Reducing the Risk of Blood Transfusion
|
|
|
- Caren Stewart
- 5 years ago
- Views:
Transcription
1 Reducing the Risk of Blood Transfusion Edward L. Snyder and Roger Y. Dodd There are continuing concerns over the safety of the nation s and the world s blood supply. The allogeneic blood supply is tested for antibodies to HIV1/2, HTLVI/II, hepatitis B, hepatitis C (HCV) and syphilis. Testing is also performed for donor ALT (SGOT) levels, for the presence of hepatitis B surface antigen, human immunodeficiency virus (HIV) p24 antigen and, using nucleic acid amplification testing (NAT), for HIV and HCV nucleic acids. Still, there are concerns regarding other pathogenic agents. Dr. Roger Dodd addresses a series of pathogens that are already known to be transmissible by transfusion. These include malaria, Chagas disease, babesiosis, bacteria and some viral agents. The need for new donor screening assays to protect the integrity and purity of the blood supply must be balanced against the loss of potential donors and the cost of developing and implementing these new screening assays. This issue will be highlighted. Dr. Edward Snyder reviews the status of research into development of systems for pathogen inactivation (PI) of blood and its components. A proactive technology wherein PI reagents such as psoralen, riboflavin, dimethylmethylene blue or inactine are added to blood collection bags could assure multiple log reduction of a variety of pathogens including viruses, bacteria, protozoa and fungi without the need to initially pre-screen the blood for a specific pathogen. Such a program could also cover new pathogens as they enter the blood supply. As a key issue relates to the toxicology of these agents, Dr. Snyder provides data on a novel carcinogenicity assay that uses a heterozygous p53 knock-out mouse model. The criteria likely to be needed for PI technology to be adopted by the transfusion community are summarized. I. EMERGING INFECTIONS AND THE SAFETY OF THE BLOOD SUPPLY Roger Y. Dodd, PhD* There has been great progress in the assurance and maintenance of the microbiological safety of the blood supply. Indeed, the risk of being infected by a unit of blood has decreased by three to four orders of magnitude over the past thirty years. 1 Particular success has been achieved for hepatitis C and HIV/AIDS, where largescale implementation of nucleic acid testing has reduced the risk to negligible levels. 2 In contrast, little effort had been directed to a variety of parasitic and arthropodborne infections. Although most of these are very rarely transmitted by transfusion in the US, their importance is increasing relative to the declining risk of the more familiar transfusion-transmitted infections. In addition, many of these agents qualify as emerging infections. * American Red Cross, Holland Laboratory, Transmissible Diseases Department, 1560 Crabbs Branch Way, Rockville MD Malaria Malaria is one of the most widespread infections globally and is undoubtedly responsible for the majority of all cases of transfusion-transmitted disease in the world. On a global basis, there may be 300 to 500 million cases of malaria each year, although as few as 10% of all cases are reported. At any one time, as many as 250 million individuals may be circulating infectious parasites. The greatest threat of transmission by transfusion obviously occurs in those regions of greatest endemicity. A number of factors are leading to an increased incidence of the disease, particularly in some areas previously thought to be free of infection. More specifically, climate change is leading to an expansion of the geographic range in which the parasite is effectively maintained and transmitted by the mosquito vectors. Further, the increased speed and range of population movement introduces many more infected individuals into areas in which conditions are suitable for the transmission of infection. Finally the geographic range of drug-resistant strains of the parasites is increasing. Currently, malaria is a minor risk of transfusion in the United States, with only about one case for every 3- Hematology
2 4 million units of blood transfused. 3 More importantly, this rate has been relatively constant for many years, as has the frequency of imported cases, which amount to about one thousand each year. This figure is strikingly low when compared to a similar number of imported cases in smaller countries such as Germany and Canada. These higher frequencies of disease represent differing travel patterns and, in the case of Canada, a liberal attitude towards refugee populations. In the US, the risk of transfusion malaria is maintained at low levels by a policy of careful questioning about donor travel history. 4 Potential donors are deferred for one year after travel in a malarious area or three years if they have lived in such an area, or if they have a history of the disease itself. In addition, individuals who emigrated from a malarious area are deferred for three years after any subsequent travel to a malarious area in order to account for the fact that partial immunity can extend the incubation period. A number of recent cases of transfusion malaria have been attributed to failure of the questioning process itself or to unexpectedly long incubation periods for Plasmodium falciparum infection. Although there has been increased regulatory attention to the risk of malaria exposure among donors, it might be more important to assure that current screening procedures are conducted properly. Concern continues about the risk of transfusion malaria in the US. Somewhat curiously, this concern has led to tightening of the requirements for deferral related to travel, with particular reference to casual travel to resort areas and cruise-ship ports of call, despite the absence of any evidence that these are a significant source of infection. Greater concern should perhaps be focused on so-called airport malaria, or mosquito transmitted infection with no ready epidemiologic explanation. Little can be done to prevent transmission in areas of high endemicity. However, transfusion recipients may be routinely treated with antimalarials as a prophylactic measure. Also, in endemic areas, the risk of malaria is not necessarily uniform, so it makes sense to avoid transferring blood from high-risk to low-risk areas. In addition, efforts are usually made to avoid collecting blood from individuals with a history of recent malaria. In some countries, serologic testing is used as an adjunct to a travel history. More specifically, those donors with a travel-related risk for malaria are tested, and their blood is used if the serologic test result is negative. Unfortunately, at the time of writing, the only test with adequate sensitivity and specificity is an immunofluorescence assay. Chagas disease Chagas disease is caused by the protozoan parasite Trypanosoma cruzi. This parasite is confined to the continental Americas, generally between latitudes 40 N and 40 S. It infects a wide range of mammalian species and is transmitted by hematophagous triatomine or reduviid bugs. Humans are accidental hosts and play little or no role in the normal cycle of the parasite. Human cases are essentially confined to parts of South and Central America and Mexico, where some million individuals are thought to be infected and there may be a million new cases each year. Natural transmission to humans occurs when living conditions permit relatively close contact between infected mammals, the insect vector and humans. This occurs most frequently in rural areas with substandard housing with crevices or thatched roofs that harbor infected bugs. These insects bite the inhabitants of the house, usually during the night. During or after feeding, the bugs defecate; the feces contain high numbers of infectious forms of the parasite. The feces may then be rubbed into the mucous membranes or the bite site and, thus, the bite results in infection. Other infection routes are congenital or by blood transfusion. Prevalence rates vary considerably between and within countries. 5 There are significant and largely successful efforts to reduce the incidence of human infection in many of the affected countries. Acute disease may occur after T. cruzi infection, but such disease is usually relatively mild, generally with flu-like symptoms. Acute disease and infection may be treated with nifurtimox or benznidazole, but both of these drugs are experimental and have severe side effects. At least 30-40% of infected individuals develop chronic, lifelong infection. Such infection is usually asymptomatic for many years, but cardiac or intestinal disease may occur and can be fatal. In particular cardiac arrhythmia is relatively common and unexpected cardiac deaths occur. Alternatively, chronically infected individuals may suffer from megacolon or megaesophagus. Population movements have resulted in the introduction of T. cruzi-infected individuals into countries where the disease does not normally infect humans. These infected persons do offer a risk of transmission of the parasite, if they donate blood. There have been 6 reported cases of transfusion-transmitted T. cruzi in the US and Canada, for example. Seroprevalence studies in the US have shown rates of 1 in 7,500 to 1 in 33,000. Case-control studies have shown that a questioning strategy to detect infected donors would neither be sensitive nor specific. In addition, there is suggestive evidence that a few seropositive donors may have been infected by the perinatal route, perhaps through as many as three family generations. 6,7 Despite the measurable seroprevalence rates for T. cruzi in the US, the frequency of transmission by transfusion is not commensurate with these data, as defined by look-back studies or direct investigation of a popula- 434 American Society of Hematology
3 tion of patients receiving over 120,000 blood units in aggregate. Although six of the patients were positive for T. cruzi, their pretransfusion specimens were also positive. 8 Further studies are required to define the factors associated with transmission of this parasite. Babesia Babesia microti is a malaria-like parasite, transmitted by small ticks of the genus Ixodes. It is endemic among certain mammals in coastal areas of the Northeastern US and may be accidentally transmitted to humans. There are at least 36 reported cases of transmission of this agent by transfusion, resulting in some fatal outcomes. 9 There is evidence that this parasite may be spreading in range; 10 other related organisms have been found in widely scattered regions of North America, and two cases of transfusion disease have been attributed to WA1, one of these organisms. 11 Babesia spp. are widespread throughout the world, and there has been a report of transfusion transmission from Japan. 12 Serologic and laboratory studies are underway to define the seroprevalence and rates of parasitemia of B. microti. These rates may exceed 1% among blood donors from areas of high endemicity, and parasitemia is quite frequent (i.e. in more than 50% of antibody-positive individuals). At this time, there do not appear to be any effective methods for donor testing, and the most effective intervention is to avoid collecting blood from donors in endemic areas. This is particularly true during the summertime, when individuals are at greatest risk of tick-bites. Other parasites Although concern has been expressed about the potential for transmission of other parasitic diseases, there are few convincing reports of such transmission in the literature. Leishmania was a matter of concern some years ago, when an unusual visceral manifestation of L. tropica was noted among a few returnees from the Gulf war. However, there was no evidence of any transmission by transfusion, and a temporary deferral policy for these individuals was eventually rescinded. Toxoplasma has properties that are consistent with an ability to be transmitted by transfusion, but there are no definitive cases of such transmission in the contemporary literature. Emerging viruses A number of emerging viral diseases should be considered in terms of their potential for transmission by transfusion. Such potential requires that there be an asymptomatic, viremic phase and that the virus can survive in blood during storage. Perhaps the most dramatic example of an emerging virus is Ebola. In most cases, it appears that an infected individual becomes symptomatic shortly after infection and would be extremely unlikely to transmit the virus by transfusion. However, asymptomatic infection with evidence of a self-limited viremia has been reported. West Nile virus has recently appeared in the US, apparently due to the increased volume of international travel. 13 This virus does have a brief period of viremia prior to the appearance of symptoms, and it is thought that 80% or more of all infections are essentially asymptomatic. Thus, the potential for transfusion transmission does exist, and there may be concern in the event of a widespread outbreak. However, closely related viruses do not appear to have been implicated in this route of transmission. The recently recognized human herpesvirus 8, thought to be the etiologic agent of Kaposi s sarcoma, may have been a human pathogen for many years, centuries or more. However, its prevalence is probably increasing in at least some populations infected with HIV. It has been shown to be transmitted by organ transplantation 14, but recipients of blood from seropositive individuals do not appear to have been infected in a limited number of cases evaluated. 15 Blood-borne transmission has been suggested to occur in the setting of injection drug use. 16 Further studies are underway. The continuing search for agents of transfusion-associated hepatitis has resulted in the recognition of at least two more groups of viruses. Again, however, these seem to be viruses that have existed in human populations for many years and are probably not emerging. The so-called hepatitis G virus is related to the flaviviruses and thus to hepatitis C virus (HCV). It is readily transmitted by transfusion but is not associated with any disease state (including liver disease). 17 Similarly, a large group of small DNA viruses, probably related to the circoviruses, has been recognized and shown to be almost ubiquitous among humans. Again, these viruses (including TTV and SEN-V) were originally isolated from patients with hepatitis, but it has not yet been possible to demonstrate the existence of any causal relationship. 17,18 Transfusion-transmissible bacteria A number of bacterial infections have been shown to be transmissible by transfusion and some others have the potential for transmission. Perhaps the best-known of this group is Treponema pallidum, the etiologic agent of syphilis. In fact, there has been no recorded case of transfusion transmitted syphilis in the US since This is likely due to the fragility of the organism in the conditions currently used to store blood and to the decreased incidence of the disease. At this time, it is not known whether routine testing has any impact upon transmission. It is of interest to note that in a small pilot study, T. Hematology
4 pallidum DNA could not be detected in 100 donor samples selected on the basis of positive syphilis serology. 19 Rickettsiae and other small intracellular bacteria could be transmitted by transfusion. There is a single case of transmission of Rocky Mountain spotted fever via this route and a potential case of transmission of Ehrlichia was reported in ,20 It is of interest that both of these agents are transmitted by ticks. In contrast, there is no evidence that Borrelia burgdorferi, the agent of Lyme disease and probably the most widespread tick-borne bacterium, has ever been transmitted by transfusion. 9 Brucella is considered to be a risk of transfusion in some locations, but there is only one report of a possible transmission in the literature of the last 25 years. Outgrowth of Bacteria in Blood Components A much more frequent and serious problem is the occurrence of septic reactions due to the presence of a substantial titer of contaminating bacteria in a blood component. Such reactions are often, but not always, dramatic, and among recognized cases, mortality is 25% or greater. A wide variety of organisms has been implicated. 21 The frequency of transfusion-induced sepsis is poorly defined. It is relatively low among red cell recipients, with a rate that is usually less than one in a million products. In almost all cases, red cell-related sepsis is due to psychrophilic organisms (i.e. those that can multiply at the low temperatures used for storage of red cells) that may reach high levels in concentrates that have been stored for two or more weeks. About half of the cases are caused by Yersinia enterocolitica and many of the remainder are due to Pseudomonas spp. The former probably enters the blood unit directly from the donor s bloodstream, while the latter appears to be an environmental contaminant. The majority of cases of bacterial sepsis are seen among recipients of platelet concentrates. This is generally attributed to the relatively fast growth of bacteria in these components during storage at 20 C. Some studies suggest that one septic event may occur in about every 10,000 transfusions, but other observations show rates that may be substantially higher or lower. A wide variety of organisms are involved, ranging from skin bacteria, such as Staphylococci, to enteric organisms such as Salmonella; there are even a few reports of Enterobacter spp. A number of instances of sepsis due to Serratia marcescens have been reported, some as a result of contamination of the blood containers themselves during the manufacturing process. There is considerable interest in the development of measures to reduce or prevent such septic events. These include use of automated culture methods to establish sterility or implementation of a number of tests designed to identify high titers of bacterial contaminants immediately prior to use of the component. In addition, it seems likely that methods designed to inactivate viruses in platelet concentrates will also be effective in eliminating contaminating bacteria. Some authorities believe that leukoreduction may have some impact upon bacterial contamination, but this approach should probably not be considered a primary defense. II. PATHOGEN INACTIVATION OF THE BLOOD SUPPLY Edward L. Snyder, MD* Although the blood supply is already extremely safe, there are still concerns regarding the potential transmission of human immunodeficiency virus (HIV) and other viruses. This is the case, despite data from the Retrovirus Epidemiology Donor Study and other studies, indicating that the risk of receiving a unit of infectious blood is less than 1:500,000 for HIV, 1:100,000 for hepatitis C and l:70,000 for hepatitis B. In the United States and in other industrialized countries several tiers of safety are employed to ensure the purity of the blood supply. 1 The donor history provides the first major level. Several types of viral testing for the various transfusion transmitted diseases, including HIV l/2, human T cell lymphomaleukemia viruses (HTLV I/II), hepatitis C and hepatitis B, provides the second major level of protection. A third major tier of safety currently being developed is pathogen inactivation technology. 2 This technology calls for addition of various additives to blood products to inactivate viruses, bacteria, fungi, protozoa and other transfusion transmitted pathogens. None of the currently available systems are able to inactivate prions such as those that are believed to cause variant Creutzfeld-Jakob disease (vcjd). Each of the three types of blood components, plasma including cryoprecipitate, platelet concentrates and red cells, requires special storage conditions. Similarly, each requires that special conditions be met to ensure that the pathogen inactivation technology does not destroy the blood component. It is easier to pathogen inactivate plasma and cryoprecipitate since there are no cellular elements contained in these blood components. However, robust technology also has been developed for inactivating viruses and other microorganisms in units of platelets and red cells. Fresh Frozen Plasma Currently, there are two approaches to plasma pathogen inactivation. The first is an FDA-licensed technique that * Blood Bank, Yale-New Haven Hospital, 20 York Street, CB- 459, New Haven CT American Society of Hematology
5 uses solvent/detergent treatment. The second is an experimental plasma treatment system that incorporates UV-A light and a psoralen compound (S-59). 2 A third technology exists, but it does not inactivate pathogens. Rather, it extends the quarantine time of the unit of plasma coupled with subsequent retesting of the specific donor. This manipulation, known as delayed release/donor retested plasma, is not in wide use in the US today. Riboflavin inactivation technology is also being evaluated. Solvent/Detergent Plasma Pathogen inactivation technology for solvent/detergent plasma (SDP) involves the addition of a solvent, tri-nbutylphosphate (TNBP), and a detergent, triton X The solvent and detergent dissolve the wall of lipid enveloped viruses which include the viruses causing hepatitis B, hepatitis C, HIV, HTLV and many other viral agents including Epstein-Barr virus (EBV) and cytomegalovirus (CMV). It does not, however, affect proteincoated (non-lipid enveloped) viruses, most importantly, hepatitis A and parvovirus B19. For the SDP treatment process, plasma units that are collected and frozen either as source plasma or derived from plasmapheresis collections are thawed and pooled in lot sizes of about 500 liters (2,500 units (donors)/pool). The pool is incubated at 30 o C for 4 hours with 1% tri-n-butylphosphate and 1% triton X-100. The solvent and detergent are then removed by extraction with vegetable oil, followed by resin chromatographic purification. The processed SDP is then aliquoted into 200 ml ABO group-specific units, labeled, and distributed for use. SDP is thus available as an ABO blood group specific, pooled human plasma product. The solvent detergent pathogen inactivation process has virtually eliminated the risk of transmitting lipid enveloped viruses such as the ones mentioned above. Solvent/detergent technology has been used for many years in the manufacture of factor VIII, and intravenous immune globulin (IVIG). This process does not substantively alter or inactivate labile or stable coagulation factors or plasma proteins such as fibrinogen or immunoglobulins. 3-6 The SDP is manufactured in a pharmaceutical plant environment following good manufacturing practices. SDP is specifically indicated for use for replacement of coagulation factor deficiencies where no factor concentrate is available, 5 for treatment of thrombotic thrombocytopenic purpura (TTP), 7 for treatment of acquired multiple factor deficiencies such as is needed for reversal of warfarin therapy, treatment of hepatic failure, or bleeding due to a dilutional coagulopathy. 5,7 These indications are identical to those for use of standard fresh frozen plasma (FFP) prepared from a single donor. In contrast to standard FFP, however, SDP lacks large von Willebrand factor multimers, provides more consistent levels of other coagulation factors since it is a pooled product, is provided in a standard volume (200 ml), and is derived from a pool up to 2,500 donors. This latter aspect has caused a problem for many physicians who feel that the use of a pooled product, despite the fact that it is pathogen inactivated, presents a greater hazard regarding transmission of hepatitis A, a non-enveloped virus. Indeed, there have been reports of hepatitis A transmission from pooled solvent/detergent treated coagulation factor concentrates. Another possible serious complication of SDP is transmission of parvovirus B19 in selected populations such as pregnant women. In this group, B19 infection is associated with spontaneous abortion and hydrops fetalis. In patients with chronic hemolytic anemia, B19 infection is associated with a higher risk of red cell aplasia or aplastic crises. Parvovirus B19 infection is also a serious concern in patients with chronic severe immunodeficiencies. However, many physicians who would not use SDP for such patients or those needing small volume transfusions would consider the use of SDP for treatment of patients with acute or recurrent TTP who require large numbers of transfusions. For such patients a pathogen inactivated product would have a safer risk-benefit profile, especially if all the plasma infused came from the same lot of SDP. Currently, lot release criteria have been established for parvovirus levels in units of SDP. Although 2500 donors is a small pool size compared to the pool size of tens of thousands of donors used in the production of factor concentrates, and immune globulins, concerns still remain. SDP is used for transfusion in Germany, France, Austria, the Netherlands, Norway and Belgium. More than 2,000,000 units of SDP have been given worldwide without reports of virus transmission. Solvent/detergent treated factor concentrates have been reported to transmit hepatitis A and parvovirus B19, and notably they do not contain neutralizing antibodies. SDP, in contrast, does contain neutralizing antibody to these pathogens, and this is believed by some scientists to provide the added degree of protection. Little data are available on the use of SDP in neonates, infants and pregnant women. The rate of allergic reaction to SDP is similar to that of FFP. Some researchers have raised concerns regarding cost effectiveness of SDP. SDP is associated with a relatively high cost and provides relatively small benefit, especially with the implementation of nucleic acid testing (NAT) programs. It is estimated that SDP costs over $200,000 for one quality adjusted lifeyear saved. Some believe that this reality may not justify its widespread use. Others believe a safer product is preferable regardless of cost. A key concept is whether SDP as derived from a pool of donors, is a safer product. SDP may be especially beneficial as protection against other lipid enveloped viruses for which there is Hematology
6 no test. Due to the pool size, however, possible contamination of the donor pool with an unknown non-lipid enveloped pathogenic agent that is not inactivated by the solvent/detergent treatment poses a serious risk. One potential group of such agents is the prions associated with vcjd. Although not a single case of transfusion transmitted vcjd has ever been reported, this issue remains a concern related to the pool size of the final product. A product, not yet licensed, referred to as Uniplas Solvent/Detergent is SDP from which anti-a and anti-b iso-agglutinin antibodies have been removed. This processing is designed to enhance the blood supply in emergency situations and in times of shortage when a specific ABO type of plasma may not be available. Uniplas Solvent/Detergent would eliminate transfusion reactions resulting from ABO mismatched plasma and would also simplify inventory logistics. In this product anti-a and anti-b IgG and IgM antibodies are removed by chromatography using A and B synthetic sugar antigens that are covalently bound to a resin. Isoagglutinin titers drop to less than 1:2; all the other specifications are identical to SDP. This material may find additional use in some situations but still retains the caveats regarding concerns about large pool size and disease transmission. The SDP treatment process cannot be used for pathogen inactivation of red blood cells and platelets since the solvent and detergent would dissolve the red cell and platelet membrane, thus rendering the cells useless. However, SDP can be added to units of both red cells and platelets. 8,9 Psoralen S-59 Compounds A photochemical treatment system using a novel psoralen material (S-59, developed by Cerus and Baxter Corporations) and UV-A (long wavelength) ultraviolet light, has been developed to inactivate viruses in single donor units of FFP. A variety of in vitro studies have shown very effective viral inactivation by psoralen-uv-a treatment of FFP, coupled with preservation of coagulation factor and plasma protein activity. When exposed to UV- A, the S-59 forms non-reversible covalent monoadducts with DNA and RNA, resulting in inactivation of viruses, bacteria and other pathogens. The process also eliminated the mitotic capacity of leukocytes. During photochemical treatment with psoralen-uv- A, individual units of plasma are either separated from whole blood or collected by apheresis technology and transferred into a plastic container. S-59 is added to a concentration 150 µm through an integral closed system into a UV-A transparent vessel containing the plasma and, after a 5 minute incubation, the S-59 treated plasma is illuminated with shaking for three minutes in a high intensity UV-A-light box. After illumination the plasma is transferred to another container with an S-59 reduction device (SRD), which is used to remove most of the photoproducts caused by light activation of the S-59. After S-59 reduction device processing for 1 hour, the plasma is transferred via a closed system into a final container for freezing at 18C and storage for up to 1 year. The system can be used to treat single ml plasma units or jumbo units containing mls of plasma. Wages et al 10 reported a phase I single blinded crossover study in 15 healthy individuals who were given both autologous FFP treated with S-59 and untreated autologous FFP as a control. No adverse events were attributed to transfusion of autologous S-59 treated FFP at any doses used up to 1 liter. Following infusion of S-59 FFP, there were no clinically significant changes in posttransfusion coagulation parameters. This phase I safety and tolerability trial in healthy subjects thus showed no adverse effects. Preclinical studies performed without the additional SRD S-59 reduction treatment have similarly shown no clinically relevant toxicity. The use of the S-59 reduction device, however, will likely further ensure the safety of the process by minimizing residual levels of S-59 and its photoproducts. The S-59 UV-A technique can reduce the titers of cell-free HIV; cell associated HIV; bovine viral diarrhea virus (BVDV), an HCV surrogate; duck hepatitis B virus (DHBV), an HCV surrogate; and other lipid-enveloped pathogens by 5-6 log 10. In addition, S- 59 inactivates a spectrum of non-lipid enveloped viruses including parvovirus B19. Following treatment and freezing, functional coagulation activity is well preserved; factor VIII demonstrates the largest decrease post treatment but remains within a therapeutically acceptable range. Fibrinogen functional activity is about 80% of control values. A phase II warfarin challenge study was completed in 27 healthy adults each of whom donated about 2000 ml of plasma. 11 One liter was processed with S-59 UV-A photochemical treatment, the other liter was maintained as a standard FFP control. Following plasmapheresis, the subjects were treated with oral warfarin, 7.5 mg per day for 4 days to reduce factor VII levels. On the fourth day, they received a transfusion of either autologous photochemically treated FFP or autologous standard FFP, in random order. Following transfusion, the effect of FFP transfusion on various clotting factors and factor recovery was determined. Due to the short half-life of factor VII, the pharmacokinetics of factor VII clearance were also measured. The study demonstrated that photochemically treated FFP was well tolerated, appropriately reversed the effects of warfarin, and provided acceptable post transfusion recovery of vitamin K dependent factors. A variety of other S-59 clinical trials have been completed. These include studies of S-59 safety; effects of S-59 FFP treatment on the kinet- 438 American Society of Hematology
7 ics of factor VII, protein C and protein S; and studies of use of S-59 processed FFP for treatment of patients with congenital coagulopathies. Not currently licensed by the FDA, S-59-treated FFP is being evaluated in large scale phase III clinical trials for treatment of patients with acquired coagulopathies and TTP. Platelet Concentrates The S-59-UV-A treatment protocol for pathogen inactivation of platelets is similar to that described above for plasma. 2,12-16 During the process of photoinactivation with S-59, the platelets are resuspended in a 300 ml volume containing a platelet additive solution known as PAS III. After illumination, the platelets are transferred to another container containing an S-59 reduction device (SRD) and incubated and agitated for 6 hours. After SRD incubation, the platelets are transferred via an integral closed system to a final container for 5 days of storage. Three clinical toxicology studies performed without the S-59 reduction device have shown no toxicity from the S-59 or its photoproducts. However, further reduction in S-59 levels should further enhance the safety margin. Photochemical treatment with S-59 showed inactivation of high levels (5 to 6 log 10 ) of HIV; CMV; DHBV, duck hepatitis B virus (an HBV surrogate); bovine viral diarrhea virus, (BVDV) an HCV surrogate; gram positive; and gram negative bacteria. Studies using a chimpanzee infectivity model, demonstrated inactivation of community acquired inocula of HBV and HCV. Other studies have demonstrated inactivation of T. cruzi, P. malariae and other organisms in platelet concentrate. Preclinical trials have shown that S-59 UV-A does not adversely affect platelet concentrates during 5 days of storage. Clinical trials in healthy volunteers demonstrated that the photochemically treated platelets were well tolerated and safe and that they provided adequate platelet viability. 17 Recent studies of S- 59 toxicity using a p53 carcinogenicity assay showed that exposure to S-59 over 6 months did not produce a greater number of tumors in heterozygote p53 knockout mice as compared to control mice without a p53 knockout. A large phase III clinical study using pooled buffy coats to assess hemostatic efficacy of photochemically treated platelets in thrombocytopenic patients, was recently completed in Europe (EuroSPRITE). 18 Another large clinical trial of S-59-treated plateletpheresis components was recently completed in the US. Results of these clinical trials are being evaluated. Riboflavin Riboflavin is another pathogen inactivation system for platelets. This compound is also activated by light. Riboflavin is an essential nutrient (vitamin B2) that absorbs visible and UV light. Upon exposure to light, riboflavin intercalates into DNA and RNA. Photolysis occurs and the complex is subject to guanine oxidation, single strand breaks and formation of covalent adducts. These processes are not oxygen dependent. As the safety record of riboflavin is well known clinically, the ability of this compound to inactivate blood pathogens including those in red cells, platelets and plasma, will be viewed with great interest. 19,20 Red Blood Cells S-303 Red blood cell pathogen inactivation programs are less clinically advanced than are plasma and platelet protocols. There are a variety of agents being studied. One approach, psoralen UV-A light, was not found to be useful since UV-A light is absorbed by hemoglobin, preventing the intercalation of the psoralen into the nucleic acid structure. Another agent currently being studied is coded as S-303. S-303 is one of a recently discovered class of compounds that inactivates viruses and bacteria in red cell concentrates by crosslinking DNA and RNA in a rapid ph-dependent light-independent reaction. The S-303 process uses a nucleic acid target system called Frangible Anchor Linker Effectors (FRALES). FRALES are tri-partite molecules with a nucleic acid anchor, an effector moiety that binds covalently to nucleic acid, and a frangible linker that breaks down, leading to compound inactivation. Upon addition to red cells that are stored at a neutral ph, FRALES react with nucleic acid and the frangible bond degrades leaving a breakdown product with a net negative charge no longer capable of nucleic acid binding. S-303 is the FRALE compound used in one major red cell pathogen inactivation program. For the treatment process the compound is added to red cell concentrates and incubated for 2 hours at room temperature to achieve pathogen inactivation. Following incubation for an additional 6 hours the red cell units are transferred to storage and stored in current generation red cell containers for up to 42 days. S-303 has demonstrated 21 robust inactivation of cell-free, cell associated, and proviral HIV; DHBV; herpes simplex virus, a model for CMV; BVDV agent; vesicular stomatitis virus and bacteriophage R-17. Multiple red cell function parameters showed no abnormal results when exposed to FRALE treatment. Cook et al 22 analyzed red cell function after exposure to S-303 using a study design in which red cells were pooled and redistributed into various containers. The red cells were pelleted, the platelet poor plasma removed and the concentrates suspended in an additive solution and then treated with either 150 µg/ml of S- 303 or left untreated as a control. No biologically significant differences were noted between the two arms Hematology
8 during 42 days of storage, including the degree of hemolysis, potassium leakage, glucose consumption, lactate generation, the levels of ATP and 2-3 DGP. Red cell recovery and life span were evaluated in vivo, in mouse and dog models. No significant differences were found in red cell recovery between units treated with 150 µg/ml S-303 versus controls. Phase I clinical trials of transfusion of S-303 treated red cells in humans have been completed as have phase II studies. Phase III clinical trials are starting in fall Dimethylmethylene Blue Another material under investigation for pathogen-inactivation of red cells is dimethylmethylene blue This material functions similarly to methylene blue to kill viruses, but dimethylmethylene blue is more hydrophobic and can more easily enter cells. Wagner et al found that dimethylmethylene blue and light inactivated several RNA and DNA model viruses and leukocytes under conditions that resulted in small decreases in RBC ATP and 2,3 DPG levels, less than 1% hemolysis, minimal alterations of osmotic fragility, negative direct antiglobulin tests in 11 of 13 samples, unchanged strength for 12 of 13 RBC antigens, unchanged RBC morphology, similar banding patterns in SDS-PAGE, and small alterations in potassium efflux during 42-day storage. Further trials will be required to determine if dimethylmethylene bluetreated cells have acceptable in vivo survival. Inactine A new pathogen inactivation technology using Inactine has been developed by the Vitex Corporation. 26 Inactine is a compound chemically related to binary ethyleneimine that is highly selective for nucleic acids. In the free state it has low reactivity for biomolecules, but electrostatic binding to nucleic acids results in the activation of Inactine to a reactive species. 26 Modifications of nucleic acids by ethyleneimines occurs principally by covalent interaction of the aziridino group with the N-7 position of guanine. 26 This results in the spontaneous opening of the imidazole ring structure. These structures are potent stop signals for DNA and RNA polymerases and the modified nucleic acids can no longer serve as templates for transcription or replication. 26 Inactine activation is not a photochemical process. It is mixed with red cells for 6-24 hours at room temperature during which time pathogen and leukocyte inactivation occurs. Residual Inactine is removed by cell washing. This process has been shown to have high efficiency kill against a broad spectrum of pathogens. Toxicity and mutagenicity studies reported to date have all been unremarkable. Recent reports by Aubuchon and others showed that Inactine is useful for pathogen inactivation of red blood cells. Accordingly, Inactine is another pathogen inactivation system which will need to be evaluated for eventual licensure for treatment of red cells. As outlined by Corash, 2 many research efforts are being put forth to develop a third level of safety for the blood supply using pathogen inactivation technologies. There are a variety of technologies available for inactivation of FFP due to the relative ease of viral inactivation of a non-cellular material. Progress is also being made in development of S-59 and riboflavin-based therapies for inactivation of platelets and S-303, riboflavin and Inactine technologies for pathogen inactivation of red cells. Despite the reality that there is a very low risk of transfusion transmitted disease, the public perception that the blood supply is still unsafe persists. This perception will likely remain a concern for the foreseeable future as bacteria, rickettsia, 31 viruses such as Ebola, Japanese encephalitis, Hanta, CMV, and parasitic diseases such as babesiosis, malaria and Chagas disease continue to plague the blood supply. Even today, new viruses unheard of by most non-virologists and certainly by the public at large, including retroviruses such as animal Simian Foamy Virus (SFV), are starting to enter the blood supply with as yet, unknown consequences. 32 Techniques aimed at developing non-human sources of blood products, i.e., red cells or other blood components made from cell culture lines in commercial processing plants, are being pursued. Other means of providing an effective increase in blood count without transfusion are also being studied, such as use of hematopoietic cytokines. Pathogen inactivation will likely be required in the future if only because there are just too many pathogens. It is too expensive for society to develop a new assay for each of the hundreds of yet to be addressed pathogens threatening the blood supply. It is also too expensive to replace lost donors. Pathogen inactivation will also address concerns in real-time. That is the pathogen inactivated material will already be in the blood bag waiting to inactivate pathogens we may not even realize are present in the donor s blood. Thus, pathogen inactivation technology will be helping to ensure the safety of the blood supply even, perhaps, against bio-terrorist attack. Hospitals can no longer afford to pay for a series of expensive tests for each pathogen du jour that makes the headlines. Pathogen inactivation of the blood supply should be in the form of a single unit treatment format for it is unlikely that a pooled approach such as was used with SDP will be acceptable due to concerns over exposure to large numbers of donors and the potential spread of prion disease. A successful pathogen inactivation technology will need to have a low toxicity and be safe for most patients including neonates, pediatric age patients, renal and hepatic failure patients, the elderly and the immunosuppressed, both congenital and acquired. It 440 American Society of Hematology
9 must also be cost-reasonable. This is a major issue and one likely to be debated for years. Development of methods for pathogen inactivation will remain an active area of research in transfusion medicine well into the 21st century. Indeed, pathogen inactivation technology is yet one more defensive maneuver developed by man in his never-ending struggle against a bewildering and likely innumerable horde of pathogens ready to, and capable of, invading the blood supply. REFERENCES I. Emerging Infections and the Safety of the Blood Supply 1. Dodd RY. Germs, gels, and genomes: A personal recollection of 30 years in blood safety testing. In: Stramer SL, ed. Blood Safety in the New Millennium. Bethesda: American Association of Blood Banks; 2001: Stramer SL, Caglioti S, Strong DM. NAT of the United States and Canadian blood supply. Transfusion. 2000;40: Nahlen BL, Lobel HO, Cannon SE, Campbell CC. Reassessment of blood donor selection criteria for United States travellers to malarious areas. Transfusion. 1991;31: Mungai M, Tegtmeier G, Chamberland M, Parise M. Transfusion-transmitted malaria in the United States from 1963 through N Engl J Med 2001;344: Schmuñis GA. Trypanosoma cruzi, the etiologic agent of Chagas disease: Status in the blood supply in endemic and nonendemic countries. Transfusion. 1991;31: Leiby DA, Read EJ, Lenes BA, et al. Seroepidemiology of Trypanosoma cruzi, etiologic agent of Chagas disease, in US blood donors. J Infect Dis. 1997;176: Leiby DA, Fucci MH, Stumpf RJ. Trypanosoma cruzi in a lowto moderate-risk blood donor population: seroprevalence and possible congenital transmission. Transfusion. 1999;39: Leiby DA, Rentas FJ, Nelson KE, et al. Evidence of Trypanosoma cruzi infection (Chagas disease) among patients undergoing cardiac surgery. Circulation. 2000;102: McQuiston JH, Childs JE, Chamberland ME, Tabor E. Transmission of tick-borne agents of disease by blood transfusion: a review of known and potential risks in the United States. Transfusion. 2000;40: Eskow ES, Krause PJ, Spielman A, Freeman K, Aslanzadeh J. Southern extension of the range of human babesiosis in the eastern United States. J Clin Microbiol. 1999;37: Herwaldt BL, Kjemtrup AM, Conrad PA, et al. Transfusiontransmitted babesiosis in Washington state: First reported case caused by a WA1-type parasite. J Infect Dis. 1997;175: Saito-Ito A, Tsuji M, Wei Q, et al. Transfusion-acquired, autochthonous human babesiosis in Japan: Isolation of Babesia microti-like parasites with hu-rbc-scid mice. J Clin Microbiol. 2000;38: Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in N Engl J Med. 2001;344: Farge D, Lebbé C, Marjanovic Z, et al. Human herpes virus-8 and other risk factors for Kaposi s sarcoma in kidney transplant recipients. Transplantation. 1999;67: Operskalski EA, Busch MP, Mosley JW, Kedes DH. Blood donations and viruses. Lancet. 1997;349: Cannon MJ, Dollard SC, Smith DK, et al. Blood-borne and sexual transmission of human herpesvirus 8 in women with or at risk for human immunodeficiency virus infection. N Engl J Med. 2001;344: Alter HJ, Nakatsuji Y, Melpolder J, et al. The incidence of transfusion-associated hepatitis G virus infection and its relation to liver disease. N Engl J Med. 1997;336: Mushahwar IK. Recently discovered blood-borne viruses: Are they hepatitis viruses or merely endosymbionts? Commentary. J Med Virol. 2000;62: Orton SL, ARCNET Program, Liu H, Cable R, Grindon A, Williams A. Prevalence of circulating T. pallidum in STS+/FTA- ABS+ blood donors [abstract]. Transfusion. 2000;39:2S. 20. Eastlund T, Persing D, Mathiesen D, et al. Human granulocytic ehrlichosis after red cell transfusion [abstract]. Transfusion. 2000;39:117S. 21. Wagner SJ, Friedman LI, Dodd RY. Transfusion-associated bacterial sepsis. Clin Microbiol Rev. 1994;7: II. Pathogen Inactivation of the Blood Supply 1. Schreiber GB, Busch MP, Kleinman S, et al. The risk of transfusion-transmitted viral infections. N Engl J Med. 1996; Corash L. Inactivation of viruses, bacteria, protozoa, and leukocytes in platelet concentrates: current research perspectives. Transfusion Med Rev. 1999;13: Klein HG, Dodd RY, Dzik WH, et al. Current status of solvent/ detergent-treated frozen plasma. Transfusion. 1998;38: Mast AE, Latham K, Stadanlik, JE, et al. Solvent-detergent treatment of fresh frozen plasma causes polymerization and inactivation of antiplasmin but not antithrombin. Blood. 1998;92 (suppl. 1):502a. Abstract # Pehta JC: Clinical studies with solvent detergent-treated products. Transfusion Med Rev. 1996;10: Lerner RG, Nelson J, Sorcia E, et al. Evaluation of solvent/ detergent-treated plasma in patients with a prolonged prothrombin time. Vox Sang. 2000;79: Evans G, Llewelyn C, Luddington R, Baglin TP, Williamson LM. Solvent/detergent fresh frozen plasma as primary treatment of acute thrombotic thrombocytopenic purpura. Clin Lab Haematol. 1999;21: Tocci LJ, Napychank PA, Cable RG, Snyder EL. The effect of solvent/detergent-treated plasma on stored platelet concentrates. Transfusion. 1993;33: Geiger TL, Pisciotto PT, Baril LL, Snyder EL. The effect of solvent/detergent-treated plasma on red cells stored in vitro. Transfusion. 1995;35: Wages D, Smith D, Walsh J, et al. Transfusion of therapeutic doses of virally inactivated fresh frozen plasma in healthy subjects. Blood. 1997;90 suppl. 1:S Wages, D, Radu-Radurescu L, Adams M, et al. Quantitative analysis of coagulation factors in response to transfusion of S- 59 photochemically treated fresh frozen plasma (S-59 FFP) and standard FFP. Blood. 1998;92 (suppl.1):503a. 12. Lin L, Cook DN, Wiesehahn GP, et al. Photochemical inactivation of viruses and bacteria in platelet concentrates by use of a novel psoralen and long-wavelength ultraviolet light. Transfusion. 1997;37: Margolis-Nunno H, Bardossy L, Robinson R, et al. Psoralenmediated photochemical decontamination of platelet concentrates: Inactivation of cell-free and cell-associated forms of human immunodeficiency virus and assessment of platelet function in vivo. Transfusion. 1997;37: Lin L, Cook DN, Wiesehahn GP, et al. Photochemical inactivation of viruses and bacteria in platelet concentrates by use of a novel psoralen and long-wavelength ultraviolet light. Transfusion. 1997;37: Hematology
Pathogen Reduction/Inactivation KABB Annual Meeting Elpidio Pena, MD, MA Norton Healthcare Transfusion Services
 Pathogen Reduction/Inactivation KABB Annual Meeting 2017 Elpidio Pena, MD, MA Norton Healthcare Transfusion Services Goals Discuss the approved methods for pathogen reduction/inactivation of blood products
Pathogen Reduction/Inactivation KABB Annual Meeting 2017 Elpidio Pena, MD, MA Norton Healthcare Transfusion Services Goals Discuss the approved methods for pathogen reduction/inactivation of blood products
EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS
 EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits
EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits
New Advances in Transfusion EM I LY CO BERLY, M D
 New Advances in Transfusion EM I LY CO BERLY, M D TRANSFUSI ON M EDI CI NE FELLO W VANDERBI LT UNI VERSITY Objectives To discuss the terminology, components, transfusion risks, and dosing guidelines for
New Advances in Transfusion EM I LY CO BERLY, M D TRANSFUSI ON M EDI CI NE FELLO W VANDERBI LT UNI VERSITY Objectives To discuss the terminology, components, transfusion risks, and dosing guidelines for
Keeping the Blood Supply Safe Current and Future Strategies. Marissa Li, MD
 Keeping the Blood Supply Safe Current and Future Strategies Marissa Li, MD Overview Pre-collection Donor Screening Collection Visual Inspection Aseptic Processing Leukoreduction of Apheresis Collections
Keeping the Blood Supply Safe Current and Future Strategies Marissa Li, MD Overview Pre-collection Donor Screening Collection Visual Inspection Aseptic Processing Leukoreduction of Apheresis Collections
Screening donors and donations for transfusion transmissible infectious agents. Alan Kitchen
 Screening donors and donations for transfusion transmissible infectious agents Alan Kitchen Aim Not to teach you microbiology To provide and awareness of the big picture To provide an understanding of
Screening donors and donations for transfusion transmissible infectious agents Alan Kitchen Aim Not to teach you microbiology To provide and awareness of the big picture To provide an understanding of
Babesia from a donor perspective
 Babesia from a donor perspective American Red Cross, Massachusetts Region Bryan Spencer, MPH Research Scientist American Society for Apheresis Annual Meeting May 8, 2015 San Antonio, TX The need is constant.
Babesia from a donor perspective American Red Cross, Massachusetts Region Bryan Spencer, MPH Research Scientist American Society for Apheresis Annual Meeting May 8, 2015 San Antonio, TX The need is constant.
Objectives. Methods. Results. Economic
 Octaplas Compared with Fresh Frozen Plasma to Reduce the Risk of Transmitting Lipid-Enveloped Viruses: An Economic Analysis and Budget Impact Analysis [Adapted from Membe SK, Coyle D, Husereau D, Cimon
Octaplas Compared with Fresh Frozen Plasma to Reduce the Risk of Transmitting Lipid-Enveloped Viruses: An Economic Analysis and Budget Impact Analysis [Adapted from Membe SK, Coyle D, Husereau D, Cimon
Be ready. The INTERCEPT Blood System for Platelets and Plasma pathogen reduction system
 Be ready. The INTERCEPT Blood System for Platelets and Plasma pathogen reduction system INTERCEPT Blood System for Platelets an Blood safety is changing Safe donated blood is critical to improving certain
Be ready. The INTERCEPT Blood System for Platelets and Plasma pathogen reduction system INTERCEPT Blood System for Platelets an Blood safety is changing Safe donated blood is critical to improving certain
Blood Transfusion. What is blood transfusion? What are blood banks? When is a blood transfusion needed? Who can donate blood?
 What is blood transfusion? A blood transfusion is a safe, common procedure in which blood is given through an intravenous (IV) line in one of the blood vessels. A blood transfusion usually takes two to
What is blood transfusion? A blood transfusion is a safe, common procedure in which blood is given through an intravenous (IV) line in one of the blood vessels. A blood transfusion usually takes two to
Blood transfusion. Dr. J. Potgieter Dept. of Haematology NHLS - TAD
 Blood transfusion Dr. J. Potgieter Dept. of Haematology NHLS - TAD General Blood is collected from volunteer donors >90% is separated into individual components and plasma Donors should be: healthy, have
Blood transfusion Dr. J. Potgieter Dept. of Haematology NHLS - TAD General Blood is collected from volunteer donors >90% is separated into individual components and plasma Donors should be: healthy, have
Surveillance Report 2014
 Surveillance Report 2014 Executive summary We are pleased to present the third report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Surveillance Report 2014 Executive summary We are pleased to present the third report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Optimal / Evidence-based Method to Prevent Transmission of Cytomegalovirus (CMV) by Transfusion
 ILABB Fall Meeting 2015 Optimal / Evidence-based Method to Prevent Transmission of Cytomegalovirus (CMV) by Transfusion Presenter Ronald G Strauss, MD Associate Medical Director, LifeSource/ITxM Terms
ILABB Fall Meeting 2015 Optimal / Evidence-based Method to Prevent Transmission of Cytomegalovirus (CMV) by Transfusion Presenter Ronald G Strauss, MD Associate Medical Director, LifeSource/ITxM Terms
Non-reproductive tissues and cells Recommending authority/ association
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Tested pathogen Donor test/ technique
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Tested pathogen Donor test/ technique
Update on Transfusion- Transmitted Infectious Diseases
 Update on Transfusion- Transmitted Infectious Diseases Scott Koepsell MD PhD Associate Professor University of Nebraska Medical Center I have no conflicts of interest to disclose. Global changes and the
Update on Transfusion- Transmitted Infectious Diseases Scott Koepsell MD PhD Associate Professor University of Nebraska Medical Center I have no conflicts of interest to disclose. Global changes and the
Update on THERAFLEX UV-Platelets: a novel system for pathogen reduction of platelets
 Update on THERAFLEX UV-Platelets: a novel system for pathogen reduction of platelets Axel Seltsam, MD, MHBA German Red Cross Blood Service NSTOB, Springe Fribourg 2011 THERAFLEX UV-Platelets Efficient
Update on THERAFLEX UV-Platelets: a novel system for pathogen reduction of platelets Axel Seltsam, MD, MHBA German Red Cross Blood Service NSTOB, Springe Fribourg 2011 THERAFLEX UV-Platelets Efficient
Keeping blood transfusions safe:
 ISP-WIV Seminar Diagnosis and surveillance of infectious diseases May 18 th, 2017 Keeping blood transfusions safe: the challenge of emerging infectious diseases Presented by Roland HÜBNER Superior Health
ISP-WIV Seminar Diagnosis and surveillance of infectious diseases May 18 th, 2017 Keeping blood transfusions safe: the challenge of emerging infectious diseases Presented by Roland HÜBNER Superior Health
For more information about how to cite these materials visit
 Author(s): Robertson Davenport, M.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Noncommercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Robertson Davenport, M.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Noncommercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Blood Components & Indications for Transfusion. Neda Kalhor
 Blood Components & Indications for Transfusion Neda Kalhor Blood products Cellular Components: Red blood cells - Leukocyte-reduced RBCs - Washed RBCs - Irradiated RBCs Platelets - Random-donor platelets
Blood Components & Indications for Transfusion Neda Kalhor Blood products Cellular Components: Red blood cells - Leukocyte-reduced RBCs - Washed RBCs - Irradiated RBCs Platelets - Random-donor platelets
The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P
 The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P Record Status This is a critical abstract of an economic evaluation that meets
The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P Record Status This is a critical abstract of an economic evaluation that meets
Outbreak Investigation Guidance for Vectorborne Diseases
 COMMUNICABLE DISEASE OUTBREAK MANUAL New Jersey s Public Health Response APPENDIX T3: EXTENDED GUIDANCE Outbreak Investigation Guidance for Vectorborne Diseases As per N.J.A.C. 8:57, viruses that are transmitted
COMMUNICABLE DISEASE OUTBREAK MANUAL New Jersey s Public Health Response APPENDIX T3: EXTENDED GUIDANCE Outbreak Investigation Guidance for Vectorborne Diseases As per N.J.A.C. 8:57, viruses that are transmitted
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING October2010. Issue Summary
 TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
Non-reproductive tissues and cells
 Colour key Minimum requirements as set out in Directive 2004/23/EC and its technical Directives (particularly 2006/17/EC) More stringent -legally binding, applies for all donations and all donor profiles
Colour key Minimum requirements as set out in Directive 2004/23/EC and its technical Directives (particularly 2006/17/EC) More stringent -legally binding, applies for all donations and all donor profiles
Blood Products & Transfusion. Karim Rafaat, M.D.
 Blood Products & Transfusion Karim Rafaat, M.D. Compatibility Testing Compatibility testing involves three separate procedures involving both donor and recipient blood. 1. ABO & Rh blood type identification
Blood Products & Transfusion Karim Rafaat, M.D. Compatibility Testing Compatibility testing involves three separate procedures involving both donor and recipient blood. 1. ABO & Rh blood type identification
Surveillance Report 2015
 Surveillance Report 2015 Executive summary We are pleased to present the fourth report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Surveillance Report 2015 Executive summary We are pleased to present the fourth report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Pathogen Safety and BSE / variant CJD
 Pathogen Safety and BSE / variant CJD Thomas R. Kreil, Global Pathogen Safety Parenteral Drug Industry Plasma Protein Industry Summit September 7, 2017; Beijing Pathogen Safety Those who cannot remember
Pathogen Safety and BSE / variant CJD Thomas R. Kreil, Global Pathogen Safety Parenteral Drug Industry Plasma Protein Industry Summit September 7, 2017; Beijing Pathogen Safety Those who cannot remember
Jovona Powelson, B.S. MLT (ASCP) Director of Laboratories
 Jovona Powelson, B.S. MLT (ASCP) Director of Laboratories Blood Products From the Donor to You Objectives Deliver a brief virtual tour of a blood center. Describe the number of donors needed to meet the
Jovona Powelson, B.S. MLT (ASCP) Director of Laboratories Blood Products From the Donor to You Objectives Deliver a brief virtual tour of a blood center. Describe the number of donors needed to meet the
Non-reproductive tissues and cells
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legally binding on national level More stringent testing - recommended on national level Not legally binding
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legally binding on national level More stringent testing - recommended on national level Not legally binding
Pathogen inactivation in platelet concentrates in France
 Swisstransfusion: Jahrestagung 2011 Fribourg, 8-9 septembre, 2011 Pathogen inactivation in platelet concentrates in France Jean-Pierre Cazenave EFS-Alsace, UMR_S949 INSERM-Université de Strasbourg, France
Swisstransfusion: Jahrestagung 2011 Fribourg, 8-9 septembre, 2011 Pathogen inactivation in platelet concentrates in France Jean-Pierre Cazenave EFS-Alsace, UMR_S949 INSERM-Université de Strasbourg, France
Guidance for Industry
 Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
DHQ Flowcharts v2.0 eff. February 2016
 Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Ring Forms in Red Blood Cells (RBCs) Babesia? from Danish Chronically Ill Patients, All Clinically Suspect of Having Persistent Active Borreliosis!
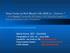 Ring Forms in Red Blood Cells (RBCs) Babesia? from Danish Chronically Ill Patients, All Clinically Suspect of Having Persistent Active Borreliosis! Marie Kroun, MD Denmark Presentation in York, UK June
Ring Forms in Red Blood Cells (RBCs) Babesia? from Danish Chronically Ill Patients, All Clinically Suspect of Having Persistent Active Borreliosis! Marie Kroun, MD Denmark Presentation in York, UK June
Babesia spp. Emerging Transfusion Dilemmas
 Babesia spp. Emerging Transfusion Dilemmas David A. Leiby, PhD Transmissible Diseases Department American Red Cross Holland Laboratory Department of Microbiology and Tropical Medicine George Washington
Babesia spp. Emerging Transfusion Dilemmas David A. Leiby, PhD Transmissible Diseases Department American Red Cross Holland Laboratory Department of Microbiology and Tropical Medicine George Washington
PATHOGEN INACTIVATION:
 PATHOGEN INACTIVATION: She s worth it. Be SURE. With INTERCEPT you know that you are doing everything in your power to deliver safe and effective blood products to patients. Transfusion-transmitted infection
PATHOGEN INACTIVATION: She s worth it. Be SURE. With INTERCEPT you know that you are doing everything in your power to deliver safe and effective blood products to patients. Transfusion-transmitted infection
Non-reproductive tissues and cells
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding on national level More stringent - recommended on national level Not legy binding and not recommended on
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding on national level More stringent - recommended on national level Not legy binding and not recommended on
CTYOMEGALOVIRUS (CMV) - BACKGROUND
 CTYOMEGALOVIRUS (CMV) - BACKGROUND PURPOSE The flowing information provides guidance on the use of CMV negative blood components provided by the blood bank at the Royal Children s Hospital (RCH) including
CTYOMEGALOVIRUS (CMV) - BACKGROUND PURPOSE The flowing information provides guidance on the use of CMV negative blood components provided by the blood bank at the Royal Children s Hospital (RCH) including
Ensuring Compliance: Regulatory guidance for virus clearance validation
 Application Note Ensuring Compliance: Regulatory guidance for virus clearance validation Introduction To assure virus safety of biological therapeutics, regulatory guidance advocates an approach in which
Application Note Ensuring Compliance: Regulatory guidance for virus clearance validation Introduction To assure virus safety of biological therapeutics, regulatory guidance advocates an approach in which
Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services
 Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services Outline 1 Current criteria Proposed criteria Stakeholder outreach Outline 2 Safety Assessment Results since implementation
Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services Outline 1 Current criteria Proposed criteria Stakeholder outreach Outline 2 Safety Assessment Results since implementation
PROPOSED TECHNICAL REQUIREMENTS BLOOD AND BLOOD COMPONENTS. File 1 DRAFT FOR CONSULTATION PURPOSES ONLY
 PROPOSED TECHNICAL REQUIREMENTS BLOOD AND BLOOD COMPONENTS File 1 DRAFT FOR CONSULTATION PURPOSES ONLY ANNEX I INFORMATION REQUIREMENTS A. INFORMATION TO BE PROVIDED TO DONORS 1. Accurate but generally
PROPOSED TECHNICAL REQUIREMENTS BLOOD AND BLOOD COMPONENTS File 1 DRAFT FOR CONSULTATION PURPOSES ONLY ANNEX I INFORMATION REQUIREMENTS A. INFORMATION TO BE PROVIDED TO DONORS 1. Accurate but generally
Magdy El Ekiaby, MD Shabrawishi Hospital BTC & HTC Cairo, Egypt
 Magdy El Ekiaby, MD Shabrawishi Hospital BTC & HTC Cairo, Egypt Are plasma and cryoprecipitate still used? They are still largely used for treatment of hereditary bleeding disorders in many resource limited
Magdy El Ekiaby, MD Shabrawishi Hospital BTC & HTC Cairo, Egypt Are plasma and cryoprecipitate still used? They are still largely used for treatment of hereditary bleeding disorders in many resource limited
GUIDELINES FOR THE TRANSFUSION OF BLOOD COMPONENTS
 CHILDREN S HOSPITALS AND CLINICS OF MINNESOTA Introduction: GUIDELINES FOR THE TRANSFUSION OF BLOOD COMPONENTS These guidelines have been developed in conjunction with the hospital Transfusion Committee.
CHILDREN S HOSPITALS AND CLINICS OF MINNESOTA Introduction: GUIDELINES FOR THE TRANSFUSION OF BLOOD COMPONENTS These guidelines have been developed in conjunction with the hospital Transfusion Committee.
Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey
 Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey This questionnaire consists of three sections: A. Collection and
Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey This questionnaire consists of three sections: A. Collection and
Changing eligibility criteria for MSM: The Canadian Perspective
 Changing eligibility criteria for MSM: The Canadian Perspective Mindy Goldman MD Canadian Blood Services ASFA & WAA Joint Meeting, San Francisco April 4, 2014 Outline - Historical background - International
Changing eligibility criteria for MSM: The Canadian Perspective Mindy Goldman MD Canadian Blood Services ASFA & WAA Joint Meeting, San Francisco April 4, 2014 Outline - Historical background - International
A Transfusion Reaction What Do I Do Now? Judith A. Sullivan, MS, MT(ASCP)SBB, CQA(ASQ) ASCLS Region III Triennial Meeting Birmingham AL
 A Transfusion Reaction What Do I Do Now? Judith A. Sullivan, MS, MT(ASCP)SBB, CQA(ASQ) ASCLS Region III Triennial Meeting Birmingham AL This promotional educational activity is brought to you by Ortho
A Transfusion Reaction What Do I Do Now? Judith A. Sullivan, MS, MT(ASCP)SBB, CQA(ASQ) ASCLS Region III Triennial Meeting Birmingham AL This promotional educational activity is brought to you by Ortho
TRANSFUSIONS FIRST, DO NO HARM
 TRANSFUSIONS FIRST, DO NO HARM BECAUSE BLOOD CAN KILL 7 TRALI DEATHS SINCE 2002 WMC 5 women BECAUSE In OB you are transfusing 2 instead of 1 BECAUSE BLOOD IS A LIQUID TRANSPLANT RISKS versus BENEFITS versus
TRANSFUSIONS FIRST, DO NO HARM BECAUSE BLOOD CAN KILL 7 TRALI DEATHS SINCE 2002 WMC 5 women BECAUSE In OB you are transfusing 2 instead of 1 BECAUSE BLOOD IS A LIQUID TRANSPLANT RISKS versus BENEFITS versus
Transfusion Reactions. Directed by M-azad March 2012
 Transfusion Reactions Directed by M-azad March 2012 Transfusion Reactions are Adverse reactions associated with the transfusion of blood and its components Transfusion reactions Non-threatening to fatal
Transfusion Reactions Directed by M-azad March 2012 Transfusion Reactions are Adverse reactions associated with the transfusion of blood and its components Transfusion reactions Non-threatening to fatal
Pretransfusion Testing
 Pretransfusion Testing Blood transfusion services The immunohematologic testing needed for By proper patient blood typing, component Dr. selection Reham and Talaat compatibility testing. Which Blood ensure
Pretransfusion Testing Blood transfusion services The immunohematologic testing needed for By proper patient blood typing, component Dr. selection Reham and Talaat compatibility testing. Which Blood ensure
Transfusion-transmitted Cytomegalovirus
 Transfusion-transmitted Cytomegalovirus Can you confidently abandon CMV seronegative products in the modern era of pre-storage leukoreduction? Jeannie Callum, BA, MD, FRCPC Really? Are we still talking
Transfusion-transmitted Cytomegalovirus Can you confidently abandon CMV seronegative products in the modern era of pre-storage leukoreduction? Jeannie Callum, BA, MD, FRCPC Really? Are we still talking
Mary Berg, M.D. Medical Director, Transfusion Services Associate Professor of Pathology University of Colorado Hospital
 Transfusion Reactions/Complications Mary Berg, M.D. Medical Director, Transfusion Services Associate Professor of Pathology University of Colorado Hospital Acute Transfusion Reactions Can be seen with
Transfusion Reactions/Complications Mary Berg, M.D. Medical Director, Transfusion Services Associate Professor of Pathology University of Colorado Hospital Acute Transfusion Reactions Can be seen with
1. Virus 2. Capsid 3. Envelope
 VIRUSES BIOLOGY II VOCABULARY- VIRUSES (22 Words) 1. Virus 2. Capsid 3. Envelope 4. Provirus 5. Retrovirus 6. Reverse transcriptase 7. Bacteriophage 8. Lytic Cycle 9. Virulent 10. Lysis 11. Lysogenic Cycle
VIRUSES BIOLOGY II VOCABULARY- VIRUSES (22 Words) 1. Virus 2. Capsid 3. Envelope 4. Provirus 5. Retrovirus 6. Reverse transcriptase 7. Bacteriophage 8. Lytic Cycle 9. Virulent 10. Lysis 11. Lysogenic Cycle
EBV and Infectious Mononucleosis. Infectious Disease Definitions. Infectious Diseases
 Infectious Disease Definitions Infection when a microorganism invades a host and multiplies enough to disrupt normal function by causing signs and symptoms Pathogencity ability of an organism to cause
Infectious Disease Definitions Infection when a microorganism invades a host and multiplies enough to disrupt normal function by causing signs and symptoms Pathogencity ability of an organism to cause
Surveillance Report 2016
 Surveillance Report 2016 Executive summary We are pleased to present the fifth report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Surveillance Report 2016 Executive summary We are pleased to present the fifth report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Answering your daily challenges. in the ELISA technology I N FECTI OUS DISEASES. bioelisa
 Answering your daily challenges in the ELISA technology I N FECTI OUS DISEASES bioelisa RETROVIRUS HIV is the cause of the most important global pandemic. Due to the lack of vaccination, early diagnosis
Answering your daily challenges in the ELISA technology I N FECTI OUS DISEASES bioelisa RETROVIRUS HIV is the cause of the most important global pandemic. Due to the lack of vaccination, early diagnosis
Emerging Pathogens that Impact the Canadian Blood Supply Alberta Vein to Vein Conference March 18-19, 2016
 Emerging Pathogens that Impact the Canadian Blood Supply Alberta Vein to Vein Conference March 18-19, 2016 Dr. Margaret Fearon Medical Director, Medical Microbiology, Canadian Blood Services March 18,
Emerging Pathogens that Impact the Canadian Blood Supply Alberta Vein to Vein Conference March 18-19, 2016 Dr. Margaret Fearon Medical Director, Medical Microbiology, Canadian Blood Services March 18,
Pathogenreduktion mittels UVC-Licht: Entwicklung des THERAFLEX UV-Plättchen-Systems
 Pathogenreduktion mittels UVC-Licht: Entwicklung des THERAFLEX UV-Plättchen-Systems Pathogen reduction by UVC light: Development of the THERAFLEX UV-Platelets system Fribourg 2011 Prof. Dr. Axel Seltsam,
Pathogenreduktion mittels UVC-Licht: Entwicklung des THERAFLEX UV-Plättchen-Systems Pathogen reduction by UVC light: Development of the THERAFLEX UV-Platelets system Fribourg 2011 Prof. Dr. Axel Seltsam,
Emerging TTIs How Singapore secure its blood supply
 Emerging TTIs How Singapore secure its blood supply Ms Sally Lam Acting Division Director I Blood Supply Management I Blood Services Group I Health Sciences Outline Risk Mitigation Strategies to secure
Emerging TTIs How Singapore secure its blood supply Ms Sally Lam Acting Division Director I Blood Supply Management I Blood Services Group I Health Sciences Outline Risk Mitigation Strategies to secure
Babesia and Blood Safety
 Babesia and Blood Safety American Red Cross, New England Region Bryan Spencer, MPH Manager of Blood Research / REDS-III Coordinator ASM 46 th Annual Region I Meeting 27 October, 2011 Randolph, MA The need
Babesia and Blood Safety American Red Cross, New England Region Bryan Spencer, MPH Manager of Blood Research / REDS-III Coordinator ASM 46 th Annual Region I Meeting 27 October, 2011 Randolph, MA The need
PLASMA PRODUSTS IN CRITICAL CARE MEDICINE
 PLASMA PRODUSTS IN CRITICAL CARE MEDICINE Paul F. W. Strengers Introduction Plasma from blood donors is the source for the preparation of a number of medicinal products, with a wide range of clinical indications.
PLASMA PRODUSTS IN CRITICAL CARE MEDICINE Paul F. W. Strengers Introduction Plasma from blood donors is the source for the preparation of a number of medicinal products, with a wide range of clinical indications.
Blood Product Modifications: Leukofiltration, Irradiation and Washing
 1. Leukocyte Reduction Definitions and Standards: o Process also known as leukoreduction, or leukofiltration o Applicable AABB Standards, 25th ed. Leukocyte-reduced RBCs At least 85% of original RBCs
1. Leukocyte Reduction Definitions and Standards: o Process also known as leukoreduction, or leukofiltration o Applicable AABB Standards, 25th ed. Leukocyte-reduced RBCs At least 85% of original RBCs
Chapter 18. Viral Genetics. AP Biology
 Chapter 18. Viral Genetics 2003-2004 1 A sense of size Comparing eukaryote bacterium virus 2 What is a virus? Is it alive? DNA or RNA enclosed in a protein coat Viruses are not cells Extremely tiny electron
Chapter 18. Viral Genetics 2003-2004 1 A sense of size Comparing eukaryote bacterium virus 2 What is a virus? Is it alive? DNA or RNA enclosed in a protein coat Viruses are not cells Extremely tiny electron
Interventions for Babesia (and Plasmodium)
 Interventions for Babesia (and Plasmodium) Susan L. Stramer, Ph.D. May 16 2017 www.aabb.org Babesiosis Malaria-like illness caused by Babesia spp. Asymptomatic fatal Non-specific symptoms (malaise, fever,
Interventions for Babesia (and Plasmodium) Susan L. Stramer, Ph.D. May 16 2017 www.aabb.org Babesiosis Malaria-like illness caused by Babesia spp. Asymptomatic fatal Non-specific symptoms (malaise, fever,
Duration: 12 months May to April
 SPECIALIST CERTIFICATE IN TRANSFUSION SCIENCE PRACTICE PROGRAMME OF STUDY OVERVIEW Example only Duration: 12 months May to April This document serves as a general programme overview only. To ensure you
SPECIALIST CERTIFICATE IN TRANSFUSION SCIENCE PRACTICE PROGRAMME OF STUDY OVERVIEW Example only Duration: 12 months May to April This document serves as a general programme overview only. To ensure you
Disease Transmission Methods
 Disease Transmission Methods In epidemiology, transmission simply means any method by which an infectious agent is spread from one host to another. Knowing the type of pathogen often, but not always, identifies
Disease Transmission Methods In epidemiology, transmission simply means any method by which an infectious agent is spread from one host to another. Knowing the type of pathogen often, but not always, identifies
LifeBridge Health Transfusion Service Sinai Hospital of Baltimore Northwest Hospital Center BQA Transfusion Criteria Version#2 POLICY NO.
 LifeBridge Health Transfusion Service Sinai Hospital of Baltimore Northwest Hospital Center BQA 1011.02 Transfusion Criteria Version#2 Department POLICY NO. PAGE NO. Blood Bank Quality Assurance Manual
LifeBridge Health Transfusion Service Sinai Hospital of Baltimore Northwest Hospital Center BQA 1011.02 Transfusion Criteria Version#2 Department POLICY NO. PAGE NO. Blood Bank Quality Assurance Manual
KNOWLEDGE INFUSION: FOCUS ON RISK-BASED DECISION-MAKING
 KNOWLEDGE INFUSION: FOCUS ON RISK-BASED DECISION-MAKING Permission to Use: Please note that the presenter has agreed to make their presentation available. However, should you want to use some of the data
KNOWLEDGE INFUSION: FOCUS ON RISK-BASED DECISION-MAKING Permission to Use: Please note that the presenter has agreed to make their presentation available. However, should you want to use some of the data
Confirmed (Laboratory Tests) Serum positive for IgM anti-hbc or, hepatitis B surface antigen (HbsAg).
 Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
Lecture 2: Virology. I. Background
 Lecture 2: Virology I. Background A. Properties 1. Simple biological systems a. Aggregates of nucleic acids and protein 2. Non-living a. Cannot reproduce or carry out metabolic activities outside of a
Lecture 2: Virology I. Background A. Properties 1. Simple biological systems a. Aggregates of nucleic acids and protein 2. Non-living a. Cannot reproduce or carry out metabolic activities outside of a
A. Plasma - A little more than half of your blood is a watery portion termed plasma.
 Lesson Three Blood and Immunity Outline II. Blood - Blood is composed of a cellular portion and a watery portion. It carries the essential life-sustaining nutrients, gases (oxygen) and wastes throughout
Lesson Three Blood and Immunity Outline II. Blood - Blood is composed of a cellular portion and a watery portion. It carries the essential life-sustaining nutrients, gases (oxygen) and wastes throughout
Seroprevalence of Babesia microti in Individuals with Lyme Disease. Sabino R. Curcio, M.S, MLS(ASCP)
 Seroprevalence of Babesia microti in Individuals with Lyme Disease Sabino R. Curcio, M.S, MLS(ASCP) Lyme Disease Most common vectorborne illness in the United States Caused by the tick-transmitted spirochete
Seroprevalence of Babesia microti in Individuals with Lyme Disease Sabino R. Curcio, M.S, MLS(ASCP) Lyme Disease Most common vectorborne illness in the United States Caused by the tick-transmitted spirochete
Non-reproductive tissues and cells
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legally binding on national level More stringent testing - recommended on national level Not legally binding
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legally binding on national level More stringent testing - recommended on national level Not legally binding
Immune System. Before You Read. Read to Learn
 Immune System 37 section 1 Infectious Diseases Biology/Life Sciences 10.d Students know there are important differences between bacteria and viruses with respect to their requirements for growth and replication,
Immune System 37 section 1 Infectious Diseases Biology/Life Sciences 10.d Students know there are important differences between bacteria and viruses with respect to their requirements for growth and replication,
MSM DEFERRAL POLICY ISSUE DOCUMENT: MSM DEFERRAL ISSUE BACKGROUND
 MSM DEFERRAL POLICY ISSUE DOCUMENT: MSM DEFERRAL ISSUE Countries around the world are reviewing the policy of deferring from blood donation men who have had sex with men (MSM). Both Canadian Blood Services
MSM DEFERRAL POLICY ISSUE DOCUMENT: MSM DEFERRAL ISSUE Countries around the world are reviewing the policy of deferring from blood donation men who have had sex with men (MSM). Both Canadian Blood Services
Transfusion Medicine Best Practices: Indications for Blood Components
 Transfusion Medicine Best Practices: 1.0 Policy Statements 1.1 Regional Health Authorities (RHAs) shall develop policies, processes and procedures for ordering, distribution, storage, transfusion and administration
Transfusion Medicine Best Practices: 1.0 Policy Statements 1.1 Regional Health Authorities (RHAs) shall develop policies, processes and procedures for ordering, distribution, storage, transfusion and administration
TRANSFUSION REACTIONS
 14 TRANSFUSION REACTIONS 14.1 INTRODUCTION Transfusion of blood and blood products are reported to cause reactions during or after procedure specially in patients who receive multiple transfusions. These
14 TRANSFUSION REACTIONS 14.1 INTRODUCTION Transfusion of blood and blood products are reported to cause reactions during or after procedure specially in patients who receive multiple transfusions. These
Consent Laboratory Transfuse RBC
 Peds Blood Product Infusion Order Set (386) [386] Blood product review will be performed unless exclusion criteria met. MD: Please note if transfusion giv en outside of parameter, please justify use in
Peds Blood Product Infusion Order Set (386) [386] Blood product review will be performed unless exclusion criteria met. MD: Please note if transfusion giv en outside of parameter, please justify use in
Blood Transfusions Danil hammoudi.md
 Whole blood transfusions are used: When blood loss is substantial In treating thrombocytopenia Packed red cells (cells with plasma removed) are used to treat anemia Blood Transfusions Danil hammoudi.md
Whole blood transfusions are used: When blood loss is substantial In treating thrombocytopenia Packed red cells (cells with plasma removed) are used to treat anemia Blood Transfusions Danil hammoudi.md
TRANSFUSION ASSOCIATED DISEASE, RECALL, OR COMPLICATION INVESTIGATION POLICY I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION:
 I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
Preventing CMV Transmission through Leukodepletion
 Preventing CMV Transmission through Leukodepletion Possibility & Facts Prof.S.B.Rajadhyaksha, MD,DTM,PGDMLS Head, Dept. of Transfusion Medicine Tata Memorial Hospital, Mumbai 1 Donor Leukocytes Linked
Preventing CMV Transmission through Leukodepletion Possibility & Facts Prof.S.B.Rajadhyaksha, MD,DTM,PGDMLS Head, Dept. of Transfusion Medicine Tata Memorial Hospital, Mumbai 1 Donor Leukocytes Linked
Non-reproductive tissues and cells Recommending authority/ association
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Non-reproductive tissues and cells VIRAL
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Non-reproductive tissues and cells VIRAL
UKGS TRANSFUSION SERVICE PRODUCTS AND AVAILABILITY
 Lexington, KY Page 1 of 13 Affected Sites: Enterprise Chandler X Good Samaritan I. PRINCIPLE: The UK Good Samaritan Hospital is dedicated to serve the patients with safe, high quality blood products and
Lexington, KY Page 1 of 13 Affected Sites: Enterprise Chandler X Good Samaritan I. PRINCIPLE: The UK Good Samaritan Hospital is dedicated to serve the patients with safe, high quality blood products and
Anticipating (re)emerging infections to ensure blood safety
 Anticipating (re)emerging infections to ensure blood safety Pierre Tiberghien Etablissement Français du Sang, St-Denis and Université de Franche-Comté, Besançon, France IPFA/PEI 23rd International Workshop
Anticipating (re)emerging infections to ensure blood safety Pierre Tiberghien Etablissement Français du Sang, St-Denis and Université de Franche-Comté, Besançon, France IPFA/PEI 23rd International Workshop
Human Immunodeficiency Virus. Acquired Immune Deficiency Syndrome AIDS
 Human Immunodeficiency Virus Acquired Immune Deficiency Syndrome AIDS Sudden outbreak in USA of opportunistic infections and cancers in young men in 1981 Pneumocystis carinii pneumonia (PCP), Kaposi s
Human Immunodeficiency Virus Acquired Immune Deficiency Syndrome AIDS Sudden outbreak in USA of opportunistic infections and cancers in young men in 1981 Pneumocystis carinii pneumonia (PCP), Kaposi s
Non-reproductive tissues and cells
 Colour key Tested pathogen VIRAL Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legy binding on national level More stringent testing - recommended on national level Not
Colour key Tested pathogen VIRAL Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legy binding on national level More stringent testing - recommended on national level Not
Non-reproductive tissues and cells
 Ministry of Health: Institute for Transplantation and Biomedicine / Colour key VIRAL HIV 1 and HIV 2 Hepatitis B Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More
Ministry of Health: Institute for Transplantation and Biomedicine / Colour key VIRAL HIV 1 and HIV 2 Hepatitis B Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More
Non-reproductive tissues and cells Recommending authority/ association
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legy binding on national level More stringent testing - recomd on national level Not legy binding and not recomd
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legy binding on national level More stringent testing - recomd on national level Not legy binding and not recomd
Guide to the preparation, use and quality assurance of blood components
 Contents Foreword...3 Members of the European Committee (Partial Agreement) on Blood Transfusion... 8 Members of the GTS working group... 22 Members of the TS066 working group... 30 Recommendation No.
Contents Foreword...3 Members of the European Committee (Partial Agreement) on Blood Transfusion... 8 Members of the GTS working group... 22 Members of the TS066 working group... 30 Recommendation No.
Table of Contents (continued)
 Emerging Molecular and Immunohematology Blood Typing, Grouping And Infectious Disease NAT Screening Assays And Companies Developing New Technologies and Products Table of Contents 1. Blood Typing and Grouping
Emerging Molecular and Immunohematology Blood Typing, Grouping And Infectious Disease NAT Screening Assays And Companies Developing New Technologies and Products Table of Contents 1. Blood Typing and Grouping
11/15/2011. Outline. Structural Features and Characteristics. The Good the Bad and the Ugly. Viral Genomes. Structural Features and Characteristics
 Chapter 19 - Viruses Outline I. Viruses A. Structure of viruses B. Common Characteristics of Viruses C. Viral replication D. HIV II. Prions The Good the Bad and the Ugly Viruses fit into the bad category
Chapter 19 - Viruses Outline I. Viruses A. Structure of viruses B. Common Characteristics of Viruses C. Viral replication D. HIV II. Prions The Good the Bad and the Ugly Viruses fit into the bad category
CIRCULAR. of information. For the use of Labile Blood Products. Edition
 For the use of Labile Blood Products CIRCULAR of information july 2009 Edition WARNING: The risk of transmitting known and unknown infectious disease agents is present in the transfusion of labile blood
For the use of Labile Blood Products CIRCULAR of information july 2009 Edition WARNING: The risk of transmitting known and unknown infectious disease agents is present in the transfusion of labile blood
8.L.1 Practice Questions
 Name: Date: 1. Why should antibiotics be given to a person who is ill with a bacterial disease like strep throat, but not to a person who has a viral disease like flu?. ntibiotics kill bacteria but not
Name: Date: 1. Why should antibiotics be given to a person who is ill with a bacterial disease like strep throat, but not to a person who has a viral disease like flu?. ntibiotics kill bacteria but not
Chapter 11 Risk, Toxicology, and Human Health
 Chapter 11 Risk, Toxicology, and Human Health Risk Expressed in terms of probability: how likely it is that some event will occur. Risk = (Exposure)(harm) Risk assessment (identifying, occurrence, assessing)
Chapter 11 Risk, Toxicology, and Human Health Risk Expressed in terms of probability: how likely it is that some event will occur. Risk = (Exposure)(harm) Risk assessment (identifying, occurrence, assessing)
Chapter 13 Viruses, Viroids, and Prions. Biology 1009 Microbiology Johnson-Summer 2003
 Chapter 13 Viruses, Viroids, and Prions Biology 1009 Microbiology Johnson-Summer 2003 Viruses Virology-study of viruses Characteristics: acellular obligate intracellular parasites no ribosomes or means
Chapter 13 Viruses, Viroids, and Prions Biology 1009 Microbiology Johnson-Summer 2003 Viruses Virology-study of viruses Characteristics: acellular obligate intracellular parasites no ribosomes or means
Zika Virus. Report to the Standing Committee on Health. Dr. Graham Sher Chief Executive Officer, Canadian Blood Services
 Dr. Graham Sher Chief Executive Officer, Services Dr. Dana Devine Chief Medical and Scientific Officer, Services Services Services is an arm s-length organization within the larger health-care system of
Dr. Graham Sher Chief Executive Officer, Services Dr. Dana Devine Chief Medical and Scientific Officer, Services Services Services is an arm s-length organization within the larger health-care system of
Crossmatching and Issuing Blood Components; Indications and Effects.
 Crossmatching and Issuing Blood Components; Indications and Effects. Alison Muir Blood Transfusion, Blood Sciences, Newcastle Trust Topics Covered Taking the blood sample ABO Group Antibody Screening Compatibility
Crossmatching and Issuing Blood Components; Indications and Effects. Alison Muir Blood Transfusion, Blood Sciences, Newcastle Trust Topics Covered Taking the blood sample ABO Group Antibody Screening Compatibility
October 26-30, 2003 Salt Lake City, Utah
 October 26-30, 2003 Salt Lake City, Utah High Energy Pulsed UV Light as a Sterilization Process American Association of Pharmaceutical Scientists Annual Meeting and Exposition October 27, 2003 Presented
October 26-30, 2003 Salt Lake City, Utah High Energy Pulsed UV Light as a Sterilization Process American Association of Pharmaceutical Scientists Annual Meeting and Exposition October 27, 2003 Presented
Transfusion transmitted infections in National Haemovigilance Systems: the Greek experience
 Transfusion transmitted infections in National Haemovigilance Systems: the Greek experience Global blood product safety 10 April 2019, Rome, Italy Constantina Politis National Coordinating Haemovigilance
Transfusion transmitted infections in National Haemovigilance Systems: the Greek experience Global blood product safety 10 April 2019, Rome, Italy Constantina Politis National Coordinating Haemovigilance
The testing of Donated Blood and Components at NHSBT
 The testing of Donated Blood and Components at NHSBT NHSBT is responsible for collecting all donated blood and platelets in England, then processing into components before issuing to client hospitals.
The testing of Donated Blood and Components at NHSBT NHSBT is responsible for collecting all donated blood and platelets in England, then processing into components before issuing to client hospitals.
Center for Biologics Evaluation and Research
 Center for Biologics Evaluation and Research Current Activities Future Directions Washington, DC January 25, 2010 Karen Midthun, MD Acting Director, CBER CBER Our Mission To ensure the safety, purity,
Center for Biologics Evaluation and Research Current Activities Future Directions Washington, DC January 25, 2010 Karen Midthun, MD Acting Director, CBER CBER Our Mission To ensure the safety, purity,
A Patient s Guide to Blood Components and Products
 2014 A Patient s Guide to Blood Components and Products Contents What is a blood transfusion?... 1 Informed consent... 1 Frequently asked questions about blood transfusions... 2 What can I expect during
2014 A Patient s Guide to Blood Components and Products Contents What is a blood transfusion?... 1 Informed consent... 1 Frequently asked questions about blood transfusions... 2 What can I expect during
Transfusion Medicine Kris0ne Kra1s, M.D.
 Transfusion Medicine Kris0ne Kra1s, M.D. Transfusion Medicine Outline Blood groups Introduc0on ABO system Rh system Other systems Blood transfusion Blood products Indica0ons Tes0ng Dangers Transfusion
Transfusion Medicine Kris0ne Kra1s, M.D. Transfusion Medicine Outline Blood groups Introduc0on ABO system Rh system Other systems Blood transfusion Blood products Indica0ons Tes0ng Dangers Transfusion
