TRANSFUSION-TRANSMITTED MALARIA IN THE UNITED STATES FROM 1963 THROUGH 1999
|
|
|
- Scott Owen
- 6 years ago
- Views:
Transcription
1 TRANSFUSION-TRANSMITTED MALARIA IN THE UNITED STATES FROM 1963 THROUGH 1999 TRANSFUSION-TRANSMITTED MALARIA IN THE UNITED STATES FROM 1963 THROUGH 1999 MARY MUNGAI, M.D., GARY TEGTMEIER, PH.D., MARY CHAMBERLAND, M.D., M.P.H., AND MONICA PARISE, M.D. ABSTRACT Background Transfusion-transmitted malaria is uncommon in the United States. After the report of three cases of complicated Plasmodium falciparum infection acquired by transfusion, we reviewed all cases of transfusion-transmitted malaria reported to the Centers for Disease Control and Prevention (CDC) from 1963 through Methods Information on the patients was from surveillance reports sent to the CDC. Information about the implicated blood donors came from the National Malaria Surveillance System. To determine whether donors should have been excluded from donating blood, we compared their characteristics with the exclusion guidelines of the Food and Drug Administration and the American Association of Blood Banks. Results Of 93 cases of transfusion-transmitted malaria reported in 28 states, 33 (35 percent) were due to P. falciparum, 25 (27 percent) were due to P. vivax, 25 (27 percent) were due to P. malariae, 5 (5 percent) were due to P. ovale, 3 (3 percent) were mixed infections, and 2 (2 percent) were due to unidentified species. Ten of the 93 patients (11 percent) died. There were potentially 91 donors (in two cases, two patients received blood from the same donor), 67 of whom (74 percent) could be identified as infective. Of 64 implicated donors whose country of origin was reported, 38 (59 percent) were foreign born. Among those for whom complete information was available, 37 of 60 donors (62 percent) would have been excluded from donating according to current guidelines (in place since 1994), and 30 of 48 donors (62 percent) should have been excluded under the guidelines in place at the time of donation. Conclusions Careful screening of donors according to the recommended exclusion guidelines remains the best way to prevent transfusion-transmitted malaria. (N Engl J Med 2001;344: ) Copyright 2001 Massachusetts Medical Society. IN the United States, the estimated incidence of transmission of malaria by blood transfusion (less than 1 case per million units collected 1 ) is less than that of hepatitis B virus (7 to 32 cases per million units) and bacterial infections (e.g., 1 case of platelet-related sepsis per 12,000 units 2 ) and is similar to that of hepatitis C or human immunodeficiency virus after the introduction of nucleic acid testing techniques. 3 There are few data on the risk of transfusion-transmitted babesiosis 4 (approximately 6 cases per million units). 5 Since there is no approved laboratory test in the United States to screen donated blood for malaria, prevention depends on the exclusion of potentially in- fected donors who are identified during the donor interview. The Food and Drug Administration (FDA) 6,7 and the American Association of Blood Banks 8 have recommendations for the exclusion of potentially infected donors (Table 1). However, it can be difficult to obtain accurate travel and immigration histories and to ascertain the areas of a country in which malaria is transmitted. From 1996 through 1998, three cases of transfusion-transmitted malaria occurred, two of which were fatal. 9 To gain a better understanding of how to prevent such cases, we reviewed the epidemiologic features of cases of transfusion-transmitted malaria in the United States, as reported to the Centers for Disease Control and Prevention (CDC) from 1963 (the first year complete records were available) through METHODS Information about the patients was obtained from reports of cases of malaria sent to the National Malaria Surveillance System at the CDC, which include information on demographic characteristics, date of the onset of illness, species responsible for the infection, history of travel or blood transfusion, type of antimalarial therapy, and outcome of the illness. Because malaria infections acquired in the United States are further investigated by the CDC (in conjunction with state or local health departments), further details on transfusion-transmitted cases were obtained by reviewing the annual malaria-surveillance summaries of the CDC. During the epidemiologic investigations that ensue after a case is detected, an attempt is made to collect serum from the donors involved so that it can be tested at the CDC for antimalarial antibodies by the indirect fluorescence antibody assay. 10 Antibody titers of 1:64 or more are considered positive and to indicate previous or current infection. When possible, donors are reinterviewed regarding their travel history, any prior diagnosis of malaria, country of birth, and date of entry into the United States, and a blood smear is obtained and reviewed at the CDC. We considered a donor to be the source of the malaria infection if at least one of three criteria was met: the donor had a positive blood smear; the donor had a positive serologic test result; or the patient had received blood from no other donor. In general, when a case of transfusion-transmitted malaria occurs, any remaining blood components from potentially infective donors are withheld from transfusion pending evaluation of the donors. Once an infective donor is identified, any recipient of his or her blood components (from the same or a prior donation) is evaluated for malaria. The cases of transfusion-transmitted malaria we reviewed occurred over a period of years, during which donor-exclusion guidelines changed several times (Table 1). During this time, the guidelines evolved to provide specific durations of deferral; a diagnosis From the Divisions of Parasitic Diseases (M.M., M.P.) and Viral and Rickettsial Diseases (M.C.), National Center for Infectious Diseases, Centers for Disease Control and Prevention, Atlanta; the Public Health Service, Department of Health and Human Services, Washington, D.C. (M.M., M.C., M.P.); and the Community Blood Center of Greater Kansas City, Kansas City, Mo. (G.T.). Address reprint requests to Dr. Parise at the Division of Parasitic Diseases, Mailstop F-22, Centers for Disease Control and Prevention, 4770 Buford Hwy., Atlanta, GA 30341, or at mep0@cdc.gov. N Engl J Med, Vol. 344, No. 26 June 28,
2 TABLE 1. GUIDELINES OF THE AMERICAN ASSOCIATION OF BLOOD BANKS AND THE FDA FOR THE EXCLUSION OF DONORS BECAUSE OF THE POSSIBILITY OF MALARIA, 1958 THROUGH YEAR RECOMMENDATIONS 1958 A donor shall be free from any disease transmissible by blood transfusion insofar as can be determined by history or inspection A donor shall be free from any infectious disease, including syphilis and malaria, transmissible by blood transfusion insofar as can be determined by history. TRAVELERS TO MALARIOUS AREAS IMMIGRANTS OR REFUGEES FROM OR RESIDENTS OF MALARIOUS AREAS PERSONS TREATED FOR MALARIA 1970 i) Travelers to malarious areas may be accepted as regular donors 6 months after return to the United States providing that they have been free of symptoms and have not taken antimalarial prophylaxis. ii) Travelers who have taken antimalarial prophylaxis shall be deferred for 2 years after cessation of suppressive therapy i) Same rule as for ii) Travelers to malarious areas who have taken antimalarial prophylaxis shall be deferred for 3 years after cessation of therapy. Military personnel who have been in a malarious area should be considered to have received suppressive therapy whether or not they report it i) Same rule as for ii) Prospective donors who have taken antimalarial prophylaxis or who have been military personnel in areas where malaria is endemic shall be deferred for 3 years after cessation of therapy or after departure from the area if they have been asymptomatic Same rule as for 1974 (except there is no separate mention of military personnel). 1994* Travelers who are residents of nonmalarious areas who have been in a malarious area may be accepted as donors 1 year after their return to the nonmalarious area (irrespective of the use of chemoprophylaxis) if they have been free of malaria symptoms. A prospective donor who has ever had definite malaria or is an immigrant or visitor from a malarious area is permanently rejected. Same rule as for Immigrants or visitors from endemic areas may be accepted as regular donors 3 years after departure from the area if they have been asymptomatic in the interim. Prospective donors who have had malaria shall be deferred for 3 years either after becoming asymptomatic or after cessation of therapy. Same rule as for Same rule as for Same rule as for Same rule as for Immigrants or visitors from endemic areas may be accepted 3 years after departure from the area if they have been asymptomatic. Former residents of malarious areas who now live in the United States but who return to visit a malarious area may be accepted 3 years after their last visit. Prospective donors who have had a diagnosis of malaria shall be deferred for 3 years after becoming asymptomatic. Same rule as for *In 1994, the FDA continued to recommend a three-year deferral for persons who had had malaria, whereas the American Association of Blood Banks during 1994 through 1996 recommended deferring such persons indefinitely. In 1996, the American Association of Blood Banks returned to recommending a three-year deferral for such persons. The 1994 guidelines of the FDA were used for donor-suitability analyses in this article. The guidelines proposed by the FDA in 2000 are currently in draft form. of malaria now requires a three-year deferral instead of a permanent exclusion, and the deferral period for travelers is now the same whether or not they have received chemoprophylaxis. To determine the suitability of donors implicated in these cases, we reviewed the reported epidemiologic characteristics of each donor in the light of the current FDA exclusion guidelines (defined as those issued in 1994) and those in effect at the time of donation. The incidence of transfusion-transmitted malaria in the United States was calculated as the number of cases divided by the number of units of whole blood and packed red cells transfused during each year. 1,11-16 Patients RESULTS Ninety-three cases of transfusion-transmitted malaria were reported from 1963 through 1999 by 28 states. Sixty (65 percent) were reported by six jurisdictions: New York City (14); Texas (13); California (13); New York State, excluding New York City (7); Pennsylvania (7); and Florida (6). Of 91 patients whose sex was reported, 53 (58 percent) were male. The patients ranged in age from 2 days to 85 years; 24 (26 percent) were older than 65 years of age; in comparison, approximately 54 percent of persons who receive red-cell transfusions in the United States are older than 65 years old. 17 Table 2 shows the species of malaria parasite identified. There was an increase in the proportion of cases caused by Plasmodium falciparum in the 1990s. The number of donors per patient ranged from 1 to 192 (median, 7). Among the 70 patients for whom information was available, the infective component was whole blood in 63 percent, packed red cells in 31 percent, and platelets (which can transmit malaria because of contamination with residual red cells) in 6 percent. The proportion of cases attributed to whole blood decreased from 88 per N Engl J Med, Vol. 344, No. 26 June 28,
3 TRANSFUSION-TRANSMITTED MALARIA IN THE UNITED STATES FROM 1963 THROUGH 1999 TABLE 2. THE CAUSATIVE SPECIES OF PLASMODIUM AND INCUBATION PERIODS IN 93 CASES OF TRANSFUSION-TRANSMITTED MALARIA IN THE UNITED STATES, 1963 THROUGH 1999.* SPECIES (N=17) (N=34) (N=28) (N=14) INCUBATION PERIOD RANGE MEAN ±SD MEDIAN days Plasmodium falciparum 8 (47) 10 (29) 5 (18) 10 (71) 8 36 (n=20) P. vivax 2 (12) 14 (41) 8 (29) 1 (7) (n=16) P. malariae 5 (29) 8 (24) 10 (36) 2 (14) 8 90 (n=15) P. ovale 1 (6) 1 (3) 2 (7) 1 (7) (n=2) Mixed 1 (6) 1 (3) 1 (4) (n=3) Unknown (7) 0 11 (n=1) 17± ± ± ± ± ±0 11 *A listing of all cases, with details on the year, the number of donors involved, demographic characteristics and travel or immigration history of the implicated donor, laboratory results, and compliance with donor-deferral guidelines is available from the authors. Values in parentheses are the numbers of cases with data on the incubation period. cent from 1963 through 1979 to 27 percent from 1980 through 1999 (P<0.001). Ten of the 93 patients (11 percent) died. Patients who died were significantly older than those who survived (mean, 71 vs. 47 years; P<0.001; range, 53 to 85 years and 2 days to 78 years, respectively). Six of the patients who died had P. falciparum infection, two had P. vivax, and two had P. malariae. The reported cause of death of the two patients infected with P. vivax was the underlying disease. The reported cause of death of the two patients with P. malariae infection involved unusually high densities of parasites, although the specific densities were unknown. The incubation period was available in 57 cases (Table 2). In 43 cases for which data were available, the number of days from the onset of symptoms to the diagnosis of malaria ranged from 1 to 180 (median, 10). Implicated Donors There were a presumed 91 infective donors for the 93 patients (in two different episodes, 2 patients had the same donor). In 67 of the 91 episodes (74 percent), an infective donor could be identified. Fiftythree of 59 donors (90 percent) whose sex was reported were male. In 37 cases in which information was available, the implicated donors ages ranged from 19 to 59 years (median, 27); 78 percent were 21 to 40 years old. In 64 cases, the country of origin of the implicated donor was known. Twenty-six (41 percent) were born in the United States, and 38 (59 percent) were born in other countries (24 in Africa, 4 in Asia, 6 in the Americas, and 4 in Europe). The proportion of implicated donors in the past 20 years who were former residents of countries where malaria was endemic (86 percent) was significantly greater than the proportion from 1963 through 1979 (26 percent, P<0.001) (Table 3). Forty-eight of the 67 implicated donors were identified by serologic tests (72 percent), 7 by blood smear (10 percent), 10 by both serologic tests and blood smear (15 percent), and 2 by the sole-donor criterion (3 percent). Serologic tests were positive in 58 of 59 implicated donors in which testing was performed (98 percent); a blood smear showed malaria parasites in 17 of 49 donors in which it was performed (35 percent). There was enough information in the records to permit a judgment to be made as to whether the donor should have been excluded according to current exclusion criteria in 60 cases and according to prior exclusion criteria in 48 cases. Thirty-seven of 60 donors (62 percent) should have been excluded according to current FDA guidelines, and 30 of 48 (62 percent) according to the guidelines in place at the time of donation. The species distributions for cases in which current guidelines were followed and those in which they were not are shown in Table 4. Of the 15 P. malariae infections that occurred when current guidelines were followed, 12 were due to foreignborn donors and 3 to U.S.-born donors. The time N Engl J Med, Vol. 344, No. 26 June 28,
4 TABLE 3. CHARACTERISTICS OF DONORS IMPLICATED IN CASES OF TRANSFUSION-TRANSMITTED MALARIA IN THE UNITED STATES, 1963 THROUGH 1999.* DONOR CHARACTERISTIC (N=11) (N=24) no. (%) (N=17) (N=12) Former resident of malarious area 4 (36) 5 (21) 15 (88) 10 (83) U.S. civilian traveler 0 2 (8) 0 1 (8) Visitor to country of origin 1 (9) 4 (17) 2 (12) 1 (8) U.S. military personnel 6 (55) 13 (54) 0 0 *Data were available for 64 of the 67 implicated donors. A visitor was defined as a person who had lived in a malarious area before coming to live in the United States and who returned to the country of origin to visit friends or relatives. TABLE 4. INFECTIVE PLASMODIUM SPECIES IN 23 CASES IN WHICH CURRENT DONOR-EXCLUSION GUIDELINES WERE CORRECTLY IMPLEMENTED AND IN 37 CASES IN WHICH THE GUIDELINES WERE NOT IMPLEMENTED CORRECTLY. SPECIES GUIDELINES CORRECTLY IMPLEMENTED (N=23) no. (%) GUIDELINES NOT CORRECTLY IMPLEMENTED (N=37) Plasmodium falciparum 4 (17) 22 (59) P. vivax 2 (9) 10 (27) P. malariae 15 (65) 3 (8) P. ovale 1 (4) 2 (5) Mixed 1 (4) 0 between the donor s immigration to the United States or last foreign travel to a malarious area and blood donation ranged from 3 to 44 years (median, 8). Of the four P. falciparum infections that occurred even though current guidelines had been followed, one was due to a foreign-born donor who had immigrated to the United States years before donation (more than the 3-year deferral period). The remaining three were due to U.S.-born donors; the time between their last travel to a malarious area and blood donation ranged from 1 to 5 years (median, 13 months). The implicated donors in two cases of P. vivax malaria that occurred despite adherence to guidelines had been born in the United States but had subsequently lived in malarious areas; one had been in the United States for one year before donation, and the other for two years. One case caused by P. ovale was associated with a U.S.-born donor who had lived in Liberia for several years but who had reportedly last been in a malarious area four years before donating blood. The implicated donor in a case of mixed infection (P. malariae and P. ovale) was foreign born and had reportedly last been in a malarious area seven years before donating. Trends in the Incidence of Transfusion-Transmitted Malaria The incidence of transfusion-transmitted malaria in the United States has decreased in the past three decades and now remains at a stable low level (Fig. 1). From 1965 through 1970, the incidence rate ranged from 0 to 1.37 cases per million units transfused; from 1993 through 1998, the incidence rate ranged from 0 to 0.18 case per million units transfused. The peak in cases of transfusion-transmitted malaria in the late 1960s and early 1970s was associated with the return of military personnel from Vietnam and was accompanied by an increase in the overall numbers of cases of malaria reported in the United States, largely caused by increased immigration from Southeast Asia. The reason for the increase in the number of cases of transfusion-transmitted malaria in the early 1980s is unknown. DISCUSSION Transfusion-transmitted malaria remains rare in the United States. Its reported incidence has not changed substantially in the past decade; however, there have been important changes in the patterns of transmission in the past three decades. Although implicated donors were approximately evenly distributed between those born in the United States and those born in other countries, in the past decade most infective donors have been immigrants. Although P. malariae and P. vivax have been reported as the species most frequently associated with transfusion-transmitted malaria, in our series the most common infecting species was P. falciparum, particularly in more recent years. This may be due to increasing immigration from malarious areas, particularly sub-saharan Africa, where P. falciparum is the most common species transmitted. Prevention of transfusion-transmitted malaria relies entirely on the exclusion of potentially infected donors. However, in approximately two thirds of cases, the donor-screening process failed, illustrating the difficulties in obtaining accurate travel and immigration histories from donors. Information on previous exposure to malaria was elicited from the potentially infected donors during the epidemiologic investigations initiated after the identification of a transfusiontransmitted case. Because not all records of the donor interviews were available to us, we do not know whether these donors had given the same history at the time of donation nor do we know exactly how the blood-bank staff may have evaluated the travel and immigration history. To assist blood banks in screening, the American Asso N Engl J Med, Vol. 344, No. 26 June 28,
5 TRANSFUSION-TRANSMITTED MALARIA IN THE UNITED STATES FROM 1963 THROUGH 1999 Number of Cases Figure 1. The Number of Cases and the Incidence of Transfusion-Transmitted Malaria in the United States, 1963 through The incidence is the number of cases per 1,000,000 units of whole blood and packed red cells transfused No. of cases Incidence ciation of Blood Banks modified its uniform donorscreening questionnaire in Blood-bank personnel inquire generally about travel outside the United States or Canada within the previous three years (instead of relying on donors to report whether they have been in a malarious area) and then probe to determine whether travel was to a malarious area. To improve the assessment of such donors, the FDA has recently proposed that donors first be asked whether they were born in the United States. 7 If they were not, they are asked when they moved to the United States and whether they have traveled outside the United States since their arrival. Approximately one third of the cases of transfusion-transmitted malaria occurred despite adherence to current guidelines. The donor-exclusion criteria have their scientific basis in the biologic behavior of the different plasmodium species. Infections with species that cause relapsing illness (P. vivax and P. ovale) rarely persist longer than three years. 22 Infections with P. falciparum rarely persist longer than one or two years, 1 and 99 percent of patients present within one year of departure from a malarious area. 23 National malaria-surveillance data from 1985 through 1997 included 7407 reported cases in U.S.- born residents and 6252 in foreign-born residents. Among 5737 cases in U.S.-born residents for which information was available, 119 cases (2.1 percent) had their onset more than one year after the patient had traveled to a malarious area. Among 4229 cases in foreign-born residents for which information was available, 7 cases (0.2 percent) had their onset more than three years after the patient left a malarious area. In U.S. surveillance data, we found transfusion-transmitted cases of P. vivax, P. ovale, and P. falciparum infections in which donors had reportedly left malarious areas five, seven, and nine years, respectively, before the diagnosis of malaria in the recipient. We also found 9 case reports in the literature of transfusion-transmitted infections by species other than P. malariae with more than three years between the donor s departure from a malarious area and the diagnosis of malaria in the recipient or the transfusion. The longest periods between the reported exposure to malaria and the donation of blood products that transmitted the infection were 13 years in the case of a P. falciparum infection, years in the case of a P. vivax infection, 24 and 7 years in the case of a P. ovale infection. 25 In our series, the longest interval between travel to a malarious area and transmission of malaria through a blood transfusion was 44 years in the case of a P. malariae infection, 5 years in the case of a P. falciparum infection, 2.5 years in the case of a P. vivax infection, and 7 years in the case of a P. ovale infection. Because P. malariae parasites can persist for decades, rare cases of transfusion-transmitted malaria will continue to occur despite the use of current exclusion guidelines. The guidelines aim to strike a balance between minimizing the risk of malaria and excluding as few uninfected donors as possible. One possible option for reducing transfusion-transmitted malaria is laboratory screening. Among potential screening tests, diagnosis on the basis of a bloodsmear examination is not sensitive enough, since donors who have transmitted the infection typically have a low level of parasitemia 22 that may not be detected even by careful examination of a blood smear. In their current stage of development, antigen-detection tests 29,30 have an even higher limit of detection (in terms of the number of parasites per cubic millimeter) than blood-smear examination and would be of limited usefulness in screening No. of Cases/Million Units Transfused N Engl J Med, Vol. 344, No. 26 June 28,
6 Another screening option is the detection of plasmodium DNA or RNA by the polymerase chain reaction (PCR), which is more sensitive and specific than microscopical examination 31 and has been used on donor blood. 32 In a recent case, 9 one implicated donor had negative results on the examination of blood smears but had parasite DNA detectable by PCR. PCR, however, is relatively cumbersome, and data to date are insufficient to show that it is sensitive enough to detect the lowest parasite densities that can cause malaria. Serologic tests, another option, could be applied either selectively (to high-risk donors) or universally. Since the presence of antibodies does not necessarily indicate the presence of parasitemia, 10 serologic screening would result in the exclusion of some uninfected donors but overall would probably increase the amount of blood available, as was noted in several European countries when serologic testing was used to screen donors who had traveled to malarious areas. 33,34 Although transfusion-transmitted malaria is reported rarely, current exclusion practices in the United States result in the loss of many potential donors. Among the 535,211 donations excluded in 1998 by blood-collection centers affiliated with America s Blood Centers (a national network of nonprofit, independent community blood centers that collect 47 percent of the U.S. blood supply), 15,338 (2.9 percent) were excluded because of the potential risk of malaria. If these proportions are extrapolated to the entire U.S. blood supply, an estimated 50,000 donations annually (of approximately 13 million donations) are excluded because the donor had a history of travel to a malarious area (Bianco C: personal communication). An analysis of the cost and benefits of various screening procedures, which takes into account the strengths and limitations of screening tests, the rates of seropositivity for malaria in the donor population, and the proportion of blood donors with a history of travel to malarious areas, will be a useful step toward addressing this problem. We are indebted to Dr. Phuc Nguyen-Dinh for technical advice and helpful suggestions; to Marianna Wilson, Katharine Grady, Dr. Norman Pieniazek, and Susan Slemenda for laboratory support at the CDC; to Kay Gregory at the American Association of Blood Banks for assistance in obtaining prior editions of the donor-exclusion guidelines; and to Drs. Mark Heintzelman, Jay Epstein, and Chiang Syin at the FDA for their collaboration. REFERENCES 1. Guerrero IC, Weniger BC, Schultz MG. Transfusion malaria in the United States, Ann Intern Med 1983;99: Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. Transfusion medicine. 1. Blood transfusion. N Engl J Med 1999;340: Busch MP. HIV, HBV, and HCV: new developments related to transfusion safety. Vox Sang 2000;78:Suppl 2: McQuiston JH, Childs JE, Chamberland ME, Tabor E. Transmission of tick-borne agents of disease by blood transfusion: a review of known and potential risks in the United States. Transfusion 2000;40: Linden JV, Wong SJ, Chu FK, Schmidt GB, Bianco C. Transfusionassociated transmission of babesiosis in New York State. Transfusion 2000; 40: Zoon K. Recommendations for deferral of donors for malaria risk: letter to all registered blood establishments. Washington, D.C.: Food and Drug Administration, July Draft guidance for industry: recommendations for donor questioning regarding possible exposure to malaria availability. Fed Regist 2000; 65(111): Standards for blood banks and transfusion services. 19th ed. Bethesda, Md.: American Association of Blood Banks, Transfusion-transmitted malaria Missouri and Pennsylvania, MMWR Morb Mortal Wkly Rep 1999;48: Sulzer AJ, Wilson M. The indirect fluorescent antibody test for the detection of occult malaria in blood donors. Bull World Health Organ 1971; 45: Lopez CE, Schultz MG. Incidence of transfusion malaria and standards for blood donor selection. J Infect Dis 1977;135: Surgenor DM, Wallace EL, Hao SHS, Chapman RH. Collection and transfusion of blood in the United States, N Engl J Med 1990;322: Wallace EL, Surgenor DM, Hao HS, An J, Chapman RH, Churchill WH. Collection and transfusion of blood and blood components in the United States, Transfusion 1993;33: Wallace EL, Churchill WH, Surgenor J, et al. Collection and transfusion of blood and blood components in the United States, Transfusion 1995;35: Wallace EL, Churchill WH, Surgenor DM, Cho GS, McGurk S. Collection and transfusion of blood and blood components in the United States, Transfusion 1998;38: Blood supply: transfusion-associated risks. Washington, D.C.: General Accounting Office, February (GAO/PEMD-97-2.) 17. Vamvakas EC, Taswell HF. Epidemiology of blood transfusion. Transfusion 1994;34: Gilles HM. Epidemiology of malaria. In: Gilles HM, Warrell DA, eds. Essential malariology. 3rd ed. London: Arnold, 1993: Bruce-Chwatt LJ. Transfusion malaria. Bull World Health Organ 1974; 50: Idem. Transfusion malaria revisited. Trop Dis Bull 1982;79: Association bulletin no Vol : (Bethesda, Md.: American Association of Blood Banks.) 22. Miller LH. Transfusion malaria. In: Greenwalt TJ, Jamieson GA, eds. Transmissible disease and blood transfusion. New York: Grune & Stratton, 1975: Williams HA, Roberts J, Kachur SP, et al. Malaria surveillance United States, MMWR CDC Surveill Summ 1999;48(1): Besson P, Robert JF, Reviron J, Richard-Lenoble D, Gentilini M. A propos de deux observations du paludisme transfusionnel: essai de prévention associant un test d immunofluorescence indirecte aux critères de sélection clinique. Rev Fr Transfus Immunohematol 1976;19: Nahlen BL, Lobel HO, Cannon SE, Campbell CC. Reassessment of blood donor selection criteria for United States travelers to malarious areas. Transfusion 1991;31: Talib VH, Prakash I. Transfusion malaria. Indian J Pathol Microbiol 1996;39: Shulman IA. Parasitic infections and their impact on blood donor selection and testing. Arch Pathol Lab Med 1994;118: Turc JM. Malaria and blood transfusion. In: Westphal RG, Carlson KB, Turc JM, eds. Emerging global patterns in transfusion-transmitted infections. Arlington, Va.: American Association of Blood Banks, 1990: Beadle C, Long GW, Weiss WR, et al. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet 1994;343: Piper R, Lebras J, Wentworth L, et al. Immunocapture diagnostic assays for malaria using Plasmodium lactate dehydrogenase (pldh). Am J Trop Med Hyg 1999;60: Kachur SP, Bloland PB. Malaria. In: Wallace RB, ed. Maxcy Rosenau Last public health and preventive medicine. 14th ed. Stamford, Conn.: Appleton & Lange, 1998: Vu TT, Tran VB, Phan NT, et al. Screening donor blood for malaria by polymerase chain reaction. Trans R Soc Trop Med Hyg 1995;89: Brasseur P, Bonneau J-C. Le paludisme transfusionnel: risque, prévention et coût (expérience d un année). Revue Francaise de Transfusion et Hematologie 1981;24: Chiodini PL, Hartley S, Hewitt PE, et al. Evaluation of a malaria antibody ELISA and its value in reducing potential wastage of red cell donations from blood donors exposed to malaria, with a note on a case of transfusion-transmitted malaria. Vox Sang 1997;73: Copyright 2001 Massachusetts Medical Society N Engl J Med, Vol. 344, No. 26 June 28,
Reducing Babesia and Malaria Risks: Testing, Donor Selection, Inactivation. Susan L. Stramer PhD IPFA 23 rd Meeting May
 Reducing Babesia and Malaria Risks: Testing, Donor Selection, Inactivation Susan L. Stramer PhD IPFA 23 rd Meeting May 26 2016 Babesiosis Malaria-like illness caused by babesia spp. Asymptomatic fatal
Reducing Babesia and Malaria Risks: Testing, Donor Selection, Inactivation Susan L. Stramer PhD IPFA 23 rd Meeting May 26 2016 Babesiosis Malaria-like illness caused by babesia spp. Asymptomatic fatal
Interventions for Babesia (and Plasmodium)
 Interventions for Babesia (and Plasmodium) Susan L. Stramer, Ph.D. May 16 2017 www.aabb.org Babesiosis Malaria-like illness caused by Babesia spp. Asymptomatic fatal Non-specific symptoms (malaise, fever,
Interventions for Babesia (and Plasmodium) Susan L. Stramer, Ph.D. May 16 2017 www.aabb.org Babesiosis Malaria-like illness caused by Babesia spp. Asymptomatic fatal Non-specific symptoms (malaise, fever,
EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS
 EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits
EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits
Babesia from a donor perspective
 Babesia from a donor perspective American Red Cross, Massachusetts Region Bryan Spencer, MPH Research Scientist American Society for Apheresis Annual Meeting May 8, 2015 San Antonio, TX The need is constant.
Babesia from a donor perspective American Red Cross, Massachusetts Region Bryan Spencer, MPH Research Scientist American Society for Apheresis Annual Meeting May 8, 2015 San Antonio, TX The need is constant.
How to design intranational deferral Malaria in Greece
 How to design intranational deferral Malaria in Greece Constantina Politis Coordinating Haemovigilance Centre (SKAE) Hellenic Centre for Disease Control and Prevention (KEELPNO) Malaria - Key Facts Malaria
How to design intranational deferral Malaria in Greece Constantina Politis Coordinating Haemovigilance Centre (SKAE) Hellenic Centre for Disease Control and Prevention (KEELPNO) Malaria - Key Facts Malaria
Outbreak Investigation Guidance for Vectorborne Diseases
 COMMUNICABLE DISEASE OUTBREAK MANUAL New Jersey s Public Health Response APPENDIX T3: EXTENDED GUIDANCE Outbreak Investigation Guidance for Vectorborne Diseases As per N.J.A.C. 8:57, viruses that are transmitted
COMMUNICABLE DISEASE OUTBREAK MANUAL New Jersey s Public Health Response APPENDIX T3: EXTENDED GUIDANCE Outbreak Investigation Guidance for Vectorborne Diseases As per N.J.A.C. 8:57, viruses that are transmitted
PARASITOLOGY CASE HISTORY #14 (BLOOD PARASITES) (Lynne S. Garcia)
 PARASITOLOGY CASE HISTORY #14 (BLOOD PARASITES) (Lynne S. Garcia) A 37-year-old woman, who had traveled to New Guinea for several weeks, presented to the medical clinic with fever, chills, and rigors within
PARASITOLOGY CASE HISTORY #14 (BLOOD PARASITES) (Lynne S. Garcia) A 37-year-old woman, who had traveled to New Guinea for several weeks, presented to the medical clinic with fever, chills, and rigors within
 Malaria parasites Malaria parasites are micro-organisms that belong to the genus Plasmodium. There are more than 100 species of Plasmodium, which can infect many animal species such as reptiles, birds,
Malaria parasites Malaria parasites are micro-organisms that belong to the genus Plasmodium. There are more than 100 species of Plasmodium, which can infect many animal species such as reptiles, birds,
T he diagnosis of malaria in clinical laboratories in the UK
 862 ORIGINAL ARTICLE Use of rapid diagnostic tests for diagnosis of malaria in the UK D Chilton, A N J Malik, M Armstrong, M Kettelhut, J Parker-Williams, P L Chiodini... See end of article for authors
862 ORIGINAL ARTICLE Use of rapid diagnostic tests for diagnosis of malaria in the UK D Chilton, A N J Malik, M Armstrong, M Kettelhut, J Parker-Williams, P L Chiodini... See end of article for authors
Persons with Early Syphilis Identified through Blood or Plasma Donation Screening in the United States
 545 CONCISE COMMUNICATION Persons with Early Syphilis Identified through Blood or Plasma Donation Screening in the United States Carolyn Gardella, 1,2 Anthony A. Marfin, 1,a Richard H. Kahn, 1 Emmett Swint,
545 CONCISE COMMUNICATION Persons with Early Syphilis Identified through Blood or Plasma Donation Screening in the United States Carolyn Gardella, 1,2 Anthony A. Marfin, 1,a Richard H. Kahn, 1 Emmett Swint,
Cases of Severe Malaria and Cerebral Malaria in Apam Catholic Hospital and Manhiya District Hospital
 Cases of Severe Malaria and Cerebral Malaria in Apam Catholic Hospital and Manhiya District Hospital CL Barba, MSIV Charles Drew University of Medicine and Science Los Angeles, CA QUESTION What pediatric
Cases of Severe Malaria and Cerebral Malaria in Apam Catholic Hospital and Manhiya District Hospital CL Barba, MSIV Charles Drew University of Medicine and Science Los Angeles, CA QUESTION What pediatric
Malaria screening of donors and donations - issues and outcomes
 Malaria screening of donors and donations - issues and outcomes lan Kitchen National Transfusion Microbiology NHS Blood & Transplant London IPF/PEI, Rome, May 2014 Blood and Transplant Key elements Nature
Malaria screening of donors and donations - issues and outcomes lan Kitchen National Transfusion Microbiology NHS Blood & Transplant London IPF/PEI, Rome, May 2014 Blood and Transplant Key elements Nature
Copyright, 1995, by the Massachusetts Medical Society
 Copyright, 1995, by the Massachusetts Medical Society Volume 333 DECEMBER 28, 1995 Number 26 ESTIMATED RISK OF TRANSMISSION OF THE HUMAN IMMUNODEFICIENCY VIRUS BY SCREENED BLOOD IN THE UNITED STATES EVE
Copyright, 1995, by the Massachusetts Medical Society Volume 333 DECEMBER 28, 1995 Number 26 ESTIMATED RISK OF TRANSMISSION OF THE HUMAN IMMUNODEFICIENCY VIRUS BY SCREENED BLOOD IN THE UNITED STATES EVE
Update on Transfusion- Transmitted Infectious Diseases
 Update on Transfusion- Transmitted Infectious Diseases Scott Koepsell MD PhD Associate Professor University of Nebraska Medical Center I have no conflicts of interest to disclose. Global changes and the
Update on Transfusion- Transmitted Infectious Diseases Scott Koepsell MD PhD Associate Professor University of Nebraska Medical Center I have no conflicts of interest to disclose. Global changes and the
Guidance for Industry
 Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Surveillance Report 2014
 Surveillance Report 2014 Executive summary We are pleased to present the third report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Surveillance Report 2014 Executive summary We are pleased to present the third report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Alberta Health Public Health Notifiable Disease Management Guidelines July 2012
 July 2012 Malaria Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) Case Definition Confirmed Case Laboratory confirmation
July 2012 Malaria Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) Case Definition Confirmed Case Laboratory confirmation
DHQ Flowcharts v2.0 eff. February 2016
 Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Page 1 of 9 13-ID ID-08. Public Health Reporting and National Notification for Malaria
 Committee: Infectious Disease Title: Public Health Reporting and National Notification for Malaria I. tatement of the Problem: Malaria is one of the nationally notifiable conditions for which CTE has a
Committee: Infectious Disease Title: Public Health Reporting and National Notification for Malaria I. tatement of the Problem: Malaria is one of the nationally notifiable conditions for which CTE has a
Original article J Bas Res Med Sci 2017; 4(2):4-8.
 Prevalence of HIV, hepatitis B and C infections among volunteer blood donors at the blood transfusion center of Ilam city, Iran Hasan Boustani 1, Enayat Anvari 2, Sedighe Saiadi Sartang 2, Mehdi Omidi
Prevalence of HIV, hepatitis B and C infections among volunteer blood donors at the blood transfusion center of Ilam city, Iran Hasan Boustani 1, Enayat Anvari 2, Sedighe Saiadi Sartang 2, Mehdi Omidi
EUROPEAN COMMISSION DIRECTORATE GENERAL FOR HEALTH AND FOOD SAFETY
 Ref. Ares(2016)5909621-13/10/2016 EUROPEAN COMMISSION DIRECTORATE GENERAL FOR HEALTH AND FOOD SAFETY Directorate B - Health systems, medical products and innovation B4 Medical products: quality, safety
Ref. Ares(2016)5909621-13/10/2016 EUROPEAN COMMISSION DIRECTORATE GENERAL FOR HEALTH AND FOOD SAFETY Directorate B - Health systems, medical products and innovation B4 Medical products: quality, safety
EPIDEMIOLOGICAL SURVEILLANCE REPORT Malaria in Greece, 2012
 HELLENIC CENTRE FOR DISEASE CONTROL AND PREVENTION MINISTRY OF HEALTH Page 1 EPIDEMIOLOGICAL SURVEILLANCE REPORT Malaria in Greece, 2012 Introduction Malaria is a parasitic infection, transmitted through
HELLENIC CENTRE FOR DISEASE CONTROL AND PREVENTION MINISTRY OF HEALTH Page 1 EPIDEMIOLOGICAL SURVEILLANCE REPORT Malaria in Greece, 2012 Introduction Malaria is a parasitic infection, transmitted through
Uses and Misuses of Viral Hepatitis Testing. Origins of Liver Science
 Uses and Misuses of Viral Hepatitis Testing Richard S Lang, MD, MPH, FACP Chairman, Preventive Medicine Vice-Chair, Wellness Institute Raul J Seballos, MD, FACP Vice-Chair, Preventive Medicine Wellness
Uses and Misuses of Viral Hepatitis Testing Richard S Lang, MD, MPH, FACP Chairman, Preventive Medicine Vice-Chair, Wellness Institute Raul J Seballos, MD, FACP Vice-Chair, Preventive Medicine Wellness
HEPATITIS C, ACUTE CRUDE DATA. Number of Cases 5 Annual Incidence a LA County 0.05 California b 0.10 United States b 0.68 Age at Diagnosis Mean 38
 2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
Guidance for Investigation and Management of Zika Virus Infection
 Guidance for Investigation and Management of Zika Virus Infection Update: February 11, 2016 Public Health Agency of Canada has issued recommendations from the Committee to Advise on Tropical Medicine and
Guidance for Investigation and Management of Zika Virus Infection Update: February 11, 2016 Public Health Agency of Canada has issued recommendations from the Committee to Advise on Tropical Medicine and
Epidemiology Update Hepatitis A
 December 2011 Epidemiology Update Hepatitis A Hepatitis A Key Points Between 2000 and 2010, 209 cases of hepatitis A were reported in Hennepin County residents. This represents 30% of the cases reported
December 2011 Epidemiology Update Hepatitis A Hepatitis A Key Points Between 2000 and 2010, 209 cases of hepatitis A were reported in Hennepin County residents. This represents 30% of the cases reported
Guidance for Industry
 Guidance for Industry Lookback for Hepatitis C Virus (HCV): Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test
Guidance for Industry Lookback for Hepatitis C Virus (HCV): Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test
Eliminating Chronic Hepatitis B Disparities among Asian Pacific Islanders: A Model for Transforming Public Health in the Pacific
 Eliminating Chronic Hepatitis B Disparities among Asian Pacific Islanders: A Model for Transforming Public Health in the Pacific Augustina Manuzak, MD, MPH, PhD Augustina.manuzak@doh.hawaii.gov 10/10/2012
Eliminating Chronic Hepatitis B Disparities among Asian Pacific Islanders: A Model for Transforming Public Health in the Pacific Augustina Manuzak, MD, MPH, PhD Augustina.manuzak@doh.hawaii.gov 10/10/2012
EPIDEMIOLOGICAL SURVEILLANCE REPORT Malaria in Greece, 2018, up to 22/10/2018
 Page 1 EPIDEMIOLOGICAL SURVEILLANCE REPORT Malaria in Greece, 2018, up to 22/10/2018 Introduction Greece was declared free from malaria in 1974, following an intense control program (1946-1960). Since
Page 1 EPIDEMIOLOGICAL SURVEILLANCE REPORT Malaria in Greece, 2018, up to 22/10/2018 Introduction Greece was declared free from malaria in 1974, following an intense control program (1946-1960). Since
Emerging TTIs How Singapore secure its blood supply
 Emerging TTIs How Singapore secure its blood supply Ms Sally Lam Acting Division Director I Blood Supply Management I Blood Services Group I Health Sciences Outline Risk Mitigation Strategies to secure
Emerging TTIs How Singapore secure its blood supply Ms Sally Lam Acting Division Director I Blood Supply Management I Blood Services Group I Health Sciences Outline Risk Mitigation Strategies to secure
23 November Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P
 The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P Record Status This is a critical abstract of an economic evaluation that meets
The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P Record Status This is a critical abstract of an economic evaluation that meets
Screening donors and donations for transfusion transmissible infectious agents. Alan Kitchen
 Screening donors and donations for transfusion transmissible infectious agents Alan Kitchen Aim Not to teach you microbiology To provide and awareness of the big picture To provide an understanding of
Screening donors and donations for transfusion transmissible infectious agents Alan Kitchen Aim Not to teach you microbiology To provide and awareness of the big picture To provide an understanding of
Invest in the future, defeat malaria
 Invest in the future, defeat malaria Malaria is caused by parasites from the genus Plasmodium, which are spread to people by infected mosquitoes. There are five species of Plasmodium that can infect humans.
Invest in the future, defeat malaria Malaria is caused by parasites from the genus Plasmodium, which are spread to people by infected mosquitoes. There are five species of Plasmodium that can infect humans.
Babesia and Blood Safety
 Babesia and Blood Safety American Red Cross, New England Region Bryan Spencer, MPH Manager of Blood Research / REDS-III Coordinator ASM 46 th Annual Region I Meeting 27 October, 2011 Randolph, MA The need
Babesia and Blood Safety American Red Cross, New England Region Bryan Spencer, MPH Manager of Blood Research / REDS-III Coordinator ASM 46 th Annual Region I Meeting 27 October, 2011 Randolph, MA The need
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING October2010. Issue Summary
 TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
International Journal of Pharma and Bio Sciences
 Research Article Medical Microbiology International Journal of Pharma and Bio Sciences ISSN 0975-6299 COMPARISON OF RAPID IMMUNOCHROMATOGRAPHIC ASSAYS (ICT MALARIA P.F. /P.V. TEST AND OPTIMAL TEST) WITH
Research Article Medical Microbiology International Journal of Pharma and Bio Sciences ISSN 0975-6299 COMPARISON OF RAPID IMMUNOCHROMATOGRAPHIC ASSAYS (ICT MALARIA P.F. /P.V. TEST AND OPTIMAL TEST) WITH
Malaria in London: Review of imported malaria cases. Data from 2000 to 2012
 : Review of imported malaria cases Data from 2000 to 2012 About Public Health England We work with national and local government, industry and the NHS to protect and improve the nation's health and support
: Review of imported malaria cases Data from 2000 to 2012 About Public Health England We work with national and local government, industry and the NHS to protect and improve the nation's health and support
Changing eligibility criteria for MSM: The Canadian Perspective
 Changing eligibility criteria for MSM: The Canadian Perspective Mindy Goldman MD Canadian Blood Services ASFA & WAA Joint Meeting, San Francisco April 4, 2014 Outline - Historical background - International
Changing eligibility criteria for MSM: The Canadian Perspective Mindy Goldman MD Canadian Blood Services ASFA & WAA Joint Meeting, San Francisco April 4, 2014 Outline - Historical background - International
DHQ v2.0, February 2016 Table Detailing Changes from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you
 DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
Prevalence of malaria as co-infection in HIV-infected individuals in a malaria endemic area of southeastern Nigeria
 J Vector Borne Dis 44, December 2007, pp. 250 254 Prevalence of malaria as co-infection in HIV-infected individuals in a malaria endemic area of southeastern Nigeria C.C. Onyenekwe a, N. Ukibe a, S.C.
J Vector Borne Dis 44, December 2007, pp. 250 254 Prevalence of malaria as co-infection in HIV-infected individuals in a malaria endemic area of southeastern Nigeria C.C. Onyenekwe a, N. Ukibe a, S.C.
Maximizing Cornea and Tissue Donation through Specimen Quality
 Maximizing Cornea and Tissue Donation through Specimen Quality Robert W. Bresler, Sydney D. Gastreich, Elias G. Koulouriotis, Linda S. Martin, Susan Diane Brockmeier, Chak-Sum Ho, PhD Abstract Purpose:
Maximizing Cornea and Tissue Donation through Specimen Quality Robert W. Bresler, Sydney D. Gastreich, Elias G. Koulouriotis, Linda S. Martin, Susan Diane Brockmeier, Chak-Sum Ho, PhD Abstract Purpose:
Malaria is a serious and potentially fatal infection. It is
 A Regional Centralized Microbiology Service in Calgary for the Rapid Diagnosis of Malaria Deirdre L. Church, MD, PhD, FRCPC; Angelika Lichtenfeld, MLT, ART; Sameer Elsayed, MD, FRCPC; Susan Kuhn, MD, FRCPC;
A Regional Centralized Microbiology Service in Calgary for the Rapid Diagnosis of Malaria Deirdre L. Church, MD, PhD, FRCPC; Angelika Lichtenfeld, MLT, ART; Sameer Elsayed, MD, FRCPC; Susan Kuhn, MD, FRCPC;
Implementation: To be determined by each Service. Change Notification UK National Blood Services No
 Issued by JPAC: 18 July 2017 Implementation: To be determined by each Service Change Notification UK National Blood Services No. 17-2017 Malaria These changes apply to the individual Tissue and Cells Donor
Issued by JPAC: 18 July 2017 Implementation: To be determined by each Service Change Notification UK National Blood Services No. 17-2017 Malaria These changes apply to the individual Tissue and Cells Donor
Viral Hepatitis. Background
 Viral Hepatitis Background Hepatitis or inflammation of the liver can be caused by infectious and noninfectious problems. Infectious etiologies include viruses, bacteria, fungi and parasites. Noninfectious
Viral Hepatitis Background Hepatitis or inflammation of the liver can be caused by infectious and noninfectious problems. Infectious etiologies include viruses, bacteria, fungi and parasites. Noninfectious
Routine Use of Mini-Pool Nucleic Acid Testing (MP-NAT) Multiplex Assay for Sero-Negative Blood Donors
 Journal of the Egyptian Society of Haematology & Research, Vol. 7, No. 2, September: 1-5, 2011 Routine Use of Mini-Pool Nucleic Acid Testing (MP-NAT) Multiplex Assay for Sero-Negative Blood Donors HISHAM
Journal of the Egyptian Society of Haematology & Research, Vol. 7, No. 2, September: 1-5, 2011 Routine Use of Mini-Pool Nucleic Acid Testing (MP-NAT) Multiplex Assay for Sero-Negative Blood Donors HISHAM
Hepatitis B: A Preventable Cause of Liver Cancer. Saira Khaderi MD, MPH Assistant Professor of Surgery Associate Director, Project ECHO June 17, 2016
 Hepatitis B: A Preventable Cause of Liver Cancer Saira Khaderi MD, MPH Assistant Professor of Surgery Associate Director, Project ECHO June 17, 2016 Overview Epidemiology HBV and cancer Screening, Diagnosis
Hepatitis B: A Preventable Cause of Liver Cancer Saira Khaderi MD, MPH Assistant Professor of Surgery Associate Director, Project ECHO June 17, 2016 Overview Epidemiology HBV and cancer Screening, Diagnosis
Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey
 Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey This questionnaire consists of three sections: A. Collection and
Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey This questionnaire consists of three sections: A. Collection and
July 14, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 July 14, 2015 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2015-D-1211
July 14, 2015 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2015-D-1211
CTYOMEGALOVIRUS (CMV) - BACKGROUND
 CTYOMEGALOVIRUS (CMV) - BACKGROUND PURPOSE The flowing information provides guidance on the use of CMV negative blood components provided by the blood bank at the Royal Children s Hospital (RCH) including
CTYOMEGALOVIRUS (CMV) - BACKGROUND PURPOSE The flowing information provides guidance on the use of CMV negative blood components provided by the blood bank at the Royal Children s Hospital (RCH) including
Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services
 Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services Outline 1 Current criteria Proposed criteria Stakeholder outreach Outline 2 Safety Assessment Results since implementation
Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services Outline 1 Current criteria Proposed criteria Stakeholder outreach Outline 2 Safety Assessment Results since implementation
Road map for malaria control (and elimination): public health priorities
 Road map for malaria control (and elimination): public health priorities Anne E McCarthy, MD, FRCPC, DTM&H Director Tropical Medicine and International Health Clinic Ottawa Hospital Professor of Medicine
Road map for malaria control (and elimination): public health priorities Anne E McCarthy, MD, FRCPC, DTM&H Director Tropical Medicine and International Health Clinic Ottawa Hospital Professor of Medicine
New Brunswick Report on Sexually Transmitted and Blood Borne Infections, 2016
 New Brunswick Report on Sexually Transmitted and Blood Borne Infections, 6 Table of Contents. Introduction.... Methodology... 3. Data Limitations.... Definitions used... 3 5. Overview of STBBI epidemiology
New Brunswick Report on Sexually Transmitted and Blood Borne Infections, 6 Table of Contents. Introduction.... Methodology... 3. Data Limitations.... Definitions used... 3 5. Overview of STBBI epidemiology
Title: Public Health Reporting and National Surveillance for Babesiosis
 10-ID-27 Committee: Infectious Disease Title: Public Health Reporting and National Surveillance for Babesiosis I. Statement of the Problem: The increasing incidence of reported cases of babesiosis, the
10-ID-27 Committee: Infectious Disease Title: Public Health Reporting and National Surveillance for Babesiosis I. Statement of the Problem: The increasing incidence of reported cases of babesiosis, the
June 8, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
Epidemiology of blood-borne Infections in Vietnam
 IPFA 4 th Asia Workshop on Plasma Quality and Supply Epidemiology of blood-borne Infections in Vietnam Nguyen Thị Lan Anh MD, PhD Center for Bio-Medical Rsearch Screening donated blood for infections:
IPFA 4 th Asia Workshop on Plasma Quality and Supply Epidemiology of blood-borne Infections in Vietnam Nguyen Thị Lan Anh MD, PhD Center for Bio-Medical Rsearch Screening donated blood for infections:
Hira L. Nakhasi, Ph.D. CBER/USFDA July 3 rd 2015
 Strategies for Implementation of Antibody and Nucleic Acid-based Testing for Babesia microti in Blood Donations: Summary of May 13 th 2015 Blood Product Advisory Committee Meeting Hira L. Nakhasi, Ph.D.
Strategies for Implementation of Antibody and Nucleic Acid-based Testing for Babesia microti in Blood Donations: Summary of May 13 th 2015 Blood Product Advisory Committee Meeting Hira L. Nakhasi, Ph.D.
Prevalence of Malaria in Blood Donors and Risk of Transfusion Transmissible Malaria
 ISSN: 2319-7706 Volume 4 Number 8 (2015) pp. 352-357 http://www.ijcmas.com Original Research Article Prevalence of Malaria in Blood Donors and Risk of Transfusion Transmissible Malaria Subh Lakshmi* and
ISSN: 2319-7706 Volume 4 Number 8 (2015) pp. 352-357 http://www.ijcmas.com Original Research Article Prevalence of Malaria in Blood Donors and Risk of Transfusion Transmissible Malaria Subh Lakshmi* and
Policy Document. Blood Donation Deferral. Background
 Policy Document Blood Donation Deferral Background The Australian Medical Students Association (AMSA) is the peak representative body for medical students in Australia. Accordingly, AMSA advocates on issues
Policy Document Blood Donation Deferral Background The Australian Medical Students Association (AMSA) is the peak representative body for medical students in Australia. Accordingly, AMSA advocates on issues
Hepatitis E in developing countries
 Hepatitis E in developing countries Hepatitis E in developing countries Rakesh Aggarwal Department of Gastroenterology Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India Forms Epidemic
Hepatitis E in developing countries Hepatitis E in developing countries Rakesh Aggarwal Department of Gastroenterology Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India Forms Epidemic
Seroprevalence of Babesia microti in Individuals with Lyme Disease. Sabino R. Curcio, M.S, MLS(ASCP)
 Seroprevalence of Babesia microti in Individuals with Lyme Disease Sabino R. Curcio, M.S, MLS(ASCP) Lyme Disease Most common vectorborne illness in the United States Caused by the tick-transmitted spirochete
Seroprevalence of Babesia microti in Individuals with Lyme Disease Sabino R. Curcio, M.S, MLS(ASCP) Lyme Disease Most common vectorborne illness in the United States Caused by the tick-transmitted spirochete
Question: 1. Are you currently taking an antibiotic?
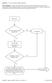 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Influenza-Associated Pediatric Mortality rev Jan 2018
 rev Jan 2018 Infectious Agent Influenza A, B or C virus BASIC EPIDEMIOLOGY Transmission Transmission occurs via droplet spread. After a person infected with influenza coughs, sneezes, or talks, influenza
rev Jan 2018 Infectious Agent Influenza A, B or C virus BASIC EPIDEMIOLOGY Transmission Transmission occurs via droplet spread. After a person infected with influenza coughs, sneezes, or talks, influenza
EPIDEMIOLOGICAL SURVEILLANCE REPORT Malaria in Greece, 2017, up to 17/08/2017
 Page 1 EPIDEMIOLOGICAL SURVEILLANCE REPORT Malaria in Greece, 2017, up to 17/08/2017 Introduction Greece was declared free from malaria in 1974, following an intense control program (1946-1960). Since
Page 1 EPIDEMIOLOGICAL SURVEILLANCE REPORT Malaria in Greece, 2017, up to 17/08/2017 Introduction Greece was declared free from malaria in 1974, following an intense control program (1946-1960). Since
DEPARTMENT OF HEALTH AND HUMAN SERVICES CENTERS FOR DISEASE CONTROL AND PREVENTION SAFTER HEALTHIER PEOPLE TM
 MALARIA TREATMENT GUIDELINES Treatment of Malaria (Guidelines For Clinicians) If you wish to share your clinical experience, please contact us at: nciddpdmalaria@cdc.gov Treatment Table The Treatment Table
MALARIA TREATMENT GUIDELINES Treatment of Malaria (Guidelines For Clinicians) If you wish to share your clinical experience, please contact us at: nciddpdmalaria@cdc.gov Treatment Table The Treatment Table
3. Rapidly recognize influenza seasons in which the impact of influenza appears to be unusually severe among children.
 07-ID-14 Committee: Title: Infectious Disease Influenza-Associated Pediatric Mortality Statement of the Problem: In 2004, CSTE adopted influenza-associated pediatric mortality reporting with a provision
07-ID-14 Committee: Title: Infectious Disease Influenza-Associated Pediatric Mortality Statement of the Problem: In 2004, CSTE adopted influenza-associated pediatric mortality reporting with a provision
Transfusion transmitted infections in National Haemovigilance Systems: the Greek experience
 Transfusion transmitted infections in National Haemovigilance Systems: the Greek experience Global blood product safety 10 April 2019, Rome, Italy Constantina Politis National Coordinating Haemovigilance
Transfusion transmitted infections in National Haemovigilance Systems: the Greek experience Global blood product safety 10 April 2019, Rome, Italy Constantina Politis National Coordinating Haemovigilance
Hospitalization Criteria in Imported Falciparum Malaria
 306 Hospitalization Criteria in Imported Falciparum Malaria Valérie Briand, MD, MPH, * Olivier Bouchaud, MD, PhD, Jérôme Tourret, MD, Charlotte Behr, PhD, Sophie Abgrall, MD, PhD, Pascal Ralaimazava, MD,
306 Hospitalization Criteria in Imported Falciparum Malaria Valérie Briand, MD, MPH, * Olivier Bouchaud, MD, PhD, Jérôme Tourret, MD, Charlotte Behr, PhD, Sophie Abgrall, MD, PhD, Pascal Ralaimazava, MD,
Updates in Infectious Diseases. Kelley Struble, DO, MS St. John Physicians Infectious Disease September 30, 2016
 Updates in Infectious Diseases Kelley Struble, DO, MS St. John Physicians Infectious Disease September 30, 2016 Disclosures No financial relationships or affiliations to disclose Overview Activity Pre-Test
Updates in Infectious Diseases Kelley Struble, DO, MS St. John Physicians Infectious Disease September 30, 2016 Disclosures No financial relationships or affiliations to disclose Overview Activity Pre-Test
INTRODUCTION PATIENTS, MATERIALS, AND METHODS
 Am. J. Trop. Med. Hyg., 66(5), 2002, pp. 492 502 Copyright 2002 by The American Society of Tropical Medicine and Hygiene A RETROSPECTIVE EXAMINATION OF SPOROZOITE-INDUCED AND TROPHOZOITE-INDUCED INFECTIONS
Am. J. Trop. Med. Hyg., 66(5), 2002, pp. 492 502 Copyright 2002 by The American Society of Tropical Medicine and Hygiene A RETROSPECTIVE EXAMINATION OF SPOROZOITE-INDUCED AND TROPHOZOITE-INDUCED INFECTIONS
Media centre. WHO Hepatitis B. Key facts. 1 of :12 AM.
 1 of 5 2013-08-02 7:12 AM Media centre Hepatitis B Share Print Fact sheet N 204 Updated July 2013 Key facts Hepatitis B is a viral infection that attacks the liver and can cause both acute and chronic
1 of 5 2013-08-02 7:12 AM Media centre Hepatitis B Share Print Fact sheet N 204 Updated July 2013 Key facts Hepatitis B is a viral infection that attacks the liver and can cause both acute and chronic
DENGUE AND BLOOD SAFETY. Ester C Sabino, MD, PhD Dep. of Infectious Disease/Institute of Tropical Medicine University of São Paulo
 DENGUE AND BLOOD SAFETY Ester C Sabino, MD, PhD Dep. of Infectious Disease/Institute of Tropical Medicine University of São Paulo Dengue virus Arbovirus (arthropod-borne virus) virus transmitted by mosquitoes:
DENGUE AND BLOOD SAFETY Ester C Sabino, MD, PhD Dep. of Infectious Disease/Institute of Tropical Medicine University of São Paulo Dengue virus Arbovirus (arthropod-borne virus) virus transmitted by mosquitoes:
Zika Virus. Report to the Standing Committee on Health. Dr. Graham Sher Chief Executive Officer, Canadian Blood Services
 Dr. Graham Sher Chief Executive Officer, Services Dr. Dana Devine Chief Medical and Scientific Officer, Services Services Services is an arm s-length organization within the larger health-care system of
Dr. Graham Sher Chief Executive Officer, Services Dr. Dana Devine Chief Medical and Scientific Officer, Services Services Services is an arm s-length organization within the larger health-care system of
Clinical Policy: Diagnostic Testing for Zika Virus Reference Number: CP.MP.111 Effective Date: 06/16
 Clinical Policy: Diagnostic Testing for Zika Virus Reference Number: CP.MP.111 Effective Date: 06/16 Last Review Date: 05/16 Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: Diagnostic Testing for Zika Virus Reference Number: CP.MP.111 Effective Date: 06/16 Last Review Date: 05/16 Revision Log See Important Reminder at the end of this policy for important
WASHINGTON STATE COMMUNICABLE DISEASES OF PUBLIC HEALTH SIGNIFICANCE FOR THE CIVIL SURGEON
 WASHINGTON STATE COMMUNICABLE DISEASES OF PUBLIC HEALTH SIGNIFICANCE FOR THE CIVIL SURGEON SCOTT LINDQUIST MD MPH WASHINGTON STATE DEPARTMENT OF HEALTH STATE EPIDEMIOLOGIST FOR COMMUNICABLE DISEASES FOREIGN-BORN
WASHINGTON STATE COMMUNICABLE DISEASES OF PUBLIC HEALTH SIGNIFICANCE FOR THE CIVIL SURGEON SCOTT LINDQUIST MD MPH WASHINGTON STATE DEPARTMENT OF HEALTH STATE EPIDEMIOLOGIST FOR COMMUNICABLE DISEASES FOREIGN-BORN
Question: 1. Are you currently taking an antibiotic?
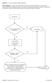 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Hot from the Tropics! Fever in the returned traveler workshop. UHN Conference 2015
 Hot from the Tropics! Fever in the returned traveler workshop UHN Conference 2015 Case 1: General approach to fever in the returning traveller Exercise 1: Location of travel and pathogens 1. Focus on what
Hot from the Tropics! Fever in the returned traveler workshop UHN Conference 2015 Case 1: General approach to fever in the returning traveller Exercise 1: Location of travel and pathogens 1. Focus on what
JMSCR Vol 05 Issue 01 Page January 2017
 www.jmscr.igmpublication.org Impact Factor 5.84 Index Copernicus Value: 83.27 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v5i1.100 Seroprevalence of Common Transfusion
www.jmscr.igmpublication.org Impact Factor 5.84 Index Copernicus Value: 83.27 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v5i1.100 Seroprevalence of Common Transfusion
HALOFANTRINE HYDROCHLORIDE - EFFICACY AND SAFETY IN CHILDREN WITH ACUTE MALARIA
 HALOFANTRINE HYDROCHLORIDE - EFFICACY AND SAFETY IN CHILDREN WITH ACUTE MALARIA Pages with reference to book, From 8 To 10 Mushtaq A. Khan, Gui Nayyer Rehman, S.A. Qazi ( Children Hospital, Pakistan Institute
HALOFANTRINE HYDROCHLORIDE - EFFICACY AND SAFETY IN CHILDREN WITH ACUTE MALARIA Pages with reference to book, From 8 To 10 Mushtaq A. Khan, Gui Nayyer Rehman, S.A. Qazi ( Children Hospital, Pakistan Institute
EPIDEMIOLOGY OF IMPORTED MALARIA CASES IN JORDAN BETWEEN 2000 AND 2005
 EPIDEMIOLOGY OF IMPORTED MALARIA CASES IN JORDAN BETWEEN 2000 AND 2005 Suleiman Meneizel MD*, Katiba Rabadi MD**, Hayel Muhareb, MD^, Ghassan Kawar MD** ABSTRACT Objectives: To determine some epidemiological
EPIDEMIOLOGY OF IMPORTED MALARIA CASES IN JORDAN BETWEEN 2000 AND 2005 Suleiman Meneizel MD*, Katiba Rabadi MD**, Hayel Muhareb, MD^, Ghassan Kawar MD** ABSTRACT Objectives: To determine some epidemiological
Armed Services Blood Program
 Armed Services Blood Program Defense Health Board Concerns Regarding the Collection and Transfusion of Non-FDA Compliant Blood Products in Theater Information Brief Defense Health Board 17 August 2009
Armed Services Blood Program Defense Health Board Concerns Regarding the Collection and Transfusion of Non-FDA Compliant Blood Products in Theater Information Brief Defense Health Board 17 August 2009
Transfusion Risk for Hepatitis B, Hepatitis C and HIV in the State of Santa Catarina, Brazil,
 236 BJID 2004; 8 (June) Transfusion Risk for Hepatitis B, Hepatitis C and HIV in the State of Santa Catarina, Brazil, 1991-2001 Emil Kupek Department of Public Health, Federal University of Santa Catarina,
236 BJID 2004; 8 (June) Transfusion Risk for Hepatitis B, Hepatitis C and HIV in the State of Santa Catarina, Brazil, 1991-2001 Emil Kupek Department of Public Health, Federal University of Santa Catarina,
Risk of Other Donor-Derived Infections (nonhiv, nonhcv) Daniel Kaul MD Associate Professor University of Michigan
 Risk of Other Donor-Derived Infections (nonhiv, nonhcv) Daniel Kaul MD Associate Professor University of Michigan Conflict of Interest Disclosure I have no relevant financial relationships to disclose
Risk of Other Donor-Derived Infections (nonhiv, nonhcv) Daniel Kaul MD Associate Professor University of Michigan Conflict of Interest Disclosure I have no relevant financial relationships to disclose
Surveillance Report 2015
 Surveillance Report 2015 Executive summary We are pleased to present the fourth report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Surveillance Report 2015 Executive summary We are pleased to present the fourth report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Annual Tuberculosis Report Oregon 2007
 Annual Tuberculosis Report Oregon 7 Oregon Department of Human Services Public Health Division TB Program April 8 Page 2 Table of Contents Charts Chart 1 TB Incidence in the US and Oregon, 1985-7.. page
Annual Tuberculosis Report Oregon 7 Oregon Department of Human Services Public Health Division TB Program April 8 Page 2 Table of Contents Charts Chart 1 TB Incidence in the US and Oregon, 1985-7.. page
Hot Topics in Tissue Safety
 Hot Topics in Tissue Safety 37 th Annual AATB Meeting National Harbor, Maryland 03 October 2013 Scott A. Brubaker, CTBS Chief Policy Officer PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis
Hot Topics in Tissue Safety 37 th Annual AATB Meeting National Harbor, Maryland 03 October 2013 Scott A. Brubaker, CTBS Chief Policy Officer PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis
In the setting of measles elimination in the United States, the current measles case definition lacks specificity.
 12-ID-07 Committee: Infectious Disease Title: Public Health Reporting and ational otification for Measles I. Statement of the Problem In the setting of measles elimination in the United States, the current
12-ID-07 Committee: Infectious Disease Title: Public Health Reporting and ational otification for Measles I. Statement of the Problem In the setting of measles elimination in the United States, the current
New recommendations for immunocompromised patients
 New recommendations for immunocompromised patients Hepatitis E Virus (HEV): Transmission, incidence and presentation Emerging evidence regarding HEV transmission from blood components and dietary consumption
New recommendations for immunocompromised patients Hepatitis E Virus (HEV): Transmission, incidence and presentation Emerging evidence regarding HEV transmission from blood components and dietary consumption
Inspections, Compliance, Enforcement, and Criminal Investigations. Central Texas Regional Blood & Tissue Center 07-Nov-03
 1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
Overview of Blood Transfusion System of Iran:
 Report Article Overview of Blood Transfusion System of Iran: 2002-2011 AM Cheraghali High Institute for Research and Education on Transfusion Medicine, Iran Blood Transfusion Organization and University
Report Article Overview of Blood Transfusion System of Iran: 2002-2011 AM Cheraghali High Institute for Research and Education on Transfusion Medicine, Iran Blood Transfusion Organization and University
Hepatitis C January 26, 2018
 Hepatitis C January 26, 2018 Case Investigation Guidelines Contents A. Purpose...2 B. Case Definitions...2 a. Acute Hepatitis C (2016...2 b. Chronic Hepatitis C (2016)...3 c. Perinatal Hepatitis C (2017
Hepatitis C January 26, 2018 Case Investigation Guidelines Contents A. Purpose...2 B. Case Definitions...2 a. Acute Hepatitis C (2016...2 b. Chronic Hepatitis C (2016)...3 c. Perinatal Hepatitis C (2017
Epidemiology of hepatitis E infection in Hong Kong
 RESEARCH FUND FOR THE CONTROL OF INFECTIOUS DISEASES Epidemiology of hepatitis E infection in Hong Kong DPC Chan *, KCK Lee, SS Lee K e y M e s s a g e s 1. The overall anti hepatitis E virus (HEV) seropositivity
RESEARCH FUND FOR THE CONTROL OF INFECTIOUS DISEASES Epidemiology of hepatitis E infection in Hong Kong DPC Chan *, KCK Lee, SS Lee K e y M e s s a g e s 1. The overall anti hepatitis E virus (HEV) seropositivity
Update: ACIP Recommendations for Hepatitis B Vaccination
 National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Update: ACIP Recommendations for Hepatitis B Vaccination Sarah Schillie, MD, MPH, MBA Summit for the Elimination of Hepatitis B and
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Update: ACIP Recommendations for Hepatitis B Vaccination Sarah Schillie, MD, MPH, MBA Summit for the Elimination of Hepatitis B and
Hepa%%s E Virus Is it a Concern?
 IPFA/PEI 17 th Workshop on Surveillance and Screening of Blood Borne Pathogens 26-27 May 2010, The Regent Esplanade Zagreb Hotel, Zagreb, Croa%a Hepa%%s E Virus Is it a Concern? Keiji Matsubayashi Hokkaido
IPFA/PEI 17 th Workshop on Surveillance and Screening of Blood Borne Pathogens 26-27 May 2010, The Regent Esplanade Zagreb Hotel, Zagreb, Croa%a Hepa%%s E Virus Is it a Concern? Keiji Matsubayashi Hokkaido
Guidance for Industry
 Reprinted from FDA s website by Guidance for Industry Recommendations for Screening, Testing, and Management of Blood Donors and Blood and Blood Components Based on Screening Tests for Syphilis DRAFT GUIDANCE
Reprinted from FDA s website by Guidance for Industry Recommendations for Screening, Testing, and Management of Blood Donors and Blood and Blood Components Based on Screening Tests for Syphilis DRAFT GUIDANCE
patients with blood borne viruses Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled Document Lead:
 CONTROLLED DOCUMENT Procedure for the management of patients with blood borne viruses CATEGORY: CLASSIFICATION: PURPOSE Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled
CONTROLLED DOCUMENT Procedure for the management of patients with blood borne viruses CATEGORY: CLASSIFICATION: PURPOSE Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled
TRAINING RELEVANT TO INTERNATIONAL TRANSFUSION SAFETY. Africa Society for Blood Transfusion (AfSBT) Mauritius 4 th -7 th June 2012
 TRAINING IN CLINICALRESEARCH RELEVANT TO INTERNATIONAL TRANSFUSION SAFETY Africa Society for Blood Transfusion (AfSBT) Mauritius 4 th -7 th June 2012 Background Research in epidemiology, virology, and
TRAINING IN CLINICALRESEARCH RELEVANT TO INTERNATIONAL TRANSFUSION SAFETY Africa Society for Blood Transfusion (AfSBT) Mauritius 4 th -7 th June 2012 Background Research in epidemiology, virology, and
Travel-Related Infections in Canadian Children
 Travel-Related Infections in Canadian Children Maryanne Crockett MD MPH FRCPC FAAP DTM&H Depts. of Pediatrics & Child Health and Medical Microbiology University of Manitoba Objectives To discuss travel-related
Travel-Related Infections in Canadian Children Maryanne Crockett MD MPH FRCPC FAAP DTM&H Depts. of Pediatrics & Child Health and Medical Microbiology University of Manitoba Objectives To discuss travel-related
*This response is constantly evolving and recommendations in this presentation may change over time, please call your district epidemiologist or a
 *This response is constantly evolving and recommendations in this presentation may change over time, please call your district epidemiologist or a GDPH epidemiologist 404-657-2588, 8-5 pm M-F for current
*This response is constantly evolving and recommendations in this presentation may change over time, please call your district epidemiologist or a GDPH epidemiologist 404-657-2588, 8-5 pm M-F for current
