Efficacy and Safety of Belimumab in the treatment of Systemic Lupus Erythematosus: a Prospective Multicenter Study.
|
|
|
- Janice Mathews
- 5 years ago
- Views:
Transcription
1 1. Title Efficacy and Safety of Belimumab in the treatment of Systemic Lupus Erythematosus: a Prospective Multicenter Study. 2. Background Systemic Lupus Erythematosus (SLE) is a chronic, autoimmune and multisystemic disease, clinically heterogenous, with remitting and relapsing periods, that predominantly affects women of childbearing age. Therapeutic approach includes antimalarials, corticosteroids, immunosuppressants and cytotoxic agents and depends on the severity of the disease, organ involvement and patient status. 1 Pathophysiologic mechanisms remains largely unknown, but studies suggest a genetic susceptibility and environmental factors (such as virus, ultraviolet light, silica dust, smoking, medications and alcohol) that may act as triggers of the disease. 2 In spite of being one of the few rheumatic diseases with a treat-to-target recommendations approach 3, SLE have one of the highest mortality rates among them. 4 This fact highlines the need for new therapeutic targets in SLE. Some studies have demonstrated the overexpression of BLyS (a B-lymphocyte stimulator) in SLE 5, which may explain the increased autoantibody production in the disease. In 2011, Belimumab, a human monoclonal antibody targeting BLyS, became the first biotechnologic drug available for SLE. Clinical trials with Belimumab showed reduction in the disease activity, reduction in the number and in the severity of flares, steroid-sparing effects and improvement in health-related quality of life and fatigue. 6-8 Belimumab showed better efectiveness in patients with higher disease activity, anti-dsdna positivity and low complement levels at baseline. 9 Belimumab is not approved for lupus nephritis or central nervous system lupus, as the clinical trials excluded these patients. A randomized, double-blinded, placebo-controlled phase III study is ongoing to evaluate the efectiveness and tolerability of Belimumab in adults with active lupus nephritis (BLISS-LN). In the European Union, Belimumab is indicated in adult patients with active and autoantibody-positive SLE with high degree of disease activity (low complement levels and high anti-dsdna antibodies), despite standard therapy. 10 With the widespread use of Belimumab, it becomes necessary to evaluate its efectiveness and safety in clinical practice. We intend to study the efectiveness and safety of the use of Belimumab in SLE patients followed in the portuguese rheumatology centers. 3. Objectives Primary Objective: - Evaluate Belimumab effectiveness and safety in the treatment of a Portuguese SLE multicenter sample. Secundary Objectives: - Identify the main indications for the treatment with Belimumab in the real world setting.
2 -Identify possible relationships between organic involvement or other patients characteristics and the response to Belimumab. - Acess the steroid-sparing effects of Belimumab. Primary Outcomes: - Efectiveness: proportion of patients that showed a >= 4-point reduction SELENA-SLEDAI score (SLE Responder Index) at 6, 12 and 24 months of treatment with Belimumab. - Safety: any adverse events during the treatment with Belimumab, including deaths and malignancies, and necessity of suspension of Belimumab. Secundary Outcomes: - Proportion of patients that showed a reduction in SELENA-SLEDAI score (SLE Responder Index) at 6, 12 and 24 months of treatment with Belimumab. - Proportion of patients that showed a reduction in SELENA-SLEDAI score (SLE Responder Index) at 6, 12 and 24 months of treatment with Belimumab according to the organic involvement. - Proportion of patients that showed a reduction in corticoid therapy dosage at 6, 12 and 24 months of treatment with Belimumab. - Proportion of patients still treated with Belimumab at 6, 12 and 24 monts of treatment. - Predictors of response to belimumab, according SELENA-SLEDAI score (SLE Responder Index), at 6, 12 and 24 months. 4. Methods Type of Study: Prospective Multicenter Cohort Study. Inclusion Criteria: Patients diagnosed with SLE (that fulfill the 2012 SLICC diagnostic criteria 11 ) treated with Belimumab. Variables to be collected - Demographic features: gender, ethnicity, age, body weight (at baseline, 6, 12 and 24 months of treatment with Belimumab) - SLE data: years of disease, age at diagnosis, SLE drugs and duration, organic involvement (musculoskeletal, mucocutaneous, immunological, hematological, serositis, nephritis); SLEDAI (at baseline, 6, 12 and 24 months of treatment with Belimumab); Immunology: AntidsDNA antibodies levels, complement levels [C3 and C4] and immunoglobulins levels [IgG, IgM and IgA] (at baseline, 6, 12 and 24 months of treatment with Belimumab); blood cells count: leukocytes, neutrophils, lymphocytes and platelets count (at baseline, 6, 12 and 24 months of treatment with Belimumab); acute phase reactants: ESR and CRP (at baseline, 6, 12 and 24 months of treatment with Belimumab); Serum creatinine (at baseline, 6, 12 and 24 months of treatment with Belimumab); Urinalysis parameters: protein, red blood cells, white blood cells and casts (at baseline, 6, 12 and 24 months of treatment with Belimumab)
3 - Belimumab data: reason of choice (which disease involvement); protocol of administration and doses; duration of treatment with Belimumab (months); glucocorticoid doses (prednisolone equivalents at baseline, 6, 12 and 24 months of treatment with Belimumab); adverse events (any, including deaths: which adverse event and when); Belimumab suspension (reason and when) 5. Description of variables Descriptive analysis: Continuous variables presented as median, mean and stardard deviation. Changes in SELENA-SLEDAI score before and after treatment with Belimumab will be calculated through Wilcoxon signed rank test. The association between baseline immunology values (complement factors, immunoglobulins levels and anti-dsdna antibodies) and changes in SLEDAI will be calculated using linear correlation. Influence of specific organ involvement and other disease or patients characteristics in the response to the treatment with Belimumab will be accessed through Chi-square test. We also aim to determine the major predictors of a 4-point reduction from baseline in the SELENA SLEDAI score. The magnitude of the associations between clinical and laboratorial variables and the presence of this SELENA SLEDAI score reduction will be assessed using logistic regression models in order to estimate crude and adjusted odds ratios (OR) and their 95% confidence intervals (CI). All analysis were two-sided and p-values <0.05 were considered statistically significant. Alternatively, the magnitude of these associations could also be estimated from linear regression coefficients and respective 95% confidence intervals. 6. Expected results and possible limitations We anticipate that missing data will be a limitation. We expect to confirm the effectiveness of Belimumab in SLE population with high disease activity and positive immunology, particularly in musculoskeletal, mucocutaneous and immunological domains. Concerning safety, we expect to confirm the good tolerability of belimumab, particularly regarding infections. 7. Calendar of tasks Data collection: October 2018 Data analysis: November Proponent Bruno Miguel Fernandes Department of Rheumatology, São João Hospital Center, Porto. 9. Research Team
4 Bruno Miguel Fernandes, Miguel Bernardes, Lúcia Costa Department of Rheumatology, São João Hospital Center, Porto. 10. Institutions The project is open to all Nacional Rheumatology Centers interested in participate. 11. Co-authors All clinicians who actively work on the project will be co-authors, with a maximum of two co-authors per participating center, according to the rules of Vancouver. 12. Conflits of interest There are no conflicts of interest to declare. 13. Ethical considerations This study will be submitted for approval to the Committee of Ethics of São João Hospital Center and will be carried out according to the Declaration of Helsinki. References 1- Bertsias G, Ioannidis JP, Boletis J, Bombardieri S, Cervera R, et al, EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics, Annals of the Rheumatic Diseases 2008;67: ; 2- Fortuna G, Brennan MT, Systemic Lupus Erythematosus Epidemiology, Phatophysiology, Manifestations, and Management, Dental Clinics of North America 2013;57(4): ; 3- Van Vollenhoven RF, Mosca M, Bertsias G, Isenberg D, Kuhn A, et al, Treat-to-target in Systemic Lupus Erythematosus Recommendations from an international task force, Annals of the Rheumatic Diseases 2014;3: Centers dor Disease Control and Prevention, Trends in Deaths from Systemic Lupus Erythematosus United States, , Journal of the American Medical Association 2002;287: Hui-Yuen JS, Li XQ, Askanase AD, Belimumab in Systemic Lupus Erythematosus: a Prespective Review, Therapeutic advances in musculoskeletal disease 2015;7(4): Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, et al, Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebocontrolled, phase 3 trial, The Lancet 2011;26;377(9767): Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, et al, A phase III, randomized, placebocontrolled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus, Arthritis & Rheumatology 2011;63(12): Blair HA, Duggan ST, Belimumab: A Review in Systemic Lupus Erythematosus, Drugs 2018;78(3): Van Vollenhoven RF, Petri MA, Cervera R, Roth DA, Ji BN, et al, Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response, Annals of the Rheumatic Diseases 2012;71(8):
5 10- European Medicines Agency - Benlysta (belimumab): summary of product characteristics. Acessed 25, July, 2018.) 11- Petri M, Orbai A-M, Alarcón GS, Gordon C, Merrill JT, et al, Derivation and Validation of Systemic Lupus International Collaborating Clinics Classification Criteria for Systemic Lupus Erythematosus, Arthritis & Rheumatology 2012;64(8):
Elevated BLyS levels in patients with systemic lupus erythematosus: Associated factors and responses to belimumab
 (2016) 25, 346 354 http://lup.sagepub.com PAPER Elevated BLyS levels in patients with systemic lupus erythematosus: Associated factors and responses to belimumab DA Roth 1, A Thompson 2, Y Tang 2*, AE
(2016) 25, 346 354 http://lup.sagepub.com PAPER Elevated BLyS levels in patients with systemic lupus erythematosus: Associated factors and responses to belimumab DA Roth 1, A Thompson 2, Y Tang 2*, AE
Committee Approval Date: May 9, 2014 Next Review Date: May 2015
 Medication Policy Manual Policy No: dru248 Topic: Benlysta, belimumab Date of Origin: May 13, 2011 Committee Approval Date: May 9, 2014 Next Review Date: May 2015 Effective Date: June 1, 2014 IMPORTANT
Medication Policy Manual Policy No: dru248 Topic: Benlysta, belimumab Date of Origin: May 13, 2011 Committee Approval Date: May 9, 2014 Next Review Date: May 2015 Effective Date: June 1, 2014 IMPORTANT
Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response
 ARD Online First, published on April 5, 2012 as 10.1136/annrheumdis-2011-200937 1 Unit for Clinical Therapy Research, Infl ammatory Diseases (ClinTRID), The Karolinska Institute, Stockholm, Sweden 2 Division
ARD Online First, published on April 5, 2012 as 10.1136/annrheumdis-2011-200937 1 Unit for Clinical Therapy Research, Infl ammatory Diseases (ClinTRID), The Karolinska Institute, Stockholm, Sweden 2 Division
Benlysta (belimumab) Prior Authorization Criteria Program Summary
 Benlysta (belimumab) Prior Authorization Criteria Program Summary This prior authorization applies to Commercial, NetResults A series, NetResults F series and Health Insurance Marketplace formularies.
Benlysta (belimumab) Prior Authorization Criteria Program Summary This prior authorization applies to Commercial, NetResults A series, NetResults F series and Health Insurance Marketplace formularies.
* Autoantibody positive (i.e. antinuclear antibody or ANA titre 1:80 or anti-double stranded (ds) DNA antibody 30 IU/mL)
 Benlysta (belimumab) Policy Number: 5.02.509 Last Review: 05/2018 Origination: 06/2011 Next Review: 05/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Benlysta
Benlysta (belimumab) Policy Number: 5.02.509 Last Review: 05/2018 Origination: 06/2011 Next Review: 05/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Benlysta
Belimumab: Present and future
 Mini Review imedpub Journals http://www.imedpub.com Journal of Autoimmune Disorders Belimumab: Present and future CD Tripathi and Preeta Kaur Chugh Abstract Belimumab, a B lymphocyte stimulator (BLyS)
Mini Review imedpub Journals http://www.imedpub.com Journal of Autoimmune Disorders Belimumab: Present and future CD Tripathi and Preeta Kaur Chugh Abstract Belimumab, a B lymphocyte stimulator (BLyS)
Which outcome measures in SLE clinical trials best reflect medical judgment?
 To cite: Thanou A, Chakravarty E, James JA, et al. Which outcome measures in SLE clinical trials best reflect medical judgment? Lupus Science & Medicine 2014;1:e000005. doi:10.1136/lupus-2013-000005 Received
To cite: Thanou A, Chakravarty E, James JA, et al. Which outcome measures in SLE clinical trials best reflect medical judgment? Lupus Science & Medicine 2014;1:e000005. doi:10.1136/lupus-2013-000005 Received
ONE of the following:
 Medical Coverage Policy Belimumab (Benlysta) EFFECTIVE DATE: 01 01 2012 POLICY LAST UPDATED: 11 21 2017 OVERVIEW Belimumab (Benlysta ) is indicated for the treatment of adult patients with active, autoantibody-positive,
Medical Coverage Policy Belimumab (Benlysta) EFFECTIVE DATE: 01 01 2012 POLICY LAST UPDATED: 11 21 2017 OVERVIEW Belimumab (Benlysta ) is indicated for the treatment of adult patients with active, autoantibody-positive,
EXTENDED REPORT. Clinical and epidemiological research
 1 Allegheny Singer Research Institute, West Penn Allegheny Health System, Temple University School of Medicine, Pittsburgh, Pennsylvania, USA 2 Instituto Nacional de Ciencias Medicas y Nutricion Salvador
1 Allegheny Singer Research Institute, West Penn Allegheny Health System, Temple University School of Medicine, Pittsburgh, Pennsylvania, USA 2 Instituto Nacional de Ciencias Medicas y Nutricion Salvador
Persistent disease activity may have significant implications for your SLE patients life journey. 1,2
 Persistent disease activity may have significant implications for your SLE patients life journey. 1,2 Is it time to increase the focus on disease activity reduction? BENLYSTA (belimumab) is indicated as
Persistent disease activity may have significant implications for your SLE patients life journey. 1,2 Is it time to increase the focus on disease activity reduction? BENLYSTA (belimumab) is indicated as
New Drug Evaluation: Belimumab Injection, Intravenous and Subcutaneous
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Market Access CTR Summary
 Market Access CTR Summary Study No.: BEL114246 Title: Efficacy of Belimumab Treatment in a Subpopulation of Systemic Lupus Erythematosus (SLE) Patients: A Pooled Analysis of the HGS1006-C1056 (BLISS-52)
Market Access CTR Summary Study No.: BEL114246 Title: Efficacy of Belimumab Treatment in a Subpopulation of Systemic Lupus Erythematosus (SLE) Patients: A Pooled Analysis of the HGS1006-C1056 (BLISS-52)
New Drug Evaluation: Belimumab Injection, Intravenous and Subcutaneous
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
New long-term organ damage analysis published for GSK s Benlysta (belimumab)
 PRESS RELEASE Issued: Thursday 3 March 2016, London, UK New long-term organ damage analysis published for GSK s Benlysta (belimumab) GSK today announced publication of a new long-term analysis showing
PRESS RELEASE Issued: Thursday 3 March 2016, London, UK New long-term organ damage analysis published for GSK s Benlysta (belimumab) GSK today announced publication of a new long-term analysis showing
Baseline Predictors of Systemic Lupus Erythematosus Flares
 ARTHRITIS & RHEUMATISM Vol. 65, No. 8, August 2013, pp 2143 2153 DOI 10.1002/art.37995 2013, American College of Rheumatology Baseline Predictors of Systemic Lupus Erythematosus Flares Data From the Combined
ARTHRITIS & RHEUMATISM Vol. 65, No. 8, August 2013, pp 2143 2153 DOI 10.1002/art.37995 2013, American College of Rheumatology Baseline Predictors of Systemic Lupus Erythematosus Flares Data From the Combined
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Belimumab Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 11/15/2017 Next
Cigna Drug and Biologic Coverage Policy Subject Belimumab Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 11/15/2017 Next
Horizon Scanning Centre May Tabalumab for systemic lupus erythematosus SUMMARY NIHR HSC ID: 5581
 Horizon Scanning Centre May 2014 Tabalumab for systemic lupus erythematosus SUMMARY NIHR HSC ID: 5581 This briefing is based on information available at the time of research and a limited literature search.
Horizon Scanning Centre May 2014 Tabalumab for systemic lupus erythematosus SUMMARY NIHR HSC ID: 5581 This briefing is based on information available at the time of research and a limited literature search.
A 6-month open-label extension study of the safety and efficacy of subcutaneous belimumab in patients with systemic lupus erythematosus
 (2018) 27, 1489 1498 journals.sagepub.com/home/lup PAPER of the safety and efficacy of subcutaneous belimumab in patients with systemic lupus erythematosus A Doria 1, D Bass 2, A Schwarting 3,4, A Hammer
(2018) 27, 1489 1498 journals.sagepub.com/home/lup PAPER of the safety and efficacy of subcutaneous belimumab in patients with systemic lupus erythematosus A Doria 1, D Bass 2, A Schwarting 3,4, A Hammer
Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta397
 Belimumab for treating active autoantibody-positive systemic lupus erythematosus Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta397 NICE 2018. All rights reserved. Subject
Belimumab for treating active autoantibody-positive systemic lupus erythematosus Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta397 NICE 2018. All rights reserved. Subject
HIGH ANTI-DSDNA CONTENT IN SLE IMMUNE COMPLEXES IS ASSOCIATED WITH CLINICAL REMISSION FOLLOWING BELIMUMAB TREATMENT
 HIGH ANTI-DSDNA CONTENT IN SLE IMMUNE COMPLEXES IS ASSOCIATED WITH CLINICAL REMISSION FOLLOWING BELIMUMAB TREATMENT A. Sohrabian 1, I. Parodis 2, N. Carlströmer-Berthén 1, C. Sjöwall 3, A. Jönsen 4, A.
HIGH ANTI-DSDNA CONTENT IN SLE IMMUNE COMPLEXES IS ASSOCIATED WITH CLINICAL REMISSION FOLLOWING BELIMUMAB TREATMENT A. Sohrabian 1, I. Parodis 2, N. Carlströmer-Berthén 1, C. Sjöwall 3, A. Jönsen 4, A.
Research Article Performance Characteristics of Different Anti-Double-Stranded DNA Antibody Assays in the Monitoring of Systemic Lupus Erythematosus
 Hindawi Immunology Research Volume 217, Article ID 17292, pages https://doi.org/.11/217/17292 Research Article Performance Characteristics of Different Anti-Double-Stranded DNA Antibody Assays in the Monitoring
Hindawi Immunology Research Volume 217, Article ID 17292, pages https://doi.org/.11/217/17292 Research Article Performance Characteristics of Different Anti-Double-Stranded DNA Antibody Assays in the Monitoring
Belimumab (Benlysta) A Breakthrough Therapy for Systemic Lupus Erythematosus
 Belimumab (Benlysta) A Breakthrough Therapy for Systemic Lupus Erythematosus Raymond Lamore III, PharmD; Sapna Parmar, PharmD; Khilna Patel, PharmD Candidate; and Olga Hilas, PharmD, MPH, BCPS, CGP INTRODUCTION
Belimumab (Benlysta) A Breakthrough Therapy for Systemic Lupus Erythematosus Raymond Lamore III, PharmD; Sapna Parmar, PharmD; Khilna Patel, PharmD Candidate; and Olga Hilas, PharmD, MPH, BCPS, CGP INTRODUCTION
Ronald F. van Vollenhoven Unit for Clinical Therapy Research, Inflammatory Diseases (ClinTRID) The Karolinska Institute Stockholm, Sweden
 Ronald F. van Vollenhoven Unit for Clinical Therapy Research, Inflammatory Diseases (ClinTRID) The Karolinska Institute Stockholm, Sweden Disclosures Research Grants: AbbVie, Amgen, BMS, GSK, Pfizer, Roche,
Ronald F. van Vollenhoven Unit for Clinical Therapy Research, Inflammatory Diseases (ClinTRID) The Karolinska Institute Stockholm, Sweden Disclosures Research Grants: AbbVie, Amgen, BMS, GSK, Pfizer, Roche,
RE: FINAL APPRAISAL DETERMINATION BELIMUMAB FOR THE TREATMENT OF SYSTEMIC LUPUS ERYTHEMATOSUS (SLE)
 GlaxoSmithKline UK Ltd Stockley Park West Uxbridge Middlesex UB11 1BT Tel: +44 (0)20 8990 9000 Fax: +44 (0)20 8990 4321 www.gsk.com BY E-MAIL Friday, May 11, 2012 XXXXXXXXXXX Chair, Appeal Committee National
GlaxoSmithKline UK Ltd Stockley Park West Uxbridge Middlesex UB11 1BT Tel: +44 (0)20 8990 9000 Fax: +44 (0)20 8990 4321 www.gsk.com BY E-MAIL Friday, May 11, 2012 XXXXXXXXXXX Chair, Appeal Committee National
Belimumab for Systemic Lupus Erythematosus
 T h e n e w e ngl a nd j o u r na l o f m e dic i n e clinical therapeutics Belimumab for Systemic Lupus Erythematosus Bevra Hannahs Hahn, M.D. This Journal feature begins with a case vignette that includes
T h e n e w e ngl a nd j o u r na l o f m e dic i n e clinical therapeutics Belimumab for Systemic Lupus Erythematosus Bevra Hannahs Hahn, M.D. This Journal feature begins with a case vignette that includes
ENFERMEDADES AUTOINMUNES SISTÉMICAS. Dr. José María Pego Reigosa
 ENFERMEDADES AUTOINMUNES SISTÉMICAS Dr. José María Pego Reigosa ABSTRACT NUMBER: 2754 Comparison of Individually Tailored vs Systematic Rituximab Regimens to Maintain ANCA-Associated Vasculitis Remissions:
ENFERMEDADES AUTOINMUNES SISTÉMICAS Dr. José María Pego Reigosa ABSTRACT NUMBER: 2754 Comparison of Individually Tailored vs Systematic Rituximab Regimens to Maintain ANCA-Associated Vasculitis Remissions:
Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December Reference : NHSCB/A3C/1b
 Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December 2012 Reference : NHSCB/A3C/1b NHS Commissioning Board Clinical Commissioning Policy Statement: Rituximab
Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December 2012 Reference : NHSCB/A3C/1b NHS Commissioning Board Clinical Commissioning Policy Statement: Rituximab
Correlation between Systemic Lupus Erythematosus Disease Activity Index, C3, C4 and Anti-dsDNA Antibodies
 Original Article Correlation between Systemic Lupus Erythematosus Disease Activity Index, C3, C4 and Anti-dsDNA Antibodies Col K Narayanan *, Col V Marwaha +, Col K Shanmuganandan #, Gp Capt S Shankar
Original Article Correlation between Systemic Lupus Erythematosus Disease Activity Index, C3, C4 and Anti-dsDNA Antibodies Col K Narayanan *, Col V Marwaha +, Col K Shanmuganandan #, Gp Capt S Shankar
Significance of Anti-C1q Antibodies in Patients with Systemic Lupus Erythematosus as A Marker of Disease Activity and Lupus Nephritis
 THE EGYPTIAN JOURNAL OF IMMUNOLOGY Vol. 23 (1), 2016 Page: 00-00 Significance of Anti-C1q Antibodies in Patients with Systemic Lupus Erythematosus as A Marker of Disease Activity and Lupus Nephritis 1
THE EGYPTIAN JOURNAL OF IMMUNOLOGY Vol. 23 (1), 2016 Page: 00-00 Significance of Anti-C1q Antibodies in Patients with Systemic Lupus Erythematosus as A Marker of Disease Activity and Lupus Nephritis 1
Forigerimod plus standard of care for systemic lupus erythematosus
 NIHR Innovation Observatory Evidence Briefing: March 2018 Forigerimod plus standard of care for systemic lupus erythematosus NIHRIO (HSRIC) ID: 2313 NICE ID: 9732 LAY SUMMARY Systemic lupus erythematosus
NIHR Innovation Observatory Evidence Briefing: March 2018 Forigerimod plus standard of care for systemic lupus erythematosus NIHRIO (HSRIC) ID: 2313 NICE ID: 9732 LAY SUMMARY Systemic lupus erythematosus
High Impact Rheumatology
 High Impact Rheumatology Systemic Lupus Erythematosus Bernard Rubin, DO MPH Case 1: History A 45-year-old woman presents with severe dyspnea and cough. She was in excellent health until 4 weeks ago when
High Impact Rheumatology Systemic Lupus Erythematosus Bernard Rubin, DO MPH Case 1: History A 45-year-old woman presents with severe dyspnea and cough. She was in excellent health until 4 weeks ago when
ENFERMEDADES AUTOINMUNES SISTÉMICAS. Dr. J. María Pego Reigosa
 ENFERMEDADES AUTOINMUNES SISTÉMICAS Dr. J. María Pego Reigosa ABSTRACT NUMBER: 888 PHASE 3 TRIAL RESULTS WITH BLISIBIMOD, A SELECTIVE INHIBITOR OF B-CELL ACTIVATING FACTOR, IN SUBJECTS WITH MODERATE-TO-SEVERE
ENFERMEDADES AUTOINMUNES SISTÉMICAS Dr. J. María Pego Reigosa ABSTRACT NUMBER: 888 PHASE 3 TRIAL RESULTS WITH BLISIBIMOD, A SELECTIVE INHIBITOR OF B-CELL ACTIVATING FACTOR, IN SUBJECTS WITH MODERATE-TO-SEVERE
Benlysta. Benlysta (belimumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.99.01 Subject: Benlysta Page: 1 of 5 Last Review Date: June 22, 2018 Benlysta Description Benlysta (belimumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.99.01 Subject: Benlysta Page: 1 of 5 Last Review Date: June 22, 2018 Benlysta Description Benlysta (belimumab)
Effect of mycophenolate mofetil on the white blood cell count and the frequency of infection in systemic lupus erythematosus.
 Thomas Jefferson University Jefferson Digital Commons Department of Medicine Faculty Papers Department of Medicine 10-22-2015 Effect of mycophenolate mofetil on the white blood cell count and the frequency
Thomas Jefferson University Jefferson Digital Commons Department of Medicine Faculty Papers Department of Medicine 10-22-2015 Effect of mycophenolate mofetil on the white blood cell count and the frequency
Development of SLE among Possible SLE Patients Seen in Consultation: Long-Term Follow-Up. Disclosures. Background. Evidence-Based Medicine.
 Development of SLE among Patients Seen in Consultation: Long-Term Follow-Up Abstract # 1699 May Al Daabil, MD Bonnie L. Bermas, MD Alexander Fine Hsun Tsao Patricia Ho Joseph F. Merola, MD Peter H. Schur,
Development of SLE among Patients Seen in Consultation: Long-Term Follow-Up Abstract # 1699 May Al Daabil, MD Bonnie L. Bermas, MD Alexander Fine Hsun Tsao Patricia Ho Joseph F. Merola, MD Peter H. Schur,
Taming the wolf: Treating to target, treating to remission new strategies for SLE
 Taming the wolf: Treating to target, treating to remission new strategies for SLE Ronald van Vollenhoven Seattle, April 28, 2017 Disclosures Research support, consultancy: Abbott (AbbVie), Biotest, BMS,
Taming the wolf: Treating to target, treating to remission new strategies for SLE Ronald van Vollenhoven Seattle, April 28, 2017 Disclosures Research support, consultancy: Abbott (AbbVie), Biotest, BMS,
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: belimumab_benlysta 6/2011 2/2018 2/2019 3/2018 Description of Procedure or Service Belimumab (Benlysta) is
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: belimumab_benlysta 6/2011 2/2018 2/2019 3/2018 Description of Procedure or Service Belimumab (Benlysta) is
Lupuzor/P140 peptide in patients with systemic lupus erythematosus: a randomised, double-blind, placebo-controlled phase IIb clinical trial
 ARD Online First, published on November 21, 2012 as 10.1136/annrheumdis-2012-202460 Clinical and epidemiological research 1 ImmuPharma, Mulhouse, France 2 Servicio de Reumatología, Hospital Interzonal
ARD Online First, published on November 21, 2012 as 10.1136/annrheumdis-2012-202460 Clinical and epidemiological research 1 ImmuPharma, Mulhouse, France 2 Servicio de Reumatología, Hospital Interzonal
Benlysta. Benlysta (belimumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Benlysta Page: 1 of 5 Last Review Date: December 3, 2015 Benlysta Description Benlysta (belimumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Benlysta Page: 1 of 5 Last Review Date: December 3, 2015 Benlysta Description Benlysta (belimumab)
Disease activity patterns in a monocentric cohort of SLE patients: a seven-year follow-up study
 Disease activity patterns in a monocentric cohort of SLE patients: a seven-year follow-up study M. Zen, N. Bassi, L. Nalotto, M. Canova, S. Bettio, M. Gatto, A. Ghirardello, L. Iaccarino, L. Punzi, A.
Disease activity patterns in a monocentric cohort of SLE patients: a seven-year follow-up study M. Zen, N. Bassi, L. Nalotto, M. Canova, S. Bettio, M. Gatto, A. Ghirardello, L. Iaccarino, L. Punzi, A.
Systemic Lupus Erythematosus (SLE) Epidemiology of SLE (United States)
 1:10-2:25pm Closing the Loop on Lupus: Primary Care s Key Role in the Elusive Diagnosis and Management of Patients SPEAKER Richard Sadovsky, MD Richard Furie, MD Disclosures This session is supported by
1:10-2:25pm Closing the Loop on Lupus: Primary Care s Key Role in the Elusive Diagnosis and Management of Patients SPEAKER Richard Sadovsky, MD Richard Furie, MD Disclosures This session is supported by
Arthritis & Rheumatology Clinics of Kansas PATIENT EDUCATION SYSTEMIC LUPUS ERYTHEMATOSUS
 Arthritis & Rheumatology Clinics of Kansas PATIENT EDUCATION SYSTEMIC LUPUS ERYTHEMATOSUS Introduction: There is perhaps no rheumatic disease that evokes so much fear and confusion among both patients
Arthritis & Rheumatology Clinics of Kansas PATIENT EDUCATION SYSTEMIC LUPUS ERYTHEMATOSUS Introduction: There is perhaps no rheumatic disease that evokes so much fear and confusion among both patients
Evidence Based Treatment of SLE with Treatment Algorithm. Dr. Md. Mujibur Rahman Professor of Medicine Shaheed Suhrawardy Medical College
 Evidence Based Treatment of SLE with Treatment Algorithm Dr. Md. Mujibur Rahman Professor of Medicine Shaheed Suhrawardy Medical College Natural Histoty Inflammatory multisystem disease Onset usually between
Evidence Based Treatment of SLE with Treatment Algorithm Dr. Md. Mujibur Rahman Professor of Medicine Shaheed Suhrawardy Medical College Natural Histoty Inflammatory multisystem disease Onset usually between
Long-term impact of belimumab on health-related quality of life and fatigue in patients with systemic lupus erythematosus: 6 years of treatment
 Article type : Original Article Long-term impact of belimumab on health-related quality of life and fatigue in patients with systemic lupus erythematosus: 6 years of treatment Vibeke Strand 1 (MD, MACR,
Article type : Original Article Long-term impact of belimumab on health-related quality of life and fatigue in patients with systemic lupus erythematosus: 6 years of treatment Vibeke Strand 1 (MD, MACR,
How the Innate Immune System Profiles Pathogens
 How the Innate Immune System Profiles Pathogens Receptors on macrophages, neutrophils, dendritic cells for bacteria and viruses Broad specificity - Two main groups of bacteria: gram positive, gram-negative
How the Innate Immune System Profiles Pathogens Receptors on macrophages, neutrophils, dendritic cells for bacteria and viruses Broad specificity - Two main groups of bacteria: gram positive, gram-negative
Immunopathogenesis of SLE and Rationale for Biologic Therapy
 Immunopathogenesis of SLE and Rationale for Biologic Therapy Peter Lipsky, MD Editor, Arthritis Research and Therapy Bethesda, MD Environmental Triggers, Gender, and Genetic Factors Contribute to SLE Pathogenesis
Immunopathogenesis of SLE and Rationale for Biologic Therapy Peter Lipsky, MD Editor, Arthritis Research and Therapy Bethesda, MD Environmental Triggers, Gender, and Genetic Factors Contribute to SLE Pathogenesis
Erythrocyte-bound C4d in combination with complement and autoantibody status for the monitoring of SLE
 To cite: Merrill JT, Petri MA, Buyon J, et al. Erythrocytebound C4d in combination with complement and autoantibody status for the monitoring of SLE. Lupus Science & Medicine 2018;5:e000263. doi:10.1136/
To cite: Merrill JT, Petri MA, Buyon J, et al. Erythrocytebound C4d in combination with complement and autoantibody status for the monitoring of SLE. Lupus Science & Medicine 2018;5:e000263. doi:10.1136/
The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters.
 Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebocontrolled, multicentre study
Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebocontrolled, multicentre study
BENLYSTA PRODUCT INFORMATION
 BENLYSTA PRODUCT INFORMATION NAME OF THE MEDICINE The active ingredient of BENLYSTA is belimumab (rmc). DESCRIPTION Belimumab is a fully human IgG1λ monoclonal antibody specific for soluble human B Lymphocyte
BENLYSTA PRODUCT INFORMATION NAME OF THE MEDICINE The active ingredient of BENLYSTA is belimumab (rmc). DESCRIPTION Belimumab is a fully human IgG1λ monoclonal antibody specific for soluble human B Lymphocyte
Belimumab for the treatment of autoantibody-positive systemic lupus erythematosus.
 Belimumab for the treatment of autoantibodypositive systemic lupus erythematosus. ERG REPORT: ERRATA SHEET Page / location Original Change / Replacement Pg 13 para 3, last line P = 0.027 P = 0.0207 Pg
Belimumab for the treatment of autoantibodypositive systemic lupus erythematosus. ERG REPORT: ERRATA SHEET Page / location Original Change / Replacement Pg 13 para 3, last line P = 0.027 P = 0.0207 Pg
UNDERSTANDING SYSTEMIC LUPUS ERYTHEMATOSUS
 UNDERSTANDING SYSTEMIC LUPUS ERYTHEMATOSUS Stacy Kennedy, M.D.,M.B.A. October 20, 2012 Agenda What is lupus Who is affected Causes of lupus Symptoms and organ involvement Diagnosis Treatment Pregnancy
UNDERSTANDING SYSTEMIC LUPUS ERYTHEMATOSUS Stacy Kennedy, M.D.,M.B.A. October 20, 2012 Agenda What is lupus Who is affected Causes of lupus Symptoms and organ involvement Diagnosis Treatment Pregnancy
9/25/2013 SYSTEMIC LUPUS ERYTHEMATOSUS (SLE)
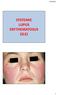 SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) 1 Other Types of Lupus Discoid Lupus Erythematosus Lupus Pernio --- Sarcoidosis Lupus Vulgaris --- Tuberculosis of the face Manifestations of SLE Fever Rashes Arthritis
SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) 1 Other Types of Lupus Discoid Lupus Erythematosus Lupus Pernio --- Sarcoidosis Lupus Vulgaris --- Tuberculosis of the face Manifestations of SLE Fever Rashes Arthritis
Late onset systemic lupus erythematosus in southern Chinese. Citation Annals Of The Rheumatic Diseases, 1998, v. 57 n. 7, p.
 Title Late onset systemic lupus erythematosus in southern Chinese Author(s) Ho, CTK; Mok, CC; Lau, CS; Wong, RWS Citation Annals Of The Rheumatic Diseases, 1998, v. 57 n. 7, p. 437-440 Issued Date 1998
Title Late onset systemic lupus erythematosus in southern Chinese Author(s) Ho, CTK; Mok, CC; Lau, CS; Wong, RWS Citation Annals Of The Rheumatic Diseases, 1998, v. 57 n. 7, p. 437-440 Issued Date 1998
Mandana Nikpour 1,2, Murray B Urowitz 1*, Dominique Ibanez 1, Paula J Harvey 3 and Dafna D Gladman 1. Abstract
 RESEARCH ARTICLE Open Access Importance of cumulative exposure to elevated cholesterol and blood pressure in development of atherosclerotic coronary artery disease in systemic lupus erythematosus: a prospective
RESEARCH ARTICLE Open Access Importance of cumulative exposure to elevated cholesterol and blood pressure in development of atherosclerotic coronary artery disease in systemic lupus erythematosus: a prospective
Systemic Lupus Erythematosus among Jordanians: A Single Rheumatology Unit Experience
 Systemic Lupus Erythematosus among Jordanians: A Single Rheumatology Unit Experience Ala M. AlHeresh MD* ABSTRACT Objectives: To study the characteristics of Systemic Lupus Erythematosus in Jordan and
Systemic Lupus Erythematosus among Jordanians: A Single Rheumatology Unit Experience Ala M. AlHeresh MD* ABSTRACT Objectives: To study the characteristics of Systemic Lupus Erythematosus in Jordan and
NIH Public Access Author Manuscript Arthritis Rheum. Author manuscript; available in PMC 2010 September 15.
 NIH Public Access Author Manuscript Published in final edited form as: Arthritis Rheum. 2009 September 15; 61(9): 1143 1151. doi:10.1002/art.24698. Novel Evidence-Based Systemic Lupus Erythematosus Responder
NIH Public Access Author Manuscript Published in final edited form as: Arthritis Rheum. 2009 September 15; 61(9): 1143 1151. doi:10.1002/art.24698. Novel Evidence-Based Systemic Lupus Erythematosus Responder
Predictors of arthritis in pediatric patients with lupus
 Sule et al. Pediatric Rheumatology (2015) 13:30 DOI 10.1186/s12969-015-0027-7 RESEARCH ARTICLE Open Access Predictors of arthritis in pediatric patients with lupus SD Sule 1*, DG Moodalbail 2, J Burnham
Sule et al. Pediatric Rheumatology (2015) 13:30 DOI 10.1186/s12969-015-0027-7 RESEARCH ARTICLE Open Access Predictors of arthritis in pediatric patients with lupus SD Sule 1*, DG Moodalbail 2, J Burnham
Policy. Background
 Last Review Status/Date: December 2016 Page: 1 of 11 Summary Systemic lupus erythematosus (SLE) is an autoimmune connective tissue disease that can be difficult to diagnose because patients often present
Last Review Status/Date: December 2016 Page: 1 of 11 Summary Systemic lupus erythematosus (SLE) is an autoimmune connective tissue disease that can be difficult to diagnose because patients often present
Definition Chronic autoimmune disease The body s immune system starts attacking itself Can affect most organs and tissues in the body Brain, lungs, he
 LIVING WITH SYSTEMIC LUPUS ERYTHEMATOSUS Stacy Kennedy, M.D.,M.B.A. Rowan Diagnostic Clinic Salisbury, N.C. May 11, 2013 Agenda What is lupus Who is affected Causes of lupus Symptoms and organ involvement
LIVING WITH SYSTEMIC LUPUS ERYTHEMATOSUS Stacy Kennedy, M.D.,M.B.A. Rowan Diagnostic Clinic Salisbury, N.C. May 11, 2013 Agenda What is lupus Who is affected Causes of lupus Symptoms and organ involvement
Prior treatment with non-biologic Disease- Modifying Antirheumatic. Not to be used in combination with another biologic DMARD
 Abatacept (Orencia) 1, 2, 7, 11, 13, 14, 18, 24, 31, 44, 48, 49, 51, 53, 55, 57 J0129 Alpha 1 - Proteinase inhibitor (Prolastin-C) 5, 6, 10, 12, 40 Medically Necessary (if all the following criteria apply):
Abatacept (Orencia) 1, 2, 7, 11, 13, 14, 18, 24, 31, 44, 48, 49, 51, 53, 55, 57 J0129 Alpha 1 - Proteinase inhibitor (Prolastin-C) 5, 6, 10, 12, 40 Medically Necessary (if all the following criteria apply):
Research Article Rituximab for Remission Induction and Maintenance in Refractory Systemic Lupus Erythematosus
 Autoimmune Diseases, Article ID 731806, 4 pages http://dx.doi.org/10.1155/2014/731806 Research Article Rituximab for Remission Induction and Maintenance in Refractory Systemic Lupus Erythematosus Fabio
Autoimmune Diseases, Article ID 731806, 4 pages http://dx.doi.org/10.1155/2014/731806 Research Article Rituximab for Remission Induction and Maintenance in Refractory Systemic Lupus Erythematosus Fabio
Association of Plasma B Lymphocyte Stimulator Levels and Disease Activity in Systemic Lupus Erythematosus
 ARTHRITIS & RHEUMATISM Vol. 58, No. 8, August 2008, pp 2453 2459 DOI 10.1002/art.23678 2008, American College of Rheumatology Association of Plasma B Lymphocyte Stimulator Levels and Disease Activity in
ARTHRITIS & RHEUMATISM Vol. 58, No. 8, August 2008, pp 2453 2459 DOI 10.1002/art.23678 2008, American College of Rheumatology Association of Plasma B Lymphocyte Stimulator Levels and Disease Activity in
MEDICAL COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 08/19/14 SECTION: DRUGS LAST REVIEW DATE: LAST CRITERIA REVISION DATE: ARCHIVE DATE:
 RITUXAN (rituximab) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
RITUXAN (rituximab) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
Belimumab for the treatment of autoantibody-active systemic lupus erythematosus
 National Institute for Health and Clinical Excellence Comment 1: the draft remit Single Technology Appraisal (STA) Belimumab for the treatment of autoantibody-active systemic lupus erythematosus Response
National Institute for Health and Clinical Excellence Comment 1: the draft remit Single Technology Appraisal (STA) Belimumab for the treatment of autoantibody-active systemic lupus erythematosus Response
Objective. To assess the efficacy/safety of the B lymphocyte stimulator inhibitor belimumab plus standard therapy compared with placebo plus standard
 ARTHRITIS & RHEUMATISM Vol. 63, No. 12, December 2011, pp 3918 3930 DOI 10.1002/art.30613 2011, American College of Rheumatology A Phase III, Randomized, Placebo-Controlled Study of Belimumab, a Monoclonal
ARTHRITIS & RHEUMATISM Vol. 63, No. 12, December 2011, pp 3918 3930 DOI 10.1002/art.30613 2011, American College of Rheumatology A Phase III, Randomized, Placebo-Controlled Study of Belimumab, a Monoclonal
REPEATED B CELL DEPLETION IN TREATMENT OF REFRACTORY SYSTEMIC LUPUS ERYTHEMATOSUS
 ARD Online First, published on November 3, 2005 as 10.1136/ard.2005.044487 REPEATED B CELL DEPLETION IN TREATMENT OF REFRACTORY SYSTEMIC LUPUS ERYTHEMATOSUS Concise report Kristine P. Ng 1 Maria J. Leandro
ARD Online First, published on November 3, 2005 as 10.1136/ard.2005.044487 REPEATED B CELL DEPLETION IN TREATMENT OF REFRACTORY SYSTEMIC LUPUS ERYTHEMATOSUS Concise report Kristine P. Ng 1 Maria J. Leandro
Lupus The Clinical Perspective
 Lupus The Clinical Perspective Anca D. Askanase, M.D., M.P.H. Associate Professor of Medicine Director Lupus Center Columbia University Medical Center New York-Presbyterian Hospital Systemic Lupus Erythematosus
Lupus The Clinical Perspective Anca D. Askanase, M.D., M.P.H. Associate Professor of Medicine Director Lupus Center Columbia University Medical Center New York-Presbyterian Hospital Systemic Lupus Erythematosus
Systemic Lupus Erythematosus in Libyan Children: Diagnosis and Management
 Research Article imedpub Journals www.imedpub.com ARCHIVES OF MEDICINE DOI: 10.21767/1989-5216.1000276 Abstract Systemic Lupus Erythematosus in Libyan Children: Diagnosis and Management Background: Childhood
Research Article imedpub Journals www.imedpub.com ARCHIVES OF MEDICINE DOI: 10.21767/1989-5216.1000276 Abstract Systemic Lupus Erythematosus in Libyan Children: Diagnosis and Management Background: Childhood
LUPUS CAN DO EVERYTHING, BUT NOT EVERYTHING IS LUPUS LUPUS 101 SLE SUBSETS AUTOIMMUNE DISEASE 11/4/2013 HOWARD HAUPTMAN, MD IDIOPATHIC DISCOID LUPUS
 LUPUS 101 LUPUS CAN DO EVERYTHING, BUT NOT EVERYTHING IS LUPUS HOWARD HAUPTMAN, MD IDIOPATHIC DISCOID LUPUS SLE SUBSETS SUBACUTE CUTANEOUS LUPUS DRUG INDUCED LUPUS NEONATAL LUPUS LATE ONSET LUPUS ANTI-PHOSPHOLIPID
LUPUS 101 LUPUS CAN DO EVERYTHING, BUT NOT EVERYTHING IS LUPUS HOWARD HAUPTMAN, MD IDIOPATHIC DISCOID LUPUS SLE SUBSETS SUBACUTE CUTANEOUS LUPUS DRUG INDUCED LUPUS NEONATAL LUPUS LATE ONSET LUPUS ANTI-PHOSPHOLIPID
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Policy. Section: Medicine Effective Date: January 15, 2015 Subsection: Pathology/Laboratory Original Policy Date: December 5, 2014 Subject:
 Last Review Status/Date: December 2014 Page: 1 of 10 Summary Systemic lupus erythematosus (SLE) is an autoimmune connective tissue disease that can be difficult to diagnose because patients often present
Last Review Status/Date: December 2014 Page: 1 of 10 Summary Systemic lupus erythematosus (SLE) is an autoimmune connective tissue disease that can be difficult to diagnose because patients often present
New Evidence reports on presentations given at ACR Improving Radiographic, Clinical, and Patient-Reported Outcomes with Rituximab
 New Evidence reports on presentations given at ACR 2009 Improving Radiographic, Clinical, and Patient-Reported Outcomes with Rituximab From ACR 2009: Rituximab Rituximab in combination with methotrexate
New Evidence reports on presentations given at ACR 2009 Improving Radiographic, Clinical, and Patient-Reported Outcomes with Rituximab From ACR 2009: Rituximab Rituximab in combination with methotrexate
Noninfectious Causes of Fever in 128 Patients with Systemic Lupus Erythematosus
 Iran J Public Health, Vol. 48, No.1, Jan 2019, pp.62-68 Original Article Noninfectious Causes of Fever in 128 Patients with Systemic Lupus Erythematosus Feng GUO 1, Jianmei CHEN 1, Yu XIE 1, *Xueping ZHOU
Iran J Public Health, Vol. 48, No.1, Jan 2019, pp.62-68 Original Article Noninfectious Causes of Fever in 128 Patients with Systemic Lupus Erythematosus Feng GUO 1, Jianmei CHEN 1, Yu XIE 1, *Xueping ZHOU
Efficacy and Safety of Subcutaneous Belimumab in Systemic Lupus Erythematosus
 ARTHRITIS & RHEUMATOLOGY Vol. 69, No. 5, May 2017, pp 1016 1027 DOI 10.1002/art.40049 VC 2017 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of
ARTHRITIS & RHEUMATOLOGY Vol. 69, No. 5, May 2017, pp 1016 1027 DOI 10.1002/art.40049 VC 2017 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of
Section: Medicine Effective Date: January 15, 2016 Subsection: Pathology/Laboratory Original Policy Date: December 5, 2014 Subject:
 Last Review Status/Date: December 2015 Page: 1 of 11 Summary Systemic lupus erythematosus (SLE) is an autoimmune connective tissue disease that can be difficult to diagnose because patients often present
Last Review Status/Date: December 2015 Page: 1 of 11 Summary Systemic lupus erythematosus (SLE) is an autoimmune connective tissue disease that can be difficult to diagnose because patients often present
Where a licence is displayed above, please note the terms and conditions of the licence govern your use of this document.
 Differences in the diagnosis and management of systemic lupus erythematosus by primary care and specialist providers in the American Indian/Alaska Native population McDougall, J. A.; Helmick, Charles G.;
Differences in the diagnosis and management of systemic lupus erythematosus by primary care and specialist providers in the American Indian/Alaska Native population McDougall, J. A.; Helmick, Charles G.;
Treatment for lupus is evolving. This review summarizes
 172 Treatment of Systemic Lupus Erythematosus A 2012 Update Joan T. Merrill, M.D. Treatment for lupus is evolving. This review summarizes some of the major recommendations for care from the time of the
172 Treatment of Systemic Lupus Erythematosus A 2012 Update Joan T. Merrill, M.D. Treatment for lupus is evolving. This review summarizes some of the major recommendations for care from the time of the
Herbert Struemper 1 Mita Thapar
 Clin Pharmacokinet (2018) 57:717 728 https://doi.org/10.1007/s40262-017-0586-5 ORIGINAL RESEARCH ARTICLE Population Pharmacokinetic and Pharmacodynamic Analysis of Belimumab Administered Subcutaneously
Clin Pharmacokinet (2018) 57:717 728 https://doi.org/10.1007/s40262-017-0586-5 ORIGINAL RESEARCH ARTICLE Population Pharmacokinetic and Pharmacodynamic Analysis of Belimumab Administered Subcutaneously
New Evidence reports on presentations given at EULAR Rituximab for the Treatment of Rheumatoid Arthritis and Vasculitis
 New Evidence reports on presentations given at EULAR 2011 Rituximab for the Treatment of Rheumatoid Arthritis and Vasculitis Report on EULAR 2011 presentations Anti-TNF failure and response to rituximab
New Evidence reports on presentations given at EULAR 2011 Rituximab for the Treatment of Rheumatoid Arthritis and Vasculitis Report on EULAR 2011 presentations Anti-TNF failure and response to rituximab
American College of Rheumatology
 SPECIAL REPORT Highlights from the American College of Rheumatology Meeting Chicago, IL 2011 > Highlights of the American College of Rheumatology 2011 Annual Scientific Meeting > First Lupus Nephritis
SPECIAL REPORT Highlights from the American College of Rheumatology Meeting Chicago, IL 2011 > Highlights of the American College of Rheumatology 2011 Annual Scientific Meeting > First Lupus Nephritis
Review B cell targeted therapies Edward Keystone
 Available online http://arthritis-research.com/contents/7/s3/s13 Review B cell targeted therapies Edward Keystone Department of Medicine, University of Toronto, Ontario, Canada Corresponding author: Edward
Available online http://arthritis-research.com/contents/7/s3/s13 Review B cell targeted therapies Edward Keystone Department of Medicine, University of Toronto, Ontario, Canada Corresponding author: Edward
Improving the Diagnosis and Treatment of Lupus Practical Guidance for the Primary Care Physician
 Improving the Diagnosis and Treatment of Lupus Practical Guidance for the Primary Care Physician Judith A. James, MD, PhD Oklahoma Medical Research Foundation Oklahoma City, Oklahoma Disclosures Judith
Improving the Diagnosis and Treatment of Lupus Practical Guidance for the Primary Care Physician Judith A. James, MD, PhD Oklahoma Medical Research Foundation Oklahoma City, Oklahoma Disclosures Judith
Systemic Lupus Erythematosus
 Systemic Lupus Erythematosus Marc C. Hochberg, MD, MPH Professor of Medicine and Head, Division of Rheumatology University of Maryland School of Medicine CASE: HISTORY A 26-year-old woman is seen for migratory
Systemic Lupus Erythematosus Marc C. Hochberg, MD, MPH Professor of Medicine and Head, Division of Rheumatology University of Maryland School of Medicine CASE: HISTORY A 26-year-old woman is seen for migratory
Systemic Lupus Erythematosus (SLE) Learning Objectives. Presenter Disclosure Information
 9:30 10:45 AM Closing the Loop on Lupus: Primary Care s Key Role in the Elusive Diagnosis and Management of Patients Presenter Disclosure Information The following relationships exist related to this presentation:
9:30 10:45 AM Closing the Loop on Lupus: Primary Care s Key Role in the Elusive Diagnosis and Management of Patients Presenter Disclosure Information The following relationships exist related to this presentation:
.,Dr Ali Alkazzaz Babylon collage of medicine 2016
 .,Dr Ali Alkazzaz Babylon collage of medicine 2016 Lupus history Lupus is the Latin word for wolf 1 st used medically in the 10 th century Described clinically in the 19 th century Butterfly rash in 1845
.,Dr Ali Alkazzaz Babylon collage of medicine 2016 Lupus history Lupus is the Latin word for wolf 1 st used medically in the 10 th century Described clinically in the 19 th century Butterfly rash in 1845
Improving the Diagnosis and Treatment of Lupus
 Improving the Diagnosis and Treatment of Lupus Michelle Petri, MD, MPH Michelle Petri is a Professor of Medicine in the Division of Rheumatology and Director of the Lupus Center at the Johns Hopkins University
Improving the Diagnosis and Treatment of Lupus Michelle Petri, MD, MPH Michelle Petri is a Professor of Medicine in the Division of Rheumatology and Director of the Lupus Center at the Johns Hopkins University
Renal Flare as a Predictor of Incident and Progressive CKD in Patients with Lupus Nephritis
 Article Renal Flare as a Predictor of Incident and Progressive in Patients with Lupus Nephritis Samir V. Parikh,* Haikady N. Nagaraja, Lee Hebert,* and Brad H. Rovin* Summary Background and objectives
Article Renal Flare as a Predictor of Incident and Progressive in Patients with Lupus Nephritis Samir V. Parikh,* Haikady N. Nagaraja, Lee Hebert,* and Brad H. Rovin* Summary Background and objectives
Therapy with belimumab may suppress the response of peripheral blood mononuclear cells to apoptotic cells.
 Biomedical Research 2017; 28 (16): 7167-7171 ISSN 0970-938X www.biomedres.info Therapy with belimumab may suppress the response of peripheral blood mononuclear cells to apoptotic cells. Yi Liu 1#, Pei
Biomedical Research 2017; 28 (16): 7167-7171 ISSN 0970-938X www.biomedres.info Therapy with belimumab may suppress the response of peripheral blood mononuclear cells to apoptotic cells. Yi Liu 1#, Pei
Quality of Life Over Time in Patients With Systemic Lupus Erythematosus
 Arthritis & Rheumatism (Arthritis Care & Research) Vol. 59, No. 2, February 15, 2008, pp 181 185 DOI 10.1002/art.23339 2008, American College of Rheumatology ORIGINAL ARTICLE Quality of Life Over Time
Arthritis & Rheumatism (Arthritis Care & Research) Vol. 59, No. 2, February 15, 2008, pp 181 185 DOI 10.1002/art.23339 2008, American College of Rheumatology ORIGINAL ARTICLE Quality of Life Over Time
ORIGINAL PAPER. autoantibodies are central to the pathogenesis of the disorder, their development must. n INTRODUCTION
 Reumatismo, 2018; 70 (2): 85-91 Antibodies to extractable nuclear antigens (ENAS) in systemic lupus erythematosus patients: correlations with clinical manifestations and disease activity Y. Emad1, T. Gheita1,
Reumatismo, 2018; 70 (2): 85-91 Antibodies to extractable nuclear antigens (ENAS) in systemic lupus erythematosus patients: correlations with clinical manifestations and disease activity Y. Emad1, T. Gheita1,
Definition and treatment of lupus flares measured by the BILAG index
 Rheumatology 2003;42:1372 1379 doi:10.1093/rheumatology/keg382, available online at www.rheumatology.oupjournals.org Advance Access publication 16 June 2003 Definition and treatment of lupus flares measured
Rheumatology 2003;42:1372 1379 doi:10.1093/rheumatology/keg382, available online at www.rheumatology.oupjournals.org Advance Access publication 16 June 2003 Definition and treatment of lupus flares measured
Advanced oxidation protein products as a biomarker of cutaneous lupus erythematosus complicated by nephritis: a case-control study
 Advanced oxidation protein products as a biomarker of cutaneous lupus erythematosus complicated by nephritis: a case-control study Z. Xie 1 *, M. Zhang 2 *, B. Zhao 1, Q. Wang 1, J. Li 1, Y.Y. Liu 1, Y.H.
Advanced oxidation protein products as a biomarker of cutaneous lupus erythematosus complicated by nephritis: a case-control study Z. Xie 1 *, M. Zhang 2 *, B. Zhao 1, Q. Wang 1, J. Li 1, Y.Y. Liu 1, Y.H.
Successful sequential therapy with rituximab and belimumab in patients with active systemic lupus erythematosus: a case series
 ; 36: 643-647. BRIEF PAPER Successful sequential therapy with rituximab and belimumab in patients with active systemic lupus erythematosus: a case series R. Gualtierotti 1, M.O. Borghi 2, M. Gerosa 1,
; 36: 643-647. BRIEF PAPER Successful sequential therapy with rituximab and belimumab in patients with active systemic lupus erythematosus: a case series R. Gualtierotti 1, M.O. Borghi 2, M. Gerosa 1,
Safety and effectiveness of biologic Disease-Modifying Antirheumatic Drugs in elderly patients with rheumatoid arthritis
 1. Title: Safety and effectiveness of biologic Disease-Modifying Antirheumatic Drugs in elderly patients with rheumatoid arthritis 2. Background: The population of older individuals with rheumatoid arthritis
1. Title: Safety and effectiveness of biologic Disease-Modifying Antirheumatic Drugs in elderly patients with rheumatoid arthritis 2. Background: The population of older individuals with rheumatoid arthritis
Study of Flare Assessment in Systemic Lupus Erythematosus Based on Paper Patients
 Arthritis Care & Research Vol. 70, No. 1, January 2018, pp 98 103 DOI 10.1002/acr.23252 2017, The Authors. Arthritis Care & Research published by Wiley Periodicals, Inc. on behalf of American College of
Arthritis Care & Research Vol. 70, No. 1, January 2018, pp 98 103 DOI 10.1002/acr.23252 2017, The Authors. Arthritis Care & Research published by Wiley Periodicals, Inc. on behalf of American College of
Corporate Presentation January 2013
 NASDAQ: ANTH Corporate Presentation January 2013 1 Forward Looking Statements This presentation contains "forward-looking" statements within the meaning of Section 27A of the Securities Act of 1933, as
NASDAQ: ANTH Corporate Presentation January 2013 1 Forward Looking Statements This presentation contains "forward-looking" statements within the meaning of Section 27A of the Securities Act of 1933, as
Review Article Targeting the B-Cell Pathway in Lupus Nephritis: Current Evidence and Future Perspectives
 The Scientific World Journal Volume 2013, Article ID 745239, 5 pages http://dx.doi.org/10.1155/2013/745239 Review Article Targeting the B-Cell Pathway in Lupus Nephritis: Current Evidence and Future Perspectives
The Scientific World Journal Volume 2013, Article ID 745239, 5 pages http://dx.doi.org/10.1155/2013/745239 Review Article Targeting the B-Cell Pathway in Lupus Nephritis: Current Evidence and Future Perspectives
2.0 Synopsis. Adalimumab DE019 OLE (5-year) Clinical Study Report Amendment 1 R&D/06/095. (For National Authority Use Only)
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Humira Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title
2.0 Synopsis Abbott Laboratories Name of Study Drug: Humira Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title
Evaluation of American College of Rheumatology Provisional Composite Response Index in Systemic Sclerosis (CRISS) in the FaSScinate Trial
 Evaluation of American College of Rheumatology Provisional Composite Response Index in Systemic Sclerosis (CRISS) in the FaSScinate Trial D Khanna 1, V Berrocal 1, C Denton 2, A Jahreis 3, H Spotswood
Evaluation of American College of Rheumatology Provisional Composite Response Index in Systemic Sclerosis (CRISS) in the FaSScinate Trial D Khanna 1, V Berrocal 1, C Denton 2, A Jahreis 3, H Spotswood
1.0 Abstract. Title. Keywords. Rationale and Background
 1.0 Abstract Title A Prospective, Multi-Center Study in Rheumatoid Arthritis Patients on Adalimumab to Evaluate its Effect on Synovitis Using Ultrasonography in an Egyptian Population Keywords Synovitis
1.0 Abstract Title A Prospective, Multi-Center Study in Rheumatoid Arthritis Patients on Adalimumab to Evaluate its Effect on Synovitis Using Ultrasonography in an Egyptian Population Keywords Synovitis
