Improved Rat Model of Pneumocystis carinii Pneumonia: Induced Laboratory Infections in Pneumocystis-Free Animals
|
|
|
- Bernadette Sullivan
- 5 years ago
- Views:
Transcription
1 INFECrION AND IMMUNITY, Apr. 1992, p /92/ $02.00/0 Copyright X) 1992, American Society for Microbiology Vol. 60, No. 4 Improved Rat Model of Pneumocystis carinii Pneumonia: Induced Laboratory Infections in Pneumocystis-Free Animals CAROLE J. BOYLAN* AND WILLIAM L. CURRENT Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, Indiana Received 23 October 1991/Accepted 14 January 1992 An immunosuppressed rat model of Pneumocystis carinii pneumonia is described that utilizes simple, noninvasive intratracheal (i.t.) inoculation of cryopreserved parasites and results in development of severe P. carinii pneumonia within 5 weeks. This is an improvement over the most commonly used models of P. carinii pneumonia that rely on immune suppression to activate latent P. carinii infections and that often require 8 to 12 weeks to produce heavy infections of P. carinii. It is also less labor intensive than more recent models requiring surgical instillation of parasites. Our report describes a series of preliminary studies to select an appropriate strain of rat; to determine suitable methods for inducing uniform immunosuppression, P. carinii inoculation, and laboratory maintenance of P. carinii; and to determine effective animal husbandry methods for maintaining animals free from serious secondary infections. Results of our more detailed studies demonstrate that animals receiving two or three i.t. inoculations of approximately 106 cryopreserved P. carinii organisms have a predictable course of disease progression which includes moderate P. carinii infections within 3 weeks, severe P. carinii pneumonia in 5 weeks, and a high percentage of mortality due to P. carinii pneumonia in 6 weeks. Parasites were distributed evenly between the right and left lungs, regardless of the number of P. carinii inoculations administered. Non-P. carinii-inoculated immunosuppressed control rats maintained in microisolator cages remained free ofp. carinii, thus providing an important control that is missing from many P. carinii pneumonia models. Most non-p. carinii-inoculated control animals and P. carinii-inoculated rats treated with trimethoprim-sulfamethoxazole that were housed in open caging in the same room containing heavily infected animals had no detectable infections after 5 to 6 weeks of immunosuppression; however, some had a small number of P. carinii in their lungs. Because heavy, reproducible infections are achieved 5 weeks after i.t. inoculation, because few animals are lost to secondary infections, and because animals can be maintained as noninfected contemporaneous controls, this animal model is useful for the maintenance of P. carinii strains, for studies of the transmission and natural history of P. carinii, for the production of large numbers of organisms for laboratory studies, and for the evaluation of potential anti-p. carinii drugs. Pneumocystis carinii pneumonia is a leading cause of morbidity and mortality in persons with AIDS. Historically, it has been the initial disease manifestation in more than 60% and ultimately occurred in at least 85% of AIDS patients in North America (4, 13). P. caninii pneumonia is fatal in 5 to 10% of initial episodes and has been the cause of death in approximately 25% of AIDS patients reported in autopsy series (13). Although recent, widespread implementation of prophylaxis for P. carinii pneumonia has apparently been successful in lowering its incidence, it remains an important disease; more deaths presently occur in the United States from P. carinii pneumonia than from all reportable diseases (8). Thus, a major goal for biomedical research is increased success in diagnosis, prevention, and treatment of P. carinji pneumonia. To meet these goals, researchers must have access to reliable animal models of P. cannii pneumonia that can be used as a source of large numbers of organisms for laboratory studies and as a tool for evaluating the efficacy of new drugs. Historically, most animal models used to study P. cannii relied on steroid-induced immunosuppression to activate latent P. carnii infections (1, 3, 6, 9-11, 14, 17, 18). The presence of latent P. carinii infections in many breeding colonies of Sprague-Dawley rats resulted in this rodent becoming the animal of choice for most investigators (1, 3, 9-11, 17). The utility of this animal model is often limited by * Corresponding author unknown baselines of P. carinii infection, the long time required for development of heavy infections (8 to 12 weeks), the lack of control over parasite strains, and often the presence of secondary infections that render many of the animals unusable. This animal model is becoming more difficult to maintain in most laboratories because of the reduced availability of rodents with latent P. cainni. Most suppliers have eliminated or are in the process of eliminating primary and opportunistic pathogens, including P. carinii, from commercial production colonies. To circumvent many of the problems associated with the conventional (dirty rat) model of P. carinii pneumonia, investigators have developed surgical procedures to introduce P. carinii into the lungs of virus-free, P. carinii-free rats (2). Although the surgical procedures are labor intensive, they provide a means of obtaining consistent, heavy P. carinii infection in immunosuppressed rats. In this communication, we describe the development of an improved animal model of laboratory-induced P. carinii infections that results in severe P. carinii pneumonia within 5 weeks after noninvasive intratracheal (i.t.) inoculation of parasites. This model produces consistent, heavy infections of P. carinii with minimal secondary bacterial infections and has been used extensively in our laboratory to evaluate candidate drugs for anti-p. carinii pneumonia activity and for propagation of a strain of P. carinii for laboratory studies. (Presented in part at the International Workshop on Pneumocystis, Cryptosporidium, and Microsporidia, Bozeman, Mont., 30 June to 2 July 1991 [abstract WS-65].)
2 1590 BOYLAN AND CURRENT MATERIALS AND METHODS Preliminary studies. A series of preliminary studies were conducted to select an appropriate strain of rat; to determine suitable methods of immunosuppression, P. carinii inoculation, and maintenance of P. carinii; and to determine effective animal husbandry methods for maintaining animals free from serious secondary bacterial infections. These studies are summarized below before a presentation of the materials and methods used in the more-detailed studies to develop the animal model. (i) Selection of rat strain. Three strains of rats, Sprague- Dawley, Fisher 344, and Lewis, were evaluated and compared to determine which was best suited for steroid-induced immunosuppression and maintenance of P. cainni pneumonia. All 20 rats in each of the three strains developed heavy P. caninii infections 6 weeks after immunosuppression and i.t. inoculation of parasites; however, the inbred, virus-free Lewis rats developed fewer secondary bacterial infections and were more docile. Female Lewis rats (Harlan Sprague- Dawley, Inc., Indianapolis, Ind.) weighing 120 to 140 g were utilized in all subsequent studies to develop the model. (ii) Immunosuppression regimen. Two immunosuppressive therapies were compared. Depo-Medrol (methlyprednisolone acetate; The Upjohn Co., Kalamazoo, Mich.; 40 and 30 pug/g for weeks 1 and 2, continuing weekly at 20,ug/g) administered subcutaneously resulted in a more controlled, uniform dose of immunosuppression than did dexamethasone (Schering Corp., Kenilworth, N.J.) administered in the drinking water. Animals immunosuppressed with methylprednisolone acetate developed heavy, consistent P. cannii infections within 6 weeks following i.t. inoculation of parasites. (iii) Preparation and maintenance of P. carinii inoculum. Parasite inoculum was prepared from the lungs of heavily infected donor rats housed in microisolator cages (Lab Products Inc., Maywood, N.J.; cage bottom no ; stainless steel wire lid no ; and filter top no ). Rats were anesthetized with approximately 135,ug of ketamine (ketamine hydrochloride injection USP, 100 mg/ml; Aveco Co., Inc., Fort Dodge, Iowa) per g and 4,ug of xylazine (20 mg/ml; Mobay Corp., Shawnee, Kans.) per g, and the thoracic and abdominal cavities were aseptically opened. After the abdominal aorta and vena cava were severed, the pulmonary circulation was aseptically perfused by using a 10-cm3 syringe and 23-gauge needle to inject approximately 7 ml of 4 C Alsever's solution (GIBCO Laboratories, Grand Island, N.Y.) into the pulmonary artery. Perfused lungs were collected, suspended in 1:40 (wt/vol) Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, glutamine (2 mm), pyruvate (1 mm), penicillin-streptomycin (100 U/100,ug/ml), and amphotericin B (Fungizone; 0.1,ug/ml) (S-DMEM), and homogenized (Brinkman Polytron homogenizer, speed 5 for 10 to 15 s). Trophozoites, precysts, and cysts of P. carinii were separated from most of the host cell debris by two repetitions of centrifugation at 40 x g and 1,000 x g for 10 min. The supernatant was saved from the 40 x g centrifugation, whereas the pellet was saved from the 1,000 x g centrifugation. The final P. carinii-enriched pellet was resuspended in S-DMEM, counted with a hemacytometer, resuspended at approximately 2 x 107 P. carinii organisms per ml in S-DMEM containing 7.5% (vol/vol) dimethyl sulfoxide, cryopreserved by using a 1 C/min cooling cycle (model 700 automated cell freezer; Cryo-Med, Division of Forma Scientific Inc., New Baltimore, Mich.), and stored as 1-ml INFECT. IMMUN. aliquots in liquid nitrogen for up to 1 year. Prior to use, each batch of inoculum was cultured for bacterial and fungal contaminants on brain heart infusion and Sabouraud dextrose agar plates. Only batches of culture-negative inocula were used. Cryopreserved inoculum was thawed at 37 C and resuspended in an equal volume of S-DMEM, and 0.1 ml containing approximately 106 P. carinii organisms was inoculated i.t. into the lungs of each immunosuppressed rat. (iv) Method of P. carinii inoculation. Two inoculation techniques for inducing P. carinii infections were evaluated. One week following initial immunosuppression, P. carnii was introduced deep into the trachea either by i.t. intubation with a 3-in. (7.6-cm), 20-gauge curved stainless steel animal feeding tube (Popper and Sons, Inc., New Hyde Park, N.Y.) or by surgical instillation into the trachea as described by Bartlett et al. (2). Inoculation i.t. following light halothane anesthesia proved to be the most satisfactory method because it was rapid and noninvasive and reduced both the anesthesia requirements and the physical stress associated with surgical exposure of the trachea. Lightly anesthetized rats were suspended by their upper incisors on a wire loop at the top of a Plexiglas slant board (8 by 4 in. [ca. 20 by 10 cm], 60-degree angle). The animal's tongue was grasped and pulled to one side of the lower incisors, and a stainless steel feeding tube attached to a 1-cm3 syringe containing 0.1 ml of inoculum and then 0.4 ml of air was directed with slight pressure along the back of the tongue into the larynx. Tracheal rings were palpated against the feeding tube to confirm correct placement before depositing the inoculum in the trachea approximately 0.5 cm above the primary bronchi. (v) Prevention of secondary infections. In most immunosuppressed animal models of P. cainni pneumonia, tetracycline is routinely added to the drinking water to control secondary bacterial infections (1-3, 6, 9-11, 17). In our preliminary studies, hyperchlorinated drinking water (approximately 8-mg/ml available chlorine) was compared with water containing tetracycline (0.5 mg/ml of water). In our animal facilities, tetracycline in the drinking water provided no apparent advantage over hyperchlorinated drinking water with respect to the occurrence of secondary bacterial infections in immunosuppressed rats housed in both microisolator and wire-bottom cages. This observation is consistent with recent reports indicating that tetracycline fails to produce effective blood levels when administered in the drinking water (15). Hyperchlorinated drinking water administered without tetracycline significantly reduced labor requirements since animals were maintained five per cage in wirebottom caging with an automatic watering system, thus reducing the potential bacterial contamination from animal caretakers who manually change water bottles. Also important in preventing serious secondary bacterial infections was handling rats infrequently (once weekly for weighing and for injection of methylprednisolone) and the use of sterilized latex gloves. In the remaining studies reported herein, tetracycline was not included in the drinking water and the rats were maintained on hyperchlorinated water and autoclaved, standard (not low protein) rodent chow (Ralston Purina Co. no. 5001). Evaluation ofp. carinii infections. The severity ofp. carinji infections was determined by monitoring percent survival and body weight loss and by evaluating parasite burdens in the lungs collected at necropsy. In our initial studies, Giemsa- and methenamine silver-stained lung impression smears were used to determine the severity of P. carinii infections. Giemsa-stained impression smears were used to
3 VOL. 60, 1992 IMPROVED RAT MODEL OF PNEUMOCYSTIS CARINII PNEUMONIA 1591 evaluate all lung stages of the organism (trophozoites, precysts, and cysts), whereas methenamine silver stain was specific for only the cyst stage. Each impression smear was assigned an infection score, a logarithmic representation of the actual number of parasites seen: 0, no parasites found in 30 microscopic fields; 1, 1 to 5 parasites per 10 microscopic fields; 2, approximately 1 parasite per field; 3, 2 to 10 parasites per field; 4, >10 but <100 parasites per field; 5, >100 but <1,000 parasites per field. Impression smears containing >1,000 parasites per field were assigned a value of 6. Giemsa- and methenamine silver-stained slides were examined by using a final magnification of x 1,000 and x 400, respectively. All slides were randomized and then examined microscopically by two persons using a blinded protocol. In rare cases in which infection scores of the two observers differed by more than 0.5, the slides were reevaluated and the actual infection score was determined. During the study to investigate the kinetics of P. carinii infection following i.t. inoculation (see below), another procedure was evaluated to determine the severity of P. carinii infections in the lung. Infection scores obtained from microscopic examination of Giemsa- and methenamine silver-stained impression smears were compared with infection scores obtained from methenamine silver-stained lung homogenates, the new evaluation procedure. Stained slides of lung homogenates were prepared at the time of necropsy and were scored by the same criteria described above for methenamine silver-stained impression smears. Rats were anesthetized with ketamine-xylazine (as described above), exsanguinated via the right atrium to remove excess blood from the pulmonary circulation, and then necropsied. The lungs were removed from each rat, weighed, and then homogenized in a 40x (wt/vol) volume of sterile water for approximately 15 s (Brinkman Polytron homogenizer, setting 5). Large particulates comprising host cell debris were allowed to settle for approximately 10 min, and then 4-,ul samples of the supematant containing P. cannii cysts were distributed evenly onto the wells of Teflon-coated, 12-well microscope slides (catalog no ; 6-mm well diameter; Shandon Inc., Pittsburgh, Pa.) and stained with methenamine silver. Infection scores from lung homogenates diluted 1:40 in water (wt/vol) were slightly lower but comparable to those of Giemsa- and methenamine silverstained lung impression smears (see Tables 2 and 3). Optimizing i.t. inoculation of P. carinii. Immunosuppressed rats were given one, two, or three i.t. inoculations of parasites (0.1 ml of S-DMEM containing 106 P. carinii parasites administered 48 h apart) to determine the optimum number of inoculations required to produce consistent, heavy P. carinii infections. To reduce the number of animals used, these rats also served as P. carinii-infected controls for therapy studies to evaluate candidate drugs for anti-p. caninii pneumonia activity. Noninfected control rats and trimethoprim-sulfamethoxazole (TMP-SMX, trimethoprim [0.2 mg/ml] and sulfamethoxazole [1 mg/ml] in hyperchlorinated water ad lib)-treated rats were also included in the therapy studies to confirm the P. carinii-free status of the rats and to demonstrate the efficacy of TMP-SMX as a positive control drug. In each of four studies, 10 rats were assigned to the following treatment groups: rats receiving one, two, or three inoculations of P. carinii; P. cariniiinfected rats (two P. carinii inoculations) treated with TMP- SMX; and nontreated, noninfected control animals. Six weeks after the initial P. carindi inoculation, rats were necropsied, and impression smears collected from the left lung were stained and evaluated for severity of P. carinii infections. Infection scores were then compared with the number of P. carinii inoculations each group received. P. carinii distribution in the lungs following i.t. inoculation. Immunosuppressed rats were given one, two, or three i.t. inoculations of P. carinii, and the distribution of parasites in the left and right lungs was determined. Ten rats were assigned to each of four treatment groups: rats receiving one, two, or three inoculations of P. carinii (0.1 ml of S-DMEM containing 106 P. carinii organisms) and noninfected controls. Six weeks after P. carindi inoculation, rats were necropsied, and Giemsa- and methenamine silverstained impression smears were prepared from the left lung of each animal. The remaining left and right lungs from each rat were separated, homogenized, and prepared for methenamine silver-stained lung homogenates as described above. Infection scores from lung impression smears and lung homogenates were evaluated and compared to determine whether the two procedures yield comparable infection scores. Lung homogenates prepared from the left and right lungs of each rat were then evaluated to determine the distribution of organisms within the lungs. Kinetics of P. carinii infection. Immunosuppressed rats given one, two, or three i.t. inoculations of P. carinii were evaluated to determine the severity of P. carinii infections during weeks 3 to 6 postinoculation (p.i.). Thirty-two rats were assigned to each of four treatment groups: rats receiving one, two, or three P. carinii inoculations and noninoculated controls. Eight rats from each treatment group were necropsied at 3, 4, 5, and 6 weeks after i.t. inoculation to determine the severity of P. carinii infections. After impression smears were collected from the left lung, the remainder of the lung tissue was weighed and prepared for methenamine silver-stained lung homogenates. Stained impression smears of the lungs and stained lung homogenates were evaluated and compared to determine the severity of P. carinii infection at 3, 4, 5, and 6 weeks p.i. The 5-week P. carinii pneumonia model for drug screening. Near the end of our studies to characterize the immunosuppressed rat model of P. carinii pneumonia, it was apparent that 5 weeks after i.t. inoculation of P. carinii was the optimal time to necropsy the animals and to evaluate the extent of P. carinii infections. This 5-week model was used in our laboratory in studies to evaluate the potential anti-p. carinii pneumonia activity of experimental compounds. These studies routinely had 8 to 10 immunosuppressed rats assigned to each of the following treatment groups: P. carinii-infected, nontreated rats (infected controls); P. caninii-infected animals given TMP-SMX in the water (positive drug treatment controls); non-p. carinii-infected control animals; and P. cannii-infected animals receiving experimental compounds. One week after initiation of immunosuppression, rats received two i.t. inoculations of P. carinii 48 h apart and were assigned to control groups or to therapy or prophylaxis treatment groups. Animals used to screen drugs for prophylactic activity against P. carinii pneumonia started receiving therapy 24 h after initial P. carinii inoculation, and animals harboring a 2-week-old P. carinii infection were used to screen drugs for therapeutic activity. Both therapeutic and prophylactic treatments were continued until week 5 of P. carinii infection. In addition to monitoring percent survival and percent body weight loss, we determined the efficacy of candidate drugs against P. carinii pneumonia by evaluating the lungs for severity of P. carinii infections at necropsy. Impression smears were collected from the left lung, and the remainder of lung tissue was weighed and homogenized for cyst quantitation as described above.
4 _~~~ ~~ I * ~~silver 1592 BOYLAN AND CURRENT INFECT. IMMUN ± 0.79 Stained lung homogenates were evaluated to determine the iipc4.9 ± 038 severity of P. carinii infection at 5 weeks. Treatment groups showing signs of anti-p. carinii pneumonia activity were 1//////////////////////////////////////////////////m S.31I *0.90 further evaluated by using stained lung impression smears. 2xPC I.I0I2I ± 0.2 I Finally, TMP-SMX-treated animals and P. carinii-infected and non-p. carinii-infected animals were evaluated and S compared to confirm the validity of the model. 3xPC 4.8S ±.', 1.71 ± RESULTS TMP/SMX ± Glmsa I... Optimizing i.t. inoculation of P. carinii. Animals receiving 0.00 two or three i.t. inoculations of P. carinii developed consis- NIC 027 i 0.47 tent, heavy P. carinii infections (mean infection scores of 5.31 to 5.59) within 6 weeks p.i. (Fig. 1; Table 1). Most rats o receiving only one inoculation also demonstrated heavy P. Infection Score carinii infections within 6 weeks; however, these animals FIG. 1. Mean infection scores (± standard deviation) 6 weeks showed an increased incidence of lower infection scores after on *e, two, or three i.t. inoculations of P. cannii (1 x PC, 2 x compared with animals receiving two to three P. carinji PC,3 x PC, respectively). Each infection score represents the mean inoculations (Table 1). Mean percent mortality and mean of four studies, 4 each with 8 to 10 rats per group. Impression smears percent body weight loss (data not shown) were higher in were sttained with Giemsa or methenamine silver. All life cycle animals receiving two or three inoculations and were attribstages (trophozoites, precysts, cysts) were scored when reading uted to heavy parasite burdens. TMP-SMX administered in Giemsa -stained smears (x1,000 magnification), whereas only cysts.. were sc-ored when reading silver-stained smears (x400 magnifica- the drinkig water (weeks 2 to 6 p.i.) was highly effective tion). P carinii-inoculated rats treated with TMP-SMX served as eradicating parasites from the lungs of infected rats, and positive drug treatment controls. Noninoculated control (NIC) rats little or no evidence of P. carinii was found in the lungs 6 were imimunosuppressed and housed in open cages in the same room weeks after parasite inoculation (Fig. 1; Table 1). Although as heavrily infected animals. Distributions of the infection scores some (approximately 15%) non-p. cainni-inoculated, immu- from the four studies are shown in Table among irats 1. TABLE 1. Distribution of infection scores 6 weeks following one, two, or three i.t. inoculations of P. carinji (1 x PC, 2 x PC, or 3 x PC, respectively) No. of rats in each Reported No. of methenamine silver-stained impression smearsb with the Study no. group/no. of rats cause of following infection score: necropsied death" < x PC 1 10/ / / /7 3 PCP x PC 1 10/ /6 2PCP /6 2PCP /5 5 PCP x PC 1 10/ /6 3 PCP / /7 3 PCP Non-P. carinii inoculatedc 1 10/ / / / TMP-SMX (positive treatment control) 1 10/ / / /9 1 KF a PCP, P. carinii pneumonia: infection scores ranged from 5.0 to 6.0 (Giemsa impression smears); KF, kidney failure resulting from immune suppressioninduced purulent streptococcal nephritis. b Impression smears collected from the center of the left lung. c Noninoculated, immunosuppressed rats housed for 6 weeks in open cages in the same room as heavily infected animals. Non-P. carinii-inoculated rats in study 3 were housed in the same cage rack as the 1, 2, and 3 x PC groups, whereas those in studies 1, 2, and 4 were housed in separate cage racks.
5 VOL. 60, 1992 IMPROVED RAT MODEL OF PNEUMOCYSTIS CARINII PNEUMONIA 1593 TABLE 2. Distribution of P. carinii in the left versus right lung and comparison of infection score evaluation procedures Mean infection scores ± SD No. of No. of rats in each Reported cause P. carinii group/no. of rats of deathc Impression smears' Silver-stained homogenate necropsiedb inoculationse5 Giemsa Silver Left lung Right lung 0 10/ ± ± ± /9 1 PCP 5.42 ± ± ± ± /5 5 PCP ± ± /6 4 PCP 5.50 ± ± ± ± 0.18 a 0, noninoculated, immunosuppressed rats housed for 6 weeks in open cages in the same room as heavily infected animals. b Animals necropsied 6 weeks p.i. C PCP, P. carinii pneumonia: infection scores ranged from 4.5 to 6.0 (Giemsa-stained impression smears). d Impression smears collected from the center of the left lung. nosuppressed rats housed in standard wire-bottom cages in carinii-infected animals housed in wire-bottom cages in the the same room as heavily infected animals had small num- same room when these animals were placed in a cage rack bers of P. carinii in their lungs after 6 weeks (Fig. 1; Table 1), without P. carinii-infected animals. the remaining animals (approximately 85%) maintained in Kinetics of the P. carinii infection. Mean P. carinii infection microisolator cages remained free of detectable P. carinii. scores and infection score incidence at different times after Housing non-p. carinii-inoculated, immunosuppressed rats i.t. inoculation of P. carinii are presented in Tables 3 and 4, in the same cage rack as heavily infected animals resulted in respectively. P. carinii infections were well established in all a higher incidence of moderate P. carinii infection compared groups by 3 weeks, regardless of the number of inoculations with noninoculated controls housed in a separate cage rack received, and mean infection scores at this time ranged from (Table 1, study 3). Mean P. carinii infection scores deter to 4.04 (Table 3). P. carinii pneumonia progressed mined by evaluation of stained lung impression smears were consistently over time for each of the inoculation groups, comparable for both Giemsa and methenamine silver stains and by week 5, mean infection scores of all animals receiving for each of the animals evaluated (Fig. 1; Table 1). two or three P. carinii inoculations were 4.97 or greater. The Distribution of P. carinii in lungs following i.t. inoculation. severity of P. carinii pneumonia at 6 weeks was directly Parasites were distributed evenly between the left and right related to the number of inoculations received; however, lungs regardless of the number of P. carinii inoculations high mortality rates resulting from heavy P. carinii pneumoadministered (Table 2). Animals receiving two or three P. nia during weeks 5 and 6 p.i. reduced the number of animals carinii inoculations had higher mortality rates and more available for evaluation. consistent, heavy mean P. carinii infections than rats receiv- The 5-week P. carinii pneumonia model for drug screening. ing one P. carinii inoculation. All mortalities were attributed Infection scores obtained from P. carinii-infected control to heavy P. carinii burdens. In addition, little or no evidence animals, non-p. carinii-infected control animals, and P. of P. carinii pneumonia was found in the lungs of non-p. carinii-infected TMP-SMX-treated animals from 10 studies TABLE 3. Mean infection scores 3, 4, 5, and 6 weeks following i.t. inoculation of P. carinji Mean infection scores ± SD Week No. of rats in each Reportedasmears'usn Impression p-i.a ~ group/no, of rats of deathb mrsio mas Lung necropsied homogenate Giemsa Silver (silver) 1 x PC 3 8/7 1 KF ± ± / ± /5 3 PCP ± ± /3 5 PCP 5.50 ± ± x PC 3 8/ ± ± ± / ± ± /6 1 PCP; 1 MAL 5.37 ± ± ± /4 4 PCP 5.40 ± ± ± x PC 3 8/ ± ± / ± ± ± /7 1 KF 5.35 ± ± ± /3 5 PCP 6.00 ± ± ± 0.19 a 1 x PC, 2 x PC, 3 x PC, one, two, or three P. carinii inoculations, respectively. b PCP, P. carinii pneumonia; KF, kidney failure resulting from immune suppression-induced purulent streptococcal nephritis; MAL, malocclusion of the front incisors. c Impression smears collected from the center of the left lung.
6 1594 BOYLAN AND CURRENT INFECT. IMMUN. TABLE 4. Distribution of infection scores 3, 4, 5, and 6 weeks following 1, 2, or 3 i.t. inoculations of P. caninii (1 x PC, 2 x PC, or 3 x PC, respectively) Week No. of rats in each group/ No. of methenamine silver-stained lung homogenates with the following infection score: p.i. no. of rats necropsied < x PC 3 8/ / / / x PC 3 8/ / / / x PC 3 8/ / / / are presented in Fig. 2 and Table 5. Heavy P. cannii infections developed within 5 weeks in all control rats inoculated twice with P. cannii (mean infection score, ), and of these rats, approximately 20% died of heavy P. carinii burdens during week 4 of infection. Some non-p. carinii-inoculated controls as well as TMP-SMX-treated rats developed very light infections if kept in open cages in different cage racks but in the same room with P. cariniiinfected animals, and some developed moderate infections when they were housed in cages close to heavily infected animals (Table 5, study 4). Non-P. carinii-inoculated, immunosuppressed rats remained P. caninii-free if kept in isolation (data not shown). 2xPC (± 0.37) 0.51 (± 0.62) 0 silver-stained lung homogenates n = 75 to 77 rats group (± 0.39) Infection Score FIG. 2. Mean infection scores (+ standard deviation) of control rats from the 5-week model of P. carinii pneumonia. Each infection score represents the mean of 10 studies, each with 8 to 10 rats per group. Only cysts were scored with methenamine silver-stained lung homogenates. P. carinii-inoculated rats treated with TMP-SMX served as positive drug treatment controls. Noninoculated control (NIC) rats were immunosuppressed and housed in open cages in the same room as heavily infected animals. Distributions of the infection scores among rats from the 10 studies are shown in Table 5. DISCUSSION The most widely used animal model of P. cannii pneumonia, first developed by Frenkel et al. (6), relies on activation of latent P. cannii infections in rats immunosuppressed by steroids. The presence of latent P. cannii infections in most breeding colonies of Sprague-Dawley rats resulted in this rodent becoming the animal of choice for most investigators (1, 3, 9, 10, 17). These rats have been used both as a source of organisms for laboratory studies and for most evaluations of drugs for efficacy against P. carinii pneumonia (1, 3, 9, 10, 12, 14, 17). Inherent to this model are the problems of inconsistent infections, the long course (8 to 12 weeks) of immunosuppression to allow development of heavy infections, a lack of control over P. cainni strains that may be present, and often a high incidence of secondary infections that render many of the animals unusable (1, 3, 16). These problems are thought to be primarily a result of variations among rats in the degree of latent infections, in the amount of steroids consumed or their susceptibility to steroids, and in the presence of opportunistic pathogens that may cause disease when the animals are immunosuppressed (1). Another aspect that has recently made this animal model increasingly difficult for many researchers is a marked reduction in the supply of rats with latent P. cannii infections. Through continuous upgrading of the pathogen-free status of most breeding stocks, animal suppliers have succeeded in reducing or eliminating inherent P. carinii infections in their laboratory animal colonies. Today, many of the commercial rodent colonies are free of viral and bacterial pathogens and do not have latent P. cannii. In the absence of substantial monetary incentives to maintain rat colonies that have latent P. caninii, it is likely that the traditional dirty rat model may soon be unavailable to many researchers. A logical way to circumvent many of the problems associated with the conventional model described above is to induce controlled laboratory infections in P. carinii-free animals. Early attempts to induce laboratory infections met with varied success. Walzer et al. (19) successfully infected nude mice by percutaneous injection of the parasites into the lung. Although intranasal inoculation of the organism was used successfully to produce P. cannii pneumonia in mice in
7 VOL. 60, 1992 IMPROVED RAT MODEL OF PNEUMOCYSTIS CARINII PNEUMONIA 1595 TABLE 5. Distribution of infection scores of control rats from the 5-week model of P. cannii pneumonia No. of rats in each No. of methenamine silver-stained lung homogenates with the Study no. group/no, of rats Reported cause following infection score: necropsied < x PCb 1 10/ /5 3 PCP /7 3 PCP /8 1 PCP / /7 1 PCP /7 1 PCP /5 3 PCP /7 1 KF /7 1 PCP TMP-SMX (positive treatment control) 1 10/9 1 MAL / / / / / / / / / Non-P. cannii-inoculatedc 1 8/ / / / / / / / / /6 1 KF; 1 MAL a PCP, P. carinii pneumonia; KF, kidney failure resulting from immune suppression-induced purulent streptococcal nephritis; MAL, malocclusion of the front incisors. bimmunosuppressed rats inoculated twice with P. carinii (P. carnii-infected control rats). c Non-P. carinii-inoculated immunosuppressed control rats housed for 5 weeks in open cages in the same room as but away from heavily infected animals. In study 4, these rats were housed in open caging near heavily infected animals. one laboratory (7), the technique did not produce P. carinii pneumonia in animal models used in two other laboratories (6, 19). More recently, a rat model was developed in which consistent laboratory-induced P. carinii infections were achieved and which eliminated some of the undesirable variables associated with activation of latent P. carinii infections (2). However, labor-intensive surgical manipulation was required to inoculate the organisms into the trachea. Our animal model of laboratory-induced P. carinii pneumonia represents an improvement over the existing models. The key features that make the model useful for a variety of studies include the use of relatively non-labor-intensive, controlled, uniform immunosuppression of P. carinii-free animals; the use of rapid, noninvasive i.t. inoculation of cryopreserved parasites; and a predictable course of disease development resulting in consistent, heavy P. carinii infections within 5 weeks p.i. Incorporation of dexamethasone in the drinking water is the immunosuppressive regimen most often used in animal models of P. carinii pneumonia (1-3, 8-11, 14, 16-19). One major shortcoming of this method is the amount of labor required to ensure that all animals receive the same dose of dexamethasone. Because there is often considerable variability in the amount of water consumed by individual rats, they must be housed individually, daily water intake must be monitored, and drug concentrations must be adjusted to compensate for differences in individual water intake. Our preliminary studies demonstrated that weekly injections of methylprednisolone resulted in a uniform level of immunosuppression and that tetracycline was not needed in the drinking water to prevent secondary bacterial infections. This allows housing of more than one animal per cage and the use of an automatic watering system, features which greatly reduce the amount of labor for animal care because changing of individual water bottles every 1 to 3 days is eliminated. The methylprednisolone dosage originally described by Cushion et al. (5) was adjusted to accommodate for body weight loss associated with steroid therapy. Feeding standard rodent chow in combination with weekly subcutaneous injections of methylprednisolone produced a balance of immunosuppression sufficient for the maintenance of P. carinii pneumonia while lessening the wasting syndrome
8 1596 BOYLAN AND CURRENT associated with the often-used combination of steroid-induced immunosuppression and low-protein diet. With the conventional steroid-induced P. carinii pneumonia model (dirty rat model), there is often a high percentage of animals which are unsuitable for use in in vitro studies because of high incidences of secondary infections, especially pulmonary bacterial infections (1, 16). P. carinii-free Lewis rats were selected for our model because these animals are more docile than the Sprague-Dawley or Fisher 344 rats and because they developed heavy P. carinii infections with minimal secondary bacterial infections. Most virus-free Lewis rats used in our studies remained free from detectable secondary bacterial and fungal infections. Deaths due to causes other than P. carinii pneumonia were uncommon in our 5-week model of P. cannii pneumonia. Of the 253 immunosuppressed animals reported in Table 5, only four deaths were attributed to causes other than P. cafinii pneumonia; two died from steroid-induced streptococcal nephritis and two died of malnutrition attributed to malocclusion of the front incisors. Although kidney lesions due to steroidinduced streptococcal nephritis were occasionally observed at necropsy, less than 1% of the immunosuppressed animals apparently died from this cause. The virus-free status of Lewis rats used in our studies, the shorter duration of immunosuppression, and the animal housing and care procedures outlined above resulted in a lower occurrence of serious secondary bacterial infections compared with most models of P. carinii pneumonia; however, complete suppression of localized bacterial infections was not achieved. Tracheal intubation with a standard 20-gauge feeding tube provides a rapid method of inoculating parasites deep into the lung without surgical manipulation, visualization of the epiglottis, or special equipment. Placement of the feeding tube i.t. is easily verified, and rats recover from light halothane anesthesia within minutes. After becoming familiar with the technique, one person can inoculate P. carindi into the lungs of 100 to 120 rats in 1 h. The use of cryopreserved parasites along with i.t. inoculation of P. carinii-free rats provides a practical means of long-term maintenance of individual P. carinii isolates or strains and a consistent source of large numbers of parasites for laboratory studies. By maintaining a small stock colony of i.t.-inoculated animals in microisolator cages, one can be assured that heavily infected animals are available for laboratory use when needed. Inoculation of five rats i.t. each week should provide an uninterrupted, adequate supply of P. carinii for most laboratory studies. A major shortcoming of the conventional model of P. carinii pneumonia that is overcome by our model is the absence of immunosuppressed, non-p. carinii-infected control animals. By using P. carinii-free rats housed in microisolators, immunosuppressed, noninfected animals can be incorporated into a variety of studies that require the presence of this type of contemporaneous control to properly interpret the data obtained. Perhaps the most important feature of our model is the predictable course of disease progression that results in most, if not all, rats having moderate infections 2 and 3 weeks after i.t. inoculation of P. cannii, severe P. carinii pneumonia 4 and 5 weeks p.i., and approximately 50% mortality due to P. carinii pneumonia at 6 weeks p.i. This is preferable to the long (8 to 12 weeks), often unpredictable course of infection that occurs in conventional dirty rat models. In the early stages of our model development, rats were necropsied at 6 weeks p.i.; however, in later studies, more than 50% of P. carinii-infected control rats died of INFECT. IMMUN. severe P. caninii pneumonia by 6 weeks p.i. Therefore, a 5-week study duration was selected to optimize percent survival among heavily infected rats used to evaluate experimental compounds for both therapeutic and prophylactic activity against P. cannii pneumonia. The therapeutic evaluation model allows 2 weeks for moderate P. cannii infections to develop and then 3 weeks to evaluate the efficacy of experimental compounds. The use of P. carinii-free rats and coordination of parasite inoculation and initiation of therapy within a 24-h period allows a more-controlled evaluation of prophylactic activity compared with conventional models that rely on activation of latent P. cannii infections. In summary, we describe an immunosuppressed rat model of P. carinii pneumonia that results in a predictable course of disease development which includes moderate P. carinii infections in 2 to 3 weeks, heavy infections in 4 to 5 weeks, and a high percentage of mortality due to P. cannii pneumonia in 6 weeks. The model also provides uninfected, immunosuppressed contemporaneous controls, an experimental compartment that is needed to correctly interpret results obtained from many different studies. Noninvasive i.t. inoculations of cryopreserved parasites into P. carinii- and virus-free rats immunosuppressed by weekly injections of methylprednisolone are key features of the model that result in the development of consistent heavy P. carinii infections and very few secondary infections that can interfere with studies. This model is useful for maintaining over time isolates or strains of P. cannii, for producing large numbers of parasites for laboratory studies, and for evaluating the anti-p. carinii activity of experimental compounds and approved drugs. REFERENCES 1. Bartlett, M. S., J. A. Fishmen, M. M. Durkin, S. F. Queener, and J. W. Smith Pneumocystis carinii: improved models to study efficacy of drugs for treatment or prophylaxis of Pneumocystis pneumonia in the rat (Rattus sp.). Exp. Parasitol. 70: Bartlett, M. S., J. A. Fishman, S. F. Queener, M. M. Durkin, M. A. Jay, and J. W. Smith New rat model of Pneumocystis carinii infection. J. Clin. Microbiol. 26: Bartlett, M. S., S. F. Queener, M. A. Jay, M. M. Durkin, and J. W. Smith Improved rat model for studying Pneumocystis carinii pneumonia. J. Clin. Microbiol. 25: Centers for Disease Control AIDS weekly surveillance report. 30 January:1-5. Centers for Disease Control, Atlanta. 5. Cushion, M. T., J. A. De Stefano, and P. D. Walzer Pneumocystis carinii: surface reactive carbohydrates detected by lectin probes. E-xp. Parasitol. 67: Frenkel, J. K., J. T. Good, and J. A. Schultz Latent Pneumocystis infection of rats, relapse, and chemotherapy. Lab. Invest. 15: Futura, T., K. Ueda, K. Fujiwara, and K. Yamanouchi Cellular and humoral immune responses of mice subclinically infected with Pneumocystis carinii. Infect. Immun. 47: Hughes, W. T Closing comments: Pneumocystis carini symposium. J. Protozool. 38:243S. 9. Hughes, W. T., V. L. Gray, W. E. Gutteridge, V. S. Latter, and M. Pudney Efficacy of a hydroxynaphthoquinone, 566C80, in experimental Pneumocystis cannii pneumonitis. Antimicrob. Agents Chemother. 34: Hughes, W. T., P. C. McNabb, and T. D. Makres Efficacy of trimethoprim and sulfamethoxazole in the prevention and treatment of Pneumocystis carinii pneumonitis. Antimicrob. Agents Chemother. 5: Jones, S. K., J. E. Hall, M. A. Allen, S. D. Morrison, K. A. Reddy, V. V. Geratz, and R. R. Tidwell Novel pentamidine analogs in the treatment of experimental Pneumocystis carinii pneumonia. Antimicrob. Agents Chemother. 34:
9 VOL. 60, 1992 IMPROVED RAT MODEL OF PNEUMOCYSTIS CARINII PNEUMONIA Kovacs, J. A., J. L. Halpern, B. Lundgren, J. C. Swan, J. E. Pamillo, and H. Masur Monoclonal antibodies to Pneumocystis carinii: identification of specific antigens and characterization of antigenic differences between rat and human isolates. J. Infect. Dis. 159: Kovacs, J. A., and H. Masur Prophylaxis of Pneumocystis carinii pneumonia: an update. J. Infect. Dis. 160: Pesanti, E. L., and C. Cox Metabolic and synthetic activities of Pneumocystis carinii in vitro. Infect. Immun. 34: Porter, W. P., Y. S. Bitar, J. D. Strandberg, and P. C. Charache Absence of therapeutic blood concentrations of tetracycline in rats after administration in drinking water. Lab. Anim. Sci. 35: Walzer, P. D., C. K. Kim, and M. T. Cushion Pneumocystis carinii, p In P. D. Walzer and R. Genta (ed.), Parasitic infections in the compromised host: immunologic mechanisms and clinical applications. Marcel Dekker, Inc., New York. 17. Walzer, P. D., C. K. Kim, H. J. Foy, M. J. Linke, and M. T. Cushion Cationic antitrypanosomal and other antimicrobial agents in the therapy of experimental Pneumocystis carinii pneumonia. Antimicrob. Agents Chemother. 32: Walzer, P. D., M. E. Rutledge, and K. Yoneda Experimental Pneumocystis carinii pneumonia in C3H/HeJ and C3HeB/FeJ mice. J. Reticuloendothel. Soc. 33: Walzer, P. D., V. Schnelle, D. Armstrong, and P. P. Rosen Nude mouse: a new experimental model for Pneumocystis cannii infection. Science 197:
Pneumocystis caninii Organisms Obtained from Rats, Ferrets,
 INFECrION AND IMMUNITY, Apr. 1993, p. 1315-1319 0019-9567/93/041315-05$02.00/0 Copyright C) 1993, American Society for Microbiology Vol. 61, No. 4 Pneumocystis caninii Organisms Obtained from Rats, Ferrets,
INFECrION AND IMMUNITY, Apr. 1993, p. 1315-1319 0019-9567/93/041315-05$02.00/0 Copyright C) 1993, American Society for Microbiology Vol. 61, No. 4 Pneumocystis caninii Organisms Obtained from Rats, Ferrets,
Immunosuppressed Mouse Models of Pneumocystis carinii Pneumonia for Evaluation of Drugs and Leukocytes
 CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, Sept. 1994, p. 511-516 Vol. 1, No. 5 1071-412X/94/$04.00+0 Copyright X 1994, American Society for Microbiology Comparison of Corticosteroid- and L3T4+ Antibody-
CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, Sept. 1994, p. 511-516 Vol. 1, No. 5 1071-412X/94/$04.00+0 Copyright X 1994, American Society for Microbiology Comparison of Corticosteroid- and L3T4+ Antibody-
Standard Operating Procedure
 1.0 Purpose: 1.1 Relaxation, dissection, weighing and fixation of heart for histological analysis. Changes in heart weight and wall thickness are linked to cardiovascular phenotypes. This protocol describes
1.0 Purpose: 1.1 Relaxation, dissection, weighing and fixation of heart for histological analysis. Changes in heart weight and wall thickness are linked to cardiovascular phenotypes. This protocol describes
In vitro cultivation of Plasmodium falciparum
 Chapter 2 In vitro cultivation of Plasmodium falciparum In vitro cultivation of Plasmodium falciparum 2.1 INTRODUCTION Malaria represents the world s greatest public health problem in terms of number of
Chapter 2 In vitro cultivation of Plasmodium falciparum In vitro cultivation of Plasmodium falciparum 2.1 INTRODUCTION Malaria represents the world s greatest public health problem in terms of number of
Influence of Water Diuresis on Antimicrobial
 Influence of Water Diuresis on Antimicrobial Treatment of Enterococcal Pyelonephritis SANDRA P. LEVISON and DONALD KAYE From the Department of Medicine, The Medical College of Pennsylvania, Philadelphia,
Influence of Water Diuresis on Antimicrobial Treatment of Enterococcal Pyelonephritis SANDRA P. LEVISON and DONALD KAYE From the Department of Medicine, The Medical College of Pennsylvania, Philadelphia,
To place an order, please visit lifelinecelltech.com or call customer service at
 Human Melanocyte Cells Instruction Manual HEMn Human Epidermal Melanocytes, Neonatal HEMn-HP Human Epidermal Melanocytes, Neonatal-Highly Pigmented HEMa Human Epidermal Melanocytes, Adult HEMa-HP Human
Human Melanocyte Cells Instruction Manual HEMn Human Epidermal Melanocytes, Neonatal HEMn-HP Human Epidermal Melanocytes, Neonatal-Highly Pigmented HEMa Human Epidermal Melanocytes, Adult HEMa-HP Human
AN ACUTE ORAL TOXICITY STUDY IN RATS WITH ADVANTRA Z
 AN ACUTE ORAL TOXICITY STUDY IN RATS WITH ADVANTRA Z AMENDED FINAL REPORT Author Deborah A Douds, M.S. Original Study Completion Date July 8, 1997 Amended Study Completion Date November 19, 1997 Performing
AN ACUTE ORAL TOXICITY STUDY IN RATS WITH ADVANTRA Z AMENDED FINAL REPORT Author Deborah A Douds, M.S. Original Study Completion Date July 8, 1997 Amended Study Completion Date November 19, 1997 Performing
Rifampin Resistance. Charlottesville, Virginia i0w organisms in Trypticase soy broth (BBL Microbiology
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Apr. 1980, p. 658-662 0066-4804/80/04-0658/05$02.00/0 Vol. 17, No. 14 Treatment of Experimental Staphylococcal Infections: Effect of Rifampin Alone and in Combination
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Apr. 1980, p. 658-662 0066-4804/80/04-0658/05$02.00/0 Vol. 17, No. 14 Treatment of Experimental Staphylococcal Infections: Effect of Rifampin Alone and in Combination
Pneumocystis. Pneumocystis BIOL Summer Introduction. Mycology. Introduction (cont.) Introduction (cont.)
 Introduction Pneumocystis Disclaimer: This lecture slide presentation is intended solely for educational purposes. Many of the images contained herein are the property of the original owner, as indicated
Introduction Pneumocystis Disclaimer: This lecture slide presentation is intended solely for educational purposes. Many of the images contained herein are the property of the original owner, as indicated
PARASITOLOGY CASE HISTORY 10 (HISTOLOGY) (Lynne S. Garcia)
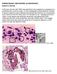 PARASITOLOGY CASE HISTORY 10 (HISTOLOGY) (Lynne S. Garcia) A 46-year-old man with AIDS was admitted to the hospital for complaints of a persisting fever and dry cough. A chest radiograph showed bilateral
PARASITOLOGY CASE HISTORY 10 (HISTOLOGY) (Lynne S. Garcia) A 46-year-old man with AIDS was admitted to the hospital for complaints of a persisting fever and dry cough. A chest radiograph showed bilateral
Product # Kit Components
 3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Pneumocystis jirovecii PCR Kit Product # 42820 Product Insert Background Information
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Pneumocystis jirovecii PCR Kit Product # 42820 Product Insert Background Information
Cryopreserved Human Hepatocytes (Cat # HP-F)
 Certificate of Analysis Cryopreserved Human Hepatocytes (Cat # HP-F) This CoA represents a single Lot specific batch of cells. For details of current Lots available please contact us. Lot#: H0434 Gender
Certificate of Analysis Cryopreserved Human Hepatocytes (Cat # HP-F) This CoA represents a single Lot specific batch of cells. For details of current Lots available please contact us. Lot#: H0434 Gender
A Change in the Contagious Character of a Strain of Swine Influenza
 SWINE INFLUENZA V. STUDIES ON CONTAGION BY RICHARD E. SHOPE, M.D. (From the Department of Animal and Plant Pathology of The Rockefeller Institute for Medical Research, Princeton, N. J.) (Received for publication,
SWINE INFLUENZA V. STUDIES ON CONTAGION BY RICHARD E. SHOPE, M.D. (From the Department of Animal and Plant Pathology of The Rockefeller Institute for Medical Research, Princeton, N. J.) (Received for publication,
Human Mammary Luminal Epithelial Cells. Manual
 Human Mammary Luminal Epithelial Cell Manual INSTRUCTION MANUAL SHIPPING CONDITIONS ZBM0071.00 Human Mammary Luminal Epithelial Cells Orders are delivered via Federal Express courier. All US and Canada
Human Mammary Luminal Epithelial Cell Manual INSTRUCTION MANUAL SHIPPING CONDITIONS ZBM0071.00 Human Mammary Luminal Epithelial Cells Orders are delivered via Federal Express courier. All US and Canada
Human Umbilical Cord Blood CD34 + /133+ Progenitor Cell Care Manual
 Human Umbilical Cord Blood CD34 + /133+ Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.04 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+/133+ Progenitor Cells, cryopreserved Cryopreserved
Human Umbilical Cord Blood CD34 + /133+ Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.04 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+/133+ Progenitor Cells, cryopreserved Cryopreserved
Human CD4+T Cell Care Manual
 Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.04 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.04 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
Method of Testing the Susceptibility of Pneumocystis carinii to Antimicrobial Agents In Vitro
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Dec. 1985, p. 796-801 0066-4804/85/120796-06$02.00/0 Copyright C) 1985, American Society for Microbiology Vol. 28, No. 6 Method of Testing the Susceptibility of Pneumocystis
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Dec. 1985, p. 796-801 0066-4804/85/120796-06$02.00/0 Copyright C) 1985, American Society for Microbiology Vol. 28, No. 6 Method of Testing the Susceptibility of Pneumocystis
Pathogenesis of Simian Foamy Virus Infection in Natural and Experimental Hosts
 INCTION AD ImmuNrry, Sept. 1975, p. 470-474 Copyright 0 1975 American Society for Microbiology Vol. 12, No. 3 Printed in U.S.A. Pathogenesis of Simian Foamy Virus Infection in Natural and Experimental
INCTION AD ImmuNrry, Sept. 1975, p. 470-474 Copyright 0 1975 American Society for Microbiology Vol. 12, No. 3 Printed in U.S.A. Pathogenesis of Simian Foamy Virus Infection in Natural and Experimental
FOCUS SubCell. For the Enrichment of Subcellular Fractions. (Cat. # ) think proteins! think G-Biosciences
 169PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS SubCell For the Enrichment of Subcellular Fractions (Cat. # 786 260) think
169PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS SubCell For the Enrichment of Subcellular Fractions (Cat. # 786 260) think
Role of Interferon in the Propagation of MM Virus in L Cells
 APPLIED MICROBIOLOGY, Oct. 1969, p. 584-588 Copyright ( 1969 American Society for Microbiology Vol. 18, No. 4 Printed in U S A. Role of Interferon in the Propagation of MM Virus in L Cells DAVID J. GIRON
APPLIED MICROBIOLOGY, Oct. 1969, p. 584-588 Copyright ( 1969 American Society for Microbiology Vol. 18, No. 4 Printed in U S A. Role of Interferon in the Propagation of MM Virus in L Cells DAVID J. GIRON
Techniques for Examining Pneumocystis carinii in Fresh Specimens
 JOURNAL OF CLINICAL MICROBIOLOGY, Jan. 1986, p. 17-21 0095-1137/86/010017-05$02.00/0 Copyright 1986, American Society for Microbiology Vol. 23, No. 1 Techniques for Examining Pneumocystis carinii in Fresh
JOURNAL OF CLINICAL MICROBIOLOGY, Jan. 1986, p. 17-21 0095-1137/86/010017-05$02.00/0 Copyright 1986, American Society for Microbiology Vol. 23, No. 1 Techniques for Examining Pneumocystis carinii in Fresh
Furazolidone and Nitrofurantoin in the Treatment of Experimental
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Jan 1991, p 158-163 66-484/91/1158-6$2/ Copyright 1991, American Society for Microbiology Vol 35, No 1 Furazolidone and Nitrofurantoin in the Treatment of Experimental
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Jan 1991, p 158-163 66-484/91/1158-6$2/ Copyright 1991, American Society for Microbiology Vol 35, No 1 Furazolidone and Nitrofurantoin in the Treatment of Experimental
1.40 Prevention of Nosocomial Pneumonia
 1.40 Prevention of Nosocomial Pneumonia Purpose Audience Policy Statement: The guideline is designed to reduce the incidence of pneumonia and other acute lower respiratory tract infections. All UTMB healthcare
1.40 Prevention of Nosocomial Pneumonia Purpose Audience Policy Statement: The guideline is designed to reduce the incidence of pneumonia and other acute lower respiratory tract infections. All UTMB healthcare
RODENT Hepatocytes Care Manual
 RODENT Hepatocytes Care Manual INSTRUCTION MANUAL ZBM0054.02 SHIPPING CONDITIONS Rodent Hepatocytes cryopreserved Orders are delivered via Federal Express courier. All US and Canada orders are shipped
RODENT Hepatocytes Care Manual INSTRUCTION MANUAL ZBM0054.02 SHIPPING CONDITIONS Rodent Hepatocytes cryopreserved Orders are delivered via Federal Express courier. All US and Canada orders are shipped
Media Fill Test Kits. Manufactured by. Making USP <797> compliance easy!
 Media Fill Test Kits Manufactured by Making USP compliance easy! Compliance was never so easy! Microbial Contamination Testing for CSPs Hardy Diagnostics offers all the products you need to easily
Media Fill Test Kits Manufactured by Making USP compliance easy! Compliance was never so easy! Microbial Contamination Testing for CSPs Hardy Diagnostics offers all the products you need to easily
RHODOCOCCUS EQUI. Post-mortem Environmental Persistence Specific Control Measures Release of Animals from Isolation
 RHODOCOCCUS EQUI Definition Clinical Signs Transmission Diagnostic Sampling, Testing and Handling Post-mortem Environmental Persistence Specific Control Measures Release of Animals from Isolation Biosecurity
RHODOCOCCUS EQUI Definition Clinical Signs Transmission Diagnostic Sampling, Testing and Handling Post-mortem Environmental Persistence Specific Control Measures Release of Animals from Isolation Biosecurity
QUALITY CONTROL IN ISOLATION AND IDENTIFICATION OF MYCOBACTERIA FROM CLINICAL SPECIMENS
 QUALITY CONTROL IN ISOLATION AND IDENTIFICATION OF MYCOBACTERIA FROM CLINICAL SPECIMENS Dr. C. N. Paramasivam Deputy Director (Bacteriology) Tuberculosis Research Centre Spurtank Road, Chetput, Madras
QUALITY CONTROL IN ISOLATION AND IDENTIFICATION OF MYCOBACTERIA FROM CLINICAL SPECIMENS Dr. C. N. Paramasivam Deputy Director (Bacteriology) Tuberculosis Research Centre Spurtank Road, Chetput, Madras
Chronic Infections by Herpes Simplex Viruses and by the Horse and Cat Herpesviruses
 INFECTION AND IMMUNITY, Apr. 70, p. 351-355 Copyright 70 American Society for Microbiology Vol. 1, No. 4 Printed in U.S.A. Chronic Infections by Herpes Simplex Viruses and by the Horse and Cat Herpesviruses
INFECTION AND IMMUNITY, Apr. 70, p. 351-355 Copyright 70 American Society for Microbiology Vol. 1, No. 4 Printed in U.S.A. Chronic Infections by Herpes Simplex Viruses and by the Horse and Cat Herpesviruses
NUMBER: The purpose of this policy is to provide guidelines for mice and rats involved in tumor studies.
 Purpose PAGE 1 OF 8 The purpose of this policy is to provide guidelines for mice and rats involved in tumor studies. I. Application A. All injectable and/or implantable materials used for establishing
Purpose PAGE 1 OF 8 The purpose of this policy is to provide guidelines for mice and rats involved in tumor studies. I. Application A. All injectable and/or implantable materials used for establishing
Prevention and Treatment of Pneumocystis carinii Pneumonitis
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Mar. 1974, p. 289-293 Copyright ( 1974 American Society for Microbiology Vol. 5, No. 3 Printed in U.S.A. Efficacy of Trimethoprim and Sulfamethoxazole in the Prevention
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Mar. 1974, p. 289-293 Copyright ( 1974 American Society for Microbiology Vol. 5, No. 3 Printed in U.S.A. Efficacy of Trimethoprim and Sulfamethoxazole in the Prevention
Human ipsc-derived Ventricular Cardiomyocytes. Protocol version 3.1
 Human ipsc-derived Ventricular Cardiomyocytes Protocol version 3.1 Protocol version 3.1 Table of Contents Product Information 2 Recommendations 2 Preparing Cardiomyocyte Maintenance Medium 3 Cardiomyocyte
Human ipsc-derived Ventricular Cardiomyocytes Protocol version 3.1 Protocol version 3.1 Table of Contents Product Information 2 Recommendations 2 Preparing Cardiomyocyte Maintenance Medium 3 Cardiomyocyte
ENG MYCO WELL D- ONE REV. 1.UN 29/09/2016 REF. MS01283 REF. MS01321 (COMPLETE KIT)
 ENG MYCO WELL D- ONE MYCO WELL D-ONE System for the presumptive identification and antimicrobial susceptibility test of urogenital mycoplasmas, Gardnerella vaginalis, Trichomonas vaginalis, Candida albicans
ENG MYCO WELL D- ONE MYCO WELL D-ONE System for the presumptive identification and antimicrobial susceptibility test of urogenital mycoplasmas, Gardnerella vaginalis, Trichomonas vaginalis, Candida albicans
Growth and Serial Passage of Pneumocystis carinii in the A549 Cell Line
 INFECTION AND IMMUNITY, May 1984, p. 245-251 0019-9567/84/050245-07$02.00/0 Copyright 1984, American Society for Microbiology Vol. 44, No. 2 Growth and Serial Passage of Pneumocystis carinii in the A549
INFECTION AND IMMUNITY, May 1984, p. 245-251 0019-9567/84/050245-07$02.00/0 Copyright 1984, American Society for Microbiology Vol. 44, No. 2 Growth and Serial Passage of Pneumocystis carinii in the A549
Large Scale Infection for Pooled Screens of shrna libraries
 Last modified 01/11/09 Large Scale Infection for Pooled Screens of shrna libraries Biao Luo, Glenn Cowley, Michael Okamoto, Tanaz Sharifnia This protocol can be further optimized if cells being used are
Last modified 01/11/09 Large Scale Infection for Pooled Screens of shrna libraries Biao Luo, Glenn Cowley, Michael Okamoto, Tanaz Sharifnia This protocol can be further optimized if cells being used are
CONSENSUS PROTOCOL FOR PBMC CRYOPRESERVATION AND THAWING Version 3.0 Revision dated 1/8/02 (Changes are in Red Type)
 CONSENSUS PROTOCOL FOR PBMC CRYOPRESERVATION AND THAWING Version 3.0 Revision dated 1/8/02 (Changes are in Red Type) 1. EQUIPMENT/SUPPLIES/REAGENTS 1.1 Gloves (latex, vinyl, nitrile) 1.2 Lab coat or protective
CONSENSUS PROTOCOL FOR PBMC CRYOPRESERVATION AND THAWING Version 3.0 Revision dated 1/8/02 (Changes are in Red Type) 1. EQUIPMENT/SUPPLIES/REAGENTS 1.1 Gloves (latex, vinyl, nitrile) 1.2 Lab coat or protective
ACTG Laboratory Technologist Committee Revised Version 2.0 ACTG Lab Man HIV Qualitative PBMC Micrococulture 1 June 2004
 HIV QUALITATIVE PBMC MICROCOCULTURE ASSAY 1. PRINCIPLE: 1.1 A co-culture of patient peripheral blood mononuclear cells (PBMC) and uninfected PHA-stimulated PBMCs is maintained under ideal conditions to
HIV QUALITATIVE PBMC MICROCOCULTURE ASSAY 1. PRINCIPLE: 1.1 A co-culture of patient peripheral blood mononuclear cells (PBMC) and uninfected PHA-stimulated PBMCs is maintained under ideal conditions to
Mouse Hepatic Progenitor Organoid Culture: Supplementary Protocols
 TECHNICAL BULLETIN Mouse Hepatic Progenitor Organoid Culture: The following are supplementary protocols for the culture of hepatic organoids with HepatiCult Organoid Growth Medium (Mouse) (Catalog #06030).
TECHNICAL BULLETIN Mouse Hepatic Progenitor Organoid Culture: The following are supplementary protocols for the culture of hepatic organoids with HepatiCult Organoid Growth Medium (Mouse) (Catalog #06030).
NONGYNECOLOGICAL CYTOLOGY FINE NEEDLE ASPIRATION SPECIMENS
 NONGYNECOLOGICAL CYTOLOGY FINE NEEDLE ASPIRATION SPECIMENS I. Purpose Fine needle aspiration of mass lesions is commonly utilized in the detection and characterization of a variety of malignant diseases.
NONGYNECOLOGICAL CYTOLOGY FINE NEEDLE ASPIRATION SPECIMENS I. Purpose Fine needle aspiration of mass lesions is commonly utilized in the detection and characterization of a variety of malignant diseases.
Telephone: Order Desk: Fax:
 Home» Newcastle-Bronchitis Vaccine (B1 Type, B1 Strain; Mass. & Conn. Types) (MERIAL SELECT, INC.) MERIAL SELECT, INC. P.O. DRAWER 2497, GAINESVILLE, GA, 30503 Telephone: 770-536-8787 Order Desk: 770-536-8787
Home» Newcastle-Bronchitis Vaccine (B1 Type, B1 Strain; Mass. & Conn. Types) (MERIAL SELECT, INC.) MERIAL SELECT, INC. P.O. DRAWER 2497, GAINESVILLE, GA, 30503 Telephone: 770-536-8787 Order Desk: 770-536-8787
Investigating respiratory disease
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Investigating respiratory disease Author : David Gibson Categories : Vets Date : August 3, 2009 David Gibson explores diagnostic
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Investigating respiratory disease Author : David Gibson Categories : Vets Date : August 3, 2009 David Gibson explores diagnostic
EXPERIMENTAL SALMONELLOSIS
 EXPERIMENTAL SALMONELLOSIS INTRACELLULAR GROWTH OF Salmonella enteritidis INGESTED IN MONONUCLEAR PHAGOCYTES OF MICE, AND CELLULAR BASIS OF IMMUNITY SUSUMU MITSUHASHI, ICHIEI SATO, AND TOKUMITSU TANAKA
EXPERIMENTAL SALMONELLOSIS INTRACELLULAR GROWTH OF Salmonella enteritidis INGESTED IN MONONUCLEAR PHAGOCYTES OF MICE, AND CELLULAR BASIS OF IMMUNITY SUSUMU MITSUHASHI, ICHIEI SATO, AND TOKUMITSU TANAKA
For research or further manufacturing use only. Not for injection or diagnostic procedures.
 PRIME-XV T cell Expansion XSFM PRIME-XV T Cell Expansion XSFM is a xeno-free, serum-free medium optimized for the activation and expansion of human T lymphocytes. This medium contains gentamicin and requires
PRIME-XV T cell Expansion XSFM PRIME-XV T Cell Expansion XSFM is a xeno-free, serum-free medium optimized for the activation and expansion of human T lymphocytes. This medium contains gentamicin and requires
Protocol for Thawing and Use of Plateable and Suspension Cryopreserved Hepatocytes
 Protocol for Thawing and Use of Plateable and Suspension Cryopreserved Hepatocytes Introduction This protocol covers the thawing and prep of cryopreserved hepatocytes for their subsequent use in applications
Protocol for Thawing and Use of Plateable and Suspension Cryopreserved Hepatocytes Introduction This protocol covers the thawing and prep of cryopreserved hepatocytes for their subsequent use in applications
Correlation of Organism Burden and Alveolar Macrophage Counts during Infection with Pneumocystis carinii and Recovery
 CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, Mar. 2003, p. 293 302 Vol. 10, No. 2 1071-412X/03/$08.00 0 DOI: 10.1128/CDLI.10.2.293 302.2003 Copyright 2003, American Society for Microbiology. All Rights
CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, Mar. 2003, p. 293 302 Vol. 10, No. 2 1071-412X/03/$08.00 0 DOI: 10.1128/CDLI.10.2.293 302.2003 Copyright 2003, American Society for Microbiology. All Rights
THE PROPAGATION OF A VIRULENT GOAT PLEUROPNEUMONIA-LIKE ORGANISM IN THE CHICK EMBRYO
 THE PROPAGATION OF A VIRULENT GOAT PLEUROPNEUMONIA-LIKE ORGANISM IN THE CHICK EMBRYO RICHARD YAMAMOTO, HENRY E. ADLER, AND DONALD R. CORDY School of Veterinary Medicine, University of California, Davis,
THE PROPAGATION OF A VIRULENT GOAT PLEUROPNEUMONIA-LIKE ORGANISM IN THE CHICK EMBRYO RICHARD YAMAMOTO, HENRY E. ADLER, AND DONALD R. CORDY School of Veterinary Medicine, University of California, Davis,
Outline. Cryptococcosis Pneumocystosis Diarrhea. Case Histories: HIV Related- Opportunistic Infections in 2015
 AU Edited: 05/06/15 Case Histories: HIV Related- Opportunistic Infections in 2015 Henry Masur, MD Clinical Professor of Medicine George Washington University School of Medicine Bethesda, Maryland Washington,
AU Edited: 05/06/15 Case Histories: HIV Related- Opportunistic Infections in 2015 Henry Masur, MD Clinical Professor of Medicine George Washington University School of Medicine Bethesda, Maryland Washington,
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 [Version 8, 10/2012] Cevac MD HVT_2016-October-18 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Cevac MD HVT suspension and solvent for suspension for injection
[Version 8, 10/2012] Cevac MD HVT_2016-October-18 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Cevac MD HVT suspension and solvent for suspension for injection
ACTG Laboratory Technologist Committee Revised Version 2.0 ACTG Lab Man HIV Quantitative PBMC culture May 2004
 HIV QUANTITATIVE PBMC MICROCOCULTURE ASSAY 1 PRINCIPLE The quantitative PBMC micrococulture assay estimates the number of infectious units of HIV per million mononuclear cells (IUPM) in peripheral blood
HIV QUANTITATIVE PBMC MICROCOCULTURE ASSAY 1 PRINCIPLE The quantitative PBMC micrococulture assay estimates the number of infectious units of HIV per million mononuclear cells (IUPM) in peripheral blood
Unidirectional Airflow Smoke Test Protocol
 Unidirectional Airflow Smoke Test Protocol Persons signing as approvers shall have examined the Unidirectional Airflow Protocol. This approval shall signify that testing of operational parameters is adequate
Unidirectional Airflow Smoke Test Protocol Persons signing as approvers shall have examined the Unidirectional Airflow Protocol. This approval shall signify that testing of operational parameters is adequate
Best Practices for PBMC Processing
 Immunology Quality Assessment Cryopreservation Proficiency Testing Program Best Practices for PBMC Processing 2015 ACTG Network Annual Meeting Presented by: Sarah Keinonen June 23, 2015 1 IQA Cryopreservation
Immunology Quality Assessment Cryopreservation Proficiency Testing Program Best Practices for PBMC Processing 2015 ACTG Network Annual Meeting Presented by: Sarah Keinonen June 23, 2015 1 IQA Cryopreservation
CULTIVATION OF VACCINE VIRUS
 Published Online: 1 October, 1930 Supp Info: http://doi.org/10.1084/jem.52.4.465 Downloaded from jem.rupress.org on December 27, 2018 CULTIVATION OF VACCINE VIRUS BY C. P. LI, M.D., Am) T. M. RIVERS, M.D.
Published Online: 1 October, 1930 Supp Info: http://doi.org/10.1084/jem.52.4.465 Downloaded from jem.rupress.org on December 27, 2018 CULTIVATION OF VACCINE VIRUS BY C. P. LI, M.D., Am) T. M. RIVERS, M.D.
Cryopreserved HepaRG Cells and Media Supplements
 Cryopreserved HepaRG Cells and Media Supplements Catalog No. MMHPR116 Catalog No. MMADD621 Catalog No. MMADD631 Catalog No. MMADD641 Catalog No. MMADD651 Catalog No. MMADD671 FOR RESEARCH USE ONLY Not
Cryopreserved HepaRG Cells and Media Supplements Catalog No. MMHPR116 Catalog No. MMADD621 Catalog No. MMADD631 Catalog No. MMADD641 Catalog No. MMADD651 Catalog No. MMADD671 FOR RESEARCH USE ONLY Not
axion Protocol Cell Culture on Microelectrode Arrays Cell Type: QBM Cell Science - E14,15 Embryonic C57 Mouse Cortical Neurons BioSystems v. 1.
 axion BioSystems Cell Culture on Microelectrode Arrays Cell Type: QBM Cell Science - E14,15 Embryonic C57 Mouse Cortical Neurons Protocol v. 1.1 Trademarks Axion BioSystems, Inc. and the logo are trademarks
axion BioSystems Cell Culture on Microelectrode Arrays Cell Type: QBM Cell Science - E14,15 Embryonic C57 Mouse Cortical Neurons Protocol v. 1.1 Trademarks Axion BioSystems, Inc. and the logo are trademarks
Page 1132
 Page 1131 Page 1132 Page 1133 Page 1134 Page 1135 Huntingdon Life Sciences 03-6140 Page 6. Final Report TABLE OF CONTENTS Page 1136 COVER PAGE...1 GLP STATEMENT...2 SIGNATURE PAGE...3 QUALITY ASSURANCE
Page 1131 Page 1132 Page 1133 Page 1134 Page 1135 Huntingdon Life Sciences 03-6140 Page 6. Final Report TABLE OF CONTENTS Page 1136 COVER PAGE...1 GLP STATEMENT...2 SIGNATURE PAGE...3 QUALITY ASSURANCE
Biosafety Level 3 (BL3)
 Biosafety Level 3 (BL3) NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (Appendix G-II-C) - April 2016 Biosafety Level 3 (BL3) Biosafety Level 3 is applicable to clinical,
Biosafety Level 3 (BL3) NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (Appendix G-II-C) - April 2016 Biosafety Level 3 (BL3) Biosafety Level 3 is applicable to clinical,
Biological Consulting Services
 Biological Consulting Services of North Florida/ Inc. May 13, 2009 Aphex BioCleanse Systems, Inc. Dear Sirs, We have completed antimicrobial efficacy study on the supplied Multi-Purpose Solution. The testing
Biological Consulting Services of North Florida/ Inc. May 13, 2009 Aphex BioCleanse Systems, Inc. Dear Sirs, We have completed antimicrobial efficacy study on the supplied Multi-Purpose Solution. The testing
Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit
 PROTOCOL Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit DESCRIPTION Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit Sufficient materials
PROTOCOL Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit DESCRIPTION Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit Sufficient materials
MALDI Sepsityper Kit
 Instructions for Use MALDI Sepsityper Kit Kit for identification of microorganisms from positive blood cultures using the MALDI Biotyper system CARE products are designed to support our worldwide customers
Instructions for Use MALDI Sepsityper Kit Kit for identification of microorganisms from positive blood cultures using the MALDI Biotyper system CARE products are designed to support our worldwide customers
SOP History Number Date Reason for Change v1 10/10/2014 Original V2 10/10/2016 Update V3 10/10/2018 Update
 Document Number: SASoM/METHOD/073.v3 Title: Version: Author: Lentivirus production from HEK293T cells v3 Paul Reynolds Effective from: 10/10/2018 Valid to: 09/10/2020 SOP History Number Date Reason for
Document Number: SASoM/METHOD/073.v3 Title: Version: Author: Lentivirus production from HEK293T cells v3 Paul Reynolds Effective from: 10/10/2018 Valid to: 09/10/2020 SOP History Number Date Reason for
Residue Chemistry Studies for Veterinary Drugs. Human Food Safety Assessment. Presentation Topic. Improving residue chemistry submissions
 Residue Chemistry Studies for Veterinary Drugs Julia A. Oriani, Ph.D. Food and Drug Administration Center for Veterinary Medicine Division of Human Food Safety Human Food Safety Assessment Risk = Hazard
Residue Chemistry Studies for Veterinary Drugs Julia A. Oriani, Ph.D. Food and Drug Administration Center for Veterinary Medicine Division of Human Food Safety Human Food Safety Assessment Risk = Hazard
I.C.E. Embryo Vitrification Kit
 I.C.E. Embryo Vitrification Kit Vitrification Media V1, V2, V3 ICE Embryo Vitrification Instructions For Use Testing and Cautions Innvative Cryo Enterprises LLC 317 Springfield Road Linden, New Jersey
I.C.E. Embryo Vitrification Kit Vitrification Media V1, V2, V3 ICE Embryo Vitrification Instructions For Use Testing and Cautions Innvative Cryo Enterprises LLC 317 Springfield Road Linden, New Jersey
Controlling cell-based bioassay performance through controlled preparation of bioassayready
 Controlling cell-based bioassay performance through controlled preparation of bioassayready cells Teresa Surowy September 2013 Promega Corporation Introduction The importance of cell-based bioassays and
Controlling cell-based bioassay performance through controlled preparation of bioassayready cells Teresa Surowy September 2013 Promega Corporation Introduction The importance of cell-based bioassays and
Many people use a combination of the standard 17mm cannula and the shorter 13mm cannula length depending where on their body they are inserting.
 There are many types of infusion sets available through AMSL Diabetes. Our infusion sets are obtained through Animas and are all manufactured by UnoMedical which has a worldwide reputation for producing
There are many types of infusion sets available through AMSL Diabetes. Our infusion sets are obtained through Animas and are all manufactured by UnoMedical which has a worldwide reputation for producing
NON-LACTOSE FERMENTING BACTERIA FROM. While B. coli is generally accepted as a satisfactory index of
 NON-LACTOSE FERMENTING BACTERIA FROM POLLUTED WELLS AND SUB-SOIL' I. J. KLIGLER From the Laboratories of the Rockefeller Institute for Medical Research, New York Received for publication February 1, 1918
NON-LACTOSE FERMENTING BACTERIA FROM POLLUTED WELLS AND SUB-SOIL' I. J. KLIGLER From the Laboratories of the Rockefeller Institute for Medical Research, New York Received for publication February 1, 1918
Predisposing Factors in Pneumocystis carinii Pneumonia: Effects of Tetracycline, Protein Malnutrition, and Corticosteroids on Hosts
 INFECTION AND IMMUNITY, Dec. 1984, p. 747-73 19-967/84/12747-7$2./ Copyright C) 1984, American Society for Microbiology Vol. 46, No. 3 Predisposing Factors in Pneumocystis carinii Pneumonia: Effects of
INFECTION AND IMMUNITY, Dec. 1984, p. 747-73 19-967/84/12747-7$2./ Copyright C) 1984, American Society for Microbiology Vol. 46, No. 3 Predisposing Factors in Pneumocystis carinii Pneumonia: Effects of
Chapter 1 The Public Health Role of Clinical Laboratories
 Chapter 1 The Public Health Role of Clinical Laboratories A. Epidemic Diarrhea The two most common types of epidemic diarrhea in developing countries are watery diarrhea caused by Vibrio cholerae serogroup
Chapter 1 The Public Health Role of Clinical Laboratories A. Epidemic Diarrhea The two most common types of epidemic diarrhea in developing countries are watery diarrhea caused by Vibrio cholerae serogroup
STERILITY IN MALE ANIMALS INDUCED BY INJECTION OF CHEMICAL AGENTS INTO THE VAS DEFERENS*
 FERTILITY AND STERILITY Copyright 1973 by The Williams & Wilkins Co. Vol. 24, No. 11, November 1973 Printed in U.S.A. STERILITY IN MALE ANIMALS INDUCED BY INJECTION OF CHEMICAL AGENTS INTO THE VAS DEFERENS*
FERTILITY AND STERILITY Copyright 1973 by The Williams & Wilkins Co. Vol. 24, No. 11, November 1973 Printed in U.S.A. STERILITY IN MALE ANIMALS INDUCED BY INJECTION OF CHEMICAL AGENTS INTO THE VAS DEFERENS*
Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia
 Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia Terry W. Wright, 1 Francis Gigliotti, 1,2 Jacob N. Finkelstein, 1 John
Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia Terry W. Wright, 1 Francis Gigliotti, 1,2 Jacob N. Finkelstein, 1 John
Natural History of Aortic Valve Endocarditis in Rats
 INFECTION AND IMMUNITY, JUlY 192, p. 127-131 19-9567/2/7127-5$2./ Vol. 37, No. 1 Natural History of Aortic Valve Endocarditis in Rats ERIC HtRAIEF, MICHEL P. GLAUSER,* AND LAWRENCE R. FREEDMANt Division
INFECTION AND IMMUNITY, JUlY 192, p. 127-131 19-9567/2/7127-5$2./ Vol. 37, No. 1 Natural History of Aortic Valve Endocarditis in Rats ERIC HtRAIEF, MICHEL P. GLAUSER,* AND LAWRENCE R. FREEDMANt Division
Product Use HPSC-CC are for research use only. It is not approved for human or animal use, or for application in in vitro diagnostic procedures.
 HPSC-derived Cardiomyocyte Cells (HPSC-CC) Catalog #6240 Cell Specification Human primary cardiomyocytes and cardiac tissue are superior modeling systems for heart disease studies, drug discovery and toxicity
HPSC-derived Cardiomyocyte Cells (HPSC-CC) Catalog #6240 Cell Specification Human primary cardiomyocytes and cardiac tissue are superior modeling systems for heart disease studies, drug discovery and toxicity
Mucomist Respirator Solution, Acetylcysteine USP 200 mg/ ml Solution for inhalation (not for injection)
 Mucomist Respirator Solution, Acetylcysteine USP 200 mg/ ml Solution for inhalation (not for injection) DESCRIPTION Mucomist Respirator Solution is a derivative of the amino acid, cysteine. It acts mostly
Mucomist Respirator Solution, Acetylcysteine USP 200 mg/ ml Solution for inhalation (not for injection) DESCRIPTION Mucomist Respirator Solution is a derivative of the amino acid, cysteine. It acts mostly
OUTLINE Laboratory Detection and Reporting of Streptococcus agalactiae
 OUTLINE Laboratory Detection and Reporting of Streptococcus agalactiae I. Importance of prenatal screening strategies II. Past approaches Erik Munson Clinical Microbiology Wheaton Franciscan Laboratory
OUTLINE Laboratory Detection and Reporting of Streptococcus agalactiae I. Importance of prenatal screening strategies II. Past approaches Erik Munson Clinical Microbiology Wheaton Franciscan Laboratory
ACTG Laboratory Technologist Committee Revised Version 2.0 ACTG Lab Man HIV Syncytium-Inducing (MT-2) assay 29 April 2004
 HIV SYNCYTIUM-INDUCING (MT-2) ASSAY 1. BACKGROUND and CLINICAL SIGNIFICANCE Host and viral factors may play a role in determining the way in which an individual responds to anti-retroviral therapy. Presence
HIV SYNCYTIUM-INDUCING (MT-2) ASSAY 1. BACKGROUND and CLINICAL SIGNIFICANCE Host and viral factors may play a role in determining the way in which an individual responds to anti-retroviral therapy. Presence
Laboratory Detection and Reporting of Streptococcus agalactiae
 Laboratory Detection and Reporting of Streptococcus agalactiae Erik Munson Clinical Microbiology Wheaton Franciscan Laboratory Milwaukee, Wisconsin The presenter states no conflict of interest and has
Laboratory Detection and Reporting of Streptococcus agalactiae Erik Munson Clinical Microbiology Wheaton Franciscan Laboratory Milwaukee, Wisconsin The presenter states no conflict of interest and has
Human Keratinocyte Manual
 INSTRUCTION MANUAL SHIPPING CONDITIONS Human Keratinocyte Cells Human Keratinocyte Manual ZBM0032.09 Orders are delivered via Federal Express courier. All US and Canada orders are shipped via Federal Express
INSTRUCTION MANUAL SHIPPING CONDITIONS Human Keratinocyte Cells Human Keratinocyte Manual ZBM0032.09 Orders are delivered via Federal Express courier. All US and Canada orders are shipped via Federal Express
Title: Column Chromatography of Green Fluorescent Protein
 Title: Column Chromatography of Green Fluorescent Protein Approvals: Preparer Date_07Oct06 Reviewer: Mary Jane Kurtz Date 09Jul13 Part I Crude Isolation of GFP from Lysed Cells q Page 1 of 6 1. Purpose:
Title: Column Chromatography of Green Fluorescent Protein Approvals: Preparer Date_07Oct06 Reviewer: Mary Jane Kurtz Date 09Jul13 Part I Crude Isolation of GFP from Lysed Cells q Page 1 of 6 1. Purpose:
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Pneumocystis Carinii Real Time PCR Kit. For In Vitro Diagnostic Use Only User Manual
 Revision No.: ZJ0003 Issue Date: Aug 7 th, 2008 Pneumocystis Carinii Real Time PCR Kit Cat. No.: QD-0082-02 For use with ABI Prism 7000/7300/7500/7900; Smart CyclerII; icycler iq 4/iQ 5; Rotor Gene 2000/3000;
Revision No.: ZJ0003 Issue Date: Aug 7 th, 2008 Pneumocystis Carinii Real Time PCR Kit Cat. No.: QD-0082-02 For use with ABI Prism 7000/7300/7500/7900; Smart CyclerII; icycler iq 4/iQ 5; Rotor Gene 2000/3000;
10.00 PBS OVA OVA+isotype antibody 8.00 OVA+anti-HMGB1. PBS Methatroline (mg/ml)
 RESEARCH ARTICLE Penh (100% of PBS) 1 PBS 8.00 +anti-hmgb1 6.00 4.00 p=0.054 Cellular & Molecular Immunology advance online publication, PBS 3.12 6.25 Methatroline (mg/ml) Neutrophil isolation and culture
RESEARCH ARTICLE Penh (100% of PBS) 1 PBS 8.00 +anti-hmgb1 6.00 4.00 p=0.054 Cellular & Molecular Immunology advance online publication, PBS 3.12 6.25 Methatroline (mg/ml) Neutrophil isolation and culture
1918 Influenza; Influenza A, H1N1. Basic agent information. Section I- Infectious Agent. Section II- Dissemination
 1918 Influenza; Influenza A, H1N1 Basic agent information Section I- Infectious Agent Risk Group: - RG3 Synonym or Cross reference: - Spanish Flu - 1918 Flu - El Grippe Characteristics: - SELECT AGENT
1918 Influenza; Influenza A, H1N1 Basic agent information Section I- Infectious Agent Risk Group: - RG3 Synonym or Cross reference: - Spanish Flu - 1918 Flu - El Grippe Characteristics: - SELECT AGENT
Rapid-VIDITEST. Strep A Blister. One step Strep A Blister for the detection of Group A Streptococcal antigen from throat swabs or culture.
 Rapid-VIDITEST Strep A Blister One step Strep A Blister for the detection of Group A Streptococcal antigen from throat swabs or culture. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II
Rapid-VIDITEST Strep A Blister One step Strep A Blister for the detection of Group A Streptococcal antigen from throat swabs or culture. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II
STUDIES ON ISOMETAMIDIUM: THE EFFECT OF ISOMETAMIDIUM, HOMIDIUM AND PYRITHIDIUM ON THE INFECTIVITY OF TRYPANOSOMES FOR MICE
 Brit. J. Pharmacol. (1965), 25, 658-663. STUDIES ON ISOMETAMIDIUM: THE EFFECT OF ISOMETAMIDIUM, HOMIDIUM AND PYRITHIDIUM ON THE INFECTIVITY OF TRYPANOSOMES FOR MICE BY J. HILL From the Research Laboratories,
Brit. J. Pharmacol. (1965), 25, 658-663. STUDIES ON ISOMETAMIDIUM: THE EFFECT OF ISOMETAMIDIUM, HOMIDIUM AND PYRITHIDIUM ON THE INFECTIVITY OF TRYPANOSOMES FOR MICE BY J. HILL From the Research Laboratories,
TECHNICAL REPORT Minitube TurboFreezer A standardised freezing process is crucial for efficient production of cryopreserved bull semen
 TECHNICAL REPORT 02 2016 Minitube TurboFreezer A standardised freezing process is crucial for efficient production of cryopreserved bull semen Dr. Monika Esch, Minitüb GmbH The objective of utilizing nitrogen
TECHNICAL REPORT 02 2016 Minitube TurboFreezer A standardised freezing process is crucial for efficient production of cryopreserved bull semen Dr. Monika Esch, Minitüb GmbH The objective of utilizing nitrogen
Intracellular (Total) ROS Activity Assay Kit (Red)
 Intracellular (Total) ROS Activity Assay Kit (Red) Catalog Number KA2525 200 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General
Intracellular (Total) ROS Activity Assay Kit (Red) Catalog Number KA2525 200 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General
Effect of Azithromycin plus Rifampin versus That of Azithromycin Alone on the Eradication of Chlamydia pneumoniae
 Antimicrobial Agents and Chemotherapy, June 1999, p. 1491-1493, Vol. 43, No. 6 0066-4804/99/$04.00+0 Copyright 1999, American Society for Microbiology. All rights reserved. Effect of Azithromycin plus
Antimicrobial Agents and Chemotherapy, June 1999, p. 1491-1493, Vol. 43, No. 6 0066-4804/99/$04.00+0 Copyright 1999, American Society for Microbiology. All rights reserved. Effect of Azithromycin plus
Acceptability of Sputum Specimens
 JOURNAL OF CLINICAL MICROBIOLOGY, Oct. 1982, p. 627-631 0095-1137/82/100627-05$02.00/0 Copyright C 1982, American Society for Microbiology Vol. 16, No. 4 Comparison of Six Different Criteria for Judging
JOURNAL OF CLINICAL MICROBIOLOGY, Oct. 1982, p. 627-631 0095-1137/82/100627-05$02.00/0 Copyright C 1982, American Society for Microbiology Vol. 16, No. 4 Comparison of Six Different Criteria for Judging
Human Influenza A (Swine Flu) Rapid test
 Human Influenza A (Swine Flu) Rapid test Cat.No: DTSXY-Z9 Lot. No. (See product label) Size 20T Intended use The Influenza A (Swine Flu) test is a rapid chromatographic immunoassay for the qualitative
Human Influenza A (Swine Flu) Rapid test Cat.No: DTSXY-Z9 Lot. No. (See product label) Size 20T Intended use The Influenza A (Swine Flu) test is a rapid chromatographic immunoassay for the qualitative
Procine sphingomyelin ELISA Kit
 Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Vaccination Recommendations Practice and Shelter-Housed Dogs
 Vaccination Recommendations Practice and Shelter-Housed Dogs 1. MIXING VACCINES. Can different types of vaccines be mixed in the same syringe? No. Unless specifically stated on the product label (package
Vaccination Recommendations Practice and Shelter-Housed Dogs 1. MIXING VACCINES. Can different types of vaccines be mixed in the same syringe? No. Unless specifically stated on the product label (package
2009 H1N1 Influenza ( Swine Flu ) Hemagglutinin ELISA kit
 2009 H1N1 Influenza ( Swine Flu ) Hemagglutinin ELISA kit Catalog Number : SEK001 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as
2009 H1N1 Influenza ( Swine Flu ) Hemagglutinin ELISA kit Catalog Number : SEK001 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as
NONGYNECOLOGICAL CYTOLOGY PULMONARY SPECIMENS (Sputum, Post-Bronchoscopy Sputum, Bronchial Brushings, Bronchial Washings, Bronchoalveolar Lavage)
 NONGYNECOLOGICAL CYTOLOGY PULMONARY SPECIMENS (Sputum, Post-Bronchoscopy Sputum, Bronchial Brushings, Bronchial Washings, Bronchoalveolar Lavage) I. Purpose The adequacy of a sputum specimen is determined
NONGYNECOLOGICAL CYTOLOGY PULMONARY SPECIMENS (Sputum, Post-Bronchoscopy Sputum, Bronchial Brushings, Bronchial Washings, Bronchoalveolar Lavage) I. Purpose The adequacy of a sputum specimen is determined
Human Induced Plutipotent Stem Cell (ipsc) Handling Protocols: Matrigel and mtesr/e8 Media
 General Guidelines for Handling Human ipsc cells ipsc are cryopreserved in plastic cryovials and shipped on dry ice. If storing the ipsc before thawing, store in liquid nitrogen vapor. Storage directly
General Guidelines for Handling Human ipsc cells ipsc are cryopreserved in plastic cryovials and shipped on dry ice. If storing the ipsc before thawing, store in liquid nitrogen vapor. Storage directly
20X Buffer (Tube1) 96-well microplate (12 strips) 1
 PROTOCOL MitoProfile Rapid Microplate Assay Kit for PDH Activity and Quantity (Combines Kit MSP18 & MSP19) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSP20 Rev.1 DESCRIPTION MitoProfile Rapid Microplate
PROTOCOL MitoProfile Rapid Microplate Assay Kit for PDH Activity and Quantity (Combines Kit MSP18 & MSP19) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSP20 Rev.1 DESCRIPTION MitoProfile Rapid Microplate
Acute Oral Toxicity Study in Rats with Argentyn 23 (EPA/OECD Guidelines) Study Title. Monica M. Vegarra, BS. Sponsor
 Note: This study was done using our Professional strength 23 ppm product (Argentyn 23), therefore making Sovereign Silver at 10 ppm, 2.3 times safer. Conclusion on page 6 shows the product to be non-toxic
Note: This study was done using our Professional strength 23 ppm product (Argentyn 23), therefore making Sovereign Silver at 10 ppm, 2.3 times safer. Conclusion on page 6 shows the product to be non-toxic
ROS Activity Assay Kit
 ROS Activity Assay Kit Catalog Number KA3841 200 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials
ROS Activity Assay Kit Catalog Number KA3841 200 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials
Pharmacy Instructions for Preparation
 MARQIBO (vincristine sulfate LIPOSOME injection) Pharmacy Instructions for Preparation Important Information for Preparation 1 The instructions for the constitution of MARQIBO are provided in each kit.
MARQIBO (vincristine sulfate LIPOSOME injection) Pharmacy Instructions for Preparation Important Information for Preparation 1 The instructions for the constitution of MARQIBO are provided in each kit.
Protocol for embryo vitrification using open pulled straws. Introduction. Reagents. Materials
 Protocol for embryo vitrification using open pulled straws Froylan Sosa and Peter J Hansen Department of Animal Sciences, University of Florida Introduction This vitrification protocol is a slight modification
Protocol for embryo vitrification using open pulled straws Froylan Sosa and Peter J Hansen Department of Animal Sciences, University of Florida Introduction This vitrification protocol is a slight modification
Pneumocystis Pneumonia. Dr. Pradeep kumar II yr Pulmonary Medicine
 Pneumocystis Pneumonia Dr. Pradeep kumar II yr Pulmonary Medicine PNEUMOCYSTIS CARINII PNEUMONIA Pneumocystis carinii pneumonia (PCP), is commonly termed Pneumocystis jiroveci pneumonia, is the 2 nd most
Pneumocystis Pneumonia Dr. Pradeep kumar II yr Pulmonary Medicine PNEUMOCYSTIS CARINII PNEUMONIA Pneumocystis carinii pneumonia (PCP), is commonly termed Pneumocystis jiroveci pneumonia, is the 2 nd most
The DTAC News CONTENTS WELCOME. From the OPTN/UNOS Ad Hoc Disease Transmission Advisory Committee
 The DTAC News From the OPTN/UNOS Ad Hoc Disease Transmission Advisory Committee FIRST EDITION FEBRUARY, 2010 Welcome to the first edition of the DTAC News. This newsletter is brought to you by the OPTN/UNOS
The DTAC News From the OPTN/UNOS Ad Hoc Disease Transmission Advisory Committee FIRST EDITION FEBRUARY, 2010 Welcome to the first edition of the DTAC News. This newsletter is brought to you by the OPTN/UNOS
