The Pennsylvania State University The Graduate School Graduate Program in Integrative Biosciences PHYSIOLOGICAL AND PATHOLOGICAL ACTIVATION OF IKK AND
|
|
|
- Basil Booth
- 6 years ago
- Views:
Transcription
1 The Pennsylvania State University The Graduate School Graduate Program in Integrative Biosciences PHYSIOLOGICAL AND PATHOLOGICAL ACTIVATION OF IKK AND IKK-RELATED KINASES A Dissertation in Integrative Biosciences by Xuefeng Wu 2008 Xuefeng Wu Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy May 2008
2 ii The thesis of Xuefeng Wu was reviewed and approved* by the following: Shao-Cong Sun Professor of Immunology Thesis Co-Advisor Co-Chair of Committee Neil Christensen Professor of Pathology, and Microbiology and Immunology Thesis Co-Advisor Co-Chair of Committee Pamela Correll Associate Professor of Immunology Channe Gowda Professor of Biochemistry and Molecular Biology Jianming Hu Associate Professor of Microbiology and Immunology Peter J. Hudson Verne M. Willaman Professor of Biology Co-Director of Graduate Program in Integrative Biosciences *Signatures are on file in the Graduate School
3 iii ABSTRACT Owing to its wide range of important biological roles, nuclear factor kappa B (NF-κB) has been intensely investigated for the past two decades. Inactive NF-κB is located in the cytosol complexed with the inhibitory IκB proteins, and its nuclear translocation is induced through phosphorylation-mediated degrdation of IκB, a molecular event that in turn is mediated by IκB kinase (IKK). NF-κB activation is generally a transient process due to its tight regulation by negative feedbacks, thus timely inducing targeted genes involved in diverse biological processes. Defect in NF-κB negative regulations, due to either genetic deficiencies or pathological actions, can lead to severe diseases, such as inflammation and cancer. Thus, deciphering the negative regulation of NF-κB represents a highly significant and challenging area of NF-κB research. The primary goal of this thesis research project is to better understand the molecular mechanisms mediating the abnormal NF-κB activation. Specifically, two aspects of abnormal NF-κB activation were focused on. One was the pathological activation of NF-κB by retroviral oncoprotein Tax encoded by the human T-cell leukemia virus type I (HTLV-I). The other aspect was the genetic deficiency of a negative regulator, cyclindromatosis (CYLD). In Chapter II, I described one novel mechanism by which Tax mediates constitutive NF-κB and IKK activation. HTLV-I Tax oncoprotein persistently activates NF-κB, which is required for HTLV-I-mediated T-cell transformation. NF-κB activation by Tax has been shown through its capacity of stimulating the activity of IκB kinase (IKK), but
4 iv the underlying mechanism remained elusive. We showed here that Tax functions as an intracellular stimulator of an IKK-activating kinase, Tak1. Additionally, Tax physically interacts with Tak1 and mediates the recruitment of IKK to Tak1. In HTLV-I-infected T cells, Tak1 is constitutively activated and complexed with both Tax and IKK. We provided genetic evidence that Tak1 is essential for Tax-induced IKK activation. Interestingly, ubiquitin-binding function of IKKγ, which is essential for cellular stimuli induced NF-κB activation, is not required for Tax-specific NF-κB signaling. These findings demonstrate a pathological mechanism of IKK activation by Tax and provide an example for how IKK is persistently activated in cancer cells. The focus of Chapter III is CYLD, a deubiquitination enzyme that regulates the ubiquitination and signaling function of key components involved in activation of NFκB. By employing CYLD knockout mice, we discovered a novel function of CYLD in the regulation of antiviral responses. CYLD deficiency causes constitutive activation of the atypical IκB kinases, IKKε and TBK1, and aberrant induction of type I interferons (IFN-Is) in virus-infected cells. CYLD acts by preventing basal ubiquitination and signaling function of an IKKε/TBK1 activator, RIG-I. Interestingly, despite their competence in IFN production, the CYLD-deficient cells and mice are considerably more susceptible to viral infection. We provided genetic evidence that IFN-induced signaling and antiviral gene expression are attenuated in the absence of CYLD. These findings suggest that CYLD mediates effective and controlled antiviral responses by preventing IKKε/TBK1 deregulation and modulating IFN receptor signaling. Since IKKε/TBK1 deregulation is associated with oncogenesis, these findings also have implications on the tumor suppressor function of CYLD.
5 v Based on that CYLD negatively regulates IKKε/TBK1, Chapter IV is dedicated to investigate the crosstalk between IKKε/TBK1 and CYLD by focusing on mechanism of CYLD phosphorylation. Previously, we observed that CYLD undergoes transient phosphorylation at an IKKγ (NEMO)-dependent manner. In this chapter, I described the identification of a critical serine site, Ser418 of CYLD as an inducible phosphorylation site using mass spectrometry analysis and a phospho-specific α-cyld antibody. Mutation of serine 418 to alanine renders CYLD superactive, since expression of the CYLD S418A mutant blocks the activation of IKK and JNK by mitogens and TNFα. Thus, CYLD phosphorylation appears to serve as a mechanism that temporally inactivates its DUB function. Furthermore, genetic evidence was provided that IKKε/TBK1 mediate phosphorylation of CYLD. Finally, given that the physiological functions of CYLD phosphorylation have not been explored, I highlighted future directions to elucidating mechanism by which CYLD, as a tumor suppressor, encounters IKKε/TBK1 in the oncogenesis process in Chapter V. Taken together, investigation on the crosstalk between CYLD and IKKε/TBK1 may provide further insights into development of novel strategies for cancer prevention and treatment.
6 vi TABLE OF CONTENTS LIST OF ABBREVIATIONS..ix Page ACKNOWLEGEMENTS xiii CHAPTER I. LITERATURE REVIEW Overview of NF-κB/IKK signaling Ubiquitination and NF-κB/IKK regulation Ubiquitination and deubiquitination Ubiquitination in NF-κB/IKK regulation Abnormal NF-κB regulation by retroviral oncoprotein HTLV-I Tax Role of ubiquitination in antiviral signaling pathways Functions of CYLD in immune responses and tumorigenesis Crosstalk between typical IKK signaling and antiviral signaling pathways Identification of TBK1/IKKε as oncogenes CHAPTER II. RETROVIAL ONCOPROTEIN TAX DEREGULATES NF-κB BY ACTIVATING TAK1 AND MEDIATING TAK1-IKK PHYSICAL ASSOCIATION...31 Abstract. 32 Introduction 33 Results and Discussion..35 Materials and Methods...40
7 vii Acknowledgements.43 Figures and Legends 44 CHAPTER III. TUMOR SUPPRESSOR CYLD REGULATES ANTIVIRAL INNATE IMMUNE RESPONSE...53 Abstract 54 Introduction.55 Results...57 Discussion...64 Experimental Procedures...67 Acknowledgements...71 Figures and Legends.72 CHAPTER IV. PHOSPHORYLATION OF DUB ENZYME CYLD BY IKKε/TBK Abstract..86 Introduction 87 Materials and Methods..89 Results...92 Discussion.97 Acknowledgements...99 Figures and Legends.100
8 viii CHAPTER V. OVERVIEW AND DISCUSSION Overview of major findings Discussion Mechanism of TAK1 activation by Tax Deregulated IFN signaling caused by loss of CYLD Defined picture of CYLD phosphorylation Future plans BIBLIOGRAPHY...120
9 ix LIST OF ABBREVIATIONS ALI ATF ATL BAFF BHK bhlh acute lung injury activating transcription factor adult T-cell leukemia B cell activating factor baby hamster kidney basic helix-loop-helix Bcl10 B-cell leukemia/lymphoma 10 CARD CEC CREB CYLD DC dsrna DUB ELISA EMSA GM-CSF HCC HECT HTLV-I IB caspase recruitment domain colonic epithelial cell cyclic AMP-responsive element-binding protein cyclindromatosis dendritic cell double stranded RNA deubiquitinating Enzyme-Linked Immuno Sorbent Assay electrophoresis mobility shift assay Granulocyte/Macrophage colony stimulating factor hepatocellular carcinoma Homologous to the E6-AP Carboxyl Terminus human T-cell leukemia virus type I immunoblot
10 x IFNAR IFN-I IκB IKK IKKε IL-1R IFNα/β receptor type I interferon inhibitory κb IκB kinase inducible IκB kinase interleukine 1 receptor IL-2 interleukin 2 i.n. IP IRAK IRF i.v. intronasal immunoprecipitation IL-1 receptor-associated kinase interferon regulatory factor intravenous JAK1 janus kinase 1 JNK LPS LTβ c-jun N-terminal kinase lipopolysaccharide lymphotoxin β MALT-1 gastric mucosa associated lymphoid tissue 1 MAP MAVS mitogen-activated protein mitochondrial antiviral signalling protein Mda5 melanoma differentiation-associated gene 5 mdc MEF myeloid DC mouse embryonic fibroblast MKK6 mitogen-activated protein kinase kinase 6
11 xi MOI Multiplicity of Infection MyD88 myeloid differentiation primary response gene (88) NAK NF-κB activating kinase NAP1 NAK-associated protein 1 NEMO NF-AT NF-κB ng NIK NF-kappa B essential modulator nuclear factor of activated T cells nuclear factor κb nanogram NF-κB-inducing kinase Nod2 nucleotide-binding oligomerization domain containing 2 NTHi NZF PAI-1 PAMP pdc pfu PHH PRR RHD RIG-I RING nontypeable Haemophilus influenzae novel zinc-finger plasminogen activator inhibitor-1 pathogen-associated molecular pattern plasmacytoid DC plaque-forming unit primary human hepatocyte pattern-recognition recptor Rel homology functional domain retinoic acid induced gene I Really Interesting New Gene RIP1 receptor interacting protein 1 SRF serum responsive factor
12 xii STAT1 signal transducer and activator of transcription 1 TAB TAK1-binding protein TAK1 TGF-β activating kinase 1 TANK TRAF family member-associated NF-κB activator TBK1 TANK-binding kinase 1 TCR TGFβ TLR TNF TNFR TRADD TRIKA TRAF TRIM T2K T-cell receptor Transforming growth factor beta Toll-like receptor tumor necrosis factor tumor necrosis factor receptor TNFR-associated death domain TRAF6-regulated IKK activator TNFR-associate factor tripartite motif TRAF2-associated kinase Tyk2 Tyrosine kinase 2 UBC UEV1A ubiquitin-conjugating enzyme ubiquitin-conjugating enzyme E2 variant 1 isoform A µg microgram µl microliter VSV vesicular stomatitis virus
13 xiii ACKNOWLEGEMENTS I would like to thank my advisor Dr. Shao-Cong Sun, who guided me into immunology field from plant biology field. The work described in this thesis would not have been possible without all his help and encouragement. His hard work and dedication is truly appreciated and set a fine example for others to follow. I would also like to thank the members of my committee, Drs. Christensen, Correll, Gowda, and Hu, for the time they took to serve on my committee and for the helpful suggestions they provided. I would like to extend thanks to the members of our laboratory, both past and present. My particular thanks go to Dr. Minying Zhang, who taught me numerous techniques during my graduate study and always provided substantial support for the experiments. I would also like to thank Dr. William Reiley, who guided me during the rotation and showed the passion for scientific research. Fellow students Wei Jin, Mikyoung Chang, Ato Wright, and Andy Lee, assisted on many projects and contributed excellent ideas from fruitful discussion we always had during lab meetings. My wife, Lihong (Kelly), deserves special thanks for all her support and understanding. During the last few years, I started my PhD study at State College, then Hershey, finally Houston. She never hesitated to move with me, although the moving interrupted her own study. My parents, who are still farming at my hometown in China, devoted themselves to providing the best educations as they could to my younger brother, sister and myself. I would like to thank them for all the supports.
14 1 CHAPTER I LITERATURE REVIEW
15 Overview of NF-κB/IKK signaling Since its discovery in 1986 (1), nuclear factor κb (NF-κB) has been one of the most remarkably studied signaling pathways during the past 20 years in immunological research (2). NF-κB was originally thought to be a B-cell specific transcription factor regulating κ light-chain enhancer, but it is now evident that NF-κB functions in almost all cell types and regulates numerous genes involved in diverse biological functions. Particularly, NF-κB is well known for its role in regulating innate and adaptive immune responses. Furthermore, NF-κB serves as a major survival factor during oncogenesis. Thus the NF-κB signaling pathway has become an attractive therapeutic target in the treatment of immunological disorders and cancers. Mammalian cells contain five NF-κB members, including RelA (p65), RelB, c- Rel, p50, and p52. These proteins share the Rel homology functional domain (RHD), which is essential for nuclear localization, DNA binding and homo- or heterodimerization (3). In resting cells, NF-κB is located in the cytosol complexed with members of the inhibitory κb (IκB) family, predominantly IκBα (3). A variety of extracellular signals, including cytokines, antigens, environmental stresses, and growth factors, can activate NF-κB. Almost all stimulations, although not exclusively, converge with the activation of the IκB kinases (IKKs) through the intermediacy of integral membrane receptors. IKK complex is composed of a heterodimer of the catalytic IKKα and IKKβ subunits and a "master" regulatory protein termed IKKγ or NEMO (NF-kappa B essential modulator), which serves as a sensing scaffold for the catalytic subunits IKKα and IKKβ (4).
16 3 Cellular stimulation by TNFα, IL-1β, or LPS initiates signaling cascades that lead to activation of TGF-β activating kinase 1 (TAK1), an activator of both IKK and MAP kinases (MAPKs). Activated TAK1 phosphorylates IKKβ, triggering the catalytic activity of this key canonical NF-κB activating kinase (5) (Fig. 1.1). Upon activation, IKKβ phosphorylates IκBα, which triggers its ubiquitination and subsequent degradation by the protesome (4). Consequently, NF-κB is liberated and translocated into the nucleus, where it transactivates a spectrum of genes involved in diverse cellular functions. One of the target genes of NF-κB is that encoding IκBα. Newly-synthesized IκBα enters the nucleus, removes NF-κB from DNA, and exports the complex back to the cytoplasm to restore the original latent state (4). Thus, this so-called canonical pathway of NF-κB activation is generally a transient process, lasting from minutes in most cells. Recent studies have led to the discovery of a noncanonical NF-κB signaling pathway, which is activated by a subset of tumor necrosis factor (TNF) superfamily members, including B cell activating factor (BAFF), lymphotoxin β (LTβ) and CD40 ligand (6) (Fig. 1.1). This noncanonical pathway depends on proteasomal processing of a NF-κB precursor protein, p100 (7). Structurally, p100 contains an IκB-like structure in its C-terminal portion and sequesters specific NF-κB members, particularly RelB, in the cytoplasm. The processing of p100 is triggered through its phosphorylation by an atypical IKK complex, composed of IKKα and NF-κB-inducing kinase (NIK) (7, 8). Once phosphorylated, the IκB-like sequence of p100 is degraded, producing a mature NF-κB protein, p52, which translocates into the nucleus as a p52/relb complex that in
17 4 turn regulates genes involved in lymphoid organogenesis, B cell maturation, and development of immune tolerance (6). Although the mechanisms by which NF-κB signaling pathways are regulated have been intensively investigated, the defined regulation of NF-κB still awaits further studies. For example, how the receptor-ligand complex leads to the activation of IKK complex remains elusive (4). Regulation of diverse kinases or other signaling molecules that are upstream of IKK needs to be characterized. In the physiological scenario, different negative or positive regulators modulate NF-κB activation, thus conferring its fine regulation on different biological processes. Particularly, deciphering the mechanisms of negative regulation represents a major challenge for NF-κB research.
18 5 Receptor specific stimulation induces the activation of the canonical IKK complex or a noncanonical NIK-IKKα complex, leading to IκB degradation or p100 processing, respectively.
19 Ubiquitination and NF-κB/IKK regulation Ubiqutin, a highly conserved small regulatory protein that is ubiquitous in eukaryotes, plays a very important regulatory role in diverse signaling pathways (9). Both NF-κB and IKK activations are modulated by ubiquitin-dependent regulation, including ubiquitination and deubiquitination Ubiquitination and deubiquitination Ubiquitination is the post-translational modification of a protein by the covalent attachment (via an isopeptide bond) of one or more ubiquitin monomers (10). The most prominent function of ubiquitin is labeling proteins for proteasomal degradation (11). Ubiquitination is a multi-step reaction catalyzed sequentially by a ubiquitin activating enzyme (E1), a ubiquitin conjugating enzyme (E2), and a ubiquitin ligase (E3) (Figure 1.2 and (12)): 1) Ubiquitin activation, which is a two-step ATP-dependent reaction regulated by an E1 ubiquitin-activating enzyme. An ubiquitin-adenylate intermediate is first produced and then ubiquitin is transferred to the E1 active site cysteine residue and AMP is released at the same time. A thioester linkage between the C-terminal carboxyl group of ubiquitin and the E1 cysteine sulfhydryl group is generated as a result of this step. 2) Ubiquitin transfer, which involves ubiquitin transfer from E1 to the active site cysteine of a ubiquitin-conjugating enzyme E2. 3) Ubiquitin conjugation to substrate, which is mediated by E3 that functions as the substrate recognition modules of the system
20 7 and are able to interact with both E2 and substrate. Mammalian genome encodes only one E1, E2s, and hundreds of E3s (10). The polyubiquitin chains that target proteins for proteasomal degradation are formed through linkage of the C-terminus of one ubiquitin with lysine 48 (K48) of another ubiquitin (13). In contrast, polyubiquitin chains linked via another internal lysine, K63, modulates protein functions in signal transduction (10). Similar to protein phosphorylation, protein ubiquitination is a reversible process, in which the ubiquitinated proteins can be deubiquitinated by a family of cysteine proteases, namely deubiquitination enzymes (DUBs), which are specialized in digestion of polyubiquitin chains as well as deconjugation of monoubiquitinted proteins (14, 15).
21 8 Ubiquitination is a multi-step reaction including ubiquitin activation, transfer, and conjugation, which involves E1, E2, and E3, respectively (12).
22 Ubiquitination in NF-κB/IKK regulation K48 ubiquitination has long been known as a mechanism that targets IκB degradation, a major step of NF-κB signaling pathway. Following stimulation by signals coming from the outside of the cell, two serine residues (S32 and 36) of IκB are phosphorylated by the IκB kinase (IKK), which creates a binding site for an E3 ubiquitin ligase SCF βtrcp, leading to K48 ubiquitination of IκB (16). The ubiquitinated IκB is then targeted to the proteasome for degradation. Similar to the degradation of IκB, NF-κB precursor proteins processing is also mediated by the ubiquitin-proteasome pathway (17). The NIK/IKKα complex phosphorylates the C-terminal region of p100, leading to its ubiquitination and degradation of the IκB-like C-terminal domain, resulting in generation of p52 and activation of the sequestered RelB (7, 8). Recent findings suggest that K63 ubiquitination serves as a key mechanism mediating activation of IKK. It is generally thought that K63-linked ubiquitin chains attached to upstream signaling components form a platform for recruiting IKK and its activating kinase, TAK1, which is important for activation of both TAK1 and IKK (18-22). The K63 ubiquitination involved in IKK activation is mediated by specific E3 ligases, most importantly members of the TNFR-associated factors (TRAFs) (3, 19). Seven TRAF family members, namely TRAF1-7, have been identified in human genome. Except for TRAF1, all TRAF proteins contain an N-terminal RING domain (characteristic domain of E3 ligases), and several zinc-finger domains. Among the TRAF proteins, TRAF2 and TRAF6 have been extensively studied and shown to mediate the ubiquitin-dependent
23 10 activation of IKK induced by diverse immune receptors, including TNFR, IL-1R, and TLRs (3, 17). Through binding to the adaptor protein TRADD (TNFR-associated death domain), TRAF2 is recruited to the TNFR complex (23). TRAF2 is a RING-domain ubiquitin ligase (24-27). Receptor aggregation triggers the self-ubiquitination of TRAF2 as well as TRAF2-mediated ubiquitination of downstream targets, particularly the adaptor kinase receptor-interacting protein 1 (RIP1) (28). The K63-linked polyubiquitin chains of RIP1 form a signaling platform that recruits IKK and TAK1, triggering activation of these downstream kinases and nuclear translocation of NF-κB (17). Additionally, TAK1 also activates the c-jun N terminal kinase (JNK). Similar signaling events are initiated by the IL-1R and TLRs, although TRAF6, in this case, serves as the E3 ligase. TRAF6 activation requires specific adaptors associated with IL-1R and TLRs, particularly, MyD88, an adaptor protein that is recruited into IL-1R and most TLRs (29, 30). MyD88 further recruits the protein kinases IRAK4 and IRAK1, which mediates TRAF6 activation and initiation of downstream signaling cascades (29, 30). Two TRAF6-regulated IKK activators (TRIKAs) were identified during the investigation of the mechanism of how TRAF6 activates IKK (19, 20). TRIKA1 was shown to be an E2 enzyme complex consisting UBC13 and a UBC-like protein, UEV1A (19, 31). This complex functions with TRAF6, a RING-domain E3, to synthesize a K63- linked polyubiquitin chain on NEMO and TRAF6 itself. TRIKA2 is a trimeric complex that is composed of the protein kinase TAK1 and two adaptor proteins TAB1 and TAB2 (TAK1-binding proteins 1 and 2). TAK1 phosphorylates IKKβ at two serine residues in the activation loop. TAB2 and its related protein TAB3 contain a highly conserved novel
24 11 zinc-finger (NZF) domain that binds preferentially to K63-linked polyubiquitin chains (24). In the current model (17), TRAF6 is recruited to the receptor complexes and forms oligomers following ligand binding to IL-1R or TLRs. TRAF6 oligomerization activates its ligase activity, causing TRAF6 K63 polyubiquitnation. Ubiquitinated TRAF6 then recruits TAB2 and activates the TAB2-associated TAK1 kinase, which acts as an IKKβ kinase. Ubiquitin-activated TAK1 also phosphoylates and activates the JNK and p38 kinase pathways (20). Interestingly, NEMO ubiquitination has been recently shown to play important roles in NF-κB/IKK activation (32). NEMO ubiquitination was first observed in TNF pathway (33), in which c-iap-1 (inhibitor of apoptosis 1), a TRAF2 binding protein, may act as NEMO E3 ligase. NEMO ubiquitination also plays a role in the T cell receptor (TCR) signaling pathway. Overexpression of B-cell leukemia/lymphoma 10 (Bcl10), which connects the TCR pathway to NF-κB activation, induces NEMO K63-linked polyubiqutination (34). Two other components are required for this modification: gastric mucosa associated lymphoid tissue 1 (MALT-1), which is a Bcl10-interacting protein and a putative E3 ligase, and Ubc13 (34). Ubiquitination of NEMO at K399 appears to be important for IKK activation (34), although a recent study has also revealed ubiquitination of NEMO at K285 in Nod2 signaling pathway (35). The phenomenon that different lysine residues are modified depending on the stimulus studied suggests that polyubiquitnation of NEMO plays an essential role for IKK activation by providing the platform for integrating diverse signaling inputs (32). Moreover, genetic evidence suggests that NEMO ubiquitination also regulates the activation of MAP kinases (36).
25 Abnormal NF-κB regulation by retroviral oncoprotein HTLV-I Tax NF-κB regulates a wide range of genes that control cell proliferation and cell survival. Constitutive activation of NF-κB is found in most human tumors. A plethora of genes that mediate cell proliferation and survival are aberrantly induced by chronically activated NF-κB. Different aspects of NF-κB signaling pathways are deregulated in tumor cells, such as mutations in genes encoding the NF-κB transcription factors or in genes that control NF-κB activity, for example, IκB genes. Additionally, some tumor cells secret cytokines or growth factors that cause NF-κB activation (37). NF-κB also serves as a cellular target of oncogenic virus, such as human T-cell leukemia virus type I (HTLV-I). HTLV-I is an oncogenic retrovirus that is etiologically associated with an acute T-cell malignancy, termed adult T-cell leukemia (ATL) (38, 39). HTLV-I transforms T cells by modulating diverse cellular genes involved in T-cell growth and survival (40) and deregulating the functions of cellular factors involved in cell cycle control and DNA damage repair (41). HTLV-I infection promotes the expression of various cytokines and their receptors, such as the T-cell growth factor interleukin-2 (IL-2) (42, 43) and the α subunit of its high-affinity receptor complex (IL- 2Rα) (43-45). In addition, a large number of genes, which encode apoptosis inhibitors, immune receptors that belong to the tumor necrosis factor receptor (TNFR) family, transcription factors and intracellular signaling molecules, were found abnormally expressed.
26 13 Several nonstructural proteins are encoded by a px region of HTLV-I genome, these include Tax (46), Rex (47), and the necessary accessory proteins p12 I, p27 I, p13 II, p30 II (48, 49). Tax, the 40kDa transactivator protein, however, serves as the primary oncogenic mediator of HTLV-I. Tax exhibits the most notable signaling functions and contributions to dysregulate cellular genes (50). Although Tax does not directly transactivate target genes because of the absence of a typical DNA-binding domain, this viral protein can modulate a number of cellular transcription factors; these include cyclic AMP-responsive element-binding protein/activating transcription factor (CREB/ATF), NF-κB, serum responsive factor (SRF), NF-AT, basic helix-loop-helix (bhlh) proteins, and p53 (51, 52). Among those, NF-κB has been demonstrated as the key mediator of Tax-stimulated cellular gene expression (51). During a normal immune response, NF-κB is transiently activated through the TCR signals and mediates the organized expression of genes involved in proliferation and survival of antigen activated T cells. However, NF-κB is constitutively activated in HTLV-I infected and Tax-expressing T cells, which contributes to the aberrant growth and immortalization of the host cells. Although precisely how HTLV-I activates NF-κB is not completely understood, it appears that TCR and its proximal signaling molecules are not involved in this pathogenic pathway. It has been suggested that HTLV-I Tax protein bypasses the TCR-proximal signaling factors and directly induces NF-κB (51). Tax binds to several NF-κB members, including RelA, p50, p52 (53-56), as well as members of the IκB family, such as IκBα and the NF-κB precursor proteins p105 and p100 (55, 57-60). However, these physical interactions are insufficient for Tax to
27 14 activate NF-κB. Genetic and biochemical evidence suggests an essential role for the cellular protein kinase IKK in Tax-mediated NF-κB activation (Fig. 1.3 and (50)). Besides inducing the canonical NF-κB members, Tax potently stimulates a noncanonical NF-κB signaling pathway, leading to generation of the NF-κB2 gene product, p52 (58, 61). This latter function of Tax is mediated through activation of a specific subunit of the IKK complex, IKKα (61). IKKα is a central component of noncanonical NF-κB signaling stimulated by both Tax and cellular signals; however, the Tax pathway does not require the upstream kinase, NIK (61). Although the mechanism by which Tax constitutively activates IKK has not been clearly demonstrated, Tax physically associates with IKK, which likely contributes to sustained IKK activation (51). Indeed, IKK remains chronically phosphorylated and activated in the Tax-IKK complex (62, 63). The formation of Tax-IKK complex relies on physical interaction between Tax and the IKK regulatory subunit, IKKγ (64-66). The association between Tax/IKKγ is required for recruiting Tax to the IKK catalytic subunits and for Tax-mediated IKK activation (67). This is further supported by genetic evidence that IKKγ is an essential factor for Tax-stimulated activation of IKK and NF-κB in both fibroblasts and T cells (68, 69).
28 15 Tax activates both NF-kB canonical and noncanonical pathways by physically targeting two different IKK complexes, both requiring the adaptor protein IKKγ. Formation of the noncanonical Tax/IKK complex requires the interaction of Tax with both IKKγ and p100 (50).
29 16 The mechanism by which Tax-IKK physical association leads to IKK activation still remains elusive. Two possibilities exist: the first is that Tax induces IKK catalytic activity through physical crosslinking the IKK complex, since Tax possesses the ability of oligomerization (70, 71); the other possibility is that Tax recruits an IKK-activating kinase to the IKK complex, thus triggering the activation of IKK by the upstream kinase (50). In support of the first hypothesis, fusion of Tax to IKK catalytic subunit confers the ability to stimulate IKK catalytic activity (72); however, this result does not exclude the possibility of the involvement of a Tax-recruited upstream kinase. Regarding the second hypothesis, possible upstream IKK kinases, such as MEKK1 (73) and NIK (74), were suggested. However, genetic evidence for the involvement of these kinases in IKK activation is still lacking (50). Recent studies suggest that ubiquitination plays an important regulatory role in Tax-mediated NF-κB activation. Tax undergoes mono and polyubiqutination in both transfected cells and HTLV-I infected T cells (75, 76). Mutation of the lysine ubiquitin acceptor sites within Tax causes the loss of its ability to activate both canonical and noncanonical NF-κB pathways (77, 78). Further studies showed that Tax undergoes predominantly K63 linkage polyubiquitnation (79). The polyubiquitin chain attached to Tax may function as a scaffold to promote the assembly of complexes that contain kinases and other signaling molecules. Although one recent study showed that Ubc13 acts as a E2 enzyme to Tax ubiquitination (79), E3 enzymes involved in this process have not been characterized. In addition, Tax was also shown to induce polyubiquitination of NEMO, and monoubiquitination of IKKβ (80). Upon chronic phosphorylation of IKKβ at Ser-
30 17 177/Ser-181, monoubiquitin is attached to T loop-proximal residue Lys-163 (80). The functional significance of Tax-mediated IKK ubiquitination is supported by the finding that a mutant Tax that is defective in NF-κB activation is unable to induce IKKβ or IKKγ ubiquitination (80). However, it is not clear whether Tax functions as an E3 ligase, since Tax lacks a conserved domain typical of E3 ligases, such as HECT (81) or RING (82). Further studies will be needed to characterize the biochemical nature of Tax-induced ubiquitination events and their roles in Tax-mediated NF-κB activation. Recent studies identified IKKγ as a ubiquitin-binding protein (22). IKKγ contains a ubiquitin-binding domain that preferentially associates with K63-linked polyubiquitin chains (22). Point mutations in IKKγ that prevent binding to Lys63-linked polyubiquitin abrogate the activation of IKK and NF-κB by T-cell mitogen and TNFα (22), demonstrating the importance of intrinsic ubiquitin-binding activity of IKKγ. Given the elusive nature of how ubiquitination regulates Tax-induced IKK activation, it would be valuable to investigate whether similar mechanism regulates this process Role of ubiquitination in antiviral signaling pathways Triggering an effective anti-viral innate immune response requires two events: 1) immune receptors detecting invading virus; 2) initiation of protein signaling cascades that regulate synthesis of type I IFN (Fig. 1.4 and (83)). Viral pathogens possess specific structures, termed pathogen-associated molecular patterns (PAMPs), which can be recognized by the host pattern-recognition receptors (PRRs) (84). The best characterized PRRs include Toll-like receptors (TLRs) and the recently identified cytosolic viral RNA
31 18 receptors RIG-I (85), and Mda5 (86). In particular, RIG-I specifically recognizes 5 triphosphate containing viral RNA and transmits signals that induces type I IFN-mediated host protective innate immunity against viral infection (87, 88). RIG-I contains two N- terminal caspase activation and recruitment domains (CARDs) and one DExD box RNA helicase activity in the C-terminus (85). RIG-I deficient mice are highly susceptible to infection with RNA viruses compared to control mice (89). Further characterization of IFN antiviral response in different cell types, like fibroblastic, epithelial and conventional dendritic cells showed that RIG-I, not TLR system, plays an essential role (89). Mitochondrial antiviral signaling adaptor (MAVS), also named as (IPS- 1/VISA/Cardif), was elucidated as the adaptor molecule linking RIG-I sensing of incoming viral RNA and downstream activation events (90-93). Structurally similar to RIG-I, MAVS also contains an N-terminal CARD domain and a C-terminal transmembrane domain, which localizes MAVS to the mitochondrial membrane. Expression of MAVS activated the IFN-α, IFN-β, and NF-κB promoters (91). MAVS interacts with RIG-I and recruits two homologues downstream kinases, TBK1 and ΙΚΚε, to the signaling complex (93, 94). Investigation on MAVS-deficient mice demonstrates its essential role in viral induction of IFN (90, 95). Except for pdc, MAVS is essential for NF-κB and IRF3 activation in multiple cell types (90, 95).
32 19 Antiviral immune response is initiated by recognition of RNA virus by cytosolic viral RNA receptor RIG-I and MAVS localized on the mitochondrial membrane, followed by signaling cascades through TRAF6 and TRAF3, which then activate the typical IKK complex and IKK-related kinases IKKε/TBK, finally leads to activations NF-κB and IRF, respectively (96).
33 20 Type I IFNs include several IFN-α subtypes and a single IFN-β subtype (97). The induction of type I IFN genes is predominantly regulated at the level of transcription, and the underlying mechanism is best studied using IFN-β promoter (83). In response to a viral challenge, a multi-protein enhanceosome is assembled at the IFN-β promoter (98). At least three classes of transcription factors, i.e. ATF-2/c-Jun, NF-κB and interferon regulatory factor 3 (IRF3) exist in the enhancesome. Similar to NF-κB, IRF3 is normally located in the cytoplasm as an inactive form. In response to a viral challenge, the IKKlike kinases TBK-1 and ΙΚΚε phosphorylate IRF3, thus leading to its dimerization and translocation into the nucleus (99, 100). ATF-2/c-Jun is phosphorylated in the nucleus by stress kinases such as JNK and p38 kinases, which are activated by viral infection. A positive feedback loop regulates type I IFNs production, that is, through binding to the IFNα/β receptor (IFNAR) in autocrine and paracrine manner (97). IFNARs trigger the janus kinase (JAK) family members JAK1 and Tyk2 activation, which further induces phosphorylation and activation of the signal transducer and activator of transcription 1 (STAT1) and STAT2 proteins. Through initiating transcription of different interferon stimulated genes (ISGs), host cells elicit an anti-viral state by inhibiting different stages of virus replication (83). Emerging lines of evidence suggest that ubiquitination plays an important role in anti-viral innate immune response ( ). First, ubiquitination is essential for the type I IFN response (101). TLR7, TLR8 and TLR9, induce antiviral responses by producing interferon-α (IFN-α) in a MyD88-dependent manner. MyD88 formed a complex specifically with IRF7, instead of IRF3. Furthermore, IRF7 is also activated through binding by the adaptor molecule TRAF6, in which ubiquitin ligase activity of
34 21 TRAF6 is essential for IRF7 activation. Second, RIG-I, a key PRR that mediates host responses to single-stranded RNA viruses, has been shown to undergo K63-linked polyubiquitination by TRIM25α, a member of the tripartite motif (TRIM) protein family involved in various cellular processes including cell proliferation and antiviral activity. TRIM25α contains a RING-finger domain, B-box/coiled-coil domain and a SPRY domain, thus confers its E3 ubiquitin ligase activity (102). Third, ubiquitination occurs on several components of the type I IFN receptor signaling pathway; these include IFNARs (103, 104) and JAK1(105). However, the physiological roles of these ubiquitination events have not been explored. Recent studies have shown that deubiquitinating enzymes (DUBs) play important roles in recycling ubiquitin and modulating signaling functions of ubiquitinated proteins. Deubiquitinating enzyme (DUBs) is a large group of proteases (more than 60 known) that regulates ubiquitin-dependent metabolic pathways by cleaving ubiquitin-protein bonds. Among them, CYLD has been intensively studied and shown to regulate diverse signaling pathways Functions of CYLD in immune responses and tumorigenesis CYLD is characterized as a member of the deubiquitinase family that can specifically hydrolyse K63-linked poly-ubiquitin chains. A prominent feature of CYLD is its specific DUB function toward signaling molecules, including NEMO, TRAF2, and TRAF6 ( ). Consistent with the critical role of these signaling components in NFκB activation, CYLD negatively regulates NF-κB induced by TLRs and TNFRs (106-
35 22 108). CYLD also has a negative role in regulating other signaling pathways, such as the c-jun N-terminal kinase (JNK) (109) and p38 (110). Additionally, CYLD positively regulates the signaling functions of some other targets, including the tyrosine kinse Lck (111) and the cell cycle regulatory kinase PLK1 (112). Studies on CYLD deficient animals have characterized the multiple physiological roles that CYLD plays, such as the normal developmental processes, inflammation, immunity, and tumorigenesis (111, ). By knocking out endogenous CYLD in mice, it has become evident that CYLD regulates T cell development (111). Compared to wildtype control, CYLD-/- mice have substantially fewer mature CD4+ and CD8+ single-positive thymocytes and peripheral T cells. The underlying mechanism appears to involve the physical association of CYLD to active Lck, which promotes the recruitment of active Lck to its substrate, Zap70. In transfected cells, CYLD de-conjugates both K48 and K63 linked polyubiquitin chains from Lck, although how CYLD modulates Lck ubiquitintion in vivo is not clear (111). In contrast to its negative role in many other pathways, CYLD plays a positive role in regulating proximal T cell receptor signaling in thymocytes. Analogous to its role in thymocyte development, CYLD also plays a positive role in male germ cell development, or spermatogenesis (114). Starting with the observation that CYLD-/- male mice are sterile, Wright et al. found severe impaired seminiferous tubule organization and dramatic reduction in the number of postmeiotic germ cells. CYLD deficiency attenuates the early wave of germ cell apoptosis, which is essential for maintaining the balance between germ cells and supporting sertoli cells. Higher basal NF-κB activation and aberrant expression of antiapoptotic genes were found in testicular cells as a result of the
36 23 loss of CYLD. In this aspect, CYLD negatively regulates ubiquitin-dependent NF-κB activator, RIP1. Given that CYLD is conserved from invertebrates to mammals (118), studies on Drosophila CYLD (dcyld) also revealed its role on regulating apoptosis during Drosophila development (115). By generating Drosophila CYLD (dcyld) mutant and transgenic flies expressing wildtype and mutant dcyld proteins, the authors showed that dcyld was required for JNK-dependent oxidative stress resistance and normal lifespan. Consistent with mammalian animals, dcyld regulates TNF-induced JNK activation and cell death through dtraf2. dcyld encodes a deubiquitinating enzyme that deubiquitinates dtraf2 and prevents dtraf2 from ubiquitin-mediated proteolytic degradation. Another aspect of CYLD s physiological functions can be demonstrated by its involvement in inflammation. An excellent example came from a series of studies on CYLD-deficient mice stands for (113, 119). First, CYLD was shown to play a pivotal role in regulating T cell activation and homeostasis. CYLD deficient mice spontaneously develop intestinal inflammation, a feature mimicking human inflammatory bowel diseases (IBDs), and this is attributed to the hyperresponsiveness of CYLD-deficient T cells. Under endogenous conditions, CYLD targets a ubiquitin-dependent kinase, transforming growth factor ß-activated kinase 1 (Tak1), and inhibits its ubiquitination and autoactivation. Therefore, loss of CYLD causes constitutively active Tak1 and its downstream kinases c-jun N-terminal kinase and IκB kinase ß in T cells (113). Second, CYLD also regulates the peripheral development and activation of B cells. CYLDdeficient mice exhibit B cell hyperplasia, lymphoid organ enlargement and increased number of marginal zone B cells. These abnormalities are also due to constitutive
37 24 activation of NF-κB (119). Consistent with this finding, another group recently generated a mouse strain that expresses a naturally occurring splice variant of CYLD (CYLD (ex7/8) mice) and observed a dramatic expansion of mature B cells in all peripheral lymphoid organs in this strain (120). Since the splice variant CYLD does not contain the TRAF2 and NEMO binding sites as full-length CYLD, the phenotype observed further supports the critical role of CYLD in preventing abnormal lymphocytes activation and provided underlying molecular mechanism. Physiological functions of CYLD have been expanded into host response to infections (121). Infection of nontypeable Haemophilus influenzae (NTHi), a major respiratory bacterial pathogen, leads to up-regulation of epithelial Toll-like receptor (TLR7) expression at a TLR2-dependent manner, in which TLR2-MyD88-IRK-TRF6- IKK-NF-κB signaling pathway is involved. Interestingly, following NTHi infection, CYLD expression is induced, thus providing a feedback mechanism to down-regulate TLR7 expression (122). The negative feedback loop executed by CYLD may represent an important mechanism by which host limits serious tissue damage caused by detrimental inflammatory responses during microbial infection (121). Not surprisingly, loss of CYLD also counts for gain of function, as demonstrated by protecting mice from Streptococcus pneumonie (S. pneumonie) pneumolysin-induced acute lung injury (ALI) and lethality (110). Following S. pneumonie infection, CYLD is substantially induced and thereby inhibits MKK3-p38 kinase-dependent expression of plasminogen activator inhibitor-1 (PAI-1) in lung, which detriments ALI and mortality. The significance of CYLD as a tumor suppressor has been further emphasized by animal studies revealing its critical involvement in the tumorigenesis (116, 117).
38 25 Initially identified as the tumor suppressor gene for familial cyclindromatosis, a rare autosomal dominant disease (118), CYLD was found mutated in those patients. The cyclindromatosis patients carry germ-line mutations in one CYLD allele (heterozygous), while the other allele is lost in the tumor cells (118, 123, 124). Since patients carry a wildtype allele in all but the tumor cells, studies on patient provided little insight into the function of CYLD in normal cells. Important functions of CYLD in tumorigenesis have been demonstrated by several recent studies using CYLD knockout mice (110, 111, 116, 117). In support with the deubiquitnation function of CYLD, CYLD-deficient mice generated by another group were more susceptible to induced colonic inflammation and showed remarkable increase in the incidence of tumors compared with controls in a colitisassociated cancer model (117). CYLD-/- mice generated by another group are more susceptible to chemically induced skin tumor (116). This high sensitivity mimics the pathogenesis of familial cyclindromatosis. In keratinocytes, CYLD deubiquitinates and negatively regulates the antiapoptotic molecule Bcl-3, thus preventing its nuclear accumulation. CYLD deficiency causes nuclear accumulation of Bcl-3, which interacts with p50 or p52, resulting NF-κB activation. Moreover, recent studies have uncovered the deregulation of CYLD in many cancer cell lines ( ). CYLD was found downregulated or ablated in different colon and hepatocellular carcinoma cell lines compared with primary human colonic epithelial cells and hepatocytes, respectively (128). Downregulation or loss CYLD at both mrna and protein levels was further revealed in most tumor samples from patients with colon carcinoma or hepatocellular carcinoma (HCC) compared with non-tumorous
39 26 tissues (128). Furthermore, deletion of CYLD was also shown in multiple myeloma (126, 127), a late stage B cell malignancy. Thus, CYLD downregulation or loss might contribute to constitutive NF-κB signaling in multiple tumor cells. Interestingly, western blot analysis of CYLD in primary human hepatocytes (PHHs) and colonic epithelial cells (CEC) showed two bands, of which the upper band might correspond to phosphorylated form (128). Phosphorylation of CYLD, in those cells, may mediate further degradation induced by higher NF-κB activation that occurs in tumor cells. Although studies on CYLD-deficient animals expanded our understanding on the physiological functions of CYLD; studies on cancer cell lines, on the other hand, provided valuable information on how CYLD is deregulated in human cells. Furthermore, studies of both aspects will lead to elucidating how the tumor suppressor CYLD might crosstalk with oncogenes or other tumor suppressors. Therefore, it would be possible to develop effective therapeutic inhibition on cancer pathogenesis by targeting CYLD. Several questions arise as we summarize our current understanding on the regulation of CYLD on diverse signaling pathways. First, it becomes clear that CYLD modulates diverse signaling pathways in a receptor and cell type-specific manner. Is this mode of action mediated through cell-type specific distribution of CYLD or yet-to-be identified CYLD regulators? Second, multiple targets of CYLD have been identified using in vivo or transfection approaches. Are all of these molecules the natural substrates of CYLD? If they are, what is the underlying mechanism by which CYLD targets different substrates in different biological processes? Another question is whether CYLD directly targets its substrates or depends on adaptor proteins, as shown for A20 (129,
40 27 130)? It seems that depending on the specific cellular context, CYLD acts as deubiquitination enzyme to remove either the K48-linked polyubiquitintion, which mediates proteasome-dependent protein degradation, or K63-linked polyubiquitination, which mediates endocytosis and signal transduction. How is the substrate specificity of CYLD determined? Is there any functional redundancy between CYLD and other DUBs, such as A20? Third, CYLD has been shown to regulate NF-κB and MAP kinase cascades in mediating inflammation and host defense against bacterial infections. However, the role of CYLD in antiviral innate immune response is not known. For example, although ubiquitintion regulates type I IFN signaling, it is unknown whether this anti-viral signaling pathway is under the regulation by CYLD. Last but not least, CYLD functions as a tumor suppressor in both human patients and animal models; however, it remains unclear whether it regulates the functions of any oncogenes or other tumor suppressors Crosstalk between typical IKK signaling and antiviral signaling pathways The IKK-related kinases IKKε and TBK1 share similar structural domains with classical IKK kinases IKKα and IKKβ; these include a catalytic kinase domain, a leucine zipper domain and a helix-loop-helix domain involved in protein-protein interactions ( ). Although IKKε and TBK1 share 64% homology to each other, they only exhibit 33% sequence identity within the kinase domain to IKKα and β kinases ( ). The different homologies indicated that TBK1 and IKKε might obtain unique function. Indeed, initial studies revealed that TBK1 and IKKε phosphorylate only one of
41 28 the two serines (i.e. Ser36) of IκBα that are phosphorylated by the typical IKK. Since phosphorylation of Ser36 is insufficient for triggering IκBα degradation, this suggests that IκBα is not a physiological substrate for TBK1 and IKKε (132, 135, 136). Interestingly, however, TBK1 deficient mice were embryonic lethal, and died at E14.5 of massive liver degeneration and apoptosis, which is similar to the mice lacking key NF-κB signaling components (136). Because of this finding, it was proposed that IKKε/TBK1 may function upstream of IKK complex (131). Both TBK1 and IKKε interact with the adaptor protein TANK, which is able to activate NF-κB (133). Overexpression of TBK1 or IKKε induces phosphorylation of TANK, leading its dissociation from TRAF2 and subsequent activation of NF-κB via the classical IKK pathway (133, 137). TBK1 and IKKε also directly associate with a homologue of TANK, namely NAP1 (NAK-associated protein 1) (138). NAP1 associated TBK1 or IKKε and the NAK-NAP1 complex-mediated NF-κB activation might protect cells from TNFα induced apoptosis (138). Similar to the regulatory role of NEMO in the classical IKK complex, TANK and NAP1, appears to be essential for the activation of TBK1 and IKKε (96). Recent studies have identified an unexpected connection between NEMO and the IFN antiviral signaling pathway (139). NEMO physically associates with TANK, and this association serves to recruit the TBK1/IKKε complex to the RNA-binding PRR, RIG-I, leading to activation of TBK1/IKKε and the downstream transcription factors, IRF3 and IRF7. Thus NEMO is an important adaptor that allows RIG-I to activate both the NF-κB and IRF signaling pathways (Fig. 1.4 and (139, 140)).
42 Identification of IKKε/TBK1 as oncogenes Despite the critical roles of IKKε/TBK1 play in the antiviral immune response, a series of recent research has identified both atypical IκB kinases as oncogenes (141, 142). RalB GTPase, a member of the Ras GTPase super family, blocks apoptosis in tumor cell lines (143). In the effort to determine which RalB effector promotes cell survival in human epithelial tumor cells, Chien et al identified Sec5 as an essential component of mediating the prosurvival function of RalB in cancer cells (141). Surprisingly, Sec5 complex was found to contain TBK1. They provided evidence that RalB activates TBK1 by promoting the association of TBK1 with Sec5. TBK1-deficient mouse embryonic fibroblasts (MEFs) were resistant to the oncogenic K-ras induced transformation. In contrast, if K-ras is co-expressed with kinase-dead TBK1 (which dominantly inhibits native TBK1 activation) in MEFs, cells undergo apoptosis. As summarized in 1.5, TBK1 activates NF-κB and IRF3 pathways in innate immune pathway. Interestingly, RalB helps trigger this response in nontumorigenic human epithelial cells following dsrna or Sendai virus stimulation, which activates TLRs. TBK1 and IKKε were known to directly phosphorylate a C-terminal domain of c- Rel, thereby triggering nuclear accumulation of crel (144). Given the oncogenic role of c-rel, this function of IKKε/TBK1 may contribute to their oncogenic action. By using three independent approaches, i.e. comparative genomics, RNAi screens, and overexpression screens, Boehm et al identified IKKε as an oncogene and activator of the NF-κB pathway in breast cancer cell. Notably, the oncogenic capacity of IKKε requires its function of activating NF-κB, but not IRF-3 (142). Deregulated IKKε leads to
43 30 upregulation of NF-κB-responsive genes, IκB destabilization and NF-κB. The transforming activity of IKKε was suppressed by expressing a degradation-resistant IκB. This might provide the molecular explanation for activation of NF-κB pathway in many breast cancer cells. Given the oncogenic functions of IKKε/TBK1, developing and testing inhibitors that block activities of IKKε/TBK1 should be therapeutically beneficial for the treatment of specific tumors. However, elucidation of the mechanism that regulates the oncogenic functions of IKKε/TBK1 would provide more insights into developing more specific and less toxic inhibitors.
44 31 CHAPTER II RETROVIRAL ONCOPROTEIN TAX DEREGULATES NF-κB BY ACTIVATING TAK1 AND MEDIATING TAK1-IKK PHYSICAL ASSOCIATION Xuefeng Wu and Shao-Cong Sun EMBO Rep. (2007) 8(5): 510-5
45 32 Abstract The Tax oncoprotein of human T-cell leukemia virus type I (HTLV-I) persistently activates NF-κB, which is required for HTLV-I-mediated T-cell transformation. Tax activates NF-κB by stimulating the activity of IκB kinase (IKK), but the underlying mechanism remains elusive. We show here that Tax functions as an intracellular stimulator of an IKK-activating kinase, Tak1. Additionally, Tax physically interacts with Tak1 and mediates the recruitment of IKK to Tak1. In HTLV-I-infected T cells, Tak1 is constitutively activated and complexed with both Tax and IKK. We provide genetic evidence that Tak1 is essential for Tax-induced IKK activation. Furthermore, unlike cellular stimuli, the Tax-specific NF-κB signaling does not require the ubiquitin-binding function of IKK. These findings demonstrate a pathological mechanism of IKK activation by Tax and provide an example for how IKK is persistently activated in cancer cells. Keywords: HTLV-I, Tax, NF-κB, Tak1, IKK
46 33 Introduction Human T-cell leukemia virus type I (HTLV-I) is an oncogenic retrovirus etiologically associated with an acute T-cell malignancy termed adult T-cell leukemia (ATL) (145). HTLV-I encodes a regulatory protein, Tax, which plays a central role in HTLV-I-induced T- cell transformation (146). The oncogenic action of Tax involves aberrant induction of a large array of cellular genes regulating cell growth and survival (51, 147). Tax induces many of these genes by activating NF-κB (50), a family of cellular transcription factors with a pivotal role in the control of cell proliferation and apoptosis inhibition (148). The activity of NF-κB is tightly regulated through its cytoplasm sequestration by the IκB family of inhibitory proteins, predominantly IκBα (149). Activation of NF-κB by diverse cellular stimuli involves stimulation of a multisubunit IκB kinase (IKK), composed of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit termed IKKγ (149). Upon activation, IKK phosphorylates IκBα, triggering its ubiquitination and degradation, which allows NFκB to move to the nucleus and exert its transcription function. The mechanism mediating IKK activation by different stimuli is not completely understood, although a number of upstream kinases have been implicated in the activation of IKK. However, recent genetic evidence suggests an essential role for TGF-beta activating kinase 1 (Tak1) in IKK activation by a number of cellular stimuli ( ). Tak1 phosphorylates IKKβ as well as MKK6, leading to activation of both NF-κB and c- Jun N-terminal kinase pathways (21). Emerging evidence also suggests that IKK activation by immune stimuli involves lysine 63 (K63)-linked polyubiquitination of IKKγ and upstream regulators, including tumor necrosis factor receptor-associated factor 6 (TRAF6)
47 34 and receptor-interacting protein 1 (RIP-1) (34, 156, 157). Ubiquitination of RIP1 and TRAF6 appears to serve as a mechanism that recruits IKK and Tak1 (22, 28, 158). Indeed, both IKKγ and a Tak1-associated protein, Tab2, are ubiquitin-binding proteins (22, 24, 28). Thus, the polyubiquitin chains may provide a platform for IKK activation by Tak1. Since the signal-induced protein ubiquitination is quickly reversed by deubiquitinating enzymes (159), this mechanism explains the rapid and transient nature of NF-κB activation by cellular stimuli. In contrast to the transient activation of NF-κB by cellular stimuli, Tax stimulates persistent NF-κB activation, which is essential for HTLV-I-induced T-cell transformation (51, 160, 161). Although precisely how Tax induces persistent NF-κB activation is enigmatic, this viral specific pathway does not require the upstream signaling factors, such as TRAFs and RIP-1 (64, 162). Instead, Tax appears to use a mechanism that involves its physical interaction with IKK via the IKKγ subunit (64-66). However, it is currently unknown how the Tax/IKK association causes the activation of IKK. We show in this study that Tax stimulates the catalytic activity of the IKK activating kinase Tak1 as well as mediates the physical recruitment of IKK to Tak1. This viral specific mechanism does not require the ubiquitin-binding function of IKKγ. These findings emphasize a pathological mechanism of IKK activation and provide an example for how IKK is persistently activated in cancer cells.
48 35 Results and Discussion Tak1 is required for Tax-induced activation of NF-κB and IKK Although Tax is known to physically interact with IKK, how Tax stimulates the catalytic activity of IKK remains unclear (50). It is generally believed that Tax may activate IKK through either inducing IKK oligomerization or recruiting IKK to an upstream kinase (51). However, neither of these hypotheses has been experimentally approved. To date, genetic evidence for the involvement of upstream kinases is lacking. To further investigate the mechanism of NF-κB activation by Tax, we examined the role of Tak1 using a genetic approach. MEFs derived from Tak1-deficient (Tak1 / ) and wildtype control (Tak1 +/+ ) mice were transfected with the Tax expression vector followed by analyzing the activation of NFκB by EMSA. As expected, expression of Tax in wildtype MEFs resulted in potent activation of NF-κB DNA binding activity (Figure 1A). Further, this function of Tax was associated with a marked loss of the NF-κB inhibitor IκBα (Figure 1B). Remarkably, the Tak1 deficiency abrogated the Tax-stimulated activation of NF-κB (Figure 1A) as well as the loss of IκBα (Figure 1B). Consistently, the loss of Tak1 also diminished Tax-stimulated expression of the TNF-α gene (Figure 1C). We next examined whether Tak1 is required for Tax activation of IKK. When expressed in wildtype cells, Tax stimulated the catalytic activity of endogenous IKK (Figure 1D, lane 2). In sharp contrast, Tax failed to enhance the IKK activity in Tak1 / cells (Figure 1D, lane 4). This signaling defect was not due to the alteration in the expression level of IKK subunits (Figure 1E). Taken together, these results demonstrate an essential role for Tak1 in mediating Tax-stimulated activation of IKK and NF-κB.
49 36 Tax stimulates the catalytic activity of Tak1 The essential role of Tak1 in Tax-induced IKK activation prompted us to examine whether Tax activates the catalytic activity of Tak1. We first analyzed whether Tax promotes the IKK-activating activity of Tak1. When expressed in 293 cells, both Tax and Tak1 were able to activate IKK, as demonstrated by immunecomplex kinase assays (Figure 2A, panel 1). Importantly, Tax significantly potentiated Tak1-mediated activation of IKK (Figure 2A, upper panel, lane 4). This effect was not due to elevation in Tak1 expression, since the level of Tak1 was even lower in the presence of Tax (Figure 2A, panel 3). Consistent with this biochemical results, Tax also synergized with Tak1 in the induction of a κb-luciferase reporter (Figure 2B). Although it is likely that the Tax/Tak1 synergy might involve other factors, these data suggest the possibility that Tax may stimulate the catalytic activity of Tak1. This idea was confirmed by directly analyzing the Tak1 activity by kinase assays. Indeed, Tax potently stimulated the catalytic activity of Tak1 (Figure 2C, panel 1, lane 3) without increasing the level of Tak1 expression (Figure 2C, panels 2 and 3, lane 3). A hallmark of HTLV-I-transformed T cells is the constitutive activation of IKK (50). It was thus important to determine whether Tak1 was also activated in these HTLV-Iinfected T cells. Compared to the HTLV-negative T-cell line SupT1 (Figure 2D, lane 1), the HTLV-I-transformed T cells exhibited markedly higher levels of Tak1 kinase activity (Figure 2D, lanes 3-4). This deregulated activation of Tak1 appeared to be mediated by Tax, since it was also detected in a T-cell line transformed by the Tax protein (Figure 2D, lane 5). To further examine this molecular connection, we expressed Tax or a control
50 37 GFP protein in the HTLV-negative SupT1 cells by retroviral transduction. Indeed, Tax expression was sufficient for stimulating the catalytic activity of Tak1 in these T cells (Figure 2E). Thus, the retroviral oncoprotein Tax is an intracellular stimulator of Tak1. Tax physically interacts with Tak1 and induces Tak1/IKK association Since Tax activation of NF-κB does not require TRAFs and RIP-1 (64, 162), which are immediate upstream regulators of Tak1 and IKK (157), it raises the intriguing question of whether Tax binds to Tak1 and mediates Tak1/IKK association. This question was addressed using both Tax-transfected and HTLV-I-transformed T cells. In transfected cells, Tax and Tak1 indeed formed a stable complex, which was precipitated by the Tak1 antibody (Figure 3A). Importantly, this molecular interaction was also readily detected in HTLV-I-transformed T cells (Figure 3E and data not shown). Since Tak1 exists in a complex, composed of the adaptor proteins TAB1 and TAB2 (157), it is unclear whether the Tax/Tak1 interaction is direct or via additional proteins, such as the TABs. Our preliminary data revealed that GST-Tax fusion protein was ineffective to pull down in vitro translated Tak1 from Wheat Germ Extract (data not shown). While this result indicates the requirement of accessory proteins in Tax/Tak1 binding, other possibilities should also be considered. For example, Tax undergoes phosphorylation in mammalian cells, which is required for its signaling function in NF-κB activation (163). It is possible that posttranslational modifications (such as phosphorylation) of Tax are required for its association with Tak1. Furthermore, it is also possible that the fusion of Tax to GST may interfere with its binding to Tak1. Notwithstanding, our data clearly demonstrate that Tax and Tak1 are assembled into a stable complex in vivo.
51 38 To assess the functional significance of Tak1/Tax interaction in Tax-mediated activation of Tak1 and IKK, we examined the Tak1-binding activity of a wellcharacterized Tax mutant, M22, known to be defective in the activation of IKK and NFκB (51, 164). Surprisingly, M22 was fully competent in binding Tak1 (Figure 3B). Further, like the wildtype Tax, M22 was able to stimulate the catalytic activity of Tak1 (Figure 3C) as well as the downstream kinase JNK (Figure 3D). These results suggest that Tax activation of Tak1 is insufficient for triggering the activity of IKK. The ability of Tax to bind both Tak1 (Figure 3A) and IKK (64-66) suggested the possibility that Tax might promote the association of Tak1 with IKK. This possibility was first examined by using control and HTLV-I-transformed T cells. Consistent with the requirement of cellular signals for triggering Tak1/IKK association, Tak1 was not associated with IKK in the HTLV-negative SupT1 cells (Figure 3E, panel 1, lane 1). Remarkably, both IKKα (data not shown) and IKKβ were coprecipitated with Tak1 in the HTLV-I-transformed SLB-1 cells (Figure 3E, panel 1, lane 2) as well as HUT102 and Tax1 cells (data not shown). Tax was also assembled into this signaling complex (panel 3). To further confirm that Tax stimulates Tak1/IKK association, we employed a transfection model. Transfected Tak1 weakly interacted with endogenous IKK in the absence of Tax (Figure 3F, lane 2). Importantly, this molecular interaction was strongly promoted by Tax (Figure 3F, lane 3). On the other hand, the M22 mutant of Tax failed to promote the binding of Tak1 to IKK (Figure 3F, lane 4). This finding explained why M22 was unable to activate IKK (51) despite its ability to stimulate the catalytic activity of Tak1 (Fig. 3C). Since Tax M22 was known to be defective in IKKγ binding (64-66), it is
52 39 logical to propose that Tax may function as an adaptor mediating Tak1/IKK association through binding to both of these cellular signaling components. Tax activation of NF-κB does not require the ubiquitin-binding function of IKKγ Recent studies have demonstrated a ubiquitin-dependent mechanism of IKK activation by immune stimuli (Chen et al., 2006). Specifically, the IKK regulatory subunit, IKKγ, has intrinsic ubiquitin-binding activity, which appears to be required for recruiting IKK complex to upstream signaling molecules, such as RIP-1 (22, 28). Indeed, IKKγ mutants lacking the ubiquitin-binding function are unable to support the activation of NF-κB when stably expressed in an IKKγ-deficient Jurkat T-cell line (22, 28). Using the same approach, we examined whether the ubiquitin-binding function of IKKγ is required for Tax-mediated NF-κB activation. As we previously reported (69), Tax was unable to induce NF-κB activation in the IKKγ-deficient Jurkat T cells (Figure 4, lane 2). Furthermore, this signaling defect could be rescued by stable expression of exogenous IKKγ (Figure 4, lane 4). Interestingly, despite their inability to mediate NF-κB activation by TNF-α (22), the ubiquitin bindingdefective IKKγ mutants, D311N and L329P, efficiently mediated Tax-stimulated NF-κB activation (Figure 4, lanes 6 and 8). This result further suggests a unique mechanism that mediates the Tax-specific pathway of NF-κB activation. In conclusion, the data presented in this study identify a novel pathological mechanism of NF-κB activation. The retroviral oncoprotein Tax physically interacts with Tak1 and stimulates its catalytic activity. Consistently, Tak1 is essential for Tax-mediated
53 40 activation of IKK and NF-κB. However, it is clear that activation of Tak1 is only part of the mechanism by which Tax activates IKK. Another important part of the mechanism is Tax-mediated recruitment of IKK to Tak1. In keeping with these biochemical results, Tax activation of NF-κB does not require the ubiquitin-binding function of IKKγ. This novel mechanism provides an explanation for how Tax persistently activates NF-κB without the requirement of more upstream signaling factors. Materials and Methods Cell lines. C8166, HUT102, and SLB-1 are IL-2-independent HTLV-I-transformed human T-cell lines, and Tax1 is an IL-2-dependent human T-cell clone immortalized by Tax in the context of a herpes saimiri vector. These cell lines have been described previously (74). Human embryonic kidney cell line 293 and an HTLV-I-negative leukemia T-cell line SupT1 (165) were from ATCC. Murine embryonic fibroblasts (MEFs) derived from Tak1- deficient (Tak1 / ) and wildtype control (Tak1+/+) mice were kindly provided by Dr. Shizuo Akira (150). IKKγ-deficient Jurkat cells (JM4.5.2) was generated by somatic cell mutagenesis (69). JM4.5.2 cell clones stably transfected with wildtype IKKγ and IKKγ mutants, D311N and L329P, were provided by Dr. Jonathan Ashwell (22). Plasmid constructs, antibodies, and other reagents. pcmv4-based expression vectors encoding Tax and Tax M22 were kindly provided by Dr. Warner Greene (164). Green fluorescence protein (GFP) expression vector (pegfp) was from CLONTECH. pclxsn
54 41 (GFP) retroviral vector was generated by replacing the neomycine-resistance gene of pclxsn (166) with EGFP cdna. The pclxsn (GFP)-Tax was cloned by inserting Tax cdna into the BamHI site of the pclxsn (GFP) vector. The pcmv-ha-tak1 vector and anti-tak1 antibody were kindly provided by Drs. Kunihiro Matsumoto and Jun Ninomiya- Tsuji (21). GST-IκBα (1-54) and GST-c-Jun (1-79) were described previously (109). Recombinant MKK6 was from Upstate. HRP-conjugated HA antibody (3F10) was from Roche Molecular Biochemicals. Anti-Tax monoclonal antibody was prepared from a hybridoma (168B ) provided by the AIDS Research and Reference Program, NIAID, NIH. Anti-IKKα (H-744), anti-ikkβ (H470), anti-ikkγ (FL-419), anti-actin (C-2), anti- JNK1 (C-17), and anti-tubulin (TU-02) were from Santa Cruz. Cell transfection and retroviral infection. MEFs and 293 cells were seeded in 6-well plates and transfected using Lipofectamine-2000 (Invitrogen). Retroviral transduction was performed using the pclxsn system provided by Dr. Inder Verma (166). The procedure for retrovirus production and infection was as previously described (167). The bulk of infected cells were used in experiments to prevent clonal variation. Immunoblotting (IB), immunoprecipitation (IP), and in vitro kinase assays. Cells were lysed in a buffer containing 20 mm Hepes (ph 7.6), 250 mm NaCl, 0.5% NP-40, 20 mm β- glycerophosphate, 1 mm EDTA, 20 mm p-nitrophenylphosphate, 0.1 mm Na 3 VO 4, 1 mm dithiothreitol (DTT) and 1 mm phenylmethylsulfonyl fluoride (PMSF). The protein complexes were isolated by IP and subjected to either IB or in vitro kinase assays essentially as described (74).
55 42 Electrophoresis mobility shift assay (EMSA). Nuclear extracts were prepared and subjected to EMSA using a 32 P-radiolabeled κb probe (5'-CAA CGG CAG GGG AAT TCC CCT CTC CTT-3') or a control probe containing the NF-Y binding site (5 -AAG AGA TTA ACC AAT CAC GTA CGG TCT-3 ) followed by resolving the DNA-protein complexes on native 5% polyacrylamide gels. Reporter gene assays. 293 cells were seeded into 24-well plates and transfected, using Lipofectamine-2000, with the indicated cdna expression vectors together with 100 ng κbluciferase reporter. For normalizing the transfection efficiency, the cells were also transfected with a control renilla luciferase reporter driven by the constitutive thymidine kinase promoter (40 ng). At 36 hr post transfection, the cells were collected for duel luciferase assays (Promega). The κb-specific luciferase activity was normalized based on the ranila luciferase activity.
56 43 Acknowledgements We thank Warner Greene for Tax expression vectors, Inder Verma for retroviral vectors, Kunihiro Matsumoto and Jun Ninomiya-Tsuji for anti-tak1 antibody and Tak1 expression vector, Shizuo Akira for Tak1 knockout MEFs, Jonathan Ashwell for IKKγrescued JM4.5.2 cells, and the AIDS Research and Reference Program of NIAID for anti- Tax hybridoma. This work was supported by research grants (R01 CA68471, R01 CA94922, and R01 AI057555) from the National Institutes of Health to S.-C. S.
57 44 Figure 1 Tak1 is required for Tax-stimulated activation of IKK and NF-κB. (A) Tak1 / and wildtype (Tak +/+ ) MEFs were transfected with expression vectors encoding GFP (pegfp) or Tax (pcmv4-tax). Around 36 hr after transfection, nuclear extracts were isolated and subjected to EMSA using κb (upper) or NF-Y (lower) probes. (B) Cytoplasmic extracts (Panels 1 and 2) and nuclear extracts (panels 3 and 4) from the cells used in A were subjected to IB using the indicated antibodies. (C) MEFs were transfected with expression vectors encoding either GFP or Tax. Around 36 hr after transfection, RNA was prepared and subjected to semi-quantitative RT-PCR to analyze the mrna levels of TNF-a and the house keeping gene GAPDH. (D) IKK complex was isolated, from the cells used in A, by IP using anti-ikkγ followed by in vitro kinase assays using GST-IκBα(1-54) as substrate (Panel 1). IB was performed to monitor the expression level of IKKβ, Tax, and the loading control tubulin (Panels 2-4). (E) Whole-cell lysates prepared from the cells used in A were subjected to IB to detect the indicated proteins.
58 45
59 46 Figure 2 Tax stimulates the catalytic activity of Tak1. (A) 293 cells were either mock transfected ( ) or transfected (+) with expression vectors encoding HA-tagged Tak1, Tax, or both expression vectors. IKK complex was isolated by IP using anti-ikkγ followed by in vitro kinase assay using GST-IκBα(1-54) as substrate (panel 1). The kinase assay membrane was blotted with anti-ikkβ to detect the IKK protein in the immune complex (Panel 2). The expression level of Tak1 and Tax in cell lysates was detected by IB (Panels 3 and 4). (B) 293 cells were transfected (in 24-well plates) with (+) or without ( ) the indicated expression vectors along with a κb-luciferase reporter (100 ng) and a control Renilla luciferase reporter driven by the constitutive thymidine kinase promoter (prl-tk-luc, 40 ng). The κb-specific luciferase activity was normalized based on the control Renilla luciferase. Data are representative of three independent experiments. (C) 293 cells were transfected with (+) or without ( ) HA-Tak1 or Tax as indicated. Tak1 was isolated by IP and subjected to in vitro kinase assays using MKK6 as substrate (panel 1). The kinase assay membrane was subjected to IB to detect Tak1 protein (Panel 2). IB was also performed with the cell lysates to detect the expression levels of Tak1 and Tax (Panels 3 and 4). (D) Tak1 was isolated by IP from the HTLV-negative SupT1 and the indicated Tax-expressing T cells transformed by either HTLV-I (C8166, SLB-1, and HUT102) or Tax (Tax1). Tak1 kinase assay (Panel 1) and IB (Panels 2-4) were performed as in B. (E) SupT1 cells were infected with retroviral vectors encoding either GFP or Tax followed by analyzing the Tak1 kinase activity and protein expression levels as in C.
60 47
61 48 Figure 3 Tax physically interacts with Tak1 and induces Tak1/IKK association. (A) 293 cells were transfected with Tax either in the presence (+) or absence (-) of HA-Tak1. The Tak1 protein complex was isolated by IP using anti-tak1 followed by IB to detect the Tak1- associated Tax (Panel 1) and level of precipitated HA-Tak1 (Panel 2). IB was also performed to detect the expression of Tax and HA-Tak1 in cell lysates (Panels 3 and 4). The small amount of Tax precipitated by anti-tak1 from HA-Tak1-negative cells (Panel 1, lane 1) was likely due to the binding of Tax to endogenous Tak1. (B) 293 cells were transfected with either an empty vector ( ) or expression vectors encoding wildtype (WT) Tax or a mutant form of Tax (M22) known to be defective in IKK activation. Endogenous Tak1 was isolated by IP followed by IB to detect the coprecipitated Tax and M22 (Panel 1) and the amount of precipitated Tak1 (Panel 2). The expression level of Tax in cell lysates was also monitored by IB (Panel 3). (C) 293 cells were transfected as in B. Endogenous Tak1 was isolated by IP and subjected to kinase assays using MKK6 as substrate (Panel 1). IB was performed to detect the indicated proteins (Panels 2-4). (D) JNK1 was isolated by IP from the transfected cells used in B and subjected to kinase assays using GST-cJun (1-79) as substrate (top panel). The expression levels of JNK1 isoforms and Tax were analyzed by IB (lower two panels). (E) Tak1 complex was isolated from either SupT1 or the HTLV-I-transformed SLB-1 T cells followed by IB to detect the Tak1-associated IKKβ (Panel 1) and Tax (Panel 3) as well as the precipitated Tak1 (Panel 2). IB was also performed with cell lysates to monitor the indicated proteins (Panels 2-4). (F) 293 cells were transfected with Tak1 (+) either in the absence ( ) or presence (+) of Tax or Tax M22. IKK complex was isolated by IP using anti-ikkγ followed by IB to detect the
62 49 coprecipitated Tak1 (Panel 1) and IKKα (Panel 2). The amounts of transfected proteins were monitored by IB using the cell lysates (Panels 3 and 4).
63 50
64 51 Figure 4 Tax activation of NF-κB does not require the ubiquitin-binding function of IKKγ. IKKγdeficient Jurkat cells (JM4.5.2) were stably transfected with either wildtype (WT) IKKγ or the indicated IKKγ mutants defective in ubiquitin binding. These different cells were infected with retroviruses encoding carrying either the pclxsn (GFP) vector ( ) or pclxsn (GFP)-Tax. Around 30 hr post infection, the cells were collected for preparation of nuclear and whole-cell extracts. Nuclear extracts were subjected to EMSA using κb or NF-Y oligonucleotide probes, and the whole-cell extracts were analyzed by IB to detect the indicated proteins.
65 52
66 53 CHAPTER III TUMOR SUPPRESSOR CYLD REGULATES ANTIVIRAL INNATE IMMUNE RESPONSE
67 54 Abstract An antiviral innate immune response involves induction of type I interferons (IFNs) and their subsequent autocrine and paracrine actions, but the underlying regulatory mechanisms are poorly understood. We show here that CYLD, a tumor suppressor with deubiquitinating enzyme (DUB) function, has dual roles in regulating antiviral responses. CYLD deficiency causes constitutive activation of the atypical IκB kinases, IKKε and TBK1, and aberrant induction of IFNs in virus-infected cells. CYLD acts by preventing basal ubiquitination and signaling function of an IKKε/TBK1 activator, RIG-I. Interestingly, despite their competence in IFN production, the CYLD-deficient cells and mice are considerably more susceptible to viral infection. We provide genetic evidence that IFN-induced signaling and antiviral gene expression are attenuated in the absence of CYLD. These findings suggest that CYLD mediates effective and controlled antiviral responses by preventing IKKε/TBK1 deregulation and modulating IFN receptor signaling. Since IKKε/TKB1 deregulation is associated with oncogenesis, these findings also have implications on the tumor suppressor function of CYLD.
68 55 Introduction Innate immunity serves as the first line of host defense against microbial infections. A central step in anti-viral innate immunity involves the production of type I interferons (IFNs), which is triggered when host pattern recognition receptors (PRRs) detect viral products(168, 169). Several members of the Toll-like receptor (TLR) family function as PRRs that detect viral nucleic acids in endosomes. In addition, viral RNAs produced during viral replication in the cytoplasm can be detected by two recently identified PRRs, retinoic acid induced gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA-5) (170). The different PRRs signal for IFN production through a common downstream signaling pathway that involves activation of the IFN-responsive factor (IRF) transcription factors, IRF3 and IRF7 (171). Once produced by virus-infected cells, the type I IFNs (IFN-α and IFN-β) bind to their receptor, the type I IFNα/β receptor (IFNAR), on both the infected cells and neighboring uninfected cells. These autocrine and paracrine actions of IFNs elicit a second-wave of signaling events, characterized by activation of Janus kinases (JAKs) and subsequent phosphorylation of their target transcription factors, signal transducer and activator of transcription 1 (STAT1) and STAT2 (172). Phosphorylated STATs form dimers and migrate into the nucleus to participate in transactivation of IFN-responsive genes involved in the establishment of an antiviral state (172). Recent studies have established two homologous protein kinases, IKKε and TBK1, as key mediators of PRR-induced IFN gene expression (99, 100, ). IKKε (also named IKKi) and TBK1 (also named T2K and NAK) are known as IκB kinase (IKK)-related kinases due to their structural homology to IKK ( ), a central
69 56 component of the NF-κB signaling pathway (3, 4). Unlike the typical IKK, which phosphorylates NF-κB inhibitors (IκBs) and mediates NF-κB activation, IKKε and TBK1 phosphorylate IRF3 and IRF7 to trigger their dimerization and nuclear translocation, thereby mediating induction of IFN gene expression (4). However, emerging evidence suggests that IKKε and TBK1 are multi-functional kinases. In addition to mediating IFN induction, IKKε/TBK1 promotes cell survival and oncogenesis(141, 142, 176, 177). The latter function of IKKε/TBK1 appears to be independent of IFNs, although the precise molecular mechanism remains unclear (142, 177). Since uncontrolled function of IKKε and TBK1 contributes to the survival and transformation of cancer cells (177), their activity must be subject to tight regulation. However, how IKKε/TBK1 is negatively regulated is currently not well understood. An emerging mechanism that regulates signal transduction in various biological processes is protein ubiquitination (178). Analogous to protein phosphorylation, which is reversibly controlled by protein kinases and phosphatases, protein ubiquitination is a reversible event mediated by the counteractive actions of ubiquitin conjugating enzymes and deubiquitinating enzymes (DUBs) (179). Although ubiquitination is traditionally known as a process that mediates protein degradation by the proteasome, it is now evident that specific ubiquitin chains facilitate protein/protein interactions that lead to the activation of signaling molecules (180). Notably, ubiquitination of RIG-I, a PRR that recognizes double-stranded and 5 -phosphorylated single-stranded viral RNAs (170), triggers its function in the activation of IKKε/TBK1 (102). This activation event is mediated by the ubiquitin ligase TRIM25 (102), a member of the tripartite motif (TRIM)
70 57 protein family (181). To date, the counteracting DUB(s) involved in negative regulation of RIG-I has not been characterized. In the present study, we show that CYLD, a recently identified DUB ( ), physically interacts with RIG-I and negatively regulates RIG-I ubiquitination. CYLD deficiency causes constitutive activation of IKKε and TBK1 along with heightened induction of IFNs by viral infection. Interestingly, CYLD has dual functions in antiviral responses. It both governs effective IFN-induced signaling and prevents deregulated activation of IKKε/TBK1. Thus, the CYLD deficiency attenuates antiviral host defense both in cell culture and in animals. Our findings establish CYLD as a novel and critical innate immune regulator that may function to maintain safe and effective antiviral immune responses. Given the oncogenic action of IKKε and TBK1, these findings also shed new light on how CYLD suppresses tumorigenesis. Results Hyperproduction of type I IFNs by CYLD / cells To investigate the role of CYLD in regulating antiviral immune responses, we analyzed the effect of CYLD deficiency on cytokine production induced by vesicular stomatitis virus (VSV) using a strain (AV-1) known to induce strong production of cytokines (182). Infection of wildtype dendritic cells (DCs) resulted in strong induction of IFN-α, as detected by ELISA (Fig. 1A). Interestingly, the level of IFN-α induction was markedly higher in CYLD / DCs (Fig. 1A), thus revealing a role for CYLD in negatively regulating virus-induced IFN production. Aberrant induction of IFN-α was
71 58 also detected in CYLD / mouse embryonic fibroblasts (MEFs) (Fig. 1A). Furthermore, the negative role of CYLD in IFN regulation occurred at the level of RNA, since the CYLD deficiency caused a hyper induction of IFN-α and IFN-β mrnas by VSV (Fig. 1B and C). To examine whether CYLD negatively regulates the transcription of IFN genes, we performed reporter gene assays using a luciferase reporter driven by the IFN-β promoter (IFNβ-luc). VSV infection induced IFNβ-luc expression in wildtype MEFs (Fig. 1D). Moreover, a strikingly higher level of IFNβ-luc induction was detected in the CYLD / MEFs (Fig. 1D). This result was not due to variations in transfection, since the data were normalized based on expression of a constitutive Renilla luciferase. Thus, CYLD deficiency causes aberrant expression of type I IFN genes in virus-infected cells. Loss of CYLD causes constitutive activation of IKKε/TBK1 Since IKKε and TBK1 are critical signaling molecules in antiviral immune responses (99, 100), we next examined whether CYLD regulates the activity of these atypical IKKs. Infection of wildtype DCs with VSV led to a dose-dependent activation of IKKε (Fig. 2A, panel 1, lanes 2 and 3). Remarkably, the CYLD deficiency caused constitutive activation of IKKε even in uninfected DCs (Fig. 2A, panel 1, lane 4). The activity of IKKε in CYLD / cells was not further induced upon VSV infection (Fig. 2A, upper panel, lanes 5 and 6). These results suggest that CYLD is critical for maintaining the inducible function of IKKε. As seen with IKKε, the other atypical IKK, TBK1, was constitutively activated in uninfected CYLD / cells (Fig. 2B). These data demonstrate an essential role for CYLD in preventing uncontrolled activation of IKKε and TBK1 and maintaining their ability to respond to viral infection.
72 59 Viral infection also activates the typical IKK complex, which contributes to the induction of various cytokines, including IFNs. We thus examined whether CYLD regulates the activity of the typical IKK in DCs. Following VSV infection, the typical IKK complex was isolated by IP using the IKKγ antibody and subjected to kinase assays using IκBα as substrate. As expected, VSV infection caused a dose-dependent induction of IKK activity in wildtype DCs (Fig. 2A, panel 3, lanes 2 and 3). However, unlike the effect on IKKε/TBK1, the CYLD deficiency did not significantly upregulate the constitutive activity of IKK (Fig. 2A, panel 3, lane 4). It is noteworthy, on the other hand, that the effect of CYLD on IKK activation appeared to be cell type specific, since loss of CYLD causes constitutive activation of IKK in lymphocytes (113, 119). Notwithstanding, these findings suggest that CYLD plays a crucial role in preventing uncontrolled activation of IKKε/TBK1 in DCs. CYLD inhibits the ubiquitination and signaling function of RIG-I Ubiquitination plays an important role in antiviral immune responses. In particular, ubiquitination controls the activity of a cytoplasmic RNA sensor and IKKε/TBK1 activator, RIG-I (102). To understand how CYLD regulates IKKε/TBK1, we examined the role of CYLD in regulating the ubiquitination of RIG-I. Consistent with a recent report, RIG-I became ubiquitinated when expressed in 293 cells (Fig. 3A, lane 2). Furthermore, a constitutively active form of RIG-I containing its N-terminal 2 CARD domains, RIG-I (2CARD), was even more robust in undergoing ubiquitination (Fig. 3B, lane 2; note the lower expression level of 2CARD than the wildtype RIG-I). Importantly, the ubiquitination of both RIG-I and RIG-I (2CARD) was efficiently
73 60 inhibited by CYLD (Fig. 3A and B, lane 3). This action of CYLD required its DUB activity, since a catalytically inactive CYLD mutant (1-932) failed to inhibit RIG-I ubiquitination (Fig. 3A and B, lane 4). To assess the physiological relevance of these findings, we examined the ubiquitination of RIG-I in wildtype and CYLD / DCs. A low level of RIG-I ubiquitination was detected in wildtype DCs (Fig. 3C, lane 1). In contrast, a notable accumulation of the ubiquitinated RIG-I was detected in the CYLD / DCs (Fig. 3C, lane 2). Parallel assays did not detect appreciable ubiquitination of a RIG-I binding protein, MAVS, in wildtype or CYLD / cells (Fig. 3C, lanes 3 and 4). Thus, we propose that CYLD is a DUB that prevents uncontrolled ubiquitination of RIG-I. To directly examine whether CYLD regulates the signaling function of RIG-I, we performed reporter gene assays. As previously reported, the constitutively active RIG- I(2CARD) induced the expression of IFN-β-luc reporter in wildtype MEFs (Fig. 3D). Consistent with the biochemical studies described above, the signaling function of RIG-I (2CARD) was greatly potentiated in CYLD / MEFs (Fig. 3D). Moreover, restoration of CYLD expression in these mutant cells led to efficient suppression of the RIG-I (2CARD) signaling function (Fig. 3D). These results suggest that CYLD negatively regulates the ubiquitination and signaling function of RIG-I, which provides an insight into the mechanism of IKKε/TBK1 regulation by CYLD. CYLD physically interacts with RIG-I To determine the mechanism by which CYLD regulates RIG-I function, we examined the potential physical association between RIG-I and CYLD. When co-expressed in 293 cells, CYLD indeed formed a complex with wildtype Flag-tagged RIG-I, which was
74 61 precipitated by the Flag antibody (Fig. 3E, lane 2). This molecular interaction was specific, since the Flag antibody did not precipitate CYLD in cells lacking Flag-RIG-I. The N-terminal CARD domain of RIG-I, forming the catalytically active region, appeared to be the binding site for CYLD, since RIG-I (2CARD) was sufficient for CYLD binding (Fig. 3E, lane 3). In fact, this RIG-I mutant exhibited strikingly higher CYLD-binding activity than the wildtype RIG-I (Fig. 3E), which may suggest that CYLD preferentially binds to activated RIG-I. We also examined the molecular interaction of CYLD with other known components of the RIG-I signaling complex, including MAVS and TRAF3 (83, 183, 184). Like the RIG-I (2CARD), MAVS strongly interacted with CYLD, whereas TRAF3 only exhibited a weak CYLD-binding activity (Fig. 3E, lanes 4 and 5). Thus, CYLD appears to physically target the RIG-I signaling complex. CYLD is required for antiviral defense in mice Since CYLD negatively regulates IFN induction, we examined whether the loss of CYLD renders mice more resistant to viral infection. Age-matched CYLD / and wildtype control mice were infected intravenously (i.v.) with a sub-lethal doses of VSV and monitored daily for mortality and survival over a course of 14 days. Under this condition, about 60% of the wildtype mice survived the infection (Fig. 4A). Surprisingly, the CYLD / mice did not show enhanced antiviral ability. In fact, the CYLD / mice were substantially more sensitive to VSV infection, with only about 20% of the mice surviving under the same conditions (Fig. 4A). To examine whether the role of CYLD in antiviral defense is route-dependent, we repeated the experiment by intranasal (i.n.) infections. We selected a dose range known
75 62 to cause a low level of mortality in wildtype mice (185, 186). At a dose of 2.5 x 10 4 pfu, more than 80% of the wildtype mice survived the infection following a 12-day period of observation (Fig. 4B). As seen with the i.v. injection, the CYLD / mice had a much higher mortality rate, with only about 20% surviving the infection (Fig. 4B). Starting from 2-4 days of infection, the mice that eventually died of the infection also displayed notable disease symptoms, including respiratory distress, piloerection, disorientation, and other neurological symptoms. These results reveal a novel and unexpected function of CYLD in regulating antiviral host defense. CYLD / dendritic cells and fibroblasts are more sensitive to VSV infection To examine whether the CYLD / cells are intrinsically more sensitive to VSV infection, we infected wildtype and CYLD / DCs with VSV and assessed the efficiency of viral infection based on the expression of a major viral glycoprotein, VSV-G. Consistent with the whole-animal studies, the CYLD / DCs were dramatically more sensitive to VSV infection compared to the wildtype DCs (Fig. 5A). Similar results were also obtained with mouse embryonic fibroblasts (MEFs) (Fig. 5B). To further confirm the hypersensitivity of CYLD / cells to VSV infection, we employed a VSV strain expressing the GFP protein (VSV-GFP). In agreement with a recent study (95), the wildtype MEFs were highly resistant to VSV-GFP infection. Importantly, the CYLD / MEFs displayed markedly higher sensitivity (Fig. 5C). Thus, despite their hyperproduction of IFNs, the CYLD / cells are more sensitive to viral infection. These results further establish the crucial role for CYLD in regulating antiviral immune response.
76 63 CYLD deficiency attenuates IFN-induced antiviral defense and signaling Since loss of CYLD does not block, but rather promotes, the induction of IFNs, the question was raised as to how the CYLD deficiency attenuates antiviral host defense. As stated above, antiviral innate immunity involves both the initial induction of type I IFNs and the subsequent effector functions of IFNs. When secreted by virus-infected cells, type I IFNs bind to their specific receptor, IFNAR, on both the infected cells and neighboring uninfected cells. Thus, IFNs not only restrain the replication of viruses in infected cells but also protect uninfected cells from viral infection. To further address the mechanism by which CYLD regulates antiviral innate immunity, we examined how CYLD deficiency affects cellular responses to IFNs. We used a well-defined method to compare the IFN responses of wildtype and CYLD / DCs. When pre-incubated with exogenous IFN-β, wildtype cells were efficiently protected from VSV infection (Fig. 6A). In contrast, IFN-β was considerably less efficient in protecting the CYLD / cells, generating a notable protective effect only at a high concentration (Fig. 6A). This finding indicates that CYLD controls the efficiency of IFN-induced cell signaling, although it is not absolutely essential for this molecular event. A critical step in IFN-induced cell signaling is tyrosine phosphorylation of STAT1, which serves as a molecular trigger for its dimerization and nuclear translocation(172). As expected, IFN-β induced rapid and sustained STAT1 phosphorylation in wildtype DCs (Fig. 6B). Consistent with the antiviral functional assays described above, the CYLD deficiency significantly attenuated, although it did not block, the inducible phosphorylation of STAT1 (Fig. 6B).
77 64 The consequence of IFN signaling is the induction of genes involved in the establishment of an antiviral state. We thus examined how the loss of CYLD affected IFN-induced gene expression. Stimulation of wildtype DCs with IFN-β led to strong induction of three known IFN-responsive genes (187), MX1, Adar1, and Ifi203 (Fig. 6C). Remarkably, the induction of all three of these genes was diminished in CYLD / DCs (Fig. 6C). Thus, CYLD is a crucial factor that mediates effective IFN signaling and antiviral gene induction. Discussion The results presented in this paper establish CYLD as a novel regulator of antiviral innate immune responses. A unique feature of CYLD is its dual roles in regulating the signaling network involved in the establishment of an antiviral state. On one hand, CYLD prevents uncontrolled activation of IKKε and TBK1, thereby ensuring normal induction of IFNs during a viral infection. On the other hand, CYLD modulates IFN-induced signaling to achieve efficient effector functions of IFNs in the antiviral host defense. We have obtained genetic evidence that CYLD is required for maintaining a normal host defense against viral infections. The CYLD / DCs and fibroblasts are hyper-sensitive to VSV infection despite their competence in producing IFNs, and the CYLD / mice display attenuated anti-vsv host defense ability. This is to our knowledge the first demonstration that a DUB functions as an essential antiviral innate immune regulator. A recent study suggests that CYLD is an evolutionarily conserved DUB that exists in lower invertebrate animals such as Drosophila. Interestingly, as seen in mice, loss of CYLD in Drosophila renders these flies more vulnerable to infections (188). The
78 65 flies do not have an adaptive immune system, but they possess innate immune mechanisms that mediate effective defense against various microbial infections (189). Although how CYLD regulates host defense in Drosophila remains unclear, our present findings in mice suggest the possibility that regulation of innate immune function may represent an evolutionarily conserved function of CYLD. We have shown that in addition to maintaining the inducible nature of IKKε/TBK1, CYLD participates in IFNinduced antiviral signaling. In the absence of CYLD, IFNs are produced but are ineffective in inducing the expression of antiviral genes. Furthermore, the CYLD deficiency attenuates IFN-induced STAT1 phosphorylation. Our finding that CYLD maintains the normal signaling function of IKKε/TBK1 suggests a role for CYLD in preventing aberrant production of IFNs, a condition that may lead to severe autoimmune disorders ( ). Additionally, our finding also sheds new light on the tumor suppressor function of CYLD. CYLD was initially discovered as a tumor suppressor mutated in cylindromatosis, a predisposition to multiple tumors of skin appendages (118). Accumulating evidence suggests that CYLD deficiency may also promote the development of other types of tumors, including multiple familial trichoepithelioma ( ), colon and hepatocellular carcinomas (128), and multiple myeloma (126, 127, 196). Interestingly, recent studies identified both IKKε and TBK1 as oncoprotein kinases (141, 142, 176, 177). The IKKε gene is amplified in breast cancer cells (142), and deregulation of IKKε and TBK1 is also involved in other cancers (141, 142, 176). Notably, IKKε promotes cell survival and oncogenesis by an IFN-independent mechanism (142), thus revealing its diverse biological functions. We have found that CYLD prevents spontaneous activation of IKKε/TBK1 not only in DCs but also in other
79 66 cell types, including T cells (data not shown). Future studies will examine whether the IKKε/TBK1 activation promotes the survival of CYLD / cells. A recent study suggests that ubiquitination plays an important role in IKKε/TBK1 activation by RIG-I (102). During viral infection, RIG-I is transiently ubiquitinated via the specific action of an E3 ubiquitin ligase, TRIM25, and this molecular event appears to serve as a trigger for initiating an antiviral signaling pathway leading to activation of IKKε/TBK1 and induction of type I IFNs. Our current work demonstrates CYLD as a DUB that negatively regulates RIG-I ubiquitination. Remarkably, loss of CYLD causes constitutive ubiquitination of RIG-I. This finding implies that RIG-I may be undergoing constant ubiquitination and deubiquitination, with deubiquitination being a dominant event. In support of the previous report (102), we have shown that the accumulation of ubiquitinated RIG-I in CYLD / cells is associated with constitutive activation of IKKε and TBK1. However, it is important to note that CYLD does not seem to regulate the magnitude or kinetics of virus-induced IKKε/TBK1 activation. In fact, in CYLD / cells, VSV infection causes downregulation of IKKε/TBK1 activity (Fig. 2A). Thus, CYLD may function to maintain the normal inducible nature of IKKε/TBK1 and prevent deregulated production of IFNs and other biological functions of IKKε/TBK1.
80 67 Experimental Procedures Mice Cyld knockout mice were generated as described (111). Heterozygous (Cyld +/ ) mice (in C57BL6/DBA mixed genetic background) were intercrossed to generate Cyld / and Cyld +/+ littermates, and genotyping was performed by PCR using tail DNA (113). All mice were housed in specific pathogen-free cages and monitored periodically (every 3 month) for the lack of common pathogens. Animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University College of Medicine and the University of Texas MD Anderson Cancer Center. Plasmids, antibodies, and cytokines pcdna-ha based expression vectors encoding CYLD, a catalytically inactive CYLD mutant (1-932), TRAF3, and ubiquitin were described previously (61, 109, 197). GST- IRF3 (amino acids ) was a gift from John Hiscott (100). GST-IκBα (amino acids 1-54 of IκBα) was described (109). Flag-tagged RIG-I and a RIG-I truncation mutant containing the N-terminal CARD domains, RIG-I(2CARD), were provided by Chris Basler, Flag-tagged MAVS was provided by Kui Li, and IFNβ-luc (luciferase driven by IFN-β promoter) was provided by Dimitris Thanos. Antibodies for STAT1 (p84/p91, M22), TRAF6 (H274), Tubulin (TU-02), IKKγ (FL419), TBK-1 (M-375), and TRAF3 (H122) were purchased from Santa Cruz. Anti- Flag, anti-ikkε (anti-ikki), anti-phospho-stat1 (Tyr701), and anti-ha-hrp (3F10) were from Sigma, ebioscience, Cell Signaling, and Roche, respectively. Rabbit
81 68 polyclonal antibodies for CYLD and RIG-I were described previously (109, 198). Antiubiquitin and anti-mavs were provided by Drs. Vincent Chau and Zhijian Chen, respectively. Recombinant IFN-β was purchased from PeproTech. Cells and transfection Human embryonic kidney cell line 293T was cultured in DMEM media containing 5% FBS. The cells were seeded in 6-well plates and transfected using Lipofectamine-2000 (Invitrogen). DCs were prepared by cultivating bone marrow cells in RPMI medium containing 10% FBS and 20% of a GM-CSF conditional medium (provided by Dr. Christopher Norbury). 50% of the culturing medium was replaced with fresh growth medium every 2 days, and cells were used for experiments between day 7 and day 9. To prepare CYLD +/+ and CYLD / MEFs, CYLD +/ mice were bred to produce CYLD / and control embryos. Cells were prepared from the embryos, cultured in DMEM medium supplemented with 10% FBS, and immortalized using SV40 large T antigen. Viruses and infection VSV (Indiana strain) and VSV carrying a GFP gene (VSV-GFP) were provided by Glen Barber (199). A VSV variant harboring a point mutation in the M gene (AV1) was provided by John Bell (182). AV1 is more potent than parental VSV in cytokine induction due to reduced activity of the mutant M in blocking nuclear export of mrna (182). Viral stock was prepared and titered by infecting baby hamster kidney (BHK) cells.
82 69 For in vitro infection, DCs were washed with and resuspended in HBSS buffer supplemented with 0.08% bovine serum avalbumin. The cells were incubated with the indicated doses of VSV strains for 30 min at 37ºC and then collected by centrifugation. Infected cells were cultured in DC growth medium for the indicated times. MEFs were seeded into 12-well plates (3.75 x 10 5 cells/well) and infected with VSV strains in serumfree medium for 1 hr. The cells were washed once and cultured in growth medium. For in vivo infection, age-matched CYLD / and wildtype mice were infected i.v. via tail veins or i.n. by placing a droplet between the nostrils using a pippetor. The infected mice were observed daily for disease symptoms and mortality. Animals displaying hindlimb paralysis or severe morbidity were noted as such and euthanized according to IACUC protocol. ELISA and Real-time quantitative RT-PCR DCs and MEFs were infected with VSV AV1 as described above. At the indicated times of postinfection, the cell culture supernatants were collected and subjected to ELISA using a commercial IFN-α kit (PBL Biomedical Laboratories). For analyzing RNA induction, total RNA was isolated from DCs and MEFs using TRIZOL reagent (Invitrogen) and subjected to cdna synthesis using RNase H-reverse transcriptase (Invitrogen) and oligo (dt) primers. Real-time quantitative PCR was performed using icycler Sequence Detection System (Bio-Rad) and RT Real-Time TM SYBR green PCR master mix (Superarray). The expression of individual genes was calculated by a standard curve method and normalized to the expression of actin. The gene-specific primer sets (all for murine genes) were: IFN-β, 5 -AGCTCCAAGAAAGGACGAACAT-3 and 5 -GCCCTGTAGGTGAGGTTGATCT-3 ;
83 70 IFN-α, 5 -TGACCTCAAAGCCTGTGTGATG-3 and 5 -AAGTATTTCCTCACAGCCAGCAG- 3 ; MX1, 5 -AAACCTGATCCGACTTCACTTCC-3 and 5 - TGATCGTCTTCAAGGTTTCCTTGT; Ifi203, 5 -CTCTCACTGATGGTAAGACTTAC-3 and 5 -CTGCAGGTACATGGTCAGTCAC-3 ; Adar1, 5 -GTGAGTTTCGAGCCATCATGG-3 and 5 -TTCAGCTGGCACTCAGTTAGC-3 ; Actin, 5 -CGTGAAAAGATGACCCAGATCA-3 and 5 -CACAGCCTGGATGGCTACGT-3. Luciferase reporter gene assays MEFs were seeded into 24-well plates (1 x 10 5 cells/well) and transfected using Lipofectamine 2000 (Invitrogen) with IFNβ-luc (300 ng) together with a control Renilla luciferase reporter driven by the constitutive thymidine kinase promoter (prl-tk-luc, 5 ng). After 24 h of transfection, the cells were either mock infected or infected with VSV AV1 for 16 h. Dual luciferase assays were performed according to manufacturer s instruction (Promega, Madison, WI). In some experiments, the reporters were cotransfected with cdna expression vectors, and luciferase assays were performed after 36 h. The IFNb-specific luciferase activity was normalized on the basis of the Renilla luciferase activity. IB, coip, protein kinase assay, and ubiquitination assay. Whole-cell lysates were prepared in a kinase cell lysis buffer supplemented with phosphatase inhibitors, and subjected to IB, coip, and in vitro kinase assays as previously described (74). For ubiquitination assays, cellls were lysed in kinase cell lysis buffer supplemented with
84 71 1mM N-ethylmaleimide (NEM). RIG-I and MAVS were isolated IP, and their ubiquitinconjugated aducts were detected by IB using anti-ubiquitin. Acknowledgements We thank Glen Barber, John Bell, Vincent Chau, Zhijian Chen, John Hiscott, Kui Li, Christopher Norbury, and Dimitris Thanos for reagents. This study was supported by grants from National Institutes of Health (AI064639, AI057555, and CA94922).
85 72 Figure 1. Aberrant induction of type I IFN expression by VSV in CYLD / cells. (A) Bone marrow derived DCs and MEFs prepared from CYLD +/+ and CYLD / mice were infected with VSV AV1 (moi: 0.1 for DCs and 0.03 for MEFs ) or treated under the same conditions in the absence of viruses (mock). Media were collected at 18 hr postinfection and subjected to ELISA to detect IFN-α. Data are presented as means ± standard deviation (s.d.). (B) and (C) CYLD +/+ and CYLD / DCs (B) and MEFs (C) were infected as in A for 12 h, and RNA was isolated for real-time PCR. RNA levels are presented as fold of induction over mock-infected CYLD +/+ cells (D) CYLD +/+ and CYLD / MEFs were transfected with IFNβ-luc and a control Renilla luciferase reporter, prl-tk-luc. At 24 h post-transfection, the cells were either mock infected or infected with VSV AV1 (moi: 0.03) and subjected to dual luciferase assays after 16 h of infection. The IFNβ-specific luciferase activity was normalized on the basis of the control Renilla luciferase and presented as relative luciferase unit (RLU). Data represent means of three independent experiments ±s.d.
86 73
87 74 Figure 2. Constitutive activation of IKKε and TBK1 in CYLD / DCs. (A) DCs derived from the bone marrow of CYLD +/+ and CYLD / mice were either mock infected or infected with VSV for 2 h at the indicated moi. IKKε was isolated by IP using anti-ikkε and subjected to in vitro kinase assays using GST-IRF-3 as substrate (panel 1). The typical IKK complex was isolated by IP using anti-ikkγ and subjected to kinase assays using GST-IκBα as substrate (panel 3). Cell lysates were subjected to IB to examine the expression of the indicated proteins (panels 2, 4, 5, and 6). (B) CYLD +/+ and CYLD / DCs were lysed without mock infection and subjected to IKKε and TBK1 kinase assays using GST-IRF-3 as substrate and IB assays.
88 75
89 76 Figure 3. CYLD physically interacts with RIG-I and inhibits the ubiquitination and signaling function of RIG-I. (A) Ubiquitination of transfected RIG-I. 293 cells were transfected with (+) or without ( ) Flag-tagged RIG-I either in the absence ( ) or presence of wildtype (Wt) CYLD or a catalytically inactive CYLD mutant (Mut, CYLD 1-932). All cells were also transfected with HA-tagged ubiquitin. RIG-I was isolated by IP using anti-flag, and the ubiquitinconjugated RIG-I was detected by IB using anti-ha (upper panel). The level of RIG-I expression was monitored by IB (lower panel). (B) Ubiquitination of RIG-I mutant. 293 cells were transfected as in A excepted for the replacement of wildtype RIG-I with a RIG-I truncation mutant encoding its N-terminal 2 CARD domains (2CARD). The ubiquitination and expression of RIG-I (2CARD) were analyzed as in A. (C) Ubiquitination of endogenous RIG-I. Endogenous RIG-I and MAVS were isolated by IP from lysates of CYLD +/+ and CYLD / DCs, and the ubiquitinated RIG-I and MAVS were detected by IB using anti-ubiquitin (upper panel). Cell lysates were subjected to IB to monitor the expression of RIG-I and MAVS. (D) CYLD +/+ and CYLD / MEFs were transfected with (+) or without ( ) the indicated expression vectors along with IFNβ-luc and the control reporter prl-tk-luc. After 36 h, cell lysates were subjected to dual luciferase assays. The IFNβ-specific luciferase activity was normalized on the basis of the control Renilla luciferase and presented as in Fig. 1D. (E) 293 cells were transfected with HA-CYLD along with an empty pcdna vector, Flag- RIG-I, Flag-2CARD, Flag-MAVS, or HA-TRAF3. The expressed proteins were isolated
90 77 by IP using anti-flag (for RIG-I, 2CARD, and MAVS) or anti-traf3 (for TRAF3), and the co-precipitated CYLD was analyzed by IB using anti-ha (top panel). The membranes were reprobed with anti-flag or anti-ha to detect the expression of RIG-I and 2CARD (left, middle panel), MAVS (right, panel 2), and TRAF3 (right, panel 3). The cell lysates were subjected to direct IB to analyze CYLD expression (bottom panel).
91 78
92 79 Figure 4. CYLD regulates antiviral host defense. (A) Age-matched CYLD +/+ (n = 16) and CYLD / (n = 16) mice were infected i.v. with VSV (Indiana strain; 5 x 10 7 pfu). Mortality was monitored for 14 days, and data are presented as percentage of survival mice. (B) Age-matched CYLD +/+ (n = 11) and CYLD / (n = 12) mice were infected i.n. with VSV (Indiana strain; 2.5 x 10 4 pfu). Mortality was monitored for 12 days, and data are presented as percentage of survival mice.
93 80
94 81 Figure 5. CYLD regulates antiviral defense in DCs and MEFs. (A) and (B) CYLD +/+ and CYLD / DCs (A) and MEFs (B) were either mock infected or infected with the indicated moi of VSV for 20 hr. The level of VSV infection was assessed by IB to detect a major VSV glycoprotein, VSV-G. Tubulin IB was used as loading control. (C) MEFs were either mock infected or infected with VSV-GFP and subjected to microscopy to monitor the total cells (Bright) and VSV-infected cells (GFP). Data are representative of 4 independent experiments.
95 82
96 83 Figure 6. CYLD deficiency attenuates IFN-induced antiviral defense, STAT1 phosphorylation, and gene expression. (A) CYLD +/+ and CYLD / DCs were pre-treated overnight with the indicated doses of IFN-β and then infected with VSV (moi: 0.1). After 20 h, the level of viral infection was assessed by IB to detect VSV-G expression. (B) CYLD +/+ and CYLD / DCs were stimulated with IFN-β (500 U/ml) for the indicated times, and STAT1 phosphorylation was analyzed by IB using a phospho-specific STAT1 antibody detecting phosphorylated tyrosines 84 and 91. Total STAT1 and tubulin were included as loading controls. (C) CYLD +/+ and CYLD / DCs were stimulated with IFN-β for 3 h. Total RNA was isolated and subjected to real-time PCR assays. Relative mrna levels are presented as fold of induction over non-treated (NT) CYLD +/+ cells, and data represent mean values ±s.d.
97 84
98 85 CHAPTER IV PHOSPHORYLATION OF DUB ENZYME CYLD BY IKKε/TBK1
99 86 Abstract CYLD is a deubiquitinating enzyme that plays an important role in diverse biological processes, including innate and adaptive immune responses, cell cycle progression, and spermatogenesis. CYLD functions by targeting different signaling molecules, particularly those involved in activation of the transcription factor NFκB and c-jun N-terminal kinase (JNK). However, it is currently unclear how the DUB function of CYLD is regulated. Nevertheless, we have recently shown that CYLD undergoes transient phosphorylation upon diverse cellular stimuli, although the underlying biochemical mechanism and functional significance remain poorly understood. In this study, we identified a critical serine site, Ser418 of CYLD as an inducible phopshorylation site by mass spectrometry analysis and phospho-specific antibody. Interestingly, mutation of this phosphorylation site of CYLD rendered it super active in the negative regulation of IKK and JNK signaling pathways. This finding is consistent with our in vitro finding that CYLD phosphorylation attenuates its DUB activity. We further show that the inducible phosphorylation of CYLD depends on the regulatory subunit of IKK complex, NEMO, and may also involve two IKK-related kinases, IKKε and TBK1. These findings provide important insights into the mechanism of CYLD regulation and shed light on how CYLD may mediate the crosstalk between diverse signaling pathways.
100 87 Introduction Protein ubiquitination is a posttranslational process that involves attachment of mono- or poly-ubiquitin chains to a protein, leading to the targeted protein degradation or modulation of its trafficking and signaling function (12, 200). This process is accomplished by a series of enzymes, namely a ubiqtuitin activating enzyme (E1), a ubiquitin conjugating enzyme (E2), and a ubiquitin ligase (E3)(12). The process of ubiquitination is reversed by deubiquitination enzymes (DUBs), which cleave ubiquitin chains from its protein substrates (15). Close to 100 DUBs have been identified, but the function of most of them is unknown. However, recent studies have characterized a number of DUBs that regulate signal transductions. One of them is CYLD, a tumor suppressor gene mutated in familial cyclindromatosis( , 118). Initial in vitro studies suggest that CYLD negatively regulates NF-κB activation by deubiquitinating the TNF receptor-associated factor 2 (TRAF2), TRAF6, and NEMO ( ). Recent studies on CYLD knock out mice uncovered several more substrates of this DUB and provided important insights into the physiological function of CYLD in vivo. Specifically, CYLD plays roles in T cell development, spermatogenesis, inflammation, and tumorigenesis by targeting and deubiqutinating Lck, RIP1, TAK1, and Bcl3, respectively (111, 113, 114, 116). Unlike other DUBs, such as A20, CYLD is a consistently expressed DUB, although its level can be further enhanced under certain conditions (122). Strong evidence suggests that CYLD is also a constitutively active DUB, since RNAi-mediated CYLD knockdown causes basal ubiquitination of TRAF2 (201). Similarly, CYLD
101 88 knockout causes spontaneous ubiquitination of several targets, such as RIP1 (114) and TAK1 (113). The question is thus raised as to how the activity of CYLD is regulated. Previously, we have shown that CYLD undergoes inducible phosphorylation and this phosphorylation appears to attenuate its DUB activity toward TRAF2 (201). Interestingly, this phosphorylation event is dependent on the regulatory subunit of IKK NEMO (201). Although this result suggests the mechanism of IKK in CYLD phosphorylation, genetic evidence is still lacking. Since NEMO is required for activation of both IKK and the IKK-related kinases, TBK1 and IKKε (139), all these kinases may have a potential role in CYLD phosphorylation. In this report, we further characterized the mechanism of CYLD phosphorylation. By mass spectrometry and phospho-specific antibody, we identified serine418 as an inducible CYLD phosphorylation site. Ser418 phosphorylation plays a regulatory role to phosphorylation on other sites within the serine cluster we identified previously. In the functional aspect, serine to alanine mutation on amino acid 418 of CYLD creates a super active DUB that, when expressed in HeLa and Jurkat T cells, strongly inhibits JNK and IKK activities. This finding indicates an important role for CYLD phosphorylation in negatively regulating its DUB function. Our transfection studies identified the IKK-related kinases, TBK1 or IKKε, as potent inducers of CYLD phosphorylation. In vitro kinase assays showed that the TBK1/IKKε induced CYLD phosphorylation predominantly occurs on serine 418. By using virus infection to activate TBK1/IKKε, we observed serine 418 phosphorylation in wild-type MEFs, while this phosphoryltaion was abrogated in TBK1/IKKε double knockout MEFs. Combined with the previous data showing CYLD regulates anti-viral immune responses (chapter III),
102 89 The phosphorylation of CYLD by TBK1/IKKε also provides valuable insights into understanding the regulation of host anti-virus process. Furthermore, given the oncogenic function of TBK1/IKKε, their role in CYLD phosphorylation has interesting implications on oncogenesis. Materials and Methods Plasmid constructs-human CYLD wildtype and mutant in pcdna-ha vector were described previously (109). Flag-tagged CYLD plasmid was produced by inserting the CYLD encoding fragment from pcdna-ha-cyld into p3xflag-cmv7 vector (Sigma), thus containing both Flag and HA tags. S418A mutant was generated by sitedirected mutagenesis (Stratagene) using the wildtype CYLD expression vector as template. To generate RNAi-resistant form of CYLD (CYLD R ) and S418A R plasmids, sense mutations (no alteration of amino acid codons) at the sirna-binding site were introduced (201). Retroviral vectors expressing CYLD and S418A mutant were obtained through transferring the corresponding cdnas from pcdna-ha to the pclxsn retroviral vector (provided by Dr. Inder Verma, see (166)). Expression vectors encoding HA-tagged NEMO, IKKβ, IKKα (74), GST-IκBα (1-54) and GST-c-Jun (1-79) (109) were previously described. Plasmids encoding for Flag-IKKε (WT) and the dominantnegative version Flag-IKKε K38A were gifts from Dr. R. Lin (McGill University, Montreal, Quebec, Canada). Flag-tagged TBK1 (WT) and Flag-tagged TBK1 (K38A) plasmids were kindly provided by Dr. James Chen (UT Southwestern Medical Center). GST-CYLD ( ) was generated by inserting the DNA fragment encoding amino
103 90 acid of human CYLD into the pgex4t-1 vector (Amersham/Pharmacia Biotech) (201). The point mutant of GST-CYLD ( ) S418A was generated by site-directed mutagenesis. All the DNA constructs were sequenced at the Core Facility of Hershey Medical Center at the Pennsylvania State University. Cell culture and antibodies-human embryonic kidney 293 cells, human cervical carcinoma HeLa cells, and Jurkat cells were obtained from ATCC. JM4.5.2 and JM4.5.2 IKKγ cells were described previously (69). TBK1+/-IKKε+/- and TBK1-/-IKKε-/- MEFs were kindly provided by Dr. S Akira (Osaka University, Osaka, Japan). The anti-cyld antibody (109) and phospho-cyld antibody detecting Ser418 phosphorylation (201) were previously described. The polyclonal antibodies for tubulin (TU-02), JNK1 (C-17), JNK2 (N-18), IKKγ (FL-419) and GST (Z-5) were purchased from Santa Cruz. Flag (M2) monoclonal antibody and HA-HRP (3F10) were from Sigma and Roche respectively. Silver staining and mass spectrometry-293 cells were transfected with plasmids encoding for Flag-CYLD and/or HA-NEMO using Lipofectamine 2000 following manufacturer s instructions. 36 hours later, cells were harvested and lysed in kinase lysis buffer as described (202). The expressed CYLD was isolated by immunoprecipitation using anti-flag antibody and separated by 10% SDS-PAGE gel. Silver staining was performed using a kit from Pharmacia Biotech (Catalog Number ). The visualized bands were excised by razor blade with as little excess empty gel as possible.
104 91 The LC/MS/MS analysis was done by the Taplin biological mass spectrometry facility at Harvard University (Boston, MA). Retroviral infection of CYLD-Stably knocking down endogenous CYLD in Jurkat or HeLa cells were previously described (201). Briefly, 293 cells were transfected with small hairpin RNA (shrna)-cyld construct (psuper-shcyld) together with retroviral packaging plasmids pcl-ampho and VSV-G to produce recombinant viruses, which were then used to infect Jurkat or HeLa cells. To reconstitute cells with CYLD and its mutant, pclxsn-gfp-cyld WT or S418A constructs were used to infect CYLD knockdown cells. Limiting dilution assays were used to enrich CYLD expressing cells as described (203). Virus infection of MEFs-VSV infection was performed as previously described (see chapter III). Briefly, MEFs were seeded one day prior to infection. Appropriate amount of VSV was diluted serum-free medium and incubated with MEFs for 1 hour at 37 ºC with 5% CO2. MEFs were then washed twice with serum free medium to rinse away non-absorbed virus and continue to culture with growth medium. Immunoblotting (IB), in vitro kinase assay-cell lysates were prepared by lysing the cells in kinase lysis buffer and immediately subjected to IB. In vitro kinase assays were performed as described (74).
105 92 Results CYLD is phosphorylated at serine 418 During the course of the previous investigation, we observed that CYLD undergoes transient phosphorylation by different stimuli, including the T-cell mitogens PMA and Ionomycine, the proinflammatory cytokine TNFα, and the TLR4 ligand LPS (201). The kinetics of CYLD phosphorylation is consistent with that of IKK activation. Interestingly, CYLD phosphorylation depends on IKKγ, as in IKKγ-deficient cells (JM 4.5.2), CYLD phosphorylation is abrogated. To examine the contribution of different IKK subunits to CYLD phosphorylation, CYLD was co-transfected with IKKβ, IKKα, or IKKβ plus IKKγ (Fig. 1A). The extent of CYLD phosphorylation was analyzed by immunoblot based on the retarded migration (band shift) of the phosphorylated CYLD. Expression of either IKKβ or IKKα only induced a weak level of CYLD phosphorylation. In contrast, a striking higher level of CYLD phosphorylation was detected when IKKβ is expressed together with IKKγ. Since overexpressed IKKβ is known to phosphorylate IκBα without the need of exogenous IKKγ, we tested whether IKKγ had a special role in mediating CYLD phosphorylation. Remarkably, expression of IKKγ alone led to strong CYLD phosphorylation (Fig.1B and 1C). Since IKKγ itself does not contain catalytic activity as IKKα or β, this indicates a kinase(s) other than IKK mediated by IKKγ might functions as the CYLD kinase. In order to characterize the functions of CYLD phosphorylation, identification of phosphorylation site(s) is critical for further experimental approaches. We utilized mass spectrometry to identify CYLD phosphorylation sites. Since the limiting factor for mass spectrometry is to get sufficient amount of protein, we scaled up the system by using up
106 93 to three 100 mm petri dishes of 293T cells and used Flag-tagged CYLD plasmid in this experiment. Compared to CYLD or HA antibodies, Flag antibody works more efficiently for immunoprecipitation (IP). Through these strategies, the phosphorylation form of CYLD was visualized on SDS-PGE gel by silver staining (Fig. 1B). Multiple phosphorylation sites were identified from mass spectrometry, among which some were basal as they were identified in the sample that expresses CYLD alone (data not shown). Interestingly, one site, serine 418, was identified as the only site that was phosphorylated induced by IKKγ (Fig. 1B). To further confirm this inducible phosphorylation site, we generated a phospho-antibody that recognizes phosphorylated serine 418. As shown in Fig. 1C, this antibody specifically detected the phsophorylated form of CYLD, which correlates with the upper band of regular CYLD blot. Furthermore, this phosphoantibody can distinguish the phosphorylated CYLD induced by PMA and Ionomycine stimulation (Fig. 1D). As shown before, signal induced CYLD phosphorylation occurred at an IKKγ-dependent manner (201). CYLD phosphorylation was not detected in the IKKγ-deficient T-cell line (JM4.5.2), but this signaling defect was efficiently rescued by reconstitution of these cells with an IKKγ expression vector (JM4.5.2 IKKγ) (Fig. 1D). Together, these results showed that CYLD serine 418 undergoes inducible phosphorylation in an IKKγ-dependent manner. CYLD serine 418 phoshorylation attenuates its negative signaling function in the IKK and JNK pathways. To pursue the physiological functions of CYLD phosphorylation, we established a system that allowed us to exclusively express CYLD phosphorylation mutant, in which
107 94 serine 418 was mutated to alanine (S418A), thereby blocking phosphorylation on amino acid 418. Endogenous CYLD was first knocked down by small hairpin RNA (shrna) as described (201), and the CYLD knocked down cells were then transduced with RNAiresistant expression vectors encoding wildtype (WT) or S418A CYLD by retroviral infection. In reconstituted Jurkat cells, PMA/Ionomycine (PI) stimulation induced CYLD phosphorylation (band shift) was abolished when serine 418 was mutated to alanine (Fig.2A). The same result was obtained using TNFα stimulated HeLa cells (Fig. 2B). Since CYLD plays an important role in both IKK and JNK signaling pathways (106, 109), we performed in vitro kinase assays to examine whether CYLD phosphorylation regulates its role in the regulation of these kinases. Compared to cells expressing wildtype CYLD, the cells expressing CYLD S418A had attenuated activation of both JNK and IKK in S418A cells when stimulated by mitogens (Fig. 2C). The reduced activation of IKK and JNK was confirmed in two independent cell clones (#1 and #2, Fig. 2C). As seen with mitogen-stimulated Jurkat T-cells, diminished JNK and IKK activations was also detected in HeLa cells expressing S418A upon activation by TNFα (Fig. 2D). Overall, these data suggested that serine 418 of CYLD is a predominant site of its phosphorylation and that phosphorylation at this site serves to inhibit the DUB function of CYLD, thereby allowing productive activation of IKK and JNK pathways. IKK-related kinase IKKε induces CYLD phosphorylation. As shown in Fig. 1, IKKγ potently induces the phosphorylation of CYLD, whereas over expressing of the catalytic subunits, IKKβ and IKKα, only led to lower level of CYLD phosphorylation (Fig. 1A, 1B, and 1C). This finding suggests that other
108 95 kinase(s) might be associated with IKKγ and phosphorylate CYLD. Indeed, IKKγ was able to induce CYLD phosphorylation in IKKβ or IKKα knockout MEFs (data not shown). Since CYLD interacts with IKKγ (201) and IKKγ functions as an adaptor of kinases other than IKKβ and IKKα (96), we attempted to identify the kinase(s) that induces CYLD phosphorylation. Of particular interest were the IKK-related kinases, IKKε and TBK1. These kinases have homology with IKKα and IKKβ and are regulated by IKKγ. When expressed in 293T cells, IKKε induced robust phosphorylation of CYLD, which was similar to that induced by IKKγ (Fig. 3A). Like IKKγ, IKKε phosphorylated CYLD at Ser418 (Fig. 3A). Similar results were obtained when the homologue of IKKε, TBK1, was expressed (data not shown). A naturally occurred CYLD mutant (1-932) that is defective in deubiquitination capacity was not phosphorylated by IKKε (Fig.3B). This result indicates that induction of CYLD phosphorylation by IKKε/TBK1 either requires the CYLD s enzymatic activity or the C- terminal structure of CYLD. It is important to note that NEMO was recently shown to regulate interferon signaling pathway by mediating IKKε/TBK1 activation (139). This finding, together with our data, suggests that IKKε/TBK1 regulate CYLD phosphorylation in vivo. Virus infection causes CYLD Ser418 phosphorylation at an IKKε/TBK1- dependent manner. To investigate that IKKε/TBK1 function as the CYLD kinases, we performed in vitro kinase assays (Fig. 4A). Immunoprecipitated TBK1 and IKKε could both phosphorylate wild-type GST-CYLD; however, the phosphorylation was significantly
109 96 diminished when GST-CYLDS418A was used as substrate (Fig. 4A). Phosphorylation of CYLD relied on IKKε/TBK1 s catalytic activity, since the kinase dead mutant (K38A) of IKKε/TBK1 failed to phosphorylate CYLD (Fig.4A). We next examined the physiological role of IKKε/TBK1 in CYLD phosphorylation in vivo. In this regard, IKKε/TBK1 are known as a target of viral infection. In particular, VSV (Vesicular Stomatitis Virus) infection activates IKKε/TBK1 in a dose-dependent manner (204) and IKKε/TBK1 regulated IRF signaling pathway is critical for host anti-viral immune response (173). It was thus important to examine whether CYLD is phosphorylated in VSV-infected cells. When we infected MEF with VSV to activate IKKε/TBK1, CYLD was indeed phosphorylated as shown by phospho-cyld blotting (Fig. 4B). In this experiment, MEFs were transfected with Flagtagged CYLD prior to virus infection because MEFs have very low level of endogenous CYLD (data not shown). In keeping with that IKKε/TBK1 phosphorylate CYLD (Fig. 3A), CYLD phosphorylation was not detectable in IKKε/TBK1 double knockout cells (Fig. 4B). This result provides the genetic evidence that IKKε/TBK1 function as the CYLD kinases.
110 97 Discussion In this study, we provided genetic evidence that IKKε/TBK1 phosphorylate CYLD at Ser418. This finding has important implications regarding the regulation and function of CYLD. Our data suggests that CYLD may play an important role in anti-virus signaling pathways. As described in the previous chapter (chapter III), CYLD has dual roles in antiviral responses. CYLD functions as a deubiquitination enzyme of RIG-I. Loss of CYLD causes aberrant activation of IKKε/TBK1 and over production of IFNs. Surprisingly, however, CYLD plays a positive role in regulating IFN-induced signaling, as CYLD-deficiency causes defect in IFN signaling, as demonstrated by attenuated STAT1 phosphorylation in CYLD-deficient cells. Interestingly, CYLD is phosphorylated along with antiviral signaling. This result not only further suggests the involvement of IKKε/TBK1 in CYLD phosphorylation, but also indicates a role for CYLD in regulating antiviral immune response. To further explore the function of CYLD phosphorylation in antiviral immune response, it is critical to generate animals expressing the phosphorylation-deficient mutant. We have shown that a CYLD mutant (1-932) does not undergo phosphorylation when expressed with IKKε. Since CYLD lacks the DUB activity, it suggests the possibility that the DUB activity may be required for its phosphorylation. However, it is also possible that the C-terminal 23 amino acids are required for CYLD/IKKε association or recruiting other molecules that facilitate the interaction between CYLD and IKKε. To
111 98 better understand the mechanism by which CYLD phosphorylation is regulated, it would be necessary to define how CYLD interacts with IKKε or TBK1. The sequence of CYLD phospho-peptide used to generate phospho-antibody is: ENRFHS 418 LPFSLTKMPN. Interestingly, this phosphorylation sequence shares strong homology with a known phosphorylation site in C-Cbl (Ser619), which belongs to a RSXSXP motif that binds to proteins (205). In fact, the phospho-cyld antibody can detect C-Cbl phosphorylation in Jurkat cells, while this phosphorylation does not require IKKγ (data not shown) proteins regulate a wide range of biological processes by interacting with different target proteins. This interaction can result in: 1) a conformational change in the target protein; 2) masking of a specific region on the target, which could be an active site, a ligand binding region, or a region that interacts with another protein; or 3) colocalization of two proteins (206). Motif scanning shows that ser418 of CYLD is localized in the binding motif ( Further study focusing on how regulates the cellular localization of CYLD and modulates its DUB activity will provide more insights into the biochemical mechanism underlying the regulation and DUB function of CYLD. Given the potential role of CYLD phosphorylation in suppressing its activity, it is also important to examine whether CYLD phosphorylation occurs chronically in tumor cells. This possibility is strongly suggested by our finding that CYLD is constitutively phosphorylated in different HTLV-I infected cell lines, including C8166, Tax-1, and SLB-1 (data not shown). In these cells, NF-κB is consistently activated, although it has yet to be examined whether CYLD phosphorylation contributes to the deregulation of NF-κB. Additionally, it remains to be investigated how CYLD is phosphorylated in
112 99 HTLV-transformed T cells. Studying whether TBK1 or IKKε is activated in these cells or Tax activates TBK1 or IKKε will provide insights into the mechanisms of CYLD phosphorylation and NF-κB deregulation. Acknowledgements We thank Drs. S Akira, J Chen, and R Lin for providing reagents. This work was supported by grants from National Institutes of Health (AI064639, AI057555, and CA94922).
113 100 Fig. 1 Phosphorylation of CYLD at serine418 by signal stimulation and under overexpression conditions. (A) CYLD phosphorylation by over-expressed IKK. 293 cells were transfected with HA-tagged CYLD together with either empty vector or expressing vectors encoding IKKβ (0.5 µg), IKKα (0.5 µg), or IKKβ (0.5 µg) plus IKKγ (25ng). CYLD phosphorylation was analyzed by IB with HA-HRP antibody. (B) Mass spectrometry identifies inducible phosphorylation at serine cells (three 100mm Petri dishes) were transfected with vector alone (10 µg), p3xflag-cyld (10µg), or p3xflag-cyld (10µg) together with pcdnaha-nemo (3µg). 36 hours after transfection, cells were lysed and CYLD protein was immunoprecipitated by Flag antibody then subjected to SDS-PAGE gel, and following by silver staining to visualize the CYLD protein (top panel). Middle panel shows the mass to charge (m/z) values of amino acids in the peptide containing serine 418 (*label). Lower panel highlights the determined phosphorylation site of serine 418 (#label), which was defined by the results from LC/MS/MS analysis. (C) Serine 418 phosphorylation induced by IKK confirmed by phospho-antibody. 293 cells were transfected with HA-CYLD (0.4 µg) or HA-CYLD (0.4 µg) plus IKKγ (100 ng), cell lysates were subjected to SDS-PAGE and IB with phospho-cyld antibody (left panel) or CYLD antibody (right panel). (D) IKKγdependent Serine 418 phosphorylation induced by PI stimulation. JM4.5.2 or JM4.5.2 IKKγ cells were treated with PMA (50 ng/ml) and Ionomycine (1µM) for 15 minutes. Cell lysates were immunoprecipitated by anti-cyld antibody and IB with phospho- CYLD antibody (top panel). CYLD protein levels were monitored by anti-cyld blot (lower panel).
114 101
115 102 Fig. 2: Serine 418 phosphorylation negatively regulates both IKK and JNK activations. (A) Serine 418 is the predominant phosphorylation site in the PI stimulation pathway. CYLD knockdown Jurkat cells (Jurkat-shCYLD) were reconstituted with RNAi-resistant form of wildtype or S418A CYLD (WT R and S418A R ), the reconstituted cells were nontreated (NT) or treated by PMA (50 ng/ml) and Ionomycine (1µM) for 15 minutes. CYLD was immunoprecipitated using anti-cyld antibody, followed by HA-HRP blot. (B) Serine 418 is the predominant phopshorylation site in the TNFα signaling pathway. HeLa-shCYLD cells were reconstituted with wildtype or S418A CYLD, which were then non-treated (NT) or stimulated by human TNFα (20ng/ml) for 15 minutes. CYLD phosphorylation was analysed by anti-cyld immunoprecipitation and HA-HRP IB. (C) JNK and IKK activations induced by PI were lower in Juarkat-shCYLD S418A reconstituted cells. Jurkat-shCYLD reconstituted with wildtype (WT R ) and S418A (S418A R, two lines #1 and #2) were non-treated (NT) or stimulated by PI (50ng/ml and 1µM respectively) for 7.5 and 15 minutes. Cell lysates were immunoprecipitated using both JNK1 and JNK2 antibodies to pull down JNK complexes. JNK catalytic activities were examined by in vitro kinase assay using GST-cJun (1-79) as substrate (top panel). IKK complex was pulled down by IKKγ immunoprecipitation. IKK catalytic activity was detected by in vitro kinase assay using substrate GST-IκBα (1-54) (middle panel). Tubulin and IκBα western blots were performed to monitor the loading control and stimulations (bottom panel). (D) JNK and IKK activations induced by TNFα were lower in HeLa-shCYLD S418A reconstituted cells. HeLa-shCYLD reconstituted with wildtype (WT R ) and S418A (S418A R ) were non-treated (NT) or stimulated by TNFα (20ng/ml) for
116 and 15 minutes. JNK (top panel) and IKK (middle) kinase assays were performed, with the loading controls shown by tubulin and IκBα western blots (bottom panel).
117 104
118 105 Fig. 3: Over-expressing IKKε induces serine418 phosphorylation. (A) 293 cells were transfected with HA-CYLD (0.4 µg) plus either vector only (V, 50 ng), either HA- NEMO (50 ng), or Flag-IKKε (50 ng). CYLD expression was shown by HA-HRP IB (top panel), serine418 phosphorylation was examined by phospho-cyld IB (second to top panel). Expression of IKKε and NEMO was monitored by Flag and HA-HRP western blot respectively (bottom two panels). (B) 293 cells were tansfected with wildtype HA-CYLD (0.4 µg) plus empty pcdna vector (0.1 µg) (lane 1) or Flag-IKKε (0.1 µg) (lane 2) or HA-CYLD mutant (0.4 µg) plus empty pcdna vector (0.1µg) (lane 3) or Flag-IKKε (0.1 µg) (lane 4). Expression of CYLD (upper panel) and phosphorylation at serine418 (lower panel) were detected by HA-HRP and phospho- CYLD IB respectively.
119 106
120 107 Fig.4: IKKε/TBK1 serves as the kinase(s) that phosphorylate serine418. (A) 293 cells were transfected with either empty vector, or Flag-TBK1, or Flag-TBK1K38A mutant, or Flag-IKKε, or Flag-IKKε K38A mutant (all with the amount of 0.3 µg). Cells lysates were used to pull down TBK1 or IKKε complexes by anti-flag antibody IP, followed by in vitro kinase assay using GST-CYLD ( ) (WT, left top panel) or GST-CYLD ( ) S418A mutant (S418A, right top panel) as substrate. Kinase assay membrane was blotted with Flag antibody to show the expression level of TBK1 or IKKε (middle panel) and GST antibody (bottom panel) to monitor substrate amount. (B) TBK1+/- IKKε+/- and TBK1-/-IKKε-/- MEFs were transfected with Flag-HA-CYLD (5 µg for one 100 mm per Petri dish of cells). 30 hours later, cells were either mock infected (0) or infected with VSV with MOI of 50 for times as indicated. Cell lysates were immunoprecipitated with Flag antibody to pull down CYLD and subjected to SDS-PAGE and blotted with phospho-cyld antibody IB (top panel). Lysates were blotted with HA- HRP (middle panel) and VSV-G (bottom panel) to detect CYLD level and virus protein expression respectively.
121 108
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION
 CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
Newly Recognized Components of the Innate Immune System
 Newly Recognized Components of the Innate Immune System NOD Proteins: Intracellular Peptidoglycan Sensors NOD-1 NOD-2 Nod Protein LRR; Ligand Recognition CARD RICK I-κB p50 p65 NF-κB Polymorphisms in Nod-2
Newly Recognized Components of the Innate Immune System NOD Proteins: Intracellular Peptidoglycan Sensors NOD-1 NOD-2 Nod Protein LRR; Ligand Recognition CARD RICK I-κB p50 p65 NF-κB Polymorphisms in Nod-2
Intrinsic cellular defenses against virus infection
 Intrinsic cellular defenses against virus infection Detection of virus infection Host cell response to virus infection Interferons: structure and synthesis Induction of antiviral activity Viral defenses
Intrinsic cellular defenses against virus infection Detection of virus infection Host cell response to virus infection Interferons: structure and synthesis Induction of antiviral activity Viral defenses
The NF- B/Rel family
 The NF-κB/Rel family The NF-κB/Rel family A family of signal-responsive transcription factors rapid response som ikke requires proteinsyntese Involved in proinflammatory response: a first line of defense
The NF-κB/Rel family The NF-κB/Rel family A family of signal-responsive transcription factors rapid response som ikke requires proteinsyntese Involved in proinflammatory response: a first line of defense
Innate Immunity & Inflammation
 Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
RAS Genes. The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes.
 ۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
Toll-like Receptor Signaling
 Toll-like Receptor Signaling 1 Professor of Medicine University of Massachusetts Medical School, Worcester, MA, USA Why do we need innate immunity? Pathogens multiply very fast We literally swim in viruses
Toll-like Receptor Signaling 1 Professor of Medicine University of Massachusetts Medical School, Worcester, MA, USA Why do we need innate immunity? Pathogens multiply very fast We literally swim in viruses
INVESTIGATING THE ROLE OF IKKε. CANCER-ASSOCIATED NF-κB ACTIVITY. Mezher Adli. Chapel Hill 2007
 INVESTIGATING THE ROLE OF IKKε (EPSILON) IN CANCER-ASSOCIATED NF-κB ACTIVITY Mezher Adli A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill in partial fulfillment
INVESTIGATING THE ROLE OF IKKε (EPSILON) IN CANCER-ASSOCIATED NF-κB ACTIVITY Mezher Adli A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill in partial fulfillment
Role of Innate Immunity in Control of Adaptive Immunity
 Role of Innate Immunity in Control of Adaptive Immunity Innate Immunity The burden of pathogen sensing is placed on the innate immune system Danger hypothesis Missing Self Based on the detection of molecular
Role of Innate Immunity in Control of Adaptive Immunity Innate Immunity The burden of pathogen sensing is placed on the innate immune system Danger hypothesis Missing Self Based on the detection of molecular
Genome of Hepatitis B Virus. VIRAL ONCOGENE Dr. Yahwardiah Siregar, PhD Dr. Sry Suryani Widjaja, Mkes Biochemistry Department
 Genome of Hepatitis B Virus VIRAL ONCOGENE Dr. Yahwardiah Siregar, PhD Dr. Sry Suryani Widjaja, Mkes Biochemistry Department Proto Oncogen and Oncogen Oncogen Proteins that possess the ability to cause
Genome of Hepatitis B Virus VIRAL ONCOGENE Dr. Yahwardiah Siregar, PhD Dr. Sry Suryani Widjaja, Mkes Biochemistry Department Proto Oncogen and Oncogen Oncogen Proteins that possess the ability to cause
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Innate Immunity. Chapter 3. Connection Between Innate and Adaptive Immunity. Know Differences and Provide Examples. Antimicrobial peptide psoriasin
 Chapter Know Differences and Provide Examples Innate Immunity kin and Epithelial Barriers Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive Immunity
Chapter Know Differences and Provide Examples Innate Immunity kin and Epithelial Barriers Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive Immunity
1. Activated receptor tyrosine kinases (RTKs) phosphorylates themselves
 Enzyme-coupled receptors Transmembrane proteins Ligand-binding domain on the outer surface Cytoplasmic domain acts as an enzyme itself or forms a complex with enzyme 1. Activated receptor tyrosine kinases
Enzyme-coupled receptors Transmembrane proteins Ligand-binding domain on the outer surface Cytoplasmic domain acts as an enzyme itself or forms a complex with enzyme 1. Activated receptor tyrosine kinases
Post-translational modifications of proteins in gene regulation under hypoxic conditions
 203 Review Article Post-translational modifications of proteins in gene regulation under hypoxic conditions 1, 2) Olga S. Safronova 1) Department of Cellular Physiological Chemistry, Tokyo Medical and
203 Review Article Post-translational modifications of proteins in gene regulation under hypoxic conditions 1, 2) Olga S. Safronova 1) Department of Cellular Physiological Chemistry, Tokyo Medical and
SUPPLEMENTARY INFORMATION
 Figure S1. Silver staining and immunoblotting of the purified TAK1 kinase complex. The TAK1 kinase complex was purified through tandem affinity methods (Protein A and FLAG), and aliquots of the purified
Figure S1. Silver staining and immunoblotting of the purified TAK1 kinase complex. The TAK1 kinase complex was purified through tandem affinity methods (Protein A and FLAG), and aliquots of the purified
NFκB What is it and What s the deal with radicals?
 The Virtual Free Radical School NFκB What is it and What s the deal with radicals? Emily Ho, Ph.D Linus Pauling Institute Scientist Department of Nutrition and Food Management Oregon State University 117
The Virtual Free Radical School NFκB What is it and What s the deal with radicals? Emily Ho, Ph.D Linus Pauling Institute Scientist Department of Nutrition and Food Management Oregon State University 117
Enzyme-coupled Receptors. Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors
 Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Biol403 MAP kinase signalling
 Biol403 MAP kinase signalling The mitogen activated protein kinase (MAPK) pathway is a signalling cascade activated by a diverse range of effectors. The cascade regulates many cellular activities including
Biol403 MAP kinase signalling The mitogen activated protein kinase (MAPK) pathway is a signalling cascade activated by a diverse range of effectors. The cascade regulates many cellular activities including
Signaling Through Immune System Receptors (Ch. 7)
 Signaling Through Immune System Receptors (Ch. 7) 1. General principles of signal transduction and propagation. 2. Antigen receptor signaling and lymphocyte activation. 3. Other receptors and signaling
Signaling Through Immune System Receptors (Ch. 7) 1. General principles of signal transduction and propagation. 2. Antigen receptor signaling and lymphocyte activation. 3. Other receptors and signaling
ROLE OF CYLD, A DEUBIQUITINATING ENZYME, IN MAST CELL FUNCTION AND SPERMATOGENESIS
 The Pennsylvania State University The Graduate School Graduate Program in Cellular and Molecular Biology ROLE OF CYLD, A DEUBIQUITINATING ENZYME, IN MAST CELL FUNCTION AND SPERMATOGENESIS A Dissertation
The Pennsylvania State University The Graduate School Graduate Program in Cellular and Molecular Biology ROLE OF CYLD, A DEUBIQUITINATING ENZYME, IN MAST CELL FUNCTION AND SPERMATOGENESIS A Dissertation
NTD Vaccine Design Toolkit and Training Workshop Providence, RI January 05, 2011 Cytokines Leslie P. Cousens, PhD EpiVax, Inc.
 NTD Vaccine Design Toolkit and Training Workshop Providence, RI January 05, 2011 Cytokines Leslie P. Cousens, PhD EpiVax, Inc. Cytokines Properties of Cytokines Cytokines are proteins with specific roles
NTD Vaccine Design Toolkit and Training Workshop Providence, RI January 05, 2011 Cytokines Leslie P. Cousens, PhD EpiVax, Inc. Cytokines Properties of Cytokines Cytokines are proteins with specific roles
KEY CONCEPT QUESTIONS IN SIGNAL TRANSDUCTION
 Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
* Kyoto Encyclopedia of Genes and Genomes.
 Supplemental Material Complete gene expression data using Affymetrix 3PRIME IVT ID Chip (54,614 genes) and human immature dendritic cells stimulated with rbmasnrs, IL-8 and control (media) has been deposited
Supplemental Material Complete gene expression data using Affymetrix 3PRIME IVT ID Chip (54,614 genes) and human immature dendritic cells stimulated with rbmasnrs, IL-8 and control (media) has been deposited
Toll-like Receptors (TLRs): Biology, Pathology and Therapeutics
 Toll-like Receptors (TLRs): Biology, Pathology and Therapeutics Dr Sarah Sasson SydPATH Registrar 23 rd June 2014 TLRs: Introduction Discovered in 1990s Recognise conserved structures in pathogens Rely
Toll-like Receptors (TLRs): Biology, Pathology and Therapeutics Dr Sarah Sasson SydPATH Registrar 23 rd June 2014 TLRs: Introduction Discovered in 1990s Recognise conserved structures in pathogens Rely
G-Protein Signaling. Introduction to intracellular signaling. Dr. SARRAY Sameh, Ph.D
 G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Scott Abrams, Ph.D. Professor of Oncology, x4375 Kuby Immunology SEVENTH EDITION
 Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 11 T-Cell Activation, Differentiation, and Memory Copyright 2013 by W. H. Freeman and
Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 11 T-Cell Activation, Differentiation, and Memory Copyright 2013 by W. H. Freeman and
T cell maturation. T-cell Maturation. What allows T cell maturation?
 T-cell Maturation What allows T cell maturation? Direct contact with thymic epithelial cells Influence of thymic hormones Growth factors (cytokines, CSF) T cell maturation T cell progenitor DN DP SP 2ry
T-cell Maturation What allows T cell maturation? Direct contact with thymic epithelial cells Influence of thymic hormones Growth factors (cytokines, CSF) T cell maturation T cell progenitor DN DP SP 2ry
The death receptors: signaling and modulation
 The death receptors: signaling and modulation 1 1 The extrinsic cell death pathway 2 Nat Rev Drug Discov. 2008 Dec;7(12):1001-12. 2 Death receptors Belong to the tumor necrosis factor (TNF) receptor gene
The death receptors: signaling and modulation 1 1 The extrinsic cell death pathway 2 Nat Rev Drug Discov. 2008 Dec;7(12):1001-12. 2 Death receptors Belong to the tumor necrosis factor (TNF) receptor gene
Cell Quality Control. Peter Takizawa Department of Cell Biology
 Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Cell Signaling part 2
 15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
number Done by Corrected by Doctor Maha Shomaf
 number 19 Done by Waseem Abo-Obeida Corrected by Abdullah Zreiqat Doctor Maha Shomaf Carcinogenesis: the molecular basis of cancer. Non-lethal genetic damage lies at the heart of carcinogenesis and leads
number 19 Done by Waseem Abo-Obeida Corrected by Abdullah Zreiqat Doctor Maha Shomaf Carcinogenesis: the molecular basis of cancer. Non-lethal genetic damage lies at the heart of carcinogenesis and leads
Chapter 11 CYTOKINES
 Chapter 11 CYTOKINES group of low molecular weight regulatory proteins secreted by leukocytes as well as a variety of other cells in the body (8~30kD) regulate the intensity and duration of the immune
Chapter 11 CYTOKINES group of low molecular weight regulatory proteins secreted by leukocytes as well as a variety of other cells in the body (8~30kD) regulate the intensity and duration of the immune
The ubiquitin-proteasome system. in bone biology. University of Nottingham. ECTS PhD Training July 5 th Dr Rob Layfield
 The ubiquitin-proteasome system in bone biology Dr Rob Layfield University of Nottingham ECTS PhD Training July 5 th 2009 Images reproduced from: http://www.bostonbiochem.com/overview.php?prod=ubchains
The ubiquitin-proteasome system in bone biology Dr Rob Layfield University of Nottingham ECTS PhD Training July 5 th 2009 Images reproduced from: http://www.bostonbiochem.com/overview.php?prod=ubchains
Chapter 3 The Induced Responses of Innate Immunity
 Chapter 3 The Induced Responses of Innate Immunity Pattern recognition by cells of the innate immune system Pattern recognition by cells of the innate immune system 4 main pattern recognition receptors
Chapter 3 The Induced Responses of Innate Immunity Pattern recognition by cells of the innate immune system Pattern recognition by cells of the innate immune system 4 main pattern recognition receptors
Identification of Microbes
 Identification of Microbes Recognition by PRR (pattern recognition receptors) Recognize conserved molecular patterns on microbes called pathogen associated molecular patterns (PAMPs) which are not present
Identification of Microbes Recognition by PRR (pattern recognition receptors) Recognize conserved molecular patterns on microbes called pathogen associated molecular patterns (PAMPs) which are not present
Interferon Induction by RNA Viruses and Antagonism by Viral Pathogens
 Viruses 2014, 6, 4999-5027; doi:10.3390/v6124999 Review OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Interferon Induction by RNA Viruses and Antagonism by Viral Pathogens Yuchen Nan
Viruses 2014, 6, 4999-5027; doi:10.3390/v6124999 Review OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Interferon Induction by RNA Viruses and Antagonism by Viral Pathogens Yuchen Nan
Allergy and Immunology Review Corner: Chapter 13 of Immunology IV: Clinical Applications in Health and Disease, by Joseph A. Bellanti, MD.
 Allergy and Immunology Review Corner: Chapter 13 of Immunology IV: Clinical Applications in Health and Disease, by Joseph A. Bellanti, MD. Chapter 13: Mechanisms of Immunity to Viral Disease Prepared by
Allergy and Immunology Review Corner: Chapter 13 of Immunology IV: Clinical Applications in Health and Disease, by Joseph A. Bellanti, MD. Chapter 13: Mechanisms of Immunity to Viral Disease Prepared by
A. Incorrect! It s not correct. Synergism of cytokines refers to two or more cytokines acting together.
 Immunology - Problem Drill 11: Cytokine and Cytokine Receptors Question No. 1 of 10 1. A single cytokine can act on several different cell types, which is known as. Question #1 (A) Synergism (B) Pleiotropism
Immunology - Problem Drill 11: Cytokine and Cytokine Receptors Question No. 1 of 10 1. A single cytokine can act on several different cell types, which is known as. Question #1 (A) Synergism (B) Pleiotropism
D2 inhibits TLR2- initiated 12p40 transcription (-) TLR2 PGN MDP. MyD88 IRAK ECSIT TRAF6 NIK. Smallest unit of PGN muramyl dipeptide IKK.
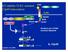 D2 inhibits TLR2- initiated 12p40 transcription CARD CARD NOD2 LRR RICK/Rip2 NIK MDP TRAF6 PGN TLR2 MyD88 IRAK ECSIT (-) IKK Smallest unit of PGN muramyl dipeptide IκB NF-κB atanabe et al, 2004 NF-κB IL-12p40
D2 inhibits TLR2- initiated 12p40 transcription CARD CARD NOD2 LRR RICK/Rip2 NIK MDP TRAF6 PGN TLR2 MyD88 IRAK ECSIT (-) IKK Smallest unit of PGN muramyl dipeptide IκB NF-κB atanabe et al, 2004 NF-κB IL-12p40
Interleukin-6; pathogenesis and treatment of autoimmune inflammatory diseases
 54 Review Article Interleukin-6; pathogenesis and treatment of autoimmune inflammatory diseases Toshio Tanaka 1, 2), Masashi Narazaki 3), Kazuya Masuda 4) and Tadamitsu Kishimoto 4, ) 1) Department of
54 Review Article Interleukin-6; pathogenesis and treatment of autoimmune inflammatory diseases Toshio Tanaka 1, 2), Masashi Narazaki 3), Kazuya Masuda 4) and Tadamitsu Kishimoto 4, ) 1) Department of
Innate immune regulation of T-helper (Th) cell homeostasis in the intestine
 Innate immune regulation of T-helper (Th) cell homeostasis in the intestine Masayuki Fukata, MD, Ph.D. Research Scientist II Division of Gastroenterology, Department of Medicine, F. Widjaja Foundation,
Innate immune regulation of T-helper (Th) cell homeostasis in the intestine Masayuki Fukata, MD, Ph.D. Research Scientist II Division of Gastroenterology, Department of Medicine, F. Widjaja Foundation,
Signaling. Dr. Sujata Persad Katz Group Centre for Pharmacy & Health research
 Signaling Dr. Sujata Persad 3-020 Katz Group Centre for Pharmacy & Health research E-mail:sujata.persad@ualberta.ca 1 Growth Factor Receptors and Other Signaling Pathways What we will cover today: How
Signaling Dr. Sujata Persad 3-020 Katz Group Centre for Pharmacy & Health research E-mail:sujata.persad@ualberta.ca 1 Growth Factor Receptors and Other Signaling Pathways What we will cover today: How
The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein
 THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
Follicular Lymphoma. ced3 APOPTOSIS. *In the nematode Caenorhabditis elegans 131 of the organism's 1031 cells die during development.
 Harvard-MIT Division of Health Sciences and Technology HST.176: Cellular and Molecular Immunology Course Director: Dr. Shiv Pillai Follicular Lymphoma 1. Characterized by t(14:18) translocation 2. Ig heavy
Harvard-MIT Division of Health Sciences and Technology HST.176: Cellular and Molecular Immunology Course Director: Dr. Shiv Pillai Follicular Lymphoma 1. Characterized by t(14:18) translocation 2. Ig heavy
Innate Immunity. Connection Between Innate and Adaptive Immunity. Know Differences and Provide Examples Chapter 3. Antimicrobial peptide psoriasin
 Know Differences and Provide Examples Chapter * Innate Immunity * kin and Epithelial Barriers * Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive
Know Differences and Provide Examples Chapter * Innate Immunity * kin and Epithelial Barriers * Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive
mirna Dr. S Hosseini-Asl
 mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
Follow this and additional works at: Part of the Medicine and Health Sciences Commons
 Washington University School of Medicine Digital Commons@Becker Open Access Publications 2009 Induction of IFN-beta and the innate antiviral response in myeloid cells occurs through an IPS-1-dependent
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2009 Induction of IFN-beta and the innate antiviral response in myeloid cells occurs through an IPS-1-dependent
Insulin Resistance. Biol 405 Molecular Medicine
 Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
p53 and Apoptosis: Master Guardian and Executioner Part 2
 p53 and Apoptosis: Master Guardian and Executioner Part 2 p14arf in human cells is a antagonist of Mdm2. The expression of ARF causes a rapid increase in p53 levels, so what would you suggest?.. The enemy
p53 and Apoptosis: Master Guardian and Executioner Part 2 p14arf in human cells is a antagonist of Mdm2. The expression of ARF causes a rapid increase in p53 levels, so what would you suggest?.. The enemy
Autoimmunity. Autoimmunity arises because of defects in central or peripheral tolerance of lymphocytes to selfantigens
 Autoimmunity Autoimmunity arises because of defects in central or peripheral tolerance of lymphocytes to selfantigens Autoimmune disease can be caused to primary defects in B cells, T cells and possibly
Autoimmunity Autoimmunity arises because of defects in central or peripheral tolerance of lymphocytes to selfantigens Autoimmune disease can be caused to primary defects in B cells, T cells and possibly
Structure and Function of Antigen Recognition Molecules
 MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
Innate immunity. Abul K. Abbas University of California San Francisco. FOCiS
 1 Innate immunity Abul K. Abbas University of California San Francisco FOCiS 2 Lecture outline Components of innate immunity Recognition of microbes and dead cells Toll Like Receptors NOD Like Receptors/Inflammasome
1 Innate immunity Abul K. Abbas University of California San Francisco FOCiS 2 Lecture outline Components of innate immunity Recognition of microbes and dead cells Toll Like Receptors NOD Like Receptors/Inflammasome
The Innate Immune Response is Conserved Throughout Evolution and is Triggered by Pattern Recognition. Lipopolysaccharide = Lipid + Polysaccharide
 The Innate Immune Response is Conserved Throughout Evolution and is Triggered by Pattern Recognition Lipopolysaccharide = Lipid + Polysaccharide E.coli Cell wall organization Lipopolysaccharide Outer membrane
The Innate Immune Response is Conserved Throughout Evolution and is Triggered by Pattern Recognition Lipopolysaccharide = Lipid + Polysaccharide E.coli Cell wall organization Lipopolysaccharide Outer membrane
10th International Rotavirus Symposium Bangkok, Thailand
 Rotavirus Host Range Restriction and Innate Immunity: Mechanisms of Vaccine Attenuation Harry Greenberg MD Stanford University 10th International Rotavirus Symposium Bangkok, Thailand 09/19/12 B dsrna
Rotavirus Host Range Restriction and Innate Immunity: Mechanisms of Vaccine Attenuation Harry Greenberg MD Stanford University 10th International Rotavirus Symposium Bangkok, Thailand 09/19/12 B dsrna
Chapter 11. B cell generation, Activation, and Differentiation. Pro-B cells. - B cells mature in the bone marrow.
 Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway
 Research article NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway Tomohiro Watanabe, 1,2 Naoki Asano, 1 Stefan
Research article NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway Tomohiro Watanabe, 1,2 Naoki Asano, 1 Stefan
Chapter 11. B cell generation, Activation, and Differentiation. Pro-B cells. - B cells mature in the bone marrow.
 Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Introduction. Cancer Biology. Tumor-suppressor genes. Proto-oncogenes. DNA stability genes. Mechanisms of carcinogenesis.
 Cancer Biology Chapter 18 Eric J. Hall., Amato Giaccia, Radiobiology for the Radiologist Introduction Tissue homeostasis depends on the regulated cell division and self-elimination (programmed cell death)
Cancer Biology Chapter 18 Eric J. Hall., Amato Giaccia, Radiobiology for the Radiologist Introduction Tissue homeostasis depends on the regulated cell division and self-elimination (programmed cell death)
Receptor mediated Signal Transduction
 Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
Chapter 15: Signal transduction
 Chapter 15: Signal transduction Know the terminology: Enzyme-linked receptor, G-protein linked receptor, nuclear hormone receptor, G-protein, adaptor protein, scaffolding protein, SH2 domain, MAPK, Ras,
Chapter 15: Signal transduction Know the terminology: Enzyme-linked receptor, G-protein linked receptor, nuclear hormone receptor, G-protein, adaptor protein, scaffolding protein, SH2 domain, MAPK, Ras,
1. TLR. TLR Toll-like receptors. Toll Toll-like receptor, TLR TLR TLR TLR. type I TLR TLR. Toll
 54pp.145 152 2004 1. TLR T B TLR Toll-like receptors TLR TLR I IFN TLR T B B T Toll NF- B 1996 565-0871 3-1 TEL 06-6879-8303 FAX 06-6879-8305 E-mail uemattsu@biken.osaka-u.ac.jp Toll Toll-like receptor,
54pp.145 152 2004 1. TLR T B TLR Toll-like receptors TLR TLR I IFN TLR T B B T Toll NF- B 1996 565-0871 3-1 TEL 06-6879-8303 FAX 06-6879-8305 E-mail uemattsu@biken.osaka-u.ac.jp Toll Toll-like receptor,
The interplay of IKK, NF- κb and RIPK1 signaling in the regulation of cell death, tissue homeostasis and inflammation
 DOI: 10.1111/imr.12550 INVITED REVIEW The interplay of IKK, NF- κb and RIPK1 signaling in the regulation of cell death, tissue homeostasis and inflammation Vangelis Kondylis 1,2,3 Snehlata Kumari 1,2,3
DOI: 10.1111/imr.12550 INVITED REVIEW The interplay of IKK, NF- κb and RIPK1 signaling in the regulation of cell death, tissue homeostasis and inflammation Vangelis Kondylis 1,2,3 Snehlata Kumari 1,2,3
Oncolytic virus strategy
 Oncolytic viruses Oncolytic virus strategy normal tumor NO replication replication survival lysis Oncolytic virus strategy Mechanisms of tumor selectivity of several, some of them naturally, oncolytic
Oncolytic viruses Oncolytic virus strategy normal tumor NO replication replication survival lysis Oncolytic virus strategy Mechanisms of tumor selectivity of several, some of them naturally, oncolytic
Antigen Presentation and T Lymphocyte Activation. Abul K. Abbas UCSF. FOCiS
 1 Antigen Presentation and T Lymphocyte Activation Abul K. Abbas UCSF FOCiS 2 Lecture outline Dendritic cells and antigen presentation The role of the MHC T cell activation Costimulation, the B7:CD28 family
1 Antigen Presentation and T Lymphocyte Activation Abul K. Abbas UCSF FOCiS 2 Lecture outline Dendritic cells and antigen presentation The role of the MHC T cell activation Costimulation, the B7:CD28 family
Alcoholic hepatitis is a drug-induced disorder
 Alcoholic hepatitis is a drug-induced disorder Gyongyi Szabo, MD, PhD Professor of Medicine University of Massachusetts Medical School Source: 2 Sobernation.com Clinical Progression of ALD Mortality Acute
Alcoholic hepatitis is a drug-induced disorder Gyongyi Szabo, MD, PhD Professor of Medicine University of Massachusetts Medical School Source: 2 Sobernation.com Clinical Progression of ALD Mortality Acute
Chapter 13: Cytokines
 Chapter 13: Cytokines Definition: secreted, low-molecular-weight proteins that regulate the nature, intensity and duration of the immune response by exerting a variety of effects on lymphocytes and/or
Chapter 13: Cytokines Definition: secreted, low-molecular-weight proteins that regulate the nature, intensity and duration of the immune response by exerting a variety of effects on lymphocytes and/or
2. Innate immunity 2013
 1 Innate Immune Responses 3 Innate immunity Abul K. Abbas University of California San Francisco The initial responses to: 1. Microbes: essential early mechanisms to prevent, control, or eliminate infection;
1 Innate Immune Responses 3 Innate immunity Abul K. Abbas University of California San Francisco The initial responses to: 1. Microbes: essential early mechanisms to prevent, control, or eliminate infection;
Signal Transduction Cascades
 Signal Transduction Cascades Contents of this page: Kinases & phosphatases Protein Kinase A (camp-dependent protein kinase) G-protein signal cascade Structure of G-proteins Small GTP-binding proteins,
Signal Transduction Cascades Contents of this page: Kinases & phosphatases Protein Kinase A (camp-dependent protein kinase) G-protein signal cascade Structure of G-proteins Small GTP-binding proteins,
TRAF molecules in cell signaling and in human diseases
 Xie Journal of Molecular Signaling 2013, 8:7 REVIEW Open Access TRAF molecules in cell signaling and in human diseases Ping Xie Abstract The tumor necrosis factor receptor (TNF-R)-associated factor (TRAF)
Xie Journal of Molecular Signaling 2013, 8:7 REVIEW Open Access TRAF molecules in cell signaling and in human diseases Ping Xie Abstract The tumor necrosis factor receptor (TNF-R)-associated factor (TRAF)
TNFα- and IKKβ-mediated TANK/I-TRAF phosphorylation: implications for interaction with NEMO/IKKγ and NF-κB activation
 Biochem. J. (2006) 394, 593 603 (Printed in Great Britain) doi:10.1042/bj20051659 593 TNFα- and IKKβ-mediated TANK/I-TRAF phosphorylation: implications for interaction with NEMO/IKKγ and NF-κB activation
Biochem. J. (2006) 394, 593 603 (Printed in Great Britain) doi:10.1042/bj20051659 593 TNFα- and IKKβ-mediated TANK/I-TRAF phosphorylation: implications for interaction with NEMO/IKKγ and NF-κB activation
Problem Set 8 Key 1 of 8
 7.06 2003 Problem Set 8 Key 1 of 8 7.06 2003 Problem Set 8 Key 1. As a bright MD/PhD, you are interested in questions about the control of cell number in the body. Recently, you've seen three patients
7.06 2003 Problem Set 8 Key 1 of 8 7.06 2003 Problem Set 8 Key 1. As a bright MD/PhD, you are interested in questions about the control of cell number in the body. Recently, you've seen three patients
Molecular biology :- Cancer genetics lecture 11
 Molecular biology :- Cancer genetics lecture 11 -We have talked about 2 group of genes that is involved in cellular transformation : proto-oncogenes and tumour suppressor genes, and it isn t enough to
Molecular biology :- Cancer genetics lecture 11 -We have talked about 2 group of genes that is involved in cellular transformation : proto-oncogenes and tumour suppressor genes, and it isn t enough to
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
TNFSF13B tumor necrosis factor (ligand) superfamily, member 13b NF-kB pathway cluster, Enrichment Score: 3.57
 Appendix 2. Highly represented clusters of genes in the differential expression of data. Immune Cluster, Enrichment Score: 5.17 GO:0048584 positive regulation of response to stimulus GO:0050778 positive
Appendix 2. Highly represented clusters of genes in the differential expression of data. Immune Cluster, Enrichment Score: 5.17 GO:0048584 positive regulation of response to stimulus GO:0050778 positive
RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh-
 1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system
 Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
Tolerance, autoimmunity and the pathogenesis of immunemediated inflammatory diseases. Abul K. Abbas UCSF
 Tolerance, autoimmunity and the pathogenesis of immunemediated inflammatory diseases Abul K. Abbas UCSF Balancing lymphocyte activation and control Activation Effector T cells Tolerance Regulatory T cells
Tolerance, autoimmunity and the pathogenesis of immunemediated inflammatory diseases Abul K. Abbas UCSF Balancing lymphocyte activation and control Activation Effector T cells Tolerance Regulatory T cells
Molecular Cell Biology - Problem Drill 19: Cell Signaling Pathways and Gene Expression
 Molecular Cell Biology - Problem Drill 19: Cell Signaling Pathways and Gene Expression Question No. 1 of 10 1. Which statement about cell signaling is correct? Question #1 (A) Cell signaling involves receiving
Molecular Cell Biology - Problem Drill 19: Cell Signaling Pathways and Gene Expression Question No. 1 of 10 1. Which statement about cell signaling is correct? Question #1 (A) Cell signaling involves receiving
Cancer. The fundamental defect is. unregulated cell division. Properties of Cancerous Cells. Causes of Cancer. Altered growth and proliferation
 Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
General information. Cell mediated immunity. 455 LSA, Tuesday 11 to noon. Anytime after class.
 General information Cell mediated immunity 455 LSA, Tuesday 11 to noon Anytime after class T-cell precursors Thymus Naive T-cells (CD8 or CD4) email: lcoscoy@berkeley.edu edu Use MCB150 as subject line
General information Cell mediated immunity 455 LSA, Tuesday 11 to noon Anytime after class T-cell precursors Thymus Naive T-cells (CD8 or CD4) email: lcoscoy@berkeley.edu edu Use MCB150 as subject line
Cytokines modulate the functional activities of individual cells and tissues both under normal and pathologic conditions Interleukins,
 Cytokines http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter22/animation the_immune_response.html Cytokines modulate the functional activities of individual cells and tissues both under
Cytokines http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter22/animation the_immune_response.html Cytokines modulate the functional activities of individual cells and tissues both under
TOLL-LIKE RECEPTORS AND CYTOKINES IN SEPSIS
 TOLL-LIKE RECEPTORS AND CYTOKINES IN SEPSIS A/PROF WILLIAM SEWELL ST VINCENT S CLINICAL SCHOOL, UNSW SYDPATH, ST VINCENT S HOSPITAL SYDNEY GARVAN INSTITUTE INNATE VERSUS ADAPTIVE IMMUNE RESPONSES INNATE
TOLL-LIKE RECEPTORS AND CYTOKINES IN SEPSIS A/PROF WILLIAM SEWELL ST VINCENT S CLINICAL SCHOOL, UNSW SYDPATH, ST VINCENT S HOSPITAL SYDNEY GARVAN INSTITUTE INNATE VERSUS ADAPTIVE IMMUNE RESPONSES INNATE
Chapter 9. Cellular Signaling
 Chapter 9 Cellular Signaling Cellular Messaging Page 215 Cells can signal to each other and interpret the signals they receive from other cells and the environment Signals are most often chemicals The
Chapter 9 Cellular Signaling Cellular Messaging Page 215 Cells can signal to each other and interpret the signals they receive from other cells and the environment Signals are most often chemicals The
Effect of viral RNA mimics and ISA virus infection. on expression of key genes of. the Atlantic salmon interferon system.
 Effect of viral RNA mimics and ISA virus infection on expression of key genes of the Atlantic salmon interferon system Øyvind Kileng PhD thesis A dissertation for the degree of philosophiae doctor UNIVERSITY
Effect of viral RNA mimics and ISA virus infection on expression of key genes of the Atlantic salmon interferon system Øyvind Kileng PhD thesis A dissertation for the degree of philosophiae doctor UNIVERSITY
Cover Page. The following handle holds various files of this Leiden University dissertation:
 Cover Page The following handle holds various files of this Leiden University dissertation: http://hdl.handle.net/188/59466 Author: Oudshoorn, D. Title: Wrapping up : nidovirus membrane structures and
Cover Page The following handle holds various files of this Leiden University dissertation: http://hdl.handle.net/188/59466 Author: Oudshoorn, D. Title: Wrapping up : nidovirus membrane structures and
Signal Transduction: Information Metabolism. Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire
 Signal Transduction: Information Metabolism Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire Introduction Information Metabolism How cells receive, process and respond
Signal Transduction: Information Metabolism Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire Introduction Information Metabolism How cells receive, process and respond
Herpesviruses. Virion. Genome. Genes and proteins. Viruses and hosts. Diseases. Distinctive characteristics
 Herpesviruses Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Virion Enveloped icosahedral capsid (T=16), diameter 125 nm Diameter of enveloped virion 200 nm Capsid
Herpesviruses Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Virion Enveloped icosahedral capsid (T=16), diameter 125 nm Diameter of enveloped virion 200 nm Capsid
Page 32 AP Biology: 2013 Exam Review CONCEPT 6 REGULATION
 Page 32 AP Biology: 2013 Exam Review CONCEPT 6 REGULATION 1. Feedback a. Negative feedback mechanisms maintain dynamic homeostasis for a particular condition (variable) by regulating physiological processes,
Page 32 AP Biology: 2013 Exam Review CONCEPT 6 REGULATION 1. Feedback a. Negative feedback mechanisms maintain dynamic homeostasis for a particular condition (variable) by regulating physiological processes,
Regulation of Gene Expression in Eukaryotes
 Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Immune Regulation and Tolerance
 Immune Regulation and Tolerance Immunoregulation: A balance between activation and suppression of effector cells to achieve an efficient immune response without damaging the host. Activation (immunity)
Immune Regulation and Tolerance Immunoregulation: A balance between activation and suppression of effector cells to achieve an efficient immune response without damaging the host. Activation (immunity)
Basis and Clinical Applications of Interferon
 Interferon Therapy Basis and Clinical Applications of Interferon JMAJ 47(1): 7 12, 2004 Jiro IMANISHI Professor, Kyoto Prefectural University of Medicine Abstract: Interferon (IFN) is an antiviral substance
Interferon Therapy Basis and Clinical Applications of Interferon JMAJ 47(1): 7 12, 2004 Jiro IMANISHI Professor, Kyoto Prefectural University of Medicine Abstract: Interferon (IFN) is an antiviral substance
Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells
 ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
Effects of Second Messengers
 Effects of Second Messengers Inositol trisphosphate Diacylglycerol Opens Calcium Channels Binding to IP 3 -gated Channel Cooperative binding Activates Protein Kinase C is required Phosphorylation of many
Effects of Second Messengers Inositol trisphosphate Diacylglycerol Opens Calcium Channels Binding to IP 3 -gated Channel Cooperative binding Activates Protein Kinase C is required Phosphorylation of many
Journal club. Lama Nazzal
 Journal club Lama Nazzal Background Kidney stone disease affects about 12% of men and 5% of women during their lifetimes in the United States Intrarenal nephrocalcinosis is often asymptomatic, but can
Journal club Lama Nazzal Background Kidney stone disease affects about 12% of men and 5% of women during their lifetimes in the United States Intrarenal nephrocalcinosis is often asymptomatic, but can
Central tolerance. Mechanisms of Immune Tolerance. Regulation of the T cell response
 Immunoregulation: A balance between activation and suppression that achieves an efficient immune response without damaging the host. Mechanisms of Immune Tolerance ACTIVATION (immunity) SUPPRESSION (tolerance)
Immunoregulation: A balance between activation and suppression that achieves an efficient immune response without damaging the host. Mechanisms of Immune Tolerance ACTIVATION (immunity) SUPPRESSION (tolerance)
Mechanisms of Immune Tolerance
 Immunoregulation: A balance between activation and suppression that achieves an efficient immune response without damaging the host. ACTIVATION (immunity) SUPPRESSION (tolerance) Autoimmunity Immunodeficiency
Immunoregulation: A balance between activation and suppression that achieves an efficient immune response without damaging the host. ACTIVATION (immunity) SUPPRESSION (tolerance) Autoimmunity Immunodeficiency
Cellular Signaling Pathways. Signaling Overview
 Cellular Signaling Pathways Signaling Overview Signaling steps Synthesis and release of signaling molecules (ligands) by the signaling cell. Transport of the signal to the target cell Detection of the
Cellular Signaling Pathways Signaling Overview Signaling steps Synthesis and release of signaling molecules (ligands) by the signaling cell. Transport of the signal to the target cell Detection of the
Introduction to Cancer Biology
 Introduction to Cancer Biology Robin Hesketh Multiple choice questions (choose the one correct answer from the five choices) Which ONE of the following is a tumour suppressor? a. AKT b. APC c. BCL2 d.
Introduction to Cancer Biology Robin Hesketh Multiple choice questions (choose the one correct answer from the five choices) Which ONE of the following is a tumour suppressor? a. AKT b. APC c. BCL2 d.
