Oncological emergencies: Tumor lysis syndrome
|
|
|
- Naomi George
- 6 years ago
- Views:
Transcription
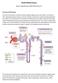
1 PRACE POGLĄDOWE Joanna Sobota Mirosława Püsküllüoğlu Aneta L. Zygulska Oncological emergencies: Tumor lysis syndrome Stany nagłe w onkologii: Zespół rozpadu guza Klinika Onkologii Szpitala Uniwersyteckiego w Krakowie Kierownik: Dr hab. med. Krzysztof Krzemieniecki Dodatkowe słowa kluczowe: zespół rozpadu guza stany nagłe w onkologii wytyczne rasburykaza Additional key words: tumor lysis syndrome oncological emergencies guidelines rasburicase Tumor lysis syndrome (TLS) occurs as a result of massive lysis of malignant cells and release of intracellular contents into the systemic circulation. It can lead to hyperuricaemia, hyperkalaemia, hyperphosphataemia and hypocalcaemia. TLS is most commonly present after initiation of anticancer therapy but it can also develop spontaneously (STLS spontaneous tumor lysis syndrome). In the article, pathophysiology, classification, risk factors and recommendations of management in TLS, with a special focus on solid tumors, are discussed. The keys to the identification of high-risk patients, prevention and management of TLS are included in presented guidelines. Zespół rozpadu guza pojawia się w wyniku masywnej lizy komórek nowotworowych i uwolnienia ich zawartości do krwi. Może to prowadzić do hyperurykemii, hyperkaliemii, hyperfosfatemii i hypokalcemii. Jest on najczęściej powikłaniem leczenia przeciwnowotworowego, ale może również rozwinąć się spontanicznie. W artykule przedstawiono patofizjologię, podział, czynniki ryzyka i zalecenia dotyczące zespołu rozpadu guza, zwracając uwagę na jego występowanie w przypadku guzów litych. Zasady klasyfikacji grup ryzyka, prewencji i leczenia są zawarte w cytowanych wytycznych. Adres do korespondencji: ul. Śniadeckich 10, IIp., Kraków tel , fax joanna.sobota.wl@gmail.com What is the tumor lysis syndrome? Tumor lysis syndrome (TLS) is an oncological emergency. It is caused by the abrupt lysis of a large amount of malignant cells and the release of cellular constituents into the bloodstream. The massive release of nucleic acids, potassium, phosphorus and proteins can result in metabolic disturbances which may have severe clinical complications [1,2]. TLS leads to the death in % of cases if undiagnosed or if diagnosed too late [3]. TLS has been recognized in association with leukaemia therapy since the 1870s. According to Malaguarnera and colleagues it was first reported by Bedrna and Polcák in adult patients with chronic leukaemia treated with irradiation in 1929 [4]. Etiology TLS most often occurs after the initiation of cytoreductive treatment (mainly chemotherapy) [1,5]. It is one of the most frequent emergencies in oncohematology [1,2]. TLS may also occur in solid tumors, especially those with a high proliferative rate and high sensitivity to anticancer therapy e.g. neuroblastoma, germ cell tumors or small cell lung cancer [6]. Occasionally, it was observed in solid malignancies with poor response to antineoplastic agents like malignant melanoma, hepatocellular carcinoma, non-small cell lung cancer and carcinoma of the vulva [8]. The number of cases TLS in solid tumors seems to grow over the last decade. It is likely due to the availability of more effective anticancer therapies and an insufficient use of prophylactic regiments [9]. Differences in TLS between hematological malignancies and solid tumors are presented in Table I [8]. The most important risk factors for TLS are included in Table II [10]. The latest TLS risk classification system is presented in Table III [6]. In LRD (low risk disease), an approximate risk of developing TLS is less than 1%. In IRD (intermediate risk disease), the risk is approximately 1 5 %. In HRD (high risk disease), the risk exceeds 5%. In the case of renal dysfunction, patients with LRD become IRD. Patients originally with IRD are classified as HRD if renal dysfunction is noticed or if uric acid, phosphate or potassium levels are elevated [6]. Spontaneous tumor lysis syndrome (STLS), occurring in the absence of anticancer therapy is rare, but may have a worse prognosis due to delayed diagnosis [11,12]. It can also be the first manifestation of malignancy [13,14]. The etiology of STLS is not fully known. Various hypotheses are taken into consideration, e.g.: increased production of glucocorticoids, hypoperfusion of the tumor (worsened by pressure from the surrounding tissues or by hypovolaemia), hyperthermia or even surgical treatment of the tumor [11,13-15]. A disappearance of lymphadenopathy or an occurrence of necrosis in the tumor may be a clinical sign of STLS [11,12,15]. Pathophysiology The rapid release of intracellular contents of malignant cells can overwhelm the excretory capacity of the kidneys and cause electrolyte and metabolic disturbances [16]. Hyperuricaemia usually occurs hours after initiation of anticancer treatment [16]. Purine nucleic acids are released from broken tumor cells and metabolized into uric acid [2]. In Figure 1, a scheme of uric acid formation is presented. Due to the acidic ph and high concentration of uric acid, crystals are formed in the renal collecting ducts. Ob- 210 J. Sobota i wsp.
2 struction of urine flow by these crystals leads to uric acid nephropathy and acute renal failure. Other factors which can promote acute renal failure in TLS are: volume depletion, precipitation of xanthine and/or calcium phosphate crystals, crystal-independent mechanisms, cytokine-mediated alteration in renal perfusion, renal vasoconstriction as a result of e.g. release of adenosine from malignant cells [2,16,17]. Hyperphosphataemia develops hours after initiation of cytoreductive treatment. High concentration of calcium-phosphate product leads to the precipitation of its crystals in the renal collecting ducts and hypocalcaemia [16]. Prior to lysis, the main mechanisms of hyperkalaemia are: disrupted functioning of a sodium/potassium adenosine triphosphatase (ATPase) due to increased cellular consumption of adenosine triphosphate (ATP) in malignant cells exposed to chemotherapy or radiation, and after lysis, the release of potassium from stores of broken cells [17]. This electrolyte disturbance appears 6 72 hours following initiation of anticancer treatment [16]. Some authors indicate the possible role of hypercytokinaemia, which can cause systemic inflammatory response syndrome and multiple organ dysfunction syndrome [18-21]. Symptoms TLS can be asymptomatic. Symptoms usually occur within 12 to 72 hours after initiation of cytoreductive therapy [1]. The clinical signs of TLS are presented in Table IV [1,16,22]. Diagnosis An identification of patients at risk of TLS is essential [6]. Not only appropriate causal therapy should be given, but also urine output, fluid balance, serum levels of electrolytes, lactate dehydrogenase (LDH), creatinine and uric acid should be monitored in this group of patients. Duration of the monitoring period and its frequency depend on the patient condition and the therapeutic regimen [2]. Based on the Cairo-Bishop definition (Table V), TLS can be classified as laboratory tumor lysis syndrome (LTLS) or clinical Table III. TLS risk classification system [6]. Klasyfikacja grup ryzyka zespołu rozpadu guza [6]. tumor lysis syndrome (CTLS) [1,2]. The latest guidelines do not regard the calcium level as a criterion for establishing LTLS. A decrease in calcium level can be associated with high phosphate levels not being a direct consequence of TLS [6]. Management The aims of TLS management is preservation of renal function, prevention of dysrhythmias and neuromuscular irritability [2]. TLS treatment principles are similar in hematological and solid tumors [8]. Aggressive hydration 2-3 L/m 2 /per day is the main point of prevention and management of TLS [1]. If it is necessary and there are no contra-indications, loop diuretics can be used to sustain a urine output of ml/ m 2 /hour [1,14]. They are essential to improve renal blood flow and glomerular filtration rate and to increase urine volume, dilution and excretion of metabolites [2]. Nephrotoxic agents (contrast media, selected antibiotics, non-steroidal anti-inflammatory drugs) and agents decreasing excretion of uric acid (aspirin, thiazide-type diuretics) should be Table I Differences in TLS between hematological malignancies and solid tumors [8]. Zespół rozpadu guza w nowotworach hematologicznych i guzach litych porównanie [8]. Tumor type hematological solid Frequency of occurrence very high very rare Time of onset Correlation with sensitivity of tumor to anticancer treatment early, even prior to anticancer therapy very high may be late, even several days to weeks after anticancer treatment variable Mortality low high Table II Risk factors for TLS [10]. Czynniki ryzyka zespołu rozpadu guza [10]. Risk Factors for Tumor Lysis Syndrome Cancer type: mostly hematological malignancies but TLS can occur also in solid tumors, e.g. hepatocellular, colon, small cell lung carcinoma High rate of proliferation of cancer cells High sensitivity to anticancer therapy/ high intensity of treatment Bulky tumor Extensive metastases In case of solid tumors: liver metastases with/ without liver function abnormalities High level of lactate dehydrogenase (LDH) High level of white blood cells count (WBC) Dehydration or volume depletion Pre-existing renal insufficiency/ kidneys involvement at diagnosis Pre-existing hyperuricaemia Acidic urine promoting uric acid crystals precipitation Hypotension Exposure to nephrotoxins Underlying problems, e.g. infection LRD IRD HRD majority of solid tumors multiple myeloma chronic myeloid leukaemia (CML) indolent non-hodgkin lymphoma (NHL) or intermediate grade NHL in adults with LDH <2 * ULN Hodgkin lymphoma (HL) chronic lymphoid leukaemia (CLL) treated with alkylating agents acute myeloid leukaemia (AML) with WBC <25 * 109/L and LDH <2 * ULN anaplastic large cell lymphoma (ALCL) in adults ULN - upper limit of normal neuroblastoma, germ cell tumors, small cell lung cancer and others rare solid tumors with bulky or advanced stage disease and high sensitivity to chemotherapy AML with WBC * 109/L or WBC <25 * 109/L and LDH 2 * ULN intermediate grade NHL in adults with LDH 2 * ULN ALCL stage III/IV in children, intermediate grade NHL stage III/IV in children with LDH <2 * ULN acute lymphoblastic leukaemia (ALL) with WBC <100 * 109/L and LDH <2 * ULN Burkitt lymphoma/ leukaemia with LDH <2 * ULN lymphoblastic lymphoma (LL) stage I/II with LDH <2 * ULN CLL treated with fludarabine, rituximab or with WBC 50 * 109/L no solid tumors AML with WBC 100 * 109/L ALL with WBC 100 * 109/L and/ or LDH 2 * ULN Burkitt lymphoma/ leukaemia stage III/IV and/ or LDH 2 * ULN LL stage III/IV and/ or LDH 2 * ULN Przegląd Lekarski 2014 / 71 / 4 211
3 Table IV. Symptoms of TLS [1,16,22]. Objawy zespołu rozpadu guza [1,16,22]. Hyperuricaemia Hyperkalaemia Hyperphosphataemia Hypocalcaemia nausea, vomiting, diarrhea, anorexia symptoms of renal insufficiency (e.g. oliguria, anuria, edema) diarrhea, vomiting, anorexia, weakness muscle cramps, paresthesia electrocardiographic changes (widening of the QRS complex and peaked T waves) cardiac arrhythmias, ventricular tachycardia, fibrillation, cardiac arrest nausea, vomiting, diarrhea lethargy, seizures cardiac arrhythmias, hypotension tetany, muscular cramps Table V. Cairo-Bishop definition laboratory and clinical TLS [1,2]. Laboratoryjny i kliniczny zespół rozpadu guza definicja według Cairo-Bishop [1,2]. LTLS (Laboratory Tumor Lysis Syndrome) Uric acid Potassium Phosphorus Calcium 8 mg/dl (476 μmol/l) 6 mg/l (6 mmol/l) 4,5 mg/dl (1.5 mmol/l) for adults 6,5 mg/dl (2.1 mmol/l) for children 7 mg/dl (1.75 mmol/l) or 25% decrease from baseline 2 of the above, 3 days before to 7 days after the initiation of anticancer treatment are required CTLS (Clinical Tumor Lysis Syndrome) ULN - upper limit of normal renal insufficiency: creatinine >1.5 * ULN cardiac arrhythmias or sudden death Seizures The presence of LTLS and 1 of the above clinical complications are required avoided [11]. In the past, urinary alkalinization was one of the steps of hyperuricaemia management. It increases uric acid solubility (from 15 mg/dl in ph 5.0 to 200 mg/dl in ph 7.0) [1]. However, in alkaline urine, greater potential of precipitation of hypoxanthine and xanthine (usually elevated in patients treated with allopurinol) and calcium phosphate in renal tubules is observed [2,23,24]. Optimal treatments for preventing uric acid precipitation are uricolytic agents and improvement of urine flow. Alkalinization of urine is no longer recommended [1,24]. Uric acid lowering agents can preserve or improve renal function and reduce - as a second beneficial effect - the serum phosphorus levels [2]. The impact of these drugs on uric acid metabolism is presented in Figure 1. Uric acid is poorly soluble in water. In many species, there is an enzyme urate oxidase which converts uric acid into much more soluble allantoin. In humans it is not present. Allopurinol is a xanthine analog which competitively blocks xanthine oxidase and thereby uric acid formation. It is ineffective in reducing pre-existing uric acid pool [2]. Allopurinol demonstrates efficacy in management of hyperuricaemia in patients at TLS risk but this drug can take several days until reductions in uric acid levels are observed [24]. An increase in the levels of xanthine and hypoxanthine and precipitation of their crystals in the renal tubules may occur after application of allopurinol. This agent also interferes with metabolism of other purinebased substances (e.g. 6-mercaptopurine, azathioprine) and methotrexate, decreasing their clearance. For that reason, lower doses of those drugs are required when used concomitantly with allopurinol. Combining allopurinol with capecitabine is contraindicated [1]. In patients with renal insufficiency, a 50% reduction of allopurinol is recommended. Allopurinol can cause hypersensitivity reactions, such as rash or fever [1,22]. Uricozyme is a nonrecombinant urate oxidase initially isolated from Aspergillus flavus. It promotes the catabolism of uric acid pool and causes a faster decrease in its serum level than allopurinol. During uric acid conversion into allantoin, H 2 O 2 is released causing oxidative stress [1]. In glucose-6-phosphate dehydrogenase (G6PD) deficiency, the breakdown of H 2 O 2 is ineffective and toxicities like hemolysis or methemoglobinaemia occur [24]. Rasburicase is a recombinant urate oxidase produced by the genetically modified Saccharomyces cerevisiae. It allows purification of the drug, potentially reducing the risk of contaminant-related hypersensitivity reactions [1]. As it has been shown in a randomized clinical trial, rasburicase is superior to allopurinol in reducing and controlling the plasma uric acid level [25]. Rasburicase has been approved to be administered at the daily dosage of 0.2 mg/kg for up to 5 days. Its Figure 1. Scheme of metabolism of nucleic acids from broken malignant cells and points of action of allopurinol and rasburicase [2]. Schemat metabolizmu kwasów nukleinowych uwalnianych z rozpadłych komórek nowotworowych i punkty uchwytu działania leków: allopurinolu i rasburykazy [2]. action occurs 4 hours after the first dose has been applied, and is effective in reducing the pre-existing uric acid pool, not causing hypoxanthine or xanthine accumulation [1]. The results of the randomized study showed that a single dose of rasburicase is effective in reducing and maintaining the uric acid level [26]. Using rasburicase, followed by allopurinol, can also effectively reduce the uric acid concentration and maintain it at a low level [25]. Known G6PD deficiency or history of hypersensitivity to urate oxidase are contraindications of using rasburicase [1]. Potential adverse reaction are: anaphylaxis, rash, hemolysis, methemoglobinaemia, fever, neutropaenia, respiratory distress, sepsis, mucositis, vomiting, diarrhea or headache. If rasburicase is contraindica- 212 J. Sobota i wsp.
4 or elevated potassium or phosphate levels and hypocalcaemia, a consultation with a nephrologist and dialysotherapy should be considered [1]. HRD patients should receive intensive nursing care in a facility with ready access to dialysis, continuous cardiac monitoring and laboratory monitoring every 4 to 6 hours. IRD patients should undergo laboratory monitoring every 8 to 12 hours, in case of LRD once daily [1,2]. If TLS has not developed within 2 days after finishing chemotherapy, the likelihood of its occurrence is minimal [1,2]. Generally, it is recommended to hold up with anticancer treatment since the prevention measures have started to act. Usually, the delay varies from 24 to 48 hours. In aggressive tumors, it is possible to administer rasburicase and start chemotherapy with only a few hours delay [27]. Patients who have a high risk of TLS may receive low-intensity initial therapy. It causes slower tumor lysis and allows the kidneys to clear metabolites more effectively [2]. Figure 2 Algorithm of management of patients at TLS risk [2]. Algorytm postępowania u pacjentów z ryzykiem wystąpienia zespołu rozpadu guza [2]. means assessment of ; TLS tumor lysis syndrome; LTLS laboratory tumor lysis syndrome; CTLS clinical tumor lysis syndrome; LRD low risk of disease; IRD intermediate risk of disease; HRD high risk of disease. Table VI. Guidelines for TLS management [1,6]. Wytyczne postępowania w zespole rozpadu guza [1,6]. TLS risk Low Intermediate High ted, allopurinol should be used. Due to the high costs of rasburicase, it is essential to monitor the serum uric acid level to adjust the optimal duration of treatment [23]. Blood samples must be immediately placed on ice until the completion of assay because rasburicase degrades uric acid ex vivo at room temperature [23]. An appropriate conventional management should be introduced for prevention and treatment of electrolytes abnormalities. It is essential to treat symptomatic hypocalcaemia. Care must be taken to avoid increased concentration of the calcium-phosphate product and its potential precipitation Action monitor for development of TLS and complications normal hydration no prophylaxis for hyperuricaemia in the case of bulky, advanced or high proliferative disease or signs of metabolic abnormalities, add allopurinol in the case of LTLS use rasburicase monitor for development of TLS and complications increased hydration (3 L/m 2 /d) allopurinol ( mg, orally, q8h, daily) in adults with hyperuricaemia use rasburicase; in children consider rasburicase in the case of LTLS, use rasburicase monitor frequently for development of TLS and complications increased hydration (3 L/m 2 /d), unless evidence of renal insufficiency and oliguria rasburicase (0,1 0,2 mg/kg), one dose repeated only if clinically necessary in the kidney and other tissues [2]. The latest recommendations for management of TLS included in Table V are based on the risk classification system presented in Table II [1,6]. In daily clinical practice, an algorithm of care based on actual patient condition seems to be more applicative (Figure 2). The assessment of TLS risk is based on a clinical judgment of actual patient s features and easily defined tumor characteristics. It is more universal as it is not based on tumor histology [2]. Prevention and treatment options in each risk group are similar to those presented in Table VI. In the case of low urine output, persistent Summary TLS is an oncological emergency. Abrupt lysis of malignant cells and release of intracellular contents into the systemic circulation can cause life-threatening metabolic and electrolyte disturbances. The prophylaxis is a cornerstone in TLS management. The lysis of tumor cells can develop as a result of anticancer treatment or may occur spontaneously. TLS affects bulky tumors with a high proliferative rate and sensitivity to chemotherapy. It is the most common of all hematologic malignancies but it can also occur in solid tumors. Patients are divided into low, intermediate and high risk groups, based on tumor type, stage and patient presentation. Different prevention and treatment options have been elaborated in this article. Knowledge about actual guidelines is essential to introduce the most appropriate management of TLS in particular groups of patients. Acknowledgments Authors would like to thank Ms Joanna Gołąb for editing the English version of the article. References 1. Coiffier B, Altman A, Pui CH, Younes A, Cairo MS: Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008; 26: Howard SC, Jones DP, Pui CH: The tumor lysis syndrome. N Engl J Med. 2011; 364: Coiffer B: Acute tumor lysis syndrome - a rare complication in the treatment of solid tumors. Onkologie 2010; 33: Malaguarnera G, Giordano M, Malaguarnera M: Rasburicase for the treatment of tumor lysis in hematological malignancies. Expert Rev Hematol. 2012; 5: Bose P, Qubaiah O: A review of tumour lysis syndrome with targeted therapies and the role of rasburicase. J Clin Pharm Ther. 2011; 36: Cairo MS, Coiffier B, Reiter A, Younes A, Baruchel A. et al: Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010; 149: Vodopivec DM, Rubio JE, Fornoni A, Lenz O: An unusual presentation of tumor lysis syndrome in a patient with advanced gastric adenocarcinoma: case report and literature review. Case Rep Med. 2012; 2012: Przegląd Lekarski 2014 / 71 / 4 213
5 8. Gemici C: Tumour lysis syndrome in solid tumours. Clin Oncol (R Coll Radiol). 2006; 18: McBride A, Westervelt P: Recognizing and managing the expanded risk of tumor lysis syndrome in hematologic and solid malignancies. J Hematol Oncol. 2012; 5: Świerkowska-Czeneszew M, Walewski J: Zespół rozpadu guza u dzieci i dorosłych zalecenia postępowania w oparciu o przegląd dowodów klinicznych. Medycyna Praktyczna Onkologia 2009/ Saini N, Pyo Lee K, Jha S, Patel S, Bonthu N. et al: Hyperuricemic renal failure in nonhematologic solid tumors: a case report and review of the literature. Case Report Med 2012; 2012: Woo IS, Kim JS, Park MJ, Lee MS, Cheon RW. et al: Spontaneous acute tumor lysis syndrome with advanced gastric cancer. J Korean Med Sci. 2001; 16: Chubb EA, Maloney D, Farley-Hills E: Tumour lysis syndrome: an unusual presentation. Anaesthesia. 2010; 65: Opyrchal M, Figanbaum T, Ghosh A, Rajkumar V, Caples S: Spontaneous tumor lysis syndrome in the setting of B-cell lymphoma. Case Report Med. 2010; 2010: Park SG, Chung CH, Park CY: Spontaneous tumor lysis syndrome with resolution of pancytopenia and disappearance of lymphadenopathy in a patient with peripheral T-cell lymphoma unspecified. Int J Hematol. 2011; 93: Davidson MB, Thakkar S, Hix JK, Bhandarkar ND, Wong A. et al: Pathophysiology, clinical consequences and treatment of tumor lysis syndrome. Am J Med. 2004; 116: Muslimani A, Chisti MM, Wills S, Nadeau L, Zakalik D. et al: How we treat tumor lysis syndrome. Oncology (Williston Park). 2011; 25: Hijiya N, Metzger ML, Pounds S, Schmidt JE, Razzouk BI. et al: Severe cardiopulmonary complications consistent with systemic inflammatory response syndrome caused by leukemia cell lysis in childhood acute myelomonocytic or monocytic leukemia. Pediatr Blood Cancer. 2005; 44: Nakamura M, Oda S, Sadahiro T, Hirayama Y, Tateishi Y. et al: The role of hypercytokinemiain the pathophysiology of tumor lysis syndrome (TLS) and the treatment with continuous hemodiafiltrationusing a polymethylmethacrylate membrane hemofilter (PMMA-CHDF). Transfus Apher Sci. 2009; 40: Soares M, Feres GA, Salluh JIF: Systemic inflammatory response syndrome and multiple organ dysfuncion in patients with acute tumor lysis syndrome. Clinics (Sao Paulo). 2009; 64: Suzuki T, Takeuchi M, Saeki H, Yamazaki S, Koga H. et al: Super-acute onset of tumor lysis syndrome accompanied by hypercytokinemia during treatment of Hodgkin s lymphoma with ABVD chemotherapy. Clin Ther. 2010; 32: Burghi G, Berrutti D, Manzanares W: Tumor lysis syndrome in intensive therapy: diagnostic and therapeutic encare. Med Intensiva. 2011; 35: Binkowska P, Zaucha JM: Niskie dawki rasburykazy w zapobieganiu zespołowi lizy guza u chorej z chłoniakiem z dużych komórek B - opis przypadku. Onkol. Prakt. Klin. 2008; 4: Zuckerman T, Ganzel Ch, Tallman MS, Rowe JM: How I treat hematologic emergencies in adults with acute leukemia. Blood. 2012; 120: Cortes J, Moore JO, Maziarz RT, Wetzler M, Craig M. et al: Control of plasma uric acid in adults at risk for tumor lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone - results of a multicenter phase III study. J Clin Oncol. 2010; 28: Vadhan-Raj S, Fayad LE, Fanale MA, Pro B, Rodriguez A. et al: A randomized trial of a singledose rasburicase versus five-daily doses in patients at risk for tumor lysis syndrome. Ann Oncol. 2011; 23: Will A, Tholouli E: The clinical management of tumour lysis syndrome in haematological malignancies. Br J Haematol. 2011; 154: J. Sobota i wsp.
Contents Page No. 1. Outline of Procedure 2. Area of Application 3. Objective 4. Stages of the Process 5. Responsibilities
 Contents Page No. 1. Outline of Procedure 3 2. Area of Application 3 3. Objective 3 4. Stages of the Process 3 5. Responsibilities 8 6. Other useful information 8 7. References 8 Page 2 of 10 Clinical
Contents Page No. 1. Outline of Procedure 3 2. Area of Application 3 3. Objective 3 4. Stages of the Process 3 5. Responsibilities 8 6. Other useful information 8 7. References 8 Page 2 of 10 Clinical
Analysis of the incidence of tumor lysis syndrome in patients with hematological malignancies treated with rasburicase
 MOLECULAR AND CLINICAL ONCOLOGY 6: 955-959, 2017 Analysis of the incidence of tumor lysis syndrome in patients with hematological malignancies treated with rasburicase EISEKI USAMI 1, MICHIO KIMURA 1,
MOLECULAR AND CLINICAL ONCOLOGY 6: 955-959, 2017 Analysis of the incidence of tumor lysis syndrome in patients with hematological malignancies treated with rasburicase EISEKI USAMI 1, MICHIO KIMURA 1,
GUIDELINE FOR THE MANAGEMENT AND PREVENTION OF ACUTE TUMOUR LYSIS SYNDROME IN HAEMATOLOGICAL MALIGNANCIES
 GUIDELINE FOR THE MANAGEMENT AND PREVENTION OF ACUTE TUMOUR LYSIS SYNDROME IN HAEMATOLOGICAL MALIGNANCIES Full Title of Guideline: Author (include email and role): Division & Speciality: Scope (Target
GUIDELINE FOR THE MANAGEMENT AND PREVENTION OF ACUTE TUMOUR LYSIS SYNDROME IN HAEMATOLOGICAL MALIGNANCIES Full Title of Guideline: Author (include email and role): Division & Speciality: Scope (Target
Tumor Lysis Syndrome Nephrology Grand Rounds Tuesday, July 27 th, 2010 Aditya Mattoo
 Tumor Lysis Syndrome Nephrology Grand Rounds Tuesday, July 27 th, 2010 Aditya Mattoo Outline Background/Definition Epidemiology/Risk Stratification Pathophysiology Treatment Renal Replacement Therapy Background/Definition
Tumor Lysis Syndrome Nephrology Grand Rounds Tuesday, July 27 th, 2010 Aditya Mattoo Outline Background/Definition Epidemiology/Risk Stratification Pathophysiology Treatment Renal Replacement Therapy Background/Definition
Oncology Emergency Essentials: Addressing Tumor Lysis Syndrome in Your Practice
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Guidelines for the Prevention of Tumour Lysis Syndrome
 1.0 Introduction For the purposes of this guideline, tumour lysis syndrome (TLS) is defined as metabolic derangement resulting from abrupt and massive breakdown of malignant cells and the release of intracellular
1.0 Introduction For the purposes of this guideline, tumour lysis syndrome (TLS) is defined as metabolic derangement resulting from abrupt and massive breakdown of malignant cells and the release of intracellular
Outcome of Tumor Lysis Syndrome with Hydration and Alkalinization in Children with Acute Lymphoblastic Leukemia
 Bangladesh Journal of Medical Science Vol. 11 No. 04 Oct 12 Original article Outcome of Tumor Lysis Syndrome with Hydration and Alkalinization in Children with Acute Lymphoblastic Leukemia Sultana A 1,
Bangladesh Journal of Medical Science Vol. 11 No. 04 Oct 12 Original article Outcome of Tumor Lysis Syndrome with Hydration and Alkalinization in Children with Acute Lymphoblastic Leukemia Sultana A 1,
Spontaneous Tumor Lysis Syndrome in Small Cell Lung Cancer
 Open Access Case Report DOI: 10.7759/cureus.1017 Spontaneous Tumor Lysis Syndrome in Small Cell Lung Cancer Venkatkiran Kanchustambham 1, Swetha Saladi 2, Setu Patolia 2, David Stoeckel 2 1. Pulmonary
Open Access Case Report DOI: 10.7759/cureus.1017 Spontaneous Tumor Lysis Syndrome in Small Cell Lung Cancer Venkatkiran Kanchustambham 1, Swetha Saladi 2, Setu Patolia 2, David Stoeckel 2 1. Pulmonary
2.1 mmol/l or 25% increase from baseline mmol/l or 25% decrease from baseline
 22.1 Hyperleukocytosis and tumour lysis syndrome The guideline is addressed for ALL patients with hyperleukocytosis (WBC 100 x109/l) only and should not be used without modifications in case of other diseases
22.1 Hyperleukocytosis and tumour lysis syndrome The guideline is addressed for ALL patients with hyperleukocytosis (WBC 100 x109/l) only and should not be used without modifications in case of other diseases
Development of tumor lysis syndrome (TLS): A potential risk factor in cancer patients receiving anticancer therapy
 www.bioinformation.net Hypothesis Volume 10(11) Development of tumor lysis syndrome (TLS): A potential risk factor in cancer patients receiving anticancer therapy Mahmood Rasool 1 *, Arif Malik 2, Muhammad
www.bioinformation.net Hypothesis Volume 10(11) Development of tumor lysis syndrome (TLS): A potential risk factor in cancer patients receiving anticancer therapy Mahmood Rasool 1 *, Arif Malik 2, Muhammad
Announcements. Learning Objectives. Introduction. Prevention and Treatment of an Oncologic Emergency: Focus on Tumor Lysis Syndrome
 Prevention and Treatment of an Oncologic Emergency: Focus on Tumor Lysis Syndrome Kamakshi V. Rao, Pharm.D., BCOP Oncology Clinical Pharmacy Specialist University of North Carolina Hospitals and Clinics
Prevention and Treatment of an Oncologic Emergency: Focus on Tumor Lysis Syndrome Kamakshi V. Rao, Pharm.D., BCOP Oncology Clinical Pharmacy Specialist University of North Carolina Hospitals and Clinics
Guidelines for use of RASBURICASE in adult Haematology and Oncology patients
 Network Guidance Document Guidelines for use of RASBURICASE in adult Haematology and Oncology patients Status: Expiry Date: Version Number: Publication Date: Final September 2013 6 September 2011 Page
Network Guidance Document Guidelines for use of RASBURICASE in adult Haematology and Oncology patients Status: Expiry Date: Version Number: Publication Date: Final September 2013 6 September 2011 Page
Avoiding Dialysis in Tumour Lysis Syndrome: Is Urate Oxidase Effective? A Case Report and Review of Literature
 Case Report 679 Avoiding Dialysis in Tumour Lysis Syndrome: Is Urate Oxidase Effective? A Case Report and Review of Literature Wan-Yee Teo, 1 MBBS, MRCPCH (Lond), Tsee-Foong Loh, 2 MBBS, MMed (Paeds),
Case Report 679 Avoiding Dialysis in Tumour Lysis Syndrome: Is Urate Oxidase Effective? A Case Report and Review of Literature Wan-Yee Teo, 1 MBBS, MRCPCH (Lond), Tsee-Foong Loh, 2 MBBS, MMed (Paeds),
Managing patients with bulky cancers
 SIOP PODC Supportive Care Education (ICON 2016) Presentation Date: 23 rd January 2016 Recording Link at www.cure4kids.org: https://www.cure4kids.org/ums/home/conference_rooms/enter.php?room=p2pjfjp8nha
SIOP PODC Supportive Care Education (ICON 2016) Presentation Date: 23 rd January 2016 Recording Link at www.cure4kids.org: https://www.cure4kids.org/ums/home/conference_rooms/enter.php?room=p2pjfjp8nha
Tumour Lysis Syndrome (TLS)
 (TLS) Overview: Tumour lysis syndrome refers to a number of metabolic disturbances (hyperuricaemia, hyperphosphataemia, hyperkalaemia and hypocalcaemia) that occur as the result of rapid cell lysis. This
(TLS) Overview: Tumour lysis syndrome refers to a number of metabolic disturbances (hyperuricaemia, hyperphosphataemia, hyperkalaemia and hypocalcaemia) that occur as the result of rapid cell lysis. This
Subject: Rasburicase (Elitek )
 09-J2000-43 Original Effective Date: 10/15/15 Reviewed: 08/01/18 Revised: 09/15/18 Subject: Rasburicase (Elitek ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION OF
09-J2000-43 Original Effective Date: 10/15/15 Reviewed: 08/01/18 Revised: 09/15/18 Subject: Rasburicase (Elitek ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION OF
Haematological Emergencies (Part 1) Ray Mun Koo Haematology Advanced Trainee Canberra Hospital
 Haematological Emergencies (Part 1) Ray Mun Koo Haematology Advanced Trainee Canberra Hospital Case Number 1 43 year old male presenting with fevers, abdominal distension and weight gain over 2 weeks.
Haematological Emergencies (Part 1) Ray Mun Koo Haematology Advanced Trainee Canberra Hospital Case Number 1 43 year old male presenting with fevers, abdominal distension and weight gain over 2 weeks.
Iranian Journal of Blood & Cancer. The Efficacy of Single Dose Rasburicase in Prevention or Treatment of Tumor Lysis Syndrome in Children
 IJBC 2016; 8(2): 33-37 Iranian Journal of Blood & Cancer Journal Home Page: www.ijbc.ir Original Article The Efficacy of Single Dose Rasburicase in Prevention or Treatment of Tumor Lysis Syndrome in Children
IJBC 2016; 8(2): 33-37 Iranian Journal of Blood & Cancer Journal Home Page: www.ijbc.ir Original Article The Efficacy of Single Dose Rasburicase in Prevention or Treatment of Tumor Lysis Syndrome in Children
Fasturtec 1.5 mg/ml powder and solvent for concentrate for solution for infusion.
 1. NAME OF THE MEDICINAL PRODUCT Fasturtec 1.5 mg/ml powder and solvent for concentrate for solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION After reconstitution, 1 ml of Fasturtec concentrate
1. NAME OF THE MEDICINAL PRODUCT Fasturtec 1.5 mg/ml powder and solvent for concentrate for solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION After reconstitution, 1 ml of Fasturtec concentrate
The pattern of metabolic derangements known as tumor. Tumor Lysis Syndrome. Resident Short Reviews
 Resident Short Reviews Tumor Lysis Syndrome Shelly M. Williams, MD; Anthony A. Killeen, MB, BCh, PhD Tumor lysis syndrome (TLS) is an acute, life-threatening disease among adults and children that is associated
Resident Short Reviews Tumor Lysis Syndrome Shelly M. Williams, MD; Anthony A. Killeen, MB, BCh, PhD Tumor lysis syndrome (TLS) is an acute, life-threatening disease among adults and children that is associated
Frequency of Tumor Lysis Syndrome in Aggressive and Slow Introduction Chemotherapy in Children with ALL
 Original Article Frequency of Tumor Lysis Syndrome in Aggressive and Slow Introduction Chemotherapy in Children with ALL Downloaded from ijpho.ssu.ac.ir at 1:59 IRDT on Friday July 1th 2018 Hashemi A 1,
Original Article Frequency of Tumor Lysis Syndrome in Aggressive and Slow Introduction Chemotherapy in Children with ALL Downloaded from ijpho.ssu.ac.ir at 1:59 IRDT on Friday July 1th 2018 Hashemi A 1,
DERBY-BURTON LOCAL CANCER NETWORK FILENAME R-CODOX-M.DOC CONTROLLED DOC NO: HCCPG B115 CSIS Regimen Name: R-CODOXM. Rituximab + CODOX-M
 Rituximab + CODOX-M Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Burkitt
Rituximab + CODOX-M Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Burkitt
Non-Hodgkin s Lymphomas Version
 NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Non-Hodgkin s Lymphomas Version 2.2015 NCCN.org Continue Supportive Care for NHL Tumor Lysis Syndrome (TLS) Laboratory hallmarks of TLS:
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Non-Hodgkin s Lymphomas Version 2.2015 NCCN.org Continue Supportive Care for NHL Tumor Lysis Syndrome (TLS) Laboratory hallmarks of TLS:
tumor lysis syndrome
 2.0 ANCC CONTACT HOURS Oncology Emergency series Recognizing and preventing tumor lysis syndrome By Roberta Kaplow, PhD, APRN-CCNS, AOCNS, CCRN, and Karen Iyere, MSN, RN TUMOR LYSIS SYNDROME (TLS) is one
2.0 ANCC CONTACT HOURS Oncology Emergency series Recognizing and preventing tumor lysis syndrome By Roberta Kaplow, PhD, APRN-CCNS, AOCNS, CCRN, and Karen Iyere, MSN, RN TUMOR LYSIS SYNDROME (TLS) is one
The clinical management of tumour lysis syndrome in
 state of the art review The clinical management of tumour lysis syndrome in haematological malignancies Andrew Will 1 and Eleni Tholouli 2 1 Department of Paediatric Haematology, Royal Manchester Children
state of the art review The clinical management of tumour lysis syndrome in haematological malignancies Andrew Will 1 and Eleni Tholouli 2 1 Department of Paediatric Haematology, Royal Manchester Children
Single-Dose Rasburicase 6 mg in the Management of Tumor Lysis Syndrome in Adults
 Single-Dose Rasburicase 6 mg in the Management of Tumor Lysis Syndrome in Adults Anne M. McDonnell, Pharm.D., Kristi L. Lenz, Pharm.D., Debra A. Frei-Lahr, M.D., John Hayslip, M.D., and Philip D. Hall,
Single-Dose Rasburicase 6 mg in the Management of Tumor Lysis Syndrome in Adults Anne M. McDonnell, Pharm.D., Kristi L. Lenz, Pharm.D., Debra A. Frei-Lahr, M.D., John Hayslip, M.D., and Philip D. Hall,
Guidelines for the Management of Tumour Lysis Syndrome in Haematological and Solid Tumour Malignancies
 Guidelines for the Management of Tumour Lysis Syndrome in Haematological and Solid Tumour Malignancies CSPM 02 Date to be reviewed: September 2017 No of pages: 7 Author(s): Dr Yvonne Jones Author(s) title:
Guidelines for the Management of Tumour Lysis Syndrome in Haematological and Solid Tumour Malignancies CSPM 02 Date to be reviewed: September 2017 No of pages: 7 Author(s): Dr Yvonne Jones Author(s) title:
Hypouricemic effect and safety of febuxostat used for prevention of tumor lysis syndrome
 Maie et al. SpringerPlus 2014, 3:501 a SpringerOpen Journal RESEARCH Hypouricemic effect and safety of febuxostat used for prevention of tumor lysis syndrome Open Access Koichiro Maie 1, Yasuhisa Yokoyama
Maie et al. SpringerPlus 2014, 3:501 a SpringerOpen Journal RESEARCH Hypouricemic effect and safety of febuxostat used for prevention of tumor lysis syndrome Open Access Koichiro Maie 1, Yasuhisa Yokoyama
Tumor lysis syndrome (TLS) is a group of PROCEEDINGS CURRENT AND EMERGING TREATMENT OPTIONS FOR PATIENTS WITH TUMOR LYSIS SYNDROME * Sima Jeha, MD
 CURRENT AND EMERGING TREATMENT OPTIONS FOR PATIENTS WITH TUMOR LYSIS SYNDROME * Sima Jeha, MD ABSTRACT Tumor lysis syndrome (TLS) is a life-threatening complication of cancer chemotherapy in which hyperuricemia
CURRENT AND EMERGING TREATMENT OPTIONS FOR PATIENTS WITH TUMOR LYSIS SYNDROME * Sima Jeha, MD ABSTRACT Tumor lysis syndrome (TLS) is a life-threatening complication of cancer chemotherapy in which hyperuricemia
Acute tumor lysis syndrome (ATLS), although
 ACUTE TUMOR LYSIS SYNDROME Michele Cohen, DVM, DACVIM (Oncology) College of Veterinary Medicine Auburn University Auburn, Alabama Acute tumor lysis syndrome (ATLS), although uncommon, can be a serious
ACUTE TUMOR LYSIS SYNDROME Michele Cohen, DVM, DACVIM (Oncology) College of Veterinary Medicine Auburn University Auburn, Alabama Acute tumor lysis syndrome (ATLS), although uncommon, can be a serious
Urate oxidase for the prevention and treatment of tumour lysis syndrome in children with cancer. Title. Cheuk, DKL; Chiang, AKS; Chan, GCF; Ha, SY
 Title Urate oxidase for the prevention and treatment of tumour lysis syndrome in children with cancer Author(s) Cheuk, DKL; Chiang, AKS; Chan, GCF; Ha, SY Citation Cochrane Database of Systematic Reviews,
Title Urate oxidase for the prevention and treatment of tumour lysis syndrome in children with cancer Author(s) Cheuk, DKL; Chiang, AKS; Chan, GCF; Ha, SY Citation Cochrane Database of Systematic Reviews,
BCCA Protocol Summary Guidelines for the Diagnosis and Management of Malignancy Related Hypercalcemia
 BCCA Protocol Summary Guidelines for the Diagnosis and Management of Malignancy Related Hypercalcemia Protocol Code Tumour Group Supportive Care Group Contacts SCHYPCAL Supportive Care Lisa Wanbon (VIC)
BCCA Protocol Summary Guidelines for the Diagnosis and Management of Malignancy Related Hypercalcemia Protocol Code Tumour Group Supportive Care Group Contacts SCHYPCAL Supportive Care Lisa Wanbon (VIC)
PRODUCT INFORMATION FASTURTEC
 PRODUCT INFORMATION FASTURTEC NAME OF THE MEDICINE NON-PROPRIETARY NAME Rasburicase rys for injection CAS REGISTRY NUMBER 134774-45-1 DESCRIPTION Fasturtec is a sterile powder supplied in a stoppered clear
PRODUCT INFORMATION FASTURTEC NAME OF THE MEDICINE NON-PROPRIETARY NAME Rasburicase rys for injection CAS REGISTRY NUMBER 134774-45-1 DESCRIPTION Fasturtec is a sterile powder supplied in a stoppered clear
Case presentation. serum uric acid = 11.5 mg/dl 24-hour uric acid excretion = 300 mg
 GOUT 55 y/o male 12 hours pain in my big toe & ankle went to bed last night feeling fine felt as if had broken toe this morning similar problems in right ankle & left wrist Case presentation lab studies
GOUT 55 y/o male 12 hours pain in my big toe & ankle went to bed last night feeling fine felt as if had broken toe this morning similar problems in right ankle & left wrist Case presentation lab studies
Tumour Lysis Syndrome: Implications for Cancer Therapy
 DOI:http://dx.doi.org/10.7314/APJCP.2012.13.8.3555 MINI-REVIEW Denish Mika, Sabrina Ahmad, C Guruvayoorappan* Abstract The tumour lysis syndrome (TLS) is a group of metabolic abnormalities caused by rapid
DOI:http://dx.doi.org/10.7314/APJCP.2012.13.8.3555 MINI-REVIEW Denish Mika, Sabrina Ahmad, C Guruvayoorappan* Abstract The tumour lysis syndrome (TLS) is a group of metabolic abnormalities caused by rapid
SPONTANEOUS TUMOR LYSIS SYNDROME: A case report and review of literature
 Case Report SPONTANEOUS TUMOR LYSIS SYNDROME: A case report and review of literature Ashraf Ahmed Hussein Hashem 1, Tarek Abdel Hameed Mostafa Dowod 2, Mohsen Mahmooud Abdelmajeed 3 ABSTRACT We report
Case Report SPONTANEOUS TUMOR LYSIS SYNDROME: A case report and review of literature Ashraf Ahmed Hussein Hashem 1, Tarek Abdel Hameed Mostafa Dowod 2, Mohsen Mahmooud Abdelmajeed 3 ABSTRACT We report
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION
 DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
Leukemias. Prof. Mutti Ullah Khan Head of Department Medical Unit-II Holy Family Hospital Rawalpindi Medical College
 Leukemias Prof. Mutti Ullah Khan Head of Department Medical Unit-II Holy Family Hospital Rawalpindi Medical College Introduction Leukaemias are malignant disorders of the haematopoietic stem cell compartment,
Leukemias Prof. Mutti Ullah Khan Head of Department Medical Unit-II Holy Family Hospital Rawalpindi Medical College Introduction Leukaemias are malignant disorders of the haematopoietic stem cell compartment,
Hematologic Emergency. Le Wang, MD, PhD Hematology & Oncology
 Hematologic Emergency Le Wang, MD, PhD Hematology & Oncology Severe Thrombocytopenia (ITP) Clinical: bleeding risk 0 no bleeding; 1 minimal bleeding after trauma; 2 spontaneous but selflimited bleeding;
Hematologic Emergency Le Wang, MD, PhD Hematology & Oncology Severe Thrombocytopenia (ITP) Clinical: bleeding risk 0 no bleeding; 1 minimal bleeding after trauma; 2 spontaneous but selflimited bleeding;
Rasburicase 1.5 mg/ml powder and solvent for concentrate for solution for infusion. FASTURTEC
 For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory This package insert is continually updated. Please read carefully before using a new pack. Rasburicase 1.5 mg/ml powder
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory This package insert is continually updated. Please read carefully before using a new pack. Rasburicase 1.5 mg/ml powder
4/20/2015. Chemotherapy in the Infusion Clinic: Patient Electrolyte Imbalance Considerations. Learning Objectives. Ambulatory Oncology Infusion Center
 Chemotherapy in the Infusion Clinic: Patient Electrolyte Imbalance Considerations Nichole Korpi-Steiner, PhD, DABCC, FACB University of North Carolina Chapel Hill, NC Learning Objectives Explore the benefits
Chemotherapy in the Infusion Clinic: Patient Electrolyte Imbalance Considerations Nichole Korpi-Steiner, PhD, DABCC, FACB University of North Carolina Chapel Hill, NC Learning Objectives Explore the benefits
Salicylate (Aspirin) Ingestion California Poison Control Background 1. The prevalence of aspirin-containing analgesic products makes
 Salicylate (Aspirin) Ingestion California Poison Control 1-800-876-4766 Background 1. The prevalence of aspirin-containing analgesic products makes these agents, found in virtually every household, common
Salicylate (Aspirin) Ingestion California Poison Control 1-800-876-4766 Background 1. The prevalence of aspirin-containing analgesic products makes these agents, found in virtually every household, common
Easy Trick to Spot Leukemia for Pediatricians
 Easy Trick to Spot Leukemia for Pediatricians Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital Most Common Pediatric Cancers Age 0-14 Leukemia 32%
Easy Trick to Spot Leukemia for Pediatricians Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital Most Common Pediatric Cancers Age 0-14 Leukemia 32%
CASE REPORT: SPONTANEOUS TUMOR LYSIS SYNDROME IN HEMATOLOGICAL MALIGNANCY
 CASE REPORT: SPONTANEOUS TUMOR LYSIS SYNDROME IN HEMATOLOGICAL MALIGNANCY Gökçen GÜNGÖR 1, Ülkü ERGENE 2, Ali İhsan GEMİCİ 2 Lütfü ÇETİNTEPE 3 1- Department of İnternal Medicine, Celal Bayar University
CASE REPORT: SPONTANEOUS TUMOR LYSIS SYNDROME IN HEMATOLOGICAL MALIGNANCY Gökçen GÜNGÖR 1, Ülkü ERGENE 2, Ali İhsan GEMİCİ 2 Lütfü ÇETİNTEPE 3 1- Department of İnternal Medicine, Celal Bayar University
Review Article Tumor Lysis Syndrome in Patients with Hematological Malignancies
 Hindawi Journal of Oncology Volume 2017, Article ID 9684909, 9 pages https://doi.org/10.1155/2017/9684909 Review Article Tumor Lysis Syndrome in Patients with Hematological Malignancies Yohannes Belay,
Hindawi Journal of Oncology Volume 2017, Article ID 9684909, 9 pages https://doi.org/10.1155/2017/9684909 Review Article Tumor Lysis Syndrome in Patients with Hematological Malignancies Yohannes Belay,
RiTUXimab 375 mg/m 2 Therapy-7 day
 RiTUXimab 375 mg/m 2 Therapy-7 day This regimen supercedes NCCP Regimen 00208 rituximab 375mg/m2 therapy-follicular lymphoma as of February 2019 INDICATIONS FOR USE: Regimen *Reimbursement INDICATION ICD10
RiTUXimab 375 mg/m 2 Therapy-7 day This regimen supercedes NCCP Regimen 00208 rituximab 375mg/m2 therapy-follicular lymphoma as of February 2019 INDICATIONS FOR USE: Regimen *Reimbursement INDICATION ICD10
Electrolyte Imbalance and Resuscitation. Dr. Mehmet Okumuş Sütçü Imam University Faculty of Medicine Department of Emergency Medicine
 Electrolyte Imbalance and Resuscitation Dr. Mehmet Okumuş Sütçü Imam University Faculty of Medicine Department of Emergency Medicine Presentation plan Definition of the electrolyte disturbances Conditions
Electrolyte Imbalance and Resuscitation Dr. Mehmet Okumuş Sütçü Imam University Faculty of Medicine Department of Emergency Medicine Presentation plan Definition of the electrolyte disturbances Conditions
LEUKAEMIA and LYMPHOMA. Dr Mubarak Abdelrahman Assistant Professor Jazan University
 LEUKAEMIA and LYMPHOMA Dr Mubarak Abdelrahman Assistant Professor Jazan University OBJECTIVES Identify etiology and epidemiology for leukemia and lymphoma. Discuss common types of leukemia. Distinguish
LEUKAEMIA and LYMPHOMA Dr Mubarak Abdelrahman Assistant Professor Jazan University OBJECTIVES Identify etiology and epidemiology for leukemia and lymphoma. Discuss common types of leukemia. Distinguish
Pitfalls, prevention, and treatment of hyperuricemia during tumor lysis syndrome in the era of rasburicase (recombinant urate oxidase)
 REVIEW Pitfalls, prevention, and treatment of hyperuricemia during tumor lysis syndrome in the era of rasburicase (recombinant urate oxidase) Andrea Pession Fraia Melchionda Claudia Castellini Oncologia
REVIEW Pitfalls, prevention, and treatment of hyperuricemia during tumor lysis syndrome in the era of rasburicase (recombinant urate oxidase) Andrea Pession Fraia Melchionda Claudia Castellini Oncologia
This is a controlled document and therefore must not be changed or photocopied L.80 - R-CHOP-21 / CHOP-21
 R- / INDICATION Lymphoma Histiocytosis Omit rituximab if CD20-negative. TREATMENT INTENT Disease modification or curative depending on clinical circumstances PRE-ASSESSMENT 1. Ensure histology is confirmed
R- / INDICATION Lymphoma Histiocytosis Omit rituximab if CD20-negative. TREATMENT INTENT Disease modification or curative depending on clinical circumstances PRE-ASSESSMENT 1. Ensure histology is confirmed
Pediatric GU Dysfunction
 Pediatric GU Dysfunction Assessment of pediatric renal function Signs and symptoms Laboratory tests Radiological tests Nursing considerations Psychosocial and developmental considerations GU Disorders
Pediatric GU Dysfunction Assessment of pediatric renal function Signs and symptoms Laboratory tests Radiological tests Nursing considerations Psychosocial and developmental considerations GU Disorders
Oncologic Emergencies
 Oncologic Emergencies Luca Delatore, MD James Emergency Department Medical Director Associate Professor Clinical Department of Emergency Medicine The Ohio State University Wexner Medical Center Prevalence
Oncologic Emergencies Luca Delatore, MD James Emergency Department Medical Director Associate Professor Clinical Department of Emergency Medicine The Ohio State University Wexner Medical Center Prevalence
Oncologic Emergencies
 Oncologic Emergencies Luca Delatore, MD James Emergency Department Medical Director Associate Professor Clinical Department of Emergency Medicine The Ohio State University Wexner Medical Center Prevalence
Oncologic Emergencies Luca Delatore, MD James Emergency Department Medical Director Associate Professor Clinical Department of Emergency Medicine The Ohio State University Wexner Medical Center Prevalence
Non-protein nitrogenous substances (NPN)
 Non-protein nitrogenous substances (NPN) A simple, inexpensive screening test a routine urinalysis is often the first test conducted if kidney problems are suspected. A small, randomly collected urine
Non-protein nitrogenous substances (NPN) A simple, inexpensive screening test a routine urinalysis is often the first test conducted if kidney problems are suspected. A small, randomly collected urine
RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University
 RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University OBJECTIVES By the end of this lecture each student should be able to: Define acute & chronic kidney disease(ckd)
RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University OBJECTIVES By the end of this lecture each student should be able to: Define acute & chronic kidney disease(ckd)
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Chronic lymphocytic leukaemia 1 Chronic lymphocytic leukemia (CLL) is a condition characterized by a progressive accumulation
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Chronic lymphocytic leukaemia 1 Chronic lymphocytic leukemia (CLL) is a condition characterized by a progressive accumulation
Drugs Used to Treat Gout. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Drugs Used to Treat Gout Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Gout is a metabolic disease characterized by recurrent episodes of acute arthritis
Drugs Used to Treat Gout Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Gout is a metabolic disease characterized by recurrent episodes of acute arthritis
DERBY-BURTON CANCER NETWORK CONTROLLED DOC NO:
 OBINUTUZUMAB+CHLORAMBUCIL Regimen RDH; Day 1 and 2 Dose to be given on Ward Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community
OBINUTUZUMAB+CHLORAMBUCIL Regimen RDH; Day 1 and 2 Dose to be given on Ward Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community
ACUTE PROMYELOCYTIC LEUKEMIA Rome (Italy), September 29-October 1, 2005
 4 th International Symposium on ACUTE PROMYELOCYTIC LEUKEMIA Rome (Italy), September 29-October 1, 2005 CORPORATE SYMPOSIUM TUMOR LYSIS SYNDROME: PATHOGENESIS AND CLINICAL ASPECTS Tosi P Seràgnoli Institute
4 th International Symposium on ACUTE PROMYELOCYTIC LEUKEMIA Rome (Italy), September 29-October 1, 2005 CORPORATE SYMPOSIUM TUMOR LYSIS SYNDROME: PATHOGENESIS AND CLINICAL ASPECTS Tosi P Seràgnoli Institute
Normal range of serum potassium is meq/l true hyperkalemia manifests clinically as : Clinical presentation : muscle and cardiac dysfunction
 Potassium Disorders hyperkalemia Potassium is mainly an cation? What is the major physiological role of potassium in the body? What is the major regulatory system of serum potassium level? Which part of
Potassium Disorders hyperkalemia Potassium is mainly an cation? What is the major physiological role of potassium in the body? What is the major regulatory system of serum potassium level? Which part of
Obinutuzumab+Bendamustine followed by Obinutuzumab Maintenance Burton in-patient Derby in-patient Burton day-case Derby day-case
 Obinutuzumab+Bendamustine followed by Obinutuzumab Maintenance Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Available
Obinutuzumab+Bendamustine followed by Obinutuzumab Maintenance Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Available
Clinical Biochemistry department/ College of medicine / AL-Mustansiriyah University
 Clinical Biochemistry department/ College of medicine / AL-Mustansiriyah University Dr. Ali al-bayati NUCLEOTIDE METABOLISM Lec. 3 The salvage pathway of purine synthesis Purines that result from the normal
Clinical Biochemistry department/ College of medicine / AL-Mustansiriyah University Dr. Ali al-bayati NUCLEOTIDE METABOLISM Lec. 3 The salvage pathway of purine synthesis Purines that result from the normal
Tumour lysis syndrome: new therapeutic strategies and classification
 review Tumour lysis syndrome: new therapeutic strategies and classification Mitchell S. Cairo 1 and Michael Bishop 2 1 Department of Pediatrics, Children s Hospital of New York Presbyterian, Columbia University,
review Tumour lysis syndrome: new therapeutic strategies and classification Mitchell S. Cairo 1 and Michael Bishop 2 1 Department of Pediatrics, Children s Hospital of New York Presbyterian, Columbia University,
uric acid Non electrolytes of the plasma
 73 uric acid Non electrolytes of the plasma 1 Purines and uric acid Fig 2 JFI Uric acid is the major product of catabolism of the purine nucleosides adenosine and guanosine, Uric acid is sparingly soluble
73 uric acid Non electrolytes of the plasma 1 Purines and uric acid Fig 2 JFI Uric acid is the major product of catabolism of the purine nucleosides adenosine and guanosine, Uric acid is sparingly soluble
Sponsor/Company: sanofi-aventis Drug substance: Elitek/Fasturtec (rasburicase, SR29142)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor/Company: sanofi-aventis Drug
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor/Company: sanofi-aventis Drug
MabThera. SC. The wait is over. MabThera delivered in just 5 minutes. SC= subcutaneous injection
 MabThera SC. The wait is over. MabThera delivered in just 5 minutes Abbreviated Prescribing Information MabThera 1400 mg solution for subcutaneous (SC) injection (Rituximab) Indications: Indicated in adults
MabThera SC. The wait is over. MabThera delivered in just 5 minutes Abbreviated Prescribing Information MabThera 1400 mg solution for subcutaneous (SC) injection (Rituximab) Indications: Indicated in adults
Hospital, Chengdu , China. Hospital, Chengdu , China
 Hyperuricemia and acute renal failure secondary to spontaneous tumor lysis syndrome in low risk myelodysplastic syndrome. Yunlin Feng, M.D. 1,, Tao Jiang 2 Correspondence: Yunlin Feng fengyunlin@tsinghua.org.cn
Hyperuricemia and acute renal failure secondary to spontaneous tumor lysis syndrome in low risk myelodysplastic syndrome. Yunlin Feng, M.D. 1,, Tao Jiang 2 Correspondence: Yunlin Feng fengyunlin@tsinghua.org.cn
SERUM PHOSPHORUS TESTING
 MEDICAL POLICY For use with the UnitedHealthcare Laboratory Benefit Management Program, administered by BeaconLBS SERUM PHOSPHORUS TESTING Policy Number: CMP - 035 Effective Date: January 21, 2017 Table
MEDICAL POLICY For use with the UnitedHealthcare Laboratory Benefit Management Program, administered by BeaconLBS SERUM PHOSPHORUS TESTING Policy Number: CMP - 035 Effective Date: January 21, 2017 Table
R-FC for Chronic Lymphocytic Leukaemia LKWOS-005/01
 West of Scotland Cancer Network Chemotherapy Protocol R-FC for Chronic Lymphocytic Leukaemia LKWOS-005/01 Indication B cell Chronic Lymphocytic Leukaemia First line therapy in patients under 70 years of
West of Scotland Cancer Network Chemotherapy Protocol R-FC for Chronic Lymphocytic Leukaemia LKWOS-005/01 Indication B cell Chronic Lymphocytic Leukaemia First line therapy in patients under 70 years of
Composition: Each Tablet contains. Pharmacokinetic properties:
 Composition: Each Tablet contains Torsemide 5/10/20/40/100mg Pharmacokinetic properties: Torsemide is well absorbed from the gastrointestinal tract. Peak serum concentrations are achieved within 1 hour
Composition: Each Tablet contains Torsemide 5/10/20/40/100mg Pharmacokinetic properties: Torsemide is well absorbed from the gastrointestinal tract. Peak serum concentrations are achieved within 1 hour
Acute Renal Failure. Dr Kawa Ahmad
 62 Acute Renal Failure Dr Kawa Ahmad Acute Renal Failure It is characterised by an abrupt reduction (usually within a 48- h period) in kidney function. This results in an accumulation of nitrogenous waste
62 Acute Renal Failure Dr Kawa Ahmad Acute Renal Failure It is characterised by an abrupt reduction (usually within a 48- h period) in kidney function. This results in an accumulation of nitrogenous waste
School of Medicine and Health Sciences Division of Basic Medical Sciences Discipline of Biochemistry and Molecular Biology PLB SEMINAR
 1 School of Medicine and Health Sciences Division of Basic Medical Sciences Discipline of Biochemistry and Molecular Biology PLB SEMINAR URINARY (RENAL) STONE FORMATION An Overview What are Urinary (Renal)
1 School of Medicine and Health Sciences Division of Basic Medical Sciences Discipline of Biochemistry and Molecular Biology PLB SEMINAR URINARY (RENAL) STONE FORMATION An Overview What are Urinary (Renal)
What is a hematological malignancy? Hematology and Hematologic Malignancies. Etiology of hematological malignancies. Leukemias
 Hematology and Hematologic Malignancies Cancer of the formed elements of the blood What is a hematological malignancy? A hematologic malignancy is a malignancy (or cancer) of any of the formed elements
Hematology and Hematologic Malignancies Cancer of the formed elements of the blood What is a hematological malignancy? A hematologic malignancy is a malignancy (or cancer) of any of the formed elements
PACKAGE LEAFLET: INFORMATION FOR THE USER. SODIPHOS 22mEq / 10ml Concentrate for solution for infusion. Disodium phosphate dihydrate
 PACKAGE LEAFLET: INFORMATION FOR THE USER SODIPHOS 22mEq / 10ml Concentrate for solution for infusion Disodium phosphate dihydrate Read all of this leaflet carefully before you start using this medicine.
PACKAGE LEAFLET: INFORMATION FOR THE USER SODIPHOS 22mEq / 10ml Concentrate for solution for infusion Disodium phosphate dihydrate Read all of this leaflet carefully before you start using this medicine.
Hematologic Emergencies. Udomsak Bunworasate Chulalongkorn University
 Hematologic Emergencies Udomsak Bunworasate Chulalongkorn University Hematologic Emergencies Hyperleukocytosis Tumor lysis syndrome SVC syndrome Spinal cord compression Hypercalcemia 1. Hyperleukocytosis
Hematologic Emergencies Udomsak Bunworasate Chulalongkorn University Hematologic Emergencies Hyperleukocytosis Tumor lysis syndrome SVC syndrome Spinal cord compression Hypercalcemia 1. Hyperleukocytosis
3/9/2017. Chapter 56. Care of the Patient with Cancer. Cancer Rates in the US. Carcinogenesis
 Chapter 56 Care of the Patient with Cancer All items and derived items 2015, 2011, 2006 by Mosby, Inc., an imprint of Elsevier Inc. All rights reserved. Cancer Rates in the US 1 in 2 men and 1 in 3 women
Chapter 56 Care of the Patient with Cancer All items and derived items 2015, 2011, 2006 by Mosby, Inc., an imprint of Elsevier Inc. All rights reserved. Cancer Rates in the US 1 in 2 men and 1 in 3 women
50% Concentrated Injection
 NAME OF THE MEDICINE. The molecular weight of the compound is 246.5 and the CAS registry number is 10034-99-8. The molecular formula is MgSO4, 7H2O. DESCRIPTION MAGNESIUM SULFATE HEPTAHYDRATE 50% CONCENTRATED
NAME OF THE MEDICINE. The molecular weight of the compound is 246.5 and the CAS registry number is 10034-99-8. The molecular formula is MgSO4, 7H2O. DESCRIPTION MAGNESIUM SULFATE HEPTAHYDRATE 50% CONCENTRATED
Professor Dr. Saiyeedur Rahman Professor and Head Department of Medicine SBMCH, Barisal
 Professor Dr. Saiyeedur Rahman Professor and Head Department of Medicine SBMCH, Barisal What is Oncologic Emergency? An oncologic emergency is an acute, potentially life-threatening event resulting from
Professor Dr. Saiyeedur Rahman Professor and Head Department of Medicine SBMCH, Barisal What is Oncologic Emergency? An oncologic emergency is an acute, potentially life-threatening event resulting from
Instruct patient and caregivers: Need for constant monitoring Potential complications of drug therapy
 Assessment Prior to administration: Assess patient for chest pain, dysrhythmias, and vital signs (initially and throughout therapy) Obtain complete medical history, including allergies, especially heart
Assessment Prior to administration: Assess patient for chest pain, dysrhythmias, and vital signs (initially and throughout therapy) Obtain complete medical history, including allergies, especially heart
Immunosuppressants. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Immunosuppressants Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Immunosuppressive Agents Very useful in minimizing the occurrence of exaggerated or inappropriate
Immunosuppressants Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Immunosuppressive Agents Very useful in minimizing the occurrence of exaggerated or inappropriate
Cisplatin / Paclitaxel Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol Cisplatin / Paclitaxel Gynaecological Cancer PROCTOCOL REF: MPHAGYNCIP (Version No: 1.0) Approved for use in: First line treatment for stage Ib-IV with minimal residual
Systemic Anti Cancer Treatment Protocol Cisplatin / Paclitaxel Gynaecological Cancer PROCTOCOL REF: MPHAGYNCIP (Version No: 1.0) Approved for use in: First line treatment for stage Ib-IV with minimal residual
Acute Kidney Injury. Eleanor Haskey BSc(hons) RVN VTS(ECC) VPAC A1
 Acute Kidney Injury Eleanor Haskey BSc(hons) RVN VTS(ECC) VPAC A1 Anatomy and Physiology The role of the kidneys is to filter the blood through the glomerulus to form filtrate. The filtrate is then reabsorbed
Acute Kidney Injury Eleanor Haskey BSc(hons) RVN VTS(ECC) VPAC A1 Anatomy and Physiology The role of the kidneys is to filter the blood through the glomerulus to form filtrate. The filtrate is then reabsorbed
GOOD MORNING! July 3, 2014
 GOOD MORNING! July 3, 2014 OUR PATIENT 4yo Female with: 2 days of fever, sore throat, swollen nodes in neck and abdominal pain PMH: Tonsillectomy age 2 Immunizations: UTD NKDA DIFFERENTIAL: OUR PATIENT
GOOD MORNING! July 3, 2014 OUR PATIENT 4yo Female with: 2 days of fever, sore throat, swollen nodes in neck and abdominal pain PMH: Tonsillectomy age 2 Immunizations: UTD NKDA DIFFERENTIAL: OUR PATIENT
R-Gemcitabine (1000mg/m 2 ) Oxaliplatin Therapy i - 14 day
 R-Gemcitabine (1000mg/m 2 ) Oxaliplatin i - 14 day INDICATIONS FOR USE: Regimen Code INDICATION ICD10 Relapsed or refractory CD20 positive diffuse large B cell lymphoma in patients ineligible for stem
R-Gemcitabine (1000mg/m 2 ) Oxaliplatin i - 14 day INDICATIONS FOR USE: Regimen Code INDICATION ICD10 Relapsed or refractory CD20 positive diffuse large B cell lymphoma in patients ineligible for stem
HTN, retenopathy, edema, encephalopathy
 ARF Uremic syndrom Uremic syndrome (uremia) is a serious complication of CRF & ARF. It occurs when urea and other waste products build up in the body because the kidneys are unable to eliminate them. These
ARF Uremic syndrom Uremic syndrome (uremia) is a serious complication of CRF & ARF. It occurs when urea and other waste products build up in the body because the kidneys are unable to eliminate them. These
Adverse effects of Immunotherapy. Asha Nayak M.D
 Adverse effects of Immunotherapy Asha Nayak M.D None Financial Disclosures Objectives Understand intensity of the AEs. Understanding unique side-effects. Develop effective monitoring and management guidelines.
Adverse effects of Immunotherapy Asha Nayak M.D None Financial Disclosures Objectives Understand intensity of the AEs. Understanding unique side-effects. Develop effective monitoring and management guidelines.
PACKAGE LEAFLET: INFORMATION FOR THE USER. Active substance: enoximone
 PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfan Injection 100 mg/20 ml Concentrate for Solution for Injection Active substance: enoximone Read all of this leaflet carefully before you
PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfan Injection 100 mg/20 ml Concentrate for Solution for Injection Active substance: enoximone Read all of this leaflet carefully before you
Carboplatin / Liposomal Doxorubicin CARBO/CAELYX Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol Carboplatin / CARBO/CAELYX Gynaecological Cancer PROCTOCOL REF: MPHAGYNCCX (Version No: 1.0) Approved for use in: Advanced ovarian cancer in women who have progressed
Systemic Anti Cancer Treatment Protocol Carboplatin / CARBO/CAELYX Gynaecological Cancer PROCTOCOL REF: MPHAGYNCCX (Version No: 1.0) Approved for use in: Advanced ovarian cancer in women who have progressed
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Abdominal tumors, in children, 530 531 Alkalinization, in tumor lysis syndrome, 516 Allopurinol, in tumor lysis syndrome, 515 Anaphylaxis, drug
Note: Page numbers of article titles are in boldface type. A Abdominal tumors, in children, 530 531 Alkalinization, in tumor lysis syndrome, 516 Allopurinol, in tumor lysis syndrome, 515 Anaphylaxis, drug
TIP Paclitaxel, Ifosfamide and Cisplatin
 Systemic Anti Cancer Treatment Protocol TIP Paclitaxel, Ifosfamide and Cisplatin PROTOCOL REF: MPHATIPGC (Version No: 1.0) Approved for use in: Second line treatment of germ cell tumours Dosage: Drug Dosage
Systemic Anti Cancer Treatment Protocol TIP Paclitaxel, Ifosfamide and Cisplatin PROTOCOL REF: MPHATIPGC (Version No: 1.0) Approved for use in: Second line treatment of germ cell tumours Dosage: Drug Dosage
Te-Fu Weng, Kung-His Wu, Ching-Tien Peng Department of Hematology and Oncology Children s Hospital of China Medical University
 Successful resuscitation using extracorporeal membrane oxygenation for fetal hyperkalemia and refractory ventricular fibrillation due to severe tumor lysis syndrome Te-Fu Weng, Kung-His Wu, Ching-Tien
Successful resuscitation using extracorporeal membrane oxygenation for fetal hyperkalemia and refractory ventricular fibrillation due to severe tumor lysis syndrome Te-Fu Weng, Kung-His Wu, Ching-Tien
Cisplatin / Capecitabine (+ Trastuzumab) in Gastric Cancer
 Cisplatin / Capecitabine (+ Trastuzumab) in Gastric Cancer Page 1 of 5 Indication: Confirmed HER2-positive (3+ or FISH+) metastatic adenocarcinoma of the stomach or gastrooesophageal junction. Patient
Cisplatin / Capecitabine (+ Trastuzumab) in Gastric Cancer Page 1 of 5 Indication: Confirmed HER2-positive (3+ or FISH+) metastatic adenocarcinoma of the stomach or gastrooesophageal junction. Patient
Tarek ElBaz, MD. Prof. Internal Medicine Chief, Division of Renal Medicine Al Azhar University President, ESNT
 The Kidney in Multiple Myeloma Tarek ElBaz, MD. Prof. Internal Medicine Chief, Division of Renal Medicine Al Azhar University President, ESNT Normal Cell Plasma cells produce antibodies that bind to antigens,
The Kidney in Multiple Myeloma Tarek ElBaz, MD. Prof. Internal Medicine Chief, Division of Renal Medicine Al Azhar University President, ESNT Normal Cell Plasma cells produce antibodies that bind to antigens,
Summary of the risk management plan (RMP) for Gazyvaro (obinutuzumab)
 EMA/319729/2014 Summary of the risk management plan (RMP) for Gazyvaro (obinutuzumab) This is a summary of the risk management plan (RMP) for Gazyvaro, which details the measures to be taken in order to
EMA/319729/2014 Summary of the risk management plan (RMP) for Gazyvaro (obinutuzumab) This is a summary of the risk management plan (RMP) for Gazyvaro, which details the measures to be taken in order to
Lymphoma: What You Need to Know. Richard van der Jagt MD, FRCPC
 Lymphoma: What You Need to Know Richard van der Jagt MD, FRCPC Overview Concepts, classification, biology Epidemiology Clinical presentation Diagnosis Staging Three important types of lymphoma Conceptualizing
Lymphoma: What You Need to Know Richard van der Jagt MD, FRCPC Overview Concepts, classification, biology Epidemiology Clinical presentation Diagnosis Staging Three important types of lymphoma Conceptualizing
Pfizer Laboratories (Pty) Ltd P&U Etoposide CSV Range Approved PI: 12 Sep 2005 Page 1 of 5
 Approved PI: 12 Sep 2005 Page 1 of 5 SCHEDULING STATUS: S4 PROPRIETARY NAME (and dosage form): P&U ETOPOSIDE CSV 100 mg/5 ml INJECTION (INJECTION CONCENTRATE FOR INTRAVENOUS INFUSION) P&U ETOPOSIDE CSV
Approved PI: 12 Sep 2005 Page 1 of 5 SCHEDULING STATUS: S4 PROPRIETARY NAME (and dosage form): P&U ETOPOSIDE CSV 100 mg/5 ml INJECTION (INJECTION CONCENTRATE FOR INTRAVENOUS INFUSION) P&U ETOPOSIDE CSV
keyword: diuretics Drug monitoring Monitoring diuretics in primary care 2 March 2009 best tests
 www.bpac.org.nz keyword: diuretics Drug monitoring Monitoring diuretics in primary care 2 March 2009 best tests Why do we monitor patients taking diuretics and what do we monitor? Monitoring a person on
www.bpac.org.nz keyword: diuretics Drug monitoring Monitoring diuretics in primary care 2 March 2009 best tests Why do we monitor patients taking diuretics and what do we monitor? Monitoring a person on
VI.2 Elements for a Public Summary. VI.2.1 Overview of disease epidemiology
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Febuxostat is a medicine used in adults with gout to reduce high levels of uric acid in the blood. Gout results from a build up
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Febuxostat is a medicine used in adults with gout to reduce high levels of uric acid in the blood. Gout results from a build up
The tumor lysis syndrome is the most common disease-related
 T h e n e w e ngl a nd j o u r na l o f m e dic i n e review article current concepts The Tumor Lysis Syndrome Scott C. Howard, M.D., Deborah P. Jones, M.D., and Ching-Hon Pui, M.D. From the Department
T h e n e w e ngl a nd j o u r na l o f m e dic i n e review article current concepts The Tumor Lysis Syndrome Scott C. Howard, M.D., Deborah P. Jones, M.D., and Ching-Hon Pui, M.D. From the Department
