The Conundrum Of Sensitization When Recording From Nociceptors
|
|
|
- Ginger Georgiana Sanders
- 5 years ago
- Views:
Transcription
1 University of New England DUNE: DigitalUNE Biomedical Sciences Faculty Publications Biomedical Sciences Faculty Works The Conundrum Of Sensitization When Recording From Nociceptors Geoffrey M. Bove University of New England, Andrew Dilley University of Sussex at Brighton Follow this and additional works at: Part of the Medical Sciences Commons Recommended Citation Bove, Geoffrey M. and Dilley, Andrew, "The Conundrum Of Sensitization When Recording From Nociceptors" (2010). Biomedical Sciences Faculty Publications This Article is brought to you for free and open access by the Biomedical Sciences Faculty Works at DUNE: DigitalUNE. It has been accepted for inclusion in Biomedical Sciences Faculty Publications by an authorized administrator of DUNE: DigitalUNE. For more information, please contact
2 NIH Public Access Author Manuscript Published in final edited form as: J Neurosci Methods May 15; 188(2): doi: /j.jneumeth The conundrum of sensitization when recording from nociceptors Geoffrey M. Bove [Associate Professor] University of New England College of Osteopathic Medicine 102 Stella Maris Hall 11 Hills Beach Rd. Biddeford, ME gbove@une.edu Andrew Dilley [Lecturer in Anatomy] Division of Clinical and Laboratory Investigation Brighton and Sussex Medical School Medical Research Building University of Sussex Falmer Brighton BN1 9PS United Kingdom Tel: +44 (0) (Office)/ (Lab) Fax: +44 (0) A.Dilley@bsms.ac.uk Abstract Nociceptors are sensory neurons that detect harmful, or potentially harmful, stimuli, and can become sensitized following injury or repetitive stimulation. When sensitized, nociceptors often exhibit activity in the absence of apparent or additional stimulation, called ongoing (or spontaneous) activity (OA). In this report, we provide evidence that OA in nociceptors can be caused by the stimuli typically used to identify and characterize the neuron, which must by definition be noxious and therefore potentially sensitizing. Such OA caused by the experimental methodology can confound interpretation. In our nerve inflammation model, OA can potentially arise from multiple sites, including the lesion site and the receptive field. We provide evidence that the OA rate recorded during these experiments may be related to the site and cause of OA generation. We suggest that there are two types of OA, characterized by their rates. Very slow rates of ongoing activity (<0.2 Hz) are likely to arise from the receptive field and may indicate sensitization during the experiment. Faster rates are likely to arise from the nerve trunk, i.e. the neuritis, or the neuronal cell body. Without appropriate methodological consideration, interpretations of results from such studies of nociceptor function may be methodologically confounded. Keywords Ongoing activity; electrophysiology; methodology; neuritis; pain; nociception 1. Introduction Understanding the function of nociceptors is an ongoing challenge. There are many ways of studying nociceptors, but arguably the most powerful method is to record from the neurons directly. The most common technique used is extracellular recording from the peripheral nerve trunk, although some researchers, including the present authors, also record from the dorsal roots. Identified neurons can be stimulated electrically, and mechanical, thermal, and chemical response properties can be tested at the receptive field or nerve trunk for individually characterized neurons. Changes in these parameters (e.g. changes in mechanical threshold or 2010 Elsevier B.V. All rights reserved Correspondence to: Geoffrey M. Bove. Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
3 Bove and Dilley Page 2 2. Methods the development of ongoing activity) are interpreted as sensitization, and may impact sensory perception. Indeed, studies of sensitization are critical to understanding the actions of many drugs and other compounds. A key feature of nociceptors is that they develop ongoing activity (OA) when they, or the structures they innervate, are injured or inflamed (Bessou and Perl, 1969; Perl, 1976; Perl, Kumazawa et al., 1976). Because noxious and potentially injurious stimulation must be used to activate or access nociceptor receptive terminals, OA can develop even in control/untreated animals. Thus, studies often report OA as a property of some normal nociceptors (Bove and Light, 1995; Bove, Ransil et al., 2003; Strassman and Levy, 2006; Dilley and Bove, 2008). Certainly, OA in a sensory element that could lead to pain is not desirable under normal circumstances. Since persistent nociceptor OA maybe the basis for some cases of acute and chronic pain, understanding the details of this characteristic is important to all studies of pain and nociception. There is a methodological conundrum in the electrophysiological approaches to studying nociceptors, especially when studying non-cutaneous neurons. To characterize a deep neuron, one must identify its receptive field, which involves noxiously mechanically stimulating peripheral structures. Each search does not lead to identification, and thus it is normal in these experiments to deliver repeated noxious stimuli to the same structures over the course of hours. We hypothesized that the cumulative stimulation throughout such experiments leads to sensitization of the neuron's receptive field, which is reflected as OA. Such OA could be expected to mask the effects of various interventions, and thus lead to false negative results. The present report will demonstrate this masking effect in a model of localized peripheral nerve inflammation (the neuritis model) Animals and surgery All experiments adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain and the Principles of laboratory animal care (NIH publication No , revised 1996), and were approved by the Animal Care and Use Committees of Beth Israel Deaconess Medical Center and University of Southern Maine. The data presented combine both published and unpublished observations from two different recording models, the methods of which have been recently detailed (Dilley and Bove, 2008; Bove, 2009). Data were gathered from adult, male Wistar and female Long Evans rats (there have been no reports of differences in peripheral neuronal sensitivities between these strains). In both models, recordings were made from normal nerves, and also from nerves that had been locally inflamed with complete Freund's adjuvant (the neuritis model). The nerve inflammation was accomplished by incising the skin over the posterior thigh, and exposing the sciatic nerve using blunt dissection. A small strip of surgical gelatin foam (5 5 10mm) was soaked in complete Freund's adjuvant and placed loosely around the nerve, and the animal allowed to recover until the desired post-operative day Dorsal root recordings Recordings were made from untreated (n=6) and neuritis animals, 1 (n=18), 4 (n=17) and 8 (n=14) weeks following surgery (details in Dilley and Bove, 2008). A lumbar laminectomy was performed from L2 to L5 to expose the contents of the spinal canal. The surrounding skin was used to form a pool that was filled with mineral oil. The dura mater was opened and the left L5 dorsal root was cut close to the dorsal root entry zone. The cut end of the root was
4 Bove and Dilley Page 3 positioned on a small black glass platform (10 mm 6 mm) for recording. Bipolar stimulating electrodes were placed under the dorsal root, distal to the platform. Recordings were made from fine filaments that were teased from the cut end of the dorsal root, using finely sharpened forceps. Filaments were split until single action potentials could be evoked using electrical stimulation of the dorsal root (square wave pulses: ms duration and V amplitude). Only filaments with clearly identifiable waveforms were studied. During dorsal root recordings, receptive fields (RF) for electrically isolated neurons with slowly conducting axons (<1.5m/s) within the sciatic nerve were searched for below the knee using intense mechanical stimuli (Fig. 1A). Neurons were only included for further study if they had mechanically sensitive RFs in the lower limb located distal to the exposed part of the sciatic nerve, i.e. the axons pass through the neuritis. Critical for the present report, the time was recorded at which noxious stimulation of the periphery commenced, i.e. the time that the first neuron's receptive field was identified. Thereafter, the time that each subsequent neuron was recorded was also noted Peripheral nerve recordings Recordings were made from untreated (n = 13) and neuritis animals 3 8 days following surgery (n = 12; details in Bove, 2009). The sciatic and sural nerves were carefully dissected from the ankle to the sciatic notch. The surrounding skin was used to form a pool that was filled with mineral oil. Recordings were made from fine filaments that were teased from the sural nerve. Following a lumbar laminectomy, another oil pool was formed over the lumbar spinal cord. The L4 L6 dorsal roots were cut close to the spinal cord and placed over bipolar stimulating electrodes. During peripheral nerve recordings, sensory neurons with slowly conducting axons within the sciatic nerve were identified by electrically stimulating the dorsal roots while recording from the distal end of the cutaneous sural nerve (Fig. 1B). Therefore, axons were stimulated in an antidromic (opposite) direction. In these recordings, no RF could be determined, because they had been disconnected from the nerve. Consequently, there could be no sensitization due to RF stimuli Outcome measurements 2.5. Statistical analysis In all experiments, ongoing activity was recorded for at least 2 minutes for each neuron. In the dorsal root model, there were three possible sources of OA: the receptive field, the neuritis lesion, and the neuronal cell body. In the peripheral nerve recording model, there were two potential sources of OA: the neuritis lesion and the neuronal cell body. Data were collected in raw form at 20 KHz for offline analysis using Spike 2 software (Cambridge Electronic Designs, UK). All comparisons between frequency data were made using multiple Chi-Square tests or, when expected frequencies were less than 5, Fisher's exact tests. Paired comparisons were against the control group unless stated. When multiple comparisons were made, Bonferroni's correction was applied in assessing the significance (e.g. between control and neuritis groups, the level of significance was p = 0.017). Since the rates of ongoing activity were negatively skewed, a Kruskal-Wallis test was used to compare between all groups. Correlation coefficients were calculated between the time from first recording and the percentage of neurons exhibiting OA and also the time from first recording and the rate of OA.
5 Bove and Dilley Page 4 3. Results 3.1. Changes in Leg Appearance 3.2. Ongoing activity In the dorsal root recordings, the lower limb was repeatedly noxiously stimulated while searching for RFs. In these experiments, it took the experimenter between 11 and 15 minutes to locate the first mechanically sensitive RF, and minutes to characterize the neuron and subsequently identify another neuron and its RF. This process resulted in visible swelling of the lower limb and foot, which increased over time Percentage of OA In both experimental setups (dorsal root and peripheral nerve recordings), OA was present in a number of neurons in each group (Fig. 2). In control peripheral nerve experiments the neurons were primarily silent, with only one of 77 neurons showing any OA. However, during neuritis, the percentage of neurons with OA increased significantly to 35% at 3 8 days (17/49; p = 0.001, Fisher Exact Test). In experiments recorded from the dorsal roots, there were no significant differences between control and neuritis groups at 1, 4, and 8 weeks (p = 0.16, Chi-Square 4 2 contingency table). However, 47% (17/36) of the neurons recorded from the dorsal root during control conditions exhibited OA, a significant difference compared to the OA incidence of the control peripheral nerve recordings (p<0.0001, Chi- Square) Rates of OA When the OA rates from the dorsal root recordings were compared, there were no significant differences between groups (untreated, 1, 4 and 8 weeks; p = 0.58, Kruskal-Wallis). There was also no change in the rates over time when all OA rates were pooled (r 2 = 0.003; p = 0.6). Figure 3A shows the rates for all neurons recorded from the dorsal root. There appears to be a bimodality in the rates, with one population having very slow OA, apparently peaking at under 1 action potential per minute (0.033 Hz), and another population with a faster peak OA rate (~0.3 Hz). These two populations overlapped near 0.2 Hz. Only 11% of neurons with OA had rates >0.5 Hz. In the peripheral nerve recordings (i.e. without the receptive field attached; Fig. 3B), rates of OA tended to be faster. Fifty three percent of neurons fired at rates >0.5 Hz. There were negligible neurons firing at 1 action potential per minute (0.033 Hz). It is clear from the peripheral nerve recordings that the neuritis caused a significant increase in OA incidence from control recordings (Fig. 2). However, this effect was absent from the dorsal root recordings (Fig. 2). Because of this discrepancy, and the apparent bimodality in the rates from the dorsal root data (Fig. 3A), we hypothesized that the high proportion of neurons with very slow OA (which were absent from the peripheral nerve recordings) may be masking the true effect of the neuritis. We hypothesized that the very slow OA rates observed in the dorsal root recordings may have come from the RFs. When we separated the neurons based on whether they were recorded in the first hour (early; see 3.2.3, below), and thus had less inflammation due to RF stimulation, an effect of the neuritis became more clear (Fig. 4A and B), in that it is more consistent with the pattern of OA observed in the peripheral nerve recordings. In 4A, OA with rates of Hz have been removed, and in 4B, OA with <0.05 Hz have been removed. In contrast, there is no clear effect seen when this analysis is performed on recordings performed one hour or longer into the recording session (late) in the experiment (Fig. 4C and D) Time trends To evaluate the possibility that OA incidence and/or rate were due to repeated RF searches, ongoing activity data for all dorsal root recordings (control and neuritis) were pooled and grouped by hour following the first recording. There was found to be a
6 Bove and Dilley Page 5 4. Discussion significant positive correlation between time after first recording and OA incidence (r 2 = 0.84, p = 0.03; Fig. 5A). Because of this observation, we separated the data into neurons recorded within the first hour (early) and those recorded in later hours (late), and found the OA incidences to be significantly different (p = , Chi-square). With the exception of the 1 week time point, there were more neurons with OA recorded late in each group (Fig. 5B). During inflammation, OA develops in skin, joint, and muscle nociceptors (Perl, Kumazawa, Lynn, and Kenins, 1976; Grigg, Schaible et al., 1986; Schaible, Schmidt et al., 1987; Berberich, Hoheisel et al., 1988; Cohen and Perl, 1988), and is an indicator of sensitization (Perl, Kumazawa et al., 1976). The present data demonstrate a methodological confound of characterizing nociceptors by their RF properties, especially when studying OA. To locate RFs, especially in deep tissues, the structures must be stimulated noxiously. Thus, when a slowly conducting neuron is isolated, the peripheral structures from the distal thigh to the toes are stimulated noxiously using fingers or forceps. However, if a mechanical receptive field is not found, another neuron is isolated, and the search repeated. This typically takes dozens of searches over the course of the recording sessions, which in these experiments were 4 7 hours long. In our experiments it was clear that the noxious mechanical stimulus necessary to identify the RFs was in itself sufficient to cause inflammation. Because there was no RF present in the peripheral nerve recordings, such inflammation was not present. Consistently, there was no OA during the control recordings. We conclude that the OA in the dorsal root recordings was at least in part due to sensitization from repeated noxious stimuli. The differences in OA rates and incidence from our previous publications describing effect of neuritis on axons (Bove, Ransil, Lin, and Leem, 2003; Dilley, Lynn et al., 2005; Dilley and Bove, 2008) are likely due to methodological artifacts. The presence of OA in normal nociceptor neurons, especially from deep structures such as muscle has been contentious for a long time. In two of our (GB) earlier papers, where we studied putatively normal nociceptors from deep paraspinal structures and from cranial dura mater (Bove and Light, 1995; Bove and Moskowitz, 1997), OA was present in the majority of nociceptors, and in the latter paper, the OA rates were higher later in the experiment. In both papers, it was suggested that the OA was likely due to methodological factors. The incidence of OA in muscle nociceptors was also reported to be higher in later recordings (from 66% to 90%). These reports consistently demonstrate that the recording methods induce cause OA, and thus sensitization. Our data from dorsal root recordings reveal two possible types of OA, very slow (peak rate ~0.33 Hz) and slow (peak rate 0.3 Hz). This was suggested both by the bimodality of the rates and because the removal of the very slow OA neurons showed a temporal relationship with inflammation-induced axonal mechanical sensitivity (Dilley and Bove, 2008). Although there were higher rates of OA in these recordings (>0.5 Hz), these only accounted for 11% of neurons with OA. Importantly, in our peripheral nerve recordings where the RF could not contribute to the OA, no neurons exhibited very slow OA. Collectively, these observations support that the very slow OA may come from the receptive field, while the faster rates of OA may come from the lesion. There are few reports that include such slow OA rates. When recording from uninjured nociceptors following injury to neighboring axons (afferent or efferent) in the same nerve, very slow rates of OA have been reported. In one of the studies, the median discharge rate from uninjured nociceptors was 0.04 Hz following injury to neighboring afferent axons (Wu, Ringkamp et al., 2001). In another report, OA rates in uninjured nociceptors were Hz following injury to neighboring efferent axons, and these rates were not different from
7 Bove and Dilley Page 6 Acknowledgments sham-operated animals (Wu, Ringkamp et al., 2002). In another study, the median rate of OA in uninjured nociceptors was not given, but only 3% of neurons had rates 0.07 Hz, with the vast majority of these exhibiting OA 0.02 Hz (Ali, Ringkamp et al., 1999). In each of these studies, sensitizing stimulation to the RF was avoided. When neighboring afferent axons were injured, the median OA rate was <0.02 Hz, but when the axons were also locally inflamed, the median rate increased to 1.8 Hz (Djouhri, Koutsikou et al., 2006). In this study, potentially sensitizing stimuli of the RF were applied throughout the experiments. We also reported OA rates following an L4 lesion while recording from uninjured L5 nociceptors (Dilley and Bove, 2008). The median rate was 0.2 Hz, with 4 neurons below this rate and 10 above this rate. These data are all consistent with our hypothesis that OA may arise from the RF. However, the data are inconsistent with our hypothesis that the very slow OA is due to cumulative noxious stimuli to the RF, since three of the studies avoided mechanical stimulation of the RF. An alternate and attractive hypothesis that would explain these disparate findings is that very slow OA may be due to cumulative antidromic discharge into the RF, resulting in neurogenic inflammation (NI). In all the experiments cited and reported presently, the nerves were repeatedly electrically stimulated at levels suprathreshold to evoke action potentials (see arrows, Fig. 1). This releases neuropeptides into the RF, leading to NI (Jancso, Janscó-Gábor et al., 1967;Kenins, 1981;Kenins, Hurley et al., 1984). It could be that NI is responsible for the very slow OA of nociceptors. Clinical studies have shown OA present in nociceptors during neuropathic pain (Campero, Serra et al., 1998), and have implicated NI as a probable mechanism of spontaneous pain at least in chronic regional pain syndromes (Birklein and Schmelz, 2008). Ongoing activity in sensory elements could be expected to impact the sensory modality of that element. Thus, OA in nociceptors could be expected to lead to the sensation of pain. However, the discharge rate that is necessary to reach perception remains unclear, and definitive studies have not been performed. Using microneurography in humans, which is similar to the methods reported here, Konietzny et al., reported that electrically evoked nociceptor discharge rates as low at 0.5 Hz could evoke pain (Konietzny, Perl et al., 1981). Another human study reported that while nociceptor discharge under 0.2 Hz usually did not evoke pain, 0.4 Hz usually did (Van Hees and Gybels, 1981), consistent with a similar report (Van Hees and Gybels, 1972). These findings are in general consistent with our findings (Fig. 3). Although it thus remains unknown whether very slow levels of OA are significant for pain, any low rate will release neurotransmitters at their synapses in the spinal cord, which may be extensive due to the high degree of branching of primary afferent neurons to spinal cord neurons. Thus, very slow rate discharge from multiple neurons, even with RFs spatially remote from each other, could potentially lead to facilitation of the sensory system. Further inquiry is thus justified before dismissing the very slow rate OA as experimental artifact in studies of primary afferent nociceptors. Moreover, there are numerous potential sites on a sensory neuron where ongoing activity could develop. Determining the site responsible for the OA will inform interpretation, and may direct treatment. It is suggested that future experiments consider these factors in their analyses until we understand more about the nature of OA in nociceptors. Financial support was provided by a grant to GMB by the Samueli Institute, and by National Institutes of Health Grant 5R01AR (GMB) through the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Center for Complementary and Alternative Medicine.
8 Bove and Dilley Page 7 Reference List Ali Z, Ringkamp M, Hartke TV, Chien HF, Flavahan NA, Campbell JN, Meyer RA. Uninjured C-fiber nociceptors develop spontaneous activity and alpha- adrenergic sensitivity following L-6 spinal nerve ligation in monkey. J Neurophysiol 1999;81: [PubMed: ] Berberich P, Hoheisel U, Mense S. Effects of a carrageenan-induced myositis on the discharge properties of group III and IV muscle receptors in the cat. J.Neurophysiol 1988;59: [PubMed: ] Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J.Neurophysiol 1969;32: [PubMed: ] Birklein F, Schmelz M. Neuropeptides, neurogenic inflammation and complex regional pain syndrome (CRPS). Neurosci.Lett 2008;437: [PubMed: ] Bove GM. Focal nerve inflammation induces neuronal signs consistent with symptoms of early complex regional pain syndromes. Exp.Neurol 2009;219: [PubMed: ] Bove GM, Light AR. Unmyelinated nociceptors of rat paraspinal tissues. J.Neurophysiol 1995;73: [PubMed: ] Bove GM, Moskowitz MA. Primary afferent neurons innervating guinea pig dura. J.Neurophysiol 1997;77: [PubMed: ] Bove GM, Ransil BJ, Lin H-C, Leem JG. Inflammation induces ectopic mechanical sensitivity in axons of nociceptors innervating deep tissues. J.Neurophysiol 2003;90: [PubMed: ] Campero M, Serra J, Marchettini P, Ochoa JL. Muscle Nerve 1998;21: [PubMed: ] Cohen RH, Perl ER. Chemical factors in the sensitization of cutaneous nociceptors. Prog.Brain Res 1988;74: [PubMed: ] Dilley A, Bove GM. Resolution of inflammation induced axonal mechanical sensitivity and conduction slowing in C-fiber nociceptors. J.Pain 2008;9: [PubMed: ] Dilley A, Lynn B, Pang SJ. Pressure and stretch mechanosensitivity of peripheral nerve fibres following local inflammation of the nerve trunk. Pain 2005;117: [PubMed: ] Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J.Neurosci 2006;26: [PubMed: ] Eliav E, Benoliel R, Herzberg U, Kalladka M, Tal M. The Role of IL-6 and IL-1beta in Painful Perineural Inflammatory Neuritis. Brain Behav.Immun 2009;24: [PubMed: ] Grigg P, Schaible HG, Schmidt RF. Mechanical sensitivity of group III and IV afferents from posterior articular nerve in normal and inflamed cat knee. J.Neurophysiol 1986;55: [PubMed: ] Jancso N, Janscó-Gábor A, Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br.J.Pharmacol.Chemother 1967;31: [PubMed: ] Kenins P. Identification of the unmyelinated sensory nerves which evoke plasma extravasation in response to antidromic stimulation. Neurosci.Lett 1981;25: [PubMed: ] Kenins P, Hurley JV, Bell C. The role of substance P in the axon reflex in the rat. Br.J.Derm 1984;111: Konietzny F, Perl ER, Trevino D, Light A, Hensel H. Sensory experiences in man evoked by intraneural electrical stimulation of intact cutaneous afferent fibers. Exp.Brain Res 1981;42: [PubMed: ] Perl, ER. Sensitization of nociceptors and its relation to sensation. In: Bonica, JJ.; Albe-Fessard, D., editors. Advances in Pain Research and Therapy. Vol. Vol. 1. Raven Press; New York: p Perl ER, Kumazawa T, Lynn B, Kenins P. Sensitization of high threshold receptors with unmyelinated (C) afferent fibers. Prog.Brain Res 1976;43: [PubMed: ] Schaible HG, Schmidt RF, Willis WD. Convergent inputs from articular, cutaneous and muscle receptors onto ascending tract cells in the cat spinal cord. Exp.Brain Res 1987;66: [PubMed: ]
9 Bove and Dilley Page 8 Strassman AM, Levy D. Response properties of dural nociceptors in relation to headache. J Neurophysiol 2006;95: [PubMed: ] Van Hees J, Gybels J. C nociceptor activity in human nerve following painful and non painful skin stimulation. J.Neurol.Neurosurg.Psychiat 1981;44: [PubMed: ] Van Hees J, Gybels JM. Pain related to single afferent c fibers from human skin. Brain Research 1972;48: [PubMed: ] Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J.Neurosci 2001;21:RC140. [PubMed: ] Wu G, Ringkamp M, Murinson BB, Pogatzki EM, Hartke TV, Weerahandi HM, Campbell JN, Griffin JW, Meyer RA. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J.Neurosci 2002;22: [PubMed: ]
10 Bove and Dilley Page 9 Figure 1. Recording methods. A. Recordings were made from dorsal rootlets, with conduction velocities determined from electrical stimulation of the dorsal root and sciatic nerve (jagged arrow). In these experiments, noxious stimuli were applied to the innervated structures to locate the receptive fields (RF). Ongoing activity (OA) sources include the RF, the neuritis lesion (N), and the cell body. B. Recordings were made from filaments of the sural nerve, and confirmed as sensory units by electrical stimuli of the dorsal roots. The RFs in these experiments could not contribute to OA.
11 Bove and Dilley Page 10 Figure 2. Percentage of neurons exhibiting OA under different recording conditions. There was negligible OA in control neurons when recorded without the contribution of the RF (peripheral nerve recordings), but 35% exhibited OA during neuritis (p<0.0001, Chi-square; all cutaneous neurons with intact RFs, black bars). There were no significant differences in the percentage of neurons with OA when recorded at the dorsal root, between any groups (gray bars).
12 Bove and Dilley Page 11 Figure 3. Ongoing activity rates of neurons recorded from the dorsal roots (A) and peripheral nerve (B).
13 Bove and Dilley Page 12 Figure 4. The effect of neuritis on OA incidence over time, comparing neurons recorded within the first hour (early) to those recorded later in the experiment (late) neurons (see text) When neurons with the slower OA rates (A and C: <0.033 Hz; B and D:<0.5 Hz) are removed, a clear pattern develops for the early-recorded neurons (A, B), revealing that neuritis most likely causes ongoing activity peaking at 1 week and becoming less at 4 and 8 weeks. The data from the later-recorded units (C, D) do not fit any pattern, regardless of whether the very slowly discharging neurons are included. * p = 0.04, One-way Fisher exact test comparing control with one week neuritis.
14 Bove and Dilley Page 13 Figure 5. A. The percentage of neurons exhibiting OA increased with time after the localization of the first unit. These data are all from dorsal root recordings, from all time points, including control and 1, 4, and 8 weeks following the induction of sciatic neuritis. B. Percentage of neurons with OA, recorded at the dorsal root, separated by time during the experiment. More neurons exhibited OA later in the experiment.
A role for uninjured afferents in neuropathic pain
 Acta Physiologica Sinica, October 25, 2008, 60 (5): 605-609 http://www.actaps.com.cn 605 Review A role for uninjured afferents in neuropathic pain Richard A. Meyer 1,2,3,*, Matthias Ringkamp 1 Departments
Acta Physiologica Sinica, October 25, 2008, 60 (5): 605-609 http://www.actaps.com.cn 605 Review A role for uninjured afferents in neuropathic pain Richard A. Meyer 1,2,3,*, Matthias Ringkamp 1 Departments
Biomechanics of Pain: Dynamics of the Neuromatrix
 Biomechanics of Pain: Dynamics of the Neuromatrix Partap S. Khalsa, D.C., Ph.D. Department of Biomedical Engineering The Neuromatrix From: Melzack R (1999) Pain Suppl 6:S121-6. NIOSH STAR Symposium May
Biomechanics of Pain: Dynamics of the Neuromatrix Partap S. Khalsa, D.C., Ph.D. Department of Biomedical Engineering The Neuromatrix From: Melzack R (1999) Pain Suppl 6:S121-6. NIOSH STAR Symposium May
Mechanism of Pain Production
 Mechanism of Pain Production Pain conducting nerve fibers are small myelinated (A-delta) or unmyelinated nerve fibers (C-fibers). Cell bodies are in the dorsal root ganglia (DRG) or sensory ganglia of
Mechanism of Pain Production Pain conducting nerve fibers are small myelinated (A-delta) or unmyelinated nerve fibers (C-fibers). Cell bodies are in the dorsal root ganglia (DRG) or sensory ganglia of
Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007)
 Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007) Introduction Adrian s work on sensory coding Spinal cord and dorsal root ganglia Four somatic sense modalities Touch Mechanoreceptors
Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007) Introduction Adrian s work on sensory coding Spinal cord and dorsal root ganglia Four somatic sense modalities Touch Mechanoreceptors
CHAPTER 10 THE SOMATOSENSORY SYSTEM
 CHAPTER 10 THE SOMATOSENSORY SYSTEM 10.1. SOMATOSENSORY MODALITIES "Somatosensory" is really a catch-all term to designate senses other than vision, hearing, balance, taste and smell. Receptors that could
CHAPTER 10 THE SOMATOSENSORY SYSTEM 10.1. SOMATOSENSORY MODALITIES "Somatosensory" is really a catch-all term to designate senses other than vision, hearing, balance, taste and smell. Receptors that could
Mechanical sensitization of cutaneous sensory fibers in the spared nerve injury mouse model
 Smith et al. Molecular Pain 2013, 9:61 MOLECULAR PAIN SHORT REPORT Open Access Mechanical sensitization of cutaneous sensory fibers in the spared nerve injury mouse model Amanda K Smith, Crystal L O Hara
Smith et al. Molecular Pain 2013, 9:61 MOLECULAR PAIN SHORT REPORT Open Access Mechanical sensitization of cutaneous sensory fibers in the spared nerve injury mouse model Amanda K Smith, Crystal L O Hara
Sensory coding and somatosensory system
 Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
Department of Neurology/Division of Anatomical Sciences
 Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Meninges. Connective tissue membranes
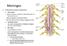 Meninges Connective tissue membranes Dura mater: -outermost layer; continuous with epineuriumof the spinal nerves - dense irregular connective tissue - from the level of the foramen magnum to S2 Arachnoid
Meninges Connective tissue membranes Dura mater: -outermost layer; continuous with epineuriumof the spinal nerves - dense irregular connective tissue - from the level of the foramen magnum to S2 Arachnoid
211MDS Pain theories
 211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
HEAD AND NECK PART 2
 HEAD AND NECK PART 2 INTEGRATED CURRICULUM = Integrate Basic Science and Clinical Training 1- ENT PATIENT EXAM IN ICS COURSE - Today and next week - Review/Preview Anatomy underlying ENT exam 2- NEUROANATOMY/NEUROLOGY
HEAD AND NECK PART 2 INTEGRATED CURRICULUM = Integrate Basic Science and Clinical Training 1- ENT PATIENT EXAM IN ICS COURSE - Today and next week - Review/Preview Anatomy underlying ENT exam 2- NEUROANATOMY/NEUROLOGY
Compound Action Potential, CAP
 Stimulus Strength UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY INTRODUCTION TO NEUROPHYSIOLOGY Spring, 2013 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition
Stimulus Strength UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY INTRODUCTION TO NEUROPHYSIOLOGY Spring, 2013 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition
Sensory information processing, somato-sensory systems
 mm? Sensory information processing, somato-sensory systems Recommended literature 1. Kandel ER, Schwartz JH, Jessel TM (2000) Principles of Neural Science, McGraw-Hill, Ch. xx. 2. Berne EM, Levy MN, Koeppen
mm? Sensory information processing, somato-sensory systems Recommended literature 1. Kandel ER, Schwartz JH, Jessel TM (2000) Principles of Neural Science, McGraw-Hill, Ch. xx. 2. Berne EM, Levy MN, Koeppen
Enhanced formalin nociceptive responses following L5 nerve ligation in the rat reveals neuropathy-induced inflammatory hyperalgesia
 University of Kentucky From the SelectedWorks of Renee R. Donahue 2001 Enhanced formalin nociceptive responses following L5 nerve ligation in the rat reveals neuropathy-induced inflammatory hyperalgesia
University of Kentucky From the SelectedWorks of Renee R. Donahue 2001 Enhanced formalin nociceptive responses following L5 nerve ligation in the rat reveals neuropathy-induced inflammatory hyperalgesia
Human Anatomy. Spinal Cord and Spinal Nerves
 Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
Somatosensation. Recording somatosensory responses. Receptive field response to pressure
 Somatosensation Mechanoreceptors that respond to touch/pressure on the surface of the body. Sensory nerve responds propotional to pressure 4 types of mechanoreceptors: Meissner corpuscles & Merkel discs
Somatosensation Mechanoreceptors that respond to touch/pressure on the surface of the body. Sensory nerve responds propotional to pressure 4 types of mechanoreceptors: Meissner corpuscles & Merkel discs
Somatosensory modalities!
 Somatosensory modalities! The somatosensory system codes five major sensory modalities:! 1. Discriminative touch! 2. Proprioception (body position and motion)! 3. Nociception (pain and itch)! 4. Temperature!
Somatosensory modalities! The somatosensory system codes five major sensory modalities:! 1. Discriminative touch! 2. Proprioception (body position and motion)! 3. Nociception (pain and itch)! 4. Temperature!
Lecture 14: The Spinal Cord
 Lecture 14: The Spinal Cord M/O Chapters 16 69. Describe the relationship(s) between the following structures: root, nerve, ramus, plexus, tract, nucleus, and ganglion. 70. Trace the path of information
Lecture 14: The Spinal Cord M/O Chapters 16 69. Describe the relationship(s) between the following structures: root, nerve, ramus, plexus, tract, nucleus, and ganglion. 70. Trace the path of information
Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon
 Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon Page 22 of 237 Conduct impulses away from cell body Impulses arise from
Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon Page 22 of 237 Conduct impulses away from cell body Impulses arise from
Somatic Sensory System I. Background
 Somatic Sensory System I. Background A. Differences between somatic senses and other senses 1. Receptors are distributed throughout the body as opposed to being concentrated at small, specialized locations
Somatic Sensory System I. Background A. Differences between somatic senses and other senses 1. Receptors are distributed throughout the body as opposed to being concentrated at small, specialized locations
Fig Cervical spinal nerves. Cervical enlargement C7. Dural sheath. Subarachnoid space. Thoracic. Spinal cord Vertebra (cut) spinal nerves
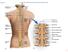 Fig. 13.1 C1 Cervical enlargement C7 Cervical spinal nerves Dural sheath Subarachnoid space Thoracic spinal nerves Spinal cord Vertebra (cut) Lumbar enlargement Medullary cone T12 Spinal nerve Spinal nerve
Fig. 13.1 C1 Cervical enlargement C7 Cervical spinal nerves Dural sheath Subarachnoid space Thoracic spinal nerves Spinal cord Vertebra (cut) Lumbar enlargement Medullary cone T12 Spinal nerve Spinal nerve
Guide to the use of nerve conduction studies (NCS) & electromyography (EMG) for non-neurologists
 Guide to the use of nerve conduction studies (NCS) & electromyography (EMG) for non-neurologists What is NCS/EMG? NCS examines the conduction properties of sensory and motor peripheral nerves. For both
Guide to the use of nerve conduction studies (NCS) & electromyography (EMG) for non-neurologists What is NCS/EMG? NCS examines the conduction properties of sensory and motor peripheral nerves. For both
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves
 Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Group IV Nociceptors Develop Axonal Chemical Sensitivity During Neuritis And Following Treatment Of The Sciatic Nerve With Vinblastine
 University of New England DUNE: DigitalUNE Biomedical Sciences Faculty Publications Biomedical Sciences Faculty Works 2017 Group IV Nociceptors Develop Axonal Chemical Sensitivity During Neuritis And Following
University of New England DUNE: DigitalUNE Biomedical Sciences Faculty Publications Biomedical Sciences Faculty Works 2017 Group IV Nociceptors Develop Axonal Chemical Sensitivity During Neuritis And Following
HUMAN MOTOR CONTROL. Emmanuel Guigon
 HUMAN MOTOR CONTROL Emmanuel Guigon Institut des Systèmes Intelligents et de Robotique Université Pierre et Marie Curie CNRS / UMR 7222 Paris, France emmanuel.guigon@upmc.fr e.guigon.free.fr/teaching.html
HUMAN MOTOR CONTROL Emmanuel Guigon Institut des Systèmes Intelligents et de Robotique Université Pierre et Marie Curie CNRS / UMR 7222 Paris, France emmanuel.guigon@upmc.fr e.guigon.free.fr/teaching.html
Peripheral Nervous System
 Peripheral Nervous System 1 Sensory Receptors Sensory Receptors and Sensation Respond to changes (stimuli) in the environment Generate graded potentials that can trigger an action potential that is carried
Peripheral Nervous System 1 Sensory Receptors Sensory Receptors and Sensation Respond to changes (stimuli) in the environment Generate graded potentials that can trigger an action potential that is carried
Note: Please refer to handout Spinal Plexuses and Representative Spinal Nerves for
 Chapter 13 Outline Note: Please refer to handout Spinal Plexuses and Representative Spinal Nerves for what you need to know from Exhibits 13.1 13.4 I. INTRODUCTION A. The spinal cord and spinal nerves
Chapter 13 Outline Note: Please refer to handout Spinal Plexuses and Representative Spinal Nerves for what you need to know from Exhibits 13.1 13.4 I. INTRODUCTION A. The spinal cord and spinal nerves
(Received 10 April 1956)
 446 J. Physiol. (I956) I33, 446-455 A COMPARISON OF FLEXOR AND EXTENSOR REFLEXES OF MUSCULAR ORIGIN BY M. G. F. FUORTES AND D. H. HUBEL From the Department ofneurophysiology, Walter Reed Army Institute
446 J. Physiol. (I956) I33, 446-455 A COMPARISON OF FLEXOR AND EXTENSOR REFLEXES OF MUSCULAR ORIGIN BY M. G. F. FUORTES AND D. H. HUBEL From the Department ofneurophysiology, Walter Reed Army Institute
Chapter 17 Nervous System
 Chapter 17 Nervous System 1 The Nervous System Two Anatomical Divisions Central Nervous System (CNS) Brain and Spinal Cord Peripheral Nervous System (PNS) Two Types of Cells Neurons Transmit nerve impulses
Chapter 17 Nervous System 1 The Nervous System Two Anatomical Divisions Central Nervous System (CNS) Brain and Spinal Cord Peripheral Nervous System (PNS) Two Types of Cells Neurons Transmit nerve impulses
Gross Anatomy of Lower Spinal Cord
 Chapter 13 Spinal Cord, Spinal Nerves and Somatic Reflexes Spinal cord Spinal nerves Somatic reflexes Gross Anatomy of Lower Spinal Cord Meninges of Vertebra & Spinal Cord Spina Bifida Congenital defect
Chapter 13 Spinal Cord, Spinal Nerves and Somatic Reflexes Spinal cord Spinal nerves Somatic reflexes Gross Anatomy of Lower Spinal Cord Meninges of Vertebra & Spinal Cord Spina Bifida Congenital defect
Chapter 9. Nervous System
 Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Electrophysiological Assessment of the Cutaneous Arborization of A -Fiber Nociceptors
 Electrophysiological Assessment of the Cutaneous Arborization of A -Fiber Nociceptors YUAN B. PENG, 1 MATTHIAS RINGKAMP, 1 JAMES N. CAMPBELL, 1,2 AND RICHARD A. MEYER 1,2 Departments of 1 Neurosurgery
Electrophysiological Assessment of the Cutaneous Arborization of A -Fiber Nociceptors YUAN B. PENG, 1 MATTHIAS RINGKAMP, 1 JAMES N. CAMPBELL, 1,2 AND RICHARD A. MEYER 1,2 Departments of 1 Neurosurgery
The Somatosensory System
 The Somatosensory System Reading: BCP Chapter 12 cerebrovortex.com Divisions of the Somatosensory System Somatosensory System Exteroceptive External stimuli Proprioceptive Body position Interoceptive Body
The Somatosensory System Reading: BCP Chapter 12 cerebrovortex.com Divisions of the Somatosensory System Somatosensory System Exteroceptive External stimuli Proprioceptive Body position Interoceptive Body
Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit. Supplementary Information. Adam W. Hantman and Thomas M.
 Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit Supplementary Information Adam W. Hantman and Thomas M. Jessell Supplementary Results Characterizing the origin of primary
Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit Supplementary Information Adam W. Hantman and Thomas M. Jessell Supplementary Results Characterizing the origin of primary
NERVOUS SYSTEM ANATOMY
 INTRODUCTION to NERVOUS SYSTEM ANATOMY M1 - Gross and Developmental Anatomy Dr. Milton M. Sholley Professor of Anatomy and Neurobiology and Dr. Michael H. Peters Professor of Chemical and Life Science
INTRODUCTION to NERVOUS SYSTEM ANATOMY M1 - Gross and Developmental Anatomy Dr. Milton M. Sholley Professor of Anatomy and Neurobiology and Dr. Michael H. Peters Professor of Chemical and Life Science
Skin types: hairy and glabrous (e.g. back vs. palm of hand)
 Lecture 19 revised 03/10 The Somatic Sensory System Skin- the largest sensory organ we have Also protects from evaporation, infection. Skin types: hairy and glabrous (e.g. back vs. palm of hand) 2 major
Lecture 19 revised 03/10 The Somatic Sensory System Skin- the largest sensory organ we have Also protects from evaporation, infection. Skin types: hairy and glabrous (e.g. back vs. palm of hand) 2 major
NERVOUS SYSTEM ANATOMY
 NTRODUCTON to NERVOUS SYSTEM ANATOMY M1 - Gross and Developmental Anatomy Dr. Milton M. Sholley Professor of Anatomy and Neurobiology and Dr. Michael H. Peters Professor of Chemical and Life Science Engineering
NTRODUCTON to NERVOUS SYSTEM ANATOMY M1 - Gross and Developmental Anatomy Dr. Milton M. Sholley Professor of Anatomy and Neurobiology and Dr. Michael H. Peters Professor of Chemical and Life Science Engineering
ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 7 THE NERVOUS SYSTEM
 ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 7 THE NERVOUS SYSTEM Introduction The nervous system is the major controlling, regulatory, and communicating system in the body. It is the center of all mental
ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 7 THE NERVOUS SYSTEM Introduction The nervous system is the major controlling, regulatory, and communicating system in the body. It is the center of all mental
Alterations in Synaptic Strength Preceding Axon Withdrawal
 Alterations in Synaptic Strength Preceding Axon Withdrawal H. Colman, J. Nabekura, J.W. Lichtman presented by Ana Fiallos Synaptic Transmission at the Neuromuscular Junction Motor neurons with cell bodies
Alterations in Synaptic Strength Preceding Axon Withdrawal H. Colman, J. Nabekura, J.W. Lichtman presented by Ana Fiallos Synaptic Transmission at the Neuromuscular Junction Motor neurons with cell bodies
Bi/CNS/NB 150: Neuroscience. November 11, 2015 SOMATOSENSORY SYSTEM. Ralph Adolphs
 Bi/CNS/NB 150: Neuroscience November 11, 2015 SOMATOSENSORY SYSTEM Ralph Adolphs 1 Menu for today Touch -peripheral -central -plasticity Pain 2 Sherrington (1948): senses classified as --teloreceptive
Bi/CNS/NB 150: Neuroscience November 11, 2015 SOMATOSENSORY SYSTEM Ralph Adolphs 1 Menu for today Touch -peripheral -central -plasticity Pain 2 Sherrington (1948): senses classified as --teloreceptive
Received 26 April 2004; revised 21 August 2004; accepted 20 September 2004 Available online 19 November 2004
 Experimental Neurology 191 (2005) 128 136 www.elsevier.com/locate/yexnr Ectopic discharges from injured nerve fibers are highly correlated with tactile allodynia only in early, but not late, stage in rats
Experimental Neurology 191 (2005) 128 136 www.elsevier.com/locate/yexnr Ectopic discharges from injured nerve fibers are highly correlated with tactile allodynia only in early, but not late, stage in rats
Spinal Cord- Medulla Spinalis. Cuneyt Mirzanli Istanbul Gelisim University
 Spinal Cord- Medulla Spinalis Cuneyt Mirzanli Istanbul Gelisim University Spinal Column Supports the skull, pectoral girdle, upper limbs and thoracic cage by way of the pelvic girdle. Transmits body weight
Spinal Cord- Medulla Spinalis Cuneyt Mirzanli Istanbul Gelisim University Spinal Column Supports the skull, pectoral girdle, upper limbs and thoracic cage by way of the pelvic girdle. Transmits body weight
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress REDUCING THE PAIN FACTOR AN UPDATE ON PERI-OPERATIVE ANALGESIA Sandra Forysth, BVSc DipACVA Institute of Veterinary,
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress REDUCING THE PAIN FACTOR AN UPDATE ON PERI-OPERATIVE ANALGESIA Sandra Forysth, BVSc DipACVA Institute of Veterinary,
12 Anatomy and Physiology of Peripheral Nerves
 12 Anatomy and Physiology of Peripheral Nerves Introduction Anatomy Classification of Peripheral Nerves Sensory Nerves Motor Nerves Pathologies of Nerves Focal Injuries Regeneration of Injured Nerves Signs
12 Anatomy and Physiology of Peripheral Nerves Introduction Anatomy Classification of Peripheral Nerves Sensory Nerves Motor Nerves Pathologies of Nerves Focal Injuries Regeneration of Injured Nerves Signs
Lecture VIII. The Spinal Cord, Reflexes and Brain Pathways!
 Reflexes and Brain Bio 3411! Monday!! 1! Readings! NEUROSCIENCE 5 th ed: Review Chapter 1 pp. 11-21;!!Read Chapter 9 pp. 189-194, 198! THE BRAIN ATLAS 3 rd ed:! Read pp. 4-17 on class web site! Look at
Reflexes and Brain Bio 3411! Monday!! 1! Readings! NEUROSCIENCE 5 th ed: Review Chapter 1 pp. 11-21;!!Read Chapter 9 pp. 189-194, 198! THE BRAIN ATLAS 3 rd ed:! Read pp. 4-17 on class web site! Look at
Section 4. Intro to Neurophysiology
 Section 4. Intro to Neurophysiology 4.1 Action potentials at work (Cockroach Receptive Fields) Overview The goals of this unit are to: 1) introduce you to the basic concepts, equipment, and methodology
Section 4. Intro to Neurophysiology 4.1 Action potentials at work (Cockroach Receptive Fields) Overview The goals of this unit are to: 1) introduce you to the basic concepts, equipment, and methodology
What is pain?: An unpleasant sensation. What is an unpleasant sensation?: Pain. - Aristotle.
 What is pain?: An unpleasant sensation. What is an unpleasant sensation?: Pain. - Aristotle. Nociception The detection of tissue damage or impending tissue damage, but There can be tissue damage without
What is pain?: An unpleasant sensation. What is an unpleasant sensation?: Pain. - Aristotle. Nociception The detection of tissue damage or impending tissue damage, but There can be tissue damage without
Chapter 16. Sense of Pain
 Chapter 16 Sense of Pain Pain Discomfort caused by tissue injury or noxious stimulation, and typically leading to evasive action important /// helps to protect us lost of pain in diabetes mellitus = diabetic
Chapter 16 Sense of Pain Pain Discomfort caused by tissue injury or noxious stimulation, and typically leading to evasive action important /// helps to protect us lost of pain in diabetes mellitus = diabetic
Spinal nerves. Aygul Shafigullina. Department of Morphology and General Pathology
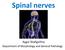 Spinal nerves Aygul Shafigullina Department of Morphology and General Pathology Spinal nerve a mixed nerve, formed in the vicinity of an intervertebral foramen, where fuse a dorsal root and a ventral root,
Spinal nerves Aygul Shafigullina Department of Morphology and General Pathology Spinal nerve a mixed nerve, formed in the vicinity of an intervertebral foramen, where fuse a dorsal root and a ventral root,
Our senses provide us with wonderful capabilities. If you had to lose one, which would it be?
 Our senses provide us with wonderful capabilities. If you had to lose one, which would it be? Neurological disorders take away sensation without a choice! http://neuroscience.uth.tmc.edu/s2/chapter04.html
Our senses provide us with wonderful capabilities. If you had to lose one, which would it be? Neurological disorders take away sensation without a choice! http://neuroscience.uth.tmc.edu/s2/chapter04.html
How strong is it? What is it? Where is it? What must sensory systems encode? 9/8/2010. Spatial Coding: Receptive Fields and Tactile Discrimination
 Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Spatial Coding: Receptive Fields and Tactile Discrimination
 Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Pain Pathways. Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH
 Pain Pathways Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH Objective To give you a simplistic and basic concepts of pain pathways to help understand the complex issue of pain Pain
Pain Pathways Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH Objective To give you a simplistic and basic concepts of pain pathways to help understand the complex issue of pain Pain
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE
 Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Sensory Assessment of Regional Analgesia in Humans
 REVIEW ARTICLE Dennis M. Fisher, M.D., Editor-in-Chief Anesthesiology 2000; 93:1517 30 2000 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Sensory Assessment of Regional
REVIEW ARTICLE Dennis M. Fisher, M.D., Editor-in-Chief Anesthesiology 2000; 93:1517 30 2000 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Sensory Assessment of Regional
Neural Integration I: Sensory Pathways and the Somatic Nervous System
 15 Neural Integration I: Sensory Pathways and the Somatic Nervous System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to Sensory Pathways and
15 Neural Integration I: Sensory Pathways and the Somatic Nervous System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to Sensory Pathways and
10.1: Introduction. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial cells) Dendrites.
 10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
Synapse Homework. Back page last question not counted. 4 pts total, each question worth 0.18pts. 26/34 students answered correctly!
 Synapse Homework Back page last question not counted 26/34 students answered correctly! 4 pts total, each question worth 0.18pts Business TASS hours extended! MWF 1-2pm, Willamette 204 T and Th 9:30-10:30am,
Synapse Homework Back page last question not counted 26/34 students answered correctly! 4 pts total, each question worth 0.18pts Business TASS hours extended! MWF 1-2pm, Willamette 204 T and Th 9:30-10:30am,
Chapter 13: The Spinal Cord and Spinal Nerves
 Chapter 13: The Spinal Cord and Spinal Nerves Spinal Cord Anatomy Protective structures: Vertebral column and the meninges protect the spinal cord and provide physical stability. a. Dura mater, b. Arachnoid,
Chapter 13: The Spinal Cord and Spinal Nerves Spinal Cord Anatomy Protective structures: Vertebral column and the meninges protect the spinal cord and provide physical stability. a. Dura mater, b. Arachnoid,
Fundamentals of the Nervous System and Nervous Tissue. Nervous System. Basic Divisions of the Nervous System C H A P T E R 12.
 C H A P T E R 12 Fundamentals of the Nervous System and Nervous Tissue Nervous System Sensory input Integration Motor output Figure 12.1 Basic Divisions of the Nervous System Brain CNS Spinal cord Nerves
C H A P T E R 12 Fundamentals of the Nervous System and Nervous Tissue Nervous System Sensory input Integration Motor output Figure 12.1 Basic Divisions of the Nervous System Brain CNS Spinal cord Nerves
Capsaicin Responses in Heat-Sensitive and Heat-Insensitive A-Fiber Nociceptors
 The Journal of Neuroscience, June 15, 2001, 21(12):4460 4468 Capsaicin Responses in Heat-Sensitive and Heat-Insensitive A-Fiber Nociceptors Matthias Ringkamp, 1 Yuan B. Peng, 1 Gang Wu, 1 Timothy V. Hartke,
The Journal of Neuroscience, June 15, 2001, 21(12):4460 4468 Capsaicin Responses in Heat-Sensitive and Heat-Insensitive A-Fiber Nociceptors Matthias Ringkamp, 1 Yuan B. Peng, 1 Gang Wu, 1 Timothy V. Hartke,
SUPPLEMENTARY INFORMATION. Supplementary Figure 1
 SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
Male circumcision decreases
 Male circumcision decreases penile sensitivity Justine Schober MD University of Pittsburgh Medical Center Hamot Hospital, Erie PA Rockefeller University, New York, NY Anatomy is not destiny but a little
Male circumcision decreases penile sensitivity Justine Schober MD University of Pittsburgh Medical Center Hamot Hospital, Erie PA Rockefeller University, New York, NY Anatomy is not destiny but a little
MANAGING CHRONIC PAIN
 George Hardas MANAGING CHRONIC PAIN The guide to understanding chronic pain and how to manage it. George Hardas MMed (UNSW) MScMed (Syd) MChiro (Macq) BSc (Syd) Grad Cert Pain Management (Syd) Cognitive
George Hardas MANAGING CHRONIC PAIN The guide to understanding chronic pain and how to manage it. George Hardas MMed (UNSW) MScMed (Syd) MChiro (Macq) BSc (Syd) Grad Cert Pain Management (Syd) Cognitive
by mapping the spinal cord in the rostro-caudal axis while recording cord dorsum
 J. Physiol. (1984), 357, pp. 357-368 357 With 8 text-figures Printed in Great Britain DORSAL ROOT POTENTIALS ARE UNCHANGED IN ADULT RATS TREATED AT BIRTH WITH CAPSAICIN BY F. CERVERO AND M. B. PLENDERLEITH
J. Physiol. (1984), 357, pp. 357-368 357 With 8 text-figures Printed in Great Britain DORSAL ROOT POTENTIALS ARE UNCHANGED IN ADULT RATS TREATED AT BIRTH WITH CAPSAICIN BY F. CERVERO AND M. B. PLENDERLEITH
Pain. Pain. Pain: One definition. Pain: One definition. Pain: One definition. Pain: One definition. Psyc 2906: Sensation--Introduction 9/27/2006
 Pain Pain Pain: One Definition Classic Paths A new Theory Pain and Drugs According to the international Association for the Study (Merskey & Bogduk, 1994), Pain is an unpleasant sensory and emotional experience
Pain Pain Pain: One Definition Classic Paths A new Theory Pain and Drugs According to the international Association for the Study (Merskey & Bogduk, 1994), Pain is an unpleasant sensory and emotional experience
Nervous system Reflexes and Senses
 Nervous system Reflexes and Senses Physiology Lab-4 Wrood Slaim, MSc Department of Pharmacology and Toxicology University of Al-Mustansyria 2017-2018 Nervous System The nervous system is the part of an
Nervous system Reflexes and Senses Physiology Lab-4 Wrood Slaim, MSc Department of Pharmacology and Toxicology University of Al-Mustansyria 2017-2018 Nervous System The nervous system is the part of an
J. Physiol. (I957) I35, (Received 20 July 1956) The interpretation ofthe experimental results ofthe preceding paper (Matthews
 263 J. Physiol. (I957) I35, 263-269 THE RELATIVE SENSITIVITY OF MUSCLE NERVE FIBRES TO PROCAINE BY PETER B. C. MATTHEWS AND GEOFFREY RUSHWORTH From the Laboratory of Physiology, University of Oxford (Received
263 J. Physiol. (I957) I35, 263-269 THE RELATIVE SENSITIVITY OF MUSCLE NERVE FIBRES TO PROCAINE BY PETER B. C. MATTHEWS AND GEOFFREY RUSHWORTH From the Laboratory of Physiology, University of Oxford (Received
Human Anatomy and Physiology I Laboratory Spinal and Peripheral Nerves and Reflexes
 Human Anatomy and Physiology I Laboratory Spinal and Peripheral Nerves and Reflexes 1 This lab involves the second section of the exercise Spinal Cord, Spinal Nerves, and the Autonomic Nervous System,
Human Anatomy and Physiology I Laboratory Spinal and Peripheral Nerves and Reflexes 1 This lab involves the second section of the exercise Spinal Cord, Spinal Nerves, and the Autonomic Nervous System,
ANAT2010. Concepts of Neuroanatomy (II) S2 2018
 ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
Nerve Conduction Studies NCS
 Nerve Conduction Studies NCS Nerve conduction studies are an essential part of an EMG examination. The clinical usefulness of NCS in the diagnosis of diffuse and local neuropathies has been thoroughly
Nerve Conduction Studies NCS Nerve conduction studies are an essential part of an EMG examination. The clinical usefulness of NCS in the diagnosis of diffuse and local neuropathies has been thoroughly
Chapter 12b. Overview
 Chapter 12b Spinal Cord Overview Spinal cord gross anatomy Spinal meninges Sectional anatomy Sensory pathways Motor pathways Spinal cord pathologies 1 The Adult Spinal Cord About 18 inches (45 cm) long
Chapter 12b Spinal Cord Overview Spinal cord gross anatomy Spinal meninges Sectional anatomy Sensory pathways Motor pathways Spinal cord pathologies 1 The Adult Spinal Cord About 18 inches (45 cm) long
Nervous system. The main regulation mechanism of organism's functions
 Nervous system The main regulation mechanism of organism's functions Questions Neuron The reflex arc The nervous centers Properties of the nervous centers The general principles of coordination Inhibition
Nervous system The main regulation mechanism of organism's functions Questions Neuron The reflex arc The nervous centers Properties of the nervous centers The general principles of coordination Inhibition
Learning Modules - Medical Gross Anatomy Nervous System Overview - Page 1 of 14
 Nervous System Overview - Page 1 of 14 Overview of the Nervous System Every minute of every day, your nervous system is sending and receiving countless messages about what is happening both inside and
Nervous System Overview - Page 1 of 14 Overview of the Nervous System Every minute of every day, your nervous system is sending and receiving countless messages about what is happening both inside and
Nerve Conduction Studies NCS
 Nerve Conduction Studies NCS Nerve conduction studies are an essential part of an EMG examination. The clinical usefulness of NCS in the diagnosis of diffuse and local neuropathies has been thoroughly
Nerve Conduction Studies NCS Nerve conduction studies are an essential part of an EMG examination. The clinical usefulness of NCS in the diagnosis of diffuse and local neuropathies has been thoroughly
NERVOUS SYSTEM. Academic Resource Center. Forskellen mellem oscillator og krystal
 NERVOUS SYSTEM Academic Resource Center Forskellen mellem oscillator og krystal Overview of the Nervous System Peripheral nervous system-pns cranial nerves spinal nerves ganglia peripheral nerves enteric
NERVOUS SYSTEM Academic Resource Center Forskellen mellem oscillator og krystal Overview of the Nervous System Peripheral nervous system-pns cranial nerves spinal nerves ganglia peripheral nerves enteric
Receptors and Neurotransmitters: It Sounds Greek to Me. Agenda. What We Know About Pain 9/7/2012
 Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
POSTSYNAPTIC INHIBITION OF CRAYFISH TONIC FLEXOR MOTOR NEURONES BY ESCAPE COMMANDS
 J. exp. Biol. (1980), 85, 343-347 343 With a figures Printed in Great Britain POSTSYNAPTIC INHIBITION OF CRAYFISH TONIC FLEXOR MOTOR NEURONES BY ESCAPE COMMANDS BY J. Y. KUWADA, G. HAGIWARA AND J. J. WINE
J. exp. Biol. (1980), 85, 343-347 343 With a figures Printed in Great Britain POSTSYNAPTIC INHIBITION OF CRAYFISH TONIC FLEXOR MOTOR NEURONES BY ESCAPE COMMANDS BY J. Y. KUWADA, G. HAGIWARA AND J. J. WINE
-12. -Osama Asa ad Khader. -Ameera Almomani. - Loay Zgoul
 -12 -Osama Asa ad Khader -Ameera Almomani - Loay Zgoul In this lecture we are going to talk about dermatomes, visceral sensation and pain disorders. Sorry for any mistakes though I tried my best to include
-12 -Osama Asa ad Khader -Ameera Almomani - Loay Zgoul In this lecture we are going to talk about dermatomes, visceral sensation and pain disorders. Sorry for any mistakes though I tried my best to include
The Nervous System. Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output
 The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
Hole s Human Anatomy and Physiology Tenth Edition. Chapter 10
 PowerPoint Lecture Outlines to accompany Hole s Human Anatomy and Physiology Tenth Edition Shier Butler Lewis Chapter 10 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or
PowerPoint Lecture Outlines to accompany Hole s Human Anatomy and Physiology Tenth Edition Shier Butler Lewis Chapter 10 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or
Nervous System. Master controlling and communicating system of the body. Secrete chemicals called neurotransmitters
 Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Chapter 13. The Spinal Cord & Spinal Nerves. Spinal Cord. Spinal Cord Protection. Meninges. Together with brain forms the CNS Functions
 Spinal Cord Chapter 13 The Spinal Cord & Spinal Nerves Together with brain forms the CNS Functions spinal cord reflexes integration (summation of inhibitory and excitatory) nerve impulses highway for upward
Spinal Cord Chapter 13 The Spinal Cord & Spinal Nerves Together with brain forms the CNS Functions spinal cord reflexes integration (summation of inhibitory and excitatory) nerve impulses highway for upward
Pathways of proprioception
 The Autonomic Nervous Assess Prof. Fawzia Al-Rouq Department of Physiology College of Medicine King Saud University Pathways of proprioception System posterior column& Spinocerebellar Pathways https://www.youtube.com/watch?v=pmeropok6v8
The Autonomic Nervous Assess Prof. Fawzia Al-Rouq Department of Physiology College of Medicine King Saud University Pathways of proprioception System posterior column& Spinocerebellar Pathways https://www.youtube.com/watch?v=pmeropok6v8
Anatomy and Physiology 1 Chapters 12 and 13 self quiz Pro, Dima Darwish,MD.
 Anatomy and Physiology 1 Chapters 12 and 13 self quiz Pro, Dima Darwish,MD. 1) Which of the following is a function of the nervous system? A) sense the internal and external environments B) integrate sensory
Anatomy and Physiology 1 Chapters 12 and 13 self quiz Pro, Dima Darwish,MD. 1) Which of the following is a function of the nervous system? A) sense the internal and external environments B) integrate sensory
Chapter 13 PNS and reflex activity
 Chapter 13 PNS and reflex activity I. Peripheral nervous system A. PNS links CNS to the body B. Sensory: the afferent division C. Motor: the efferent division D. Ganglia: collections of cell bodies in
Chapter 13 PNS and reflex activity I. Peripheral nervous system A. PNS links CNS to the body B. Sensory: the afferent division C. Motor: the efferent division D. Ganglia: collections of cell bodies in
Coding of Sensory Information
 Coding of Sensory Information 22 November, 2016 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Sensory Systems Mediate Four Attributes of
Coding of Sensory Information 22 November, 2016 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Sensory Systems Mediate Four Attributes of
The Nervous System PART A
 7 The Nervous System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Structural Classification
7 The Nervous System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Structural Classification
ANAT2010. Concepts of Neuroanatomy (II) S2 2018
 ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
Nervous System C H A P T E R 2
 Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
MYOFASCIAL PAIN. Dr. Janet Travell ( ) credited with bringing MTrPs to the attention of healthcare providers.
 Myofascial Trigger Points background info Laurie Edge-Hughes BScPT, MAnimSt (Animal Physio), CAFCI, CCRT History lesson Dr. Janet Travell (1901 1997) credited with bringing MTrPs to the attention of healthcare
Myofascial Trigger Points background info Laurie Edge-Hughes BScPT, MAnimSt (Animal Physio), CAFCI, CCRT History lesson Dr. Janet Travell (1901 1997) credited with bringing MTrPs to the attention of healthcare
The Pain System The Neural Basis of Nociceptive Transmission in the Mammalian Nervous System
 The Pain System The Neural Basis of Nociceptive Transmission in the Mammalian Nervous System Pain and Headache Vol. 8 Series Editor Philip L. Gildenberg, Houston, Tex. KARGER S.Karger Basel Miinchen: Paris
The Pain System The Neural Basis of Nociceptive Transmission in the Mammalian Nervous System Pain and Headache Vol. 8 Series Editor Philip L. Gildenberg, Houston, Tex. KARGER S.Karger Basel Miinchen: Paris
Impact of Demyelination Disease on Neuronal Networks
 Impact of Demyelination Disease on Neuronal Networks Sandeep Adem Chiyuan Chang Mark Fleming sadem@eng.ucsd.edu chc418@eng.ucsd.edu m3flemin@eng.ucsd.edu 1. Abstract Demyelination has a detrimental impact
Impact of Demyelination Disease on Neuronal Networks Sandeep Adem Chiyuan Chang Mark Fleming sadem@eng.ucsd.edu chc418@eng.ucsd.edu m3flemin@eng.ucsd.edu 1. Abstract Demyelination has a detrimental impact
Omar Sami. Muhammad Abid. Muhammad khatatbeh
 10 Omar Sami Muhammad Abid Muhammad khatatbeh Let s shock the world In this lecture we are going to cover topics said in previous lectures and then start with the nerve cells (neurons) and the synapses
10 Omar Sami Muhammad Abid Muhammad khatatbeh Let s shock the world In this lecture we are going to cover topics said in previous lectures and then start with the nerve cells (neurons) and the synapses
ANATOMY OF SPINAL CORD. Khaleel Alyahya, PhD, MEd King Saud University School of
 ANATOMY OF SPINAL CORD Khaleel Alyahya, PhD, MEd King Saud University School of Medicine @khaleelya OBJECTIVES At the end of the lecture, students should be able to: Describe the external anatomy of the
ANATOMY OF SPINAL CORD Khaleel Alyahya, PhD, MEd King Saud University School of Medicine @khaleelya OBJECTIVES At the end of the lecture, students should be able to: Describe the external anatomy of the
SENSORY NERVOUS SYSTEM & SENSORY RECEPTORS. Dr. Ayisha Qureshi Professor MBBS, MPhil
 SENSORY NERVOUS SYSTEM & SENSORY RECEPTORS Dr. Ayisha Qureshi Professor MBBS, MPhil Sensory Deprivation Tank Is the world really as we perceive it? Is the world really as we perceive it? NO. The world
SENSORY NERVOUS SYSTEM & SENSORY RECEPTORS Dr. Ayisha Qureshi Professor MBBS, MPhil Sensory Deprivation Tank Is the world really as we perceive it? Is the world really as we perceive it? NO. The world
Response of Cutaneous A- and C-Fiber Nociceptors in the Monkey to Controlled-Force Stimuli
 Response of Cutaneous A- and C-Fiber Nociceptors in the Monkey to Controlled-Force Stimuli R. M. SLUGG, 1 R. A. MEYER, 1,2 AND J. N. CAMPBELL 1,2 1 Department of Neurosurgery and 2 Applied Physics Laboratory,
Response of Cutaneous A- and C-Fiber Nociceptors in the Monkey to Controlled-Force Stimuli R. M. SLUGG, 1 R. A. MEYER, 1,2 AND J. N. CAMPBELL 1,2 1 Department of Neurosurgery and 2 Applied Physics Laboratory,
