed essentially as described previously (17). Whole cell lysates and precipitates were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes (Millipore), and analyzed by Western blotting. Bound antibodies were detected using the ECL detection system from GE Healthcare. Immunohistochemistry, electron microscopy, confocal microscopy, and flow cytometry Electron microscopy and immunohistochemistry were performed using standard procedures. For indirect immunofluorescence, cryosections from patient biopsies or coverslips with cells were incubated with antibodies as previously described (17). Images were acquired at room temperature with a confocal Leica TCS NT or AOBS microscope using 20x (NA 0.7) dry, 40x (NA 1.25) oil and 63x (NA 1.32) oil objectives (Leica) and AxioVision 4 software (Carl Zeiss MicroImaging). Pictures were processed using Photoshop 7.0 and ImageJ. Flow cytometry and cell sorting were performed as previously described (17). acknowledgements We are grateful to Drs C. Antignac, I. Braakman, F. Berditchevski, B. Chan, W. Carter, A. Cress, E. Engvall, M. Hemler, E. Rubenstein, T. Sasaki, K. Sekiguchi, and G. Tarone for their generous gifts of reagents. We thank Dr. A. Perrakis for help with the modeling of the a3b1 ectodomain, and Drs A.Y. Konijnenberg, E.J. D Haens, R.A. van Lingen, and G. Shabo for taking care of the patient. This work was financially supported by the Dutch Kidney Foundation with a grant to KR (KSTP10.004) and AS (C ). ABBREVIatIons CNV, copy number variations; Col, collagen; ER, endoplasmic reticulum; FA, focal adhesion; GBM, glomerular basement membrane; LCSH, long continuous stretch of homozygosity; LN, laminin; SNP, single-nucleotide polymorphism 100
103 RefeRences 1. Campbell ID, and MJ Humphries (2011) Integrin structure, activation and interactions. Cold Spring Harb Perspect Biol 3, doi: /cshperspect.a Geiger B, and KM Yamada (2011) Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol 3, doi: /cshperspect. a Kim C, et al (2011) Regulation of integrin activation. Annu Rev Cell Dev Biol 27, Caswell PT, et al (2009) Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol 10, Margadant C, et al (2011) Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol 23, Lissitzky JC, et al (2000) Endoproteolytic processing of integrin pro-a subunits involves the redundant function of furin and proprotein convertase (PC) 5A, but not paired basic amino acid converting enzyme (PACE) 4, PC5B or PC7. Biochem J 346, Delwel GO, et al (1997) Identification of the cleavage sites in the a6a integrin subunit: structural requirements for cleavage and functional analysis of the uncleaved a6ab1 integrin. Biochem J 324, Pulkkinen L, and J Uitto (1999) Mutation analysis and molecular genetics of epidermolysis bullosa. Matrix Biol 18, Nurden AT, et al (2011) Glanzmann thrombasthenia: a review of ITGA2B and ITGB3 defects with emphasis on variants, phenotypic variability, and mouse models. Blood 118, Hayashi YK, et al (2011) Mutations in the integrin a7 gene cause congenital myopathy. Nat Genet 19, Hogg N, et al (2011) The insider s guide to leukocyte integrin signalling and function. Nat Rev Immunol 11, Wickström SA, et al (2011) Genetic analyses of integrin signaling. Cold Spring Harb Perspect Biol Kreidberg JA, et al (1996) a3b1 integrin has a crucial role in kidney and lung organogenesis. Development 122, DiPersio CM, et al (1997) a3b1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol 137, Hodivala-Dilke KM, et al (1998) Novel roles for a3b1 integrin as a regulator of cytoskeletal assembly and as a trans-dominant inhibitor of integrin receptor function in mouse keratinocytes. J Cell Biol 142, Conti FJ, et al (2003) a3b1 integrin regulates hair follicle but not interfollicular morphogenesis in adult epidermis. J Cell Sci 116, Margadant C, et al (2009) Integrin a3b1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci 122, Yauch RL, et al (1998) Highly stoichiometric, stable, and specific association of integrin a3β1 with CD151 provides a major link to phosphatidylinositol 4-kinase, and may regulate cell migration. Mol Biol Cell 9, Sachs N, et al (2006) Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol 175, El-Aouni C, et al (2006) Podocytespecific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J Am Soc Nephrol 17, Miner JH and C Li (2000) Defective glomerulogenesis in the absence of laminin a5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev Biol 217, Kanasaki K, et al (2008) Integrin b1- mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol 313, Pozzi A, et al (2008) b1-integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol 316, Sachs N, et al (2012) Blood pressure influences end-stage renal disease of Cd151 knockout mice. J Clin Invest 122, Baleato RM, et al (2008) Deletion of CD151 results in a strain-dependent glomerular disease due to severe alterations of the glomerular basement membrane. Am J Pathol 173, Mutation in a3b1 causes lung and kidney disease 101
104 26. Kaplan JM, et al (2000) Mutations in ACTN4, encoding a-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24, Zenker M, et al (2004) Human laminin b2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet 13, Karamatic Crew V, et al (2004) CD151, the first member of the tetraspanin (TM4) superfamily detected on erythrocytes, is essential for the correct assembly of human basement membranes in kidney and skin. Blood 104, Takemoto M, et al (2002) A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161, Kazarov AR, et al (2002) An extracellular site on tetraspanin CD151 determines a3 and a6 integrin-dependent cellular morphology. J Cell Biol 158, Serru V, et al (1999) Selective tetraspanintegrin complexes (CD81/a4b1, CD151/a3b1, CD151/a6b1) under conditions disrupting tetraspan interactions. Biochem J 340, Yauch RL, et al (2000) Direct extracellular contact between integrin a3b1 and TM4SF protein CD151. J Biol Chem 275, Nagae M, et al (2012) Crystal structure of a5b1 integrin ectodomain: Atomic details of the fibronectin receptor. J Cell Biol 197, Berditchevski F, et al (2001) Analysis of the CD151-a3b1 integrin and CD151- tetraspanin interactions by mutagenesis. J Biol Chem 276, Caramelo JJ, and AJ Parodi (2008) Getting in and out from calnexin/calreticulin cycles. J Biol Chem 283, Lenter M, and D Vestweber (1994) The integrin chains b1 and a6 associate with the chaperone calnexin prior to integrin assembly. J Biol Chem 269, Egan ME, et al (2004) Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 304, Wang X, et al (2008) Chemical and biological folding contribute to temperature-sensitive DeltaF508 CFTR trafficking. Traffic 9, Bernier V, et al (2004) Pharmacological chaperone action on G-protein-coupled receptors. Curr Opin Pharmacol 4, Smith MH, et al (2011) Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science 334, Lederkremer GZ (2009) Glycoprotein folding, quality control and ER-associated degradation. Curr Opin Struct Biol 19, Has C, et al (2012) Integrin a3 mutations with kidney, lung, and skin disease. N Engl J Med 366, Margadant C, et al (2008) Regulation of hemidesmosome (dis-)assembly by growth factor receptors. Curr Opin Cell Biol 20, Margadant C, et al (2010) Unique and redundant functions of integrins in the epidermis. FASEB J 24, Byron A, et al (2009) Anti-integrin monoclonal antibodies. J Cell Sci 122, Nelson EJ, et al (2005) Three novel b- propeller mutations causing Glanzmann thrombasthenia result in production of normally stable pro-aiib, but variably impaired progression of pro-aiibb3 from endoplasmic reticulum to Golgi. J Thromb Haemost 3, Mitchell WB, et al (2006) aiibb3 biogenesis is controlled by engagement of aiib in the calnexin cycle via the N15- linked glycan. Blood 107, Vogt G, et al (2007) Gain-of-glycosylation mutations. Curr Opin Genet Dev 17, Vogt G, et al (2005) Gains of glycosylation comprise an unexpectedly large group of pathogenic mutations. Nat Genet 37, Back AL, et al (1993) A point mutation associated with leukocyte adhesion deficiency type 1 of moderate severity. Biochem Biophys Res Commun 193, Delwel GO, et al (1994) Distinct and overlapping ligand specificities of the a3ab1 and a6ab1 integrins: recognition of laminin isoforms. Mol Biol Cell 5, De Melker AA, et al (1997) The A and B variants of the a3 integrin subunit: tissue distribution and functional characterization. Lab Invest 76, Ports MO, et al (2009) Extracellular engagement of a6 integrin inhibited urokinase-type plasminogen activatormediated cleavage and delayed human prostate bone metastasis. Cancer Res 69,
105 Table S1 Primers used for ITGA3 mutation analysis. EXon FORWARD PRIMERS 5 3 REVERse PRIMERS 5 3 PRODUct SIZE (bp) 1a cgtcacatccatcttgctc atccgtgggtctatcttcct 460 1b ctccctcctgtcctccttg cgaccgagtagccgaagag 539 1c acgcctgatgctctgtgc gactaccagcgaggtgctta ttttccttgcctgccttac gcacctcacccatacttcag actcactgcccacaaggat gacacacagccacaggaag gtctctcatccttccctgct aagtcatggtggttgctgat ggcaaaatgctcaccaataa ttccaagtagggcaagaaag ctactttcttgccctacttgg ataaagcctgactgcaaacc atattggcatctccatgtcc acatctgcacatcctctctc ctctgtccctgatgctctg gcttctctccatggattacc cccagcaggtacagagagac gagacaacagagccagacag tcttcttcatctttgtctgcac aatgaggttgggtagagagg cagacctgctttgtggactc acaccaatagccttccaaac agtaggaagtcgcaatttgg catctgcaagttgctctcac atcctcaaccaggcacag gcacctggaggagaaagc aggtgggatggtcagaaac attctccaagcagcagagac ctctggtctgggccttc ggcctcttctcaccctctac gtggggtggggaggtagag cagaggagtttgggagatagc gctatctcccaaactcctctg aaccacctccatcttaccaac ctgtggaggatgtaggaagc gaggaagaattgggagcag caaccctctcaacctcactc gcctcatcacctcatcacac tgtgcatgagtgaaaggaag acacatccatgcaaagacac ctggctgacagatcctttg agacaccagaactcctccag agcaggacaaacagcaggt gtgtggtcagaagccagag cttctgaccacaccaccaa cttgcccttgaccttgttc tctggctttgaggagttctg gctctttggcttgttttgg Mutation in a3b1 causes lung and kidney disease 103
106
107 Integrin α3β1 inhibits directional migration and wound re-epithelialization in the skin Coert Margadant, Karine Raymond #, Maaike Kreft, Norman Sachs, Hans Janssen, and Arnoud Sonnenberg Division of Cell Biology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands. # Present address: UMR 144 CNRS - Institut Curie, Centre de recherche, 26 Rue d Ulm, Paris, France. J Cell Sci 122, (2009)
108
109 ABSTRAct Re-epithelialization after skin wounding requires both migration and hyperproliferation of keratinocytes. During migration over the provisional matrix, laminin-332 is deposited. To investigate the function of the laminin-332 binding integrin a3b1 in wound re-epithelialization, we generated Itgα3 flox/flox ; K14-Cre mice lacking the a3 subunit specifically in the basal layer of the epidermis. These mice are viable but display several skin defects including local inflammation, hair loss, basement membrane duplication and microblistering at the dermal-epidermal junction, whereas hemidesmosome assembly and keratinocyte differentiation are not impaired. Wound healing is faster in the absence of a3b1 while proliferation, the distribution of other integrins and the deposition of basement membrane proteins in the wound bed are unaltered. In vitro, cell spreading is rescued by increased surface expression of a6b1 in the absence of a3. The a3-deficient keratinocytes migrate with an increased velocity and persistence, whereas proliferation, growth factor signaling, hemidesmosome assembly, and laminin-332 deposition are normal. We suggest that α3b1 delays keratinocyte migration during wound re-epithelialization, by binding the laminin-332 that is newly deposited into the wound bed. 3 IntRODUctIon The skin is composed of a layer of stratified squamous epithelium (the epidermis), and an underlying layer of connective tissue (the dermis), which are separated by a basement membrane (BM) consisting primarily of laminins and collagens (1). Attachment of basal epidermal keratinocytes to the BM is mediated by members of the integrin family. The integrin repertoire in basal keratinocytes is restricted to α2β1, α3β1 and α6β4, while de novo expression of integrins α5β1, αvβ5, αvβ6, and probably αvβ1, is induced upon wounding (2). Whereas α2β1 mediates attachment to collagens, adhesion to the main BM component laminin (Ln)-332 (previously named Ln-5, nicein, kalinin, or epiligrin) is established by the predominant epidermal integrins α3β1 and α6β4 (3-5). Integrin α3β1 links the extracellular matrix (ECM) to the actin cytoskeleton and is localized at the basolateral membrane. It is often found in clusters surrounding HDs (HDs), and in focal adhesions in cultured cells (6,7). In contrast, integrin α6β4 associates with the intermediate filament system, and its distribution is restricted to the basal surface of keratinocytes. There, it governs the assembly of hemidesmosomes (HDs), which consist further of the cytoskeletal linker proteins plectin and bullous pemphigoid (BP) antigen 230, and the transmembrane proteins BP180 and CD151 (8). Ablation in mice of the gene encoding α6 or β4 impairs HD formation and results in severe epidermal blistering and perinatal death, demonstrating the importance of α6β4 in the maintenance of skin integrity at the dermal-epidermal junction (DEJ; 9-11). Similarly, humans carrying a mutation in either of these genes or in the genes encoding the a3, b3, or g2 chains of Ln-332 suffer from a skin blistering disease referred to as junctional epidermolysis bullosa (12-14). Integrin α3β1 Inhibits Wound Healing 107
110 Deletion of the gene encoding the integrin β1 subunit is lethal in the early embryo (15,16). Evidence for the function of b1 in skin stems from conditional knockout mouse models in which b1 ablation is restricted to the basal epidermal keratinocytes, resulting in skin blistering at the DEJ, a reduced number of HDs, failure of BM assembly, impaired invagination of hair follicles and eventual hair loss (17,18). This suggests that β1 integrins are required for hair growth, BM assembly, and HD formation. Furthermore, epidermal migration during wound healing is impaired in the absence of b1 (19). Since a2-null mice do not have any obvious skin defects, neither under steady-state conditions nor during wound healing (20,21), the wound healing defect caused by b1 deletion is possibly due to loss-of-function of a5β1 or avβ1, whose expression is induced upon wounding, or loss-of-function of a3β1, which most likely explains the other features of the phenotype as well. Indeed, a3-null mice are born with a disorganized BM membrane and microblistering at the DEJ, although the skin phenotype is much less severe than that observed in the absence of a6, b1, or b4 (22). However, these mice die neonatally as a consequence of defective kidney and lung organogenesis (23), complicating the study of α3β1 in adult skin and wound healing. In vitro evidence of the role of a3b1 in keratinocyte migration is controversial; whereas some studies have suggested that a3b1 promotes keratinocyte migration (24,25), the opposite has also been reported (26-29). To address the role of a3b1 in adult skin homeostasis and wound healing, we have generated epidermis-specific a3 knockout mice by crossing Itga3 flox/flox mice with mice expressing Cre-recombinase under the control of the K14 promoter. The resulting Itga3 flox/flox ; K14-Cre mice are viable but display several skin abnormalities including local inflammation, microblistering at the DEJ, and BM duplication, whereas HD assembly and keratinocyte differentiation are normal. Skin wounds close faster in these animals whereas proliferation rates are similar, suggesting accelerated keratinocyte migration in the absence of a3. This is confirmed by in vitro observations; a3-deficient keratinocytes migrate with increased velocity and persistence compared to a3-expressing cells. Taken together, these results show that the integrin α3b1 is required for the maintenance of dermal-epidermal integrity but not for keratinocyte proliferation, differentiation, or HD formation. Furthermore, α3b1 delays keratinocyte migration during wound re-epithelialization, by binding the Ln-332 that is newly deposited into the wound bed. ResUlts Generation and characterization of epidermis-specific Itga3 knockout mice Integrin a3-null mice die shortly after birth, displaying aberrant kidney and lung organogenesis and moderate skin blistering at the DEJ (22,23). To address the role of the a3 subunit in adult skin, specifically in adhesion and wound healing, we created epidermis-specific a3 knockout mice by crossing mice homozygous for a floxed 108
111 Itga3 allele (Itga3 flox/flox ) (30) with mice expressing Cre-recombinase under the control of the K14 promoter (Figure S1). Deletion of the a3 subunit from the epidermis had no obvious effects on overall skin structure; the proliferative layer of the epidermis was typically 2-3 cell layers thick, and keratinization and HD assembly seemed to be normal (Figure 1A). However, microblistering at the DEJ was occasionally observed, which was associated with epidermal hyperthickening, and 3 Figure 1 Skin phenotype of Itga3 flox/flox ; K14-Cre mice. (A) Upper panel, cryosections from back-skin of neonatal Itga3 flox/flox and Itga3 flox/flox ; K14-Cre mice were stained with antibodies directed against the a3 subunit and Ln-332. Bar, 100 mm. Middle panel, haematoxylin/eosin (H/E) stainings of back-skin of 4-month old Itga3 flox/flox and Itga3 flox/flox ; K14-Cre mice. Bar, 200 mm. Lower panels, EM pictures and schematic representations of back-skin of 1-year old Itga3 flox/flox and Itga3 flox/flox ; K14-Cre mice, showing the BM and HDs (indicated by arrowheads). Bar, 200 nm. (B) Top panel, H/E staining of back-skin of a 1-year old Itga3 flox/flox ; K14-Cre mouse. Arrowheads indicate microblisters at the DEJ. Bar, 500 mm. Lower panels, cryosections of a region containing microblisters were stained with antibodies against Ln-332 and b4. Arrowheads point toward blisters. Bar, 50 mm. (C) Ultrastructural analysis and schematic representation of back-skin of a 1-year old Itga3 flox/flox ; K14-Cre mouse, showing BM duplication. The 2 BMs are marked by the thick and dotted lines, and collagen fibers extending throughout the 2 BMs are indicated. Bar, 200 nm. (D) Regions of inflammation in a 4-month old Itga3 flox/flox ; K14-Cre mouse are denoted by arrows. Back skin sections were stained with an antibody against CD3. Arrows point to infiltrated lymphocytes. (E) Alopecia in a 1-year old Itga3 flox/flox ; K5-Cre mouse (right), as compared to an Itga3 flox/flox mouse of the same age. BM, basement membrane; Col, collagen; D, dermis; E, epidermis. Integrin α3β1 Inhibits Wound Healing 109
112 the presence of an inflammatory infiltrate (Figure 1B). Ln-332 was distributed at both the roof and floor of the blisters, while localization of b4 was restricted to the roof (Figure 1B). Ultrastructural analysis revealed duplication of the BM (Figure 1C). Inflammation occurred frequently around 3-4 months after birth, especially at the ears and around the eyes (Figure 1D). The inflammatory infiltrate was mainly observed in regions where the epidermis was abnormally thick (Figure 1D). Around the same time, these mice developed alopecia (local hair loss), which progressed with age (Figure 1E). Differentiation in the epidermis was essentially normal, as assessed by staining of cryosections for keratins 10 and 14, filaggrin, and involucrin (Figure 2, and data not shown). Similar results were obtained when Itga3 flox/ flox mice were crossed with mice expressing Cre-recombinase under the control of the K5 promoter (data not shown). Summarizing, the targeted deletion of Itga3 in mouse epidermis causes several skin abnormalities associated with reduced adhesion of the epidermis to the dermis. Wound closure is accelerated in α3-null skin due to increased migration but not proliferation To investigate the role of integrin α3b1 in wound healing, 2 full-thickness excision wounds were inflicted on either side of the dorsal midline in 4-months old Itga3 flox/flox and Itga3 flox/flox ; K14-Cre mice. Re-epithelialization of such wounds occurs by migration and hyperproliferation of keratinocytes from outside the wound bed over the dermis and granulation tissue (Figure 3A). In the tip of the advancing epidermis, integrin α3β1 is strongly upregulated and Ln-332 is deposited, whereas deposition of other ECM proteins such as Ln-511, Collagen (Col)-IV, and Nidogen (Nd) occurs behind the tip, to restore the damaged BM (Figure S2A). Upregulation and distribution of b1 in the epidermal tip appeared normal in Itga3 flox/flox ; K14-Cre mice, suggesting that another α-subunit binds β1 Figure 2 Deletion of integrin a3 in the epidermis does not inhibit keratinocyte differentiation. Skin cryosections from neonatal Itga3 flox/flox and Itga3 flox/flox ; K14-Cre mice were stained with antibodies directed against keratin- 14, filaggrin, or involucrin, and counterstained with Ln-332 and DAPI to visualize the BM and nuclei, respectively. Bar, 50 mm. 110
113 3 Figure 3 Wound closure is accelerated in the absence of α3b1. Full-thickness wounds were generated on either side of the dorsal midline in Itga3 flox/flox or Itga3 flox/flox ; K14-Cre mice, and excised 3 or 7 days after injury. (A) Schematic representation of a wound. D, dermis; E, epidermis; Es, eschar; F, fat tissue; G, granulation tissue. (B) H/E stainings depicting wound closure 7 days after wounding in Itga3 flox/flox (top) and Itga3 flox/flox ; K14-Cre mice (bottom). Arrows mark the edges of the migrating epidermis. The number of closed wounds after 7 days of migration is represented in the bar graph. Depicted are the means ± SEM of at least 40 wounds pooled from 4 independent experiments (* p < 0.05). Bar, 500 mm. (C) The distance covered by the migrating epidermis (indicated by the dotted line) was quantified 3 days after wounding in Itga3 flox/flox (top) and Itga3 flox/flox ; K14-Cre mice (bottom). The graph indicates the means ± SEM of at least 35 wounds pooled from 3 experiments (* p < 0.05). Bar, 250 mm. (D) Proliferation in the re-epithelializing wounds was assessed by injecting BrdU 2 or 4 days after wounding, and determining the ratio of BrdU(+) cells over the total number of cells using ImageJ. Depicted are the means ± SEM of at least 30 images. Bar, 150 mm. Integrin α3β1 Inhibits Wound Healing 111
114 in the absence of a3 in the leading keratinocytes (Figure S2B). Furthermore, the localization of integrins a5, a6, and β4 as well as the BM proteins Col-IV, Ln-511, Nd, and Ln-332 was comparable to that in Itga3 flox/flox mice, suggesting that the distribution of other integrins and the deposition of BM proteins are not affected by the deletion of α3 (Figure S2B). We next determined the rate of wound closure on H/E stained paraffin sections. Wound healing was not impaired in Itga3 flox/flox ; K14-Cre mice but was in fact accelerated compared to that in Itga3 flox/ flox skin, as determined both by the number of wounds that had completely closed after a week, or by the extent of re-epithelialization at earlier time points (Figures 3B,C). To assess whether this was due to increased proliferation, BrdU was injected intraperitoneally at several timepoints after wounding, and skin sections were stained to detect BrdU incorporation. There was no significant difference in the number of proliferating cells in the advancing epidermis of Itga3 flox/flox versus Itga3 flox/flox ; K14-Cre mice, indicating that α3 does not affect proliferation during wound healing (Figure 3D). Taken together, these results suggest that deletion of integrin α3 in skin promotes wound healing by accelerating keratinocyte migration. In vitro adhesion to Ln-332 is mediated by α6 integrins in the absence of α3 To confirm the migration data in vitro and to investigate the underlying mechanism, we isolated keratinocytes from newborn Itga3 flox/flox mice and cultured them on Col-I (3 μg/ml). Several spontaneously immortalized clones were obtained, which we named mouse keratinocytes (MK)a3(+). The clones were unable to grow in Ca 2+ -rich medium and did not give rise to tumors when injected subcutaneously into nude mice (10 7 cells/ injection, 8 injections in 2 independent experiments), indicating that they are not transformed. To generate α3-null cells MKa3(-), α3 was efficiently deleted by adenoviral delivery of Cre-recombinase (Figures 4A, S3A), thus the α3-knockout cells were derived directly from the same clones. There was no significant difference in proliferation rates between MKα3(+) and MKα3(-) cells (Figure S3B). Moreover, both MKα3(+) and MKα3(-) cells responded similarly to transfer to Ca 2+ -rich medium, by initiating the formation of cell-cell contacts such as adherens junctions and tight junctions, as suggested by zonula occludens (ZO)-1, occludin, b-catenin, and E-cadherin stainings (Figure S3C), and the assembly of HD-like structures (Figure S3D). These results are in line with the in vivo observations and suggest that the integrin α3β1 is not per se required for the formation of cell-cell contacts, HD assembly, or proliferation. To determine whether a3 deletion affected the expression of other integrins, we determined integrin cell surface levels by flow cytometry. Expression of β1 on the cell surface was downregulated in the knockout cells whereas the expression of α5, αv, α2, α6, and β4 was not significantly different (Figure 4B). To investigate whether cell adhesion was compromised, we performed adhesion assays on Col-1 or Ln-332. Adhesion of MKa3(-) cells to Ln-332 was slightly reduced (~70% of control cells) but decreased dramatically in the presence of the a6-blocking antibody GoH3, indicating that adhesion 112
115 3 Figure 4 In vitro adhesion to Ln-332 is rescued by α6 integrins in the absence of a3b1. Keratinocytes were isolated from newborn Itga3 flox/flox mice and designated MKα3(+). MKα3(-) cells were then generated by in vitro deletion of Itga3. (A) Immunoblot depicting the expression of α3 in MKα3(+) and MKα3(-) cells. Murine mammary cell line Rac-11P and murine fibroblast cell line NIH3T3 were included as a positive and negative control, respectively. (B) Cell surface expression of integrin subunits α5, α2, α6, αv, β4 and β1 in MKα3(+) and MKα3(-) was determined by FACS analysis. Negative control (only secondary antibody) is indicated by the black line. (C) MKa3(+) and MKa3(-) cells were seeded onto Col-1 or Ln-332 in K-SFM without supplements in the absence or the presence of a6-blocking antibody GoH3 (10 μg/ml). After 30 min, non-adherent cells were washed away. Values shown represent the average percentages of adherent cells from 3 experiments performed in triplicate (* p < 0.05, *** p < ). (D) Immunoprecipitations of integrin subunits α6 and β1 were performed on lysates of MKα3(+) and MKα3(-) cells. After fractionation by SDS-PAGE, Western blots were analyzed for integrins β1 and β4, and the light chains (L) of α3 and α6. to Ln-332 in the absence of a3 is mediated by a6 integrins (Figure 4C). Adhesion to Col-1 was similar for MKa3(+) and MKa3(-) cells, and was not blocked by GoH3 in either cell line, as expected (Figure 4C). Immunoprecipitation experiments revealed increased association of a6 with b1 in the knockout cells, whereas a6b1 levels in MKα3(+) cells were hardly detectable (Figure 4D). Together, these data show that keratinocyte adhesion to Ln-332 is rescued by a6 integrins, and that surface expression of integrin a6b1 is upregulated in the absence of α3. Cell spreading on endogenous Ln-332 is mediated by α6β1 in the absence of α3 Next, we analyzed whether cell spreading was affected by the deletion of a3. Consistent with the adhesion data, cell spreading on Ln-332 was slightly reduced in MKα3(-) cells compared to control cells, Integrin α3β1 Inhibits Wound Healing 113
116 and addition of the α6-blocking antibody GoH3 decreased cell spreading significantly in the MKa3(-) but not MKa3(+) cells (Figure 5A). Surprisingly, the same results were obtained on Col-I, suggesting that cell spreading of MKα3(-) cells on Col-I is also mediated by α6 integrins. We assume that Col-I is used for initial adhesion and spreading but that spreading on the patch of Ln-332 that is deposited over time underneath the cell is maintained by Ln-332-binding integrins, i.e. α3β1 in the control cells and α6β1 in the absence of α3. To exclude that Ln-332 deposition itself was affected by the loss of a3, we next compared Ln-332 deposition by Western blotting and immunofluorescence. Ln-332 deposition by knockout cells was not impaired over time (Figure 6A), and the pattern of deposition was similar to that of MKa3(+) cells (Figure 6B). However, trails of Ln-332 that were left behind by migrating MKα3(-) cells were strikingly longer, suggesting increased motility and migration in the absence of a3 (Figure 6C). To verify that the observed effects Figure 5 Integrin α6β1 mediates cell spreading on endogenous Ln-332 in the absence of α3b1. MKa3(+) and MKa3(-) cells were seeded on Ln-332 (A) or Col-1 (B), allowed to spread, and then incubated with α6-blocking antibody GoH3 (10 μg/ml). After 3 hrs, the number of spread cells was scored and expressed as a percentage of the total number of cells. In each independent experiment, approximately 500 cells were counted per condition. The graphs depict the averages of 3 experiments (* p < 0.05, *** p < ). Bar, 50 μm. 114
117 are not due to adaptation in culture, we also performed adhesion and cell spreading assays in primary, non-immortalized keratinocytes isolated from neonatal Itga3 flox/flox and Itga3 flox/flox ; K14-Cre mice. These assays yielded essentially the same results (Figure S4). In conclusion, these data show that the deletion of a3 in keratinocytes does not affect the deposition of endogenous Ln-332, and that cell spreading over endogenous Ln-332 is maintained by upregulation of integrin a6b1. Deletion of α3 enhances velocity and directionality of keratinocyte migration We next mimicked wound healing in an in vitro assay. Upon wounding, quiescent keratinocytes become activated by the release of growth factors such as epidermal growth factor (EGF), inducing hyperproliferation and migration over exposed dermal collagens. Therefore, MKα3(+) and MKα3(-) cells were grown to confluency on Col-I, deprived of supplements and growth factors overnight, and then scratched with the tip of a pipette, after which they were allowed to migrate into the artificial wound in the presence of EGF (5 ng/ml). Proliferation was inhibited with mitomycin C. Consistent with the in vivo observations, the MKα3(-) cells migrated significantly faster into the denuded area than the MKa3(+) cells (Figure 7A). This was probably not due to altered growth factor signaling, as stimulation with EGF after growth factor depletion induced similar activation of the ERK pathway (Figure 7B). To rule 3 Figure 6 Ln-332 synthesis and deposition are not affected by the deletion of integrin α3. (A) MKα3(+) and MKα3(-) cells were seeded on Col-I and detached with EDTA at the indicated time points thereafter. The ECM was then dissolved in sample buffer, subjected to SDS-PAGE, and the g2 chain of Ln-332 was detected by Western blotting. (B) Immunofluorescence images demonstrating patches of deposited Ln-332 in spread MKα3(+) cells (left panel) and MKα3(-) cells (right panel). Bar, 10 μm. (C) Lowmagnification immunofluorescence images demonstrating Ln-332 trails left behind by MKα3(+) cells (left), and MKα3(-) cells (right) migrating over Col-I. Bar, 10 μm. Integrin α3β1 Inhibits Wound Healing 115
118 116 Figure 7 Loss of α3 enhances directionality and velocity of keratinocyte migration. (A) Confluent MKα3(+) and MKα3(-) were deprived of growth factors, incubated for 2 hrs with mitomycin C (10 mg/ml), and scratched with the tip of a pipette prior to EGF stimulation. The black bars indicate the wound edges at t = 0. Scale bar, 100 mm. Wound areas were determined using Image J, and the ratio of the wound area after overnight migration over the wound area at t = 0 was calculated and expressed in the bar graph. Values shown represent the means ± SEM of 3 independent experiments (*** p < ). (B) EGF-induced phosphorylation of ERK-1/2 was monitored by Western blotting in lysates of MKα3(+) and MKα3(-) cells, that were deprived of growth factors prior to EGF stimulation for the indicated time points. (C) Cells were sparsely seeded on Col-I and monitored in time-lapse recordings during 16 hrs. An image was captured every 5 min. Cell tracks were then determined using ImageJ. The migration plots indicate tracks from 10 individual cells out of 4 independent experiments. To quantify average velocity and directionality (D/T ratio), data from 4 independent experiments were pooled. The graphs represent the means ± SEM from ~50 cells (* p < 0.05, ** p < 0.005, *** p < ). To determine stable polarization, cells were sparsely seeded on Col-I and monitored by time-lapse recordings. Images were captured every 5 min. Cells were considered stably polarized when maintaining a leading lamellipodium during 1 hr. The number of polarized cells was counted and expressed as a percentage of the total number of cells. The graph shows the means ± SEM from 250 cells pooled from 4 independent experiments (* p < 0.05). (D) Cells were sparsely seeded on Ln-332 or Col-I, serum-starved, and then stimulated with 5 ng/ml EGF at the indicated time points. An image was captured every 5 min. Cell tracks were determined using Matlab (Mathworks), and average velocity was plotted over time. The graphs depict the average velocity of ~50 cells from a representative experiment.
119 out that the observed effects were due to differences in cell-cell adhesion, we next assessed single-cell migration on Col-I by time-lapse video microscopy. Consistent with the observed differences in length of the Ln-332 trails (Figure 6C), the migration tracks of MKα3(-) cells were clearly longer than those of MKα3(+) cells within the same time-frames (Figure 7C). Quantification of the average velocity confirmed that MKα3(-) cells migrated faster (~80 mm/h vs ~60 mm/h). To analyze the persistence of migration, the direct distance from start to end point (D) was divided by the total track distance (T). The resulting D/T ratio was almost 2-fold higher in a3(-) cells, indicating that not only migration speed but also the mode of migration is modulated by integrin a3: whereas MKa3(+) cells moved randomly and in a back-and-forth fashion, deletion of a3 stimulated cell migration in a more persistent manner (Figure 7C). In line with these observations, a significantly larger fraction of the MKα3(-) cells was stably polarized throughout time, as determined by the adoption of a fan-shaped morphology in time-lapse movies (Figure 7C). The effect of EGF was also investigated in single-cell migration assays. The EGF-induced increase in migration velocity followed comparable kinetics in the absence and the presence of a3. However, the average velocity of migration was always higher in MKa3(-) cells, which was observed both on Col-1 and on Ln-332 (Figure 7D). To verify that the observed effects were indeed due to the absence of α3, MKα3(-) cells were reconstituted with the gene encoding human a3a (ha3a) by retroviral transduction followed by FACS sorting, generating a cell line which we designated MKα3(R) (Figure 8A). Addition of GoH3 to MKα3(R) monolayers did not induce cell detachment, neither on Col-1 nor on Ln-332, which confirms that a6b1 was no longer required to maintain cell spreading as in a3(-) cells (Figures 8B,C). As expected, MKα3(R) cells migrated more slowly in scratch assays, although the rate of scratch closure was higher than in MKa3(+) cells, probably because the expression of ha3a was much lower than the expression of endogenous a3 (Figure 8D). Altogether, these data are consistent with the results of the in vivo wound healing assays, and confirm that the loss of a3 promotes keratinocyte migration. 3 DIscUssIon Here, we show that loss of a3b1 from the epidermis causes skin abnormalities, including microblistering at the DEJ, BM duplication, and progressive hair loss. Although the microblistering suggests that a3b1 is only a minor contributor to adhesion, fragile and inflamed skin areas were nevertheless frequently observed. We assume that mechanical trauma, e.g. as applied during cleansing, causes repeated dissociation of the epidermis from the dermis because of reduced adhesion strength. BM duplication is either a compensation mechanism, or a result of rupture within the plane of the BM in regions where blistering occurs. The relative mildness of the blisters is probably explained by rescue of adhesion by Integrin α3β1 Inhibits Wound Healing 117
120 118 Figure 8 The phenotype of MKa3(-) cells is reversed by reconstitution with human α3a. (A) MKα3(-) cells were transduced with the gene encoding for hα3a, and cell surface expression was verified by FACS analysis using monoclonal antiserum J143 against ha3a. Negative control (secondary antibody only) is indicated by the black line. (B) MKα3(R) cells were seeded on Ln-332 (left panel) or Col-I (right panel), allowed to spread, and then incubated for 3 hrs with GoH3 (10 mg/ml). Bar, 20 mm. (C) The number of spread cells was scored and expressed as a percentage of the total number of cells. In each independent experiment, approximately 500 cells per condition were counted. The graphs depict the averages of 3 experiments (*** p < ). (D) Lysates of MKα3(+), MKα3(-), and MKα3(R) cells were analyzed by SDS- PAGE, and expression of integrin a3 was determined by Western blotting using mab 29A3 recognizing both human and murine a3 (L = light chain). Confluent MKα3(+), MKα3(-), and MKα3(R) cells were deprived of growth factors, incubated for 2 hrs with mitomycin C (10 mg/ml), and scratched with the tip of a pipette prior to EGF stimulation. Wound areas were determined using Image J, and the ratio of the wound area after overnight migration over the wound area at t = 0 was calculated and expressed in a bar graph. Values shown represent the means ± SEM of 3 independent experiments (* p < 0.05, *** p < ).
121 a6-containing integrins, as HD assembly is normal in these mice. The progressive hair loss is reminiscent of mice deficient for Ln-511, a high-affinity ligand for a3b1 and the major Ln isoform expressed in hair follicles (31). Several abnormalities in hair follicle morphology have previously been described in the absence of a3, in transplantation studies grafting skin from newborn a3-deficient mice onto wild-type recipients (32). The observed phenotype was partly due to aberrant organization of the actin cytoskeleton. We are currently investigating the cause of hair loss in our system. b1 integrins are required for keratinocyte migration in vitro and during wound healing (19). Since deletion of a2 has no effect on wound re-epithelialization (20), we expected a3b1 to be essential for keratinocyte migration. Surprisingly, wounds heal faster in Itga3 flox/flox ; K14-Cre mice than in Itga3 flox/flox mice, suggesting an inhibitory effect of a3 on wound healing. Differences in the distribution of integrins during re-epithelialization were not detected, suggesting that the loss of a3 does not affect other integrins. A similar conclusion was reached previously when analyzing the skin of neonatal a3-null animals under steady-state conditions (28). This suggests that a3b1 inhibits or at least delays keratinocyte migration during wound healing. Possibly, the b1-integrin that drives epidermal migration is a5b1, avb1, or a combination of these. Integrins a5b1 and avb1 are de novo expressed upon wounding (33-35), and bind fibronectin (FN) that is deposited in the provisional matrix. Since deletion of a5 is embryonically lethal (36), mice with a specific deletion of a5 in the epidermis should shed light on this matter. To confirm our observations in vitro, we isolated keratinocytes from newborn Itga3 flox/flox mice and deleted Itga3, thus generating cells identical to those of the original clones except for the presence of a3. Although a role of a3b1 in the establishment of cell-cell contacts has been suggested (37,38), we found no obvious defects in the localization of tight junction or adherens junction proteins in knockout cells. Adhesion to Ln-332, but not to Col-1, was somewhat compromised whereas cell spreading was slightly reduced on both matrices. Both adhesion on Ln-322 and cell spreading over exogenous Ln-332 or Col-I were mediated by a6 integrins in the knockout cells. Increased surface expression of a6b1, which also binds Ln-332 (39), probably rescues cell spreading both on exogenous Ln-332 as well as on endogenous Ln-332 that is deposited on top of an exogenous substrate such as Col-I. Previously, a3b1 has been implicated in Ln-332 deposition via Tiam1-Rac signaling (40). From the results presented here, it is obvious that a3b1 is not necessary for Ln-332 deposition. Likely, there are more integrins that can create a platform for Rac activity and thus, in this case, for Ln-332 deposition. Indeed, Rac activity levels were the same in the presence or absence of a3 (data not shown). Whereas in vitro adhesion and cell spreading was rescued by increased surface expression of a6b1, we only detected very low amounts of a6b1 in vivo in the epidermis of both Itga3 flox/ flox ; K14-Cre and Itga3 flox/flox animals, and there was no upregulation in the absence of a3 (data not shown). Keratinocyte migration was investigated in more detail in vitro. Whereas MKa3(+) keratinocytes moved in a 3 Integrin α3β1 Inhibits Wound Healing 119
122 random fashion, mainly in circular and back and forth patterns, a3(-) cells migrated faster and with higher directional persistence. This is a direct consequence of the loss of a3b1, which would implicate that a3b1 under normal conditions retards keratinocyte migration, or an effect of the upregulation of a6b1, suggesting that a6b1 specifically enhances the velocity and directionality of migration. Alternatively, a6b4 could drive migration in the absence of a3, as several studies have implicated this integrin in migration (41). However, this is probably not the case, because 1) we did not observe a difference in cell surface levels of a6b4 in the absence or the presence of a3, 2) we did not detect a redistribution of a6b4 from HDs into migration-associated structures, and 3) the enhanced migration of the a3-null cells is not only observed on Ln-332 but also on Col-1, which is not a substrate for a6b4. The phenotype can probably also not be attributed to the upregulation of a6b1, since increased migration velocity was also reported in keratinocytes derived from a3-null mice, in which a6b1 was not upregulated (28,29). Similarly, inhibiting a3 function with blocking antibodies in human keratinocytes increased migration, without affecting the levels of other integrins (26,27). In addition, affinity of a3b1 for Ln-332 is higher than of a6b1 (39,42). This is reflected in the reduced adhesion and cell spreading of the MKa3(-) cells, and indicates that MKa3(+) cells are more tightly ligated to Ln-332 than knockout cells. Finally, enhanced migration is observed not only on Ln-332, but also on Col-1, which is not a substrate for a6b1. Integrin binding to deposits of endogenously produced Ln-332 can inhibit migration, but cannot drive migration on an exogenous substrate. This is underlined by the fact that Ln-332 is always observed in the rear of migrating cells, and not in the front. We therefore conclude that the enhanced motility is mainly a direct effect of the loss of inhibition by a3b1, which is supported by previous reports suggesting that a3b1 inhibits keratinocyte migration on FN, Col-I, or Col-IV (26-29), caused by ligation to endogenous Ln-332 deposits (27,43,44). Consistent with this idea is the hypermotility observed in keratinocytes isolated from an epidermolysis bullosa patient that do not express the g2 chain of Ln-332 (45), which is reversed by the restoration of Ln-332 expression (46). However, the exact effect of the a3b1-ln-332 interaction on keratinocyte migration is controversial, as promotion of migration by this interaction was also reported for normal human keratinocytes (24,25). Similarly, human keratinocytes defective in Ln-332 expression due to a deletion in the LAMB3 gene were found to migrate with decreased velocity and directional persistence (47). The apparent paradox is most likely explained by differences in the matrices used; on a matrix that consists exclusively of Ln-332, a3b1 supports polarization, cell spreading and migration (48), whereas on a different ligand or a complex matrix offering various ECM components, as in vivo when keratinocytes migrate over a provisionally formed matrix, migration is driven by other integrins while a3b1 maintains adhesion to endogenous Ln-332 deposits at the rear of the cell. Interaction with these deposits regulates cell polarization (49), and is crucial to maintain adhesion 120
123 during migration. This is illustrated by the observation that in the MKa3(-) cells, GoH3 induced detachment not only of sessile but also of migrating cells (data not shown). Thus, keratinocytes require both a motogenic factor at the cell front and adhesion to Ln-332 deposits at the rear in order to polarize and maintain adhesion during migration. MateRIals and MetHODS Generation of Itga3 conditional knockout mice A genomic fragment of 15 kb encompassing exons 1-3 of Itga3 was isolated from a Lambda-FixII SV129 library and subcloned into plasmid vector pbs-sk +. A single loxp site and a loxp-pgkneo r -PGKtkloxP (floxed neo/tk cassette) were inserted into HpaI and BamH1, respectively. The targeting construct (excised from the plasmid with NotI and SwaI) was electroporated into 129/Ola-derived embryonic stem cells. Colonies resistant to geneticin (G418) were screened for the desired homologous recombination by Southern blotting. The floxed neo-tk cassette was deleted by transient transfection of Creexpression plasmid pog231. A recombinant clone harboring the conditional Itga3 allele was injected into mouse C57BL/6 blastocysts, which were transferred to mothers of the same strain. The chimeric male offspring was mated with FVB/N females. Agouti coat-colored offspring was screened for presence of the conditional allele by genotyping tail DNA with primers P1 (GAACAACATCTGCCT- GGAGT) and P2 (GTATGACTTCT- GCCATGTAGC). Heterozygous mice were intercrossed and homozygous mice were used to generate animals that were transgenic for the K14-Cre recombinase and carried the conditional Itga3 alleles. The K14-Cre transgene was detected by PCR with primers K14-Cre3 (CGATG- CAACGAGTGATGAGGTTC) and -Cre5 (G CACGTTCACCG G CATCA AC). Removal of exon 1 by Cre-mediated recombination was confirmed by PCR using primers P1 and P3 (CAACAG- CACTGCTGTAGCA). All animal experiments were carried out with approval from the relevant institutional animal ethics committees. Establishment of keratinocyte cell lines and cell culture Isolation of primary keratinocytes from neonatal Itga3 flox/flox mice and Cre-mediated deletion of the Itga3 flox/flox allele was performed essentially as described previously (50). For all experiments, 3 clones were used with similar results. Throughout this manuscript, results obtained with 1 clone (K3) are presented. Retroviral delivery of the human α3a subunit cloned into plzrs-ms-ires-zeo/pbr vector was established according to previously described protocols (51). Cells were cultured at 37 C and 5% CO 2 in keratinocyte serum-free medium (K-SFM; Gibco BRL) supplemented with 50 μg/ml bovine pituitary extract, 5 ng/ml EGF, 100 U/ml penicillin and 100 U/ml streptomycin. To induce differentiation, keratinocytes were maintained for up to 48 hrs in DMEM (Gibco BRL) containing 10% FCS and 100 U/ml penicillin/streptomy- 3 Integrin α3β1 Inhibits Wound Healing 121
124 cin. Rac-11P cells (52) and NIH3T3 cells were cultured in DMEM with 10% FCS and antibiotics. Antibodies and other materials Mouse mabs used in this study were directed against: α-catenin, β-catenin, p120 catenin, and E-cadherin (Transduction laboratories, Lexington, KY), BrdU (Bu20a from DAKO Corp., Carpinteria, CA), integrin α3a (29A3) (53), human integrin a3a ( J143) (54), integrin b1 (U19, from U. Mayer, University of Manchester, Manchester, UK) and pan-actin (Chemicon international). Rat mabs were: GoH3 against α6 (55), 4G6 against Ln-511 from L. Sorokin (University of Muenster, Muenster, Germany), A against β4 from S.J. Kennel (Oak Ridge Laboratories, Oak Ridge, TN), and BMA5 against α5 and MB1.2 against β1, both from B.M.C. Chan (University of Ontario, Ontario, Canada). Hamster mabs against integrins α2 (HMα2) and αv (H9.2B8) were from PharMingen (San Diego, CA), and goat mab against integrin a3a was from R&D systems. Rabbit polyclonal antibodies were directed against ZO-1 and occludin (Zymed laboratories), BP180 (mo-nc16a) from L. Bruckner-Tuderman (University of Freiburg, Freiburg, Germany), Col-IV from E. Engvall (The Burnham Institute, La Jolla, CA), Ln-332 and Nd from T. Sasaki (Shriners Hospital for Children Research Center, Portland, OR), CD3 from Thermo Fisher Scientific Inc. (Fremont, California), and filaggrin, involucrin and keratin-5, -6, -10, and -14 from Covance Research Products Inc. Texas Red-, TRITC- and FITC-conjugated secondary antibodies, phalloidin and DAPI were from Molecular Probes (Eugene, OR), HRP-conjugated secondary antibodies were from Amersham, BrdU was from Sigma-Aldrich (Steinheim, Germany), and Col-I was from Vitrogen (Nutacon, Leimuiden, The Netherlands). Preparation of ecm matrices Culture dishes were coated with Col-1 (3 mg/ml) or 2% BSA for 30 min at 37 C. Ln-332-containing matrix was prepared by growing Rac-11P cells to confluency, prior to overnight detachment with 10 mm EDTA at 4 C. The plates were then washed twice with PBS, blocked with 2% BSA for 1 hr at 37 C, and washed twice with PBS before use. In vivo wound healing and proliferation experiments Wound healing experiments were conducted as previously described (40). Sections were photographed on an Axiovert S100 widefield system equipped with an Axiocam CCD camera (Zeiss). Wound closure was determined by counting the number of closed wounds 7 days after wounding, and expressing the number of closed wounds as a percentage of the total number of wounds. Alternatively, the length of the neo-epidermis was determined 3 days after wounding using ImageJ. The graphs depict the average values of approximately 40 wounds pooled from 3-4 independent experiments. To analyze proliferation, BrdU was injected intraperitoneally (50 mg/g body weight) at 2 or 4 days after wounding, 3 hrs before sacrifice. The number of BrdU(+) cells was quantified from sections using ImageJ and expressed as a percentage of the total number of cells. At least 30 sections per condition were analyzed. 122
125 Immunoprecipitations and Western blotting Immunoprecipitation of integrins was performed as described previously (51). Total cell lysates were prepared in SDS sample buffer, resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes (Millipore), and analyzed by Western blotting followed by ECL using the SuperSignal system (Pierce Chemical Co.). Ultrastructural analysis, immunofluorescence microscopy, and flow cytometry Electron microscopy on mouse skin and immunofluorescence labeling of cryosections and cells on coverslips were carried out as previously reported (50). Images were acquired at RT with a confocal Leica TCS NT or AOBS microscope using 20x (NA 0.7) dry, 40x (NA 1.25) oil, and 63x (NA 1.32) oil objectives (Leica) and AxioVision 4 software (Carl Zeiss Micro- Imaging, Inc.). Pictures were processed and cell debris was masked using Photoshop 7.0 and ImageJ. Flow cytometry and cell sorting were performed as previously described (51). Adhesion assays Subconfluent cells were trypsinized, resuspended in K-SFM with or without GoH3 (10 mg/ml), and then seeded in 96-well plates coated with BSA, Col-I, or Ln-332 at a density of 3x10 4 cells per well. After 30 min at 37 C, nonadherent cells were washed away with PBS. The adherent cells were fixed in 4% PFA, washed twice with H 2 O, stained for 10 min with crystal violet, washed twice with H 2 O, and then lysed in 2% SDS. Absorbance was measured at 490 nm on a microplate reader. Background values (binding to BSA) were subtracted from all other values, and the number of adherent MKa3(+) cells was set to 100%. Cell spreading assays Cells were seeded in K-SFM on 24-well plates coated with Col-I or Ln-332 and cell spreading was allowed for 5 hrs, after which the cells were maintained in the absence or presence of GoH3 (10 mg/ ml) for an additional 3 hrs. Cells were then photographed on a Widefield CCD system using 10x and 20x dry lens objectives (Carl Zeiss MicroImaging, Inc.) and images were processed using Photoshop 7.0. The number of spread cells was counted and expressed as a percentage of the total number of cells. Values shown represent the averages of 3 experiments. In each experiment, approximately 500 cells were analyzed for each condition. Single-cell migration, scratch assays, and polarization assays For scratch assays, cells were grown to confluency and starved overnight. Mitomycin C (Nycomed Inc., Breda, The Netherlands; 10 μg/ml) was added 2 hrs prior to scratching with a yellow pipette tip. After 2 washings with K-SFM, cells were stimulated with 5 ng/ml EGF. To analyze single-cell migration and polarization, cells were seeded sparsely on Col-I or Ln-332 in K-SFM with or without supplements, covered with mineral oil, and EGF (5 ng/ml) was added when appropriate at the indicated time-point after seeding. Phase/contrast images were captured every 5 min at 37 C and 5% CO 2 on a Widefield CCD system using a 10x dry lens objective (Carl Zeiss MicroImaging, Inc.). Images were processed using 3 Integrin α3β1 Inhibits Wound Healing 123
126 Photoshop 7.0, and migration tracks or scratch areas were analyzed using ImageJ or MatLab (Mathworks). Scratch closure is represented as the ratio of the wound area after overnight migration over the wound area at t = 0. Values shown represent the means ± SEM of 3 independent experiments. The number of polarized cells was counted from ~250 cells pooled from 4 independent experiments, and expressed as a percentage of the total number of cells. Cells were considered stably polarized when they maintained a leading lamellipodium for at least 1 hr. Average velocity and persistence of single-cell migration were calculated from ~50 cells per experiment using ImageJ or Matlab, and the graphs represent either the averages ± SEM pooled from 4 independent experiments, or the averages of 1 representative experiment as indicated. Statistical analysis Data were analyzed using a homoscedastic 2-tailed t test, and p<0.05 was considered statistically significant. ACKnoWleDGEMents We thank colleagues for their generous gifts of antibodies and other materials. We thank Lauran Oomen and Lenny Brocks for excellent technical assistance with microscopy, and Anita Pfauth and Frank van Diepen for expert technical assistance with FACS. Johan de Rooij is thanked for critical reading of the manuscript. ABBREVIatIons BM, basement membrane; BP, bullous pemphigoid; Col-I, collagen-i; Col-IV, collagen- IV; DEJ, dermal-epidermal junction; EGF, epidermal growth factor; FN, Fibronectin; HD, hemidesmosome; H/E, haematoxylin/eosin; K-SFM, keratinocyte serum-free medium; Ln-332, laminin-332; Ln-511, laminin-511; MK, mouse keratinocytes; Nd, nidogen; ZO-1, zonula occludens-1 RefeRences 1. Burgeson RE and AM Christiano (1997) The dermal-epidermal junction. Curr Opin Cell Biol 9, Watt FM (2002) Role of integrins in regulating epidermal adhesion, growth, and differentiation. EMBO J 21, Carter WG, et al (1991) Epiligrin, a new cell adhesion ligand for integrin α3β1 in epithelial basement membranes. Cell 65, Rousselle P, et al (1991) Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol 114, Aumailley M and P Rousselle (1999) Laminins of the dermo-epidermal junction. Matrix Biol 18, Carter WG, et al (1990) Distinct functions for integrins α3β1 in focal adhesions and α6β4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to HDs. J Cell Biol 111, DiPersio CM, et al (1995) α3aβ1 integrin localizes to focal contacts in response to 124
127 diverse extracellular matrix proteins. J Cell Sci 108, Borradori L and A Sonnenberg (1999) Structure and function of HDs: more than simple adhesion complexes. J Invest Dermatol 112, Dowling J, et al (1996) β4 integrin is required for HD formation, cell adhesion and cell survival. J Cell Biol 134, Georges-Labouesse E, et al (1996) Absence of integrin a6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet 13, Van der Neut R, et al (1996) Epithelial detachment due to absence of HDs in integrin b4 null mice. Nat Genet 13, Niessen CM, et al (1996) Deficiency of the integrin b4 subunit in junctional epidermolysis bullosa associated with pyloric atresia: consequences for HD formation and adhesion properties. J Cell Sci 109, Ashton GHS, et al (2001) a6b4 integrin abnormalities in junctional epidermolysis bullosa with pyloric atresia. Br J Dermatol 144, Castiglia D, et al (2001) Novel mutations in the LAMC2 gene in non-herlitz junctional epidermolysis bullosa: effects on laminin-5 assembly, secretion, and deposition. J Invest Dermatol 117, Fassler R, and M Meyer (1995) Consequences of lack of b1 integrin gene expression in mice. Genes Dev 9, Stephens LE, et al (1995) Deletion of b1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev 9, Raghavan S, et al (2000) Conditional ablation of β1 integrin in skin: severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol 150, Brakebusch C, et al (2000) Skin and hair follicle integrity is crucially dependent on b1 integrin expression in keratinocytes. EMBO J 19, Grose R, et al (2002) A crucial role of b1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 129, Chen J, et al (2002) The α2 integrin subunitdeficient mouse. Am J Pathol 161, Zweers MC, et al (2006) Integrin a2b1 is required for regulation of murine wound angiogenesis but is dispensable for reepithelialization. J Invest Dermatol 127, DiPersio CM, et al (1997) α3β1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol 137, Kreidberg JA, et al (1996). α3β1 integrin has a crucial role in kidney and lung organogenesis. Development 122, Zhang K, and RH Kramer (1996) Laminin-5 deposition promotes keratinocyte motility. Exp Cell Res 227, Nguyen BP, et al (2000) Deposition of laminin-5 in epidermal wounds regulates integrin signaling and adhesion. Curr Opin Cell Biol 12, Kim JP, et al (1992) Integrin receptors and RGD sequences in human keratinocyte migration: unique anti-migratory function of α3β1 epiligrin receptor. J Invest Dermatol 98, O Toole EA, et al (1997) Laminin-5 inhibits human keratinocyte migration. Exp Cell Res 233, Hodivala-Dilke KM, et al (1998) Novel roles for α3β1 integrin as a regulator of cytoskeletal assembly and as a transdominant inhibitor of integrin receptor function in mouse keratinocytes. J Cell Biol 142, DeHart GW, et al (2003) The role of α3β1 integrin in determining the supramolecular organization of laminin-5 in the extracellular matrix of keratinocytes. Exp Cell Res 283, Sachs N, et al (2006) Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol 175, Li J, et al (2003) Laminin-10 is crucial for hair follicle morphogenesis. EMBO J 22, Conti FJA, et al (2003) a3b1 integrin regulates hair follicle but not interfollicular morphogenesis in adult epidermis. J Cell Sci 116, Cavani A, et al (1993) Distinctive integrin expression in the newly forming epidermis during wound healing in humans. J Invest Dermatol 101, Juhasz I, et al (1993) Regulation of extracellular matrix proteins and integrin cell substratum adhesion receptors on epithelium during cutaneous human wound healing in vivo. Am J Pathol 143, Integrin α3β1 Inhibits Wound Healing 125
128 35. Larjava H, et al (1993) Expression of integrins and basement membrane components by wound keratinocytes. J Clin Invest 92, Yang JT, et al (1993) Embryonic mesodermal defects in a5 integrin-deficient mice. Development 119, Carter WG, et al (1990) The role of integrins a2b1 and a3b1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol 110, Wang Z, et al (1999) a3b1 integrin regulates epithelial cytoskeletal organization. J Cell Sci 112, Delwel GO, et al (1994) Distinct and overlapping ligand specificities of the α3aβ1 and α6aβ1 integrins: recognition of laminin isoforms. Mol Biol Cell 5, Hamelers IH, et al (2005) The Rac activator Tiam1 is required for α3β1-mediated laminin-5 deposition, cell spreading, and cell migration. J Cell Biol 171, Margadant C, et al (2008) Regulation of HD disassembly by growth factor receptors. Curr Opin Cell Biol 20, Kikkawa Y, et al (2000) Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by a3b1, a6b1, and a6b4 integrins. J Cell Sci 113, Decline F, and P Rouselle (2000) Keratinocyte migration requires α2β1 integrin-mediated interaction with the laminin-5 γ2 chain. J Cell Sci 114, Decline F, et al (2003) Keratinocyte motility induced by TGF-b1 is accompanied by dramatic changes in cellular interactions with laminin-5. Cell Motil Cytoskeleton 54, Miquel C, et al (1996) Establishment and characterization of a cell line LSV5 that retains the altered adhesive properties of human junctional epidermolysis bullosa keratinocytes. Exp Cell Res 224, Gagnoux-Palacios L, et al (1996) Functional re-expression of laminin-5 in laminin-γ-2- deficient human keratinocytes modifies cell morphology, motility, and adhesion. J Biol Chem 271, Hartwig B, et al (2007) Laminin-5- deficient human keratinocytes: defective adhesion results in a saltatory and inefficient mode of migration. Exp Cell Res 313, Choma DP, et al (2004) Integrin α3β1 directs the stabilization of a polarized lamellipodium in epithelial cells through activation of Rac1. J Cell Sci 117, Frank DE, and WG Carter (2004) Laminin-5 deposition regulates keratinocyte polarization and persistent migration. J Cell Sci 117, Raymond K, et al (2005) Keratinocytes display normal proliferation, survival and differentiation in conditional b4-integrin knockout mice. J Cell Sci 118, Sterk LM, et al (2000) The tetraspan molecule CD151, a novel constituent of hemidesmomes, associates with the integrin a6b4 and may regulate the spatial organization of HDs. J Cell Biol 149, Sonnenberg A, et al (1993) Formation of HDs in cells of a transformed murine mammary tumor cell line and mechanisms involved in adherence of these cells to laminin and kalinin. J Cell Sci 106, de Melker AA, et al (1997) The A and B variants of the α3 integrin subunit: tissue distribution and functional characterization. Lab Invest 76, Kantor RR, et al (1987) Biochemical analysis of two cell surface glycoprotein complexes, very common antigen 1 and very common antigen 2. Relationship to very late activation T cell antigens. J Biol Chem 262, Sonnenberg A, et al (1988) Laminin receptor on platelets is the VLA-6 integrin. Nature 336,
129
130
131 Mechanisms of integrin activation and trafficking Coert Margadant 1, Hanneke N. Monsuur 1, Jim C. Norman 2, and Arnoud Sonnenberg 1 1 Division of Cell Biology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands. 2 Beatson Institute for Cancer Research, Cancer Research UK, Glasgow, G61 3BX, Scotland. Curr Opin Cell Biol 23, (2011)
132
133 ABSTRAct Integrin adhesion receptors are essential for the normal function of most multicellular organisms, and defective integrin activation or -signaling is associated with an array of pathological conditions. Integrins are regulated by conformational changes, clustering, and trafficking, and regulatory mechanisms differ strongly between individual integrins and between cell types. Whereas integrins in circulating blood cells are activated by an inside-out-induced conformational change which favors high-affinity ligand binding, β1-integrins in adherent cells can be activated by force or clustering. In addition, endocytosis and recycling play an important role in the regulation of integrin-turnover and -redistribution in adherent cells, especially during dynamic processes such as cell migration and invasion. Integrin trafficking is strongly regulated by their cytoplasmic tails, and the mechanisms are now being identified. IntRODUctIon Integrins are heterodimeric αβ transmembrane receptors that connect the extracellular matrix (ECM) to the cytoskeleton. In mammals, 18 α- and eight β-subunits assemble into 24 different integrins, which bind collagens, laminins, or proteins containing an Arg-Gly-Asp (RGD) sequence. In addition, several integrins bind soluble ligands or cellular receptors (Figure 1). Many integrins are known to adopt low-, intermediate-, and high-affinity conformations, and these exist in a dynamic equilibrium with one another. An increase in the proportion of heterodimers adopting high-affinity conformations is termed integrin activation, and can be induced either by cytoplasmic events ( inside-out activation; Figure 2A), or by extracellular factors ( outside-in activation). Ligand-binding triggers integrin clustering (avidity), integrin connection to the cytoskeleton, and the formation of macromolecular adhesion complexes (Figure 2A). Moreover, integrin-ligand interactions induce a plethora of outside-in events such as cell spreading and migration, ECM assembly, and the activation of several signal transduction pathways that regulate cell proliferation, survival, and gene expression (1). Most integrins engage the actin cytoskeleton, and a range of integrin-containing actin-associated adhesive structures has been described, including focal complexes, focal adhesions (FAs), fibrillar adhesions (FBs), podosomes, and invadopodia (2) (Figure 2B). In contrast, integrin α6β4 connects to the intermediate filament system, and localizes to hemidesmosomes (3). The relatively short α- and β-cytoplasmic tails (13-70 amino acids, except for β4) contain docking sites for a variety of proteins that control integrin activation, recruitment to adhesion sites, and trafficking. Here, we discuss recent advances in our understanding of how these processes are regulated by integrin cytoplasmic tails, with emphasis on differences between adherent and non-adherent cells, and between individual integrins. 4 Integrin activation and trafficking 131
134 Figure 1 Mammalian integrins and their ligands. Ligands for leukocyte integrins include E-cadherin, fibrinogen, factor X, intercellular adhesion molecule, inactive complement factor 3b, vascular cell adhesion molecule, and von Willebrand Factor. RGD-containing ligands include bone sialoprotein, fibronectin, latency-associated-peptide of transforming growth factor-β1/3, nephronectin, osteopontin, thrombospondin, and vitronectin. InteGRIN activation MecHanISMS Integrin activation in non-adherent cells such as leukocytes and platelets is rapid, reversible, and tightly-controlled, and this process is best-exemplified by the rapid enhancement of ligand-binding capacity of integrin αiibβ3 following platelet activation with agonists such as thrombin. In resting platelets, the bent, low-affinity conformation of αiibβ3 is stabilized by a clasp formed between the GFFKR sequence in αiib and the HDRxE motif in β3, most importantly a salt bridge between R995 and D723 (Figure 3) (4), and this mechanism is thought to help prevent inappropriate platelet aggregation which could lead to thrombosis. Activation of cytoplasmic signaling downstream of G-protein coupled receptors (such as the thrombin receptor) leads to disruption of the salt bridge, and the subsequent separation ( unclasping ) of the cytoplasmic tails triggers an allosteric change to favor the extended, high-affinity integrin conformation. The last step of this inside-out mechanism is the binding of the four-point-one/ezrin/radixin/moesin (FERM)-containing head-domain of talin to the membrane-proximal (MP)-NPxY motif and an additional MP region of the β3 cytotail (Figures 2A, 3) (4,5). Insideout activation also requires kindlin-3, a member of the kindlin family of proteins whose FERM domains bind the membrane-distal (MD)-NxxY motif in β-tails (6). Loss of talin or kindlin-3 from platelets prevents platelet adhesion and aggre- 132
135 4 Figure 2 Integrin activation and adhesion assembly. (A) In leukocytes and platelets, inside-out activation is triggered by a cytoplasmic chain-of-events that terminates in the binding of talin and kindlin-3 to the β-subunit, triggering a shift from the low-affinity conformation (bent with a closed head) toward the intermediate- (extended with closed head) and high- (extended with open head) affinity conformation. Ligand-binding induces outside-in events including talin-mediated connection to the actin cytoskeleton and cell spreading, integrin clustering, and activation of various signal transduction cascades. (B) Types of integrincontaining adhesion complexes: (1) Focal complexes (red arrows) are Rac-induced peripheral adhesions ( 1 µm) that stabilize membrane protrusions such as lamellipodia, whereas focal adhesions (yellow arrows) are Rhoinduced tensioned structures (1-5 µm) that represent major sites of signaling. Red, F-actin; green, phosphotyrosines; blue, nuclei. (2) Fibrillar adhesions (arrows) are central adhesions of 1-10 µm in length, contain integrin α5β1 and tensin but few tyrosine-phosphorylated proteins, and align with FN fibrils. Red, F-actin; green, tensin; blue, FN. (3) Podosomes are punctate structures with an actin core (indicated by white arrows), whereas hemidesmosomes are huge assemblies formed by integrin α6β4. Red, F-actin; green, β4. gation in mice, and kindlin-3-deficient leukocytes fail to adhere to endothelial cells (7-10). The phenotypes of talin- or kindlin-3-knockout mice resemble those of the human disorders Glanzmann s thrombasthenia (haemorrhages due to defects in αiibβ3 on platelets), leukocyte adhesion deficiency syndrome-i (LAD-I; immunodeficiency due to defects in β2-integrins on leukocytes), and LAD-III (immunodeficiency and haemorrhages due to combined defects in function of β1-, β2-, and β3-integrins). Indeed, since the discovery of kindlin-3, mutations in its gene have been disclosed in several LAD-III patients (11). Tyrosine-phosphorylation of the NPxY/NxxY motifs modulates the affinity of integrin-binding proteins in vitro and has been proposed to act as a molecular switch to inhibit talin-binding and allow for the binding of tensin or Dok-1, a negative regu- Integrin activation and trafficking 133
136 lator of integrin activation (12). The motifs are phosphorylated in β3 after ligandbinding, and prevention of this event by Y>F substitutions causes defects in platelet aggregation and a bleeding defect in mice (13). However, Y>F mutations in the NPxY motifs of β1 in mice do not cause any abnormalities whereas Y>A mutations lead to a complete loss-of-function, suggesting that in β1-integrins, structural integrity of the NPxY motifs is required but tyrosinephosphorylation is dispensable. Furthermore, mice carrying a D759A substitution in β1 (which prevents formation of a salt bridge) are normal, suggesting that also the salt bridge is not essential for β1-integrin function in vivo (14,15). As integrins in adherent cells need generally to be in an on - rather than off -state, the bent conformation is less likely to predominate in these cells. Nevertheless, the turnover of adhesion complexes that occurs for example during cell migration, requires dynamic regulation of integrin-ligand binding. Conformational changes in α5β1 from the bent to the extended conformation have been observed in FAs, but the mechanism that triggers the unbending remains to be elucidated (16). Probably, integrin activation in adherent cells occurs by an outside-in mechanism (e.g. the high concentration of available ligand in the ECM), by regulation of avidity (integrin clustering), or by force. For example, α5β1 can be activated by cytoskeletal tension generated by myosin-ii, in response to a stiff ECM. Under low tension, α5β1 forms relaxed bonds to the RGD-site of fibronectin (FN), whereas under high tension, α5β1 binds both the RGD and the synergy site, leading to stronger, tensioned bonds (17). The lifetime of a5b1-fn bonds is thus increased by tensile force (18). Interestingly, neither force nor high avidity seems to influence talin-induced molecular extension of αiibβ3 (19). This indicates that talin-regulated αiibβ3 activation may solely occur through conformational changes, whereas integrin-mediated adhesion in adherent cells may depend principally on talin-dependent increases in integrin avidity through the formation of antiparallel talin homodimers, and/or connection to the actin cytoskeleton via the talin rod-domain (Figure 2A). The latter is strongly suggested by intriguing observations in Drosophila; demonstrating that both talin- and integrin-null mutations cause wing blistering, however in the absence of talin, integrin-ligand binding is not impaired but integrins fail to connect to the cytoskeleton (20). Talin may also indirectly promote integrin function, by preventing the binding of the negative regulator filamin (21). Filamin competes with talin for binding of the β-tail, and its affinity is decreased by phosphorylation of the threonine/serine (T/S)-rich region that separates the two NPxY motifs (Figure 3) (22). T/S phosphorylation is thus thought to act as a molecular switch that increases talin-binding and integrin activation, and this is supported by observations that 1) T/S-phosphorylation occurs in β1, β2, β3, and β7 after stimulation with integrinactivating agonists, and 2) conservative and non-conservative mutations of the T/S residues in β3 or β1a decrease integrin activation and cell adhesion (4). The importance of T/S-phosphorylation in vivo remains to be determined. The kindlins are also important for integrin function in adherent cells. In humans, 134
137 4 Figure 3 Sequences of integrin cytoplasmic tails. Depicted are the sequences of the human β1a, β1d, β2, β3, β5, β6, and β7 subunits (β4 and β8 have been omitted because of poor sequence homology), and the human αiib, α2, and α5 subunits. Numbers indicate the first and last residues. The green box represents the clasp that maintains the low-affinity conformation, formed between the HDRxE region (with the D-residue essential for the salt bridge in red), and the GFFKR motif in the α-subunit. Yellow boxes indicate the membraneproximal (MP)-NPxY and membrane-distal (MD)-NxxY motifs, and the red box indicates the intervening T/S region. Shown are the binding sites for key regulators of integrin activation and trafficking including Arf-GAP with coiled-coil, ANK repeat and PH domain-containing protein1 (ACAP1), β3-endonexin, Disabled (Dab)-1/- 2, Dok-1, filamin, G-protein signaling GAIP-interacting protein COOH terminus (GIPC), integrin cytoplasmic domain associated protein-1α (ICAP-1α), kindlins, Numb, p120rasgap, protein kinase C (PKC), protein kinase D1 (PKD1), Rab21, Rab25, Src, talin, and tensin. The subunits to which binding has been demonstrated are indicated in brackets, and dashed lines indicate that the exact binding sites have not been mapped. kindlin-1 mutations cause Kindler syndrome, a skin-blistering disorder that resembles loss-of-function of α3β1 in the epidermis, and gene targeting of kindlin-1 and -2 compromises integrin-mediated adhesion in mice (23-25). However, the molecular mechanism remains elusive and requires further investigation. First, the effects of kindlins are cell-type-specific, as kindlin-3 overexpression enhances integrin activation in macrophages but not in CHO cells (9). Second, kindlins exert integrin-specific effects, because co-expression of kindlin-1 or kindlin-2 with talin results in activation of αiibβ3 but inhibition of α5β1 in CHO cells, and kindlin-2 regulates adhesion to vitronectin whereas kindlin-3 regulates adhesion to FN in endothelial cells (26,27). Third, kindlins can regulate integrins independent of activation, as cell adhesion and cell spreading were compromised in kindlin-1- deficient-keratinocytes, whereas integrin activation was not impaired (28). In summary, it is clear that mechanisms of activation are not generic for all Integrin activation and trafficking 135
138 integrins. Instead, different integrins are subject to different regulatory mechanisms, which have evolved to meet varying biological requirements. Such diversity is at least in part accomplished through sequence differences among β-tails, which can strongly influence the affinities for binding proteins (29). This is illustrated by the recently resolved structures of various β-tails in complex with talin-1 or talin-2; the tightest link is formed between β1d and talin-2, which are predominant in the myotendinous junctions of striated muscle, and has to withstand the forces of muscle contraction (30). InteGRIN CYtoPlasMIC MotIfs and THE REGUlatIon of InteGRIN TRAffICKING Over the past years, it has been firmly established that integrin trafficking in adherent cells is important for integrindependent cell adhesion, spreading and migration, as well as cancer cell invasion. Integrin trafficking regulates FA disassembly, matrix turnover, and localized integrin redistribution to new adhesion sites, for example at the leading edge in migrating cells (31). Trafficking mechanisms include the delivery of newly synthesized integrins via the biosynthetic-secretory pathway, integrin internalization, and recycling of internalized integrins. Early reports have suggested that talin-binding at the endoplasmic reticulum (ER) may regulate delivery of newly synthesized integrins to the membrane via myosin-mediated vesicular transport along actin filaments (32). Talin-binding exposes the GFFKR sequence which is a suggested ER-export signal, and mutations in this sequence cause reduced surface expression, as observed in some GT patients. It remains unclear how talinintegrin binding at the ER is regulated, and how this affects integrin conformation. Using conformation-specific antibodies, it was recently suggested that β1-integrins are in the bent conformation after association with α-subunits at the ER, and that they stay like this as they journey through the Golgi to the plasma membrane (33). Integrin internalization occurs through clathrin-dependent and clathrin-independent (via caveolae or macropinocytosis) mechanisms, and many integrins can follow more than one route into the cell. Caveolar internalization of α5β1 and FN regulates matrix turnover, and early reports have shown that also α2β1 is endocytosed in a caveolin-dependent manner, which may involve a direct association of protein kinase Cα (PKCα) with the β1-tail (34,35). Recently, it has become clear that αvβ3 can be internalized by macropinocytosis from dorsal ruffles that are induced by platelet-derived growth factor (PDGF) stimulation. The internalized integrins then transit through recycling endosomal compartments to repopulate newly formed FAs on the ventral surface (36). Clathrin-mediated integrin endocytosis is emerging as an important mechanism of adhesion disassembly during cell migration, and recent studies have identified many of the molecules involved in this process. Although there is an early study suggesting that αvβ5 binds clathrin directly, it is gener- 136
139 ally thought that most integrins require adaptor proteins to recruit them into clathrin-coated pits. Indeed, clathrin adaptors such as Disabled (Dab)-2 and adaptor protein-2 (AP-2) accumulate at or near FAs shortly before their disassembly. Furthermore, dynamin-dependent integrin internalization and transport along microtubules to Rab5-positive endosomes has been reported following manipulations that lead to synchronous disruption of FAs (37,38). Accordingly, depletion of clathrin or any of these adaptors leads to increased integrin surface expression and reduced migration (37-39). In addition to Dab-2, the clathrin adaptor Numb localizes in FAs at the leading edge of migrating cells to mediate integrin endocytosis and migration (40). Studies in Drosophila now indicate that clathrinand Rab5-dependent integrin turnover is also important in vivo, in particular for the maintenance of the myotendinous junction (41). NPxY motifs are canonical signals for clathrin-mediated endocytosis of plasma membrane receptors, and Numb and Dab-2 bind respectively to the MP-NPxY and the MD-NxxY of β-tails (29). Several observations suggest that mutations in these motifs impair integrin internalization. First, Y>A substitution in the MD-NxxY of β3a impairs the β3-endonexin-mediated internalization of ligand-bound integrins. Second, F>A substitutions in either one of the NPxF motifs of β2-integrins compromises their endocytosis. Third, Y>F mutations in both NPxY motifs of β1 reduces clathrindependent integrin endocytosis in fibroblasts (42). The latter result is surprising as β1 YY/FF mice are apparently normal, and it suggests that another endocytic mechanism can compensate for loss of clathrindependent internalization of β1-integrins in vivo (14,15). Indeed, endocytosis of β1 YY/FF can be rescued by overexpression of Rab21 and this is most likely via the upregulation a clathrin-independent pathway (42). Despite these advances, it remains unclear how the individual NPxY motifs regulate integrin trafficking, and through which proteins. Furthermore, the interplay between internalization and activation requires further investigation. It has been suggested that upon microtubule-induced FA disassembly, active integrins endocytose more rapidly than inactive integrins (38). In addition, whereas some reports find Dab-2 enrichment in FAs, others suggest that Dab-2 is required for bulk endocytosis of inactive integrins, since it colocalizes with β1-integrins distributed over the entire cell surface (39). The possibility that internalization of active and inactive integrins is regulated by separate pathways is intriguing, but has so far only been substantiated for α5β1. Indeed, selective internalization of active α5β1 from FBs and vesicular intracellular transport along actin filaments is mediated by Neuropilin1- and GIPC-mediated connection to myosinvi, whereas endocytosis of inactive α5β1 occurs by Neuropilin1-independent mechanisms (43). Internalized integrins may be either returned to the plasma membrane or routed to lysosomes for degradation, and the decision to recycle or degrade internalized a5b1 is likely influenced by its ubiquitination. In migrating fibroblasts, the α5-tail is ubiquitinated upon FN binding, and this is required to direct FN-a5b1 complexes to lysosomes. It was suggested that ubiquitination functions to prevent 4 Integrin activation and trafficking 137
140 endosomal accumulation of ligated integrins, which may interfere with cell signaling and migration (44). However, most internalized integrins are not degraded, but are returned to the plasma membrane, and a number of growth factors, kinases, and GTPases of the Rab and Arf families are now known to influence this process. For example, PDGF stimulates rapid, short-loop recycling of internalized αvβ3 from early endosomes (EEs), whereas α5β1 travels from EEs to the perinuclear recycling compartment (PNRC) and is then recycled via a longer loop to the plasma membrane (Figure 4) (45). Short-loop recycling depends on Rab4 and protein kinase D1 (PKD1), the latter of which binds directly to the β3-tail (Figure 3). By contrast, long-loop recycling requires Rab11, Arf6, and protein kinase B (PKB)/Akt (46,47). PKB phosphorylates the Arf-GAP with coiled-coil, ANK repeat and PH domain-containing protein1 (ACAP1), which then associates directly with the β1-tail and mediates recycling of β1-integrins from recycling endosomes (Figure 4) (47). Different routes of integrin recycling can exert differences in Rho GTPase signaling and directional migration (48). The α-tails of integrins are now known to recruit GTPases and other factors that control their trafficking. Rab21 is a Rab5- related GTPase which associates directly with a region of the α2- and α5-tails that is close to the GFFKR motif (49). Very recently, it has become clear that p120rasgap also binds to this portion of the α-tail, and does so in a way to replace Rab21, which then mediates integrin Figure 4 Integrin trafficking mechanisms regulate adhesion (dis-)assembly and migration. Integrin endocytosis is mediated by Rab5 and Rab21. From early endosomes, integrins are recycled to the membrane either via a short loop that involves Rab4, or via the perinuclear recycling compartment in a Rab11- or Rab25- dependent manner. Alternatively, they are delivered to late endosomes/lysosomes for degradation, which may require α-chain ubiquitination. Integrins can also be redistributed to dorsal ruffles after PDGF-stimulation, after which uptake occurs by macropinocytosis. They are then delivered to endosomes and exocytosed at the ventral surface. ACAP1, Arf-GAP with coiled-coil, ANK repeat and PH domain-containing protein1; EE, early endosome; MPS, macropinosome; PKB, protein kinase B; PKD1, protein kinase D1; RE, recycling endosome; Ub, ubiquitination. 138
141 recycling from EEs (Figure 4) (50). These results indicate that as integrins travel from one intracellular compartment to the other they are able to swap proteins that are associated with their tails in a way that supports their recycling. Possibly, other Rabs also bind directly to the α-tail, and it is conceivable that sequence differences in the region adjacent to the GFFKR are responsible for the integrin-specific effects of Rabs on individual heterodimers (31,42,45,48,49). Moreover, this might explain the long-standing observation that various integrins traffic with different kinetics depending on the α-subunit; whereas rapid and constitutive endocytosis occurs for α5β1 and α M β2, no or slow endocytosis is observed for α3β1, α4β1 and α L β2 (31). Interestingly, the Rabs contain phosphotyrosine-binding domains that recognize NPxY motifs, and can thus potentially also bind to β-tails. Indeed, Rab25 interacts with the β1-tail, and drives recycling of a5b1 to the tips of pseudopods in invading cancer cells (51). In conclusion, motifs in the cytotails of integrins are key to the regulation of integrin endocytosis and recycling via a number of pathways, and although many players in these processes have now been identified, major issues still need to be resolved. These include the function of the individual NPxY motifs in trafficking of various integrins, the role of the α-subunit, and the interplay between activation and internalization. 4 CONCLUSIons Our knowledge of the diverse mechanisms of integrin regulation has steadily increased over the years, and it is now clear that mechanisms of integrin activation and trafficking differ between different integrins and across cell types. Future work requires more focus on how integrin trafficking is regulated by the α-subunits and associated proteins, and by the two NPxY motifs in the b-subunits. Furthermore, the interplay between trafficking and activation merits further exploration, which will reveal how differential trafficking of active and inactive integrins is regulated, and via which proteins. ACKnoWleDGEMents We apologise to all authors whose work has been omitted due to space restrictions, and for not always citing primary literature. This study was funded by Cancer Research, UK ( J.C.N.) and a grant from the Dutch Cancer Society (A.S.). ABBREVIatIons ACAP1, Arf-GAP with coiled-coil, ANK repeat and PH domain-containing protein1; AP-2, adaptor protein-2; Dab, disabled; ECM, extracellular matrix; EE, early endosome; ER, endoplasmic reticulum; FA, focal adhesion; FB, fibrillar adhesion; FERM, four-point-one/ezrin/radixin/moesin; FN, fibronectin; GIPC, G-protein signaling Integrin activation and trafficking 139
142 GAIP-interacting protein COOH terminus; GT, Glanzmann s thrombasthenia; ICAP-1, integrin cytoplasmic domain-associated protein; LAD, leukocyte adhesion deficiency; MD, membrane-distal; MP, membrane-proximal; PDGF, platelet-derived growth factor; PKB, protein kinase B; PKC, protein kinase C; PKD1, protein kinase D1; PNRC, perinuclear recycling compartment; RGD, Arg-Gly-Asp RefeRences 1. Campbell ID, and MJ Humphries (2011) Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol 3, doi: /cshperspect.a Geiger B, and KM Yamada (2011) Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol 3, doi: /cshperspect.a Margadant C, et al (2008) Regulation of hemidesmosome disassembly by growth factor receptors. Curr Opin Cell Biol 20, Kim C, et al (2011) Regulation of integrin activation. Annu Rev Cell Dev Biol 27, Anthis NJ, et al (2009) The structure of an integrin/talin complex reveals the basis of inside-out signal transduction. EMBO J 28, Moser M, et al (2009) The tail of integrins, talin, and kindlins. Science 324, Nieswandt B, et al (2007) Loss of talin-1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J Exp Med 204, Petrich BG, et al (2007) Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med 204, Moser M, et al (2008) Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med 14, Moser M, et al (2009) Kindlin-3 is required for b2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med 15, Hogg N, et al (2011) The insider s guide to leukocyte integrin signalling and function. Nat Rev Immunol 11, Anthis NJ, et al (2009) b-integrin tyrosine phosphorylation is a conserved mechanism for regulating talin-induced integrin activation. J Biol Chem 284, Law DA, et al (1999) Integrin cytoplasmic tyrosine motif is required for outside-in aiibb3 signalling and platelet function. Nature 401, Chen H, et al (2006) In vivo b1 integrin function requires phosphorylationindependent regulation by cytoplasmic tyrosines. Genes Dev 20, Czuchra A, et al (2006) Genetic analysis of b1 integrin activation motifs in mice. J Cell Biol 174, Askari JA, et al (2010) Focal adhesions are sites of integrin extension. J Cell Biol 188, Friedland JC, et al (2009) Mechanically activated integrin switch controls a5b1 function. Science 323, Kong F, et al (2010) Demonstration of catch bonds between an integrin and its ligand. J Cell Biol 185, Ye F, et al (2010) Recreation of the terminal events in physiological integrin activation. J Cell Biol 188, Brown NH, et al (2002) Talin is essential for integrin function in Drosophila. Dev Cell 3, Nieves B, et al (2010) The NPIY motif in the integrin b1 tail dictates the requirement for talin-1 in outside-in signaling. J Cell Sci 123, Kiema T, et al (2006) The molecular basis of filamin binding to integrins and competition with talin. Mol Cell 21, Margadant C, et al (2009) Integrin a3b1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci 122, Margadant C, et al (2010) Unique and redundant functions of integrins in the epidermis. FASEB J 24, Meves A, et al (2009) The kindlin protein family: new members to the club of focal adhesion proteins. Trends Cell Biol 19,
143 26. Harburger DS, et al (2009) Kindlin-1 and -2 directly bind the C-terminal region of b integrin cytoplasmic tails and exert integrin-specific activation effects. J Biol Chem 284, Bialkowska K, et al (2010) The integrin co-activator kindlin-3 is expressed and functional in a non-hematopoietic cell, the endothelial cell. J Biol Chem 285, Ussar S, et al (2008) Loss of kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet 4, e Calderwood DA, et al (2003) Integrin b cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. PNAS 100, Anthis NJ, et al (2010) Structural diversity in integrin/talin interactions. Structure 18, Caswell PT, et al (2009) Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol 10, Martel V, et al (2000) Talin controls the exit of the integrin a5b1 from an early compartment of the secretory pathway. J Cell Sci 113, Tiwari S, et al (2011) Divalent cations regulate the folding and activation status of integrins during their intracellular trafficking. J Cell Sci Shi F, and J Sottile (2008) Caveolin-1- dependent b1 integrin endocytosis is a critical regulator of fibronectin turnover. J Cell Sci 121, Pellinen T, and J Ivaska (2006) Integrin traffic. J Cell Sci 119, Gu Z, et al (2011) Integrins traffic rapidly via circular dorsal ruffles and macropinocytosis during stimulated cell migration. J Cell Biol 193, Ezratty EJ, et al (2009) Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol 187, Chao W-T, and J Kunz (2009) Focal adhesion disassembly requires clathrindependent endocytosis of integrins. FEBS Lett 583, Teckchandani A, et al (2009) Quantitative proteomics identifies a Dab-2/integrin module regulating cell migration. J Cell Biol 186, Nishimura T, and K Kaibuchi (2007) Numb controls integrin endocytosis for directional cell migration with apkc and PAR-3. Dev Cell 13, Yuan L, et al (2010) Analysis of integrin turnover in fly myotendinous junctions. J Cell Sci 123, Pellinen T, et al (2008) Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev Cell 15, Valdembri D, et al (2009) Neuropilin-1/GIPC1 signaling regulates a5b1 integrin traffic and function in endothelial cells. PLoS Biol 7, e Lobert VH, et al (2010) Ubiquitination of a5b1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev Cell 19, Woods AJ, et al (2004) PKD1/PKCµ promotes avb3 integrin recycling and delivery to nascent focal adhesions. EMBO J 23, di Blasio L, et al (2010) Protein kinase D1 regulates VEGF-A-induced avb3 integrin trafficking and endothelial cell migration. Traffic 11, Li J, et al (2005) Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin β1 to control cell migration. Dev Cell 9, White DP, et al (2007) avb3 and a5b1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J Cell Biol 177, Pellinen T, et al (2006) Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of b1-integrins. J Cell Biol 173, Mai A, et al (2011) Competitive binding of Rab21 and p120rasgap to integrins regulates receptor traffic and migration. J Cell Biol 194, Caswell PT, et al (2007) Rab25 associates with a5b1 integrin to promote invasive migration in 3D microenvironments. Dev Cell 13, Integrin activation and trafficking 141
144
145 Kindlin-1 regulates integrin dynamics and adhesion turnover Coert Margadant 1, Maaike Kreft 1, Giovanna Zambruno 2, and Arnoud Sonnenberg 1 1 Division of Cell Biology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands. 2 Laboratory of Molecular and Cell Biology, IDI-IRCCS, Rome, Italy. Submitted for publication
146
147 ABSTRAct Loss-of-function mutations in the gene encoding the integrin co-activator kindlin-1 cause Kindler syndrome. We report a novel kindlin-1-deficient keratinocyte cell line derived from a Kindler syndrome patient. Despite the expression of kindlin-2, the patient s cells display several hallmarks related to reduced function of b1 integrins, including abnormal cell morphology, cell adhesion, cell spreading, focal adhesion assembly, and cell migration. Defective cell adhesion was aggravated by kindlin-2 depletion, indicating that kindlin-2 can compensate for the loss of kindlin-1 to a certain extent. In the epidermis, kindlin-1 and kindlin-2 are localized in different subcellular compartments, both in the patient and in an unaffected individual. Intriguingly, β1-integrins at the cell-surface were aberrantly glycosylated in the patient s cells, and β1 expression was considerably reduced, both in vitro and in the patient s epidermis. Reconstitution with wild-type kindlin-1 but not with a b1-binding defective mutant restored the aberrant b1 expression and glycosylation, and normalized cell morphology, adhesion, spreading, and migration. Furthermore, expression of wild-type kindlin-1, but not of the integrin-binding-defective mutant, increased the stability of integrin-mediated cell-matrix adhesions and enhanced the redistribution of internalized integrins to the cell surface. Thus, these data uncover a role for kindlin-1 in the regulation of integrin trafficking and adhesion turnover. IntRODUctIon 5 Integrins are ab heterodimeric transmembrane glycoproteins that link the extracellular matrix to the cytoskeleton. Integrinligand binding triggers the recruitment of a variety of adaptor, structural, and signalling proteins, and the formation of adhesion complexes such as focal adhesions (FAs) (1). Most integrins reside in focal adhesions and connect to the actin cytoskeleton, with the exception of integrin a6b4 which is localized in hemidesmosomes and connects to intermediate filaments (2). Many integrins can tune their affinity for ligand by conformational changes, and the switch from the low- to the high-affinity conformation is called integrin activation (3). Integrin activation is promoted by the binding of talin-1 or talin-2 and any of the 3 kindlin isoforms to the cytoplasmic tail of the b-subunit (3-5). The kindlins consist of an F0-F3 four-point-one/ezrin/ radixin/moesin (FERM) domain, that contains the integrin-binding site in F3, and a pleckstrin homology (PH) domain inserted into F2. Kindlin-1 is highly expressed in epithelia, in particular in the epidermis and the gastro-intestinal tract, and loss-of-function mutations in KIND1, the gene encoding kindlin-1, cause Kindler syndrome (KS), a congenital bullous disorder of the epidermolysis bullosa-family (6-8). KS is characterized by skin fragility and blistering, photosensitivity and poikiloderma, and some patients suffer from colitis (9-12). The defects result from reduced b1-integrin-mediated adhesion of the epidermis to the basement membrane (BM), and are reminiscent of the abnormalities in mice lacking the a3 or the b1 subunit in the epidermis, as well as of Kindlin-1 regulates integrin dynamics 145
148 patients carrying mutations in the ITGA3 gene encoding a3 (9,10,13-18). In vitro, keratinocytes isolated from KS patients or keratinocytes in which kindlin-1 expression is suppressed, display several abnormalities related to defects in b1 integrin function including reduced cell adhesion, cell spreading, and polarity (19-21). In this study, we describe a novel kindlin-1-deficient keratinocyte cell line derived from an Italian KS patient, which expresses kindlin-2 but not kindlin-1. We study the functional redundancy between the kindlins, and identify a role for kindlin-1 in the regulation of adhesion turnover and integrin trafficking. ResUlts and DIscUssIon Defects in b1 integrin function in Kindler syndrome cells that express kindlin-2 but not kindlin-1. We isolated kindlin-1-deficient keratinocytes from a previously described male KS patient from Italy (22). This patient is homozygous for the mutation c.1161dela within exon 10, which is predicted to cause a complete loss of expression due to nonsense-mediated RNA decay, or to cause the expression of a protein product that is truncated in the PH-domain (Figure 1A). We first investigated kindlin-1 protein expression by Western blotting, using an antibody directed against an epitope in the F1 domain (20). Full-length kindlin-1 was clearly detected at the expected size (~75 kda) in normal human keratinocytes (NHK), isolated from a healthy individual (23). In KS cells, a very faint band of the same size was detected but smaller proteins were not observed, suggesting that the c.1161dela mutation causes the near-complete loss of expression of full-length kindlin-1, whereas truncated protein products are not synthesized (Figure 1B). Kindlin-2 expression was detected in both NHK and KS keratinocytes (Figure 1B). The morphology of the KS cells was highly aberrant, as compared to that of NHK (Figure 1C). In addition, cell growth was poor and large numbers of dead cells were regularly observed (data not shown). To establish whether the observed abnormalities were due to defects in integrin-mediated adhesion, we analyzed adhesion to collagen (Col)-1 in adhesion assays. Adhesion of KS cells was indeed significantly impaired, and only a fraction of the cells that adhered was able to spread (Figures 1D,E). Consistent with reduced integrin-mediated adhesion, cell motility was significantly enhanced (Figures 1F,G). Finally, we analysed the organization of the actin cytoskeleton and the presence of FAs, using phalloidin and an antibody against phosphotyrosines P(Y). P(Y)-staining was generally weak in KS keratinocytes, and actin filaments appeared abnormal (Figure 1H). In contrast, the assembly of hemidesmosomelike structures upon transfer to Ca 2+ rich medium, and the synthesis and deposition of laminin (Ln)-332 in KS cells appeared to be normal (Figures S1A,B). In summary, we have isolated a kindlin-1-deficient keratinocyte cell line that displays defects in b1 integrin function, despite the presence of kindlin
149 Figure 1 Abnormalities in KS cells. (A) Schematic representation of the KIND1 gene (top), indicating the position of the mutation, and kindlin-1 protein (bottom). Exons are represented by boxes, introns are not to scale. (B) Western blot showing the expression of kindlin-1 and kindlin-2 in NHK and KS cells. (C) Phase/contrast images of NHK and KS cells. Bar, 20 mm. (D) Adhesion of NHK and KS cells to Col-1. (E) Cell spreading of NHK and KS cells on Col-1. (F) Rose-plots depicting migration tracks of NHK and KS cells. (G) Quantification of the velocity of cell migration (~250 cells). (H) Confocal images of FAs, visualized using an antibody against P(Y) (green), and F-actin (red). Bar, 10 mm. 5 Kindlin-1 and kindlin-2 are partially redundant, and b1 expression is decreased in kindlin-1-deficient keratinocytes and epidermis. We next investigated the cell-surface expression and activation status of b1-integrins in KS and NHK cells by flow cytometry. Interestingly, b1 cell-surface expression was significantly reduced, whereas the activation status, as judged by the ratio of 9EG7 staining over total b1 staining, was slightly (but not significantly) increased (Figure 2A). Decreased expression of b1 in KS cells was further confirmed by Western blotting (Figure 2B). We then analysed the expression of b1 in skin biopsies of the same patient. Ln-332 staining revealed BM abnormalities and detachment of keratinocytes in the patient s epidermis (Figure 2C; indicated by arrows), typical of KS (22,24), and the expression of b1 was strikingly decreased (Figure 2C). Thus, whereas there is a clear reduction in b1 expression, both in vivo and in vitro, integrin activation in the KS cells is not impaired. The latter finding is reminiscent of keratinocytes derived from the kindlin-1 knockout mice, in which integrin-mediated cell adhesion and cell spreading were compromised whereas Kindlin-1 regulates integrin dynamics 147
150 there was no significant reduction in integrin activation, due to the expression of kindlin-2 (25). We therefore introduced shrnas directed against kindlin-2 into KS cells by lentiviral transduction. Depletion of kindlin-2 caused complete detachment of KS cells (Figure 2D). Previous studies have reported both overlapping and distinct functions of kindlin-1 and kindlin-2 in keratinocytes (26,27). Our results are in line with these findings as kindlin-2 can apparently partially rescue cell adhesion in the absence of kindlin-1 in KS cells, but there are still considerable defects in cell adhesion and spreading. In vivo, kindlin-2 cannot completely compensate for the loss of kindlin-1, neither in the epidermis of KS patients, nor in the colon of kindlin- 1(-/-) mice (25,28), which is probably due to differences in subcellular localization (8). We therefore also investigated kindlin-2 distribution in vivo. Consistent with its expression in NHK and KS cells, kindlin-2 was detected both in the patient s epidermis and the epidermis of a normal individual. In basal keratino- Figure 2 Decreased integrin expression in the absence of kindlin-1. (A) FACS histograms and quantification (n=3) of NHK and KS cells showing cell-surface expression of b1 (left) and active b1 (right), as measured by 9EG7 staining. (B) Western blot showing the precursor (110 kda) and the mature form of b1 (130 kda) in NHK and KS cells. (C) Expression of b1 (green) and Ln-332 (red) in the skin of an unaffected individual (normal) and the KS patient. Outline indicates the upper border of the epidermis. Bar, 50 mm. (D) Depletion of kindlin-2 in KS cells causes complete cell detachment. Bar, 20 mm. (E) Expression of kindlin-1 (top) and kindlin-2 (bottom) in the skin. Bar, 50 mm. d; dermis, e; epidermis. 148
151 cytes kindlin-2 localization was exclusively lateral, while kindlin-1 distribution in normal epidermis was predominantly basal, in line with previous observations (Figure 2E) (8,28). Interestingly, kindlin-2 staining at the lateral membranes was weak and occasionally completely absent from the basal keratinocyte layer of the patient (Figure 2E; indicated by arrows), which most likely reflects defects in cellcell contacts, as described in Chapter 6. Together, these data show that b1 expression is reduced in KS cells and epidermis, and that kindlin-2 compensates only partially for reduced cell adhesion in the absence of kindlin-1. Stable re-expression of full-length kindlin-1 in KS cells restores the defects in integrin function. The previous sections have shown that in the absence of kindlin-1, integrin-dependent events are disturbed despite the presence of kindlin-2. To investigate whether the observed defects in KS cells are a direct consequence of the loss of kindlin- 1, kindlin-1 expression was restored in KS cells by retroviral delivery of egfp-conjugated kindlin-1 followed by FACS sorting, creating a stable cell line that we designated KSK. Expression of egfp-kindlin-1 was confirmed by Western blotting (Figure 3A). Re-expression of kindlin-1 reversed the aberrant morphology of KS cells, and significantly enhanced cell proliferation (Figures 3B,C). Kindlin-1 was diffusely distributed in the cytoplasm, while some enrichment in P(Y)-positive FAs was observed. In addition, a re-organization of the actin cytoskeleton into stress fibers and/or circumferential actin bundles was observed in KSK cells (Figure 3D). Reexpression of kindlin-1 clearly enhanced integrin-mediated cell adhesion, both to Col-1 and to a Ln-332-containing matrix derived from Rac-11P cells (Figure 3E). In addition, cell spreading on these substrates was significantly increased (Figures 3F,G) and the hypermotility observed in KS cells was reversed by re-introduction of kindlin-1 (Figures 3H,I). Finally, b1 cell-surface levels were increased in KSK cells with respect to KS, confirming that kindlin-1 enhances b1 expression (Figure 3J). This notion is consistent with several previous studies (29-31), and was further supported by the observation that overexpression of kindlin-1 also enhanced b1 cell-surface expression in NHK cells, which was accompanied by enhanced cell spreading (Figure S2). Together, these data show that restoration of kindlin-1 expression in KS cells rescues the defects in b1 integrin function. Regulation of b1 expression and function by kindlin-1 requires the F3 domain. We next investigated whether the effects of kindlin-1 depend on a direct interaction with the integrin b1-tail. The integrinbinding site in kindlin-1 resides in the C-terminal F3 domain, and a mutation that causes the expression of a protein lacking the F3 and part of the F2 has been identified in a KS patient, demonstrating the vital importance of this region (32). In addition, we have recently isolated a zebrafish mutant with KS-like epidermal defects, which expresses a truncated kindlin-1 protein lacking the F3 domain (described in Chapter 6). To delete the integrinbinding site, we truncated the F3 region after residue 581, and stably expressed 5 Kindlin-1 regulates integrin dynamics 149
152 Figure 3 Re-expression of kindlin-1 in KS cells. (A) Western blot showing expression of egfp-kindlin-1. (B) Morphology of KS and KSK cells. Bar, 20 mm. (C) Proliferation of KS and KSK cells. (D) egfp-kindlin-1 (green), FAs (blue) and F-actin (red) in KSK cells. Bar, 5 mm. (E) Cell adhesion to Col-1 and Ln-332 in KS and KSK cells. (F) Number of KS and KSK cells spread on Ln-332 and Col-1. (G) Surface area of KS and KSK cells on Ln-332 and Col-1. (H) Rose-plots depicting migration tracks of KS and KSK cells generated by time-lapse video microscopy. (I) Quantification of the velocity of cell migration (±300 cells). (J) FACS histograms of NHK and KS cells showing b1 cell-surface expression (left) and quantification (n=3) (right). egfp-kindlin-1 del581 into KS cells, creating a cell line designated KSK del581 (Figure 4A). Western blotting revealed a band of the expected size of ~95 kda in KSK del581 cells (Figure 4B). Intriguingly, increased expression of mature b1, but not precursor b1, was observed in KSK cells but not in KSK del581, suggesting that a direct interaction is required for the stimulation of b1 cell-surface expression by kindlin- 1. Furthermore, the mobility of mature b1 in gel electrophoresis was reduced in lysates of KS and KSK del581 with respect to KSK cells (Figure 4C). This was reversed after treatment with neuraminidase, which specifically removes monosaccharides called sialic acids, indicating that kindlin-1 regulates b1 sialylation, depending on the F3 domain (Figure 4D). Flow cytometry analysis confirmed that b1 cell-surface levels were increased in KSK, but not in KSK del581 cells (Figure 4E). Furthermore, the expression of egfp-kindlin-1 del581 did not promote cell spreading of KS cells (Figure 4F). Consistently, FAs seemed less pronounced in KSK del581 than in KSK cells, and the subcellular distribution of mutant kindlin-1 was different from that of fulllength kindlin-1; kindlin-1 del581 localization seemed predominantly cytoplasmic, with no clear enrichment in adhesions (Figure 4G). Similarly, kindlin-1 mutants 150
153 5 Figure 4 Regulation of b1 expression and cell spreading by kindlin-1 require the F3 domain. (A) Schematic representation of full-length kindlin-1 (top) and kindlin-1 del581 (bottom). (B) Expression of fulllength egfp-kindlin-1 and egfp-kindlin-1 del581 in KSK and KSK del581 cells. (C) Expression of precursor (110 kda) and mature b1 (130 kda) in KS, KSK, and KSK del581 cells. (D) Immunoprecipitated b1 was treated with neuraminidase and analyzed by Western blotting. (E) FACS histograms (left) and quantification (right) of b1 cell-surface expression in KS, KSK and KSK del581 cells. (F) Phase-contrast images of KS, KSK, and KSK del581 cells on Col-1 (left), and surface area of KS, KSK and KSK del581 cells (n ~250) (right). Bar, 10 mm. (G) Subcellular distribution of egfp-kindlin-1 and egfp-kindlin-1 del581 (green). FAs (blue), F-actin (red). Bar, 5 mm. carrying a Q611A or W612A mutation, that prevents interaction with integrins, are not targeted to FAs and do not increase cell-surface expression of a5b1, when overexpressed in fibroblasts (29). Together, these data suggest that a direct interaction between kindlin-1 and b1 is required for the targeting of kindlin-1 to cell-matrix adhesions, and for the effects of kindlin-1 on b1 cell-surface expression and glycosylation. Kindlin-1 targeting to adhesions and adhesion dynamics depend on the F3 domain. We next addressed the relationship between kindlin-1 targeting to adhesions and adhesion dynamics in living cells. To this end, we introduced the FA marker vinculin, fused to mcherry, into KS, KSK, and KSK del581 cells by lentiviral transduction. The dynamics of egfp-kindlin-1 and mcherry-vinculin were then monitored by total internal reflection (TIRF) Kindlin-1 regulates integrin dynamics 151
154 microscopy. Consistent with the results of the cell migration assays, KS cells were very motile and displayed many rapid cell shape changes. Imaging of vinculin revealed few FAs that had a high turnover rate (Figure 5A). In contrast, KSK cells were considerably more static, in line with the reduced velocity of migration, and their adhesions were much more stable than those in KS cells (Figure 5B). Interestingly, egfp-kindlin-1 was clearly enriched in adhesions, some of which were surprisingly large, but many of these clusters did not contain mcherry-vinculin, suggesting that kindlin-1 and vinculin can reside in distinct pools of adhesions. Furthermore, kindlin-1 was strongly concentrated in retraction fibers, consistent with the role of kindlin-1 in delaying cell migration (Figure 5B). In KSK del581 cells, we also observed a rapid turnover of mcherry-vinculin-containing adhesions, Figure 5 Kindlin-1 targeting to adhesions and adhesion stability depend on the F3 domain. (A) Stills from a TIRF movie, showing the dynamics of mcherry-vinculin in KS cells. (B) Dynamics of mcherry-vinculin (top), and egfp-kindlin-1 (bottom) in KSK cells. (C) Dynamics of mcherry-vinculin (top), and egfp-kindlin-1 (bottom) in KSK del581 cells. Look-up table fire was used to enhance visibility of adhesions. Shown are images at 0, 7.5, 15, 22.5, and 30 min. Boxed regions are enlarged. Bar, 10 mm. 152
155 as well as fast cell shape changes. Consistent with the images acquired by confocal microscopy, there was some diffuse localization of egfp-kindlin-1 del581 at the basal cell-surface, but clearly no enrichment in adhesions or retraction fibers (Figure 5C). Thus, kindlin-1 controls the dynamics of integrin-mediated cell-matrix adhesions, which is dependent on an intact F3 region. Kindlin-1 interaction with b1 regulates integrin traffic We next investigated whether integrin trafficking plays a role in the regulation of cell-matrix adhesion dynamics and b1 surface expression by kindlin-1. Integrins undergo continuous internalization, and the recycling of internalized integrins is important for integrin-mediated processes such as cell spreading (33,34). Internalization and recycling were determined according to a well-established protocol (35). First, we labeled cell-surface b1 with the antibody K20, conjugated to DyLight 649 (10 mg/ml), which clearly revealed localisation of b1 integrins at the membrane in KS, KSK, and KS cells (Figure 6, top). The cells were then transferred to serum-free medium at 37ºC, which allows internalization but not recycling of internalized integrins. The labeled cellsurface pool underwent internalization in all cell lines, with no apparent differences depending on kindlin-1 (Figure 6, middle panel). Recycling of internalized integrins was subsequently induced by stimulation with 20% FCS, which in KSK cells triggered the rapid return of b1 integrins to the plasma membrane and their delivery to peripheral adhesions (Figure 6, bottom). In contrast, redistribution of the internal integrin pool to the plasma membrane was not evident in KS cells or KS cells expressing truncated kindlin- 1, suggesting that kindlin-1 regulates the redistribution of internalized integrins, depending on the F3 domain. We did not detect kindlin-1 in vesicles, in line with similar observations for kindlin-2 (30,36). Therefore, kindlin-1 probably regulates integrin routing indirectly, i.e. by sorting integrins at the plasma membrane to a specific internalization and recycling pathway. This is conceivable as the kindlin-binding site in b1 is largely defined by the membrane-distal NPxY motif, which is also a canonical signal for clathrinmediated endocytosis. In summary, the results presented here suggest that kindlin-1 regulates the redistribution of internalized integrins, which requires a direct kindlin-integrin interaction. 5 MateRIals and MetHODS Antibodies, plasmids and other materials Plasmids encoding egfp-kindlin-1 or mcherry-vinculin were generously donated by Dr. Reinhard Fassler and Dr. Johan de Rooij, respectively. Antibodies used in this study were directed against actin (clone C4; Chemicon), a-tubulin (clone B5-1-2; Sigma-Aldrich), GFP (Covance), the integrin a6-subunit (GoH3), the integrin β1-subunit (clone TS2/16; Developmental Studies Hybridoma Bank, clone 9EG7; a kind gift from Dr. Dietmar Vestweber, clone K-20; a kind gift from Dr. Andre van Agthoven; and clone 18 from BD Transduction laboratories), kindlin-1 (KS-4; a kind gift from Dr. Kindlin-1 regulates integrin dynamics 153
156 Figure 6 Kindlin-1 interaction with b1 regulates integrin trafficking. Cell-surface b1 integrins on KS, KSK, and KSK del581 cells were labelled with DyLight 649-conjugated K-20 at 4ºC (top panel), after which they were allowed to internalize in serum-free medium at 37ºC for 2 hrs (middle panel). Recycling of the internal pool was induced with 20% FCS for 7.5 min (bottom panel). Cells were then fixed and processed for confocal microscopy. b1 is pseudo-colored green, nuclei were counterstained with DAPI (pseudocolored red). Arrows indicate delivery of recycled b1 to adhesions. Bar, 10 mm. Cristina Has), kindlin-2 (Sigma-Aldrich), kindlin-2 (a kind gift from Dr. Reinhard Fassler), Ln-332 (a kind gift from Dr. Takako Sasaki), plectin (a kind gift from Dr. Katsushi Owaribe), and P(Y) (clone 4G10; a kind gift from Dr. Kevin Wilhelmsen). Neuraminidase, puromycin and zeocin were from Sigma-Aldrich. TRITC-, FITC-, and Cy5-conjugated secondary antibodies, phalloidin, and DAPI were purchased from Molecular Probes (Eugene, OR), HRP-conjugated secondary antibodies were from Amersham, and Col-I was from Vitrogen (Nutacon, Leimuiden, The Netherlands). K-20 was conjugated to DyLight 649 (Thermo Scientific) at the NKI. Patient material, cell culture, cloning, retroviral and lentiviral transductions Skin cryosections and primary KS keratinocytes were obtained after informed consent from a previously described patient (22), and immortalized by SV40 infection. NHK cells have been described previously (23). KS and NHK cells were routinely cultured on Col-1 (3 mg/ml) in keratinocyte serum-free medium (K-SFM; Gibco BRL), supplemented with 50 μg/ml bovine pituitary extract, 5 ng/ml EGF, 154
157 100 U/ml penicillin and 100 U/ml streptomycin. Rac-11P cells were cultured in DMEM supplemented with 10% FCS, 100 U/ml penicillin and 100 U/ml streptomycin. All cells were maintained at 37 C and 5% CO 2. egfp-kindlin-1 del581 was generated using egfp-kindlin-1 in C1. Full-length or truncated kindlin-1 were recloned into LZRS-IRES-zeo, and transfected into Phoenix packaging cells using the Calcium Phosphate method. Viruscontaining supernatant was isolated 48 hrs later and stable expression in KS cells was achieved by retroviral transduction, followed by selection with zeocin and cell sorting. Expression of mcherry-vinculin was established by lentiviral transduction of the plv-cmv-mcherry-vinculin-ires- Puro-construct, followed by selection with 5 mg/ml puromycin. Knockdown of kindlin-2 in KS cells Short hairpins against human kindlin-2 (target sequence CGACTGATATA- ACTCCTGAAT), cloned into plko.1, were obtained from the TRC shrna Open Biosystems library and transfected into HEK 293FT cells together with the Virapower TM Packaging mix (Invitrogen), using Lipofectamine 2000 according to the manufacturers instructions. Viral supernatant was harvested 48 hrs later, transduced into KS cells, and positive cells were selected with puromycin. Flow cytometry and cell sorting For flow cytometry and cell sorting, cultured cells were trypsinized, washed twice in PBS containing 2% FCS, and incubated with primary antibodies for 45 min at 4 C. Cells were then washed twice in 2% FCS/ PBS, incubated with appropriate secondary antibodies for 45 min at 4 C, washed twice in 2% FCS/PBS, and analyzed on a FACS Calibur (BD Biosciences). Alternatively, the cells were sorted on a MoFlo High Speed Cell Sorter (Beckman Coulter). Immunoprecipitations and Western blotting Cells were washed in ice-cold PBS and lysed on ice in RIPA buffer (25 mm Tris/ HCl ph 7.6, 150 mm NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS), supplemented with protease inhibitor cocktail (Sigma). Immunoprecipitation of b1 was performed essentially as described earlier (16), using TS2/16. For Western blotting of whole-cell extracts, cell lysates were cleared by centrifugation at 13,000xg, heated at 95ºC in SDS sample buffer (50 mm Tris-HCl ph 6.8, 2% SDS, 10% glycerol, 1% β-mercaptoethanol, 12.5 mm EDTA, 0.02 % bromophenol blue), and proteins were resolved by SDS- PAGE, after which they were transferred to polyvinylidene difluoride membranes (Millipore) and analyzed by Western blotting followed by ECL using the SuperSignal system (Pierce Chemical Co.). Microscopy Phase-contrast images were acquired on a Zeiss microscope (Axiovert 25) at 10x (NA 0.25) or 20x (NA 0.3) magnification, using a Zeiss CCD camera (Axiocam MRC) and Zeiss Mr. Grab 1.0 software. For confocal microscopy, cryosections of human skin or cells cultured on coverslips were prepared as previously described (16), and images were acquired on an inverted confocal microscope (Leica AOBS) using 20x (NA 0.7) dry, 40x (NA 1.25) oil, and 63x (NA 1.32) oil objectives 5 Kindlin-1 regulates integrin dynamics 155
158 (Leica). For TIRF microscopy, cells were seeded on glass coverslips and videos were acquired using Leica application suite software on a Leica DMI600B system with a 63X objective (NA 1.47), at 37 C in an atmosphere containing 5% CO 2. Images and videos were processed using Photoshop 7.0 and ImageJ Adhesion, migration, cell spreading, and proliferation assays For adhesion assays, 96-well plates were coated with 2% BSA or 3 mg/ml Col-1 for 1 hr at 37 C. Ln-332-containing matrix was prepared by growing Rac-11P cells to confluency, prior to overnight detachment with 10 mm EDTA at 4 C. The plates were then washed twice with PBS, blocked with 2% BSA for 1 hr at 37 C, and washed twice with PBS before use. Subconfluent cells were trypsinized, resuspended in K-SFM and seeded at a density of 3x10 4 cells per well. After 30 min at 37 C, nonadherent cells were washed away with PBS. The adherent cells were fixed in 4% PFA, washed with H2O, stained for 10 min with crystal violet, washed with H2O, and then lysed in 2% SDS. Absorbance was measured at 490 nm on a microplate reader. Background values (binding to BSA) were subtracted from all other values. To determine cell spreading, subconfluent cells were trypsinized, resuspended in K-SFM, and then seeded in 12-well plates coated with Col-1 or Rac11P matrix. Cells were photographed on a Widefield CCD system using 10x and 20x dry lens objectives (Carl Zeiss MicroImaging, Inc.). The number of spread cells was counted and expressed as a percentage of the total number of cells. Alternatively, the surface area was determined using ImageJ. Values shown represent the averages of 3 experiments. In each experiment, approximately 500 cells were analyzed for each condition. For single-cell migration assays, cells were seeded sparsely on 3 mg/ml Col-1, and phase-contrast images were captured every 15 min at 37 C and 5% CO 2 on a Widefield CCD system using a 10x dry lens objective (Carl Zeiss MicroImaging). Migration tracks were generated using ImageJ 1.44, and the average velocity was calculated from approximately 250 cells out of 3 independent experiments. Proliferation was investigated by seeding cells in 6-well plates, coated with 3 mg/ml Col-1, at a density of 5x10 4 cells per well, whereafter they were trypsinized and counted every day. Values shown represent the averages of 3 experiments. Integrin internalization and recycling assays Integrin internalization and recycling were investigated essentially as described earlier with some modifications (34). Briefly, cells on glass coverslips were incubated for 2 hrs at 37 C in serum-free medium, after which they were washed twice in the same medium at 4 C. Cellsurface b1 was then labeled with DyLight 649-conjugated K-20 (10 mg/ml) for 1 hr at 4 C. Immediately after labeling, some coverslips were fixed, and the rest was transferred to 37 C to undergo endocytosis. After 2 hrs, some coverslips were fixed, and the rest was stimulated with 20% FCS for 7.5 min to stimulate recycling of internalized integrins. The cells were fixed, permeabilized with 0.5% Triton and 0.01% saponin, and then processed for confocal microscopy as described above. 156
159 ACKnoWleDGEMents We are grateful to Andre van Agthoven, Reinhard Fassler, Cristina Has, Katsushi Owaribe, Johan de Rooij, Takako Sasaki, Dietmar Vestweber, and Kevin Wilhelmsen for their generous gifts of antibodies or constructs. We thank Lauran Oomen and Lenny Brocks for their excellent assistance with TIRF and confocal microscopy, and Anita Pfauth and Frank van Diepen for expert technical assistance with FACS. Many thanks to Ana Jimenez Orgaz and Tomas Meijer for technical support. This work was financially supported by DEBRA UK. ABBREVIatIons BM, basement membrane; Col, collagen; FA, focal adhesion; FERM, four-point-one/ ezrin/radixin/moesin; KS, Kindler syndrome; Ln-332, laminin-332; NHK, normal human keratinocytes; PH, pleckstrin homology, P(Y), phosphotyrosine; TIRF, total internal reflection microscopy RefeRences 1. Legate KR, and R Fassler (2009) Mechanisms that regulate adaptor binding to b-integrin cytoplasmic tails. J Cell Sci 122, Margadant C, et al (2008) Regulation of hemidesmosome disassembly by growth factor receptors. Curr Opin Cell Biol 20, Kim C, et al (2011) Regulation of integrin activation. Annu Rev Cell Dev Biol 27, Larjava H, et al (2008) Kindlins: essential regulators of integrin signalling and cellmatrix adhesion. EMBO Rep 9, Meves A, et al (2009) The kindlin protein family: new members to the club of focal adhesion proteins. Trends Cell Biol 19, Jobard F, et al (2003) Identification of mutations in a new gene encoding a FERM family protein with a pleckstrin homology domain in Kindler syndrome. Hum Mol Genet 12, Siegel DH, et al (2003) Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin-extracellular matrix linker protein UNC-112, causes Kindler syndrome. Am J Hum Genet 73, Ussar S, et al (2006) The kindlins: subcellular localization and expression during murine development. Exp Cell Res 312, Ashton GHS (2004) Kindler syndrome. Clin Exp Dermatol 29, White SJ, and WH McLean (2005) Kindler surprise: mutations in a novel actin-associated protein cause Kindler syndrome. J Dermatol Sci 38, Sadler E, et al (2006) Novel KIND1 gene mutation in Kindler syndrome patients with severe gastro-intestinal tract involvement. Arch Dermatol 142, Kern JS, et al (2007) Chronic colitis due to an epithelial barrier defect: the role of kindlin-1 isoforms. J Pathol 213, DiPersio CM, et al (1997) a3b1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol 137, Brakebusch C, et al (2000) Skin and hair follicle integrity is crucially dependent on b1 integrin expression in keratinocytes. EMBO J 19, Raghavan S, et al (2000) Conditional ablation of β1 integrin in skin: severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol 150, Margadant C, et al (2009) Integrin α3β1 inhibits directional migration and wound 5 Kindlin-1 regulates integrin dynamics 157
160 re-epithelialization in the skin. J Cell Sci 122, Margadant C, et al (2010) Unique and redundant functions of integrins in the epidermis. FASEB J 24, Has C, et al (2012) Integrin a3 mutations with kidney, lung, and skin disease. N Engl J Med 366, Kloeker S, et al (2004) The Kindler syndrome protein is regulated by transforming growth factor-b and involved in integrin-mediated adhesion. J Biol Chem 279, Herz C, et al (2006) Kindlin-1 is a phosphoprotein involved in regulation of polarity, proliferation, and motility of epidermal keratinocytes. J Biol Chem 281, Has C, et al (2009) Kindlin-1 Is required for RhoGTPase-mediated lamellipodia formation in keratinocytes. Am J Pathol 175, Has C et al (2006) Molecular basis of Kindler syndrome in Italy: novel and recurrent Alu/ Alu recombination, splice site, nonsense, and frameshift mutations in the KIND1 gene. J Invest Dermatol 126, Niessen CM, et al (1996) Deficiency of the integrin b4 subunit in junctional epidermolysis bullosa with pyloric atresia: consequences for hemidesmosome formation and adhesion properties. J Cell Sci 109, Lai-Cheong JE, et al (2009) Loss-offunction FERMT1 mutations in Kindler syndrome implicate a role for fermitin family homolog-1 in integrin activation. Am J Pathol 175, Ussar S, et al (2008) Loss of kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet 4, e He Y, et al (2011) Kindlin-1 and -2 have overlapping functions in epithelial cells; implications for phenotype modification. Am J Pathol 178, Bandyopadhyay A, et al (2012) Uncovering functional differences between kindlin-1 and kindlin-2 in keratinocytes. J Cell Sci 125, Lai-Cheong JE, et al (2008) Colocalization of kindlin-1, kindlin-2, and migfilin at keratinocyte focal adhesion and relevance to the pathophysiology of Kindler syndrome. J Invest Dermatol 128, Harburger DS, et al (2009) Kindlin-1 and -2 directly bind the C-terminal region of b integrin cytoplasmic tails and exert integrin-specific activation effects. J Biol Chem 284, Böttcher RT, et al (2012) Sorting nexin-17 prevents lysosomal degradation of β1 integrins by binding to the β1-integrin tail. Nat Cell Biol 14, Margadant C, et al (2012) Distinct roles of talin and kindlin in regulating integrin α5β1 function and trafficking. Curr Biol 22, Has C, et al (2008) C-terminally truncated kindlin-1 leads to abnormal adhesion and migration of keratinocytes. Br J Dermatol 159, Caswell PT, et al (2009) Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol 10, Margadant C, et al (2011) Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol 23, Powelka AM, et al (2004) Stimulationdependent recycling of integrin b1 regulated by ARF6 and Rab11. Traffic 5, Steinberg F, et al (2012) SNX17 protects integrins from degradation by sorting between lysosomal and recycling pathways. J Cell Biol 197,
161
162
163 Kindlin-1 mutant zebrafish as an in vivo model sytem to study adhesion mechanisms in the epidermis Coert Margadant 1,*, Ruben Postel 1,*, Maaike Kreft 1, Hans Janssen 1, Pablo Secades 1, Giovanna Zambruno 2, and Arnoud Sonnenberg 1 1 Division of Cell Biology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands. 2 Laboratory of Molecular and Cell Biology, IDI-IRCCS, Rome, Italy. *equal contribution. Submitted for publication
164
165 ABSTRAct From a forward genetic screen for epidermal defects in zebrafish, we identified a loss-offunction mutation in Kindlin-1, an essential regulator of integrin function. The mutation generates a premature stop codon, deleting the integrin-binding site. The mutant zebrafish develop cell-matrix and cell-cell adhesion defects in the basal epidermis leading to progressive fin rupturing, and was therefore designated rupturing-of-fins (rof). Similar defects were observed in the epidermis of Kindler syndrome patients, carrying a loss-of-function mutation in kindlin-1. Mutational analysis and rescue experiments in zebrafish revealed that residues K610, W612, and I647 in the F3 domain are essential for Kindlin-1 function in vivo, and that Kindlin-2 can functionally compensate for the loss of Kindlin-1. The fin phenotype of rof/kindlin-1 mutants resembles that of badfin mutants, carrying a mutation in Integrin α3. We show here that this mutation impairs the biosynthesis of Integrin α3β1, and causes cell-matrix and cell-cell defects in vivo. Whereas both Integrin-linked kinase and Kindlin-1 cooperate with Integrin α3β1 to resist trauma-induced epidermal defects, Kindlin-1 and Ilk surprisingly do not act synergistically but in parallel. Thus, the rof/kindlin-1 mutant zebrafish provides a unique model system to study epidermal adhesion mechanisms in vivo. IntRODUctIon Integrins are αβ heterodimeric transmembrane receptors that connect the extracellular matrix to the cytoskeleton. Ligand binding triggers integrin clustering and the recruitment of a variety of signaling, structural, and adaptor proteins, leading to the formation of multi-protein adhesion complexes (1). In the skin, the main integrin-based complex that mediates adhesion of the epidermis to the underlying basement membrane (BM) is the hemidesmosome (HD). HDs are assembled by the laminin (Ln)-binding integrin α6β4 and consist further of plectin, CD151, and the bullous pemphigoid antigens 180 and 230 (2). While the binding to intermediate filaments provides for the mechanical stability of the HD-BM connection, the BM is anchored to the dermis by anchoring fibrils, consisting of collagen (Col)-VII. A defect in any of these components leads to skin blistering, as illustrated by a variety of mouse models and a group of human bullous disorders classified as epidermolysis bullosa (EB) (3). In contrast to α6β4, β1-containing integrins connect to the actin cytoskeleton, and assemble adhesion structures designated focal adhesions (FAs). The predominant β1-integrin in the epidermis is α3β1, which is involved in epidermal adhesion, BM organization, and stem cell maintenance (4-7). Consequently, mice that lack the α3 or the β1 subunit in the epidermis, in addition to human patients that carry mutations in the ITGA3 gene encoding α3, develop BM abnormalities and skin blisters though the blistering is less severe than in the absence of α6β4, because adhesion to the BM is partially rescued by HDs (8-12). The abnormalities caused by loss of α3β1 are partially recapitulated in mice that lack the FA protein integrin-linked kinase (ILK), and 6 Studying epidermal adhesion in vivo 163
166 by the human disease Kindler syndrome (KS), a congenital disorder characterized by trauma-induced skin fragility and blistering, photosensitivity, and poikiloderma (13-16). A subset of KS patients also suffers from lesions in the gastro-intestinal tract (17,18). KS is caused by mutations in the KIND1 gene, encoding the protein kindlin-1 that is highly expressed in epithelia, predominantly in the skin and the intestine (17-20). The kindlins are essential regulators of integrin function that contain a four-point-one/ezrin/radixin/ moesin (FERM) domain, consisting of subdomains F0-F3, with a pleckstrin homology (PH) domain inserted into F2. While the PH domain regulates the recruitment to the plasma membrane, the kindlins bind directly to the cytoplasmic tail of integrin β-subunits through their C-terminal F3 region (21,22). In this study, we identified a zebrafish Kindlin-1 loss-of-function mutant that lacks the F3 region, and develops mechanical trauma-induced epidermal fragility and fin rupturing during embryonic development. The zebrafish epidermis consists of a basal layer (the basal epidermis), surrounded by the periderm. During early embryonic development, adhesion of the basal epidermis to the underlying BM is mediated by Integrin α3β1 binding to Ln-511, whereas HD assembly starts not until 5 to 6 days after fertilization (23-26). Therefore, zebrafish embryos constitute a unique model sytem to study β1-integrindependent phenomena in vivo in the absence of HDs. Here, we study the regulation of adhesion in the developing epidermis in zebrafish, with specific focus on Integrin α3β1, Kindlin-1, and Ilk. ResUlts and DIscUssIon A loss-of-function mutation in Kindlin-1 causes epidermal fragility and progressive fin rupturing in zebrafish From a forward genetic screen in zebrafish for epidermal defects during embryonic development, we isolated a mutant (hu8654) which developed epidermal fragility at 2 days post-fertilization (dpf) (Figure 1A). The mutant was designated rupturing-of-fins (rof), because progressive rupturing of the medial fins occurred at later stages of development, eventually resulting in their complete loss (Figure 1B). The rof mutant embryos are not viable, and die around 10 dpf. Meiotic mapping positioned the causal mutation on chromosome 20, close to marker z22041 (Figure 1C). One of the genes within the corresponding genomic interval is kindlin-1 (FERMT1, LOC ), which encodes Kindlin-1, the zebrafish orthologue of human kindlin-1. Whole-mount in situ hybridization in wild-type embryos revealed kindlin-1 mrna expression at the medial and pectoral fin folds, the branchial arches, and in the intestinal epithelium (Figure 1D). Next, we depleted kindlin-1 mrna levels in wild-type embryos using a morpholino targeting the translation start-site or splice-site. Both approaches recapitulated the epidermal fragility and fin rupturing of rof mutants, indicating that the rof phenotype is likely due to a mutation in kindlin-1 (Figure 1E; Table S1). Sequencing the kindlin-1 cdna of rof mutants revealed a G>T mutation in exon 164
167 13 of the kindlin-1 coding region, which is predicted to introduce a premature stop codon at amino acid residue E565 (Figure 1F). Due to a lack of available antibodies that recognize zebrafish Kindlin-1, we could not determine whether the mutation affects protein expression. However, the stop codon does not affect the stability of the transcript, because kindlin-1 mrna expression in rof/kindlin-1 mutants was equal to that in wild-type embryos (Figure 1G). We therefore assume that the mutation causes the expression of a truncated Kindlin-1 protein (Kindlin- 1 del565 ) that lacks the C-terminal 96 amino acids, which comprise almost the entire F3 domain (Figure 1H). Of note, a mutation in KIND1 that introduces a premature stop codon has been identified in a KS patient, which leads to the expression of a nonfunctional protein product lacking 145 amino acids at the C-terminus (27). Injection of kindlin-1 G1693T RNA, encoding the putative truncated Kindlin-1 del565 mutant, did not induce developmental defects in wild-type embryos (Table S2), excluding the possibility that the rof/kindlin-1 phenotype is caused by dominant-negative effects. Expression of full-length Kindlin-1 but not Kindlin-1 del565 in rof/kindlin-1 mutants rescued the epidermal fragility and fin rupturing completely (Figure 1I; Table S2). In addition, human kindlin-1 also rescued the epidermal defects (Figure 1I; Table S2), in line with the high sequence conservation between human kindlin-1 and zebrafish Kindlin-1 (approximately 70%; Figure S1). The F3 domain of kindlins contains the integrin-binding site, and overexpression studies in cultured cells indicate that amino acids K610, W612, and I647 in kindlin-1 are crucial for the interaction with β1 and its recruitment to FAs (28). These residues are conserved in zebrafish Kindlin-1, and are lost in the putative Kindlin-1 del565 mutant (Figure S1). To study the importance of the 3 residues in vivo, we replaced them for alanines, either individually or in combination, and performed reconstitution experiments in rof/ kindlin-1 embryos. Intriguingly, the WI/ AA and either single point-mutant completely rescued the phenotype, whereas the KI/AA and KW/AA mutants induced only partial rescue and the KWI/AAA mutant did not rescue at all (Figure 1I; Table S2), indicating that K610, together with either W612 or I647, is required for kindlin-1 function in vivo. Loss-of-function mutations in kindlin-1 cause cell-matrix and cell-cell defects in the basal epidermis of zebrafish and in KS patients To examine the epidermal abnormalities in rof/kindlin-1 mutants in more detail, we performed transmission electron microscopy on embryos at 2 dpf. Compared to wild-type embryos, the basal epidermis of rof/kindlin-1 mutants was severely malformed, and microblisters were observed between the basal epidermis and the BM (Figure 2A, upper and lower panels). Interestingly, we also observed gaps between cells in the basal epidermis, suggesting defects in cell-cell adhesion (Figure 2A, middle panel). We therefore investigated the formation of cell-cell contacts by immunostaining of E-cadherin in wholemounts of wild-type and rof/kindlin-1 embryos, which was visualized by confocal microscopy. Compared to wild-type embryos, E-cadherin appeared more diffusely distributed in basal epidermal cells 6 Studying epidermal adhesion in vivo 165
168 166 Figure 1 A mutation in kindlin-1 causes progressive rupturing-of-fins (rof) in zebrafish. (A) Medial fins of wild-type and rof mutant zebrafish. (B) Quantification of medial fin outgrowth. (C) Linkage analysis of the rof locus. Arrows, direction of the mutation; red line, open reading frame of the transcript. (D) In situ hybridisation for kindlin-1 in wild-type embryos at 24, 48, and 72 hpf. Inset, gut epithelium. (E) Medial fins of non-injected control (nic) and wild-type embryos injected with a kindlin-1 ATG-morpholino or splice-site morpholino. (F) Sequence of wild-type and rof/kindlin-1 cdna. Arrow indicates the mutation. (G) mrna expression of kindlin-1 (ef1α = cdna input control) in wild-type and rof/kindlin-1 embryos. (H) Structure of wild-type and mutant zebrafish Kindlin-1. (I) Medial fins of rof/kindlin-1 embryos expressing zebrafish (zf) Kindlin-1, zfkindlin-1 del565, human (hu)kindlin-1, or hukindlin-1 KWI>AAA.
169 in rof/kindlin-1 mutants, and a large fraction appeared to be localized in vesicles, which is probably due to increased cell-cell contact dissociation leading to E-cadherin internalization (Figure 2B). Because the E-cadherin/β-catenin complex plays a critical role in the assembly of the cortical actin cytoskeleton, we also stained embryos with phalloidin to visualize filamentous actin (F-actin), using p63 as a counterstaining to identify basal epidermal cells. In rof/kindlin-1 embryos, cortical F-actin appeared both less abundant and less organized in the basal epidermis but not in the periderm, in line with the aberrant organization of cell-cell contacts (Figure 2C). We next investigated whether lossof-function mutations in kindlin-1 also cause defects in the organization of cellcell contacts in human epidermis. Therefore, we examined biopsies of two KS patients from Italy, a male homozygous for mutation c.1161dela, and a male homozygous for mutation IVS7-1G>A (29). Immunolabeling of Ln-332 revealed BM abnormalities, including blisters and interrupted or increased BM deposition, typical of KS (Figure 3). Intriguingly, E-cadherin was hardly detected at the lateral cell membranes in basal keratinocytes in the two KS patients, suggesting a loss of cell-cell contacts, whereas its localization in the suprabasal layers of the epidermis appeared normal (Figure 3). The badfin mutation disrupts Integrin α3β1 biosynthesis and causes cell-matrix and cell-cell adhesion defects in the basal epidermis of zebrafish In a previous screen for epidermal defects, a missense mutation was identified in itga3, the gene encoding the Integrin α3 subunit in zebrafish, causing defects in fin morphogenesis for which the mutant was designated badfin (bdf) (23). The mutation leads to the substitution of serine 427 for proline, and the corresponding residue in the human α3 sequence is serine 433 (S433) (Figures 4A,S2). S433 is located in the β-propeller region, as shown by homology modeling based on the crystal structure of α5β1 (Figure 4B) (30). The β-propeller contains seven β-sheets formed by FG-GAP repeats (Figure 4C), which are strongly conserved among integrin α-subunits and across species, and the region is pivotal for proper protein folding of integrin α-subunits. To investigate the effects of S433P substitution on α3 biosynthesis, we introduced the mutation in human α3 cdna, and stably expressed either wild-type α3 wt or mutant α3 S433P in α3-deficient murine MKα3 - keratinocytes (10). We first analyzed the expression of precursor and mature α3 by Western blotting. Like most integrin α-subunits, α3 is synthesized as a precursor of ~150 kda. After association with β1, the α3β1 heterodimer is transported to the Golgi network where the α3 precursor is cleaved into a heavy (~115 kda) and a light chain (~35 kda), and the mature α3β1 translocates to the plasma membrane (31,32). Although protein expression of precursor α3 S433P was detected, it was considerably reduced as compared to that of α3 wt. Furthermore, the mature product was completely absent, as evidenced by the lack of the α3 light chain (Figure 4D). Flow cytometry confirmed that α3 S433P was not expressed at the cell-surface (Figure 4E). Presumably, the mutation disrupts the folding of the α3 precursor, thereby preventing α/β heterodimerization, transport to the Golgi, and to the plasma membrane. The low expression of the 6 Studying epidermal adhesion in vivo 167
170 168
171 Figure 2 rof/kindlin-1 mutants develop cell-matrix and cell-cell contact defects in the basal epidermis. (A) Electron microscopy of wild-type (left) and rof/kindlin-1 mutant (right) embryos at 2 dpf. Black arrows mark the sites of cell-cell contact, arrowheads mark the basement membrane, asterisks mark defects in cell-matrix and cell-cell contacts. e, epidermis; ecm, extracellular matrix; pe, periderm; be, basal epidermis; a, actinotrichia. (B) Whole-mount immuno-staining of E-cadherin in wild-type (left) and rof/ kindlin-1 (right) mutant embryos at 6 dpf. (C) Labeling of F-actin (green) and p63 (red) in basal epidermis and periderm of wild-type and rof/kindlin-1 mutant embryos at 6 dpf. precursor is likely due to the endoplasmic reticulum-associated degradation pathway, which clears misfolded proteins. Thus, the S433P mutation is a true loss-of-function mutation. Consequently, the α3 S433P keratinocytes behaved as α3-deficient cells, as cell adhesion to human Ln-511 was decreased by the α6-blocking antibody GoH3 in MKα3 and MKα3 S433P, but not in MKα3 wt cells, indicating that α6 integrins mediate adhesion to Ln-511 in the absence of α3. In contrast, adhesion to Col-1 was not affected by GoH3 (Figure 4F). GoH3 also reduced cell spreading of MKα3 and MKα3 S433P cells but not of MKα3 + cells, both on Ln-511 and to a lesser extent also on Col-I, which is due to cell spreading on deposits of endogenous Ln-332 as we have described previously (Figures 4G,S3) (10). We then investigated the epidermal defects in bdf/itga3 embryos by electron microscopy. Similar to in rof/kindlin-1 mutants, gaps were observed between basal epidermal cells, in addition to blisters at the basal side (Figure 4H). Confocal microscopy confirmed that also in bdf/itga3 mutants, E-cadherin and β-catenin were diffusely distributed, with a large fraction seemingly localized in vesicles (Figure 4I). It is well-established that integrin α3β1 localizes basolaterally in epithelia such as the epidermis and in epithelial cells in vitro, and a number of studies have shown that α3β1 can promote cell-cell adhesion (33-35). However, it is unclear whether this is a direct effect. Importantly, a mutation in lama5, the gene encoding the α5 chain of Ln-511, also causes both cellmatrix and cell-cell adhesion defects in zebrafish (26). Furthermore, knockdown of either Ln-511 or α3 expression disrupts cell-cell contacts in vitro (36). The cell-cell contact defects observed in rof/kindlin-1 and bdf/itga3 mutants are therefore probably an indirect consequence of reduced cell-matrix adhesion. It should be noted that cell-cell contact defects have not been observed in newborn mice lacking α3 in the epidermis, and that newborn kindlin- 1-deficient mice develop skin atrophy, but no noticeable skin fragility or blistering (10,37). This is probably due to the fact that compared to zebrafish embryos, mice hardly encounter mechanical stress during embryonic development, and kindlin-1- deficient mice die before stress-induced skin defects can develop in neonatal life. Nevertheless, the clear defects in E-cadherin localization in the basal epidermis of KS patients indicate that the rof/kindlin-1 mutant is a suitable model for KS, and that defects in cell-cell contacts represent a hitherto unrecognized feature of KS. Fin rupturing in rof/kindlin-1 mutants is induced by mechanical trauma, and is rescued by expression of Kindlin-2 To investigate whether the epidermal fragility and fin rupturing observed in rof/ 6 Studying epidermal adhesion in vivo 169
172 Figure 3 Loss-of-function mutations in kindlin-1 cause cell-matrix and cell-cell defects in the epidermis in KS patients. Immunostaining of E-cadherin (red) and Ln-332 (green) in the skin of two KS patients and an unaffected individual (normal). Nuclei were counter-stained with DAPI. Arrows indicate BM abnormalities, arrowheads mark defects in cell-cell contacts. Figure 4 Disruption of α3β1 biosynthesis causes cell-matrix and cell-cell defects in zebrafish. (A) The S427 residue in α3, mutated in bdf/itga3, and its human homologue S433. (B) Position of S433 in the β-propeller of α3. (C) Position of S433 in the FG-GAP repeats. (D) Western blot demonstrating the expression of precursor and mature α3 in MKα3 -, MKα3 wt, and MKα3 S433P keratinocytes. (E) FACS histogram showing cell-surface expression of α3 in MKα3 -, MKα3 wt, and MKα3 S433P. (F) Adhesion to Ln-511 and Col-1 in the absence (black bars) or the presence (white bars) of GoH3 (10 µg/ml). Values represent the averages of 3 independent experiments. (G) Cell spreading over Ln-511 and Col-1 in the absence (black bars) or the presence (white bars) of GoH3 (10 µg/ml). Values represent the averages from ~500 cells. (H) Electron microscopy of bdf/itga3 mutant embryo at 2 dpf. Arrows, cell-cell defects; asterisks, cell-matrix defects. ecm, extracellular matrix; pe, periderm; be, basal epidermis. (I) E-cadherin and β-catenin distribution in the basal epidermis of wild-type and bdf/itga3 embryos at 6 dpf. Statistically significant differences (unpaired t-test) are indicated by * (p<0.05), ** (p<0.01), or *** (p<0.005). ns, not significant. 170
173 6 Studying epidermal adhesion in vivo 171
174 kindlin-1 mutants are indeed induced by mechanical trauma, we raised rof/ kindlin-1 embryos in 1% methyl-cellulose supplemented with 0.001% tricaine, which restricts movement of the embryos and prevents direct contact with the culture dish. A significant reduction of fin rupturing was observed at 3 dpf and 6 dpf compared to untreated rof/kindlin-1 embryos, indicating that the defects are indeed induced by mechanical trauma (Figures 5A,B). To determine whether overexpression of Kindlin-2 can compensate for the loss of Kindlin-1 during embryonic development, kindlin-2 RNA was injected in rof/ kindlin-1 embryos. Intriguingly, expression of zebrafish Kindlin-2 or human kindlin-2 completely rescued the fin defects of rof/kindlin-1 mutants (Figure 5C; Table S2). Although functional redundancy of kindlin-1 and kindlin-2 has been shown in cultured keratinocytes, they also seem to have unique functions, both in vitro but particularly in vivo, since kindlin-2 expression in the intestinal epithelium cannot rescue the intestinal defects in kindlin-1- deficient mice, and KS patients develop defects despite the presence of kindlin-2 in the epidermis (37-39). It is assumed that functional differences in vivo arise at least in part from the differential distribution of the kindlins in epithelia; whereas kindlin-1 is predominantly localized in the basal layer of the epidermis at the interface with the BM, kindlin-2 is enriched at the lateral membranes in the basal as well as the suprabasal layers (40). The observed redundancy of Kindlin-1 and Kindlin-2 in zebrafish embryos may indicate that both kindlins fulfill similar roles during early development whereas they acquire unique functions later. Alternatively, the kindlins may function similarly in a one-cell layered epithelium such as the zebrafish embryonic epidermis, whereas their functions are distinct in a fully differentiated multi-layered epithelium as in mammals. Kindlin-1 and ILK act synergistically with Integrin α3β1 but not with each other We have previously described a nonsense mutation in ilk, the gene encoding Ilk in zebrafish, which was designated lostcontact (loc) because it causes skin blistering (41,42). Compared to rof/kindlin-1 and bdf/itga3 mutants, the phenotype of loc/ilk embryos is slightly different; fin rupturing is less pronounced and blisters develop at the tip of the tail, suggesting that the functions of Ilk do not completely overlap with those of Kindlin-1 and Integrin α3β1 (Figure S4). To study genetic interactions between ilk and kindlin-1, we intercrossed heterozygous loc/ilk with rof/kindlin-1 mutants. Compared to single homozygous mutants, compound homozygous mutants displayed more severe epidermal fragility and rupturing of the medial fins whereas compound heterozygotes were indistinguishable from wildtype embryos, suggesting that Ilk and Kindlin-1 act in a parallel fashion rather than synergistically (Figures 6A-D). However, a potential genetic interaction between ilk and kindlin-1 in the compound heterozygous embryos could be masked by dosage compensation. Therefore, synergistic interaction experiments were performed by co-injecting suboptimal doses of morpholinos directed against ilk, kindlin-1, or itga3 in wild-type embryos. We first determined the suboptimal dose for each morpholino, at which no epider- 172
175 6 Figure 5 Kindlin-2 expression rescues the trauma-induced fin rupturing in rof/kindlin-1 mutants. (A) Medial fins of wild-type and rof/kindlin-1 embryos at 3 dpf and 6 dpf cultured in viscous medium (VM), supplemented with a low dose of anaesthetics (LA). (B) Quantification of the outgrowth of the medial fins. Statistically significant differences (unpaired t-test) are indicated by * (p<0.05) or ** (p<0.01). (C) Rescue of medial fin rupturing at 3 dpf in rof/kindlin-1 embryos by ectopic expression of zebrafish Kindlin-2 or human kindlin-2. mal defects occur (Figures 6E-G; Table S3). Co-injection of suboptimal doses of morpholinos against itga3 and kindlin- 1, or against itga3 and ilk resulted in fin rupturing, revealing that Integrin α3β1 acts synergistically with both Kindlin-1 and Ilk (Figures 6H,I; Table S3). The defects were rescued by co-injection of the itga3/kindlin-1 morpholinos with kindlin-1 RNA, and the itga3/ilk morpholinos with ilk RNA, respectively (Figure 6J,K; Table S3). Intriguingly, co-injection Studying epidermal adhesion in vivo 173
176 of suboptimal doses of ilk/kindlin-1 morpholinos did not cause epidermal defects, suggesting that Ilk and Kindlin-1 do not act synergistically but in parallel (Figure 6L; Table S3). Experiments using the kindlin-1 ATG-morpholino, an itga3 ATGmorpholino, or an ilk splice-site morpholino yielded similar results (Table S3). Although ILK can directly bind to β1, previous studies indicate that kindlin-2 binds ILK and is required for targeting ILK to FAs (43). Furthermore, UNC- 112, the kindlin homologue in C. elegans, recruits ILK to sites of muscle attachment (44). It is possible that ILK interacts only with kindlin-2 but not with kindlin-1, or that interaction between ILK and kindlins occurs in a tissue-specific manner, e.g. ILK and kindlin-2 interaction in muscle. This hypothesis is underlined by the similar phenotypes caused by loss-of-function mutations in ilk or kindlin-2 in zebrafish; both develop defects in cardiac and skeletal muscles (41,42,45,46). Figure 6 Ilk and Kindlin-1 do not act synergistically in the basal epidermis of zebrafish. (A-D) Morphology of the medial fins of homozygous wild-type (A), loc/ilk (B), rof/kindlin-1 (C), and loc/ilk // rof/ kindlin-1 (D) embryos at 3 dpf. (E-I) Morphology of the medial fins of wild-type embryos at 3 dpf after injection of suboptimal doses of morpholinos against kindlin-1 (E), itga3 (F), ilk (G), itga3 and kindlin-1 (H), or itga3 and ilk (I). (J-L) Morphology of the medial fins of wild-type embryos at 3 dpf after co-injection of morpholinos against itga3 and kindlin-1 together with kindlin-1 RNA (J), morpholinos against itga3 and ilk together with ilk RNA (K), or morpholinos against kindlin-1 and ilk (l). MateRIals and MetHODS Antibodies and other materials Antibodies used in this study were directed against human α3a ( J143; hybridoma from American Type Culture Collection, and a home-made antibody (47), β-catenin (BD bioscience), E-cadherin (BD bioscience), Ln-332 (a kind gift from Dr. T. Sasaki), and p63 (Santa Cruz Biotechnology). TRITC-, FITC-, and Cy5-conjugated secondary antibodies, phalloidin, and DAPI were purchased from Molecular Probes (Eugene, OR), HRP-conjugated secondary antibodies were from Amersham, and Col-I was from Vitrogen (Nutacon, Leimuiden, The Netherlands). Ln-511 was acquired from BioLamina AB (Sweden). 174
177 Zebrafish strains and forward genetic screening Fish were raised and maintained under standard laboratory conditions. Fish experiments were performed in accordance with institutional guidelines and as approved by the Animal Experimentation Committee of the Royal Netherlands Academy of Arts and Sciences. The rof/ kindlin-1 mutant was identified during a forward genetic screen performed at the Hubrecht Institute, Utrecht, The Netherlands. ENU mutagenesis was performed as previously described for the creation of the Hubrecht Institute target selected mutagenesis library (483). F1 progeny of mutagenised males were outcrossed to create approximately 300 F2 families, which were then incrossed. F3 progeny were screened for epidermal integrity defects at 2 to 3 dpf. Meiotic mapping of the rof/kindlin-1 mutation was performed using standard simple sequence length polymorphisms (SSLP). SSLP primers sequences can be found on org. Genotyping PCR and subsequent sequencing of the kindlin-1 g1693t mutation on finclip DNA or DNA of single embryos was performed with the following primers: 5 -aacttgcctagttaacctttaagtc-3 (f ) and 5 -acaaagtctaacgcgacctc-3 (r). Methylcellulose treatment and fin outgrowth measurements Embryos were cultured from 2 dpf onwards in 1% methylcellulose solution and 0.001% tricaine. The medial fins were imaged with a Leica stereo microscope. Measuring the average fin outgrowth was performed using ImageJ at 4 different positions as indicated in Fig 1B. Rna and morpholino injections RNA and morpholino (MO) solutions (~1 nl) were injected at the one-cell stage. MO s were obtained from Gene Tools (Philomath, OR). The following MO s were used: kindlin-1 splice-mo: cttctaatgtctgtaaacagagtta and ATG-MO: gccgaggccatgattctgcctgaaa; ilk splice-mo (41), and itga3 splice-mo and ATG-MO (23). In situ hybridisation, cdna constructs and Rna synthesis Whole-mount in situ hybridization (ISH) was performed essentially as described previously (49). Briefly, embryos were fixed with 4% PFA/PBS and stored in 100% methanol. After ISH, embryos were cleared in methanol and mounted in benzylbenzoate/benzylalcohol (2:1 v/v). The following primers were used to produce the kindlin-1 cdna fragment: 5 -tttaaactgcgggtcaaag-3 (f); 5 -cgtaccacagactggtggag-3 (r). Fragments were cloned into the pcrii-topo vector (Invitrogen) and antisense diglabelled probes were synthesised according to standard protocols. Full-length zebrafish kindlin-1 and kindlin-2 cdna were derived by PCR on cdna using primers 5 -gaattccaccatggcctcggccggtgaacat-3 (f); and 5 -ctcgagtcagtcttgtcctcctgtgag-3 (r); or 5 -ggatccaccatggcgctggacggtata-3 (f); and 5 -gaattcttaaacccagccgctggtca-3 (r), and cloned into the PCS2+ vector with EcoR1/ Xho1 and BamH1/EcoR1, respectively. Mutations were introduced in cdna using the QuickChange kit (Stratagene). Full-length RNA was synthesized in vitro using the SP6 mmessage mmachine kit (Ambion). 6 Studying epidermal adhesion in vivo 175
178 Patient material, cell culture, cloning, and retroviral transduction Skin cryosections from KS patients and an unaffected individual were obtained after informed consent. The S433P mutation was generated by PCR overlap extension method using a cdna encoding human full-length α3a as a template. Wild-type ITGA3 was isolated by digestion with SacI and ligated into puc18-a3. After digestion with SphI, wild-type or mutant ITGA3 was ligated into LZRS-IRES-zeo and transfected into Phoenix packaging cells using the Calcium Phosphate method. Virus-containing supernatant was isolated after 48 hrs and stable expression in α3-deficient MKα3 - cells was achieved by retroviral transduction, followed by selection with 200 μg/ml zeocin (Sigma-Aldrich). All cells were maintained at 37 C and 5% CO 2 and cultured routinely on Col-1 (3 µg/ml) in keratinocyte serum-free medium (K-SFM; Gibco BRL), supplemented with 50 μg/ ml bovine pituitary extract, 5 ng/ml epidermal growth factor, 100 U/ml penicillin and 100 U/ml streptomycin. Flow cytometry and microscopy For flow cytometry, cultured cells were trypsinized, washed twice in PBS containing 2% FCS, and incubated with primary antibodies for 45 min at 4 C. Cells were then washed twice in 2% FCS/PBS, incubated with appropriate secondary antibodies for 45 min at 4 C, washed twice in 2% FCS/PBS, and analyzed on a FACS Calibur (BD Biosciences). Alternatively, the cells were sorted on a MoFlo High Speed Cell Sorter (Beckman Coulter). For confocal microscopy, wholemounts of zebrafish, cryosections of human skin or cells cultured on coverslips were prepared essentially as described previously (10), and images were acquired on an inverted confocal microscope (Leica AOBS) using 20x (NA 0.7) dry, 40x (NA 1.25) oil, and 63x (NA 1.32) oil objectives (Leica). Images were processed using Photoshop 7.0 and ImageJ Phase-contrast images were acquired on a Zeiss microscope (Axiovert 25) at 10x (NA 0.25) or 20x (NA 0.3) magnification, using a Zeiss CCD camera (Axiocam MRC) and Zeiss Mr. Grab 1.0 software. Electron microscopy was performed essentially as described earlier (10). Adhesion and cell spreading assays For adhesion assays, 96-well plates were coated with 2% BSA, 3 µg/ml Col-1, or 5 µg/ml Ln-511 for 1 hr at 37 C. The plates were then washed twice with PBS, blocked with 2% BSA for 1 hr at 37 C, and washed twice with PBS before use. Subconfluent cells were trypsinized, resuspended in K-SFM and seeded at a density of 3x10 4 cells per well. After 30 min at 37 C, nonadherent cells were washed away with PBS. Adherent cells were fixed in 4% PFA, washed with H2O, stained for 10 min with crystal violet, washed with H2O, and then lysed in 2% SDS. Absorbance was measured at 490 nm on a microplate reader. Background values (binding to BSA) were subtracted from all other values. To determine cell spreading, subconfluent cells were trypsinized, resuspended in K-SFM, and then seeded in 12-well plates coated with Col-1 or Ln-511. Cells were photographed on a Widefield CCD system using 10x and 20x dry lens objectives (Carl Zeiss MicroImaging, Inc.). The number of spread cells was 176
179 counted and expressed as a percentage of the total number of cells. Values shown represent the averages of 3 experiments. In each experiment, approximately 500 cells were analyzed for each condition. Cell lysis and Western blotting Cells were washed in ice-cold PBS and lysed on ice in RIPA buffer (25 mm Tris/HCl ph 7.6, 150 mm NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS), supplemented with protease inhibitor cocktail (Sigma). Cell lysates were cleared by centrifugation at 13,000xg, heated at 95ºC in SDS sample buffer (50 mm Tris-HCl ph 6.8, 2% SDS, 10% glycerol, 1% β-mercaptoethanol, 12.5 mm EDTA, 0.02 % bromophenol blue), and proteins were resolved by SDS-PAGE, after which they were transferred to polyvinylidene difluoride membranes (Millipore) and analyzed by Western blotting followed by ECL using the SuperSignal system (Pierce Chemical Co.). ACKnoWleDGEMents We are grateful to Dr. J. Bakkers, Dr. S. Schulte-Merker, S. Chocron, and M. Witte for organizing the forward mutagenesis screen at the Hubrecht Institute. We thank Dr. M. Hammerschmidt for his generous gift of the bdf/itga3 mutant, and Dr. T. Sasaki for kindly providing us with the antibody against Ln-332. This work was financially supported by DEBRA UK. ABBREVIatIons bdf, badfin; BM, basement membrane; Col, collagen; dpf, days post-fertilization; EB, epidermolysis bullosa; FA, focal adhesion; FERM, four-point-one/ezrin/radixin/moesin; HD, hemidesmosome; hpf, hrs post-fertilization; ILK, integrin-linked kinase; KS, Kindler syndrome; Ln, laminin; loc, lost-contact; PH, pleckstrin homology; rof, rupturing-of-fins 6 RefeRences 1. Geiger B, and KM Yamada (2011) Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol 3, doi: /cshperspect. a Margadant C, et al (2008) Regulation of hemidesmosome disassembly by growth factor receptors. Curr Opin Cell Biol 20, Natsuga K, et al (2010) Animal models of epidermolysis bullosa. Dermatol Clin 28, DiPersio CM, et al (1997) a3b1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol 137, Hodivala-Dilke KM, et al (1998) Novel roles for a3b1 integrin as a regulator of cytoskeletal assembly and as a transdominant inhibitor of integrin receptor function in mouse keratinocytes. J Cell Biol 142, Margadant C, et al (2010) Unique and redundant functions of integrins in the epidermis. FASEB J 24, Has C, et al (2012) Integrin a3 mutations with kidney, lung, and skin disease. N Engl J Med 366, Brakebusch C, et al (2000) Skin and hair follicle integrity is crucially dependent on β1 integrin expression in keratinocytes. EMBO J 19, Studying epidermal adhesion in vivo 177
180 9. Raghavan S, et al (2000) Conditional ablation of β1 integrin in skin: severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol 150, Margadant C, et al (2009) Integrin α3β1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci 122, Lorenz K, et al (2007) Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J Cell Biol 177, Nakrieko KA, et al (2008) Impaired hair follicle morphogenesis and polarized keratinocyte movement upon conditional inactivation of integrin-linked kinase in the epidermis. Mol Biol Cell 19, Ashton GHS (2004) Kindler syndrome. Clin Exp Dermatol 29, White SJ, and WH McLean (2005) Kindler surprise: mutations in a novel actin-associated protein cause Kindler syndrome. J Dermatol Sci 38, Sadler E, et al (2006) Novel KIND1 gene mutation in Kindler syndrome patients with severe gastro-intestinal tract involvement. Arch Dermatol 142, Kern JS, et al (2007) Chronic colitis due to an epithelial barrier defect: the role of kindlin-1 isoforms. J Pathol 213, Jobard F, et al (2003) Identification of mutations in a new gene encoding a FERM family protein with a pleckstrin homology domain in Kindler syndrome. Hum Mol Genet 12, Siegel DH, et al (2003) Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin-extracellular matrix linker protein UNC-112, causes Kindler syndrome. Am J Hum Genet 73, Herz C, et al (2006) Kindlin-1 is a phosphoprotein involved in regulation of polarity, proliferation, and motility of epidermal keratinocytes. J Biol Chem 281, Ussar S, et al (2006) The kindlins: subcellular localization and expression during murine development. Exp Cell Res 312, Larjava H, et al (2008) Kindlins: essential regulators of integrin signalling and cellmatrix adhesion. EMBO Rep 9, Meves A, et al (2009) The kindlin protein family: new members to the club of focal adhesion proteins. Trends Cell Biol 19, Carney TJ, et al (2010) Genetic analysis of fin development in zebrafish identifies furin and hemicentin1 as potential novel fraser syndrome disease genes. PLoS Genet 6, e Sonawane M, et al (2005) Zebrafish penner/ lgl2 functions in hemidesmosome formation, maintenance of cellular morphology and growth regulation in the developing basal epidermis. Development 132, Sonawane M, et al (2009) Lgl2 and E- cadherin act antagonistically to regulate hemidesmosome formation during epidermal development in zebrafish. Development 136, Webb AE, et al (2007) Laminin-a5 is essential for the formation of the zebrafish fins. Dev Biol 311, Has C, et al (2008) C-terminally truncated kindlin-1 leads to abnormal adhesion and migration of keratinocytes. Br J Dermatol 159, Harburger DS, et al (2009) Kindlin-1 and -2 directly bind the C-terminal region of b-integrin cytoplasmic tails and exert integrin-specific activation effects. J Biol Chem 284, Has C et al (2006) Molecular basis of Kindler syndrome in Italy: novel and recurrent alu/ alu recombination, splice site, nonsense, and frameshift mutations in the KIND1 gene. J Invest Dermatol 126, Nagae M, et al (2012) Crystal structure of α5β1 integrin ectodomain: Atomic details of the fibronectin receptor. J Cell Biol 197, Delwel GO, et al (1997) Identification of the cleavage sites in the a6a integrin subunit: structural requirements for cleavage and functional analysis of the uncleaved a6ab1 integrin. Biochem J 324, Lissitzky JC, et al (2000) Endoproteolytic processing of integrin pro-a subunits involves the redundant function of furin and proprotein convertase (PC) 5A, but not paired basic amino acid converting enzyme (PACE) 4, PC5B or PC7. Biochem J 346, Chattopadhyay N, et al (2003). a3b1 integrin-cd151, a component of the cadherin-catenin complex, regulates PTPmu expression and cell-cell adhesion. J Cell Biol 163, Shigeta M, et al (2003) CD151 regulates epithelial cell-cell adhesion through PKC- 178
181 and Cdc42-dependent actin cytoskeletal reorganization. J Cell Biol 163, Zhang F, et al (2003) Distinct ligand binding sites in integrin a3b1 regulate matrix adhesion and cell-cell contact. J Cell Biol 163, Greciano PG, et al (2012) Laminin-511 partners with laminin-332 to mediate directional migration of Madin-Darby canine kidney epithelial cells. Mol Biol Cell 23, Ussar S, et al (2008) Loss of kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet 4, e He Y, et al (2011) Kindlin-1 and -2 have overlapping functions in epithelial cells; implications for phenotype modification. Am J Pathol 178, Bandyopadhyay A, et al (2012) Uncovering functional differences between kindlin-1 and kindlin-2 in keratinocytes. J Cell Sci 125, Lai-Cheong JE, et al (2008) Colocalization of kindlin-1, kindlin-2, and migfilin at keratinocyte focal adhesion and relevance to the pathophysiology of Kindler syndrome. J Invest Dermatol 128, Knoll R, et al (2007) Laminin-α4 and integrin-linked kinase mutations cause human cardiomyopathy via simultaneous defects in cardiomyocytes and endothelial cells. Circulation 116, Postel R, et al (2008) Zebrafish integrinlinked kinase is required in skeletal muscles for strengthening the integrin- ECM adhesion complex. Dev Biol 318, Montanez E, et al (2008) Kindlin-2 controls bidirectional signaling of integrins. Genes Dev 22, Mackinnon AC, et al (2002) C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr Biol 12, Dowling JJ, et al (2008) Kindlin-2 is an essential component of intercalated discs and is required for vertebrate cardiac structure and function. Circ Res 102, Dowling JJ, et al (2008) Kindlin-2 is required for myocyte elongation and is essential for myogenesis. BMC Cell Biol 9, Sachs N, et al (2006) Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol 175, Wienholds E, et al (2003) Efficient targetselected mutagenesis in zebrafish. Genome Res 13, Thisse C, et al (1993) Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development 119, Studying epidermal adhesion in vivo 179
182 Table S3 Quantification of synergistic interactions. Injected morpholino at the 1-cell stage n % wild-type phenotype % fin rupturing uninjected kindlin-1 splice-mo (1:4) kindlin-1 ATG-MO (1:12) itga3 splice-mo (1:6) itga3 ATG-MO (1:12) ilk splice-mo (1:30) itga3 splice-mo (1:6) + kindlin-1 splice-mo (1:4) itga3 splice-mo (1:6) + ilk splice-mo (1:30) ilk splice-mo (1:30) + kindlin-1 splice-mo (1:4) itga3 ATG-MO (1:12) + kindlin-1 splice-mo (1:4) ilk splice-mo (1:30)+ itga3 ATG-MO (1:12) kindlin-1 ATG-MO (1:12) + itga3 splice-mo (1:12) kindlin-1 ATG-MO (1:12) + ilk splice-mo (1:30) itga3 splice-mo (1:6) + kindlin-1 splice-mo (1:4) + kindlin-1 RNA (100pg) itga3 splice-mo (1:6) + ilk splice-mo (1:30) + ilk RNA (100pg)
183
184
185 Distinct roles of talin and kindlin in regulating integrin α5β1 function and trafficking Coert Margadant 1, Maaike Kreft 1, Dirk-Jan de Groot 1, Jim C. Norman 2, and Arnoud Sonnenberg 1 1 Division of Cell Biology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX, Amsterdam, The Netherlands. 2 Integrin Cell Biology laboratory, Beatson Institute for Cancer Research, Cancer Research UK, Glasgow, G61 3BX, Scotland. Curr Biol 22, 1-10 (2012)
186
187 ABSTRAct Integrins are heterodimeric αβ transmembrane receptors that play key roles in cellular physiology and pathology. Accumulating data indicate that two NPxY motifs in the b-subunit synergistically promote integrin activation through the binding of talin and kindlin. However, it is unclear how the individual motifs regulate integrin function and trafficking. To investigate how the NPxY motifs individually control integrin a5b1 function and trafficking, we introduced Y>A mutations in either motif. Disruption of the membrane-proximal NPxY completely prevented a5b1-induced morphological changes, cell scattering and migration, and fibronectin fibrillogenesis. In addition, it reduced a5b1 internalization but not its recycling. In contrast, disruption of the membrane-distal NPxY promoted degradation of a5b1 in late endosomes/lysosomes, but did not prevent a5b1-dependent cell scattering, migration, or fibronectin fibrillogenesis. Whereas depletion of either talin-1 or kindlin-2 reduced a5b1 binding to fibronectin and cell adhesion, talin-1 depletion recapitulated the loss-of-function phenotype of the membrane-proximal NPxY mutation, while kindlin-2 depletion induced a5b1 accumulation in lysosomes and degradation. Thus, the two NPxY motifs of β1 play distinct and separable roles in controlling the function and trafficking of a5b1. Whereas talin-binding to the membrane-proximal NPxY is crucial for connecting a5b1 to the actin cytoskeleton and thus to permit the tension required for fibronectin fibrillogenesis and cell migration, kindlin-binding to the membrane-distal NPxY is dispensable for these events but regulates a5b1 surface expression and degradation. IntRODUctIon Integrins constitute a family of 24 heterodimeric αβ transmembrane receptors that connect the extracellular matrix (ECM) to the cytoskeleton and thus regulate cell adhesion, spreading and migration, and ECM organization. Integrins exist in low- and high-affinity conformations for ligand, and the allosteric change favouring high-affinity can be induced either by cytoplasmic events ( insideout activation) or by extracellular factors ( outside-in activation) (1). Ligand-binding triggers integrin clustering (avidity), connection to the cytoskeleton, and the assembly of adhesion complexes including focal adhesions (FAs) and fibrillar adhesions (FBs) (2). Integrins in circulating cells such as the platelet-integrin aiibb3 are kept in the low-affinity conformation predominantly by a salt bridge between the a- and b-subunit, which is disrupted upon talin-binding to a conserved membrane-proximal (MP)-NPxY motif in the β-cytoplasmic tail (1). Furthermore, aiibb3 activation requires kindlin-3- binding to a membrane-distal (MD)- NxxY motif (3). Whereas tyrosine-phosphorylation of these motifs is important for aiibb3 function in vivo, mutations that impair phosphorylation of both motifs in the b1-tail do not cause abnormalities in mice, and neither does a D>A mutation that prevents the formation of the salt- 7 NPxY-regulated integrin activation and trafficking 185
188 bridge (4-6). Indeed, it is increasingly recognized that activation-mechanisms differ across cell types and between individual integrins, and inside-out activation may be less important for b1-integrins in adherent cells (7,8). Integrin function in adherent cells depends strongly on talinmediated increase in avidity and connection to the actin cytoskeleton, as well as on the dynamic regulation of integrin internalization and recycling (9-12). NPxY motifs are canonical signals for clathrin-mediated endocytosis, but it is controversial to what extent the NPxY motifs in integrin b-subunits control integrin internalization, as integrins can internalize via both clathrin-dependent and clathrin-independent mechanisms (13). Clathrin adaptors including Dab-2 and Numb bind to the NPxY motifs, and depletion of Dab-2, Numb, or clathrin leads to decreased integrin endocytosis (14-17). Accordingly, endocytosis of b1-integrins is reduced by Y>F substitutions in both NPxY motifs (18). However, the same mutations cause no phenotype in mice. In fact, more disruptive Y>A mutations in both NPxY motifs dramatically decrease, in addition to integrin activation, also integrin surface expression (5,6). The latter observation is intriguing and suggests that the NPxY motifs may actually promote integrin recycling, rather than internalization. It is likely that the regulation of integrin activation and trafficking by the NPxY motifs is interconnected, as accumulating evidence indicates that active and inactive integrins are internalized and recycled via separate pathways and through different compartments (19-23). Here, we investigate how the individual NPxY motifs in the b1-tail coordinate activation, internalization, and subsequent intracellular sorting and recycling of the fibronectin (FN)-binding integrin a5b1. We find that whereas both contribute to internalization, disruption of the MP-NPxY leads to a complete loss-offunction, but does not impair recycling. By contrast, disruption of the MD-NPxY leads to lysosomal degradation. Nevertheless, it does not abolish a5b1 function. The loss-of-function phenotype of the MP-NPxY mutant is recapitulated by depletion of talin-1, whereas depletion of kindlin-2 recapitulates the reduction in a5b1 surface levels, accumulation in lysosomes, and increased degradation. ResUlts The MP-NPxY but not the MD-NPxY motif in b1 is required for a5b1-induced disruption of cell-cell junctions and cell scattering. Expression of the integrin b1-subunit into b1-null epithelial GE11 cells, creating GEb1 cells, results primarily in surface expression of a5b1 and causes the loss of cell-cell contacts, cell scattering and fast cell migration. In addition, a5b1 induces the assembly of FBs, FN fibrillogenesis, and a morphological change toward a contractile, fibroblast-like phenotype with multiple protrusions and a few large FAs (Figure S1) (25-27). We introduced Y>A mutations in b1a, either in the MP-NPxY motif (b1 Y783A ), the MD-NPxY motif (b1 Y795A ), or both (b1 Y783/795A ), and then stably expressed these mutants in GE11 (Figure 1A). Strikingly, cells expressing 186
189 a5b1 Y783A or a5b1 Y783/795A completely failed to induce cell scattering and morphological changes, and thus resembled the knockout cells (Figures 1B-D). In contrast, a5b1 Y795A caused cell scattering, disruption of cell-cell contacts, and a flattened, mesenchymal morphology, albeit with less-pronounced protrusions than in GEb1 cells (Figures 1B-D). In fact, cell spreading of GEb1 Y795A cells was on average increased two-fold compared to GEb1 (2005 versus 870 mm 2 ). FAs were visualized using an antibody against phosphotyrosine (PY), which co-localizes with several FA proteins (Figure S2A). The number of FAs was up to 5 times higher (315 versus 64 FAs/cell), although individual adhesions were on average slightly smaller (0.53 versus 0.64 mm 2 ) (Figures 1E-G). The total adhesive area of GEb1 Y795A was almost two-fold larger than that of GEb1 (8% versus 5%; Figure 1H). We then generated mcherryvinculin-expressing GEb1 and GEb1 Y795A cells, and analysed FAs by live-cell imaging using total internal reflection microscopy (TIRF). The number of FAs was clearly increased in GEb1 Y795A cells, as compared to that in GEb1 (Figure S2B). Together, these 7 Figure 1 Disruption of the MP- but not the MD-NPxY in b1 prevents a5b1-induced dissociation of cell-cell contacts and cell scattering. (A) Amino-acid sequences of the cyto-tail of wild-type b1a and mutants. The MP- and MD-NPxY motifs are underlined. (B) Phase-contrast images of GE11, GEb1, GEb1 Y783A, GEb1 Y795A, and GEb1 Y783/795A cells. Bar, 40 mm. (C) ZO-1 (green), pan-cadherin (Cadh; red), and DAPI (blue). Bar, 20 mm. (D) P(Y) (green), F-actin (red), and nuclei (blue). Bar, 20 mm. (E-H) Confocal images of GEb1 and GEb1 Y795A cells (n~30 cells) were analyzed to quantify cell area (E), the average number of FAs per cell (F), and the average FA size (G). The adhesive area was calculated as the ratio total FA area / cell area (H). Statistically significant differences are indicated by ** (p<0.01) or **** (p<0.0001). NPxY-regulated integrin activation and trafficking 187
190 data show that disruption of the MP-NPxY prevents a5b1-induced cell scattering and morphological changes, whereas disruption of the MD-NPxY does not. The MP-NPxY but not the MD-NPxY motif in b1 is required for a5b1- induced fn fibrillogenesis, fibrillar adhesion formation, and cell migration. We next investigated FN fibrillogenesis in confluent cell cultures by confocal microscopy. Both b1 Y783A and b1 Y783/795A failed to support FN fibrillogenesis, whereas cells expressing b1 Y795A formed FN fibrils, similarly to GEb1 cells (Figure 2A). Transient transfection of GFP-tensin-1 and staining of FAs revealed that GEb1 and GEb1 Y795A cells contained centrally-located tensin-1- positive FBs (Figure 2B, dashed arrows) that were clearly separated from PY-positive FAs at the cell periphery, which was not observed in GE11, GEb1 Y783A, and GEb1 Y783/795A cells (solid arrows). In addition, GEb1 and GEb1 Y795A cells were fastmigrating cells, whereas GE11, GEb1 Y783A and b1 Y783/795A were significantly less motile (Figures 2C-E). We then introduced the same b1-mutants in a different b1-deficient cell line, GD25. Like in GE11, both wild-type b1 and b1 Y795A induced a loss of cadherinbased cell-cell junctions, cell scattering, and a redistribution of FAs to the tips of protrusions. In contrast, GDb1 Y783A and GDb1 Y783/795A cells grow in islands like GD25, with cadherin-based cell- Figure 2 Disruption of the MP- but not the MD-NPxY in b1 prevents a5b1-induced FN fibrillogenesis, assembly of FBs, and cell migration. (A) FN (green) and nuclei (blue) visualized by confocal microscopy. Bar, 100 mm. (B) GFP-tensin-1 (green), P(Y) (red), and F-actin (blue) visualized by confocal microscopy. Bar, 20 mm. (C) Images from time-lapse series with overlaid migration tracks. Bar, 40 mm. (D) Individual migration tracks from ~15 cells. (E) Velocity of cell migration (averages ± SEM from ~250 cells, 3 independent experiments). Statistically significant differences are indicated by ** (p<0.01) or **** (p<0.0001). ns; not significant. 188
191 cell junctions and many small FAs dispersed over the basal cell surface (Figures S3A-C). Furthermore, both GDb1 and GDb1 Y795A cells, but not GDb1 Y783A and GDb1 Y783/795A, supported FN fibrillogenesis (Figure S3D) and displayed large, central FBs (Figure S3E, dashed arrows), and small peripheral FAs (Figure S3E, solid arrows). Last, both GDb1 and GDb1 Y795A cells migrated significantly faster over FN than did GD25, GDb1 Y783A, and GDb1 Y783/795A cells (Figures S4A,B). Taken together, these data strongly suggest that the MP-NPxY motif in b1 is absolutely required for a5b1 function, whereas the MD-NPxY motif is not. The MP-NPxY motif in b1 is required for growth factor-induced cell scattering and loss of cell-cell contacts. The previous sections have shown that the MP-NPxY motif in b1 is essential for a5b1-induced changes reminiscent of those that occur during epithelial-tomesenchymal transition (EMT), such as disruption of cell-cell contacts and the stimulation of cell scattering, migration, and FN fibrillogenesis. We next investigated whether well-established physiological inducers of cell scattering and other EMT-related events, such as hepatocyte growth factor (HGF) or transforming growth factor-b (TGF-b), can overcome the requirement for the MP-NPxY motif. Stimulation with either HGF or TGF-b triggered morphological changes in all cell lines (Figure 3). However, although the islands formed by GEb1 Y783A and (to a lesser extent) GEb1 Y783/795A cells seemed less coherent, a dramatic loss of cell-cell adhesion and concomitant cell scattering was not observed, even after prolonged incubation (up to 72 hrs) with either factor (Figure 3). This was not due to an intrinsic defect in the signaling machinery to respond to these growth factors, as HGF- and TGF-b-induced changes in gene expression were detected in all cell lines (data not shown). Thus, these data show that disruption of the MP-NPxY motif in b1 prevents cell scattering, even in the presence of known inducers of cell scattering and EMT. The MP-NPxY motif in b1 is required for a5b1 activation, whereas the MD-NPxY motif prevents accumulation in late endosomes/lysosomes and subsequent degradation. We next analyzed the cell-surface expression and activation status of the mutant integrins. Interestingly, although all cell lines had been sorted for equal expression levels shortly after retroviral delivery, FACS-analysis consistently revealed that surface expression of the a5b1 Y795A and a5b1 Y783/795A mutants was dramatically reduced (to 50% of that of wild-type a5b1), both in GE11 and in GD25 cells, whereas expression of a5b1 Y783A was not reduced (Figures 4A, S4C). Consistent with the biological phenotype, the activation-index (ratio active b1/total b1) of both a5b1 Y783A and a5b1 Y783/795A at the cell-surface was low (~30% of that of wild-type b1; Figure 4A). In contrast, the activation-index of a5b1 Y795A was slightly decreased in GE11 (~70% of that of wild-type b1; Figure 4A) and unaltered in GD25 cells (Figure S4C). Consistent with these results, FN-binding to cellsurface a5b1 was significantly reduced in GEb1 Y783A and GEb1 Y783/795A cells, but not in GEb1 Y795A. The differences in affinity 7 NPxY-regulated integrin activation and trafficking 189
192 Figure 3 Disruption of the MP-NPxY in b1 prevents dissociation of cell-cell contacts and cell scattering induced by HGF or TGF-b. (A) Images of GE11, GEb1, GEb1 Y783A, GEb1 Y795A, and GEb1 Y783/795A cells that were untreated (Ctrl), or stimulated for 48 hrs with HGF (5 ng/ml) or TGF-b (2 ng/ml). Bar, 100 mm. (B) Cell scattering was quantified as the average number of neighbouring cells per cell at 60 hrs of stimulation with TGF-b (2 ng/ml) or HGF (5 ng/ml). Data represent the means ± SEM from ~150 cells per condition. Statistically significant differences are indicated by * (p<0.05). ns; not significant. could not be attributed to altered expression of talins or kindlins (Figure S4D). We next assessed whether the NPxY motifs regulate the subcellular distribution of a5b1, using live-cell confocal microscopy on cells transiently transfected with egfp-a5. Wild-type a5b1 was distributed all over the membrane. In addition, a large pool was found in vesicles, and co-transfection of mcherrytagged lysosomal-associated membrane protein (LAMP)-1 revealed that a proportion of these were late endosomes (LE)/ lysosomes (Figure 4B). Localization of a5b1 Y783A was also observed at the membrane, but was virtually absent from vesicles (Figure 4B). In contrast, a5b1 Y795A and a5b1 Y783/795A were hardly visible at the membrane, but accumulated dramatically in LE/lysosomes (Figure 4B). Western blotting for the b1-subunit supported the results obtained by FACS; the mature pool of b1 (130 kd) was clearly reduced in GEb1 Y795A and GEb1 Y783/795A, but not in GEb1 Y783A cells (Figure 4B). The precursor b1 (110 kd) was reduced in all mutants. Treatment with the lysosomal inhibitor Bafilomycin A1 strongly increased cellsurface b1 expression in all cell lines, but most prominently in GEb1 Y795A and GEb1 Y783/795A cells. In contrast, expression of the transferrin receptor (TfnR), which is not routed to lysosomes, was unaffected (Figure 4C). We then investigated the stability of a5b1 over time by surfacelabeling with biotin, followed by capture- ELISA. With increasing incubation time, both a5b1 Y795A and a5b1 Y783/795A were 190
193 degraded significantly more rapidly than wild-type a5b1, whereas degradation of a5b1 Y783A occurred initially much more slowly but tended toward wild-type levels after 6 hrs (Figure 4D). Together, these results indicate that the MP-NPxY motif in b1 primarily regulates integrin a5b1 activation, while the MD-NPxY motif regulates cell-surface expression, by preventing lysosomal degradation. Integrin activation triggers a5b1 accumulation in late endosomes/ lysosomes, but not cell scattering, upon disruption of the MP-NPxY Because it is primarily the active, FNbound fraction of a5b1 that traffics to the LE/lysosomal system (22,23), we reasoned that a5b1 Y795A and a5b1 Y783/795A in LE/lysosomes are in the active conformation. Colocalisation of b1 with 9EG7 staining supported this hypothesis (Figure 5A). To confirm that integrin activation triggers lysosomal routing, we 7 Figure 4 The MP-NPxY motif is required for a5b1 activation, whereas the MD-NPxY motif regulates a5b1 surface expression, accumulation in LE/lysosomes, and degradation. (A) Quantification of b1 surfacelevels, the active b1/total b1 ratio, and relative FN binding (FN/b1), as determined by FACS. Data represent averages ± SEM of 3 independent experiments. (B) Live-cell confocal microscopy of egfp-a5 (green; upper panel) and mcherry-lamp-1 (red; middle panel). The degree of co-localization is indicated. Bar, 10 mm. (C) Expression of b1 and TfnR in cells cultured for 8 hrs in the absence or the presence of Bafilomycin A1 (100 nm). (D) Degradation of a5b1 (averages ± SEM of 3 independent experiments). Statistically significant differences with GEb1 cells are indicated by * (p<0.05), ** (p<0.01), *** (p<0.001), or **** (p<0.0001). ns; not significant. NPxY-regulated integrin activation and trafficking 191
194 used a constitutively-active a5-mutant (CA-a5) containing an F1025A mutation in the GFFKR sequence (Figure 5B) (27). Subcellular distribution of CA-a5b1 was visualized in cells co-expressing egfp- CA-a5 and mcherry-lamp-1. In GEb1 cells, CA-a5b1 was detected at the membrane and in LE/lysosomes (Figure 5B). Expression of CA-a5 in GEb1 Y783A cells directed a significant fraction of a5b1 to LE/lysosomes, but had no obvious effect on the subcellular distribution of a5b1 Y795A and a5b1 Y783/795A (Figure 5B). We next incubated cells either with the b1-activating antibody TS2/16 (10 mg/ ml), or with K-20 (10 mg/ml), which recognizes both active and inactive b1-integrins and does not induce integrin activation. Whereas TS2/16 accumulated dramatically in LE/lysosomes in all cell lines, K-20 was predominantly detected in LE/ lysosomes in GEb1 Y795A and GEb1 Y793/795A cells, but not in GEb1 and GEb1 Y783A, as in steady-state conditions (Figure 5C). Importantly, neither expression of CA-a5 nor incubation with TS2/16 induced cell scattering or the concomitant morphological changes in GEb1 Y783 and GEb1 Y783/795A cells (Figure 5C and data not shown). We then tested the effects of TS2/16 on a5b1-mediated cell adhesion to FN, in the presence of GRGDSP-peptide (0.5 mg/ ml) to block FN-binding by endogenous avb3 (28). Basal a5b1-mediated cell adhesion to FN was comparable in GEb1 and GEb1 Y795A cells, but was considerably reduced in GEb1 Y783A and completely prevented in GEb1 Y793/795A cells. Interestingly, TS2/16 significantly increased cell adhesion in all cell lines except in GEb1 Y795A (Figure 5D). In summary, these results show that lysosomal a5b1 Y795A and a5b1 Y783/795A are in active conformation, that activated integrins from the cellsurface accumulate in LE/lysosomes, and that outside-in activation can modestly promote cell adhesion, but not cell scattering and concomitant morphological changes, in the MP-NPxY mutants. The NPxY motifs regulate a5b1 internalization and recycling We next addressed how a5b1 internalization and recycling are regulated by the NPxY motifs by biotin-labeling of cell-surface integrins followed by capture- ELISA, according to established methods (10). Interestingly, internalization of all b1-mutants in the presence of the recycling inhibitor primaquine was reduced about two-fold compared to that of wildtype a5b1, whereas internalization of TfnR in the same cells was not (Figures 6A,S5A). A large fraction of internalized a5b1 (up to 60%) was rapidly degraded in GEb1 Y795A and GEb1 Y793/795A cells, but not in GEb1 Y783A (Figure 6B), while degradation of internalized TfnR was negligible in all cell lines (data not shown). Importantly, despite the dramatic degradation of internalized a5b1 Y795A and a5b1 Y793/795, there was still measurable recycling of the two mutants, albeit less than of wildtype a5b1. Very rapid recycling was observed for a5b1 Y783A (Figure 6C). In contrast, recycling of TfnR was similar in all mutant cell lines, though unexpectedly higher than in GEb1 cells (Figure S5B). Together, these results demonstrate that both NPxY motifs contribute to a5b1 internalization, and that the MD-NPxY but not the MP-NPxY is critical to allow internalized a5b1 to escape lysosomal routing and degradation. Importantly, the 192
195 igure 5 Disruption of the MP-NPxY does not abolish activation-triggered a5b1 routing to LE / lysosomes, but prevents cell scattering. (A) Integrin b1 (green), active b1 (9EG7; red), and F-actin (blue) in GEb1 Y795A and GEb1 Y783/795A cells. Bar, 10 mm. (B) Top, amino-acid sequences of the cyto-tail of human a5 and constitutively-active a5 (CA-a5). Bottom, confocal images of egfp-ca-a5 (green) and mcherry- LAMP-1 (red). The degree of co-localization is indicated. Bar, 20 mm. (C) Cells were incubated overnight with TS2/16 or K-20, after which they were stained using an antibody against endogenous LAMP-1, followed by a FITC-conjugated antibody against TS2/16 or K-20 (green), and a TRITC-conjugated antibody to visualize LAMP-1 (red). F-actin, blue. Bar, 10 mm. The degree of co-localization is indicated. (D) Effects of TS2/16 on a5b1-mediated cell adhesion to FN (averages ± SEM of 3 independent experiments). Statistically significant differences are indicated by * (p<0.05), *** (p<0.001), or **** (p<0.0001). 7 MD-NPxY mutant that escapes lysosomal degradation can still be recycled. Depletion of b1 or talin-1 prevents a5b1-mediated cell scattering and morphological changes, whereas depletion of kindlin-2 induces a5b1 degradation We next investigated whether the phenotypes of the Y>A mutants can be recapitulated by the depletion of talin or kindlin. To this end, we introduced shrnas against talin-1, kindlin-2 or b1 into GEb1 cells. Knockdown of protein expression was verified by Western blotting and was highly efficient (more than 90%; Figure 7A). Depletion of b1 clearly reversed the a5b1-mediated changes; cell scattering was prevented and cells grew in GE11- like islands with cadherin-based cell-cell contacts and many small and randomly distributed FAs, rather than a few large FAs at the cell periphery (Figures 7B,C). NPxY-regulated integrin activation and trafficking 193
196 Figure 6 Both NPxY motifs promote a5b1 internalization, and integrity of the MD-NPxY is required for recycling. (A) Internalization of a5b1 in the presence of 0.6 mm primaquine (averages ± SEM of 3 independent experiments). (B) Degradation of internalized a5b1 (averages ± SEM of 3 independent experiments). (C) Recycling of internalized a5b1 (averages ± SEM of 3 independent experiments). Statistically significant differences with GEb1 cells are indicated by * (p<0.05), ** (p<0.01), *** (p<0.001), or **** (p<0.0001). Talin-1 depletion also reduced cell scattering and caused at least a partial reversal toward the formation of islands with cadherin-based cell-cell contacts. On the contrary, kindlin-2 depletion seemed to have no apparent effect on cell scattering or morphology (Figures 7B,C). We then assessed b1 expression at the cell-surface as well as a5b1 binding to FN by FACS. While talin-1 depletion seemed to have no apparent effect on b1 cell-surface expression, binding of soluble FN was strongly decreased. By contrast, kindlin-2 knockdown decreased b1 cell-surface expression while modestly reducing FN binding (Figures 7D,E). Consistent with this result, cell adhesion to FN was strongly decreased by depletion of talin-1 and to a lesser extent by kindlin-2 depletion (Figure 7F). Live-cell microscopy on GFP-a5 and mcherry-lamp-1 co-expressing cells revealed a reduction of a5b1 at the plasma membrane in kindlin-2-depleted cells, confirming the results obtained by FACS, and increased a5b1 accumulation was observed in LE/lysosomes (Figure 7G). Moreover, degradation assays revealed that the degradation rate of cell-surface a5b1 is increased in kindlin-2-depleted cells (Figure 7H). In conclusion, these results suggest that whereas both talin-1 and kindlin-2 increase a5b1 activation and cell adhesion to FN, depletion of talin-1 in GEb1 cells causes a reversal of the a5b1- dependent phenotype but depletion of kindlin-2 does not. Instead, knockdown of kindlin-2 expression leads to reduced cell-surface levels of a5b1, increased accumulation in LE/lysosomes, and enhanced a5b1 degradation. 194
197 FFigure 7 Depletion of b1 or talin-1 in GEb1 cells induces MET, whereas depletion of kindlin-2 triggers a5b1 accumulation in LE / lysosomes and degradation. (A) Western blots showing depletion of the indicated proteins in GEb1 cells expressing shrnas against b1, talin-1 or kindlin-2. (B) Phase-contrast images of GEb1 and GEb1 cells expressing shrnas against b1, talin-1 or kindlin-2. Bar, 30 mm. (C) Confocal images showing P(Y) (green), F-actin (red), and pan-cadherin (blue). Bar, 20 mm. (D) Expression of b1 at the cell surface as determined by FACS. Graphs represent the averages ± SEM of 3 experiments. (E) FN binding was determined by FACS. Depicted is the FN/b1 ratio, averages ± SEM of 3 experiments. (F) Cell adhesion to 10 mg/ml FN. (G) Live-cell confocal microscopy of egfp-a5 (green) and mcherry-lamp-1 (red) in GEb1 and GEb1_shkind-2 cells. The degree of co-localization is indicated. Bar, 10 mm. (H) Degradation of a5b1 (averages ± SEM of 3 experiments). Statistically significant differences are indicated by * (p<0.05), ** (p<0.01), *** (p<0.001). ns; not significant. 7 DIscUssIon Here, we show that Y>A mutation in the talin-binding MP-NPxY (b1 Y783A ) completely abolishes a5b1 function, whereas the recycling of internalized integrins from endosomes is not impaired. In contrast, Y>A mutation in the kindlin-binding MD-NPxY (b1 Y795A ) leads to dramatic lysosomal degradation, but surprisingly does not abolish a5b1 activation or function. These data imply that the two NPxY motifs play quite distinct and separable roles with regard to controlling a5b1 activation and trafficking. The loss-of-function phenotype of the a5b1 Y783A mutant is not rescued by forced integrin activation, because neither NPxY-regulated integrin activation and trafficking 195
198 ectopic expression of CA-a5 nor treatment with TS2/16 induces b1-dependent cell scattering and morphological changes. Moreover, although TS2/16 increases a5b1 Y783A -mediated cell adhesion to FN, adhesion to FN in wild-type cells treated with TS2/16 is still higher, suggesting that even when activated, a5b1 Y783A does not optimally support cell adhesion. These findings emphasize the importance of the MP-NPxY motif for post-activation events. The Y>A mutation in the MP-NPxY motif disrupts the binding of both talin and tensin, which are the principal connectors of a5b1 to the actin cytoskeleton (29,30). Hence, simultaneous loss of talin- and tensin-binding will prevent all a5b1-induced events that require cytoskeletal contractility, including assembly of FAs and FBs, FN fibrillogenesis, and cell spreading, scattering, and migration (31-34). In addition, it likely also compromises cell adhesion, which is supported by the finding in D. melanogaster that integrins can bind ECM without talin but blister formation occurs nonetheless because there is no connection to the cytoskeleton (9). Talin-binding downstream of integrin activation is furthermore important to prevent recruitment of the negative regulator filamin (35,36). The pivotal role of talin was confirmed by talin-1 depletion in GEb1 cells, which prevented cell scattering and induced a reversal to islands with intact cell-cell junctions. However, the islands formed by talin-1-depleted cells still contained some protrusions and occasional large peripheral FAs, presumably due to residual talin expression or partial compensation for the loss of talin by tensin. Surprisingly, mutation of the MD-NPxY did not abolish a5b1 function, despite massively increased degradation. In fact, with only 50% of wildtype levels, a5b1 Y795A induced the whole gamut of a5b1-induced events, probably because there is still some residual recycling. Importantly, these data show that an intact MD-NPxY is not absolutely required for a5b1 function. A large accumulation of active integrins was detected in LE/lysosomes, both in GEb1 Y795A and in GEb1 Y783/795A cells, suggesting that even simultaneous disruption of both NPxY motifs does not completely prevent the adoption of the high-affinity conformation. However, the MP-NPxY is clearly dominant for integrin function, because the phenotype of GEb1 Y783/795A resembled that of GEb1 Y783, and expression of CA-a5 or treatment with TS2/16 also failed to induce cell scattering in GEb1 Y783/795A. Thus, GEb1 Y783/795A cells contain the combined effects of both mutations; impaired cell scattering, migration and FN fibrillogenesis due to disruption of the MP-NPxY, and increased degradation leading to low integrin expression, due to disruption of the MD-NPxY. Together, the two mutations reduce cell adhesion to that of b1-null cells. An important observation is that the absolute number of active conformers on the membrane, as judged by 9EG7 staining, is the same in GEb1 Y783A and GEb1 Y795A cells (about 30% of wild-type levels), whereas the phenotypes are strikingly different. This clearly demonstrates that the number of integrins in high-affinity conformation on the membrane does not predict their biological functionality, and emphasizes the importance of the internal integrin pool, as well as the dynamic regulation of integrin internal- 196
199 ization and recycling. We show here that both NPxY motifs in b1 contribute to a5b1 internalization, complementing a previous observation that integrin internalization is reduced upon simultaneous mutation of the NPxY motifs (18). Nevertheless, there is still considerable residual internalization, supporting earlier findings that many integrins can be internalized in both a clathrin-dependent and a clathrinindependent fashion, and in particular with the observation that a5b1 and FN are internalized via caveolae (21). Intriguingly, in addition to a role in integrin internalization, both NPxY motifs in b1 regulate integrin recycling. Whereas the a5b1 Y783A mutant is primarily localized at the membrane, the internal pool is small, which is in part caused by its decreased internalization, and in part by fast recycling back to the membrane. These data fit well with a recent study showing that inactive integrins are primarily at the membrane and hardly in vesicles, due to rapid recycling (20). Thus, the rapid recycling of a5b1 Y783A is possibly an indirect effect of its low activation-status. Our data support the well-established view of kindlins as co-activators for talininduced integrin activation, as knockdown of both talin-1 and kindlin-2 reduced FN-binding by a5b1 as well as cell adhesion, but depletion of talin-1 had a much stronger effect than depletion of kindlin-2. However, downstream of integrin activation, talin-1 is important for cell scattering while kindlin-2 is dispensable. Furthermore, depletion of kindlin-2 but not talin-1 reduced cell-surface expression of a5b1, consistent with a number of observations that cell-surface expression of several integrins is low in the absence of kindlins, whereas it is enhanced by kindlin overexpression (37-39). The decrease in cell-surface levels was concomitant with enhanced a5b1 accumulation in LE/ lysosomes and degradation, but the reduction of total a5b1 protein levels was not as prominent as in GEb1 Y795A, suggesting that kindlin-2 knockdown does not completely recapitulate the MD-NPxY mutation. Integrin routing is likely regulated by distinct factors in different subcellular compartments. Indeed, whereas kindlin-2 operates at the plasma membrane, two very recent reports have shown that upon internalization, sorting nexin-17 binds the MD-NPxY motif in endosomes to prevent b1-containing integrins from being delivered to LE/ lysosomes (40,41). Knockdown of sorting nexin-17 leads to reduced integrin recycling, enhanced lysosomal degradation, and reduced cell-surface levels, much like mutation of the MD-NPxY motif. Nevertheless, the two studies differ as to whether sorting nexin-17 is important for the recycling of inactive integrins only, or whether it mediates the recycling of both active and inactive integrins (40,41). Our data confirm the importance of the MD-NPxY in integrin trafficking and lysosomal degradation, and extend these observations to the role of the MP-NPxY, as well as the combined effects of both motifs in the regulation of integrin trafficking and integrin-regulated events such as cell scattering and migration. However, whereas our results indicate that depletion of kindlin-2 increases a5b1 degradation, one of the mentioned reports did not find that kindlin-2 regulates the degradation of b1-integrins. Rather, the effects of kindlin-2 on integrin cell-surface levels were attributed to transcriptional upregu- 7 NPxY-regulated integrin activation and trafficking 197
200 lation of the b1-subunit (41). The mechanisms of how kindlins regulate integrin expression will require further study. Of note, it was previously reported that the kindlin-mediated increase in a5b1 cellsurface levels requires an intact integrinbinding site in kindlin, suggesting that a direct interaction between kindlin and b1 is involved, at least for a5b1 (37). It will be important to resolve whether the mechanisms identified here apply only for a5b1 or also for other b1-integrins. The rates of internalization and recycling differ greatly between different integrins, suggesting that integrin-specific effects are regulated in part by differences in the sequences of the a-subunits (42). Indeed, accumulating evidence indicates that these recruit GTPases and other factors that control integrin trafficking and activation. For example, Rab21 GTPase binds to a2 and a5 and promotes integrin recruitment to the endocytic machinery, and subsequent displacement of Rab21 by p120rasgap leads to integrin recycling from early endosomes (43,44). Future work should focus more on the differences in regulation between individual integrin heterodimers. MateRIals and MetHODS Antibodies, plasmids and other materials Plasmids encoding egfp-a5 and egfp- CAa5 were generously donated by Dr. Donna Webb, GFP-tensin-1 was a kind gift from Dr. Ken Yamada, and mcherry- Vinculin was a kind gift from Dr. Johan de Rooij. Antibodies used were directed against actin (clone C4; Chemicon), FN (clone 10; BD Transduction Laboratories), the integrin a5-subunit (clone MFR5; Pharmingen), the integrin β1-subunit (clone TS2/16; Developmental Studies Hybridoma Bank, clone 9EG7; a kind gift from Dr. Dietmar Vestweber, clone K-20; a kind gift from Dr. Andre van Agthoven; and clone 18 from BD Transduction laboratories), LAMP-1 (clone 1D4B; Abcam), pan-cadherin (clone CH19; Sigma- Aldrich), P(Y) (clone 4G10; a kind gift from Dr. Kevin Wilhelmsen), talin (clone 8D4; Sigma-Aldrich), TfnR (clone C2; Pharmingen and clone H68.4; Invitrogen), and ZO-1 (clone ZMD.436; Zymed), and polyclonal antibodies against P(Y118) paxillin and P(Y397)FAK (Invitrogen), vinculin and kindlin-2 (Sigma-Aldrich). NHS-SS-biotin was from Pierce, and puromycin, zeocin, primaquine-bisphosphate, Bafilomycin A1, HGF, TGF-b, and FN were from Sigma-Aldrich. TRITC-, FITC-, and Cy5-conjugated secondary antibodies, lysotracker-red, phalloidins, and DAPI were from Molecular Probes (Eugene, OR), HRP-conjugated secondary antibodies and HRP-streptavidin were from Amersham, and GRGDSP peptide and Cy5-conjugated FN were generated at the NKI. Constructs, cell culture, transient transfections, retroviral and lentiviral transductions Cells were maintained in DMEM supplemented with 10% FCS, 100 U/ml penicillin and 100 U/ml streptomycin, at 37 C in a humidified atmosphere containing 5% CO 2. b1 Y783A, b1 Y795A, and b1 Y783/795A mutations were generated by PCR overlap 198
201 extension method using a cdna encoding human full-length b1a as a template. Wild-type b1a was isolated by digestion with XbaI and ligated into the pcdna3 vector. HindIII fragments were used to exchange wild-type b1a with b1a mutants. The mutants were then recloned into LZRS-IRES-zeo, which was transfected into Phoenix packaging cells using the Calcium Phosphate method. Viruscontaining supernatant was isolated 48 hrs later and stable expression in GE11 and GD25 was achieved by retroviral transduction, followed by selection with zeocin (200 mg/ml) and cell sorting. Transient transfection was performed 1 day prior to experiment using the Amaxa Nucleofector system (solution T, program T-13), according to the manufacturer s instructions. Stable cell lines expressing mcherryvinculin were generated by lentiviral transduction of the plv-cmv-mcherry- Vinculin-Ires-Puro-construct, followed by selection with 5 mg/ml puromycin. Generation of stable knockdown cell lines Short hairpins against the human integrin b1-subunit, mouse talin-1, and mouse kindlin-2, cloned into plko.1, were obtained from the TRC shrna Open Biosystems library. They were transfected into HEK 293FT cells together with the Virapower TM Packaging mix (Invitrogen), using Lipofectamine 2000 according to the manufacturers instructions. Viruscontaining supernatant was harvested 48 hrs later, transduced into GEb1 cells, and positive cells were selected with 5 mg/ml puromycin. Target sequences included C C A A ATC ATGTG G A G A ATGTA, G CCCTCC A G ATG A C ATA G A A A, G C C T TG C AT TA C TG C TG ATAT, CCTGT T TAC A AG G AG CTG A A A, and GCAAATTGTCAGAAGGAGTAA against b1, GCCACCAACAATCTGT- GTGAA, CGCTCCAAGAGTATTATTA- AT, and GCCCATTGTAATCTCTGC- TAA against talin-1; GCCTACCTTG- ATATGCCGTAT, GAAGTATAAGAGC- AAACAGAT, GATGAGGAGATGTTC- TACAAA, and GCCTTTGTGTTACA- ATATGAA against kindlin-2. Flow cytometry and cell sorting For flow cytometry and cell sorting, trypsinized cells were washed twice in PBS containing 2% FCS, and incubated with primary antibodies for 45 min at 4 C. Cells were then washed twice in 2% FCS/ PBS, incubated with appropriate secondary antibodies for 45 min at 4 C, washed twice in 2% FCS/PBS, and analyzed on a FACS Calibur (BD Biosciences). Alternatively, the cells were sorted on a MoFlo High Speed Cell Sorter (Beckman Coulter). To assess FN binding, cells were incubated with Cy5-conjugated FN (5 mg/ml) in the presence of GRGDSP peptide (500 mg/ml), to block FN-binding by endogenous avb3. Cell lysis and Western blotting Cells were washed in ice-cold PBS and lysed on ice in RIPA buffer (25 mm Tris/ HCl ph 7.6, 150 mm NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS), supplemented with protease inhibitor cocktail (Sigma). Cell lysates were centrifuged at 13,000xg, heated at 95ºC in SDS sample buffer (50 mm Tris- HCl ph 6.8, 2% SDS, 10% glycerol, 1% β-mercaptoethanol, 12.5 mm EDTA, 0.02 % bromophenol blue), and proteins were resolved by SDS-PAGE, after which they were transferred to polyvinylidene 7 NPxY-regulated integrin activation and trafficking 199
202 difluoride membranes (Millipore) and analyzed by Western blotting followed by ECL using the SuperSignal system (Pierce Chemical Co.). Phase-contrast microscopy, confocal microscopy and total internal reflection microscopy Phase-contrast images were acquired on a Zeiss microscope (Axiovert 25) at 10x (NA 0.25) or 20x (NA 0.3) magnification, using a Zeiss CCD camera (Axiocam MRC) and Zeiss Mr. Grab 1.0 software. For confocal microscopy of fixed cells, cells were prepared on coverslips as previously described (25), and images were acquired on an inverted confocal microscope (Leica AOBS) using 20x (NA 0.7) dry, 40x (NA 1.25) oil, and 63x (NA 1.32) oil objectives (Leica). Live-cell confocal movies of cells on glass-bottomed 3-cm plates were acquired using a 64x objective on an inverted confocal microscope (Fluoview FV1000, Olympus), at 37 C in an atmosphere containing 5% CO 2. TIRF movies of cells on glass coverslips were acquired using Leica application suite software on a Leica DMI600B system with a 63X objective (NA 1.47), at 37 C in an atmosphere containing 5% CO 2. Images and movies were processed using Photoshop 7.0 and ImageJ Quantification of co-localization, fas, and cell spreading Colocalization between a5 and LAMP-1 was determined from confocal images as described previously (23). Confocal images of P(Y)/F-actin stainings were acquired using fixed settings, backgroundsubtracted and thresholded in ImageJ 1.44, and FA size and number were then determined using the analyze particles function. Cell area was quantified from the F-actin-channel, and the adhesive area was determined as the total FA area / cell area. Values shown represent the averages of FAs from ~30 cells. Migration and adhesion assays For single-cell migration assays, cells were sparsely seeded on 10 mg/ml FN, and phase-contrast images were captured every 15 min at 37 C and 5% CO 2 on a Widefield CCD system using a 10x dry lens objective (Carl Zeiss MicroImaging). Migration tracks were generated using ImageJ 1.44, and the average velocity was calculated from ~250 cells out of 3 independent experiments. For adhesion assays, 96-well plates were coated with 10 mg/ml FN, washed with PBS, and blocked with 2% BSA. Cells were seeded at a density of 3x10 4 per well in DMEM with GRGDSP peptide (500 mg/ml), with or without TS2/16 (10 mg/ml). After 30 min at 37 C, nonadherent cells were washed away with PBS. Remaining cells were fixed in 4% PFA, washed with H2O, stained with crystal violet, washed, and lysed in 2% SDS. Absorbance was measured at 490 nm. Residual adhesion of GE11 cells was subtracted from all other cell lines. Integrin internalization, recycling, and degradation assays Integrin internalization, recycling, and degradation were investigated essentially as described earlier (10,23). Briefly, cells were stimulated with fresh DMEM/FCS 1 hr prior to the assay, transferred to ice, washed twice in PBS and surface-labeled at 4ºC with 130 mg/ml NHS-SS-biotin for 30 min. For internalization, cells were transferred to DMEM/FCS at 37ºC in the presence of 0.6 mm primaquine, 200
203 after which they were washed twice with ice-cold PBS, and remaining cell-surface biotin was removed by reduction with sodium 2-mercaptoethanesulphonate (MesNa). After washing in PBS, cells were lysed in 1.5 NDLB buffer (150 mm NaCl, 50 mm Tris, 10 mm NaF, 1.5 mm Na3VO3, 5 mm EDTA, 5 mm EGTA, 1.5% Triton X-100, 0.75% Igepal CA-630, 50 mg/ml leupeptin, 50 mg/ml aprotinin, and 1 mm 4-(2-aminoethyl) benzynesulphonyl fluoride). For recycling assays, internalization was performed for 15 min in the absence of primaquine, after which the cells were washed with PBS, and remaining cell-surface biotin was removed with MesNa. Recycling of internalized proteins was then induced in DMEM/FCS at 37ºC, whereafter biotin was removed from recycled proteins by a second reduction with MEsNa, which was quenched with 20 mm iodoacetamide. The second reduction was omitted to detect degradation of the internal pool. Cells were then washed twice in PBS and lysed in 1.5 NDLB buffer. To measure degradation in steady-state conditions, cells were washed in PBS, surface-labeled at 4ºC with NHS-SS-biotin, washed in PBS, and transferred to DMEM/FCS at 37ºC, after which they were lysed in 1.5 NDLB buffer at the indicated time-points. Capture elisa Maxisorb 96-well plates (Life Technologies) were coated overnight with 5 μg/ ml anti-integrin a5 or anti-tfnr in 0.05 M Na 2 CO 3 ph 9.6 at 4ºC and blocked in PBS/0.05% Tween-20 (PBS-T) with 5% BSA. Plates were incubated overnight with cell lysates at 4ºC, washed with PBS-T, and incubated with HRP-streptavidin in PBS- T/1% BSA for 1 hr at 4ºC. After washing, biotinylated proteins were detected with ortho-phenylenediamine in a buffer containing 25.4 mm Na 2 HPO 4, 12.3 mm citric acid (ph 5.4), and 0.003% H 2 O 2. The reaction was terminated with 8 M H 2 SO 4 and absorbance was read at 490 nm. Statistical analysis Graphs were generated either in Excel or in GraphPad Prism 5.01, and statistical analysis was perfomed using unpaired t-tests. Throughout the paper, statistically significant differences are indicated by * (p<0.05), ** (p<0.01), *** (p<0.001), or **** (p<0.0001). 7 ACKnoWleDGEMents We are grateful to Donna Webb, Ken Yamada, Reinhard Fässler, Kevin Wilhelmsen, and Johan de Rooij for their generous gifts of reagents, and Henk Hilkmann and Patrick Celie for generation of the GRGDSP peptide and Cy5-conjugated FN. We thank Lauran Oomen, Lenny Brocks, Anita Pfauth and Frank van Diepen for expert technical assistance. We gratefully acknowledge the Rene Vogels-Stichting / Dutch foundation for Oncology for financial support. Many thanks to Ana Jimenez Orgaz for technical assistance. Work in JCN s laboratory is funded by Cancer Research UK. NPxY-regulated integrin activation and trafficking 201
204 ABBREVIatIons CA, constitutively active; ECM, extracellular matrix; FA, focal adhesion; FAK, focal adhesion kinase; FB, fibrillar adhesion; FN, fibronectin; EMT, epithelial-to-mesenchymal transition; HGF, hepatocyte growth factor; LAMP-1, lysosomal-associated membrane protein-1; LE, late endosome; MD, membrane-distal; MP, membrane-proximal; TfnR, transferrin receptor; TGF-b, transforming growth factor-b; ZO-1, zonula occludens-1 RefeRences 1. Kim C, et al (2011) Regulation of integrin activation. Annu Rev Cell Dev Biol 27, Geiger B, and KM Yamada (2011) Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol 3, doi: /cshperspect.a Moser M, et al (2009) The tail of integrins, talin, and kindlins. Science 324, Law DA, et al (1999) Integrin cytoplasmic tyrosine motif is required for outside-in aiibb3 signalling and platelet function. Nature 401, Chen H, et al (2006) In vivo b1-integrin function requires phosphorylationindependent regulation by cytoplasmic tyrosines. Genes Dev 20, Czuchra A, et al (2006) Genetic analysis of b1-integrin activation motifs in mice. J Cell Biol 174, Margadant C, et al (2011) Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol 23, Wickström SA, et al (2011) Genetic analyses of integrin signaling. Cold Spring Harb Perspect Biol 3, doi: / cshperspect.a Brown NH, et al (2002) Talin is essential for integrin function in Drosophila. Dev Cell 3, Roberts M, et al (2001) PDGFregulated rab4-dependent recycling of avb3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr Biol 11, Caswell PT, et al (2007) Rab25 associates with a5b1 integrin to promote invasive migration in 3D microenvironments. Dev Cell 13, Caswell PT, et al (2008) Rab-coupling protein coordinates recycling of a5b1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol 183, Caswell PT, et al (2009) Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol 10, Nishimura T, and K Kaibuchi (2007) Numb controls integrin endocytosis for directional cell migration with apkc and PAR-3. Dev Cell 13, Chao W-T, and J Kunz (2009) Focal adhesion disassembly requires clathrindependent endocytosis of integrins. FEBS Lett 583, Ezratty EJ, et al (2009) Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol 187, Teckchandani A, et al (2009) Quantitative proteomics identifies a Dab2/integrin module regulating cell migration. J Cell Biol 186, Pellinen T, et al (2008) Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev Cell 15, Valdembri D, et al (2009) Neuropilin-1/ GIPC1 signaling regulates a5b1 integrin traffic and function in endothelial cells. PLoS Biol 7, e Arjonen A, et al (2012) Distinct recycling of active and inactive β1 integrins. Traffic, doi: /j x 21. Shi F, and J Sottile (2008) Caveolin-1- dependent b1-integrin endocytosis is a critical regulator of fibronectin turnover. J Cell Sci 121, Lobert VH, et al (2010) Ubiquitination of a5b1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev Cell 19, Dozynkiewicz MA, et al (2012) Rab25 and the Chloride Intracellular Channel 202
205 Protein-3 collaborate to promote integrin recycling from late endosomal/lysosomal compartments and to drive cancer progression. Dev Cell 22, Gimond C, et al (1999) Induction of cell scattering by expression of b1-integrins in b1- deficient epithelial cells requires activation of members of the rho family of GTPases and downregulation of cadherin and catenin function. J Cell Biol 147, Danen EH, et al (2002) The fibronectinbinding integrins a5b1 and avb3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol 159, Danen EH, et al (2005) Integrins control motile strategy through a Rho-cofilin pathway. J Cell Biol 169, Webb DJ, et al (2007) a5 integrin signaling regulates the formation of spines and synapses in hippocampal neurons. J Biol Chem 282, Wennerberg K, et al (1996) b1 integrindependent and -independent polymerization of fibronectin. J Cell Biol 132, Calderwood DA, et al (2003) Integrin b-cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc Natl Acad Sci USA 100, McCleverty CJ, et al (2007) Structure of the PTB domain of tensin1 and a model for its recruitment to fibrillar adhesions. Protein Sci 16, Pankov R, et al (2000) Integrin dynamics and matrix assembly: tensin-dependent translocation of a5b1 integrins promotes early fibronectin fibrillogenesis. J Cell Biol 148, Wu C, et al (1995) Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell 83, Monkley SJ, et al (2000) Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev Dyn 219, de Rooij J, et al (2005) Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J Cell Biol 171, Nieves B, et al (2010) The NPIY motif in the integrin b1 tail dictates the requirement for talin-1 in outside-in signaling. J Cell Sci 123, Kiema T, et al (2006) The molecular basis of filamin binding to integrins and competition with talin. Mol Cell 21, Harburger DS, et al (2009) Kindlin-1 and -2 directly bind the C-terminal region of b-integrin cytoplasmic tails and exert integrin-specific activation effects. J Biol Chem 284, Qu H, et al (2011) Kindlin-2 regulates podocyte adhesion and fibronectin matrix deposition through interactions with phosphoinositides and integrins. J Cell Sci 124, Schmidt S, (2011) Kindlin-3-mediated signaling from multiple integrin classes is required for osteoclast-mediated bone resorption. J Cell Biol 192, Steinberg F, et al (2012) SNX17 protects integrins from degradation by sorting between lysosomal and recycling pathways. J Cell Biol 197, Böttcher RT, et al (2012) Sorting nexin 17 prevents lysosomal degradation of β1 integrins by binding to the β1-integrin tail. Nat Cell Biol 14, Bretscher MS (1992) Circulating integrins: a5b1, a6b4 and Mac-1, but not a3b1, a4b1 or LFA-1. EMBO J 11, Pellinen T, et al (2006) Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of b1-integrins. J Cell Biol 173, Mai A, et al (2011) Competitive binding of Rab21 and p120rasgap to integrins regulates receptor traffic and migration. J Cell Biol 194, NPxY-regulated integrin activation and trafficking 203
206
207 Epigenetic regulation of galectin-3 expression by β1 integrins promotes cell adhesion and migration Coert Margadant 1,5, Iman van den Bout 1,2,5, Antonius L. van Boxtel 3,5, Victor L. Thijssen 4, and Arnoud Sonnenberg 1 1 From the Division of Cell Biology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands. 2 Inositide Laboratory, Paterson Institute for Cancer Research, Wilmslow Road, Withington, Manchester, M20 4BX, UK. 3 Institute for Environmental Studies, VU University, 1081 HV Amsterdam, The Netherlands. Present address: Cancer Research UK, 44 Lincoln s Inn Fields, London, WC2A 3LY, UK. 4 Departments of Medical Oncology and Radiation Oncology, VU University medical center, De Boelelaan 1118, 1081 HV, Amsterdam, The Netherlands. 5 equal contribution. J Biol Chem, in press (2012)
208
209 ABSTRAct Introduction of the integrin b1- but not the b3-subunit in GE11 cells induces an epithelial-to-mesenchymal-transition (EMT)-like phenomenon that is characterized by the loss of cell-cell contacts, cell scattering, increased cell migration and RhoA activity, and fibronectin fibrillogenesis. Because galactose-binding lectins (galectins) have been implicated in these phenomena, we investigated here whether galectins are associated with the b1-induced phenotype. Intriguingly, we found that out of 9 galectins examined, the expression of galectin-3 (Gal-3) is specifically induced by b1 but not by b3. Using b1-b3 chimeric integrins, we show that the induction of Gal-3 expression requires the hypervariable region in the extracellular domain of b1, but not its cytoplasmic tail. Furthermore, Gal-3 expression does not depend on RhoA signaling, serum factors, or any of the major signal transduction pathways involving protein kinase-c (PKC), p38 mitogen-activated protein kinase (p38mapk), extracellular signal-regulated kinase-1/-2 (ERK-1/2), phosphatidylinositol-3-oh kinase (PI3-K), or Src kinases. Instead, Gal-3 expression is controlled in an epigenetic manner. Whereas DNA methylation of the Lgals3 promoter maintains Gal-3 silencing in GE11 cells, expression of b1 causes its demethylation, leading to transcriptional activation of Gal-3. In turn, Gal-3 expression enhances b1 integrinmediated cell adhesion to fibronectin (FN) and laminin (LN), as well as cell migration. Gal-3 also promoted b1-mediated cell adhesion to LN and Collagen-1 (Col)-1 in cells that endogenously express Gal-3 and b1 integrins. In conclusion, we identify a functional feedback-loop between b1 integrins and Gal-3, that involves the epigenetic induction of Gal-3 expression during integrin-induced EMT and cell scattering. IntRODUctIon Integrins are transmembrane receptors composed of an a- and a b-subunit that connect the extracellular matrix (ECM) to the cytoskeleton, thus integrating the cell interior with its environment. In this way, integrins regulate cell adhesion and cell spreading, as well as cell migration, proliferation, differentiation, and ECM remodeling (1-2). Upon integrin-ligand binding, a variety of proteins associates with the cytoplasmic tail of the b-subunit, initiating cytoskeletal reorganization, assembly of macro-molecular adhesion complexes such as focal adhesions (FAs), and signaling through Rho GTPases and kinase-regulated pathways (3-6). However, individual integrins can trigger very distinct cellular responses, even if they bind the same ligand. We have previously characterized the cellular phenotypes induced by the b1 versus the b3 subunit in the b1-deficient murine epithelial cell line GE11 (7,8). GE11 cells grow in well-defined epithelial islands and express relatively low levels of avb3. Introduction of the b1-subunit, which results primarily in cell-surface expression of the fibronectin (FN) receptor a5b1, causes dramatic morphological changes reminiscent of an epithelial-tomesenchymal transition (EMT), including the loss of cell-cell contacts, cell scat- 8 Epigenetic regulation of galectin-3 by b1 integrins 207
210 tering, and a contractile, fibroblast-like phenotype with high cytoskeletal tension, large peripheral FAs, and multiple protrusions. The b1-induced phenotype is associated with high RhoA activity, fast but random cell migration, FN fibrillogenesis, and the assembly of fibrillar adhesions distributed along FN fibrils (7). In contrast, overexpression of the β3 subunit, leading to cell-surface expression of the alternate FN-receptor αvβ3, increases cell spreading but induces only a modest loss of cell-cell contacts and a pancake -like morphology with many small, randomly distributed FAs (8-9). Moreover, b3 stimulates Rac but not Rho activity and promotes directional rather than random migration. GE11 cells therefore constitute a unique model to study differential effects of integrins on Rho GTPase activation, cell migration, and other integrinregulated processes. Several of the b1-induced phenomena are associated with the actions of a family of matricellular proteins, the b-galactoside-binding lectins (galectins). The galectins comprise 15 highly conserved proteins that bind through a carbohydrate-recognition domain to N-glycosylated proteins at the cell surface, including integrins and several of their ligands such as laminin (LN) and FN (10-14). Galectins can thus modulate integrin-mediated events, and this has been most extensively documented for galectin-1 (Gal-1), galectin-3 (Gal-3), and galectin-8 (Gal-8). For instance, Gal-1 stimulates integrinmediated adhesion and signaling in platelets and smooth muscle cells, and Gal-8 modulates cell adhesion and cell spreading in a number of cell types including neutrophils, fibroblasts, and several tumor cell lines (15-19). The best-characterized family member, Gal-3, has an important role in a wide variety of physiological and pathological phenomena, which is at least in part mediated through its interactions with integrins. Gal-3 regulates the adhesion of epithelial cells to collagens and laminins, and promotes keratinocyte migration over LN-332 and wound re-epithelialization in mice (14,20-23). Furthermore, Gal-3 stimulates neutrophil adhesion and migration, as well as eosinophil adhesion and rolling (24-25). Gal-3 also regulates adhesion and cell spreading of a number of cancer cell lines (26-28). In addition, Gal-3 promotes FA turnover and integrin a5b1-dependent assembly of fibrillar adhesions and FN fibrillogenesis in tumor cells (29-30). Finally, Gal-3 increases the adhesion of disseminating cancer cells to endothelium, thereby protecting them from anoikis (12,31). In this study, we investigated whether galectins are involved in the b1-induced phenotype in GE11 cells. Intriguingly, we found that b1 but not b3 specifically triggers transcriptional activation of Gal-3, through a mechanism that involves the demethylation of the Lgals3 promoter. In turn, Gal-3 promotes b1-mediated cell adhesion and cell migration. Thus, we identify a functional feedback-loop between b1 integrins and Gal
211 ResUlts Gal-3 expression is induced by the integrin b1, but not the b3 subunit To determine the expression of galectin family members in GE11 cells and GE11 cells stably expressing the b1-subunit (GEb1), we performed quantitative- PCR (Q-PCR) analysis of 9 galectins. The only galectin that was expressed at a detectable level in GE11 cells was Gal-1, and its expression was increased 4-fold in GEb1 cells. Intriguingly, Gal-3 was hardly expressed in GE11 cells but its expression increased dramatically (28-fold) in GEb1 cells (Figure 1A). Next, we analyzed protein levels of Gal-1 and Gal-3 by Western blotting. To determine if the regulation of these galectins was specific for the b1 subunit, Gal-1 and Gal-3 expression was also analyzed in GE11 cells that overexpress the b3 subunit (GEb3), which leads to elevated cell-surface expression of avb3 (8). Whereas Gal-1 protein was detected in GE11 cells and its expression was enhanced in both GEb1 and GEb3 cells, high expression of Gal-3 protein was only detected in GEb1 cells but not in GE11 or GEb3 (Figure 1B). Thus, whereas both b1 and b3 enhance Gal-1 expression, high Gal-3 expression is induced by b1 but not by b3. The hypervariable region of the I-like domain of b1 is required for Gal-3 expression Since b1 but not b3 induces Gal-3 expression, and these integrin subunits trigger very different morphological responses, we investigated whether the induction of Gal-3 expression correlates with cell morphology. Furthermore, we addressed the importance of the b1-cytoplasmic tail in the regulation of Gal-3 expression. We therefore used the b1-3 chimeric integrin, which consists of the extracellular and transmembrane regions of b1, fused to the cytoplasmic domain of b3 8 Figure 1 b1 integrins induce expression of Gal-3. (A) Expression of the indicated galectins in GE11 and GEb1 cells was investigated by Q-PCR analysis. Represented are the normalized averages of 3 independent experiments, and statistically significant differences (p<0.05) are denoted by an asterisk. (B) Protein expression of Gal-1 and Gal-3 was analyzed by Western blotting in GE11, GEb1, and GEb3 cells. Epigenetic regulation of galectin-3 by b1 integrins 209
212 (Figure 2A). Expression of this chimera in GE11 cells induces a phenotype similar to that of wild-type b1, including the loss of cell-cell contacts, cell scattering, and a fibroblast-like morphology with multiple protrusions and peripheral FAs (Figure 2A) (8). Thus, the extracellular but not the cytoplasmic domain of b1 is required for these events, and the extracellular domain of b3 cannot substitute for it. The extracellular domains of integrin b-subunits vary widely in a region contained within the I-like domain, called the hypervariable region. This region affects ligand specificity and regulates the activity of Rho GTPases (32,33). Replacing the hypervariable region in b1 with that of β3 (creating b1-3-1) prevents RhoA-mediated contractility and FN fibrillogenesis, and induces a GEb3-like morphology (Figure 2A) (34). Intriguingly, we detected high levels of Gal-3 expression in GEb1 and GEb1-3 cells, but not in GEb3 or GEb1-3-1 cells. In contrast, the level of Gal-1 expression was similar in GEb1, GEb1-3 and GEb1-3-1 cells and was only marginally lower in GEb3 cells (Figure 2B). Thus, these findings show that Gal-3 expression specifically correlates with the contractile, mesenchymal-like phenotype, and does not depend on the cytoplasmic tail of b1. Rather, the hypervariable region in the extracellular domain of b1 is required for both the b1-induced cell morphology, and the induction of Gal-3 expression. b1-induced Gal-3 expression is not regulated by RhoA signaling or kinaseregulated signal transduction pathways As the hypervariable region of b1 is required for both Gal-3 expression and RhoA activity, we reasoned that RhoA signaling might regulate Gal-3 expression downstream of b1. A major effector of RhoA is Rho kinase (ROCK). To test if ROCK is involved in Gal-3 expression, GEb1 cells were incubated for 48 hrs with the ROCK-inhibitor Y This treatment efficiently inhibited ROCK activity, as judged by the dissolution of actin stress fibers and the induction of long, thin protrusions (Figure S1A). However, Gal-3 expression was not affected by ROCK inhibition at any concentration tested (Figure S1B). To assess whether RhoA regulates Gal-3 expression through another pathway, we depleted RhoA using RNAi. Whereas RhoA was completely absent 48 hrs after transfection, Gal-3 expression levels remained unchanged (Figure S1C). We also investigated if active RhoA can induce Gal-3 expression independently of b1, by introducing a constitutively active RhoA mutant fused to GFP (GFP-RhoA-Q63L) (35) into GEb3 cells. Transfected cells were FACSsorted for GFP and subsequently grown overnight, after which Gal-3 protein levels were determined by Western blotting. Expression of GFP-RhoA-Q63L did not induce a GEb1-like phenotype in GEb3 cells, indicating that the activation of Rho signaling alone is not sufficient for the induction of EMT-like changes, and that b1 is absolutely required for this morphology (data not shown). In line with the previous results, expression of GFP-RhoA- Q63L did not induce Gal-3 expression in the absence of b1 (Figure S1D). Taken together, these results suggest that RhoA signaling is not involved in the induction of Gal-3 expression by b1. Besides Rho GTPases, b1-integrins activate a variety of signal transduction 210
213 Figure 2 Gal-3 expression correlates with mesenchymal morphology and depends on the hypervariable region in the I-like domain of b1. (A) Top: schematic presentation of the b1- and the b3-subunit, as well as the b1-3 and b1-3-1 swap mutants. Indicated are the extracellular (ex), transmembrane (TM), and intracellular (in) domains, as well as the I-like domain. The box represents the amino acid sequence of the hypervariable regions of b1 and b3. Bottom; GEb1, GEb3, GEb1-3 and GEb1-3-1 cells were cultured on coverslips and processed for immunofluorescence. FAs were visualized using an antibody against paxillin (green), and the actin cytoskeleton was stained with phalloidin (red). Scalebar, 10 mm. (B) Gal-1 and Gal-3 expression in GEb1, GEb3, GEb1-3 and GEb1-3-1 cells was investigated by Western blotting. pathways (4,5). In an attempt to identify a b1-induced signaling pathway that regulates Gal-3 expression, we treated GEb1 cells with standard concentrations of a variety of compounds to inhibit signal transduction pathways that are commonly activated by b1-integrins, including PD98059 and UO126 to inhibit the extracellular signal-regulated kinase-1/-2 (ERK-1/-2) cascade, SB to inhibit p38 mitogen-activated protein kinase (p38mapk), PI-103 and LY to inhibit phosphatidylinositol-3-oh kinase (PI3-K), PP1 to inhibit the Fyn/Src/Yes family of kinases, and Gö6983 to inhibit all isoforms of protein kinase-c (PKC). In addition, we used the broad-spectrum tyrosine kinase inhibitor genistein, and the 8 Epigenetic regulation of galectin-3 by b1 integrins 211
214 broad-spectrum serine/threonine kinase inhibitor staurosporine. None of the used compounds prevented Gal-3 expression in GEb1 cells although genistein did reduce Gal-3 expression to some 70% of control levels, which is possibly due to cytotoxic side-effects (Figure S2A). Notably, whereas some inhibitors seemed to enhance Gal-3 expression in GEb1 cells, they failed to do so in GE11, further underlining that expression of Gal-3 cannot be activated in the absence of b1. We then analyzed whether Gal-3 expression in GEb1 cells depends on soluble factors that can trigger signaling pathways at the cell-surface, by culturing GE11 and GEb1 cells in the absence or the presence of serum for 24 to 48 hrs. Gal-3 expression was only slightly elevated by serum but was not prevented in its absence, suggesting that although serumcomponents may potentiate b1-induced Gal-3 expression, they are not absolutely required (Figure S2B). b1 integrins induce Gal-3 expression by demethylation of the Lgals3 promoter The previous sections indicate that Gal-3 expression in GEb1 cells is not maintained by RhoA signaling, serum factors, or kinase-regulated signaling. In addition, the b1-cytoplasmic tail, which contains docking sites for numerous signaling proteins, is dispensable for Gal-3 expression. It therefore seems unlikely that Gal-3 expression in GEb1 depends on a classic integrin signaling event. The expression of Gal-3 and other galectins is often silenced epigenetically by DNA methylation, but can be reversed to induce de novo expression, for example during tumor progression (36-38). We therefore investigated the DNA methylation status of the promoter region of Lgals3, the gene encoding Gal-3, by bisulfite sequencing. The murine Lgals3 gene contains a CpG island in the promoter region, which overlaps the start of exon 1 (Figure 3A). Following bisulfiteconversion of genomic DNA, a 268 bp Lgals3 promoter fragment containing 21 CpG sites was amplified using a bisulfite treatment-specific primer set (Figure 3A). The purified products were cloned into a TOPO vector, and sequencing of multiple individual clones (n > 9) revealed that virtually all of the CpG sites were methylated in GE11 and GEb3 cells, corresponding with the lack of Gal-3 expression (Figure 3B). In contrast, the Lgals3 promoter was predominantly demethylated in GEb1 cells (Figure 3B). We subsequently employed an alternative strategy to confirm the differences in methylation status of the Lgals3 promoter, making use of the selective digestive properties of the HpaII/MspI restriction enzyme pair. Whereas both enzymes recognize the CCGG sequence, digestion by HpaII but not MspI is prevented by DNA methylation. The Lgals3 promoter contains 3 HpaII/MspI sites. When fully methylated, digestion of genomic DNA with HpaII, but not MspI, should generate a PCR fragment of 466 bp with primers overlapping the restriction sites (Figure 3C). Therefore, genomic DNA was treated with HpaII or MspI, and the 466 bp fragment was amplified by PCR and analyzed on gel. The expected fragment was generated from HpaII-digested genomic DNA isolated from GE11 and GEb3, but not from GEb1 cells (Figure 3C). In contrast, digestion of genomic DNA with MspI never resulted in an amplified product, thus confirming that 212
215 the Lgals3 promoter is indeed methylated in GE11 and GEb3 cells, but not in GEb1. These findings strongly suggest that the induction of Gal-3 expression in GEb1 cells is caused by demethylation of the Lgals3 promoter. DNA methylation is maintained by DNA methyltransferases (Dnmts). To investigate whether the demethylation of the Lgals3 promoter in GEb1 cells was the result of decreased overall expression or activity of Dnmts, we tested Dnmt expression by Western blot- Figure 3 Transcriptional activation of Gal-3 by b1-integrins is caused by demethylation of the Lgals3 promoter. (A) Relative distribution of 21 CpG sites located in the promoter region of the Lgals3 gene. Shown is the region from -164 bp upstream to +125 bp downstream of the start of exon 1. Arrows indicate start positions of amplification. (B) The methylation status of CpG sites in the Lgals3 promoter was determined in GE11, GEb1, and GEb3 cells by bisulfite modification followed by methylation-specific PCR and sequencing. Depicted is the representative methylation-status of 21 CpG sites for each cell line, determined from at least 9 individual clones. Closed circles represent methylated CpG sites, whereas open circles represent unmethylated CpG sites. The start of exon 1 is indicated by (0). (C) Top; schematic representation of the Lgals3 promoter, showing the location of 3 HpaII/MspI sites. Bottom; genomic DNA of GE11, GEb1, and GEb3 cells was treated with HpaII or MspI, subjected to PCR, and reaction products were analyzed on gel. Shown is a representative experiment (n=3). (D) Expression of Dnmt3b in GE11 and GEb1 cells was investigated by Western blotting (left). Content of 5-Me-dC, expressed as a percentage of total dc content, in GE11 and GEb1 cells was determined by HPLC (right). (E) GE11 cells were incubated for 48 hrs with 1 mm decitabine, and the methylation status of the Lgals3 promoter was investigated using digestion with HpaII or MspI, followed by PCR. (F) GE11 cells were incubated for 48 hrs with the indicated concentrations of decitabine, and expression of Gal-3 was investigated by Q-PCR (left) and Western blotting (right). (G) GEb3 cells were incubated for 48 hrs with the indicated concentrations of decitabine, and expression of Gal-3 was investigated by Q-PCR (left; expressed relative to GE11 without decitabine) and Western blotting (right). 8 Epigenetic regulation of galectin-3 by b1 integrins 213
216 ting. Whereas Dnmt1 and Dnmt3a were not detected, the expression of Dnmt3b was not decreased in GEb1 cells with respect to GE11 and perhaps even slightly elevated (Figure 3D). As a readout for global Dnmt activity, we then tested the overall content of methylated cytosines in GEb1 cells by HPLC (36). The ratio of methylated/unmethylated cytosines was not decreased in GEb1 cells, suggesting that demethylation of the Lgals3 promoter is a specific event, and is not the result of decreased overall Dnmt activity (Figure 3D). To confirm that DNA methylation is the cause for Gal-3 silencing in GE11, we incubated GE11 cells for 48 hrs with the Dnmt inhibitor decitabine, and performed digestion of genomic DNA with HpaII/MspI as described above. The 466 bp fragment was hardly amplified from GE11 cells after treatment with decitabine, indicating that the Lgals3 promoter was demethylated by this treatment (Figure 3E). In addition, decitabine caused mrna and protein expression of Gal-3 in GE11 cells in a dose-dependent manner, confirming that demethylation of the Lgals3 promoter leads to transcriptional activation of the Lgals3 gene (Figure 3F). Finally, decitabine treatment also increased Gal-3 mrna and protein in GEb3 cells (Figure 3G). These findings unambiguously demonstrate that 1) DNA methylation maintains Gal-3 silencing in GE11 and GEb3 cells, and 2) demethylation is sufficient to activate Gal-3 transcription, even in the absence of b1. Gal-3 promotes b1 integrin-mediated cell adhesion We next investigated whether Gal-3 contributes to b1-mediated adhesion of GEb1 cells. The b1-integrin repertoire in GEb1 cells consists primarily of the FNbinding integrin a5b1, whereas low levels of FN-binding avb1, as well as the LNbinding integrins a3b1 and a6b1 are also expressed (7,8). We first tested whether adhesion to FN was affected by knockdown of Gal-3 expression with sirna. Because b1 also enhanced Gal-1 levels, Gal-1 was depleted in parallel. Both sirnas caused a strong and specific reduction of their respective targets within 24 hrs after transfection, while a scrambled sirna-control had no effect (Figure 4A). Interestingly, cell adhesion to FN was reduced to 50% of that of control cells by Gal-3 depletion, but was not affected by the loss of Gal-1 expression (Figures 4B,C). Thus, Gal-3 but not Gal-1 contributes to adhesion of GEb1 cells to FN. Similar results were obtained using stable cell lines that were infected with shrnas directed against Gal-3, and subsequently selected with puromycin (data not shown). Because the low amounts of a3b1 and a6b1 on GEb1 cells allow weak adhesion to laminins, we next tested whether Gal-3 also promotes cell adhesion to LN-511, a ligand for both a3b1 and a6b1. In line with the results obtained on FN, Gal-3 depletion reduced cell adhesion to LN-511, albeit to a lesser extent (Figure 4D). Thus, Gal-3 promotes b1-integrin-mediated cell adhesion to FN and LN-511 in GEb1 cells. We then determined whether Gal-3 affects b1-mediated adhesion in epithelial cells that endogenously express Gal-3 and b1-integrins. For this purpose we used a keratinocyte cell line designated MKa3, which we have described previously (39). MKa3 cells are non-transformed keratinocytes isolated from mouse epidermis, 214
217 and their predominant b1-integrins are a2b1 and a3b1, whereas a5b1 is not expressed (39). Knockdown of Gal-3 expression in MKa3 cells reduced a2b1- mediated cell adhesion to Col-1 (Figure 4E). In addition, MKa3 adhesion to LN-511, which is primarily mediated by a3b1, was also decreased though less prominently than on Col-1 (Figure 4F). Together, these results show that Gal-3 promotes b1-integrin-mediated cell adhesion to FN and LN-511 in GEb1 cells, and to Col-1 and LN-511 in MKa3. b1-induced Gal-3 expression promotes cell migration, but not fn fibrillogenesis or RhoA-mediated contractility Next, we investigated whether Gal-3 is involved in other b1-induced phenomena in GEb1 cells such as cell migration, FN fibrillogenesis, and RhoA activity. To analyze cell migration, GEb1 cells 8 Figure 4 Gal-3 promotes b1-integrin-mediated cell adhesion in GEb1 and MKa3 cells. (A) GEb1 cells were transfected with Gal-1, Gal-3 or control sirnas, and protein expression was analyzed 24 hrs after transfection by Western blotting. (B) Cell adhesion to FN was investigated in GEb1 cells treated with control sirnas (squares) or an sirna directed against Gal-3 (circles). (C) Cell adhesion to FN was investigated in GEb1 cells treated with control sirnas (squares) or sirnas directed against Gal-1 (circles). (D) Cell adhesion to LN-511 was investigated in GEb1 cells and GEb1 cells depleted of Gal-3. (E) Gal-3 expression was knocked down in mouse keratinocytes MKa3, and cell adhesion to LN-511 was investigated in an adhesion assay. (F) Adhesion of MKa3 cells and MKa3 cells depleted of Gal-3 to Col-1. Graphs represent the average of 3 independent experiments. Statistically significant differences are indicated by * (p<0.05) or ** (p<0.01). Epigenetic regulation of galectin-3 by b1 integrins 215
218 were transfected with control or Gal-3 sirnas and grown to confluence, and 24 hrs after transfection the monolayers were scratched with a pipette tip. Migration into the wound was then monitored by time-lapse microscopy, and migration speed was calculated from the movies. The knockdown of Gal-3 expression delayed migration speed by 30%, compared to cells transfected with control sirnas (Figure 5A). Thus, in addition to cell adhesion, Gal-3 expression in GEb1 cells also promotes cell migration. We then investigated FN fibrillogenesis in cell monolayers 24 hrs after sirna transfection by confocal microscopy. Whereas FN fibrils in GE11 cells were not detected, GEb1 cells transfected with control or Gal-3 sirnas both demonstrated abundant FN fibrillogenesis (Figure 5B), and quantification demonstrated that the extent of fibril formation was not significantly decreased upon Gal-3 depletion (Figure 5C). Furthermore, Gal-3 knock- Figure 5 Gal-3 promotes cell migration, but not FN fibrillogenesis or RhoA activity in GEb1 cells. (A) Monolayers of GEb1 cells transfected with control or Gal-3 sirnas were wounded with a yellow pipette tip and then imaged overnight. The relative migration speed was determined as the ratio of the wound area after overnight migration t(end) over the wound area at the start of the experiment t(0), and expressed relative to the control-transfected cells. The graph represents the averages of 3 independent experiments (p<0.05). Scalebar, 100 mm. AU, arbitrary units. (B) Monolayers of GE11 cells and GEb1 cells transfected with control or Gal-3 sirnas were incubated in FN-depleted medium, supplemented with biotinylated FN. FN fibrils were visualized with FITC-conjugated streptavidin (green), and nuclei were stained with DAPI (blue). Scalebar, 50 mm. (C) The ratio between total FN fluorescence and the total number of cells was determined using ImageJ, and the averages of 3 independent experiments are depicted in the graph. AU, arbitrary units. (D) GEb1 cells were transfected with control or Gal-3 sirnas and seeded on tissue-culture plates. After 24 hrs of incubation, RhoA activity was assessed using a G-LISA. AU, arbitrary units. (E) GEb1 cells were transfected with control or Gal-3 sirnas, seeded on coverslips, and after overnight incubation they were processed for immunofluorescence. FAs were visualized using an antibody against paxillin (green), phalloidin was used to stain F-actin (red), and DAPI to stain nuclei (blue). Scalebar, 20 mm. 216
219 down in GEb1 cells did not affect RhoA activity, as determined by a RhoA G-LISA (Figure 5D). Consistent with this finding, Gal-3 knockdown did also not disrupt the RhoA-mediated contractile phenotype of GEb1 cells, characterized by stress fibers and FAs located in the periphery (Figure 5E). Summarizing, these data indicate that Gal-3 expression in GEb1 cells promotes b1-mediated cell adhesion and migration, but not RhoA-mediated contractility or FN fibrillogenesis. DIscUssIon In this study, we identify a functional synergistic loop between b1-integrins and Gal-3, consisting of the epigenetic activation of Gal-3 expression during b1-induced EMT, which enhances b1-mediated cell adhesion and migration. A number of reports demonstrate that galectins can regulate integrin-mediated cell adhesion. However, the exact mechanism underlying this phenomenon is poorly understood and galectins seem to have both promoting and inhibiting effects. For example, whereas overexpression of Gal-3 promotes adhesion and invasion in tumor cells, their adhesion is inhibited by addition of exogenous Gal-3 (27,28). Similarly, soluble Gal-8 has been shown to inhibit cell adhesion, whereas Gal-8-coated surfaces support cell adhesion, cell spreading and integrin signaling in the same cells (15,16). It is conceivable that soluble galectins perform different functions than endogenously expressed galectins that are associated with membrane proteins, or immobilized galectins that are present in the ECM. However, additional complexity arises from observations that galectins can have opposing effects across different cell types (which may have different integrin repertoires), and even on different integrins within the same cell type. An example of the former is that while soluble Gal-8 inhibits the adhesion of CHO, HeLa, and HaCaT cells, it stimulates adhesion of neutrophils (15,17). An example of the latter is that Gal-3 regulates a2b1-mediated adhesion to collagens, but has no effect on FN- or LN-binding integrins in MDCK cells (22). We find that Gal-3 promotes b1-mediated cell adhesion to FN and LN in GEb1 cells, and to Col-1 and LN in mouse keratinocytes. Earlier reports have documented that Gal-3 regulates surface expression of a6b1 and a4b7 on cancer cells (26,28,31), but we did not detect differences in b1 expression by flow cytometry upon knockdown of Gal-3 expression (data not shown). Moreover, we found that FN fibrillogenesis was not affected upon Gal-3 depletion, while a previous study has shown that soluble Gal-3 can promote this process in mammary tumor cells (30). The discrepancy between the latter study and ours probably reflects differences in the used cell system, as well as differences in the effects of soluble Gal-3 versus endogenous Gal-3. It is clear that the effects of galectins on integrin function are complex and depend on the integrin repertoire, the cell type, and the particular galectin involved. Accumulating evidence suggests that the pattern of glycosylation is key to the interactions of galectins with their ligands, and thus to the effects of galectins on cell 8 Epigenetic regulation of galectin-3 by b1 integrins 217
220 behavior. Galectins bind to N-acetyllactosamine (typically Galβ1,4GlcNAc), which is a recurring sequence in branched N-glycans, and the affinity of galectins increases with the number of branches. Affinity is also regulated by adjacent saccharides, as different galectins show specificity for different oligosaccharides (11,40). Many galectins exist as dimers or oligomers, and Gal-3 can form up to pentamers, which are able to cross-link multiple ligands at the cell surface to form lattices of glycoproteins (41,42). In this way, galectins can cluster integrins but they can also form heterogeneous lattices consisting of integrins, non-integrin proteins that regulate adhesion such as MUC1, and other glycoproteins including cytokine- and growth factor-receptors (43,44). Thus, it is increasingly recognized that the effects of galectins can only be understood completely with knowledge of the molecular composition of the glycoproteome at the cell surface, and its pattern and degree of glycosylation (40). Irrespective of the mechanism whereby Gal-3 enhances b1 function, our data reveal a new aspect of galectinintegrin biology, as we document the transcriptional activation of a galectin by an integrin, which clearly has significance for integrin function. Gal-3 was specifically induced by b1, whereas neither endogenous avb3 in GE11 cells, nor overexpression of the b3-subunit in GEb3 cells was able to activate Gal-3 expression. Moreover, introduction of the b4-subunit in GE11 also failed to induce Gal-3 (data not shown). Neither of these integrins supports the EMT-like phenotypic changes induced by b1 integrins, and the correlation between this phenotype and Gal-3 expression is further strengthened by our observations using chimeric integrins. Surprisingly, the induction of Gal-3 expression was independent of the b1 cytoplasmic tail, RhoA signaling, or common integrin-induced signal transduction pathways. Rather, its expression in GEb1 cells is regulated in an epigenetic fashion by the demethylation of DNA sequences around the transcription-initiation site of the Lgals3 gene, which are methylated in GE11 and GEb3. Demethylation of the Lgals3 promoter is a specific event, and is not the result of a loss or reduction of global methylation in GEb1 cells. Our data emphasize the importance of DNA methylation in the regulation of the expression of various galectins, in line with previous reports (36-38). In light of our data, 2 previous studies are particularly interesting. First, it has been shown that the cell-surface glycoprotein MUC1 controls Gal-3 expression in an epigenetic manner in cancer cells, be it through a mirna-dependent mechanism rather than DNA-methylation. In turn, Gal-3 binds MUC1 at the cell surface and bridges it with epidermal growth factor receptor, thereby establishing a functional feedback-loop (44). Second, integrin a6b4 activates the expression of a number of target genes involved in invasion and metastasis, by demethylation of their promoters in MDA-MB-435 human mammary carcinoma cells (45). Thus, these reports provide additional evidence that integrins, as well as other cell-surface receptors, can function in a regulatory feedback-loop that involves the activation of expression of accessory proteins via epigenetic mechanisms rather than through a conventional signal transduction cascade. 218
221 Future research should aim at the identification of regulators between the integrin and Lgals3 methylation, and the link between Gal-3 expression and the acquirement of a mesenchymal phenotype. As Gal-3 is considered to be an important player in cancer progression and metastasis, and its expression is frequently regulated by DNA demethylation in tumor cells, it will be interesting to determine whether integrins are responsible for de novo expression of Gal-3 during tumor progression. MateRIals and MetHODS Antibodies and other materials Antibodies used in this study were directed against actin (Millipore), Gal-1 (R&D systems), Gal-3 (Abcam), integrin β1 (TS2/16) and paxillin (both from BD Transduction Labs), RhoA (Santa Cruz), Dnmt3b (Abcam), and tubulin (Sigma). Texas Red-conjugated phalloidin and DAPI were from Molecular Probes, FITC- or Texas Red-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories, decitabine (5-azadeoxycitidine), FN, Y-27632, PD98059, UO126, SB203580, LY , PP1, Gö6983, genistein, staurosporine and puromycin were from Sigma, PI-103 was from Merck, and Texas Red- and FITCconjugated Streptavidin were from Pierce Chemical Co. Collagen (Col)-1 was purchased from Vitrogen and LN-511 from BioLamina. Biotinylated-FN was prepared as described previously (8). Cells, plasmids, and transient transfections The pcdna3-gfp-rhoa-q63l construct was kindly provided by Dr. Sylvio Gutkind (NIH, Bethesda, Maryland, USA). The b1-3 and b1-3-1 expression plasmids were a kind gift from Dr. Yoshikazu Takada (UC Davis, Sacramento, CA, USA). GE11 cells are epithelial in origin and were obtained by injecting β1-null mouse embryonic stem cells into blastocysts, which were allowed to develop into whole chimeric embryos until day E10.5. Cells were then retrieved from these embryos, immortalized with SV-40, and selected with G418. Polarized cells which had formed small colonies were cloned (7). GE11 cells expressing human b1, human b3, or the chimeric human integrins b1-3 and β1-3-1 were generated by transfecting Phoenix packaging cells with retroviral constructs encoding the indicated integrins, to produce culture supernatants containing virus. GE11 cells were infected with the supernatants, selected with zeocin, and FACS-sorted for expression of the respective integrins at the cell surface (7,8,34). All GE-derived cell lines were cultured in DMEM supplemented with 10% FCS and 100 U/ml penicillin/streptomycin (Gibco BRL). Mouse keratinocytes MKa3 were isolated as described previously (39). Briefly, skin was obtained from newborn mice and epidermis and dermis were separated with trypsin. Keratinocytes were retrieved by gentle shaking and centrifugation, and incubation in keratinocyte serumfree medium (Gibco) supplemented with 50 μg/ml bovine pituitary extract, 5 ng/ml epidermal growth factor, and penicillin/ streptomycin. Spontaneously immortalized clones were obtained after several weeks. 8 Epigenetic regulation of galectin-3 by b1 integrins 219
222 These were named MKa3 and cultured routinely on Col-1 (3 mg/ml). All cells were maintained at 37 o C in a humidified atmosphere containing 5% CO 2. Transient transfections were performed using the Amaxa nucleofector, according to the manufacturers instructions. Western blotting Cells were washed with ice-cold PBS and lysed in protein loading buffer (62.5 mm Tris-HCl, ph 6.8, 20% glycerol, 2% SDS, 5% mercaptoethanol) at 4 o C. Proteins were separated on SDS-PAGE gels, transferred to polyvinylidene difluoride membranes (Millipore), and analyzed by Western blotting followed by ECL using the Super signal system (Pierce Chemical Co.). Q-PCR analysis Q-PCR was performed using the icycler (Biorad) in a total volume of 25 ml containing 1.5 ml cdna, 12.5 ml iq SYBR Green Supermix (Biorad) and 400 nm of both the forward and reverse primer. The primers used were designed and validated according to a previously described protocol (46), and included primers for Gal-1 ( 5 -TGTGTGTA AC ACC A AG G A A- GAT-3 forward and 3 -ACCTCTGTG- ATGCTCCCG-5 reverse), Gal-2 (5 -CA- ATGATGTGGACCGCTTC-3 forward and 3 -ACAGACAATGGTGGATTCATC- -5 reverse), Gal-3 (5 -CAGGATTGTTCT- AGATTTCAGG-3 forward and 3 -TTGT- CCTGCTTCGTGTTACAC-5 reverse, or alternatively 5 -GCCTACCCCAGTGCT- CCT-3 forward and 3 -GGTCATAGG- GCACCGTCA-5 reverse), Gal-4 (5 - - G A AG AG AG G A AG GTG G CCT- 3 forward and 3 -CCATTTGGAATGCT- TGGAAC-5 reverse), Gal-7 5 -AACAC- CAAAGAACAAGGCAA-3 forward and 3 -TGGAAGTGGAGATATTCGTCA-5 reverse), Gal-8 (5 -CTGAGCCTGCCA- TTTGAA-3 forward and 3 -AAGCTTC- GGGCATTGGTG-5 reverse), Gal-9 (5 - - CTTTCTACACCCCCATTCCA-3 forward and 3 -GATATGGAACCTCG- TAGCATCT-5 reverse), Gal-10 (5 -CTG- A G TG TA C T T TG G TC AT TG G - 3 forward and 3 - AGATGCTCAGATTA- AATGGTTTA-5 reverse), Gal-12 (5 -C- CTGAGCCTGAAGGATGG-3 forward and 3 -CGGAGACAGCTTCTTTCGT-5 reverse). As a negative control, the cdna was replaced by milliq. Cyclophilin A, b-actin and HPRT-1 were used as reference genes. Each sample was analyzed in quadruplicate. Differences in mrna expression were calculated by normalizing the Ct value of each sample to the geometric mean of the 3 reference genes ([delta] C t = C t,sample - (C t,cyclo *C t,actin *C t,hprt 1/3 ). Subsequently, the fold difference in expression was calculated as 2 -[delta][delta]ct, with [delta][delta]c t = [delta]c t,ge11 - [delta] C t,geb1. The Mann-Whitney rank sum test was used to identify statistically significant differences between [delta]c t -values of GE11 and GEb1. All statistical analyses were performed in SPSS Confocal microscopy Cells on glass coverslips were fixed in 2% paraformaldehyde, washed with PBS, permeabilized with 0.2% Triton X-100, and washed with PBS. Coverslips were subsequently blocked with 2% BSA in PBS for 1 hr, incubated with primary antibodies for 1 hr at RT, washed with PBS, and incubated with secondary antibodies for 1 hr at RT. After washing with PBS, coverslips were mounted in Mowiol supplemented with 220
223 DABCO (Calbiochem), and examined with a confocal Leica TCS NT microscope. Rna interference For Gal-1 and RhoA knockdown, a SMARTpool from Dharmacon consisting of 4 sirnas was used (catalog number M against Gal-1 and M against RhoA). For Gal-3 knockdown, a custom sirna was ordered from Dharmacon with the target sequence GUAACACGAAGCAGGACAA. The standard sicontrol sirna from Dharmacon was used as negative control. Cells were transfected using the transfection reagent DharmaFECT according to the manufacturers instructions. Experiments were performed hrs post-transfection and knockdown was verified in each experiment by Western blotting. Alternatively, short hairpins against Gal-3 (target sequences included GCAGTACAACCATCGGAT- GAA, CCCGCTTCAATGAGAACAACA, C C G C ATG C TG ATC A C A ATC AT, CCT T TG AG AGTG G C A A ACC AT, CCCA ACGCA A ACAGG ATTGTT), cloned into plko.1, were obtained from the TRC shrna Open Biosystems library and transfected into HEK 293FT cells together with the Virapower TM Packaging mix (Invitrogen), using Lipofectamine 2000 according to the manufacturers instructions. Viral supernatant was harvested 48 hrs later, transduced into GEb1 or MKa3 cells, and positive cells were selected with puromycin. Adhesion assays 96-well plates were coated at 37 C with 1 mg/ml Col-1 (5 min), 1 mg/ml FN (1 hr), or 8 mg/ml LN-511 (overnight). The plates were washed twice with PBS, blocked in 2% BSA for 1 hr at 37 C, and washed twice with PBS. Cells were seeded at a density of 3x10 4 1x10 5 per well and allowed to adhere at 37 C for the indicated time-points. Non-adherent cells were washed away with PBS before the addition of substrate buffer (7.5 mm p-nitrophenyl N-acetyl-beta-D-glucosaminide (NPAG) in 0.1 M sodium citrate ph 5, 0.5% Triton-X100). Plates were then incubated overnight at 37 C, after which stop buffer (50 mm glycine, ph 10.4, 5 mm EDTA) was added and the absorbance was measured at 405 nm on a BioRad microplate reader. Alternatively, cells were fixed after washing and processed as described previously (33). Each condition was analyzed in triplicate, and graphs represent the average of 3 independent experiments. Scratch assays Cells in 24-well plates were grown to confluency and subsequently serum-starved overnight. Proliferation was inhibited with 10 μg/ml mitomycin C (Nycomed Inc.) 2 hrs before scratching the monolayers with a yellow pipette tip. Cells were then washed twice in serum-free medium to remove cell debris, stimulated with fresh medium, and incubated at 37 C. Scratched areas were photographed overnight at 10x magnification. To determine the relative migration, the area that was not covered by cells at the end of the assay t(end) was calculated over the open area at the beginning of the assay t(0). The resulting ratio in untreated cells was set to 1. Experiments were performed 3 times in triplicate. RhoA assay Cells were transfected with control or Gal-3 sirnas, plated in tissue-culture plates, and RhoA activity was assessed 8 Epigenetic regulation of galectin-3 by b1 integrins 221
224 24 hrs post-transfection using the Rho G-LISA kit (Cytoskeleton), according to the manufacturers instructions. fn fibrillogenesis assay Cells on coverslips were grown to confluency and then incubated overnight in DMEM containing FN-depleted serum, supplemented with 10 mg/ml biotinylated FN. Thereafter, they were processed for immunofluorescence as described above. FN was visualized with Texas Red-conjugated strepavidin and nuclei were stained with TOPRO-3. Several fields were photographed, and total fluorescence for each field was measured using ImageJ software and divided by the number of cells. Graphs represent the averages of 3 independent experiments. Analysis of promoter methylation For methylation analysis of the Lgals3 promoter, genomic DNA was isolated using the nucleospin tissue DNA isolation kit (Machery Nagel). The purified DNA was bisulfite-converted using the Epitect Bisulfite Kit (Qiagen) according to the manufacturers instructions. Next, PCR for regions of the Lgals3 promoter was performed using the following mouse- and bisulfite-specific primers: 5 -TGTTTTAGTT- TATTGTGGGGAAGTT-3 (forward); 5 -AAAACCCAACCCTTTCTTAATA- CAC-3 (reverse), amplifying a 268-bp product which contained 21 CpGs located adjacent to the start of exon 1. PCR products were isolated from gel using a gel extracion kit (Qiagen), cloned into the pcrii-topo TA cloning vector (invitrogen), and transformed into Top10 E. coli competent cells (Invitrogen). Positive clones were selected by blue-white screening and sequenced using M-13 reverse primer (Invitrogen). At least 9 individual clones per cell line, derived from independent experiments, were analyzed. CpG island prediction and primer design were performed using MethPrimer (47). For DNA methylation analysis using restriction enzyme digestion, genomic DNA was treated with HpaII or MspI, after which 30 cycles of PCR were performed using the following primers: 5 -TTGGGT- GAGTCTGAGAGTCTG-3 (forward); 5 -CATCCCAAAGAGACTTAGTATG-3 (reverse), to amplify a 466 bp product. Reaction products were normalized for the amount of input DNA and analyzed on gel. All experiments were performed in triplicate. Analysis of global genomic methylation by HPlc To assess global genomic methylation levels, the ratio of 5-methyl-deoxycytosine (5-Me-dC) over total deoxycytosine (dc) was determined using HPLC (48). Cell lysates were depleted of RNA with 1 mg/ml RNAse A (Sigma) for 5 min, after which DNA was extracted as described above. Next, 1 µg DNA was digested to single nucleotides using a combination of DNAse I (Sigma), Nuclease P1 (Sigma) and alkaline phosphatase (New England Biolabs) treatment, and dc and 5-Me-dC content were quantified with an HPLC-UV system (Shimadzu) equipped with a 125x4mm Nucleosil SA column (Macherey-Nagel). The mobile phase consisted of 40 mm acetic acid in 15% acetonitrile (ph 4.8), and the flow rate was 0.6 ml/min. Global 5-Me-dC content was expressed as a percentage of the total dc content. 6 replicates were analyzed per condition. 222
225 ACKnoWleDGEMents We thank Yoshikazu Takada and Sylvio Gutkind for their generous gifts of constructs. Stephan Huveneers is acknowledged for assistance with experiments, Jorke Kamstra and Juliette Legler are thanked for assistance with HPLC, Lauran Oomen and Lenny Brocks are thanked for expert assistance with microscopy, and Anita Pfauth and Frank van Diepen for expert assistance with flow cytometry. This work was supported by a grant from the Dutch Cancer Society (NKI ). A.L. van Boxtel was partly funded by the Netherlands Organization for Scientific Research ( ). ABBREVIatIons Collagen-1, Col-1; ECM, extracellular matrix; EMT, epithelial-to-mesenchymal transition; ERK, extracellular signal-regulated kinase; FA, focal adhesion; FN, fibronectin; Gal-1, galectin-1; Gal-3, galectin-3; Gal-8, galectin-8; LN, laminin; MAPK, mitogen-activated protein kinase; PI3-K, phosphatidylinositol-3-oh kinase; PKC, protein kinase-c; ROCK, Rho kinase RefeRences 1. Campbell ID, and MJ Humphries (2011) Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol 3, doi: /cshperspect.a Geiger B, and KM Yamada (2011) Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol 3, doi: /cshperspect.a Huveneers S, and EH Danen (2009) Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci 122, Legate KR, et al (2009) Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev 23, Margadant C, et al (2010) Unique and redundant functions of integrins in the epidermis. FASEB J 24, Margadant C, et al (2011) Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol 23, Gimond C, et al (1999) Induction of cell scattering by expression of b1 integrins in b1- deficient epithelial cells requires activation of members of the Rho family of GTPases and downregulation of cadherin and catenin function. J Cell Biol 147, Danen EH, et al (2002) The fibronectinbinding integrins a5b1 and avb3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol 159, Danen EH, et al (2005) Integrins control motile strategy through a Rho-cofilin pathway. J Cell Biol 169, Woo HJ, et al (1990) The major nonintegrin laminin binding protein of macrophages is identical to carbohydrate binding protein 35 (Mac-2). J Biol Chem 265, Hughes RC (2001) Galectins as modulators of cell adhesion. Biochimie 83, Liu FT, and GA Rabinovich (2005) Galectins as modulators of tumour progression. Nat Rev Cancer 5, Dumic J, et al (2006) Galectin-3: an openended story. Biochim Biophys Acta 1760, Kariya Y, et al (2010) Bisecting GlcNAc residues on laminin-332 down-regulate galectin-3-dependent keratinocyte motility. J Biol Chem 285, Levy Y, et al (2001) Galectin-8 functions as a matricellular modulator of cell adhesion. J Biol Chem 276, Levy Y, et al (2003) Sustained induction of ERK, protein kinase B, and p70 S6 kinase 8 Epigenetic regulation of galectin-3 by b1 integrins 223
226 regulates cell spreading and formation of F-actin microspikes upon ligation of integrins by galectin-8, a mammalian lectin. J Biol Chem 278, Nishi N, et al (2003) Galectin-8 modulates neutrophil function via interaction with integrin am. Glycobiology 13, Pacienza N, et al (2008) The immunoregulatory glycan-binding protein galectin-1 triggers human platelet activation. FASEB J 22, Moiseeva EP, et al (2003) Galectin-1 interacts with b1 subunit of integrin. Biochem Biophys Res Commun 310, Cao Z, et al (2002) Galectins-3 and -7, but not galectin-1, play a role in reepithelialization of wounds. J Biol Chem 277, Friedrichs J, et al (2007) Contributions of galectin-3 and -9 to epithelial cell adhesion analyzed by single cell force spectroscopy. J Biol Chem 282, Friedrichs J, et al (2008) Galectin-3 regulates integrin a2b1-mediated adhesion to collagen-i and -IV. J Biol Chem 283, Saravanan C, et al (2009) Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on a3b1 integrin. J Cell Sci 122, Kuwabara I, and FT Liu (1996) Galectin-3 promotes adhesion of human neutrophils to laminin. J Immunol 156, Rao SP, et al (2007) Galectin-3 functions as an adhesion molecule to support eosinophil rolling and adhesion under conditions of flow. J Immunol 179, Furtak V, et al (2001) Galectin-3 mediates the endocytosis of b1 integrins by breast carcinoma cells. Biochem Biophys Res Commun 289, Ochieng J, et al (1998) Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem Biophys Res Commun 246, Warfield PR, et al (1997) Adhesion of human breast carcinoma to extracellular matrix proteins is modulated by galectin-3. Invasion Metastasis 17, Goetz JG, et al (2008) Concerted regulation of focal adhesion dynamics by galectin-3 and tyrosine-phosphorylated caveolin-1. J Cell Biol 180, Lagana A, et al (2006) Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol Cell Biol 26, Matarrese P, et al (2000) Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int J Cancer 85, Takagi J, et al (1997) Changing ligand specificities of avb1 and avb3 integrins by swapping a short diverse sequence of the b- subunit. J Biol Chem 272, Miao H, et al (2002) Differential regulation of Rho GTPases by b1 and b3 integrins: the role of an extracellular domain of integrin in intracellular signaling. J Cell Sci 115, Huveneers S, et al (2008) Binding of soluble fibronectin to integrin a5b1 - link to focal adhesion redistribution and contractile shape. J Cell Sci 121, Teramoto H, et al (1996) Signaling from the small GTP-binding proteins Rac1 and Cdc42 to the c-jun N-terminal kinase/ stress-activated protein kinase pathway. J Biol Chem 271, Benvenuto G, et al (1996) Cell-specific transcriptional regulation and reactivation of galectin-1 gene expression are controlled by DNA methylation of the promoter region. Mol Cell Biol 16, Chiarotti L, et al (2004) Galectin genes: regulation of expression. Glycoconj J 19, Ruebel KH, et al (2005) Effects of DNA methylation on galectin-3 expression in pituitary tumors. Cancer Res 65, Margadant C, et al (2009) Integrin a3b1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci 122, Dennis JW, et al (2009) Adaptive regulation at the cell surface by N- glycosylation. Traffic 10, Ahmad N, et al (2004) Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem 279, Nieminen J, et al (2007) Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J Biol Chem 282,
227 43. Partridge EA, et al (2004) Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 306, Ramasamy S, et al (2007) The MUC1 and galectin-3 oncoproteins function in a microrna-dependent regulatory loop. Mol Cell 27, Chen M, et al (2009) Integrin a6b4 controls the expression of genes associated with cell motility, invasion, and metastasis, including S100A4/metastasin. J Biol Chem 284, Thijssen VL, et al (2004) Angiogenesis gene expression profiling in xenograft models to study cellular interactions. Exp Cell Res 299, Li LC, and R Dahiya (2002) MethPrimer: designing primers for methylation PCRs. Bioinformatics 18, Rozhon W, et al (2008) Rapid quantification of global DNA methylation by isocratic cation exchange high-performance liquid chromatography. Anal Biochem 375, Epigenetic regulation of galectin-3 by b1 integrins 225
228
229 MAPK uncouples cell cycle progression from cell spreading and cytoskeletal organization in cycling cells Coert Margadant 1,2, Lobke Cremers 1, Arnoud Sonnenberg 2, and Johannes Boonstra 1 1 Cell Biology, Faculty of Sciences, University of Utrecht, Padualaan 8, 3584 CH Utrecht, The Netherlands. 2 Department of Cell Biology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands. Cell Mol Life Sci, in press (2012)
230
231 ABSTRAct Integrin-mediated cytoskeletal tension supports growth-factor-induced proliferation, and disruption of the actin cytoskeleton in growth factor-stimulated cells prevents the re-expression of cyclin D and cell cycle re-entry from quiescence. In contrast to cells that enter the cell cycle from G0, cycling cells continuously express cyclin D, and are subject to major cell shape changes during the cell cycle. Here, we investigated the cell cycle requirements for cytoskeletal tension and cell spreading in cycling mammalian cells that enter G1-phase from mitosis. Disruption of the actin cytoskeleton at progressive time-points in G1-phase induced cell rounding, FA disassembly, and attenuated both integrin signaling and growth factor-induced p44/p42 mitogen-activated protein kinase activation. Although cyclin D expression was reduced, the expression of cyclin A and entry into S-phase were not affected. Moreover, expression of cyclin B1, progression through G2- and M-phase, and commitment to a new cell cycle occurred normally. In contrast, cell cycle progression was strongly prevented by inhibition of MAPK activity in G1-phase, whereas cell spreading, cytoskeletal organization, and integrin signaling were not impaired. MAPK inhibition also prevented cytoskeleton-independent cell cycle progression. Thus, these results uncouple the requirements for cell spreading and cytoskeletal organization from MAPK signaling, and show that cycling mammalian cells can proliferate independently of actin stress fibers, focal adhesions, or cell spreading, as long as a threshold level of MAPK activity is sustained. IntRODUctIon Cell cycle progression in normal mammalian cells requires both growth factor stimulation and cell adhesion to the extracellular matrix (ECM) mediated by transmembrane heterodimeric αβ receptors called integrins (1,2). Engagement of virtually all integrins leads to the assembly of large macromolecular complexes called focal adhesions (FAs), at least in cells in culture, where they connect to actin stress fibers. In this way, integrins generate intracellular tension, mediate cell spreading over the substrate, and initiate several signaling pathways (3,4). Disruption of stress fibers with pharmacological agents prevents integrin-mediated cell spreading, and inhibits growth-factorinduced re-entry into G1-phase from quiescence (5-14). Cell cycle arrest upon cytoskeletal disruption is characterized by a failure to downregulate the cell cycle inhibitor p27 KIP1, to induce sustained p44/ p42 mitogen-activated protein kinase (MAPK) phosphorylation and activation, and to induce expression of D-type cyclins, which together with cdk4/6 are essential factors for promoting G1/Sphase progression (12-15). The same is observed upon loss of adhesion, or restriction of cell spreading on micro-patterned surfaces (16-18). Induction of cyclin D expression is crucial for growth factor-stimulated reentry into G1-phase from quiescence, and is the main step that is sensitive to cytoskeletal tension (5,6,19-22). Over- 9 MAPK permits cell cycle progression without cell spreading 229
232 expression of cyclin D1 can rescue proliferation in non-adherent cells, and is often associated with the anchorage-independent growth of tumor cells (23-26). It thus seems that the requirement of cell spreading for proliferation is not absolute, and that cell cycle progression and cell spreading can be disconnected as long as cyclin D1 is expressed. Indeed, normal G0- to S-phase progression in fibroblasts depends on integrin-sustained Rho activation, stress fiber formation and cell spreading, which leads to mid-g1-phase induction of cyclin D expression (27,28). However, upon inhibition of Rho signaling, a Rac/Cdc42-driven pathway induces early expression of cyclin D and shortening of G1 phase, and allows for cell cycle progression in the absence of stress fibers or cell spreading (28). Hence, depending on the circumstances, cells may switch between a tension-dependent and -independent mode of proliferation, provided that there is a signal to induce cyclin D. Consistent with such a model, we have previously shown that in cycling cells (i.e. as opposed to cells entering G1-phase from quiescence), which express cyclin D and contain low levels of cell cycle inhibitors, prevention of post-mitotic stress fiber assembly and concomitant cell spreading does not inhibit progression through G1-phase (29). We hypothesize that after cytokinesis, cells can commit to a new cycle either in a high-tensional state with extensive stress fibers and FAs, or rounded without extensive stress fibers and FAs, depending on the environmental requirements and/or restraints. Possibly, cell spreading elicits an irreversible cell-cycle program so that artificial cell rounding will inhibit cell cycle progression, which would explain why quiescent, spread cells do not enter the cell cycle upon cytoskeletal disruption. In addition, M-to-S-phase progression may only require cytoskeletal integrity and cell spreading during a discrete time-window, as has been observed for capillary endothelial cells that re-enter G1-phase from quiescence (30). In the present study, we explored the requirements for cell spreading, stress fibers, FAs and MAPK signaling in cell cycle progression in cycling cells, as well as the cross-talk between MAPK, cell spreading and the cytoskeleton. We have focused on cell cycle progression supported by fibronectin (FN), as most studies concerning integrin-mediated cytoskeletal tension and proliferation center on FN-binding integrins, most importantly integrin a5b1 (27,28,31,32). To obtain synchronized cycling cells, we have collected cells from asynchronously growing cultures of N2A and CHO cells by mitotic shake-off, which yields high numbers of cells in cytokinesis without the use of metaphase-blocking agents such as nocodazole, and we have previously extensively characterized cell cycle regulation in these cells (29,33,34). Although both cell lines are mitogen- and adhesiondependent, we also investigated cell cycle progression in non-transformed mouse GEb1 cells, as the tensional requirements for cell cycle progression are frequently lost in transformed cells (5). 230
233 ResUlts Disruption of the actin cytoskeleton in G1-phase reverses post-mitotic cell spreading and fa assembly Here we employ the mitotic shake-off method to collect cells in cytokinesis from asynchronously growing cell cultures. Released cells reattach to the substratum within 15 min and most of them complete cytokinesis within 1 hr after synchronization, whereafter cell spreading increases progressively throughout G1-phase (Figure 1A). Whereas D-type cyclins are expressed in mitotic cells and throughout the entire G1-phase, expression of S-phase promoting cyclin A is induced about 5 hrs after mitosis, and the G2/M-phase cyclin B1 becomes detectable from 10 hrs after mitosis (Figure 1B). The population undergoes the next cytokinesis approximately 16 hrs after synchronization (data not shown). The induction of cyclin A expression marks the onset of S-phase, and progression to S-phase requires the sustained activity and nuclear translocation of p44/p42 MAPK, as these events are blocked using UO126, an inhibitor of the MAPK pathway (Figures 1C,D) (33). To investigate whether cytoskeletal organization and cell spreading are required for cell cycle progression in G1-phase in cycling N2A cells, we used the actin-destabilizing drugs cytochalasin D (CCD) and latrunculin B (LB). Both drugs favor depolymerization of actin filaments but they act by distinct mechanisms; whereas CCD caps the growing ends of actin polymers, LB sequesters actin monomers. Mitotic cells were allowed to re-adhere and spread on FN-coated dishes in fresh medium with serum, after which they were treated with 500 ng/ml CCD or 100 ng/ml LB at progressive time-points in G1-phase. Post-mitotic cell spreading was quantified by measuring the apparent surface area from phase/contrast images. Cell spreading of untreated cells increased from mitosis into G1, and treatment of spread cells in G1-phase (from 3 hrs after mitosis) with either CCD or LB for 1 hr (M + 3_1) or 3 hrs (M + 3_3) induced cell rounding (Figures 1E,F). We next analysed cytoskeletal organization and FA assembly in G1-phase, using the filamentous actin (F-actin)-binding compound phalloidin and an antibody against the FA marker vinculin, which is involved in linking integrins to the actin cytoskeleton. Actin stress fibers associated with FAs were observed in spread cells in G1-phase, and treatment with either drug induced FA loss and dissolution of actin stress fibers, whereas some F-actin was retained in membrane structures left behind after cell rounding (Figure 1G). Thus, treatment with CCD or LB in G1-phase cells disrupts the normal organization of the actin cytoskeleton, and induces cell rounding and FA disassembly. Disruption of the actin cytoskeleton in G1-phase inhibits integrin signaling and growth factor signaling Integrin-mediated re-adhesion and cell spreading after mitotis triggers the autophosphorylation of focal adhesion kinase (FAK) on Y397 (29,35). Autophosphorylation of FAK triggers the recruitment of Src, which subsequently phosphorylates FAK on Y925. In turn, FAK interacts with the signal transduction adapter protein paxillin, and induces its phosphorylation at Y118 and Y31. To determine whether 9 MAPK permits cell cycle progression without cell spreading 231
234 Figure 1 Disruption of actin stress fibers in G1-phase causes cell rounding and FA disassembly. (A) Stills from a time-lapse movie showing N2A cells undergoing cytokinesis and post-mitotic cell spreading. (B) N2A cells were synchronized by mitotic shake-off and released in fresh medium. Cells were lysed at the indicated time-points and the sequential expression of cyclin D1/D2, cyclin A, and cyclin B1 was determined by Western blotting. (C) N2A cells were synchronized by mitotic shake-off and released in the presence of 10 mm BrdU. Progression into S-phase was determined at the indicated time-points by analyzing BrdU incorporation by immunofluorescence. (D) Progression from mitosis to S-phase is largely prevented by inhibition of the MAPK pathway with the inhibitor UO126 (20 mm). (E) N2A cells were synchronized by mitotic shake-off and released in fresh medium. After 3 hrs, cells were either left untreated (upper panel), treated with 500 ng/ml CCD (mid-panel) or 100 ng/ml LB (lower panel), and photographed at 1 or 3 hrs thereafter. Bar, 10 mm. (F) Cell area was determined at the indicated time-points from phase/contrast images using ImageJ, and expressed relative to the cell area of mitotic cells. AU, arbitrary units. (G) N2A cells were synchronized by mitotic shake-off and treated with 500 ng/ml CCD or 100 ng/ml LB 3 hrs thereafter. Cells were fixed after 3 hrs of incubation and the nuclei (blue), F-actin (red), and vinculin (green) were visualized with confocal microscopy. Bar, 10 mm. cytoskeletal disruption and cell rounding induced by CCD or LB in G1-phase cells affects integrin signaling, we treated G1-phase cells 3 hrs after mitosis with the actin inhibitors, and investigated the phosphorylation of paxillin and FAK by immunofluorescence and Western blotting. Whereas paxillin phosphorylation on Y118 and Y31 and FAK phosphorylation on Y397 and Y925 were clearly visible in FAs in untreated cells, reduced or no phosphorylation on these residues was observed in the CCD- or LB-treated cells (Figures 2A,B), indicating that disruption of the actin cytoskeleton in G1-phase inhibits integrin-mediated signaling events. Because integrin-mediated cell spreading and organization of 232
235 the actin cytoskeleton support growth factor signaling, we next investigated whether disruption of actin stress fibers in G1-phase cells also impedes growth factor-induced MAPK phosphorylation. MAPK phosphorylation in untreated cells was detected throughout G1, but was considerably reduced upon treatment with CCD or LB (Figure 2B), confirming that growth factor signaling in G1 depends Figure 2 Disruption of actin stress fibers in G1-phase inhibits integrin signaling and growth factor signaling. (A) N2A cells were synchronized by mitotic shake-off, released in fresh medium, and 3 hrs after synchronization they were either left untreated (top row), treated with 500 ng/ml CCD (middle row), or treated with 100 ng/ml LB (bottom row). Cells were fixed 3 hrs later and the nuclei (blue), F-actin (red), and phosphorylation of FAK and paxillin at the indicated residues (green) were visualized with confocal microscopy. Bar, 10 mm. (B) N2A cells were synchronized by mitotic shake-off and 3 hrs later treated as described. Cells were lysed 2, 3, 4 and 5 hrs thereafter and autophosphorylation of (Y397)FAK, phosphorylation of (Y118)paxillin, and phosphorylation of p44/p42 MAPK, as well as total levels of FAK, paxillin, and p42 MAPK were investigated by Western blotting. 9 MAPK permits cell cycle progression without cell spreading 233
236 on intact actin filaments. Taken together, these results indicate that disruption of the actin cytoskeleton and cell spreading in G1-phase cells attenuates both integrin signaling and growth factor signaling. G1/S-phase progression in cycling N2A and CHO cells is not inhibited by disruption of the actin cytoskeleton and cell spreading We next examined whether cell cycle progression into S-phase was affected by the disruption of actin filaments. First, we treated cells with the drugs starting at 3 hrs after mitosis until well into S-phase (8 hrs post-mitosis), and then analysed the expression of cyclin D by Western blotting. Consistent with previous observations, a progressive decline in cyclin D levels was observed in CCD- or LB-treated cells, but expression was not abolished completely (Figure 3A). Moreover, cytoskeletal disruption did also not impair the induction of cyclin A expression (Figure 3A). We then analyzed whether these cells progressed through S-phase by releasing synchronized cells in medium containing the thymidine analogue BrdU, and measuring BrdU incorporation 14 hrs after mitosis using an ELISA. To assess whether there is a specific time-frame in G1 that requires cytoskeletal integrity, CCD or LB were added at 0, 2, 4, or 6 hrs after synchronization. Interestingly, drug addition at neither time-point decreased BrdU incorporation as compared to untreated cells, indicating that also in the presence of the drugs, the entire cell population had entered and completed S-phase (Figure 3B). In contrast, disruption of the actin cytoskeleton did prevent S-phase entry in quiescent cells that were serum-stimulated to reenter the cell cycle (Figure S1), as has been documented extensively in a variety of cell types (7,9,10,12,13,15). We then investigated whether the drugs caused a delay or acceleration in the rate of progression from mitosis to S-phase, by analyzing BrdU incorporation at several time-points for up to 10 hrs after mitotis. BrdU incorporation was identical in all conditions, suggesting that disruption of the cytoskeleton does not affect the rate of G1-phase progression (Figure 3C). To exclude that the observed phenomenon is restricted to N2A cells, we next analysed the same events in CHO cells isolated by mitotic shake-off. Similar to in N2A cells, incubation of spread post-mitotic CHO cells with CCD or LB induced cell rounding and disruption of stress fibers and FAs (data not shown). Correspondingly, FAK autophosphorylation and MAPK phosphorylation were also reduced in CHO cells (Figure S2A). However, as in N2A, progression to S-phase was not prevented, as indicated by expression levels of cyclin A and BrdU incorporation, and no actin cytoskeletondependent time-window for G1/S-phase progression was detected (Figure S2B). Together, these results suggest that disruption of the actin cytoskeleton during G1-phase does not inhibit progression from mitosis to S-phase in cycling N2A and CHO cells, despite decreased integrin signaling and growth factor-stimulated MAPK phosphorylation. G1/S-phase progression in the absence of cell spreading or stress fibers depends on MAPK signaling The previous sections have shown that in the absence of cell spreading, actin stress 234
237 Figure 3 Disruption of actin stress fibers in G1-phase does not inhibit S-phase entry in continuously cycling N2A cells. (A) N2A cells were synchronized by mitotic shake-off and 3 hrs later treated as descibed. Cells were lysed 2, 3, 4 and 5 hrs thereafter and expression of cyclin D and cyclin A was investigated by Western blotting (p42 MAPK=loading control). (B) N2A cells were synchronized by shake-off, released in fresh medium containing 10 µm BrdU, and CCD or LB were added at the indicated time-points. After 14 hrs, cells were fixed and BrdU incorporation was determined with an ELISA. Incorporation in untreated cells was set to 100%. The graph represents the averages ± s.e.m. from 3 independent experiments. (C) Mitotic N2A cells were released on coverslips in fresh medium containing 10 µm BrdU, and CCD or LB were added at 0, 2, 4, or 6 hrs after mitosis. Incorporation of BrdU at the indicated time-points was determined by immunofluorescence. fibers or detectable FAs, cycling N2A and CHO cells can progress from mitosis to S-phase, despite strongly reduced MAPK activity. Because normal progression from mitosis to S-phase requires sustained MAPK activity throughout G1-phase (Figure 1D) (33), we next explored the cross-talk between MAPK signaling and cytoskeletal organization, and the requirement for MAPK activity in cytoskeletondisrupted cells. We therefore synchronized N2A cells by mitotic shake-off and released them in medium containing 500 ng/ml CCD, 100 ng/ml LB, or 20 mm UO126. Western blotting for phosphorylated p44/p42 MAPK levels revealed that UO126 inhibited MAPK activity much more rigorously than CCD and LB, as P-MAPK was virtually absent after prolonged incubation with UO126, even on overexposed blots (Figure 4A). In contrast, P(Y397)FAK was strongly inhibited by CCD or LB but not by UO126, indicating that integrin-dependent events do not require MAPK activity (Figure 4A). Consistent with this notion, cell spreading, stress fiber formation, or FA assembly (visualized using an antibody against phosphotyrosines) were not affected by treatment with UO126 (Figure 4B). We 9 MAPK permits cell cycle progression without cell spreading 235
238 then incubated cells in the presence of CCD or LB together with UO126, and determined whether these cells progressed into S-phase by measuring BrdU incorporation as described above. Intriguingly, the addition of UO126 strongy prevented BrdU incorporation, indicating that cell cycle progression in cytoskeleton-disrupted cells critically depends on MAPK activity (Figure 4C). Taken together, these data indicate that MAPK activity is not required for cell spreading, cytoskeletal organization, and integrin signaling. However, MAPK is crucial for cell cycle progression, both in untreated and cytoskeleton-disrupted cells. Figure 4 Progression through G1-phase in the absence of cell spreading and stress fibers depends on MAPK signaling. (A) N2A cells were synchonized by shake-off and released in fresh medium supplemented with 500 ng/ml CCD, 100 ng/ml LB, or 20 mm UO126. Cells were lysed at the indicated time-points after synchronization, and phosphorylation of p44/p42 MAPK and (Y397)FAK were investigated by Western blotting (p42 MAPK=loading control). (B) Top; phase-contrast images of N2A cells that were incubated for up to 5 hrs after mitosis in the absence (left) or the presence of 20 mm UO126 (right). Bar, 10 mm. Bottom; Confocal images showing F-actin (red), FAs (visualized with an antibody against phosphotyrosines, green) and nuclei (blue) in N2A cells that were incubated for up to 5 hrs after mitosis in the absence (left) or the presence of 20 mm UO126 (right). Bar, 10 mm. (C) Mitotic N2A cells were released on coverslips in fresh medium containing either 20 mm UO126 alone, 20 mm UO126 and CCD (500 ng/ml), or 20 mm UO126 and LB (100 ng/ml). BrdU incorporation was then determined 14 hrs later by immunofluorescence. The graph represents the averages from ~300 cells ± s.e.m. from 3 independent experiments. 236
239 Cycling N2A cells progress through G2- and M-phase in the absence of cell spreading, actin stress fibers, or fas We next investigated whether cell cycle progression proceeded after S-phase through G2 and the next M-phase. For this purpose, synchronized cells were treated with the actin inhibitors at several timepoints in G1 as described above, and the expression of the G2/M markers cyclin B1, which together with cdk1 promotes entry into mitosis, and securin, an essential modulator of metaphase-anaphase transition, was determined by Western blotting. Intriguingly, both cyclin B1 and securin were detectable from 10 hrs after mitosis in all conditions, suggesting that cells with disrupted cytoskeletons progress normally though G2-phase and complete their whole cycle (Figure 5A and data not shown). We then incubated synchronized cells for the length of more than an entire cell cycle with the actin inhibitors. Suppression of actin polymerization during mitosis leads to cleavage failure, creating bi-nucleated cells. We therefore used bi-nucleation as a parameter for cell cycle completion in the presence of CCD or LB. After 20 hrs of incubation, cells were still rounded, indicating that the inhibitors were still functional (data not shown). The drugs were then washed out, and the cells were allowed to recover for 1 hr in fresh medium, in which cell spreading was resumed (Figure 5B). Cell spreading was accompanied by de novo re-organization of the actin cytoskeleton into stress fibers, as well as FA assembly and integrin signaling, as judged by staining for phosphorylated (Y118)paxillin (Figure 6). In line with the expression of cyclin B1 and securin, the vast majority of the CCD- and LB-treated N2A cells was bi-nucleated, confirming that they had indeed progressed through G2- and M-phase (Figure 5B). Moreover, immunofluorescence analysis revealed that all bi-nucleated cells had incorporated BrdU (Figure S3). This is in line with the results obtained with the ELISAs, and reaffirms that the bi-nucleated cells have gone through an entire cycle. Intriguingly, nuclear localization of cyclin D was detected in bi-nucleated cells (Figure 6). Nuclear import of cyclin D occurs in growth-committed cells in G1-phase and is necessary for passage of the restriction point, whereas its subsequent export and degradation in the cytoplasm is required during S-phase (38). During the ongoing cell cycle, nuclear cyclin D is detectable starting 2 hrs after mitosis until the onset of S-phase (~6 hrs after mitosis), whereafter nuclear cyclin D levels decrease (Figure S4). Quantification of cyclin D-positive nuclei revealed that cyclin D accumulation in the nucleus of CCD- or LB-treated cells was similar to that in untreated cells (Figure 7), suggesting that these cells not only completed an entire cycle, but even committed to a new cycle in the presence of the drugs. However, during prolonged treatment with the inhibitors over several days, the multi-nucleated population underwent massive apoptosis, possibly due to genomic instability (data not shown). Summarizing, the data presented here show that N2A and CHO cells can progress through the continuous cell cycle in the absence of extensive cell spreading, stress fibers, or FAs. 9 MAPK permits cell cycle progression without cell spreading 237
240 Cycling GEb1 cells do not require cell spreading, actin stress fibers, or fas In the previous sections, we have shown that FN-supported cell cycle progression in cycling N2A and CHO cells does not depend on an intact cytoskeleton or cell spreading, which is in apparent contrast to studies using non-transformed capillary endothelial cells and fibroblasts, in which cell cycle re-entry from quiescence is inhibited upon disruption of the actin cytoskeleton (7-14). The tensional Figure 5 Cycling N2A cells progress through G2- and M-phase in the absence of cell spreading or cytoskeletal integrity. (A) N2A cells were collected by shake-off and treated with CCD or LB at 2 or 4 hrs after synchronization. Cells were lysed at 8, 10, or 14 hrs after synchronization, and expression of cyclin B1 and securin were detected by Western blotting. (B) N2A cells were collected by shake-off and treated with CCD or LB at the indicated time-points after synchronization. The drugs were washed away after 20 hrs, after which the cells were released in fresh medium for 1 hr. They were then fixed, stained with DAPI, and the percentage of mono- and bi-nucleated cells was determined from ~300 cells per experiment. The graph represents the averages ± s.e.m. from 3 independent experiments. 238
241 requirements for cell cycle progression may differ between non-transformed and transformed cells. We therefore analyzed FN-supported cell cycle progression under the same conditions in GEb1 cells, which predominantly express FN-binding integrin a5b1, and do not display typical hallmarks of oncogenic transformation (they do not grow without growth factors or in soft agar, and do not form tumors in mice unless transformed with oncogenic Ras) (36-38). Mitotic GEb1 cells were isolated by shake-off and released on FN, after which they were treated with CCD (250 ng/ml) or LB (100 ng/ml) at appropriate time-points in G1. Cytoskeletal organization, FA assembly, and integrininduced signaling events were visualized using phalloidin and an antibody against tyrosine-phosphorylated proteins. Postmitotic GEb1 cells resumed cell spreading and re-gained stress fibers and FAs within 2 hrs, and cell spreading increased progressively thereafter (Figure 8A). Treat- Figure 6 Cell spreading and actin stress fibers are dispensable for nuclear translocation of cyclin D1/D2 in cycling N2A cells. N2A cells were synchonized by shake-off and treated with CCD or LB at the indicated time-points after synchronization. After 20 hrs, the drugs were washed away and the cells were fixed 1 hr later, after which they were processed for microscopy using phalloidin-tritc and DAPI, as well as antibodies against cyclin D1/D2 and P(Y118)paxillin. Pictures were obtained on a confocal microscope. Top; nuclei (blue), F-actin (red), P(Y118)paxillin (green). Bottom; cyclin D1/D2. Bar, 10 mm. 9 MAPK permits cell cycle progression without cell spreading 239
242 Figure 7 Disruption of cytoskeletal integrity and cell spreading does not prevent entry into a new cell cycle. N2A cells were collected by shake-off and treated with CCD or LB at the indicated time-points after synchronization. After 20 hrs, the cells were fixed and cyclin D1/D2 (green) and the nuclei (blue) were visualized with confocal microscopy. The percentage of cyclin D(+) cells was quantified using ImageJ from confocal images acquired with the same settings. For each condition, ~250 cells were analyzed and the graph represents the averages ± s.e.m. from 3 independent experiments. Bar, 50 mm. ment with either drug in G1-phase abolished actin stress fibers, cell spreading, FA assembly, and tyrosine-phoshorylations within 1 hr (Figure 8A). We next investigated cell cycle progression in the presence of the inhibitors, by determining the bi-nucleation index as described above. Drugs were washed away 20 hrs after mitosis, and cells were allowed to recover in fresh medium. A complete recovery of actin stress fibers, cell spreading, and FA assembly was observed 1,5 hrs after drug wash-out, and the vast majority of the cells appeared to have 2 nuclei (Figure 8B). Indeed, bi-nucleation indices clearly show that disruption of the actin cytoskeleton at neither time-point in G1-phase induced a cell cycle arrest (Figure 8C). Taken together, these data show that similar to in N2A and CHO cells, cell cycle progression in cycling GEb1 cells does not depend on actin stress fibers, FAs, or extensive cell spreading. 240
243 Figure 8 Cell cycle progression in GEb1 cells does not require cell spreading, actin stress fibers, or FAs. (A) GEb1 cells were synchronized by mitotic shake-off, released in fresh medium on FN-coated coverslips, and then fixed in G1-phase at the indicated time-points. Alternatively, they were treated with CCD (250 ng/ml) or LB (100 ng/ml) 3 hrs after mitosis, and fixed 1 hr later. The nuclei (blue), F-actin (red), and FAs (stained with an antibody against phosphotyrosines; green) were visualized with confocal microscopy. Bar, 10 mm. (B) GEb1 cells were synchonized by shake-off and treated with CCD or LB at the indicated timepoints after synchronization. After 20 hrs, the drugs were washed away and the cells were released in fresh medium. They were fixed 1 hr later, and nuclei (blue), F-actin (red), and P(Y118)paxillin (green) were visualized with confocal microscopy. Bar, 10 mm. (C) GEb1 cells were treated as in (B), fixed, and stained with DAPI. The percentage of mono- and bi-nucleated cells was determined from ~300 cells per experiment. The graph represents the averages ± s.e.m. from 3 independent experiments. DIscUssIon In this study, we investigated the requirements for FA assembly, cytoskeletal integrity, cell spreading, and MAPK activity in FN-supported progression through the ongoing cell cycle. Mitotic N2A and CHO cells were collected by shake-off and released on FN-coated dishes, after which post-mitotic actin stress fiber formation was abolished with pharmacological agents at progressive time-points 9 MAPK permits cell cycle progression without cell spreading 241
UvA-DARE (Digital Academic Repository) What tumor cells cannot resist Ebbing, E.A. Link to publication
 UvA-DARE (Digital Academic Repository) What tumor cells cannot resist Ebbing, E.A. Link to publication Citation for published version (APA): Ebbing, E. A. (2018). What tumor cells cannot resist: Mechanisms
UvA-DARE (Digital Academic Repository) What tumor cells cannot resist Ebbing, E.A. Link to publication Citation for published version (APA): Ebbing, E. A. (2018). What tumor cells cannot resist: Mechanisms
Citation for published version (APA): Zeddies, S. (2015). Novel regulators of megakaryopoiesis: The road less traveled by
 UvA-DARE (Digital Academic Repository) Novel regulators of megakaryopoiesis: The road less traveled by Zeddies, S. Link to publication Citation for published version (APA): Zeddies, S. (2015). Novel regulators
UvA-DARE (Digital Academic Repository) Novel regulators of megakaryopoiesis: The road less traveled by Zeddies, S. Link to publication Citation for published version (APA): Zeddies, S. (2015). Novel regulators
Building blocks for return to work after sick leave due to depression de Vries, Gabe
 UvA-DARE (Digital Academic Repository) Building blocks for return to work after sick leave due to depression de Vries, Gabe Link to publication Citation for published version (APA): de Vries, G. (2016).
UvA-DARE (Digital Academic Repository) Building blocks for return to work after sick leave due to depression de Vries, Gabe Link to publication Citation for published version (APA): de Vries, G. (2016).
UvA-DARE (Digital Academic Repository) Falling: should one blame the heart? Jansen, Sofie. Link to publication
 UvA-DARE (Digital Academic Repository) Falling: should one blame the heart? Jansen, Sofie Link to publication Citation for published version (APA): Jansen, S. (2015). Falling: should one blame the heart?
UvA-DARE (Digital Academic Repository) Falling: should one blame the heart? Jansen, Sofie Link to publication Citation for published version (APA): Jansen, S. (2015). Falling: should one blame the heart?
Studies on inflammatory bowel disease and functional gastrointestinal disorders in children and adults Hoekman, D.R.
 UvA-DARE (Digital Academic Repository) Studies on inflammatory bowel disease and functional gastrointestinal disorders in children and adults Hoekman, D.R. Link to publication Citation for published version
UvA-DARE (Digital Academic Repository) Studies on inflammatory bowel disease and functional gastrointestinal disorders in children and adults Hoekman, D.R. Link to publication Citation for published version
Enzyme replacement therapy in Fabry disease, towards individualized treatment Arends, M.
 UvA-DARE (Digital Academic Repository) Enzyme replacement therapy in Fabry disease, towards individualized treatment Arends, M. Link to publication Citation for published version (APA): Arends, M. (2017).
UvA-DARE (Digital Academic Repository) Enzyme replacement therapy in Fabry disease, towards individualized treatment Arends, M. Link to publication Citation for published version (APA): Arends, M. (2017).
Tumor control and normal tissue toxicity: The two faces of radiotherapy van Oorschot, B.
 UvA-DARE (Digital Academic Repository) Tumor control and normal tissue toxicity: The two faces of radiotherapy van Oorschot, B. Link to publication Citation for published version (APA): van Oorschot, B.
UvA-DARE (Digital Academic Repository) Tumor control and normal tissue toxicity: The two faces of radiotherapy van Oorschot, B. Link to publication Citation for published version (APA): van Oorschot, B.
UvA-DARE (Digital Academic Repository) The systemic right ventricle van der Bom, T. Link to publication
 UvA-DARE (Digital Academic Repository) The systemic right ventricle van der Bom, T. Link to publication Citation for published version (APA): van der Bom, T. (2014). The systemic right ventricle. General
UvA-DARE (Digital Academic Repository) The systemic right ventricle van der Bom, T. Link to publication Citation for published version (APA): van der Bom, T. (2014). The systemic right ventricle. General
Dissecting Lyme borreliosis; Clinical aspects, pathogenesis and prevention Coumou, J.
 UvA-DARE (Digital Academic Repository) Dissecting Lyme borreliosis; Clinical aspects, pathogenesis and prevention Coumou, J. Link to publication Citation for published version (APA): Coumou, J. (2016).
UvA-DARE (Digital Academic Repository) Dissecting Lyme borreliosis; Clinical aspects, pathogenesis and prevention Coumou, J. Link to publication Citation for published version (APA): Coumou, J. (2016).
Tobacco control policies and socio-economic inequalities in smoking cessation Bosdriesz, J.R.
 UvA-DARE (Digital Academic Repository) Tobacco control policies and socio-economic inequalities in smoking cessation Bosdriesz, J.R. Link to publication Citation for published version (APA): Bosdriesz,
UvA-DARE (Digital Academic Repository) Tobacco control policies and socio-economic inequalities in smoking cessation Bosdriesz, J.R. Link to publication Citation for published version (APA): Bosdriesz,
UvA-DARE (Digital Academic Repository) Obesity, ectopic lipids, and insulin resistance ter Horst, K.W. Link to publication
 UvA-DARE (Digital Academic Repository) Obesity, ectopic lipids, and insulin resistance ter Horst, K.W. Link to publication Citation for published version (APA): ter Horst, K. W. (2017). Obesity, ectopic
UvA-DARE (Digital Academic Repository) Obesity, ectopic lipids, and insulin resistance ter Horst, K.W. Link to publication Citation for published version (APA): ter Horst, K. W. (2017). Obesity, ectopic
Citation for published version (APA): van der Paardt, M. P. (2015). Advances in MRI for colorectal cancer and bowel motility
 UvA-DARE (Digital Academic Repository) Advances in MRI for colorectal cancer and bowel motility van der Paardt, M.P. Link to publication Citation for published version (APA): van der Paardt, M. P. (2015).
UvA-DARE (Digital Academic Repository) Advances in MRI for colorectal cancer and bowel motility van der Paardt, M.P. Link to publication Citation for published version (APA): van der Paardt, M. P. (2015).
Use of the comprehensive geriatric assessment to improve patient-centred care in complex patient populations Parlevliet, J.L.
 UvA-DARE (Digital Academic Repository) Use of the comprehensive geriatric assessment to improve patient-centred care in complex patient populations Parlevliet, J.L. Link to publication Citation for published
UvA-DARE (Digital Academic Repository) Use of the comprehensive geriatric assessment to improve patient-centred care in complex patient populations Parlevliet, J.L. Link to publication Citation for published
UvA-DARE (Digital Academic Repository) Anorectal malformations and hirschsprung disease Witvliet, M.J. Link to publication
 UvA-DARE (Digital Academic Repository) Anorectal malformations and hirschsprung disease Witvliet, M.J. Link to publication Citation for published version (APA): Witvliet, M. J. (2017). Anorectal malformations
UvA-DARE (Digital Academic Repository) Anorectal malformations and hirschsprung disease Witvliet, M.J. Link to publication Citation for published version (APA): Witvliet, M. J. (2017). Anorectal malformations
UvA-DARE (Digital Academic Repository) Functional defecation disorders in children Kuizenga-Wessel, S. Link to publication
 UvA-DARE (Digital Academic Repository) Functional defecation disorders in children Kuizenga-Wessel, S. Link to publication Citation for published version (APA): Kuizenga-Wessel, S. (2017). Functional defecation
UvA-DARE (Digital Academic Repository) Functional defecation disorders in children Kuizenga-Wessel, S. Link to publication Citation for published version (APA): Kuizenga-Wessel, S. (2017). Functional defecation
Citation for published version (APA): Braakhekke, M. W. M. (2017). Randomized controlled trials in reproductive medicine: Disclosing the caveats
 UvA-DARE (Digital Academic Repository) Randomized controlled trials in reproductive medicine Braakhekke, M.W.M. Link to publication Citation for published version (APA): Braakhekke, M. W. M. (2017). Randomized
UvA-DARE (Digital Academic Repository) Randomized controlled trials in reproductive medicine Braakhekke, M.W.M. Link to publication Citation for published version (APA): Braakhekke, M. W. M. (2017). Randomized
Citation for published version (APA): Owusu, E. D. A. (2018). Malaria, HIV and sickle cell disease in Ghana: Towards tailor-made interventions
 UvA-DARE (Digital Academic Repository) Malaria, HIV and sickle cell disease in Ghana Owusu, E.D.A. Link to publication Citation for published version (APA): Owusu, E. D. A. (2018). Malaria, HIV and sickle
UvA-DARE (Digital Academic Repository) Malaria, HIV and sickle cell disease in Ghana Owusu, E.D.A. Link to publication Citation for published version (APA): Owusu, E. D. A. (2018). Malaria, HIV and sickle
The role of media entertainment in children s and adolescents ADHD-related behaviors: A reason for concern? Nikkelen, S.W.C.
 UvA-DARE (Digital Academic Repository) The role of media entertainment in children s and adolescents ADHD-related behaviors: A reason for concern? Nikkelen, S.W.C. Link to publication Citation for published
UvA-DARE (Digital Academic Repository) The role of media entertainment in children s and adolescents ADHD-related behaviors: A reason for concern? Nikkelen, S.W.C. Link to publication Citation for published
Functional abdominal pain disorders in children: therapeutic strategies focusing on hypnotherapy Rutten, J.M.T.M.
 UvA-DARE (Digital Academic Repository) Functional abdominal pain disorders in children: therapeutic strategies focusing on hypnotherapy Rutten, J.M.T.M. Link to publication Citation for published version
UvA-DARE (Digital Academic Repository) Functional abdominal pain disorders in children: therapeutic strategies focusing on hypnotherapy Rutten, J.M.T.M. Link to publication Citation for published version
Citation for published version (APA): Diederen, K. (2018). Pediatric inflammatory bowel disease: Monitoring, nutrition and surgery.
 UvA-DARE (Digital Academic Repository) Pediatric inflammatory bowel disease Diederen, K. Link to publication Citation for published version (APA): Diederen, K. (2018). Pediatric inflammatory bowel disease:
UvA-DARE (Digital Academic Repository) Pediatric inflammatory bowel disease Diederen, K. Link to publication Citation for published version (APA): Diederen, K. (2018). Pediatric inflammatory bowel disease:
UvA-DARE (Digital Academic Repository) Toothbrushing efficacy Rosema, N.A.M. Link to publication
 UvA-DARE (Digital Academic Repository) Toothbrushing efficacy Rosema, N.A.M. Link to publication Citation for published version (APA): Rosema, N. A. M. (2015). Toothbrushing efficacy. General rights It
UvA-DARE (Digital Academic Repository) Toothbrushing efficacy Rosema, N.A.M. Link to publication Citation for published version (APA): Rosema, N. A. M. (2015). Toothbrushing efficacy. General rights It
UvA-DARE (Digital Academic Repository) Mucorales between food and infection Dolat Abadi, S. Link to publication
 UvA-DARE (Digital Academic Repository) Mucorales between food and infection Dolat Abadi, S. Link to publication Citation for published version (APA): Dolatabadi, S. (2015). Mucorales between food and infection
UvA-DARE (Digital Academic Repository) Mucorales between food and infection Dolat Abadi, S. Link to publication Citation for published version (APA): Dolatabadi, S. (2015). Mucorales between food and infection
Molecular and mechanical functions of the intermediate filament protein GFAP Stassen, O.M.J.A.
 UvA-DARE (Digital Academic Repository) Molecular and mechanical functions of the intermediate filament protein GFAP Stassen, O.M.J.A. Link to publication Citation for published version (APA): Stassen,
UvA-DARE (Digital Academic Repository) Molecular and mechanical functions of the intermediate filament protein GFAP Stassen, O.M.J.A. Link to publication Citation for published version (APA): Stassen,
Citation for published version (APA): van de Vijver, S. J. M. (2015). Cardiovascular disease prevention in the slums of Kenya
 UvA-DARE (Digital Academic Repository) Cardiovascular disease prevention in the slums of Kenya van de Vijver, Steven Link to publication Citation for published version (APA): van de Vijver, S. J. M. (2015).
UvA-DARE (Digital Academic Repository) Cardiovascular disease prevention in the slums of Kenya van de Vijver, Steven Link to publication Citation for published version (APA): van de Vijver, S. J. M. (2015).
Index. derm.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Adhesion and migration, the diverse functions of the laminin a3 subunit, 79 87 Alopecia in epidermolysis bullosa, 165 169 Amblyopia and inherited
Note: Page numbers of article titles are in boldface type. A Adhesion and migration, the diverse functions of the laminin a3 subunit, 79 87 Alopecia in epidermolysis bullosa, 165 169 Amblyopia and inherited
Antimicrobial drug resistance at the human-animal interface in Vietnam Nguyen, V.T.
 UvA-DARE (Digital Academic Repository) Antimicrobial drug resistance at the human-animal interface in Vietnam Nguyen, V.T. Link to publication Citation for published version (APA): Nguyen, V. T. (2017).
UvA-DARE (Digital Academic Repository) Antimicrobial drug resistance at the human-animal interface in Vietnam Nguyen, V.T. Link to publication Citation for published version (APA): Nguyen, V. T. (2017).
Citation for published version (APA): Donker, M. (2014). Improvements in locoregional treatment of breast cancer
 UvA-DARE (Digital Academic Repository) Improvements in locoregional treatment of breast cancer Donker, Mila Link to publication Citation for published version (APA): Donker, M. (2014). Improvements in
UvA-DARE (Digital Academic Repository) Improvements in locoregional treatment of breast cancer Donker, Mila Link to publication Citation for published version (APA): Donker, M. (2014). Improvements in
Citation for published version (APA): Tjon-Kon-Fat, R. I. (2017). Unexplained subfertility: Illuminating the path to treatment.
 UvA-DARE (Digital Academic Repository) Unexplained subfertility Tjon-Kon-Fat, R.I. Link to publication Citation for published version (APA): Tjon-Kon-Fat, R. I. (2017). Unexplained subfertility: Illuminating
UvA-DARE (Digital Academic Repository) Unexplained subfertility Tjon-Kon-Fat, R.I. Link to publication Citation for published version (APA): Tjon-Kon-Fat, R. I. (2017). Unexplained subfertility: Illuminating
Citation for published version (APA): Kruizinga, R. (2017). Out of the blue: Experiences of contingency in advanced cancer patients
 UvA-DARE (Digital Academic Repository) Out of the blue Kruizinga, R. Link to publication Citation for published version (APA): Kruizinga, R. (2017). Out of the blue: Experiences of contingency in advanced
UvA-DARE (Digital Academic Repository) Out of the blue Kruizinga, R. Link to publication Citation for published version (APA): Kruizinga, R. (2017). Out of the blue: Experiences of contingency in advanced
Citation for published version (APA): van Es, N. (2017). Cancer and thrombosis: Improvements in strategies for prediction, diagnosis, and treatment
 UvA-DARE (Digital Academic Repository) Cancer and thrombosis van Es, N. Link to publication Citation for published version (APA): van Es, N. (2017). Cancer and thrombosis: Improvements in strategies for
UvA-DARE (Digital Academic Repository) Cancer and thrombosis van Es, N. Link to publication Citation for published version (APA): van Es, N. (2017). Cancer and thrombosis: Improvements in strategies for
UvA-DARE (Digital Academic Repository) Bronchial Thermoplasty in severe asthma d'hooghe, J.N.S. Link to publication
 UvA-DARE (Digital Academic Repository) Bronchial Thermoplasty in severe asthma d'hooghe, J.N.S. Link to publication Citation for published version (APA): d'hooghe, J. N. S. (2018). Bronchial Thermoplasty
UvA-DARE (Digital Academic Repository) Bronchial Thermoplasty in severe asthma d'hooghe, J.N.S. Link to publication Citation for published version (APA): d'hooghe, J. N. S. (2018). Bronchial Thermoplasty
UvA-DARE (Digital Academic Repository) Intraarterial treatment for acute ischemic stroke Berkhemer, O.A. Link to publication
 UvA-DARE (Digital Academic Repository) Intraarterial treatment for acute ischemic stroke Berkhemer, O.A. Link to publication Citation for published version (APA): Berkhemer, O. A. (2016). Intraarterial
UvA-DARE (Digital Academic Repository) Intraarterial treatment for acute ischemic stroke Berkhemer, O.A. Link to publication Citation for published version (APA): Berkhemer, O. A. (2016). Intraarterial
Citation for published version (APA): de Groof, E. J. (2017). Surgery and medical therapy in Crohn s disease: Improving treatment strategies
 UvA-DARE (Digital Academic Repository) Surgery and medical therapy in Crohn s disease de Groof, E.J. Link to publication Citation for published version (APA): de Groof, E. J. (2017). Surgery and medical
UvA-DARE (Digital Academic Repository) Surgery and medical therapy in Crohn s disease de Groof, E.J. Link to publication Citation for published version (APA): de Groof, E. J. (2017). Surgery and medical
The Beauty of the Skin
 The Beauty of the Skin Rose-Anne Romano, Ph.D Assistant Professor Department of Oral Biology School of Dental Medicine State University of New York at Buffalo The Big Question How do approximately 50 trillion
The Beauty of the Skin Rose-Anne Romano, Ph.D Assistant Professor Department of Oral Biology School of Dental Medicine State University of New York at Buffalo The Big Question How do approximately 50 trillion
UvA-DARE (Digital Academic Repository) On the innovative genius of Andreas Vesalius Brinkman, R.J.C. Link to publication
 UvA-DARE (Digital Academic Repository) On the innovative genius of Andreas Vesalius Brinkman, R.J.C. Link to publication Citation for published version (APA): Brinkman, R. J. C. (2017). On the innovative
UvA-DARE (Digital Academic Repository) On the innovative genius of Andreas Vesalius Brinkman, R.J.C. Link to publication Citation for published version (APA): Brinkman, R. J. C. (2017). On the innovative
Skin (Integumentary System) Wheater, Chap. 9
 Skin (Integumentary System) Wheater, Chap. 9 Skin (Integument) Consists of skin and associated derivatives Largest organ of body (21 ft 2 ; 9 lbs.; has 11 miles of blood vessels) Functions: Protection
Skin (Integumentary System) Wheater, Chap. 9 Skin (Integument) Consists of skin and associated derivatives Largest organ of body (21 ft 2 ; 9 lbs.; has 11 miles of blood vessels) Functions: Protection
UvA-DARE (Digital Academic Repository) Hip and groin pain in athletes Tak, I.J.R. Link to publication
 UvA-DARE (Digital Academic Repository) Hip and groin pain in athletes Tak, I.J.R. Link to publication Citation for published version (APA): Tak, I. J. R. (2017). Hip and groin pain in athletes: Morphology,
UvA-DARE (Digital Academic Repository) Hip and groin pain in athletes Tak, I.J.R. Link to publication Citation for published version (APA): Tak, I. J. R. (2017). Hip and groin pain in athletes: Morphology,
This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur.
 Background Knowledge Functions of normal skin Background Knowledge This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur. Learning
Background Knowledge Functions of normal skin Background Knowledge This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur. Learning
UvA-DARE (Digital Academic Repository) Therapeutic targets in sickle cell disease Sins, J.W.R. Link to publication
 UvA-DARE (Digital Academic Repository) Therapeutic targets in sickle cell disease Sins, J.W.R. Link to publication Citation for published version (APA): Sins, J. W. R. (2017). Therapeutic targets in sickle
UvA-DARE (Digital Academic Repository) Therapeutic targets in sickle cell disease Sins, J.W.R. Link to publication Citation for published version (APA): Sins, J. W. R. (2017). Therapeutic targets in sickle
Finding the balance between overtreatment and undertreatment of ductal carcinoma in situ Elshof, L.E.
 UvA-DARE (Digital Academic Repository) Finding the balance between overtreatment and undertreatment of ductal carcinoma in situ Elshof, L.E. Link to publication Citation for published version (APA): Elshof,
UvA-DARE (Digital Academic Repository) Finding the balance between overtreatment and undertreatment of ductal carcinoma in situ Elshof, L.E. Link to publication Citation for published version (APA): Elshof,
Skin. Kristine Krafts, M.D.
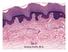 Skin Kristine Krafts, M.D. Skin Lecture Objectives Describe the functions of skin. Describe the structure, location and function of the cell types found in epidermis: keratinocytes, melanocytes, Langerhans
Skin Kristine Krafts, M.D. Skin Lecture Objectives Describe the functions of skin. Describe the structure, location and function of the cell types found in epidermis: keratinocytes, melanocytes, Langerhans
Percutaneous coronary intervention in acute myocardial infarction: from procedural considerations to long term outcomes Delewi, R.
 UvA-DARE (Digital Academic Repository) Percutaneous coronary intervention in acute myocardial infarction: from procedural considerations to long term outcomes Delewi, R. Link to publication Citation for
UvA-DARE (Digital Academic Repository) Percutaneous coronary intervention in acute myocardial infarction: from procedural considerations to long term outcomes Delewi, R. Link to publication Citation for
Integumentary System
 Integumentary System Overview Functions 1. Protection 2. Excretion of wastes 3. Maintenance of T b 4. Synthesis of vitamin D 3 5. Storage of lipids 6. Detection of sensory stimuli Epidermis Tissue types
Integumentary System Overview Functions 1. Protection 2. Excretion of wastes 3. Maintenance of T b 4. Synthesis of vitamin D 3 5. Storage of lipids 6. Detection of sensory stimuli Epidermis Tissue types
Cell Cell Communication
 IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
Novel insights into the complexity of ischaemic heart disease derived from combined coronary pressure and flow velocity measurements van de Hoef, T.P.
 UvA-DARE (Digital Academic Repository) Novel insights into the complexity of ischaemic heart disease derived from combined coronary pressure and flow velocity measurements van de Hoef, T.P. Link to publication
UvA-DARE (Digital Academic Repository) Novel insights into the complexity of ischaemic heart disease derived from combined coronary pressure and flow velocity measurements van de Hoef, T.P. Link to publication
Histology = the study of tissues. Tissue = a complex of cells that have a common function
 { EPITHELIAL TISSUE Histology = the study of tissues Tissue = a complex of cells that have a common function The Four Primary Tissue Types: Epithelium (epithelial tissue) covers body surfaces, lines body
{ EPITHELIAL TISSUE Histology = the study of tissues Tissue = a complex of cells that have a common function The Four Primary Tissue Types: Epithelium (epithelial tissue) covers body surfaces, lines body
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/23937 holds various files of this Leiden University dissertation. Author: Liu, Zhen Title: Exploring the interplay between TGF-β and VEGF signalling in
Cover Page The handle http://hdl.handle.net/1887/23937 holds various files of this Leiden University dissertation. Author: Liu, Zhen Title: Exploring the interplay between TGF-β and VEGF signalling in
Cell Cell Communication
 IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
Chapter 4 Opener Pearson Education, Inc.
 Chapter 4 Opener Introduction The integumentary system is composed of: Skin Hair Nails Sweat glands Oil glands Mammary glands The skin is the most visible organ of the body Clinicians can tell a lot about
Chapter 4 Opener Introduction The integumentary system is composed of: Skin Hair Nails Sweat glands Oil glands Mammary glands The skin is the most visible organ of the body Clinicians can tell a lot about
Animal Tissue Culture SQG 3242 Biology of Cultured Cells. Dr. Siti Pauliena Mohd Bohari
 Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
The Integumentary System: An Overview
 The Integumentary System: An Overview Functions: Protective covering Helps regulate body temperature Retards water loss from deeper tissues Houses sensory receptors Synthesizes biochemicals Excretes small
The Integumentary System: An Overview Functions: Protective covering Helps regulate body temperature Retards water loss from deeper tissues Houses sensory receptors Synthesizes biochemicals Excretes small
DEBRIDEMENT: ANATOMY and PHYSIOLOGY. Professor Donald G. MacLellan Executive Director Health Education & Management Innovations
 DEBRIDEMENT: ANATOMY and PHYSIOLOGY Professor Donald G. MacLellan Executive Director Health Education & Management Innovations ANATOMY and PHYSIOLOGY Epidermal Layers ECM Structure Dermis Structure Skin
DEBRIDEMENT: ANATOMY and PHYSIOLOGY Professor Donald G. MacLellan Executive Director Health Education & Management Innovations ANATOMY and PHYSIOLOGY Epidermal Layers ECM Structure Dermis Structure Skin
Anatomy and Physiology Homework: Chapters 3-4
 Anatomy and Physiology Homework: Chapters 3-4 CHAPTER 3: Cells and Tissues 1. The smallest unit of living tissue is called a. All living organisms are composed of these basic units where all life processes
Anatomy and Physiology Homework: Chapters 3-4 CHAPTER 3: Cells and Tissues 1. The smallest unit of living tissue is called a. All living organisms are composed of these basic units where all life processes
Lecture 13 - Intermediate filaments
 02.12.10 Lecture 13 - Intermediate filaments Intermediate filaments Present in nearly all animals, but absent from plants and fungi Rope-like network of filaments in the cell Principle function is maintenance
02.12.10 Lecture 13 - Intermediate filaments Intermediate filaments Present in nearly all animals, but absent from plants and fungi Rope-like network of filaments in the cell Principle function is maintenance
Integument. Squamous Cell Carcinoma. Melanoma. Largest organ 30% of all clinical diagnoses 1/3 of all tumors
 Squamous Cell Carcinoma Integument Largest organ 30% of all clinical diagnoses 1/3 of all tumors Melanoma Epidermis Stratified, squamous keratinized epithelium Derived from ectoderm Appendages hair follicles
Squamous Cell Carcinoma Integument Largest organ 30% of all clinical diagnoses 1/3 of all tumors Melanoma Epidermis Stratified, squamous keratinized epithelium Derived from ectoderm Appendages hair follicles
Lab 7: Integumentary System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS
 Lab 7: Integumentary System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS Pre-Lab Activity 1: 1. a. epidermis b. dermis c. hypodermis d. adipose tissue e. hair f. sebaceous gland g. sweat gland 2. a Pre-Lab
Lab 7: Integumentary System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS Pre-Lab Activity 1: 1. a. epidermis b. dermis c. hypodermis d. adipose tissue e. hair f. sebaceous gland g. sweat gland 2. a Pre-Lab
Study Guide for Bio 101 Lecture Exam 3
 Study Guide for Bio 101 Lecture Exam 3 Please note that this study guide is a listing of objectives that you are required to master for this course. However, items mentioned in class or in laboratory as
Study Guide for Bio 101 Lecture Exam 3 Please note that this study guide is a listing of objectives that you are required to master for this course. However, items mentioned in class or in laboratory as
Dr Mahmood S Choudhery, PhD, Postdoc (USA) Assistant Professor Tissue Engineering and Regenerative Medicine King Edward Medical University/Mayo
 Integration of Cells into Tissues Dr Mahmood S Choudhery, PhD, Postdoc (USA) Assistant Professor Tissue Engineering and Regenerative Medicine King Edward Medical University/Mayo Hospital Lahore 1. How
Integration of Cells into Tissues Dr Mahmood S Choudhery, PhD, Postdoc (USA) Assistant Professor Tissue Engineering and Regenerative Medicine King Edward Medical University/Mayo Hospital Lahore 1. How
Anatomy Ch 6: Integumentary System
 Anatomy Ch 6: Integumentary System Introduction: A. Organs are body structures composed of two or more different tissues. B. The skin and its accessory organs make up the integumentary system. Types of
Anatomy Ch 6: Integumentary System Introduction: A. Organs are body structures composed of two or more different tissues. B. The skin and its accessory organs make up the integumentary system. Types of
UvA-DARE (Digital Academic Repository) Gastrointestinal consequences of bariatric surgery Boerlage, T.C.C. Link to publication
 UvA-DARE (Digital Academic Repository) Gastrointestinal consequences of bariatric surgery Boerlage, T.C.C. Link to publication Citation for published version (APA): Boerlage, T. C. C. (2018). Gastrointestinal
UvA-DARE (Digital Academic Repository) Gastrointestinal consequences of bariatric surgery Boerlage, T.C.C. Link to publication Citation for published version (APA): Boerlage, T. C. C. (2018). Gastrointestinal
INTEGUMENTARY 1-Epidermis, 2-Dermis, Structure of thick and thin skin I- Epidermis . Stratum basale
 INTEGUMENTARY The skin (integument, cutis ) and its derivatives constitute the integumentary system. It form the external covering of the body and is the largest organ of the body. The skin consists of
INTEGUMENTARY The skin (integument, cutis ) and its derivatives constitute the integumentary system. It form the external covering of the body and is the largest organ of the body. The skin consists of
Integumentary System. Integumentary System
 1. General aspects a. The integumentary system consists of several organs major organ of the system is the skin other organs are relatively small and they can be considered as specialized structures of
1. General aspects a. The integumentary system consists of several organs major organ of the system is the skin other organs are relatively small and they can be considered as specialized structures of
Integumentary System and Body Membranes
 Integumentary System and Body Membranes The Skin and its appendages hair, nails, and skin glands Anatomy/Physiology NHS http://www.lab.anhb.uwa.edu.au/mb140/corepages/integumentary/integum.htm I. System
Integumentary System and Body Membranes The Skin and its appendages hair, nails, and skin glands Anatomy/Physiology NHS http://www.lab.anhb.uwa.edu.au/mb140/corepages/integumentary/integum.htm I. System
Healing & Repair. Tissue Regeneration
 Healing & Repair Dr. Srikumar Chakravarthi Repair & Healing: Are they same? Repair :Regeneration of injured cells by cells of same type, as with regeneration of skin/oral mucosa (requires basement membrane)
Healing & Repair Dr. Srikumar Chakravarthi Repair & Healing: Are they same? Repair :Regeneration of injured cells by cells of same type, as with regeneration of skin/oral mucosa (requires basement membrane)
Patient reported outcomes in chronic skin diseases: ehealth applications for clinical practice van Cranenburgh, O.D.
 UvA-DARE (Digital Academic Repository) Patient reported outcomes in chronic skin diseases: ehealth applications for clinical practice van Cranenburgh, O.D. Link to publication Citation for published version
UvA-DARE (Digital Academic Repository) Patient reported outcomes in chronic skin diseases: ehealth applications for clinical practice van Cranenburgh, O.D. Link to publication Citation for published version
Introduction. Skin and Body Membranes. Cutaneous Membranes Skin 9/14/2017. Classification of Body Membranes. Classification of Body Membranes
 Introduction Skin and Body Membranes Body membranes Cover surfaces Line body cavities Form protective and lubricating sheets around organs Classified in 5 categories Epithelial membranes 3 types- cutaneous,
Introduction Skin and Body Membranes Body membranes Cover surfaces Line body cavities Form protective and lubricating sheets around organs Classified in 5 categories Epithelial membranes 3 types- cutaneous,
B. Incorrect! The ectoderm does not produce the dermis. C. Incorrect! The dermis is derived from the mesoderm.
 Human Anatomy - Problem Drill 04: The Integumentary System Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as 1. From the inner cell
Human Anatomy - Problem Drill 04: The Integumentary System Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as 1. From the inner cell
Signaling Vascular Morphogenesis and Maintenance
 Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Dr Narmeen S. Ahmad. Lab 1
 Dr Narmeen S. Ahmad Lab 1 1 Tissues are groups of cells with a common structure (form) and function (job). There are (4) types of tissue: 1. Epithelial 2. Connective 3. Muscle 4. Nervous 2 Epithelial cells
Dr Narmeen S. Ahmad Lab 1 1 Tissues are groups of cells with a common structure (form) and function (job). There are (4) types of tissue: 1. Epithelial 2. Connective 3. Muscle 4. Nervous 2 Epithelial cells
Effects of biological response modifiers in psoriasis and psoriatic arthritis Goedkoop, A.Y.
 UvA-DARE (Digital Academic Repository) Effects of biological response modifiers in psoriasis and psoriatic arthritis Goedkoop, A.Y. Link to publication Citation for published version (APA): Goedkoop, A.
UvA-DARE (Digital Academic Repository) Effects of biological response modifiers in psoriasis and psoriatic arthritis Goedkoop, A.Y. Link to publication Citation for published version (APA): Goedkoop, A.
UvA-DARE (Digital Academic Repository) Clinical issues in the surgical treatment of colon cancer Amri, R. Link to publication
 UvA-DARE (Digital Academic Repository) Clinical issues in the surgical treatment of colon cancer Amri, R. Link to publication Citation for published version (APA): Amri, R. (2015). Clinical issues in the
UvA-DARE (Digital Academic Repository) Clinical issues in the surgical treatment of colon cancer Amri, R. Link to publication Citation for published version (APA): Amri, R. (2015). Clinical issues in the
Esthetic and bonding enhancements of tooth colored indirect restorations El-Zohairy, A.A.
 UvA-DARE (Digital Academic Repository) Esthetic and bonding enhancements of tooth colored indirect restorations El-Zohairy, A.A. Link to publication Citation for published version (APA): El-Zohairy, A.
UvA-DARE (Digital Academic Repository) Esthetic and bonding enhancements of tooth colored indirect restorations El-Zohairy, A.A. Link to publication Citation for published version (APA): El-Zohairy, A.
CHAPTER 5 INTEGUMENTARY
 CHAPTER 5 INTEGUMENTARY skin under the skin other stuff cutaneous layer hypodermis (subcutaneous) accessory structures Cutaneous layer = skin epithelial layers = connective tissue layer = dermis Subcutaneous
CHAPTER 5 INTEGUMENTARY skin under the skin other stuff cutaneous layer hypodermis (subcutaneous) accessory structures Cutaneous layer = skin epithelial layers = connective tissue layer = dermis Subcutaneous
The Integumentary System
 The Integumentary System Integument is skin Skin and its appendages make up the integumentary system A fatty layer (hypodermis) lies deep to it Two distinct regions Epidermis Dermis PHL 212 1 Function
The Integumentary System Integument is skin Skin and its appendages make up the integumentary system A fatty layer (hypodermis) lies deep to it Two distinct regions Epidermis Dermis PHL 212 1 Function
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 5 The Integumentary System Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails,
Principles of Anatomy and Physiology 14 th Edition CHAPTER 5 The Integumentary System Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails,
Ex. 7: Integumentary
 Collin County Community College BIOL. 2401 Ex. 7: Integumentary. Skin or Integument Consists of three major regions Epidermis outermost superficial region Dermis middle region Hypodermis (superficial fascia)
Collin County Community College BIOL. 2401 Ex. 7: Integumentary. Skin or Integument Consists of three major regions Epidermis outermost superficial region Dermis middle region Hypodermis (superficial fascia)
Hole s Essentials of Human Anatomy & Physiology
 Hole s Essentials of Human Anatomy & Physiology David Shier Jackie Butler Ricki Lewis Created by Dr. Melissa Eisenhauer Head Athletic Trainer/Assistant Professor Trevecca Nazarene University Chapter 6
Hole s Essentials of Human Anatomy & Physiology David Shier Jackie Butler Ricki Lewis Created by Dr. Melissa Eisenhauer Head Athletic Trainer/Assistant Professor Trevecca Nazarene University Chapter 6
The Integumentary System
 The Integumentary System The Integumentary System Integument is skin Skin and its appendages make up the integumentary system (See if you can name some appendages) A fatty layer (hypodermis) lies deep
The Integumentary System The Integumentary System Integument is skin Skin and its appendages make up the integumentary system (See if you can name some appendages) A fatty layer (hypodermis) lies deep
Anatomy and Physiology I Student Outline The Integumentary System. Integumentary System. Page 1
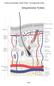 Anatomy and Physiology I Student Outline The Integumentary System Integumentary System Page 1 Have a very clear understanding of the each particular tissue and their unique functions in each layer of the
Anatomy and Physiology I Student Outline The Integumentary System Integumentary System Page 1 Have a very clear understanding of the each particular tissue and their unique functions in each layer of the
1. Introduction (Open your text to the image of a cross section of skin) i. Organ of the Integument. Connective Tissues. Epithelial Tissues
 Integumentary System 1. Introduction (Open your text to the image of a cross section of skin) A. Integumentary System i. Organ of the Integument a. Tissues Connective Tissues * Tissue / Location Relationships
Integumentary System 1. Introduction (Open your text to the image of a cross section of skin) A. Integumentary System i. Organ of the Integument a. Tissues Connective Tissues * Tissue / Location Relationships
Principles of Genetics and Molecular Biology
 Cell signaling Dr. Diala Abu-Hassan, DDS, PhD School of Medicine Dr.abuhassand@gmail.com Principles of Genetics and Molecular Biology www.cs.montana.edu Modes of cell signaling Direct interaction of a
Cell signaling Dr. Diala Abu-Hassan, DDS, PhD School of Medicine Dr.abuhassand@gmail.com Principles of Genetics and Molecular Biology www.cs.montana.edu Modes of cell signaling Direct interaction of a
Epidermis. Integumentary system
 Epidermis the doctor mentioned at the begging of the lecture that the slides is from different sources and has information and details that is enough for us so we don t have to go back and read from the
Epidermis the doctor mentioned at the begging of the lecture that the slides is from different sources and has information and details that is enough for us so we don t have to go back and read from the
****************************************************************************************************** INTEGUMENTARY SYSTEM
 BIOLOGY 211: HUMAN ANATOMY & PHYSIOLOGY ****************************************************************************************************** INTEGUMENTARY SYSTEM ******************************************************************************************************
BIOLOGY 211: HUMAN ANATOMY & PHYSIOLOGY ****************************************************************************************************** INTEGUMENTARY SYSTEM ******************************************************************************************************
UvA-DARE (Digital Academic Repository) Marfan syndrome: Getting to the root of the problem Franken, Romy. Link to publication
 UvA-DARE (Digital Academic Repository) Marfan syndrome: Getting to the root of the problem Franken, Romy Link to publication Citation for published version (APA): Franken, R. (2016). Marfan syndrome: Getting
UvA-DARE (Digital Academic Repository) Marfan syndrome: Getting to the root of the problem Franken, Romy Link to publication Citation for published version (APA): Franken, R. (2016). Marfan syndrome: Getting
UvA-DARE (Digital Academic Repository) Hypercoagulability in hematological malignancies Lauw, M.N. Link to publication
 UvA-DARE (Digital Academic Repository) Hypercoagulability in hematological malignancies Lauw, M.N. Link to publication Citation for published version (APA): Lauw, M. N. (2015). Hypercoagulability in hematological
UvA-DARE (Digital Academic Repository) Hypercoagulability in hematological malignancies Lauw, M.N. Link to publication Citation for published version (APA): Lauw, M. N. (2015). Hypercoagulability in hematological
UvA-DARE (Digital Academic Repository) Genetic basis of hypertrophic cardiomyopathy Bos, J.M. Link to publication
 UvA-DARE (Digital Academic Repository) Genetic basis of hypertrophic cardiomyopathy Bos, J.M. Link to publication Citation for published version (APA): Bos, J. M. (2010). Genetic basis of hypertrophic
UvA-DARE (Digital Academic Repository) Genetic basis of hypertrophic cardiomyopathy Bos, J.M. Link to publication Citation for published version (APA): Bos, J. M. (2010). Genetic basis of hypertrophic
Human Anatomy & Physiology
 PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 5 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 5 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
Tissue renewal and Repair. Nisamanee Charoenchon, PhD Department of Pathobiology, Faculty of Science
 Tissue renewal and Repair Nisamanee Charoenchon, PhD Email: nisamanee.cha@mahidol.ac.th Department of Pathobiology, Faculty of Science Topic Objectives 1. Describe processes of tissue repair, regeneration
Tissue renewal and Repair Nisamanee Charoenchon, PhD Email: nisamanee.cha@mahidol.ac.th Department of Pathobiology, Faculty of Science Topic Objectives 1. Describe processes of tissue repair, regeneration
SKIN HISTOLOGY the microscopic anatomy of the Integument. Mikrogeo. com
 SKIN HISTOLOGY the microscopic anatomy of the Integument Mikrogeo. com Hair follicles, sweat glands, sebaceous glands (even teeth) are products of the epidermis,embryologically speaking ectododerm, that
SKIN HISTOLOGY the microscopic anatomy of the Integument Mikrogeo. com Hair follicles, sweat glands, sebaceous glands (even teeth) are products of the epidermis,embryologically speaking ectododerm, that
Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs
 Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs Classification of Body Membranes Epithelial membranes Cutaneous
Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs Classification of Body Membranes Epithelial membranes Cutaneous
Intercellular indirect communication
 Intercellular indirect communication transmission of chemical signals: sending cell signal transmitting tissue hormone medium receiving cell hormone intercellular fluid blood neurocrine neurotransmitter
Intercellular indirect communication transmission of chemical signals: sending cell signal transmitting tissue hormone medium receiving cell hormone intercellular fluid blood neurocrine neurotransmitter
G-Protein Signaling. Introduction to intracellular signaling. Dr. SARRAY Sameh, Ph.D
 G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
Chapter 5. Integumentary System 5-1
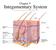 Chapter 5 Integumentary System 5-1 Structures that are part of the integument Skin Hair Nails Glands Overview of Functions Protection Sensation Temperature regulation Vitamin D production Excretion Immunity
Chapter 5 Integumentary System 5-1 Structures that are part of the integument Skin Hair Nails Glands Overview of Functions Protection Sensation Temperature regulation Vitamin D production Excretion Immunity
INTEGUMENTARY SYSTEM PART I: FUNCTIONS & EPIDERMIS
 INTEGUMENTARY SYSTEM PART I: FUNCTIONS & EPIDERMIS Integumentary System Cutaneous membrane Epidermis (5-layers) made up of epithelial tissue only Dermis (2-layers) contains connective tissue, vessels,
INTEGUMENTARY SYSTEM PART I: FUNCTIONS & EPIDERMIS Integumentary System Cutaneous membrane Epidermis (5-layers) made up of epithelial tissue only Dermis (2-layers) contains connective tissue, vessels,
Unit 4 The Integumentary System
 Unit 4 The Integumentary System I. Classification of Body Membranes A. Epithelial Membranes (3) 1. Cutaneous Membrane > Stratified Squamous > Sits on Dense Connective Tissue > Skin: Epidermis & Dermis
Unit 4 The Integumentary System I. Classification of Body Membranes A. Epithelial Membranes (3) 1. Cutaneous Membrane > Stratified Squamous > Sits on Dense Connective Tissue > Skin: Epidermis & Dermis
UvA-DARE (Digital Academic Repository) Improving aspects of palliative care for children Jagt, C.T. Link to publication
 UvA-DARE (Digital Academic Repository) Improving aspects of palliative care for children Jagt, C.T. Link to publication Citation for published version (APA): Jagt, C. T. (2017). Improving aspects of palliative
UvA-DARE (Digital Academic Repository) Improving aspects of palliative care for children Jagt, C.T. Link to publication Citation for published version (APA): Jagt, C. T. (2017). Improving aspects of palliative
Four Types of Vertebrate Tissue
 BIO 121 Molecular Cell Biology Lecture Section IV A. Cells in the Context of Tissue, Organ and Organismal Architecture B. Wound Healing Four Types of Vertebrate Tissue 1.Epithelium 2.Connective Tissue
BIO 121 Molecular Cell Biology Lecture Section IV A. Cells in the Context of Tissue, Organ and Organismal Architecture B. Wound Healing Four Types of Vertebrate Tissue 1.Epithelium 2.Connective Tissue
Chapter 4 :Organization & Regulation of Body Systems
 Chapter 4 :Organization & Regulation of Body Systems 4.1 Types of tissues What is a tissue? A collection of cells of the same type that perform a common function There are 4 major tissue types in the body:
Chapter 4 :Organization & Regulation of Body Systems 4.1 Types of tissues What is a tissue? A collection of cells of the same type that perform a common function There are 4 major tissue types in the body:
11/8/2012. Chapter 6 Part 1 Objectives: Skin = Integument = Cutaneous Membrane. The Structure of Skin. Epidermis
 Chapter 6 Part 1 Objectives: Define organ, and associate the skin as an organ of the integumentary system. List the general functions of the skin. Describe the structure of the layers of the skin. Summarize
Chapter 6 Part 1 Objectives: Define organ, and associate the skin as an organ of the integumentary system. List the general functions of the skin. Describe the structure of the layers of the skin. Summarize
Levels of Organization
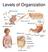 Levels of Organization Oklahoma Laws Violators can be fined, arrested or jailed for making ugly faces at a dog. Females are forbidden from doing their own hair without being licensed by the state. Dogs
Levels of Organization Oklahoma Laws Violators can be fined, arrested or jailed for making ugly faces at a dog. Females are forbidden from doing their own hair without being licensed by the state. Dogs
