Cigna Drug and Biologic Coverage Policy
|
|
|
- Gervase Griffith
- 6 years ago
- Views:
Transcription
1 Cigna Drug and Biologic Coverage Policy Subject Belimumab Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 11/15/2017 Next Review Date... 11/15/2018 Coverage Policy Number Related Coverage Resources NSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna companies. Coverage Policies are intended to provide guidance in interpreting certain standard Cigna benefit plans. Please note, the terms of a customer s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer s benefit plan document may contain a specific exclusion related to a topic addressed in a Coverage Policy. In the event of a conflict, a customer s benefit plan document always supersedes the information in the Coverage Policies. In the absence of a controlling federal or state coverage mandate, benefits are ultimately determined by the terms of the applicable benefit plan document. Coverage determinations in each specific instance require consideration of 1) the terms of the applicable benefit plan document in effect on the date of service; 2) any applicable laws/regulations; 3) any relevant collateral source materials including Coverage Policies and; 4) the specific facts of the particular situation. Coverage Policies relate exclusively to the administration of health benefit plans. Coverage Policies are not recommendations for treatment and should never be used as treatment guidelines. In certain markets, delegated vendor guidelines may be used to support medical necessity and other coverage determinations. Proprietary information of Cigna. Copyright 2017 Cigna Coverage Policy Cigna covers belimumab (Benlysta ) intravenously or subcutaneously as medically necessary for the treatment of active systemic lupus erythematosus (SLE) in an adult when all of the following criteria are met: Positive autoantibody test (i.e., anti-nuclear antibody [ANA] greater than or equal to 1:80 and/or antidouble-stranded DNA [anti-dsdna] greater than or equal to 30 IU/ml) Failure/inadequate response to standard therapy for SLE with any of the following: o Hydroxychloroquine o Immunosuppressant agents (i.e., oral cyclophosphamide, azathioprine, mycophenolate, methotrexate, and cyclosporine) o Corticosteroids (for example, methylprednisolone, prednisone) Absence of severe active lupus nephritis or severe active central nervous system lupus Cigna does not cover the use of belimumab (Benlysta ) for any other indication including the following because it is considered experimental, investigational or unproven (this list may not be all-inclusive): Anti-neutrophil cytoplasmic antibodies (ANCA) positive vasculitis (i.e., Wegener's granulomatosis and microscopic polyangiitis) Primary Sjogren s syndrome Rheumatoid arthritis Waldenstrom macroglobulinemia When coverage is available and medically necessary, the dosage, frequency, duration of therapy, and site of care should be reasonable, clinically appropriate, and supported by evidence-based literature and adjusted based upon severity, alternative available treatments, and previous response to belimumab (Benlysta) therapy. Page 1 of 5
2 Note: Receipt of sample product does not satisfy any criteria requirements for coverage FDA Approved Indications Benlysta is indicated for the treatment of adult patients with active, autoantibody-positive, systemic lupus erythematosus (SLE) who are receiving standard therapy. Limitations of Use: The efficacy of Benlysta has not been evaluated in patients with severe active lupus nephritis or severe active central nervous system lupus. Benlysta has not been studied in combination with other biologics or intravenous cyclophosphamide. Use of Benlysta is not recommended in these situations. FDA Recommended Dosing Formulation Intravenous FDA Recommended Dosing Recommended Dosage Vials are intended for intravenous use only (not for subcutaneous use) and autoinjectors and prefilled syringes are intended for subcutaneous use only (not for intravenous use). The recommended intravenous dosage regimen is 10 mg/kg at 2-week intervals for the first 3 doses and at 4-week intervals thereafter. Reconstitute, dilute, and administer as an intravenous infusion over a period of 1 hour. The infusion rate may be slowed or interrupted if the patient develops an infusion reaction. The infusion must be discontinued immediately if the patient experiences a serious hypersensitivity reaction. Subcutaneous Prior to intravenous dosing with Benlysta, consider administering premedication for prophylaxis against infusion reactions and hypersensitivity reactions. Recommended Dosage The recommended dosage is 200 mg once weekly given as a subcutaneous injection in the abdomen or thigh. Subcutaneous dosing is not based on weight. If transitioning from intravenous therapy with Benlysta to subcutaneous administration, administer the first subcutaneous dose 1 to 4 weeks after the last intravenous dose. Drug Availability Formulation Intravenous Subcutaneous It is recommended that the first subcutaneous injection of Benlysta should be under the supervision of a healthcare professional. The healthcare provider should provide proper training in subcutaneous technique and education about signs and symptoms of hypersensitivity reactions. A patient may self-inject or the patient caregiver may administer Benlysta subcutaneously after the healthcare provider determines it is appropriate. Instruct the patient to administer Benlysta 200 mg once a week, preferably on the same day each week. Drug Availability For injection: 120 mg or 400 mg lyophilized powder in single-dose vials for reconstitution and dilution prior to intravenous infusion Injection: 200 mg/ml as a clear to opalescent, and colorless to pale yellow solution in a single-dose prefilled autoinjector or a single-dose prefilled glass syringe. General Background Pharmacology Belimumab is a human IgG 1-lambda monoclonal antibody that binds to the B-lymphocyte stimulator (BLyS) protein to inhibit it from binding to B cells. B-lymphocyte stimulator protein plays a role in the survival of B-cells. Without the BLyS protein, fewer B-cells differentiate into plasma cells that produce autoantibodies. (McEvoy, 2017) Page 2 of 5
3 Guidelines American College of Rheumatology (ACR) The ACR criteria for classification of SLE are used to identify patients for clinical studies and requires 4 of 11 criteria at some point in their medical history for an individual to be considered as having SLE. Additionally, these criteria are often used in clinical practice as a guide to help facilitate the diagnosis of SLE. The criteria are contained in the following table: Item Malar Rash Discoid rash Photosensitivity Oral ulcers Nonerosive arthritis Pleuritis or Pericarditis ACR SLE Criteria Definition/Description Fixed erythema, flat or raised, over the malar eminences, tending to spare the nasolabial folds Erythematosus raised patches with adherent keratotic scaling and follicular plugging: atrophic scarring may occur in older lesions Skin rash as a result of unusual reaction to sunlight, by patient history or physician observation Oral or nasopharyngeal ulceration, usually painless, observed by a physician Involving 2 or more peripheral joints, characterized by tenderness, swelling or effusion Pleuritis convincing history of pleuritic pain or rub heard by a physician or evidence of pleural effusion Pericarditis documented by electrocardiogram or rub or evidence or pericardial effusion Renal disorder Neurologic disorder Hematologic Disorder Immunologic Disorder Positive antinuclear antibody Persistent proteinuria > 0.5gm/d or >3+ if quantitation not performed Cellular casts may be red cell, hemoglobin, granular, tubular, or mixed Seizures in the absence of offending drugs or known metabolic derangement, e.g., uremia, ketoacidosis, or electrolyte imbalance Psychosis in the absence of offending drugs or known metabolic derangement, e.g., uremia, ketoacidosis, or electrolyte imbalance Hemolytic anemia with reticulocytosis Leukopenia - < 4,000/mm 3 on 2 occasions Lymphopenia - < 1,500/mm 3 on 2 occasions Thrombocytopenia - < 100,000/mm 3 in the absence of offending drug Anti-DNA: antibody to native DNA in abnormal titer Anti-Sm: presence of antibody to Sm nuclear antigen Positive finding of antiphospholipid antibodies based on: o Abnormal serum level of IgG or IgM anticardiolipin antibodies o o A positive test result for lupus anticoagulant using a standard method A false-positive test result for at least 6 months and confirmed by Treponema pallidum immobilization or fluorescent treponemal antibody absorption tests An abnormal titer of antinuclear antibody by immunofluorescence or an equivalent assay at any point in time in the absence of drug (ACR, 1997; ACR, 1999) Page 3 of 5
4 Clinical Efficacy FDA Approved Indications To date there are no clinical trials comparing belimumab with other agents used to treat SLE. A phase II dose finding study compared standard treatment plus belimumab 1 mg/kg, 4 mg/kg, 10 mg/kg, and placebo in 449 patients. Primary outcomes evaluated the percent change in SELENA-SLEDAI score at week 24 and time to first flare at week 52. No dose of belimumab was significantly different compared to placebo in either primary efficacy endpoint. However, a subgroup analysis of percent change in SELENA-SLEDAI at week 52 showed the combined belimumab groups to be significantly different compared to placebo in 321 patients who were serologically active. (Wallace, 2009) This significant difference led to the development of two phase III trials. The two phase III, multicenter, randomized, double blind, placebo-controlled trials which were conducted are Belimumab International SLE Study (BLISS)-52 and BLISS-76. BLISS-52 evaluated 865 patients. BLISS-76 evaluated 819 patients. Both trials compared standard treatment plus either belimumab 1 mg/kg, 10 mg/kg, or placebo. The primary outcome in both trials was the SRI response rate at week 52. The SRI response rate for belimumab 10 mg/kg was significantly improved compared to placebo at week 52 in both trials. A secondary endpoint for BLISS-76 was the SRI response rate at week 76 which was not significantly different compared to placebo. This result may be due to an increased rate of dropouts in the belimumab group compared to the placebo group between week 52 and week 76. Response rates for belimumab 1 mg/kg were not consistent in both BLISS trials. Belimumab 1 mg/kg was significantly improved compared to placebo for SRI response rate at week 52 in BLISS-52; however, there was no difference in SRI response rate between belimumab 1 mg/kg and placebo at week 52 in BLISS-76. (CDER 2011; Navarra, 2011) Experimental, Investigational, or Unproven Uses Belimumab has been evaluated in a phase II clinical trial for individuals with rheumatoid arthritis who have failed at least one disease-modifying antirheumatic drug (DMARD). Compared to placebo significantly more belimumab-treated patients (dose groups 1 mg/kg and 10 mg/kg) had a 20% improvement in American College of Rheumatology criteria (ACR20). (Stohl, 2013) Belimumab was also evaluated and found to be effective in a small (n = 30), phase II, open-label trial in primary Sjogren s syndrome. (Mariette, 2015) A phase II, single-arm trial in 12 patients with Waldenstrom macroglobulinemia did not produce any disease responses with belimumab (10 of the patients had stable disease for a median duration of 6.75 months). (Bishton, 2013) Phase III studies to evaluate belimumab in ANCA positive vasculitis (Wegener s granulomatosis and microscopic polyangiitis) and in active lupus nephritis are ongoing. (Human Genome Sciences Inc.) Coding/Billing Information Note: 1) This list of codes may not be all-inclusive. 2) Deleted codes and codes which are not effective at the time the service is rendered may not be eligible for reimbursement HCPCS Codes J0490 Description Injection, belimumab, 10 mg References 1. American College of Rheumatology Update of the 1982 American College of Rheumatology Revised Criteria for Classification of Systemic Lupus Erythematosus. Available at evised_criteria_for_classification_of_sle.pdf#toolbar=1. Accessed October 14, American College of Rheumatology. Guidelines for Referral and Management of Systemic Lupus Erythematosus in Adults. Arthritis Rheum 1999; 42 (9): Page 4 of 5
5 3. American College of Rheumatology. Systemic Lupus Erythematosus Disease Activity Index SELENA Modification. Available at Accessed September 14, Bishton M, Spencer A, Dickinson M, Ritchie D. A single-arm, phase II study of the anti-blys monoclonal antibody belimumab in symptomatic Waldenstrom macroglobulinemia (WM). Clin Lymphoma Myeloma Leuk 2013; 13 (5): GlaxoSmithKline. Benlysta (belimumab) [product information]. Research Triangle Park, NC: GlaxoSmithKline. September Mariette X, Seror R, Quartuccio L. Efficacy and safety of belimumab in primary Sjogren s syndrome: results of the BELISS open-label phase II study. Ann Rheum Dis Mar,74(3): McEvoy GK, ed. AHFS 2015 Drug Information. Bethesda, MD: American Society of Health-Systems Pharmacists, Inc Navarra SV, Guzman RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. Feb ;377(9767): Stohl W, Merrill JT, McKay JD, et al. Efficacy and safety of belimumab in patients with rheumatoid arthritis: a phase II, randomized, double-blind, placebo-controlled, dose-ranging study. J Rheumatol 2013; 40 (5): The registered marks "Cigna" and the "Tree of Life" logo are owned by Cigna Intellectual Property, Inc., licensed for use by Cigna Corporation and its operating subsidiaries. All products and services are provided by or through such operating subsidiaries and not by Cigna Corporation. Such operating subsidiaries include Connecticut General Life Insurance Company, Cigna Health and Life Insurance Company, Cigna Behavioral Health, Inc., Cigna Health Management, Inc., and HMO or service company subsidiaries of Cigna Health Corporation. Page 5 of 5
Benlysta (belimumab) Prior Authorization Criteria Program Summary
 Benlysta (belimumab) Prior Authorization Criteria Program Summary This prior authorization applies to Commercial, NetResults A series, NetResults F series and Health Insurance Marketplace formularies.
Benlysta (belimumab) Prior Authorization Criteria Program Summary This prior authorization applies to Commercial, NetResults A series, NetResults F series and Health Insurance Marketplace formularies.
ONE of the following:
 Medical Coverage Policy Belimumab (Benlysta) EFFECTIVE DATE: 01 01 2012 POLICY LAST UPDATED: 11 21 2017 OVERVIEW Belimumab (Benlysta ) is indicated for the treatment of adult patients with active, autoantibody-positive,
Medical Coverage Policy Belimumab (Benlysta) EFFECTIVE DATE: 01 01 2012 POLICY LAST UPDATED: 11 21 2017 OVERVIEW Belimumab (Benlysta ) is indicated for the treatment of adult patients with active, autoantibody-positive,
Committee Approval Date: May 9, 2014 Next Review Date: May 2015
 Medication Policy Manual Policy No: dru248 Topic: Benlysta, belimumab Date of Origin: May 13, 2011 Committee Approval Date: May 9, 2014 Next Review Date: May 2015 Effective Date: June 1, 2014 IMPORTANT
Medication Policy Manual Policy No: dru248 Topic: Benlysta, belimumab Date of Origin: May 13, 2011 Committee Approval Date: May 9, 2014 Next Review Date: May 2015 Effective Date: June 1, 2014 IMPORTANT
* Autoantibody positive (i.e. antinuclear antibody or ANA titre 1:80 or anti-double stranded (ds) DNA antibody 30 IU/mL)
 Benlysta (belimumab) Policy Number: 5.02.509 Last Review: 05/2018 Origination: 06/2011 Next Review: 05/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Benlysta
Benlysta (belimumab) Policy Number: 5.02.509 Last Review: 05/2018 Origination: 06/2011 Next Review: 05/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Benlysta
LUPUS CAN DO EVERYTHING, BUT NOT EVERYTHING IS LUPUS LUPUS 101 SLE SUBSETS AUTOIMMUNE DISEASE 11/4/2013 HOWARD HAUPTMAN, MD IDIOPATHIC DISCOID LUPUS
 LUPUS 101 LUPUS CAN DO EVERYTHING, BUT NOT EVERYTHING IS LUPUS HOWARD HAUPTMAN, MD IDIOPATHIC DISCOID LUPUS SLE SUBSETS SUBACUTE CUTANEOUS LUPUS DRUG INDUCED LUPUS NEONATAL LUPUS LATE ONSET LUPUS ANTI-PHOSPHOLIPID
LUPUS 101 LUPUS CAN DO EVERYTHING, BUT NOT EVERYTHING IS LUPUS HOWARD HAUPTMAN, MD IDIOPATHIC DISCOID LUPUS SLE SUBSETS SUBACUTE CUTANEOUS LUPUS DRUG INDUCED LUPUS NEONATAL LUPUS LATE ONSET LUPUS ANTI-PHOSPHOLIPID
The only biologic approved to treat SLE: now with multiple delivery options
 The only biologic approved to treat SLE: now with multiple delivery options BENLYSTA (belimumab) Autoinjector SC Prefilled syringe IV Intravenous infusion Consider the options: visit Belimumab.com INDICATION
The only biologic approved to treat SLE: now with multiple delivery options BENLYSTA (belimumab) Autoinjector SC Prefilled syringe IV Intravenous infusion Consider the options: visit Belimumab.com INDICATION
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: belimumab_benlysta 6/2011 2/2018 2/2019 3/2018 Description of Procedure or Service Belimumab (Benlysta) is
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: belimumab_benlysta 6/2011 2/2018 2/2019 3/2018 Description of Procedure or Service Belimumab (Benlysta) is
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Romiplostim Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 12/15/2017 Next
Cigna Drug and Biologic Coverage Policy Subject Romiplostim Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 12/15/2017 Next
CIBMTR Center Number: CIBMTR Recipient ID: RETIRED. Today s Date: Date of HSCT for which this form is being completed: &
 Systemic Lupus Erythematosus Pre-HSCT Data Sequence Number: Date Received: Registry Use Only Today s Date: Date of HSCT for which this form is being completed: & HSCT type: o autologous o allogeneic, o
Systemic Lupus Erythematosus Pre-HSCT Data Sequence Number: Date Received: Registry Use Only Today s Date: Date of HSCT for which this form is being completed: & HSCT type: o autologous o allogeneic, o
Systemic lupus erythematosus in a male patient
 IOP Conference Series: Earth and Environmental Science PAPER OPEN ACCESS Systemic lupus erythematosus in a male patient To cite this article: H Sibarani and Z Zubir 2018 IOP Conf. Ser.: Earth Environ.
IOP Conference Series: Earth and Environmental Science PAPER OPEN ACCESS Systemic lupus erythematosus in a male patient To cite this article: H Sibarani and Z Zubir 2018 IOP Conf. Ser.: Earth Environ.
Benlysta. Benlysta (belimumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.99.01 Subject: Benlysta Page: 1 of 5 Last Review Date: June 22, 2018 Benlysta Description Benlysta (belimumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.99.01 Subject: Benlysta Page: 1 of 5 Last Review Date: June 22, 2018 Benlysta Description Benlysta (belimumab)
Systemic lupus erythematosus (SLE) ... PRESENTATIONS... Epidemiology of Systemic Lupus Erythematosus. Based on a presentation by Susan Manzi, MD, MPH
 ... PRESENTATIONS... Epidemiology of Systemic Lupus Erythematosus Based on a presentation by Susan Manzi, MD, MPH Presentation Summary Tracking the epidemiology of systemic lupus erythematosus is problematic
... PRESENTATIONS... Epidemiology of Systemic Lupus Erythematosus Based on a presentation by Susan Manzi, MD, MPH Presentation Summary Tracking the epidemiology of systemic lupus erythematosus is problematic
LUPUS (SLE) MEDICAL SOURCE STATEMENT
 LUPUS (SLE) MEDICAL SOURCE STATEMENT From: Re: (Name of Patient) (Social Security No.) Please answer the following questions concerning your patient s impairments. Attach relevant treatment notes, radiologist
LUPUS (SLE) MEDICAL SOURCE STATEMENT From: Re: (Name of Patient) (Social Security No.) Please answer the following questions concerning your patient s impairments. Attach relevant treatment notes, radiologist
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Interleukin (IL)-5 Antagonists: Mepolizumab and Reslizumab Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 5
Cigna Drug and Biologic Coverage Policy Subject Interleukin (IL)-5 Antagonists: Mepolizumab and Reslizumab Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 5
Benlysta. Benlysta (belimumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Benlysta Page: 1 of 5 Last Review Date: December 3, 2015 Benlysta Description Benlysta (belimumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Benlysta Page: 1 of 5 Last Review Date: December 3, 2015 Benlysta Description Benlysta (belimumab)
Cigna Drug and Biologic Policy
 Cigna Drug and Biologic Policy Subject Collagenase clostridium histolyticum Effective Date... 11/15/2017 Next Review Date... 11/15/2018 Coverage Policy Number... 1021 Table of Contents Coverage Policy...
Cigna Drug and Biologic Policy Subject Collagenase clostridium histolyticum Effective Date... 11/15/2017 Next Review Date... 11/15/2018 Coverage Policy Number... 1021 Table of Contents Coverage Policy...
MANAGING THE PATIENT WITH POSITIVE ANA
 MANAGING THE PATIENT WITH POSITIVE ANA Rafael F. Rivas-Chacon, M.D. Disclosures Grant/Research support for: Pfizer Study JIA A3921104 Tofacitinib not related to this presentation 1 Positive Antinuclear
MANAGING THE PATIENT WITH POSITIVE ANA Rafael F. Rivas-Chacon, M.D. Disclosures Grant/Research support for: Pfizer Study JIA A3921104 Tofacitinib not related to this presentation 1 Positive Antinuclear
Elevated BLyS levels in patients with systemic lupus erythematosus: Associated factors and responses to belimumab
 (2016) 25, 346 354 http://lup.sagepub.com PAPER Elevated BLyS levels in patients with systemic lupus erythematosus: Associated factors and responses to belimumab DA Roth 1, A Thompson 2, Y Tang 2*, AE
(2016) 25, 346 354 http://lup.sagepub.com PAPER Elevated BLyS levels in patients with systemic lupus erythematosus: Associated factors and responses to belimumab DA Roth 1, A Thompson 2, Y Tang 2*, AE
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Antiemetic Therapy Table of Contents Coverage Policy... 1 General Background... 6 Coding/Billing Information... 8 References... 8 Effective Date... 1/1/2018
Cigna Drug and Biologic Coverage Policy Subject Antiemetic Therapy Table of Contents Coverage Policy... 1 General Background... 6 Coding/Billing Information... 8 References... 8 Effective Date... 1/1/2018
Efficacy and Safety of Belimumab in the treatment of Systemic Lupus Erythematosus: a Prospective Multicenter Study.
 1. Title Efficacy and Safety of Belimumab in the treatment of Systemic Lupus Erythematosus: a Prospective Multicenter Study. 2. Background Systemic Lupus Erythematosus (SLE) is a chronic, autoimmune and
1. Title Efficacy and Safety of Belimumab in the treatment of Systemic Lupus Erythematosus: a Prospective Multicenter Study. 2. Background Systemic Lupus Erythematosus (SLE) is a chronic, autoimmune and
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Apremilast Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 1/1/2018 Next
Cigna Drug and Biologic Coverage Policy Subject Apremilast Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 1/1/2018 Next
Autoimmune Disease. Autoimmunity. Epidemiology. ACR Criteria for Diagnosis. Signs and Symptoms. Autoreactivity: Reactivity to self antigens:
 Autoimmunity Reactivity to self antigens: Autoreactivity: Autoimmune Disease T cells B cells Leading to tissue damage or dysfunction Occurring in the absence of ongoing infection Epidemiology SLE Pathogenesis
Autoimmunity Reactivity to self antigens: Autoreactivity: Autoimmune Disease T cells B cells Leading to tissue damage or dysfunction Occurring in the absence of ongoing infection Epidemiology SLE Pathogenesis
Autoimmunity. Autoimmune Disease
 Autoimmunity Reactivity to self antigens: T cells B cells Autoimmune Disease Autoreactivity: Leading to tissue damage or dysfunction Occurring in the absence of ongoing infection 1 SLE Pathogenesis Immune
Autoimmunity Reactivity to self antigens: T cells B cells Autoimmune Disease Autoreactivity: Leading to tissue damage or dysfunction Occurring in the absence of ongoing infection 1 SLE Pathogenesis Immune
Systemic Lupus Erythematosus
 Systemic Lupus Erythematosus Marc C. Hochberg, MD, MPH Professor of Medicine and Head, Division of Rheumatology University of Maryland School of Medicine CASE: HISTORY A 26-year-old woman is seen for migratory
Systemic Lupus Erythematosus Marc C. Hochberg, MD, MPH Professor of Medicine and Head, Division of Rheumatology University of Maryland School of Medicine CASE: HISTORY A 26-year-old woman is seen for migratory
Early diagnosis of systemic lupus erythematosus in primary care by family doctors
 ISSN 2229-5518 2,302 Early diagnosis of systemic lupus erythematosus in primary care by family doctors Saad Ali Alalyani, Abdullah Saad Alalyani, Sultan Salem Algethami, Hamad Sulayyih Alosaimi, Abdullah
ISSN 2229-5518 2,302 Early diagnosis of systemic lupus erythematosus in primary care by family doctors Saad Ali Alalyani, Abdullah Saad Alalyani, Sultan Salem Algethami, Hamad Sulayyih Alosaimi, Abdullah
MEDICAL COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 08/19/14 SECTION: DRUGS LAST REVIEW DATE: LAST CRITERIA REVISION DATE: ARCHIVE DATE:
 RITUXAN (rituximab) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
RITUXAN (rituximab) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Alpha1-Proteinase Inhibitors Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 5 References... 6 Effective Date...
Cigna Drug and Biologic Coverage Policy Subject Alpha1-Proteinase Inhibitors Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 5 References... 6 Effective Date...
Market Access CTR Summary
 Market Access CTR Summary Study No.: BEL114246 Title: Efficacy of Belimumab Treatment in a Subpopulation of Systemic Lupus Erythematosus (SLE) Patients: A Pooled Analysis of the HGS1006-C1056 (BLISS-52)
Market Access CTR Summary Study No.: BEL114246 Title: Efficacy of Belimumab Treatment in a Subpopulation of Systemic Lupus Erythematosus (SLE) Patients: A Pooled Analysis of the HGS1006-C1056 (BLISS-52)
CSTAR CASE STUDIES: BLOCK F Type 3 Hypersensitivity Reaction
 CSTAR CASE STUDIES: BLOCK F Type 3 Hypersensitivity Reaction Setting: ER Mr. Smith I ve just felt so weak for so long, and I ve lost so much weight, and now I m having trouble breathing it s affecting
CSTAR CASE STUDIES: BLOCK F Type 3 Hypersensitivity Reaction Setting: ER Mr. Smith I ve just felt so weak for so long, and I ve lost so much weight, and now I m having trouble breathing it s affecting
and rheumatism arthritis THE 1982 REVISED CRITERIA FOR THE CLASSIFICATION OF SYSTEMIC LUPUS ERYTHEMATOSUS SPECIAL ARTICLE
 arthritis and rheumatism * Official Journal of the American Rheumatism Association Section of the Arthritis Foundation SPECIAL ARTICLE THE 1982 REVISED CRITERIA F THE CLASSIFICATION OF SYSTEMIC LUPUS ERYTHEMATOSUS
arthritis and rheumatism * Official Journal of the American Rheumatism Association Section of the Arthritis Foundation SPECIAL ARTICLE THE 1982 REVISED CRITERIA F THE CLASSIFICATION OF SYSTEMIC LUPUS ERYTHEMATOSUS
Dr. Venkateswari. R. Dr. Janani Sankar s unit Kanchi Kamakoti CHILDS Trust Hospital
 Dr. Venkateswari. R. Dr. Janani Sankar s unit Kanchi Kamakoti CHILDS Trust Hospital Acknowledgements: KKCTH Dr. Ramkumar Consultant Dermatologist Dr. Ramprakash Consultant Ophthalmologist Dr. Prasad Manne
Dr. Venkateswari. R. Dr. Janani Sankar s unit Kanchi Kamakoti CHILDS Trust Hospital Acknowledgements: KKCTH Dr. Ramkumar Consultant Dermatologist Dr. Ramprakash Consultant Ophthalmologist Dr. Prasad Manne
Definition Chronic autoimmune disease The body s immune system starts attacking itself Can affect most organs and tissues in the body Brain, lungs, he
 LIVING WITH SYSTEMIC LUPUS ERYTHEMATOSUS Stacy Kennedy, M.D.,M.B.A. Rowan Diagnostic Clinic Salisbury, N.C. May 11, 2013 Agenda What is lupus Who is affected Causes of lupus Symptoms and organ involvement
LIVING WITH SYSTEMIC LUPUS ERYTHEMATOSUS Stacy Kennedy, M.D.,M.B.A. Rowan Diagnostic Clinic Salisbury, N.C. May 11, 2013 Agenda What is lupus Who is affected Causes of lupus Symptoms and organ involvement
Belimumab: Present and future
 Mini Review imedpub Journals http://www.imedpub.com Journal of Autoimmune Disorders Belimumab: Present and future CD Tripathi and Preeta Kaur Chugh Abstract Belimumab, a B lymphocyte stimulator (BLyS)
Mini Review imedpub Journals http://www.imedpub.com Journal of Autoimmune Disorders Belimumab: Present and future CD Tripathi and Preeta Kaur Chugh Abstract Belimumab, a B lymphocyte stimulator (BLyS)
UNDERSTANDING SYSTEMIC LUPUS ERYTHEMATOSUS
 UNDERSTANDING SYSTEMIC LUPUS ERYTHEMATOSUS Stacy Kennedy, M.D.,M.B.A. October 20, 2012 Agenda What is lupus Who is affected Causes of lupus Symptoms and organ involvement Diagnosis Treatment Pregnancy
UNDERSTANDING SYSTEMIC LUPUS ERYTHEMATOSUS Stacy Kennedy, M.D.,M.B.A. October 20, 2012 Agenda What is lupus Who is affected Causes of lupus Symptoms and organ involvement Diagnosis Treatment Pregnancy
New Drug Evaluation: Belimumab Injection, Intravenous and Subcutaneous
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Policy. Section: Medicine Effective Date: January 15, 2015 Subsection: Pathology/Laboratory Original Policy Date: December 5, 2014 Subject:
 Last Review Status/Date: December 2014 Page: 1 of 10 Summary Systemic lupus erythematosus (SLE) is an autoimmune connective tissue disease that can be difficult to diagnose because patients often present
Last Review Status/Date: December 2014 Page: 1 of 10 Summary Systemic lupus erythematosus (SLE) is an autoimmune connective tissue disease that can be difficult to diagnose because patients often present
Arthritis & Rheumatology Clinics of Kansas PATIENT EDUCATION SYSTEMIC LUPUS ERYTHEMATOSUS
 Arthritis & Rheumatology Clinics of Kansas PATIENT EDUCATION SYSTEMIC LUPUS ERYTHEMATOSUS Introduction: There is perhaps no rheumatic disease that evokes so much fear and confusion among both patients
Arthritis & Rheumatology Clinics of Kansas PATIENT EDUCATION SYSTEMIC LUPUS ERYTHEMATOSUS Introduction: There is perhaps no rheumatic disease that evokes so much fear and confusion among both patients
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Voriconazole Effective Date... 3/15/2018 Next Review Date... 3/15/2019 Coverage Policy Number... 4004 Table of Contents Coverage Policy... 1 General Background...
Cigna Drug and Biologic Coverage Policy Subject Voriconazole Effective Date... 3/15/2018 Next Review Date... 3/15/2019 Coverage Policy Number... 4004 Table of Contents Coverage Policy... 1 General Background...
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Omalizumab Table of Contents Coverage Policy... 1 General Background... 4 Coding/Billing Information... 6 References... 7 Effective Date... 3/15/2018 Next
Cigna Drug and Biologic Coverage Policy Subject Omalizumab Table of Contents Coverage Policy... 1 General Background... 4 Coding/Billing Information... 6 References... 7 Effective Date... 3/15/2018 Next
BENLYSTA PRODUCT INFORMATION
 BENLYSTA PRODUCT INFORMATION NAME OF THE MEDICINE The active ingredient of BENLYSTA is belimumab (rmc). DESCRIPTION Belimumab is a fully human IgG1λ monoclonal antibody specific for soluble human B Lymphocyte
BENLYSTA PRODUCT INFORMATION NAME OF THE MEDICINE The active ingredient of BENLYSTA is belimumab (rmc). DESCRIPTION Belimumab is a fully human IgG1λ monoclonal antibody specific for soluble human B Lymphocyte
Summary Article: Lupus (Systemic Lupus Erythematosus) from Harvard Medical School Health Topics A-Z
 Topic Page: Systemic Lupus Erythematosus Summary Article: Lupus (Systemic Lupus Erythematosus) from Harvard Medical School Health Topics A-Z What Is It? Lupus is thought to develop when the immune system
Topic Page: Systemic Lupus Erythematosus Summary Article: Lupus (Systemic Lupus Erythematosus) from Harvard Medical School Health Topics A-Z What Is It? Lupus is thought to develop when the immune system
Rheumatology Primer: What Labs and When
 Rheumatology Primer: What Labs and When Irina Konon, MD Department of Internal Medicine Division of Rheumatology Medical College of Wisconsin Disclosures None 1 Objective Discuss principles of laboratory
Rheumatology Primer: What Labs and When Irina Konon, MD Department of Internal Medicine Division of Rheumatology Medical College of Wisconsin Disclosures None 1 Objective Discuss principles of laboratory
Lupus. Fast facts. What is lupus? What causes lupus? Who gets lupus?
 Lupus Systemic lupus erythematosus, referred to as SLE or lupus, is sometimes called the "great imitator." Why? Because of its wide range of symptoms, people often confuse lupus with other health problems.
Lupus Systemic lupus erythematosus, referred to as SLE or lupus, is sometimes called the "great imitator." Why? Because of its wide range of symptoms, people often confuse lupus with other health problems.
High Impact Rheumatology
 High Impact Rheumatology Systemic Lupus Erythematosus Bernard Rubin, DO MPH Case 1: History A 45-year-old woman presents with severe dyspnea and cough. She was in excellent health until 4 weeks ago when
High Impact Rheumatology Systemic Lupus Erythematosus Bernard Rubin, DO MPH Case 1: History A 45-year-old woman presents with severe dyspnea and cough. She was in excellent health until 4 weeks ago when
New Drug Evaluation: Belimumab Injection, Intravenous and Subcutaneous
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Policy. Background
 Last Review Status/Date: December 2016 Page: 1 of 11 Summary Systemic lupus erythematosus (SLE) is an autoimmune connective tissue disease that can be difficult to diagnose because patients often present
Last Review Status/Date: December 2016 Page: 1 of 11 Summary Systemic lupus erythematosus (SLE) is an autoimmune connective tissue disease that can be difficult to diagnose because patients often present
Section: Medicine Effective Date: January 15, 2016 Subsection: Pathology/Laboratory Original Policy Date: December 5, 2014 Subject:
 Last Review Status/Date: December 2015 Page: 1 of 11 Summary Systemic lupus erythematosus (SLE) is an autoimmune connective tissue disease that can be difficult to diagnose because patients often present
Last Review Status/Date: December 2015 Page: 1 of 11 Summary Systemic lupus erythematosus (SLE) is an autoimmune connective tissue disease that can be difficult to diagnose because patients often present
EXTENDED REPORT. Clinical and epidemiological research
 1 Allegheny Singer Research Institute, West Penn Allegheny Health System, Temple University School of Medicine, Pittsburgh, Pennsylvania, USA 2 Instituto Nacional de Ciencias Medicas y Nutricion Salvador
1 Allegheny Singer Research Institute, West Penn Allegheny Health System, Temple University School of Medicine, Pittsburgh, Pennsylvania, USA 2 Instituto Nacional de Ciencias Medicas y Nutricion Salvador
Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta397
 Belimumab for treating active autoantibody-positive systemic lupus erythematosus Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta397 NICE 2018. All rights reserved. Subject
Belimumab for treating active autoantibody-positive systemic lupus erythematosus Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta397 NICE 2018. All rights reserved. Subject
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Mecasermin Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 5 References... 5 Effective Date... 5/15/2017 Next
Cigna Drug and Biologic Coverage Policy Subject Mecasermin Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 5 References... 5 Effective Date... 5/15/2017 Next
Autoimmune diseases. SLIDE 3: Introduction to autoimmune diseases Chronic
 SLIDE 3: Introduction to autoimmune diseases Chronic Autoimmune diseases Sometimes relapsing : and remitting. which means that they present as attacks Progressive damage Epitope spreading more and more
SLIDE 3: Introduction to autoimmune diseases Chronic Autoimmune diseases Sometimes relapsing : and remitting. which means that they present as attacks Progressive damage Epitope spreading more and more
Systemic Lupus Erythematosus (SLE) Epidemiology of SLE (United States)
 1:10-2:25pm Closing the Loop on Lupus: Primary Care s Key Role in the Elusive Diagnosis and Management of Patients SPEAKER Richard Sadovsky, MD Richard Furie, MD Disclosures This session is supported by
1:10-2:25pm Closing the Loop on Lupus: Primary Care s Key Role in the Elusive Diagnosis and Management of Patients SPEAKER Richard Sadovsky, MD Richard Furie, MD Disclosures This session is supported by
To live with lupus, we need to know about lupus.
 To live with lupus, we need to know about lupus. Zineb Aouhab, MD Assistant Professor of Medicine Loyola University Medical Center Division of Rheumatology 1 Where did the word lupus come from? The word
To live with lupus, we need to know about lupus. Zineb Aouhab, MD Assistant Professor of Medicine Loyola University Medical Center Division of Rheumatology 1 Where did the word lupus come from? The word
Persistent disease activity may have significant implications for your SLE patients life journey. 1,2
 Persistent disease activity may have significant implications for your SLE patients life journey. 1,2 Is it time to increase the focus on disease activity reduction? BENLYSTA (belimumab) is indicated as
Persistent disease activity may have significant implications for your SLE patients life journey. 1,2 Is it time to increase the focus on disease activity reduction? BENLYSTA (belimumab) is indicated as
Controversies in Women s Health: Clinical Dilemmas in Arthritis
 Controversies in Women s Health: Clinical Dilemmas in Arthritis Jonathan Graf, M.D. Assistant Professor of Medicine, UCSF Division of Rheumatology, SFGH December, 2008 Approximate Prevalence of Rheumatic
Controversies in Women s Health: Clinical Dilemmas in Arthritis Jonathan Graf, M.D. Assistant Professor of Medicine, UCSF Division of Rheumatology, SFGH December, 2008 Approximate Prevalence of Rheumatic
UPDATES ON PEDIATRIC SLE
 UPDATES ON PEDIATRIC SLE BY ANGELA MIGOWA, PEDIATRIC RHEUMATOLOGIST/SENIOR INSTRUCTOR AKUHN MBCHB-UON, MMED-AKUHN,PEDIATRIC RHEUMATOLOGY- MCGILL UNIVERSITY HEALTH CENTRE ROSA PARKS OBJECTIVES RECOGNIZE
UPDATES ON PEDIATRIC SLE BY ANGELA MIGOWA, PEDIATRIC RHEUMATOLOGIST/SENIOR INSTRUCTOR AKUHN MBCHB-UON, MMED-AKUHN,PEDIATRIC RHEUMATOLOGY- MCGILL UNIVERSITY HEALTH CENTRE ROSA PARKS OBJECTIVES RECOGNIZE
9/25/2013 SYSTEMIC LUPUS ERYTHEMATOSUS (SLE)
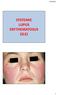 SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) 1 Other Types of Lupus Discoid Lupus Erythematosus Lupus Pernio --- Sarcoidosis Lupus Vulgaris --- Tuberculosis of the face Manifestations of SLE Fever Rashes Arthritis
SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) 1 Other Types of Lupus Discoid Lupus Erythematosus Lupus Pernio --- Sarcoidosis Lupus Vulgaris --- Tuberculosis of the face Manifestations of SLE Fever Rashes Arthritis
.,Dr Ali Alkazzaz Babylon collage of medicine 2016
 .,Dr Ali Alkazzaz Babylon collage of medicine 2016 Lupus history Lupus is the Latin word for wolf 1 st used medically in the 10 th century Described clinically in the 19 th century Butterfly rash in 1845
.,Dr Ali Alkazzaz Babylon collage of medicine 2016 Lupus history Lupus is the Latin word for wolf 1 st used medically in the 10 th century Described clinically in the 19 th century Butterfly rash in 1845
10/6/08. Systemic Lupus Erythematosus. SLE Epidemiology: who is at risk? Margrit Wiesendanger Division of Rheumatology, CUMC.
 Systemic Lupus Erythematosus SLE Epidemiology: who is at risk? One of the most common autoimmune diseases affecting women of all ages Predominantly women in child-bearing years (M:F ratio is 1:10) Incidence
Systemic Lupus Erythematosus SLE Epidemiology: who is at risk? One of the most common autoimmune diseases affecting women of all ages Predominantly women in child-bearing years (M:F ratio is 1:10) Incidence
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Eculizumab Table of Contents Coverage Policy... 1 General Background... 4 Coding/Billing Information... 5 References... 6 Effective Date... 8/15/2018 Next
Cigna Drug and Biologic Coverage Policy Subject Eculizumab Table of Contents Coverage Policy... 1 General Background... 4 Coding/Billing Information... 5 References... 6 Effective Date... 8/15/2018 Next
Evidence Based Treatment of SLE with Treatment Algorithm. Dr. Md. Mujibur Rahman Professor of Medicine Shaheed Suhrawardy Medical College
 Evidence Based Treatment of SLE with Treatment Algorithm Dr. Md. Mujibur Rahman Professor of Medicine Shaheed Suhrawardy Medical College Natural Histoty Inflammatory multisystem disease Onset usually between
Evidence Based Treatment of SLE with Treatment Algorithm Dr. Md. Mujibur Rahman Professor of Medicine Shaheed Suhrawardy Medical College Natural Histoty Inflammatory multisystem disease Onset usually between
2017 Blue Cross and Blue Shield of Louisiana
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
SLE and the Antiphospholipid Syndrome
 SLE and the Antiphospholipid Syndrome Susan Y. Ritter MD, PhD Associate Physician Division of Rheumatology, Immunology and Allergy Department of Medicine Brigham and Women s Hospital Instructor in Medicine
SLE and the Antiphospholipid Syndrome Susan Y. Ritter MD, PhD Associate Physician Division of Rheumatology, Immunology and Allergy Department of Medicine Brigham and Women s Hospital Instructor in Medicine
Learning about Lupus. Learning About Lupus. Lupus Society of Illinois
 Learning About Lupus Learning about Lupus Lupus Society of Illinois 525 W. Monroe Street, Suite 900 Chicago, Illinois 60661 Robert S. Katz, M.D. Professor of Medicine Rush University Medical Center Northwestern
Learning About Lupus Learning about Lupus Lupus Society of Illinois 525 W. Monroe Street, Suite 900 Chicago, Illinois 60661 Robert S. Katz, M.D. Professor of Medicine Rush University Medical Center Northwestern
Living with Lupus: An Insider s Perspective
 Living with Lupus: An Insider s Perspective Pamela Thorpe, MD, FACP Lupus Foundation of America, Inc. Philadelphia Tri-State Chapter Volunteer May 2014 My Own Story Is it Lupus Yet? The What What is this?
Living with Lupus: An Insider s Perspective Pamela Thorpe, MD, FACP Lupus Foundation of America, Inc. Philadelphia Tri-State Chapter Volunteer May 2014 My Own Story Is it Lupus Yet? The What What is this?
Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response
 ARD Online First, published on April 5, 2012 as 10.1136/annrheumdis-2011-200937 1 Unit for Clinical Therapy Research, Infl ammatory Diseases (ClinTRID), The Karolinska Institute, Stockholm, Sweden 2 Division
ARD Online First, published on April 5, 2012 as 10.1136/annrheumdis-2011-200937 1 Unit for Clinical Therapy Research, Infl ammatory Diseases (ClinTRID), The Karolinska Institute, Stockholm, Sweden 2 Division
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Canakinumab Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 5 References... 5 Effective Date... 7/15/2017 Next
Cigna Drug and Biologic Coverage Policy Subject Canakinumab Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 5 References... 5 Effective Date... 7/15/2017 Next
Insights into the DX of Pediatric SLE
 Insights into the DX of Pediatric SLE Dr. John H. Yost Pediatric Rheumatology Children s Hospital at Dartmouth Assistant Professor of Medicine Geisel School of Medicine at Dartmouth john.h.yost@hitchcock.org
Insights into the DX of Pediatric SLE Dr. John H. Yost Pediatric Rheumatology Children s Hospital at Dartmouth Assistant Professor of Medicine Geisel School of Medicine at Dartmouth john.h.yost@hitchcock.org
New long-term organ damage analysis published for GSK s Benlysta (belimumab)
 PRESS RELEASE Issued: Thursday 3 March 2016, London, UK New long-term organ damage analysis published for GSK s Benlysta (belimumab) GSK today announced publication of a new long-term analysis showing
PRESS RELEASE Issued: Thursday 3 March 2016, London, UK New long-term organ damage analysis published for GSK s Benlysta (belimumab) GSK today announced publication of a new long-term analysis showing
Conflict of Interest. Systemic Lupus Erythematosus and the Antiphospholipid Syndrome Bonnie L. Bermas, MD Brigham and Women s Hospital.
 Systemic Lupus Erythematosus and the Antiphospholipid Syndrome Bonnie L. Bermas, MD Brigham and Women s Hospital Conflict of Interest Disclosures: None Overview Diagnostic Classification Criteria of SLE
Systemic Lupus Erythematosus and the Antiphospholipid Syndrome Bonnie L. Bermas, MD Brigham and Women s Hospital Conflict of Interest Disclosures: None Overview Diagnostic Classification Criteria of SLE
Serum Biomarker Panel Testing for Systemic Lupus Erythematosus
 Serum Biomarker Panel Testing for Systemic Lupus Erythematosus Policy Number: 2.04.123 Last Review: 8/2017 Origination: 8/2015 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Serum Biomarker Panel Testing for Systemic Lupus Erythematosus Policy Number: 2.04.123 Last Review: 8/2017 Origination: 8/2015 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
RITUXAN (rituximab and hyaluronidase human)
 Drug Prior Authorization Guideline RITUXIMAB products J9310 RITUXAN (rituximab and hyaluronidase human) PA9847 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria
Drug Prior Authorization Guideline RITUXIMAB products J9310 RITUXAN (rituximab and hyaluronidase human) PA9847 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria
Development of SLE among Possible SLE Patients Seen in Consultation: Long-Term Follow-Up. Disclosures. Background. Evidence-Based Medicine.
 Development of SLE among Patients Seen in Consultation: Long-Term Follow-Up Abstract # 1699 May Al Daabil, MD Bonnie L. Bermas, MD Alexander Fine Hsun Tsao Patricia Ho Joseph F. Merola, MD Peter H. Schur,
Development of SLE among Patients Seen in Consultation: Long-Term Follow-Up Abstract # 1699 May Al Daabil, MD Bonnie L. Bermas, MD Alexander Fine Hsun Tsao Patricia Ho Joseph F. Merola, MD Peter H. Schur,
Systemic Lupus Erythematosus (SLE) Learning Objectives. Presenter Disclosure Information
 9:30 10:45 AM Closing the Loop on Lupus: Primary Care s Key Role in the Elusive Diagnosis and Management of Patients Presenter Disclosure Information The following relationships exist related to this presentation:
9:30 10:45 AM Closing the Loop on Lupus: Primary Care s Key Role in the Elusive Diagnosis and Management of Patients Presenter Disclosure Information The following relationships exist related to this presentation:
India; Tamil Nadu, India; Received on: Accepted on:
 ISSN: 0975-766X CODEN: IJPTFI Available Online through Research Article www.ijptonline.com DECISION SUPPT SYSTEM F SYSTEMIC LUPUS ERYTHEMATOSUS USING MACHINE LEARNING TECHNIQUES S. Samundeswari 1,2*, V.
ISSN: 0975-766X CODEN: IJPTFI Available Online through Research Article www.ijptonline.com DECISION SUPPT SYSTEM F SYSTEMIC LUPUS ERYTHEMATOSUS USING MACHINE LEARNING TECHNIQUES S. Samundeswari 1,2*, V.
Horizon Scanning Centre May Tabalumab for systemic lupus erythematosus SUMMARY NIHR HSC ID: 5581
 Horizon Scanning Centre May 2014 Tabalumab for systemic lupus erythematosus SUMMARY NIHR HSC ID: 5581 This briefing is based on information available at the time of research and a limited literature search.
Horizon Scanning Centre May 2014 Tabalumab for systemic lupus erythematosus SUMMARY NIHR HSC ID: 5581 This briefing is based on information available at the time of research and a limited literature search.
A 6-month open-label extension study of the safety and efficacy of subcutaneous belimumab in patients with systemic lupus erythematosus
 (2018) 27, 1489 1498 journals.sagepub.com/home/lup PAPER of the safety and efficacy of subcutaneous belimumab in patients with systemic lupus erythematosus A Doria 1, D Bass 2, A Schwarting 3,4, A Hammer
(2018) 27, 1489 1498 journals.sagepub.com/home/lup PAPER of the safety and efficacy of subcutaneous belimumab in patients with systemic lupus erythematosus A Doria 1, D Bass 2, A Schwarting 3,4, A Hammer
MEDICATION GUIDE. BENLYSTA (ben-list-ah) (belimumab) Injection for intravenous use
 MEDICATION GUIDE BENLYSTA (ben-list-ah) (belimumab) Injection for intravenous use Read this Medication Guide before you start receiving BENLYSTA and before each treatment. There may be new information.
MEDICATION GUIDE BENLYSTA (ben-list-ah) (belimumab) Injection for intravenous use Read this Medication Guide before you start receiving BENLYSTA and before each treatment. There may be new information.
Kevzara (sarilumab) NEW PRODUCT SLIDESHOW
 Kevzara (sarilumab) NEW PRODUCT SLIDESHOW Introduction Brand name: Kevzara Generic name: Sarilumab Pharmacological class: Interleukin-6 antagonist Strength and Formulation: 150mg/1.14mL, 200mg/1.14mL;
Kevzara (sarilumab) NEW PRODUCT SLIDESHOW Introduction Brand name: Kevzara Generic name: Sarilumab Pharmacological class: Interleukin-6 antagonist Strength and Formulation: 150mg/1.14mL, 200mg/1.14mL;
Belimumab (Benlysta) A Breakthrough Therapy for Systemic Lupus Erythematosus
 Belimumab (Benlysta) A Breakthrough Therapy for Systemic Lupus Erythematosus Raymond Lamore III, PharmD; Sapna Parmar, PharmD; Khilna Patel, PharmD Candidate; and Olga Hilas, PharmD, MPH, BCPS, CGP INTRODUCTION
Belimumab (Benlysta) A Breakthrough Therapy for Systemic Lupus Erythematosus Raymond Lamore III, PharmD; Sapna Parmar, PharmD; Khilna Patel, PharmD Candidate; and Olga Hilas, PharmD, MPH, BCPS, CGP INTRODUCTION
Staff-Assisted Home Hemodialysis
 Medical Coverage Policy Staff-Assisted Home Hemodialysis Table of Contents Coverage Policy... 1 Overview... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date...11/15/2017
Medical Coverage Policy Staff-Assisted Home Hemodialysis Table of Contents Coverage Policy... 1 Overview... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date...11/15/2017
Neuropsychiatric Systemic Lupus Erythematosus (NPSLE) Case presentations and topic discussion The Rheumatology Unit UMMC experience
 Neuropsychiatric Systemic Lupus Erythematosus (NPSLE) Case presentations and topic discussion The Rheumatology Unit UMMC experience References Sanna G, Bertolaccini ML. Neuropsychiatric manifestations
Neuropsychiatric Systemic Lupus Erythematosus (NPSLE) Case presentations and topic discussion The Rheumatology Unit UMMC experience References Sanna G, Bertolaccini ML. Neuropsychiatric manifestations
Which outcome measures in SLE clinical trials best reflect medical judgment?
 To cite: Thanou A, Chakravarty E, James JA, et al. Which outcome measures in SLE clinical trials best reflect medical judgment? Lupus Science & Medicine 2014;1:e000005. doi:10.1136/lupus-2013-000005 Received
To cite: Thanou A, Chakravarty E, James JA, et al. Which outcome measures in SLE clinical trials best reflect medical judgment? Lupus Science & Medicine 2014;1:e000005. doi:10.1136/lupus-2013-000005 Received
Living with Lupus. LFA - Georgia Gary E Myerson MD Arthritis and Rheumatology of GA
 Living with Lupus LFA - Georgia Gary E Myerson MD Arthritis and Rheumatology of GA LUPUS A REALITY CHECK LUPUS A REALITY CHECK LUPUS A REALITY CHECK LUPUS A REALITY CHECK SLE 1.5 million Americans: some
Living with Lupus LFA - Georgia Gary E Myerson MD Arthritis and Rheumatology of GA LUPUS A REALITY CHECK LUPUS A REALITY CHECK LUPUS A REALITY CHECK LUPUS A REALITY CHECK SLE 1.5 million Americans: some
New Onset Arthritis. Clinical Dilemmas in Arthritis and Rheumatology. Physical Examination. Other Pertinent History
 New Onset Arthritis Clinical Dilemmas in Arthritis and Rheumatology Primary Care Principles and Practice October 2008 Jonathan Graf, MD Assistant Professor of Medicine, UCSF Division of Rheumatology, SFGH
New Onset Arthritis Clinical Dilemmas in Arthritis and Rheumatology Primary Care Principles and Practice October 2008 Jonathan Graf, MD Assistant Professor of Medicine, UCSF Division of Rheumatology, SFGH
LABORATORY STANDARDS IN THE DIAGNOSIS AND THERAPY MONITORING OF SYSTEMIC LUPUS ERYTHEMATOSUS
 LABORATORY STANDARDS IN THE DIAGNOSIS AND THERAPY MONITORING OF SYSTEMIC LUPUS ERYTHEMATOSUS Prof. Sandor Sipka, M.D., Ph.D. 3rd Department of Medicine, Institute for Internal Medicine, Medical and Health
LABORATORY STANDARDS IN THE DIAGNOSIS AND THERAPY MONITORING OF SYSTEMIC LUPUS ERYTHEMATOSUS Prof. Sandor Sipka, M.D., Ph.D. 3rd Department of Medicine, Institute for Internal Medicine, Medical and Health
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked
ENFERMEDADES AUTOINMUNES SISTÉMICAS. Dr. J. María Pego Reigosa
 ENFERMEDADES AUTOINMUNES SISTÉMICAS Dr. J. María Pego Reigosa ABSTRACT NUMBER: 888 PHASE 3 TRIAL RESULTS WITH BLISIBIMOD, A SELECTIVE INHIBITOR OF B-CELL ACTIVATING FACTOR, IN SUBJECTS WITH MODERATE-TO-SEVERE
ENFERMEDADES AUTOINMUNES SISTÉMICAS Dr. J. María Pego Reigosa ABSTRACT NUMBER: 888 PHASE 3 TRIAL RESULTS WITH BLISIBIMOD, A SELECTIVE INHIBITOR OF B-CELL ACTIVATING FACTOR, IN SUBJECTS WITH MODERATE-TO-SEVERE
SYNOPSIS. Clinical Study Report IM Double-blind Period
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier SYNOPSIS (For National Authority
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier SYNOPSIS (For National Authority
Clinical Policy: Etanercept (Enbrel) Reference Number: PA.CP.PHAR.250 Effective Date: 01/18 Last Review Date: 08/17 Line of Business: Medicaid
 Clinical Policy: (Enbrel) Reference Number: PA.CP.PHAR.250 Effective Date: 01/18 Last Review Date: 08/17 Line of Business: Medicaid Coding Implications Revision Log Description (Enbrel ) is tumor necrosis
Clinical Policy: (Enbrel) Reference Number: PA.CP.PHAR.250 Effective Date: 01/18 Last Review Date: 08/17 Line of Business: Medicaid Coding Implications Revision Log Description (Enbrel ) is tumor necrosis
Methotrexate Injectable Step Therapy Program Summary
 Methotrexate Injectable Step Therapy Program Summary This prior authorization applies to Commercial, SourceRx and Health Insurance Marketplace formularies. OBJECTIVE The intent of the methotrexate injectable
Methotrexate Injectable Step Therapy Program Summary This prior authorization applies to Commercial, SourceRx and Health Insurance Marketplace formularies. OBJECTIVE The intent of the methotrexate injectable
Synopsis Style Clinical Study Report SAR ACT sarilumab Version number : 1 (electronic 1.0)
 SYNOPSIS Title of the study: A randomized, double-blind, parallel-group, placebo- and active calibrator-controlled study assessing the clinical benefit of SAR153191 subcutaneous (SC) on top of methotrexate
SYNOPSIS Title of the study: A randomized, double-blind, parallel-group, placebo- and active calibrator-controlled study assessing the clinical benefit of SAR153191 subcutaneous (SC) on top of methotrexate
Pharmacy Management Drug Policy
 SUBJECT: : Nucala (mepolizumab), Cinqair (reslizumab), & Fasenra (benralizumab) POLICY NUMBER: Pharmacy-62 EFFECTIVE DATE: 12/15 LAST REVIEW DATE: 3/5/2018 If the member s subscriber contract excludes
SUBJECT: : Nucala (mepolizumab), Cinqair (reslizumab), & Fasenra (benralizumab) POLICY NUMBER: Pharmacy-62 EFFECTIVE DATE: 12/15 LAST REVIEW DATE: 3/5/2018 If the member s subscriber contract excludes
MP Serum Biomarker Panel Testing for Systemic Lupus Erythematosus and Other Connective Tissue Diseases
 Medical Policy MP 2.04.123 Serum Biomarker Panel Testing for Systemic Lupus Erythematosus and Other Connective Tissue Diseases BCBSA Ref. Policy: 2.04.123 Last Review: 06/27/2018 Effective Date: 06/27/2018
Medical Policy MP 2.04.123 Serum Biomarker Panel Testing for Systemic Lupus Erythematosus and Other Connective Tissue Diseases BCBSA Ref. Policy: 2.04.123 Last Review: 06/27/2018 Effective Date: 06/27/2018
Belimumab for Systemic Lupus Erythematosus
 T h e n e w e ngl a nd j o u r na l o f m e dic i n e clinical therapeutics Belimumab for Systemic Lupus Erythematosus Bevra Hannahs Hahn, M.D. This Journal feature begins with a case vignette that includes
T h e n e w e ngl a nd j o u r na l o f m e dic i n e clinical therapeutics Belimumab for Systemic Lupus Erythematosus Bevra Hannahs Hahn, M.D. This Journal feature begins with a case vignette that includes
Systemic examination
 PROLONGED FEVER IN AN ADOLESCENT BOY Dr.Praveena Lionel, DNB PG, Dr.Kannan (HOD) Railway Hospital, Perambur History 11 yrs old adolescent boy was admitted with c/o Fever -1 wk Myalgia -1 wk Arthralgia
PROLONGED FEVER IN AN ADOLESCENT BOY Dr.Praveena Lionel, DNB PG, Dr.Kannan (HOD) Railway Hospital, Perambur History 11 yrs old adolescent boy was admitted with c/o Fever -1 wk Myalgia -1 wk Arthralgia
Systemic Lupus Erythematosus. Margrit Wiesendanger Division of General Medicine, CUMC
 Systemic Lupus Erythematosus Margrit Wiesendanger Division of General Medicine, CUMC October 13, 2009 SLE Epidemiology: who is at risk? One of the most common autoimmune diseases affecting women of all
Systemic Lupus Erythematosus Margrit Wiesendanger Division of General Medicine, CUMC October 13, 2009 SLE Epidemiology: who is at risk? One of the most common autoimmune diseases affecting women of all
ACTEMRA (tocilizumab)
 ACTEMRA (tocilizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
ACTEMRA (tocilizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Clinical Policy: Etanercept (Enbrel) Reference Number: CP.PHAR.250 Effective Date: 08/16 Last Review Date: 08/17 Line of Business: Medicaid
 Clinical Policy: (Enbrel) Reference Number: CP.PHAR.250 Effective Date: 08/16 Last Review Date: 08/17 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of this
Clinical Policy: (Enbrel) Reference Number: CP.PHAR.250 Effective Date: 08/16 Last Review Date: 08/17 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of this
Scleritis LEN V KOH OD
 Scleritis LEN V KOH OD 2014 PUCO 1 Introduction A painful, destructive, and potentially blinding disorder Highly symptomatic High association with systemic disease Immunosuppresssive agents 2014 PUCO 2
Scleritis LEN V KOH OD 2014 PUCO 1 Introduction A painful, destructive, and potentially blinding disorder Highly symptomatic High association with systemic disease Immunosuppresssive agents 2014 PUCO 2
