Role of Matrix Metalloproteinase 13 in Both Endochondral and Intramembranous Ossification during Skeletal Regeneration
|
|
|
- Agnes Gregory
- 5 years ago
- Views:
Transcription
1 Role of Matrix Metalloproteinase 13 in Both Endochondral and Intramembranous Ossification during Skeletal Regeneration Danielle J. Behonick 1, Zhiqing Xing 2, Shirley Lieu 2, Jenni M. Buckley 3, Jeffrey C. Lotz 3, Ralph S. Marcucio 2, Zena Werb 1, Theodore Miclau 2,Céline Colnot 2 * 1 Department of Anatomy and Biomedical Sciences Graduate Program, University of California at San Francisco, San Francisco, California, United States of America, 2 Cellular and Molecular Biology Laboratory, Department of Orthopaedic Surgery, University of California at San Francisco, San Francisco General Hospital, San Francisco, California, United States of America, 3 Orthopaedic Bioengineering Laboratory, Department of Orthopaedic Surgery, University of California at San Francisco, San Francisco, California, United States of America Extracellular matrix (ECM) remodeling is important during bone development and repair. Because matrix metalloproteinase 13 (MMP13, collagenase-3) plays a role in long bone development, we have examined its role during adult skeletal repair. In this study we find that MMP13 is expressed by hypertrophic chondrocytes and osteoblasts in the fracture callus. We demonstrate that MMP13 is required for proper resorption of hypertrophic cartilage and for normal bone remodeling during non-stabilized fracture healing, which occurs via endochondral ossification. However, no difference in callus strength was detected in the absence of MMP13. Transplant of wild-type bone marrow, which reconstitutes cells only of the hematopoietic lineage, did not rescue the endochondral repair defect, indicating that impaired healing in Mmp13 2/2 mice is intrinsic to cartilage and bone. Mmp13 2/2 mice also exhibited altered bone remodeling during healing of stabilized fractures and cortical defects via intramembranous ossification. This indicates that the bone phenotype occurs independently from the cartilage phenotype. Taken together, our findings demonstrate that MMP13 is involved in normal remodeling of bone and cartilage during adult skeletal repair, and that MMP13 may act directly in the initial stages of ECM degradation in these tissues prior to invasion of blood vessels and osteoclasts. Citation: Behonick DJ, Xing Z, Lieu S, Buckley JM, Lotz JC, et al (2007) Role of Matrix Metalloproteinase 13 in Both Endochondral and Intramembranous Ossification during Skeletal Regeneration. PLoS ONE 2(11): e1150. doi: /journal.pone INTRODUCTION Bone is noteworthy in that it does not heal through the formation of scar tissue, but rather through a regenerative process that resembles fetal skeletal development. During bone healing much of the initial developmental program is conserved, from the various cell types involved to the genetic mechanisms regulating cell differentiation [1 3]. As in development, repair of long bones by endochondral ossification begins with the production of cartilage at the injury site. The chondrocytes of the fracture callus deposit an extracellular matrix (ECM) comprised of type II collagen (Col2) and aggrecan, then differentiate into hypertrophic chondrocytes that deposit an ECM comprised of type X collagen (Col10). This ECM is then partially mineralized, resorbed and replaced by a matrix comprised predominantly of type I collagen (Col1). This process is regulated by the action of invading osteoblasts and osteoclasts. These cells, whose development and function are intimately linked, continue to remodel this regenerated tissue into mature bone until the fracture is consolidated. Other aspects of skeletal repair differ from skeletal development. For example, skeletal repair may be influenced by the mechanical environment. While non-stabilized fractures heal via endochondral ossification, stabilized fractures heal via intramembranous ossification. In this process, mesenchymal precursors recruited to the site of injury differentiate only along the osteoblastic lineage and produce both compact (cortical) and spongy (cancellous) bone in the absence of cartilage production [4,5]. Skeletal elements are rich in ECM and the remodeling of this ECM is central to both development and repair [3,5]. Matrix remodeling in both of these processes is regulated by many of the same proteases, and these enzymes may determine the rate and effectiveness of the development and repair programs [6]. The role of matrix metalloproteinases (MMPs) during bone development has been studied extensively [7 12]. MMP13 promotes both the resorption of hypertrophic cartilage from the growth plate and the remodeling of newly deposited trabecular bone during long bone development [9,10]. Moreover, other work has pointed to the requirement for MMPs used in bone development for bone repair [3,5,13,14]. These reports have led us to ask whether MMP13 also participates in skeletal repair. In the present study, we employed several models of repair by both endochondral and intramembranous ossification, including non-stabilized and stabilized fracture models as well as a cortical defect model, to elucidate the role of MMP13 during the various stages of healing. RESULTS Mmp13 is expressed in the non-stabilized fracture callus We first established which cells express Mmp13 during endochondral ossification in non-stabilized fractures. We performed in situ Academic Editor: Richard Steinhardt, University of California at Berkeley, United States of America Received August 13, 2007; Accepted October 7, 2007; Published November 7, 2007 Copyright: ß 2007 Behonick et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: This work was funded by grants from the National Institutes of Health (AG and AR to ZW, AR to TM, RM and CC, DE to CC), the Musculoskeletal Transplant Foundation and the UCSF Research Evaluation and Allocation Committee (CC). Competing Interests: The authors have declared that no competing interests exist. * To whom correspondence should be addressed. colnotc@orthosurg. ucsf.edu PLoS ONE 1 November 2007 Issue 11 e1150
2 hybridization analyses on histological sections through fracture calluses from wild-type (WT) mice using probes for Mmp13 and a number of cell type-specific marker genes (Fig. 1). At 3 days postfracture, we observed Mmp13 expression in regions of activated periosteum that also expressed Col1 (data not shown). Likewise, at 6 days post-fracture, we observed Mmp13-expressing cells within the Col1 expression domain in Osteocalcin (Oc)-negative portions of the periosteum, indicating that Mmp13 is expressed during the early stages of healing by immature osteoblasts (Fig. 1A D and data not shown). The Mmp13 expression pattern differed from that of Mmp9, indicating that Mmp13 was not upregulated in newly recruited inflammatory cells and osteoclasts (Fig. 1B D) as observed previously (Colnot et al., 2003). At 10 days post-fracture, Mmp13 expression in the cartilage overlapped with Col10 and Vascular endothelial growth factor (Vegf) expression, indicating that Mmp13 is expressed by hypertrophic and late hypertrophic chondrocytes (Fig. 1E H). At 14 days post-fracture, Mmp13 expression colocalized with Col1 and Oc expression, suggesting that by this time point Mmp13 is expressed in both immature and mature osteoblasts within the callus (Fig. 1I L). Mmp13 expression was detected in osteoblasts throughout the remodeling phase of healing and was not associated with osteoclasts (Fig. 1A D and data not shown). These results demonstrate that the expression pattern of Mmp13 in both cartilage and bone tissues during fracture healing parallels that seen during development via endochondral ossification [10,15,16] and suggest that MMP13 may play a role in both cartilaginous and bony tissues during fracture healing. Mmp13 2/2 mice accumulate cartilage during nonstabilized fracture healing but cartilage differentiation to hypertrophy is normal Cartilage begins to develop in the non-stabilized fracture callus by days 3 to 7 post-fracture (soft callus phase), peaks at day 10, is being remodeled by day 14 (hard callus phase) and is fully resorbed by day 28 (remodeling phase; [5]). To study the effect of MMP13 deficiency on this process, we created closed, non-stabilized fractures in WT and Mmp13 2/2 adult mice and examined the timing and extent of cartilage and bone formation throughout the healing process. Histological analyses indicated that cartilage is formed in Mmp13 2/2 calluses, but its remodeling and removal are delayed (Fig 2A). Quantitative analyses confirmed these observations. Histomorphometric analyses revealed that there was no significant difference in total callus volume between WT and Mmp13 2/2 samples at any time point examined (Fig. 2B), but there was a significantly greater volume of cartilage and of cartilage as a proportion of total callus volume in Mmp13 2/2 calluses at 7, 14 and 21 days post-fracture (Fig. 2B). Examination of the percentage of samples that exhibited cartilage confirmed the delay in cartilage removal in Mmp13 2/2 calluses. At day 21, all Mmp13 2/2 calluses contained cartilage while only one third of WT calluses did. At day 28, cartilage was still present in one third of Mmp13 2/2 calluses but was never observed in WT samples. To characterize the differences observed during the early stages of healing, we analyzed Col2 expression in fracture calluses at day 5 by in situ hybridization. We did not detect an alteration in the initial differentiation of chondrocytes in Mmp13 2/2 calluses as Figure 1. Mmp13 expression during non-stabilized fracture healing. (Left column) Safranin-O/Fast Green (SO) and Trichrome (TC) stained sagittal sections through the WT callus at 6 (A), 10 (E) and 14 (I) days post-fracture. Cartilage (red) develops during the soft callus phase of healing (A), is resorbed during the hard callus phase (E) and is replaced by bone (blue, I). (Middle/Right column) In situ hybridization analyses of Mmp13 expression and osteoblast/chondrocyte differentiation markers. (B D) At 6 days post-fracture, Mmp13 is expressed in the callus and overlaps with Col1- expressing cells (early osteoblasts) but not Mmp9-expressing cells (osteoclasts and inflammatory cells). (F H) At day 10, Mmp13 mrna is detected in hypertrophic chondrocytes also expressing Col10 and Vegf. (J L) At day 14, Mmp13 is expressed in mature osteoblasts co-expressing Col1 and/or Oc. Scale bars: A, E, I = 1mm; B-D, F-H, J-L-200 mm. doi: /journal.pone g001 PLoS ONE 2 November 2007 Issue 11 e1150
3 Figure 2. Mmp13 2/2 mice display an accumulation of cartilage during non-stabilized fracture healing. (A) SO staining of WT and Mmp13 2/2 fracture callus at 7, 10, 14, and 21 days post-fracture shows that cartilage persists in the Mmp13 2/2 callus from 14 through 21 days post-fracture. Scale bar = 1 mm (B) Histomorphometric measurements of total callus volume (TV), total cartilage volume (CV) and total cartilage volume as a proportion of total callus volume (CV/TV) in WT and Mmp13 2/2 mice at day 7 (WT n =6, Mmp13 2/2 n = 6), 10 (WT n =8,Mmp13 2/2 n = 6), 14 (WT n =8,Mmp13 2/2 n = 6), 21 (WT n =6,Mmp13 2/2 n = 6) and 28 (WT n =6,Mmp13 2/2 n = 6). There is a statistically significant increase in total cartilage volume in Mmp13 2/2 calluses compared with WT at day 7 (**p,0.01), 14 (*p,0.05) and 21 (*p,0.05). There is a statistically significant increase in total cartilage volume as a proportion of total callus volume in Mmp13 2/2 calluses compared with WT at day 7 (**p,0.01), 14 (*p,0.05) and 21 (*p,0.05). Wilcoxon test, bars represent means 6 S.D. At 21 days post-fracture, all Mmp13 2/2 calluses contained cartilage as compared to 1/3 of WT. At 28 days post-fracture, only Mmp13 2/2 calluses (1/3) still contained cartilage. doi: /journal.pone g002 compared to WT (Fig. 3A), indicating that the difference in cartilage volume observed by day 7 was transient. We next examined chondrocyte hypertrophy in the Mmp13 2/2 calluses by investigating Col10 expression (Fig. 3A). The Col10-expression domain was comparable between WT and Mmp13 2/2 calluses at days 10 and 14 post-fracture, suggesting that chondrocyte hypertrophy was not altered in the absence of MMP13. These studies demonstrate that the absence of MMP13 did not affect the overall amount of cartilage produced in the callus or its differentiation to hypertrophy, but did affect the removal of hypertrophic cartilage. To understand the basis for the delayed hypertrophic cartilage removal, we examined angiogenesis and ECM remodeling, two key components of endochondral ossification (Fig. 3B; [5,10,12,17]). Platelet endothelial cell adhesion marker (PECAM) immunostaining (left column) demonstrated that the observed delay in cartilage remodeling/removal was not due to delayed vascular invasion into the Mmp13 2/2 callus. Staining for Tartrateresistant acid phosphate (TRAP)-positive cells (Fig. 3B, middle column) demonstrated the delay was also not due to delayed osteoclast recruitment into the Mmp13 2/2 callus. However, these recruited blood vessels and osteoclasts did not invade the hypertrophic cartilage matrix, suggesting the inability of the cartilage matrix itself to become invaded. We next stained for an epitope of aggrecan, the major proteoglycan of the cartilage ECM [18] which is degraded during the very last stages of chondrocyte differentiation/removal [19]. Cleavage of aggrecan by MMPs, as assessed by immunostaining for the cryptic DIPEN epitope of aggrecan (Fig. 3B, right column; [20]), decreased in the Mmp13 2/2 callus indicating that processing of the cartilage ECM in the Mmp13 2/2 callus was delayed. Mmp13 2/2 mice have increased bone density during non-stabilized fracture healing Since hypertrophic cartilage remodeling is necessary for the replacement of cartilage by bone during healing [5], we asked whether delayed removal of hypertrophic cartilage affects ossification of the fracture callus in Mmp13 2/2 mice. Histomorphometric analyses revealed a significantly smaller bone volume in Mmp13 2/2 samples at day 7 post-fracture (Fig. 4B), suggesting that the increase in cartilage formation at this early time point may have compromised the initial stages of bone formation. By day 14, however, we did not detect a significantly decreased bone volume PLoS ONE 3 November 2007 Issue 11 e1150
4 Figure 3. Integral steps in endochondral ossification are unperturbed during non-stabilized fracture repair in Mmp13 2/2 mice. (A) Overlay of SO stained sections with in situ hybridization for Col2 (red) indicate no difference in the early differentiation of chondrocytes in WT and Mmp13 2/2 calluses at day 5. Overlay of SO stained sections with in situ hybridization for Col10 (yellow) shows a delay in hypertrophic chondrocyte removal in the Mmp13 2/2 callus at day 14. Scale bar = 1 mm (B) Cellular analyses of WT and Mmp13 2/2 calluses at day 14 show that blood vessels (PECAM) and osteoclasts (TRAP) are present in the Mmp13 2/2 callus while aggrecan cleavage by MMPs (DIPEN epitope) is reduced in the Mmp13 2/2 callus. Scale bar = 1 mm doi: /journal.pone g003 nor a significantly decreased bone volume as a proportion of total callus volume in Mmp13 2/2 samples compared to WT (Fig. 4B). This suggests that the increased cartilage deposition/decreased cartilage removal observed at this time point does not impair new bone deposition. At later stages of repair, during active remodeling of the new bone in WT calluses, Mmp13 2/2 calluses had significantly greater callus bone volume and a significantly greater bone volume as a proportion of total callus volume, both at days 28 and 56 post-fracture (Fig. 4B). By histological examination, we observed that spongy bone accumulated in Mmp13 2/2 calluses while the reconstitution of the bone marrow cavity was more advanced in WT calluses (Fig. 4A, day 28, middle column; day 56, right column). Histomorphometric analyses performed on the volumes of new bone in WT and Mmp13 2/2 calluses at 21 and 28 days post-fracture confirmed differences in the spongy and compact bone in Mmp13 2/2 calluses. At 28 days post-fracture, there was a significantly greater volume of spongy bone in Mmp13 2/2 calluses as compared to WT (Fig. 4C). By contrast, there was no significant difference observed in compact bone volume, regardless of timepoint or genotype. Taken together, these results suggest that the increased bone volume observed in the Mmp13 2/2 non-stabilized fracture callus resulted from an increase in the spongy bone of the callus. Figure 4. Mmp13 2/2 mice display increased bone volume during nonstabilized fracture healing. (A) Trichrome staining of WT and Mmp13 2/2 non-stabilized fracture calluses shows an increase in the amount of bone in the Mmp13 2/2 callus compared to WT at days 28 and 56 post-fracture. Scale bar = 1 mm (B) Histomorphometric measurements of total bone volume (BV) and total bone volume as a proportion of total callus volume (BV/TV) in WT and Mmp13 2/2 mice at days 7 (WT n =6,Mmp13 2/2 n =6), 10 (WT n =8, Mmp13 2/2 n = 6), 14 (WT n =8, Mmp13 2/2 n = 6), 21 (WT n =6,Mmp13 2/2 n = 6), 28 (WT n =6,Mmp13 2/2 n = 6) and 56 (WT n =5, Mmp13 2/2 n = 4) confirm this observation There is a statistically significant decrease in BV in Mmp13 2/2 calluses compared with WT at day 7 (*p,0.05), but a statistically significant increase in BV and BV/TV in Mmp13 2/2 calluses compared with WT at days 28 (**p,0.01 and p,0.05 respectively) and 56 (*p,0.05). Wilcoxon test, bars represent means 6 S.D. (C) Histomorphometric measurements indicate a statistically significant difference in total spongy bone volume in Mmp13 2/2 calluses compared with WT at day 28 (**p,0.01) but no difference is detected in total compact bone volume. (D) Micro-CT analyses show that bone mineral density is significantly increased in the Mmp13 2/2 callus compared to WT at 21 (**p,0.01) and 28 (*p,0.05) days post-fracture. Bonferroni corrected t-test, bars represent means 6 SD. doi: /journal.pone g004 Furthermore, we performed micro-ct analyses on nonstabilized fracture calluses from WT and Mmp13 2/2 mice at 21 and 28 days post-fracture. While no difference in overall callus volume was detected by micro-ct regardless of healing time point or genotype (data not shown), a significant increase in bone mineral density was observed in Mmp13 2/2 calluses as compared to WT at 21 and 28 days post-fracture (Fig. 4D). Given that histomorphometric analyses indicated no significant increase in bone volume at 21 days, these results suggest that the bone matrix PLoS ONE 4 November 2007 Issue 11 e1150
5 was not fully mineralized in Mmp13 2/2 calluses at this time point. By 28 days, the micro-ct results correlated with that of histomorphometry confirming that MMP13 is required for proper bone remodeling in the non-stabilized fracture callus. Since the increase in Mmp13 2/2 callus bone volume observed by histomorphometry persisted through 8 weeks post-fracture, these results reflected a prolonged change in the bone volume of the mutant callus. We considered the possibility that the impact of MMP13 deficiency on bone volume and bone mineral density in the non-stabilized fracture callus could affect the mechanical properties of these calluses. To test this, we performed mechanical analyses on non-stabilized fracture calluses from WT and Mmp13 2/2 mice at 14, 21 and 28 days post-fracture (Table 1). We observed no significant impact on the maximum force at failure of fracture calluses regardless of healing timepoint or genotype (Table 1). Therefore, increased bone volume within the spongy bone compartment of the callus did not change the overall mechanical properties of the tissue. WT bone marrow-derived cells do not rescue the Mmp13 2/2 non-stabilized fracture healing phenotype Osteoclasts are the major bone resorbing cells. Although we did not detect MMP13 in osteoclasts, other cells of hematopoietic origin, specifically in the monocyte/macrophage lineage, can express MMP13 [21]. To distinguish the effects of the Mmp13 mutation on osteoblasts and chondrocytes from its effects on cells derived from the hematopoietic lineage, we asked whether transplantation of WT bone marrow could rescue the Mmp13 2/2 phenotype. As previously observed in WT hosts [22], we confirmed that bone marrow was derived from donor mice (Fig. 5A left panel), while chondrocytes (Fig. 5A, middle panel) and osteoblasts/osteocytes in the callus (Fig. 5A, right panel) were host derived. Histology (Fig. 5B) and histomorphometric analyses (Fig. 5C) showed that there was no difference in the proportion of cartilage in the callus at 14 days post-fracture, or in the proportion of bone in the callus at 28 days post-fracture between Mmp13 2/2 mice that received WT (WTRMmp13 2/2 ) and Mmp13 2/2 (Mmp13 2/2 RMmp13 2/2 ) bone marrow. These results demonstrate that MMP13-competent hematopoietically derived cells, including osteoclasts are insufficient to restore the timely resorption of cartilage and bone in Mmp13 2/2 fracture callus. This suggests that there is an intrinsic defect in chondrocytes, osteoblasts and/or their matrices as the source of the Mmp13 2/2 healing phenotype. Mmp13 is required for healing by intramembranous ossification To differentiate the consequences of the Mmp13 2/2 mutation on cartilage and bone during repair, we examined healing via intramembranous ossification in fractures that were stabilized with a rigid external fixator [4,5] and in a cortical defect model [23,24]. WT and Mmp13 2/2 mice that received stabilized fractures were examined at 10 and 28 days post-fracture (Fig. 6A). Unlike the Mmp9 2/2 mice, which display aberrant cartilage formation at 10 days post-fracture (Fig. 6A bottom left panel; [5]), Mmp13 2/2 stabilized fracture calluses (Fig. 6A, middle left panel) did not display cartilage and were comparable to WT (Fig. 6A, top left panel). At 28 days post-fracture, histological analyses suggested that Mmp13 2/2 stabilized fracture calluses (Fig. 6A, middle right panel), were larger and contained more bone as compared to WT. However, since differences in callus sizes and bone content may result from variability in the alignment of bone ends at the fracture site, we turned to another model of intramembranous bone healing for quantification. During healing of cortical defects in long bones, no cartilage is formed [23,24]. Healing produces new compact bone to repair the damaged compact bone as well as spongy bone in the marrow space underlying the cortical defect. This spongy bone is eventually remodeled and removed [24,25]. Cortical defects were produced in WT and Mmp13 2/2 mice and assessed at 21 or 28 days by histology and histomorphometry. SO staining confirmed that no cartilage was present in the defects at the time points examined regardless of genotype (data not shown). TC staining suggested that there was an increased amount of spongy bone in Mmp13 2/2 mice as compared to WT at 21 and 28 days post-injury (Fig. 6B). Histomorphometric analyses of these samples confirmed that while there was no measurable difference in the ratio of compact bone volume as a proportion of total defect volume in these defects, there was a significant increase in the ratio of spongy bone volume as a proportion of defect volume in Mmp13 2/2 mice as compared to WT at 21 and 28 days postsurgery (Fig. 6B). These results indicate that the defects observed during late stages of cortical defect healing are similar to the bone remodeling defects observed in non-stabilized fractures. Since cortical defect healing occurs without cartilage intermediates, this model allowed us to produce a bone phenotype in the absence of cartilage perturbations. We conclude that the Mmp13 2/2 repair defect occurring in bone can be uncoupled from the defect occurring in cartilage; therefore the Mmp13 2/2 mutation overall affected cartilage and bone remodeling independently of one another during fracture repair. DISCUSSION MMP13 is required for cartilage resorption We demonstrate here that the absence of MMP13 does not affect the overall amount of cartilage produced in the callus during nonstabilized fracture healing, but rather affects the removal of hypertrophic cartilage from the callus. This is reminiscent of skeletal development in the absence of MMP13, where an accumulation of hypertrophic cartilage results from its delayed... Table 1. Mechanical properties of intact tibiae and unstabilized fracture calluses.... Healing time (days) Intact Moment (N*mm) WT WT WT WT Mmp13 2/ Mmp13 2/ Mmp13 2/ Mmp13 2/ Results are given as mean 6 SD of sample group (intact WT n = 10, Mmp13 2/2 n = 10; 14 days post fracture WT n =7, Mmp13 2/2 n = 5; 21 days WT n =2, Mmp13 2/2 n = 7; 28 days WT n =6, Mmp13 2/2 n =2). doi: /journal.pone t001 PLoS ONE 5 November 2007 Issue 11 e1150
6 Figure 5. Transplant of WT bone marrow does not rescue the Mmp13 2/2 non-stabilized fracture healing phenotype. (A) Immunostaining for GFP on callus tissues from Mmp13 2/2 mice transplanted with bone marrow from b-actin GFP mice (GFPRMmp13 2/2 mice). (Left panel) Bone marrow cells (bm) are positive for GFP (black staining) showing they are donor-derived but the adjacent cortex (c) is negative. (Middle panel) Chondrocytes at day 14 and (Right panel) osteocytes embedded in the new bone (arrows) at day 28 do not stain for GFP, showing they are host-derived. (B, Left column) SO and (Right column) Masson s Trichrome staining of non-stabilized fracture calluses from Mmp13 2/2 mice transplanted with WT bone marrow (WT R Mmp13 2/2 ) and Mmp13 2/2 mice transplanted with Mmp13 2/2 bone marrow (Mmp13 2/2 R Mmp13 2/2 ) show no difference in the amount of cartilage volume at 14 days post-fracture (WT R Mmp13 2/2 n =6, Mmp13 2/2 R Mmp13 2/2 n = 5) and no difference in the amount bone at day 28 (WT R Mmp13 2/2 n =7, Mmp13 2/2 R Mmp13 2/2 n = 4). (C) Histomorphometric analyses of total cartilage volume as a proportion of total callus volume (CV/TV; day 14) and total bone volume as a proportion of total callus volume (BV/TV; day 28) demonstrate no significant difference between WT R Mmp13 2/2 and Mmp13 2/2 R Mmp13 2/2 animals, suggesting that bone marrow transplant does not rescue the Mmp13 2/ non-stabilized fracture healing phenotype. Bonferroni corrected t-test, bars represent means 6 SD. Scale bars: (A, left and middle) = 50 mm, (A, right) = 25 mm, B = 1 mm. doi: /journal.pone g005 removal from the endochondral growth plate [9,10]. Our study has provided evidence for the importance of MMP13 activity during skeletal repair, which is both complementary to and distinct from its role during development. Previous studies have also revealed a mechanistic link between fetal skeletal development and adult skeletal repair [3,5]. The delayed cartilage removal in Mmp13 2/2 mice resembles the phenotype that was observed in Mmp9 2/2 mice during develop- Figure 6. MMP13 is required for normal healing by intramembranous ossification. (A, Left column) SO stain of stabilized fracture calluses at day 10 post-fracture show that unlike Mmp9 2/2 mice, no cartilage is formed during stabilized fracture healing in Mmp13 2/2 mice (n =14) comparedto WT mice (n =3).(A, Right column) At day 28, stabilized fracture calluses in Mmp13 2/2 mice (n = 12) appear to have increased bone volume as compared to WT (n = 14) by histology. (B) Masson s Trichrome staining of cortical defect samples at 21 and 28 days post-surgery suggests an increased amount of bone in Mmp13 2/2 mice. Labels designate compact (Co) and spongy (S) regions of defect. Histomorphometric analyses (within the boxed area) of WT (d21 n =5,d28n =6)andMmp13 2/2 (d21 n =6,d28 n = 6) cortical defect samples confirm that there is an increase in spongy bone volume (SV/DV) but not compact volume (CoV/DV) in the defect area measured at day 21 and 28 (*p,0.05) in Mmp13 2/2 as compared to WT. Bonferroni corrected t-test, bars represent means 6 SD. Scale bars: A = 1 mm, B = 500 mm. doi: /journal.pone g006 PLoS ONE 6 November 2007 Issue 11 e1150
7 Figure 7. A model for MMP13 action in the cartilage and bone compartments of the non-stabilized fracture callus. MMP13 secreted from hypertrophic chondrocytes (HC) and osteoblasts (OB) acts upon the ECM to produce a pre-processed ECM in both compartments. This pre-processed ECM is then invaded by blood vessels and further modified by osteoclasts (OC) secreting MMP9, leading to the production of a processed ECM. This processed ECM then promotes further steps of callus maturation including hypertrophic chondrocyte apoptosis, replacement of cartilage by bone and new bone remodeling. doi: /journal.pone g007 ment and repair of long bones [5,8]. While MMP9 is strongly expressed by osteoclasts in the fracture callus, we observed that MMP13 was confined to chondrocytes and osteoblasts, suggesting that the Mmp13 2/2 and Mmp9 2/2 phenotypes may differ at the cellular level. Further analyses of these phenotypes confirmed this assumption. While both angiogenesis and osteoclast recruitment, two events essential for proper and timely resorption of endochondral cartilage during healing are delayed in Mmp9 2/2 calluses [5,8,15,26,27], in contrast, there was no disruption in the recruitment of mature osteoclasts or blood vessels in Mmp13 2/2 fracture calluses. However, consistent with our observations, providing a source of WT osteoclasts by bone marrow transplant did not rescue the Mmp13 2/2 repair phenotype. This is distinct from the Mmp9 2/2 phenotype where the delay in cartilage resorption during fetal bone development was rescued by transplant of WT bone marrow [8]. In addition, the Mmp9 2/2 fracture phenotype could be rescued by exogenous VEGF, which stimulated the invasion of the callus by blood vessels and osteoclasts [5]. These results suggest a role for MMP13 prior to vascular invasion and osteoclast recruitment in the process of hypertrophic cartilage remodeling (Fig. 7). The observed decrease in aggrecan cleavage in the absence of MMP13 activity suggests impaired degradation of the cartilage ECM, which slowly resolves over time, in Mmp13 2/2 mice. Thus, MMP13 produced by hypertrophic chondrocytes appears to be directly involved in the initiation of hypertrophic cartilage degradation, independent from MMP9 and the recruitment of matrix resorbing cells and blood vessels. MMP13 is required in bone remodeling We found other differences between Mmp13 2/2 and Mmp9 2/2 phenotypes when we analyzed the production and remodeling of new bone in the fracture callus. In Mmp9 2/2 mice, the delayed removal of hypertrophic cartilage correlated with a delay in ossification. On the contrary, Mmp13 2/2 mice did not exhibit a delay in the deposition of new bone as a consequence of the cartilage phenotype. Instead, we observed an increase in bone volume at later stages of healing. This increase was restricted to the areas of spongy bone, much like those observed in Mmp13 2/2 bones during development [10]. Since spongy bone is lower in density and strength than compact bone, this result may explain why no significant difference was detected when we compared the strength of WT and Mmp13 2/2 calluses at late stages of healing. Examination of stabilized fractures and cortical defects also revealed a role for MMP13 in the timely resorption of the transient compartments of spongy bone during healing via intramembranous ossification. Unlike development, where MMP13 activity is required for the formation of endochondral but not intramembranous bone, these observations suggest that its proteolytic activity is required during repair regardless of the type of ossification involved. These results support the idea that the bone defect observed during adult skeletal repair in the absence of MMP13 is caused by a defect intrinsic to osteoblasts or the matrix they produce. Furthermore, WT osteoclasts did not accelerate the resorption of spongy bone in the Mmp13 2/2 callus. Similar to its role in hypertrophic cartilage, MMP13 present in new bone matrix may be required for the initial degradation of ECM components allowing the timely remodeling of the bone callus (Fig. 7). Cartilage-bone interaction in the Mmp13 2/2 fracture callus That bone remodeling was delayed in both intramembranous and endochondral ossification during bone repair demonstrates that in the absence of MMP13 the cartilage phenotype manifests independently of the bone phenotype. These results are in accordance with the observations reported for skeletal development in the absence of MMP13 by Stickens et al. (2004). In addition, the observed delay in cartilage resorption did not affect the deposition of bone in the Mmp13 2/2 callus at day 14 postfracture. While bone and cartilage have been shown to provide regulatory feedback to each other [28], these results indicate that proper remodeling of the ECM depends on unique signals or properties intrinsic to the bone and cartilage. Regulatory interactions between bone and cartilage also take place during the early stages of fracture healing [5]. During the early stages of non-stabilized fracture healing in the absence of MMP13, we observed a delay in initial bone deposition correlated with an increase in cartilage formation. This might indicate a transient inhibition of osteogenesis due to increased chondrogenesis or a decreased ability of MMP13-deficient mesenchymal precursor cells to differentiate into fully mature osteoblasts [29]. This early imbalance was not observed during stabilized fracture healing in either WT or Mmp13 2/2 mice, which healed via intramembranous ossification. This stands in contrast with Mmp9 2/2 mice that heal stabilized fractures via an aberrant endochondral process [5]. A role for MMP13 in osteoclasts? The independent manifestation of the cartilage and bone phenotypes may be due to the fact that osteoclasts are not affected in Mmp13 2/2 mice. Several studies point to MMPs, possibly even MMP13, as potentially important mediators of osteoclast recruitment during development and repair [30 33] and a number of factors that promote bone resorption/remodeling are able to affect Mmp13 gene expression [34 38]. Our results indicate that Mmp13 is not co-expressed with Mmp9 in osteoclasts. Furthermore, our bone marrow transplantation studies indicate that WT osteoclasts were not able to support normal ECM remodeling in the Mmp13 2/2 environment, suggesting that they did not provide PLoS ONE 7 November 2007 Issue 11 e1150
8 a source of MMP13. This result points again to a defect that is intrinsic to the ECM. MMP13 activity may be required prior to the onset of osteoclastic and MMP9 activity to prepare the bone matrix for resorption (Fig. 7). Indeed, prior work has suggested that MMP13 does participate in the resorption of the organic bone matrix, but that this is through the action of mesenchymal stem cell-derived cells, not osteoclasts [39]. Consequently, WT (and therefore MMP13-competent) osteoclasts are unable to perform their normal function due to a deficiency in this preparatory step in the Mmp13 2/2 ECM. Implications for normal skeletal repair In humans, although the mode of healing may vary depending on the site of injury, extent of trauma, and fixation, most fractures heal through endochondral ossification. Therefore in the majority of clinical cases, remodeling of both cartilage and bone is critical for bone bridging and full recovery of mechanical integrity. While most fractures heal spontaneously, 5 to 10% fail to heal in a timely manner [40]. Risk factors such as age, injury type and socioeconomic conditions have been shown to impact the outcome of healing, yet little is known about the genetic predispositions to impaired healing. Although mutations in MMP genes have been linked to skeletal defects in humans [12,41], none have been associated with delayed healing. Our results show that MMP13 and other MMPs such as MMP9 play a role in all stages of fracture healing from the early deposition of cartilage and bone to the late stages of callus remodeling. Perturbations in any of these processes may compromise healing. Although Mmp13 2/2 mice have phenotypic manifestations similar to Mmp9 2/2 mutants, such as late onset of hypertrophic cartilage removal, we show that there are intrinsic differences in the way these two proteases act in fracture repair and that treatment strategies to overcome these defects differ. These observations point to the need for a better understanding of the underlying causes of delayed healing in humans in order to plan efficient therapies. From this perspective, the detailed analyses of Mmp9 2/2 and Mmp13 2/2 mutant mice shed light into the normal process of bone healing and potential approaches to treat impaired healing. MATERIALS AND METHODS Non-stabilized and stabilized fractures Mmp13 2/2 mice (3- to 6-month-old males; grams (g)) and their WT littermates were anesthetized with an intraperitoneal injection of 50 mg/ml Ketamine/0.5 mg/ml Medetomidine (0.03 ml/mouse). Closed, standardized non-stabilized fractures were produced as previously described [5]. For stabilized fractures, an external fixator was placed as previously described [4]. Mice were sacrificed by cervical dislocation following an intraperitoneal injection of 2% Avertin (0.5 ml/mouse). Non-stabilized fractures were collected at 5, 10, 14, 21, 28, and 56 days post-fracture. Stabilized fractures were collected at 10 and 28 days post-fracture. All protocols were approved by the Institutional Animal Care and Use Committee at UCSF. Bone marrow transplantation 10-week-old male Mmp13 2/2 mice were lethally irradiated with two 6 Gy doses of c-irradiation 3 4 hours apart. Bone marrow cells from WT, Mmp13 2/2 and b-actin Green Fluorescent Protein (GFP) mice were transplanted into irradiated Mmp13 2/2 mice from the same FVB/N background as previously described (Colnot et al., 2006). Following a recovery period (Colnot et al. 2006), nonstabilized fractures were produced in chimeric mice as described above. Fracture tissues were collected at 14 and 28 days postfracture and processed for histological analyses. Cortical defects Monocortical defects (1mm in diameter) were produced on the anterior-proximal tibia as previously described [23,24]. Samples were collected at 21 and 28 days post-surgery and processed for paraffin embedding. Longitudinal sections parallel to the plane of the defect were collected and processed for histological analyses. Histology and immunohistochemistry Callus tissues were fixed overnight at 4uC in 4% paraformaldehyde, decalcified at 4uC in 19% EDTA (ph 7.4) for days, then dehydrated in a graded ethanol series and embedded in paraffin. Sections (10 mm thick) were stained with Safranin-O/ Fast Green (SO) to detect cartilage formation as described [4]. A modified Milligan s Trichrome (TC) staining using Analine Blue was performed to analyze bone formation in the fracture callus. Tartrate-resistant acid phosphatase (TRAP) staining was performed using a leukocyte acid phosphatase kit (Sigma, St. Louis, MO) as previously described [16]. Immunohistochemistry for platelet endothelial cell adhesion molecule-1 (PECAM) was done as previously described [5,8]. Immunohistochemistry for the DIPEN epitope was performed as described previously [10]. Calluses collected from Mmp13 2/2 mice transplanted with bone marrow from b-actin GFP mice were fixed and decalcified as mentioned above, then cryo-embedded in OCT. Sections (8 mm) were cut on a cryostat (Leica). For GFP immunostaining, cryosections were treated with 0.3%H 2 O 2 in methanol, digested with ficin (Zymed), and blocked with 5% milk in PBS and 5% normal goat serum in PBS. Antibody staining was done using rabbit anti-gfp antibody (Abcam) followed by goat anti-rabbit secondary antibody conjugated to horseradish peroxidase. Slides were developed with diaminobenzidine and counterstained with 0.1% fast green. In situ hybridization In situ hybridization was performed using 35 S-labeled probes from mouse cdnas for Mmp13, Col1 a1 chain (Col1a1 Mouse Genome Informatics), Mmp9, Col10 (Col10a1 Mouse Genome Informatics), Vegf (Vegfa Mouse Genome Informatics), Oc (Tcirg1 Mouse Genome Informatics) and Col2 (Col2a1 Mouse Genome Informatics) as previously described [16]. Emulsion coating and image analyses were performed as described previously [2,42]. Histomorphometric measurements Histomorphometry on fracture samples was performed as previously described [5,43]. To determine the amount of cartilage within each callus, every thirtieth section (300 mm) was stained with SO. Adobe Photohsop was used to capture images from a Leica DM 5000 B light microscope (Leica Microsystems GmbH, Wetzler, Germany) that was equipped with a camera (Diagnostic Instruments, Inc., Sterling Heights, MI). To determine the amount of bone within each callus, adjacent sections were stained with TC and photographed. The area of the callus, cartilage and bone (including compact and spongy bone [44] was determined using Adobe PhotoShop. To determine the amount of bone within cortical defects, 2 central sections within the defect were selected and stained with TC. In Adobe PhotoShop, a standard box (as shown in Figure 6) was used to select an area in the center of the defect, including the damaged cortical region and the underlying bone marrow space. The total area of bone within the defect, as well as the areas of PLoS ONE 8 November 2007 Issue 11 e1150
9 compact and spongy bone (Steele, 1988) within each standard box were quantified and the ratios of compact repair/defect volume and spongy repair/defect volume were calculated. Mechanical testing Mechanical testing was performed on intact tibiae from 12-weekold mice and tibiae from mice that had received non-stabilized fractures collected at 14, 21 and 28 days post-fracture. The proximal and distal end of each tibia was potted in a cryovial cap (Corning Incorporated Life Sciences, Lowell, MA) in polymethylmethacrylate (PMMA) bone cement at room temperature. Potted samples were stored overnight at 4uC until testing [45]. Prior to testing, samples were warmed to room temperature for at least 30 minutes; any intact fibulae were removed and mineral-free PBS was applied to the calluses after mounting into the testing apparatus. Tests were performed at room temperature. Data was collected using LabView4 (National Instruments Corporation, Austin, TX). Each potted mouse tibia specimen was placed horizontally into a uniaxial test frame to prevent gravitational loading of the specimen by the PMMA endcaps [46]. The distal end of the specimen was rigidly held to the test frame while the proximal end was attached to a single-cable pulley system. Movement of the test frame actuator caused tension in the cable, which was positioned around a loading ring so as to generate a pure moment, or couple, loading condition. A uniaxial load cell (Sensotec Model 129, 25 lb capacity, Honeywell International, Inc., Columbus, OH) was used to monitor cable tension, and the moment applied to the specimen was calculated as the product of the cable tension and the diameter of the loading ring (N*mm). Moment and crosshead displacement were monitored, and the ultimate moment over the entire destructive test cycle was recorded as the failure strength of the specimen. Statistical analyses For non-stabilized fracture studies, Wilcoxon rank sum tests were used at each timepoint to examine whether differences between WT and Mmp13 2/2 samples in cartilage, callus, and bone volumes were statistically significant (a,0.05). Bonferroni adjustments were performed. For mechanical testing of non-stabilized fracture calluses, force data were analyzed using GraphPad Prism 4 by one-way analysis of variance (ANOVA). For bone marrow transplant and cortical defect studies, histomorphometry data were analyzed using ANOVA followed by Bonferroni corrected t-tests for data sets where ANOVA, p,0.05. ACKNOWLEDGMENTS The authors thank Andrew Burghardt for technical assistance with the micro-ct study, Kirk Pedersen for assistance with mechanical testing, and Pia Ghosh and Kathy Tsui for help with histological and histomorphometric analyses. The authors would also like to thank Jill Helms for her support with in situ hybridization data and Chuanyong Lu for helpful comments on the manuscript. Author Contributions Conceived and designed the experiments: CC. Performed the experiments: CC ZX SL DB. Analyzed the data: CC DB. Contributed reagents/ materials/analysis tools: JB JL ZW. Wrote the paper: CC DB. Other: Critically revised the manuscript: ZX JB RM ZW TM. REFERENCES 1. Vortkamp A, Pathi S, Peretti GM, Caruso EM, Zaleske DJ, et al. (1998) Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech Dev 71: Ferguson C, Alpern E, Miclau T, Helms JA (1999) Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev 87: Uusitalo M, Mikkila H, Karma A, Kivela T (2000) Search for autoantibodies against the HNK-1 carbohydrate epitope in the human eye in intermediate uveitis. Acta Ophthalmol Scand 78: Thompson Z, Miclau T, Hu D, Helms JA (2002) A model for intramembranous ossification during fracture healing. J Orthop Res 20: Colnot C, Thompson Z, Miclau T, Werb Z, Helms JA (2003) Altered fracture repair in the absence of MMP9. Development 130: Henle P, Zimmermann G, Weiss S (2005) Matrix metalloproteinases and failed fracture healing. Bone 37: Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, et al. (1999) MT1- MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99: Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, et al. (1998) MMP-9/ gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93: Inada M, Wang Y, Byrne MH, Rahman MU, Miyaura C, et al. (2004) Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci U S A 101: Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, et al. (2004) Altered endochondral bone development in matrix metalloproteinase 13- deficient mice. Development 131: Ortega N, Behonick DJ, Werb Z (2004) Matrix remodeling during endochondral ossification. Trends Cell Biol 14: Egeblad M, Shen HC, Behonick DJ, Wilmes L, Eichten A, et al. (2007) Type I collagen is a genetic modifier of matrix metalloproteinase 2 in murine skeletal development. Dev Dyn 236: Yamagiwa H, Tokunaga K, Hayami T, Hatano H, Uchida M, et al. (1999) Expression of metalloproteinase-13 (Collagenase-3) is induced during fracture healing in mice. Bone 25: Lehmann W, Edgar CM, Wang K, Cho TJ, Barnes GL, et al. (2005) Tumor necrosis factor alpha (TNF-alpha) coordinately regulates the expression of specific matrix metalloproteinases (MMPS) and angiogenic factors during fracture healing. Bone 36: Engsig MT, Chen QJ, Vu TH, Pedersen AC, Therkidsen B, et al. (2000) Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol 151: Colnot CI, Helms JA (2001) A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech Dev 100: Ortega N, Behonick DJ, Colnot C, Cooper DN, Werb Z (2005) Galectin-3 is a downstream regulator of matrix metalloproteinase-9 function during endochondral bone formation. Mol Biol Cell 16: Doege KJ (1999) Aggrecan. In: Kreis VR, ed (1999) Guidebook to the Extracellular Matrix, Anchor and Adhesion Proteins. New York: Oxford University Press. pp Lee ER, Murphy G, El-Alfy M, Davoli MA, Lamplugh L, et al. (1999) Active gelatinase B is identified by histozymography in the cartilage resorption sites of developing long bones. Dev Dyn 215: Singer II, Kawka DW, Bayne EK, Donatelli SA, Weidner JR, et al. (1995) VDIPEN, a metalloproteinase-generated neoepitope, is induced and immunolocalized in articular cartilage during inflammatory arthritis. J Clin Invest 95: Fallowfield JA, Mizuno M, Kendall TJ, Constandinou CM, Benyon RC, et al. (2007) Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol 178: Colnot C, Huang S, Helms J (2006) Analyzing the cellular contribution of bone marrow to fracture healing using bone marrow transplantation in mice. Biochem Biophys Res Commun 350: Lu C, Huang S, Miclau T, Helms JA, Colnot C (2004) Mepe is expressed during skeletal development and regeneration. Histochem Cell Biol 121: Colnot C, Romero DM, Huang S, Helms JA (2005) Mechanisms of action of demineralized bone matrix in the repair of cortical bone defects. Clin Orthop Relat Res. pp Roberts WE (1988) Bone tissue interface. J Dent Educ 52: Gerber HP, Ferrara N (2000) Angiogenesis and bone growth. Trends Cardiovasc Med 10: Weizmann S, Tong A, Reich A, Genina O, Yayon A, et al. (2005) FGF upregulates osteopontin in epiphyseal growth plate chondrocytes: implications for endochondral ossification. Matrix Biol 24: Goldring MB, Goldring SR (1990) Skeletal tissue response to cytokines. Clin Orthop Relat Res. pp PLoS ONE 9 November 2007 Issue 11 e1150
Effects of Delayed Stabilization on Fracture Healing
 Effects of Delayed Stabilization on Fracture Healing Theodore Miclau, 1 Chuanyong Lu, 1 Zachary Thompson, 1 Paul Choi, 1 Christian Puttlitz, 2 Ralph Marcucio, 1 Jill A. Helms 3 1 Department of Orthopaedic
Effects of Delayed Stabilization on Fracture Healing Theodore Miclau, 1 Chuanyong Lu, 1 Zachary Thompson, 1 Paul Choi, 1 Christian Puttlitz, 2 Ralph Marcucio, 1 Jill A. Helms 3 1 Department of Orthopaedic
Supplemental Data. Wnt/β-Catenin Signaling in Mesenchymal Progenitors. Controls Osteoblast and Chondrocyte
 Supplemental Data Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis Timothy F. Day, Xizhi Guo, Lisa Garrett-Beal, and
Supplemental Data Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis Timothy F. Day, Xizhi Guo, Lisa Garrett-Beal, and
Impaired remodeling phase of fracture repair in the absence of matrix metalloproteinase-2
 Disease Models & Mechanisms 4, 203-211 (2011) doi:10.1242/dmm.006304 Impaired remodeling phase of fracture repair in the absence of matrix metalloproteinase-2 Shirley Lieu 1, Erik Hansen 1, Russell Dedini
Disease Models & Mechanisms 4, 203-211 (2011) doi:10.1242/dmm.006304 Impaired remodeling phase of fracture repair in the absence of matrix metalloproteinase-2 Shirley Lieu 1, Erik Hansen 1, Russell Dedini
BONE TISSUE. Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology
 BONE TISSUE Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology BONE FUNCTION Support Protection (protect internal organs) Movement (provide leverage system for skeletal muscles, tendons, ligaments
BONE TISSUE Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology BONE FUNCTION Support Protection (protect internal organs) Movement (provide leverage system for skeletal muscles, tendons, ligaments
For more information about how to cite these materials visit
 Author(s): University of Michigan Medical School, Department of Cell and Developmental Biology License: Unless otherwise noted, the content of this course material is licensed under a Creative Commons
Author(s): University of Michigan Medical School, Department of Cell and Developmental Biology License: Unless otherwise noted, the content of this course material is licensed under a Creative Commons
Skeletal Tissues. Skeletal tissues. Frame; muscles, organs and CT attach. Brain, spinal cord, thoracic organs; heart and lungs.
 Skeletal Tissues Functions 1) support 2) protection 3) movement Skeletal tissues Frame; muscles, organs and CT attach. Brain, spinal cord, thoracic organs; heart and lungs. Aids muscle contraction; generate
Skeletal Tissues Functions 1) support 2) protection 3) movement Skeletal tissues Frame; muscles, organs and CT attach. Brain, spinal cord, thoracic organs; heart and lungs. Aids muscle contraction; generate
SKELETAL TISSUES CHAPTER 7 INTRODUCTION TO THE SKELETAL SYSTEM TYPES OF BONES
 SKELETAL TISSUES CHAPTER 7 By John McGill Supplement Outlines: Beth Wyatt Original PowerPoint: Jack Bagwell INTRODUCTION TO THE SKELETAL SYSTEM STRUCTURE Organs: Bones Related Tissues: Cartilage and Ligaments
SKELETAL TISSUES CHAPTER 7 By John McGill Supplement Outlines: Beth Wyatt Original PowerPoint: Jack Bagwell INTRODUCTION TO THE SKELETAL SYSTEM STRUCTURE Organs: Bones Related Tissues: Cartilage and Ligaments
Derived copy of Bone *
 OpenStax-CNX module: m57739 1 Derived copy of Bone * Shannon McDermott Based on Bone by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By
OpenStax-CNX module: m57739 1 Derived copy of Bone * Shannon McDermott Based on Bone by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By
Altered fracture repair in the absence of MMP9
 Development 130, 4123-4133 2003 The Company of Biologists Ltd doi:10.1242/dev.00559 4123 Altered fracture repair in the absence of MMP9 Céline Colnot 1, Zachary Thompson 1, Theodore Miclau 1, Zena Werb
Development 130, 4123-4133 2003 The Company of Biologists Ltd doi:10.1242/dev.00559 4123 Altered fracture repair in the absence of MMP9 Céline Colnot 1, Zachary Thompson 1, Theodore Miclau 1, Zena Werb
Bone. Development. Tim Arnett. University College London. Department of Anatomy and Developmental Biology
 Bone Development Tim Arnett Department of Anatomy and Developmental Biology University College London Bone development Outline Bone composition matrix + mineral Bone formation - intramembranous & endochondral
Bone Development Tim Arnett Department of Anatomy and Developmental Biology University College London Bone development Outline Bone composition matrix + mineral Bone formation - intramembranous & endochondral
Re-growing the Skeleton: Approaches in Tissue Engineering and Regenerative Medicine
 Re-growing the Skeleton: Approaches in Tissue Engineering and Regenerative Medicine How we fix things now Total Knee Replacements Fracture Plates Fusing Joints Defining Regenerative Medicine restore form
Re-growing the Skeleton: Approaches in Tissue Engineering and Regenerative Medicine How we fix things now Total Knee Replacements Fracture Plates Fusing Joints Defining Regenerative Medicine restore form
Peggers Super Summaries Basic Sciences Bone
 Bone Overview & Turnover BONES Function o Support o Protection o Assisting movement o Storage of minerals o Production of red blood cells from marrow Types o Cancellous o Compact with Haversian systems
Bone Overview & Turnover BONES Function o Support o Protection o Assisting movement o Storage of minerals o Production of red blood cells from marrow Types o Cancellous o Compact with Haversian systems
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
Fig Articular cartilage. Epiphysis. Red bone marrow Epiphyseal line. Marrow cavity. Yellow bone marrow. Periosteum. Nutrient foramen Diaphysis
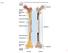 Fig. 7.1 Articular cartilage Epiphysis Red bone marrow Epiphyseal line Marrow cavity Yellow bone marrow Nutrient foramen Diaphysis Site of endosteum Compact bone Spongy bone Epiphyseal line Epiphysis Articular
Fig. 7.1 Articular cartilage Epiphysis Red bone marrow Epiphyseal line Marrow cavity Yellow bone marrow Nutrient foramen Diaphysis Site of endosteum Compact bone Spongy bone Epiphyseal line Epiphysis Articular
Chapter 6: Skeletal System: Bones and Bone Tissue
 Chapter 6: Skeletal System: Bones and Bone Tissue I. Functions A. List and describe the five major functions of the skeletal system: 1. 2. 3.. 4. 5.. II. Cartilage A. What do chondroblasts do? B. When
Chapter 6: Skeletal System: Bones and Bone Tissue I. Functions A. List and describe the five major functions of the skeletal system: 1. 2. 3.. 4. 5.. II. Cartilage A. What do chondroblasts do? B. When
CHAPTER 6 LECTURE OUTLINE
 CHAPTER 6 LECTURE OUTLINE I. INTRODUCTION A. Bone is made up of several different tissues working together: bone, cartilage, dense connective tissue, epithelium, various blood forming tissues, adipose
CHAPTER 6 LECTURE OUTLINE I. INTRODUCTION A. Bone is made up of several different tissues working together: bone, cartilage, dense connective tissue, epithelium, various blood forming tissues, adipose
Regulation of the skeletal mass through the life span
 Regulation of the skeletal mass through the life span Functions of the skeletal system Mechanical protection skull Movement leverage for muscles Mineral metabolism calcium store Erythropoiesis red blood
Regulation of the skeletal mass through the life span Functions of the skeletal system Mechanical protection skull Movement leverage for muscles Mineral metabolism calcium store Erythropoiesis red blood
Distinct Roles Of CCN1 And CCN2 In Limb Development
 Distinct Roles Of CCN1 And CCN2 In Limb Development Jie Jiang, PhD 1, Jessica Ong, BS 1, Faith Hall-Glenn, PhD 2, Teni Anbarchian, BS 1, Karen M. Lyons, PhD 1. 1 University of California, Los Angeles,
Distinct Roles Of CCN1 And CCN2 In Limb Development Jie Jiang, PhD 1, Jessica Ong, BS 1, Faith Hall-Glenn, PhD 2, Teni Anbarchian, BS 1, Karen M. Lyons, PhD 1. 1 University of California, Los Angeles,
Ossification = Osteogenesis
 Ossification = Osteogenesis Ossification = Osteogenesis Parts of the fetal skeleton form during the first few weeks after conception By the end of the 8 th week, the skeletal pattern is formed : cartilage
Ossification = Osteogenesis Ossification = Osteogenesis Parts of the fetal skeleton form during the first few weeks after conception By the end of the 8 th week, the skeletal pattern is formed : cartilage
Functions of the Skeletal System. Chapter 6: Osseous Tissue and Bone Structure. Classification of Bones. Bone Shapes
 Chapter 6: Osseous Tissue and Bone Structure Functions of the Skeletal System 1. Support 2. Storage of minerals (calcium) 3. Storage of lipids (yellow marrow) 4. Blood cell production (red marrow) 5. Protection
Chapter 6: Osseous Tissue and Bone Structure Functions of the Skeletal System 1. Support 2. Storage of minerals (calcium) 3. Storage of lipids (yellow marrow) 4. Blood cell production (red marrow) 5. Protection
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 6 The Skeletal System: Bone Tissue Introduction The skeletal system has 6 important functions: Provides support Protects the internal organs (brain,
Principles of Anatomy and Physiology 14 th Edition CHAPTER 6 The Skeletal System: Bone Tissue Introduction The skeletal system has 6 important functions: Provides support Protects the internal organs (brain,
The Skeletal System:Bone Tissue
 The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose, and dense
The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose, and dense
Pathophysiology of fracture healing
 Pathophysiology of fracture healing Bone anatomy and biomechanics Fracture patterns Bone healing and blood supply Influence of implants 1 What is the structure of bone? 2 Bone structure Four levels: Chemical
Pathophysiology of fracture healing Bone anatomy and biomechanics Fracture patterns Bone healing and blood supply Influence of implants 1 What is the structure of bone? 2 Bone structure Four levels: Chemical
Chapter 7. Skeletal System
 Chapter 7 Skeletal System 1 Introduction: A. Bones are very active, living tissues B. Each bone is made up of several types of tissues and so is an organ. C. Bone functions include: muscle attachment,
Chapter 7 Skeletal System 1 Introduction: A. Bones are very active, living tissues B. Each bone is made up of several types of tissues and so is an organ. C. Bone functions include: muscle attachment,
OSSEOUS TISSUE & BONE STRUCTURE PART I: OVERVIEW & COMPONENTS
 OSSEOUS TISSUE & BONE STRUCTURE PART I: OVERVIEW & COMPONENTS The Skeletal System Skeletal system includes: bones of the skeleton, cartilages, ligaments, and connective tissues What are the functions of
OSSEOUS TISSUE & BONE STRUCTURE PART I: OVERVIEW & COMPONENTS The Skeletal System Skeletal system includes: bones of the skeleton, cartilages, ligaments, and connective tissues What are the functions of
Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury
 Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury Bastian OW, Koenderman L, Alblas J, Leenen LPH, Blokhuis TJ. Neutrophils contribute to
Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury Bastian OW, Koenderman L, Alblas J, Leenen LPH, Blokhuis TJ. Neutrophils contribute to
SKELETAL SYSTEM I NOTE: LAB ASSIGNMENTS for this topic will run over 3 Weeks. A SEPARATE WORKSHEET WILL BE PROVIDED.
 BIO 211; Anatomy and Physiology I REFERENCE: CHAPTER 07 1 Dr. Lawrence Altman Naugatuck Valley Community College LECTURE TOPICS OUTLINE SKELETAL SYSTEM I NOTE: LAB ASSIGNMENTS for this topic will run over
BIO 211; Anatomy and Physiology I REFERENCE: CHAPTER 07 1 Dr. Lawrence Altman Naugatuck Valley Community College LECTURE TOPICS OUTLINE SKELETAL SYSTEM I NOTE: LAB ASSIGNMENTS for this topic will run over
FORMATION OF BONE. Intramembranous Ossification. Bone-Lec-10-Prof.Dr.Adnan Albideri
 FORMATION OF BONE All bones are of mesodermal origin. The process of bone formation is called ossification. We have seen that formation of most bones is preceded by the formation of a cartilaginous model,
FORMATION OF BONE All bones are of mesodermal origin. The process of bone formation is called ossification. We have seen that formation of most bones is preceded by the formation of a cartilaginous model,
Rama Nada. - Mousa Al-Abbadi. 1 P a g e
 - 1 - Rama Nada - - Mousa Al-Abbadi 1 P a g e Bones, Joints and Soft tissue tumors Before we start: the first 8 minutes was recalling to Dr.Mousa s duties, go over them in the slides. Wherever you see
- 1 - Rama Nada - - Mousa Al-Abbadi 1 P a g e Bones, Joints and Soft tissue tumors Before we start: the first 8 minutes was recalling to Dr.Mousa s duties, go over them in the slides. Wherever you see
Chapter 6 Bones and Bone Tissue Chapter Outline
 Chapter 6 Bones and Bone Tissue Chapter Outline Module 6.1: Introduction to Bones as Organs (Figures 6.1, 6.2, 6.3, 6.4) A. The skeletal system includes the bones, joints, and their associated supporting
Chapter 6 Bones and Bone Tissue Chapter Outline Module 6.1: Introduction to Bones as Organs (Figures 6.1, 6.2, 6.3, 6.4) A. The skeletal system includes the bones, joints, and their associated supporting
Module 2:! Functional Musculoskeletal Anatomy A! Semester 1! !!! !!!! Hard Tissues, Distal Upper Limb & Neurovascular Supply of Upper Limb!
 Functional Musculoskeletal Anatomy A Module 2: Hard Tissues, Distal Upper Limb & Neurovascular Supply of Upper Limb Semester 1 1 18. Bone Tissue & Growth of Bones 18.1 Describe the structure of bone tissue
Functional Musculoskeletal Anatomy A Module 2: Hard Tissues, Distal Upper Limb & Neurovascular Supply of Upper Limb Semester 1 1 18. Bone Tissue & Growth of Bones 18.1 Describe the structure of bone tissue
NOTES: Skeletal System (Ch 5, part 1)
 NOTES: Skeletal System (Ch 5, part 1) Individual bones are the organs of the skeletal system. A bone contains very active tissues. BONE STRUCTURE: *Bone structure reflects its function. Parts of a long
NOTES: Skeletal System (Ch 5, part 1) Individual bones are the organs of the skeletal system. A bone contains very active tissues. BONE STRUCTURE: *Bone structure reflects its function. Parts of a long
Skeletal System. The skeletal System... Components
 Skeletal System The skeletal System... What are the general components of the skeletal system? What does the skeletal system do for you & how does it achieve these functions? Components The skeletal system
Skeletal System The skeletal System... What are the general components of the skeletal system? What does the skeletal system do for you & how does it achieve these functions? Components The skeletal system
Skeletal System worksheet
 Skeletal System worksheet Name Section A: Intro to Skeletal System The skeletal system performs vital functions that enable us to move through our daily lives. Support - The skeleton provides support and
Skeletal System worksheet Name Section A: Intro to Skeletal System The skeletal system performs vital functions that enable us to move through our daily lives. Support - The skeleton provides support and
SKELETAL SYSTEM CHAPTER 07. Bone Function BIO 211: ANATOMY & PHYSIOLOGY I. Body Movement interacts with muscles bones act as rigid bar of a lever
 Page 1 BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 07 SKELETAL SYSTEM Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill. Some illustrations are courtesy of
Page 1 BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 07 SKELETAL SYSTEM Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill. Some illustrations are courtesy of
SKELETAL SYSTEM CHAPTER 07 BIO 211: ANATOMY & PHYSIOLOGY I
 BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 07 SKELETAL SYSTEM Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill. Some illustrations are courtesy of McGraw-Hill.
BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 07 SKELETAL SYSTEM Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill. Some illustrations are courtesy of McGraw-Hill.
Lecture 2: Skeletogenesis
 Jilin University School of Stomatology Skeletogenesis Lecture 2: Skeletogenesis Aug. 18, 2015 Yuji Mishina, Ph.D. mishina@umich.edu Student will describe Development of Bone - the general anatomy of bone
Jilin University School of Stomatology Skeletogenesis Lecture 2: Skeletogenesis Aug. 18, 2015 Yuji Mishina, Ph.D. mishina@umich.edu Student will describe Development of Bone - the general anatomy of bone
Outline. Skeletal System. Functions of Bone. Bio 105: Skeletal System 3/17/2016. The material from this lecture packet will be on the lecture exam
 Bio 105: Skeletal System Lecture 8 Chapter 5 The material from this lecture packet will be on the lecture exam The identification that you do after this lecture will be on the lab exam Outline I. Overview
Bio 105: Skeletal System Lecture 8 Chapter 5 The material from this lecture packet will be on the lecture exam The identification that you do after this lecture will be on the lab exam Outline I. Overview
AAO Foundation Awards Final Report
 401 N. Lindbergh Blvd. St. Louis, MO 63141 Tel.: 314.993.1700, #546 Toll Free: 800.424.2841, #546 Fax: 800.708.1364 Cell: 314.283.1983 E-Mail: rhazel@aaortho.org AAO Foundation Awards Final Report In an
401 N. Lindbergh Blvd. St. Louis, MO 63141 Tel.: 314.993.1700, #546 Toll Free: 800.424.2841, #546 Fax: 800.708.1364 Cell: 314.283.1983 E-Mail: rhazel@aaortho.org AAO Foundation Awards Final Report In an
Human Anatomy & Physiology
 PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 6 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 6 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
BONE LABORATORY DEMONSTRATIONS. These demonstrations are found on the bulletin boards outside the MCO Bookstore.
 BONE LABORATORY DEMONSTRATIONS These demonstrations are found on the bulletin boards outside the MCO Bookstore. COMPACT & TRABECULAR BONE - LM When viewed under the polarizing light microscope, the layering
BONE LABORATORY DEMONSTRATIONS These demonstrations are found on the bulletin boards outside the MCO Bookstore. COMPACT & TRABECULAR BONE - LM When viewed under the polarizing light microscope, the layering
Ossification and Bone Remodeling
 Ossification and Bone Remodeling Pre-natal Ossification Embryonic skeleton: fashioned from fibrous membranes or cartilage to accommodate mitosis. 2 types of pre-natal ossification (bone formation) 1.
Ossification and Bone Remodeling Pre-natal Ossification Embryonic skeleton: fashioned from fibrous membranes or cartilage to accommodate mitosis. 2 types of pre-natal ossification (bone formation) 1.
Do Now: 1. Where, specifically, is blood created? Which part of the long bone? 2. Which structures are primarily associated with growth? 3.
 Do Now: 1. Where, specifically, is blood created? Which part of the long bone? 2. Which structures are primarily associated with growth? 3. How could damage to these areas impact bone growth? WRITE AND
Do Now: 1. Where, specifically, is blood created? Which part of the long bone? 2. Which structures are primarily associated with growth? 3. How could damage to these areas impact bone growth? WRITE AND
Bone healing, delayed fracture healing and nonunion. Norbert Wiegand
 Bone healing, delayed fracture healing and nonunion Norbert Wiegand 2/3 of traumatology: treatment of fractures We have to understand: Structure of the bone Biology of the bone Bone healing Types of Bone
Bone healing, delayed fracture healing and nonunion Norbert Wiegand 2/3 of traumatology: treatment of fractures We have to understand: Structure of the bone Biology of the bone Bone healing Types of Bone
Anabolic Therapy With Teriparatide Indications Beyond Osteoporosis
 Anabolic Therapy With Teriparatide Indications Beyond Osteoporosis Andreas Panagopoulos MD, PhD Upper Limb & Sports Medicine Orthopaedic Surgeon Assistant Professor, University of Patras Outline Teriparatide
Anabolic Therapy With Teriparatide Indications Beyond Osteoporosis Andreas Panagopoulos MD, PhD Upper Limb & Sports Medicine Orthopaedic Surgeon Assistant Professor, University of Patras Outline Teriparatide
Supplementary Data. Supplementary Methods:
 Supplementary Data Supplementary Methods: Release kinetics of from collagen sponges in vivo. 2μg of human recombinant 165 was labeled with lexafluor 555 (Microscale Protein Labeling Kit; Invitrogen) as
Supplementary Data Supplementary Methods: Release kinetics of from collagen sponges in vivo. 2μg of human recombinant 165 was labeled with lexafluor 555 (Microscale Protein Labeling Kit; Invitrogen) as
Compact bone; Many parallel Haversian canals contain: small blood vessels. very small nerve. Interconnected by Volkmann s canals.
 Special characteristics of COMPACT BONE (dense bone) Thick; well vascularized Osteocytes and lamellae Concentric rings around blood vessels Most bones: outer compact bone inner spongy bone Marrow cavity
Special characteristics of COMPACT BONE (dense bone) Thick; well vascularized Osteocytes and lamellae Concentric rings around blood vessels Most bones: outer compact bone inner spongy bone Marrow cavity
Oral Histology. Alveolar bone or process: Functions of alveolar bone: Chemical composition: Development of the alveolar process: Dr.
 Oral Histology Lec.12 Alveolar bone or process: Dr. Nada Al-Ghaban Alveolar bone is a specialized part of the mandibular and maxillary bones that forms the primary support structure for teeth. Although
Oral Histology Lec.12 Alveolar bone or process: Dr. Nada Al-Ghaban Alveolar bone is a specialized part of the mandibular and maxillary bones that forms the primary support structure for teeth. Although
BIOL 2457 CHAPTER 6 SI 1. irregular ectopic: sutural (Wormian) The is between the shaft and end. It contains cartilage that is
 BIOL 2457 CHAPTER 6 SI 1 1. List 5 functions of bones: 2. Classify bones according to shape: give descriptions and examples: long short flat irregular ectopic: sutural (Wormian) ectopic: sesamoid 3. The
BIOL 2457 CHAPTER 6 SI 1 1. List 5 functions of bones: 2. Classify bones according to shape: give descriptions and examples: long short flat irregular ectopic: sutural (Wormian) ectopic: sesamoid 3. The
Skeletal Tissues Dr. Ali Ebneshahidi
 Skeletal Tissues Dr. Ali Ebneshahidi Functions of Bones 1. Support and protection: Bones give shape to body structure. Bones provide support to body weight. Certain bones protect vital internal organs
Skeletal Tissues Dr. Ali Ebneshahidi Functions of Bones 1. Support and protection: Bones give shape to body structure. Bones provide support to body weight. Certain bones protect vital internal organs
ANATOMY & PHYSIOLOGY 1 ( ) For Intensive Nursing PAUL ANDERSON SAMPLE TEST
 ANATOMY & PHYSIOLOGY 1 (101-805) For Intensive Nursing PAUL ANDERSON SAMPLE TEST 3 2011 1. If calcium levels in the extracellular fluid are too low, parathyroid hormone secretion would and osteoclast activity
ANATOMY & PHYSIOLOGY 1 (101-805) For Intensive Nursing PAUL ANDERSON SAMPLE TEST 3 2011 1. If calcium levels in the extracellular fluid are too low, parathyroid hormone secretion would and osteoclast activity
Bones. The division of bones anatomically is : long, short, irregular, flat and sesamoid.
 Bones Osteocytes : Are responsible for maintenance of bones Present in lacunae, and send processes. Unable to divide. The division of bones anatomically is : long, short, irregular, flat and sesamoid.
Bones Osteocytes : Are responsible for maintenance of bones Present in lacunae, and send processes. Unable to divide. The division of bones anatomically is : long, short, irregular, flat and sesamoid.
The Skeletal System:Bone Tissue
 The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose, and dense
The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose, and dense
SKELETAL SYSTEM. Introduction Notes (pt 1)
 SKELETAL SYSTEM Introduction Notes (pt 1) I. INTRODUCTION 1. Bones include active, living tissues: bone tissue, cartilage, dense connective tissue, blood, and nervous tissue. 2. Bones: support and protect
SKELETAL SYSTEM Introduction Notes (pt 1) I. INTRODUCTION 1. Bones include active, living tissues: bone tissue, cartilage, dense connective tissue, blood, and nervous tissue. 2. Bones: support and protect
Joint and Epiphyseal Progenitor Cells Revitalize Tendon Graft and Form Mineralized Insertion Sites in Murine ACL Reconstruction Model
 Joint and Epiphyseal Progenitor Cells Revitalize Tendon Graft and Form Mineralized Insertion Sites in Murine ACL Reconstruction Model Yusuke Hagiwara 1,2, Nathaniel A. Dyment 3, Douglas J. Adams 3, Shinro
Joint and Epiphyseal Progenitor Cells Revitalize Tendon Graft and Form Mineralized Insertion Sites in Murine ACL Reconstruction Model Yusuke Hagiwara 1,2, Nathaniel A. Dyment 3, Douglas J. Adams 3, Shinro
KEY CONCEPTS Unit 6 THE SKELETAL SYSTEM
 ANATOMY & PHYSIOLOGY 1 (101-805 - AB) PAUL ANDERSON 2011 KEY CONCEPTS Unit 6 THE SKELETAL SYSTEM A Overview of The Skeletal System 1. Definition: Anatomically the SKELETAL SYSTEM consists of bones, cartilages,
ANATOMY & PHYSIOLOGY 1 (101-805 - AB) PAUL ANDERSON 2011 KEY CONCEPTS Unit 6 THE SKELETAL SYSTEM A Overview of The Skeletal System 1. Definition: Anatomically the SKELETAL SYSTEM consists of bones, cartilages,
Tissue repair. (3&4 of 4)
 Tissue repair (3&4 of 4) What will we discuss today: Regeneration in tissue repair Scar formation Cutaneous wound healing Pathologic aspects of repair Regeneration in tissue repair Labile tissues rapid
Tissue repair (3&4 of 4) What will we discuss today: Regeneration in tissue repair Scar formation Cutaneous wound healing Pathologic aspects of repair Regeneration in tissue repair Labile tissues rapid
Paracrine Effect of Mesenchymal Stem Cells derived from Human Adipose tissue in Bone Regeneration.
 Paracrine Effect of Mesenchymal Stem Cells derived from Human Adipose tissue in Bone Regeneration. By Linero I., Chaparro O., 2014 Suzana Tufegdzic 1 Background 2 Background Stem Cells: undifferentiated
Paracrine Effect of Mesenchymal Stem Cells derived from Human Adipose tissue in Bone Regeneration. By Linero I., Chaparro O., 2014 Suzana Tufegdzic 1 Background 2 Background Stem Cells: undifferentiated
Chapter 6: SKELETAL SYSTEM
 Chapter 6: SKELETAL SYSTEM I. FUNCTIONS A. Support B. Protection C. Movement D. Mineral storage E. Lipid storage (Fig. 6.8b) F. Blood cell production (Fig. 6.4) II. COMPONENTS A. Cartilage 1. Hyaline 2.
Chapter 6: SKELETAL SYSTEM I. FUNCTIONS A. Support B. Protection C. Movement D. Mineral storage E. Lipid storage (Fig. 6.8b) F. Blood cell production (Fig. 6.4) II. COMPONENTS A. Cartilage 1. Hyaline 2.
BONE REMODELLING. Tim Arnett. University College London. Department of Anatomy and Developmental Biology
 BONE REMODELLING Tim Arnett Department of Anatomy and Developmental Biology University College London The skeleton, out of sight and often out of mind, is a formidable mass of tissue occupying about 9%
BONE REMODELLING Tim Arnett Department of Anatomy and Developmental Biology University College London The skeleton, out of sight and often out of mind, is a formidable mass of tissue occupying about 9%
Dr. Heba Kalbouneh. Saba Alfayoumi. Heba Kalbouneh
 11 Dr. Heba Kalbouneh Saba Alfayoumi Heba Kalbouneh 2- Bone Bone tissue is also classified into primary bone and secondary bone. In the beginning, the first bone that is deposited by the osteoblasts is
11 Dr. Heba Kalbouneh Saba Alfayoumi Heba Kalbouneh 2- Bone Bone tissue is also classified into primary bone and secondary bone. In the beginning, the first bone that is deposited by the osteoblasts is
2 PROCESSES OF BONE OSSIFICATION
 2 PROCESSES OF BONE OSSIFICATION ENDOCHONDRAL OSSIFICATION 6 STEPS 1. CARTILAGE ENLARGES, BY APPOSITIONAL GROWTH; CHONDROCYTES AT CENTER OF CARTILAGE GROW IN SIZE; MATRIX REDUCES IN SIZE & SPICULES CALCIFY;
2 PROCESSES OF BONE OSSIFICATION ENDOCHONDRAL OSSIFICATION 6 STEPS 1. CARTILAGE ENLARGES, BY APPOSITIONAL GROWTH; CHONDROCYTES AT CENTER OF CARTILAGE GROW IN SIZE; MATRIX REDUCES IN SIZE & SPICULES CALCIFY;
Blood. Hematopoietic Tissue
 Blood Hematopoietic Tissue Is a type of connective tissue in which its cells are suspended in a circulating fluid. Erythrocytes+ leukocytes + platelets (thrombocytes) =formed elements of blood. These formed
Blood Hematopoietic Tissue Is a type of connective tissue in which its cells are suspended in a circulating fluid. Erythrocytes+ leukocytes + platelets (thrombocytes) =formed elements of blood. These formed
Bone Tissue- Chapter 5 5-1
 Bone Tissue- Chapter 5 5-1 Bone Functions Support Protection Assistance in movement Mineral storage and release Blood cell production Triglyceride storage 5-2 Bone Chemistry Water (25%) Organic Constituent
Bone Tissue- Chapter 5 5-1 Bone Functions Support Protection Assistance in movement Mineral storage and release Blood cell production Triglyceride storage 5-2 Bone Chemistry Water (25%) Organic Constituent
Osteology. Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College
 Osteology Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College Functions of the Skeletal System: Support Movement Protection Hemopoiesis Electrolyte balance (Ca ++ /PO -3 4 ) Acid-base balance Storage
Osteology Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College Functions of the Skeletal System: Support Movement Protection Hemopoiesis Electrolyte balance (Ca ++ /PO -3 4 ) Acid-base balance Storage
Supplemental Table 1. Primer sequences for transcript analysis
 Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
ANATOMY & PHYSIOLOGY - CLUTCH CH. 8 - BONE AND CARTILAGE.
 !! www.clutchprep.com CONCEPT: BONE CLASSIFICATIONS There are four classifications of bones based on their 1. Long bones are greater in length than in width - Found in the upper and lower limbs (ex: arm,
!! www.clutchprep.com CONCEPT: BONE CLASSIFICATIONS There are four classifications of bones based on their 1. Long bones are greater in length than in width - Found in the upper and lower limbs (ex: arm,
OpenStax-CNX module: m Bone Structure * Ildar Yakhin. Based on Bone Structure by OpenStax. Abstract
 OpenStax-CNX module: m63474 1 Bone Structure * Ildar Yakhin Based on Bone Structure by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By
OpenStax-CNX module: m63474 1 Bone Structure * Ildar Yakhin Based on Bone Structure by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By
Calcium Phosphate Cement
 Calcium Phosphate Cement Fast-Setting Bone Graft and AutoGraft Extender. * Ossilix is a high performance next generation calcium phosphate cement indicated for filling bony defects in cancellous bone.
Calcium Phosphate Cement Fast-Setting Bone Graft and AutoGraft Extender. * Ossilix is a high performance next generation calcium phosphate cement indicated for filling bony defects in cancellous bone.
Siglec-15 Is A Potential Therapeutic Target For Postmenopausal Osteoporosis
 Siglec-15 Is A Potential Therapeutic Target For Postmenopausal Osteoporosis Yusuke Kameda, Masahiko Takahata, Tomohiro Shimizu, Hiroki Hamano, Norimasa Iwasaki. Department of Orthopedic Surgery, Hokkaido
Siglec-15 Is A Potential Therapeutic Target For Postmenopausal Osteoporosis Yusuke Kameda, Masahiko Takahata, Tomohiro Shimizu, Hiroki Hamano, Norimasa Iwasaki. Department of Orthopedic Surgery, Hokkaido
Finite-element study of vibration effect to fracture healing of a human tibia
 Finite-element study of vibration effect to fracture healing of a human tibia Leonid Maslov 1, Jean-Baptiste Etheve 2, Nikolay Sabaneev 3 Ivanovo State Power Engineering University, Ivanovo, Russia 1 Corresponding
Finite-element study of vibration effect to fracture healing of a human tibia Leonid Maslov 1, Jean-Baptiste Etheve 2, Nikolay Sabaneev 3 Ivanovo State Power Engineering University, Ivanovo, Russia 1 Corresponding
Due in Lab. Due next week in lab - Scientific America Article Select one article to read and complete article summary
 Due in Lab 1. Skeletal System 33-34 2. Skeletal System 26 3. PreLab 6 Due next week in lab - Scientific America Article Select one article to read and complete article summary Cell Defenses and the Sunshine
Due in Lab 1. Skeletal System 33-34 2. Skeletal System 26 3. PreLab 6 Due next week in lab - Scientific America Article Select one article to read and complete article summary Cell Defenses and the Sunshine
Osseous Tissue and Bone Structure
 C h a p t e r 6 Osseous Tissue and Bone Structure PowerPoint Lecture Slides prepared by Jason LaPres Lone Star College - North Harris Copyright 2009 Pearson Education, Inc., publishing as Pearson Benjamin
C h a p t e r 6 Osseous Tissue and Bone Structure PowerPoint Lecture Slides prepared by Jason LaPres Lone Star College - North Harris Copyright 2009 Pearson Education, Inc., publishing as Pearson Benjamin
An Introduction to the Skeletal System Skeletal system includes Bones of the skeleton Cartilages, ligaments, and connective tissues
 An Introduction to the Skeletal System Skeletal system includes Bones of the skeleton Cartilages, ligaments, and connective tissues Functions of the Skeletal System Support Storage of minerals (calcium)
An Introduction to the Skeletal System Skeletal system includes Bones of the skeleton Cartilages, ligaments, and connective tissues Functions of the Skeletal System Support Storage of minerals (calcium)
Indian hedgehog synchronizes skeletal angiogenesis and perichondrial maturation with cartilage development
 Research article 1057 Indian hedgehog synchronizes skeletal angiogenesis and perichondrial maturation with cartilage development Céline Colnot 1, Luis de la Fuente 1, Steve Huang 1, Diane Hu 1, Chuanyong
Research article 1057 Indian hedgehog synchronizes skeletal angiogenesis and perichondrial maturation with cartilage development Céline Colnot 1, Luis de la Fuente 1, Steve Huang 1, Diane Hu 1, Chuanyong
Healing & Repair. Tissue Regeneration
 Healing & Repair Dr. Srikumar Chakravarthi Repair & Healing: Are they same? Repair :Regeneration of injured cells by cells of same type, as with regeneration of skin/oral mucosa (requires basement membrane)
Healing & Repair Dr. Srikumar Chakravarthi Repair & Healing: Are they same? Repair :Regeneration of injured cells by cells of same type, as with regeneration of skin/oral mucosa (requires basement membrane)
Nanomechanical Symptoms in Cartilage Precede Histological Osteoarthritis Signs after the Destabilization of Medial Meniscus in Mice
 Nanomechanical Symptoms in Cartilage Precede Histological Osteoarthritis Signs after the Destabilization of Medial Meniscus in Mice Basak Doyran 1, Wei Tong 2, Qing Li 1, Haoruo Jia 2, Xianrong Zhang 3,
Nanomechanical Symptoms in Cartilage Precede Histological Osteoarthritis Signs after the Destabilization of Medial Meniscus in Mice Basak Doyran 1, Wei Tong 2, Qing Li 1, Haoruo Jia 2, Xianrong Zhang 3,
Estrogen receptor α- (ERα), but not ERβ-signaling, is crucially involved in mechanostimulation of bone fracture healing by whole-body vibration
 Estrogen receptor α- (ERα), but not ERβ-signaling, is crucially involved in mechanostimulation of bone fracture healing by whole-body vibration Melanie Haffner-Luntzer et.al. published in BONE 1 Abstract
Estrogen receptor α- (ERα), but not ERβ-signaling, is crucially involved in mechanostimulation of bone fracture healing by whole-body vibration Melanie Haffner-Luntzer et.al. published in BONE 1 Abstract
Regulation of the IGF axis by TGF-b during periosteal chondrogenesis: implications for articular cartilage repair
 Regulation of the IGF axis by TGF-b during periosteal chondrogenesis: implications for articular cartilage repair Chapter 04 Boek 1_Gie.indb 55 21-05-2007 12:27:33 Chapter 04 Abstract Goal: TGF-b and IGF-I
Regulation of the IGF axis by TGF-b during periosteal chondrogenesis: implications for articular cartilage repair Chapter 04 Boek 1_Gie.indb 55 21-05-2007 12:27:33 Chapter 04 Abstract Goal: TGF-b and IGF-I
Tissue renewal and Repair. Nisamanee Charoenchon, PhD Department of Pathobiology, Faculty of Science
 Tissue renewal and Repair Nisamanee Charoenchon, PhD Email: nisamanee.cha@mahidol.ac.th Department of Pathobiology, Faculty of Science Topic Objectives 1. Describe processes of tissue repair, regeneration
Tissue renewal and Repair Nisamanee Charoenchon, PhD Email: nisamanee.cha@mahidol.ac.th Department of Pathobiology, Faculty of Science Topic Objectives 1. Describe processes of tissue repair, regeneration
Skeletal System. Bio 105
 Skeletal System Bio 105 Outline I. Overview of the skeletal system II. Function of bones III. Bone structure IV. Bone cells V. Cartilage VI. Tendons and Ligaments VII. Joints VIII. Bone development IX.
Skeletal System Bio 105 Outline I. Overview of the skeletal system II. Function of bones III. Bone structure IV. Bone cells V. Cartilage VI. Tendons and Ligaments VII. Joints VIII. Bone development IX.
Outline. Skeletal System. Tendons link the skeletal and the muscular systems.
 Outline Skeletal System Bio 105 I. Overview of the skeletal system II. Function of bones III. Bone structure IV. Bone cells V. Cartilage VI. Tendons and Ligaments VII. Joints VIII. Bone development IX.
Outline Skeletal System Bio 105 I. Overview of the skeletal system II. Function of bones III. Bone structure IV. Bone cells V. Cartilage VI. Tendons and Ligaments VII. Joints VIII. Bone development IX.
Species differences in histomorphometry
 Species differences in histomorphometry Reinhold G. Erben Department of Biomedical Sciences Institute of Physiology and Pathophysiology University of Veterinary Medicine Vienna Purpose of histomorphometry
Species differences in histomorphometry Reinhold G. Erben Department of Biomedical Sciences Institute of Physiology and Pathophysiology University of Veterinary Medicine Vienna Purpose of histomorphometry
The Skeletal System Vertebral column Sacrum. Osseous tissue For the body and soft organs. Magnesium, sodium, fluoride Levers for muscle action
 10/1/2016 Cranium Facial s Skull Clavicle Scapula Sternum Rib Humerus Vertebra Radius Ulna Carpals Thoracic cage (ribs and sternum) The Skeletal System Vertebral column Sacrum Phalanges Metacarpals Femur
10/1/2016 Cranium Facial s Skull Clavicle Scapula Sternum Rib Humerus Vertebra Radius Ulna Carpals Thoracic cage (ribs and sternum) The Skeletal System Vertebral column Sacrum Phalanges Metacarpals Femur
The purpose of this practical session is to demonstrate cartilage and bone as specialized connective tissues to the student.
 1 CARTILAGE AND BONE The purpose of this practical session is to demonstrate cartilage and bone as specialized connective tissues to the student. 1. Hyaline cartilage Slide 73 This is a cross section through
1 CARTILAGE AND BONE The purpose of this practical session is to demonstrate cartilage and bone as specialized connective tissues to the student. 1. Hyaline cartilage Slide 73 This is a cross section through
Introduction to Biomedical Engineering: Basic concepts and Bone Biomechanics
 Introduction to Biomedical Engineering: Basic concepts and Bone Biomechanics Fall 2016, AKUT G. Rouhi Biomechanics The field of biomechanics applies principles of mechanics to study the structure and function
Introduction to Biomedical Engineering: Basic concepts and Bone Biomechanics Fall 2016, AKUT G. Rouhi Biomechanics The field of biomechanics applies principles of mechanics to study the structure and function
Biology. Dr. Khalida Ibrahim
 Biology Dr. Khalida Ibrahim BONE TISSUE Bone tissue is a specialized form of connective tissue and is the main element of the skeletal tissues. It is composed of cells and an extracellular matrix in which
Biology Dr. Khalida Ibrahim BONE TISSUE Bone tissue is a specialized form of connective tissue and is the main element of the skeletal tissues. It is composed of cells and an extracellular matrix in which
The formation of blood cells is called. hemopoiesis. What does our bone store? Where do our bones store fat? yellow marrow.
 What are the 5/6 functions of the skeletal system? support, protection, movement, blood cell formation, storage, homeostasis The formation of blood cells is called hemopoiesis What does our bone store?
What are the 5/6 functions of the skeletal system? support, protection, movement, blood cell formation, storage, homeostasis The formation of blood cells is called hemopoiesis What does our bone store?
b. Adult bones produce 2.5 million RBCs each second.
 Ch 6 Skeletal System I. Functions of the Skeletal System A. The skeletal system consists of: 1. bones, cartilage, tendons and ligaments B. Living bone is not Gr. dried up 1. It is dynamic and adaptable
Ch 6 Skeletal System I. Functions of the Skeletal System A. The skeletal system consists of: 1. bones, cartilage, tendons and ligaments B. Living bone is not Gr. dried up 1. It is dynamic and adaptable
PRO-STIM Injectable Inductive Graft TECHNICAL MONOGRAPH
 PRO-STIM Injectable Inductive Graft TECHNICAL MONOGRAPH PRO-STIM Injectable Inductive Graft TECHNICAL MONOGRAPH Headline Contents Headline Appendix 4 5 7 8 13 14 Clinical Benefits Self-Forming Porous Scaffold
PRO-STIM Injectable Inductive Graft TECHNICAL MONOGRAPH PRO-STIM Injectable Inductive Graft TECHNICAL MONOGRAPH Headline Contents Headline Appendix 4 5 7 8 13 14 Clinical Benefits Self-Forming Porous Scaffold
Trauma-Induced Inflammation and Fracture Healing
 REVIEW ARTICLE Hans-Christophe Pape, MD,* Ralph Marcucio, PhD, Catherine Humphrey, MD, Celine Colnot, PhD, Matthias Knobe, MD,* and Edward J. Harvey, MDCM, MSc, FRCSC Summary: Fracture healing is an extremely
REVIEW ARTICLE Hans-Christophe Pape, MD,* Ralph Marcucio, PhD, Catherine Humphrey, MD, Celine Colnot, PhD, Matthias Knobe, MD,* and Edward J. Harvey, MDCM, MSc, FRCSC Summary: Fracture healing is an extremely
Skeletal Tissues. Dr. Ali Ebneshahidi
 Skeletal Tissues Dr. Ali Ebneshahidi Functions of Bones 1. Support and protection : Bones give shape to body structure. Bones provide support to body weight. Certain bones protect vital internal organs
Skeletal Tissues Dr. Ali Ebneshahidi Functions of Bones 1. Support and protection : Bones give shape to body structure. Bones provide support to body weight. Certain bones protect vital internal organs
Promoting Fracture Healing Through Systemic or Local Administration of Allogeneic Mesenchymal Stem Cells
 Promoting Fracture Healing Through Systemic or Local Administration of Allogeneic Mesenchymal Stem Cells Gang Li Dept. of Orthopaedics and Traumatology School of Biomedical Sciences, The Chinese University
Promoting Fracture Healing Through Systemic or Local Administration of Allogeneic Mesenchymal Stem Cells Gang Li Dept. of Orthopaedics and Traumatology School of Biomedical Sciences, The Chinese University
Mutation in Osteoactivin Enhances RANKL-Mediated Signaling, Promoting Osteoclast Differentiation, Survival and Inhibiting Bone Resorption
 Mutation in Osteoactivin Enhances RANKL-Mediated Signaling, Promoting Osteoclast Differentiation, Survival and Inhibiting Bone Resorption Samir Abdelmagid, MD, PhD, Fouad Moussa, BS, Sondag Gregory, MS,
Mutation in Osteoactivin Enhances RANKL-Mediated Signaling, Promoting Osteoclast Differentiation, Survival and Inhibiting Bone Resorption Samir Abdelmagid, MD, PhD, Fouad Moussa, BS, Sondag Gregory, MS,
What are the parts of the skeletal system? Chapter 6- Part I Bones and Skeletal Tissues. Growth of Cartilage. Bones come in many shapes
 Chapter 6- Part I Bones and Skeletal Tissues Components of the skeletal system Classification of Bone (bone shapes) Functions of bone Bone structure Microscopic structure of bone and bone cells What are
Chapter 6- Part I Bones and Skeletal Tissues Components of the skeletal system Classification of Bone (bone shapes) Functions of bone Bone structure Microscopic structure of bone and bone cells What are
Orthopedic & Sports Medicine, Bay Care Clinic, 501 N. 10th Street, Manitowoc, WI Procedure. Subtalar arthrodesis
 OSTEOAMP Allogeneic Morphogenetic Proteins Subtalar Nonunions OSTEOAMP Case Report SUBTALAR NONUNIONS Dr. Jason George DeVries and Dr. Brandon M. Scharer Orthopedic & Sports Medicine, Bay Care Clinic,
OSTEOAMP Allogeneic Morphogenetic Proteins Subtalar Nonunions OSTEOAMP Case Report SUBTALAR NONUNIONS Dr. Jason George DeVries and Dr. Brandon M. Scharer Orthopedic & Sports Medicine, Bay Care Clinic,
The Skeletal System PART A
 5 The Skeletal System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Skeletal System
5 The Skeletal System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Skeletal System
36 1 The Skeletal System Slide 1 of 40
 1 of 40 The Skeleton All organisms need structural support. Unicellular organisms have a cytoskeleton. Multicellular animals have either an exoskeleton (arthropods) or an endoskeleton (vertebrates). 2
1 of 40 The Skeleton All organisms need structural support. Unicellular organisms have a cytoskeleton. Multicellular animals have either an exoskeleton (arthropods) or an endoskeleton (vertebrates). 2
supplementary information
 DOI:.38/ncb1963 a wild 5.3kb 11.2kb targeting vector stop PCR primer KKpn NNhe targeted allele 5.7kb 6.8kb probe b d (g) 35 3 25 2 genomic Southern blot Kpn I digest Nhe I digest / / / / / / 11.2 kb 5.7
DOI:.38/ncb1963 a wild 5.3kb 11.2kb targeting vector stop PCR primer KKpn NNhe targeted allele 5.7kb 6.8kb probe b d (g) 35 3 25 2 genomic Southern blot Kpn I digest Nhe I digest / / / / / / 11.2 kb 5.7
